
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 29 January 2021
Sec. Ethnopharmacology
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.631170
 Mengwei Ni1†
Mengwei Ni1† Haojia Wang1†
Haojia Wang1† Miaomiao Wang1
Miaomiao Wang1 Wei Zhou1
Wei Zhou1 Jingyuan Zhang1
Jingyuan Zhang1 Jiarui Wu1*
Jiarui Wu1* Dan Zhang1
Dan Zhang1 Zhiwei Jing2
Zhiwei Jing2 Xinkui Liu1
Xinkui Liu1 Zhishan Wu1
Zhishan Wu1 Siyu Guo1
Siyu Guo1 Shanshan Jia1
Shanshan Jia1 Xiaomeng Zhang1
Xiaomeng Zhang1 Xiaoguang Sheng1
Xiaoguang Sheng1Background: As non-small cell lung cancer (NSCLC) seriously threatens human health, several clinical studies have reported that Chinese herbal injections (CHIs) combined with vinorelbine and cisplatin (NP) are beneficial. This multidimensional network meta-analysis was performed to explore the preferable options among different CHIs for treating NSCLC.
Methods: A literature search was performed in several databases to identify randomized controlled trials (RCTs) of CHIs in the treatment of NSCLC from inception to January 31, 2019. Final included studies met the eligibility criteria and methodological quality recommendations. Data analysis was performed using Stata 13.0 and WinBUGS 14.0 software. Each outcome was presented as an odds ratio and the surface under the cumulative ranking curve value (SCURA). The “scatterplot3d” package in R 3.6.1 software was used to perform multidimensional cluster analysis.
Results: Ultimately, 97 eligible RCTs involving 7,440 patients and 14 CHIs were included in this network meta-analysis. Combined with NP chemotherapy, Kanglaite injection plus NP exhibited a better impact on the clinical effectiveness rate (SCURA probability: 78.34%), and Javanica oil emulsion injection plus NP was better in the performance status (95.44%). Huachansu injection plus NP was dominant in reducing thrombocytopenia (92.67%) and gastrointestinal reactions (92.52%). As to multidimensional cluster analysis, Shenmai injection plus NP was superior considering improving the clinical effectiveness rate, performance status and relieving leukopenia.
Conclusions: The combination of CHIs and NP has a better impact on patients with NSCLC than NP alone. Among them, Shenmai injection plus NP, Kanglaite injection plus NP and Javanica oil emulsion injection plus NP were notable. Nevertheless, more multicenter and better designed RCTs are needed to validate our findings.
The global cancer statistics suggests that the number of new cancer cases and cancer deaths in 2018 are 18.1 million and 9.6 million. Lung cancer, the most commonly diagnosed cancer (11.6%) and the leading cause of cancer death (18.4%), has a poor prognosis, with a five-year survival of only 16.8% (Ferlay et al., 2015; Bray et al., 2018; Ettinger et al., 2013). Based on histology, lung cancer is separated into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) (Siegel et al., 2018). Approximately 85% of lung cancer patients are NSCLC, and in which 50% present at advanced stages at the time of their first diagnosis (La Fleur et al., 2019). With treatments for NSCLC improving in recent years, platinum-based two-drug combination chemotherapy regimens have become the primary therapeutic regimens, including vinorelbine plus cisplatin (NP), paclitaxel plus cisplatin, and gemcitabine plus cisplatin (Maione et al., 2011). NP is one of the most commonly used chemotherapy drugs for the clinical treatment of NSCLC, and its antineoplastic efficacy has been endorsed (Rinaldi et al., 2006; Li et al., 2014). However, this treatment is often accompanied by a highly toxic physiological environment and adverse events (Pfister et al., 2004).
In China, the method of using a combination of traditional Chinese medicine (TCM) and chemotherapy has been widely adopted in the treatment of cancer (Qi et al., 2010). The beneficial role of TCM in adjuvant treatment of cancer has been reported in many studies, specifically in retarding cancer progression and ameliorating chemotherapy-induced complications and adverse events (Hofseth and Wargovich, 2007; Konkimalla and Efferth, 2008). Chinese herbal injections (CHIs), an indispensable part of TCM, is considered more and more important in treating cancer. Many studies have shown that the active ingredients of the Aidi injection, such as cantharidin, ginsenosides, and astragalosides, have antitumor and immunomodulatory effects in lung cancer (Huang et al., 2011; Zhang et al., 2014; Cichello et al., 2015; Xiao et al., 2016; Ge et al., 2017; Hsieh et al., 2017; Meng et al., 2018). The active ingredients in ginseng can increase the accumulation of apoptotic proteins in the mitochondrial and downregulate the expression of antiapoptotic proteins. Studies have shown that ginseng can activate macrophages and NK cells, which may be related to its antitumor effect (Yoon et al., 2004; Hsia et al., 2016; Dai et al., 2017; Jiang et al., 2017; Wang J et al., 2018a; Wang JJ et al., 2018b; Majeed et al., 2018).
However, there are various types of CHIs, and the optimal strategy for combining CHIs with chemotherapy for treating NSCLC remains inconclusive, which may cause difficulty for clinicians in clinical treatment. Network meta-analyses (NMAs) can integrate the available comparisons based on clinical trials and simultaneous various interventions to assess their comparative efficacy (Salanti et al., 2014). Recently, NMA has increasingly been considered as a vital methodology by health care decision makers and clinical researchers (Ioannidis, 2006; Edwards et al., 2009; Piccini and Kong, 2011; Laws et al., 2019). This NMA compared the efficacy of combining 14 CHIs with NP for treating NSCLC by quantitatively synthesizing the evidence. The objective of this NMA was to supplement the optimal strategy of NSCLC treatment and to strengthen additional insights for clinical practice in the future. The graphical abstract of this NMA is presented in Figure 1.
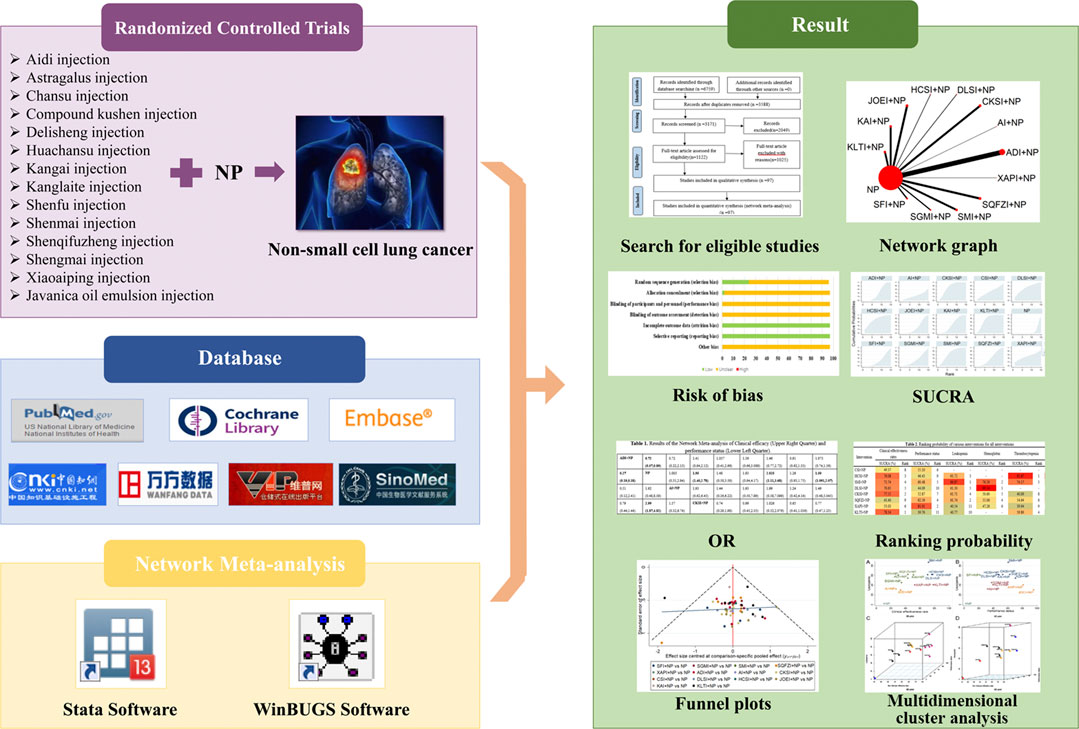
FIGURE 1. Graphical abstract of the network meta-analysis. Note: NP, vinorelbine plus cisplatin; SUCRA, surface under the cumulative ranking curve; OR, odds ratio.
The procedure of the current NMA was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions (Hutton et al., 2015). A completed PRISMA check list is included as Supplementary material Presentation File 1.
In this NMA, a comprehensive literature search was performed using electronic databases including embase, PubMed, the Cochrane Library, the China National Knowledge Infrastructure database, the Wanfang database, the CQVIP database and the China Biology Medicinedisc without restrictions on the publication year, language, or blinding methods. The retrieval period was from inception to January 31, 2019. To identify relevant publications, the method of combining MeSH terms with free text search terms was applied to the search, which focused on three themes: 1) NSCLC, 2) CHIs, and 3) study type (randomized controlled trials (RCTs)). Using PubMed as an example, the following terms were used for NSCLC: “Non-Small-Cell Lung Carcinomas [MeSH Terms],” “Non-Small-Cell Lung Carcinoma,” “Nonsmall Cell Lung Cancer,” “Non Small Cell Lung Carcinoma,” “Non-Small Cell Lung Carcinoma,” and “Non-Small Cell Lung Cancer.” Details on the retrieval strategies are provided in Supplementary material Presentation File 1.
The study was an RCT that compared the relative outcomes of CHIs combined with NP for treating NSCLC. No limitations on language, publication year, or publication status was applied. Only the first publication will be included if there are duplicate studies. Studies were excluded if the study designs and publication types were duplicates, and the full text was unavailable.
The study included patients with NSCLC in stage Ⅲ or Ⅳ and were diagnosed by cytology or pathology without limitations on sex, age, race, region or nationality. Patients with NSCLC who had other tumors were excluded.
The study conducted a comparison with NSCLC patients receiving NP alone or in combination with another CHI, disregarding its course or dosage. Interventions included the combined application of CHIs and NP in either arm of treatment, and the CHIs used (such as Shenfu injection, Shenmai injection, and Shenqifuzheng injection) were applied in clinics for treating tumors and authorized by the China Food and Drug Administration. If patients had complications during the treatment, then some appropriate mitigation measures could be taken. The interventions included surgery, radiotherapy or other cancer treatments were excluded.
The study described efficacy outcomes including the clinical effectiveness rate and the performance status, and adverse drug reactions and adverse drug events (ADRs/ADEs), such as leukopenia, hemoglobin reduction, thrombocytopenia and gastrointestinal reactions (Sylvester, 1980; People's Republic of China Department of Health Management, 1991). 1) Clinical effectiveness rate. According to the WHO criteria for evaluating the efficacy of solid tumors, the clinical effectiveness rate can be divided into four levels: complete response (CR), which means that patients’ visible lesions disappeared completely >1 month after the end of treatment; partial response (PR) means that the tumor area of a single lesion was reduced by ≥ 50%, or the sum of the vertical diameter products of the two largest tumors in multiple lesions was reduced by >50%; stable disease (SD), with no significant change within at least 4 weeks, and estimated tumor size increased by <25% or decreased by <50%; and progressive disease (PD), which means that the estimated size of the new or original lesion had increased by ≥ 25%. The clinical effectiveness rate of this study was calculated by the following formula: the clinical effectiveness rate = (CR + PR)/total number of patients × 100%. 2) Performance status. The performance status was evaluated by Karnofsky performance tstatus (KPS) score. The improvement of KPS score by ≥ 10 points after treatment was considered to improve the motor state; a decrease of ≥10 points in KPS score was considered to reduce the performance status; while the increase or decrease of KPS score <10 points was considered to be stable. Performance improvement rate = number of patients with improved performance/total number of patients × 100%. 3) Leukopenia, hemoglobin reduction, thrombocytopenia and gastrointestinal reactions. They were evaluated according to the “Acute and Subacute Toxicity Standards of Chemotherapy Drugs” formulated by the WHO in 1981. The ADRs/ADEs were divided into 5 grades. The incidence of ADR/ADEs = number of patients with ADRs/total number of patients × 100%.
All citations were managed and organized via NoteExpress software. After duplicate records being removed, the two investigators independently screened the titles and abstracts of the articles. Further assessment of the potential articles was based on full-text versions. Any discrepancies of opinions were resolved by discussion or consultating a third reviewer. The following data were recorded in a form predesigned. 1) Publication information: the first author name and the publication year. 2) Number, age, sex and other characteristics of the enrolled patients with NSCLC, as well as the cancer type and stage. 3) Dosage, duration, treatment cycle and other information of interventions. 4) The measured data on the efficacy outcomes. 5) Important items for quality evaluation, such as blinding, randomized allocation methods, and so on.
The quality of eligible RCTs were evaluate using the Cochrane risk of bias tool (Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0) (Higgins and Green, 2011). The quality assessment was performed by two reviewers, and any discrepancies during this process were solved by discussion or through adjudication by a third investigator. The following domains were assessed: selection bias (random sequence generation and allocation concealment), performance bias (blinding of the participants and personnel), detection bias (blinding of the outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting) and other bias. Each bias has three levels: “low risk”, “unclear risk” and “high risk”.
For each outcome, we carried out a Bayesian NMA to compare the efficacy among the eligible CHIs combined with NP for treating NSCLC. WinBUGS 14.0 software (MRC Biostatistics Unit, Cambridge, UK) was used to perform statistical analysis. The comparative efficacies of the treatments are expressed as odds ratios (ORs) with 95% confidence intervals (95% CIs) for dichotomous outcomes. The differences between the compared groups were deemed significant when the 95% CI of the OR did not contain 1.00. The results of the analysis procedure were based on 200,000 simulation iterations, and 10,000 adaptation iterations were used for the annealing algorithm to eliminate the impact of the initial value. Additionally, all graphs of the NMA were presented using Stata 13.1 software (Stata Corp, College Station, TX) (Shim et al., 2017). The network graph illustrates the relationships of interventions. The width of the line in the network graph is proportional to the number of RCTs included in this comparison, and the node size corresponds to the total sample size of this intervention (Chaimani et al., 2013; Donegan et al., 2013). The surface under the cumulative ranking curve (SUCRA) was used to estimate the ranking probabilities for different CHIs, which ranged from 0 to 100%. A better treatment was indicated by a higher SUCRA (Rücker and Schwarzer, 2015; Trinquart et al., 2016; Chang and Guo, 2017; Cai et al., 2018). A comparison-adjusted funnel plot was also constructed to graphically estimate the publication bias. If the comparison-adjusted funnel plot was symmetrical, there was no obvious publication bias (Trinquart et al., 2012; Krahn et al., 2014). A cluster analysis was also performed to determine the most efficacious injection in the treatment of NSCLC. Interventions located in the upper-right corner were superior to others (Veroniki et al., 2015).
In order to detect the amount and source of heterogeneity among included RCTs, we used the Review Manager 5.3 software (The Nordic Cochrane Center, Copenhagen, Denmark) to conduct a meta-analysis of the results of direct comparison between CHI + NP and NP analysis for each outcome. The heterogeneity within each injection subgroup was analyzed by Cochrane's Q test, and the p-value was used to evaluate the degree of heterogeneity. When p > 0.05, the difference within a group is considered small and the heterogeneity is not obvious, while p < 0.05 is considered that there is obvious heterogeneity among studies. For outcomes with obvious heterogeneity, covariates that may have an impact on heterogeneity were selected, and were analyzed in meta-regression. Use Stata 13.1 software to implement meta-regression, and specify the restricted maximum likelihood (REML) method to estimate the variance component τ2 between studies. Finally, each covariate is introduced into the regression model to determine its relationship with heterogeneity.
The present NMA did not require ethical approval because data was gathered from previously published RCTs.
In this study, the “scatterplot3d” package in R 3.6.1 software (Mathsoft, Cambridge, United States) was used to perform multidimensional cluster analysis of the outcomes. The k-means method was used to cluster these interventions, and the number of clusters was adjusted according to the actual problem. First, all interventions were randomly divided into k initial classes, and the average data of these k classes were used as initial aggregation points. Next, one intervention was classified as the category of the aggregation point closest to it, and the aggregation point of this category updated to the average of the current outcome indicators. Then re-categorize and classify all interventions. Repeat the above steps until all interventions have been assigned. Finally, a three-dimensional stereo Gram that can visually display clustering results of three outcomes is produced. Different categories of interventions were marked with different colors.
Initially, the search strategy yielded 6,759 prospective articles from the electronic databases. After excluding 3,588 duplicates and screening the titles and abstracts, 1,122 articles remained for further evaluation. After detailed examination, a total of 97 RCTs involving 14 CHIs met our selection criteria. Information about the included injections were shown in Supplementary material Presentation File 1. The study identification, screening, and inclusion process is illustrated in Figure 2. The number of studies included for different CHIs was as follows: Aidi injection (20 RCTs), Astragalus injection (1 RCT), Chansu injection (1 RCT), Compound kushen injection (8 RCTs), Delisheng injection (2 RCTs), Huachansu injection (3 RCTs), Kangai injection (7 RCTs), Kanglaite injection (12 RCTs), Shenfu injection (9 RCTs), Shenma injection (9 RCTs), Shenqifuzheng injection (11 RCTs), Shengmai injection (5 RCTs), Xiaoaiping injection (1 RCTs), and Javanica oil emulsion injection (8 RCTs).
The baseline characteristics of each included RCT are summarized in Table 1. Overall, the 97 RCTs enrolled 7,440 patients with NSCLC; 3,747 of them received a combination of CHIs and NP in the experimental group, and 3,693 patients received only NP in the control group. All RCTs reported the sample size and the patients’ age, sex, tumor node metastasis (TNM) stage, expected survival time, and Karnofsky performance status (KPS) score before treatment. Figure 3 presents the network plot of the interventions included in the Bayesian analysis.
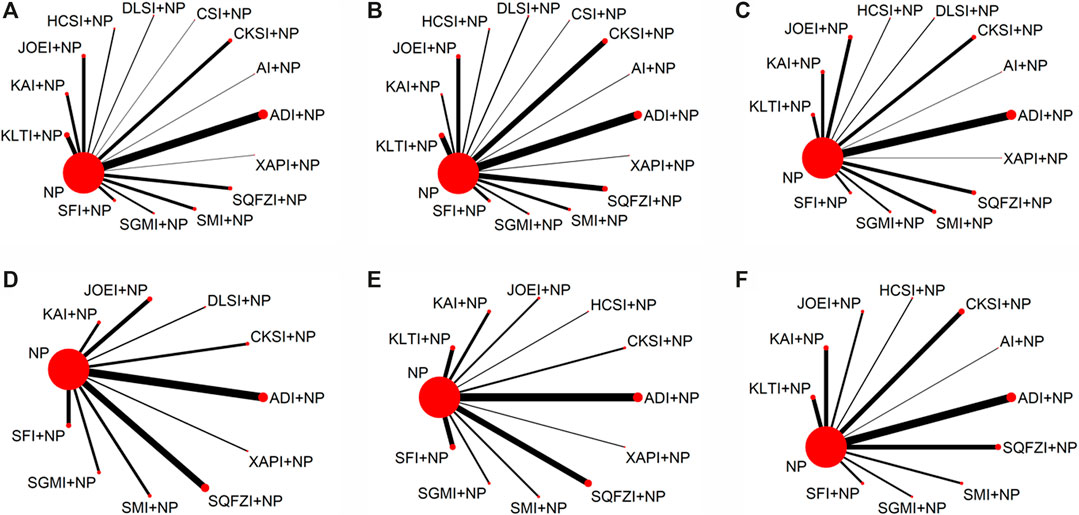
FIGURE 3. Network graph of outcomes. (A) Clinical effectiveness rate; (B) Performance status; (C) Leukopenia; (D) Hemoglobin reduction; (E) Thrombocytopenia; (F) Gastrointestinal reactions. Note: The width of the lines in the network graph is proportional to the number of RCTs used for the comparisons, and the node sizes correspond to the total sample sizes for the treatments. NP, vinorelbine and cisplatin; ADI, Aidi injection; AI, Astragalus injection; CSI, Chansu injection; CKSI, Compound kushen injection; DLSI, Delisheng injection; HCSI, Huachansu injection; JOEI, Javanica oil emulsion injection; KAI, Kangai injection; KLTI, Kanglaite injection; SFI, Shenfu injection; SMI, Shenmai injection; SQFZI, Shenqifuzheng injection; SGMI, Shengmai injection; XAPI, Xiaoaiping injection.
The quality of the RCTs was evaluated by the Cochrane risk of bias tool. Regarding selection bias, 24 of 97 RCTs were rated as “low risk” because 21 RCTs adopted random number tables and three RCTs used lot-drawing for randomization. Three of the included RCTs described allocation concealment by the envelope method. The risk of the remaining RCTs was deemed “unclear”. Not all provided information on the blinding methods, so the performance bias and detection bias were assessed as “unclear”. In addition, since all RCTs did not have incomplete data and selective reporting, their attrition bias and reporting bias were evaluated as “low risk”. For the other bias assessment of the quality of the RCTs, the original studies did not mention the details about other problems that were relative to a high risk of bias; hence, the other bias was remarked as “unclear”. Overall, the quality of the enrolled RCTs was not high, and specific information about the risk of bias is shown in Figure 4.
This study conducted a direct comparison and subgroup analysis on the six outcomes involved. The three outcomes (performance status, leukopenia, and gastrointestinal reaction) with significant heterogeneity in the subgroup analysis were further analyzed by meta regression. Then three factors of age, DDP dose and cancer grade were explored relationship with heterogeneity. The tumor stages included in this study were all stage Ⅲ-Ⅳ, which were consistent and had no obvious clinical heterogeneity. Therefore, this study only performed regression analysis on age and DDP dose. The regression results showed that age was not a source of heterogeneity. The dose of the chemotherapy drug DDP was one of the sources of heterogeneity, which could explain part of the inter-study variation (Adj R-squared = 8.55%) (The details were shown in the Supplementary material Presentation File 1.
A total of 85 RCTs with 14 types of CHIs reported information on the clinical effectiveness rate. The results suggested that six types of CHIs: Aidi injection, Compound kushen injection, Huachansu injection, Kangai injection, Kanglaite injection, and Shenmai injection, in combination with NP presented higher clinical effectiveness rates than NP alone. In addition, significant differences were detected across these five types of CHIs compared with NP (Table 2).
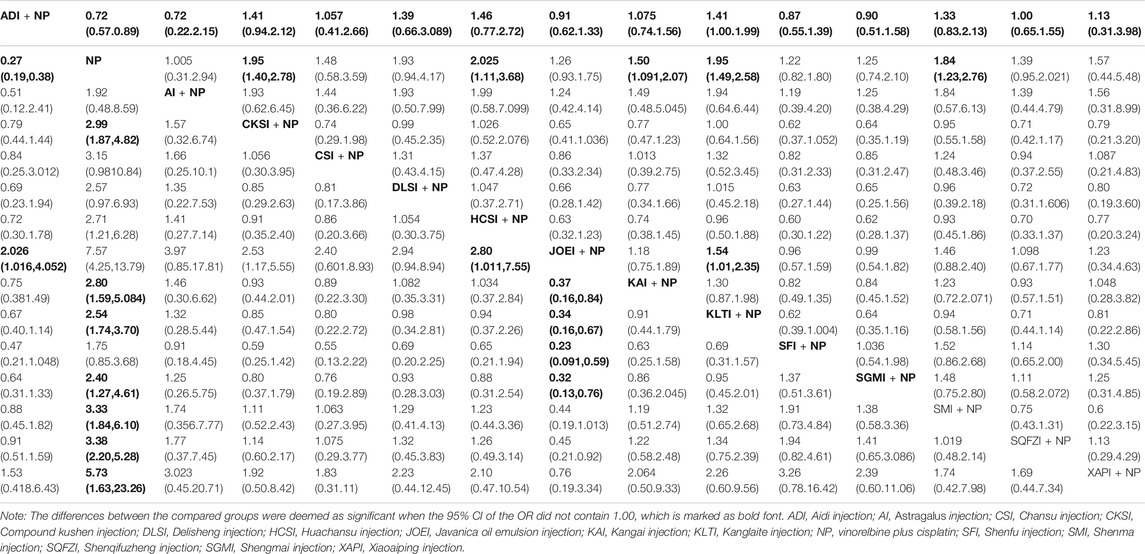
TABLE 2. Results of the network meta-analysis of the clinical effectiveness rate (upper right quarter) and the performance status (lower left quarter).
Compared with Javanica oil emulsion injection plus NP, Kanglaite injection plus NP might hold greater potential for increasing the clinical effectiveness rate (Table 2). Figure 5A presents the cumulative probabilities (SUCRA values) that 14 types of CHIs combined with NP improve the clinical effectiveness rate. The combination of Kanglaite injection and NP was associated with the highest probability of being the best option for improving the clinical effectiveness rate (78.34%), followed by Compound kushen injection plus NP (77.55%) and Huachansu injection plus NP (76.08%). The SUCRA values of each intervention in terms of different outcomes are presented in Table 3.
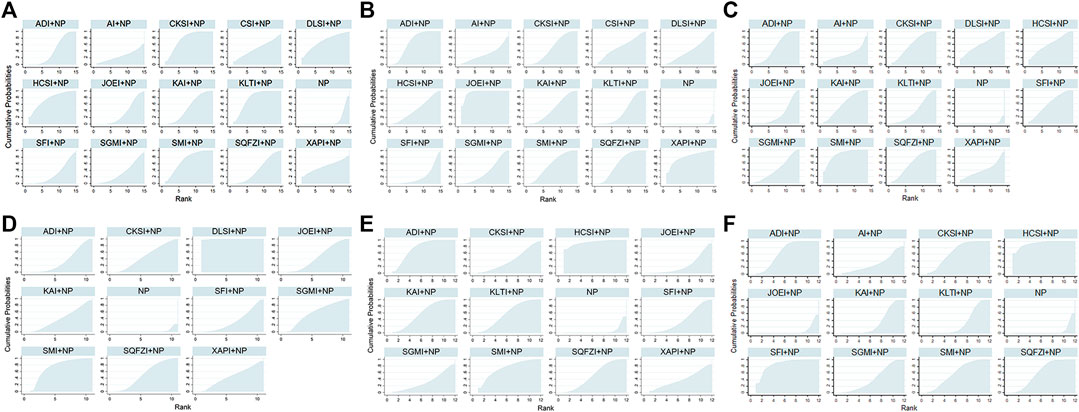
FIGURE 5. Plot of the surface under the cumulative ranking curves for outcomes. (A) Clinical effectiveness rate; (B) Performance status; (C) leukopenia; (D) hemoglobin reduction; (E) thrombocytopenia; (F) gastrointestinal reactions. Note: The surface under the cumulative ranking curve (SUCRA) was used to estimate the ranking probabilities for different CHIs, which ranged from 0% to 100%. A better treatment is indicated by a higher SUCRA value. NP, vinorelbine and cisplatin; ADI, Aidi injection; AI, Astragalus injection; CSI, Chansu injection; CKSI, Compound kushen injection; DLSI, Delisheng injection; HCSI, Huachansu injection; JOEI, Javanica oil emulsion injection; KAI, Kangai injection; KLTI, Kanglaite injection; SFI, Shenfu injection; SMI, Shenmai injection; SQFZI, Shenqifuzheng injection; SGMI, Shengmai injection; XAPI, Xiaoaiping injection.
A total of 63 RCTs contributed to the evidence network for performance status across the 14 types of CHIs. The results indicated that patients receiving 10 types of CHIs: Aidi injection, Compound kushen injection, Huachansu injection, Javanica oil emulsion injection, Kangai injection, Kanglaite injection, Shengmai injection, Shenmai injection, Shenqifuzheng injection, and Xiaoaiping injection, plus NP exhibited considerable improvements in performance status relative to those receiving NP alone. There were significant differences between these groups (Table 3). Compared with Aidi injection plus NP, Compound kushen injection plus NP, Huachansu injection plus NP, Kangai injection plus NP, Kanglaite injection plus NP, Shenfu injection plus NP and Shengmai injection plus NP, Javanica oil emulsion injection plus NP might hold greater potential for improvements in performance status (Table 2). The combination of Javanica oil emulsion injection and NP seemed to be the best intervention for performance status, with a SUCRA value of 95.44%, followed by Xiaoaiping injection plus NP (81.01%) and Aidi injection plus NP (70.37%) (Figure 5B). The SUCRA values of the other CHIs are summarized in Table 3.
There were 78 RCTs with 13 CHIs involved in the NMA concerning leukopenia. As shown in Table 4, Aidi injection, Compound kushen injection, Delisheng injection, Huachansu injection, Javanica oil emulsion injection, Kangai injection, Kanglaite injection, Shenfu injection, Shengmai injection, Shenmai injection and Shenqifuzheng injection were 11 injections combined with NP, which demonstrated a favorable trend for relieving leukopenia compared with NP alone. There were significant differences between these groups. Compared with Javanica oil emulsion injection plus NP, Shenmai injection plus NP might hold greater potential for relieving leukopenia. The results of the SUCRA analysis showed that Shenmai injection plus NP (86.87%) seemed to be the most tolerable option for significantly relieving leukopenia, followed by Shenqifuzheng injection plus NP (61.74%) and Huachansu injection plus NP (61.72%) (Figure 5C). The SUCRA values of each treatment in terms of different outcomes are displayed in Table 3.
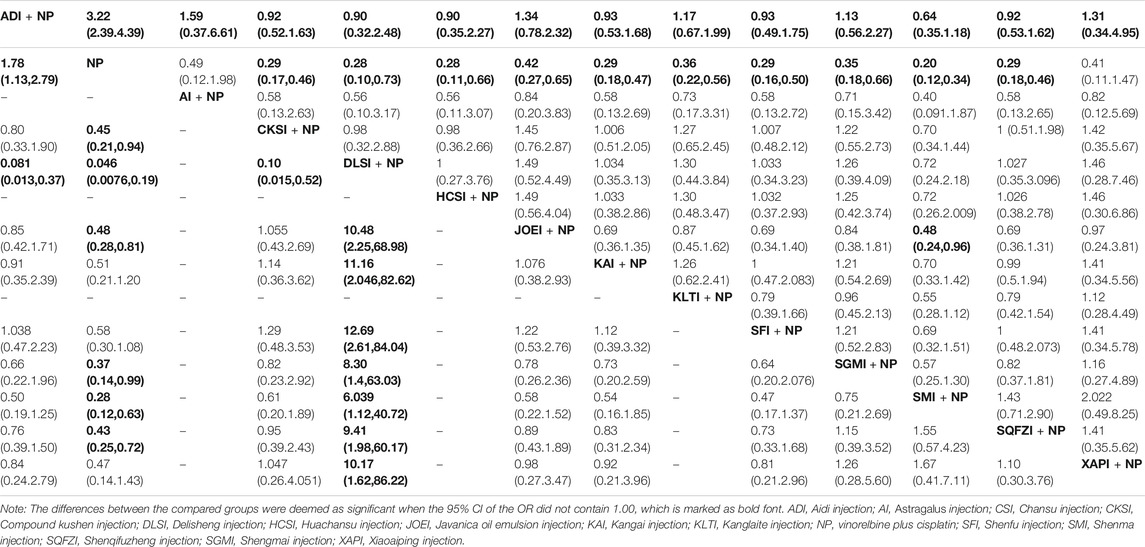
TABLE 4. Results of the network meta-analysis of leukopenia (upper-right quarter) and hemoglobin reduction (lower left quarter).
A total of 27 RCTs with 10 CHIs provided information on hemoglobin reduction in the NMA. The results indicated that Aidi injection, Compound kushen injection, Delisheng injection, Javanica oil emulsion injection, Shengmai injection, Shenmai injection and Shenqifuzheng injection in combination with NP were significantly different from NP alone with respect to lowering the level of hemoglobin (Table 4). The SUCRA results showed that Delisheng injection combined with NP (99.54%) might be a more suitable option than the other types of CHIs for patients with NSCLC experiencing hemoglobin reduction, followed by Shenmai injection plus NP (76.26%) and Shengmai injection plus NP (60.46%) (Figure 5D). The SUCRA values of the other options are shown in Table 3.
Data on thrombocytopenia were available for 36 RCTs involving 11 CHIs. The results showed that the combination of Aidi injection, Huachansu injection, Kangai injection, Kanglaite injection, Shenfu injection, Shenmai injection or Shenqifuzheng injection with NP could significantly improve thrombocytopenia compared with NP alone (Table 5). The SUCRA results illustrated that Huachansu injection plus NP (92.67%), Aidi injection plus NP (76.20%) and Shenmai injection plus NP (74.17%) were regarded as more efficient in relieving thrombocytopenia than the other types of CHIs. (Figure 5E). The SUCRA values of each intervention in terms of different outcomes are shown in Table 3.
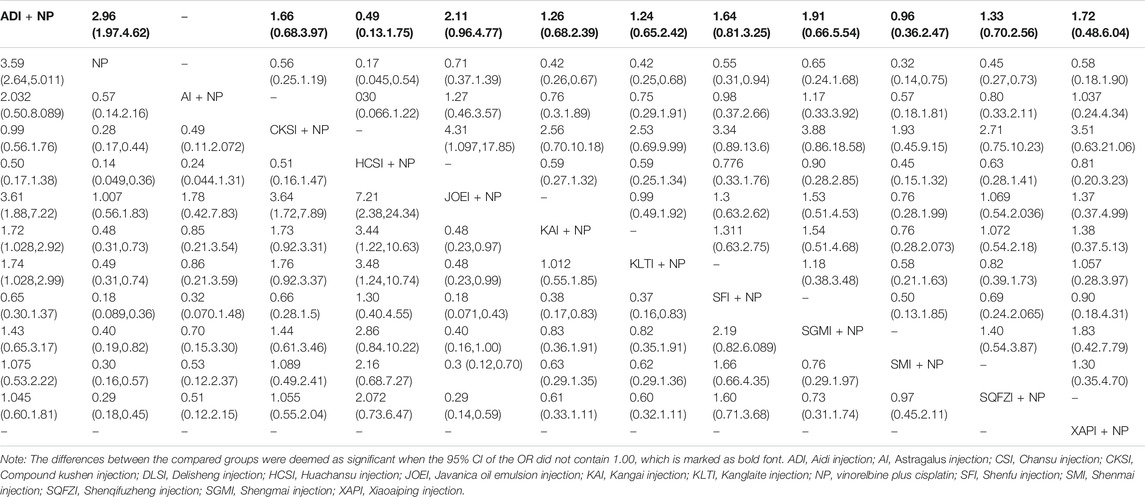
TABLE 5. Results of the network meta-analysis of thrombocytopenia (upper-right quarter) and gastrointestinal reaction (lower left quarter).
A total of 62 RCTs with 11 CHIs were included in the NMA focused on gastrointestinal reactions. Table 5 presents evidence that the combination of Aidi injection, Compound kushen injection, Huachansu injection, Kangai injection, Kanglaite injection, Shenfu injection, Shengmai injection, Shenmai injection or Shenqifuzheng injection with NP can reduce the risk of gastrointestinal reactions compared with NP alone. The superiority of Huachansu injection plus NP (92.52%), Shenfu injection plus NP (87.61%) and Compound kushen injection plus NP (68.01%) over other types of CHIs in relieving gastrointestinal reactions was further confirmed via SUCRA analysis (Figure 5F). The SUCRA values of the other CHIs are summarized in Table 3.
The cluster analysis was conducted based on the SUCRA values to estimate the safest and most effective treatments. Concerning the efficacy outcomes, the results of the cluster analysis (Figure 6A B) illustrated that among the interventions, Shenmai injection plus NP was superior in improving the clinical effectiveness rate and the performance status and relieving leukopenia. In addition, Kanglaite injection plus NP exhibited a better impact on the clinical effectiveness rate, and Javanica oil emulsion injection plus NP was associated with a preferable response in performance status.
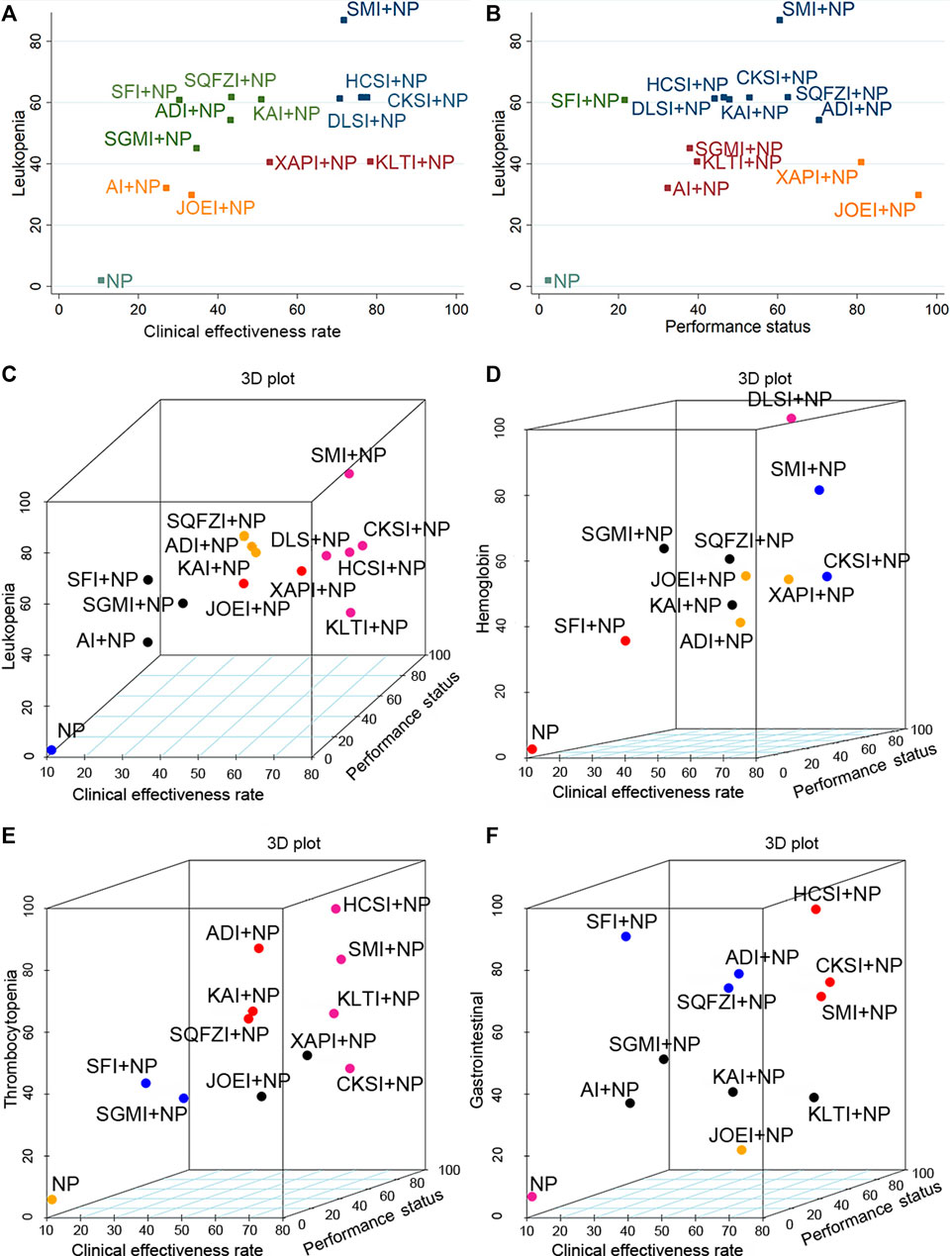
FIGURE 6. Plots of the cluster analyses for all types of outcomes. (A) Clinical effectiveness rate (x-axis) and leukopenia (y-axis); (B) Performance status (x-axis) and leukopenia (y-axis); (C) Clinical effectiveness rate (x-axis), performance status (y-axis), and leukopenia (z-axis); (D) Clinical effectiveness rate (x-axis), performance status (y-axis), and hemoglobin (z-axis); (E) Clinical effectiveness rate (x-axis), performance status (y-axis), and thrombocytopenia (z-axis); (F) Clinical effectiveness rate (x-axis), performance status (y-axis), and gastrointestinal reactions (z-axis). Note: Interventions with the same color belong to the same cluster, and interventions located in the upper-right corner indicate optimal therapy for two different outcomes. NP, vinorelbine and cisplatin; ADI, Aidi injection; AI, Astragalus injection; CSI, Chansu injection; CKSI, Compound kushen injection; DLSI, Delisheng injection; HCSI, Huachansu injection; JOEI, Javanica oil emulsion injection; KAI, Kangai injection; KLTI, Kanglaite injection; SFI, Shenfu injection; SMI, Shenmai injection; SQFZI, Shenqifuzheng injection; SGMI, Shengmai injection; XAPI, Xiaoaiping injection.
In the multidimensional cluster analysis of the three outcomes of the clinical effectiveness rate, the performance status, and alleviation of leukopenia, NP ranked the worst in relative ranking, and Shenmai injection combined with NP was the most noteworthy (Figure 6C). When it comes to the cluster analysis of interventions that reported the clinical effectiveness rate, the performance status, and alleviation of hemoglobin reduction, NP performed the worst in the comprehensive ranking, and Delisheng injection combined with NP chemotherapy may be the best intervention (Figure 6D). Among the interventions that simultaneously reported the clinical effectiveness rate, the performance status, and relieving thrombocytopenia, the combination of Huachansu injection and NP performed best in the cluster analysis (Figure 6E). And it showed that, Huachansu injection combined with NP were dominant in the comprehensive ranking of the clinical effectiveness rate, the performance status, and alleviation of gastrointestinal reactions (Figure 6F).
Publication bias was measured by comparison-adjusted funnel plots for efficacy outcomes in this NMA. Visual inspections of the clinical effectiveness rate and performance status revealed that the included RCTs were distributed relatively symmetrically and based on the zero line, and the angle between the adjusted auxiliary line and the zero line was small. Therefore, there may be minor publication bias (Figure 7).
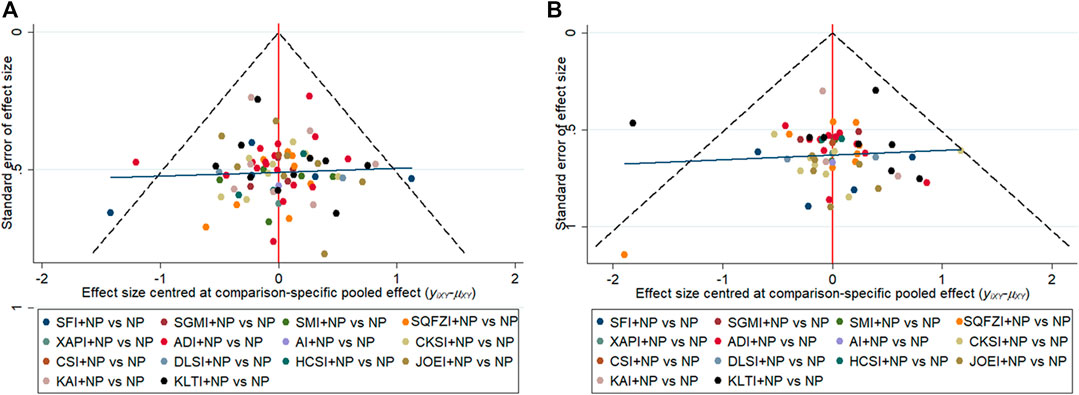
FIGURE 7. Comparison-adjusted funnel plot for outcomes. (A) Clinical effectiveness rate; (B) Performance status. Note: NP, vinorelbine and cisplatin; ADI, Aidi injection; AI, Astragalus injection; CSI, Chansu injection; CKSI, Compound kushen injection; DLSI, Delisheng injection; HCSI, Huachansu injection; JOEI, Javanica oil emulsion injection; KAI, Kangai injection; KLTI, Kanglaite injection; SFI, Shenfu injection; SMI, Shenma injection; SQFZI, Shenqifuzheng injection; SGMI, Shengmai injection; XAPI, Xiaoaiping injection.
Lung cancer, one of the most malignant tumors, seriously threatens human health (Sun, 2004). As many studies have shown, the combination of CHIs and NP is widely adopted in China and has achieved the desired efficacy (Hofseth and Wargovich, 2007; Konkimalla and Efferth, 2008; Qi et al., 2010). This NMA evaluated 14 types of CHIs combined with NP in the treatment of NSCLC and indicated that all eligible CHIs combined with NP have a positive effect on NSCLC patients. According to the results of the cluster analysis and the ORs, all eligible CHIs combined with NP were associated with a more beneficial effect than NP alone. Moreover, Shenmai injection combined with NP was associated with a preferable response in improving the clinical effectiveness rate and performance status and relieving leukopenia. Hence, the efficacy of Shenmai injection combined with NP should be considered for patients with NSCLC. Moreover, Kanglaite injection plus NP might be a suitable option regarding the clinical effectiveness rate, and Javanica oil emulsion injection plus NP was considered more efficient regarding the performance status. However, clinicians should choose different treatments adhering to the specific requirements of the patients.
Shenmai injection is derived from a famous traditional Chinese herbal prescription and has been used as an injection since 1995, in which the primary pharmacological activity constituents are ginsenosides and Ophiopogon (Xia et al., 2008; Lu et al., 2014; Zhou et al., 2018). Several pharmacological studies have shown that Shenmai injection has immunomodulatory effects on tissue damage by inhibiting the release of the inflammatory products of macrophages, such as tumor necrosis factor alpha (TNF-α) and nitric oxide (NO). It also confers protective effects by expanding coronary arteries, which can increase blood supply throughout the body and improve capillary blood circulation (Bai et al., 2011). Javanica oil emulsion injection combined with NP is estimated as the best intervention in improving the performance status. Pharmacological studies have shown that it has good anti-tumor and anti-malarial effects, which may be due to its main active ingredients including fatty acids, such as oleic acid, palmitic acid, and arachidonic acid (Sun et al., 1981; Li et al., 2004). Their anti-tumor mechanisim may be that they can inhibit the growth of tumor cells, block their cell cycle, and specifically destroy the membrane and mitochondria of cancer cells. And it is worth noting that they have the potential to reverse drug resistance (Ding and Suo, 2006; Jiang et al., 2011).
Currently, there has been only one previous NMA that evaluated CHIs combined with NP for treating NSCLC, which was published in 2015 and included 167 RCTs involving 4,480 participants (Tian et al., 2015). In contrast, this NMA has the following merits. First, this NMA included comprehensive coverage of the current and latest research findings with the aim of ensuring the objectivity and authenticity of the results. Second, the eligibility criteria were formulated and established strictly in this NMA. Specifically, the patients of the included RCTs were restricted to having stage Ⅲ or Ⅳ NSCLC to reduce the interference of clinical heterogeneity. Finally, in this NMA, hemoglobin reduction and thrombocytopenia were added as outcome indicators for patients, and the cluster analysis was performed based on the SUCRA values to select the superior CHIs in terms of efficacy.
Several unavoidable limitations of the current NMA should be noted. First, this NMA did not estimate the results of long-term endpoint outcomes due to insufficient information, while the survival time or survival rate were regarded as vital in identifying and judging the therapeutic effects of patients with cancer. Second, some of the included RCTs did not perform a dialectical analysis. Systematic reviews should synthesize the data according different types of symptoms and provide clearer guidelines for clinical practice. Third, the methodological quality of the included RCTs was not high. The percentage of RCTs that provided details in regard to the randomization method, allocation concealment and blinding methods and the sample size in each study were relatively small. For this reason, we recommend that RCTs should be registered ahead of time to ensure the transparency of the process and improve the methodological quality. Second, the implementation of RCTs should abide by the latest clinical diagnosis and treatment guidelines. Third, RCTs of patients with cancer should focus on long-term and valuable endpoints. In terms of the above limitations, more rigorous RCTs with high quality are needed to verify the value of CHIs combined with NP for patients with NSCLC.
In general, the current evidence indicates that CHIs combined with NP might have a more beneficial effect on NSCLC patients than NP alone. Among the 14 interventions, Shenmai injection plus NP, Kanglaite injection plus NP and Javanica oil emulsion injection plus NP were found to be preferable interventions for NSCLC. Owing to dialectical analysis within the study populations and the methodological quality of the included RCTs, more multicenter and better designed RCTs are needed to support the findings in this NMA.
JW, MN, and MW done conception and design of the network meta-analysis; MN, HW, WZ, and JZ performed the network meta-analysis; MN, XL, ZJ, DZ, and XZ assessed the quality of the network meta-analysis; MN, HW, SJ, ZW, and XS analyzed study data; MN, HW, ZW, and SG wrote the paper. All authors read and approved the final version of the manuscript.
The authors acknowledge receipt of the following financial support for the research, authorship, and/or publication of this article: the National Natural Science Foundation of China (grant numbers 81473547, 81673829) and Young Scientists Training Program of Beijing University of Chinese Medicine (grant number BUCM-QNLJ 2019001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.631170/full#supplementary-material.
95% CI, 95% credible interval; ADRs/ADEs, adverse drug reactions/adverse drug events; CHIs, Chinese herbal injections; CR, complete response; KPS, Karnofsky performance status; NMA, network meta-analysis; NSCLC, non-small cell lung cancer; NP, vinorelbine and cisplatin; OR, odds ratio; PD, progressive disease; PR, partial response; RCTs, randomized controlled trials; SD, stable disease; SUCRA, surface under the cumulative ranking curve.
Bai, Y., Yao, H. X., Hu, M. L., Wang, L. R., Jin, L. D., Wang, W. T., et al. (2011). Effects of shenmai injection on pulmonary aquaporin 1 in rats following traumatic brain injury. Chin. Med. J. 124 (3), 457–460. doi:10.3760/cma.j.issn.0366-6999.2011.03.024
Bian, L., Liu, C., Zhong, W., Chen, Y., and Liu, P. (2005). Therapeutic effect of Shenma injection combined with chemotherapy on advanced non-small cell lung cancer. (Chinese). Shandong Med. J. 34, 37–38. doi:10.3969/j.issn.1002-266X.2005.34.018
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68 (6), 394–424. doi:10.3322/caac.21492
Cai, W. Y., Gu, Y. Y., Cui, H. Q., Cao, Y. Y., Wang, X. L., Yao, Y., et al. (2018). The efficacy and safety of mainstream medications for patients with cDMARD-naïve rheumatoid arthritis: a network meta-analysis. Front. Pharmacol. 9, 138. doi:10.3389/fphar.2018.00138
Cao, C. (2009). Clinical study of Huachansu injection combined with NP regimen in the treatment of non-small cell lung cancer. (Chinese). J. Med. Theory Pract. 22 (8), 935–936. doi:10.3969/j.issn.1001-7585.2009.08.025
Chaimani, A., Higgins, J. P., Mavridis, D., Spyridonos, P., and Salanti, G. (2013). Graphical tools for network meta-analysis in STATA. PloS One 8 (10), e76654. doi:10.1371/journal.pone.0076654
Chang, L., and Guo, R. (2017). Comparison of the efficacy among multiple chemotherapeutic interventions combined with radiation therapy for patients with cervix cancer after surgery: a network meta-analysis. Oncotarget 8 (30), 49515–49533. doi:10.18632/oncotarget.17259
Chen, H. A. (2010). Clinical analysis of Shenfu injection combined with NP regimen in the treatment of non-small cell lung cancer. (Chinese). Heb. Med. J. 32 (20), 2892–2893. doi:10.3969/j.issn.1002-7386.2010.20.058
Chen, Q., Chen, Y., Yang, Z., Liu, J., and Fei, R. (2011). The effect on quality of life to advanced stage non-small cell lung cancer patient treated withShengmai injection combined chemotherapy. (Chinese). Chin. J. Chin. Med. 26 (1), 13–15. doi:CNKI:SUN:HNZK.0.2011-01-006
Chen, Q., Yang, Z., Ou, W., Deng, C., and Fei, R. (2010). Effect of Shenma injection combined with chemotherapy on quality of life of patients with advanced non - small cell lung cancer. (Chinese). Hebei. J. TCM 32 (10), 1535–1536.
Chen, X., and Cai, Y. (2007). Clinical observation of Brucea javanica oil emulsion combined with chemotherapy in the treatment of advanced non-small cell lung cancer. (Chinese). J. Clin. Int. Med. 8, 556–557. doi:10.3969/j.issn.1001-9057.2007.08.018
Chen, Y. (2008). Clinical observation of Aidi injection combined with NP regimen in the treatment of advanced non-small cell lung cancer. (Chinese). Nei Mongol. J. Tradit. Chin. Med. 27 (21), 70–71. doi:10.3969/j.issn.1006-0979.2008.11.046
Chen, Y., Li, P., Yang, L., Xue, D., Sun, H., and Yang, H. (2004). “Analysis of therapeutie efect of shengmai injection in decreasing chemothearpeutic toxicity of advanced non-small-cell lung cancer,” in Proceedings of the 2nd international symposium on integrated traditional Chinese and western medicine and TCM oncology. Guangzhou, China, October 1, 2004.
Cichello, S. A., Yao, Q., Dowell, A., Leury, B., and He, X. Q. (2015). Proliferative and inhibitory activity of siberian ginseng (eleutherococcus senticosus) extract on cancer cell lines; A-549, XWLC-05, HCT-116, CNE and beas-2b. Asian Pac. J. Cancer Prev. APJCP 16 (11), 4781–4786. doi:10.7314/apjcp.2015.16.11.4781
Dai, G., and Kong, F. (2008). Clinical observation on 60 cases of non-small-cell lung cancer treated by Aidi injection combined with NP regimen. (Chinese). Chin. J. Clin. Ratl. Drug Use 2 (17), 65–66. doi:10.3969/j.issn.1674-3296.2009.17.039
Dai, P. C., Liu, D. L., Zhang, L., Ye, J., Wang, Q., Zhang, H. W., et al. (2017). Astragaloside IV sensitizes non-small cell lung cancer cells to gefitinib potentially via regulation of SIRT6. Tumour Biol. 39 (4), 1010428317697555. doi:10.1177/1010428317697555
Deng, L. (2014). “Therapeutic effect of Kangai injection combined with chemotherapy on advanced non-small cell lung cancer,” in 2014 Annual conference of chinese medicine, Guangzhou, China, April 6, 2014,
Ding, C. X., and Suo, Y. R. (2006). Research progress on chemical constituents and pharmacology of traditional Chinese medicine Brucea javanica. (Chinese). Chin. Tradit. Pat. Med. 28 (1), 117–120. doi:10.3969/j.issn.1001-1528.2006.01.036
Donegan, S., Williamson, P., D’Alessandro, U., and Tudur Smith, C. (2013). Assessing key assumptions of network meta-analysis: a review of methods. Res. Synth. Methods 4 (4), 291–323. doi:10.1002/jrsm.1085
Dong, X., Wang, Y., Zhou, J., Xu, J., and Li, J. (2009). Brucea javanica oil emulsion combined with NP regimen chemotherapy for non-small cell lung cancer. (Chinese). J. Xi'an Jiaot. Univ. 30 (2), 244–246. doi:CNKI:SUN:XAYX.0.2009-02-028
Du, M., and Shi, M. (2006). Therapeutic effect of chemotherapy combined with brucea javanica oil emulsion on advanced non-small cell lung cancer. (Chinese). J. Basic Clin. Oncol. 2, 151–152. doi:CNKI:SUN:HLZL.0.2006-02-035
Edwards, S. J., Clarke, M. J., Wordsworth, S., and Borrill, J. (2009). Indirect comparisons of treatments based on systematic reviews of randomised controlled trials. Int. J. Clin. Pract. 63 (6), 841–854. doi:10.1111/j.1742-1241.2009.02072.x
Ettinger, D. S., Akerley, W., Borghaei, H., Chang, A. C., Cheney, R. T., Chirieac, L. R., et al. (2013). Non-small cell lung cancer, version 2.2013. J. Natl. Compr. Canc. Netw. 11 (6), 645–653. doi:10.6004/jnccn.2013.0084
Farley, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., et al. (2015). Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Canc. 136 (5), E359–E386. doi:10.1002/ijc.29210
Gao, Y. (2008). Clinical analysis of Aidi injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer. (Chinese). J. Mudanjiang Med. Univ. 3, 67–68. doi:10.1016/j.jep.2018.09.024
Ge, G., Yan, Y., and Cai, H. (2017). Ginsenoside Rh2 inhibited proliferation by inducing ROS mediated ER stress dependent apoptosis in lung cancer cells. Biol. Pharm. Bull. 40 (12), 2117–2124. doi:10.1248/bpb.b17-00463
Geng, L. (2004). Shenqi Fuzheng Injection combined with chemotherapy Treatment of non-small cell lung cancer. (Chinese). J. Med. Forum 25 (17), 29–31. doi:10.3969/j.issn.1672-3422.2004.17.015
Gong, H., and Luo, X. (2008). Clinical observation of Shenfu injection adjuvant chemotherapy in the treatment of elderly patients with advanced non-small cell lung cancer. (Chinese). J. Emerg. Tradit. Chin. Med. 6, 782–783. doi:10.3969/j.issn.1004-745X.2008.06.030
Guo, J., Lu, H., and Wang, L. (2007). Clinical observation on treatment of 63 cases of advanced non-small-cell lung cancer with compound kushen injection combined with NP regimen. (Chinese). J. Xinjiang Med. Univ. 9, 1018–1019. doi:10.3969/j.issn.1009-5551.2007.09.034
Han, Y. (2012). Clinical observation of Aidi injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer. (Chinese). China Pract. Med 7 (17), 166–167. doi:10.3969/j.issn.1673-7555.2012.17.124
Higgins, J. P. T., and Green, S. (2011). Cochrane Handbook for systematic reviews of interventions. New Jersey, NJ, USA; John wiley and sons, 729.
Hofseth, L. J., and Wargovich, M. J. (2007). Inflammation, cancer, and targets of ginseng. J. Nutr. 137 (1 Suppl. l), 183s–185s. doi:10.1093/jn/137.1.183S
Hou, E. (2008). A clinical study on kanglaite injection combined with chemical therapy in the treatment of patients with advanced non–small cell lung cancer. (Chinese). Tradit. Chin. Drug Res. Clin. Pharmacol. 6, 504–506. doi:10.3321/j.issn:1003-9783.2008.06.028
Hsia, T. C., Yu, C. C., Hsiao, Y. T., Wu, S. H., Bau, D. T., Lu, H. F., et al. (2016). Cantharidin impairs cell migration and invasion of human lung cancer NCI-H460 cells via UPA and MAPK signaling pathways. Anticancer Res. 36 (11), 5989–5997. doi:10.21873/anticanres.11187
Hsiah, F. S., Hung, M. H., Wang, C. Y., Chen, Y. L., Hsiao, Y. J., Tsai, M. H., et al. (2017). Inhibition of protein phosphatase 5 suppresses non-small cell lung cancer through AMP-activated kinase activation. Lung Canc. 112, 81–89. doi:10.1016/j.lungcan.2017.07.040
Hu, L., Liao, T., and Liu, S. (2010). Clinical observation on treating Non-small cell lung cancer with Shenma injection plus chemotherapy. (Chinese). Clin. J. Chin. Med. 2 (10), 74–75. doi:10.3969/j.issn.1674-7860.2010.10.046
Hu, W., Ding, J., Jia, S., and Lin, Z. (2008). Therapeutic effect of Shenma injection combined with chemotherapy on advanced lung cancer. (Chinese). J. Liaoning Univ. Tradit. Chin. Med. 11, 108–109. doi:CNKI:SUN:LZXB.0.2008-11-064
Hu, Z., and Sun, S. (2005). Clinical observation of AiDi pro injection plus NP regimen combination in the treatment of advanced non-small-cell lung cancer. (Chinese). Clin. Med. J. China 3, 530–532. doi:10.3969/j.issn.1008-6358.2005.03.064
Huang, D., Zhu, Z., Li, L., and Xiao, B. (2013). Kanglaite injection combined with NP regimen in the treatment of advanced non - small cell lung cancer (NSCLC). (Chinese). Mod. Oncol. 21 (2), 321–323. doi:10.3969/j.issn.1672-4992.2013.02.31
Huang, L., Zhao, H., Huang, B., Zheng, C., Peng, W., and Qin, L. (2011). Acanthopanax senticosus: review of botany, chemistry and pharmacology. Pharmazie 66 (2), 83–97. doi:10.1691/ph.2011.0744
Huang, R., Liu, H., Li, Y., and Chen, S. (2006). Clinical observation of Kangai injection combined with NP regimen in the treatment of advanced non-small cell lung cancer. (Chinese). Mod. Med. J. China 8 (5), 22–24. doi:10.3969/j.issn.1672-9463.2006.05.008
Huang, S., Li, J., Zou, Y., and Guo, Y. (2011). Therapeutic effect of Delisheng combined with NP regimen in the treatment of advanced non-small cell lung cancer. Chinese) China Foreign Med. Treat. 30 (8), 112. doi:10.3969/j.issn.1674-0742.2011.08.084
Huang, X., Huang, C., Lu, Q., and Liao, Z. (2008). Therapeutic effect of Aidi combined with NP regimen in the treatment of 60 elderly patients with non-small cell lung cancer. (Chinese). J. Emerg. Tradit. Chin. Med. 1, 20–21. doi:10.3969/j.issn.1004-745X.2008.01.011
Huang, Y. (2011). Kangai Injection’s enhancement in patients with advanced non-small cell lung cancer. (Chinese). J. China Trait. China Inf. 3 (6), 20–22.
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 162 (11), 777–784. doi:10.7326/M14-2385
Ioannidis, J. P. (2006). Indirect comparisons: the mesh and mess of clinical trials. Lancet 368 (9546), 1470–1472. doi:10.1016/S0140-6736(06)69615-3
Jiang, L., Zhou, Y. M., Chen, M., Zhou, C., and Li, B. (2011). Early evaluation of the response of breast cancer to neoadjuvant chemotherapy with functional MRI. (Chinese). Chin. J. Breast Dis. (Electronic Version) 5 (3), 5–10. doi:10.3969/j.issn.1674-0807.2011.03.002
Jiang, P., and Zhang, X. (2008). Observation on the attenuating effect of Shenqi Fuzheng injection on NP regimen for NSCLC. (Chinese). Chin. Prac. Med. 3 (12), 32–33. doi:10.3969/j.issn.1673-7555.2008.12.017
Jiang, Z., Yang, Y., Yang, Y., Zhang, Y., Yue, Z., Pan, Z., et al. (2017). Ginsenoside Rg3 attenuates cisplatin resistance in lung cancer by downregulating PD-L1 and resuming immune. Biomed. Pharmacother. 96, 378–383. doi:10.1016/j.biopha.2017.09.129
Konkimalla, V. B., and Efferth, T. (2008). Evidence-based Chinese medicine for cancer therapy. J. Ethnopharmacol. 116 (2), 207–210. doi:10.1016/j.jep.2007.12.009
Krahn, U., Binder, H., and Konig, J. (2014). Visualizing inconsistency in network meta-analysis by independent path decomposition. BMC Med. Res. Methodol. 14, 131. doi:10.1186/1471-2288-14-131
La Fleur, L., Falk-Sörqvist, E., Smeds, P., Berglund, A., Sundström, M., Mattsson, J. S., et al. (2019). Mutation patterns in a population-based non-small cell lung cancer cohort and prognostic impact of concomitant mutations in KRAS and TP53 or STK11. Lung Canc. 130, 50–58. doi:10.1016/j.lungcan.2019.01.003
Laws, A., Tao, R., Wang, S., Padhiar, A., and Goring, S. (2019). A comparison of national guidelines for network meta-analysis. Value Health 22 (10), 1178–1186. doi:10.1016/j.jval.2019.05.013
Li, C. H., Liu, M. Y., Liu, W., Li, D. D., and Cai, L. (2014). Randomized control study of nedaplatin or cisplatin concomitant with other chemotherapy in the treatment of advanced non-small cell lung cancer. Asian Pac. J. Cancer Prev. APJCP 15 (2), 731–736. doi:10.7314/apjcp.2014.15.2.731
Li, F., and Cao, H. (2007). Astragalus injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer. (Chinese). Chin. J. Coal Ind. Med. 6, 652. doi:10.3969/j.issn.1007-9564.2007.06.019
Li, H., Li, G., and Yang, F. (2005a). Aidi injection combined with non-small cell lung cancer. (Chinese). J. Med. Forum 16, 55–57. doi:10.3969/j.issn.1672-3422.2005.16.032
Li, J., Cai, H., and Chen, Z. (2018). Effects of kanglaite injection adjuvant NP chemotherapy on lung cancer. (Chinese). J. Gannan Med. Univ. 38 (3), 232–235. doi:10.3969/j.issn.1001-5779.2018.03.008
Li, J. (2014). Clinical study of Shenfu injection combined with NP regimen in the treatment of non-small cell lung cancer. (Chinese). World. J. Integr. Tradit. West. Med. 9 (10), 1081–1082. doi:10.13935/j.cnki.sjzx.141019
Li, S., Ma, N., Wang, G., Zhang, H., and Li, H. (2005b). Shenma injection combined with chemotherapy in the treatment of 30 cases of advanced non-small cell lung cancer. (Chinese). Shandong. J. Tradit. Chin. Med. 24 (9), 532–533. doi:10.3969/j.issn.0257-358X.2005.09.008
Li, X., and Dong, L. (2016). Effect of kanglaite injection combined with chemotherapy on the quality of life and arrest of bone marrow in patients with advanced non –small cell lung cancer. (Chinese) Hebei Med. 22 (4), 543–545. doi:10.3969/j.issn.1006-6233.2016.04.006
Li, X., and Wu, H. (2010). Clinical observation of brucea javanica oil emulsion combined with chemotherapy in the treatment of advanced non-small cell lung cancer. (Chinese). J. Clin. Pulm. Med. 15 (2), 266–267. doi:10.3969/j.issn.1009-6663.2010.02.072
Li, X. W., Wang, H., Qin, W. J., Li, X., Liu, T., and Fan, C. M. (2004). Necrosis and apoptosis induced by brucea javanica oil emulsion in bladder cancer cell. (Chinese)Chin. J. Rehabil. Theory Pract. 10, 163–164. doi:10.3969/j.issn.1006-9771.2004.03.012
Li, Y., Chen, S., and Huang, R. (2007). Clinical observation of Shenqi Fuzheng injection combined with NP regimen in the treatment of advanced non-small cell lung cancer. (Chinese). Mod. Med. J. China 9 (3), 40–41. doi:10.3969/j.issn.1672-9463.2007.03.014
Li, Y. F. (2010). Compound matrine injection combined with chemotherapy (NP) for 32 patients with advanced non-small cellular lung cancer. (Chinese). Medical Inform. 8, 26–28.
Li, Y., Zhou, X., Zhang, T., and Chen, H. (2010). Clinical study of shenqi fuzheng injection on attenuation of non-small cell lung cancer with NP chemotherapy. (Chinese). Chin. J. New Drug 19 (2), 123–126. doi:CNKI:SUN:ZXYZ.0.2010-02-013
Liang, B., Yang, J., and Liu, P. (2005). Effect of Aidi injection plus NP regimen in treatment of advanced non-small-cell lung cancer. (Chinese). J. Med. Forum 1, 28–29. doi:10.3969/j.issn.1672-3422.2005.01.013
Liao, G., Wang, G., Liu, P., Wang, H., Qu, Y., and Xie, G. (2009). Clinical observation of Kanglaite injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer. (Chinese). J. Pract. Ontol. 24 (6), 595–598. doi:CNKI:SUN:SYZZ.0.2009-06-023
Liao, Y. (2016). The clinical effect observation of brucea javanica combined with NP chemotherapy on treatment of advanced non-small-cell lung cancer. (Chinese). J. Liaoning Med. Univ. 37 (2), 37–43. doi:CNKI:SUN:JZYX.0.2016-02-013
Lin, Q., Zhang, M., Sun, Y., Xian, T., Lin, Q. J., Zhang, M., et al. (2007). Clinical observation on 40 cases of advanced non-small-cell lung cancer treated with Aidi injection combined with chemotherapy. (Chinese). J. Beijing Univ. Tradit. Chin. Med. 2, 18–20. doi:10.3969/j.issn.1672-2205.2007.02.008
Liu, J., and Huang, C. (2011). Effect of Shenfu injection on hematopoietic function and quality of life in patients with advanced non-small-cell lung cancer. (Chinese). Chin. Med. Herald 8 (20), 119–120. doi:10.4103/0973-1482.187299
Liu, Y., and Wang, X. (2009). Clinical observation of Aidi combined with chemotherapy in the treatment of non-small cell lung cancer. (Chinese). Chin. Commun. Doctors 11 (15), 95. doi:CNKI:SUN:ZGSQ.0.2009-15-117
Lu, L. Y., Zheng, G. Q., and Wang, Y. (2014). An overview of systematic reviews of shenmai injection for healthcare. Evid. Based Complement. Alternat. Med. 2014, 840650. doi:10.1155/2014/840650
Lu, Q. (2010). Effect of shenqi fuzheng injection on reducing chemotoxicity and quality of life in patients with advanced non-small cell lung cancer. (Chinese). Mod. Tradit. Chin. Med. 30 (6), 43–45. doi:CNKI:SUN:XDZY.0.2010-06-026
Lu, Q., Lei, W., Huang, D., Liu, J., and Huang, C. (2011). The effect of Shenma injection combined with chemotherapy on life quality of patients with advanced non-small cell lung cancer. (Chinese). Chin. J. Chin. Med. 3 (5), 65–66.
Lu, X., Yang, Z., and Deng, C. (2010). Influence of Shenfu injection with chemotherapy on advanced non-small-cell lung cancer immmune function. (Chinese). J. Chin. Med. 25 (6), 1049–1051.
Lv, J. (2008). Shenqi Fuzheng Injection adjuvant chemotherapy Clinical observation of advanced non-small cell lung cancer. (Chinese). Chin. Med. Herald 5 (36), 73–74. doi:10.3969/j.issn.1673-7210.2008.36.045
Lv, X., Zhang, X., and Li, Q. (2004). Clinical study of Kanglaite combined with chemotherapy on quality of life in patients with NSCLC. (Chinese). J. Clin. Pulm. Med. 4, 342–343. doi:10.3969/j.issn.1009-6663.2004.04.014
Ma, R., Dong, W., Hu, X., Yan, F., and Liu, X. (2005). Clinical observation of Delisheng injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer. (Chinese). J. Mod. Oncol. 6, 111–112. doi:10.3969/j.issn.1672-4992.2005.06.051
Maione, P., Rossi, A., Bareschino, M. A., Sacco, P. C., Schettino, C., Falanga, M., et al. (2011). Factors driving the choice of the best second-line treatment of advanced NSCLC. Rev. Recent Clin. Trials 6 (1), 44–51. doi:10.2174/157488711793980192
Majeed, F., Malik, F. Z., Ahmed, Z., Afreen, A., Afzal, M. N., and Khalid, N. (2018). Ginseng phytochemicals as therapeutics in oncology: recent perspectives. Biomed. Pharmacother. 100, 52–63. doi:10.1016/j.biopha.2018.01.155
Meng, Q., Pan, J., Liu, Y., Chen, L., and Ren, Y. (2018). Anti-tumour effects of polysaccharide extracted from Acanthopanax senticosus and cell-mediated immunity. Exp. Ther. Med. 15 (2), 1694–1701. doi:10.3892/etm.2017.5568
Miao, C., Yu, Q., and Liang, H. (2007). Clinical observation of Huachansu injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer. (Chinese). Chin. J. Integr. Trad. West. Med. 7, 657–658. doi:10.3321/j.issn:1003-5370.2007.07.027
Miao, S., Yang, W., and Geng, C. (2010). Clinical observation of Shenqi Fuzheng injection combined with NP regimen in the treatment of advanced non-small cell lung cancer. (Chinese). Chin. Prac. Med. 5 (11), 16–17. doi:10.3969/j.issn.1673-7555.2010.11.007
People's Republic of China Department of Health Management (1991). Guidelines for the diagnosis and treatment of cancer. Beijing, China: Pecking Union Medical College Press, 11–15.
Pfister, D. G., Johnson, D. H., Azzoli, C. G., Sause, W., Smith, T. J., Baker, S., et al. (2004). American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J. Clin. Oncol. 22 (2), 330–353. doi:10.1200/JCO.2004.09.053
Piccini, J. P., and Kong, D. F. (2011). Mixed treatment comparisons for atrial fibrillation: evidence network or bewildering entanglement? Europace 13 (3), 295–296. doi:10.1093/europace/eur029
Qi, F., Li, A., Inagaki, Y., Gao, J., Li, J., Kokudo, N., et al. (2010). Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. Biosci. Trends 4 (6), 297–307.
Ran, R., Liu, H., and Huang, J. (2009). Clinical observation of NPK chemotherapy in the treatment of non-small cell lung cancer. (Chinese). J. Hubei Inst. Nat. 26 (4), 34–35. doi:10.3969/j.issn.1008-8164.2009.04.012
Ren, Y., Liu, H., and Quan, W. (2012). Effect of fructus brucea oil emulsion combined eith chemotherapy of NP regimen in patients with advanced NSCLC. (Chinese). Prog. Mod. Biomed. 12 (21), 4079–4082. doi:10.1155/2016/5928562
Rinaldi, M., Cauchi, C., and Gridelli, C. (2006). First line chemotherapy in advanced or metastatic NSCLC. Ann. Oncol. 17 (Suppl. 5), v64–v67. doi:10.1093/annonc/mdj953
Rücker, G., and Schwarzer, G. (2015). Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med. Res. Methodol. 15, 58. doi:10.1186/s12874-015-0060-8
Salanti, G., Del Giovane, C., Chaimani, A., Caldwell, D. M., and Higgins, J. P. (2014). Evaluating the quality of evidence from a network meta-analysis. PloS One 9 (7), e99682. doi:10.1371/journal.pone.0099682
Sang, X. (2012). Addition of compound kushen injection to vinorelbine plus cisplatin for patients with non-small-cell lung cancer: clinical observation. (Chinese). Eval. Anal. Drug Use Hosp. China 12 (7), 645–648. doi:CNKI:SUN:YYPF.0.2012-07-026
Shen, J., Qin, H., Liu, J., and Huang, C. (2012). Clinical observation on 30 cases of advanced non-small cell lung cancer treated with Shenma injection combined with NP regimen. Xianyang, China: (Chinese) The First National TCM Cancer Summit Forum.
Shi, X., Ding, Q., and Yang, Q. (2007). Shenqi Fuzheng Injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer. (Chinese) Cancer. Res. Clin. 19 (12), 823–824. doi:10.3760/cma.j.issn.1006-9801.2007.12.011
Shi, Z. (2010). 20 cases of advanced non-small lung cancer treated by herbal preparation eddy injection with chemotherapy. (Chinese) China. J. Chin. Med. 25 (4), 624–625. doi:CNKI:SUN:HNZK.0.2010-04-012
Shim, S., Yoon, B. H., Shin, I. S., and Bae, J. M. (2017). Network meta-analysis: application and practice using STATA software. Epidemiol. Health 39, e2017047. doi:10.4178/epih.e2017047
Siegel, R.L., Miller, K. D., and Jemal, A. (2018). Cancer statistics for hispanics/latinos, 2018. CA Cancer J. Clin. 68 (1), 425–445. doi:10.3322/caac.21494
Song, X. (2010). Clinical observation of compound Kushen injection combined with NP regimen in the treatment of advanced non-small cell lung cancer. (Chinese). Nei Mongol. J. Tradit. Chin. Med. 29 (07), 34–35. doi:10.7150/jca.40267
Su, W. Z., and Li, X. N. (2007). Therapeutic effect of Yanshu injection combined with NP regimen in the treatment of advanced non-small cell lung cancer. (Chinese). Med. Recapit. 13 (23), 1891–1892. doi:10.3969/j.issn.1006-2084.2007.23.065
Su, Z., Liang, J., and Huang, Y. (2005). Aidi injection combined with chemotherapy Clinical observation of advanced non-small cell lung cancer. (Chinese). J. Guangxi Tradit. Chin. Med. Univ. 2, 22–23. doi:10.1016/j.jep.2018.04.013
Sun, Y. (2004). The pathogenesis, prevention, early diagnosis and treatment for lung cancer. (Chinese). Chin. Med. Tribune 30, 13.
Sun, Y. Q., Wang, L. Q., and Zhao, L. P. (1981). Study on the Antitumor Effect of Brucea javanica: chromatography-mass spectrometry analysis of 11-deesterified burcea oil. (Chinese). J. Shenyang Pharm. Univ. 12 (14), 13–23.
Sylvester, R. (1980). Swiss franc.WHO handbook for reporting results of cancer treatment WHO offset publication #48 World Health Organization, Geneva, 19796. Contr. Clin. Trials 1, 45–277.
Tian, J. H., Zhao, Y., Li, J. L., Ge, L., and Yang, K. H. (2015). Network meta-analysis of 10 Chinese herb injections combined with vinorelbine and cisplatin for non small cell lung cancer. (Chinese). Chin. J. Drug Eval. 32 (1), 45–49. doi:10.3969/j.issn.2095-3593.2015.01.014
Trinquart, L., Attiche, N., Bafeta, A., Porcher, R., and Ravaud, P. (2016). Uncertainty in treatment rankings: reanalysis of network meta-analyses of randomized trials. Ann. Intern. Med. 164 (10), 666–673. doi:10.7326/M15-2521
Trinquart, L., Chatellier, G., and Ravaud, P. (2012). Adjustment for reporting bias in network meta-analysis of antidepressant trials. BMC Med. Res. Methodol. 12, 150. doi:10.1186/1471-2288-12-150
Veroniki, A. A., Soobiah, C., Tricco, A. C., Elliott, M. J., and Straus, S. E. (2015). Methods and characteristics of published network meta-analyses using individual patient data: protocol for a scoping review. BMJ Open 5 (4), e007103. doi:10.1136/bmjopen-2014-007103
Wang, C., and Feng, J. (2010). Treatment of 80 cases of advanced non-small-cell lung cancer with compound kushen injection combined with NP regimen. Cancer Res. Clinic. 22 (z1), 53–54. doi:10.3760/cma.j.issn.1006-9801.2010.z1.025
Wang, C., and Han, Y. (2009). Clinical study of coicis oil ijnection combined with chemotherapy in the treatmeat for advanced non-mall cell lung cancer. (Chinese). Oncol. Prog 7 (2), 217–220. doi:10.3969/j.issn.1672-1535.2009.02.022
Wang, D., Chen, Y., Ren, J., Cai, Y., Liu, M., and Zhan, Q. (2004). Randomized clinical study of Aidi injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer. (Chinese). Chin. J. Lung Cancer 3, 247–249. doi:10.3779/j.issn.1009-3419.2004.03.16
Wang, J., Li, G., Yu, L., Mo, T., Wu, Q., and Zhou, Z. (2018a). Aidi injection plus platinum-based chemotherapy for stage IIIB/IV non-small cell lung cancer: a meta-analysis of 42 RCTs following the PRISMA guidelines. J. Ethnopharmacol. 221, 137–150. doi:10.1016/j.jep.2018.04.013
Wang, J., Tian, L., Khan, M. N., Zhang, L., Chen, Q., Zhao, Y., et al. (2018b). Ginsenoside Rg3 sensitizes hypoxic lung cancer cells to cisplatin via blocking of NF-κB mediated epithelial-mesenchymal transition and stemness. Canc. Lett. 415, 73–85. doi:10.1016/j.canlet.2017.11.037
Wang, K., and Guo, Z. (2009). Xiaoaiping injection combining with NP regiment in the treatment of 56 patients with advanced lung cancer. (Chinese). J. Basic Clin. Oncol. 22 (1), 47–48. doi:10.3969/j.issn.1673-5412.2009.01.018
Wang, K., Long, X., Yang, W., and Wang, H. (2011). Short-term efficacy of Brucea javanica oil emulsion combined with NP regimen in the treatment of advanced lung cancer in 72 cases. (Chinese). Nei Mongol. J. Tradit. Chin. Med. 30 (7), 26–27. doi:10.3969/j.issn.1006-0979.2011.07.030
Wang, Q., Qin, J., and Nong, Y. (2007). Clinical observation of shenqi fuzheng injection combined with NP chemotherapy in the treatment of advanced non-small cell lung cancer. (Chinese). Mod. J. Integr. Tradit. Chin. West. Med. 16 (26), 3797–3798. doi:10.3969/j.issn.1008-8849.2007.26.012
Wang, Z., and Zhang, Z. (2007). Clinical observation of Kanglaite injection combined with NP regimen in the treatment of advanced non-small cell lung cancer. (Chinese). Heb. Med. J. 1, 47–48. doi:10.1155/2020/8586596
Wu, D., and Chen, M. (2014). Clinical analyses of chemotherapy combined with compound Sophora injection in the treatment of advanced non-small-cell lung cancer. China Health Care Nutr. 24 (3), 1223–1224.
Wu, H., Zou, H., and Liu, T. (2006a). Clinical observation of Yanshu injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer. (Chinese). Eval. Anal. Drug Use Hosp. China 6 (1), 43–45. doi:10.3969/j.issn.1672-2124.2006.01.013
Wu, W. Y., Wang, B., Zhang, H. B., Chai, X. S., Long, S. Q., Deng, H., et al. (2006b). Clinical study of Shenfu injection on the attenuation of non-small-cell lung cancer by NP regimen. (Chinese). J. new Chin. Med. 10, 20–23+27. doi:10.3969/j.issn.0256-7415.2006.10.012
Wu, W. Y., Wang, B., Zhang, H. B., Chai, X. S., Long, S. Q., Deng, H., et al. (2007). Effect of Shenfu injection on quality of life of patients with non-small-cell lung cancer treated with NP regimen. (Chinese). Chin. Tradit. Patent Med. 1, 14–18. doi:10.4103/0973-1482.187299
Wu, X., Qian, Y., and Fu, Z. (2005). Clinical observation on 60 cases of advanced non-small cell lung cancer treated with Aidi injection. Chinese). Guizhou Med. J. 11, 993–994. doi:10.3969/j.issn.1000-744X.2005.11.014
Wu, Y. (2008). Treatment of 32 cases of advanced non-small cell lung cancer with Brucea javanica oil emulsion combined with chemotherapy. (Chinese). Mod. J. Integr. Tradit. Chin. West. Med. 14, 2180–2181. doi:10.3969/j.issn.1008-8849.2008.14.056
Xia, C., Wang, G., Sun, J., Hao, H., Xiong, Y., Gu, S., et al. (2008). Simultaneous determination of ginsenoside Rg1, Re, Rd, Rb1 and ophiopogonin D in rat plasma by liquid chromatography/electrospray ionization mass spectrometric method and its application to pharmacokinetic study of 'SHENMAI' injection. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 862 (1-2), 72–78. doi:10.1016/j.jchromb.2007.11.020
Xiao, Z., Wang, C., Sun, Y., Li, N., Li, J., Chen, L., et al. (2016). Can Aidi injection restore cellular immunity and improve clinical efficacy in non-small-cell lung cancer patients treated with platinum-based chemotherapy? A meta-analysis of 17 randomized controlled trials following the PRISMA guidelines. Medicine (Baltim.) 95 (44), e5210. doi:10.1097/MD.0000000000005210
Xie, X. D., Zheng, Z. D., Gao, Y. L., Liu, D. W., Liu, Y. Y., Shan, X. J., et al. (2003a). A clinical controlled study of Kanglaite on quality of life in patients with locally advanced non-small cell lung cancer. (Chinese). Chin. J. Prac. Intern. Med. 23 (11), 677–678. doi:10.3969/j.issn.1005-2194.2003.11.017
Xie, X. D., Zheng, Z. D., Gao, Y. L., Liu, D. W., and Liu, Y. Y. (2003b). A random control study: effect of Konglotite on life quality of patient with lung cancer in advanced stage. (Chinese). Chin. J. Clin. Rehabil. 11, 1672–1673. doi:10.3321/j.issn:1673-8225.2003.11.029
Xing, H., Wang, L., Lv, H., Zou, Q., and Qi, X. (2014). Application of Aidi combined with NP regimen in the treatment of elderly patients with non-small cell lung cancer. (Chinese). Chin. J. Prim. Med. Pharm. 21 (2), 280–281. doi:10.3760/cma.j.issn.1008-6706.2014.02.056
Xu, L., Wu, B., and Hong, W. (2013). Effect of Aidi combined with Vinorelbine and Cisplatin in patients with non-small cell lung cancer. (Chinese). Chin. Med. Herald 10 (05), 77–79. doi:10.3969/j.issn.1673-7210.2013.05.030
Xu, X., and Li, D. (2010). Therapeutic effect of Kanglaite on chemotherapy in elderly patients with advanced non-small cell lung cancer. (Chinese). J. Mod. Oncol. 18 (10), 1959–1960. doi:10.3969/j.issn.1672-4992.2010.10.26
Xu, X., Zhang, Y., and Fu, Z. (2007). Clinical study on efficacy of Aidi injection plus NP regimen in treatment of non-small-cell lung cancer. (Chinese) Herald Med. 3, 244–245. doi:10.3870/j.issn.1004-0781.2007.03.009
Xue, R. (2007). Shenma injection adjuvant chemotherapy for 30 cases of non-small cell lung cancer. (Chinese). J. Med. Theory Pract. 20 (12), 1409. doi:10.3969/j.issn.1001-7585.2007.12.023
Yang, G., Peng, Q., and Dong, Q. (2017). Analysis and study on the effect of Aidi injection combined with chemotherapy in the treatment of non-small cell lung cancer. (Chinese). Chin. Foreign Med. Res. 15 (15), 3–4. doi:10.14033/j.cnki.cfmr.2017.15.002
Yang, X., and Xi, J. (2006). Therapeutic effect of Huachansu combined with chemotherapy on non-small cell lung cancer. Herald Med 12, 1287–1288. doi:10.3870/j.issn.1004-0781.2006.12.021
Yang, Z., Deng, C., Qiu, Y., and Mo, B. (2012). Shengmai injection combined with NP regimen in treating 30 cases of advanced non-small cell lung cancer. (Chinese). World Health. Digest Med. Periodieal 9 (26), 124–125. doi:10.3969/j.issn.1672-5085.2012.26.096
Yin, B., Li, D. H., Zhang, L., and Zhang, Q. F. (2007). Clinical observation on 31 cases of advanced non-small-cell carcinoma treated with Kangai injection combined with chemotherapy. (Chinese). Harbin Med. J. 1, 32–33. doi:10.3969/j.issn.1001-8131.2007.01.026
Yoon, T. J., Yoo, Y. C., Lee, S. W., Shin, K. S., Choi, W. H., Hwang, S. H., et al. (2004). Anti-metastatic activity of Acanthopanax senticosus extract and its possible immunological mechanism of action. J. Ethnopharmacol. 93 (2-3), 247–253. doi:10.1016/j.jep.2004.03.052
Yu, Q. (2007). Shenqi Fuzheng Injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer. (Chinese). Chin. J. Integr. Trad. West. Med. 27 (5), 473–474. doi:10.1371/journal.pone.0185254
Zhang, A., Zheng, Y., Que, Z., Zhang, L., Lin, S., Le, V., et al. (2014). Astragaloside IV inhibits progression of lung cancer by mediating immune function of Tregs and CTLs by interfering with Ido. J. Canc. Res. Clin. Oncol. 140 (11), 1883–1890. doi:10.1007/s00432-014-1744-x
Zhang, H., and Qi, R. (2010). Clinical observation of Kanglaite injection combined with NP regimen in the treatment of advanced non-small cell lung cancer. (Chinese). J. Mod. Oncol. 18 (5), 927–928. doi:10.3969/j.issn.1672-4992.2010.05.31
Zhang, J., Zhu, J., and Zhang, L. (2006). Effect of Aidi injection combined with NP regimen on advanced non-small-cell lung cancer. (Chinese). Med. J. Commun. 5, 528–529. doi:10.3969/j.issn.1006-2440.2006.05.024
Zhang, L., Jia, Y., and Chen, L. (2013). Clinical observation of Shenma injection in relieving bone marrow suppression in chemotherapy for non-small cell lung cancer. (Chinese). Jilin Med. J. 34 (15), 2951. doi:10.3969/j.issn.1004-0412.2013.15.076
Zhang, Q. (2008a). Clinical observation of shengmai injection in the treatment of non-small cell lung cancer. (Chinese). mod. J. Integr. Tradit. Chin. West. Med. 24, 3827–3828. doi:10.3969/j.issn.1008-8849.2008.24.075
Zhang, S. (2012). Clinical observation of Aidi injection combined with NP regimen in the treatment of non-small cell lung cancer. (Chinese). Chin. Comm. Doctors 14 (29), 190–191. doi:10.3969/j.issn.1007-614x.2012.32.126
Zhang, X., and Luan, X. (2005). Therapeutic effect of Kangai injection combined with chemotherapy on advanced non-small cell lung cancer. (Chinese). Chin. J. Integr. Trad. West. Med. 6, 543–544. doi:10.3321/j.issn:1003-5370.2005.06.017
Zhang, Y. (2008b). Clinical observation of Shenfu injection combined with NP regimen in the treatment of advanced non-small cell lung cancer [M.D], Guangxi College Trad. Chin. med.
Zhao, J., Xing, L., Li, W., Zhang, S., Li, G., and Qian, H. (2006). Clinical study of Huachansu injection combined with chemotherapy in the treatment of advanced non-small-cell lung cancer. (Chinese). Lishizhen. Med. Mater. Med. Res. 8, 1604–1605.
Zhao, L., Yin, W., Jiang, A., and Zhang, Y. (2017). Clinical observation of Shenfu injection combined with NP regimen in the treatment of quality of life in patients with non-small cell lung cancer. (Chinese). J. Emerg. Tradit. Chin. Med. 26 (4), 730–732. doi:10.3969/j.issn.1004-745X.2017.04.054
Zhao, Z., Wu, D., Chen, M., Jiang, H., and Yan, G. (2007). Short-term efficacy of Shenqi Fuzheng Injection-assisted NP regimen in the treatment of elderly patients with advanced non-small cell lung cancer. (Chinese). Mode. Oncol. 15 (1), 42–43. doi:10.3969/j.issn.1672-4992.2007.01.017
Zhou, G., and Li, A. (2003). Shengmai injection improves 30 cases of non-small cell lung cancer patients with poor treatment. (Chinese). Chin. J. Integr. Trad. West. med. 3, 231–232. doi:10.3321/j.issn:1003-5370.2003.03.026
Zhou, Y. S., Zhao, B. M., Wu, W. Y., Yang, X. B., Long, S. Q., Deng, H., et al. (2018). Shenmai injection for the treatment of canceZou, Y. (Kangai injection combined with NP regimen for advanced treatment Clinical observation of patients with NSCLC. (Chinese). Zhejiang. Clin. Med. J. 5, 639–641. doi:10.1186/s13063-018-2845-7
Keywords: Chinese herbal injection, vinorelbine plus cisplatin, non-small cell lung cancer, network meta-analysis, systematic review, multidimensional cluster analysis
Citation: Ni M, Wang H, Wang M, Zhou W, Zhang J, Wu J, Zhang D, Jing Z, Liu X, Wu Z, Guo S, Jia S, Zhang X and Sheng X (2021) Investigation on the Efficiency of Chinese Herbal Injections for Treating Non-small Cell Lung Cancer With Vinorelbine and Cisplatin Based on Multidimensional Bayesian Network Meta-Analysis. Front. Pharmacol. 11:631170. doi: 10.3389/fphar.2020.631170
Received: 19 November 2020; Accepted: 29 December 2020;
Published: 29 January 2021.
Edited by:
Ke-Wu Zeng, Peking University, ChinaCopyright © 2021 Ni, Wang, Wang, Zhou, Zhang, Wu, Zhang, Jing, Liu, Wu, Guo, Jia, Zhang and Sheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiarui Wu, ZXhvZ2FteUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.