
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 15 December 2020
Sec. Inflammation Pharmacology
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.615972
 Qi Han1,2,3,4†
Qi Han1,2,3,4† Mingyue Guo1,2,3,4†
Mingyue Guo1,2,3,4† Yue Zheng1,2,3,4†
Yue Zheng1,2,3,4† Ying Zhang1,2,3,4
Ying Zhang1,2,3,4 Yanshan De1,2,3,4
Yanshan De1,2,3,4 Changchang Xu1,2,3,4
Changchang Xu1,2,3,4 Lin Zhang1,2,3,4
Lin Zhang1,2,3,4 Ruru Sun1,2,3,4
Ruru Sun1,2,3,4 Ying Lv1,2,3,4
Ying Lv1,2,3,4 Yan Liang1,2,3,4
Yan Liang1,2,3,4 Feng Xu1,2,3,4
Feng Xu1,2,3,4 Jiaojiao Pang1,2,3,4*
Jiaojiao Pang1,2,3,4* Yuguo Chen1,2,3,4*
Yuguo Chen1,2,3,4*Background: Interleukin-6 (IL-6) is known to be detrimental in coronavirus disease 2019 (COVID-19) because of its involvement in driving cytokine storm. This systematic review and meta-analysis aimed to assess the safety and efficacy of anti-IL-6 signaling (anti-IL6/IL-6R/JAK) agents on COVID-19 based on the current evidence.
Methods: Studies were identified through systematic searches of PubMed, EMBASE, ISI Web of Science, Cochrane library, ongoing clinical trial registries (clinicaltrials.gov), and preprint servers (medRxiv, ChinaXiv) on August 10, 2020, as well as eligibility checks according to predefined selection criteria. Statistical analysis was performed using Review Manager (version 5.3) and STATA 12.0.
Results: Thirty-one studies were included in the pooled analysis of mortality, and 12 studies were identified for the analysis of risk of secondary infections. For mortality analysis, 5630 COVID-19 cases including 2,132 treated patients and 3,498 controls were analyzed. Anti-IL-6 signaling agents plus standard of care (SOC) significantly decreased the mortality rate compared to SOC alone (pooled OR = 0.61, 95% CI 0.45–0.84, p = 0.002). For the analysis of secondary infection risk, 1,624 patients with COVID-19 including 639 treated patients and 985 controls were included, showing that anti-IL-6 signaling agents did not increase the rate of secondary infections (pooled OR = 1.21, 95% CI 0.70–2.08, p = 0.50). By contrast, for patients with critical COVID-19 disease, anti-IL-6 signaling agents failed to reduce mortality compared to SOC alone (pooled OR = 0.75, 95% CI 0.42–1.33, p = 0.33), but they tended to increase the risk of secondary infections (pooled OR = 1.85, 95% CI 0.95–3.61, p = 0.07). A blockade of IL-6 signaling failed to reduce the mechanical ventilation rate, ICU admission rate, or elevate the clinical improvement rate.
Conclusion: IL-6 signaling inhibitors reduced the mortality rate without increasing secondary infections in patients with COVID-19 based on current studies. For patients with critical disease, IL-6 signaling inhibitors did not exhibit any benefit.
The COVID-19 pandemic has resulted in numerous deaths, and continues to circulate worldwide (World Health Organization, 2019). Effective treatment is still urgently needed. In addition to novel therapeutic regimes, repurposing available pharmaceuticals based on key pathological changes is extremely important for the treatment of COVID-19.
After invading the body, SARS-CoV-2 binds to angiotensin-converting enzyme 2 on cell membranes, enters cells by membrane infusion, completes self-replication, and triggers complex responses. Inflammatory responses induced in immune cells, and epithelial and endothelial cells were demonstrated to be crucial in triggering “cytokine storm” and lead to severe injury in the context of COVID-19 (Azkur et al., 2020). Cytokine storm was also proven to play an important role in severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (Liu et al., 2020a), characterized by robust amplification of proinflammatory cytokines including tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β), and others (Liu et al., 2020b; Chen et al., 2020; Huang et al., 2020). Cytokine storm further leads to inflammatory filtration (e.g., exudative lung lesions), tissue destruction, and even multi-organ dysfunction. Cytokine storm is more destructive than the virus itself. The latest World Health Organization (WHO) guideline recommends systemic corticosteroids for the treatment of severe and critical COVID-19 patients (World Health Organization, 2020). Corticosteroids are powerful drugs used to fight cytokine storm, but its side effects should not be ignored, including secondary infections, hyperglycemia, peptic ulcer, sterile necrosis of the femoral head, and so on. Antibodies that can accurately combat cytokine storm might be a perspective choice.
IL-6 is thought to be a key mediator of cytokine storm, causing tissue injury and the progression of COVID-19. Levels of serum IL-6 and IL-6 receptors (IL-6R) were significantly elevated in patients with COVID-19 (Liu et al., 2020a), and were closely associated with respiratory failure, acute respiratory distress syndrome, secondary infections, and death (Somers et al., 2020). A meta-analysis of 21 studies involving 3,377 patients with COVID-19 supported the notion that IL-6 was a significant indicator for the severity of COVID-19 (Henry et al., 2020).
IL-6 functions via a signaling network to amplify inflammation and induce other damage. Under stimuli, IL-6 can be generated from various types of cells including monocytes, macrophages, T cells, B cells, epithelial and endothelial cells, and fibroblasts (Tanaka et al., 2014). There are two types of IL-6 receptors: membrane-bound IL-6 receptors (mIL-6R) and soluble IL-6 receptors (sIL-6R). Membrane-bound IL-6R is localized mainly in immune cells, while soluble IL-6R is produced through the cleavage of mIL-6R, which displays a biological effect in various ways (Kang et al., 2019; Tanaka et al., 2016; Moore & June, 2020). When IL-6 binds to mIL-6R or sIL-6R, the complex triggers the dimerization of transmembrane glycol protein 130 (gp130), followed by the activation of Janus kinases (JAK) that subsequently initiates intracellular signaling, including mitogen-activated protein kinases (MAPK) and signal transducers and activators of transcription (STAT) pathways (Heinrich et al., 2003). The activation of the MAPK cascade, including Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK) and p38, mediates the expression of multiple pro-inflammatory genes as transcriptional factors (Arthur and Ley, 2013). For example, JNK signaling upregulates the transcription of M1 macrophage-specific genes including CD86, iNOS, and IL-1β in bone marrow-derived macrophages. The ERK pathway triggers the transcription of cytokine genes such as IL-1β, IL-8, and TNF-α in macrophages and mesenchymal stem cells. p38 signaling controls the strength and duration of inflammatory responses by MAPK activated kinase 2. The activation of STAT3 is essential for the transformation of Th0 cells to Th17 cells, which can produce IL-17, IL-6, and TNF-α which promotes an inflammatory response (Luo et al., 2020a). (Figure 1). Previous studies demonstrated that IL-6, together with IL-1β and TNF-α, damage the cellular basement membrane and extracellular matrix, leading to high tissue permeability and edema via upregulating trypsin and activating matrix metalloproteinases (Indalao et al., 2017). Moreover, IL-6 was found to be related to the exhaustion of CD4+ and CD8+ T cells in patients with COVID-19, evidenced by a negative association of IL-6 with the numbers of T lymphocytes (Zhang et al., 2020).
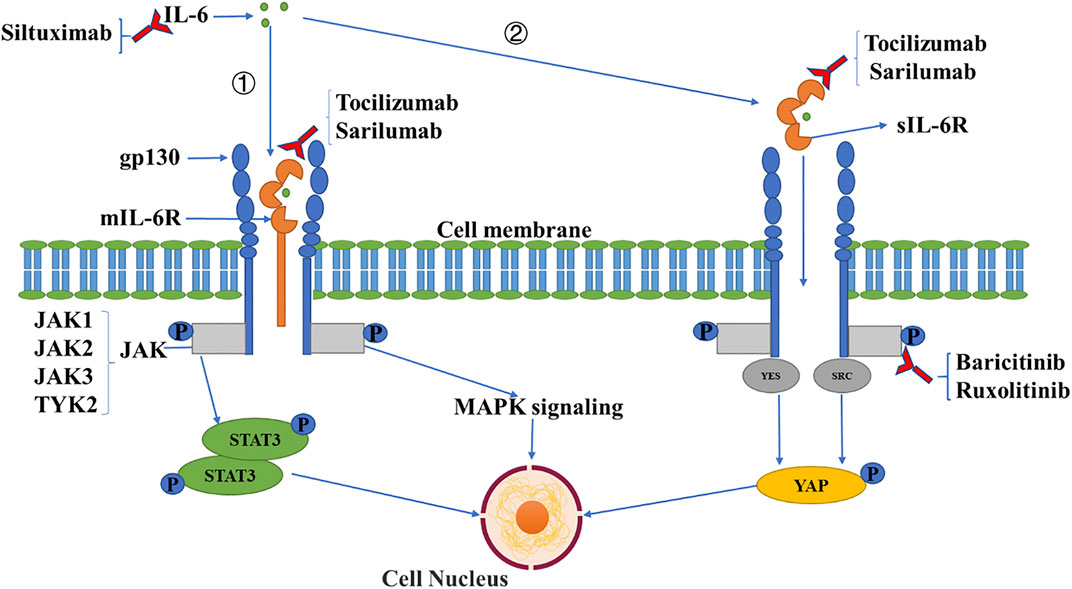
FIGURE 1. IL-6 signaling pathway and inhibitory agents. IL-6 binds to mIL-6R (①) or sIL-6R (②), which triggers the dimerization of transmembrane gp130, followed by the activation of JAK which induces intracellular signaling including MAPK and STAT signaling pathways, and the activation of the SRC family kinase (YES, SRC), with the YES-associated protein signaling pathway subsequently induced. Three types of antibodies have been applied clinically against IL-6 signaling including anti-IL-6 (siltuximab), anti-IL-6R (tocilizumab, sarilumab), and anti-JAK (baricitinib, ruxolitinib) monoclonal antibodies.
To date, three categories of antibodies have been developed against IL-6 signaling, including anti-IL-6, anti-IL-6R, and anti-JAK monoclonal antibodies. These are used to treat autoimmune diseases such as rheumatoid arthritis. Based on the crucial role of IL-6 in SARS-CoV-2-induced injury, investigation of the safety and efficacy of anti-IL-6 signaling agents is extremely important for patients with COVID-19.
In this meta-analysis and systemic review, we pooled data from all available studies with acceptable quality to assess the effects of anti-IL-6/IL-6R/JAK antibodies on COVID-19.
We identified studies involving antibodies that block IL-6 signaling for the treatment of patients confirmed to have COVID-19 using a systematic search on PubMed, EMBASE, ISI Web of Science, Cochrane library, ongoing clinical trial registries (clinicaltrials.gov), and preprint servers including medRxiv, ChinaXiv on August 10, 2020. The search algorithm combining relevant key words were as follows: “anti-interleukin 6 receptor OR anti-interleukin 6 OR anti-IL-6R OR anti-IL-6 OR anti-JAK OR tocilizumab OR sarilumab OR siltuximab OR sirukumab OR olokizumab OR clazakizumab OR sylvant OR EBI-031 OR actemra OR kevzara NI-1201 OR vobarilizumab OR olamkicept OR tofacitinib OR XELJANZ OR ruxolitinib OR jakavi OR filgotinib OR baricitinib OR olumiant OR upadacitinib OR Rinvoq OR PF-04965842” AND “COVID-19 OR coronavirus OR SARS-CoV-2 OR COVID”. We did not limit our search by language. The aim of our systematic review and meta-analysis was to assess clinical outcomes and adverse events in patients with COVID-19 treated with anti-IL-6/IL-6R/JAK signaling antibodies.
Eligibility was checked carefully in accordance with predefined selection criteria by reading titles, abstracts, and full manuscripts. We included studies that investigated the safety and efficacy of anti-IL-6/IL-6R/JAK antibodies on adult patients with confirmed SARS-CoV-2 infections. Controlled studies, including randomized controlled trials, cohort studies, and case-control studies, were incorporated in the meta-analysis. Uncontrolled studies were descriptively reviewed. We excluded case reports, letters without original data, reviews, systematic reviews, protocols, non-human studies, duplicates, and studies without complete data.
Two independent investigators reviewed the full texts of relevant studies, extracting essential features of each study, including the name of the first author, type of study, performed country, the number of patients, and baseline characteristics of participants such as age, sex, comorbidities including hypertension, chronic kidney disease, diabetes, obesity, heart and lung diseases, and clinical outcomes. The primary endpoint of interest was the mortality rate in patients confirmed with COVID-19. Secondary outcomes included the requirement of mechanical ventilation, intensive care unit (ICU) admission, clinical improvement, and secondary infections. The methodological quality of case-control studies and cohort studies were assessed using the Newcastle-Ottawa Scale (NOS) by two independent investigators. Cochrane’s bias risk assessment tool was used to evaluate the quality of the randomized controlled trial. The MINORS index was used to evaluate the quality of single-arm research.
We used the Mantel-Haenszel method for the analysis of categorical variables. Statistical heterogeneity among studies was measured using Cochrane Q statistics and heterogeneity index I2. If the heterogeneity was high (I2 > 50%), we used the random-effects model. Otherwise, the fixed-effects model was used for analysis. The subgroup analyses were implemented according to performed continents, the severity of COVID-19 and the type of anti-IL-6 signaling agent. Sensitivity analysis was carried out by excluding studies one by one and observing whether the heterogeneity changed. Publication bias was evaluated using funnel plot analysis, which was considered to indicate no statistical difference if p > 0.05 in Begg’s and Egger’s tests. Statistical analysis was performed using Review Manager (version 5.3) and STATA 12.0.
Clinical improvement was defined as discharge from hospital, a decrease of at least two points from baseline on the seven-category ordinal scale, or both. The seven-category ordinal scale as recommended by the WHO R&D Blueprint Group (https://www.who.int/teams/blueprint/covid-19) is as follows: 1) not hospitalized and able to resume normal activities; 2) not hospitalized, but unable to resume normal activities; 3) hospitalized, not requiring supplemental oxygen; 4) hospitalized, requiring supplemental oxygen; 5) hospitalized, requiring nasal high-flow oxygen therapy, non-invasive ventilation, or both; 6) hospitalized, requiring extracorporeal membrane oxygenation, invasive mechanical ventilation, or both; and 7) death.
The severity of disease has no pre-defined and unified definition. We accepted the classification of severity in each included study. The definition of SOC was also according to every specific study.
We identified 59 studies according to the selection criteria. We performed meta-analysis with one randomized controlled trial and 32 controlled studies. Others were single-arm studies, which we did not use in the production of conclusions, but only briefly described as part of the systematic review to introduce readers to the progress in this field. The flowchart of the literature search and screening process are listed in Figure 2.
The characteristics of the 33 controlled studies are shown in Table 1. Regarding types of anti-IL-6 signaling agents, 29 studies used anti-IL-6R antibodies (tocilizumab in 28 studies and sarilumab in one study). One study involved an anti-IL-6 antibody (siltuximab), and three studies involved anti-JAK-1/2 antibodies (baricitinib in two studies and ruxolitinib in one study).
For the countries where the studies were implemented, European countries were involved in 21 studies (Italy 14, France three, Spain three, and Sweden one), the US conducted ten studies, and Asian countries conducted two studies (China one and India one).
Thirty-one studies reported mortality as an outcome, involving 2,132 treated patients and 3,498 controls. The results of pooled analysis showed that anti-IL-6 signaling agents plus SOC significantly decreased the mortality rate relative to SOC alone in patients with COVID-19 (pooled OR = 0.61, 95% CI 0.45–0.84, p = 0.002). However, the heterogeneity of these studies was high (I2 = 73%, p < 0.00001) (Figure 3).
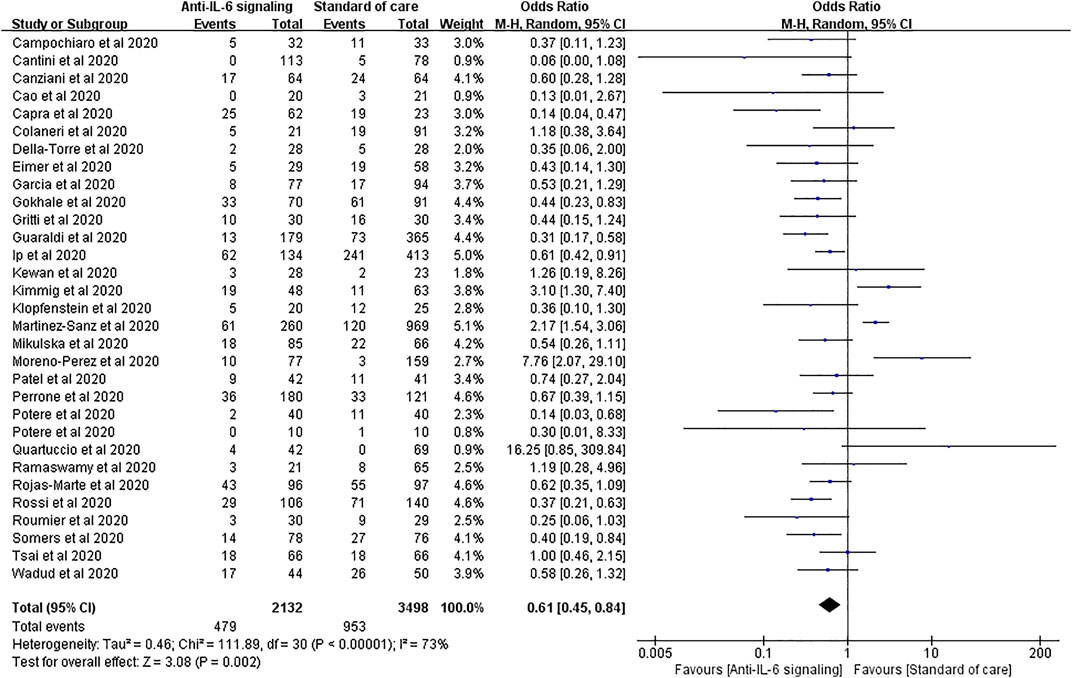
FIGURE 3. Pooled odds ratio and forest plot of mortality between the anti-IL-6 signaling treatment and standard of care (SOC) groups among patients with COVID-19. Thirty-one studies including 5,630 patients with COVID-19 were included in the statistical analysis, with 2,132 treated patients and 3,498 controls. The result showed that anti-IL-6 signaling agents plus SOC significantly decreased mortality relative to SOC alone in patients with COVID-19 (pooled OR = 0.61, 95% CI 0.45–0.84, p = 0.002).
To reduce the high heterogeneity, we performed sensitivity analysis and found that the study by Martinez-Sanz et al. had a substantial effect (Supplementary Figure S1). When this study was excluded, statistically significant decreases in mortality were still observed in the treated group compared with the SOC group, with lower heterogeneity (pooled OR = 0.57, 95% CI 0.44–0.74, p < 0.0001; I2 = 54%, p = 0.0002).
We performed a subgroup analysis according to the severity of COVID-19 by sorting severe and critical patients. For patients with severe COVID-19, anti-IL-6 signaling agents displayed remarkable advantages in reducing mortality compared to SOC, consistent with the result above (pooled OR = 0.49, 95% CI 0.32–0.74, p = 0.0007). Notably, for patients with critical disease, anti-IL-6 signaling agents failed to reduce the mortality rate compared to SOC (pooled OR = 0.75, 95% CI 0.42–1.33, p = 0.33) (Figure 4).
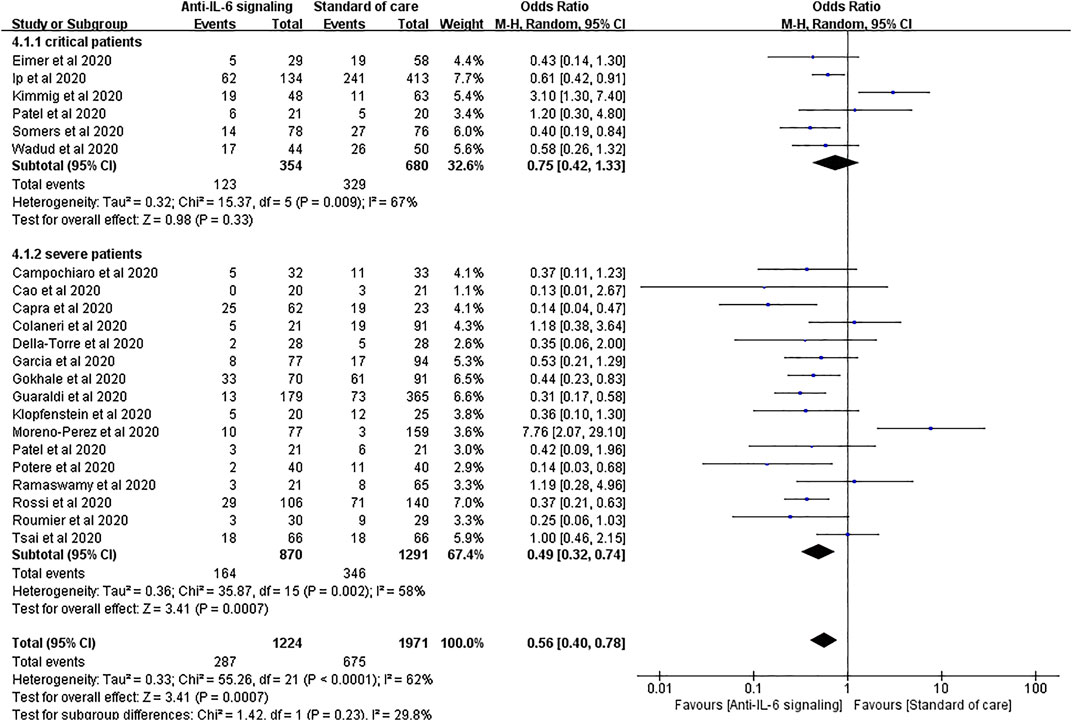
FIGURE 4. Subgroup analysis according to severity of patients with COVID-19 for pooled odds ratio of mortality. Subgroup analysis compared the risk of mortality in groups of severe or critical patients with COVID-19 separately. Six studies were included in the subgroup of critical patients, including 354 treated patients and 680 controls. There was no statistical difference in the risk of mortality between the two groups of critical patients (pooled OR = 0.75, 95% CI 0.42–1.33, p = 0.33). Sixteen studies were included in the subgroup of severe patients, including 870 treated patients and 1,291 controls. The mortality rate in the anti-IL-6 signaling treatment group was remarkably reduced (pooled OR = 0.49, 95% CI 0.32–0.74, p = 0.0007) compared to the SOC group in patients with severe disease.
According to the types of anti-IL-6 signaling drugs (i.e., IL-6 neutralizing, IL-6 receptor blockers, and JAK inhibitors), we did a subgroup analysis showing that both IL-6 receptor blockers and JAK inhibitors showed superiority in reducing death rate compared to SOC (OR = 0.64, 95% CI 0.47–0.89, p = 0.007; OR = 0.09, 95% CI 0.01–0.70, p = 0.02, respectively), while IL-6 neutralizing drug tended to reduce deaths but did not reach a significant difference (OR = 0.44, 95% CI 0.15–1.24, p = 0.12) (Supplementary Figure S2).
Subgroup analysis according to various continents where these studies were implemented showed that studies in American patients did not support the effectiveness of anti-IL-6 signaling agents on mortality from COVID-19 (Supplementary Figure S3).
Secondary infections are among the most reported adverse effects of anti-IL-6 signaling agents. Twelve studies reported secondary infections, involving 639 treated patients and 985 controls. No significant difference in the rate of secondary infections was found between the two groups (pooled OR = 1.21, 95% CI 0.70–2.08, p = 0.50) (Figure 5).
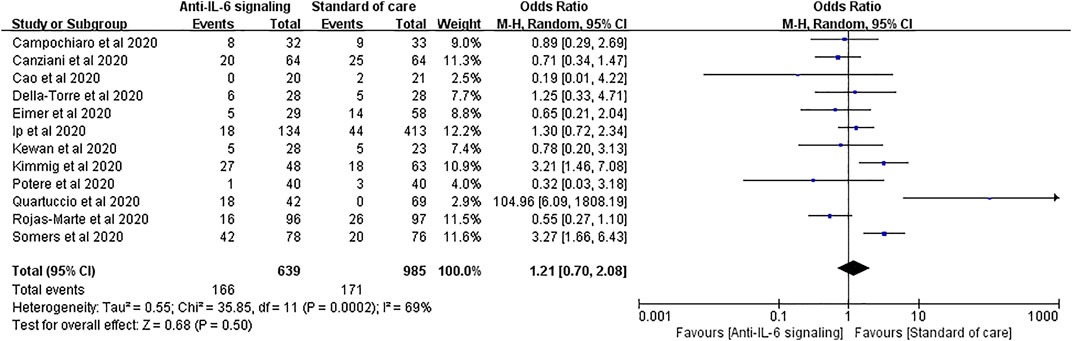
FIGURE 5. Pooled odds ratio and forest plot of secondary infections between the anti-IL-6 signaling treatment group and standard of care group in patients with COVID-19. Twelve studies were included in the statistical analysis on secondary infections, including 639 patients treated with IL-6 signaling inhibitors and 985 patients with SOC. There was no significant difference in terms of secondary infection rates between patients with COVID-19 treated with anti-IL-6 signaling agents and those treated with SOC (pooled OR = 1.21, 95% CI 0.70–2.08, p = 0.50).
We performed a further subgroup analysis according to the severity of disease. For patients with severe disease, the risk of secondary infections did not substantially increase with anti-IL-6 signaling treatment compared to SOC alone (pooled OR = 0.81, 95% CI 0.37–1.75, p = 0.59). Again, it is noteworthy that the rate of secondary infections tended to increase in the group treated with anti-IL-6 signaling agents compared to the SOC group in patients with critical disease (pooled OR = 1.85, 95% CI 0.95–3.61, p = 0.07), despite not reaching statistical difference (Figure 6).
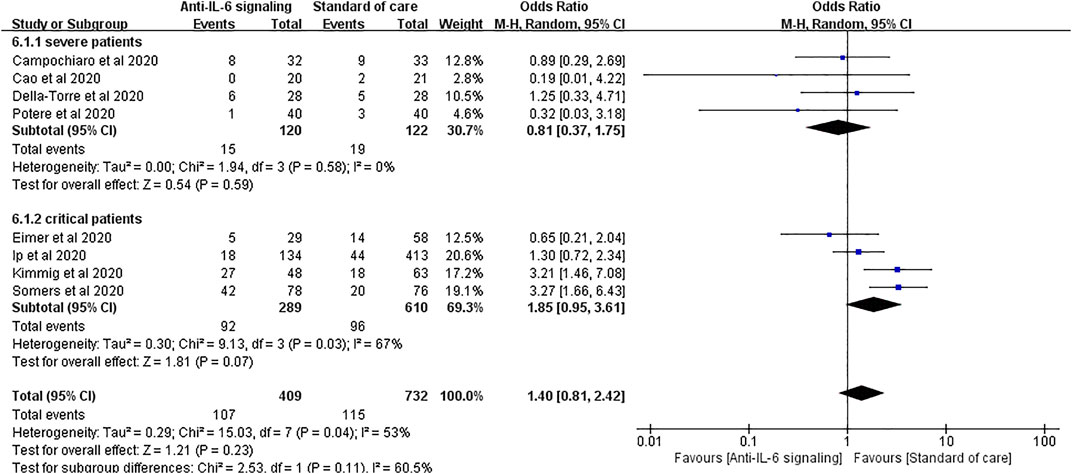
FIGURE 6. Subgroup analysis according to severity of patients with COVID-19 for pooled odds ratio of secondary infections. Four studies were included in the subgroup of patients with severe disease, including 120 patients treated with IL-6 signaling inhibitors and 122 patients treated with SOC. There was no significant difference in terms of risk of infection between groups (pooled OR = 0.81, 95% CI 0.37–1.75, p = 0.59). Four studies were included in the subgroup of patients with critical disease, including 289 patients treated with IL-6 signaling inhibitors and 610 patients treated with SOC. The risk of secondary infections tended to be greater in the subgroup of patients with critical disease treated with anti-IL-6 signaling agents than in those treated with SOC (pooled OR = 1.85, 95% CI 0.95–3.61, p = 0.07), though it did not reach statistical significance.
Seventeen studies applied mechanical ventilation rate as an outcome, with 1,166 treated cases and 2,623 controls. The pooled analysis showed that anti-IL-6 signaling agents did not reduce the rate of mechanical ventilation relative to SOC for patients with severe disease (pooled OR = 0.81, 95% CI 0.42–1.57, p = 0.53) (Figure 7).
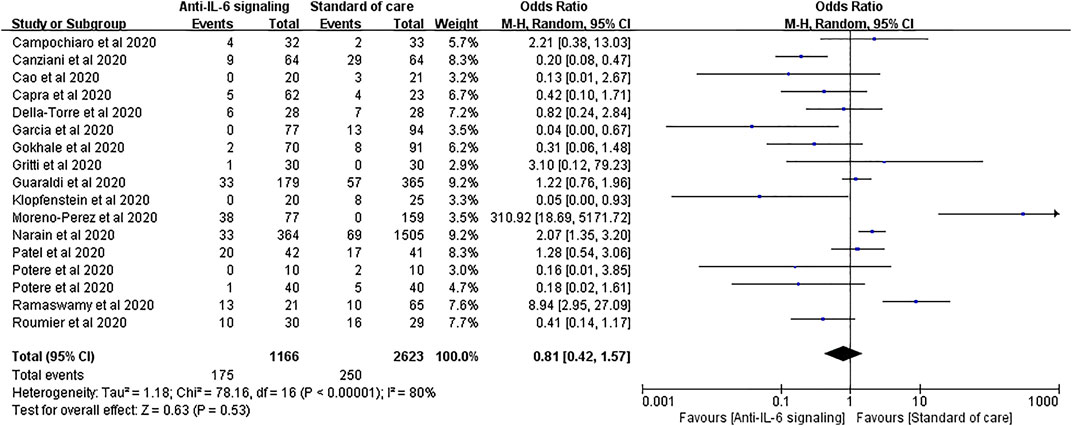
FIGURE 7. Pooled odds ratio and forest plot of mechanical ventilation rate between the anti-IL-6 signaling treatment group and the standard of care group among patients with COVID-19. Seventeen studies were included in the statistical analysis of the mechanical ventilation rate including 1,166 patients treated with IL-6 signaling inhibitors and 2,623 patients treated with SOC. Anti-IL-6 signaling treatment did not have a beneficial effect on the mechanical ventilation rate relative to SOC (pooled OR = 0.81, 95% CI 0.42–1.57, p = 0.53).
Similar results were found in the outcomes of ICU admission. Nine studies with 643 treated patients and 1,534 controls were pooled for analysis, showing no statistical difference between the two groups (pooled OR = 1.02, 95% CI 0.27–3.85, p = 0.98) (Figure 8). However, in patients treated with JAK inhibitors, the ICU admission rate was significantly reduced compared to SOC (OR = 0.04, 95% CI 0.00–0.29, p = 0.002) (Supplementary Figure S2).
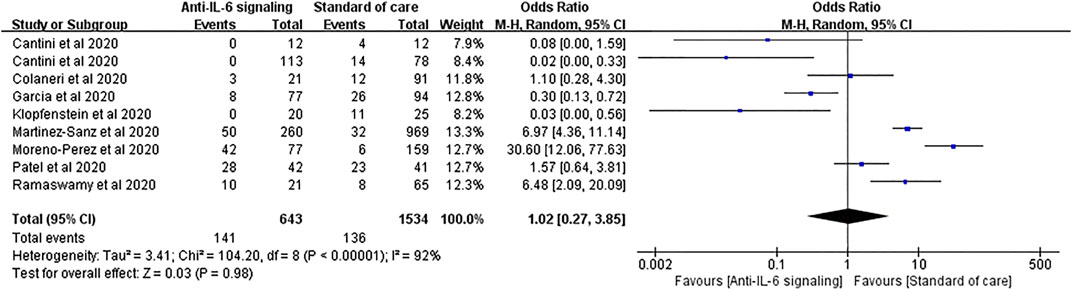
FIGURE 8. Pooled odds ratio and forest plot of ICU admission rate between the anti-IL-6 signaling treatment group and the standard of care group among patients with COVID-19. Nine studies were included in the statistical analysis with 643 patients treated with IL-6 signaling inhibitors and 1,534 patients with SOC. Anti-IL-6 signaling treatment did not show a beneficial effect on the ICU admission rate compared to SOC in patients with COVID-19 (pooled OR = 1.02, 95% CI 0.27–3.85, p = 0.98).
For the clinical improvement rate, pooled analysis of six studies involving 210 treated patients and 168 controls showed that 67.6% and 62.5% patients in the anti-IL-6 signaling and the SOC groups, respectively, displayed clinical improvement (pooled OR = 1.81, 95% CI 0.75–4.39, p = 0.19) (Figure 9).
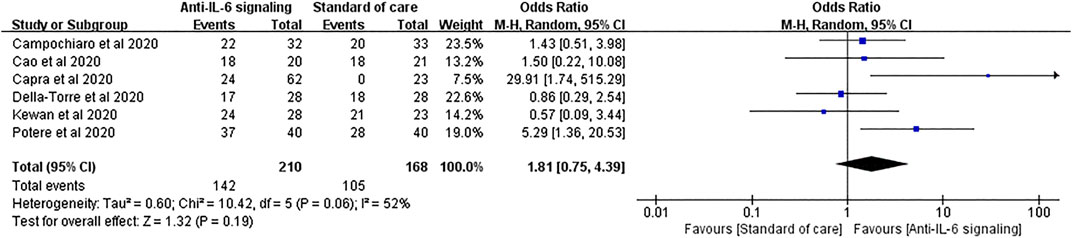
FIGURE 9. Pooled odds ratio and forest plot of clinical improvement rate between the anti-IL-6 signaling treatment group and the standard of care group among patients with COVID-19. Six studies were included in the analysis of clinical improvement, including 210 patients treated with IL-6 signaling inhibitors and 168 patients treated with SOC. Anti-IL-6 signaling treatment did not show any beneficial effects on the clinical improvement rate compared to SOC in patients with COVID-19 (pooled OR = 1.81, 95% CI 0.75–4.39, p = 0.19).
Randomized and non-randomized controlled trials were assessed using Cochrane’s bias risk assessment tool and NOS, respectively (Supplementary Table S1). We found that all the controlled studies were of satisfactory high quality for meta-analysis.
We utilized the funnel plot to analyze publication bias in the 31 studies included in the pooled analysis of mortality. The dots symbolizing the studies mostly clustered in the middle and at the top of the funnel plot, with both sides nearly symmetrical (Figure 10). Only eight articles presented outside the funnel plot. Begg’s and Egger’s tests further demonstrated no significant publication bias (Begg’s test, p = 0.892; Egger’s test, p = 0.158).
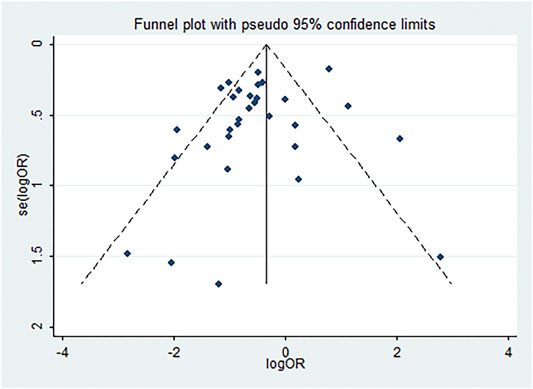
FIGURE 10. Funnel plot analysis of publication bias. Publication bias of the 31 studies. Dots symbolizing studies mostly clustered in the middle and at the top of the funnel plot; both sides are nearly symmetrical, and only eight articles are outside the funnel plot.
We reviewed 26 single-arm studies including 1,565 patients. Characteristics of the studies are shown in Supplementary Table S2. Two individual researchers performed quality evaluation with the MINORS index shown in Supplementary Table S1.
In these single-arm studies, mortality ranged from 0 to 43% in patients with COVID-19 treated with anti-IL-6 signaling agents, and the raw overall mortality was 15.27%, which was significantly lower than the pooled mortality of the SOC group (15.27 vs. 27.24%, p < 0.0001) and also the anti-IL-6 signaling group (15.27 vs. 22.47%, p < 0.0001) in the meta-analysis. These differences may be partially derived from the different baseline characteristics of patients with COVID-19. Six single-arm studies reported secondary bacterial infections, with an overall rate of 14.35%, which was also remarkably lower than that of the SOC group (14.35 vs. 17.36%, p < 0.0001) and the anti-IL-6 signaling group (14.35 vs. 25.98%, p < 0.0001) in the meta-analysis. Four single-arm studies reported increased hepatic enzyme levels, and other studies reported increased creatinine, thrombocytopenia, or neutropenia.
The largest single-arm study was reported by Sinha et al. with 255 patients with COVID-19 enrolled; the overall mortality was 10.98%. Patients with COVID-19 were divided into two groups according to the fraction of inspiration O2 (FiO2 < 45% or >45%) at the time of administration of IL-6R antibody treatment (sarilumab or tocilizumab). The mortality rate was significantly lower in the FiO2 < 45% group compared to the FiO2 > 45% group (adjusted hazard ration 0.24, 95% CI 0.08–0.74). Similarly, patients in the FiO2 < 45% group had a higher discharge rate and a lower intubation rate. This suggests that the early use of IL-6R antibodies in less severe patients was more beneficial.
In this meta-analysis and systematic review, we analyzed 33 controlled studies, including 31 studies for pooled analysis of mortality (5,630 patients with COVID-19), 12 studies for pooled analysis of secondary infections (1,624 patients with COVID-19), and appropriate studies for the assessment of mechanical ventilation, ICU admission, and clinical improvement. Our results showed that a blockade of IL-6 signaling by anti-IL-6/IL-6R/JAK antibodies plus SOC significantly reduced the mortality rate but did not increase the risk of secondary infections compared to SOC alone in patients with COVID-19. By contrast, in patients with critical disease, the beneficial effect of anti-IL-6/IL-6R/JAK antibodies on mortality became indistinct, and resulted in an evident trend of increased secondary infection rates. Blockade of IL-6 signaling did not show a beneficial effect on reducing the mechanical ventilation rate, decreasing ICU admission, or increasing clinical improvement.
IL-6 plays a key role in immune cell proliferation and differentiation through the IL-6/IL-6R/JAK pathway. To date, clinically available antibodies targeting the IL-6/IL-6R/JAK pathway include anti-IL-6 antibodies (e.g., siltuximab), anti-IL-6R antibodies (e.g., tocilizumab and sarilumab), and anti-JAK antibodies (e.g., ruxolitinib and baricitinib), all of which have been successfully used to treat autoimmune diseases such as rheumatoid arthritis. Tocilizumab and sarilumab are both humanized anti-IL-6 receptor monoclonal antibodies, capable of binding to either mIL-6R or sIL-6R. Tocilizumab has been approved in several countries since 2005 and is currently used to treat rheumatoid arthritis, giant cell arteritis, and juvenile idiopathic arthritis (Tanaka et al., 2014). Sarilumab was approved in the USA for the treatment of refractory rheumatoid arthritis in 2017 (Lamb and Deeks, 2018). Siltuximab is an anti-IL-6 immunoglobulin G-k monoclonal antibody that specifically binds to IL-6 and neutralizes its biological activity; it was approved in the USA in 2014 with restriction to the treatment of Castleman disease (Lyseng-Williamson, 2015). Baricitinib and ruxolitinib, both targeting JAK1 and JAK2 which are the dominant kinases downstream of IL-6, have been approved for the treatment of myelofibrosis and rheumatoid arthritis, respectively (Garbers et al., 2018).
Mortality was set as the primary outcome in this meta-analysis. Except for two studies that reported no specific data concerning death rate, 31 controlled studies were involved in the pooled analysis of mortality, including 5,630 patients with COVID-19. We found that anti-IL-6 signaling agents significantly decreased mortality in patients with COVID-19. There was high heterogeneity (I2 = 73%, p < 0.00001), partially due to varying study designs, patient severity, agent dosages, and follow-up durations. In the sensitivity analysis, removal of the largest study (Martinez-Sanz et al.) generated lower heterogeneity with no substantial alteration of the pooled OR.
Strikingly, we found that blockade of IL-6 signaling failed to effectively reduce mortality in patients with critical disease. Previous studies reported that serum IL-6 levels were elevated in patients with critical disease in comparison with severe patients in the early stages of SARS-CoV-2 infection (Cummings et al., 2020; Han et al., 2020). One study illustrated the predictive ability of IL-6 for prognosis in patients with COVID-19 (Han et al., 2020). Therefore, we speculated that IL-6 in critical patients was not sufficiently neutralized. On the other hand, more complex pathophysiological changes may occur in the critical stage that limit the therapeutic effect of anti-IL-6 signaling agents. This supports the early application of IL-6 blockade treatment for patients with COVID-19.
Of note, Marfella et al. found that patients with COVID-19 who had hyperglycemia at admission had five-fold higher plasma IL-6 levels than non-hyperglycemia cases, and hyperglycemia diminished the effect of tocilizumab on disease progression, possibly through the upregulation of IL-6 (Marfella et al., 2020; Sardu et al., 2020a; Sardu et al., 2020b). Sardu et al. discovered that an early glucose control within the first 24 hours significantly slowed COVID-19 progression and improved survival (Sardu et al., 2020c). In our hands, critical patients have a higher rate of previously diagnosed diabetes than severe patients (311/940, 33.09% vs. 429/1971, 21.77%, p < 0.0001), and in severe patients who received tocilizumab treatment, diabetic patients tended to have higher mortality compared to non-diabetic patients, but without statistical significance (OR = 1.64, 95% CI 0.81–3.32, p = 0.17) (Supplementary Figure S4). Therefore, hyperglycemia may contribute to the unsatisfactory effect of tocilizumab on patients with critical disease, and early glucose management could be a perspective strategy to improve the effect of tocilizumab on critical cases. It was also shown that patients with critical COVID-19 had higher morbidity of hypertension than severe cases (570/940, 60.64% vs. 794/1971, 40.28%, p < 0.0001), and in severe patients who received tocilizumab treatment, hypertensive patients with COVID-19 tended to have a high death rate compared to non-hypertensive patients (OR = 1.85, 95% CI 0.93–3.67, p = 0.08) (Supplementary Figure S5). However, whether hypertension has a negative effect on tocilizumab function still needs to be confirmed (Sardu et al., 2020d). Moreover, ABO blood types might be associated with outcomes in patients with critical disease. As reported by Sardu et al., non-O blood types in hypertensive patients were closely related to pro-thrombotic status, higher rate of cardiac injury, and deaths compared to O blood type patients with COVID-19 (Sardu et al., 2020e). Finally, how to achieve the optimal effect of anti-IL-6 signaling agents in patients with critical COVID-19 is still worth further study.
Subgroup analysis also revealed that studies in the USA did not support the effectiveness of anti-IL-6 signaling agents on mortality in patients with COVID-19, which was different from the pooled results of the European studies or Asian studies. This may result from disparate patient severities, diverse SOC regimens, and varying study methods.
The issue of the secondary infection rate was prioritized in this meta-analysis. IL-6/IL-6R/JAK blockade therapeutics were reported to increase the risk of secondary bacterial infection (Rose-John et al., 2017). Both anti-IL-6R antibodies tocilizumab and sarilumab received black box warnings regarding the risks of serious infections for patients with pulmonary diseases, issued by the U.S. Food and Drug Administration (Food and Drug Administration, 2010; Food and Drug Administration, 2018). Studies indicated that approximately 8% of rheumatoid arthritis patients treated with tocilizumab had serious infections (Smolen et al., 2007; Campbell et al., 2011). Thus, whether the off-label use of anti-IL-6 signaling agents for COVID-19 could increase secondary infections is indeed a matter of concern. We analyzed 12 studies that collected data about secondary infections, and found no significant difference between anti-IL-6 signaling agents and SOC.
However, in a subgroup analysis in patients with severe and critical disease, we found a substantial tendency toward an increased rate of secondary infections owing to anti-IL-6 signaling treatment in the critical group. This might be interpreted as immunosuppression status giving a higher probability of occurrence and higher severity in patients with critical disease. This gives us a hint that, for patients with critical disease, treatment with IL-6 inhibitors needs to be considered in the context of immune status.
Our study has several limitations. One limitation is that only one randomized controlled study was included, because the implementation of this type of study is confronted with many obstacles in the pandemic situation. The second limitation is the high heterogeneity in some results. Non-randomized methods, diverse severities, different dosages and routes of administration of anti-IL-6 signaling agents, and various regimens of SOC (e.g., anti-viral agents and corticosteroids) may contribute to the heterogeneity of the studies. Third, baseline characteristics such as age, gender, hypertension, and obesity of the two groups of patients did not match (Table 2). Therefore, randomized trials with relatively large sample sizes and rational designs are still needed. Moreover, due to the absence of data, our study did not consider or analyze the baseline levels of IL-6; therefore, we could not illustrate the specific function of IL-6/IL-6R/JAK pathway antibodies on patients with COVID-19 with different baseline IL-6 levels.
Despite these limitations, our review and meta-analysis summarized the available studies to some extent, and provided some basis for clinical practice and further large-scale studies. The forthcoming results of a phase III randomized controlled study sponsored by Hoffmann-La Roche (COVACTA, NCT04320615) may provide more valid clinical evidence and understanding of anti-IL-6 signaling agent usage in patients with COVID-19.
Under conditions where no specific medication proves to be efficacious, anti-IL-6 signaling agents are promising for the treatment of COVID-19. However, for patients with critical disease, the risk-benefit ratio of IL-6 signaling blockade agents should be carefully evaluated. Randomized controlled trials, trials on patients with critical disease, and studies on the effect of a blockade of IL-6 signaling on laboratory parameters remain in urgent need.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
QH and MG contributed to the literature research and data extraction. QH and YuZ contributed to the quality evaluation. YiZ, CX, and LZ helped with the literature search. RS, YD, YiL, and YaL helped with data extraction. FX provided valuable advice for the methods and manuscript writing. QH and YuZ wrote the first version of the manuscript. JP and YC contributed to the study design and manuscript revision.
This study was supported by the National Key R&D Program of China (2020YFC0846600), the Shandong Provincial Key R&D Program (2020SFXGFY03), the National Natural Science Foundation of China (81701952), the Taishan Pandeng Scholar Program of Shandong Province (tspd20181220), the Taishan Young Scholar Program of Shandong Province (tsqn20161065, tsqn201812129), and the Qilu Young Scholar Program.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All authors would like to pay tribute to all healthcare workers for their arduous efforts to combat the COVID-19 pandemic.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.615972/full#supplementary-material.
COVID-19, coronavirus disease 2019; ERK, extracellular signal-regulated kinase; FiO2, fraction of inspired oxygen; gp130, glycol protein 130; ICU, intensive care unit; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-6R, IL-6 receptor; JAK, Janus kinases; JNK, Jun n-terminal kinase; MAPK, mitogen-activated protein kinases; mIL-6R, membrane-bound IL-6 receptor; NOS, Newcastle-Ottawa scale; SaO2, oxygen saturation; SARS, severe acute respiratory syndrome; sIL-6R, soluble IL-6 receptor; SOC, standard of care; STAT, signal transducer and activator of transcription; TNF-α, tumor necrosis factor-alpha; WHO, World Health Organization.
Alattar, R., Ibrahim, T. B. H., Shaar, S. H., Abdalla, S., Shukri, K., Daghfal, J. N., et al. (2020). Tocilizumab for the treatment of severe COVID-19. J. Med. Virol. 92, 2042–2049. doi:10.1002/jmv.25964
Antony, S. J., Davis, M. A., Davis, M. G., Almaghlouth, N. K., Guevara, R., Omar, F., et al. (2020). Early use of tocilizumab in the prevention of adult respiratory failure in SARS-CoV-2 infections and the utilization of interleukin-6 levels in the management. J. Med. Virol. [Epub ahead of print]. doi:10.1002/jmv.26288
Arthur, J. S., and Ley, S. C. (2013). Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 13 (9), 679–692. doi:10.1038/nri3495
Azkur, A. K., Akdis, M., Azkur, D., Sokolowska, M., Van De Veen, W., Bruggen, M. C., et al. (2020). Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 75 (7), 1564–1581. doi:10.1111/all.14364
Borku Uysal, B., Ikitimur, H., Yavuzer, S., Ikitimur, B., Uysal, H., Islamoglu, M. S., et al. (2020). Tocilizumab challenge: a series of cytokine storm therapy experiences in hospitalized COVID-19 pneumonia patients. J. Med. Virol. [Epub ahead of print]. doi:10.1002/jmv.26111
Campbell, L., Chen, C., Bhagat, S. S., Parker, R. A., and Ostor, A. J. (2011). Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatology 50 (3), 552–562. doi:10.1093/rheumatology/keq343
Campins, L., Boixeda, R., Perez-Cordon, L., Aranega, R., and Lopera, C. (2020). Early tocilizumab treatment could improve survival among COVID-19 patients. Clin. Exp. Rheumatol. 38 (3), 578. [Epub ahead of print].
Campochiaro, C., Della-Torre, E., Cavalli, G., De Luca, G., Ripa, M., Boffini, N., et al. (2020). Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur. J. Intern. Med. 76, 43–49. doi:10.1016/j.ejim.2020.05.021
Cantini, F., Niccoli, L., Matarrese, D., Nicastri, E., Stobbione, P., and Goletti, D. (2020a). Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J. Infect. 81 (2), 318–356. doi:10.1016/j.jinf.2020.04.017
Cantini, F., Niccoli, L., Nannini, C., Matarrese, D., Natale, M. E. D., Lotti, P., et al. (2020b). Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J. Infect. 81, 647–679. doi:10.1016/j.jinf.2020.06.052
Canziani, L. M., Trovati, S., Brunetta, E., Testa, A., De Santis, M., Bombardieri, E., et al. (2020). Interleukin-6 receptor blocking with intravenous tocilizumab in COVID-19 severe acute respiratory distress syndrome: a retrospective case-control survival analysis of 128 patients. J. Autoimmun. 114, 102511. doi:10.1016/j.jaut.2020.102511
Cao, Y., Wei, J., Zou, L., Jiang, T., Wang, G., Chen, L., et al. (2020). Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J. Allergy Clin. Immunol. 146 (1), 137–146. doi:10.1016/j.jaci.2020.05.019
Capra, R., De Rossi, N., Mattioli, F., Romanelli, G., Scarpazza, C., Sormani, M. P., et al. (2020). Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur. J. Intern. Med. 76, 31–35. doi:10.1016/j.ejim.2020.05.009
Chen, G., Wu, D., Guo, W., Cao, Y., Huang, D., Wang, H., et al. (2020). Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 130 (5), 2620–2629. doi:10.1172/JCI137244
Colaneri, M., Bogliolo, L., Valsecchi, P., Sacchi, P., Zuccaro, V., Brandolino, F., et al. (2020). Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE). Microorganisms 8 (5), 695. doi:10.3390/microorganisms8050695
Cummings, M. J., Baldwin, M. R., Abrams, D., Jacobson, S. D., Meyer, B. J., Balough, E. M., et al. (2020). Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 395 (10239), 1763–1770. doi:10.1016/S0140-6736(20)31189-2
Della-Torre, E., Campochiaro, C., Cavalli, G., De Luca, G., Napolitano, A., La Marca, S., et al. (2020). Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Ann. Rheum. Dis. 79, 218122. doi:10.1136/annrheumdis-2020-218122
Eimer, J., Vesterbacka, J., Svensson, A. K., Stojanovic, B., Wagrell, C., Sönnerborg, A., et al. (2020). Tocilizumab shortens time on mechanical ventilation and length of hospital stay in patients with severe COVID-19: a retrospective cohort study. J. Intern. Med. [Epub ahead of print]. doi:10.1111/joim.13162
Fernández-Ruiz, M., López-Medrano, F., Pérez-Jacoiste Asín, M. A., Maestro De La Calle, G., Bueno, H., Caro-Teller, J. M., et al. (2020). Tocilizumab for the treatment of adult patients with severe COVID-19 pneumonia: a single-center cohort study. J. Med. Virol. [Epub ahead of print]. doi:10.1002/jmv.26308
Food and Drug Administration, and KEVZARA (KEV-za-ra) (2018). (Sarilumab) injection, for intravenous or subcutaneous use Initial U.S. Approval:2010. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761037s001lbl.pdf#page=27 (Accessed April 2018).
Food and Drug Administration (2010). ACTEMRA (tocilizumab) injection, for intravenous or subcutaneous use Initial U.S. Approval. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125276s129125472s042lbl.pdf#page=46 (Accessed May 2020).
Formina, D. S., Lysenko, M. Y. A., Beloglazova, I. P., Mutinova, Z. Y., Poteshkina, N. G., Samsonova, I. V., et al. (2020). Temporal clinical and laboratory response to interleukin-6 receptor blockade with Tocilizumab in 89 hospitalized patients with COVID-19 pneumonia. medRxiv: the preprint server for health sciences [Preprint]. Available at: https://doi.org/10.1101/2020.06.12.20122374 (Accessed June 12, 2020).
Garbers, C., Heink, S., Korn, T., and Rose-John, S. (2018). Interleukin-6: designing specific therapeutics for a complex cytokine. Nat. Rev. Drug Discov. 17 (6), 395–412. doi:10.1038/nrd.2018.45
Gokhale, Y., Mehta, R., Karnik, N., Kulkarni, U., and Gokhale, S. (2020). Tocilizumab improves survival in patients with persistent hypoxia in severe COVID-19 pneumonia. EClinicalMedicine 24, 100467. doi:10.1016/j.eclinm.2020.100467
Gorgolas, M., Cabello, A., Prieto Perez, L., Villar Alvarez, F., Alvarez Alvarez, B., Rodriguez Nieto, M. J., et al. (2020). Compassionate Use of Tocilizumab in Severe SARS-CoV2 Pneumonia. When late administration is too late. medRxiv: the preprint server for health sciences [Preprint]. Available at: https://doi.org/10.1101/2020.06.13.20130088 (Accessed June 16, 2020).
Gritti, G., Raimondi, F., Ripamonti, D., Riva, I., Landi, F., Alborghetti, L., et al. (2020). IL-6 signalling pathway inactivation with siltuximab in patients with COVID-19 respiratory failure: an observational cohort study. medRxiv: the preprint server for health sciences [Preprint]. Available at: https://doi.org/10.1101/2020.04.01.20048561 (Accessed June 20, 2020).
Guaraldi, G., Meschiari, M., Cozzi-Lepri, A., Milic, J., Tonelli, R., Menozzi, M., et al. (2020). Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol 2 (8), e474–e484. doi:10.1016/S2665-9913(20)30173-9
Han, H., Ma, Q., Li, C., Liu, R., Zhao, L., Wang, W., et al. (2020). Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microb. Infect. 9 (1), 1123–1130. doi:10.1080/22221751.2020.1770129
Hassoun, A., Thottacherry, E. D., Muklewicz, J., Aziz, Q. U., and Edwards, J. (2020). Utilizing tocilizumab for the treatment of cytokine release syndrome in COVID-19. J. Clin. Virol. 128, 104443. doi:10.1016/j.jcv.2020.104443
Heinrich, P. C., Behrmann, I., Haan, S., Hermanns, H. M., M¨Uller-Newen, G., and Schaper, F. (2003). Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374, 1–200. doi:10.1042/BJ20030407
Henry, B. M., De Oliveira, M. H. S., Benoit, S., Plebani, M., and Lippi, G. (2020). Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chem. Lab. Med. 58 (7), 1021–1028. doi:10.1515/cclm-2020-0369
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395 (10223), 497–506. doi:10.1016/s0140-6736(20)30183-5
Indalao, I. L., Sawabuchi, T., Takahashi, E., and Kido, H. (2017). IL-1β is a key cytokine that induces trypsin upregulation in the influenza virus-cytokine-trypsin cycle. Arch. Virol. 162 (1), 201–211. doi:10.1007/s00705-016-3093-3
Ip, A., Berry, D. A., Hansen, E., Goy, A. H., Pecora, A. L., Sinclaire, B. A., et al. (2020). Hydroxychloroquine and tocilizumab therapy in COVID-19 patients - an observational study. medRxiv: the preprint server for health sciences [Preprint]. Available at: https://doi.org/10.1101/2020.05.21.20109207 (Accessed May 25, 2020).
Issa, N., Dumery, M., Guisset, O., Mourissoux, G., Bonnet, F., and Camou, F. (2020). Feasibility of tocilizumab in ICU patients with COVID-19. J. Med. Virol. [Epub ahead of print]. doi:10.1002/jmv.26110
Jiménez-Brítez, G., Ruiz, P., and Soler, X. (2020). Tocilizumab plus glucocorticoids in severe and critically COVID-19 patients. A single center experience. Med. Clin. 155, 410–411. doi:10.1016/j.medcli.2020.07.001
Jordan, S. C., Zakowski, P., Tran, H. P., Smith, E. A., Gaultier, C., Marks, G., et al. (2020). Compassionate use of tocilizumab for treatment of SARS-CoV-2 pneumonia. Clin. Infect. Dis. [Epub ahead of print]. doi:10.1093/cid/ciaa812
Kang, S., Tanaka, T., Narazaki, M., and Kishimoto, T. (2019). Targeting interleukin-6 signaling in clinic. Immunity 50 (4), 1007–1023. doi:10.1016/j.immuni.2019.03.026
Keske, Ş., Tekin, S., Sait, B., İrkören, P., Kapmaz, M., Çimen, C., et al. (2020). Appropriate use of tocilizumab in COVID-19 infection. Int. J. Infect. Dis. 99, 338–343. doi:10.1016/j.ijid.2020.07.036
Kewan, T., Covut, F., Al-Jaghbeer, M. J., Rose, L., Gopalakrishna, K. V., and Akbik, B. (2020). Tocilizumab for treatment of patients with severe COVID-19: a retrospective cohort study. EClinicalMedicine 24, 100418. doi:10.1016/j.eclinm.2020.100418
Kimmig, L. M., Wu, D., Gold, M., Pettit, N. N., Pitrak, D., Mueller, J., et al. (2020). IL6 inhibition in critically ill COVID-19 patients is associated with increased secondary infections. medRxiv: the preprint server for health sciences [Preprint]. Available at: https://doi.org/10.1101/2020.05.15.20103531 (Accessed September 12, 2020).
Klopfenstein, T., Zayet, S., Lohse, A., Balblanc, J. C., Badie, J., Royer, P. Y., et al. (2020). Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med. Maladies Infect. 50 (5), 397–400. doi:10.1016/j.medmal.2020.05.001
Knorr, J. P., Colomy, V., Mauriello, C. M., and Ha, S. (2020). Tocilizumab in patients with severe COVID-19: a single-center observational analysis. J. Med. Virol. 92, 2813–2820. doi:10.1002/jmv.26191
La Rosée, F., Bremer, H. C., Gehrke, I., Kehr, A., Hochhaus, A., Birndt, S., et al. (2020). The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation. Leukemia 34 (7), 1805–1815. doi:10.1038/s41375-020-0891-0
Lamb, Y. N., and Deeks, E. D. (2018). Sarilumab: a review in moderate to severe rheumatoid arthritis. Drugs 78 (9), 929–940. doi:10.1007/s40265-018-0929-z
Liu, B. W., Li, M., Zhou, Z. G., Guan, X., and Xiang, Y. F. (2020a). Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)?. J. Autoimmun. 111, 102452. doi:10.1016/j.jaut.2020.102452
Liu, J., Li, S., Liu, J., Liang, B., Wang, X., Wang, H., et al. (2020b). Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 55, 102763. doi:10.1016/j.ebiom.2020.102763
Lohse, A., Klopfenstein, T., Balblanc, J. C., Royer, P. Y., Bossert, M., Gendrin, V., et al. (2020). Predictive factors of mortality in patients treated with tocilizumab for acute respiratory distress syndrome related to coronavirus disease 2019 (COVID-19). Microbes Infect. 22, 500–506. doi:10.1016/j.micinf.2020.06.005
Luo, P., Liu, Y., Qiu, L., Liu, X. L., Liu, D., and Li, J. (2020b). Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. 92 (7), 814–818. doi:10.1002/jmv.25801
Luo, W., Li, Y. X., Jiang, L. J., Chen, Q., Wang, T., and Ye, D. W. (2020a). Targeting JAK-STAT signaling to control cytokine release syndrome in COVID-19. Trends Pharmacol. Sci. 41 (8), 531–543. doi:10.1016/j.tips.2020.06.007
Lyseng-Williamson, K. A. (2015). Siltuximab: a review in idiopathic (human herpesvirus-8-negative) multicentric castleman disease. BioDrugs 29 (6), 399–406. doi:10.1007/s40259-015-0142-5
Marfella, R., Paolisso, P., Sardu, C., Bergamaschi, L., D'angelo, E. C., Barbieri, M., et al. (2020). Negative impact of hyperglycaemia on tocilizumab therapy in Covid-19 patients. Diabetes Metab. 46 (5), 403–405. doi:10.1016/j.diabet.2020.05.005
Martinez-Sanz, J., Muriel, A., Ron, R., Herrera, S., Ron, R., Perez-Molina, J. A., et al. (2020). Effects of tocilizumab on mortality in hospitalized patients with COVID-19: a multicenter cohort study. medRxiv: the preprint server for health sciences [Preprint]. Available at: https://doi.org/10.1101/2020.06.08.20125245 (Accessed June 9, 2020).
Mastroianni, A., Greco, S., Apuzzo, G., De Santis, S., Oriolo, C., Zanolini, A., et al. (2020). Subcutaneous tocilizumab treatment in patients with severe COVID-19–related cytokine release syndrome: an observational cohort study. EClinicalMedicine 24, 100410. doi:10.1016/j.eclinm.2020.100410
Mikulska, M., Nicolini, L. A., Signori, A., Di Biagio, A., Sepulcri, C., Russo, C., et al. (2020). Tocilizumab and steroid treatment in patients with severe. medRxiv: the preprint server for health sciences [Preprint]. Available at: https://doi.org/10.1101/2020.06.22.20133413 (Accessed June 26, 2020).
Moore, J. B., and June, C. H. (2020). Cytokine release syndrome in severe COVID-19. Science 368 (6490), 473–474. doi:10.1126/science.abb8925
Morena, V., Milazzo, L., Oreni, L., Bestetti, G., Fossali, T., Bassoli, C., et al. (2020). Off-label use of tocilizumab for the treatment of SARS-CoV-2 pneumonia in Milan, Italy. Eur. J. Intern. Med. 76, 36–42. doi:10.1016/j.ejim.2020.05.011
Moreno Garcia, E., Rico Caballero, V., Albiach, L., Aguero, D., Ambrosioni, J., Bodro, M., et al. (2020). Tocilizumab is associated with reduction of the risk of ICU admission and mortality in patients with SARS-CoV-2 infection. medRxiv: the preprint server for health sciences [Preprint]. Available at: https://doi.org/10.1101/2020.06.05.20113738 (Accessed June 5, 2020).
Moreno-Pérez, O., Andres, M., Leon-Ramirez, J. M., Sánchez-Payá, J., Rodríguez, J. C., Sánchez, R., et al. (2020). Experience with tocilizumab in severe COVID-19 pneumonia after 80 days of follow-up: a retrospective cohort study. J. Autoimmun. 114, 102523. doi:10.1016/j.jaut.2020.102523
Morrison, A. R., Johnson, J. M., Griebe, K. M., Jones, M. C., Stine, J. J., Hencken, L. N., et al. (2020). Clinical characteristics and predictors of survival in adults with coronavirus disease 2019 receiving tocilizumab. J. Autoimmun. 114, 102512. doi:10.1016/j.jaut.2020.102512
Narain, S., Stefanov, D., Chau, A. S., Weber, A. G., Marder, G. S., Kaplan, B., et al. (2020). Comparative survival analysis of immunomodulatory therapy for COVID-19 'cytokine storm': a retrospective observational cohort study. medRxiv: the preprint server for health sciences [Preprint]. Available at: https://doi.org/10.1101/2020.06.16.20126714 (Accessed June 19, 2020).
Patel, A., Shah, K., Dharsandiya, M., Patel, K., Patel, T., Patel, M., et al. (2020b). Safety and efficacy of tocilizumab in the treatment of severe acute respiratory syndrome coronavirus-2 pneumonia: a retrospective cohort study. Indian J. Med. Microbiol. 38 (1), 117–123. doi:10.4103/ijmm.IJMM_20_298
Patel, K., Gooley, T. A., Bailey, N., Bailey, M., Hegerova, L., Batchelder, A., et al. (2020a). Use of the IL-6R antagonist tocilizumab in hospitalized COVID-19 patients. J. Intern. Med. https://doi.org/10.1111/joim.13163
Perrone, F., Piccirillo, M. C., Ascierto, P. A., Salvarani, C., Parrella, R., Marata, A. M., et al. (2020). Tocilizumab for patients with COVID-19 pneumonia. The TOCIVID-19 prospective phase 2 trial. medRxiv: the preprint server for health sciences [Preprint]. Available at: https://doi.org/10.1101/2020.06.01.20119149 (Accessed July 2, 2020).
Potere, N., Di Nisio, M., Cibelli, D., Scurti, R., Frattari, A., Porreca, E., et al. (2020a). Interleukin-6 receptor blockade with subcutaneous tocilizumab in severe COVID-19 pneumonia and hyperinflammation: a case–control study. Ann. Rheum. Dis. [Epub ahead of print]. doi:10.1136/annrheumdis-2020-218243
Potere, N., Di Nisio, M., Rizzo, G., La Vella, M., Polilli, E., Agostinone, A., et al. (2020b). Low-Dose subcutaneous tocilizumab to prevent disease progression in patients with moderate COVID-19 pneumonia and hyperinflammation. Int. J. Infect. Dis. 100, 421–424. doi:10.1016/j.ijid.2020.07.078
Price, C. C., Altice, F. L., Shyr, Y., Koff, A., Pischel, L., Goshua, G., et al. (2020). Tocilizumab treatment for cytokine release syndrome in hospitalized COVID-19 patients: survival and clinical outcomes. Chest 158, 1397–1408. doi:10.1016/j.chest.2020.06.006
Quartuccio, L., Sonaglia, A., Mcgonagle, D., Fabris, M., Peghin, M., Pecori, D., et al. (2020). Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: results from a single Italian Centre study on tocilizumab versus standard of care. J. Clin. Virol. 129, 104444. doi:10.1016/j.jcv.2020.104444
Ramaswamy, M., Mannam, P., Comer, R., and Sinclair, E. (2020). Off-label real World experience using tocilizumab for patients hospitalized with COVID-19 disease in a regional community health system: a case-control study. medRxiv: the preprint server for health sciences [Preprint]. Available at: https://doi.org/10.1101/2020.05.14.20099234
Rojas-Marte, G. R., Khalid, M., Mukhtar, O., Hashmi, A. T., Waheed, M. A., Ehrlich, S., et al. (2020). Outcomes in patients with severe COVID-19 disease treated with tocilizumab - a case- controlled study. QJM 113, 546–550. doi:10.1093/qjmed/hcaa206
Rose-John, S., Winthrop, K., and Calabrese, L. (2017). The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat. Rev. Rheumatol. 13 (7), 399–409. doi:10.1038/nrrheum.2017.83
Rossi, B., Nguyen, L. S., Zimmermann, P., Boucenna, F., Baucher, L., Dubret, L., et al. (2020). Effect of tocilizumab in hospitalized patients with severe pneumonia COVID-19: a cohort study. medRxiv: the preprint server for health sciences [Preprint]. Available at: https://doi.org/10.1101/2020.06.06.20122341 (Accessed June 9, 2020).
Roumier, M., Paule, R., Groh, M., Vallee, A., and Ackermann, F. (2020). Interleukin-6 blockade for severe COVID-19. medRxiv: the preprint server for health sciences [Preprint]. Available at: https://doi.org/10.1101/2020.04.20.20061861 (Accessed April 22, 2020).
Sardu, C., D'onofrio, N., Balestrieri, M. L., Barbieri, M., Rizzo, M. R., Messina, V., et al. (2020c). Hyperglycaemia on admission to hospital and COVID-19. Diabetologia 63 (11), 2486–2487. doi:10.1007/s00125-020-05216-2
Sardu, C., D'onofrio, N., Balestrieri, M. L., Barbieri, M., Rizzo, M. R., Messina, V., et al. (2020a). Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control?. Diabetes Care 43 (7), 1408–1415. doi:10.2337/dc20-0723
Sardu, C., Gargiulo, G., Esposito, G., Paolisso, G., and Marfella, R. (2020b). Impact of diabetes mellitus on clinical outcomes in patients affected by Covid-19. Cardiovasc. Diabetol. 19 (1), 76. doi:10.1186/s12933-020-01047-y
Sardu, C., Maggi, P., Messina, V., Iuliano, P., Sardu, A., Iovinella, V., et al. (2020d). Could anti-hypertensive drug therapy affect the clinical prognosis of hypertensive patients with COVID-19 infection? Data from centers of southern Italy. J. Am. Heart Assoc. 9 (17), e016948. doi:10.1161/JAHA.120.016948
Sardu, C., Marfella, R., Maggi, P., Messina, V., Cirillo, P., Codella, V., et al. (2020e). Implications of AB0 blood group in hypertensive patients with covid-19. BMC Cardiovasc. Disord. 20 (1), 373. doi:10.1186/s12872-020-01658-z
Sciascia, S., Aprà, F., Baffa, A., Baldovino, S., Boaro, D., Boero, R., et al. (2020). Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin. Exp. Rheumatol. 38 (3), 529–532. [Epub ahead of print].
Sinha, P., Mostaghim, A., Bielick, C. G., Mclaughlin, A., Hamer, D. H., Wetzler, L., et al. (2020). Early administration of Interleukin-6 inhibitors for patients with severe Covid-19 disease is associated with decreased intubation, reduced mortality, and increased discharge. Int. J. Infect. Dis. 99, 28–33. doi:10.1016/j.ijid.2020.07.023
Smolen, J. S., Aletaha, D., Koeller, M., Weisman, M. H., and Emery, P. (2007). New therapies for treatment of rheumatoid arthritis. Lancet 370 (9602), 1861–1874. doi:10.1016/S0140-6736(07)60784-3
Somers, E. C., Eschenauer, G. A., Troost, J. P., Golob, J. L., Gandhi, T. N., Wang, L., et al. (2020). Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin. Infect. Dis. ciaa954. doi:10.1093/cid/ciaa954
Tanaka, T., Narazaki, M., and Kishimoto, T. (2014). IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 6 (10), a016295. doi:10.1101/cshperspect.a016295
Tanaka, T., Narazaki, M., and Kishimoto, T. (2016). Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy 8 (8), 959–970. doi:10.2217/imt-2016-0020
Tomasiewicz, K., Piekarska, A., Stempkowska-Rejek, J., Serafińska, S., Gawkowska, A., Parczewski, M., et al. (2020). Tocilizumab for patients with severe COVID-19: a retrospective, multi-center study. Expert Rev. Anti Infect. Ther. 2020, 1–7 doi:10.1080/14787210.2020.1800453
Toniati, P., Piva, S., Cattalini, M., Garrafa, E., Regola, F., Castelli, F., et al. (2020). Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 19, 102568. doi:10.1016/j.autrev.2020.102568
Tsai, A., Diawara, O., Nahass, R. G., and Brunetti, L. (2020). Impact of tocilizumab administration on mortality in severe COVID-19. medRxiv: the preprint server for health sciences [Preprint]. Available at: https://doi.org/10.1101/2020.07.30.20114959 (Accessed August 2, 2020).
Wadud, N., Ahmed, N., Mannu Shergil, M., Khan, M., Krishna, M. G., Gilani, A., et al. (2020). Improved survival outcome in SARs-CoV-2 (COVID-19) Acute Respiratory Distress Syndrome patients with Tocilizumab administration. medRxiv: the preprint server for health sciences [Preprint]. Available at: https://doi.org/10.1101/2020.05.13.20100081 (Accessed May 16, 2020).
World Health Organization (2019) Coronavirus disease (COVID-19) pandemic. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (Accessed December 31, 2019).
World Health Organization (2020) Corticosteroids for COVID-19. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1 (Accessed September 2, 2020).
Keywords: COVID-19, anti-IL-6 signaling agents, mortality, secondary infections, mechanical ventilation, ICU admission, clinical improvement
Citation: Han Q, Guo M, Zheng Y, Zhang Y, De Y, Xu C, Zhang L, Sun R, Lv Y, Liang Y, Xu F, Pang J and Chen Y (2020) Current Evidence of Interleukin-6 Signaling Inhibitors in Patients With COVID-19: A Systematic Review and Meta-Analysis. Front. Pharmacol. 11:615972. doi: 10.3389/fphar.2020.615972
Received: 10 October 2020; Accepted: 17 November 2020;
Published: 15 December 2020.
Edited by:
Stefania Tacconelli, University of Studies G. d’Annunzio Chieti and Pescara, ItalyReviewed by:
Raffaele Marfella, University of Campania Luigi Vanvitelli, ItalyCopyright © 2020 Han, Guo, Zheng, Zhang, De, Xu, Zhang, Sun, Lv, Liang, Xu, Pang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuguo Chen, Y2hlbjkxOTA4NUBzZHUuZWR1LmNu; Jiaojiao Pang, amlhb2ppYW9wYW5nQDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.