- Department of Clinical Chinese Pharmacy, School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, China
Background: Considering the limitations of broad-spectrum antiviral drugs for the treatment of herpangina and the extensive exploration of Chinese herbal injections (CHIs), systematic evaluation of the efficacy of different CHIs in the treatment of herpangina is a key imperative. In this study, we performed a network meta-analysis to investigate the efficacy of CHIs, including Reduning injection (RDN), Shuanghuanglian injection (SHL), Tanreqing injection (TRQ), Xiyanping injection (XYP), and Yanhuning injection (YHN), in the treatment of herpangina.
Methods: A systematic literature review including studies published before December 17, 2018, was conducted in several databases. The quality of the included studies was assessed using the Cochrane risk of bias tool. Data were analyzed using STATA 13.0 and WinBUGS 1.4.3 software. Surface under the cumulative ranking curve (SUCRA) probability values were applied to rank the examined treatments. Clustering analysis was performed to compare the effects of CHIs between two different outcomes.
Results: A total of 72 eligible randomized controlled trials involving 8,592 patients and five CHIs were included. All patients were under the age of 15 years, and most were under 7 years. The results of the network meta-analysis showed that RDN, XYP, and YHN had significantly better treatment performance than ribavirin. SHL (OR: 0.18; 95% CI: 0.09–0.34) and TRQ (OR: 0.18; 95% CI: 0.10–0.31) were obviously superior to ribavirin with respect to total clinical effectiveness. The results of SUCRA and cluster analysis indicated that RDN is the best intervention with respect to total clinical effectiveness, antipyretic time, and blebs disappearing time. Fifty-four studies described adverse drug reactions/adverse drug events (ADRs/ADEs), and 32 studies reported ADRs/ADEs in detail.
Conclusions: CHIs were found to be superior to ribavirin in terms of treatment performance and may be beneficial for patients with herpangina. RDN had the potential to be the best CHI with respect to all outcome measures. More evidence is needed to assess the safety aspects of CHIs.
Introduction
Herpangina is a common pediatric disease that is mainly caused by Coxsackie A virus; respiratory and fecal-oral routes are the main routes of transmission. Coxsackie A virus is a small RNA virus that is present in the intestines. The virus exhibits rapid transmission, especially in summer and early autumn. Children in the age group of 1–7 years are particularly vulnerable to infection (Jiang et al., 2015; Huang, 2016). Children infected with herpangina can manifest sore throat, excessive salivation, fever, oral herpes, anorexia, and other symptoms. Enteroviruses are also known to cause serious diseases such as myocardial damage or myocarditis (Wu, 2018; Guo and Li, 2019). Currently, there is no specific treatment for herpangina. Antiviral drugs, symptomatic supportive care, and prevention of complications are the mainstays of treatment (Guo and Li, 2019). Ribavirin is a broad-spectrum antiviral drug that is commonly used for the treatment of herpangina. However, the mechanism of action of ribavirin is highly dependent on viral adenosine kinase; this results in a high probability of the development of drug resistance, which in turn affects the therapeutic effect (Li and Zhan, 2017; Liu et al., 2019). Several recent studies have documented the efficacy of Chinese herbal injections (CHIs) in the treatment of herpangina (Zhu et al., 2014; Xu et al., 2016; Xia, 2016). However, several varieties of CHIs have been used to treat herpangina, and further research is required to identify the best type of CHI for this purpose. Therefore, in this study, we used the network meta-analysis (NMA) method to systematically evaluate the efficacy of different CHIs in the treatment of herpangina. The objective was to identify an optimal intervention measure and provide a basis for clinical drug use.
Methods
This study is reported in strict accordance with the standard format of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Specification: PRISMA Extension Statement specification (Hutton et al., 2015; Ge et al., 2017).
Search Strategy
PubMed, the Cochrane Library, Embase, the Chinese Biological Medicine Literature Service System (SinoMed), the China National Knowledge Infrastructure (CNKI) database, the Chinese Scientific Journal Database (VIP), and the Wanfang Database were searched for randomized controlled trials (RCTs) of CHIs for the treatment of herpangina. Studies published as of December 17, 2018 were eligible for inclusion. In addition, the reference lists of the included studies were manually searched to identify relevant literature. There were three parts of the search strategy, including herpangina, Chinese herbal injection, and random controlled trial. A total of 132 types of CHIs incorporating national standards of the Chinese Food and Drug Administration and 36 kinds of Chinese medicine-derived chemical injections were included in the prescreening. The five CHIs that were finally included in the analysis were Reduning injection (RDN), Shuanghuanglian injection (SHL), Tanreqing injection (TRQ), Xiyanping injection (XYP), and Yanhuning injection (YHN). The detailed search strategy is described in Presentation File.
Inclusion Criteria
Types of Studies
RCTs of CHIs for the treatment of herpangina were eligible if they were referred to as “random,” with or without blinding.
Types of Participants
All patients included were clinically diagnosed with herpangina according to clear diagnostic criteria, with no limitations of sex, race, or age.
Types of Interventions
The interventions included were comparisons between CHIs and ribavirin or between different types of CHIs. Ribavirin and CHIs were administered intravenously; in addition, according to the patient’s condition, certain symptomatic supportive treatments were adopted (e.g., cooling, rehydration, maintenance of water and electrolyte balance, and antibiotic therapy for concurrent bacterial infection). No limitations were imposed with respect to the dosage or treatment course. No other Chinese medicine or remedies were used, such as decoction, proprietary Chinese medicine, acupuncture, or massage.
Types of Outcomes
Outcome indicators included total clinical effectiveness, antipyretic time, blebs disappearing time, and adverse reactions (ADRs)/adverse events (ADEs). Total clinical effectiveness = (total number of patients—;number of patients in whom treatment was ineffective)/total number of patients×100%. The evaluation criteria for efficacy were based on the posttreatment recovery of clinical symptoms and signs; ineffective treatment implies deterioration or no change in symptoms and signs after the treatment course.
Data Extraction and Quality Assessment
All retrieved studies were managed using NoteExpress software. After excluding duplicates, two researchers independently screened the retrieved studies based on the inclusion and exclusion criteria and extracted the data from the included RCTs. The titles and abstracts of retrieved studies were screened to exclude animal studies, literature reviews, and other unrelated articles. Subsequently, studies that met the inclusion criteria were identified, and their full texts were reviewed. A specially designed form (created using Microsoft Excel 2016 software) was used to extract data pertaining to the following information from the included studies: (1) name of first author and the year of publication; (2) basic characteristics of patients: the numbers of patients in the treatment group and the control group, sex distribution, average age or age range, interventions, and treatment details; (3) outcome measures; and (4) study types and main factors affecting the risk of bias. Any disagreement between two researchers during the screening of studies and extraction of data was resolved by consensus or by consulting a third researcher.
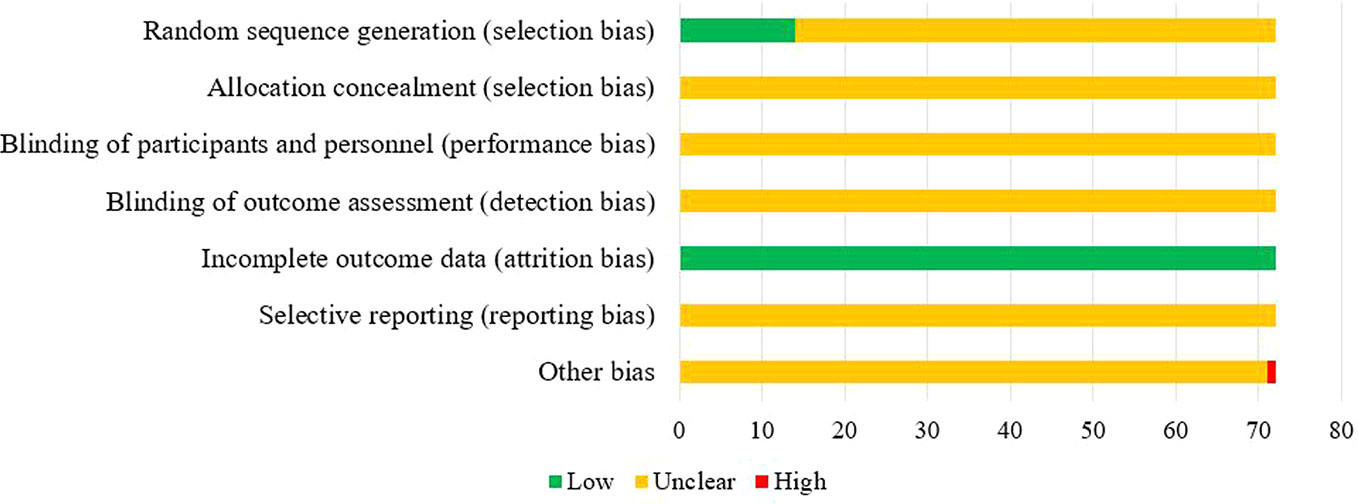
Two authors independently assessed the risk of bias in the included studies in accordance with the risk of bias assessment tool recommended in the Cochrane Handbook 5.1 (Higgins and Green, 2010). The following elements were assessed: (1) selection bias associated with random sequence generation; (2) selection bias associated with allocation concealment; (3) performance bias: blinding of participants and personnel; (4) detection bias: blinding of outcome assessment; (5) attrition bias: integrity of outcome data; (6) reporting bias: selective reporting; and (7) bias from other sources. Each element was categorized as “low risk,” “high risk,” or “unclear.” “Low risk” implies that the implementation method is correct or does not affect the result; “high risk” implies that the implementation method is incorrect and affects the measurement of the result; “unclear” means that the information is insufficient, and the risk of bias cannot be judged. Consensus was attained by discussion or involving a third researcher.
Data Analysis
WinBUGS 1.4.3 software was used to perform NMA, and the Markov chain Monte Carlo method with random-effects model was performed for Bayesian inference. In the WinBUGS software, the number of iterations was set as 200,000, with the first 10,000 iterations used for burn-in to eliminate the impact of the initial value. On NMA, the odds ratio (OR) and 95% confidence intervals (95% CI) were calculated for the binary outcomes; the mean difference (MD) and 95% CI were calculated for continuous outcomes. When the 95% CI for the OR value did not contain 1 and the 95% CI for MD value did not contain 0, the difference between groups was deemed to be statistically significant. Stata 13.0 software was used to map the network of different interventions for each outcome measure, showing the results of the direct and indirect comparison of CHIs. When using the results of WinBUGS software with Stata software, the surface under the cumulative ranking probability (SUCRA) of different CHIs in each outcome index was obtained. The larger the SUCRA and the higher the ranking, the greater the probability that the CHI is the best intervention. A comparison-adjusted funnel plot was used to assess potential publication bias. If points on both sides of the midline in the funnel diagram were symmetric, which meant the correction guideline was at right angles to the midline, it was considered indicative of no significant publication bias. The cluster analysis method was used to comprehensively analyze and compare interventions for two different outcome indicators; then, the optimal injection variety for the two outcome indicators was obtained. The farther from the origin in the cluster map, the better the effect is in these two outcome indicators. If there was a closed loop, the inconsistency test was used to evaluate the consistency of each closed loop, and the inconsistency factors (IFs) and 95% CI were calculated. When the 95% CI contained 0, the consistency was good; otherwise, the closed loop was considered to exhibit significant inconsistency.
Results
Search Results
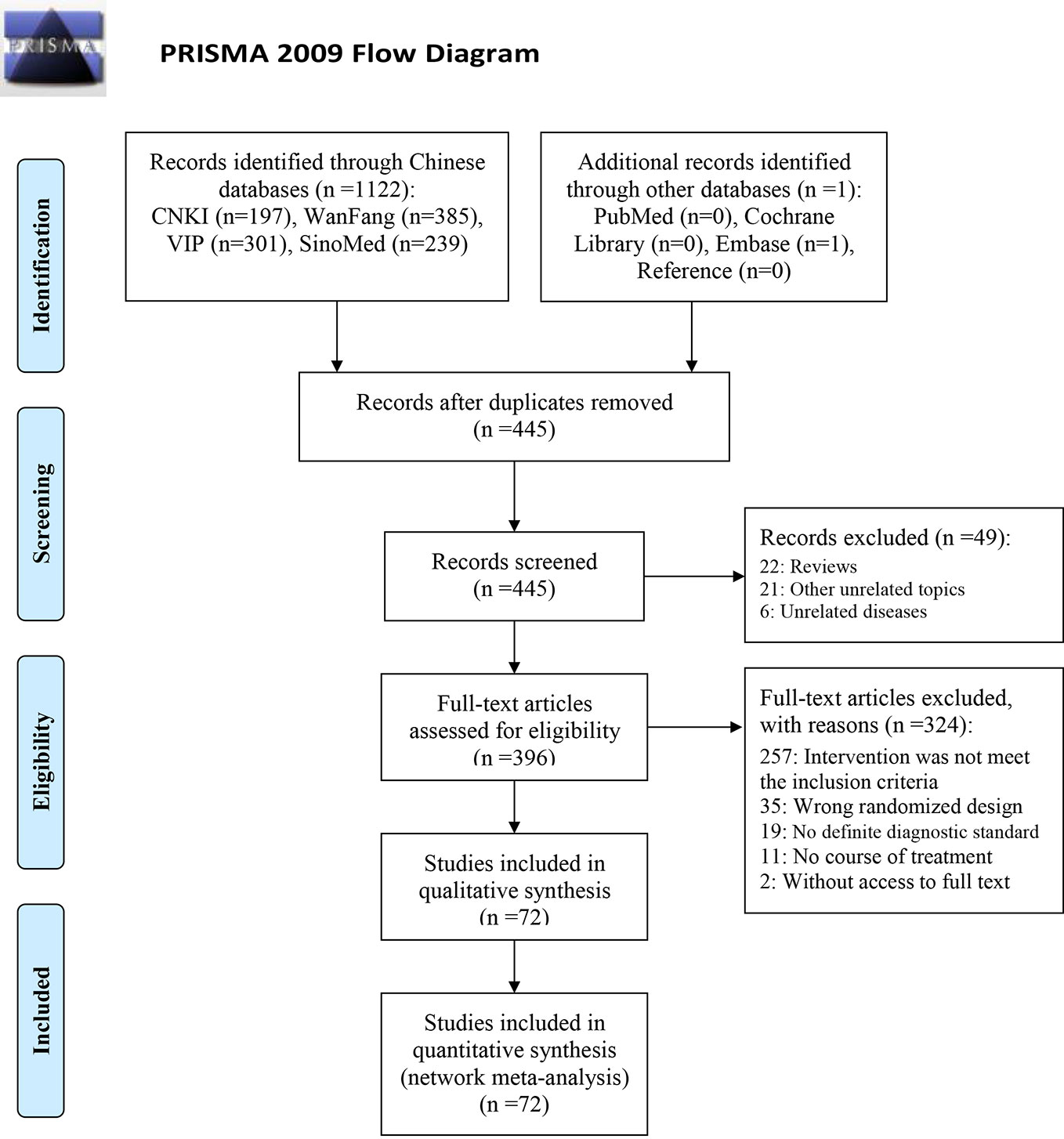
Out of the 1,123 retrieved articles, 72 RCTs (shown in Table 1) were selected and included in the NMA. Further details of the literature screening process are presented in Figure 1. Two studies were three-arm studies (RDN vs. XYP vs. YHN, and RDN vs. XYP vs. ribavirin), while all other studies were two-arm studies. Among these, 67 RCTs investigated CHIs vs. ribavirin as the intervention, including five kinds of CHIs: RDN (27 RCTs), SHL (4 RCTs), TRQ (5 RCTs), XYP (18 RCTs), and YHN (13 RCTs). The remaining three RCTs investigated CHI vs. another CHI as the intervention: RDN vs. YHN (2 RCTs) and TRQ vs. SHL (1 RCT). All included studies were published in Chinese, and the year of publication ranged from 2007 to 2018.
Inclusion Studies and Characteristics
The 72 RCTs included 8,592 patients; of these, 1,866 patients were treated with RDN, 270 patients received SHL, 390 patients received TRQ, 1,211 patients received XYP, 896 patients received YHN, and 3,959 patients received ribavirin. Six studies did not report the sex distribution in the study population; the remaining studies enrolled 4,320 male patients, which accounted for 54.50% (4,320/7,927). All included patients were under the age of 15 years, and most were under 7 years. The maximum sample size of the included RCTs was 195, and the minimum sample size was 18. Sixty-nine RCTs (95.83%, five CHIs) reported total clinical effectiveness, 45 RCTs (62.50%, five CHIs) reported antipyretic time, and 38 RCTs (52.78%, three CHIs) reported blebs disappearing time. The network graph of CHIs with different outcomes is shown in Figure 2. All treatment courses lasted < 7 days. The details of the included studies are shown in Table 1.
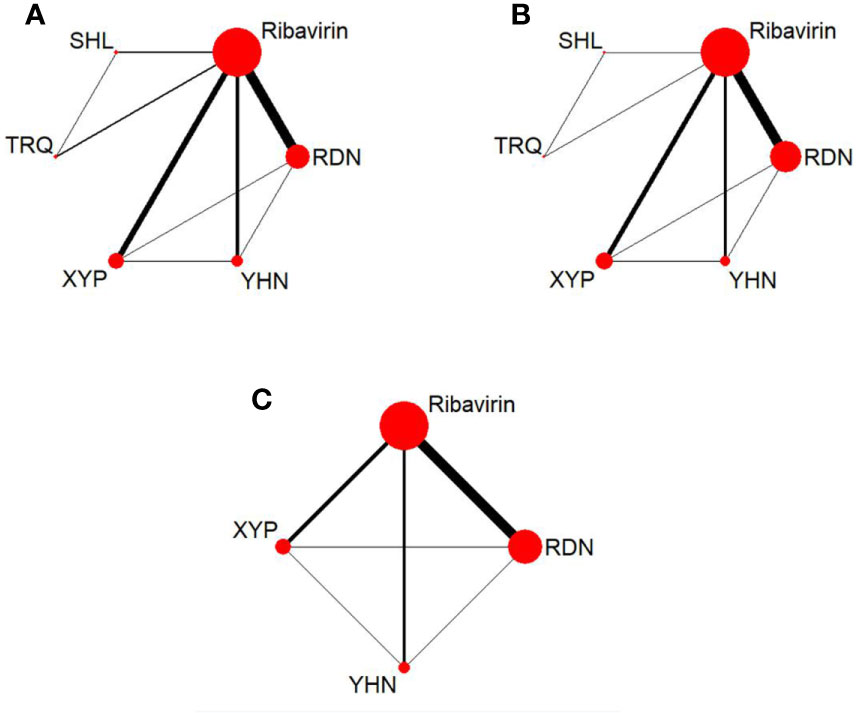
Figure 2 Network graph for different outcomes. (A) Total clinical effectiveness; (B) antipyretic time; (C) Blebs disappearing time. RDN, Reduning injection; SHL, Shuanghuanglian injection; TRQ, Tanreqing injection; XYP, Xiyanping injection; YHN, Yanhuning injetion.
Methodological Quality
Of the 72 included studies, 12 RCTs used a random number table for group allocation, while two RCTs used a random sampling method. The selection bias associated with random sequence generation of the above studies was evaluated as “low risk.” All studies reported complete data, and their attrition bias was evaluated as “low risk.” One RCT did not indicate whether the baseline characteristics of the two groups were comparable at the time of grouping, which may have impacted the results, and other corresponding biases were evaluated as “high risk.” The risk of bias entries for the remaining studies was rated as “unclear” due to insufficient information. The results of the risk of bias evaluation are shown in Figure 3.
Network Meta-Analysis
Total Clinical Effectiveness
Sixty-nine RCTs reported the total clinical effectiveness, involving five CHIs and six interventions. The network graph is shown in Figure 2. The OR value of the NMA is shown in Table 2. Compared with ribavirin treatment, RDN, SHL, TRQ, XYP, and YHN were found to have greater total clinical effectiveness in patients with herpangina; the between-group differences were statistically significant. There were no significant differences between the remaining intervention groups.
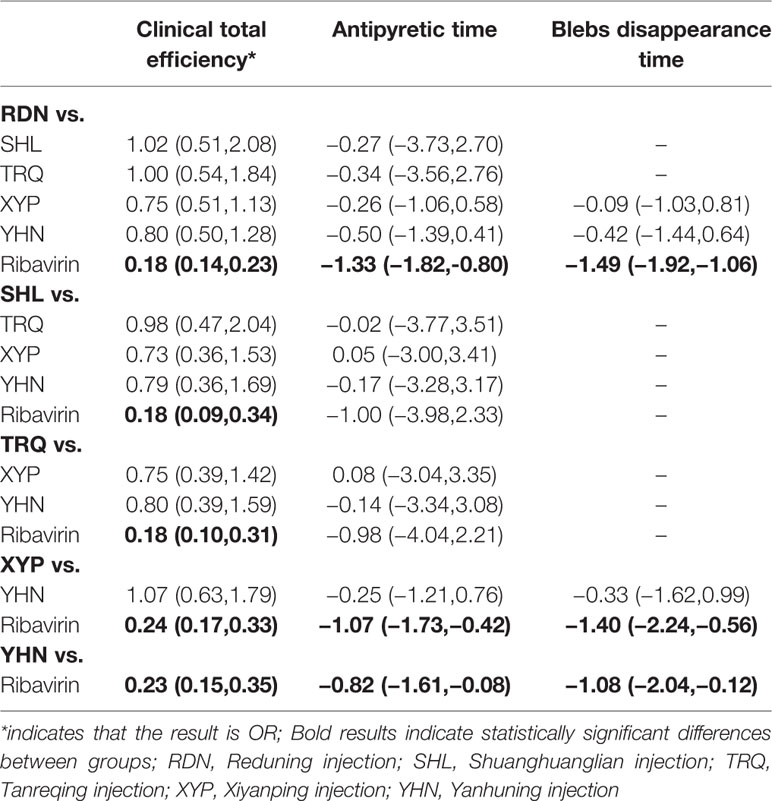
Table 2 Statistical results of network meta-analysis for the outcomes [odds ratio (OR)/mean difference (MD) value, 95% CI].
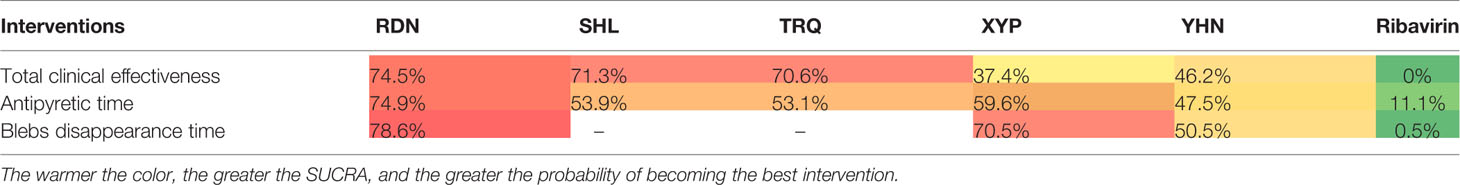
The SUCRA ordering and probability value results (Figure 4, Table 3) indicate that RDN is the most likely to improve total clinical effectiveness in herpangina patients compared with ribavirin, followed by SHL and TRQ.
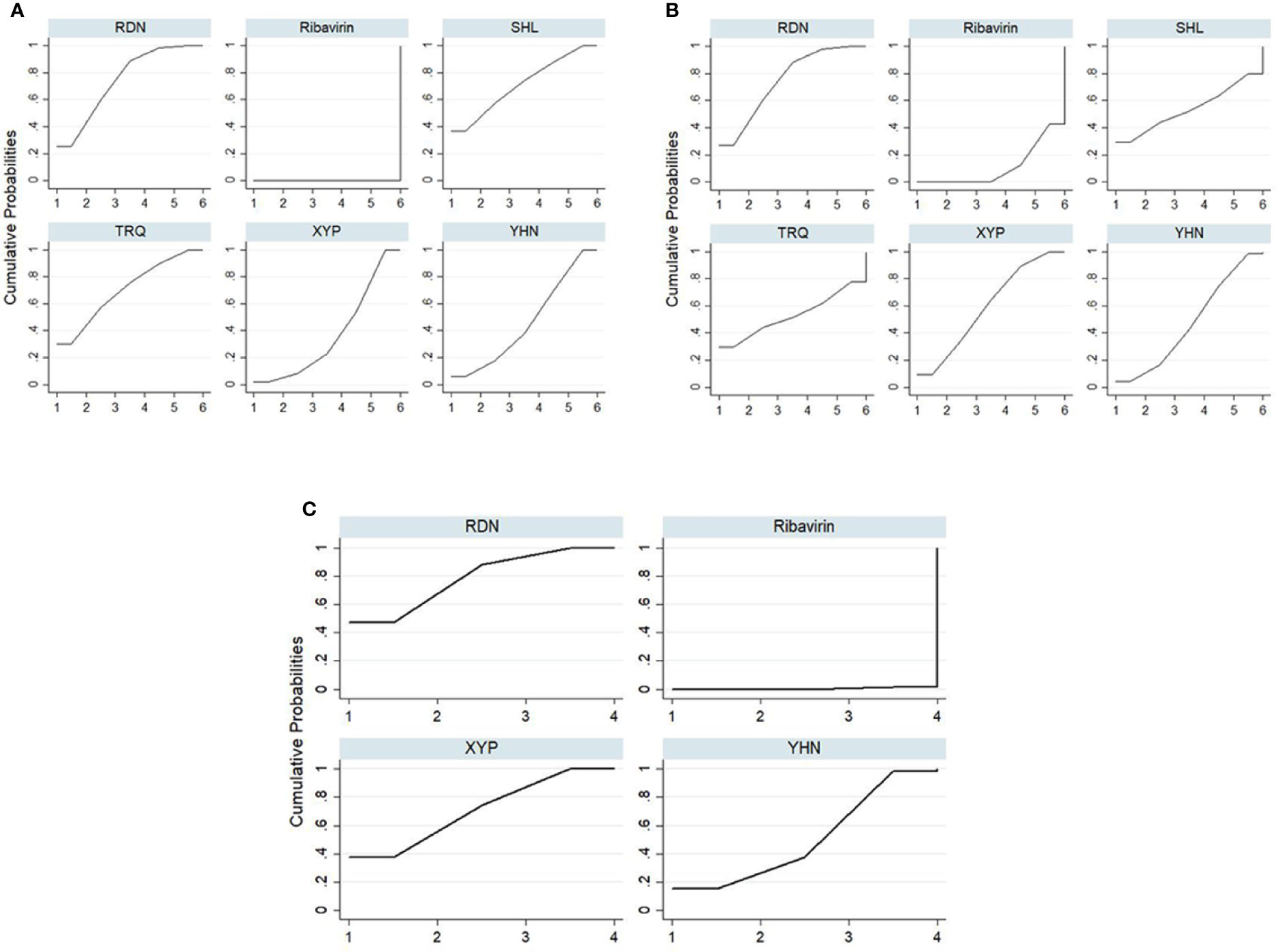
Figure 4 Plot of the surface under the cumulative ranking curves for all treatments. (A) Total clinical effectiveness; (B) antipyretic time; (C) Blebs disappearing time. RDN, Reduning injection; SHL, Shuanghuanglian injection; TRQ, Tanreqing injection; XYP, Xiyanping injection; YHN, Yanhuning injection.
Antipyretic Time
Forty-five RCTs reported antipyretic time, involving five kinds of CHIs and six interventions. The network diagram is shown in Figure 2. The results of NMA (Table 2) showed that RDN, XYP, and YHN can shorten the antipyretic time compared with ribavirin; between-group differences in this respect were statistically significant. The difference between the remaining interventions was not statistically significant. The SUCRA ordering and probability value results (Figure 4, Table 3) indicated that RDN has the best treatment effect, followed by XYP and SHL.
Blebs Disappearing Time
Thirty-eight RCTs reported the blebs disappearing time; these involved four interventions (RDN, XYP, YHN, and ribavirin). The network diagram is shown in Figure 2. On NMA (Table 2), RDN, XYP, and YHN were found to be associated with a shorter blebs disappearing time compared with ribavirin; the between-group difference in this respect was statistically significant. No significant between-group differences were observed for other interventions. The SUCRA ordering and probability value results (Figure 4, Table 3) indicated that RDN has the best treatment effect, followed by XYP and YHN.
Cluster Analysis
The cluster analysis method allowed for a comprehensive comparison of the effects of different interventions on total clinical effectiveness, antipyretic time, and blebs disappearing time. The results showed (Figure 5) that RDN was the best intervention in terms of total clinical effectiveness and antipyretic time, total clinical effectiveness and blebs disappearing time; these findings suggest that the efficacy of RDN in the treatment of herpangina is worthy of attention.
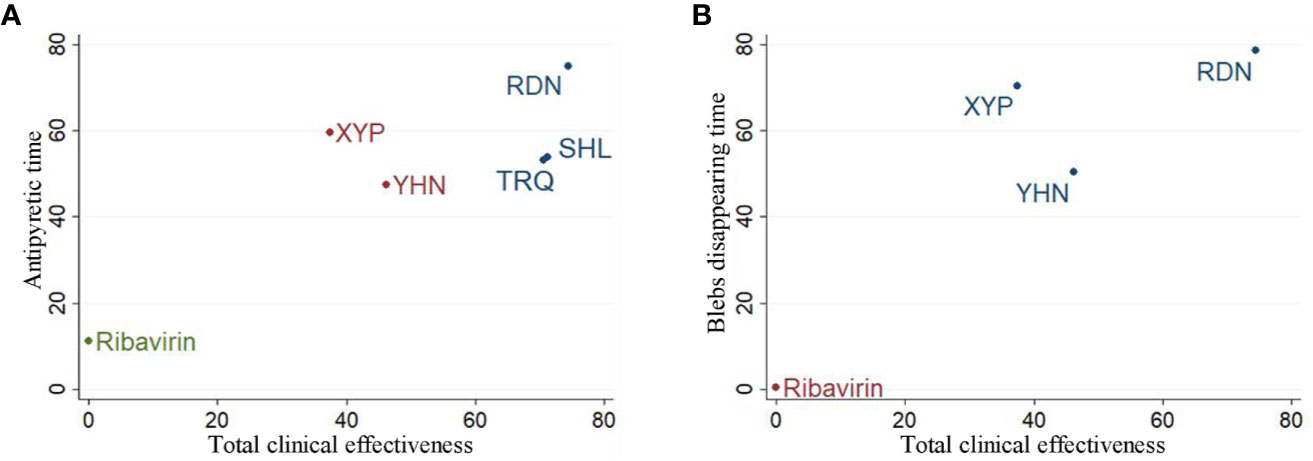
Figure 5 Cluster analysis plot for three outcomes. (A) Cluster analysis plot of total clinical effectiveness and antipyretic time; (B) cluster analysis plot of Total clinical effectiveness and blebs disappearing time. Interventions with the same color belonged to the same cluster, and interventions located in the upper right corner indicate optimal therapy for two different outcomes; RDN, Reduning injection; SHL, Shuanghuanglian injection; TRQ, Tanreqing injection; XYP, Xiyanping injection; YHN, Yanhuning injection.
Publication Bias
Figure 6 shows the comparison-correction funnel plot for total clinical effectiveness to assess potential publication bias. The points on both sides of the centerline of the funnel plot are not completely symmetrical, and there is a large angle between the correction guideline and the centerline. This suggests that our results may have been affected by publication bias to some extent.
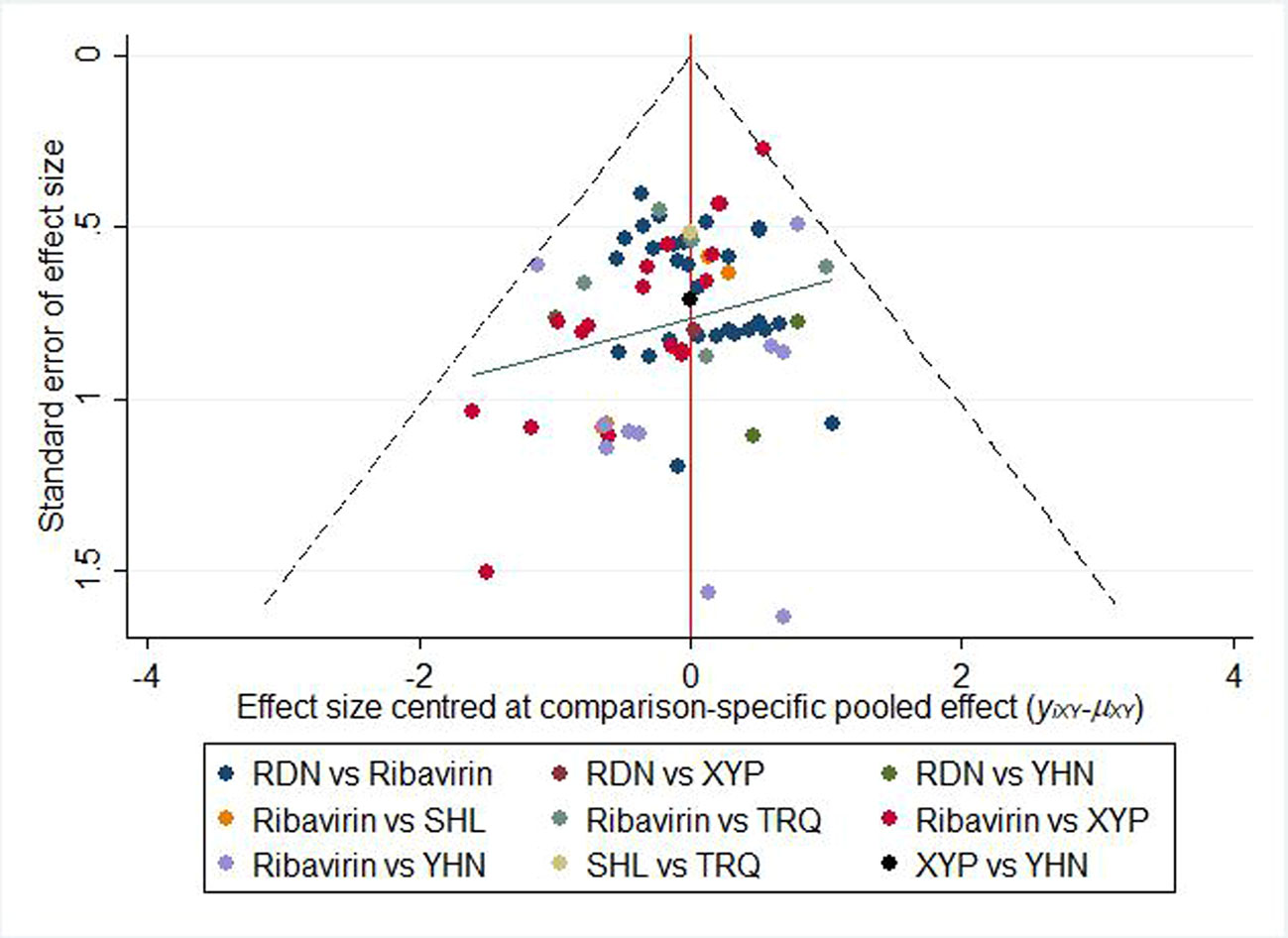
Figure 6 Funnel plot of the clinical effectiveness. RDN, Reduning injection; SHL, Shuanghuanglian injection; TRQ, Tanreqing injection; XYP, Xiyanping injection; YHN, Yanhuning injection.
Consistency Test
To evaluate the consistency of each closed loop, the IF and its 95% CI were calculated using Stata software. When the 95% CI contained 0, it was considered to be consistent; otherwise, there was a significant inconsistency in the closed loop. For example, an inconsistency plot of total clinical effectiveness is shown in Figure 7. The inconsistency test results showed the inclusion of five rings, and only the 95% CI of 1 ring did not contain 0; this indicates that there was a small inconsistency in the included studies and that the results were relatively reliable.
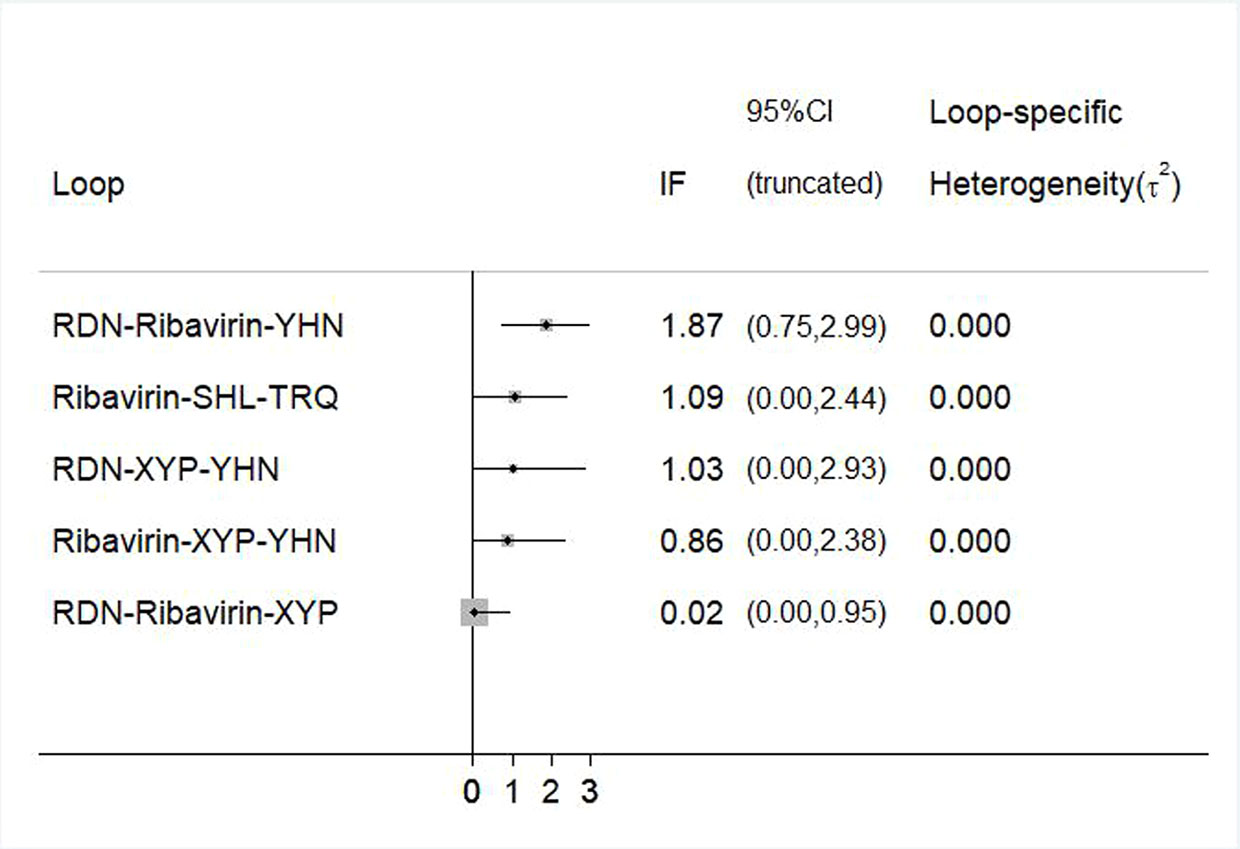
Figure 7 Inconsistency test for the clinical effectiveness. RDN, Reduning injection; SHL, Shuanghuanglian injection; TRQ, Tanreqing injection; XYP, Xiyanping injection; YHN, Yanhuning injection.
Adverse Drug Reactions/Adverse Drug Events
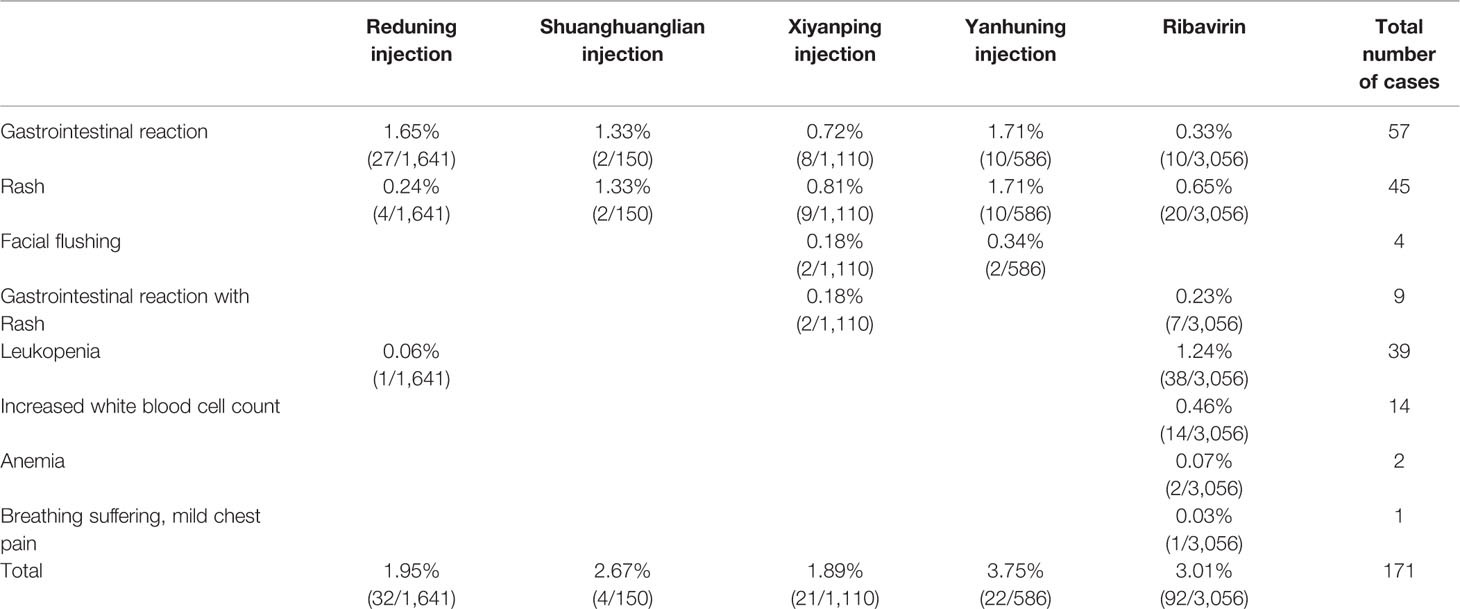
Of the 72 included studies, 18 (25.00%) did not monitor ADRs/ADEs during treatment. Out of the 54 (75.00%) studies that described ADRs/ADEs, 22 studies recorded no ADRs/ADEs, while 32 studies reported the occurrence and the number of affected patients in detail. The total number of patients who experienced ADRs/ADEs was 6,647, which accounted for 77.36% of the total patients. No ADRs/ADEs on TRQ were reported in the currently included studies; ADRs/ADEs of other interventions are shown in Table 4.
Discussion
In this study, we evaluated the use of five types of commonly used CHIs (RDN, SHL, TRQ, XYP, YHN) and ribavirin for the treatment of herpangina. The efficacy of the CHIs was systematically evaluated based on the results of 72 included studies and three outcomes. The results of NMA indicated that the efficacy of RDN, XYP, and YHN was better than that of ribavirin with respect to all outcome measures. With respect to total clinical effectiveness, the efficacy of SHL and TRQ was better than that of ribavirin, and the between-group difference was statistically significant. From the results of SUCRA ordering, among the three outcome indicators, RDN ranked as the best intervention, while all CHIs showed better efficacy than ribavirin. On cluster analysis, RDN was found to be the best intervention with respect to all three outcome measures. Our results highlight the efficacy of RDN in the treatment of herpangina. However, the effect of publication bias on our results cannot be ruled out; therefore, treatment decision-making in individual cases should be guided by specific situations and the experience of clinicians.
In terms of safety, 75% of the included studies monitored ADRs/ADEs. Compared with the medication monitoring of other common respiratory diseases, the RCTs included in this study were better with regard to monitoring the safety of drug use. Among the patients monitored, no significant ADRs occurred in patients treated with TRQ; therefore, its safety needs to be further confirmed by observational studies. In the reported ADRs/ADEs, except for one case of dyspnea and mild chest pain in the ribavirin group, no serious cases occurred in the other groups. The most frequently reported ADRs/ADEs of CHIs were gastrointestinal reactions, followed by rash and leukopenia. Leukopenia occurred primarily in the ribavirin group. The incidence of ADRs was most common in the YHN group, followed by the ribavirin group; the XYP group had the lowest incidence of ADRs/ADEs. Therefore, due care should be taken to avoid ADRs, especially when using YHN and ribavirin.
This is the first study that used the NMA method to evaluate the efficacy and safety of CHIs in the treatment of herpangina and ranked the results of clinical total effectiveness and the disappearing time of two main clinical symptoms. The objective was to provide evidence and recommendations for the clinical selection of drugs. However, some limitations of this study should be considered when interpreting our results: (1) The methodological quality of the included studies was not very high. Only 14 of the 72 RCTs described the correct generation of random sequences. None of the studies mentioned allocation concealment and blinding, and one study did not describe whether the two groups had comparable baseline characteristics. (2) All the included studies were published in Chinese journals; therefore, the findings may not be entirely generalizable to other settings. (3) Most of the included RCTs compared CHIs versus ribavirin, and there was a lack of a more direct comparison of two or more CHIs. (4) This meta-analysis has not been registered online.
Based on the above limitations, we make the following recommendations: (1) For future clinical RCTs, the registration of the protocol should be carried out in advance, and the study should strictly adhere to the protocol to ensure transparency of the implementation process and avoid selective reporting. (2) Future studies should use robust methods for random sequence generation (such as the use of a random number table), implement allocation concealment (e.g., with the use of opaque envelopes), and implement strict blinding to ensure the reliability of the results. (3) More studies should be conducted to evaluate the efficacy of CHIs.
Conclusion
In conclusion, the use of CHIs was associated with improved treatment performance and could be beneficial for patients with herpangina compared to ribavirin. RDN showed the best efficacy with respect to all three outcome measures. However, more direct comparison studies of two or more CHIs are needed to further confirm the results. Future studies should include meticulous monitoring of the safety of CHIs.
Author Contributions
JW and XD done conception and design of the network meta-analysis. XD, HW and KW performed the network meta-analysis. XD, WZ and XL assessed the quality of the network meta-analysis. XD, HW and KW analyzed study data. XD and HW wrote the paper. All authors read and approved the final version of the manuscript.
Funding
The National Natural Science Foundation of China (grant numbers 81473547, 81673829) Young Scientists Training Program of Beijing University of Chinese Medicine.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.00693/full#supplementary-material
Presentation File | This file contains three parts, which includes items regarding the PRISMA checklist for network meta-analysis and corresponding pages of this study, the search strategy of traditional Chinese medicine injections in PubMed database, and details about the product information of five CHIs.
Abbreviations
CHIs, Chinese herbal injections; NMA, network meta-analysis; RDN, Reduning injection; SHL, Shuanghuanglian injection; TRQ, Tanreqing injection; XYP, Xiyanping injection; YHN, Yanhuning injection; SUCRA, Surface under the cumulative ranking curve; ADRs/ADEs, Adverse drug reactions/Adverse drug events; RCTs, Randomized controlled trials; OR, Odds ratio; MD, mean difference; IF, Inconsistency factors.
References
Cai, C. Y. (2011). Clinical Observation of Reduning Injection in Treating Herpangina in Children. Strait Pharm. J. 23 (6), 187–188.
Cai, H. B. (2012). Treating 108 Cases of Herpangina with Tanreqing Injection. Chin. J. Pract. Med. 39 (12), 114.
Cao, X. C. (2008). Clinical Effect of Shuanghuanglian Injection in Child Herpangina. China Foreign Med. Treat. 2791 (15), 99.
Cao, Y. (2015). Clinical Observation of Xiyanping Injection in the Treatment of Herpangina. J. Yichun Coll. 37 (12), 82–83.
Chen, G. B., Lin, S. Z., Ye, Z. L. (2008). Evaluation of the efficiency and safety of Xiyanping in treating Herpangina of children. Int. Med. Health Guid. News 14 (12), 82–85.
Chen, H. J. (2012). Clinical Observation of Reduning in Treating Herpangina in Children. China Pract. Med. 7 (29), 178–179.
Deng, Z. L., Tang, D. S. (2014). Discussion on Reduning Injection in the treatment of herpangina. Med. Aesthetics Cosmetol. 3 (5), 554–555.
Dong, C. Y. (2014). Clinical analysis of treatment of herpangina in children with Reduning. For All Health 8 (2), 196.
Dong, X. J., Feng, T. (2013). Therapeutic effect of Yanhuning injection on herpangina. Guide China Med. 11 (33), 207–208.
Fang, Y. (2011). Therapeutic effect of Yanhuning on children with herpetic angina. Mod. J. Integr. Tradit. Chin. West. Med. 20 (5), 584–610.
Feng, C. X., Peng, S. H. (2013). Treating 80 Cases of Herpangina in Children with Tanreqing Injection. Guiding J. Tradit. Chin. Med. Pharm. 19 (5), 101–102.
Feng, J. H., Zhang, Y. S., Feng, X. C. (2015). Clinical efficacy of Reduning Combined with Ribavirin in the treatment of herpangina. J. China Prescript. Drug 13 (10), 90–91.
Ge, L., Pan, B., Pan, J. X., Li, Y. N., Wu, Y. T., Guo, S. J., et al. (2017). An Introduction of AMSTAR-2 : A Quality Assessment Instrument of Systematic Reviews Including Randomized or Non-randomized Controlled Trials or Both. Chin. J. Drug Eval. 34 (5), 334–338.
Guo, Q., Li, S. S. (2019). Recombinant Human Interferon α-1b Spray in the Treatment of Children with Herpangina. J. Pedia. Pharm. 25 (2), 31–33.
Guo, Y. B., Gui, J. G., Wu, H. Z., Cui, Y. C. (2014). Therapeutic effect of Reduning injection on Yanhuning in the treatment of herpes angina. Clin. J. Tradit. Chin. Med. 26 (6), 586–587.
Guo, J. H. (2009). Therapeutic effect of Xiyanping on children with herpangina. Public Med. Forum Mag. 13 (S1), 133–134.
Guo, Q. J. (2010). Therapeutic effect of Reduning injection on 60 cases of herpangina. Mod. J. Integr. Tradit. Chin. West. Med. 19 (8), 933–934.
Guo, L. J. (2011). Yanhuning for the treatment of herpes angina 44 cases. Chin. Med. Mod. Dis. Educ. China 9 (15), 19.
He, M. L., Peng, F. (2010). Therapeutic effect of Xiyanping injection on herpetic angina. Chin. J. Misdiagn. 10 (10), 2349–2350.
Higgins, J., Green, S. E. (2010). Cochrane handbook for systematic reviews of interventions Version 5.1.0. Cochrane Collab., 202–206.
Hu, Z. S. (2008). Evaluation of the efficacy of Yanhuning in the treatment of herpetic angina. China Contemp. Med. 14 (13), 138–139.
Hu, W. X. (2014). Application of Reduning Injection in Pediatric Herpangina. Neimonggu J. Tradit. Chin. Med. 33 (15), 9–10.
Huang, L. M., Meng, X. P., Meng, S. Y., Li, Z. P., Hu, F. E. (2008). Analysis of 68 cases of herpes angina treated with Xiyanping. J. Chin. Mod. Pediatr. 5 (1), 58–59.
Huang, R. (2016). Clinical observation of riboflavin sodium phosphate combined with interferon in treatment of children herpangina. Drugs Clin. 31 (2), 186–189.
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 162 (11), 777–784. doi: 10.7326/M14-2385
Ji, X. Z., Chen, Z. H., Chen, X. J. (2014). Clinical Observation on 190 Cases of Reduning Injection in Children with Herpangina. Med. Info. 15 (39), 401.
Jia, M., Tian, L. Y. (2012). Therapeutic effect of Xiyanping injection on 70 cases of herpetic angina. Xinli Yesheng 1 (8), 281.
Jiang, Z. F., Shen, K. L., Shen, Y., Zhu, F. T. (2015). Practical pediatrics. Eighth edition (Beijing: People’s Medical Publishing House), 901–902.
Jiang, Z. E. (2009). Clinical Observation on Treatment of Herpangina with Tanreqing Injection. J. Liaoning Coll. Tradit. Chin. Med. 11 (6), 148.
Ke, H. Y. (2014). Clinical research of Reduning Injection in the treatment of children with herpetic angina. J. Pediatr. Tradit. Chin. Med. 10 (5), 18–20.
Li, Q. X., Zhan, H. Y. (2017). Clinical Study of the Effects and Mechanism of Qingfei Liyan Decoction Combined with Acupoint Exter-nal Application on Herpangina in Children. J. Emerg. Tradit. Chin. Med. 26 (6), 1022–1025.
Li, J. Y., Dai, Z. L., Gao, C. H. (2011). Application of Xiyanping injection on Herpesangina. China Pract. Med. 6 (14), 30–31.
Li, X. P. (2011). Therapeutic effect of Yanhuning on herpes angina. World Health Digest 8 (7), 380–381.
Li, H. B. (2012). Clinical observation of Yanhuning in treating 42 cases of herpetic angina in children. Jilin Med. J. 33 (1), 132–133.
Lin, J. Y. (2014). Treatment of 48 cases of herpetic angina with Xiyanping injection. China Naturopathy 22 (7), 44–45.
Liu, A. L., Li, L. (2011). Therapeutic effect of Xiyanping injection and Reduning injection on herpes angina. Chin. J. Clin. 5 (4), 1152–1154.
Liu, J., Xiong, Z. Y., Ao, X. D. (2019). Clinical analysis of treatment of herpangina with different administration routes and different doses of interferon. Contemp. Med. 25 (5), 146–147.
Liu, W. (2015). Therapeutic effect of Reduning on the treatment of herpangina in children. Yiyao Qianyan 5 (30), 109–110.
Lv, J. (2009). Clinical Observation on 30 Cases of Herpangina Treated by Yanhuning. J. Commun. Doctors 11 (13), 112.
Pang, G. X., Lin, S. Z., Zhang, Z. H. (2008). Evaluation of the efficiency and safety of Redunin injection in treating Herpangina of children. Neimonggu J. Tradit. Chin. Med. 20 (10), 8–9.
Peng, Y. L., Tao, Y. (2010). Clinical Analysis of Shuanghuanglian Freeze-dried Powder Injection in Treating 66 Cases of Herpangina in Children. J. North China Coal Med. Coll. 12 (6), 825.
Pu, H. X. (2012). Therapeutic effect of Reduning injection on children with herpangina. Yiyao Qianyan 2 (24), 185–186.
Qu, D. Y., Li, Z. F., Liu, Z. L. (2016). Therapeutic effect of Yanhuning on herpes angina. World Latest Med. Info. 16 (73), 139.
Shen, X. Y. (2010). Clinical Observation on 50 Cases of Herpangina in Treating Xiyanping. China Health Care Nutr. 2 (10), 139–140.
Song, X. G., Fan, J. (2013). Therapeutic effect of Yanhuning injection on herpes simplex angina in infants. Guide China Med. 11 (11), 273–274.
Su, Z. Q., Ke, D. H. (2012). Changes of immune function in children with herpangina and observation of curative effect of Xiyanping. Strait Pharm. J. 24 (9), 132–133.
Sun, L. M., Chang, X. C., Zhao, R. B., Cui, Y. L., Wang, L. J. (2011). Reduning treatment of children with herpes angina efficacy. China Med. Herald 8 (6), 149–150.
Tan, G. M. (2011). Therapeutic Efficacy of Tanreqing Injection for Herpangina. Guangming J. Chin. Med. 26 (6), 1137–1138.
Tan, F. Z. (2014). Therapeutic effect of Reduning on the treatment of herpangina in children. Cardiovasc. Dis. J. Integr. Tradit. Chin. West. Med. 2 (7), 87–88.
Wang, Z. Y., Li, J. (2015). Therapeutic effect of Reduning on herpangina. World Latest Med. Info. 4 (41), 134–136.
Wang, Z. J., Xiang, J. J., Pang, W. J. (2012). Clinical application of Yanhuning in herpangina. J. China Tradit. Chin. Med. Info. 4 (1), 64.
Wang, H. R. (2012). Clinical Observation on 92 Cases of Herpangina in Children Treated with Reduning Injection. Chin. Commun. Doct. 14 (32), 174–176.
Wang, H. Y. (2012). Clinical Observation on Treatment of Herpangina in Children with Traditional Chinese Medicine Injection. Guide China Med. 10 (5), 232–233.
Wang, L. X. (2013). Therapeutic effect of Shuanghuanglian injection on children with herpetic angina. Med. Info. 26 (2), 160–161.
Wang, Y. M. (2013). Application of Xiyanping in the treatment of herpangina. World Health Digest 6 (40), 83–84.
Wei, K. C. (2007). Therapeutic effect of Yanhuning on herpes angina. Youjiang Med. J. 35 (6), 668–669.
Wen, J. F. (2012). Therapeutic effect of Tanreqing injection on herpetic angina. Health Guide 2 (2), 1–2.
Wu, Q. (2018). Efficacy and safety of treatment of Herpangina with elevated C-reactive protein in children. J. Clin. Med. Lit. 5 (97), 31–32.
Xia, J. W. (2016). Observation on the Therapeutic Effect of Xiyanping Injection in Treatment of Herpes. China Continuing Med. Edu. 8 (30), 173–175.
Xia, W. H. (2016). Meta analysis of curative effect of reduning injection in the treatment of herpangina in children. Chin. Commun. Doct. 138-139 (34), 113.
Xiao, X. L. (2010). Effective observation on treating pediatric herpetic angina with Redu-ning. Clin. J. Chin. Med. 2 (13), 50.
Xiao, X. Q. (2016). Influence of Reduning injection on immune function in children with blister measles angina. Int. Med. Health Guid. News 22 (20), 3141–3143.
Xie, B. Q. (2011). Therapeutic effect of Reduning injection on children with herpangina. J. China Tradit. Chin. Med. Info. 3 (7), 124.
Xie, J. (2017). Clinical Observation of Reduning in Treating Herpangina in Children. Oriental Diet Ther. Health Care 10 (4), 132.
Xu, X. Y., Hu, Y., Xue, J., Xu, W. Q., Xu, X. L., Dai, T. J. (2009). Efficacy and safety of Reduning injection in children with herpangina. World Clin. Drugs 30 (10), 609–611.
Xu, J. L., Liang, Z. R., Guo, Z. L., Li, Z. C., Deng, L. M. (2016). Xiyanping Combined with Ribavirin in Treatment of Children with Pediatric Herpangina. Chin. J. Exp. Tradit. Med. Form. 22 (4), 195–200.
Yang, M. L., Tang, H., He, H. (2013). Observe the efficacy of Xiyanping injection in treatment of children with herpangina in 123 cases. Natl. Med. Front. China 8 (22), 63–64.
Yang, L. (2011). Clinical observation of Xiyanping in the treatment of 61 cases of herpetic angina in children. World Health Digest 8 (36), 182.
Yang, L. N. (2013). Therapeutic effect of Reduning on the treatment of herpangina in children. China Hwalth Care Nutr. 2 (11), 475.
Yang, F. J. (2014). Effect of Yanhuning on the treatment of herpangina in children. China Pract. Med. 9 (35), 99–100.
Yin, Z. X. (2011). Therapeutic effect of Yanhuning on herpes angina. J. China Tradit. Chin. Med. Info. 3 (21), 129.
Yu, J. T., Qian, C. Y. (2014). Clinical Observation of Reduning in Treating Herpangina in Children. Acta Chin. Med. Pharm. 42 (2), 127–128.
Zeng, H. J., Li, J., Huang, Y. B. (2013). To explore the efficacy of Xiyanping injection in the treatment of herpes angina. Healthmust-Readmagazine 12 (1), 293–294.
Zeng, Y. (2011). Therapeutic effect of Reduning and ribavirin on children with herpangina. Med. Info. 24 (12), 780.
Zhang, S. C., Liu, T., Zhang, W. P. (2012). Clinical observation on Reduning Injection in the treatment of 96 children with herpes angina. China Modern Med. 19 (27), 25–26.
Zhang, F. R. (2011). Clinical analysis of Xiyanping injection in the treatment of herpetic angina. Mod. Med. Health 27 (18), 2832.
Zhang, L. (2011). Therapeutic effect of Xiyanping on children with herpetic angina. Gansu. Med. J. 30 (3), 164–165.
Zhang, Z. M. (2012). Clinical Observation of Reduning Injection in Treating Herpangina in Children. J. Med. Theory Pract. 25 (12), 1481–1482.
Zhang, Y. L. (2013). Therapeutic effect of Reduning injection on children with herpangina. Yiyao Qianyan 3 (6), 164–165.
Zhao, B. Y. (2012). Clinical treatment of herpetic angina in children. Guide China Med. 10 (31), 547–548.
Zhou, S. Y. (2012). Therapeutic effect of Reduning injection combined with Yanhuning injection on herpes angina. China Mod. Doctor 50 (11), 142–144.
Zhou, H. (2013). Clinical Observation of Xiyanping Injection in Treating Pediatric Herpangina. Med. Info. 26 (8), 418–419.
Zhu, Q. L., Yang, S. P., Ye, X. H., Chen, H. (2014). A Systematic Review of Yanhuning versus Ribabirin for Herpangina in Children. J. Pediatr. Pharm. 20 (4), 4–9.
Keywords: network meta-analysis, Bayesian model, Chinese herbal injections, herpangina, ribavirin
Citation: Duan X, Wang H, Wu J, Zhou W, Wang K and Liu X (2020) Comparative Efficacy of Chinese Herbal Injections for the Treatment of Herpangina: A Bayesian Network Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 11:693. doi: 10.3389/fphar.2020.00693
Received: 27 December 2019; Accepted: 27 April 2020;
Published: 15 May 2020.
Edited by:
Yonggang Zhang, Sichuan University, ChinaReviewed by:
Priyia Pusparajah, Monash University Malaysia, MalaysiaWisit Cheungpasitporn, University of Mississippi Medical Center, United States
Copyright © 2020 Duan, Wang, Wu, Zhou, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiarui Wu, ZXhvZ2FteUAxNjMuY29t
†These authors have contributed equally to this work
 Xiaojiao Duan†
Xiaojiao Duan† Jiarui Wu
Jiarui Wu Xinkui Liu
Xinkui Liu