- 1Department of Pharmacy, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Objective: Prevalence of osteoporosis in Chinese postmenopausal women has significantly increased over the past decade and oral bisphosphonates are the most potent antiresorptive drugs. The purpose of the present research was to evaluate the cost-effectiveness of oral alendronate for individuals with osteoporosis. We also assessed the impact of medication compliance and persistence on economic outcomes of alendronate and potential economic evaluations of persistence-enhancing interventions.
Methods: We constructed an individual-level state-transition model to project health outcomes and costs of oral alendronate for Chinese postmenopausal osteoporotic women. The impact of medication compliance and persistence on economic evaluation was addressed in various scenario analyses. Model inputs were derived from clinical trials and published sources, where available. Deterministic and probabilistic sensitivity analyses were conducted to explore the impact of uncertainties and assumptions on the cost-effectiveness results.
Results: Compared with no treatment, alendronate treatment was associated with an additional 0.052 QALYs (quality-adjusted life-years) at an additional cost of USD 738, which yielded an incremental cost-effectiveness ratio (ICER) of USD 14,192.308/QALY. The ICER for the different scenarios (full compliance, full persistence, and both full persistence and full compliance) was USD 4,933.333/QALY, USD 3,006.849/QALY, and USD 2,049.822/QALY, respectively. One-way sensitivity analysis showed the ICER was most sensitive to variations in time horizon and residual effect. Probabilistic sensitivity analysis demonstrated that, at a willingness to pay of USD 29,340/QALY, the probability that oral alendronate therapy will be cost-effective is approximately 80%.
Conclusion: The findings support the view that oral alendronate is cost-effective for the treatment of osteoporotic fractures in Chinese postmenopausal women. Medication persistence is found to have a greater impact on cost-effectiveness than compliance and interventions to improve persistence to be an efficient use of resources.
Introduction
Osteoporosis, or porous bone, is a disease characterized by low bone mass and structural deterioration of bone tissues, leading to bone fragility and an increased risk of fractures (Watts, 2018). The International Osteoporosis Foundation (IOF) estimates that, by 2050, more than 50% of all osteoporotic fractures will occur in Asia, and China will be most severely affected due to its large population of seniors (Pisani et al., 2016). Fractures significantly affect patients by impairing their ability to perform daily activities. Moreover, a health economics model was developed and forecasted that the costs of osteoporotic fractures in China will double by 2035, rising to approximately USD 25.58 billion by 2050, indicating that, in addition to morbidity and mortality, osteoporotic fractures are also associated with a significant health care expenditure to the society (Si et al., 2015; Liu et al., 2018).
Fortunately, medical advancements have increased the range of therapeutic options available for the prevention and treatment of fractures (Iolascon et al., 2020). Currently, oral bisphosphonates are the most potent antiresorptive drugs for the treatment of osteoporosis in postmenopausal women (Maraka and Kennel, 2015). Multiple meta-analyses and systematic reviews have shown that bisphosphonates are effective in decreasing the risk of various types of bone fractures (Feng et al., 2013; Brown et al., 2014). However, it is widely acknowledged that compliance (the extent to which a patient acts in accordance with the prescribed interval and dose of a dosing regimen) and persistence (duration of time from initiation to discontinuation of the therapy) with oral osteoporosis medications are poor (Imaz et al., 2010; Hiligsmann et al., 2012a; Hiligsmann et al., 2012b; Fatoye et al., 2019). A recent observational study estimated that 53% of the study population achieved a medication possession ratio (MPR) of 80% or higher 6 months after initiating therapy, and the equivalent value for 7–12 months was only 43% (Kothawala et al., 2007). Persistence, or the length of time a patient continues therapy, is similarly poor. It has been reported that the rate of persistence among new users was 46% after 7–12-month treatment period (Kothawala et al., 2007).
Although poor compliance and persistence decrease the medicine costs, the effectiveness of treatment is also reduced, which reduces bone mineral density and in turn leads to a higher risk of fractures (Hiligsmann et al., 2010; Hiligsmann et al., 2012a; Hiligsmann et al., 2012b). Hence, in order to estimate the cost-effectiveness of the intervention in real-world settings, it is important that economic evaluations take compliance and persistence into account.
In our recent study (You et al., 2020), we demonstrated the cost-effectiveness of yearly intravenous zoledronic acid, which has a higher persistence and compliance rate. Further economic evaluation of alendronate is an evidence gap that could inform prescribers about the potential loss of benefits resulting from poor compliance and persistence. More specifically, we first compared the clinical and economic outcomes derived from a real-life setting with those expected with full compliance and persistence. In addition, we further evaluated the potential economic value of persistence-enhancing interventions.
Methods
Overview
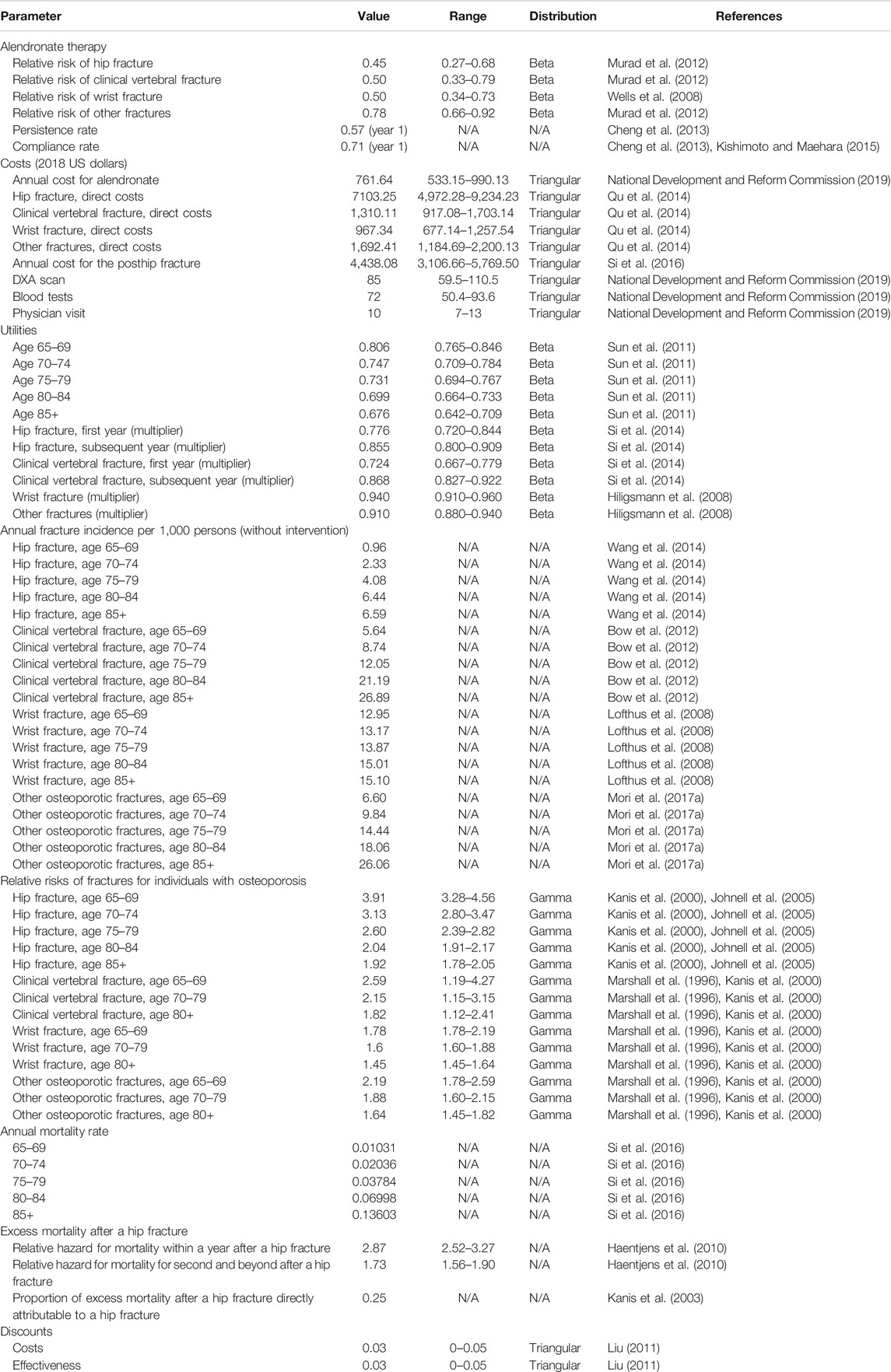
The development of this model adhered to the recommendations for the conduct of economic evaluations in osteoporosis (Hiligsmann et al., 2019). We used an updated version of the previously validated individual-level state-transition model (You et al., 2020) to estimate the impact of the compliance and persistence on the cost-effectiveness of alendronate treatment for Chinese postmenopausal osteoporotic women aged 65 and older. The model estimated the outcomes including the number of fracture quality-adjusted life-years (QALYs); direct societal costs in 2018 US dollars (USD); and incremental cost-effectiveness ratios (ICERs) per QALY gained. Costs and health outcomes beyond the first year were discounted at an annual rate of 3%, which is consistent with Chinese guidelines for pharmacoeconomic evaluations (Liu, 2011). We assessed cost-effectiveness from the health care payer perspectives and considered three times per capita gross domestic product of China in 2018 (USD 29,340) as the threshold. We used TreeAge Pro 2018 (TreeAge Software Inc., Williamston, MA, USA) to perform our analyses.
Model Structure
We modeled the disease progression of osteoporosis through six states: no fracture, hip fracture, clinical vertebral fracture, wrist fracture, other osteoporotic fractures, and death. The other osteoporotic fractures (i.e., humerus, distal forearm other than wrist, tibia/fibula, pelvis, or femur other than hip) as defined by the IOF-EFPIA report (Svedbom et al., 2013). The cycle length of the model was 1 year which was chosen to represent a clinically meaningful time interval. Each individual can sustain only one fracture per cycle and can experience up to two hip fractures but unlimited clinical vertebral, wrist, and other osteoporotic fractures during the entire study period. We used tracker variables to record individual characteristics and disease histories, which adjusted transition probabilities, costs, and utilities. Table 1 shows the key parameters used in the health economics model. A more detailed description of the model can be found in our previously published work (You et al., 2020).
Fracture Incidence and Mortality Rates
Hip and vertebral fracture incidences were derived from reported epidemiological data in China (Bow et al., 2012; Wang et al., 2014). Estimation of the incidence rates of the wrist and other osteoporotic fractures in the Chinese context was not available; hence, we utilized data collected from an Asian population (Melton et al., 1999; Lofthus et al., 2008). The incidence of fracture in the general population was further adjusted to accurately reflect the fracture risks of women with osteoporosis. The method calculated the relative risks for bone mineral density using a method previously described (Marshall et al., 1996; Kanis et al., 2000; Johnell et al., 2005).
Baseline mortality rates for age-stratified Chinese women were retrieved from the China Public Health Statistical Yearbook (National Health Committee of the People's Republic of China, 2018) and increased mortality was assumed for individuals who experienced the hip fracture (Haentjens et al., 2010). Because excess mortality may be attributable to comorbidities in this older population, only 25% of the excess mortality was considered to be attributable to the fractures themselves (Kanis et al., 2003). There was no increase in mortality following clinical vertebral, wrist, and other fractures (Mori et al., 2017a; Mori et al., 2017b).
Treatment
We assumed that treated women received alendronate 70 mg once a week for 5 years. Relative risks for fractures in women taking alendronate were based on the recent systematic reviews (Wells et al., 2008; Murad et al., 2012). It was assumed that reductions in fracture risk during therapy were consistent regardless of patients’ age and there was no significant change in bioequivalence between brand name and generic drugs. We also assigned the cost of one general consultation visit, bone mineral density, and biochemical test per year, as suggested by the Chinese guidelines for the diagnosis and treatment of primary osteoporosis (Chinese Society of Osteoporosis and Bone Mineral Research, 2019).
Inadequate medication compliance and persistence are known to be major problems in all patients with osteoporotic disease (Stevenson and Selby, 2014). We considered compliance and persistence rates of alendronate obtained on the observational studies in the Chinese or Asian population (Cheng et al., 2013; Kishimoto and Maehara, 2015). Compliance rates with oral alendronate were higher in clinical than observational studies. The influence of their difference was incorporated into the microsimulation model by assuming a linear relationship between the relative risk reduction and medication compliance (Mori et al., 2017a; Mori et al., 2017b). In addition, we modeled the residual effects of alendronate for those who discontinue therapy (called offset-time effect). We assumed that if individuals stopped treatment, they received no further therapy and offset time was assumed to be equal to their treatment period (Hiligsmann et al., 2012a; Hiligsmann et al., 2012b).
Costs
The cost of alendronate was based on different brand prices and corresponding market share in China. Total medication costs were multiplied by their compliance and persistence levels. We charged the cost of 6-month alendronate supply for individuals who discontinued alendronate within the first year. The estimated annual costs related to hip fracture of the first year and long-term care costs were obtained from previously published studies in Chinese setting (Qu et al., 2014; Si et al., 2016). Costs of physician visits, DXA scan, laboratory tests, and nursing home residence were collected from the health system or the National Development and Reform Commission of China (National Development and Reform Commission, 2019). All original costs were converted to a common currency and price year, 2018 United States dollars (USD), given the latest version of a web-based cost converter (CCEMG-EPPI-Centre Cost Converter, 2008).
Utilities
The Chinese National Health Services Survey in China has established the utility values in osteoporosis (Sun et al., 2011). No disutilities were assumed for simulated individuals without fractures. Fracture events were associated with decrements in utility values which differed between the fracture sites and time. The quality-of-life multipliers were based on a recent meta-analysis (Hiligsmann et al., 2008; Si et al., 2014).
Model Simulation and Sensitivity Analysis
We performed base-case, deterministic (one-way) sensitivity, probabilistic sensitivity, and scenario analyses. For baseline analysis, we ran the model with 100,000 iterations (100,000 individuals through the model one at a time). One-way sensitivity analysis was undertaken to examine the effect of each key model parameter, including fracture costs and disutilities, medication costs, initial age of treatment, time horizon, residual effect, and discount rates. Probabilistic sensitivity analysis was conducted to evaluate the impact of the joint uncertainty surrounding the model variables using Monte-Carlo simulations (1,000 simulations and 10,000 trials per simulation). We also examined different scenarios: A) the individuals with full compliance, B) the individuals with full persistence, C) the individuals with both full persistence and full compliance, and D) potential persistence-enhancing interventions.
Results
Model Validation
The probability of dying by 105 years for untreated individuals at the ages of 65, 70, 75, and 80 predicted by our model was 99.0%, 98.8%, 98.5%, and 98.5%, respectively. Model-predicted mortality risks were comparable to the Chinese life table (National Health Committee of the People's Republic of China, 2018). We also projected that, without intervention, the cumulative probability of having at least one hip fracture or clinical vertebral fracture is equal to 11.099% and 39.693%, respectively, which is comparable to the epidemiological data in China (Chinese Society of Osteoporosis and Bone Mineral Research, 2019).
Base-Case Findings
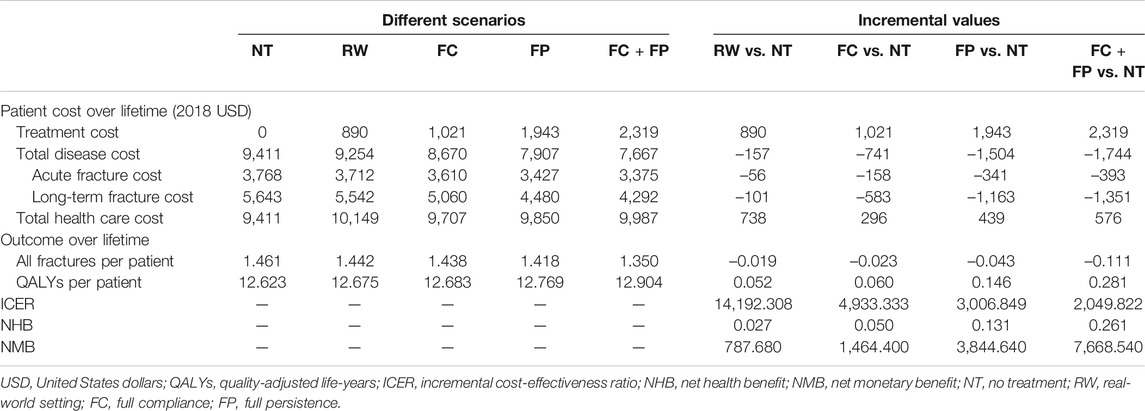
Table 2 presented the total health care costs, the number of fractures, QALYs, and ICER estimated by the model. Compared with no treatment (mean cost USD 9,411; mean effect 12.623 QALYs), alendronate treatment in the real-world setting (mean cost USD 10,149; mean effect 12.675 QALYs) was associated with an overall increase in total health care cost of USD 738 and in QALYs of 0.052, yielded in an ICER of USD 14,192.308/QALY gained. Besides, both NMB and NHB were positive, and further indicated oral alendronate is more cost-effective than no intervention.
Sensitivity Analysis Findings
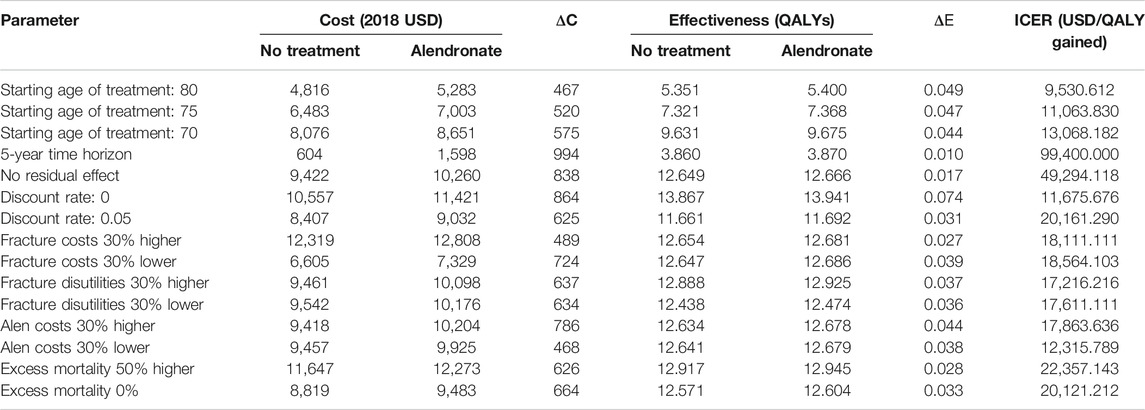
Deterministic sensitivity analysis showed that the most impactful parameters in the model were the time horizon and the residual effect. The ICER was markedly increased to USD 994,000/QALY when reducing the time horizon from lifetime to 5 years. Assuming no residual effect following treatment resulted in ICER increase to USD 49,294.118/QALY (Table 3).
Probabilistic sensitivity analysis confirmed the aforementioned results (Figure 1). At a threshold of USD 29,340/QALY, the probability that alendronate would be cost-effective was approximately 80% for individuals aged 65.
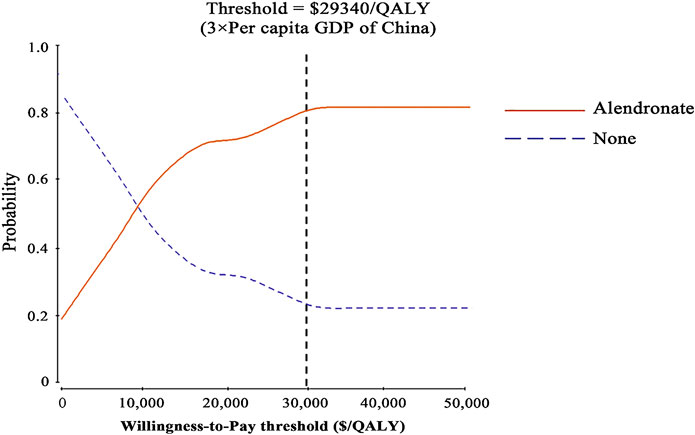
FIGURE 1. Results of probabilistic sensitivity analyses. The cost-effectiveness acceptability curves represent probabilities of being cost-effective achieved by the alendronate strategy compared to no treatment at thresholds for postmenopausal osteoporotic women.
Scenario Analysis Findings
The results of the scenario analysis considering alendronate therapy compliance and persistence were shown in Table 2 and Figure 2. The lifetime cost per person was USD 9,707 for the full compliance scenario, USD 9,850 for the full persistence scenario, and USD 9,987 for both full persistence and full compliance scenario. Total cost was lower in the scenario analysis than in the real-world setting, as the prevented costs of treating additional osteoporotic fractures resulting from noncompliance and persistence exceed the cost of the additional therapy induced by the improved compliance and persistence.
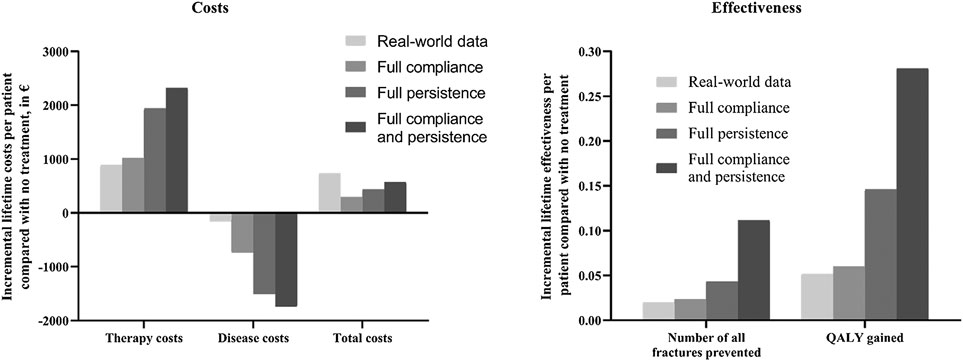
FIGURE 2. Impact of medication compliance and persistence on therapy, disease, total costs, and health outcomes (expressed as number of fractures prevented and QALY gained). QALY, quality-adjusted life-year.
Effectiveness was measured as the number of all osteoporotic fractures and quality-adjusted life-years. The lifetime number of all fractures per person was 1.438 for the full compliance scenario, 1.418 for the full persistence, and 1.350 for both full compliance and full persistence. Hence, the number of osteoporotic fractures prevented in real-world setting represented 81.2%, 43.8%, and 17.1% to that estimated with full compliance, full persistence, and both full compliance and full persistence scenario, respectively. Mean lifetime QALYs were estimated at 12.683, 12.769, and 12.904 in all scenarios tested, respectively. The QALYs gained in the real-world scenario represent 86.7%, 35.6%, and 18.5% to that obtained under the above three scenarios, respectively.
Compared with no treatment, the ICER for the three scenarios ranged from USD 2019.822/QALY to USD 4933.333/QALY. These results were all lower than those derived from real-world analysis. It should be noted that three different scenarios were associated with lower costs and great QALYs than the real-world setting, indicating that the improvement of compliance and persistence was found to be cost-saving.
Figure 3 displayed the economic assessment of persistence-enhancing interventions based on differential reduction in treatment discontinuation and their corresponding cost. When the reductions in treatment discontinuation were high (> 30%) and the invention costs were low (< USD 100), the ICER was less than USD 9,780/QALY (1 × GDP per capita) and could be considered highly cost-effective. Conversely, when the invention costs were high (> USD 400) and the reductions in treatment discontinuation were low (< 10%), the ICER was more than USD 29,340/QALY (3 × GDP per capita) and could be considered not cost-effective. For other potential combinations of values within the given range, the ICER between USD 9,780/QALY and USD 29,340/QALY is regarded as acceptable cost-effectiveness limit.
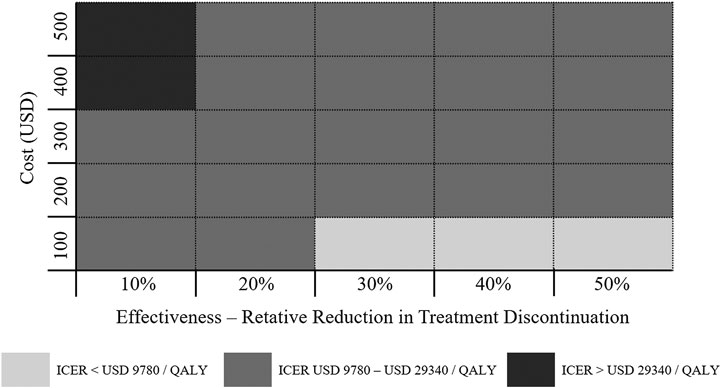
FIGURE 3. Economic value of persistence-enhancing interventions according to a given range of their costs and effectiveness values. Each block represents a possible intervention characterized by its cost and effectiveness. The color coding denotes the cost-effectiveness of the intervention.
Discussions
In this study, we used a modeling approach incorporating the medication compliance and persistence to examine the cost-effectiveness of oral alendronate treatment versus no intervention in the treatment of osteoporosis in Chinese postmenopausal women. Our base-case analysis revealed that, compared with no treatment, oral alendronate therapy 70 mg once a week for 5 years was a high-value treatment at a threshold of USD 29,340/QALY.
The key variable in the current research was the medication persistence and compliance. Although oral alendronates have been demonstrated to be high value with current medication discipline, they are more cost-effective with full compliance and persistence. In addition, persistence was found to have a greater impact on cost-effectiveness than compliance. Full persistence in our model would yield an ICER of USD 3,006.849/QALY, lower than the equivalent value for the full compliance (USD 4,933.333/QALY). It should be noted that this heightened persistence rate of oral alendronate was emphasized by our assumption of a residual effect from treatment; the risk for fracture returned to rates in the absence of therapy over the same years as the treatment duration in a gradual linear fashion after completing the therapy. This is also examined by deterministic sensitivity analyses, in which we assumed no residual effect after the treatment; the ICER of oral alendronate was sharply increased to USD 9,294.118/QALY. Hence, interventions to enhance persistence are necessary to decrease the considerable economic burden caused by the nonpersistence with oral alendronate.
Our results confirmed prior work that it is important to include medication persistence and compliance in pharmacoeconomic analysis of osteoporosis treatment. The two studies of Hiligsmann and colleagues (Hiligsmann et al., 2010; Hiligsmann et al., 2012a; Hiligsmann et al., 2012b) which were focused on oral bisphosphonates suggested that poor adherence with osteoporosis medications results in approximately a 50% reduction in the potential benefits observed in clinical trials and a doubling of the cost per QALY gained from these medications. Programs to improve compliance were considered to be an efficient use of resources. In contrast, the study of Chen and colleagues (Si et al., 2016) in the China setting which compared raloxifene treatment with conventional treatment (alendronate, calcitonin, and calcium combined with vitamin D) found opposite results. In this study, although high persistence and compliance increased both clinical effectiveness and average costs, the improvement in effectiveness was marginal in their research, thus resulting in higher ICER compared with the real-world scenario. The main reasons for such a difference could be attributed to the costs for fracture inpatients and the comparator.
In our previous study (You et al., 2020), in which we examined the cost-effectiveness of once-yearly injection of zoledronic acid compared with oral alendronate once a week for postmenopausal osteoporotic women without prior history of fracture in China, we concluded that zoledronic acid was cost-effective at all starting ages and even cost-saving in scenario analysis mainly based on zoledronic acid’s higher persistence leading to higher efficacy. In this study, we came to a similar conclusion that medication persistence plays a key role in shaping perceptions of fracture risk and osteoporosis drug effectiveness. In addition, we extend the prior work by designing a meaningful framework for assessing the economic value of persistence-enhancing interventions. We assessed the potential combination of the intervention costs from USD 100 to 500 and the relative reduction in discontinuation from 10% to 50%.
There are limitations associated with the current study. First, like all models, the generalizability of the results to the target population of other races/ethnicities or in other countries may be uncertain due to the heterogeneity of payer perspectives and the country-specific epidemiologic data used. Moreover, although much of the data which constructed the model were obtained from the Chinese context, some data were also extrapolated from other countries. An updated pharmacoeconomic analysis should be explored when these data are available in Chinese setting. Second, compliance and persistence rates were derived from a retrospective study (Cheng et al., 2013) in which whether patients actually took the dispensed drug is unknown. The study assumed that patients who obtain prescription refills do take their medications based on chart review. As a result, compliance may be overestimated. Third, our analysis did not examine the impact of restart therapy after discontinuation. We assumed that those who did not take alendronate continued not to take the medication in this model, which may not always mimic treatment in the real world because some patients might return to treatment after this period. Finally, we did not perform a budget impact analysis to assess the potential cost-savings of this strategy. Due to the enormous amount of osteoporosis cases in China, the financial burdens for the health care system might be heavy.
Despite these limitations, our research has several key strengths. First, to the best of our knowledge, this is the first pharmacoeconomic analysis that compared oral alendronate to no treatment in a Chinese population. Second, we incorporated medication persistence and compliance, which are considered to be critical impedance to osteoporosis management, into our hybrid modeling and extensively examined how these changes in parameters have an impact on model results. We further assessed the potential cost-effectiveness of persistence-enhancing interventions according to a given range of their costs and effectiveness values.
In conclusion, oral alendronate is considered to be a high-value therapy option for postmenopausal osteoporotic women from the perspective of Chinese health care payers, and further interventions to improve osteoporosis medication persistence will likely have favorable ICERs.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
ZL conceived and designed the research. RY collected and analyzed the data. All authors wrote the manuscript.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge Takahiro Mori for the contribution to build the Markov model, which we updated and used in our analysis.
References
Bow, C. H., Cheung, E., Cheung, C. L., Xiao, S. M., Loong, C., Soong, C., et al. (2012). Ethnic difference of clinical vertebral fracture risk. Osteoporos. Int. 23 (3), 879–885. doi:10.1007/s00198-011-1627-9
Brown, J. P., Morin, S., Leslie, W., Papaioannou, A., Cheung, A. M., Davison, K. S., et al. (2014). Bisphosphonates for treatment of osteoporosis: expected benefits, potential harms, and drug holidays. Can. Fam. Physician 60 (4), 324–333.
CCEMG-EPPI-Centre Cost Converter (2008). Available at: http://eppi.ioe.ac.uk/costconversion/default.aspx (Accessed December 18 2019) [Online].
Cheng, T., Yu, S., Hsu, C., Chen, S., Su, B. Y., and Yang, T. (2013). Differences in adherence to osteoporosis regimens: a 2-year analysis of a population treated under specific guidelines. Clin. Ther. 35 (7), 1005–1015. doi:10.1016/j.clinthera.2013.05.019
Chinese Society of Osteoporosis and Bone Mineral Research (2019). Guidelines for the diagnosis and management of primary osteoporosis. Chin. J. Osteoporos. 25 (3), 281–309. doi:10.3969/j.issn.1006-7108
Fatoye, F., Smith, P., Gebrye, T., and Yeowell, G. (2019). Real-world persistence and adherence with oral bisphosphonates for osteoporosis: a systematic review. BMJ Open 9 (4), e27049. doi:10.1136/bmjopen-2018-027049
Feng, Z., Zeng, S., Wang, Y., Zheng, Z., and Chen, Z. (2013). Bisphosphonates for the prevention and treatment of osteoporosis in patients with rheumatic diseases: a systematic review and meta-analysis. PLoS One 8 (12), e80890. doi:10.1371/journal.pone.0080890
Haentjens, P., Magaziner, J., Colón-Emeric, C. S., Vanderschueren, D., Milisen, K., Velkeniers, B., et al. (2010). Meta-analysis: excess mortality after hip fracture among older women and men. Ann. Intern. Med. 152 (6), 380–390. doi:10.7326/0003-4819-152-6-201003160-00008
Hiligsmann, M., Boonen, A., Rabenda, V., and Reginster, J. Y. (2012a). The importance of integrating medication adherence into pharmacoeconomic analyses: the example of osteoporosis. Expert Rev. Pharmacoecon. Outcomes Res. 12 (2), 159–166. doi:10.1586/erp.12.8
Hiligsmann, M., Ethgen, O., Richy, F., and Reginster, J. Y. (2008). Utility values associated with osteoporotic fracture: a systematic review of the literature. Calcif. Tissue Int. 82 (4), 288–292. doi:10.1007/s00223-008-9117-6
Hiligsmann, M., McGowan, B., Bennett, K., Barry, M., and Reginster, J. Y. (2012b). The clinical and economic burden of poor adherence and persistence with osteoporosis medications in Ireland. Value Health 15 (5), 604–612. doi:10.1016/j.jval.2012.02.001
Hiligsmann, M., Rabenda, V., Bruyere, O., and Reginster, J. Y. (2010). The clinical and economic burden of non-adherence with oral bisphosphonates in osteoporotic patients. Health Policy 96 (2), 170–177. doi:10.1016/j.healthpol.2010.01.014
Hiligsmann, M., Reginster, J. Y., Tosteson, A., Bukata, S. V., Saag, K. G., Gold, D. T., et al. (2019). Recommendations for the conduct of economic evaluations in osteoporosis: outcomes of an experts' consensus meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the US branch of the International Osteoporosis Foundation. Osteoporos. Int. 30 (1), 45–57. doi:10.1007/s00198-018-4744-x
Imaz, I., Zegarra, P., Gonzalez-Enriquez, J., Rubio, B., Alcazar, R., and Amate, J. M. (2010). Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta-analysis. Osteoporos. Int. 21 (11), 1943–1951. doi:10.1007/s00198-009-1134-4
Iolascon, G., Moretti, A., Toro, G., Gimigliano, F., Liguori, S., and Paoletta, M. (2020). Pharmacological therapy of osteoporosis: what's new? Clin. Interv. Aging 15, 485–491. doi:10.2147/CIA.S242038
Johnell, O., Kanis, J. A., Oden, A., Johansson, H., De Laet, C., Delmas, P., et al. (2005). Predictive value of BMD for hip and other fractures. J. Bone Miner. Res. 20 (7), 1185–1194. doi:10.1359/JBMR.050304
Kanis, J. A., Johnell, O., Oden, A., Jonsson, B., De Laet, C., and Dawson, A. (2000). Risk of hip fracture according to the World Health Organization criteria for osteopenia and osteoporosis. Bone 27 (5), 585–590. doi:10.1016/s8756-3282(00)00381-1
Kanis, J. A., Oden, A., Johnell, O., De Laet, C., Jonsson, B., and Oglesby, A. K. (2003). The components of excess mortality after hip fracture. Bone 32 (5), 468–473. doi:10.1016/S8756-3282(03)00061-9
Kishimoto, H., and Maehara, M. (2015). Compliance and persistence with daily, weekly, and monthly bisphosphonates for osteoporosis in Japan: analysis of data from the CISA. Arch. Osteoporos. 10, 231. doi:10.1007/s11657-015-0231-6
Kothawala, P., Badamgarav, E., Ryu, S., Miller, R. M., and Halbert, R. J. (2007). Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin. Proc. 82 (12), 1493–1501. doi:10.1016/S0025-6196(11)61093-8
Liu, R., Chao, A., Wang, K., and Wu, J. (2018). Incidence and risk factors of medical complications and direct medical costs after osteoporotic fracture among patients in China. Arch. Osteoporos. 13 (1), 12. doi:10.1007/s11657-018-0429-5
Lofthus, C. M., Frihagen, F., Meyer, H. E., Nordsletten, L., Melhuus, K., and Falch, J. A. (2008). Epidemiology of distal forearm fractures in Oslo, Norway. Osteoporos. Int. 19 (6), 781–786. doi:10.1007/s00198-007-0499-5
Maraka, S., and Kennel, K. A. (2015). Bisphosphonates for the prevention and treatment of osteoporosis. BMJ 351, h3783. doi:10.1136/bmj.h3783
Marshall, D., Johnell, O., and Wedel, H. (1996). Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312 (7041), 1254–1259. doi:10.1136/bmj.312.7041.1254
Melton, L. J., Crowson, C. S., and O Fallon, W. M. (1999). Fracture incidence in Olmsted county, Minnesota: comparison of urban with rural rates and changes in urban rates over time. Osteoporos. Int. 9 (1), 29–37. doi:10.1007/s001980050113
Mori, T., Crandall, C. J., and Ganz, D. A. (2017a). Cost-effectiveness of denosumab versus oral alendronate for elderly osteoporotic women in Japan. Osteoporos. Int. 28 (5), 1733–1744. doi:10.1007/s00198-017-3940-4
Mori, T., Crandall, C. J., and Ganz, D. A. (2017b). Cost-effectiveness of combined oral bisphosphonate therapy and falls prevention exercise for fracture prevention in the USA. Osteoporos. Int. 28 (2), 585–595. doi:10.1007/s00198-016-3772-7
Murad, M. H., Drake, M. T., Mullan, R. J., Mauck, K. F., Stuart, L. M., Lane, M. A., et al. (2012). Clinical review. Comparative effectiveness of drug treatments to prevent fragility fractures: a systematic review and network meta-analysis. J. Clin. Endocrinol. Metab. 97 (6), 1871–1880. doi:10.1210/jc.2011-3060
National Development and Reform Commission (2019). Available at: http://www.ndrc.gov.cn/ (Accessed December 25 2019) [Online].
National Health Committee of the People's Republic of China. (2018). China health statistical yearbook. Beijing, China: Peking Union Medical College Publishing House.
Pisani, P., Renna, M. D., Conversano, F., Casciaro, E., Di Paola, M., Quarta, E., et al. (2016). Major osteoporotic fragility fractures: risk factor updates and societal impact. World J. Orthop. 7 (3), 171–181. doi:10.5312/wjo.v7.i3.171
Qu, B., Ma, Y., Yan, M., Wu, H. H., Fan, L., Liao, D. F., et al. (2014). The economic burden of fracture patients with osteoporosis in western China. Osteoporos. Int. 25 (7), 1853–1860. doi:10.1007/s00198-014-2699-0
Si, L., Winzenberg, T. M., Chen, M., Jiang, Q., Neil, A., and Palmer, A. J. (2016). Screening for osteoporosis in Chinese post-menopausal women: a health economic modelling study. Osteoporos. Int. 27 (7), 2259–2269. doi:10.1007/s00198-016-3502-1
Si, L., Winzenberg, T. M., de Graaff, B., and Palmer, A. J. (2014). A systematic review and meta-analysis of utility-based quality of life for osteoporosis-related conditions. Osteoporos. Int. 25 (8), 1987–1997. doi:10.1007/s00198-014-2636-2
Si, L., Winzenberg, T. M., Jiang, Q., Chen, M., and Palmer, A. J. (2015). Projection of osteoporosis-related fractures and costs in China: 2010-2050. Osteoporos. Int. 26 (7), 1929–1937. doi:10.1007/s00198-015-3093-2
Stevenson, M. D., and Selby, P. L. (2014). Modelling the cost effectiveness of interventions for osteoporosis: issues to consider. Pharmacoeconomics 32 (8), 735–743. doi:10.1007/s40273-014-0156-8
Sun, S., Chen, J., Johannesson, M., Kind, P., Xu, L., Zhang, Y., et al. (2011). Population health status in China: EQ-5D results, by age, sex and socio-economic status, from the National Health Services Survey 2008. Qual. Life Res. 20 (3), 309–320. doi:10.1007/s11136-010-9762-x
Svedbom, A., Hernlund, E., Ivergård, M., Compston, J., Cooper, C., Stenmark, J., et al. (2013). Osteoporosis in the European Union: a compendium of country-specific reports. Arch. Osteoporos. 8 (1), 137. doi:10.1007/s11657-013-0137-0
Wang, J., Wang, Y., Liu, W. D., Wang, F., and Yin, Z. S. (2014). Hip fractures in Hefei, China: the Hefei osteoporosis project. J. Bone Miner. Metabol. 32 (2), 206–214. doi:10.1007/s00774-013-0484-3
Watts, N. B. (2018). Postmenopausal osteoporosis: a clinical review. J. Womens Health (Larchmt) 27 (9), 1093–1096. doi:10.1089/jwh.2017.6706
Wells, G., Cranney, A., Peterson, J., Boucher, M., Shea, B., Robinson, V., et al. (2008). Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst. Rev. (1), CD004523. doi:10.1002/14651858.CD004523.pub3
Keywords: economic analysis, postmenopausal osteoporosis, alendronate, compliance, persistence
Citation: You R and Liu Z (2020) Economic Evaluation of Oral Alendronate Therapy for Osteoporosis in Chinese Postmenopausal Women: The Impact of Medication Compliance and Persistence. Front. Pharmacol. 11:575893. doi: 10.3389/fphar.2020.575893
Received: 24 June 2020; Accepted: 30 September 2020;
Published: 16 November 2020.
Edited by:
Jean-Marie Boeynaems, Université libre de Bruxelles, BelgiumReviewed by:
Adina Turcu-Stiolica, University of Medicine and Pharmacy of Craiova, RomaniaBrian Godman, Karolinska Institutet, Sweden
Copyright © 2020 You and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zijie Liu, bGl1emlqaWVAaW1tLmFjLmNu
 Ruxu You
Ruxu You Zijie Liu
Zijie Liu