
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 17 September 2019
Sec. Neuropharmacology
Volume 10 - 2019 | https://doi.org/10.3389/fphar.2019.01045
This article is part of the Research Topic Pharmacology of BPSD (Behavioral and Psychological Symptoms of Dementia) View all 31 articles
Antipsychotic drugs are often used for the treatment of behavioral and psychological symptoms of dementia (BPSD), especially psychosis and behavioral disturbances (e.g., aggression and agitation). They are prescribed alone or in conjunction with anti-dementia (e.g., anti-Alzheimer’s disease drugs) and other psychotropic drugs (e.g., antidepressants). However, antipsychotic drugs frequently produce serious extrapyramidal side effects (EPS) including Parkinsonian symptoms (e.g., bradykinesia, akinesia, tremor, and muscle rigidity). Therefore, appropriate drug choice and combination strategy are important in the treatment of BPSD. Among anti-Alzheimer’s disease drugs, cholinesterase inhibitors (ChEIs, e.g., donepezil and galantamine) have a propensity to potentiate EPS associated with antipsychotic treatment in a synergistic manner. In contrast, the NMDA receptor antagonist memantine reduces antipsychotic-induced EPS. Antidepressant drugs, which inhibit 5-HT reuptake into the nerve terminals, also synergistically augment antipsychotic-induced EPS, while mirtazapine (α2, 5-HT2 and 5-HT3 antagonist) reduces the EPS induction. Importantly, previous studies showed that multiple 5-HT receptors play crucial roles in modulating EPS associated with antipsychotic treatment. Specifically, activation of 5-HT1A receptors or blockade of 5-HT2, 5-HT3 and 5-HT6 receptors can alleviate EPS induction both by antipsychotics alone and by combined antipsychotic treatments with ChEIs or 5-HT reuptake inhibitors. In this article, we review antipsychotic use in treating BPSD and discuss the favorable drug selection in terms of the management of antipsychotic-induced EPS.
Dementia is a neurodegenerative brain disorder with diverse clinical symptoms including cognitive impairment (e.g., memory loss and learning deficits) and non-cognitive disorders (e.g., behavioral and psychological deficits). Nearly 50 million patients worldwide develop dementia and this population is expected to exceed 130 million in 2050 (Prince et al., 2015; Jin and Liu, 2019). The global cost associated with dementia was about 1,000 billion dollars in 2015, and this continues to increase rapidly. There are numerous causes of dementia including Alzheimer’s disease, cerebrovascular diseases, Parkinson’s disease, Lewy body disease, and mixed types, among which Alzheimer’s disease is the most frequent (Lee et al., 2004; Prince et al., 2015; Sturm et al., 2018).
Behavioral and psychological symptoms of dementia (BPSD) occur in the majority (up to 90%) of dementia patients, and this causes significant distress to both patients and caretakers (O’Donnell et al., 1992; O’Brien, 2003; Rosdinom et al., 2013). BPSD includes behavioral excitement (e.g., agitation and aggression), mood disorders (e.g., apathy, depression and anxiety), psychosis (e.g., hallucinations and delusions) and other symptoms (e.g., eating disturbances and sleep disorders) (Figure 1). Although the prevalence of BPSD varies among reported studies, hallucinations occur in 15–50% of patients with dementia, delusions in 10–75% and behavioral disturbances (e.g., agitation and aggression) in about 50%, while affective symptoms are less common (van der Linde et al., 2014; Devshi et al., 2015). To treat BPSD, non-pharmacological interventions such as cognitive stimulation training, exercise, music therapy, light therapy and aromatherapy are recommended as first-line treatments. Nonetheless, pharmacological treatments with antipsychotics and other psychotropic drugs are necessary to treat BPSD (Brimelow et al., 2019; Jin and Liu, 2019; Kales et al., 2019) (Figure 1). Specifically, antipsychotic drugs are the first choice to reduce psychosis and behavioral disturbances despite their frequent side effects (Lee et al., 2004; Trifirò et al., 2009; Brimelow et al., 2019; Sturm et al., 2018).
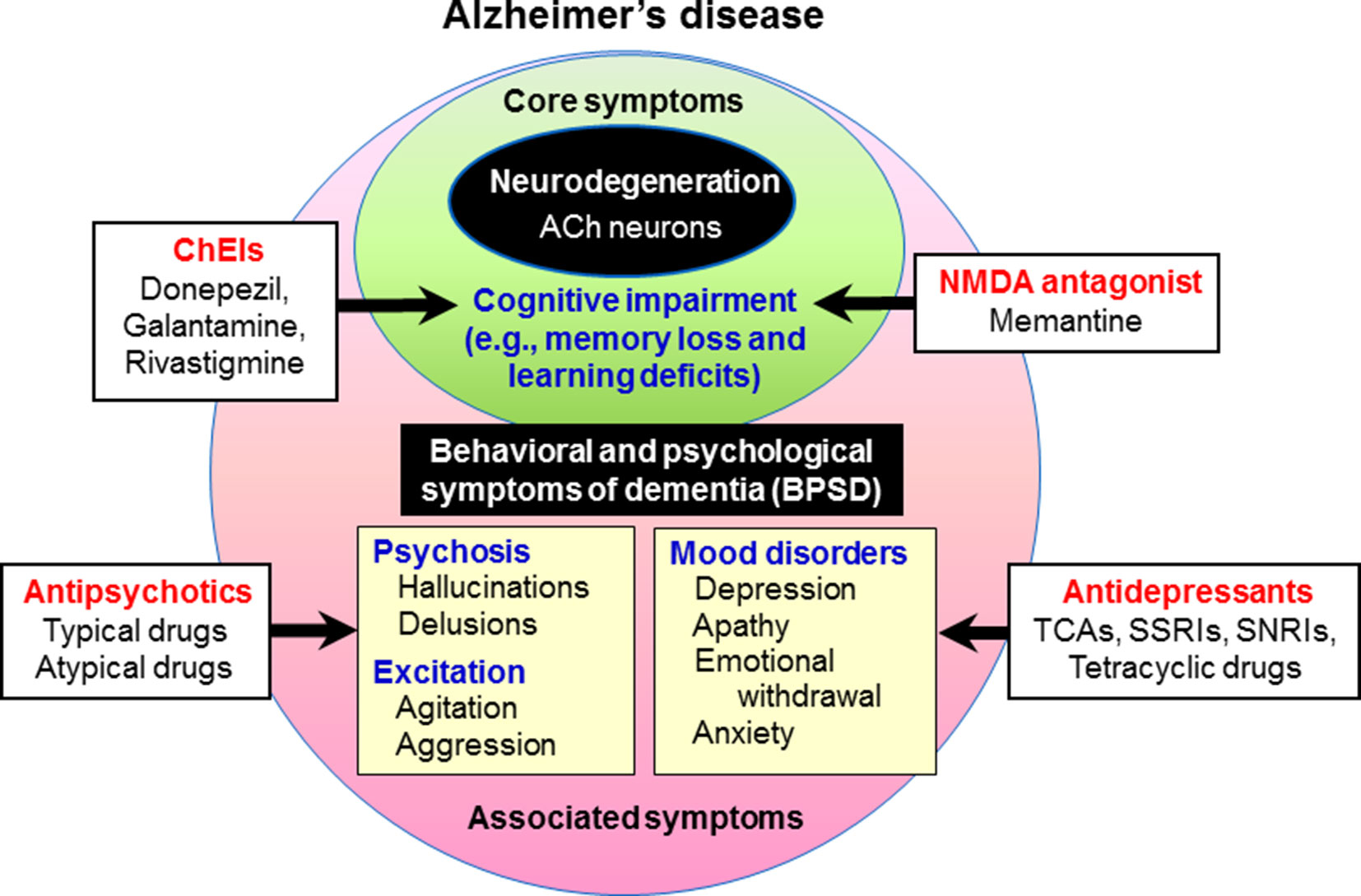
Figure 1 Behavioral and psychological symptoms of dementia (BPSD) in Alzheimer’s disease. Patients with dementia show not only core symptoms of dementia (e.g., memory loss and learning deficits), but also various associated symptoms including BPSD (e.g., psychosis, behavioral excitation, and mood disorders). Cognitive impairment in Alzheimer’s disease is usually treated with cognitive enhancers such as the cholinesterase inhibitors (ChEIs, e.g., donepezil, galantamine, and rivastigmine) and the NMDA antagonist (e.g., memantine). In the treatment of BPSD, antipsychotic drugs are used for psychosis and behavioral disturbances, and antidepressants for depressive mood.
It is well known that antipsychotic drugs commonly cause serious extrapyramidal side effects (EPS) (e.g., bradykinesia, muscle rigidity, tremor, and akathisia) by blocking dopamine D2 receptors in the striatum (Remington and Kapur, 1999; Kapur and Remington, 2001; Ohno et al., 2013; Ohno et al., 2015; Ohno, 2019). Antipsychotic-induced EPS often leads to suboptimal treatment of BPSD or treatment discontinuation. In addition, recent studies showed that cholinesterase inhibitors (ChEIs), licensed drugs for cognitive impairment due to Alzheimer’s disease, potentiate EPS induction with antipsychotic treatments (Shimizu et al., 2015). It is therefore important to understand the mechanism underlying antipsychotics-induced EPS and antipsychotic drug interactions with other medications in the treatment of BPSD.
In this article, we review the pharmacological features of antipsychotic drugs, especially those related to EPS, and discuss the proper usage and selection of antipsychotics in treating BPSD in terms of EPS management.
Antipsychotic drugs are used to treat BPSD with a prescription rate of about 20–50% (Lee et al., 2004; Brimelow et al., 2019; Sturm et al., 2018). The target symptoms of antipsychotic drugs include agitation, aggression, psychosis, and inappropriate behaviors (Figure 1). None of the antipsychotics, except for haloperidol and risperidone in several countries, are approved to treat BPSD; therefore, these drugs are generally prescribed as off-label. Nonetheless, antipsychotic drugs are reported to produce significantly better improvements than placebos in treating BPSD (Lee et al., 2004; Brimelow et al., 2019; Sturm et al., 2018).
Antipsychotic drugs commonly possess dopamine D2 blocking actions. It is known that D2 receptor blockade by antipsychotics in the cortico-limbic regions (e.g., nucleus accumbens) contributes to antipsychotic activities, which alleviates psychosis (e.g., hallucinations and delusions) and behavioral excitation (e.g., agitation, aggression and hyperactivity) (Figure 2). However, it should be noted that all antipsychotic drugs frequently cause extrapyramidal motor disorders due to the striatal D2 receptor blockade, which disrupts the effective treatment of BPSD.
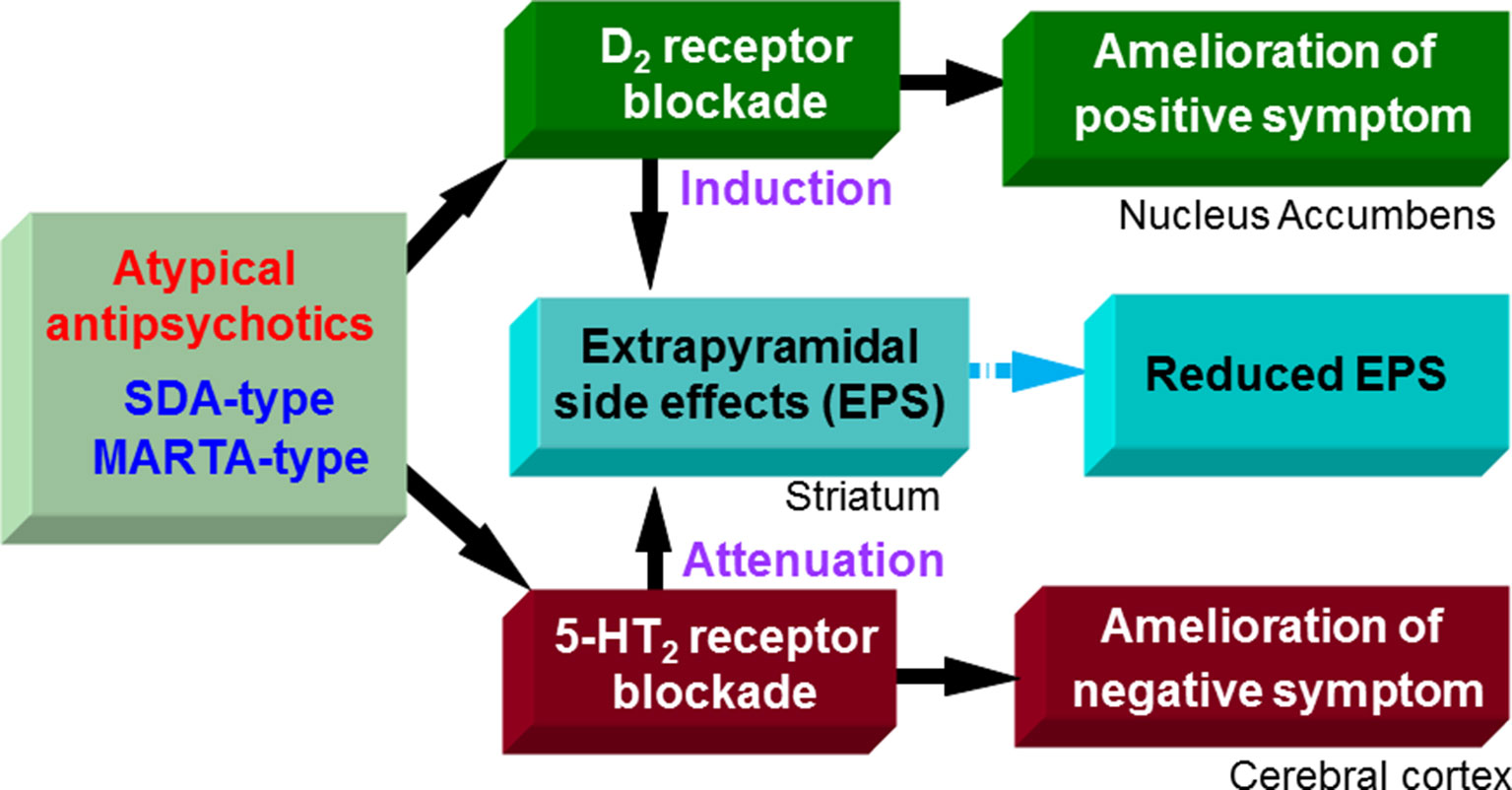
Figure 2 Pharmacological actions of atypical antipsychotics. Likely typical antipsychotic drugs, D2 blocking actions of serotonin and dopamine antagonists (SDA)-type and multiple-acting receptor targeted antipsychotics (MARTA)-type antipsychotics ameliorate positive symptoms (e.g., hallucination, delusions, and excitation) in schizophrenia, but induce extrapyramidal side effects (EPS). On the other hand, SDA-type and MARTA-type antipsychotics show higher 5-HT2 than D2 binding affinities and possess potent 5-HT2 antagonistic actions. The 5-HT2 blocking activities of SDA-type antipsychotics ameliorate negative symptoms (e.g., apathy and social withdrawal) in schizophrenia and can reduce EPS. Thereby, overall EPS liability of SDA-type antipsychotics is lower than typical antipsychotics (D2 antagonists).
Antipsychotic drugs are generally classified into two groups, typical and atypical (Ohno et al., 1997; Ohno et al., 2012). Typical antipsychotics are the classic standard drugs and frequently cause severe EPS. Based on their chemical structures, they are grouped into several classes, phenothiazines (e.g., chlorpromazine and fluphenazine), butyrophenones (e.g., haloperidol and spiperone), benzamides (e.g., sulpiride and tiapride), and others. On the other hand, atypical antipsychotics were developed as second generation, and are generally less potent than typical ones in inducing EPS (Figures 2 and 3). These include the serotonin and dopamine antagonists (SDAs) with potent blocking action for 5-HT2 receptors, the multiple-acting receptor targeted antipsychotics (MARTAs) and the dopamine D2 partial agonists (Ohno et al., 2012). Besides reduced EPS, these drugs were originally expected be superior to typical antipsychotics in terms of their efficacy to treat negative symptoms (e.g., apathy and emotional withdrawal) (Figure 2). However, comprehensive clinical studies including the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) and European First-Episode Schizophrenia Trial (EUFEST), revealed no clear advantages of atypical over typical drugs in terms of efficacy (Lieberman et al., 2005; Keefe et al., 2007; Davidson et al., 2009). Nonetheless, due to the reduced side effect profile, atypical antipsychotics are widely used as a first line drug in BPSD treatment as well as schizophrenia treatment.
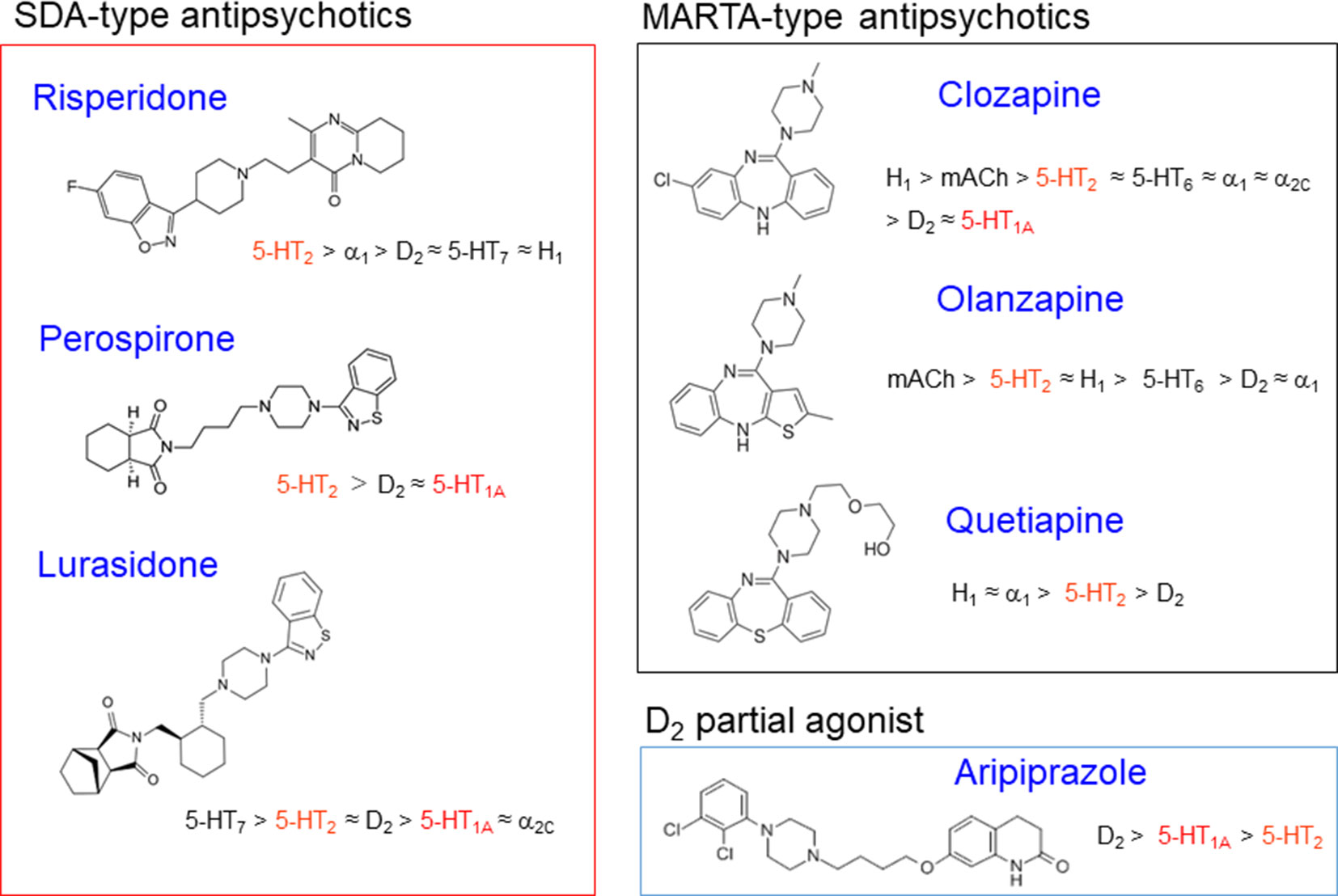
Figure 3 Classification and characteristics of atypical antipsychotic drugs. Figure shows chemical structures of atypical antipsychotics with their receptor binding profiles (affinities). Most atypical antipsychotics possess potent 5-HT2 blocking actions and act as serotonin and dopamine antagonists (SDAs). Among SDAs, clozapine derivatives (e.g., olanzapine and quetiapine) show various actions on other receptors than D2 and 5-HT2 receptors, including histamine H1, adrenergic α1, and muscarinic blocking actions. Thereby, they are sometimes called as multi-acting receptor-targeted antipsychotics (MARTAs) and differentiated from SDAs (e.g., risperidone, perospirone, and lurasidone). Although aripiprazole possess moderate 5-HT2 blocking activities, it primarily acts as a dopamine D2 partial agonist. Furthermore, several atypical antipsychotics have own characteristics such as 5-HT1A partial agonistic actions for perospirone, lurasidone and aripiprazole, 5-HT6 blocking actions for olanzapine and quetiapine, and 5-HT3 blocking actions for olanzapine.
Major EPS symptoms associated with antipsychotic treatment of BPSD include Parkinsonian symptoms, akathisia, and dystonia. Tardive dyskinesia (repeated abnormal involuntary movements) is another antipsychotic-induced EPS, but is rare during the relatively short-term BPSD treatment as it is a chronic side effect associated with long-term antipsychotic treatment and usually appears upon the cessation of treatment.
Antipsychotic-induced Parkinsonian symptoms are involuntary movement disorders including bradykinesia, tremor and muscle rigidity (Samii et al., 2004; Haddad and Dursun, 2008; Ohno et al., 2015). Parkinsonian symptoms usually occur in a few weeks after starting the antipsychotic treatment. Bradykinesia refers to reduced motor activity and slowing movements, which leads to akinesia in more severe cases. Tremor is an involuntary, rhythmic muscle contraction and relaxation (oscillation or twitching movements), affecting the hands, feet and head especially during resting state. In addition, affected patients often exhibit a stooped posture with increased muscle tone (rigidity) and a slow gait without arm swing.
Patients with akathisia suffer from restlessness and repetitive movements of the legs and feet (Keefe et al., 2007; Haddad and Dursun, 2008). As a result, they cannot keep sitting and frequently shift their body position. Akathisia usually appears soon after starting antipsychotics or after increasing the dose.
Dystonia causes sustained muscle contraction, often leading to postural distortion (Haddad and Dursun, 2008). Dystonia often attacks the neck muscles, tongue, trunk, and limbs. Acute dystonia usually appears in the first week after starting or increasing the dose of antipsychotics.
It is well known that antipsychotic-induced EPS are caused by the blockade of dopamine D2 receptors in the striatum (caudate-putamen) (Ohno et al., 1997; Ohno et al., 2013; Ohno et al., 2015; Ohno, 2019) (Figure 4). The GABAergic medium spiny neurons in the striatum receive excitatory glutamatergic inputs from the cerebral cortex and acetylcholinergic inputs from striatal interneurons. The medium spiny neurons also receive inhibitory dopaminergic inputs from the substantia nigra pars compacta (SNc) and express a high density of D2 receptors (Ohno et al., 2015). In addition, the dopaminergic neurons from the SNc also negatively regulate activities of the acetylcholinergic interneuron via D2 receptors. Most antipsychotic drugs commonly act as dopamine D2 receptor antagonists and activate the medium spiny neurons and acetylcholinergic interneurons in the striatum, eliciting various EPS symptoms (Ohno et al., 2013) (Figure 4).
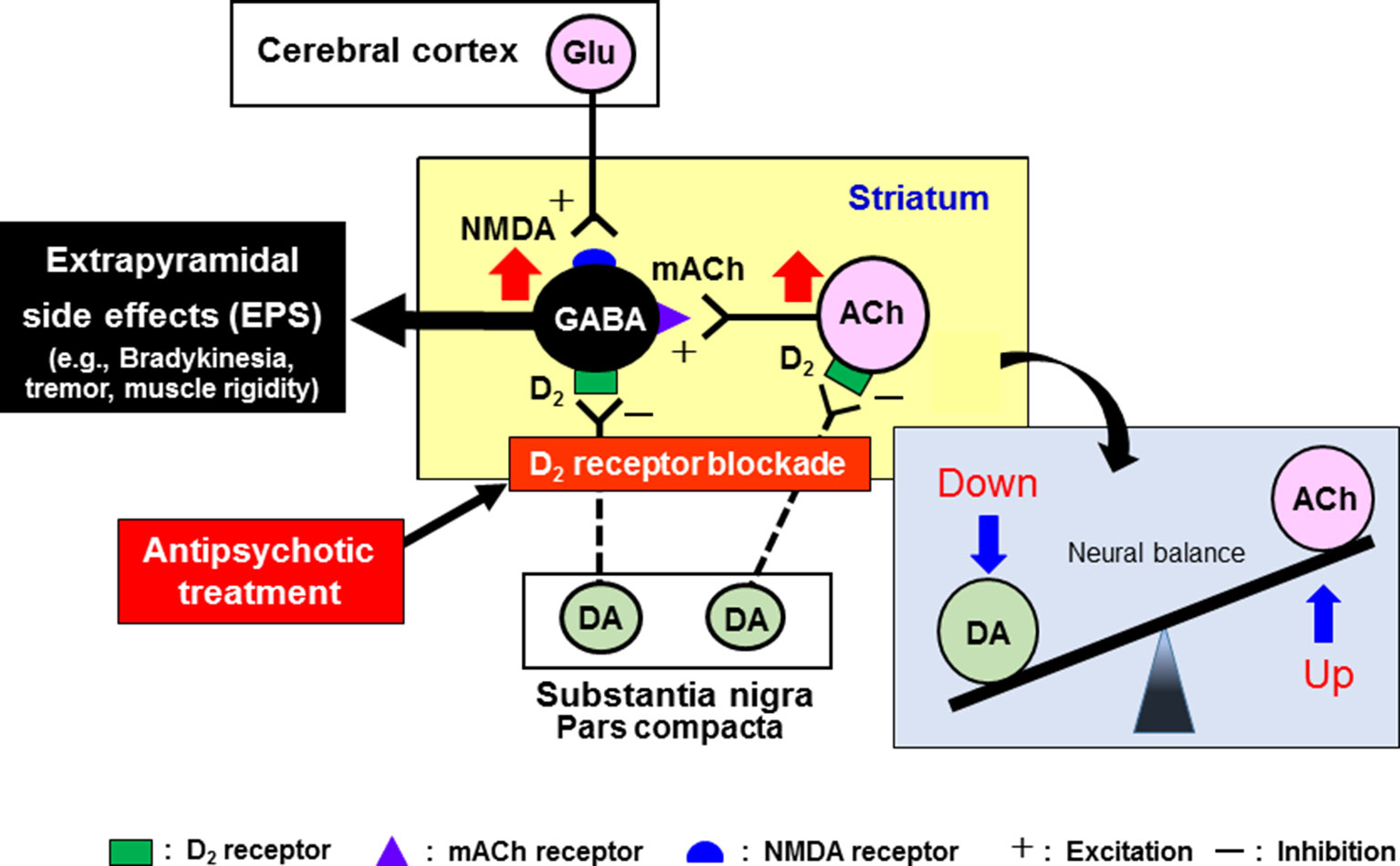
Figure 4 Pathophysiological mechanisms underlying the induction of extrapyramidal side effects (EPS) with antipsychotic treatments. Antipsychotic drugs commonly exert dopamine D2 blocking actions in the striatum, which relieves the striatal neurons (GABA-containing medium spiny neurons and acetylcholine (ACh)-containing interneurons) from negative regulation by the nigrostriatal dopaminergic neurons. Thus, overall activation of striatal medium spiny neurons by antipsychotics evokes EPS (e.g., bradykinesia, tremor, and muscle rigidity). Antipsychotic-induced EPS can be alleviated by anti-muscarinic drugs (e.g., trihexyphenidyl and biperidene), which reverses the imbalance between dopamine and ACh neuron activities in the striatum. However, due to the side effects, these agents are not recommended for the elderly patients.
To reduce EPS, a series of atypical antipsychotics, that show potent 5-HT2 blocking activities have been developed in the last three decades (Ohno et al., 1997; Ohno et al., 2012) (Figures 2 and 3). These agents include risperidone, perospirone, olanzapine, quetiapine, lurasidone, and paliperidone, and they commonly exhibit higher 5-HT2 than D2 affinities. Since olanzapine and quetiapine also show high affinities for other multi-receptors (e.g., histamine H1, adrenergic α1, and muscarinic acetylcholine (mACh) receptors), these drugs are sometimes called as MARTAs and distinguished from SDAs.
It is well documented that blockade of 5-HT2 receptors attenuates antipsychotic-induced EPS associated with the striatal D2 receptor blockade (Figure 2). 5-HT2 receptors are located on nerve terminals and cell bodies of dopaminergic neurons in the striatum and the SNc, respectively, and inhibit dopaminergic neuron activities (Ohno et al., 1997; Ohno et al., 2012; Ohno et al., 2013). It is therefore proposed that blockade of 5-HT2 receptors relieves 5-HT2 receptor-mediated inhibition of dopamine release in the striatum and of dopamine neuron firing in the SNc, which leads to alleviation of EPS (Figure 5) (Remington and Kapur, 1999; Kapur and Remington, 2001). In fact, blockade of 5-HT2 receptors can reverse various responses of striatal neurons to antipsychotics (D2 receptor blockade), such as the enhancement of acetylcholine (ACh) release, the increase in metabolic turnover rate of dopamine and the induction of Fos protein expression, in the striatum Ohno et al., 1997; Ohno et al., 2013).
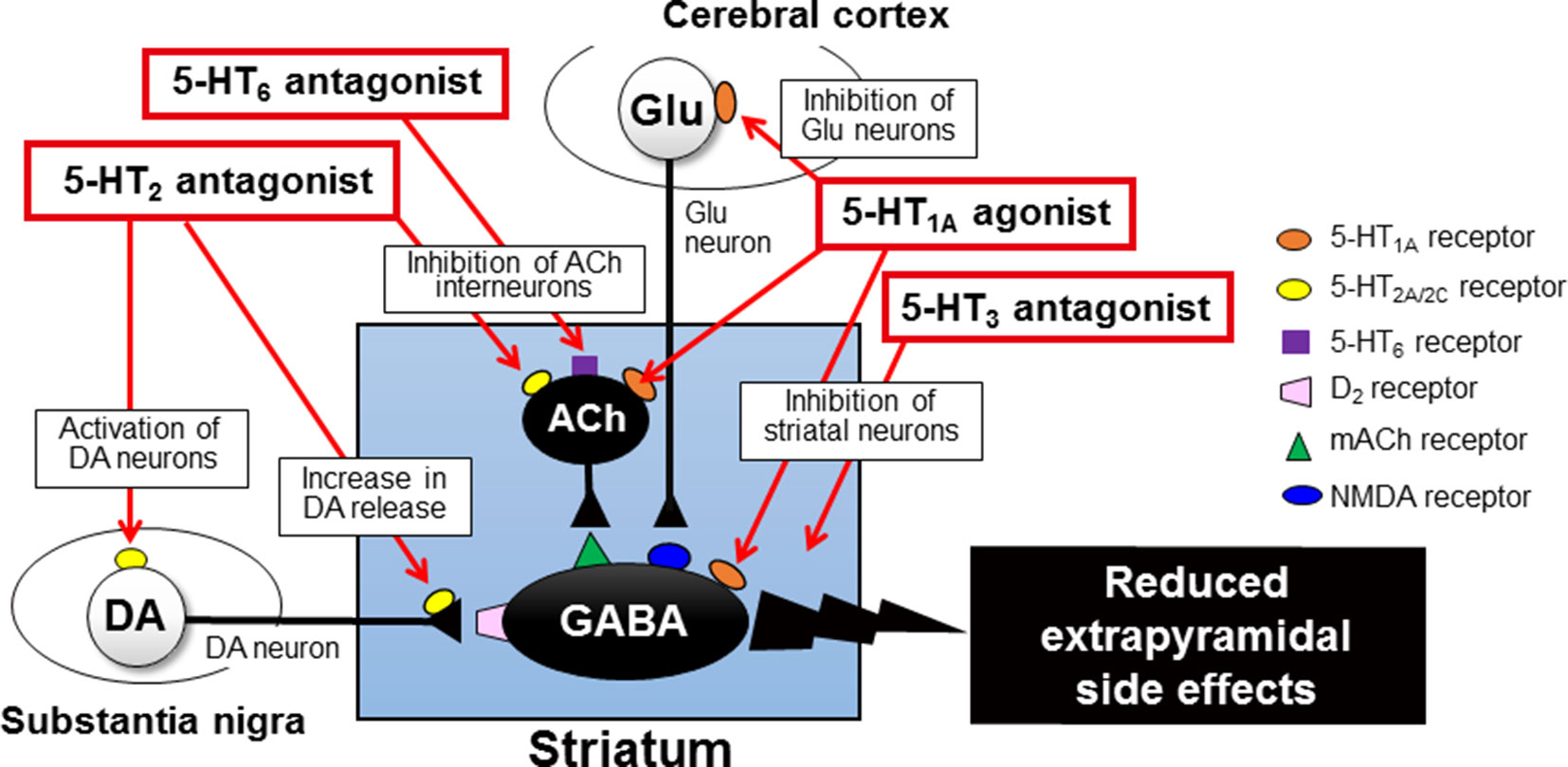
Figure 5 Mechanisms underlying serotonergic modulation of antipsychotic-induced extrapyramidal side effects (EPS). Activation of 5-HT1A receptors, especially postsynaptic 5-HT1A receptors in the striatum and cerebral cortex, alleviates antipsychotic-induced EPS. Blockade of 5-HT2 receptors on nigral dopamine neurons and their nerve terminals in the striatum can relieve the negative serotonergic regulation and thereby can increase the dopaminergic activities, which contributes to EPS reduction. Similarly, blockade of 5-HT3 and 5-HT6 receptors attenuates antipsychotic-induced EPS possibly via acting in the striatum. This figure is quoted and arranged from Biol. Pharm. Bull. 36, 1396, 2013.
As described previously, the serotonergic nervous system plays an important role in modulating EPS induction. Specifically, antipsychotic-induced EPS is augmented by stimulation of 5-HT2 receptors and attenuated by 5-HT2 receptor blockade. Besides 5-HT2 receptors, several 5-HT receptor subtypes, including 5-HT1A, 5-HT3 and 5-HT6 receptors, are involved in regulation of EPS induction associated with antipsychotic treatment (Ohno et al., 2013; Ohno et al., 2015).
5-HT1A receptors function as both presynaptic autoreceptors and postsynaptic receptors, which inhibits neural activities via activating G-protein-gated inwardly rectifying K+ channels (Baumgarten and Grozdanovic, 1995; Barnes and Sharp, 1999; Shimizu et al., 2013a; Shimizu et al., 2013b; Ohno, 2019). Activation of 5-HT1A receptors is known to reduce antipsychotic-induced EPS and motor disorders in animal models of Parkinson’s disease (Neal-Beliveau et al., 1993; Wadenberg et al., 1999; Mignon and Wolf, 2002; Ohno et al., 2008a; Ohno et al., 2008b; Ohno et al., 2009; Shimizu et al., 2010). Our previous studies showed that selective 5-HT1A agonists (e.g., 8-OH-DPAT) ameliorated haloperidol-induced EPS (e.g., bradykinesia and catalepsy) and reversed the striatal Fos protein expression by the haloperidol treatment (Ohno et al., 2008a; Ohno et al., 2008b; Ohno et al., 2009). In addition, the anti-EPS action of 5-HT1A agonists persisted against the denervation of 5-HT neurons with p-chlorophenylalanine treatment, illustrating that postsynaptic 5-HT1A receptors are responsible for EPS reduction (Neal-Beliveau et al., 1993; Mignon and Wolf, 2002; Ohno et al., 2008a; Ohno et al., 2008b) Furthermore, microinjection of 5-HT1A agonists into the striatum or the cerebral cortex (i.e., motor cortex) also attenuated extrapyramidal disorders (Shimizu et al., 2010). Therefore, it is likely that activation of 5-HT1A receptors reduces antipsychotic-induced EPS by inhibiting neural activity in the striatum and motor cortex (Figure 5). Nonetheless, several studies suggest that presynaptic 5-HT1A autoreceptors are also involved to reduce EPS (Wadenberg et al., 1999; Mombereau et al., 2017).
5-HT3 receptors function as cation (Na+, K+, and Ca2+)-permeable ion channels and excite target neurons (Barnes and Sharp, 1999; Ohno, 2019). Several studies demonstrated that blockade of 5-HT3 receptors reduced haloperidol-induced EPS (e.g., catalepsy and bradykinesia) (Silva et al., 1995; Ohno et al., 2011; Tatara et al., 2012) (Figure 5). Clinical studies also showed that the selective 5-HT3 antagonist, ondansetron, reduced the incidence and severity of antipsychotic-induced EPS in the schizophrenia treatment (Zhang et al., 2006; Akhondzadeh et al., 2009).
5-HT6 receptors are highly expressed in the basal ganglia (e.g., striatum), as well as the limbic (e.g., olfactory tubercles and hippocampus) and cortical regions (Barnes and Sharp, 1999; Ohno, 2019). We previously showed that the selective 5-HT6 antagonist, SB-258585, alleviated haloperidol-induced bradykinesia and catalepsy (Ohno et al., 2011; Tatara et al., 2012). In addition, EPS induction was also reduced by microinjection of SB-258585 into the striatum, implying that blockade of the striatal 5-HT6 receptors is at least partly involved in alleviating EPS. Since 5-HT6 receptors positively regulate the neural activities of the striatal ACh interneurons (Bonsi et al., 2007), it is conceivable that 5-HT6 antagonists reduce antipsychotic-induced EPS by inhibiting them (Figure 5).
Regarding other 5-HT receptor subtypes, neither 5-HT4 (GR-125487), 5-HT5a (SB-699551), nor 5-HT7 (SB-269970) antagonists affected antipsychotic-induced EPS (Ohno et al., 2011). Therefore, the modulatory roles of these 5-HT receptors in modulating EPS appear to be minimal.
Alzheimer’s disease is the major component of elderly dementia. Since Alzheimer’s disease accompanies the loss of ACh neurons (Fibiger, 1991; Silva et al., 2014), several ChEIs such as donepezil, galantamine, and rivastigmine, which can increase the ACh level by inhibiting cholinesterase, are widely used to treat the cognitive impairment in Alzheimer’s disease. In addition, an NMDA receptor antagonist, memantine, is also used to alleviate the cognitive impairment. These anti-Alzheimer’s disease drugs are often prescribed in combination with antipsychotic drugs which can reduce BPSD (Salamone et al., 2001; Kozman et al., 2006), giving greater efficacy than monotherapy (Schmitt et al., 2004).
Although information on the drug interactions between antipsychotic and anti-Alzheimer’s disease drugs is limited, our previous study revealed that they markedly potentiated antipsychotic-induced EPS induction (Shimizu et al., 2015). Specifically, donepezil and galantamine rarely induce EPS signs when taken alone; however, they markedly potentiated bradykinesia induction by low dose of haloperidol in a dose-dependent and synergistic manner (Figure 6). In addition, the bradykinesia potentiation by galantamine was significantly reversed by a 5-HT1A agonist (8-OH-DPAT), a 5-HT2 antagonist (ritanserin) and a 5-HT6 antagonist (SB-258585) (Shimizu et al., 2015). These findings indicate that caution is needed in the combined usage of antipsychotics and ChEIs in BPSD treatment. Furthermore, antipsychotics that can stimulate 5-HT1A receptors or antagonize 5-HT2 and 5-HT6 receptors appear favorable as an adjunctive therapy for BPSD. Interestingly, in contrast to ChEIs, memantine, which antagonizes NMDA receptors, attenuated antipsychotic-induced EPS (Figure 6). Therefore, it seems likely that memantine is more favorable than ChEIs in the combined therapy of BPSD with antipsychotics.
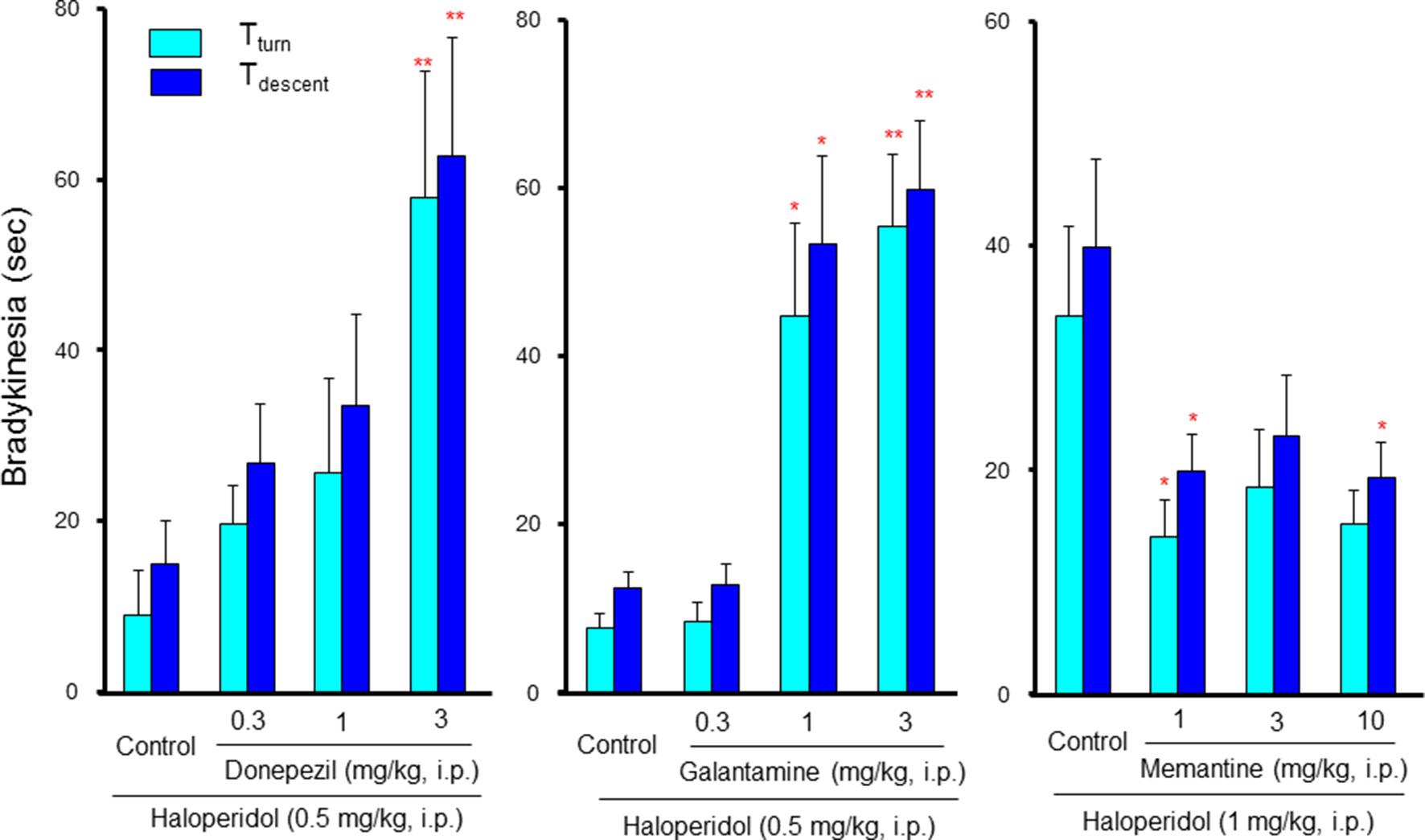
Figure 6 Interactions between anti-Alzheimer’s disease drugs and antipsychotics in induction of extrapyramidal side effects (EPS). Bradykinesia was estimated by the pole test, where mice were placed head-upward at the top of a pole (45 cm in height) and the time for mice to rotate downward (Tturn) and to descend to the floor (Tdescent) was measured (Ohno et al., 2008a). Bradykinesia was evaluated as the prolongation of Tturn or Tdescent Values. Although low dose (0.5 mg/kg) of haloperidol showed marginal effects in the pole test, combined treatment with cholinesterase inhibitors, donepezil, and galantamine, markedly potentiated haloperidol-induced bradykinesia in a synergistic manner. By contrast, the NMDA antagonist, memantine, significantly reduced bradykinesia induced by a high dose (1 mg/kg) of haloperidol. *P<0.05, **P,0.01; Significantly different from the control values. This figure is partly quoted and arranged from J. Pharmacol. Sci. 127, 439, 2015.
Precise mechanisms underlying the synergistic potentiation of EPS by ChEIs is still unknown. However, the action of antipsychotics on cholinergic interneurons in the striatum seems to be involved since the firing of striatal cholinergic interneurons is negatively regulated by dopaminergic neurons and is reportedly facilitated by antipsychotics, increasing the ACh release (Damsma et al., 1990; DeBoer and Abercrombie, 1996). Therefore, ChEIs may augment the induction of EPS more potently in the presence of antipsychotics than their monotherapy.
Antidepressant drugs, as well as antipsychotic drugs, are often used to treat BPSD, especially the mood disorders such as apathy, depression and emotional withdrawal (Lee et al., 2004; Trifirò et al., 2009; Brimelow et al., 2019; Sturm et al., 2018; Jin and Liu, 2019; Kales et al., 2019) (Figure 6). The majority of antidepressant drugs commonly inhibit neural reuptake of 5-HT and/or noradrenaline, and increase the synaptic levels of 5-HT and/or noradrenaline (Ohno, 2019). These drugs are generally classified as tricyclic antidepressants (TCAs) (e.g., nortriptyline, clomipramine, and imipramine), selective serotonin reuptake inhibitors (SSRIs) (e.g., fluoxetine, sertraline, and paroxetine) and serotonin noradrenaline reuptake inhibitors (SNRIs) (e.g., milnacipran, duloxetine, and venlafaxine). In addition, tetracyclic antidepressant drugs (e.g., mirtazapine and mianserin), which block adrenergic α2, 5-HT2 and 5-HT3 receptors without affecting 5-HT or noradrenaline transporters (Wood et al., 1993; Anttila and Leininen, 2001; Wikström et al., 2002; Fernández et al., 2005; Gillman, 2006), are also used to treat BPSD (Figure 1). These agents enhance noradrenaline and 5-HT release by inhibiting α2 autoreceptors on adrenergic nerve terminals and α2 heteroreceptors on serotonergic nerve terminals, respectively.
Neither SSRIs nor TCAs induced EPS by themselves; however, they markedly potentiated antipsychotic-induced bradykinesia and catalepsy in a dose-dependent manner (Tatara et al., 2012; Shimizu et al., 2013a; Shimizu et al., 2013b) (Figure 7). Clinical studies also showed that antidepressants worsen extrapyramidal motor disorders (Gill et al., 1997; Govoni et al., 2001; DeBattista and DeBattista, 2010). Therefore, caution should be taken in the combined usage of antidepressants with antipsychotics in BPSD treatment even though antidepressants do not cause EPS by themselves. Since both SSRIs and TCAs commonly enhance serotonergic activity, these agents potentiate antipsychotic-induced EPS probably by stimulating 5-HT2, 5-HT3 and 5-HT6 receptors. In addition, although the synergistic mechanism in potentiating EPS remains uncertain, antipsychotic-induced activation of striatal cholinergic interneurons may be involved since 5-HT excites the cholinergic neurons via 5-HT2C and 5-HT6 receptors (Bonsi et al., 2007). In fact, blockade of 5-HT2 receptors by ritanserin, 5-HT3 receptors by ondansetron (5-HT3 antagonist), and 5-HT6 receptors by SB-258585 (5-HT6 antagonist), significantly attenuated the EPS augmentation by SSRIs (Tatara et al., 2012). In addition, stimulation of postsynaptic 5-HT1A receptors by 8-HO-DPAT also alleviated SSRIs-induced EPS augmentation (Shimizu et al., 2013a; Shimizu et al., 2013b). This implies that antipsychotics which possess 5-HT1A stimulating actions or 5-HT2, 5-HT3, and 5-HT6 blocking actions, could be useful as adjunctive therapies for BPSD.
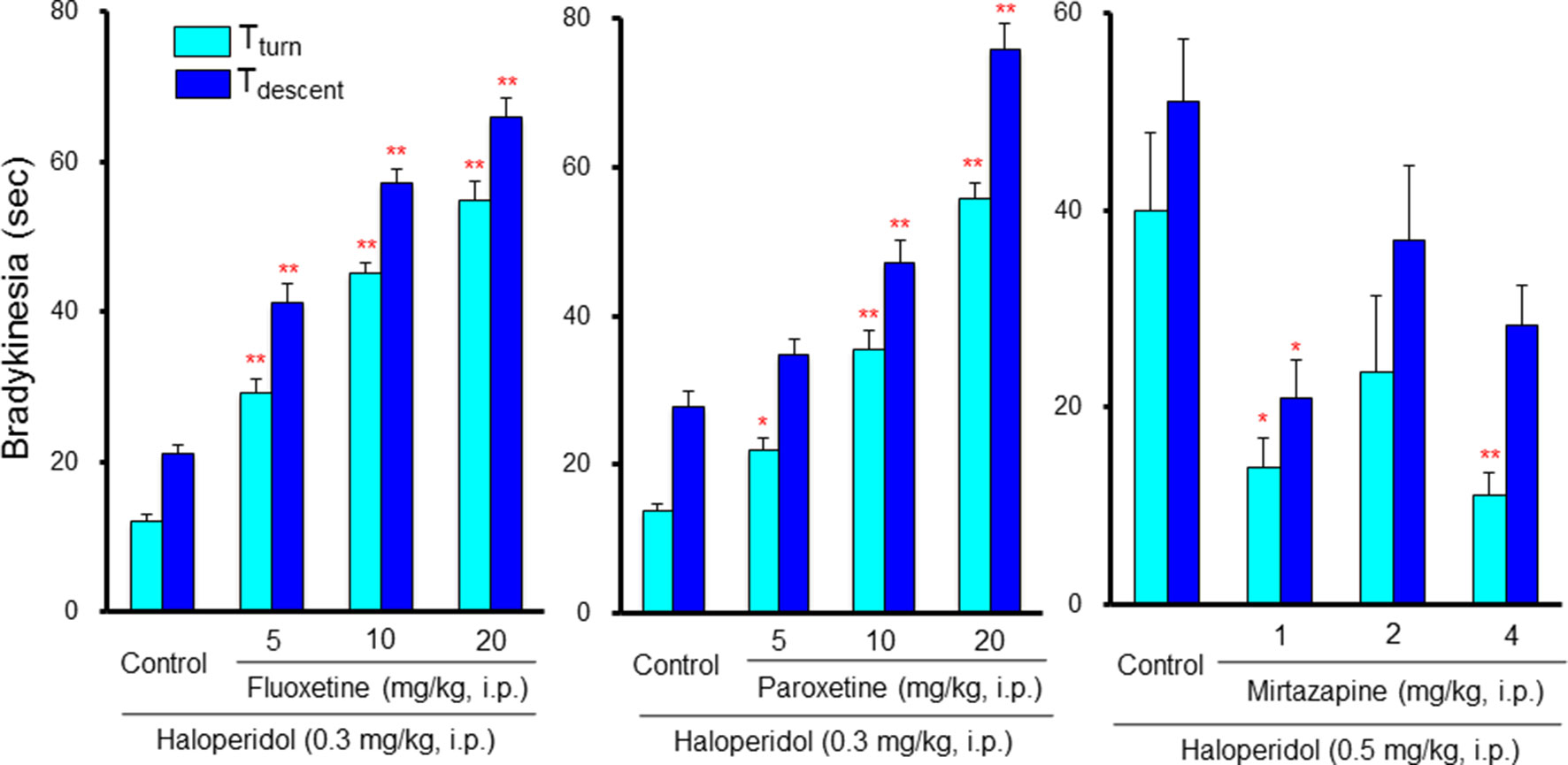
Figure 7 Interactions between antidepressants and antipsychotics in induction of extrapyramidal side effects (EPS). Bradykinesia was estimated by the pole test as described in Figure 6 legend. Although low dose (0.3 mg/kg) of haloperidol showed only weak effects in the pole test, combined treatment with selective serotonin reuptake inhibitors, fluoxetine and paroxetine, markedly potentiated haloperidol-induced bradykinesia in a synergistic manner. By contrast, the tetracyclic antidepressant mirtazapine, which possesses α2, 5-HT2 and 5-HT3 antagonistic actions, reduced bradykinesia induced by a moderate dose (0.5 mg/kg) of haloperidol. *P<0.05, **P,0.01; Significantly different from the control values. This figure is quoted and arranged from Prog. Neuro-Psychopharmacol. Biol. Psychiatry 38, 252, 2012.
In contrast to SSRIs and TCAs, tetracyclic antidepressants (mirtazapine and mianserin) did not augment, but rather attenuated antipsychotic-induced EPS (Tatara et al., 2012) (Figure 7). Thus, it seems likely that tetracyclic antidepressants are superior to SSRIs or TCAs in modulating EPS in combined treatment of BPSD with antipsychotics. Since the blockade of α2 receptors reportedly reduced antipsychotic-induced EPS (Imaki et al., 2009), EPS reduction by tetracyclic antidepressants is probably due to the α2 blocking action in addition to their 5-HT2 and 5-HT3 blocking activities.
We reviewed antipsychotic use in BPSD treatment focusing on EPS, the most frequent side effects associated with the striatal D2 receptor blockade. Antipsychotic-induced EPS significantly disrupts activities of daily life and impairs the quality of life in the elderly patients with dementia. Therefore, information on the mechanisms and the drug interactions in modulating EPS induction are necessary to achieve proper pharmacotherapy of BPSD. In this regard, we should be very careful not only about EPS liability of antipsychotics by itself, but also about the interaction of antipsychotics with anti-Alzheimer’s disease drugs and antidepressant drugs.
Atypical antipsychotics (e.g., SDAs, MARTAs, and D2 partial agonists) are now the first line drug to treat psychosis and inappropriate behaviors (e.g., agitation and aggression) in patients with dementia. But, we should pay more attention to individual pharmacological characteristics of the atypical drug, especially their interactions with 5-HT receptor subtypes. Although most SDAs or MARTAs commonly possess high affinities to 5-HT2 receptors, many atypical antipsychotics shows a differential binding profile each other, interacting with various monoamine receptors (Farah, 2005). In fact, olanzapine additionally show high affinities for 5-HT3 and 5-HT6 receptors and acts as antagonist (Bymaster et al., 2001). In addition to 5-HT2 receptors, the SDA antagonist lurasidone also binds to 5-HT1A receptors and acts as a partial agonist (Ishibashi et al., 2010). Furthermore, the dopamine D2 partial agonist aripiprazole also binds to 5-HT1A and 5-HT2 receptors, and acts as a partial agonist and an antagonist, respectively (Stark et al., 2007). Since the actions of these agents with 5-HT receptor subtypes can reduce EPS caused by combined treatment of antipsychotics with anti-Alzheimer’s disease drugs and antidepressants, they could be a favorable BPSD treatment in terms of EPS management.
Among anti-Alzheimer’s disease drugs, the NMDA antagonist memantine appears superior to ChEIs in the combined BPSD therapy with antipsychotics as it attenuates antipsychotic-induced EPS. Likewise, the tetracyclic antidepressants (mirtazapine and mianserin) are recommended for combined use with antipsychotics to treat BPSD. Unlike 5-HT reuptake inhibitors (e.g., SSRIs, SNRI, and TCAs), these agents do not augment EPS induction, but alleviate antipsychotic-induced EPS, which is possibly by blocking α2, 5-HT2 and 5-HT3 receptors (Imaki et al., 2009; Ohno et al., 2011).
This article provides information on the safe usage of antipsychotics in adjunctive therapy for BPSD in patients with dementia. The crucial roles of 5-HT receptors, especially 5-HT1A, 5-HT2, 5-HT3, and 5-HT6 receptors, in modulating antipsychotic-induced EPS were revealed. Although antipsychotic drugs are effective for psychosis, agitation, excitation, and abnormal behaviors, we should be very careful about drug selection in the combined use of antipsychotics with anti-Alzheimer’s disease drugs or antidepressants. Specifically, ChEIs and 5-HT reuptake inhibitors (SSRIs, SNRI, and TCAs) markedly potentiate antipsychotic-induced EPS in a synergistic manner. In contrast, the NMDA antagonist (memantine) or the tetracyclic antidepressants (mirtazapine and mianserin) seem to be more suitable for adjunctive therapy of cognitive impairment and mood disorders of BPSD, respectively. Furthermore, antipsychotics which have 5-HT1A agonistic actions or 5-HT2, 5-HT3, and 5-HT6 antagonistic actions appear to be useful for adjunctive BPSD treatment.
YO drafted the initial manuscript. All authors (YO, NK, SS) improved, contributed to and agreed on the final version of the manuscript.
This study was partly supported by a research grant from by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (YO:17K08324, SS:16K21501) and from the Smoking Research Foundation (YO).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Akhondzadeh, S., Mohammadi, N., Noroozian, M., Karamghadiri, N., Ghoreishi, A., Jamshidi, A. H., et al. (2009). Added ondansetron for stable schizophrenia: a double blind, placebo controlled trial. Schizophr. Res. 107, 206–212. doi: 10.1016/j.schres.2008.08.004
Anttila, S. A., Leininen, E. V. (2001). A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 7, 249–264. doi: 10.1111/j.1527-3458.2001.tb00198.x
Barnes, N. M., Sharp, T. (1999). A review of central 5-HT receptors and their function. Neuropharmacology 38, 1083–1152. doi: 10.1016/S0028-3908(99)00010-6
Baumgarten, H. G., Grozdanovic, Z. (1995). Psychopharmacology of central serotonergic systems. Pharmacopsychiatry 28, 73–79. doi: 10.1055/s-2007-979623
Bonsi, P., Cuomo, D., Ding, J., Sciamanna, G., Ulrich, S., Tscherter, A., et al. (2007). Endogenous serotonin excites striatal cholinergic interneurons via the activation of 5-HT2C, 5-HT6, and 5-HT7 serotonin receptors: implications for extrapyramidal side effects of serotonin reuptake inhibitors. Neuropsychopharmacology 32, 1840–1854. doi: 10.1038/sj.npp.1301294
Brimelow, R. E., Wollin, J. A., Byrne, G. J., Dissanayaka, N. N. (2019). Prescribing of psychotropic drugs and indicators for use in residential aged care and residents with dementia. Int. Psychogeriatr. 31, 837–847. doi: 10.1017/S1041610218001229
Bymaster, F. P., Falcone, J. F., Bauzon, D., Kennedy, J. S., Schenck, K., DeLapp, N. W., et al. (2001). Potent antagonism of 5-HT3 and 5-HT6 receptors by olanzapine. Eur. J. Pharmacol. 430, 341–349. doi: 10.1016/S0014-2999(01)01399-1
Damsma, G., de Boer, P., Westerink, B. H., Fibiger, H. C. (1990). Dopaminergic regulation of striatal cholinergic interneurons: an in vivo microdialysis study. Naunyn Schmiedebergs Arch. Pharmacol. 342, 523–527. doi: 10.1007/BF00169040
Davidson, M., Galderisi, S., Weiser, M., Werbeloff, N., Fleischhacker, W. W., Keefe, R. S., et al. (2009). Cognitive effects of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: a randomized, open-label clinical trial (EUFEST). Am. J. Psychiatry 166, 675–682. doi: 10.1176/appi.ajp.2008.08060806
DeBattista, C., DeBattista, K. (2010). Safety considerations of the use of second generation antipsychotics in the treatment of major depression: extrapyramidal and metabolic side effects. Curr. Drug Saf. 5, 263–266. doi: 10.2174/157488610791698325
DeBoer, P., Abercrombie, E. D. (1996). Physiological release of striatal acetylcholine in vivo: modulation by D1 and D2 dopamine receptor subtypes. J. Pharmacol. Exp. Ther. 277, 775–783.
Devshi, R., Shaw, S., Elliott-King, J., Hogervorst, E., Hiremath, A., Velayudhan, L., et al. (2015). Prevalence of Behavioural and Psychological Symptoms of Dementia in Individuals with Learning Disabilities. Diagnostics 5, 564–576. doi: 10.3390/diagnostics5040564
Farah, A. (2005). Atypicality of atypical antipsychotics. Prim. Care Companion J. Clin. Psychiatry 7, 268–274. doi: 10.4088/PCC.v07n0602
Fernández, J., Alonso, J. M., Andrés, J. I., Cid, J. M., Díaz, A., Iturrino, L., et al. (2005). Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. J. Med. Chem. 48, 1709–1712. doi: 10.1021/jm049632c
Fibiger, H. C. (1991). Cholinergic mechanisms in learning, memory and dementia: a review of recent evidence. Trends Neurosci. 14, 220–223. doi: 10.1016/0166-2236(91)90117-D
Gill, H. S., DeVance, C. L., Risch, S. C. (1997). Extrapyramidal symptoms associated with cyclic antidepressant treatment: a review of the literature and consolidating hypotheses. J. Clin. Psychopharmacol. 17, 377–389. doi: 10.1097/00004714-199710000-00007
Gillman, P. K. (2006). A systematic review of the serotonergic effects of Mirtazapine in humans: implications for its dual action status. Hum. Psychopharmacol. 21, 117–125. doi: 10.1002/hup.750
Govoni, S., Racchi, M., Masoero, E., Zamboni, M., Ferini-Strambi, L. (2001). Extrapyramidal symptoms and antidepressant drugs: neuropharmacological aspects of a frequent interaction in the elderly. Mol. Psychiatry 6, 134–142. doi: 10.1038/sj.mp.4000801
Haddad, P. M., Dursun, S. M. (2008). Neurological complications of psychiatric drugs: clinical features and management. Hum. Psychopharmacol. Suppl 1, 15–26. doi: 10.1002/hup.918
Imaki, J., Mae, Y., Shimizu, S., Ohno, Y. (2009). Therapeutic potential of α2 adrenoceptor antagonism for antipsychotic-induced extrapyramidal motor disorders. Neurosci. Lett. 454, 143–147. doi: 10.1016/j.neulet.2009.03.001
Ishibashi, T., Horisawa, T., Tokuda, K., Ishiyama, T., Ogasa, M., Tagashira, R., et al. (2010). Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J. Pharmacol. Exp. Ther. 334, 171–181. doi: 10.1124/jpet.110.167346
Jin, B., Liu, H. (2019). Comparative efficacy and safety of therapy for the behavioral and psychological symptoms of dementia: a systemic review and Bayesian network meta-analysis. J. Neurol. doi: 10.1007/s00415-019-09200-8
Kales, H. C., Lyketsos, C. G., Miller, E. M., Ballard, C. (2019). Management of behavioral and psychological symptoms in people with Alzheimer’s disease: an international Delphi consensus. Int. Psychogeriatr. 31, 83–90. doi: 10.1017/S1041610218000534
Kapur, S., Remington, G. (2001). Atypical antipsychotics: new directions and new challenges in the treatment of schizophrenia. Ann. Rev. Med. 52, 503–517. doi: 10.1146/annurev.med.52.1.503
Keefe, R. S., Bilder, R. M., Davis, S. M., Harvey, P. D., Palmer, B. W., Gold, J. M., et al. (2007). Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Arch. Gen. Psychiatry 64, 633–647. doi: 10.1001/archpsyc.64.6.633
Kozman, M. N., Wattis, J., Curran, S. (2006). Pharmacological management of behavioural and psychological disturbance in dementia. Hum. Psychopharmacol. 21, 1–12. doi: 10.1002/hup.745
Lee, P. E., Gill, S. S., Freedman, M., Bronskill, S. E., Hillmer, M. P., Rochon, P. A. (2004). Atypical antipsychotic drugs in the treatment of behavioural and psychological symptoms of dementia: systematic review. BMJ 329, 75. doi: 10.1136/bmj.38125.465579.55
Lieberman, J. A., Stroup, T. S., McEvoy, J. P., Swartz, M. S., Rosenheck, R. A., Perkins, D. O., et al. (2005). Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 353, 1209–1223. doi: 10.1056/NEJMoa051688
Mignon, L., Wolf, W. A. (2002). Postsynaptic 5-HT1A receptors mediate an increase in locomotor activity in the monoamine-depleted rat. Psychopharmacology 163, 85–94. doi: 10.1007/s00213-002-1121-3
Mombereau, C., Arnt, J., Mørk, A. (2017). Involvement of presynaptic 5-HT1A receptors in the low propensity of brexpiprazole to induce extrapyramidal side effects in rats. Pharmacol. Biochem. Behav. 153, 141–146. doi: 10.1016/j.pbb.2016.12.015
Neal-Beliveau, B. S., Joyce, J. N., Lucki, I. (1993). Serotonergic involvement in haloperidol-induced catalepsy. J. Pharmacol. Exp. Ther. 265, 207–217.
O’Brien, J. (2003). Behavioral symptoms in vascular cognitive impairment and vascular dementia. Int. Psychogeriatr. 15 Suppl 1, 133–138. doi: 10.1017/S1041610203009098
O’Donnell, B. F., Drachman, D. A., Barnes, H. J., Peterson, K. E., Swearer, J. M., Lew, R. A. (1992). Incontinence and troublesome behaviors predict institutionalization in dementia. J. Geriatr. Psychiatry Neurol. 5, 45–52. doi: 10.1177/002383099200500108
Ohno, Y., Ishida-Tokuda, K., Ishibashi, T., Sakamoto, H., Tagashira, R., Horisawa, T., et al. (1997). Potential role of 5-HT2 and D2 receptor interaction in the atypical antipsychotic action of the novel succimide derivative, perospirone. Pol. J. Pharmacol. 49, 213–219.
Ohno, Y., Shimizu, S., Imaki, J., Ishihara, S., Sofue, N., Sasa, M., et al. (2008a). Anticataleptic 8-OH-DPAT preferentially counteracts with haloperidol-induced Fos expression in the dorsolateral striatum and the core region of the nucleus accumbens. Neuropharmacology 55, 717–723. doi: 10.1016/j.neuropharm.2008.06.005
Ohno, Y., Shimizu, S., Imaki, J., Ishihara, S., Sofue, N., Sasa, M., et al. (2008b). Evaluation of the antibradykinetic actions of 5-HT1A agonists using the mouse pole test. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 1302–1307. doi: 10.1016/j.pnpbp.2008.04.005
Ohno, Y., Shimizu, S., Imaki, J. (2009). Effects of tandospirone, a 5-HT1A agonistic anxiolytic agent, on haloperidol-induced catalepsy and forebrain Fos expression in mice. J. Pharmacol. Sci. 109, 593–599. doi: 10.1254/jphs.08313FP
Ohno, Y., Imaki, J., Mae, Y., Takahashi, T., Tatara, A. (2011). Serotonergic modulation of extrapyramidal motor disorders in mice and rats: role of striatal 5-HT3 and 5-HT6 receptors. Neuropharmacology 60, 201–208. doi: 10.1016/j.neuropharm.2010.08.019
Ohno, Y., Tatara, A., Shimizu, S., Sasa, M., (2012). “Management of cognitive impairments in schizophrenia: the therapeutic role of 5-HT receptors,” in Schizophrenia Research: Recent Advances. Editor Sumiyoshi, T. (NY: Nova Science Publishers, Inc.), 323–338.
Ohno, Y., Shimizu, S., Tokudome, K. (2013). Pathophysiological roles of serotonergic system in regulating extrapyramidal motor function. Biol. Pharm. Bull. 36, 1396–1400. doi: 10.1248/bpb.b13-00310
Ohno, Y., Shimizu, S., Tokudome, K., Kunisawa, N., Sasa, M. (2015). New insight into the therapeutic role of the serotonergic system in Parkinson’s disease. Prog. Neurobiol. 134, 104–121. doi: 10.1016/j.pneurobio.2015.09.005
Ohno, Y. (2019). “Serotonin receptors as the therapeutic target for central nervous system disorders,” in Serotonin: The mediator that spans evolution. Eds. Pilowsky, P. M. (London: Elsevier), 369–390. doi: 10.1016/B978-0-12-800050-2.00018-8
Prince, M., Wimo, A., Guerchet, M., Ali, G. C., Wu, Y. T., Prina, M., (2015). “World alzheimer report 2015 — The global impact of dementia: an analysis of prevalence, incidence, cost and trends,” (Alzheimer’s Disease International, London).
Remington, G., Kapur, S. (1999). D2 and 5-HT2 receptor effects of antipsychotics: bridging basic and clinical findings using PET. J. Clin. Psychiatry 60 Suppl 10, 15–19.
Rosdinom, R., Zarina, M. Z., Zanariah, M. S., Marhani, M., Suzaily, W. (2013). Behavioural and psychological symptoms of dementia, cognitive impairment and caregiver burden in patients with dementia. Prev. Med. 57 Suppl, S67–S69. doi: 10.1016/j.ypmed.2012.12.025
Salamone, J. D., Correa, M., Carlson, B. B., Wisniecki, A., Mayorga, A. J., Nisenbaum, E., et al. (2001). Neostriatal muscarinic receptor subtypes involved in the generation of tremulous jaw movements in rodents implications for cholinergic involvement in parkinsonism. Life Sci. 68, 2579–2584. doi: 10.1016/S0024-3205(01)01055-4
Samii, A., Nutt, J. G., Ransom, B. (2004). Parkinson’s disease. Lancet 363, 1783–1793. doi: 10.1016/S0140-6736(04)16305-8
Schmitt, B., Bernhardt, T., Moeller, H. J., Heuser, I., Frölich, L. (2004). Combination therapy in Alzheimer’s disease: a review of current evidence. CNS Drugs 18, 827–844. doi: 10.2165/00023210-200418130-00001
Shimizu, S., Tatara, A., Imaki, J., Ohno, Y. (2010). Role of cortical and striatal 5-HT1A receptors in alleviating antipsychotic-induced extrapyramidal disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 877–881. doi: 10.1016/j.pnpbp.2010.04.005
Shimizu, S., Mizuguchi, Y., Ohno, Y. (2013a). Improving the treatment of schizophrenia: role of 5-HT receptors in modulating cognitive and extrapyramidal motor functions. CNS Neurol. Disord. Drug Targets 12, 861–869. doi: 10.2174/18715273113129990088
Shimizu, S., Mizuguchi, Y., Tatara, A., Kizu, T., Andatsu, S., Sobue, A., et al. (2013b). 5-HT1A agonist alleviates serotonergic potentiation of extrapyramidal disorders via postsynaptic mechanisms. Prog. Neuropsychopharmacol. Biol. Psychiatry 46, 86–91. doi: 10.1016/j.pnpbp.2013.06.016
Shimizu, S., Mizuguchi, Y., Sobue, A., Fujiwara, M., Morimoto, T., Ohno, Y. (2015). Interaction between anti-Alzheimer and antipsychotic drugs in modulating extrapyramidal motor disorders in mice. J. Pharmacol. Sci. 127, 439–445. doi: 10.1016/j.jphs.2015.03.004
Silva, S. R., Futuro-Neto, H. A., Pires, J. G. (1995). Effects of 5-HT3 receptor antagonists on neuroleptic-induced catalepsy in mice. Neuropharmacology 34, 97–99. doi: 10.1016/0028-3908(94)00146-J
Silva, T., Reis, J., Teixeira, J., Borges, F. (2014). Alzheimer’s disease, enzyme targets and drug discovery struggles: from natural products to drug prototypes. Ageing Res. Rev. 15, 116–145. doi: 10.1016/j.arr.2014.03.008
Stark, A. D., Jordan, S., Allers, K. A., Bertekap, R. L., Chen, R., Mistry Kannan, T., et al. (2007). Interaction of the novel antipsychotic aripiprazole with 5-HT1A and 5-HT2A receptors: functional receptor-binding and in vivo electrophysiological studies. Psychopharmacology 190, 373–382. doi: 10.1007/s00213-006-0621-y
Sturm, A. S., Trinkley, K. E., Porter, K., Nahata, M. C. (2018). Efficacy and safety of atypical antipsychotics for behavioral symptoms of dementia among patients residing in long-term care. Int. J. Clin. Pharm. 40, 135–142. doi: 10.1007/s11096-017-0555-y
Tatara, A., Shimizu, S., Shin, N., Sato, M., Sugiuchi, T., Imaki, J., et al. (2012). Modulation of antipsychotic-induced extrapyramidal side effects by medications for mood disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 38, 252–259. doi: 10.1016/j.pnpbp.2012.04.008
Trifirò, G., Spina, E., Gambassi, G. (2009). Use of antipsychotics in elderly patients with dementia: do atypical and conventional agents have a similar safety profile? Pharmacol. Res. 59, 1–12. doi: 10.1016/j.phrs.2008.09.017
van der Linde, R. M., Dening, T., Matthews, F. E., Brayne, C. (2014). Grouping of behavioural and psychological symptoms of dementia. Int. J. Geriatr. Psychiatry 29, 562–568. doi: 10.1002/gps.4037
Wadenberg, M. L., Young, K. A., Richter, J. T., Hicks, P. B. (1999). Effects of local application of 5-hydroxytryptamine into the dorsal or median raphe nuclei on haloperidol-induced catalepsy in the rat. Neuropharmacology 38, 151–156. doi: 10.1016/S0028-3908(98)00162-2
Wikström, H. V., Mensonides-Harsema, M. M., Cremers, T. I., Moltzen, E. K., Arnt, J. (2002). Synthesis and pharmacological testing of 1,2,3,4,10,14b– hexahydro-6-methoxy-2-methyldibenzo[c,f]pyrazino[1,2-a]azepin and its enantiomers in comparison with the two antidepressants mianserin and mirtazapine. J. Med. Chem. 45, 3280–3285. doi: 10.1021/jm010566d
Wood, M. D., Thomas, D. R., Watkins, C. J., Newberry, N. R. (1993). Stereoselective interaction of mianserin with 5-HT3 receptors. J. Pharm. Pharmacol. 45, 711–714. doi: 10.1111/j.2042-7158.1993.tb07094.x
Zhang, Z. J., Kang, W. H., Li, Q., Wang, X. Y., Yao, S. M., Ma, A. Q. (2006). Beneficial effects of ondansetron as an adjunct to haloperidol for chronic, treatment-resistant schizophrenia: a double-blind, randomize, placebo-controlled study. Schizophr. Res. 88, 102–110 doi: 10.1016/j.schres.2006.07.010.
Keywords: behavioral and psychological symptoms of dementia (BPSD), extrapyramidal side effects (EPS), antipsychotics, anti-Alzheimer’s disease drugs, antidepressants, 5-HT receptors
Citation: Ohno Y, Kunisawa N and Shimizu S (2019) Antipsychotic Treatment of Behavioral and Psychological Symptoms of Dementia (BPSD): Management of Extrapyramidal Side Effects. Front. Pharmacol. 10:1045. doi: 10.3389/fphar.2019.01045
Received: 14 June 2019; Accepted: 19 August 2019;
Published: 17 September 2019.
Edited by:
Lydia Gimenez-Llort, Autonomous University of Barcelona, SpainCopyright © 2019 Ohno, Kunisawa and Shimizu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yukihiro Ohno, eW9obm9AZ2x5Lm91cHMuYWMuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.