- Research Center of TCM Processing Engineering, College of Pharmaceutical Sciences, Zhejiang Chinese Medical University, Hangzhou, China
Coicis semen, a medicinal food, is derived from the dried and mature seeds of Coix lacryma-jobi L. var. ma-yuen (Rom.Caill.) Stapf, a member of the Gramineae family. Lipids are its main constituents. Previous literature reported that coicis semen contains twenty triglycerides and twelve diglycerides. However, we identified thirty-five triglycerides, sixteen diglycerides, four monoglycerides, and two sterols under the preoptimized conditions of UPCC-Xevo G2-XS QTOF combined with a personalized TCM database. Furthermore, we successfully determined glycerol trioleate content to evaluate quality differences. Finally, we identified the fatty acid compositions of seven out of nine differential markers via Progenesis QI using principal component analysis, orthogonal projection to latent structures–discriminant analysis, and the LipidMaps database. In addition, we applied a software-based classification, a method that was previously developed by our team, to verify and predict structurally similar compounds. Our findings confirmed that UPCC-Xevo G2-XS QTOF combined with software-based group classification could be used as an efficient method for exploring the potential lipid markers of seed medicine.
Introduction
Common sense dictates that various natural ingredients exist in TCM. However, most reports on the active components of TCM have focused on polysaccharides, alkaloids, and flavonoids. Fatty oils are widely available ingredients of herbs, and the limited attention that they have received may restrict their further development and application. Fatty oils can be obtained as an active ingredient from animals and plants (Liu et al., 2015; Chen et al., 2016; Hong et al., 2016). A number of TCM contain fatty oils, which are mainly derived from the seeds and fruits of herbs. Diverse fatty oils comprise of glycerol and different types of saturated, monounsaturated, and polyunsaturated fatty acids that each exert therapeutic effects.
Coicis semen (Job’s tears seed or adlay), which has been documented in the 2015 edition of the Chinese pharmacopoeia, is the dry and mature seed of Coix lacryma-jobi L. var. ma-yuen (Rom.Caill.) Stapf. It is not only a commonly used TCM, but it is also a commonly consumed food. Coicis semen has been proven to have numerous functions, such as detoxification and dampness and arthralgia removal; it also reduces cancer risk (Yu et al., 2011; Bai et al., 2018; Duan, 2018; Xu et al., 2018; Choi et al., 2019; Huang Q. et al., 2019; Huang Y. L. et al., 2019; Li et al., 2019; Liu H. Q. et al., 2019; Liu Y. N. et al., 2019; Manosroi et al., 2019; Qian et al., 2019; Zhang et al., 2019). Moreover, it has a wide range of anti-inflammatory, antioxidation, analgesic, and sedative effects, as well as pharmacological effects against gastric cancer, hepatocellular cancer, Lewis lung cancer, non-small cell lung cancer, pancreatic cancer, and pulmonary cancer (“Fuzheng” category represented by coicis semen oil) (Manosroi et al., 2016a; Manosroi et al., 2016b; Qian et al., 2016; Xi et al., 2016; Wang et al., 2016; Han et al., 2017; Qu et al., 2017a; Schwartzberg et al., 2017; Jang et al., 2018; Kim et al., 2018; Zhang et al., 2018). It has a variety of clinical dosage forms, such as microporous microspheres, microemulsions, and intravenous emulsions (Qu et al., 2016; Qu et al., 2017b; Qu et al., 2017c; Trinh et al., 2017; Chen et al., 2018; Guo et al., 2019). Discrepancies in curative effects can be attributed to the various types, contents, and other nutrients of different fatty oils.
Recent research shows that the variety and content of the active components of different fatty oils remarkably influence human health and have their own specific advantages in treatment. Near-infrared spectroscopy analysis showed that the lipid contents of forty-one polished coicis semen samples range from 5.14% to 9.40%. Coicis semen oils contain seven types of triglycerides (trilinolein, 1,2-linolein-3-olein, 1-palmitin-2-linolein-3-olein, 1-palmitin-2,3-linolein, 1-palmitin-2, 3-olein, triolein, and 1,2-olein-3-linolein). Hou et al. identified twenty triglycerides and twelve diglycerides in the lipid profile of coicis semen and developed a green quantification strategy for simultaneously determining the content of 7 TGs (LLL, LLP, LLO, POL, OOL, OOP, and OOO) by combining core–shell column technology and SSDMCs. Lin et al. found β-sitosterol and stigmasterol in the ethyl acetate fraction of an adlay hull extract. Dong et al. established a rapid and reliable m-SPE approach using magnetic multiwalled carbon nanotubes as the adsorbent for the purification of type A trichothecenes, including T-2 toxins, HT-2 toxins, diacetoxyscirpenol, and neosolaniol, in coicis semen (Dong et al., 2016; Hou et al., 2018a; Hou et al., 2018b; Lin et al., 2019).
However, several components, especially glycerides, of coicis semen oil still require analysis, and the identification of the active ingredients of this material needs in-depth research. Therefore, our team used Acquity UPCC-Xevo G2-XS QTOF coupled with software-based group classification to further excavate, identify, and visually classify active ingredients in coicis semen oils. Coicis semen oils have boundless development prospects and need to be explored in-depth to lay a foundation for its new preparation, development, and extensive clinical application.
Materials and Methods
Materials and Reagents
Glycerol trioleate with the purity of more than 99.9% as determined via HPLC–ELSD was purchased from the Nature Standard Co. Ltd (Shanghai, China). Acetonitrile and methanol (HPLC–MS grade) were purchased from Merck (Darmstadt, Germany). Ammonium formate (HPLC grade) was purchased from Sigma-Aldrich. High-purity CO2 (99.999%) was purchased from the Shanghai Yizhi Industry Gases Co., Ltd. (Shanghai, China). All other reagents used in sample preparation were of analytical grade. Seven batches of dried coicis semen were purchased from different TCM enterprises in China. The manufacturers and batch numbers of the samples were as follows: batch number 180901 (Zhejiang Chinese Medical University Medical Pieces Co., Ltd., Hangzhou); batch number 190101 (Jirentang Pharmaceutical Co., Ltd., Guiyang); batch number 190105 (Zuoli Baicao Herbal Pieces Co., Ltd., Jiangxi); batch number 181201 (Huadong Herbal Pieces Co., Ltd., Hangzhou); batch number 181206 (Zuoli Baicao Herbal Pieces Co., Ltd., Zhejiang); batch number 190122 (Haiyuan Prepared Slices of Chinese Crude Drugs Co., Ltd., Nanjing); and batch number 190216 (Haichang Chinese Medicine Group Co., Ltd., Nanjing).
Preparation of Reference and Sample Solutions
The appropriate amount of glyceryl trioleate, which was used as the reference substance, was weighed accurately and diluted with n-hexane to prepare a series of working solutions with concentrations of 0.0099, 0.0988, 0.9881, 4.9405, and 9.8810 μg/mL.
All samples were ground and passed through a No. 3 sieve (355 ± 13 μm). The passing rate of the particles was maintained at more than 80%. The sample preparation procedure was as follows:
A total of 50.0 mL of n-hexane was added to 0.6 g of powdered coicis semen. The seed powder was soaked for 2 h and then sonicated (50 kHz, 250 W, KQ-500DB) for 30 min. The supernatant was filtered to obtain the sample solution. The filtrate was diluted with n-hexane 5 times and 100-fold for the analysis of different components of different samples and the quantitative analysis of glyceride trioleate, respectively. A total of 200 μL solution of each batch was mixed together and used as a pooled QC sample solution for the analysis of free fatty acids.
UPCC-Xevo G2-XS QTOF Parameters
On the basis of preliminary experiments, the final experimental conditions were determined as follows: Liquid phase system: ACQUITY UPCC; column: Torus 2-PIC, 3.0 × 100 mm, 1.7 μm; mobile phase A: CO2, mobile phase B: methanol acetonitrile = 9:1; column temperature: 55°C, ABPR: 2600 psi; compensation solution: methanol solution with 0.5 mM ammonium formate; flow rate: 0.5 mL/min; and injection volume: 0.3 μL. The gradient program was used with a flow rate of 1.0 mL/min and was as follows: 0–2 min (1% B), 2–7 min (1%–5% B), 7–10 min (5% B), and 10–13 min (5%–1% B).
The conditions for mass spectrometry are as follows: Mass spectrometry system: Xevo G2-XS QTOF; ionization method: ESI+/−; data acquisition mode: MSE; MSE impact energy: low, off, high, 20–50 eV; quantitative ion: m/z 902.8177; collection mass range: 100–1200 Da; capillary voltage: 2.0 kV; cone-hole voltage: 40 V; ion source temperature: 120°C; atomization temperature: 500°C; cone-hole gas flow rate: 50 L/h; and atomized gas flow rate: 1000 L/h. Data calibration was performed by using an external reference (LockSpray) with the constant infusion of leucine–enkephalin solution (200 pg/μL) at a flow rate of 5 μL/min.
The data processing software included UNIFI 1.9.4 and Masslynx V 4.1.
Data Acquisition and Analysis
Xevo G2-XS QTOF uses the patented LockSpray technology to ensure the accuracy of the collected data in real time. High-accuracy mass numbers can be obtained, and the combination of high-accuracy mass numbers and isotope distribution and secondary fragment information accurately provides molecular formulas. Xevo G2-XS QTOF applies patented MSE technology to obtain the primary and secondary mass spectral information of the compounds for further structural confirmation with one injection at the same time.
The ESI+/− mode was used for the analysis of glycerides and free fatty acids. The first step involved understanding the fragmentation pattern of glycerides as a whole. In the second step, by combining the fragmentation patterns and consulting related literature, a UNIFI database for the glyceride analysis of coicis semen was established. In the third step, the self-built database was imported into UNIFI in addition to the ChemSpider online database, and the appropriate analysis method was set. Furthermore, the software automatically analyzed primary and secondary mass spectral information. Finally, it quickly screened out the target through a unique workflow.
Our team developed a classification program in the Visual Basic for Applications (VBA; Microsoft, USA) and MATLAB v7.1 (The Mathworks, Natick, USA) environments. The classification program consisted of three parts (Shan et al., 2012).
A total of 2,916 features were introduced into the SIMCA-P 13.5 software (Umetrics, Umeå, Sweden) for principal component analysis (PCA) and orthogonal projection to latent structures–discriminant analysis (OPLS–DA). The corresponding variable importance in the projection value (VIP value) was calculated in the OPLS–DA model. A potential differential marker was selected when its VIP value exceeded 2.00 and its S-Plot exceeded 0.95.
Results and Discussion
Qualitative Results of Glycerides and Free Fatty Acids in Coicis Semen Oils
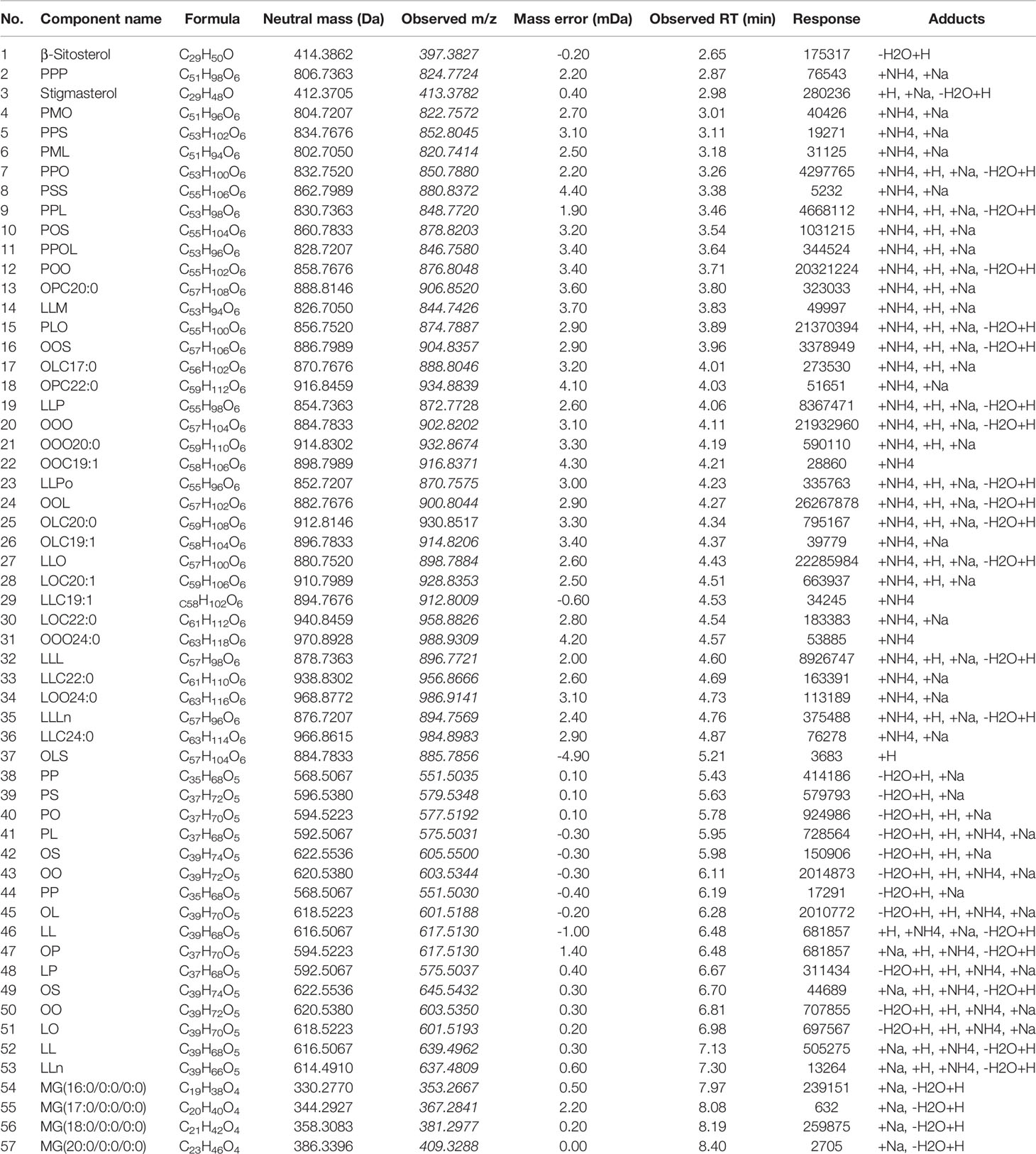
In the ESI+ mode, fifty-six and fifty-seven compounds were identified in five (No. 180901, No. 181201, No. 181206, No. 190101, and No. 190122) and two (No. 190105 and No. 190216) batches of samples, respectively. These compounds were mainly composed of glycerides. The total ion chromatogram of all samples is provided in Figure 1A, and the corresponding identified glycerides are listed in Table 1. Among them, fifty-six common compounds, including thirty-five triglycerides, fifteen diglycerides, four glycerides, and two sterols, were identified in comparison with the corresponding results of twenty triglycerides and twelve diglycerides (Hou et al., 2018a). However, the OP of diglycerides was identified only in two batches of samples, namely 190105 and 190216, likely because of the different processing technologies of different medical enterprises. The QC sample mentioned in Figure 1B was overlaid in the following differential component analysis.
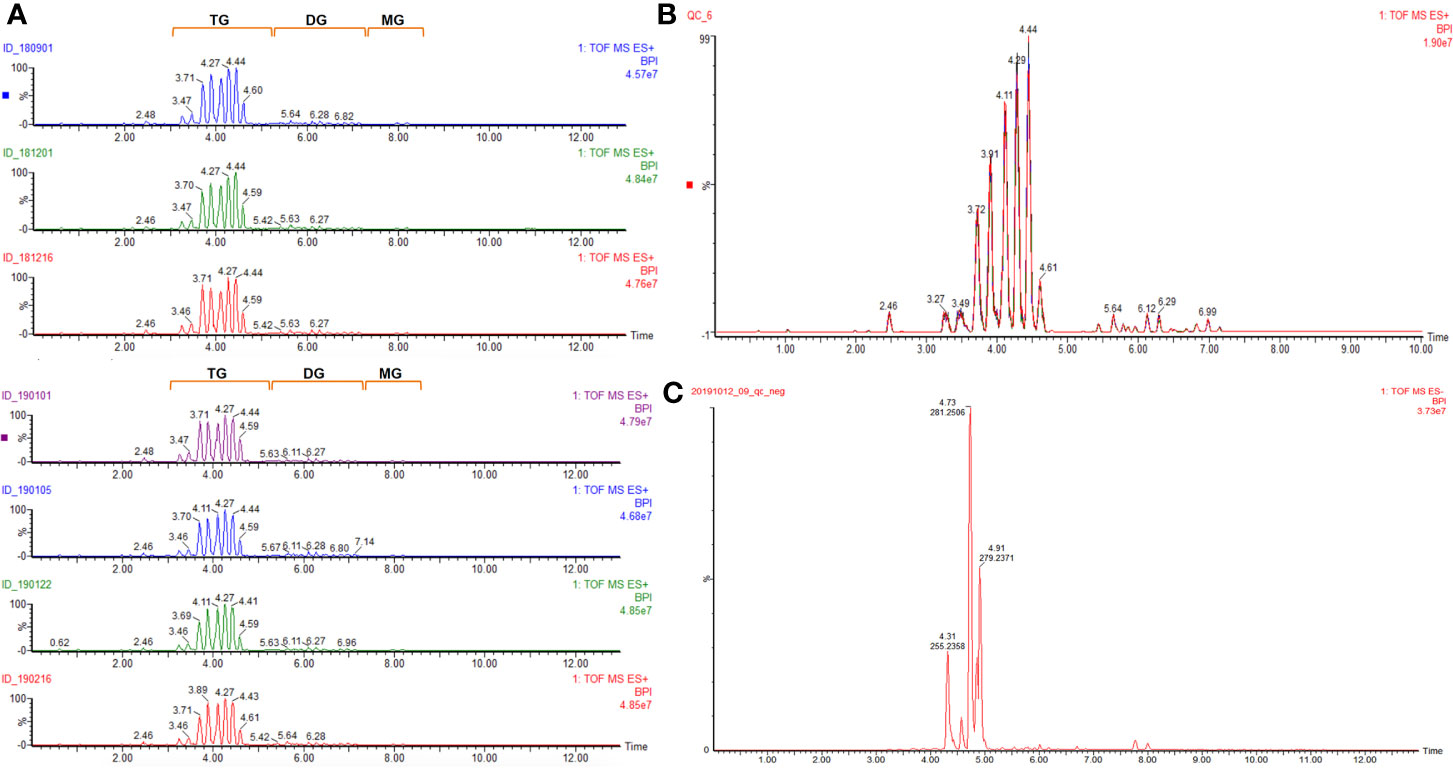
Figure 1 Total ion chromatogram of (A) (qualitative analysis of glyceride contents of seven batches of coicis semen oils in ESI+); (B) (overlay of the QC sample for the differential component analysis of different samples in ESI+); (C) (qualitative analysis of free fatty acids of QC sample in ESI−).
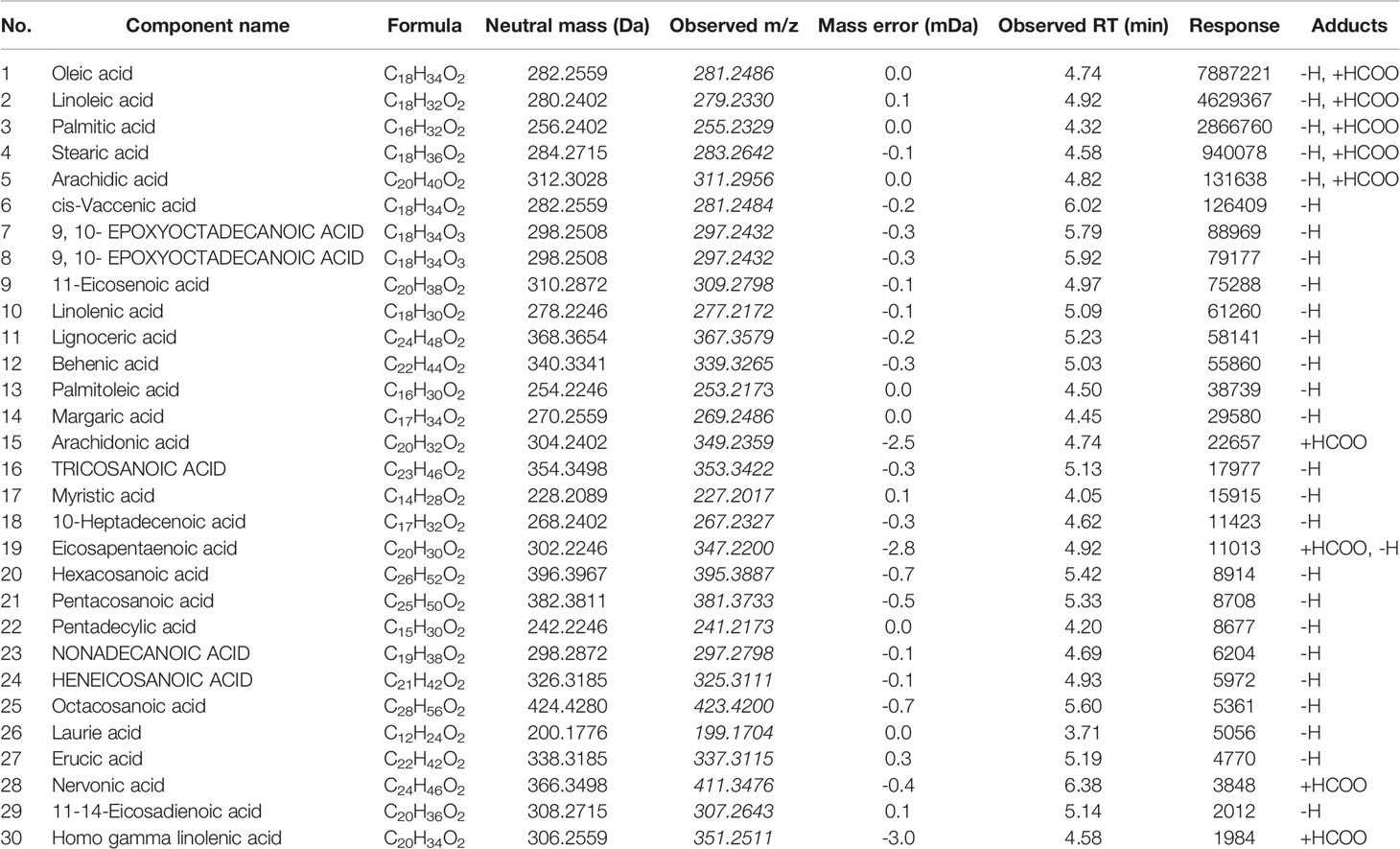
A total of thirty free fatty acids were identified in the ESI− mode, and some unsaturated fatty acids could have had isomers, which needed to be confirmed further by using a reference substance. The total ion chromatogram of the QC sample is given in Figure 1C, and the corresponding identified free fatty acids are listed in Table 2.
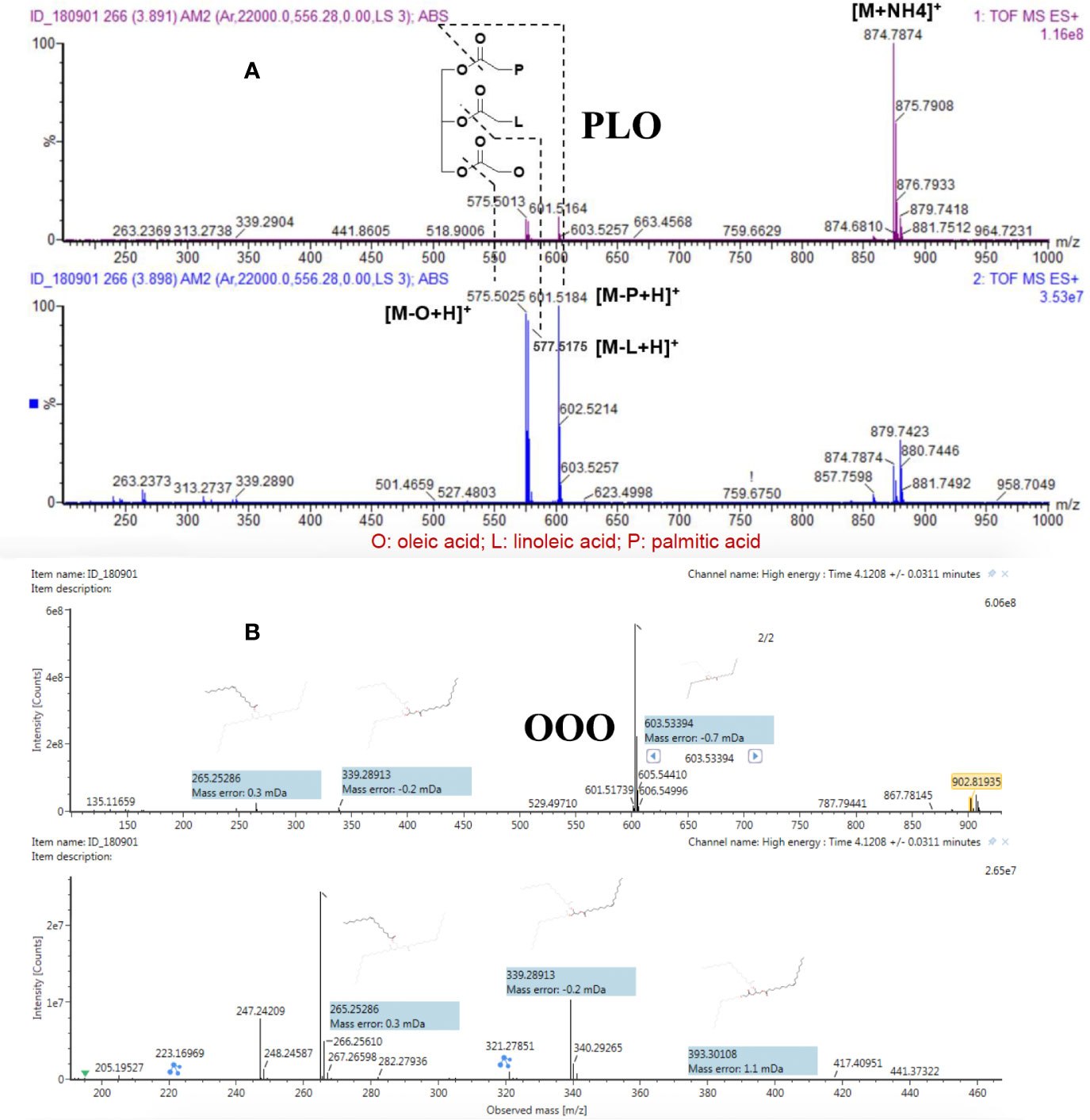
As shown in Figure 2A, PLO (tR 3.90 min) provided a precursor ion ([M+NH4]+) at m/z 874.7874 with a double-bond equivalent. The MS/MS product ions at m/z 601.5184 ([M-P+H]+ palmitic acid), m/z 577.5175 ([M-L+H]+ linoleic acid), and m/z 575.5025 ([M-O+H]+ oleic acid) resulted from the sn-1, sn-2, and sn-3 cleavages of the ester groups, respectively. Figure 2B shows the secondary fragment matching diagram for OOO generated by the UNIFI software.
Software-Based Group Classification of Glycerides
Our teams previously developed a program in VBA and MATLAB for the classification of multiple complex components. This program successfully grouped the constituents in the n-hexane extract of coicis semen. Through the comprehensive analysis of herbal samples, fifty-seven peaks were identified and divided into four groups as shown in Figures 3, 4. Three of these groups consisted of triglycerides and diglycerides. The remaining group was composed of four monoglycerides, two diglycerides, and two sterols. The chemical structures and special MS fragmentation pathways of these compounds indicated that the same group might have similar features, and unknown ingredients could be identified through the comprehensive software-based group classification of these compounds.
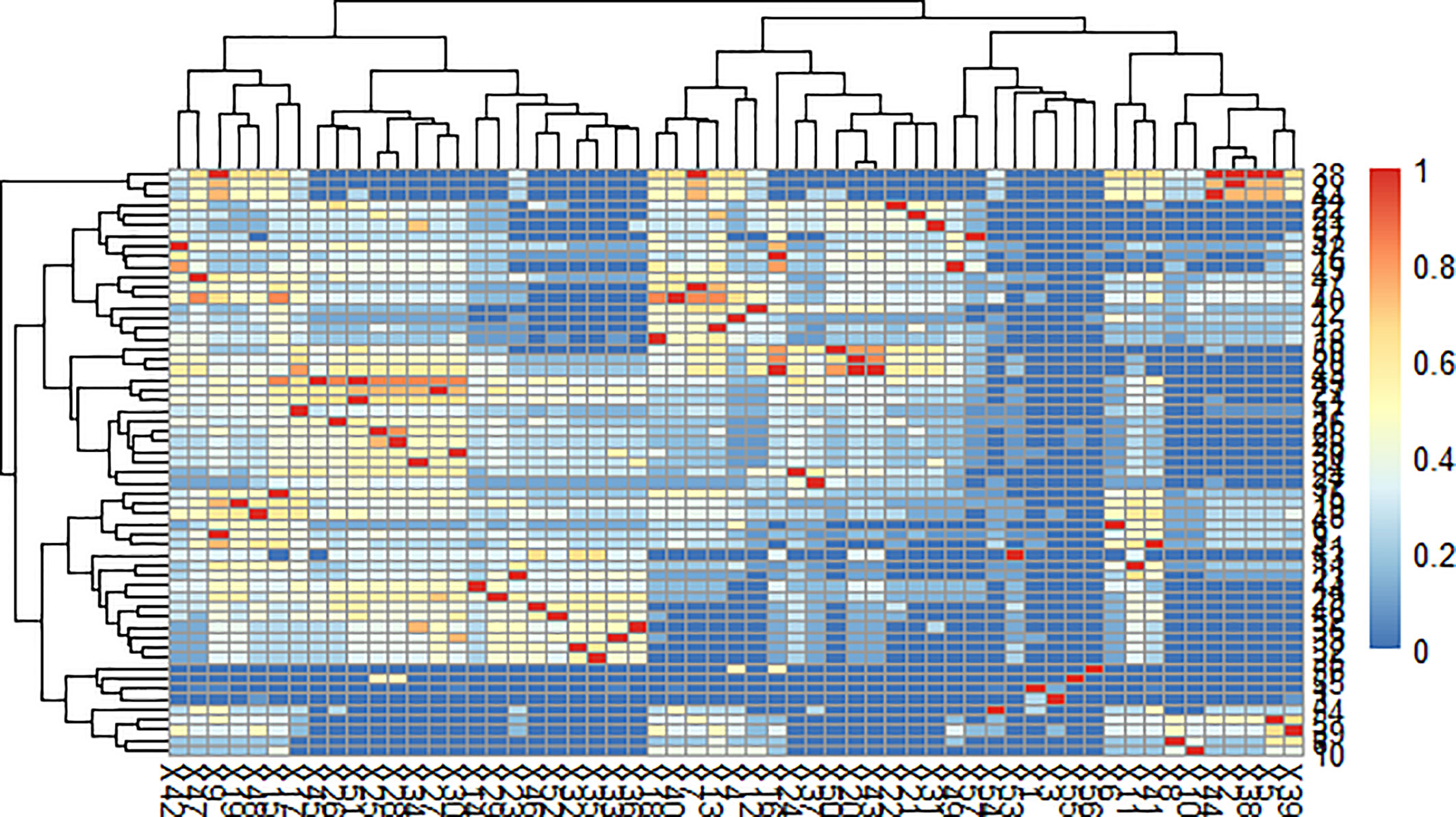
Figure 3 Affinity diagram of the mass spectra of glycerides with software-based group classification.
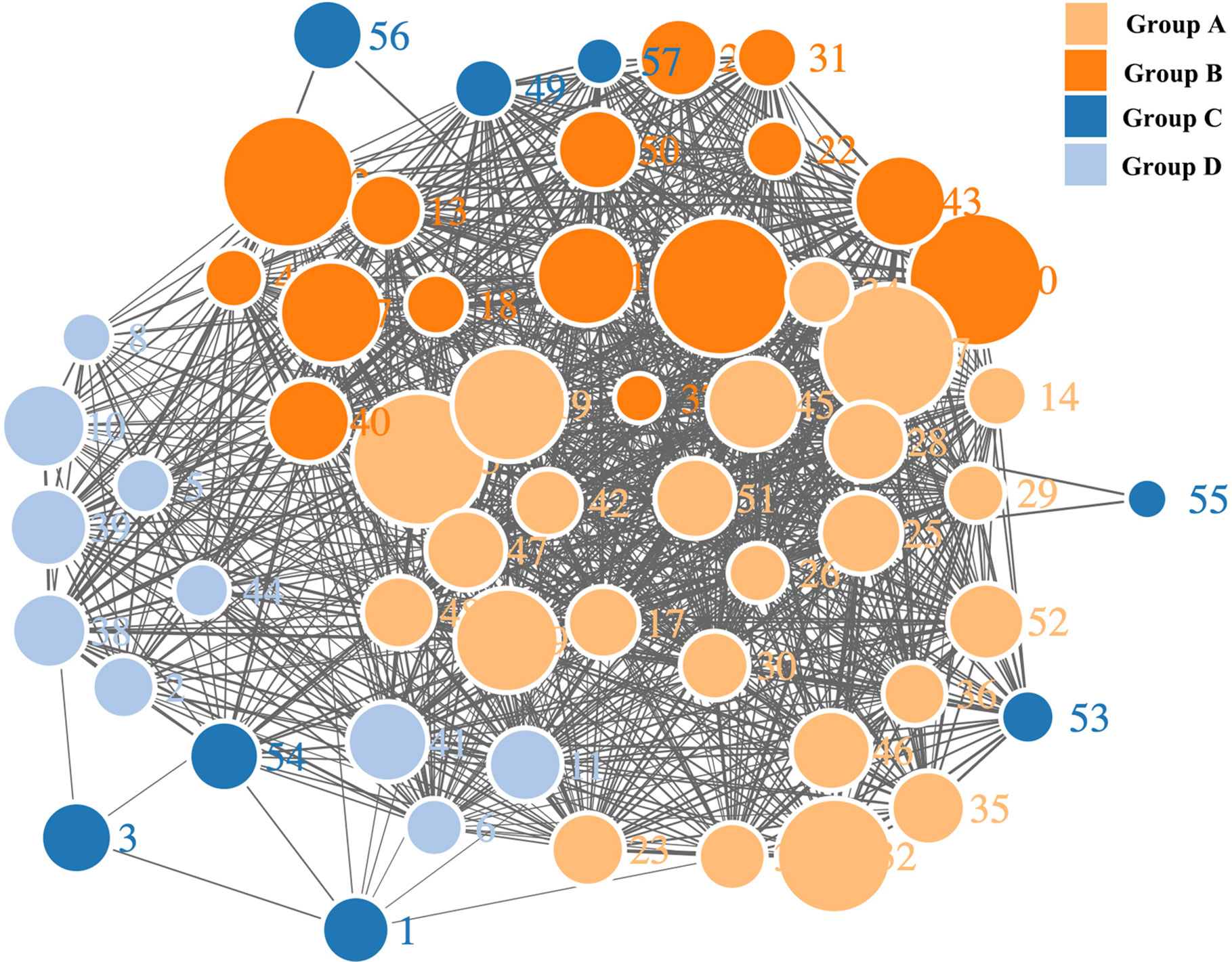
Figure 4 Network diagram of the mass spectra of glycerides with software-based group classification.
Differential Component Analysis of Different Samples
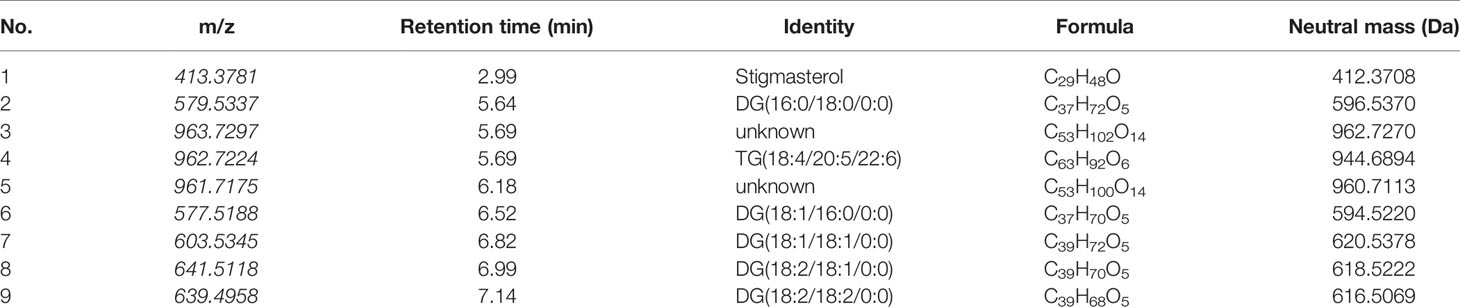
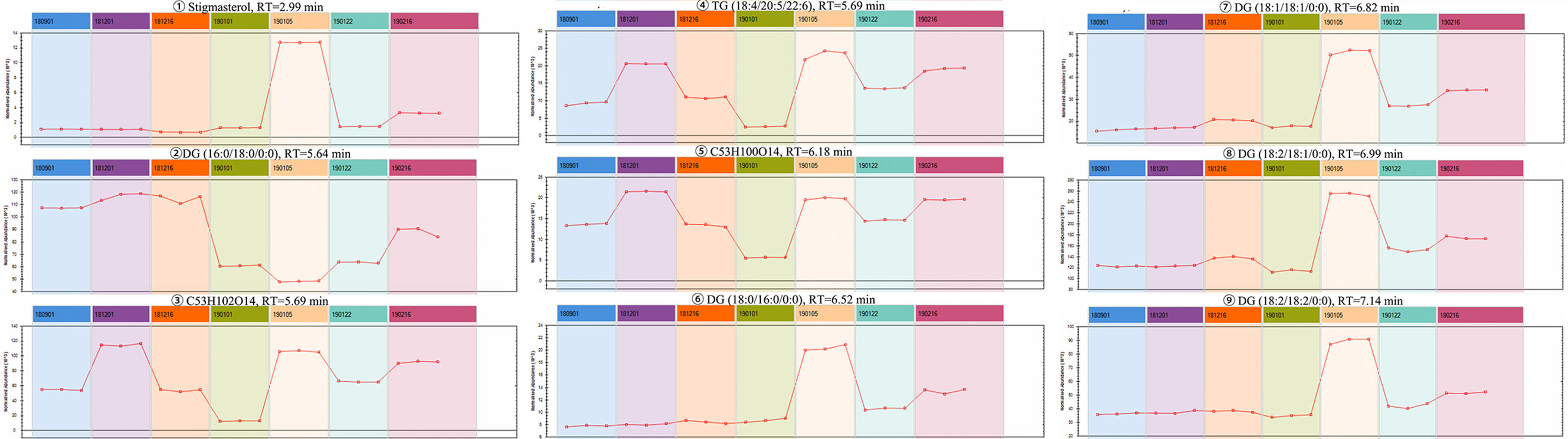
The Progenesis QI omics analysis software was used for differential component research. Before exploring quality markers, the analytical system was first validated for repeatability upon the injection of six QC samples. A total of 2,916 features were extracted and then imported into EZinfo for multivariate statistical analysis. PCA was used to study the variations in the oils of seven batches of coicis semen (Figure 5A). The differences between the groups of samples, namely No. 181216 and No.190122, were large. Furthermore, No. 190105 and No. 190101, which showed the largest differences, were subjected to OPLS–DA analysis (Figure 5B). These two groups were clearly distinct. Furthermore, we selected compounds with S Plot ≥ 0.95 and VIP ≥ 2 as markers (Figures 5C, D), and transferred them back to the QI for identification. Finally, nine markers were found (Table 3). Then, the LipidBlast, LipidMaps, and Chemspider databases were searched in QI for further identification. Among the nine markers found, seven were identified (five diglycerides, one triglyceride, and one stigmasterol), and the molecular formulas of the remaining two unknown compounds were estimated using an elemental composition tool. The abundance distribution of the nine markers in all the samples is shown in Figure 6. The abundance of markers, except diglycerides (16:0/18:0/0:0), in No. 190105 were remarkably higher than that in No. 190101. This result could be related to the largest differences between No. 190105 and No. 190101 and indicated that massive differences in resources and processing technologies existed among medical enterprises.
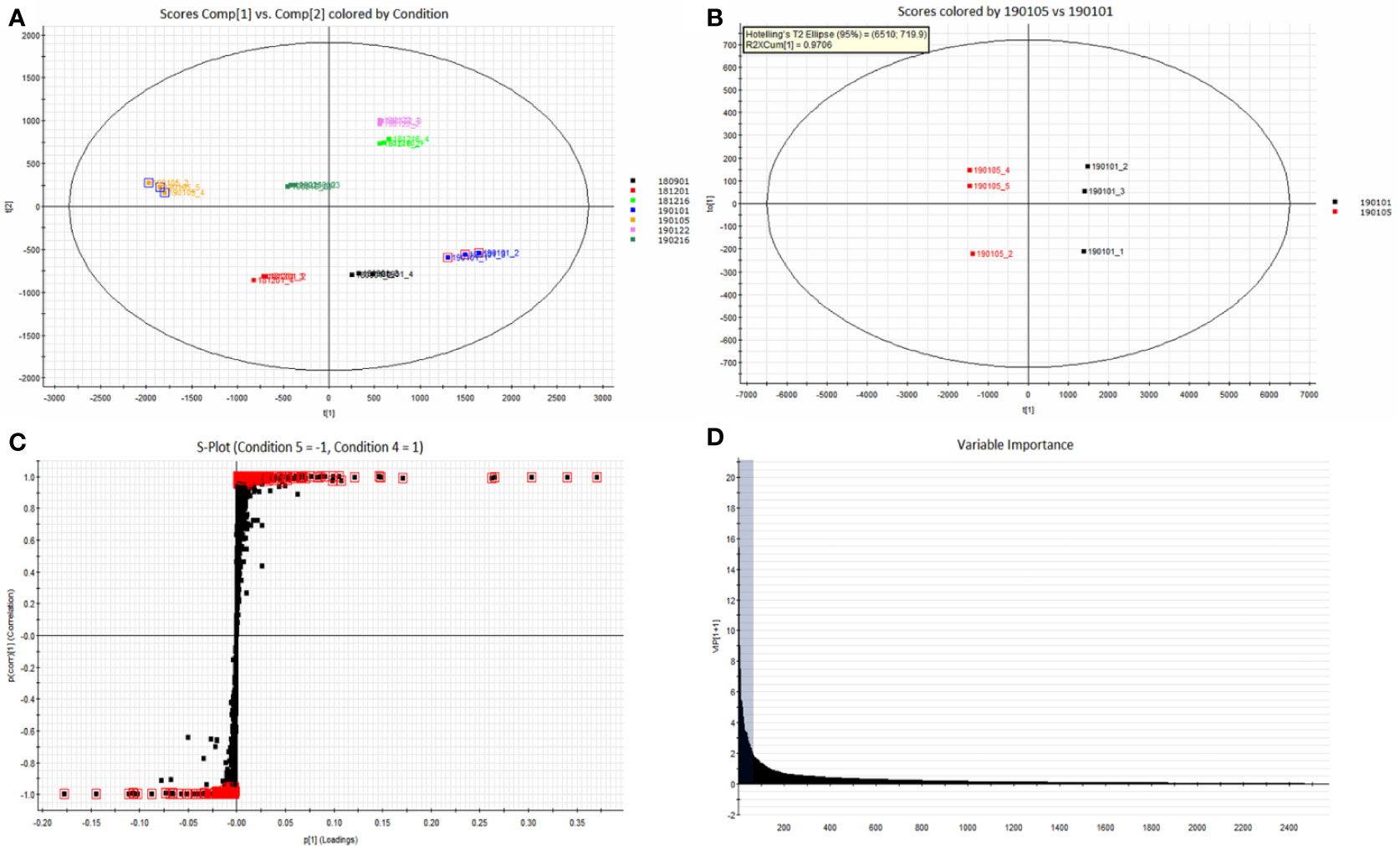
Figure 5 Differential component analysis of different samples. (A): PCA classification of seven batches of samples; (B) OPLS–DA analysis of No. 190101 and No. 190105 with significant differences; (C) S-Plot of No. 190101 and No. 190105; (D) VIP diagram of No. 190101 and No. 190105.
Quantitation of Glycerol Trioleate in Coicis Semen Oils
Investigation of Linear Relations
Reference solutions with concentrations of 0.0099, 0.0988, 0.9881, 4.9405, and 9.8810 μg/mL were used to perform three consecutive injections. The results showed that glyceryl trioleate had a good linear relationship in the range of 0.0090–9.8810 μg/mL, r2> 0.9990.
Quantitative Limit Investigation
The reference solution was diluted stepwise at certain multiples until glyceride trioleate presented S/N ≈ 10. The results showed that the quantitative limit was 4.94 ng/mL.
Instrument Precision Inspection
The low, middle, and high concentrations (0.0099, 0.9881, and 9.8810 μg/mL) of the reference solution on the calibration curve were taken and used in six consecutive injections to check instrument precision. The RSD value was less than 3%, which indicated good precision.
Repeatability Test
Six powder samples (0.6 g each) of the same batch (No. 180901) were weighed and prepared via the sample solution preparation method. The average content of glyceryl trioleate was determined and calculated as 0.91%. The results showed that the RSD value was 4.12% (n = 6).
Recovery Rate Test
ine powder samples (0.3 g each) of the same batch of known content were weighed, and then low, medium, and high levels of the three different concentrations of the reference substance were added precisely. The reference substance/sample ratio was controlled at 0.5:1, 1:1, 1.5:1, and each concentration level was tested in triplicate. The results showed a high average recovery of 102.28%.
Sample Measurement Results
The content of glyceryl trioleate in seven batches of the samples was determined using the established method above. The representative chromatogram is shown in Figure 7. Each batch was replicated in triplicate. The average content ranged from 0.84% to 1.05%. The RSD value was less than 5%.
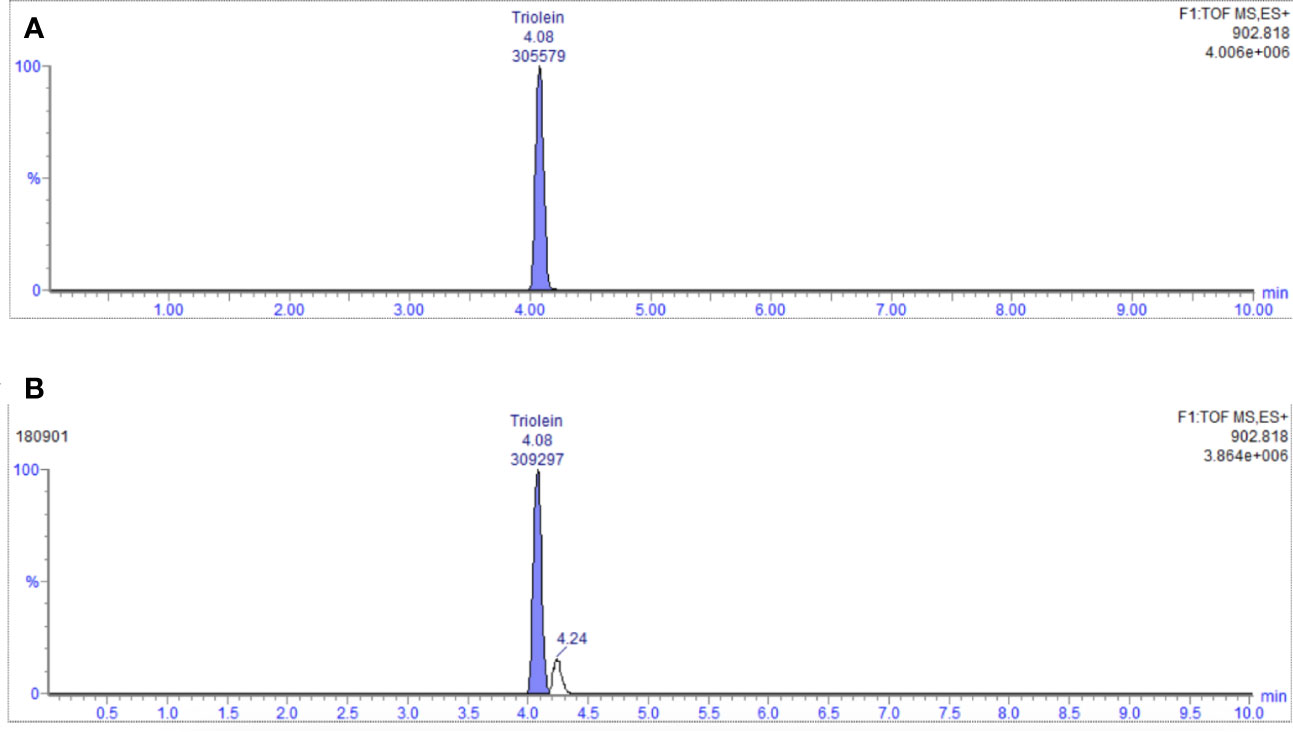
Figure 7 (A) Chromatogram of reference (glycerol trioleate); (B) EIC diagram of representative sample (No. 180901).
Conclusion
The established analytical method fully demonstrated that ACQUITY UPCC enabled the fast and efficient chromatographic separation of lipids in coicis semen. Xevo G2-XS QTOF combined with LockSpray real-time external standard mass calibration technology ensured mass accuracy. The data collection method based on MSE tandem mass spectrometry without content discrimination ensured the full collection of information, and one-shot collection could obtain precursor ion and fragment ion information simultaneously with convenient, fast, and high-throughput characteristics.
By using the ACQUITY UPCC/Xevo G2-XS QTOF system combined with the UNIFI software, fifty-seven compounds of glycerides were identified and divided into four groups on the basis of their similar features via software-based group classification in the ESI+ mode. Moreover, thirty free fatty acids were identified in ESI−. In addition, QI omics analysis software found nine differential compounds between No. 190101 and No. 190105, and seven of these compounds were identified. Finally, the quantitative analysis of glyceryl trioleate (quality control component in the 2015 Edition of the Chinese Pharmacopoeia) and methodological verification were performed, and the results showed that the linearity, precision, reproducibility, recovery, and other parameters of the method were good. The established quantitative method determined that the glyceryl trioleate contents of the seven batches of samples ranged from 0.84% to 1.05%.
In summary, we identified additional glycerides and free fatty acids in coicis semen oils. Our results could supplement corresponding component research. Furthermore, nine differential components were found to be potential markers of quality for differentiating coicis semen with different origins. Finally, glyceryl trioleate was determined to evaluate its pros and cons. This approach might be useful for assessing the quality of TCM.
Data Availability Statement
All datasets presented in this study are included in the article/Supplementary Material.
Author Contributions
Conceptualization: XW and GC. Data curation: RZ, XX, and QS. Formal analysis: KW. Funding acquisition: GC. Investigation: RZ, XX, and KW. Writing—original draft: RZ and XX. Writing—review and editing: XW and GC. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Zhejiang Public Welfare Technology Application Research Project (2017C33175) and the National Natural Science Foundation of China (No. 81703707).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.549181/full#supplementary-material
References
Bai, C. Q., Zheng, J. X., Zhao, L., Chen, L. L., Xiong, H., McClements, D. J. (2018). Development of oral delivery systems with enhanced antioxidant and anticancer activity: Coix seed oil and -carotene co-loaded liposomes. J. Agr. Food Chem. 67, 406–414. doi: 10.1021/acs.jafc.8b04879
Chen, C., Zhang, Y. Y., Gao, Y. L., Xu, Q., Ju, X. R., Wang, L. F. (2016). Identification and anti-tumour activities of phenolic compounds isolated from defatted adlay (Coix lachryma-jobi L . var. ma-yuen Stapf) seed meal. J. Funct. Foods 26, 394–405. doi: 10.1016/j.jff.2016.08.016
Chen, Y. Y., Qu, D., Fu, R. P., Guo, M. F., Qin, Y., Guo, J., et al. (2018). A Tf-modified tripterine-loaded coix seed oil microemulsion enhances anti-cervical cancer treatment. Int. J. Nanomed. 13, 7275–7287. doi: 10.2147/IJN.S182475
Choi, Y. H., Choi, C. W., Hong, S. H., Park, S. K., Oh, J. S., Lee, D. H., et al. (2019). Coixlachryside B: a new benzoxazinoid glycoside from the roots of Coix lachryma-jobi var. ma-yuen (Gramineae). J. Asian Nat. Prod. Res. 21, 806–812. doi: 10.1080/10286020.2018.1497016
Dong, M. F., Si, W. S., Wang, W. M., Bai, B., Nie, D. X., Song, W. G., et al. (2016). Determination of type A trichothecenes in coix seed by magnetic solid-phase extraction based on magnetic multi-walled carbon nanotubes coupled with ultra-high performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 408, 6823–6831. doi: 10.1007/s00216-016-9809-0
Duan, G. Q. (2018). The Effects of Combination of Coix Seed Extract and Cisplatin on TAM and Expression of HIF-1α in Vivo in Lewis Lung Carcinoma. Iran J. Public Health 47, 838–843.
Guo, M. F., Qu, D., Qin, Y., Chen, Y. Y., Liu, Y. P., Huang, M. M., et al. (2019). Transferrin-Functionalized Microemulsions Coloaded with Coix Seed Oil and Tripterine Deeply Penetrate To Improve Cervical Cancer Therapy. Mol. Pharmaceut. 16, 4826–4835. doi: 10.1021/acs.molpharmaceut.9b00717
Han, X. C., Ji, X. M., Zhao, H. J., Zhang, Y. N., Liu, G. W., Wang, Y. F., et al. (2017). Mechanisms of coix seed compositions in the treatment of spleen deficiency and wet dampness zheng. Afr. J. Tradit. Complem. 14, 239–246. doi: 10.21010/ajtcam.v14i4.26
Hong, S. S., Choi, C. W., Choi, Y. H., Oh, J. S. (2016). Coixlachryside A: A new lignan glycoside from the roots of Coix lachryma-jobi L. var. ma-yuen Stapf. Phytochem. Lett. 17, 152–157. doi: 10.1016/j.phytol.2016.07.004
Hou, J. J., Cao, C. M., Xu, Y. W., Yao, S., Cai, L. Y., Long, H. L., et al. (2018a). Exploring lipid markers of the quality of coix seeds with different geographical origins using supercritical fluid chromatography mass spectrometry and chemometrics. Phytomedicine 45, 1–7. doi: 10.1016/j.phymed.2018.03.010
Hou, J. J., Guo, J. L., Cao, C. M., Yao, S., Long, H. L., Cai, L. Y., et al. (2018b). Green Quantification Strategy Combined with Chemometric Analysis for Triglycerides in Seeds Used in Traditional Chinese Medicine. Planta Med. 84, 457–464. doi: 10.1055/s-0044-100723
Huang, Q., Xu, M. Y., Zhang, H. L., He, D., Kong, Y. T., Chen, L., et al. (2019). Transcriptome and proteome analyses of the molecular mechanisms associated with coix seed nutritional quality in the process of breeding. Food Chem. 272, 549–558. doi: 10.1016/j.foodchem.2018.07.116
Huang, Y. L., Zhu, J. Y., Lin, X., Hong, Y. L., Feng, Y., Shen, L. (2019). Potential of Fatty Oils from Traditional Chinese Medicine in Cancer Therapy: A Review for Phytochemical, Pharmacological and Clinical Studies. Am. J. Chin. Med. 47, 727–750. doi: 10.1142/S0192415X19500381
Jang, J. W., Lim, D. W., Chang, J. U., Kim, J. E. (2018). The Combination of Ephedrae herba and Coicis semen in Gambihwan Attenuates Obesity and Metabolic Syndrome in High-Fat Diet-Induced Obese Mice. Evid. Based Compl. Alt. 2018:5614091. doi: 10.1155/2018/5614091
Kim, H. Y., Song, H. N., Davaatseren, M., Chang, H. J., Chun, H. S. (2018). Endoplasmic reticulum stress induced by an ethanol extract of Coicis semen in Chang liver cells. BMC Complem. Altern. M. 18, 100. doi: 10.1186/s12906-018-2175-z
Li, Y. L., Tian, X. D., Li, S. C., Chang, L. J., Sun, P., Lu, Y. B., et al. (2019). Total polysaccharides of adlay bran (Coix lachryma-jobi L.) improve TNF-β induced epithelial barrier dysfunction in Caco-2 cells via inhibition of the inflammatory response. Food Funct. 10, 2906–2913. doi: 10.1039/C9FO00590K
Lin, P. H., Shih, C. K., Yen, Y. T., Chiang, W. C., Hsia, S. M. (2019). Adlay (Coix lachryma-jobi L. var. ma-yuen Stapf.) Hull Extract and Active Compounds Inhibit Proliferation of Primary Human Leiomyoma Cells and Protect against Sexual Hormone-Induced Mice Smooth Muscle Hyperproliferation. Molecules 24:1556. doi: 10.3390/molecules24081556
Liu, X., Zhang, X., Rong, Y. Z., Wu, J. H., Yang, Y. J., Wang, Z. W. (2015). Rapid determination of fat, protein and amino acid content in coix seed using near-infrared spectroscopy technique. Food Anal. Method. 8, 334–342. doi: 10.1007/s12161-014-9897-4
Liu, H. Q., Li, L., Zou, J., Zhou, T., Wang, B. Y., Sun, H. H., et al. (2019). Coix seed oil ameliorates cancer cachexia by counteracting muscle loss and fat lipolysis. BMC Complem. Altern. M. 19, 267. doi: 10.1186/s12906-019-2684-4
Liu, Y. N., Tong, T., Zhang, R. R., Liu, L. M., Shi, M. L., Ma, Y. C., et al. (2019). Interdependent nitric oxide and hydrogen peroxide independently regulate the coix seed oil-induced triterpene acid accumulation in Ganoderma lingzhi. Mycologia 111, 529–540. doi: 10.1080/00275514.2019.1615816
Manosroi, A., Sainakham, M., Chankhampan, C., Abe, M., Manosroi, W., Manosroi, J. (2016a). Potent in vitro anti-proliferative, apoptotic and anti-oxidative activities of semi-purified Job’s tears (Coix lachryma-jobi Linn.) extracts from different preparation methods on 5 human cancer cell lines. J. Ethnopharmacol. 187, 281–292. doi: 10.1016/j.jep.2016.04.037
Manosroi, A., Sainakham, M., Chankhampan, C., Manosroi, W., Manosroi, J. (2016b). In vitro anti-cancer activities of Job’s tears (Coix lachryma-jobi Linn.) extracts on human colon adenocarcinoma. Saudi J. Biol. Sci. 23, 248–256. doi: 10.1016/j.sjbs.2015.03.008
Manosroi, J., Chankhampan, C., Kitdamrongtham, W., Manosroi, W., Manosroi, A. (2019). Potent in vitro Anti-mouth Cancer (KB) and Immunostimulating Activities of the Job’s Tears (Coix lachryma-jobi Linn.) Seed Semi-purified Extract Cocktails Containing Linoleic Acid. J. Oleo Sci. 68, 351–359. doi: 10.5650/jos.ess18255
Qian, Y. F., Yang, B., Xiong, Y., Gu, M. C. (2016). Coix seed emulsion synergistically enhances the antitumor activity of gemcitabine in pancreatic cancer through abrogation of NF-kB signaling. Oncol. Rep. 36, 1517–1525. doi: 10.3892/or.2016.4958
Qian, Y. F., Xiong, Y., Feng, D., Wu, Y. L., Zhang, X., Chen, L. P., et al. (2019). Coix Seed Extract Enhances the Anti-Pancreatic Cancer Efficacy of Gemcitabine through Regulating ABCB1- and ABCG2- Mediated Drug Efflux: A Bioluminescent Pharmacokinetic and Pharmacodynamic Study. Int. J. Mol. Sci. 20:5250. doi: 10.3390/ijms20215250
Qu, D., Sun, W. J., Liu, M. J., Liu, Y. P., Zhou, J., Chen, Y. (2016). Bitargeted microemulsions based on coix seed ingredients for enhanced hepatic tumor delivery and synergistic therapy. Int. J. Pharmaceut. 503, 90–101. doi: 10.1016/j.ijpharm.2016.03.001
Qu, D., Guo, M. F., Qin, Y., Wang, L. X., Zong, B., Chen, Y. Y., et al. (2017a). A multicomponent microemulsion using rational combination strategy improves lung cancer treatment through syn-ergistic effects and deep tumor penetration. Drug deliv. 24, 1179–1190. doi: 10.1080/10717544.2017.1365394
Qu, D., Liu, M. J., Huang, M. M., Wang, L. X., Chen, Y., Liu, C. Y., et al. (2017b). Octanoyl galactose ester-modified microemulsion system self-assembled by coix seed components to enhance tumor targeting and hepatoma therapy. Int. J. Nanomed. 12, 2045–2059. doi: 10.2147/IJN.S125293
Qu, D., Wang, L. X., Liu, M., Shen, S. Y., Li, T., Liu, Y. P., et al. (2017c). Oral Nanomedicine Based on Multicomponent Microemulsions for Drug-Resistant Breast Cancer Treatment. Biomacromolecules 18, 1268–1280. doi: 10.1021/acs.biomac.7b00011
Schwartzberg, L. S., Arena, F. P., Bienvenu, B. J., Kaplan, E. H., Camacho, L. H., Campos, L. T., et al. (2017). A Randomized, Open-Label, Safety and Exploratory Efficacy Study of Kanglaite Injection (KLTi) plus Gemcitabine versus Gemcitabine in Patients with Advanced Pancreatic Cancer. J. Cancer 8, 1872–1883. doi: 10.7150/jca.15407
Shan, Q. Y., Cao, G., Cai, H., Cong, X. D., Cai, B. C. (2012). Novel software-based method to classify structurally similar compounds combined with high performance liquid chromatography–quadrupole time of flight mass spectrometry to identify complex components of herbal medicines. J. Chromatogr. A. 1264, 13–21. doi: 10.1016/j.chroma.2012.09.045
Trinh, T. A., Park, S. C., Oh, J. H., Kim, C. E., Kang, K. S., Yoo, H. S., et al. (2017). Preventive Effect and Safety of a Follicle Stimulating Hormone Inhibitory Formulation Containing a Mixture of Coicis Semen and Artemisia capillaris for Precocious Puberty: A Preliminary Experimental Study Using Female Rats. Evid. Based Compl. Alt. 2017:2906014. doi: 10.1155/2017/2906014
Wang, L. F., Chen, C., Su, A. X., Zhang, Y. Y., Yuan, J., Ju, X. R. (2016). Structural characterization of phenolic compounds and antioxidant activity of the phenolic-rich fraction from defatted adlay (Coix lachryma-jobi L . var. ma-yuen Stapf) seed meal. Food Chem. 196, 509–517. doi: 10.1016/j.foodchem.2015.09.083
Xi, X. J., Zhu, Y. G., Tong, Y. P., Yang, X. L., Tang, N. N., Ma, S. M., et al. (2016). Assessment of the Genetic Diversity of Different Job’s Tears (Coix lacryma-jobi L.) Accessions and the Active Composition and Anticancer Effect of Its Seed Oil. PloS One 11, e153269. doi: 10.1371/journal.pone.0153269
Xu, M. Y., He, D., Teng, H., Chen, L., Song, H. B., Huang, Q. (2018). Physiological and proteomic analyses of coix seed aging during storage. Food Chem. 260, 82–89. doi: 10.1016/j.foodchem.2018.03.129
Yu, F., Gao, J., Zeng, Y., Liu, C. X. (2011). Effects of adlay seed oil on blood lipids and antioxidant capacity in hyperlipidemic rats. J. Sci. Food Agric. 91, 1843–1848. doi: 10.1002/jsfa.4393
Zhang, P. R., Meng, X. Y., Tang, X. H., Ren, L., Liang, J. (2018). The effect of a coix seed oil injection on cancer pain relief. Support. Care Cancer. 27, 461–465. doi: 10.1007/s00520-018-4313-z
Keywords: markers, coicis semen, triglyceride, qualitative and quantitative, MATLAB
Citation: Zhu R, Xu X, Shan Q, Wang K, Cao G and Wu X (2020) Determination of Differentiating Markers in Coicis Semen From Multi-Sources Based on Structural Similarity Classification Coupled With UPCC-Xevo G2-XS QTOF. Front. Pharmacol. 11:549181. doi: 10.3389/fphar.2020.549181
Received: 05 April 2020; Accepted: 18 August 2020;
Published: 16 October 2020.
Edited by:
Anthony Booker, University of Westminster, United KingdomReviewed by:
Jianxin Chan, Beijing University of Chinese Medicine, ChinaFrancisc Dulf, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, Romania
Copyright © 2020 Zhu, Xu, Shan, Wang, Cao and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Cao, Y2FvZ2FuZzMzQDE2My5jb20=; Xin Wu, d3Voc2luQHFxLmNvbQ==
†These authors have contributed equally to this work
 Ruyi Zhu†
Ruyi Zhu† Xiaofen Xu
Xiaofen Xu Qiyuan Shan
Qiyuan Shan Gang Cao
Gang Cao Xin Wu
Xin Wu