- Department of Public Health, Nanjing University of Chinese Medicine, Nanjing, China
Background: As a traditional Chinese medicine (TCM) prescription for acute stroke, Liangxue Tongyu formula (LXTYF) was widely used as auxiliary treatment measure in some clinical practice. This study aimed to evaluate the clinical efficacy and safety of LXTYF combined western conventional medicine (WCM) with WCM only for acute intracerebral hemorrhage (ICH).
Methods: We systematically searched PubMed, Embase, Cochrane Library, CMB (Chinese biomedicine database), CNKI (China National Knowledge Infrastructure), WanFang, and VIP until August 2019 to confirm relevant randomized controlled trials (RCTs) compared the combination of LXTYF and WCM with WCM alone for the treatment of acute ICH. Two investigators independently assessed the risk of bias, and extracted and analyzed the data from the identified studies using RevMan 5.3.0 software following Cochrane’s standard and PRISMA guidelines. The herbal compositions of LXTYF were also assessed.
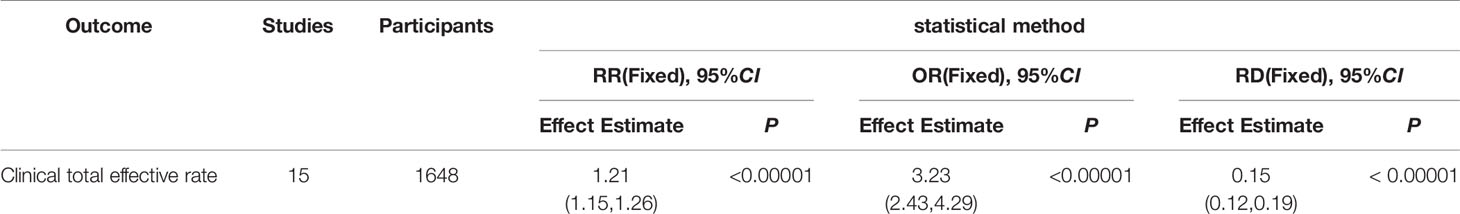
Results: 15 RCTs were identified, totally recruiting 1648 patients with acute intracerebral hemorrhage. Compared with the WCM alone, the combination therapy of LXTYF with WCM could improve the clinical effective rate (RR, 1.21; 95% CI, 1.15–1.25, P < 0.05) and ADL score (MD, 18.09; 95% CI, 12.11–24.07; P < 0.05), and reduce syndrome scores of the TCM (MD, −4.11; 95% CI, −4.69 to −3.53; P < 0.05) and the Glasgow outcome score(GOS) (MD=0.43, 95%CI: 0.06 to 0.79, P=0.02) Moreover, there was no sufficient evidence to indicate the adverse effects would increase compared with WCM alone.
Conclusion: Based on current evidence, we concluded that the combined therapy had some benefits in treating acute intracerebral hemorrhage. However, considering the potential biases and limitations of our study, additional large, high-quality RCTs are required in the future to confirm or refute the effects of LFTYF combined with WCM in acute stroke.
Introduction
Intracerebral hemorrhage (ICH) is the non-traumatic hemorrhage caused by rupture of blood vessels in brain parenchyma, which is one of most fatal subtypes of stroke (Wang and Talked, 2009). It has attracted board attention because of its high morbidity and mortality worldwide (Adeoye and Broderick, 2010; Luo, 2010). Especially in Chinese population, the overall incidence of stroke and the proportion of ICH is higher than white population (Tsai et al., 2013). Acute phase refers to the first 3 weeks of illness. In this period, the brain tissue is damaged and has severe cerebral edema, brain dysfunction and the body is in stress state. The death rate of the acute stage is 30% to 40% (Qureshi et al., 2009). In routine clinic, the treatment of acute ICH mainly adopts the western conventional medicine (WCM), such as preventing further bleeding, controlling brain edema, decreasing intracranial pressure, maintaining life function, and preventing complications (Dimitre et al., 2010). Obviously, the actual efficacy of the WCM alone does not meet expectations (Andaluz and Zuccarello, 2009; Zhou et al., 2014). Therefore, new treatment drugs need to be developed under the guidance of evidence-based medicine. Several studies reported that traditional Chinese medicine (TCM) was benefit for stroke treatment (Li et al., 2015). As a classical TCM prescription for acute ICH, Liangxue Tongyu formula (LXTYF), originating from ‘Xi Jiao Di Huang Tang’ recorded in the ancient medical book ‘Bei Ji Qian Jing Yao Fang’ of AD 652, was used as auxiliary treatment measure in some clinical practice in China. But there is no systematic review to evaluate the quality of these studies and synthesize evidence on the effects of this formula. Therefore, the objective of this article is to systematically collect randomized controlled trials (RCTs) to evaluate the efficacy and safety of LXTYF in the treatment of acute ICH.
Materials and Methods
This systematic review and meta-analysis was performed following the guidelines of PRISMA (the Preferred Reporting Items for System Reviews and Meta-Analysis) (Moher et al., 2015) and was registered in PROSPERO. The registration identifier of the protocol was CRD42018093324 (Jiang et al., 2018).
Types of Studies
All clinical RCTs adopting WCM combined with LXTYF to treat acute ICH were included, which was not limited to publish language, publish form, and whether to adopt blind method.
Types of Participants
The patients with ICH were included according to the diagnosis criteria of western medicine: Diagnostic points of various cerebrovascular diseases revised at the Fourth National Conference of the China Society of Medicine on Cerebrovascular Diseases in 1995 (Chen, 1996). The diagnosis criteria of stroke in Chinese medicine are as follows: Evaluation criteria for diagnosis and efficacy of apoplexy established by State Administration of Traditional acute encephalopathy research collaborative groups in 1996 (The State Administration of Traditional acute encephalopathy research collaborative groups, 1996). Patients who had the syndromes of TCM in fengyang, fire-heat, blood stasis, stagnant heat, phlegm-heat, yin-deficiency, and yang-predominance were also included.
Types of Interventions
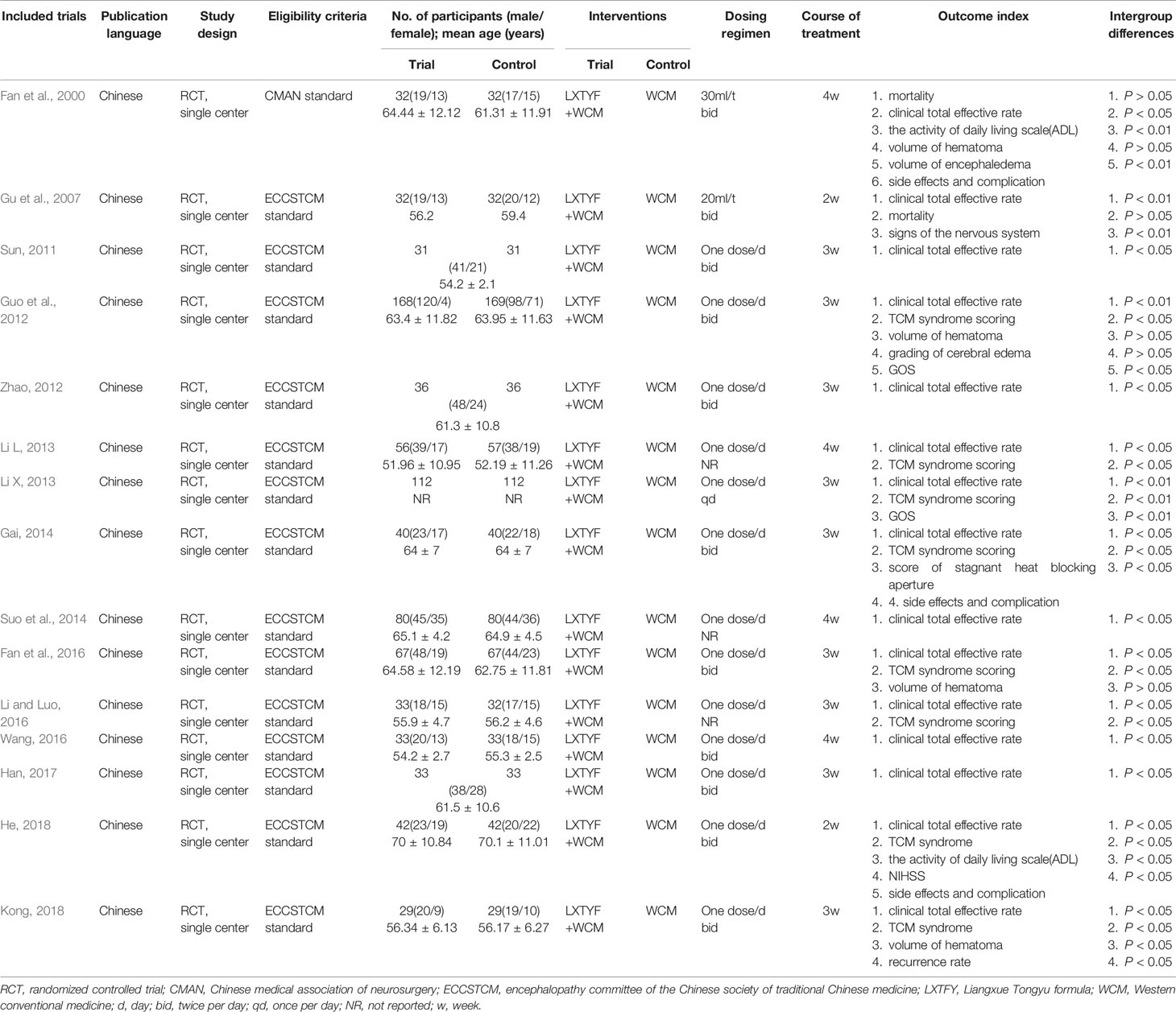
Eligible comparisons were LXTYF+WCM versus the WCM alone. There was no limitation on the dosages or dosage form (oral medicine or injection) or treatment courses. The control groups were adopted WCM, such as decreasing intracranial pressure, regulating blood pressure, keeping water electrolyte balance, preventing the occurrence of stress ulcers, anti-infection, and other symptomatic treatment. The treatment groups were adopted LXTYF combined with WCM, whether it was an oral agent or an injections. The formula of prescription mainly contains six ingredients: Rheum officinale Baill 10 g, Bubali cornu 30 g, Rehmannia glutinosa Libosch 20 g, Paeonia lactiflora Pall 15 g, Paeonia suffruticosa Andrews 10 g, Acoru tatarinowii Schott 10 g.
Outcome Indicator
With reference to the diagnostic scores of stroke in evaluation criteria for diagnosis and efficacy of apoplexy (The State Administration of Traditional acute encephalopathy research collaborative groups, 1996). To evaluate the therapeutic effect by the percentage of TCM syndrome scoring improving(S) and the TCM syndrome scoring after treatment (F) of the patients. S≥81%, F≤6 Basic recovery; 81% > S≥56% Significant progress; 56% > S≥36% Progress; 36% > S≥11% Slight progress; 11% > S≥0% No change; S < 0% deterioration. Total efficiency = (basic recovery + significant progress + progress + slight progress)/n×100%. The primary outcome was clinical total effective rate after the treatment. Other assessment outcomes also included volume of cerebral hematoma and brain edema, Glasgow outcome scale (GOS), Activities of daily living(ADL) score, TCM syndrome score, and number of adverse events.
Exclusion Criteria
The following studies were excluded: (1) the full text was not available through electronic retrieval, manual retrieval, and the authors’ email; (2) repetitive publication; (3) Targeted interventions were not LXTYF combined with WCM. (4) Although it reported to use LXTYF, the composition has significant difference. (5) Data was incorrect, incomplete or unavailable. (6) The patient with intracranial hemorrhage caused by transient ischemic attack, cerebral infarction, subarachnoid hemorrhage, blood disease, tumorous trauma, with cerebral hernia or deep coma, had serious complication in heart, lung, liver, kidney; had the syndrome of Chinese medicine in qi-deficiency, phlegm-dampness, prostration syndrome. (7) Reviews or meta-analysis, retrospective studies, case reports, experimental research, and conference abstracts. (8) RCTs with wrong allocation sequence generation method. For example, the patients enrolled into the different group according to their will.
Search Strategy and Methods for Identification of Studies
Two independent researchers (CJ and XY) searched the electronic databases thoroughly including PubMed, EMBASE, the Cochrane Library, CNKI, the Wanfang database, and VIP Journals Database from inception to March 2018. The following multiple combinations of search keywords were used: (Liangxue Tongyu formula) AND (cerebral hemorrhage OR stroke OR cerebral apoplexy OR hemorrhagic stroke) for English databases and (Liang xue tong yu (the Yu have two different Chinese characters)) AND (Chu xue zhong feng OR Nao chu xue OR Nao zu zhong) in Chinese phonetic alphabets for Chinese database. In order to avoid omission, the references to the articles and reviews we retrieved were also examined. Publication date or country were not limited.
Literature Selection and Data Extraction
After literatures duplicate checking, two reviewers (CJ and XY) screened all articles according to the inclusion/exclusion criteria, extracted the data and evaluated the risk of bias, independently. Then checked each other. Disagreement were resolved through discussion with a third party (GL). The extracted data mainly included: author names, publication year, study design, sample size, detail of intervention: names, dosage form, dosage, ingredient, treatment courses, outcomes, and quality assessment.
Risk of Bias Assessment
Two researchers (CJ and XY) independently assessed the risk of bias in identified RCTs with the reference to risk of bias tool of Cochrane Collaboration (Higgins and Green, 2011). The assessment tool of risk of bias contains the following evaluation contents: random sequence generation; allocation concealment; blinding; incomplete data; selective reporting; and other bias. According to the detailed rules, each item could be divided into high risk, low risk, unclear risk. When there was no enough information to determine whether a study satisfies the scoring criteria, the study was considered as an unclear risk. If there was a difference in the judgement between two researchers, it would be settled by discussion with a third party.
LXTYF Composition
The frequency of most commonly used ingredients of LXTYF was calculated, and those herbs or ingredients were described in detail.
The Quality of the Included RCT
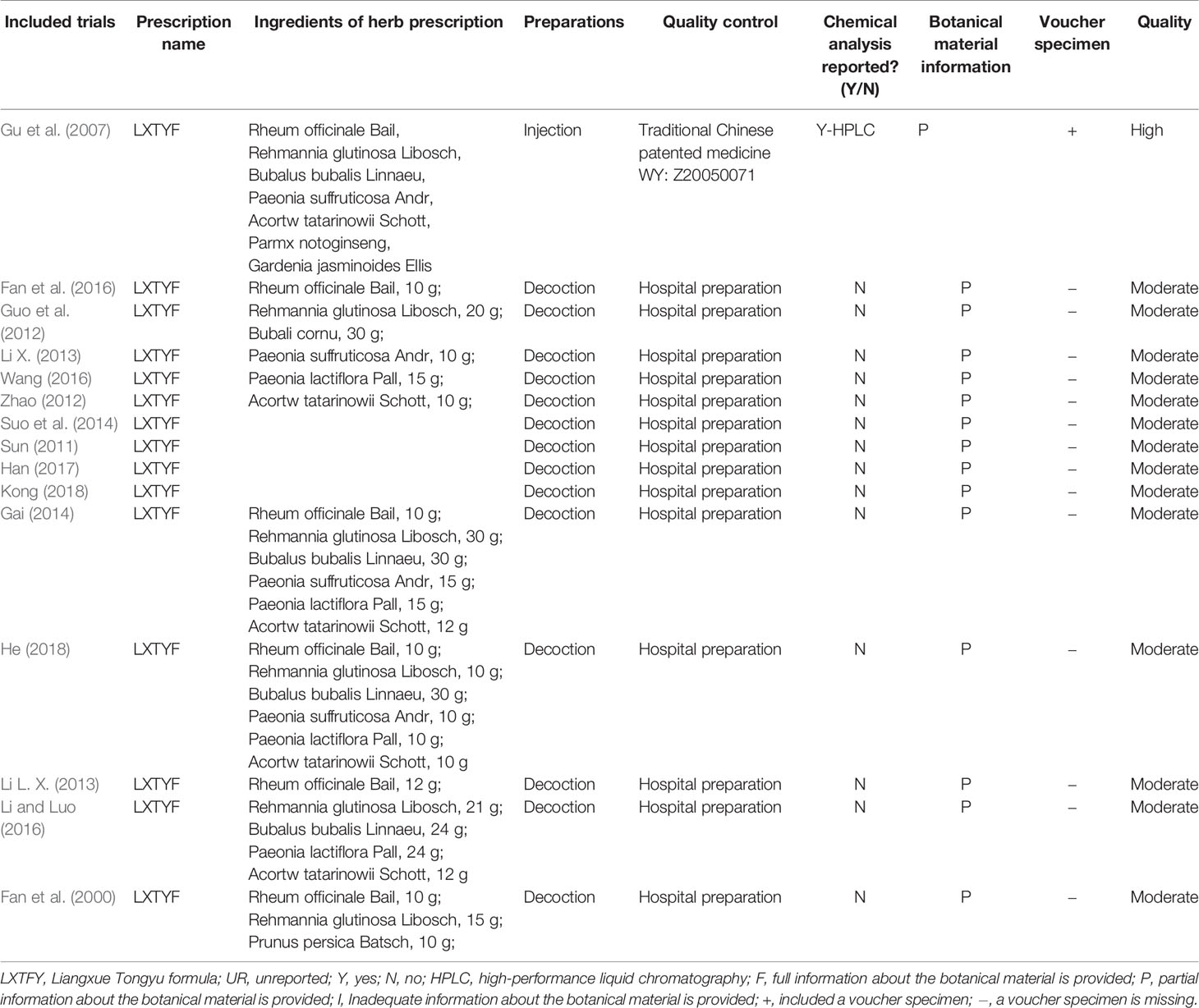
In order to assess the quality of the included RCTs, we used a rating system (Wang et al., 2019) as follows: (1) high quality, full information about the botanical material is provided, including a voucher specimen; (2) moderate quality, only partial information about the botanical material is provided, and a voucher specimen is missing; there are taxonomic inaccuracies; (3) low quality, inadequate information and overall taxonomically is inadequate.
Statistics Analysis
Review Manager 5.3 (Cochrane, 2012) was used to merge the outcomes of clinical trials and analysis data. Quantitative outcomes were expressed as weighted mean difference (WMD), while qualitative outcomes were expressed as risk ratio (RR). Both 95% confidence interval was also calculated. Chi-square test and I-square (I2) index were used to test heterogeneity. The result were considered as having high heterogeneity if P value of Chi-square test less than 0.05 and I2 values greater than 50%. At this time, the random effects model was used for analysis, otherwise the fixed effects model was adopted. Publication bias was inspected by using funnel plots.
Results
Procedure for Study Selection
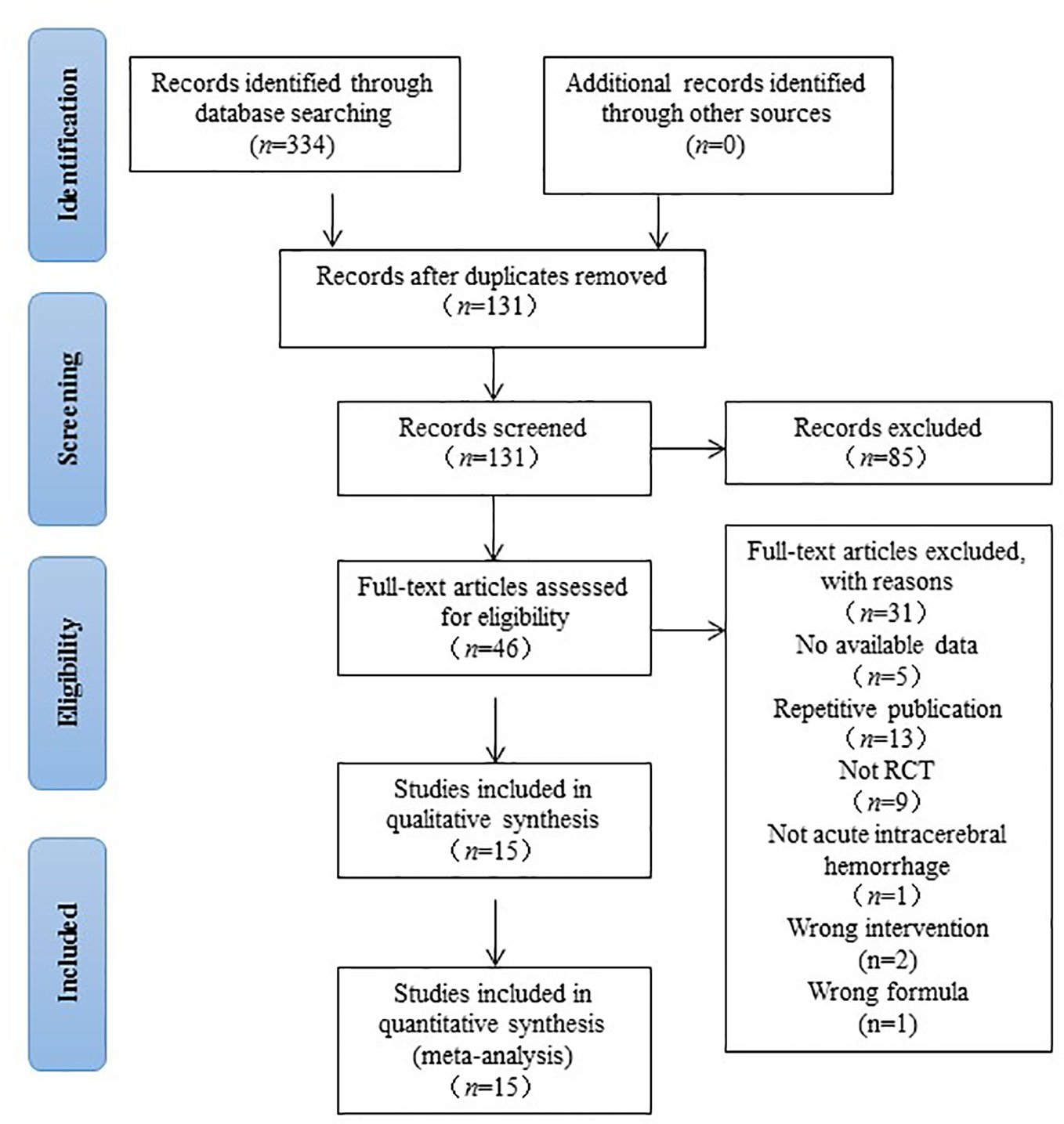
After a comprehensive searched in multiple databases we initially identified 334 studies. After deleting the duplicate articles, 131 studies were retained for further confirmation. By reading the titles and abstracts, 85 studies that did not conform to inclusion criteria were eliminated. The remaining 46 studies were read in full texts, and 31 studies were ultimately excluded by the following reasons: (1) the article was a review or theoretical summary; (2) not a clinical trial; (3) no available data; (4) repetitive publication; (5) not acute ICH; (6) wrong comparison; (7) wrong formula. The screening procedure was illustrated in Figure 1.
Characteristics of Included Studies
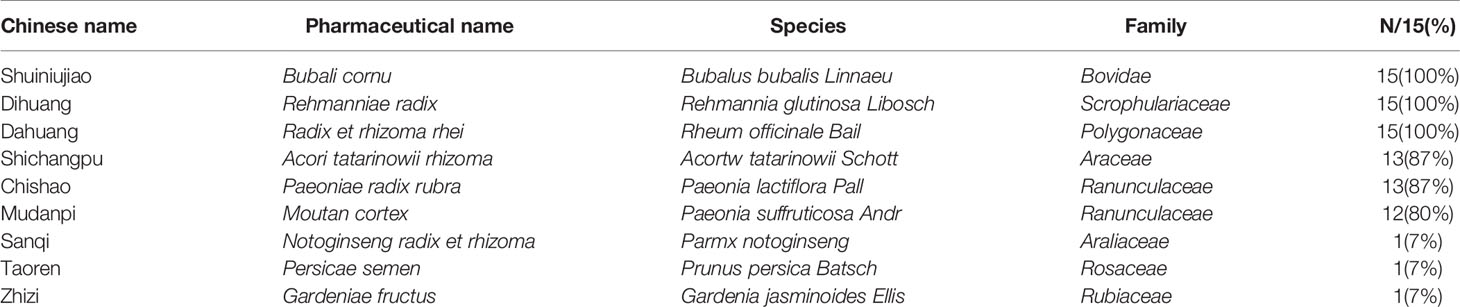
This systematic review eventually included 15 studies published in Chinese from 2000 to 2019. The sample size varied from 58 to 337, with a total of 1648 patients with acute ICH (823 patients were in experimental group and 825 patients were in control group). The course of treatment lasted from 14 d to 28d in 15 studies. The characteristics of the 15 RCTs were summarized in Table 1. Of the 15 studies, 1 study compared Liangxue Tongyu injection plus WCM versus WCM alone (Gu et al., 2007). The remaining studies were all compared decoction of LXTYF plus WCM with WCM alone. One RCT (Gu et al., 2007) used a commercial preparation. In the other 14 RCTs (Fan et al., 2000; He, 2018), the preparations were made in hospital including the associated pharmaceutical quality control. The ingredients of the prescription and pharmaceutical quality control in each included RCT was illustrated in detail in Table 2.
The Reporting Completeness of the Material in Included Studies
We accessed the reporting completeness of the material in each study with a rating system, which is related to the information about the botanical material and voucher specimens. Only one RCT (Guo et al., 2012) are of high quality, which provided the full information about the botanical material and included voucher specimens. The remaining 14 RCTs are of moderate quality, which provided partial information about botanical material and did not provided voucher specimens. We confirmed that only one RCT (Guo et al., 2012) did chemical analysis though high performance liquid chromatography (HPLC). The remaining RCT did not report the relevant information. However, our team did the same analysis in LXTYF and reported in previous articles (Li et al., 2018). Meanwhile, we also report the results in the Supplementary Material. The detail was summarized in Table 2.
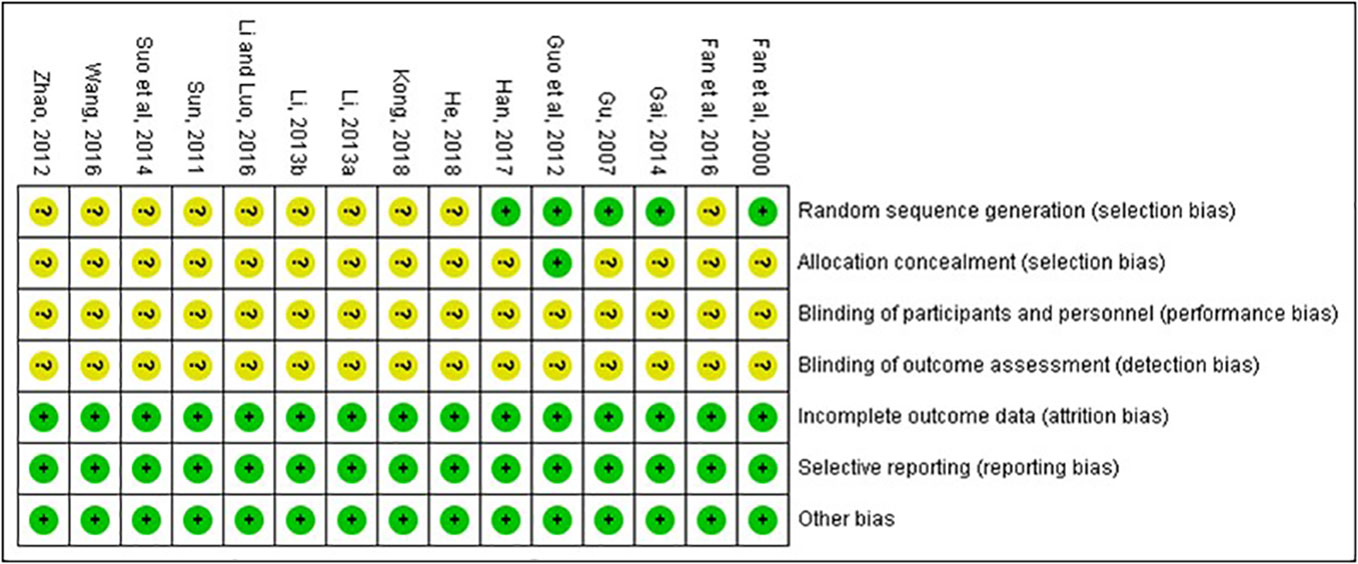
Risk of Bias and Quality of Included Studies
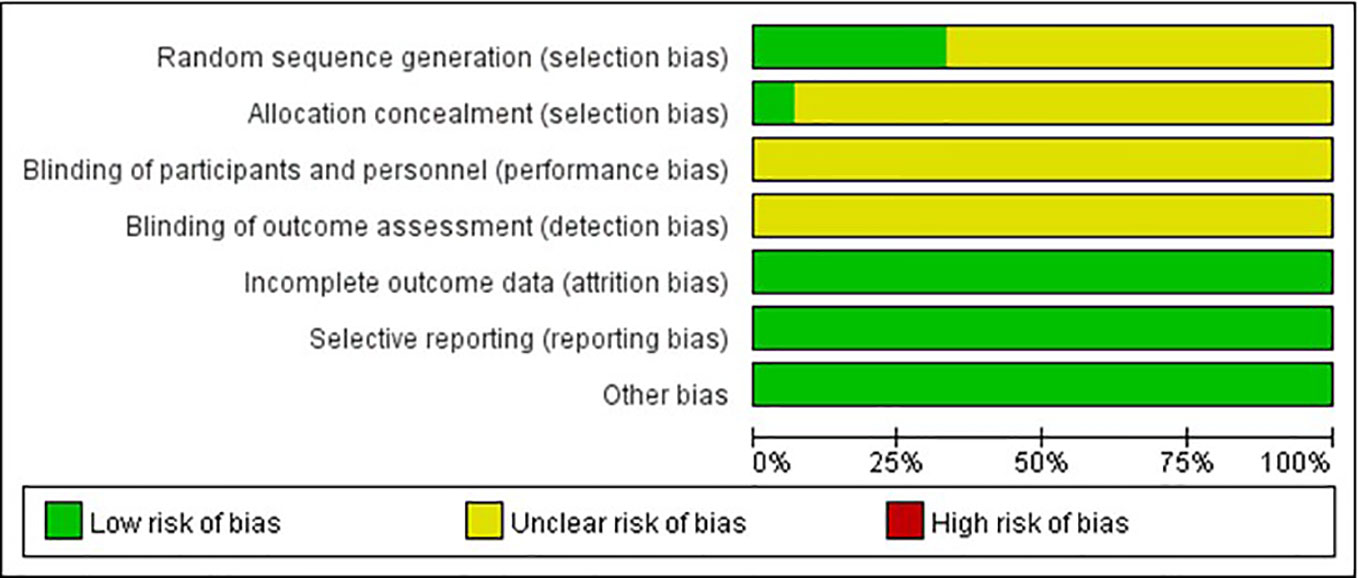
The quality of the included studies was also evaluated according to the risk of bias assessment tool of standard RCTs evidence recommended by Cochrane Collaboration. One study used computer to generate random number for random allocation (Guo et al., 2012) and two studies used paired randomization (Fan et al., 2000; Gu et al., 2007). Two studies used random number table (Gai, 2014; Han, 2017). All these five studies were regarded as low risk of bias, and the others claimed that they used randomization but did not report the details of how to randomize, which we considered to have unclear risk of bias. Except Guo’s research, none of the other studies mentioned method of allocation concealment (Guo et al., 2012). Besides, none of the included RCTs assessed had incomplete data and selective report, so these items were appraised as low risk. There is no evidence of other biases, so this item was evaluated as low risk. The detailed quality evaluation of the included studies were shown in Figures 2 and 3. Funnel plots were shown in Figure 4.
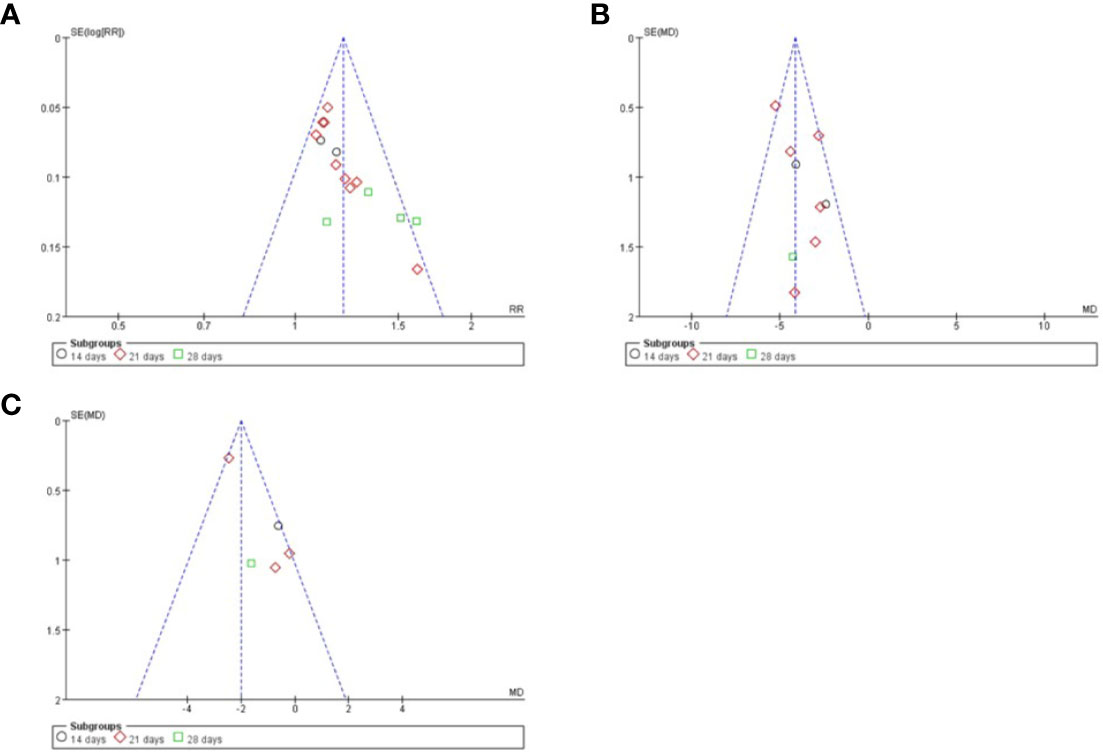
Figure 4 Funnel plots of different index. (A) clinical total effective rate; (B) TCM syndrome score; (C) volume of cerebral hematoma.
Efficacy Assessment
Clinical Total Effective Rate
The clinical total effective rate was assessed in all included studies. The calculations of all of them was based on the same criteria. It was calculated at 14, 21, and 28 days after treatment. The meta-analysis indicated that the combination of LXTYF and WCM significantly improved the clinical total effective rate compared with WCM alone. The relative ratio (RR) (relative benefit) in 15 studies ranged from 1.09 to 1.61. The overall RR was 1.21 (95% CI, 1.15 to 1.25; P < 0.05, I2 = 32%; Figure 5; Table 3). In all included studies, Guo et al. had the highest quality and sufficient sample size (Guo et al., 2012). The RR value of their study was 1.14, which was lower than the combined overall RR. Furthermore, the clinical effective rate in experimental group was significantly higher than control group at 14 d (RR=1.15, 95%CI: 1.03 to 1.28, P < 0.05, I2 = 0%), 21 d (RR=1.17, 95%CI: 1.11 to 1.23, P < 0.05, I2 = 0%) and 28 d (RR=1.41, 95%CI: 1.24 to 1.60, P < 0.05, I2 = 31%).
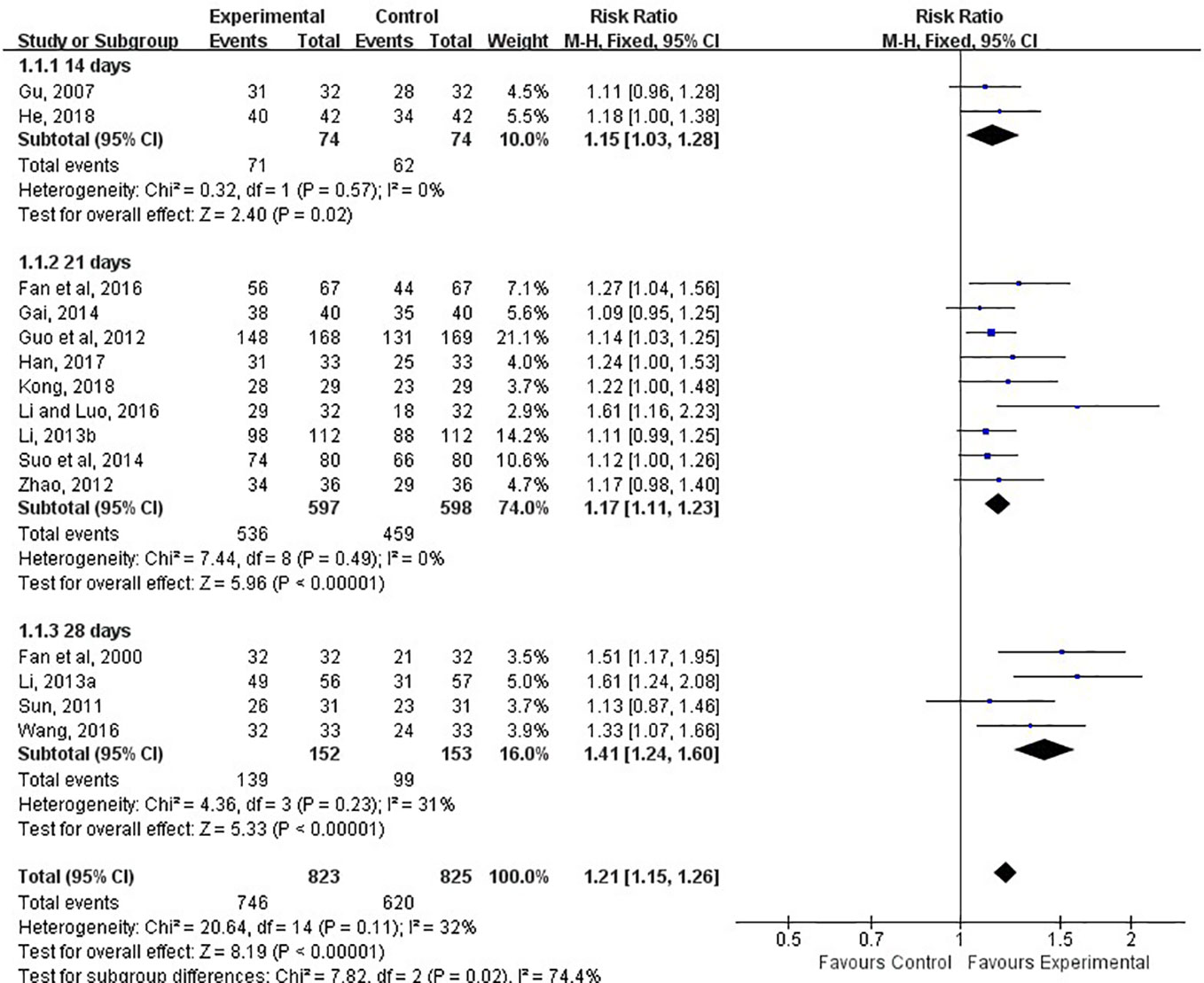
Figure 5 Forest plots showing a improvement of clinical total effective rate in experimental group compared with control group.
TCM Syndrome Score
The TCM syndrome scoring was reported in 9 of the 15 studies and assessed at 14th, 21st, 28th days after treatment (Gu et al., 2007; Guo et al., 2012; Gai, 2014; Li and Luo, 2016; He, 2018; Kong, 2018; Li L. X. 2013; Li X. 2013; Fan et al., 2016). The meta-analysis showed the combination of LXTYF and WCM prominently decreased the TCM syndrome score compared with the WCM alone (MD, −4.11; 95% CI, −4.69 to −3.53; P < 0.05; I2 = 37%; Figure 6). After treatment, remarkable difference between two comparison groups were observed at all evaluation time points (14 d [MD, −3.84; 95% CI, −4.90 to −2.05; P < 0.05; I2 = 22%]; 21 d [MD, −4.24; 95% CI, −4.89 to −3.59; P < 0.05; I2 = 53%]; 28 d [MD, −4.27; 95% CI, −7.34 to −1.20, P < 0.05]).
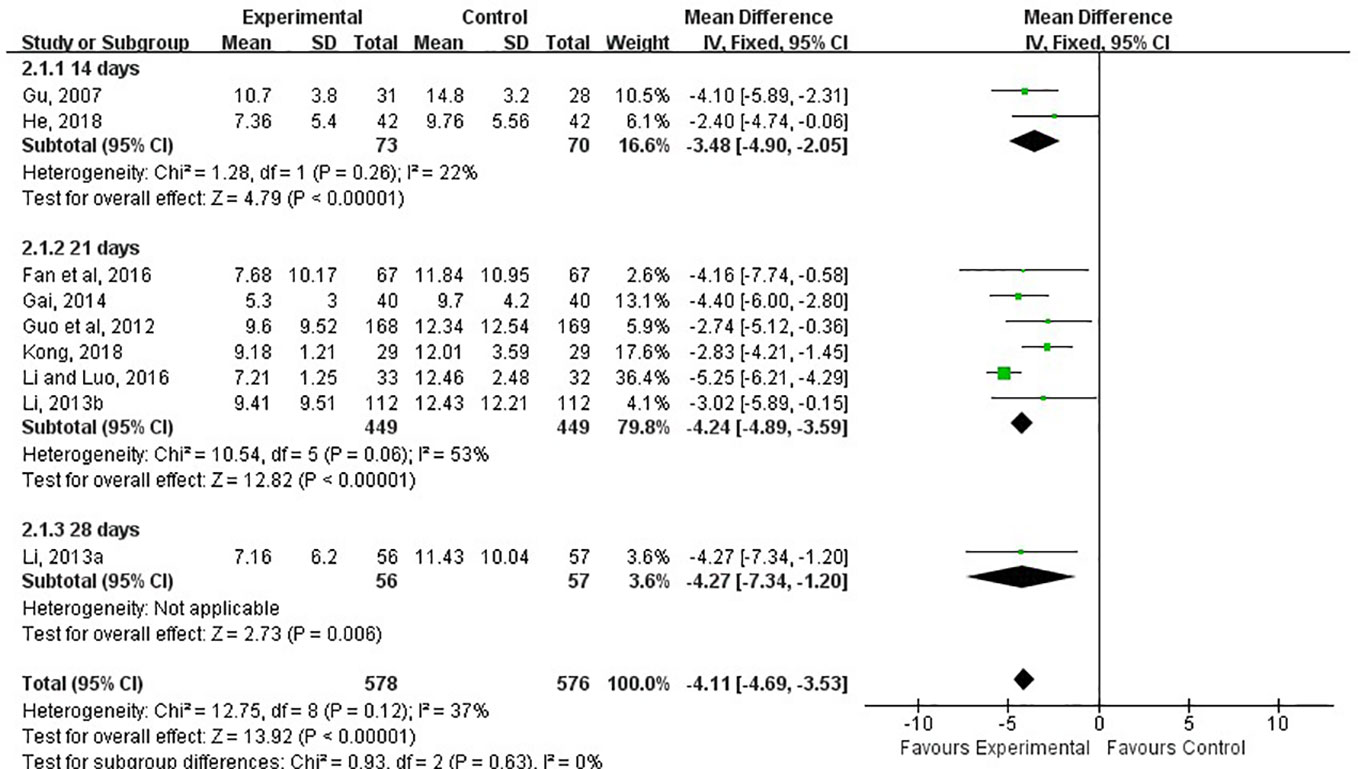
Figure 6 Forest plots showing a reduction of TCM syndrome score in experimental group compared with control group.
Volume of Cerebral Hematoma
The volume of the cerebral hemorrhage after treatment was assessed in five studies (Fan et al., 2000; Gu et al., 2007; Guo et al., 2012; Fan et al., 2016; Kong, 2018). Although the combination of LXTYF and WCM prominently decreased the volume of cerebral hematoma compared with the WCM alone, there were significant differences between two comparison groups (MD, −1.31; 95% CI, −2.40 to −0.22; P = 0.02; I2 = 64%, Figure 7).
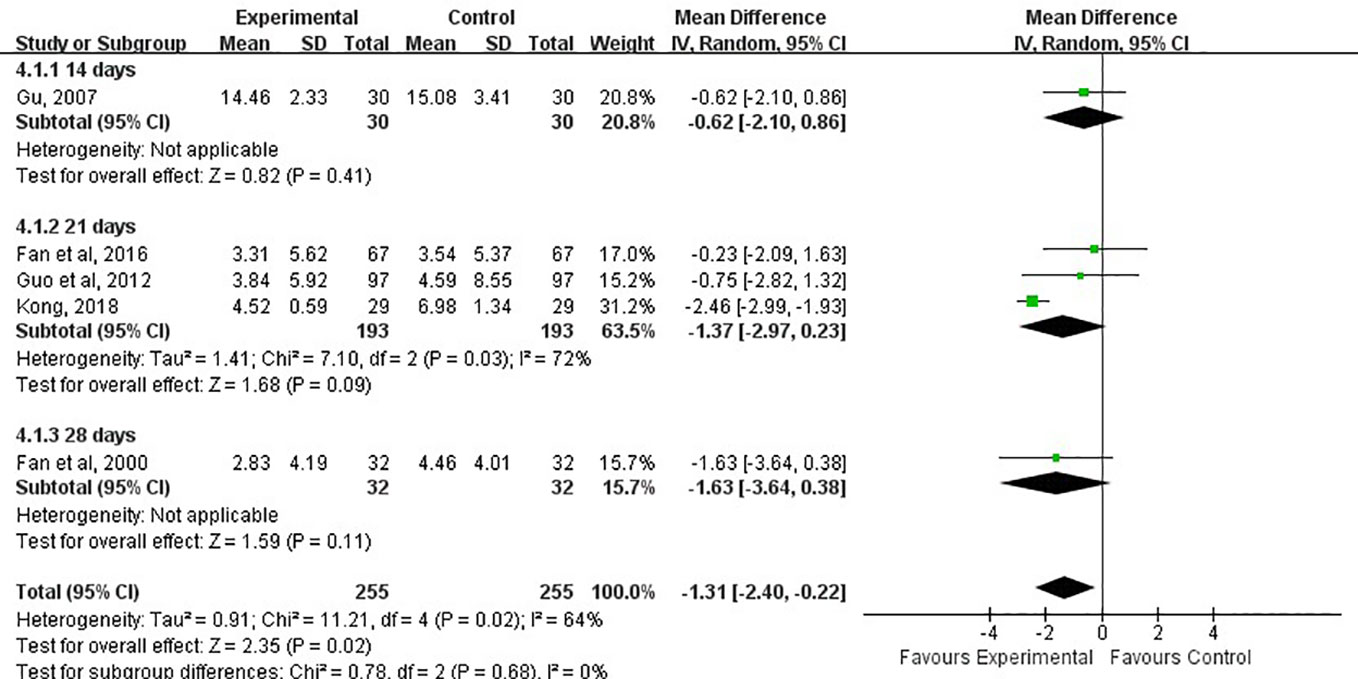
Figure 7 Forest plots showing a reduction of volume of cerebral hematoma in experimental group compared with control group.
GOS Score Difference
Glasgow outcome score (GOS) were divided into five levels: good recovery, moderate disability, severe disability, vegetative state, and death. The higher the score, the worse the prognosis. Two of the 13 studies used GOS to reflect the recovery of physical sign and symptoms of neurological deficits in patients with cerebral apoplexy (Guo et al., 2012; Li L. X., 2013). The GOS in two studies were all evaluated at 21st days after treatment. The overall results indicated that the experimental group had a measurably better recovery of neurological functions than the control group (MD, 0.43; 95% CI, 0.06 to 0.79, P=0.02, I2 = 91%, Figure 8) with random effect model. Compared with control group, the experimental group showed a greater decrease in GOS score after treatment.
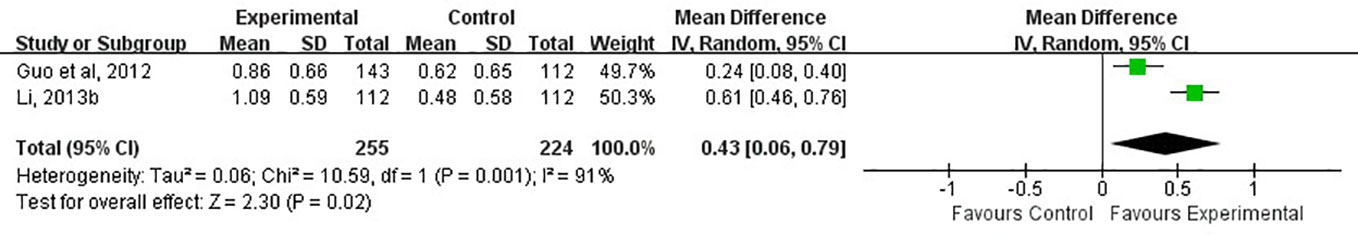
Figure 8 Forest plots showing a reduction of GOS score in experimental group compared with control group.
ADL Score
Activities of daily living (ADL) score refers to daily living ability, which reflects people’s most basic ability in the family(or medical institution) and community. It is the most important index in rehabilitation medicine. The higher the ADL score, the more self-care ability you have. Of 15 RCTs, Only two studies used this index to evaluate the impairment of nerve function. The ADL score in two studies were assessed at 14 and 28 days, respectively (Fan et al., 2000; He, 2018). The results showed that the experiment group had a higher score than the control group (MD, 18.09; 95% CI, 12.11 to 24.07; P < 0.05; I2 = 0%; Figure 9).
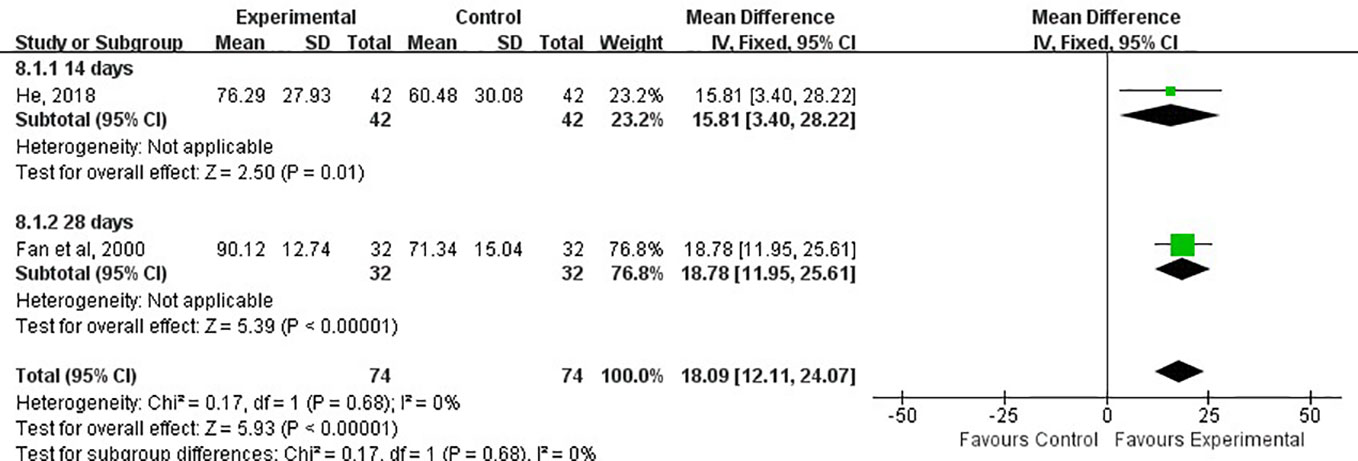
Figure 9 Forest plots showing a improvement of ADL score in experimental group compared with control group.
Adverse Events
There were three studies (Fan et al., 2000; Gai, 2014; He, 2018) reported adverse events. Fan et al. reported that there were two cases of mild diarrhea and three cases of stomach upset in the experiment group. The symptoms disappeared after taking the LXTYF after the meal. In the He’s report, there were three adverse events in control group and four in experimental group, all of which were mild impairment of liver function and slight elevation of creatine kinase. Under close monitoring, all patients with adverse events did not take targeted drugs and successfully completed the treatment as plan. Gai reported that there was one patient had nausea, and two patients had headache in the control group, while two cases of nausea observed in the experimental group. After stopping the medication, the symptoms relieve spontaneously without no treatment. These reports showed that the addition of LXTYF is safe and reliable, without increasing the burden of liver and kidney functions and binging toxic and side effects.
Sensitivity Analysis
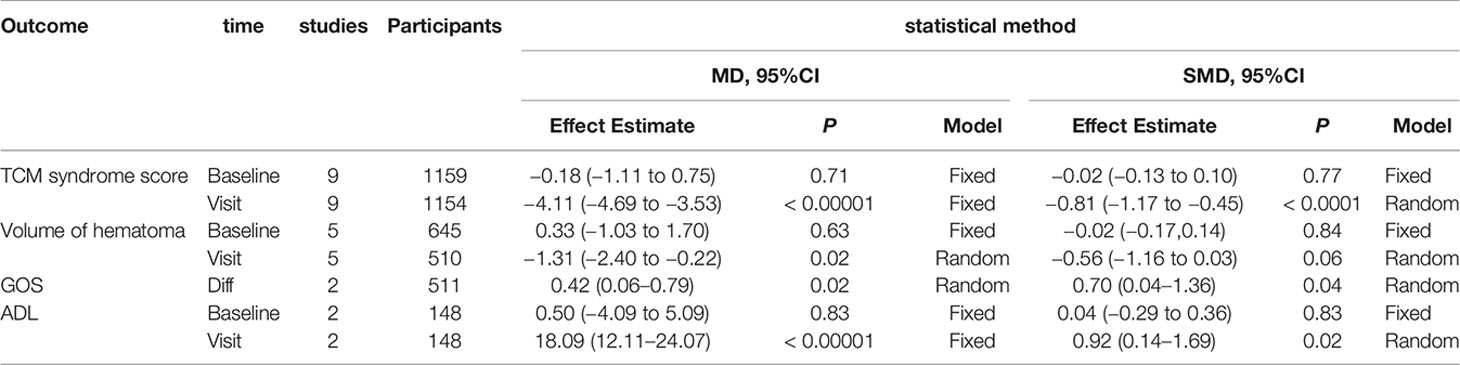
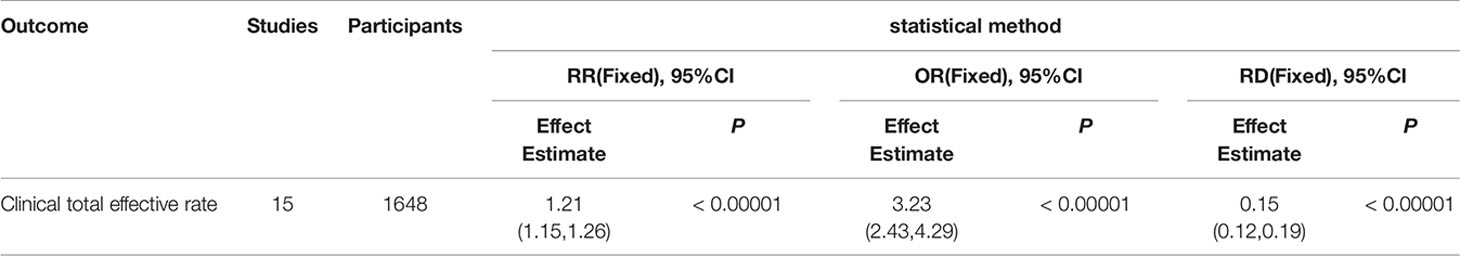
Through sensitivity analysis, we found that our conclusions were robust regardless of whether one study which treatment was Liangxue Tongyu injection included or not, and our overall estimates of the total effective rate and TCM syndrome score remained unchanged. In addition, we used different analytical models, which also showed that our conclusion was robust. We provided these results of analysis in the appendices (Appendices 1 and 2).
Description of the LXTYF
Nine ingredients were included in the 15 RCTs. The mainly used TCMs were Bubali cornu, Rehmanniae radix, Radix et rhizoma rhei, Acori tatarinowii rhizome, Paeoniae radix rubra, Moutan cortex. Based on these ingredients, Notoginseng radix et rhizome, Persicae semen, Gardeniae fructus were also added in one RCT (Gu et al., 2012).The full and validated botanical names of herbs or constituents were listed in Table 4.
Discussion
Summary of Main Results
We included in this systematic review 15 randomized controlled trials of 1648 patients with acute ICH. Except for Gu’s study, which used injection, all other studies used LXTYF orally combined with WCM as the intervention. The meta-analysis indicated that combination of LXTYF and WCM could increase 15% of the clinical total effective rate and reduce 4.11 of TCM syndrome score and 0.42 of GOS score and 1.31 ml of volume of hematoma and 18.09 of ADL score. In terms of safety, adverse events were reported in about 1.3% of participants who received the combination therapy. Unfortunately, reporting of adverse events was incomplete.
Quality of the Evidence
Overall, of the included 15 RCTs, 8 RCTs reported the method of randomization (Gu et al., 2007; Guo et al., 2012; Gai, 2014; Han, 2017; He, 2018; Li X. 2013; Li L. X. 2013; Fan et al., 2000), 5 reported the generation method of random sequence (Fan et al., 2000; Gu et al., 2007; Guo et al., 2012; Gai, 2014; Han, 2017). Only Guo’s Study reported the allocation concealment. No one studies reported the details of the Blinding to participants or outcome assessors. The largest quantity of study was from Guo’s RCTs, which has the largest sample size and the most standard trials protocol.
Potential Biases in the Review Process
We prepared a funnel plot for the primary outcome of clinical total effective rate and found asymmetrical funnel distribution, which indicated the possibility of publication bias. In addition, although the main ingredients of LXTYF in included studies were not significantly different, the weight of some ingredients were adjusted according to clinical practice, which would introduce heterogeneity and lead to bias.
Implications for Practice
The treatment of acute stage of ICH in western medicine is just symptomatic treatment, and there are no specific drugs for absorption of hematoma, not to say it reflects the principle of individualized treatment.
Many studies have showed that TCM can not only improve brain microcirculation, but also promote the absorption of hematoma effectively and improve the prognosis if neurological function. However, due to the lack of multi-centers RCTs, many TCMs have not been widely used. In addition, most of the current clinical guidelines on the treatment of cerebral hemorrhage by TCM are expert consensus and experience, and lack of evidence-based evidence (Gao, 2016).
LXTYF includes eight ingredients and has been used to treat ICH in the clinical. Some pharmacological studies have shown that LXTYF had integrated therapeutic effect on ICH due to activities of anti-inflammatory, anti-coagulation, blood vessel protection, and protection neuron from excitotoxicity. The compound combination in LXYTF, including Taurine, Paeonol, and Ginsenoside Rb1, can offer protection neuron from excitotoxicity at the low concentration by activation of the PI3K/AKT pathway (Li et al., 2018).
In this review, we focused on the clinical efficacy and safety of LXTYF. Through this work, we hope to inherit the experience of famous TCM doctors and to provide evidence-based evidence for traditional Chinses medicine and integrated traditional Chinses and western medicine in treating acute stroke.
Limitation of the Research
The enrolled patients in RCTs were merely Chinese. Therefore, we could not confirm whether there was a similar effect for non-Chinese. The included studies in this review did not have long-term follow-up data after the end of 14 to 28 days of treatment. Thus, we could not evaluate the long-term effect. In this review, we found that only two RCT reported the GOS and ADL scores, which mainly reflect the neurological impairment. It will not help us to evaluate the real effect of LXTYF in improving prognosis.
Conclusion
In summary, based on current evidence, we concluded the combined therapy could increase the clinical total effective rate, reduce the degree of neurological deficit, and improve prognosis, and there was no evidence to show that the combination therapy would lead to safety problems. A number of sensitivity analysis have shown that our conclusions are robust. However, considering the potential biases and limitations of our study, additional large, high-quality RCTs are required in the future to confirm or refute the effects of LFTYF combined with WCM in acute stroke.
Author Contributions
GL and JD designed the study. CJ and XY contributed to data collection and statistical analysis. Results were interpreted by GL and CJ. The report was drafted by CJ. Critical revision of the report for important intellectual content was done by all the investigators.
Funding
This study was funded by the Jiangsu Natural Science Foundation (No. SBK2016022369) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (integration of Chinese and Western medicine).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.00437/full#supplementary-material
References
Adeoye, O., Broderick, J. P. (2010). Advances in the management of intracerebral hemorrhage. Nat. Rev. Neurol. 6, 593–601. doi: 10.1038/nrneurol.2010.146
Andaluz, N., Zuccarello, M. (2009). Recent trends in the treatment of spontaneous intracerebral hemorrhage: analysis of a nationwide inpatient database. J. Neurosurg. 110, 403–410. doi: 10.3171/2008.5.17559
Chen, Q. T. (1996). Classification, diagnostic criteria and evaluation of neurological impairment for stroke patients. Chin J. Neurol. 29, 379.
Dimitre, S., Hagen, B. H., Martin, K., Juergen, B., Peter, D. S. (2010). Nobel approaches to the treatment of intracerebral hemorrhage. Int. J. Stroke. 5, 457–465. doi: 10.1111/j.1747-4949.2010.00487.x
Fan, Y., Zhou, Z. Y., Jin, M. W., Li, J. Y., Gu, X. Z., Shen, W. P., et al. (2000). Effects of Liangxue Tongyu oral liquid on the early rehabilitation of patients with spontaneous hypertensive intracerebral hemorrhage. Chin J. Integr. Trad West Med. 20, 138–140. doi: 10.7661/CJIM.2000.2.138
Fan, P., Fan, C. H., Qin, L., Qin, H. (2016). Clinical research on acute cerebral hemorrhage treated with blood-cooling and blood stasis-removing decoction. Henan Tradit Chin Med. 36, 74–76. doi: 10.16367/j.issn.1003-5028.2016.01.0032
Gai, C. A. (2014). The efficacy and safety evaluation of Liangxue Tongyu formula in treating the symptom of stagnant heat blocking aperture in the acute stage of cerebral hemorrhage. Shanxi Med. J. 43, 2091–2092.
Gao, L. (2016). Expert consensus on diagnosis and treatment of acute hypertensive cerebral hemorrhage with integrated traditional Chinese and western medicine. Chin Gen. Prac. 19, 3641–3648. doi: 10.3969/j.issn.1007-9572.2016.30.001
Gu, N., Wang, C. S., Zhou, Z. Y. (2007). Effect of Liangxue Tongyu injection on the short-term effect of acute intracerebral hemorrhage. J. New Chin. Medicine. 39, 40–41. doi: 10.13457/j.cnki.jncm.2007.01.020
Guo, W. F., Zhang, L. K., Wu, M. H., Li, G. C., Zhou, X. P., Ye, F., et al. (2012). Liangxue Tongyu Fang for treating acuter-phase cerebral hemorrhage in 168 cases. J. Beijing Univ. traditional Chin. medicine. 35, 603–606. doi: CNKI:SUN:JZYB.0.2012-09-005
Han, X. W. (2017). Treatment of acute cerebral hemorrhage by cold blood stasis. Cardiovasc. Dis. J. Integrated Traditional Chin. Western Medicine. 5, 75–76. doi: 10.16282/j.cnki.cn11-9336/r.2017.06.063
He, X. G. (2018). Clinical study of L Liangxue Tongyu prescription in treating acute cerebral infraction and its mechanism of anti-inflammatory: [D] (Nanjing University of Chinese Medicine).
Higgins, J. P. T., Green, S. (2011). Cochrane handbook for systematic reviews of interventions version. Cochrane collaboration. http://handbook-5-1.cochrane.org.
Jiang, C., Yang, X. J., Li, G. C. (2018). Systematic review and meta-analysis of curative effect and safety of Liangxue Tongyu formula on patients with acute intracerebral hemorrhage. York: Univ. York (UK). https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=93324.
Kong, Q. X. (2018). Effect of Liangxue Tongyu decoction in treating 58 cases of acute cerebral hemorrhage. World Latest Med. Inf. (Electronic Version). 18, 152. doi: 10.19613/j.cnki.1671-3141.2018.20.108
Li, Y., Luo, J. (2016). To investigate the clinical effect of Liangxue Tongyu therapy in the treatment of acute cerebral hemorrhage. China Health Care Nutrition. 26, 85.
Li, H. Q., Wei, J. J., Xia, W., Li, J. H., Liu, A. J., Yin, S. B., et al. (2015). Promoting blood circulation for removing blood stasis therapy for acute intracerebral hemorrhage: a systematic review and meta-analysis. Acta Pharmacol. Sin. 36, 659–675. doi: 10.1038/aps.2014.139
Li, X., Huang, X., Tang, Y., Zhao, F., Gao, Y., Yin, L., et al. (2018). Assessing the pharmacological and therapeutic efficacy of traditional Chinese medicine liangxue tongyu prescription for intracerebral hemorrhagic stroke in neurological disease models. Front. Pharmacol. 9, 1169. doi: 10.3389/fphar.2018.01169
Li, L. X. (2013). Clinical curative effect of Liangxue Tongyu combined with western medicine for 224cases of acute cerebral hemorrhage. Healthmust-Readmagazine 12, 90–91.
Li, X. (2013). Cool through blood stasis Chinese medicine treatment of acute phase of cerebral hemorrhage,56 cases of clinical observation. World Latest Med. Information. 13, 281–282. doi: 10.3969/j.issn.1671-3141.2013.08.211
Luo, J. N. (2010). New progress on treating acute cerebral infarction. Pract. J. Cardiac Cereb Pneumal Vasc. Dis. 18, 1546–1547. doi: 110.3969/j.issn.1008-5971.2010.10.113
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Rev. 4 (1), 1. doi: 10.1186/2046-4053-4-1
Qureshi, A. I., Mendelow, A. D., Hanley, D. F. (2009). Intracerebral haemorrhage. Lancet. 373 (9675), 1632–1644. doi: 10.1016/S0140-6736(09)60371-8
Sun, Z. T. (2011). Analysis of the clinical effect of Liangxue Tongyu formula in the treatment of cerebral hemorrhage. Chin. foreign Women Health 19, 335.
Suo, R. B., Jiao, H. F., Liu, W. M. (2014). Discussion on the clinical efficacy of TCM Liangxue Tongyu formula in in treating the symptom of stagnant heat blocking aperture in the acute stage of cerebral hemorrhage. Global traditional Chin. medicine. 7, 48. doi: 10.3969/j.issn.1006-1959.2013.25.686
The State Administration of Traditional acute encephalopathy research collaborative groups, (1996). Evaluation criteria for diagnosis and efficacy apoplexy. J. Beijing Univ. Traditional Chin. Medicine. 19, 55–56.
Tsai, C. F., Thomas, B., Sudlow, C. L. M. (2013). Epidemiology of stroke and its subtypes in Chinese vs white population: A systematic review. Neurology. 81, 264–272. doi: 10.1212/WNL.0b013e31829bfde3
Wang, D. Z., Talked, A. V. (2009). Treatment of intracerebral hemorrhage: what should we do now? Curr. Neurol. Neurosci. 9, 13–18. doi: 10.1007/s11910-009-0003-z
Wang, Y., Lou, X. T., Shi, Y. H., Tong, Q., Zheng, G. Q. (2019). Erxian decoction, a Chinese herbal formula, for menopausal syndrome: an updated systematic review. J. Ethnopharmacol. 15, 8–20. doi: 10.1016/j.jep.2019.01.010
Wang, Q. (2016). Clinical study on the treatment of intracerebral hemorrhage by Liangxue Tongyu formula. Healthy people. 10, 44.
Zhao, B. S. (2012). Observation on the curative effect of Liangxue Tongyu formula in the treatment of the symptom of stagnant heat blocking aperture in the acute stage of cerebral hemorrhage. Jilin Med. J. 33, 6567. doi: CNKI:SUN:JLYX.0.2012-30-062
Zhou, H. J., Tang, T., Zhong, J. X., Luo, J. K., Cui, H. J., Zhang, Q. M., et al. (2014). Electroacupuncture improves recovery after hemorrhagic brain injury by inducing the expression of angiopoietin-1 and -2 in rats. BMC Complem Altern. M. 14, 127–132. doi: 10.1186/1472-6882-14-127
Appendix 1. Summary of sensitivity analysis (continuous outcomes).
Appendix 2. Summary of sensitivity analysis (categories outcome).
Keywords: acute intracerebral haemorrhage, Liangxue Tongyu formula, systematic review, meta-analysis, randomized controlled trials (RCT), traditional Chinese medicine
Citation: Jiang C , Yang X, Dong J and Li G (2020) Systematic Review and Meta-Analysis of Randomized Controlled Trials of Liangxue Tongyu Formula on Patients With Acute Intracerebral Hemorrhage. Front. Pharmacol. 11:437. doi: 10.3389/fphar.2020.00437
Received: 17 September 2019; Accepted: 20 March 2020;
Published: 15 April 2020.
Edited by:
Pulok Kumar Mukherjee, Jadavpur University, IndiaReviewed by:
Francis-Alfred Unuagbe Attah, University of Ilorin, NigeriaAmit Krishna De, Indian Science Congress Association (ISCA), India
Copyright © 2020 Jiang, Yang, Dong and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guochun Li, bGlndW9jaHVuY25AMTI2LmNvbQ==
†These authors share first authorship
 Chao
Jiang
Chao
Jiang Xiaojuan Yang
Xiaojuan Yang Ju Dong
Ju Dong Guochun Li
Guochun Li