- 1School of International Pharmaceutical Business, China Pharmaceutical University, Nanjing, China
- 2College of Pharmacy, Jinan University, Guangzhou, China
- 3International Cooperative Laboratory of Traditional Chinese Medicine Modernization and Innovative Drug Development of Chinese Ministry of Education (MOE), Jinan University, Guangzhou, China
- 4Department of Pharmacy, Guangzhou General Hospital of Guangzhou Military Command, Guangzhou, China
- 5Guangzhou Huabo Biopharmaceutical Research Institute, Guangzhou, China
- 6Dongguan Institute of Jinan University, Guangzhou, China
Background: Endoscopic submucosal dissection (ESD) was commonly used for en bloc resection in gastric cancer and adenoma with the risk of delayed bleeding after ESD. We conducted a direct and indirect comparison meta-analysis to evaluate the best choice in preventing post-ESD bleeding among proton pump inhibitors (PPIs), histamine2-receptor antagonists (H2RAs), and the most widely used potassium-competitive acid blocker, vonoprazan.
Methods: The Pubmed, Cochrane Library, and Embase were searched for randomized trials. We pooled odds ratios (OR) for preventing post-ESD bleeding using meta-analysis.
Results: Sixteen randomized trials met the inclusion criteria including 2,062 patients. Direct comparisons showed slightly significant efficacy in PPIs rather than H2RAs in preventing post-ESD bleeding [OR: 1.83; 95% confidence interval (CI): 1.10 to 3.05] and vonoprazan was better than PPIs (OR: 0.46; 95% CI: 0.25 to 0.86). The adjusted indirect comparison indicated vonoprazan was superior to H2RAs (OR: 0.30, 95% CI: 0.12 to 0.74). In subgroup analysis, PPIs had similar efficacy as H2RAs in 4 weeks, while PPIs were better than H2RAs in 8 weeks’ treatment (OR: 1.91; 95% CI: 1.08 to 3.40). The superiority of vonoprazan than PPIs was more significant in combination therapy (OR: 0.18; 95% CI: 0.04 to 0.69). There was a significant difference in vonoprazan for 8 weeks of medication (OR: 0.44; 95% CI: 0.21 to 0.92).
Conclusions: The effects of vonoprazan is better than PPIs than H2RAs in preventing bleeding after ESD. When vonoprazan combined with mucosal protective antiulcer drug in treatment or used in 8 weeks of medication, the efficacy may be even better.
Introduction
Endoscopic submucosal dissection (ESD), as an endoscopic procedure, commonly used to treat gastric cancer and adenoma, was first reported in Japan and Korea in the late 1990s (Lightdale, 2004; Fujishiro, 2006). ESD is performed using a variety of electrosurgical knives such as the insulated-tip or the triangle-tip models as to make gastrointestinal mucosal incisions and submucosal dissections (Miyamoto et al., 2002; Yamamoto et al., 2003; Miyashita et al., 2003). Unlike conventional techniques such as the strip biopsy or cap endoscopic mucosal resection (EMR), the en bloc resection can be achieved by ESD (Rosch et al., 2004). Nowadays, it has gained popularity in Asian countries. With technical improvements and structured training, ESD is increasingly used in Western countries, and it is necessary to master the operation of this technology and vigorously promote it (Fukami, 2014).
However, ESD, as a more complex and time-consuming procedure, has a higher risk of complications than classic EMR, mainly related to bleeding (Oka et al., 2006). Delayed bleeding occurs mostly within 24 h after ESD, and the incidence rate of delayed bleeding is approximately 5% (Takizawa et al., 2008). Although rare, it can develop severe complications such as delayed perforation, and blood will interfere with subsequent endoscopic procedures. Hence, prevention of bleeding after ESD has been an important clinical issue.
The bleeding was affected by pH levels, and the gastric pH may affect the efficiency of blood coagulation and platelet aggregation at the bleeding site (Green et al., 1978; Barer et al., 1983). To neutralize pH level and prevent bleeding after ESD, there are some alternative medication options that include proton pump inhibitors (PPIs), histamine2-receptor antagonists (H2RAs), and so on. PPIs have been reported to be preferred than H2RAs treatment in some previous studies (Daneshmend et al., 1992; Esaki et al., 2002; Abe et al., 2004; Ye et al., 2006). Conversely, other studies showed that PPIs are not significantly effective than H2RAs (Ohya et al., 2010; Imaeda et al., 2011; Jang et al., 2012; Tomita et al., 2012). Vonoprazan (Takeda Pharmaceutical co., Tokyo, Japan) is a new potassium-competitive acid blocker (P-CAB). Its pharmaceutical advantages over PPIs include more rapid, stronger, and long-lasting inhibition of gastric acid secretion after administration (Jenkins et al., 2015; Sakurai et al., 2015). Moreover, vonoprazan effect is neither affected by CYP2C19 polymorphisms in its pharmacokinetics (Kagami et al., 2016) nor affected by food intake (Sachs et al., 2000; Ozawa et al., 2003). As a new class of acid-suppressing agents, it is expected to reduce the incidence of delayed bleeding after ESD better than conventional PPIs and serve as an alternative to PPIs (Hori et al., 2011; Jenkins et al., 2015; Sakurai et al., 2015).
The relative efficacy of PPIs, H2RAs, and the P-CAB (vonoprazan) has been assessed in randomized trials. However, the most appropriate management in preventing post-ESD bleeding remains controversial and to be defined. Moreover, the consumption of PPIs is especially high nowadays, and the overuse of PPIs has been an international concern (Lanas, 2016). Hence, in our study, we performed a meta-analysis among H2RAs, PPIs, and vonoprazan to find out the best regimen to prevent bleeding after ESD. Despite the absence of head-to-head randomized control trials (RCTs), we conducted indirect comparison meta-analysis to estimate the efficacy of vonoprazan and H2RAs using PPI as the common comparator.
Methods
Data Sources and Searches
The manuscript was drafted by the preferred reporting items for systematic reviews and meta-analyses statement (Moher et al., 2009). We searched three electronic databases: Pubmed, Cochrane Library, and Embase, from their inception date to 15th March 2019. There are no restrictions on abstracts and conference proceedings. We hand searched the relevant articles’ references to extend the literature search. The following terms were used in the search, which were included in their titles, abstracts, or keyword lists: “endoscopic submucosal dissection,” “ESD,” “proton pump inhibitors,” “PPI,” “vonoprazan,” “Potassium-Competitive Acid Blocker,” “H2Ras.” Only English publications were included.
Study Selection and Endpoints
The inclusion criteria used to select studies are predetermined. The eligible studies are as follows: 1) RCTs that compared the efficacy of H2RAs or vonoprazan against PPIs on prevention of bleeding after ESD, not during the operation procedure, 2) the study population are adults aging from 18 years old, 3) studies must report the data of delayed bleeding incidence after ESD, and 4) trials were excluded by not about the prevention of bleeding. We chose the delayed bleeding as the primary outcome. Two independent reviewers (Tiantian Zhang and Jiahao Li) retrieved all the potentially relevant articles. Any discrepancies between the two independent reviewers were resolved by a third investigator (Ning Wan).
Data Extraction and Quality Assessment
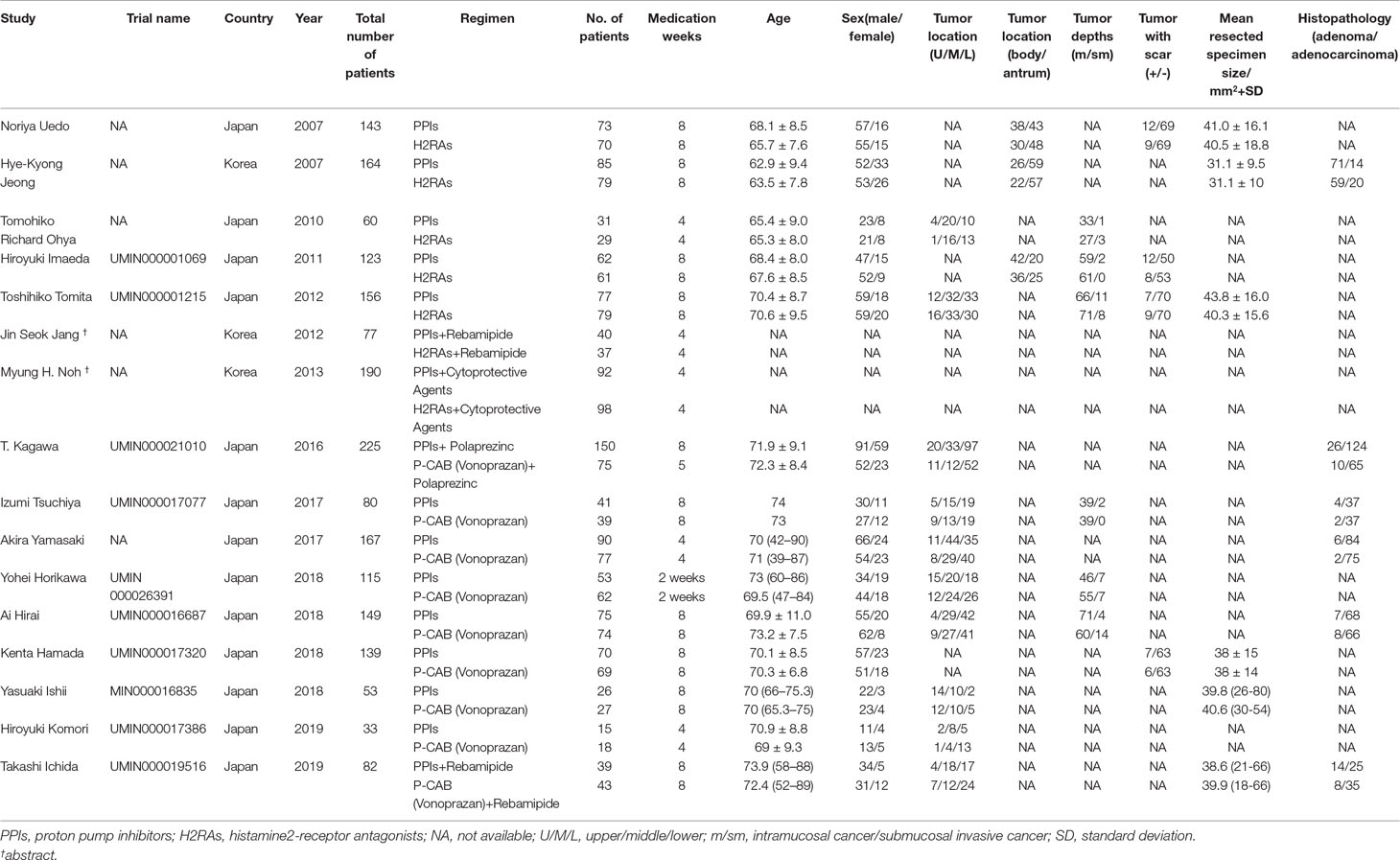
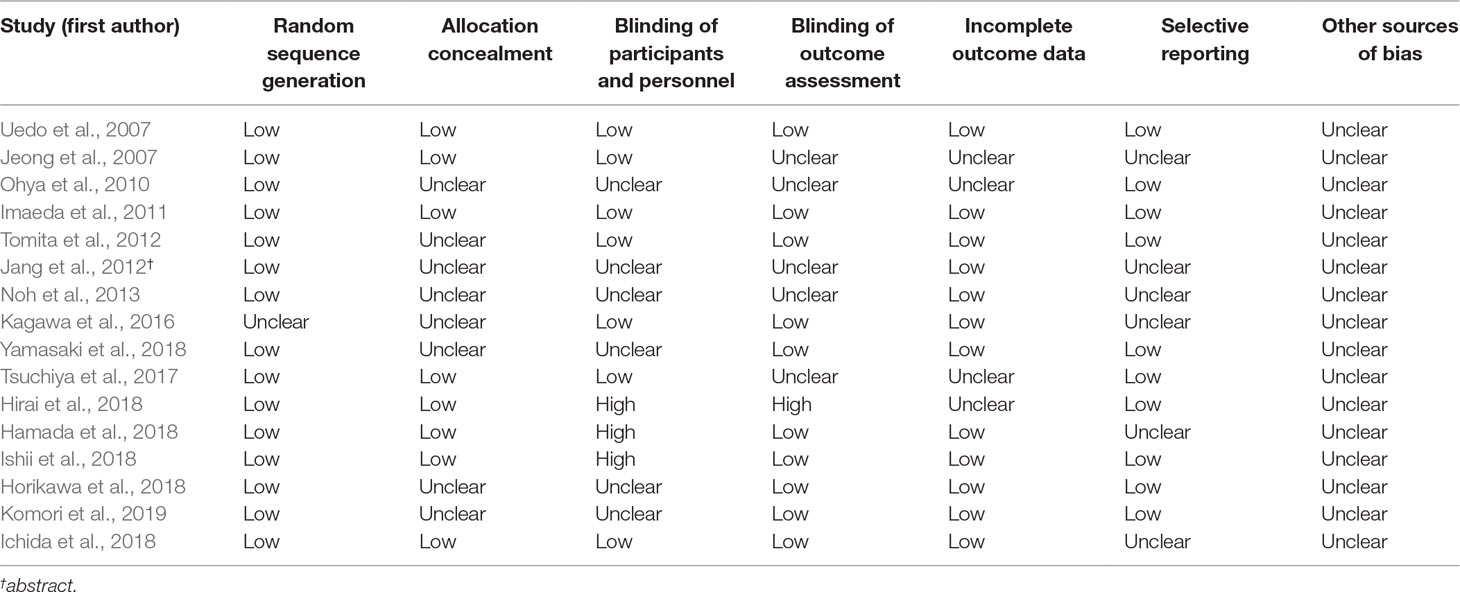
The data were extracted by two reviewers (Tiantian Zhang and Jiahao Li) independently and included the name of the first author, year of publication, country, a total number of patients, age, sex of patients, therapeutic regimen, trial name, tumor location, tumor depths, tumor with scar, mean resected specimen, and histopathology. Each study was judged on the potential bias including low, high, and unclear as outlined in the Cochrane Collaboration Handbook (Higgins et al., 2011).
Data Analysis
In the absence of trials making head to head comparisons between H2RAs and vonoprazan, we then performed an adjusted indirect comparison using a common comparator—that is, PPIs—as described by the method of Bucher (Glenny et al., 2005). When few direct studies are available and direct comparative studies have not been performed, indirect meta-analysis is used to estimate treatment effect and to strengthen the power of comparisons instead of replacing randomized trials of direct comparison (Collaborative overview of randomised trials of antiplatelet therapy–II, 1994; Song et al., 2000; Fisher et al., 2001; Packer et al., 2001). We performed traditional meta-analysis combining trials of H2RAs versus PPIs, vonoprazan versus PPIs, to obtain the estimated odds ratios (OR) with 95% confidence interval (CI). We calculated the I2 statistic and the Chi-square test to examine statistical heterogeneity among studies. If the heterogeneity results were considered to be statistically significant at a P value < 0.1 and an I2 statistic > 50%, the random-effects model would be much more appropriate. Otherwise, the fixed-effects model was used. To strengthen the reliability of these pooled results and explore possible reasons for heterogeneity, we performed a sensitivity analysis using the leave-one-out method and examined the publication bias by funnel plot and Egger’s test. All tests were two-sided, and a P < 0.05 was considered statistically significant (Higgins et al., 2003; Fox et al., 2012; Loke et al., 2014). We used the STATA statistical software system v14.0 for statistical analysis.
Results
Literature Search
The process of study selection in our meta-analysis is shown in Figure 1. Overall, the literature search identified 197 potentially relevant studies in our initial search. We excluded 130 articles for the following reasons: not involving prevention after ESD, review articles, duplicate articles, and retrospective studies. The remaining 67 articles were retrieved for further consideration. There were 51 articles that were excluded for not involving delayed bleeding and without sufficient data. Only 16 articles were included in the analysis including two conference abstracts (Jeong et al., 2007; Uedo et al., 2007; Ohya et al., 2010; Imaeda et al., 2011; Tomita et al., 2012; Jang et al., 2012; Kagawa et al., 2016; Ichida et al., 2018; Ishii et al., 2018; Hamada et al., 2018; Horikawa et al., 2018; Yamasaki et al., 2018).
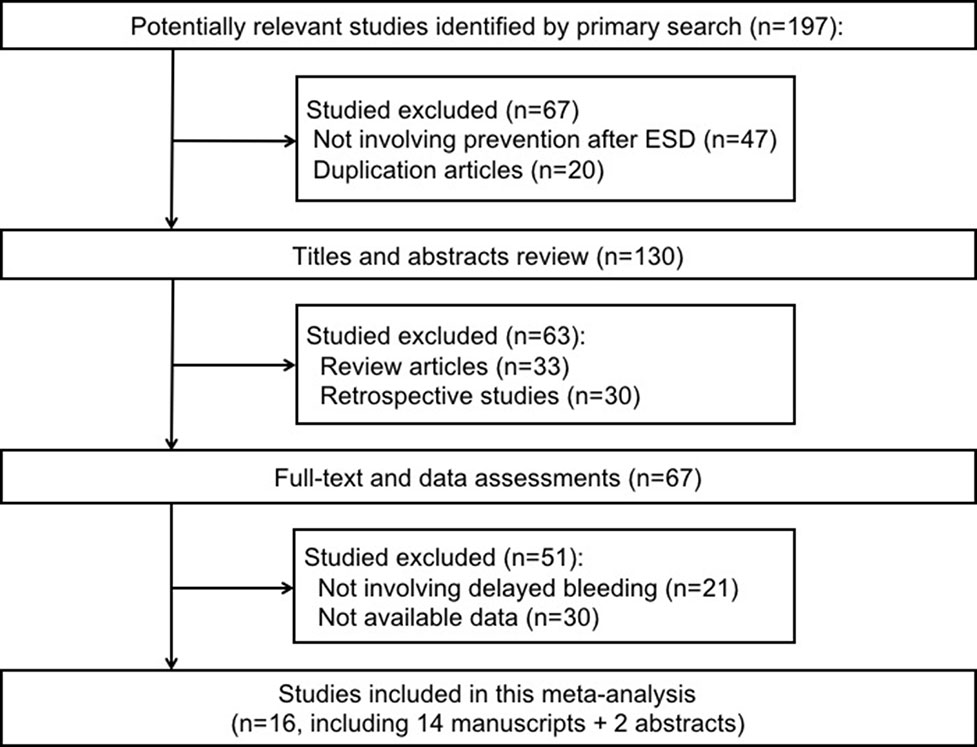
Figure 1 Preferred reporting items for systematic reviews and meta-analyses flowchart of the studies included in the meta-analysis.
Study Characteristics and Qualities
A total of 1,956 patients were enrolled in the meta-analysis including 16 trials. The characteristic of the 16 included articles is shown in Table 1. The quality of the randomized studies is shown in Table 2.
Conventional Meta-Analysis
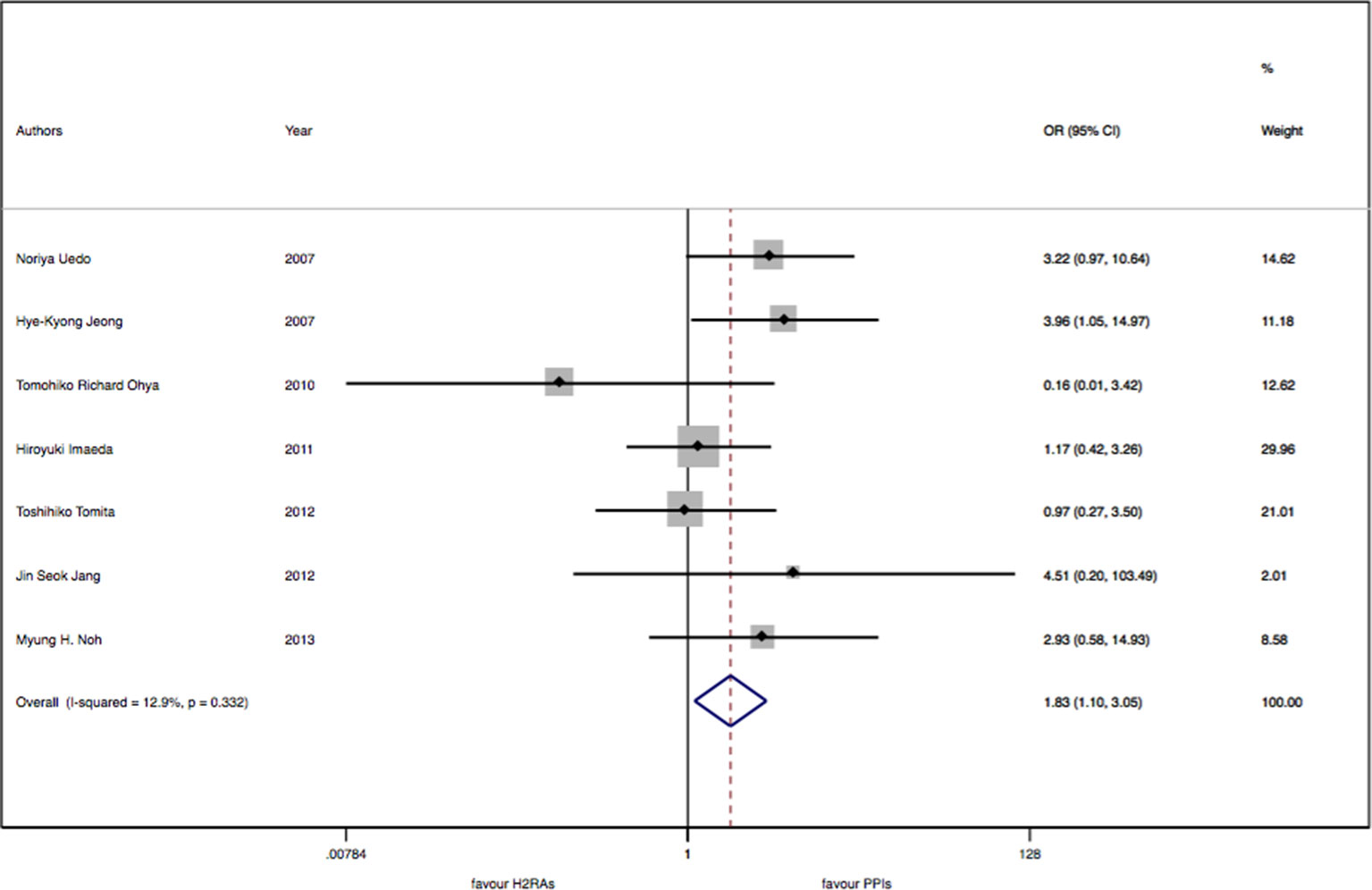
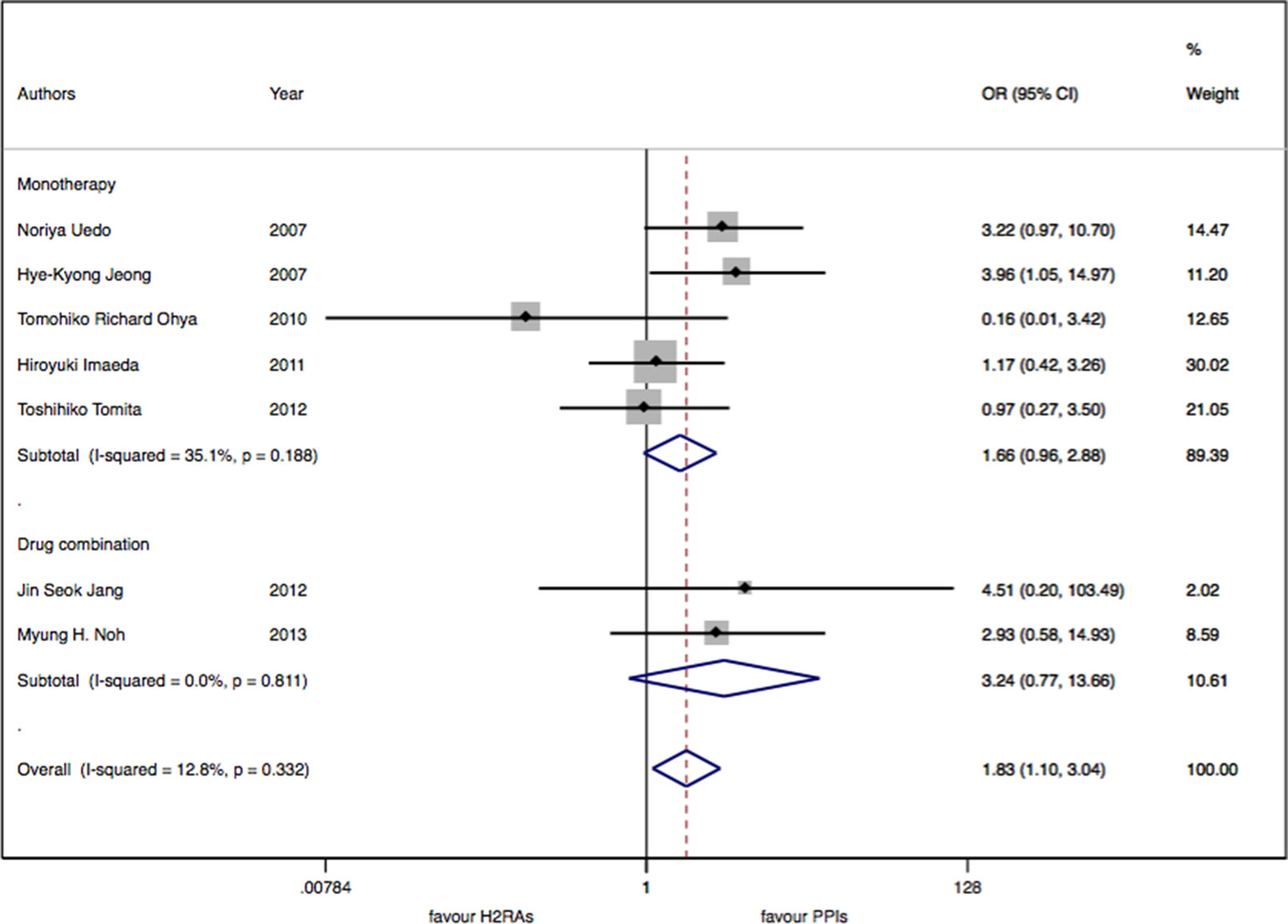
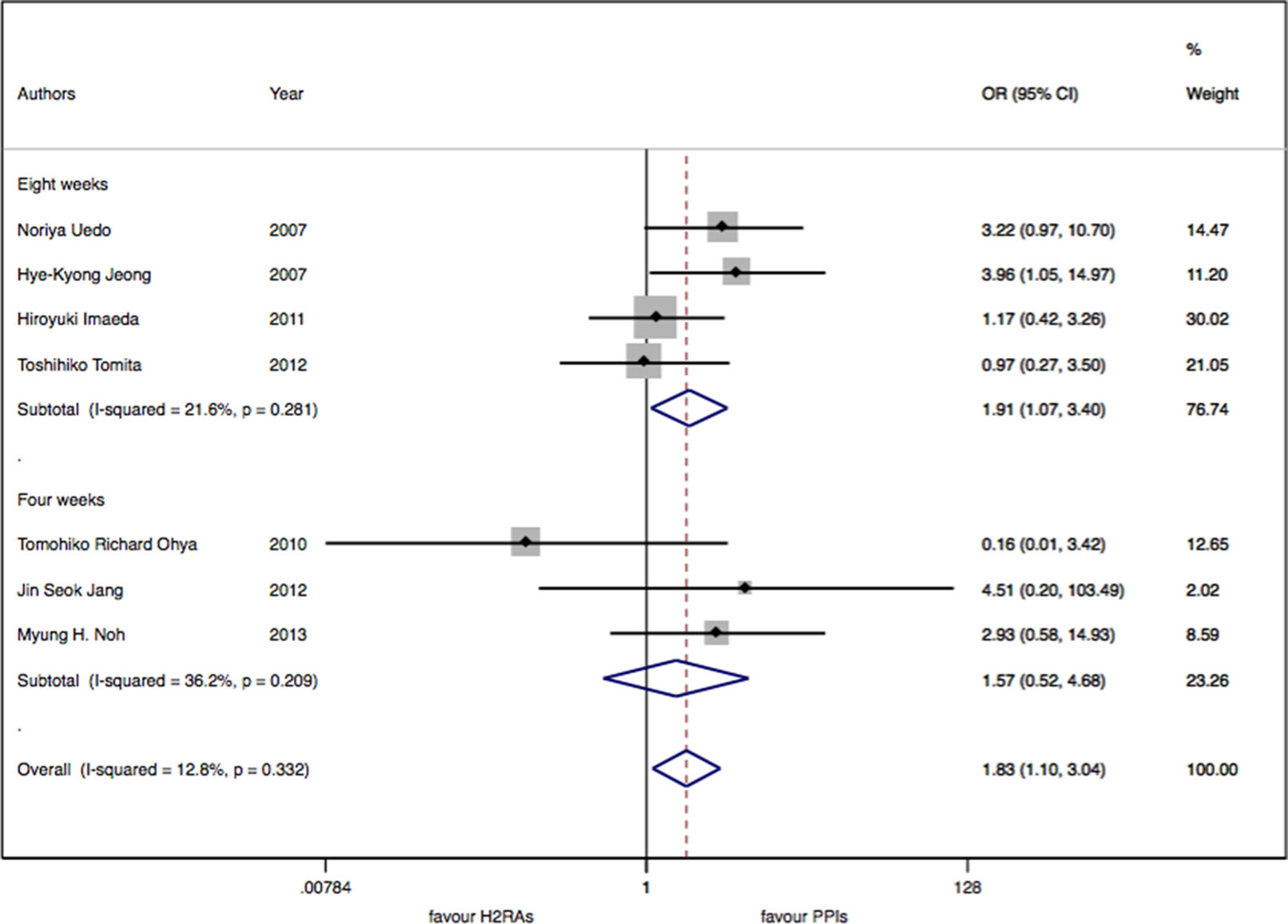
There were seven studies with 913 patients about H2RAs versus PPIs. No significant statistical heterogeneity among trials was detected (I2 = 12.9%, P = 0.33). There was a slightly significant difference in delayed bleeding between the H2RAs and PPIs groups (OR: 1.83; 95% CI: 1.10 to 3.05, P = 0.02). However, this difference was not significant for 4 weeks (OR: 1.57; 95% CI: 0.52 to 4.68, P = 0.42). It was only for 8 weeks; PPIs were superior to H2RAs in delayed bleeding (OR: 1.91; 95% CI: 1.08 to 3.40, P = 0.028). Compared with the OR of PPIs monotherapy, the OR of H2RAs monotherapy was 1.66 (95% CI: 0.96 to 2.88). When combined with protective agents, the OR increased to 3.24 (95% CI: 0.77 to 13.66) (see Figures 2–4).
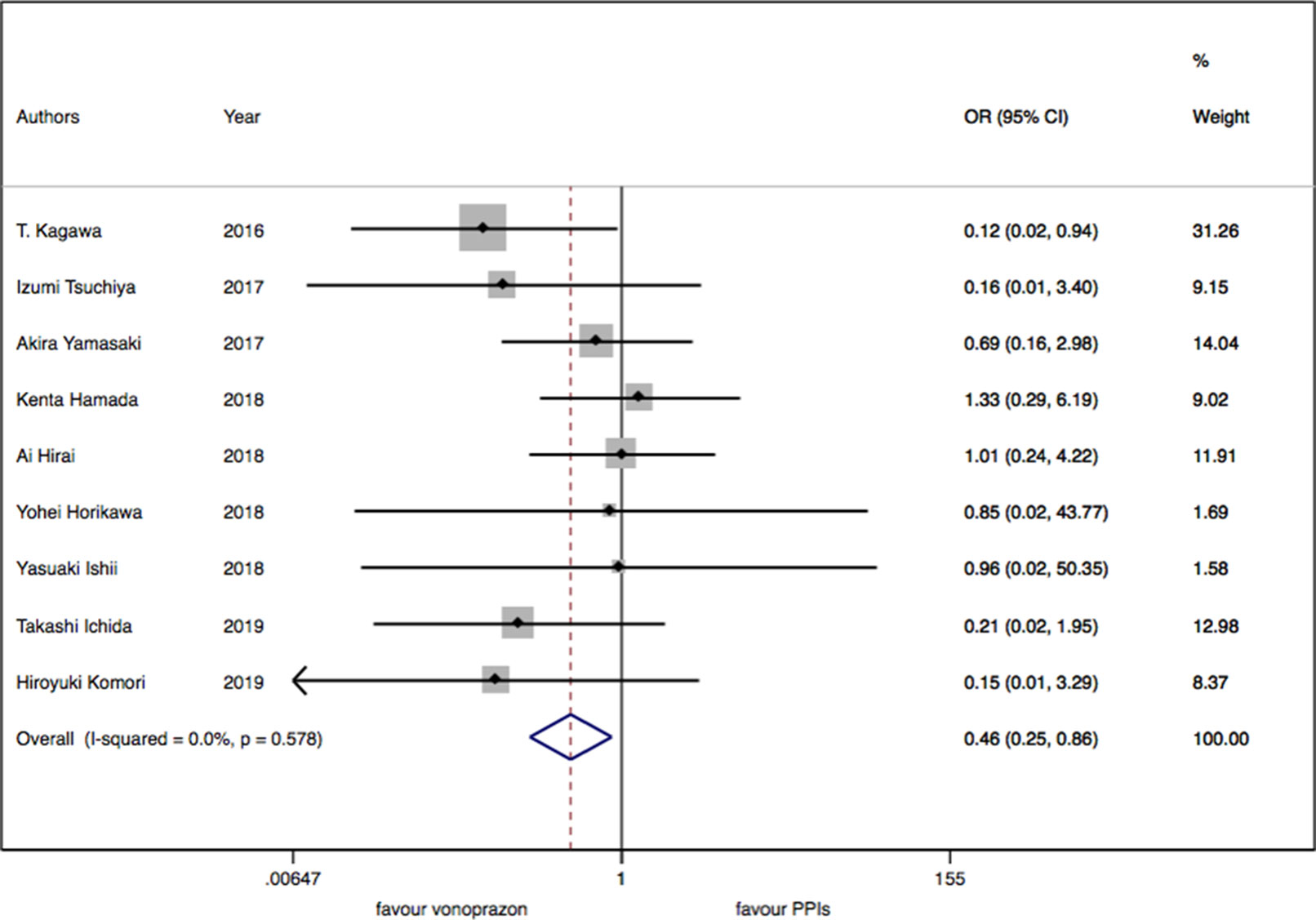
As for the nine trials of vonoprazan versus PPIs including 1,043 patients, a significant difference in delayed bleeding (OR: 0.46; 95% CI: 0.25 to 0.86, P = 0.015) was found in vonoprazan versus PPIs. In the subgroup analysis, there existed a significant difference in delayed bleeding when vonoprazan was combined with mucosal protective antiulcer drug in treatment compared with PPI combination therapy (OR: 0.18; 95% CI: 0.04 to 0.69, P = 0.013), while the OR was 0.70 for vonoprazan monotherapy (95% CI: 0.33 to 1.47). Additionally, vonoprazan showed better efficacy in delayed bleeding in 8 weeks’ medication than PPIs (OR: 0.44; 95% CI: 0.21 to 0.92) (see Figures 5–7).
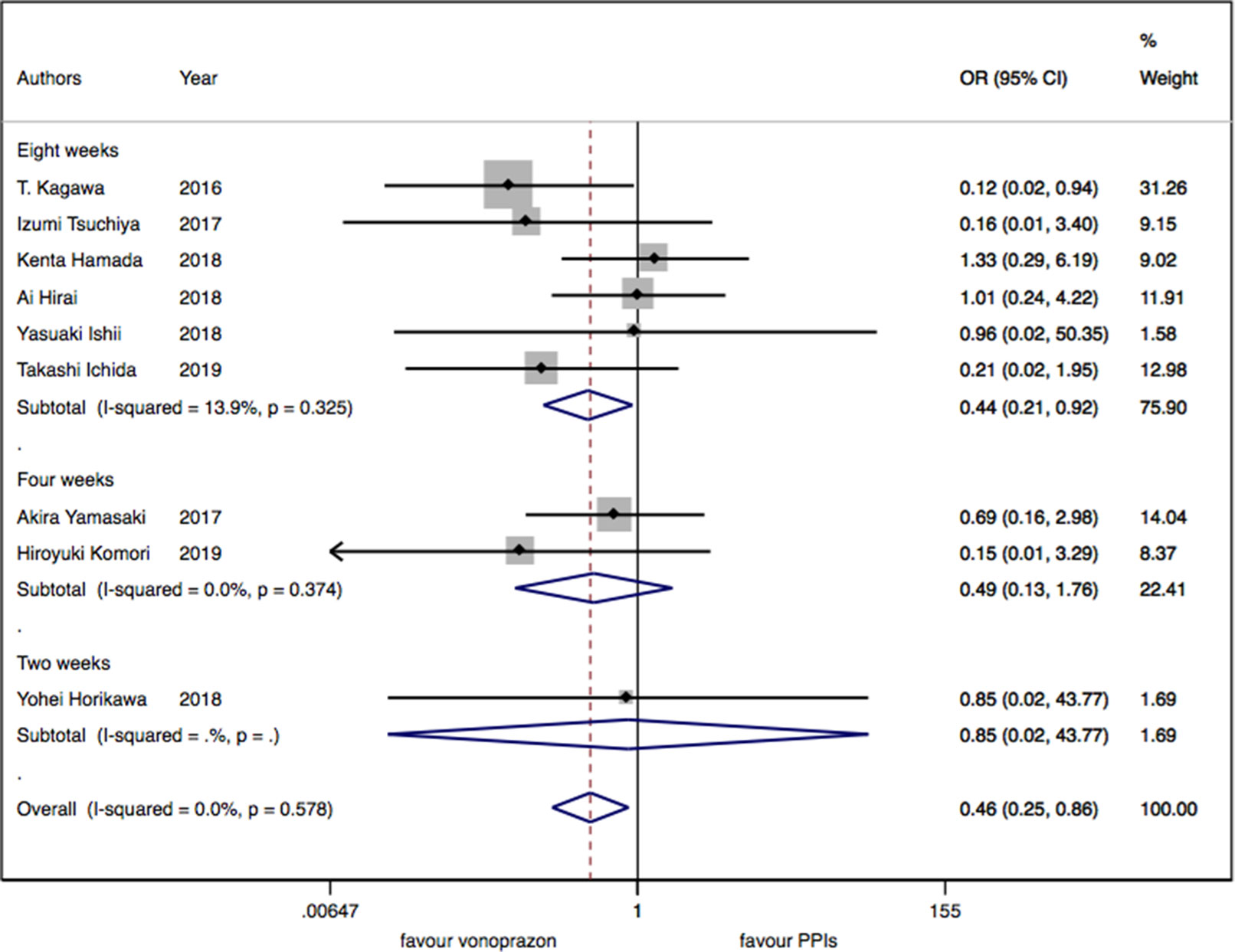
Figure 6 The medication duration subgroup meta-analysis of delayed bleeding for vonoprazan with PPIs.
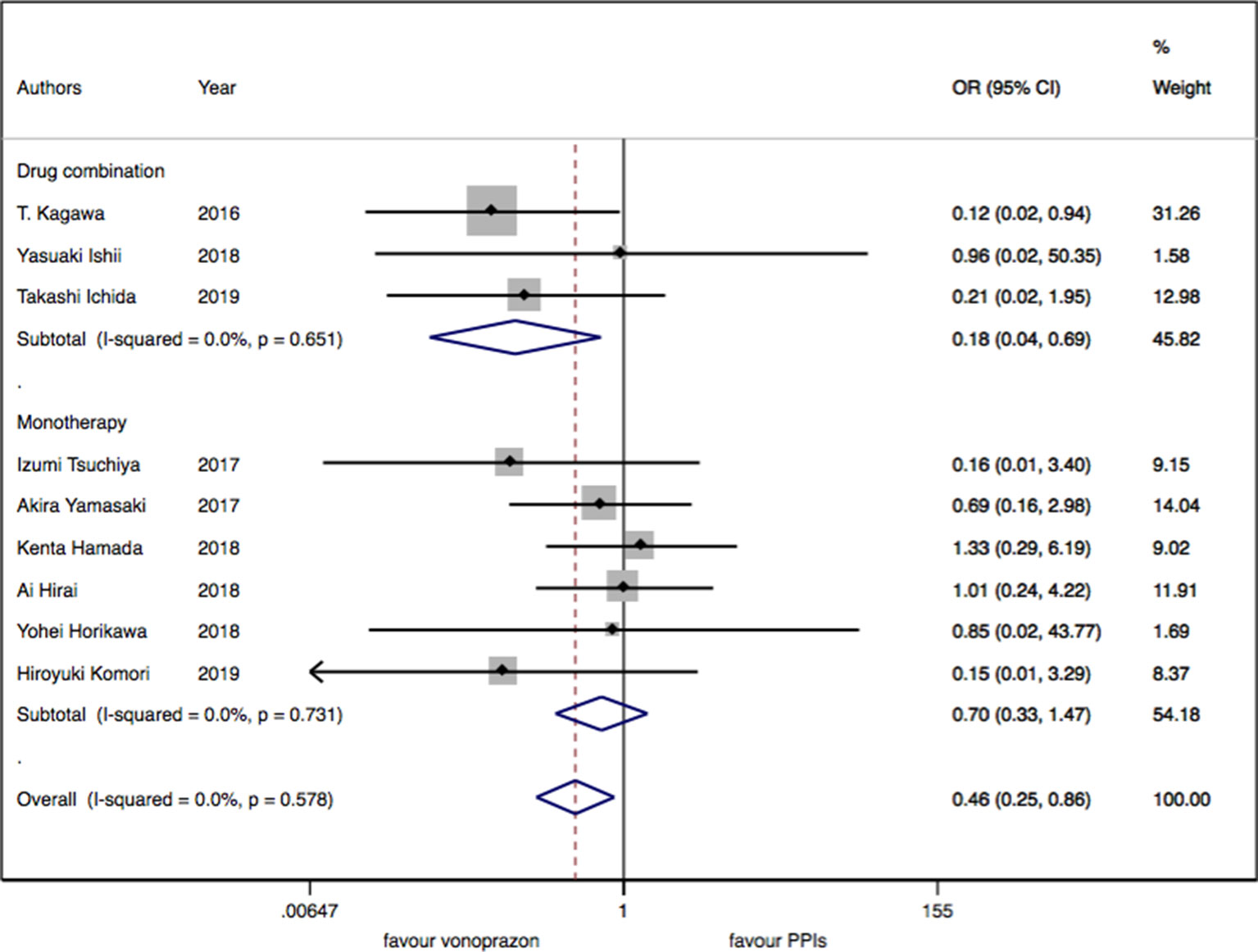
Figure 7 The medication regimen subgroup meta-analysis of delayed bleeding for vonoprazan with PPIs.
Adjusted Indirect Comparison Based on Common Controls
The adjusted indirect comparison of delayed bleeding was performed for vonoprazan and H2RAs using PPIs as the comparator. This demonstrated that vonoprazan was superior to H2RAs (OR: 0.30, 95% CI: 0.12 to 0.74). The same trend was found in the subgroup analysis of vonoprazan compared with H2RAs. The OR between vonoprazan and H2RAs of 8 weeks’ medication duration was 0.29 (95% CI: 0.10 to 0.86). The OR between vonoprazan and H2RAs in a combination regimen with protective agents was 0.061 (95% CI: 0.006 to 0.458).
Sensitivity Analysis
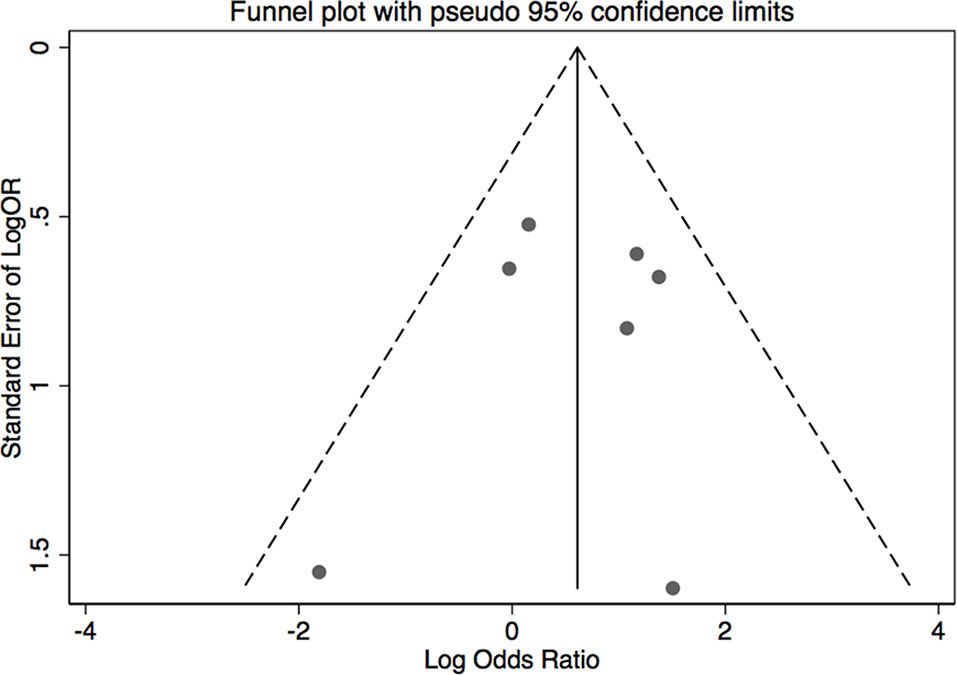
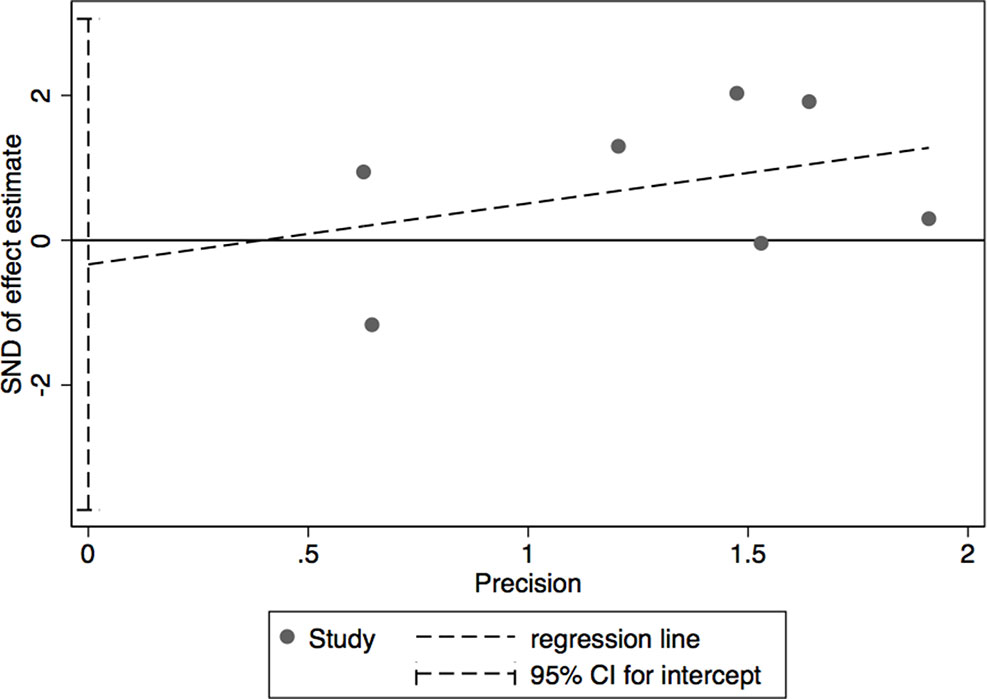
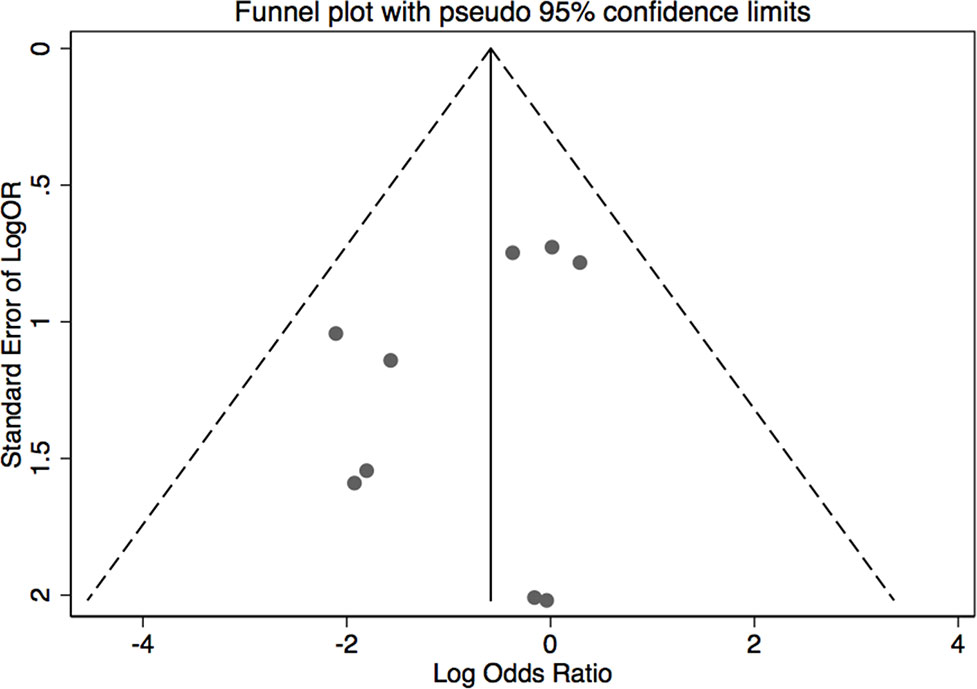
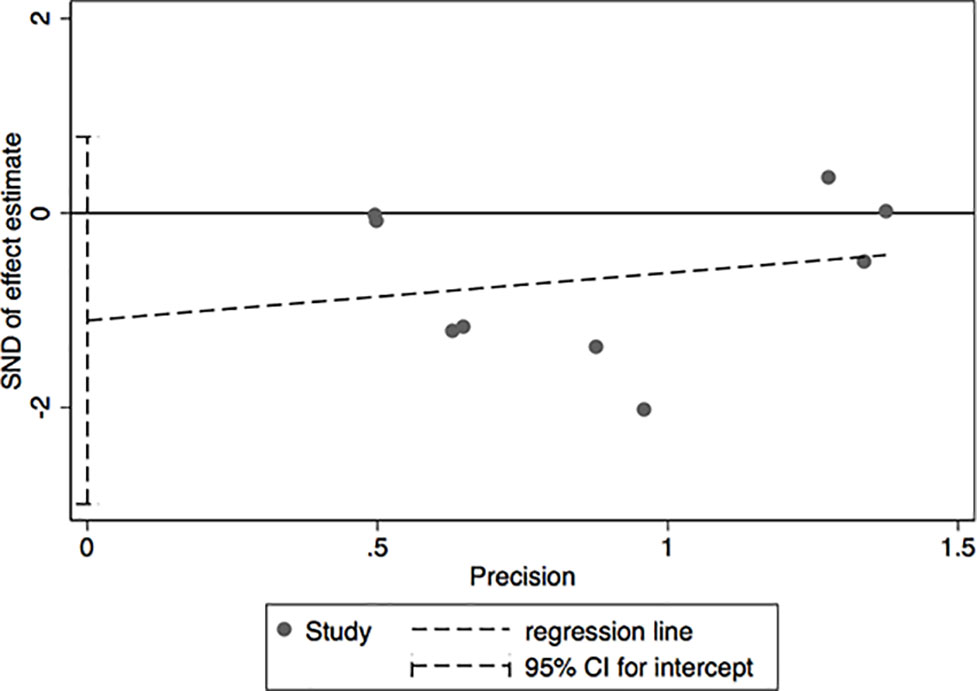
The results of leave-one-out sensitivity analysis indicated that no individual studies significantly influenced the OR (see Figure 8). Based on the funnel plot and Egger’s test, no significant publication bias was found that may confirm the stability of our results (see Figures 9–12). The P values for Egger’s test were 0.810 for H2RAs with PPIs and 0.209 for vonoprazan with PPIs.
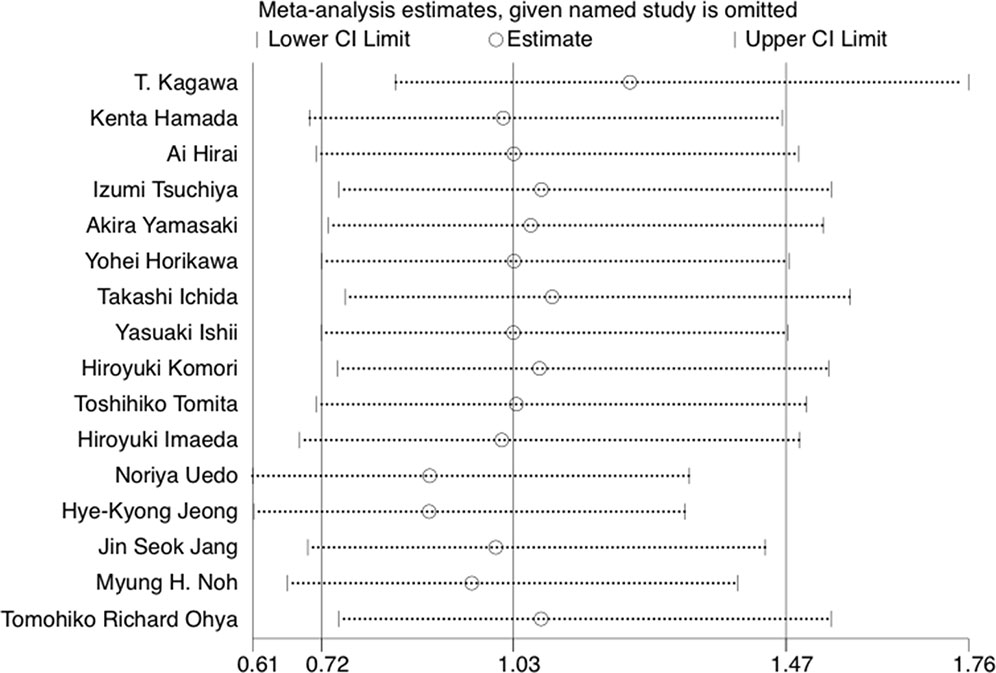
Figure 8 The leave-one-out sensitivity analysis of preventing bleeding after ESD per medication option.
Discussion
Based on the traditional meta-analysis, PPIs was inferior to vonoprazan in the prevention of bleeding after ESD, while it was superior to H2RAs. In the result of indirect comparison meta-analysis by using PPIs as an intermediary, there was a significant decrease in delayed bleeding in vonoprazan versus H2RAs. In the subgroup analysis, the superiority of PPIs than H2RAs was more obvious in 8 weeks of medication duration than 4 weeks. Meanwhile, the significant effect of vonoprazan versus PPIs was more obvious under the situation of combination therapy than monotherapy and 8 weeks medication duration than 4 weeks.
The rapid promotion of ESD calls for quality improvement and precise management in preventing post-ESD bleeding. To our knowledge, we represented the first step at finding out a more appropriate management system for delayed bleeding prevention including choice of drug, combination of medication, and medication duration by conducting both direct and indirect meta-analysis.
According to our research results, either vonoprazan or PPIs may have better efficacy in preventing post-ESD bleeding in 8 weeks of medication duration than 4 weeks. Additionally, vonoprazan–mucosal protective antiulcer drugs regimen was better than PPIs–mucosal protective antiulcer drugs. The probable explanation was that it may exist as a much stronger synergistic effect between the vonoprazan and mucosal protective antiulcer drugs than PPIs. Therefore, we recommended that the patients who have a high risk of bleeding including long operation time, large resection range, deeper tumor location, and the combination treatment with drugs of potential bleeding take vonoprazan–mucosal protective antiulcer drugs for 8 weeks. However, for patients under low risk of bleeding, shorter duration may be a better choice, taking both cost and adverse events into consideration.
In one previous meta-analysis (Yang et al., 2011), it indicated that PPIs were superior to H2RAs for the prevention of delayed bleeding. However, the author mixed the EMR and ESD studies to assess the delayed bleeding in the ESD subgroup analysis and may lead to some controversial results. Instead, our study focused on ESD specifically and compared the prevention efficacy of PPIs with H2RAs and vonoprazan for delaying the bleeding after ESD. In the medication duration of H2RA and PPI subgroup analysis, our results were consistent with the former study that revealed that PPIs exerted a better influence in 8 weeks’ medication duration than in 4 weeks. The reason may be that the actions of H2RAs are significantly faster than those of PPIs (Ahmad et al., 2002; van Rensburg et al., 2003). A recent meta-analysis compared the rate of healing of ulcers caused by ESD and post-ESD delayed bleeding between vonoprazan and PPIs (Jaruvongvanich et al., 2018). However, since three eligible studies were not included, the research concluded that no difference was found between vonoprazan and PPIs.
There were several limitations associated with our study. Firstly, we should not neglect the heterogeneity among different clinical trials. In our study, the regimens of the RCTs were diverse from each other. As the multivariate analysis indicated, the scar in the tumor as well as the size of the tumor, and the endoscopists’ skill, were independent predictors for bleeding (Uedo et al., 2007; Chung et al., 2009; Jang et al., 2009; Okada et al., 2011). All these factors may have an impact on clinical results. Further studies with a larger number of patients are required to clarify the association between the comorbidities and complications after ESD. Secondly, we could not analyze the safety of the treatments without enough adverse events data. Thirdly, there may exist a population bias in our study. All trials included in our research were implemented in Asia, and only one evidence was from Italy (Catalano et al., 2009). Therefore, the generalization of results should be taken in caution. On the other hand, the most appropriate drug administration time (before ESD or after ESD) and method (orally or intravenously) of PPIs still remained controversial. Meanwhile, even vonoprazan and PPI were preferable in efficacy; it may not be optimal choices in terms of their high cost compared with H2RAs. Therefore, more high-quality researches including cost-effectiveness analysis should be conducted to evaluate these issues and give some further guidance for clinical application.
In conclusion, our results show better effects of vonoprazan over PPIs over H2RAs in preventing bleeding after ESD. When vonoprazan was combined with mucosal protective antiulcer drug in treatment or used in 8 weeks of medication, the efficacy may be even better. So, as for the high-bleeding risk patients, it was recommended to take vonoprazan combination therapy or take vonoprazan for 8 weeks. However, due to the lack of adverse events data, more studies are needed on safety profiles of the previously discussed options and should be conducted on the Western population for the generalization of our results.
Author Contributions
All authors were responsible for the structure of this paper. YW and TZ contributed to the conception and design. XJ and JL conducted the literature search and data analysis. JX, ZL, NW, and JJ checked for statistical inconsistency and interpreted data. XJ and JL drafted the paper. YW and TZ conducted critical revisions of the paper. All authors approved the final version for submission.
Funding
This study was supported by the National Natural Science Foundation of China (grant no. 71704064), the Natural Science Foundation of Guangdong Province, China (grant no. 2017A030310174), and the Fundamental Research Funds for the Central Universities (grant no. 21616324).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abe, Y., Inamori, M., Togawa, J., Kikuchi, T., Muramatsu, K., Chiguchi, G., et al. (2004). The comparative effects of single intravenous doses of omeprazole and famotidine on intragastric pH. J. Gastroenterol. 39 (1), 21–25. doi: 10.1007/s00535-003-1240-6
Ahmad, N. A., Kochman, M. L., Long, W. B., Furth, E. E., Ginsberg, G. G. (2002). Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 55 (3), 390–396. doi: 10.1067/mge.2002.121881
Barer, D., Ogilvie, A., Henry, D., Dronfield, M., Coggon, D., French, S., et al. (1983). Cimetidine and tranexamic acid in the treatment of acute upper-gastrointestinal-tract bleeding. New Eng. J. Med. 308 (26), 1571–1575. doi: 10.1056/NEJM198306303082606
Catalano, F., Trecca, A., Rodella, L., Lombardo, F., Tomezzoli, A., Battista, S., et al. (2009). The modern treatment of early gastric cancer: our experience in an Italian cohort. Surg. Endosc. 23 (7), 1581–1586. doi: 10.1007/s00464-009-0350-5
Chung, I. K., Lee, J. H., Lee, S. H., Kim, S. J., Cho, J. Y., Cho, W. Y., et al. (2009). Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 69 (7), 1228–1235. doi: 10.1016/j.gie.2008.09.027
Collaborative overview of randomised trials of antiplatelet therapy–II (1994). Maintenance of vascular graft or arterial patency by antiplatelet therapy. Antiplatelet Trialists’ Collaboration. BMJ 308 (6922), 159–168. doi: 10.1136/bmj.308.6922.159
Daneshmend, T. K., Hawkey, C. J., Langman, M. J., Logan, R. F., Long, R. G., Walt, R. P. (1992). Omeprazole versus placebo for acute upper gastrointestinal bleeding: randomised double blind controlled trial. BMJ 304 (6820), 143–147. doi: 10.1136/bmj.304.6820.143
Esaki, M., Aoyagi, K., Matsumoto, T., Kuwano, Y., Shimizu, M., Fujishima, M. (2002). Effects of omeprazole and famotidine on fibroblast growth factor-2 during artificial gastric ulcer healing in humans. Eur. J. Gastroenterol. Hepatol. 14 (4), 365–369. doi: 10.1097/00042737-200204000-00005
Fisher, L. D., Gent, M., Buller, H. R. (2001). Active-control trials: how would a new agent compare with placebo? A method illustrated with clopidogrel, aspirin, and placebo. Am. Heart J. 141 (1), 26–32. doi: 10.1067/mhj.2001.111262
Fox, B. D., Kahn, S. R., Langleben, D., Eisenberg, M. J., Shimony, A. (2012). Efficacy and safety of novel oral anticoagulants for treatment of acute venous thromboembolism: direct and adjusted indirect meta-analysis of randomised controlled trials. BMJ 345, e7498. doi: 10.1136/bmj.e7498
Fujishiro, M. (2006). Endoscopic submucosal dissection for stomach neoplasms. World J. Gastroenterol. 12 (32), 5108–5112. doi: 10.3748/wjg.v12.i32.5108
Fukami, N. (2014). ESD around the world: United States. Gastrointest. Endosc. Clin. N. America 24 (2), 313–320. doi: 10.1016/j.giec.2013.12.004
Glenny, A. M., Altman, D. G., Song, F., Sakarovitch, C., Deeks, J. J., D’Amico, R., et al. (2005). Indirect comparisons of competing interventions. Health Technol. Assess. 9 (26), 1–134, iii-iv. doi: 10.3310/hta9260
Green, F. W., Jr., Kaplan, M. M., Curtis, L. E., Levine, P. H. (1978). Effect of acid and pepsin on blood coagulation and platelet aggregation. A possible contributor prolonged gastroduodenal mucosal hemorrhage. Gastroenterology 74 (1), 38–43. doi: 10.1016/0016-5085(78)90352-9
Hamada, K., Uedo, N., Tonai, Y., Arao, M., Suzuki, S., Iwatsubo, T., et al. (2018). Efficacy of vonoprazan in prevention of bleeding from endoscopic submucosal dissection-induced gastric ulcers: a prospective randomized phase II Study. J. Gastroenterol. 54 (2), 122–130. doi: 10.1007/s00535-018-1487-6
Higgins, J. P., Thompson, S. G., Deeks, J. J., Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327 (7414), 557–560. doi: 10.1136/bmj.327.7414.557
Higgins, J. P., Altman, D. G., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi: 10.1136/bmj.d5928
Hirai, A., Takeuchi, T., Takahashi, Y., Kawaguchi, S., Ota, K., Harada, S., et al. (2018) Comparison of the effects of vonoprazan and lansoprazole for treating endoscopic submucosal dissection-induced artificial ulcers. Dig. Dis. Sci. 63 (4), 974–981. doi: 10.1007/s10620-018-4948-0
Hori, Y., Matsukawa, J., Takeuchi, T., Nishida, H., Kajino, M., Inatomi, N. (2011). A study comparing the antisecretory effect of TAK-438, a Novel Potassium-Competitive Acid Blocker, with Lansoprazole in Animals. J. Pharmacol. Exp. Ther. 337 (3), 797–804. doi: 10.1124/jpet.111.179556
Horikawa, Y., Mizutamari, H., Mimori, N., Kato, Y., Fushimi, S., Sato, S., et al. (2018). Short-term efficacy of potassium-competitive acid blocker following gastric endoscopic submucosal dissection: a propensity score analysis. Scand. J. Gastroenterol. 53 (2), 243–251. doi: 10.1080/00365521.2017.1410569
Ichida, T., Ueyama, S., Eto, T., Kusano, F., Sakai, Y. (2018). Randomized controlled trial comparing the effects of vonoprazan plus rebamipide and esomeprazole plus rebamipide on gastric ulcer healing induced by endoscopic submucosal dissection. Int. Med. doi: 10.2169/internalmedicine.1146–1118
Imaeda, H., Hosoe, N., Suzuki, H., Saito, Y., Ida, Y., Nakamura, R., et al. (2011). Effect of lansoprazole versus roxatidine on prevention of bleeding and promotion of ulcer healing after endoscopic submucosal dissection for superficial gastric neoplasia. J. Gastroenterol. 46 (11), 1267–1272. doi: 10.1007/s00535-011-0447-1
Ishii, Y., Yamada, H., Sato, T., Sue, S., Kaneko, H., Irie, K., et al. (2018). Effects of vonoprazan compared with esomeprazole on the healing of artificial postendoscopic submucosal dissection ulcers: a prospective, multicenter, two-arm, randomized controlled trial. Gastroenterol. Res. Pract. doi: 10.1155/2018/1615092
Jang, J. S., Choi, S. R., Graham, D. Y., Kwon, H. C., Kim, M. C., Jeong, J. S., et al. (2009). Risk factors for immediate and delayed bleeding associated with endoscopic submucosal dissection of gastric neoplastic lesions. Scand. J. Gastroenterol. 44 (11), 1370–1376. doi: 10.3109/00365520903194609
Jang, J. S., Han, J. S., Choi, S. R. (2012). Interim analysis of preventive effect of PPI alone, PPI plus rebamipide and H2RA plus rebamipide on bleeding from after endoscopic submucosal dissection: a prospective randomized controlled trial. Gastrointest Endosc. 75 (4), 236–237. doi: 10.1016/j.gie.2012.04.181
Jaruvongvanich, V., Poonsombudlert, K., Ungprasert, P. (2018). Vonoprazan versus proton-pump inhibitors for gastric endoscopic submucosal dissection-induced ulcers: a systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. doi: 10.1097/MEG.0000000000001204
Jenkins, H., Sakurai, Y., Nishimura, A., Okamoto, H., Hibberd, M., Jenkins, R., et al. (2015). Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharm Ther. 41 (7), 636–648. doi: 10.1111/apt.13121
Jeong, H. K., Park, C. H., Jun, C. H., Lee, G. H., Kim, H. I., Kim, H. S., et al. (2007). A prospective randomized trial of either famotidine or pantoprazole for the prevention of bleeding after endoscopic submucosal dissection. J. Korean Med. Sci. 22 (6), 1055–1059. doi: 10.3346/jkms.2007.22.6.1055
Kagami, T., Sahara, S., Ichikawa, H., Uotani, T., Yamade, M., Sugimoto, M., et al. (2016). Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP2C19 genotype. Aliment Pharmacol. Ther. 43 (10), 1048–1059. doi: 10.1111/apt.13588
Kagawa, T., Iwamuro, M., Ishikawa, S., Ishida, M., Kuraoka, S., Sasaki, K., et al. (2016). Vonoprazan prevents bleeding from endoscopic submucosal dissection-induced gastric ulcers. Aliment Pharm Ther. 44 (6), 583–591. doi: 10.1111/apt.13747
Komori, H., Ueyama, H., Nagahara, A., Akazawa, Y., Takeda, T., Matsumoto, K., et al. (2019) A prospective randomized trial of a potassium competitive acid blocker vs proton pump inhibitors on the effect of ulcer healing after endoscopic submucosal dissection of gastric neoplasia. J. Int. Med. Res. 47 (4), 1441–1452. doi: 10.1177/0300060519828514
Lanas, A. (2016). We are using too many PPIs, and we need to stop: a european perspective. Am. J. Gastroenterol. 111 (8), 1085–1086. doi: 10.1038/ajg.2016.166
Lightdale, C. J. (2004). Endoscopic mucosal resection: This is our turf. Endoscopy 36 (9), 808–810. doi: 10.1055/s-2004-825829
Loke, Y. K., Pradhan, S., Yeong, J. K., Kwok, C. S. (2014). Comparative coronary risks of apixaban, rivaroxaban and dabigatran: a meta-analysis and adjusted indirect comparison. Br J. Clin. Pharmacol. 78 (4), 707–717. doi: 10.1111/bcp.12376
Miyamoto, S., Muto, M., Hamamoto, Y., Boku, N., Ohtsu, A., Baba, S., et al. (2002). A new technique for endoscopic mucosal resection with an insulated-tip electrosurgical knife improves the completeness of resection of intramucosal gastric neoplasms. Gastrointest Endosc. 55 (4), 576–581. doi: 10.1067/mge.2002.122579
Miyashita, M., Tajiri, T., Maruyama, H., Makino, H., Nomura, T., Sasajima, K., et al. (2003). Endoscopic mucosal resection scissors for the treatment of early gastric cancer. Endoscopy 35 (7), 611–612. doi: 10.1055/s-2003-40238
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., Group, P. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med. 6 (7), e1000097. doi: 10.1371/journal.pmed.1000097
Noh, M. H., Jang, J. S., Han, J. S., Choi, S. R. (2013). Analysis of preventive effect of PPI alone, PPI plus cytoprotective agents and H2RA plus cytoprotective agents on bleeding after endoscopic submucosal dissection: prospective randomized controlled trial. Gastrointest Endosc. 77 (58), AB252–AB253. doi: 10.1016/j.gie.2013.03.616
Ohya, T. R., Endo, H., Kawagoe, K., Yanagawa, T., Hanawa, K., Ohata, K., et al. (2010). A prospective randomized trial of lafutidine vs rabeprazole on post-ESD gastric ulcers. World J. Gastrointest. Endosc. 2 (1), 36–40. doi: 10.4253/wjge.v2.i1.36
Oka, S., Tanaka, S., Kaneko, I., Mouri, R., Hirata, M., Kawamura, T., et al. (2006). Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 64 (6), 877–883. doi: 10.1016/j.gie.2006.03.932
Okada, K., Yamamoto, Y., Kasuga, A., Omae, M., Kubota, M., Hirasawa, T., et al. (2011). Risk factors for delayed bleeding after endoscopic submucosal dissection for gastric neoplasm. Surg. Endosc. 25 (1), 98–107. doi: 10.1007/s00464-010-1137-4
Ozawa, T., Yoshikawa, N., Tomita, T., Akita, Y., Mitamura, K. (2003). The influence of feeding on gastric acid suppression in Helicobacter pylori-positive patients treated with a proton pump inhibitor or an H2-receptor antagonist after bleeding from a gastric ulcer. J. Gastroenterol. 38 (9), 844–848. doi: 10.1007/s00535-003-1159-y
Packer, M., Antonopoulos, G. V., Berlin, J. A., Chittams, J., Konstam, M. A., Udelson, J. E. (2001). Comparative effects of carvedilol and metoprolol on left ventricular ejection fraction in heart failure: results of a meta-analysis. Am. Heart J. 141 (6), 899–907. doi: 10.1067/mhj.2001.115584
Rosch, T., Sarbia, M., Schumacher, B., Deinert, K., Frimberger, E., Toermer, T., et al. (2004). Attempted endoscopic en bloc resection of mucosal and submucosal tumors using insulated-tip knives: a pilot series. Endoscopy 36 (9), 788–801. doi: 10.1055/s-2004-825838
Sachs, G., Shin, J. M., Munson, K., Vagin, O., Lambrecht, N., Scott, D. R., et al. (2000). Review article: the control of gastric acid and Helicobacter pylori eradication. Aliment Pharm. Ther. 14 (11), 1383–1401. doi: 10.1046/j.1365-2036.2000.00837.x
Sakurai, Y., Mori, Y., Okamoto, H., Nishimura, A., Komura, E., Araki, T., et al. (2015). Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects–a randomised open-label cross-over study. Aliment Pharmacol. Ther. 42 (6), 719–730. doi: 10.1111/apt.13325
Song, F., Glenny, A. M., Altman, D. G. (2000). Indirect comparison in evaluating relative efficacy illustrated by antimicrobial prophylaxis in colorectal surgery. Controlled Clin. Trials 21 (5), 488–497. doi: 10.1016/S0197-2456(00)00055-6
Takizawa, K., Oda, I., Gotoda, T., Yokoi, C., Matsuda, T., Saito, Y., et al. (2008). Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection. An analysis of risk factors. Endoscopy 40 (3), 179–183. doi: 10.1055/s-2007-995530
Tomita, T., Kim, Y., Yamasaki, T., Okugawa, T., Kondo, T., Toyoshima, F., et al. (2012). Prospective randomized controlled trial to compare the effects of omeprazole and famotidine in preventing delayed bleeding and promoting ulcer healing after endoscopic submucosal dissection. J. Gastroenterol. Hepatol. 27 (9), 1441–1446. doi: 10.1111/j.1440-1746.2012.07144.x
Tsuchiya, I., Kato, Y., Tanida, E., Masui, Y., Kato, S., Nakajima, A., et al. (2017) Effect of vonoprazan on the treatment of artificial gastric ulcers after endoscopic submucosal dissection: prospective randomized controlled trial. Dig. Endosc. 29 (5), 576–583. doi: 10.1111/den.12857
Uedo, N., Takeuchi, Y., Yamada, T., Ishihara, R., Ogiyama, H., Yamamoto, S., et al. (2007). Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am. J. Gastroenterol. 102 (8), 1610–1616. doi: 10.1111/j.1572-0241.2007.01197.x
van Rensburg, C. J., Hartmann, M., Thorpe, A., Venter, L., Theron, I., Luhmann, R., et al. (2003). Intragastric pH during continuous infusion with pantoprazole in patients with bleeding peptic ulcer. Am. J. Gastroenterol. 98 (12), 2635–2641. doi: 10.1111/j.1572-0241.2003.08723.x
Yamamoto, H., Kawata, H., Sunada, K., Sasaki, A., Nakazawa, K., Miyata, T., et al. (2003). Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy 35 (8), 690–694. doi: 10.1055/s-2003-41516
Yamasaki, A., Yoshio, T., Muramatsu, Y., Horiuchi, Y., Ishiyama, A., Hirasawa, T., et al. (2018). Vonoprazan is superior to rabeprazole for healing endoscopic submucosal dissection: Induced Ulcers. Digestion. 97 (2), 170–176. doi: 10.1159/000485028
Yang, Z. P., Wu, Q., Liu, Z. G., Wu, K. C., Fan, D. M. (2011). Proton pump inhibitors versus histamine-2-receptor antagonists for the management of iatrogenic gastric ulcer after endoscopic mucosal resection or endoscopic submucosal dissection: a meta-analysis of randomized trials. Digestion. 84 (4), 315–320. doi: 10.1159/000331138
Ye, B. D., Cheon, J. H., Choi, K. D., Kim, S. G., Kim, J. S., Jung, H. C., et al. (2006). Omeprazole may be superior to famotidine in the management of iatrogenic ulcer after endoscopic mucosal resection: a prospective randomized controlled trial. Aliment Pharm. Ther. 24 (5), 837–843 doi: 10.1111/j.1365-2036.2006.03050.x.
Keywords: endoscopic submucosal dissection, proton pump inhibitors, histamine2-receptor antagonists, vonoprazan, delayed bleeding
Citation: Jiang X, Li J, Xie J, Liang Z, Wan N, Jiang J, Zhang T and Wu Y (2019) Histamine2-Receptor Antagonists, Proton Pump Inhibitors, or Potassium-Competitive Acid Blockers Preventing Delayed Bleeding After Endoscopic Submucosal Dissection: A Meta-Analysis. Front. Pharmacol. 10:1055. doi: 10.3389/fphar.2019.01055
Received: 10 June 2019; Accepted: 20 August 2019;
Published: 19 September 2019.
Edited by:
Tahir Mehmood Khan, University of Veterinary and Animal Sciences, PakistanReviewed by:
Robert L. Lins, Retired, Antwerp, BelgiumAdina Turcu-Stiolica, University of Medicine and Pharmacy of Craiova, Romania
Copyright © 2019 Jiang, Li, Xie, Liang, Wan, Jiang, Zhang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tiantian Zhang, ztt_84@126.com; Yingyu Wu, 1020132388@cpu.edu.cn
†These authors have contributed equally to this work
 Xin Jiang1†
Xin Jiang1† Jiahao Li
Jiahao Li Tiantian Zhang
Tiantian Zhang