
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 26 August 2020
Sec. Ethnopharmacology
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.01068
Abelmoschus manihot, an annual herbal flowering plant, is widely distributed throughout eastern Europe and in temperate and subtropical regions of Asia. Its flowers have been traditionally used for the treatment of chronic kidney disease in China. Currently, more than 128 phytochemical ingredients have been obtained and identified from the flowers, seeds, stems, and leaves of A. manihot. The primary components are flavonoids, amino acids, nucleosides, polysaccharides, organic acids, steroids, and volatile oils. A. manihot and its bioactive constituents possess a plethora of biological properties, including antidiabetic nephropathy, antioxidant, antiadipogenic, anti-inflammatory, analgesic, anticonvulsant, antidepressant, antiviral, antitumor, cardioprotective, antiplatelet, neuroprotective, immunomodulatory, and hepatoprotective activities, and have effects on cerebral infarction, bone loss, etc. However, insufficient utilization and excessive waste have already led to a rapid reduction of resources, meaning that a study on the sustainable use of A. manihot is urgent and necessary. Moreover, the major biologically active constituents and the mechanisms of action of the flowers have yet to be elucidated. The present paper provides an early and comprehensive review of the traditional uses, chemical constituents, pharmacological activities, and pharmaceutical, quality control, toxicological, and clinical settings to emphasize the benefits of this plant and lays a solid foundation for further development of A. manihot.
Abelmoschus manihot (L.) Medicus (syn.: Hibiscus manihot; Figure 1), which belongs to the Malvaceae family and is commonly called Huang Shu Kui Hua (in Chinese), Dakpul (in Korean), and Aibika (in Indonesian), is an annual or perennial herbaceous flowering plant and an edible form of hibiscus. It is distributed widely throughout eastern Europe and Asia, including China, Papua New Guinea, eastern Indonesia, Nepal, Fiji, India, Sri Lanka, Vanuatu, New Caledonia, and northern Australia (Park et al., 2015; Prabawardani et al., 2016; Rubiang-Yalambing et al., 2016). A. manihot, which has been demonstrated to have high nutritional value for human health, is commonly used as a green vegetable and is very popular in the South Pacific Islands, Papua New Guinea, and eastern Indonesia (Prabawardani et al., 2016; Rubiang-Yalambing et al., 2016). In China, numerous health foods have been commercially developed using the roots, stems, and leaves of A. manihot (Du L. Y. et al., 2015; Du L. et al., 2015). For hundreds of years, A. manihot has been widely used as a folk medicine in clinical practice in China to cure forms of chronic kidney disease (CKD) and exerts significant effects by decreasing the protein content in urine and protecting kidney function (Peng et al., 2010; Wang and Gao, 2010; Zhu and Bi, 2010; Tsumbu et al., 2012). According to the Compendium of Materia Medica, a famous classical book of Chinese materia medica compiled by Shizhen Li (1518–1593 CE), the flowers of A. manihot have been recorded as an effective herb to treat malignant sores, cellulitis, and burns (Jiang et al., 2012; Cai et al., 2017a; Hou et al., 2020). Importantly, A. manihot flowers are listed in the 2015 edition of the Pharmacopoeia of the People’s Republic of China (a.k.a. the Chinese Pharmacopoeia) for treating many diseases, such as chronic glomerulonephritis and diabetic nephropathy (DN), in clinical practice. The Chinese Pharmacopoeia states that the content of hyperoside, which is used as a standard for quality control of A. manihot and its compound preparations, in A. manihot flowers should not be less than 0.5% (Guo et al., 2013; Committee for the Pharmacopoeia of P.R. China, 2015). Furthermore, Huangkui capsule (HKC), a Chinese patent medicine, is a single plant-based drug extracted from the dry flowers of A. manihot. HKC was approved by the State Food and Drug Administration of China (Z19990040) in 1999 for the treatment of CKD, such as DN, chronic glomerulonephritis, membranous nephropathy, and other inflammatory diseases, in clinical practice (Zhang et al., 2014; Du L. Y. et al., 2015; Li et al., 2017). Its main mechanisms of action include improving the immune response, decreasing inflammation, improving renal fibrosis, and protecting renal tubular epithelial cells (Chen et al., 2012).

Figure 1 Photograph of Abelmoschus manihot (L.) Medicus. Courtesy of Huachun Xu, Lengshui, Bijie, Guizhou Province of China.
Modern pharmacological findings have shown that the extracts and active constituents of A. manihot possess various biological properties, including anti-DN (Tu et al., 2013), anticonvulsant (Guo et al., 2011), antioxidant (Zhang et al., 2013), antiadipogenic, anti-inflammatory (Jain and Bari, 2010), analgesic (Fan et al., 2003), antidepressant (Guo et al., 2011), antiviral (Wu et al., 2007), antitumor (Zheng et al., 2016), antiplatelet (Guo et al., 2005), anti-Crohn’s disease (Yang B. L. et al., 2018), anti-poststroke depression (Liu et al., 2009), proangiogenic (Zhu et al., 2018), cardioprotective (Lv et al., 2017), neuroprotective (Cheng et al., 2006), immunomodulatory (Pan et al., 2018), and hepatoprotective (Ai et al., 2013) properties, and are effective against cerebral infarction, bone loss (Guo and Chen, 2002; Puel et al., 2005), etc. Many of these activities are consistent with those of A. manihot in traditional medicine and support traditional usage. Overall, these investigations summarize the pharmacological activities of A. manihot flowers in many medical situations.
Because of their marked clinical therapeutic effects and nutritional value, increasing numbers of researchers have widely and intensively studied the chemical components of the flowers of A. manihot. Phytochemical investigations found that flavonoids, amino acids, nucleosides, polysaccharides, organic acids, steroids, and volatile oils were the main components present in the flowers, seeds, stems, and leaves of A. manihot. Among these, flavonoids have been regarded as the active components and are officially used as markers to monitor the quality of the herb and preparations containing extracts of A. manihot according to the 2015 edition of the Chinese Pharmacopoeia; therefore, flavonoids have been the most widely studied components (Chi et al., 2009; Yu et al., 2014; Xia et al., 2019). However, conclusions about the pharmacological activities of a majority of bioactive compounds derived from traditional Chinese Medicine or natural products have been strongly questioned in the literature on pan-assay interference compounds (PAINS) (Baell and Walters, 2014; Baell and Nissink, 2018; Heinrich et al., 2020). As a result, these flavonoids should be assessed and distinguished in the future.
In recent decades, many attempts have been made to investigate the phytochemistry and pharmacology of A. manihot. Several authors have published reviews regarding the chemical constituents and pharmacological effects of A. manihot components only (Liu et al., 2010; Todarwal et al., 2011; Wen et al., 2015). However, the published works do not contain comprehensive and up-to-date information on A. manihot. We have therefore reviewed and discussed in detail the complete range of recent advances in scientific information on the botanical details, traditional uses, chemical constituents, pharmacological activities, clinical settings, pharmacokinetics and metabolism, qualitative and quantitative analysis, and toxicity of A. manihot to support its therapeutic potential. We believe this article will be a guide for the full utilization of this plant in the development of novel candidate drugs and therapies for various diseases, especially CKD.
A. manihot is widely distributed in valleys and grasslands, at the edges of fields, and in or near ditches. It is 1–2 m high and the whole plant is sparsely hirsute. The leaves are nearly circular, with five to nine palmate lobes, and are 10–30 cm in diameter; the lobes are oblong–lanceolate, 8–18 cm long, and 1–6 cm wide with an acuminate apex and coarse blunt teeth; the length of the petiole is 6–20 cm; and the stipules are lanceolate and 0.8–1.5 cm long. The flowers are solitary and borne on the axils of terminal leaves on the branches. The pedicel is 1–3 cm long; there are four or five ovate–lanceolate bracts that are 1.2–2.5 cm in length, 0.4–1 cm wide, and sparsely hirsute; the calyx is spathulate, subentire, and apically five-toothed; and the smaller bracts are slightly longer and pubescent and are shed with the fruit. The corolla is funnel-shaped and yellow with a purple inner surface at the base and is 7–12 cm in diameter; there are five petals, which are broadly obovate; the stamen columns are 1.2–2 cm in length with basal anthers, which are subsessile; the ovary has five chambers, each of which contains multiple ovules; the style has five branches; and the stigma is purple and in the form of a spoon-shaped disk. The capsule is ovate–elliptical, covered in bristles, 4–6 cm long, and 2–3 cm in diameter, and the length of the stalk is 8 cm. Most seeds are kidney-shaped with several vertical stripes of pubescence. The plant flowers from July to October (http://ppbc.iplant.cn/sp/22499; Chen et al., 2016).
Traditional Chinese medicine has served the Chinese people for 2000 years, preventing and treating diseases and maintaining health; it remains an important part of the provision of medical services (Dhiman and Chawla, 2005; Stickel and Schuppan, 2007; Yan et al., 2015). Currently, attracted by herbal medicines’ properties of high efficiency, low toxicity, and low cost with respect to chemical drugs, increasing numbers of scientists are turning to supplementary medicine with great interest to study kidney-protecting agents (Yan et al., 2015).
In ancient China, A. manihot was first described in the two oldest books on classical medicine; namely, Jia You Ben Cao (simplified Chinese: 嘉佑本草) and Ban Cao Gang Mu (simplified Chinese: 本草纲目). It was officially listed in the 2015 edition of the Chinese Pharmacopoeia because of its medical and economic value and because it is widely distributed in many provinces of China, including Shanxi, Guangdong, Fujian, Guizhou, Guangxi, Hebei, Henan, Hubei, Hunan, Shandong, Sichuan, Yunnan, and Taiwan (Yan et al., 2015; Chen et al., 2016). It is described as sweet and cold in nature, and it acts on the kidney and bladder meridians (Committee for the Pharmacopoeia of P.R. China, 2015). The flowers, roots, leaves, and seeds of A. manihot have traditionally been widely used to treat edema and damp heat and to heal ulcers by expelling toxins; they have also been used to treat burns due to water and fire (Committee for the Pharmacopoeia of P.R. China, 2015; Pan et al., 2018). In particular, since the Song dynasty (960–1279 BC) the flowers of A. manihot have been widely regarded as a staple food and folk medicine in China, and have been used to alleviate inflammation, protect against kidney injuries, and restore tissue affected by ulcers and burns (Tu et al., 2013; Zhang et al., 2014). In addition, the flowers of A. manihot, when administered in the form of a healthy beverage, have been proved to possess a wide range of pharmacological properties such as activity against DN and CKD (Zhou et al., 2012; Du et al., 2017; Wan et al., 2019). In addition, a decoction of A. manihot flowers is traditionally used to treat jaundice and acute and chronic hepatitis in the Anhui and Jiangsu Provinces of China (Wang et al., 2004). Importantly, HKC has been widely used for many years in clinical practice to effectively treat chronic glomerulonephritis (Tu et al., 2013; Mao et al., 2015; Ge et al., 2016), alleviate proteinuria, and relieve kidney insufficiency in patients with early-stage CKD in clinical practice (Han and Qiu, 2010; Liu et al., 2011). Simultaneously, many functional foods have been obtained from the roots, stems, and leaves of A. manihot because of their abundant nucleotides, nucleosides, and nucleobases (Du L. Y. et al., 2015). Furthermore, nucleotides, nucleosides, and nucleobases have been regarded as quality control markers for many traditional Chinese medicines, such as royal jelly, Mactra veneriformis, and Cordyceps sinensis (Liu R. et al., 2012; Wu et al., 2015; Lin et al., 2018; Zhou et al., 2018).
A. manihot is mainly distributed in tropical areas, especially Asia and the Pacific Islands. Its flowers and leaves are edible and have medicinal effects, and they have been traditionally used to treat inflammation, pain, urinary infections, and chronic bronchitis because of their anti-inflammatory, antiviral, antibacterial, and wound-healing properties (Yang et al., 2015; Rubiang-Yalambing et al., 2016; Kim et al., 2018). In eastern Indonesia, the South Pacific Islands, and Papua New Guinea, A. manihot is regarded as an edible hibiscus and is consumed as a popular leafy vegetable because it contains high levels of nutrients such as Ca, Fe, K, Mg, Mn, Na, Zn, Cu, folate, and β-carotene (Rubiang-Yalambing et al., 2016). In particular, in Papua New Guinea and the Pacific Islands, the flower of A. manihot is employed as a staple folk medicine to alleviate kidney pain, lower high cholesterol levels, impede menorrhagia, induce abortions, ease childbirth, stimulate lactation, treat diarrhea, and prevent osteoporosis (Bourdy and Walter, 1992; Puel et al., 2005; Prabawardani et al., 2016). Interestingly, as a green leafy vegetable A. manihot is an integral part of the main daily meal in rural and urban areas of Papua New Guinea, and approximately 75% of local people consume this vegetable because it is rich in micronutrients (Tsumbu et al., 2011). In India, A. manihot is used as a source of traditional medicines for treating kidney pain, osteoporosis, high cholesterol levels, and heartburn (Todarwal et al., 2011). In Nepal, the juice from the leaves and roots of A. manihot, which has remarkable analgesic effects, is commonly and traditionally used for the treatment of sprains (Todarwal et al., 2011; Taroreh et al., 2016). In Korea, A. manihot has been used to make the traditional form of paper known as hanji. It was previously recognized as a species of hibiscus, although it is now classified in the genus Abelmoschus and its scientific name is Abelmoschus manihot (Kim et al., 2018).
In African states such as the Democratic Republic of the Congo, Cameroon, Uganda, Nigeria, Gabon, and Angola, A. manihot is very important in the local economy because it is the principal green vegetable for local residents in these nations (Almazan and Theberge, 1989; Kubo et al., 2006). The seeds and leaves of A. manihot have traditionally been used in Africa to treat rheumatism, fever, headache, and hemorrhoids in folk medicine (Miladiyah et al., 2011). In particular, A. manihot is utilized for the treatment of ringworm, tumors, conjunctivitis, sores, and abscesses in Nigeria (Miladiyah et al., 2011).
According to the available literature, approximately 128 chemical constituents have been isolated from A. manihot, most of which were purified from the flowers. Here, these constituents are classified into eight groups; namely, flavonoids, amino acids, nucleosides, polysaccharides, organic acids, steroids, volatile oils, and others (Table 1 and Figure 2). We also list five reported polysaccharides obtained from A. manihot and provide comprehensive information with regard to their molecular weights, monosaccharide compositions, structural features, and bioactivities, as well as associated references, in Table 2.
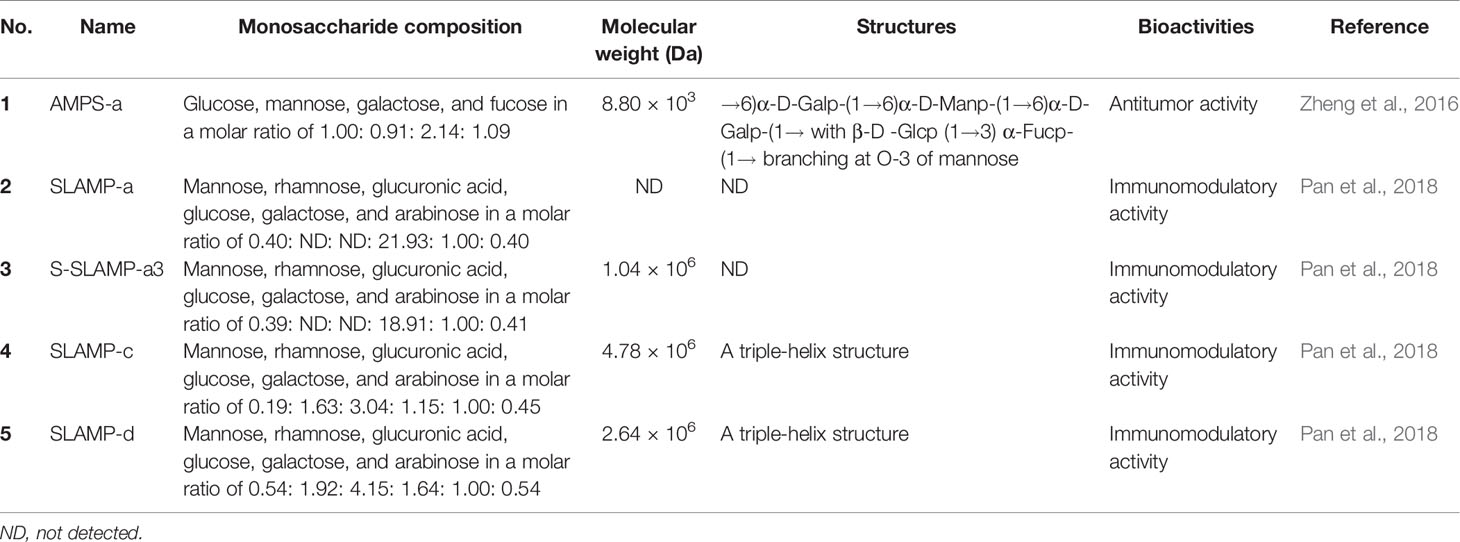
Table 2 Monosaccharide composition, molecular weight, structures, and bioactivities of polysaccharides purified from Abelmoschus manihot.
Flavonoids, as important secondary metabolites, are widespread throughout the plant kingdom, either in their free form or in the form of glycosides (Liao et al., 2012; He et al., 2017). Since the first study of A. manihot flowers in 1981, several flavonoids and their derivatives have been successively purified and identified. Currently, a total of 49 flavonoids (1–49) have been isolated and identified from the flowers of A. manihot. Phytochemical studies have indicated that the total flavonoids extracted from the flowers of A. manihot (TFAM) are their major pharmacologically active constituents and include seven chemically identified flavone glycosides. TFAM have been proved to exhibit a broad range of pharmacological activities (Yan et al., 2015). The seven main compounds with their contents identified in TFAM using high-performance liquid chromatography (HPLC) are hyperoside (43.2%), hibifolin (27.1%), isoquercitrin (13.7%), quercetin-3′-O-β-D-glucopyranoside (8.8%), quercetin-3-O-robinobioside (3.8%), myricetin (3.2%), and quercetin (0.2%) (Trendafilova et al., 2011; Xue et al., 2011b; Zhou et al., 2012). In addition, floramanosides A, B, C, D, E, and F (34–39) from A. manihot have also been demonstrated to display multiple biological effects in vivo and in vitro (Table 1 and Figure 2).
Most studies have focused on the flowers of A. manihot, whereas the compounds from other parts of A. manihot, such as the roots, stems, and leaves, have been little reported. Currently, 22 amino acids (50–71), namely phenylalanine (50), leucine (51), isoleucine (52), tryptophan (53), γ-aminobutyric acid (54), methionine (55), valine (56), proline (57), tyrosine (58), alanine (59), hydroxyproline (60), threonine (61), glycine (62), glutamate (63), glutamine (64), lysine (65), serine (66), asparagine (67), aspartic acid (68), citrulline (69), arginine (70), and ornithine (71), have been obtained from the flowers, roots, stems, and leaves of A. manihot (Du L. et al., 2015; Liu et al., 2016). Furthermore, it is recognized that amino acids from A. manihot contribute to the regulation of whole-body metabolism and play a key role in neurotransmission and lipid transport, as well as being involved in many pharmacological activities (Wang et al., 2010; Zhou et al., 2013; Refaey et al., 2015; Zhang et al., 2015).
Nucleotides, nucleosides, and nucleobases are basic components of all cells and form the various types of nucleic acids. They have been proved to be significant biological components related to many physiological processes (Du L. Y. et al., 2015). To date, 16 nucleotides, nucleosides, and nucleobases (72–87) have been isolated and identified from the leaves and flowers of A. manihot, namely adenosine (72), 5′-deoxy-5′-methylsulfinyl adenosine (73), nicotinamide (74), thymidine (75), thymine (76), 2′-deoxyuridine (77), uracil (78), 2′-deoxyadenosine (79), 2′-deoxyinosine (80), adenine (81), inosine (82), cytidine (83), guanine (84), 2′-deoxyadenosine-5′-monophosphate (85), cytidine-5′-monophosphate (86), and guanosine (87) (Du L. Y. et al., 2015). These compounds, which have nutraceutical and bioactive properties, might be developed in the future.
Thus far, there have been many studies on flavonoids but few studies on polysaccharides from A. manihot. Zheng et al. (2016) isolated a low-molecular-weight polysaccharide, named AMPS-a, from the ethanolic extract of the flowers of A. manihot. The results showed that AMPS-a is composed of glucose (88), mannose (89), galactose (90), and fucose (91) in a molar ratio of 1.00 : 0.91 : 2.14 : 1.09 (Zheng et al., 2016). Moreover, three polysaccharides, S-SLAMP-a3, SLAMP-c, and SLAMP-d, were obtained from the stems and leaves of A. manihot. These polysaccharides are mainly composed of mannose (89), rhamnose (92), glucuronic acid (93), glucose (88), galactose (90), and arabinose (94) (Pan et al., 2018). In addition, the monosaccharide compositions, molecular weights, structural characteristics, and biological activities of polysaccharides purified from A. manihot are listed in Table 2.
Currently, 13 organic acids (95–107) have been obtained from the flowers of A. manihot. Specifically, 2,4-dihydroxybenzoic acid (95), 4-hydroxybenzoic acid β-D-glucose ester (96), protocatechuic acid (97), protocatechuic acid 3-O-β-D-glucoside (98), caffeic acid (99), palmitic acid (100), hexacosoic acid (101), gallic acid (102), 3-O-caffeoylquinic acid (103), 3,5-di-O-caffeoylquinic acid (104), 4,5-di-O-caffeoylquinic acid (105), 3,4-di-O-caffeoylquinic acid (106), and (E)-9-octadecenoic acid (107) were isolated from the ethanolic extract of the flowers of A. manihot (Chen, 2006; Lai et al., 2006; Chen et al., 2007; Li et al., 2011). However, the bioactivity of these compounds has not been thoroughly studied and needs further investigation.
To date, only five steroids (108–112) have been purified and characterized from the flowers of A. manihot. Stigmasterol (108) has been obtained and identified from the petroleum ether extract of the woody stems of A. manihot and identified (Jain et al., 2009). The compounds α-spinasterol (109), β-sitosterol (110), β-sitosterol-3-O-β-D-glucopyranoside (111), and β-daucosterol (112) have been isolated and identified from the ethanolic extract of the flowers of A. manihot (Chen, 2006; Lai et al., 2006; Chen et al., 2007).
The compositions and contents of volatile oils from the flowers of A. manihot have been investigated. Lai et al. (2006) and Zhang et al. (2008) analyzed the major constituents of the volatile oil from the ethanol extract of the flowers of A. manihot by gas chromatography–mass spectrometry (MS). The results demonstrated that volatile oil was mainly composed of maleic acid (113, 2.46%), tetracosane (114, 11.02%), hexadecane (115, 1.96%), heneicosane (116, 1.38%), octadecane (117, 1.34%), allyl undecylenate (118, 1.41%), docosane (119, 15.06%), hexadecanoic acid (120, 53.37%), tetradecanoic acid (122, 3.15%), undecanone, 6,10-dimethyl (123, 2.06%), heptadecane, 2,6,10,15-tetramethyl (124, 2.84%), and 9,12-octadecadienoic acid (125, 6.41%) (Lai et al., 2006; Zhang et al., 2008).
Scopoletin (126), glycerol monopalmitate (127), and 1-triacontanol (128) have been reported to be present in A. manihot (Chen, 2006; Lai et al., 2006). Rao et al. (1990) reported that the leaves of A. manihot contained 1.77% lipids, 2.20% proteins, and 1.61% ash, and the content of water reached 88.4%. The lipids consisted of non-polar lipids, glycolipids, and phospholipids (Rao et al., 1990). Jarret et al. (2011) reported that the oil content in the seeds of A. manihot reached 16.1–22.0%. Lin et al. (2002) and Wu J. F. et al. (2019) found that a large content of unsaturated fatty acids (91.815%) was present in the seed oil of A. manihot, including oleic acid (82.179%), stearic acid (9.195%), linolenic acid (4.756%), palmitoleic acid (2.681%), palmitic acid (0.441%), linoleic acid (0.328%), and other unknown acids (0.748%). Importantly, there are 24 mineral elements (K, Ca, Fe, Mn, Cu, Zn, Mo, etc.) in the seed oil, and the contents of some harmful elements, such as Hg, As, and Cr, were far less than the maximum set by food standards (Lin et al., 2002; Wu J. F. et al., 2019). Therefore, the seeds of A. manihot have high nutritional and healthcare value and have vast potential for the development of a series of functional foods.
Various pharmacological activities have been reported for the extracts and active compounds from A. manihot both in vitro and in vivo (Table 3). Monomer compounds and extracts from different parts of this plant exhibited potent antidiabetic nephropathy, antioxidant, antiadipogenic, anti-inflammatory, analgesic, antiviral, anticonvulsant, antidepressant, antitumor, cardioprotective, antiplatelet, neuroprotective, immunomodulatory, and hepatoprotective activities. The pharmacological properties of the monomer compound and extracts from A. manihot as well as a schematic depiction of the possible mechanism of action are presented in Figures 3 and 4, respectively.
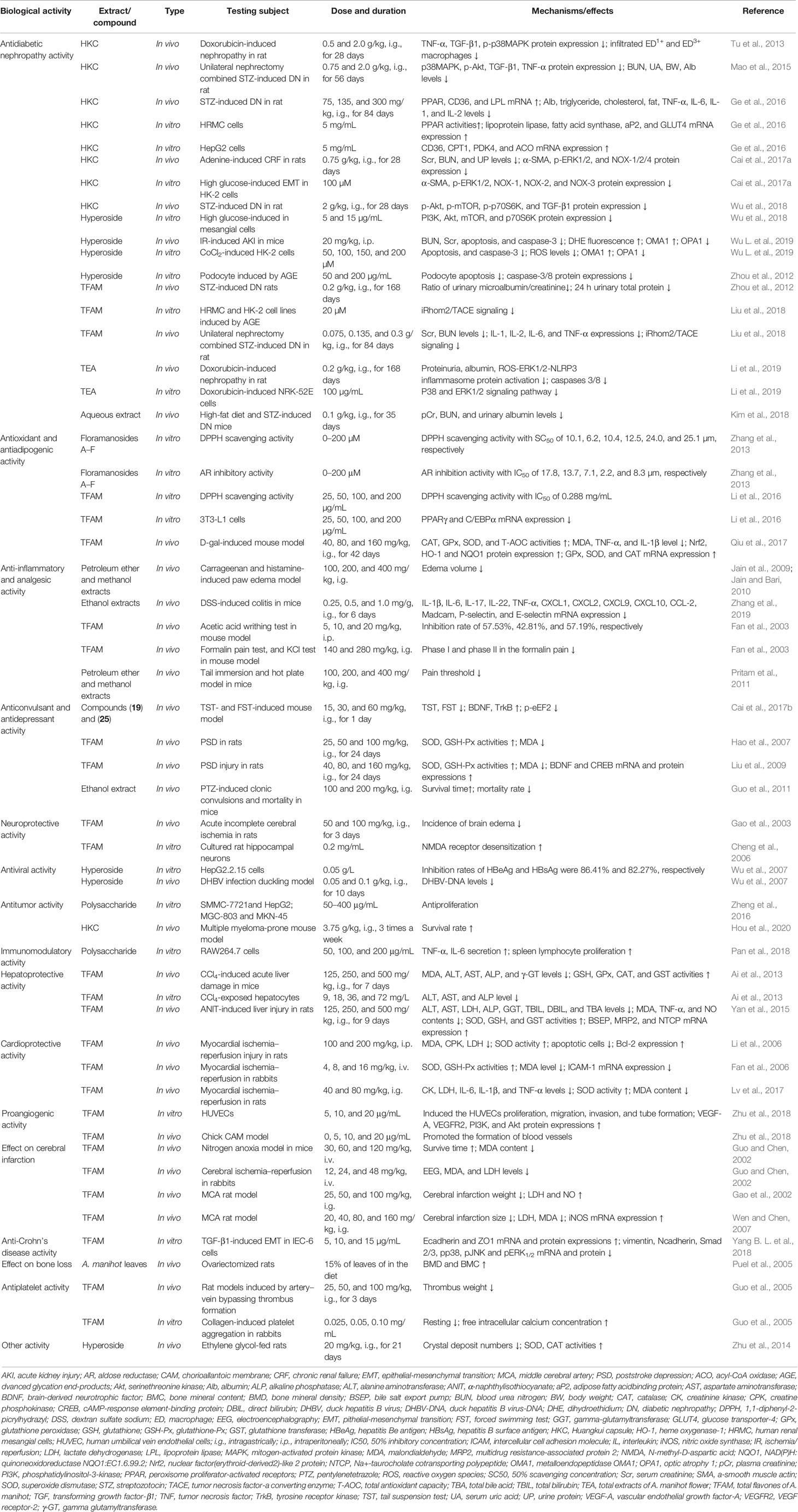
Table 3 Biological properties of extracts or compounds from Abelmoschus manihot L. and their possible mechanisms of action observed in the literature.
DN is a common complication of diabetes that has become a serious threat to human health and life and is expected to become the commonest cause of end-stage renal disease and cardiovascular events (Balakumar et al., 2012; Lv et al., 2015; Ge et al., 2016). In a rat model of unilateral nephrectomy and doxorubicin-induced nephropathy, HKC (at doses of 0.5 and 2.0 g/kg by intragastric [i.g.] administration for 28 days) notably improved the general status of rats; alleviated renal histological changes, proteinuria, albuminuria, and glomerulosclerosis; decreased the infiltration of ED1+ and ED3+ macrophages into the glomeruli; and inhibited the protein expression of tumor necrosis factor (TNF)-α in the kidney (Tu et al., 2013; Zhao et al., 2019). Moreover, mechanism studies showed that HKC significantly downregulated the protein expression of transforming growth factor (TGF)-β1 and p38-mitogen-activated protein kinase (MAPK) by suppressing the p38/MAPK signaling pathway in a rat model of doxorubicin-induced nephropathy (Tu et al., 2013; Zhao et al., 2019). In a rat model of DN induced by unilateral nephrectomy and streptozotocin (STZ) injections in comparison with α-lipoic acid, HKC at doses of 0.75 and 2.0 g/kg (i.g.) for 56 days significantly reduced urinary albumin levels; improved renal function by decreasing the blood urea nitrogen (BUN) and serum uric acid levels; alleviated kidney fibrosis by reducing the number of cells and the amount of extracellular matrix in the glomeruli; and reversed increases in markers of oxidative stress, such as malondialdehyde (MDA), 8-hydroxy-2′-deoxyguanosine, total superoxide dismutase (SOD), and nicotinamide adenine dinucleotide phosphate oxidase-4 (Mao et al., 2015). Further mechanism studies proved that HKC simultaneously decreased the protein expression of p-p38MAPK, p-Akt, TGF-β1, and TNF-α by inhibiting the p38MAPK and Akt signaling pathways in the kidney in a rat model of DN (Mao et al., 2015). Later, in in vitro and in vivo studies, HKC (at doses of 75, 135, and 300 mg/kg [i.g.] for 84 days) increased the mRNA expression of peroxisome proliferator-activated receptor (PPAR)-α and PPAR-γ in the livers and kidneys of rats with DN. HKC also increased serum albumin levels and decreased levels of serum triglycerides, cholesterol, and total fats in a dose-dependent manner in the livers of rats with DN in comparison with irbesartan (Ge et al., 2016). Moreover, HKC decreased the expression of interleukin (IL)-1, IL-2, IL-6, and TNF-α by suppressing the inflammatory reaction in the kidneys of rats with DN. Strikingly, HKC alleviated endoplasmic reticulum stress and decreased the activation of c-Jun NH2-terminal kinase in the livers and kidneys of rats with DN and subsequently reduced renal injury (Ge et al., 2016). The results of the above studies demonstrate that HKC might be a candidate agent for treating DN in humans.
HKC (at a dose of 0.75 g/kg [i.g.] for 28 days) significantly decreased the levels of BUN, serum creatinine, and urine protein in plasma, and molecular mechanisms demonstrated that HKC notably downregulated the protein expression of NADPH oxidase (NOX)-1, NOX-2, NOX-4, α-smooth muscle actin (α-SMA), and p-extracellular signal-regulated kinase (ERK)1/2 by inhibiting the NADPH oxidase/ROS/ERK signaling pathways in renal tissue in rats with chronic renal failure induced by adenine in vivo (Cai et al., 2017a). Phytochemical investigations showed the main bioactive components of HKC; namely, quercetin, quercetin-3′-O-glucoside, isoquercitrin, and hyperoside. In particular, gossypetin-8-O-β-D-glucuronide, at a concentration of 100 µM, significantly inhibited the protein expression of α-smooth muscle actin, p-ERK1/2, NOX-1, NOX-2, and NOX-4 in HK-2 cells induced by high glucose levels in the same way as the NOX inhibitor diphenyleneiodonium (Cai et al., 2017a). These in vivo and in vitro results suggest that HKC and its major flavonoid components protect against tubulointerstitial fibrosis in rats with chronic renal failure by suppressing the NOX/reactive oxygen species (ROS)/ERK signaling pathway. Furthermore, HKC (at a dose of 2 g/kg [i.g.] for 28 days) dramatically reduced urinary microalbumin levels, increased body weight and serum albumin levels, improved renal morphology and kidney weight, reduced the kidney hypertrophy index, alleviated glomerular hypertrophy, decreased the expression of α-smooth muscle actin and proliferating nuclear cell antigen, and decreased thickening of the glomerular basement membrane. The mechanism explored demonstrated that HKC downregulated the protein expression of p-p70S6K, p-mammalian target of rapamycin (mTOR), TGF-β1, and p-Akt by repressing the phosphoinositide-3-kinase (PI3K)/Akt/mTOR/p70S6K signaling pathway in the kidneys of rats in a model of early DN (Wu et al., 2018). In agreement with the results of the in vivo study, hyperoside, which is a bioactive component of HKC, at concentrations of 5 and 15 µg/mL significantly downregulated the protein expression of p-Akt, p-mTOR, p-PI3K, and p-p70S6K in murine mesangial cells induced by high glucose levels in vitro (Wu et al., 2018), which suggested that HKC safely and efficiently alleviates early pathological changes in the glomeruli in DN by inhibiting the PI3K/Akt/mTOR/p70S6K signaling pathway in vivo and in vitro and provides reliable evidence of the prevention of early DN.
In an in vitro study, TFAM at a concentration of 20 μM suppressed the activation of iRhom2/TNF-α-converting enzyme in human renal mesangial cells and HK-2 cells induced by advanced glycation end products (Liu et al., 2018). Similarly, the results of an in vivo study demonstrated that TFAM (at doses of 75, 135, and 300 mg/kg [i.g.] for 84 days) notably decreased the levels of serum creatinine and BUN by downregulating the expression of IL-1, IL-2, IL-6, and TNF-α in a dose-dependent manner by suppressing the activation of the iRhom2/TNF-α-converting enzyme signaling pathway in a rat model of DN induced by unilateral nephrectomy and STZ injections in comparison with 4-phenylbutanoic acid (2.5 mg/kg) as a positive control (Liu et al., 2018). Kim et al. (2018) established a mouse model of DN induced by a high-fat diet and STZ after unilateral nephrectomy. The results showed that extracts of A. manihot (100 mg/kg [i.g.] for 35 days) markedly reduced the levels of plasma creatinine, BUN, and urinary albumin. Moreover, the kidney/body weight ratio also increased in mice with DN in comparison with control mice (Kim et al., 2018).
The total extract of A. manihot flowers (TEA) at a concentration of 100 μg/mL reduced doxorubicin-induced changes in cellular histology, decreases in cell viability, and apoptosis in NRK-52E cells by suppressing protein oxidation and the p38 and ERK1/2 signaling pathways in vitro (Li et al., 2019). Similarly, the results of an in vivo study proved that TEA at a dose of 1.5 g/kg (i.g.) significantly reduced proteinuria and low serum albumin levels, alleviated lesions of the renal tubules, and inhibited the expression of ROS, ERK1/2, and NLRP3 inflammasome proteins in the renal tubules of rats with doxorubicin-induced nephropathy in comparison with a doxorubicin model group (Li et al., 2019), which indicates that TEA protects renal tubular cells against doxorubicin-induced injury by suppressing the ROS-ERK1/2-NLRP3 inflammasome signaling pathway both in vitro and in vivo.
In addition, oral administration of TFAM at a dose of 200 mg/kg for 24 weeks significantly decreased the ratio of urinary microalbumin to creatinine and 24 h urinary total protein and reduced the apoptosis of glomerular cells in a rat model of STZ-induced DN (Zhou et al., 2012). Moreover, one of the major active constituents of TFAM, namely hyperoside, at concentrations of 50 and 200 µg/mL, significantly decreased the apoptosis of podocytes induced by advanced glycation end products by suppressing the protein expression of caspase-3 and caspase-8 (Zhou et al., 2012). Simultaneously, in a mouse model of ischemia–reperfusion-induced acute kidney injury, pretreatment with hyperoside (20 mg/kg by intraperitoneal [i.p.] administration) reduced the extent of injury to the renal tubules, decreased the levels of BUN and serum creatinine, reduced the apoptosis of tubular cells, and suppressed the production of ROS and the expression of caspase-3 in the kidneys (Wu L. et al., 2019). Hyperoside also suppressed mitochondrial fission by suppressing OMA1-mediated proteolysis of optic atrophy 1. In agreement with the findings of the in vivo study, hyperoside (at concentrations of 50, 100, 150, and 200 μM) prevented cobalt chloride (CoCl2)-induced apoptosis and inhibited the cleavage of caspase-3 in HK-2 cells by modulating the OMA1–optic atrophy 1 axis in an in vitro study (Wu L. et al., 2019). According to these findings, hyperoside exerts its renoprotective activity partly because of its antiapoptotic and antioxidant activities and might have potential as a novel therapeutic agent for the treatment of acute kidney injury.
Oxidative stress has been implicated in the pathophysiology of various ocular diseases, including cerebral ischemia, atherosclerosis, inflammation, diabetes, and cancer (Ghanem et al., 2010; Zhang et al., 2013). Zhang et al. (2013) found that floramanosides A, B, C, D, E, and F, isolated from the flowers of A. manihot, have strong antioxidative and radical scavenging activities against DPPH with 50% scavenging concentrations (SC50) of 10.1, 6.2, 10.4, 12.5, 24.0, and 25.1 µM, respectively. Moreover, floramanosides A, B, C, D, and E exhibited significant inhibitory activity against aldose reductase, with half maximal inhibitory concentration (IC50) values of 17.8, 13.7, 7.1, 2.2, and 8.3 µM, respectively (Zhang et al., 2013). In addition, hyperoside exhibited notable DPPH scavenging activity, with an IC50 value of 0.288 mg/mL. Furthermore, hyperoside at a concentration of 100 µg/mL exhibited significant antiadipogenic activity and downregulated the mRNA expression of PPAR-γ and CCAAT/enhancer binding protein-α in a 3T3-L1 cell line (Li et al., 2016). These results indicate that A. manihot flowers may have potential as an agent for treating oxidative stress-related diseases.
In a D-galactose-induced mouse model, TFAM at doses of 40, 80, and 160 mg/kg (i.g.) for 42 consecutive days significantly boosted the activities of catalase (CAT), glutathione peroxidase (GPx), and SOD; increased total antioxidant capacity; and decreased the content of MDA in the liver with a dose-dependent manner when compared with ascorbic acid (80 mg/kg) as a positive control (Qiu et al., 2017). Furthermore, after treatment with TFAM in Nrf2-mediated antioxidant responses, the protein expression of Nrf2, heme oxygenase-1, and NAD(P)H quinone oxidoreductase-1 as well as the mRNA expression of GPx, SOD, and CAT dramatically increased, whereas the TNF-α and IL-1β expression significantly decreased (Qiu et al., 2017). However, investigations into the antioxidant activity of TFAM and the precise mechanism of action involved in vivo are very limited.
Petroleum ether and methanolic extracts of the woody stems of A. manihot at doses of 100, 200, and 400 mg/kg (i.g.) displayed notable anti-inflammatory activity in the rat model of carrageenan- and histamine-induced paw edema by dose-dependently reducing the percentage increase in the volume of edema in comparison with the standard drug diclofenac sodium (10 mg/kg) as a positive control (Jain et al., 2009; Jain and Bari, 2010). The ethanolic extracts of A. manihot at doses of 0.25, 0.5, and 1.0 mg/g (i.g.) for 6 days significantly alleviated dextran sulfate sodium-induced colitis in mice by regulating the composition of the gut microbiota, boosted microbial diversity, and increased the abundance and levels of gut microbiota that produce straight-chain fatty acids, especially butyric acid and acetic acid (Zhang et al., 2019). In addition, treatment with ethanolic extracts of A. manihot dramatically decreased the mRNA levels of proinflammatory cytokines such as IL-22, IL-17, IL-6, IL-1β, and TNF-α, chemokines such as CXCL-1, CXCL-2, CXCL-9, CXCL-10, and CCL-2, and adhesion molecules such as mucosal address in cell adhesion molecule, P-selectin, and E-selectin (Zhang et al., 2019), which mainly acted via the PPAR-γ signaling pathway and resulted in an increase in the generation of Treg cells and inhibition of the development of Th17 cells.
Petroleum ether and methanolic extracts of A. manihot leaves (at doses of 100, 200, and 400 mg/kg, i.g.) exhibited significant analgesic activity in mouse tail immersion and hot plate models by markedly increasing the pain threshold in a dose-dependent manner in comparison with pentazocine (10 mg/kg) as a control (Pritam et al., 2011). TFAM has displayed remarkable analgesic effects in models of acetic acid-induced writhing, formalin-induced pain, and KCl-induced reactions. TFAM at doses of 5, 10, and 20 mg/kg (i.p.) significantly suppressed acetic acid-induced writhing in mice, with inhibition rates of 57.53%, 42.81%, and 57.19%, respectively. TFAM at doses of 140 and 280 mg/kg (i.g.) markedly reduced acetic acid-induced writhing in mice, with inhibition rates of 62.16% and 42.34%, respectively (Fan et al., 2003). Moreover, TFAM markedly alleviated the reaction induced by KCl in rabbits after intra-arterial injection at 200 mg/kg (Fan et al., 2003). Advanced studies should be carried out to further investigate the related biochemical pathways and develop safe and effective analgesic agents.
Depression is characterized by a disturbance of mood associated with alterations in behavior, energy, and appetite and is a widespread incapacitating psychiatric disorder (Nemeroff, 2007). In pentylenetetrazole-induced clonic convulsions and associated mortality in mice, Guo et al. (2011) found that oral administration of ethanolic extract of A. manihot flowers at a dose of 200 mg/kg (i.g.) greatly prolonged the time to death and decreased the mortality rate and the immobility time, but it had no effect on the latency time in comparison with fluoxetine (20 mg/kg, i.g.) (Guo et al., 2011). In addition, TFAM (at doses of 25, 50, and 100 mg/kg [i.g.] for 24 days) significantly increased the crossing and rearing scores in an open-field test; suppressed increases in the viscosity of whole blood, as well as plasma, at high, moderate, and low shear rates; decreased the deformation of erythrocytes; enhanced the activities of SOD and GPx; and decreased the MDA content in the brains of rats with poststroke depression in comparison with fluoxetine (1.8 mg/kg) (Hao et al., 2007). Most importantly, A. manihot potentiated the antimobility effect of fluoxetine and fluoxetine hydrochloride (conventional antidepressants), which confirmed the therapeutic effect of this plant against depression.
TFAM at doses of 40, 80, and 160 mg/kg (i.g.) for 24 days dramatically alleviated escape-directed behavioral impairment in mice induced by poststroke depression, significantly decreased the content of MDA, and increased the activities of SOD and GPx in comparison with fluoxetine (2.5 mg/kg). It also alleviated poststroke depression-induced neuronal death and loss by upregulating the mRNA and protein expressions of brain-derived neurotrophic factor and CREB, which suggests that TFAM has a protective effect against poststroke depression-induced injury in mice (Liu et al., 2009). Further studies demonstrated that the active compounds gossypetin-8-O-β-D-glucuronide and quercetin-3’-O-glucoside (at doses of 15, 30, and 60 mg/kg, i.g.) of TFAM dramatically shortened the immobility time in the tail suspension and forced-swimming tests, notably increased the expression of brain-derived neurotrophic factor (BDNF) and tropomyosin receptor kinase-B (TrkB), and decreased the expression of p-eukaryotic elongation factor-2 (p-eEF2) in the hippocampus in comparison with ketamine (10 mg/kg) as a positive control (Cai et al., 2017b). These results will provide new insights into the development of antidepressant agents for the treatment of major depressive disorder.
TFAM at doses of 50 and 100 mg/kg (i.g.) for 3 days significantly reduced the incidence of brain edema and alleviated pathological changes in brain tissues in rats with acute incomplete cerebral ischemia in comparison with Ginkgo biloba extract (50 mg/kg) as a positive control (Gao et al., 2003). In cultured rat hippocampal neurons, TFAM quickly and reversibly suppressed the N-methyl-D-aspartate (NMDA)-activated current in a concentration-dependent manner with an IC50 value of 0.46 mg/mL, and also non-competitively suppressed the NMDA-activated current by strengthening the desensitization of NMDA receptors in the presence of TFAM (0.2 mg/mL), which suggests that TFAM exerts a neuroprotective function by inhibiting the NMDA receptor response (Cheng et al., 2006).
Wu et al. (2007) found that hyperoside at a concentration of 0.05 g/L notably suppressed the secretion of HBeAg and HBsAg in HepG2.2.15 human hepatoma cells with inhibition rates of 86.41% and 82.27%, respectively (Wu et al., 2007). In addition, hyperoside (at doses of 0.05 and 0.1 g/kg [i.g.[for 10 days) significantly reduced the DNA levels of duck HBV in a duckling model of duck HBV infection, and the decreases in peak viremia reached 60.79% on day 10 and 69.78% on day 13, respectively. These results suggest that hyperoside is an effective inhibitor of the secretion of HBsAg and HBeAg in HepG2.2.15 cells and decreases DNA levels of duck HBV in a duck model of HBV infection (Wu et al., 2007). Further studies should be performed to elucidate the molecular mechanism of hyperoside for HBV.
AMPS-a, a polysaccharide purified from the ethanolic extract of A. manihot flowers, at concentrations ranging from 50 to 400 μg/mL, remarkably suppressed the proliferation of human hepatic carcinoma cells (SMMC-7721 and HepG2) and gastric cancer cells (MGC-803 and MKN-45) (Zheng et al., 2016). However, more evidence is needed to obtain detailed structural information as well as the mechanism of action of AMPS-a and explore it as a potential antitumor agent. In a recent study, Hou et al. (2020) found that HKC, at 3.75 g/kg/day, prominently prolonged the survival rate of a multiple myeloma-prone mouse model. Further phytochemical investigations proved that four bioactive ingredients from HKC, namely hyperoside (28), cannabiscitrin (29), 8-(2′′-pyrrolidione-5-yl)-quercetin (48), and 3-O-kaempferol-3-O-acetyl-6-O-(p-coumaroyl)-β-D-glucopyranoside (49), at concentrations of 0.05 and 5 μM, notably promoted the differentiation of murine pre-osteoblast MC3T3-E1 cells (Hou et al., 2020). Furthermore, compound (49) suppressed the proliferation of multiple myeloma ARP1 and H929 cells and induced cell cycle arrest at G0/G1 phase, which may be related to inhibition of the β-catenin protein, upregulation of the expressions of IL-6 and TNF-α, as well as activation of mature TGF-β1 (Hou et al., 2020). The results from this study indicated that HKC exerts protective effects and may serve as a promising anti-multiple myeloma drug.
Four polysaccharides, SLAMP-a, S-SLAMP-a3, SLAMP-c, and SLAMP-d, isolated from the stems and leaves of A. manihot, at concentrations of 50, 100, and 200 μg/mL, exhibited significant immunomodulatory activity by promoting the proliferation of spleen lymphocytes and stimulating the secretion of TNF-α and IL-6 in RAW264.7 cells. Especially, the sulfated derivatives S-SLAMP-a3 exhibited the highest immunomodulatory activities and the proliferation rate reached 1.47 (Pan et al., 2018), which indicates that sulfated derivatives of polysaccharides might be promising candidates for the development of antitumor drugs. This also provides new insights into the utilization of enormous amounts of discarded resources to avoid extensive waste, as well as environmental pollution.
The antihepatotoxic activity of crude extracts of A. manihot (TFAM) has been demonstrated both in vitro and in vivo. In acute liver damage induced by CCl4 (0.12%, v/v, dissolved in olive oil, 10 mL/kg body weight) in mice, oral administration of TFAM at doses of 125, 250, and 500 mg/kg daily for 7 days significantly decreased the contents of AST, ALT, ALP, and γ-GT in serum in comparison with biphenyldicarboxylate (150 mg/kg) as a positive control (Ai et al., 2013). In addition, TFAM decreased the MDA content and increased the activities of SOD, GPx, CAT, and glutathione-S-transferase in the liver in a dose-dependent manner in comparison with a control group treated with CCl4. Histological analyses of the liver also showed that TFAM reduced the extent of liver lesions induced by CCl4 (Ai et al., 2013). Moreover, treatment with different concentrations of TFAM (i.e., 9, 18, 36, and 72 mg/L) significantly decreased the levels of ALT, AST, and ALP in the medium in hepatocytes exposed to CCl4 in an in vitro study (Ai et al., 2013). Similarly, TFAM (at doses of 125, 250, and 500 mg/kg [i.g.] for 9 consecutive days) in a dose-dependent manner notably reduced the levels of AST, AST, LDH, γ-GT, total bilirubin, direct bilirubin, and total bile acids in serum, decreased the contents of MDA, TNF-α, and NO, and increased the activities of SOD, glutathione, and glutathione-S-transferase in liver tissue in cholestatic liver injury induced by α-naphthylisothiocyanate in rats (Yan et al., 2015). Further studies demonstrated that pretreatment with TFAM significantly upregulated the protein and mRNA expression of the bile salt export pump multidrug resistance-associated protein-2 and Na+-taurocholate-cotransporting polypeptide in liver injury with cholestasis induced by α-naphthylisothiocyanate (Yan et al., 2015).
TFAM at doses of 100 and 200 mg/kg (i.p.) notably reduced the content of MDA in the myocardium and the production of creatine phosphokinase in serum, increased the activity of SOD and Bcl-2 expression, and decreased the number of apoptotic cells and LDH release in rat myocardium injured by ischemia–reperfusion when compared with nifedipine (2 mg/kg) as a positive control (Li et al., 2006). Intravenous (i.v.) administration of TFAM at doses of 4, 8, and 16 mg/kg significantly alleviated myocardial injury induced by ischemia–reperfusion in rabbits, increased the activities of SOD and GPx, and reduced the level of MDA in plasma. Moreover, the mRNA expression of intercellular adhesion molecule-1 (ICAM-1) in the myocardium was significantly downregulated in rabbits in comparison with verapamil (0.8 mg/kg) as a positive control (Fan et al., 2006). TFAM at doses of 40 and 80 mg/kg (i.p.) exhibited notable cardioprotective activity by decreasing the levels of creatinine kinase, LDH, IL-6, IL-1β, and TNF-α in serum, increasing the activity of SOD, and reducing the MDA content in a rat model of myocardial ischemia–reperfusion. In addition, TFAM alleviated myocardial injury induced by ischemia–reperfusion in rats by suppressing the NLRP3 inflammasome (Lv et al., 2017). Taken together, these results suggest that TFAM might be a candidate drug for treating cardiovascular diseases.
TFAM at concentrations of 5, 10, and 20 μg/mL significantly induced the proliferation, migration, invasion, and formation of human umbilical vein endothelial cells by boosting the phosphorylation of PI3K and Akt and intensifying the expression of vascular endothelial growth factor (VEGF)-A and VEGF receptor-2 in these cells in vitro. In addition, a study using a chick model of the chorioallantoic membrane demonstrated that TFAM clearly promoted the formation of branched blood vessels in vivo (Zhu et al., 2018). These results indicate that TFAM may exert proangiogenic activity by activating the VEGF-A/VEGF receptor-2–PI3K/Akt signaling pathway both in vitro and in vivo.
TFAM (at doses of 30, 60, and 120 mg/kg, i.v.) prolonged the survival time, increased the survival rate, and decreased the MDA content of the cerebral cortex in a mouse model of nitrogen anoxia. In addition, TFAM (at doses of 12, 24, and 48 mg/kg, i.v.) remarkably reduced electroencephalogram activity and the levels of MDA and LDH in rabbits subjected to cerebral ischemia–reperfusion (Guo and Chen, 2002). In addition, TFAM (at doses of 25, 50, and 100 mg/kg, i.g.) markedly decreased the extent of cerebral infarction and increased the contents of LDH and NO in a rat model of occlusion of the right middle cerebral artery (Gao et al., 2002). These findings indicate that TFAM has protective effects against cerebral ischemia–reperfusion injury, and its mechanism of action may be related to the inhibition of free radicals and lipid peroxidation.
Pretreatment with TFAM (at doses of 20, 40, 80, and 160 mg/kg, i.g.) led to a remarkable percentage reduction in the extent of cerebral infarction. Pretreatment with TFAM also significantly reduced the activity of LDH and the MDA content, increased the serum levels of NO, and upregulated the cerebral mRNA expression of inducible nitric oxide synthase (iNOS) in rats with ischemia–reperfusion-induced cerebral infarction subjected to occlusion of the right middle cerebral artery in comparison with nimodipine (2 mg/kg) as a control (Wen and Chen, 2007). These findings indicate that TFAM reduces the extent of cerebral infarction, which is partly due to its antioxidant and anti-inflammatory activities.
TFAM at concentrations of 5, 10, and 15 μg/mL effectively suppressed TGF-β1-induced morphological changes, migration, and invasion; increased the mRNA and protein levels of Ecadherin and ZO-1; decreased the mRNA and protein levels of vimentin and Ncadherin; and reduced the levels of Smad 2/3, p-p38, p-c-Jun N-terminal kinase, and p-ERK1/2, which involved the Smad/MAPK signaling pathway. In addition, si-Smad and MAPK inhibitors effectively suppressed the TGF-β1-induced epithelial–mesenchymal transition in IEC-6 cells (Yang B. L. et al., 2018). Thus, a combination of TFAM with si-Smad or MAPK inhibitors had greater inhibitory effects on the TGF-β1-induced epithelial–mesenchymal transition in IEC-6 cells, which suggests that TFAM might represent a novel therapeutic strategy for the treatment of intestinal fibrosis in Crohn’s disease.
The ability of A. manihot leaves to inhibit bone loss in ovariectomized rats was investigated by Puel et al. (2005). The results of this study showed that osteopenia was significantly reduced after the administration of the highest dose of the leaves of A. manihot, namely 15%, in the diet, although the lowest dose did not produce any effects: bone-sparing effects were produced, which enabled improvements in bone mineral density and bone mineral content (Puel et al., 2005). Therefore, A. manihot, as an edible plant, has been used in folk medicine to alleviate symptoms of sex hormone imbalances and can help to reduce bone loss in conditions of estrogen deficiency and thus provide some protection against osteoporosis (Puel et al., 2005). Further study data are required for the development of potential alternative nutraceutical agents.
TFAM at doses of 25, 50, and 100 mg/kg (i.g.) for 3 days notably decreased the weight of thrombi in a rat model of thrombus formation induced by arteriovenous bypass in a dose-dependent manner. Moreover, TFAM at concentrations of 0.025, 0.05, and 0.10 mg/mL displayed dose-dependent inhibitory effects on platelet aggregation induced by collagen in rabbits. In addition, TFAM markedly reduced the resting and CaCl2-induced increased concentrations of free intracellular calcium ions in rabbit platelets in vitro (Guo et al., 2005). Therefore, TFAM may have wide therapeutic potential for treating various circulatory diseases, such as cerebral infarction.
The effect of a combination of quercetin and hyperoside (at a dose of 20 mg/kg [i.g.] for 21 days) on the formation of calcium oxalate deposits in rats fed ethylene glycol was studied. The results showed that the quercetin–hyperoside combination remarkably reduced the number of crystal deposits and increased the activities of SOD and CAT in comparison with those in the untreated group (Zhu et al., 2014). Therefore, quercetin in combination with hyperoside may act as a complementary effective drug for preventing stone formation.
In 2003, high-throughput screening technology as a normal method was established to identify novel lead compounds to increase the number of validated drug targets in drug screening (Baell and Walters, 2014; Baell, 2016; Baell and Nissink, 2018). However, it also introduced many special molecules that interfere with drug screening, such as pan-assay interference compounds (PAINS), which resulted in false-positive assay readouts in pharmacological investigations, and ignored bioactive compounds with real potential (Baell and Walters, 2014), suggesting that the structural context in which PAINS are presented may play an important role for eliciting false-positive activities. Moreover, at least 10 chemical constituent classes have been considered to be PAINS, including rhodanines, phenolic Mannich bases, 1,2,3-aralkylpyrroles, hydroxyphenylhydrazones, alkylidene barbiturates, alkylidene heterocycles, 2-amino-3-carbonylthiophenes, activated benzofurazans, catechols, and quinones. These should be avoided in further studies as they largely reduced the false-positive readouts of the lead compound (Baell and Holloway, 2010). Hence, PAINS have already become a major point for discussion in drug discovery, especially in the fields of medicinal plant research, ethnopharmacology, and natural product research with respect to their pharmacological investigations (Baell and Walters, 2014; Heinrich et al., 2020).
In the present review, flavonoid compounds, as major bioactive ingredients purified from different medicinal parts of A. manihot, exhibited a variety of pharmacological activities. However, these activities may have been affected by PAINS; consequently, the results reported should be regarded with skepticism. Simultaneously, the screening approach should be studied carefully during drug screening to reduce the interference of PAINS. In addition, PAINS should be completely eliminated by various methods, including computer prediction and biochemical methods, especially in the early stages of drug research and development. It is worth noting that, in view of the complexity of biological systems and the characteristics of the interactions between drugs and the body, in the process of predicting the effects of different compounds, accurate elimination of PAINS still needs to be specifically investigated in future studies.
The metabolites of bioactive flavonoids from A. manihot have been investigated over the past decade. Hibifolin, isolated from A. manihot flowers, was incubated with human intestinal bacteria, and then four metabolites, namely gossypetin 8-O-β-D-4′-deoxy-Δ4′′-glucuropyranoside, quercetin, 8-methoxyquercetin, and gossypetin, were detected in the incubation solution on the basis of ultraviolet, MS, and nuclear magnetic resonance data (Xu et al., 2009). Pharmacokinetics studies were carried out after the administration of HKC at a dose of 0.75 g/kg (i.g.) for 10 consecutive days to rats in combination with 1 mg/kg glibenclamide, which was administered before the first time and after the last time that HKC was given. The results of this study showed that the plasma concentration of glibenclamide was markedly reduced when it was coadministered with HKC, and the maximum serum concentration and area under the concentration–time curve (AUC) significantly decreased, which suggests that HKC can decrease the absorption and accelerate the metabolism of glibenclamide by inducing the enzyme cytochrome P450 (Liu Z. X. et al., 2012). Therefore, when glibenclamide and HKC are combined to treat patients with nephropathy, the glibenclamide concentration in plasma should be carefully monitored (Liu Z. X. et al., 2012).
Guo et al. (2010) developed a rapid analytical method using ultra-performance liquid chromatography (UPLC)–quadrupole time-of-flight MS in combination with automated data analysis software to determine the metabolic profiles of flavonoids from A. manihot in plasma and urine samples from rats. The results showed that 16 and 38 metabolites were identified in plasma and urine, respectively, in comparison with the corresponding blank samples, which suggests that methylation and glucuronidation after deglycosylation are the main metabolic pathways of flavonoid glycosides (Guo et al., 2010). Xue et al. (2011a) used the same method to determine the metabolic profile of an A. manihot extract in intestinal flora. A total of 14 compounds, including six native chemical constituents and eight metabolites, were identified in incubation solutions from humans and rats. Taken together, these findings clearly suggest that flavonoids in A. manihot extracts can be metabolized in intestinal bacteria to generate metabolites via hydrolysis, hydroxylation, acetylation, methylation, and deoxygenation (Xue et al., 2011a). Xue et al. (2011b) also analyzed potential active agents in the blood and kidney of rats after oral administration of an A. manihot extract at a dose of 6.8 g/kg (equivalent to 5.8, 6.0, 12.2, and 5.1 mg/kg isoquercitrin, hyperoside, hibifolin, and quercetin-3′-O-glucoside, respectively) using microdialysis in combination with UPLC–quadrupole time-of-flight MS. The results showed that unbound compounds in rat blood included quercetin-3′-O-glucoside, hibifolin, hyperoside, myricetin, quercetin, isoquercitrin, and quercetin monoglucuronide, whereas unbound compounds in the kidney included hyperoside, isoquercitrin, and quercetin monoglucuronide (Xue et al., 2011b).
Cao et al. (2010) devised a method that used HPLC to determine the concentrations of quercetin-3′-O-glucoside, hyperoside, isoquercitrin, and hibifolin in rat plasma after i.g. and i.p. administration of extracts of A. manihot flowers to rats. The results showed that high linearity was achieved in the concentration range of 0.1–8 μg/mL for flavonoids in rat plasma, with a value of r > 0.99. The limit of quantification was 0.1 μg/mL and the recovery rate exceeded 70%. The intra- and inter-day relative standard deviations were both less than 15%. Pharmacokinetic parameters of flavonoids differed from each other after i.g. and i.p. administration. The absolute bioavailability of the above flavonoids was 12.9%, 10.8%, 2.2%, 10.2%, and 5.9%, respectively (Cao et al., 2010). Similarly, Guo et al. (2012) compared the effects of the two different administration routes on the brains of rats after i.g. and i.p. administration of hyperoside in vivo. Interestingly, pharmacokinetics results showed that hyperoside and its metabolite 3′-O-methylhyperoside were detected using UPLC–tandem mass spectrometry (MS/MS) and microdialysis in rat brains after i.p. administration but not after i.g. administration. Moreover, i.p. administration of hyperoside resulted in higher bioavailability and biological activity in rat brains. Furthermore, the forms of hyperoside present in rat brains were hyperoside and its methylated metabolite, with maximum concentrations of 63.78 ng/mL and 24.66 ng/mL, respectively, after i.p. administration at 20 mg/kg (Guo et al., 2012). These results might be used to further study the bioavailability of TFAM and establish a reasonable dosage for clinical use.
In addition, Guo et al. (2016) further investigated the pharmacokinetic profile of the flavonoid fraction of A. manihot at a dose of 400 mg/kg after oral administration to normal rats and a rat model of CKD. The results showed that the AUC values for quercetin glucuronide conjugates, isorhamnetin glucuronide conjugates, quercetin sulfate conjugates, and isorhamnetin sulfate conjugates in plasma from normal rats were 459.45, 1153.01, 417.81, and 2475.19 μmol h/L, respectively (Guo et al., 2016). In contrast, the AUC values for quercetin and isorhamnetin were 5.47 and 30.73 μmol h/L, respectively (Guo et al., 2016). Therefore, the AUC values for the glucuronide and sulfate conjugates of quercetin and isorhamnetin were approximately 125 times those for the corresponding aglycones (i.e., quercetin and isorhamnetin), which indicates that glucuronide and sulfate conjugates represent the main circulating forms of the flavonoid fraction of A. manihot in vivo (Guo et al., 2016). Moreover, the AUC values for isorhamnetin glucuronide conjugates and quercetin sulfate conjugates were 719.65 and 275.49 μmol h/L, respectively, which suggests that few conjugated metabolites were formed in rats with CKD in comparison with normal rats. The AUCglucuronide/sulfate/AUCaglycone ratio decreased from 125 to 104, which suggests that the ability to perform phase II metabolism was impaired in rats with CKD (Guo et al., 2016).
DN is one of the most common primary glomerular diseases worldwide and is a major cause of cardiovascular events. It has thus become a major threat to human health and cause of mortality, but effective therapies remain limited and many patients progress to end-stage renal disease (Balakumar et al., 2012). However, the use of traditional Chinese medicines and natural products has attracted the attention of scientists involved in research into kidney disease. The results of the first randomized controlled trial carried out to examine the safety and efficacy of HKC in patients with primary glomerular disease showed that HKC (at a dose of 2.5 g [i.g.[three times per day) significantly decreased 24 h urinary protein after treatment for 24 weeks. This suggests that HKC might be a promising agent for patients with primary kidney disease and moderate proteinuria, especially in CKD stages 1 and 2 (Carney, 2014; Zhang et al., 2014). Li et al. (2017) assessed the efficacy and safety of HKC for treating immunoglobulin A (IgA) nephropathy in a multicenter, prospective, double-blind, and double-dummy randomized controlled clinical trial by enrolling approximately 1600 patients with biopsy-proven IgA nephropathy at 100 centers and following them for up to 48 weeks. The results of this study indicated that HKC (at a dose of 2.5 g [i.g.] three times per day) markedly decreased 24 h urinary protein and the estimated glomerular filtration rate with respect to baseline after treatment for 48 weeks (Li et al., 2017). In addition, two systematic reviews and a meta-analysis were conducted to determine the safety and efficacy of HKC for the treatment of DN and type 2 DN, respectively. The results showed that HKC remarkably reduced 24 h urinary protein, proteinuria, the 24 h urinary protein reduction rate, the urinary albumin excretion rate, and serum creatinine levels; restored a normal urinary albumin excretion rate; and protected kidney function in patients with DN and type 2 DN (Chen et al., 2015; Chen et al., 2016; Shi et al., 2019). The results of these studies demonstrate that A. manihot is a potential promising candidate agent for the treatment of patients with CKD (Guo et al., 2015).
Although A. manihot has been used as a staple food or medicine for a long time, its systematic safety and toxicity remain unclear. Only two tests of the acute toxicity of A. manihot have been performed; specifically, by i.g. administration to Swiss albino mice and Wistar rats at a dose of 2.0 g/kg for 14 days. The results found no mortality or significant changes in the eyes; mucous membranes; skin; respiratory, circulatory, autonomic and central nervous systems; or in behavior, food intake, and body weight, which further proved that A. manihot is non-toxic (Jain et al., 2009; Pritam et al., 2011). Although these toxicity studies were conducted on animals, no safety assessments have been carried out on dogs, monkeys, or even humans in clinical practice.
Significantly, flavonoids are considered to be the major effective constituents of A. manihot. Lai et al. (2007) devised and validated a method for the simultaneous determination of five flavonols, namely isoquercitrin (1), hibifolin (2), myricetin (3), quercetin-3′-O-D-glucoside (4), and quercetin (5), in rat plasma and urine after oral administration of TFAM. Analysis of the plasma and urinary extracts was performed with a YMC-Pack ODS-A C18 column and a Thermo ODS-2 Hypersil C18 reversed-phase column, respectively. A mobile phase consisting of acetonitrile–0.1% phosphoric acid was employed. HPLC analysis was conducted with different elution gradients. The flow rate was 1.0 mL/min, and the detection wavelength was set at 370 nm. The calibration ranges for flavonols 2–5 in plasma were 0.011–2.220 μg/mL, 0.014–2.856 μg/mL, 0.022–4.320 μg/mL, and 0.028–5.600 μg/mL, respectively. In urine, the calibration ranges for flavonols 1, 2, 4, and 5 were 2.00–16.00 μg/mL, 8.56–102.72 μg/mL, 2.70–21.60 μg/mL, and 3.00–24.00 μg/mL, respectively. The intra- and inter-day relative standard deviations were less than 5.40% and 4.89%, respectively, for plasma and less than 3.96% and 6.85%, respectively, for urine (Lai et al., 2007).
Later, Lai et al. (2009) developed an HPLC method for the simultaneous quantification of seven flavonols, namely quercetin-3-O-robinobioside, hyperoside, isoquercitrin, hibifolin, myricetin, quercetin-3′-O-glucoside, and quercetin, in A. manihot flowers. HPLC separation was performed using a linear gradient at room temperature (25 °C) and a flow rate of 1.0 mL/min with a Thermo ODS-2 Hypersil reversed-phase column connected to a Phenomenex C18 guard column. The gradient elution started with a solution of acetonitrile and phosphoric acid (15 : 85, v/v), the acetonitrile content was increased to 40% within 40 min, and the detection wavelength was 370 nm. All seven flavonols were effectively separated and identified. The recovery rate achieved using this method was 94.31–107.08% with a relative standard deviation of ≤3.14%, and high linearity (R2 > 0.9996) was achieved for all the analytes (Lai et al., 2009).
In addition, Du et al. (2015a) developed a UPLC–triple-quadrupole MS/MS method to detect 12 nucleotides, nucleosides, and nucleobases present in the roots, stems, leaves, and flowers of A. manihot and successfully identified these 12 analytes in different parts of A. manihot harvested during 10 growth periods. The results showed that the distribution and concentration of the 12 compounds in the four plant parts occurred in decreasing order as follows: leaves > flowers > stems > roots, which indicates that the leaves and flowers of A. manihot might be developed in the future into health products with nutraceutical and bioactive properties. Moreover, this method could be used for the quality control of A. manihot leaves and other herbal medicines that are rich in nucleotides, nucleosides, and nucleobases (Du L. Y. et al., 2015). Subsequently, Pan et al. (2017) devised a rapid and highly sensitive UPLC–MS/MS method, which was successfully used for the simultaneous determination of five flavonoids (rutin, hyperoside, isoquercitrin, quercetin, and myricetin) in different parts of A. manihot harvested during 10 growth periods. The results indicated that the total contents of these five flavonoids in the roots, stems, leaves, and flowers of A. manihot ranged from 2.86 to 123.7 μg/g, 46.39 to 141.0 μg/g, 929.4 to 3096 μg/g, and 10 150 to 19 390 μg/g, respectively, which suggests that the total flavonoids were present in decreasing order as follows: flowers > leaves > stems > roots (Pan et al., 2017).
Wan et al. (2018) developed novel molecularly imprinted polymers (MIPs) based on bifunctional monomers and successfully isolated and identified myricetin from A. manihot. The results of this study revealed that these MIPs exhibited high adsorption ability and selectivity toward myricetin. Finally, the MIPs were employed as adsorbents for the solid-phase extraction of myricetin from safflower and the flowers of A. manihot. Further analysis was conducted using HPLC–diode array detection. The recovery of myricetin from safflower and the flowers of A. manihot ranged from 79.82% to 83.91% and from 81.50% to 84.32%, respectively, which suggests that MIPs can be used for the extraction and separation of myricetin from various complex matrices (Wan et al., 2019).
In a recent study, a highly efficient and ecofriendly extraction method using deep eutectic solvents was devised to isolate bioactive flavonoids from A. manihot. The extraction conditions were as follows: solid to solvent ratio, 35 : 1 (mg/mL); extraction time, 30 min; and extraction temperature, 30 °C. Moreover, qualitative and quantitative analyses were carried out using UPLC–MS/MS and HPLC. The results showed that the extraction efficiencies for hyperoside, isoquercitrin, and myricetin under the optimal extraction conditions were calculated to be 11.57 mg/g, 5.64 mg/g, and 1.11 mg/g, respectively, which were much higher than those achieved using traditional extraction solvents (Wan et al., 2019).
As an important traditional medicine in eastern Europe and Asia, the flowers of A. manihot have been used in a variety of clinical applications and also as a vegetable by local people for thousands of years. Numerous studies have been conducted and have shown that A. manihot flowers exhibit many biological effects, many of which are in accordance with their traditional uses. Flavonoids are used to monitor the quality of A. manihot, and the hyperoside content should be a minimum of 0.5% according to the 2015 edition of the Chinese Pharmacopoeia (Committee for the Pharmacopoeia of P.R. China, 2015). In traditional Chinese medicine, the flowers of A. manihot are mainly applied topically to treat CKD and related inflammatory diseases. In modern medical practice, A. manihot flowers exhibited promising renoprotective effects, and thus the medicinal value of A. manihot for the treatment of kidney diseases and syndromes has been fundamentally proved. In addition, many studies on chemical constituents and pharmacological effects have validated ethnomedicinal uses of A. manihot.
Taken together, A. manihot is a valuable medicinal resource with specific biological activities demonstrated in in vivo studies. However, there are also some shortcomings and further improvement and research are needed, as follows. (1) As important traditional Chinese medicines, the flowers, roots, stems, leaves, and seeds of A. manihot possess a wide range of biological properties. However, only the flowers of A. manihot are regarded as medicinal parts in the commonest modes of application, whereas the roots, stems, leaves, and seeds are discarded and burned, which undoubtedly causes serious environmental pollution and resource waste and also results in sharp reductions in resources of A. manihot. Further phytochemical studies of these parts are necessary, especially with regard to their polysaccharide constituents, which are used as stabilizers and thickeners in the food industry. (2) The flavonoids, as major active components, have received attention from scientists because of their renoprotective activity and high nutritional value, and further investigations into these active constituents should be a priority. Moreover, the mechanisms of action responsible for the anti-DN, anticonvulsant, antioxidant, antidepressant, anti-inflammatory, cardioprotective, and neuroprotective effects of these active flavonoids need to be scientifically investigated in depth to clarify the precise mechanisms responsible for their various activities both in vivo and in vitro. Meanwhile, efforts should be put into the elimination of false negatives, such as PAINS. (3) Although the flowers of A. manihot are listed in the 2015 edition of the Chinese Pharmacopoeia and are widely used in clinical practice, animal experiments and randomized clinical trials involving this plant and its derivatives are still in the exploratory stage, and the precise mechanisms responsible for their therapeutic effects remain unknown. Therefore, multicenter clinical trials with large samples need to be conducted and high-level clinical evidence needs to be obtained urgently to explain the mechanisms of action and to identify potential lead compounds for further development of A. manihot constituents and guarantee their safety and effectiveness in clinical practice. (4) HKC has been demonstrated to effectively alleviate proteinuria and improve kidney function in CKD in clinical practice and flavonoids are responsible for protecting renal function. However, the dosages of HKC used (7.5 g/day for an adult by oral administration) are still relatively high, which might affect patient compliance. Therefore, more advanced purification techniques need to be rapidly developed and more in-depth studies should be conducted to purify and enrich the flavonoids and thus enable a decrease in the dosage of the respective remedy obtained from A. manihot. For example, Box–Behnken response surface methodology experiments and static adsorption–desorption experiments should be employed to identify the optimal process parameters for the extraction of flavonoids, which would enable the further purification and enrichment of flavonoids, improve patient compliance, and eventually lead to effective treatment in clinical practice. (5) Importantly, large numbers of amino acids and nucleotides have been demonstrated to be present in the stems and leaves of A. manihot. Hence, the stems and leaves of A. manihot might be developed in the future into health products that possess nutraceutical and biological properties. (6) In regard to its toxicity, some studies have suggested that the A. manihot has low toxicity. To conclude, future investigations should include the identification of any side effects or toxicity. Meanwhile, more systematic and comprehensive investigations of pharmacokinetics, metabolism, and clinical settings will be indispensable to demonstrate the safety and efficacy of the main biologically active compounds from different medicinal parts of A. manihot before proceeding to the development of pharmaceutical formulations. (7) Future studies on A. manihot should focus on establishing the links between traditional uses, bioactive ingredients, and claimed pharmacological properties. We expect to obtain molecules with new skeletons and new activities from different parts of A. manihot.
QW, DL, and ZG obtained the literatures. FL, HL, and NZ wrote the manuscript. FL, HL, and YY revised the manuscript and NZ contributed ideas and edited the manuscript. All authors contributed to the article and approved the submitted version.
This study was financially supported by the National Natural Science Foundation of China (no. 81473399).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to Huachun Xu from http://www.plantphoto.cn for providing Figure 1.
CKD, chronic kidney disease; DN, diabetic nephropathy; HKC, Huangkui capsule; TCM, traditional Chinese medicine; HPLC, high-performance liquid chromatography; MS, mass spectrometry; TNF-α, tumor necrosis factor-α; TGF-β1, transforming growth factor-β1; p38MAPK, p38 mitogen-activated protein kinase; BUN, blood urea nitrogen; STZ, streptozotocin; Akt, serine–threonine kinase; PPAR-α/-γ, peroxisome proliferator-activated receptors-α; Alb, albumin; IL-1/1β/2/6/17/22, interleukin-1/1β/2/6/17/22; UP, urine protein; Scr, serum creatinine; NOX-1/2/4, NADPH oxidase 1/2/4; α-SMA, α-smooth muscle actin; p-ERK1/2, phosphorylation–extracellular signal-regulated kinase; CRF, chronic renal failure; PCNA, proliferating cell nuclear antigen; GBM, glomerular basement membrane; mTOR, mammalian target of rapamycin; p70S6K, 70-kDa ribosomal protein S6 kinase; PI3K, phosphatidylinositol-3-kinase; TFAM, total flavones of A. manihot; AGEs, advanced glycation end-products; TACE, tumor necrosis factor-a converting enzyme; ROS, reactive oxygen species; NLRP3, nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain containing-3; IR, ischemia–reperfusion; AKI, acute kidney injury; CAT, catalase; SOD, superoxide dismutase; T-AOC, total antioxidant capacity; MDA, malondialdehyde; GPx, glutathione peroxidase; DSS, dextran sulfate sodium; PSD, poststroke depression; HBV; hepatitis B virus; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; γ-GT, gamma glutamyltransferase; GST, glutathione transferase; TBIL, total bilirubin; DBIL, direct bilirubin; TBA, total bile acid; NO, nitric oxide; LDH, lactate dehydrogenase; CK, creatinine kinase; CAM, chick chorioallantoic membrane; VEGF, vascular endothelial growth factor; MCA, middle cerebral artery; EMT, epithelial–mesenchymal transition; BMD, bone mineral density; BMC, bone mineral content; OPA1, optic atrophy 1; NMR, nuclear magnetic resonance; UPLC-Q-TOF/MS, ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry; UPLC-MS/MS, ultra-high-performance liquid chromatography coupled with tandem mass spectrometry; ESRD, end-stage renal disease; eGFR, estimated glomerular filtration rate; HPLC-DAD, high-performance liquid chromatography-diode array detection; MIPs, molecularly imprinted polymers; TST, tail suspension test; FST, forced swimming test; BDNF, brain-derived neurotrophic factor; TrkB, tyrosine receptor kinase; p-eEF2, phosphorylation eukaryotic elongation factor 2; CREB, cAMP-response element-binding protein.
Ai, G., Liu, Q. C., Hua, W., Huang, Z. M., Wang, D. W. (2013). Hepatoprotective evaluation of the total flavonoids extracted from flowers of Abelmoschus manihot (L.) Medic: in vitro and in vivo studies. J. Ethnopharmacol. 146, 794–802. doi: 10.1016/j.jep.2013.02.005
Almazan, A. M., Theberge, R. L. (1989). Influence of cassava mosaic virus on cassava leaf-vegetable quality. Trop. Agric. 66, 305–308. doi: 10.1111/j.1744-7348.1989.tb03396.x
An, Y. T., Zhang, Y., Li, C. M., Qian, Q., He, W., Wang, T. (2011). Inhibitory effects of flavonoids from Abelmoschus manihot flowers on triglyceride accumulation in 3T3-L1 adipocytes. Fitoterapia 82, 595–600. doi: 10.1016/j.fitote.2011.01.010
Baell, J. B., Holloway, G. A. (2010). New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 53, 2719–2740. doi: 10.1021/jm901137j
Baell, J. B., Nissink, J. W. M. (2018). Seven Year Itch: Pan-Assay Interference Compounds (PAINS) in 2017-Utility and Limitations. ACS Chem. Biol. 13, 36–44. doi: 10.1021/acschembio.7b00903
Baell, J., Walters, M. A. (2014). Chemistry: Chemical con artists foil drug discovery. Nature 513, 481–483. doi: 10.1038/513481a
Baell, J. B. (2016). Feeling Nature’s PAINS: Natural Products, Natural Product Drugs, and Pan Assay Interference Compounds (PAINS). J. Nat. Prod. 79, 616–628. doi: 10.1021/acs.jnatprod.5b00947
Balakumar, P., Kadian, S., Mahadevan, N. (2012). Are PPAR alpha agonists a rational therapeutic strategy for preventing abnormalities of the diabetic kidney? Pharmacol. Res. 65, 430–436. doi: 10.1016/j.phrs.2012.01.004
Bourdy, G., Walter, A. (1992). Maternity and medicinal plants in Vanuatu. I. The cycle of reproduction. J. Ethnopharmacol. 37, 179–196. doi: 10.1016/0378-8741(92)90033-n
Cai, H. D., Su, S. L., Qian, D. W., Guo, S., Tao, W. W., Cong, X. D., et al. (2017a). Renal protective effect and action mechanism of Huangkui capsule and its main five flavonoids. J. Ethnopharmacol. 206, 152–159. doi: 10.1016/j.jep.2017.02.046
Cai, H. D., Tao, W. W., Su, S. L., Guo, S., Zhu, Y., Guo, J. M., et al. (2017b). Antidepressant activity of flavonoid ethanol extract of Abelmoschus manihot corolla with BDNF up-regulation in the hippocampus. Acta Pharm. Sin. 52 (2), 222–228. doi: 10.16438/j.0513-4870.2016-0764
Cao, X. S., Sha, M., Ou, Y. Q., Pan, J. H., Lin, P. (2010). In vivo integrated pharmacokinetics of four flavonoids of Abelmoschus manihot extract in rats. Chin. Tradit. Herb. Drugs 41, 255–259.
Carney, E. F. (2014). Antiproteinuric efficacy of A. manihot superior to losartan. Nat. Rev. Nephrol. 10, 300. doi: 10.1038/nrneph.2014.63
Chen, G., Zhang, H. J., Tu, A. P., Dong, J. X. (2007). Studies on the chemical constituents of Abelmoschus manihot. Chin. Tradit. Herb. Drugs 38, 827–828.
Chen, P., Wan, Y. G., Wang, C. J., Zhao, Q., Wei, Q. X., Tu, X., et al. (2012). Mechanisms and effects of Abelmoschus manihot preparations in treating chronic kidney disease. China J. Chin. Mater. Med. 37, 2252–2256. doi: 10.4268/cjcmm20121514
Chen, Y. Z., Gong, Z. X., Cai, G. Y., Gao, Q., Chen, X. M., Tang, L., et al. (2015). Efficacy and safety of Flos Abelmoschus manihot (Malvaceae) on type 2 diabetic nephropathy: A systematic review. Chin. J. Integr. Med. 21, 464–472. doi: 10.1007/s11655-014-1891-6
Chen, Y. Z., Cai, G. Y., Sun, X. F., Chen, X. M. (2016). Treatment of chronic kidney disease using a traditional Chinese medicine, Flos Abelmoschus manihot (Linnaeus) Medicus (Malvaceae). Clin. Exp. Pharmacol. Physiol. 43, 145–148. doi: 10.1111/1440-1681.12528
Chen, G. (2006). Studies on the chemical constituents and antihyperglycemic action of Abelmoschus manihot L. Medic [D]. PLA Acad. Milit. Med. Sci.
Cheng, X. P., Qin, S., Dong, L. Y., Zhou, J. N. (2006). Inhibitory effect of total flavone of Abelmoschus manihot L. Medic on NMDA receptor-mediated current in cultured rat hippocampal neurons. Neurosci. Res. 55, 142–145. doi: 10.1016/j.neures.2006.02.011
Chi, Y. M., Zhu, H. Y., Ju, L., Zhang, Y., Shen, X. N., Hua, X. Y., et al. (2009). Analysis of flavonols compounds in Flos Abelmoschus manihot by high performance liquid chromatography-electrospray ionization/quadrupole-time of flight-mass/mass spectrometry. Chin. J. Anal. Chem. 37, 227–231.
Committee for the Pharmacopoeia of P.R. China (2015). Pharmacopoeia of P. R. China Vol. 1 (China: China Medical Science and Technology Press), 306.
Dhiman, R. K., Chawla, Y. K. (2005). Herbal medicines for liver diseases. Dig. Dis. Sci. 50, 1807–1812. doi: 10.1007/s10620-005-2942-9
Du, L. Y., Qian, D. W., Jiang, S., Shang, E. X., Guo, J. M., Liu, P., et al. (2015). Comparative characterization of nucleotides, nucleosides and nucleobases in Abelmoschus manihot roots, stems, leaves and flowers during different growth periods by UPLC-TQ-MS/MS. J. Chromatogr. B. 1006, 130–137. doi: 10.1016/j.jchromb.2015.10.021
Du, L., Qian, D., Jiang, S., Guo, J., Su, S., Duan, J. (2015). Comparative characterization of amino acids in Abelmoschus manihot roots, stems and leaves during different growth periods by UPLC-TQ-MS/MS. Anal. Methods 7, 10280–10290. doi: 10.1039/C5AY02103K
Du, L. Y., Tao, J. H., Jiang, S., Qian, D. W., Guo, J. M., Duan, J. A. (2017). Metabolic profiles of the Flos Abelmoschus manihot extract by intestinal bacteria from the normal and CKD model rats based on UPLC-Q-TOF/MS. Biomed. Chromatogra. 31, 1–12. doi: 10.1002/bmc.3795
Fan, L., Dong, L. Y., Chen, Z. W., Cen, D. Y., Jiang, Q., Ma, C. G. (2003). Analgesic effect of total flavone of Abelmoschl manihot L medic. Pharmacol. Clin. Chin. Mater. Med. 19, 12–14. doi: 10.13412/j.cnki.zyyl.2003.01.007
Fan, L., Chen, Z. W., Ma, C. G. (2006). Protective effects of pharmacological preconditioning of total flavone of Ablemoschl manihot L. Medic on myocardial reperfusion injury in rabbits. Chin. Pharmacol. Bull. 22, 106–109.
Fan, Y. F., Chen, Z. W., Guo, Y., Wang, Q. H., Song, B. (2011). Cellular mechanisms underlying hyperin-induced relaxation of rat basilar artery. Fitoterapia 82, 626–631. doi: 10.1016/j.fitote.2011.01.023
Gao, S., Dong, L. Y., Cen, D. Y., Jiang, Q., Fang, M., Chen, Z. W. (2002). Protection effects of the total flavone from Abelmoschus manihot L medic on cerebral ischemia injure. Chin. J. Basic Med. Tradit. Chin. Med. 8, 19–20.
Gao, S., Fan, L., Dong, L. Y., Cen, D. Y., Jiang, Q., Fang, M., et al. (2003). Protection effects of the total flavone from Abelmoschus manihot L medic on acute incompletely cerebral ischemia in rats. Chin. J. Clin. Pharmacol. Ther. 8, 167–169.
Ge, J., Miao, J. J., Sun, X. Y., Yu, J. Y. (2016). Huangkui capsule, an extract from Abelmoschus manihot (L.) medic, improves diabetic nephropathy via activating peroxisome proliferator-activated receptor (PPAR)-α/γ and attenuating endoplasmic reticulum stress in rats. J. Ethnopharmacol. 189, 238–249. doi: 10.1016/j.jep.2016.05.033
Ghanem, A. A., Arafa, L. F., El-Baz, A. (2010). Oxidative stress markers in patients with primary open-angle glaucoma. Curr. Eye Res. 35, 295–301. doi: 10.3109/02713680903548970
Guo, Y., Chen, Z. W. (2002). Protective effect of Abelmischl manihot l. medic against cerebral ischemia-reperfusion injury. Chin. Pharmacol. Bull. 18, 692–695.
Guo, Y., Fan, L., Dong, L. Y., Chen, Z. W. (2005). Effects of total flavone of Abelmoschl manihot L. Medic on the function of platelets and its mechanism. Chin. J. Integr. Med. 11, 57–59. doi: 10.1007/BF02835752
Guo, J., Shang, E. X., Duan, J. A., Tang, Y., Qian, D., Su, S. (2010). Fast and automated characterization of major constituents in rat biofluid after oral administration of Abelmoschus manihot extract using ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry and MetaboLynx. Rapid. Commun. Mass Spectrom. 24, 443–453. doi: 10.1002/rcm.4416
Guo, J. M., Xue, C. F., Duan, J. A., Qian, D. W., Tang, Y. P., You, Y. (2011). Anticonvulsant, antidepressant-like activity of Abelmoschus manihot ethanol extract and its potential active components in vivo. Phytomedicine 18, 1250–1254. doi: 10.1016/j.phymed.2011.06.012
Guo, J. M., Lin, P., Duan, J. A., Shang, E. X., Qian, D. W., Tang, Y. P. (2012). Application of microdialysis for elucidating the existing form of hyperoside in rat brain: comparison between intragastric and intraperitoneal administration. J. Ethnopharmacol. 144, 664–670. doi: 10.1016/j.jep.2012.10.008
Guo, J. M., Lin, P., Lu, Y. W., Duan, J. A., Shang, E. X., Qian, D. W., et al. (2013). Investigation of in vivo metabolic profile of Abelmoschus manihot based on pattern recognition analysis. J. Ethnopharmacol. 148, 297–304. doi: 10.1016/j.jep.2013.04.029
Guo, J. M., Lu, Y. W., Shang, E. X., Li, T., Liu, Y., Duan, J. A., et al. (2015). Metabolite identification strategy of non-targeted metabolomics and its application for the identification of components in Chinese multicomponent medicine Abelmoschus manihot L. Phytomedicine 22, 579–587. doi: 10.1016/j.phymed.2015.02.002
Guo, J. M., Du, L. Y., Shang, E. X., Li, T., Liu, Y., Qian, D. W., et al. (2016). Conjugated metabolites represent the major circulating forms of Abelmoschus manihot in vivo and show an altered pharmacokinetic profile in renal pathology. Pharm. Biol. 54, 595–603. doi: 10.3109/13880209.2015.1068337
Han, Y. R., Qiu, Z. Y. (2010). Clinical study of huang kui capsule combined with benazepril in the treatment of primary IgA nephropathy. Chin. J. Integr. Tradit. West. Nephrol. 11, 998–999.
Hao, J. L., Zhou, L. L., Si, L., Jiang, Q. H., Ma, Y., Yan, H. Y. (2007). Effects of total flavone of Abelmoschus manihot L. Medic on post-stroke depression in rats. China Pharm. 18, 885–887.
He, X. R., Luan, F., Zhao, Z. F., Ning, N., Li, M. X., Jin, L., et al. (2017). The genus Patrinia: A review of traditional uses, phytochemical and pharmacological Studies. Am. J. Chin. Med. 45, 637–666. doi: 10.1142/S0192415X17500379
Heinrich, M., Appendino, G., Efferth, T., Fürst, R., Izzo, A. A., Kayser, O., et al. (2020). Best practice in research-Overcoming common challenges in phytopharmacological research. J. Ethnopharmacol. 246:112230. doi: 10.1016/j.jep.2019.112230
Hou, J. H., Qian, J. J., Li, Z. L., Gong, A. X., Zong, S. X., Qiao, L., et al. (2020). Bioactive compounds from Abelmoschus manihot L. alleviate the progression of multiple myeloma in mouse model and improve bone marrow microenvironment. Onco. Targets Ther. 13, 959–973. doi: 10.2147/OTT.S235944
Jain, P. S., Bari, S. B. (2010). Anti-inflammatory activity of Abelmoschus manihot extracts. Int. J. Pharmacol. 6, 505–509. doi: 10.3923/ijp.2010.505.509
Jain, P. S., Bari, S. B., Surana, S. J. (2009). Isolation of stigmasterol and γ-sitosterol from petroleum ether extract of woody stem of Abelmoschus manihot. Asian J. Biol. Sci. 2, 112–117. doi: 10.3923/ajbs.2009.112.117
Jarret, R. L., Wang, M. L., Levy, I. J. (2011). Seed oil and fatty acid content in okra (Abelmoschus esculentus) and related species. J. Agric. Food Chem. 59, 4019–4024. doi: 10.1021/jf104590u
Jiang, Y. R., Zhang, Z. Y., Wen, J., Li, Q., Zhang, C. L., Xie, X. S., et al. (2012). Effects of okra capsule for IgA nephropathy: a systematic review. Chin. J. Evid. Based Med. 12, 1135–1140.
Kim, H., Dusabimana, T., Kim, S. R., Je, J., Jeong, K., Kang, M. C., et al. (2018). Supplementation of Abelmoschus manihot ameliorates diabetic nephropathy and hepatic steatosis by activating autophagy in mice. Nutrients 10:1703. doi: 10.3390/nu10111703
Kubo, I., Masuoka, N., Nihei, K., II, Burgheim, B. (2006). Maniçoba, a quercetin-rich Amazona dish. J. Food Compos. Anal. 19, 579–588. doi: 10.1016/j.jfca.2006.02.007
Lai, X. Y., Zhao, Y. Y., Liang, H. (2006). Studies on chemical constituents in flower of Abelmoschus manihot. China J. Chin. Mater. Med. 31, 1597–1600.
Lai, X. Y., Zhao, Y. Y., Liang, H., Bai, Y. J., Wang, B., Guo, E. A. (2007). SPE-HPLC method for the determination of four flavonols in rat plasma and urine after oral administration of Abelmoschus manihot extract. J. Chromatogr. B. 852, 108–114. doi: 10.1016/j.jchromb.2006.12.043
Lai, X. Y., Liang, H., Zhao, Y. Y., Wang, B. (2009). Simultaneous determination of seven active flavonols in the flowers of Abelmoschus manihot by HPLC. J. Chromatogr. Sci. 47, 206–210. doi: 10.1093/chromsci/47.3.206
Li, Q. L., Wang, C. Y., Peng, D. Y., Meng, G., Chen, Z. W., Ma, C. G. (2006). Protective effects of total flavone from the flowers of Ablemoschl manihot L. Medic on the myocardial ischemia reperfusion injury. Chin. J. Exp. Tradit. Med. Formul. 12, 39–42.
Li, C. M., Wang, T., Zhang, W., Gao, X. M., Li, T. X. (2010a). Isolation and identification of chemical constituents of flowers of Abelmoschus manihot (L.) Medic. Flos (I). J. Shenyang Pharm. Univ. 27, 711–714.
Li, C. M., Wang, T., Zhang, W., Han, L. F., Liu, E. W., Gao, X. M. (2010b). Isolation and identification of chemical constituents from the flowers of Abelmoschus manihot (L.) Medic. (II). J. Shenyang Pharm. Univ. 27, 803–807.
Li, C. M., An, Y. T., Wang, T., Shang, H. W., Gao, X. M., Zhang, W. (2011). Isolation and identification of chemical constituents from the flowers of Abelmoschus manihot (L.) Medic (III). J. Shenyang Pharm. Univ. 28, 520–525.
Li, J. J., Zhang, J., Wang, M. (2016). Extraction of flavonoids from the flowers of Abelmoschus manihot (L.) Medic by modified supercritical CO2 extraction and determination of antioxidant and anti-adipogenic activity. Molecules 21:810. doi: 10.3390/molecules21070810
Li, P., Chen, Y. Z., Lin, H. L., Ni, Z. H., Zhan, Y. L., Wang, R., et al. (2017). Abelmoschus manihot-a traditional Chinese medicine versus losartan potassium for treating IgA nephropathy: study protocol for a randomized controlled trial. Trials 18, 170. doi: 10.1186/s13063-016-1774-6
Li, W., He, W. M., Xia, P., Sun, W., Shi, M., Zhou, Y., et al. (2019). Total extracts of Abelmoschus manihot L. attenuates adriamycin-induced renal tubule injury via suppression of ROS- ERK1/2-mediated NLRP3 inflammasome activation. Front. Pharmacol. 10:567. doi: 10.3389/fphar.2019.00567
Liao, J. C., Deng, J. S., Chiu, C. S., Huang, S. S., Hou, W. C., Lin, W. C., et al. (2012). Chemical compositions, anti-inflammatory, antiproliferative and radical-scavenging activities of Actinidia callosavar ephippioides. Am. J. Chin. Med. 40, 1047–1062. doi: 10.1142/S0192415X12500772
Lin, W. Q., Chen, Z., Chen, J. L., Wu, H. P., Liu, J. Q. (2002). Study on the morphology and chemical composition of Abelmoschus manihot (L.) seeds. Nat. Prod. Res. Dev. 14, 41–44. doi: 10.16333/j.1001-6880.2002.03.012
Lin, J., Liang, Q., Jiang, F., Li, M., An, C. H. (2018). Determination of flavonoids fingerprints and content of Abelmoschus manihot leaf in different origin in Guizhou. J. Chin. Med. Mater. 41, 317–321.
Liu, M., Jiang, Q. H., Hao, J. L., Zhou, L. L. (2009). Protective effect of total flavones of Abelmoschus manihot L. Medic against poststroke depression injury in mice and its action mechanism. Anat. Rec. 292, 412–422. doi: 10.1002/ar.20864
Liu, S., Jiang, W. X., Wu, B. (2010). Advance research on chemical constituents and pharmacological activities of Abelmoschus manihot (L.) Medic. Modern Chin. Med. 12, 5–9. doi: 10.13313/j.issn.1673-4890.2010.08.006
Liu, Z. Z., Kong, X. J., Liu, Y. S. (2011). Effect of Huangkui capsule on serum Lkn-1 and TNF-α of patients with chronic glomerulonephritis. Chin. J. Integr. Tradit. West. Nephrol. 12, 78–79.
Liu, R., Ji, J., Wang, L. C., Chen, S. Y., Guo, S., Wu, H. (2012). Characterisation of nucleosides and nucleobases in Mactra veneriformis by high performance liquid chromatography coupled with diode array detector-mass spectrometry (HPLC-DAD-MS). Food Chem. 135, 548–554. doi: 10.1016/j.foodchem.2012.05.019
Liu, Z. X., Liu, S. J., Zhou, L., Gao, X. J., Ju, W. Z., Tan, H. S., et al. (2012). Effects of HuangKui capsules on glibenclamide pharmacokinetics in rats. J. Ethnopharmacol. 139, 1–5. doi: 10.1016/j.jep.2011.03.043
Liu, J., Guo, S., Duan, J. A., Yan, H., Qian, D. W., Tang, H. T., et al. (2016). Analysis and utilization value discussion of multiple chemical composition in different tissues of Abelmoschus manihot. China J. Chin. Mater. Med. 41, 3782–3791.
Liu, S., Ye, L. F., Tao, J., Ge, C., Huang, L. J., Yu, J. Y. (2018). Total flavones of Abelmoschus manihot improve diabetic nephropathy by inhibiting the iRhom2/TACE signalling pathway activity in rats. Pharm. Biol. 56, 1–11. doi: 10.1080/13880209.2017.1412467
Lv, M., Chen, Z., Hu, G., Li, Q. (2015). Therapeutic strategies of diabetic nephropathy: recent progress and future perspectives. Drug Discov. Today 20, 332–346. doi: 10.1016/j.drudis.2014.10.007
Lv, D. L., Cheng, X. H., Tang, L. Y., Jiang, M. (2017). The cardioprotective effect of total flavonoids on myocardial ischemia/reperfusion in rats. Biomed. Pharmacother. 88, 277–284. doi: 10.1016/j.biopha.2017.01.060
Mao, Z. M., Shen, S. M., Wan, Y. G., Sun, W., Chen, H. L., Huang, M. M., et al. (2015). Huangkui capsule attenuates renal fibrosis in diabetic nephropathy rats through regulating oxidative stress and p38MAPK/Akt pathways, compared to α-lipoic acid. J. Ethnopharmacol. 173, 256–265. doi: 10.1016/j.jep.2015.07.036
Miladiyah, I., Dayi, F., Desrini, S. (2011). Analgesic activity of ethanolic extract of Manihot esculenta Crantz leaves in mice. Univ. Med. 30, 3–10.
Nemeroff, C. B. (2007). The burden of severe depression: a review of diagnostic challenges and treatment alternatives. J. Psychiatr. Res. 41, 189–206. doi: 10.1016/j.jpsychires.2006.05.008
Pan, X. X., Du, L. Y., Tao, J. H., Jiang, S., Qian, D. W., Duan, J. N. (2017). Dynamic changes of flavonoids in Abelmoschus manihot different organs at different growth periods by UPLC-MS/MS. J. Chromatogr. B. 1059, 21–26. doi: 10.1016/j.jchromb.2017.05.020
Pan, X. X., Tao, J. H., Jiang, S., Zhu, Y., Qian, D. W., Duan, J. A. (2018). Characterization and immunomodulatory activity of polysaccharides from the stems and leaves of Abelmoschus manihot and a sulfated derivative. Int. J. Biol. Macromol. 107, 9–16. doi: 10.1016/j.ijbiomac.2017.08.130
Park, J. H., Cho, S. E., Hong, S. H., Shin, H. D. (2015). Choanephora flower rot caused by choanephora cucurbitarum on Abelmoschus manihot. Trop. Plant Pathol. 40, 147–149. doi: 10.1007/s40858-015-0016-x
Peng, T., Yang, X. D., Li, D. R., Guo, L., Xia, Q., Hu, Z. (2010). Observation of effect of Huang Kui capsule combined with valsartan in the treatment of IgA nephropathy. Chin. J. Integr. Tradit. West. Nephrol. 11, 723–724.
Prabawardani, S., Djuuna, I. A. F., Asyerem, F., Yaku, A., Lyons, G. (2016). Morphological diversity and the cultivation practice of Abelmoschus manihot in West Papua, Indonesia. Biodiversitas 17, 894–999. doi: 10.13057/biodiv/d170267
Pritam, S. J., Amol, A. T., Sanjay, B. B., Sanjay, J. S. (2011). Analgesic activity of Abelmoschus manihot extracts. Int. J. Pharmacol. 7, 716–720. doi: 10.3923/ijp.2011.716.720
Puel, C., Mathey, J., Kati-Coulibaly, S., Davicco, M. J., Lebecque, P., Chanteranne, B., et al. (2005). Preventive effect of Abelmoschus manihot (L.) Medik. on bone loss in the ovariectomised rats. J. Ethnopharmacol. 99, 55–60. doi: 10.1016/j.jep.2005.01.047
Qiu, Y., Ai, P. F., Song, J. J., Liu, C., Li, Z. W. (2017). Total flavonoid extract from Abelmoschus manihot (L.) Medic flowers attenuates D-galactose-induced oxidative stress in mouse liver through the nrf2 pathway. J. Med. Food 20, 557–567. doi: 10.1089/jmf.2016.3870
Rao, K. S., Dominic, R., Singh, K., Kaluwin, C., Rivett, D. E., Jones, G. P. (1990). Lipid, fatty acid, amino acid, and mineral compositions of five edible plant leaves. J. Agric. Food Chem. 38, 2137–2139. doi: 10.1021/jf00102a007
Refaey, E. M., Watkins, C. P., Kennedy, E. J., Chang, A., Zhong, Q., Ding, K. H., et al. (2015). Oxidation of the aromatic amino acids tryptophan and tyrosine disrupts their anabolic effects on bone marrow mesenchymal stem cells. Mol. Cell. Endocrinol. 410, 87–96. doi: 10.1016/j.mce.2015.01.034
Rubiang-Yalambing, L., Arcot, J., Greenfield, H., Holford, P. (2016). Aibika (Abelmoschus manihot L.): genetic variation, morphology and relationships to micronutrient composition. Food Chem. 193, 62–68. doi: 10.1016/j.foodchem.2014.08.058
Shi, L. W., Feng, L., Zhang, M. Z., Li, X. W., Yang, Y. A., Zhang, Y. Y., et al. (2019). Abelmoschus manihot for diabetic nephropathy: a systematic review and meta-analysis. Evid. Based Complement. Alternat. Med. 2019:9679234. doi: 10.1155/2019/9679234
Stickel, F., Schuppan, D. (2007). Herbal medicine in the treatment of liver diseases. Dig. Liver Dis. 39, 293–304. doi: 10.1016/j.dld.2006.11.004
Taroreh, M., Raharjo, S., Hastuti, P., Murdiati, A. (2016). Antioxidative activities of various fractions of Gedi’s leaf extracts (Abelmoschus manihot L. Medik). Agric. Agric. Sci. Proc. 9, 271–278. doi: 10.1016/j.aaspro.2016.02.112
Todarwal, A., Jain, P. S., Bari, S. B. (2011). Abelmoschus manihot Linn: ethnobotany, phytochemistry and pharmacology. Asian J. Tradit. Med. 6, 1–7.
Trendafilova, A., Todorova, M., Nikolova, M., Gavrilova, A., Vitkova, A. (2011). Flavonoid constituents and free radical scavenging activity of Alchemilla mollis. Nat. Prod. Commun. 6, 1851–1854. doi: 10.1177/1934578X1100601216
Tsumbu, C. N., Deby-Dupont, G., Tits, M., Angenot, L., Franck, T., Serteyn, D., et al. (2011). Antioxidant and antiradical activities of Manihot esculenta crantz (Euphorbiaceae) leaves and other selected tropical green vegetables investigated on lipoperoxidation and phorbol-12- myristate-13-acetate (PMA) activated monocytes. Nutrients 3, 818–838. doi: 10.3390/nu3090818
Tsumbu, C. N., Deby-Dupont, G., Tits, M., Angenot, L., Frederich, M., Kohnen, S., et al. (2012). Polyphenol content and modulatory activities of some tropical dietary plant extracts on the oxidant activities of neutrophils and myeloperoxidase. Int. J. Mol. Sci. 13, 628–650. doi: 10.3390/ijms13010628
Tu, Y., Sun, W., Wan, Y. G., Che, X. Y., Pu, H. P., Yin, X. J., et al. (2013). Huangkui capsule, an extract from Abelmoschus manihot (L.) medic, ameliorates adriamycin-induced renal inflammation and glomerular injury via inhibiting p38MAPK signaling pathway activity in rats. J. Ethnopharmacol. 147, 311–320. doi: 10.1016/j.jep.2013.03.006
Wan, Y. Y., Wang, M., Fu, Q. F., Wang, L. J., Wang, D. D., Zhang, K. L., et al. (2018). Novel dual functional monomers based molecularly imprinted polymers for selective extraction of myricetin from herbal medicines. J. Chromatogr. B., 1097–1098, 1–9. doi: 10.1016/j.jchromb.2018.08.033
Wan, Y. Y., Wang, M., Zhang, K. L., Fu, Q. F., Wang, L. J., Gao, M. J., et al. (2019). Extraction and determination of bioactive flavonoids from Abelmoschus manihot (Linn.) Medicus flowers using deep eutectic solvents coupled with high-performance liquid chromatography. J. Sep. Sci. 42, 2044–2052. doi: 10.1002/jssc.201900031
Wang, X. C., Gao, F. (2010). Observation of effect of Huang Kui capsule combined with valsartan in the treatment of diabetic nephropathy. J. Hebei Med. Univ. 31, 733–734.
Wang, X. R., Wang, Z. Q., Li, Y. (1981). Studies on the chemical constituents of Abelmoschus manihot L. Medic. Acta Bot. Sin. 23, 222–227.
Wang, X. R., Zhou, Z. H., Du, A. Q., Huang, Z. M. (2004). Studies on the flavonol constituents of Abelmoschus manihot L. Medic. Chin. J. Nat. Med. Mar. 2, 91–93.
Wang, L., Xu, R. J., Hu, B., Li, W., Sun, Y., Tu, Y. Y., et al. (2010). Analysis of free amino acids in Chinese teas and flower of tea plant by high performance liquid chromatography combined with solid-phase extraction. Food Chem. 123, 1259–1266. doi: 10.1016/j.foodchem.2010.05.063
Wen, J. Y., Chen, Z. W. (2007). Protective effect of pharmacological preconditioning of total flavones of Abelmoschl manihot on cerebral ischemic reperfusion injury in rats. Am. J. Chin. Med. 35, 653–661. doi: 10.1142/S0192415X07005144
Wen, R., Xie, G. Y., Li, X. S., Qin, B. J. (2015). Advance research on chemical constituents and pharmacological activities of Abelmoschus manihot (L.) Medic. Chin. Wild Plant Resour. 34, 37–44. doi: 10.3969/j.issn.1006-9690.2015.02.010
Wu, L. L., Yang, X. B., Huang, Z. M., Liu, H. Z., Wu, G. X. (2007). In vivo and in vitro antiviral activity of hyperoside extracted from Abelmoschus manihot (L) medik. Acta Pharmacol. Sin. 28, 404–409. doi: 10.1111/j.1745-7254.2007.00510.x
Wu, L. M., Chen, L. Z., Selvaraj, J. N., Wei, Y., Wang, Y., Li, Y., et al. (2015). Identification of the distribution of adenosine phosphates, nucleosides and nucleobases in royal jelly. Food Chem. 2015 (173), 1111–1118. doi: 10.1016/j.foodchem.2014.10.137
Wu, W., Hu, W., Han, W. B., Liu, Y. L., Tu, Y., Yang, H. M., et al. (2018). Inhibition of Akt/mTOR/p70S6K signaling activity with Huangkui capsule alleviates the early glomerular pathological changes in diabetic nephropathy. Front. Pharmacol. 9, 443. doi: 10.3389/fphar.2018.00443
Wu, J. F., Liang, Q., Jiang, F., Li, M., An, C. H., Lin, J. (2019). Identification of fatty acid and determination of three constituents from the seeds of Abelmoschus manihot. Chin. Tradit. Pat. Med. 32, 1–7.
Wu, L., Li, Q., Liu, S. M., An, X. F., Huang, Z. M., Zhang, B., et al. (2019). Protective effect of hyperoside against renal ischemia-reperfusion injury via modulating mitochondrial fission, oxidative stress, and apoptosis. Free Radic. Res. 53, 727–736. doi: 10.1080/10715762.2019.1623883
Xia, K. Y., Zhang, C. L., Cao, Z. Y., Ge, H. T., Tang, H. T. (2019). Chemical constituents from Corolla abelmoschi. J. Strait Pharm. 31, 58–61.
Xu, T. T., Yang, X. W., Wang, B., Xu, W., Zhao, Y. Y., Zhang, Q. Y. (2009). Metabolism of hibifolin by human intestinal bacteria. Planta Med. 75, 483–487. doi: 10.1055/s-0029-1185317
Xue, C. F., Jiang, S., Guo, J. M., Qian, D. W., Duan, J. A., Shang, E. X. (2011a). Screening for in vitro metabolites of Abelmoschus manihot extract in intestinal bacteria by ultra- performance liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Chromatogr. B. 879, 3901–3908. doi: 10.1016/j.jchromb.2011.10.043
Xue, C. F., Guo, J. M., Qian, D. W., Duan, J. A., Shang, E. X., Shu, Y., et al. (2011b). Identification of the potential active components of Abelmoschus manihot in rat blood and kidney tissue by microdialysis combined with ultra-performance liquid chromatography/ quadrupole time-of-flight mass spectrometry. J. Chromatogr. B. 879, 317–325. doi: 10.1016/j.jchromb.2010.12.016
Yan, J. Y., Ai, G., Zhang, X. J., Xu, H. J., Huang, Z. M. (2015). Investigations of the total flavonoids extracted from flowers of Abelmoschus manihot (L.) Medic against α- naphthylisothiocyanate-induced cholestatic liver injury in rats. J. Ethnopharmacol. 172, 202–213. doi: 10.1016/j.jep.2015.06.044
Yang, G., Zhang, M., Zhang, M., Chen, S., Chen, P. (2015). Effect of huangshukuihua (flos Abelmoschi manihot) on diabetic nephropathy: A meta-analysis. J. Tradit. Chin. Med. 35, 15–20. doi: 10.1016/s0254-6272(15)30003-0
Yang, S. Y., Li, Z. L., Zhao, Y. M., Zuo, Q. Y., Lv, X. H., Wang, X. J., et al. (2017). Chemical constituents from flowers of Abelmoschus manihot. Chin. Tradit. Herb. Drugs 48, 2827–2831. doi: 10.7501/j.issn.0253-2670.2017.14.004
Yang, Z. Z., Tang, H. T., Shao, Q., Bilia, A. R., Wang, Y., Zhao, X. P. (2018). Enrichment and purification of the bioactive flavonoids from flower of Abelmoschus manihot (L.) Medic using macroporous resins. Molecules 23:2649. doi: 10.3390/molecules23102649
Yang, B. L., Zhu, P., Li, Y. R., Xu, M. M., Wang, H., Qiao, L. C., et al. (2018). Total flavone of Abelmoschus manihot suppresses epithelial-mesenchymal transition via interfering transforming growth factor-β1 signaling in Crohn’s disease intestinal fibrosis. World J. Gastroenterol. 24, 3414–3425. doi: 10.3748/wjg.v24.i30.3414
Yu, Y. H., Liu, H. Q., Liu, J., Tang, H. T., Zhang, P., Zhang, J. (2014). Purification of total flavonoids from Abelmoschus manihot (L.) Medic by D-101 microporous resin. Chin. Tradit. Pat. Med. 36, 520–525. doi: 10.3969/j.issn.1001-1528.2014.03.016
Zhang, Y. Y., Jia, X. N., Cao, Y. X., Wang, J. X. (2008). Studies on the chemical constituents of Abelmoschus manihot. J. Northwest Pharm. 23, 80–82.
Zhang, Y., He, W., Li, C. M., Chen, Q., Han, L. F., Liu, E. W., et al. (2013). Antioxidative flavonol glycosides from the flowers of Abelmouschus manihot. J. Nat. Med. 67, 78–85. doi: 10.1007/s11418-012-0651-1
Zhang, L., Li, P., Xing, C. Y., Zhao, J. Y., He, Y. N., Wang, J. Q., et al. (2014). Efficacy and safety of Abelmoschus manihot for primary glomerular disease: a prospective, multicenter randomized controlled clinical trial. Am. J. Kidney Dis. 64, 57–65. doi: 10.1053/j.ajkd.2014.01.431
Zhang, H., Dong, H., Lei, S. (2015). Neurotensinergic augmentation of glutamate release at the perforant path-granule cell synapse in rat dentate gyrus: Roles of L-Type Ca2+ channels, calmodulin and myosin light-chain kinase. Neuropharmacology 95, 252–260. doi: 10.1016/j.neuropharm.2015.03.028
Zhang, W., Cheng, C., Han, Q., Chen, Y. G., Guo, J. M., Wu, Q. N., et al. (2019). Flos Abelmoschus manihot extract attenuates DSS-induced colitis by regulating gut microbiota and Th17/Treg balance. Biomed. Pharmacother. 117:109162. doi: 10.1016/j.biopha.2019.109162
Zhao, Q., Wan, Y. G., Sun, W., Wang, C. J., Wei, Q. X., Chen, H. L., et al. (2019). Effects of Huangkui capsule on renal inflammatory injury by intervening p38MAPK signaling pathway in rats with adriamycin-induced nephropathy. China J. Chin. Mater. Med. 37, 2926–2934. doi: 10.4268/cjcmm20121920
Zheng, X., Liu, Z. H., Li, S., Wang, L. L., Lv, J. J., Li, J. S., et al. (2016). Identification and characterization of a cytotoxic polysaccharide from the flower of Abelmoschus manihot. Int. J. Biol. Macromol. 82, 284–290. doi: 10.1016/j.ijbiomac.2015.10.004
Zhou, L., An, X. F., Teng, S. C., Liu, J. S., Shang, W. B., Zhang, A. H., et al. (2012). Pretreatment with the total flavone glycosides of Flos Abelmoschus manihot and hyperoside prevents glomerular podocyte apoptosis in streptozotocin-induced diabetic nephropathy. J. Med. Food. 15, 461–468. doi: 10.1089/jmf.2011.1921
Zhou, G., Pang, H., Tang, Y., Yao, X., Mo, X., Zhu, S., et al. (2013). Hydrophilic interaction ultra- performance liquid chromatography coupled with triple-quadrupole tandem mass spectrometry for highly rapid and sensitive analysis of underivatized amino acids in functional foods. Amino Acids 44, 1293–1305. doi: 10.1007/s00726-013-1463-7
Zhou, D., Zeng, J., Fu, Q. F., Gao, D., Zhang, K. L., Ren, X. J., et al. (2018). Preparation and evaluation of a reversed-phase/hydrophilic interaction/ion-exchange mixed-mode chromatographic stationary phase functionalized with dopamine-based dendrimers. J. Chromatogr. A. 1571, 165–175. doi: 10.1016/j.chroma.2018.08.018
Zhu, K. Y., Bi, C. Y. (2010). Observation of effects of Huang Kui capsule in the treatment of chronic glomerulonephritis with proteinuria. Chin. Pract. Med. 5, 122–123.
Zhu, W., Xu, Y. F., Feng, Y., Peng, B., Che, J. P., Liu, M., et al. (2014). Prophylactic effects of quercetin and hyperoside in a calcium oxalate stone forming rat model. Urolithiasis 42, 519–526. doi: 10.1007/s00240-014-0695-7
Keywords: Abelmoschus manihot L., traditional uses, total flavones, antidiabetic nephropathy activity, clinical settings, toxicological
Citation: Luan F, Wu Q, Yang Y, Lv H, Liu D, Gan Z and Zeng N (2020) Traditional Uses, Chemical Constituents, Biological Properties, Clinical Settings, and Toxicities of Abelmoschus manihot L.: A Comprehensive Review. Front. Pharmacol. 11:1068. doi: 10.3389/fphar.2020.01068
Received: 02 December 2019; Accepted: 30 June 2020;
Published: 26 August 2020.
Edited by:
Rudolf Bauer, University of Graz, AustriaReviewed by:
Liang Feng, China Pharmaceutical University, ChinaCopyright © 2020 Luan, Wu, Yang, Lv, Liu, Gan and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haizhen Lv, bGh6MDI4QGFsaXl1bi5jb25t; Nan Zeng, emVuZ25hbjY2NkAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.