
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 21 October 2020
Sec. Drugs Outcomes Research and Policies
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.568477
Background: Camrelizumab (SHR1210) is a high-affinity, humanized immunoglobulin programmed cell death 1 (PD-1) monoclonal antibody. It was developed by Jiangsu Hengrui Medicine Co. Ltd. and has been approved for relapsed or refractory classical Hodgkin lymphoma patients and hepatocellular carcinoma patients in China. Apatinib is an orally administered vascular endothelial growth factor receptor-2 (VEGFR-2) tyrosine kinase inhibitor and has been approved for advanced gastric adenocarcinoma or gastroesophageal junction adenocarcinoma in China. Camrelizumab alone and its combination with apatinib have been used in the treatment of various solid cancers.
Methods: We searched Embase, PubMed, and other databases with the keyword “camrelizumab” or “SHR1210,” and evaluated the safety and efficacy data of the involved studies. Adverse events (AEs) mentioned in at least two studies were summarized, including any grade and grade ≥3 treatment-related AEs. Meanwhile, efficacy data were collected, such as overall response rate (ORR), disease control rate (DCR), duration of response, 6-month progression-free survival (PFS) rate, median PFS time, 12-month overall survival rate, and median overall survival time.
Results: The major AEs of camrelizumab alone were reactive cutaneous capillary endothelial proliferation, fatigue, aspartate aminotransferase increase, proteinuria, pruritus, and alanine transaminase increase. The ORR and DCR were 20.2% (95% CI: 15.1–26.6%, p = 0.000, I2 = 70.360) and 45.8% (95% CI: 39.0–52.7%, p = 0.256, I2 = 58.661), respectively. In the three studies of combination therapy, two studies were combined with apatinib and one combined with chemotherapy. For these studies, common AEs were hypertension, platelet count decrease, nausea, proteinuria, aspartate aminotransferase increase, and white blood cell count decrease. The pooled ORR, DCR, and 6-month PFS rate were 41.8% (95% CI: 29.7–54.9%, p = 0.220, I2 = 86.265), 82.4% (95% CI: 75.9–87.4%, p = 0.000, I2 = 55.207), and 56.2% (95% CI: 35.8–74.6%, p = 0.559, I2 = 79.739), respectively.
Conclusion: Camrelizumab and its combination are tolerable and appear to be efficient in treating numerous solid cancers. The combination therapy appears to have better efficacy with durable toxicity. However, these remain to be shown in future studies. Besides, baseline lactate dehydrogenase, programmed cell death ligand 1 (PD-L1) expression, tumor mutation burden, and the incidence of reactive cutaneous capillary endothelial proliferation may be efficacy predictors and need to be clarified in further studies.
Immune checkpoint inhibitors (ICIs) targeting the programmed death 1/programmed death ligand 1 (PD-1/PD-L1) pathway have become the hottest therapeutics for various tumors. Tumor cells are able to escape immune surveillance through the interaction between immune checkpoints and their ligands (Pardoll 2012). Inhibitors targeting these pathways could break immunity evasion, enhance antitumor immunity, and produce durable clinical responses (Sharma and Allison, 2015). A lot of PD-1 or PD-L1 inhibitors have been developed and showed promising efficacy results.
Camrelizumab (SHR1210, AiRuiKaTM) is a high-affinity, selective, humanized immunoglobulin G4/k PD-1 monoclonal antibody. It interacts with PD-1 on immunity cells (including activated T lymphocytes, B cells, and natural killer cells) and programmed cell death ligand 2 (PD-L2) on antigen-presenting cells (Sharpe and Pauken, 2017). It was developed by Jiangsu Hengrui Medicine Co. Ltd. in China (Markham and Keam, 2019) and has been approved as a third-line treatment for relapsed or refractory classical Hodgkin lymphoma patients and second-line treatment for hepatocellular carcinoma (HCC) patients by the China Food and Drug Administration (Markham and Keam, 2019).
As a highly selective vascular endothelial growth factor receptor-2 (VEGFR-2) tyrosine kinase inhibitor, apatinib (YN968D1) has been approved as a third-line and subsequent treatment for advanced gastric adenocarcinoma or gastroesophageal junction adenocarcinoma in China (Zhang, 2015). The binding of VEGF and its receptors (VEGFR-1, VEGFR-2, or VEGFR-3) plays an important part in tumor-associated angiogenesis (Mi et al., 2010). Apatinib interacts with VEGFR-2, locks the VEGF signaling pathway, and thus inhibits tumor growth and metastasis (Scott et al., 2015). Besides, apatinib could promote immune response (Zhao et al., 2017; Zheng et al., 2018), overcome resistance to immunotherapy (Wang et al., 2020), and produce synergistic antitumor effects when combined with immunotherapy in the mouse model (Wang, et al., 2020). The combination has recently been reported in a few case reports (Yang et al., 2018; Zhao et al., 2019) and clinical trials (Liang et al., 2019; Liu et al., 2019; Xie et al., 2019).
So far, the safety and efficacy of camrelizumab and its combination with chemotherapy (and apatinib) have been assessed in patients with esophageal squamous cell carcinoma (ESCC), gastric cancer, HCC, nasopharyngeal carcinoma (NPC), non-small cell lung cancer (NSCLC), and so on. In addition, the optimal efficacy predictors, such as PD-L1 expression and tumor mutation burden (TMB), were mentioned in a few studies. In this study, we analyzed the safety and efficacy of camrelizumab and its combination in solid cancers, and determined the potential predictive biomarkers to accurately classify the most efficient patients based on published clinical trials.
Studies were searched in Embase, PubMed, and the Cochrane Library databases with the keywords “camrelizumab” and “SHR1210” (publications up to February 31, 2020). The American Society of Clinical Oncology, European Society for Medical Oncology, World Conference on Lung Cancer, World Organization for Specialized Studies on Diseases of the Esophagus World Conference database, and Gastrointestinal Cancers Symposium were also searched for relevant publications. After excluding the duplicated articles, full-text articles and conference abstracts were reviewed by two reviewers (HY and KW) for eligibility independently. We solved all the disagreements by a discussion with the third author.
Included articles had to satisfy the following criteria: 1) clinical trials concerning the safety or efficacy of camrelizumab or camrelizumab plus other drugs; 2) enrolled patients had pathologically confirmed solid cancers; 3) the safety or efficacy data were available; and 4) published in English.
The exclusion criteria were as follows: 1) studies were not related to camrelizumab or cancers; 2) studies lacked adequate safety or efficacy data; 3) studies enrolled less than ten patients; 4) studies conducted in hematological malignancy; and 5) retrospective studies, reviews, reports, comments, meta-analyses, letters, case reports, correction, or guideline.
The following data were extracted from the included articles: 1) the basic information of studies, including the first author, published year, clinical trial number, study design (including study phase), number of patients, cancer types, treatments, follow-up time, and so on; 2) adverse events (AEs) mentioned in at least two studies, including any grade and grade ≥3 treatment-related AEs; 3) efficacy data, such as overall response rate (ORR), disease control rate (DCR), duration of response, 6-month progression-free survival (PFS) rate, median PFS time, 12-month overall survival (OS) rate, and median OS time. To avoid duplication of data, we chose articles with more useful data rather than the most recent publications or those including more patients.
Statistical analyses were performed by Comprehensive Meta-Analysis V3 (Biostat, Englewood, NJ, United States) and Review Manager 5.3 (RevMan; The Cochrane Collaboration, Oxford, England) software. Safety was evaluated by calculating the proportion and derived 95% CI of any grade and grade ≥3 AEs in at least two studies. The efficacy of camrelizumab and the combination therapy was evaluated by calculating the proportion and derived 95% CI of the ORR, DCR, and pooled 6-month PFS rate. The odds ratio (OR) and corresponding 95% CIs were calculated to compare the ORR, DCR, and PFS rate. The hazard ratio (HR) and their 95% CIs were used to compare PFS and OS time. All statistical analyses were two-sided, and p values <0.05 were identified as statistically significant. A fixed-effects model was applied when inconsistency index (I2) <50% or else a random-effects model was used.
Systematic biases of the involved randomized controlled trials (RCTs) were evaluated using the Cochrane risk-of-bias tool (Review Manager 5.3). Non-randomized trials were evaluated according to the methodological index for non-randomized studies score (Slim et al., 2003). The scoring system has eight items for non-comparative studies, and each item is scored 0, 1, or 2. Score 0 means the studies did not mention an item; score 1 means the item was mentioned but not adequately; and score 2 means the item was adequately reported. Two researchers scored each trial for the risk of bias independently. All the disagreements were solved by a discussion with the third author.
A total of 189 articles were assessed. After carefully screening the full text or conference abstracts, 24 articles were eligible. Finally, 15 articles with 1,390 patients were included after removing nine articles with duplicate data (Fang et al., 2018; Huang et al., 2019a; Huang et al., 2019b; Liu et al., 2019; Qin et al., 2019; Shen et al., 2019; Wu et al., 2019; Xie et al., 2019; Xu et al., 2019; Zhang et al., 2019; Zhou et al., 2019a; Zhou et al., 2019b; Chen et al., 2020a; Lickliter et al., 2020; Qin et al., 2020). Trial NCT03121716 had both a single-agent cohort (camrelizumab alone) and a combination cohort (camrelizumab plus other therapies), and the two cohorts were enrolled separately as two studies (Fang et al., 2018). Trial NCT03394287 had a continuous cohort and an intermittent cohort (Liu et al., 2019). Patients received camrelizumab and apatinib for 14 days in the continuous cohort and were treated with only 7 days of apatinib in the intermittent cohort. Since only nine patients were evaluable in the intermittent cohort, we abandoned this cohort in our analysis. Finally, six studies on camrelizumab treatment and 10 studies on combination therapy were eligible, and the detailed selection procedure is shown in Figure 1.
All eligible studies had ClinicalTrials.gov numbers. Among these studies, there were six phase I studies, seven phase II studies, one phase I/II studies, and two phase III studies. Most studies were published in 2019, three studies were published in 2020, and only two studies were published in 2018. Two studies were RCTs, and the other 14 articles were single-arm trials. Camrelizumab was administered intravenously at a dose of 200 mg every 2 or 3 weeks in most studies. Studies were conducted in patients with biliary tract cancer (BTC), ESCC, gastric cancer, HCC, NPC, NSCLC, osteosarcoma, and triple-negative breast cancer. The combination options were chemotherapy and apatinib. Basic information of the included studies is provided in Table 1.
Seven studies were enrolled to assess the AE rate, with three studies of combination therapy. The results are presented in Figures 2, 3. Figure 2 shows the result of camrelizumab treatment, including all-grade AEs (A, fixed model; B, random model) and grade ≥3 AEs (C, fixed model). Figure 3 shows the results of all-grade AEs in combination therapy (A, fixed model; B, random model) and the results of grade ≥3 AEs (C, fixed model; D, random model). Reactive cutaneous capillary endothelial proliferation (RCCEP) was the most common AE of camrelizumab treatment and occurred in 76.6% of patients. Hypertension ranked first in combination therapy and occurred in 61.0% of patients. Table 2 shows the top five most frequent AEs in the two groups, respectively.
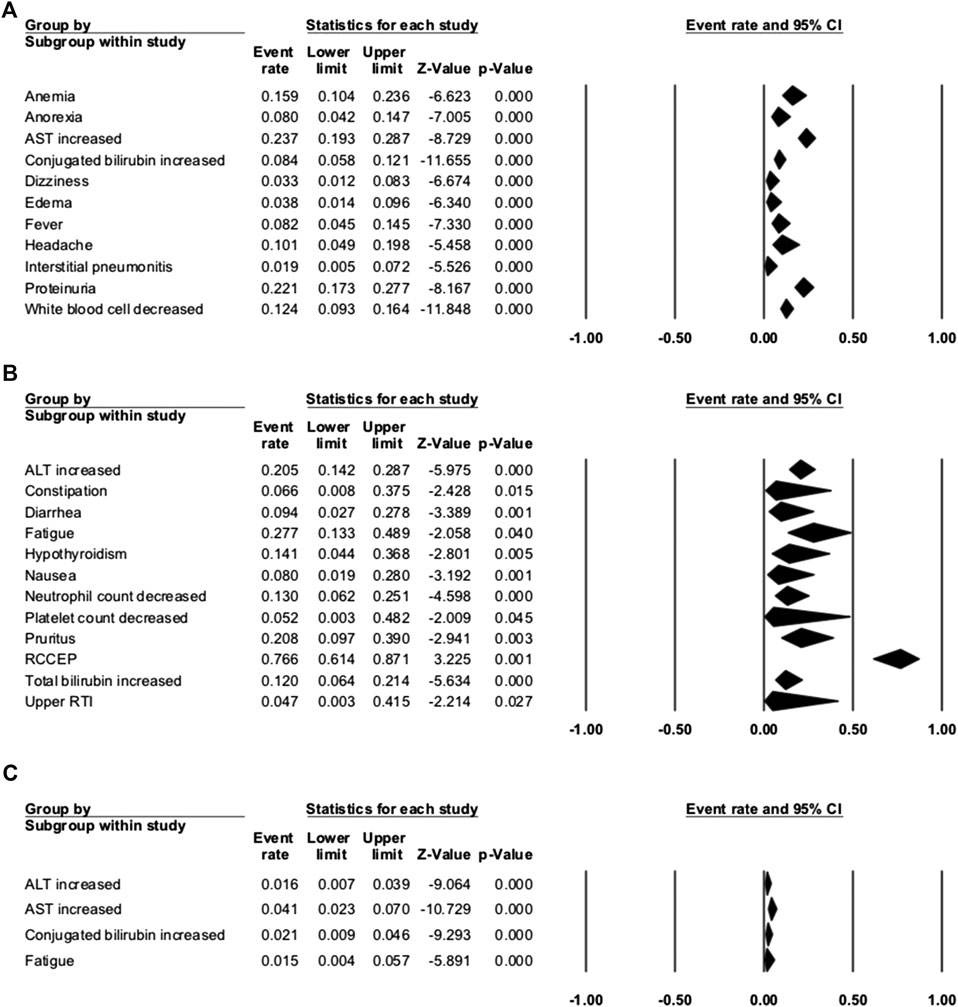
FIGURE 2. Adverse events (AEs) of camrelizumab alone. (A) The fixed model of all-grade AEs; (B) the random model of all-grade AEs; and (C) the fixed model of grade ≥3 AEs.
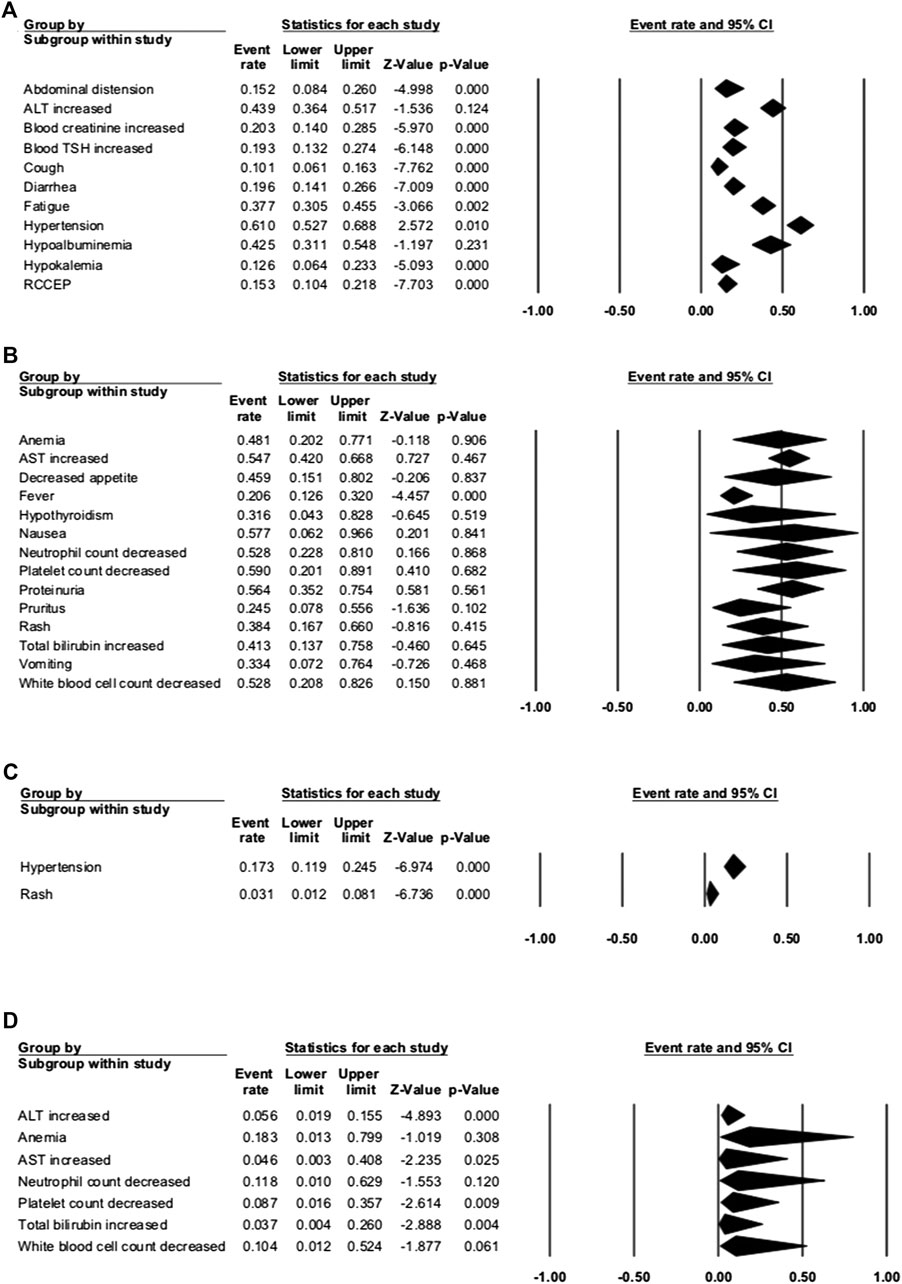
FIGURE 3. Adverse events (AEs) of combination therapy. (A) The fixed model of all-grade AEs; (B) the random model of all-grade AEs; (C) the fixed model of grade ≥3 AEs; and (D) the random model of grade ≥3 AEs.
Among the enrolled articles, four studies were subsumed in any-grade AE analysis, and three articles were incorporated in the grade ≥3 AE analysis because some articles had no applicable data. The major AEs of any grade occurred in over 20% of patients with camrelizumab treatment, including RCCEP, fatigue, aspartate aminotransferase (AST) increase, proteinuria, pruritus, and alanine transaminase (ALT) increase. RCCEP was the most frequent AE with an incidence range from 61.2 to 88.2%, and the overall event rate was 76.6% (95% CI: 61.4–87.1%). Fatigue occurred in 27.7% (95% CI: 13.3–48.9%) of the patients, and AST increase occurred in 23.7% of the patients. Compared with the high incidence of any-grade AEs, the incidence of grade ≥3 AE was rare. The incidence of grade ≥3 AST increase was 4.1%. Other grade ≥3 AEs were conjugated bilirubin increase, ALT increase, and fatigue. These occurred in 2.1, 1.6, and 1.5% of the patients, respectively. Serious AEs that led to therapy discontinuation were reported in five studies, and treatment-associated death happened in two studies. The incidence of therapy discontinuation ranged from 3.7 to 7.7%, and death was reported in 0.3% of gastric cancer patients (one patient with interstitial lung disease) and 3.1% of ESCC patients (Table 3). Interstitial pneumonitis has a low incidence of 1.9% (95% CI: 0.5–7.2%), but it caused one death in gastric cancer patients. When using camrelizumab, doctors should take care of patients with poor lung function or interstitial lung disease.
In the three studies of combination therapy, combination with apatinib was used in two studies and combination with chemotherapy was used in one study. Among them, hypertension had the highest incidence of 61.0% (95% CI: 52.7–68.8%). Other common any-grade AEs occurred in more than 50% of the patients, including platelet count decrease (59.0%), nausea (57.7%), proteinuria (56.4%), AST increase (54.7%), and white blood cell count decrease (52.8%). The most common grade ≥3 AE was anemia, with the incidence of 18.3% (95% CI: 1.3–79.9%). Other grade ≥3 AEs that occurred in over 10% of patients were hypertension (17.3%), neutrophil count decrease (11.8%), and white blood cell count decrease (10.4%). AEs that led to therapy discontinuation were reported in three studies, and treatment-associated death happened in one study. The incidence of therapy discontinuation was 1.2–13.0%, and death happened in 2.4% NSCLC patients with camrelizumab and chemotherapy.
When comparing the incidence of any-grade AEs between camrelizumab alone and combination therapy, we found that most AEs occurred more frequently with combination therapy. The incidences of some AEs with camrelizumab alone were more than twice the incidences with combination therapy, like hepatic function (ALT, AST, or total bilirubin increase), hematologic toxicities (neutrophil, platelet, or white blood cell count), anemia, diarrhea, fever, nausea, and proteinuria. These were obviously associated with chemotherapy or apatinib. ALT increase and AST increase were common grade ≥3 AEs in both groups. This should remind doctors of the importance of hepatic function examination and liver-protective drugs during treatment. The incidence of RCCEP dramatically decreased with combination therapy (76.6 vs. 15.3%), ensured the safety of camrelizumab, and made this combination reasonable.
In our analysis, the pooled ORR, DCR, and 6-month PFS rate were used to judge the efficacy of camrelizumab and the combination therapy. Six studies of camrelizumab alone and 10 studies of combination therapy were tested.
For camrelizumab alone, six studies were included in the ORR analysis, and five studies were enrolled in the DCR analysis. The pooled ORR and DCR were 20.2% (95% CI: 15.1–26.6%, p = 0.000, I2 = 70.360) and 45.8% (95% CI: 39.0–52.7%, p = 0.256, I2 = 58.661), respectively (Figure 4). The 6-month PFS rate was reported as 48.2% in trial NCT02721589 of NPC patients. The median TTR ranged from 1.83 to 1.9 months, the median PFS varied from 1.87 to 5.6 months, and the median OS varied from 8.3 to 19.4 months (Table 4).
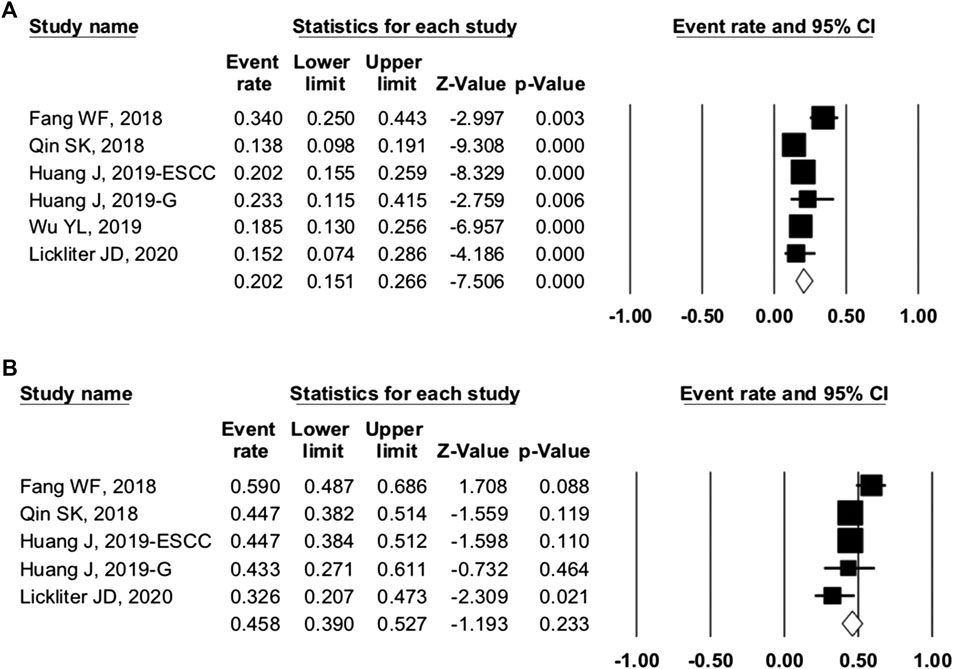
FIGURE 4. Efficacy of camrelizumab alone. (A) overall response rate and (B) disease control rate of included studies.
Ten studies were included in the efficacy analysis of combination therapy. The pooled ORR, DCR, and 6-month PFS rate were 41.8% (95% CI: 29.7–54.9%, p = 0.220, I2 = 86.265), 82.4 (95% CI: 75.9–87.4%, p = 0.000, I2 = 55.207), and 56.2% (95% CI: 35.8–74.6%, p = 0.559, I2 = 79.739) (Figure 5), respectively. So, the ORR and DCR were twice higher in patients with combination therapy than camrelizumab alone. Combination therapy appears to have better efficacy than camrelizumab alone, but a direct comparison between the two groups was lacking.
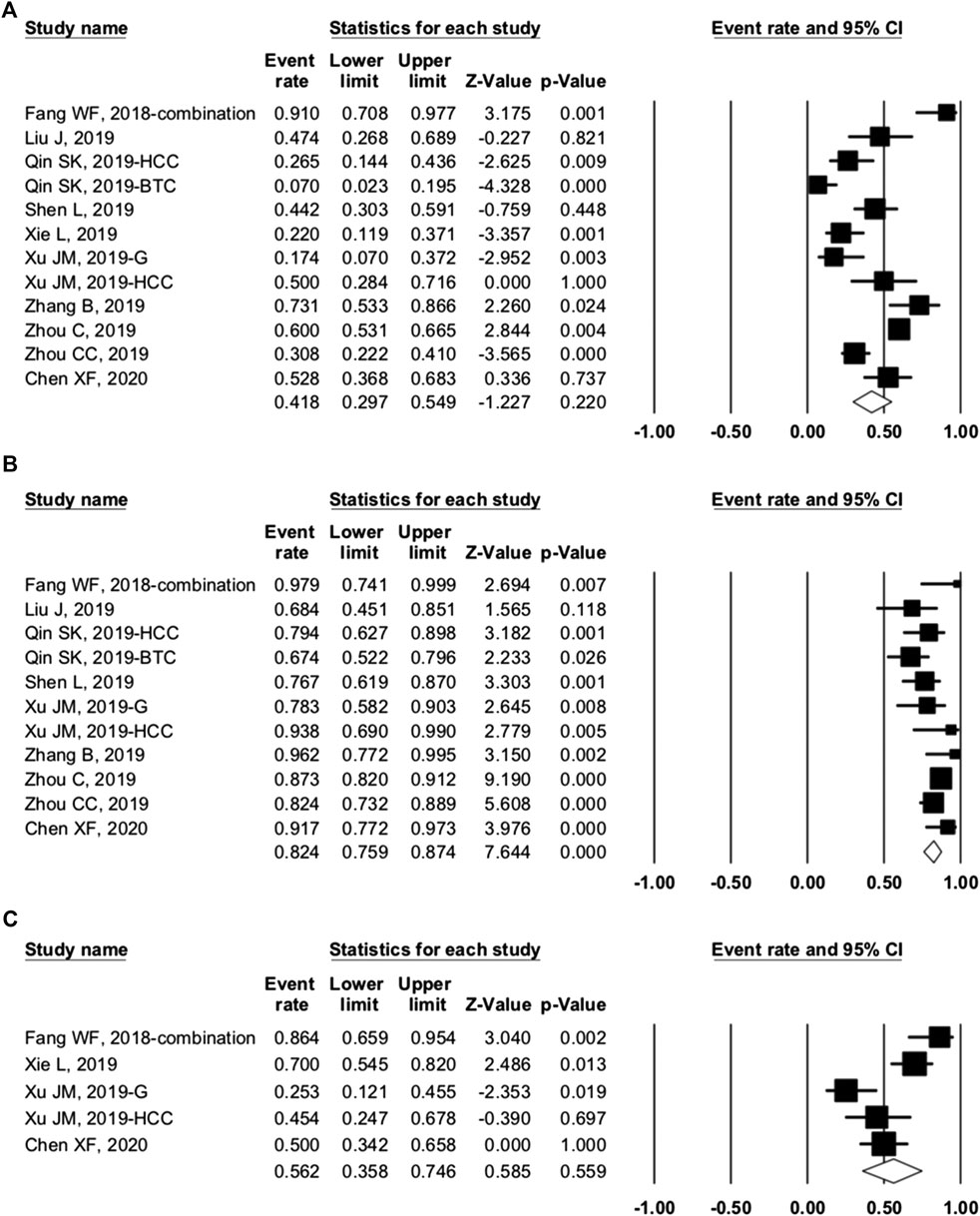
FIGURE 5. Efficacy of combination therapy. (A) overall response rate; (B) disease control rate; and (C) 6-month progression-free survival rate of included studies.
The efficacy data of two RCTs are displayed in Table 5. In the second-line treatment of ESCC, camrelizumab was better than chemotherapy in Chinese patients (NCT03099382). The camrelizumab group had better ORR (20.2 vs. 6.4%), better 12-month survival rate (33.7 vs. 22.3%), and longer OS (8.3 vs. 6.2 months, HR = 0.71, 95% CI: 0.57–0.87, p = 0.0010). NCT03134872 compared chemotherapy and camrelizumab plus chemotherapy as a first-line treatment in advanced or metastatic non-squamous NSCLC patients with negative epithelial growth factor receptor (EGFR) and anaplastic lymphoma kinase. The combination group showed superior ORR (60.0 vs. 39.1%) and PFS (11.3 vs. 8.3 months, HR = 0.61, 95% CI: 0.46–0.80, p = 0.0002).
To investigate the source of heterogeneity among studies, we conducted subgroup analyses. In trials of camrelizumab alone, NCT02742935 and NCT03085069 showed the ORR in different PD-L1 expression subgroups. Taking 1% as the cutoff value of PD-L1 positivity, we found the OR was 1.931 (95% CI: 0.883–4.223, p = 0.099) between PD-L1–positive and negative patients (Figure 6). Camrelizumab seems to have an effect regardless of PD-L1 expression, but this result had no significant difference.
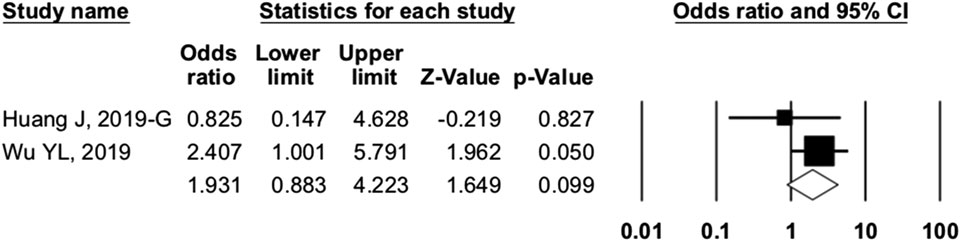
FIGURE 6. Comparison of programmed cell death ligand 1–positive and programmed cell death ligand 1–negative patients. Our study showed that PD-L1 expression (cutoff value: 1%) was not associated with overall response rate (odds ratio = 1.931, 95% CI: 0.883–4.223, p = 0.099).
In trial NCT02742935, elevated baseline lactate dehydrogenase (LDH) was associated with lower ORR (8.3 vs. 32.3%, p = 0.02), shorter PFS (1.8 vs. 4.0 months; HR = 0.39, p = 0.002), and shorter OS (4.2 vs. 10.2 months; HR = 0.22, p<0.0001) in ESCC patients (Wang et al., 2019). In another trial of advanced BTC patients (NCT03486678), increased baseline LDH level was also associated with poor PFS (5.0 vs. 6.2 months, p = 0.053) and shorter OS (6.8 vs. 12.6 months, p < 0.001) (Chen et al., 2020b). Besides, BTC patients with high TMB (cutoff value: 8.6 muts/Mb) had a significantly higher ORR (100 vs. 26%, p = 0.0294) (Chen et al., 2019b; Wu et al., 2019). In NSCLC patients treated with camrelizumab and apatinib, high TMB (cutoff value: 1.54 muts/Mb) was associated with higher ORR (52.6 vs. 17.1%) and better PFS (7.8 vs. 5.2 months). Of note, for HCC patients treated with camrelizumab, patients who developed RCCEP had better objective response (19·3 vs. 5.6%) (Qin et al., 2020).
We grouped trials with combination therapy by cancer types (Figure 7). The pooled ORR of BTC, gastric cancer, HCC, and NSCLC was 23.3% (95% CI: 2.1–81.0%, p = 0.377), 30.5% (95% CI: 10.7–61.4%, p = 0.210), 36.7% (95% CI: 17.6–61.1%, p = 0.284), and 45.3% (95% CI: 20.1–73.1%, p = 0.755), respectively. The DCR was 81.6% (95% CI: 46.3–95.8%, p = 0.075), 77.3% (95% CI: 65.9–85.7%, p = 0.000), 85.2% (95% CI: 62.9–95.1%, p = 0.005), and 85.5% (95% CI: 80.4–89.5%, p = 0.0.000), respectively. When grouped by treatment frequency, the pooled ORR of treatment every 2 weeks and every 3 weeks was 30.2% (95% CI: 20.4–42.2%, p = 0.002) and 65.4% (95% CI: 48.5–79.1%, p = 0.074), respectively. The DCR of the two groups was 79.4% (95% CI: 71.3–85.7%, p = 0.000) and 87.5% (95% CI: 76.2–93.8%, p = 0.000), respectively. Data were insufficient for the analysis of PFS, OS, and others.
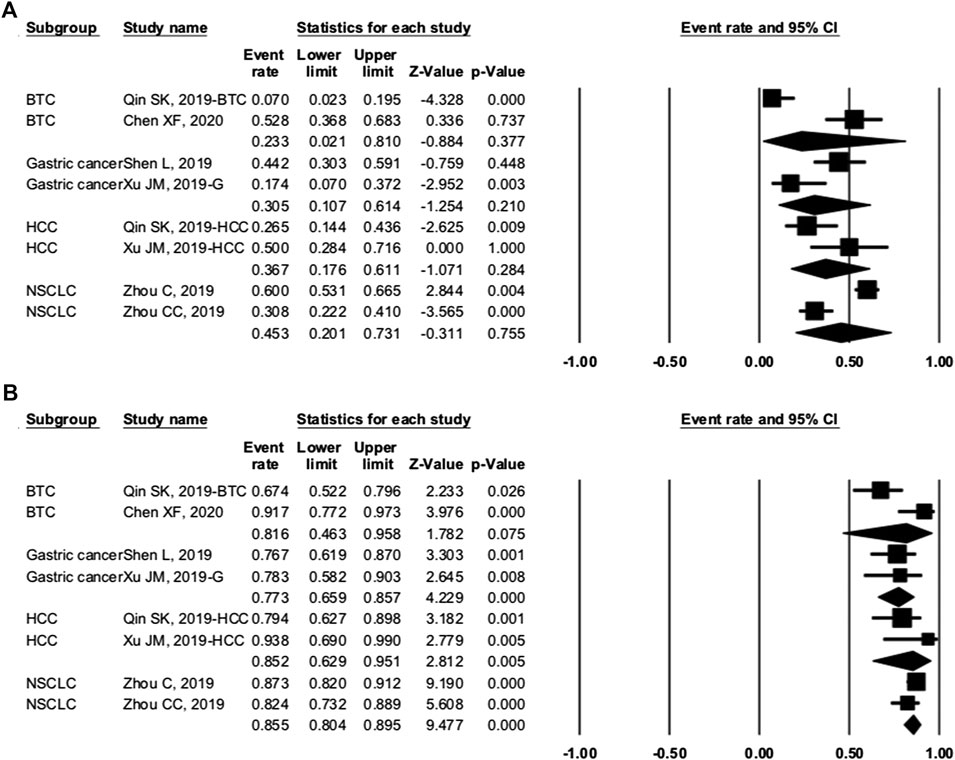
FIGURE 7. Subgroup analysis of cancer types. (A) overall response rate and (B) disease control rate of different cancers.
The methodological quality of the enrolled RCT study was assessed by Review Manager 5.3. The risk of bias graph and risk of bias summary are shown in Figure 8. The non-randomized studies were assessed by the methodological index for non-randomized studies score (Table 1).
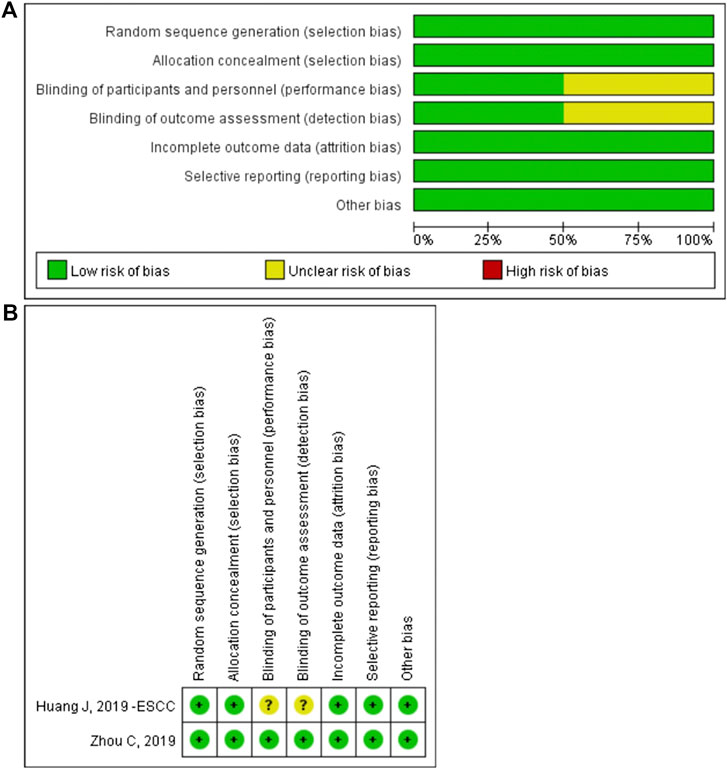
FIGURE 8. The risk of bias graph (A) and the risk of bias summary (B).The overall risk of bias was evaluated as low risk.
Camrelizumab was the first ICI independently developed by Chinese biopharma. It has been approved for classical Hodgkin lymphoma and HCC patients in China (Markham and Keam, 2019). The recommended dose is 200 mg via intravenous infusion once every 2 weeks until intolerable toxicity or disease progression occurs. This study investigated the efficacy and safety of camrelizumab and its combination in solid cancers.
As we know, ICIs could enhance the nonspecific immune response of hosts. In our pooled analysis, the most common AEs for camrelizumab alone were RCCEP, fatigue, AST increase, proteinuria, and pruritus. RCCEP has been considered as a unique toxicity of camrelizumab treatment (Chen et al., 2019a; Teng et al., 2019), but it has also been reported in 2.4% of patients treated with nivolumab and pembrolizumab for advanced melanoma (Hwang et al., 2016). In our study, RCCEPs occurred in as high as 76.6% of various solid cancer patients, and there was no grade ≥3 RCCEPs. The median time from the camrelizumab treatment to the onset of RCCEP was 2–4 weeks in previous studies (Chen et al., 2019a; Chen et al., 2020a). Most RCCEPs occurred on the skin (mainly on the face and trunk), and a few were found in oral and nasal mucosa. RCCEPs were mild and self-limiting. Apparent involution and complete regression of RCCEP could be observed both during and after treatment. Treatment was not necessary in most cases, except for patients with a high risk of bleeding.
The probable mechanism of RCCEP remains unclear now. The binding epitope of camrelizumab differs from other PD-1 inhibitors, which might influence the regulation of PD-1 signaling (Qin et al., 2020). A study reported that camrelizumab can increase VEGF expression on human umbilical vein endothelial cells and promote the proliferation and migration of human umbilical vein endothelial cells by activating hypoxia-inducible factor-1 α (HIF-1 α)/VEGF pathway in vivo (Wu et al., 2020). Another study performed human receptor proteome screening and identified that camrelizumab has a low affinity, highly selective interaction with VEGFR-2 (Finlay et al., 2019). The off-target binding and agonism of VEGFR-2 drive angiogenesis via vascular endothelial cell activation and lead to an abnormal proliferation of cutaneous capillary endothelial cells. When combined with apatinib, the anti-angiogenesis function of apatinib dramatically helps decrease the incidence of RCCEP to 15.3% and further ensures the safety of camrelizumab in combination therapy.
Meanwhile, apatinib has some common toxicity, including hypertension, hand–foot syndrome, proteinuria, and hematologic toxicity in treating solid cancers. Hypertension is the most common AE in combination therapy and is thought to be associated with the anti-angiogenesis function of apatinib on the normal vasculature. In previous studies of apatinib alone, incidences of hypertension and grade ≥3 hypertension were about 40.0 and 10.0%, respectively, in advanced gastric cancer patients (Li et al., 2013; Yang et al., 2019). It mostly occurred in 2 weeks of treatment and was controllable. The incidence of hypertension increased to 61.0% after combining with camrelizumab. Blood pressure should be actively monitored during and after patients received combination treatment. Once it happens, antihypertensive drugs should be used to avoid treatment disruption.
Proteinuria always occurred 3 weeks after apatinib treatment, with an incidence of about 50.0% in gastric patients (Li et al., 2016). The incidence was 56.4% in patients with combination treatment in our study, which was similar to the previous report. Routine urinalysis should be performed every 2 weeks to monitor proteinuria. If it occurs, dose decrease or treatment suspension may be required.
Both apatinib and camrelizumab can cause hematologic toxicity and hepatic function abnormality. Hepatic function abnormality could manifest as ALT elevation, AST elevation, or an increase in total bilirubin. The incidences of associated AEs with combination therapy were more than twice the incidences of associated AEs with camrelizumab alone. Routine blood and liver function examination tests at least every 2 weeks are necessary to timely detect the AEs.
For camrelizumab alone, the pooled ORR and DCR were 20.2 and 45.8%, respectively. The median PFS varied from 1.87 to 5.6 months, and the median OS was 8.3 to 19.4 months. According to NCT03099382, camrelizumab was better than chemotherapy in Chinese patients with ESCC (Huang et al., 2019b; Xu et al., 2019). The camrelizumab group showed better ORR and longer PFS and OS. More RCTs in different cancers would further ensure the efficacy of camrelizumab. The ORR and DCR of combination therapy increased to 41.8 and 82.4%, respectively, which were twice of those of camrelizumab alone. The combination of camrelizumab and apatinib is safe and appears to have better efficacy than camrelizumab alone. Future trials will provide further evidence.
To investigate the source of heterogeneity among studies, we conducted subgroup analyses. In studies of camrelizumab alone, PD-L1 expression was not associated with better ORR. Taking 1% as the cutoff value of PD-L1 positivity, we found the OR was 1.931 (95% CI: 0.883–4.223, p = 0.099) between PD-L1–positive and negative patients (Figure 6). However, there were only two associated trials, and further studies are required. Other possible predictive biomarkers included TMB, LDH, and the incidence of RCCEP. The association between these biomarkers and prognosis needs to be further studied.
In the enrolled cancer patients receiving combination therapy, the ORR ranged from 45.3% for NSCLC to 23.3% for BTC (Figure 7). The DCR varied between 77.3 and 85.5%. NSCLC patients had the highest ORR and DCR in our study. When grouped by treatment frequency, the pooled ORR was higher in treatment every 3 weeks (65.4 vs. 30.2%) (Figure 8). The DCRs were prominent in both groups (79.4 vs. 87.5%). Regardless of whether the patients received camrelizumab every 2 or 3 weeks, both frequencies could provide clinical benefits.
In this meta-analysis, several limitations are unavoidable. First, patients included in this study were heterogeneous with different cancer types and stages. The incidence of AEs may be totally different in different cancers. Second, the enrolled clinical trials used different doses, frequencies, and treatment lines of camrelizumab. These factors may affect the incidence of AEs and efficacy data. Third, some trials were published as conference abstracts and introduced some biases to the analysis. Most trials reported ORR, DCR, or PFS instead of OS because of insufficient follow-up time. A large meta-analysis revealed that PFS was not a surrogate endpoint for OS (Cortazar et al., 2014; Prasad et al., 2015; Paoletti et al., 2020). Evidences for the efficacy of camrelizumab were unsatisfactory without OS data. Finally, most of the included studies were single-arm studies without enough double-blinded RCTs, and some trials were in phase I or II. The failure of olaratumab in the treatment of soft tissue sarcoma showed the inconformity of phase II and phase III clinical trials (Pontes et al., 2020). A lot of new medicines were unable to translate promising phase II results into positive results in phase III trials. The effectiveness of camrelizumab also must be tested and verified in phase III comparative confirmatory trials. Considering those uncertainties in trials, a larger homogeneous patient pooled analysis and more phase III confirmatory trials with more survival results are needed to verify our conclusions.
Camrelizumab and its combination with chemotherapy and apatinib appeared to have durable efficacy with tolerable toxicity in patients with various solid cancers. Combination therapy appeared to provide more clinical benefit with increasing AEs. However, these remain to be proven in future studies. RCCEP is the most common AE in camrelizumab treatment, and the incidence appears to appreciably decrease after combining with apatinib. Nevertheless, the incidences of hypertension, proteinuria, and hepatic function abnormality should be noted and regularly monitored during and after treatment. Baseline LDH may be a predictor of efficacy, and the role of PD-L1 and TMB, and the incidence of RCCEP may be clarified in further studies.
HY generated the idea. KW and BL collected the data and wrote the manuscript. HY and LY analyzed the data. ML and LY helped in correcting the grammar and editing the pictures. All authors read and approved the final version of the manuscript.
This work was supported by the Science and Technology Department, Henan Province (grant numbers: 152300410164, 192102310048, and SB201901113).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Chen, X., Ma, L., Wang, X., Mo, H., Wu, D., Lan, B., et al. (2019a). Reactive capillary hemangiomas: a novel dermatologic toxicity following anti-PD-1 treatment with SHR-1210. Cancer Biol. Med. 16(1), 173–181. doi:10.20892/j.issn.2095-3941.2018.0172
Chen, X., Wu, H., Wu, X., Shao, Q., Zhu, F., Qian, X., et al. (2020a). Biomarker exploration for SHR-1210 plus GEMOX as first-line treatment in advanced biliary tract cancer. J. Clin. Oncol. 38(Suppl. 4), 536. doi:10.1200/JCO.2020.38.4_suppl.536
Chen, X., Wu, X., Wu, H., Shao, Q., Zhu, F., Qian, X., et al. (2019b). SHR-1210 plus GEMOX as first line treatment in biliary tract cancer: results from a single-arm exploratory study. J. Clin. Oncol. 37(Suppl. 4), 4092. doi:10.1200/JCO.2019.37.15_suppl.4092
Chen, X., Wu, X., Wu, H., Shao, Q., Zhu, F., Qian, X., et al. (2020b). SHR-1210 combined with GEMOX as first-line treatment in patients with advanced biliary tract cancer. J. Clin. Oncol. 38(Suppl. 4), 535. doi:10.1200/JCO.2020.38.4_suppl.535
Cortazar, P., Zhang, L., Untch, M., Mehta, K., Costantino, J. P., Wolmark, N., et al. (2014). Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384, 164–172. doi:10.1016/S0140-6736(13)62422-8
Fang, W., Yang, Y., Ma, Y., Hong, S., Lin, L., He, X., et al. (2018). Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol. 19(10), 1338–1350. doi:10.1016/S1470-2045(18)30495-9
Finlay, W. J. J., Coleman, J. E., Edwards, J. S., and Johnson, K. S. (2019). Anti-PD1 ‘SHR-1210’ aberrantly targets pro-angiogenic receptors and this polyspecificity can be ablated by paratope refinement. MAbs 11(1), 26–44. doi:10.1080/19420862.2018.1550321
Huang, J., Mo, H., Zhang, W., Chen, X., Qu, D., Wang, X., et al. (2019a). Promising efficacy of SHR‐1210, a novel anti-programmed cell death 1 antibody, in patients with advanced gastric and gastroesophageal junction cancer in China. Cancer 125(5), 742–749. doi:10.1002/cncr.31855
Huang, J., Xu, J., Chen, Y., Zhuang, W., Zhang, Y., Chen, Z., et al. (2019b). “Phase 3 study of camrelizumab vs chemotherapy for locally advanced/metastatic esophageal cancer: the ESCORT Study,” in Oral presentation at the 15th OESO world conference for esophaeal diseases. November 7–9, 2019. Beijing, China
Hwang, S. J. E., Carlos, G., Wakade, D., Byth, K., Kong, B. Y., Chou, S., et al. (2016). Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J. Am. Acad. Dermatol. 74(3), 455–461. doi:10.1016/j.jaad.2015.10.029
Li, J., Qin, S., Xu, J., Guo, W., and Xiong, J., Bai, Y., et al. (2013). Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J. Clin. Oncol. 31(26), 3219–3225. doi:10.1200/JCO.2013.48.8585
Li, J., Qin, S., Xu, J., Xiong, J., Wu, C., Bai, Y., et al. (2016). Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J. Clin. Oncol. 34 (13), 1448–1454. doi:10.1200/JCO.2015.63.5995
Liang, L., Wen, Y., Hu, R., Wang, L., Xia, Y., Hu, C., et al. (2019). Safety and efficacy of PD-1 blockade-activated multiple antigen-specific cellular therapy alone or in combination with apatinib in patients with advanced solid tumors: a pooled analysis of two prospective trials. Cancer Immunol. Immunother. 68 (9), 1467–1477. doi:10.1007/s00262-019-02375-z
Lickliter, J. D., Gan, H. K., Voskoboynik, M., Arulananda, S., Gao, B., Nagrial, A., et al. (2020). A first-in-human dose finding study of camrelizumab in patients with advanced or metastatic cancer in Australia. Drug Des. Devel. Ther. 14, 1177–1189. doi:10.2147/dddt.s243787
Liu, J., Jiang, Z., Li, Q., Li, Y., Liu, Q., and Song, E. (2019). Efficacy and safety of anti-PD-1 antibody SHR-1210 combined with apatinib in patients with advanced triple-negative breast cancer. J. Clin. Oncol. 37(Suppl. 15), 1066. doi:10.1200/JCO.2019.37.15_suppl.1066
Markham, A., and Keam, S. J. (2019). Camrelizumab: first global approval. Drugs 79, 1355–1361. doi:10.1007/s40265-019-01167-0
Mi, Y. J., Liang, Y. J., Huang, H. B., Zhao, H. Y., Wu, C. P., Wang, F., et al. (2010). Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res. 70(20), 7981–7991. doi:10.1158/0008-5472.CAN-10-0111
Mo, H., Huang, J., Xu, J., Chen, X., Wu, D., Qu, D., et al. (2018). Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: a dose-escalation, phase 1 study. Br. J. Cancer 119(5), 538–545. doi:10.1038/s41416-018-0100-3
Paoletti, X., Lewsley, L.-A., Daniele, G., Cook, A., Yanaihara, N., Tinker, A., et al. (2020). Assessment of progression-free survival as a surrogate end point of overall survival in first-line treatment of ovarian cancer. JAMA Netw. Open 3(1), e1918939. doi:10.1001/jamanetworkopen.2019.18939
Pardoll, D. M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264. doi:10.1038/nrc3239
Pontes, C., Zara, C., Torrent-Farnell, J., Obach, M., Nadal, C., Vella-Bonanno, P., et al. (2020). Time to review authorisation and funding for new cancer medicines in Europe? Inferences from the case of Olaratumab. Appl. Health Econ. Health Policy 18(1), 5–16. doi:10.1007/s40258-019-00527-x
Prasad, V., Kim, C., Burotto, M., and Vandross, A. (2015). The strength of association between surrogate end points and survival in oncology: a systematic review of trial-level meta-analyses. JAMA Intern. Med. 175(8), 1389–1398. doi:10.1001/jamainternmed.2015.2829
Qin, S., Chen, Z., Liu, Y., Xiong, J., Ren, Z., Meng, Z., et al. (2019). A phase II study of anti-PD-1 antibody camrelizumab plus FOLFOX4 or GEMOX systemic chemotherapy as first-line therapy for advanced hepatocellular carcinoma or biliary tract cancer. J. Clin. Oncol. 37(Suppl. 15), 4074. doi:10.1200/jco.2019.37.15_suppl.4074
Qin, S., Ren, Z., Meng, Z., Chen, Z., Chai, X., Xiong, J., et al. (2020). Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 21(4), 571–580. doi:10.1016/S1470-2045(20)30011-5
Scott, A. J., Messersmith, W. A., and Jimeno, A. (2015). Apatinib: a promising oral antiangiogenic agent in the treatment of multiple solid tumors. Drugs Today 51(4), 223–229. doi:10.1358/dot.2015.51.4.2320599
Sharma, P.,and Allison, J. P. (2015). The future of immune checkpoint therapy. Science 348, 56–61. doi:10.1126/science.aaa8172
Sharpe, A. H.,and Pauken, K. E. (2017). The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 18, 153. doi:10.1038/nri.2017.108
Shen, L., Peng, Z., Zhang, Y.-Q., Wei, J., Wang, F., Ying, J., et al. (2019). Camrelizumab combined with capecitabine and oxaliplatin followed by camrelizumab and apatinib as first-line therapy for advanced or metastatic gastric or gastroesophageal junction cancer: updated results from a multicenter, open label phase II trial. J. Clin. Oncol. 37(Suppl. 15), 4031. doi:10.1200/JCO.2019.37.15_suppl.4031
Slim, K., Nini, E., Forestier, D., Kwiatkowski, F., Panis, Y., and Chipponi, J. (2003). Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J. Surg. 73 (9), 712–716. doi:10.1046/j.1445-2197.2003.02748.x
Teng, Y., Guo, R., Sun, J., Jiang, Y., and Liu, Y. (2019). Reactive capillary hemangiomas induced by camrelizumab (SHR-1210), an anti-PD-1 agent. Acta Oncol. 58, 388–389. doi:10.1080/0284186X.2019.1567935.
Wang, Q., Gao, J., Di, W., and Wu, X. (2020). Anti-angiogenesis therapy overcomes the innate resistance to PD-1/PD-L1 blockade in VEGFA-overexpressed mouse tumor models. Cancer Immunol. Immunother.69, 1781. doi:10.1007/s00262-020-02576-x
Wang, X., Zhang, B., Chen, X., Mo, H., Wu, D., Lan, B., et al. (2019). Lactate dehydrogenase and baseline markers associated with clinical outcomes of advanced esophageal squamous cell carcinoma patients treated with camrelizumab (SHR‐1210), a novel anti‐PD‐1 antibody. Thorac. Cancer. 10(6), 1395–1401. doi:10.1111/1759-7714.13083
Wu, X., Zhang, X., Shu, P., Ma, Q., Chen, Y., Li, D., et al. (2020). A39 reactive cutaneous capillary endothelial proliferation caused by camrelizumab (SHR-1210) through activation of HIF-1α/VEGF signaling pathway. J. Thorac. Oncol. 15(2), S25–S26. doi:10.1016/j.jtho.2019.12.068
Wu, Y., Huang, C., Fan, Y., Feng, J., Pan, H., Jiang, L., et al. (2019). JCSE01.09 a phase II umbrella study of camrelizumab in different PD-L1 expression cohorts in pre-treated advanced/metastatic non-small cell lung cancer. J. Thorac. Oncol. 14(10), S128. doi:10.1016/j.jtho.2019.08.267
Xie, L., Guo, W., Xu, J., Sun, X., Liu, K., Zheng, B., et al. (2019). Apatinib plus camrelizumab (SHR-1210) for unresectable high-grade osteosarcoma (APFAO) progressing after chemotherapy: a prospective, open label, phase II trial. J. Clin. Oncol. 37(Suppl. 15), 11013. doi:10.1200/JCO.2019.37.15_suppl.11013
Xu, J., Zhang, Y., Jia, R., Yue, C., Chang, L., Liu, R., et al. (2019). Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin. Cancer Res. 25(2), 515–523. doi:10.1158/1078-0432.CCR-18-2484
Yang, C., Feng, W., and Wu, D. (2018). Apatinib for advanced non-small cell lung cancer: a retrospective case series analysis. J. Cancer Res. Ther. 14(1), 159–162. doi:10.4103/jcrt.JCRT_258_17
Yang, Y., Wu, X., Li, F., Wang, N., Zhang, M., Sun, T., et al. (2019). Evaluation of efficacy and safety of apatinib treatment in advanced gastric cancer. J. Cancer Res. Ther. 15(2), 365–369. doi:10.4103/jcrt.JCRT_297_18
Zhang, B., Qi, L., Wang, X., Jiang, J., Zhang, X., Liu, Y., et al. (2019). Phase 2 study of camrelizumab (anti-PD-1 antibody) combined with apatinib and chemotherapy for the first-line treatment of advanced esophageal squamous cell carcinoma. J. Clin. Oncol. 37(Suppl. 15), 4033. doi:10.1200/JCO.2019.37.15_suppl.4033
Zhang, H. (2015). Apatinib for molecular targeted therapy in tumor. Drug Des. Devel. Ther. 9, 6075–6081. doi:10.2147/DDDT.S97235
Zhao, L., Yang, Y., and Gao, Q. (2019). Efficacy and safety of nivolumab plus apatinib in advanced liver carcinosarcoma: a case report. Immunotherapy 11(8), 651–656. doi:10.2217/imt-2018-0214
Zhao, S., Jiang, T., Li, X., and , C. (2017). OA11.07 combining anti-angiogenesis and immunotherapy enhances antitumor effect by promoting immune response in lung cancer. J. Thorac. Oncol. 12(1), S288. doi:10.1016/j.jtho.2016.11.293
Zhao, S., Ren, S., Jiang, T., Zhu, B., Li, X., Zhao, C., et al. (2019). Low-dose apatinib optimizes tumor microenvironment and potentiates antitumor effect of PD-1/PD-L1 blockade in lung cancer. Cancer Immunol. Res. 7(4), 630–643. doi:10.1158/2326-6066.CIR-17-0640
Zheng, B., Ren, T., Huang, Y., and Guo, W. (2018). Apatinib inhibits migration and invasion as well as PD-L1 expression in osteosarcoma by targeting STAT3. Biochem. Biophys. Res. Commun. 495(2), 1695–1701. doi:10.1016/j.bbrc.2017.12.032
Zhou, C., Chen, G., Huang, Y., Zhou, J., Lin, L., Feng, J., et al. (2019a). OA04.03 A randomized phase 3 study of camrelizumab plus chemotherapy as 1st line therapy for advanced/metastatic non-squamous non-small cell lung cancer. J. Thorac. Oncol. 14(10), S215–S216. doi:10.1016/j.jtho.2019.08.425
Keywords: camrelizumab, apatinib, safety, efficacy, solid cancers, meta-analysis
Citation: Wang K, Li B, Li M, Li S, Yang H and Yuan L (2020) The Safety and Efficacy of Camrelizumab and Its Combination With Apatinib in Various Solid Cancers. Front. Pharmacol. 11:568477. doi:10.3389/fphar.2020.568477
Received: 01 June 2020; Accepted: 18 September 2020;
Published: 21 October 2020.
Edited by:
Tanveer Ahmed Khan, National Institute of Health, PakistanReviewed by:
Muhammad Usman, University of Veterinary and Animal Sciences, PakistanCopyright © 2020 Wang, Li, Li, Li, Yang and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Yang, ZHIuaHVpeWFuZ0BnbWFpbC5jb20=, Ling Yuan, SE5ITllMQDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.