- 1Department of Internal Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 2Pharmaceutical Sciences Research Center, Health Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 3Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences, Sofia, Bulgaria
- 4Departamento de Ciencias del Ambiente, Facultad de Química y Biología, Universidad de Santiago de Chile, Santiago, Chile
Background: Pulmonary hypertension (PH) is a progressive disease that is associated with pulmonary arteries remodeling, right ventricle hypertrophy, right ventricular failure and finally death. The present study aims to review the medicinal plants and phytochemicals used for PH treatment in the period of 1994 – 2019.
Methods: PubMed, Cochrane and Scopus were searched based on pulmonary hypertension, plant and phytochemical keywords from August 23, 2019. All articles that matched the study based on title and abstract were collected, non-English, repetitive and review studies were excluded.
Results: Finally 41 studies remained from a total of 1290. The results show that many chemical treatments considered to this disease are ineffective in the long period because they have a controlling role, not a therapeutic one. On the other hand, plants and phytochemicals could be more effective due to their action on many mechanisms that cause the progression of PH.
Conclusion: Studies have shown that herbs and phytochemicals used to treat PH do their effects from six mechanisms. These mechanisms include antiproliferative, antioxidant, antivascular remodeling, anti-inflammatory, vasodilatory and apoptosis inducing actions. According to the present study, many of these medicinal plants and phytochemicals can have effects that are more therapeutic than chemical drugs if used appropriately.
Introduction
A Brief Overview of Lung Circulatory System
The lung is a metabolic organ with unique circulatory system. It has two types of blood circulation: 1) pulmonary circulation that is responsible for gas exchange and 2) bronchial circulation (called pulmonary collateral circulation, systemic circulation) that carries oxygenated blood to the bronchi and trachea, extra pulmonary and intra pulmonary airways, nerves and lymph nodes. Pulmonary arteries have thinner walls without basal tone and with less vascular smooth muscle compared to other arteries. Factors that affect lung circulation are vascular structure, hormonal and neuronal agents and oxygen uptake from the environment. The most important function of the pulmonary circulation is gas exchange to provide blood-containing oxygen for organs consumption. CO2-rich blood from the right atrium and right ventricle and the pulmonary artery is transferred to the lung for gas exchange; then oxygen-rich blood is transferred via pulmonary vein to the left atrium and left ventricle and then delivered to the body through the aortic artery. Bronchial circulation provides oxygen and nutrients for airway wall and inflammatory cells needed for immune response in airway mucosa, and cleaning the airway from inflammatory mediators and infected particles (Suresh and Shimoda, 2016).
Pulmonary Hypertension: General Definition and Epidemiology
Pulmonary hypertension (PH) is a disease associated with increase in pulmonary vasculature resistance due to increase in vascular tone and remodeling the structure of pulmonary arteries (PAs). PH occurs predominantly in women (80%), over the mean age of 53 years (Simonneau et al., 2013). It has been reported about 100 million people around the world suffering from PH (Hui-li, 2011). Approximately every untreated patient with PH could survive for about 2.8 years. Some of the PH symptoms include permanent hypoxia, inflammation of lungs, oxidative stress, increase of proliferation of endothelial cells in pulmonary arteries and inhibition of apoptosis, which leads to pulmonary vascular remodeling (Schermuly et al., 2011).
One of the results of PH is an increase in the volume of pumped blood from the heart and increased pulmonary vasoconstriction to compensate the lack of oxygen in the lungs (Reyes et al., 2018); when blood volume increased, it leads to increased right ventricular pressure and right ventricular hypertrophy and finally leads to right ventricular failure (Julian and Mirsalimi, 1992). Increase the pulmonary arterial pressure leads to right ventricle hypertrophy (RV/TV ratios) and increase circulating blood volume of the lungs, compared to normal conditions. Rapid blood flow moving through the lungs vasculature causes disturbance in O2 and CO2 exchange (Powell et al., 1985).
The most important indexes for PH are fatigue and dyspnea during walking or moderate physical activity. The inability to do daily functions in these people leads to decrease their quality of life. PH is difficult to diagnose because of its similarities of symptoms to the other cardiopulmonary disease; as a result, the disease progresses and leads to increased mortality (Benza et al., 2010). The final diagnosis is through clinical assays for the detection of hemodynamic abnormalities. In most patients that suffering from PH, right ventricular hypertrophy is seen in the electrocardiogram and pulmonary function tests, confirm a decrease in lung capacity (Galie et al., 2009).
Classification and Etiology
PH is classified into five groups based on its etiology: i) Hypertension in the pulmonary arteries that lead to pulmonary arterial hypertension (PAH); ii) Left-sided heart failure that leads to PH; iii) Hypoxic pulmonary vasoconstriction that leads to PH; iv) High pressure in the blood vessels of the lungs that leads to chronic thromboembolic pulmonary hypertension (CTEPH), and v) Idiopathic PH (unknown reason).
Many studies suggested the effect of various factors such as high altitude, low temperatures, intense light, air pollution, type of nutrition, oxidative stress and gene mutations on the progression of PH (Baghbanzadeh and Decuypere, 2008). Based on these studies, the use of antioxidant compounds could be effective in treatment and control of PH (Wong et al., 2013).
Approximately 50% of patients suffer from idiopathic PAH. Diverse mutations are responsible for PH including ALK-I, BMPR2, endoglin, and CAV-I (Simonneau et al., 2013) too. Studies have proven the effects of nutrition on the progression of PH, such as calorie intake, amount of minerals and pure proteins (Khajali and Fahimi, 2010). The cellular and molecular reasons presented bellow could explain occurrence of PH.
Cellular/Molecular Changes during PH Progression
Some of the most important cellular and molecular changes during PH include smooth muscle cells remodeling, uncontrolled proliferation of endothelial cells, inflammation and the presence of proinflammatory cells, platelet aggregation and thrombosis (Humbert et al., 2004).
Smooth Muscle Cells and Remodeling
During the progression of PH, smooth muscle cells are remodeling to small peripheral, pulmonary arteries inside the respiratory acinus are observed; in other words, increase of smooth muscle cells of the distal pulmonary arteries, also known as pathological remodeling. The cellular process of this mechanism is incompletely understood. Anyway, during the process, a layer of myofibroblasts and extracellular matrix are created between the endothelium and internal elastic lamina (Stenmark et al., 2002). The first mechanism of fibroblast to media migration is increased expression of matrix metalloproteinases (MMP2 and MMP9). In summary, all the mechanisms involved in these processes lead to increase the thickness and obstruction of the pulmonary arteries (Davie et al., 2004).
Uncontrolled Proliferation of Endothelial Cells
All of the reasons for abnormal proliferation of endothelial cells occur on genetic background, but some of them are due to oxidative stress, hypoxia conditions, inflammation, allergy of drugs or toxins. It has been proven that patients with idiopathic PAH have defects in growth-suppressive genes such as BAX and TGF-β (Yeager et al., 2001). Approximately 30% of lesions, a mutation in the TGF-βR2 gene and 90% inhibition of expression of TGF-βR2 protein were observed (Yeager et al., 2001). Studies show that vasculotropic viruses such as human herpesvirus 8 infection could be effective in idiopathic PAH by changing the signaling pathways involved in endothelial cells proliferation (Cool et al., 2003). TGF-β1 is an important factor in the process of PH that is associated with endothelial injury and vascular remodeling. Studies suggest that TGF-β1 stimulates the production of ET-1 and that leads to increase of the smooth muscle cells proliferation (Star et al., 2009). Also, TGF-β1 initiates endothelium-smooth muscle transformation (Masszi et al., 2004).
Inflammation and Proinflammatory Cells
Inflammatory factors are the important ones in the progression of PH of human and animal models (Dorfmüller et al., 2003). In some patients, the suppression of the immune system shows positive results in reduction of the inflammation (Dorfmüller et al., 2003). Also, it has been reported that there are elevation of proinflammatory cytokines (IL-1 and IL-6) levels in patients with idiopathic PH. Histological studies of the lungs confirmed the presence of macrophages and lymphocytes and increased expression of chemokines CCL5 and fractalkine in severe PH (Balabanian et al., 2002).
Platelets Aggregation and Thrombosis
A notable mechanism in PH progression is thrombosis lesions due to platelet aggregation. This pathway can be initiated by endothelial cells, inflammatory mediators or by the platelets. Blood clot formation in PH patients is characterized by increase of fibrinopeptide A- and D-dimers levels in the plasma. Current studies show that stress itself and pulmonary vascular injury can initiate the process. Other evidence suggests that vascular abnormalities in PH leads to release of procoagulant, vasoactive, and mitogenic mediators by platelets. Some contributing factors in pulmonary vasoconstriction like serotonin, TXA2, TGF-β, AGEPC, PDGF and VPF can be store and release by platelet (Herve et al., 2001).
Animal Models
Briefly, animal models used in PH studies are divided into several groups according to the type of induction: hypoxia induces PH in mice and rats (Rabinovitch et al., 1979) and various types of cattle with genetic background (Shirley et al., 2008); fawn-hooded rat (FHR) that spontaneously develop PH in mild hypoxia conditions (Nagaoka et al., 2001); monocrotaline that induces PH by increased inflammation in mice and rats (Kay et al., 1967), genetically pulmonary hypertensive rats and mice that develop PH by loss-of-function mutation of BMPR2 gene in smooth muscle (Beppu et al., 2004).
Restrictions of Current Treatments of PH
Some of the commonly used drugs for the treatment of PH are phosphodiesterase-5 inhibitors such as sildenafil (Pepke-Zaba et al., 2008), prostacyclin analogs such as epoprostenol (Sitbon et al., 2002), and antagonists of endothelin receptor such as bosentan (McLaughlin et al., 2006). Some of the effective oral medications include anticlotting drugs, urine-enhancing drugs, oxygen-enhancing drugs, vasorelaxant drugs and calcium channel inhibitors. These treatments improve the quality of life during the first 3 years, but there is no cure for improvement of patient's quality of life for 5 years (Waxman and Zamanian, 2013). Also, these cures have not been completely successful because of side effects or incomplete efficacy (Humbert et al., 2004). Due to the important role of ROS and oxidative stress in PH progression, the intake of antioxidant compounds, which aims to reduce oxidative damage, is one of the notable issues in the treatment of PH (Wong et al., 2013).
Methods
Study Design
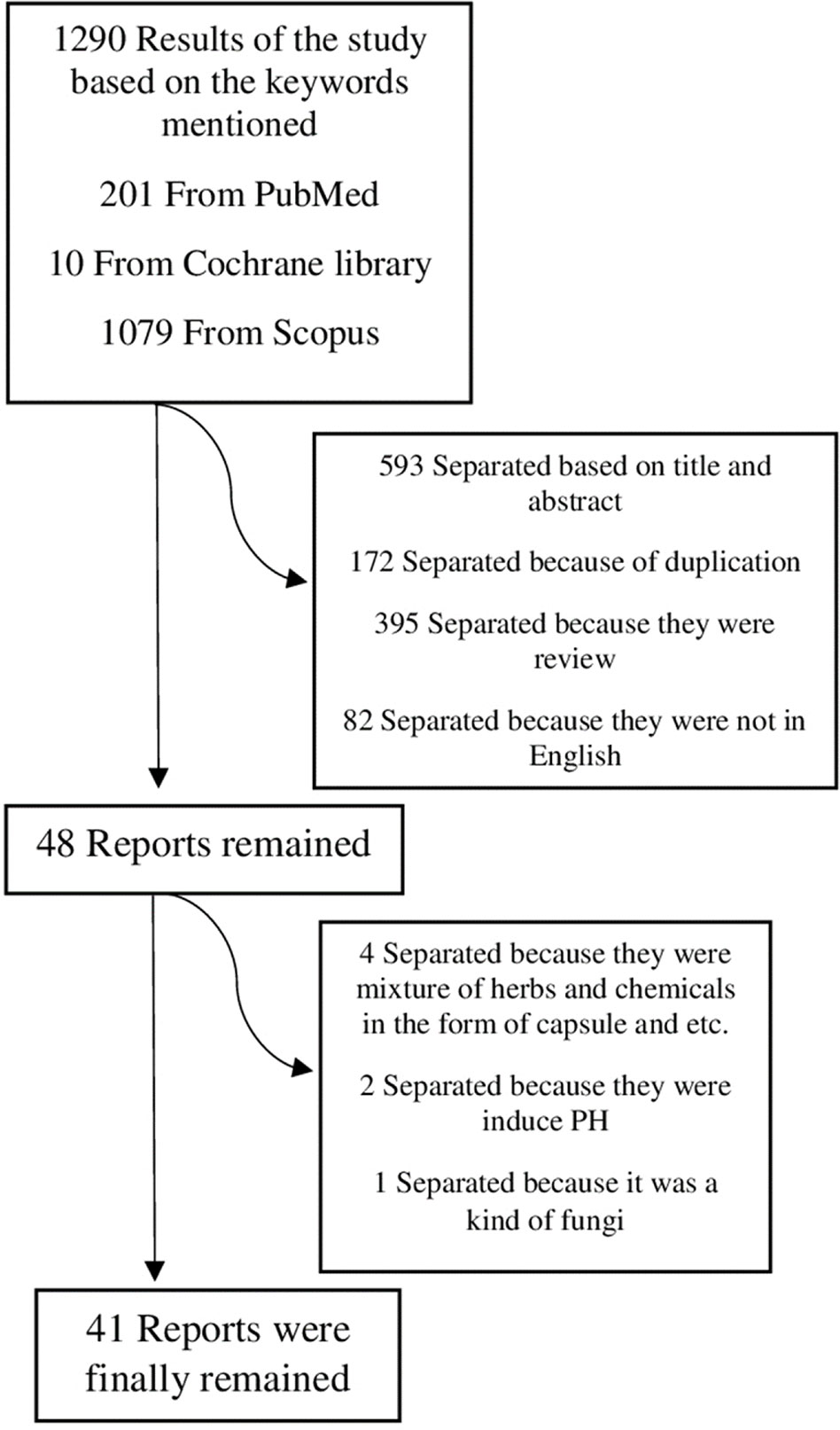
Electronic databases used in this review study were PubMed, Scopus, and Cochrane library. The keywords used were ‘pulmonary hypertension' in the title/abstract and ‘plant', ‘herb', ‘phytochemical' in the whole text. The period of articles reviewed is between 1979 and 2019. The primary search was performed by two researchers separately, and unrelated articles were separated based on their title and abstract. In the present study, both clinical and animal studies were included in the search but the number of clinical trials were very limited, and therefore the number of the results from the Cochrane library are fewer than these from Scopus and PubMed. Unrelated articles based on title and abstract, non-English and duplicate ones were excluded in the first step, then remaining articles were reviewed in full text. In the second step, several articles were excluded based on the full text. Study design diagram is available in the results section.
Data Extraction
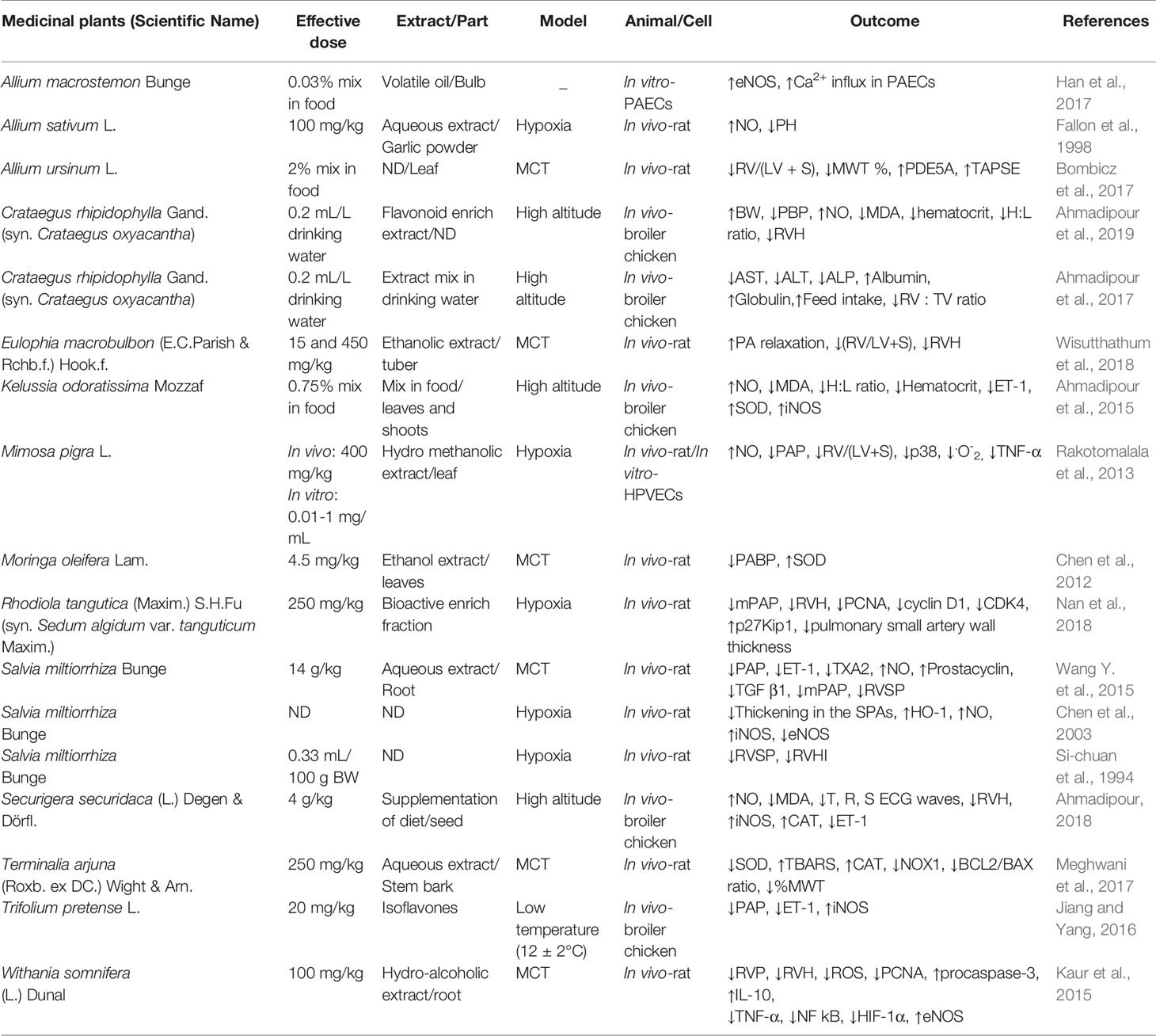
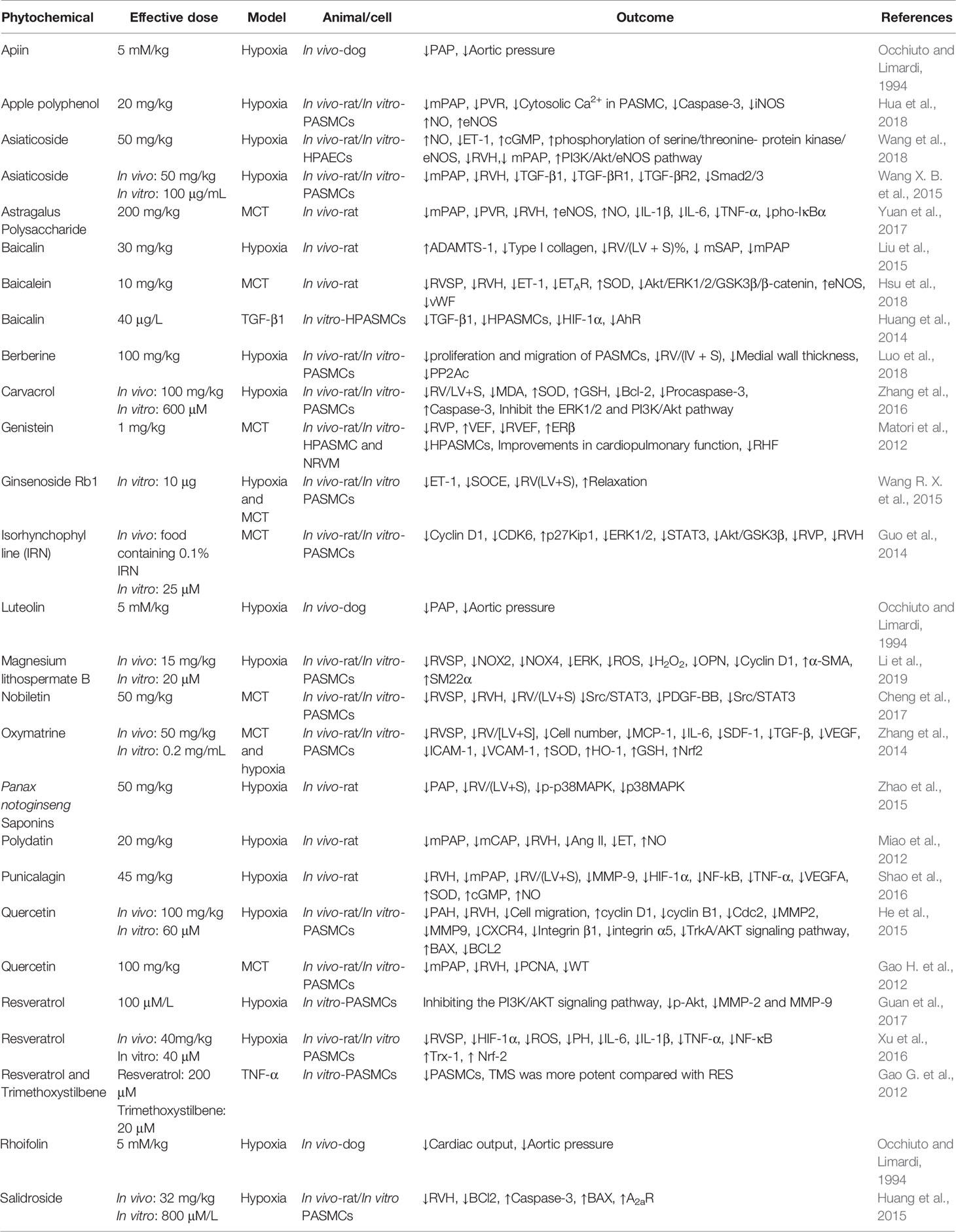
All data were extracted and studied by two researchers and were summarized in Tables 1 and 2. Abstract information of articles, including the name of the plant and the category of phytochemicals, effective dose, the type of PH model, and outcomes of the treatments, were categorized in Tables 1 and 2. The ↑ and ↓ signs show significant increase and significant decrease respectively of the evaluated factors during the mentioned studies.
Results
Study Selection and Characteristics
From 1290 studies, 172 studies were separated because of duplication; 395 studies were separated because they were reviews; 593 studies were separated based on their title and abstract; 82 studies were separated because they had not full text in English. From 48 remaining reports, 4 studies were separated because they were mixture of herbs and chemical compounds in the form of capsule; 2 studies were separated because they were induced PH and 1 study was separated because it was for fungus species (not plant). Figure 1 shows the method of the study and criteria for article separation. The general information of the articles were summarized in Table 1 and 2.
Medicinal Plants used for PH Treatment
Allium sativum L.
Allium sativum L. (Garlic) is a herbal remedy used in traditional medicine for treatment of a wide range of diseases. The main constituents of A. sativum that are responsible for its medicinal actions are sulfur-containing compounds including crysteine sulfoxides. Alliin is part of this group, which could be transformed into thiosulfinates (Reuter et al., 1996; Horníčková et al., 2010).
Some of the therapeutic actions of A. sativum such as lowering blood glucose levels, reducing oxidative stress, anticancer, reducing inflammation, immune system enhancer, anti-infectious diseases and cardiovascular protective were demonstrated (Lanzotti et al., 2014). There is evidence suggesting that A. sativum could relax vascular smooth muscles, to increase the activity of eNOS, and to hyperpolarize the smooth muscle cell membrane. Fallon et al. (1998) demonstrated that aqueous extract of A. sativum powder could prevent the progression of PH induced by hypoxia in rats due to its vasorelaxant activity.
Allium macrostemon Bunge
Allium macrostemon Bunge is a member of Amaryllidaceae family and used in traditional Chinese medicine with many pharmacological activities: antitumor, antioxidant, antiasthmatic (due to its ability to relax the bronchial smooth muscle, reducing blood perfusion and the mean PAs pressure), enhancing immune system (Tan et al., 2012). Dimethyl disulfide (DMDS) is the main constituent of A. macrostemon (34.93%) that possesses many biological activities such as regulation of melanin formation, anti-inflammatory and anti-hypertensive, and therefore it is used for treatment of myocardial ischemia (Chu et al., 2017).
The study of Han et al. (2017) demonstrated that use of volatile oil of A. macrostemon in PAECs causes increase eNOS expression and serine 1177 phosphorylation. Serine 1177 is a residue for phosphorylation of eNOS in PKA, Akt, and Calmodulin processes (Fleming, 2010). Also, volatile oil of A. macrostemon induces relaxation in PAs by activation of intracellular Ca2+/PKA/eNOS signaling pathway. Therefore, volatile oil of A. macrostemon and its active compound DMDS can improve PH by activation Ca2+/PKA/eNOS signaling pathway in PAECs (Han et al., 2017).
Allium ursinum L.
Allium ursinum L. (Wild garlic or bear's garlic), a member of Amaryllidaceae family, is a native plant of Eurasia. It is widely used in traditional medicine (Sobolewska et al., 2015). A. ursinum produce a variety of chemical structures responsible for its biological activities. One of the main compounds are flavonoid glycosides and sulfur constituents responsible for its garlic-like fragrance. Some of the A. ursinum compounds like galactolipids and phytosterols are species-specific (Oszmianski et al., 2012). Some of the most important pharmacological activities of A. ursinum are antiaggregatory effects on human platelets, inhibition effect on 5-lipoxygenase (5-LOX) and prostaglandin-endoperoxide synthase (PTGS) enzymes in vitro, high antioxidant activity and inhibition of in vitro cholesterol biosynthesis. Its action is also associated with a decrease in blood pressure and phosphodiesterase enzyme (PDE 5A) inhibition. The saponins and flavonoids found in A. ursinum could inhibit PDE5A activity (Lines and Ono, 2006). Upregulation of PDE5 has been reported in PH (Kass et al., 2007). The treatment of pulmonary hypertensive rats with A. ursinum reverses RVH and the pulmonary vascular remodeling evaluated through the increasing of RV/(LV+S) ratio and the pulmonary arterial wall thickness respectively compared to untreated pulmonary hypertensive rats (Bombicz et al., 2017).
Crataegus rhipidophylla Gand.
Crataegus rhipidophylla Gand. (syn. Crataegus oxyacantha L.; Hawthorn) is a member of Rosaceae family and it is native to Europe, Africa and Asia (Chang et al., 2002). Some of the bioactive compounds found in C. rhipidophylla (fruits, leaves, and flowers) are epicatechin, hyperoside, and chlorogenic acid. Its extracts have many pharmacological activities such as neuroprotective, hepatoprotective, cardioprotective, nephroprotective (Salehi et al., 2009).
Flavonoid extracts of C. rhipidophylla cause improvement in body weight gain, reduction of heterophil to lymphocyte ratio and an increase of NO levels on PH induced by high altitude in broiler chickens (Ahmadipour et al., 2019). Treatment of broiler chickens with these extracts could overexpress the iNOS and SOD-1, and reduce the expression of ET-1 in the heart tissue.
Polyphenols (mainly flavonoids) and oligometric proanthocyanidins (OPCs) compounds of C. rhipidophylla are responsible for some of these activities, such as antioxidant, anti-anxiety, anti-cancer and growth inducer. (Surai, 2014).
Treatment with C. rhipidophylla extract mix in drinking water of broiler chickens with pulmonary hypertension syndrome (PHS) induced by high altitude suggested that the extract cause an increase in albumin, globulin, food intake and body weight and a decrease in AST, ALT, ALP, and RV : TV ratio (Ahmadipour et al., 2017). ALP is one of the serum's enzymes which is a marker of liver tissue damage and the two liver enzymes, AST and ALT, increase their concentrations in oxidative stress conditions and hypoxic state before PHS. These liver enzymes are sensitive markers for measuring oxidative stress, which is an important factor during PHS progression (Fathi et al., 2011). Therefore, the decrease in ALP, ALT and AST levels show reduction of tissue damage due to oxidative stress and that could lead to inhibition of PHS progression (Ahmadipour et al., 2017).
Eulophia macrobulbon (E.C.Parish & Rchb.f.) Hook.f.
Eulophia macrobulbon (E.C. Parish & Rchb. f.) Hook.f. (Corduroy orchid) is a member of Orchidaceae family. Orchids are commonly used in traditional medicine in Asia, Europe and America. Their pharmacological activities include the treatment of pulmonary, autoimmune and inflammatory diseases, cancer, and diabetes (Narkhede et al., 2016). The ethanolic extract of E. macrobulbon contains flavonoids, terpenoids and phosphodiesterase type 5 (PDE5) inhibitor, so it could be a pulmonary vasodilator (Temkitthawon et al., 2017).
Wisutthathum et al. (2018) during a study on MCT induced PH in rats, demonstrated that the treatment with ethanolic extract of E. macrobulbon inhibits CaCl2-induced contraction in PA rings in vitro and reduces (RV/LV+S) ratio. Stilbenes and their derivatives found in E. macrobulbon extracts are responsible for inducing relaxation in coronary arteries in hypercholesterolemic pigs (Vilahur et al., 2015). Therefore, it has been suggested that E. macrobulbon extract could induce relaxation in PAs (like coronary arteries) and modulate PH (Wisutthathum et al., 2018).
Kelussia odoratissima Mozzaf
Kelussia odoratissima Mozzaf. (Wild celery) is a member of Umbelliferae family, that grows at high altitudes (more than 2000 m a.s.l.), mainly in Iran. It is commonly used in Iranian traditional medicine for hypertension and inflammation treatments. Active compounds of its essential oil are phthalides, (Z)-ligustilide and terpenes (Shojaei et al., 2011). The alcoholic extract includes components with high antioxidant activity such as flavonoids and polyphenols (Pirbalouti et al., 2013).
Results of the study of Ahmadipour et al. (2015) on broiler chickens with PH shows improvement in body weight gain, an increase in NO levels and heterophil/lymphocyte ratio and a decrease in MDA levels in serum. In the broiler chickens overexpression of SOD1 and iNOS and suppression of the expression of ET-1 gene in the heart tissue were observed due to treatment with K. odoratissima. Thus, this herb could improve the outcomes of PH in broiler chickens.
Mimosa pigra L.
Mimosa pigra L. (giant sensitive tree) is a member of Fabaceae family, popular with its therapeutic application in the countries of Africa, America and Asia. It is used for treatment of many diseases such as cardiovascular diseases, digestive disorders and infections (Rosado-Vallado et al., 2000).
Rakotomalala et al. (2013) in a study on therapeutic potential of M. pigra against hypoxia-induced PH in rats, indicated that this plant can increase NO production, reduce PAP and RV/(LV+S) and p38 MAPK expression and phosphorylation in lung tissue. P38 MAPK activated in pulmonary hypertensive rats (Welsh et al., 2005). The results of these studies suggested that M. pigra has ameliorative effect on PH outcomes.
Moringa oleifera Lam.
Moringa oleifera Lam. (Drumstick tree) belongs to the Moringaceae family and it is widely cultivated in tropical and subtropical areas. It is native to Northwestern India. Some therapeutic applications of the herb are associated with a decrease in blood pressure, reduction of cholesterol levels, with its high antioxidant, hypoglycemic, anti-ulcer and anticancer activities (Stohs and Hartman, 2015). Nitryl glycosides compounds of M. oleifera leaves show hypotensive and antioxidant activities (Gupta et al., 2007). Other studies show that M. oleifera can increase the GSH levels in liver tissue (Fakurazi et al., 2008).
Chen et al. (2012) found an increase in SOD levels and a decrease in pulmonary pressure and in pulmonary arterial wall thickness due to treatment with M. oleifera in MCT induced PH in rats. It has been demonstrated that niazirin and thiocarbamates (two of the active compounds of M. oleifera) have antihypertensive effects on spontaneous hypertension in rats (Faizi et al., 1998). So it could be considered as an useful treatment for PH.
Rhodiola tangutica (Maxim.) S.H.Fu
Rhodiola tangutica (Maxim.) S.H.Fu (syn. Sedum algidum var. tanguticum Maxim.) (Rhodiola, Golden root) is an important herb used in traditional medicine in Qinghai and Tibetan. It is used for treatment of many diseases especially to prevent colds caused by high altitude (Li et al., 2009). Some of the biologically active substances of enrich fraction of R. tangutica are phenylethanols, flavonoids, terpenoids (Nan et al., 2018).
Nan et al. (2018) during a study on hypoxia-induced PH in rats marked that the use of bioactive enriched fraction of R. tangutica can decrease mPAP, RV/BW, RV/LV+S, hematocrit and p27Kip1 protein expression and therefore could increase protein expression of PCNA, CDK4, and Cyclin D. These three proteins have key roles in cell cycle switching of G0/G1 phase to S phase. P27kip1 is a CDK inhibitor. Therefore, regulation of these factors can inhibit cell proliferation and following that could inhibit medial vessel thickness, which is a factor for structural changes in pulmonary hypertension.
Vasorelaxant activity of R. tangutica was confirmed by in vitro study on isometric tension changes induced by a force transducer in rat pulmonary artery (Li et al., 2016). Therefore, according to these studies R. tangutica could be considered as an effective treatment for PH.
Salvia miltiorrhiza Bunge
Salvia miltiorrhiza Bunge (Red sage, Danshen) is a member of Lamiaceae family and it is highly valued for its roots. In traditional Chinese medicine, it is used to increase blood flow and cooling the blood in abscess treatment. The main chemicals of aqueous extract of S. miltiorrhiza are caffeic acid metabolites (Lu and Foo, 2002; Wang, 2010). Some of the pharmacological activities of S. miltiorrhiza in traditional medicine are protection of myocardium derangement induced by ischemia, protection of neural cells injuries induced by anoxia, inhibition of the platelet aggregation, hepatic fibrosis and HIV-1 (Lay et al., 2003). Also, this plant is used as a protective agent on vascular endothelial cells (Wang et al., 2010). It has protective effect on disorders that contributed to endothelial dysfunction or vascular endothelial injury, like focal cerebral infarction and cerebral ischemia (Cai et al., 2007).
Wang Y. et al. (2015) suggested that treatment of pulmonary hypertensive rats with S. miltiorrhiza aqueous extract could improve hemodynamic parameters such as mPAP and RVSP. It also increased NO and 6-Keto-PGF1α and decreased ET-1 and TXB2 levels in the plasma and down-regulated the expression of TGF-β1 in lung tissue which is one of the important factors in inducing vascular remodeling and consequently, PH progression. Endothelial injury causes imbalance of vasoactive substances such as vasorelaxant (PGI2 and NO), and vasoconstrictors (ET-1 and TXA2). These results suggest that S. miltiorrhiza can be considered as a treatment for PH by increasing NO levels and decreasing ET-1, TXA2, and TGF-β1 expression in lung tissue and improvement of hemodynamic indices.
Si-chuan et al. (1994) in a study on hypoxia induced pulmonary hypertensive rats suggested that S. miltiorrhiza can be an effective treatment for PH due to its reducing activity on RVSP and RVHI. Chen et al. (2003) demonstrated that S. miltiorrhiza in pulmonary hypertensive rats could reduce thickening in the SPAs, could increase the levels of HO-1 and iNOS and decrease the expression of eNOS. In high altitude conditions, HO-CO pathway is up-regulated and the expression of HO-1 protein and the CO production increased. In other words, HO-CO is a pathway related to pulmonary arterial vasodilation and protective from pathological remodeling (Llanos et al., 2012). Therefore, according to the available evidence, S. miltiorrhiza could ameliorate PH.
Securigera securidaca (L.) Degen & Dörfl.
Securigera securidaca (L.) Degen & Dörfl. (Goat pea), a member of Fabaceae family, that grows at West Asia, Europe and Africa. It is used in traditional medicine for treatment of many diseases such as chronic obesity, diabetes, central nervous system diseases, high blood pressure, and digestive problems (Hosseinzadeh et al., 2002). Flavonoids, alkaloids and saponins are found in its aqueous and alcoholic extracts (Ahmadi et al., 2016). Ahmadipour (2018) reported that the treatment with S. securidaca in broiler chickens with PH could increase NO levels in plasma. NO is a vasorelaxant agent and can improve PH. The flavonoids found in S. securidaca could protect NO from inactivation by scavenging superoxide anions. The results of Ahmadipour's study show that the treatment with S. securidaca could improve hemodynamic parameters and can decrease R, S and T wave amplitudes of the electrocardiogram. This plant can reduce oxidative stress by reducing MDA levels. It has been suggested that the antioxidant properties of S. securidaca are due to phytosterols, 4‐Methyl‐2,6‐di‐tertbutylphenol, palmitic acid, 9,12-octadecadienoic acid and alkaloidal compounds. Therefore, these compounds could be considered as an appropriate treatment for PH (Ahmadipour, 2018). Several studies demonstrated that there is a strong relationship between ET-1 expression and PH. ET-1 expression in PH broilers chicken increased compared with normal broilers (Gómez et al., 2007). Studies show that S. securidaca can reduce ET-1 expression in high altitude-induced PH in broiler chicken (Ahmadipour, 2018). Also, flavonoids found in S. securidaca can reduce transmembrane 45Ca2+ absorption or inhibit protein kinase C activity, inhibit cAMP‐ and cGMP-phosphodiesterase (PDE) (Beretz et al., 1982) and that eventually leads to reduction in contraction of the vascular system and PH improvement. Therefore, S. securidaca with its vasorelaxant and antioxidant activities can ameliorate outcomes of PH (Ahmadipour, 2018).
Terminalia arjuna (Roxb. ex DC.) Wight & Arn.
Terminalia arjuna (Roxb. ex DC.) Wight & Arn. (Arjuna) is an evergreen tree grow in India, a member of the Combretaceae family. It is used in traditional medicine to improve cardiovascular disease (Dwivedi, 2007). Some of the active compounds of T. arjuna include flavonoids, terpenoids, tannins and minerals (Varghese et al., 2015). Some of the pharmacological activities of T. arjuna are anti-ischemic, antioxidant, hypolipidemi, increasing cardiac contraction and decreasing blood pressure, (Pawar and Bhutani, 2005; Kapoor et al., 2014).
Meghwani et al. (2017) found that the aqueous extract of T. arjuna stem bark can prevent the decrease of relative weight of lungs in pulmonary hypertensive rats. The study was performed on MCT-induced PH rats. The effects of T. arjuna are associated also with reduction of RV hypertrophy and %MWT of pulmonary artery, with decrease lipid peroxidation and NOX1 protein expression in lung and with increase the SOD and CAT, also with decrease in BCL2/BAX ratio mRNA demonstrating antiapoptotic effect of the T. arjuna in pulmonary hypertensive rats. The results of these studies suggest that T. arjuna can be considered as an effective treatment for PH.
Trifolium pratense L.
Trifolium pratense L. (Red clover) belongs to Fabaceae family. It is native to Europe, West Asia and Northwest Africa. Several types of flavonoids are essential constituents of T. pratense (Boué et al., 2003). A study on animal models suggested that isoflavones in T. pratense can reduce contractions of the smooth muscle in ileum, uterus and bladder (Wang et al., 2008). Jiang and Yang (2016) demonstrated that the use of isoflavones of T. pratense extract, mixed in food, in broiler chickens with PH could reduce ET-1 in serum and lungs and could increase the secretion of NOS. ET-1 can increase the MMP-2 expression that is related to pulmonary vascular remodeling (Tan et al., 2012). T. pratense has phytoestrogen activity and can increase NOS and NO levels in the serum (Simoncini et al., 2005). All of these findings suggested that T. pratense isoflavones have therapeutic potential for PH in bird models by increasing in NO and decreasing in ET-1 expression.
Withania somnifera (L.) Dunal
Withania somnifera (L.) Dunal (Ashwagandha) is a herbal remedy belongs to Solanaceae family which is used for treatment of many diseases especially in India (Mohanty et al., 2004). HPLC analysis shows withaferin A and withanolide A as the most important active components of W. somnifera root powder extract (Kaur et al., 2015). Pharmacological uses of W. somnifera include anti-fatigue, bone enhancer, anti-aging, anti-Alzheimer, anti-inflammatory, antioxidant, hypoglycemic and anticancer (Ojha and Arya, 2009). It is used also to lower blood pressure and to lower cholesterol levels in the blood (Mohanty et al., 2008).
Kaur et al. (2015) during a study on MCT induced pulmonary hypertensive rats shows that the treatment with W. somnifera can reduce RVP and RVH, also could reduce the expression of PCNA. The treatment with W. somnifera causes an increase in procaspase-3 expression, and therefore inducement of apoptosis in pulmonary vessels. In addition, treatment with W. somnifera could reduce ROS levels in lung tissue. It can increase the levels of IL-10 and decrease in levels of TNF-α and NFkB, which is in connection with its anti-inflammatory effect. Withanolides found that W. somnifera inhibit the activation of NFkB by inhibition of TNF-α and show the anti-inflammatory effect of W. somnifera (Ichikawa et al., 2006). W. somnifera also increases eNOS expression and decreases HIF-1α expression in lung tissue (HIF-1α increases in hypoxia conditions). Increased eNOS activity can increase NO levels, which cause vasorelaxation (Kaur et al., 2015). According to the mentioned studies, W. somnifera could be a potential herb in the treatment of PH by its antioxidant, vasorelaxant and anti-inflammatory activities.
Phytochemicals Used for PH Treatment
Apple Polyphenol
Apple polyphenol is a compound that derived from different types of apple fruits (Malus spp.), with many pharmacological activities for treatment of diseases such as gastric mucosal damage, cancers, inflammatory and cardiovascular diseases (Espley et al., 2013).
Hua et al. (2018) during a study on hypoxia-induced PH in rats found that treatment with apple polyphenol can modulate hemodynamic indicators such as mPAP and PVR and therefore could decrease the contraction of pulmonary vessel rings. In vitro it decreases cytosolic Ca2+ in PASMCs. At the genetic level, apple polyphenol causes an increase of eNOS expression, an increase of NO levels and decrease in caspase-3 and iNOS expression in PAEC. Therefore, all of these findings confirm that apple polyphenol can be considered as a treatment for PH that induces its effect by reducing of contraction and increasing of NO levels.
Asiaticoside
Asiaticoside is a saponin derived from Centella asiatica (L.) Urb. and it has many applications in traditional medicine with high range of biological activities such as antioxidant, anti-inflammatory, anti-hepatofibrotic (Dong et al., 2004) and neuroprotection effect on transient cerebral ischemia and reperfusion (Chen et al., 2014).
Wang et al. (2018) during a study on hypoxia induced PH shows that treatment with asiaticoside decreased the RVH and mPAP, also increase the concentration of cGMP and NO and decrease circulating concentration of ET-1. Additionally, this compound increase the activity of AKT, the phosphorylation and therefore leads to activation of eNOS and to inhibition of endothelial cells apoptosis. Studies suggested that asiaticoside can enhance phosphorylation and activation of serine/threonine-proteinn kinase/eNOS pathway and therefore to increase NO production that inhibits the progression of PH.
Wang X. B. et al. (2015) during an in vivo and in vitro investigation on hypoxia induced PH in rats and PASMCs demonstrated that treatment with asiaticoside improves the mPAP and RVH, also reduces the expression of TGF-β1, TGF-βR1, and TGF-βR2 and inhibits the phosphorylation of Smad2/3 in lung tissue of animals. It has been shown that Smad2/3 phosphorylation raises during PH progression (Richter et al., 2004). In PASMCs asiaticoside reduces the cell number and migration, also reduces the TGF-β1 expression. So asiaticoside do its therapeutic effect by reducing proliferation and raising apoptosis (Wang X. B. et al., 2015).
Astragalus Polysaccharides
Astragalus polysaccharides are active compounds derived from Astragalus membranaceus Fisch. ex Bunge, that have many pharmacological activities especially anti-inflammatory and antioxidant (Auyeung et al., 2016).
Yuan et al. (2017) during a study on MCT induced PH in rats demonstrated that treatment with Astragalus polysaccharides causes improvement in hemodynamic indicators such as decreasing mPAP, PVR, RVH and medial wall thickness. In addition, overexpression of eNOS and increase of NO levels were observed in treatment groups. Decrease in pro-inflammatory mediators levels were observed due to treatment with Astragalus polysaccharides. Also, decrease in the expression of pho-IκBα was confirmed. Pho-IκBα is a marker for assessment of inflammation, so according to the studies Astragalus polysaccharides can be a useful treatment for PH by inhibition of the inflammation and by increasing of NO levels leading to improvement of hemodynamic indicators.
Baicalin
Baicalin is a flavonoid derived from Scutellaria baicalensis Georgi roots and it is used in traditional medicine with many pharmacological activities such as antioxidant, anticancer, anti-inflammatory and inducing apoptosis (Dong et al., 2010). Studies show that baicalin could inhibit the expression of collagen I due to its anti-fibrotic activity (Hu et al., 2009). Wang et al. (2013) demonstrated that pulmonary arterial collagen is an important factor in PH due to hypoxic conditions.
Liu et al. (2015) during a study on hypoxia-induced PH shows that treatment with baicalin cause improvement of hemodynamic factors including mPAP, mSAP, and RV/(LV+S)%. Also, baicalin reduces protein and mRNA expression of collagen I in lung tissue, which is an index for PH progression. It should be noted that baicalin has small effect on collagen III protein and mRNA expression compared with its great effect on collagen I mRNA and protein expression. Baicalin increases the expression of ADAMTS1 and therefore inhibits the expression of collagen I. Therefore, baicain can be considered as a treatment for PH due to its inhibitory effect on collagen I synthesis and improvement in hemodynamic factors (Liu et al., 2015).
Huang et al. (2014), during a study on HPASMCs exposed to TGF-β1, demonstrated that treatment with baicalin decrease the expression of HIF-1α and AhR that are responsible for cell proliferation. So treatment with baicalin, in vitro, cause decrease of proliferation and it can be considered for improving the conditions caused by PH. Studied prove that treatment with baicalin can improve PH by anti-proliferation effect mediated by the AhR pathway.
Hsu et al. (2018) during a study on MCT induced PH in rats shows that treatment with baicalin causes improvement of hemodynamic indicators such as RVSP and RVH, also reduction of ET-1 and ETA (ETAR) protein receptor expression in lungs and therefore leads to suppression of the Akt/ERK1/2/GSK3β/β catenin signaling pathway. Activation of this pathway cause increase of the ET-1 expression and progression of PH. Additionally, the reduction of superoxide production and increase the expression of eNOS were observed in treated rats. It has been demonstrated that the treatment with baicalin causes decrease of von Willebrand factor (vWF), which is a marker of endothelial injury (Hsu et al., 2018). These results suggest that baicalin can be considered as a potent treatment for PH.
Berberine
Berberine is an isoquinoline alkaloid derived from some Chinese herbs, such as Coptidis rhizome (Zhu and Qian, 2006). It has many pharmacological activities: antimicrobial, antiproliferative, cardioprotective, antidiabetic, anticancer and others (Gao et al., 2017; Neag et al., 2018; Belwal et al., 2020).
Luo et al. (2018) during a study shows that treatment with berberine cause decrease of protein phosphatase 2A (PP2Ac) phosphorylation, which has an important role in the progression of PH. Therefore the decrease of PP2Ac expression cause decrease in the proliferation and migration of PASMCs which leads to improvement of PH; also treatment with berberine leads to improvement in hemodynamic indicators such as RV/(LV + S) and to reduction of medial wall thickness percentage.
Carvacrol
Carvacrol is a monoterpenoid phenol, derived from oregano (Origanum spp.) and thyme (Thymus spp.) leaves. Some of the most important pharmacological activities of carvacrol are antibacterial, antiviral, antioxidant and anticancer (Sökmen et al., 2004).
Zhang et al. (2016) during a study on hypoxia-induced PH in rats demonstrated that carvacrol reduces RVH and the thickening of pulmonary arteries. Also, it can reduce oxidative stress by increasing SOD and GSH activity and decreasing MDA levels in PASMCs. Treatment with carvacrol reduces the levels of mRNA and protein expression of Bcl-2 (anti-apoptotic protein) and reduces the Procaspase-3 expression, raises the caspase-3 expression and so it can increase PASMCs apoptosis. Also, ERK1/2 and Akt increase cell survival and decrease apoptosis. Additionally, carvacrol treatment inhibits the ERK1/2 and PI3K/Akt pathway. All of these findings demonstrate that carvacrol can ameliorate PH by increasing apoptosis and decreasing oxidative stress in PASMCs.
Genistein
Genistein is a phytoestrogen which has higher affinity to ERβ compared with ERα. This natural compound has many pharmacological activities such as vasodilator, cardioprotective, and anti-inflammatory. It has been suggested that genistein has protective effect on PH progression (Homma et al., 2006).
Matori et al. (2012) during a study on MCT induced PH shows that treatment with genistein can improve hemodynamic indicators including RVP, RVH, RVEF, and RV dilatation. Genistein also decreases pulmonary fibrosis and tissue injuries due to PH. Genistein causes increase in protein expression of ERβ in lung and RV. In vitro studies show that genistein inhibits the proliferation of HPASMCs and hypertrophy of NRVM.
All of the protective effects of genistein on PH are related to its phytoestrogenic activity. Various studies have shown that estrogens have protective effects against cardiovascular and PH disease and this effect is via estrogen receptor-β (ERβ) (Umar et al., 2011). Therefore, genistein as a phytoestrogen, can modulate PH outcomes by affecting on ERβ expression.
Ginsenoside Rb1
Ginsenoside Rb1 is one of the phytochemicals derived from Panax ginseng that shows many pharmacological activities especially in the treatment of heart and lung diseases like treatment of endothelial injuries and inflammation disorders in endothelial smooth muscle cells (Yu et al., 2007; Li et al., 2011).
Wang R. X. et al. (2015) during an investigation on hypoxia and MCT induced PH in rats demonstrated that treatment with Ginsenoside Rb1 improves PH in vivo and in vitro by improving RV/(LV+S) ratio, reducing the expression of ET-1 and SOCE and induces relaxation in PAs. The concentration of cytosolic Ca2+ adjusted by SOCE in PASMCs and it's a key factor for contraction and proliferation of PASMCs. Ginsenoside Rb1 do its therapeutic effect by reducing the ET-1 and SOCE expression and reducing contraction, so it can be considered as a treatment for PH.
Isorhynchophylline
Isorhynchophylline is an alkaloid derived from Uncaria rhynchophylla (Miq.) Miq. ex Havil. and it is widely used in traditional medicine especially for treatment of heart and brain diseases (Zhou and Zhou, 2012). Some of the pharmacological activities of isorhynchophylline include reducing oxidative stress, apoptosis, anticancer and anti-inflammatory (Kawakami et al., 2011).
Guo et al. (2014) during a study on MCT induced PH in rats shows that treatment with isorhynchophylline can improve RVP and RVH. Also, isorhynchophylline can reduce PASMCs proliferation in vitro. They also demonstrated that isorhynchophylline can reduce the expression of Cyclin D1 and CDK6, and can increase the expression of p27Kip1 and therefore it can regulate cell cycle and could reduce proliferation. Isorhynchophylline inhibits ERK1/2 and STAT3 signaling pathways and this way it can suppress cell growth (Guo et al., 2014). All of these findings suggested that isorhynchophylline can be considered as a potent treatment for PH that causes regulation of cell cycle and inhibit cell growth and proliferation.
Magnesium lithospermate B
Magnesium lithospermate B is a phytochemical isolated from Salvia miltiorrhiza. Studies shows that magnesium lithospermate B is a potent antioxidant that do its effect through inhibition of NADPH oxidase; NADPH oxidase (NOX) is a factor contributing to increased ROS and cell damage (Lou et al., 2015; Jin et al., 2016).
Li et al. (2019) during an in vivo and in vitro investigation shows that treatment of pulmonary hypertensive rats and PASMCs with magnesium lithospermate B reduces the expression of NOX2 and NOX4 and ERK in PAs, also reduces the ROS and H2O2 levels. In PASMCs, it reduces OPN and Cyclin D1 and raises α-SMA and SM22α expression (contractile genes with high and low rate of proliferation, respectively). So, based on these findings magnesium lithospermate B can be considered as a potent treatment of PH by modulating oxidative stress in PAs of animals and modulating contractile genes in PASMCs.
Nobiletin
Nobiletin is a flavonoid derived from peels of citrus fruits. This compound has many applications due to its biological activities such as anti-inflammatory, antioxidant and anti-tumor (Huang et al., 2016).
Cheng et al. (2017) during a study on MCT induced pulmonary hypertensive rats suggested that treatment with nobiletin leads to improvement of hemodynamic indicators such as RVSP and RVH and therefore has a positive effect on pulmonary vascular remodeling induced by hypoxia. In in vitro conditions, nobiletin suppresses PDGF-BB, Src and STAT3 phosphorylation, after that it suppresses Pim1 and NFATc2, which are target genes of Src and STAT3. Finally, this gene cascade inhibits proliferation. Therefore, nobiletin can prevent the progression of PH by its inhibition effect on Src/STAT3 axis that is one of the pathways of proliferation.
Oxymatrine
Oxymatrine is a phytochemical found in Sophora flavescens Ait. that shows many pharmacological activities such as anti-inflammatory and antioxidant activities especially in lung and heart tissues, compared to other organs (Jun-xue and Guo-jun, 2000); studies shows its protective effect against lung injury (Xu et al., 2005).
Zhang et al. (2014) during an investigation on hypoxia and MCT induced PH in rats (in vivo) and PASMCs (in vitro) shows that it cause improvement of PH by reducing the mRNA expression of inflammatory factors, chemokines and cytokines, growth and adhesion factors including MCP-1, IL-6, SDF-1, TGF-β, VEGF, ICAM-1, and VCAM-1. It also improves hemodynamic indicators. In addition, oxymatrine decreases the expression of HIF-1α and p-NF-κB in lung tissue and increased antioxidant factors including Nrf2, SOD, Ho-1 and GSH in PASMCs. This phytochemical can modulate PH in vitro and in vivo and in both hypoxia and MCT models by its antioxidant, antiproliferative and antiinflammatory effects.
Panax notoginseng Saponins
Panax notoginseng Saponins (PNS) are compounds that are used in traditional Chinese medicine due to their biological and therapeutic activities such as vasorelaxant, antioxidant and inhibitory effect on vascular smooth muscle receptor of the voltage-gated Ca2+ channels (Chu et al., 2007).
Zhao et al. (2015) during a study on PNS injection on pulmonary hypertensive rats induced by hypoxia, shows that PNS can improve hemodynamic indicators such as mPAP, mCAP and RV/(LV+S) ratio and therefore can reduce the expression of p38MAPK mRNA in lung tissue of pulmonary hypertensive rats. P38MAPK is an important index for physiological stress and its expression increases during PH progression. So according to the studies, PNS could be considered as a treatment for PH.
Polydatin
Polydatin is a phytochemical derived from Polygonum cuspidatum and has many pharmacological and therapeutic activities such as antiinflammatory, antioxidant, anticancer and inhibition of platelet aggregation (Miao et al., 2011).
Studies shows that during the progression of PH, increase in the levels of endothelin and angiotensin II leads to reduce in the bioavailability of NO (Berger et al., 2009). Miao et al. (2012) during an investigation on hypoxia induced PH in rats showed that treatment with polydatin increases the NO levels via reduction of endothelin and angiotensin II levels in lung and serum. It also cause improvement of hemodynamic indicators like mPAP, mCAP and RVH.
Punicalagin
Punicalagin is a phytochemical isolated from pomegranate juice and has many pharmacological activities due to its high antioxidant content (Xu et al., 2015); also it has been reported that punicalagin used for treatment of lung diseases in animal models (Peng et al., 2015).
Shao et al. (2016) during an investigation on hypoxia induced PH in rats shows that treatment with punicalagin improves hemodynamic indicators and induce relaxation by enhancing NO-cGMP signaling pathway in PAs. Also this phytochemical reduces oxidative stress and raises mRNA expression of MMP-9, HIF-1α, VEGFA, NF-kB and TNF-α in lung tissue of animals. All of these factors increase in hypoxia conditions and as mentioned, they returned to their normal level by punicalagin treatment. So this phytochemical can be considered as an effective treatment for PH.
Quercetin
Quercetin is a flavonoid found in a wide range of plants and fruits with many biological activities such as antioxidant, anti-inflammatory, antitumor, antiproliferative, inducing apoptosis, antimetastatic, vasodilator and decreasing blood pressure (Muhammad et al., 2015). Also, it has been suggested that quercetin inhibits the progression of PH in rats (Morales-Cano et al., 2014).
He et al. (2015) during a study on hypoxia induced PH in rats demonstrated that treatment with quercetin improves hemodynamic indicators such as PAH and RVH. Quercetin can inhibit the proliferation of PASMCs and can induce apoptosis in cells by inhibiting the TrkA/AKT signaling pathway. It was confirmed that quercetin increases the BAX expression and decreases the expression of Bcl-2. Additionally, quercetin causes an increase of cyclin D1 and a decrease of cyclin B1 and Cdc2 protein expression. They are responsible for cell cycle regulation and reduce the expression of MMP2, MMP9, CXCR4, integrin β1 and integrin α5 which are responsible for cell migration. Gao, H. et al (2012) during an investigation on MCT induced pulmonary hypertensive rats demonstrated that treatment with quercetin reduces the proliferation in PASMCs via reducing the expression of PCNA gene also quercetin reduces mPAP and RVH and WT in treated rats. So they shows that quercetin do its therapeutic effect by inhibition of proliferation in PASMCs. Therefore, quercetin can be considered as a treatment for PH by its prevention effect on migration and by its apoptosis inducing effect.
Resveratrol
Resveratrol is a powerful antioxidant with a polyphenolic structure. It has many biological activities and therefore has many therapeutic applications such as antiinflammatory, antioxidant and antiaging (Ji et al., 2013; Yeung et al., 2019). The anticancer activity of resveratrol is due to its inhibition of ACT, which leads to increase of apoptosis in human uterine cancer cells (Testa and Bellacosa, 2001).
Guan et al. (2017) during a study on pulmonary artery vascular smooth muscle cells (PASMCs) which have been exposed to hypoxia, demonstrated that treatment with resveratrol could inhibit proliferation and migration of PASMCs and after that could reduce AKT and p-AKT expression in PAMSCs. Also, resveratrol decreases the expression of MMP-2 and MMP-9. MMPs are responsible for increased migration and proliferation of PASMCs by activating the PI3K/AKT signaling pathway (Shivakrupa et al., 2003).
It has been suggested that resveratrol can increase the expression of NO, decrease inflammation, cell proliferation and oxidative stress. Therefore, it could improve the function of pulmonary artery endothelium and PH. Additionally, resveratrol can inhibit dysfunctions of rat pulmonary artery vessels (like inflammation and contraction that leads to PAH) and cardiomyocyte hypertrophy (Paffett et al., 2012).
Xu et al. (2016) during a study on hypoxia-induced PH in vivo and in vitro, demonstrated that resveratrol could improve PH and could reduce inflammatory mediators in the lungs of rats. Also treatments with resveratrol cause decrease of H2O2 in rats lungs. HIF-1α is an important gene in PH progression, NO and ROS activate HIF-1α expression (Prabhakar and Semenza, 2012) and resveratrol decreases its expression by its antioxidant activity. Trx-1 and Nrf-2 proteins expression were increased by resveratrol. Nrf2 is an antioxidant marker that has balancing effects on antioxidant proteins like Trx-1. Resveratrol increases GSH and SOD activities, Trx-1 and Nrf-2 protein levels in PASMCs and it decreases the proliferation of PASMCs and ROS, H2O2 production and expression of HIF-1α. All of these markers shows that resveratrol is a powerful antioxidant (Xu et al., 2016).
Trimethoxystilbene (TMS) is a synthesized compound from methylation of resveratrol that has more activities and low toxicity compared with resveratrol (Ma et al., 2009). Gao, G., et al (2012) during an in vitro study on PASMCs shows that TMS is more potent in treatment of PH compared to resveratrol, by increasing induction of apoptosis and decreasing cell proliferation. Therefore, it is suggested that resveratrol can be a potent treatment for PH due to its antioxidant and anti-proliferative activities.
Rhoifolin, Apiin and Luteolin
Rhoifolin, apiin and luteolin are three types of flavonoids used in traditional medicine. Studies show that flavonoids are present in the majority of vegetables and plants. Some of the biological and pharmacological activities of these flavonoids are anti-inflammatory, antiallergic and spasmolytic (Gabor, 1986). It has been suggested that luteolin, apiin and rhoifolin have inhibitory effects on slow fibers of the dog heart; also they can reduce aortic pressure and capillary pulmonary pressure in normal animals (Occhiuto et al., 1988).
Occhiuto and Limardi (1994), during a study on hypoxia-induced PH in dogs, demonstrated that structural changes of flavonoids (luteolin, apiin and rhoifolin) cause significant changes in antihypertensive activity. They showed that rhoifolin reduces aortic pressure but has not effect on pulmonary vascular resistance while luteolin and apiin have antihypertensive properties.
Salidroside
Salidroside is a phytochemical isolated from Rhodiola rosea L. that widely used for PH treatment and has many pharmacological applications such as antioxidant, anticancer, antiinflammation and immune system enhancer (Panossian and Wagner, 2005; Guan et al., 2011).
Huang et al. (2015) during an investigation on pulmonary hypertensive mice demonstrated that treatment with salidroside reduces RVH in treated mice and raises Caspase-3, BAX, A2aR and reduces BCL2 expression in PASMCs. Adenosine A2aR has protective effect in PH progression and inhibits vascular remodeling process. So according to this study, salidroside can be considered as a cure for PH by regulating apoptosis pathway.
Discussion
PH is a progressive disease characterized by increase in pulmonary blood pressure, right ventricular hypertrophy and thickening of the vascular intima, media, and adventitia (Voelkel et al., 2012). Vascular thickening is due to increased proliferation and resistant to apoptosis in PASMCs (Xu et al., 2016). Many signaling pathways are responsible in this regard such as Ca2+ signaling pathway (Reyes et al., 2018); JAK/STAT (Wang et al., 2005); RhoA/ROCKs (Antoniu, 2012); Notch (Yamamura et al., 2014), and Src/STAT3 (Paulin et al., 2011).
Despite recent advances in medicine and pharmacy, industrial remedies are not sufficient to prevent the progression of the PH (Lythgoe et al., 2016). Therefore, introduction and evaluation of natural compounds effective in the treatment of PH seems necessary.
According to our study, chemical compounds that are effective in treatment of PH include flavonoids, isoflavonoids, monoterpenoid phenolics DMDS, polymethoxylated flavones, terpenoids, phytoesterogens, isoquinoline alkaloids, saponins, polysaccharides, tetracyclic oxindole alkaloid.
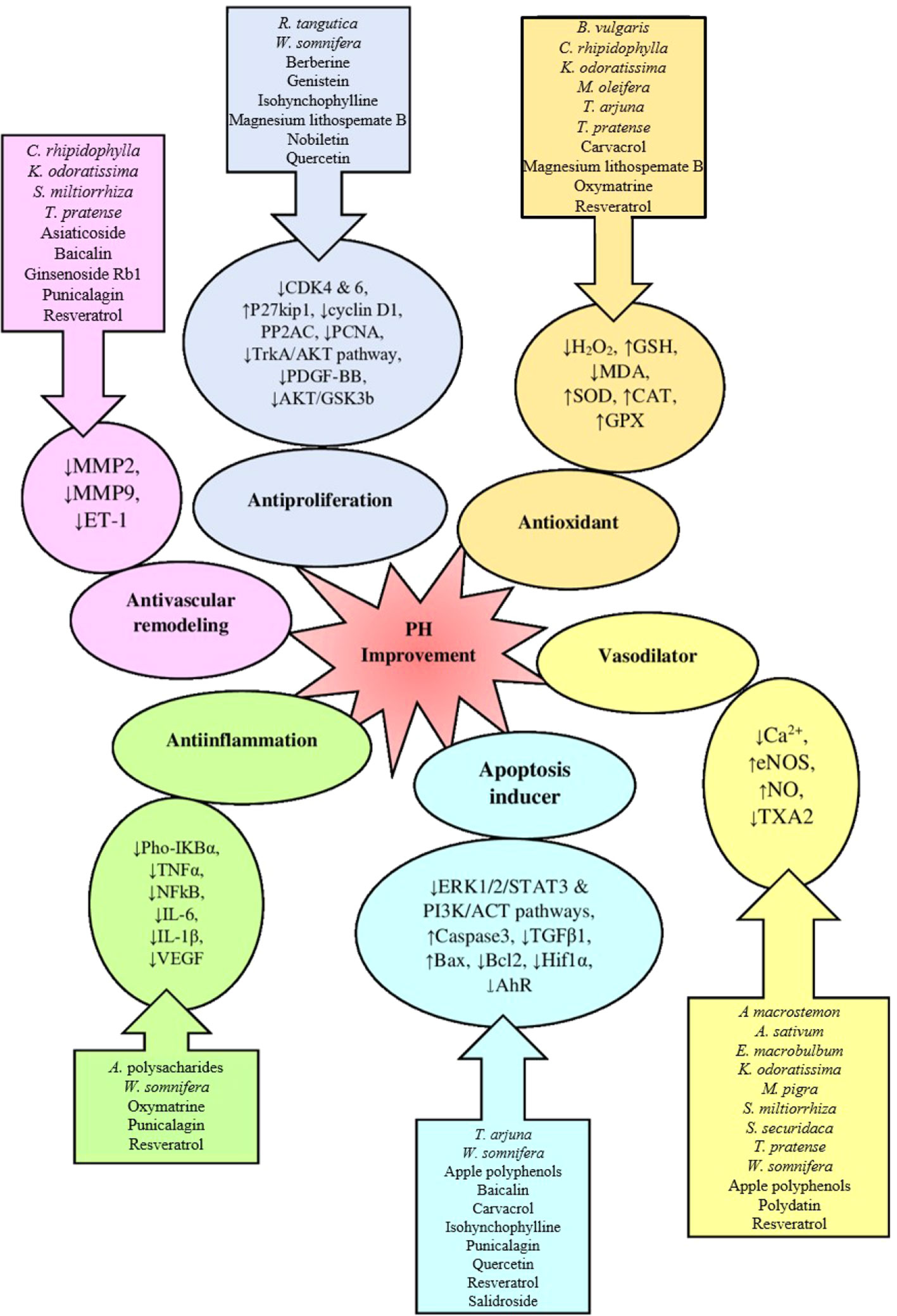
All herbs and phytochemicals induce their therapeutic effect through one of the 6 pathways (Figure 2):
a. Antiproliferation: Plant extracts and phytochemicals that have antiproliferative effects include R. tangutica, W. somnifera, berberine, genistein, isohynchophylline, magnesium lithospemate B, nobiletin, and quercetin, which do their effects by reducing expression or suppressing the pathways involved in cell proliferation. Some of these markers and pathways decreases the expression of cyclin D1, CDK 4 and 6, PP2AC, PDGF-BB, and PCNA markers and increases the p27kip1 (cyclin inhibitor) marker, that suppresses Trk A/ACT and ACT/GSK3b pathways; the final result is reduction of proliferation (Guo et al., 2014; He et al., 2015; Kaur et al., 2015; Cheng et al., 2017; Luo et al., 2018; Nan et al., 2018).
b. Antioxidant: Plant extracts and phytochemicals with antioxidant properties on treatment of PH include B. vulgaris, C. rhipidophylla, K. odoratissima, M. oleifera, T. arjuna, T. pratense, carvacrol, magnesium lithospemate B, oxymatrine and resveratrol, which do their effect by increasing CAT, SOD, GPx, GSH levels and reducing H2O2, ROS, and MDA levels. All of these indicators show decrease oxidative stress and reduce tissue damage (Chen et al., 2012; Ahmadipour et al., 2015; Kaur et al., 2015; Jiang and Yang, 2016; Xu et al., 2016; Zhang et al., 2016; Guan et al., 2017; Meghwani et al., 2017; Ahmadipour et al., 2019).
c. Antivascular remodeling: Vascular remodeling is one of the important events during PH progression that has been explained before. Many plant extracts and phytochemicals do its effects by suppressing ET-1 expression and following that MMP-2 and MMP-9 expression which cause inhibition of the progression of vascular remodeling. Some of these plant extracts and phytochemicals include C. rhipidophylla, K. odoratissima, S. miltiorrhiza, T. pratense, asiaticoside, baicalin, ginsenoside Rb1, punicalagin and resveratrol; that have vascular antiremodeling effect and this way inhibit the progression of PH (Chen et al., 2003; Ahmadipour et al., 2015; He et al., 2015; Jiang and Yang, 2016; Xu et al., 2016; Ahmadipour et al., 2017; Guan et al., 2017; Hsu et al., 2018; Wang et al., 2018; Ahmadipour et al., 2019).
d. Vasodilator: One of the important reasons for PH is increased blood vessel contraction and after that PH. The most important vascular relaxation inducers do their effect by increasing NO levels due to increasing eNOS expression and decreasing the levels of TXA2 and cytosolic Ca2+. Plant extracts and phytochemicals with these properties include A macrostemon, A. sativum, E. macrobulbum, K. odoratissima, M. pigra, S. miltiorrhiza, S. securidaca, T. pratense, W. somnifera, Apple polyphenols, polydatin and resveratrol (Fallon et al., 1998; Chen et al., 2003; Rakotomalala et al., 2013; Kaur et al., 2015; Ahmadipour et al., 2015; Jiang and Yang, 2016; Han et al., 2017; Hua et al., 2018).
e. Apoptosis inducer: Some of the plant extracts and phytochemicals cause improvement in PH by increasing apoptosis such as T. arjuna, W. somnifera, apple polyphenols, baicalin, carvacrol, isohynchophylline, punicalagin, quercetin, resveratrol and salidroside. They act by inhibiting of growth factors such as TGF-β1, AhR and HIF-1α or suppressing PI3K/ACT, ERK1/2/STAT3 pathways, or increase the expression of BAX and caspase-3 (apoptosis inducers) and decrease the expression of BCL2 (anti-apoptosis factor). All of these agents drive the cells (lung tissue cells, PAECs and PASMCs) toward apoptosis and inhibit the progression of PH (Guo et al., 2014; Huang et al., 2014; Kaur et al., 2015; Wang Y. et al., 2015; He et al., 2015; Zhang et al., 2016; Guan et al., 2017; Meghwani et al., 2017; Hua et al., 2018).
f. Anti-inflammation: One of the notable processes in PH is increased inflammation. Some of the plant extracts and phytochemicals such as Astragalus polysacharides, W. somnifera, oxymatrine, punicalagin and resveratrol, can reduce this inflammation by decreasing inflammation factors such as IL-10, IL-6, IL-1β, TNF-α, NFkB, VEGF, pho-IkBα. Therefore, they can inhibit inflammation and following that can reduce the progression of PH (Kaur et al., 2015; Xu et al., 2016; Yuan et al., 2017).
Future Direction
The present study provides comprehensive information about medicinal plants and phytochemicals used for PH treatment, as discussed, all these herbs and phytochemicals have therapeutic effects on PH but exert their effects in different ways. There are still unknown mechanisms to industrialization of these medicinal plants and phytochemicals including purification of active compounds of herbs, combining several herbs or phytochemicals to improve their effectiveness (in this case, pharmacological activities, toxicity and herb/food/drug interaction should be completely investigated). Bioinformatics studies are suggested to determine the interaction between active compounds or phytochemicals and their target receptors or target cells. Further studies to identify the signaling pathways are suggested. Making suitable formulations with optimal bioavailability and clinical trials to evaluating the effect of herbs and phytochemicals on patients suffering from PH and investigating the side effects, toxicity, safely and effectiveness of these treatments can provide the field for use in pharmacy.
Conclusion
According to this review study, as described, many herbs and natural compounds have the potential for adjuvant therapy of PH treatment without side effects of chemical drugs. In addition, as mentioned, there is no definitive cure for PH, so use of herbs and phytochemicals can help to improve the quality of life in patients with this disorder.
Author Contributions
MF and SJ designed the study and collected the information. JE extracted articles and HK wrote the article. IA and JE revised the text.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thanks to Pharmaceutical Sciences Research Center of Kermanshah University of Medical Sciences for their supports. JE gratefully acknowledges support from CONICYT (PAI/ACADEMIA No. 79160109). IA expresses her thanks for the support provided by the project DN 16/3 (National Science Fund of Bulgaria).
References
Ahmadi, A., Khalili, M., Margedari, S. H., Nahri-Niknafs, B. (2016). The effects of solvent polarity on hypoglycemic and hypolipidemic activities of Securigera securidaca (L.) seeds. Drug Res. 66 (03), 130–135. doi: 10.1055/s-0035-1555773
Ahmadipour, B., Hassanpour, H., Asadi, E., Khajali, F., Rafiei, F., Khajali, F. (2015). Kelussia odoratissima Mozzaf–A promising medicinal herb to prevent pulmonary hypertension in broiler chickens reared at high altitude. J. Ethnopharmacol. 159, 49–54. doi: 10.1016/j.jep.2014.10.043
Ahmadipour, B., Kalantar, M., Hosseini, S., Yang, L., Kalantar, M., Raza, S., et al. (2017). Hawthorn (Crataegus oxyacantha) Extract in the drinking water of broilers on growth and incidence of Pulmonary Hypertension Syndrome (PHS). Braz. J. Poultry Sci. 19 (4), 639–644. doi: 10.1590/1806-9061-2017-0558
Ahmadipour, B., Kalantar, M., Hosseini, S. M., Ur Rehman, Z., Farmanullah, F., Kalantar, M. H., et al. (2019). Hawthorn (Crataegus oxyacantha) Flavonoid Extract as an Effective Medicinal Plant Derivative to Prevent Pulmonary Hypertension and Heart Failure in Broiler Chickens. Kafkas Univ. Vet. Fak. Derg. 25 (3), 321–328. doi: 10.9775/kvfd.2018.20930
Ahmadipour, B. (2018). Securigera securidaca seed medicinal herb supplementation of diets improves pulmonary hypertensive response in broiler chickens reared at high altitude. J. Anim. Physiol. Anim. Nutr. 102 (6), 1601–1607. doi: 10.1111/jpn.12981
Antoniu, S. A. (2012). Targeting RhoA/ROCK pathway in pulmonary arterial hypertension. Expert Opin. Ther. Targets 16 (4), 355–363. doi: 10.1517/14728222.2012.671811
Auyeung, K. K., Han, Q. B., Ko, J. K. (2016). Astragalus membranaceus: a review of its protection against inflammation and gastrointestinal cancers. Am. J. Chin. Med. 44(01), 1–22. doi: 10.1142/S0192415X16500014
Baghbanzadeh, A., Decuypere, E. (2008). Ascites syndrome in broilers: physiological and nutritional perspectives. Avian Pathol. 37 (2), 117–126. doi: 10.1080/03079450801902062
Balabanian, K., Foussat, A., Dorfm̈ller, P., Durand-Gasselin, I., Capel, F., Bouchet-Delbos, L., et al. (2002). CX3C chemokine fractalkine in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 165 (10), 1419–1425. doi: 10.1164/rccm.2106007
Belwal, T., Bisht, A., Devkota, H. P., Ullah, H., Khan, H., Bhatt, I. D., et al. (2020). Phytopharmacology and clinical updates of Berberis species against diabetes and other metabolic diseases. Front. Pharmacol. 11, 41. doi: 10.3389/fphar.2020.00041
Benza, R. L., Miller, D. P., Gomberg-Maitland, M., Frantz, R. P., Foreman, A. J., Coffey, C. S., et al. (2010). Predicting survival in pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL). Circulation 122 (2), 164–172. doi: 10.1161/CIRCULATIONAHA.109.898122
Beppu, H., Ichinose, F., Kawai, N., Jones, R. C., Yu, P. B., Zapol, W. M., et al. (2004). BMPR-II heterozygous mice have mild pulmonary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. Am. J. Physiol. Lung. Cell. Mol. Physiol. 287 (6), 1241–1247. doi: 10.1152/ajplung.00239.2004
Beretz, A., Stierle, A., Anton, R., Cazenave, J. P. (1982). Role of cyclic AMP in the inhibition of human platelet aggregation by quercetin, a flavonoid that potentiates the effect of prostacyclin. Biochem. Pharmacol. 31 (22), 3597–3600. doi: 10.1016/0006-2952(82)90581-0
Berger, M. M., Dehnert, C., Bailey, D. M., Luks, A. M., Menold, E., Castell, C., et al. (2009). Transpulmonary plasma ET-1 and nitrite differences in high altitude pulmonary hypertension. High Alt. Med. Biol. 10 (1), 17–24. doi: 10.1089/ham.2008.1053
Bombicz, M., Priksz, D., Varga, B., Kurucz, A., Kertész, A., Takacs, A., et al. (2017). A novel therapeutic approach in the treatment of pulmonary arterial hypertension: Allium ursinum liophylisate alleviates symptoms comparably to sildenafil. Int. J. Mol. Sci. 18 (7), 1436. doi: 10.3390/ijms18071436
Boué, S. M., Wiese, T. E., Nehls, S., Burow, M. E., Elliott, S., Carter-Wientjes, C. H., et al. (2003). Evaluation of the estrogenic effects of legume extracts containing phytoestrogens. J. Agric. Food Chem. 51 (8), 2193–2199. doi: 10.1021/jf021114s
Cai, H., Li, D., Xia, Z. (2007). Effects of Salvia Miltiorrhiza Bge. f. alba aqueous extract on cerebral ischemia. Chin. Traditional Patent Med. 29, 1082–1084.
Chang, Q., Zuo, Z., Harrison, F., Chow, M. S. S. (2002). Hawthorn. J. Clin. Pharm. 42 (6), 605–612. doi: 10.1177/00970002042006003
Chen, Y., Ruan, Y., Li, L., Chu, Y., Xu, X., Wang, Q., et al. (2003). Effects of Salvia miltiorrhiza extracts on rat hypoxic pulmonary hypertension, heme oxygenase-1 and nitric oxide synthase. Chin. Med. J. 116 (5), 757–760.
Chen, K.-H., Chen, Y.-J., Yang, C.-H., Liu, K.-W., Chang, J.-L., Pan, S.-F., et al. (2012). Attenuation of the extract from Moringa oleifera on monocrotaline-induced pulmonary hypertension in rats. Chin. J. Physiol. 55 (1), 22–30. doi: 10.4077/CJP.2012.AMM104
Chen, S., Yin, Z.-J., Jiang, C., Ma, Z.-Q., Fu, Q., Qu, R., et al. (2014). Asiaticoside attenuates memory impairment induced by transient cerebral ischemia–reperfusion in mice through anti-inflammatory mechanism. Pharmacol. Biochem. Behav. 122, 7–15. doi: 10.1016/j.pbb.2014.03.004
Cheng, X., Li, Q., Liu, J., Wu, G., Wang, T. (2017). Nobiletin protects against monocrotaline-induced pulmonary arterial hypertension in rats by regulating Src/STAT3 signaling pathway. Int. J. Clin. Exp. Med. 10 (7), 10342–10350.
Chu, M.-P., Xu, Z.-X., Zhang, X.-L. (2007). Changes of expression of ICAM-1 in autoimmune myocarditis in mice and the interfering effect of Panax notoginseng saponin. J. Wenzhou Med. College 37, 203–205.
Chu, C.-C., Wu, W.-S., Shieh, J.-P., Chu, H.-L., Lee, C.-P., Duh, P.-D. (2017). The anti-inflammatory and vasodilating effects of three selected dietary organic sulfur compounds from Allium species. J. Funct. Biomater. 8 (1), 5. doi: 10.3390/jfb8010005
Cool, C. D., Rai, P. R., Yeager, M. E., Hernandez-Saavedra, D., Serls, A. E., Bull, T. M., et al. (2003). Expression of human herpesvirus 8 in primary pulmonary hypertension. N. Engl. J. Med. 349 (12), 1113–1122. doi: 10.1056/NEJMoa035115
Davie, N. J., Crossno, J. T., Jr., Frid, M. G., Hofmeister, S. E., Reeves, J. T., Hyde, D. M., et al. (2004). Hypoxia-induced pulmonary artery adventitial remodeling and neovascularization: contribution of progenitor cells. Am. J. Physiol. Lung. Cell. Mol. Physiol. 286 (4), L668–L678. doi: 10.1152/ajplung.00108.2003
Dong, M.-S., Jung, S.-H., Kim, H.-j., Kim, J.-R., Zhao, L.-X., Lee, E.-S., et al. (2004). Structure-related cytotoxicity and anti-hepatofibric effect of asiatic acid derivatives in rat hepatic stellate cell-line, HSC-T6. Arch. Pharm. Res. 27 (5), 512–517. doi: 10.1007/bf02980124
Dong, L.-H., Wen, J.-K., Miao, S.-B., Jia, Z., Hu, H.-J., Sun, R.-H., et al. (2010). Baicalin inhibits PDGF-BB-stimulated vascular smooth muscle cell proliferation through suppressing PDGFRβ-ERK signaling and increase in p27 accumulation and prevents injury-induced neointimal hyperplasia. Cell Res. 20 (11), 1252–1262. doi: 10.1038/cr.2010.111
Dorfmüller, P., Perros, F., Balabanian, K., Humbert, M. (2003). Inflammation in pulmonary arterial hypertension. Eur. Respir. J. 22 (2), 358–363. doi: 10.1183/09031936.03.00038903
Dwivedi, S. (2007). Terminalia arjuna Wight and Arn. a useful drug for cardiovascular disorders. J. Ethnopharmacol. 114 (2), 114–129. doi: 10.1016/j.jep.2007.08.003
Espley, R. V., Butts, C. A., Laing, W. A., Martell, S., Smith, H., McGhie, T. K., et al. (2013). Dietary flavonoids from modified apple reduce inflammation markers and modulate gut microbiota in mice. J. Nutr. 144 (2), 146–154. doi: 10.3945/jn.113.182659
Faizi, S., Siddiqui, B. S., Saleem, R., Aftab, K., Shaheen, F. (1998). Hypotensive constituents from the pods of Moringa oleifera. Planta Med. 64 (03), 225–228. doi: 10.1055/s-2006-957414
Fakurazi, S., Hairuszah, I., Nanthini, U. (2008). Moringa oleifera Lam prevents acetaminophen induced liver injury through restoration of glutathione level. Food Chem. Toxicol. 46 (8), 2611–2615. doi: 10.1016/j.fct.2008.04.018
Fallon, M. B., Abrams, G. A., Abdel-Razek, T. T., Dai, J., Chen, S.-J., Chen, Y.-F., et al. (1998). Garlic prevents hypoxic pulmonary hypertension in rats. Am. J. Physiol. Lung Cell Mol. Physiol. 275 (2), L283–L287. doi: 10.1152/ajplung.1998.275.2.L283
Fathi, M., Nazer, A., Nezhad, Y. E., Shahryar, H. A., Daneshyar, M., Tanha, T. (2011). The role of oxidative stress in development of congestive heart failure (CHF) in broiler with pulmonary hypertension syndrome (PHS). J. Anim. Vet. Advan. 10 (20), 2724–2729.
Fleming, I. (2010). Molecular mechanisms underlying the activation of eNOS. Pflugers Arch. 459 (6), 793–806. doi: 10.1007/s00424-009-0767-7
Gómez, A., Moreno, M., Iglesias, A., Coral, P., Hernández, A. (2007). Endothelin 1, its endothelin type A receptor, connective tissue growth factor, platelet-derived growth factor, and adrenomedullin expression in lungs of pulmonary hypertensive and nonhypertensive chickens. Poult. Sci. 86 (5), 909–916. doi: 10.1093/ps/86.5.909
Gabor, M. (1986). Anti-inflammatory and anti-allergic properties of flavonoids. Prog. Clin. Biol. Res. 213, 471.
Galie, N., Hoeper, M. M., Humbert, M., Torbicki, A., Vachiery, J.-L., Barbera, J. A., et al. (2009). Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur. Heart J. 30 (20), 2493–2537. doi: 10.1093/eurheartj/ehp297
Gao, G., Wang, X., Qin, X., Jiang, X., Xiang, D., Xie, L., et al. (2012). Effects of trimethoxystilbene on proliferation and apoptosis of pulmonary artery smooth muscle cells. Cell Biochem. Biophys. 64 (2), 101–106. doi: 10.1007/s12013-012-9377-7
Gao, H., Chen, C., Huang, S., Li, B. (2012). Quercetin attenuates the progression of monocrotaline-induced pulmonary hypertension in rats. Int. J. Biomed. Res. 26 (2), 98–102. doi: 10.1016/S1674-8301(12)60018-9
Gao, Z., Li, Q., Wu, X., Zhao, X., Zhao, L., Tong, X. (2017). New insights into the mechanisms of chinese herbal products on diabetes: a focus on the “Bacteria-Mucosal Immunity-Inflammation-Diabetes” axis. J. Immunol. Res. 1813086. doi: 10.1155/2017/1813086
Guan, S., Feng, H., Song, B., Guo, W., Xiong, Y., Huang, G., et al. (2011). Salidroside attenuates LPS-induced pro-inflammatory cytokine responses and improves survival in murine endotoxemia. Int. Immunopharmacol. 11 (12), 2194–2199. doi: 10.1016/j.intimp.2011.09.0
Guan, Z., Shen, L., Liang, H., Yu, H., Hei, B., Meng, X., et al. (2017). Resveratrol inhibits hypoxia-induced proliferation and migration of pulmonary artery vascular smooth muscle cells by inhibiting the phosphoinositide 3-kinase/protein kinase B signaling pathway. Mol. Med. Rep. 16 (2), 1653–1660. doi: 10.3892/mmr.2017.6814
Guo, H., Zhang, X., Cui, Y., Deng, W., Xu, D., Han, H., et al. (2014). Isorhynchophylline protects against pulmonary arterial hypertension and suppresses PASMCs proliferation. Biochem. Biophys. Res. Commun. 450 (1), 729–734. doi: 10.1016/j.bbrc.2014.06.044
Gupta, R., Dubey, D., Kannan, G., Flora, S. (2007). Concomitant administration of Moringa oleifera seed powder in the remediation of arsenic-induced oxidative stress in mouse. Cell Biol. 31 (1), 44–56. doi: 10.1016/j.cellbi.2006.09.007
Han, C., Qi, J., Gao, S., Li, C., Ma, Y., Wang, J., et al. (2017). Vasodilation effect of volatile oil from Allium macrostemon Bunge are mediated by PKA/NO pathway and its constituent dimethyl disulfide in isolated rat pulmonary arterials. Fitoterapia 120, 52–57. doi: 10.1016/j.fitote.2017.05.007
He, Y., Cao, X., Liu, X., Li, X., Xu, Y., Liu, J., et al. (2015). Quercetin reverses experimental pulmonary arterial hypertension by modulating the TrkA pathway. Exp. Cell Res. 339 (1), 122–134. doi: 10.1016/j.yexcr.2015.10.013
Herve, P., Humbert, M., Sitbon, O., Parent, F., Nunes, H., Legal, C., et al. (2001). Pathobiology of pulmonary hypertension. The role of platelets and thrombosis. Clin. Chest Med. 22 (3), 451–458. doi: 10.1016/s0272-5231(05)70283-5
Homma, N., Morio, Y., Takahashi, H., Yamamoto, A., Suzuki, T., Sato, K., et al. (2006). Genistein, a phytoestrogen, attenuates monocrotaline-induced pulmonary hypertension. Respiration 73 (1), 105–112. doi: 10.1159/000088946
Horníčková, J., Kubec, R., Cejpek, K., Velíšek, J., Ovesna, J., Stavělíková, H. (2010). Profiles of S-alk (en) ylcysteine sulfoxides in various garlic genotypes. Czech J. Food Sci. 28 (4), 298–308.
Hosseinzadeh, H., Ramezani, M., Danaei, A. (2002). Antihyperglycaemic effect and acute toxicity of Securigera securidaca L. seed extracts in mice. Phytother. Res. 16 (8), 745–747. doi: 10.1002/ptr.1020
Hsu, W.-L., Lin, Y.-C., Jeng, J.-R., Chang, H.-Y., Chou, T.-C. (2018). Baicalein Ameliorates Pulmonary Arterial Hypertension Caused by Monocrotaline through Downregulation of ET-1 and ETAR in Pneumonectomized Rats. Am. J. Chin. Med. 46 (04), 769–783. doi: 10.1142/S0192415X18500404
Hu, Q., Noor, M., Wong, Y. F., Hylands, P. J., Simmonds, M. S., Xu, Q., et al. (2009). In vitro anti-fibrotic activities of herbal compounds and herbs. Nephrol. Dial. Transplant 24 (10), 3033–3041. doi: 10.1093/ndt/gfp245
Hua, C., Zhao, J., Wang, H., Chen, F., Meng, H., Chen, L., et al. (2018). Apple polyphenol relieves hypoxia-induced pulmonary arterial hypertension via pulmonary endothelium protection and smooth muscle relaxation: In vivo and in vitro studies. Biomed. Pharmacother. 107, 937–944. doi: 10.1016/j.biopha.2018.08.080
Huang, S., Chen, P., Shui, X., He, Y., Wang, H., Zheng, J., et al. (2014). Baicalin attenuates transforming growth factor-β1-induced human pulmonary artery smooth muscle cell proliferation and phenotypic switch by inhibiting hypoxia inducible factor-1α and aryl hydrocarbon receptor expression. J. Pharm. Pharmacol. 66 (10), 1469–1477. doi: 10.1111/jphp.12273
Huang, X., Zou, L., Yu, X., Chen, M., Guo, R., Cai, H., et al. (2015). Salidroside attenuates chronic hypoxia-induced pulmonary hypertension via adenosine A2a receptor related mitochondria-dependent apoptosis pathway. J. Mol. Cell. Cardiol. 82, 153–166. doi: 10.1016/j.yjmcc.2015.03.005
Huang, H., Li, L., Shi, W., Liu, H., Yang, J., Yuan, X., et al. (2016). The multifunctional effects of nobiletin and its metabolites in vivo and in vitro. Evid. Based. Complement. Alternat. Med. 2016, 2918796. doi: 10.1155/2016/2918796
Hui-li, G. (2011). The management of acute pulmonary arterial hypertension. Cardiovasc. Ther. 29 (3), 153–175. doi: 10.1111/j.1755-5922.2009.00095.x
Humbert, M., Morrell, N. W., Archer, S. L., Stenmark, K. R., MacLean, M. R., Lang, I. M., et al. (2004). Cellular and molecular pathobiology of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 43 (12), S13–S24. doi: 10.1016/j.jacc.2004.02.029
Ichikawa, H., Takada, Y., Shishodia, S., Jayaprakasam, B., Nair, M. G., Aggarwal, B. B. (2006). Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-κB (NF-κB) activation and NF-κB–regulated gene expression. Mol. Cancer Ther. 5(6), 1434–1445. doi: 10.1158/1535-7163
Ji, Q., Liu, X., Fu, X., Zhang, L., Sui, H., Zhou, L., et al. (2013). Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/β-catenin signal pathway. PloS One 8 (11), e78700. doi: 10.1371/journal.pone.0078700
Jiang, Y., Yang, Y. (2016). Trifolium pratense isoflavones improve pulmonary vascular remodelling in broiler chickens. J. Anim. Physiol. Anim. Nutr. 100 (6), 1159–1168. doi: 10.1111/jpn.12424
Jin, H., Liu, M., Zhang, X., Pan, J., Han, J., Wang, Y., et al. (2016). Grape seed procyanidin extract attenuates hypoxic pulmonary hypertension by inhibiting oxidative stress and pulmonary arterial smooth muscle cells proliferation. J. Nutr. Biochem. 36, 81–88. doi: 10.1016/j.jnutbio.2016.07.006
Julian, R., Mirsalimi, S. (1992). Blood oxygen concentration of fast-growing and slow-growing broiler chickens, and chickens with ascites from right ventricular failure. Avian Dis. 36 (3), 730–732. doi: 10.2307/1591774
Jun-xue, W., Guo-jun, W. (2000). Pharmacological effects and clinical application of matrine and oxymatrine. Chin. Hepatol. 5 (2), 116–117.
Kapoor, D., Vijayvergiya, R., Dhawan, V. (2014). Terminalia arjuna in coronary artery disease: ethnopharmacology, pre-clinical, clinical and safety evaluation. J. Ethnopharmacol. 155 (2), 1029–1045. doi: 10.1016/j.jep.2014.06.056
Kass, D. A., Champion, H. C., Beavo, J. A. (2007). Phosphodiesterase type 5: expanding roles in cardiovascular regulation. Circ. Res. 101 (11), 1084–1095. doi: 10.1161/CIRCRESAHA.107.162511
Kaur, G., Singh, N., Samuel, S. S., Bora, H. K., Sharma, S., Pachauri, S. D., et al. (2015). Withania somnifera shows a protective effect in monocrotaline-induced pulmonary hypertension. Pharm. Biol. 53 (1), 147–157. doi: 10.3109/13880209.2014.912240
Kawakami, Z., Kanno, H., Ikarashi, Y., Kase, Y. (2011). Yokukansan, a kampo medicine, protects against glutamate cytotoxicity due to oxidative stress in PC12 cells. J. Ethnopharmacol. 134 (1), 74–81. doi: 10.1016/j.jep.2010.11.063
Kay, J. M., Harris, P., Heath, D. (1967). Pulmonary hypertension produced in rats by ingestion of Crotalaria spectabilis seeds. Thorax 22 (2), 176–179. doi: 10.1136/thx.22.2.176
Khajali, F., Fahimi, S. (2010). Influence of dietary fat source and supplementary α-tocopheryl acetate on pulmonary hypertension and lipid peroxidation in broilers. J. Anim. Physiol. Anim. Nutr. 94 (6), 767–772. doi: 10.1111/j.1439-0396.2009.00959.x
Lanzotti, V., Scala, F., Bonanomi, G. (2014). Compounds from Allium species with cytotoxic and antimicrobial activity. Phytochem. Rev. 13 (4), 769–791. doi: 10.1007/s11101-014-9366-0
Lay, S., Chiu, J. H., Shiao, M. S., Lui, W. Y., Wu, C. W. (2003). Crude extract of Salvia miltiorrhiza and salvianolic acid B enhance in vitro angiogenesis in murine SVR endothelial cell line. Planta Med. 69 (01), 26–32. doi: 10.1055/s-2003-37034
Li, H., Sze, S., Tong, Y., Ng, T. (2009). Production of Th1-and Th2-dependent cytokines induced by the Chinese medicine herb, Rhodiola algida, on human peripheral blood monocytes. J. Ethnopharmacol. 123 (2), 257–266. doi: 10.1016/j.jep.2009.03.009
Li, Q. Y., Chen, L., Fu, W. H., Li, Z. D., Wang, B., Shi, X. J., et al. (2011). Ginsenoside Rb1 inhibits proliferation and inflammatory responses in rat aortic smooth muscle cells. J. Agric. Food. Chem. 59 (11), 6312–6318. doi: 10.1021/jf200424k
Li, G., Gai, X., Li, Z., Chang, R., Qi, Y., Zhaxi, D., et al. (2016). Preliminary study of active component and mechanism of Rhodiola algida var. tangutica on inducing rat pulmonary artery vasorelaxation. J. Qin. Med. Coll. 01, 40–45.
Li, T., Luo, X. J., Wang, E. L., Li, N. S., Zhang, X. J., Song, F. L., et al. (2019). Magnesium lithospermate B prevents phenotypic transformation of pulmonary arteries in rats with hypoxic pulmonary hypertension through suppression of NADPH oxidase. Europ. J. Pharmacol. 847, 32–41. doi: 10.1016/j.ejphar.2019.01.020
Lines, T., Ono, M. (2006). FRS 1000, an extract of red onion peel, strongly inhibits phosphodiesterase 5A (PDE 5A). Phytomedicine 13 (4), 236–239. doi: 10.1016/j.phymed.2004.12.001
Liu, P., Yan, S., Chen, M., Chen, A., Yao, D., Xu, X., et al. (2015). Effects of baicalin on collagen I and collagen III expression in pulmonary arteries of rats with hypoxic pulmonary hypertension. Int. J. Mol. Med. 35 (4), 901–908. doi: 10.3892/ijmm.2015.2110
Llanos, A. J., Ebensperger, G., Herrera, E. A., Reyes, R. V., Cabello, G., Díaz, M., et al. (2012). The heme oxygenase–carbon monoxide system in the regulation of cardiorespiratory function at high altitude. Respir. Physiol. Neurobiol. 184 (2), 186–191. doi: 10.1016/j.resp.2012.05.003
Lou, Z., Ren, K. D., Tan, B., Peng, J. J., Ren, X., Yang, Z. B., et al. (2015). Salviaolate protects rat brain from ischemia-reperfusion injury through inhibition of NADPH oxidase. Planta Med. 81 (15), 1361–1369. doi: 10.1055/s-0035-1557774
Lu, Y., Foo, L. Y. (2002). Polyphenolics of Salvia—a review. Phytochemistry 59 (2), 117–140. doi: 10.1016/S0031-9422(01)00415-0
Luo, J., Gu, Y., Liu, P., Jiang, X., Yu, W., Ye, P., et al. (2018). Berberine attenuates pulmonary arterial hypertension via protein phosphatase 2A signaling pathway both in vivo and in vitro. J. Cell. Physiol. 233 (12), 9750–9762. doi: 10.1002/jcp.26940
Lythgoe, M. P., Rhodes, C. J., Ghataorhe, P., Attard, M., Wharton, J., Wilkins, M. R. (2016). Why drugs fail in clinical trials in pulmonary arterial hypertension, and strategies to succeed in the future. Pharmacol. Therapeut. 164, 195–203. doi: 10.1016/j.pharmthera.2016.04.012
Ma, N., Li, J., Xu, F., Xiang, D.-X. (2009). Studies on metabolic structures and pathways of resveratrol derivative in rat urine. Chin. J. Pharm. Anal. 29 (12), 1994–2001.
Masszi, A., Fan, L., Rosivall, L., McCulloch, C. A., Rotstein, O. D., Mucsi, I., et al. (2004). Integrity of cell-cell contacts is a critical regulator of TGF-β1-induced epithelial-to-myofibroblast transition: role for β-catenin. Am. J. Pathol. 165 (6), 1955–1967. doi: 10.1016/S0002-9440(10)63247-6
Matori, H., Umar, S., Nadadur, R. D., Sharma, S., Partow-Navid, R., Afkhami, M., et al. (2012). Genistein, a soy phytoestrogen, reverses severe pulmonary hypertension and prevents right heart failure in rats. Hypertension 60 (2), 425–430. doi: 10.1161/HYPERTENSIONAHA.112.191445
McLaughlin, V. V., Oudiz, R. J., Frost, A., Tapson, V. F., Murali, S., Channick, R. N., et al. (2006). Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 174 (11), 1257–1263. doi: 10.1164/rccm.200603-358OC
Meghwani, H., Prabhakar, P., Mohammed, S. A., Seth, S., Hote, M. P., Banerjee, S. K., et al. (2017). Beneficial effects of aqueous extract of stem bark of Terminalia arjuna (Roxb.), An ayurvedic drug in experimental pulmonary hypertension. J. Ethnopharmacol. 197, 184–194. doi: 10.1016/j.jep.2016.07.029
Miao, Q., Wang, S., Miao, S., Wang, J., Xie, Y., Yang, Q. (2011). Cardioprotective effect of polydatin against ischemia/reperfusion injury: roles of protein kinase C and mito KATP activation. Phytomedicine 19 (1), 8–12. doi: 10.1016/j.phymed.2011.06.023
Miao, Q., Shi, X. P., Ye, M. X., Zhang, J., Miao, S., Wang, S. W., et al. (2012). Polydatin attenuates hypoxic pulmonary hypertension and reverses remodeling through protein kinase C mechanisms. Int. J. Mol. Sci. 13 (6), 7776–7787. doi: 10.3390/ijms13067776
Mohanty, I., Arya, D. S., Dinda, A., Talwar, K. K., Joshi, S., Gupta, S. K. (2004). Mechanisms of cardioprotective effect of Withania somnifera in experimentally induced myocardial infarction. Basic Clin. Pharmacol. Toxicol. 94 (4), 184–190. doi: 10.1111/j.1742-7843.2004.pto940405.x
Mohanty, I. R., Arya, D. S., Gupta, S. K. (2008). Withania somnifera provides cardioprotection and attenuates ischemia–reperfusion induced apoptosis. Clin. Nutr. 27 (4), 635–642. doi: 10.1016/j.clnu.2008.05.006
Morales-Cano, D., Menéndez, C., Moreno, E., Moral-Sanz, J., Barreira, B., Galindo, P., et al. (2014). The flavonoid quercetin reverses pulmonary hypertension in rats. PloS One 9 (12), e114492. doi: 10.1371/journal.pone.0114492
Muhammad, D., Hubert, J., Lalun, N., Renault, J. H., Bobichon, H., Nour, M., et al. (2015). Isolation of Flavonoids and Triterpenoids from the Fruits of Alphitonia Neocaledonica and Evaluation of their Anti-oxidant, Anti-tyrosinase and Cytotoxic Activities. Phytochem. Anal. 26 (2), 137–144. doi: 10.1002/pca.2545
Nagaoka, T., Muramatsu, M., Sato, K., McMurtry, I., Oka, M., Fukuchi, Y. (2001). Mild hypoxia causes severe pulmonary hypertension in fawn-hooded but not in Tester Moriyama rats. Resp. Physiol. 127 (1), 53–60. doi: 10.1016/S0034-5687(01)00217-1
Nan, X., Su, S., Ma, K., Ma, X., Wang, X., Zhaxi, D., et al. (2018). Bioactive fraction of Rhodiola algida against chronic hypoxia-induced pulmonary arterial hypertension and its anti-proliferation mechanism in rats. J. Ethnopharmacol. 216, 175–183. doi: 10.1016/j.jep.2018.01.010
Narkhede, A. N., Kasote, D. M., Kuvalekar, A. A., Harsulkar, A. M., Jagtap, S. D. (2016). Amarkand: A comprehensive review on its ethnopharmacology, nutritional aspects, and taxonomy. J. Intercult. Ethnopharmacol. 5 (2), 198–204. doi: 10.5455/jice.20160324054420
Neag, M. A., Mocan, A., Echeverría, J., Pop, R. M., Bocsan, C. I., Crişan, G., et al. (2018). Berberine: Botanical occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic, and renal disorders. Front. Pharmacol. 9, 557. doi: 10.3389/fphar.2018.00557
Occhiuto, F., Limardi, F. (1994). Comparative effects of the flavonoids luteolin, apiin and rhoifolin on experimental pulmonary hypertension in the dog. Phytother. Res. 8 (3), 153–156. doi: 10.1002/ptr.2650080307
Occhiuto, F., Busà, G., De, A. P. (1988). Study on the action mechanism of some flavones through registration of intracardiac potentials in anesthetized dogs. Pharmacol. Res. Commun. 20 (12), 1073–1074. doi: 10.1016/s0031-6989(88)80732-x
Ojha, S. K., Arya, D. S. (2009). Withania somnifera Dunal (Ashwagandha): a promising remedy for cardiovascular diseases. World J. Med. Sci. 4(2), 156–158.
Oszmianski, J., Kolniak-Ostek, J., Wojdyło, A. (2012). Characterization and content of flavonol derivatives of Allium ursinum L. plant. J. Agric. Food Chem. 61 (1), 176–184. doi: 10.1021/jf304268e
Paffett, M. L., Lucas, S. N., Campen, M. J. (2012). Resveratrol reverses monocrotaline-induced pulmonary vascular and cardiac dysfunction: a potential role for atrogin-1 in smooth muscle. Vasc. Pharmacol. 56 (1-2), 64–73. doi: 10.1016/j.vph.2011.11.002
Panossian, A., Wagner, H. (2005). Stimulating effect of adaptogens: an overview with particular reference to their efficacy following single dose administration. Phytother. Res. 19 (10), 819–838. doi: 10.1002/ptr.1751
Paulin, R., Courboulin, A., Meloche, J., Mainguy, V., Dumas de la Roque, E., Saksouk, N., et al. (2011). Signal transducers and activators of transcription-3/pim1 axis plays a critical role in the pathogenesis of human pulmonary arterial hypertension. Circulation 123 (11), 1205–1215. doi: 10.1161/CIRCULATIONAHA.110.963314
Pawar, R., Bhutani, K. (2005). Effect of oleanane triterpenoids from Terminalia arjuna a cardioprotective drug on the process of respiratory oxyburst. Phytomedicine 12 (5), 391–393. doi: 10.1016/j.phymed.2003.11.007
Peng, J., Wei, D., Fu, Z., Li, D., Tan, Y., Xu, T., et al. (2015). Punicalagin ameliorates lipopolysaccharide-induced acute respiratory distress syndrome in mice. Inflammation 38 (2), 493–499. doi: 10.1007/s10753-014-9955-5
Pepke-Zaba, J., Gilbert, C., Collings, L., Brown, M. C. (2008). Sildenafil improves health-related quality of life in patients with pulmonary arterial hypertension. Chest 133 (1), 183–189. doi: 10.1378/chest.07-0592
Pirbalouti, A. G., Setayesh, M., Siahpoosh, A., Mashayekhi, H. (2013). Antioxidant activity, total phenolic and flavonoids contents of three herbs used as condiments and additives in pickles products. Herb. Pol. 59 (3), 51–62. doi: 10.2478/hepo-2013-0016
Powell, F. L., Hastings, R. H., Mazzone, R. W. (1985). Pulmonary vascular resistance during unilateral pulmonary arterial occlusion in ducks. Am. J. Physiol. Regul. Integr. Comp. Physiol. 249 (1), R39–R43. doi: 10.1152/ajpregu.1985.249.1.R39
Prabhakar, N. R., Semenza, G. L. (2012). Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol. Rev. 92 (3), 967–1003. doi: 10.1152/physrev.00030.2011
Rabinovitch, M., Gamble, W. J., Nadas, A. S., Miettinen, O. S., Reid, L. (1979). Rat pulmonary circulation after chronic hypoxia: Hemodynamic and structural features. Am. J. Physiol. 236 (6), 818–827. doi: 10.1152/ajpheart.1979.236.6.H818
Rakotomalala, G., Agard, C., Tonnerre, P., Tesse, A., Derbré, S., Michalet, S., et al. (2013). Extract from Mimosa pigra attenuates chronic experimental pulmonary hypertension. J. Ethnopharmacol. 148 (1), 106–116. doi: 10.1016/j.jep.2013.03.075
Reuter, H., Koch, H., Lawson, L. (1996). The Science and Therapeutic Application of Allium sativum L. and Related Species (Baltimore, MD: Williams and Wilkins).
Reyes, R. V., Castillo-Galán, S., Hernandez, I., Herrera, E. A., Ebensperger, G., Llanos, A. J. (2018). Revisiting the role of TRP, orai, and ASIC channels in the pulmonary arterial response to hypoxia. Front. Physiol. 9, 486. doi: 10.3389/fphys.2018.00486
Richter, A., Yeager, M. E., Zaiman, A., Cool, C. D., Voelkel, N. F., Tuder, R. M. (2004). Impaired transforming growth factor-β signaling in idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 170 (12), 1340–1348. doi: 10.1164/rccm.200311-1602OC
Rosado-Vallado, M., Brito-Loeza, W., Mena-Rejon, G., Quintero-Marmol, E., Flores-Guido, J. (2000). Antimicrobial activity of Fabaceae species used in Yucatan traditional medicine. Fitoterapia 71 (5), 570–573. doi: 10.1016/S0367-326X(00)00200-8
Sökmen, M., Serkedjieva, J., Daferera, D., Gulluce, M., Polissiou, M., Tepe, B., et al. (2004). In vitro antioxidant, antimicrobial, and antiviral activities of the essential oil and various extracts from herbal parts and callus cultures of Origanum acutidens. J. Agric. Food Chem. 52 (11), 3309–3312. doi: 10.1021/jf049859g
Salehi, S., Long, S. R., Proteau, P. J., Filtz, T. M. (2009). Hawthorn (Crataegus monogyna Jacq.) extract exhibits atropine-sensitive activity in a cultured cardiomyocyte assay. J. Nat. Med. 63 (1), 1–8. doi: 10.1007/s11418-008-0278-4
Schermuly, R. T., Ghofrani, H. A., Wilkins, M. R., Grimminger, F. (2011). Mechanisms of disease: pulmonary arterial hypertension. Nat. Rev. Cardiol. 8 (8), 443. doi: 10.1038/nrcardio.2011.87
Shao, J., Wang, P., Liu, A., Du, X., Bai, J., Chen, M. (2016). Punicalagin prevents hypoxic pulmonary hypertension via anti-oxidant effects in rats. Am. J. Chin. Med. 44 (04), 785–801. doi: 10.1142/S0192415X16500439
Shirley, K. L., Beckman, D. W., Garrick, D. J. (2008). Inheritance of pulmonary arterial pressure in Angus cattle and its correlation with growth. J. Anim. Sci. 86 (4), 815–819. doi: 10.2527/jas.2007-0270
Shivakrupa, R., Bernstein, A., Watring, N., Linnekin, D. (2003). Phosphatidylinositol 3′-kinase is required for growth of mast cells expressing the kit catalytic domain mutant. Cancer Res. 63 (15), 4412–4419.
Shojaei, Z. A., Ebrahimi, A., Salimi, M. (2011). Chemical composition of three ecotypes of wild celery (Kelussia odoratissima). J. Herb. Spices Med. Plants 17 (1), 62–68. doi: 10.1080/10496475.2011.560089
Si-chuan, X., Dong-yuan, C., Wan-rong, Z. (1994). The inhibitory effect of radix salviae miltiorrhizae on hypoxic structural remodeling of intra-acinar pulmonary arteries. J. Tongji Med. Univ. 14 (3), 148–152. doi: 10.1007/bf02886795
Simoncini, T., Fornari, L., Mannella, P., Caruso, A., Garibaldi, S., Baldacci, C., et al. (2005). Activation of nitric oxide synthesis in human endothelial cells by red clover extracts. Menopause 12 (1), 69–77.
Simonneau, G., Gatzoulis, M. A., Adatia, I., Celermajer, D., Denton, C., Ghofrani, A., et al. (2013). Updated clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 62, 34–41. doi: 10.1016/j.jacc.2013.10.029
Sitbon, O., Humbert, M., Nunes, H., Parent, F., Garcia, G., Hervé, P., et al. (2002). Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J. Am. Coll. Cardiol. 40 (4), 780–788. doi: 10.1016/s0735-1097(02)02012-0
Sobolewska, D., Podolak, I., Makowska-Wąs, J. (2015). Allium ursinum: botanical, phytochemical and pharmacological overview. Phytochem. Rev. 14 (1), 81–97. doi: 10.1007/s11101-013-9334-0
Star, G. P., Giovinazzo, M., Langleben, D. (2009). Effects of bone morphogenic proteins and transforming growth factor-beta on In-vitro production of endothelin-1 by human pulmonary microvascular endothelial cells. Vasc. Pharmacol. 50 (1-2), 45–50. doi: 10.1016/j.vph.2008.09.001
Stenmark, K. R., Gerasimovskaya, E., Nemenoff, R. A., Das, M. (2002). Hypoxic activation of adventitial fibroblasts: role in vascular remodeling. Chest 122 (6), 326S–334S. doi: 10.1378/chest.122.6_suppl.326s
Stohs, S. J., Hartman, M. J. (2015). Review of the safety and efficacy of Moringa oleifera. Phytother. Res. 29 (6), 796–804. doi: 10.1002/ptr.5325
Surai, P. (2014). Polyphenol compounds in the chicken/animal diet: from the past to the future. J. Anim. Physiol. Anim. Nutr. 98 (1), 19–31. doi: 10.1111/jpn.12070
Suresh, K., Shimoda, L. A. (2016). Lung circulation. Compr. Physiol. 6 (2), 897–943. doi: 10.1002/cphy.c140049
Tan, X., Chai, J., Bi, S.-C., Li, J.-J., Li, W.-W., Zhou, J.-Y. (2012). Involvement of matrix metalloproteinase-2 in medial hypertrophy of pulmonary arterioles in broiler chickens with pulmonary arterial hypertension. Vet. J. 193 (2), 420–425. doi: 10.1016/j.tvjl.2012.01.017
Temkitthawon, P., Changwichit, K., Khorana, N., Viyoch, J., Suwanborirux, K., Ingkaninan, K. (2017). Phenanthrenes from Eulophia macrobulbon as novel phosphodiesterase-5 inhibitors. Nat. Prod. Commun. 12 (1), 79–82. doi: 10.1177/1934578X1701200121
Testa, J. R., Bellacosa, A. (2001). AKT plays a central role in tumorigenesis. Proc. Natl. Acad. Sci. U. S. A. 98 (20), 10983–10985. doi: 10.1073/pnas.211430998
Umar, S., Iorga, A., Matori, H., Nadadur, R. D., Li, J., Maltese, F., et al. (2011). Estrogen rescues preexisting severe pulmonary hypertension in rats. Am. J. Respir. Crit. Care Med. 184 (6), 715–723. doi: 10.1164/rccm.201101-0078OC
Varghese, A., Savai, J., Pandita, N., Gaud, R. (2015). In vitro modulatory effects of Terminalia arjuna, arjunic acid, arjunetin and arjungenin on CYP3A4, CYP2D6 and CYP2C9 enzyme activity in human liver microsomes. Toxicol. Rep. 2, 806–816. doi: 10.1016/j.toxrep.2015.02.008
Vilahur, G., Padró, T., Casaní, L., Mendieta, G., López, J. A., Streitenberger, S., et al. (2015). Polyphenol-enriched diet prevents coronary endothelial dysfunction by activating the Akt/eNOS pathway. Rev. Esp. Cardiol. 68 (3), 216–225. doi: 10.1016/j.rec.2014.04.021
Voelkel, N. F., Gomez-Arroyo, J., Abbate, A., Bogaard, H. J., Nicolls, M. R. (2012). Pathobiology of pulmonary arterial hypertension and right ventricular failure. Eur. Respir. J. 40 (6), 1555–1565. doi: 10.1183/09031936.00046612
Wang, G. S., Qian, G. S., Zhou, D. S., Zhao, J. Q. (2005). JAK—STAT signaling pathway in pulmonary arterial smooth muscle cells is activated by hypoxia. Cell Biol. Int. 29 (7), 598–603. doi: 10.1016/j.cellbi.2005.03.014
Wang, L.-D., Qiu, X.-Q., Tian, Z.-F., Zhang, Y.-F., Li, H.-F. (2008). Inhibitory effects of genistein and resveratrol on guinea pig gallbladder contractility in vitro. World J. Gastroenterol. 14 (31), 4955. doi: 10.3748/wjg.14.4955
Wang, Z., Lakes, R. S., Eickhoff, J. C., Chesler, N. C. (2013). Effects of collagen deposition on passive and active mechanical properties of large pulmonary arteries in hypoxic pulmonary hypertension. Biomech. Model. Mechan. 12 (6), 1115–1125. doi: 10.1007/s10237-012-0467-7
Wang, Y., Cao, S.-H., Cui, Y.-J., Kong, L.-K., Tian, H., Cai, H.-X., et al. (2015). Salvia miltiorrhiza Bge. f. alba Ameliorates the Progression of Monocrotaline-Induced Pulmonary Hypertension by Protecting Endothelial Injury in Rats. Tohoku J. Exp. Med. 236 (2), 155–162. doi: 10.1620/tjem.236.155
Wang, X. B., Wang, W., Zhu, X. C., Ye, W. J., Cai, H., Wu, P. L., et al. (2015). The potential of asiaticoside for TGF-β1/Smad signaling inhibition in prevention and progression of hypoxia-induced pulmonary hypertension. Life Sci. 137, 56–64. doi: 10.1016/j.lfs.2015.07.016
Wang, R. X., He, R. L., Jiao, H. X., Dai, M., Mu, Y. P., Hu, Y., et al. (2015). Ginsenoside Rb1 attenuates agonist-induced contractile response via inhibition of store-operated calcium entry in pulmonary arteries of normal and pulmonary hypertensive rats. Cell. Physiol. Biochem. 35 (4), 1467–1481. doi: 10.1159/000373966
Wang, X., Cai, X., Wang, W., Jin, Y., Chen, M., Huang, X., et al. (2018). Effect of asiaticoside on endothelial cells in hypoxia−induced pulmonary hypertension. Mol. Med. Rep. 17 (2), 2893–2900. doi: 10.3892/mmr.2017.8254
Wang, B. Q. (2010). Salvia miltiorrhiza: Chemical and pharmacological review of a medicinal plant. J. Med. Plants Res. 4 (25), 2813–2820.
Waxman, A. B., Zamanian, R. T. (2013). Pulmonary arterial hypertension: new insights into the optimal role of current and emerging prostacyclin therapies. Am. J. Cardiol. 111 (5), 1–16. doi: 10.1016/j.amjcard.2012.12.002
Welsh, D., Mortimer, H., Kirk, A., Peacock, A. (2005). The role of p38 mitogen-activated protein kinase in hypoxia-induced vascular cell proliferation: an interspecies comparison. Chest 128 (6), 573–574. doi: 10.1378/chest.128.6_suppl.573S
Wisutthathum, S., Demougeot, C., Totoson, P., Adthapanyawanich, K., Ingkaninan, K., Temkitthawon, P., et al. (2018). Eulophia macrobulbon extract relaxes rat isolated pulmonary artery and protects against monocrotaline-induced pulmonary arterial hypertension. Phytomedicine 50, 157–165. doi: 10.1016/j.phymed.2018.05.014
Wong, C.-M., Bansal, G., Pavlickova, L., Marcocci, L., Suzuki, Y. J. (2013). Reactive oxygen species and antioxidants in pulmonary hypertension. Antioxid. Redox Signal. 18 (14), 1789–1796. doi: 10.1089/ars.2012.4568
Xu, G. L., Yao, L., Rao, S. Y., Gong, Z. N., Zhang, S. Q., Yu, S. Q. (2005). Attenuation of acute lung injury in mice by oxymatrine is associated with inhibition of phosphorylated p38 mitogen-activated protein kinase. J. Ethnopharmacol. 98 (1-2), 177–183. doi: 10.1016/j.jep.2005.01.026
Xu, X., Li, H., Hou, X., Li, D., He, S., Wan, C., et al. (2015). Punicalagin induces Nrf2/HO-1 expression via upregulation of PI3K/AKT pathway and inhibits LPS-induced oxidative stress in RAW264. 7 macrophages. Mediators Inflamm. 2015, 705–715. doi: 10.1155/2015/380218
Xu, D., Li, Y., Zhang, B., Wang, Y., Liu, Y., Luo, Y., et al. (2016). Resveratrol alleviate hypoxic pulmonary hypertension via anti-inflammation and anti-oxidant pathways in rats. Int. J. Med. Sci. 13 (12), 942–954. doi: 10.7150/ijms.16810
Yamamura, H., Yamamura, A., Ko, E. A., Pohl, N. M., Smith, K. A., Zeifman, A., et al. (2014). Activation of Notch signaling by short-term treatment with Jagged-1 enhances store-operated Ca2+ entry in human pulmonary arterial smooth muscle cells. Am. J. Physiol. Cell Physiol. 306 (9), C871–C878. doi: 10.1152/ajpcell.00221.2013
Yeager, M. E., Halley, G. R., Golpon, H. A., Voelkel, N. F., Tuder, R. M. (2001). Microsatellite instability of endothelial cell growth and apoptosis genes within plexiform lesions in primary pulmonary hypertension. Circ. Res. 88 (1), e2–e11. doi: 10.1161/01.res.88.1.e2
Yeung, A. W. K., Aggarwal, B. B., Balacheva, A., Barreca, D., Battino, M., Belwal, T., et al. (2019). Resveratrol, a popular dietary supplement for human and animal health: Quantitative research literature analysis. Anim. Sci. Pap. Rep. 37 (2), 103–118.
Yu, J., Eto, M., Akishita, M., Kaneko, A., Ouchi, Y., Okabe, T. (2007). Signaling pathway of nitric oxide production induced by ginsenoside Rb1 in human aortic endothelial cells: a possible involvement of androgen receptor. Biochem. Biophys. Res. Commun. 353 (3), 764–769. doi: 10.1016/j.bbrc.2006.12.119
Yuan, L.-B., Hua, C.-Y., Gao, S., Yin, Y.-L., Dai, M., Meng, H.-Y., et al. (2017). Astragalus polysaccharides attenuate monocrotaline-induced pulmonary arterial hypertension in rats. Am. J. Chin. Med. 45 (04), 773–789. doi: 10.1142/S0192415X17500410
Zhang, B., Niu, W., Xu, D., Li, Y., Liu, M., Wang, Y., et al. (2014). Oxymatrine prevents hypoxia-and monocrotaline-induced pulmonary hypertension in rats. Free Radic. Biol. Med. 69, 198–207. doi: 10.1016/j.freeradbiomed.2014.01.013
Zhang, Q., Fan, K., Wang, P., Yu, J., Liu, R., Qi, H., et al. (2016). Carvacrol induces the apoptosis of pulmonary artery smooth muscle cells under hypoxia. Eur. J. Pharmacol. 770, 134–146. doi: 10.1016/j.ejphar.2015.11.037
Zhao, S., Zheng, M.-X., Chen, H.-e., Wu, C.-Y., Wang, W. (2015). Effect of Panax notoginseng saponins injection on the p38MAPK pathway in lung tissue in a rat model of hypoxic pulmonary hypertension. Chin. J. Integr. Med. 21 (2), 147–151. doi: 10.1007/s11655-014-1790-2
Zhou, J.-Y., Zhou, S.-W. (2012). Isorhynchophylline: A plant alkaloid with therapeutic potential for cardiovascular and central nervous system diseases. Fitoterapia 83 (4), 617–626. doi: 10.1016/j.fitote.2012.02.010
Zhu, F., Qian, C. (2006). Berberine chloride can ameliorate the spatial memory impairment and increase the expression of interleukin-1beta and inducible nitric oxide synthase in the rat model of Alzheimer's disease. BMC Neurosci. 7 (1), 78. doi: 10.1186/1471-2202-7-78
Appendix.
Keywords: pulmonary hypertension, medicinal plants, phytochemicals, herbal medicine, phytopharmacological review
Citation: Jasemi SV, Khazaei H, Aneva IY, Farzaei MH and Echeverría J (2020) Medicinal Plants and Phytochemicals for the Treatment of Pulmonary Hypertension. Front. Pharmacol. 11:145. doi: 10.3389/fphar.2020.00145
Received: 27 November 2019; Accepted: 04 February 2020;
Published: 12 March 2020.
Edited by:
Banasri Hazra, Jadavpur University, IndiaReviewed by:
Wesam Kooti, Kurdistan University of Medical Sciences, IranRoberto V. Reyes, University of Chile, Chile
Copyright © 2020 Jasemi, Khazaei, Aneva, Farzaei and Echeverría. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Hosein Farzaei, bWguZmFyemFlaUBnbWFpbC5jb20=; Javier Echeverría, amF2aWVyLmVjaGV2ZXJyaWFtQHVzYWNoLmNs
 Seyed Vahid Jasemi1
Seyed Vahid Jasemi1 Hosna Khazaei
Hosna Khazaei Ina Yosifova Aneva
Ina Yosifova Aneva Mohammad Hosein Farzaei
Mohammad Hosein Farzaei Javier Echeverría
Javier Echeverría