- 1Unit of Pharmacology, Faculty of Medicine and Defence Health, Universiti Pertahanan Nasional Malaysia (National Defence University of Malaysia), Kuala Lumpur, Malaysia
- 2Department of Periodontology and Implantology, Karnavati University, Gandhinagar, India
- 3Department of Pharmacology, All India Institute of Medical Sciences, Jodhpur, India
- 4Department of Pediatric Dentistry, Karnavati University, Gandhinagar, India
- 5Department of Microbiology, Jahangirnagar University, Savar, Bangladesh
- 6Department of Conservative Dentistry and Endodontics, Rajasthan University of Health Sciences, Jaipur, India
- 7Pharmacy Department, Ghana Health Service, Keta Municipal Hospital, Keta-Dzelukope, Ghana
- 8Pharmacy Practice Department, School of Pharmacy, University of Health and Allied Sciences, Volta Region, Ghana
- 9Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow, United Kingdom
- 10Department of Pharmacology, College of Pharmacy, Hawler Medical University, Erbil, Iraq
- 11Independent Consumer Advocate, Brunswick, VIC, Australia
- 12Division of Clinical Pharmacology, Karolinska Institute, Karolinska University Hospital Huddinge, Stockholm, Sweden
- 13School of Pharmacy, Sefako Makgatho Health Sciences University, Ga-Rankuwa, South Africa
- 14School of Pharmaceutical Sciences, Universiti Sains Malaysia, Penang, Malaysia
Background: COVID-19 has already claimed a considerable number of lives worldwide. However, there are concerns with treatment recommendations given the extent of conflicting results with suggested treatments and misinformation, some of which has resulted in increased prices and shortages alongside increasing use and prices of personal protective equipment (PPE). This is a concern in countries such as India where there have been high patient co-payments and an appreciable number of families going into poverty when members become ill. However, balanced against pricing controls. Community pharmacists play a significant role in disease management in India, and this will remain. Consequently, there is a need to review prices and availability of pertinent medicines during the early stages of the COVID-19 pandemic in India to provide future direction.
Objective: Assess current utilisation and price changes as well as shortages of pertinent medicines and equipment during the early stages of the pandemic.
Our Approach: Multiple approach involving a review of treatments and ongoing activities across India to reduce the spread of the virus alongside questioning pharmacies in selected cities from early March to end May 2020.
Our Activities: 111 pharmacies took part, giving a response rate of 80%. Encouragingly, no change in utilisation of antimalarial medicines in 45% of pharmacies despite endorsements and for antibiotics in 57.7% of pharmacies, helped by increasing need for a prescription for dispensing. In addition, increased purchasing of PPE (over 98%). No price increases were seen for antimalarials and antibiotics in 83.8 and 91.9% of pharmacies respectively although shortages were seen for antimalarials in 70.3% of pharmacies, lower for antibiotics (9.9% of pharmacies). However, price increases were typically seen for PPE (over 90% of stores) as well as for analgesics (over 50% of pharmacies). Shortages were also seen for PPE (88.3%).
Conclusion: The pandemic has impacted on utilisation and prices of pertinent medicines and PPE in India but moderated by increased scrutiny. Key stakeholder groups can play a role with enhancing evidenced-based approaches and reducing inappropriate purchasing in the future.
Introduction/Background
Healthcare System in India and Role of Community Pharmacists
Until recently, healthcare in India has largely been funded through out-of-pocket payments, with payments relatively stagnant at between 69% to over 75% of total healthcare expenditure (Kastor and Mohanty, 2018; Selvaraj et al., 2018; Sood and Wagner, 2020). The cost of medicines accounted for an appreciable proportion of this at over 60% of total expenditure and over 70% of out-of-pocket expenditures (Selvaraj et al., 2018; Satheesh et al., 2019), which is similar to other lower- and middle-income countries (LMICs) (Cameron et al., 2009). These out-of-pocket expenses have potentially catastrophic consequences if family members become ill, exacerbated by an increase in non-communicable diseases (NCDs) in India in recent years. As a result, up to 39 million people are pushed into poverty each year in India due to healthcare payments (Garg and Karan, 2009; Kastor and Mohanty, 2018; Reddy, 2018; Selvaraj et al., 2018). This is beginning to change with ongoing reforms to provide health insurance coverage for up to 100 million families in poverty (Reddy, 2018; Sood and Wagner, 2020); however, this will take time in view of a number of challenges including patient enrollment, agreeing the future role of physicians, necessary strengthening of regulatory systems and increasing the percentage of gross domestic product (GDP) spent on health (Reddy, 2018; Editorial, 2019; Sood and Wagner, 2020). These reforms will also involve improving healthcare delivery in rural areas to address current inadequacies, for instance through the instigation of 150,000 health and wellness centers as healthcare facilities (Kasthuri, 2018; Sood and Wagner, 2020).
In view of the contribution of the costs of medicines to total healthcare costs in India, coupled with existing high co-payments for visiting physicians and funding medicines, community pharmacies have and will continue to play an appreciable role in managing patients in India (Abdulsalim et al., 2018; Daftary et al., 2019; Nafade et al., 2019; Abdulsalim et al., 2020; Sousa Pinto et al., 2020). Key reasons include avoiding paying physician fees, especially for milder symptoms, accessibility with long opening hours, and a lack of queues compared with ambulatory care clinics (Soumya et al., 2016; Mohathasim Billah and Venkatesan, 2017; Daftary et al., 2019). As a result, community pharmacists in India are the first point of healthcare professional contact for up to 40% of patients with tuberculosis (TB) symptoms and an appreciable number of patients continue to seek their advice after diagnosis (Daftary et al., 2019).
Overall, community pharmacists in India play a crucial role in optimizing the use of medicines and improving patient outcomes whilst seeking to prevent the misuse of medicines (Mohathasim Billah and Venkatesan, 2017; Abdulsalim et al., 2018). This is similar to their roles in other countries where they are often the first healthcare professional patients consult regarding their illness, and they are increasingly involved in the management of chronic and other diseases (Khanal et al., 2016; Markovic-Pekovic et al., 2017; Akutey et al., 2018; Sousa Pinto et al., 2020). The cost of medicines to patients in India have been helped in recent years by ongoing reforms since 2013 to fix prices for essential medicines as well as encourage the prescribing of generic medicines where possible (HealthWorld, 2016; BioVoice, 2018; Pavithra, 2019). However, concerns have been raised about the knowledge of pharmacists regarding pharmaceutical care, the actual extent of pharmaceutical care activities undertaken in practice, and the continued self-purchasing of antibiotics that exists despite legislation, which along with increased utilisation rates of antibiotics in recent years has led to increased antimicrobial resistance (AMR) (Satheesh et al., 2019; Nafade et al., 2019; Farooqui et al., 2018; pal Jeyamani et al., 2018).
Reforms are also needed in India regarding the management of NCDs, with NCDs now the leading cause of death in India. NCDs accounted for 60% of all deaths in India in 2014 alongside continued high rates of infectious diseases including TB and growing AMR rates (Farooqui et al., 2018; Kastor and Mohanty, 2018; Daftary et al., 2019). The growing burden of NCDs including cardiovascular disease (CVD), hypertension, and diabetes, coupled with the associated need for medicines, will appreciably increase healthcare costs unless addressed, alongside increasing expenditure on antimicrobials with increasing utilisation (Farooqui et al., 2018; India State-Level Disease Burden Initiative CVDC, 2018; India State-Level Disease Burden Initiative Diabetes C, 2018; Ramakrishnan et al., 2019; Vijayakumar et al., 2019).
It is likely therefore that any Government activities to transfer funds and personnel to tackle COVID-19 will impact on planned activities to reduce the growing burden of NCDs in India as well as any planned activities surrounding other infectious diseases including reducing AMR rates. Consequently, existing healthcare needs and activities need careful monitoring to reduce the extent any unintended consequences arising from the pandemic.
COVID-19 and Risk Factors
COVID-19 was first identified Wuhan in China in December 2019, quickly spreading to all continents (Kumar et al., 2020; Li et al., 2020; Wu and McGoogan, 2020). By September 27, 2020 there were 32.73 million cases with over 990,000 deaths worldwide, giving a case fatality ratio (CFR) among confirmed cases of 3.03% (WHO, 2020a). This included over 6.72 million confirmed cases in the WHO South East Asian Region including India, with over 110,000 deaths, giving a CFR of 1.65% (WHO, 2020a). It is recognised that there has been appreciable under-reporting of prevalence rates and deaths in a number of South East Asian countries with a lack of testing capabilities, particularly at the beginning (Anwar et al., 2020; Associated Press, 2020; Lee, 2020).
COVID-19 is transmitted from person to person principally through respiratory droplets and aerosol transmission, alongside direct contact with contaminated surfaces, forming the basis of preventative measures (Anderson et al., 2020; Haque, 2020; Klompas et al., 2020; Ng et al., 2020; Pradhan et al., 2020; WHO, 2020b; World Health Organisation, 2020). Increased morbidity and mortality from COVID-19 appears to be associated with a number of underlying health conditions including CVD, hypertension, diabetes, chronic obstructive pulmonary disease (COPD), shortness of breath, smoking and blood types (Aghagoli et al., 2020; Alqahtani et al., 2020; Ellinghaus et al., 2020; Huang et al., 2020; Richardson et al., 2020; Vardavas and Nikitara, 2020; Zhao et al., 2020; Zheng et al., 2020). Ethnicity may also be important with patients in the United Kingdom of Indian origin at appreciably increased risk of dying from COVID-19 vs. those of white ethnicity (Khunti et al., 2020; Kirby, 2020; Public Health England, 2020; Sonwalkar, 2020). Smoking is also an issue in India with high rates particularly among men (Mishra et al., 2016; Mohan et al., 2018; WHO, 2018); however, rates appear to be decreasing following the instigation of the WHO’s framework convention on tobacco control and other measures, which is encouraging (Suliankatchi Abdulkader et al., 2019).
Response to COVID-19 and Concerns in India
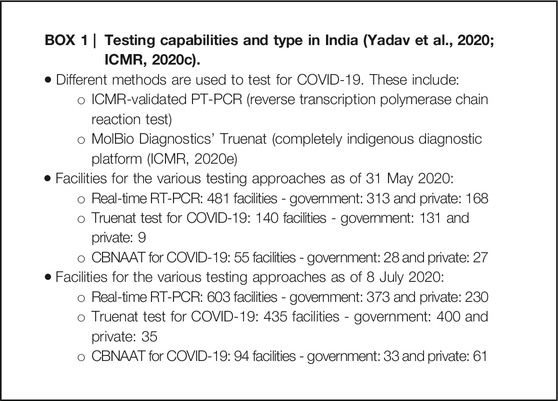
The first reported cases in India for COVID-19 were in Kerala, on January 30, 2020 (Kamath et al., 2020; Kumar et al., 2020; WHO India, 2020a). As of September 27, 2020, 5.992 million confirmed cases and over 94,000 deaths have been reported in India due to COVID-19, the highest rates in the WHO South East Asia region and giving a CFR of 1.58% (WHO, 2020a). This is likely to be an underestimate though with lack of testing and capacity issues certainly during the early stages of the pandemic until the number of testing facilities appreciably increased with the help of the private sector Box 1, exacerbated in some cases by patients having to cover the high costs of tests themselves although this is now changing (Alluri and Pathi, 2020; Associated Press, 2020). By late March, there were only 18 tests being undertaken per million population in India vs. 6,931 per million in South Korea (Kamath et al., 2020). By 25 April, over 5.79 million tests had been undertaken from data supplied by the Indian Council of Medical Research (ICMR) (Pai et al., 2020). Other reports suggest a cumulative total of 3.8 million tests by June 1, 2020 also citing ICMR. The testing rates at the end of May/beginning of June were still only 0.08 per 1,000 people in India vs. 1.02 in Italy and 1.16 in the USA (Yadav et al., 2020). Since then, with the help of an appreciable increase in the number of testing facilities and increased production of testing equipment (ICMR, 2020a; Yadav et al., 2020), the cumulative number of tests as of July 8, 2020 was over 110 million with 1,132 laboratories now involved with testing throughout India (ICMR, 2020b; ICMR, 2020c). ICMR has also recently approved the use of a point-of-care rapid antigen test to aid early detection of COVID-19 to further enhance the numbers tested to aid the “test-track-treat” strategy (Bhargava, 2020; ICMR, 2020d).
India faces ongoing challenges with preventing the spread of COVID-19, including issues with social distancing in big cities with crowded streets, lack of access to clean water, lack of regular hand washing facilities in an appreciable number of households, lack of physicians, lack of hospital beds (at 0.5 to 0.7 beds per 1,000 compared with 4.3 per 1,000 in China) and ICUs vs. higher income countries, just 20,000 ventilators in the country, and a lack of personal protective equipment (PPE) among healthcare professionals (Bhattacharya et al., 2020; Dutta, 2020; Ganapathy, 2020; Garattini et al., 2020; Kamath et al., 2020; Ma and Vervoort, 2020; Roy et al., 2020; Saaliq, 2020).
There are also challenges with high co-payments for treating existing infectious and non-infectious diseases, which will be exacerbated by diverting expenditures towards prevention and management of COVID-19, as well as purchasing basic necessities if incomes are reduced as a result of the pandemic (Ganapathy, 2020; Kamath et al., 2020). This is in addition to concerns about the routine availability of medicines to treat priority diseases in India. Medicine prices are typically lower in India compared with a number of other LMICs although appreciable variation in prices has been seen among pharmacies (Millard et al., 2018; Babar et al., 2019; Ray et al., 2020). Having said this, as mentioned, there are ongoing activities by the Indian Government to regulate the prices of medicines in India to help with co-payments for essential medicines (HealthWorld, 2016; Pavithra, 2019). Purchasing personal protective equipment (PPE) remains an issue with the costs of the materials to make PPE appreciably increasing following the pandemic (Business Today, 2020); however, prices and shortages may be alleviated by increased local production along with pricing and manufacturing regulations (Bhattacharya et al., 2020; GMA Consulting Group, 2020; Pandey, 2020).
Ongoing national and regional activities during the early stages of the pandemic to help reduce the spread of COVID-19, as well as the financial consequences, are contained in Table 1. It was perceived that timely decisions to introduce lockdowns helped with controlling infection rates initially although at a negative socioeconomic cost (Pai et al., 2020). However, we are now seeing a spike in infection rates as lockdown measures are relaxed despite warnings to maintain social distancing and as a consequence of the recent monsoons (Associated Press, 2020; Siddiqui, 2020). Consequently, there has been a re-instigation of such measures although rail and other services have resumed and places of worship have opened (ANI, 2020; The Hindu, 2020).

TABLE 1. Range of national and regional activities in India in 2020 from January 2020 up to June 2020 to help reduce the spread of COVID 19 and its impact.
Currently, there is not a cure for COVID-19; however, a number of medicines have been proposed and are undergoing trials (Sanders et al., 2020; Scavone et al., 2020). Recently, dexamethasone has been shown in the UK Recovery Trials to reduce deaths in ventilated patients and in those receiving oxygen only (Horby et al., 2020a). Remdesivir has shown encouraging results in one study after earlier concerns over an underpowered study (Beigel et al., 2020; ECDC, 2020; Wang et al., 2020). However a recent study among moderate patients failed to show similar benefit with remdesivir; consequently, still being considered as experimental (Dyer, 2020; McCreary and Angus, 2020; Spinner et al., 2020). Triple antiviral therapy is also showing promise in the management of COVID-19 patients although numbers are small (Hung et al., 2020); however, more studies are needed before any recommendations can be made. In addition, an appreciable number of vaccines are now in development (Checcucci et al., 2020; ECDC, 2020).
However, there is still considerable controversy surrounding the use of chloroquine and hydroxychloroquine with or without azithromycin for both prevention and treatment of COVID-19, following initial studies in China (Boulware et al., 2020; Cortegiani et al., 2020; Gao et al., 2020; Gautret et al., 2020). Internationally, concerns were raised about the lack of comparisons in the initial studies as well as potential harm including cardiac side-effects (Abena et al., 2020; Borba et al., 2020; Gautret et al., 2020; International Society of Antimicrobial Chemotherapy, 2020; ISAC/Elsevier, 2020; Ferner & Aronson, 2020; Littlejohn, 2020). Subsequent studies, including registry studies, have enhanced these concerns for hydroxychloroquine for both the prevention and treatment of COVID-19 (Boulware et al., 2020; Das et al., 2020; Geleris et al., 2020; Horby et al., 2020b; Littlejohn, 2020; Rosenberg et al., 2020). Consequently, the European Medicines Agency has advised against its prescribing outside of clinical trials (European Medicine Agency, 2020) and Das et al. (2020) in India also advised caution (Das et al., 2020). The study by Mehra et al. (2020) also showed increased mortality with chloroquine or hydroxychloroquine; however, this paper has now been retracted and is currently subject to external auditing (Editorial, 2020; Mehra et al., 2020a; Mehra et al., 2020b). In view of recent studies including the UK Recovery trial, the WHO has now halted the hydroxychloroquine arm in the ongoing Solidarity Trial and the National Institute of Health in the US has also halted the use of hydroxychloroquine in its studies (ECDC, 2020; Horby et al., 2020b; NIH, 2020; WHO, 2020c).
Having said this, the ICMR under the Ministry of Health and Family Welfare in India continues to recommend hydroxychloroquine for prophylaxis despite potential concerns (Kumar et al., 2020; Natnayas et al., 2020; Pulla, 2020; Rathi et al., 2020; Tilangi et al., 2020). In a recently published case controlled study, Chatterjee et al. (2020) demonstrated that the prescribing of four or more maintenance doses of hydroxychloroquine was associated with a significant reduction in the odds of healthcare workers getting COVID-19 (Chatterjee et al., 2020). Other treatments initially recommended by ICMR and others include lopinavir-ritonavir, although there is contrasting data regarding its effectiveness in COVID-19 patients (Bhatnagar et al., 2020; Cao et al., 2020; Kumar et al., 2020). More recently, the WHO has discontinued the lopinavir-ritonavir arm of the Solidarity trial with the interim results showing this combination demonstrated little or no reduction in the mortality of hospitalized COVID-19 patients when compared to standard of care (WHO, 2020c). Overall, further studies are needed before specific treatments can be robustly recommended given the continuing concerns with a number of treatments, the redaction of recent studies and issues with study design and setting (Bae et al., 2020; Godman, 2020; International Society of Antimicrobial Chemotherapy, 2020; Mehra et al., 2020b).
We are aware the endorsement of hydroxychloroquine has resulted in appreciably increased use in a number countries and localities along with increased prices, although there have been hospitalisations and deaths from poisoning (Abena et al., 2020; Buasi and Adebayo, 2020; Haque et al., 2020; Kwasi, 2020; Mendel et al., 2020; Salo, 2020; Topp, 2020; Vaduganathan et al., 2020). Increased prices can be a concern in India with potentially detrimental consequences for families if limited available funds for priority disease areas including NCDs are being diverted towards purchasing treatments where there are ongoing controversy along with purchasing of PPE at increased prices (Business Today, 2020).
Study Aims and Objectives
In view of the current situation in India, we believed there was an urgent need to assess the impact of COVID-19 on the availability and prices of medicines and other technologies to prevent and treat COVID-19 in community pharmacies. The key considerations are the high rates of both infectious and non-infectious diseases in India, issues with sanitation and crowded streets, the catastrophic consequences for families when members become ill, and fears regarding the availability of regular medicines during the pandemic (Kastor & Mohanty, 2018; Selvaraj et al., 2018; Statista, 2020). Community pharmacists are a particular target since they currently play a key role in the management of diseases in India, with ongoing issues with access to physicians and high co-payments especially in rural areas (Abdulsalim et al., 2018; Daftary et al., 2019; Nafade et al., 2019). They are also in a good position to suggest to patients with more severe symptoms that they seek additional help (Amariles et al., 2020). This is important since it is known it can be difficult to differentiate respiratory tract infections from COVID-19 in patients presenting with coughs and fever (Ongole et al., 2020).
We have seen prices rise for essential medicines in LMICs over time and for treatments for COVID-19, as well as appreciable price increases for the materials to make PPE in India following the start of the pandemic. In view of this, we believe it is important to evaluate the current situation in India regarding pertinent medicines and PPE to provide future guidance at this critical time (Kasonde et al., 2019; Buasi & Adebayo, 2020; Business Today, 2020). Consequently, we sought to address this through direct contact with community pharmacists across India. However, we are aware of government regulations regarding the pricing of essential medicines in India (HealthWorld, 2016; Pavithra, 2019). As a result, in this initial study we sought to assess the utilisation, availability and price changes of medicines and PPE for COVID-19 among a number of cities across India to provide future direction to key stakeholder groups in India. Senior level co-authors would also be targeted to provide guidance based on their experiences across LMICs.
We have not assessed the influence of the various Government initiatives (Table 1) on the prevalence and mortality of COVID-19 in India. We are aware of the concerns about the testing rates for COVID-19 certainly initially as well as evolving strategies to address ongoing spikes in infection rates as lockdown measures are eased and with the advent of the monsoons (Associated Press, 2020; Siddiqui, 2020). We will though be monitoring morbidity and mortality rates alongside the unintended consequences of lockdown and other measures given rising prevalence rates of COVID-19 in India and existing high prevalence rates for both infectious and non-infectious diseases, and will be reporting on this in future studies. Unintended consequences include issues of increased gender violence as well as mental health issues associated with any stigma with COVID-19 as well as lockdown measures (IFRC & WHO, 2020; Rajkumar, 2020; SADC, 2020). In addition, rising rates of NCDs especially if patients cannot attend clinics or receive their medicines due to lockdown measures as well as having affordability issues with medicines through lack of earnings due to lockdown and other measures (Basu, 2020; Kluge et al., 2020).
Methodology
We initially undertook a narrative review of the current situation regarding COVID-19 in India and on suggested treatments. We subsequently undertook quantitative research in the form of a survey. The narrative review included a review of current and proposed treatment approaches including vaccines and recommendations for preventing and managing COVID-19, including the role of community pharmacists as well as issues of misinformation. We did not systematically review the papers or other information sources for their quality using well-known scales such as the Newcastle-Ottawa scale as some of the papers quoted are in pre-publication format and we have used a considerable number of internet sources (Almeida et al., 2018). However, the publications and internet sources were filtered by the co-authors to add robustness to the paper and its suggestions.
The information sourced from the pragmatic review of the literature was combined with a questionnaire survey among community pharmacies (Appendix 1) to assess the situation regarding prices, availability and usage patterns of carefully selected medicines that could potentially be used in the management of COVID-19, as well as PPE, soon after the start of the pandemic.
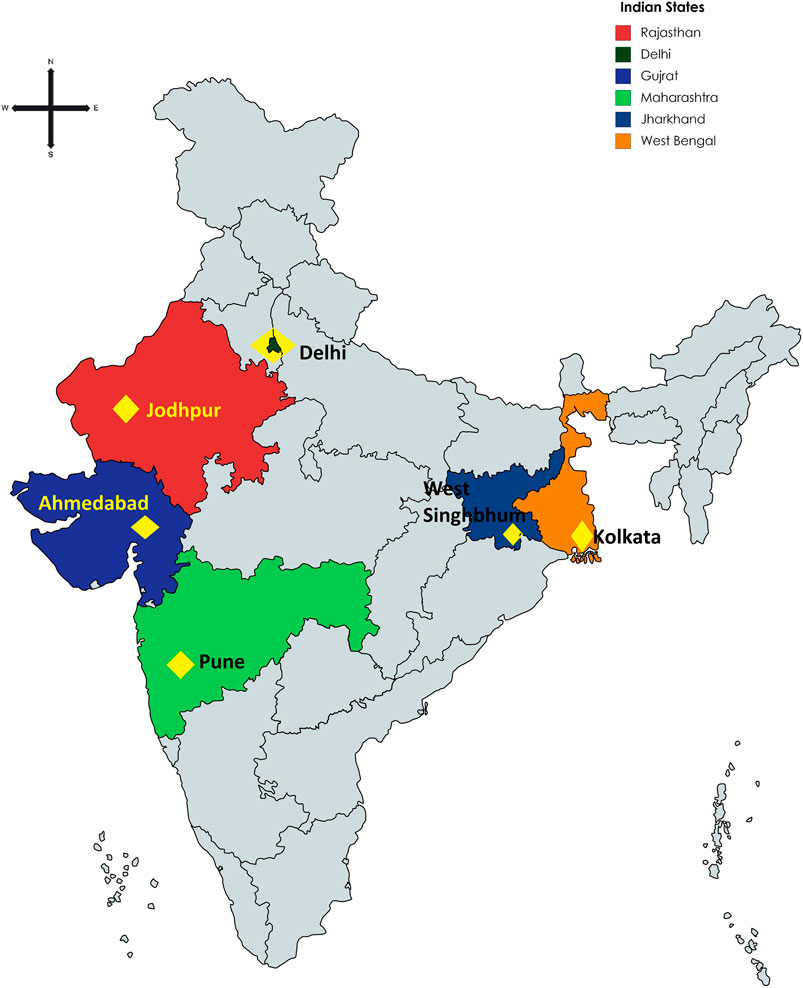
For this rapid analysis, we selected via purposive sampling representation of pharmacies from across India. This included Ahmedabad, Jodhpur, and Pune as part of Western India, West Singhbhum and Kolkata as part of Eastern India, and Delhi in northern India. Convenience sampling in these cities was used to select pharmacists through emails, telephone contact, personal contacts and other mechanisms. There was no sample size calculation as there was no previous data in India to base calculations upon. However, the intention was to undertake the research among an appreciable number of community pharmacies across India to gain good insights and provide a basis for future studies if needed.
Key questions were to assess patterns of demand, availability, and price changes of selected medicines and equipment, as well as the potential future role of pharmacists to reduce misinformation. These are contained in Box 2 (building on Appendix 1). Those conducting the research were provided with an Excel spreadsheet of the questions to complete. The questions were open ended as we were aware that in a number of situations we would be unable to obtain exact details of changes in utilisation patterns and prices; however, we wanted to capture data including more general information for this initial study. The answers were collated where possible into logical bands for comparisons with other countries such as Bangladesh (Haque et al., 2020). These bands were not pre-defined as this was an exploratory rapid pilot study, with changes in prices based on local prices. In addition, general information would be sufficient if the pharmacists were unable to be specific given the exploratory nature of this study.

Box 2. Open ended questions to community pharmacists in India regarding pertinent medicines and equipment to prevent and treat COVID-19.
The pharmacists were briefed on the objectives of the study with the option to participate or not, with confidentiality maintained throughout. Our hypothesis, based on findings in other countries, was that there would be shortages of some of the medicines, although countered in India as a chief producer of medicines with export bans in place certainly initially (Duffy & Hussain, 2020) Price rises especially for medicines potentially tempered though by the Ministry of Pharma and Consumer Affairs being instructed to take necessary action to regulate these for PPE and other health related materials alongside existing regulations for (Table 1) (WHO India, 2020e). The findings were compiled into a tabular format. No formal statistical analysis was performed as the level of detail varied considerably across the pharmacies.
We subsequently combined the data collected using the experience of the co-authors regarding key issues including pharmaceutical care, health policy and self-purchasing in LMICs to provide future direction, building on comments from the interviewed pharmacists. We have previously successfully used this approach to provide future direction in LMICs (Godman et al., 2018; Godman et al., 2019a; Godman et al., 2020a; Godman et al., 2020b; Godman et al., 2020c; Godman et al., 2020d).
Ethical approval for this study was not required according to national legislation and institutional guidelines. However, all pharmacists freely provided the requested information having been given the opportunity to refuse to participate if wished. This is in line with previous studies undertaken by the co-authors in related areas including analysis of policies to enhance the rationale use of medicines and biosimilars, pricing policies and issues surrounding generics, which involved direct contact with health authority personnel and other key stakeholders (Moorkens et al., 2017; Godman et al., 2019b; Gad et al., 2020; Godman et al., 2020a; Godman et al., 2020c; Miljković et al., 2020).
Results
Overall, 135 pharmacies were visited to give a response rate of 82% (Table 2).
We first report on changes in utilisation patterns before reporting on any price changes seen with the various medicines and equipment as well as any shortages. The reported figures are absolute figures based on interviewee feedback.

TABLE 2. Details of responses among pharmacy/drugs stores approached. NB—Groups 1 and 2 refer to the consolidation co-ordination of the findings by the principal co-author involved. The pharmacies were located throughout India (Figure 1; Table 3).
Utilisation
Table 4 depicts changes in utilisation patterns for the various medicines, vitamins and PPE equipment and hand sanitisers from the beginning of March until the end of May 2020. Encouragingly, no change or decreased utilisation of antimalarials was seen in 45% of pharmacies taking part, with similarly limited increases in the utilisation of antibiotics (42.3% of pharmacies taking part). An appreciable increase in antibiotic utilisation was seen though in a small number of pharmacies.
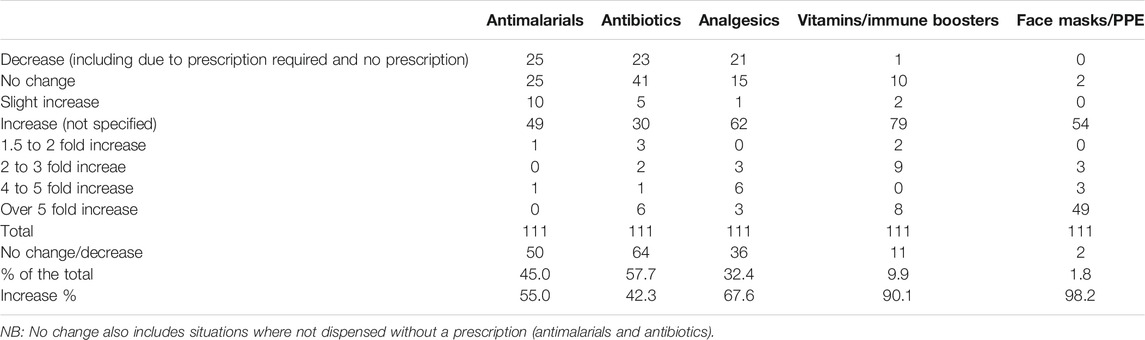
TABLE 4. Utilisation changes for medicines and PPE between beginning March 2020 and end May 2020 among 111 pharmacies in India.
Encouragingly, there was an increase in the purchasing of PPE and vitamins to boost the immune system in over 90% of pharmacies taking part, nearing 100% for PPE. It is likely that we will continue to see such increases in the purchasing of both of these if COVID-19 infection rates continue to increase following any easing of lockdown measures.
Price Changes
Table 5 depicts price changes for pertinent medicines and PPE. Encouragingly, there were limited price changes for antimalarials (only 16.2% of the pharmacies) and antibiotics (8.1% of the pharmacies). Contrasting with this, price increases were seen for PPE among over 90% of the pharmacies taking part in the study. This was only a slight increase, or an increase in only one of the three months, in 39.6% of pharmacies taking part.
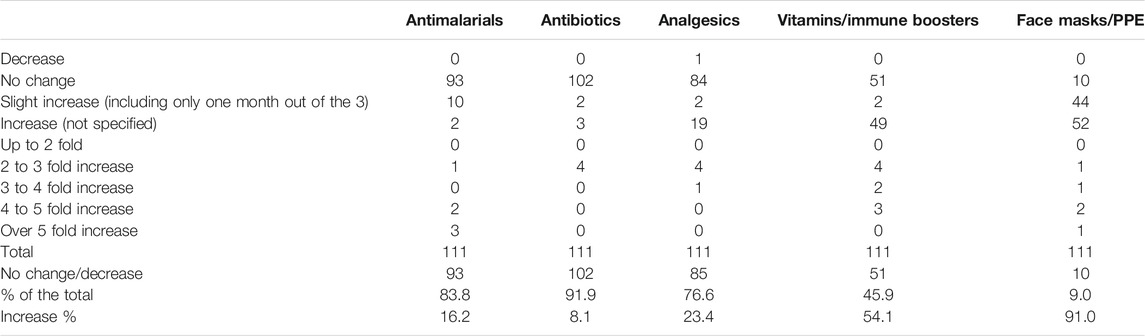
TABLE 5. Price changes for medicines and PPE between beginning March 2020 and end May 2020 among 111 pharmacies in India.
Medicine and Prevention Shortages
Perhaps not surprisingly, shortages of some medicines and PPE were seen among the pharmacies in India (Table 6). These were principally confined to antimalarial medicines (70.3%) and PPE (88.3%).
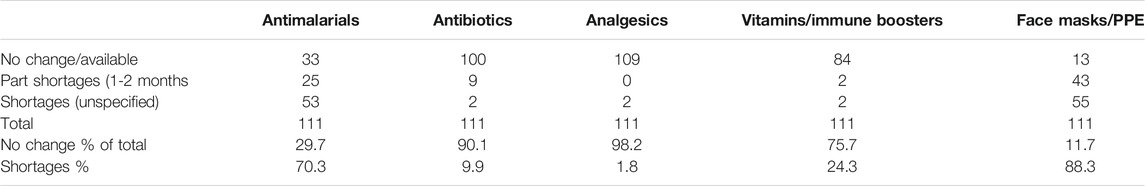
TABLE 6. Shortages for medicines and PPE between beginning March 2020 and end May 2020 among 111 pharmacies in India.
Potential Ways Forward to Address Misinformation and Enhance Appropriate Use of Medicines and Equipment Across Sectors
Possible strategies based on interviewee and co-author feedback to address misinformation and other concerns regarding the management of COVID-19 and any unintended consequences are included in Table 7. We believe this is especially important for patients with NCDs given rising rates in India, and we will be researching this further.

TABLE 7. Key activities among stakeholder groups to improve prevention and management of patients with COVID-19.
Discussion
We believe this is one of the first comprehensive studies worldwide to assess the impact of COVID-19 on the utilisation, availability and price changes of pertinent medicines and PPE used to prevent and treat COVID-19 among LMICs with a high response rate (82%) among those taking part. In India, high patient co-payments have contributed to potentially catastrophic consequences for families when members become ill, although this is beginning to change helped by ongoing legislation to moderate prices of essential medicines (Kastor and Mohanty, 2018; Reddy, 2018; Pavithra, 2019; Sood and Wagner, 2020).
As expected, our study demonstrates an increase in the utilisation of a number of suggested medicines and PPE, enhanced by endorsement of certain approaches including increased protection with PPE (Table 4). However, increases in utilisation were not as great for antimalarial treatments (55% of pharmacies noting an increase) and antibiotics (42.3% of pharmacies noting an increase) compared with analgesics (67.6%) and vitamins (90.1%). This is in line initiatives by the government advising pharmacists not to sell antimalarials and antibiotics without a prescription. The utilisation patterns seen in India appear similar to the situation in Bangladesh with antimalarial tablets (48.8% of pharmacies and drug stores seeing an increase), analgesics (97.6% of pharmacies and drug stores seeing an increase) and vitamins (90.6% seeing an increase), but lower than in Bangladesh with respect to antibiotics (with 70.6% of stores noting an increase), perhaps reflecting greater scrutiny over the need for patients to have a prescription for an antibiotic in India (Haque et al., 2020).
Price increases were also seen for pertinent medicines (Table 5), which was expected given some of the shortages seen (Table 6). However, price increases appeared appreciably lower than seen in Bangladesh with only 16.2% of pharmacies in India reporting a price increase for antimalarials vs. 50% in Bangladesh and only 8.1% of pharmacies in India reporting price increases for antibiotics vs. 34.7% in Bangladesh (Haque et al., 2020). We believe this reflects greater price controls for medicines in India compared with Bangladesh, providing direction to countries such as Bangladesh during the current pandemic and after (HealthWorld, 2016; Pavithra, 2019). A lower number of pharmacies in India (23.4%) also reported price increases for analgesics vs. 45.3% in Bangladesh (Haque et al., 2020). This may reflect greater production of ingredients for medicines (API) in India than seen in Bangladesh coupled with Government price controls (Pavithra, 2019). However, further research will be needed before any definitive statement can be made especially as shortages of antimalarials were seen in India during the study period (70.3% of pharmacies), although this was only short term in a third of these (Table 6). Shortages of antimalarial medicines were seen in 54.1% of pharmacies and drug stores in Bangladesh during the same period (Haque et al., 2020).
As mentioned, we observed an appreciable increase in the use of PPE in both Bangladesh (over 95% of pharmacies and drug stores respectively) and India (98.2% respectively) during the study period (Haque et al., 2020). This was typically accompanied by shortages and price rises in both countries, some of which were substantial (Haque et al., 2020). We have also seen substantial increase in the price of PPE in other LMICs as the pandemic progresses (Weston, 2020).
Table 7 highlights potential activities that can be undertaken by key stakeholder groups in India and more widely to address issues and concerns with COVID-19. This includes addressing the unintended consequences of COVID-19 including issues of stigma as well as addressing concerns with appropriate identification and management of other infectious diseases apart from COVID-19 as well as among patients with chronic NCDs given rising rates of CVD and diabetes in India. We will be monitoring the situation with respect to hydroxychloroquine given ongoing controversies across countries.
We are aware of a number of limitations with this study. These include the fact that we were unable to obtain exact details on changes in the utilisation and prices of pertinent medicines and PPEs from all the pharmacists visited due to issues of confidentiality and having the data readily to hand. We also did not cover all the regions in India. In addition, we did not break down antibiotics into azithromycin and other antibiotics such as amoxycillin in view of the study constraints. We also did not ask about shortages of other medicines such as those to treat patients with NCDs. Alongside this, we are aware that some of the shortages initially may have been due to transport and logistic issues. A number of these issues will be addressed in future studies. However, we are confident our findings can be helpful for future planning purposes in India and wider given the number of community pharmacies that were involved.
Conclusion
In our study we have seen increases in utilisation and prices as well as shortages of pertinent medicines and PPE used to prevent and treat COVID-19 in India. Encouragingly, the extent of shortages and price increases in India were not as high as originally expected, potentially helped by local production of medicines, including the active ingredients, greater scrutiny over the dispensing of antimalarials and antibiotics without a prescription, as well as government control over prices including recently; with similarities with Bangladesh. There are ongoing concerns and challenges regarding potential treatments including antimalarials, which needs urgent addressing. Increasing recognition of the need for evidence-based medicine in terms of treatment recommendations and prescribing can help further reduce inappropriate treatments being recommended, prescribed or dispensed. Patient organisations can also play a role through social media and other platforms to reduce the extent of misinformation and its potential damaging consequences. We will be monitoring this in the future.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
All authors contributed to the study. MH, IS, AK and BBG designed the questionnaire with MH, SK, JC, RB, SI, SD, JPA, and YS. BBG developed the initial draft manuscript with all authors helping to refine the paper in successive drafts before submission
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor declared a past co-authorship with one of the authors (BG).
Acknowledgments
We thank Nor Azlina A. Rahman for helpful comments regarding the sample and size of the study. We would like to acknowledge ‘mapchart.net’ to prepare a map diagram for our study, a free service.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.582154/full#supplementary-material.
Appendix 1—Questionnaire to community pharmacists in India.
The Questionnaire is designed to ascertain changes in the utilisation, prices and shortages of possible medicines and equipment to prevent and treat patients with COVID-19. You are free to participate or not, and confidentiality will be maintained.
Question 1: What is your location (City and region)?
Question 2: What changes in medicine purchasing patterns have you noticed in your pharmacy from the beginning of March, i.e. soon after the start of the pandemic but before major travel and other restrictions until the end of May, for antimalarials (hydroxychloroquine), antibiotics (e.g. azithromycin and co-amoxiclav), multivitamins including Vitamin C and analgesics [Please note - This is based on invoices where possible from prior to the beginning of March or other information sources; alternatively impressions (free text)]
Question 3: What changes in prices have you noticed in your pharmacy from the beginning of March until the end of May, for antimalarials (hydroxychloroquine), antibiotics (e.g. azithromycin and co-amoxiclav), multivitamins including Vitamin C and analgesics [Please note - This is based on invoices where possible from prior to the beginning of March or other information sources; alternatively impressions (free text)]
Question 4: Have there been any shortages for antimalarials (hydroxychloroquine), antibiotics (e.g. azithromycin and co-amoxiclav), multivitamins including Vitamin C or analgesics in your pharmacy from the beginning of March until the end of May, for antimalarials (hydroxychloroquine), antibiotics (e.g. azithromycin and co-amoxiclav), multivitamins including Vitamin C and analgesics? If so, what is the extent of any shortages (free text)?
Question 5: What has been the changes in utilisation, prices and potential shortages for personal protection equipment (PPE) such as hand sanitisers and face masks in your pharmacy from the beginning of March until the end of May? [[Please note - This is based on invoices where possible from prior to the beginning of March or other information sources; alternatively impressions (free text)]
Question 6: What suggestions do you have for the authorities in India to reduce misinformation regarding different management and treatment approaches for new pandemics as well as reduce inappropriate self-medication with antimicrobials (free text)?
References
Abdulsalim, S., Unnikrishnan, M. K., Manu, M. K., Alrasheedy, A. A., Godman, B., and Morisky, D. E. (2018). Structured pharmacist-led intervention programme to improve medication adherence in COPD patients: a randomized controlled study. Res. Soc. Adm. Pharm. 14 (10), 909–914. doi:10.1016/j.sapharm.2017.10.008
Abdulsalim, S., Unnikrishnan, M. K., Manu, M. K., Alsahali, S., Alrasheedy, A. A., Martin, A. P., et al. (2020). Impact of a clinical pharmacist intervention on medicine costs in patients with chronic obstructive pulmonary disease in India. Pharmacoecon Open 4 (2):331–342. doi:10.1007/s41669-019-0172-x
Abena, P. M., Decloedt, E. H., Bottieau, E., Suleman, F., Adejumo, P., Sam-Agudu, N. A., et al. (2020). Chloroquine and hydroxychloroquine for the prevention or treatment of COVID-19 in Africa: caution for inappropriate off-label use in healthcare settings. Am. J. Trop. Med. Hyg. 102 (6), 1184–1188. doi:10.4269/ajtmh.20-0290
Aghagoli, G., Gallo Marin, B., Soliman, L. B., and Sellke, F. W. (2020). Cardiac involvement in COVID-19 patients: risk factors, predictors, and complications: a review. J. Card. Surg. 35 (6), 1302–1305. doi:10.1111/jocs.14538
Ahmed, A. (2020). India outlines $23 billion stimulus to help poor hit by lockdown. Available at URL: https://www.weforum.org/agenda/2020/03/india-stimulus-support-lockdown-pandemic-covid19-epidemic-economics/ (Accessed March 13 2013).
Akutey, R., Der, R., Owusu-Daaku, F., and Baiden, F. (2018). Using community pharmacies to expand access to screening for noncommunicable diseases in suburban Ghana-A facility-based survey on client needs and acceptability. Health Sci Rep. 1 (9), e79. doi:10.1002/hsr2.79
Alluri, A., and Pathi, K. (2020). India coronavirus: should people pay for their own Covid-19 tests? Available at URL: https://www.bbc.co.uk/news/world-asia-india-52322559 (Accessed April 20 2020).
Almeida, P. H. R. F., Silva, T. B. C., de Assis Acurcio, F., Guerra Júnior, A. A., Araújo, V. E., Diniz, L. M., et al. (2018). Quality of life of patients with type 1 diabetes mellitus using insulin analog glargine compared with NPH insulin: a systematic review and policy implications. Patient 11 (4), 377–389. doi:10.1007/s40271-017-0291-3
Alqahtani, J. S., Oyelade, T., Aldhahir, A. M., Alghamdi, S. M., Almehmadi, M., Alqahtani, A. S., et al. (2020). Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One 15 (5), e0233147. doi:10.1371/journal.pone.0233147
Amariles, P., Ledezma-Morales, M., Salazar-Ospina, A., and Hincapié-García, J. A. (2020). How to link patients with suspicious COVID-19 to health system from the community pharmacies? A route proposal. Res Social Adm Pharm. S1551 (20), 30248. doi:10.1016/j.sapharm.2020.03.007
Anderson, E. L., Turnham, P., Griffin, J. R., and Clarke, C. C. Consideration of the aerosol transmission for COVID-19 and public health (2020). Risk Anal. 40 (5). 902–907. doi:10.1111/risa.13500
ANI (2020). COVID-19 measures extended till Sept 20 in WB. Avaialable at: https://www.aninews.in/news/national/general-news/covid-19-measures-extended-till-sept-20-in-wb-complete-lockdown-on-sept-7-11-1220200826223016/ (Accessed September 26 2020).
Anwar, S., Nasrullah, M., and Hosen, M. J. (2020). COVID-19 and Bangladesh: challenges and how to address them. Frontiers. Public Health 8 (154). doi:10.3389/fpubh.2020.00154
Associated Press (2020). Asia Today: India’s cases spike again to near half-million. Available at URL: https://www.msn.com/en-gb/news/world/asia-today-india-s-cases-spike-again-to-near-half-million/ar-BB15YLo3?ocid=spartan-dhp-feeds (Accessed June 26 2020).
Babar, Z. U., Ramzan, S., El-Dahiyat, F., Tachmazidis, I., Adebisi, A., and Hasan, S. S. (2019). The availability, pricing, and affordability of essential diabetes medicines in 17 low-, middle-, and high-income countries. Front. Pharmacol. 10 (1375), 1375. doi:10.3389/fphar.2019.01375
Bae, S., Kim, M. C., Kim, J. Y., Cha, H. H., Lim, J. S., Jung, J., et al. (2020). Notice of retraction: effectiveness of surgical and cotton masks in blocking SARS-CoV-2. Ann. Intern. Med., L20-0745. doi:10.7326/L20-0745
Basu, S. Non-communicable disease management in vulnerable patients during Covid-19 (2020). Indian J. Med. Ethics 5 (2), 103–105. doi:10.20529/IJME.2020.041
Beigel, J. H., Tomashek, K. M., Dodd, L. E., Mehta, A. K., Zingman, B. S., Kalil, A. C., et al. (2020). Remdesivir for the treatment of covid-19-preliminary report. N. Engl. J. Med. 383 (19), 1813–1826. doi:10.1056/NEJMoa2007764
Bhargava, B. (2020). Sudan P—Ministry of Health and Welfare India. Empowering citizens for testing of SARS-CoV-2virus to save precious lives and contain the virus. Available at URL: https://www.icmr.gov.in/pdf/covid/strategy/Joint_Letter_Test_Track_Treat.pdf (Accessed July 01 2020).
Bhatnagar, T., Murhekar, M. V., Soneja, M., Gupta, N., Giri, S., Wig, N., et al. (2020). Lopinavir/ritonavir combination therapy amongst symptomatic coronavirus disease 2019 patients in India: protocol for restricted public health emergency use. Indian J. Med. Res. 151 (2&3), 184–189. doi:10.4103/ijmr.IJMR_502_20
Bhattacharya, S., Hossain, M. M., and Singh, A (2020)Addressing the shortage of personal protective equipment during the COVID-19 pandemic in India-A public health perspective. AIMS Public Health 7 (2), 223–227. doi:10.3934/publichealth.2020019
BioVoice (2018). Fixing medicine prices saved 11,462 crore for patients: Govt. Available at URL: https://www.biovoicenews.com/fixing-medicine-prices-saved-%E2%82%B911462-crore-for-patients-govt/ (Accessed December 20 2018).
Borba, M. G. S., Almeida Val, F. F., Sampaio, V. S., Alexandre, M. A. A., Melo, G. C., Brito, M., et al. (2020). Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS-CoV-2) infection: preliminary safety results of a randomized, double-blinded, phase IIb clinical trial (CloroCovid-19 Study). MedRxiv preprint. Available at: https://doi.org/10.1101/2020.04.07.20056424
Boulware, D. R., Pullen, M. F., Bangdiwala, A. S., Pastick, K. A., Lofgren, S. M., Okafor, E. C., et al. (2020). A randomized trial of hydroxychloroquine as postexposure prophylaxis for covid-19. N Engl J Med. 383 (6), 517–525. doi:10.1056/NEJMoa2016638
Buasi, S., and Adebayo, B. (2020). Nigeria records chloroquine poisoning after Trump endorses it for coronavirus treatment. Available at URL: https://edition.cnn.com/2020/03/23/africa/chloroquine-trump-nigeria-intl/index.html (Accessed March 23 2020).
Business Today (2020). Coronavirus: protective health gear, N-95 masks, coveralls in short supply. Available at URL: https://www.businesstoday.in/current/economy-politics/coronavirus-protective-health-gear-n-95-masks-coveralls-in-short-supply/story/398955.html (Accessed March 25 2020).
Cameron, A., Ewen, M., Ross-Degnan, D., Ball, D., and Laing, R. (2020). Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet 373 (9659), 240–249. doi:10.1016/S0140-6736(08)61762-6
Cao, B., Wang, Y., Wen, D., Liu, W., Wang, J., Fan, G., et al. (2020). A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 382 (19), 1787–1799. doi:10.1056/NEJMoa2001282
Chatterjee, P., Anand, T., Singh, K. J., Rasaily, R., Singh, R., Das, S., et al. (2020). Healthcare workers & SARS-CoV-2 infection in India: a case-control investigation in the time of COVID-19. Indian J. Med. Res. 151 (5), 459–467. doi:10.4103/ijmr.IJMR_2234_20
Checcucci, E., Piramide, F., Pecoraro, A., Amparore, D., Campi, R., Fiori, C., et al. (2020). The vaccine journey for COVID-19: a comprehensive systematic review of current clinical trials in humans. Panminerva Med. doi:10.23736/S0031-0808.20.03958-0
Cortegiani, A., Ingoglia, G., Ippolito, M., Giarratano, A., and Einav, S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19 (2020). J. Crit. Care 57, 279–283. doi:10.1016/j.jcrc.2020.03.005
Council for International Organizations of Medical Sciences (2020). Medicines assessment during public health emergencies needs good science, best practices and proper communication. Available at URL: https://cioms.ch/wp-content/uploads/2020/06/CIOMS_WGXII_Statement.pdf (Accessed June 3 2020).
Daftary, A., Satyanarayana, S., Jha, N., Singh, M., Mondal, S., Vadnais, C., et al. (2019). Can community pharmacists improve tuberculosis case finding? A mixed methods intervention study in India. BMJ global health 4 (3), e001417.
Das, S., Bhowmick, S., Tiwari, S., and Sen, S. (2020). An updated systematic review of the therapeutic role of hydroxychloroquine in coronavirus disease-19 (COVID-19). Clin. Drug Invest. 40 (7), 591–601. doi:10.1007/s40261-020-00927-1
Davis, R. (2020). Viral outbreak: fake news spreads in SA in tandem with Covid-19. Available at URL: https://www.dailymaverick.co.za/article/2020-03-31-viral-outbreak-fake-news-spreads-in-sa-in-tandem-with-covid-19/ (Accessed March 31 2020).
Duffy, J. P., and Hussain, I. (2020). India rescinds exemptions to hydroxychloroquine export restrictions and increases the probability of international arbitration claims. Available at URL: https://www.reedsmith.com/en/perspectives/2020/04/india-rescinds-exemptions-to-hydroxychloroquine-export-restriction (Accessed April 26 2020).
Dutta, P. K. Challenge to fighting coronavirus in India: 36% wash hands with soap before a meal. 2020. Available at URL: https://www.indiatoday.in/coronavirus-outbreak/story/challenge-to-fighting-coronavirus-in-india-36-wash-hands-with-soap-before-a-meal-1660295-2020-03-27 (Accessed March 27 2020).
Dyer, O. (2020). Covid-19: Remdesivir has little or no impact on survival, WHO trial shows. BMJ 371, m4057.
Dzirutwe, M. (2020). Zimbabwe president threatens 20 years jail over fake Covid-19 statement. Available at URL: https://www.sowetanlive.co.za/news/africa/2020-04-14-zimbabwe-president-threatens-20-years-jail-over-fake-covid-19-statement/ (Accessed April 14 2020).
ECDC (2020). ECDC—Vaccines and treatment of COVID-19. Available at URL: https://www.ecdc.europa.eu/en/covid-19/latest-evidence/vaccines-and-treatment (Accessed June 30 2020).
Editorial (2019). Bill of health—India’s doctors and its government are at loggerheads over much-needed reforms. Nature 572, 415.
Editorial (2020). Expression of concern: hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet 395 (10240), e102. doi:10.1016/S0140-6736(20)31290-3
Ellinghaus, D., Degenhardt, F., Bujanda, L., Buti, M., Albillos, A., Invernizzi, P., et al. (2020). Genomewide association study of severe covid-19 with respiratory failure. N. Engl. J. Med. 383 (16), 1522–1534. doi:10.1056/NEJMoa2020283
European Medicine Agency (2020). COVID-19: reminder of risk of serious side effects with chloroquine and hydroxychloroquine. Available at URL: https://www.ema.europa.eu/en/news/covid-19-reminder-risk-serious-side-effects-chloroquine-hydroxychloroquine (Accessed April 23 2020).
Farooqui, H. H., Selvaraj, S., Mehta, A., and Heymann, D. L. (2020). Community level antibiotic utilization in India and its comparison vis-à-vis European countries: evidence from pharmaceutical sales data. PLoS One 13 (10), e0204805. doi:10.1371/journal.pone.0204805
Ferner, R. E., and Aronson, J. K. (2020). Chloroquine and hydroxychloroquine in covid-19. BMJ 369, m1432. doi:10.1136/bmj.m1432
Gad, M., Salem, A., Oortwijn, W., Hill, R., and Godman, B. (2020). Mapping of current obstacles for rationalizing use of medicines (CORUM) in europe: current situation and potential solutions. Front. Pharmacol. 11, 144. doi:10.3389/fphar.2020.00144
Ganapathy, N. (2020). Coping with Covid-19: lockdown unleashes India’s community spirit. Available at URL: https://www.straitstimes.com/asia/south-asia/lockdown-unleashes-indias-community-spirit (Accessed April 11 2020).
Gandhi, J. (2020). What does Covid-19 mean for India?. Available at URL: https://www.schroders.com/en/insights/economics/what-does-covid-19-mean-for-india/ (Accessed April 8 2020).
Gao, J., Tian, Z., and Yang, X. (2020) Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends 14 (1), 72–73. doi:10.5582/bst.2020.01047
Garattini, L., Zanetti, M., and Freemantle, N. (2020). The Italian NHS: what lessons to draw from COVID-19? Appl. Health Econ. Health Pol. 18 (4), 463–466. doi:10.1007/s40258-020-00594-5
Garg, C. C., and Karan, A. K. (2009). Reducing out-of-pocket expenditures to reduce poverty: a disaggregated analysis at rural-urban and state level in India. Health Pol. Plann. 24 (2), 116–128. doi:10.1093/heapol/czn046
Gautret, P., Lagier, J. C., Parola, P., Hoang, V. T., Meddeb, L., Mailhe, M., et al. (2020). Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 56, 105949. doi:10.1016/j.ijantimicag.2020.105949
Geleris, J., Sun, Y., Platt, J., Zucker, J., Baldwin, M., Hripcsak, G., et al. (2020). Observational study of hydroxychloroquine in hospitalized patients with covid-19. N. Engl. J. Med. 382 (25), 2411–2418. doi:10.1056/NEJMoa2012410
GMA Consulting Group (2020). India implements new regulations for personal protective equipment. Available at URL: https://www.gma.trade/single-post/India-Implements-New-Regulation-for-Personal-Protective-Equipment (Accessed March 27 2020).
Godman, B., Bucsics, A., Vella Bonanno, P., Oortwijn, W., Rothe, C. C., Ferrario, A., et al. (2018). Barriers for access to new medicines: searching for the balance between rising costs and limited budgets. Front. Public Health 6, 328. doi:10.3389/fpubh.2018.00328
Godman, B., Grobler, C., Van-De-Lisle, M., Wale, J., Barbosa, W. B., Massele, A., et al. (2019a). Pharmacotherapeutic interventions for bipolar disorder type II: addressing multiple symptoms and approaches with a particular emphasis on strategies in lower and middle-income countries. Expet Opin. Pharmacother. 20 (18), 2237–2255. doi:10.1080/14656566.2019.1684473
Godman, B., Hill, A., Simoens, S., Kurdi, A., Gulbinovič, J., Martin, A. P., et al. (2019b). Pricing of oral generic cancer medicines in 25 European countries; findings and implications. Generics and Biosimilars Initiative Journal (GaBI Journal) 8 (2), 49–70.
Godman, B., Haque, M., McKimm, J., Abu Bakar, M., Sneddon, J., Wale, J., et al. (2020a). Ongoing strategies to improve the management of upper respiratory tract infections and reduce inappropriate antibiotic use particularly among lower and middle-income countries: findings and implications for the future. Curr. Med. Res. Opin. 36 (2), 301–327. doi:10.1080/03007995.2019.1700947
Godman, B., Basu, D., Pillay, Y., and Mwita, J. C., Rwegerera, G. M., Anand Paramadhas, B. D., et al. (2020b). Review of ongoing activities and challenges to improve the care of patients with type 2 diabetes across Africa and the implications for the future. Front. Pharm. 11, 108. doi:10.3389/fphar.2020.00108
Godman, B., McCabe, H., Leong, T. D., Mueller, D., Martin, A. P., Hoxha, I., et al. (2020c). Fixed dose drug combinations—are they pharmacoeconomically sound? Findings and implications especially for lower- and middle-income countries. Expert Rev. Pharmacoeconomics Outcomes Res. 20 (1), 1–26. doi:10.1080/14737167.2020.1734456
Godman, B., Basu, D., Pillay, Y., Almeida, P. H. R. F., Mwita, J. C., Rwegerera, G. M., et al. (2020d). Ongoing and planned activities to improve the management of patients with Type 1 diabetes across Africa; implications for the future. Hosp. Pract. 48 (2), 51–67. doi:10.1080/21548331.2020.1745509
Godman, B. (2020). Combating COVID-19: lessons learnt particularly among developing countries and the implications. Bangladesh J. of Med. Sci., Special Iss. on Covid19, S103–S108. doi:10.3329/bjms.v19i0.48413
Gupta, S., and Sud, V. (2020). India’s Modi promises $266 billion to protect economy from Covid-19. Available at URL: https://edition.cnn.com/2020/05/13/business/india-stimulus-covid-19-intl-hnk/index.html (Accessed May 13 2020).
Habersaat, K. B., Betsch, C., Danchin, M., Sunstein, C. R., Böhm, R., Falk, A., et al. (2020). Ten considerations for effectively managing the COVID-19 transition. Nat Hum Behav 4, 677–687.
Haque, M. (2020). Combating COVID-19: a coordinated efforts of healthcare providers and policy makers with global participation are needed to achieve the desired goals. Bangladesh. J. of Med. Sci., Special Iss. on Covid19, 1–5 doi:10.3329/bjms.v19i0.47610
Haque, M., Islam, S., Iqbal, S., Urmi, U. L., Kamal, Z. M., Shuvo, S. A., et al. (2020). Availability and price changes of potential medicines and equipment for the prevention and treatment of COVID-19 among pharmacy and drug stores in Bangladesh; findings and implications. Bangladesh J. Med. Sci. 19, S36–S50.
HealthWorld (2016). Fixing drug prices helped consumers save Rs 5,060 crore: Government. Available at URL: https://health.economictimes.indiatimes.com/news/pharma/fixing-drug-prices-helped-consumers-save-rs-5060-crore-government/55562102.
Horby, P., Lim, W. S., Emberson, J. R., Mafham, M., Bell, J. L., Linsell, L., et al. (2020a). Dexamethasone in hospitalized patients with covid-19 - preliminary report. N. Engl. J. Med.
Horby, P., Mafham, M., Linsell, L., Bell, J. L., Staplin, N., Emberson, J. R., et al. (2020b). Effect of Hydroxychloroquine in hospitalized patients with Covid-19. N. Engl. J. Med. 2020
Huang, I., Lim, M. A., and Pranata, R. (2020). Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia—a systematic review, meta-analysis, and meta-regression. Diabetes & metabolic syndrome 14 (4), 395–403.doi:10.1016/j.dsx.2020.04.018
Hung, I. F., Lung, K. C., Tso, E. Y., Liu, R., Chung, T. W., Chu, M. Y., et al. (2020). Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 395 (10238), 1695–1704. doi:10.1016/S0140-6736(20)31042-4
ICMR (2020a). How India ramped up COVID-19 testing capacity. Available at URL: https://www.icmr.gov.in/pdf/press_realease_files/ICMR_Press_Release_India_testing_story_20052020.pdf.
ICMR (2020b). SARS-CoV-2 (COVID-19) testing status. Available at URL: https://www.icmr.gov.
ICMR (2020c). Total Operational (initiated independent testing) Laboratories reporting to ICMR. Available at URL: https://www.icmr.gov.in/pdf/covid/labs/archive/COVID_Testing_Labs_08072020.pdf (Accessed July 8 2020).
ICMR (2020d). Advisory on use of rapid antigen detection test for COVID-19. Available at URL: https://www.icmr.gov.in/pdf/covid/strategy/Advisory_for_rapid_antigen_test14062020.pdf (Accessed June 14 2020).
ICMR (2020e). ICMR validates completely indigenous diagnostic platform for COVID-19 diagnosis. Available at URL: https://www.icmr.gov.in/pdf/press_realease_files/ICMR_Press_Release_TruNat_21052020.pdf.
IFRC, UNICEF and WHO (2020). Social stigma associated with COVID-19. Available at URL: https://reliefweb.int/sites/reliefweb.int/files/resources/covid19-stigma-guide.pdf.
India State-Level Disease Burden Initiative Diabetes C (2018). The increasing burden of diabetes and variations among the states of India: the Global Burden of Disease Study 1990–2016. Lancet Global Health 6 (12), e1352–e1362.
India State-Level Disease Burden Initiative CVDC (2018). The changing patterns of cardiovascular diseases and their risk factors in the states of India: the Global Burden of Disease Study 1990–2016. Lancet Global Health 6 (12), e1339.
Indian Council of Medical Research (2020). National guidelines for ethics committees reviewing biomedical & health research during COVID-19 pandemic. Availble at URL: https://www.icmr.gov.in/pdf/covid/techdoc/EC_Guidance_COVID19_06052020.pdf.
International Society of Antimicrobial Chemotherapy. Official Statement from International Society of Antimicrobial Chemotherapy (ISAC) - hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Available at URL: https://www.isac.world/news-and-publications/official-isac-statement.
ISAC/Elsevier (2020). Joint ISAC and Elsevier statement on Gautret et al. paper [PMID 32205204]. Available at URL: https://www.isac.world/news-and-publications/isac-elsevier-statement.
Kamath, S., Kamath, R., and Salins, P. (2020). COVID-19 pandemic in India: challenges and silver linings. Postgrad. Med.
Kasonde, L., Tordrup, D., Naheed, A., Zeng, W., Ahmed, S., and Babar, Z. U. (2019). Evaluating medicine prices, availability and affordability in Bangladesh using World health organisation and health action international methodology. BMC Health Serv. Res. 19 (1), 383. doi:10.1186/s12913-019-4221-z
Kasthuri, A. (2018). Challenges to healthcare in India—the five A’s. Indian J. Community Med. 43 (3), 141–143. doi:10.4103/ijcm.IJCM_194_18
Kastor, A., and Mohanty, S. K. (2018). Disease-specific out-of-pocket and catastrophic health expenditure on hospitalization in India: do Indian households face distress health financing? PLoS One 13 (5), e0196106. doi:10.1371/journal.pone.0196106
Khanal, S., Nissen, L., Veerman, L., and Hollingworth, S. (2016). Pharmacy workforce to prevent and manage non-communicable diseases in developing nations: the case of Nepal. Res. Soc. Adm. Pharm. 12 (4), 655–659. doi:10.1016/j.sapharm.2015.09.005
Khunti, K., Singh, A. K., Pareek, M., and Hanif, W. (2020). Is ethnicity linked to incidence or outcomes of covid-19? BMJ 369, m1548. doi:10.1136/bmj.m1548
Kirby, T. (2020). Evidence mounts on the disproportionate effect of COVID-19 on ethnic minorities. Lancet Respir. Med. 8 (6), 547–548. doi:10.1016/S2213-2600(20)30228-9
Klompas, M., Baker, M. A., and Rhee, C. (2020). Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. Jama 324 (5), 441–442. doi:10.1001/jama.2020.12458
Kluge, H. H. P., Wickramasinghe, K., Rippin, H. L., Mendes, R., Peters, D. H., Kontsevaya, A., et al. (2020). Prevention and control of non-communicable diseases in the COVID-19 response. Lancet 395(10238), 1678–1680. doi:10.1016/S0140-6736(20)31067-9
Kumar, S. U., Kumar, D. T., Christopher, B. P., and Doss, C. G. P. (2020). The rise and impact of COVID-19 in India. Front. Med. 7, 250. doi:10.3389/fmed.2020.00250
Kwasi, E. D. (2020). Chloroquine, coronavirus and Ghana’s preparedness: old hero to the rescure. Available at URL: https://www.myjoyonline.com/news/health/chloroquine-coronavirus-and-ghanas-preparedness-old-hero-to-the-rescue/.
Lee, Y. N. (2020). Southeast Asia could be the next coronavirus hot spot—these charts show why Available at URL: https://www.cnbc.com/2020/04/20/southeast-asia-could-be-the-next-coronavirus-hot-spot-these-charts-show-why.html.
Li, Q., Guan, X., Wu, P., Wang, X., Zhou, L., Tong, Y., et al. (2020). Early transmission dynamics in wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 382 (13), 1199–1207. doi:10.1056/NEJMoa2001316
Ma, X., and Vervoort, D. (2020). Critical care capacity during the COVID-19 pandemic: global availability of intensive care beds. J. Crit. Care 58, 96–97. doi:10.1016/j.jcrc.2020.04.012
Markovic-Pekovic, V., Grubisa, N., Burger, J., Bojanic, L., and Godman, B. (2017). Initiatives to reduce nonprescription sales and dispensing of antibiotics: findings and implications. J. Res. Pharm. Pract. 6 (2), 120–125. doi:10.4103/jrpp.JRPP_17_12
McCreary, E. K., and Angus, D. C. (2020). Efficacy of remdesivir in COVID-19. Jama 324 (11), 1041–1042. doi:10.1001/jama.2020.16337
Mehndiratta, A., Sharma, S., Gupta, N. P., Sankar, M. J., and Cluzeau, F. (2017). Adapting clinical guidelines in India-a pragmatic approach. BMJ 359, j5147. doi:10.1136/bmj.j5147
Mehra, M. R., Desai, S. S., Ruschitzka, F., and Patel, A. N. (2020a). Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. The Lancet 22. doi:10.1016/S0140-6736(20)31180-6
Mehra, M. R., Ruschitzka, F., and Patel, A. N. (2020b). Retraction—hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. doi:10.1016/S0140-6736(20)31324-6
Mendel, A., Bernatsky, S., Thorne, J. C., Lacaille, D., Johnson, S. R., and Vinet, É. (2020). Hydroxychloroquine shortages during the COVID-19 pandemic. Ann. Rheu. Dis., doi:10.1136/annrheumdis-2020-217835
Miljković, N., Godman, B., van Overbeeke, E., Kovačević, M., Tsiakitzis, K., Apatsidou, A., et al. (2020). Risks in antibiotic substitution following medicine shortage: a health-care failure mode and effect analysis of six European hospitals. Front. Med. 7, 157. doi:10.3389/fmed.2020.00157
Millard, C., Kadam, A. B., Mahajan, R., Pollock, A. M., and Brhlikova, P. (2018). Availability of brands of six essential medicines in 124 pharmacies in Maharashtra. J Glob Health 8 (1), 010402. doi:10.7189/jogh.08.010402
Ministry of Health and Family Welfare Government of India (2020). COVID-19 India. Available at URL: https://www.mohfw.gov.
Ministry of Home Affairs India (2020a). Extension of lockdown for a further period of two weeks with effect from May 4, 2020. Available at URL: https://pib.gov.in/PressReleasePage.aspx?PRID=1620095 (Accessed May 01 2020).
Ministry of Home Affairs India (2020b). New guidelines to fight COVID-19. Available at URL: https://pib.gov.in/PressReleasePage.aspx?PRID=1627965 (Accessed June 01 2020).
Mishra, S., Joseph, R. A., Gupta, P. C., Pezzack, B., Ram, F., Sinha, D. N., et al. (2016) Trends in bidi and cigarette smoking in India from 1998 to 2015, by age, gender and education. BMJ Glob Health 1 (1), e000005. doi:10.1136/bmjgh-2015-000005
Mohan, P., Lando, H. A., and Panneer, S. (2018). Assessment of tobacco consumption and control in India. Indian J. Clin. Med. 9, 1–8. doi:10.1177/1179916118759289
Mohathasim Billah, A., and Venkatesan, P. (2017). A self-limited survey on community pharmacies in India, the services offered, facilities available to make ease of compliance for the medication prescribed and over the counter medication in view of pharmacists. J. Pharmaceut. Sci. Res. 9 (3), 314–317.
Moorkens, E., Vulto, A. G., Huys, I., Dylst, P., Godman, B., Keuerleber, S., et al. (2017). Policies for biosimilar uptake in Europe: an overview. PloS One 12 (12), e0190147. doi:10.1371/journal.pone.0190147
Nafade, V., Huddart, S., Sulis, G., Daftary, A., Miraj, S. S., Saravu, K., et al. (2019). Over-the-counter antibiotic dispensing by pharmacies: a standardised patient study in Udupi district, India. BMJ Glob Health 4 (6), e001869. doi:10.1136/bmjgh-2019-001869
Natnayas, E., Gaikwad, V. E., and Jain, Y. (2020). Rolling out mass hydroxychloroquine prophylaxis for covid-19 in India’s slums risks eroding public trust. Available at URL: https://blogs.bmj.com/bmj/2020/05/01/rolling-out-mass-hydroxychloroquine-prophylaxis-for-covid-19-in-indias-slums-risks-public-trust/.
Ng, Y., Li, Z., Chua, Y. X., Chaw, W. L., Zhao, Z., Er, B., et al. (2020). Evaluation of the effectiveness of surveillance and containment measures for the first 100 patients with COVID-19 in Singapore - January 2-February 29, 2020. MMWR Morb. Mortal. Wkly. Rep. 69 (11), 307–311. doi:10.15585/mmwr.mm6911e1
NIH, (2020). NIH halts clinical trial of hydroxychloroquine. Available at URL: https://www.nhlbi.nih.gov/news/2020/nih-halts-clinical-trial-hydroxychloroquine (Accessed June 20 2020).
Ogunleye, O. O., Basu, D., Mueller, D., Sneddon, J., Seaton, R. A., Yinka-Ogunleye, A. F., et al. (2020). Response to the novel corona virus (COVID-19) pandemic across Africa: successes, challenges, and implications for the future. Front. Pharmacol. 11 (1205).
Ongole, J. J., Rossouw, T. M., Fourie, P. B., Stoltz, A. C., Hugo, J., and Marcus, T. S. (2020). Sustaining essential healthcare in Africa during the COVID19 pandemic. Available at URL: https://www.theunion.org/news-centre/news/body/IJTLD-June-0214_Ongole.pdf.
Pai, C., Bhaskar, A., and Rawoot, V. (2020). Investigating the dynamics of COVID-19 pandemic in India under lockdown. Chaos, Solit. Fractals 138, 109988. doi:10.1016/j.chaos.2020.109988
pal Jeyamani, S. V., Rajan, A. K., Sahayamary, F. M., and Magesh, M. (2018). Knowledge, attitude and perception of community pharmacists about the professional standards and responsibilities entrusted by pharmacy practice regulation, 2015- A cross sectional survey in the state of Tamil nadu. Acta Sci. Pharma. Sci. 2 (7), 55–59.
Pandey, V. (2020). Coronavirus: India’s race against time to save doctors. Available at URL: https://www.bbc.co.uk/news/world-asia-india-52215071 (Accessed April 13 2020).
Pavithra, K. M. (2019). Explainer: are medicine prices controlled in India? Available at URL: https://factly.in/explainer-are-medicine-prices-controlled-in-india/.
PIBG (2020). Press Information Bureau Government of India Ministry of Home Affairs - Government of India issues Orders prescribing lockdown for containment of COVID-19 Epidemic in the country. Available at URL: https://pib.gov.in/newsite/PrintRelease.aspx?relid=200655.
Pradhan, D., Biswasroy, P., Kumar Naik, P., Ghosh, G., and Rath, G. (2020). A review of current interventions for COVID-19 prevention. Arch. Med. Res. 51 (5), 363–374. doi:10.1016/j.arcmed.2020.04.020
Prime Minister’s Office (2020). Text of PM’s address to the Nation. Available at URL: https://pib.gov.in/PressReleseDetail.aspx?PRID=1614215.
Public Health England (2020). Disparities in the risk and outcomes of COVID-19. Available at URL:. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/892085/disparities_review.pdf.
Pulla, P. (2020). ICMR’s latest clarification on its hydroxychloroquine policy is just baffling. Available at URL: https://science.thewire.in/health/covid-19-hydroxychloroquine-policy-raman-gangakhedkar-icmr-observational-study/.
Rajkumar, R. P. (2020). COVID-19 and mental health: a review of the existing literature. Asian J Psychiatr 52, 102066. doi:10.1016/j.ajp.2020.102066
Ramakrishnan, S., Zachariah, G., Gupta, K., Shivkumar Rao, J., Mohanan, P. P., Venugopal, K., et al. (2019). Prevalence of hypertension among Indian adults: results from the great India blood pressure survey. Indian Heart J. 71 (4), 309–313. doi:10.1016/j.ihj.2019.09.012
Rathi, S., Ish, P., Kalantri, A., and Kalantri, S. (2020). Hydroxychloroquine prophylaxis for COVID-19 contacts in India. The lancet infectious diseases.
Ray, A., Najmi, A., Khandelwal, G., and Sadasivam, B. (2020). A cost variation analysis of drugs available in the indian market for the management of thromboembolic disorders. Cureus 12 (5), e7964. doi:10.7759/cureus.7964
Reddy, K. S. (2018). Health care reforms in India. JAMA 319 (24), 2477–2478. doi:10.1001/jama.2018.5284
Richardson, S., Hirsch, J. S., Narasimhan, M., Crawford, J. M., McGinn, T., Davidson, K. W., et al. (2020). Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA 323 (20), 2052–2059. doi:10.1001/jama.2020.6775
Rosenberg, E. S., Dufort, E. M., Udo, T., Wilberschied, L. A., Kumar, J., Tesoriero, J., et al. (2020). Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA 323 (24):, 2493–2502. doi:10.1001/jama.2020.8630
Roy, A., Ghoshal, D., and Kalra, A. (2020). More patients than beds in Mumbai as India faces surge in virus cases. Available at URL: https://uk.reuters.com/article/uk-health-coronavirus-india-medics-insig/more-patients-than-beds-in-mumbai-as-india-faces-surge-in-virus-cases-idUKKBN23103D.
Saaliq, S. (2020). Limited access to clean water among India's poor spawns coronavirus concerns. Available at URL: https://news.yahoo.com/limited-access-clean-water-among-130525398.html.
SADC (2020). Statement by the SADC executive secretary, H.E. Dr stergomena lawrence tax on covid-19 and gender based violence and domestic violence. Available at URL: https://www.sadc.int/news-events/news/statement-sadc-executive-secretary-he-dr-stergomena-lawrence-tax-covid-19-and-gender-based-violence-and-domestic-violence/.
Salo, J. (2020). Nigeria reports poisonings from possible coronavirus drug chloroquine. Available at URL: https://nypost.com/2020/03/22/nigeria-reports-poisonings-from-possible-coronavirus-drug-chloroquine/ (Accessed March 22 2020).
Sanders, J. M., Monogue, M. L., Jodlowski, T. Z., and Cutrell, J. B. (2020). Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. Jama.
Satheesh, G., Puthean, S., and Chaudhary, V. (2019). E-pharmacies in India: can they improve the pharmaceutical service delivery? J Glob Health 10 (1), 010301. doi:10.7189/jogh.10.010302
Scavone, C., Brusco, S., Bertini, M., Sportiello, L., Rafaniello, C., Zoccoli, A., et al. (2020). Current pharmacological treatments for COVID-19: what’s next?. Br. J. Pharmacol. 177 (21). 4813–4824.doi:10.1111/bph.15072
Selvaraj, S., Farooqui, H. H., and Karan, A. (2018). Quantifying the financial burden of households' out-of-pocket payments on medicines in India: a repeated cross-sectional analysis of National Sample Survey data, 1994–2014. BMJ open, 8 (5). e018020. doi:10.1136/bmjopen-2017-018020
Sibbal, S. (2020). India approves Malaysia’s request for supplying hydroxychloroquine drug to deal with COVID-19 crisis. Available at URL: https://zeenews.india.com/india/india-approves-malaysia-s-request-for-supplying-hydroxychloroquine-drug-to-deal-with-covid-19-crisis-2279664.html (Accessed April 28 2020).
Siddiqui, Z. (2020). India coronavirus cases hit record high amid monsoon rains. Available at URL: https://www.msn.com/en-gb/news/world/india-coronavirus-cases-hit-record-high-amid-monsoon-rains/ar-BB16k1PG?ocid=msedgdhp (Accessed July 4 2020).
Sonwalkar, P. (2020). Covid-19: Indians, non-whites in UK more at risk of death. Available at URL: https://www.hindustantimes.com/indians-abroad/covid-19-indians-non-whites-in-uk-more-at-risk-of-death/story-aDtiNXMxE1xJJaPwSX9t4O.html.
Sood, N., and Wagner, Z. (2020). JAMA health forum. India’s historic effort to expand health insurance to individuals living below the poverty line. Available at URL: https://jamanetwork.com/channels/health-forum/fullarticle/2763530.
Soon, J. M. Consumers’ awareness and trust toward food safety news on social media in Malaysia (2020). J. Food Protect. 83 (3), 452–459. doi:10.4315/0362-028X.JFP-19-415
Soumya, R., Devarashetty, V., Jayanthi, C. R., and Sushma, M. (2016). Drug dispensing practices at pharmacies in Bengaluru: a cross-sectional study. Indian J. Pharmacol. 48 (4), 360–364. doi:10.4103/0253-7613.186204
Sousa Pinto, G., Bader, L., Billberg, K., Criddle, D., Duggan, C., El Bizri, L., et al. (2020). Beating non-communicable diseases in primary health care: the contribution of pharmacists and guidance from FIP to support WHO goals. Res. Soc. Adm. Pharm. 16 (7), 974–977. doi:10.1016/j.sapharm.2019.10.008
Spinner, C. D., Gottlieb, R. L., Criner, G. J., Arribas López, J. R., Cattelan, A. M., Soriano Viladomiu, A., et al. (2020). Effect of remdesivir vs standard care on clinical status at 11 Days in patients with moderate COVID-19: a randomized clinical trial. Jama 324 (11), 1048–1057. doi:10.1001/jama.2020.16349
Statista, (2020) Fears and concerns arising from the coronavirus (COVID-19) across India in April 2020. Available at URL: https://www.statista.com/statistics/1111149/india-opinion-on-coronavirus-fears-and-concerns/ (Accessed April 26 2020).
Suliankatchi Abdulkader, R., Sinha, D. N., Jeyashree, K., Rath, R., Gupta, P. C., Kannan, S., et al. (2020). Trends in tobacco consumption in India 1987-2016: impact of the World health organization framework convention on tobacco control. Int J Public Health 64 (6), 841–851. doi:10.1007/s00038-019-01252-x
The Hindu (2020). Coronavirus India lockdown Day 159 updates. Available at URL: https://www.thehindu.com/news/national/coronavirus-india-lockdown-august-30-2020-live-updates/article32478249.ece#! (Accessed August 30 2020).
Tilangi, P., Desai, D., Khan, A., and Soneja, M. (2020). Hydroxychloroquine prophylaxis for high-risk COVID-19 contacts in India: a prudent approach. The Lancet Infectious diseases.
Topp, S. Malaysia’s youngest hospital CEO on fighting coronavirus on the front lines. 4 April 2020. Available at URL: https://generationt.asia/leaders/malaysias-youngest-hospital-ceo-on-fighting-coronavirus-on-the-front-lines (Accessed April 4 2020).
Vaduganathan, M., van Meijgaard, J., Mehra, M. R., Joseph, J., O'Donnell, C. J., and Warraich, H. J. (2020). Prescription fill patterns for commonly used drugs during the COVID-19 pandemic in the United States. Jama 323 (24), 2524–2526. doi:10.1001/jama.2020.9184
Vardavas, C. I., and Nikitara, K. (2020). COVID-19 and smoking: a systematic review of the evidence. Tob. Induc. Dis. 18, 20.
Vijayakumar, G., Manghat, S., Vijayakumar, R., Simon, L., Scaria, L. M., Vijayakumar, A., et al. (2019) Incidence of type 2 diabetes mellitus and prediabetes in Kerala, India: results from a 10-year prospective cohort. BMC Public Health 19 (1), 140. doi:10.1186/s12889-019-6445-6
Wang, Y., Zhang, D., and Du, G., (2020). Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 395 (10236), 1569–1578. doi:10.1016/S0140-6736(20)31022-9
Weston, M. (2020). Coronavirus reaches Sudan, one of the countries least equipped to cope with it. Available at URL: https://mg.co.za/africa/2020-03-24-coronavirus-reaches-sudan-one-of-the-countries-least-equipped-to-cope-with-it/ (Accessed June 24 2020).
WHO (2018). Fact Sheet India 2018. Heart disease and stroke are the commonest ways by which tobacco kills people. Available at URL: https://apps.who.int/iris/bitstream/handle/10665/272672/wntd_2018_india_fs.pdf?sequence=1.
WHO (2020a). Coronavirus disease (COVID-19) Situation Report- 27 September 2020. Available at URL: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200928-weekly-epi-update.pdf?sfvrsn=9e354665_4 (Accessed October 1 2020).
WHO (2020b). Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. Available at URL: https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations.
WHO (2020c). WHO discontinues hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID-19. Available at URL: https://www.who.int/news-room/detail/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19 (Accessed July 4 2020).
WHO India (2020a). Novel coronavirus disease (COVID-19) situation update report-1. Available at URL: https://www.who.int/docs/default-source/wrindia/india-situation-report-1.pdf?sfvrsn=5ca2a672_0.
WHO India (2020b). Novel coronavirus disease (COVID-19) situation update report-2. Available at URL: https://www.who.int/docs/default-source/wrindia/india-situation-report-2.pdf?sfvrsn=962f294b_0.
WHO India (2020c). Novel coronavirus disease (COVID-19) situation update report - 6. Available at URL:https://www.who.int/docs/default-source/wrindia/situation-report/india-situation-report-6606711da860b4d38b266c91265952977.pdf?sfvrsn=2f6c5c95_2.
WHO India (2020d). Novel coronavirus disease (COVID-19) situation update report - 7. Available at URL: https://www.who.int/docs/default-source/wrindia/situation-report/india-situation-report-7.pdf?sfvrsn=cf4a7312_2.
WHO India (2020e). Novel coronavirus disease (COVID-19) situation update report - 8. Available at URL: https://www.who.int/docs/default-source/wrindia/situation-report/india-situation-report-8bc9aca340f91408b9efbedb3917565fc.pdf?sfvrsn=5e0b8a43_2.
World Health Organisation (2020). Operational considerations for case management of COVID-19 in health facility and community: interim guidance. Available at URL: https://apps.who.int/iris/handle/10665/331492 (Accessed March 19 2020).
Wu, Z., and McGoogan, J. M. (2020). Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 323 (13), 1239–1242. doi:10.1001/jama.2020.2648
Yadav, P., Mehndiratta, A., Chalkidou, K., Rupani, S., and Reddy, K. (2020). India’s COVID-19 testing capacity must grow by a factor of 10: here’s how that can happen. Available at URL: https://www.cgdev.org/publication/indias-covid-19-testing-capacity-must-grow-factor-10-heres-how-can-happen.
Zhao, J., Yang, Y., Huang, H., Li, D., Gu, D., Lu, X., et al. (2020). Relationship between the ABO blood group and the COVID-19 susceptibility. MedRxiv preprint. Available at: https://www.medrxiv.org/content/10.1101/2020.03.11.20031096v2.full.pdf (Accessed March 11 2020).
Keywords: community pharmacists, India, lower- and middle-income countries, medicines, protective equipment, price rises, shortages
Citation: Haque M, Kumar S, Charan J, Bhatt R, Islam S, Dutta S, Abhayanand JP, Sharma Y, Sefah I, Kurdi A, Wale J and Godman B (2021) Utilisation, Availability and Price Changes of Medicines and Protection Equipment for COVID-19 Among Selected Regions in India: Findings and Implications. Front. Pharmacol. 11:582154. doi: 10.3389/fphar.2020.582154
Received: 10 July 2020; Accepted: 14 October 2020;
Published: 14 January 2021.
Edited by:
Debjani Mueller, Charlotte Maxeke Medical Research Center (CMeRC), South AfricaReviewed by:
Gouri Shankar Bhattacharyya, Independent Researcher, Kolkata, IndiaKedar Joshi, Hanul Medizin LLP, India
Copyright © 2021 Haque, Kumar, Charan, Bhatt, Islam, Dutta, Abhayanand, Sharma, Sefah, Kurdi, Wale and Godman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brian Godman, YnJpYW4uZ29kbWFuQHN0cmF0aC5hYy51aw==
 Mainul Haque
Mainul Haque Santosh Kumar
Santosh Kumar Jaykaran Charan
Jaykaran Charan Rohan Bhatt4
Rohan Bhatt4 Salequl Islam
Salequl Islam Siddhartha Dutta
Siddhartha Dutta Israel Sefah
Israel Sefah Amanj Kurdi
Amanj Kurdi Brian Godman
Brian Godman