- 1Department of Infectious Diseases, First Hospital of Jilin University, Changchun, China
- 2Department of Pediatrics, University of Oklahoma Health Sciences Center, Oklahoma City, OK, United States
Biochanin A (BCA) is an isoflavone mainly found in red clover with poor solubility and oral absorption that is known to have various effects, including anti-inflammatory, estrogen-like, and glucose and lipid metabolism modulatory activity, as well as cancer preventive, neuroprotective, and drug interaction effects. BCA is already commercially available and is among the main ingredients in many types of supplements used to alleviate postmenopausal symptoms in women. The activity of BCA has not been adequately evaluated in humans. However, the results of many in vitro and in vivo studies investigating the potential health benefits of BCA are available, and the complex mechanisms by which BCA modulates transcription, apoptosis, metabolism, and immune responses have been revealed. Many efforts have been exerted to improve the poor bioavailability of BCA, and very promising results have been reported. This review focuses on the major effects of BCA and its possible molecular targets, potential uses, and limitations in health maintenance and treatment.
Introduction
Phytoestrogens are compounds found in plants with a molecular structure and size resembling those of estrogens. Plant flavonoid isoflavones are the most popular among the many estrogenic compounds (Heinonen et al., 1999). In humans, the main dietary sources of isoflavones are soybean and soybean products. When these types of food are consumed, they have multiple effects (Vitale et al., 2013). Epidemiological studies have indicated that populations with a high isoflavone intake through soy consumption have lower rates of several cancers, such as breast, prostate, bladder, gastric, and colon cancer (Kweon et al., 2013; Zhang et al., 2017; Perez-Cornago et al., 2018; Wada et al., 2018; You et al., 2018; Grainger et al., 2019). Isoflavones are considered chemoprotective and can be used as an alternative therapy for a wide range of hormonal disorders (van Duursen, 2017; Křížová et al., 2019).
Biochanin A (5,7-dihydroxy-4’-methoxy-isoflavone, BCA) (Figure 1A) is an isoflavone present in red clover, cabbage, alfalfa, and many other herbal products (Cassady et al., 1988). BCA may occur as an aglycon and can also be used as a hormone alternative therapy. BCA plays complex roles in the regulation of multiple biological functions by binding DNA and some specific proteins or acting as a competitive substrate for some enzymes (Roberts et al., 2004; Křížová et al., 2019; Liang et al., 2019; Luo et al., 2019). BCA is the methylated precursor of the isoflavone genistein (GEN), which is another well-studied isoflavone. In the gut, intestinal bacteria convert BCA to its demethylated form (Setchell et al., 2001). However, the biological effects of BCA observed in vitro and in vivo are not identical to those of GEN. Recently, medical research focusing on BCA has increased because of its various purported biological activities, including its antioxidant, anti-inflammatory, anti-infective, and anticarcinogenic effects, and BCA has been used for several purposes, such as to treat estrogen deficiency and pain and reduce the severity of nerve damage (Puli et al., 2006; Medjakovic and Jungbauer, 2008). This extract from plants is already commercially available because of its potential benefits to human health and because it is considered innocuous (Howes et al., 2002; Atkinson et al., 2004; Beck et al., 2005; Sklenickova et al., 2010). Most commercial products are composed of several isoflavone contents, including BCA (Booth et al., 2006; Ahmad et al., 2013). These botanical dietary supplements are sold in tablet form in several countries and are commonly used to alleviate postmenopausal symptoms in women. The use of these products is clearly increasing. However, BCA is a Biopharmaceutics Classification System Class II drug because of its poor water solubility. Given that studies are increasingly focusing on the effects of BCA (Table S1), it is timely and appropriate to obtain in-depth knowledge of the effects of BCA and critically evaluate the paradoxical observations in the published literature.
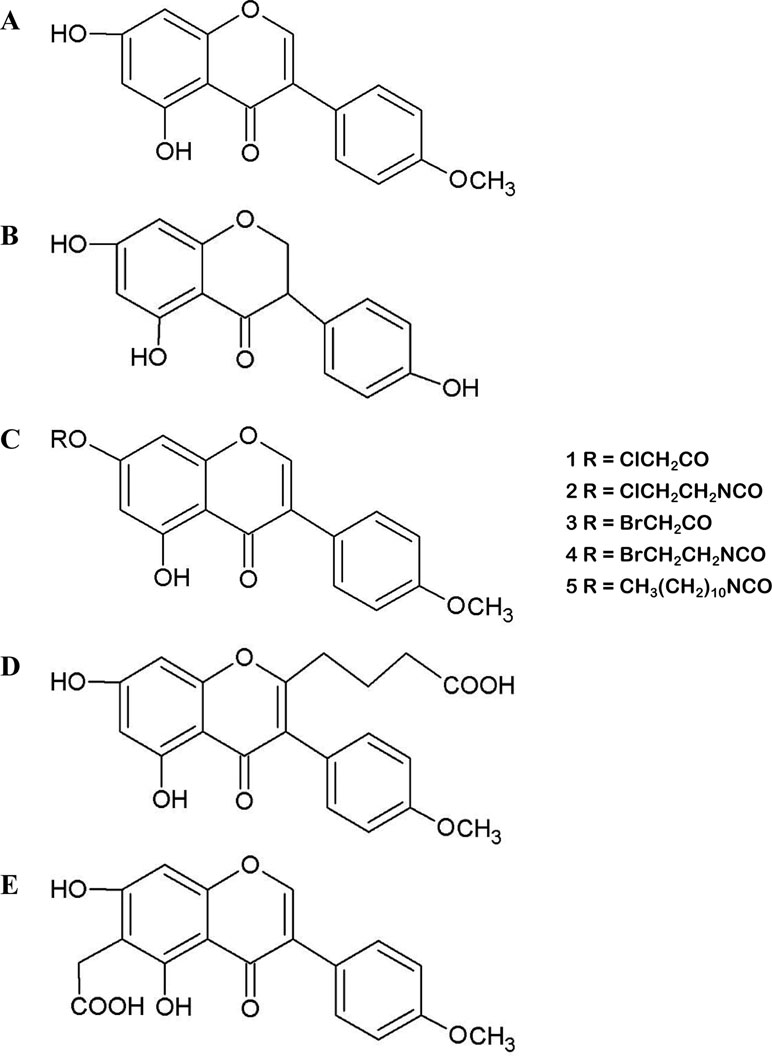
Figure 1 (A) Molecular structure of biochanin A (BCA). (B) Molecular structure of genistein (GEN). (C) Structures of synthesized esters (1, 3) and carbamate esters (2, 4, 5), which are BCA derivatives. (D and E) Molecular structures of carboxyalkyl BCA.
BCA Has Chemopreventive Activity Against Various Cancers
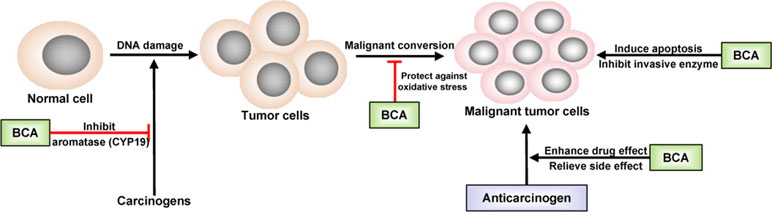
Inspired by epidemiological evidence suggesting that a relationship exists between the consumption of certain foods containing isoflavones and decreased cancer incidence in humans, BCA has been evaluated in many studies related to cancer treatment. The first study was performed in 1988 in hamster embryo cell cultures and found that BCA inhibited carcinogen activation (Cassady et al., 1988). Subsequently, studies investigating the anticancer activity of BCA were carried out in different cancer cell lines, followed by animal models. Many types of tumors could be inhibited by BCA, such as lung cancer (Lee et al., 1991), prostate cancer (Peterson and Barnes, 1993; Sun et al., 1998), gastrointestinal tract cancer (Yanagihara et al., 1993), pancreatic cancer (Bhardwaj et al., 2014), breast cancer (Balabhadrapathruni et al., 2000; Sehdev et al., 2009), osteosarcoma (Hsu et al., 2018; Zhao et al., 2018), malignant melanoma (Xiao et al., 2017), and tumors of the central nervous system (Sehm et al., 2014). However, the ability of BCA to inhibit the growth of some types of cancer cells was weaker than that of GEN (Peterson and Barnes, 1991), but the anticancer usage of BCA might be broader because of its targeting of anticancer activity, especially in malignant brain tumors (Sehm et al., 2014). BCA is a potent inhibitor of cytochrome P450 (CYP) and, thus, may be useful as a chemopreventive agent against hydrocarbon-induced carcinogenesis, and BCA has an inhibitory effect on the metabolism of some carcinogens, such as benzo(a)pyrene, by binding DNA (Chae et al., 1991; Lee et al., 1991; Lee et al., 1992). BCA significantly reduces the synthesis of prostaglandin E2 and thromboxane B2 and the activity of CYP19/aromatase (Almstrup et al., 2002; Wang et al., 2008), leading to cyclooxygenase-2 (COX-2) inhibition (Lam et al., 2004; Lim et al., 2013). The chronic activation or overexpression of COX-2 has been shown to be correlated with the development of cancer, particularly at sites of inflammation. The inhibition of COX-2 has been linked to the decreased development of some types of cancer (Dannenberg and Subbaramaiah, 2003). BCA provides protection against oxidative stress and inhibits the expression and activity of invasive enzymes (Ullah et al., 2009; Sehdev et al., 2009). In earlier studies, apoptosis was regarded as the major mechanism underlying the antitumor activity of BCA (Yanagihara et al., 1993; Yanagihara et al., 1996; Balabhadrapathruni et al., 2000; Puthli et al., 2013). In recent studies, more details regarding the antitumor effects of BCA have been discovered, such as the signaling pathways and effects on vascular invasion (Xiao et al., 2017; Lai et al., 2018; Hsu et al., 2018). BCA could effectively inhibit the proliferation of lung cancer cells by downregulating Ki-67, induce apoptosis by activating the cleavage of caspase-3 and caspase-9, and suppress cell migration by downregulating matrix metallopeptidase-2 (MMP-2) and vascular endothelial growth factor (VEGF) (Lai et al., 2018; Hsu et al., 2018). BCA inhibited cell migration and invasion in a dose-dependent manner and upregulated the expression of key proteins in the NF-κB and mitogen-activated protein kinase (MAPK) signaling pathways (Xiao et al., 2017). BCA acts as a remarkable pro-oxidant factor, significantly enhancing radiotoxicity in colon cancer cells in vitro (Puthli et al., 2013). The anticancer effects of BCA are presented in Figure 2. BCA also enhances the effects of some anticarcinogens and relieves their side effects. The most important point is that BCA showed no such effects on normal tissues and cells at the moderate dose at which it inhibited cancer cells (Sehdev et al., 2009; Sehm et al., 2014; Hsu et al., 2018). BCA is considered a potent chemopreventive and/or therapeutic agent against cancer.
BCA May Play a Therapeutic Role in Metabolic Disorders
BCA is metabolized in the gut to GEN or formononetin, which is converted to daidzein and then to equol (Knight and Eden, 1996). BCA is an estrogen receptor (ER) α and ERβ agonist that promotes transcriptional repression and activation at physiological levels. BCA may act as a natural selective ER modulator that elicits distinct clinical effects from estrogens used for hormone replacement by selectively recruiting coregulatory proteins to ERβ to trigger transcriptional pathways. As a promising alternative estrogen therapy, BCA might be used for the management of the renal and cutaneous changes observed in postmenopausal women while preventing bone loss (An et al., 2001; Beck et al., 2003; Hellström and Muntzing, 2012; Elsherbini et al., 2017; Galal et al., 2018). BCA is well known for its regulation of blood glucose and has significant effects in type 2 diabetes mellitus in vivo by affecting mechanisms that influence autophagy, differentiation, inflammation, and metabolism (Mehrabadi et al., 2018; Nikolic et al., 2018; Oza and Kulkarni, 2018). BCA exerts lipid-lowering effects by increasing the cholesterol efflux and preventing cholesterol ester transport (Xue et al., 2017). BCA also has a gastroprotective effect through the enhancement of cellular metabolic cycles, as evidenced by increases in superoxide dismutase (SOD) and nitric oxide (NO) activity, decreases in the malondialdehyde (MDA) and Bax levels, and increases in Hsp70 expression (Hajrezaie et al., 2015). Ovariectomy results in a marked increase in body weight and a decrease in femoral bone mineral density and trabecular bone, which are common findings after 17β-estradiol (E2) treatment. BCA treatment can effectively prevent the ovariectomy-induced increases in bone loss and bone turnover possibly by increasing osteoblast activity and decreasing osteoclast activity. All stages of bone formation, including osteoblast proliferation, differentiation, and mineralization, are influenced by BCA (Su et al., 2013a; Kaczmarczyk-Sedlak et al., 2015; Mohamed et al., 2018). BCA has been reported to stimulate endothelial NO synthase (eNOS) and the release of NO, which is vasodilatory and vasoprotective. BCA has been shown to attenuate hypertension in ovariectomized rats by decreasing the systolic, diastolic, and mean arterial blood pressures; decreasing oxidative stress and the tumor necrosis factor-α (TNF-α) levels; and increasing the NO levels in an eNOS-dependent manner (Sachdeva et al., 2016). BCA regulates bone formation by preventing adipogenesis and enhancing osteoblast differentiation in mesenchymal stem cells and has beneficial regulatory effects on bone formation. BCA may be a useful agent in the treatment and prevention of osteoarthritis (Su et al., 2013b; Wu et al., 2014). BCA is well known for its antidiabetic and hypolipidemic effects. Its hypolipidemic effect in diabetes is achieved at least partially by the activation of hepatic peroxisome proliferator-activated receptor α (PPARα) (Qiu et al., 2012). BCA increases the circulating insulin levels and improves insulin sensitivity, leading to body weight control, an increase in liver glycogen, and a decrease in plasma glucose (Harini et al., 2012; Oza and Kulkarni, 2018). BCA also has protective effects on β cells in diabetic rats (Azizi et al., 2014). BCA ameliorates hepatic steatosis and insulin resistance by modulating lipid and glucose metabolism in obese rats (Park et al., 2016). Moreover, BCA helps prevent diabetic complications because it is an excellent inhibitor of insulin and hemoglobin glycosylation and has anti-inflammatory activity (Asgary et al., 2002; Chundi et al., 2016; Patil et al., 2016; Mehrabadi et al., 2018). BCA inhibits fatty acid amide hydrolase and may be used as a novel analgesic agent (Thors et al., 2010). BCA has been shown to inhibit melanogenesis in vitro and in vivo because of its tyrosinase inhibitory effect and could be a promising candidate as a skin-whitening agent for the treatment of skin hyperpigmentation disorders (Lin et al., 2011). Therefore, BCA may have wide application prospects in the treatment of metabolic diseases.
BCA Affects Proinflammatory Responses
Numerous studies have indicated the anti-inflammatory effects of BCA, which were first demonstrated in microglia in 2007, when BCA was shown to inhibit lipopolysaccharide (LPS)-induced activation of microglia (Chen et al., 2007). The anti-inflammatory effect of BCA has been demonstrated in many other types of cells, including macrophages, various cancer cells, and endothelial cells, in numerous in vivo experiments (Lee and Choi, 2005; Park et al., 2006; Kole et al., 2011; Ming et al., 2015). BCA inhibits the production of inflammatory mediators, such as TNF-α, interleukin-1β (IL-1β), IL-6, iNOS, COX-2, MMP-9, and NO, in various inflammatory responses and tissue injury by attenuating the ERK-MAPK/MSK1 cascade, inhibiting the TLR/TIRAP/MyD88 pathway, inhibiting IκB kinase (IKK) activity, and activating PPARα as an estrogen at low concentrations or PPARγ by binding PPARγ at high concentrations, leading to the NF-κB-driven inhibition of gene transcription and decreased expression of TNF-α, IL-1β, IL-6, iNOS, COX-2, and MMP-9 (Figure 3) (Lee and Choi, 2005; Vanden Berghe et al., 2006; Mueller et al., 2010; Kole et al., 2011; Qiu et al., 2012; Breikaa et al., 2013a; Wang et al., 2015c; Zhang and Chen, 2015; Wu et al., 2018). A study claimed that BCA upregulated the production of IL-4 via the activation of the PKC/p38/AP-1 and PI3K/PKC/NF-AT pathways (Park et al., 2006). However, some recent studies drew completely different conclusions, namely, BCA did not increase the production of IL-4 and rather suppressed its increase upon stimulation (Ko et al., 2011; Chung et al., 2013). BCA also inhibits AKT/MAPK (ERK, JNK, and p38)/mTOR activation (Chung et al., 2013; Bhardwaj et al., 2014; Jain et al., 2015); this pathway is involved in the regulation of NF-κB and other transcription factors (such as MSK1 and AP1). Reactive oxygen species (ROS) and COX-2 are important proinflammatory factors that can stimulate transcription factors to increase inflammatory mediator expression. BCA scavenges ROS and increases SOD activity (Xue et al., 2017; Zhao et al., 2018). BCA significantly reduces the synthesis of prostaglandin E2 and/or thromboxane B2 by inhibiting COX-2 expression (Lam et al., 2004; Lim et al., 2013). Several BCA targets exert anti-inflammatory effects on the pathways triggered by different inducers in various types of cells (Figure 3). In animal models of acute and chronic inflammation, BCA protects against organ injury by exerting robust anti-inflammatory and antioxidant effects (Ko et al., 2011; Breikaa et al., 2013b; Oh et al., 2016).
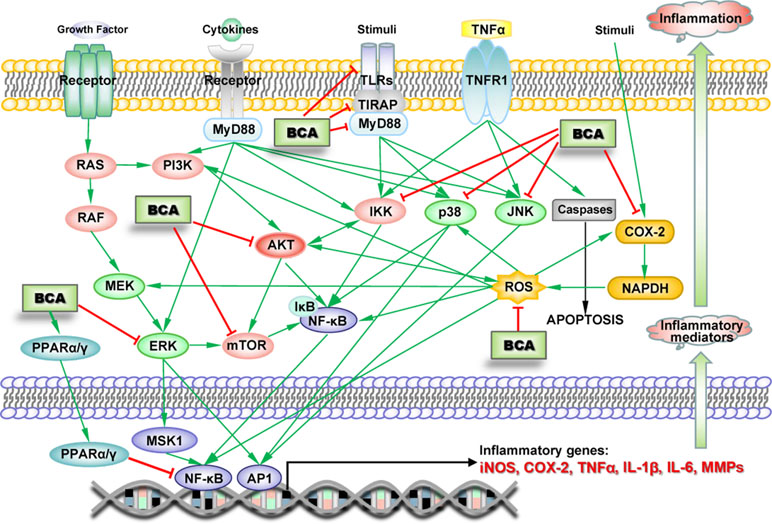
Figure 3 Schematic of the BCA targets (proteins and genes) in key inflammation-associated signaling pathways. →, direct stimulation; ⊥, direct inhibition.
BCA could influence many types of diseases associated with inflammation because of its effects on several inflammatory signaling pathways. Further research is needed to obtain an in-depth understanding of its impact on these diseases.
BCA Influences Pathogen Infection
BCA was found to have an antiviral potential in 1996; BCA inhibited human herpesvirus 6 antigen expression by suppressing the phosphorylation of protein tyrosine kinases (Cirone et al., 1996). BCA also inhibited influenza A nucleoprotein production, reduced virus-induced caspase 3 cleavage and the nuclear export of viral RNP complexes, and enhanced the effects of the neuraminidase inhibitor zanamivir in influenza H5N1 virus-infected lung epithelial cells by affecting signaling pathways to ultimately reduce the virus-induced activation of AKT, ERK½, and NF-κB. BCA also inhibits the virus-induced production of cytokines, such as IL-6, IL-8, TNF-α, and IP-10 (Sithisarn et al., 2013). BCA enhances H5N1-induced ROS formation, whereas antioxidant use suppresses BCA-induced ROS formation and strongly increases its anti-H5N1 activity in H5N1-infected human alveolar basal epithelial cells (Michaelis et al., 2014). However, BCA does not have broad-spectrum antiviral activity, and it has been demonstrated that BCA does not exhibit anti-enterovirus 71 activity (Li et al., 2017).
Some researchers have studied BCA in the context of antibacterial treatment, but most results of treatment with BCA alone have been negative. However, a previous study found that BCA had selective antibacterial action; BCA inhibited all clostridia, which may be responsible for severe intestinal infections, but not bifidobacteria, which are regarded as probiotic microorganisms (Sklenickova et al., 2010). Another study found that BCA has an inhibitory effect on intracellular bacteria belonging to the genus Chlamydia and is a potent inhibitor of Chlamydia spp. (Hanski et al., 2014). A recent study demonstrated that BCA induced AMPK/ULK1/mTOR-mediated autophagy and macrophage extracellular traps (METs), which enhanced defense against Salmonella infection in vitro and in vivo. In addition, BCA inhibits both inflammatory and anti-inflammatory responses when the body is infected by bacteria. These findings provide basic data regarding the control of infections by enhancing the host immune defense and indicate a potential new strategy to overcoming the desperate scarcity of new therapeutic approaches.
Neuroprotective Effects of BCA
Microglia, which are the resident immune cells in the brain, play a role in immune surveillance and host defense against infectious agents under normal conditions. Activated microglia produce a variety of proinflammatory factors, including cytokines, such as TNF-α, and the free radicals NO and superoxide. The accumulation of these factors is deleterious to neurons (Huang et al., 2005). The abnormal activation of microglia is closely associated with some neurodegenerative diseases, such as Parkinson’s disease (PD), Alzheimer’s disease (AD), and frontotemporal dementia (FTD) (Bachiller et al., 2018). Accumulating evidence suggests that estrogen inhibits the LPS-induced inflammatory response in microglia and has a neuroprotective effect (Suuronen et al., 2005; Pozzi et al., 2006; Vegeto et al., 2006). As a promising phytoestrogen, many studies have focused on the effect of BCA on neurodegenerative diseases, especially PD and AD (Figure 4).
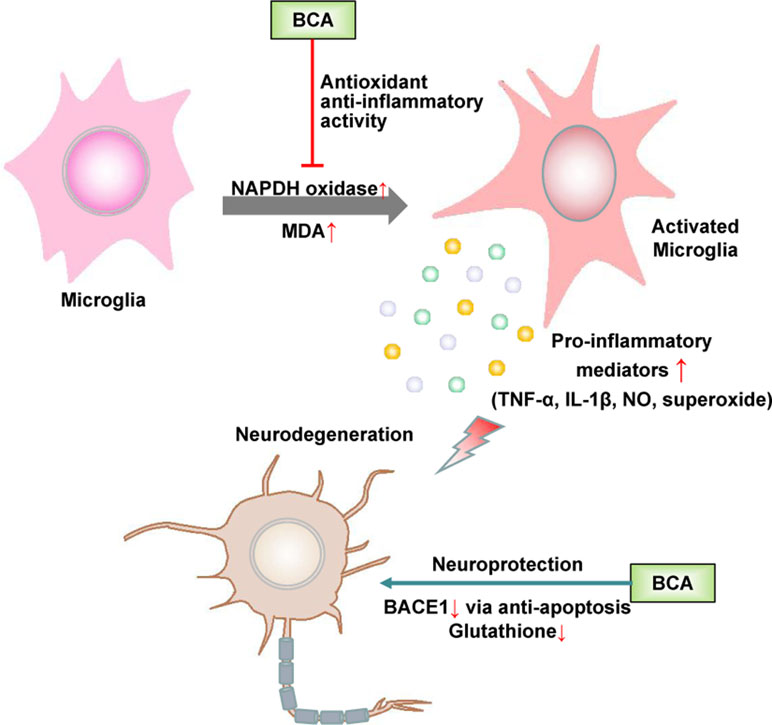
Figure 4 Schematic of the neuroprotective effects of BCA. →, direct stimulation; ⊥, direct inhibition.
BCA has been shown to protect dopaminergic neurons against LPS-induced damage by inhibiting the activation of microglia; the generation of proinflammatory factors, such as TNF-α, IL-1β, NO, and superoxide (Chen et al., 2007); and MAPK signaling pathways in microglia (Wu et al., 2015; Wang et al., 2016). BCA inhibits nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase) activation and malondialdehyde (MDA) production, thereby increasing SOD and glutathione peroxidase (GPx) activity in the brain. The neuroprotective effect of BCA is partially associated with its antioxidant activity and ability to maintain a redox imbalance (Occhiuto et al., 2009; Wang et al., 2015b; Yu et al., 2017). BCA also exerts a neuroprotective effect against L-glutamate-induced cytotoxicity, which plays a crucial role in neuronal cell death in various neurodegenerative diseases and reduces glutathione levels (Tan et al., 2013; Biradar et al., 2014). BCA is a potent, reversible, and selective oxidase-B (MAO-B) inhibitor because of the hydrophobic interactions between BCA and MAO-B, and MAO-B inhibitors are widely used in the treatment of PD and have potential in the future treatment of AD (Zarmouh et al., 2017). BCA effectively inhibits the activity of beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) not only via a mitochondria-dependent apoptosis pathway but also by binding the allosteric site of BACE1; BACE1 accumulation is among the major histological hallmarks of AD (Youn et al., 2016). BCA may be used as a preventative and/or therapeutic agent for AD by binding the preformed fibril structure of β-amyloid25–35 and inhibiting β-amyloid25–35-induced apoptosis by suppressing caspase activity (Ghobeh et al., 2014; Tan and Kim, 2016). Furthermore, BCA has been shown to have neuroprotective effects in cerebral ischemia/reperfusion and subarachnoid hemorrhage based on the inhibition of inflammatory injury and neuronal apoptosis and the induction of glutamate oxaloacetate transaminase-mediated glutamate metabolism (Wang et al., 2015c; Khanna et al., 2017; Wu et al., 2018).
BCA Plays Complex Roles in Paradoxical Drug–Drug Interactions
Multidrug resistance (MDR) is a major obstacle to the success of cancer chemotherapy and is a complex and multifactorial phenomenon. One important classical mechanism of MDR is the overexpression of drug efflux transporters, such as P-glycoprotein (P-gp). P-gp confers resistance by actively pumping cytotoxic drugs out of cancer cells (Savas et al., 1992). As a multidrug transporter, P-gp also influences the distribution of many other types of drugs (Singh et al., 2012; Stępień et al., 2012). However, BCA can inhibit P-gp-mediated cellular efflux by modulating P-gp ATPase activity without changing the cellular P-gp level (Zhang and Morris, 2003; Zhang et al., 2010; Chung et al., 2005; Dash and Konkimalla, 2017). Interestingly, BCA has been found to stimulate P-gp in some studies (An and Morris, 2010). Therefore, the effect of BCA on P-gp may be substrate dependent. BCA differentially affects the oral bioavailability of some P-gp substrates (Peng et al., 2006; An and Morris, 2010; Singh et al., 2012; Li et al., 2016). BCA can also inhibit non-P-gp-mediated pathways in MDR (Versantvoort et al., 1993), such as MDR-associated protein 1 (MRP1)-mediated drug transport (Versantvoort et al., 1993; Nguyen et al., 2003) and breast cancer resistance protein (BCRP)-mediated cellular efflux, because BCA sulfate is a substrate of BCRP (An and Morris, 2010). Oatp3 is a highly expressed influx/efflux transporter in the rat small intestine that plays an important role in limiting the absorption and, therefore, bioavailability of its substrates. BCA has been shown to inhibit Oatp3, causing a decrease in drug bioavailability (Peng et al., 2006). BCA synergizes with quinolones to inhibit Staphylococcus aureus by increasing the accumulation of ciprofloxacin and suppressing the bacterial expression of the norA protein and the efflux system [adenosine triphosphate (ATP)-binding ABC transporters] but has no inhibitory effect on the bacteria alone (Liu et al., 2011; Zou et al., 2014). Synergy between quinolones and BCA has also been observed in the treatment of pathogenic mycoplasma and Mycobacterium avium (Jin et al., 2017; Cannalire et al., 2017). The most common mechanism underlying these drug–drug interactions is the inhibition of the CYP system, which is responsible for the metabolism of nearly 90% of drugs in humans. BCA exerts minimal effects on CYP isoforms other than CYP1A2 and CYP3A4. The consumption of BCA along with other drugs is assumed to be safe with a minimal possibility of alterations in the pharmacokinetics of the coadministered drugs (Arora et al., 2015; Kopečná-Zapletalová et al., 2017). However, BCA was found to enhance the distribution and cytotoxicity of some drugs in vivo and cause unwanted pharmacokinetic interactions (Zhang and Morris, 2003; An and Morris, 2010; Li et al., 2016). BCA ameliorated the adverse effects of some anticarcinogens by increasing their cellular uptake and efficacy to reverse drug resistance, significantly improving serum oxidant/antioxidant activity or modulating the proliferation and apoptosis of cancer cells (Youssef et al., 2016; Galal et al., 2018). BCA acts as a nephroprotective agent in the presence of certain chemotherapeutics, such as cisplatin, because of its anti-inflammatory and antiapoptotic activities (Suliman et al., 2018) and protects heart tissue and the kidney against arsenic toxicity because of its antioxidant characteristics (Jalaludeen et al., 2015, Jalaludeen et al., 2016). The complex roles of BCA in paradoxical drug–drug interactions are summarized in Table S2.
It is possible that BCA could be used alone or in combination with other drugs to reverse MDR. However, the probability of pharmacokinetic interactions must be carefully considered before the coadministration of BCA with other drugs.
Bioavailability of BCA
Because of its potential benefits, BCA has been studied in many in vitro and in vivo experiments. However, BCA is a poorly soluble bioflavonoid, and this characteristic prevents its oral absorption. BCA has a high clearance and a large apparent volume of distribution, and its bioavailability is poor. BCA (Figure 1A) has been reported to undergo extensive metabolism in vivo; GEN (Figure 1B) and sulfate and glucuronide conjugates are the major metabolites in the blood of humans. Significant levels of BCA and GEN conjugates were detected in plasma and bile in vivo (Moon et al., 2006). These metabolites may contribute to the chemopreventive effects of BCA and might have longer exposure periods depending on enterohepatic recycling. The administration of multiple flavonoids, including BCA, leads to increased flavonoid bioavailability and decreased clearance potentially caused by increased enterohepatic cycling (Moon and Morris, 2007).
However, the low biological availability and poor aqueous solubility of BCA limit its usefulness as a chemotherapeutic agent. Various attempts have been made to improve the solubility and bioavailability of BCA, including the use of liposomes (Hendrich et al., 2002), dispersion agents (Han et al., 2011), silver nanoparticles (Sekine et al., 2011), different film formulations for buccal delivery (Hanski et al., 2014), nanostructured lipid carriers (Wang et al., 2013), nanostructured lipid carriers modified with polyethylene glycol (PEG) (Wang et al., 2015a), enteric-coated microparticles (Sachdeva et al., 2016), micelles (Wu et al., 2017), and inclusion complexes with cyclodextrins (Nikolic et al., 2018). Ester and carbamate ester derivatives of BCA (Figure 1C) and several carboxy–BCA compounds (Figures 1D, E) have been synthesized. These derivatives maintain estrogenic and cancer chemopreventive activities, and some have better metabolic stability than BCA in cells (Somjen et al., 2005; Kohen et al., 2007; Somjen et al., 2011; Fokialakis et al., 2012). These efforts have enhanced the solubility and bioavailability of BCA while maintaining its efficacy and activity. These findings provide excellent prospects for the application of BCA in the treatment of various diseases.
Conclusion
BCA has shown many potential benefits in numerous in vitro and in vivo studies. However, the safety of supplements containing BCA is unknown, and actual evidence from patients is limited; therefore, more research needs to be performed in this field.
Author Contributions
YW conceived the general idea. CY, LL, and PZ wrote the first draft. YW revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant Number 81801972).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.00793/full#supplementary-material
References
Ahmad, A., Biersack, B., Li, Y., Bao, B., Kong, D., Ali, S., et al. (2013). Perspectives on the role of isoflavones in prostate cancer. AAPS J. 15 (4), 991–1000. doi: 10.1208/s12248-013-9507-1
Almstrup, K., Fernández, M. F., Petersen, J. H., Olea, N., Skakkebaek, N. E., Leffers, H. (2002). Dual effects of phytoestrogens result in u-shaped dose-response curves. Environ. Health Perspect. 110 (8), 743–748. doi: 10.1289/ehp.02110743
An, G., Morris, M. E. (2010). Effects of single and multiple flavonoids on BCRP-mediated accumulation, cytotoxicity and transport of mitoxantrone in vitro. Pharm. Res. 27 (7), 1296–1308. doi: 10.1007/s11095-010-0108-8
An, J., Tzagarakis-Foster, C., Scharschmidt, T. C., Lomri, N., Leitman, D. C. (2001). Estrogen receptor beta-selective transcriptional activity and recruitment of coregulators by phytoestrogens. J. Biol. Chem. 276 (21), 17808–17814. doi: 10.1074/jbc.M100953200
Arora, S., Taneja, I., Challagundla, M., Raju, K. S., Singh, S. P., Wahajuddin, M. (2015). In vivo prediction of CYP-mediated metabolic interaction potential of formononetin and biochanin A using in vitro human and rat CYP450 inhibition data. Toxicol. Lett. 239 (1), 1–8. doi: 10.1016/j.toxlet.2015.08.202
Asgary, S., Naderi, G. A., Zadegan, N. S., Vakili, R. (2002). The inhibitory effects of pure flavonoids on in vitro protein glycosylation. J. Herb Pharmacother. 2 (2), 47–55. doi: 10.1080/J157v02n02_05
Atkinson, C., Compston, J. E., Day, N. E., Dowsett, M., Bingham, S. A. (2004). The effects of phytoestrogen isoflavones on bone density in women: a double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 79 (2), 326–333. doi: 10.1093/ajcn/79.2.326
Azizi, R., Goodarzi, M. T., Salemi, Z. (2014). Effect of biochanin a on serum visfatin level of streptozocin-induced diabetic rats. Iran Red Crescent Med. J. 16 (9), e15424. doi: 10.5812/ircmj.15424
Bachiller, S., Jiménez-Ferrer, I., Paulus, A., Yang, Y., Swanberg, M., Deierborg, T., et al. (2018). Microglia in neurological diseases: a road map to brain-disease dependent-inflammatory response. Front. Cell. Neurosci. 12, 488. doi: 10.3389/fncel.2018.00488
Balabhadrapathruni, S., Thomas, T. J., Yurkow, E. J., Amenta, P. S., Thomas, T. (2000). Effects of genistein and structurally related phytoestrogens on cell cycle kinetics and apoptosis in MDA-MB-468 human breast cancer cells. Oncol. Rep. 7 (1), 3–12. doi: 10.3892/or.7.1.3
Beck, V., Rohr, U., Jungbauer, A. (2005). Phytoestrogens derived from red clover: an alternative to estrogen replacement therapy? J. Steroid Biochem. Mol. Biol. 94 (5), 499–518. doi: 10.1016/j.jsbmb.2004.12.038
Beck, V., Unterrieder, E., Krenn, L., Kubelka, W., Jungbauer, A. (2003). Comparison of hormonal activity (estrogen, androgen and progestin) of standardized plant extracts for large scale use in hormone replacement therapy. J. Steroid Biochem. Mol. Biol. 84 (2–3), 259–268. doi: 10.1016/S0960-0760(03)00034-7
Bhardwaj, V., Tadinada, S. M., Jain, A., Sehdev, V., Daniels, C. K., Lai, J. C., et al. (2014). Biochanin A reduces pancreatic cancer survival and progression. Anticancer Drugs 25 (3), 296–302. doi: 10.1097/CAD.0000000000000044
Biradar, S. M., Joshi, H., Chheda, T. K. (2014). Biochanin-A ameliorates behavioural and neurochemical derangements in cognitive-deficit mice for the betterment of Alzheimer’s disease. Hum. Exp. Toxicol. 33 (4), 369–382. doi: 10.1177/0960327113497772
Booth, N. L., Piersen, C. E., Banuvar, S., Geller, S. E., Shulman, L. P., Farnsworth, N. R. (2006). Clinical studies of red clover (Trifolium pratense) dietary supplements in menopause: a literature review. Menopause 13 (2), 251–264. doi: 10.1097/01.gme.0000198297.40269.f7
Breikaa, R. M., Algandaby, M. M., El-Demerdash, E., Abdel-Naim, A. B. (2013a). Biochanin A protects against acute carbon tetrachloride-induced hepatotoxicity in rats. Biosci. Biotechnol. Biochem. 77 (5), 909–916. doi: 10.1271/bbb.120675
Breikaa, R. M., Algandaby, M. M., El-Demerdash, E., Abdel-Naim, A. B. (2013b). Multimechanistic antifibrotic effect of biochanin a in rats: implications of proinflammatory and profibrogenic mediators. PLoS One 8 (7), e69276. doi: 10.1371/journal.pone.0069276
Cannalire, R., Machado, D., Felicetti, T., Santos Costa, S., Massari, S., Manfroni, G., et al. (2017). Natural isoflavone biochanin A as a template for the design of new and potent 3-phenylquinolone efflux inhibitors against Mycobacterium avium. Eur. J. Med. Chem. 140, 321–330. doi: 10.1016/j.ejmech.2017.09.014
Cassady, J. M., Zennie, T. M., Chae, Y. H., Ferin, M. A., Portuondo, N. E., Baird, W. M. (1988). Use of a mammalian cell culture benzo(a)pyrene metabolism assay for the detection of potential anticarcinogens from natural products: inhibition of metabolism by biochanin A, an isoflavone from Trifolium pratense L. Cancer Res. 48 (22), 6257–6261.
Chae, Y. H., Marcus, C. B., Ho, D. K., Cassady, J. M., Baird, W. M. (1991). Effects of synthetic and naturally occurring flavonoids on benzo[a]pyrene metabolism by hepatic microsomes prepared from rats treated with cytochrome P-450 inducers. Cancer Lett. 60 (1), 15–24. doi: 10.1016/0304-3835(91)90044-I
Chen, H. Q., Jin, Z. Y., Li, G. H. (2007). Biochanin A protects dopaminergic neurons against lipopolysaccharide-induced damage through inhibition of microglia activation and proinflammatory factors generation. Neurosci. Lett. 417 (2), 112–117. doi: 10.1016/j.neulet.2006.11.045
Chundi, V., Challa, S. R., Garikapati, D. R., Juvva, G., Jampani, A., Pinnamaneni, S. H., et al. (2016). Biochanin-A attenuates neuropathic pain in diabetic rats. J. Ayurveda Integr. Med. 7 (4), 231–237. doi: 10.1016/j.jaim.2016.08.001
Chung, M. J., Sohng, J. K., Choi, D. J., Park, Y. I. (2013). Inhibitory effect of phloretin and biochanin A on IgE-mediated allergic responses in rat basophilic leukemia RBL-2H3 cells. Life Sci. 93 (9-11), 401–4108. doi: 10.1016/j.lfs.2013.07.019
Chung, S. Y., Sung, M. K., Kim, N. H., Jang, J. O., Go, E. J., Lee, H. J. (2005). Inhibition of P-glycoprotein by natural products in human breast cancer cells. Arch. Pharm. Res. 28 (7), 823–828. doi: 10.1007/BF02977349
Cirone, M., Zompetta, C., Tarasi, D., Frati, L., Faggioni, A. (1996). Infection of human T lymphoid cells by human herpesvirus 6 is blocked by two unrelated protein tyrosine kinase inhibitors, biochanin A and herbimycin. AIDS Res. Hum. Retroviruses 12 (17), 1629–1634. doi: 10.1089/aid.1996.12.1629
Dannenberg, A. J., Subbaramaiah, K. (2003). Targeting cyclooxygenase-2 in human neoplasia: rationale and promise. Cancer Cell 4 (6), 431–436. doi: 10.1016/S1535-6108(03)00310-6
Dash, T. K., Konkimalla, V. B. (2017). Formulation and optimization of doxorubicin and biochanin a combinational liposomes for reversal of chemoresistance. AAPS PharmSciTech. 18 (4), 1116–1124. doi: 10.1208/s12249-016-0614-z
Elsherbini, A. M., Mohammed, M. A. R., Ibrahim, F. M. (2017). Effect of biochanin a versus 17β estradiol in rat submandibular salivary gland. J. Oral Sci. 59 (4), 579–588. doi: 10.2334/josnusd.16-0651
Fokialakis, N., Alexi, X., Aligiannis, N., Siriani, D., Meligova, A. K., Pratsinis, H., et al. (2012). Ester and carbamate ester derivatives of biochanin A: synthesis and in vitro evaluation of estrogenic and antiproliferative activities. Bioorg. Med. Chem. 20 (9), 2962–2970. doi: 10.1016/j.bmc.2012.03.012
Galal, A. A. A., Mohamed, A. A., Khater, S. I., Metwally, M. M. M. (2018). Beneficial role of biochanin A on cutaneous and renal tissues of ovariectomized rats treated with anastrozole. Life Sci. 201, 9–16. doi: 10.1016/j.lfs.2018.03.037
Ghobeh, M., Ahmadian, S., Meratan, A. A., Ebrahim-Habibi, A., Ghasemi, A., Shafizadeh, M., et al. (2014). Interaction of Aβ(25-35) fibrillation products with mitochondria: effect of small-molecule natural products. Biopolymers 102 (6), 473–86. doi: 10.1002/bip.22572
Grainger, E. M., Moran, N. E., Francis, D. M., Schwartz, S. J., Wan, L., Thomas-Ahner, J., et al. (2019). A novel tomato-soy juice induces a dose-response increase in urinary and plasma phytochemical biomarkers in men with prostate cancer. J. Nutr. 149 (1), 26–35. doi: 10.1093/jn/nxy232
Hajrezaie, M., Salehen, N., Karimian, H., Zahedifard, M., Shams, K., Al Batran, R., et al. (2015). Biochanin a gastroprotective effects in ethanol-induced gastric mucosal ulceration in rats. PLoS One 10 (3), e0121529. doi: 10.1371/journal.pone.0121529
Han, H. K., Lee, B. J., Lee, H. K. (2011). Enhanced dissolution and bioavailability of biochanin A via the preparation of solid dispersion: in vitro and in vivo evaluation. Int. J. Pharm. 415 (1–2), 89–94. doi: 10.1016/j.ijpharm.2011.05.055
Hanski, L., Genina, N., Uvell, H., Malinovskaja, K., Gylfe, Å., Laaksonen, T., et al. (2014). Inhibitory activity of the isoflavone biochanin A on intracellular bacteria of genus Chlamydia and initial development of a buccal formulation. PLoS One 9 (12), e115115. doi: 10.1371/journal.pone.0115115
Harini, R., Ezhumalai, M., Pugalendi, K. V. (2012). Antihyperglycemic effect of biochanin A, a soy isoflavone, on streptozotocin-diabetic rats. Eur. J. Pharmacol. 676 (1–3), 89–94. doi: 10.1016/j.ejphar.2011.11.051
Heinonen, S., Wähälä, K., Adlercreutz, H. (1999). Identification of isoflavone metabolites dihydrodaidzein, dihydrogenistein, 6′-oh-o-dma, and cis-4-oh-equol in human urine by gas chromatography–mass spectroscopy using authentic reference compounds. Anal. Biochem. 274, 211–219. doi: 10.1006/abio.1999.4279
Hellström, A. C., Muntzing, J. (2012). The pollen extract Femal–a nonestrogenic alternative to hormone therapy in women with menopausal symptoms. Menopause 19 (7), 825–829. doi: 10.1097/gme.0b013e31824017bc
Hendrich, A. B., Zugaj, J., Michalak, K. (2002). Biochanin A similarly influences the fluidity of liposomes formed from charged and zwitterionic lipids. Cell. Mol. Biol. Lett. 7, 284.
Howes, J., Waring, M., Huang, L., Howes, L. G. (2002). Long-term pharmacokinetics of an extract of isoflavones from red clover (Trifolium pratense). J. Altern. Complement. Med. 8 (2), 135–142. doi: 10.1089/107555302317371424
Hsu, Y. N., Shyu, H. W., Hu, T. W., Yeh, J. P., Lin, Y. W., Lee, L. Y., et al. (2018). Anti-proliferative activity of biochanin A in human osteosarcoma cells via mitochondrial-involved apoptosis. Food Chem. Toxicol. 112, 194–204. doi: 10.1016/j.fct.2017.12.062
Huang, Y., Erdmann, N., Peng, H., Zhao, Y., Zheng, J. (2005). The role of TNF related apoptosis-inducing ligand in neurodegenerative diseases. Cell. Mol. Immunol. 2 (2), 113–122.
Jain, A., Lai, J. C., Bhushan, A. (2015). Biochanin A inhibits endothelial cell functions and proangiogenic pathways: implications in glioma therapy. Anticancer Drugs 26 (3), 323–30. doi: 10.1097/CAD.0000000000000189
Jalaludeen, A. M., Ha, W. T., Lee, R., Kim, J. H., Do, J. T., Park, C., et al. (2016). Biochanin A ameliorates arsenic-induced hepato- and hematotoxicity in rats. Molecules 21 (1), 69. doi: 10.3390/molecules21010069
Jalaludeen, A. M., Lee, W. Y., Kim, J. H., Jeong, H. Y., Ki, K. S., Kwon, E. G., et al. (2015). Therapeutic efficacy of biochanin A against arsenic-induced renal and cardiac damage in rats. Environ. Toxicol. Pharmacol. 39 (3), 1221–1231. doi: 10.1016/j.etap.2015.04.020
Jin, H., Qi, C., Zou, Y., Kong, Y., Ruan, Z., Ding, H., et al. (2017). Biochanin A partially restores the activity of ofloxacin and ciprofloxacin against topoisomerase IV mutation-associated fluoroquinolone-resistant Ureaplasma species. J. Med. Microbiol. 66 (11), 1545–1553. doi: 10.1099/jmm.0.000598
Kaczmarczyk-Sedlak, I., Zych, M., Wojnar, W., Ozimina-Kamińska, E., Dudek, S., Chadała, N., et al. (2015). Biochanin A shows no effect on skeletal system in ovariectomized rats, when administered in moderate dose. Acta Pol. Pharm. 72 (3), 587–596.
Khanna, S., Stewart, R., Gnyawali, S., Harris, H., Balch, M., Spieldenner, J., et al. (2017). Phytoestrogen isoflavone intervention to engage the neuroprotective effect of glutamate oxaloacetate transaminase against stroke. FASEB J. 31 (10), 4533–4544. doi: 10.1096/fj.201700353
Knight, D. C., Eden, J. A. (1996). A review of the clinical effects of phytoestrogens. Obstet. Gynecol. 87 (5 Pt 2), 897–904.
Ko, W. C., Lin, L. H., Shen, H. Y., Lai, C. Y., Chen, C. M., Shih, C. H. (2011). Biochanin a, a phytoestrogenic isoflavone with selective inhibition of phosphodiesterase 4, suppresses ovalbumin-induced airway hyperresponsiveness. Evid. Based Complement. Alternat. Med. 2011, 635058. doi: 10.1155/2011/635058
Kohen, F., Gayer, B., Kulik, T., Frydman, V., Nevo, N., Katzburg, S., et al. (2007). Synthesis and evaluation of the antiproliferative activities of derivatives of carboxyalkyl isoflavones linked to N-t-Boc-hexylenediamine. J. Med. Chem. 50 (25), 6405–6410. doi: 10.1021/jm070727z
Kole, L., Giri, B., Manna, S. K., Pal, B., Ghosh, S. (2011). Biochanin-A, an isoflavon, showed anti-proliferative and anti-inflammatory activities through the inhibition of iNOS expression, p38-MAPK and ATF-2 phosphorylation and blocking NF-κB nuclear translocation. Eur. J. Pharmacol. 653 (1-3), 8–15. doi: 10.1016/j.ejphar.2010.11.026
Kopečná-Zapletalová, M., Krasulová, K., Anzenbacher, P., Hodek, P., Anzenbacherová, E. (2017). Interaction of isoflavonoids with human liver microsomal cytochromes P450: inhibition of CYP enzyme activities. Xenobiotica 47 (4), 324–331. doi: 10.1080/00498254.2016.1195028
Křížová, L., Dadáková, K., Kašparovská, J., Kašparovský, T. (2019). Isoflavones. Molecules 24 (6), E1076. doi: 10.3390/molecules24061076
Kweon, S. S., Shu, X. O., Xiang, Y., Cai, H., Yang, G., Ji, B. T., et al. (2013). Intake of specific nonfermented soy foods may be inversely associated with risk of distal gastric cancer in a Chinese population. J. Nutr. 143 (11), 1736–1742. doi: 10.3945/jn.113.177675
Lai, X., Li, Y., Gao, M. (2018). Biochanin A regulates the growth and migration of NSCLC through suppressing the VEGF/VEGFR2 signaling pathway. Oncol. Res. doi: 10.3727/096504018X15321979274728
Lam, A. N., Demasi, M., James, M. J., Husband, A. J., Walker, C. (2004). Effect of red clover isoflavones on cox-2 activity in murine and human monocyte/macrophage cells. Nutr. Cancer 49 (1), 89–93. doi: 10.1207/s15327914nc4901_12
Lee, K. H., Choi, E. M. (2005). Biochanin A stimulates osteoblastic differentiation and inhibits hydrogen peroxide-induced production of inflammatory mediators in MC3T3-E1 cells. Biol. Pharm. Bull. 28 (10), 1948–1953. doi: 10.1248/bpb.28.1948
Lee, Y. S., Kim, T. H., Cho, K. J., Jang, J. J. (1992). Inhibitory effects of biochanin A on benzo(a)pyrene induced carcinogenesis in mice. In Vivo 6 (3), 283–286.
Lee, Y. S., Seo, J. S., Chung, H. T., Jang, J. J. (1991). Inhibitory effects of biochanin A on mouse lung tumor induced by benzo(a)pyrene. J. Korean Med. Sci. 6 (4), 325–328. doi: 10.3346/jkms.1991.6.4.325
Li, G., Gao, Q., Yuan, S., Wang, L., Altmeyer, R., Lan, K., et al. (2017). Characterization of three small molecule inhibitors of enterovirus 71 identified from screening of a library of natural products. Antiviral Res. 143, 85–96. doi: 10.1016/j.antiviral.2017.04.006
Li, J., Liu, Y., Yu, X., Zhang, J., Gu, J., Zhao, L. (2016). reduced oral bioavailability and altered pharmacokinetics of saquinavir by co-administration with biochanin a in rats. Drug Res. (Stuttg) 66 (9), 484–488. doi: 10.1055/s-0042-110393
Liang, F., Cao, W., Huang, Y., Fang, Y., Cheng, Y., Pan, S., et al. (2019). Isoflavone biochanin A, a novel nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response element activator, protects against oxidative damage in HepG2 cells. Biofactors 1–12. doi: 10.1002/biof.1514
Lim, T. G., Kim, J. E., Jung, S. K., Li, Y., Bode, A. M., Park, J. S., et al. (2013). MLK3 is a direct target of biochanin A, which plays a role in solar UV-induced COX-2 expression in human keratinocytes. Biochem. Pharmacol. 86 (7), 896–903. doi: 10.1016/j.bcp.2013.08.002
Lin, V. C., Ding, H. Y., Tsai, P. C., Wu, J. Y., Lu, Y. H., Chang, T. S. (2011). In vitro and in vivo melanogenesis inhibition by biochanin A from Trifolium pratense. Biosci. Biotechnol. Biochem. 75 (5), 914–918. doi: 10.1271/bbb.100878
Liu, G., Liang, J. C., Wang, X. L., Li, Z. H., Wang, W., Guo, N., et al. (2011). In Vitro Synergy of Biochanin A and ciprofloxacin against clinical isolates of staphylococcus aureus. Molecules 16 (8), 6656–6666. doi: 10.3390/molecules16086656
Luo, Q., Shi, X., Ding, J., Ma, Z., Chen, X., Leng, Y., et al. (2019). Network pharmacology integrated molecular docking reveals the antiosteosarcoma mechanism of biochanin A. Evid. Based Complement. Alternat. Med. 2019, 1410495. doi: 10.1155/2019/1410495
Medjakovic, S., Jungbauer, A. (2008). Red clover isoflavones biochanin A and formononetin are potent ligands of the human aryl hydrocarbon receptor. J. Steroid Biochem. Mol. Biol. 108, 171–177. doi: 10.1016/j.jsbmb.2007.10.001
Mehrabadi, M. E., Salemi, Z., Babaie, S., Panahi, M. (2018). Effect of Biochanin A on retina levels of vascular endothelial growth factor, tumor necrosis factor-alpha and interleukin-1beta in rats with streptozotocin-induced diabetes. Can. J. Diabetes 42 (6), 639–644. doi: 10.1016/j.jcjd.2018.03.008
Michaelis, M., Sithisarn, P., Cinatl, J., Jr. (2014). Effects of flavonoid-induced oxidative stress on anti-H5N1 influenza a virus activity exerted by baicalein and biochanin A. BMC Res. Notes 7, 384. doi: 10.1186/1756-0500-7-384
Ming, X., Ding, M., Zhai, B., Xiao, L., Piao, T., Liu, M. (2015). Biochanin A inhibits lipopolysaccharide-induced inflammation in human umbilical vein endothelial cells. Life Sci. 136, 36–41. doi: 10.1016/j.lfs.2015.06.015
Mohamed, A. A., Ahmed, M. M., Gomaa, M., Ebraheim, L. L. M. (2018). Bone health consequence of adjuvant anastrozole in monotherapy or associated with biochanin-A in ovariectomized rat model. Life Sci. 212, 159–167. doi: 10.1016/j.lfs.2018.09.059
Moon, Y. J., Morris, M. E. (2007). Pharmacokinetics and bioavailability of the bioflavonoid biochanin A: effects of quercetin and EGCG on biochanin A disposition in rats. Mol. Pharm. 4 (6), 865–872. doi: 10.1021/mp7000928
Moon, Y. J., Sagawa, K., Frederick, K., Zhang, S., Morris, M. E. (2006). Pharmacokinetics and bioavailability of the isoflavone biochanin A in rats. AAPS J. 8 (3), E433–E442. doi: 10.1208/aapsj080351
Mueller, M., Hobiger, S., Jungbauer, A. (2010). Red clover extract: a source for substances that activate peroxisome proliferator-activated receptor alpha and ameliorate the cytokine secretion profile of lipopolysaccharide-stimulated macrophages. Menopause 17 (2), 379–387. doi: 10.1097/gme.0b013e3181c94617
Nguyen, H., Zhang, S., Morris, M. E. (2003). Effect of flavonoids on MRP1-mediated transport in Panc-1 cells. J. Pharm. Sci. 92 (2), 250–257. doi: 10.1002/jps.10283
Nikolic, I. L., Savic, I. M., Popsavin, M. M., Rakic, S. J., Mihajilov-Krstev, T. M., Ristic, I. S., et al. (2018). Preparation, characterization and antimicrobial activity of inclusion complex of biochanin A with (2-hydroxypropyl)-β-cyclodextrin. J. Pharm. Pharmacol. 70 (11), 1485–1493. doi: 10.1111/jphp.13003
Occhiuto, F., Palumbo, D. R., Samperi, S., Zangla, G., Pino, A., De Pasquale, R., et al. (2009). The isoflavones mixture from Trifolium pratense L. Phytother. Res. 23 (2), 192–196. doi: 10.1002/ptr.2584
Oh, J. S., Cho, I. A., Kang, K. R., You, J. S., Yu, S. J., Lee, G. J., et al. (2016). Biochanin-A antagonizes the interleukin-1β-induced catabolic inflammation through the modulation of NFκB cellular signaling in primary rat chondrocytes. Biochem. Biophys. Res. Commun. 477 (4), 723–730. doi: 10.1016/j.bbrc.2016.06.126
Oza, M. J., Kulkarni, Y. A. (2018). Biochanin A improves insulin sensitivity and controls hyperglycemia in type 2 diabetes. Biomed. Pharmacother. 107, 1119–1127. doi: 10.1016/j.biopha.2018.08.073
Park, H. S., Hur, H. J., Kim, S. H., Park, S. J., Hong, M. J., Sung, M. J., et al. (2016). Biochanin A improves hepatic steatosis and insulin resistance by regulating the hepatic lipid and glucose metabolic pathways in diet-induced obese mice. Mol. Nutr. Food Res. 60 (9), 1944–1955. doi: 10.1002/mnfr.201500689
Park, J., Chung, S. W., Kim, S. H., Kim, T. S. (2006). Up-regulation of interleukin-4 production via NF-AT/AP-1 activation in T cells by biochanin A, a phytoestrogen and its metabolites. Toxicol. Appl. Pharmacol. 212 (3), 188–199. doi: 10.1016/j.taap.2005.08.001
Patil, K. K., Meshram, R. J., Dhole, N. A., Gacche, R. N. (2016). Role of dietary flavonoids in amelioration of sugar induced cataractogenesis. Arch. Biochem. Biophys. 593, 1–11. doi: 10.1016/j.abb.2016.01.015
Peng, S. X., Ritchie, D. M., Cousineau, M., Danser, E., Dewire, R., Floden, J. (2006). Altered oral bioavailability and pharmacokinetics of P-glycoprotein substrates by coadministration of biochanin A. J. Pharm. Sci. 95 (9), 1984–1993. doi: 10.1002/jps.20664
Perez-Cornago, A., Appleby, P. N., Boeing, H., Gil, L., Kyrø, C., Ricceri, F., et al. (2018). Circulating isoflavone and lignan concentrations and prostate cancer risk: a meta-analysis of individual participant data from seven prospective studies including 2,828 cases and 5,593 controls. Int. J. Cancer 143 (11), 2677–2686. doi: 10.1002/ijc.31640
Peterson, G., Barnes, S. (1993). Genistein and biochanin A inhibit the growth of human prostate cancer cells but not epidermal growth factor receptor tyrosine autophosphorylation. Prostate 22 (4), 335–345. doi: 10.1002/pros.2990220408
Peterson, G., Barnes, S. (1991). Genistein inhibition of the growth of human breast cancer cells: independence from estrogen receptors and the multi-drug resistance gene. Biochem. Biophys. Res. Commun. 179 (1), 661–667. doi: 10.1016/0006-291X(91)91423-A
Pozzi, S., Benedusi, V., Maggi, A., Vegeto, E. (2006). Estrogen action in neuroprotection and brain inflammation. Ann. N. Y. Acad. Sci. 1089, 302–323. doi: 10.1196/annals.1386.035
Puthli, A., Tiwari, R., Mishra, K. P. (2013). Biochanin A enhances the radiotoxicity in colon tumor cells in vitro. J. Environ. Pathol. Toxicol. Oncol. 32 (3), 189–203. doi: 10.1615/JEnvironPatholToxicolOncol.2013007280
Puli, S., Lai, J. C., Bhushan, A. (2006). Inhibition of matrix degrading enzymes and invasion in human glioblastoma (U87MG) cells by isoflavones. J. Neurooncol. 79 (2), 135–142. doi: 10.1007/s11060-006-9126-0
Qiu, L., Lin, B., Lin, Z., Lin, Y., Lin, M., Yang, X. (2012). Biochanin A ameliorates the cytokine secretion profile of lipopolysaccharide-stimulated macrophages by a PPARγ-dependent pathway. Mol. Med. Rep. 5 (1), 217–222. doi: 10.3892/mmr.2011.599
Roberts, D. W., Doerge, D. R., Churchwell, M. I., Da Costa, G. G., Marques, M. M., Tolleson, W. H. (2004). Inhibition of extrahepatic human cytochromes P450 1A1 and 1B1 by metabolism of isoflavones found in Trifolium pratense (red clover). J. Agric. Food Chem. 52 (21), 6623–32. doi: 10.1021/jf049418x
Sachdeva, C., Mishra, N., Sharma, S. (2016). Development and characterization of enteric-coated microparticles of biochanin A for their beneficial pharmacological potential in estrogen deficient-hypertension. Drug Deliv. 23 (6), 2044–2057. doi: 10.3109/10717544.2015.1114046
Savas, B., Cole, S. P., Akoglu, T. F., Pross, H. F. (1992). P-glycoprotein-mediated multidrug resistance and cytotoxic effector cells. Nat. Immun. 11 (4), 177–192.
Sehdev, V., Lai, J. C., Bhushan, A. (2009). Biochanin A modulates cell viability, invasion, and growth promoting signaling pathways in her-2-positive breast cancer cells. J. Oncol. 2009, 121458. doi: 10.1155/2009/121458
Sehm, T., Fan, Z., Weiss, R., Schwarz, M., Engelhorn, T., Hore, N., et al. (2014). The impact of dietary isoflavonoids on malignant brain tumors. Cancer Med. 3 (4), 865–877. doi: 10.1002/cam4.265
Sekine, R., Vongsvivut, J., Robertson, E. G., Spiccia, L., McNaughton, D. (2011). Analysis of 5-hydroxyisoflavones by surface-enhanced Raman spectroscopy: genistein and methoxy derivatives. J. Phys. Chem. B. 115 (47), 13943–13954. doi: 10.1021/jp207730g
Setchell, K. D., Brown, N. M., Desai, P., Zimmer-Nechemias, L., Wolfe, B. E., Brashear, W. T., et al. (2001). Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J. Nutr. 131 (4 Suppl), 1362S–75S. doi: 10.1093/jn/131.4.1362S
Singh, S. P., Wahajuddin, Raju, K. S., Ali, M. M., Kohli, K., Jain, G. K. (2012). Reduced bioavailability of tamoxifen and its metabolite 4-hydroxytamoxifen after oral administration with biochanin A (an isoflavone) in rats. Phytother. Res. 26 (2), 303–307. doi: 10.1002/ptr.3652
Sithisarn, P., Michaelis, M., Schubert-Zsilavecz, M., Cinatl, J., Jr. (2013). Differential antiviral and anti-inflammatory mechanisms of the flavonoids biochanin A and baicalein in H5N1 influenza A virus-infected cells. Antiviral Res. 97 (1), 41–48. doi: 10.1016/j.antiviral.2012.10.004
Sklenickova, O., Flesar, J., Kokoska, L., Vlkova, E., Halamova, K., Malik, J. (2010). Selective growth inhibitory effect of biochanin A against intestinal tract colonizing bacteria. Molecules 15 (3), 1270–1279. doi: 10.3390/molecules15031270
Somjen, D., Katzburg, S., Kohen, F., Gayer, B., Posner, G. H., Yoles, I., et al. (2011). The effects of native and synthetic estrogenic compounds as well as vitamin D less-calcemic analogs on adipocytes content in rat bone marrow. J. Endocrinol. Invest. 34 (2), 106–110. doi: 10.1007/BF03347039
Somjen, D., Kohen, F., Lieberherr, M., Gayer, B., Schejter, E., Katzburg, S., et al. (2005). Membranal effects of phytoestrogens and carboxy derivatives of phytoestrogens on human vascular and bone cells: new insights based on studies with carboxy-biochanin A. J. Steroid Biochem. Mol. Biol. 93 (2–5), 293–303. doi: 10.1016/j.jsbmb.2004.12.029
Stępień, K. M., Tomaszewski, M., Tomaszewska, J., Czuczwar, S. J. (2012). The multidrug transporter P-glycoprotein in pharmacoresistance to antiepileptic drugs. Pharmacol. Rep. 64 (5), 1011–1019. doi: 10.1016/S1734-1140(12)70900-3
Su, S. J., Yeh, Y. T., Su, S. H., Chang, K. L., Shyu, H. W., Chen, K. M., et al. (2013a). Biochanin a promotes osteogenic but inhibits adipogenic differentiation: evidence with primary adipose-derived stem cells. Evid. Based Complement. Alternat. Med. 2013, 846039. doi: 10.1155/2013/846039
Su, S. J., Yeh, Y. T., Shyu, H. W. (2013b). The preventive effect of biochanin a on bone loss in ovariectomized rats: involvement in regulation of growth and activity of osteoblasts and osteoclasts. Evid. Based Complement. Alternat. Med. 2013, 594857. doi: 10.1155/2013/594857
Suliman, F. A., Khodeer, D. M., Ibrahiem, A., Mehanna, E. T., El-Kherbetawy, M. K., Mohammad, H. M. F., et al. (2018). Renoprotective effect of the isoflavonoid biochanin A against cisplatin induced acute kidney injury in mice: Effect on inflammatory burden and p53 apoptosis. Int. Immunopharmacol. 61, 8–19. doi: 10.1016/j.intimp.2018.05.010
Sun, X. Y., Plouzek, C. A., Henry, J. P., Wang, T. T., Phang, J. M. (1998). Increased UDP-glucuronosyltransferase activity and decreased prostate specific antigen production by biochanin A in prostate cancer cells. Cancer Res. 58 (11), 2379–2384.
Suuronen, T., Nuutinen, T., Huuskonen, J., Ojala, J., Thornell, A., Salminen, A. (2005). Anti-inflammatory effect of selective estrogen receptor modulators (SERMs) in microglial cells. Inflamm. Res. 54 (5), 194–203. doi: 10.1007/s00011-005-1343-z
Tan, J. W., Kim, M. K. (2016). Neuroprotective Effects of Biochanin A against β-Amyloid-Induced Neurotoxicity in PC12 Cells via a Mitochondrial-Dependent Apoptosis Pathway. Molecules 21 (5), E548. doi: 10.3390/molecules21050548
Tan, J. W., Tham, C. L., Israf, D. A., Lee, S. H., Kim, M. K. (2013). Neuroprotective effects of biochanin A against glutamate-induced cytotoxicity in PC12 cells via apoptosis inhibition. Neurochem. Res. 38 (3), 512–518. doi: 10.1007/s11064-012-0943-6
Thors, L., Burston, J. J., Alter, B. J., McKinney, M. K., Cravatt, B. F., Ross, R. A., et al. (2010). Biochanin A, a naturally occurring inhibitor of fatty acid amide hydrolase. Br. J. Pharmacol. 160 (3), 549–560. doi: 10.1111/j.1476-5381.2010.00716.x
Ullah, M. F., Shamim, U., Hanif, S., Azmi, A. S., Hadi, S. M. (2009). Cellular DNA breakage by soy isoflavone genistein and its methylated structural analogue biochanin A. Mol. Nutr. Food Res. 53 (11), 1376–1385. doi: 10.1002/mnfr.200800547
Van Duursen, M. B. M. (2017). Modulation of estrogen synthesis and metabolism by phytoestrogens in vitro and the implications for women’s health. Toxicol. Res. (Camb) 6 (6), 772–794. doi: 10.1039/C7TX00184C
Vanden Berghe, W., Dijsselbloem, N., Vermeulen, L., Ndlovu, M. N., Boone, E., Haegeman, G. (2006). Attenuation of mitogen- and stress-activated protein kinase-1-driven nuclear factor-kappaB gene expression by soy isoflavones does not require estrogenic activity. Cancer Res. 66 (9), 4852–4862. doi: 10.1158/0008-5472.CAN-05-2957
Vegeto, E., Belcredito, S., Ghisletti, S., Meda, C., Etteri, S., Maggi, A. (2006). The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology 147 (5), 2263–2272. doi: 10.1210/en.2005-1330
Versantvoort, C. H., Schuurhuis, G. J., Pinedo, H. M., Eekman, C. A., Kuiper, C. M., Lankelma, J., et al. (1993). Genistein modulates the decreased drug accumulation in non-P-glycoprotein mediated multidrug resistant tumour cells. Br. J. Cancer 68 (5), 939–946. doi: 10.1038/bjc.1993.458
Vitale, D. C., Piazza, C., Melilli, B., Drago, F., Salomone, S. (2013). Isoflavones: estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 38 (1), 15–25. doi: 10.1007/s13318-012-0112-y
Wada, K., Tsuji, M., Tamura, T., Konishi, K., Goto, Y., Mizuta, F., et al. (2018). Soy Isoflavone Intake and Bladder Cancer Risk in Japan: From the Takayama Study. Cancer Epidemiol. Biomarkers Prev. 27 (11), 1371–1375. doi: 10.1158/1055-9965.EPI-18-0283
Wang, L., Luo, Q., Lin, T., Li, R., Zhu, T., Zhou, K., et al. (2015a). PEGylated nanostructured lipid carriers (PEG-NLC) as a novel drug delivery system for biochanin A. Drug Dev. Ind. Pharm. 41 (7), 1204–1212. doi: 10.3109/03639045.2014.938082
Wang, J., He, C., Wu, W. Y., Chen, F., Wu, Y. Y., Li, W. Z., et al. (2015b). Biochanin A protects dopaminergic neurons against lipopolysaccharide-induced damage and oxidative stress in a rat model of Parkinson’s disease. Pharmacol. Biochem. Behav. 138, 96–103. doi: 10.1016/j.pbb.2015.09.013
Wang, J., Wu, W. Y., Huang, H., Li, W. Z., Chen, H. Q., Yin, Y. Y. (2016). Biochanin A protects against lipopolysaccharide-induced damage of dopaminergic neurons both in vivo and in vitro via inhibition of microglial activation. Neurotox. Res. 30 (3), 486–498. doi: 10.1007/s12640-016-9648-y
Wang, Q., Cheng, H., Zhou, K., Wang, L., Dong, S., Wang, D., et al. (2013). Nanostructured lipid carriers as a delivery system of biochanin A. Drug Deliv. 20 (8), 331–337. doi: 10.3109/10717544.2013.838716
Wang, W., Tang, L., Li, Y., Wang, Y. (2015c). Biochanin A protects against focal cerebral ischemia/reperfusion in rats via inhibition of p38-mediated inflammatory responses. J. Neurol. Sci. 348 (1–2), 121–125. doi: 10.1016/j.jns.2014.11.018
Wang, Y., Man Gho, W., Chan, F. L., Chen, S., Leung, L. K. (2008). The red clover (Trifolium pratense) isoflavone biochanin A inhibits aromatase activity and expression. Br. J. Nutr. 99 (2), 303–310. doi: 10.1017/S0007114507811974
Wu, D. Q., Zhong, H. M., Ding, Q. H., Ba, L. (2014). Protective effects of biochanin A on articular cartilage: in vitro and in vivo studies. BMC Complement. Altern. Med. 14, 444. doi: 10.1186/1472-6882-14-444
Wu, L. Y., Ye, Z. N., Zhuang, Z., Gao, Y., Tang, C., Zhou, C. H., et al. (2018). Biochanin A reduces inflammatory injury and neuronal apoptosis following subarachnoid hemorrhage via suppression of the TLRs/TIRAP/MyD88/NF-κB pathway. Behav. Neurol. 2018, 1960106. doi: 10.1155/2018/1960106
Wu, W. Y., Wu, Y. Y., Huang, H., He, C., Li, W. Z., Wang, H. L., et al. (2015). Biochanin A attenuates LPS-induced pro-inflammatory responses and inhibits the activation of the MAPK pathway in BV2 microglial cells. Int. J. Mol. Med. 35 (2), 391–398. doi: 10.3892/ijmm.2014.2020
Wu, X., Ge, W., Shao, T., Wu, W., Hou, J., Cui, L., et al. (2017). Enhancing the oral bioavailability of biochanin A by encapsulation in mixed micelles containing Pluronic F127 and Plasdone S630. Int. J. Nanomedicine 12, 1475–1483. doi: 10.2147/IJN.S125041
Xiao, P., Zheng, B., Sun, J., Yang, J. (2017). Biochanin A induces anticancer effects in SK-Mel-28 human malignant melanoma cells via induction of apoptosis, inhibition of cell invasion and modulation of NF-κB and MAPK signaling pathways. Oncol. Lett. 14 (5), 5989–5993. doi: 10.3892/ol.2017.6945
Xue, Z., Zhang, Q., Yu, W., Wen, H., Hou, X., Li, D., et al. (2017). Potential lipid-lowering mechanisms of biochanin A. J. Agric. Food Chem. 65 (19), 3842–3850. doi: 10.1021/acs.jafc.7b00967
Yanagihara, K., Ito, A., Toge, T., Numoto, M. (1993). Antiproliferative effects of isoflavones on human cancer cell lines established from the gastrointestinal tract. Cancer Res. 53 (23), 5815–5821.
Yanagihara, K., Numoto, M., Tauchi, H., Akama, Y., Yokozaki, H., Tahara, E., et al. (1996). Genetic status of p53 and induction of apoptosis by radiation or isoflavones in human gastric carcinoma cell lines. Int. J. Oncol. 9 (1), 95–102. doi: 10.3892/ijo.9.1.95
You, J., Sun, Y., Bo, Y., Zhu, Y., Duan, D., Cui, H., et al. (2018). The association between dietary isoflavones intake and gastric cancer risk: a meta-analysis of epidemiological studies. BMC Public Health 18 (1), 510. doi: 10.1186/s12889-018-5424-7
Youn, K., Park, J. H., Lee, J., Jeong, W. S., Ho, C. T., Jun, M. (2016). The identification of biochanin a as a potent and selective β-site app-cleaving enzyme 1 (bace1) inhibitor. Nutrients 8 (10), E637. doi: 10.3390/nu8100637
Youssef, M. M., Tolba, M. F., Badawy, N. N., Liu, A. W., El-Ahwany, E., Khalifa, A. E., et al. (2016). Novel combination of sorafenib and biochanin-A synergistically enhances the anti-proliferative and pro-apoptotic effects on hepatocellular carcinoma cells. Sci. Rep. 6, 30717. doi: 10.1038/srep30717
Yu, L., Wang, X., Chen, H., Yan, Z., Wang, M., Li, Y. (2017). Neurochemical and behavior deficits in rats with iron and rotenone co-treatment: role of redox imbalance and neuroprotection by biochanin A. Front. Neurosci. 11, 657. doi: 10.3389/fnins.2017.00657
Zarmouh, N. O., Eyunni, S. K., Soliman, K. F. (2017). The Benzopyrone Biochanin-A as a reversible, competitive, and selective monoamine oxidase B inhibitor. BMC Complement. Altern. Med. 17 (1), 34. doi: 10.1186/s12906-016-1525-y
Zhang, F. F., Haslam, D. E., Terry, M. B., Knight, J. A., Andrulis, I. L., Daly, M. B., et al. (2017). Dietary isoflavone intake and all-cause mortality in breast cancer survivors: the breast cancer family registry. Cancer 123 (11), 2070–2079. doi: 10.1002/cncr.30615
Zhang, S., Morris, M. E. (2003). Effects of the flavonoids biochanin A, morin, phloretin, and silymarin on P-glycoprotein-mediated transport. J. Pharmacol. Exp. Ther. 304 (3), 1258–1267. doi: 10.1124/jpet.102.044412
Zhang, S., Sagawa, K., Arnold, R. D., Tseng, E., Wang, X., Morris, M. E. (2010). Interactions between the flavonoid biochanin A and P-glycoprotein substrates in rats: in vitro and in vivo. J. Pharm. Sci. 99 (1), 430–441. doi: 10.1002/jps.21827
Zhang, Y., Chen, W. A. (2015). Biochanin A inhibits lipopolysaccharide-induced inflammatory cytokines and mediators production in BV2 microglia. Neurochem. Res. 40 (1), 165–171. doi: 10.1007/s11064-014-1480-2
Zhao, X., Tang, X., Guo, N., An, Y., Chen, X., Shi, C., et al. (2018). Biochanin a Enhances the defense against salmonella enterica infection through AMPK/ULK1/mTOR-mediated autophagy and extracellular traps and reversing SPI-1-dependent macrophage (MΦ) M2 polarization. Front. Cell. Infect. Microbiol. 8, 318. doi: 10.3389/fcimb.2018.00318
Keywords: biochanin A, chemopreventive, inflammation, neuroprotective effect, bioavailability
Citation: Yu C, Zhang P, Lou L and Wang Y (2019) Perspectives Regarding the Role of Biochanin A in Humans. Front. Pharmacol. 10:793. doi: 10.3389/fphar.2019.00793
Received: 01 May 2019; Accepted: 18 June 2019;
Published: 12 July 2019.
Edited by:
Jiangjiang Qin, Zhejiang Chinese Medical University, ChinaCopyright © 2019 Yu, Zhang, Lou and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Wang, d3lhbmdAamx1LmVkdS5jbg==
 Chen Yu1
Chen Yu1 Yang Wang
Yang Wang