- Phase I Clinical Trial Center, Beijing Shijitan Hospital, Capital Medical University, Beijing, China
Objective: This study compared the pharmacokinetics (PK), safety, and immunogenicity of the biosimilar TAB008 monoclonal antibody to bevacizumab (Avastin®) in normal healthy Chinese male volunteers.
Methods: In this randomized, double-blind, parallel controlled study, a total of 100 healthy Chinese male subjects were randomized (1:1) to receive a single 1 mg/kg intravenous dose of TAB008 or Avastin® over a 90-min infusion. The subjects were followed for 99 days after drug administration. Primary endpoints were bioequivalence of major pharmacokinetic parameters (AUC0-t and AUC0-∞) and maximum observed serum concentration (Cmax). Secondary endpoints included safety and immunogenicity parameters.
Results: The two groups of test subjects (49 subjects in the TAB008 group and 50 subjects in the Avastin® group) were well matched in regards to all demographic and baseline characteristics. The treatment group ratios of LS geometric means for the three primary PK parameters were fully contained within the bioequivalence limits of 80.00–125.00% (90% CI was 103.66–118.33% for Cmax, 94.32–111.72% for AUC0-t, and 94.69–112.23% for AUC0-∞). Treatment-emergent adverse events (TEAEs) were reported for 24 (49.0%) subjects in the TAB008 group and 22 (44.0%) subjects in the Avastin® group. TEAEs related to the study drug were reported for 19 (38.8%) subjects in the TAB008 group and 19 (38.0%) subjects in the Avastin® group. National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) Grade 3 TEAEs were reported for 1 (2.0%) subject in the TAB008 group and 3 (6.0%) subjects in the Avastin® group. There were no Grade 4 or 5 TEAEs or serious adverse events (SAEs) during the study. Anti-drug antibody generation was reported once only in each group, and neutralizing antibody (Nab) analysis was negative upon follow-up.
Conclusion: TAB008 attained pharmacokinetic similarity to bevacizumab, and was safe and well tolerated.
Introduction
Bevacizumab (Avastin®) is a recombinant humanized monoclonal IgG1 antibody that effectively binds to and inhibits vascular endothelial growth factor (VEGF), thereby reducing new blood vessel formation (Avastin: EPAR-Product Information. EMA; US FDA Avastin®). Avastin® was first approved by the United States Food and Drug Administration (FDA) in February 2004 for first-line treatment of metastatic colorectal cancer in combination with chemotherapy, and then for first-line treatment of advanced non-squamous non-small cell, non-EGFR-mutant lung cancer (NSCLC) in combination with paclitaxel and carboplatin. In 2009, Avastin was approved for glioblastoma and renal cell carcinoma after prior therapy. In 2014, significant efficacy was demonstrated against cervical and ovarian cancer. All these approvals were based on stringent randomized clinical trials with significant overall survival improvement as the cornerstone for approval. In the European Union, aside from the previously mentioned indications, the EMA also approved Avastin® in combination with EGFR tyrosine kinase inhibitors as first-line therapy in EGFR-mutant NSCLCs, and first-line treatment in combination with paclitaxel or capecitabine (if paclitaxel-intolerant) for triple-negative breast cancer. Avastin® has since been approved in many countries and regions in the world (Avastin: EPAR-Product Information. EMA; US FDA Avastin®).
In China, a phase I dose escalation study (5, 10, 15 mg/kg) was performed in 39 refractory solid tumor patients. Bevacizumab pharmacokinetics were dose-linear at 5 mg/kg every 2 weeks and 15 mg/kg every 3 weeks (Wu et al., 2010). In February 2010, Avastin® (5 mg/kg every 2 weeks) was approved in combination with irinotecan, 5-fluorouracil, and leucovorin for first-line management of metastatic colorectal cancer after the registration ARTIST trial (214 patients) demonstrated improved efficacy compared with patients who received chemotherapy alone; outcomes included overall response rate (ORR) (35% vs. 17%, respectively; p = 0.013), progression-free survival (PFS) (8.3 vs. 4.2 months, respectively; p < 0.01), and overall survival (OS) (18.7 vs. 13.4 months, respectively; p = 0.014) (Guan et al., 2011). In July 2015, the China Food and Drug Administration approved bevacizumab (15 mg/kg every 3 weeks) in combination with paclitaxel and carboplatin for first-line management of NSCLC after the registration BEYOND study (276 patients) demonstrated improved ORR (54% vs. 26%, p < 0.01), PFS (9.2 vs. 6.5 months, p < 0.01), and OS (24.3 vs. 17.7 months, HR = 0.68, 95% CI 0.5–0.93, p = 0.0154) compared with patients who received chemotherapy alone (Zhou et al., 2015).
Bevacizumab has demonstrated powerful anticancer efficacy when added to chemotherapy. Recent research suggests that in combination with checkpoint inhibitors, Avastin further expands the landscape of cancer control (Socinski et al., 2018). As a result of bevacizumab’s great potential, many biosimilars have been developed globally.
The abbreviated licensure pathway for development of biosimilars has been clearly outlined by regulatory authorities, including the EMA (issued October 23, 2014) (Avastin: EPAR-Product Information. EMA), the US FDA (issued April 2015) (US FDA Avastin®), and China (Technical Guidelines for Development and Evaluation of Biosimilars, issued February 28, 2015 by the Center for Drug Evaluation) (Technical guidelines for research and evaluation of biosimilars. NMPA). China also issued an additional directive on July 18, 2017, specifically for bevacizumab (Recommendations on the design and review of clinical study for bevacizumab injection biosimilar drug). All guidelines require a phase I comparative pharmacokinetic study to the originator, with concurrent evaluation of the safety and immunogenicity profile of the biosimilar product. Phase I clinical trials should be followed by phase III clinical trials with overall response rate as primary endpoint.
TAB008 is a biosimilar monoclonal antibody to bevacizumab developed by TOT BIOPHARM. Analytical similarities have been demonstrated in comparative physical, chemical, and extensive structural and functional characterization studies of TAB008 and the originator bevacizumab. Pre-clinical, pharmacokinetic, pharmacologic, and toxicologic studies (in mice, rats, and cynomolgus monkeys), with Avastin® as the control, confirmed TAB008 to be a biosimilar.
Pharmacokinetic studies demonstrated that bevacizumab showed dose linearity within the dose range of 1–20 mg/kg, with a terminal t½ of around 20 days (Han et al., 2016). As such, a single 1 mg/kg dose over a 90-min infusion was selected for administration in a phase I randomized, double-blind, bevacizumab (EU-sourced) controlled study to compare the pharmacokinetics, safety, and immunogenicity of TAB008 versus Avastin® in healthy Chinese male subjects. The 1 mg/kg dose was selected to minimize drug exposure in normal healthy volunteers, so such a low dose over a 90-min infusion will be a very stringent test for the biosimilarity of TAB008. Since the main purpose of any biosimilar study is to measure similarities, the FDA, EMA, and National Medical Products Administration (NMPA) suggest choosing healthy homogeneous male population to describe PK characteristics (Avastin: EPAR-Product Information. EMA; Recommendations on the design and review of clinical study for bevacizumab injection biosimilar drug; US FDA Avastin®).
Materials, Subjects, and Methods
Study Population
The study was registered in the Chinese Clinical Trials Registry (ChiCTR-IIR-16009827). The trial protocol was approved by the Drug Clinical Trial Ethics Committee of Beijing Shijitan Hospital of Capital Medical University. All subjects provided written informed consent. A total of 100 healthy Chinese male subjects were randomized (1:1) to receive a single 1 mg/kg intravenous dose of either TAB008 or Avastin®. Inclusion criteria were as follows: 18–45 years of age, body mass index (BMI) of 19–28 kg/m2 (inclusive), and body weight of 50–75 kg (inclusive). The subjects were evaluated through medical history, physical examination, laboratory tests, and electrocardiogram (ECG). Exclusion criteria included history of significant illness, alcohol or drug abuse (all volunteers underwent a drug abuse screen), prior exposure to bevacizumab or any VEGF- or VEGF receptor-targeting drug, or participation in clinical trials within the past 3 months. Patients with detectable baseline anti-bevacizumab antibody were also excluded.
Study Design
The study was a phase I, single-dose, randomized, double-blind, parallel controlled study designed to investigate the pharmacokinetics, safety and immunogenicity of TAB008 Monoclonal Antibody Injection compared to Avastin® in healthy Chinese male subjects.
Pharmacokinetics Analysis
Pharmacokinetic parameters were calculated using non-compartmental methods (Phoenix® WinNonlin® 6.4) and summarized using descriptive statistics. The primary PK parameters Cmax, AUC0-t, and AUC0-∞ were compared between treatment groups using a linear model. Secondary endpoints evaluated included tmax (time to maximum serum concentration), CL (systemic clearance), z (terminal rate constant), t½ (terminal half-life), Vss (volume of distribution at steady state), and Vz (volume of distribution).
Blood samples were collected from each subject at pre-determined time points (pre-dosing; 0.75 h after initiation of infusion; 0, 1, 2, 4, and 8 h from the end of infusion; and at 24, 48, and 96 h; and days 8, 11, 15, 22, 29, 36, 43, 57, 71, 85, and 99 from the start of infusion). Blood (4 ml) was drawn at each time point; 21 blood samples were collected (84 ml in total). Serum drug concentration was measured using a validated enzyme-linked immunosorbent assay. The quantification range of the assay was 39.06–10,000.00 ng/ml, with lower limit of quantification of 78.13 ng/ml.
Safety Evaluation
Safety parameters included reporting of adverse events (AE) and abnormal clinical laboratory test values, ECG, vital signs, physical examinations, and immunogenicity tests. The number of subjects with AEs was summarized, coded, and classified according to the Medical Dictionary for Regulatory Activities (MedDRA) (version 20.0). AEs were graded according to CTCAE (version 4.03). Descriptive statistical analyses were applied.
Immunogenicity
TAB008 Monoclonal Antibody Injection and Avastin® were compared in terms of immunogenicity. Anti-drug antibody (ADA) was screened for as scheduled (pre-dosing and days 15, 43, and 85 from the start of infusion). Samples were first tested for binding to TAB008 Monoclonal Antibody Injection to identify potential presence of ADAs; if the result was positive, a confirmatory test based on TAB008 Monoclonal Antibody Injection and the reference Avastin® was conducted to confirm the positive status. A third test was performed on the confirmed ADA-positive samples to test the relative titer of ADA. If the sample tested was confirmed as ADA-positive, Nab analysis was conducted.
Sample Size Estimation
When calculating the main endpoints of AUC0-∞, AUC0-t, and Cmax, an inter-individual difference of 25% was presumed so that the ratio of the true mean would be 1.05. Considering 80.00–125.00% equivalence margin for the main PK parameters and α = 0.05 (double unilateral, t-test), 36 effective subjects were required in each treatment group. Considering prior clinical trial data and a 10% dropout rate, 45 subjects were needed per arm. After 90 subjects were treated under blinded conditions, the sample size was re-estimated. If the coefficient of variation (cv) was ≤28%, recruitment continued up to a total of no more than 100 subjects, but if the cv was >28%, and the recalculated sample size was ≤285, the study continued to recruit up to the recalculated sample number. Sample size estimation was performed using SAS 9.2.
Statistical Considerations
Pharmacokinetic similarity between two drugs can be declared if the 90% CIs of the ratios for the geometric least-squares (LS) means of the primary PK parameters (AUC0-∞, AUC0-t, and Cmax) are within the range 80.00–125.00%.
Results
Disposition and General Characteristics of the Study Subjects
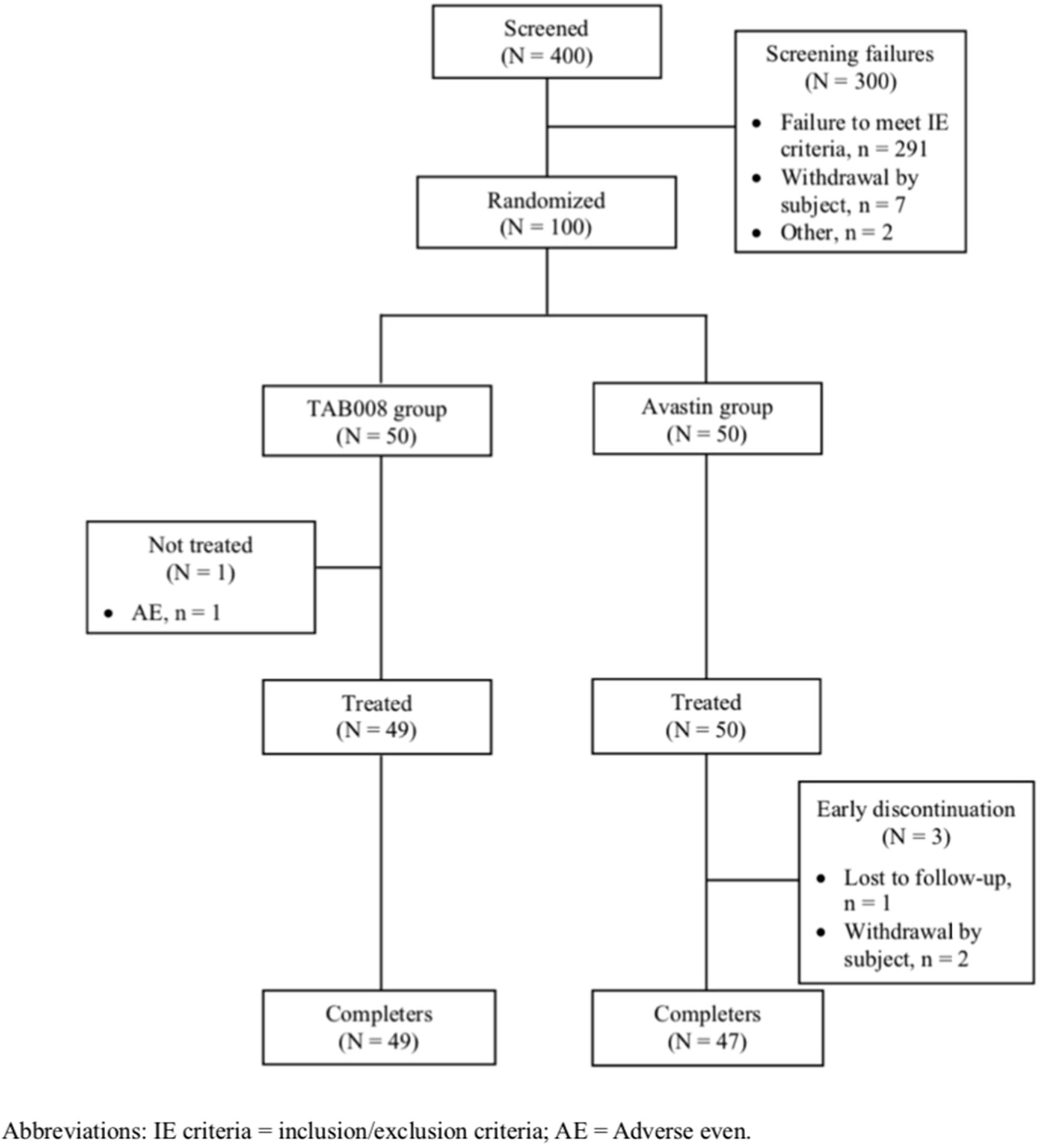
A total of 400 subjects signed the informed consent form. There were 300 screen failures (291 failed to fulfill enrollment criteria and 7 withdrew after providing consent). Of the 100 subjects randomized, 49 received TAB008 (one subject experienced a non-drug related AE) and 50 received Avastin® and were included in the full analysis set (FAS) (Figure 1). All subjects in the FAS were also included in the pharmacokinetics analysis set (PKAS) and safety analysis set (SAF). Eventually, 49 subjects in the TAB008 group and 47 subjects in the Avastin® group completed the study. There were three early discontinuations in the Avastin® group: withdrawal of consent (Avastin: EPAR-product Information. EMA) and loss to follow-up (US FDA Avastin®).
The two groups were well matched with regard to all demographic and baseline characteristics. Mean age was 29.5 (± 5.42) years in the TAB008 group and 29.3 (± 5.59) years in the Avastin® group. Mean BMI was 22.40 (± 1.90) kg/m2 in the TAB008 group and 22.14 (± 1.89) kg/m2 in the Avastin® group. The two groups had a comparable history of tobacco and alcohol use. No abnormalities were with regard to baseline vital signs or ECG or physical examination results.
Pharmacokinetic Parameters
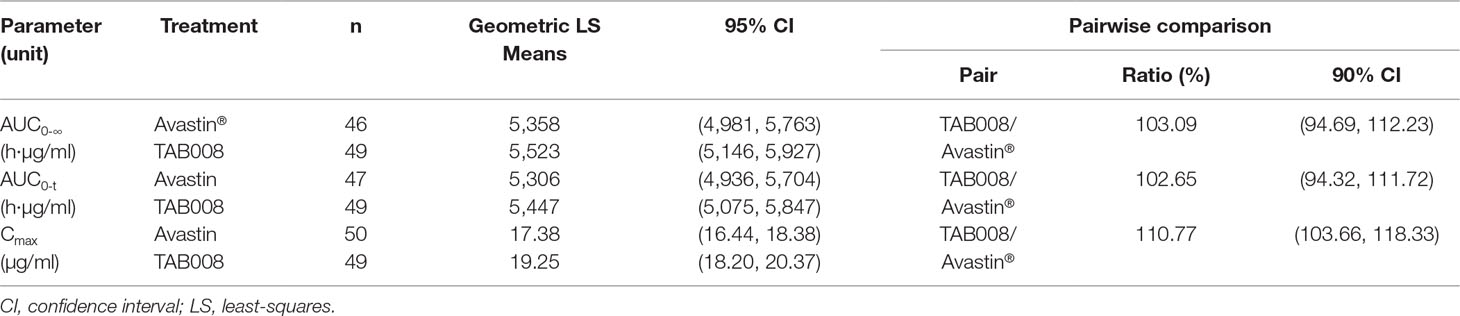
All PK analyses were carried out using the PKAS, which comprised subjects who received a full dose of study drug and had at least one valid post-dose concentration detected without significant protocol deviations or events that could seriously impact PK concentration. PK parameters are shown in Figure 2 and Table 1. The data show that the primary and secondary PK parameters were similar between the two treatment groups.
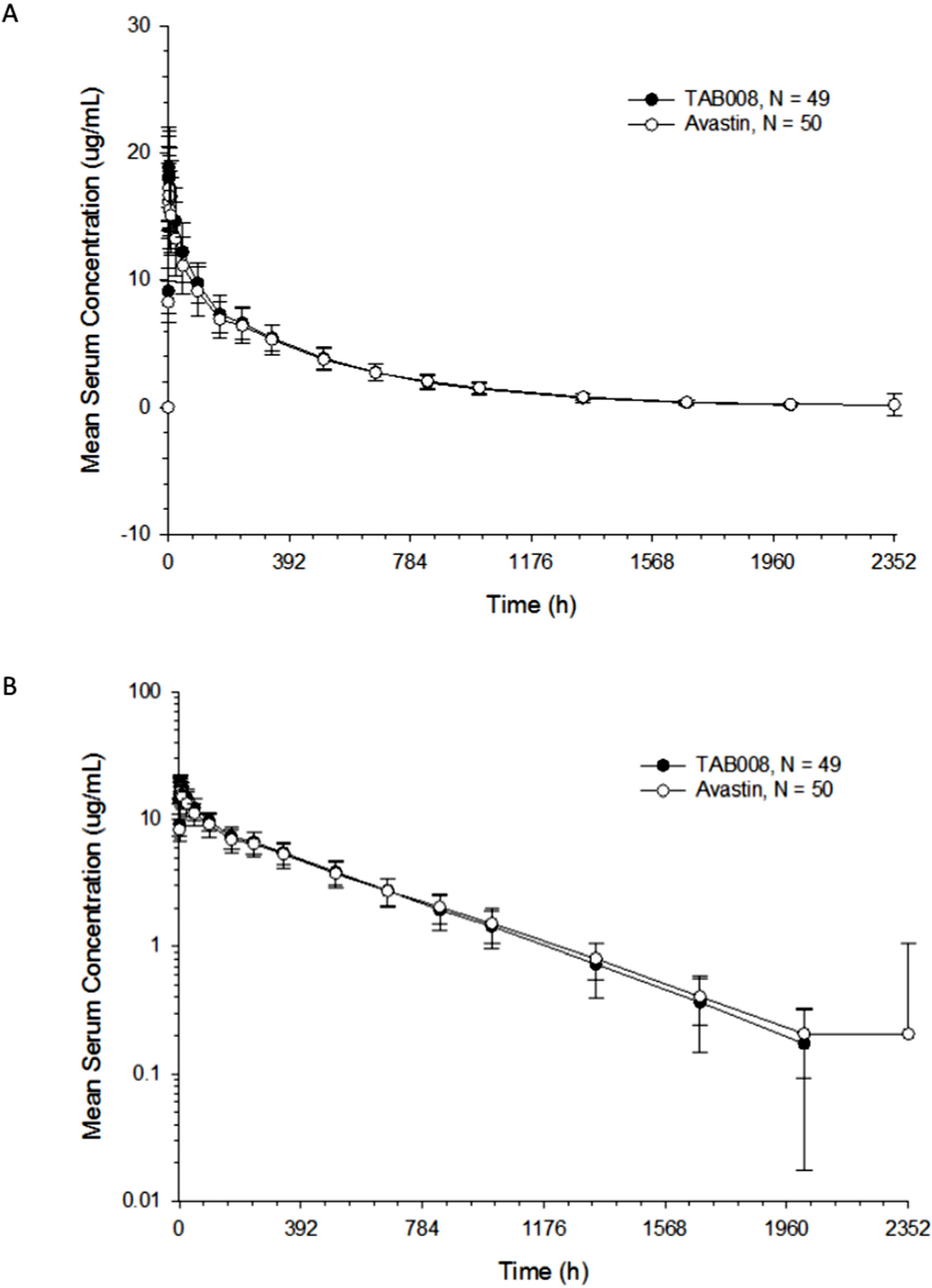
Figure 2 Arithmetic mean (± SD) bevacizumab serum concentration–time profiles for TAB008 versus Avastin® on linear (A) and semilogarithmic scales (B).
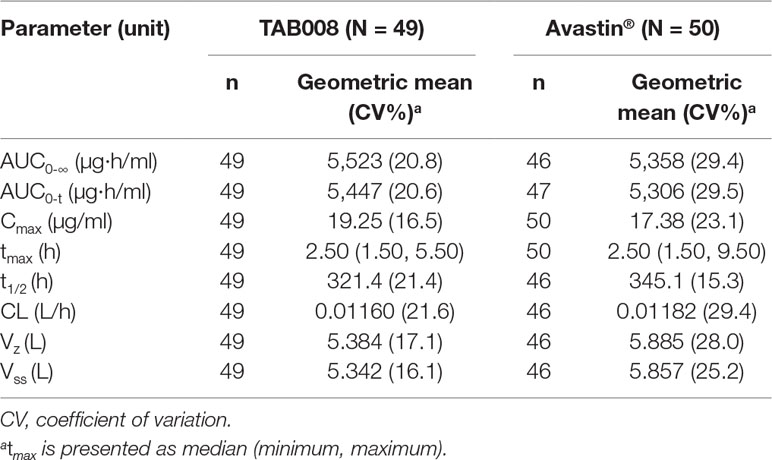
Table 1 Descriptive statistics for geometric mean and geometric CV of pharmacokinetic parameters for each treatment group.
Bioequivalence Statistical Results
The mean concentration profiles between TAB008 Monoclonal Antibody Injection and Avastin® were similar over the profiling interval. The treatment group ratios of LS geometric means for the three primary PK parameters were fully contained within the bioequivalence limits of 80.00–125.00% (90% CI was 103.66–118.33% for Cmax, 94.32–111.72% for AUC0-t, and 94.69–112.23% for AUC0-∞) (Table 2). Based on the result of the nonparametric analysis, the median tmax values of TAB008 Monoclonal Antibody Injection and Avastin® were the same.
Safety Analysis Results
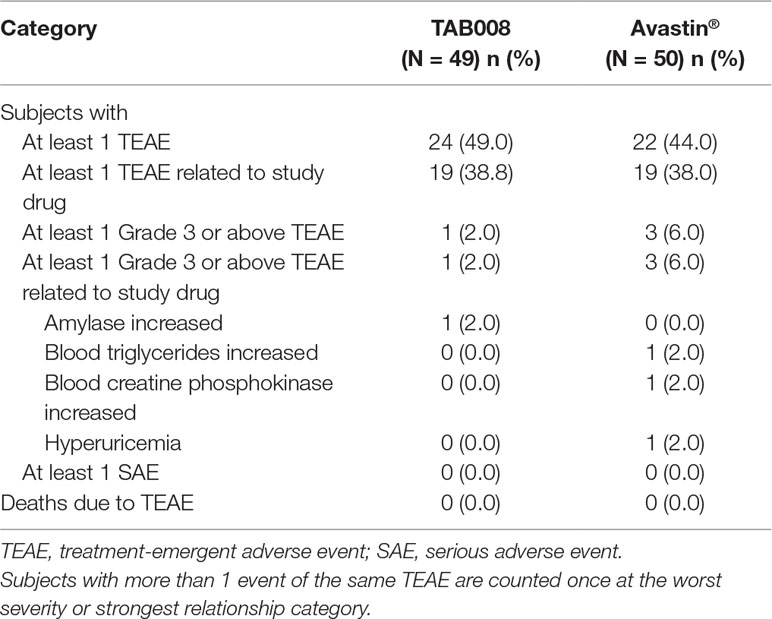
TAB008 Monoclonal Antibody Injection was safe and well tolerated in healthy Chinese male subjects and demonstrated safety profiles comparable with those of Avastin®. TEAEs were reported for 24 (49.0%) subjects in the TAB008 group and 22 (44.0%) subjects in the Avastin® group. TEAEs considered by the investigator to be related to the study drug were reported for 19 (38.8%) subjects in the TAB008 group and 19 (38.0%) subjects in the Avastin® group. NCI-CTCAE Grade 3 TEAEs were reported for one (2.0%) subject in the TAB008 group and three (6.0%) subjects in the Avastin® group. There were no Grade 4 or 5 TEAEs, SAEs, or deaths during the study, and no TEAEs leading to treatment discontinuation (Table 3).
Hypertriglyceridemia was the most frequently reported treatment-related AE in both the TAB008 (10.2%) and Avastin® (8.0%) groups. Clinically significant increases in triglycerides, uric acid, and liver function test results (alanine aminotransferase, total bilirubin, and direct bilirubin) were observed in both groups. Clinically significant hypertension was observed for both groups (Table 4). ECG and physical examination did not reveal any clinically relevant abnormalities in either group.
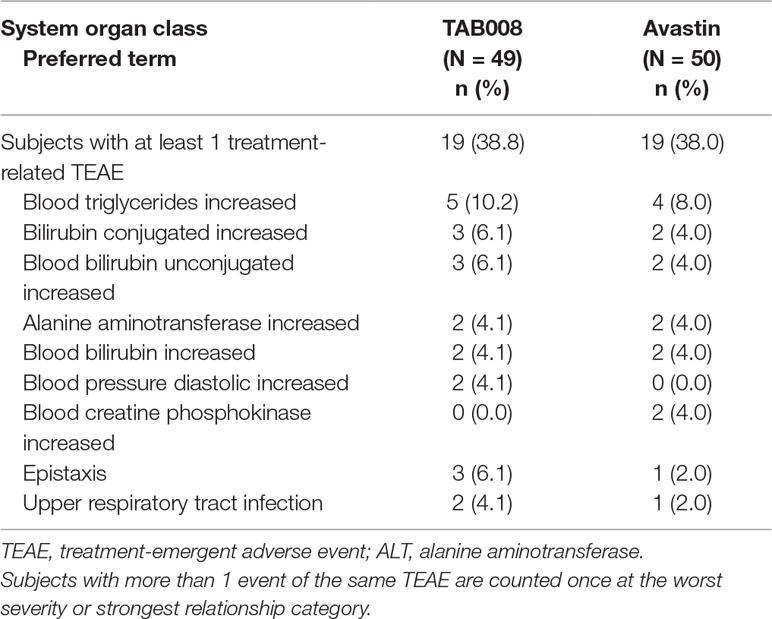
Table 4 Summary of most frequent (>2%) treatment-related treatment-emergent adverse events by system organ class and preferred term for each treatment group (safety analysis set).
Immunogenicity Examination Findings
All subjects tested negative for ADA before treatment. One subject in the TAB008 group and one in the Avastin® group showed positive ADA on days 15 and 85, respectively, but positivity disappeared at the subsequent testing time point. The ADAs were not neutralizing antibodies. Neutralizing antibody titers were not assessed for other subjects.
Discussion
According to the FDA, EMA, and NMPA guidelines, all biosimilars of bevacizumab used in phase I trials have to be compared to the originator, which can be sourced from Europe and/or the US. Avastin® (EU-sourced) was approved by NMPA in 2010 for the treatment of colorectal cancer, NSCLC, malignant glioma, and other tumors in China; therefore, Avastin® (EU-sourced) was chosen as the control for this study.
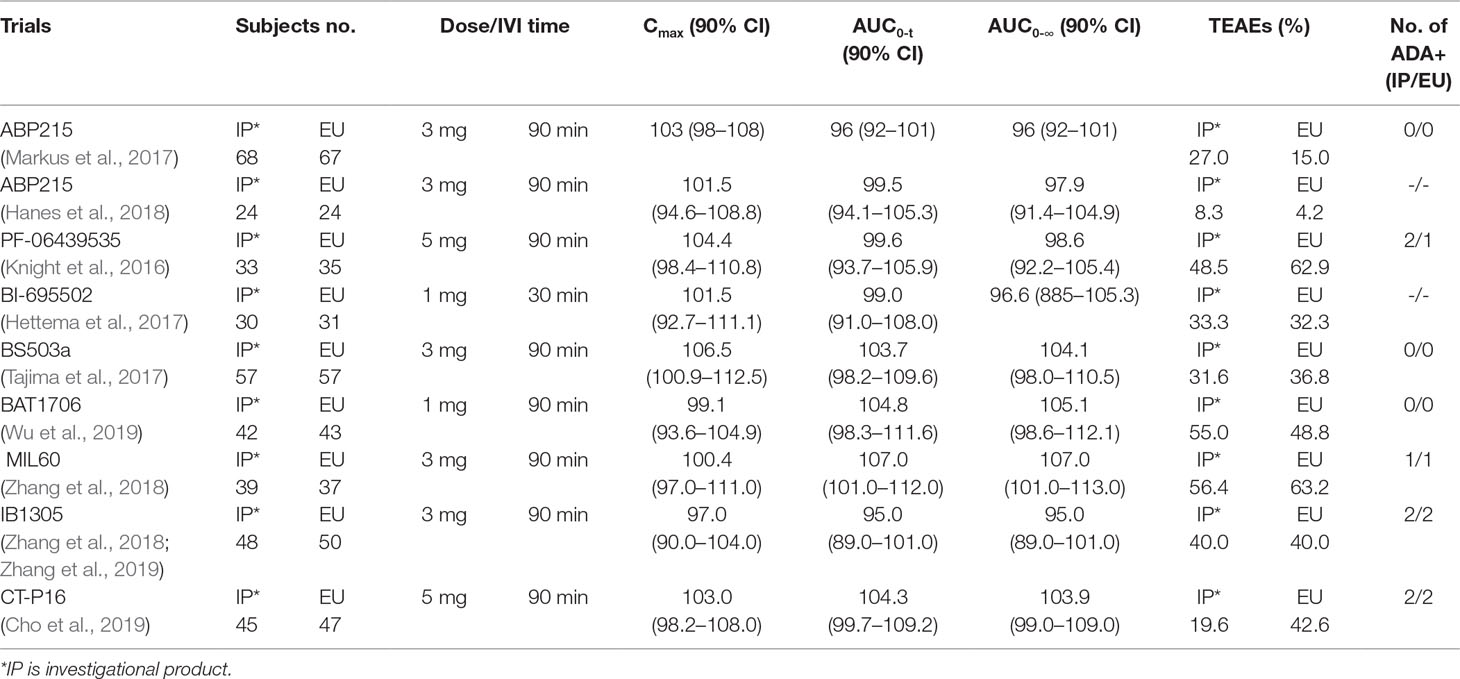
Phase I studies for evaluating biosimilarity to originator molecules have been conducted in some countries. The doses ranged from 1–5 mg/kg with infusion times of 30–90 min, and the number of normal healthy male volunteers ranged from 24–68 per arm of treatment. Since the PK profile of Avastin® was linear for doses of 1–10 mg/kg (Avastin: EPAR-Scientific Discussion. EMA; Hettema et al., 2017; Wu et al., 2019), 1 mg/kg was selected to minimize drug exposure. The number of healthy male volunteers in this study was 50 per arm of treatment.
The mean concentration profiles of TAB008 Monoclonal Antibody Injection and Avastin® were similar over the profiling interval. The treatment group ratios of LS geometric means for the three primary PK parameters were fully contained within the bioequivalence limits of 80–125%. These results were consistent with other similar studies in Caucasian, Japanese, Korean, and Chinese healthy volunteers (Table 5) (Knight et al., 2016; Hettema et al., 2017; Markus et al., 2017; Tajima et al., 2017; Hanes et al., 2018; Zhang et al., 2018; Cho et al., 2019; Wu et al., 2019; Zhang et al., 2019).
TEAEs considered related to the study drug in this study were reported for 19 (38.8%) subjects in the TAB008 group and 19 (38.0%) subjects in the Avastin® group. AE seemed to vary widely between these studies (Knight et al., 2016; Hettema et al., 2017; Markus et al., 2017; Tajima et al., 2017; Hanes et al., 2018; Zhang et al., 2018; Cho et al., 2019; Wu et al., 2019; Zhang et al., 2019), but there was no significant correlation with the dose. The %AE with 1 mg/kg ranged across studies from 33.3% to 55.0% for biosimilars and 32.3% to 48.8% for Avastin® (EU-sourced). The %AE with 3 mg/kg ranged across studies from 8.3% to 56.4% for biosimilars and 4.2% to 63.2% for Avastin® (EU-sourced). The %AE with 5 mg/kg ranged across studies from 19.6% to 48.5% for biosimilars and 42.6% to 62.9% for Avastin® (EU-sourced) (Table 5) (Knight et al., 2016; Hettema et al., 2017; Markus et al., 2017; Tajima et al., 2017; Hanes et al., 2018; Zhang et al., 2018; Cho et al., 2019; Wu et al., 2019; Zhang et al., 2019).
Here, we found that one subject in the TAB008 group and one in the Avastin® group were positive for ADA after treatment; however, positivity disappeared upon subsequent testing. ADA levels were generally low or undetectable (previous studies report equally high values for both the biosimilar and originator).
Conclusion
The present study shows that bevacizumab is pharmacokinetically similar to TAB008; indeed, the treatment group ratios of LS geometric means for the three primary PK parameters (Cmax, AUC0-t, and AUC0-∞) were fully contained within the bioequivalence limits of 80–125%. TAB008 Monoclonal Antibody Injection was safe and well tolerated by healthy Chinese male subjects (although evaluation of safety and tolerability at 1 mg/kg might not be sufficient to estimate the safety profile at the therapeutic dose), with no notable difference in immunogenicity.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The trial protocol was approved by the Drug Clinical Trial Ethics Committee of Beijing Shijitan Hospital of Capital Medical University.
Author Contributions
JW and XW made substantial contributions to the conception and design of this study, to the acquisition of data, and to the analysis and interpretation of the data. LQ, LL, ZW, GC, YW, XL YL, HL, YT, CLi, and CLe made substantial contributions to the implementation of the study. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Avastin: EPAR-Product Information. EMA. https://www.ema.europa.eu/en/medicines/human/EPAR/avastin/, Accessed 2018/8/31.
Avastin: EPAR-Scientific Discussion. EMA. https://www.ema.europa.eu/en/documents/scientific-discussion/avastin-epar-scientific-discussion_en.pdf. 2006/1/24.
Cho, S. H., Han, S., Ghim, J. L., Nam, M.S., Yu, S., Park, T. (2019). A randomized, double-blind trial comparing the pharmacokinetics of CT-P16, a candidate bevacizumab biosimilar, with its reference product in healthy adult males. Bio. Drugs 33 (2), 173–181. doi: 10.1007/s40259-019-00340-x
Guan, Z. Z., Xu, J. M., Luo, R. C., Feng, F.Y., Wang, L.W., Shen, L. (2011). Efficacy and safety of bevacizumab plus chemotherapy in Chinese patients with metastatic colorectal cancer: a randomized phase III ARTIST trial. Chin. J. Cancer 30 (10), 682–9. doi: 10.5732/cjc.011.10188
Han, K., Peyret, T., Marchand, M., Quartino, A., Gosselin, N.H., Girish, S. (2016). Population pharmacokinetics of bevacizumab in cancer patients with external validation. Cancer Chemother. Pharmacol. 78, 341–351. doi: 10.1007/s00280-016-3079-6
Hanes, V., Chow, V., Pan, Z., Markus, R. (2018). A randomized, single-blind, single-dose study to assess the pharmacokinetic equivalence of the biosimilar ABP 215 and bevacizumab in healthy Japanese male subjects. Cancer Chemother. Pharmacol. 82, 899–905. doi: 10.1007/s00280-018-3695-4
Hettema, W., Wynne, C., Lang, B., Altendorfer, M., Czeloth, N., Lohmann, R. (2017). A randomized, single-blind, Phase I trial (INVICTAN-1) assessing the bioequivalence and safety of BI 695502, a bevacizumab biosimilar candidate, in healthy subjects. Expert. Opin. Investig. Drugs 26 (8), 889–896. doi: 10.1080/13543784.2017.1347635
Knight, B., Rassam, D., Liao, S., Ewesuedo, R. (2016). A phase I pharmacokinetics study comparing PF-06439535 (a potential biosimilar) with bevacizumab in healthy male volunteers. Cancer Chemother. Pharmacol. 77, 839–846. doi: 10.1007/s00280-016-3001-2
Markus, R., Chow, V., Pan, Z., Hanes, V. (2017). A phase I, randomized, single-dose study evaluating the pharmacokinetic equivalence of biosimilar ABP 215 and bevacizumab in healthy adult men. Cancer Chemother. Pharmacol. 80, 755–763. doi: 10.1007/s00280-017-3416-4
Recommendations on the design and review of clinical study for bevacizumab injection biosimilar drug. http://www.cde.org.cn/dzkw.domethod=largePage&id=313923. 2017/7/18
Technical guidelines for research and evaluation of biosimilars. NMPA. http://www.cde.org.cn/zdyz.do?method=largePage&id=243. 2015/2/28.
Socinski, M. A., Jotte, R. M., Cappuzzo, F., Orlandi, F., Stroyakovskiy, D., Nogami, N. (2018). Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Engl. J. Med. 378 (24), 2288–2301. doi: 10.1056/NEJMoa1716948
Tajima, N., Martinez, A., Kobayashi, F., He, L., Dewland, P. (2017). A phase 1 study comparing the proposed biosimilar BS-503a with bevacizumab in healthy male volunteers. Pharma. Res. Per. 5 (2), e00286. doi: 10.1002/prp2.286
US FDA Avastin® package insert https://druginserts.com/lib/rx/meds/avastin-1/ accessed 2019/1/20.
Wu, J. Y., Wu, X. N., Li, Ding, Zhao, Y.B., Ai, B., Li, Y. (2010). Phase I safety and pharmacokinetic study of bevacizumab in Chinese patients with advanced cancer. Chin. Med. J. 123 (7), 901–906. doi: 10.3760/cma.j.issn.0366-6999.2010.07.025
Wu, X., Wynne, C., Xu, C., Gan, Y., Wang, C., Thomas, B.E. (2019). A global phase I clinical study comparing the safety and pharmacokinetics of proposed biosimilar BAT1706 and bevacizumab (Avastin®) in healthy male subjects. Bio. Drugs 33 (3), 335–342. doi: 10.1007/s40259-019-00352-7
Zhang, H., Li, Q., Zhu, X., Li, C., Li, X., Liu, C. (2018). Tolerance, variability, and pharmacokinetics of bevacizumab biosimilars in Chinese healthy male subjects. Cancer Chemother. Pharmacol. 82, 615–623. doi: 10.1007/s00280-018-3645-1
Zhang, H., Zhu, X., Wei, H., Li, C., Chen, H., Li, X. (2019). A phase I, randomized, double-blinded, single-dose study evaluating the pharmacokinetic equivalence of the biosimilar IBI305 and bevacizumab in healthy male subjects. Int. J. Clin. Pharmacol. Ther. 57 (3), 167–174. doi: 10.5414/CP203349
Zhou, C., Wu, Y.-L., Chen, G., Liu, X., Zhu, Y., Lu, S. (2015). Beyond: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non–small-cell lung cancer. J. Clin. Oncol. 33, 2197–2204. doi: 10.1200/JCO.2014.59.4424
Keywords: TAB008 monoclonal antibody injection, Avastin®, pharmacokinetics, biosimilarity, immunogenicity
Citation: Wang J, Qi L, Liu L, Wang Z, Chen G, Wang Y, Liu X, Liu Y, Liu H, Tong Y, Liu C, Lei C and Wang X (2019) A Phase I, Randomized, Single-Dose Study Evaluating the Biosimilarity of TAB008 to Bevacizumab in Healthy Volunteers. Front. Pharmacol. 10:905. doi: 10.3389/fphar.2019.00905
Received: 22 April 2019; Accepted: 18 July 2019;
Published: 15 August 2019.
Edited by:
Fabrizio Marcucci, University of Milan, ItalyReviewed by:
Md Kamal Hossain, University of Toledo, United StatesGuang Xu, Marian University, United States
Copyright © 2019 Wang, Qi, Liu, Wang, Chen, Wang, Liu, Liu, Liu, Tong, Liu, Lei and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinghe Wang, d2FuZ3hoQGJqc2p0aC5jbg==
†These authors have contributed equally to this work and share first authorship.
 Jin Wang
Jin Wang Lu Qi†
Lu Qi†