
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 08 December 2020
Sec. Ethnopharmacology
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.590453
This article is part of the Research Topic Assessing the Pharmacological Effects and Therapeutic Potential of Traditional Chinese Medicine in Neurological Disease Models: An Update View all 18 articles
 Mengmeng Wang1,2†
Mengmeng Wang1,2† Zhuqing Liu1,2†
Zhuqing Liu1,2† Shoushan Hu1,2
Shoushan Hu1,2 Xianchun Duan1
Xianchun Duan1 Yanyan Zhang3
Yanyan Zhang3 Can Peng1,2
Can Peng1,2 Daiyin Peng1,2,4
Daiyin Peng1,2,4 Lan Han1,2*
Lan Han1,2*Objective: Taohong Siwu decoction (THSWD) is one of the classic prescriptions for promoting blood circulation and removing blood stasis, and it has a good therapeutic effect on ischemic stroke. We sought to explore the therapeutic effects of THSWD on pyroptosis in rats with middle cerebral artery occlusion-reperfusion (MCAO/R).
Methods: MCAO/R model of rats were established by suture-occluded method. MCAO/R rats were randomly divided into five groups, which were model group, nimodipine group, THSWD high, medium and low dose group (18, 9, and 4.5 g/kg, respectively), rats of sham group without thread embolus. All rats were treated by intragastric administration for 7 days. We detected the level of inflammatory factors. NLRP3 and Caspase-1 were detected by immunofluorescence. Western blot was used to detect NLRP3, Caspase-1, ASC, and GSDMD in penumbra. Also, the expression of TXNIP, HMGB1, toll-like receptors (TLR4), NF-κB, and MAPK were detected.
Results: THSWD treatment improved the behavioral function and brain pathological damage. These results showed that the levels of TNF-α, TGF-β, IL-2, IL-6, IL-1β, and IL-18 were significantly reduced in THSWD treatment groups. THSWD could significantly decrease the expression levels of NLRP3, Caspase-1, Caspase-1 p10, ASC, TXNIP, GSDMD, HMGB1, TLR4/NFκB, p38 MAPK, and JNK in penumbra.
Conclusion: Our results showed that THSWD could reduce the activation level of NLRP3 inflammatory corpuscle, down-regulate GSDMD, and inhibit pyroptosis in MCAO/R rats. These may be affected by inhibiting HMGB1/TLR4/NFκB, MAPK signaling pathways.
In 2016, The Lancet reported that about 90.7% of strokes were related to 10 risk factors, including hypertension, smoking, dyslipidemia, alcohol intake, unhealthy diet, etc (O’Donnell et al., 2016). Although the mortality rate of stroke has shown a downward trend in China (Wang et al., 2017). However, stroke was an important cause of death and disability at present. The prevalence rate continues to increased, and the affected population has shown a younger trend (Fu et al., 2020). Therefore, the prevention and treatment of stroke was a very serious problem in China.
Mitochondrial dysfunction triggers cascade reactions after ischemia stroke, such as the generation of large amounts of endogenous ROS, inflammation, and autophagy. Inflammatory corpuscle and pyroptosis are important in stroke (Fann et al., 2013; Barrington et al., 2017; Dong et al., 2018). The activated NF-κB pathway up-regulates the gene expression of NLRP3, pro-IL-1β, and pro-IL-18 (Bauernfeind et al., 2009). The production of endogenous ROS and cathepsin B could stimulate self-assembly of NLRP3 inflammatory corpuscle (Chen and Sun, 2013; Latz et al., 2013). NLRP3, ASC, and pro-Caspase-1 have been assembled to form a protein complex, which promoted cleavage to produce activated Caspase-1. GSDMD is cleaved by mature Caspase-1. GSDMD N-terminal domain assembles membrane pores to induce pyroptosis (Shi et al., 2015; Mulvihill et al., 2018). Mature IL-1β and IL-18 are released extracellularly through GSDMD membrane pores (He et al., 2015). At the same time, the contents such as HMGB1 are released to the outside of the cell. HMGB1 binds to the transmembrane receptors RAGE and toll-like receptors (TLR4), and further activates the nuclear transcription factor NF-κB, which further enhances the inflammatory response (Paudel et al., 2019).
Ischemic stroke is considered to be the cerebrovascular disease (Rutten-Jacobs and Rost, 2019). Traditional Chinese medicine has been widely used to treat vascular diseases for nearly 2000 years. Taohong Siwu decoction (THSWD) originated from the traditional Chinese medicine book Yizong Jinjian of the Qing Dynasty, which is composed of Prunus persica (L.) Batsch, Carthamus tinctorius L, Angelica sinensis (Oliv.) Diels, Rehmannia glutinosa (Gaertn.) DC., Ligusticum chuanxiong Hort, Paeonia lactiflora Pall. (Table 1). THSWD has been widely used clinically to treat vascular diseases in the past. Our research showed that THSWD could reduce oxidative stress injury, improve learning and memory function, and promote angiogenesis in ischemic stroke (Han et al., 2015; Chen et al., 2020; Wang M. et al., 2020). It is well known that inflammation and pyroptosis are involved in ischemic stroke injury (Barrington et al., 2017; Dong et al., 2018). There is no reported about the effect of THSWD on pyroptosis. The mechanisms whereby THSWD is of value has not been fully elucidated in the treatment of ischemic stroke. Therefore, we hypothesized that THSWD could reduce pyroptosis in ischemic stroke.
In present study, we studied the effect of THSWD on inflammatory factors in rats with middle cerebral artery occlusion-reperfusion (MCAO/R). More importantly, we further explored the effect of THSWD on pyroptosis. This is more conducive to the research and development of THSWD as a candidate drug for the treatment of stroke.
Tao Ren (Prunus persica (L.) Batsch, 1702181), Hong Hua (Carthamus tinctorius L., 17072135.), Dang Gui (Angelica sinensis (Oliv.) Diels, 1611085), Shu Di Huang (Rehmannia glutinosa (Gaertn.) DC., 1705312), Chuan Xiong (Ligusticum chuanxiong Hort, 17010335), Bai Shao (Paeonia lactiflora Pall., 17110114) were purchased from Bozhou Yonggang Pieces Factory Co., Ltd. (Bozhou, China). They were verified by Qingshan Yang (Anhui University of Chinese Medicine, Hefei, China). Nimodipine (State Food and Drug Administration approval number: H14022821) was purchased from Yabao Pharmaceutical Group CO., Ltd. (Yuncheng, China).
Anti-body (GSDMD:ab219800, Caspase-1:ab1872, Caspase-1 p10:ab179515, HMGB1:ab77302, JNK:ab124956, p38:ab45381, TLR4:ab217274) were purchased from Abcam (Cambridge, MA, United States). Anti-body (ASC:sc-514414, TXNIP:sc-271238) were bought from Santa Cruz Biotechnology (Santa Cruz, CA, United States). Anti-NLRP3 (NBP2-12446) was bought from Novus (Colorado, United States). Anti-NF-κB (bs-0465R) was bought from Bioss (Beijing, China). Anti-GAPDH (19AF0406) was purchased from ZSGB-BIO (Beijing, China). Rat TNF-α, IL-6, IL-1β, and IL-18 Elisa kits (201903) were purchased from Meimian (Jiangsu, China), rat IL-2, and TGF-β Elisa kits (GR2019-03) were purchased from JYM (Wuhan China).
These THSWD were mixed of six herbs in the proportion of Table 1, soaked in 10 times (v/w) 75% ethanol for 2 h, boiled and refluxed for 2 h. Then the filtrate was collected, and the residue was refluxed with 8 times the amount of 75% ethanol for 2 h. The two filtrates were mixed and concentrate to 1.8 g/ml by rotary evaporation. According to the published standard experimental procedure, UPLC was used to ensure the quality and stability of the THSWD (Han et al., 2017; Chen et al., 2020). The assay chromatogram of THSWD of the same batch number and preparation has been published (Chen et al., 2020).
Healthy male Sprague–Dawley rats weighing 270 ± 20 g were provided by the Experimental Animal Center of Anhui Medical University (Hefei, China). All rats were housed in a polypropylene cage (25 ± 5°C, 50–60% relative humidity) under controlled lighting (12 h light/dark cycle), and allowed free access to food and water.
All experimental rats were anesthetized by pentobarbital (50 mg/kg, i.p.). The common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were carefully separated from the middle cervical incision of the rat neck. To ensure the middle cerebral artery (MCA) was occluded, an incision was made in the CCA, and the nylon suture was inserted to about 18–20 mm through the ICA. The nylon suture was a polylysine coated monofilament nylon with a diameter of 0.285 mm. After 2 h of surgery, the nylon suture was withdrawn from MCA and reperfused. After 2 h of surgery, the nylon suture was withdrawn to allow reperfused. The sham rats only performed the same process of separating blood vessels. The temperature was kept at 37°C in the experimental process.
After 24 h of ischemia-reperfusion, the behavioral scores were performed according to the method of Zea Longa, and rats were randomly divided into six groups: Sham, MCAO, THSWD (18, 9 and 4.5 g/kg, respectively, equivalent to the dry weight of the raw materials), nimodipine (20 mg/kg) groups, and treated (i.g.) for 7 days.
On the 7th day after the rats were given treatment, all rats completed the Bederson scores. 0, no observable deficit. 1, forelimb flexion when suspended by the tail. 2, decreased resistance to push. 3, counterclockwise circling. 4, unconsciousness, including death within 24 h.
After killing the rats, brains were isolated and sectioned into five coronal slices in 2 mm thickness. Which were stained with 2,3,5-Triphenyltetrazolium chloride (TTC, 2%, T8170, Solarbio, Beijing, China) for 30 min under dark conditions of 37°C. The coronal slices were taken pictures through digital camera and analyze the infarct volume by Image J (NIH, Bethesda, MD, United States).
The brains were fixed in 4% paraformaldehyde, and paraffin sections were prepared for HE staining (BA-4041, BA-4024, BASO, Zhuhai China). After sealed with neutral resin, and images were captured using an optical microscope (SYZX6061, Nikon, Tokyo, Japan). The histomorphological analysis was evaluated by two examiners blinded to the treatment groups.
Rat penumbra tissues were separated and homogenized with 10 times PBS (v/w) on ice. The homogenates were centrifuged at 4,000 rpm for 10 min at 4°C, and supernatants were collected, then stored in −80°C until future use. The secretion levels of inflammatory cytokines (TNF-α, IL-2, IL-6, TGF-β, IL-1β, IL-18) were analyzed by ELISA. According to the manufacturer’s protocol above in the ELISA kit instructions, the optical density (OD) at 450 nm was measured by enzyme-labeled instrument (Multiskan GO, Thermo, Waltham, MA, United States).
The brain tissues were quickly removed and frozen, and frozen sections were made. The sections were reacted with a primary antibody and subsequently reacted with a fluorescently labeled secondary antibody. Then, which were sealed with a sealer containing a quencher. Two examiners blinded to the treatment groups were observed and photographed using a fluorescence microscope (ECLIPSE TI-SR, Nikon, Tokyo, Japan), and analyzed by ImageJ.
Rat penumbra tissues were separated and homogenized with 10 times ice-cold lysis buffer containing protein inhibitor. The homogenates were centrifuged at 12,000 rpm for 10 min at 4°C, and supernatants were collected. The protein concentration of supernatants were measured by BCA kit (PICPI23223, Thermo, Waltham, MA, United States). In order to denature the protein, the protein samples were added to buffer and boiled for 10 min at 100°C. Equal amounts of proteins were separated by electrophoresis, transferred to NC membranes at low temperatures. After blocking with 5% skimmed milk powder for 2 h, membranes were incubated overnight at 4°C with primary antibodies (anti-NLRP3, anti-ASC, anti-Caspase-1, anti-TXNIP, anti-Caspase-1 p10, anti-GAPDH). The NC membranes were then incubated with the secondary antibody (HRP-conjugated anti-rabbit and anti-mouse secondary antibody, A21010, Abbkine, Wuhan China), and developed with enhanced chemiluminescence (ECL, 32109, Thermo, Waltham, MA, United States).
The data were analyzed with SPSS 23.0 software and expressed as the mean ± SD. Statistical analyses were performed using one-way ANOVA, followed by LSD tests for the significance of the difference between groups. p < 0.05 was considered statistically significant.
As shown in Figure 1B, after 7 days of treatment with THSWD and nimodipine, the behavioral function were significantly improved of MCAO/R rats. Compared with model group, the Berderson scores of THSWD and nimodipine groups were significantly reduced (p < 0.05, p < 0.01). Sham group rats had no behavioral function impairment. TTC staining was used to calculate the infarct volume of rats. The brain of sham group rats had no infarction volume. Compared with model group, the infarct volume were significantly reduced of THSWD and the nimodipine treatment group (Figures 1C,D).
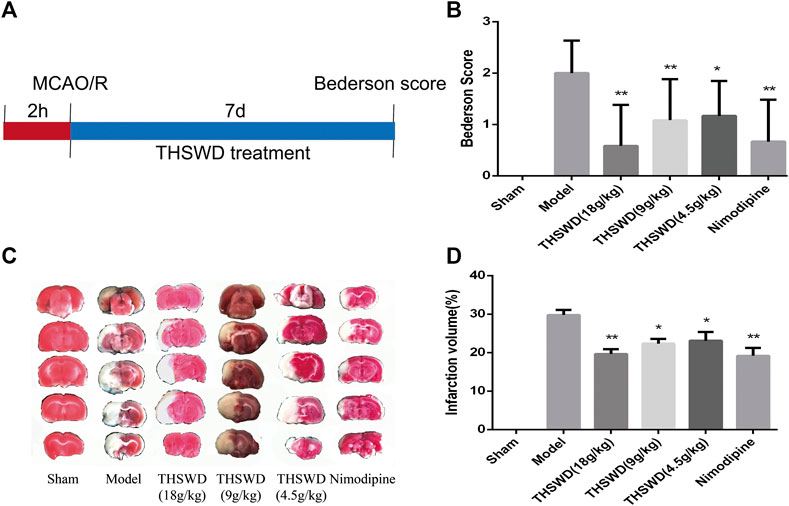
FIGURE 1. Taohong Siwu decoction (THSWD) alleviated middle cerebral artery occlusion-reperfusion (MCAO/R) induced brain damage. (A) The flow diagram of the experiment. (B) Neurological deficits scores. (C) 2,3,5-Triphenyltetrazolium chloride staining of representative sections. (D) Quantification of infarction volume rates. The results were presented as the mean ± SD (n = 6). Compared with sham group, #p < 0.05, ##p < 0.01. Compared with model group, *p < 0.05, **p < 0.01.
The pathological damage of brain tissue was observed by HE staining. The specific results showed in Figure 2. The positive cells were intact and abundant, and no infiltration of inflammatory cells in sham group. The model group showed that the number of positive cells nuclei were significantly reduced, most cells exhibited visible disorder. There were phenomena, such as nuclear shrinkage, nuclear rupture, and inflammatory cells infiltrated. Advantageously, THSWD treatment group significantly alleviated the abnormal phenomena caused by MCAO/R.
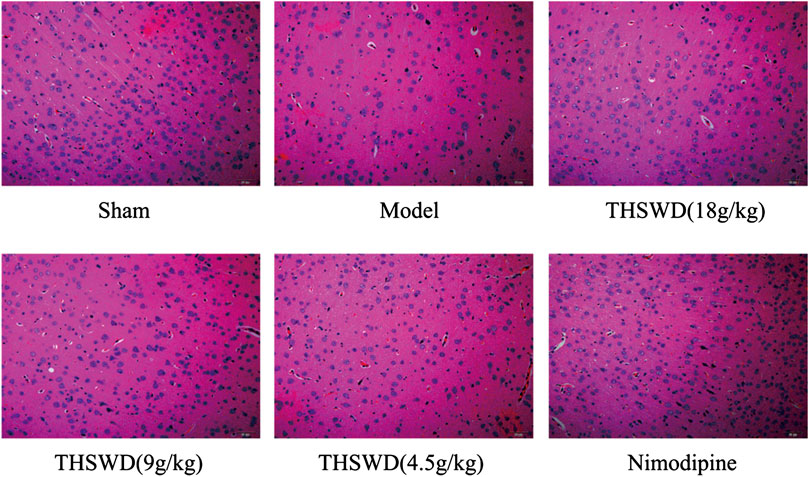
FIGURE 2. Effect of Taohong Siwu decoction (THSWD) on the level of pathological damage in middle cerebral artery occlusion-reperfusion (MCAO/R) rats (×200, n = 3).
Pro-inflammatory factors increased after a stroke. Besides, plenty of inflammatory factors were detrimental to the function of tissues and cells. We detected the levels of inflammatory factors in penumbra by ELISA. Compared with sham group, the levels of TNF-α, IL-2, IL-6, TGF-β, IL-1β, and IL-18 were significantly increased in model group (p < 0.05, p < 0.01). Compared with model group, THSWD and nimodipine treatment groups significantly reduced the level of inflammatory factors (p < 0.05, p < 0.01). These showed that THSWD could attenuate inflammatory response of MCAO/R rats (Figure 3). During the occurrence of pyroptosis, IL-1β and IL-18 were secreted to enhance the inflammatory response. Therefore, we further investigated the effect of THSWD on pyroptosis in MCAO/R rats.
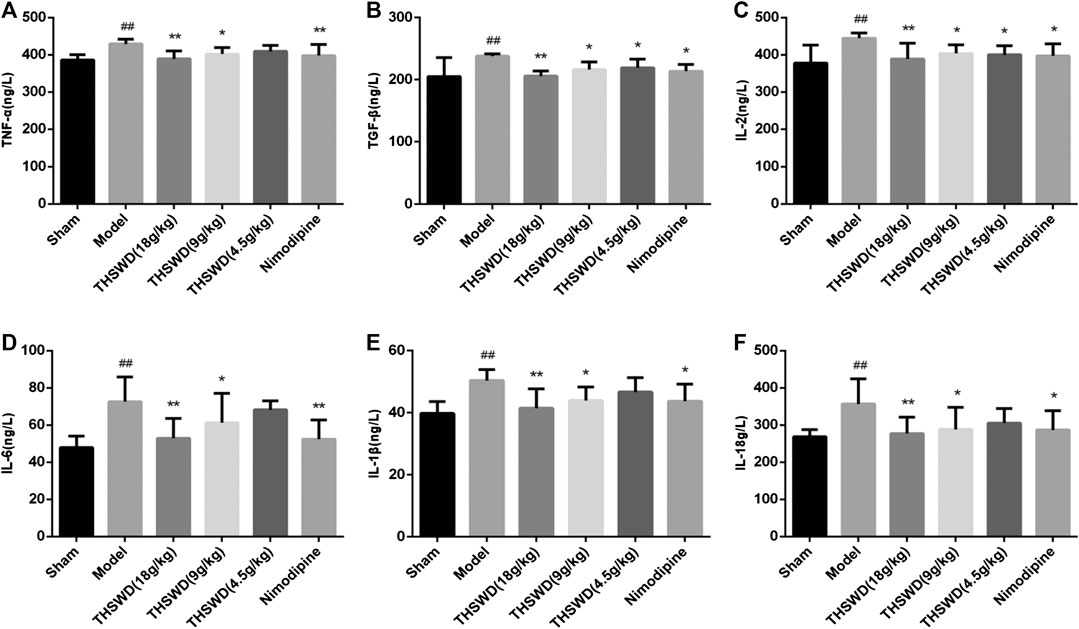
FIGURE 3. Taohong Siwu decoction (THSWD) reduced the levels of inflammatory factors in middle cerebral artery occlusion-reperfusion (MCAO/R) rats. (A) TNF-α, (B) TGF-β, (C) IL-2, (D) IL-6, (E) IL-1β, (F) IL-18. The results were presented as the mean ± SD (n = 6). Compared with sham group, #p < 0.05, ##p < 0.01. Compared with model group, *p < 0.05, **p < 0.01.
Immunofluorescence was used to detect the expression of NLRP3 and Caspase-1 in brain. We observed the picture qualitatively and draw the following preliminary results. As shown in Figure 4, compared with sham group, the fluorescence intensity of NLRP3 and Caspase-1 increased significantly in model group. Compared with model group, the fluorescence intensity of NLRP3 and Caspase-1 decreased in THSWD (18 g/kg) treatment groups. We also advanced quantitative analysis of NLRP3 and Caspase-1 by western blot.
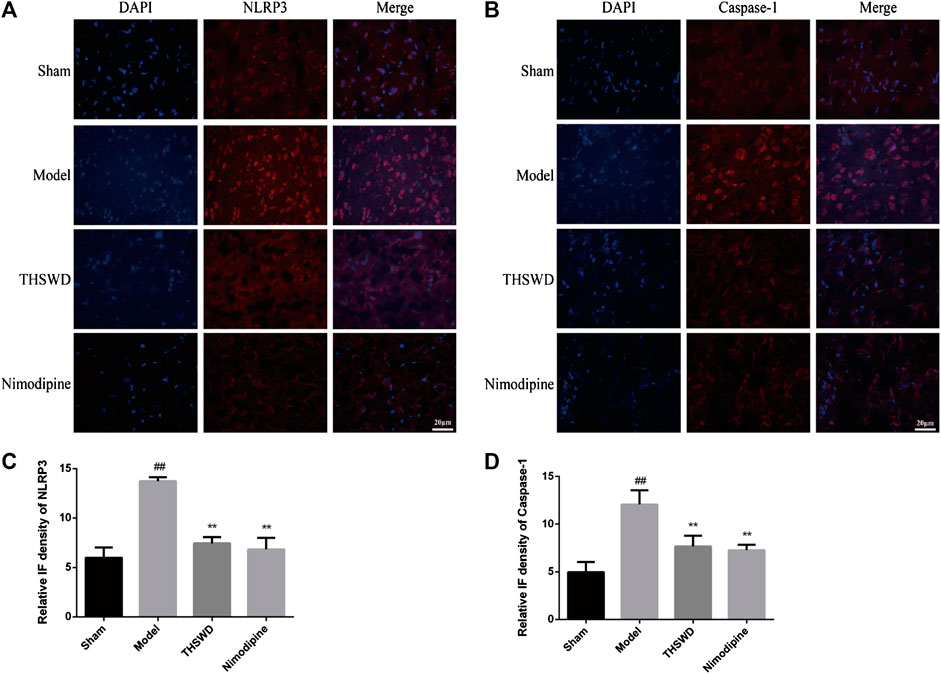
FIGURE 4. Taohong Siwu decoction (THSWD) inhibited NLRP3 and Caspase-1 expression (×400). (A) NLRP3, (B) Caspase-1. The nuclei were stained blue by DAPI, and NLRP3 and Caspase-1 were red. (C,D) Fluorescence intensity analysis of NLRP3 and Caspase-1 staining (n = 3).
We detected the expression levels of NLRP3 inflammatory corpuscle constituent protein and pyroptosis executive protein in penumbra. As shown in Figure 5, these results showed that compared with sham group, the levels of NLRP3, Caspase-1, Caspase-1 p10, ASC, and GSDMD were significantly increased (p < 0.01) in model group. Compared with model group, the levels of NLRP3, Caspase-1, Caspase-1 p10, ASC, and GSDMD were significantly reduced (p < 0.05, p < 0.01) of THSWD and nimodipine treatment groups in penumbra. Our results indicated that THSWD could inhibit the activation of NLRP3 inflammatory corpuscle and inhibit pyroptosis in MCAO/R rats.
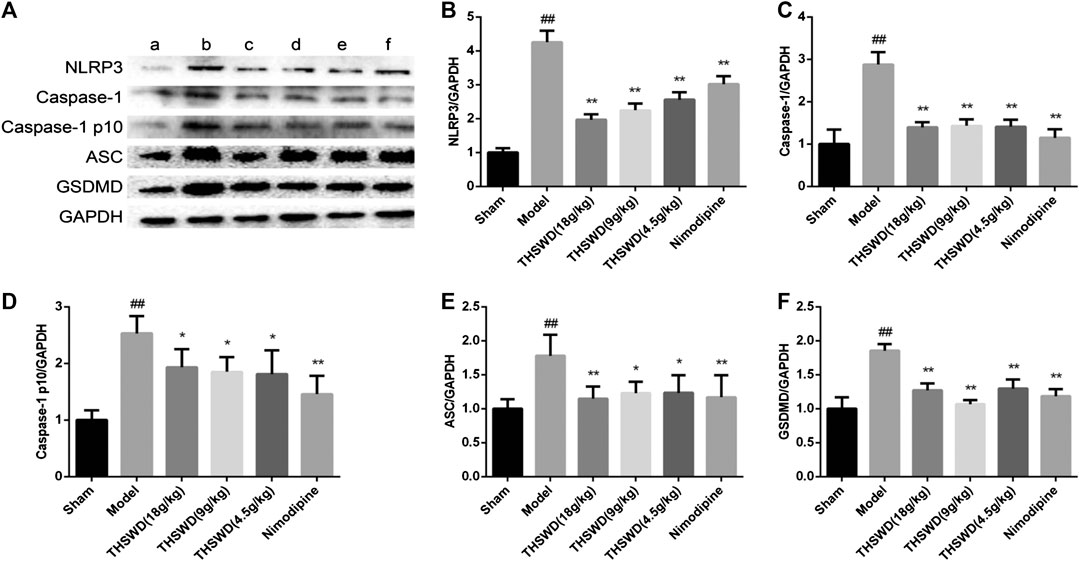
FIGURE 5. Effect of Taohong Siwu decoction (THSWD) on the characteristic protein of pyroptosis in middle cerebral artery occlusion-reperfusion (MCAO/R) rats. (A) Photographs of western blots, (B) NLRP3, (C) Caspase-1, (D) Caspase-1 p10, (E) ASC, (F) GSDMD. a: Sham, b: Model, c: THSWD (18 g/kg), d: THSWD (9 g/kg), e: THSWD (4.5 g/kg), f: nimodipine. The results were presented as the mean ± SD (n = 3). Compared with sham group, #p < 0.05, ##p < 0.01. Compared with model group, *p < 0.05, **p < 0.01.
To explore how THSWD inhibits pyroptosis, western blot was used to detect the signaling pathway in penumbra of the brain. These results showed that compared with sham group, the levels of TXNIP, HMGB1, TLR4 and NF-κB p65 were significantly increased (p < 0.01) in model group. Compared with model group, the levels of TXNIP, HMGB1, TLR4, and NF-κB p65 were significantly reduced (p < 0.05, p < 0.01) in THSWD and nimodipine treatment groups (Figures 6B–D). This indicated that THSWD inhibited pyroptosis through down-regulating the HMGB1-TLR4-NFκB pathway.
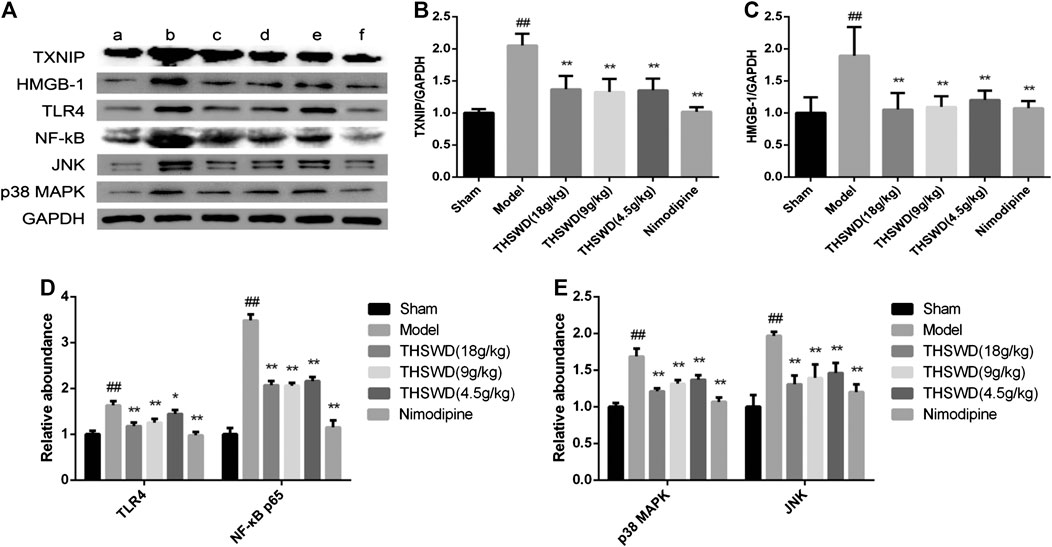
FIGURE 6. Effect of Taohong Siwu decoction (THSWD) on the pathways of regulation of pyroptosis in middle cerebral artery occlusion-reperfusion (MCAO/R) rats. (A) Photographs of western blots, (B) TXNIP, (C) HMGB-1, (D) toll-like receptors-NFκB, (E) MAPK. a: Sham, b: Model, c: THSWD (18 g/kg), d: THSWD (9 g/kg), e: THSWD (4.5 g/kg), f: nimodipine. The results were presented as the mean ± SD (n = 3). Compared with sham group, #p < 0.05, ##p < 0.01. Compared with model group, *p < 0.05, **p < 0.01.
In order to study the effect of THSWD on the MAPK pathway of MCAO/R rats, the expression of JNK and p38 MAPK were detected in penumbra. These results showed compared with sham group, the levels of JNK and p38 MAPK were significantly increased (p < 0.01) in MCAO/R rats. Compared with model group, the levels of JNK and p38 MAPK were significantly reduced (p < 0.05, p < 0.01) in THSWD and nimodipine treatment groups (Figure 6E). This indicated that THSWD inhibited pyroptosis through down-regulating the MAPK pathway.
The inflammatory response is activated due to vascular occlusion in ischemic stroke. Leukocytes are recruited into endothelial cells, which damages the blood-brain barrier (BBB) and a large number of inflammatory mediators are released (Anrather and Iadecola, 2016; De Meyer et al., 2016). Intravascular inflammation is the basis of BBB breakdown and leukocyte invasion. At the same time, the inflammatory cascade process started in brain parenchyma. Microglia are recruited near damaged blood vessels and they are activated quickly (Szalay et al., 2016). Inflammatory factors are released such as IL-1β and TNF-α and fed back into the inflammatory cascade through other immune cells (Anrather and Iadecola, 2016; Szalay et al., 2016). Our previous research has proved that THSWD has a good therapeutic effect on MCAO/R. This study also confirms previous conclusions. In this study, the results showed that THSWD could reduce the levels of inflammatory factors in MCAO/R rats. This provides a positive signal for exploring the role of THSWD in pyroptosis.
Pyroptosis is widely involved in central nervous system diseases. Unlike apoptosis, pyroptosis occurred faster and accompanied by the release of a large number of inflammatory factors. Many studies have shown that almost all N-terminal domains of Gasdermin family proteins could induce pyroptosis. GSDMD is the common substrate for activated Caspase-1 and Caspase-4/5/11 (Wang K. et al., 2020). The domain of GSDMD between N-terminal domain and C-terminal domain is cleaved by activated Caspase. GSDMD N-terminal domain specifically is bound to cardiolipin and phosphoinositide, recruited oligomerization on the plasma membrane to form a membrane pore. GSDMD C-terminal domain could inhibit GSDMD N-terminal domain to maintain GSDMD inhibition state (Liu et al., 2018). NLRP3 inflammatory corpuscle is the most widely studied all inflammatory corpuscles. A large number of studies have shown that NLRP3 is expressed among neurons, endothelial cells, and microglia. TXNIP is an endogenous inhibitor of TRX. After cells are stimulated by inflammatory corpuscle activators (ROS), the oxidized TRX causes TXNIP/TRX decomposition. TXNIP is shuttled to cytoplasmic mitochondria in the ROS-dependent manner, which bound to NLRP3, and activates NLRP3 inflammatory corpuscle (Zhou et al., 2011; Nasoohi et al., 2018). Our direct observation of immunofluorescence results showed that compared with sham group, the expression levels of NLRP3 and Caspase-1 increased in MCAO/R group. Compared with MCAO/R group, the expression of NLRP3 and Caspase-1 decreased in the THSWD (18 g/kg) treatment group. Further research confirmed that THSWD could significantly decrease the expression levels of NLRP3, Caspase-1, GSDMD, TXNIP, ASC. These results proved that pyroptosis is activated by MCAO/R. THSWD could reduce the activation of NLRP3 inflammatory corpuscle and inhibit pyroptosis.
HMGB1 is transferred from the nucleus to the cytoplasm, which is secreted extracellularly by activating inflammatory corpuscles (Vande Walle et al., 2011). Outside the cell, HMGB1 bound to its receptor (TLR2, TLR4, TLR9, RAGE), mediated the production of downstream inflammatory factors and expanded the inflammatory response. Studies have shown that HMGB1 could induce the formation of NLRP3 inflammatory corpuscle through TLRs (Song et al., 2017; Yu et al., 2019). The pro-inflammatory HMGB1-TLR4-NLRP3-GSDMD signal axis could induce Caspase-1 mediated pyroptosis (Dong et al., 2019). Signaling molecules downstream of TLR4/MyD88 pathway include NF-κB, JNK, p38 MAPK, and ERK1/2. JNK and p38 MAPK are mainly activated by various cellular stress signals and pro-inflammatory cytokines (Kim and Choi, 2015). In ischemic stroke, MAPK and NF-κB signaling pathways are key links in the expression and activation of NLRP1 and NLRP3 inflammatory corpuscles (Fann et al., 2018). Their involvement is widely recognized in activating inflammatory corpuscles. In this study, we have demonstrated that HMGB1/TLR4/NFκB and MAPK were activated in the MCAO/R rats. THSWD could inhibit the activation of HMGB1/TLR4/NFκB and MAPK.
Our team conducted the THSWD fingerprint study by UPLC. A total of fifteen compounds were identified. The six compounds were initially compared by standard product, including hydroxysafflor yellow a, 5-hydroxymethyl furfuraldehyde, ferulic acid, ligustilide, amygdalin and paeoniflorin (Han et al., 2017). The assay chromatogram of THSWD of the same batch number and preparation has been published (Chen et al., 2020). Their respective contents of hydroxysafflor yellow A, amygdalin, paeoniflorin, ferulic acid, verbascoside, and ligustilide in THSWD were identified as 0.198, 0.45, 0.602, 0.031, 0.014, and 0.256 mg ml−1 (Chen et al., 2020). Also, our previous report has investigated the major constituents of THSWD by UPLC-Q-TOF-MS. A total of 95 components have been identified, including aromatic acids, flavonoids, polysaccharides, volatile oils, monoterpene glycosides, aromatic cyanoglycosides (Duan et al., 2019). Many published articles have confirmed that they are the basis of THSWD inhibitors of pyroptosis (Liu et al., 2017; Ye et al., 2020; Yin et al., 2020).
In summary, the findings showed that THSWD could significantly reduce the level of inflammatory factors. Additionally, this study demonstrated that pyroptosis is involved in MCAO/R rats. THSWD exerts significant effects on ischemic brain injury through a mechanism closely related to reduce the activation of NLRP3 inflammatory corpuscle and inhibit pyroptosis. These may be achieved by down-regulating the HMGB1-TLR4-NFκB and MAPK pathways. This study is of great significance to verify the main efficacy of traditional Chinese medicine THSWD, as it confirms the mechanism of action of the THSWD on pyroptosis. This work is conducive to the research and development of the stroke candidate drugs of THSWD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The principles of laboratory animal care followed the guiding principles for the care and use of laboratory animals. All experimental procedures were authorized by the Committee on the Ethics of Animal Experiments of Anhui University of Chinese Medicine.
MW, CP, DP, and LH designed and supervised the study. MW and ZL performed the experiments. SH, XD, and YZ analyzed the data. MW and ZL wrote the paper.
This work was supported by grants from the National Natural Science Foundation of China (Nos. 82074152, 81903953, 81503291, and 81473387).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Anrather, J., and Iadecola, C. (2016). Inflammation and stroke: an overview. Neurotherapeutics 13 (4), 661–670. doi:10.1007/s13311-016-0483-x
Barrington, J., Lemarchand, E., and Allan, S. M. (2017). A brain in flame; do inflammasomes and pyroptosis influence stroke pathology? Brain Pathol. 27 (2), 205–212. doi:10.1111/bpa.12476
Bauernfeind, F. G., Horvath, G., Stutz, A., Alnemri, E. S., Macdonald, K., Speert, D., et al. (2009). Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183 (2), 787–791. doi:10.4049/jimmunol.0901363
Chen, F.-F., Wang, M.-M., Xia, W.-W., Peng, D.-Y., and Han, L. (2020). Tao-Hong-Si-Wu decoction promotes angiogenesis after cerebral ischaemia in rats via platelet microparticles. Chin. J. Nat. Med. 18 (8), 620–627. doi:10.1016/s1875-5364(20)30074-1
Chen, S., and Sun, B. (2013). Negative regulation of NLRP3 inflammasome signaling. Protein Cell 4 (4), 251–258. doi:10.1007/s13238-013-2128-8
De Meyer, S. F., Denorme, F., Langhauser, F., Geuss, E., Fluri, F., and Kleinschnitz, C. (2016). Thromboinflammation in stroke brain damage. Stroke 47 (4), 1165–1172. doi:10.1161/strokeaha.115.011238
Dong, W., Zhu, Q., Yang, B., Qin, Q., Wang, Y., Xia, X., et al. (2019). Polychlorinated biphenyl quinone induces caspase 1-mediated pyroptosis through induction of pro-inflammatory HMGB1-TLR4-NLRP3-GSDMD signal axis. Chem. Res. Toxicol. 32 (6), 1051–1057. doi:10.1021/acs.chemrestox.8b00376
Dong, Z., Pan, K., Pan, J., Peng, Q., and Wang, Y. (2018). The possibility and molecular mechanisms of cell pyroptosis after cerebral ischemia. Neurosci. Bull. 34 (6), 1131–1136. doi:10.1007/s12264-018-0294-7
Duan, X., Pan, L., Bao, Q., and Peng, D. (2019). UPLC-Q-TOF-MS study of the mechanism of THSWD for breast cancer treatment. Front. Pharmacol. 10, 1625. doi:10.3389/fphar.2019.01625
Fann, D. Y., Lee, S. Y., Manzanero, S., Tang, S. C., Gelderblom, M., Chunduri, P., et al. (2013). Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis. 4, e790. doi:10.1038/cddis.2013.326
Fann, D. Y.-W., Lim, Y.-A., Cheng, Y.-L., Lok, K.-Z., Chunduri, P., Baik, S.-H., et al. (2018). Evidence that NF-κB and MAPK signaling promotes NLRP inflammasome activation in neurons following ischemic stroke. Mol. Neurobiol. 55 (2), 1082–1096. doi:10.1007/s12035-017-0394-9
Fu, H., Zhang, D., Zhu, R., Cui, L., Qiu, L., Lin, S., et al. (2020). Association between lipoprotein(a) concentration and the risk of stroke in the Chinese Han population: a retrospective case-control study. Ann. Transl. Med. 8 (5), 212. doi:10.21037/atm.2020.01.38
Han, L., Ji, Z., Chen, W., Yin, D., Xu, F., Li, S., et al. (2015). Protective effects of tao-Hong-si-Wu decoction on memory impairment and hippocampal damage in animal model of vascular dementia. Evid Based Compl. Alternat. Med. 2015, 195835. doi:10.1155/2015/195835
Han, L., Qiao, O., Wu, H., Wu, S., Zhang, Y., Yao, L., et al. (2017). Chromatographic fingerprint analysis is feasible for comprehensive quality control of Taohongsiwu. Int. J. Pharmacol. 13 (5), 488–494. doi:10.3923/ijp.2017.488.494
He, W.-T., Wan, H., Hu, L., Chen, P., Wang, X., Huang, Z., et al. (2015). Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 25 (12), 1285–1298. doi:10.1038/cr.2015.139
Kim, E. K., and Choi, E.-J. (2015). Compromised MAPK signaling in human diseases: an update. Arch. Toxicol. 89 (6), 867–882. doi:10.1007/s00204-015-1472-2
Latz, E., Xiao, T. S., and Stutz, A. (2013). Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13 (6), 397–411. doi:10.1038/nri3452
Liu, Y.-M., Shen, J.-D., Xu, L.-P., Li, H.-B., Li, Y.-C., and Yi, L.-T. (2017). Ferulic acid inhibits neuro-inflammation in mice exposed to chronic unpredictable mild stress. Int. Immunopharm. 45, 128–134. doi:10.1016/j.intimp.2017.02.007
Liu, Z., Wang, C., Rathkey, J. K., Yang, J., Dubyak, G. R., Abbott, D. W., et al. (2018). Structures of the gasdermin D C-terminal domains reveal mechanisms of autoinhibition. Structure 26 (5), 778.e773–784.e773. doi:10.1016/j.str.2018.03.002
Mulvihill, E., Sborgi, L., Mari, S. A., Pfreundschuh, M., Hiller, S., and Müller, D. J. (2018). Mechanism of membrane pore formation by human gasdermin‐D. EMBO J. 37 (14), e98321. doi:10.15252/embj.201798321
Nasoohi, S., Ismael, S., and Ishrat, T. (2018). Thioredoxin-interacting protein (TXNIP) in cerebrovascular and neurodegenerative diseases: regulation and implication. Mol. Neurobiol. 55 (10), 7900–7920. doi:10.1007/s12035-018-0917-z
O’donnell, M. J., Chin, S. L., Rangarajan, S., Xavier, D., Liu, L., Zhang, H., et al. (2016). Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet 388 (10046), 761–775. doi:10.1016/s0140-6736(16)30506-2
Paudel, Y. N., Angelopoulou, E., Piperi, C., Balasubramaniam, V. R. M. T., Othman, I., and Shaikh, M. F. (2019). Enlightening the role of high mobility group box 1 (HMGB1) in inflammation: updates on receptor signalling. Eur. J. Pharmacol. 858, 172487. doi:10.1016/j.ejphar.2019.172487
Rutten-Jacobs, L. C. A., and Rost, N. S. (2020). Emerging insights from the genetics of cerebral small-vessel disease. Ann. N. Y. Acad. Sci. 1471 (1), 5–17. doi:10.1111/nyas.13998
Shi, J., Zhao, Y., Wang, K., Shi, X., Wang, Y., Huang, H., et al. (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526 (7575), 660–665. doi:10.1038/nature15514
Song, E., Jahng, J. W., Chong, L. P., Sung, H. K., Han, M., Luo, C., et al. (2017). Lipocalin-2 induces NLRP3 inflammasome activation via HMGB1 induced TLR4 signaling in heart tissue of mice under pressure overload challenge. Am. J. Transl. Res. 9 (6), 2723–2735.
Szalay, G., Martinecz, B., Lenart, N., Kornyei, Z., Orsolits, B., Judak, L., et al. (2016). Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat. Commun. 7, 11499. doi:10.1038/ncomms11499
Vande Walle, L., Kanneganti, T.-D., and Lamkanfi, M. (2011). HMGB1 release by inflammasomes. Virulence 2 (2), 162–165. doi:10.4161/viru.2.2.15480
Wang, K., Sun, Q., Zhong, X., Zeng, M., Zeng, H., Shi, X., et al. (2020). Structural mechanism for GSDMD targeting by autoprocessed Caspases in pyroptosis. Cell 180 (5), 941–955. doi:10.1016/j.cell.2020.02.002
Wang, M., Wang, F., Peng, D., Duan, X., Chen, W., Xu, F., et al. (2020). Tao-Hong Si-Wu decoction alleviates cerebral ischemic damage in rats by improving anti-oxidant and inhibiting apoptosis pathway. Int. J. Pharmacol. 16 (3), 214–222. doi:10.3923/ijp.2020.214.222
Wang, W., Wang, D., Liu, H., Sun, H., Jiang, B., Ru, X., et al. (2017). Trend of declining stroke mortality in China: reasons and analysis. Stroke Vasc Neurol. 2 (3), 132–139. doi:10.1136/svn-2017-000098
Ye, J.-X., Wang, M., Wang, R.-Y., Liu, H.-T., Qi, Y.-D., Fu, J.-H., et al. (2020). Hydroxysafflor yellow A inhibits hypoxia/reoxygenation-induced cardiomyocyte injury via regulating the AMPK/NLRP3 inflammasome pathway. Int. Immunopharm. 82, 106316. doi:10.1016/j.intimp.2020.106316
Yin, N., Gao, Q., Tao, W., Chen, J., Bi, J., Ding, F., et al. (2020). Paeoniflorin relieves LPS‐induced inflammatory pain in mice by inhibiting NLRP3 inflammasome activation via transient receptor potential vanilloid 1. J. Leukoc. Biol. 108 (1), 229–241. doi:10.1002/jlb.3ma0220-355r
Yu, R., Jiang, S., Tao, Y., Li, P., Yin, J., and Zhou, Q. (2019). Inhibition of HMGB1 improves necrotizing enterocolitis by inhibiting NLRP3 via TLR4 and NF‐κB signaling pathways. J. Cell. Physiol. 234 (8), 13431–13438. doi:10.1002/jcp.28022
Keywords: MAPK, HMGB1/toll-like receptors/NFκB, pyroptosis, ischemic stroke, Taohong Siwu decoction
Citation: Wang M, Liu Z, Hu S, Duan X, Zhang Y, Peng C, Peng D and Han L (2020) Taohong Siwu Decoction Ameliorates Ischemic Stroke Injury Via Suppressing Pyroptosis. Front. Pharmacol. 11:590453. doi: 10.3389/fphar.2020.590453
Received: 01 August 2020; Accepted: 28 October 2020;
Published: 08 December 2020.
Edited by:
Jiahong Lu, University of Macau, ChinaReviewed by:
Chun Yang, Nanjing Medical University, ChinaCopyright © 2020 Wang, Liu, Hu, Duan, Zhang, Peng, Peng and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lan Han, aGFubGFuNTZAYWh0Y20uZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.