- 1Laboratory of Environmental Medicine and Developmental Toxicology, Shantou University Medical College, Shantou, China
- 2Pharmacy Department, Cancer Hospital of Shantou University Medical College, Shantou, China
- 3Digestive Medical Oncology, Cancer Hospital of Shantou University Medical College, Shantou, China
- 4Department of Clinical Laboratory Medicine, Cancer Hospital of Shantou University Medical College, Shantou, China
- 5Pharmacy Intravenous Admixture Service, Cancer Hospital of Shantou University Medical College, Shantou, China
Previous studies only focused on different adverse reactions caused by various platinum drugs, but not on common immunotoxicity caused by the accumulation of elemental platinum. Here, we determined the serum platinum concentrations of cancer patients after a metabolism period of platinum drug chemotherapy, in addition to hematological indices and subsequent immune-related adverse reactions, then analyzed the correlations between platinum accumulation, immune cell levels, and immune-toxicity. We chose the day before the next round of chemotherapy as the specified time point for blood sampling. Samples were collected at five time points, separately in oxaliplatin and cisplatin groups. The median serum platinum concentrations in all patients was 294.8 (205.6, 440.3) μg/L, and was approximately two-fold greater in the cisplatin group than in the oxaliplatin group (429.3 vs. 211.7 μg/L). The platinum level of both groups peaked at the third time point, with the average of females being higher than males (383.9 vs. 266.5 μg/L), and was positively correlated with leukocyte and platelet counts, but negatively correlated with erythrocyte counts and concentration of hemoglobin. The risks of anemia and adverse reactions were individually increased by 0.002- and 0.007-fold for every μg/L increase of platinum concentration. To our knowledge, this is the first study on the relationship between platinum accumulation, immune cell levels and toxicity, showing that drug-induced platinum accumulation may interfere with immune cells and thus increase the risk of toxicity.
Introduction
There are 18.1 million new cancer cases and 9.6 million cancer deaths every year, as estimated by WHO (Bray et al., 2018). Platinum is the most widely used antitumor drug with a long history of drug development, including the first-generation cisplatin, second-generation carboplatin and nedaplatin, third-generation oxaliplatin and fourth-generation lobaplatin. Although more and more alternative strategies, such as derived nanoparticles, have been produced and implemented (Garofalo et al., 2019), platinum-based drugs still play a dominant role in clinical therapy, including basic, combination therapy and even radiotherapy (Li et al., 2019). It has been reported that platinum antitumor drugs can cause adverse reactions related to neuro-, nephritic, hematic and immune toxicity (Yamauchi et al., 2015; Chopra et al., 2016), with immunotoxicity, including allergy, pruritus, diarrhea, nausea, and emesis, being the most common adverse reaction in our previous investigation, and may be due to the effects of immune system by killing cancer cells (Manohar and Leung, 2018). Prior research has demonstrated inhibition of immune function by chemotherapeutic drugs, but some researchers have shown immune enhancement by platinum drugs (Zitvogel et al., 2008; Bezu et al., 2015; Emens and Middleton, 2015). No matter whether inhibiting or enhancing the immune system, most of the research only focuses on the adverse reactions from one kind of platinum drug, but not the common toxicity from platinum drugs in general, and never consider whether the adverse reactions are due to platinum accumulation. Moreover, some patients display a poorer therapeutic effect after several courses of chemotherapy with the same dosage of platinum drugs, which also may relate to platinum accumulation. Reports indicate that the platinum level is 100–1,000 times higher in cancer patients 20 years after platinum chemotherapy than people without platinum therapy (Chovanec et al., 2017), and type I allergic reactions mediated by IgE in workers may be caused by platinum intake through breathing (Linde et al., 2017). Therefore, we believe that the adverse reactions related to platinum drugs may be due to accumulation of elemental platinum, especially for patients undergoing more courses of therapy, even though the original drug had been completely metabolized. There have been few studies on immune interference or its mechanisms by platinum, although studies of other heavy metals, such as lead and mercury, have appeared. For instance, lead can reduce the CD4+ subsets of T cells and the CD4/CD8 ratio, and influence expression of cytokines by Th1, Th2, and T cells. Chronic lead nitrate exposure results in decreased rat erythrocytes, hemoglobin, lymphocytes, and monocytes, and mercury exposure results in release of ROS, which inhibits IL-2-dependent signal transduction and reduces proliferation and survival of T cells (Biswas et al., 2008; Mishra, 2009; Sharma et al., 2010; Jorissen et al., 2013). Concerning erythrocytes mediating immune regulation, the concentration of blood lead negatively correlates with CD44 and CD58 adhesion molecule expression (Huo et al., 2019). Lead also has been reported to correlate with anemia (Hsieh et al., 2017), and it has been suggested that cisplatin, as well as other metals, may participate in oxidative stress, causing inflammation by cytokines (Manohar and Leung, 2018), and finally interfering with immune function. As another heavy metal, platinum could perhaps also interfere with the immune system. We therefore explored the change of related hemocyte levels, as well as adverse reactions, in response to platinum accumulation after complete metabolism of the drug, to establish a mechanism of platinum immune response toxicity and offer insight on immunological mechanisms of platinum toxicity.
Methods and Materials
Sample Collection
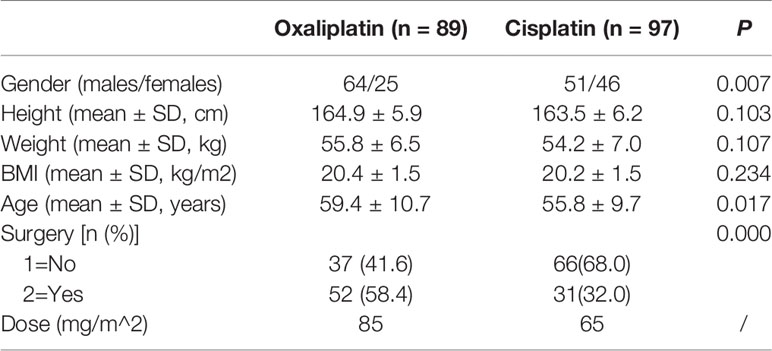
Cases of cancer patients with platinum drug chemotherapy were randomly recruited after diagnosis by a doctor according to the NCCN (National Comprehensive Cancer Network) in the Cancer Hospital of Shantou University Medical College. All patients were eligible for enrollment and exclusion conditions before medication shown in Table 1.
Oxaliplatin has a half-life of 46 h, whereas cisplatin has 72 h. In theory, both drugs should be cleared completely after 5.5 times the half-life, which would be 10.5 days for oxaliplatin and 16.5 days for cisplatin. The time interval of chemotherapy was set at three weeks between rounds, according to drug elimination and the clinical requirements of the NCCN. We chose the day before the next round of chemotherapy as the specified time point for blood sampling and collected samples at five time points for the corresponding courses. Finally, we obtained 187 cases after eliminating invalid samples, 89 for oxaliplatin and 97 for cisplatin, from January to December in 2018. The dosage of oxaliplatin and cisplatin was set by the oncologist according to body surface area and the NCCN, as shown in Table 3.
Whole blood was extracted by elbow venous in all cases. A total of 1 ml was extracted in a heparin anticoagulant tube for hematological index analysis, and 3 ml was centrifuged (1,200 g, 3 min) to obtain serum for platinum measurement. Adverse reactions were simultaneously recorded. All protocols in this investigation were approved by the Human Ethics Committee of the Cancer Hospital of Shantou University Medical College, China (2015030907). All patients signed informed consent.
Hematological Index Analysis
Hematological indices were measured with an automatic blood analyzer (Beckman-LH780, USA) immediately after sample collection by the methods on impedance measurement and cyanated methemoglobin colorimetric procedure.
Measurement of Serum Platinum
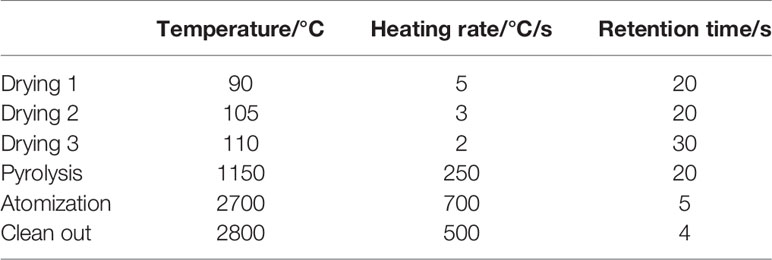
A total of 100 μl serum sample and 900 μl 0.5% nitric acid (65%, guarantee) were mixed by vortexing and quantified by graphite furnace atomic absorption spectrophotometry (Jena ZEEnit 650, Germany). Parameters for the AAS-650 were a 265.9 nm wavelength, 0.2 nm slit width, 8 mA Pt-lamp current and the temperature program was shown in Table 2. The limit of detection (LOD) was 31.16 μg/L and accuracy of this method was verified by recoveries between 91.73 and 98.31% from spiked serum samples.
Adverse Reaction Record at Each Corresponding Time Point
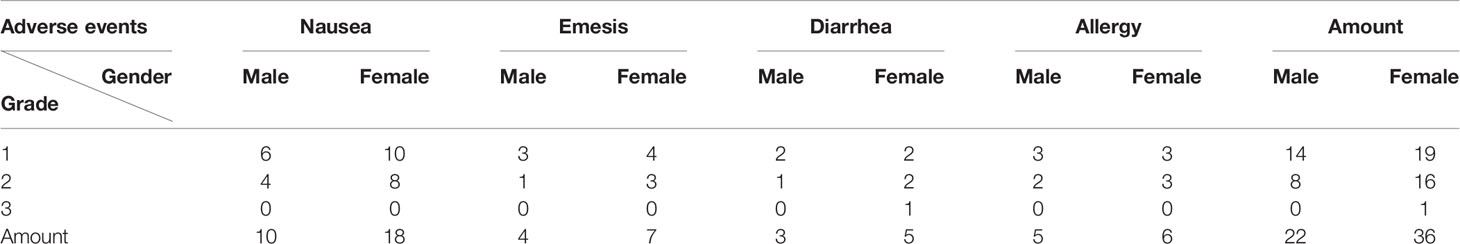
Adverse reactions were assessed and recorded by a clinical doctor at each time point, based on The Common Terminology Criteria for Adverse Events (CTCAE, 5.0). We recorded the adverse reactions, including diarrhea, nausea, emesis and allergy, and classified them into five grades (from 0-no symptoms to 4-severe life-threatening).
Statistical Analysis
Nonparametric analysis was performed, using the Kolmogorov-Smirnov test, for frequency distribution; data with skewed distributions was represented by median and 25th–75th percentile, the comparison of rates was performed by the chi-square test, skewed data was compared by non-parametric testing (Mann Whitney U test), and correlation analysis was performed by Spearman rank correlation analysis. The concentration-response relationship was analyzed by binary logistic regression. All analyses were performed with SPSS 22.0 (IBM Corporation, USA) and GraphPad Prism 7.0 (GraphPad, CA) software. A P<0.05 was considered as statistically significant in all analyses.
Results
General Characteristics of the Study Population
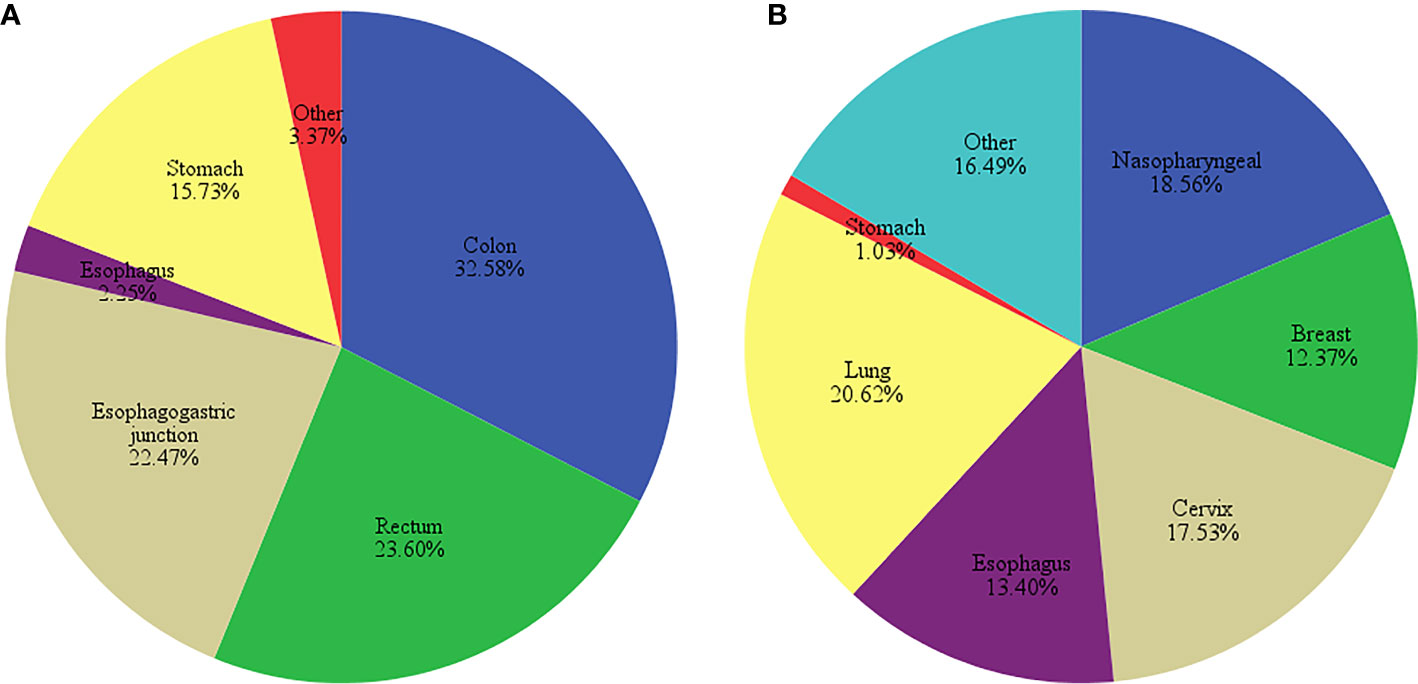
In this study, there were 89 patients who were treated with oxaliplatin and 97 with cisplatin, with a higher proportion of female patients and lower average age in the cisplatin-treated group. No significant differences were found in height, weight and BMI between the patients in both groups (P>0.05, Table 3). Oxaliplatin-treated patients had mostly colon, rectum, and gastroesophageal junction cancers, whereas cisplatin-treated patients had lung, nasopharyngeal and cervix cancers (Figure 1).
Platinum Accumulation
Serum Platinum of Patients With Different Drugs
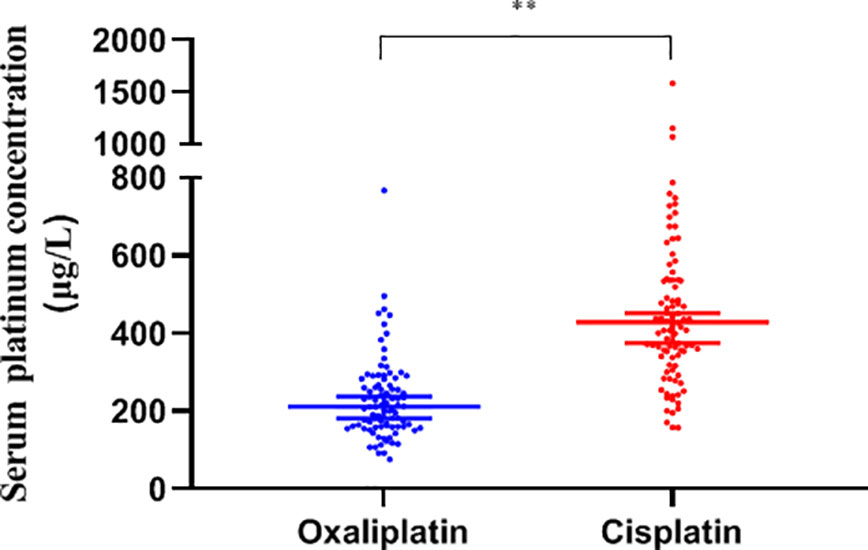
The serum platinum concentration of the two groups showed a non-normal distribution and was analyzed by the median. The concentration of platinum displayed a high degree of dispersion with a median of 294.8 (205.6, 440.3) μg/L in all subjects, with the cisplatin group being significantly higher than oxaliplatin, 429.3 (340.3, 539.5) vs. 211.7 (160.9, 275.5) μg/L, (P<0.01, Figure 2).
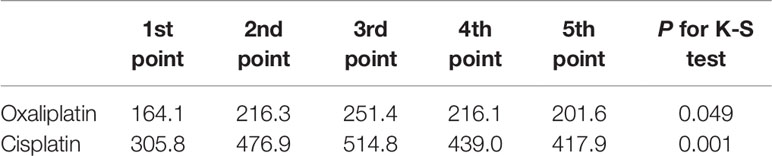
Serum Platinum at Different Time Points
After analysis of the platinum concentration at each of the five time points, we found significantly different concentrations by the Kolmogorov-Smirnov test between each time point, with peak values obtained at the third point for both oxaliplatin and cisplatin treatments (P<0.05, Table 4).
Serum Platinum in Different Genders
We grouped the serum platinum data by gender and found the median level was significantly higher in females than males, with median values being 383.9 (240.0, 496.7) vs. 266.5 (181.9,400.8) μg/L (P <0.05) for females and males, respectively.
Correlations Between Serum Platinum and Hematological Indices
Hematological Indices in Two Groups
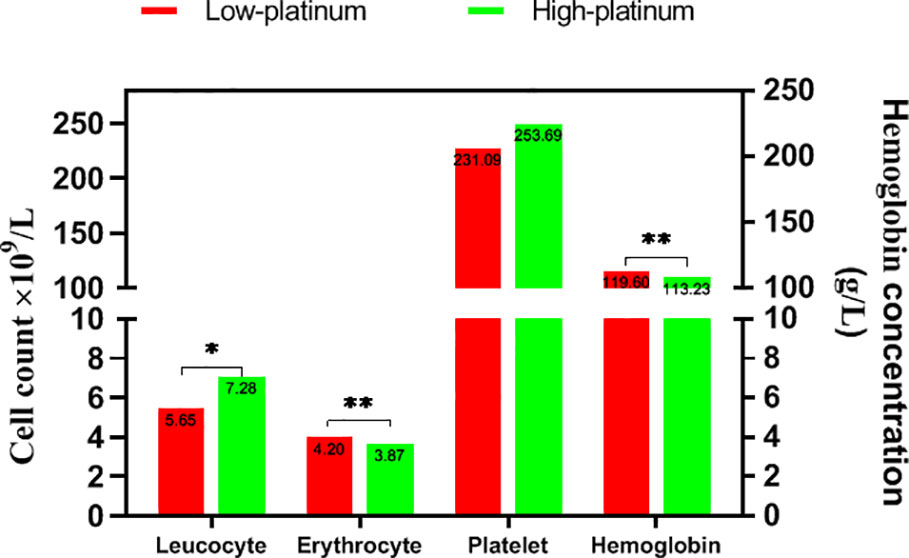
We divided the cases into low- and high-platinum groups by the mean of the natural logarithm of platinum concentration, compared the hematological index in the two groups (Figure 3). The count of leucocyte was lower in the low platinum group than that in the high platinum group (P<0.05). On the contrary, erythrocyte count and hemoglobin took higher levels in the low platinum group (P<0.01). There is no significant difference on platelet between the two groups.
Correlations Between Serum Platinum and Hematological Indices
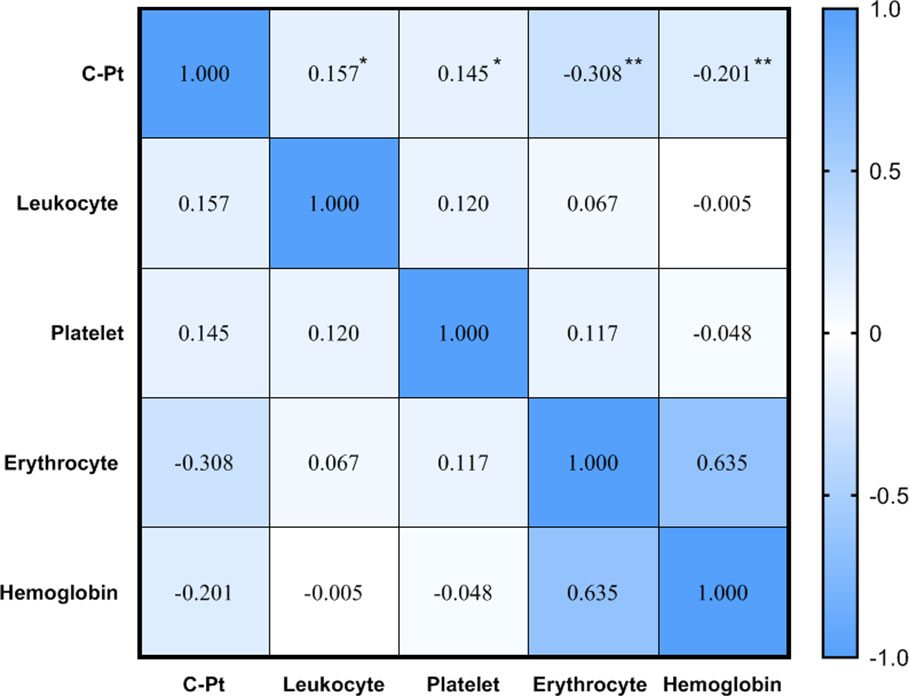
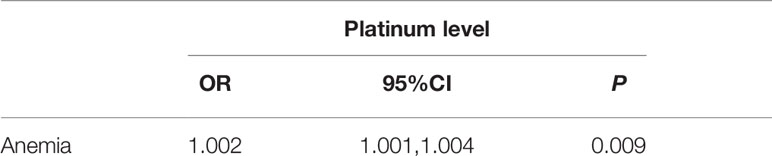
Spearman correlation analysis showed a positive correlation between platinum level and leukocyte and platelet counts, but negative correlations between platinum level and erythrocyte counts and hemoglobin (P<0.05, Figure 4). The current definition of anemia is hemoglobin <120 g/L in adult males, or hemoglobin <110 g/L in adult females (non-pregnancy). In this study, the hemoglobin mean of male and female patients was 119.1 ± 16.4 vs.112.4 ± 12.0 g/L, respectively, with the incidence of anemia being 51.3 vs. 39.4% (no significant difference between male and female groups, P>0.05). To determine the correlation between anemia and platinum, a binary logistic regression model was used with occurrence of anemia as a dependent variable (1=no, 2=yes) and platinum as an independent variable. The results showed that the risk of anemia increased 0.002-fold for every unit (μg/L) increase of serum platinum concentration (P<0.05, Table 5).
Correlations Between Adverse Reactions and Serum Platinum
We recorded the adverse reactions related to immune toxicity of tumor patients and observed almost no serious events (Table 6). However, the chi-square test showed that there was a significant difference between males and females, with the latter displaying a higher incidence of more serious cases (50.7% vs.19.1%, χ2 = 20.393, p=0.000).
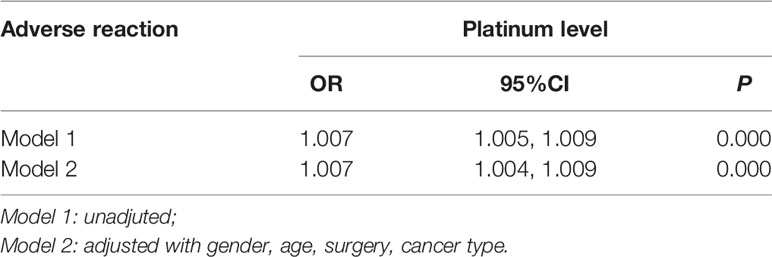
Spearman correlation analysis showed that serum platinum was positively correlated with the incidence and degree of adverse reactions (rs=0.466, P<0.01; rs=0.490, P<0.01). In order to further analyze the concentration-response relationship between serum platinum and adverse reactions, a binary logistic regression model was used with the occurrence of adverse reaction as a dependent variable (1=no, 2=yes), serum platinum concentration as an independent variable, and following adjustment for gender and age. The results showed that the risk of adverse reactions was increased by 0.007 times for every unit (μg/L) increase of serum platinum concentration, after adjustment with gender, age, surgery, cancer types (P <0.05, Table 7).
Discussion
Platinum Accumulation
In this study, we found accumulation of platinum in cancer patients after rational interval of chemotherapy and was higher in cisplatin- than oxaliplatin-treated patients (429.3 vs. 211.7 μg/L). It is the first time to compare platinum accumulation of these two drugs, although a study on cisplatin has been reported indicating its continued presence 20 years after therapy (Linde et al., 2017). The original levels of platinum in the two groups were consistent, based on calculations of drug structure and dosage. Differences in platinum accumulation may be due to different drug structures, which would have different binding rates of metabolites with glutathione, cysteine, and ABCC protein (Boisdron-Celle et al., 2001; Wang et al., 2017). Hence, we attribute the difference of platinum accumulation, between cisplatin and oxaliplatin, to molecular structural differences. Moreover, we found large individual and gender differences in platinum levels, perhaps related to polymorphisms of the GSTP1 and XRCC1 genes, and drug metabolic diversity in vivo (Sawers et al., 2014; Gu et al., 2015). Our finding is consistent with prior research showing that the elimination time for platinum is more than 25 times that for the intact platinum-based drug in the rat (Qin et al., 2019). In addition, there was an increasing trend over the first three time points, which reflected the platinum accumulation. A decreasing trend was observed from the fourth time point, which we suspect was due to greater clearance of the original drug because of the development of resistance to platinum drugs in patients. Most reports have attributed the resistance mechanism to gene mutation or methylation (Alkema et al., 2016; Yan et al., 2017), autophagy induction by cisplatin (Grimaldi et al., 2019), and acquisition of resistance to oxaliplatin accompanied by cross-resistance to another metal such as copper (Martinez-Balibrea et al., 2015). As a result, we consider the trend of platinum distribution as a drug resistance phenomenon caused by platinum accumulation.
Associations Between Immune Toxicity and Platinum Accumulation
Adverse reactions, such as diarrhea and myelosuppression, in chemotherapy of some platinum drugs (for example: cisplatin, oxaliplatin, or carboplatin) result from toxicity to rapidly dividing cells (Bezu et al., 2015), but more and more data has shown interference of immune system function by platinum drugs. For instance, oxaliplatin and cisplatin can increase production of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), in vivo and stimulate the immune response (Manohar and Leung, 2018; Stojanovska et al., 2019).
Heavy metals can interfere with inflammation mediated by leukocytes and their subsets. For instance, cadmium affects the telomere length of leukocytes and immune regulation (Zota et al., 2015), lead and cadmium may change the counts of leukocytes and their subsets (Zhang et al., 2017). In our research, there were almost no serious adverse reactions of patients, and the more frequent and serious cases were exhibited by the female patients. We also found that residual serum platinum was positively correlated with leukocyte and thrombocyte counts, as well as the incidence of adverse reactions, such as diarrhea, nausea, emesis, and allergy after a period sufficient for drug elimination. Regression analysis further showed the increasing risk of platinum toxicity on immune inflammation mediated by leukocytes.
On the other hand, serum platinum may result in anemia, as shown by the negative correlation with erythrocytes and hemoglobin, and regression analysis showed the risk of increasing platinum on anemia, which is similar to other divalent metals due to a competitive inhibition of iron ion (Hsieh et al., 2017; Weinhouse et al., 2017). Erythrocyte counts are traditionally considered an index of anemia, but more and more research has pointed out its role in immunoregulation. Blood lead has been reported to negatively correlate with adhesion molecules (CD44 and CD58) of erythrocytes (Huo et al., 2019), which hints toward a correlation between platinum accumulation, similar to lead, and immune toxicity mediated by erythrocytes.
In all, we conducted a study on the relationship between platinum accumulation, immunocytes and subsequent adverse reactions. The results show significant correlations between all three, as well as differing toxicities in different genders. All results demonstrate that drug-induced platinum accumulation may stimulate the immune system, as is the case with other heavy metals, and further lead to immune toxicity. Influences on adverse reactions and anemia risk mediated by platinum accumulation suggests that quelation therapy and nourishment to enhance immune function for patients should be considered. The mechanism of platinum resistance is still not clear, but our study suggests that it may due to the platinum accumulation resulting from subsequent rounds of chemotherapy. More and intensive study on the mechanism of immune toxicity caused by platinum accumulation should be carried out in the future.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Human Ethics Committee of the Cancer Hospital of Shantou University Medical College, China (2015030907). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LF is the corresponding author of this manuscript. He conceptualized, analyzed the platinum-immune toxicity assessment, performed and wrote the statistical analysis described in this manuscript. YZ is the first author of this manuscript. She measured the concentrations of platinum and analyzed the relationships between the platinum and adverse reactions. JZ collected and stored the blood samples, and administered the questionnaires with patients in the hospital. YJ is the physician of medical oncology. He implemented the therapeutic regimen and assessed adverse reactions of patients. XH is the clinical laboratory doctor. He took responsibilities for the hematological index analysis. All authors reviewed the manuscript.
Funding
This work was supported by the Science and Technology Plan of Shantou (2016-6) and the Youth Research Fund of the Cancer Hospital of Shantou University Medical College.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
We would like to thank Dr. Stanley Lin for his constructive comments and English language editing.
References
Alkema, N. G., Wisman, G. B., van der Zee, A. G., van Vugt, M. A., de Jong, S. (2016). Studying platinum sensitivity and resistance in high-grade serous ovarian cancer: Different models for different questions. Drug Resist. Update 24, 55–69. doi: 10.1016/j.drup.2015.11.005
Bezu, L., Gomes-da-Silva, L. C., Dewitte, H., Breckpot, K., Fucikova, J., Spisek, R., et al. (2015). Combinatorial strategies for the induction of immunogenic cell death. Front. Immunol. 6, 187. doi: 10.3389/fimmu.2015.00187
Biswas, R., Ghosh, P., Banerjee, N., Das, J. K., Sau, T., Banerjee, A., et al. (2008). Analysis of T-cell proliferation and cytokine secretion in the individuals exposed to arsenic. Hum. Exp. Toxicol. 27 (5), 381–386. doi: 10.1177/0960327108094607
Boisdron-Celle, M., Lebouil, A., Allain, P., Gamelin, E. (2001). [Pharmacokinetic properties of platinium derivatives]. Bull. Cancer 88 Spec No, S14–S19. doi: 10.1054/brst.2001.0348
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68 (6), 394–424. doi: 10.3322/caac.21492
Chopra, D., Rehan, H. S., Sharma, V., Mishra, R. (2016). Chemotherapy-induced adverse drug reactions in oncology patients: A prospective observational survey. Indian J. Med. Paediatr. Oncol. 37 (1), 42–46. doi: 10.4103/0971-5851.177015
Chovanec, M., Abu Zaid, M., Hanna, N., El-Kouri, N., Einhorn, L. H., Albany, C. (2017). Long-term toxicity of cisplatin in germ-cell tumor survivors. Ann. Oncol. 28 (11), 2670–2679. doi: 10.1093/annonc/mdx360
Emens, L. A., Middleton, G. (2015). The Interplay of Immunotherapy and Chemotherapy: Harnessing Potential Synergies. Cancer Immunol. Res. 3 (5), 436–443. doi: 10.1158/2326-6066.Cir-15-0064
Garofalo, M., Villa, A., Rizzi, N., Kuryk, L., Rinner, B., Cerullo, V., et al. (2019). Extracellular vesicles enhance the targeted delivery of immunogenic oncolytic adenovirus and paclitaxel in immunocompetent mice. J. Control Release 294, 165–175. doi: 10.1016/j.jconrel.2018.12.022
Grimaldi, M., Bo, V. D., Ferrari, B., Roda, E., De Luca, F., Veneroni, P., et al. (2019). Long-term effects after treatment with platinum compounds, cisplatin and [Pt(O,O’-acac)(gamma-acac)(DMS)]: Autophagy activation in rat B50 neuroblastoma cells. Toxicol. Appl. Pharmacol. 364, 1–11. doi: 10.1016/j.taap.2018.12.005
Gu, A. Q., Wang, W. M., Chen, W. Y., Shi, C. L., Lu, J. H., Han, J. Q. (2015). XRCC1 genetic polymorphisms and sensitivity to platinum-based drugs in non-small cell lung cancer: an update meta-analysis based on 4708 subjects. Int. J. Clin. Exp. Med. 8 (1), 145–154.
Hsieh, N. H., Chung, S. H., Chen, S. C., Chen, W. Y., Cheng, Y. H., Lin, Y. J., et al. (2017). Anemia risk in relation to lead exposure in lead-related manufacturing. BMC Public Health 17 (1), 389. doi: 10.1186/s12889-017-4315-7
Huo, X., Dai, Y., Yang, T., Zhang, Y., Li, M., Xu, X. (2019). Decreased erythrocyte CD44 and CD58 expression link e-waste Pb toxicity to changes in erythrocyte immunity in preschool children. Sci. Total Environ. 664, 690–697. doi: 10.1016/j.scitotenv.2019.02.040
Jorissen, A., Plum, L. M., Rink, L., Haase, H. (2013). Impact of lead and mercuric ions on the interleukin-2-dependent proliferation and survival of T cells. Arch. Toxicol. 87 (2), 249–258. doi: 10.1007/s00204-012-0926-z
Li, Y., Yun, K. H., Lee, H., Goh, S. H., Suh, Y. G., Choi, Y. (2019). Porous platinum nanoparticles as a high-Z and oxygen generating nanozyme for enhanced radiotherapy in vivo. Biomaterials 197, 12–19. doi: 10.1016/j.biomaterials.2019.01.004
Linde, S. J. L., Franken, A., du Plessis, J. L. (2017). Occupational Respiratory Exposure to Platinum Group Metals: A Review and Recommendations. Chem. Res. Toxicol. 30 (10), 1778–1790. doi: 10.1021/acs.chemrestox.7b00184
Manohar, S., Leung, N. (2018). Cisplatin nephrotoxicity: a review of the literature. J. Nephrol. 31 (1), 15–25. doi: 10.1007/s40620-017-0392-z
Martinez-Balibrea, E., Martinez-Cardus, A., Gines, A., Ruiz de Porras, V., Moutinho, C., Layos, L., et al. (2015). Tumor-Related Molecular Mechanisms of Oxaliplatin Resistance. Mol. Cancer Ther. 14 (8), 1767–1776. doi: 10.1158/1535-7163.MCT-14-0636
Mishra, K. P. (2009). Lead exposure and its impact on immune system: a review. Toxicol. In Vitro 23 (6), 969–972. doi: 10.1016/j.tiv.2009.06.014
Qin, Z., Ren, G., Yuan, J., Chen, H., Lu, Y., Li, N., et al. (2019). Systemic Evaluation on the Pharmacokinetics of Platinum-Based Anticancer Drugs From Animal to Cell Level: Based on Total Platinum and Intact Drugs. Front. Pharmacol. 10, 1485. doi: 10.3389/fphar.2019.01485
Sawers, L., Ferguson, M. J., Ihrig, B. R., Young, H. C., Chakravarty, P., Wolf, C. R., et al. (2014). Glutathione S-transferase P1 (GSTP1) directly influences platinum drug chemosensitivity in ovarian tumour cell lines. Br. J. Cancer 111 (6), 1150–1158. doi: 10.1038/bjc.2014.386
Sharma, V., Sharma, A., Kansal, L. (2010). The effect of oral administration of Allium sativum extracts on lead nitrate induced toxicity in male mice. Food Chem. Toxicol. 48 (3), 928–936. doi: 10.1016/j.fct.2010.01.002
Stojanovska, V., Prakash, M., McQuade, R., Fraser, S., Apostolopoulos, V., Sakkal, S., et al. (2019). Oxaliplatin Treatment Alters Systemic Immune Responses. BioMed. Res. Int. 2019, 4650695. doi: 10.1155/2019/4650695
Wang, Z., Sun, X., Feng, Y., Liu, X., Zhou, L., Sui, H., et al. (2017). Dihydromyricetin reverses MRP2-mediated MDR and enhances anticancer activity induced by oxaliplatin in colorectal cancer cells. Anticancer Drugs 28 (3), 281–288. doi: 10.1097/CAD.0000000000000459
Weinhouse, C., Ortiz, E. J., Berky, A. J., Bullins, P., Hare-Grogg, J., Rogers, L., et al. (2017). Hair Mercury Level is Associated with Anemia and Micronutrient Status in Children Living Near Artisanal and Small-Scale Gold Mining in the Peruvian Amazon. Am. J. Trop. Med. Hygiene 97 (6), 1886–1897. doi: 10.4269/ajtmh.17-0269
Yamauchi, H., Goto, T., Takayoshi, K., Sagara, K., Uoi, M., Kawanabe, C., et al. (2015). A retrospective analysis of the risk factors for allergic reactions induced by the administration of oxaliplatin. Eur. J. Cancer Care (Engl.) 24 (1), 111–116. doi: 10.1111/ecc.12156
Yan, D., Tu, L., Yuan, H., Fang, J., Cheng, L., Zheng, X., et al. (2017). WBSCR22 confers oxaliplatin resistance in human colorectal cancer. Sci. Rep. 7 (1), 15443. doi: 10.1038/s41598-017-15749-z
Zhang, Y., Xu, X., Sun, D., Cao, J., Zhang, Y., Huo, X. (2017). Alteration of the number and percentage of innate immune cells in preschool children from an e-waste recycling area. Ecotoxicol. Environ. Saf. 145, 615–622. doi: 10.1016/j.ecoenv.2017.07.059
Zitvogel, L., Apetoh, L., Ghiringhelli, F., Kroemer, G. (2008). Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 8 (1), 59–73. doi: 10.1038/nri2216
Zota, A. R., Needham, B. L., Blackburn, E. H., Lin, J., Park, S. K., Rehkopf, D. H., et al. (2015). Associations of cadmium and lead exposure with leukocyte telomere length: findings from National Health and Nutrition Examination Survey 1999-2002. Am. J. Epidemiol. 181 (2), 127–136. doi: 10.1093/aje/kwu293
Keywords: platinum, accumulation, immune, toxicity, drug-induced
Citation: Zhang Y, Zheng J, Jiang Y, Huang X and Fang L (2020) Neglected, Drug-Induced Platinum Accumulation Causes Immune Toxicity. Front. Pharmacol. 11:1166. doi: 10.3389/fphar.2020.01166
Received: 27 April 2020; Accepted: 17 July 2020;
Published: 12 August 2020.
Edited by:
Ilaria Dando, University of Verona, ItalyReviewed by:
Mariangela Garofalo, University of Padua, ItalyEduardo J. Salustiano, Federal University of Rio de Janeiro, Brazil
Copyright © 2020 Zhang, Zheng, Jiang, Huang and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Fang, a3VhbmdyZW5iYWppYW9Ac2luYS5jb20=
†These authors have contributed equally to this work
 Yuling Zhang1†
Yuling Zhang1† Ling Fang
Ling Fang