
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 09 October 2019
Sec. Drugs Outcomes Research and Policies
Volume 10 - 2019 | https://doi.org/10.3389/fphar.2019.01158
Aim: To assess platelet (PLT) function and bleeding risks in patients with acute coronary syndrome after tirofiban infusion.
Methods: Patients diagnosed with acute coronary syndrome from May 2016 to February 2018 in the Department of Cardiac Intensive Care Unit, Zhongshan Hospital, were enrolled. They were symmetrically allocated into two groups: tirofiban treatment group or control group (without tirofiban treatment). Blood samples were collected 24 h postoperation for the evaluation of antiplatelet effect of tirofiban. We applied thromboelastography to detect on-treatment PLT reactivity and conducted laboratory tests to assess the risk of bleeding following tirofiban treatment. After discharge, telephone follow-up and outpatient interview were carried out. The primary clinical endpoint was major adverse cardiovascular events, including cardiovascular death, cardiovascular mortality, myocardial infarction, and revascularization for the targeted vascular lesion.
Results: There were a total of 196 patients with acute coronary syndrome after screening with the inclusion criteria and the exclusion criteria. Ninety-eight patients were assigned to receive either tirofiban treatment or control treatment. Patients treated with tirofiban had more coronary lesions and stents implanted compared with the control group (P = 0.000). After tirofiban infusion, inhibition of platelet aggregation induced by thromboxane A2 and adenosine diphosphate was significantly higher compared to patients without tirofiban infusion (80.3% ± 19.6% vs. 72.6% ± 13.0%, P = 0.002; and 81.0% ± 19.8% vs. 75.4% ± 12.4%, P = 0.020, respectively). There was no significant difference in the reduction of hemoglobin (Hb), hematocrit (Hct), and PLT after administration of tirofiban, compared with baseline (P > 0.05). In addition, no significant differences were identified between the two groups with respect to Hb, Hct, and PLT after tirofiban infusion. However, C-reactive protein level, referred to as an inflammation marker, was significantly lowered after infusion tirofiban compared with the control group (11.9 ± 14.2 vs. 17.9 ± 21.2, P = 0.020). During the 1-year follow-up, the incidence rate of major adverse cardiovascular events remains indiscriminate between the two groups (P = 0.208). The assessments of cardiac biomarkers showed that tirofiban could decrease incidence of procedural myocardial infarction (odds ratio [OR] = 0.250, 95% confidence interval [CI] = 0.067–0.925, P = 0.027). At follow-up, the morbidity of left atrial dilation in tirofiban-treated patients, defined as enlargement of left atrial diameter >40mm, was lower compared to the control group (OR = 0.533, 95% CI = 0.301–0.945, P = 0.031).
Conclusion: Tirofiban infusion could decrease PLT activation in patients with acute coronary syndrome without increasing the risk of bleeding. As a concomitant medication, tirofiban shows no benefit in reducing the occurrence of major adverse cardiovascular events.
Acute coronary syndrome (ACS) has become the life-threatening disorder with high mortality and disability rate, which also causes significant economic burden annually (Turpie, 2006; Gao et al., 2018 ). The pathophysiology of ACS is in most cases due to the erosion or rupture of a plaque with consequent thrombotic obstruction of coronary artery (Seguchi and Kitakaze, 2005). Upon plaque rupture and erosion, platelet (PLT) activation and aggregation will lead to the coronary thrombosis. As PLTs play an important role in the progression of ACS, antiplatelet therapy could decrease the incidence and mortality of ACS (Amsterdam et al., 2014; Bittl et al., 2016).
Regular dual antiplatelet agents, aspirin and clopidogrel, could inhibit PLT activation and aggregation by different pathways on the PLT receptor of thromboxane and adenosine diphosphate (ADP) (Lamberts et al., 2014). Many current clinical trials indicated that the addition of tirofiban, a glycoprotein (GP) IIb/IIIa receptor antagonist, could effectively reduce ischemic events for patients with high risk of developing thrombosis (Januzzi et al., 2002; Servoss et al., 2004). Tirofiban is a nonpeptide drug with an l-tyrosine–modified structure widely used as a GPIIb/IIIa receptor antagonist. It inhibits the formation of PLT-specific integrin, which binds to protein ligands with adhesion properties, namely, fibrinogen, fibronectin, von Willebrand factor, and vitronectin (Choi et al., 2013). Tirofiban can provide a rapid, intensive PLT inhibition through reversible antagonist of fibrinogen binding to GPIIb/IIIa receptor, thereby blocking the final common pathway of PLT aggregation (Centurión, 2016).
Although several meta-analyses of randomized trials (Montalescot et al., 2007; De Luca et al., 2009) showed that periprocedural administration of GPIIb/IIIa inhibitor can improve the outcome of patients in terms of reinfarction and survival in relation to the patient risk profile, other clinical trials did not show any differences in mortality and reinfarction or major bleeding events in patients undergoing primary angioplasty. Current guidelines recommend that GPIIb/IIIa inhibitors should be taken into consideration in cases of no-reflow or a thrombotic complication (class IIa) (Roffi et al., 2015). There is currently paucity of large-scale studies comparing the clinical antiplatelet effect and safety of GPIIb/IIIa antagonists as well as studies investigating the effect of GPIIb/IIIa antagonists on the prognosis of myocardial infarction. This study evaluated the antiplatelet effect, risk of bleeding and major adverse cardiovascular events (MACEs) after intravenous tirofiban infusion.
This single-center, prospective cohort study recruited patients who were diagnosed with ACS according to the European Society of Cardiology (ESC) guidelines (Roffi et al., 2015) in the Department of Cardiac Intensive Care Unit, Zhongshan Hospital, Fudan University, between May 2016 and February 2018. All the patients presented with the symptoms of chest pain or discomfort, shortness of breath, dizziness, nausea, or sweating and were planned to undergo percutaneous coronary intervention (PCI). To be enrolled in this study, the inclusion criteria are as follows: 1) age ≥18 years, 2) no antiplatelet treatment history, 3) chest pain ≥10 min, 4) change in ST segment and T wave of electrocardiogram, 5) elevation of cardiac biomarkers, and 6) receiving loading dose of aspirin and clopidogrel before PCI operation. The exclusion criteria are as follows: 1) history of bleeding or hemorrhagic disease, 2) upstream use of antiplatelet drugs, 3) claiming coronary artery bypass surgery history, 4) PLT count <150,000/mm3, 5) prothrombin time >1.5 times control, and 6) hematocrit (Hct) < 30%. Clopidogrel at a loading dose (LD) of 300 mg was administrated before PCI, and a maintenance dose (MD) of 75 mg/d was administrated after PCI. Besides, all the enrolled patients were prescribed with the concomitant drugs including aspirin (LD/MD: 300/100 mg), low-molecular-weight heparin, renin–angiotensin–aldosterone system antagonists, statins, and β-blockers after admission to our hospital.
The eligible ACS patients were admitted to cardiac catheterization room for PCI operation, and stents were implanted on the occlusion coronary artery with stenosis >70%. Intravenous tirofiban was administrated according to the surgeon’s evaluation of thrombus burden and blood flow based on the level of stenosis in culprit vessels and multicoronary lesions. Patients were allocated 1:1 to the tirofiban group and control group.
In the tirofiban group, intravenous loading bolus 25 µg/kg of tirofiban was administrated within 5 min for the high-risk thrombosis patients during the operator progress, and postinfusion dose was 0.15 µg/kg/min for up to 18 h. Blood samples were collected for the evaluation of the antiplatelet effect of tirofiban 24 h after PCI operation.
Detailed information about each subject, including history of smoking or alcohol consumption, comorbidity disease, levels of hemoglobin (Hb) and Hct, PLT count, alanine aminotransferase, estimated glomerular filtration rate, and cardiac biomarkers as well as concomitant drugs in use, were collected through electronic medical records. We applied echocardiography to evaluate cardiac function including left atrial diameter and left ventricular ejection fraction (LVEF) during follow-up.
This study was conducted in compliance with the Declaration of Helsinki and Good Clinical Practice and approved by the Ethic Committee of Zhongshan Hospital. The written informed consent was signed by every participant.
Approximately 4 ml of whole venous blood samples were collected into Vacutainers containing anticoagulant ethylene diaminetetraacetic acid (EDTA) from all patients upon recruitment to detect the on-treatment PLT reactivity with thromboelastography (TEG; Haemoscope Corp, Niles, IL, USA).
Inhibition of PLT aggregation was analyzed according to manufacturer’s instructions. We added the following agonists as PLT stimulators: ADP (20 μmol/L) and thromboxaneA2 (TXA2; 1 mmol/L). The largest change in the value of the amplitude of coagulation intensity was defined as the maximum clot strength (MA). The MA was classified into the thrombin-induced maximum coagulation strength (MAthrombin), the activator-induced maximum coagulation strength (MA), and the fibrin-induced maximum coagulation strength (MAFibrin), according to different types of activators (ADP and TXA2) added to the blood sample. The IPA was calculated by the formula: inhibition of PLT aggregation (IPA) (%) = (MA − MAFibrin)/(MAThrombin − MAFibrin) (Lu et al., 2013).
Follow-ups were made via telephone and outpatient interview after discharge. The primary clinical endpoint was MACE, including cardiovascular death, cardiovascular mortality, myocardial infarction, and revascularization for the targeted vascular lesion (Park et al., 2011). Thrombolysis in Myocardial Infarction (TIMI) bleeding was defined as a bleeding event associated with decrease in Hb or Hct values (Hochholzer et al., 2011).
The primary endpoint definition was comparison of IPA stimulated by ADP and TXA2 24 h after PCI operation. According to previous studies, IPA(ADP) < 30% and IPA(TXA2) < 50% were defined as high on-treatment PLT reactivity (HPR) (Lv et al., 2016). The Hb, Hct, and PLT levels after PCI as compared with baseline in each group were evaluated between two groups. The second endpoint was the occurrence of target vessel revascularization or stent thrombosis at 12 months and bleeding complications according to TIMI criteria.
Our study was designed on the basis of the noninferiority principle (the without-tirofiban group was considered noninferior to the tirofiban group) (Marian et al., 2017). For the sample size calculation, the primary endpoint (IPA after tirofiban infusion) was set at 95%. Based on a power of 85% and an α level of 0.05, at least 89 patients were required in each group to reach statistical significance. Considering a 10% dropout rate, 98 patients were enrolled in each group, and they all met the inclusion criteria and exclusion criteria.
The numerical data are presented as mean ± SD. Categorical variables are expressed with frequencies ± percentage. Independent t test was used in parametric data, and Mann–Whitney U test was used in nonparametric data. The baseline and angiography procedural characteristics were compared between two groups. The cutoff value of TXA2 and ADP high sensitive test from TEG was considered to be 50% and 30%, respectively (Lu et al., 2013). Results of antiplatelet effect induced by TXA2 and ADP were compared by t test.
The comparison of Hb and Hct levels changes, C-reactive protein, and bleeding complications were analyzed by Student t test. We analyzed MACE using multiple statistical models. Variables including occurrence of new abnormal Q-waves, cardiac biomarkers, LVEF, and left atrial dilation were analyzed. Hazard ratios (HRs) with two-sided 95% confidence intervals (CIs) were calculated for the risk factors of MACE. Results are presented as HRs along with 95% CI. Survival curves were constructed using Kaplan–Meier estimates. A two-sided P value was used to determine significance (threshold, P < 0.05). Statistical analysis was performed using SPSS (IBM SPSS Statistics 22.0) and Prism 5 (GrandPad Software). A P value of 0.05 was considered to be the threshold for statistical significance.
Our enrollment began in May 2016 and ended in February 2018. A total of 196 ACS patients who met both the inclusion criteria and the exclusion criteria were enrolled and symmetrically divided to two groups: 98 patients were assigned to receive tirofiban and 98 were in the control group. All the clinical data were presented in Supplementary Table 1. Comparison of baseline characteristics is shown in Table 1. There was no significant difference between the two groups with respect to age, gender, body mass index, cardiovascular risk factors, cardiac biomarkers, concomitant drug, and other characteristics.
All enrolled patients underwent PCI operation, and stents were implanted on culprit lesions. There was no significant difference between the two groups (tirofiban group and control group) with respect to coronary occlusion, unfractionated heparin dose, number of stents, total stent length, lesion vessels, cardiac biomarker levels, days of hospitalization, and MACE ratio. Patients who received tirofiban were at higher risk of left anterior descending culprit lesions (59.2% vs. 30.6%, P = 0.000; Table 2). In addition, ACS patients receiving tirofiban treatment had more coronary lesions and stent implantations compared with the control group (P = 0.000; Table 2).
The percentage of HPR stimulated with TXA2 1 mmol/L were 3.1% and 4.1% for the tirofiban and control groups, respectively (P = 0.997). As shown in Figure 1A, after tirofiban infusion, IPA induced by TXA2 was significantly higher compared to that in the control group (80.3% ± 19.6% vs. 72.6% ± 13.0%, P = 0.002). Meanwhile, the percentage of HPR stimulated with ADP 20 μmol/L were 1.0% and 1.0% for the tirofiban group and control group, respectively (P = 1.000). As shown in Figure 1B, ADP-induced IPA was significantly higher in the tirofiban group than that in the control group 24 h after PCI operation (81.0% ± 19.8% vs. 75.4% ± 12.4%, P = 0.020).
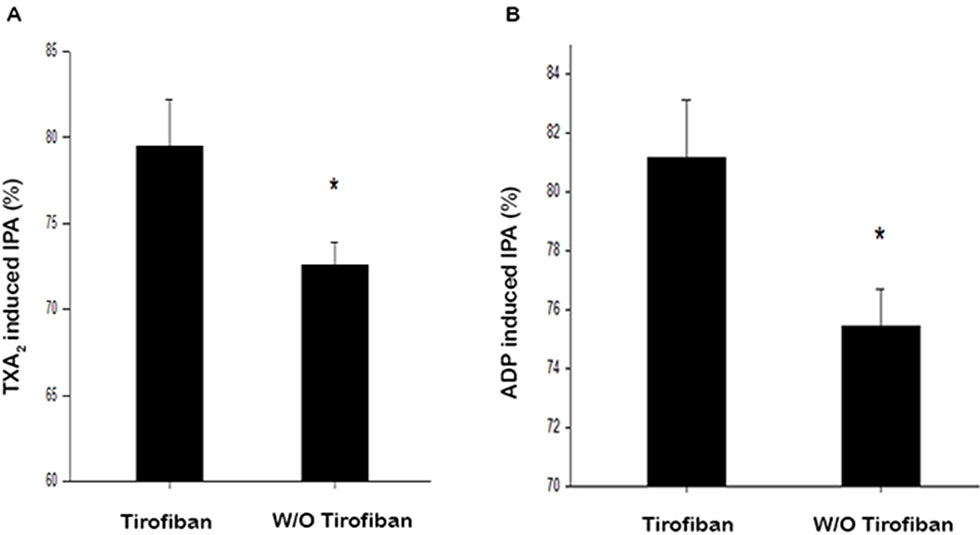
Figure 1 Antiplatelet effect of IPA with addition of TXA2(A) and ADP. (B) TXA2, 1 mmol/L; ADP, 20 μmol/L; IPA, inhibition of platelet aggregation; *P < 0.05.
As shown in Figure 2, the baseline values of Hb, Hct, and PLT were not significantly different between the groups (P > 0.05). After administration of tirofiban, decreases in Hb, Hct, and PLT were not significantly different compared with baseline (P > 0.05). There was no significant difference between the two groups with respect to the levels of Hb, Hct, and PLT 24 h after PCI.
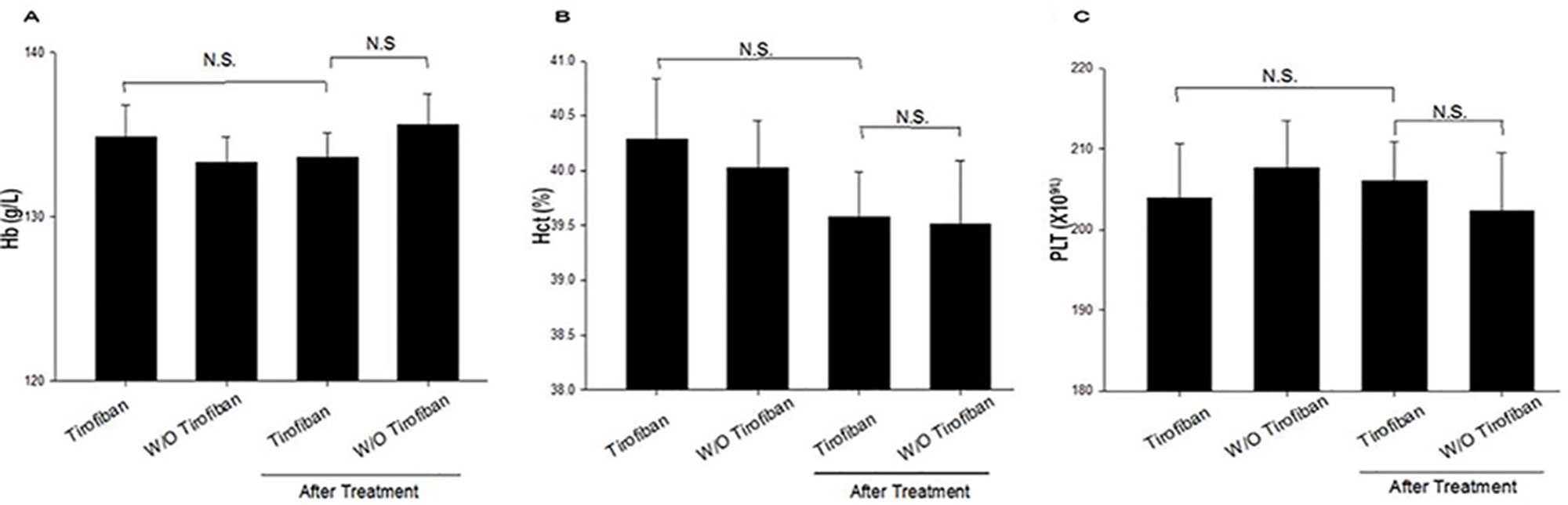
Figure 2 Laboratory characteristic comparison between baseline and treatment for the two groups (A–C). Hb, hemoglobin; Hct, hematocrit; PLT, platelet; N.S., no significance.
As shown in Figure 3, inflammation biomarker and C-reactive protein values were significantly lower in the tirofiban group than that in the control group 24 h after PCI operation (11.9 ± 14.2 vs. 17.9 ± 21.2, P = 0.020).
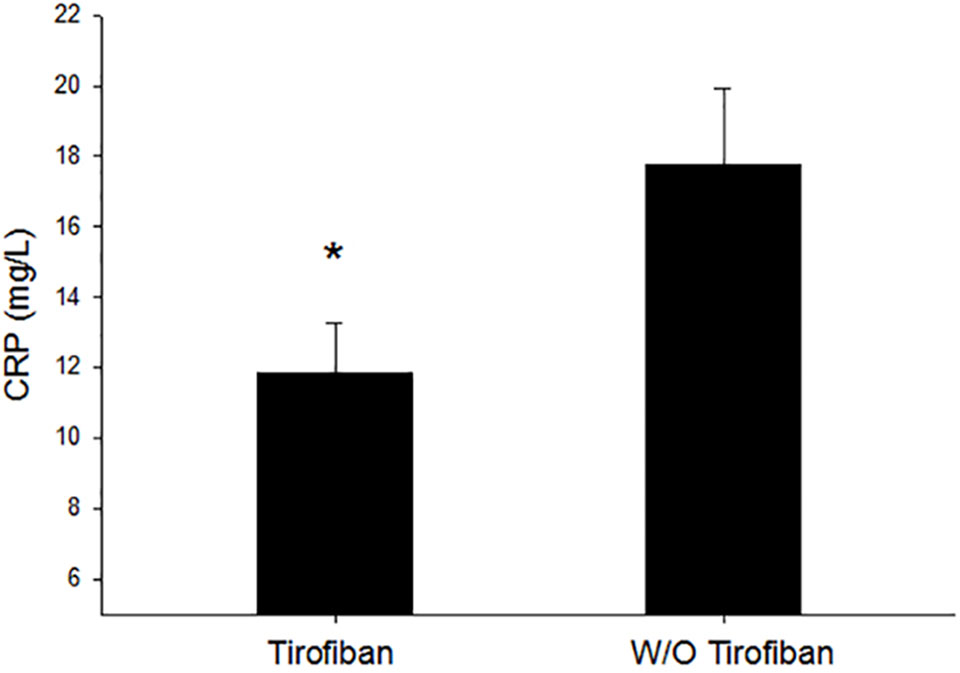
Figure 3 Inflammation comparison between the tirofiban group and control group. CRP, C-reactive protein; *P < 0.05.
During the 1-year follow-up, the primary rates of MACE occurrence including the rehospitalization for the revascularization between the tirofiban group and control group were 11.2% and 17.3%, respectively. There was no significant difference after tirofiban administration in terms of MACE occurrence ratio compared with the control group (P = 0.208) As shown in Figure 4.
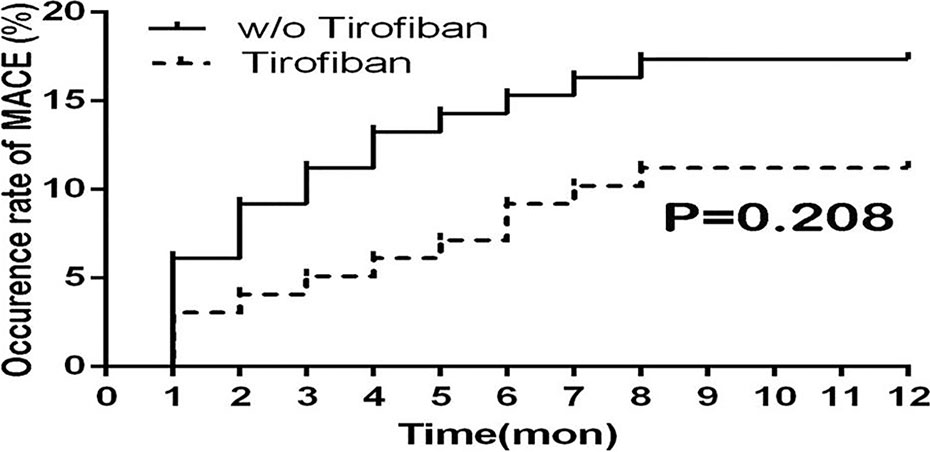
Figure 4 Survival curve of MACE ratio for 1-year follow-up between groups. MACE, major adverse cardiovascular events.
The assessments of cardiac biomarkers showed that tirofiban could decrease the incidence of procedural myocardial infarction [odds ratio (OR) = 0.250, 95% CI = 0.067–0.925, P = 0.027] (Table 3). Also for the follow-up, occurrence ratio of left atrial dilation defined as enlargement of left atrial diameter >40 mm was lower in the tirofiban group than in the control group (OR = 0.533, 95% CI = 0.301–0.945, P = 0.031) (Table 3).
A large body of evidence is now available about the benefits of tirofiban application, in terms of the prevention of stent thrombosis and the reduction of MACE, especially among ACS patients undergoing PCI operation (Januzzi et al., 2002). Although the GPIIb/IIIa antagonists were developed to prevent PLT aggregation, increasing evidence supports the concept that GPIIb/IIIa antagonists can exert further biological effects and that PLTs are not the only targeted cells. Such effects may be, in part, dependent on integrin–transmembrane receptor triggering. In particular, the integrin-mediated induction of vascular endothelial growth factor (VEGF) stimulated by the arginine–glycine–aspartic acid (RGD) mimetic tirofiban appeared to be of considerable interest (Giordano et al., 2016).
Our study found that administration with IIb/IIIa blocker antagonist, tirofiban, could provide a stronger effect on PLT inhibition induced by TXA2 and ADP compared with treatment not using a GP inhibitor (GPI). In addition, the risk of bleeding was not aggravayed by tirofiban. Moreover, as shown in previous overall clinical trials (Januzzi et al., 2002; Servoss et al., 2004), ACS patients treated with tirofiban had a lower rate of MACE. Platelet GPIIb/IIIa receptor blocker can inhibit the formation of thromboembolism by preventing fibrinogen binding to PLT IIb/IIIa receptors, which is the final pathway for PLT aggregation (Starnes et al., 2011). In our study, tirofiban infusion had benefit on patients who were prone to develop restenosis after PCI operation. One registry reported that ACS patients treated with GPI had longer stents with larger diameters and more complexed lesions (Safley et al., 2015). A meta-analysis showed that mortality could be reduced by 25% for patients undergoing PCI who were confined at the highest risk (Sethi et al., 2013). Considering the high thrombosis burden mediated with PLT aggregation, current guidelines (Roffi et al., 2015; Bittl et al., 2016) recommend tirofiban infusion for high-risk ACS patients after PCI operation. However, most clinical trials mainly focused on the occurrence of adverse cardiovascular events. On top of this, our study also showed the effect of tirofiban on the inhibition of PLT aggregation induced by TXA2 and ADP. Our result demonstrated that administration of tirofiban induced a robust inhibition of PLT aggregation induced by TXA2. Various stimulations, including TXA2 and ADP, mediate PLT activity and are upstream pathways that can be modulated by specific antiplatelet therapy. Tirofiban, acting on final downstream pathway of PLT aggregation, can regulate PLT activation induced by TXA2. Patients medicated with tirofiban achieved statistically higher ADP-induced IPA. This phenomenon (Valgimigli et al., 2012) might be explained by the fact that tirofiban provides complementary antiplatelet effect through GPIIb/IIIa receptor affecting both the magnitude and the consistency of ADP-induced IPA. One randomized clinical trial (Holmes et al., 2015) revealed that after 24-h tirofiban infusion, ADP-induced PLT aggregation decreased from 95.5% to 28%. Another clinical trial (Marian et al., 2017) reported that ADP-induced PLT aggregation dropped from 99.8% to 59.4% in the high-risk non–ST-segment ACS patients 24 h after administration with GPI. Our results did not reflect such a significant drop compared with previously reported result (Marian et al., 2017), possibly because the P2Y12 receptor antagonist used in our study was clopidogrel, whereas in the previous study, novel oral antiplatelet drugs, such as prasugrel and ticagrelor, were used.
In this study, tirofiban inhibited the release of inflammatory cytokines such as CRP. One possible explanation might be that GPI can improve the function of vascular endothelium and increase the level of circulating endothelial progenitor cells mediated by inhibition of PLT aggregation (Jeong et al., 2017). Our result demonstrated that tirofiban infusion had potent and sustained suppressive effects on CRP levels following PCI. The main mechanism might be that elevated CRP levels had been correlated with vascular injury and PLT aggregation. The inflammatory sequence that follows vessel injury involved PLTs as well as leukocytes and is reflected in the temporal sequence of elevated serologic markers, including sCD40L, IL-6, and CRP (Kereiakes, 2006). The CRP levels might be directly correlated with the incidence of adverse ischemic outcomes in patients with ACS and could be a powerful predictor for clinical benefit associated with tirofiban therapy.
Besides the antithrombosis effect, the safety profile such as bleeding complications of tirofiban needs to be taken into account. Our results showed that no significant difference was found between groups with respect to laboratory biomarkers such as Hb, Hct, and PLT (P > 0.05). One meta-analysis reported (Winchester et al., 2012) that the use of tirofiban was not associated with an increase of major bleeding risk for ACS patients. Tirofiban has been shown to reduce MACEs for high-risk ACS patients when administrated in addition to aspirin and clopidogrel (Servoss et al., 2004). Previous study indicated that endothelial cells cultured with tirofiban showed increased proliferation. The growth-stimulating effect of tirofiban was mediated by VEGF production (Giordano et al., 2014). This cytokine was also responsible for increased endothelial cell migration, thus explaining the effect of the increased wound healing capability of endothelial cells stimulated with tirofiban. The stimulation of VEGF production was obtained at a dose of 0.25 μg/ml tirofiban (Giordano et al., 2016).
Tirofiban could provide a rapid onset of antiplatelet aggregation, which is a crucial benefit for a better clinical outcome of myocardial ischemia reperfusion. Moreover, it could provide 80% inhibition of PLT aggregation (Authors/Task Force Members et al., 2011; Wenger, 2012 ). Our results showed tirofiban could decrease the incidence of procedural myocardial infarction (OR = 0.250, 95% CI = 0.067–0.925, P = 0.027). Left atrial size enlargement was a predictor of mortality for both cardiovascular issues and all-cause mortality (Patel et al., 2009). We demonstrated that tirofiban decreased the rate of left atrial enlargement, which might be attributed to the reduced ratio of myocardial infarction.
The results of our study showed that administration of tirofiban, when infused immediately after PCI operation, could decrease the rate of reinfarction and mortality during the following 1 year. But no significant difference was found between the two groups (P = 0.208). It might be explained that the novel P2Y12 receptor blockers ticagrelor that provided faster, greater, and more consistent inhibition of PLT aggregation can obviate use of tirofiban for high-risk ACS patients (Wallentin et al., 2009). One clinical trial (ten Berg et al., 2010) reported that there was no significant difference in the decrease of mortality (3.7 vs. 5.8%, P = 0.08) after administration with tirofiban during 1-year follow-up. Our findings were consistent with previous reports.
Our study showed that tirofiban infusion contributes to the inhibition of PLT aggregation in ACS patients 24 h after PCI operation, without elevating the risk of bleeding and concomitant administration of tirofiban with limited effect on decreasing the incidence of MACE in 1-year follow-up.
Our study had some limitations: First, it is a prospective single-center cohort study with a small sample size, which reduces the significance of our study. Second, the enrolled patients were not randomized to assigned groups. In the future, large prospective, randomized controlled trials are needed to evaluate clinical effect and adverse drug reaction of tirofiban administration. Third, patients with culprit vessel stenosis and multi–coronary lesions tended to receive medication with tirofiban. Fourth, long-term follow-up should be taken into consideration in future study. Fifth, we obtained some MACE information via telephone that could only roughly reflect the recovery of function.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
This study was carried out in accordance with the recommendations of “Comparison of antiplatelet regimens post percutaneous coronary intervention operation for acute coronary syndrome patients, approved no. B2016-002” with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the ethic committee of Zhongshan Hospital, Fudan University.
XyeL, SZ, and QL conceived the study and wrote the paper. ZW and QJ performed the experiments and contributed to the manuscript. QW and SZ enrolled the patients and collect information. XyuL contributed to the data statistical analysis.
This study was supported by the Project of Shanghai Municipal health planning commission (NO.2016ZB0301).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.01158/full#supplementary-material
Amsterdam, E. A., Wenger, N. K., Brindis, R. G., Casey, D. E., Jr., Ganiats, T. G., Holmes, D. R., et al. (2014). AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: executive summary. Circulation 130 (25), 2354–2394. doi: 10.1161/CIR.0000000000000133
Authors/Task Force Members, Hamm, C. W., Bassand, J. P., Agewall, S., Bax, J., Boersma, E., et al (2011). ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the Task Force for the Management of Acute Coronary Syndromes (ACS) in Patients Presenting Without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 32 (23), 2999–3054. doi: 10.1093/eurheartj/ehr236
Bittl, J. A., Baber, U., Bradley, S. M., Wijeysundera, D. N. (2016). Duration of dual antiplatelet therapy: a systematic review for the 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 134 (10), e156–e178. doi: 10.1161/CIR.0000000000000453
Centurión, O. A. (2016). Current role of platelet glycoprotein IIB/IIIA inhibition in the therapeutic management of acute coronary syndromes in the stent era. J. Cardiol. Curr. Res. 5 (5), 00175. doi: 10.15406/jccr.2016.05.00175
Choi, W. S., Rice, W. J., Stokes, D. L., Coller, B. S. (2013). Three-dimensional reconstruction of intact human integrin αIIbβ3: new implications for activation-dependent ligand binding. Blood 122 (26), 4165–4171. doi: 10.1182/blood-2013-04-499194
De Luca, G., Ucci, G., Cassetti, E., Marino, P. (2009). Benefits from small molecule administration as compared with abciximab among patients with ST-segment elevation myocardial infarction treated with primary angioplasty, a meta-analysis. J. Am. Coll. Cardiol. 53 (18), 1668–1673. doi: 10.1016/j.jacc.2009.01.053
Gao, R., Wu, Y., Liu, H., Su, G., Yuan, Z., Zhang, A., et al. (2018). Safety and incidence of cardiovascular events in Chinese patients with acute coronary syndrome treated with ticagrelor: the 12-month, phase IV, multicenter, single-arm DAYU study. Cardiovasc. Drugs Ther. 32 (1), 47–56. doi: 10.1007/s10557-018-6772-3
Giordano, A., D’Angelillo, A., Romano, S., D'Arrigo, P., Corcione, N., Bisogni, R., et al. (2014). Tirofiban induces VEGF production and stimulates migration and proliferation of endothelial cells. Vascul. Pharmacol. 61 (2-3), 63–71. doi: 10.1016/j.vph.2014.04.002
Giordano, A., Musumeci, G., D’Angelillo, A., Rossini, R., Zoccai, G. B., Messina, S., et al. (2016). Effects of glycoprotein IIb/IIIa antagonists: anti platelet aggregation and beyond. Curr. Drug Metab. 17 (2), 194–203. doi: 10.2174/1389200217666151211121112
Hochholzer, W., Wiviott, S. D., Antman, E. M., Contant, C. F., Guo, J., Giugliano, R. P., et al. (2011). Predictors of bleeding and time dependence of association of bleeding with mortality: insights from the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38). Circulation. 123, 2681–9. doi: 10.1161/CIRCULATIONAHA.110.002683
Holmes, L. E., Gupta, R., Rajendran, S., Luu, J., French, J. K., Juergens, C. P. (2015). A randomized trial assessing the impact of three different glycoprotein IIb/IIIa antagonists on glycoprotein IIb/IIIa platelet receptor inhibition and clinical endpoints in patients with acute coronary syndromes. Cardiovasc. Ther. 24 (5), S168–S168. doi: 10.1016/j.hlc.2015.06.136
Januzzi, J. L., Snapinn, S. M., DiBattiste, P. M., Jang, I. K., Theroux, P. (2002). Benefits and safety of tirofiban among acute coronary syndrome patients with mild to moderate renal insufficiency: results from the Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS) trial. Circulation 105 (20), 2361–2366. doi: 10.1161/01.CIR.0000016359.94919.16
Jeong, H. S., Hong, S. J., Cho, S. A., Kim, J. H., Cho, J. Y., Lee, S. H., et al. (2017). Comparison of ticagrelor versus prasugrel for inflammation, vascular function, and circulating endothelial progenitor cells in diabetic patients with non–ST-segment elevation acute coronary syndrome requiring coronary stenting: a prospective, randomized, crossover trial. JACC Cardiovasc. Interv. 10 (16), 1646–1658. doi: 10.1016/j.jcin.2017.05.064
Kereiakes, D. J. (2006). Effects of GP IIb/IIIa inhibitors on vascular inflammation, coronary microcirculation, and platelet function. Rev. Cardiovasc. Med. 7 Suppl 4, S3–11.
Lamberts, M., Gislason, G. H., Lip, G. Y. H., Lassen, J. F., Olesen, J. B., Mikkelsen, A. P., et al. (2014). Antiplatelet therapy for stable coronary artery disease in atrial fibrillation patients on oral anticoagulant: a nationwide cohort study. Circulation. 129, 1577–85. 113004834. doi: 10.1161/CIRCULATIONAHA.113.004834
Lu, D., Owens, J., Kreutz, R. P. (2013). Plasma and whole blood clot strength measured by thrombelastography in patients treated with clopidogrel during acute coronary syndromes. Thromb. Res. 132 (2), e94–e98. doi: 10.1016/j.thromres.2013.07.012
Lv, H. H., Wu, S., Liu, X., Yang, X. L., Xu, J. F., Guan, Y. T., et al. (2016). Comparison of VerifyNow P2Y12 and thrombelastography for assessing clopidogrel response in stroke patients in China. Neurol. Sci. 37 (2), 277–282. doi: 10.1007/s10072-015-2407-7
Marian, M. J., Alli, O., Al Solaiman, F., Brott, B. C., Sasse, M., Leesar, T., et al. (2017). Ticagrelor and eptifibatide bolus versus ticagrelor and eptifibatide bolus with 2-hour infusion in high-risk acute coronary syndromes patients undergoing early percutaneous coronary intervention. J. Am. Heart Assoc. 6 (6), e005562. doi: 10.1161/JAHA.117.005562
Montalescot, G., Antoniucci, D., Kastrati, A., Neumann, F. J., Borentain, M., Migliorini, A., et al. (2007). Abciximab in primary coronary stenting of ST-elevation myocardial infarction: a European meta-analysis on individual patients’ data with long-term follow-up. Eur. Heart J. 28 (4), 443–449. doi: 10.1093/eurheartj/ehl472
Park, S. J., Kim, Y. H., Park, D. W., Yun, S. C., Ahn, J. M., Song, H. G., et al. (2011). Randomized trial of stents versus bypass surgery for left main coronary artery disease. N. Engl. J. Med. 364 (18), 1718–1727. doi: 10.1056/NEJMoa1100452
Patel, D. A., Lavie, C. J., Milani, R. V., Shah, S., Gilliland, Y., et al. (2009). Clinical implications of left atrial enlargement: a review. Ochsner. J. 9 (4), 191–196.
Roffi, M., Patrono, C., Collet, J. P., Mueller, C., Valgimigli, M., Andreotti, F., et al. (2015). ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting Without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37(3):267–315. doi: 10.1093/eurheartj/ehv320
Safley, D. M., Venkitachalam, L., Kennedy, K. F., Cohen, D. J. (2015). Impact of glycoprotein IIb/IIIa inhibition in contemporary percutaneous coronary intervention for acute coronary syndromes: insights from the National Cardiovascular Data Registry. JACC Cardiovasc. Interv. 8 (12), 1574–1582. doi: 10.1016/j.jcin.2015.04.031
Seguchi, O., Kitakaze, M. (2005). Pathophysiology of acute coronary syndrome. Kokyu To Junkan 53 (1), 7–14.
Servoss, S. J., Wan, Y., Snapinn, S. M., DiBattiste, P. M., Zhao, X. Q., Theroux, P., et al. (2004). Tirofiban therapy for patients with acute coronary syndromes and prior coronary artery bypass grafting in the PRISM-PLUS trial. Am. J. Cardiol. 93 (7), 843–847. doi: 10.1016/j.amjcard.2003.12.021
Sethi, A., Bajaj, A., Bahekar, A., Bhuriya, R., Singh, M., Ahmed, A., et al. (2013). Glycoprotein IIb/IIIa inhibitors with or without thienopyridine pretreatment improve outcomes after primary percutaneous coronary intervention in high-risk patients with ST elevation myocardial infarction—a meta-regression of randomized controlled trials. Catheter. Cardiovasc. Interv. 82 (2), 171–181. doi: 10.1002/ccd.24653
Starnes, H. B., Patel, A. A., Stouffer, G. A. (2011). Optimal use of platelet glycoprotein IIb/IIIa receptor antagonists in patients undergoing percutaneous coronary interventions. Drugs 71 (15), 2009–2030. doi: 10.2165/11595010-000000000-00000
ten Berg, J. M., van ‘tHof, A. W., Dill, T., Heestermans, T., van Werkum, J. W., Mosterd, A., et al. (2010). Effect of early, pre-hospital initiation of high bolus dose tirofiban in patients with ST-segment elevation myocardial infarction on short- and long-term clinical outcome. J. Am. Coll. Cardiol. 55 (22), 2446–2455. doi: 10.1016/j.jacc.2009.11.091
Turpie, A. G. G. (2006). Burden of disease: medical and economic impact of acute coronary syndromes. Am. J. Manag. Care 12 (16), S430.
Valgimigli, M., Tebaldi, M., Campo, G., Gambetti, S., Bristot, L., Monti, M., et al. (2012). Prasugrel versus tirofiban bolus with or without short post–bolus infusion with or without concomitant prasugrel administration in patients with myocardial infarction undergoing coronary stenting: the FABOLUS PRO (Facilitation through Aggrastat By dropping or shortening Infusion Line in patients with ST-segment elevation myocardial infarction compared to or on top of Prasugrel given at loading dose) trial. JACC Cardiovasc. Interv. 5 (3), 268–277. doi: 10.1016/j.jcin.2012.01.006
Wallentin, L., Becker, R. C., Budaj, A., Cannon, C. P., Emanuelsson, H., Held, C., et al. (2009). Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 361 (11), 1045–1057. doi: 10.1056/NEJMoa0904327
Wenger, N. K. (2012). ACCF/AHA focused update of the guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction (updating the 2007 Guideline): highlights for the clinician. Clin. Cardiol. 126 (7), 3–8. doi: 10.1002/clc.20964
Winchester, D. E., Brearley, W. D., Wen, X., Park, K. E., Bavry, A. A. (2012). Efficacy and safety of unfractionated heparin plus glycoprotein IIb/IIIa inhibitors during revascularization for an acute coronary syndrome: a meta-analysis of randomized trials performed with stents and thienopyridines. Clin. Cardiol. 35 (2), 93–100. doi: 10.1002/clc.20974
Keywords: tirofiban, acute coronary syndrome, antiplatelet, bleeding risks, major adverse cardiovascular events
Citation: Li X, Zhang S, Wang Z, Ji Q, Wang Q, Li X and Lv Q (2019) Platelet Function and Risk of Bleeding in Patients With Acute Coronary Syndrome Following Tirofiban Infusion. Front. Pharmacol. 10:1158. doi: 10.3389/fphar.2019.01158
Received: 19 February 2019; Accepted: 09 September 2019;
Published: 09 October 2019.
Edited by:
Olayinka Olabode Ogunleye, Lagos State University, NigeriaReviewed by:
Domenico Criscuolo, Italian Society of Pharmaceutical Medicine, ItalyCopyright © 2019 Li, Zhang, Wang, Ji, Wang, Li and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qianzhou Lv, MTM5MTYwODg5MzhAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.