- Department of Respiratory and Critical Care Medicine, West China Hospital/West China School of Medicine, Sichuan University, Chengdu, China
Introduction: The role of combination treatment in the management of carbapenem-resistant Enterobacteriaceae infections (CRE) is still unclear. There have been no meta-analysis comparing the efficiency of triple therapy in treating CRE infections with that of double therapy. In this perspective, we conducted a meta-analysis to clarify whether triple therapy is superior to double therapy in treating patients with CRE infections.
Methods: We performed a systematic review, using PubMed and Embase without any restrictions until October 2019. Risk ratio (RR) with 95% CI were pooled to evaluate the effect of intervention.
Results: A total of 33 studies with 1,441 subjects were identified. Pooled analysis showed that triple therapy was not associated with a reduced mortality compared with double therapy (HR 0.99 95% CI 0.85–1.14, P = 0.85).
Conclusions: This meta-analysis suggests that triple therapy is not superior to double therapy in the treatment of patients with CRE infections, although the quality of evidence is generally low based on current literatures. Future well-defined, randomized controlled trials will be required to elucidate the role of triple therapy in the treatment of CRE infections.
Introduction
The use of carbapenems has led to the surge of carbapenem-resistant Enterobacteriaceae (CRE), which represents a threat to global public health (McLaughlin et al., 2013; van Duin et al., 2013). These isolates that produce carbapenemase are usually resistant to many non-β-lactams classes of antibiotics such as fluoroquinolones, aminoglycosides, and co-trimoxazole (Bratu et al., 2005; Cuzon et al., 2008; Marchaim et al., 2008). Recent data suggests that the prevalence of CRE is increasing across the world (Lee et al., 2016; Logan and Weinstein, 2017). This is particularly worrisome because the infections caused by CRE are associated with a high mortality.
To date, the optimal treatment of CRE infections remains unknown. The treatment of CRE infections is challenging because only few therapeutic options are available, including colistin, tigecycline, aminoglycoside, and carbapenem in selected cases. This situation has forced clinicians to search for optimal combination strategy to maximize bacterial killing. In vitro data also showed that combination therapy is associated with various degrees of synergy and increased bactericidal activity compared with monotherapy (Brennan-Krohn et al., 2017). However, many clinical studies have been carried out to evaluate the efficacy of combination therapy in the treatment of CRE infections, with conflicting results. Tumbarello et al. found that patients with bloodstream infections due to Klebsiella pneumoniae carbapenemase-producing K. pneumoniae receiving two or more drugs had a lower 30-day mortality compared with those taking monotherapy (Tumbarello et al., 2012). On the contrary, another retrospective study including 256 patients claimed that combination therapy was not superior to monotherapy for treatment of infections caused by carbapenem-resistant Enterobacteriaceae (Alexander et al., 2017). Moreover, a recent randomized controlled trial including 406 patients observed that colistin plus meropenem was not superior to colistin monotherapy for treatment of infections caused by carbapenem-resistant Gram-negative bacteria (Paul et al., 2018). It is still unclear whether combination therapy could improve the clinical outcome of patients with CRE infections. Previous trials and reviews exclusively focused on the differences between monotherapy and combination therapy in these patients. In fact, many patients in the combination group actually received two or more antibiotic and the clinical difference between triple therapy and double therapy has not yet been systematically reviewed. The primary goal of this systematic review and meta-analysis was to compare triple therapy with double therapy for the treatment of severe infections caused by CRE.
Methods
Data Source and Search Strategy
We performed a systematic literature search in the PubMed and Embase without any restrictions up to October 2019. We used the following search strategy (“extensively drug-resistant” or “multidrug drug-resistant” or “carbapenem-resistant” or “carbapenemase” or “carbapenemase producing” or “Klebsiella pneumoniae carbapenemase” or “NDM” or “VIM” or “IMP” or “OXA”) and (“Gram-negative” or “Enterobacteriaceae” or “Escherichia” or “Klebsiella” or “Enterobacter” or “Proteus” or “Serratia” or “Citrobacter” or “Salmonella” or “Shigella”) and (“survival” or “mortality” or “fatality” or “death” or “lethality” or “predictor” or “prognosis”). To identify more pertinent publications, the reference lists of selected articles were also hand searched.
Inclusion and Exclusion Criteria
Screening of potentially eligible studies was conducted independently by two authors, and disagreement being resolved by consensus. Articles must have evaluated a therapeutic intervention for the treatment of patients with CRE infections. Studies provided clinical outcome regarding the efficiency of triple and double therapy were considered eligible. Triple and double therapy was defined as any three and two antibiotic combination, respectively. The primary outcome was 30-day mortality and if not reported at day 30 we extracted and documented the closest timepoint. Studies were excluded if any of the following existed: (1) essential data could not be extracted from the published articles; (2) studies reporting on the clinical outcomes of patients colonized with carbapenemase-producing Enterobacteriaceae or CRE were excluded; (3) studies were excluded if they were in vitro study, study protocol, letter, note, review, commentary, conference abstract, animal, and children study; (4) case reports and case-series including fewer than 10 infected patients were also excluded. For studies that reported the same cohort, only the study with more patients was considered. All analyses were based on previously published studies; thus, no ethical approval and patient consent are required.
Data Extraction and Risk of Bias Assessment
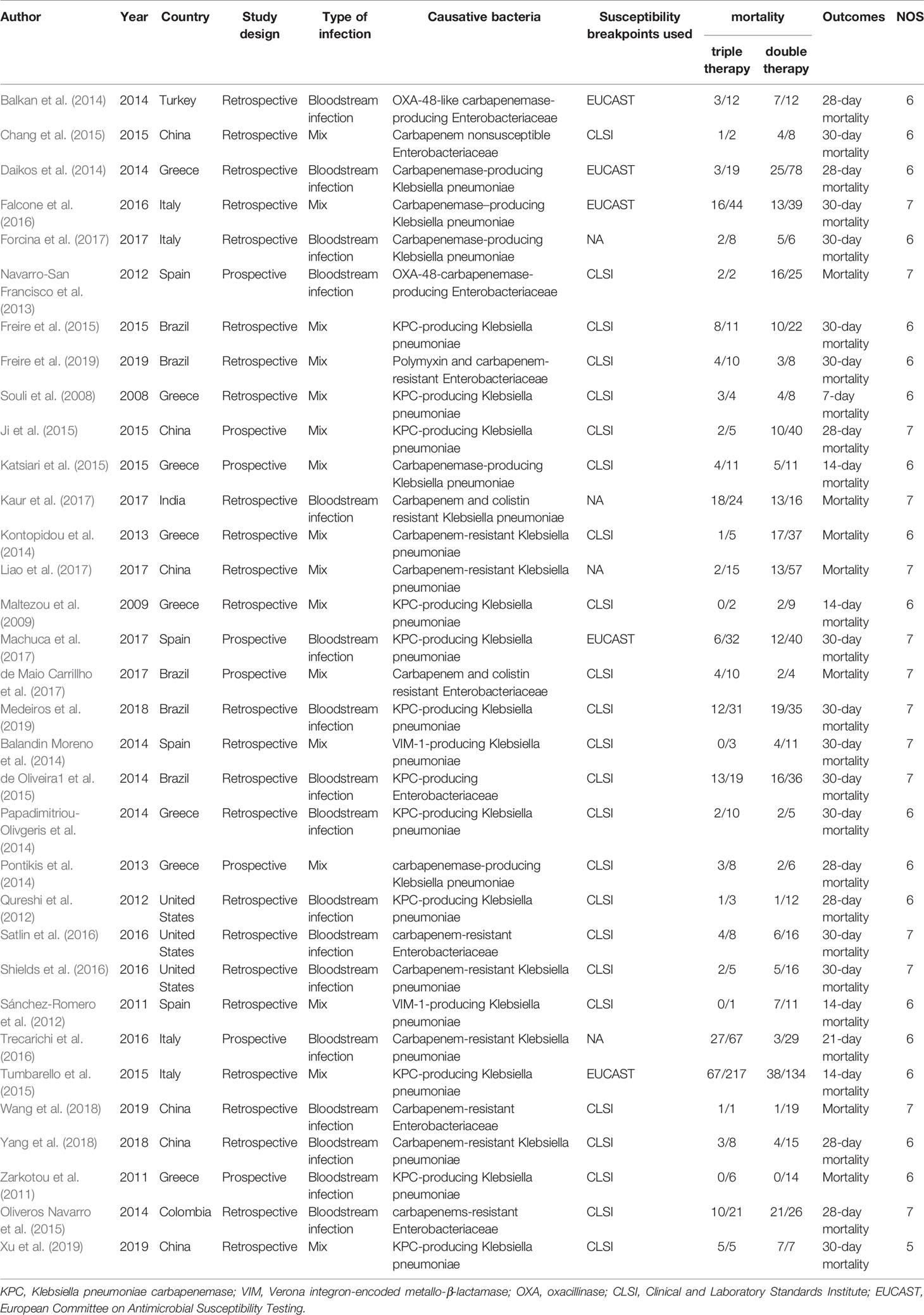
Two independent reviewers extracted the information from each study and used a predesigned data extraction excel form. If there was a disagreement, a third reviewer further assessed these articles. The following data were extracted from each included study: first author, publication year, study design, type of infection, causative pathogens, susceptibility breakpoints used for carbapenem and data on mortality for patients with CRE infections. Newcastle-Ottawa Scale was used to evaluate the quality of nonrandomized studies (Wells et al.,).
Data Analysis
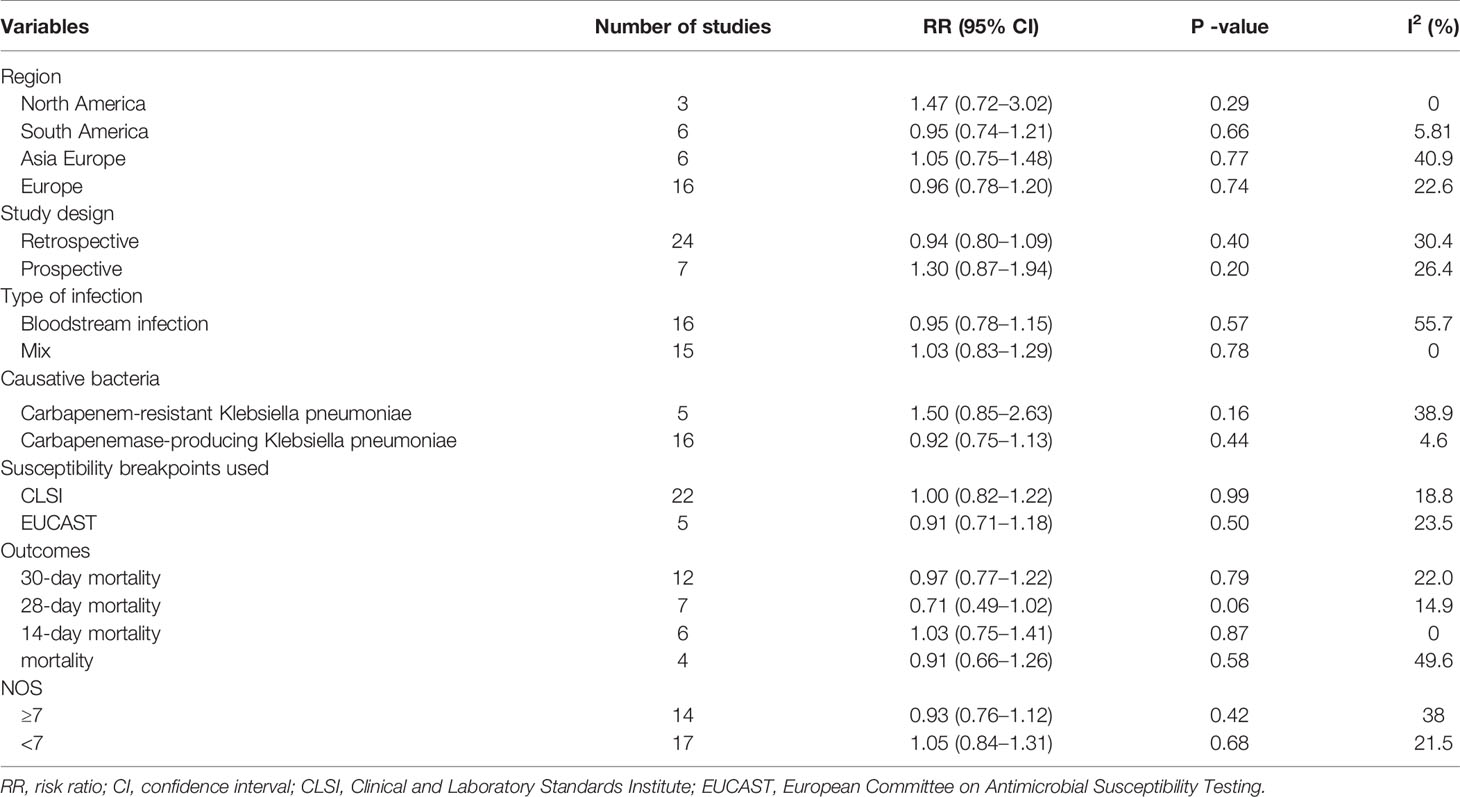
The pooled risk ratio (RR) and 95% confidence interval (CI) was calculated for outcome analysis. Heterogeneity across studies was tested by using the I2 statistic, which is a quantitative measure of inconsistency across studies (Higgins and Thompson, 2002). If the P < 0.10 and I2 > 50%, there was obvious between-study heterogeneity and a random-effects model was used; otherwise, the fixed-effects model was used. Begg’s test as well as the funnel plot were used to evaluate publication bias. Subgroup analysis were performed according to region, study design, causative pathogens, type of infections, susceptibility breakpoints used, and NOS. All statistical analyses were performed with the use of STATA 12.0 (Stata Corp, College Station, Texas, USA). All P less than 0.05 were considered as significant unless otherwise specified.
Results
Description of the Search and Selection of Trials
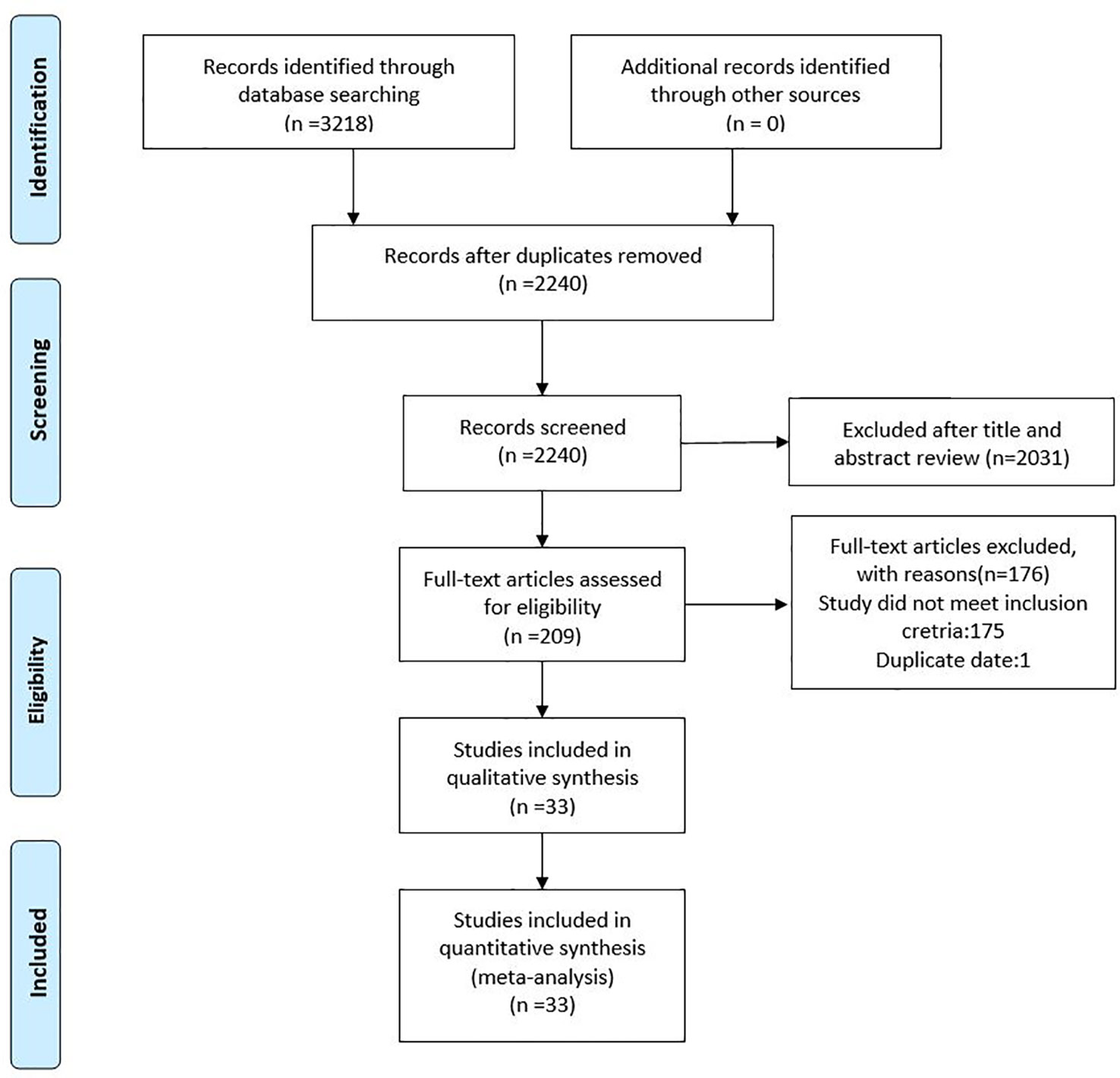
As shown in Figure 1, the literature search strategy initially identified 3,218 articles. 978 studies were excluded because they were duplicated studies. A total of 2,031 articles were removed after initial screening of titles and abstracts. Among the 209 remaining articles, 132 studies provided unclear antibiotic treatment, 17 included patients less than 10 people, 14 did not report clinical outcomes of interest, 12 treated all patients with monotherapy and/or double therapy, and 1 used duplicated data. Hence, a total of 33 eligible studies were included in this current meta-analysis (Souli et al., 2008; Maltezou et al., 2009; Zarkotou et al., 2011; Qureshi et al., 2012; Sanchez-Romero et al., 2012; Navarro-San Francisco et al., 2013; Balandin Moreno et al., 2014; Balkan et al., 2014; Daikos et al., 2014; Kontopidou et al., 2014; Papadimitriou-Olivgeris et al., 2014; Pontikis et al., 2014; Chang et al., 2015; de Oliveira et al., 2015; Freire et al., 2015; Ji et al., 2015; Katsiari et al., 2015; Oliveros Navarro et al., 2015; Tumbarello et al., 2015; Falcone et al., 2016; Satlin et al., 2016; Shields et al., 2016; Trecarichi et al., 2016; de Maio Carrillho et al., 2017; Forcina et al., 2017; Kaur et al., 2017; Liao et al., 2017; Machuca et al., 2017; Wang et al., 2018; Yang et al., 2018; Freire et al., 2019; Medeiros et al., 2019; Xu et al., 2019).
Characteristics and Risk of Bias Assessment of Included Trials
The basic characteristics of all the included studies are presented in Table 1. In total, the 33 retrospective and prospective studies were all published between 2011 and 2019. All studies included patients suffering from severe infections caused by CRE or carbapenemase-producing Enterobacteriaceae (CPE). In particular, two studies focused on oxacillinase (OXA)-48-CPE and Verona integron-encoded metallo-β-lactamase (VIM-1) producing K pneumoniae, respectively. All studies interpreted carbapenem resistance according to the Clinical and Laboratory Standards Institute (CLSI) or European Committee on Antimicrobial Susceptibility Testing (ECAST) except four studies. All articles reported the primary outcomes of interest, 13 reporting 30-day mortality, 7 reporting overall mortality, 7 reporting 28-day mortality, 1 reporting 21-day mortality, 4 reporting 14-day mortality and the remaining 1 reported 7-day mortality. Risk of bias assessment of included trials is summarized in Table 1. Overall methodological quality of included studies was moderate, and the NOS scores ranged from five to seven (Supplementary Table S1).
Mortality
Meta-analysis of these studies showed that mortality did not significantly differ between triple therapy and double therapy based on fixed effect model (HR 0.99 95% CI 0.85–1.14, P = 0.85; Figure 2). The heterogeneity across studies was low (I2 = 27.6%, P = 0.08).
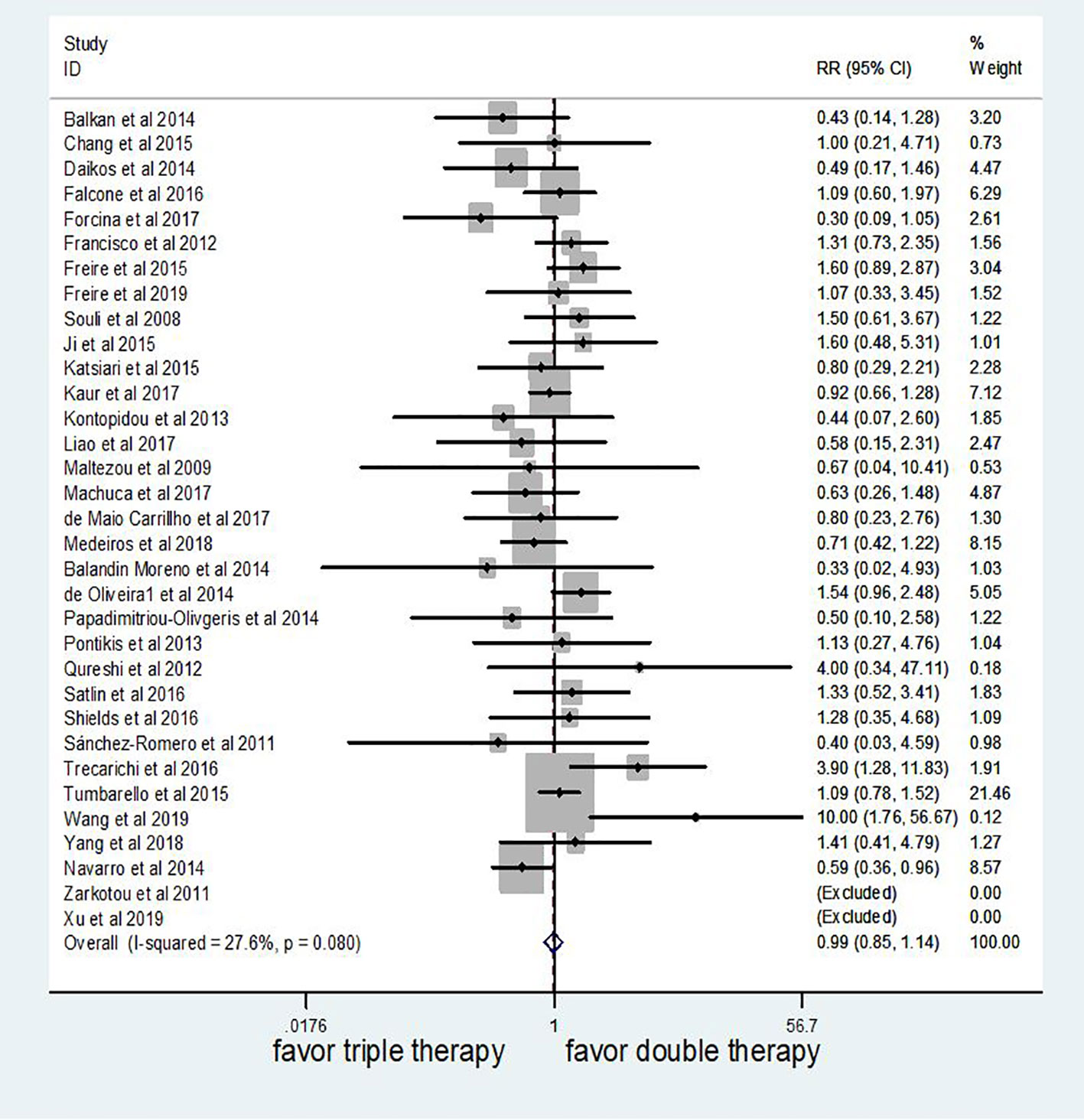
Figure 2 The efficacy of triple therapy, as compared with double therapy, in the treatment of infections due to carbapenem-resistant Enterobacteriaceae (CRE).
The subgroup analysis by study characteristics is shown in Table 2. Our meta-analysis showed that the outcome did not differ significantly on the basis of any of the following individual study characteristics: region, study design, type of infection, causative bacteria, susceptibility breakpoints used, outcome, as well as NOS.
Publication Bias
No significant statistical bias was detected by Begg’s methods and the funnel plot demonstrated no marked evidence of asymmetry (P = 0.66, Figure 3).
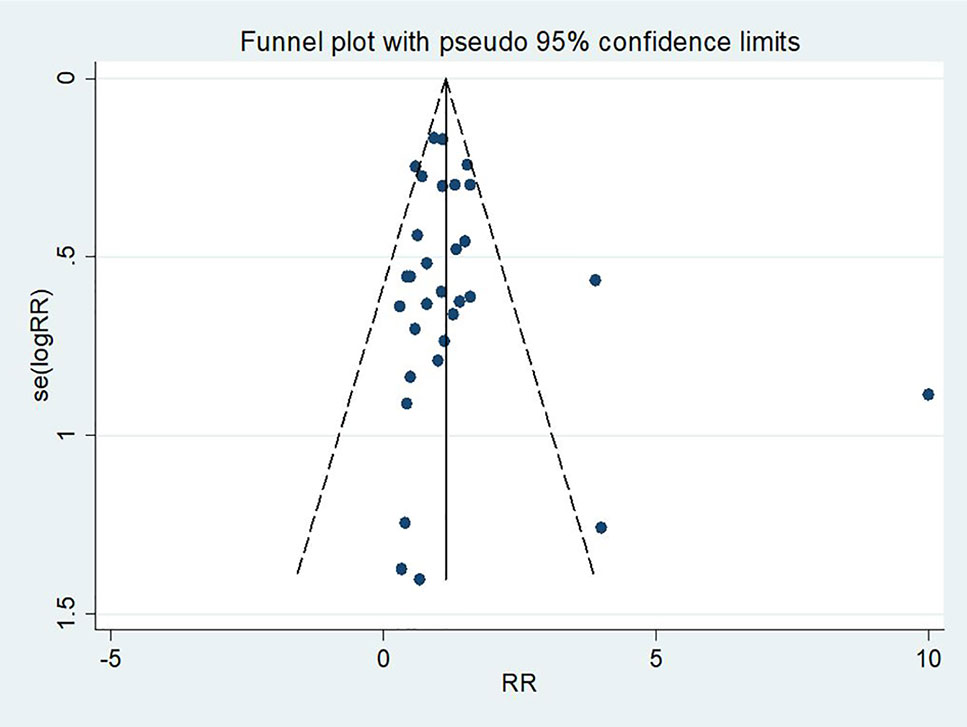
Figure 3 A funnel plot of mortality rate in patients treated with triple therapy compared with that in patients treated with double therapy for infections caused by carbapenem-resistant Enterobacteriaceae (CRE).
Discussion
To our knowledge, this is the first systematic review and meta-analysis to compare triple versus double antibiotic therapy among patients with CRE infections who have limited treatment options. Our meta-analysis failed to show the superiority of triple over double antibiotic therapy in the treatment of these patients.
There is no doubt that antibiotics have revolutionized medical practice. However, the widespread use of antibiotics has spurred the emergence of CRE. Since the first case was reported in 2001, CRE have spread worldwide (Munoz-Price et al., 2013). Moreover, infections due to CRE are associated with an alarming mortality rate, with an estimated fatality of more than 50% (Falagas et al., 2014). As a result, the US Centers for Disease Control and Prevention (CDC) recognized CRE as one of the three most urgent antimicrobial resistant threats. New therapeutic strategies are desperately needed to address this increasingly important global public health problem. Although we made a comprehensive subgroup analysis, no individual study characteristic was associated with an improved clinical outcome among patients receiving triple antibiotic therapy. This finding is essential because the adoption of triple therapy for CRE might lead to excessive use of antibiotics, resulting in a vicious cycle of antibiotic use and antibiotic resistance. A previous meta-analysis involving 3,627 participants demonstrated that prior antibiotic use such as carbapenem and aminoglycoside was an important risk factor of carbapenem-resistant Klebsiella pneumoniae infection (Liu et al., 2018). Given that roughly 30%–50% of the antibiotic use in hospitals is unnecessary and the limitation of antimicrobial drugs has the potential to reduce the prevalence of CRE, strong antibiotic stewardship policies are urgently needed to curb unnecessary prescribing to ensure more judicious use of antibiotics (Yong et al., 2010; Fleming-Dutra et al., 2016; Tacconelli et al., 2018). Our meta-analysis is in accord with a previous in vitro study which observed that adding a third antibiotic did not enhance synergistic effect in multidrug-resistant Klebsiella pneumonia isolates (Stein et al., 2015). It seems plausible that the use of triple therapy might act synergistically to kill bacteria and hopefully improve clinical outcomes. Unfortunately, in our meta-analysis, this supposed synergy failed to translate into improved clinical outcome. One potential explanation for this finding is that triple therapy might have little if any additional pharmacologic effect compared with double therapy. Another potential explanation relates to an unfavorable balance between positive and negative effects of triple therapy. Although there have been cases successfully treated with triple therapy, there is currently no large clinal trial specifically investigating the safety profile of triple therapy among patients with severe infections. Moreover, giving a noncovering antibiotic, especially carbapenem, to patients is not clinically harmless. The added antibiotics might favor the development of Clostridium difficile infection, which is a common healthcare-associated disease worldwide (Vuotto et al., 2018). Therefore, our meta-analysis does not support the routine triple therapy in the management of CRE infections based on currently available evidence.
With the increasing prevalence of CRE, it is urgent for the medical community to develop novel antibiotics. The recent introduction of β-lactamase inhibitors such as avibactam provided alternative treatment options for severe infections due to CRE. In 2015, the US Food and Drug Administration (FDA) approved ceftazidime-avibactam for the treatment of complicated intraabdominal and urinary tract infections (López-Hernández et al., 2017). Another promising agent is plazomicin, a novel aminoglycoside with in vitro activity against CRE. However, future large clinical trials are desperately needed to clarify the efficiency of these drugs in the treatment of serious infections due to CRE.
Our study has several strengths. First, this systematic review focuses on a precise clinical question that deserves future research among patients with CRE infections. Second, our study includes a total of 33 retrospective and prospective studies covering 1,441 patients with CRE infections. Third, it takes several important factors into consideration in the analysis of our data, which adds to the robustness of this study. Several limitations should be considered when interpreting our finding. First, although we observed a noninferiority of double therapy in the treatment of CRE infections compared with triple therapy, this conclusion is exclusively from retrospective and prospective studies. Result from nonrandomized controlled trials is susceptible to bias and confounders. Second, the study failed to compare different triple therapies because there are many combinations in terms of triple versus double antibiotic and some articles do not provide detailed information about the antibiotics used, it is unclear whether a specific triple regimen would improve the clinical outcomes of these patients. Finally, the quality of the included studies was not homogeneous, which may originate from differences in study design and data analysis. As a result, the confidence in estimates of effect in this meta-analysis was generally low. Despite these limitations, our meta-analysis has rigorously compared the efficiency of triple therapy in the treatment of patients with CRE infections with that of double therapy.
Conclusion
In summary, by applying a comprehensive search strategy, we found that triple antibiotic therapy is not superior to double antibiotic therapy in the treatment of patients with CRE infections, although this result requires cautious interpretation. Triple therapy may be a suboptimal choice for infections due to CRE and the optimal treatment of such conditions remains unknown. To date, there have been no randomized controlled trial to address whether triple therapy improves clinical outcomes among patients with infections caused by CRE. Future well-defined, randomized controlled trials will be required to elucidate the role of triple therapy in the treatment of CRE infections.
Author Contributions
HF conceived the studies. LW, XT, and HJ carried out the literature search and interpretation of the data. LZ and DW helped conduct the analyses. MW and TL assessed the risk of bias. LW wrote the manuscript. All authors approved the final manuscript.
Funding
This study was supported by the National Key R&D Program of China (2017YFC1309703).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Materials
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.01673/full#supplementary-material
References
Alexander, E. L., Loutit, J., Tumbarello, M., Wunderink, R., Felton, T., Daikos, G., et al. (2017). Carbapenem-resistant enterobacteriaceae infections: results from a retrospective series and implications for the design of prospective clinical trials. Open Forum Infect. Dis. 4, ofx063. doi: 10.1093/ofid/ofx063
Balandin Moreno, B., Fernandez Simon, I., Pintado Garcia, V., Sanchez Romero, I., Isidoro Fernandez, B., Romera Ortega, M. A. (2014). Alcantara Carmona, S.; Perez Redondo, M.; Galdos Anuncibay, P. Tigecycline therapy for infections due to carbapenemase-producing Klebsiella pneumoniae in critically ill patients. Scand. J. Infect. Dis. 46, 175–180. doi: 10.3109/00365548.2013.861608
Balkan, I. I., Aygun, G., Aydin, S., Mutcali, S. I., Kara, Z., Kuskucu, M., et al. (2014). Blood stream infections due to OXA-48-like carbapenemase-producing Enterobacteriaceae: treatment and survival. Int. J. Infect. Dis. 26, 51–56. doi: 10.1016/j.ijid.2014.05.012
Bratu, S., Landman, D., Haag, R., Recco, R., Eramo, A., Alam, M., et al. (2005). Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City. Arch. Intern. Med. 165, 1430–1435. doi: 10.1001/archinte.165.12.1430
Brennan-Krohn, T., Truelson, K. A., Smith, K. P., Kirby, J. E. (2017). Screening for synergistic activity of antimicrobial combinations against carbapenem-resistant Enterobacteriaceae using inkjet printer-based technology. J. Antimicrob. Chemother. 72, 2775–2781. doi: 10.1093/jac/dkx241
Chang, Y. Y., Chuang, Y. C., Siu, L. K., Wu, T. L., Lin, J. C., Lu, P. L., et al. (2015). Clinical features of patients with carbapenem nonsusceptible Klebsiella pneumoniae and Escherichia coli in intensive care units: a nationwide multicenter study in Taiwan. J. Microbiol. Immunol. Infect. 48, 219–225. doi: 10.1016/j.jmii.2014.05.010
Cuzon, G., Naas, T., Demachy, M. C., Nordmann, P. (2008). Plasmid-mediated carbapenem-hydrolyzing β-lactamase KPC in Klebsiella pneumoniae isolate from Greece. Antimicrob. Agents Chemother. 52, 796–797. doi: 10.1128/AAC.01180-07
Daikos, G. L., Tsaousi, S., Tzouvelekis, L. S., Anyfantis, I., Psichogiou, M., Argyropoulou, A., et al. (2014). Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob. Agents Chemother. 58, 2322–2328. doi: 10.1128/AAC.02166-13
de Maio Carrillho, C. M., Gaudereto, J. J., Martins, R. C., de Castro Lima, V. A., de Oliveira, L. M., Urbano, M. R., et al. (2017). Colistin-resistant Enterobacteriaceae infections: clinical and molecular characterization and analysis of in vitro synergy. Diagn. Microbiol. Infect. Dis. 87, 253–257. doi: 10.1016/j.diagmicrobio.2016.11.007
de Oliveira, M. S., de Assis, D. B., Freire, M. P., Boas do Prado, G. V., Machado, A. S., Abdala, E., et al. (2015). Treatment of KPC-producing Enterobacteriaceae: suboptimal efficacy of polymyxins. Clin. Microbiol. Infect. 21, 179 e1–179 e7. doi: 10.1016/j.cmi.2014.07.010
Falagas, M. E., Tansarli, G. S., Karageorgopoulos, D. E., Vardakas, K. Z. (2014). Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg. Infect. Dis. 20 (7), 1170–1175. doi: 10.3201/eid2007.121004
Falcone, M., Russo, A., Iacovelli, A., Restuccia, G., Ceccarelli, G., Giordano, A., et al. (2016). Predictors of outcome in ICU patients with septic shock caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin. Microbiol. Infect. 22, 444–450. doi: 10.1016/j.cmi.2016.01.016
Fleming-Dutra, K. E., Hersh, A. L., Shapiro, D. J., Bartoces, M., Enns, E. A., File, TM, Jr, et al. (2016). Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA 315, 1864–1873. doi: 10.1001/jama.2016.4151
Forcina, A., Baldan, R., Marasco, V., Cichero, P., Bondanza, A., Noviello, M., et al. (2017). Control of infectious mortality due to carbapenemase-producing Klebsiella pneumoniae in hematopoietic stem cell transplantation. Bone Marrow Transplant 52, 114–119. doi: 10.1038/bmt.2016.234
Freire, M. P., Pierrotti, L. C., Filho, H. H., Ibrahim, K. Y., Magri, A. S., Bonazzi, P. R., et al. (2015). Infection with Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae in cancer patients. Eur. J. Clin. Microbiol. Infect. Dis. 34, 277–286. doi: 10.1007/s10096-014-2233-5
Freire, M. P., de Oliveira Garcia, D., Cury, A. P., Francisco, G. R., Dos Santos, N. F., Spadao, F., et al. (2019). The role of therapy with aminoglycoside in the outcomes of kidney transplant recipients infected with polymyxin- and carbapenem-resistant Enterobacteriaceae. Eur. J. Clin. Microbio. Infect. Dis. 38, 755–765. doi: 10.1007/s10096-019-03468-4
Higgins, J., Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558. doi: 10.1002/sim.1186
Ji, S., Lv, F., Du, X., Wei, Z., Fu, Y., Mu, X., et al. (2015). Cefepime combined with amoxicillin/clavulanic acid: a new choice for the KPC-producing K. pneumoniae infection. Int. J. Infect. Dis. 38, 108–114. doi: 10.1016/j.ijid.2015.07.024
Katsiari, M., Panagiota, G., Likousi, S., Roussou, Z., Polemis, M., Alkiviadis Vatopoulos, C., et al. (2015). Carbapenem-resistant Klebsiella pneumoniae infections in a Greek intensive care unit: molecular characterisation and treatment challenges. J. Glob. Antimicrob. Resist. 3, 123–127. doi: 10.1016/j.jgar.2015.01.006
Kaur, A., Gandra, S., Gupta, P., Mehta, Y., Laxminarayan, R., Sengupta, S. (2017). Clinical outcome of dual colistin- and carbapenem-resistant Klebsiella pneumoniae bloodstream infections: A single-center retrospective study of 75 cases in India. Am. J. Infect. Control 45, 1289–1291. doi: 10.1016/j.ajic.2017.06.028
Kontopidou, F., Giamarellou, H., Katerelos, P., Maragos, A., Kioumis, I., Trikka-Graphakos, E., et al. (2014). Infections caused by carbapenem-resistant Klebsiella pneumoniae among patients in intensive care units in Greece: a multi-centre study on clinical outcome and therapeutic options. Clin. Microbiol. Infect. 20, O117–O123. doi: 10.1111/1469-0691.12341
Lee, C. R., Lee, J. H., Park, K. S., Kim, Y. B., Jeong, B. C., Lee, S. H. (2016). Global dissemination of carbapenemase-producing Klebsiella pneumoniae: epidemiology, genetic context, treatment options, and detection methods. Front. Microbiol. 7, 895. doi: 10.3389/fmicb.2016.00895
Liao, Y., Hu, G. H., Xu, Y. F., Che, J. P., Luo, M., Zhang, H. M., et al. (2017). Retrospective analysis of fosfomycin combinational therapy for sepsis caused by carbapenem-resistant Klebsiella pneumoniae. Exp. Ther. Med. 13, 1003–1010. doi: 10.3892/etm.2017.4046
Liu, P., Li, X., Luo, M., Xu, X., Su, K., Chen, S., et al. (2018). Risk factors for carbapenem-resistant Klebsiella pneumoniae infection: a meta-analysis. Microb. Drug Resist. 24, 190–198. doi: 10.1089/mdr.2017.0061
Logan, L. K., Weinstein, R. A. (2017). The epidemiology of carbapenem-resistant Enterobacteriaceae: The impact and evolution of a global menace. J. Infect. Dis. 215, S28–S36. doi: 10.1093/infdis/jiw282
López-Hernández, I., Alonso, N., Fernández-Martínez, M., Zamorano, L., Rivera, A., Oliver, A., et al. (2017). Activity of ceftazidime-avibactam against multidrug-resistance Enterobacteriaceae expressing combined mechanisms of resistance. Enferm. Infecc. Microbiol. Clin. 35, 499–504. doi: 10.1016/j.eimc.2016.09.013
Machuca, I., Gutiérrez-Gutiérrez, B., Gracia-Ahufinger, I., Rivera Espinar, F., Cano, Á, Guzmán-Puche, J., et al. (2017). Mortality associated with bacteremia due to colistin-resistant Klebsiella pneumoniae with high-level meropenem resistance: importance of combination therapy without colistin and carbapenems. Antimicrob. Agents Chemother. 61, e00406–e00417. doi: 10.1128/AAC.00406-17
Maltezou, H. C., Giakkoupi, P., Maragos, A., Bolikas, M., Raftopoulos, V., Papahatzaki, H., et al. (2009). Outbreak of infections due to KPC-2-producing Klebsiella pneumoniae in a hospital in Crete (Greece). J. Infect. 58, 213–219. doi: 10.1016/j.jinf.2009.01.010
Marchaim, D., Navon-Venezia, S., Schwaber, M. J., Carmeli, Y. (2008). Isolation of imipenem-resistant Enterobacter species: emergence of KPC-2 carbapenemase, molecular characterization, epidemiology, and outcomes. Antimicrob. Agents Chemother. 52, 1413–1418. doi: 10.1128/AAC.01103-07
McLaughlin, M., Advincula, M. R., Malczynski, M., Qi, C., Bolon, M., Scheetz, M. H. (2013). Correlations of antibiotic use and carbapenem resistance in enterobacteriaceae. Antimicrob. Agents Chemother. 57, 5131–5133. doi: 10.1128/AAC.00607-13
Medeiros, G. S., Rigatto, M. H., Falci, D. R., Zavascki, A. P. (2019). Combination therapy with polymyxin B for carbapenemase-producing Klebsiella pneumoniae bloodstream infection. Int. J. Antimicrob. Agents 53, 152–157. doi: 10.1016/j.ijantimicag.2018.10.010
Munoz-Price, L. S., Poirel, L., Bonomo, R. A., Schwaber, M. J., Daikos, G. L., Cormican, M., et al. (2013). Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 13, 785–796. doi: 10.1016/S1473-3099(13)70190-7
Navarro-San Francisco, C., Mora-Rillo, M., Romero-Gomez, M. P., Moreno-Ramos, F., Rico-Nieto, A., Ruiz-Carrascoso, G., et al. (2013). Bacteraemia due to OXA-48-carbapenemase-producing Enterobacteriaceae: a major clinical challenge. Clin. Microbiol. Infect. 19, E72–E79. doi: 10.1111/1469-0691.12091
Oliveros Navarro, A., Uribe, N., Sierra, P., Jaimes, F., González, J. M. (2015). Bacteriemia por enterobacterias resistentesa carbapenems. Un estudio transversal. Infectio 19, 60–66. doi: 10.1016/j.infect.2014.11.006
Papadimitriou-Olivgeris, M., Marangos, M., Christofidou, M., Fligou, F., Bartzavali, C., Panteli, E. S., et al. (2014). Risk factors for infection and predictors of mortality among patients with KPC-producing Klebsiella pneumoniae bloodstream infections in the intensive care unit. Scand. J. Infect. Dis. 46, 642–648. doi: 10.3109/00365548.2014.923106
Paul, M., Daikos, G. L., Durante-Mangoni, E., Yahav, D., Carmeli, Y. (2018). Benattar YD Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect. Dis. 18, 391–400. doi: 10.1016/S1473-3099(18)30099-9
Pontikis, K., Karaiskos, I., Bastani, S., Dimopoulos, G., Kalogirou, M., Katsiari, M., et al. (2014). Outcomes of critically ill intensive care unit patients treated with fosfomycin for infections due to pandrug-resistant and extensively drug-resistant carbapenemase-producing Gram-negative bacteria. Int. J. Antimicrob. Agents 43, 52–59. doi: 10.1016/j.ijantimicag.2013.09.010
Qureshi, Z. A., Paterson, D. L., Potoski, B. A., Kilayko, M. C., Sandovsky, G., et al. (2012). Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob. Agents Chemother. 56, 2108–2113. doi: 10.1128/AAC.06268-11
Sanchez-Romero, I., Asensio, A., Oteo, J., Munoz-Algarra, M., Isidoro, B., Vindel, A., et al. (2012). Nosocomial outbreak of VIM-1-producing Klebsiella pneumoniae isolates of multilocus sequence type 15: molecular basis, clinical risk factors, and outcome. Antimicrob. Agents Chemother. 56, 420–427. doi: 10.1128/AAC.05036-11
Satlin, M. J., Cohen, N., Ma, K. C., Gedrimaite, Z., Soave, R., Askin, G., et al. (2016). Bacteremia due to carbapenem-resistant Enterobacteriaceae in neutropenic patients with hematologic malignancies. J. Infect. 73, 336–345. doi: 10.1016/j.jinf.2016.07.002
Shields, R. K., Clancy, C. J., Press, E. G., Nguyen, M. H. (2016). Aminoglycosides for treatment of bacteremia due to carbapenem-resistant Klebsiella pneumoniae. Antimicrob. Agents Chemother. 60, 3187–3192. doi: 10.1128/AAC.02638-15
Souli, M., Kontopidou, F. V., Papadomichelakis, E., Galani, I., Armaganidis, A., Giamarellou, H. (2008). Clinical experience of serious infections caused by Enterobacteriaceae producing VIM-1 metallo-beta-lactamase in a Greek University Hospital. Clin. Infect. Dis. 46, 847–854. doi: 10.1086/528719
Stein, C., Makarewicz, O., Bohnert, J. A., Pfeifer, Y., Kesselmeier, M., Hagel, S., et al. (2015). Three dimensional checkerboard synergy analysis of colistin, meropenem, tigecycline against multidrug-resistant clinical Klebsiella pneumonia Isolates. PLoS One 10, e0126479. doi: 10.1371/journal.pone.0126479
Tacconelli, E., Sifakis, F., Harbarth, S., Schrijver, R., van Mourik, M., Voss, A., et al. (2018). Surveillance for control of antimicrobial resistance. Lancet Infect. Dis. 18, e99–e106. doi: 10.1016/S1473-3099(17)30485-1
Trecarichi, E. M., Pagano, L., Martino, B., Candoni, A., Di Blasi, R., Nadali, G., et al. (2016). Bloodstream infections caused by Klebsiella pneumoniae in onco-hematological patients: clinical impact of carbapenem resistance in a multicentre prospective survey. Am. J. Hematol. 91, 1076–1081. doi: 10.1002/ajh.24489
Tumbarello, M., Viale, P., Viscoli, C., Trecarichi, E. M., Tumietto, F., Marchese, A., et al. (2012). Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin. Infect. Dis. 55, 943–950. doi: 10.1093/cid/cis588
Tumbarello, M., Trecarichi, E. M., De Rosa, F. G., Giannella, M., Giacobbe, D. R., Bassetti, M., et al. (2015). Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J. Antimicrob. Chemother. 70, 2133–2143. doi: 10.1093/jac/dkv086
van Duin, D., Kaye, K. S., Neuner, E. A., Bonomo, R. A. (2013). Carbapenem-resistant Enterobacteriaceae: a review oftreatment and outcomes. Diagn. Microbiol. Infect. Dis. 75, 115–120. doi: 10.1016/j.diagmicrobio.2012.11.009
Vuotto, C., Donelli, G., Buckley, A., Chilton, C. (2018). Clostridium difficile Biofilm. Adv. Exp. Med. Biol. 1050, 97–115. doi: 10.1007/978-3-319-72799-8_7
Wang, X., Wang, Q., Cao, B., Sun, S., Zhang, Y., Gu, B., et al. (2018). Retrospective observational study from a Chinese network of the impact of combination therapy versus monotherapy on mortality from carbapenem-resistant enterobacteriaceae bacteremia. Antimicrob. Agents Chemother. 63, e01511–e01518. doi: 10.1128/AAC.01511-18
Wells, G. A., Shea, B., O’Connell, D., Peterson, J., Welch, V., Losos, M., et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Xu, M., Fu, Y., Fang, Y., Xu, H., Kong, H., Liu, Y., et al. (2019). High prevalence of KPC-2-producing hypervirulent Klebsiella pneumoniae causing meningitis in Eastern China. Infect. Drug Resist. 12, 641–653. doi: 10.2147/IDR.S191892
Yang, X., Cui, J., Zhao, J., Ni, W. (2018). Clinical characteristics of carbapenem-resistant Klebsiella pneumoniae bloodstream infection and the risk factors of mortality. Chin. J. Infect. Chemother. 18, 142–149. doi: 10.16718/j.1009-7708.2018.02.004
Yong, M. K., Buising, K. L., Cheng, A. C., Thursky, K. A. (2010). Improved susceptibility of Gram-negative bacteria in an intensive care unit following implementation of a computerized antibiotic decision support system. J. Antimicrob. Chemother. 65, 1062–1069. doi: 10.1093/jac/dkq058
Zarkotou, O., Pournaras, S., Tselioti, P., Dragoumanos, V., Pitiriga, V., Ranellou, K., et al. (2011). Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin. Microbiol. Infect. 17, 1798–1803. doi: 10.1111/j.1469-0691.2011.03514.x
Keywords: carbapenem-resistant Enterobacteriaceae, infection, therapy, survival, meta-analysis
Citation: Wang L, Tong X, Huang J, Zhang L, Wang D, Wu M, Liu T and Fan H (2020) Triple Versus Double Therapy for the Treatment of Severe Infections Caused by Carbapenem-Resistant Enterobacteriaceae: A Systematic Review and Meta-Analysis. Front. Pharmacol. 10:1673. doi: 10.3389/fphar.2019.01673
Received: 29 July 2019; Accepted: 23 December 2019;
Published: 30 January 2020.
Edited by:
Stefania Tacconelli, Università degli Studi G. d’Annunzio Chieti e Pescara, ItalyCopyright © 2020 Wang, Tong, Huang, Zhang, Wang, Wu, Liu and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Fan, ZmFuaG9uZ2ZhbkBxcS5jb20=
 Lei Wang
Lei Wang Xiang Tong
Xiang Tong Jizhen Huang
Jizhen Huang Li Zhang
Li Zhang Dongguang Wang
Dongguang Wang Man Wu
Man Wu Tao Liu
Tao Liu Hong Fan
Hong Fan