
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 22 January 2021
Sec. Drugs Outcomes Research and Policies
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.539545
Background: The efficacy and safety of immunosuppressive monotherapy agents were evaluated for immunoglobulin A nephropathy (IgAN) using a network meta-analysis approach.
Methods: Randomized controlled trials (RCTs) published prior to October 1, 2019, using immunosuppressive agents for treating IgAN, were systematically searched in PubMed, Embase, Cochrane Library, and Web of Science databases. Relative risks (RRs) or standard mean differences with 95% confidence intervals (CIs) were estimated using the random-effects model. The primary outcomes were clinical remission, end-stage renal disease (ESRD), and serious adverse events (SAEs). The secondary outcomes were urinary protein excretion and serum creatinine.
Results: Twenty-five RCTs with 2,005 participants were deemed eligible. Six medications were evaluated: corticosteroids, mycophenolate mofetil (MMF), tacrolimus (TAC), cyclosporine, leflunomide, and hydroxychloroquine (HCQ). Steroids (RR 1.50, 95% CI 1.17–1.93), MMF (RR 2.05, 95% CI 1.15–3.65), TAC (RR 3.67, 95% CI 1.06–12.63), and HCQ (RR 3.25, 95% CI 1.05–10.09) significantly improved clinical remission rates compared to supportive care alone. Only steroids reduced the risk of ESRD (RR 0.35, 95% CI 0.12–0.98); however, there were significantly more SAEs than in the control group (RR 2.90, 95% CI 1.37–6.13). No significantly different effects in serum creatinine levels were found among the therapies. MMF showed no significant improvement in remission when excluding studies with a follow-up of fewer than 2 years in the sensitivity analysis (RR 1.41, 95% CI 0.40–4.92). The effect of TAC in the decrease of proteinuria was reversed after discontinuing medication for 3 months; the long-term effects of HCQ could not be evaluated due to the short follow-up duration.
Conclusion: Corticosteroids might induce remission and increase renal survival in IgAN; however, adverse reactions should be taken into consideration. MMF, TAC, and HCQ might improve the remission of proteinuria when treating IgAN, but showed no superiority compared to steroids, and the long-term effects require further study.
Immunoglobulin A nephropathy (IgAN) is one of the most common glomerular diseases and a leading cause of end-stage renal disease (ESRD) worldwide (Rodrigues et al., 2017). Approximately 20–40% of patients progress to ESRD within 10–20 years after diagnosis (Magistroni et al., 2015). Currently, the inhibition of the renin-angiotensin system (RAS) for supportive care is the preferred treatment for IgAN and corticosteroids are recommended for patients with proteinuria greater than 1.0 g/24 h and an estimated glomerular filtration rate (eGFR) greater than 50 ml min−1·1.73 m2, despite the fact that standard therapies are prescribed according to the Kidney Disease Improving Global Outcomes (KDIGO) guidelines (Radhakrishnan and Cattran, 2012). However, in light of the KDIGO Controversies Conference in 2017, this recommendation may need to be revisited (Floege et al., 2019). More recently, steroids were shown to be potentially beneficial for clinical remission of IgAN, despite being associated with a significant increase in serious adverse events (SAEs) (Lv et al., 2017). Corticosteroid-based immunosuppressive treatments have also been used as a potential treatment for remission in IgAN; however, they are also limited by their high risks of adverse events (Rauen et al., 2015). Immunosuppressive monotherapy is an optional treatment for IgAN and includes calcineurin inhibitors and mycophenolate mofetil (MMF) (Zhang and Zhang, 2018). Previous pairwise meta-analyses indicated that calcineurin inhibitors and MMF could be effective in the treatment of IgAN (Peng et al., 2016; Du et al., 2017), although their independent effects are controversial and their relative effects among different immunosuppressive agents are unknown. As network meta-analysis (NMA) can compare the effects of all these drugs under a coherent framework, in addition to the probability of optimal treatment, we conducted an NMA to determine the effect of monotherapy for IgAN using different immunosuppressive agents and, if possible, predicted the best candidate for treatment.
This work was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for NMA (Hutton et al., 2015) (see Supplementary Table S1). The NMA protocol was registered in PROSPERO: CRD42019147935.
Eligible studies published prior to October 1, 2019, were searched through PubMed, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), and the Web of Science. Medical subject headings and all-fields searches consisted of three parts without language restrictions: immunosuppressive agent (cyclophosphamide [CYC], azathioprine [AZA], cyclosporine [CsA], tacrolimus [TAC], leflunomide (LEF), hydroxychloroquine [HCQ], MMF, and steroid), IgAN, and randomized controlled trial (RCT). The search strategies are shown in Supplementary Text 1. The related systematic reviews and meta-analyses on immunosuppressive agents for IgAN were also checked (Peng et al., 2016; Du et al., 2017; Qian et al., 2019; Zhang et al., 2019).
The inclusion criteria were as follows: 1) participants: patients with biopsy-proven IgAN; 2) different immunosuppressive monotherapy agents: CYC, AZA, MMF, CsA, TAC, LEF, HCQ, or steroids that were compared with each other or with non-immunosuppressive treatments. In addition, supportive therapies that were administered to both groups; 3) outcomes: the primary outcomes were clinical remission, including complete remission (CR), or partial remission (PR), which were provided in each original study, and the endpoint of the ESRD and SAEs, including death, serious infection, new diabetes mellitus, and other SAEs defined by the original studies, and the secondary outcomes included urinary protein excretion and serum creatinine; and 4) study design: RCTs.
The exclusion criteria included: 1) secondary IgAN; or 2) no data available for analysis.
Two investigators (S.-S. Han and T.-W. Yao) performed the study selection process and extracted the data independently and any disagreement was solved via discussion with a third reviewer (Y. Wang). The process of study selection is summarized in the PRISMA flowchart (Figure 1). The following data were extracted: first author, year of publication, location, baseline information for all groups (sample size, age, and sex), intervention details, duration of follow-up, loss to follow-up, and outcomes.
The risk of bias was assessed by the Cochrane Handbook (version 5.1.0) for each trial by two investigators independently (Y. Lu and M. Chen). This tool consists of seven items—the assessments of selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias, with each domain at three levels—low risk, high risk, and unclear risk (Higgins et al., 2011).
All statistical analyses were performed using the Stata software (version 14.0) and the network command (White, 2015) utilizing previously reported routines (Chaimani et al., 2013). Continuous data were compared using standardized mean differences (SMDs) and corresponding 95% confidence intervals (CIs) because they could be detected at different follow-up times. Relative risks (RRs) with 95% CIs were calculated for discrete data. Random-effects models of pairwise meta-analyses were performed for each outcome of direct contrast. The heterogeneity was estimated by a common heterogeneity variance (tau). Values of 0.1–0.5, 0.5–1.0, and >1.0 represented low, high, and extreme heterogeneity, respectively (Turner et al., 2012). An inconsistency test (based on the design-by-treatment interaction approach) was applied to evaluate the inconsistency of the entire network (Higgins et al., 2012). In the case in which there was no inconsistency, a random-effects NMA was performed to compare all the interventions for each predefined outcome in the frequentist framework. The surface under the cumulative ranking curve (SUCRA) value was used to rank the medications. A sensitivity analysis was conducted by excluding studies with follow-ups of fewer than 2 years. A subgroup analysis was performed by stratifying the groups according to the eGFR and histological lesions to clarify potential variables related to immunosuppressive therapy responsiveness and renal outcomes. The small-study effect was assessed by a comparison-adjusted funnel plot.
One thousand two hundred and fifty-one publications were retrieved after removing duplications. From a total of 29 publications, 25 RCTs were identified for meta-analyses (Lai et al., 1986; Lai et al., 1987; Julian and Barker, 1993; Pozzi et al., 1999; Shoji et al., 2000; Locatelli et al., 2001; Chen et al., 2002; Katafuchi et al., 2003; Lee et al., 2003; Maes et al., 2004; Pozzi et al., 2004; Frisch et al., 2005; Kim et al., 2005; Tang et al., 2005; Hogg et al., 2006; Lou et al., 2006; Koike et al., 2008; Lv et al., 2009; Manno et al., 2009; Tang et al., 2010; Kim et al., 2013; Cheng et al., 2015; Hogg et al., 2015; Wu et al., 2016; Fellstrom et al., 2017; Lv et al., 2017; Yu et al., 2017; Tang et al., 2018; Liu et al., 2019) (Figure 1). There were 2,005 participants (1,195 males and 810 females) enrolled in the 25 RCTs. In these studies, six immunosuppressive agents, including corticosteroids, MMF, TAC, CsA, LEF, and HCQ, were reported. A novel targeted-release formulation of the steroid (targeted-release formulation (TRF)-budesonide) was included; therefore, it was presented separately to differentiate it from conventional formulations of steroids. Supportive therapies were administrated to both groups and comparisons were performed between immunosuppressive drugs and controls (placebo, 11; supportive care, 12; dipyridamole, 1), except one that compared MMF with prednisone (Chen et al., 2002). The network graphs are shown in Figure 2. The median duration of each drug was as follows: steroids, 8.5 (minimum 4, maximum 24) months; MMF, 12 (minimum 6, maximum 36) months; TAC, 16 weeks; CsA, 12 weeks; LEF, 6 (minimum 6, maximum 24) months; and HCQ, six months. The median follow-up was as follows: steroids, 35 (minimum 12, maximum 120) months; MMF, 24 (minimum 18, maximum 72) months; TAC, five years; CsA, 24 weeks; LEF, 7 (minimum 6, maximum 24) months; and HCQ, six months. Characteristics of the selected RCTs are presented in Supplementary Table S2.
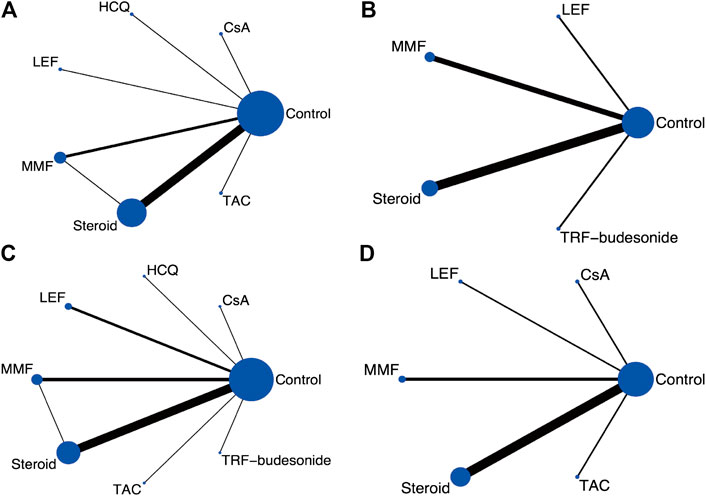
FIGURE 2. Network graphs for the predefined outcome (A) Clinical remission (B) ESRD (C) Serious adverse events (D) Serum creatinine. (CsA: Cyclosporine; ESRD: End-stage renal disease; HCQ: Hydroxychloroquine; LEF: Leflunomide; MMF: Mycophenolate mofetil; TRF: Targeted-release formulation; TAC: Tacrolimus).
Fifteen of the 25 (60%) trials described their procedures of random sequences generation appropriately and were considered to have low risk selection bias (Lai et al., 1987; Julian and Barker, 1993; Pozzi et al., 1999; Shoji et al., 2000; Frisch et al., 2005; Hogg et al., 2006; Lv et al., 2009; Manno et al., 2009; Kim et al., 2013; Cheng et al., 2015; Hogg et al., 2015; Wu et al., 2016; Fellstrom et al., 2017; Lv et al., 2017; Liu et al., 2019). Eleven (44%) trials used placebo-controlled blinding; thus, their performance bias risk was considered low (Lai et al., 1987; Maes et al., 2004; Frisch et al., 2005; Hogg et al., 2006; Kim et al., 2013; Cheng et al., 2015; Hogg et al., 2015; Wu et al., 2016; Fellstrom et al., 2017; Lv et al., 2017; Liu et al., 2019). Thirteen (52%) trials reported the processes for allocation of concealment (Lai et al., 1987; Julian and Barker, 1993; Pozzi et al., 1999; Frisch et al., 2005; Koike et al., 2008; Manno et al., 2009; Kim et al., 2013; Cheng et al., 2015; Hogg et al., 2015; Wu et al., 2016; Fellstrom et al., 2017; Lv et al., 2017; Liu et al., 2019). Nine (36%) studies reported the assessment of a blinded outcome, and their detection bias was classified as low risk (Shoji et al., 2000; Frisch et al., 2005; Hogg et al., 2006; Cheng et al., 2015; Hogg et al., 2015; Wu et al., 2016; Fellstrom et al., 2017; Lv et al., 2017; Liu et al., 2019). All (100%) studies possessed complete data. Six (24%) studies had a low reporting bias because of early registration (Kim et al., 2013; Hogg et al., 2015; Wu et al., 2016; Fellstrom et al., 2017; Lv et al., 2017; Liu et al., 2019). Three (12%) trials were terminated early (Frisch et al., 2005; Hogg et al., 2015; Lv et al., 2017), and one (4%) was a preliminary analysis (Julian and Barker, 1993); thus, other biases were classified as high risk (Figure 3).
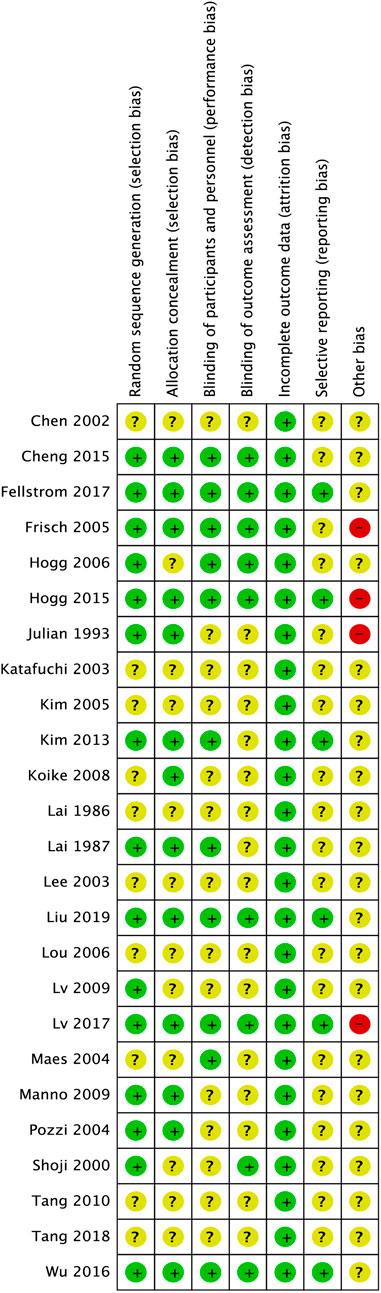
FIGURE 3. Risk of bias assessment for included studies. The green circles with “+” indicate a low risk of bias, yellow circles with “?” indicate an unclear risk of bias, and red circles with “-” indicate a high risk of bias.
Seventeen studies reported CR or PR. The NMA results indicated that steroids (RR 1.50, 95% CI 1.17–1.93), MMF (RR 2.05, 95% CI 1.15–3.65), TAC (RR 3.67, 95% CI 1.06–12.63), and HCQ (RR 3.25, 95% CI 1.05–10.09) significantly improved the clinical remission rate in patients with IgAN compared to the control group, as did the results of the pairwise meta-analyses. However, no significant differences were found in the clinical remission rates among these immunosuppressive agents for patients with IgAN (Table 1).
Ten studies, involving LEF, MMF, steroids, and TRF-budesonide, reported the hard endpoint of ESRD. No significant differences in the incidences of ESRD were determined among these groups, except the conventional steroids group (RR 0.35, 95% CI 0.12–0.98), which showed better renal survival than the control group (Table 2).
SAEs were reported in 22 studies for all included medications for IgAN. The results showed that patients with IgAN receiving steroids had higher risks of SAEs than the control group (RR 2.90, 95% CI 1.37–6.13) and similar results were observed in the pairwise meta-analysis (RR 4.27, 95% CI 1.79–10.18). All the remaining immunosuppressive agents studied showed no significant difference when compared to the controls or each other (Table 3).
Nineteen RCTs reported urinary protein excretion. The network indicated low heterogeneity (tau = 0.43) but significant inconsistency (p = 0.04); therefore, the results were analyzed only using a pairwise meta-analysis. The patients with IgAN receiving steroids (SMD: −0.69, 95% CI: −0.98–-0.41), LEF (SMD: −0.58, 95% CI: −0.89–-0.27), and HCQ (SMD: −1.09, 95% CI: −1.64–-0.55) had significantly lower levels of urinary protein excretion than the control group. Patients in the MMF groups showed lower urinary protein excretion than those in the steroid treatment groups (SMD: −0.77, 95% CI: −1.28–0.25) (see Supplementary Table S3). Eleven RCTs, including five immunosuppressive agents (LEF, CsA, TAC, MMF, and steroids), reported serum creatinine levels. The serum creatinine levels among the groups showed no significant differences, either in network or pairwise meta-analyses (see Supplementary Table S4).
As follow-up time might influence the main outcomes, we performed a sensitivity analysis to exclude studies with less than 2-years follow-ups. The sensitivity analysis for determining clinical remission was conducted among MMF, steroids, and the control group. Steroids improved clinical remission compared to non-immunosuppressive therapy (RR 1.47, 95% CI 1.10–1.96); however, MMF showed no significant improvement on remission in the sensitivity analysis compared to controls (RR 1.41, 95% CI 0.40–4.92). The results of the ESRD were stable in this sensitivity analysis among LEF, MMF, steroids, and the control group (see Supplementary Table S5).
Small study effects might exist according to the comparison-adjusted funnel plot (Figure 4); therefore, we next performed a sensitivity analysis to compare the main outcomes after omitting studies with less than 100 participants and the same results were documented (see Supplementary Table S6).
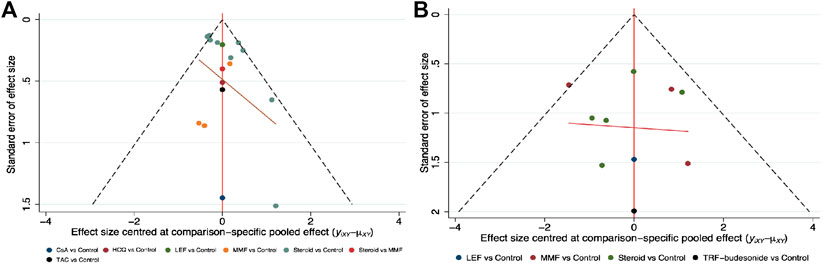
FIGURE 4. Comparison-adjusted funnel plots for clinical remission and end-stage renal disease (A) Clinical remission (B) ESRD. (CsA: Cyclosporine; ESRD: End-stage renal disease; HCQ: Hydroxychloroquine; LEF: Leflunomide; MMF: Mycophenolate mofetil; TRF: Targeted-release formulation; TAC: Tacrolimus).
Considering that baseline eGFR and histological lesions might be associated with the response to immunosuppressive therapy and clinical outcomes (Nagaraju et al., 2017; Trimarchi et al., 2017), we attempted to explore the correlations by stratifying according to eGFR ≥50 ml min−1·1.73 m2 or <50 ml min−1·1.73 m2 and the Oxford classification (Trimarchi et al., 2017). Patients with eGFR <50 ml min−1·1.73 m2 showed a significantly higher risk of eGFR loss, ESRD, or death owing to renal failure than those with eGFR ≥50 ml min−1·1.73 m2 in the steroids arm (7/52 vs. 1/83, RR = 11.17, 95% CI 1.41–88.23, p = 0.02). Although all the included studies reported homogeneity in histological baseline, only a few studies focused on the correlations between histopathology and outcomes/therapy responsiveness. Only one among the four trials using the Oxford MEST/-C scores [MEST-C, mesangial hypercellularity (score <0.5 M0, >0.5 M1), endocapillary hypercellularity (absent E0, present E1), segmental glomerular sclerosis (absent S0, present S1), tubular atrophy/interstitial fibrosis (≤25% T0, 26–50% T1, >50% T2), and cellular/fibrocellular crescents (absent C0, ≥1 glomeruli, C1, >25% glomeruli C2)] reported the association between histological lesions and outcomes. In the steroid group, patients with E1 showed better composite outcome defined as 40% eGFR decrease, ESRD, or death than those with E0, but the differences were not significant (1/43 vs. 7/93, RR 0.31, 95% CI 0.04–2.43, p = 0.26). Furthermore, there were no differences between the steroid and placebo arms in the E1 or E0 scores (Lv et al., 2017). Pozzi et al. reported that the S, T, or C score had no effect on eGFR loss (Pozzi et al., 1999). However, Katafuchi et al. reported that higher E score in the steroid group was associated poor CR, and there were no differences in the M, S, T, and C scores the groups (Katafuchi et al., 2003). It should be noted that the criterion in the above two studies was not the Oxford classification.
The ranking of treatments among MMF, steroids, and controls indicated that MMF might be the best treatment to induce remission (SUCRA 91.7%), followed by steroids and controls (SUCRA 57.9 and 0.4%, respectively). Steroids were ranked as the best intervention to prevent ESRD (SUCRA 91.4%) but the worst treatment when SAEs were considered (SUCRA 3.7%, MMF 76.2%) (Table 4).
IgAN is a common glomerulonephritis worldwide and the cause of ESRD (Moriyama, 2019). Several risk factors have correlations with the renal outcome of IgAN, of which, proteinuria has been reported as the most valuable marker for the prognosis of the treatment response, as well as deteriorated renal function (Barbour and Reich, 2018). Steroids and immunosuppressive regimens are potential optimal therapies to reduce proteinuria and improve renal survival; however, adverse events, especially serious infections, are significant (Barratt and Tang, 2018). Another choice for IgAN might be the use of immunosuppressive monotherapy agents; nevertheless, the efficacy and adverse reactions have not been determined.
This NMA was an effort to compare the direct and indirect effects of single therapy with different immunosuppressive agents, besides corticosteroids, with and without supportive care, in treating patients with IgAN. The study included 25 RCTs with 2,005 subjects, involving corticosteroids, MMF, TAC, CsA, LEF, and HCQ. The results indicated that steroids, MMF, TAC, and HCQ might improve clinical remission rates for IgAN; however, the beneficial effect of MMF for remission was not significant in studies with follow-up timepoints of more than 2 years, suggesting that the long-term efficacy of MMF for IgAN might be poor. Although TAC showed a beneficial effect in inducing remission, the 5-years follow-up of the same participants presented negative results (Yu et al., 2017). The follow-up times of CsA and HCQ for IgAN in the included studies were less than 1 year; thus, the long-term effect is unknown. Only steroids decreased the risk of ESRD but there were significantly more SAEs. All immunosuppressants exhibited no superiority compared to glucocorticoids, whether in terms of clinical efficacy or adverse reactions.
The results of this NMA were consistent with the recent TESTING study comparing oral methylprednisolone with placebo, which showed a higher rate of remission in proteinuria and lower occurrence of ESRD in the methylprednisolone group than in the placebo group (48.2 vs. 21.8%; 2.9 vs. 7.9%) but was discontinued because of excess SAEs (14.7 vs. 3.2%) (Lv et al., 2017). Thus, the safety profiles of steroid regimens in IgAN should be considered carefully.
The use of MMF in IgAN is still controversial—this study suggested higher clinical remission in MMF monotherapy groups but no beneficial effect when the follow-up time was more than 2 years in clinical remission, ESRD, or serum creatinine level. These results were consistent with Hogg et al. (Kim et al., 2013), which was terminated early because of the lack of benefit (PR, 14 vs. 9%). Conversely, a 6-years study by Tang et al. (Tang et al., 2010) showed that patients receiving MMF have better renal survival than those receiving placebo (90 vs. 55%). These paradoxical results might be due to different follow-up times or races, as the trials were conducted in North America and Asia, respectively. Notably, the adverse reactions of MMF seem to be tolerable. The results also provided a possibility for combined therapy of MMF and low-dose glucocorticoid to reach clinical remission with fewer adverse reactions. A recent trial compared 1.5 g/d of MMF with 0.4–0.6 mg/kg·d of prednisolone against 0.8–1.0 mg/kg·d of prednisolone alone in treating IgAN (Hou et al., 2017). Although the CR rates showed no significant differences, there were fewer steroid-associated adverse events in the MMF group than the prednisone group; however, the follow-up time of this study was only 12 months. Further studies are required to confirm the long-term effects of MMF for IgAN.
We showed that TAC could be an effective treatment to induce remission in patients with IgAN within 16 weeks; however, the sample size was limited (overall 40 subjects) and the 5-years follow-up of the same cohorts showed that the anti-proteinuria effect was promptly reversed 3 months after discontinuing the drug (Yu et al., 2017). HCQ has been little studied in IgAN—a recent RCT compared the effect of a 6 months prescription of HCQ with placebo in patients with IgAN (Liu et al., 2019) and suggested that HCQ effectively reduces proteinuria and increases PR in proteinuria. In addition, HCQ was well tolerated and no SAEs were reported. As the study was an early-phase trial, the long-term renoprotective efficacy and safety still require confirmation. A recent systematic review assessed the effect of LEF in treating IgAN (Yi et al., 2019) and showed significantly lower urine protein and serum creatinine in patients treated with LEF and corticosteroids or angiotensin-converting enzyme inhibitor (ACEI) than in patients treated with corticosteroids or ACEI alone. Our results indicate that LEF monotherapy had no superiority in achieving remission of proteinuria or renal survival when compared with supportive care alone, although the direct comparison suggested that LEF might have lower proteinuria-causing activity.
Our results showed that patients with IgAN and eGFR <50 ml min−1·1.73 m2 are at a higher risk of eGFR loss, ESRD, or death owing to renal failure than those with eGFR ≥50 ml min−1·1.73 m2 when treated with steroids. This indicated that patients with IgAN and eGFR ≥50 ml min−1·1.73 m2 show a better treatment effect in preventing disease progression than those with lower eGFR; the results are consistent with those of Nagaraju et al. (Nagaraju et al., 2017). The Oxford classification of IgAN has been widely adopted in clinical practice, and it was first published in 2009 and updated in 2016 using the MEST-C scores (Mubarak, 2018). Several validation studies have reported varying results regarding the predictive value of the Oxford classification in patients with IgAN; furthermore, the correlation between MEST-C scores and immunosuppressive therapy response has not been determined (Moriyama et al., 2020). An exploratory analysis of MEST-C scores and renal outcomes in the STOP-IgAN trial showed that ESRD occurred more frequently in patients with higher T scores when additional immunosuppressants were administrated. However, the T scores did not correlate with CR or eGFR loss rate, and high M scores indicated the trend of poor CR and renal survival with no statistical significance. The E, S, and C scores were not associated with any clinical outcomes in the group administered immunosuppressants (Schimpf et al., 2018). A validation study in Japan demonstrated that corticosteroids/immunosuppressants improved renal prognosis, based on the E1, S1, and C1 scores (Moriyama et al., 2020). However, a meta-analysis based on cohort study and retrospective study showed that the M1, S1, and T1/2 scores were strongly associated with a poor response to steroid therapy (Yang et al., 2017) and progression to kidney failure (Lv et al., 2013). Our results indicated that patients with higher E scores showed better renal outcomes than those with lower E scores, but the difference was not significant. Therefore, these contradictory results could not reveal the correlation between the Oxford classification and glucocorticoid responsiveness, necessitating further research.
Several limitations of this study should be considered. First, the quality of the included trials varied, leading to significant heterogeneity. Second, the lack of reporting of main outcomes in many trials was a potential limitation. Third, some contributing studies had small sample sizes, such as the results of the funnel plot, which might have resulted in more uncertainty and less precision of the findings. Finally, the correlations between pathological lesions or other baseline characteristics and renal outcomes or immunosuppressive therapy responsiveness were not clear because of insufficient data.
In conclusion, steroids might be an effective intervention strategy for IgAN to induce remission and increase renal survival; however, the adverse reactions cannot be ignored. Calcineurin inhibitors, LEF, HCQ, and MMF might improve remission of proteinuria in treating IgAN but they showed no superiority compared to steroids and the long-term effects, in particular, still require further study.
SS and TW conducted the literature retrieval, data extraction, data analyses, and manuscript writing; YL and MC assessed the risk of bias; YQ and YW designed this study, participated in the whole process. All authors approved the final manuscript.
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81403361, 81774106), and three-year projects for TCM development in Shanghai (Grant Nos. ZY (2018-2020)-RCPY-3006, ZY (2018-2020)-FWTX-4027) and project supported by Shanghai Committee of Science and Technology, China (Grant Nos.20Y21902100).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The initial version of this manuscript has been released as a pre-print at ResearchSquare, (S.-S. Han et al.) (Han et al., 2020).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.539545/full#supplementary-material.
AZA, Azathioprine; ACEI, Angiotensin-converting enzyme inhibitor; CENTRAL, Cochrane Central Register of Controlled Trials; CYC, Cyclophosphamide; CsA, Cyclosporine; CR, Complete remission; CI, Confidence interval; eGFR, estimated glomerular filtration rate; ESRD, End-stage renal disease; HCQ, Hydroxychloroquine; IgAN, Immunoglobulin A nephropathy; KDIGO, Kidney Disease Improving Global Outcomes; LEF, Leflunomide; MMF, Mycophenolate mofetil; NMA, Network meta-analysis; PR, Partial remission; RAS, Renin-angiotensin system; RCT, Randomised controlled trials; RR, Relative risk; SMD, Standard mean differences; SUCRA, Surface under the cumulative ranking curve; SAE, Serious adverse event; TRF, Targeted-release formulation; TESTING, Therapeutic Evaluation of Steroids in IgA Nephropathy Global study; TAC, Tacrolimus.
Barbour, S., and Reich, H. (2018). An update on predicting renal progression in IgA nephropathy. Curr. Opin. Nephrol. Hypertens. 27, 214–220. doi:10.1097/MNH.0000000000000405
Barratt, J., and Tang, S. C. W. (2018). Treatment of IgA Nephropathy: evolution over half a century. Semin. Nephrol. 38, 531–540. doi:10.1016/j.semnephrol.2018.05.023
Chaimani, A., Higgins, J. P., Mavridis, D., Spyridonos, P., and Salanti, G. (2013). Graphical tools for network meta-analysis in STATA. Plos One 8, e76654. doi:10.1371/journal.pone.0076654
Chen, X., Chen, P., Cai, G., Wu, J., Cui, Y., Zhang, Y., et al. (2002). A randomized control trial of mycophenolate mofeil treatment in severe IgA nephropathy. Zhonghua Yixue Zazhi 82, 796–801.
Cheng, G., Liu, D., Margetts, P., Liu, L., Zhao, Z., Liu, Z., et al. (2015). Valsartan combined with clopidogrel and/or leflunomide for the treatment of progressive immunoglobulin a nephropathy. Nephrology 20, 77–84. doi:10.1111/nep.12359
Du, B., Jia, Y., Zhou, W., Min, X., Miao, L., and Cui, W. (2017). Efficacy and safety of mycophenolate mofetil in patients with IgA nephropathy: an update meta-analysis. BMC Nephrol. 18, 245. doi:10.1186/s12882-017-0647-x
Fellström, B. C., Barratt, J., Cook, H., Coppo, R., Feehally, J., de Fijter, J. W., et al. (2017). Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): a double-blind, randomised, placebo-controlled phase 2b trial. Lancet 389, 2117–2127. doi:10.1016/S0140-6736(17)30550-0
Floege, J., Barbour, S. J., Cattran, D. C., Hogan, J. J., Nachman, P. H., Tang, S. C. W., et al. (2019). Management and treatment of glomerular diseases (part 1): conclusions from a kidney disease: improving global outcomes (KDIGO) Controversies conference. Kidney Int. 95, 268–280. doi:10.1016/j.kint.2018.10.018
Frisch, G., Lin, J., Rosenstock, J., Markowitz, G., D'Agati, V., Radhakrishnan, J., et al. (2005). Mycophenolate mofetil (MMF) vs placebo in patients with moderately advanced IgA nephropathy: a double-blind randomized controlled trial. Nephrol. Dial. Transplant. 20, 2139–2145. doi:10.1093/ndt/gfh974
Han, S. S., Yao, T. W., Lu, Y., Chen, M., Xu, Y. Q., and Wang, Y. (2020). Efficacy and safety of immunosuppressive agent monotherapy for IgA nephropathy: a network meta-analysis. ResearchSquare [Preprint]Available at: https://www.researchsquare.com/article/rs-10870/v1 (Accessed January 07 2020).
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Higgins, J. P., Jackson, D., Barrett, J. K., Lu, G., Ades, A. E., and White, I. R. (2012). Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res. Synth. Methods 3, 98–110. doi:10.1002/jrsm.1044
Hogg, R. J., Bay, R. C., Jennette, J. C., Sibley, R., Kumar, S., Fervenza, F. C., et al. (2015). Randomized controlled trial of mycophenolate mofetil in children, adolescents, and adults with IgA nephropathy. Am. J. Kidney Dis. 66, 783–791. doi:10.1053/j.ajkd.2015.06.013
Hogg, R. J., Lee, J., Nardelli, N., Julian, B. A., Cattran, D., Waldo, B., et al. (2006). Clinical trial to evaluate omega-3 fatty acids and alternate day prednisone in patients with IgA nephropathy: report from the Southwest Pediatric Nephrology study group. Clin. J. Am. Soc. Nephrol. 1, 467–474. doi:10.2215/CJN.01020905
Hou, J. H., Le, W. B., Chen, N., Wang, W. M., Liu, Z. S., Liu, D., et al. (2017). Mycophenolate mofetil combined with prednisone versus full-dose prednisone in IgA nephropathy with active proliferative lesions: a randomized controlled trial. Am. J. Kidney Dis. 69, 788–795. doi:10.1053/j.ajkd.2016.11.027
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 162, 777–784. doi:10.7326/M14-2385
Julian, B. A., and Barker, C. (1993). Alternate-day prednisone therapy in IgA nephropathy. Preliminary analysis of a prospective, randomized, controlled trial. Contrib. Nephrol. 104, 198–206.
Katafuchi, R., Ikeda, K., Mizumasa, T., Tanaka, H., Ando, T., Yanase, T., et al. (2003). Controlled, prospective trial of steroid treatment in IgA nephropathy: a limitation of low-dose prednisolone therapy. Am. J. Kidney Dis. 41, 972–983.
Kim, Y. C., Chin, H. J., Koo, H. S., and Kim, S. (2013). Tacrolimus decreases albuminuria in patients with IgA nephropathy and normal blood pressure: a double-blind randomized controlled trial of efficacy of tacrolimus on IgA nephropathy. Plos One 8, e71545. doi:10.1371/journal.pone.0071545
Kim, Y. K., Lee, S. H., Lee, T. W., Kim, M. J., Yang, M. H., and Ihm, C. G. (2005). Renoprotective effect of the combined use of steroid and angiotensin II receptor blocker in IgA Nephropathy. Korean J. Nutr. 24, 71–79.
Koike, M., Takei, T., Uchida, K., Honda, K., Moriyama, T., Horita, S., et al. (2008). Clinical assessment of low-dose steroid therapy for patients with IgA nephropathy: a prospective study in a single center. Clin. Exp. Nephrol. 12, 250–255. doi:10.1007/s10157-008-0036-7
Lai, K. N., Lai, F. M., Ho, C. P., and Chan, K. W. (1986). Corticosteroid therapy in IgA nephropathy with nephrotic syndrome: a long-term controlled trial. Clin. Nephrol. 26, 174–180.
Lai, K. N., Lai, F. M., Li, P. K., and Vallance-Owen, J. (1987). Cyclosporin treatment of IgA nephropathy: a short term controlled trial. Br. Med. J. 295, 1165–1168. doi:10.1136/bmj.295.6607.1165
Lee, S. H., Shim, J. J., Lee, S. H., Lee, T. W., Kim, M. J., and Yang, M. H. (2003). Effect of combined treatment of steroid and angiotensin II receptor blocker (ARB) in proteinuric IgA nephropathy. Korean J. Nutr. 22, 539–545.
Liu, L. J., Yang, Y. Z., Shi, S. F., Bao, Y. F., Yang, C., Zhu, S. N., et al. (2019). Effects of hydroxychloroquine on proteinuria in IgA nephropathy: a randomized controlled trial. Am. J. Kidney Dis. 74, 15–22. doi:10.1053/j.ajkd.2019.01.026
Locatelli, F., Pozzi, C., Del Vecchio, L., Bolasco, P. G., Fogazzi, G. B., Andrulli, S., et al. (2001). Role of proteinuria reduction in the progression of IgA nephropathy. Ren. Fail. 23, 495–505. doi:10.1081/jdi-100104732
Lou, T., Wang, C., Chen, Z., Shi, C., Tang, H., Liu, X., et al. (2006). Randomised controlled trial of leflunomide in the treatment of immunoglobulin A nephropathy. Nephrology 11, 113–116. doi:10.1111/j.1440-1797.2006.00547.x
Lv, J., Shi, S., Xu, D., Zhang, H., Troyanov, S., Cattran, D. C., et al. (2013). Evaluation of the Oxford Classification of IgA nephropathy: a systematic review and meta-analysis. Am. J. Kidney Dis. 62, 891–899. doi:10.1053/j.ajkd.2013.04.021
Lv, J., Zhang, H., Chen, Y., Li, G., Jiang, L., Singh, A. K., et al. (2009). Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: a randomized controlled trial. Am. J. Kidney Dis. 53, 26–32. doi:10.1053/j.ajkd.2008.07.029
Lv, J., Zhang, H., Wong, M. G., Jardine, M. J., Hladunewich, M., Jha, V., et al. (2017). Effect of oral methylprednisolone on clinical outcomes in patients with IgA Nephropathy: the testing randomized clinical trial. J. Am. Med. Assoc. 318, 432–442. doi:10.1001/jama.2017.9362
Maes, B. D., Oyen, R., Claes, K., Evenepoel, P., Kuypers, D., Vanwalleghem, J., et al. (2004). Mycophenolate mofetil in IgA nephropathy: results of a 3-year prospective placebo-controlled randomized study. Kidney Int. 65, 1842–1849. doi:10.1111/j.1523-1755.2004.00588.x
Magistroni, R., D'Agati, V. D., Appel, G. B., and Kiryluk, K. (2015). New developments in the genetics, pathogenesis, and therapy of IgA nephropathy. Kidney Int. 88, 974–989. doi:10.1038/ki.2015.252
Manno, C., Torres, D. D., Rossini, M., Pesce, F., and Schena, F. P. (2009). Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol. Dial. Transplant. 24, 3694–3701. doi:10.1093/ndt/gfp356
Moriyama, T. (2019). Clinical and histological features and therapeutic strategies for IgA nephropathy. Clin. Exp. Nephrol. 23, 1089–1099. doi:10.1007/s10157-019-01735-4
Moriyama, T., Karasawa, K., Miyabe, Y., Akiyama, K., Ogura, S., Takabe, T., et al. (2020). Validation of the revised Oxford classification for IgA nephropathy considering treatment with corticosteroids/immunosuppressors. Sci. Rep. 10, 11151. doi:10.1038/s41598-020-68087-y
Mubarak, M. (2018). Updated Oxford classification of IgA nephropathy: expanding scope of the schema. J. Ren. Inj. Prev. 7, 53–55.
Nagaraju, S. P., Laxminarayana, S. L. K., Mareddy, A. S., Rao, S. P., Kaza, S., Shenoy, S., et al. (2017). Role of corticosteroid therapy in IgA nephropathy; where do we stand? J. Nephropathol 6, 368–373. 10.15171/jnp.2017.61
Peng, W., Tang, Y., Jiang, Z., Li, Z., Mi, X., and Qin, W. (2016). The effect of calcineurin inhibitors in the treatment of IgA nephropathy: a systematic review and meta-analysis (PRISMA). Medicine (Baltim.) 95, e4731. doi:10.1097/MD.0000000000004731
Pozzi, C., Andrulli, S., Del Vecchio, L., Melis, P., Fogazzi, G. B., Altieri, P., et al. (2004). Corticosteroid effectiveness in IgA nephropathy: long-term results of a randomized, controlled trial. J. Am. Soc. Nephrol. 15, 157–163. doi:10.1097/01.asn.0000103869.08096.4f
Pozzi, C., Bolasco, P. G., Fogazzi, G. B., Andrulli, S., Altieri, P., Ponticelli, C., et al. (1999). Corticosteroids in IgA nephropathy: a randomised controlled trial. Lancet 353, 883–887. doi:10.1016/s0140-6736(98)03563-6
Qian, G., Zhang, X., Xu, W., Zou, H., and Li, Y. (2019). Efficacy and safety of glucocorticoids for patients with IgA nephropathy: a meta-analysis. Int. Urol. Nephrol. 51, 859–868. doi:10.1007/s11255-019-02094-5
Radhakrishnan, J., and Cattran, D. C. (2012). The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines—application to the individual patient. Kidney Int. 82, 840–856. doi:10.1038/ki.2012.280
Rauen, T., Eitner, F., Fitzner, C., Sommerer, C., Zeier, M., Otte, B., et al. (2015). Intensive supportive care plus immunosuppression in IgA Nephropathy. N. Engl. J. Med. 373, 2225–2236. doi:10.1056/NEJMoa1415463
Rodrigues, J. C., Haas, M., and Reich, H. N. (2017). IgA nephropathy. Clin. J. Am. Soc. Nephrol. 12, 677–686. doi:10.2215/CJN.07420716
Schimpf, J. I., Klein, T., Fitzner, C., Eitner, F., Porubsky, S., Hilgers, R.-D., et al. (2018). Renal outcomes of STOP-IgAN trial patients in relation to baseline histology (MEST-C scores). BMC Nephrol. 19, 328. doi:10.1186/s12882-018-1128-6
Shoji, T., Nakanishi, I., Suzuki, A., Hayashi, T., Togawa, M., Okada, N., et al. (2000). Early treatment with corticosteroids ameliorates proteinuria, proliferative lesions, and mesangial phenotypic modulation in adult diffuse proliferative IgA nephropathy. Am. J. Kidney Dis. 35, 194–201. doi:10.1016/s0272-6386(00)70326-x
Tang, S., Leung, J. C., Chan, L. Y., Lui, Y. H., Tang, C. S., Kan, C. H., et al. (2005). Mycophenolate mofetil alleviates persistent proteinuria in IgA nephropathy. Kidney Int. 68, 802–812. doi:10.1111/j.1523-1755.2005.00460.x
Tang, S. C., Tang, A. W., Wong, S. S., Leung, J. C., Ho, Y. W., and Lai, K. N. (2010). Long-term study of mycophenolate mofetil treatment in IgA nephropathy. Kidney Int. 77, 543–549. doi:10.1038/ki.2009.499
Tang, Y., He, H., Sun, W., Hu, P., Chen, X., and Xu, X. (2018). Corticosteroid therapy in IgA nephropathy with minimal proteinuria and high renal pathological score: a single‑center cohort study. Mol. Med. Rep. 18, 4103–4112. doi:10.3892/mmr.2018.9413
Trimarchi, H., Barratt, J., Cattran, D. C., Cook, H. T., Coppo, R., Haas, M., et al. (2017). Oxford classification of IgA nephropathy 2016: an update from the IgA nephropathy classification working group. Kidney Int. 91, 1014–1021. doi:10.1016/j.kint.2017.02.003
Turner, R. M., Davey, J., Clarke, M. J., Thompson, S. G., and Higgins, J. P. (2012). Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int. J. Epidemiol. 41, 818–827. doi:10.1093/ije/dys041
Wu, J., Duan, S. W., Sun, X. F., Li, W. G., Wang, Y. P., Liu, W. H., et al. (2016). Efficacy of leflunomide, telmisartan, and clopidogrel for immunoglobulin A nephropathy: a randomized controlled trial. Chin. Med. J. 129, 1894–1903. doi:10.4103/0366-6999.187848
Yang, P., Chen, X., Zeng, L., Hao, H., and Xu, G. (2017). The response of the Oxford classification to steroid in IgA nephropathy: a systematic review and meta-analysis. Oncotarget 8, 59748–59756. doi:10.18632/oncotarget.19574
Yi, J., He, Z., Xu, S., and Feng, S. (2019). Efficacy and safety of leflunomide in IgA nephropathy: a systematic review and meta-analysis. Int. Urol. Nephrol. 51, 1987–1998. doi:10.1007/s11255-019-02255-6
Yu, M. Y., Kim, Y. C., Koo, H. S., and Chin, H. J. (2017). Short-term anti-proteinuric effect of tacrolimus is not related to preservation of the glomerular filtration rate in IgA nephropathy: a 5-year follow-up study. Plos One 12, e0188375. doi:10.1371/journal.pone.0188375
Zhang, Y. M., and Zhang, H. (2018). Update on treatment of immunoglobulin A nephropathy. Nephrology 23 (Suppl. 4), 62–67. doi:10.1111/nep.13453
Keywords: immunosuppressive therapy, IgA nephropathy, network meta-analysis, systematic review, renoprotective
Citation: Han S, Yao T, Lu Y, Chen M, Xu Y and Wang Y (2021) Efficacy and Safety of Immunosuppressive Monotherapy Agents for IgA Nephropathy: A Network Meta-Analysis. Front. Pharmacol. 11:539545. doi: 10.3389/fphar.2020.539545
Received: 05 June 2020; Accepted: 26 November 2020;
Published: 22 January 2021.
Edited by:
Jean-Marie Boeynaems, Université libre de Bruxelles, BelgiumReviewed by:
Hamid Nasri, Isfahan University of Medical Sciences, IranCopyright © 2021 Han, Yao, Lu, Chen, Xu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Wang, ZHJ3YW5neWkwMTEwQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.