- 1Department of Pharmacy, Xijing Hospital, Fourth Military Medical University, Xi'an, China
- 2Department of Pharmacy, Shaanxi University of Chinese Medicine, Xi'an, China
- 3Department of Medicinal Chemistry, School of Pharmacy, Fourth Military Medical University, Xi'an, China
- 4Department of Pharmacy, 940 Hospital of PLA Joint Logistics Support Forces, Lanzhou, China
Resveratrol is a natural polyphenol in lots of foods and traditional Chinese medicines, which has shown promising treatment for neurodegenerative diseases (NDs). However, the molecular mechanisms of its action have not been systematically studied yet. In order to elucidate the network pharmacological prospective effects of resveratrol on NDs, we assessed of pharmacokinetics (PK) properties of resveratrol, studied target prediction and network analysis, and discussed interacting pathways using a network pharmacology method. Main PK properties of resveratrol were acquired. A total of 13,612 genes related to NDs, and 138 overlapping genes were determined through matching the 175 potential targets of resveratrol with disease-associated genes. Gene Ontology (GO) function analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment were performed to obtain more in-depth understanding of resveratrol on NDs. Accordingly, nodes with high degrees were obtained according using a PPI network, and AKT1, TP53, IL6, CASP3, VEGFA, TNF, MYC, MAPK3, MAPK8, and ALB were identified as hub target genes, which showed better affinity with resveratrol in silico studies. In addition, our experimental results demonstrated that resveratrol markedly enhanced the decreased levels of Bcl-2 and significantly reduced the increased expression of Bax and Caspase-3 in hippocampal neurons induced by glutamate exposure. Western blot results confirmed that resveratrol inhibited glutamate-induced apoptosis of hippocampal neurons partly by regulating the PI3K/AKT/mTOR pathway. In conclusion, we found that resveratrol could target multiple pathways forming a systematic network with pharmacological effects.
Introduction
Neurodegenerative diseases (NDs) are multifactorial debilitating disorders that are characterized by progressive dysfunction and neuronal injury, which preferentially affect the normal functioning of the brain including learning and memory (Jakaria et al., 2019). Examples of NDs are Alzheimer's diseases (AD), Parkinson's disease (PD), Huntington's diseases (HD), amyotrophic lateral sclerosis (ALS), and spinocerebellar ataxia (SCA) (Rahman et al., 2019). Notwithstanding, although the different clinical and neuropathological characteristics of each ND (Raza et al., 2018), there are several common pathological mechanisms of NDs, which are characterized by multiple targets. Most importantly, NDs are typically characterized by loss of neurons (Velmurugan et al., 2018). Some studies suggested that the pathogenesis of these kinds of diseases is the result of multiple pathological mechanisms or processes, such as glutamate excitotoxicity, oxidative stress, and abnormal apoptosis (Huang et al., 2018). Some symptomatic treatments are offered, but particular therapies have not been found owing to conventional philosophy of “one gene, one drug, one disease” (Esteves, 2017). Accordingly, the multitargeted natural products with substantial pharmacological activities are most likely to have potential advantages. Resveratrol is a natural polyphenol with a stilbene scaffold (Figure 1) in numerous foods including grapes, mulberries, and blueberries, which has shown many beneficial properties including antioxidant, antiinflammation, neuroprotective, and even antiaging activities. And resveratrol has revealed appealing outcomes as treatment for several NDs in preclinical studies (Penalver et al., 2018). But the mechanism by which resveratrol displays its protective function is not extremely well comprehended yet (Sanchez-Melgar et al., 2019).
Network pharmacology as an emerging discipline located on the general concepts of systems biology (Zhou et al., 2019), which was utilized to systematically evaluate pharmacological effects of multiingredient medicine (Zhang R. et al., 2019). In addition, it similarly has been provided lately for revealing the molecular mechanisms of various complicated chronic diseases, such as NDs and cardiocerebral vascular diseases (Wang W. et al., 2019). Therefore, network analysis based on lots of existing databases enables us to produce a preliminary understanding of the mechanisms by which multitarget drugs treat complex diseases.
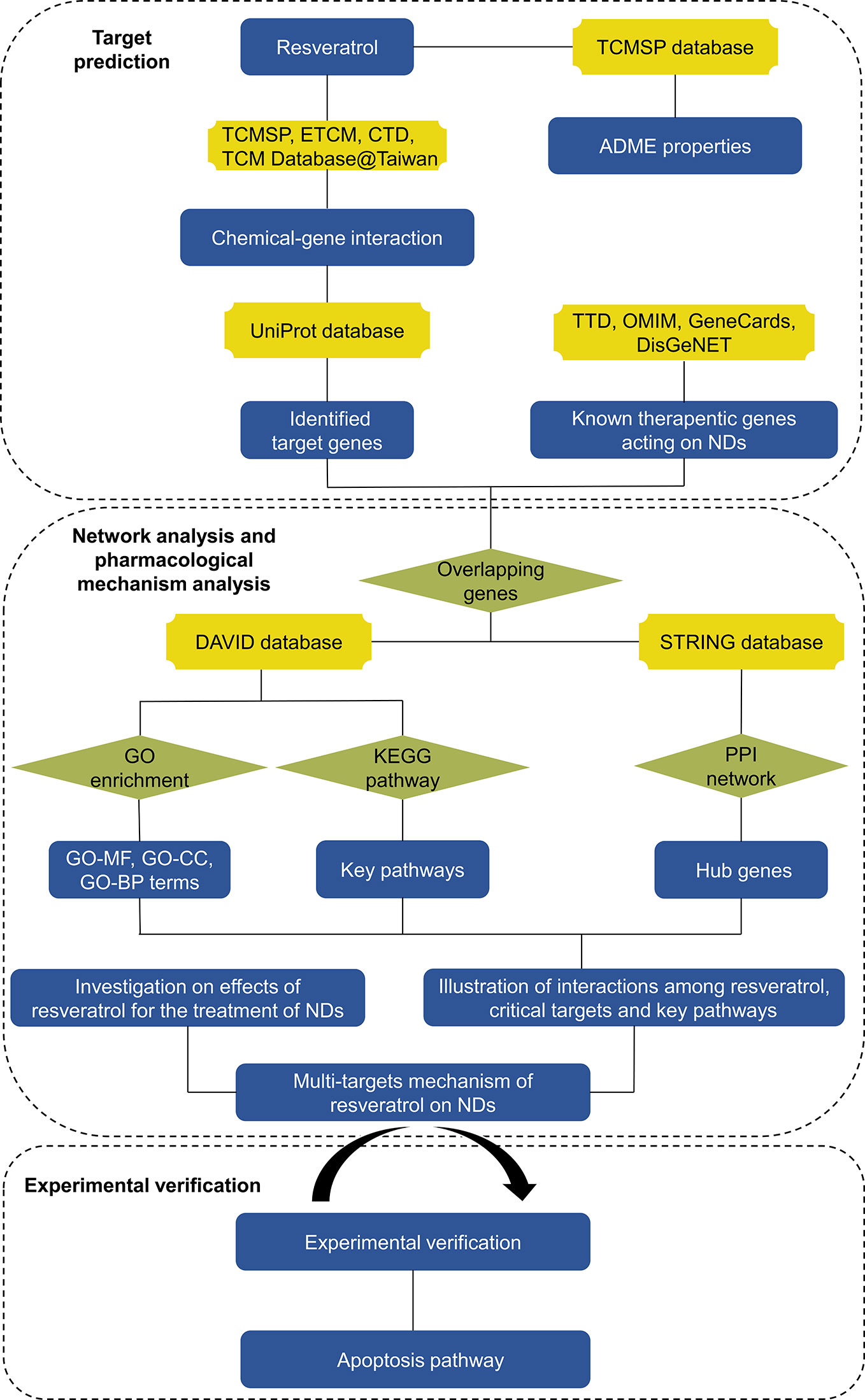
In present study, we illuminated the pharmacological actions of resveratrol on NDs systematically utilizing network pharmacology methods. Resveratrol was hypothesized to have therapeutic effects on NDs through multiple-targets mechanism. First, pharmacokinetic (PK) parameters of resveratrol were acquired from Traditional Chinese Medicine Systems Pharmacology Database (TCMSP) server. Potential candidate targets of resveratrol and therapeutic target genes of NDs were gathered respectively. Then, the potential candidate target genes were predicted via network pharmacology databases. In addition, multitarget of resveratrol network was constructed to supply a methodical overview. Furthermore, pivotal target genes, Gene Ontology (GO) function analysis and KEGG pathway enrichment were evaluated by STRING database and DAVID database. Finally, key targets and signaling pathways were identified by western blot. Network pharmacology analysis workflow was shown in Figure 2.
Material and Methods
Assessment of PK Parameters
PK parameters of resveratrol were acquired from TCMSP database version 2.3 (http://tcmspw.com/tcmsp.php) (Ru et al., 2014), which is a phytochemical database for TCMs or related ingredients. Meanwhile, the information of absorption, distribution, metabolism, and excretion (ADME) properties of a drug with potential biological activities also can be acquired, such as oral bioavailability (OB), drug likeness (DL), Caco-2 permeability (Caco-2), blood-brain barrier (BBB). In this study, with the chemical name “resveratrol” as the keyword, and PK properties of resveratrol were searched in the search box.
Construction and Identification of Target Genes
All of genes associated to resveratrol were gathered from the databases: TCMSP database version 2.3 (http://tcmspw.com/tcmsp.php), TCM Database@Taiwan (http://tcm.cmu.edu.tw/) (Chen, 2011), the Comparative Toxicogenomics Database (CTD, http://ctdbase.org/) (Davis et al., 2019), and the Encyclopedia of Traditional Chinese Medicine (ETCM, www.nrc.ac.cn:9090/ETCM/) (Xu et al., 2019). Subsequently, the official names of gene were drawn from UniProt database (http://www.uniprot.org/) (Uniprot, 2019) by restricting the types to “Homo sapiens.” Then, different genes' ID terms were converted into UniProt IDs. And a resveratrol-targets relationship dataset was constructed.
Gene Dataset Acquisition of NDs
With “NDs,” “ADs,” “PD,” “HDs,” “ALS,” and “SCA” as the keywords, then therapeutic target genes of NDs were acquired from the Therapeutic Target Database (TTD, https://db.idrblab.org/ttd/) (Wang et al., 2020), the Online Mendelian Inheritance in Man (OMIM, http://www.omim.org/) (Amberger et al., 2015), GeneCards (https://www.genecards.org/) (Fishilevich et al., 2016) and a database of gene-disease associations (DisGeNET, http://www.disgenet.org/) (Pinero et al., 2017), and just “Homo sapiens” proteins linked to NDs were selected.
GO Function Enrichment and KEGG Pathway Analysis
A pharmacology network is comprised of nodes and edges. The entities that make up the nodes of the networks are resveratrol NDs and related target genes. The Cytoscape version 3.7.2 was used to constructed networks, which is a java based open source software (Demchak et al., 2014). Functional pathways of resveratrol related to NDs were analyzed using GO enrichment and KEGG pathways analysis based upon the database for Annotation, Visualization and Integrated Discovery (DAVID) version 6.8 (https://david.ncifcrf.gov/) (Ke et al., 2019). P < 0.05 suggested the enrichment degree had statistically significant and the pathway results might be essential functional mechanisms of resveratrol in the treatment of NDs.
Construction of Target Protein-Protein Interaction (PPI) Data
The potential interprotein interactions were obtained from STRING database version 11.0 (https://string-db.org/), which is a database of known and predicted protein-protein interactions (Ge et al., 2018). The software produces scores information for each pair of protein. The higher the score, the more confident the target protein's interactions were. Thus, the potential targets of resveratrol on NDs were imported into STRING tool to acquire potential interprotein interactions. We selected a high confidence score > 0.7 with the species restricted to “Homo sapiens” (Szklarczyk et al., 2019). Then, target genes with high degree, betweenness, and closeness were selected as the hub genes of NDs.
In Silico Docking Study of Resveratrol With Key Targets
A study of in silico docking of resveratrol with key targets was conducted by Autodock Vina (Trott and Olson, 2010). For this purpose, resveratrol was prepared and optimized using the PubChem database (https://www.ncbi.nlm.nih.gov/), then converted to the PDB file format. Receptor structures were downloaded from the RCSB Web site (http://www.rcsb.org/pdb) in PDB format. Before docking, the original crystal ligands and water molecules were removed from the protein-ligand complexes. Hydrogen atoms and charge were added, and default settings were selected for other parameters. The theoretical binding affinities of resveratrol to proteins are predicted based on docking score.
Cell Culture and Treatments
The study was reviewed and approved by Ethics Committee of Animal Experimentation of the Fourth Military Medical University (Xi'an China). Primary hippocampal neurons were prepared from embryonic d15 mouse embryos. Embryonic brain tissue was mechanically triturated and centrifuged. Neurons were cultured in the atmosphere of 5%/95% CO2/air at 37°C using the Dulbecco's modified Eagle's medium (DMEM) which contains 10% fetal bovine serum, 100 U/ml of penicillin, and 100 μg/ml streptomycin. The coincubation model incorporating samples and glutamate (25 mM) was used to evaluate the protective effects of resveratrol (50 mg/ml) on glutamate-induced apoptotic cells. The equivalent volume of PBS and resveratrol were used in the control groups, respectively. To investigate the involvement of PI3K/AKT/mTOR in the effects of resveratrol, phosphatidylinositol-3-kinase (PI3K) inhibitors, LY294002 (10 μM) was added to glutamate-stimulated hippocampal neurons. All operations were repeated over three times.
Western Blot Analysis
Primary hippocampal neurons were lysed at indicated time points with 40-μl RIPA ice-cold lysis buffer (Rockford, IL, USA) supplemented with protease inhibitor (Vazyme Biotech, Nanjing, China) for 30 min and centrifuged at 9,000 g for 10 min at 4°C. The protein concentrations were analyzed by the BCA (Rockford, IL, USA). Equal amounts of proteins were separated and transferred to a PVDF membrane (Millipore, USA). The membranes were blocked with 3% BSA in TBS/T and stained with primary antibodies (1:1,000) and antibodies for β-actin (1:10,000) overnight at 4°C. Membranes were then probed with peroxidase conjugated secondary antibody at a 1:10,000 dilution. The antigen-antibody complexes were detected with an ECL reagent (Rockford, IL, USA). Primary antibodies used for western blotting were mouse antibodies. Secondary antibodies used were peroxidase conjugated goat antimouse antibodies.
Statistical Analysis
The data of the experiment was expressed as means ± SEM and analyzed using one-way ANOVA. P < 0.05 was a significant difference and P < 0.01 is a very significant difference.
Results
PK Parameters
The information of resveratrol on 12 main characteristics like Caco-2 and BBB for drug screening and evaluation (Table 1). Significantly, OB is the primary feature of oral medications because it plays a critical part in assessing the effectiveness of drug distribution for systemic circulation. In spite of the low bioavailability of resveratrol (its OB was calculated to be 19.07%), there are lots of evidence that resveratrol has the therapeutic potential on neurodegeneration (Yang et al., 2017). Furthermore, resveratrol has a relatively small molecular weight and moderate blood-brain barrier permeability with a BBB value of −0.01.
Potential Target Genes and Network Analysis
A total of 182 candidate target genes were identified (Supplementary Table 1). Afterwards, seven were duplicated and therefore removed, 175 unique target genes for resveratrol remained (Figure 3A). Based on gene databases, we determined 13,612 genes concerned NDs (Supplementary Table 2). Then, 138 overlapping genes were identified through matching the potential targets of resveratrol with disease-associated genes (Figure 3B). Despite the different clinical and neuropathological characteristics of each ND, which are characterized by multiple targets, the underlying causes at the molecular level are almost similar (Suresh et al., 2020). Therefore, resveratrol reveled multitarget effects, which can regulate the variety of changes associated with NDs, unlike specific target drugs.
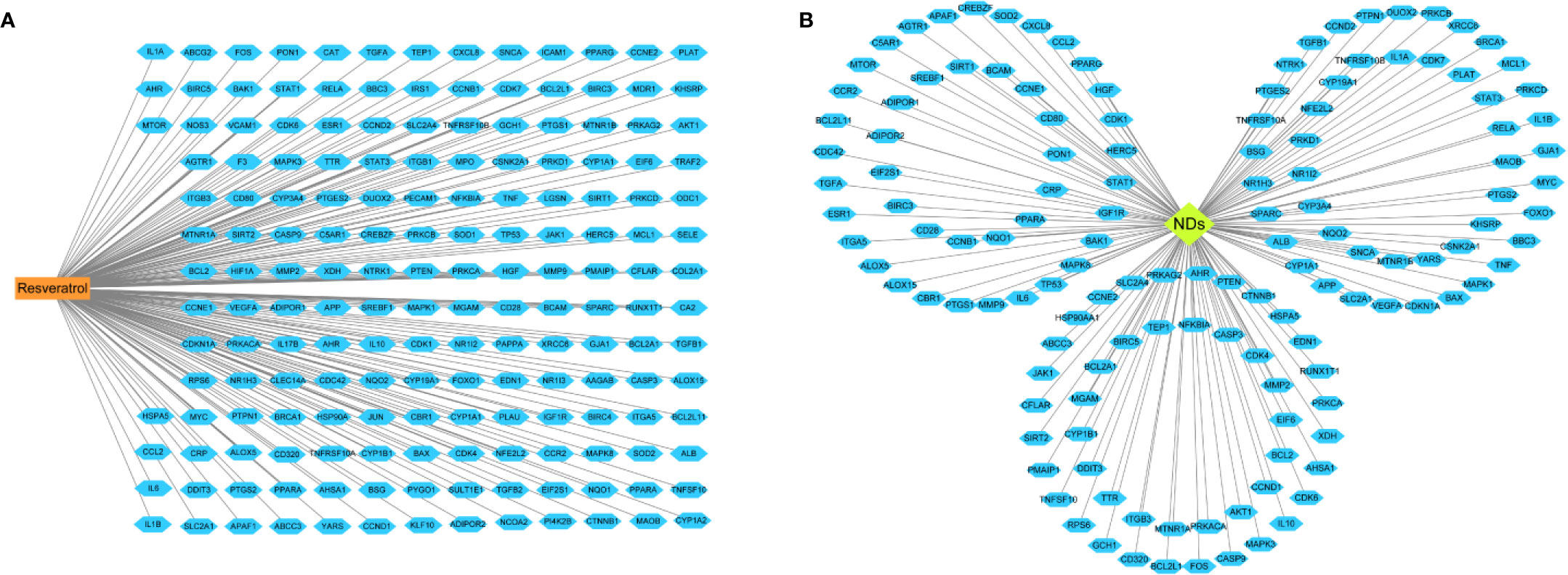
Figure 3 Linkage of drug, disease, and target genes. (A) The network of resveratrol- candidate targets. (B) Network of 138 common potential protein targets related to neurodegenerative diseases (NDs). The orange rectangle represents the resveratrol, green diamond means NDs and blue hexagon represent the target genes on which the drug acts.
GO Term Enrichment and Pathway Analysis
To elucidate the function and pharmacological mechanism of resveratrol, we conducted GO enrichment and KEGG pathway analysis of the 138 determined targets. GO analysis (Supplementary Table 3) is determined by the biological process (BP), cell component (CC), and molecular function (MF) terms. An introduction of the GO enrichment was discovered with the leading five enriched entries in the BP, CC, and MF terms (Figure 4A, P < 0.05). Especially, the enriched BP ontologies were dominated by positive regulation of transcription from RNA polymerase II promoter, DNA-templated transcription, and negative regulation of apoptotic process, etc. The enriched MF ontologies were dominated by ATP binding, DNA binding, and so on. The nucleus accounted for the largest proportion in CC analysis (42 target genes). Then, a total of 119 significant pathways were acquired (P < 0.05). Among them, 20 significant pathways were showed in Figure 4B. As shown, FoxO, PI3K-Akt, p53 and apoptosis signaling pathways may be the interaction pathways to apply their combined results versus NDs.
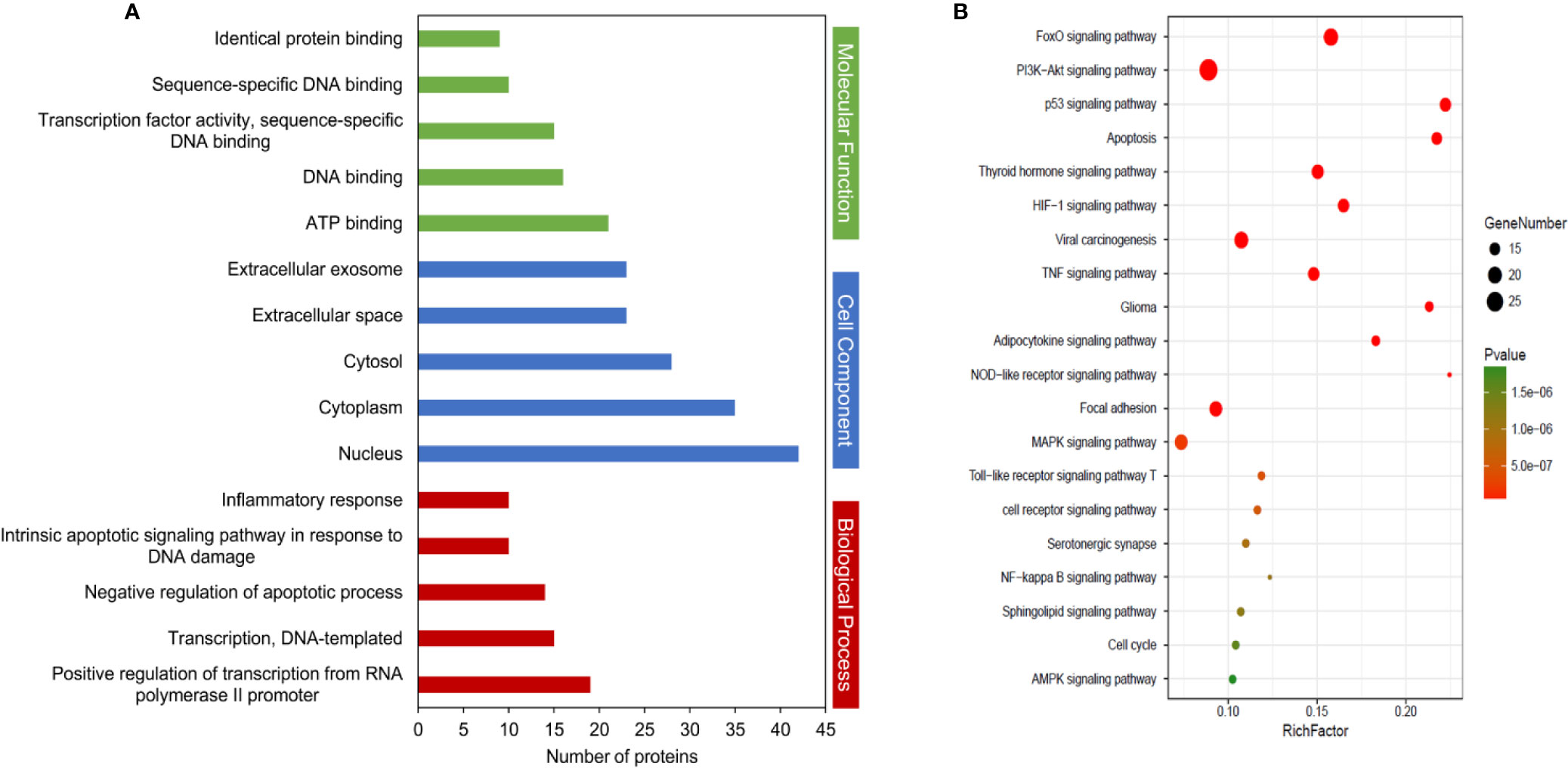
Figure 4 Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways and Gene Ontology (GO) analyses by database for Annotation, Visualization and Integrated Discovery (DAVID) database. (A) GO enrichment analysis of target proteins. The number of GO entries in the functional categories of cell composition (CC), molecular function (MF), and biological process (BP) (P < 0.05). (B) KEGG pathways of target genes (P < 0.05).
PPI Network of Target Genes
The 138 target genes were submitted to STRING tool to acquire PPI relationships. We selected high-confidence target protein based on interaction with a score of > 0.7 to ensure the dependability of the study, and got the network of PPI relationships (Figure 5A). The targets with high degree, betweenness, and closeness were chosen as the hub genes for NDs (Figure 5B). And the predicted mode of action of 10 hub genes was shown in Figure 5C. The hub genes were including RAC-alpha serine/threonine-protein kinase (AKT1), cellular tumor antigen p53 (TP53), interleukin 6 (IL6), caspase-3 (CASP3), vascular endothelial growth factor A (VEGFA), tumor necrosis factor (TNF), myc proto-oncogene protein (MYC), mitogen-activated protein kinase 3 (MAPK3), mitogen-activated protein kinase 8 (MAPK8), and serum albumin (ALB). Among the above 10 hub proteins, such as AKT1, TP53, CASP3, and TNF, are involved in external or internal apoptotic pathways. Therefore, Resveratrol may play a protective role in NDs by regulating the apoptotic pathways involved in AKT1, TP53, CASP3, and TNF proteins (Figure 6).
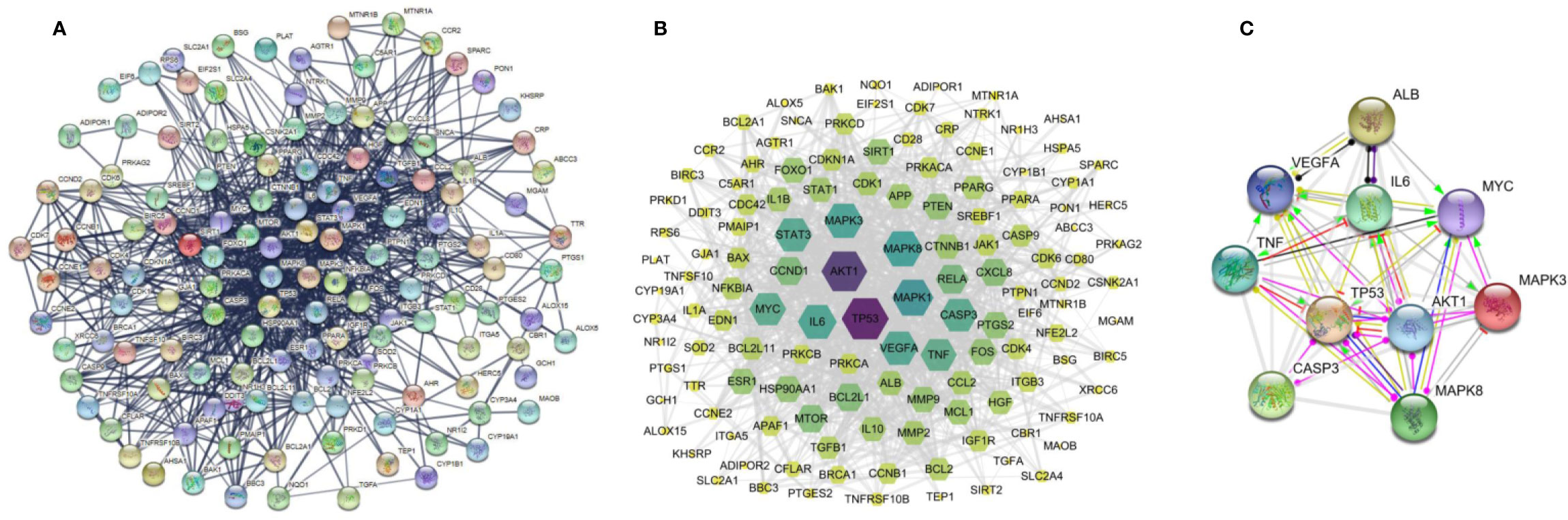
Figure 5 Protein-protein interaction (PPI) networks of resveratrol for the treatment of neurodegenerative diseases (NDs). (A) All nodes represent the relevant to genes, the edge means line thickness indicates the strength of data support. (B) The target genes with high degree, betweenness, and closeness. And (C) the predicted mode of action of 10 hub genes, “→” represents activation, “—|” means inhibition, and “—•” represents unspecified.
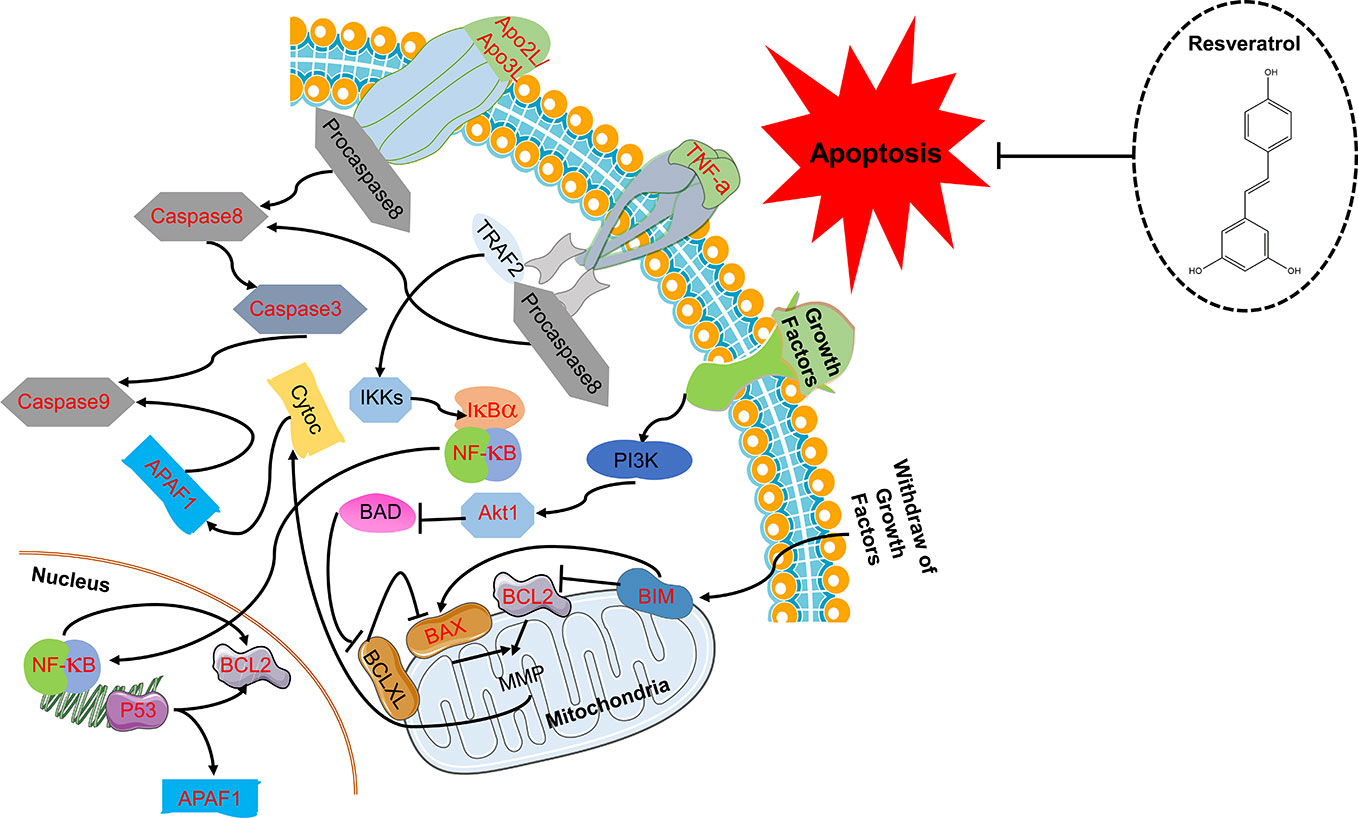
Figure 6 Resveratrol plays a protective role in neurodegenerative diseases (NDs) by regulating the apoptotic pathways involved in AKT1, TP53, CASP3, and TNF proteins.
Molecular Docking Analysis
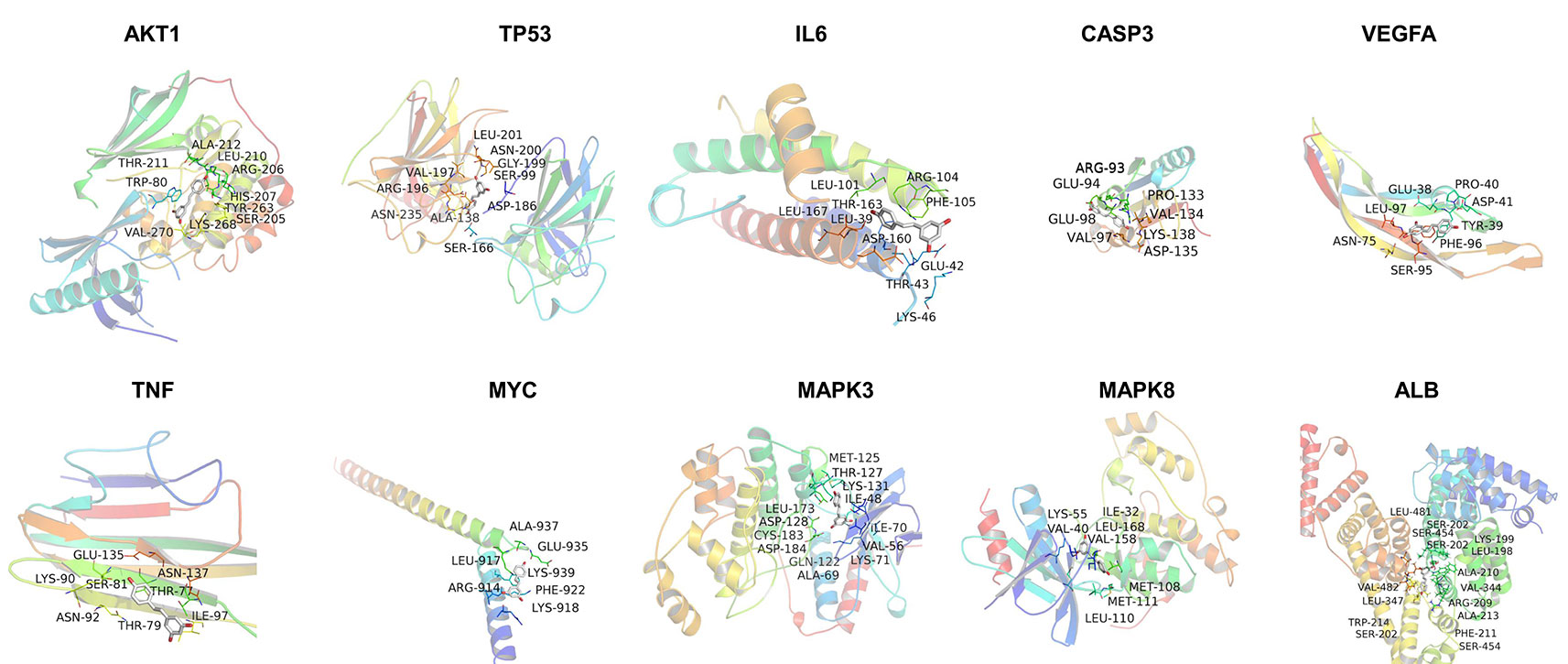
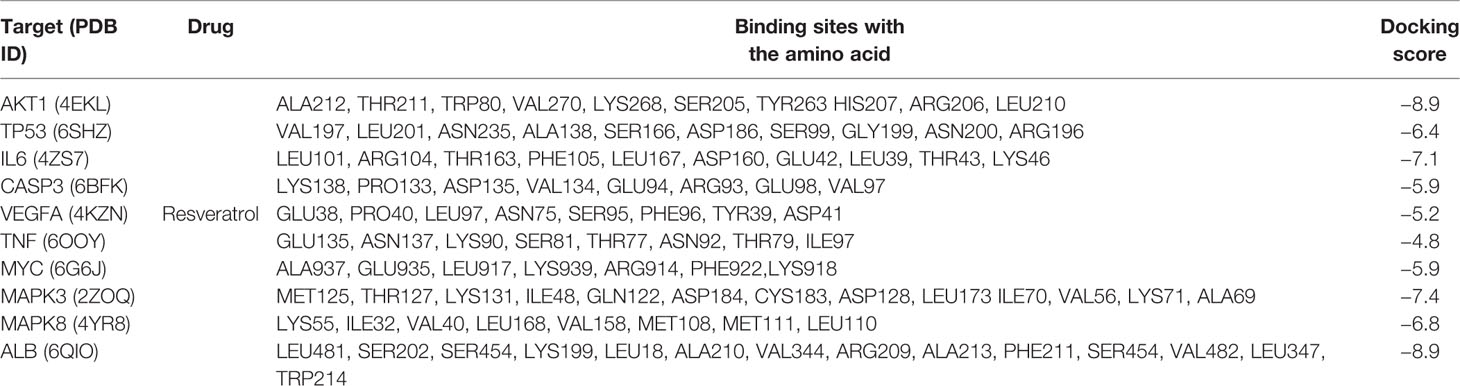
In silico studies were conducted to study the binding affinity of resveratrol with key target receptors. The results revealed that docking scores of resveratrol with AKT1, TP53, IL6, CASP3, VEGFA, TNF, MYC, MAPK3, MAPK8, and ALB ranged from −4.8 to −8.9. Particularly, resveratrol had the highest docking score with AKT1 (docking score: −8.9), which indicated that resveratrol was well located inside the binding site with AKT1. Other hub proteins also showed better affinity with resveratrol, as shown in Figure 7 and Table 2.
Experimental Validation
The protective effects of resveratrol on glutamate−induced injury in the culture model in vitro were determined. In fact, FoxO, PI3K-Akt, and p53 signaling pathways are also involved in regulating apoptosis (Akhter et al., 2014). Thus, based on the hub targets and KEGG pathway enrichment, we chose the apoptosis-related protein Bax, Bcl-2, and Caspase-3 for validation the mechanisms underlying the neuroprotective effects of resveratrol. Resveratrol markedly reinforced the decreased levels of Bcl-2 in glutamate treated hippocampal neurons (P < 0.01). Meanwhile, resveratrol significantly reduced the increased expression of Bax and Caspase-3 induced by glutamate exposure (P < 0.01, Figure 8).
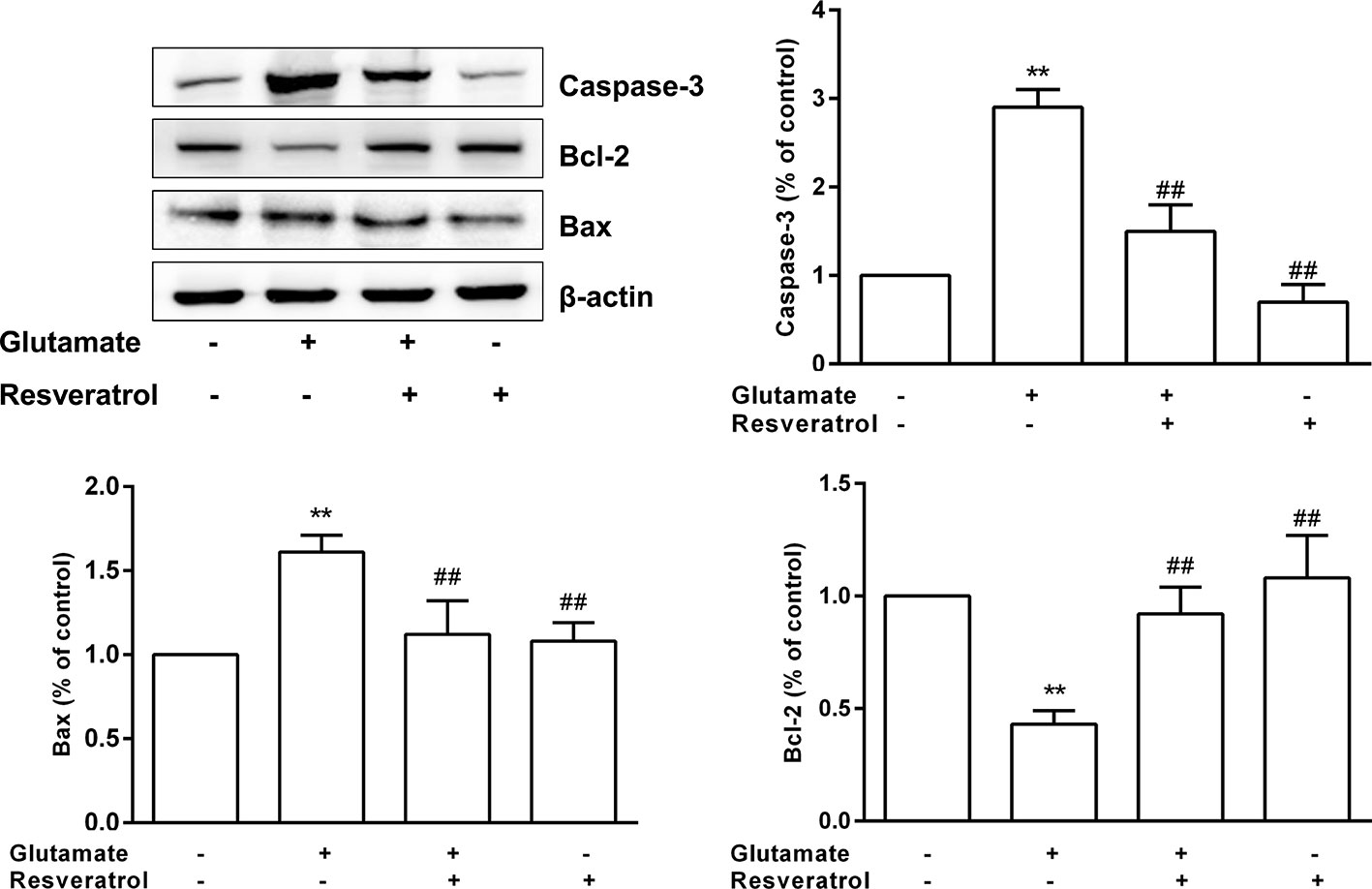
Figure 8 Effects of resveratrol on the expression of Caspase-3, Bcl-2, and Bax induced by glutamate exposure in hippocampal neurons. **P < 0.01 compared with control; ##P < 0.01 compared with glutamate alone.
The results of molecular docking showed that resveratrol was well located inside the binding site with AKT and PI3K (docking score: −7.5, Figure 9A), the upstream protein of AKT. It is well known that PI3K/AKT/mTOR signaling pathway is an important pathway mediating cell survival and differentiation, proliferation, apoptosis, and metastasis (Hou et al., 2018). Then we further confirmed whether resveratrol inhibited glutamate-induced apoptosis of hippocampal neurons by regulating the PI3K/AKT/mTOR pathway. The results revealed that resveratrol significantly reinforced the decreased the levels of p-PI3K, p-AKT, and p-mTOR after glutamate exposure (P <0.05, P <0.01). Additionally, LY294002 (PI3K inhibitor) significantly attenuated the effects of resveratrol (P <0.05, P <0.01), which can be observed from the protein expression of p-PI3K, p-AKT, p-mTOR and Bcl-2, Bax, and Caspase-3 (Figure 9). These results suggested that resveratrol plays a neuroprotective role partly by activating the PI3K/AKT/mTOR pathway to inhibit the apoptosis of neurons.
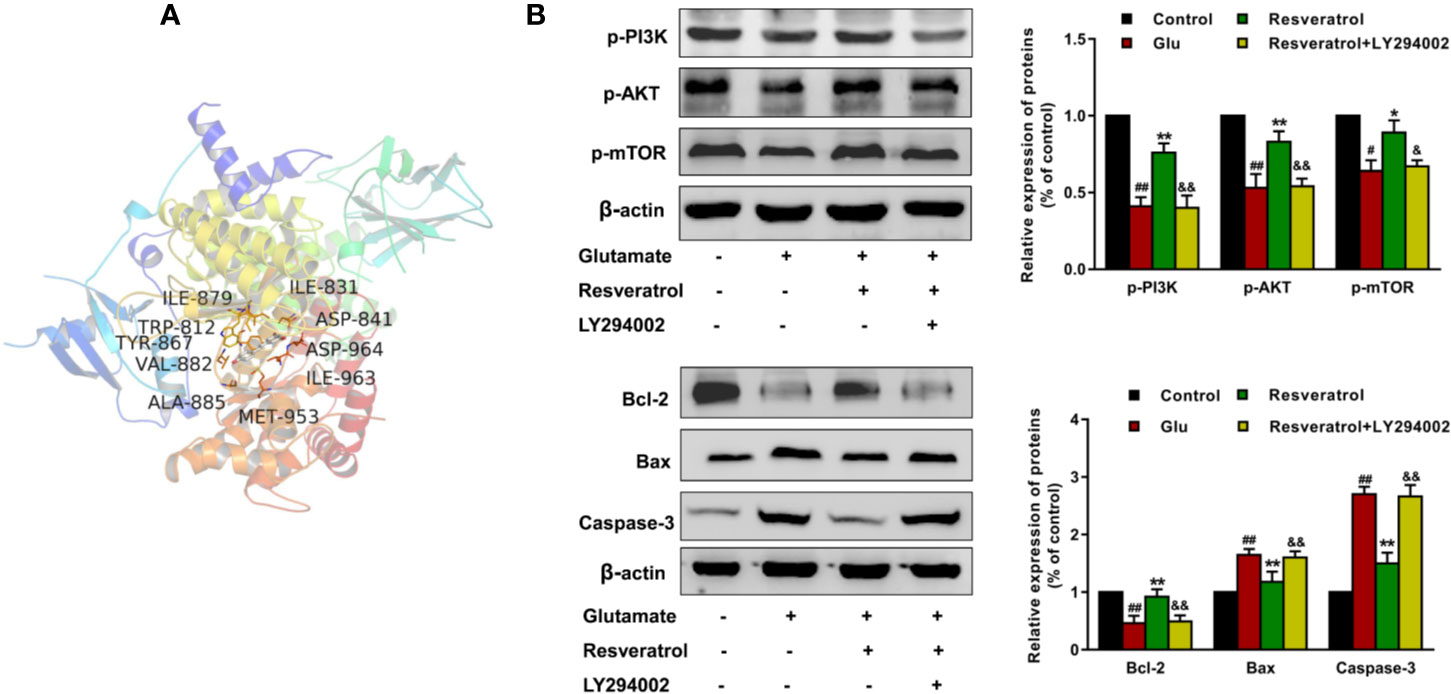
Figure 9 Resveratrol inhibited glutamate-induced apoptosis of hippocampal neurons by regulating the PI3K/AKT/mTOR pathway. (A) Structural interactions of resveratrol with PI3K (PDB ID: 6AUD) in silico studies. (B) Effects of resveratrol on protein expression of p-PI3K, p-AKT, p-mTOR, Bcl-2, Bax, and Caspase-3 after glutamate-induced apoptosis of hippocampal neurons. ##P < 0.01 compared with control; *P < 0.05 and **P < 0.01 compared with glutamate alone; &P < 0.05 and &&P < 0.01 compared with resveratrol treated.
Discussion and Conclusion
NDs are common ailments in the elderly, and they are rapidly rising in prevalence as members of society age (Bianchi et al., 2019). And NDs vary in pathophysiology, such as memory and cognitive impairments, and the ability to move, speak, and breathe is affected. There is an urgent need to search for more effective therapeutic strategies to curb the progress of NDs. Moreover, it is important to gain insight into the causes and mechanisms of each disease (Gitler et al., 2017). Current advances in network pharmacology have provided brand-new chances to elucidate the treatment of certain complex diseases with certain drugs (Zhang et al., 2016). In this study, 12 very important PK properties of resveratrol were acquired from the TCMSP database. A total of 13,612 genes relevant to NDs, and 138 overlapping genes were identified through matching the potential targets of resveratrol with disease-associated genes. To acquire a more in-depth understanding of resveratrol on NDs, we performed GO function analysis and KEGG pathway enrichment. Accordingly, genes with degree, betweenness and closeness of differential expression were gotten according utilizing a PPI network, and AKT1, TP53, IL6, CASP3, VEGFA, TNF, MYC, MAPK3, MAPK8, and ALB were identified as hub nodes. These hub targets showed better affinity with resveratrol in silico studies. In addition, our experimental results demonstrated that suggested that resveratrol plays a neuroprotective role partly by activating the PI3K/AKT/mTOR pathway to inhibit the apoptosis of neurons.
Toxicity and PK are the most important characteristics should be given priority in drug research (Zhang Y.F. et al., 2019). Resveratrol is a natural nonflavonoid polyphenol, but its intense metabolism and particularly low OB seem to limit its application in human therapeutics. However, resveratrol nanoformulations are being looked upon as a resolution to these PK issues (Summerlin et al., 2015). Additionally, Lipinski's rule of five can determine some main drug properties: the compounds with molecular weight from 180 to 500 Dalton are viewed as more druggable, AlogP value is less than 5, numbers of possible hydrogen-bond donors and acceptors are less than 5 and 10, respectively (Chagas et al., 2018). And resveratrol could be up to these standards, suggesting it could be considered a lead compound that can be structurally optimized. Thus, resveratrol is a prospect for drug development.
A growing number of studies have suggested that resveratrol is a multitarget treatment for NDs. In this study, AKT1, TP53, IL6, CASP3, VEGFA, TNF, MYC, MAPK3, MAPK8, and ALB were identified as hub genes in the PPI analysis. Similarly, several previous studies have addressed gene expression changes. The majority of these studies compared the transcriptional levels of genes in cells or animals. AKT1 exerts major influences on the regulation of cell proliferation, cell survival, and protein synthesis (Ahmad et al., 2014; Palmieri et al., 2017). In fact, resveratrol has been shown to be protective in cellular and animal models of neurodegeneration by enhancing AKT1 activity (Zhang et al., 2014; Wang et al., 2018). TP53 is a crucial protein in NDs (Goiran et al., 2018). Several studies consistently suggested that TP53-dependent apoptotic cells are detectable in specific locations in PD and AD (Checler et al., 2018). Neuroinflammation is intensively demonstrated to be related with various NDs (Qi et al., 2019). Especially, the findings of the meta-analysis demonstrated higher peripheral concentrations of IL-6 and TNF in patients with PD (Qin et al., 2016). CASP3 plays a crucial function in intrinsic and extrinsic pathways of programed cell death and in cell proliferation. Previous study reported that the administration of resveratrol could increase proinflammatory cytokine levels and inhibit apoptosis in the hippocampus (Shen et al., 2019). VEGFA is a proangiogenic factor, which also involves in neuroprotection, neurogenesis, synaptic plasticity, and modulation of inflammation and astrocyte proliferation (Harris et al., 2018; Wei et al., 2018). Recently, human induced pluripotent stem cells, which are produced from somatic cells by overexpressing four reprogramming factors (including MYC), were applying in the cellular therapy of NDs (Berry et al., 2018). ALB is the most plentiful protein in blood plasma and cerebrospinal fluid, where it contributes substantially to regulate the osmotic pressure and metabolic processes (Ts et al., 2017). Studies have demonstrated that resveratrol stabilizes the protein structure and regulates the development of fibrils along the preliminary stage of the ALB aggregation pathway (Stirpe et al., 2016). Simultaneously, MAPK is a crucial target for chronic inflammatory diseases such as AD (Criscuolo et al., 2017). It was reported that resveratrol reduced the upregulated protein expression of AMPK to prevent alcohol−induced neurodegeneration (Gu et al., 2018).
GO and KEGG pathway analyses were performed to better understand the interactions of the target genes. In results, GO analysis revealed that target genes were majorly related to the BPs of positive regulation of transcription from RNA polymerase II promoter, DNA-templated transcription, and negative regulation of apoptotic process, etc. The enriched MF ontologies were dominated by ATP binding, DNA binding, and so on. The nucleus accounted for the largest proportion in CC analysis. Furthermore, KEGG pathway analysis indicated that FoxO signaling pathway, PI3K-Akt signaling pathway and apoptosis, etc. may be the interaction pathways to apply their combined results versus NDs. These results followed the previous reports that above pathways participate in crucial functions in the progression of NDs (Huang et al., 2019; Qi et al., 2019; Rai et al., 2019).
NDs are typically characterized by loss of neurons. Studies have confirmed that apoptosis is an important molecular biological mechanism that is closely related to NDs (Ghavami et al., 2014). Wang N. et al. (2019) reported that resveratrol improves cognitive function of rats and reduces oxidative stress-induced neuronal damage in the frontal cortex and hippocampus by inhibiting neuronal apoptosis. In present study, among the 10 hub proteins from PPI network, such as AKT1, TP53, CASP3, and TNF, are involved in external or internal apoptotic pathways (Figure 6). And FoxO, PI3K-Akt, and p53 signaling pathways are also involved in regulating apoptosis. Apoptosis is a process of programmed cell death, which is vital for normal neural development (Ghavami et al., 2014). Under pathologic conditions, apoptosis also coresponsible for the loss of neurons associated with NDs (Maino et al., 2017). The proteins of Bcl-2 family, caspases and Apaf1 are main molecular components of the apoptosis program (Burek et al., 2006). Particularly, Bax gene is the first known proapoptotic member (Li et al., 2017). It has been reported that Bax promotes the release of cytochrome C by transferring it to the mitochondrial membrane, accordingly promoting downstream cell apoptosis (Kosten et al., 2008). Bcl-2 is a key member of the antiapoptotic Bcl-2 family (Garner et al., 2017). Overexpressed Bcl-2 has been demonstrated to protect nerve cells from damage by neurotoxins (Laulier and Lopez, 2012). Caspase-3 is a type of proapoptotic enzyme that plays the role of the apoptotic executor (Zhang Y. et al., 2019). Our results demonstrated that resveratrol markedly enhanced the decreased levels of Bcl-2 and significantly reduced the increased expression of Bax and Caspase-3 in hippocampal neurons induced by glutamate exposure. Then, Western blot results further confirmed that resveratrol inhibited glutamate-induced apoptosis of hippocampal neurons partly by regulating the PI3K/AKT/mTOR pathway. This further suggested that resveratrol plays a protective role against NDs through the apoptosis pathway.
In summary, resveratrol is an active and promising compound that is expected to be developed as a safe and effective multitarget drug against NDs. Our network pharmacological analysis of resveratrol predicted that the therapeutic effects of resveratrol related to NDs through mechanisms regulated by active compounds and apoptosis associated signaling pathways, such as PI3K/AKT/mTOR pathway. Further verification experiments study is necessary to explore and the key mechanisms resveratrol.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The study was reviewed and approved by Ethics Committee of Animal Experimentation of the Fourth Military Medical University (Xi'an China).
Author Contributions
YD, WW, and AW conceived and proposed the idea. WW, SW, YM, and TL designed the research, analyzed the data and wrote the paper. SH, LL, and YD revised the manuscript. All authors read the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81603385) and the Booster Plan of Xijing Hospital (XJZT18D06).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.00694/full#supplementary-material
Supplementary Table 1 | The identified 175 potential targets of resveratrol.
Supplementary Table 2 | The identified 13612 genes relevant to NDs.
Supplementary Table 3 | The enriched GO terms and KEGG pathways based upon DAVID database.
References
Ahmad, F., Nidadavolu, P., Durgadoss, L., Ravindranath, V. (2014). Critical cysteines in Akt1 regulate its activity and proteasomal degradation: implications for neurodegenerative diseases. Free Radic. Biol. Med. 74, 118–128. doi: 10.1016/j.freeradbiomed.2014.06.004
Akhter, R., Sanphui, P., Biswas, S. C. (2014). The essential role of p53-up-regulated modulator of apoptosis (Puma) and its regulation by FoxO3a transcription factor in beta-amyloid-induced neuron death. J. Biol. Chem. 289, 10812–10822. doi: 10.1074/jbc.M113.519355
Amberger, J. S., Bocchini, C. A., Schiettecatte, F., Scott, A. F., Hamosh, A. (2015). OMIM.org: Online Mendelian Inheritance in Man (OMIM(R)), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 43, D789–D798. doi: 10.1093/nar/gku1205
Berry, B. J., Smith, A. S. T., Young, J. E., Mack, D. L. (2018). Advances and Current Challenges Associated with the Use of Human Induced Pluripotent Stem Cells in Modeling Neurodegenerative Disease. Cells Tissues Organs 205, 331–349. doi: 10.1159/000493018
Bianchi, V. E., Herrera, P. F., Laura, R. (2019). Effect of nutrition on neurodegenerative diseases. A systematic review. Nutr. Neurosci. 4, 1–25. doi: 10.1080/1028415X.2019.1681088
Burek, M., Maddika, S., Burek, C. J., Daniel, P. T., Schulze-Osthoff, K., Los, M. (2006). Apoptin-induced cell death is modulated by Bcl-2 family members and is Apaf-1 dependent. Oncogene 25, 2213–2222. doi: 10.1038/sj.onc.1209258
Chagas, C. M., Moss, S., Alisaraie, L. (2018). Drug metabolites and their effects on the development of adverse reactions: Revisiting Lipinski's Rule of Five. Int. J. Pharmaceutics 549, 133–149. doi: 10.1016/j.ijpharm.2018.07.046
Checler, F., Goiran, T., Alves Da Costa, C. (2018). Nuclear TP53: An unraveled function as transcriptional repressor of PINK1. Autophagy 14, 1099–1101. doi: 10.1080/15548627.2018.1450022
Chen, C. Y. (2011). TCM Database@Taiwan: the world's largest traditional Chinese medicine database for drug screening in silico. PloS One 6, e15939. doi: 10.1371/journal.pone.0015939
Criscuolo, C., Fabiani, C., Cerri, E., Domenici, L. (2017). Synaptic Dysfunction in Alzheimer's Disease and Glaucoma: From Common Degenerative Mechanisms Toward Neuroprotection. Front. Cell Neurosci. 11, 53. doi: 10.3389/fncel.2017.00053
Davis, A. P., Grondin, C. J., Johnson, R. J., Sciaky, D., Mcmorran, R., Wiegers, J., et al. (2019). The Comparative Toxicogenomics Database: update 2019. Nucleic Acids Res. 47, D948–D954. doi: 10.1093/nar/gky868
Demchak, B., Hull, T., Reich, M., Liefeld, T., Smoot, M., Ideker, T., et al. (2014). Cytoscape: the network visualization tool for GenomeSpace workflows. F1000Res 3, 151. doi: 10.12688/f1000research.4492.2
Esteves, A. R. (2017). Editorial: New Avenues and Therapeutic Strategies for the Treatment of Neurodegenerative Diseases. Curr. Pharm. Des. 23, 667–668. doi: 10.2174/1381612823999170201153809
Fishilevich, S., Zimmerman, S., Kohn, A., Iny Stein, T., Olender, T., Kolker, E., et al. (2016). Genic insights from integrated human proteomics in GeneCards. Database (Oxford) 2016, baw030. doi: 10.1093/database/baw030
Garner, T. P., Lopez, A., Reyna, D. E., Spitz, A. Z., Gavathiotis, E. (2017). Progress in targeting the BCL-2 family of proteins. Curr. Opin. Chem. Biol. 39, 133–142. doi: 10.1016/j.cbpa.2017.06.014
Ge, Q., Chen, L., Tang, M., Zhang, S., Liu, L., Gao, L., et al. (2018). Analysis of mulberry leaf components in the treatment of diabetes using network pharmacology. Eur. J. Pharmacol. 833, 50–62. doi: 10.1016/j.ejphar.2018.05.021
Ghavami, S., Shojaei, S., Yeganeh, B., Ande, S. R., Jangamreddy, J. R., Mehrpour, M., et al. (2014). Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol. 112, 24–49. doi: 10.1016/j.pneurobio.2013.10.004
Gitler, A. D., Dhillon, P., Shorter, J. (2017). Neurodegenerative disease: models, mechanisms, and a new hope. Dis. Model Mech. 10, 499–502. doi: 10.1242/dmm.030205
Goiran, T., Duplan, E., Rouland, L., El Manaa, W., Lauritzen, I., Dunys, J., et al. (2018). Nuclear p53-mediated repression of autophagy involves PINK1 transcriptional down-regulation. Cell Death Differ. 25, 873–884. doi: 10.1038/s41418-017-0016-0
Gu, X., Cai, Z., Cai, M., Liu, K., Liu, D., Zhang, Q., et al. (2018). AMPK/SIRT1/p38 MAPK signaling pathway regulates alcoholinduced neurodegeneration by resveratrol. Mol. Med. Rep. 17, 5402–5408. doi: 10.3892/mmr.2018.8482
Harris, R., Miners, J. S., Allen, S., Love, S. (2018). VEGFR1 and VEGFR2 in Alzheimer's Disease. J. Alzheimers Dis. 61, 741–752. doi: 10.3233/JAD-170745
Hou, X., Xiao, H., Zhang, Y., Zeng, X., Huang, M., Chen, X., et al. (2018). Transient receptor potential channel 6 knockdown prevents apoptosis of renal tubular epithelial cells upon oxidative stress via autophagy activation. Cell Death Dis. 9 1015. doi: 10.1038/s41419-018-1052-5
Huang, L., Wang, S., Ma, F., Zhang, Y., Peng, Y., Xing, C., et al. (2018). From stroke to neurodegenerative diseases: The multi-target neuroprotective effects of 3-n-butylphthalide and its derivatives. Pharmacol. Res. 135, 201–211. doi: 10.1016/j.phrs.2018.08.007
Huang, Y., Zhu, X., Chen, K., Lang, H., Zhang, Y., Hou, P., et al. (2019). Resveratrol prevents sarcopenic obesity by reversing mitochondrial dysfunction and oxidative stress via the PKA/LKB1/AMPK pathway. Aging (Albany NY) 11, 2217–2240. doi: 10.18632/aging.101910
Jakaria, M., Kim, J., Karthivashan, G., Park, S. Y., Ganesan, P., Choi, D. K. (2019). Emerging signals modulating potential of ginseng and its active compounds focusing on neurodegenerative diseases. J. Ginseng Res. 43, 163–171. doi: 10.1016/j.jgr.2018.01.001
Ke, Z. B., Cai, H., Wu, Y. P., Lin, Y. Z., Li, X. D., Huang, J. B., et al. (2019). Identification of key genes and pathways in benign prostatic hyperplasia. J. Cell Physiol. 234, 19942–19950. doi: 10.1002/jcp.28592
Kosten, T. A., Galloway, M. P., Duman, R. S., Russell, D. S., D'sa, C. (2008). Repeated unpredictable stress and antidepressants differentially regulate expression of the bcl-2 family of apoptotic genes in rat cortical, hippocampal, and limbic brain structures. Neuropsychopharmacology 33, 1545–1558. doi: 10.1038/sj.npp.1301527
Laulier, C., Lopez, B. S. (2012). The secret life of Bcl-2: apoptosis-independent inhibition of DNA repair by Bcl-2 family members. Mutat. Res. 751, 247–257. doi: 10.1016/j.mrrev.2012.05.002
Li, M., Qi, Y., Wei, J., Lu, L., Zhao, X., Zhou, L. (2017). N6-Isopentenyladenosine promoted HeLa cell apoptosis through inhibitions of AKT and transforming growth factor beta-activated kinase 1 activation. Tumour Biol. 39, 1010428317695966. doi: 10.1177/1010428317695966
Maino, B., Paparone, S., Severini, C., Ciotti, M. T., D'agata, V., Calissano, P., et al. (2017). Drug target identification at the crossroad of neuronal apoptosis and survival. Expert Opin. Drug Discovery 12, 249–259. doi: 10.1080/17460441.2017.1280023
Palmieri, M., Pal, R., Nelvagal, H. R., Lotfi, P., Stinnett, G. R., Seymour, M. L., et al. (2017). mTORC1-independent TFEB activation via Akt inhibition promotes cellular clearance in neurodegenerative storage diseases. Nat. Commun. 8, 14338. doi: 10.1038/ncomms14338
Penalver, P., Belmonte-Reche, E., Adan, N., Caro, M., Mateos-Martin, M. L., Delgado, M., et al. (2018). Alkylated resveratrol prodrugs and metabolites as potential therapeutics for neurodegenerative diseases. Eur. J. Med. Chem. 146, 123–138. doi: 10.1016/j.ejmech.2018.01.037
Pinero, J., Bravo, A., Queralt-Rosinach, N., Gutierrez-Sacristan, A., Deu-Pons, J., Centeno, E., et al. (2017). DisGeNET: a comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 45, D833–D839. doi: 10.1093/nar/gkw943
Qi, G., Mi, Y., Fan, R., Li, R., Liu, Z., Liu, X. (2019). Nobiletin Protects against Systemic Inflammation-Stimulated Memory Impairment via MAPK and NF-kappaB Signaling Pathways. J. Agric. Food Chem. 67, 5122–5134. doi: 10.1021/acs.jafc.9b00133
Qin, X. Y., Zhang, S. P., Cao, C., Loh, Y. P., Cheng, Y. (2016). Aberrations in Peripheral Inflammatory Cytokine Levels in Parkinson Disease: A Systematic Review and Meta-analysis. JAMA Neurol. 73, 1316–1324. doi: 10.1001/jamaneurol.2016.2742
Rahman, S., Datta, M., Kim, J., Jan, A. T. (2019). CRISPR/Cas: An intriguing genomic editing tool with prospects in treating neurodegenerative diseases. Semin. Cell Dev. Biol. 96, 22–31. doi: 10.1016/j.semcdb.2019.05.014
Rai, S. N., Dilnashin, H., Birla, H., Singh, S. S., Zahra, W., Rathore, A. S., et al. (2019). The Role of PI3K/Akt and ERK in Neurodegenerative Disorders. Neurotox. Res. 35, 775–795. doi: 10.1007/s12640-019-0003-y
Raza, S. S., Wagner, A. P., Hussain, Y. S., Khan, M. A. (2018). Mechanisms underlying dental-derived stem cell-mediated neurorestoration in neurodegenerative disorders. Stem Cell Res. Ther. 9, 245. doi: 10.1186/s13287-018-1005-z
Ru, J., Li, P., Wang, J., Zhou, W., Li, B., Huang, C., et al. (2014). TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 6, 13. doi: 10.1186/1758-2946-6-13
Sanchez-Melgar, A., Albasanz, J. L., Palomera-Avalos, V., Pallas, M., Martin, M. (2019). Resveratrol Modulates and Reverses the Age-Related Effect on Adenosine-Mediated Signalling in SAMP8 Mice. Mol. Neurobiol. 56, 2881–2895. doi: 10.1007/s12035-018-1281-8
Shen, J., Qu, C., Xu, L., Sun, H., Zhang, J. (2019). Resveratrol exerts a protective effect in chronic unpredictable mild stress-induced depressive-like behavior: involvement of the AKT/GSK3beta signaling pathway in hippocampus. Psychopharmacol. (Berl) 236, 591–602. doi: 10.1007/s00213-018-5087-1
Stirpe, A., Pantusa, M., Rizzuti, B., De Santo, M. P., Sportelli, L., Bartucci, R., et al. (2016). Resveratrol induces thermal stabilization of human serum albumin and modulates the early aggregation stage. Int. J. Biol. Macromol. 92, 1049–1056. doi: 10.1016/j.ijbiomac.2016.08.014
Summerlin, N., Soo, E., Thakur, S., Qu, Z., Jambhrunkar, S., Popat, A. (2015). Resveratrol nanoformulations: challenges and opportunities. Int. J. Pharm. 479, 282–290. doi: 10.1016/j.ijpharm.2015.01.003
Suresh, S. N., Chakravorty, A., Giridharan, M., Garimella, L., Manjithaya, R. (2020). Pharmacological tools to modulate autophagy in neurodegenerative diseases. J. Mol. Biol. 432, 2822–2842. doi: 10.1016/j.jmb.2020.02.023
Szklarczyk, D., Gable, A. L., Lyon, D., Junge, A., Wyder, S., Huerta-Cepas, J., et al. (2019). STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47, D607–D613. doi: 10.1093/nar/gky1131
Trott, O., Olson, A. J. (2010). AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461. doi: 10.1002/jcc.21334
Ts, C., Hj, L., Jy, H., Mh, L., Hi, K. (2017). Molecular Insights into Human Serum Albumin as a Receptor of Amyloid-β in the Extracellular Region. J. Am. Chem. Soc. 139, 15437–15445. doi: 10.1021/jacs.7b08584
Uniprot, C. (2019). UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 47, D506–D515. doi: 10.1093/nar/gky1049
Velmurugan, B. K., Rathinasamy, B., Lohanathan, B. P., Thiyagarajan, V., Weng, C. F. (2018). Neuroprotective Role of Phytochemicals. Molecules 23, 2485. doi: 10.3390/molecules23102485
Wang, H., Dong, X., Liu, Z., Zhu, S., Liu, H., Fan, W., et al. (2018). Resveratrol Suppresses Rotenone-induced Neurotoxicity Through Activation of SIRT1/Akt1 Signaling Pathway. Anat. Rec. (Hoboken) 301, 1115–1125. doi: 10.1002/ar.23781
Wang, N., He, J., Pan, C., Wang, J., Ma, M., Shi, X., et al. (2019). Resveratrol Activates Autophagy via the AKT/mTOR Signaling Pathway to Improve Cognitive Dysfunction in Rats With Chronic Cerebral Hypoperfusion. Front. Neurosci. 13, 859. doi: 10.3389/fnins.2019.00859
Wang, W., Liu, T., Yang, L., Ma, Y., Dou, F., Shi, L., et al. (2019). Study on the multi-targets mechanism of triphala on cardio-cerebral vascular diseases based on network pharmacology. BioMed. Pharmacother. 116, 108994. doi: 10.1016/j.biopha.2019.108994
Wang, Y., Zhang, S., Li, F., Zhou, Y., Zhang, Y., Wang, Z., et al. (2020). Therapeutic target database 2020: enriched resource for facilitating research and early development of targeted therapeutics. Nucleic Acids Res. 48, D1031–D1041. doi: 10.1093/nar/gkz981
Wei, H., Zhu, X., Li, Y. (2018). Application value of serum biomarkers for choosing memantine therapy for moderate AD. J. Neurol. 265, 1844–1849. doi: 10.1007/s00415-018-8926-4
Xu, H. Y., Zhang, Y. Q., Liu, Z. M., Chen, T., Lv, C. Y., Tang, S. H., et al. (2019). ETCM: an encyclopaedia of traditional Chinese medicine. Nucleic Acids Res. 47, D976–D982. doi: 10.1093/nar/gky987
Yang, X., Xu, S., Qian, Y., Xiao, Q. (2017). Resveratrol regulates microglia M1/M2 polarization via PGC-1alpha in conditions of neuroinflammatory injury. Brain Behav. Immun. 64, 162–172. doi: 10.1016/j.bbi.2017.03.003
Zhang, J., Feng, X., Wu, J., Xu, H., Li, G., Zhu, D., et al. (2014). Neuroprotective effects of resveratrol on damages of mouse cortical neurons induced by beta-amyloid through activation of SIRT1/Akt1 pathway. Biofactors 40, 258–267. doi: 10.1002/biof.1149
Zhang, W., Bai, Y., Wang, Y., Xiao, W. (2016). Polypharmacology in Drug Discovery: A Review from Systems Pharmacology Perspective. Curr. Pharm. Des. 22, 3171–3181. doi: 10.2174/1381612822666160224142812
Zhang, R., Zhu, X., Bai, H., Ning, K. (2019). Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front. Pharmacol. 10, 123. doi: 10.3389/fphar.2019.00123
Zhang, Y., Li, Y., Wang, Y., Wang, G., Mao, L., Zhang, D., et al. (2019). Effects of resveratrol on learning and memory in rats with vascular dementia. Mol. Med. Rep. 20, 4587–4593. doi: 10.3892/mmr.2019.10723
Zhang, Y. F., Huang, Y., Ni, Y. H., Xu, Z. M. (2019). Systematic elucidation of the mechanism of geraniol via network pharmacology. Drug Des. Devel. Ther. 13, 1069–1075. doi: 10.2147/DDDT.S189088
Keywords: resveratrol, neurodegenerative diseases (NDs), network pharmacology, multitargets, apoptosis
Citation: Wang W, Wang S, Liu T, Ma Y, Huang S, Lei L, Wen A and Ding Y (2020) Resveratrol: Multi-Targets Mechanism on Neurodegenerative Diseases Based on Network Pharmacology. Front. Pharmacol. 11:694. doi: 10.3389/fphar.2020.00694
Received: 28 November 2019; Accepted: 27 April 2020;
Published: 14 May 2020.
Edited by:
Wei Zhou, The Affiliated Hospital of Shenzhen University, ChinaReviewed by:
Jianhua Chen, Shanghai Jiao Tong University, ChinaJianping Chen, Guangzhou University of Chinese Medicine, China
Copyright © 2020 Wang, Wang, Liu, Ma, Huang, Lei, Wen and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aidong Wen, YWR3ZW4tMjAwNEBob3RtYWlsLmNvbQ==; Yi Ding, ZGluZ3lpLjAwN0AxNjMuY29t
†These authors have contributed equally to this work
 Wenjun Wang
Wenjun Wang Shengzheng Wang3†
Shengzheng Wang3† Tianlong Liu
Tianlong Liu Yang Ma
Yang Ma Shaojie Huang
Shaojie Huang Aidong Wen
Aidong Wen Yi Ding
Yi Ding