
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 04 February 2020
Sec. Experimental Pharmacology and Drug Discovery
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.00024
This article is part of the Research Topic Emerging Micro- and Nanotechnologies for Medical and Pharmacological Applications View all 26 articles
Cell membrane (CM)-camouflaged nanocarriers (CMNPs) are the tools of a biomimetic strategy that has attracted significant attention. With a wide range of nanoparticle cores and CMs available, various creative CMNP designs have been studied for cancer diagnosis and therapy. The various functional CM molecules available allow CMNPs to demonstrate excellent properties such as prolonged circulation time, immune escape ability, reduced systemic toxicity, and homologous targeting ability when camouflaged with CMs derived from various types of natural cells including red and white blood cells, platelets, stem cells, and cancer cells. In this review, we summarize various CMNPs employed for cancer chemotherapy, immunotherapy, phototherapy, and in vivo imaging. We also predict future challenges and opportunities for fundamental and clinical studies.
Cancer is a worldwide health problem and is currently the most important hindrance to life expectancy improvement. In 2015, the World Health Organization (WHO) estimated that cancer is the first or second most common cause of death among people under age 70 in most countries (Bray et al., 2018). According to updated nationwide cancer statistics from the National Central Cancer Registry of China (NCCRC), there were 3.8 million new cancer cases (crude incidence rate: 278.07/100,000) and 2.3 million cancer deaths (crude incidence rate: 278.07/100,000) (Chen et al., 2018). Cancer cells exhibit uncontrollably rapid proliferation via growth signal self-sufficiency, resisting growth inhibitory, and escaping from apoptotic signals (Saez-Rodriguez et al., 2015). Cancer cells can escape from immune surveillance and metastasize from origin sites to other organs. They can even generate adaptive strategies in response to effective treatments via further gene mutation (Gatenby and Brown, 2018). Chemotherapy, radiotherapy, and immunotherapy are presently the main clinical cancer treatment methods. Other treatment strategies such as phototherapy and gene therapy are also being developed. However, these treatment strategies have demonstrated unsatisfactory results due to poor pharmacokinetics, low permeability, low targeting ability, and severe side-effects.
Due to the previously mentioned shortcomings of cancer treatment strategy, nanoparticle (NP)-based drug delivery systems (DDSs) have been widely studied for tumor diagnosis and treatment (Dan et al., 2007). Particles 1–1,000 nm in size are defined as nanoparticles and offer good drug delivery characteristics. Nanoparticles 10–100 nm in size are proven to offer the highest delivery efficacy (Narain et al., 2017). Various types of nanomaterials such as polymers, liposomes, and metals have been developed for the delivery of therapeutic agents for cancer treatment. The cooperation between payload and nanomaterial can produce effective drug delivery via passive or active targeting strategies and can offer high drug loading capacities, increased circulation times, and reduced systemic toxicities. In the passive approach, a nanoparticle-based DDS can deliver therapeutic agents effectively via the enhanced permeation and retention (EPR) effect. An active antitumor agent approach can be used when the nanomaterials are synthesized to exhibit environmentally responsive characteristics or targeting ligands. Meanwhile, the cooperation of varying payloads via nanoparticle-based DDS can elicit diversification effects, like the implementation of chemoimmunotherapy (Fang et al., 2018).
However, the foreign nature of nanoparticles makes it easy for the immune system to recognize and eliminate them easily. In order to achieve more efficient drug delivery with a low clearance rate, biomimetic nanoparticles have been designed to prolong circulation time and evade clearance by the immune system. PEGylation has been widely used to decrease nanoparticle elimination. However, it has been reported that anti-PEG antibodies can be produced after repeated administration of PEGylated nanoparticles, and this might promote the elimination these nanoparticles (Lubich et al., 2016). In contrast, lipids are a major part of the cell membrane (CM). They have been used to produce biomimetic liposomes in order to mimic biological membranes. However, these liposomes lack structural integrity and stability. This restricts their application as DDSs (Maurer et al., 2001).
A range of new biomimetic nanoparticle-based DDSs have recently been developed to combine the benefits of natural and synthetic nanomaterials (Kroll et al., 2017; Bose et al., 2018). CMs are natural-source ingredients that can be coated onto nanoparticles to produce CM-coated nanoparticles (CMNPs) with cell-like behaviors. The nanoparticle core can protect various therapeutic cargos via its high structural integrity and stability. In addition, CMs can provide CMNPs with prolonged circulation time, targeting ability, and other source cell properties. For instance, red blood cell (RBC) membrane could be used for immune evasion and prolonging circulation time. White blood cell (WBC) membranes could be employed to camouflage nanoparticle for evading opsonization and reticuloendothelial system (RES) clearance and targeting inflamed sites. Cancer cell membrane (CCM) could act as the tumor-targeting navigator and the source of tumor-associated antigens (TAAs). In Table 1, we summarize various CM-coated nanoparticles developed for cancer diagnosis and treatment. In this review, we provide an overview of CMNP-based DDSs as tumor diagnostic and therapeutic agents and discuss potential clinical applications (Scheme 1).
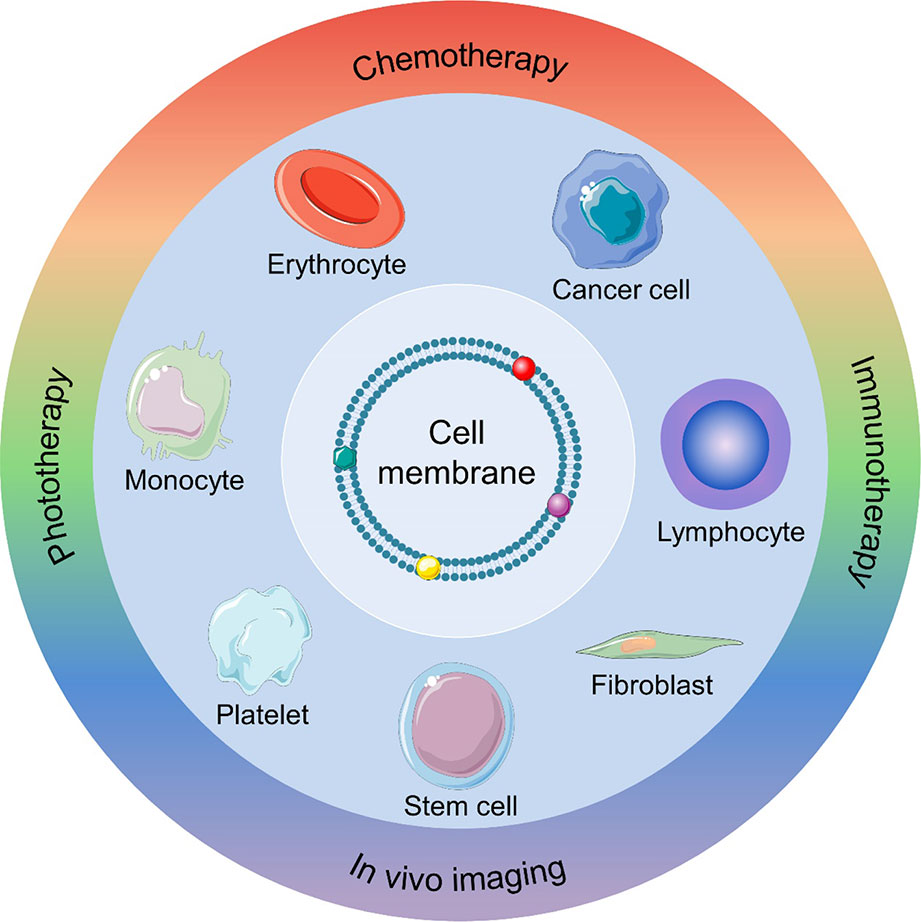
Scheme 1 Schematic illustration of a nanocarrier-assisted cell membrane designed for cancer diagnosis and treatment.
Chemotherapy is a class of traditional cancer treatments with broad clinical implementation. Classic chemotherapy can interfere with cell proliferation to achieve cancer treatment. Systemic toxicity and low bioavailability have always been disadvantages that limit further application of chemotherapy. Furthermore, many chemotherapeutic drugs are hydrophobic, which can lead to poor absorption and bioavailability. New chemotherapeutics and existing chemotherapeutics with new formulations have recently been developed to address these limitations (Qi et al., 2017; Guo et al., 2018). Application of nanomaterials as chemotherapeutic DDSs has also been studied frequently. Therapeutic efficacy has been enhanced using nanoparticle-based DDSs. Various CMs have been coated onto nanoparticle surfaces in order to improve the biocompatibility, targeting ability, and circulation time of nanoparticles loaded with chemotherapeutic drugs.
CCM is commonly coated on nanoparticles to endow them with prolonged blood circulation, effective immune escape, and homologous targeting ability. Xu et al. prepared CCM-coated NPs by coating poly(lactide-co-glycolide)-doxorubicin (PLGA-DOX) NPs with HepG2 cell-derived CCM. The resulting CCM-coated nanoparticle size and zeta potential were approximately 100 nm and −29.49 mV, respectively. Due to the tumor specificity derived from homologous binding to CCM molecules, these biomimetic nanoparticles significantly enhanced the cellular endocytosis of DOX toward HepG2 cells when compared to nanoparticles without CCM in vitro. Compared to free DOX, the CCM-coated NPs exhibited enhanced antitumor efficacy and reduced system toxicity in HepG2 xenograft mouse models. They benefited from prolonged circulation, immune evasion, and enhanced DOX accumulation at the tumor site (Xu et al., 2019). Tian et al. produced polymer NPs that were co-loaded with hemoglobin (Hb) and DOX and camouflaged with CCM. The resulting nanoparticles exhibited high tumor-targeting capacities. In addition, the nanoparticles could suppress the expression of a series of genes such as hypoxia-inducible factor-1α to abate the exocytosis of DOX, a property that may lead to safe, efficient chemotherapy (Tian et al., 2017).
Blood cells include erythrocytes, leukocytes, and platelets and also serve as possible membrane vehicles for CM-coated NPs. Several studies consider blood cell membrane-coated NPs for cancer chemotherapy. Zhang et al. reported RBC membrane (RBM)-coated, DOX-loaded poly(lactic acid) (PLA) NPs. In this study, the authors compared two strategies (physical encapsulation and chemical conjugation) for loading DOX into the PLA NPs. For physical encapsulation, DOX was loaded into the PLA NPs via nanoprecipitation. In chemical conjugation, ring-opening polymerization was performed to produce a DOX-PLA polymer conjugate. The resulting DOX-PLA polymer conjugate was added to an aqueous phase to produce nanoparticles. The chemical conjugation strategy led to higher drug loading and more sustained drug release. Furthermore, RBM-coated DOX-loaded NPs exhibited higher toxicity toward acute myeloid leukemia cells than free DOX (Aryal et al., 2013). WBC membranes were shown to act as camouflaged surfaces that help to evade opsonization and RES clearance, as well as inflamed site-targeting navigators. This characteristic may endow WBC membrane-coated NPs with the ability to target some tumors (Fang et al., 2018). Thus, numerous WBC membrane-coated NPs have been developed for cancer chemotherapy, including monocyte cell membrane-coated PLGA NPs (Krishnamurthy et al., 2016), macrophage membrane-coated NPs (Zhang et al., 2018), platelet membrane (PM)-coated core-shell nanovehicles (Hu et al., 2015), and composite CM (derived from leukocytes and tumor cells)-camouflaged liposomal NPs (He et al., 2018). For instance, it was reported that macrophage-membrane-coated NPs could perform step-by-step release of Paclitaxel (PTX) in response to the tumor microenvironment, resulting in tumor-targeting chemotherapy. Macrophage membranes with membrane molecules that exhibit inflammatory tumor-homing abilities were coated onto the nanoparticles. After injection, the macrophage membrane-coated nanocarriers could evade immune clearance and achieve tumor targeting. In an extracellular microenvironment, the nanoparticles could discharge from the outer membrane and further be taken up by cancer cells with help from the targeting peptide on the nanoparticle surface. Then, PTX could be released from the nanoparticles in response to the acidic environment of the endosome. This macrophage-membrane-coated nanoparticle exhibited potent antitumor efficacy (Figure 1) (Zhang et al., 2018).
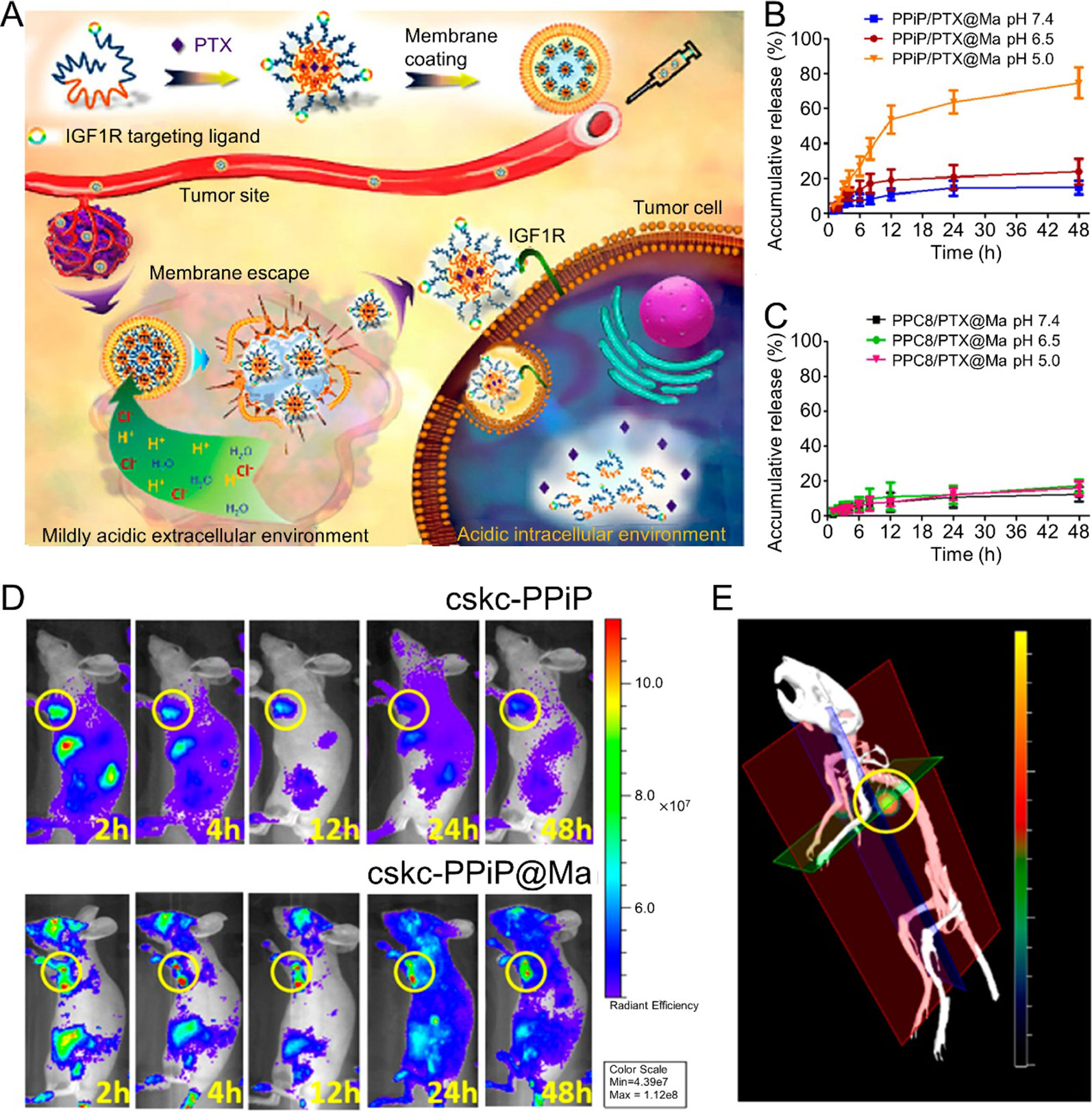
Figure 1 (A) Schematic illustrations of membrane-coated nanoparticle synthesis, membrane escape, and drug-release mechanisms. Cumulative drug-release profile of (B) PPiP/PTX@Ma and (C) PPC8/PTX@Ma in various pH environments. (D) In Vivo imaging system images of mice after injection of near-infrared probe-loaded cskc-PPiP and cskc-PPiP@Ma at different times. (E) 3D reconstruction of the 48 h fluorescence signal of a cskc-PPiP@Ma group. Reproduced with permission from (Zhang et al., 2018). Copyright @ American Chemical Society.
Use of CM-coated NPs to co-deliver chemotherapy drugs and photothermal agents has been studied for cooperative cancer therapy. Ding et al. exploited CCM to enhance the tumor-targeting ability of anticancer drug-loaded mesoporous silica nanoparticles (MSNs). MSNs were co-loaded with indocyanine green (ICG) and DOX, which are a near-infrared photothermal agent and a chemotherapy drug, respectively. CCM was coated onto drug-loaded MSNs to produce CCM-coated MSNs. The CCMs reduced drug leakage during delivery and accumulated at the tumor site efficiently. The photothermal effect of ICG led to CCM fusion and thus accelerated DOX release. This type of CCM-coated MSN could achieve synergistic treatment via chemotherapy and photothermal therapy (Ding et al., 2019). Similarly, Zhai et al. developed a cytotoxic T lymphocyte (CTL)-inspired nanovesicle (MPV) with a RBC membrane-derived shell. The CM-coated MPV possessed a gelatin nanogel core, which was co-loaded with methylene blue (MB) and cisplatin (Pt). The CM-coated MPV could achieve deep tumor penetration and produce hyperthermia when stimulated via laser irradiation. In addition, photoacoustic imaging (PAI) could be employed to monitor MPV accumulation after injection. Local irradiation could then be used at the time of maximum accumulation at tumor sites. The combination of localized hyperthermia and chemotherapy led by CM-coated MPV inhibited tumor progression while maintaining low systemic toxicity (Zhai et al., 2018). RBM-decorated hollow mesoporous Prussian blue (PB) NPs were also employed for cooperative cancer therapy. These nanoparticles also encapsulated a large quantity of DOX. The authors found that this type of nanoparticle exhibited synergistic photothermal-chemotherapeutic anticancer properties with low toxicity and high efficacy (Chen et al., 2017a).
Cancer immunotherapy can inhibit tumor progression by stimulating immune responses (Li et al., 2018c). In 1986, recombinant interferon-α (IFN-α) became the first immunotherapeutic agent marketed for hairy cell leukemia. After 6 years, recombinant interleukin-2 (IL-2) was also proven effective for metastatic renal cancer by the US Food and Drug Administration (FDA) (Ribas and Wolchok, 2018). Unfortunately, the short half-life of IL-2 can result in serious adverse effects such as vascular leak syndrome. Recent cancer immunotherapy strategies have focused on inducing specific antitumor immune responses. Sipuleucel-T (an autologous active cellular immunotherapy) has been used clinically since 2010. Checkpoint blockade cancer immunotherapies such as cytotoxic T lymphocyte antigen 4 antibody (anti-CTLA-4) and programmed cell death 1 antibody (anti-PD-1) were also demonstrated for clinical applications (Riley et al., 2019). Even though substantial advances have been achieved in cancer immunotherapy, exploring a preventive or therapeutic agent that controls modulation of the immune system, low systemic toxicity, and high antitumor efficiency remains a challenge in cancer immunotherapy applications. This is because such therapeutic agents exhibit serious adverse effects such as nonspecific inflammation and autoimmunity (Qian et al., 2018; Riley et al., 2019). Several strategies have been used to improve therapeutic efficacy and reduce side effects in order to manage cancer immunotherapy in a more controlled manner. Nanoparticle-based DDS can protect immune-related components during circulation and deliver TAAs and immune-modulating agents efficiently. Furthermore, some types of nanoparticle-based DDSs can achieve controlled drug release in response to stimuli like pH in order to harness immunotherapy and reduce systemic toxicity (Li et al., 2018c; Qian et al., 2018). A range of CMs can be used on nanoparticle surfaces to enhance the delivery efficacies of antigens and immune-modulating molecules. In the study reported by Lang et al., platelet and WBC membranes were blended and then coated onto immunomagnetic beads (IMBs). The resulting nanoparticles (HM-IMBs) were modified with anti-epithelial cell adhesion molecules (anti-EpCAMs). The PLT-WBC hybrid membranes could enhance tumor cell binding ability and reduce the homologous leukocyte interaction of the resulting nanoparticles. This can be employed to isolate circulating tumor cells efficiently. Upon testing spiked blood samples, it was found the HM-IMBs exhibited a cell separation efficiency of 91.77%, which compares favorably to 66.68% for IMBs. The cell purity of the HM-IMBs was 96.98%, which is much higher than that of the IMBs (66.53%). HM-IMBs also proved effective in detection of PIK3CA gene mutations (Rao et al., 2018). Similarly, neutrophil-membrane-coated PLGA NPs (NM-NPs) were also developed. The authors found that NM-NPs exhibited enhanced circulating tumor cell (CTC)-capture efficiency in vivo. Carfilzomib-loaded NM-NPs could deplete CTCs during circulation and prevent early metastasis (Kang et al., 2017).
CCM not only acts on the nanoparticle surface to enhance cargo delivery efficacy but also can be the source of multiple tumor-specific antigens, which are the components of antitumor vaccines. Yang et al. developed CCM-coated PLGA NPs that were loaded with imiquimod (R837). The resulting CCM-coated nanoparticles were further modified with mannose moiety. This nanovaccine exhibited improved uptake by antigen present cells (APCs), which can induce a potent antitumor immune response. When acting as a therapeutic vaccine, the nanovaccine exhibited efficient therapeutic efficacy when combined with checkpoint-blockade therapy (Figure 2) (Yang et al., 2018). Furthermore, Manolova et al. found that the nanoparticle size could influence in vivo particle migration (Manolova et al., 2008). Li et al. prepared immunoadjuvant-loaded multiantigenic NPs (MANPs/R837) with various diameters. Smaller CCM-coated PLGA NPs exhibited more efficient delivery of antigens and R837 to APCs in draining lymph nodes (LNs) to induce a stronger antitumor immune response. When combined with checkpoint blockade therapy such as the anti-PD1 strategy, MANPs/R837 (especially smaller MANPs/R837) exhibited enhanced tumor progression inhibition (Li et al., 2019).
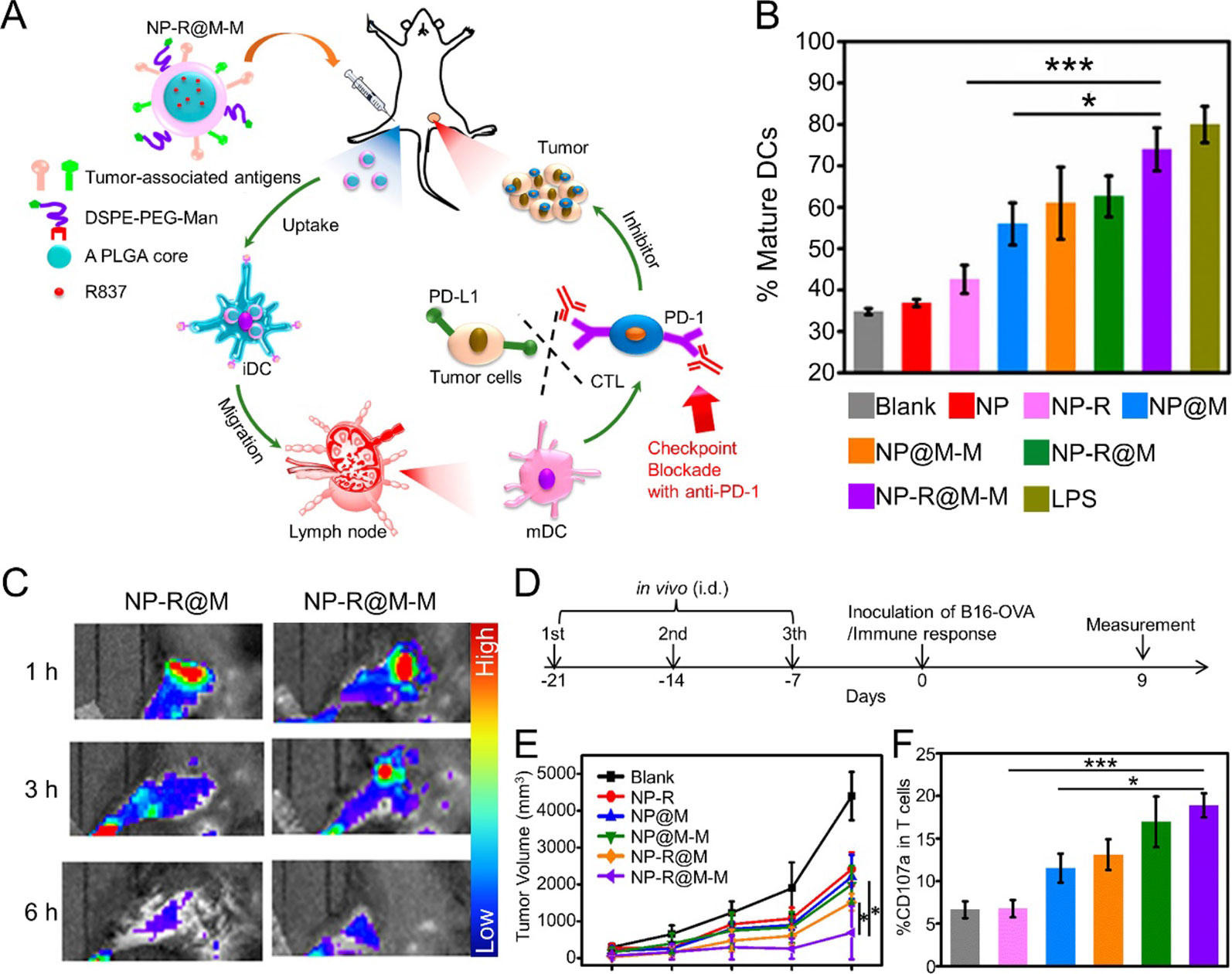
Figure 2 (A) Schematic illustration to demonstrate the structures of CCM-coated, R837-loaded, mannose-modified PLGA nanoparticles (NP-R@M-M) and their immune-stimulant functions as a nanovaccine. (B) In vitro DC activation by various nanovaccine formulations. (C) In vivo fluorescence images of mouse hind legs after intradermal injection of fluorescent-labeled NP-R@M or NP-R@M-M at three different times. (D) Schematic illustration of a tumor challenge experimental design. (E) B16-OVA tumor volume curves after pretreatment with various nanovaccine formulations (n ≥ 5). (F) Percentages of CD107a+ cells among all T cells. (***P < 0.001, *P < 0.05). Reproduced with permission from (Yang et al., 2018). Copyright @ American Chemical Society.
Synergistic treatment that combines immunotherapy with photothermal therapy or chemotherapy via nanoparticle-based DDSs has developed rapidly. Antitumor immune response can be induced via photothermal therapy, which can generate TAAs near ablated tumor cells. When photothermal therapy was combined with immunotherapy via nanoparticle-based DDSs, potent vaccine-like behavior by therapeutic agents could elicit the elimination of residual and metastatic tumor cells (Chen et al., 2016a). For instance, Liang et al. reported a biomimetic black phosphorus quantum dot (BPQD) formulation that could induce tumor ablation via near-infrared (NIR) laser irradiation to elicit an antitumor immune response that further inhibited tumor progression, metastasis, and rechallenge. RBMs were coated onto BPQDs (BPQD-RMNVs) to prolong circulation time and promote tumor accumulation. Moreover, the combination of BPQD-RMNVs and the PD-1 antibody could induce an enhanced antitumor immune response to eliminate cancer cells (Liang et al., 2019). In another study, the authors obtained CCMs via surgical removal of tumors. The resulting CCMs were then coated onto BPQDs to get BPQD-CCNVs. BPQD-CCNVs, granulocyte-macrophage colony-stimulating factor (GM-CSF), and lipopolysaccharide (LPS) were loaded into a thermosensitive hydrogel (Gel-BPQD-CCNVs). Dendritic cells (DCs) could be recruited by the GM-CSF released from Gel-BPQD-CCNVs to uptake TAAs. NIR irradiation and LPS could then induce DC maturation. The mature DCs traveled through lymphatic capillaries to LNs to induce a potent antitumor immune response. This synergistic treatment strategy could be combined with checkpoint blockade treatment to improve the antitumor efficacies of tumor-specific CD 8+ T cells (Ye et al., 2019). Similarly, chemoimmunotherapy was proven efficient for cancer treatment. In chemoimmunotherapy, low doses of chemotherapeutic agents can induce immunogenic cell death (ICD) of tumor cells to release TAAs, thus avoiding severe side effects. Immunomodulatory agents can enhance antigen presentation and induce APC and cytotoxic CD8+ T cell maturation. Thus, use of nanoparticle-based DDSs to perform a combination of chemotherapy and immunotherapy was studied and proven efficient (Calleja et al., 2017; Feng et al., 2019). It was reported that a dual pH-responsive multifunctional DDS based on poly(L-histidine) and hyaluronic acid was designed for the delivery of resiquimod (R848, a TLR7/8 agonist) and DOX to achieve synergistic effects of immunotherapy and chemotherapy against breast cancer (Liu et al., 2018). Furthermore, excellent treatment effects might be achieved when CMs are employed to improve the delivery efficacies of synergistic therapeutic nano-agents.
Phototherapy is an effective, noninvasive cancer treatment strategy (Chen et al., 2017b). Phototherapy can be initiated via laser irradiation to induce selective, localized therapeutic effects. Photothermal therapy (PTT) and photodynamic therapy (PDT) are the two major categories of phototherapy (Vijayan et al., 2018).
PTT uses heat ablation generated via light-absorbing agents to execute a new, minimally invasive cancer treatment strategy with low systemic toxicity (Chen et al., 2016a). With the development of nanotechnology, it was found that nanoparticle-based DDS could improve therapeutic agent tumor accumulation and the bioavailabilities of water-insoluble cargos. Thus, a large range of nanoparticle-based DDSs have been employed to enhance the delivery efficacies of the light-absorbing agents needed for PTT. CM-coated NPs were recently employed to further improve PTT agent delivery and enhance the therapeutic efficacy of PTT. Meng et al. developed a macrophage membrane-coated magnetic iron oxide NP (Fe3O4@MM NP) that exhibited excellent biocompatibility, prolonged circulation time, tumor-targeting ability, and effective PTT for breast cancer in vivo (Meng et al., 2018). RBC-cancer cell hybrid membranes can also be employed to camouflage melanin NPs, producing Melanin@RBC-M in order to improve therapeutic PTT efficacy. RBMs can prolong nanoparticle circulation times and CCMs can endow nanoparticles with tumor-targeting abilities. The authors studied the delivery efficacy of Melanin@RBC-M formulations with various RBM-to-CCM ratios. The in vivo biodistribution and therapeutic efficiency were investigated after intravenous injection of various therapeutic formulations into MCF-7 tumor-bearing athymic nude mice. Melanin@RBC-M with a 1:1 membrane protein weight ratio of RBMs to CCMs exhibited better delivery efficacy and a more potent PTT effect than formulations with other membrane protein weight ratios. Moreover, the authors found that Melanin@RBC-M exhibited an enhanced photoacoustic signal when the nanoparticle size increased from 64 to 148 nm. The photoacoustic amplitude increased linearly with the nanoparticle concentration in the 680 to 800 nm excitation wavelength range. This phenomenon could be employed to quantify Melanin@RBC-M in vivo (Jiang et al., 2019).
In addition, PTT can be used therapeutically in combination with chemotherapy or immunotherapy. Liu et al. used hyaluronic acid (HA)-decorated, RBM-camouflaged PB NPs to carry gamabufotalin (CS-6). The study showed that RBM can prolong the circulation time to about 10 h and enhance immune evasion ability. This nanotherapeutic agent could accumulate at tumor sites efficiently because of the HA. Meanwhile, CS-6 exhibited potent antitumor efficacy by inhibiting the expression of HSP70, which can weaken the PTT effect. The resulting nanoparticles exhibited synergistic photothermal-chemotherapy leading to potent in vivo antitumor efficacy (Liu et al., 2019). In another study, RGD peptide [c(RGDyC)]-modified platelet vesicles were employed to co-load melanin nanoparticles (MNPs) and DOX to achieve chemo-photothermal therapy for drug-resistant tumors (Jing et al., 2018). In addition, numerous CM-coated NPs have been developed for cancer photothermal-chemotherapy including RBC-melanoma cell hybrid membrane-coated, DOX-loaded hollow copper sulfide nanoparticles (Wang et al., 2018); RBC-camouflaged, PTX-loaded polymeric NPs (Su et al., 2016); RBC-mimetic hollow mesoporous PB NPs (Chen et al., 2017a); and RBC-coated, PTX-loaded gold nanocages (Zhu et al., 2018). Furthermore, synergistic treatment using PTT and immunotherapy was investigated. Liang et al. prepared RBM-coated BPQD (BPQD-RMNV) for combined cancer photothermal- and immunotherapy. Cancer progression and metastasis were substantially delayed via improved infiltration of CD8+ T cells into the tumor site when the BPQD-RMNV-mediated treatment was combined with checkpoint blocked therapy (Liang et al., 2019).
PDT can generate singlet oxygen to kill cancer cells when a photosensitizer (PS) is excited by a specific wavelength of light (Feng et al., 2017). RBM (Ding et al., 2015), PM (Xu et al., 2018), stem-cell membrane (Gao et al., 2016), and CCM (Qiu et al., 2018)-coated NP delivery systems were employed to carry PDT agents for cancer treatment. Ding and his colleagues developed an RBM-camouflaged upconversion NP (UCNP). The inner cores were loaded with merocyanine 540 (MC540) and the RBM surface was decorated with targeting moieties including folate (FA) and the triphenylphosphonium (TPP) cation. This formulation could generate 1O2 under 980 nm irradiation. Meanwhile, the resulting RBM-coated nanovectors could improve singlet oxygen infiltration relative to PDT agents with other surface coatings because of the unique nature of RBC as an oxygen (O2) carrier. The combination of the RBM coating and targeting ability significantly enhanced the PDT therapeutic efficiency (Ding et al., 2015). In another study, a PM-coated, verteporfin-loaded photodynamic NP (NP-Ver@P) was developed. P-selectin on a platelet surface could specifically bind with the CD44 receptor that is heavily expressed on cancer cell surfaces. Thus, PM could endow the nanoparticle with long circulation times, good targeting ability, and a higher tumor uptake rate than RBM. Under 680–730 nm solar irradiation, NP-Ver@P exhibited a therapeutic effect against tumors without damaging skin at tumor sites (Xu et al., 2018). CCM-coated NPs have attracted significant attention due to their homologous targeting capabilities. Yang reported coating CCM onto chlorins e6 (Ce6)-loaded silica NPs (CM/SLN/Ce6). CM/SLN/Ce6 exhibited excellent stability in physiological conditions and homologous targeting ability. The aforementioned features make CM/SLN/Ce6 a promising cancer-targeting PDT platform (Yang et al., 2019).
Cooperative therapy that combines PDT with other cancer treatment strategies has been studied widely. Pei et al. reported RBM-coated NPs (RBC(M(TPC-PTX))) for PDT combined with chemotherapy. Reactive oxygen species (ROS)-responsive PTX dimer (PTX2-TK) and 5,10,15,20-tetraphenylchlorin (a type of photosensitizer, TPC) were used to compose the inner core. Light could cause TPC to generate ROS for PDT. The resulting ROS could also elicit PTX2-TK cleavage, releasing PTX for chemotherapy. In this platform, PDT and chemotherapy were integrated to achieve high drug loading capabilities and light-induced drug release. Synergetic treatment with PDT and chemotherapy improved antitumor efficacy and on-demand PTX release reduced systemic toxicity (Figure 3) (Pei et al., 2018). In another study, a CCM-coated porphyrin metal-organic framework (MOF) was used to load glucose oxidase (GOx) and catalase. After accumulating at tumor sites efficiently, the resulting nanoparticles could enhance singlet oxygen production and intracellular glucose decomposition by catalyzing endogenous hydrogen peroxide (H2O2) to produce O2. Furthermore, this O2 could promote singlet oxygen production. Induced cooperative therapy using PDT and starvation treatment could inhibit tumor progression efficiently (Li et al., 2017). Furthermore, MR/NIR fluorescence dual-modal imaging could be combined with PDT via a CCM-coated nanoparticle delivery system. In a study reported by Li et al., Ce6-loaded magnetic nanobeads were camouflaged using CCM to get SSAP-Ce6@CCM, which exhibited excellent PDT efficacy and MR/NIR fluorescence imaging ability under 670 nm laser irradiation. SSAP-Ce6@CCM might be a promising theranostic platform for tumor treatment (Li et al., 2018a). Li et al. reported a cancer-associated fibroblast cell membrane-coated polymer NP (AF-SPN). AF-SPN demonstrated homologous targeting ability derived from the activated fibroblast cell membrane and used it to target cancer-associated fibroblasts. The AF-SPN core structure is made from poly(cyclopentadithiophene-alt-benzothiadiazole), which is an NIR-absorbing, semiconducting polymer. Thus, AF-SPN can accumulate at tumor sites to induce enhanced NIR fluorescence and PTT and PDT effects for cancer treatment (Li et al., 2018b).
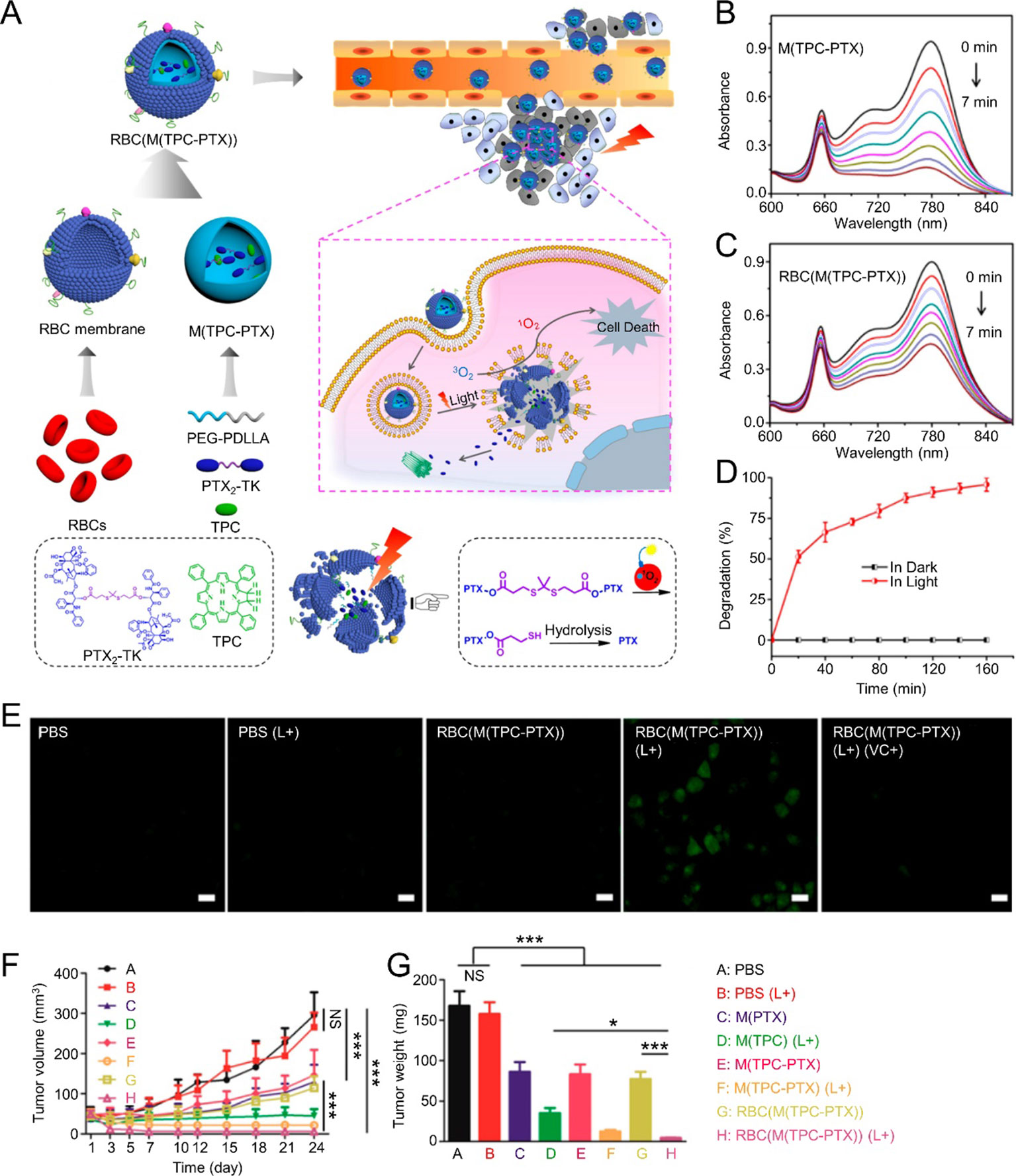
Figure 3 (A) Schematic illustration of RBC(M(TPC-PTX)) light-triggered, on-demand drug release for a combination of PDT and chemotherapy. Time-dependent UV absorption spectra of ICG in (B) M(TPC-PTX) and (C) RBC(M(TPC-PTX)) solutions under 638 nm irradiation (100 mW/cm2) for 7 min. (D) Degradation of PTX2-TK in RBC(M(TPC-PTX)) under 638 nm irradiation (100 mW/cm2) over time. (E) Generation of intracellular ROS in HeLa cells incubated with various therapeutic formulations. Scale bar = 20 μm. (F) Tumor volume curves after treatment with various therapeutic strategies (n = 6). (G) Quantitative analysis of tumor weights among various groups. (***P < 0.001, *P < 0.05). Reproduced with permission from (Pei et al., 2018). Copyright @ American Chemical Society.
In addition to acting as therapeutic drug delivery systems, CM-based NPs are also employed in biomedical imaging applications such as magnetic resonance imaging (MRI), computed tomography (CT), and fluorescence imaging.
Fe3O4 NPs are a type of novel functional material with low systemic toxicity, high stability, good biocompatibility, and the ability to act as magnetic resonance imaging (MRI) contrast agents (Ren et al., 2016). The delivery efficacies of Fe3O4 NPs improve when combined with a CM-based membrane. Rao prepared magnetic Fe3O4 NPs camouflaged with RBM (Fe3O4@RBC NP). RBM clearly reduced the Fe3O4 NP RES uptake. Fe3O4@RBC NPs exhibited excellent potential for MRI and drug delivery applications (Rao et al., 2016b). In addition to MRI applications, superparamagnetic iron oxide NPs (SPIO NPs) were also used for magnetic hyperthermia therapy by their photothermal conversion abilities. Lai et al. prepared stem cell membrane (STM)-coated SPIO NPs for tumor theranostic applications. STM-SPIO NPs acted as potential MRI agents and exhibited excellent magnetization (65.9 emu g-1) and dose-dependent T2-weighted imaging contrast (R2 = 653.3 s-1 mM-1) in vitro. Furthermore, STM-SPIO NPs also exhibited magnetic hyperthermia capabilities for cancer treatment (Lai et al., 2015).
Radiolabeled nanocarriers derived from CMs have been studied frequently for non-invasive imaging. Radiolabeled exosome mimetics (EMs) made from RBCs have been used for in vivo imaging. RBC-EMs were labeled with technetium-99m (99mTc- RBC-EMs) and exhibited nearly 100% radiochemical purity until 2 h had passed. Furthermore, 99mTc- RBC-Ems exhibited higher liver and spleen uptakes, but no thyroid uptake, unlike free 99mTc (Gangadaran et al., 2018). In another study, Yu et al. developed 89Zr-labeled multicompartment membrane-derived liposomes (MCLs). MCLs derived from CCMs were loaded with tetrakis(4-carboxyphenyl) porphyrin. The resulting 89Zr-Df-MCLs were used for non-invasive quantitative tracing via positron emission tomography (PET) imaging and PDT in vivo. 89Zr-Df-MCLs demonstrated good radiochemical stability, tumor-targeting ability, and long-term, effective PDT, as well as low systemic toxicity. Specifically, 89Zr-Df-MCLs achieved rapid, highly sensitive LN localization (Yu et al., 2018).
Fluorescence imaging is one of the most efficient cancer imaging strategies used in biological studies and clinical applications (Rao et al., 2016a). Rao and his colleagues prepared a CCM-cloaked UCNP (CC-UCNP) that exhibited prolonged blood circulation, immune escape ability, and homologous targeting ability. CC-UCNPs could convert NIR fluorescence into visible light and be used for in vivo tumor imaging. They also exhibited potential for tumor diagnosis and treatment (Figure 4) (Rao et al., 2016a). Biomimetic fluorescent nanoprobes were developed in another study. These could convert NIR radiation (λmax ≈ 720 nm) into 800 nm light. The nanoprobes exhibited ideal NIR-incoming-NIR-outgoing fluorescence features. The CCM surface imparted the nanoprobes with excellent biocompatibilities and homologous targeting abilities (Lv et al., 2018). Fluorescence imaging could be combined with other treatment strategies using the CM-based delivery system. Chen et al. reported a CCM-camouflaged, ICG-loaded NP as a theranostic nanoplatform (ICNPs). The ICNPs exhibited tumor-targeting abilities derived from the CCM, fluorescence and PAI, and PTT. Benefiting from fluorescence-photoacoustic dual imaging and PTT, ICNPs could achieve real-time tumor monitoring with high spatial resolution and effective tumor treatment (Chen et al., 2016b). In addition to ICG, PB (Xiao et al., 2019), and Ce6 (Li et al., 2018a) were tested in combination with other agents using CM-based nanoplatforms in order to support a theranostic strategy. For instance, Xiao et al. developed RBM-coated, DOX-loaded PB NPs that were modified with FA. The resulting nanoparticles could achieve chemo-photothermal therapy alongside tumor fluorescence and PAI (Xiao et al., 2019).
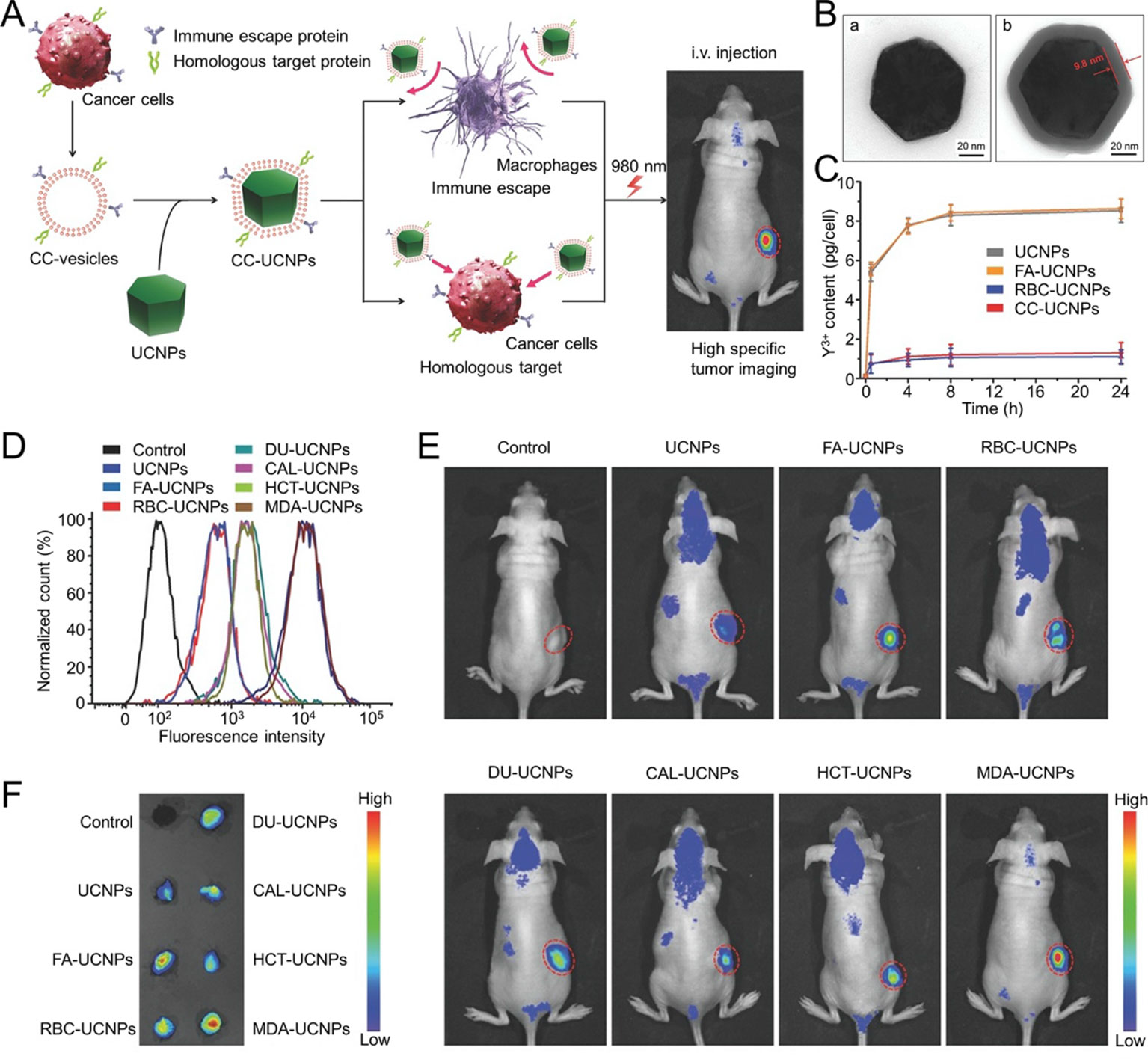
Figure 4 (A) Schematic illustration of CC‐UCNP preparation and application. (B) TEM images of (a) UCNPs and (b) CC‐UCNPs. (C) Quantitative analysis of various nanoparticle uptakes at various times. (D) Flow cytometry analysis of MDA‐MB‐435 cells after incubation with various Cy5‐labeled nanoparticles. (E) In vivo upconversion luminescence (UCL) images of MDA‐MB‐435-bearing mice 24 h after intravenous injection of various nano-formulations (tumor sites are indicated by red circles). (F) Ex vivo UCL images of tumors 24 h after injection. Reproduced with permission from (Rao et al., 2016a).
CM-based nanoparticle delivery systems have been explored in order to improve on the limitations of traditional nanomedicines. These systems exhibit excellent potential in cancer theranostic applications including chemotherapy, immunotherapy, phototherapy, and in vivo imaging. Various CM coatings have attracted significant attention due to the natural features derived from their source cells. CM coatings have been derived from various cells, including RBCs, macrophages, monocytes, neutrophils, platelets, stem cells, and cancer cells. A top-down strategy was used to develop the CM-coating nanoplatform, in which the natural functions of existing cells can be directly transferred to make the ultimate nanomedicine (Narain et al., 2017). RBM can endow RBM-coated NPs with prolonged circulation times, immune evasion, and reduced RES uptake (Gao et al., 2017). WBC membrane-coated NPs exhibit macrophage internalization inhibition because of the WBC membrane (Zhang et al., 2018). CCM-coated NPs have been proven to possess tumor-targeting abilities for drug delivery and vaccine-like functions (Fang et al., 2014; Ding et al., 2019).
Despite current progress in the field of CM-based nanoplatforms for cancer diagnosis and treatment, many challenges remain before they can be used in clinical applications. First, complex, inefficient CM-based nanomedicine preparation processes restrict their development. Coating techniques should be reformed for higher throughputs to satisfy the needs of future clinical applications. In addition, some of the specific functional proteins and structural units in CMs remain uncertain. For example, there are various proteins on the surfaces of CCM-based NPs. Only a few of them act as cancer-specific antigens for cancer immunotherapy. Others are common in human cells. The ability to identify these cancer-specific antigens and remove unwanted antigens or even enrich the expression of cancer-specific antigens through transgenic technology might allow CCM-based NPs to provide better therapeutic effects. Some types of cells such as fibroblasts, which have been used in nanoparticle engineering, have proven difficult to amplify and apply practically in clinical settings. Finally, cancer cell heterogeneity can be a significant factor behind insufficient cancer treatment of CCM-based NPs. Thus, CCMs derived from autologous tumors may serve to provide specific CCM-based NPs for cancer treatment.
In conclusion, research on CM-based nanoplatforms for cancer diagnosis and treatment is still in its infancy. Numerous challenges must be overcome before the translation from bench to bedside. More innovative, efficacious CM-based nanoplatform strategies will be developed to support cancer treatments that benefit human health.
SL and JL produced the first draft. MS and JW revised the manuscript. CW and YS proposed the outline of the article and revised the draft before submission. In addition, all authors provided final approval of the manuscript.
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 5177030177).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Aryal, S., Hu, C.-M. J., Fang, R. H., Dehaini, D., Carpenter, C., Zhang, D.-E., et al. (2013). Erythrocyte membrane-cloaked polymeric nanoparticles for controlled drug loading and release. Nanomedicine 8, 1271–1280. doi: 10.2217/nnm.12.153
Bose, R. J. C., Paulmurugan, R., Moon, J., Lee, S.-H., Park, H. (2018). Cell membrane-coated nanocarriers: the emerging targeted delivery system for cancer theranostics. Drug Discovery Today 23, 891–899. doi: 10.1016/j.drudis.2018.02.001
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. doi: 10.3322/caac.21492
Calleja, P., Irache, J. M., Zandueta, C., Martinez-Oharriz, C., Espuelas, S. (2017). A combination of nanosystems for the delivery of cancer chemoimmunotherapeutic combinations: 1-Methyltryptophan nanocrystals and paclitaxel nanoparticles. Pharmacol. Res. 126, 77–83. doi: 10.1016/j.phrs.2017.09.004
Chen, Q., Xu, L., Liang, C., Wang, C., Peng, R., Liu, Z. (2016a). Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 7, 13193. doi: 10.1038/ncomms13193
Chen, Z., Zhao, P., Luo, Z., Zheng, M., Tian, H., Gong, P., et al. (2016b). Cancer cell membrane-biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy. ACS Nano. 10, 10049–10057. doi: 10.1021/acsnano.6b04695
Chen, W., Zeng, K., Liu, H., Ouyang, J., Wang, L., Liu, Y., et al. (2017a). Cell membrane camouflaged hollow prussian blue nanoparticles for synergistic photothermal-/chemotherapy of cancer. Adv. Funct. Mater. 27, 1605795. doi: 10.1002/adfm.201605795
Chen, X., Lee, D., Yu, S., Kim, G., Lee, S., Cho, Y., et al. (2017b). In vivo near-infrared imaging and phototherapy of tumors using a cathepsin B-activated fluorescent probe. Biomaterials 122, 130–140. doi: 10.1016/j.biomaterials.2017.01.020
Chen, W., Sun, K., Zheng, R., Zeng, H., Zhang, S., Xia, C., et al. (2018). Cancer incidence and mortality in China, 2014. Chin. J. Cancer Res. 30, 1–12. doi: 10.21147/j.issn.1000-9604.2018.01.01
Dan, P., Karp, J. M., Hong, S., Farokhzad, O. C., Margalit, R., Langer, R. (2007). Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2, 751. doi: 10.1038/nnano.2007.387
Ding, H., Lv, Y., Ni, D., Wang, J., Tian, Z., Wei, W., et al. (2015). Erythrocyte membrane-coated NIR-triggered biomimetic nanovectors with programmed delivery for photodynamic therapy of cancer. Nanoscale 7, 9806–9815. doi: 10.1039/C5NR02470F
Ding, Y., Zhu, Y., Wei, S., Zhou, J., Shen, J. (2019). Cancer cell membrane as gate keeper of mesoporous silica nanoparticles and photothermal-triggered membrane fusion to release the encapsulated anticancer drug. J. Mater. Sci. 54, 12794–12805. doi: 10.1007/s10853-019-03788-y
Fang, R. H., Hu, C.-M. J., Luk, B. T., Gao, W., Copp, J. A., Tai, Y., et al. (2014). cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano. Lett. 14, 2181–2188. doi: 10.1021/nl500618u
Fang, R. H., Kroll, A. V., Gao, W., Zhang, L. (2018). Cell membrane coating nanotechnology. Adv. Mater. 30, 1706759. doi: 10.1002/adma.201706759
Feng, L., Tao, D., Dong, Z., Chen, Q., Chao, Y., Liu, Z., et al. (2017). Near-infrared light activation of quenched liposomal Ce6 for synergistic cancer phototherapy with effective skin protection. Biomaterials 127, 13–24. doi: 10.1016/j.biomaterials.2016.11.027
Feng, X., Xu, W., Li, Z., Song, W., Ding, J., Chen, X. (2019). Immunomodulatory nanosystems. Adv. Sci. 6, 1900101. doi: 10.1002/advs.201900101
Gangadaran, P., Hong, C. M., Oh, J. M., Rajendran, R. L., Kalimuthu, S., Son, S. H., et al. (2018). In vivo non-invasive imaging of radio-labeled exosome-mimetics derived from red blood cells in mice. Front. Pharmacol. 9, 817. doi: 10.3389/fphar.2018.00817
Gao, C., Lin, Z., Wu, Z., Lin, X., He, Q. (2016). Stem-cell-membrane camouflaging on near-infrared photoactivated upconversion nanoarchitectures for in vivo remote-controlled photodynamic therapy. ACS Appl. Mater. Interfaces 8, 34252–34260. doi: 10.1021/acsami.6b12865
Gao, L., Wang, H., Nan, L., Peng, T., Sun, L., Zhou, I., et al. (2017). Erythrocyte membrane-wrapped ph sensitive polymeric nanoparticles for non-small cell lung cancer therapy. Bioconjugate Chem. 28, 2591–2598. doi: 10.1021/acs.bioconjchem.7b00428
Gatenby, R., Brown, J. (2018). The evolution and ecology of resistance in cancer therapy. Cold Spring Harbor Perspect. Med. 8, a033415. doi: 10.1101/cshperspect.a033415
Guo, H., Li, F., Xu, W., Chen, J., Hou, Y., Wang, C., et al. (2018). Mucoadhesive cationic polypeptide nanogel with enhanced penetration for efficient intravesical chemotherapy of bladder cancer. Adv. Sci. 5, 1800004. doi: 10.1002/advs.201800004
He, H., Guo, C., Wang, J., Korzun, W. J., Wang, X.-Y., Ghosh, S., et al. (2018). Leutusome: a biomimetic nanoplatform integrating plasma membrane components of leukocytes and tumor cells for remarkably enhanced solid tumor homing. Nano. Lett. 18, 6164–6174. doi: 10.1021/acs.nanolett.8b01892
Hu, Q., Sun, W., Qian, C., Wang, C., Bomba, H. N., Gu, Z. (2015). Anticancer platelet-mimicking nanovehicles. Adv. Mater. 27, 7043–704+. doi: 10.1002/adma.201503323
Jiang, Q., Liu, Y., Guo, R., Yao, X., Sung, S., Pang, Z., et al. (2019). Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials 192, 292–308. doi: 10.1016/j.biomaterials.2018.11.021
Jing, L., Qu, H., Wu, D., Zhu, C., Yang, Y., Jin, X., et al. (2018). Platelet-camouflaged nanococktail: Simultaneous inhibition of drug-resistant tumor growth and metastasis via a cancer cells and tumor vasculature dual-targeting strategy. Theranostics 8, 2683–2695. doi: 10.7150/thno.23654
Kang, T., Zhu, Q., Wei, D., Feng, J., Yao, J., Jiang, T., et al. (2017). Nanoparticles coated with neutrophil membranes can effectively treat cancer metastasis. ACS Nano. 11, 1397–1411. doi: 10.1021/acsnano.6b06477
Krishnamurthy, S., Gnanasammandhan, M. K., Xie, C., Huang, K., Cui, M. Y., Chan, J. M. (2016). Monocyte cell membrane-derived nanoghosts for targeted cancer therapy. Nanoscale 8, 6981–6985. doi: 10.1039/C5NR07588B
Kroll, A. V., Fang, R. H., Zhang, L. (2017). Biointerfacing and applications of cell membrane-coated nanoparticles. Bioconjugate Chem. 28, 23–32. doi: 10.1021/acs.bioconjchem.6b00569
Lai, P.-Y., Huang, R.-Y., Lin, S.-Y., Lin, Y.-H., Chang, C.-W. (2015). Biomimetic stem cell membrane-camouflaged iron oxide nanoparticles for theranostic applications. Rsc Adv. 5, 98222–98230. doi: 10.1039/C5RA17447C
Li, S. Y., Cheng, H., Xie, B. R., Qiu, W. X., Zeng, J. Y., Li, C. X., et al. (2017). Cancer cell membrane camouflaged cascade bioreactor for cancer targeted starvation and photodynamic therapy. ACS Nano. 11, 7006–7018. doi: 10.1021/acsnano.7b02533
Li, J., Wang, X., Zheng, D., Lin, X., Wei, Z., Zhang, D., et al. (2018a). Cancer cell membrane-coated magnetic nanoparticles for MR/NIR fluorescence dual-modal imaging and photodynamic therapy. Biomater. Sci. 6, 1834–1845. doi: 10.1039/C8BM00343B
Li, J., Zhen, X., Lyu, Y., Jiang, Y., Huang, J., Pu, K. (2018b). Cell membrane coated semiconducting polymer nanoparticles for enhanced multimodal cancer phototheranostics. ACS Nano. 12, 8520–8530. doi: 10.1021/acsnano.8b04066
Li, S., Feng, X., Wang, J., He, L., Wang, C., Ding, J., et al. (2018c). Polymer nanoparticles as adjuvants in cancer immunotherapy. Nano. Res. 11, 5769–5786. doi: 10.1007/s12274-018-2124-7
Li, S., Feng, X., Wang, J., Xu, W., Islam, M. A., Sun, T., et al. (2019). multiantigenic nanoformulations activate anticancer immunity depending on size. Adv. Funct. Mater. 29, 1903391. doi: 10.1002/adfm.201903391
Liang, X., Ye, X., Wang, C., Xing, C., Miao, Q., Xie, Z., et al. (2019). Photothermal cancer immunotherapy by erythrocyte membrane-coated black phosphorus formulation. J. Control Release 296, 150–161. doi: 10.1016/j.jconrel.2019.01.027
Liu, Y., Qiao, L., Zhang, S., Wan, G., Chen, B., Zhou, P., et al. (2018). Dual pH-responsive multifunctional nanoparticles for targeted treatment of breast cancer by combining immunotherapy and chemotherapy. Acta Biomaterialia 66, 310–324. doi: 10.1016/j.actbio.2017.11.010
Liu, B., Wang, W., Fan, J., Long, Y., Xiao, F., Daniyal, M., et al. (2019). RBC membrane camouflaged prussian blue nanoparticles for gamabutolin loading and combined chemo/photothermal therapy of breast cancer. Biomaterials 217, 119301. doi: 10.1016/j.biomaterials.2019.119301
Lubich, C., Allacher, P., De La Rosa, M., Bauer, A., Prenninger, T., Horling, F. M., et al. (2016). The mystery of antibodies against polyethylene glycol (PEG) - what do we know? Pharm. Res. 33, 2239–2249. doi: 10.1007/s11095-016-1961-x
Lv, Y., Liu, M., Zhang, Y., Wang, X., Zhang, F., Li, F., et al. (2018). Cancer cell membrane-biomimetic nanoprobes with two-photon excitation and near-infrared emission for intravital tumor fluorescence imaging. ACS Nano. 12, 1350–1358. doi: 10.1021/acsnano.7b07716
Manolova, V., Flace, A., Bauer, M., Schwarz, K., Saudan, P., Bachmann, M. F. (2008). Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 38, 1404–1413. doi: 10.1002/eji.200890018
Maurer, N., Fenske, D. B., Cullis, P. R. (2001). Developments in liposomal drug delivery systems. Expert Opin. Biol. Ther. 1, 923–947. doi: 10.1517/14712598.1.6.923
Meng, Q.-F., Rao, L., Zan, M., Chen, M., Yu, G.-T., Wei, X., et al. (2018). Macrophage membrane-coated iron oxide nanoparticles for enhanced photothermal tumor therapy. Nanotechnology 29, 134004. doi: 10.1088/1361-6528/aaa7c7
Narain, A., Asawa, S., Chhabria, V., Patil-Sen, Y. (2017). Cell membrane coated nanoparticles: next-generation therapeutics. Nanomedicine 12, 2677–2692. doi: 10.2217/nnm-2017-0225
Pei, Q., Hu, X., Zheng, X., Liu, S., Li, Y., Jing, X., et al. (2018). Light-activatable red blood cell membrane-camouflaged dimeric prodrug nanoparticles for synergistic photodynamic/chemotherapy. ACS Nano. 12, 1630–1641. doi: 10.1021/acsnano.7b08219
Qi, S. S., Sun, J. H., Yu, H. H., Yu, S. Q. (2017). Co-delivery nanoparticles of anti-cancer drugs for improving chemotherapy efficacy. Drug Deliv. 24, 1909–1926. doi: 10.1080/10717544.2017.1410256
Qian, H., Liu, B., Jiang, X. (2018). Application of nanomaterials in cancer immunotherapy. Mater. Today Chem. 7, 53–64. doi: 10.1016/j.mtchem.2018.01.001
Qiu, W.-X., Zhang, M.-K., Liu, L.-H., Gao, F., Zhang, L., Li, S.-Y., et al. (2018). A self-delivery membrane system for enhanced anti-tumor therapy. Biomaterials 161, 81–94. doi: 10.1016/j.biomaterials.2018.01.037
Rao, L., Bu, L.-L., Cai, B., Xu, J.-H., Li, A., Zhang, W.-F., et al. (2016a). Cancer cell membrane-coated upconversion nanoprobes for highly specific tumor imaging. Adv. Mater. 28, 3460–346+. doi: 10.1002/adma.201506086
Rao, L., Xu, J.-H., Cai, B., Liu, H., Li, M., Jia, Y., et al. (2016b). Synthetic nanoparticles camouflaged with biomimetic erythrocyte membranes for reduced reticuloendothelial system uptake. Nanotechnology 27, 085106. doi: 10.1088/0957-4484/27/8/085106
Rao, L., Meng, Q.-F., Huang, Q., Wang, Z., Yu, G.-T., Li, A., et al. (2018). Platelet-leukocyte hybrid membrane-coated immunomagnetic beads for highly efficient and highly specific isolation of circulating tumor cells. Adv. Funct. Mater. 28, 1803531. doi: 10.1002/adfm.201803531
Ren, X., Zheng, R., Fang, X., Wang, X., Zhang, X., Yang, W., et al. (2016). Red blood cell membrane camouflaged magnetic nanoclusters for imaging-guided photothermal therapy. Biomaterials 92, 13–24. doi: 10.1016/j.biomaterials.2016.03.026
Ribas, A., Wolchok, J. D. (2018). Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355. doi: 10.1126/science.aar4060
Riley, R. S., June, C. H., Langer, R., Mitchell, M. J. (2019). Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discovery 18, 175–196. doi: 10.1038/s41573-018-0006-z
Saez-Rodriguez, J., Macnamara, A., Cook, S. (2015). Modeling signaling networks to advance new cancer therapies. Annu. Rev. Biomed. Eng. 17, 143–163. doi: 10.1146/annurev-bioeng-071813-104927
Su, J., Sun, H., Meng, Q., Yin, Q., Zhang, P., Zhang, Z., et al. (2016). Bioinspired nanoparticles with NIR-controlled drug release for synergetic chemophotothermal therapy of metastatic breast cancer. Adv. Funct. Mater. 26, 7495–7506. doi: 10.1002/adfm.201603381
Tian, H., Luo, Z., Liu, L., Zheng, M., Chen, Z., Ma, A., et al. (2017). Cancer cell membrane-biomimetic oxygen nanocarrier for breaking hypoxia-induced chemoresistance. Adv. Funct. Mater. 27, 1703197. doi: 10.1002/adfm.201703197
Vijayan, V., Uthaman, S., Park, I.-K. (2018). Cell membrane-camouflaged nanoparticles: a promising biomimetic strategy for cancer theragnostics. Polymers 10, 983. doi: 10.3390/polym10090983
Wang, D., Dong, H., Li, M., Cao, Y., Yang, F., Zhang, K., et al. (2018). Erythrocyte-cancer hybrid membrane camouflaged hollow copper sulfide nanoparticles for prolonged circulation life and homotypic-targeting photothermal/chemotherapy of melanoma. ACS Nano. 12, 5241–5252. doi: 10.1021/acsnano.7b08355
Xiao, F., Fan, J., Tond, C., Xiao, C., Wang, Z., Liu, B., et al. (2019). An erythrocyte membrane coated mimetic nano-platform for chemo-phototherapy and multimodal imaging. Rsc Adv. 9, 27911–27926. doi: 10.1039/C9RA05867B
Xu, L., Gao, F., Fan, F., Yang, L. (2018). Platelet membrane coating coupled with solar irradiation endows a photodynamic nanosystem with both improved antitumor efficacy and undetectable skin damage. Biomaterials 159, 59–67. doi: 10.1016/j.biomaterials.2017.12.028
Xu, L., Wu, S., Wang, J. (2019). Cancer cell membrane-coated nanocarriers for homologous target inhibiting the growth of hepatocellular carcinoma. J. Bioact. Compat. Polym. 34, 58–71. doi: 10.1177/0883911518819107
Yang, R., Xu, J., Xu, L., Sun, X., Chen, Q., Zhao, Y., et al. (2018). Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano. 12, 5121–5129. doi: 10.1021/acsnano.7b09041
Yang, J., Teng, Y., Fu, Y., Zhang, C. (2019). Chlorins e6 loaded silica nanoparticles coated with gastric cancer cell membrane for tumor specific photodynamic therapy of gastric cancer. Int. J. Nanomed. 14, 5061–5071. doi: 10.2147/IJN.S202910
Ye, X., Liang, X., Chen, Q., Miao, Q., Chen, X., Zhang, X., et al. (2019). Surgical tumor-derived personalized photothermal vaccine formulation for cancer immunotherapy. ACS Nano. 13, 2956–2968. doi: 10.1021/acsnano.8b07371
Yu, B., Goel, S., Ni, D., Ellison, P. A., Siamof, C. M., Jiang, D., et al. (2018). Reassembly of Zr-89-labeled cancer cell membranes into multicompartment membrane-derived liposomes for PET-trackable tumor-targeted theranostics. Adv. Mater. 30, 1704934. doi: 10.1002/adma.201704934
Zhai, Y., Ran, W., Su, J., Lang, T., Meng, J., Wang, G., et al. (2018). Traceable bioinspired nanoparticle for the treatment of metastatic breast cancer via NIR-trigged intracellular delivery of methylene blue and cisplatin. Adv. Mater. 30, 1802378. doi: 10.1002/adma.201802378
Zhang, Y., Gai, K., Li, C., Guo, Q., Chen, Q., He, X., et al. (2018). Macrophage-membrane-coated nanoparticles for tumor-targeted chemotherapy. Nano. Lett. 18, 1908–1915. doi: 10.1021/acs.nanolett.7b05263
Keywords: biological membrane, nanoparticle, drug delivery, cancer treatment, imaging
Citation: Li S, Liu J, Sun M, Wang J, Wang C and Sun Y (2020) Cell Membrane-Camouflaged Nanocarriers for Cancer Diagnostic and Therapeutic. Front. Pharmacol. 11:24. doi: 10.3389/fphar.2020.00024
Received: 30 November 2019; Accepted: 08 January 2020;
Published: 04 February 2020.
Edited by:
Chao Wang, Soochow University, ChinaReviewed by:
Quanyin Hu, Massachusetts Institute of Technology, United StatesCopyright © 2020 Li, Liu, Sun, Wang, Wang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunxi Wang, Y2h1bnhpX3dhbmdAMTI2LmNvbQ==; Yinghao Sun, c3VueWhzbW11QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.