- Quantitative Sciences, Bill & Melinda Gates Medical Research Institute, Cambridge, MA, United States
Introduction
The widespread global interest in electronic health records (EHRs) has created an impression that the requisite engagement of caregivers, providers, patients, and the institutions that rely on EHRs is well-established and in place (Pagliari et al., 2007). Meaningful Use regulation is similarly well-intended but also gives the impression that proper linkages are in place and that the relationships are optimized to create value. The point made a decade ago that “inevitability does not mean easy transition” (Blumenthal and Tavenner, 2010) is still true today. For pediatrics this is especially true. While recent legislation has obligated pharmaceutical sponsors and manufacturers (PhRMA) to be more proactive in the planning, design, and conduct of pediatric clinical trials in populations that would stand to benefit from potential new medicines, the totality of data that would support meaningful dosing guidance as well as long-term safety experience is still lacking (Barrett et al., 2018). As the assumption that adult and pediatric disease progression is similar is often not valid, bridging experiments and trials are likewise not always appropriate with the default scenario that dosing in children is heavily reliant on the adult experience. EHRs have the potential to generate meaningful real-world data (RWD) in children if a mechanism can be adopted that both captures the relevant outcome data and decision support systems built on such data become a part of the practice of medicine and not just a reference.
EHRs Past, Present, and Future
Since the 1960s, medical records were primarily paper-base with various raw data listings capturing test results and procedures in addition to prescribing (medicine orders) and billing information. The source of these record was often not centralized and based on chart-based collection managed by the group collecting and responsible for the data (e.g., Pharmacy/formulary, Pathology, diagnostics). In addition, what we now refer to as personal health information (PHI) including social security numbers, names, and addresses was scattered all of the source documents. Lockheed's initial EHR offering developed in the 1960's became the anchor for future systems developed in the 1980's with the support and guidance from the Institute of Medicine (IoM). Since the early 2000's there has been great motivation to convert all paper-based records to EHRs with incentive provided by the Office of the National Coordinator (ONC) of Health Information Technology (IT). The subsequent Health Information Technology for Economic and Clinical Health Act (HITECH) promoted the concept of “meaningful use” criteria with the intention of providing definitive outputs and incentives for EHR-adopting institutions (CDC, 2019).
On a personal level I was fortunate to receive grant funding from the American Reinvestment & Recovery Act (ARRA) which was enacted on February 17, 2009. As ARRA sought, in part, to provide opportunities to demonstrated meaningful use for electronic health records under the umbrella of the “Health Information Technology for Economic and Clinical Health (HITECH) Act,” our grant was focused on developing prototype drug dashboards which provided dosing guidance for therapeutic agents for which therapeutic dug monitoring was employed to manage pharmacotherapy (Barrett et al., 2008). While there was engagement with the hospital governance committees on the development of the dashboard prototypes the incentives for implementing the systems were not able to convince these committees on adopting or even testing the prototypes in actual patients despite retrospective evaluation of their performance and acknowledgment of their accuracy (Barrett et al., 2008). It became apparent that the value of the record systems as billing and documentation of services rendered represented too great a value to overcome the potential benefit of the prototype systems which required prospective clinical validation to fully demonstrate their clinical value. While the HER systems offer great potential clinical functionality, their implementation is still guided primarily by IT and financial motivations. Hence, the potential to guide the practice of medicine has been there for some time, though the habit and culture of doing so has not been in place.
Hospital Culture for Guidance and Therapeutics Standards
Most hospitals maintain some level of hierarchy regarding therapeutic decision making particularly pediatric institutions where formulary and standards recommendations are often made in the absence of actual pediatric-specific labeling for old and new drugs. For new drugs in particular, while there may be great motivation to initiate therapy with the new agent in children, there is often a prevailing conservativism especially if the sponsor has little data to support pediatric claims or if there is only a rationale with no claims or data available. For new drug candidates this is often under the jurisdiction of the drug use evaluation (DUE) or similar committee and/or the therapeutic standards or similar committee (TSC) with the distinction being the former is more pharmacy-based and informed about clinical pharmacology and the latter is more physician-based with emphasis on clinical outcomes. Both are intimately concerned with patient safety and typically, the DUE will provide recommendations for the TSC's approval.
Simply building decision support systems or outcome-based dashboards is not enough. There is a long history of efforts to create such tools, but they have not been sustainable even when internal champions exist at the host institutions (Jelliffe, 1968; Barrett et al., 2008; Jelliffe et al., 2009; Felton et al., 2014; Mould et al., 2014, 2018; Neely, 2017). This is also not an issue of regulatory compliance or providing guidance outside the drug label though this is often cited as a barrier for adoption.
Continued Lack of Guidance for Pediatrics
While a premium is placed on the safe use of medicines in children, the data useful to guide safe practices is seldom discussed in the proper context. The historical argument is that we need more studies in children with pediatric-specific formulations and better dosing guidance derived from pediatric trials and we need to more closely monitor off-label usage (Barrett et al., 2011). Thus, we keep looking to the pharmaceutical industry to fill in gaps in our knowledge. It is clear that pharmaceutical sponsors still struggle with demonstrating similarity of disease progression (or not) and the subject of pediatric extrapolation (Barrett et al., 2018) continues to be a topic of interest among industry and regulatory scientists. As real-world data (RWD) sources are often lacking, EHRs have been discussed as one potential source for such analyses. More importantly however, EHRs are not considered as sources of information from which dosing guidance can be derived or even modified. Why not?
Adult medicine will always have the benefit of more choices with respect to medicine per condition and suitable data on pharmacokinetics, pharmacodynamics, and pharmacogenomics based on a marketplace economy that drives the industry and the clinical development plans of pharmaceutical sponsors. Likewise, the current paradigm for pediatric drug development is tied to a decision tree that relies heavily on adult experience regarding the therapeutic window in aggregate and extrapolation approaches leveraging the adult data (Barrett et al., 2018). Pediatric doses are calculated mostly using body weight to produce a dose for the individual child though oncology still uses body surface area to a large extent. This is not true personalization of course as the dose suggested (usually in mg/kg) can be the same for a large age range of children. More recent practice with staggered dosing across age-weight bands is a step in the right direction but still stops short of individual dosing guidance.
Precision medicine in pediatrics will require an appropriate evidence base (particularly for unlicensed and off label medications), with relevant patient-specific data (e.g., genotype, environmental, and lifestyle data) added to guide both medication selection and the dose required (Hawcutt et al., 2016). The choice of the source of this data should not be the issue as long as it possesses the appropriate information value.
Discussion
What would it look like to realize the work of Mould et al. (Barrett et al., 2008; Mould et al., 2014, 2018; Hawcutt et al., 2016; Strik et al., 2018, 2019) in a more meaningful and sustainable way? Fundamentally, this will require more engaged hospital governance committees comfortable with technology, underlying models and the quality of the data collected in conjunction with a culture that is more open to seeing technology built on EHRs as an extension of their practice and not a mere record keeping or billing service. Figure 1 describes a workflow proposal more consistent with establishing clinical value and return on investment for dashboard-informed EHRs with commitment to evolve and maintain the system and capture the relevant outcomes for hospitalized children. We can't keep looking to PhRMA or regulators to solve this. It is neither their problem nor is the scope of the work to be done in a place where their experience is valuable. The practice of medicine for children has to change and confidence with a new tool set must evolve to the point where precision dosing for children is an expectation and not an exception.
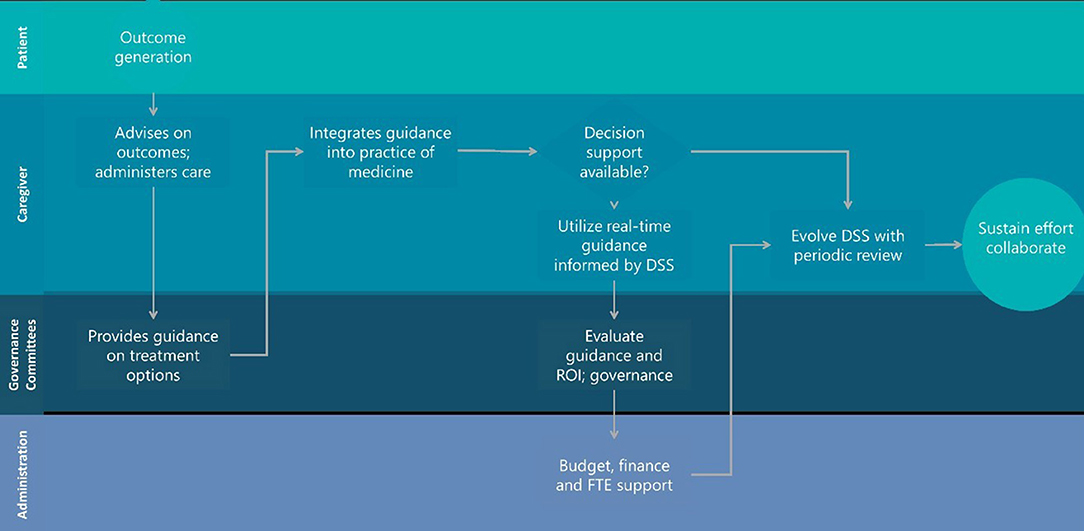
Figure 1. Hospital landscape for sustainable decision support engagement that evolves with the practice of medicine during habitual utilization.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Barrett, J. S., Bishai, R., Bucci-Rechtweg, C., Cheung, S., Corriol-Rohou, S., Haertter, S, et al. (2018). Challenges and opportunities in the development of medical therapies for pediatric populations and the role of extrapolation. Clin. Pharmacol. Ther. 103, 419–433. doi: 10.1002/cpt.1000
Barrett, J. S., Mondick, J. T., Narayan, M., Vijayakumar, K., and Vijayakumar, S. (2008). Integration of modeling and simulation into hospital-based decision support systems guiding pediatric pharmacotherapy. BMC Med. Inform. Decis. Making 8:6. doi: 10.1186/1472-6947-8-6
Barrett, J. S., Zuppa, A. F., Adamson, P. C., Patel, D., and Narayan, M. (2011). Prescribing habits and caregiver satisfaction with resources for dosing children: rationale for more informative dosing guidance. BMC Pediatr. 11:25. doi: 10.1186/1471-2431-11-25
Blumenthal, D., and Tavenner, M. (2010). The “meaningful use” regulation for electronic health records. N. Engl. J. Med. 363, 501–504. doi: 10.1056/NEJMp1006114
CDC (2019). Public Health and Promoting Interoperability Programs (Formerly, Known as Electronic Health Records Meaningful Use). Center for Surveillance, Epidemiology, and Laboratory Services. Available online at: https://www.cdc.gov/ehrmeaningfuluse/introduction.html (accessed September 9, 2019).
Felton, T. W., Roberts, J. A., Lodise, T. P., Van Guilder, M., Boselli, E., Neely, M. N., et al. (2014). Individualization of dosing of piperacillin for critically ill patients: dosing software to optimize antimicrobial therapy. Antimicrob. Agents Chemother. 58, 4094–4102. doi: 10.1128/AAC.02664-14
Hawcutt, D. B., Cooney, L., Oni, L., and Pirmohamed, M. (2016). Precision dosing in children. Expert Rev. Precis. Med. Drug Dev. 1, 69–78. doi: 10.1080/23808993.2016.1138845
Jelliffe, R., Schumitzky, A., Bayard, D., Leary, R., Botnen, A., Guilder, M. V., et al. (2009). Human genetic variation, population pharmacokinetic-dynamic models, bayesian feedback control, and maximally precise individualized drug dosage regimens. Curr. Pharmacogenom. Personal. Med. 7, 249–262. doi: 10.2174/187569209790112382
Jelliffe, R. W. (1968). An improved method of digoxin therapy. Ann. Intern. Med. 69, 703–717. doi: 10.7326/0003-4819-69-4-703
Mould, D. R., Upton, R. N., and Wojciechowski, J. (2014). Dashboard systems: implementing pharmacometrics from bench to bedside. AAPS J. 16, 925–937. doi: 10.1208/s12248-014-9632-5
Mould, D. R., Upton, R. N., Wojciechowski, J., Phan, B. L., Tse, S., and Dubinsky, M. C. (2018). Dashboards for therapeutic monoclonal antibodies: learning and confirming. AAPS J. 20:76. doi: 10.1208/s12248-018-0237-2
Neely, M. (2017). Scalpels not hammers: the way forward for precision drug prescription. Clin. Pharmacol. Ther. 101, 368–372. doi: 10.1002/cpt.593
Pagliari, C., Detmer, D., and Singleton, P. (2007). Potential of electronic personal health records. BMJ 335:330. doi: 10.1136/bmj.39279.482963.AD
Strik, A., Berends, S., Mould, D., Mathôt, R., Ponsioen, C., van den Brande, J., et al. (2019). DOP56 dashboard driven vs. conventional dosing of infliximab in inflammatory bowel disease patients: the PRECISION trial. J. Crohns Colitis 13:S063. doi: 10.1093/ecco-jcc/jjy222.090
Keywords: EHRs, precision dosing, decision support, dashboards, pediatric patients, hospital
Citation: Barrett JS (2020) Asking More of Our EHR Systems to Improve Outcomes for Pediatric Patients. Front. Pharmacol. 11:253. doi: 10.3389/fphar.2020.00253
Received: 18 January 2020; Accepted: 24 February 2020;
Published: 12 March 2020.
Edited by:
Jeannine S. McCune, Beckman Research Institute of the City of Hope, United StatesReviewed by:
Anne Zajicek, National Institutes of Health (NIH), United StatesGeorg Hempel, University of Münster, Germany
Copyright © 2020 Barrett. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeffrey S. Barrett, amVmZi5iYXJyZXR0QGdhdGVzbXJpLm9yZw==
 Jeffrey S. Barrett
Jeffrey S. Barrett