
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Pharmacol. , 09 December 2020
Sec. Obstetric and Pediatric Pharmacology
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.597550
 Fang Chen1†
Fang Chen1† Cheng-Yu Wang1†
Cheng-Yu Wang1† Jianmin Zhang2
Jianmin Zhang2 Fang Wang2
Fang Wang2 Mazhong Zhang3
Mazhong Zhang3 Hongbin Gu3
Hongbin Gu3 Xingrong Song4
Xingrong Song4 Jia Chen1
Jia Chen1 Yang Li1
Yang Li1 Yu-Hang Cai1
Yu-Hang Cai1 Jun Li1
Jun Li1 Qing-Quan Lian1
Qing-Quan Lian1 Junzheng Wu5*
Junzheng Wu5* Hua-Cheng Liu1*
Hua-Cheng Liu1*Objective: There is no universal agreement on optimal pharmacological regimens for pain management during surgeries. The aim of this study to compare the postoperative analgesic effects of nalbuphine with fentanyl in children undergoing adenotonsillectomy.
Design, Setting, Participants: We conducted a prospective, randomized, double-blind, non-inferiority and multicenter trial in 311 patients admitted to four different medical facilities in China from October 2017 to November 2018.
Main Outcome Measure: The primary outcome was postoperative pain score. The secondary outcomes were as follows: the numbers of patients who developed moderate or severe pain (FLACC ≥4 points); time to first rescue analgesic top up and the actual number of rescue pain medicine given in pain control in post-anesthesia care unit (PACU), and additional analgesics requirement (received ≥2 rescue analgesics or/and other analgesics except study medications administered in PACU and ward); emergence and extubation time; Waking up time; time of PACU stay, and other side effects (desaturation, nausea/vomiting etc.).
Results: A total of 356 children were screened and 322 patients were randomized. The mean age was 5.8 (5.5, 6.1) in the nalbuphine group and 5.6 (5.3, 5.8) in the fentanyl group (p = 0.2132). FLACC score of nalbuphine group was lower than that of fentanyl group upon patients' arrival at PACU (p < 0.05). The time to first required rescue dose of pain drug for nalbuphine group was longer than for the fentanyl group (2.5 vs 1.2 h, p < 0.0001). Only one patient (0.6%) in nalbuphine group presented a slow respiratory rate (RR) at 9/min while 29 patients (18.5%) in fentanyl group developed slow RR ≤10/min in PACU. Meanwhile, SpO2 was lower in the fentanyl group at 10 min after patients’ arrival in PACU (p < 0.05). The other profiles observed from these two drug groups were similar.
Conclusion: Nalbuphine provided better pain relief with minimal respiration depression than fentanyl in children undergoing Adenotonsillectomy.
Adenotonsillectomy or tonsillectomy is the most commonly performed procedure for the treatment of obstructive sleep disorder, especially in the pediatric population (Reckley et al., 2018). Upper airway obstruction is the most frequent and serious postoperative complication following this procedure (Isaiah et al., 2018). There are plenty of attributes to this situation, including the tissue swelling, hemorrhage and the side effects of general anesthesia. It is also noteworthy that large dose of opioids may suppress respiration and precipitate airway obstruction after surgery (Overdyk and Hillman, 2011), but suboptimal dose of opioids may has left patients restless and agitated due to poor pain control in post-anesthesia care unit (PACU).
There is no universal agreement on attenuating reflex responses to nociceptive stimuli during tonsillo-adenoidectomy. However, the common practice is to administer opioids or non-steroidal anti-inflammatory agents (NSAID), alone or in combination. The extensive studies concerning the pharmaceutical management of postoperative pain and pain-related restlessness in the recovery room can be found throughout the literature. Opioids and/or NSAIDs may be administered as premedication (Atkinson et al., 1987; Bone and Fell, 1988; Nordbladh et al., 1991; Rusy et al., 1995), during induction of anesthesia (Watters et al., 1988; Thiagarajan et al., 1993), in the middle or at the end of surgery (Nordbladh et al., 1991; Gunter et al., 1995; Sutters et al., 1995).
Nalbuphine, an active agonist–antagonist opioid on mu receptors in the medulla and on kappa receptors in the cerebral cortex (Martinelli et al., 2014) has been demonstrated to provide effective analgesic (Bahar et al., 1985). It has been well-known that its analgesic potency is equivalent to that of morphine and its onset time is similar to fentanyl (Beaver and Feise, 1978; Dick et al., 1992; Zeng et al., 2015). Also, nalbuphine possesses a ceiling effect regarding respiratory depression at 0.2–0.4 mg kg−1 (Gal et al., 1982; Bahar et al., 1985) cardiovascular stability and rapid recovery to wakefulness (Lee et al., 1981; Zsigmond et al., 1987). These properties may make nalbuphine a more ideal and safer analgesic for children undergoing adenotonsillectomy.
Fentanyl, a potent, short-acting agonist opioid, has been widely used as one of the main anesthetic agents during cardiac surgeries and as an important component in balanced anesthesia during general surgeries (Stanley and Webster, 1978; Bovill et al., 1984; Flacke et al., 1985; Pandit et al., 1987). However, fentanyl is associated with an increased risk of respiratory depression at the end of surgery (Adams and Pybus, 1978). Nalbuphine has the same effect as morphine for analgesia (Beaver and Feise, 1978), but has barely been studied in children undergoing adenotonsillectomy in which fentanyl is the main choice of the drug for perioperative pain management (Vittinghoff et al., 2018).
The aim of this study was to compare the analgesic effectiveness and the impact on respiration between nalbuphine and fentanyl in children undergoing adenotonsillectomy.
This was a prospective, randomized, double-blind, non-inferiority multicenter study, which were carried out together by four different medical facilities led by the Second Affiliated Hospital and Yuying Children’s Hospital, Wenzhou Medical University of China from October 2017 to November 2018. This clinical trial was approved by institutional review board prior to start (Reference No. 2015-09) and was registered at www.chictr.org.cn on October 13, 2017 (ChiCTR-IPR-17012969). A written informed consent for participants was provided by the parents or a legal guardian.
A total of four hundred children, scheduled for combined Tonsillectomy and Adenoidectomy under general anesthesia were enrolled initially. Inclusion criteria were as follows: Aged 3–10 years old male or female; Body mass index (BMI) ≥15.5 kg/m2 and ≤24.5 kg/m2 and body weight above 12 kg; American Society of Anesthesiologists physical status (ASA) Class I or II. Exclusion criteria are as follows: Allergy to nalbuphine or its components; preoperative use of any analgesics, sedatives, anti-emetics or antipruritic 72 h before surgery; running fever with body temperature higher than 38°C 24 h before surgery or display of the symptoms of acute upper respiratory tract infection and bronchial asthma; severe obstructive sleep apnea syndrome and arrhythmia.
A case report form (CRF) was designed for the registration of clinical data and study results. Data were stored in a password-protected computer for the concealment of patients’ confidentiality. The guideline of good clinical practice (GCP) was closely followed during the study. One investigator was specifically assigned to the job for data collection, filing, and transfer and another one to verify the data’s accuracy and safety.
Eligible recruits were randomly assigned into either nalbuphine or fentanyl group in a 1:1 ratio by a computer-generated digit-number program (SAS PLAN; SAS Institute Inc.) in each individual participating medical center. An assignment number was sealed in an envelope and was revealed just before the drug’s administration.
The medications were prepared according to the group allocation before the surgery started. For the intraoperative dosing, nalbuphine (100 µg·kg−1) or fentanyl (1 µg·kg−1) (Yichang Humanwell Pharmaceutical CO) was diluted up to 10 ml in a syringe with isotonic saline. For postoperative rescue dosing, nalbuphine (50 µg·kg−1) or fentanyl (0.5 µg·kg−1) was made up to 5 ml in a syringe with normal saline.
Each syringe was labeled with an individual assignment number, which matched a pre-sealed envelope. The anesthesiologist who administered the injections, the outcomes evaluator, the parents or guardian and the children were blinded to the allocation of study drugs.
Patients’ preoperative fasting status was confirmed to meet the ASA guideline and a peripheral venous access was established prior to surgery. No premedication was used according to the study protocol. Noninvasive blood pressure (NIBP), heart rate (HR), and oxygen saturation (SpO2) were continually monitored 30 min before surgery. Anesthesia was induced with propofol 2.5 mg kg−1 and remifentanil 2.5 µg·kg−1 intravenously. Endotracheal intubation was facilitated with cisatracurium 0.6 mg kg−1. Mechanical ventilation was carried out with a 1:1 mixture of nitrous oxide and oxygen at a total flow of 2 L/min.
Anesthesia was maintained with propofol 3–15 mg kg−1·h−1 and remifentanil with 0.1–0.5 µg·kg−1·min−1. Heart rate and mean arterial blood pressure were kept within 80–120% of baseline values. The end-expiratory carbon dioxide partial pressure (PETCO2) was maintained around 35–45 mmHg by adjusting the respiratory rate (RR) and tidal volume. Repetitive cisatracurium injection was given as needed. Intravenous infusion of propofol and remifentanil were discontinued right away after tonsillar and adenoid were removed. At the same time, nalbuphine 100 μg/kg or fentanyl 1 μg/kg, in 10 ml normal saline was slowly injected intravenously over 1 min. Ondansetron (0.1 mg/kg, max 4 mg) was given and patents were reversed with neostigmine 0.02 mg/kg and atropine 0.01 mg/kg, and then, were allowed to emerge from anesthesia. A wake-extubation technique was performed in the operating room. Children were sent to PACU and were observed for at least 1 h before the discharge to ward after having met a modified Aldrete score 9 (Aldrete and Kroulik, 1970). The postoperative pain was scored with FLACC (Merkel et al., 2002) (Table 1).
For children who suffered moderate or severe pain (FALCC ≥4 points) in PACU, a pain rescue drug, either nalbuphine or fentanyl in line with intraoperative group allocation, was given intravenously. Then, FLACC score was assessed again 10–15 min later. If the score remained ≥4 points, the second dose of rescue analgesic was administered. After that, the decision to give additional analgesic (either narcotics or NSAIDS) based on the reevaluation of FLACC score, and was totally at the discretion of anesthesiologist who was in charge of patient care. The recorded drug dose and frequency of drug administration was shown in Figure 1.
The primary outcome was postoperative pain score. All children were assessed by FLACC at the following time point: 0, 10, 20, 30, 45 min, 1, 2, 6, 12 and 24 h after PACU admission. The secondary outcomes were as follows: the number of cases with moderate or severe pain (FLACC ≥4 points); the number of rescue pain medication administered in PACU and ward; time of emergence and extubation; time of recovery; vital signs (blood pressure, heart rate, RR, oxygen saturation) in PACU; parent’s overall satisfaction with analgesia over 24 h postoperatively.
Based on the preliminary study data of 30 children from the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, the effect size of non-inferiority analgesia between the nalbuphine group and the fentanyl group was 1.02. A sample size of 296 children (149 children per group) provided 90% power at a two-sided α of 0.05 to detect the difference between the two groups. We planned to have 356 enrollments (178 per group) to allow for up to 20% dropout of children who failed to complete the study.
The anesthesia emergence and extubation time meant the time span from the end of the surgery to the extubation. The waking up time was the time span from the moment to discontinue anesthetics to the point when child was fully conscious.
Statistics analysis was conducted by SPSS version 24.0 for windows (SPSS lnc., Chicago, IL, United States). The normality of distribution of continuous variables was tested by one-sample Kolmogorov-Smirnov test. The continuous variables of normal distribution were expressed as mean (SD); non-normal variables were reported as median (interquartile range [IQR]). Means of two continuous normal distributed variables were compared by independent samples student’s test. Mann-Whitney U test and Kruskal-Wallis test were used to compare two groups of non-normally distributed variables respectively. Data were expressed in the number and percentage of categorical variables. The frequencies of categorical variables were compared with Pearson χ2 or Fisher’s exact test whenever it was appropriate. The preoperative pre-dosing baseline data was compared with post-dosing data by ANOVA test through repeated measures. Only when ANOVA test was significant, the p value for pairwise comparisons was computed with Student’s test with Bonferroni correction. All tests were two-sided, and p < 0.05 was considered to be statistically significant.
Three hundred fifty six children were initially assessed for eligibility and 20 were excluded prior to randomization because of unsatisfied inclusion requirement or refusing to participate. A total of 336 children was evenly and randomly assigned to each group. 14 children in nalbuphine group and 11 in Fentanyl group were removed from the study later for following reasons: additional analgesia having either received additional opioids or other pain medicine beyond the study protocol; withdrawal consent, surgical cancellation and etc. In the end, 154 patients were treated with nalbuphine and 157 with fentanyl (Figure 2). Demographic and clinical data were summarized in Table 2. The median age of participants was 5.2 years (IQR, 4.3–6.8 years) and the median weight of participants was 20.0 kg (IQR, 11.5–27.3 kg). There were no differences in children characteristics between nalbuphine and fentanyl group.
Complete sets of FLACC scores were collected in 311 patients. The average of FLACC scores among the patients at the arrival of PACU (0 min) was significantly higher in fentanyl group than that in nalbuphine group, but no differences were found for the rest of the assessment time-points (Figure 3). However, the total number of cases with FLACC ≥4 at 0 min of PACU was not significantly different between two groups. At 1 and 2 h PACU time points, Nalbuphine group had significantly fewer children (10 and 10) with FLACC scores ≥4 than fentanyl group (51 and 26 respectively) (Table 3). The time to first required rescue (The time from arriving PACU to first required dose of pain drug) dose of pain drug in nalbuphine group was longer than for the fentanyl group (Table 4). Six children in the fentanyl group and five in the nalbuphine group required more than two doses of rescue pain drugs and other analgesics. All patients had satisfactory pain management before discharge to ward.
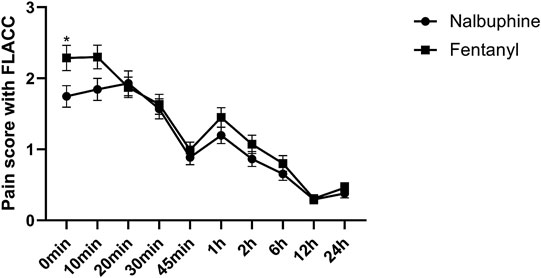
FIGURE 3. Postoperative FLACC score for pain relief receiving Nalbuphine (blue line) or Fentanyl (red line) (Mean ± SD). The average of FLACC scores among the patients at the arrival of PACU (0 min) was significantly higher in fentanyl group than that in nalbuphine group (p = 0.0364), but no differences were found for the rest of the assessment time-points.
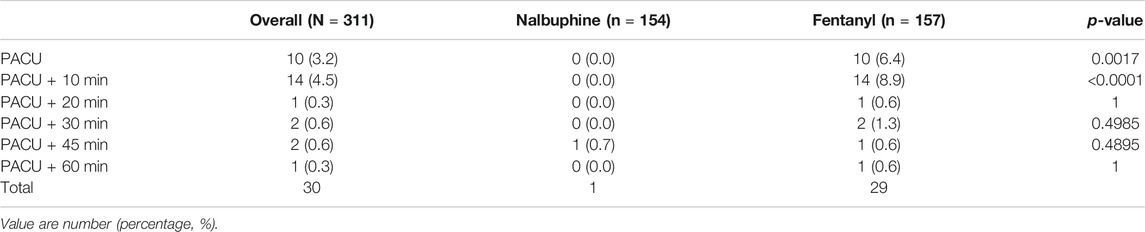
TABLE 4. Number of patients at each time point in which the respiratory rate was below 10 breath/min.
Since patients were mechanically ventilated during the surgery, only post-extubation data were used to assess the effect of analgesics on respiration. Overall, there was no significant difference in postoperative RR between two groups (Figure 4). However, the total number of cases with RR lower than l0 breath/min was significantly fewer in nalbuphine group than in fentanyl group both at the arrival of PACU and 10 min after arrival of PACU (Table 5). Only one patient (0.6%) in nalbuphine group displayed a RR of 9 breath/min at 1 h after entering the PACU, while 29 children (18.5%) in the fentanyl group developed a RR ≤10 breath/min in PACU (Table 5). At 10 min after entering the PACU, 15 patients, all from the fentanyl group, were observed with a RR ≤10 breath/min. Among those patients, 10 experienced episodes of hypoxemia (SpO2 <90 lasted for ≥5 s in PACU; eight were rapidly normalized with oxygen supplement through face mask and two were relieved after brief assisted mask ventilation with 100% oxygen. None of the children in the nalbuphine group developed hypoxemia. There was remarked difference in recorded SpO2 levels between the two groups, in which the averaged SpO2 was significant lower in Fentanyl group than in nalbuphine group at 10 min after arriving PACU (Figure 4).
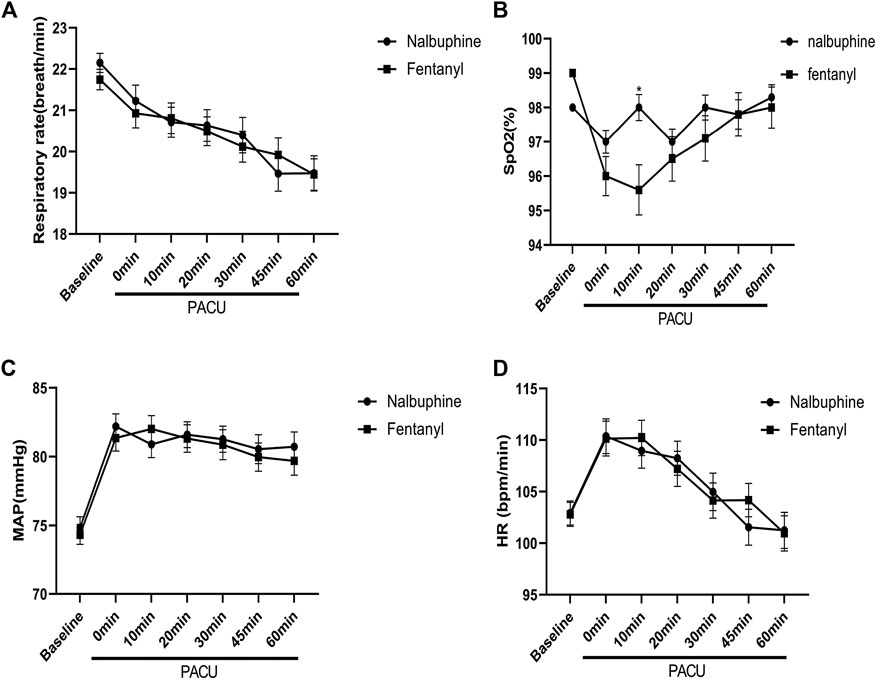
FIGURE 4. Perioperative feature. (A): Respiratory rate (RR) vs. time in patients receiving Nalbuphine or Fentanyl (Mean ± SD). No significant difference between groups. (B): SpO2 vs. time in patients receiving Nalbuphine or Fentanyl (Mean ± SD). There was marked difference in recorded SP02 levels between the two groups, in which the averaged SpO2 was significant lower in Fentanyl group than in nalbuphine group at 10 min after arriving PACU (p = 0.0009), but no differences were found for the rest of the assessment time-points. (C): Mean arterial pressure (MAP) vs. time in patients receiving Nalbuphine or Fentanyl (Mean ± SD). No significant difference between groups. D: Heart rate (HR) vs. time in patients receiving Nalbuphine or Fentanyl (Mean ± SD). No significant difference between groups.
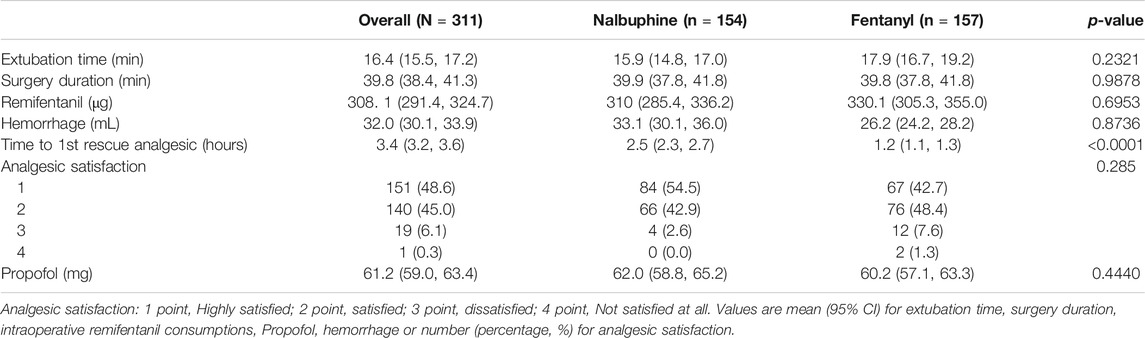
TABLE 5. Extubation time, surgery duration, intraoperative remifentanil consumptions, hemorrhage during operation, time to 1st rescue analgesic and analgesic satisfaction.
There were no significant differences found in other comparable parameters, including: the time span of emergence and extubation, surgery duration, intraoperative remifentanil consumptions, time to first rescue analgesic, intraoperative hemorrhage and analgesic satisfaction (Table 4). There were also no statistically significant differences in mean arterial pressure (MAP) and heart rate (HR) between two groups (Figure 4).
Delayed recovery, waking time, hypotension, bradycardia, nausea, vomiting, and pruritus were similar between the two groups throughout the study course. (Table 6).
This was a prospective, randomized double-blind, multicenter study designed to evaluate the effectiveness of postoperative pain relief and the safety of nalbuphine and fentanyl. Our study results have shown that Nalbuphine, an opioid agonist–antagonist, presented a better pain-relief effect with minimal respiratory depression than fentanyl in children undergoing adenotonsillectomy.
Adenotonsillectomy is one of the most performed surgeries in children, and postoperative pain, such as sore throats and earaches, are common and could persist for up to 2 weeks (Reckley et al., 2018). To date, pharmaceutical approaches remain the mainstream of pain management for adenotonsillectomy and the commonly used medications include opioids, non-steroidal anti-inflammatory drugs (NSAIDS) and local anesthetics etc. Morphine, one of the most classic and consumed opioids, has a strong pain-relief effect, but with high incidence of postoperative nausea and vomiting (PONV), and respiratory depression compared with other forms of analgesics (Zeng et al., 2015). Fentanyl, another potent, short-acting opioid agonist, is also a widely used pain-killer during short surgical procedures and however, the major worry to clinicians is that the utility of fentanyl has been noted to be quite narrow in comparison of its plasma levels between effective analgesia and significant respiratory depression (Yassen et al., 2006; Yassen et al., 2007; Yassen et al., 2008; Boom et al., 2013), By contrast, NSAIDS would be an ideal option of treatment for tonsillectomy pain with a lower incidence of PONV and non-detrimental impact on respiration, but the concern over the potential postoperative hemorrhage caused by NSAIDS has limited its clinical application (Marret et al., 2003; Møiniche et al., 2003; Lake and Khater, 2004). Nalbuphine is a 6-transmembrane MOR agonist as is buprenorphine and butorphanol all of which have a ceiling on respiratory depression (Davis et al., 2018). In addition, Nalbuphine is an opioid agonist–antagonist, active on mu and kappa receptors (Martinelli et al., 2014) to provide analgesia and certain anti-pruritic effects (Romagnoli and Keats, 1980) and have less undesirable outcomes. It has been shown to be safe and effective when used in variety of surgical procedures. The aims of our study were to assess and compare nalbuphine to fentanyl regarding their pain-relief efficiency and the impact on respiration in pediatric patients underwent adenotonsillectomy.
Studies have shown that the ratio of potency between nalbuphine and fentanyl is about 1:100 on a milligram basis, the same as the ratio between morphine and fentanyl. Therefore, we used the equipotent dose of 0.1 mg/kg nalbuphine vs. 1 µg/kg fentanyl as the initial analgesics separately in our study groups (Beaver and Feise, 1978; Twycross, 2007).
Our study showed that patients in nalbuphine group had overall relatively lower average of FLACC scores during the entire course of PACU than ones in fentanyl group, and the statistical significance for the difference can be seen particularly at the time of arriving PACU (0 min). The total numbers of administered rescue analgesics were 80 vs. 156 when comparing nalbuphine and fentanyl groups. Those results indicated that nalbuphine provided a better postoperative pain-relief effect than fentanyl in children undergoing adenotonsillectomy. Hari Prasad et al. compared these two drugs in other surgical patients under general anesthesia and he found that the time to first rescue dose analgesic top up was significantly longer in nalbuphine group than in fentanyl group. They concluded that nalbuphine provides excellent postoperative analgesia which is comparable to fentanyl at a less frequent dosing thus decreasing the overall opioid requirement (Prasad et al., 2016). By acting on μ1 and μ2 receptors, fentanyl is a stronger agonist than nalbuphine to produce analgesia and respiratory depression, and by acting on kappa receptors, nalbuphine may provide better effects of analgesia and sedation (Chen et al., 1993). Specifically, nalbuphine acts as a moderate-efficacy partial agonist or antagonist of the µ-opioid receptor (MOR) and as a high-efficacy partial agonist of the κ-opioid receptor (KOR) (Schmidt et al., 1985; Chen et al., 1993), which has the potential to maintain or even enhance μ-opioid-based analgesia while simultaneously mitigating the µ-opioid side effects (Prabhakaraiah et al., 2017).
Our results revealed that patients in both group had comparably satisfactory pain control in the first 45 min or so after PACU arrival and then, there were significantly increased case numbers with FLACC scores ≥4 in fentanyl group at 1 and 2 h time points, which is consistent with N. Rawal’s study results (Rawal and Wennhager, 1990). A rational explanation for this phenomenon was that the analgesic duration of fentanyl is about 30–60 min and its action began to wear off within 1 or 2 h after patient’s arrival in PACU while nalbuphine’s lasts approximately 3–6 h (slightly longer than morphine) and would provide sufficient pain-relief for the patients during their entire PACU stay and even beyond. The reasons for the increase in number of children who FLACC ≥4 may be multi-factorial.
Nalbuphine has been known to cause respiratory depression by a dose-related ceiling effect (Romagnoli and Keats, 1980) in which, repeated dosing or increased dose of nalbuphine will not further aggregate depressed respiration. Our study showed that none of patients developed hypoxemia and only one had slow RR ≤10/min in nalbuphine group vs. 10 patients developed hypoxemia and 29 had slow RR ≤10/min in fentanyl group. 25 out of 29 patients in fentanyl group developed slow RR within the first 20 min during their PACU stay when fentanyl had reached its peaking effect.
The statistically significant low average SpO2 level and slow RR in fentanyl group indicated that fentanyl causes greater respiratory depression than nalbuphine does. The more frequent and marked episodes of hypoxemia observed in the fentanyl group also support this finding. Most likely, this phenomenon is attributed by nalbuphine’s ceiling effect on respiratory depression which will not be worsen further when the dose of nalbuphine has reached a certain threshold. One study has found that nalbuphine, as high as 0.8 mg/kg, will not aggregate respiration depression (Bone and Tooley, 1989).
The main drawback of nalbuphine was the markedly prolonged awaking time postoperatively as reported by some investigators (Vatashsky and Haskel, 1986; Pugh and Drummond, 1987). It is generally believed that nalbuphine is a longer-acting opioid with a half-life of approximately 4 h and it is expected to extend the recovery time significantly. However, we found that the recovery time of nalbuphine group was not significantly different from the fentanyl group.
In summary, nalbuphine provides more effective pain relief than fentanyl in children underwent adenotonsillectomy, which was demonstrated by less intensity of the pain and longer duration of its analgesic effect. Clinical nalbuphine dosage only induces mild respiration depression and does not slow down anesthesia emergence and extubation process, and it will not delay PACU discharge, or increase the incidence of PONV.
Nalbuphine is an agonist/antagonist opioid and is only used as a sole opioid analgesic. And it also has analgesic “ceiling effect” and may not be sufficient to relieve severe pain encountered in other types of surgeries. In this study, we only observed the effect of one fixed dosage of nalbulphine on postoperative pain in children undergoing adenotonsillectomy and future study to find out the optimal dosage(s) for pain management is warranted.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Second Affiliated Hospital and Yuying Children’s Hospital, Wenzhou Medical University. The patients/participants provided their written informed consent to participate in this study.
All authors listed have made substantial, direct, and intellectual contribution to the work and approved it for publication.
The Special Project for Significant New Drug Research and Development in the Major National Science and Technology Projects of China (2020ZX09201002) and Clinical Research Foundation of the Second Affiliated Hospital of Wenzhou Medical University (SAHoWMU-CR2018-03-126).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.597550/full#supplementary-material
Adams, A. P., and Pybus, D. A. (1978). Delayed respiratory depression after use of fentanyl during anaesthesia. Br. Med. J. 1 (6108), 278–279. doi:10.1136/bmj.1.6108.278
Aldrete, J. A., and Kroulik, D. (1970). A postanesthetic recovery score. Anesth. Analg. 49 (6), 924–934.
R., Atkinson, G., Rushman, and J., Lee (1987). Surgical operations and choice of technique. A synopsis of anaesthesia. 10th Edn. Bristol, England: Wright, 444–492.
Bahar, M., Rosen, M., and Vickers, M. D. (1985). Self-administered nalbuphine, morphine and pethidine. Comparison, by intravenous route, following cholecystectomy. Anaesthesia 40 (6), 529–532.
Beaver, W. T., and Feise, G. A. (1978). A comparison of the analgesic effect of intramuscular nalbuphine and morphine in patients with postoperative pain. J. Pharmacol. Exp. Ther. 204 (2), 487–496.
Bone, M. E., and Fell, D. (1988). A comparison of rectal diclofenac with intramuscular papaveretum or placebo for pain relief following tonsillectomy. Anaesthesia 43 (4), 277–280. doi:10.1111/j.1365-2044.1988.tb08972.x
Bone, M. E. W. D., and Tooley, M. (1989). High dose of nalbuphine 0.8mg/kg will not produce apnea. Anaesth. Analg. 47, 147–149.
Boom, M., Olofsen, E., Neukirchen, M., Fussen, R., Hay, J., Groeneveld, G. J., et al. (2013). Fentanyl utility function: a risk-benefit composite of pain relief and breathing responses. Anesthesiology 119 (3), 663–674. doi:10.1097/ALN.0b013e31829ce4cb
Bovill, J. G., Sebel, P. S., and Stanley, T. H. (1984). Opioid analgesics in anesthesia: with special reference to their use in cardiovascular anesthesia. Anesthesiology 61 (6), 731–755.
Chen, J. C., Smith, E. R., Cahill, M., Cohen, R., and Fishman, J. B. (1993). The opioid receptor binding of dezocine, morphine, fentanyl, butorphanol and nalbuphine. Life Sci. 52 (4), 389–396. doi:10.1016/0024-3205(93)90152-s
Davis, M. P., Fernandez, C., Regel, S., and McPherson, M. L. (2018). Does nalbuphine have a niche in managing pain? J. Opioid Manag. 14 (2), 143–151. doi:10.5055/jom.2018.0441
Dick, W., Lorenz, W., Heintz, D., Sitter, H., and Doenicke, A. (1992). [Histamine release during induction of combination anesthesia using nalbuphine or fentanyl. Modulation of the reaction by premedication with promethazine/pethidine]. Anaesthesist 41 (5), 239–247 [in German].
Flacke, J. W., Bloor, B. C., Kripke, B. J., Flacke, W. E., Warneck, C. M., Van Etten, A. P., et al. (1985). Comparison of morphine, meperidine, fentanyl, and sufentanil in balanced anesthesia: a double-blind study. Anesth. Analg. 64 (9), 897–910.
Gal, T. J., DiFazio, C. A., and Moscicki, J. (1982). Analgesic and respiratory depressant activity of nalbuphine: a comparison with morphine. Anesthesiology 57 (5), 367–374. doi:10.1097/00000542-198211000-00004
Gunter, J. B., Varughese, A. M., Harrington, J. F., Wittkugel, E. P., Patankar, S. S., Matar, M. M., et al. (1995). Recovery and complications after tonsillectomy in children: a comparison of ketorolac and morphine. Anesth. Analg. 81 (6), 1136–1141. doi:10.1097/00000539-199512000-00004
Isaiah, A., Kiss, E., Olomu, P., Koral, K., and Mitchell, R. B. (2018). Characterization of upper airway obstruction using cine MRI in children with residual obstructive sleep apnea after adenotonsillectomy. Sleep Med. 50, 79–86. doi:10.1016/j.sleep.2017.10.006
Lake, A. P. J., and Khater, M. (2004). Effects of postoperative nonsteroidal antiinflammatory drugs on bleeding risk after tonsillectomy. Anesthesiology 100 (3), 748–752. doi:10.1097/00000542-200403000-00050
Lee, G., Low, R. I., Amsterdam, E. A., DeMaria, A. N., Huber, P. W., and Mason, D. T. (1981). Hemodynamic effects of morphine and nalbuphine in acute myocardial infarction. Clin. Pharmacol. Ther. 29 (5), 576–581. doi:10.1038/clpt.1981.80
Marret, E., Flahault, A., Samama, C-M., and Bonnet, F. (2003). Effects of postoperative, nonsteroidal, antiinflammatory drugs on bleeding risk after tonsillectomy: meta-analysis of randomized, controlled trials. Anesthesiology 98 (6), 1497–1502. doi:10.1097/00000542-200306000-00030.
Martinelli, S. M., DiLorenzo, A. N., and Schell, R. M. (2014) Stoelting’s pharmacology and physiology in anesthetic practice. Anesth. Analg. 121 (6), 1674–1675.
Merkel, S., Voepel-Lewis, T., and Malviya, S. (2002). Pain assessment in infants and young children: the FLACC scale. Am. J. Nurs. 102 (10), 55–58. doi:10.1097/00000446-200210000-00024
Møiniche, S., Rømsing, J., Dahl, J. B., and Tramèr, M. R. (2003). Nonsteroidal antiinflammatory drugs and the risk of operative site bleeding after tonsillectomy: a quantitative systematic review. Anesth. Analg. 96 (1), 68. doi:10.1097/00000539-200301000-00015
Nordbladh, I., Ohlander, B., and Björkman, R. (1991). Analgesia in tonsillectomy: a double-blind study on pre and post-operative treatment with diclofenac. Clin. Otolaryngol. Allied Sci. 16 (6), 554–558. doi:10.1111/j.1365-2273.1991.tb00973.x
Overdyk, F. J., and Hillman, D. R. (2011). Opioid modeling of central respiratory drive must take upper airway obstruction into account. Anesthesiology 114 (1), 219–221. doi:10.1097/ALN.0b013e3181fef383
Pandit, S. K., Kothary, S. P., Pandit, U. A., and Mathai, M. K. (1987). Comparison of fentanyl and butorphanol for outpatient anaesthesia. Can. J. Anaesth. 34 (2), 130–134. doi:10.1007/BF03015329
Prabhakaraiah, U. N., Narayanappa, A. B., Gurulingaswamy, S., Kempegowda, K., Vijaynagar, K. A., Hanumantharayappa, N. B., et al. (2017). “Comparison of nalbuphine hydrochloride and fentanyl as an adjuvant to bupivacaine for spinal anesthesia in lower abdominal surgeries:” A randomized, double-blind study. Anesth. Essays Res. 11 (4), 859–863. doi:10.4103/aer.AER_40_17
Prasad, K. H., Kumar, R., Rajagokilam, R., and Varadarajan, V. (2016). A comparative study of analgesic potential of nalbuphine versus fentanyl during general anaesthesia. Int. J. Contem. Med. Res. 3 (10), 2815–2821.
Pugh, G. C., and Drummond, G. B. (1987). A dose-response study with nalbuphine hydrochloride for pain in patients after upper abdominal surgery. Br. J. Anaesth. 59 (11), 1356–1363.
Rawal, N., and Wennhager, M. (1990). Influence of perioperative nalbuphine and fentanyl on postoperative respiration and analgesia. Acta Anaesthesiol. Scand. 34 (3), 197–202. doi:10.1111/j.1399-6576.1990.tb03069.x
Reckley, L. K., Fernandez-Salvador, C., and Camacho, M. (2018). The effect of tonsillectomy on obstructive sleep apnea: an overview of systematic reviews. Nat. Sci. Sleep 10, 105–110. doi:10.2147/NSS.S127816
Romagnoli, A., and Keats, A. S. (1980). Ceiling effect for respiratory depression by nalbuphine. Clin. Pharmacol. Ther. 27 (4), 478–485. doi:10.1038/clpt.1980.67
Rusy, L. M., Houck, C. S., Sullivan, L. J., Ohlms, L. A., Jones, D. T., McGill, T. J., et al. (1995). A double-blind evaluation of ketorolac tromethamine versus acetaminophen in pediatric tonsillectomy: analgesia and bleeding. Anesth. Analg. 80 (2), 226–229. doi:10.1097/00000539-199502000-00004
Schmidt, W. K., Tam, S. W., Shotzberger, G. S., Smith, D. H., Clark, R., and Vernier, V. G. (1985). Nalbuphine. Drug Alcohol Depend 14 (3–4), 339–362. doi:10.1016/0376-8716(85)90066-3
Stanley, T. H., and Webster, L. R. (1978). Anesthetic requirements and cardiovascular effects of fentanyl-oxygen and fentanyl-diazepam-oxygen anesthesia in man. Anesth. Analg. 57 (4), 411–416. doi:10.1213/00000539-197807000-00008
Sutters, K. A., Levine, J. D., Dibble, S., Savedra, M., and Miaskowski, C. (1995). Analgesic efficacy and safety of single-dose intramuscular ketorolac for postoperative pain management in children following tonsillectomy. Pain 61 (1), 145–153. doi:10.1016/0304-3959(94)00166-c
Thiagarajan, J., Bates, S., Hitchcock, M., and Morgan-Hughes, J. (1993). Blood loss following tonsillectomy in children. A blind comparison of diclofenac and papaveretum. Anaesthesia 48 (2), 132–135. doi:10.1111/j.1365-2044.1993.tb06851.x
Vatashsky, E., and Haskel, Y. (1986). Effect of nalbuphine on intrabiliary pressure in the early postoperative period. Can. Anaesth. Soc. J. 33 (4), 433–436. doi:10.1007/BF03010967
Vittinghoff, M., Lönnqvist, P. A., Mossetti, V., Heschl, S., Simic, D., Colovic, V., et al. (2018). Postoperative pain management in children: guidance from the pain committee of the European society for paediatric anaesthesiology (ESPA pain management ladder initiative). Paediatr. Anaesth. 28 (6), 493–506. doi:10.1111/pan.13373
Watters, C. H., Patterson, C. C., Mathews, H. M., and Campbell, W. (1988). Diclofenac sodium for post-tonsillectomy pain in children. Anaesthesia 43 (8), 641–643. doi:10.1111/j.1365-2044.1988.tb04147.x
Yassen, A., Kan, J., Olofsen, E., Suidgeest, E., Dahan, A., and Danhof, M. (2006). Mechanism-based pharmacokinetic-pharmacodynamic modeling of the respiratory-depressant effect of buprenorphine and fentanyl in rats. J. Pharmacol. Exp. Ther. 319 (2), 682–692. doi:10.1124/jpet.106.107953
Yassen, A., Olofsen, E., Kan, J., Dahan, A., and Danhof, M. (2008). Pharmacokinetic-pharmacodynamic modeling of the effectiveness and safety of buprenorphine and fentanyl in rats. Pharmaceutical research 25 (1), 183–193. doi:10.1007/s11095-007-9440-z
Yassen, A., Olofsen, E., Romberg, R., Sarton, E., Teppema, L., Danhof, M., et al. (2007). Mechanism-based PK/PD modeling of the respiratory depressant effect of buprenorphine and fentanyl in healthy volunteers. Clin. Pharmacol. Ther. 81 (1), 50–58. doi:10.1038/sj.clpt.6100025
Zeng, Z., Lu, J., Shu, C., Chen, Y., Guo, T., Wu, Q. P., et al. (2015). A comparision of nalbuphine with morphine for analgesic effects and safety : meta-analysis of randomized controlled trials. Sci. Rep. 5, 10927. doi:10.1038/srep10927
Keywords: nalbuphine, fentanyl, analgesic, adenotonsillectomy, children
Citation: Chen F, Wang C-Y, Zhang J, Wang F, Zhang M, Gu H, Song X, Chen J, Li Y, Cai Y-H, Li J, Lian Q-Q, Wu J and Liu H-C (2020) Comparison of Postoperative Analgesic Effects Between Nalbuphine and Fentanyl in Children Undergoing Adenotonsillectomy: A Prospective, Randomized, Double-Blind, Multicenter Study. Front. Pharmacol. 11:597550. doi: 10.3389/fphar.2020.597550
Received: 21 August 2020; Accepted: 16 October 2020;
Published: 09 December 2020.
Edited by:
Venkata Kashyap Yellepeddi, The University of Utah, United StatesReviewed by:
Mellar Pilgrim Davis, Geisinger Health System, United StatesCopyright © 2020 Chen, Wang, Zhang, Wang, Zhang, Gu, Song, Chen, Li, Cai, Li, Lian, Wu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junzheng Wu, SnVuemhlbmcuV3VAY2NobWMub3Jn; Hua-Cheng Liu, aHVhY2hlbmdsaXVAMTYzLmNvbQ==
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.