
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pharmacol., 03 March 2020
Sec. Ethnopharmacology
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.00171
This article is part of the Research TopicMedicinal Plants for Cardiovascular and Neurodegenerative Aging-related Diseases: From Bench to BedsideView all 20 articles
Convolvulus prostratus Forssk., a nootropic herb used in traditional medicinal systems, is also frequently known by its taxonomic synonym Convolvulus pluricaulis. In Indian medicinal system – Ayurveda – it is named as Shankhpushpi. According to the ancient literature, this herb has been attributed with several therapeutic properties, such as anxiolytic, neuroprotective, antioxidant, analgesic, immunomodulatory, antimicrobial, antidiabetic and cardioprotective activities. This medicinal herb has been reported to contain many bioactive phytoconstituents, such as, alkaloid (convolamine), flavonoid (kaempferol) and phenolics (scopoletin, β-sitosterol and ceryl alcohol), that have been ascribed to the observed medicinal properties. Several research teams across the globe have highlighted the neuro-pharmacological profile of C. prostratus, wherein, the neuroprotective, nootropic and neuro-modulatory roles have been described. Besides, role of C. prostratus extracts in neurodegeneration has been well demonstrated. Despite of such elaborative preclinical pharmacological profile, detailed clinical investigations and mechanistic mode-of-action studies of this important herb are yet to be executed. The present review is attempted to showcase the phytochemical profile, pharmacological attributes and medicinal information of C. prostratus; with comprehensive research gap analysis. It is hoped that the scientific update on the ethnomedicinal aspects of this herb would thrive research propagation and development of the CNS phytopharmaceuticals, originated from C. prostratus.
In recent years, non-communicable diseases (NCDs) have become an emerging cause of morbidity and mortality (~ 12% global prevalence) (Islam et al., 2014). Neurological disorders constitute a significant proportion as the leading causes of death among all the other non-communicable diseases (Gourie-Devi, 2014). The most common disorders of the nervous system are schizophrenia (~ 40% prevalence in India) and epilepsy (~ 45% prevalence in India), which are clinically presented by dysfunction of the interneurons, misbalancing neuronal homeostasis, ultimately leading to neurophysiological disintegration (Gururaj et al., 2005). It is estimated that by next decade, mental and behavioural disorders will lead to a reduction in the average life expectancy, nearly by one-fifth proportion (Lopez and Murray, 1998). In India, an average of 65 people out of 1,000 inhabitants suffer from mental and behavioural disorders, wherein, maximum prevalence has been recorded for alcohol dependency afflicted mental disorders (~ 6% prevalence), child and adolescent behavioural disorders (~ 4.3% prevalence) and mood disorders (~ 1.6% prevalence). These statistics makes it obvious that the prevalence of neurodegenerative disorders is escalating, however, effective and safe treatment modalities are still under infancy (Gururaj et al., 2005).
The chemotherapeutic moieties used currently for the treatment of neurological disorders face a daunting challenge of systemic delivery of the drugs to the central nervous system. Moreover, crossing the blood brain barrier after strenuous systemic administration is another challenge. Ultimately, the bioavailability of these drugs becomes low and therefore, lead to below- optimal efficacies (Tonda-Turo et al., 2018). Several drug delivery vehicles and nano-formulations have been devised so as to facilitate the targeted delivery of the drug to the site of action, i.e., the central nervous system, thereby bypassing the blood brain barrier (Saraiva et al., 2016; Tonda-Turo et al., 2018). However, these strategies have also shown little success. Most of the neurodegenerative disorders are progressive in nature, and the cycle of etiological events usually have an early-onset which might get unnoticed or under-detected. In such a case, the therapies initiated after the onset of neuropathological asymptomatic etiologies will only have limited value for the patients (Solanki et al., 2016). Moreover, the foresaid neuropathological conditions are manifested with several additional etiologies, wherein the chemotherapeutic modalities act typically on a single target, thereby providing only palliative care (Chen and Pan, 2014). Hence, natural compounds serve as the holistic option which act on multiple neural targets and are enriched with free radical scavenging polyphenolic compounds (Park et al., 2018). These phytoconstituents have well-described antioxidant and anti-inflammatory properties which aids in protecting the neuronal cells against oxidative stress. Additionally, the natural compounds, such as flavonoids also aid in modulating the neuronal cell signalling pathways (Solanki et al., 2016).
There are various herbs which are used in traditional medicine for the management of neurodegenerative diseases, for instance: Centella asiatica (L.) Urb., Glycyrrhiza glabra L., Tinospora cordifolia (Willd.) Hook. f. & Thomson, Bacopa monnieri (L.) Wettst. and Nardostachys jatamansi (D. Don) DC. (Kulkarni et al., 2012). One such example is Convolvulus prostratus Forssk. (Syn. Convolvulus pluricaulis Choisy), which is commonly known as Shankhpushpi in Ayurveda and is widely recognized for its anxiolytic, antidepressant and nootropic activities (Kulkarni et al., 2012). As per an ancient Indian medicinal scripture—Charaka Samhita—this plant is superior to other nootropic drugs (Medhya rasayana) of Ayurveda, however, a detailed ethnomedicinal update yet needs to be presented (Dev, 2006). The ensuing sections will provide an ethnomedicinal, phytochemical and pharmacological update of this cognitive booster herb, C. prostratus (CP).
C. prostratus belongs to Convolvulaceae family and is ubiquitous in the north-western regions of India (Nisar et al., 2012). In Ayurveda, this herb is classically described as a memory and intellect booster. Moreover, it is employed in a variety of formulations used for the treatment of nervous disorders, such as insanity, epilepsy, hysteria, insomnia, and psycho-neurosis (Khare, 2004). Mechanistically, it reduces the spontaneous motor activity, thereby controlling the refluxes and frightening responses. It ultimately acts as a sedative moiety which initiates a persistent fall in blood pressure and cardiac contraction, thereby managing neurological pathologies, such as anxiety, insanity and epilepsy (Chaudhary, 1996). The neuro-mechanistic aspects of this herb has been elucidated in Figure 1A. Besides, this plant has manifold therapeutic utilities, wherein, a decoction of its shoots is used as a remedy for anaemia and weakness (Singh et al., 2003). More specifically, in ancient texts, this plant has been mentioned as sara, medhya, vrsya and rasayana, which refers to the laxative, nootropic, aphrodisiac and rejuvenator properties of this herb, respectively. Additionally, one of the revered ancient Indian medical practitioner, Acharya Charaka had used white-flowered variety of C. prostratus (Shankhpushpi) along with the juice of Bacopa monnieri (Brahmi), Acorus calamus (Vacha) and Saussurea lappa (Kushtha) for alleviating insanity and epilepsy. Similar views had been presented in Chikitsasangraha written by Chakradatta; Kaideva Nighantu; and Ayurveda Saar Sangraha (Khare, 2004).
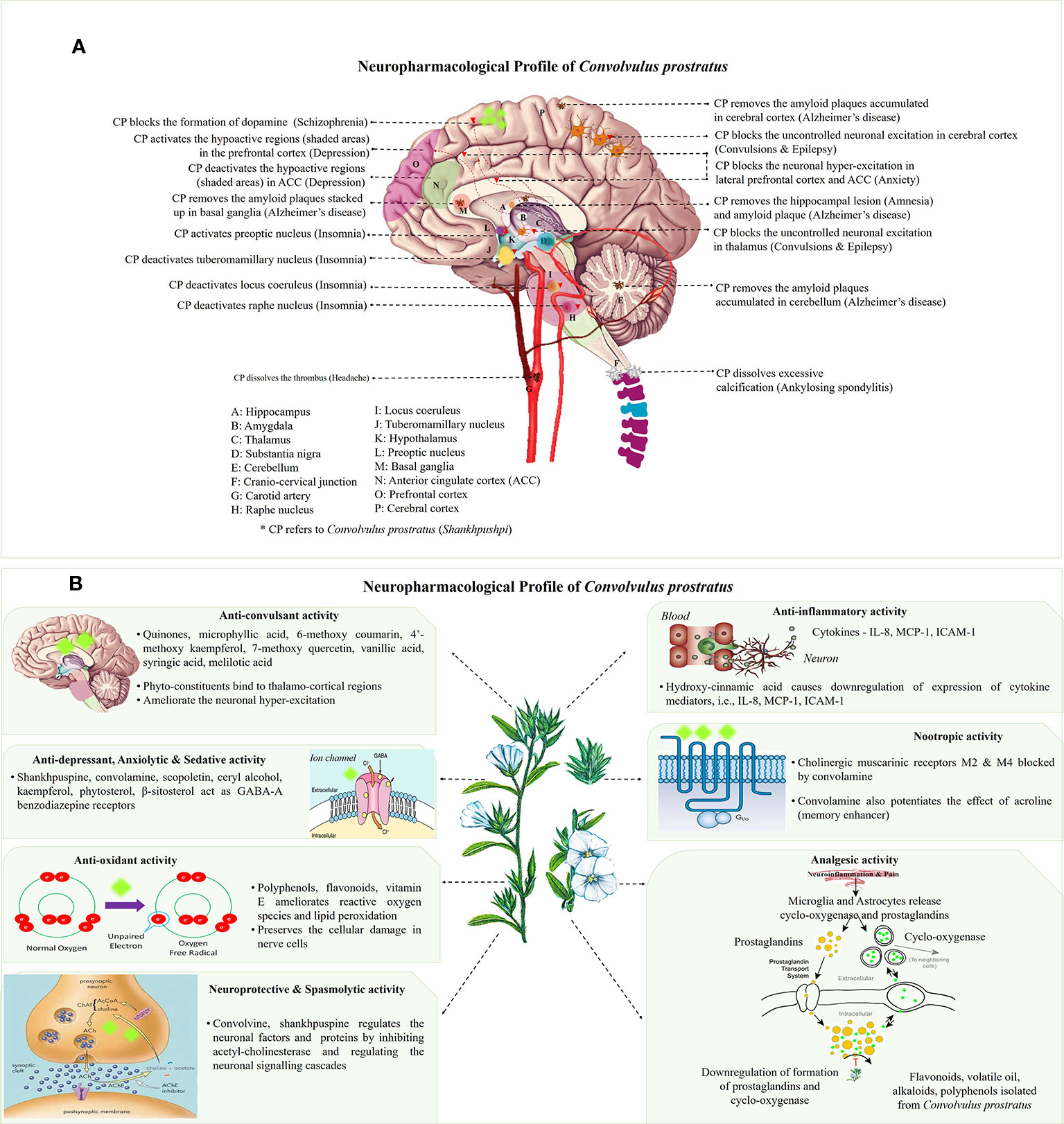
Figure 1 (A) Neuro-mechanistic aspects of Convolvulus prostratus (CP). Schematic representations of C. prostratus (CP) target and mode of action in neural system. CP reduces the lesions formed in the hippocampus (A); and amyloid plaque accumulation in hippocampus (A), cortex (P), basal ganglia (M) and cerebellum (E). CP also acts as GABA-A agonists and binds to lateral prefrontal cortex (O) and anterior cingulate cortex (N). CP deactivates the wake promoting areas, namely, tuberomamillary nucleus (J) located in the hypothalamus (K), locus coeruleus (I) and raphe nucleus (H). It also activates the sleep inducing areas of the brain, namely, preoptic nucleus (L). Moreover, CP activates the hypoactive regions situated in the prefrontal cortex (shaded pink area; O), and deactivates hyperactive regions situated in the anterior cingulate cortex (shaded green area; N). CP blocks the excessive production of dopamine as produced via substantia nigra (D). CP also removes the excessive calcification formed at the cranio-cervical junction (F). Additionally, CP removes the formation of thrombus in the carotid artery (G). (B) Main mechanisms and phytoconstituents responsible for neuro-pharmacological activities of C. prostratus (CP). This herb exhibits anti-convulsant, anti-depressant, anxiolytic, sedative, anti-inflammatory, anti-oxidant, analgesic, nootropic, spasmolytic and neuroprotective activities. Phyto-constituents belonging to diverse chemical families, namely, coumarins, alkaloids and polyphenols (see Table 2) are responsible for such evident neuro-pharmacological profile of the CP plant (Nolte, 1999; Carter, 2014). Reported mechanisms of CNS action of CP phyto-constituents has been depicted in these schematics, with putative site of action has been marked with fluorescent green blobs.
Besides Ayurveda, C. prostratus (CP) has also been used in Siddha system of medicine, wherein an oil obtained from this plant is used as a keratogenic agent for promoting hair growth (Gogte, 2012). It is also believed that a paste prepared from its roots and flowers act as anti-aging agents, thereby indicating its apparent anti-oxidant activity (Adams et al., 2007). Furthermore, in Unani medicinal system, a syrup prepared with C. prostratus and Piper nigrum is prescribed in bleeding piles and venereal diseases (Khare, 2004). All the above mentioned ethnomedicinal uses of C. prostratus have been tabulated in Table 1.
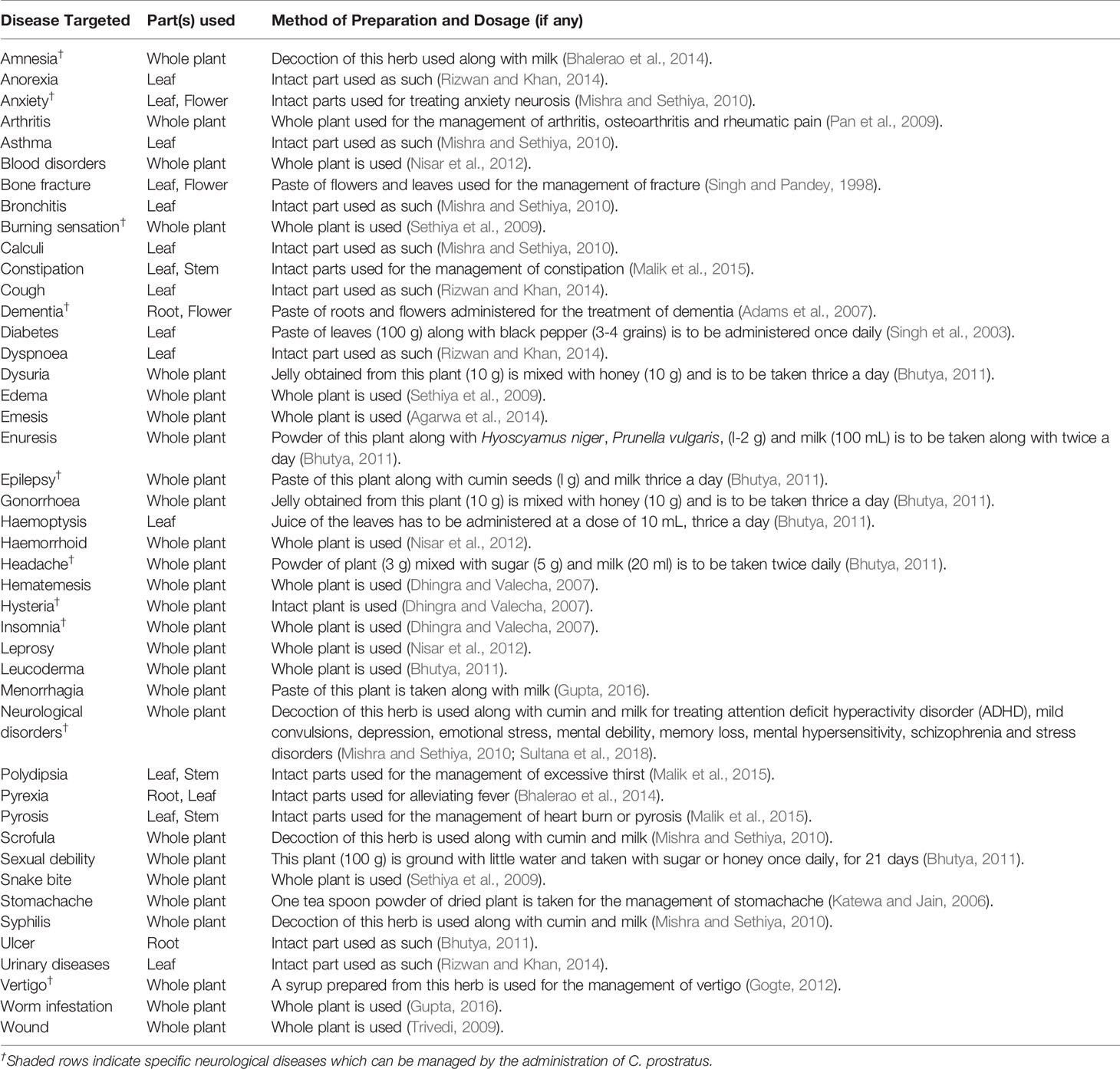
Table 1 Ethnomedicinal uses of Convolvulus prostratus (CP) with predominant plant parts and mode of herbal preparation.
C. prostratus is extensively used in pharmaceutical, cosmeceutical and nutraceutical industries (Jalwal et al., 2016). In the pharmaceutical industry, various extracts, syrups and tablets are produced, specifically for targeting neurodegenerative diseases, hypertension, hypercholesterolemia and gastric ulcers. A few examples of such marketed herbal pharmaceutical formulations include Patanjali Divya Shankhpushpi Churna™, Divya Pharmacy Shankhpushpi Sharbat™, Baidyanath Shankhpushpi Sharbat™, Dabur Shankhpushpi Syrup™, Biotrex Shankhpushpi™, Maxmind capsule™, Herbal Hills Shankhpushpi Tablets™ and many more (Bhowmik et al., 2012). Similarly, in the cosmeceutical industry, the CP herb is used as a general tonic for rejuvenating the skin and hairs, thereby treating skin related ailments as well as keratogenic disorders. A few examples for elucidating the use of CP (Shankhpushpi) as cosmeceutical ingredients include Econature Shankhpushpi Hair oil™, Khadi Natural Shankhpushpi oil™ and Alps Shankhpushpi hair Mask Powder™. Moreover, the CP powder and juice, such as, Jain Shankhpushpi Powder™ and Axiom Jeevan Ras Shankhpushpi Juice™, are also being used as skin mask for rejuvenating the skin and managing skin problems such as acne, blemishes and sun spots (Hindu, 2012). Additionally, food grade CP powder and syrups are also available in the market for being used as a nutraceutical nootropic supplement, for example, Divya Pharmacy Shankhpushpi Sharbat™, Baidyanath Shankhpushpi Sharbat™, Shivalik Herbals Shankhpushpi Nutraceutical Capsules™ and Veg E Wagon Shankhpushpi Powder (Bhowmik et al., 2012). Such extensive industrial uses of CP further confirms the holistic significance of this nontoxic wonder herb (Jalwal et al., 2016).
The CP herb has also been used as a phyto-ingredient of a polyherbal medicinal formulation: Sankhahauli, which contains leaves of C. prostratus (15 g); seeds of Piper nigrum (3 g) and Papaver somniferum (20 g); whole plant of Prunus amygdalus (10 g), Vitis vinifera (20 g) and Coriandrum sativum (10 g). This formulation is mainly used for the management of insomnia, drug addiction and hypertension (Bhutya, 2011). Several such marketed herbal formulations containing CP are being used for the management of a variety of neurological ailments in India, for example., Divya Medha Vati, Divya Medha Kwath (Patanjali Ayurved Ltd.); BR-16A (Himalaya Drug Co. Ltd.); Dimagheen (Dawakhana Tibiya College); Shankhpushpi syrup (Unjha); Shankhavali Churna (Narnaryan Pharmacy); Brain tab and Shankhpushpi syrup (Baidyanath Pharmaceuticals) (Aggarwal et al., 2011; Sethiya et al., 2013; Amin et al., 2014).
All of these stated medicinal utilities of C. prostratus (CP) have been attributed to various phytoconstituents, belonging to the chemical family of alkaloids, flavonoids, coumarins and polyphenols. Among these phytoconstituents, certain compounds are known to be present at a higher concentration (almost 20% w/w) and are known as major phyto-constituents. CP plant is known to contain kaempferol, β-sitosterol, N-hexacosanol, taraxerol, taraxerone, delphinidine and hydroxy-cinnamic acid as the major phyto-constituents, as depicted in Table 2 (Billore et al., 2005; Amin et al., 2014). Moreover, an alkaloid, namely, Sankhpuspine has also been isolated from this plant and is known as a chemotaxonomic marker for this species (Basu and Dandiya, 1948; Saroya and Singh, 2018). CP plant also contains other alkaloids (convolamine, convosine, convoline, convolidine, convolvine, confoline, evolvine, phyllabine, subhirsine, sankhpuspine) (Agarwa et al., 2014; Balaji et al., 2014); anthroquinones; carbohydrates (D-glucose, sucrose, rhamnose, maltose) (Dhingra and Valecha, 2007; Bhowmik et al., 2012; Agarwa et al., 2014); coumarins (ayapanin, scopolin, scopoletin); flavonoids (kaempferol, quercetin) (Lal, 2014); glycosides (geranilan-3-ol-1-carboxylate-1-O-β-D-xylopyranosyl-(2′→1′′)-O-β-D-xylopyranoside (Sultana et al., 2018); phenolic compounds; steroids; tannins; and terpenoids (Ravichandra et al., 2013; Agarwa et al., 2014; Balaji et al., 2014; Malik et al., 2016).
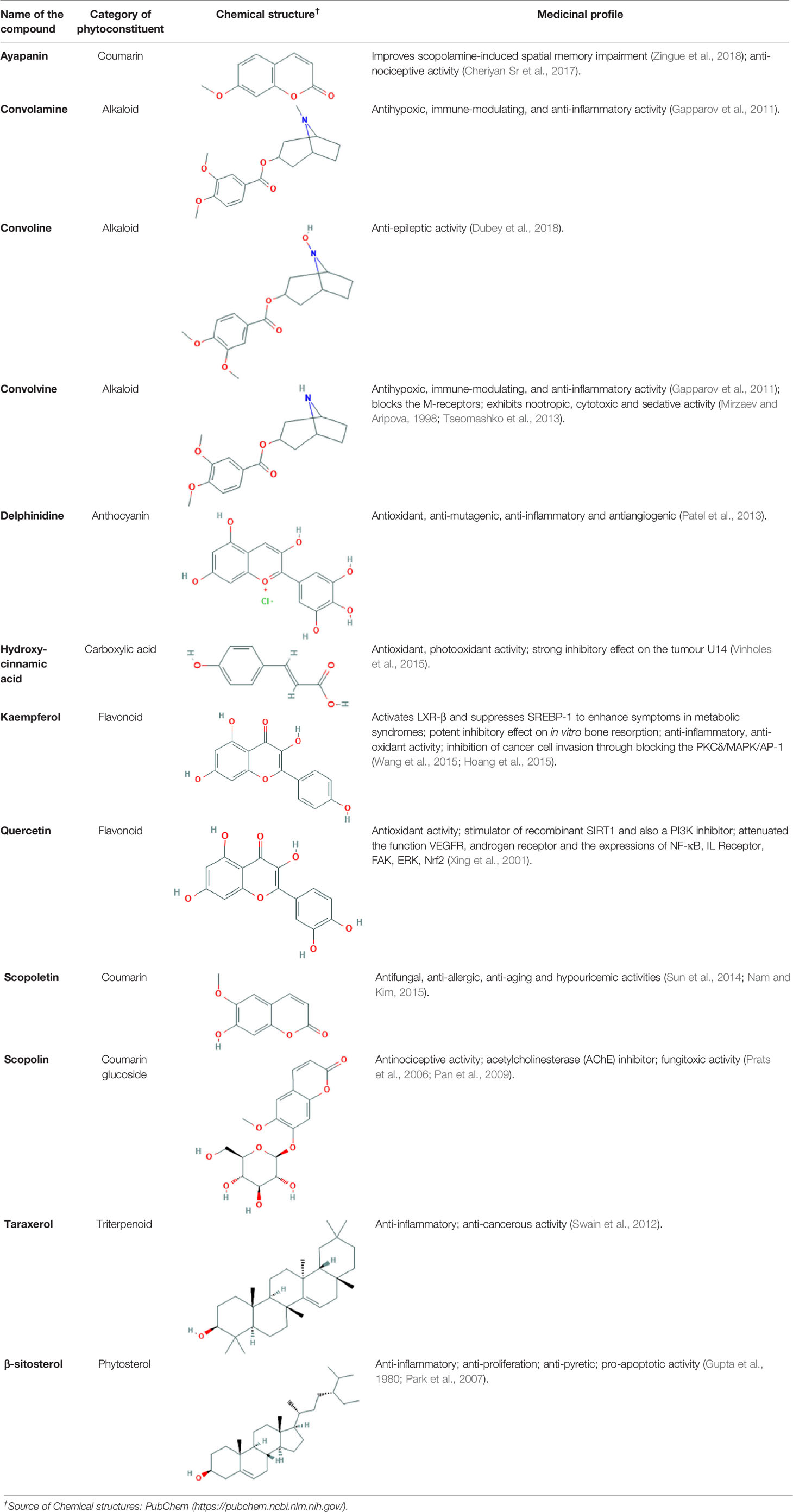
Table 2 Major phyto-constituents of Convolvulus prostratus (CP) with their reported medicinal utility.
Several other hydrocarbons, namely, 1- pentyl-2-tridecanyl cyclopentyl cyclohexane carboxylate, 1,2-benzenedicarboxylic acid, 10-bromodecanoic acid, 1-octadecanesulphonyl chloride, 2-butanone, 2-pentanol, 7- hydroxyheptadecanyl-1,7, 17-tricarboxylic acid, ascorbic acid, cyclononasiloxane, cyclo-octadecanyl methanol, decanoic acid, dicyclohexyl cyclo-octyl acetic acid, eicosane, heneicosane, hydroxy cinnamic acid, octatriacontyl pentafluoropropionate, pentadecyl 2-propyl ester, pentanoic acid, pentyl hexacosanoate, phthalic acid, silane, squalene, tetracyclohexanyl caproate and tridecane are also found in the extract of the CP plant (Bhalerao et al., 2014; Malik et al., 2016; Rachitha et al., 2018; Sultana et al., 2018).
In addition, CP is known to be a good source of vitamins and minerals, namely, calcium, copper, iron, magnesium, manganese, phosphorus, potassium, sulphur, zinc, vitamin C and E (Sethiya et al., 2010; Babu et al., 2015).
C. prostratus (CP) contains volatile oil; fatty alcohols; flavonoids, i.e. kaempferol; hydroxy cinnamic acid; β-sitosterol; and carbohydrates such as glucose, rhamnose, sucrose etc., which endow this plant with nootropic capabilities. Moreover, an alkaloid, namely, convolvine present in this herb has also been found to block cholinergic muscarinic receptors: M2 and M4. Convolvine also aids in potentiating the effect of another muscarinic memory enhancer, namely, arecoline, thereby imparting nootropic abilities to CP (Sethiya et al., 2009). In a study, Rawat and Kothiyal have also found that the aquo-methanolic, ethanolic and petroleum ether extracts isolated from CP (50–400 mg/Kg) exhibited anxiolytic, memory-enhancing and nootropic activity as evaluated by using Elevated Plus Maze (EPM) and step-down models in mice. EPM test has mainly been used to investigate the interactions between aversive memory and anxiety responses of the mice. CP effects on EPM activity has been found to be comparable to the standard of care drug, Piracetam (Rawat and Kothiyal, 2011; Kaushik, 2017). Moreover, treatment with alcoholic extract of CP plant led to an increase in the average time-span spent by mice in the enclosed arm of plus maze model, and an escalation in the mean avoidance response on the jumping box model (Rawat and Kothiyal, 2011).
The aqueous extract of the roots of C. prostratus inhibited the activity of acetylcholinesterase (AChE) within the cortex and hippocampus of male Wistar rats, that have been intoxicated with scopolamine. CP extract also posed evident anti-oxidant activity and elevated the levels of glutathione reductase, superoxide dismutase and reduced glutathione within the cortex and hippocampus (Kaushik, 2017). Similar results have been observed in case of aluminium chloride induced neurotoxicity in rat cerebral cortex. Regular administration of the CP root extracts (150 mg/Kg) for 3 months inhibited the decline in Na+/K+ ATPase activity and also preserved the mRNA expression levels of muscarinic acetylcholine receptor 1 (M1 receptor), choline acetyl transferase (ChAT) and nerve growth factor-tyrosine kinase A receptor (NGF-TrkA) (Bihaqi et al., 2009; Kaushik, 2017). The Na+/K+ ATPase pump aids in maintaining the osmotic equilibrium and membrane potential in neuronal cells (Forrest, 2014). Secondly, the muscarinic receptors bind to the neurotransmitter acetylcholine, thereby facilitating the transmission of electrical signals within the central nervous system (Brown, 2018). Furthermore, the choline acetyl transferase enzyme is essential for the synthesis of neurotransmitter acetylcholine; and the nerve growth factor-tyrosine kinase A receptor is necessary for binding of the neuronal trophic factors, thereby ensuring the survival of the neurons (Johnson et al., 2018; Indo, 2018).
In another experiments, it has been shown that the oral administration of the aqueous extract of the CP roots (150 mg/Kg) to scopolamine induced rats causes a marked reduction in the mRNA levels of tau protein (Bihaqi et al., 2012). Such reduction in the tau protein expression is responsible for causing an amelioration in the amyloid β-induced deficits in case of neurodegenerative disorders such as Alzheimer's disease (Vossel et al., 2010). Hence, the presence of phytoconstituents such as convolvine might be responsible for endowing CP with the abilities to regulate all the neuronal factors/proteins and enzymes, thereby showcasing its evident neuroprotective status (Sethiya et al., 2009; Ray and Ray, 2015).
The ethanolic and chloroform extracts isolated from the aerial parts of CP showed significant anxiolytic activity as recorded using elevated plus maze test on experimental mice. There was increase in the time spent in open arms; and in the number of open arm entries upon the CP oral administration to mice at a dose of 200 mg/Kg (Bhalerao et al., 2014). Similar results have also been observed in case of the ethanolic extract of the CP flower petals at doses 200–400 mg/Kg in mice (Kaushik, 2017).
In another study, CP methanolic extract was evaluated for anxiolytic activity on Obsessive Compulsive Disorders (OCDs) in mice by employing marble burying behaviour analysis, hole board and rota-rod tests. The results have shown that the mice group treated with 200–400 mg/Kg CP methanolic extracts can modulate serotonin or dopaminergic levels, thereby harmonizing the major pathway involving serotonergic or dopaminergic receptors manifesting obsessive compulsive disorders (Subramani et al., 2013). Such anxiolytic activity can be linked with the hypotensive effect of this herb, which in turn is attributed to the presence of GABA-A-benzodiazepine agonists, such as convolamine and scopoletin (Figure 1B) (Malik et al., 2011; Amin et al., 2014; Siddiqui et al., 2014).
The chloroform, ethanol and aqueous extracts of CP have been evaluated for anti-convulsant activity against strychnine induced as well as pentylene tetrazol (PTZ) induced convulsive seizures in different animal models. Five hundred mg/Kg concentration of the CP extracts have shown statistically significant (p < 0.001) protection against strychnine and PTZ induced clonic convulsions (Ratha and Mishra, 2012; Siddiqui et al., 2014). Methanolic extract of this plant (500–1000 mg/Kg) also exhibited anti-convulsant activity, as characterized by reduction in the mean recovery time of convulsions in case of maximal electroshock seizure model in mice (Kaushik, 2017).
The fundamental mechanism behind such evident anti-convulsing activity of CP might be the presence of coumarins and triterpenoids (Quintans Júnior et al., 2008). Moreover, it has also been proposed that the anti-convulsant activity of a phyto-medicine is escalated by the presence of certain functional groups like, quinoline, quinazoline, thiazole, benzothiazines, oxadiazole, pyridine, pyrazole, imidazole, pyrimidine, phthalazine, triazine, triazoles, cyclopropane carboxylate, and oxime ether (Wei et al., 2015; Song and Deng, 2018). Indeed, many such functional groups have been found in the CP phyto-constituents (Table 2) for e.g., quinones, microphyllic acid, 6-methoxy coumarin, 4'-methoxy kaempferol, 7-methoxy quercetin, vanillic acid, syringic acid and melilotic acid (Daniel, 2016).
Bhalerao and co-workers have found that the chloroform fraction isolated from the CP ethanolic extract reversed the reserpine-induced extension of immobility period of mice in Forced Swim Test (FST), and elicited a significant antidepressant effect by interaction with adrenergic, dopaminergic and serotonergic systems (Bhalerao et al., 2014). Similarly, a polyherbal formulation (Trans-01) containing C. prostratus (30%), Valeriana wallichii (45%), Plumbago zeylanica (7.5%), Boswellia serrata (15%) and Acorus calamus (3.5%) also exhibited similar anti-depressant properties as tested by employing the forced swim test (FST), tail suspension test (TST) and forced swimming stress (FSS)-induced alterations in serum corticosterone levels. In TST and FST, Trans-01 showed a dose-dependent decrease in immobility time. Moreover, Trans-01 significantly attenuated the elevated corticosteroid levels, thereby indicating a significant anti-depressant activity of this formulation (Shalam et al., 2007). CP herb is known to contain alkaloids (convolamine and scopoletin), flavonoids (kaempferol), and steroids (phytosterol and β-sitosterol). These phytoconstituents most probably act as GABA-A-benzodiazepine agonists and bind to the GABA-A-benzodiazepine receptors, thereby causing an increase in the chloride ion flux and consequent hyperpolarization of the postsynaptic membrane. Such hyperpolarization leads to a hypnotic effect and may alleviate depression (Siddiqui et al., 2014).
Hydroxy-cinnamic acid is a phenyl-propanoid compound found in CP. It is known to cause a downregulation in the expression of cytokine mediators such as IL-8, MCP-1 and ICAM-1, thereby blocking the expression of cytokine-mediated adhesion molecules and therefore the fundamental process of leukocyte–endothelial cell adhesion is deterred (Billore et al., 2005; Rathee et al., 2009). Hence, the CP herb may aid in ameliorating the conditions of neuro-inflammation and consequent cognitive impairment. Indeed, oral administration of the ethanolic extract of the CP leaves at dose of 800 mg/Kg, showed significant inhibition of rat paw edema, in the Carrageenan-induced paw edema and Cotton pellet-induced granuloma animal models (Agarwal et al., 2014).
The reactive oxygen species are known to deteriorate the cellular physiology of nerve cells and ultimate lead to neurodegenerative disorders. Polyphenols, flavonoids and vitamin E present in the CP plant act as reactive oxygen species (ROS) scavengers and also ameliorate the lipid peroxidation, thereby attributing towards the anti-oxidant activity of CP (Nasri et al., 2015). It has also been observed that the ethyl acetate and methanolic extract of CP have shown appreciable results (IC50 ~ 0.07 mg/mL and 0.075 mg/mL, respectively), as observed in 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. Similarly, in Ferric Reducing Antioxidant Power (FRAP) analysis, aqueous CP extract has been found to be potentially active with anti-oxidant capacity of 460 ± 8 ascorbic acid equivalent/mg of the extract (Jain et al., 2011). Interestingly, the Shankhpushpi syrup and its isolated compounds (Scopoletin and Bacoside A) also exhibited evident anti-oxidant activity as evaluated by using DPPH assay with average IC50 value ranging from 0.94 - 2.39% v/v (Jain et al., 2017; Rachitha et al., 2018). Furthermore, the aqueous extract of CP roots diminished the endogenous levels of reactive oxygen species in tauopathy flies as induced by overexpression of τ-protein, thereby substantiating its oxidative stress ameliorative effect (Olakkaran and Antony, 2017).
The ethanolic extract of CP at dose 750 mg/Kg showed statistically significant analgesic activity as compared to the standard analgesics like, morphine sulphate, when tested in hot plate method and tail-flick assays in rats (Agarwal et al., 2014). Such evident analgesic activity is cohesively attributed by flavonoids, volatile oils, alkaloids, polyphenols and organic acids by means of prevention of the formation of cyclooxygenase enzyme and prostaglandins, i.e., mediators of pain sensitization. Hence, these CP phyto-constituents ultimately aid in ameliorating the neuronal pain and headache (Aleebrahim-Dehkordy et al., 2017).
The CP ethanolic extract has exhibited spasmolytic activity in isolated rabbit ileum, isolated rat uterus, intact intestine and tracheal muscles of dog (Barar and Sharma, 1965). Such anti-spasmodic action is linked to the inhibition of acetylcholine production which is mainly brought about by the specific alkaloid present in this CP herb, convolvine (Amin et al., 2014).
Interestingly, the ethanolic and aqueous extracts of the CP aerial parts showed statistical significant potentiation of sleeping time in rats induced with thiopental sodium (Siddiqui et al., 2014). In another similar experiment, the aqueous extract of the CP leaves and flowers showed an evident barbiturate hypnosis potentiation in albino rats at a dose of 300 mg/Kg (Mudgal, 1975). Such sedative activity is directly linked to the presence of phytoconstituents like convolamine and scopoletin which act similarly to GABA-A agonists, thereby bringing about the effects of sedation (Figure 1B) (Siddiqui et al., 2014).
This CP herb has also been reported for several other pharmacological activities, including anti-diabetic, anti-hyperlipidemic, anti-hypertensive, anti-microbial, anti-platelet aggregation, anti-ulcer, cardio-vascular, hepatoprotective, and hypothyroidism (Barar and Sharma, 1965; Mudgal, 1975; Rizk et al., 1985; Mali, 1995; Jain et al., 2011; Ravichandra et al., 2013; Jalwal et al., 2016). Anti-diabetic activities of this plant might be attributed to the presence of tropane alkaloids which are known as potent inhibitor of α-glucosidases and R-galactosidases (Gaikwad et al., 2014; Rachitha et al., 2018). The polyphenols present in this species act as reactive oxygen species (ROS) quenchers, thereby ameliorating the oxidative stress that is generated as a diabetic manifestation. Additionally, the presence of vitamin E in this herb also aids in controlling the levels of protein oxidation and lipid peroxidation, thereby leading to an escalation in the antioxidant defense system (Cowan, 1999; Nasri et al., 2015; Domínguez-Avila et al., 2016; Foretz et al., 2018). Furthermore, the presence of GABA-A-benzodiazepine agonists, such as convolamine, scopoletin, ceryl alcohol, kaempferol, phytosterol, and β-sitosterol endow this herb with hypotensive and sedative activities (Malik et al., 2011; Siddiqui et al., 2014). More specifically, a compound, namely, 29-oxodotriacontanol, isolated from the CP herb has also been assessed to possess antimicrobial and anti-fungal activity (Amin et al., 2014). Certain flavonoids and phenyl-propanoids from CP have been shown to provide anti-platelet aggregation and anti-ulcerogenic activity by means of inhibition of cyclic nucleotide phosphodiesterase enzyme and clot retraction capabilities (Beretz and Cazenave, 1991; Tognolini et al., 2006). The anti-ulcerogenic effect was largely observed due to upregulation of mucosal defensive factors such as mucin and glycoprotein secretion, which in turn was induced by the flavonoids and steroids present in this herb (Srinivas et al., 2013). These flavonoids also pose the profound effects on the thyroid hormone regulation and deiodinase-1 inhibition, thereby endowing this herb with anti-thyroid activity (Nagarathna and Jha, 2013). Additionally, the fundamental principle responsible for the cardio-vascular activity of this herb has been proposed to be linked with it alkaloid derivative, evolvine hydrochloride, which is known to exhibit lobeline-like action on the cardiovascular system. This phytoconstituent acts as a cardiac depressant, ultimately leading to a fall in blood pressure, which gets gradually normalised (Dwoskin and Crooks, 2002; Sethiya et al., 2009).
The ethanolic and aqueous extracts of the CP leaves have been evaluated for acute oral toxicity study in albino Wistar rats. The animals did not show any toxicity or behavioural changes up to the dose of 5,000 mg/Kg (Agarwal et al., 2014). Similarly, the iron oxide nanoparticles of the CP herb exhibited maximum tolerable dose up to 2,000 mg/Kg in Swiss albino mice with no clinical signs of toxicity. The histopathology of brain also did not show any aberrations or degeneration of neurons. Furthermore, no inflammation was observed in the heart and liver (Ravichandra et al., 2013). These toxicological studies, therefore, confirmed that the administration of CP is safe for the vital organs within the respective treatment durations.
The use of herbal medicines continues to escalate rapidly with about 70% of the world population still relying upon traditional medicines for their primary healthcare needs. The natural plant products have negligible toxicities, if any, and are endowed with a multitude of phytoconstituents which are responsible for their holistic therapeutic action. Cognitive dysfunction is one of the major health problem in today's world, wherein the available synthetic chemotherapeutic modalities have proven to be non-absolute and, at times toxic in nature. In such a scenario, safer herbal alternative medicines play a vital role in managing the neurological etiologies. One such cognitive booster herb is C. prostratus Forssk., commonly known as Shankhpushpi, which is mainly endowed with neuroprotective, nootropic and neuro-modulatory activities (Figure 1B). Besides, it also possesses several other therapeutic properties, such as immunomodulatory, antimicrobial, antidiabetic and cardioprotective activities. The fundamental bioactive compounds responsible for the nootropic activities of this herb have been identified as 4'-methoxy kaempferol, 7-methoxy quercetin, convolamine, scopoletin, ceryl alcohol, β-sitosterol and hydroxy-cinnamic acid. Additionally, this herb did not exhibit any signs of toxicity and neurodegeneration up to a dose of 2000 mg/Kg in mice, thereby indicating its safety profile. A few initial clinical trials have conducted for CP, however, more detailed and controlled clinical trials are needed to establish and validate the neuro-pharmacological profile of C. prostratus. In addition, detailed mechanistic studies are yet to be executed to unravel the underlying mechanism of action for this cognition enhancing herb. Taken together, C. prostratus is likely to be the front runner for the clinical phyto-pharmaceutical status for treatment of neurological ailments.
AB conceived the presented research. PT analyzed the information, generated the artwork, and co-wrote the manuscript. AV investigated and supervised the findings of the work. AB and AV provided critical revision of this review article, and approved the manuscript for submission. All authors agreed with the final version of this manuscript.
The presented research work been funded by the research funds of Patanjali Research Foundation Trust (PRFT), Haridwar, India.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are grateful to Param Pujya Swami Ramdev Ji for institutional research facilities and supports. Authors gratefully acknowledge the efforts of Dr. Shivam Singh and Dr. Swami Narsingh Dev at Patanjali Research Institute, for their help in data collection and processing. We are also thankful to Mr. Gagan Kumar and Mr. Lalit Mohan for their swift administrative supports and encouragements.
Adams, M., Gmünder, F., Hamburger, M. (2007). Plants traditionally used in age related brain disorders—A survey of ethnobotanical literature. J. Ethnopharmacol. 113, 363–381. doi: 10.1016/j.jep.2007.07.016
Agarwa, P., Sharma, B., Fatima, A., Jain, S. K. (2014). An update on Ayurvedic herb Convolvulus pluricaulis Choisy. Asian Pac. J. Trop. Biomed. 4, 245–252. doi: 10.1016/S2221-1691(14)60240-9
Agarwal, P., Sharma, B., Alok, S. (2014). Screening of anti-inflammatory and anti-analgesic activity of Convolvulus pluricaulis choisy. Int. J. Pharm. Sci. Res. 5, 2458–2463. doi: 10.13040/IJPSR.0975-8232.5(6).2458-63
Aggarwal, B. B., Prasad, S., Reuter, S., Kannappan, R., R Yadav, V., Park, B., et al. (2011). Identification of novel anti-inflammatory agents from Ayurvedic medicine for prevention of chronic diseases: “reverse pharmacology“ and “bedside to bench” approach. Curr. Drug Targets 12, 1595–1653. doi: 10.2174/138945011798109464
Aleebrahim-Dehkordy, E., Tamadon, M. R., Nasri, H., Baradaran, A., Nasri, P., Beigrezaei, S. (2017). Review of possible mechanisms of analgesic effect of herbs and herbal active ingredient. J. Young Pharm. 9, 303–306. doi: 10.5530/jyp.2017.9.60
Amin, H., Sharma, R., Vyas, M., Prajapati, P. K., Dhiman, K. (2014). Shankhapushpi (Convolvulus pluricaulis Choisy): Validation of the Ayurvedic therapeutic claims through contemporary studies. Int. J. Green Pharm. 8, 193–200. doi: 10.4103/0973-8258.142666
Babu, N. G., Raju, T. P., Srinivasu, Ch. Ch., Ramanamam, V., Ram, S. S., Sudershan, M., et al. (2015). Estimation of elemental concentrations of Indian medicinal plants using Energy dispersive X-ray fluorescence (EDXRF). Int. J. Sci. Eng. Res. 6, 1379–1387. Available at https://www.ijser.org/paper/Estimation-of-elemental-concentrations-of-Indian-medicinal-plants-using-Energy-dispersive-X-ray-fluorescence.html
Balaji, K., Hean, K. C., Ravichandran, K., Shikarwar, M. (2014). In-Vitro Evaluation of Antioxidant Activity and Total Phenolic Content of Methanolic Extract of Convolvulus pluricaulis. Res. J. Pharm. Biol. Chem. Sci. 5, 959–964. doi: 10.1080/19390211.2018.1470126
Barar, F. S., Sharma, V. N. (1965). Preliminary pharmacological studies on Convolvulus pluricaulis chois–an Indian indigenous herb. Indian J. Physiol. Pharmacol. 9, 99–102. Available at https://www.ijpp.com/IJPP%20archives/1965_9_2/99-102.pdf
Basu, N. K., Dandiya, P. C. (1948). Chemical investigation of Convolvulus pluricaulis Chois. J Am. Pharm. Assoc. 37: 27–28. doi: 10.1002/jps.3030370108
Beretz, A., Cazenave, J. P. (1991). Old and new natural products as the source of modern antithrombotic drugs. Planta Med. 57, S68–S72. doi: 10.1055/s-2006-960232
Bhalerao, S. A., Verma, D. R., Teli, N. C., Trikannad, A. A. (2014). Ethnobotany, phytochemistry and pharmacology of Convolvulus pluricaulis, Choisy. Res. J. Pharm. Biol. Chem. Sci. 5, 629–636. Available at https://www.semanticscholar.org/paper/Ethnobotany%2C-Phytochemistry-and-Pharmacology-of-Bhalerao-Verma/62602df9affb6d3a5a27699015e1506aa34ef1bf
Bhowmik, D., Kumar, K., Paswan, S., Srivatava, S., Yadav, A., Dutta, A. (2012). Traditional Indian herb Convolvulus Pluricaulis and its medicinal importance. J. Pharmacogn. Phytochem. 1, 44–51. Available at http://www.phytojournal.com/vol1Issue1/Issue_may_2012/4.pdf
Bihaqi, S. W., Sharma, M., Singh, A. P., Tiwari, M. (2009). Neuroprotective role of Convolvulus pluricaulis on aluminium induced neurotoxicity in rat brain. J. Ethnopharmacol. 124, 409–415. doi: 10.1016/j.jep.2009.05.038
Bihaqi, S. W., Singh, A. P., Tiwari, M. (2012). Supplementation of Convolvulus pluricaulis attenuates scopolamine-induced increased tau and Amyloid precursor protein (AβPP) expression in rat brain. Indian J. Pharmacol. 44, 593–598. doi: 10.4103/0253-7613.100383
Billore, K. V., Yelne, M. B., Dennis, T. J., Chaudhari, B. G. (2005). Database on medicinal plants used in Ayurveda (Vol. 7) (New Delhi, India: Central Council for Research in Ayurveda & Siddha).
Brown, D. A. (2018). Regulation of neural ion channels by muscarinic receptors. Neuropharmacol. 136, 383–400. doi: 10.1016/j.neuropharm.2017.11.024
Carter, R. (2014). The human brain book: An illustrated guide to its structure, function, and disorders (London: Penguin publishers).
Chen, X., Pan, W. (2014). “The treatment strategies for neurodegenerative diseases by integrative medicine. ,”, vol. 1. Integr. Med. Int, 223–225. doi: 10.1159/000381546
Cheriyan, B. V., Sr., Kadhirvelu, P., Sr., Nadipelly, J., Jr., Shanmugasundaram, J., Sayeli, V., Sr., Subramanian, V., Sr. (2017). Anti-nociceptive effect of 7-methoxy coumarin from Eupatorium Triplinerve vahl (Asteraceae). Pharmacogn. Mag. 13, 81–84. doi: 10.4103/0973-1296.197650
Cowan, M. M. (1999). Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12, 564–582. doi: 10.1128/CMR.12.4.564
Daniel, M. (2016). “Medicinal plants: chemistry and properties,” (Boca Raton, Florida, United States: CRC press).
Dhingra, D., Valecha, R. (2007). Screening for antidepressant-like activity of Convolvulus pluricaulis Choisy in mice. Pharmacol. Online 1, 262–278. Available at https://www.semanticscholar.org/paper/SCREENING-FOR-ANTIDEPRESSANT-LIKE-ACTIVITY-OF-IN-Dhingra-Valecha/51168240f3c88647a52ce21a54878606c61a7c66
Domínguez-Avila, J., González-Aguilar, G., Alvarez-Parrilla, E., de la Rosa, L. (2016). Modulation of PPAR expression and activity in response to polyphenolic compounds in high fat diets. Int. J. Mol. Sci. 17, E1002 1002. doi: 10.3390/ijms17071002
Dubey, S. K., Singhvi, G., Krishna, K. V., Agnihotri, T., Saha, R. N., Gupta, G. (2018). Herbal medicines in neurodegenerative disorders: an evolutionary approach through novel drug delivery system. J. Environ. Pathol. Toxicol. 37, 199–208. doi: 10.1615/JEnvironPatholToxicolOncol.2018027246
Dwoskin, L. P., Crooks, P. A. (2002). A novel mechanism of action and potential use for lobeline as a treatment for psychostimulant abuse. Biochem. Pharmacol. 63, 89–98. doi: 10.1016/s0006-2952(01)00899-1
Foretz, M., Even, P., Viollet, B. (2018). AMPK activation reduces hepatic lipid content by increasing fat oxidation in vivo. Int. J. Mol. Sci. 19, E2826. doi: 10.3390/ijms19092826
Forrest, M. D. (2014). The sodium-potassium pump is an information processing element in brain computation. Front. Physiol. 5, 472–475. doi: 10.3389/fphys.2014.00472
Gaikwad, S. B., Krishna Mohan, G., Sandhya Rani, M. (2014). Phytochemicals for diabetes management. Pharm. Crop 5, 11–28. doi: 10.2174/2210290601405010011
Gapparov, A. M., Okhunov, I. I., Aripova, S. F., Nabiev, A., Khuzhaev, V. U. (2011). Derivatives of the alkaloid convolvine and their pharmacological activity. Chem. Nat. Compd. 47, 608–611. doi: 10.1007/s10600-011-0007-1
Gogte, V. M. (2012). Ayurvedic pharmacology & therapeutic uses of medicinal plants (Pune, India: Chaukhambha Sanskrit Sansthan).
Gourie-Devi, M. (2014). Epidemiology of neurological disorders in India: Review of background, prevalence and incidence of epilepsy, stroke, Parkinson's disease and tremors. Neurol. India 62, 588–598. doi: 10.4103/0028-3886
Gupta, M. B., Nath, R., Srivastava, N., Shanker, K., Kishor, K., Bhargava, K. P. (1980). Anti-inflammatory and antipyretic activities of β-sitosterol. Planta Med. 39, 157–163. doi: 10.1055/s-2008-1074919
Gupta, V. K. (2016). Traditional and folk herbal medicine: Recent researches (Vol. 3) (New Delhi, India: Daya Publishing House).
Gururaj, G., Girish, N., Isaac, M. K. (2005). Mental, neurological and substance abuse disorders: Strategies towards a systems approach. Report submitted to the National Commission of Macroeconomics and Health (Government of India, New Delhi: Ministry of Health and Family Welfare).
Hindu, V. (2012). Shanka Pushpi: A short review. Int. Res. J. Pharm. 3, 81–83. Available at https://irjponline.com/admin/php/uploads/794_pdf.pdf
Hoang, M. H., Jia, Y., Mok, B., Jun, H. J., Hwang, K. Y., Lee, S. J. (2015). Kaempferol ameliorates symptoms of metabolic syndrome by regulating activities of liver X receptor-β. J. Nutr. Biochem. 26, 868–875. doi: 10.1016/j.jnutbio.2015.03.005
Indo, Y. (2018). NGF-dependent neurons and neurobiology of emotions and feelings: Lessons from congenital insensitivity to pain with anhidrosis. Neurosci. Biobehav. Rev. 87, 1–16. doi: 10.1016/j.neubiorev.2018.01.013
Islam, S. M. S., Purnat, T. D., Phuong, N. T. A., Mwingira, U., Schacht, K., Fröschl, G. (2014). Non-Communicable Diseases (NCDs) in developing countries: a symposium report. Global. Health 10, 81–88. doi: 10.1186/s12992-014-0081-9
Jain, R., Pancholi, B., Jain, S. C. (2011). Radical scavenging and antimicrobial activities of Convolvulus microphyllus. Asian J. Chem. 23, 4591–4594. Available at https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/1109757
Jain, A., Kaur, J., Bansal, Y., Saini, B., Bansal, G. (2017). WHO guided real time stability testing on Shankhpushpi Syrup. J. Adv. Pharm. Technol. Res. 5, 1–19. doi: 10.15415/jptrm.2017.51001
Jalwal, P., Singh, B., Dahiya, J., Khokhara, S. (2016). A comprehensive review on shankhpushpi a morning glory. Pharma Innov. 5, 14–18. Available at http://www.thepharmajournal.com/archives/?year=2016&vol=5&issue=1&ArticleId=695
Johnson, C. D., Barlow-Anacker, A. J., Pierre, J. F., Touw, K., Erickson, C. S., Furness, J. B., et al. (2018). Deletion of choline acetyltransferase in enteric neurons results in postnatal intestinal dysmotility and dysbiosis. FASEB J. 32, 4744–4752. doi: 10.1096/fj.201701474RR
Katewa, S. S., Jain, A. (2006). Traditional folk herbal medicines (Udaipur, India: Apex Publishing House).
Kaushik, R. (2017). Studying the pharmacological basis of an antiepileptic ayurvedic formulation-Sarasvata churna. Int. J. Green Pharm. 11, 62–68. Available at https://pdfs.semanticscholar.org/1dc0/39b0046a0caa7e211b7a64f0ec91596d4fef.pdf
Kulkarni, R., Girish, K. J., Kumar, A. (2012). Nootropic herbs (Medhya Rasayana) in Ayurveda: an update. Pharmacogn. Rev. 6, 147–153. doi: 10.4103/0973-7847.99949
Lal, B. (2014). Development of high performance thin layer chromatography method for the determination of scopolin in convolvulus pluricaulis chois. PharmaTutor Mag. 2 (11), 77–83. Available at https://www.pharmatutor.org/articles/development-high-performance-thin-layer-chromatography-method-for-determination-scopolin-convolvulus-pluricaulis-chois?page=1%2C1
Lopez, A. D., Murray, C. C. (1998). The global burden of disease 1990–2020. Nat. Med. 4, 1241–1243. doi: 10.1038/3218
Mali, P. C. (1995). Hypolipidemic effect of Convolvulus microphyllus in cholesterol fed gerbils (Meriones hurrinae Jerdon). J. Phytol. Res. 8, 153–155. Available at https://www.researchgate.net/publication/266140204_Hypolipidemic_effect_of_Convolvulus_microphyllus_in_cholesterol_fed_gerbils_Meriones_hurrinae_Jerdon
Malik, J., Karan, M., Vasisht, K. (2011). Nootropic, anxiolytic and CNS-depressant studies on different plant sources of shankhpushpi. Pharm. Biol. 49, 1234–1242. doi: 10.3109/13880209.2011.584539
Malik, S., Ahmad, S., Sadiq, A., Alam, K., Wariss, H. M., Ahmad, I., et al. (2015). A comparative ethno-botanical study of Cholistan (an arid area) and Pothwar (a semi-arid area) of Pakistan for traditional medicines. J. Ethnobiol. Ethnomed. 11, 31. doi: 10.1186/s13002-015-0018-2
Malik, J., Karan, M., Vasisht, K. (2016). Attenuating effect of bioactive coumarins from Convolvulus pluricaulis on scopolamine-induced amnesia in mice. Nat. Prod. Res. 30, 578–582. doi: 10.1080/14786419.2015.1025398
Mirzaev, Y. R., Aripova, S. F. (1998). ). Neuro-and psychopharmacological investigation of the alkaloids convolvine and atropine. Chem. Nat. Compd. 34, 56–58. doi: 10.1007/BF02249687
Mishra, S. H., Sethiya, N. K. (2010). Review on ethnomedicinal uses and phytopharmacology of memory boosting herb ‘Convolvulus pluricaulis' Choisy. Aust. J. Med. Herb. 22 (1), 19–25. Available at https://www.semanticscholar.org/paper/Review-on-ethnomedicinal-uses-and-of-memory-herb-Sethiya-Mishra/427692942318bd7bf5c9cd65e2cf8627aaa9c61f
Mudgal, V. (1975). Studies on medicinal properties of Convolvulus pluricaulis and Boerhaavia diffusa. Planta Med. 28, 62–68. doi: 10.1055/s-0028-1097830
Nagarathna, P. K. M., Jha, D. K. (2013). Study on antithyroid property of some herbal plants. Int. J. Pharm. Sci. Rev. Res. 23, 203–211. Available at http://globalresearchonline.net/journalcontents/v23-2/36.pdf
Nam, H., Kim, M. M. (2015). Scopoletin has a potential activity for anti-aging via autophagy in human lung fibroblasts. Phytomedicine 22, 362–368. doi: 10.1016/j.phymed.2015.01.004
Nasri, H., Shirzad, H., Baradaran, A., Rafieian-Kopaei, M. (2015). Antioxidant plants and diabetes mellitus. J. Res. Med. Sci. 20, 491–502. doi: 10.4103/1735-1995.163977
Nisar, S. S., Khan, A. A., Maaz, M., Shiffa, M. (2012). Review of sankhahauli (Convolvulus pluricaulis choisy) from traditional medicine to modern science. Int. J. Institut. Pharm. Life Sci. 2, 94–101. Available at https://www.semanticscholar.org/paper/REVIEW-OF-SANKHAHAULI-(CONVOLVULUS-PLURICAULIS-FROM-Nisar-Khan/0994b296aed35c7b9ccaa97be1505fddf55218e8
Nolte, J. (1999). The human brain: an introduction to its functional anatomy (No. 798) (United States: Mosby Inc.).
Olakkaran, S., Antony, A. (2017). Convolvulus pluricaulis (Shankhapushpi) ameliorates human microtubule-associated protein tau (hMAPτ) induced neurotoxicity in Alzheimer's disease Drosophila model. J. Chem. Neuroanat. 95, 115–122. doi: 10.1016/j.jchemneu.2017.10.002
Pan, R., Dai, Y., Gao, X., Xia, Y. (2009). Scopolin isolated from Erycibe obtusifolia Benth stems suppresses adjuvant-induced rat arthritis by inhibiting inflammation and angiogenesis. Int. Immunopharmacol. 9, 859–869. doi: 10.1016/j.intimp.2009.02.019
Park, C., Moon, D. O., Rhu, C. H., Choi, B. T., Lee, W. H., Kim, G. Y., et al. (2007). β-Sitosterol induces anti-proliferation and apoptosis in human leukemic U937 cells through activation of caspase-3 and induction of Bax/Bcl-2 ratio. Biol. Pharm. Bull. 30, 1317–1323. doi: 10.1248/bpb.30.1317
Park, G., Kusuma, I. W., Kim, Y. U. (2018). Multiple Bioactivities of Traditional Medicinal Herbs for Treatment of Neurodegenerative Diseases. Evid. Based Complement. Alternat. Med. ,2018, 1–2. doi: 10.1155/2018/3075458
Patel, K., Jain, A., Patel, D. K. (2013). Medicinal significance, pharmacological activities, and analytical aspects of anthocyanidins ‘delphinidin': A concise report. J. Acute Dis. 2, 169–178. doi: 10.1016/S2221-6189(13)60123-7
Prats, E., Bazzalo, M. E., León, A., Jorrín, J. V. (2006). Fungitoxic effect of scopolin and related coumarins on Sclerotinia sclerotiorum - A way to overcome sunflower head rot. Euphytica 147, 451–460. doi: 10.1007/s10681-005-9045-8
Quintans Júnior, L. J., Almeida, J. R., Lima, J. T., Nunes, X. P., Siqueira, J. S., Oliveira, L. E. G. D., et al. (2008). Plants with anticonvulsant properties: a review. Rev. Bras. Farmacogn. 18, 798–819. doi: 10.1590/S0102-695X2008000500026
Rachitha, P., Krupashree, K., Jayashree, G. V., Kandikattu, H. K., Amruta, N., Gopalan, N., et al. (2018). Chemical composition, antioxidant potential, macromolecule damage and neuroprotective activity of Convolvulus pluricaulis. J. Tradit. Complement. Med. 8, 483–496. doi: 10.1016/j.jtcme.2017.11.002
Ratha, K. K., Mishra, S. S. (2012). Anticonvulsant activity of Shankhapuspi (Convolvulus pluricaulis Chois) on Strychnine induced seizure in experimental animals. Int. J. Ayurvedic Med. 3, 82–87. Available at https://www.ijam.co.in/index.php/ijam/article/view/163
Rathee, P., Chaudhary, H., Rathee, S., Rathee, D., Kumar, V., Kohli, K. (2009). Mechanism of action of flavonoids as anti-inflammatory agents: a review. Inflamm. Allergy Drug Targets 8, 229–235. doi: 10.2174/187152809788681029
Ravichandra, V. D., Ramesh, C., Sridhar, K. A. (2013). Hepatoprotective potentials of aqueous extract of Convolvulus pluricaulis against thioacetamide induced liver damage in rats. Biomed. Aging Pathol. 3, 131–135. doi: 10.1016/j.biomag.2013.06.005
Rawat, M. S. M., Kothiyal, P. (2011). Comparative nootropic effect of Evolvulus alsinoides and Convolvulus pluricaulis. Int. J. Pharma. Bio. Sci. 2, 616–621. Available at https://ijpbs.net/abstract.php?article=NTk2
Ray, S., Ray, A. (2015). Medhya rasayanas in brain function and disease. Med. Chem. 5, 505–511. doi: 10.4172/2161-0444.1000309
Rizk, A. M., Williamson, E. M., Evans, F. J. (1985). Constituents of Plants Growing in Qatar VII An examination of Certain Plants for Anti-Inflammatory Activity. Int. J. Crude Drug Res. 23, 1–4. doi: 10.3109/13880208509070677
Rizwan, M., Khan, A. A. (2014). Assessment of efficacy of Sankhahuli (Convolvulus pluricaulis Chois.) and gokhru (Tribulus terrestris L.) in the management of hypertension. Indian J. Trad. Know. 13, 313–318. Available at http://nopr.niscair.res.in/handle/123456789/27919
Saraiva, C., Praça, C., Ferreira, R., Santos, T., Ferreira, L., Bernardino, L. (2016). Nanoparticle-mediated brain drug delivery: overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 235, 34–47. doi: 10.1016/j.jconrel.2016.05.044
Saroya, A. S., Singh, J. (2018). Phytopharmacology of Indian Nootropic Convolvulus pluricaulis, in Pharmacotherapeutic Potential of Natural Products in Neurological Disorders. (Singapore:Springer), 141–144. doi: 10.1007/978-981-13-0289-3_13
Sethiya, N. K., Nahata, A., Mishra, S. H., Dixit, V. K. (2009). An update on Shankhpushpi, a cognition-boosting Ayurvedic medicine. J. Chin. Integr. Med. 7, 1001–1022. doi: 10.3736/jcim20091101
Sethiya, N. K., Trivedi, A., Patel, M. B., Mishra, S. H. (2010). Comparative pharmacognostical investigation on four ethanobotanicals traditionally used as Shankhpushpi in India. J. Adv. Pharm. Technol. Res. 1, 388–395. doi: 10.4103/0110-5558.76437
Sethiya, N. K., Raja, M. M. M., Mishra, S. H. (2013). Antioxidant markers based TLC-DPPH differentiation on four commercialized botanical sources of Shankhpushpi (A Medhya Rasayana): A preliminary assessment. J. Adv. Pharm. Technol. Res. 4, 25–30. doi: 10.4103/2231-4040.107497
Shalam, M. D., Shantakumar, S. M., Narasu, M. L. (2007). Pharmacological and biochemical evidence for the antidepressant effect of the herbal preparation Trans-01. Indian J. Pharmacol. 39 (5), 231–234. doi: 10.4103/0253-7613.37273
Siddiqui, N. A., Ahmad, N., Musthaq, N., Chattopadhyaya, I., Kumria, R., Gupta, S. (2014). Neuropharmacological profile of extracts of aerial parts of Convolvulus pluricaulis Choisy in mice model. Open Neurol. J. 8, 11–14. doi: 10.2174/1874205X01408010011
Singh, V. K., Govil, J. N., Hashmi, S., Singh, G. (2003). Recent progress in medicinal plants: Ethnomedicine & pharmacognosy II (Vol. 7) (Texas, USA: Studium Press).
Solanki, I., Parihar, P., Parihar, M. S. (2016). Neurodegenerative diseases: from available treatments to prospective herbal therapy.Neurochem. Int. 95, 100–108. doi: 10.1016/j.neuint.2015.11.001
Song, M. X., Deng, X. Q. (2018). Recent developments on triazole nucleus in anticonvulsant compounds: a review. J. Enzyme Inhib. Med. Chem. 33, 453–478. doi: 10.1080/14756366.2017.1423068
Srinivas, T. L., Lakshmi, S. M., Shama, S. N., Reddy, G. K., Prasanna, K. R. (2013). Medicinal plants as anti-ulcer agents. J. Pharmacogn. Phytochem. 2, 91–97. Available at http://www.phytojournal.com/archives/2013/vol2issue4/PartB/23.1.pdf
Subramani, R., Anand, M., Muralidharan, P. (2013). Effect of Convolvulus pluricaulis Choisy in obsessive compulsive disorder using animal models (India: PharmaTutor EduLabs Publishing).
Sultana, S., Ali, M., Mir, S. R., Iqbal, D. (2018). Isolation and characterization of glycosides from Convolvulus prostratus, Ficus virens, Phoenix dactifera, Spondias mangifera and Terminalia belerica. Eur. J. Pharm. Med. Res. 5, 310–318. Available at https://pdfs.semanticscholar.org/696f/2e4d7280727d2baba08bc7c6e3a7cd03ef3a.pdf
Sun, H., Wang, L., Zhang, B., Ma, J., Hettenhausen, C., Cao, G., et al. (2014). Scopoletin is a phytoalexin against Alternaria alternata in wild tobacco dependent on jasmonate signalling. J. Exp. Bot. 65, 4305–4315. doi: 10.1093/jxb/eru203
Swain, S. S., Rout, K. K., Chand, P. K. (2012). Production of triterpenoid anti-cancer compound taraxerol in Agrobacterium-transformed root cultures of butterfly pea (Clitoria ternatea L.). Appl. Biochem. Biotechnol. 168, 487–503. doi: 10.1007/s12010-012-9791-8
Tognolini, M., Barocelli, E., Ballabeni, V., Bruni, R., Bianchi, A., Chiavarini, M., et al. (2006). Comparative screening of plant essential oils: phenylpropanoid moiety as basic core for antiplatelet activity. Life Sci. 78, 1419–1432. doi: 10.1016/j.lfs.2005.07.020
Tonda-Turo, C., Origlia, N., Mattu, C., Accorroni, A., Chiono, V. (2018). Current Limitations in the Treatment of Parkinson's and Alzheimer's Diseases: State-of-the-Art and Future Perspective of Polymeric Carriers. Curr. Med. Chem. 25, 5755–5771. doi: 10.2174/0929867325666180221125759
Trivedi, P. C. (2009). Medicinal plants: Utilization and conservation. 2nd (Jaipur, India: Aavishkar Publishers).
Tseomashko, N. E., Terent'Eva, E. O., Kodirova, D. B., Okhunov, I. I., Aripova, S. F., Khashimova, Z. S., et al. (2013). Synthesis of convolinine and cytotoxic activity of alkaloids of the genus Convolvulus and their derivatives. Chem. Nat. Compd. 48, 1039–1041. doi: 10.1007/s10600-013-0459-6
Vinholes, J., Silva, B. M., Silva, L. R. (2015). “Hydroxycinnamic acids (HCAS): Structure, biological properties and health effects,” in Advances in Medicine and Biology. Ed. Berhardt , LV (India: Nova Science Publishers), 1–5.
Vossel, K. A., Zhang, K., Brodbeck, J., Daub, A. C., Sharma, P., Finkbeiner, S., et al. (2010). Tau reduction prevents Aβ-induced defects in axonal transport. Science 330, 198–198. doi: 10.1126/science.1194653
Wang, S. B., Jang, J. Y., Chae, Y. H., Min, J. H., Baek, J. Y., Kim, M., et al. (2015). Kaempferol suppresses collagen-induced platelet activation by inhibiting NADPH oxidase and protecting SHP-2 from oxidative inactivation. Free Radic. Biol. Med. 83, 41–53. doi: 10.1016/j.freeradbiomed.2015.01.018
Wei, C. X., Bian, M., Gong, G. H. (2015). Current research on antiepileptic compounds. Molecules 20, 20741–20776. doi: 10.3390/molecules201119714
Xing, N., Chen, Y., Mitchell, S. H., Young, C. Y. (2001). Quercetin inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Carcinogenesis 22, 409–414. doi: 10.1093/carcin/22.3.409
Zingue, S., Foyet, H. S., Djiogue, S., Ezo'o Ezo'o, Y., Abaïssou, H. H. N., Fachagbo, P., et al. (2018). Effects of Ficus umbellata (Moraceae) Aqueous Extract and 7-Methoxycoumarin on Scopolamine-Induced Spatial Memory Impairment in Ovariectomized Wistar Rats. Behav. Neurol. 2018, 5751864. doi: 10.1155/2018/5751864
Keywords: Convolvulus prostratus, Shankhpushpi, natural product, Ayurveda, neuroprotective, nootropic
Citation: Balkrishna A, Thakur P and Varshney A (2020) Phytochemical Profile, Pharmacological Attributes and Medicinal Properties of Convolvulus prostratus – A Cognitive Enhancer Herb for the Management of Neurodegenerative Etiologies. Front. Pharmacol. 11:171. doi: 10.3389/fphar.2020.00171
Received: 16 September 2019; Accepted: 07 February 2020;
Published: 03 March 2020.
Edited by:
Valentina Echeverria Moran, Bay Pines VA Healthcare System, United StatesReviewed by:
Sefirin Djiogue, University of Yaounde I, CameroonCopyright © 2020 Balkrishna, Thakur and Varshney. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anurag Varshney, YW51cmFnQHByZnQuY28uaW4=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.