- 1Department of Mental Disorder Research, National Institute of Neuroscience, National Center of Neurology and Psychiatry, Tokyo, Japan
- 2Medical Genome Center, National Center of Neurology and Psychiatry, Tokyo, Japan
- 3Department of Psychiatry, National Center Hospital, National Center of Neurology and Psychiatry, Tokyo, Japan
- 4Department of Psychiatry, Teikyo University School of Medicine, Tokyo, Japan
Aim: Accumulating evidence suggests that neural inflammation plays an important role in psychiatric disorders. We aimed to identify inflammatory cytokines involved in the pathophysiology of such disorders by quantifying them in cerebrospinal fluid (CSF) samples from a large sample of patients with major psychiatric disorders and healthy controls.
Methods: The subjects included 94 patients with schizophrenia, 68 with bipolar disorder, 104 with major depressive disorder, and 118 healthy controls, matched for age, sex, and ethnicity (Japanese). Lumbar puncture was performed to collect these CSF samples. A multiplex immunoassay was then performed to measure CSF cytokine levels using magnetic on-bead antibody conjugation for 19 inflammatory cytokines.
Results: CSF interferon-β level was significantly higher in total psychiatric patients than in healthy controls (corrected p = 0.000029). In diagnostic group comparisons, CSF interferon-β level was significantly higher in patients with schizophrenia, or bipolar disorder (corrected p = 0.000047 or 0.0034) than in healthy controls.
Conclusion: We present novel evidence that CSF IFN-β level showed prominent statistical differences between psychiatric groups and healthy controls. This suggests IFN-β as the most important player among the 19 cytokines tested here in the inflammation-related pathophysiology of major psychiatric disorders.
Introduction
Inflammation has been suggested to play a key role in the pathogenesis and pathophysiology of psychiatric disorders (Khandaker et al., 2017; Bauer and Teixeira, 2018), including schizophrenia (Horvath and Mirnics, 2014; Na et al., 2014; Muller et al., 2015; Khandaker and Dantzer, 2016; Muller, 2018), bipolar disorder (BD) (Rosenblat and McIntyre, 2015; Walther et al., 2017), and major depressive disorder (MDD) (Sperner-Unterweger et al., 2014; Kunugi et al., 2015; Adzic et al., 2018; Misiak et al., 2018). In the pathophysiology of these disorders, a bidirectional interplay between the brain and the immune system has been suggested (Dantzer, 2018).
Cerebrospinal fluid (CSF) is the optimal biomaterial to examine the molecular status of the central nervous system (CNS) (Veening and Barendregt, 2010; Sakka et al., 2011). Previous meta-analyses have reported altered CSF cytokine levels (e.g., interleukin [IL]-1β, IL-6, and IL-8) in patients with schizophrenia, BD, or MDD (Orlovska-Waast et al., 2018; Wang and Miller, 2018). Our research group has also reported elevations in inflammation-related molecules in the CSF of patients with schizophrenia, BD, or MDD using the enzyme-linked immunosorbent assays (Sasayama et al., 2013; Hattori et al., 2015; Ishii et al., 2018). However, there are many other candidate molecules related to inflammation.
Multiplex immunoassays (Bio-Plex and V-plex®) have been validated for the simultaneous measurement of multiple cytokine levels using blood samples from patients with MDD and healthy control subjects (Belzeaux et al., 2017). The bead-based Luminex® (Bio-Rad Laboratories, Inc.) platform has been used for CSF sample to measure 8 cytokine levels in 43 patients with BD or MDD (Haroon et al., 2016), while the immunoassay-based protein array multiplex system has been used for CSF samples to measure 10 cytokine levels in 30 patients with BD and 30 controls (Soderlund et al., 2011). The electro-chemiluminescence-based V-plex® (MesoScale Discovery) platform has also been used for CSF samples to measure 10 cytokine levels in 23 patients with chronic schizophrenia and 37 controls (Schwieler et al., 2015), 5 cytokine levels in 11 patients with recent-onset schizophrenia and 12 controls (Coughlin et al., 2016), 2 cytokine levels in 19 patients with current depression and 67 subjects without depression among older women (Kern et al., 2014), and 11 cytokine levels in 16 patients with schizophrenia and 15 with affective disorders (Maxeiner et al., 2014).
Although prior studies have conducted multiplex immunoassays to measure CSF cytokine levels in psychiatric disorders (Soderlund et al., 2011; Kern et al., 2014; Maxeiner et al., 2014; Schwieler et al., 2015; Coughlin et al., 2016; Haroon et al., 2016), the highest number of cytokines measured was at once was 11, and sample sizes of patients and controls were not very large (at most 43 and 67, respectively). This warrants to obtain more solid evidence for the role of inflammatory cytokines in the pathophysiology of major psychiatric disorders. We aimed to accomplish simultaneous quantification of a much larger number of cytokines using the bead-based Luminex® in a much larger CSF sample from patients with schizophrenia, BD, or MDD and healthy controls. We hypothesized that a variety of inflammatory changes would be detected in the CSF of patients with psychiatric disorders.
Materials and Methods
Participants
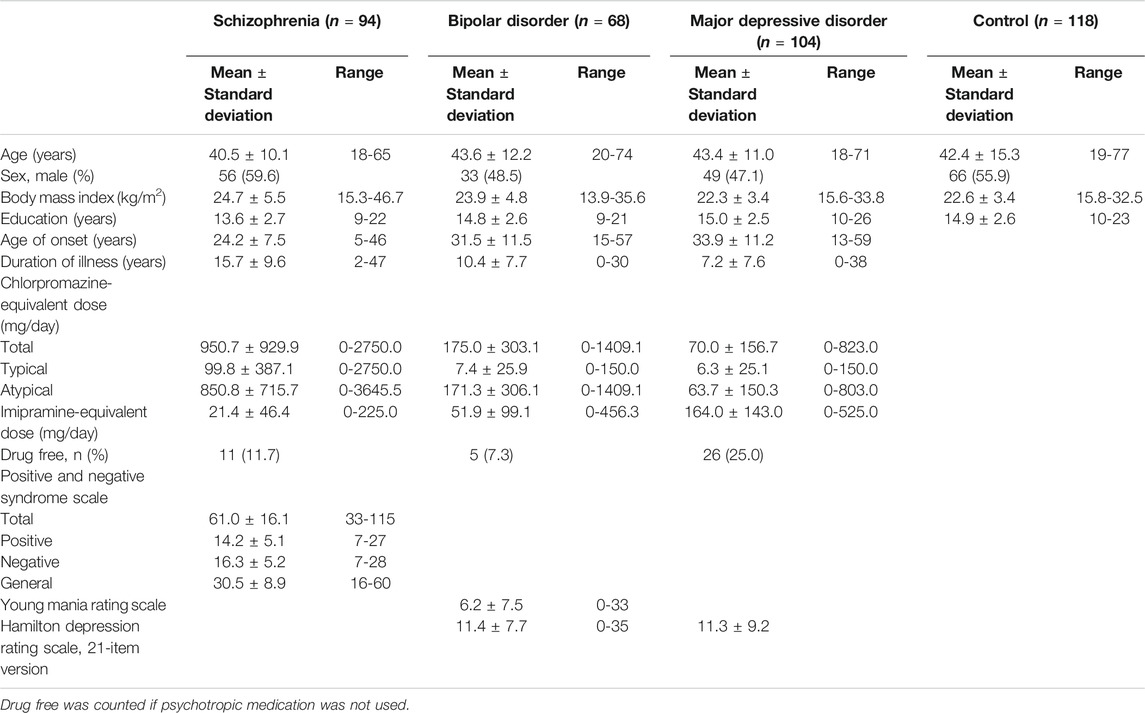
This study involved 94 patients with schizophrenia (mean age: 40.5 ± 10.1 years, 56 males and 38 females), 68 with BD (mean age: 43.6 ± 12.2 years, 33 males and 35 females), 104 with MDD (mean age: 43.4 ± 11.0 years, 49 males and 55 females), and 118 healthy controls (mean age: 42.4 ± 15.3 years, 66 males and 52 females) who were matched for age, sex, and ethnicity (Japanese). The BD group included 22 patients with BD I and 46 with BD II. A total of 384 samples were not based on any power analyses since we have not had data on pre-obtained effect size. All participants were recruited at the National Center of Neurology (NCNP), via advertisements at the NCNP Hospital, on our website, and in local free magazines. Participants were screened for psychiatric disorders by qualified psychiatrists using the Japanese version of the Mini International Neuropsychiatric Interview (Sheehan et al., 1998; Otsubo et al., 2005). Consensus diagnoses were determined according to the criteria laid out in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (American Psychiatric Association, 1994), based on the information from the Mini International Neuropsychiatric Interview, additional unstructured interviews, and medical records, if available. Healthy controls had no history of contact with any psychiatric services. Participants were excluded if they had a medical history of CNS diseases, severe head injury, substance abuse, or mental retardation. After providing them with a description of the study, written informed consent was obtained from every participant. The study protocol was approved by the ethics committee of the NCNP. The study was performed in accordance with the Declaration of Helsinki (World Medical Association, 2013).
Clinical Assessments
The Positive and Negative Syndrome Scale (PANSS) was used to evaluate symptoms in patients with schizophrenia (Kay et al., 1987), and the Young Mania Rating Scale was used to evaluate manic symptoms in patients with BD (Young et al., 1978). The 21-item version of the GRID Hamilton Depression Rating Scale was used to assess depressive symptoms in patients with BD and MDD (Williams et al., 2008). Symptoms were assessed by board-qualified psychiatrists. Daily doses of antipsychotics were converted to chlorpromazine-equivalent doses, and doses of antidepressants were converted to imipramine-equivalent doses according to a published guideline (Inada and Inagaki, 2015). Every patient’s medication status was recorded at the time of the lumbar puncture.
Lumbar Puncture
Lumbar puncture was performed in the left lateral decubitus or a sitting position. Each participant received local anesthesia by lidocaine hydrochloride injection before the puncture. CSF was withdrawn from the L3–L4 or L4–L5 interspace using an atraumatic pencil-point needle (Universe 22 or 23G, 75 mm, Unisis Corp., Tokyo, Japan). The CSF was collected in a low protein absorption tube (PROTEOSAVE SS, 15 ml Conicaltube, Sumitomo Bakelite Co., Tokyo, Japan) and immediately transferred on ice. The CSF was centrifuged (4,000 × g for 10 min) at 4°C and the supernatant was dispended in 0.5-ml aliquots and stored at −80°C in a deep freezer. After a single melting and re-freeze of the sample for the preparation of the 96-well plates, multiplex immunoassays were performed.
Multiplex Immunoassays
CSF protein levels were measured with the MAGPIX CCD imaging system (Bio-Rad Laboratories, Inc.) using magnetic on-bead antibody conjugation for 37 inflammatory cytokines (171AL001M, Bio-Plex Pro™ Human Inflammation Panel 1, 37-Plex, Bio-Rad Laboratories, Inc.) according to the manufacturer's instructions. CSF samples were diluted to 1:2, and 2 standard samples (S9 and S10) were newly created to extend the assay working range on the basis of the results of a verification assay. The assay was performed on the same day using 384 single CSF samples to secure a large number after confirming that the intra-run and inter-run coefficients of variance for the 37 proteins accounted for less than 10% of the variance in the verification assay (intra-run: one set, maximum 9.7%, quadruplicate; inter-run: 16 sets, maximum 5.7%, duplicate). A VIAFLO 96/384 system (INTEGRA Biosciences, Corp.) was used to apply samples simultaneously into the 96-well plates. To adjust the inter-assay variations between the 96-well plates, 13 randomly selected CSF diluted to 1:2, 2 standards (S4 and S10), and one blank were used as margin samples to fit measures of four plates to those of 1 standard plate that included 8 standard dilution (S4–10) and blank samples. Based on the measures of the margin samples, regression equations were calculated for the 37 proteins using 2-dimensional scatter diagrams between the standard and the other four plates for the inter-plate adjustment. Among the 37 assayed proteins, the measurements of 19 cytokines satisfied the following three criteria and were thus deemed reliable: 1) within the assay working range, 2) coefficients accounting for less than 15% median inter-run variance, and 3) strong Pearson’s correlation coefficients (r > 0.70) in the regression equations of the inter-plate adjustment. The assay data for the 18 remaining cytokines (i.e., chitinase3-like 1, gp130/soluble IL-6 receptor β, interferon[IFN]-γ, IL-2, IL-12(p70), IL-20, IL-22, IL-27(p28), IL-28A/IFN-λ1, IL-32, IL-34, IL-35, LIGHT/tumor necrosis factor superfamily (TNFSF) 14, matrix metalloproteinases-1, matrix metalloproteinases-2, osteopontin, pentraxin-3, and tumor necrosis factor-related weak inducer of apoptosis/TNFSF12) did not meet some of the criteria and were thus excluded from the following statistical analyses. CSF cytokine levels are represented as pg/ml.
Statistical Analyses
Categorical and continuous variables were compared between the four diagnostic groups using chi-square tests and analysis of variance (ANOVA), respectively. CSF cytokine levels were compared between patient (both three diagnoses combined and each diagnosis) and control groups and between drug free and non-drug free groups using Mann–Whitney U tests, while effect sizes are shown using r. Kruskal-Wallis tests were used when comparing CSF cytokine levels across the four diagnostic groups. Correlation between CSF cytokine levels and clinical variables were assessed using Pearson’s correlation coefficients, while CSF cytokine levels were compared using unpaired (Student’s or Welch’s) -t tests between sexes. Correlations between CSF cytokine levels and symptom scores or CSF total protein level were assessed using Pearson’s partial correlation coefficient controlled for age, sex, and drug use (only for patients). The correlation matrix for CSF cytokine levels was also assessed with Pearson’s partial correlation coefficient controlled for age, sex, and drug use (only for patients). Bonferroni corrections for multiple testing were applied for 3 ANOVAs for comparison of clinical variables (i.e., age, BMI, and education level) between each diagnostic and control groups (p < 0.05/3 = 0.016), Mann–Whitney U tests, Kruskal-Wallis tests, and correlational analyses on measured 19 CSF cytokine levels (p < 0.05/19 = 0.0026). Hence, corrected p-values were calculated 19 x nominal p-values for group comparisons and correlational analyses on the CSF cytokine levels. All statistical tests were 2-tailed and p < 0.05 was considered significant. Statistical analyses were performed using the Statistical Package for the Social Sciences version 25.0 and 27.0 (IBM Japan, Ltd., Tokyo, Japan).
Results
Association Between Clinical Variables and Cerebrospinal Fluid Cytokine Levels
The clinical characteristics of the participants are shown in Table 1. There were no significant differences in the distributions of age and sex, while body mass index (BMI) and education level were significantly higher and lower, respectively, in patients with schizophrenia than in healthy controls (corrected p = 0.004 and 0.006). There were 11, 5, and 26 drug-free patients with schizophrenia, BD, and MDD, respectively. Correlations between CSF cytokine levels and clinical variables in patients with schizophrenia, BD, or MDD and healthy controls are shown in Supplementary Tables S1–S4, respectively. BMI was significantly and negatively correlated with CSF IL-12(p40) level in patients with schizophrenia (corrected p < 0.05). Age was significantly and positively correlated with CSF soluble CD163 and soluble TNF-receptor one levels, while age of onset was significantly and positively correlated with CSF soluble CD163 level in patients with MDD (corrected p < 0.05).
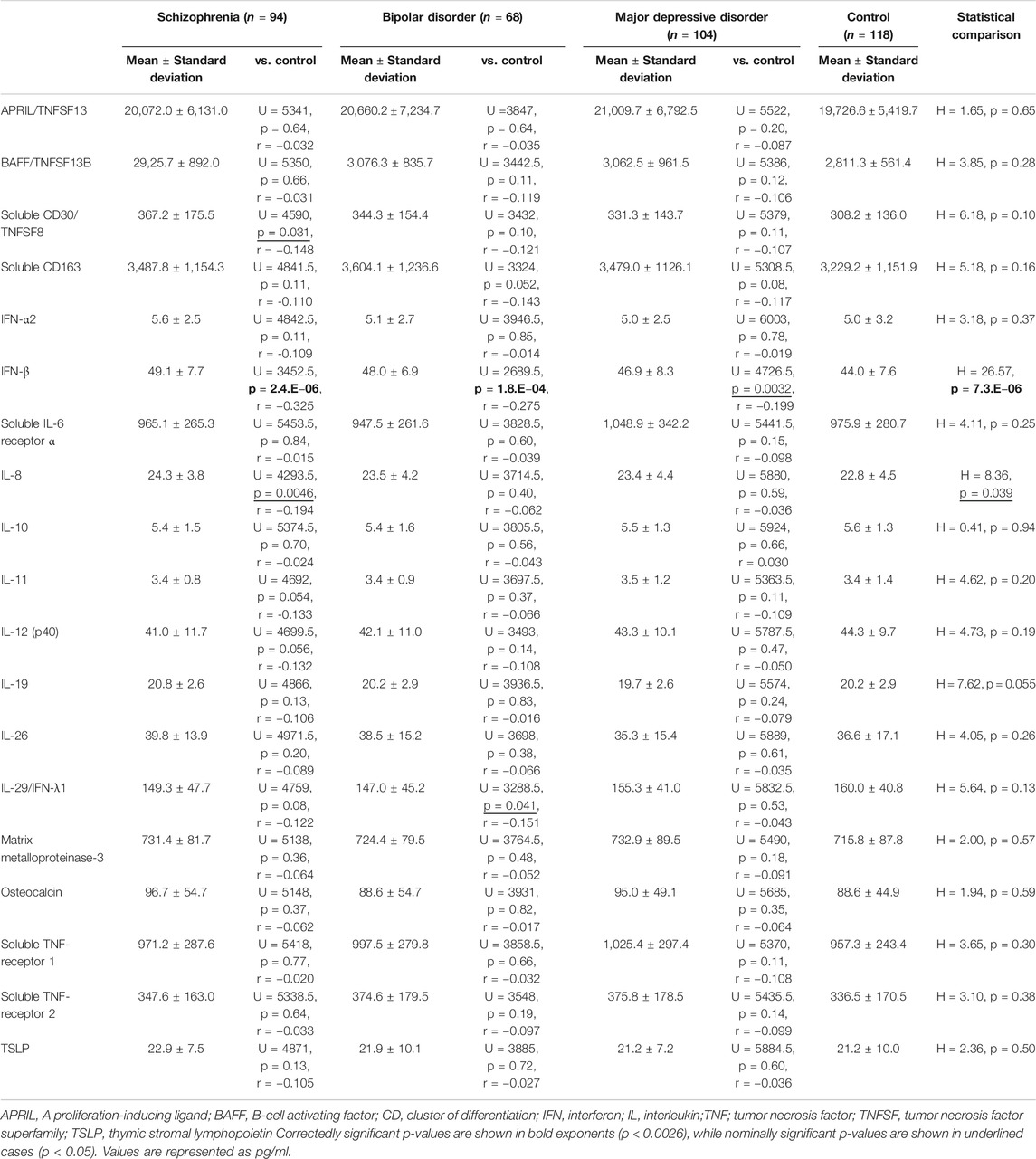
Group Comparisons of Cerebrospinal Fluid Cytokine Levels
Comparisons of CSF cytokine levels between the patient group (3 diagnoses combined; n = 266) and the control group are shown in Table 2. CSF IFN-β level was significantly higher in the psychiatric patients than in the controls (corrected p = 0.000029, Figure 1A). Comparisons across the four diagnostic groups are shown in Table 3. CSF IFN-β level was significantly higher in patients with schizophrenia or BD than in healthy controls (corrected p = 0.000047 or 0.0034, respectively, Figure 1B). Comparisons of CSF cytokine levels between drug free and non-drug free patients with schizophrenia, BD, or MDD are shown in Supplementary Tables S5–S7, respectively. CSF IL-11 level was significantly lower in drug free patients than in non-drug free patients with schizophrenia (corrected p < 0.05).
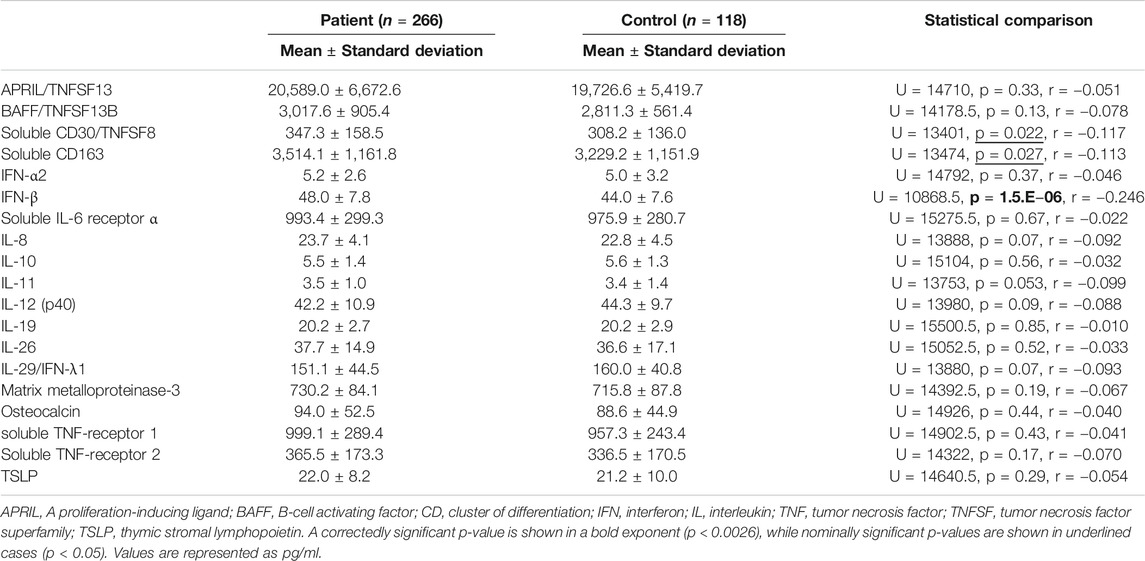
TABLE 2. Comparisons of cerebrospinal fluid cytokine levels between patient (three diagnoses combined) and control groups.
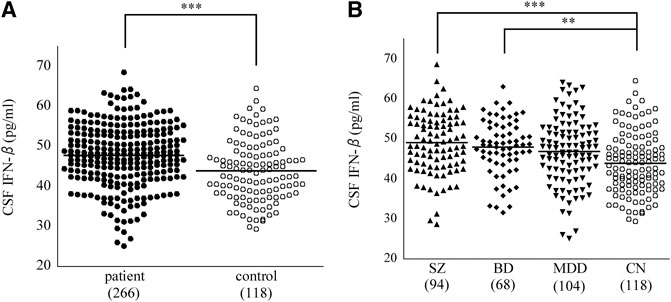
FIGURE 1. Dot plots showing that cerebrospinal fluid (CSF) cytokine interferon (IFN)-β level. CSF IFN-β level was significantly higher in the patient (3 diagnoses combined) group than in the control group (A). CSF IFN-β level was significantly higher in patients with SZ, or BD than in healthy controls (B). Horizontal lines in the dot plots signify mean values, while numbers in parentheses represent those of the participants. **p < 0.01, ***p < 0.001 (corrected). BD, bipolar disorder; CN, control; MDD, major depressive disorder; SZ, schizophrenia.
Correlational Analyses of Cerebrospinal Fluid Cytokine Levels
Correlations between CSF cytokine levels and symptom scores are shown in Supplementary Table S8. Significantly negative correlations between IL-11, IL-29/IFN-λ1, and thymic stromal lymphopoietin (TSLP) levels and PANSS scores were found in patients with schizophrenia (corrected p < 0.05), while no significant correlations were found in patients with BD or MDD. Correlations between CSF cytokine and total protein levels are shown in Supplementary Table 9S. There were no significant correlations in patients with schizophrenia or BD or in healthy controls, while CSF osteocalcin level was significantly and positively corelated with CSF total protein level in patients with MDD (corrected p < 0.05). Correlation matrices for CSF cytokine levels are shown in Supplementary Tables 10S–S13. As expected, many CSF cytokine levels showed significantly positive correlation with each other in patients with schizophrenia, those with BD, those with MDD, and healthy controls (corrected p < 0.05).
Discussion
To our knowledge, this is the largest multiplex immunoassay study in terms of both the number of cytokines measured (n = 19) and the patient (schizophrenia: n = 94, BD: n = 68, MDD: n = 104) and controls (n = 118) sample size. Among the 19 reliably measured cytokines, CSF IFN-β level was higher in psychiatric patients than in healthy controls. Even when comparing across all four diagnostic groups, we found that CSF IFN-β level was higher in patients with schizophrenia and in those with BD, in this order, than in healthy controls. Notably, CSF IFN-α2 and IFN-λ1 levels were not significantly altered. These results suggest that CSF IFN-β could be a useful biomarker for psychiatric disorders.
When all four diagnostic groups were compared with the controls, the elevation in IFN-β level reached the statistical significance even after corrections for multiple testing, suggesting that elevation of IFN-β could be involved in the pathophysiology of schizophrenia. Some earlier studies suggested that IFN-γ, rather than IFN-β, could be a trait maker of schizophrenia; the source, however, included peripheral IFN-γ (Na et al., 2014; Steiner et al., 2014). A meta-analysis (Miller et al., 2011) and a review (Mohammadi et al., 2018) reported CSF cytokine levels in patients with schizophrenia, but did not include data on CSF IFN-β level. Regarding other cytokines, CSF IL-6 level was found to be elevated (Coughlin et al., 2016), while CSF IL-8 level was unaltered in patients with schizophrenia compared to controls (Schwieler et al., 2015) in the V-plex® assays, which, since IL-6 is synonymous to IFN-β2, is consistent with the findings of our study.
CSF IFN-β level was higher in patients with BD than in healthy controls. Although IFN has been suggested as an inflammatory cytokine in patients with BD (Rosenblat and McIntyre, 2015), IFN-β was not identified as a significantly elevated cytokine in a recent systematic review (Knorr et al., 2018). The present study indicates that CSF IFN-β could be involved in the pathophysiology of BD as well. Regarding other cytokines, increased CSF IL-1β and decreased CSF IL-6 levels in patients with BD compared with healthy volunteers were found in a multiplex assay (Soderlund et al., 2011). While IL-1β was not assayed in this study, our finding on IL-6 (i.e., IFN-β2) are in contrast to this earlier study. This inconsistency may be, due at least in part, to the fact that the study of Soderlund et al. (2011) examined euthymic patients with BD. To draw any firm conclusions, further studies in a large sample with the stratification by patients’ state will be necessary.
Although statistical significance could not be obtained (corrected p = 0.061), CSF IFN-β level was tended to be higher in patients with MDD than in healthy controls. In line with this finding, CSF IL-6 (i.e., IFN-β2) level was increased in a geriatric depression group compared with a non-depression group in an earlier V-plex® assay (Kern et al., 2014). Remarkably, exogenous IFN-α and IFN-β used for hepatitis C treatment have been reported to induce depressive states as a side effect (Loftis and Hauser, 2004; Machado et al., 2017); this is partially in accordance with our data, since CSF IFN-α2 and IFN-λ1 levels showed no significant differences between our patients and controls. Two multiplex immunoassays (Luminex® and V-plex®) reported CSF cytokine levels (Maxeiner et al., 2014; Haroon et al., 2016) in patients with affective/mood disorder; however, levels for healthy controls were not reported.
We found, for the first time, that soluble CD163 was elevated in the total psychiatric group, compared with the controls, although this finding was statistically nominal. A previous clinical study reported that CD163-positive perivascular macrophages, which is a feasible origin of soluble CD163, were abundantly detected in the brain of patients with schizophrenia with high inflammation (Cai et al., 2018). Relatedly, a macrophage-derived product neopterin (Oxenkrug, 2011) level were reported to increase in the CSF of patients with psychiatric disorders (Bechter et al., 2010; Kuehne et al., 2013; Ghisoni and Latini, 2015). Our results of increases in CD163 thus imply activation of monocytes-macrophages and may provide additional support for the involvement of inflammation in psychiatric disorders.
Negative correlations were observed between CSF IL-11, IL-29/IFN-λ1, and TSLP levels and PANSS scores in patients with schizophrenia. Unexpectedly, these results suggest that brain inflammation is not positively associated with symptom severity in patients with psychiatric disorders, which is inconsistent with previous reports including animal models (Loftis et al., 2010; Na et al., 2014; Muller et al., 2015; Rosenblat and McIntyre, 2015; Adzic et al., 2018; Misiak et al., 2018; Muller, 2018). Considering the fact that the correlations were sporadic and that significant cytokines that were identified as significant in group comparisons (i.e., IFN-β) were not included, the effects of CSF cytokines on symptom severity in schizophrenia are considerably limited. Taken together with the results of our group comparisons, these findings suggest that IFN-β is related with the pathology, but not with symptom severity, in patients with psychiatric disorders.
No significant correlations were observed between CSF cytokine and total protein levels in patients and controls, despite CSF osteocalcin level showing a positive correlation only in patients with MDD. Although direct relationship between CSF and plasma cytokine levels could not presented in this study, blood-brain barrier dysfunction and CSF total protein elevation have been reported in psychiatric disorders (Orlovska-Waast et al., 2018; Pollak et al., 2018). Since plasma cytokine levels are generally equal or higher than CSF cytokine levels (Maxeiner et al., 2014; Coughlin et al., 2016; Haroon et al., 2016), the absence of a correlation between CSF cytokine and total protein levels may suggest that the origin of most of cytokines measured in this study was central rather than peripheral. This supports a role of central inflammation in the pathology of psychiatric disorders (Na et al., 2014; Anderson and Maes, 2017).
There are following limitations. First, the majority of patients (schizophrenia: 88.3%, BD: 92.7%, MDD: 75.0%) had taken some form of psychotropic medication, although the effects on CSF cytokines were confirmed to be small in statistical comparisons. Second, 19 (51.4%) among the 37 cytokines could only be measured using a Bio-Plex assay kit. Similarly, prior studies have reported that a variety of targeted cytokines cannot be measured with V-plex® (Schwieler et al., 2015; Coughlin et al., 2016) or multiplex (Soderlund et al., 2011) assays. This implies that multiplex immunoassays are not flawless when it comes to measuring, especially if they have been designed for measurements of plasma or other tissues that contain higher levels of target molecules than the CSF. Third, significant biomarkers were rarely reported among limited cytokines measured in this multiplex immunoassay, suggesting that relatively lower number of immunocompetent cells may be involved in the pathology of psychiatric disorders. Otherwise, any prominent biomarkers may exist among cytokines which have not been included in the measurement of this study. Fourth, CSF may not be the best biological sample for seeking cytokine abnormalities in patients with psychiatric disorders. Peripheral metabolic and microbiome alternations (Aizawa et al., 2016; Rosenblat and McIntyre, 2017; Hidese et al., 2018; Horne and Foster, 2018; Kraeuter et al., 2020) may rather be more sensitive than central immunological disturbances to elucidate inflammatory changes in the pathogenesis of psychiatric disorders. Fifth, since general laboratory data about inflammatory status (e.g., CSF or plasma C-reactive protein levels) were not included, this study could not address systemic inflammation in patient groups. Sixth, this study revealed that IFN-β was significantly increased in patients with psychiatric disorders; however, dot plots of patient (both all and each diagnostic) and control groups are widely overlapped. Therefore, IFN-β will not still be used as a biomarker to distinguish ‘psychiatric disease’ from ‘normal population’. Finally, the cross-sectional nature of this study does not allow us to clearly link the etiology of psychiatric disorders to CSF inflammatory cytokines. Further studies are warranted to address the pathogenesis of brain inflammation in patients with psychiatric disorders.
In conclusion, CSF IFN-β level showed most prominent increases in psychiatric groups compared with healthy controls. Our data suggest that IFN-β could be a useful biomarker in the inflammation-related pathophysiology of major psychiatric disorders.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of the NCNP. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SH and KH designed and HK supervised, the study. SH, KH, DS, and MO evaluated the symptoms and determined the diagnoses. SY helped to recruit participants among clinic patients. YY, II, and JM conducted interviews and psychological assessments of the participants. SH, KH, and DS conducted the lumbar punctures to collect CSF samples. KH selected and TM prepared the CSF sample set. RM created and maintained the biobank database system. SH performed laboratory experiments with the assistance of TT. SH performed the statistical analyses and wrote the manuscript, which was revised and approved by all authors.
Funding
This work was supported by Intramural Research Grants for Neurological and Psychiatric Disorders from the NCNP (27-6, 30-1, and 30-7 for KH and HK) and by grants from the Japan Agency for Medical Research and Development (18dk0307062h0003, 18dk0307081h0001, 18dm0107100h0003, and 19ak0101043h0205 for KH and HK). These funding sources were only financially involved in the study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Masashi Hashimoto, Chie Kimizuka, Takahiro Tomizawa, Naoko Ishihara, Tomoko Kuroshio, Moeko Hiraishi, and Chiori Maeda for their assistance in the recruitment of participants, the clinical assessments, and the lumbar punctures. We also thank Yuko Yamaoka, Ayumi Fujisawa, Izumi Sato, Misao Nakano, Yurika Adachi, and Megumi Tatsumi for their sample management. This paper was proofread by a scientific editor at Editage (Tokyo, Japan).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.594394/full#supplementary-material.
Abbreviations
ANOVA, analysis of variance; BD, bipolar disorder; BMI, body mass index; CD, cluster of differentiation; CNS, central nervous system; CSF, cerebrospinal fluid; IFN, interferon; IL, interleukin; MDD, major depressive disorder; NCNP, National Center of Neurology; PANSS, Positive and Negative Syndrome Scale; TNFSF, tumor necrosis factor superfamily; TSLP, thymic stromal lymphopoietin.
References
Adzic, M., Brkic, Z., Mitic, M., Francija, E., Jovicic, M. J., Radulovic, J., et al. (2018). Therapeutic strategies for treatment of inflammation-related depression. Curr. Neuropharmacol. 16, 176–209. doi:10.2174/1570159X15666170828163048
Aizawa, E., Tsuji, H., Asahara, T., Takahashi, T., Teraishi, T., Yoshida, S., et al. (2016). Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 202, 254–257. doi:10.1016/j.jad.2016.05.038
American Psychiatric Association (1994). Diagnostic and statistical manual of mental disorders. 4th Ed. Washington, DC: American Psychiatric Association.
Anderson, G., and Maes, M. (2017). How immune-inflammatory processes link CNS and psychiatric disorders: classification and treatment implications. CNS Neurol. Disord. 16, 266–278. doi:10.2174/1871527315666161122144659
Bauer, M. E., and Teixeira, A. L. (2018). Inflammation in psychiatric disorders: what comes first?. Ann. N. Y. Acad. Sci. 1437 (1), 57–67. doi:10.1111/nyas.13712
Bechter, K., Reiber, H., Herzog, S., Fuchs, D., Tumani, H., and Maxeiner, H. G. (2010). Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J. Psychiatr. Res. 44, 321–330. doi:10.1016/j.jpsychires.2009.08.008
Belzeaux, R., Lefebvre, M. N., Lazzari, A., Le Carpentier, T., Consoloni, J. L., Zendjidjian, X., et al. (2017). How to: measuring blood cytokines in biological psychiatry using commercially available multiplex immunoassays. Psychoneuroendocrinology 75, 72–82. doi:10.1016/j.psyneuen.2016.10.010
Cai, H. Q., Catts, V. S., Webster, M. J., Galletly, C., Liu, D., O’donnell, M., et al. (2018). Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol. Psychiatr. 25 (4), 761–775. doi:10.1038/s41380-018-0235-x
Coughlin, J. M., Wang, Y., Ambinder, E. B., Ward, R. E., Minn, I., Vranesic, M., et al. (2016). In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [(11)C]DPA-713 PET and analysis of CSF and plasma. Transl. Psychiatry 6, e777. doi:10.1038/tp.2016.40
Dantzer, R. (2018). Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol. Rev. 98, 477–504. doi:10.1152/physrev.00039.2016
Ghisoni, K., Latini, A., Kuehne, L. K., Reiber, H., Bechter, K., and Hagberg, L. (2015). Cerebrospinal fluid neopterin is brain-derived and not associated with blood-CSF barrier dysfunction in non-inflammatory affective and schizophrenic spectrum disorders. J. Psychiatr. Res. 63 (Issue 10), 141–142. doi:10.1016/j.jpsychires.2015.02.002
Haroon, E., Fleischer, C. C., Felger, J. C., Chen, X., Woolwine, B. J., Patel, T., et al. (2016). Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol. Psychiatr. 21, 1351–1357. doi:10.1038/mp.2015.206
Hattori, K., Ota, M., Sasayama, D., Yoshida, S., Matsumura, R., Miyakawa, T., et al. (2015). Increased cerebrospinal fluid fibrinogen in major depressive disorder. Sci. Rep. 5, 11412. doi:10.1038/srep11412
Hidese, S., Asano, S., Saito, K., Sasayama, D., and Kunugi, H. (2018). Association of depression with body mass index classification, metabolic disease, and lifestyle: a web-based survey involving 11,876 Japanese people. J. Psychiatr. Res. 102, 23–28. doi:10.1016/j.jpsychires.2018.02.009
Horne, R., and Foster, J. A. (2018). Metabolic and microbiota measures as peripheral biomarkers in major depressive disorder. Front. Psychiatr. 9, 513. doi:10.3389/fpsyt.2018.00513
Horváth, S., and Mirnics, K. (2014). Immune system disturbances in schizophrenia. Biol. Psychiatr. 75, 316–323. doi:10.1016/j.biopsych.2013.06.010
Inada, T., and Inagaki, A. (2015). Psychotropic dose equivalence in Japan. Psychiatr. Clin. Neurosci. 69, 440–447. doi:10.1111/pcn.12275
Ishii, T., Hattori, K., Miyakawa, T., Watanabe, K., Hidese, S., Sasayama, D., et al. (2018). Increased cerebrospinal fluid complement C5 levels in major depressive disorder and schizophrenia. Biochem. Biophys. Res. Commun. 497, 683–688. doi:10.1016/j.bbrc.2018.02.131
Kay, S. R., Fiszbein, A., and Opler, L. A. (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 13, 261–276. doi:10.1093/schbul/13.2.261
Kern, S., Skoog, I., Börjesson-Hanson, A., Blennow, K., Zetterberg, H., Ostling, S., et al. (2014). Higher CSF interleukin-6 and CSF interleukin-8 in current depression in older women. Results from a population-based sample. Brain Behav. Immun. 41, 55–58. doi:10.1016/j.bbi.2014.05.006
Khandaker, G. M., and Dantzer, R. (2016). Is there a role for immune-to-brain communication in schizophrenia?. Psychopharmacology 233, 1559–1573. doi:10.1007/s00213-015-3975-1
Khandaker, G. M., Dantzer, R., and Jones, P. B. (2017). Immunopsychiatry: important facts. Psychol. Med. 47, 2229–2237. doi:10.1017/S0033291717000745
Knorr, U., Simonsen, A. H., Zetterberg, H., Blennow, K., Hasselbalch, S. G., and Kessing, L. V. (2018). Biomarkers in cerebrospinal fluid of patients with bipolar disorder versus healthy individuals: a systematic review. Eur. Neuropsychopharmacol 28, 783–794. doi:10.1016/j.euroneuro.2018.04.002
Kraeuter, A. K., Phillips, R., and Sarnyai, Z. (2020). The gut microbiome in psychosis from mice to men: a systematic review of preclinical and clinical studies. Front. Psychiatr. 11, 799. doi:10.3389/fpsyt.2020.00799
Kuehne, L. K., Reiber, H., Bechter, K., Hagberg, L., and Fuchs, D. (2013). Cerebrospinal fluid neopterin is brain-derived and not associated with blood-CSF barrier dysfunction in non-inflammatory affective and schizophrenic spectrum disorders. J. Psychiatr. Res. 47, 1417–1422. doi:10.1016/j.jpsychires.2013.05.027
Kunugi, H., Hori, H., and Ogawa, S. (2015). Biochemical markers subtyping major depressive disorder. Psychiatr. Clin. Neurosci. 69, 597–608. doi:10.1111/pcn.12299
Loftis, J. M., and Hauser, P. (2004). The phenomenology and treatment of interferon-induced depression. J. Affect. Disord. 82, 175–190. doi:10.1016/j.jad.2004.04.002
Loftis, J. M., Huckans, M., and Morasco, B. J. (2010). Neuroimmune mechanisms of cytokine-induced depression: current theories and novel treatment strategies. Neurobiol. Dis. 37, 519–533. doi:10.1016/j.nbd.2009.11.015
Machado, M. O., Oriolo, G., Bortolato, B., Köhler, C. A., Maes, M., Solmi, M., et al. (2017). Biological mechanisms of depression following treatment with interferon for chronic hepatitis C: a critical systematic review. J. Affect. Disord. 209, 235–245. doi:10.1016/j.jad.2016.11.039
Maxeiner, H. G., Marion Schneider, E., Kurfiss, S. T., Brettschneider, J., Tumani, H., and Bechter, K. (2014). Cerebrospinal fluid and serum cytokine profiling to detect immune control of infectious and inflammatory neurological and psychiatric diseases. Cytokine 69, 62–67. doi:10.1016/j.cyto.2014.05.008
Miller, B. J., Buckley, P., Seabolt, W., Mellor, A., and Kirkpatrick, B. (2011). Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol. Psychiatr. 70, 663–671. doi:10.1016/j.biopsych.2011.04.013
Misiak, B., Beszłej, J. A., Kotowicz, K., Szewczuk-Bogusławska, M., Samochowiec, J., Kucharska-Mazur, J., et al. (2018). Cytokine alterations and cognitive impairment in major depressive disorder: from putative mechanisms to novel treatment targets. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 80, 177–188. doi:10.1016/j.pnpbp.2017.04.021
Mohammadi, A., Rashidi, E., and Amooeian, V. G. (2018). Brain, blood, cerebrospinal fluid, and serum biomarkers in schizophrenia. Psychiatr. Res. 265, 25–38. doi:10.1016/j.psychres.2018.04.036
Müller, N. (2018). Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophr. Bull. 44, 973–982. doi:10.1093/schbul/sby024
Müller, N., Weidinger, E., Leitner, B., and Schwarz, M. J. (2015). The role of inflammation in schizophrenia. Front. Neurosci. 9, 372. doi:10.3389/fnins.2015.00372
Na, K. S., Jung, H. Y., and Kim, Y. K. (2014). The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 48, 277–286. doi:10.1016/j.pnpbp.2012.10.022
Orlovska-Waast, S., Kohler-Forsberg, O., Brix, S. W., Nordentoft, M., Kondziella, D., Krogh, J., et al. (2018). Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol. Psychiatr. 24 (6), 869–887. doi:10.1038/s41380-018-0220-4
Otsubo, T., Tanaka, K., Koda, R., Shinoda, J., Sano, N., Tanaka, S., et al. (2005). Reliability and validity of Japanese version of the mini-international neuropsychiatric interview. Psychiatr. Clin. Neurosci. 59, 517–526. doi:10.1111/j.1440-1819.2005.01408.x
Oxenkrug, G. F. (2011). Interferon-gamma-inducible kynurenines/pteridines inflammation cascade: implications for aging and aging-associated psychiatric and medical disorders. J. Neural. Transm. 118, 75–85. doi:10.1007/s00702-010-0475-7
Pollak, T. A., Drndarski, S., Stone, J. M., David, A. S., Mcguire, P., and Abbott, N. J. (2018). The blood-brain barrier in psychosis. Lancet Psychiatry 5, 79–92. doi:10.1016/S2215-0366(17)30293-6
Rosenblat, J. D., and Mcintyre, R. S. (2015). Are medical comorbid conditions of bipolar disorder due to immune dysfunction?. Acta Psychiatr. Scand. 132, 180–191. doi:10.1111/acps.12414
Rosenblat, J. D., and Mcintyre, R. S. (2017). Bipolar disorder and immune dysfunction: epidemiological findings, proposed pathophysiology and clinical implications. Brain Sci. 7, 144. doi:10.3390/brainsci7110144
Sakka, L., Coll, G., and Chazal, J. (2011). Anatomy and physiology of cerebrospinal fluid. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 128, 309–316. doi:10.1016/j.anorl.2011.03.002
Sasayama, D., Hattori, K., Wakabayashi, C., Teraishi, T., Hori, H., Ota, M., et al. (2013). Increased cerebrospinal fluid interleukin-6 levels in patients with schizophrenia and those with major depressive disorder. J. Psychiatr. Res. 47, 401–406. doi:10.1016/j.jpsychires.2012.12.001
Schwieler, L., Larsson, M. K., Skogh, E., Kegel, M. E., Orhan, F., Abdelmoaty, S., et al. (2015). Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia--significance for activation of the kynurenine pathway. J. Psychiatry Neurosci. 40, 126–133. doi:10.1503/jpn.140126
Sheehan, D. V., Lecrubier, Y., Sheehan, K. H., Amorim, P., Janavs, J., Weiller, E., et al. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatr. 59 (Suppl. 20), 22–57.
Söderlund, J., Olsson, S. K., Samuelsson, M., Walther-Jallow, L., Johansson, C., Erhardt, S., et al. (2011). Elevation of cerebrospinal fluid interleukin-1ss in bipolar disorder. J. Psychiatry Neurosci. 36, 114–118. doi:10.1503/jpn.100080
Sperner-Unterweger, B., Kohl, C., and Fuchs, D. (2014). Immune changes and neurotransmitters: possible interactions in depression?. Prog. Neuro Psychopharmacol. Biol. Psychiatry 48, 268–276. doi:10.1016/j.pnpbp.2012.10.006
Steiner, J., Bernstein, H. G., Schiltz, K., Müller, U. J., Westphal, S., Drexhage, H. A., et al. (2014). Immune system and glucose metabolism interaction in schizophrenia: a chicken-egg dilemma. Prog. Neuro Psychopharmacol. Biol. Psychiatry 48, 287–294. doi:10.1016/j.pnpbp.2012.09.016
Veening, J. G., and Barendregt, H. P. (2010). The regulation of brain states by neuroactive substances distributed via the cerebrospinal fluid; a review. Cerebrospinal Fluid Res. 7, 1. doi:10.1186/1743-8454-7-1
Walther, A., Penz, M., Ijacic, D., and Rice, T. R. (2017). Bipolar spectrum disorders in male youth: the interplay between symptom severity, inflammation, steroid secretion, and body composition. Front. Psychiatr. 8, 207. doi:10.3389/fpsyt.2017.00207
Wang, A. K., and Miller, B. J. (2018). Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr. Bull. 44, 75–83. doi:10.1093/schbul/sbx035
Williams, J. B., Kobak, K. A., Bech, P., Engelhardt, N., Evans, K., Lipsitz, J., et al. (2008). The GRID-HAMD: standardization of the Hamilton depression rating scale. Int. Clin. Psychopharmacol. 23, 120–129. doi:10.1097/YIC.0b013e3282f948f5
World Medical Association (2013). World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J. Am. Med. Assoc. 310, 2191–2194. doi:10.1001/jama.2013.281053
Keywords: bipolar disorder, cerebrospinal fluid, cytokine, major depressive disorder, multiplex, schizophrenia
Citation: Hidese S, Hattori K, Sasayama D, Tsumagari T, Miyakawa T, Matsumura R, Yokota Y, Ishida I, Matsuo J, Yoshida S, Ota M and Kunugi H (2021) Cerebrospinal Fluid Inflammatory Cytokine Levels in Patients With Major Psychiatric Disorders: A Multiplex Immunoassay Study. Front. Pharmacol. 11:594394. doi: 10.3389/fphar.2020.594394
Received: 13 August 2020; Accepted: 29 December 2020;
Published: 01 February 2021.
Edited by:
Mariela Fernanda Perez, National University of Cordoba, ArgentinaReviewed by:
Dietmar Fuchs, Innsbruck Medical University, AustriaAnindya Bhattacharya, Janssen Research and Development, United States
Agustin Montivero, CCT Conicet Córdoba, Argentina
Copyright © 2021 Hidese, Hattori, Sasayama, Tsumagari, Miyakawa, Matsumurra, Yokota, Ishida, Matsuo, Yoshida, Ota and Kunugi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroshi Kunugi, aGt1bnVnaUBuY25wLmdvLmpw
 Shinsuke Hidese
Shinsuke Hidese Kotaro Hattori
Kotaro Hattori Daimei Sasayama1
Daimei Sasayama1 Sumiko Yoshida
Sumiko Yoshida Miho Ota
Miho Ota Hiroshi Kunugi
Hiroshi Kunugi