
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 24 November 2020
Sec. Neuropharmacology
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.603445
This article is part of the Research Topic Stimulant Use and Addictive Disorder View all 22 articles
The emphasis of neuronal alterations and adaptations have long been the main focus of the studies of the mechanistic underpinnings of drug addiction. Recent studies have begun to appreciate the role of innate immune system, especially toll-like receptor 4 (TLR4) signaling in drug reward-associated behaviors and physiology. Drugs like opioids, alcohol and psychostimulants activate TLR4 signaling and subsequently induce proinflammatory responses, which in turn contributes to the development of drug addiction. Inhibition of TLR4 or its downstream effectors attenuated the reinforcing effects of opioids, alcohol and psychostimulants, and this effect is also involved in the withdrawal and relapse-like behaviors of different drug classes. However, conflicting results also argue that TLR4-related immune response may play a minimal part in drug addiction. This review discussed the preclinical evidence that whether TLR4 signaling is involved in multiple drug classes action and the possible mechanisms underlying this effect. Moreover, clinical studies which examined the potential efficacy of immune-base pharmacotherapies in treating drug addiction are also discussed.
Neuronal alterations and adaptations have long been the main focus of the studies of the mechanistic underpinnings of drug addiction (Kalivas and O'Brien, 2008; Otis and Mueller, 2017). The emphasis of dopaminergic and glutamatergic signaling in brain reward circuits yield extensive important progress in the study of drug addiction (Sesack and Grace, 2010; Lee et al., 2013; Ma et al., 2014; Zhang et al., 2016). However, they ignore the potential contributions of non-neuronal cells (e.g., microglia and astrocytes) to the synaptic and behavioral adaptations underlying addiction-like behaviors (Kashima and Grueter, 2017). Recent studies have begun to illustrate the role of innate immune system, especially toll-like receptor 4 (TLR4) in drug reward associated behaviors and physiology (Hutchinson et al., 2012; June et al., 2015; Northcutt et al., 2015). This review will briefly discuss the innate immune system and TLR4 signaling. Different classes of drugs including opioids, alcohol and psychostimulants will be reviewed to discuss whether TLR4 signaling can be used as a potential therapeutic target for the treatment of drug addiction. Furthermore, clinical studies which examined the potential efficacy of immune-base pharmacotherapies in treating drug addiction are also discussed.
The innate immune system, an evolutionary defense strategy, has been well characterized (Chaplin, 2010; Yu et al., 2018). TLRs are a group of pattern recognition receptors (PRRs) in the innate immune system which detect and respond not only to exogenous pathogen associated molecular patterns (PAMPs), but also to endogenous danger associated molecular patterns (DAMPs) (Koropatnick et al., 2004; Hennessy et al., 2010; Dunne et al., 2011). Activation of TLRs promotes the maturation of antigen presenting cells, like dendritic cells (DC), which subsequently directs the induction of adaptive immunity (Apetoh et al., 2007; Liu et al., 2010; Gaudino and Kumar, 2019). In this regard, TLR agonists have been studied as vaccine adjuvants for cancer or infectious disease (Caron et al., 2005; Sfondrini et al., 2006; Kronenberger and Zeuzem, 2009). However, considering the fact that activation of TLRs leads to the promotion of inflammatory cytokine production, the inhibitors of TLRs also have significant potential as therapeutic agents for inflammatory disorders, such as rheumatoid arthritis (Klareskog et al., 2004; Feldmann, 2009). As a result, the exploitation of TLRs-based therapeutics may be promising for the treatment of multiple infectious and inflammatory diseases.
Apart from their crucial roles in immune system-related diseases, recent studies also suggest that TLRs, especially TLR4, was widely involved in drug addiction-related behaviors (Hennessy et al., 2010; Crews et al., 2017b; Kashima and Grueter, 2017). We will discuss this topic later in detail. In response to pathogen and danger signals, TLR4 and its co-receptor MD-2 can signal through two different pathways, the myeloid differentiation primary response protein 88 (MyD88)-dependent and MyD88-independent pathway (Takeda and Akira, 2004) (Figure 1). In MyD88-dependent pathway, the signal transduces through Interleukin 1 receptor associated kinase 4 and 1 (IRAK4 and IRAK1) and the following TNF receptor associated factor 6 (TRAF6). The activation of TRAF6 leads to phosphorylation of inhibitors of nuclear factor κB Kinases (IKKs), which in turn activates the IκB. The activation of IκB leads to its degradation and the initiation of activation of NFκB and the production of proinflammatory cytokines, for example, Tumor Necrosis Factor (TNF), IL-1β, and IL-6 (Kawai and Akira, 2007). In contrast, MyD88-independent pathway adopts the adaptor protein TRIF and transduces the signal through TRAF3, TBK1, and IKKε, which then phosphorylates interferon regulatory factor 3 (IRF3). IRF3 then translocates to the nucleus and promotes the transcription of type 1 interferons (Takeda and Akira, 2005).
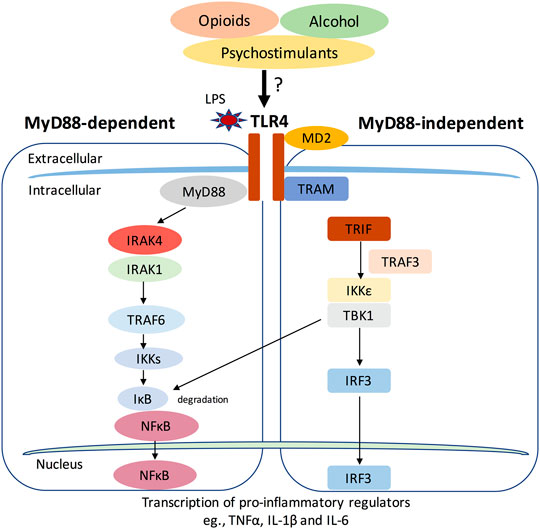
FIGURE 1. The Toll-like receptor 4 (TLR4) signaling pathway. TLR4 and its co-receptor MD-2 can signal through two different pathways, the myeloid differentiation primary response protein 88 (MyD88)-dependent and MyD88-independent pathway. In MyD88-dependent pathway, the signal transduces through Interleukin 1 receptor associated kinase 4 and 1 (IRAK4 and IRAK1) and the following TNF receptor associated factor 6 (TRAF6). The activation of TRAF6 leads to phosphorylation of inhibitors of nuclear factor κB Kinases (IKKs), which in turn activates the IκB. The activation of IκB leads to its degradation and the initiation of activation of NFκB and the production of proinflammatory cytokines, for example, Tumor Necrosis Factor (TNF), IL-1β and IL-6 (Kawai and Akira, 2007). In contrast, MyD88-independent pathway adopts the adaptor protein TRIF and transduces the signal through TRAF3, TBK1 and IKKε, which then phosphorylates interferon regulatory factor 3 (IRF3). IRF3 then translocates to the nucleus and promotes the transcription of type 1 interferons. Drugs of abuse like opioids, alcohol and psychostimulants may activate TLR4 signaling and induce pro-inflammatory responses.
The involvement of TLR4 signaling has been suggested in several neuronal disorders, including neurodegenerative disorders, depression, impulsive behaviors and addiction (Landreth and Reed-Geaghan, 2009; Gesuete et al., 2014; Aurelian et al., 2016; Garcia Bueno et al., 2016; Crews et al., 2017b; Gasiorowski et al., 2018; Nie et al., 2018; Liu et al., 2019). TLR4 is mainly expressed in cells of innate immune system, including microglia and astrocytes (Vaure and Liu, 2014). Consistent to this, several microglia inhibitors attenuated some drug addiction-related behaviors in animal studies. In this review, we will focus on the role of TLR4 in regulating addiction-related behaviors from different drug classes.
It is reported that opioids such as morphine can induce neuroinflammation in the central nervous system (Narita et al., 2006; Yang et al., 2010). Furthermore, this neuroinflammation has been associated with morphine analgesia, dependence, tolerance and withdrawal effects (Eidson and Murphy, 2013; Jacobsen et al., 2014; Mattioli et al., 2014; Eidson et al., 2017; Shah and Choi, 2017). It has been shown that morphine can directly bind to myeloid differentiation protein 2 (MD-2), the accessory receptor of TLR4, and activate TLR4 signaling by inducing the oligomerization of TLR4/MD-2. TLR4/MD-2 knockout animals showed enhanced morphine-induced analgesia, suggesting that blockade of TLR4/MD-2 inhibited morphine-induced proinflammatory responses (Wang et al., 2012b). Inhibition of TLR4 by the levo-isomer of naloxone, (+)-naloxone, attenuated morphine-induced conditioned place preference (CPP). This isomer of naloxone (26.3 mg/kg), which is inactive at opioid receptors, also reduced remifentanil self-administration. Furthermore, genetic knockout of TLR4 or MyD88 decreased oxycodone-induced CPP (Wang et al., 2012b). These results suggest that activation of TLR4 is involved in the rewarding effect of opioids. In addition, in vivo microdialysis study showed that (+)-naloxone decreased morphine-induced elevation of dopamine concentration in the shell region of nucleus accumbens (NAc) (Hutchinson et al., 2012). Together, they suggested that the TLR4/MD-2 signaling, along with classic opioid receptors, mediates opioid reward-related behaviors.
However, recent studies which contradict the role of TLR4 in opioid addiction add more complexity to this hypothesis. For example, Phil et al. reported that neither (+)-naloxone nor (+)-naltrexone (3 and 100 μM) inhibit LPS induced TLR4 activation in vitro (Skolnick et al., 2014). Stevens et al. also reported that morphine inhibits LPS-induced activation of TLR4 in a concentration-dependent manner, furthermore, this effect was not affected by naltrexone (Stevens et al., 2013). In correspondence of this discrepancy, Watkins lab pointed out the lack of translational potential of (+)-naloxone and (+)-naltrexone from in vivo studies considering the lack of biotransformation in in vitro systems (Watkins et al., 2014). In addition, apart from the difference in methodology, they also mentioned that not all agonist-antagonist relationships are equal under different conditions (Watkins et al., 2014). This explanation somewhat makes sense since antagonists could not bind to the receptors if they are fully occupied by respective agonists. Another explanation is that there might be different signaling pathways involved in these interactions thus much effort should be spent to determine the exact signaling underlying the test agents (Watkins et al., 2014). Moreover, mixed results from in vivo studies need further consideration. The most intriguing finding is that TLR4 mutant and null mice maintained opioid induced tolerance, hyperalgesia and physical dependence, suggesting a minimal role of TLR4 in opioid actions (Mattioli et al., 2014). Additionally, acute injection of (+)-naltrexone immediately before extinction test had no effect in opioid-seeking behaviors. Meanwhile, acute or chronic delivery of (+)-naltrexone did not affect the extended access heroin self-administration behavior either (Theberge et al., 2013). More importantly, it was found that (+)-naltrexone or (+)-naloxone also reduced food self-administration (Tanda et al., 2016; Yue et al., 2020), suggesting a lack of behavioral specificity of TLR4 antagonists on drug-maintained operant behaviors (Tanda et al., 2016). These results question the validity of TLR4 hypothesis and should be further addressed before it is translated to the clinic. One possible explanation is the selectivity of TLR4 (+)-isomer ligands. Studies have identified non-stereoselective actions of naloxone at sites other than TLR4 (Wang and Burns, 2009; Burns and Wang, 2010; Wang et al., 2012a). Future studies that carefully examine how (+)-isomers act on non-TLR4 are needed to dissect the exact role of TLR4 in opioid addiction.
Neuronal mechanism of opioid addiction involves the inhibition of GABAergic tone on the mesolimbic dopaminergic reward pathway from ventral tegmental area (VTA) to nucleus accumbens (NAc), resulting in an increase in dopamine release in the NAc (Coller and Hutchinson, 2012; Fields and Margolis, 2015; Volkow and Morales, 2015). Meanwhile, opioids induced glia activation was thought to contribute to their reinforcing and rewarding-like effects, which is achieved possibly through the modulation of TLR4 (Jacobsen et al., 2014). A recent study showed that opioids, like morphine, interacts with MD2 and this binding is TLR4 dependent (Shah et al., 2016). Once activated by opioids, TLR4 increases the levels of pro-inflammatory cytokines and chemokines, which subsequently affect the neuronal transmission and plasticity that is associated with opioid-induced reward (Langlois and Nugent, 2017; Zhang et al., 2020). Therefore, immune factors like TNFα or IL-1β that can modulate synaptic functions may participate in opioid reward. TNFα is a downstream effector of TLR4 signaling and inhibition of TNFα blocked TLR4-mediated morphine-induced neuroinflammation (Kawai and Akira, 2010; Eidson et al., 2017). TNFα is shown to regulate synaptic transmission by affecting the activity of GABAA receptors, AMPA receptors and presynaptic metabotropic glutamate receptors (Bezzi et al., 2001; Stellwagen et al., 2005; Domercq et al., 2006; Stellwagen and Malenka, 2006; Pascual et al., 2012; Lewitus et al., 2014). It may also contribute to opioid reward by altering the opioid sensitivity as shown by a genetic human study (Reyes-Gibby et al., 2008). Like TNFα, activation of TLR4 also leads to an increase in IL-1β expression (Latz et al., 2013). IL-1β mediates long-term potentiation (LTP) which is important to learning and memory (Rizzo et al., 2018). As addiction can be viewed as a type of aberrant reward memory, IL-1β may play a role in the perception of opioid reward (Song et al., 2006). On the other hand, it has been shown that IL-1β suppresses postsynaptic GABA receptor activities through the activation of protein kinase C (PKC) in neurons. Meanwhile, IL-1β inhibits glial glutamate transporter activity, resulting in a deficiency in glutamate supply. This shortage in turn leads to the attenuation of glutamate-glutamine cycle-dependent GABA synthesis. These processes are widely involved in synaptic plasticity which may underlie TLR4-related drug actions (Wang et al., 2000). Though the exact role of TLR4 signaling in mediating opioid addiction remains unclear, it should be noted that activation of TLR4 mediated central immune response by drug of abuse can only work in concert with the well-established neuronal mechanisms of reward, as the central immune signaling alone cannot produce related behavioral effects (Coller and Hutchinson, 2012).
With the knowledge that TLR4-related glial activity may play a role in opioid addiction preclinically, exploration of novel pharmacological treatments for opioid addiction by targeting the glial activity remains a promising choice (Zhang et al., 2020). One of the most studied existing medication is ibudilast, which is a non-selective phosphodiesterase inhibitor and TLR4 antagonist (Ruiz-Perez et al., 2016). Ibudilast is widely used in Asia for the treatment of asthma and post-stroke dizziness (Gibson et al., 2006), which showed a well safety and tolerability of a single dose (30 mg) and a 30-mg twice daily 2-week regimen in healthy subjects (Rolan et al., 2008). Recently, Comer lab has carefully examined the potential of ibudilast on opioid-induced analgesia, subjective and withdrawal symptoms in opioid-dependent volunteers. They found that ibudilast (40 mg, bid, 1 week) enhanced the oxycodone-induced analgesia as measured by subjective pain ratings (Cooper et al., 2017). Moreover, volunteers who received ibudilast (20 and 40 mg, bid, 2 weeks) also had lower ratings of withdrawal symptoms (Cooper et al., 2016). However, ibudilast did not affect oxycodone-induced subjective drug effect ratings (e.g., “high, good effect, I would pay”) (Cooper et al., 2017). In contrary, another study by Metz et al. reported that ibudilast decreased the rating of drug like following 15 mg oxycodone in opioid-dependent volunteers (Metz et al., 2017). Ibudilast also significantly decreased the drug breakpoint value under 15 mg oxycodone condition, but not under 30 mg oxycodone condition. They also observed similar results that craving for heroin, cocaine and tobacco was also reduced under active ibudilast compared with placebo (Metz et al., 2017). It seems contradicting on whether ibudilast could decrease the subjective and reinforcing effects based on these results. However, they may reconcile at some point since Metz et al. found ibudilast reduced the craving following 15 mg, but not 30 mg oxycodone, while Comer lab examined higher doses of opioid (e.g., 30 mg morphine or 25, 50 mg/70 kg oxycodone). The discrepancy may also attribute to the limited sample volume and too few trails (Zhang et al., 2020). Meanwhile, considerable individual variability may also add up to weaken the power of these studies. Therefore, future investigations with increased sample size are urgently needed to verify the clinical potential of glia modulators on opioid addiction.
Despite the clinical results of ibudilast are conflicting, the therapeutic potential of glial modulators in preventing opioid abuse should not be underestimated. Currently, other glial modulators are being examined for their ability in treating opioid use disorders as well. For example, minocycline increased accuracy on a cognition task in individuals with opioid use disorder, suggesting an effect like cognition enhancement. However, the pain threshold or tolerance, opioid craving and withdrawal weren’t changed by minocycline treatment (Arout et al., 2019). Another glial modulator, cannabidiol, was shown to decrease opioid-induced craving and anxiety in drug-abstinent individuals with opioid use disorder (Hurd et al., 2015; Hurd et al., 2019). These results, albeit complex, suggesting a promising role of glial modulators in treating opioid addiction. Further studies with more dose regimen, greater sample size and prolonged trials are needed to figure out their exact roles.
Studies have suggested that TLR4 affects some behavioral effects of ethanol (Pandey, 2012; Wu et al., 2012; Pascual et al., 2015; Blednov et al., 2017a). Both pharmacological inhibition of TLR4 and genetic deficiency of TLR4 or MyD88 significantly decreased the duration of loss of righting reflex (LORR) and reduced recovery time in motor impairment (rotarod test). Importantly, these effects were not due to changes of ethanol pharmacokinetics (Wu et al., 2012; Blednov et al., 2017a). In addition, TLR4-deficient mice showed lower sensitivity to pentobarbital-induced sedative effect and faster recovery from diazepam-induced motor impairment, suggesting a crosstalk between TLR4 and GABAergic functions (Blednov et al., 2017a). Chronic exposure of ethanol increases the expression of many cytokines (TNF-α, IL-1β) and chemokines (CX3CL1, MCP-1) in the mice striatum and serum (Pascual et al., 2015). Interestingly, mice lacking TLR4 or TLR2 receptors are protected against ethanol-induced cytokine release (Pascual et al., 2015). These mice also showed less ethanol abstinence-induced behavioral changes such as increased anxiety (Pascual et al., 2015). Combined, these results suggest a clear involvement of TLR4 signaling in some acute and chronic effects of ethanol.
Binge drinking represents the initial stage of alcohol addiction, which has a link with anxiety (Chikritzhs et al., 2001; Naimi et al., 2003; Edenberg et al., 2004; Ducci et al., 2007). It is also suggested that TLR4-GABAA α2 subunit pathway regulates alcohol binge drinking in rodents (Spanagel et al., 1995; Roberts et al., 1996; Foster et al., 2004). By infusing a GABAA α2 siRNA vector in central nucleus of amygdala (CeA) of alcohol-preferring rats (P rats), Juan et al. reported a significant and specific reduction of alcohol binge drinking, reduced α2 subtype GABAA receptor expression, decreased GABAA receptor density and inhibition of TLR4(Liu et al., 2011). Moreover, TLR4 siRNA infusion to the CeA also decreased binge drinking behaviors without affecting sucrose intake, suggesting a specificity on alcohol-related behaviors (Liu et al., 2011). Similarly, another study showed that TLR4 or MCP-1 siRNA in CeA or VTA of P rats decreased the corresponding gene expression and binge drinking behavior (June et al., 2015). A further study also showed that α2 subtype of GABAA receptor activates TLR4 signals in neurons in VTA (Balan et al., 2018). Studies from inhibitors also support the central role of TLR4 in binge drinking as TLR4 inhibitor T5342126 decreased ethanol drinking (Bajo et al., 2016).
However, a recent comprehensive study has shown that manipulations of TLR4 may have minimal impact on excessive ethanol drinking behavior (Harris et al., 2017). By using the multiple models: TLR4-KO rats, selective Tlr4 knockdown in mouse NAc and inhibitor (+)-naloxone in different species, Harris et al. demonstrated that either genetic deletion of TLR4 or pharmacological inhibition of TLR4 or Tlr4 knockdown did not affect alcohol intake using two-bottle choice procedure and drinking-in-the-dark assay (Harris et al., 2017). Meanwhile, specific Tlr4 knockdown in mouse NAc did not alter ethanol intake and preference for ethanol in the 24 h continuous access two-bottle test. These results suggest that TLR4 was not important to the excessive drinking behavior and subsequently question the hypothesis that TLR4 is a critical component in mediating alcohol response. One explanation is that Harris et al. examined a Tlr4 knockdown in the NAc while previous studies tested in the CeA or VTA. The difference in brain regions tested may lead to discrepancy in findings. In addition, while Harris used two-bottle and drinking-in-the dark tests, previous studies adopted binge-drinking model in P rats. It seems reasonable that increased GABAergic responses in P rats contribute to their altered binge-drinking behaviors. Nevertheless, they also reported consistent results that TLR4-KO rats had reduced duration of LORR, and CeA deletion of Tlr4 changed GABAA α2 subtype receptor function (Harris et al., 2017). These results at least suggest essential role of TLR4 signaling in mediating acute behavioral effects of ethanol (Alfonso-Loeches et al., 2010; Pascual et al., 2015; Blednov et al., 2017b). More studies are needed to disentangle the exact role of TLR4 signaling in alcohol addiction-related effects.
Alcohol intake increases gut permeability, allowing translocation of bacterial toxins like LPS through the intestines into blood stream (Parlesak et al., 2000; Leclercq et al., 2012; Leclercq et al., 2014). LPS in the bloodstream reaches the liver and stimulate TLR4 in liver Kupffer cells, resulting in an increase in pro-inflammatory cytokines and chemokines (Roh and Seki, 2013) which can cross the blood-brain barrier and activate the glia cells in the brain (Montesinos et al., 2016). On the other hand, alcohol can activate glial TLR4 (Blanco et al., 2005; Fernandez-Lizarbe et al., 2009) and induce translocation to the lipid raft and promoting the activation of downstream effectors (Blanco et al., 2005; Blanco et al., 2008; Fernandez-Lizarbe et al., 2009). This activation contributes to ethanol-induced neuroinflammation and neurodegeneration (Alfonso-Loeches et al., 2010; Pascual et al., 2011; Alfonso-Loeches et al., 2012). Both chronic and acute exposure to ethanol cause TLR4-associated signaling response in vivo and in vitro (Blanco et al., 2004; Valles et al., 2004; Blanco et al., 2005; Blanco and Guerri, 2007). Conversely, inhibition of TLR4 blocks the proinflammatory responses and prevents cell damage (Blanco et al., 2005).
Indeed, the activation of innate immunity and TLR4 signaling appear to be essential for alcohol addiction-like behaviors (Pascual et al., 2011). The persistent activation of neuroinflammation exacerbates the neurodegeneration of key brain regions involved with excessive alcohol consumption, thus underlying at least partly the mechanisms that regulate the development of alcohol addiction (Crews et al., 2015; Flores-Bastias and Karahanian, 2018). Alcohol consumption promotes innate immune activation that are linked to alterations in executive function, reward and negative affect-craving-anxiety that contribute to alcohol use disorders (Vetreno and Crews, 2014; Crews et al., 2017a). Alcohol-induced cell damage in brain regions like prefrontal cortex may cause an executive dysfunction over behavioral inhibition (like binge drinking) and also a lack of inhibition in mesolimbic areas, which is turn increase drinking motivation (Crews et al., 2011; Crews et al., 2015). The loss of control over progression from initial intoxication and binge drinking stage to compulsive drinking stage may lead to the development of alcohol addiction.
Most recent studies have examined the potential of ibudilast in treating alcohol use disorders (AUD). In a recent randomized, double-blinded and placebo-control study, ibudilast was tested for its safety, tolerability and initial efficacy in mild-severe AUD outpatients (Ray et al., 2017). Ibudilast (50 mg, bid) was well-tolerated with no severe adverse events in the trial. However, ibudilast was not able to affect subjective response to alcohol as shown by craving, stimulation, sedation, positive or negative mood, “like” or “wanting” alcohol (Ray et al., 2017). Nevertheless, ibudilast was associated with mood improvements and decreased tonic level of craving after stress and alcohol cue exposure (Ray et al., 2017). Further analysis revealed that ibudilast attenuated the stimulating and mood-altering effects of alcohol among individuals with higher depressive ratings (Ray et al., 2017). This study suggested a possible mood-modulating effect of ibudilast in treating AUD and which may contribute to the reduced alcohol craving after stress or cue exposure. A more recent study further examined whether ibudilast affect other appetitive behavior, like food craving in AUD participants (Cummings et al., 2018). They found that ibudilast did not affect tonic high-fat/high-sugar food craving, indicating a specificity of modulating drinking behaviors (Cummings et al., 2018). These results provide the first evidence of whether ibudilast could be used for the treatment of alcohol addiction. However, it is still unclear whether ibudilast could decrease the subjective effect or alcohol intake since only few studies have examined this effect with limited participants and trails. More extensive studies are warranted to examine the potential of ibudilast on alcohol intake, withdrawal and relapse. Meanwhile, other neuroimmune modulators, like minocycline, PDE-4 inhibitor apremilast or selective PPARα agonist fenofibrate are undergoing clinical trials to determine their efficacy in reducing alcohol use, craving and related neuroinflammation (Erickson et al., 2019). More importantly, future studies that determine the effect of combination of neuroimmune pharmacotherapies with established medications for alcohol addiction are also warranted (Stopponi et al., 2013).
There is a large body of literature that psychostimulants like cocaine and methamphetamine can activate and modulate neuroimmune responses (Loftis and Janowsky, 2014; Lacagnina et al., 2017). In vitro studies suggest that psychostimulants directly modulate the TLR4 signaling activity. For example, cocaine exposure increases the expression of TLR4 in BV-2 cells in a dose-dependent manner (Periyasamy et al., 2018), and brain TLR4 expression was higher in mice self-administering cocaine than in those self-administering saline (Northcutt et al., 2015; Brown et al., 2018; Periyasamy et al., 2018), indicating an upregulation of TLR4 signaling by cocaine exposure. Similarly, methamphetamine treatment increases the expression of TLR4 in cultured astrocytes (Du et al., 2017) while silencing TLR4 expression using siRNA abolishes methamphetamine-induced expression of IL-1β and IL-18 (Du et al., 2017). In vivo evidence are consistent with these findings in that mice pretreated with the TLR4 inhibitor TAK-242 showed significantly decreased expression of IL-1β and IL-18 in striatum induced by methamphetamine (Du et al., 2017). All these results suggest that psychostimulants can activate TLR4 which may contribute to the behavioral effects of the drug.
Pharmacological antagonism of TLR4 also showed consistent results. Indeed, (+)-naloxone blocked cocaine-induced proinflammatory signaling both in vitro and in vivo (Northcutt et al., 2015). More importantly, evidence showed that TLR4 signaling at least partially contributed to cocaine-induced elevation of NAc dopamine (Northcutt et al., 2015). (+)-Naloxone ameliorated the robust increase in NAc dopamine induced by cocaine while it alone did not produce any effect (Ducci et al., 2007). Conversely, activation of TLR4 in the VTA by local LPS injection was sufficient to produce an elevation of dopamine in the NAc (Nie et al., 2018), suggesting a mediating role of TLR4 in cocaine-induced dopamine release. Behavioral studies further strengthened this notion as pretreatment of (+)-naloxone blocked the development of cocaine CPP and responding for cocaine injection (Northcutt et al., 2015). However, (+)-naloxone did not decrease the responding for food, which suggests that general operant behaviors are intact (Northcutt et al., 2015). In addition, a recent study has shown that TLR4 contributes to the drug-induced reinstatement of cocaine seeking (Brown et al., 2018). Local antagonism of TLR4 in the VTA decreased cocaine-seeking but not sucrose-seeking behavior (Brown et al., 2018). Collectively, these results showed that psychostimulant drugs activate TLR4 signaling which in turn contributes to the reinforcing and relapse-related effects of the drugs.
However, inconsistent evidence exists that pharmacological blockade of TLR4 by (+)-naloxone and (+)-naltrexone which attenuated cocaine self-administration also decreased food-maintained responding, suggesting a non-specific effect (Tanda et al., 2016). Meanwhile, pretreatment with (+)-naloxone and (+)-naltrexone did not affect the increased dopamine levels induced by cocaine (Tanda et al., 2016). Furthermore, a more recent study shows that TNF-α, an inflammatory cytokine downstream TLR4, suppresses cocaine-induced behavioral sensitization by depressing cocaine-induced synaptic changes in NAc core (Lewitus et al., 2016). Indeed, activation of microglia by cocaine increases TNF-α production, which subsequently limits the cocaine-induced changes to NAc circuity, and finally restrains the development of cocaine-induced behavioral sensitization. More importantly, after a period of abstinence, mild activation of TLR4 can reactivate microglia and reduce both synaptic strength in the NAc and locomotor activity to cocaine (Lewitus et al., 2016). Thus, it suggests that augmenting microglia responses through TLR4 or others might be a reasonable approach to treat addiction. Nonetheless, another study showed that TLR4 knockout (KO) mice had a deficit in low-frequency stimulation-induced NMDAR-dependent long-term depression (LTD) in NAc core, which contributed to an attenuation in drug reward learning (Kashima and Grueter, 2017). These mixed results about the role of TLR4 in psychostimulants action make it difficult to draw specific conclusions here. Explanations for this discrepancy may involve differences in addiction-related behaviors and stages of addiction studied. These differences promote continued examination of the effect of TLR4 in drug addiction.
Studies have shown that cocaine and methamphetamine bind to the accessory receptor of TLR4, MD-2, which stabilizes the conformation of TLR4/MD-2 heterodimers. Methamphetamine binding activates TLR4 and NF-Kβ and upregulates the microglia activation marker CD11b and IL-6 in the VTA, which can be abolished by TLR4 antagonists LPS-RS and TAK-242 (Bachtell et al., 2015; Wang et al., 2019). Meanwhile, the TLR4 antagonist (+)-naloxone or (+)-naltrexone docked to the same pocket of MD-2, competing with other molecules, suggesting a potential modulatory role of TLR4 antagonist in psychostimulants-induced TLR4 activation (Northcutt et al., 2015). TLR4 activation by cocaine or methamphetamine leads to the increased levels of proinflammatory cytokines or chemokines, which subsequently contributes to abnormal neuronal excitatory and toxicity. This non-neuronal mechanism is believed to work in combination with the well-known neuronal circuity, such as psychostimulants-induced alterations of dopamine transporters functions (Hall et al., 2004), to achieve the associated development of drug addiction. It remains unclear how these two mechanisms synergize and result in addiction-like behaviors. However, alterations in synaptic plasticity and neuronal transmission induced by immune response are believed to play a part.
Currently, there are no FDA-approved medications for the treatment of psychostimulants addiction. However, accumulating evidence suggests that targeting neuroinflammation might be a promising strategy for developing a potential pharmacotherapy to treat stimulants addiction. In 2010, a case study reported that minocycline improved the psychotic symptoms in a female patient who had methamphetamine use disorder, suggesting a promising role of minocycline in treating methamphetamine addiction (Tanibuchi et al., 2010). However, there were no follow-up clinical studies which further examined the potential of minocycline in methamphetamine use disorders since then. On the other hand, ibudilast, which has been shown to reduce methamphetamine self-administration and reinstatement in animals, was examined for its efficacy clinically. Ibudilast was able to reduce several methamphetamine–related subjective effects (Worley et al., 2016). Further study also demonstrated that ibudilast may improve attention during early abstinence from methamphetamine dependence (Birath et al., 2017). Despite the limitations of these early-stage studies, they provide first evidence that ibudilast might serve as a potential pharmacotherapy for methamphetamine use disorders. However, a most recent study showed that ibudilast did not affect methamphetamine abstinence (Heinzerling et al., 2020). This randomize trials included 64 participants for ibudilast group and 61 for placebo. Urine specimens for drug screens were collected twice a week. Nonetheless, there was no correlation between serum ibudilast levels and methamphetamine use during treatment. This study suggests that ibudilast might not be able to affect methamphetamine abstinence, yet it’s hard to conclude that ibudilast has no effect on methamphetamine action since no further evidence reported whether ibudilast could affect methamphetamine intake or craving. Actually, a pilot randomized clinical research demonstrated that ibudilast reduced the increased levels of peripheral markers of inflammation induced by methamphetamine treatment in patients, which have implications for the development of treatment for psychostimulants addiction (Li et al., 2020). These results are encouraging, though more studies are needed to examine the long-term effect of ibudilast on both peripheral and central neuroinflammation markers and how these modulations link to clinical outcomes.
While significant effort has been made to illustrate the role of TLR4-related immune response in drug addiction, it is early to reach a solid conclusion. Since debates are remained about whether TLR4 is essential to drugs of abuse, further studies should further examine the link between TLR4-related immune activation and different stages of drug addiction. Meanwhile, it is generally believed that TLR4-related immune response activated by drugs of abuse work in concert with established neuronal mechanisms, which contribute to the rewarding and reinforcing effects. However, it remains elusive how non-neuronal activation communicates with the mesocorticolimbic reward system which underlies drug addiction-related behaviors. Thus, examinations of the interactions between these two systems would add valuable information to the knowledge of the mechanism underlying drug addiction. More importantly, these studies would further suggest the potential and novel therapeutic targets for the treatments of drug addiction. Moreover, randomized clinical trial which examines the potential efficacy of immune-based pharmacotherapies in drug addiction is in its infancy as conflicting results from clinical data weakens the translational value of the immune-based therapies. Current clinical trials have limited sample size and test restricted time window, dosage effects and drug actions. Consequently, future clinical studies including more participants, examining long-term efficacy and multiple dose-effects of immune-based pharmacotherapies for different stages of drug addiction are warranted.
Emerging evidence suggest an important role of the neuroimmune system, especially TLR4, in addiction-related effects of different classes of drugs such as opioids, alcohol and psychostimulants. Drugs of abuse activate TLR4 signaling and the modulation of TLR4 signaling has been shown to be involved in different stages of drug addiction (binge or intoxication, withdrawal and relapse). Accordingly, pharmacological strategies such as non-specific microglia inhibition is a potentially promising approach to treat drug abuse. This is a burgeoning field that requires more mechanistically based studies for target validation and future clinical trials with clinically approved drugs to repurpose for the treatment of drug addiction.
RW: conception, design, gathering, interpretation of data and writing; JL: conception, interpretation of data and writing.
RW was partially supported by the National Natural Science Foundation of China [Grant 81701340] and Natural Science Foundation of Jiangsu Province [Grant BK 20170517].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Alfonso-Loeches, S., Pascual, M., Gomez-Pinedo, U., Pascual-Lucas, M., Renau-Piqueras, J., and Guerri, C. (2012). Toll-like receptor 4 participates in the myelin disruptions associated with chronic alcohol abuse. Glia 60, 948–964. doi:10.1002/glia.22327
Alfonso-Loeches, S., Pascual-Lucas, M., Blanco, A. M., Sanchez-Vera, I., and Guerri, C. (2010). Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J. Neurosci. 30, 8285–8295. doi:10.1523/JNEUROSCI.0976-10.2010
Apetoh, L., Ghiringhelli, F., Tesniere, A., Obeid, M., Ortiz, C., Criollo, A., et al. (2007). Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 13, 1050–1059. doi:10.1038/nm1622
Arout, C. A., Waters, A. J., Maclean, R. R., Compton, P., and Sofuoglu, M. (2019). Minocycline does not affect experimental pain or addiction-related outcomes in opioid maintained patients. Psychopharmacology (Berl) 236, 2857–2866. doi:10.1007/s00213-018-5146-7
Aurelian, L., Warnock, K. T., Balan, I., Puche, A., and June, H. (2016). TLR4 signaling in VTA dopaminergic neurons regulates impulsivity through tyrosine hydroxylase modulation. Transl. Psychiatry 6, e815.
Bachtell, R., Hutchinson, M. R., Wang, X., Rice, K. C., Maier, S. F., and Watkins, L. R. (2015). Targeting the toll of drug abuse: the translational potential of toll-like receptor 4. CNS Neurol. Disord. Drug Targets 14, 692–699. doi:10.2174/1871527314666150529132503
Bajo, M., Montgomery, S. E., Cates, L. N., Nadav, T., Delucchi, A. M., Cheng, K., et al. (2016). Evaluation of TLR4 inhibitor, T5342126, in modulation of ethanol-drinking behavior in alcohol-dependent mice. Alcohol Alcohol. 51, 541–548. doi:10.1093/alcalc/agw026
Balan, I., Warnock, K. T., Puche, A., Gondre-Lewis, M. C., June, H., and Aurelian, L. (2018). The GABAA receptor alpha2 subunit activates a neuronal TLR4 signal in the ventral tegmental area that regulates alcohol and nicotine abuse. Brain Sci. 8, 72. doi:10.3390/brainsci8040072
Bezzi, P., Domercq, M., Brambilla, L., Galli, R., Schols, D., De Clercq, E., et al. (2001). CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat. Neurosci. 4, 702–710. doi:10.1038/89490
Birath, J. B., Briones, M., Amaya, S., Shoptaw, S., Swanson, A. N., Tsuang, J., et al. (2017). Ibudilast may improve attention during early abstinence from methamphetamine. Drug Alcohol Depend. 178, 386–390. doi:10.1016/j.drugalcdep.2017.05.016
Blanco, A. M., and Guerri, C. (2007). Ethanol intake enhances inflammatory mediators in brain: role of glial cells and TLR4/IL-1RI receptors. Front. Biosci. 12, 2616–2630. doi:10.2741/2259
Blanco, A. M., Pascual, M., Valles, S. L., and Guerri, C. (2004). Ethanol-induced iNOS and COX-2 expression in cultured astrocytes via NF-kappa B. Neuroreport 15, 681–685. doi:10.1097/00001756-200403220-00021
Blanco, A. M., Perez-Arago, A., Fernandez-Lizarbe, S., and Guerri, C. (2008). Ethanol mimics ligand-mediated activation and endocytosis of IL-1RI/TLR4 receptors via lipid rafts caveolae in astroglial cells. J. Neurochem. 106, 625–639. doi:10.1111/j.1471-4159.2008.05425.x
Blanco, A. M., Valles, S. L., Pascual, M., and Guerri, C. (2005). Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. J. Immunol. 175, 6893–6899. doi:10.4049/jimmunol.175.10.6893
Blednov, Y. A., Black, M., Benavidez, J. M., Da Costa, A., Mayfield, J., and Harris, R. A. (2017a). Sedative and motor incoordination effects of ethanol in mice lacking CD14, TLR2, TLR4, or MyD88. Alcohol Clin. Exp. Res. 41, 531–540. doi:10.1111/acer.13314
Blednov, Y. A., Black, M., Chernis, J., Da Costa, A., Mayfield, J., and Harris, R. A. (2017b). Ethanol consumption in mice lacking CD14, TLR2, TLR4, or MyD88. Alcohol Clin. Exp. Res. 41, 516–530. doi:10.1111/acer.13316
Brown, K. T., Levis, S. C., O'neill, C. E., Northcutt, A. L., Fabisiak, T. J., Watkins, L. R., et al. (2018). Innate immune signaling in the ventral tegmental area contributes to drug-primed reinstatement of cocaine seeking. Brain Behav. Immun. 67, 130–138. doi:10.1016/j.bbi.2017.08.012
Burns, L. H., and Wang, H. Y. (2010). PTI-609: a novel analgesic that binds filamin A to control opioid signaling. Recent Pat. CNS Drug Discov. 5, 210–220. doi:10.2174/157488910793362386
Caron, G., Duluc, D., Fremaux, I., Jeannin, P., David, C., Gascan, H., et al. (2005). Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J. Immunol. 175, 1551–1557. doi:10.4049/jimmunol.175.3.1551
Chaplin, D. D. (2010). Overview of the immune response. J. Allergy Clin. Immunol. 125, S3–S23. doi:10.1016/j.jaci.2009.12.980
Chikritzhs, T. N., Jonas, H. A., Stockwell, T. R., Heale, P. F., and Dietze, P. M. (2001). Mortality and life-years lost due to alcohol: a comparison of acute and chronic causes. Med. J. Aust. 174, 281–284. doi:10.5694/j.1326-5377.2001.tb143269.x
Coller, J. K., and Hutchinson, M. R. (2012). Implications of central immune signaling caused by drugs of abuse: mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol. Ther. 134, 219–245. doi:10.1016/j.pharmthera.2012.01.008
Cooper, Z. D., Johnson, K. W., Pavlicova, M., Glass, A., Vosburg, S. K., Sullivan, M. A., et al. (2016). The effects of ibudilast, a glial activation inhibitor, on opioid withdrawal symptoms in opioid-dependent volunteers. Addict. Biol. 21, 895–903. doi:10.1111/adb.12261
Cooper, Z. D., Johnson, K. W., Vosburg, S. K., Sullivan, M. A., Manubay, J., Martinez, D., et al. (2017). Effects of ibudilast on oxycodone-induced analgesia and subjective effects in opioid-dependent volunteers. Drug Alcohol Depend. 178, 340–347. doi:10.1016/j.drugalcdep.2017.04.029
Crews, F. T., Lawrimore, C. J., Walter, T. J., and Coleman, L. G. (2017a). The role of neuroimmune signaling in alcoholism. Neuropharmacology 122, 56–73. doi:10.1016/j.neuropharm.2017.01.031
Crews, F. T., Sarkar, D. K., Qin, L., Zou, J., Boyadjieva, N., and Vetreno, R. P. (2015). Neuroimmune function and the consequences of alcohol exposure. Alcohol Res. 37, 331–341, 344–351.
Crews, F. T., Walter, T. J., Coleman, L. G., and Vetreno, R. P. (2017b). Toll-like receptor signaling and stages of addiction. Psychopharmacology (Berl) 234, 1483–1498. doi:10.1007/s00213-017-4560-6
Crews, F. T., Zou, J., and Qin, L. (2011). Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav. Immun. 25 (Suppl. 1), S4–S12. doi:10.1016/j.bbi.2011.03.003
Cummings, J. R., Tomiyama, A. J., and Ray, L. A. (2018). Does the neuroimmune modulator ibudilast alter food craving? Results in a sample with alcohol use disorder. J Addict. Med. 12, 410–417. doi:10.1097/ADM.0000000000000416
Domercq, M., Brambilla, L., Pilati, E., Marchaland, J., Volterra, A., and Bezzi, P. (2006). P2Y1 receptor-evoked glutamate exocytosis from astrocytes: control by tumor necrosis factor-alpha and prostaglandins. J. Biol. Chem. 281, 30684–30696. doi:10.1074/jbc.M606429200
Du, S. H., Qiao, D. F., Chen, C. X., Chen, S., Liu, C., Lin, Z., et al. (2017). Toll-like receptor 4 mediates methamphetamine-induced neuroinflammation through caspase-11 signaling pathway in astrocytes. Front. Mol. Neurosci. 10, 409. doi:10.3389/fnmol.2017.00409
Ducci, F., Enoch, M. A., Funt, S., Virkkunen, M., Albaugh, B., and Goldman, D. (2007). Increased anxiety and other similarities in temperament of alcoholics with and without antisocial personality disorder across three diverse populations. Alcohol 41, 3–12. doi:10.1016/j.alcohol.2007.02.005
Dunne, A., Marshall, N. A., and Mills, K. H. (2011). TLR based therapeutics. Curr. Opin. Pharmacol. 11, 404–411. doi:10.1016/j.coph.2011.03.004
Edenberg, H. J., Dick, D. M., Xuei, X., Tian, H., Almasy, L., Bauer, L. O., et al. (2004). Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am. J. Hum. Genet. 74, 705–714. doi:10.1086/383283
Eidson, L. N., Inoue, K., Young, L. J., Tansey, M. G., and Murphy, A. Z. (2017). Toll-like receptor 4 mediates morphine-induced neuroinflammation and tolerance via soluble tumor necrosis factor signaling. Neuropsychopharmacology 42, 661–670. doi:10.1038/npp.2016.131
Eidson, L. N., and Murphy, A. Z. (2013). Blockade of toll-like receptor 4 attenuates morphine tolerance and facilitates the pain relieving properties of morphine. J. Neurosci. 33, 15952–15963. doi:10.1523/JNEUROSCI.1609-13.2013
Erickson, E. K., Grantham, E. K., Warden, A. S., and Harris, R. A. (2019). Neuroimmune signaling in alcohol use disorder. Pharmacol. Biochem. Behav. 177, 34–60. doi:10.1016/j.pbb.2018.12.007
Feldmann, M. (2009). Translating molecular insights in autoimmunity into effective therapy. Annu. Rev. Immunol. 27, 1–27. doi:10.1146/annurev-immunol-082708-100732
Fernandez-Lizarbe, S., Pascual, M., and Guerri, C. (2009). Critical role of TLR4 response in the activation of microglia induced by ethanol. J. Immunol. 183, 4733–4744. doi:10.4049/jimmunol.0803590
Fields, H. L., and Margolis, E. B. (2015). Understanding opioid reward. Trends Neurosci. 38, 217–225. doi:10.1016/j.tins.2015.01.002
Flores-Bastias, O., and Karahanian, E. (2018). Neuroinflammation produced by heavy alcohol intake is due to loops of interactions between toll-like 4 and TNF receptors, peroxisome proliferator-activated receptors and the central melanocortin system: a novel hypothesis and new therapeutic avenues. Neuropharmacology 128, 401–407. doi:10.1016/j.neuropharm.2017.11.003
Foster, K. L., Mckay, P. F., Seyoum, R., Milbourne, D., Yin, W., Sarma, P. V., et al. (2004). GABA(A) and opioid receptors of the central nucleus of the amygdala selectively regulate ethanol-maintained behaviors. Neuropsychopharmacology 29, 269–284. doi:10.1038/sj.npp.1300306
Garcia Bueno, B., Caso, J. R., Madrigal, J. L., and Leza, J. C. (2016). Innate immune receptor toll-like receptor 4 signalling in neuropsychiatric diseases. Neurosci. Biobehav. Rev. 64, 134–147. doi:10.1016/j.neubiorev.2016.02.013
Gasiorowski, K., Brokos, B., Echeverria, V., Barreto, G. E., and Leszek, J. (2018). RAGE-TLR crosstalk sustains chronic inflammation in neurodegeneration. Mol. Neurobiol. 55, 1463–1476. doi:10.1007/s12035-017-0419-4
Gaudino, S. J., and Kumar, P. (2019). Cross-talk between antigen presenting cells and T cells impacts intestinal homeostasis, bacterial infections, and tumorigenesis. Front. Immunol. 10, 360. doi:10.3389/fimmu.2019.00360
Gesuete, R., Kohama, S. G., and Stenzel-Poore, M. P. (2014). Toll-like receptors and ischemic brain injury. J. Neuropathol. Exp. Neurol. 73, 378–386. doi:10.1097/NEN.0000000000000068
Gibson, L. C., Hastings, S. F., Mcphee, I., Clayton, R. A., Darroch, C. E., Mackenzie, A., et al. (2006). The inhibitory profile of Ibudilast against the human phosphodiesterase enzyme family. Eur. J. Pharmacol. 538, 39–42. doi:10.1016/j.ejphar.2006.02.053
Hall, F. S., Sora, I., Drgonova, J., Li, X. F., Goeb, M., and Uhl, G. R. (2004). Molecular mechanisms underlying the rewarding effects of cocaine. Ann. N. Y. Acad. Sci. 1025, 47–56. doi:10.1196/annals.1316.006
Harris, R. A., Bajo, M., Bell, R. L., Blednov, Y. A., Varodayan, F. P., Truitt, J. M., et al. (2017). Genetic and pharmacologic manipulation of TLR4 has minimal impact on ethanol consumption in rodents. J. Neurosci. 37, 1139–1155. doi:10.1523/JNEUROSCI.2002-16.2016
Heinzerling, K. G., Briones, M., Thames, A. D., Hinkin, C. H., Zhu, T., Wu, Y. N., et al. (2020). Randomized, placebo-controlled trial of targeting neuroinflammation with ibudilast to treat methamphetamine use disorder. J. Neuroimmune Pharmacol. 15, 238–248. doi:10.1007/s11481-019-09883-w
Hennessy, E. J., Parker, A. E., and O'neill, L. A. (2010). Targeting toll-like receptors: emerging therapeutics? Nat. Rev. Drug Discov. 9, 293–307. doi:10.1038/nrd3203
Hurd, Y. L., Spriggs, S., Alishayev, J., Winkel, G., Gurgov, K., Kudrich, C., et al. (2019). Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: a double-blind randomized placebo-controlled trial. Am. J. Psychiatry 176, 911–922. doi:10.1176/appi.ajp.2019.18101191
Hurd, Y. L., Yoon, M., Manini, A. F., Hernandez, S., Olmedo, R., Ostman, M., et al. (2015). Early phase in the development of cannabidiol as a treatment for addiction: opioid relapse takes initial center stage. Neurotherapeutics 12, 807–815. doi:10.1007/s13311-015-0373-7
Hutchinson, M. R., Northcutt, A. L., Hiranita, T., Wang, X., Lewis, S. S., Thomas, J., et al. (2012). Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J. Neurosci. 32, 11187–11200. doi:10.1523/JNEUROSCI.0684-12.2012
Jacobsen, J. H., Watkins, L. R., and Hutchinson, M. R. (2014). Discovery of a novel site of opioid action at the innate immune pattern-recognition receptor TLR4 and its role in addiction. Int. Rev. Neurobiol. 118, 129–163. doi:10.1016/B978-0-12-801284-0.00006-3
June, H. L., Liu, J., Warnock, K. T., Bell, K. A., Balan, I., et al. (2015). CRF-amplified neuronal TLR4/MCP-1 signaling regulates alcohol self-administration. Neuropsychopharmacology 40, 1549–1559. doi:10.1038/npp.2015.4
Kalivas, P. W., and O'brien, C. (2008). Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology 33, 166–180. doi:10.1038/sj.npp.1301564
Kashima, D. T., and Grueter, B. A. (2017). Toll-like receptor 4 deficiency alters nucleus accumbens synaptic physiology and drug reward behavior. Proc. Natl. Acad. Sci. U. S. A. 114, 8865–8870. doi:10.1073/pnas.1705974114
Kawai, T., and Akira, S. (2007). Signaling to NF-kappaB by toll-like receptors. Trends Mol. Med. 13, 460–469. doi:10.1016/j.molmed.2007.09.002
Kawai, T., and Akira, S. (2010). The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384. doi:10.1038/ni.1863
Klareskog, L., Van Der Heijde, D., De Jager, J. P., Gough, A., Kalden, J., Malaise, M., et al. (2004). Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 363, 675–681. doi:10.1016/S0140-6736(04)15640-7
Koropatnick, T. A., Engle, J. T., Apicella, M. A., Stabb, E. V., Goldman, W. E., and Mcfall-Ngai, M. J. (2004). Microbial factor-mediated development in a host-bacterial mutualism. Science 306, 1186–1188. doi:10.1126/science.1102218
Kronenberger, B., and Zeuzem, S. (2009). Current and future treatment options for HCV. Ann. Hepatol. 8, 103–112. doi:10.1016/S1665-2681(19)31786-7
Lacagnina, M. J., Rivera, P. D., and Bilbo, S. D. (2017). Glial and neuroimmune mechanisms as critical modulators of drug use and abuse. Neuropsychopharmacology 42, 156–177. doi:10.1038/npp.2016.121
Landreth, G. E., and Reed-Geaghan, E. G. (2009). Toll-like receptors in Alzheimer's disease. Curr. Top. Microbiol. Immunol. 336, 137–153. doi:10.1007/978-3-642-00549-7_8
Langlois, L. D., and Nugent, F. S. (2017). Opiates and plasticity in the ventral tegmental area. ACS Chem. Neurosci. 8, 1830–1838. doi:10.1021/acschemneuro.7b00281
Latz, E., Xiao, T. S., and Stutz, A. (2013). Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13, 397–411. doi:10.1038/nri3452
Leclercq, S., Cani, P. D., Neyrinck, A. M., Starkel, P., Jamar, F., Mikolajczak, M., et al. (2012). Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav. Immun. 26, 911–918. doi:10.1016/j.bbi.2012.04.001
Leclercq, S., Matamoros, S., Cani, P. D., Neyrinck, A. M., Jamar, F., Starkel, P., et al. (2014). Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc. Natl. Acad. Sci. U. S. A. 111, E4485–E4493. doi:10.1073/pnas.1415174111
Lee, B. R., Ma, Y. Y., Huang, Y. H., Wang, X., Otaka, M., Ishikawa, M., et al. (2013). Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat. Neurosci. 16, 1644–1651. doi:10.1038/nn.3533
Lewitus, G. M., Konefal, S. C., Greenhalgh, A. D., Pribiag, H., Augereau, K., and Stellwagen, D. (2016). Microglial TNF-alpha suppresses cocaine-induced plasticity and behavioral sensitization. Neuron 90, 483–491. doi:10.1016/j.neuron.2016.03.030
Lewitus, G. M., Pribiag, H., Duseja, R., St-Hilaire, M., and Stellwagen, D. (2014). An adaptive role of TNFalpha in the regulation of striatal synapses. J. Neurosci. 34, 6146–6155. doi:10.1523/JNEUROSCI.3481-13.2014
Li, M. J., Briones, M. S., Heinzerling, K. G., Kalmin, M. M., and Shoptaw, S. J. (2020). Ibudilast attenuates peripheral inflammatory effects of methamphetamine in patients with methamphetamine use disorder. Drug Alcohol Depend. 206, 107776. doi:10.1016/j.drugalcdep.2019.107776
Liu, G., Zhang, L., and Zhao, Y. (2010). Modulation of immune responses through direct activation of toll-like receptors to T cells. Clin. Exp. Immunol. 160, 168–175. doi:10.1111/j.1365-2249.2010.04091.x
Liu, J. F., Wu, R., and Li, J. X. (2019). Toll of mental disorders: TLR-mediated function of the innate immune system. Neurosci. Bull. 35, 771–774. doi:10.1007/s12264-018-00335-8
Liu, J., Yang, A. R., Kelly, T., Puche, A., Esoga, C., June, H. L., et al. (2011). Binge alcohol drinking is associated with GABAA alpha2-regulated toll-like receptor 4 (TLR4) expression in the central amygdala. Proc. Natl. Acad. Sci. U. S. A. 108, 4465–4470. doi:10.1073/pnas.1019020108
Loftis, J. M., and Janowsky, A. (2014). Neuroimmune basis of methamphetamine toxicity. Int. Rev. Neurobiol. 118, 165–197. doi:10.1016/B978-0-12-801284-0.00007-5
Ma, Y. Y., Lee, B. R., Wang, X., Guo, C., Liu, L., Cui, R., et al. (2014). Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron 83, 1453–1467. doi:10.1016/j.neuron.2014.08.023
Mattioli, T. A., Leduc-Pessah, H., Skelhorne-Gross, G., Nicol, C. J., Milne, B., Trang, T., et al. (2014). Toll-like receptor 4 mutant and null mice retain morphine-induced tolerance, hyperalgesia, and physical dependence. PloS One 9, e97361. doi:10.1371/journal.pone.0097361
Metz, V. E., Jones, J. D., Manubay, J., Sullivan, M. A., Mogali, S., Segoshi, A., et al. (2017). Effects of ibudilast on the subjective, reinforcing, and analgesic effects of oxycodone in recently detoxified adults with opioid dependence. Neuropsychopharmacology 42, 1825–1832. doi:10.1038/npp.2017.70
Montesinos, J., Alfonso-Loeches, S., and Guerri, C. (2016). Impact of the innate immune response in the actions of ethanol on the central nervous system. Alcohol Clin. Exp. Res. 40, 2260–2270. doi:10.1111/acer.13208
Naimi, T. S., Brewer, R. D., Mokdad, A., Denny, C., Serdula, M. K., and Marks, J. S. (2003). Binge drinking among US adults. J. Am. Med. Assoc. 289, 70–75. doi:10.1001/jama.289.1.70
Narita, M., Miyatake, M., Narita, M., Shibasaki, M., Shindo, K., Nakamura, A., et al. (2006). Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacology 31, 2476–2488. doi:10.1038/sj.npp.1301007
Nie, X., Kitaoka, S., Tanaka, K., Segi-Nishida, E., Imoto, Y., Ogawa, A., et al. (2018). The innate immune receptors TLR2/4 mediate repeated social defeat stress-induced social avoidance through prefrontal microglial activation. Neuron 99, 464–479.e7. doi:10.1016/j.neuron.2018.06.035
Northcutt, A. L., Hutchinson, M. R., Wang, X., Baratta, M. V., Hiranita, T., Cochran, T. A., et al. (2015). DAT isn't all that: cocaine reward and reinforcement require toll-like receptor 4 signaling. Mol Psychiatry 20, 1525–1537. doi:10.1038/mp.2014.177
Otis, J. M., and Mueller, D. (2017). Reversal of cocaine-associated synaptic plasticity in medial prefrontal cortex parallels elimination of memory retrieval. Neuropsychopharmacology 42, 2000–2010. doi:10.1038/npp.2017.90 | 10.1038/npp.2017.90CrossRef Full Text
Pandey, S. C. (2012). TLR4-MyD88 signalling: a molecular target for alcohol actions. Br. J. Pharmacol. 165, 1316–1318. doi:10.1111/j.1476-5381.2011.01695.x
Parlesak, A., Schafer, C., Schutz, T., Bode, J. C., and Bode, C. (2000). Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J. Hepatol. 32, 742–747. doi:10.1016/s0168-8278(00)80242-1
Pascual, M., Balino, P., Alfonso-Loeches, S., Aragon, C. M., and Guerri, C. (2011). Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav. Immun. 25 (Suppl. 1), S80–S91. doi:10.1016/j.bbi.2011.02.012
Pascual, M., Balino, P., Aragon, C. M., and Guerri, C. (2015). Cytokines and chemokines as biomarkers of ethanol-induced neuroinflammation and anxiety-related behavior: role of TLR4 and TLR2. Neuropharmacology 89, 352–359. doi:10.1016/j.neuropharm.2014.10.014
Pascual, O., Ben Achour, S., Rostaing, P., Triller, A., and Bessis, A. (2012). Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc. Natl. Acad. Sci. U. S. A. 109, E197–E205. doi:10.1073/pnas.1111098109
Periyasamy, P., Liao, K., Kook, Y. H., Niu, F., Callen, S. E., Guo, M. L., et al. (2018). Cocaine-mediated downregulation of miR-124 activates microglia by targeting KLF4 and TLR4 signaling. Mol. Neurobiol. 55, 3196–3210. doi:10.1007/s12035-017-0584-5
Ray, L. A., Bujarski, S., Shoptaw, S., Roche, D. J., Heinzerling, K., and Miotto, K. (2017). Development of the neuroimmune modulator ibudilast for the treatment of alcoholism: a randomized, placebo-controlled, human laboratory trial. Neuropsychopharmacology 42, 1776–1788. doi:10.1038/npp.2017.10
Reyes-Gibby, C. C., El Osta, B., Spitz, M. R., Parsons, H., Kurzrock, R., Wu, X., et al. (2008). The influence of tumor necrosis factor-alpha -308 G/A and IL-6 -174 G/C on pain and analgesia response in lung cancer patients receiving supportive care. Cancer Epidemiol. Biomarkers Prev. 17, 3262–3267. doi:10.1158/1055-9965.EPI-08-0125
Rizzo, F. R., Musella, A., De Vito, F., Fresegna, D., Bullitta, S., Vanni, V., et al. (2018). Tumor necrosis factor and interleukin-1beta modulate synaptic plasticity during neuroinflammation. Neural Plast. 2018, 8430123. doi:10.1155/2018/8430123
Roberts, A. J., Cole, M., and Koob, G. F. (1996). Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin. Exp. Res. 20, 1289–1298. doi:10.1111/j.1530-0277.1996.tb01125.x
Roh, Y. S., and Seki, E. (2013). Toll-like receptors in alcoholic liver disease, non-alcoholic steatohepatitis and carcinogenesis. J. Gastroenterol. Hepatol. 28 (Suppl. 1), 38–42. doi:10.1111/jgh.12019
Rolan, P., Gibbons, J. A., He, L., Chang, E., Jones, D., Gross, M. I., et al. (2008). Ibudilast in healthy volunteers: safety, tolerability and pharmacokinetics with single and multiple doses. Br. J. Clin. Pharmacol. 66, 792–801. doi:10.1111/j.1365-2125.2008.03270.x
Ruiz-Perez, D., Benito, J., Polo, G., Largo, C., Aguado, D., Sanz, L., et al. (2016). The effects of the toll-like receptor 4 antagonist, ibudilast, on sevoflurane's minimum alveolar concentration and the delayed remifentanil-induced increase in the minimum alveolar concentration in rats. Anesth. Analg. 122, 1370–1376. doi:10.1213/ANE.0000000000001171
Sesack, S. R., and Grace, A. A. (2010). Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology 35, 27–47. doi:10.1038/npp.2009.93
Sfondrini, L., Rossini, A., Besusso, D., Merlo, A., Tagliabue, E., Menard, S., et al. (2006). Antitumor activity of the TLR-5 ligand flagellin in mouse models of cancer. J. Immunol. 176, 6624–6630. doi:10.4049/jimmunol.176.11.6624
Shah, M., Anwar, M. A., Yesudhas, D., Krishnan, J., and Choi, S. (2016). A structural insight into the negative effects of opioids in analgesia by modulating the TLR4 signaling: an in silico approach. Sci. Rep. 6, 39271. doi:10.1038/srep39271
Shah, M., and Choi, S. (2017). Toll-like receptor-dependent negative effects of opioids: a battle between analgesia and hyperalgesia. Front. Immunol. 8, 642. doi:10.3389/fimmu.2017.00642
Skolnick, P., Davis, H., Arnelle, D., and Deaver, D. (2014). Translational potential of naloxone and naltrexone as TLR4 antagonists. Trends Pharmacol. Sci. 35, 431–432. doi:10.1016/j.tips.2014.06.008
Song, C., Horrobin, D. F., and Leonard, B. E. (2006). The comparison of changes in behavior, neurochemistry, endocrine, and immune functions after different routes, doses and durations of administrations of IL-1beta in rats. Pharmacopsychiatry 39, 88–99. doi:10.1055/s-2006-941557
Spanagel, R., Montkowski, A., Allingham, K., Stohr, T., Shoaib, M., Holsboer, F., et al. (1995). Anxiety: a potential predictor of vulnerability to the initiation of ethanol self-administration in rats. Psychopharmacology (Berl) 122, 369–373. doi:10.1007/BF02246268
Stellwagen, D., Beattie, E. C., Seo, J. Y., and Malenka, R. C. (2005). Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J. Neurosci. 25, 3219–3228. doi:10.1523/JNEUROSCI.4486-04.2005
Stellwagen, D., and Malenka, R. C. (2006). Synaptic scaling mediated by glial TNF-alpha. Nature 440, 1054–1059. doi:10.1038/nature04671
Stevens, C. W., Aravind, S., Das, S., and Davis, R. L. (2013). Pharmacological characterization of LPS and opioid interactions at the toll-like receptor 4. Br. J. Pharmacol. 168, 1421–1429. doi:10.1111/bph.12028
Stopponi, S., De Guglielmo, G., Somaini, L., Cippitelli, A., Cannella, N., Kallupi, M., et al. (2013). Activation of PPARgamma by pioglitazone potentiates the effects of naltrexone on alcohol drinking and relapse in msP rats. Alcohol Clin. Exp. Res. 37, 1351–1360. doi:10.1111/acer.12091
Takeda, K., and Akira, S. (2005). Toll-like receptors in innate immunity. Int. Immunol. 17, 1–14. doi:10.1093/intimm/dxh186
Tanda, G., Mereu, M., Hiranita, T., Quarterman, J. C., Coggiano, M., and Katz, J. L. (2016). Lack of specific involvement of (+)-Naloxone and (+)-Naltrexone on the reinforcing and neurochemical effects of cocaine and opioids. Neuropsychopharmacology 41, 2772–2781. doi:10.1038/npp.2016.91
Tanibuchi, Y., Shimagami, M., Fukami, G., Sekine, Y., Iyo, M., and Hashimoto, K. (2010). A case of methamphetamine use disorder treated with the antibiotic drug minocycline. Gen. Hosp. Psychiatry 32, 559.e1-3. doi:10.1016/j.genhosppsych.2009.12.005
Theberge, F. R., Li, X., Kambhampati, S., Pickens, C. L., St Laurent, R., Bossert, J. M., et al. (2013). Effect of chronic delivery of the toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biol. Psychiatry 73, 729–737. doi:10.1016/j.biopsych.2012.12.019
Valles, S. L., Blanco, A. M., Pascual, M., and Guerri, C. (2004). Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain Pathol. 14, 365–371. doi:10.1111/j.1750-3639.2004.tb00079.x
Vaure, C., and Liu, Y. (2014). A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol. 5, 316. doi:10.3389/fimmu.2014.00316
Vetreno, R. P., and Crews, F. T. (2014). Current hypotheses on the mechanisms of alcoholism. Handb. Clin. Neurol. 125, 477–497. doi:10.1016/B978-0-444-62619-6.00027-6
Volkow, N. D., and Morales, M. (2015). The brain on drugs: from reward to addiction. Cell 162, 712–725. doi:10.1016/j.cell.2015.07.046
Wang, H. Y., and Burns, L. H. (2009). Naloxone's pentapeptide binding site on filamin A blocks Mu opioid receptor-Gs coupling and CREB activation of acute morphine. PloS One 4, e4282. doi:10.1371/journal.pone.0004282
Wang, Q., Zhou, H., Gao, H., Chen, S. H., Chu, C. H., Wilson, B., et al. (2012a). Naloxone inhibits immune cell function by suppressing superoxide production through a direct interaction with gp91phox subunit of NADPH oxidase. J. Neuroinflammation 9, 32. doi:10.1186/1742-2094-9-32
Wang, S., Cheng, Q., Malik, S., and Yang, J. (2000). Interleukin-1beta inhibits gamma-aminobutyric acid type A (GABA(A)) receptor current in cultured hippocampal neurons. J. Pharmacol. Exp. Ther. 292, 497–504.
Wang, X., Loram, L. C., Ramos, K., De Jesus, A. J., Thomas, J., Cheng, K., et al. (2012b). Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc. Natl. Acad. Sci. U. S. A. 109, 6325–6330. doi:10.1073/pnas.1200130109
Wang, X., Northcutt, A. L., Cochran, T. A., Zhang, X., Fabisiak, T. J., Haas, M. E., et al. (2019). Methamphetamine activates toll-like receptor 4 to induce central immune signaling within the ventral tegmental area and contributes to extracellular dopamine increase in the nucleus accumbens shell. ACS Chem. Neurosci. 10, 3622–3634. doi:10.1021/acschemneuro.9b00225
Watkins, L. R., Wang, X., Mustafa, S., and Hutchinson, M. R. (2014). In vivo veritas: (+)-Naltrexone's actions define translational importance: a letter in response to Skolnick et al. ‘Translational potential of naloxone and naltrexone as TLR4 antagonists.’ Trends Pharmacol. Sci. 35, 432–433. doi:10.1016/j.tips.2014.07.002
Worley, M. J., Swanson, A. N., Heinzerling, K. G., Roche, D. J., and Shoptaw, S. (2016). Ibudilast attenuates subjective effects of methamphetamine in a placebo-controlled inpatient study. Drug Alcohol Depend. 162, 245–250. doi:10.1016/j.drugalcdep.2016.02.036
Wu, Y., Lousberg, E. L., Moldenhauer, L. M., Hayball, J. D., Coller, J. K., Rice, K. C., et al. (2012). Inhibiting the TLR4-MyD88 signalling cascade by genetic or pharmacological strategies reduces acute alcohol-induced sedation and motor impairment in mice. Br. J. Pharmacol. 165, 1319–1329. doi:10.1111/j.1476-5381.2011.01572.x
Yang, H., Hreggvidsdottir, H. S., Palmblad, K., Wang, H., Ochani, M., Li, J., et al. (2010). A critical cysteine is required for HMGB1 binding to toll-like receptor 4 and activation of macrophage cytokine release. Proc. Natl. Acad. Sci. U. S. A. 107, 11942–11947. doi:10.1073/pnas.1003893107
Yu, J. C., Khodadadi, H., Malik, A., Davidson, B., Salles, E., Bhatia, J., et al. (2018). Innate immunity of neonates and infants. Front. Immunol. 9, 1759. doi:10.3389/fimmu.2018.01759
Yue, K., Tanda, G., Katz, J. L., and Zanettini, C. (2020). A further assessment of a role for toll-like receptor 4 in the reinforcing and reinstating effects of opioids. Behav. Pharmacol. 31, 186–195. doi:10.1097/FBP.0000000000000474
Zhang, H., Largent-Milnes, T. M., and Vanderah, T. W. (2020). Glial neuroimmune signaling in opioid reward. Brain Res. Bull. 155, 102–111. doi:10.1016/j.brainresbull.2019.11.012
Keywords: toll-like receptor 4, opioids, alcohol, psychostimulants, drug reward, reinstatement, withdrawal
Citation: Wu R and Li J-X (2020) Toll-Like Receptor 4 Signaling and Drug Addiction. Front. Pharmacol. 11:603445. doi: 10.3389/fphar.2020.603445
Received: 06 September 2020; Accepted: 22 October 2020;
Published: 24 November 2020.
Edited by:
Rosa PoggianiQi Wang, Southern Medical University, ChinaReviewed by:
Wu Xu, China Medical University, ChinaCopyright © 2020 Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruyan Wu, cnV5YW53dTA5MDhAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.