- Department of Nano-Therapeutics, Institute of Nano Science and Technology, Mohali, India
The movement of micro and macro molecules into and within a cell significantly governs several of their pharmacokinetic and pharmacodynamic parameters, thus regulating the cellular response to exogenous and endogenous stimuli. Trafficking of various pharmacological agents and other bioactive molecules throughout and within the cell is necessary for the fidelity of the cells but has been poorly investigated. Novel strategies against cancer and microbial infections need a deeper understanding of membrane as well as subcellular trafficking pathways and essentially regulate several aspects of the initiation and spread of anti-microbial and anti-cancer drug resistance. Furthermore, in order to avail the maximum possible bioavailability and therapeutic efficacy and to restrict the unwanted toxicity of pharmacological bioactives, these sometimes need to be functionalized with targeting ligands to regulate the subcellular trafficking and to enhance the localization. In the recent past the scenario drug targeting has primarily focused on targeting tissue components and cell vicinities, however, it is the membranous and subcellular trafficking system that directs the molecules to plausible locations. The effectiveness of the delivery platforms largely depends on their physicochemical nature, intracellular barriers, and biodistribution of the drugs, pharmacokinetics and pharmacodynamic paradigms. Most subcellular organelles possess some peculiar characteristics by which membranous and subcellular targeting can be manipulated, such as negative transmembrane potential in mitochondria, intraluminal delta pH in a lysosome, and many others. Many specialized methods, which positively promote the subcellular targeting and restrict the off-targeting of the bioactive molecules, exist. Recent advancements in designing the carrier molecules enable the handling of membrane trafficking to facilitate the delivery of active compounds to subcellular localizations. This review aims to cover membrane trafficking pathways which promote the delivery of the active molecule in to the subcellular locations, the associated pathways of the subcellular drug delivery system, and the role of the carrier system in drug delivery techniques.
Introduction
For a cell to live, it needs a constant flow of nutrients and the removal of unwanted or used materials. Formation, fusion, and trafficking of membranous vesicles are processes that commonly occur within the cell, which regulate the fate of cells (Tokarev et al., 2013). These processes control vital cellular activities such as: cellular uptake, transportation of nutrients for metabolism and pathogens for degradation, trafficking of moieties to the desired location, transportation of signalling molecules, communication within or outside cells, and many others (Muro, 2018). In order to maintain the transportation of proteins, lipids, and solutes, there are specialized pathways that work within the cells called membrane trafficking pathways. Membrane trafficking is a key process for maintaining the sustainability of the cell by transporting these nutrients and other solutes to all parts of the cellular system (Ebine and Ueda, 2015). Membrane trafficking is divided into two basic movement pathways viz. endocytosis and exocytosis. Their function is to control the movement of cargo and drug payloads to the plasma membrane or out of the cell. These specialized pathways also deliver the newly synthesized proteins to their desired locations inside the cells (Figure 1) (Watson et al., 2005).
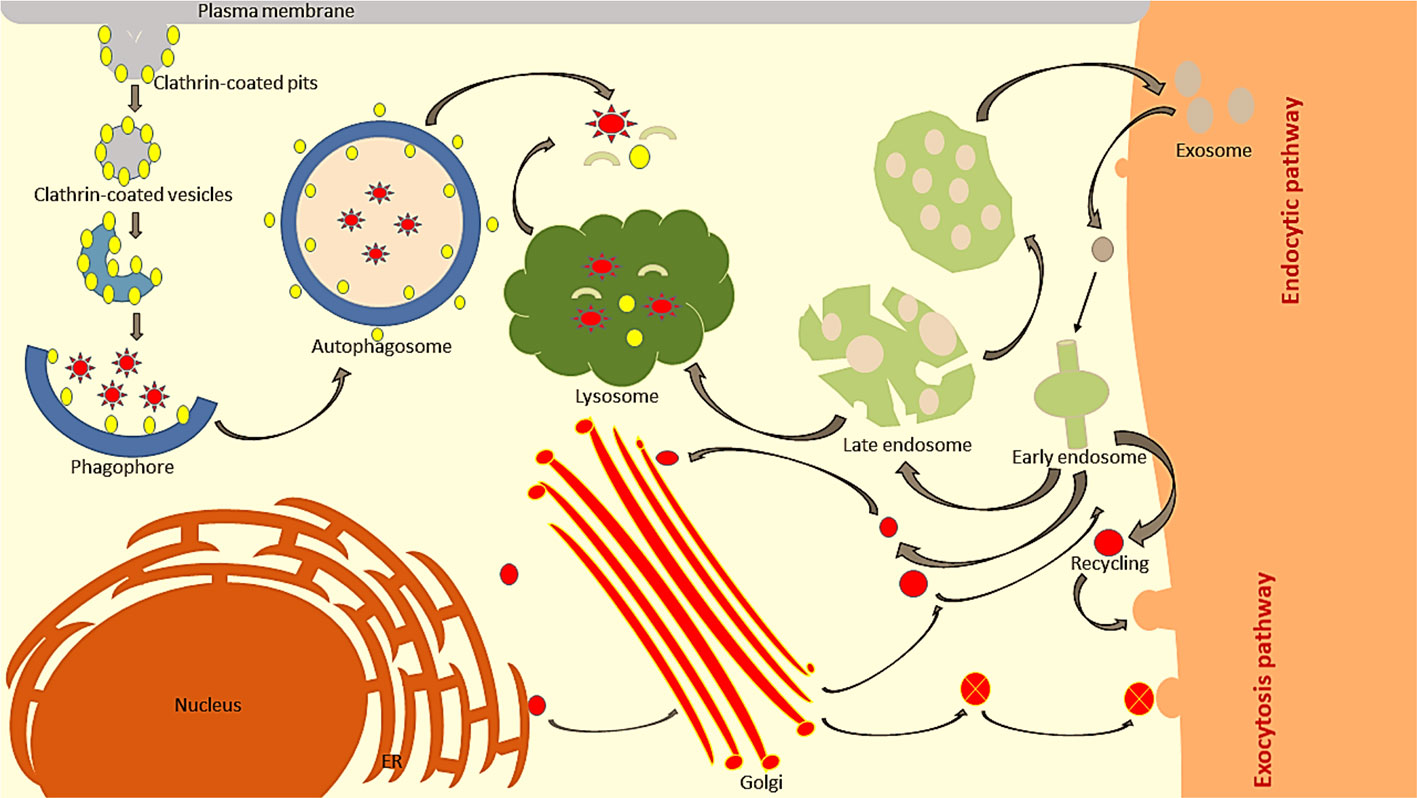
Figure 1 In eukaryotic cells, membrane trafficking is divided into two major pathways. In endocytosis, proteins are internalized from the outer cell surface into the early endosomes. The early endosome determines the trafficking route for the internalized material, either by sending it to the plasma membrane for recycling, degradation via the lysosome, or for retrieval by the trans-Golgi network. On the other hand, exocytosis translocates newly synthesized proteins into the ER, entering further into the cis-Golgi complex and is transported via the trans-Golgi network.
These trafficking phenomena exist both at the cell membrane as well as subcellular levels and have been exploited directly, or otherwise, to regulate the localization and placement of both endogenous and exogenous molecules. The attributes of the cell membrane, which govern the membrane trafficking, include, but is not limited to membrane potential, its fluidity, and alterations in its competency (Xue et al., 2013; Staudt et al., 2016). Drug resistance develops frequently as a result of altered membrane trafficking, which culminates into reduced drug uptake by membranous transporters localized at the cell surface (Xue et al., 2013). In other instances, subcellular trafficking mechanism, organelle function, and other complex molecular interplays are manipulated to achieve the maximum potency of drugs and other agents (Striebinger et al., 2015). The major characteristics which regulate subcellular trafficking include modification in the subcellular pH and modification in the cell cycle parameters. (Abdrabou and Wang, 2018) Furthermore, membrane trafficking is a complicated channelization phenomena composed of multiple trafficking vesicles and some extra vesicular networks that are involved in endocytosis, exocytosis, as well as cellular autophagy. These processes necessitate the aid of several subcellular transportation components like early endosomes, late endosomes, lysosomal networks etc (Zhang et al., 2016).
Strategies are being employed to design formulations that act as prodrugs, bearing the components that closely resemble the cell membrane constituents so as to trace and monitor their cellular handling viz. membrane and subcellular trafficking events like fusion, translocation, internalization, carrier sequestration, perinuclear localizations, drug release etc (Maji et al., 2018). The selective delivery of therapeutic molecules to specific diseased sites also involves these membranous and subcellular trafficking components. For example, in the case of cancer therapy, delivering a therapeutic payload to the vicinity of the nucleus demands not only the preferential recognition of cancerous cells and the nucleus but also the payload entry and its subsequent transportation via membranous and subcellular trafficking. This will impart selective damage to the targeted cancer cells by delivering the drug in its extreme vicinity while causing minimal harm to the adjacent normal cells and tissues (Rosenkranz et al., 2019). It is only the membrane trafficking, by which cellular and subcellular transport of micro and macromolecules takes place, that substantially governs the essential cellular functions and responses like drug interaction with plasmid DNA, transfection efficiencies, cellular uptake capabilities, internalization mechanisms, cellular and subcellular localizations, and other pharmacokinetic and pharmacodynamic paradigms of the therapeutic molecules (Mazzaglia et al., 2018).
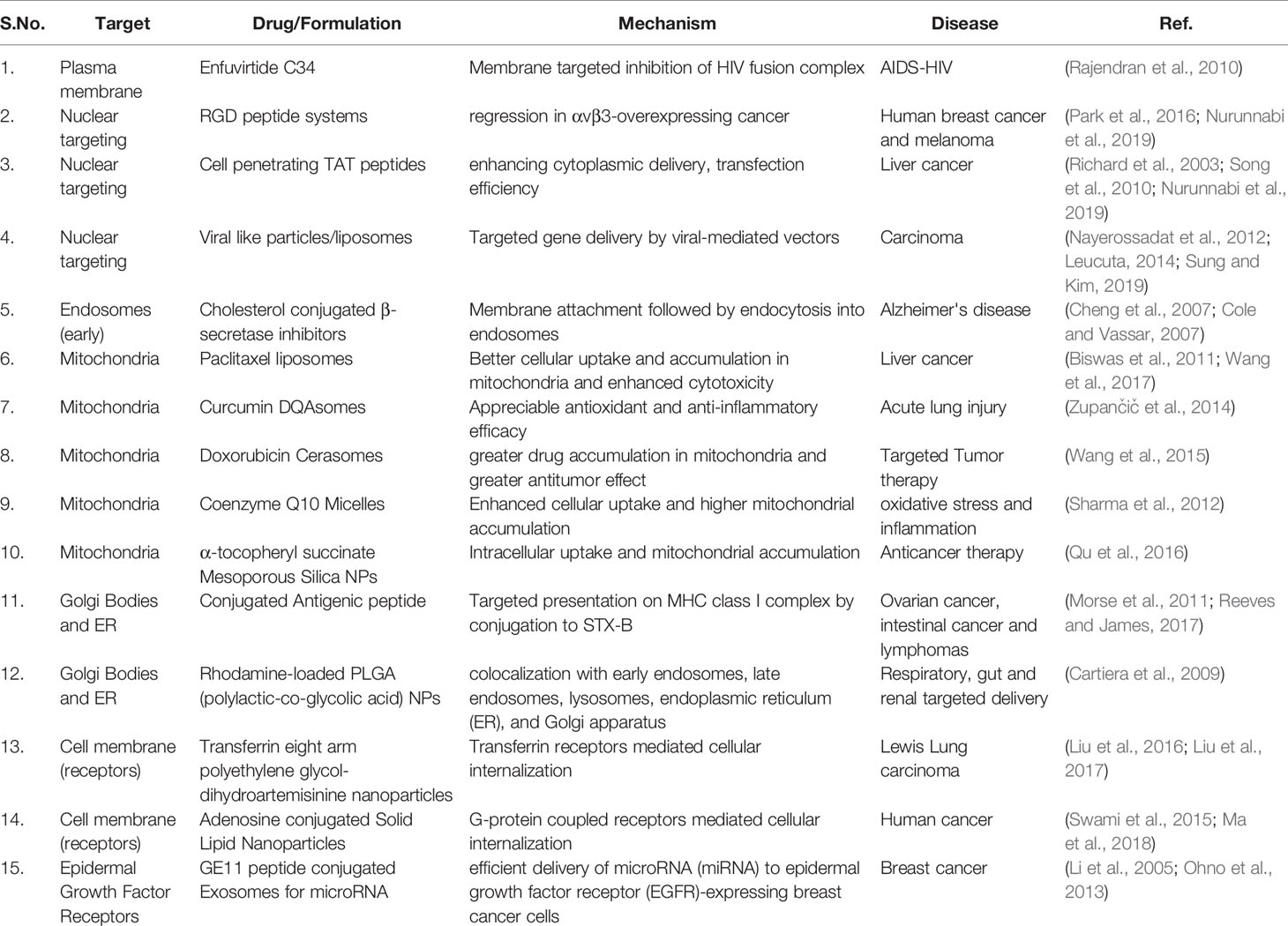
Targeting the membrane trafficking of enzymes involved in the disease pathology is another perspective which exploits the subcellular localization mechanism. Rajendran et al., described a protocol which targeted the membrane bound β-secretase transition state by an inhibitor rather than targeting the active binding site of the enzyme. This membrane targeting involves endosomes and enhances the local membrane density of inhibitor and imparts more enzyme inhibitory efficiency both in vitro and in vivo. The authors suggested that this scheme can significantly benefit the formulation of potent therapeutic molecules against membrane protein targets (Rajendran et al., 2008).
Another aspect of membrane trafficking constitutes the design of targeting strategies such that some very specific molecules viz. folate receptors (Slastnikova et al., 2017), epidermal growth factor receptros (Rosenkranz et al., 2018), melanocortin receptor-1 (Durymanov et al., 2012), and α-melanocyte stimulating hormone (Rosenkranz et al., 2003), overexpressed on the diseased (e.g. cancerous) cells, act as the target and receptor-specific ligand bearing transportation carriers that are designed for the delivery of therapeutic payloads. The carriers aid in the receptor binding, internalization, endosomal escape, and nuclear internalization. As the nucleus is the most sensitive and vulnerable subcellular locality, the translocation domain of the carrier helps the drug disposition in the vicinity of nuclear DNA of the receptor overexpressing cells and significantly enhances the cancer cell lethality as a result of the increased therapeutic efficacy of drugs (Rosenkranz et al., 2003; Durymanov et al., 2012; Slastnikova et al., 2017; Rosenkranz et al., 2018).
Although a number of reviews have previously been published, these reviews have mainly been restricted in their focus on either only subcellular drug targeting or subcellular drug targeting with respect to drug delivery. The study by Harisa and Faris (2019) mainly emphasizes the limitations of drug targeting into intracellular compartments and factors affecting molecular entry into the compartment. Likewise, Liu et al. (2020) focus on the limitations of traditional nanocarriers by explaining their pathways and principles with respect to drug targeting. The emphasis is made on challenges which arise in cancer therapy by the nanocarriers. Similarly, Nag and Delehanty (2019) discussed drug delivery with respect to cellular and subcellular targeting. The review mainly focused on recent advancements in nanoparticle mediated drug delivery techniques. Donahue et al. (2019) discussed the physiochemical properties of nanoparticles affecting cellular internalization and cellular interaction. As the movement of molecules within the cell is vitally governed by the complex process of membrane trafficking, and in order to achieve subcellular drug targeting, an in-depth understanding of membrane trafficking is of utmost significance. The present review therefore focuses, in detail, on the role of membrane trafficking in subcellular drug targeting along with various trafficking mechanisms and the carrier mediated targeting of cellular and subcellular trafficking pathways.
Membrane Trafficking
Overview
The complexity of mammalian cells is accompanied by the compartmentalization and flow of newly synthesized proteins, nutrients, and molecules and is maintained by the inbuilt trafficking system. There are a number of pathways that control the movements, like the biosynthetic pathway for proteins, or the endocytic pathway for molecules such as ligands and solutes entering cells (Watson et al., 2005). Membrane trafficking provides the fundamental needs of cells to maintain homeostasis and creates a flow of materials for the signalling process. The eukaryotic system of membrane trafficking started from endoplasmic reticulum, followed by Golgi apparatus, endosomes and lysosomes (Cheung and de Vries, 2008).
When the drug binds to its receptors, the internalization of the ligand takes place through endocytosis. One of the main steps that involves the intracellular delivery of molecules includes the formation of vesicles which squeeze out from the plasma membrane to deliver intracellularly. Mainly through the activity of GTPase, dynamin is required for this step and it is therefore the mechanochemical enzyme that initiates the formation and membrane fission of vesicles. According to another model GTPases hydrolysis dismisses the communication with the effector (Vincent, 2003).
The major pathways governing the trafficking of molecules such as the receptor, ligands, and solutes are known as endocytic pathways which control the entry into cells. The trafficking of pharmacological agents via endocytotic pathways requires intracellular membrane trafficking and molecular dynamics of the concerned organelles. This flux is maintained by the individual compartments and endocytic pathways. The trafficking of proteins and lipids is maintained by balanced targeting, retention, and retrieval mechanisms. The most effective site for delivering drugs to the targeted location is the endocytotic pathway. Nowadays, targeting of drugs to the specific location requires actively checking the exclusivity of the desired action either within the organelles or it requires the delocalization of endosomes or lysosomes prior to the targeted location. Drug release therefore depends on the early endosomal escape as compared to the utilization of lysosomal protease mediated drug release (Watson et al., 2005).
Components of Membrane Trafficking
Intracellular membrane transport systems responsible for trafficking nearly all the molecules are expressed in mammalian cells. To perform this orchestra in a pristine manner, some major components such as: endoplasmic reticulum, Golgi apparatus, endolysosomal platform, and a molecular machinery to hold approximately 2,000 proteins, actively partakes. It begins with endoplasmic reticulum, a complex system consisting of tubules and cisternae. It prepares the protein for its journey by glycosylation and folding. Sometimes it unfolds the folded protein in certain stress periods and governs the fate of the cell. After the complex folding step is completed the protein is ready to move to its next location by exiting the endoplasmic reticulum as vesicles, which are either small or large, with the help of the coat protein complex II (COPII). With the help of COPI, the cargo reaches the Golgi complex and takes part in glycosylation from the cis part of Golgi to trans part and be packed, according to their cargo, by specialized membranous carriers like- lysosomes or uncoated pleomorphic carriers in the form of secretory granules destined to plasma membrane (De Matteis and Luini, 2011).
Regulating Membrane Trafficking
In order to maintain homeostasis, cell needs to sense the surrounding environment and acts accordingly by communicating via the release of mediators and the signalling molecule. Multiple receptor mediated signalling pathways are coordinated for many important physiological processes. One of them, which belongs to the largest family of signalling receptors, is G Protein Coupled Receptors (GPCRs). The endocytic trafficking of GPCR is a result of studies related to ligand-induced desensitization of receptor-mediated signalling. There have been several studies on GPCRs, especially the β2-adrenergic receptor, which showed rapid desensitization via receptor phosphorylation. The study showed trafficking of agonist-bound receptor signalling via the upregulation of heterotrimeric G proteins which rapidly and selectivity undergo phosphorylation by GPCR kinases (GRKs) over the agonist-activated receptor via phosphorylation. Phosphorylation of the receptor and binding with β-arrestin prevents interaction between the receptor and G-protein, leading to termination of G-protein-mediated signalling. The sorting of the signalling receptor can occur at any point in the endocytic pathway. First, the plasma membrane GPCRs undergo ligand-induced concentration in clathrin-coated pits and some selective endocytosis via clathrin-independent mechanisms, which later affects endocytic trafficking (Hanyaloglu and von Zastrow, 2008).
For the complex regulatory mechanism of sorting, the intracellular membrane trafficking pathway is accompanied by multi-level specific components of transport including proteins such as SNAREs, Rab GTPases, and Sec1 homologs, for the maintenance of vesicle-docking and vascular-fusion reactions. The function of phosphatidylinositol and its phosphorylated derivatives, known as phosphoinositides, are primarily introduced as second messengers in signal transduction pathways. Recently, however, membrane trafficking regulated by the phosphoinositide-signalling pathway, has come under attention. The genetic study conducted for protein transport to the yeast vacuole or lysosome showed the presence of the VPS34 gene encoded for PI 3-kinase, involved in the phosphorylation of PTDIns at the n-3 position of the inositol ring, for the production of PtdIns3P. Vps34p is activated by the VPS15 gene product and acts as a membrane-associated serine/threonine protein kinase which catalyzes an auto-phosphorylation reaction (Odorizzi et al., 2000).
Targeted Drug Delivery Techniques and Pathways
Overview
The human body is a complex network of different organs, tissues, cells, and different organelles, with various pathways that control the cellular activities of the human body. However, sometimes, due to some internal or external factors, the cellular environment becomes disturbed and the body tries to maintain homeostasis but is unable to do so. This results in a disease of that particular organ. To treat that particular disease condition, drugs are available to antagonize the processes and causes of the disease and to normalize the biological conditions. These drugs are administered via various routes to target the particular organ affected, but, due to a complex cellular network, the administered medication cannot reach the desired target if it is located at subcellular levels (Manish and Vimukta, 2011). To target subcellular components, researchers need to develop certain carriers which can encapsulate the drug inside it and protect it from various barriers such as pH, efflux pump proteins, enzymes, and also from the body's immune responses. Earlier, various drug delivery systems were developed. Very few of these drug delivery systems successfully reached the market and many failed at various levels of clinical settings (Rani and Paliwal, 2014). Targeting a particular gene, receptor, ion channel, or enzyme through a particular moiety, which is responsive to those stimuli, could be a more effective way to tackle these delivery barriers. The course of travelling of NPs into and then further inside the cell is very complex and is mainly governed by unique endocytic pathways, which mainly depend on the particular cell type and ligand (Chou et al., 2011). Transferrin (Tf), is an iron binding protein which selectively activates transferrin receptors and becomes internalized through the clathrin-mediated endocytic pathway (Foroozandeh and Aziz, 2018). Following the course of endocytosis, researchers are more inspired by targeting intracellular trafficking networks or pathways. Research is therefore nowadays focused on the development of intracellular targeted drug delivery techniques.
Types of Drug Delivery Systems
Various membrane trafficking pathways control the cellular behaviour, and, by exploiting these pathways researchers have tried to develop novel ways to tackle problems such as drug resistance, e.g. by targeting the efflux pump inhibitor cyclosporine A encapsulated into the polymeric PEO-b-PCL micelles (Aliabadi et al., 2005; Yang et al., 2008). Mitochondria are key regulators of the energy and power house organelles inside the cells. These are involved in various biological processes such as fatty acid metabolism, Kreb's cycle, and oxidative phosphorylation. These are also involved in apoptosis by activating the cell death process through different mechanisms. Delocalized lipophilic cations (DLC's) e.g. Methyltriphenylphosphonium (Murphy, 1997) are highly lipophilic and cationic in nature and readily pass through the mitochondrial membrane, due to negative membrane potential (Chen et al., 2016). Liming Wang et al., developed serum protein coated Au-Nanorods (Au-NRs) for the treatment of cancer. They studied the effect of Au-NRs on A549 cell lines and found that Au NRs are selectively lethal for the cells. TEM analysis showed that these enter through the plasma membrane and are internalized in A549 cells and then into the endosomes, lysosomes, and mitochondria. The nanoformulations are reported to inhibit the growth of cells by increasing the ROS level in cancer cell mitochondria (Wang et al., 2011).
Advantages of Targeted Drug Delivery Techniques
The nanocarrier system for intracellular targeting has emerged as the most preferred vehicle for drug delivery in the case of cancer treatment. There are various advantages of intracellular targeting and these include the ability to bypass the mononuclear phagocyte system (MPS), the triggered release of payloads, selective targeting, improved pharmacokinetics, longer half-life, improved bioavailability, efficient navigation, and multivalent surface modification (Rejman et al., 2004; Xu et al., 2015; Jhaveri and Torchilin, 2016). This recent approach deals with the functionalization of nanoparticles with multiple functional moieties so as to fulfill multiple purposes. The multivalent functionalization platforms perform various functions like enhancing the cellular uptake, more transfection efficiencies, and precise and effective targeting of subcellular organelles etc. (Chen et al., 2017; Peng et al., 2017; Sun et al., 2017). Sometimes the nanoparticulate platforms are used for minimization of the reaction distances between different reactants such that the reaction efficiencies can be maximized. In one such instance, novel upconversion nanoparticles (UCN) were used for a photosensitizer protoporphyrin IX (PpIX) to enhance the ROS production to gain the advantage of subcellular positioning and irradiation productivity. This platform was used for mitochondrial targeting and exhibited significantly enhanced tumor cell killing and the inhibition of tumor growth (Chen et al., 2017).
The functionalization of nanoparticles with a single ligand is often not sufficient enough to achieve the desired level of cell surface or subcellular organelle targeting which ultimately hampers the best therapeutic efficiency of the delivered drug payload. Therefore, nanoparticles conjugated with dual peptides e.g. galectin-3-targeted G3-C12 peptide and D(KLAKLAK)2 (KLA) peptide were employed to target the mitochondria after undergoing receptor mediated internalization into galectin-3-overexpressing PC-3 cells. This approach provides multiple advantages such as enhanced mitochondrial membrane disruption, significantly more ROS generation, and much better cytotoxic effects. It also resulted in better tumor accumulation of the delivery system and the best therapeutic efficiencies were achieved with a better animal survival rate (Sun et al., 2017). Another similar approach for particles decorated with two targeting moieties involves one of these binding to folate membrane cell surface receptors and the second (triphenylphosphine, TPP) targeting the mitochondrial membrane. This approach offers the benefits of fine control in the targeting process through asymmetric attachment of ligands on each side of the particles. The folate receptor targeting offers enhanced cellular uptake and accumulation inside tumor cells followed by mitochondrial targeting with TPP and improved the therapeutic efficacy of the nanomedicine (López et al., 2017).
Sometimes the nanoparticulate platform in itself is composed of a self-assembling or self-aggregating moiety such as triphenylphosphonium-appended coumarin and loaded with an anticancer drug, e.g. doxorubicin, to achieve the selective mitochondrial targeting. These nanocarriers show the benefits of selective mitochondrial accumulation and possess the advantage of efficient doxorubicin delivery to mitochondria. This kind of nanomedicinal platform possesses significant clinical potential in being a specific sub-organelle targeted delivery system for anti-cancer therapeutics (Lee et al., 2018). Along with these benefits, the nanoparticles targeting cellular and subcellular locations offer other distinct advantages in terms of their physicochemical, morphological, and functional properties, which are of specific interests to researchers. Further advancements in terms of their improved selectivity, maximum therapeutic outcome, precise entry into cancer cells via targeting ligands, and responsiveness to physical or biological stimuli help in their enhanced subcellular performances (Liu et al., 2020).
Subcellular Drug Delivery Pathways
Successful treatment of any disease can only be achieved when the drug, molecules, proteins, si-RNAs, DNAs, via efficient carrier systems, are effectively administered at the subcellular level of the targeted cells. Various drug delivery techniques are followed to reach the subcellular level. One effective way to reach the subcellular level is achieved by targeting the membrane trafficking pathways (Hayes et al., 2006). The therapeutic effects will appear as a result of target binding of the specialized drug molecule at subcellular levels. The subcellular targeting system requires one to follow some of the criteria - they should not be captured by degradation pathways, capable of receptor mediated endocytosis and target recognition (Table 1). It should be able to release the drug at the desired site and also maintain the therapeutic concentration in the cells (Chou et al., 2011). In an attempt Durymanov et al., synthesized a new PEI-PEG based polyplexes loaded with a MC1SP-peptide which are specific for melanocortin receptor – 1 and investigated its potential. The synthesized and targeted polyplexes showed greater efficacy in receptor-mediated transfection of cloned (Cloudman S91) murine melanoma cells as compared to non-targeted polyplexes. In addition, the inhibition of transfection was mediated by chlorpromazine, a positive inhibitor of the clathrin-mediated endocytosis pathway. The synthesized polyplexes also showed rapid nuclear uptake as compared to non-targeted polyplexes (Durymanov et al., 2012).
The natural cell transport process was exploited for intracellular targeting of the auger electron emitter. The advantage of auger electrons, found in the decay product of many radionuclides, is the damage produced by these short range auger electrons in the local vicinity with minimal damage to adjacent normal cells, and potentiates its use for targeting cancer cells. The ability of auger emitters to localize preferentially in the cellular nuclei is exploited by researchers in cancer therapy (Rosenkranz et al., 2019). Rosenkranz et al., developed a specific transporter for cell specific nuclear delivery for locally acting drug therapy. The transporters were comprised of a melanocyte stimulating hormone, a nuclear localization sequence, and a protein carrier such that it can translocate into the nucleus and deliver the desired drug at the vulnerable site of nuclear DNA. This paradigm was able to deliver the drug to cell specific particular subcellular locations and resulted in enhanced therapeutic efficacy (Rosenkranz et al., 2003). Similar targeted therapeutic delivery platforms were exploited via other targeting approaches, formulations, and characterization of folate receptor targeted nanotransporters for cancer specific cell accumulation and cancer cell nuclei eradication (Slastnikova et al., 2017). In a similar fashion, EGFR over expressing bladder cancer cells were targeted for precise drug delivery in the vicinity of nuclear DNA such that high toxicity is precipitated in the cancer cells. The nanoformulation employed here significantly enhanced in vitro and in vivo therapeutic effects of the delivered molecules (Rosenkranz et al., 2018). There are various strategies that are discussed under this topic.
Passive Targeting
Passive drug targeting takes advantage of the biological system but does not attach any active moiety or targeting molecule in the drug delivery system. Some of these are used to target the drug molecule at the mononuclear phagocytic system, enhanced permeability, and retention effect or to target the local organ. In another way it can be the chemoembolization or the pegylation might enhance the stability and stealth effect. Hepatic chemoembolization is one of the methods used to target liver cancer or liver metastasis by injecting chemotherapeutic drug containing particles, which are specially made to halt the supply of nutrients and oxygen directly into the cancer cell arteries, and are able to inhibit the growth of cancer cells (Bruix et al., 2004). Enhanced permeability and retention effect (EPR) occurs mainly due to leakiness of the micro-vascular structure of tumor blood vessels. By administering drugs, it can therefore easily enhance the retention of the drug.
Active Targeting
Active targeting consists of targeting only diseased cells and rendering healthy cells unaffected. The main targets are overexpressed markers such as enzymes, proteins, receptors, and ion channels. To target these overexpressed markers researchers conjugated a drug carrier system with a specific ligand or substrate of that particular marker which mediates some ligand-receptor, enzyme-substrate interactions (Zheng et al., 2006; Gao et al., 2019). Lysosomal targeting is also an effective way of treating defective cells. Lysosomes contain a variety of hydrolases which are mainly involved in degradation, autophagy, recirculation, membrane repair, and secretions. The acidic environment (pH~5) of lysosomes makes it suitable for target therapies that control the release of drugs at acidic pH. Ligand conjugated nanoformulations majorly target the lysosomes via receptor mediated endocytosis (Shen et al., 2017). Mitochondria are known as the power house of cells, and researchers have therefore tried to enhance the ROS (reactive oxygen species) level, to induce mitochondrial damage, and blockage of the respiration in mitochondria, for the treatment of cancerous cells. But due to the subcellular level, these approaches were significantly less effective. Nowadays more research is focused on mitochondrial targeting by developing formulations to obtain effective access of the mitochondria. One scientist developed an iron oxide nanoparticle for the mitochondria targeted PTT (Photo-thermal therapy), the iron oxide NPs coumarin functionalized found to be primarily accumulated in endoplasmic reticulum. They demonstrated higher in vivo efficacy of the formulation (Jung et al., 2017).
Factors Affecting the Intracellular Targeted Drug Delivery Systems
There are various factors that affect intracellular targeted drug delivery, such as size, shape, hydrophobicity, surface charge, and surface modification (Qiu et al., 2010; Foroozandeh and Aziz, 2018). The endocytic uptake of NPs is mostly affected by the above mentioned parameters. In this section a brief explanation has been provided on some of the factors that govern uptake and intracellular targeting of drugs.
Size and Shape of NPs
Effective NPs uptake is highly dependent on the size and shape of the Nanomaterials (Zhu et al., 2013). It also governs the cytotoxic potential of nanomaterials (Nel et al., 2009). The optimum size of NPs for efficient uptake into the cell membrane is 50 nm (Chou et al., 2011). NPs with a size ranging from 120-150 nm primarily get internalized through caveolin and clathrin mediated endocytosis pathways (Figures 2A, B). Direct entry of NPs into the bilayer of cells can be enhanced by reducing the size of NPs (Zhu et al., 2013). Along with size, the shape of NP also plays a vital role in the absorption and trafficking of molecules through various compartments. Researchers have observed the consequences of shape of AuNP on uptake into HeLa cells and found that the spherical shaped AuNPs shows five times higher uptake than rod shaped AuNPs. Filomicelles have a role in transport and trafficking, and it has been found that they have a greater circulation half-life than a spherically shaped NP (Foroozandeh and Aziz, 2018).
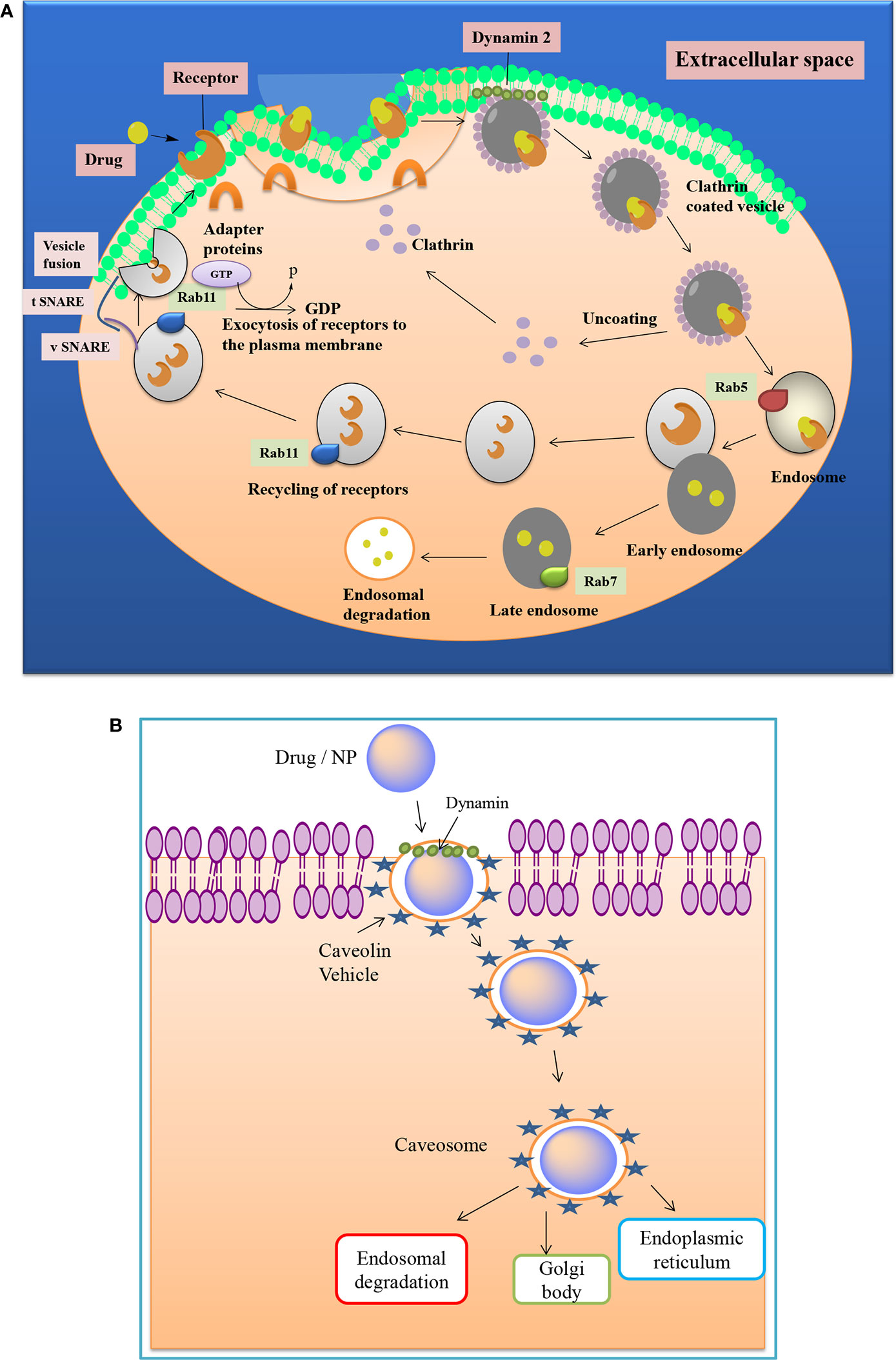
Figure 2 (A) Clathrin mediated drug transport across the cell membrane. Cell surface receptors bind the exogenous ligand and internalize the moiety by formation of clathrin, which implicates the role of various proteins like Rab, GTPases, and SNARE proteins etc. (B) Caveolae mediated transport of a drug or nanoparticle. It involves the formation of bulb-shaped, 50-60 nm plasma membrane invaginations, called caveolae, whose formation is driven by integral membrane proteins and peripheral membrane proteins.
Surface Charge
Surface charge is the most crucial factor which readily determines the cellular uptake of nanoparticles. The surface charge can be determined by Zeta potential measurement and can be positive or negative. The highly negatively charged cell membrane enhances the uptake of the positive charge nanoparticles. The higher uptake of the positive charge particles may create distortion of the membrane integrity and this can lead to an increase in cellular toxicity. The uptake of AuNPs via different pathways depends on the surface charge. The positively charge AuNPs can be internalized via micropinocytosis and clathrin mediated endocytosis (Panariti et al., 2012).
Surface Modification
The surface modification can be of great potential in increasing the uptake of NP into the cell via various trafficking pathways; and can be helpful in reducing the toxicity of NPs, governing the stability and their fate. The uptake can be increased by increasing the positive charge on NPs via surface modification, adding an amino (-NH2) group on the surface. The negative charge can be enhanced via carboxylation (-COOH) which increases the cellular uptake (Alexis et al., 2008).
Significance of Subcellular Drug Targeting
The distribution of drugs and its subsequent access to the targeted location is the main aim of drug targeting. More often than not, the target location plays a very crucial role in drug delivery, as sometimes the target location is inside the cells, or more precisely, within cellular organelles. The significance of subcellular targeting is expected to improve potency and to minimize related side effects. There are some properties which significantly influence targeting and subsequent organ accumulation of molecules viz. surrounding pH, resident molecules itself, electronic potential, lipid-bilayer composition, and membrane bound proteins (Duvvuri et al., 2004). Nowadays, pharmaceutical nanotechnology can overlook the limitations of conventional administration and poor bioavailability of drug molecules, and by using new and improved nanoformulations, which include different kinds of carriers and conjugates, overall improvement in the pharmacokinetics and pharmacodynamics behavior has been observed, facilitating the implementation of novel DDS in clinical use.
Organ targeting by transforming a drug molecule into polymer coated microspheres improves the pharmacokinetic parameters for local pulmonary therapy, minimizing drug accumulation in the heart, followed by minimizing cardiotoxicity. Incorporating polyethylene glycol (PEG) into nanoformulations overcomes the first-pass elimination from blood and bypasses the reticuloendothelial (RES) system. PEGylated liposomes of doxorubicin presented modified pharmacokinetic data as compared to plain doxorubicin. Targeting molecules into subcellular locations significantly improves the bioavailability of drugs. Internalization of drug molecules depends on the polymer-ligand interaction. The main aim of a targeted drug delivery system is to control biodistribution and to improve pharmacokinetics of the drug by controlling physiochemical and biochemical properties (Leucuta, 2014).
Role of Carriers in Drug Targeting
Although great efforts have been practiced in targeting cell surface receptors, very limited therapeutic advantages have been availed. The recent focus has since, therefore, shifted to strategies of targeting subcellular components to achieve specificity and maximum therapeutic benefits (Rajendran et al., 2010; Leucuta, 2014). The maximum therapeutic efficacy of a drug (either macromolecular or DNA, protein and peptide based drugs, oligonucleotides, siRNA etc.) can be exploited by maneuvering these with the help of some specialized carriers to various subcellular compartments like nuclear and mitochondrial targeting, to achieve safe and efficacious carriage to the desired sites of actions (Lamade et al., 2019; Nurunnabi et al., 2019; Sun et al., 2019; Wang D. et al., 2019). In this context a number of carriers have been designed, sometimes to exploit either a particular subcellular component or a drug delivery process (Huang et al., 2016). Various carriers have been formulated for the effective delivery of drug molecules across a wide spectrum of subcellular components which regulate the drug's subcellular pharmacokinetics and pharmacodynamics (Table 2) (Paillard et al., 2010; Leucuta, 2014; Sun et al., 2019).
In general, carriers have been conceptualized with the purpose of delivering drugs by targeting nuclear components such that these molecules directly regulate gene expression or protein transcription processes (Figure 3) (Ahmad et al., 2019). In other instances, a specific subcellular component, such as early or late endosomes, help drug carriers in one way or the other e.g. in pH mediated drug release, exocytosis, and subcellular drug distribution etc. (Bae et al., 2005; Yuanjie Zhu and Yuanjie Zhu, 2019). A significant example is the designing library of mitochondrial targeting tetra-peptides employed in targeting mitochondrial dysfunction in neurological and cardiovascular disorders. These molecules exhibit limited toxicity profiles with significantly higher efficacies in providing protection against exogenously-induced damages. Along with these characters, targeting agents possessed enhanced colocalization with mitochondria and regulated subcellular accumulation and eminent capacity as a targeted delivery vehicle (Sun et al., 2019).
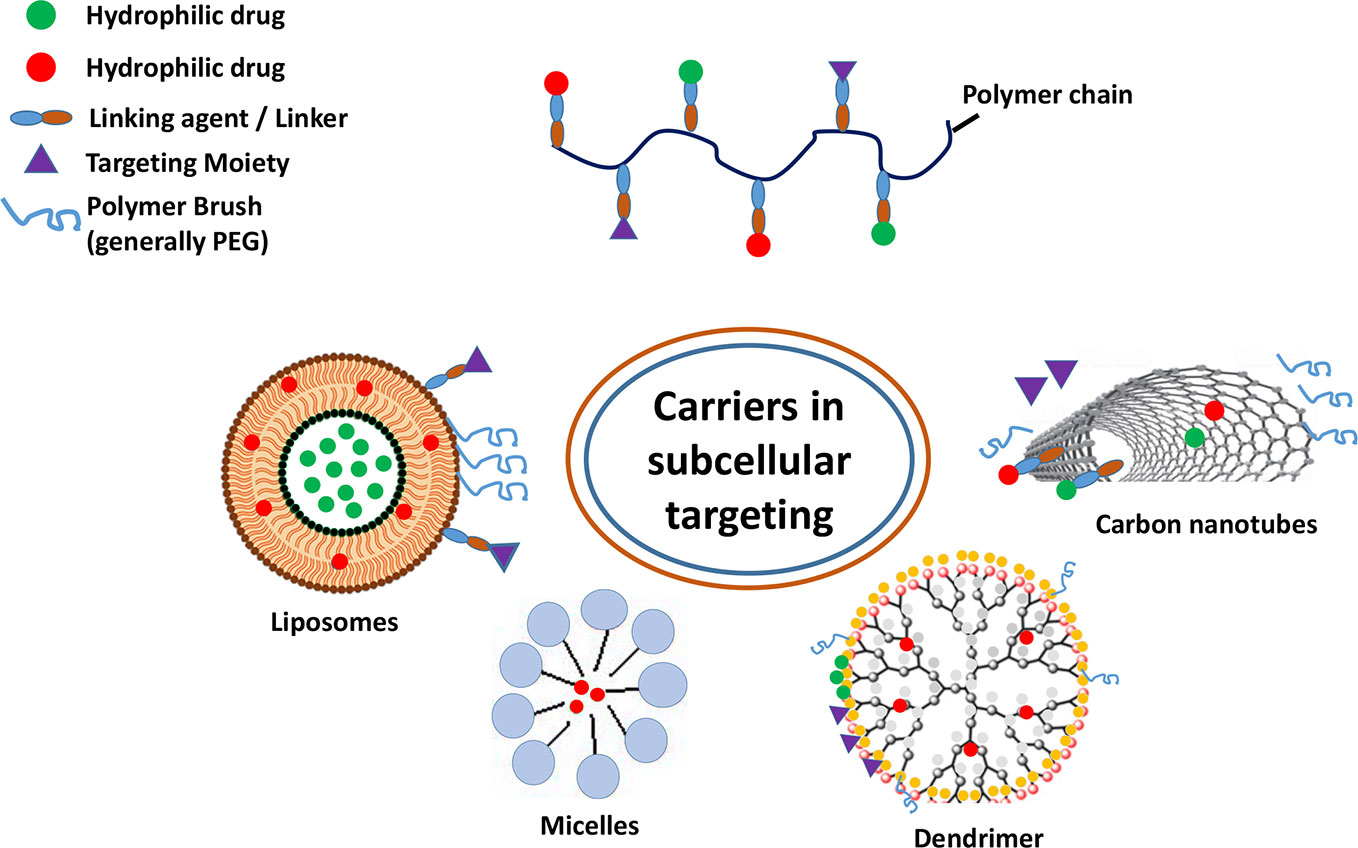
Figure 3 Various carriers and their components employed in subcellular targeting. The nanocarriers are composed of hydrophobic and/or hydrophilic polymeric chains capable of encapsulating and delivering both kinds of therapeutic payloads.
Other studies include the designing of mitochondria targeted mesoporous silica nanoparticles containing the hydrophobic anticancer agent α-Tocopheryl Succinate. An effective mitochondria targeting ligand, triphenylphosphonium (TPP), was employed for surface functionalization. After conducting various experiments the authors concluded that the nanocarriers imparted higher efficacy to drugs indicating a promising approach of targeting the mitochondria (Qu et al., 2016). In another approach, multifunctional nanocarriers were designed for the selective delivery of coenzyme Q10 to mitochondria and the efficacy of nanocarriers was assessed in terms of two different experimental paradigms viz. oxidative stress and inflammation. The authors suggested that the nanocarriers were effective and preserved and protected the drug effectiveness during its delivery (Sharma et al., 2012). Likewise, other nanocarrier mediated mitochondrial targeting paradigms like curcumin DQAsomes, rhodamine-123-conjugated polymeric liposomes were also designed for different aims. Curcumin loaded DQAsomes potentiated the antioxidant efficacy of curcumin (Zupančič et al., 2014), while modified liposomes exhibited better cellular uptake by HeLa cells. A high degree of accumulation of these rhodamine-123-conjugated polymeric liposomes could also be observed in mitochondria. In terms of cytotoxic efficacy, appreciably enhanced cell cytotoxicity could be observed with modified liposomes as compared to non-targeted liposomes (Biswas et al., 2011). Along with the mitochondrial targeting, nuclear targeting strategies such as RGD peptide systems, cell penetrating TAT peptides etc. have been introduced, which can carry and deliver the therapeutics and exhibit high promises in nuclear targeted drug delivery paradigms (Nurunnabi et al., 2019). In a recent study, a RGD peptide was used in combination with other peptides and a nuclear localization sequence, specifically for nuclear targeting, where researchers delivered a precise transportation of a tracing reagent to the nucleus. A multifunctional fluorescent probe was combined with the RGD peptide and the nuclear localization sequence, after binding to cell surface receptors, effectively being internalized into the nucleus (Cheng et al., 2017). In another concept, Yang et al. functionalized nanoparticles with the RGD peptide to enhance cellular uptake with an aim to enhance therapeutic efficacy of nanoparticles through their effective targeting into the nucleus. These peptide coated gold NPs exhibited five-fold enhancement in the nanoparticle uptake and effective nuclear localization. This effective cellular uptake and nuclear localization imparted more therapeutic efficiency to gold NPs in their anti-cancer approach (Figure 4) (Yang et al., 2014). Colin et al. report that a major barrier for efficient gene therapy is gene transit across the nuclear envelope. The RGD peptide is shown to mediate the enhanced internalization of the plasmid and synergistic gene expression. This enhanced internalization is due to increased nuclear transfer, which is an energy dependent mechanism. The authors concluded that the oligolysine-RGD peptide acts as a nuclear localization signal and RGD aids it by binding to RGD and recognizing integrin proteins (Colin et al., 2001). Along with all these approaches, the RGD peptide has extensively been used with other nanomedicine based platforms viz. RGD mediated delivery of quantum dots for tumor cell imaging (Cai et al., 2006; Lieleg et al., 2007; Smith et al., 2008), iron-oxide NPs with RGD peptide for endothelial cell labeling and imaging (Montet et al., 2006; van Tilborg et al., 2008; Almutairi et al., 2009; Schottelius et al., 2009; Sugahara et al., 2009), and RGD conjugated dendrimers for angiogenesis imaging etc. (Almutairi et al., 2009).
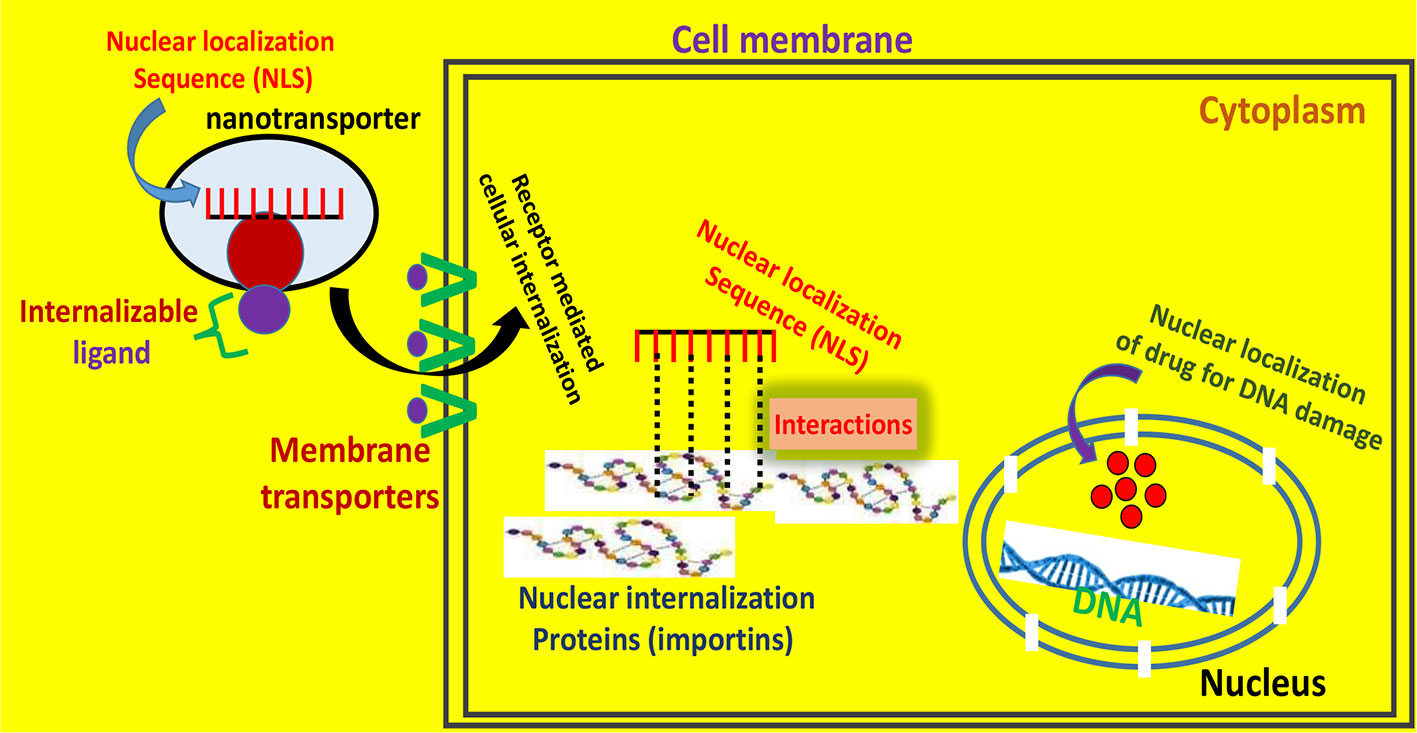
Figure 4 Membrane and subcellular trafficking implicated in the nuclear localization mechanism of drugs. The nanotransporter comprises an internalizable ligand which facilitates the receptor mediated cell entry, and a nuclear targeting moiety (nuclear localization sequence; NLS) which enters the nuclear region and regulates the gene expression.
Apart from these, certain plasma membrane targeting approaches have also been designed, which include drug Enfuvirtide-C34 against HIV-AIDS, where the lipid linkage enhances the membrane targeting and better inhibition of HIV fusion complex, Myr-proS1 for HBV fusion inhibition by targeting the HBV fusion complex, and Pepducin as GPCR modulator for an effective modulation of the GPCR signaling (Rajendran et al., 2010). Apart from nuclear, mitochondria, and plasma membrane targeting, Golgi apparatus and endoplasmic reticulum have also been the targets of drug delivery approaches. Biodegradable Rhodamine-loaded PLGA (polylactic-co-glycolic acid) NPs were assessed in epithelial cells to demonstrate colocalization with specific organelles: early endosomes, late endosomes, lysosomes, endoplasmic reticulum (ER), and Golgi apparatus (Cartiera et al., 2009). This platform was employed for respiratory, gut, and renal targeted delivery. Another system for targeting endoplasmic reticulum and Golgi apparatus was composed of conjugated Antigenic peptide directed against ovarian cancers, intestinal cancers, and lymphomas by targeting the presentation on the MHC class I complex. These approaches offered the advantage of considerably enhanced cellular uptake, colocalization with the organelles of interest, and offering biocompatible and relatively tolerable subcellular targeting delivery ssytems (Morse et al., 2011).
In the context of receptor targeting, discovery of human epidermal growth factor receptor 2 (HER2) has revolutionized anti-cancer therapies, in particular for personalized medicine in HER2 receptor amplified breast cancer patients. Recent research on HER2 receptor targeted anti-HER2 drug conjugate (HER2-ADC) has become the pillars of treatment against advanced HER2 positive breast cancers. These are composed of antibodies against the extracellular domain of human HER2 receptors and contain the monoclonal antibody (e.g. trastuzumab) that binds to HER2 and fuses with a cleavable linkage to another cytotoxic anti-microtubule reagent (e.g. tubulysin) with very potent anti-neoplastic antibodies. Upon binding to the target receptors HER2, antibody drug conjugate is internalized into cancer cells and is translocated to lysosomes where the linker is cleaved. The drug is then delivered to HER2 expressing cancer cells and finally blocks the cancer cell division, resulting in G2/M phase arresting, apoptosis, and reduced proliferation of HER2 positive cancer (Next-Generation Anti–HER2-Antibody Drug Conjugates, ; Rinnerthaler et al., 2019; D'Amico et al., 2019).
Targeting transferrin receptors in the brain, for the delivery of a drug payload across the blood brain barrier (BBB), has always been a considerable challenge for researchers working on central nervous system diseases. Although antibodies have been employed against transferrin receptors, nothing could be achieved by the mere use of naïve antibodies. Research on the engineering of those antibodies against transferrin receptors then started and various approaches were undertaken. One concept describes the conjugation of mouse antibody to another anti-amyloid plaque antibody for targeting the brain side of the BBB (Niewoehner et al., 2014). Another strategy suggests that engineering of anti-transferrin antibodies, to reduce their affinity against transferrin receptors, lead to the significant enhancement of their accumulation in the brain parenchyma (Paterson and Webster, 2016).
At molecular levels, few specialized nanoparticulate drug delivery nanocarriers have evolved that can safely and effectively carry drugs for nuclear targeting in such a way that the subcellular internalization can surpass the biological blockades either passively or via active targeting (e.g. receptor mediated drug delivery). These approaches of intracellular drug distribution with specialized drug delivery systems is expected to be clinically beneficial and will be a great boon for pharmaceutical nanotechnological platforms (Leucuta, 2014). Different criteria have been set for the drug carriers with subcellular targeting purposes, e.g. it should be inert, which means the drug should be released in its active form after reaching subcellular drug targets, the drug and carrier linkage should be strong and stable, it must get through the biological barrier points, and should be non-toxic and non-immunogenic etc (Schneider et al., 1984). Sometimes the drug carrier geometry also influences its uptake or endocytosis and ultimately the subcellular targeting, lysosomal transport, its residency in the prelysosomal compartments, the half-life of the drug in circulation, as well its specificity etc. (Muro et al., 2008). The capacity of a medicinally active compound to bind to its specific target in the cell is intimately associated with its potential to target the molecular events inside the cell organelles. However, often the compound itself does not have the potential to permeate inside the cell and therefore requires the aid of a carrier molecule, sometimes to attain metabolic stability, to attain maximal therapeutic efficacy, to control subcellular disposition, or to achieve organelle oriented targeting effects (D'Souza and Weissig, 2009). Different internalization mechanisms have also been proposed for the carriers directed to the subcellular targets viz. phagocytosis, pinocytosis, clathrin mediated endocytosis, caveolae mediated endocytosis, via formation of early and late endosomes, and macropinocytosis etc (Guo et al., 2020).
Endosomal Escape of Drugs and Carrier Systems for Subcellular Targeting
In spite of all the benefits of targeting drugs to cellular and subcellular locations, the targeting carriers may undergo entrapment followed by degradation in the acidic chambers of endosomal or lysosomal pathways. Optimization measures have been carried out to escape the endosomes and a greater understanding of measures that govern endosomal escape is required, as this is the most crucial and rate determining step in the delivery of therapeutics (Smith et al., 2019). These measures include incorporation of functional groups with positive charge, pH sensitive moieties, utilization cationic polymers and lipids e.g. poly (ethylene imine), endosomal swelling strategies, and disrupting the endosomal membrane etc. Recent approaches also undertake the use of bacterial toxins, e.g. purified Listeriolysin O (LLO) toxin, as a medium to promote escape from the degrading environment of lysosomes and to avoid the breakdown and degradation of the delivery system through the endosomal lysosomal network in the cell (Plaza-GA et al., 2019). However all of these strategies are associated with their respective limitations and designing a carrier system which can achieve a 100% endosomal escape is still a far flung achievement (Cupic et al., 2018).
Some recent studies, in the context of endosomal escape, include the formulation of a drug carrier made up of albumin combined with palmitoyl-cyclic-(D-Arg)12. This carrier was used as endosome mimicking liposomes and data further suggested that palmitoyl-cyclic-(D-Arg)12/HAS is internalized inside the cell through induction of micropinocytosis, and exhibits endosomal escape (Ichimizu et al., 2019). Another strategy describes conjugation of stromal-derived factor 1α (SN21) with membrane lytic peptides which induces micropinocytosis or membrane lysis and aids in the successful delivery of functional siRNAs and proteins. It also gives the loaded drug cargo the ability to be released from endosomes or endosomal escape (Arafiles et al., 2020). Fraire et al., describe the escape from endosomes in terms of endosomal rupture through the photothermal properties of gold nanoparticles by vapor bubble formation and heat induction. In this approach the loaded cargo of siRNA is released intact and with equal efficacy, and heat mediated endosomal escape is dependent on the number of gold nanoparticles per endosome. This approach also had the advantage of not disturbing the cellular homeostasis (Fraire et al., 2020).
To overcome the major challenge of endosomal sequestration of RNAs therapeutics Orellana et al., exploited the difference in solute concentration between nascent endosomes and cytoplasm which resulted in osmotic swelling and the bursting of endosomes. They employed the nigericin molecule to exchange potassium and hydrogen ions and enabled the folate-RNA conjugates to escape from endosomal entrapment or capture (Orellana et al., 2019). Some other recently described approaches for endosomal escape include, but are not limited to, the formulation of pH buffering particles which build up the osmotic pressure and exhibit osmolytic endosomal escape - membrane disrupting particles which destabilize the membrane and gelatinous nanoparticles which exhibit endosomal escape via swelling and endosomal rupturing mechanisms (Cupic et al., 2018). Especially for charge containing drugs and carriers, the incorporation of a charged molecule inside the membrane which leads to the breakdown of membrane symmetry, creating alterations and disturbances in the electrostatic interactions, alterations of the protonation levels of pH responsive drug delivery systems, charge mediated changes in the membrane tension and consequent membrane ruptures, and ion mediated changes in the osmotic pressure inside endosomes are some of the recently studied mechanisms for endosomal escape (Tian and Ma, 2012; Lönn et al., 2016; Allen et al., 2018).
Current Scenario of Subcellular Drug Targeting
With the advent of serious and toxic side effects associated with current conventional drugs with generalized distribution and non-specific targeted therapies, researchers have directed their focus on developing specific and subcellular targeted therapies so as to maximally enhance therapeutic benefits and to minimize off-target effects. Recently wide spectrums of subcellular targeting strategies have come into practice, which can dispose drugs at therapeutic targets and organelles (e.g. lysosomes, mitochondria and nucleus etc.) via multiple mechanisms (Chakraborty et al., 2018; Wang F. et al., 2019; Yuanjie Zhu and Yuanjie Zhu, 2019; Guo et al., 2020).
Recently, researchers formulated a reaction system comprised of copper catalyzed azide-alkyne cycloaddition to specifically target the preferential accumulation of the resveratrol derived drug in the mitochondria of living cells. The system possessed high catalytic efficiency and exhibited focused drug synthesis through site-directed specificity. The reaction system developed various advantages such as the avoidance of the troublesome delivery process and non-specific distribution of obnoxious moieties, possibilities of multi-drug synthesis, and high biocompatibility etc. (Wang F. et al., 2019). Similarly, another functionalized colloidal nanoparticulate system with chemical and biochemical surface functionalization was developed, which took into account the recent concept of nanoprobes. Polyacrylate material was employed to form colloidal nanoprobes with the specific aim of targeting subcellular compartments such that the nanobioconjugates can facilitate nanoparticle-cell interaction, helping the endocytosis lead to localized subcellular disposition of the active molecule (Chakraborty et al., 2018).
Another functional subcellular targeting strategy describes the DNA origami based nanostructures for targeting late endosomal and lysosomal drug distribution in subcellular localities. Fluorescent triangular structures were designed which were safe, biocompatible, efficient, and evaluated in U87MG cells. These nanostructures were modified with Cy5 dye, targeted against endosomes and Golgi apparatus and showed respective efficient colocalization under a confocal laser scanning microscope (Yuanjie Zhu and Yuanjie Zhu, 2019).
Recently, Kang et al., developed a mitochondrial targeting strategy by bypassing the multi-drug resistance, by formulating liposomes which carried resveratrol and targeted mitochondria. These were aimed at providing enhanced circulation of drugs in the blood and exhibited an enhanced retention and permeability (EPR) effect. The liposomes were 120 nm and possessed positive zeta potential, and when loaded with rhodamine, showed effective uptake in the cells under confocal microscope. The enhanced uptake was also demonstrated by LCMS/MS quantified resveratrol in the subcellular organelles. The mitochondrial membrane dissipation, ROS production, and cell cytotoxicity results demonstrated that these liposomes can provide a vital subcellular targeting strategy in the cancer treatment (Kang and Ko, 2019).
When the administered drug meets various biological and physiological barriers, only a fraction of drugs reach the target site and exhibits a pharmacological response, while the majority is disposed to off target sites and leads to undesired and harmful effects. To overcome these limitations another strategy suggests the design of cleavable nitrogen-terminal extensions in drug carriers such that it allows the subcellular translocation of nuclear targeting compounds such as TAT or RGD peptides into the nucleus. The desired drug can also be introduced along with these nuclear targeting proteins or peptides which exhibit a significant benefit in nuclear targeting drug delivery practices (Nurunnabi et al., 2019).
Babikova et al., recently developed a multi-functional triblock copolymer for mitochondrial targeting application which comprised of a saccharide attached polyoxyethylene block, an amphiphilic diblock copolymer, and a biodegradable hydrophobic component. The polymeric carriers were loaded with anti-cancer drugs of plant origin i.e. curcumin. The mitochondrial targeting ability was assessed in terms of the cell cytotoxicity, apoptosis potential, and NF-κB inhibition potential and all of these parameters were found to be superior in the polymeric carriers than in the naïve drug alone or in simple unconjugated carriers (Babikova et al., 2019). Another recent mitochondrial targeting strategy takes into account the enhanced temperatures of mitochondria to develop a finely tuned thermoresponsive drug carrier for paclitaxel, an anti-cancer drug. The carrier comprised of polymeric components like polyacrylamide and ply(N-isopropylacrylamide) and in all the in vitro experimentation results established the superior efficacy at higher temperatures. The nanocarriers exhibited superior efficacy in terms of cell cytotoxicity, colocalization, and cellular uptake of drug loaded nanocarriers over the naïve drug alone (Wang D. et al., 2019).
It is known that photocaging helps in the accurately site specific and non-invading release of drug molecules inside the cell, however, subcellular organelles in the cells are placed in such a way that precise targeting of these with light becomes non-workable. Henceforth, organ specific photocages can facilitate the release of these pharmaceutical compounds in subcellular localities. Kand et al., chemically functionalized and conjugated the BODIPY based photocages and their derivatives for the specific targeting of lysosomes, endoplasmic reticulum, and mitochondria. They also demonstrated that visible light could cause the selective photorelease of 2,4 DNPH, a mitochondrial uncoupler and puromycin, a protein synthesis inhibitor. These photocages also provide enhanced efficacy to pharmacological compounds because of the localized and precipitous delivery (Kand et al., 2019).
Ender et al. report that certain subcellular vesicles produced by the cancerous cell arbitrate cancer resistance against anti-cancer drug molecules through different mechanisms. These vesicles are either exosomes or ectosomes, as categorized depending on their size, shape, content, and mechanism of production. The recent advances in subcellular targeted therapies against these vesicles and an in-depth knowledge about the mechanism of this subcellular targeting strategy will be a great boon in the fight against cancer resistance (Ender et al., 2019).
Conclusion and Future Perspective
In this review we have summarized some aspects of membrane trafficking and subcellular targeting phenomena. How membrane trafficking and its various aspects and components help in the regulation of drug targeting have been briefly described. Different sub-components of drug targeting schemes have been shortly touched on and how the optimization of different drug related parameters can aid in the formulation of subcellular targeting approaches is also discussed. Lastly, how various molecules and particulate carriers aid in the drug delivery to subcellular targets has been briefly discussed. Once pharmacologically active biomolecules are delivered to the organs, tissues, and cell vicinities is accomplished, the next big task to achieve is the drug delivery inside the cells and to the specific subcellular organelles. A great deal of achievements have been fulfilled recently as far as the targeted delivery of bioactives to the subcellular compartments is concerned. Researchers have carried out exhaustive evaluation of diverse and versatile physicochemical and biological parameters for the targeted delivery of drugs to the subcellular localizations. Many techniques and processes have been standardized and established with regards to subcellular drug targeting so as to obtain the maximal therapeutic efficacy and to minimize the off-target effects, unwanted, and adverse drug reactions. These approaches will be greatly helpful in the development of personalized and precision medicine. Among the entire subcellular targeting paradigm, nuclear, mitochondrial, and lysosomal targeting strategies have achieved great accomplishments and have provided great advantages. Still, some lacunae remain and will be filled to maximally exploit subcellular targeting strategies. A lot of work remains to be done as far as the prediction of subcellular drug localization is concerned. The use of multi-labelled proteins in this context has increased significantly but has not been tapped to its maximum potential. The development of drugs with multiple subcellular targeting possibilities is still in its initial stages. An in depth understanding of drug internalization, accumulation, biotransformation, and excretion mechanisms is also needed. We hope this review will be of great help to researchers working in the field of targeted drug delivery and personalized medicine.
Author Contributions
AK, AA, AV, and RK have written the manuscript and prepared figures and tables. RK has finally edited the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
AK is thankful to Indian Council of Medical Research (ICMR) for providing a Senior Research Fellowship (No. 45/6/2019-Nan/BMS). AA is thankful to Institute of Nano Science and Technology, Mohali for providing a Senior Research Fellowship. AV is thankful to Institute of Nano Science and Technology, Mohali for providing Junior Research fellowship. This work is supported by the Department of Science and Technology (DST), SERB with grant No. CRG/2019/004018 and YSS/2015/001851. DST-Nanomission with Grant No. SR/NM/NB-1044/2016(G).
References
Abdrabou, A., Wang, Z. (2018). Post-Translational Modification and Subcellular Distribution of Rac1: An Update. Cells 7 (12), 1–16. doi: 10.3390/cells7120263
Ahmad, A., Khan, F., Mishra, R. K., Khan, R. (2019). Precision Cancer Nanotherapy: Evolving Role of Multifunctional Nanoparticles for Cancer Active Targeting. J. Med. Chem. 62 (23), 10475–10496. doi: 10.1021/acs.jmedchem.9b00511
Alexis, F., Pridgen, E., Molnar, L. K., Farokhzad, O. C. (2008). Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 5 (4), 505–515. doi: 10.1021/mp800051m
Aliabadi, H. M., Mahmud, A., Sharifabadi, A. D., Lavasanifar, A. (2005). Micelles of Methoxy Poly(Ethylene Oxide)-b-Poly(ε-Caprolactone) as Vehicles for the Solubilization and Controlled Delivery of Cyclosporine A. J. Controlled Release 104 (2), 301–311. doi: 10.1016/j.jconrel.2005.02.015
Allen, J. K., Brock, D. J., Kondow-McConaghy, H. M., Pellois, J.-P. (2018). Efficient Delivery of Macromolecules into Human Cells by Improving the Endosomal Escape Activity of Cell-Penetrating Peptides: Lessons Learned from DfTAT and Its Analogs. Biomolecules 8 (3), 1–13. doi: 10.3390/biom8030050
Almutairi, A., Rossin, R., Shokeen, M., Hagooly, A., Ananth, A., Capoccia, B., et al. (2009). Biodegradable Dendritic Positron-Emitting Nanoprobes for the Noninvasive Imaging of Angiogenesis. Proc. Natl. Acad. Sci. U. S. A 106 (3), 685–690. doi: 10.1073/pnas.0811757106
Arafiles, J. V. V., Hirose, H., Akishiba, M., Tsuji, S., Imanishi, M., Futaki, S. (2020). Stimulating Macropinocytosis for Intracellular Nucleic Acid and Protein Delivery: A Combined Strategy with Membrane-Lytic Peptides To Facilitate Endosomal Escape. Bioconjugate Chem. 31 (3), 547–553. doi: 10.1021/acs.bioconjchem.0c00064
Babikova, D., Kalinova, R., Momekova, D., Ugrinova, I., Momekov, G., Dimitrov, I. (2019). Multifunctional Polymer Nanocarrier for Efficient Targeted Cellular and Subcellular Anticancer Drug Delivery. ACS Biomater. Sci. Eng. 5 (5), 2271–2283. doi: 10.1021/acsbiomaterials.9b00192
Bae, Y., Nishiyama, N., Fukushima, S., Koyama, H., Yasuhiro, M., Kataoka, K. (2005). Preparation and Biological Characterization of Polymeric Micelle Drug Carriers with Intracellular PH-Triggered Drug Release Property: Tumor Permeability, Controlled Subcellular Drug Distribution, and Enhanced in Vivo Antitumor Efficacy. Bioconjugate Chem. 16 (1), 122–130. doi: 10.1021/bc0498166
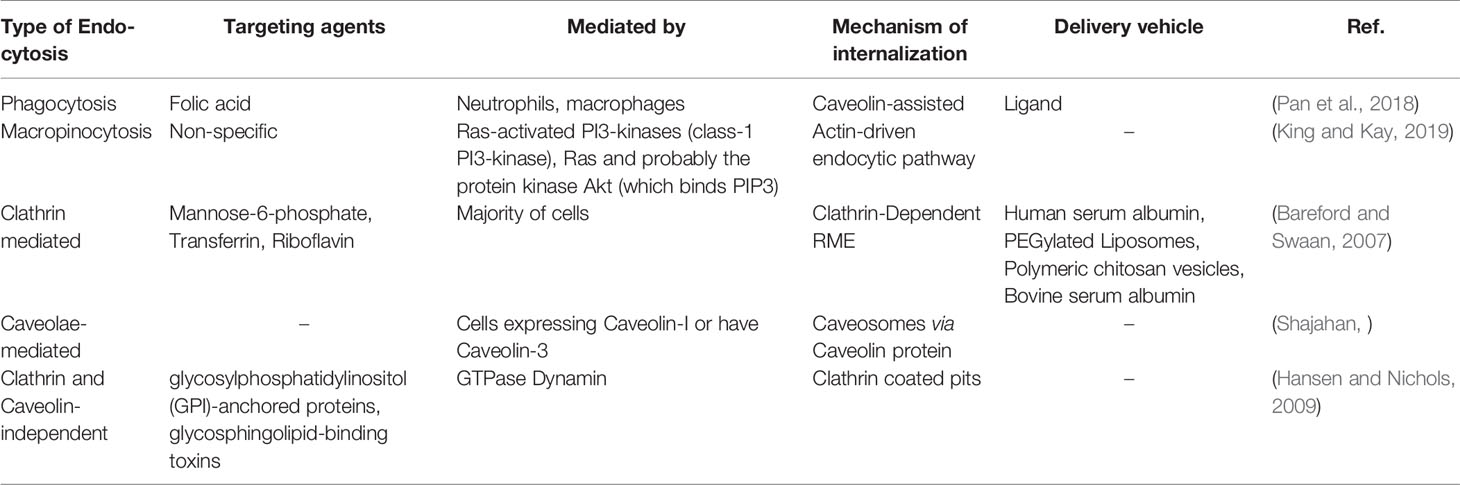
Bareford, L. M., Swaan, P. W. (2007). Endocytic Mechanisms for Targeted Drug Delivery. Adv. Drug Delivery Rev. 59 (8), 748–758. doi: 10.1016/j.addr.2007.06.008
Biswas, S., Dodwadkar, N. S., Sawant, R. R., Koshkaryev, A., Torchilin, V. P. (2011). Surface Modification of Liposomes with Rhodamine-123-Conjugated Polymer Results in Enhanced Mitochondrial Targeting. J. Drug Target 19 (7), 552–561. doi: 10.3109/1061186X.2010.536983
Bruix, J., Sala, M., Llovet, J. M. (2004). Chemoembolization for Hepatocellular Carcinoma. Gastroenterology 127 (5, Supplement 1), S179–S188. doi: 10.1053/j.gastro.2004.09.032
Cai, W., Shin, D.-W., Chen, K., Gheysens, O., Cao, Q., Wang, S. X., et al. (2006). Peptide-Labeled near-Infrared Quantum Dots for Imaging Tumor Vasculature in Living Subjects. Nano. Lett. 6 (4), 669–676. doi: 10.1021/nl052405t
Cartiera, M. S., Johnson, K. M., Rajendran, V., Caplan, M. J., Saltzman, W. M. (2009). The Uptake and Intracellular Fate of PLGA Nanoparticles in Epithelial Cells. Biomaterials 30 (14), 2790–2798. doi: 10.1016/j.biomaterials.2009.01.057
Chakraborty, A., Dalal, C., Jana, N. R. (2018). Colloidal Nanobioconjugate with Complementary Surface Chemistry for Cellular and Subcellular Targeting. Langmuir 34 (45), 13461–13471. doi: 10.1021/acs.langmuir.8b00376
Chen, Z.-P., Li, M., Zhang, L.-J., He, J.-Y., Wu, L., Xiao, Y.-Y., et al. (2016). Mitochondria-Targeted Drug Delivery System for Cancer Treatment. J. Drug Target 24 (6), 492–502. doi: 10.3109/1061186X.2015.1108325
Chen, D., Tao, R., Tao, K., Chen, B., Choi, S. K., Tian, Q., et al. (2017). Efficacy Dependence of Photodynamic Therapy Mediated by Upconversion Nanoparticles: Subcellular Positioning and Irradiation Productivity. Small 13 (13), 1602053. doi: 10.1002/smll.201602053
Cheng, H., Vetrivel, K. S., Gong, P., Parent, A., Thinakaran, G. (2007). Mechanisms of Disease: New Therapeutic Strategies for Alzheimer's Disease—Targeting Amyloid Precursor Protein Processing in Lipid Rafts. Nat. Clin. Pract. Neurol. 3 (7), 374–382. doi: 10.1038/ncpneuro0549
Cheng, Y., Sun, C., Ou, X., Liu, B., Lou, X., Xia, F. (2017). Dual-Targeted Peptide-Conjugated Multifunctional Fluorescent Probe with AIEgen for Efficient Nucleus-Specific Imaging and Long-Term Tracing of Cancer Cells †Electronic Supplementary Information (ESI) Available: Experimental Procedures, Structural Characterization Data, and Additional Figures and Scheme. See DOI: 10.1039/C7sc00402h Click Here for Additional Data File. Chem. Sci. 8 (6), 4571–4578. doi: 10.1039/c7sc00402h
Cheung, A. Y., de Vries, S. C. (2008). Membrane Trafficking: Intracellular Highways and Country Roads. Plant Physiol. 147 (4), 1451–1453. doi: 10.1104/pp.104.900266
Chou, L. Y. T., Ming, K., Chan, W. C. W. (2011). Strategies for the Intracellular Delivery of Nanoparticles. Chem. Soc. Rev. 40 (1), 233–245. doi: 10.1039/c0cs00003e
Cole, S. L., Vassar, R. (2007). The Alzheimer's Disease β-Secretase Enzyme, BACE1. Mol. Neurodegener. 2, 22. doi: 10.1186/1750-1326-2-22
Colin, M., Moritz, S., Fontanges, P., Kornprobst, M., Delouis, C., Keller, M., et al. (2001). The Nuclear Pore Complex Is Involved in Nuclear Transfer of Plasmid DNA Condensed with an Oligolysine-RGD Peptide Containing Nuclear Localisation Properties. Gene Ther. 8 (21), 1643–1653. doi: 10.1038/sj.gt.3301572
Cupic, K. I., Rennick, J. J., Johnston, A. P., Such, G. K. (2018). Controlling Endosomal Escape Using Nanoparticle Composition: Current Progress and Future Perspectives. Nanomedicine 14 (2), 215–223. doi: 10.2217/nnm-2018-0326
D'Amico, L., Menzel, U., Prummer, M., Müller, P., Buchi, M., Kashyap, A., et al. (2019). Novel Anti-HER2 Anthracycline-Based Antibody-Drug Conjugate Induces Adaptive Anti-Tumor Immunity and Potentiates PD-1 Blockade in Breast Cancer. J. ImmunoTher. Cancer 7 (1), 16. doi: 10.1186/s40425-018-0464-1
D'Souza, G. G., Weissig, V. (2009). Subcellular Targeting: A New Frontier for Drug-Loaded Pharmaceutical Nanocarriers and the Concept of the Magic Bullet. Expert Opin. Drug Delivery 6 (11), 1135–1148. doi: 10.1517/17425240903236101
De Matteis, M. A., Luini, A. (2011). Mendelian Disorders of Membrane Trafficking. N. Engl. J. Med. 365 (10), 927–938. doi: 10.1056/NEJMra0910494
Donahue, N. D., Acar, H., Wilhelm, S. (2019). Concepts of Nanoparticle Cellular Uptake, Intracellular Trafficking, and Kinetics in Nanomedicine. Adv. Drug Delivery Rev. 143, 68–96. doi: 10.1016/j.addr.2019.04.008
Durymanov, M. O., Beletkaia, E. A., Ulasov, A. V., Khramtsov, Y. V., Trusov, G. A., Rodichenko, N. S., et al. (2012). Subcellular Trafficking and Transfection Efficacy of Polyethylenimine-Polyethylene Glycol Polyplex Nanoparticles with a Ligand to Melanocortin Receptor-1. J. Control Release 163 (2), 211–219. doi: 10.1016/j.jconrel.2012.08.027
Duvvuri, M., Feng, W., Mathis, A., Krise, J. P. A. (2004). Cell Fractionation Approach for the Quantitative Analysis of Subcellular Drug Disposition. Pharm. Res. 21 (1), 26–32. doi: 10.1023/b:pham.0000012148.12516.3f
Ebine, K., Ueda, T. (2015). Roles of Membrane Trafficking in Plant Cell Wall Dynamics. Front. Plant Sci. 6, 1–7. doi: 10.3389/fpls.2015.00878
Ender, F., Bubnoff, N. V., Gieseler, F. (2019). Extracellular Vesicles: Subcellular Organelles With the Potential to Spread Cancer Resistance. Anticancer Res. 39 (7), 3395–3404. doi: 10.21873/anticanres.13483
Foroozandeh, P., Aziz, A. A. (2018). Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 13, 1–12. doi: 10.1186/s11671-018-2728-6
Fraire, J. C., Houthaeve, G., Liu, J., Raes, L., Vermeulen, L., Stremersch, S., et al. (2020). Vapor Nanobubble Is the More Reliable Photothermal Mechanism for Inducing Endosomal Escape of SiRNA without Disturbing Cell Homeostasis. J. Controlled Release 319, 262–275. doi: 10.1016/j.jconrel.2019.12.050
Gao, P., Pan, W., Li, N., Tang, B. (2019). Boosting Cancer Therapy with Organelle-Targeted Nanomaterials. ACS Appl. Mater. Interfaces 11 (30), 26529–26558. doi: 10.1021/acsami.9b01370
Guo, X., Wei, X., Chen, Z., Zhang, X., Yang, G., Zhou, S. (2020). Multifunctional Nanoplatforms for Subcellular Delivery of Drugs in Cancer Therapy. Prog. Mater. Sci. 107, 100599. doi: 10.1016/j.pmatsci.2019.100599
Hansen, C. G., Nichols, B. J. (2009). Molecular Mechanisms of Clathrin-Independent Endocytosis. J. Cell Sci. 122 (11), 1713–1721. doi: 10.1242/jcs.033951
Hanyaloglu, A. C., von Zastrow, M. (2008). Regulation of GPCRs by Endocytic Membrane Trafficking and Its Potential Implications. Annu. Rev. Pharmacol. Toxicol. 48, 537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830
Harisa, G. I., Faris, T. M. (2019). Direct Drug Targeting into Intracellular Compartments: Issues, Limitations, and Future Outlook. J. Membr. Biol. 252 (6), 527–539. doi: 10.1007/s00232-019-00082-5
Hayes, M. E., Drummond, D. C., Kirpotin, D. B., Zheng, W. W., Noble, C. O., Park, J. W., et al. (2006). Genospheres: Self-Assembling Nucleic Acid-Lipid Nanoparticles Suitable for Targeted Gene Delivery. Gene Ther. 13 (7), 646–651. doi: 10.1038/sj.gt.3302699
Huang, L., Tao, K., Liu, J., Qi, C., Xu, L., Chang, P., et al. (2016). Design and Fabrication of Multifunctional Sericin Nanoparticles for Tumor Targeting and PH-Responsive Subcellular Delivery of Cancer Chemotherapy Drugs. ACS Appl. Mater. Interfaces 8 (10), 6577–6585. doi: 10.1021/acsami.5b11617
Ichimizu, S., Watanabe, H., Maeda, H., Hamasaki, K., Ikegami, K., Chuang, V. T. G., et al. (2019). Cell-Penetrating Mechanism of Intracellular Targeting Albumin: Contribution of Macropinocytosis Induction and Endosomal Escape. J. Controlled Release 304, 156–163. doi: 10.1016/j.jconrel.2019.05.015
Jhaveri, A., Torchilin, V. (2016). Intracellular Delivery of Nanocarriers and Targeting to Subcellular Organelles. Expert Opin. Drug Delivery 13 (1), 49–70. doi: 10.1517/17425247.2015.1086745
Jung, H. S., Lee, J.-H., Kim, K., Koo, S., Verwilst, P., Sessler, J. L., et al. (2017). Mitochondria-Targeted Cryptocyanine-Based Photothermogenic Photosensitizer. J. Am. Chem. Soc 139 (29), 9972–9978. doi: 10.1021/jacs.7b04263
Kand, D., Pizarro, L., Angel, I., Avni, A., Friedmann-Morvinski, D., Weinstain, R. (2019). Organelle-Targeted BODIPY Photocages: Visible-Light-Mediated Subcellular Photorelease. Angewandte Chem. Int. Ed. 58 (14), 4659–4663. doi: 10.1002/anie.201900850
Kang, J. H., Ko, Y. T. (2019). Enhanced Subcellular Trafficking of Resveratrol Using Mitochondriotropic Liposomes in Cancer Cells. Pharmaceutics 11 (8), 423. doi: 10.3390/pharmaceutics11080423
King, J. S., Kay, R. R. (2019). The Origins and Evolution of Macropinocytosis. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 374 (1765), 1–12. doi: 10.1098/rstb.2018.0158
López, V., Villegas, M. R., Rodríguez, V., Villaverde, G., Lozano, D., Baeza, A., et al. (2017). Janus Mesoporous Silica Nanoparticles for Dual Targeting of Tumor Cells and Mitochondria. ACS Appl. Mater. Interfaces 9 (32), 26697–26706. doi: 10.1021/acsami.7b06906
Lönn, P., Kacsinta, A. D., Cui, X.-S., Hamil, A. S., Kaulich, M., Gogoi, K., et al. (2016). Enhancing Endosomal Escape for Intracellular Delivery of Macromolecular Biologic Therapeutics. Sci. Rep. 6, 1–9. doi: 10.1038/srep32301
Lamade, A. M., Kenny, E. M., Anthonymuthu, T. S., Soysal, E., Clark, R. S. B., Kagan, V. E., et al. (2019). Aiming for the Target: Mitochondrial Drug Delivery in Traumatic Brain Injury. Neuropharmacology 145, 209–219. doi: 10.1016/j.neuropharm.2018.07.014
Lee, J. H., Kim, K. Y., Jin, H., Baek, Y. E., Choi, Y., Jung, S. H., et al. (2018). Self-Assembled Coumarin Nanoparticle in Aqueous Solution as Selective Mitochondrial-Targeting Drug Delivery System. ACS Appl. Mater. Interfaces 10 (4), 3380–3391. doi: 10.1021/acsami.7b17711
Leucuta, S. E. (2014). Subcellular Drug Targeting, Pharmacokinetics and Bioavailability. J. Drug Targeting 22 (2), 95–115. doi: 10.3109/1061186X.2013.848453
Li, Z., Zhao, R., Wu, X., Sun, Y., Yao, M., Li, J., et al. (2005). Identification and Characterization of a Novel Peptide Ligand of Epidermal Growth Factor Receptor for Targeted Delivery of Therapeutics. FASEB J. 19 (14), 1978–1985. doi: 10.1096/fj.05-4058com
Lieleg, O., López-García, M., Semmrich, C., Auernheimer, J., Kessler, H., Bausch, A. R. (2007). Specific Integrin Labeling in Living Cells Using Functionalized Nanocrystals. Small 3 (9), 1560–1565. doi: 10.1002/smll.200700148
Liu, C.-G., Han, Y.-H., Kankala, R. K., Wang, S.-B., Chen, A.-Z. (2020). Subcellular Performance of Nanoparticles in Cancer Therapy. Int. J. Nanomed. (15), 675–704. doi: 10.2147/IJN.S226186.
Liu, K., Dai, L., Li, C., Liu, J., Wang, L., Lei, J. (2016). Self-Assembled Targeted Nanoparticles Based on Transferrin-Modified Eight-Arm-Polyethylene Glycol–Dihydroartemisinin Conjugate. Sci. Rep. 6, 1–12. doi: 10.1038/srep29461
Liu, Y., Qi, Q., Li, X., Liu, J., Wang, L., He, J., et al. (2017). Self-Assembled Pectin-Conjugated Eight-Arm Polyethylene Glycol–Dihydroartemisinin Nanoparticles for Anticancer Combination Therapy. ACS Sustain. Chem. Eng. 5 (9), 8097–8107. doi: 10.1021/acssuschemeng.7b01715
Liu, C.-G., Han, Y.-H., Kankala, R. K., Wang, S.-B., Chen, A.-Z. (2020). Subcellular Performance of Nanoparticles in Cancer Therapy. Int. J. Nanomed. 15, 675–704. doi: 10.2147/IJN.S226186
Ma, X., Xiong, Y., Lee, L. T. O. (2018). Application of Nanoparticles for Targeting G Protein-Coupled Receptors. Int. J. Mol. Sci. 19 (7), 1–17. doi: 10.3390/ijms19072006
Maji, D., Lu, J., Sarder, P., Schmieder, A. H., Cui, G., Yang, X., et al. (2018). Cellular Trafficking of Sn-2 Phosphatidylcholine Prodrugs Studied with Fluorescence Lifetime Imaging and Super-Resolution Microscopy. Precis. Nanomed. 1 (2), 128–145. doi: 10.33218/prnano1(2).180724.1
Manish, G., Vimukta, S. (2011). Targeted Drug Delivery System: A Review. Res. J. Chem. Sci. 1 (2), 135–138.
Mazzaglia, A., Scala, A., Sortino, G., Zagami, R., Zhu, Y., Sciortino, M. T., et al. (2018). Intracellular Trafficking and Therapeutic Outcome of Multiwalled Carbon Nanotubes Modified with Cyclodextrins and Polyethylenimine. Colloids Surf. B. Biointerfaces 163, 55–63. doi: 10.1016/j.colsurfb.2017.12.028
Montet, X., Montet-Abou, K., Reynolds, F., Weissleder, R., Josephson, L. (2006). Nanoparticle Imaging of Integrins on Tumor Cells. Neoplasia 8 (3), 214–222. doi: 10.1593/neo.05769
Morse, M. A., Secord, A. A., Blackwell, K., Hobeika, A. C., Sinnathamby, G., Osada, T., et al. (2011). MHC Class I-Presented Tumor Antigens Identified in Ovarian Cancer by Immunoproteomic Analysis Are Targets for T-Cell Responses against Breast and Ovarian Cancer. Clin. Cancer Res. 17 (10), 3408–3419. doi: 10.1158/1078-0432.CCR-10-2614
Muro, S., Garnacho, C., Champion, J. A., Leferovich, J., Gajewski, C., Schuchman, E. H., et al. (2008). Control of Endothelial Targeting and Intracellular Delivery of Therapeutic Enzymes by Modulating the Size and Shape of ICAM-1-Targeted Carriers. Mol. Ther. 16 (8), 1450–1458. doi: 10.1038/mt.2008.127
Muro, S. (2018). Alterations in Cellular Processes Involving Vesicular Trafficking and Implications in Drug Delivery. Biomimetics (Basel) 3 (3), 1–37. doi: 10.3390/biomimetics3030019
Murphy, M. P. (1997). Selective Targeting of Bioactive Compounds to Mitochondria. Trends Biotechnol. 15 (8), 326–330. doi: 10.1016/S0167-7799(97)01068-8
Nag, O. K., Delehanty, J. B. (2019). Active Cellular and Subcellular Targeting of Nanoparticles for Drug Delivery. Pharmaceutics 11 (10), 1–27. doi: 10.3390/pharmaceutics11100543
Nayerossadat, N., Maedeh, T., Ali, P. A. (2012). Viral and Nonviral Delivery Systems for Gene Delivery. Adv. BioMed. Res. 1, 1–11. doi: 10.4103/2277-9175.98152
Nel, A. E., Mädler, L., Velegol, D., Xia, T., Hoek, E. M. V., Somasundaran, P., et al. (2009). Understanding Biophysicochemical Interactions at the Nano-Bio Interface. Nat. Mater. 8 (7), 543–557. doi: 10.1038/nmat2442
Next-Generation Anti–HER2-Antibody Drug Conjugates. Extending the Actionability to “HER2-Low” Breast Cancer https://dailynews.ascopubs.org/do/10.1200/ADN.19.190401/full/ (accessed Apr 13, 2020).
Niewoehner, J., Bohrmann, B., Collin, L., Urich, E., Sade, H., Maier, P., et al. (2014). Increased Brain Penetration and Potency of a Therapeutic Antibody Using a Monovalent Molecular Shuttle. Neuron 81 (1), 49–60. doi: 10.1016/j.neuron.2013.10.061
Nurunnabi, M., Khatun, Z., Badruddoza, A. Z. M., McCarthy, J. R., Lee, Y., Huh, K. M. (2019). Biomaterials and Bioengineering Approaches for Mitochondria and Nuclear Targeting Drug Delivery. ACS Biomater. Sci. Eng. 5 (4), 1645–1660. doi: 10.1021/acsbiomaterials.8b01615
Odorizzi, G., Babst, M., Emr, S. D. (2000). Phosphoinositide Signaling and the Regulation of Membrane Trafficking in Yeast. Trends Biochem. Sci. 25 (5), 229–235. doi: 10.1016/s0968-0004(00)01543-7
Ohno, S., Takanashi, M., Sudo, K., Ueda, S., Ishikawa, A., Matsuyama, N., et al. (2013). Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor MicroRNA to Breast Cancer Cells. Mol. Ther. 21 (1), 185–191. doi: 10.1038/mt.2012.180
Orellana, E. A., Abdelaal, A. M., Rangasamy, L., Tenneti, S., Myoung, S., Low, P. S., et al. (2019). Enhancing MicroRNA Activity through Increased Endosomal Release Mediated by Nigericin. Mol. Ther. - Nucleic Acids 16, 505–518. doi: 10.1016/j.omtn.2019.04.003
Paillard, A., Hindré, F., Vignes-Colombeix, C., Benoit, J.-P., Garcion, E. (2010). The Importance of Endo-Lysosomal Escape with Lipid Nanocapsules for Drug Subcellular Bioavailability. Biomaterials 31 (29), 7542–7554. doi: 10.1016/j.biomaterials.2010.06.024
Pan, M., Neilson, M. P., Grunfeld, A. M., Cruz, P., Wen, X., Insall, R. H. (2018). Jin, T. A G-Protein-Coupled Chemoattractant Receptor Recognizes Lipopolysaccharide for Bacterial Phagocytosis. PloS Biol. 16 (5), 1–20. doi: 10.1371/journal.pbio.2005754
Panariti, A., Miserocchi, G., Rivolta, I. (2012). The Effect of Nanoparticle Uptake on Cellular Behavior: Disrupting or Enabling Functions? Nanotechnol. Sci. Appl. 5, 87–100. doi: 10.2147/NSA.S25515
Park, S.-H., Zheng, J. H., Nguyen, V. H., Jiang, S.-N., Kim, D.-Y., Szardenings, M., et al. (2016). RGD Peptide Cell-Surface Display Enhances the Targeting and Therapeutic Efficacy of Attenuated Salmonella-Mediated Cancer Therapy. Theranostics 6 (10), 1672–1682. doi: 10.7150/thno.16135
Paterson, J., Webster, C. I. (2016). Exploiting Transferrin Receptor for Delivering Drugs across the Blood-Brain Barrier. Drug Discovery Today: Technol. 20, 49–52. doi: 10.1016/j.ddtec.2016.07.009
Peng, H., Tang, J., Zheng, R., Guo, G., Dong, A., Wang, Y., et al. (2017). Nuclear-Targeted Multifunctional Magnetic Nanoparticles for Photothermal Therapy. Adv. Healthcare Mater. 6 (7), 1601289. doi: 10.1002/adhm.201601289
Plaza-GA, I., Manzaneda-González, V., Kisovec, M., Almendro-Vedia, V., Muñoz-Úbeda, M., Anderluh, G., et al. (2019). PH-Triggered Endosomal Escape of Pore-Forming Listeriolysin O Toxin-Coated Gold Nanoparticles. J. Nanobiotechnol. 17 (1), 108. doi: 10.1186/s12951-019-0543-6
Qiu, Y., Liu, Y., Wang, L., Xu, L., Bai, R., Ji, Y., et al. (2010). Surface Chemistry and Aspect Ratio Mediated Cellular Uptake of Au Nanorods. Biomaterials 31 (30), 7606–7619. doi: 10.1016/j.biomaterials.2010.06.051
Qu, Q., Ma, X., Zhao, Y. (2016). Anticancer Effect of α-Tocopheryl Succinate Delivered by Mitochondria-Targeted Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interfaces 8 (50), 34261–34269. doi: 10.1021/acsami.6b13974
Rajendran, L., Schneider, A., Schlechtingen, G., Weidlich, S., Ries, J., Braxmeier, T., et al. (2008). Efficient Inhibition of the Alzheimer's Disease Beta-Secretase by Membrane Targeting. Science 320 (5875), 520–523. doi: 10.1126/science.1156609
Rajendran, L., Knölker, H.-J., Simons, K. (2010). Subcellular Targeting Strategies for Drug Design and Delivery. Nat. Rev. Drug Discovery 9 (1), 29–42. doi: 10.1038/nrd2897
Rani, K., Paliwal, S. A. (2014). Review on Targeted Drug Delivery: Its Entire Focus on Advanced Therapeutics and Diagnostics. Sch. J. App. Med. Sci. 2 (1C), 328–1.
Reeves, E., James, E. (2017). Antigen Processing and Immune Regulation in the Response to Tumours. Immunology 150 (1), 16–24. doi: 10.1111/imm.12675
Rejman, J., Oberle, V., Zuhorn, I. S., Hoekstra, D. (2004). Size-Dependent Internalization of Particles via the Pathways of Clathrin- and Caveolae-Mediated Endocytosis. Biochem. J. 377 (Pt 1), 159–169. doi: 10.1042/BJ20031253
Richard, J. P., Melikov, K., Vives, E., Ramos, C., Verbeure, B., Gait, M. J., et al. (2003). Cell-Penetrating Peptides A Reevaluation of the Mechanism of Cellular Uptake. J. Biol. Chem. 278 (1), 585–590. doi: 10.1074/jbc.M209548200
Rinnerthaler, G., Gampenrieder, S. P., Greil, R. (2019). HER2 Directed Antibody-Drug-Conjugates beyond T-DM1 in Breast Cancer. Int. J. Mol. Sci. 20 (5), 1–17. doi: 10.3390/ijms20051115
Rosenkranz, A. A., Lunin, V. G., Gulak, P. V., Sergienko, O. V., Shumiantseva, M. A., Voronina, O. L., et al. (2003). Recombinant Modular Transporters for Cell-Specific Nuclear Delivery of Locally Acting Drugs Enhance Photosensitizer Activity. FASEB J. 17 (9), 1121–1123. doi: 10.1096/fj.02-0888fje
Rosenkranz, A. A., Slastnikova, T. A., Karmakova, T. A., Vorontsova, M. S., Morozova, N. B., Petriev, V. M., et al. (2018). Antitumor Activity of Auger Electron Emitter 111In Delivered by Modular Nanotransporter for Treatment of Bladder Cancer With EGFR Overexpression. Front. Pharmacol. 9, 45–56 doi: 10.3389/fphar.2018.01331
Rosenkranz, A. A., Slastnikova, T. A., Georgiev, G. P., Zalutsky, M. R., Sobolev, A. S. (2019). Delivery Systems Exploiting Natural Cell Transport Processes of Macromolecules for Intracellular Targeting of Auger Electron Emitters. Nucl. Med. Biol. 2 (1C), 328–1. doi: 10.1016/j.nucmedbio.2019.11.005
Schneider, Y.-J., Abarca, J., Aboud-Pirak, E., Baurain, R., Ceulemans, F., Deprez-De Campeneere, D., et al. (1984). “Drug Targeting in Human Cancer Chemotherapy,” in Receptor-Mediated Targeting of Drugs. Eds. Gregoriadis, G., Poste, G., Senior, J., Trouet, A. (Boston, MA: Springer US), 1–25. doi: 10.1007/978-1-4684-4862-7_1
Schottelius, M., Laufer, B., Kessler, H., Wester, H.-J. (2009). Ligands for Mapping Avβ3-Integrin Expression in Vivo. Acc. Chem. Res. 42 (7), 969–980. doi: 10.1021/ar800243b
Sharma, A., Soliman, G. M., Al-Hajaj, N., Sharma, R., Maysinger, D., Kakkar, A. (2012). Design and Evaluation of Multifunctional Nanocarriers for Selective Delivery of Coenzyme Q10 to Mitochondria. Biomacromolecules 13 (1), 239–252. doi: 10.1021/bm201538j
Shen, Y., Sun, Y., Yan, R., Chen, E., Wang, H., Ye, D., et al. (2017). Rational Engineering of Semiconductor QDs Enabling Remarkable 1O2 Production for Tumor-Targeted Photodynamic Therapy. Biomaterials 148, 31–40. doi: 10.1016/j.biomaterials.2017.09.026
Slastnikova, T. A., Rosenkranz, A. A., Khramtsov, Y. V., Karyagina, T. S., Ovechko, S. A., Sobolev, A. S. (2017). Development and Evaluation of a New Modular Nanotransporter for Drug Delivery into Nuclei of Pathological Cells Expressing Folate Receptors. Drug Des. Devel. Ther. 11, 1315–1334. doi: 10.2147/DDDT.S127270
Smith, B. R., Cheng, Z., De, A., Koh, A. L., Sinclair, R., Gambhir, S. S. (2008). Real-Time Intravital Imaging of RGD-Quantum Dot Binding to Luminal Endothelium in Mouse Tumor Neovasculature. Nano. Lett. 8 (9), 2599–2606. doi: 10.1021/nl080141f
Smith, S. A., Selby, L. I., Johnston, A. P. R., Such, G. K. (2019). The Endosomal Escape of Nanoparticles: Toward More Efficient Cellular Delivery. Bioconjugate Chem. 30 (2), 263–272. doi: 10.1021/acs.bioconjchem.8b00732
Song, H. P., Yang, J. Y., Lo, S. L., Wang, Y., Fan, W. M., Tang, X. S., et al. (2010). Gene Transfer Using Self-Assembled Ternary Complexes of Cationic Magnetic Nanoparticles, Plasmid DNA and Cell-Penetrating Tat Peptide. Biomaterials 31 (4), 769–778. doi: 10.1016/j.biomaterials.2009.09.085
Staudt, C., Puissant, E., Boonen, M. (2016). Subcellular Trafficking of Mammalian Lysosomal Proteins: An Extended View. Int. J. Mol. Sci. 18 (1), 1–25. doi: 10.3390/ijms18010047
Striebinger, H., Zhang, J., Ott, M., Funk, C., Radtke, K., Duron, J., et al. (2015). Subcellular Trafficking and Functional Importance of Herpes Simplex Virus Type 1 Glycoprotein M Domains. J. Gen. Virol. 96 (11), 3313–3325. doi: 10.1099/jgv.0.000262
Sugahara, K. N., Teesalu, T., Karmali, P. P., Kotamraju, V. R., Agemy, L., Girard, O. M., et al. (2009). Tissue-Penetrating Delivery of Compounds and Nanoparticles into Tumors. Cancer Cell 16 (6), 510–520. doi: 10.1016/j.ccr.2009.10.013
Sun, W., Li, L., Li, L., Yang, Q., Zhang, Z., Huang, Y. (2017). Two Birds, One Stone: Dual Targeting of the Cancer Cell Surface and Subcellular Mitochondria by the Galectin-3-Binding Peptide G3-C12. Acta Pharmacol. Sin. 38 (6), 806–822. doi: 10.1038/aps.2016.137
Sun, Y., Zhan, A., Zhou, S., Kuang, X., Shen, H., Liu, H., et al. (2019). Novel Mitochondria-Targeting Tetrapeptide for Subcellular Delivery of Nanoparticles. Chin. Chem. Lett. 30 (7), 1435–1439. doi: 10.1016/j.cclet.2019.05.001
Sung, Y., Kim, S. (2019). Recent Advances in the Development of Gene Delivery Systems. Biomater. Res. 23 (1), 8. doi: 10.1186/s40824-019-0156-z
Swami, R., Singh, I., Jeengar, M. K., Naidu, V. G. M., Khan, W., Sistla, R. (2015). Adenosine Conjugated Lipidic Nanoparticles for Enhanced Tumor Targeting. Int. J. Pharm. 486 (1–2), 287–296. doi: 10.1016/j.ijpharm.2015.03.065
Tian, W., Ma, Y. (2012). Insights into the Endosomal Escape Mechanism via Investigation of Dendrimer–Membrane Interactions. Soft. Matter 8 (23), 6378–6384. doi: 10.1039/C2SM25538C
Tokarev, A. A., Alfonso, A., Segev, N. (2013). Overview of Intracellular Compartments and Trafficking Pathways [Austin (TX):Landes Bioscience].
van Tilborg, G. A., Mulder, W. J., van der Schaft, D. W., Reutelingsperger, C. P., Griffioen, A. W., Strijkers, G. J., et al. (2008). Improved Magnetic Resonance Molecular Imaging of Tumor Angiogenesis by Avidin-Induced Clearance of Nonbound Bimodal Liposomes. Neoplasia 10 (12), 1459–1469. doi: 10.1593/neo.08858
Vincent, J.-P. (2003). Membranes, Trafficking, and Signaling during Animal Development. Cell 112 (6), 745–749. doi: 10.1016/s0092-8674(03)00198-3
Wang, L., Liu, Y., Li, W., Jiang, X., Ji, Y., Wu, X., et al. (2011). Selective Targeting of Gold Nanorods at the Mitochondria of Cancer Cells: Implications for Cancer Therapy. Nano. Lett. 11 (2), 772–780. doi: 10.1021/nl103992v
Wang, Y., Wang, B., Liao, H., Song, X., Wu, H., Wang, H., et al. (2015). Liposomal Nanohybrid Cerasomes for Mitochondria-Targeted Drug Delivery. J. Mater. Chem. B. 3 (36), 7291–7299. doi: 10.1039/C5TB01197C
Wang, Z., Guo, W., Kuang, X., Hou, S., Liu, H. (2017). Nanopreparations for Mitochondria Targeting Drug Delivery System: Current Strategies and Future Prospective. Asian J. Pharm. Sci. 12 (6), 498–508. doi: 10.1016/j.ajps.2017.05.006
Wang, D., Huang, H., Zhou, M., Lu, H., Chen, J., Chang, Y.-T., et al. (2019). Thermoresponsive Nanocarrier for Mitochondria-Targeted Drug Delivery. Chem. Commun. 55 (28), 4051–4054. doi: 10.1039/C9CC00603F
Wang, F., Zhang, Y., Liu, Z., Du, Z., Zhang, L., Ren, J., et al. (2019). Biocompatible Heterogeneous MOF–Cu Catalyst for In Vivo Drug Synthesis in Targeted Subcellular Organelles. Angewandte Chem. 131 (21), 7061–7066. doi: 10.1002/ange.201901760
Watson, P., Jones, A. T., Stephens, D. J. (2005). Intracellular Trafficking Pathways and Drug Delivery: Fluorescence Imaging of Living and Fixed Cells. Adv. Drug Deliv. Rev. 57 (1), 43–61. doi: 10.1016/j.addr.2004.05.003
Xu, X., Ho, W., Zhang, X., Bertrand, N., Farokhzad, O. (2015). Cancer Nanomedicine: From Targeted Delivery to Combination Therapy. Trends Mol. Med. 21 (4), 223–232. doi: 10.1016/j.molmed.2015.01.001
Xue, X., Hall, M. D., Zhang, Q., Wang, P. C., Gottesman, M. M., Liang, X.-J. (2013). Nanoscale Drug Delivery Platforms Overcome Platinum-Based Resistance in Cancer Cells Due to Abnormal Membrane Protein Trafficking. ACS Nano. 7 (12), 10452–10464. doi: 10.1021/nn405004f
Yang, X., Deng, W., Fu, L., Blanco, E., Gao, J., Quan, D., et al. (2008). Folate-Functionalized Polymeric Micelles for Tumor Targeted Delivery of a Potent Multidrug-Resistance Modulator FG020326. J. BioMed. Mater. Res. A 86 (1), 48–60. doi: 10.1002/jbm.a.31537
Yang, C., Uertz, J., Yohan, D., Chithrani, B. D. (2014). Peptide Modified Gold Nanoparticles for Improved Cellular Uptake, Nuclear Transport, and Intracellular Retention. Nanoscale 6 (20), 12026–12033. doi: 10.1039/c4nr02535k
Yuanjie Zhu, P. W., Yuanjie Zhu, P. W. (2019). Subcellular Distribution of DNA Origami Nanostructures. Med. Res. 2 (4), 1–5. doi: 10.21127/yaoyimr20190100
Zhang, J., Zhang, X., Liu, G., Chang, D., Liang, X., Zhu, X., et al. (2016). Intracellular Trafficking Network of Protein Nanocapsules: Endocytosis, Exocytosis and Autophagy. Theranostics 6 (12), 2099–2113. doi: 10.7150/thno.16587
Zheng, C. J., Han, L. Y., Yap, C. W., Ji, Z. L., Cao, Z. W., Chen, Y. Z. (2006). Therapeutic Targets: Progress of Their Exploration and Investigation of Their Characteristics. Pharmacol. Rev. 58 (2), 259–279. doi: 10.1124/pr.58.2.4
Zhu, M., Nie, G., Meng, H., Xia, T., Nel, A., Zhao, Y. (2013). Physicochemical Properties Determine Nanomaterial Cellular Uptake, Transport and Fate. Acc. Chem. Res. 46 (3), 622–631. doi: 10.1021/ar300031y
Keywords: membrane trafficking, drug targeting pathways, membrane vesicle (MV), nanocarrier and delivery, subcellular transport
Citation: Kumar A, Ahmad A, Vyawahare A and Khan R (2020) Membrane Trafficking and Subcellular Drug Targeting Pathways. Front. Pharmacol. 11:629. doi: 10.3389/fphar.2020.00629
Received: 28 February 2020; Accepted: 21 April 2020;
Published: 27 May 2020.
Edited by:
Alexander S. Sobolev, Lomonosov Moscow State University, RussiaReviewed by:
Qiong Gao, University of Michigan, United StatesJoEllyn McMillan, University of Nebraska Medical Center, United States
Hideyoshi Harashima, Hokkaido University, Japan
Copyright © 2020 Kumar, Ahmad, Vyawahare and Khan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rehan Khan, cmVoYW5raGFuQGluc3QuYWMuaW4=
 Ajay Kumar
Ajay Kumar Anas Ahmad
Anas Ahmad Akshay Vyawahare
Akshay Vyawahare Rehan Khan
Rehan Khan