- 1College of First Clinical Medicine, Shandong University of Traditional Chinese Medicine, Jinan, China
- 2Department of Geratology, Xiyuan Hospital, China Academy of Chinese Medical Science, Beijing, China
- 3Institute of Basic Theory for Chinese Medicine, China Academy of Chinese Medical Science, Beijing, China
Background: Pharmacological treatments play a significant role in treating mild to moderate Alzheimer’s disease (AD), but the optimal doses of various drugs used for these treatments are unknown. Our study compared the efficacy, acceptability, and safety of different doses of pharmacological treatments for mild to moderate AD.
Methods: Randomized controlled trials (RCTs) were identified by searching the PubMed, EMBASE, and Cochrane Library databases (all RCTs published from the date of inception of the databases until September 19, 2019). Trials comparing the efficacy, acceptability, and safety of pharmacological interventions involving donepezil, galantamine, rivastigmine, memantine, huperzine A, and Ginkgo biloba extract EGb761, alone or in combination, were identified. The primary outcomes were efficacy, acceptability, and safety.
Results: Our meta-analysis included 37 studies involving 14,705 participants. In terms of improving cognitive function, galantamine 32 mg, galantamine 24 mg, donepezil 5 mg, and donepezil 10 mg were more effective than other interventions, with the surface under the cumulative ranking curve (SUCRA) values of 93.2, 75.5, 73.3, and 65.6%, respectively. According to the SUCRA values, EGb761 240 mg was considered to be the optimal intervention in terms of both acceptability and safety. With regard to clinical global impression, rivastigmine 12 mg had the highest probability of being ranked first (83.7%). The rivastigmine 15 cm2 patch (SUCRA = 93.7%) may be the best choice for daily living. However, there were no interventions that could significantly improve neuropsychiatric symptoms, compared with the placebo.
Conclusions: Different doses of the tested pharmacological interventions yielded benefits with regard to cognition, acceptability, safety, function, and clinical global impressions, but not effective behaviors.
Background
There were an estimated 50 million dementia patients worldwide in 2018. Although this disease currently represents an enormous public health problem, the number of dementia patients is predicted to rise to 152 million by 2050 (Alzheimer’s Disease International, 2018). Alzheimer’s disease (AD) is an irreversible neurodegenerative disease that manifests as progressive memory loss and cognitive dysfunction, and is the leading cause of dementia, accounting for 50–75% of all cases globally (International., Alzheimer’s disease, 2019). There is currently no cure for AD; the typical pharmacological therapeutic goals are to delay disease progression and to improve the patients’ quality of life. Pharmacological treatments approved by the US Food and Drug Administration are mainly grouped into two classes by their differing mechanisms of action: acetylcholinesterase inhibitors (AChEIs), such as donepezil, galantamine, and rivastigmine, which are widely used treatments for mild to moderate disease stages (NICE; Corbett et al., 2012); and N-methyl-D-aspartate receptor antagonists, typically memantine, for moderate to severe disease stages (Kishi et al., 2017).
Donepezil is the primary treatment for mild to moderate AD; it is well tolerated and results in cognitive improvement (Rogers et al., 1998b; Rogers et al., 1998a; Burns et al., 1999). Moreover, evidence suggests that donepezil has dose-dependent effects: with increasing doses, its efficacy improves, although more adverse events also occur. Increased improvements in cognition are indicated for donepezil 10 mg but not donepezil 5 mg, especially at 18 and 24 weeks, based on the meta-analysis of Whitehead et al., which included 10 clinical trials (Whitehead et al., 2004). In routine practice, the variety of different drug preparations and dosages poses a challenge for physicians responsible for decision-making with regard to treatment options for AD.
EGb761, extracted from Ginkgo biloba, is a common herbal treatment for AD (Akram and Nawaz, 2017). A previous systematic review and meta-analysis demonstrated that compared with placebo, the Ginkgo biloba extract EGb761 appeared to have stronger cognitive effects (standard mean difference [SMD] = −0.58, 95% confidence interval [CI]: −1.14, −0.01) (Weinmann et al., 2010). Although the efficacy of the Ginkgo biloba extract EGb761 was confirmed, when compared with donepezil, the results were not conclusive (Mazza et al., 2006; Yancheva et al., 2009; Nasab et al., 2012). In addition, a Cochrane systematic review of six trials suggested that huperzine A, a reversible and selective AChEI, is likely beneficial to AD patients and resulted in no apparent serious adverse events (Li et al., 2008). To date, a direct comparison of huperzine A, EGb761, an AChEI, or memantine has not been conducted in the same study.
It should be noted that a previous network meta-analysis focused on the comparative effectiveness of different anti-dementia treatments by using direct or indirect evidence, but did not consider different drug doses (Thancharoen and Limwattananon, 2019) or include comprehensive pharmacological interventions (Dou et al., 2018). A network meta-analysis allows the summation of direct and indirect evidence from relevant randomized controlled trials (RCTs) and the performance of an integrated analysis to determine the optimal pharmacological therapy for mild to moderate AD (Higgins and Whitehead, 1996). Therefore, this study aimed to comprehensively evaluate the efficacy (i.e., improvements in cognitive function), acceptability (i.e., completion of treatment), and safety (i.e., number of adverse events) of different doses of pharmacological agents used for treating mild to moderate AD, which can be used to inform clinical practice.
Methods
Search Strategy
This network meta-analysis was performed in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) extension for network meta-analysis (Hutton et al., 2015). Relevant RCTs were identified in titles and abstracts in the PubMed, EMBASE, and the Cochrane Library databases. Results were restricted to English language publications from the date of the database inception to September 19, 2019. No restrictions were placed on publication dates or status. We adopted the MeSH and Emtree terms “Alzheimer’s disease,” “donepezil,” “galantamine,” “rivastigmine,” “memantine,” “huperzine A,” “Ginkgo biloba extract,” and “randomized controlled trials” combined with the corresponding free words adapted appropriately for each of the databases in the search algorithm. Additionally, we manually searched the references from the cited articles to identify meta-analyses and RCTs to avoid missing potentially eligible clinical trials. The details of the search strategies involving different databases are described in the Additional file: Supplementary 1.
Selection Criteria
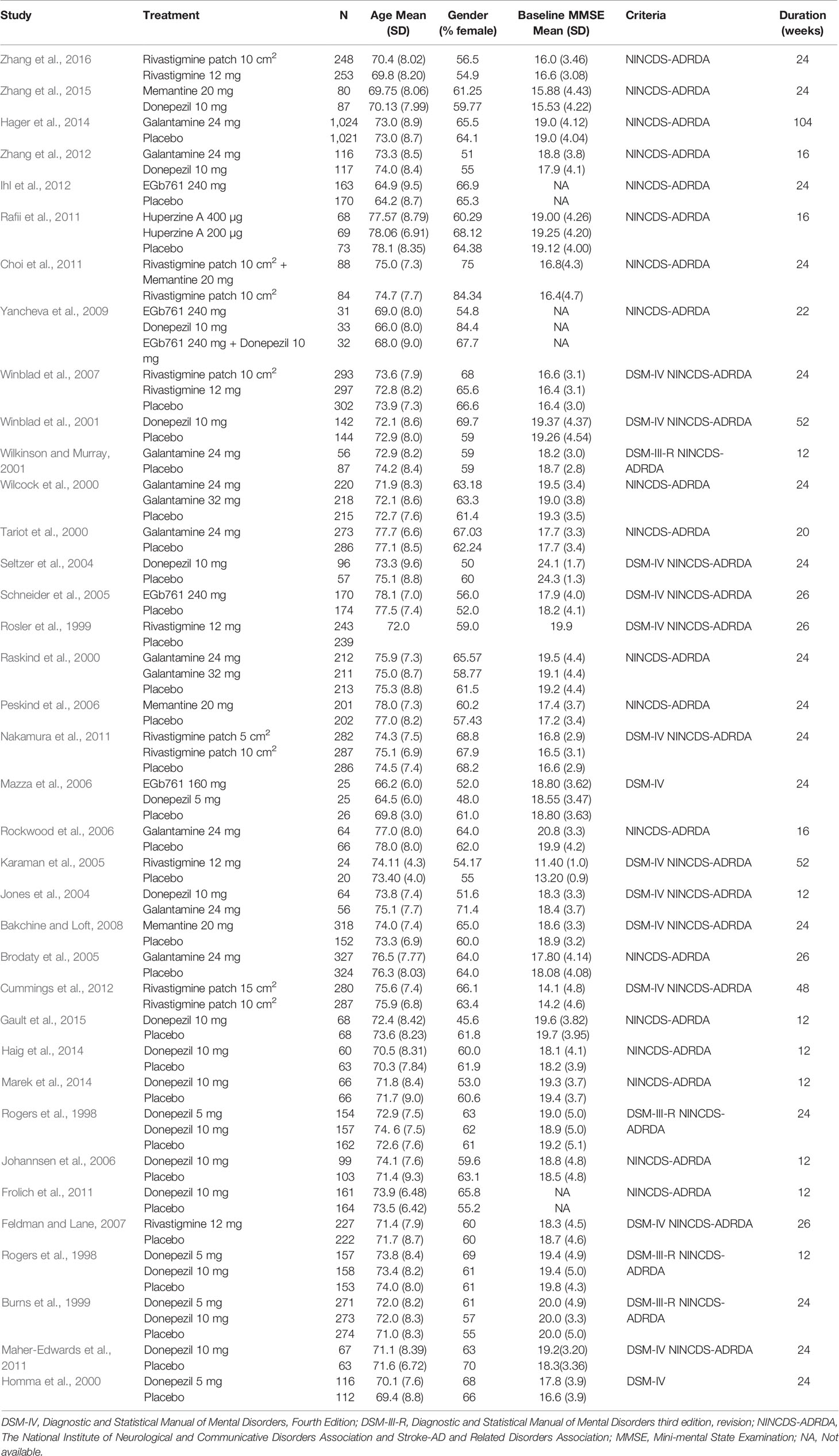
The selection criteria were based on the principle of the Population-Intervention-Comparator-Outcomes-Study design (PICOS) (Costantino et al., 2015). The eligible studies were RCTs and had to meet the following criteria: 1) participants were clinically diagnosed with AD in accordance with the criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM) or the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) (McKhann et al., 1984). Mild to moderate AD was classified by a score of 10–26 (inclusive) in the Mini-Mental State Examination (MMSE) (Folstein et al., 1975); 2) trials compared the effectiveness of pharmacological interventions using donepezil, galantamine, rivastigmine, memantine, huperzine A, or Ginkgo biloba extract alone or in combination, and drug dosages were not only within the therapeutic range but were also specific; 3) outcome measures covered at least one of the following outcomes: cognitive, global assessment, behavior, function, acceptability, or safety; and 4) the duration of follow-up was between 12 and 104 weeks. The following exclusion criteria were applied: 1) RCTs that recruited fewer than 10 participants in each group; 2) unavailability of the full text of the study, even with the support of expert librarians; and 3) participants diagnosed with other types of dementia or neurological disorders unrelated to AD, or outcome data for participants with AD that could not be independently assessed apart from data for participants diagnosed with other types of dementia.
Data Extraction
Two investigators (LN and CH) independently extracted the relevant data from all eligible studies published in English using predefined standardized spreadsheets. All extracted data were based on intention-to-treat analysis. Any discrepancies were resolved to consensus by two investigators (LN and CH) or arbitrated by a third investigator (ZT). The following information was documented for every study: first author, publication year, detailed trial information, diagnostic criteria, patient characteristics (i.e., age, gender, race, and baseline MMSE scores), treatment (dose, frequency), sample size, outcomes of the change from baseline (cognitive, global assessment, behavior, function), number of treatment completion, incidences of adverse events, and the duration of follow-up. Finally, all extracted data were cross-checked by two investigators (LN and CH) to ensure accuracy.
Quality Assessment
We evaluated the quality of the included trials using the Cochrane Collaboration’s risk of bias assessment tool (Higgins et al., 2011), and the trials were judged to have a low risk of bias, an unclear risk of bias, or a high risk of bias. Any discrepancies between the two authors’ evaluations (ZT and LN) were resolved by discussion.
Outcome Measures
We considered the overall mean change in cognitive function from the baseline to the study endpoint, the number of patients who completed the trial during the treatment period, and the number of patients who experienced any adverse events for our primary outcomes, as these were the most consistently reported estimates of efficacy, acceptability, and safety of interventions for mild to moderate AD. Cognitive function was primarily appraised by the Alzheimer’s Disease Assessment Scale-cognition subscale (ADAS-cog), and the MMSE. For secondary outcome measures, we also estimated the changes from baseline to the endpoints of cognitive function, behavioral symptoms, and the clinical global impressions of patients, which were assessed by the Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL) scale, the Neuropsychiatric Inventory (NPI), and the Clinician’s Interview-Based Impression of Change plus Caregiver Input (CIBIC-Plus) scale, respectively.
Statistical Analysis
First, we estimated the SMD for continuous outcomes and odds ratios (ORs) for dichotomous outcomes along with the corresponding 95% confidence interval (CI) by using a random-effects model, which served as the pooled effect sizes in conventional pair-wise meta-analysis. To assess the statistical heterogeneity of the direct comparison in the quantitative analysis, we used the I2 statistic and p values. Stata software version 14.0 (Stata Corporation, College Station, TX, USA) was used for all analyses.
Second, for all collected outcomes, we performed a Bayesian network meta-analysis combining direct and indirect comparisons based on a random-effect model considering the smaller deviance information criteria (DIC) value. The data analysis used OpenBUGS software (version 3.2.3), and the network diagram was produced using Stata software (version 14.0). We chose various initial values at random with the run of three Markov chains simultaneously. The total number of iterations was 30,000. The median of the calculated data served as pooled estimated effect sizes (SMD or OR), and the 2.5 and 97.5 percentiles served as the corresponding 95% credible interval (CrI). The statistical significance was evaluated in line with whether the CrI included 0 or 1. Moreover, we also calculated the surface under the cumulative ranking curve (SUCRA) to rank the interventions for each outcome in which the SUCRA value was closely related to the rank of each intervention. In addition, if the network of interventions had closed loops, the node-splitting method and loop-specific method were performed to evaluate the statistical inconsistency (Salanti et al., 2008; Dias et al., 2010; Veroniki et al., 2013). The determination of whether the loop consistency was significant depended on the CI of the inconsistency factor (IF) value containing 0. Finally, for the small-sample effect assessment of intervention networks, we constructed a comparison-adjusted funnel plot and performed a visual assessment.
Results
Literature Search Results
In total, 4,567 citations were identified by searching the PubMed, EMBASE, and the Cochrane Library databases. After 1,133 duplicate citations were removed using Endnote X7 software, the titles and abstracts for 3,434 citations were retrieved. Subsequently, the full text of 121 potentially eligible studies were reviewed further. From these, 85 publications were excluded primarily because they included other diseases (n = 18), did not report the desired intervention agents (n = 20), reported undesired outcomes (n = 5), were not RCTs (n = 9), were duplicate studies (n = 9), were not in English (n = 4), or were conference abstracts without available full texts (n = 20). Finally, 36 eligible studies met the inclusion criteria. In addition, we identified an additional publication from the references. Overall, 37 studies (Rogers et al., 1998a; Rogers et al., 1998b; Burns et al., 1999; Rosler et al., 1999; Homma et al., 2000; Raskind et al., 2000; Tariot et al., 2000; Wilcock et al., 2000; Wilkinson and Murray, 2001; Winblad et al., 2001; Jones et al., 2004; Seltzer et al., 2004; Brodaty et al., 2005; Karaman et al., 2005; Schneider et al., 2005; Johannsen et al., 2006; Mazza et al., 2006; Peskind et al., 2006; Rockwood et al., 2006; Feldman and Lane, 2007; Winblad et al., 2007; Bakchine and Loft, 2008; Yancheva et al., 2009; Choi et al., 2011; Frolich et al., 2011; Maher-Edwards et al., 2011; Nakamura et al., 2011; Rafii et al., 2011; Cummings et al., 2012; Ihl et al., 2012; Zhang et al., 2012; Hager et al., 2014; Haig et al., 2014; Marek et al., 2014; Gault et al., 2015; Zhang et al., 2015; Zhang et al., 2016) were available for inclusion in the network meta-analysis. The PRISMA flowchart detailing the literature search process is shown in Figure 1.
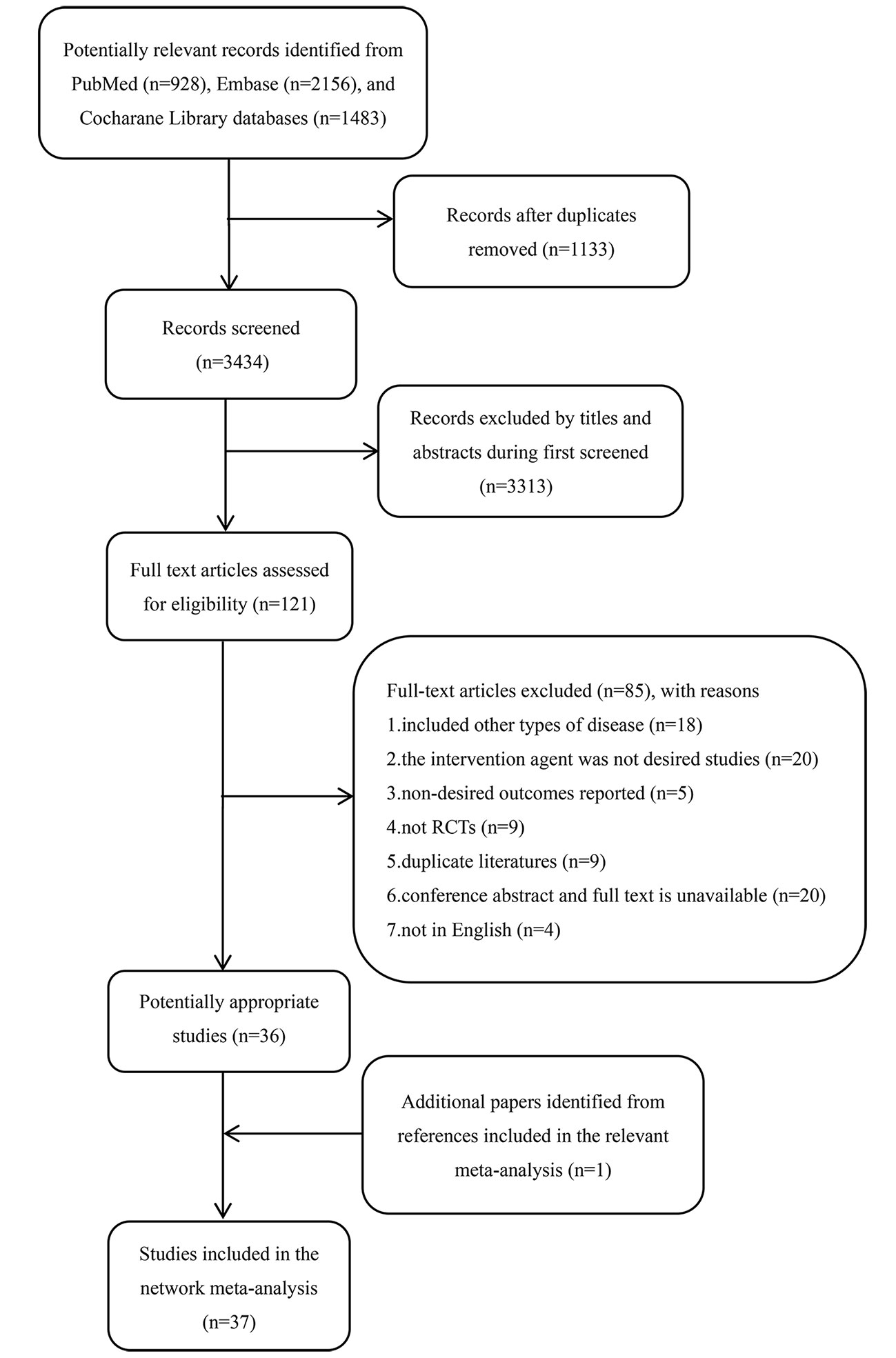
Figure 1 The PRISMA flowchart for detailed search results and selection. RCT, randomized controlled trial.
Characteristics of the Eligible Studies
The characteristics of the included studies and details of the patients are shown in Table 1. The 37 studies involving 14,705 participants contributed to the network meta-analysis. Across all trials, the year of RCT publication ranged from 1998 to 2016. The mean study sample size was 175 participants in each group, with a range between 20 and 1,024 patients. The mean (SD) age of participants was between 64.2 (8.4) and 78.1 (8.35) years of age. The minimum percentage of females was 45.6%, and the maximum percentage was 84.4%. Most trials (35 [94.6%] of 37) adopted the NINCDS-ADRDA diagnostic criteria. Follow-up data was available for all patients for a minimum of 12 weeks and a maximum of 104 weeks.
Quality of the Assessment
Detailed information regarding the risk of bias in all 37 studies is presented in Figure 2 and Additional file: Supplementary 2. It was difficult to assess the risk of selection bias in most studies, owing to the absence of adequate details recorded for randomization and allocation concealment. We identified one study with a high risk of bias associated with the blinding of participants and personnel. As for the blinding of the outcome assessment, 29 trials were rated as having an unclear risk of bias, and only eight studies had evidence indicating a low risk of bias. Most studies (36 of 37) had a low risk of bias for incomplete outcome data. The percentage of studies with unclear bias was 70.3. In addition, a high risk of bias was noted in six studies. In total, the overall quality of the studies was judged to be good.
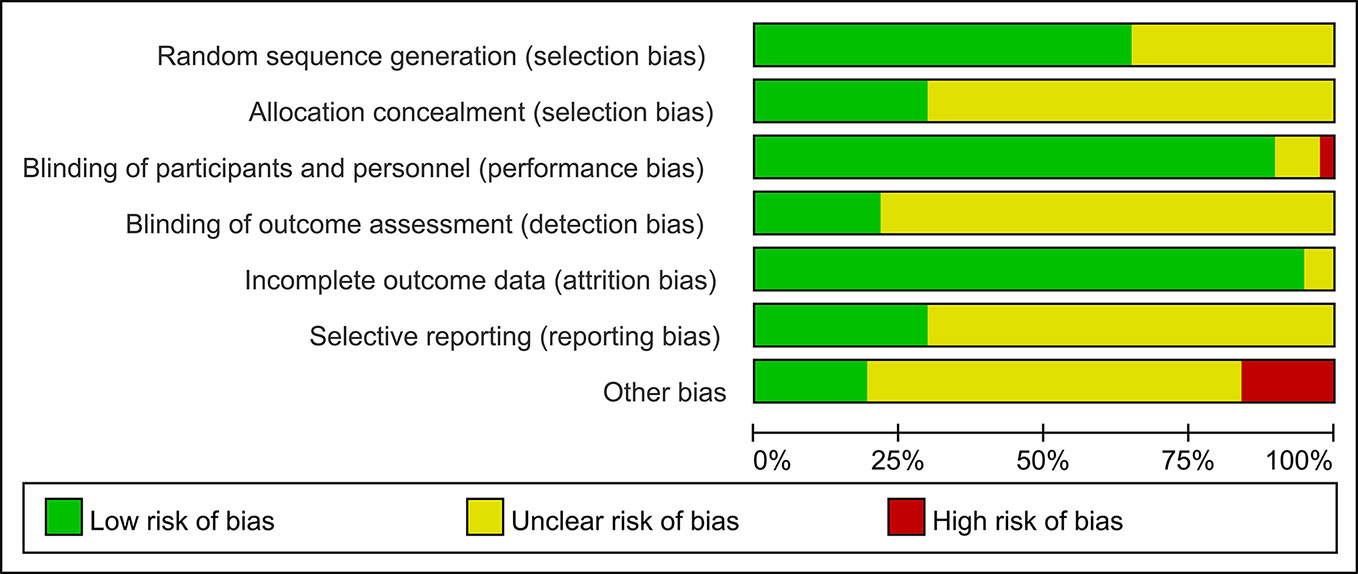
Figure 2 Risk of bias graph presented as percentage across all studies (green represents low risk of bias; red represents high risk of bias; and yellow represents an unclear risk of bias).
Pair-Wise Meta-Analysis
The tested interventions, except for rivastigmine 12 mg, the rivastigmine 5 cm2 patch, huperzine A 400 µg, and huperzine A 200 µg, showed statistically significant differences with regard to the ADCS-cog assessment for mild to moderate AD when compared with the placebo. However, in the MMSE, donepezil 10 mg, donepezil 5 mg, rivastigmine 10 cm2, galantamine 24 mg, huperzine A 400 µg, and huperzine A 200 µg was superior to the placebo. In terms of acceptability, well-tolerated interventions included rivastigmine 12 mg, rivastigmine 10 cm2 patch, rivastigmine 5 cm2 patch, galantamine 24 mg, and galantamine 32 mg compared with the placebo. For all interventions, except for donepezil 5 mg, rivastigmine 10 cm2 patch, memantine 20 mg, and EGb761 240 mg, adverse events occurred more often than that with the placebo. For secondary outcomes, in terms of daily living, either the rivastigmine 10 cm2 patch or galantamine 24 mg was superior to placebo. Compared with placebo, donepezil 10 mg, donepezil 5 mg, rivastigmine 12 mg, the rivastigmine 10 cm2 patch, and the rivastigmine 5 cm2 patch showed statistically significant differences with regard to the clinical global assessment in patients with mild to moderate AD. Compared with the placebo, only galantamine 24 mg and EGb761 240 mg improved behavioral symptoms. Heterogeneity was found only in the direct comparisons of memantine 20 mg vs. placebo (I2 = 83.1%), galantamine 24 mg vs. placebo (I2 = 78.0%), and rivastigmine 12 mg vs. placebo (I2 = 76.9%), with I2 values greater than 70%. These results of the pair-wise meta-analyses are outlined in detail in Additional file: Supplementary 3.
Network Meta-Analysis—Primary Outcomes
A network diagram of all the eligible comparisons involving 24 trials of cognitive function based on the ADAS-cog scale is presented in Figure 3A. As outlined in Figure 3A, the placebo was the most common comparator in all interventions comparisons; only the rivastigmine 15 cm2 patch and the combination of rivastigmine 10 cm2 and memantine 20 mg were not directly compared with the placebo. Six closed loops existed across all comparisons. Based on the inconsistency factors (IFs) and 95% CIs, we concluded that the direct and indirect evidence was consistent. The relevant inconsistency results and the figures are shown in Additional file: Supplementary 4. In terms of improving cognitive function, galantamine 24 mg, galantamine 32 mg, donepezil 10 mg, and donepezil 5 mg were more effective than placebo, with SMDs of −0.39 (95% CrI: [−0.65, −0.12]) for galantamine 24 mg, −0.62 (−1.01, −0.24) for galantamine 32 mg, −0.30 (−0.52, −0.07) for donepezil 10 mg, and −0.37 (−0.69, −0.04) for donepezil 5 mg. Galantamine 32 mg was superior to rivastigmine 12 mg (SMD = −0.65, 95% CrI: [−0.17, −0.20]) and the rivastigmine 10 cm2 patch (SMD = −0.52, 95% CrI: [−1.06, −0.02]). However, for other interventions, there were no statistically significant differences. In addition, when compared with rivastigmine 12 mg, galantamine 24 mg was more efficacious (SMD = −0.41, 95% CrI: [−0.85, −0.05]). The informative results for mild to moderate AD are shown in Table 2 (in the top right corner). As shown in Figure 4A and Additional file: Supplementary 5, the five most efficient interventions were ranked as galantamine 32 mg (SUCRA = 93.2%), galantamine 24 mg (SUCRA = 75.5%), donepezil 5 mg (SUCRA = 73.3%), donepezil 10 mg (SUCRA = 65.6%), and memantine 20 mg (SUCRA = 57.0%). Furthermore, we also assessed cognitive function using the MMSE. The network plot, including a total of 17 studies, is presented in Figure 3B. We noted consistent results in both direct and indirect comparisons. In the network meta-analysis, no interventions were associated with statistically significant differences compared with placebo (Figure 5A). Furthermore, rivastigmine 12 mg had the highest probability of being ranked first according to SUCRA (72.9%), followed closely by the combination of the rivastigmine 10 cm2 patch and memantine 20 mg (SUCRA = 63.1%) and the rivastigmine 5 cm2 patch (SUCRA = 60.7%) (Additional file: Supplementary 5).
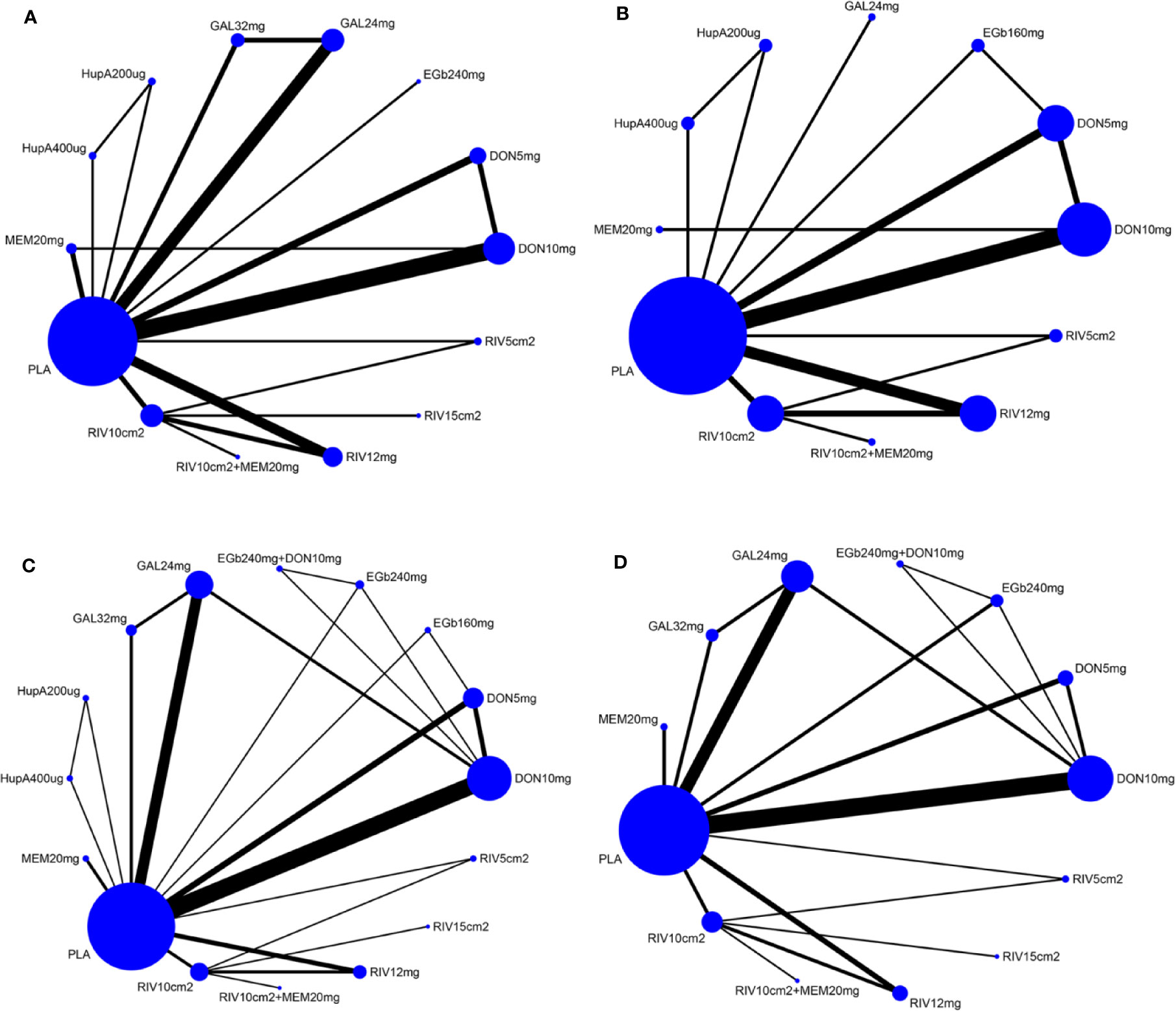
Figure 3 Network of eligible comparisons for all pharmacological treatments included in the analyses [(A) according to ADAS-cog scale, (B) MMSE results, (C) acceptability, (D) safety]. Treatments with direct comparisons are linked with a black line; its width is proportional to the number of trials evaluating every pair of the comparison. Blue Nodes represent different treatments. Node size is proportional to the total number of patients for each treatment in the network. MMSE, Mini-Mental State Examination; ADAS-cog, Alzheimer’s Disease Assessment Scale-cognition subscale; PLA, Placebo; RIV10cm2, Rivastigmine patch 10 cm2; RIV10cm2+MEM20mg, Rivastigmine patch 10 cm2 + Memantine 20 mg; RIV12mg, Rivastigmine 12 mg; RIV15cm2, Rivastigmine patch 15 cm2; RIV5cm2, Rivastigmine patch 5 cm2; DON10mg, Donepezil 10 mg; DON5mg, Donepezil 5 mg; EGb240mg, EGb761 240 mg; GAL24mg, Galantamine 24 mg; GAL32mg, Galantamine 32 mg; HupA200µg, Huperzine A 200 µg; HupA400µg, Huperzine A 400 µg; MEM20mg, Memantine 20 mg; EGb160mg, EGb761 160 mg; EGb240mg+DON10mg, EGb761 240 mg + Donepezil 10 mg.
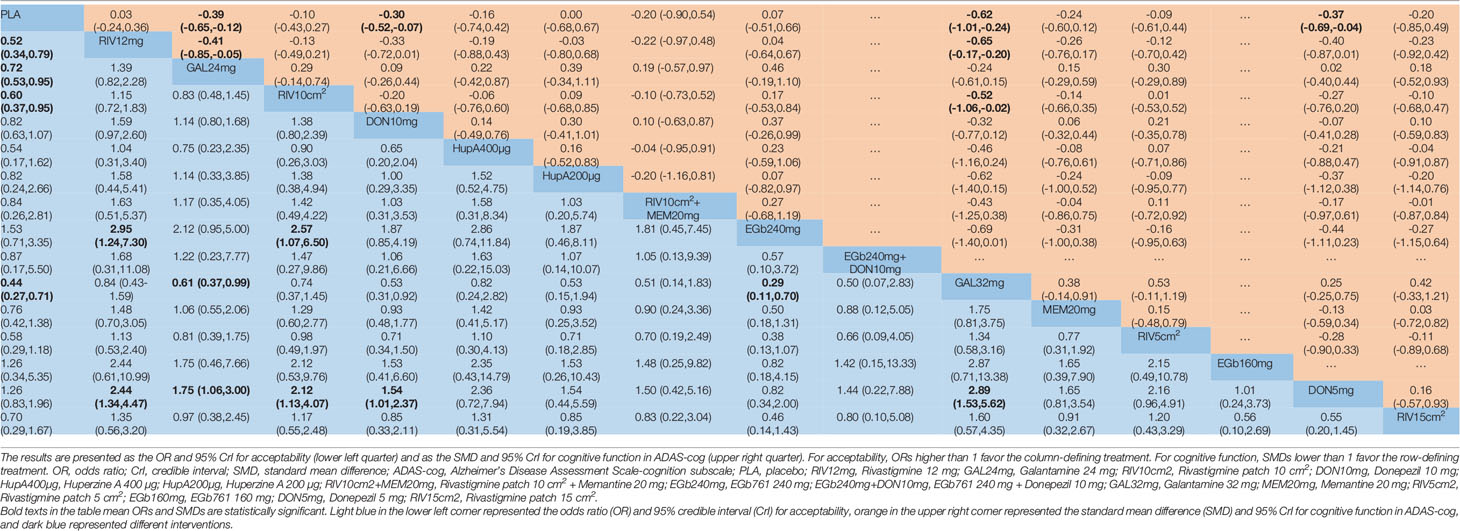
Table 2 Network meta-analysis comparison of 16 pharmacological treatments for mild to moderate Alzheimer’s disease.
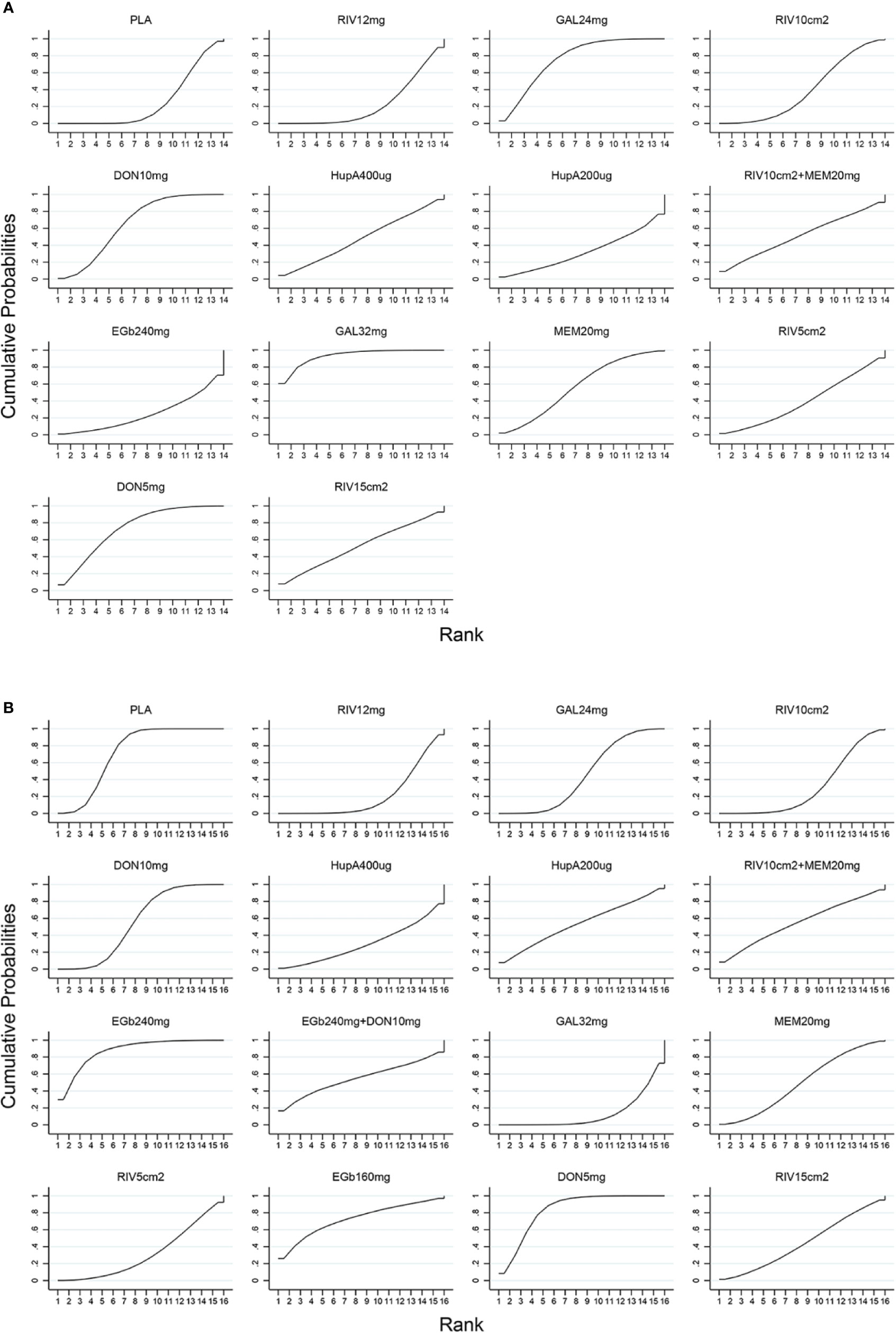
Figure 4 SUCRA for cognitive function based on ADAS-cog scale (A) and acceptability (B). The larger the SUCRA, the higher the ranking. ADAS-cog, Alzheimer’s Disease Assessment Scale-cognition subscale; SUCRA, surface under the cumulative ranking curve; PLA, Placebo; RIV12mg, Rivastigmine 12 mg; GAL24mg, Galantamine 24 mg; RIV10cm2, Rivastigmine patch 10 cm2; DON10mg, Donepezil 10 mg; HupA400µg, Huperzine A 400 µg; HupA200µg, Huperzine A 200 µg; RIV10cm2+MEM20mg, Rivastigmine patch 10 cm2 + Memantine 20 mg; EGb240mg, EGb761 240 mg; GAL32mg, Galantamine 32 mg; MEM20mg, Memantine 20 mg; RIV5cm2, Rivastigmine patch 5 cm2; DON5mg, Donepezil 5 mg; RIV15cm2, Rivastigmine patch 15 cm2; EGb240mg+DON10mg, EGb761 240 mg + Donepezil 10 mg; EGb160mg, EGb761 160 mg.
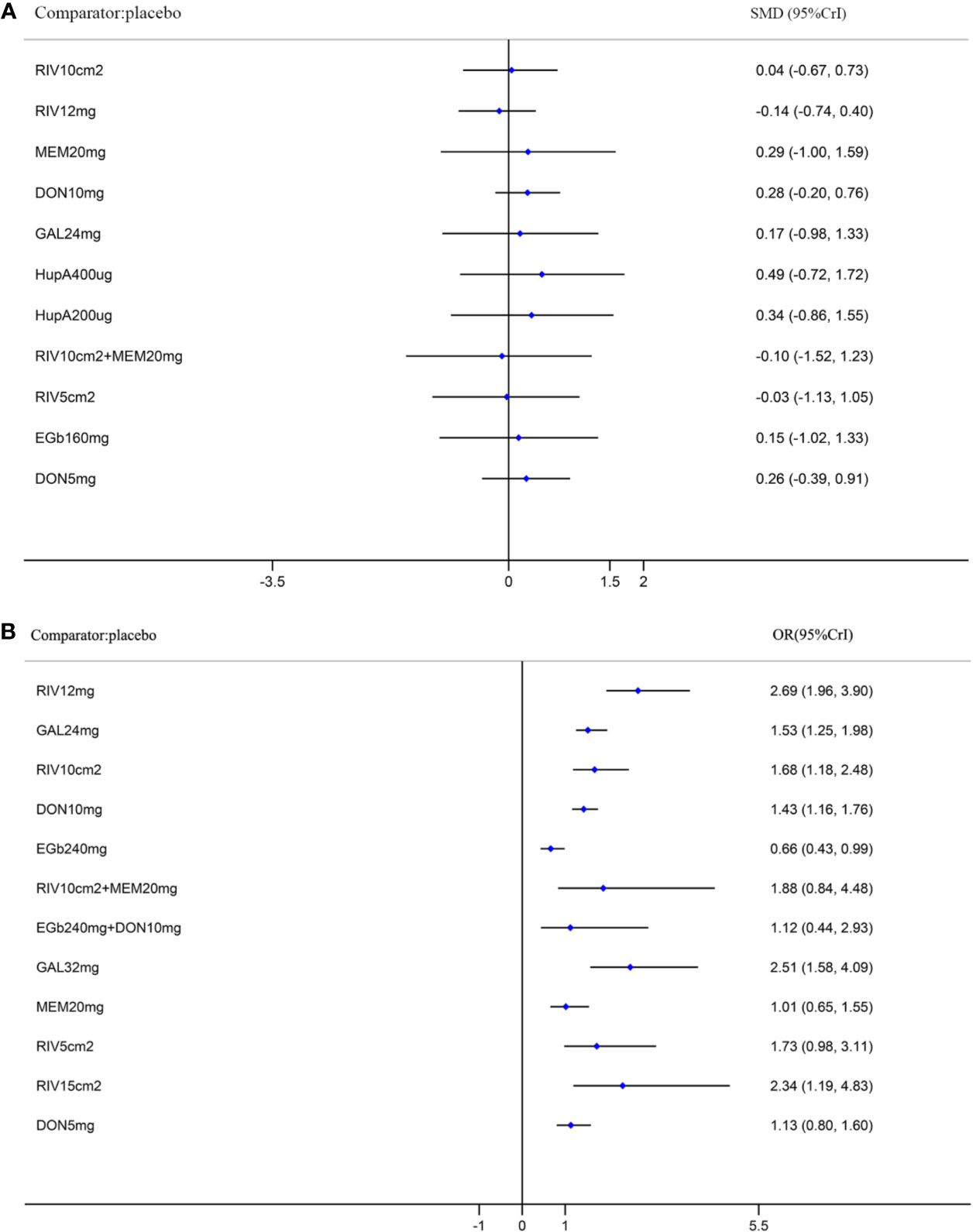
Figure 5 Forest plots of the results of network meta-analysis for function in the MMSE (A) and for safety (B) compared with placebo. SMD, standardized mean difference; OR, odds ratio; CrI, credible interval; MMSE, Mini-Mental State Examination; RIV10cm2, Rivastigmine patch 10 cm2; RIV12mg, Rivastigmine 12 mg; MEM20mg, Memantine 20 mg; DON10mg, Donepezil 10 mg; GAL24mg, Galantamine 24 mg; HupA400µg, Huperzine A 400 µg; HupA200µg, Huperzine A 200 µg; RIV10cm2+MEM20mg, Rivastigmine patch 10 cm2 + Memantine 20 mg; RIV5cm2, Rivastigmine patch 5 cm2; EGb160mg, EGb761 160 mg; DON5mg, Donepezil 5 mg; EGb240mg, EGb761 240 mg; EGb240mg+DON10mg, EGb761 240 mg + Donepezil 10 mg; GAL32mg, Galantamine 32 mg; RIV15cm2, Rivastigmine patch 15 cm2.
The network of eligible comparisons for the assessment of acceptability is shown in Figure 3C. In total, 33 trials and 16 treatments were included; most treatments were monotherapies, except for the combinations of EGb761 240 mg and donepezil 10 mg and the rivastigmine 10 cm2 patch and memantine 20 mg. We found no evidence indicating an inconsistency between direct and indirect evidence via the IF and 95% CIs of nine closed loops (Additional file: Supplementary 4). Our analysis showed that the interventions of rivastigmine 12 mg (OR = 0.52, 95% CrI: [0.34, 0.79]), galantamine 24 mg (OR = 0.72, 95% CrI: [0.53, 0.95]), rivastigmine 10 cm2 patch (OR = 0.60, 95% CrI: [0.37, 0.95]), and galantamine 32 mg (OR = 0.44, 95% CrI: [0.27, 0.71]) were associated with a significantly increased probability of treatment completion compared with placebo. In addition, EGb761 240 mg was superior to the rivastigmine 10 cm2 patch (OR = 2.57, 95% CrI: [1.07, 6.50]) and rivastigmine 12 mg (OR = 2.95, 95% CrI: [1.24, 7.30]). Moreover, galantamine 32 mg was inferior to EGb761 240 mg (OR = 0.29, 95% CrI: [0.11,0.70]) (see the left corner of Table 2). We also ranked all treatments and found that EGb761 240 mg (SUCRA = 87.5%), donepezil 5 mg (SUCRA = 83.4%), and EGb761 160 mg (SUCRA = 72.5%) were most likely to be ranked first (Figure 4B).
A total of 32 trials with 13 interventions presented data on adverse events. The network diagram is presented in Figure 3D. The direct and indirect evidence was consistent (Additional file: Supplementary 4). Our network meta-analysis demonstrated that only EGb761 240 mg was better tolerated than placebo for safety (OR = 0.66, 95% CrI: [0.43, 0.99]). Rivastigmine 12 mg, galantamine 24 mg, the rivastigmine 10 cm2 patch, donepezil 10 mg, galantamine 32 mg, and the rivastigmine 15 cm2 patch were associated with a significantly increased risk of adverse events compared with placebo (OR = 2.69, 95% CrI: [1.96, 3.90], OR = 1.53, 95% CrI: [1.25, 1.98], OR = 1.68, 95% CrI: [1.18, 2.48], OR = 1.43, 95% CrI: [1.16, 1.76], OR = 2.51, 95% CrI: [1.58, 4.09], OR = 2.34, 95% CrI: [1.19, 4.83], respectively; Figure 5B). Other drugs, such as donepezil 5 mg as a monotherapy, and the combinations of the rivastigmine 10 cm2 patch with memantine 20 mg as well as EGb761 240 mg with donepezil 10 mg showed no statistical differences when compared with placebo. Based on SUCRA values, the optimal acceptable intervention was likely to be EGb761 240 mg (SUCRA = 97.8%). Memantine 20 mg and donepezil 5 mg followed closely behind as the second (SUCRA = 78.9%) and third (SUCRA = 71.7%) most acceptable interventions (Additional file: Supplementary 5).
Network Meta-Analysis—Secondary Outcomes
Networks of eligible comparisons of the secondary outcomes are presented in Additional file: Supplementary 6, demonstrating predominantly head-to-head comparisons of drugs with active drugs or placebo. Regardless of whether the CIBIC-plus scale, ADCS-ADL, or NPI scales were used, the direct and indirect evidence indicated consistent results. (Additional file: Supplementary 4). For the assessment of clinical global impressions via the CIBIC-plus scale, memantine 20 mg, donepezil 10 mg, rivastigmine 12 mg, and donepezil 5 mg were significantly superior to placebo (SMD = −0.27, 95% CrI: [−0.48, −0.07]; SMD = −0.34, 95% CrI: [−0.50, −0.17]; SMD = −0.40, 95% CrI: [−0.62, −0.18]; SMD = −0.29, 95% CrI: [−0.53, −0.06]) (Additional file: Supplementary 7). The SUCRAs ranged from 83.7% for the highest-ranked treatment strategy (rivastigmine 12 mg) to 40.3% for the lowest-ranked agent (rivastigmine 5 cm2) (Additional file: Supplementary 5). In the assessment for improvements daily living using the ADCS-ADL scale, donepezil 10 mg, galantamine 24 mg, and the rivastigmine 15 cm2 patch were statistically more efficacious than placebo, with SMDs and 95% CrIs of 0.21 (0.02, 0.40) for donepezil 10 mg, 0.22 (0.06, 0.37) for galantamine 24 mg, and 0.51 (0.17, 0.81) for the rivastigmine 15 cm2 patch (Additional file: Supplementary 7). As shown in Additional file: Supplementary 5, the rank of the three most efficient interventions was the rivastigmine 15 cm2 (SUCRA = 93.7%), the combination of rivastigmine 10 cm2 and memantine 20 mg (SUCRA = 71.1%), followed by galantamine 24 mg (SUCRA = 60.3%). Twelve studies assessed neuropsychiatric symptoms using the NPI scale for nine different treatment interventions and placebo. However, in our network meta-analysis, there were no interventions that significantly improved neuropsychiatric symptoms compared with placebo.
Publication Bias
We produced comparison-adjusted funnel plots, with different colors representing different comparisons. Through a visual inspection, we found that the funnel plots presented an essentially symmetrical distribution, indicating that there were no small-sample effects for any outcomes (Additional file: Supplementary 8).
Discussion
This comprehensive network meta-analysis was based on 37 trials, which included 14,705 patients with mild to moderate AD randomly assigned to currently available active agents or placebo, and compared the efficacy, acceptability, and safety of various regimens. The magnitude of intervention ranking varied enormously across different cognitive enhancers and doses, especially in different assessment outcomes. The results suggested that for patients with mild to moderate AD, galantamine 32 mg, galantamine 24 mg, donepezil 5 mg, donepezil 10 mg, and memantine 20 mg were more efficacious for cognitive improvements than other pharmacotherapies. The EGb761 240 mg treatment appeared to be the most optimal in terms of both acceptability and safety. Moreover, of the current treatment therapies, rivastigmine 12 mg offered a more favorable profile with benefits in the clinical global impression. The rivastigmine 15 cm2 patch, another rivastigmine dosage form, had the highest probability of functional improvement. However, we did not find any effective interventions resulting in behavioral improvements. This project extends a previous network meta-analysis that addressed ten interventions with data for direct and indirect comparisons (Dou et al., 2018). Our study can assist in the provision of relevant options for clinical pharmacotherapies for patients with mild to moderate AD.
Galantamine is a reversible and competitive AChEI (Bores et al., 1996). A previous meta-analysis concluded that galantamine was an effective therapeutic agent and was a preferred treatment for AD compared with donepezil, memantine, and rivastigmine (Li et al., 2019). Galantamine 32 mg was associated with a significant improvement in cognitive function; however, owing to poor acceptability and adverse events, its practical use may be limited. Based on the overall evidence, galantamine 24 mg may therefore, be the optimal treatment option for patients with mild to moderate AD. In addition, the major therapeutic effect of EGb761 240 mg is based on its acceptability and fewer associated adverse events. Although some studies have shown that EGb761 was favorable for cognitive, behavioral, and functional improvements, and clinical global impressions (Yancheva et al., 2009; Ihl et al., 2012; Yang et al., 2016), their sample sizes were much smaller, and the results were mixed. Thus, we propose that EGb761 should be researched further in large-scale randomized controlled trials. It has been reported that huperzine A is a well-tolerated intervention leading to improvements in cognitive impairment; however, until now, the evidence from our network meta-analysis did not recommend its use (Xing et al., 2014). A secondary analysis showed that regardless of dosage form and dose, rivastigmine produced a relatively marked improvement in both clinical global impression and daily living. The rivastigmine patch is frequently used in patients with mild to moderate AD because the adverse events associated with the patch are greatly reduced compared with that of the capsule form (Winblad et al., 2007). It is a novel drug delivery method that allows continuous drug administration.
We carefully monitored quality between the included trials and found that the majority of trials were considered to be unclear with regard to selection bias, especially, allocation concealment. Additionally, open-label trials were included. Nevertheless, our analysis could still be powered to provide objective evaluations for unclear factors given the even distribution of patient characteristics and the objective method adopted in each treatment group. Through the node-splitting method and loop-specific method, we noticed no significant differences between consistency in terms of the concerned evaluated outcomes. To assess the bias of small-sample effects, we also produced a comparison-adjusted funnel plot, and the findings were reassuring.
We are aware of three studies associated with AD that also integrated direct and indirect comparisons simultaneously in one network meta-analysis (Dou et al., 2018; Thancharoen and Limwattananon, 2019; Tsoi et al., 2019). In contrast to these previous studies, our study included new interventions and integrated all available high-quality RCTs with regard to the effectiveness, acceptability, and safety of cognitive enhancers in treating mild to moderate AD in one analysis, while examining different doses of treatments as independent interventions.
As with any network meta-analysis, our study has some limitations. Although we tried our best to include all eligible literature through comprehensive and systematic review, the sample size was still small for some interventions in individual RCTs. Furthermore, not all studies reported data for each outcome measure. However, it is essential to include all eligible studies in a network meta-analysis to reduce potential biases. Finally, this study primarily compared the efficacy, acceptability, and safety of pharmacological treatments for mild to moderate AD but did not include an analysis of cost-effectiveness. It is known that AD poses an enormous economic burden, and it is necessary to consider the balance of the therapeutic effects and costs. However, there was a lack of primary data involving cost-effectiveness in the included studies.
Conclusions
In summary, our network meta-analysis findings suggested that galantamine (32 mg and 24 mg) and donepezil (5 mg and 10 mg) were the most effective strategies for improving the cognitive symptoms of patients with mild to moderate AD. We posit our findings, which we believe can support clinical decision-making. When taking acceptability and safety into account, EGb761 240 mg may be the optimal therapeutic choice. Rivastigmine 12 mg achieved the highest level of clinical global impression, and in terms of function, rivastigmine 15 cm2 patch is likely to be the best intervention. Nevertheless, none of the interventions effectively improved behavior. We hope that our study contributes markedly to the process of making accurate and efficient clinical decisions with regard to AD treatment.
Data Availability Statement
All datasets for this study are included in the Supplementary Material.
Author Contributions
TZ and HL were involved in the concept and design of the study. TZ drafted the manuscript. All authors were involved in acquisition, analysis, and interpretation of the data, revised the manuscript, and approved the final version.
Funding
This research was supported by the National Science and Technology Major Project for “Essential new drug research and development” (No. 2019ZX09301114), the National Natural Science Foundation of China (NO. 81873350) and the Beijing Natural Science Foundation (NO. 7202174).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.00778/full#supplementary-material
Abbreviations
AD, Alzheimer’s disease; AChEIs, acetylcholinesterase inhibitors; PRISRM, Preferred Reporting Items for Systematic Reviews and Meta-Analysis; RCTs, randomized controlled trials; DSM, Diagnostic and Statistical Manual of Mental Disorders; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association; MMSE, minimental state examination; ADAS-cog, Alzheimer’s Disease Assessment Scale-cognition subscale; ADCS-ADL, Alzheimer’s Disease Cooperative Study-Activities of Daily Living; NPI, Neuropsychiatric Inventory; CIBIC-Plus, Clinician’s Interview-Based Impression of Change plus Caregiver Input; SMD, standard mean difference; ORs, odds ratios; DIC, Deviance Information Criteria; SUCRA, surface under the cumulative ranking curve.
References
Akram, M., Nawaz, A. (2017). Effects of medicinal plants on Alzheimer’s disease and memory deficits. Neural Regener. Res. 12 (4), 660–670. doi: 10.4103/1673-5374.205108
Alzheimer’s Disease International. (2018). World Alzheimer Report 2018. The State of the Art of Dementia Research: New Frontiers. Accessed November 2, 2019. https://www.alz.co.uk/research/WorldAlzheimerReport2018.pdf.
Bakchine, S., Loft, H. (2008). Memantine treatment in patients with mild to moderate Alzheimer’s disease: results of a randomised, double-blind, placebo-controlled 6-month study. J. Alzheimer’s Dis. 13 (1), 97–107. doi: 10.3233/JAD-2008-13110
Bores, G. M., Huger, F. P., Petko, W., Mutlib, A. E., Camacho, F., Rush, D. K., et al. (1996). Pharmacological evaluation of novel Alzheimer’s disease therapeutics: acetylcholinesterase inhibitors related to galanthamine. J. Pharmacol. Exp. Ther. 277 (2), 728–738.
Brodaty, H., Corey-Bloom, J., Potocnik, F. C., Truyen, L., Gold, M., Damaraju, C. R. (2005). Galantamine prolonged-release formulation in the treatment of mild to moderate Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 20 (2-3), 120–132. doi: 10.1159/000086613
Burns, A., Rossor, M., Hecker, J., Gauthier, S., Petit, H., Möller, H. J., et al. (1999). The effects of donepezil in Alzheimer’s disease - Results from a multinational trial. Dement. Geriatr. Cogn. Disord. 10 (3), 237–244. doi: 10.1159/000017126
Choi, S. H., Park, K. W., Na, D. L., Han, H. J., Kim, E. J., Shim, Y. S., et al. (2011). Tolerability and efficacy of memantine add-on therapy to rivastigmine transdermal patches in mild to moderate Alzheimer’s disease: A multicenter, randomized, open-label, parallel-group study. Curr. Med. Res. Opin. 27 (7), 1375–1383. doi: 10.1185/03007995.2011.582484
Corbett, A., Pickett, J., Burns, A., Corcoran, J., Dunnett, S. B., Edison, P., et al. (2012). Drug repositioning for Alzheimer’s disease. Nat. Rev. Drug Discovery 11 (11), 833–846. doi: 10.1038/nrd3869
Costantino, G., Montano, N., Casazza, G. (2015). When should we change our clinical practice based on the results of a clinical study? Searching for evidence: PICOS and PubMed. Intern. Emerg. Med. 10 (4), 525–527. doi: 10.1007/s11739-015-1225-5
Cummings, J., Froelich, L., Black, S. E., Bakchine, S., Bellelli, G., Molinuevo, J. L., et al. (2012). Randomized, double-blind, parallel-group, 48-week study for efficacy and safety of a higher-dose rivastigmine patch (15 vs. 10 cm(2)) in Alzheimer’s disease. Dement. Geriatr. Cognit. Disord. 33 (5), 341–353. doi: 10.1159/000340056
Dias, S., Welton, N. J., Caldwell, D. M., Ades, A. E. (2010). Checking consistency in mixed treatment comparison meta-analysis. Stat. Med. 29 (7-8), 932–944. doi: 10.1002/sim.3767
Dou, K. X., Tan, M. S., Tan, C. C., Cao, X. P., Hou, X. H., Guo, Q. H., et al. (2018). Comparative safety and effectiveness of cholinesterase inhibitors and memantine for Alzheimer’s disease: a network meta-analysis of 41 randomized controlled trials. Alzheimers Res. Ther. 10 (1), 126. doi: 10.1186/s13195-018-0457-9
Feldman, H. H., Lane, R. (2007). Rivastigmine: a placebo controlled trial of twice daily and three times daily regimens in patients with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 78 (10), 1056–1063. doi: 10.1136/jnnp.2006.099424
Folstein, M. F., Folstein, S. E., McHugh, P. R. (1975). Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12 (3), 189–198. doi: 10.1016/0022-3956(75)90026-6
Frolich, L., Ashwood, T., Nilsson, J., Eckerwall, G. (2011). Effects of AZD3480 on cognition in patients with mild-to-moderate Alzheimer’s disease: a phase IIb dose-finding study. J. Alzheimers Dis. 24 (2), 363–374. doi: 10.3233/jad-2011-101554
Gault, L. M., Ritchie, C. W., Robieson, W. Z., Pritchett, Y., Othman, A. A., Lenz, R. A. (2015). A phase 2 randomized, controlled trial of the alpha7 agonist ABT-126 in mild-to-moderate Alzheimer’s dementia. Alzheimer’s Dement.: Trans. Res. Clin. Interventions 1 (1), 81–90. doi: 10.1016/j.trci.2015.06.001
Hager, K., Baseman, A. S., Nye, J. S., Brashear, H. R., Han, J., Sano, M., et al. (2014). Effects of galantamine in a 2-year, randomized, placebo-controlled study in Alzheimer’s disease. Neuropsychiatr. Dis. Treat 10, 391–401. doi: 10.2147/NDT.S57909
Haig, G. M., Pritchett, Y., Meier, A., Othman, A. A., Hall, C., Gault, L. M., et al. (2014). A randomized study of H3 antagonist ABT-288 in mild-to-moderate Alzheimer’s dementia. J. Alzheimer’s Dis. 42 (3), 959–971. doi: 10.3233/JAD-140291
Higgins, J. P., Whitehead, A. (1996). Borrowing strength from external trials in a meta-analysis. Stat. Med. 15 (24), 2733–2749. doi: 10.1002/(sici)1097-0258(19961230)15:24<2733::aid-sim562>3.0.co;2-0
Higgins, J. P., Altman, D. G., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj 343, d5928. doi: 10.1136/bmj.d5928
Homma, A., Takeda, M., Imai, Y., Udaka, F., Hasegawa, K., Kameyama, M., et al. (2000). Clinical efficacy and safety of donepezil on cognitive and global function in patients with Alzheimer’s disease: A 24-week, multicenter, double-blind, placebo-controlled study in Japan. Dement. Geriatr. Cogn. Disord. 11 (6), 299–313. doi: 10.1159/000017259
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 162 (11), 777–784. doi: 10.7326/m14-2385
Ihl, R., Tribanek, M., Bachinskaya, N. (2012). Efficacy and tolerability of a once daily formulation of Ginkgo biloba extract EGb 761® in Alzheimer’s Disease and vascular dementia: Results from a randomised controlled trial. Pharmacopsychiatry 45 (2), 41–46. doi: 10.1055/s-0031-1291217
International., Alzheimer’s disease (2019). “Alzheimers-Disease Fact Sheet”. accessed November 2. https://www.alz.co.uk/info/alzheimers-disease.
Johannsen, P., Salmon, E., Hampel, H., Xu, Y., Richardson, S., Qvitzau, S., et al. (2006). Assessing therapeutic efficacy in a progressive disease: A study of donepezil in Alzheimer’s disease. CNS Drugs 20 (4), 311–325. doi: 10.2165/00023210-200620040-00005
Jones, R. W., Soininen, H., Hager, K., Aarsland, D., Passmore, P., Murthy, A., et al. (2004). A multinational, randomised, 12-week study comparing the effects of donepezil and galantamine in patients with mild to moderate Alzheimer’s disease. Int. J. Geriatr. Psychiatry 19 (1), 58–67. doi: 10.1002/gps.1038
Karaman, Y., Erdoğan, F., Köseoğlu, E., Turan, T., Ersoy, A. O. (2005). A 12-month study of the efficacy of rivastigmine in patients with advanced moderate Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 19 (1), 51–56. doi: 10.1159/000080972
Kishi, T., Matsunaga, S., Oya, K., Nomura, I., Ikuta, T., Iwata, N. (2017). Memantine for Alzheimer’s Disease: An Updated Systematic Review and Meta-analysis. J. Alzheimers Dis. 60 (2), 401–425. doi: 10.3233/jad-170424
Li, J., Wu, H. M., Zhou, R. L., Liu, G. J., Dong, B. R. (2008). Huperzine A for Alzheimer’s disease. Cochrane Database Syst. Rev. (2), Cd005592. doi: 10.1002/14651858.CD005592.pub2
Li, D. D., Zhang, Y. H., Zhang, W., Zhao, P. (2019). Meta-Analysis of Randomized Controlled Trials on the Efficacy and Safety of Donepezil, Galantamine, Rivastigmine, and Memantine for the Treatment of Alzheimer’s Disease. Front. Neurosci. 13, 472. doi: 10.3389/fnins.2019.00472
Maher-Edwards, G., Dixon, R., Hunter, J., Gold, M., Hopton, G., Jacobs, G., et al. (2011). SB-742457 and donepezil in Alzheimer disease: A randomized, placebo-controlled study. Int. J. Geriatr. Psychiatry 26 (5), 536–544. doi: 10.1002/gps.2562
Marek, G. J., Katz, D. A., Meier, A., Greco, N., Zhang, W., Liu, W., et al. (2014). Efficacy and safety evaluation of HSD-1 inhibitor ABT-384 in Alzheimer’s disease. Alzheimer’s Dement. 10 (5), S364–S373. doi: 10.1016/j.jalz.2013.09.010
Mazza, M., Capuano, A., Bria, P., Mazza, S. (2006). Ginkgo biloba and donepezil: a comparison in the treatment of Alzheimer’s dementia in a randomized placebo-controlled double-blind study. Eur. J. Neurol. 13 (9), 981–985. doi: 10.1111/j.1468-1331.2006.01409.x
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34 (7), 939–944. doi: 10.1212/wnl.34.7.939
Nakamura, Y., Imai, Y., Shigeta, M., Graf, A., Shirahase, T., Kim, H., et al. (2011). A 24-week, randomized, double-blind, placebo-controlled study to evaluate the efficacy, safety and tolerability of the rivastigmine patch in Japanese patients with Alzheimer’s disease. Dement. Geriatr. Cognit. Dis. Extra 1 (1), 163–179. doi: 10.1159/000328929
Nasab, N. M., Bahrammi, M. A., Nikpour, M. R., Rahim, F., Naghibis, S. N. (2012). Efficacy of rivastigmine in comparison to ginkgo for treating Alzheimer’s dementia. J. Pak Med. Assoc. 62 (7), 677–680.
NICE. “Donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer’s disease. London: National Institute for Health and Care Excellence”. accessed November 2. https://www.nice.org.uk/guidance/ta217.
Peskind, E. R., Potkin, S. G., Pomara, N., Ott, B. R., Graham, S. M., Olin, J. T., et al. (2006). Memantine treatment in mild to moderate Alzheimer disease: A 24-week randomized, controlled trial. Am. J. Geriatr. Psychiatry 14 (8), 704–715. doi: 10.1097/01.JGP.0000224350.82719.83
Rafii, M. S., Walsh, S., Little, J. T., Behan, K., Reynolds, B., Ward, C., et al. (2011). A phase II trial of huperzine A in mild to moderate Alzheimer disease. Neurology 76 (16), 1389–1394. doi: 10.1212/WNL.0b013e318216eb7b
Raskind, M. A., Peskind, E. R., Wessel, T., Yuan, W. (2000). Galantamine in AD: a 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology 54 (12), 2261–2268. doi: 10.1212/wnl.54.12.2261
Rockwood, K., Fay, S., Song, X., MacKnight, C., Gorman, M. (2006). Attainment of treatment goals by people with Alzheimer’s disease receiving galantamine: A randomized controlled trial. CMAJ 174 (8), 1099–1105. doi: 10.1503/cmaj.051432
Rogers, S. L., Doody, R. S., Mohs, R. C., Friedhoff, L. T. (1998a). Donepezil improves cognition and global function in Alzheimer disease: A 15-week, double-blind, placebo-controlled study. Arch. Internal Med. 158 (9), 1021–1031. doi: 10.1001/archinte.158.9.1021
Rogers, S. L., Farlow, M. R., Doody, R. S., Mohs, R., Friedhoff, L. T. (1998b). A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Neurology 50 (1), 136–145. doi: 10.1212/WNL.50.1.136
Rosler, M., Anand, R., Cicin-Sain, A., Gauthier, S., Agid, Y., Dal-Bianco, P., et al. (1999). Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. Bmj 318 (7184), 633–638. doi: 10.1136/bmj.318.7184.633
Salanti, G., Higgins, J. P., Ades, A. E., Ioannidis, J. P. (2008). Evaluation of networks of randomized trials. Stat. Methods Med. Res. 17 (3), 279–301. doi: 10.1177/0962280207080643
Schneider, L. S., DeKosky, S. T., Farlow, M. R., Tariot, P. N., Hoerr, R., Kieser, M. (2005). A randomized, double-blind, placebo-controlled trial of two doses of Ginkgo biloba extract in dementia of the Alzheimer’s type. Curr. Alzheimer Res. 2 (5), 541–551. doi: 10.2174/156720505774932287
Seltzer, B., Zolnouni, P., Nunez, M., Goldman, R., Kumar, D., Ieni, J., et al. (2004). Efficacy of donepezil in early-stage Alzheimer disease: A randomized placebo-controlled trial. Arch. Neurol. 61 (12), 1852–1856. doi: 10.1001/archneur.61.12.1852
Tariot, P. N., Solomon, P. R., Morris, J. C., Kershaw, P., Lilienfeld, S., Ding, C. (2000). A 5-month, randomized, placebo-controlled trial of galantamine in AD. Neurology 54 (12), 2269–2276. doi: 10.1212/WNL.54.12.2269
Thancharoen, O., Limwattananon, C. (2019). Ginkgo biloba Extract (EGb761), Cholinesterase Inhibitors, and Memantine for the Treatment of Mild-to-Moderate Alzheimer’s Disease: A Network Meta-Analysis. Drugs Aging 36 (5), 435–452. doi: 10.1007/s40266-019-00648-x
Tsoi, K. K., Chan, J. Y., Chan, F. C., Hirai, H. W., Kwok, T. C., Wong, S. Y. (2019). Monotherapy Is Good Enough for Patients with Mild-to-Moderate Alzheimer’s Disease: A Network Meta-analysis of 76 Randomized Controlled Trials. Clin. Pharmacol. Ther. 105 (1), 121–130. doi: 10.1002/cpt.1104
Veroniki, A. A., Vasiliadis, H. S., Higgins, J. P., Salanti, G. (2013). Evaluation of inconsistency in networks of interventions. Int. J. Epidemiol. 42 (1), 332–345. doi: 10.1093/ije/dys222
Weinmann, S., Roll, S., Schwarzbach, C., Vauth, C., Willich, S. N. (2010). Effects of Ginkgo biloba in dementia: systematic review and meta-analysis. BMC Geriatr. 10, 14. doi: 10.1186/1471-2318-10-14
Whitehead, A., Perdomo, C., Pratt, R. D., Birks, J., Wilcock, G. K., Evans, J. G. (2004). Donepezil for the symptomatic treatment of patients with mild to moderate Alzheimer’s disease: a meta-analysis of individual patient data from randomised controlled trials. Int. J. Geriatr. Psychiatry 19 (7), 624–633. doi: 10.1002/gps.1133
Wilcock, G. K., Lilienfeld, S., Gaens, E. (2000). Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: Multicentre randomised controlled trial. Br. Med. J. 321 (7274), 1445–1449. doi: 10.1136/bmj.321.7274.1445
Wilkinson, D., Murray, J. (2001). Galantamine: A randomized, double-blind, dose comparison in patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatry 16 (9), 852–857. doi: 10.1002/gps.409
Winblad, B., Engedal, K., Soininen, H., Verhey, F., Waldemar, G., Wimo, A., et al. (2001). A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology 57 (3), 489–495. doi: 10.1212/WNL.57.3.489
Winblad, B., Cummings, J., Andreasen, N., Grossberg, G., Onofrj, M., Sadowsky, C., et al. (2007). A six-month double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer’s disease–rivastigmine patch versus capsule. Int. J. Geriatr. Psychiatry 22 (5), 456–467. doi: 10.1002/gps.1788
Xing, S. H., Zhu, C. X., Zhang, R., An, L. (2014). Huperzine a in the treatment of Alzheimer’s disease and vascular dementia: a meta-analysis. Evid. Based. Complement Alternat. Med. 2014, 363985. doi: 10.1155/2014/363985
Yancheva, S., Ihl, R., Nikolova, G., Panayotov, P., Schlaefke, S., Hoerr, R. (2009). Ginkgo biloba extract EGb 761, donepezil or both combined in the treatment of Alzheimer’s disease with neuropsychiatric features: a randomised, double-blind, exploratory trial. Aging Ment. Health 13 (2), 183–190. doi: 10.1080/13607860902749057
Yang, G., Wang, Y., Sun, J., Zhang, K., Liu, J. (2016). Ginkgo Biloba for Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr. Top. Med. Chem. 16 (5), 520–528. doi: 10.2174/1568026615666150813143520
Zhang, Z., Yu, L., Gaudig, M., Schäuble, B., Richarz, U. (2012). Galantamine versus donepezil in Chinese patients with Alzheimer’s disease: Results from a randomized, double-blind study. Neuropsychiatr. Dis. Treat 8, 571–577. doi: 10.2147/NDT.S38747
Zhang, N., Wei, C., Du, H., Shi, F. D., Cheng, Y. (2015). The Effect of Memantine on Cognitive Function and Behavioral and Psychological Symptoms in Mild-to-Moderate Alzheimer’s Disease Patients. Dement. Geriatr. Cogn. Disord. 40 (1-2), 85–93. doi: 10.1159/000430808
Zhang, Z. X., Hong, Z., Wang, Y. P., He, L., Wang, N., Zhao, Z. X., et al. (2016). Rivastigmine Patch in Chinese Patients with Probable Alzheimer’s disease: A 24-week, Randomized, Double-Blind Parallel-Group Study Comparing Rivastigmine Patch (9.5 mg/24 h) with Capsule (6 mg Twice Daily). CNS Neurosci. Ther. 22 (6), 488–496. doi: 10.1111/cns.12521
Keywords: Alzheimer’s disease, donepezil, network meta-analysis, pharmacological treatment, randomized controlled trial
Citation: Zhang T, Liu N, Cao H, Wei W, Ma L and Li H (2020) Different Doses of Pharmacological Treatments for Mild to Moderate Alzheimer’s Disease: A Bayesian Network Meta-Analysis. Front. Pharmacol. 11:778. doi: 10.3389/fphar.2020.00778
Received: 03 February 2020; Accepted: 11 May 2020;
Published: 26 May 2020.
Edited by:
Jos Tournoy, KU Leuven, BelgiumCopyright © 2020 Zhang, Liu, Cao, Wei, Ma and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Li, eHlocGxpaGFvMTk2NUAxMjYuY29t
 Tingting Zhang1
Tingting Zhang1 Wei Wei
Wei Wei Lina Ma
Lina Ma Hao Li
Hao Li