- 1Department of Neurobiology, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States
- 2Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States
- 3Hillman Cancer Center, University of Pittsburgh Medicine Center, Pittsburgh, PA, United States
The incidence of pain in cancer patients during diagnosis and treatment is exceedingly high. Although advances in cancer detection and therapy have improved patient prognosis, cancer and its treatment-associated pain have gained clinical prominence. The biological mechanisms involved in cancer-related pain are multifactorial; different processes for pain may be responsible depending on the type and anatomic location of cancer. Animal models of cancer-related pain have provided mechanistic insights into the development and process of pain under a dynamic molecular environment. However, while cancer-evoked nociceptive responses in animals reflect some of the patients’ symptoms, the current models have failed to address the complexity of interactions within the natural disease state. Although there has been a recent convergence of the investigation of carcinogenesis and pain neurobiology, identification of new targets for novel therapies to treat cancer-related pain requires standardization of methodologies within the cancer pain field as well as across disciplines. Limited success of translation from preclinical studies to the clinic may be due to our poor understanding of the crosstalk between cancer cells and their microenvironment (e.g., sensory neurons, infiltrating immune cells, stromal cells etc.). This relatively new line of inquiry also highlights the broader limitations in translatability and interpretation of basic cancer pain research. The goal of this review is to summarize recent findings in cancer pain based on preclinical animal models, discuss the translational benefit of these discoveries, and propose considerations for future translational models of cancer pain.
Introduction
Cancer-related pain can occur at any time during the evolution of the disease (Caraceni and Shkodra, 2019). Many patients present with pain as the first sign of cancer, and 30–50% of all cancer patients will experience moderate to severe pain; frequency and intensity of pain can increase with cancer progression (Mercadante, 1997; Mercadante and Arcuri, 1998). Despite significant advances in cancer treatment as well as early detection, cancer-related pain treatment strategies remain limited. Opioids remain the current therapeutic regimen for cancer-related pain, based on the World Health Organization (WHO) analgesic ladder (Nersesyan and Slavin, 2007), despite debilitating side effects and inadequate efficacy (Mandala et al., 2006). The limited neurobiological understanding in analgesia pharmacology has restricted novel therapeutic development; cancer pain treatments have relied largely on scientific advancements in other pain conditions.
Animal models of cancer-related pain have provided mechanistic insights into how cancer pain is generated and progresses under a constantly changing molecular architecture. Cancer pain is thought to result from processes involving crosstalk between neoplastic cells, the host’s immune system, and peripheral and central nervous systems (Jimenez Andrade and Mantyh, 2010; Lozano-Ondoua et al., 2013; Schmidt, 2014). The field of cancer pain is just beginning to apply nociceptive behavioral assays to established rodent cancer models to reflect the symptoms experienced by patients. The goal of this review is to assess translational ability of the current animal models of cancer pain to the clinical presentation and provide a reference and direction for researchers studying cancer pain.
Clinical Assessment of Cancer Pain
Assessment of cancer-related pain is broken down into that arising directly from the tumor (85%), pain due to disease progression (9%), pain as a side effect of treatment (17%) (e.g., chemotherapy, surgical resection), and pain from other causes not related to malignancy (Grond et al., 1996). Cancer can affect any type of tissue, including viscera, bone, soft and nervous tissue. Pain can arise from the original site of the cancer (e.g., pancreas, head and neck) or from distant sites (e.g., bone), where common cancers metastasize (Coleman, 2006). Cancer patients often experience pain at multiple sites. Focal pain is experienced at a single site, usually in the region of the underlying lesion. Referred pain is denoted as progressive pain in a site lacking focal pathology (Caraceni and Portenoy, 1999; Portenoy and Ahmed, 2018). Physiological mechanisms in focal cancer pain are broadly described as nociceptive, inflammatory, or neuropathic (Falk and Dickenson, 2014). Nociceptive pain can be further classified into somatic and visceral. Somatic nociceptive pain is usually well localized and described as sharp, aching, throbbing, or pressure-like. When caused by obstruction, visceral nociceptive pain is often described as gnawing or crampy; when caused by involvement of organ capsules or mesentery, visceral pain may be aching, sharp, or throbbing. Neuropathic pain, defined as pain caused by a lesion or damage to the nervous system, is present in about 39% of patients including those with mixed pain (i.e., including both a nociceptive and neuropathic component) (Bennett et al., 2012); it is described as burning, tingling, or shock-like (lancinating) (Caraceni and Portenoy, 1999; Portenoy and Ahmed, 2018).
Due to disease progression and often resulting tissue damage, the temporal variation of cancer-related pain is classified as acute, chronic, or intermittent. Acute pain is defined by recent onset and brevity. Chronic cancer pain persists for three or more months, often increases with disease progression, and may regress with tumor shrinkage. Chronic pain may be associated with affective disturbances (e.g., anxiety, depression) as well as vegetative symptoms (e.g., anorexia, sleep disturbances) (Bennett et al., 2019). In the cancer population, a brief increase of intense pain in the presence of cancer pain successfully managed with opioid drugs is common and has been defined as “breakthrough” pain; it is estimated that more than one in two patients with cancer pain will also experience breakthrough pain (Portenoy and Hagen, 1990; Deandrea et al., 2014).
Advances in cancer treatment have significantly prolonged survival, and thus the appearance of pain as a sequela is becoming more prominent (Bennett et al., 2012; Liu et al., 2017). Cancer treatment-related pain may include bone pain immediately after radiotherapy (Loblaw et al., 2007; Hird et al., 2009) and post-surgical pain due to mastectomy, neck lymph node dissection, laparotomy, and thoracotomy or due to nerve sacrifice during surgery, such as post-thoracotomy pain syndrome (Brown et al., 2014; Liu et al., 2017). Mixed pain is common when caused by cancer treatments (Urch and Dickenson, 2008; Fallon, 2013); however, pain resulting from chemotherapy, termed chemotherapy-induced peripheral neuropathy (CIPN), is the most common and purely neuropathic in nature (Lema et al., 2010). CIPN usually exhibits dose-dependence and distribution through the upper and lower extremities. CIPN can persist for several months to years, even after discontinuation of chemotherapy. Symptoms can include sensory loss, paresthesia, dysesthesia, and pain (Loprinzi et al., 2007; Grisold et al., 2012; Sisignano et al., 2014).
Animal Models of Cancer-Related Pain
To study cancer pain in the laboratory setting, several animal models have been developed over the last 2 decades to assess pain related to cancers in somatic and visceral tissues as well as cancer treatment related pain. These models seek to reflect the complex pain state observed clinically by measuring mechanical, thermal, and spontaneous pain-related behaviors.
Cancer-Related Pain Models
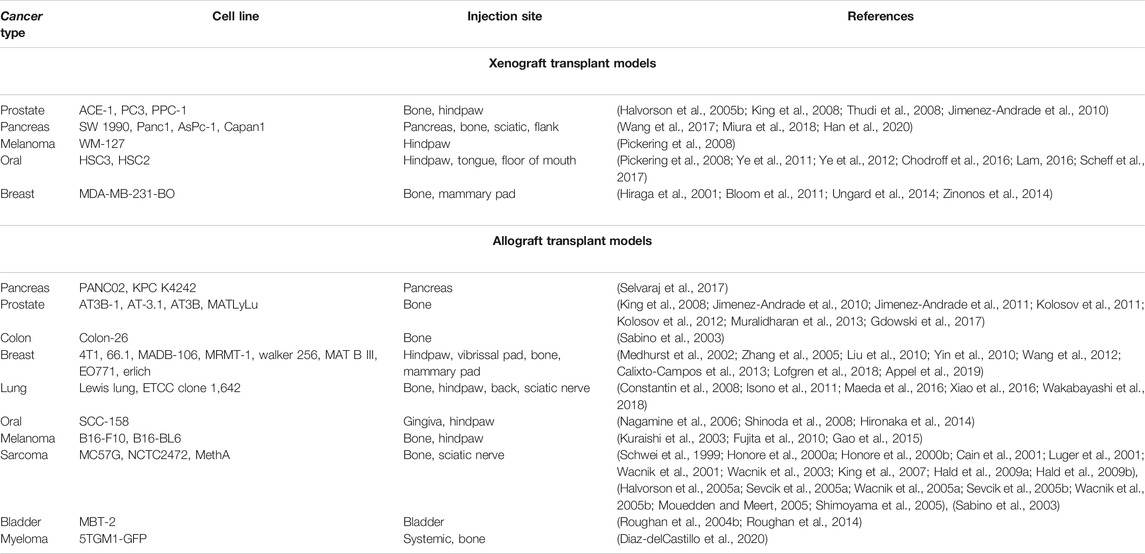
The transplantation model is the most popular model in focal and metastatic cancer pain research, and investigators have utilized several permutations. The two broad categories of transplant models are xenograft and allograft (i.e., syngeneic). Xenografts utilize commercially available human tumor cell lines; allografts use tumor cell lines derived from species with the same genetic background (e.g., mouse, rat). A variety of cell lines have been used, all of which have provided insights into the similarities and differences by which different tumors drive cancer pain; a comprehensive list is compiled in Table 1. The major benefits of transplantation models are easy replication, high modeling success rate, and high stability to facilitate the generation of abundant experimental mice. Orthotopic tumors provide a more comprehensive assessment of nociceptive behavior in response to tumor growth within an experimental environment more like the origin site (i.e. anatomical structure and sensory fiber innervation). Immunodeficiency is required for xenograft models, which is an important component when considering the multifaceted cancer pain phenotype. While immune cells are not absent in the cancer microenvironment (Chodroff et al., 2016), the loss of important signaling lymphocytes may lead to clinically irrelevant infiltration and neuro-immune communication.
To date, genetic cancer models have only been utilized to study pancreatic cancer pain [e.g., SV40 (Saenz Robles and Pipas, 2009), KPC (Biankin et al., 2012; Singhi et al., 2019)]. Genetically engineered models allow for the study of pain and neuroplasticity throughout the initiation and transformation of normal cells to malignancy as well as natural dissemination and metastasis. Typically, genetic models closely recapitulate human disease because they are based on specific genetic mutations that have been documented in patient populations. One of the greatest benefits of genetically engineered models is that the natural microenvironment remains intact, which permits modeling of complex processes that require interactions between multiple cells types (e.g., axonogenesis, metastasis, immune regulation). However, maintaining genetic models presents practical challenges. Several models have an average age of onset between 40 weeks and 20 months (Ding et al., 2016). Furthermore, as the number of transgenic alleles in a model increases, the cost-effectiveness and breeding efficiency decrease, making them expensive and time-consuming. Thus, while genetic models can play an important part in understanding biological mechanisms and pharmacology, they may not be ideal for high throughput testing (Webster et al., 2020).
Chemical-induced carcinogenesis has been utilized to study oral cancer pain (e.g., 4-nitroquinoline 1-oxide (4NQO) (Lam et al., 2012; Scheff et al., 2017; Scheff et al., 2018)) and colon cancer pain [e.g., azoxymethane (AOM) and dextran sulfate sodium (DSS) (Chartier et al., 2020)]. Like genetic models, chemical models allow for the study of pain development during the multistage dynamic carcinogenicity from initiation and progression. While some studies have employed local exposure (Bersch et al., 2009), a major benefit of these models is that the chemical is typically given systemically (e.g., drinking water) and can be used across multiple species. However, to date, cancer-related pain behavior has only been assessed in mouse and rat models. Additionally, due to uncontrolled exposure to the chemical, not all animals develop the same lesion at the same time, and a variety of lesions can be seen in a single rodent. While this can be clinically relevant as multiple primaries do occur in patients, high variability in the site and number of lesions between animals can also severely limit interpretations of pharmacology and behavioral studies. Unintended esophageal and gastrointestinal lesions are also possible and may confound results using spontaneous pain behavior assays.
The most common treatment-related cancer pain modeled in animals is CIPN, wherein chemotherapeutic agents (e.g., paclitaxel, oxaliplatin) are administered to animals resulting in dose-dependent damage to peripheral nervous system. The severity and temporal dynamics of neuropathy depend on the type of neurotoxic antineoplastic agent, dosage, and route of administration. CIPN rodent models have been extensively devised and studied in the last 30 years (Cavaletti et al., 2019). There is a wide variety of strains utilized, chemotherapeutic agents and routes of administration employed which have been systematically reviewed here (Gadgil et al., 2019). Many advantages are typical of these models: small size and prolific nature of the animals, ease of handling, along with the availability of reliable methods for peripheral nerve assessment. One of the most important benefits of this model is reproducibility within consistent methodology. Mouse CIPN models utilizing either paclitaxel or cisplatin have high efficacy in causing CIPN across sex and strains which is similar to clinical studies demonstrating a high incidence of CIPN in patients treated with these agents (Seretny et al., 2014; Molassiotis et al., 2019). However, one of the model’s major limitations is the high degree of variability regarding the chemotherapeutic agents used as well as the dose and route of administration chosen. Inconsistency in the model characteristics makes pharmacological conclusions across publications impossible to interpret.
Cancer-Related Nociceptive Behavior Assays
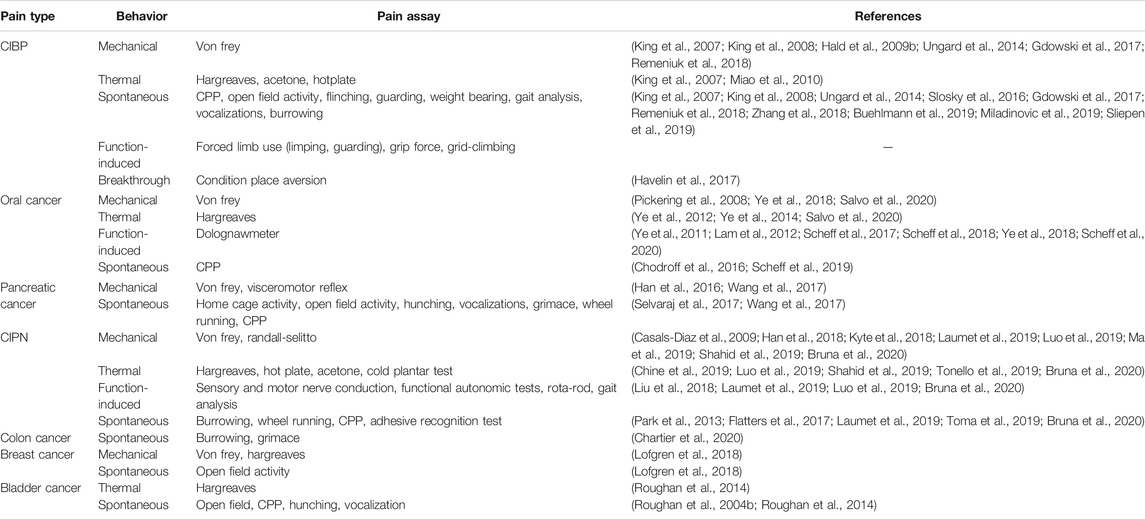
Due to the variety of clinical presentations and comorbidities (e.g., affective components) in cancer-related pain, choosing among nociceptive assays remains challenging. A comprehensive list is compiled in Table 2. The most used behavioral assay for cancer pain is evoked mechanical sensitivity measured by quantification of responses to the application of standardized von Frey monofilaments to or near the site of inoculation (e.g., hindpaw, pancreas, vibrissal pad). One benefit of this assay is its common use in non-cancer pain literature. However, subjectivity limits interpretation of results. An animal’s lack of response to a noxious stimulus could indicate analgesia, paralysis, sedation, or lack of motivation; additional tests must screen for side effects that might confound the behavioral result. The alignment of animal research and clinical practice requires the use of similar behavioral endpoints. Hence, function-based tests, which facilitate the identification of drugs that inhibit nociception in the absence of disruptive side effects, are growing in popularity. Operant and function-related assays [e.g., gnawing (Dolan et al., 2010), wheel running (Tang et al., 2016), grid climbing (Falk et al., 2017)] have been used as an index of cancer-related pain; many of these behaviors and their clinical relevance have been thoroughly reviewed here (Tappe-Theodor et al., 2019).
Spontaneous pain behavior is one of the most difficult components of cancer pain to manage and is therefore the most consistent end point against which the efficacy of a candidate drug is tested. The major limitation to using spontaneous pain as the end point is that the methodology differs substantially in the literature; spontaneous cancer-related pain has been measured indirectly using hunching (Lindsay et al., 2005a; Sevcik et al., 2006; Stopczynski et al., 2014; Wang et al., 2017; Kajiwara et al., 2020), open field activity (Stopczynski et al., 2014; Selvaraj et al., 2017; Hirth et al., 2020), home cage activity (Selvaraj et al., 2017; Hirth et al., 2020), voluntary wheel running (Selvaraj et al., 2017), vocalizations (Lindsay et al., 2005a; Sevcik et al., 2006), conditioned place preference (Selvaraj et al., 2017) as well as impressions of appearance [e.g., grimace scale (Kajiwara et al., 2020), coat condition (Roughan et al., 2004a)]. While many of these natural animal behaviors have minimal operator influence and can be translated to clinical representation of cancer-related pain, high variability of scoring criteria across studies and indirect output greatly limits the ability to assess the therapeutic impact. Additionally, distinguishing between spontaneous and “breakthrough” pain remains challenging, though recently an adaptation to the conditioned place aversion assay has been validated to measure movement-evoked breakthrough pain specifically (King et al., 2007; Havelin et al., 2017).
Pharmacology From Bench to Bedside
Nerve growth factor (NGF) has been considered the most potent pain inducer across multiple cancer models and currently holds the most promise for management of pain associated with cancer initiation and progression. Inhibition of NGF binding to the receptor TrkA in preclinical models strongly reduced mechanical, thermal and spontaneous facets of cancer-induced bone pain (Halvorson et al., 2005b; Jimenez-Andrade et al., 2011; Buehlmann et al., 2019), pancreatic cancer pain (Stopczynski et al., 2014; Amit et al., 2019), and oral cancer pain (Ye et al., 2011). A phase II clinical trial using anti-NGF antibody, fulranumab, as adjunctive therapy for cancer-related pain found no significant effect on pain intensity via visual analog scale; however, significant improvement on the Brief Pain Inventory subscales suggested improved quality of life (Slatkin et al., 2019). Additionally, there are two ongoing clinical trials, one testing the analgesic efficacy of a TrkA inhibitor on cancer patients with solid tumors or lymphoma (phase I, NCT03556228) and the other measuring the efficacy of anti-NGF monoclonal antibody tanezumab in the treatment of cancer pain due to bone metastasis in patients already taking background opioid therapy (phase III, NCT02609828).
For treatment-related cancer pain, accumulating evidence indicates that the initiation and progression of CIPN are tightly related with chemotherapeutic agent-induced impairment of intraepidermal nerve fibers (IENF) (Koskinen et al., 2011), oxidative stress (Butturini et al., 2013), abnormal spontaneous discharge, ion channel activation (Zhang and Dougherty, 2014), up-regulation of various pro-inflammatory cytokines, and activation of the neuro-immune system (Sisignano et al., 2014; Makker et al., 2017). A phase III clinical trial using duloxetine, a serotonin and norepinephrine reuptake inhibitor, for treatment of pain associated with CIPN found that the use of duloxetine compared with placebo for 5 weeks resulted in a greater reduction in pain (NCT00489411). Calmangafodipir, mimicking the mitochondrial enzyme manganese superoxide dismutase, is currently involved in two ongoing clinical trials to establish the efficacy in prevention of chronic CIPN induced by oxaliplatin (phase III, NCT03654729 and NCT04034355).
Considerations for Reverse Translation
Despite the large amount of human and experimental studies, no effective prophylactic treatment exists for cancer-related pain, and treatments (e.g., opioids) remain flawed. Identification of new targets for novel therapies to treat cancer-related pain requires models that better recapitulate interactions between cancer and its microenvironment, along with standardization of such assays and methodologies. Since so many promising studies fail in clinical translation, one must question the inherent translatability of the models themselves. While cancer-evoked nociceptive responses in animals echo some of the patients’ symptoms, the current models fail to address the complexity of interactions within the natural disease state. One of the major hypotheses for the etiology of focal cancer pain is cancer-secreted mediator-induced activation of the sensory nerve fibers innervating the cancer microenvironment (Jimenez Andrade and Mantyh, 2010; Schmidt, 2014; Lam, 2016). Therefore, anatomic site and neoplastic cell type should not be taken for granted when considering the translational relevance of the cancer pain model. Secreted mediators can differ depending on the cancer cell type (Sabino et al., 2003; Scheff et al., 2017; Scheff et al., 2020). Cancer-induced bone pain literature includes the most heterogeneity regarding cancer cell lines used (Currie et al., 2013). Substantial variability in pain behaviors and pharmacology may be attributed to the cancer cells selected to interact with peripheral nociceptive neurons; for this model multiple cancer cell lines might be required to determine if the findings are specific to one type of cancer or can be generalized to all bone metastasis. Similarly, variability in dosing and chemotherapeutic agent used in CIPN animal models could affect the consistency of findings (Gadgil et al., 2019).
To replicate symptoms observed in patients, reverse translation requires characterization of the pain associated with cancer or cancer treatment as either nociceptive, neuropathic, or mixed. Measures like numbness, tingling and ongoing pain rely on verbal report from the patient and often occur spontaneously. Fortunately, investigations into novel measures of ongoing pain in rodents are emerging (Tappe-Theodor and Kuner, 2014). A combination of pain assays including spontaneous pain should be used to demonstrate the translation of the cancer pain model to the clinical representation. However, consistency in criteria to score spontaneous pain across models is needed. Additionally, the stage of cancer progression at which pain develops in the animal model should align with the clinical representation. For example, oral squamous cell carcinoma pain in patients is thought to develop during the transition from precancerous lesion to malignancy (Lam and Schmidt, 2011). The 4NQO carcinogen model appears to be clinically representative with function-induced nociceptive behavior initiating at early stages in tumorigenesis (Scheff et al., 2017; Scheff et al., 2018); however, nociceptive behavior in an orthotopic xenograft mouse model does not develop for up to 14 days after inoculation (Ye et al., 2018; Scheff et al., 2019) suggesting that this model is more appropriate for pharmacological approaches to treat cancer-related pain at later stages in the disease when tumor burden is an active component. Lastly, the impact of age (Fujii, 1991; Lindsay et al., 2005b; Oh et al., 2018), sex (Scheff et al., 2018; Scheff et al., 2019; Rubin et al., 2020) and rodent strain (Vermeirsch and Meert, 2004; Zhang and Lao, 2012; Ono et al., 2015) can greatly impact both tumor development and nociceptive behavior and should be taken into consideration when designing a study. The cancer-related pain field needs to work together to standardize the methodology regarding both animal models and pain assays to increase the potential for reproducibility and clinical translation.
The cancer biology field is rapidly growing, and advances in cancer detection and therapy have improved patient prognosis. Preclinical models of several cancers [e.g., oral (Li et al., 2020), pancreas (Yin et al., 2015; Bisht and Feldmann, 2019), breast (Whittle et al., 2015; Holen et al., 2017)] have been extensively studied to determine a suitable research animal model that reflects the intricacies of cancer biology. In order to fully match the achievements in the cancer field broadly and understand the pain that may develop prior to detection or in response to treatments, we need to integrate the animal models most commonly used in cancer biology into the cancer-related pain field. Assimilation and standardization across both fields will allow for better translational findings across preclinical and clinical modalities. For example, patient-derived xenograft models (Aparicio et al., 2015) present a potential opportunity for patient-reported pain to be recapitulated in a transplantation mouse model with preservation of the genotypic and phenotypic diversity of the original tumor tissue. It is also imperative to consider facets of tumor biology beyond the nociceptive system (i.e., tumor growth, immune response) in pharmacological studies related to cancer pain; novel cancer pain therapies should not exacerbate cancer progression and interpretation of analgesia should be considered along with tumor size. Lastly, reverse translation could also be improved through the inclusion of large animal models [i.e., porcine (Robertson et al., 2020), canine (Kamano et al., 1988)]. There have been significant advances in veterinary oncology as well as validation of pain scales for companion animals (Brown et al., 2015; Lascelles et al., 2019) which provides an opportunity to study spontaneous cancer pain in larger species (Brown et al., 2015; Brown, 2016; Monteiro et al., 2018). To date, the cancer-related pain field has yet to integrate standardized pain assessment instruments for large animals (Henze and Urban, 2010; Viscardi et al., 2017; Lascelles et al., 2019; Luna et al., 2020).
Conclusion
All cancer-related pain models have advantages and disadvantages, and there is no ideal cancer-related pain model that will perfectly recapitulate the human experience. The appropriate model depends on the cancer-related pain condition and the specific methods used. Despite limitations, the field has begun to provide insight into the mechanisms that generate and maintain cancer-related pain while discovering potential therapeutic strategies to treat it. Additionally, there has been a recent surge in data suggesting that manipulation of neuronal activity by cancer cells may be a central mechanism for cancer progression (Faulkner et al., 2019; Zahalka and Frenette, 2020). Thus, targeting sensory neurons in the cancer microenvironment may be a potentially actionable therapeutic strategy to stop cancer pain as well as slow cancer growth. As this new field of cancer neurobiology emerges, full collaboration between cancer biologists, neurobiologists and immunologists is pivotal for success.
Author Contributions
All authors, JP-F, JS, NS, drafted the work, contributed to work design, revised it, and approved the final version to be published and are accountable for all aspects of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Amit, M., Na'ara, S., Fridman, E., Vladovski, E., Wasserman, T., Milman, N., et al. (2019). RET, a targetable driver of pancreatic adenocarcinoma. Int. J. Canc. 144 (12), 3014–3022. doi:10.1002/ijc.32040
Aparicio, S., Hidalgo, M., and Kung, A. L. (2015). Examining the utility of patient-derived xenograft mouse models. Nat. Rev. Canc. 15 (5), 311–316. doi:10.1038/nrc3944
Appel, C. K., Scheff, N. N., Viet, C. T., Schmidt, B. L., and Heegaard, A. M. (2019). Decitabine attenuates nociceptive behavior in a murine model of bone cancer pain. Pain 160 (3), 619–631. doi:10.1097/j.pain.0000000000001442
Bennett, M. I., Kaasa, S., Barke, A., Korwisi, B., Rief, W., Treede, R. D., et al. (2019). The IASP classification of chronic pain for ICD-11: chronic cancer-related pain. Pain 160 (1), 38–44. doi:10.1097/j.pain.0000000000001363
Bennett, M. I., Rayment, C., Hjermstad, M., Aass, N., Caraceni, A., and Kaasa, S. (2012). Prevalence and aetiology of neuropathic pain in cancer patients: a systematic review. Pain 153 (2), 359–365. doi:10.1016/j.pain.2011.10.028
Bersch, V. P., Osvaldt, A. B., Edelweiss, M. I., Schumacher Rde, C., Wendt, L. R., Abreu, L. P., et al. (2009). Effect of nicotine and cigarette smoke on an experimental model of intraepithelial lesions and pancreatic adenocarcinoma induced by 7,12-dimethylbenzanthracene in mice. Pancreas 38 (1), 65–70. doi:10.1097/MPA.0b013e318184d330
Biankin, A. V., Waddell, N., Kassahn, K. S., Gingras, M. C., Muthuswamy, L. B., Johns, A. L., et al. (2012). Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 491 (7424), 399–405. doi:10.1038/nature11547
Bisht, S., and Feldmann, G. (2019). Animal models for modeling pancreatic cancer and novel drug discovery. Expet Opin. Drug Discov. 14 (2), 127–142. doi:10.1080/17460441.2019.1566319
Bloom, A. P., Jimenez-Andrade, J. M., Taylor, R. N., Castaneda-Corral, G., Kaczmarska, M. J., Freeman, K. T., et al. (2011). Breast cancer-induced bone remodeling, skeletal pain, and sprouting of sensory nerve fibers. J. Pain 12 (6), 698–711. doi:10.1016/j.jpain.2010.12.016
Brown, D. C., Agnello, K., and Iadarola, M. J. (2015). Intrathecal resiniferatoxin in a dog model: efficacy in bone cancer pain. Pain 156 (6), 1018–1024. doi:10.1097/j.pain.0000000000000115
Brown, D. C. (2016). Resiniferatoxin: the evolution of the “molecular scalpel” for chronic pain relief. Pharmaceuticals (Basel) 9 (3), 47. doi:10.3390/ph9030047
Brown, M. R., Ramirez, J. D., and Farquhar-Smith, P. (2014). Pain in cancer survivors. Br J Pain 8 (4), 139–153. doi:10.1177/2049463714542605
Bruna, J., Alberti, P., Calls-Cobos, A., Caillaud, M., Damaj, M. I., and Navarro, X. (2020). Methods for in vivo studies in rodents of chemotherapy induced peripheral neuropathy. Exp. Neurol. 325, 113154. doi:10.1016/j.expneurol.2019.113154
Buehlmann, D., Ielacqua, G. D., Xandry, J., and Rudin, M. (2019). Prospective administration of anti-nerve growth factor treatment effectively suppresses functional connectivity alterations after cancer-induced bone pain in mice. Pain 160 (1), 151–159. doi:10.1097/j.pain.0000000000001388
Butturini, E., Carcereri de Prati, A., Chiavegato, G., Rigo, A., Cavalieri, E., Darra, E., et al. (2013). Mild oxidative stress induces S-glutathionylation of STAT3 and enhances chemosensitivity of tumoural cells to chemotherapeutic drugs. Free Radic. Biol. Med. 65, 1322–1330. doi:10.1016/j.freeradbiomed.2013.09.015
Cain, D. M., Wacnik, P. W., Turner, M., Wendelschafer-Crabb, G., Kennedy, W. R., Wilcox, G. L., et al. (2001). Functional interactions between tumor and peripheral nerve: changes in excitability and morphology of primary afferent fibers in a murine model of cancer pain. J. Neurosci. 21 (23), 9367–9376. doi:10.1523/JNEUROSCI.21-23-09367.2001
Calixto-Campos, C., Zarpelon, A. C., Correa, M., Cardoso, R. D., Pinho-Ribeiro, F. A., Cecchini, R., et al. (2013). The Ehrlich tumor induces pain-like behavior in mice: a novel model of cancer pain for pathophysiological studies and pharmacological screening. BioMed Res. Int. 2013, 624815. doi:10.1155/2013/624815
Caraceni, A., and Portenoy, R. K. (1999). An international survey of cancer pain characteristics and syndromes. IASP task force on cancer pain. international association for the study of pain. Pain 82 (3), 263–274. doi:10.1016/s0304-3959(99)00073-1
Caraceni, A., and Shkodra, M. (2019). Cancer pain assessment and classification. Cancers (Basel) 11 (4), 510. doi:10.3390/cancers11040510.
Casals-Diaz, L., Vivo, M., and Navarro, X. (2009). Nociceptive responses and spinal plastic changes of afferent C-fibers in three neuropathic pain models induced by sciatic nerve injury in the rat. Exp. Neurol. 217 (1), 84–95. doi:10.1016/j.expneurol.2009.01.014
Cavaletti, G., Alberti, P., Argyriou, A. A., Lustberg, M., Staff, N. P., Tamburin, S., et al. (2019). Chemotherapy-induced peripheral neurotoxicity: a multifaceted, still unsolved issue. J. Peripher. Nerv. Syst. 24 (Suppl. 2), S6–S12. doi:10.1111/jns.12337
Chartier, L. C., Hebart, M. L., Howarth, G. S., Whittaker, A. L., and Mashtoub, S. (2020). Affective state determination in a mouse model of colitis-associated colorectal cancer. PloS One 15 (1), e0228413. doi:10.1371/journal.pone.0228413
Chine, V. B., Au, N. P. B., Kumar, G., and Ma, C. H. E. (2019). Targeting axon integrity to prevent chemotherapy-induced peripheral neuropathy. Mol. Neurobiol. 56 (5), 3244–3259. doi:10.1007/s12035-018-1301-8
Chodroff, L., Bendele, M., Valenzuela, V., Henry, M., and Ruparel, S. (2016). Express: BDNF signaling contributes to oral cancer pain in a preclinical orthotopic rodent model. Mol. Pain 12, 1744806916666841. doi:10.1177/1744806916666841
Coleman, R. E. (2006). Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Canc. Res. 12 (20 Pt 2), 6243s–6249s. doi:10.1158/1078-0432.CCR-06-0931
Constantin, C. E., Mair, N., Sailer, C. A., Andratsch, M., Xu, Z. Z., Blumer, M. J., et al. (2008). Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model. J. Neurosci. 28 (19), 5072–5081. doi:10.1523/JNEUROSCI.4476-07.2008
Currie, G. L., Delaney, A., Bennett, M. I., Dickenson, A. H., Egan, K. J., Vesterinen, H. M., et al. (2013). Animal models of bone cancer pain: systematic review and meta-analyses. Pain 154 (6), 917–926. doi:10.1016/j.pain.2013.02.033
Deandrea, S., Corli, O., Consonni, D., Villani, W., Greco, M. T., and Apolone, G. (2014). Prevalence of breakthrough cancer pain: a systematic review and a pooled analysis of published literature. J. Pain Symptom Manag. 47 (1), 57–76. doi:10.1016/j.jpainsymman.2013.02.015
Diaz-delCastillo, M., Kamstrup, D., Olsen, R. B., Hansen, R. B., Pembridge, T., Simanskaite, B., et al. (2020). Differential pain-related behaviors and bone disease in immunocompetent mouse models of myeloma. JBMR Plus 4 (2), e10252. doi:10.1002/jbm4.10252
Ding, L., El Zaatari, M., and Merchant, J. L. (2016). Recapitulating human gastric cancer pathogenesis: experimental models of gastric cancer. Adv. Exp. Med. Biol. 908, 441–478. doi:10.1007/978-3-319-41388-4_22
Dolan, J. C., Lam, D. K., Achdjian, S. H., and Schmidt, B. L. (2010). The dolognawmeter: a novel instrument and assay to quantify nociception in rodent models of orofacial pain. J. Neurosci. Methods 187 (2), 207–215. doi:10.1016/j.jneumeth.2010.01.012
Falk, S., and Dickenson, A. H. (2014). Pain and nociception: mechanisms of cancer-induced bone pain. J. Clin. Oncol. 32 (16), 1647–1654. doi:10.1200/JCO.2013.51.7219
Falk, S., Gallego-Pedersen, S., and Petersen, N. C. (2017). Grid-climbing behaviour as a pain measure for cancer-induced bone pain and neuropathic pain. In Vivo 31 (4), 619–623. doi:10.21873/invivo.11102
Fallon, M. T. (2013). Neuropathic pain in cancer. Br. J. Anaesth. 111 (1), 105–111. doi:10.1093/bja/aet208
Faulkner, S., Jobling, P., March, B., Jiang, C. C., and Hondermarck, H. (2019). Tumor neurobiology and the war of nerves in cancer. Canc. Discov. 9 (6), 702–710. doi:10.1158/2159-8290.CD-18-1398
Flatters, S. J. L., Dougherty, P. M., and Colvin, L. A. (2017). Clinical and preclinical perspectives on chemotherapy-induced peripheral neuropathy (CIPN): a narrative review. Br. J. Anaesth. 119 (4), 737–749. doi:10.1093/bja/aex229
Fujii, K. (1991). Evaluation of the newborn mouse model for chemical tumorigenesis. Carcinogenesis 12 (8), 1409–1415. doi:10.1093/carcin/12.8.1409
Fujita, M., Andoh, T., Sasaki, A., Saiki, I., and Kuraishi, Y. (2010). Involvement of peripheral adenosine 5'-triphosphate and P2X purinoceptor in pain-related behavior produced by orthotopic melanoma inoculation in mice. Eur. J. Neurosci. 31 (9), 1629–1636. doi:10.1111/j.1460-9568.2010.07185.x
Gadgil, S., Ergun, M., van den Heuvel, S. A., van der Wal, S. E., Scheffer, G. J., and Hooijmans, C. R. (2019). A systematic summary and comparison of animal models for chemotherapy induced (peripheral) neuropathy (CIPN). PloS One 14 (8), e0221787. doi:10.1371/journal.pone.0221787
Gao, L., Bo, H., Wang, Y., Zhang, J., and Zhu, M. (2015). Neurotrophic factor Artemin promotes invasiveness and neurotrophic function of pancreatic adenocarcinoma in vivo and in vitro. Pancreas 44 (1), 134–143. doi:10.1097/MPA.0000000000000223
Gdowski, A. S., Ranjan, A., Sarker, M. R., and Vishwanatha, J. K. (2017). Bone-targeted cabazitaxel nanoparticles for metastatic prostate cancer skeletal lesions and pain. Nanomedicine 12 (17), 2083–2095. doi:10.2217/nnm-2017-0190
Grisold, W., Cavaletti, G., and Windebank, A. J. (2012). Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro Oncol. 14 (Suppl. 4), iv45-54. doi:10.1093/neuonc/nos203
Grond, S., Zech, D., Diefenbach, C., Radbruch, L., and Lehmann, K. A. (1996). Assessment of cancer pain: a prospective evaluation in 2266 cancer patients referred to a pain service. Pain 64 (1), 107–114. doi:10.1016/0304-3959(95)00076-3
Hald, A., Hansen, R. R., Thomsen, M. W., Ding, M., Croucher, P. I., Gallagher, O., et al. (2009a). Cancer-induced bone loss and associated pain-related behavior is reduced by risedronate but not its phosphonocarboxylate analog NE-10790. Int. J. Canc. 125 (5), 1177–1185. doi:10.1002/ijc.24436
Hald, A., Nedergaard, S., Hansen, R. R., Ding, M., and Heegaard, A. M. (2009b). Differential activation of spinal cord glial cells in murine models of neuropathic and cancer pain. Eur. J. Pain 13 (2), 138–145. doi:10.1016/j.ejpain.2008.03.014
Halvorson, K. G., Kubota, K., Sevcik, M. A., Lindsay, T. H., Sotillo, J. E., Ghilardi, J. R., et al. (2005a). A blocking antibody to nerve growth factor Attenuates skeletal pain induced by prostate tumor cells growing in bone. Canc. Res. 65 (20), 9426–9435. doi:10.1158/0008-5472.can-05-0826
Halvorson, K. G., Kubota, K., Sevcik, M. A., Lindsay, T. H., Sotillo, J. E., Ghilardi, J. R., et al. (2005b). A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res 65 (20), 9426–9435. doi:10.1158/0008-5472.CAN-05-0826
Han, F. Y., Kuo, A., Nicholson, J. R., Corradinni, L., and Smith, M. T. (2018). Comparative analgesic efficacy of pregabalin administered according to either a prevention protocol or an intervention protocol in rats with cisplatin-induced peripheral neuropathy. Clin. Exp. Pharmacol. Physiol. 45 (10), 1067–1075. doi:10.1111/1440-1681.12971
Han, L., Jiang, J., Xue, M., Qin, T., Xiao, Y., Wu, E., et al. (2020). Sonic hedgehog signaling pathway promotes pancreatic cancer pain via nerve growth factor. Reg. Anesth. Pain Med. 45 (2), 137–144. doi:10.1136/rapm-2019-100991
Han, L., Ma, J., Duan, W., Zhang, L., Yu, S., Xu, Q., et al. (2016). Pancreatic stellate cells contribute pancreatic cancer pain via activation of sHH signaling pathway. Oncotarget 7 (14), 18146–18158. doi:10.18632/oncotarget.7776
Havelin, J., Imbert, I., Sukhtankar, D., Remeniuk, B., Pelletier, I., Gentry, J., et al. (2017). Mediation of movement-induced breakthrough cancer pain by IB4-binding nociceptors in rats. J. Neurosci. 37 (20), 5111–5122. doi:10.1523/JNEUROSCI.1212-16.2017
Henze, D. A., and Urban, M. O. (2010). “Large animal models for pain therapeutic development,” in Translational pain research: from mouse to man. Editors L. Kruger, and A. R. Light, (Boca Raton, FL).
Hiraga, T., Williams, P. J., Mundy, G. R., and Yoneda, T. (2001). The bisphosphonate ibandronate promotes apoptosis in MDA-MB-231 human breast cancer cells in bone metastases. Cancer Res 61 (11), 4418–4424.
Hird, A., Chow, E., Zhang, L., Wong, R., Wu, J., Sinclair, E., et al. (2009). Determining the incidence of pain flare following palliative radiotherapy for symptomatic bone metastases: results from three canadian cancer centers. Int. J. Radiat. Oncol. Biol. Phys. 75 (1), 193–197. doi:10.1016/j.ijrobp.2008.10.044
Hironaka, K., Ozaki, N., Hattori, H., Nagamine, K., Nakashima, H., Ueda, M., et al. (2014). Involvement of glial activation in trigeminal ganglion in a rat model of lower gingival cancer pain. Nagoya J. Med. Sci. 76 (3–4), 323–332.
Hirth, M., Gandla, J., Hoper, C., Gaida, M. M., Agarwal, N., Simonetti, M., et al. (2020). CXCL10 and CCL21 promote migration of pancreatic cancer cells toward sensory neurons and neural remodeling in tumors in mice, associated with pain in patients. Gastroenterology 159, 665–681. doi:10.1053/j.gastro.2020.04.037
Holen, I., Speirs, V., Morrissey, B., and Blyth, K. (2017). In vivo models in breast cancer research: progress, challenges and future directions. Dis Model Mech 10 (4), 359–371. doi:10.1242/dmm.028274
Honore, P., Luger, N. M., Sabino, M. A., Schwei, M. J., Rogers, S. D., Mach, D. B., et al. (2000a). Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nat. Med. 6 (5), 521–528. doi:10.1038/74999
Honore, P., Rogers, S. D., Schwei, M. J., Salak-Johnson, J. L., Luger, N. M., Sabino, M. C., et al. (2000b). Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience 98 (3), 585–598. doi:10.1016/s0306-4522(00)00110-x
Isono, M., Suzuki, T., Hosono, K., Hayashi, I., Sakagami, H., Uematsu, S., et al. (2011). Microsomal prostaglandin E synthase-1 enhances bone cancer growth and bone cancer-related pain behaviors in mice. Life Sci. 88 (15–16), 693–700. doi:10.1016/j.lfs.2011.02.008
Jimenez Andrade, J. M., and Mantyh, P. (2010). “Cancer pain: from the development of mouse models to human clinical trials,” in Translational pain research: from mouse to man. Editors L. Kruger, and A. R. Light, (Boca Raton, FL).
Jimenez-Andrade, J. M., Bloom, A. P., Stake, J. I., Mantyh, W. G., Taylor, R. N., Freeman, K. T., et al. (2010). Pathological sprouting of adult nociceptors in chronic prostate cancer-induced bone pain. J. Neurosci. 30 (44), 14649–14656. doi:10.1523/JNEUROSCI.3300-10.2010
Jimenez-Andrade, J. M., Ghilardi, J. R., Castaneda-Corral, G., Kuskowski, M. A., and Mantyh, P. W. (2011). Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain 152 (11), 2564–2574. doi:10.1016/j.pain.2011.07.020
Kajiwara, I., Sano, M., Ichimaru, Y., Oshima, Y., Kitajima, O., Hao, H., et al. (2020). Duloxetine improves cancer-associated pain in a mouse model of pancreatic cancer via stimulation of noradrenaline pathway and its antitumor effects. Pain 161, 2909–2919. doi:10.1097/j.pain.0000000000001997
Kamano, T., Azuma, N., Katami, A., Tamura, J., Sakakibara, N., Matsumoto, M., et al. (1988). Preliminary observation on pancreatic duct adenocarcinoma induced by intraductal administration of N-ethyl-N'-nitro-N-nitrosoguanidine in dogs. Jpn. J. Canc. Res. 79 (1), 1–4. doi:10.1111/j.1349-7006.1988.tb00001.x
King, T. E., Pawar, S. C., Majuta, L., Sroka, I. C., Wynn, D., Demetriou, M. C., et al. (2008). The role of alpha 6 integrin in prostate cancer migration and bone pain in a novel xenograft model. PloS One 3 (10), e3535. doi:10.1371/journal.pone.0003535
King, T., Vardanyan, A., Majuta, L., Melemedjian, O., Nagle, R., Cress, A. E., et al. (2007). Morphine treatment accelerates sarcoma-induced bone pain, bone loss, and spontaneous fracture in a murine model of bone cancer. Pain 132 (1–2), 154–168. doi:10.1016/j.pain.2007.06.026
Kolosov, A., Aurini, L., Williams, E. D., Cooke, I., and Goodchild, C. S. (2011). Intravenous injection of leconotide, an omega conotoxin: synergistic antihyperalgesic effects with morphine in a rat model of bone cancer pain. Pain Med. 12 (6), 923–941. doi:10.1111/j.1526-4637.2011.01118.x
Kolosov, A., Goodchild, C. S., Williams, E. D., and Cooke, I. (2012). Flupirtine enhances the anti-hyperalgesic effects of morphine in a rat model of prostate bone metastasis. Pain Med. 13 (11), 1444–1456. doi:10.1111/j.1526-4637.2012.01502.x
Koskinen, M. J., Kautio, A. L., Haanpaa, M. L., Haapasalo, H. K., Kellokumpu-Lehtinen, P. L., Saarto, T., et al. (2011). Intraepidermal nerve fibre density in cancer patients receiving adjuvant chemotherapy. Anticancer Res. 31 (12), 4413–4416.
Kuraishi, Y., Iida, Y., Zhang, H. W., Uehara, S., Nojima, H., Murata, J., et al. (2003). Suppression by gabapentin of pain-related mechano-responses in mice given orthotopic tumor inoculation. Biol. Pharm. Bull. 26 (4), 550–552. doi:10.1248/bpb.26.550
Kyte, S. L., Toma, W., Bagdas, D., Meade, J. A., Schurman, L. D., Lichtman, A. H., et al. (2018). Nicotine prevents and reverses paclitaxel-induced mechanical allodynia in a mouse model of CIPN. J. Pharmacol. Exp. Therapeut. 364 (1), 110–119. doi:10.1124/jpet.117.243972
Lam, D. K., Dang, D., Zhang, J., Dolan, J. C., and Schmidt, B. L. (2012). Novel animal models of acute and chronic cancer pain: a pivotal role for PAR2. J. Neurosci. 32 (41), 14178–14183. doi:10.1523/JNEUROSCI.2399-12.2012
Lam, D. K. (2016). Emerging factors in the progression of cancer-related pain. Pain Manag. 6 (5), 487–496. doi:10.2217/pmt-2015-0003
Lam, D. K., and Schmidt, B. L. (2011). Orofacial pain onset predicts transition to head and neck cancer. Pain 152 (5), 1206–1209. doi:10.1016/j.pain.2011.02.009
Lascelles, B. D. X., Brown, D. C., Conzemius, M. G., Gill, M., Oshinsky, M. L., and Sharkey, M. (2019). Measurement of chronic pain in companion animals: discussions from the pain in animals workshop (PAW) 2017. Vet. J. 250, 71–78. doi:10.1016/j.tvjl.2019.07.001
Laumet, G., Edralin, J. D., Dantzer, R., Heijnen, C. J., and Kavelaars, A. (2019). Cisplatin educates CD8+ T cells to prevent and resolve chemotherapy-induced peripheral neuropathy in mice. Pain 160 (6), 1459–1468. doi:10.1097/j.pain.0000000000001512
Lema, M. J., Foley, K. M., and Hausheer, F. H. (2010). Types and epidemiology of cancer-related neuropathic pain: the intersection of cancer pain and neuropathic pain. Oncol. 15 (Suppl. 2), 3–8. doi:10.1634/theoncologist.2009-S505
Li, Q., Dong, H., Yang, G., Song, Y., Mou, Y., and Ni, Y. (2020). Mouse tumor-bearing models as preclinical study platforms for oral squamous cell carcinoma. Front Oncol 10, 212. doi:10.3389/fonc.2020.00212
Lindsay, T. H., Jonas, B. M., Sevcik, M. A., Kubota, K., Halvorson, K. G., Ghilardi, J. R., et al. (2005a). Pancreatic cancer pain and its correlation with changes in tumor vasculature, macrophage infiltration, neuronal innervation, body weight and disease progression. Pain 119 (1–3), 233–246. doi:10.1016/j.pain.2005.10.019
Lindsay, T. H., Jonas, B. M., Sevcik, M. A., Kubota, K., Halvorson, K. G., Ghilardi, J. R., et al. (2005b). Pancreatic cancer pain and its correlation with changes in tumor vasculature, macrophage infiltration, neuronal innervation, body weight and disease progression. Pain 119 (1–3), 233–246. doi:10.1016/j.pain.2005.10.019
Liu, C. N., Berryman, E., Zakur, D., Shoieb, A. M., Pardo, I. D., Boucher, M., et al. (2018). A novel endpoint for the assessment of chemotherapy-induced peripheral neuropathy in rodents: biomechanical properties of peripheral nerve. J. Appl. Toxicol. 38 (2), 193–200. doi:10.1002/jat.3513
Liu, S., Yang, J., Wang, L., Jiang, M., Qiu, Q., Ma, Z., et al. (2010). Tibia tumor-induced cancer pain involves spinal p38 mitogen-activated protein kinase activation via TLR4-dependent mechanisms. Brain Res. 1346, 213–223. doi:10.1016/j.brainres.2010.05.014
Liu, W. C., Zheng, Z. X., Tan, K. H., and Meredith, G. J. (2017). Multidimensional treatment of cancer pain. Curr. Oncol. Rep. 19 (2), 10. doi:10.1007/s11912-017-0570-0
Loblaw, D. A., Wu, J. S., Kirkbride, P., Panzarella, T., Smith, K., Aslanidis, J., et al. (2007). Pain flare in patients with bone metastases after palliative radiotherapy--a nested randomized control trial. Support. Care Canc. 15 (4), 451–455. doi:10.1007/s00520-006-0166-y
Lofgren, J., Miller, A. L., Lee, C. C. S., Bradshaw, C., Flecknell, P., and Roughan, J. (2018). Analgesics promote welfare and sustain tumour growth in orthotopic 4T1 and B16 mouse cancer models. Lab. Anim 52 (4), 351–364. doi:10.1177/0023677217739934
Loprinzi, C. L., Maddocks-Christianson, K., Wolf, S. L., Rao, R. D., Dyck, P. J., Mantyh, P., et al. (2007). The Paclitaxel acute pain syndrome: sensitization of nociceptors as the putative mechanism. Cancer J 13 (6), 399–403. doi:10.1097/PPO.0b013e31815a999b
Lozano-Ondoua, A. N., Symons-Liguori, A. M., and Vanderah, T. W. (2013). Cancer-induced bone pain: mechanisms and models. Neurosci. Lett. 557 (Pt A), 52–59. doi:10.1016/j.neulet.2013.08.003
Luger, N. M., Honore, P., Sabino, M. A., Schwei, M. J., Rogers, S. D., Mach, D. B., et al. (2001). Osteoprotegerin diminishes advanced bone cancer pain. Cancer Res 61 (10), 4038–4047.
Luna, S. P. L., de Araujo, A. L., da Nobrega Neto, P. I., Brondani, J. T., de Oliveira, F. A., Azeredo, L., et al. (2020). Validation of the UNESP-Botucatu pig composite acute pain scale (UPAPS). PloS One 15 (6), e0233552. doi:10.1371/journal.pone.0233552
Luo, X., Huh, Y., Bang, S., He, Q., Zhang, L., Matsuda, M., et al. (2019). Macrophage toll-like receptor 9 contributes to chemotherapy-induced neuropathic pain in male mice. J. Neurosci. 39 (35), 6848–6864. doi:10.1523/JNEUROSCI.3257-18.2019
Ma, J., Trinh, R. T., Mahant, I. D., Peng, B., Matthias, P., Heijnen, C. J., et al. (2019). Cell-specific role of histone deacetylase 6 in chemotherapy-induced mechanical allodynia and loss of intraepidermal nerve fibers. Pain 160 (12), 2877–2890. doi:10.1097/j.pain.0000000000001667
Maeda, T., Yamada, D., and Kawahara, K. (2016). Cancer pain relief achieved by disrupting tumor-driven semaphorin 3A signaling in mice. Neurosci. Lett. 632, 147–151. doi:10.1016/j.neulet.2016.08.060
Makker, P. G., Duffy, S. S., Lees, J. G., Perera, C. J., Tonkin, R. S., Butovsky, O., et al. (2017). Characterisation of immune and neuroinflammatory changes associated with chemotherapy-induced peripheral neuropathy. PloS One 12 (1), e0170814. doi:10.1371/journal.pone.0170814.
Mandala, M., Moro, C., Labianca, R., Cremonesi, M., and Barni, S. (2006). Optimizing use of opiates in the management of cancer pain. Therapeut. Clin. Risk Manag. 2 (4), 447–453. doi:10.2147/tcrm.2006.2.4.447
Medhurst, S. J., Walker, K., Bowes, M., Kidd, B. L., Glatt, M., Muller, M., et al. (2002). A rat model of bone cancer pain. Pain 96 (1–2), 129–140. doi:10.1016/s0304-3959(01)00437-7
Mercadante, S., and Arcuri, E. (1998). Breakthrough pain in cancer patients: pathophysiology and treatment. Canc. Treat Rev. 24 (6), 425–432. doi:10.1016/s0305-7372(98)90005-6
Mercadante, S. (1997). Malignant bone pain: pathophysiology and treatment. Pain 69 (1–2), 1–18. doi:10.1016/s0304-3959(96)03267-8
Miao, X. R., Gao, X. F., Wu, J. X., Lu, Z. J., Huang, Z. X., Li, X. Q., et al. (2010). Bilateral downregulation of Nav1.8 in dorsal root ganglia of rats with bone cancer pain induced by inoculation with Walker 256 breast tumor cells. BMC Canc. 10, 216. doi:10.1186/1471-2407-10-216
Miladinovic, T., Sharma, M., Phan, A., Geres, H., Ungard, R. G., Linher-Melville, K., et al. (2019). Activation of hippocampal microglia in a murine model of cancer-induced pain. J. Pain Res. 12, 1003–1016. doi:10.2147/JPR.S191860
Miura, T., Mitsunaga, S., Ikeda, M., Ohno, I., Takahashi, H., Kuwata, T., et al. (2018). Neural invasion spreads macrophage-related allodynia via neural root in pancreatic cancer. Anesth. Analg. 126 (5), 1729–1738. doi:10.1213/ANE.0000000000002803
Molassiotis, A., Cheng, H. L., Lopez, V., Au, J. S. K., Chan, A., Bandla, A., et al. (2019). Are we mis-estimating chemotherapy-induced peripheral neuropathy? Analysis of assessment methodologies from a prospective, multinational, longitudinal cohort study of patients receiving neurotoxic chemotherapy. BMC Canc. 19 (1), 132. doi:10.1186/s12885-019-5302-4
Monteiro, B. P., de Lorimier, L. P., Moreau, M., Beauchamp, G., Blair, J., Lussier, B., et al. (2018). Pain characterization and response to palliative care in dogs with naturally-occurring appendicular osteosarcoma: an open label clinical trial. PloS One 13 (12), e0207200. doi:10.1371/journal.pone.0207200
Mouedden, M. E., and Meert, T. F. (2005). Evaluation of pain-related behavior, bone destruction and effectiveness of fentanyl, sufentanil, and morphine in a murine model of cancer pain. Pharmacol. Biochem. Behav. 82 (1), 109–119. doi:10.1016/j.pbb.2005.07.016
Muralidharan, A., Wyse, B. D., and Smith, M. T. (2013). Optimization and characterization of a rat model of prostate cancer-induced bone pain using behavioral, pharmacological, radiological, histological and immunohistochemical methods. Pharmacol. Biochem. Behav. 106, 33–46. doi:10.1016/j.pbb.2013.02.020
Nagamine, K., Ozaki, N., Shinoda, M., Asai, H., Nishiguchi, H., Mitsudo, K., et al. (2006). Mechanical allodynia and thermal hyperalgesia induced by experimental squamous cell carcinoma of the lower gingiva in rats. J. Pain 7 (9), 659–670. doi:10.1016/j.jpain.2006.02.013
Nersesyan, H., and Slavin, K. V. (2007). Current aproach to cancer pain management: availability and implications of different treatment options. Therapeut. Clin. Risk Manag. 3 (3), 381–400.
Oh, J., Magnuson, A., Benoist, C., Pittet, M. J., and Weissleder, R. (2018). Age-related tumor growth in mice is related to integrin alpha 4 in CD8+ T cells. JCI Insight 3 (21), e122961. doi:10.1172/jci.insight.122961
Ono, K., Ye, Y., Viet, C. T., Dang, D., and Schmidt, B. L. (2015). TRPV1 expression level in isolectin B(4)-positive neurons contributes to mouse strain difference in cutaneous thermal nociceptive sensitivity. J. Neurophysiol. 113 (9), 3345–3355. doi:10.1152/jn.00973.2014
Park, H. J., Stokes, J. A., Pirie, E., Skahen, J., Shtaerman, Y., and Yaksh, T. L. (2013). Persistent hyperalgesia in the cisplatin-treated mouse as defined by threshold measures, the conditioned place preference paradigm, and changes in dorsal root ganglia activated transcription factor 3: the effects of gabapentin, ketorolac, and etanercept. Anesth. Analg. 116 (1), 224–231. doi:10.1213/ANE.0b013e31826e1007
Pickering, V., Jay Gupta, R., Quang, P., Jordan, R. C., and Schmidt, B. L. (2008). Effect of peripheral endothelin-1 concentration on carcinoma-induced pain in mice. Eur. J. Pain 12 (3), 293–300. doi:10.1016/j.ejpain.2007.06.001
Portenoy, R. K., and Ahmed, E. (2018). Cancer pain syndromes. Hematol. Oncol. Clin. N. Am. 32 (3), 371–386. doi:10.1016/j.hoc.2018.01.002
Portenoy, R. K., and Hagen, N. A. (1990). Breakthrough pain: definition, prevalence and characteristics. Pain 41 (3), 273–281. doi:10.1016/0304-3959(90)90004-w
Remeniuk, B., King, T., Sukhtankar, D., Nippert, A., Li, N., Li, F., et al. (2018). Disease modifying actions of interleukin-6 blockade in a rat model of bone cancer pain. Pain 159 (4), 684–698. doi:10.1097/j.pain.0000000000001139
Robertson, N., Schook, L. B., and Schachtschneider, K. M. (2020). Porcine cancer models: potential tools to enhance cancer drug trials. Expet Opin. Drug Discov. 15 (8), 893–902. doi:10.1080/17460441.2020.1757644
Roughan, J. V., Coulter, C. A., Flecknell, P. A., Thomas, H. D., and Sufka, K. J. (2014). The conditioned place preference test for assessing welfare consequences and potential refinements in a mouse bladder cancer model. PloS One 9 (8), e103362. doi:10.1371/journal.pone.0103362
Roughan, J. V., Flecknell, P. A., and Davies, B. R. (2004a). Behavioural assessment of the effects of tumour growth in rats and the influence of the analgesics carprofen and meloxicam. Lab. Anim 38 (3), 286–296. doi:10.1258/002367704323133673
Roughan, J. V., Flecknell, P. A., and Davies, B. R. (2004b). Behavioural assessment of the effects of tumour growth in rats and the influence of the analgesics carprofen and meloxicam. Lab. Anim 38 (3), 286–296. doi:10.1258/002367704323133673
Rubin, J. B., Lagas, J. S., Broestl, L., Sponagel, J., Rockwell, N., Rhee, G., et al. (2020). Sex differences in cancer mechanisms. Biol. Sex Differ. 11 (1), 17. doi:10.1186/s13293-020-00291-x
Sabino, M. A., Luger, N. M., Mach, D. B., Rogers, S. D., Schwei, M. J., and Mantyh, P. W. (2003). Different tumors in bone each give rise to a distinct pattern of skeletal destruction, bone cancer-related pain behaviors and neurochemical changes in the central nervous system. Int. J. Canc. 104 (5), 550–558. doi:10.1002/ijc.10999
Saenz Robles, M. T., and Pipas, J. M. (2009). T antigen transgenic mouse models. Semin. Canc. Biol. 19 (4), 229–235. doi:10.1016/j.semcancer.2009.02.002
Salvo, E., Campana, W. M., Scheff, N. N., Tu, N. H., Jeong, S. H., Wall, I., et al. (2020). Peripheral nerve injury and sensitization underlie pain associated with oral cancer perineural invasion. Pain 161, 2592–2602. doi:10.1097/j.pain.0000000000001986
Scheff, N. N., Alemu, R. G., Klares, R., Wall, I. M., Yang, S. C., Dolan, J. C., et al. (2019). Granulocyte-colony stimulating factor-induced neutrophil recruitment provides opioid-mediated endogenous anti-nociception in female mice with oral squamous cell carcinoma. Front. Mol. Neurosci. 12, 217. doi:10.3389/fnmol.2019.00217
Scheff, N. N., Bhattacharya, A., Dowse, E., Dang, R. X., Dolan, J. C., Wang, S., et al. (2018). Neutrophil-mediated endogenous analgesia contributes to sex differences in oral cancer pain. Front. Integr. Neurosci. 12, 52. doi:10.3389/fnint.2018.00052
Scheff, N. N., Ye, Y., Bhattacharya, A., MacRae, J., Hickman, D. N., Sharma, A. K., et al. (2017). Tumor necrosis factor alpha secreted from oral squamous cell carcinoma contributes to cancer pain and associated inflammation. Pain 158 (12), 2396–2409. doi:10.1097/j.pain.0000000000001044
Scheff, N. N., Ye, Y., Conley, Z., Quan, J. W., Ronald Lam, Y. V., Klares, R., et al. (2020). ADAM17-EGFR signaling contributes to oral cancer pain. Pain 161, 2330–2343. doi:10.1097/j.pain.0000000000001926
Schmidt, B. L. (2014). The neurobiology of cancer pain. Neuroscientist 20 (5), 546–562. doi:10.1177/1073858414525828
Schwei, M. J., Honore, P., Rogers, S. D., Salak-Johnson, J. L., Finke, M. P., Ramnaraine, M. L., et al. (1999). Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J. Neurosci. 19 (24), 10886–10897. doi:10.1523/JNEUROSCI.19-24-10886.1999
Selvaraj, D., Hirth, M., Gandla, J., and Kuner, R. (2017). A mouse model for pain and neuroplastic changes associated with pancreatic ductal adenocarcinoma. Pain 158 (8), 1609–1621. doi:10.1097/j.pain.0000000000000956
Seretny, M., Currie, G. L., Sena, E. S., Ramnarine, S., Grant, R., MacLeod, M. R., et al. (2014). Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain 155 (12), 2461–2470. doi:10.1016/j.pain.2014.09.020
Sevcik, M. A., Ghilardi, J. R., Halvorson, K. G., Lindsay, T. H., Kubota, K., and Mantyh, P. W. (2005a). Analgesic efficacy of bradykinin B1 antagonists in a murine bone cancer pain model. J. Pain 6 (11), 771–775. doi:10.1016/j.jpain.2005.06.010
Sevcik, M. A., Ghilardi, J. R., Peters, C. M., Lindsay, T. H., Halvorson, K. G., Jonas, B. M., et al. (2005b). Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain 115 (1–2), 128–141. doi:10.1016/j.pain.2005.02.022
Sevcik, M. A., Jonas, B. M., Lindsay, T. H., Halvorson, K. G., Ghilardi, J. R., Kuskowski, M. A., et al. (2006). Endogenous opioids inhibit early-stage pancreatic pain in a mouse model of pancreatic cancer. Gastroenterology 131 (3), 900–910. doi:10.1053/j.gastro.2006.06.021
Shahid, M., Subhan, F., Ahmad, N., and Sewell, R. D. E. (2019). Efficacy of a topical gabapentin gel in a cisplatin paradigm of chemotherapy-induced peripheral neuropathy. BMC Pharmacol Toxicol 20 (1), 51. doi:10.1186/s40360-019-0329-3
Shimoyama, M., Tatsuoka, H., Ohtori, S., Tanaka, K., and Shimoyama, N. (2005). Change of dorsal horn neurochemistry in a mouse model of neuropathic cancer pain. Pain 114 (1–2), 221–230. doi:10.1016/j.pain.2004.12.018
Shinoda, M., Ogino, A., Ozaki, N., Urano, H., Hironaka, K., Yasui, M., et al. (2008). Involvement of TRPV1 in nociceptive behavior in a rat model of cancer pain. J. Pain 9 (8), 687–699. doi:10.1016/j.jpain.2008.02.007
Singhi, A. D., George, B., Greenbowe, J. R., Chung, J., Suh, J., Maitra, A., et al. (2019). Real-time targeted genome profile Analysis of pancreatic ductal adenocarcinomas identifies genetic alterations that might Be targeted with existing drugs or used as biomarkers. Gastroenterology 156 (8), 2242–2253. doi:10.1053/j.gastro.2019.02.037
Sisignano, M., Baron, R., Scholich, K., and Geisslinger, G. (2014). Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat. Rev. Neurol. 10 (12), 694–707. doi:10.1038/nrneurol.2014.211
Slatkin, N., Zaki, N., Wang, S., Louie, J., Sanga, P., Kelly, K. M., et al. (2019). Fulranumab as adjunctive therapy for cancer-related pain: a phase 2, randomized, double-blind, placebo-controlled, multicenter study. J. Pain 20 (4), 440–452. doi:10.1016/j.jpain.2018.09.014
Sliepen, S. H. J., Diaz-Delcastillo, M., Korioth, J., Olsen, R. B., Appel, C. K., Christoph, T., et al. (2019). Cancer-induced bone pain impairs burrowing behaviour in mouse and rat. In Vivo 33 (4), 1125–1132. doi:10.21873/invivo.11582
Slosky, L. M., BassiriRad, N. M., Symons, A. M., Thompson, M., Doyle, T., Forte, B. L., et al. (2016). The cystine/glutamate antiporter system xc- drives breast tumor cell glutamate release and cancer-induced bone pain. Pain 157 (11), 2605–2616. doi:10.1097/j.pain.0000000000000681
Stopczynski, R. E., Normolle, D. P., Hartman, D. J., Ying, H., DeBerry, J. J., Bielefeldt, K., et al. (2014). Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res 74 (6), 1718–1727. doi:10.1158/0008-5472.CAN-13-2050
Tang, Y., Peng, H., Liao, Q., Gan, L., Zhang, R., Huang, L., et al. (2016). Study of breakthrough cancer pain in an animal model induced by endothelin-1. Neurosci. Lett. 617, 108–115. doi:10.1016/j.neulet.2016.01.053
Tappe-Theodor, A., King, T., and Morgan, M. M. (2019). Pros and cons of clinically relevant methods to assess pain in rodents. Neurosci. Biobehav. Rev. 100, 335–343. doi:10.1016/j.neubiorev.2019.03.009
Tappe-Theodor, A., and Kuner, R. (2014). Studying ongoing and spontaneous pain in rodents--challenges and opportunities. Eur. J. Neurosci. 39 (11), 1881–1890. doi:10.1111/ejn.12643
Thudi, N. K., Martin, C. K., Nadella, M. V., Fernandez, S. A., Werbeck, J. L., Pinzone, J. J., et al. (2008). Zoledronic acid decreased osteolysis but not bone metastasis in a nude mouse model of canine prostate cancer with mixed bone lesions. Prostate 68 (10), 1116–1125. doi:10.1002/pros.20776
Toma, W., Kyte, S. L., Bagdas, D., Jackson, A., Meade, J. A., Rahman, F., et al. (2019). The alpha7 nicotinic receptor silent agonist R-47 prevents and reverses paclitaxel-induced peripheral neuropathy in mice without tolerance or altering nicotine reward and withdrawal. Exp. Neurol. 320, 113010. doi:10.1016/j.expneurol.2019.113010
Tonello, R., Lee, S. H., and Berta, T. (2019). Monoclonal antibody targeting the matrix metalloproteinase 9 prevents and reverses paclitaxel-induced peripheral neuropathy in mice. J. Pain 20 (5), 515–527. doi:10.1016/j.jpain.2018.11.003
Ungard, R. G., Seidlitz, E. P., and Singh, G. (2014). Inhibition of breast cancer-cell glutamate release with sulfasalazine limits cancer-induced bone pain. Pain 155 (1), 28–36. doi:10.1016/j.pain.2013.08.030
Urch, C. E., and Dickenson, A. H. (2008). Neuropathic pain in cancer. Eur. J. Canc. 44 (8), 1091–1096. doi:10.1016/j.ejca.2008.03.015
Vermeirsch, H., and Meert, T. F. (2004). Morphine-induced analgesia in the hot-plate test: comparison between NMRI(nu/nu) and NMRI mice. Basic Clin. Pharmacol. Toxicol. 94 (2), 59–64. doi:10.1111/j.1742-7843.2004.pto940202.x
Viscardi, A. V., Hunniford, M., Lawlis, P., Leach, M., and Turner, P. V. (2017). Development of a piglet grimace scale to evaluate piglet pain using facial expressions following castration and tail docking: a pilot study. Front Vet Sci 4, 51. doi:10.3389/fvets.2017.00051
Wacnik, P. W., Baker, C. M., Herron, M. J., Kren, B. T., Blazar, B. R., Wilcox, G. L., et al. (2005a). Tumor-induced mechanical hyperalgesia involves CGRP receptors and altered innervation and vascularization of DsRed2 fluorescent hindpaw tumors. Pain 115 (1–2), 95–106. doi:10.1016/j.pain.2005.02.024
Wacnik, P. W., Eikmeier, L. J., Ruggles, T. R., Ramnaraine, M. L., Walcheck, B. K., Beitz, A. J., et al. (2001). Functional interactions between tumor and peripheral nerve: morphology, algogen identification, and behavioral characterization of a new murine model of cancer pain. J. Neurosci. 21 (23), 9355–9366. doi:10.1523/JNEUROSCI.21-23-09355.2001
Wacnik, P. W., Eikmeier, L. J., Simone, D. A., Wilcox, G. L., and Beitz, A. J. (2005b). Nociceptive characteristics of tumor necrosis factor-α in naive and tumor-bearing mice. Neuroscience 132 (2), 479–491. doi:10.1016/j.neuroscience.2004.12.035
Wacnik, P. W., Kehl, L. J., Trempe, T. M., Ramnaraine, M. L., Beitz, A. J., and Wilcox, G. L. (2003). Tumor implantation in mouse humerus evokes movement-related hyperalgesia exceeding that evoked by intramuscular carrageenan. Pain 101 (1–2), 175–186. doi:10.1016/s0304-3959(02)00312-3
Wakabayashi, H., Wakisaka, S., Hiraga, T., Hata, K., Nishimura, R., Tominaga, M., et al. (2018). Decreased sensory nerve excitation and bone pain associated with mouse Lewis lung cancer in TRPV1-deficient mice. J. Bone Miner. Metabol. 36 (3), 274–285. doi:10.1007/s00774-017-0842-7
Wang, L. N., Yang, J. P., Ji, F. H., Zhan, Y., Jin, X. H., Xu, Q. N., et al. (2012). Brain-derived neurotrophic factor modulates N-methyl-D-aspartate receptor activation in a rat model of cancer-induced bone pain. J. Neurosci. Res. 90 (6), 1249–1260. doi:10.1002/jnr.22815
Wang, L., Xu, H., Ge, Y., Zhu, H., Yu, D., Yu, W., et al. (2017). Establishment of a murine pancreatic cancer pain model and microarray analysis of painassociated genes in the spinal cord dorsal horn. Mol. Med. Rep. 16 (4), 4429–4436. doi:10.3892/mmr.2017.7173
Webster, J. D., Santagostino, S. F., and Foreman, O. (2020). Applications and considerations for the use of genetically engineered mouse models in drug development. Cell Tissue Res. 380 (2), 325–340. doi:10.1007/s00441-019-03101-y
Whittle, J. R., Lewis, M. T., Lindeman, G. J., and Visvader, J. E. (2015). Patient-derived xenograft models of breast cancer and their predictive power. Breast Cancer Res. 17, 17. doi:10.1186/s13058-015-0523-1
Xiao, W. F., Li, Y. S., Lou, W., Cai, T., Zhang, S., Hu, X. Y., et al. (2016). MicroRNA-93-5p may participate in the formation of morphine tolerance in bone cancer pain mouse model by targeting Smad5. Oncotarget 7 (32), 52104–52114. doi:10.18632/oncotarget.10524
Ye, Y., Bae, S. S., Viet, C. T., Troob, S., Bernabe, D., and Schmidt, B. L. (2014). IB4(+) and TRPV1(+) sensory neurons mediate pain but not proliferation in a mouse model of squamous cell carcinoma. Behav. Brain Funct. 10, 5. doi:10.1186/1744-9081-10-5
Ye, Y., Dang, D., Viet, C. T., Dolan, J. C., and Schmidt, B. L. (2012). Analgesia targeting IB4-positive neurons in cancer-induced mechanical hypersensitivity. J. Pain 13 (6), 524–531. doi:10.1016/j.jpain.2012.01.006
Ye, Y., Dang, D., Zhang, J., Viet, C. T., Lam, D. K., Dolan, J. C., et al. (2011). Nerve growth factor links oral cancer progression, pain, and cachexia. Mol. Canc. Therapeut. 10 (9), 1667–1676. doi:10.1158/1535-7163.MCT-11-0123
Ye, Y., Scheff, N. N., Bernabe, D., Salvo, E., Ono, K., Liu, C., et al. (2018). Anti-cancer and analgesic effects of resolvin D2 in oral squamous cell carcinoma. Neuropharmacology 139, 182–193. doi:10.1016/j.neuropharm.2018.07.016
Yin, Q., Cheng, W., Cheng, M. Y., Fan, S. Z., and Shen, W. (2010). Intrathecal injection of anti-CX3CR1 neutralizing antibody delayed and attenuated pain facilitation in rat tibial bone cancer pain model. Behav. Pharmacol. 21 (7), 595–601. doi:10.1097/FBP.0b013e32833e7e2a
Yin, X., Su, J., Zhou, X., Guo, J., Wei, W., and Wang, Z. (2015). K-ras-driven engineered mouse models for pancreatic cancer. Discov. Med. 19 (102), 15–21.
Zahalka, A. H., and Frenette, P. S. (2020). Nerves in cancer. Nat. Rev. Canc. 20 (3), 143–157. doi:10.1038/s41568-019-0237-2
Zhang, H., and Dougherty, P. M. (2014). Enhanced excitability of primary sensory neurons and altered gene expression of neuronal ion channels in dorsal root ganglion in paclitaxel-induced peripheral neuropathy. Anesthesiology 120 (6), 1463–1475. doi:10.1097/ALN.0000000000000176
Zhang, H., Lund, D. M., Ciccone, H. A., Staatz, W. D., Ibrahim, M. M., Largent-Milnes, T. M., et al. (2018). Peripherally restricted cannabinoid 1 receptor agonist as a novel analgesic in cancer-induced bone pain. Pain 159 (9), 1814–1823. doi:10.1097/j.pain.0000000000001278
Zhang, R., and Lao, L. (2012). A new rat model of bone cancer pain. Methods Mol. Biol. 851, 261–273. doi:10.1007/978-1-61779-561-9_20
Zhang, R. X., Liu, B., Wang, L., Ren, K., Qiao, J. T., Berman, B. M., et al. (2005). Spinal glial activation in a new rat model of bone cancer pain produced by prostate cancer cell inoculation of the tibia. Pain 118 (1–2), 125–136. doi:10.1016/j.pain.2005.08.001
Zinonos, I., Luo, K. W., Labrinidis, A., Liapis, V., Hay, S., Panagopoulos, V., et al. (2014). Pharmacologic inhibition of bone resorption prevents cancer-induced osteolysis but enhances soft tissue metastasis in a mouse model of osteolytic breast cancer. Int. J. Oncol. 45 (2), 532–540. doi:10.3892/ijo.2014.2468
Keywords: cancer pain, animal model, cancer treatment, nociception, behavior
Citation: Pineda-Farias JB, Saloman JL and Scheff NN (2020) Animal Models of Cancer-Related Pain: Current Perspectives in Translation. Front. Pharmacol. 11:610894. doi: 10.3389/fphar.2020.610894
Received: 27 September 2020; Accepted: 30 October 2020;
Published: 26 November 2020.
Edited by:
E. Alfonso Romero-Sandoval, Wake Forest School of Medicine, United StatesReviewed by:
Ohannes K. Melemedjian, University of Maryland, United StatesJuan Miguel Jiménez, Universidad Autónoma de Tamaulipas, Mexico
Copyright © 2020 Pineda-Farias, Saloman and Scheff. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicole N. Scheff, bm5zMThAcGl0dC5lZHU=
 Jorge B. Pineda-Farias
Jorge B. Pineda-Farias Jami L. Saloman
Jami L. Saloman Nicole N. Scheff
Nicole N. Scheff