- 1Department of Urology, First People’s Hospital of Shangqiu City, Shangqiu, China
- 2Department of Obstetrics and Gynecology, The General Hospital of Western Theater Command, Chengdu, China
- 3Department of Urology, Panzhihua Central Hospital, Panzhihua, China
- 4Department of Urology, The General Hospital of China National Petroleum Corporation in Jilin, Jilin, China
- 5Department of Urology, Shanxi Provincial Cancer Hospital, Taiyuan, China
Background: The prognostic role of programmed cell death-ligand 1 (PD-L1) in bladder cancer has been investigated in previous studies, but the results remain inconclusive. Therefore, we carried out a meta-analysis to evaluate the prognostic significance of PD-L1 in patients with bladder cancer.
Methods: The electronic databases PubMed, Embase, Web of Science, and Cochrane Library were searched. The association between PD-L1 expression and survival outcomes and clinicopathological factors was analyzed by hazard ratios (HRs) or odds ratios (ORs) and 95% confidence intervals (CIs).
Results: A total of 11 studies containing 1,697 patients were included in the meta-analysis. High PD-L1 expression was associated with poor overall survival (OS) (HR = 1.83, 95% CI = 1.24–2.71, p = 0.002). There was nonsignificant association between PD-L1 and recurrence-free survival (RFS) (HR = 1.43, 95% CI = 0.89–2.29, p = 0.134), cancer-specific survival (CSS) (HR = 1.51, 95% CI = 0.80–2.87, p = 0.203), or disease-free survival (DFS) (HR = 1.53, 95% CI = 0.88–2.65, p = 0.13). Furthermore, high PD-L1 was significantly correlated with higher tumor stage (OR = 3.9, 95% CI = 2.71–5.61, p < 0.001) and distant metastasis (OR = 2.5, 95% CI = 1.22–5.1, p = 0.012), while PD-L1 overexpression was not correlated with sex, tumor grade, lymph node status, and multifocality.
Conclusions: The meta-analysis suggested that PD-L1 overexpression could predict worse survival outcomes in bladder cancer. High PD-L1 expression may act as a potential prognostic marker for patients with bladder cancer.
Introduction
Bladder cancer is the most common malignancy of the urinary tract, accounting for 80,470 new cases and 17,670 deaths in 2019 alone in the United States (Siegel et al., 2019). When diagnosed, up to 75% of patients present with non-muscle-invasive bladder cancer (NMIBC), about 20% present with muscle-invasive bladder cancer (MIBC), and 5% would have metastatic disease. Although patients with NMIBC have a relatively good prognosis, the prognosis of regional and distant metastatic disease is poor, with 5-year survival rates of 35% and 5%, respectively (National Cancer Institute SEER Program). Therefore, investigation of novel biomarkers to stratify patients is important for clinical management (Slovin, 2017).
Cancer immunoediting is a process consisting of immunosurveillance and tumor development (Mittal et al., 2014). Programmed cell death-1 (PD-1) and its ligand programmed cell death-ligand 1 (PD-L1) have an important role in the regulation of responses of our immune system (Errico, 2015). PD-L1 is also known as B7-H1, CD274, which is expressed on many cancer cells. PD-L1 expression has shown prognostic value in various tumors including pancreatic cancer (Gao et al., 2018), colorectal cancer (Shen et al., 2019), and non-small cell lung cancer (Ma et al., 2018). Recently, many studies (Nakanishi et al., 2007; Boorjian et al., 2008; Wang et al., 2009; Xylinas et al., 2014; Bellmunt et al., 2015; Wu et al., 2016; Noro et al., 2017; Li et al., 2018b; Pichler et al., 2018; Owyong et al., 2019; Wang et al., 2019) also investigated the prognostic significance of PD-L1 expression in bladder cancer, but the results remain controversial. Therefore, we collected relevant data and performed a meta-analysis to quantify the prognostic role of PD-L1 and analyze the relationship of PD-L1 and clinicopathological parameters in bladder cancer.
Methods
Literature Search
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2009). The research of PubMed, Embase, Web of Science, and Cochrane Library identified relevant studies published in English. The last search was updated on March 2019. A comprehensive search strategy was performed based on the following terms: “programmed death ligand-1,” “PD-L1,” “B7-H1,” “CD274,” “bladder cancer,” “bladder neoplasm,” “bladder tumor,” and “bladder urothelial carcinoma.” The references of the included studies were also manually checked to identify relevant publications. Ethical approval was waived because we just collected the data from available publications.
Eligibility Criteria
The inclusion criteria were as follows: 1) patients were histologically diagnosed to have bladder cancer; 2) PD-L1 was detected via immunohistochemical staining (IHC); 3) the relationship between PD-L1 and survival of bladder cancer was studied; and 4) references are published in English. Exclusion criteria were as follows: 1) duplicate studies; 2) studies provided incomplete data; and 3) meeting abstracts, case reports, reviews, or animal studies.
Data Extraction and Quality Assessment
Two independent investigators extracted the following information from the eligible studies: first author, publication year, country, detection method, sample size, study design, survival analysis, age, and study period. Any disagreement was resolved by discussion. The quality of the selected articles was assessed according to the Newcastle-Ottawa Scale (NOS) (Wells et al., 2009). Total quality score of NOS was ranged from 0 to 9, and studies that scored ≥6 were considered as high-quality studies.
Statistical Analysis
Hazard ratios (HRs) and their 95% confidence intervals (CIs) were searched in the original articles or calculated by methods described by Tierney et al. (2007). The survival outcomes included overall survival (OS), recurrence-free survival (RFS), cancer-specific survival (CSS), and disease-free survival (DFS). The logHR and standard error (SE) were used to present the survival results. An observed HR > 1 implied a poorer prognosis in patients with high PD-L1 expression, while HR < 1 indicated a better prognosis. The relationship between PD-L1 expression and clinicopathological features was evaluated by odds ratios (ORs) and corresponding 95% CIs. Cochran’s Q test and Higgins I-squared statistic (I2) were used to measure the heterogeneity of the combined HRs (Higgins and Thompson, 2002). I2 > 50% and/or p < 0.1 suggested significant heterogeneity in terms of statistics, and a random-effects model was utilized. Alternatively, a fixed-effects model was applied. Begg’s test was used to detect potential publication bias (Begg and Mazumdar, 1994). All statistical analyses were conducted by using Stata version 12.0 (Stata Corporation, College Station, TX, USA). A two-sided p < 0.05 was considered statistically significant.
Results
Study Selection
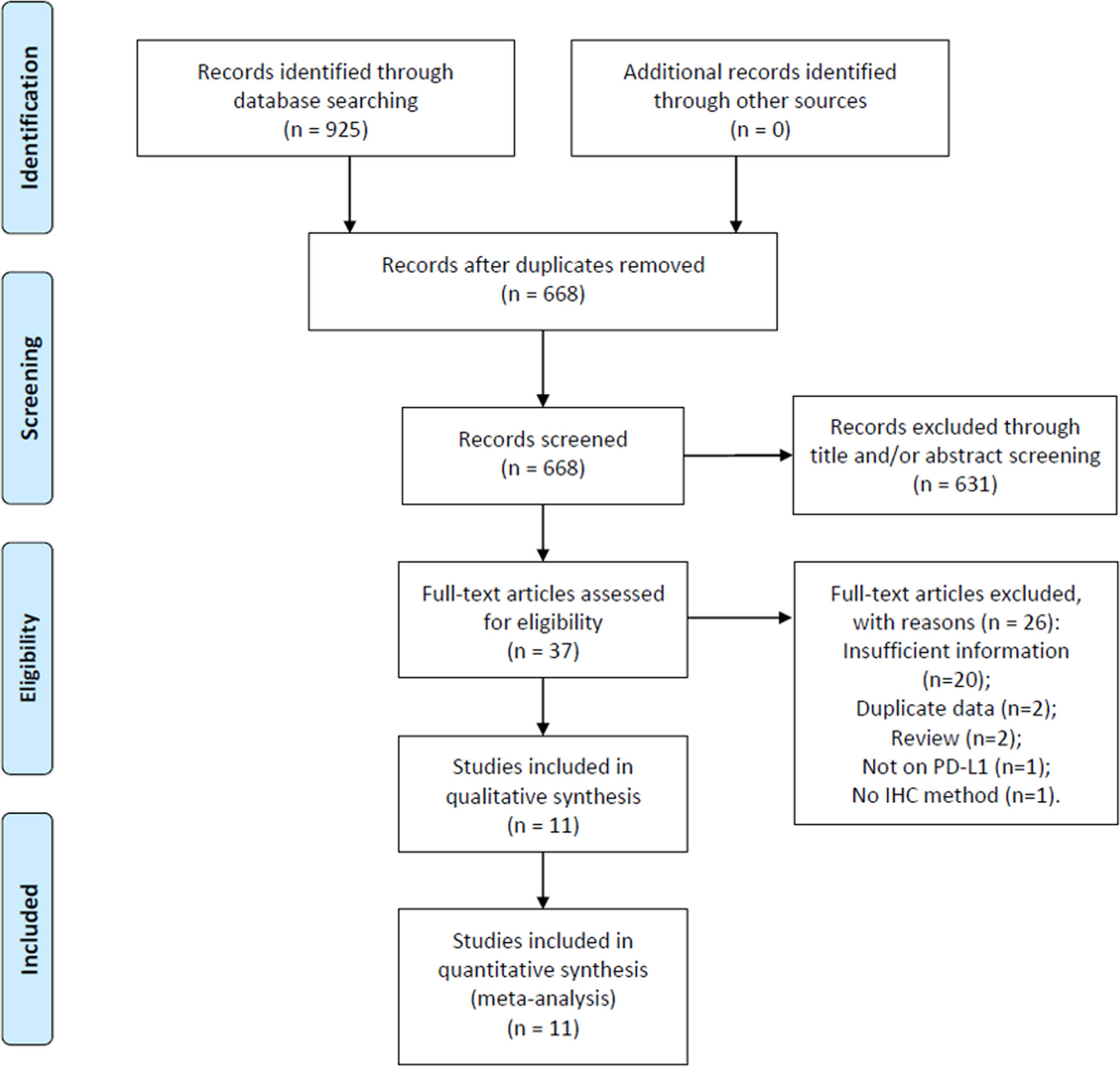
Initial literature search identified 925 records. After removal of duplicate records, 668 studies remained for further evaluation. Then, 631 recorded were excluded by scanning title and/or abstract. Thirty-seven studies were screened by full-text examination, and 26 studies were excluded for following reasons: 20 studies did not provide sufficient for analysis, 2 studies recruited overlapped patients, 2 studies were reviews, 1 study did not focus on PD-L1, and 1 study did not use IHC method for PD-L1 detection. Ultimately, 11 studies (Nakanishi et al., 2007; Boorjian et al., 2008; Wang et al., 2009; Xylinas et al., 2014; Bellmunt et al., 2015; Wu et al., 2016; Noro et al., 2017; Li et al., 2018b; Pichler et al., 2018; Owyong et al., 2019; Wang et al., 2019) were included in this meta-analysis. The flow diagram is shown in Figure 1.
Study Characteristics
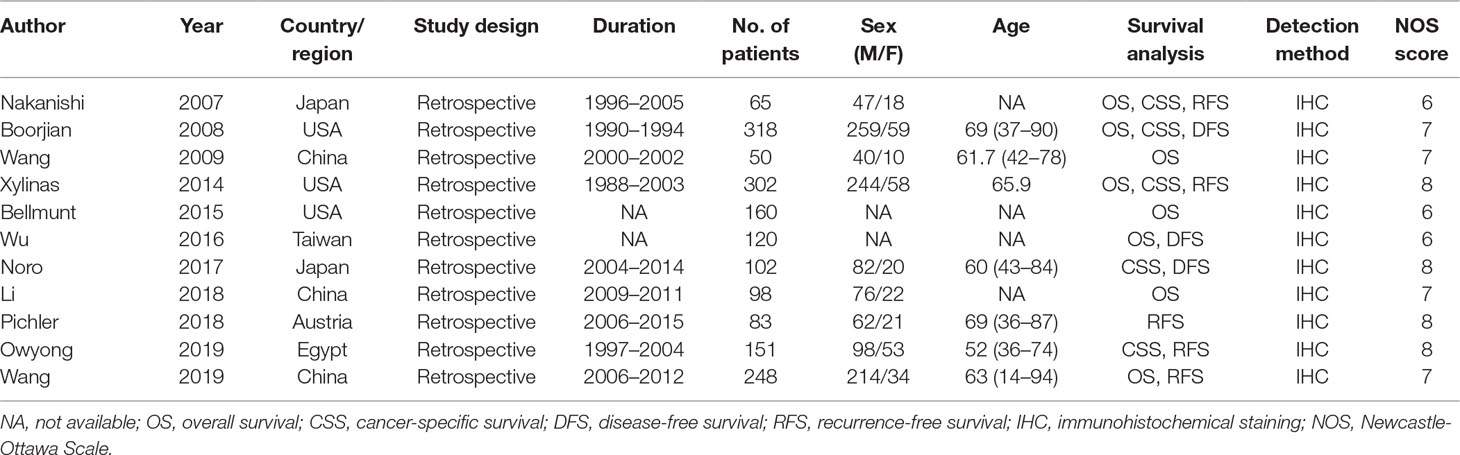
The main characteristics of eligible articles are listed in Table 1. The studies were published from 2007 to 2019. Three studies (Wang et al., 2009; Li et al., 2018b; Wang et al., 2019) were conducted in China, three were performed in United States (Boorjian et al., 2008; Xylinas et al., 2014; Bellmunt et al., 2015), two were in Japan (Nakanishi et al., 2007; Noro et al., 2017), and one each in Taiwan (Wu et al., 2016), Austria (Pichler et al., 2018) and Egypt (Owyong et al., 2019). The total sample size was 1,697, ranging from 50 to 318. All studies were a retrospective study design. Regarding clinical outcomes, eight studies reported clinicopathological factors (Boorjian et al., 2008; Wang et al., 2009; Xylinas et al., 2014; Bellmunt et al., 2015; Wu et al., 2016; Li et al., 2018b; Owyong et al., 2019; Wang et al., 2019), eight studies reported OS (Nakanishi et al., 2007; Boorjian et al., 2008; Wang et al., 2009; Xylinas et al., 2014; Bellmunt et al., 2015; Wu et al., 2016; Li et al., 2018b; Wang et al., 2019), five studies described RFS (Nakanishi et al., 2007; Xylinas et al., 2014; Pichler et al., 2018; Owyong et al., 2019; Wang et al., 2019), five studies reported CSS (Nakanishi et al., 2007; Boorjian et al., 2008; Xylinas et al., 2014; Noro et al., 2017; Owyong et al., 2019), and three studies presented DFS (Boorjian et al., 2008; Wu et al., 2016; Noro et al., 2017). Furthermore, all studies were with NOS score ≥ 6, indicating that the studies were of high quality.
Impact of PD-L1 on OS, RFS, CSS, and DFS
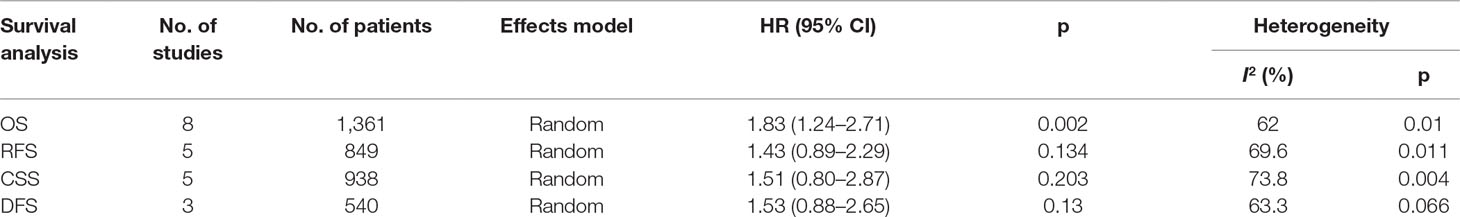
Eight studies (Nakanishi et al., 2007; Boorjian et al., 2008; Wang et al., 2009; Xylinas et al., 2014; Bellmunt et al., 2015; Wu et al., 2016; Li et al., 2018b; Wang et al., 2019) reported data on PD-L1 and OS in bladder cancer. As shown in Figure 2 and Table 2, high PD-L1 was associated with poorer OS (HR = 1.83, 95% CI = 1.24–2.71, p = 0.002). Because of significant heterogeneity (I2 = 62%, p = 0.01), a random-effects model was applied. Five studies (Boorjian et al., 2008; Xylinas et al., 2014; Pichler et al., 2018; Owyong et al., 2019; Wang et al., 2019) showed the relationship between PD-L1 and RFS. The pooled results were HR = 1.43, 95% CI = 0.89–2.29, p = 0.134, with significant heterogeneity (I2 = 69.6%, p = 0.011) (Table 2, Figure 2). The pooled data from five studies (Nakanishi et al., 2007; Boorjian et al., 2008; Xylinas et al., 2014; Noro et al., 2017; Owyong et al., 2019) suggested nonsignificant association between PD-L1 and CSS in bladder cancer (HR = 1.51, 95% CI = 0.80–2.87, p = 0.203; I2 = 73.8%, p = 0.004, Table 2, Figure 2). Moreover, three studies reported the correlation of PD-L1 and DFS (Boorjian et al., 2008; Wu et al., 2016; Noro et al., 2017). The random-effects model was applied because there was significant heterogeneity (I2 = 63.3%, p = 0.066) across the studies. The pooled HR and 95%CI were HR = 1.53, 95% CI = 0.88–2.65, p = 0.013 (Table 2, Figure 2), suggesting PD-L1 was not correlated to worse DFS.
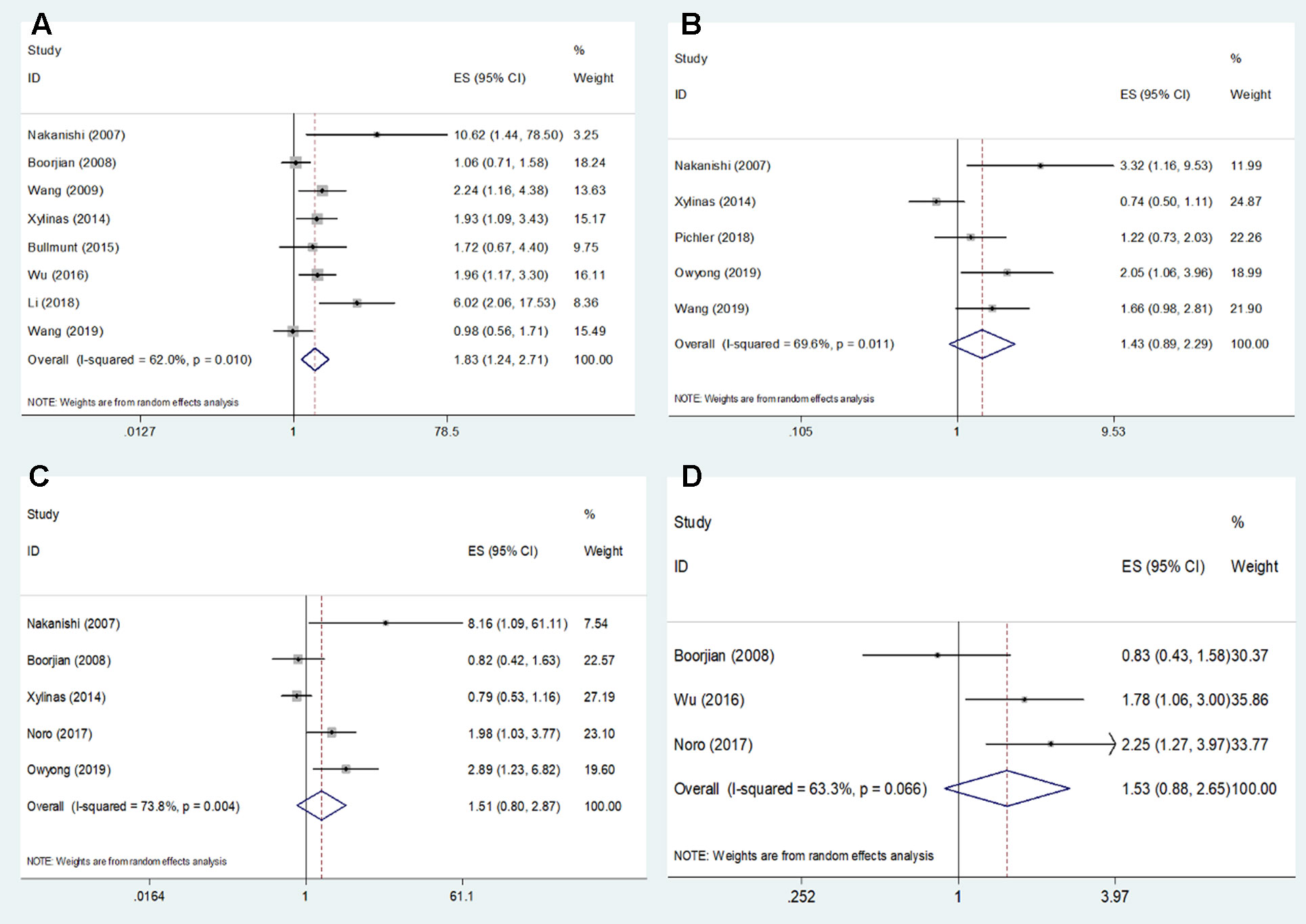
Figure 2 Forest plots describing the association between PD-L1 expression and (A) OS, (B) RFS, (C) CSS, and (D) DFS of patients with bladder cancer.
PD-L1 and Clinicopathological Features
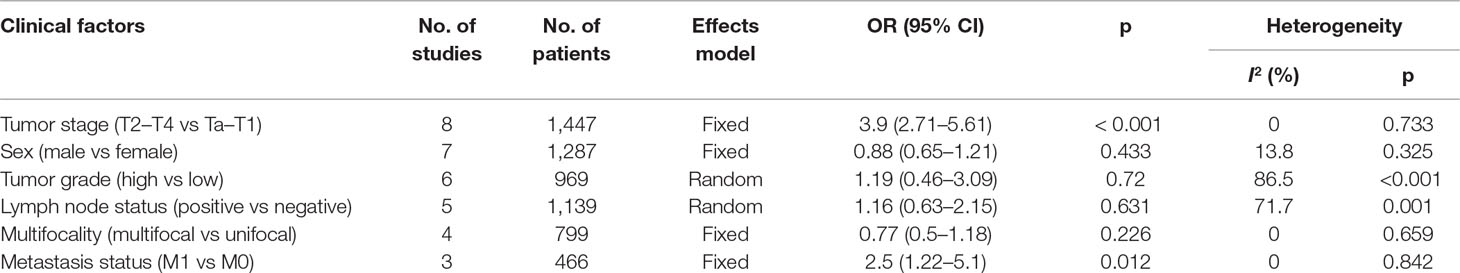
Eight studies (Boorjian et al., 2008; Wang et al., 2009; Xylinas et al., 2014; Bellmunt et al., 2015; Wu et al., 2016; Li et al., 2018b; Owyong et al., 2019; Wang et al., 2019) explored the association between PD-L1 and clinicopathological characteristics. The pooled data demonstrated that high PD-L1 was significantly correlated with higher tumor stage (OR = 3.9, 95% CI = 2.71–5.61, p < 0.001) and distant metastasis (OR = 2.5, 95% CI = 1.22–5.1, p = 0.012). However, PD-L1 overexpression was not correlated with other clinicopathological factors including sex (OR = 0.88, 95% CI = 0.65–1.21, p = 0.433), tumor grade (OR = 1.19, 95% CI = 0.46–3.09, p = 0.72), lymph node status (OR = 1.16, 95% CI = 0.63–2.15, p = 0.631), and multifocality (OR = 0.77, 95% CI = 0.5–1.18, p = 0.226). The correlation between PD-L1 and clinicopathological parameters is presented in Table 3.
Publication Bias
The assessment of the publication bias was carried out by using Begg’s funnel plot test. Begg’s p values for OS, RFS, CSS, and DFS were 0.063, 0.086, 0.221, and 0.602, respectively. Begg’s funnel plot was found to be symmetrical (Figure 3), indicating no significant publication bias in this meta-analysis.
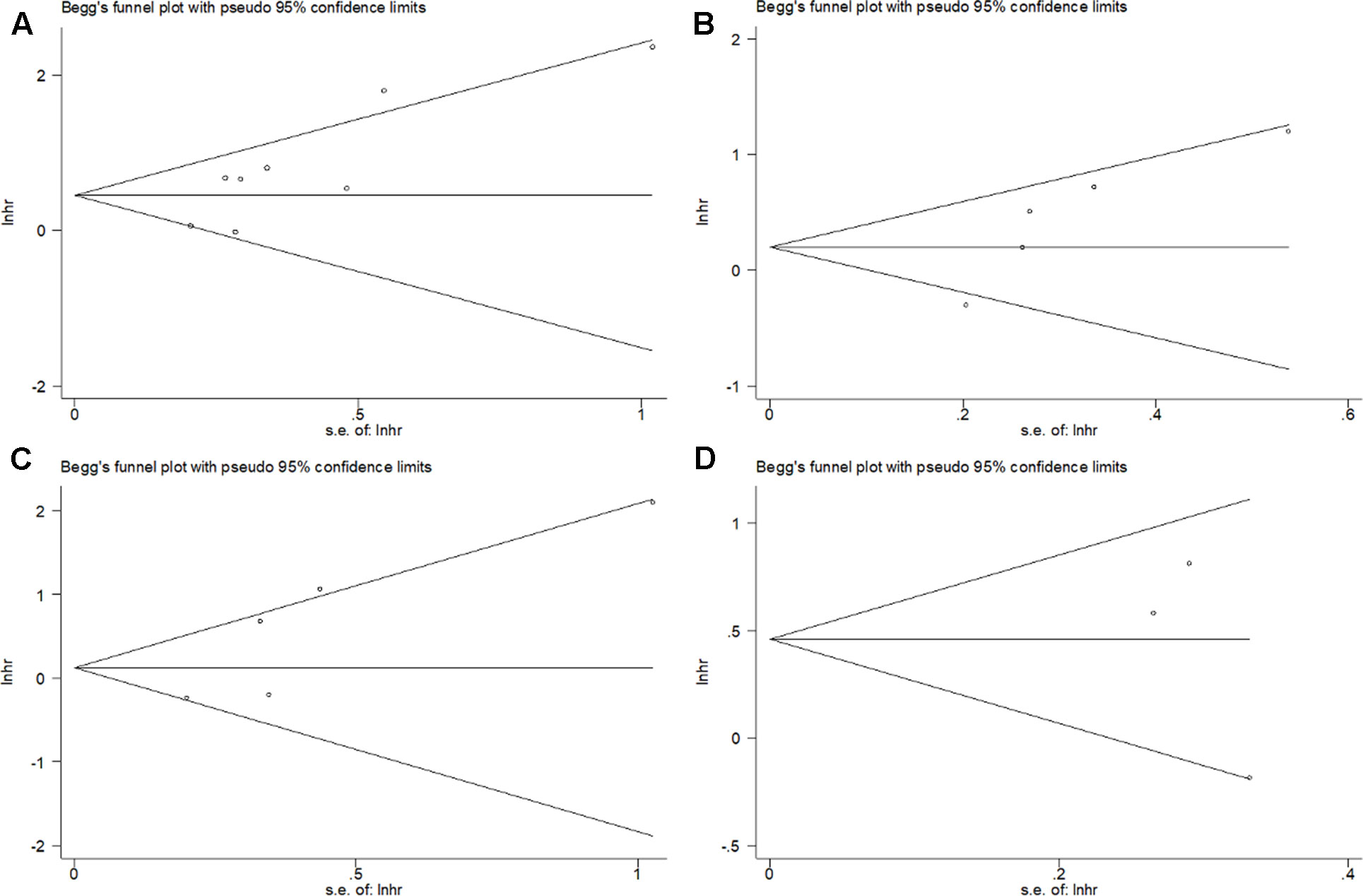
Figure 3 Begg’s funnel plot for publication bias test including PD-L1 expression and (A) OS, (B) RFS, (C) CSS, and (D) DFS in bladder cancer patients.
Discussion
In the present study, we collected information from 11 recent studies with 1,697 patients and combined the data. The results showed that elevated PD-L1 expression was associated with poorer OS. In addition, PD-L1 overexpression was also connected with higher tumor stage and distant metastasis. There was no obvious evidence of publication bias. The results suggested that PD-L1 expression may be associated with tumor progression and metastasis and could be used as a potential prognostic biomarker. To the best of our knowledge, this is the first pointed meta-analysis investigating the prognostic value of PD-L1 in patients with bladder cancer.
PD-1 and its ligands, PD-L1 and PD-L2, overexpressed in the tumor microenvironment (Riley, 2009). The interaction of PD-1/PD-L1 can inhibit T-cell activation and proliferation, cytokine production, and cytolytic function (Riley, 2009). In addition, PD-L1 can also stimulate IL-10 production in T cells to mediate immune suppression (Dong et al., 1999). PD-L1 was found to be overexpressed in multiple solid tumor types to generate an immunosuppressive tumor microenvironment (Iwai et al., 2002; Blank et al., 2005; Wang et al., 2017). In the present study, we found the association of PD-L1 and higher tumor stage and distant metastasis, which implied the role of PD-L1 in tumor development. A recent study showed that PD-L1 played a critical role in promoting epithelial-to-mesenchymal transition (EMT) phenotype of esophageal cancer (Chen et al., 2017). Another study also suggested that PD-L1 expression was a significant risk factor for nodal metastasis in cutaneous squamous cell carcinoma (Garcia-Pedrero et al., 2017). The activation of IL-6/STAT3/PD-L1 pathway was found to be involved in the EMT process in bladder cancer (Zhang et al., 2019).
A number of previous studies also reported the prognostic significance of PD-L1 in various cancers. A recent meta-analysis including 2,005 patients showed that high PD-L1 expression was associated with a poor prognosis (HR = 2.04, 95% CI = 1.18–3.54, p = 0.01) in non-Hodgkin lymphoma (Zhao et al., 2018). Li’s study showed that PD-L1 overexpression could foresee worse OS and DFS in hepatocellular carcinoma (Li et al., 2018a). In addition, another meta-analysis comprising a total of nine studies with 993 patients demonstrated that elevated PD-L1 expression was related with poor OS (HR = 1.63, 95% CI = 1.34–1.98, p < .001) and CSS (HR = 1.86, 95% CI = 1.34–2.57, p < .001) in pancreatic cancer (Hu et al., 2019). High PD-L1 expression was also correlated with poor OS in breast cancer (Zhang et al., 2017). The results of our study were in line with previous studies, suggesting the prognostic value of PD-L1 in bladder cancer. Furthermore, we also found the connection between PD-L1 and distant metastasis in bladder cancer, which may be explained by the role of PD-L1 in EMT process (Zhang et al., 2019). Recently, many studies also reported the effectiveness and patient-reported outcomes in clinical trials of PD-L1 inhibitors. Madore et al. showed that PD-L1 expression in melanoma showed marked heterogeneity within and between patients, which supported the therapeutic strategies of melanoma patients in a PD-L1-based manner (Madore et al., 2015). In addition, stage III melanoma patients with negative PD-L1 expression is associated with worse survival and immune response (Madore et al., 2016). A recent meta-analysis demonstrated that PD-L1 expression was significantly associated with mortality and clinical response to anti-PD-1/PD-L1 antibodies in metastatic melanoma patients (Gandini et al., 2016). The health-related quality of life was also better in advanced cancer patients receiving PD-1/PD-L1 inhibitors than in those receiving standard-of-care therapy (Nishijima et al., 2019). Those studies suggest that the clinical management of PD-1/PD-L1 inhibitors is complex and should be adjusted in the individual patient level.
Notably, age is also a risk factor for bladder cancer patients. In the included studies, five studies (Xylinas et al., 2014; Wu et al., 2016; Li et al., 2018b; Owyong et al., 2019; Wang et al., 2019) provided the data on age in PD-L1 (+) and PD-L1 (−) groups. However, three studies (Xylinas et al., 2014; Wu et al., 2016; Owyong et al., 2019) presented age in the format of median (range). One study (Li et al., 2018b) reported the number of patients in PD-L1 (+) and PD-L1 (−) groups using 65 years as threshold. One study used 60 years (Wang et al., 2019) to divide patients. Therefore, the quantitative analysis of PD-L1 expression and age could not be performed because of different cutoff values of age (65 and 60 years). In spite of this, we can find that patients with PD-L1 (+) expression are older than patients with PD-L1 (−) expression in four studies (Xylinas et al., 2014; Wu et al., 2016; Li et al., 2018b; Wang et al., 2019). All five studies (Xylinas et al., 2014; Wu et al., 2016; Li et al., 2018b; Owyong et al., 2019; Wang et al., 2019) reported nonsignificant association between age and PD-L1 expression (all p > 0.05). Moreover, in the analysis of association between PD-L1 expression and clinical factors, heterogeneity was found on sex, tumor grade, and lymph node status (Table 3). Because different studies may select patients with various criteria, the heterogeneity among studies may be inherent and may exist. In this occasion, we applied different effects model according to different heterogeneity.
Some limitations need to be mentioned in this meta-analysis. First, the determination of high expression of PD-L1 might vary in the studies because of different cutoff values, which may introduce potential bias. Second, the sample size was relatively small. Only 11 studies with 1,697 patients were included for analysis. For example, for CSS and DFS analysis, only five and three studies were included; the small study may compromise the credibility of the results. Third, although we did not find publication bias in the meta-analysis, the publication bias and selection bias could possibly exist. As we know, studies with significant results are inclined to be published (Koletsi et al., 2009). Therefore, the results should be treated with caution.
Conclusion
In summary, the findings of this meta-analysis suggest that elevated PD-L1 expression is associated with poor survival, higher tumor stage, and distant metastasis in bladder cancer. PD-L1 may be useful in the future as a novel prognostic factor in bladder cancer. Nevertheless, due to some limitations, well-designed, multicenter randomized controlled trials should be performed.
Data Availability
All datasets generated for this study are included in the manuscript/supplementary files.
Author Contributions
LZ, JS, and ZBL designed the study. LZ, JS, LiW, ZGL, and LeW performed the research. LZ and JS collected and analyzed the data. LZ and JS wrote the paper. LeW amended the article. ZBL acts as the submission’s guarantor and takes responsibility for the integrity of the work as a whole, from inception to published article. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Begg, C. B., Mazumdar, M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics 50 (4), 1088–1101. doi: 10.2307/2533446
Bellmunt, J., Mullane, S. A., Werner, L., Fay, A. P., Callea, M., Leow, J. J., et al. (2015). Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann. Oncol. 26 (4), 812–817. doi: 10.1093/annonc/mdv009
Blank, C., Gajewski, T. F., Mackensen, A. (2005). Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol. Immunother. 54 (4), 307–314. doi: 10.1007/s00262-004-0593-x
Boorjian, S. A., Sheinin, Y., Crispen, P. L., Farmer, S. A., Lohse, C. M., Kuntz, S. M., et al. (2008). T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin. Cancer Res. 14 (15), 4800–4808. doi: 10.1158/1078-0432.CCR-08-0731
Chen, L. J., Xiong, Y. Q., Li, J., Zheng, X., Zhou, Q., Turner, A., et al. (2017). PD-L1 expression promotes epithelial to mesenchymal transition in human esophageal cancer. Cell. Physiol. Biochem. 42 (6), 2267–2280. doi: 10.1159/000480000
Dong, H. D., Zhu, G. F., Tamada, K., Chen, L. P. (1999). B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 5 (12), 1365–1369. doi: 10.1038/70932
Errico, A. (2015). PD-1–PD-L1 axis: efficient checkpoint blockade against cancer. Nat. Rev. Clin. Oncol. 12 (2), 63. doi: 10.1038/nrclinonc.2014.221
Gandini, S., Massi, D., Mandala, M. (2016). PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: a systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 100, 88–98. doi: 10.1016/j.critrevonc.2016.02.001
Gao, H. L., Liu, L., Qi, Z. H., Xu, H. X., Wang, W. Q., Wu, C. T., et al. (2018). The clinicopathological and prognostic significance of PD-L1 expression in pancreatic cancer: a meta-analysis. Hepatobiliary and Pancreatic Dis. Int. 17 (2), 95–100. doi: 10.1016/j.hbpd.2018.03.007
Garcia-Pedrero, J. M., Martinez-Camblor, P., Diaz-Coto, S., Munguia-Calzada, P., Vallina-Alvarez, A., Vazquez-Lopez, F., et al. (2017). Tumor programmed cell death ligand 1 expression correlates with nodal metastasis in patients with cutaneous squamous cell carcinoma of the head and neck. J. Am. Acad. Dermatol. 77 (3), 527–533. doi: 10.1016/j.jaad.2017.05.047
Higgins, J. P. T., Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Stat. Med. 21 (11), 1539–1558. doi: 10.1002/sim.1186
Hu, Y., Chen, W., Yan, Z., Ma, J., Zhu, F., Huo, J. (2019). Prognostic value of PD-L1 expression in patients with pancreatic cancer: a PRISMA-compliant meta-analysis. Medicine (Baltimore) 98 (3), e14006. doi: 10.1097/MD.0000000000014006
Iwai, Y., Ishida, M., Tanaka, Y., Okazaki, T., Honjo, T., Minato, N. (2002). Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Nat. Acad. Sci. U. S. A. 99 (19), 12293–12297. doi: 10.1073/pnas.192461099
Koletsi, D., Karagianni, A., Pandis, N., Makou, M., Polychronopoulou, A., Eliades, T. (2009). Are studies reporting significant results more likely to be published? Am. J. Orthodontics and Dentofacial Orthopedics 136 (5), 632.e631–635; discussion 632–633. doi: 10.1016/j.ajodo.2009.02.024
Li, J. H., Ma, W. J., Wang, G. G., Jiang, X., Chen, X., Wu, L., et al. (2018a). Clinicopathologic significance and prognostic value of programmed cell death ligand 1 (PD-L1) in patients with hepatocellular carcinoma: a meta-analysis. Front. Immunol. 9, 2077. doi: 10.3389/fimmu.2018.02077
Li, Q., Li, F., Che, J., Zhao, Y., Qiao, C. (2018b). Expression of B7 homolog 1 (B7H1) is associated with clinicopathologic features in urothelial bladder cancer. Med. Sci. Monit. 24, 7303–7308. doi: 10.12659/MSM.910956
Ma, G. Z., Deng, Y. F., Jiang, H., Li, W., Wu, Q., Zhou, Q. H. (2018). The prognostic role of programmed cell death-ligand 1 expression in non-small cell lung cancer patients: an updated meta-analysis. Clin. Chim. Acta 482, 101–107. doi: 10.1016/j.cca.2018.03.038
Madore, J., Strbenac, D., Vilain, R., Menzies, A. M., Yang, J. Y., Thompson, J. F., et al. (2016). PD-L1 negative status is associated with lower mutation burden, differential expression of immune-related genes, and worse survival in stage III melanoma. Clin. Cancer Res. 22 (15), 3915–3923. doi: 10.1158/1078-0432.CCR-15-1714
Madore, J., Vilain, R. E., Menzies, A. M., Kakavand, H., Wilmott, J. S., Hyman, J., et al. (2015). PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigm. Cell Melanoma Res. 28 (3), 245–253. doi: 10.1111/pcmr.12340
Mittal, D., Gubin, M. M., Schreiber, R. D., Smyth, M. J. (2014). New insights into cancer immunoediting and its three component phases elimination, equilibrium and escape. Curr. Opin. Immunol. 27, 16–25. doi: 10.1016/j.coi.2014.01.004
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., Grp, P. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med. 6 (7), e1000097. doi: 10.1371/journal.pmed.1000097
Nakanishi, J., Wada, Y., Matsumoto, K., Azuma, M., Kikuchi, K., Ueda, S. (2007). Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol. Immunother. 56 (8), 1173–1182. doi: 10.1007/s00262-006-0266-z
National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts: Bladder Cancer. Available at: https://seer.cancer.gov/statfacts/html/urinb.html (Accessed November 30, 2017).
Nishijima, T. F., Shachar, S. S., Muss, H. B., Tamura, K. (2019). Patient-reported outcomes with PD-1/PD-L1 inhibitors for advanced cancer: a meta-analysis. Oncologist 24 (7), e565–e573. doi: 10.1634/theoncologist.2018-0449
Noro, D., Hatakeyama, S., Yoneyama, T., Hashimoto, Y., Koie, T., Kawaguchi, T., et al. (2017). Post-chemotherapy PD-L1 expression correlates with clinical outcomes in Japanese bladder cancer patients treated with total cystectomy. Med. Oncol. 34 (6), 117. doi: 10.1007/s12032-017-0977-3
Owyong, M., Lotan, Y., Kapur, P., Panwar, V., McKenzie, T., Lee, T. K., et al. (2019). Expression and prognostic utility of PD-L1 in patients with squamous cell carcinoma of the bladder. Urol. Oncol. 37 (7), 478–484. doi: 10.1016/j.urolonc.2019.02.017
Pichler, R., Fritz, J., Lackner, F., Sprung, S., Brunner, A., Horninger, W., et al. (2018). Prognostic value of testing PD-L1 expression after radical cystectomy in high-risk patients. Clin. Genitourin Cancer 16 (5), e1015–e1024. doi: 10.1016/j.clgc.2018.05.015
Riley, J. L. (2009). PD-1 signaling in primary T cells. Immunol. Rev. 229, 114–125. doi: 10.1111/j.1600-065X.2009.00767.x
Shen, Z., Gu, L., Mao, D., Chen, M., Jin, R. (2019). Clinicopathological and prognostic significance of PD-L1 expression in colorectal cancer: a systematic review and meta-analysis. World J. Surg. Oncol. 17 (1), 4. doi: 10.1186/s12957-018-1544-x
Siegel, R. L., Miller, K. D., Jemal, A. (2019). Cancer statistics, 2019. Cancer J. Clin. 69 (1), 7–34. doi: 10.3322/caac.21551
Slovin, S. F. (2017). The need for immune biomarkers for treatment prognosis and response in genitourinary malignancies. Biomark Med. 11 (12), 1149–1159. doi: 10.2217/bmm-2017-0138
Tierney, J. F., Stewart, L. A., Ghersi, D., Burdett, S., Sydes, M. R. (2007). Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8, 16. doi: 10.1186/1745-6215-8-16
Wang, B., Pan, W., Yang, M., Yang, W., He, W., Chen, X., et al. (2019). Programmed death ligand-1 is associated with tumor infiltrating lymphocytes and poorer survival in urothelial cell carcinoma of the bladder. Cancer Sci. 110 (2), 489–498. doi: 10.1111/cas.13887
Wang, Q. Q., Liu, F., Liu, L. (2017). Prognostic significance of PD-L1 in solid tumor: an updated meta-analysis. Medicine 96 (18), e6369. doi: 10.1097/MD.0000000000006369
Wang, Y., Zhuang, Q., Zhou, S., Hu, Z., Lan, R. (2009). Costimulatory molecule B7-H1 on the immune escape of bladder cancer and its clinical significance. J. Huazhong Univ. Sci. Technol. Med. Sci. 29 (1), 77–79. doi: 10.1007/s11596-009-0116-2
Wells, G. A., Shea, B., O’Connell, D., Peterson, J., Welch, V., Losos, M., et al.(2009). The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Wu, C. T., Chen, W. C., Chang, Y. H., Lin, W. Y., Chen, M. F. (2016). The role of PD-L1 in the radiation response and clinical outcome for bladder cancer. Scient. Rep. 6, 19740. doi: 10.1038/srep19740
Xylinas, E., Robinson, B. D., Kluth, L. A., Volkmer, B. G., Hautmann, R., Kufer, R., et al. (2014). Association of T-cell co-regulatory protein expression with clinical outcomes following radical cystectomy for urothelial carcinoma of the bladder. Ejso 40 (1), 121–127. doi: 10.1016/j.ejso.2013.08.023
Zhang, M. H., Sun, H. B., Zhao, S., Wang, Y., Pu, H. H., Wang, Y., et al. (2017). Expression of PD-L1 and prognosis in breast cancer: a meta-analysis. Oncotarget 8 (19), 31347–31354. doi: 10.18632/oncotarget.15532
Zhang, W. T., Zhang, J. F., Zhang, Z. W., Guo, Y. D., Wu, Y., Wang, R. L., et al. (2019). Overexpression of indoleamine 2, 3-dioxygenase 1 promotes epithelial–mesenchymal transition by activation of the IL-6/STAT3/PD-L1 pathway in bladder cancer. Transl. Oncol. 12 (3), 485–492. doi: 10.1016/j.tranon.2018.11.012
Keywords: meta-analysis, prognosis, PD-L1, bladder cancer, survival
Citation: Zhu L, Sun J, Wang L, Li Z, Wang L and Li Z (2019) Prognostic and Clinicopathological Significance of PD-L1 in Patients With Bladder Cancer: A Meta-Analysis. Front. Pharmacol. 10:962. doi: 10.3389/fphar.2019.00962
Received: 11 May 2019; Accepted: 29 July 2019;
Published: 30 August 2019.
Edited by:
Jie Xu, Shanghai Jiao Tong University, ChinaReviewed by:
Gunjan Arora, National Institutes of Health (NIH), United StatesHebao Yuan, University of Michigan, United States
Copyright © 2019 Zhu, Sun, Wang, Li, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhibin Li, ZHoxNDc4OTJAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Lei Zhu1†
Lei Zhu1† Zhibin Li
Zhibin Li