
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 29 January 2021
Sec. Renal Pharmacology
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.627185
This article is part of the Research Topic Applications of Herbal Medicine to Control Chronic Kidney Disease View all 38 articles
 Wei Mao1
Wei Mao1 Nizhi Yang1
Nizhi Yang1 Lei Zhang1
Lei Zhang1 Chuang Li1
Chuang Li1 Yifan Wu1
Yifan Wu1 Wenwei Ouyang1,2
Wenwei Ouyang1,2 Peng Xu1
Peng Xu1 Chuan Zou1
Chuan Zou1 Chunpeng Pei3
Chunpeng Pei3 Wei Shi4
Wei Shi4 Jihong Zhan5
Jihong Zhan5 Hongtao Yang6
Hongtao Yang6 Hongyu Chen7
Hongyu Chen7 Xiaoqin Wang8
Xiaoqin Wang8 Yun Tian9
Yun Tian9 Fang Yuan10
Fang Yuan10 Wei Sun11
Wei Sun11 Guoliang Xiong12
Guoliang Xiong12 Ming Chen13
Ming Chen13 Jianguo Guan14
Jianguo Guan14 Shuifu Tang15
Shuifu Tang15 Chunyan Zhang16
Chunyan Zhang16 Yuning Liu17
Yuning Liu17 Yueyi Deng18
Yueyi Deng18 Qizhan Lin1
Qizhan Lin1 Fuhua Lu1
Fuhua Lu1 Weihong Hong19
Weihong Hong19 Aicheng Yang20
Aicheng Yang20 Jingai Fang21
Jingai Fang21 Jiazhen Rao22
Jiazhen Rao22 Lixin Wang1
Lixin Wang1 Kun Bao1
Kun Bao1 Feng Lin23
Feng Lin23 Yuan Xu1
Yuan Xu1 Zhaoyu Lu1
Zhaoyu Lu1 Guobin Su2
Guobin Su2 La Zhang1,24
La Zhang1,24 David W Johnson25
David W Johnson25 Daixin Zhao1
Daixin Zhao1 Haijing Hou1
Haijing Hou1 Lizhe Fu1
Lizhe Fu1 Xinfeng Guo1
Xinfeng Guo1 Lihong Yang1
Lihong Yang1 Xindong Qin1
Xindong Qin1 Zehuai Wen1,26*
Zehuai Wen1,26* Xusheng Liu1*
Xusheng Liu1*Chinese herbal medicine (CHM) might have benefits in patients with non-diabetic chronic kidney disease (CKD), but there is a lack of high-quality evidence, especially in CKD4. This study aimed to assess the efficacy and safety of Bupi Yishen Formula (BYF) vs. losartan in patients with non-diabetic CKD4. This trial was a multicenter, double-blind, double-dummy, randomized controlled trial that was carried out from 11-08-2011 to 07-20-2015. Patients were assigned (1:1) to receive either BYF or losartan for 48 weeks. The primary outcome was the change in the slope of the estimated glomerular filtration rate (eGFR) over 48 weeks. The secondary outcomes were the composite of end-stage kidney disease, death, doubling of serum creatinine, stroke, and cardiovascular events. A total of 567 patients were randomized to BYF (n = 283) or losartan (n = 284); of these, 549 (97%) patients were included in the final analysis. The BYF group had a slower renal function decline particularly prior to 12 weeks over the 48-week duration (between-group mean difference of eGFR slopes: −2.25 ml/min/1.73 m2/year, 95% confidence interval [CI]: −4.03,−0.47), and a lower risk of composite outcome of death from any cause, doubling of serum creatinine level, end-stage kidney disease (ESKD), stroke, or cardiovascular events (adjusted hazard ratio = 0.61, 95%CI: 0.44,0.85). No significant between-group differences were observed in the incidence of adverse events. We conclude that BYF might have renoprotective effects among non-diabetic patients with CKD4 in the first 12 weeks and over 48 weeks, but longer follow-up is required to evaluate the long-term effects.
Clinical Trial Registration:http://www.chictr.org.cn, identifier ChiCTR-TRC-10001518.
Chronic kidney disease (CKD) is a major global health problem with poor outcomes and high medical costs, especially when patients progress to end-stage kidney disease (ESKD) (GBD Chronic Kidney Disease Collaboration, 2020). In China, the prevalence of CKD ranges from 9.4% to 13.0% (Zhang et al., 2012). Patients with stage 4 CKD accounts for 0.16% of the world's population (GBD Chronic Kidney Disease Collaboration, 2020). The principles of treatment in CKD4 include statins to control blood lipids, low-salt, high-quality, and low-protein diet, and anti-inflammatory drugs (de Zeeuw et al., 2013), but efficacy is low.
The mainstay of medical management to prevent CKD progression is to achieve optimal control of blood pressure (BP) with antihypertensive agents, particularly angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB) (Palmer et al., 2015). Nevertheless, the use of ACEI/ARB is limited because of high potassium (Ahuja et al., 2000) and aggravated renal failure (Tomlinson et al., 2013) in stage 4 CKD. More than half of users discontinued ACEI/ARB within 5 years of therapy initiation (Qiao et al., 2019).
The limitations in managing CKD4 drive numerous patients to seek Chinese herbal medicines (CHMs). Western guidelines advise that herbal supplements should not be used in CKD, partially due to concerns about a lack of supportive clinical evidence (Levin and Stevens, 2014). Nevertheless, CHMs are commonly used worldwide (Eisenberg et al., 1998). In China and other Asian countries, CHMs are widely used in patients with CKD to delay renal failure (Chen et al., 2007). Nearly half (45.3%) of the patients with CKD in Taiwan use CHM (Lin et al., 2015). Although evidence for the renoprotective benefits of CHM has been gradually accumulating (Lin et al., 2015), these studies have mainly focused on patients with early (stage 1–3) CKD or diabetes (Chen et al., 2013; Zhang et al., 2014b; Zhang et al., 2014c), but there are few high-quality studies in CKD4.
The Bupi Yishen Formula (BYF) is a patent CHM originating from the historical Si-jun-zi Decoction. It was developed based on the text mining result of 10,000 medical records from Guangdong Provincial Hospital of Chinese Medicine (GPHCM), a tertiary hospital in southern China. Radix astragali and Salvia miltiorrhiza can delay CKD (Feng et al., 2013; Care, 2017) and are the main components of BYF. BYF is composed of 86 compounds, including flavonoids, saponins, and phenolic acids (Zhang et al., 2018b). A previous study by our group (Li et al., 2013) showed an association between BYF use and preservation of renal function in patients with advanced CKD.
The aim of this multicenter randomized controlled trial (RCT) was to assess the efficacy and safety of BYF in renal progression vs. losartan. The results could help ease the burden of renal failure in patients with CKD4 without diabetes. In China, ARB, especially losartan, is used more frequently than ACEI due to the side effects such as cough and was selected for the control group.
This trial was a multicenter, double-blind, double-dummy, randomized controlled trial that was carried out from November 8, 2011, to July 20, 2015. Twenty-one hospitals in mainland China participated in this trial (Supplementary Table S1). The eligible patients were consecutively enrolled and randomized 1:1 ratio to receive BYF or losartan for 48 weeks.
The study protocol was approved by the Institutional Ethics Committee of GPHCM (approval ref. B2010-11-03) and registered at the Chinese Clinical Trial Registry Number: ChiCTR-TRC-10001518. All participants provided written informed consent before randomization.
Detailed inclusion and exclusion criteria for this trial are described in the protocol (Mao et al., 2015). We included eligible non-diabetic adult patients (18–80 years) diagnosed with CKD4 (Hou et al., 2007) for at least 3 months, with well-controlled blood pressure (<140/90 mmHg) and the Chinese Medicine syndrome of Spleen and Kidney Qi deficiency.
Randomization was conducted by the personnel from the Key Unit of Methodology in Clinical Research of the GPHCM. Randomization number list was generated using the PROC PLAN procedure in SAS 9.2 (SAS Institute, Cary, NC, United States). The randomization was stratified according to the center with confidential block size. The blinding codes were delivered to each site via a web-based system to conceal allocation.
Patients, investigators, monitors, outcome assessors, and statisticians were blinded. Masking for patients and site personnel was achieved by double-dummy placebo design. The placebos of BYF and losartan were matched to their corresponding drug in appearance, color, and taste.
The BYF herbs were extracted with hot water, concentrated, spray-dried, made into granules, and packed in sealed opaque sachets. Production was performed by Pura Pharm Pharmaceuticals Co Ltd (Nanning, Guangxi, China) and controlled rigorously according to good manufacturing practice (GMP) standards. The BYF placebo was produced by the same manufacturer as the original using a concentrate of Colyx Tea (leaf of Broadleaf Holly), caramel pigments, gardenia yellow pigment, sunset yellow pigment, saccharose, and dextrin. The BYF placebo was similar in color, smell, taste, appearance, and packaging with the BYF granule. Qualities of BYF and placebo granules, such as appearance, determination of water, size of granule, solubility, hygroscopicity, heavy metals, toxic elements, pesticide residues, and microbial limit, were controlled rigorously according to the 2010 Chinese pharmacopeia and Chinese Medicine Council of Hong Kong.
After enrollment and prior to randomization, the participants who were taking ACEi and/or ARB before enrollment were administered alternative antihypertensive agents during a 3 week run-in period according to the protocol (Mao et al., 2015). In the BYF group, the participants were instructed to dissolve 15 g of BYF granules (Supplementary Tables S2,S3) in 150 ml of boiled water and to take this solution orally thrice daily, and losartan matched placebo tablet once daily for 48 weeks. The participants in the losartan group were instructed to take 100 mg losartan orally once daily along with 15 g of BYF matched placebo thrice daily for 48 weeks. There were no dose adjustments.
All participants received conventional CKD management according to guidelines (Levin and Stevens, 2014). Any ACEi/ARB or CHM (other than the study medication) was prohibited during the trial. Keto-amino acids were originally prohibited. However, as most participants refused to stop keto-amino acids, the protocol was subsequently amended to allow their concurrent use. Compliance was monitored by dose counting.
Patients were examined at baseline and at weeks 2, 4, 6, and 8, and thereafter every 4 weeks until 48 weeks. At each visit within the first 8 weeks, serum creatinine and urea nitrogen were measured. Thereafter, urinary protein-to-creatinine ratio, serum creatinine, urea nitrogen, calcium, phosphate, serum albumin, liver enzymes (aspartate aminotransferase and alanine aminotransferase), blood lipids (cholesterol, triglycerides, and low-density lipoprotein cholesterol), intact parathyroid hormone, and blood cell counts were measured at weeks 12, 24, 36, and 48. Serum potassium was examined at each time point for safety monitoring. BP was monitored at home for one week prior to each visit. BP was also measured at each visit, in the sitting position, after a 5 min rest. Two measurements were taken; if they were >10 mmHg apart, a third measurement was taken.
The primary outcome was the difference in the slope of CKD-EPI eGFR (Inker et al., 2012) between the two groups over 48 weeks. The laboratory assays for serum creatinine measurement were standardized across all sites and repeatedly cross-checked to ensure the comparability of eGFR results.
The secondary outcomes were time to the first occurrence of a composite outcome (death from any cause, doubling of serum creatinine level, ESKD, stroke, or cardiovascular events), and time to each of the individual components of the composite outcome. ESKD was defined as eGFR decreasing to <15 ml/min/1.73 m2 for >4 weeks, or requiring permanent dialysis or kidney transplantation. Stroke was defined as either ischemic cerebral infarction or cerebral hemorrhage. Cardiovascular events were defined as acute myocardial infarction and heart failure. Urinary protein-to-creatinine ratio, serum creatinine, urea nitrogen, serum albumin, blood lipids profile (cholesterol, triglycerides, and low-density lipoprotein cholesterol), calcium, phosphate, and intact parathyroid hormone were measured as secondary outcomes.
The participants were asked to report any symptoms and adverse events (AEs) at each follow-up visit or immediately when they happened. In addition, serum potassium, liver enzymes of aspartate aminotransferase and alanine aminotransferase, blood cell counts, routine urine and stool tests, and electrocardiography were checked as safety indices.
Based on the results of previous retrospective data and trial by Hou et al., (2006), a sample size of 221 patients per group was estimated to provide 90% power and a one-sided significance level of 2.5% to detect a between-group difference of mean eGFR change of 2.3 ml/min/1.73 m2 per year with a superiority test, assuming a standard deviation of 4.2 and a clinically important difference of 1 ml/min/1.73 m2. The final sample size was adjusted to 554 assuming 20% dropout.
The primary outcome was analysed according to the Intent-to-Treat (ITT) principle using the full analysis set (FAS), i.e. all treated patients with a baseline eGFR and at least one eGFR value on treatment. Safety analyses were performed on the safety set (SS). The change of eGFR at week 48 from baseline was analyzed by fitting a mixed-effects model in which treatment and the interaction of treatment and time were treated as a fixed effect while the time effect as a random effect.
Randomization should mitigate differences in characteristics between the two groups, but because of missing data, the mixed-effects model was used instead of repeated measure ANOVA, and adjustments were made for the sake of conservativeness and insurance.
The log-rank test was used to compare time to composite outcome between the two groups, followed by unadjusted and adjusted Cox proportional hazards model estimations. Other continuous outcomes were assessed using the t-test or Mann-Whitney U-test, as appropriate. Categorical variables were compared using Fisher’s exact test or Mann-Whitney U-test, as appropriate. Subgroup analyses were repeated in strata according to sex, age (≤45 years or >45 years), hypertension, and hyperuricemia.
Sensitivity analyses were conducted: 1) To test the robust of the primary analysis, a mixed-effects model using baseline eGFR, sex, age, mean change of weight, history of hypertension, and history of gout or hyperuricemia as covariates; 2) Missing data on the primary outcome were imputed using the multiple imputation method under the missing at random assumption. 3) to detect whether there was a change in slope before and after 12 weeks, a piecewise linear mixed-effects model was used; 4) using different methods of imputing missing outcome data: multiple imputations and mixed model for repeated measures; 5) excluding patients who experienced a serum creatinine increase more than 30% within the first 12 weeks; and 6) excluding patients who took keto-amino acids during the trial.
All analyses were performed using the PASS 18.0 (IBM Corporation, Armonk, New York, USA) and STATA 11.0 (Stata Corp. College Station, Texas, USA) with a two-sided p-value <0.05 considered statistically significant.
A total of 1120 patients were screened at 21 centers; 567 agreed to participate and were randomized to BYF (n = 283) or losartan (n = 284) (Figure 1); 549 participants were included in the FAS since 18 participants were excluded due to no available post-baseline data. The compliance with study medication was 90.1% (246/273) in the BYF group and 94.6% (261/276) in the losartan group. The median follow-up was 47.4 weeks.
The baseline characteristics are shown in Table 1. The mean age was 52.2 ± 14.1 years, 250 (44.1%) were female, and 529 (93.3%) were of Han ethnicity. The most common primary renal disease was chronic glomerulonephritis (377 participants, 66.5%), and the mean eGFR at baseline was 22.4±5.8 ml/min/1.73 m2. During the run-in period, antihypertensive drugs except for ACEi and ARB were prescribed to control BP. Of 567 participants, only 23 used ACEi or ARB before enrollment and were shifted to other types of antihypertensive drugs.
In the FAS, the unadjusted mean slopes of eGFR were −4.53 (standard error [SE] 0.64) and −2.30 (SE 0.63) ml/min/1.73 m2/year in the losartan and BYF groups, respectively. The difference of adjusted mean slopes of eGFR between the two groups was −2.24 ml/min/1.73 m2 (95% confidence interval [CI]: −4.01,−0.46; p=0.014) over the 48 week interval (Table 2). As shown in Figure 2, the difference of mean slopes of eGFR between the two groups was about 4.23 ml/min/1.73 m2 prior to 12 weeks, and evidently attenuated to less than 3 ml/min/1.73 m2 post to 12 weeks (Supplementary Table S4). Correspondingly, the difference of unadjusted mean slopes of eGFR between the two groups was -2.25 ml/min/1.73 m2 (95%CI: −4.01,−0.46; p=0.013) over the first 12 weeks.
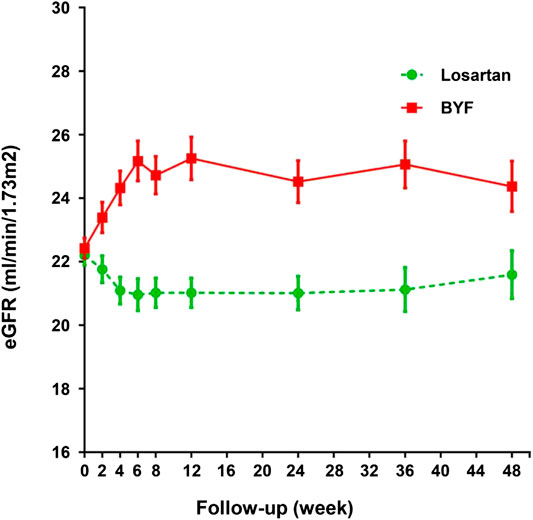
FIGURE 2. Changes in estimated glomerular filtration rate (eGFR) in the losartan and Bupi Yishen Formula (BYF) groups over 48 weeks.
The composite endpoint was achieved by 73 (28.1%) participants in the BYF group and 95 (36.4%) participants in the losartan group. The corresponding figures for individual endpoints were 71 (27.3%) vs. 91 (34.9%) for ESKD, 0 (0%) vs. 1 (0.4%) for doubling of serum creatinine, 1 (0.4%) vs. 0 (0%) for death and 1 (0.4%) vs. 3 (1.1%) for stroke or cardiovascular events (Table 2).
The mean time to the composite endpoint was significantly longer in the BYF group (49.3 weeks, 95%CI: 46.8,51.9) than in the losartan group (46.8 weeks, 95%CI: 43.6,49.9, p = 0.044) (Figure 3). Survival analysis suggested a 39% lower risk of composite endpoints in the BYF group compared with the losartan group (hazard ratio [HR]=0.73, 95%CI: 0.54,0.99, p = 0.045), consistent with the adjusted HR of the Cox model (HR=0.61, 95%CI: 0.44,0.85, p = 0.003). Most of the composite events were dominated by entering ESKD, and the adjusted risk of ESKD was significantly lower in the BYF group (HR=0.61, 95%CI: 0.43,0.85, p = 0.004). There were no between-group differences with respect to the other endpoints.
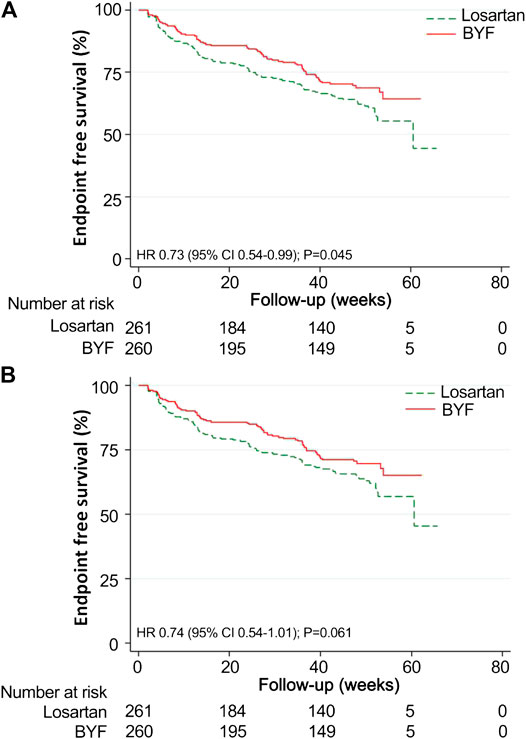
FIGURE 3. Kaplan-Meier survival curves for the Bupi Yishen Formula (BYF) and Losartan groups. Twenty-eight participants were excluded from the survival analyses as they reached end-stage kidney disease (ESKD) after randomization but before the initiation of treatment. (A) Composite endpoint (composite of death, doubled serum creatinine, ESKD, and cardiovascular or cerebrovascular events). (B) ESKD.
In the subgroup analyses, no statistically significant interactions were found between the intervention and any of the subgroups including age, sex, hypertension and hyperuricemia (Figure 4).
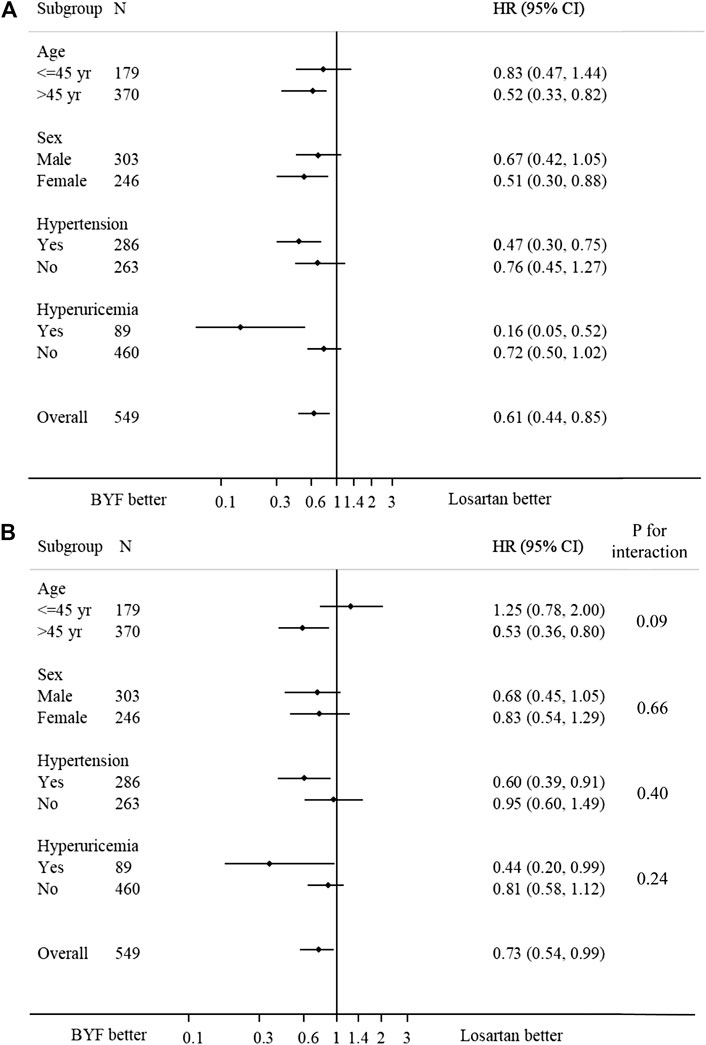
FIGURE 4. Forest plot of the effect of Bupi Yishen Formula (BYF) on the composite endpoint by subgroups. (A) Model 1: no adjustment. (B) Model 2: adjusted for center effect, sex, age, change of weight over 48 weeks, baseline estimated glomerular filtration rate (eGFR), and morbidities (hypertension, gout, or hyperuricemia).
Compared with baseline, urinary protein-creatinine-ratio decreased by 0.76 mg/mg in the losartan group and 0.34 mg/mg in the BYF group (p > 0.05). The serum creatinine reduced in the BYF group but increased in the losartan group (p = 0.041). Over the course of the study, there were rising trends of total serum cholesterol, low-density lipoprotein, and phosphate levels in the losartan group, in contrast to declines in the BYF group. The body weight tended to decrease in the losartan group but remained stable in the BYF group. There were no significant between-group differences observed in any other laboratory parameters (Supplementary Table S5,S6).
The frequencies of AEs, serious AEs, and AEs leading to withdrawal were similar in the two groups (Table 3). Hyperkalemia was the most common AE reported and was less commonly observed in the BYF group than in the losartan group (rate ratio 0.83, 95%CI: 0.67,1.03). Serious AEs leading to hospitalization occurred in six patients in the BYF group vs. ten in the losartan group. Of note, two cases of death occurred during follow-up: one patient in the BYF group died from an uncertain cause, and one patient in the losartan group died from gastrointestinal bleeding.
The results showed that eGFR at 48 weeks adjusted for baseline eGFR was higher in the BYF group (p < 0.0001). Similarly, decline in the adjusted eGFR slope using the mixed-effects model was comparably attenuated in BYF group (p = 0.013, Supplementary Table S7). To examine the potential impact of a possible early acute effect of therapy on renal function with respect to the overall result, sensitivity analyses were conducted. Thirty-three (12%) patients in the losartan group and thirteen (4.8%) patients in the BYF group experienced a serum creatinine increase of more than 30% in the first 12 weeks (p = 0.002). After excluding these patients, we observed a higher mean of eGFR at the end of follow-up and a slower decline of eGFR over 48 weeks in the BYF group (Supplementary Table S8), which remained consistent with that in FAS population. Lower risks of the composite endpoint and ESKD were still found in the BYF group (Supplementary Table S9). The results of the sensitivity analyses remained robust after excluding patients who took keto-amino acids during the trial and employing multiple imputation methods to deal with missing data (Supplementary Table S10–S13).
Our results demonstrated that, compared with losartan, BYF (the main of components were listed in Supplementary Table S2) resulted in a significantly slower decline of eGFR, particularly in the first 12 weeks, and lower risks of the composite outcome and ESKD in non-diabetic CKD4 patients over 48 weeks of follow-up. These results remained robust to a series of sensitivity analyses. In the subgroup analyses, no statistically significant interactions were found between the intervention and any of the subgroups including age, sex, hypertension and hyperuricemia. Serious adverse events and adverse events leading to withdrawal were uncommon and the incidences were similar between the two groups.
The ACEi or ARB of conventional CKD strategies is administered to achieve optimal BP control and proteinuria reduction, factors possibly associated with retarded CKD progression and decreased mortality risk (Li et al., 2013; Bhandari et al., 2016). ACEi and ARB are thought to produce long-term renoprotection through reduction of intra-glomerular pressure, which also results in a reversible early drop in kidney function. However, these effects may be harmful in patients with advanced CKD with seriously compromised kidney function, and has led to ongoing, multi-centre, randomized controlled STOP-ACEi trial which is assessing the effects of withdrawal of ACEi/ARB treatment in 410 CKD4-5 patients (Bhandari et al., 2016). Since the use of ACEi or ARBs in the CKD4-5 population is controversial and associated with a higher risk of adverse events(e.g. hyperkalaemia and acute kidney injury) (Strippoli et al., 2006; Bhandari et al., 2016), the presented study attempted to determine if there was a safe, more effective alternative in patients with stage 4 CKD using BYF. We found that, compared with the ARB losartan, BYF had favourable effects on kidney function decline and resulted in a 39% reduction in the composite outcome of death from any cause, doubling of serum creatinine level, end-stage kidney disease (ESKD), stroke, or cardiovascular events.
Comparable beneficial renal outcomes have also been previously reported for CHM products administered to patients with advanced CKD. Further study of the same cohort found that use of certain herbs contained within BYF, such as Astragalus membranaceus and Salvia miltiorrhiza, were associated with a lower risk of all-cause mortality (Hsieh et al., 2017). A systematic review of 22 studies including more than one thousand CKD participants supported the use of Astragalus preparation (the main herb of BYF) in reducing serum creatinine and urinary protein excretion, and improving creatinine clearance, though the quality of evidence was low (Zhang et al., 2014). Subgroup analysis found that the favourable effect of Astragalus preparation on serum creatinine was particularly evident in patients with later stage CKD.
Due to the multi-ingredients nature of the CHM formula, the exact mechanism of action and targets of BYF in patients with CKD are difficult to fully elucidate. In previous studies by our group, ultra-high performance liquid chromatography-mass spectrometry was used to identify compounds contained in BYF, and 86 compounds were detected with seven representative constituents (Zhang et al., 2018b). Pharmacological experiments with selected chemicals suggested that the renoprotective effects of BYF were probably mediated via anti-inflammatory, antioxidant, anti-fibrotic pathways, and gut microbiota modulatory effects (Supplementary Table S3) (Zhang et al., 2012b; Wang et al., 2014; Zhang et al., 2018). In addition, renal ischemia-reperfusion-induced injury can be attenuated by some of the compounds (Zhang et al., 2012b). Further studies are still needed to clarify the mechanism of action and related targets of BYF in CKD.
The beneficial effect of BYF on kidney function was observed early in the first 12 weeks and was sustained thereafter. It is conceivable that BYF may have early haemodynamic effects that contributed to this observation given that the BYF components of Astragalus, Salvia extract, Atractylodes, Dioscoreaop and Cuscuta have been shown to attenuate pulmonary arterial hypertension (Yuan et al., 2017), lower left ventricular end-diastolic pressure (Wang et al., 2017), inhibit the activation of rennin-angiotensin-aldosterone system(RAAS) (Cui et al., 2018), reduce glomerular volume (Guo et al., 2016), and decrease BP respectively (Patel et al., 2012). Variability in glomerular haemodynamics is a crucial prognostic risk factor for renal endpoints (Tsai et al., 2019). Nevertheless, most subjects recruited in our trial experienced little BP fluctuation to achieve BP goal after enrollment due to their intensive BP control management, and we did not obtain relevant vasodilatory cues for further investigation. Further study of intrarenal hemodynamic measurements are warranted in future studies.
The herbal ingredients of BYF were free from aristolochic acid, and hyperkalaemia was less common in the BYF group than in the losartan group. BYF appeared to be well tolerated among participants and no safety signals were observed. The result of our study suggested that not all herbal remedies were harmful to the kidney and that some, such as BYF, were beneficial to the kidney when used under the guidance of Chinese medicine professionals. As complementary and alternative medicines, including CHM, are a potentially important and inexpensive means of meeting kidney health care needs globally, the rigorous evaluation of therapy regarding complementary and alternative medicine, such as occurred in this RCT, is fundamental and meaningful, especially for low and middle-income countries.
This study was designed as a double-dummy, double-blind RCT, with strict quality control processes for sourcing and manufacturing BYF and matched identical placebo. These strengths should be balanced against the study’s limitations. Firstly, the clinical utility of serum creatinine-based eGFR may have been influenced by body mass, diet and creatinine measurement techniques. To address this issue, our results remained robust after adjustment for change of body weight over 12 months. Secondly, since our study duration was relatively short (48 weeks), further investigation with longer follow-up is warranted to validate the impact of BYF in terms of eGFR trajectory and important clinical outcomes. Thirdly, patients who reached the composite end-point (36.4% in Losartan group and 28.1% in BYF group, p = 0.003), were not continued to be followed, which may have introduced informative censoring and contributed to missing data during the later follow up. The mixed-effects model was used in this study, as recommended in Karen et al.’s paper (Leffondre et al., 2015), to make the results robust. Fourthly, urinary creatinine(UCr) and creatinine clearance (CCr) measurements were not available. Finally, only patients with stage 4 CKD were recruited into the study, such that the results may not have been generalizable to patients with earlier stage CKD.
BYF might have renoprotective effects among non-diabetic patients with CKD4 in the first 12 weeks and over 48 weeks, but longer follow-up is required to evaluate the long-term effects. This could represent an alternative for patients reluctant to take renin-angiotensin blocking medications or in low-income settings.
Data cannot be made publicly available as the dataset contains sensitive and identifying information. The authors confirm that the data will be made available upon request. Requests should be sent to both two corresponding authors, Zehuai Wen (d2VuemhAZ3p1Y20uZWR1LmNu) and Xusheng Liu (bGl1eHVzaGVuZ0BnenVjbS5lZHUuY24=).
The studies involving human participants were reviewed and approved by the Institutional Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine (approval ref. B2010-11-03). The patients/participants provided their written informed consent to participate in this study.
All authors contributed to the study design, data acquisition, or data analysis and interpretation. Specifically, WM, NY, XG, ZW, and XL obtained funding, conceived the study, organised and supervised the study. PX, CZ, CP, WS, JZ, HY, HC, XW, YT, FY, WS, GX, MC, JG, ST, CZ, YL, YD, QL, FL, HH, AY, JF, JR, LW, KB, FL, YX, DZ, HH, LF, and XQ collected the data. LZ, YW, LC, ZC, and MW participated in the study design and clinical data monitoring or data interpretation. OW, LY, and GS performed the biostatistics analysis. LZ, CL, YW, LZ, ZL, GS, and DJ drafted the manuscript. All authors were involved either in the drafting or the revision of the manuscript. All authors have approved the final version of the manuscript.
This work was supported by the State Administration of Traditional Chinese Medicine, China (No. 201007005), the United Project of Guangdong Provincial Department of Science and Technology and Guangdong Provincial Academy of Chinese Medical Science (No. 2011B032200011), and Research Project for Practice Development of National TCM Clinical Research Base. The funders/sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
BYF was patented as a treatment for CKD by the Chinese State Intellectual Property Office in April 2014 (No. 2012100298628). This issued patent is owned by Guangdong Provincial Hospital of Chinese Medicine, and XL, WM, NY, CZo, CL, LeZ, YW, FLu, QL, LW, KB, DZ, PX are the inventors. DJ is a current recipient of an Australian National Health and Medical Research Council Practitioner Fellowship.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.627185/full#supplementary-material.
Ahuja, T. S., Freeman, D., Mahnken, J. D., Agraharkar, M., Siddiqui, M., and Memon, A. (2000). Predictors of the development of hyperkalemia in patients using angiotensin-converting enzyme inhibitors. Am. J. Nephrol. 20, 268–272. doi:10.1159/000013599
Bhandari, S., Ives, N., Brettell, E. A., Valente, M., Cockwell, P., Topham, P. S., et al. (2016). Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol. Dial. Transplant. 3, 255–261. doi:10.1093/ndt/gfv346
Care, J. (2017). Evaluation of Salvia miltiorrhiza radix (Danshen) in the treatment of chronic kidney disease: a literature review. Aust. J. Herb. Med. 29, 96–106. Available at: https://eprints.utas.edu.au/25035/.
Chen, F. P., Chen, T. J., Kung, Y. Y., Chen, Y. C., Chou, L. F., Chen, F. J., et al. (2007). Use frequency of traditional Chinese medicine in Taiwan. BMC Health Serv. Res. 7, 26. doi:10.1186/1472-6963-7-26
Chen, Y., Deng, Y., Ni, Z., Chen, N., Chen, X., Shi, W., et al. (2013). Efficacy and safety of traditional Chinese medicine (Shenqi particle) for patients with idiopathic membranous nephropathy: a multicenter randomized controlled clinical trial. Am. J. Kidney Dis. 62, 1068–1076. doi:10.1053/j.ajkd.2013.05.005
Cui, X. H., Wang, H. L., Wu, R., Yao, P. A., Wei, K. Z., and Gao, J. P. (2018). Effect of Atractylodes macrocephala rhizoma on isoproterenol-induced ventricular remodeling in rats. Mol. Med. Rep. 17, 2607–2613. doi:10.3892/mmr.2017.8121
de Zeeuw, D., Akizawa, T., Audhya, P., Bakris, G. L., Chin, M., Christ-Schmidt, H., et al. (2013). Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 369, 2492–2503. doi:10.1056/NEJMoa1306033
Eisenberg, D. M., Davis, R. B., Ettner, S. L., Appel, S., Wilkey, S., Rompay, M. V., et al. (1998). Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. J. Am. Med. Assoc. 280, 1569–1575. doi:10.1001/jama.280.18.1569
Feng, M., Yuan, W., Zhang, R., Fu, P., and Wu, T. (2013). Chinese herbal medicine Huangqi type formulations for nephrotic syndrome. Cochrane Database Syst. Rev. 5, CD006335. doi:10.1002/14651858.CD006335.pub3
GBD Chronic Kidney Disease Collaboration (2020). Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395, 709–733. doi:10.1016/S0140-6736(20)30045-3
Guo, C., Ding, G., Huang, W., Wang, Z., Meng, and Z., and Xiao, W. (2016). Total saponin of Dioscoreae hypoglaucae rhizoma ameliorates streptozotocin-induced diabetic nephropathy. Drug Des. Devel. Ther. 10, 799–810. doi:10.2147/DDDT.S99670
Hou, F. F., Xie, D., Zhang, X., Chen, P. Y., Zhang, W. R., Liang, M., et al. (2007). Renoprotection of Optimal Antiproteinuric Doses (ROAD) Study: a randomized controlled study of benazepril and losartan in chronic renal insufficiency. J. Am. Soc. Nephrol. 18, 1889–1898. doi:10.1681/ASN.2006121372
Hou, F. F., Zhang, X., Zhang, G. H., Xie, D., Chen, P. Y., Zhang, W. R., et al. (2006). Efficacy and safety of benazepril for advanced chronic renal insufficiency. N. Engl. J. Med. 354, 131–140. doi:10.1056/NEJMoa053107
Hsieh, C., Chang, H., Huang, S., Chen, C. L., Chen, W. T., and Yang, C. C. (2017). Prescribed renoprotective Chinese herbal medicines were associated with a lower risk of all-cause and disease-specific mortality among patients with chronic kidney disease: a population-based follow-up study in Taiwan. Evid. Based Complement. Alternat. Med. 2017, 5632195. doi:10.1155/2017/5632195
Inker, L. A., Shaffi, K., and Levey, A. S. (2012). Estimating glomerular filtration rate using the chronic kidney disease-epidemiology collaboration creatinine equation: better risk predictions. Circ. Heart Fail. 5, 303–306. doi:10.1161/CIRCHEARTFAILURE.112.968545
Leffondre, K., Boucquemont, J., Tripepi, G., Stel, V. S., Heinze, G., and Dunkler, D. (2015). Analysis of risk factors associated with renal function trajectory over time: a comparison of different statistical approaches. Nephrol. Dial. Transplant. 30, 1237–1243. doi:10.1093/ndt/gfu320
Levin, A., and Stevens, P. E. (2014). Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 85, 49–61. doi:10.1038/ki.2013.444
Li, C., Xu, P., Mao, W., and Liu, X. (2013). The necessity of spleen and kidney tonification in the treatment of stage 4 chronic kidney disease. World Science and Technology/Modernization of Traditional Chinese Medicine and Materia Medica 15, 987–989. doi:10.11842/wst.2013.5
Lin, M. Y., Chiu, Y. W., Chang, J. S., Lin, H. L., Lee, C. T., Chiu, G. F., et al. (2015). Association of prescribed Chinese herbal medicine use with risk of end-stage renal disease in patients with chronic kidney disease. Kidney Int. 88, 1365–1373. doi:10.1038/ki.2015.226
Mao, W., Zhang, L., Zou, C., Li, C., Wu, Y., Su, G., et al. (2015). Rationale and design of the Helping Ease Renal failure with Bupi Yishen compared with the Angiotensin II Antagonist Losartan (HERBAAL) trial: a randomized controlled trial in non-diabetes stage 4 chronic kidney disease. BMC Complement. Altern. Med. 15, 316. doi:10.1186/s12906-015-0830-1
Palmer, S. C., Mavridis, D., Navarese, E., Craig, J. C., Tonelli, M., Salanti, G., et al. (2015). Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet 385, 2047–2056. doi:10.1016/S0140-6736(14)62459-4
Patel, S., Sharma, V., Chauhan, N. S., and Dixit, V. K. (2012). An updated review on the parasitic herb of Cuscuta reflexa Roxb. Zhong Xi Yi Jie He Xue Bao. 10, 249–255. doi:10.3736/jcim20120302
Qiao, Y., Shin, J. I., Sang, Y., Inker, L. A., Secora, A., Luo, S., et al. (2019). Discontinuation of angiotensin converting enzyme inhibitors and angiotensin receptor blockers in chronic kidney disease. Mayo Clin. Proc. 94, 2220–2229. doi:10.1016/j.mayocp.2019.05.031
Strippoli, G., Bonifati, C., Craig, M., Navaneethan, S. D., and Craig, J. C. (2006). Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database Syst. Rev. 4, CD006257. doi:10.1002/14651858.CD006257
Tomlinson, L. A., Abel, G. A., Chaudhry, A. N., Tomson, C. R., Wilkinson, I. B., Roland, M. O., et al. (2013). ACE inhibitor and angiotensin receptor-II antagonist prescribing and hospital admissions with acute kidney injury: a longitudinal ecological study. PLoS One 8, e78465. doi:10.1371/journal.pone.0078465
Tsai, C. W., Huang, H. C., Chiang, H. Y., Chung, C. W., Chiu, H. T., Liang, C. C., et al. (2019). First-year estimated glomerular filtration rate variability after pre-end-stage renal disease program enrollment and adverse outcomes of chronic kidney disease. Nephrol. Dial. Transplant. 34, 2066–2078. doi:10.1093/ndt/gfy200
Wang, L., Chi, Y. F., Yuan, Z. T., Zhou, W. C., Yin, P. H., Zhang, X. M., et al. (2014). Astragaloside IV inhibits renal tubulointerstitial fibrosis by blocking TGF-beta/Smad signaling pathway in vivo and in vitro. Exp. Biol. Med. (Maywood) 239, 1310–1324. doi:10.1177/1535370214532597
Wang, L., Yu, J., Fordjour, P. A., Xing, X., Gao, H., Li, Y., et al. (2017). Danshen injection prevents heart failure by attenuating post-infarct remodeling. J. Ethnopharmacol. 9, 22–32. doi:10.1016/j.jep.2017.04.027
Yuan, L. B., Hua, C. Y., Gao, S., Yin, Y. L., Dai, M., Meng, H. Y., et al. (2017). Astragalus polysaccharides attenuate monocrotaline-induced pulmonary arterial hypertension in rats. Am. J. Chin. Med. 45, 773–789. doi:10.1142/S0192415X17500410
Zhang, H., Lin, Z., Xu, C., Leung, C., and Chan, L. S. (2014). Astragalus (a traditional Chinese medicine) for treating chronic kidney disease. Cochrane Database Syst. Rev. 10, CD008369. doi:10.1002/14651858.CD008369.pub2
Zhang, H., Wang, Y., Gao, C., Gu, Y., Huang, J., Wang, J., et al. (2018). Salvianolic acid A attenuates kidney injury and inflammation by inhibiting NF-κB and p38 MAPK signaling pathways in 5/6 nephrectomized rats. Acta. Pharmacol. Sin. 39, 1855–1864. doi:10.1038/s41401-018-0026-6
Zhang, H. W., Lin, Z. X., Tung, Y. S., Kwan, T. H., Mok, C. K., Leung, C., et al. (2014). Cordyceps sinensis (a traditional Chinese medicine) for treating chronic kidney disease. Cochrane Database. Syst. Rev. 18, CD008353. doi:10.1002/14651858.CD008353.pub2
Zhang, J., Xu, W., Wang, P., Huang, J., Bai, J. Q., Huang, Z. H., et al. (2018). Chemical analysis and multi-component determination in Chinese medicine preparation Bupi yishen formula using ultra-high performance liquid chromatography with linear ion Trap-orbitrap mass spectrometry and Triple-quadrupole Tandem mass spectrometry. Front. Pharmacol. 9, 568. doi:10.3389/fphar.2018.00568
Zhang, L., Li, P., Xing, C. Y., Zhao, J. Y., He, Y. N., Wang, J. Q., et al. (2014). Efficacy and safety of Abelmoschus manihot for primary glomerular disease: a prospective, multicenter randomized controlled clinical trial. Am. J. Kidney. Dis. 64, 57–65. doi:10.1053/j.ajkd.2014.01.431
Zhang, L., Wang, F., Wang, L., Wang, W., Liu, B., Liu, J., et al. (2012). Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 379, 815–822. doi:10.1016/S0140-6736(12)60033-6
Keywords: traditional Chinese medicine, losartan, chronic kidney disease, randomized controlled trial, glomerular filtration rate
Citation: Mao W, Yang N, Zhang L, Li C, Wu Y, Ouyang W, Xu P, Zou C, Pei C, Shi W, Zhan J, Yang H, Chen H, Wang X, Tian Y, Yuan F, Sun W, Xiong G, Chen M, Guan J, Tang S, Zhang C, Liu Y, Deng Y, Lin Q, Lu F, Hong W, Yang A, Fang J, Rao J, Wang L, Bao K, Lin F, Xu Y, Lu Z, Su G, Zhang L, Johnson DW, Zhao D, Hou H, Fu L, Guo X, Yang L, Qin X, Wen Z and Liu X (2021) Bupi Yishen Formula Versus Losartan for Non-Diabetic Stage 4 Chronic Kidney Disease: A Randomized Controlled Trial. Front. Pharmacol. 11:627185. doi: 10.3389/fphar.2020.627185
Received: 08 November 2020; Accepted: 29 December 2020;
Published: 29 January 2021.
Edited by:
Ying-Yong Zhao, Northwest University, ChinaReviewed by:
Gao Zhu Ye, China Academy of Chinese Medical Sciences, ChinaCopyright © 2021 Mao, Yang, Zhang, Li, Wu, Ouyang, Xu, Zou, Pei, Shi, Zhan, Yang, Chen, Wang, Tian, Yuan, Sun, Xiong, Chen, Guan, Tang, Zhang, Liu, Deng, Lin, Lu, Hong, Yang, Fang, Rao, Wang, Bao, Lin, Xu, Lu, Su, Zhang, Johnson, Zhao, Hou, Fu, Guo, Yang, Qin, Wen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zehuai Wen, d2VuemhAZ3p1Y20uZWR1LmNu Xusheng Liu, bGl1eHVzaGVuZ0BnenVjbS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.