
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 05 July 2019
Sec. Cardiovascular and Smooth Muscle Pharmacology
Volume 10 - 2019 | https://doi.org/10.3389/fphar.2019.00753
Bioactive chemical constitutes from the root of Salvia miltiorrhiza classified in two major groups, viz., liposoluble tanshinones and water-soluble phenolics. Tanshinone IIA is a major lipid-soluble compound having promising health benefits. The in vivo and in vitro studies showed that the tanshinone IIA and salvianolate have a wide range of cardiovascular and other pharmacological effects, including antioxidative, anti-inflammatory, endothelial protective, myocardial protective, anticoagulation, vasodilation, and anti-atherosclerosis, as well as significantly help to reduce proliferation and migration of vascular smooth muscle cells. In addition, some of the clinical studies reported that the S. miltiorrhiza preparations in combination with Western medicine were more effective for treatment of various cardiovascular diseases including angina pectoris, myocardial infarction, hypertension, hyperlipidemia, and pulmonary heart diseases. In this review, we demonstrated the potential applications of S. miltiorrhiza, including pharmacological effects of salvianolate, tanshinone IIA, and its water-soluble derivative, like sodium tanshinone IIA sulfonate. Moreover, we also provided details about the clinical applications of S. miltiorrhiza preparations in controlling the cardiovascular diseases.
Cardiovascular diseases (CVDs) cause nearly one third of all deaths in human beings worldwide (Wong, 2014). Coronary atherosclerotic heart disease, also known as CHD, is the most common type of CVD and one of the deadly diseases among humans (Gao et al., 2017; Liu et al., 2018). The mortality rate of CHD is the highest among all CVDs, accounting for about 40% of all CVD-related deaths (Rezaei-Hachesu et al., 2017; Zhu et al., 2018). Numerous studies have shown that diabetes, hypertension, dyslipidemias, abdominal obesity, unhealthy diet, smoking, and psychosocial stress are major risk factors for CHD (Chow et al., 2007; Gupta et al., 2016; Ajith and Jayakumar, 2018). CHD events were defined as hospitalization for unstable angina pectoris (UAP), myocardial infarction (MI), percutaneous coronary intervention (PCI) or coronary artery bypass grafting, and cardiovascular death (Makino et al., 2015). A number of multicenter randomized clinical trials have been conducted and provided more evidence for the treatment of CVDs by traditional Chinese medicine (TCM) (Chen et al., 2006; Lu et al., 2008; Li et al., 2013; Yu et al., 2018).
Salvia miltiorrhiza Bunge (SM), known as Danshen, belongs to the family Labiatae and is widely used in TCM as a traditional natural medicine in clinics for several decades in various parts of China (Zhou et al., 2017; Cao et al., 2018). Danshen has curative effect alone or in combination with other TCM groups. SM is used to treat malignant tumors, neurological, metabolic disorders, lung diseases, CVDs, inflammatory diseases, gynecological diseases, liver diseases, and renal diseases (Cao et al., 2012; Chen et al., 2013; Yang et al., 2013; Qiang et al., 2015). The chemical constituents from the root extract of SM are divided into two categories: liposoluble tanshinones and water-soluble phenolics (Deng et al., 2019; Huang et al., 2019b; Sun et al., 2019), most of which have been identified and purified using various chromatographic and spectroscopic methods (Zhou et al., 2006). SM contains more than 40 lipophilic constituents and 50 hydrophilic constituents (Zhang et al., 2012; Fang et al., 2018); which are mainly extracted as tanshinone I, tanshinone IIA (TsIIA), tanshinone IIB, cryptotanshinone, and dihydrotanshinone I (Figure 1) (Ma et al., 2015). The major phenolic acid constituents among salvianolic acids are salvianolic acid A (Sal A), salvianolic acid B (Sal B), lithospermic acid, danshensu, caffeic acid, and rosmarinic acid (Figure 2) (Ho and Hong, 2011; Ma et al., 2013; Shi et al., 2019). Salvianolates are the main water-soluble bioactive compounds extracted from SM and are composed of Sal B, rosmarinic acid, and lithospermic acid, which are widely used in the treatment of CHD (Qi et al., 2017; Qiu et al., 2018). Tanshinones from SM are more effective against treatment of CVDs and cerebrovascular diseases, including atherosclerosis (AS), MI, cardiac hypertrophy (Gao et al., 2012), myocardial ischemia reperfusion (I/R) (Li et al., 2016), and chronic heart failure (HF) (He et al., 2016). The most studied class of tanshinones is TsIIA, which is one of the major bioactive components of SM having less water solubility compared with other tanshinones (Zhu et al., 2017). Sodium TsIIA sulfonate (STS) (Figure 3) is a water-soluble derivative of TsIIA, which has been widely used in China for the treatment of CVDs safely and effectively, including MI and angina pectoris (AP) (Zhang et al., 2014a; Mao et al., 2015; Zhu et al., 2017). Previous studies provided information on SM and its bioactive constituents for their various pharmacological activities, so in this review, we outlined the cardiovascular protective effects of TsIIA and STS, providing details about therapeutic mechanisms of CVDs, as well as the application of SM in clinical CVDs.
The Gene Cloud of Biotechnology Information (GCBI) and PubMed databases were used to search for antioxidative, anti-inflammatory, endothelial protective, ischemia/reperfusion, myocardial, anticoagulation, vasodilating, smooth muscle cell, anti-AS, and tanshinones, respectively. The literature data related to the pharmacological effects of TsIIA and STS were manually screened out. Furthermore, the GCBI and PubMed databases were used to search for Danshen, Salvia miltiorrhiza, tanshinones, TsIIA, sodium TsIIA sulfonate, salvianolate, and CVDs, respectively.
Recent research has demonstrated that TsIIA or STS or salvianolate has numerous cardioprotective effects, including antioxidative (Fei et al., 2013; Xuan et al., 2017), inhibition of apoptosis (Chen et al., 2017c; Yue et al., 2017b), anti-inflammatory (Meng et al., 2014; Feng et al., 2016), anti-cardiac fibrosis (Wu et al., 2018), anti-cardiac hypertrophy (Feng et al., 2017), anticoagulation (Maione et al., 2014), anti-AS (Liu et al., 2014b; Meng et al., 2014), and vasodilating (Li et al., 2015b); also reduction of macrophage derived foam cell formation (Liu et al., 2014b), inhibition of proliferation, and migration of vascular smooth muscle cells (VSMCs) (Wang et al., 2013a; Fang et al., 2018) (Figure 4). Therefore, TsIIA and STS can be used as a promising candidate for treating CVDs (Tables 1 and 2).
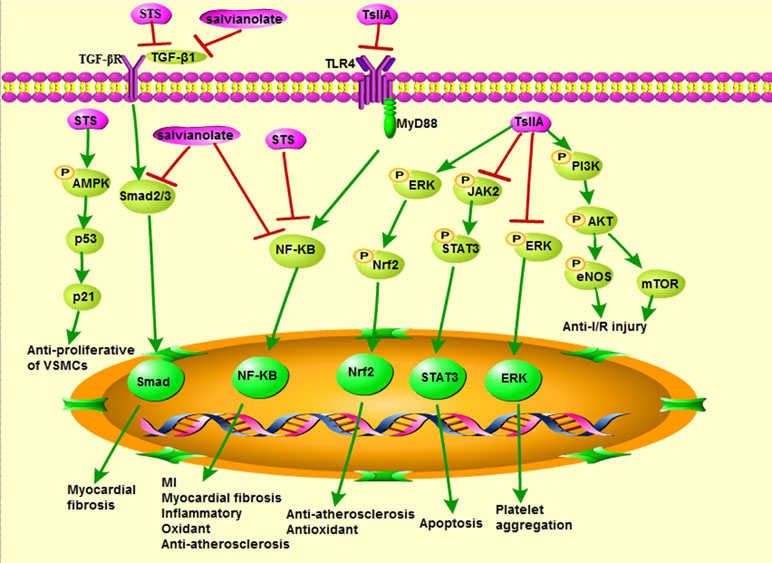
Figure 4 Cardiovascular pharmacological effects of TsIIA, STS, and salvianolate (modified based on Li et al., 2018).
Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) is a central regulator of cellular responses to oxidative stress, which plays a critical role in maintaining normal cardiac function (Guo et al., 2018). Nrf2-dependent antioxidant response mediates the protective effect of TsIIA on doxorubicin (DOX)-induced cardiotoxicity, suggesting that TsIIA may be a promising therapeutic adjuvant that prevents the serious side effects of DOX in the heart (Guo et al., 2018). Differentiation of atrial fibroblasts into myofibroblasts plays a pivotal role in atrial fibrosis (Chen et al., 2018). Studies have shown that STS prevents (angiotensin II) Ang II-induced myofibroblast differentiation through inhibiting oxidative stress and suppressing transforming growth factor-β1 (TGF-β1) signaling pathway in human atrial fibroblasts (Chen et al., 2018). STS may also protect cells from X-ray-induced cell cycle arrest, oxidative stress, and apoptosis during the treatment of radiation-induced cardiovascular damage (Zhang et al., 2017b). In addition, STS acts as an antioxidant for inhibiting hemorrhagic shock (HS)-induced organ failure by inhibiting the nuclear factor kappa B (NF-κB) pathway (Qian et al., 2017). Excessive amounts of reactive oxygen species (ROS) cause irreversible damage to DNA, cell membranes, and other cellular structures by oxidizing proteins, lipids, and nucleic acids (Farias et al., 2017; Zhu et al., 2017). Salvianolate may inhibit the production of ROS and increase the antioxidant capacity of cardiomyocytes (Fei et al., 2013). Salvianolate also improved microvascular reflow by inhibiting oxidative stress and apoptosis (Han et al., 2011).
AS is a chronic inflammatory disease of the arterial wall, which is characterized by progressive lipid accumulation in the aortic intima leading to endothelial cell dysfunction and further destruction of the endothelial barrier and vascular tone (Pang et al., 2019). Its pathogenesis is maladaptive immune response and cholesterol metabolism disorder (Sanz and Fayad, 2008; Chan et al., 2017). Inflammation is dominant in AS and CVDs (Tian et al., 2018). Recent studies showed that TsIIA and its derivatives are able to treat CVDs by decreasing the associated inflammatory responses. TsIIA significantly alleviated transverse aortic constriction (TAC)-induced myocardial remodeling by activating the silent information regulator 1 (SIRT1) signaling pathway, probably because it exerts strong antioxidant and anti-inflammatory activities (Feng et al., 2016). STS reduced the expression of tumor necrosis factor-α (TNF-α), interleukin (IL)-6, chloride intracellular channel 1 (CLIC1), vascular cell adhesion molecule 1 (VCAM-1), and intercellular adhesion molecule 1 (ICAM-1) in the atherosclerotic mice; also the antioxidant and anti-inflammatory properties are mediated by inhibiting the expression of CLIC1 and membrane translocation (Zhu et al., 2017). Salvianolate can reduce serum IL-6 and C-reactive protein (CRP) levels in AS rats in a dose-dependent manner (Meng et al., 2014). In addition, the level of IL-6 in macrophages after salvianolate treatment was also significantly reduced (Stumpf et al., 2013).
Endothelial nitric oxide synthase (eNOS) is the key enzyme that plays an important role in maintaining the homeostasis of vascular endothelial cells (Li et al., 2015b), as the endothelial cell dysfunction is the basis for the development of various cardiovascular complications of diabetes (Gilbert, 2014). Also, the TsIIA inhibited the decrease of eNOS expression and the generation of nitric oxide (NO) induced by high glucose, which exerted this effect through a variety of post-transcriptional mechanisms (Li et al., 2015b). TsIIA plays a protective role by inhibiting strain-induced endothelin-1 (ET-1) expression, increasing the endothelin type B (ETB) receptors, reducing the ETA receptors, upregulating eNOS, and increasing the formation of NO during chronic intermittent hypoxia (CIH)-induced endothelial dysfunction (Chen et al., 2017b). Furthermore, STS has multiple functions in vascular endothelial cells; it inhibits the apoptosis of human umbilical vein endothelial cells (HUVECs) induced by heat stress through phosphoinositide-3-kinase (PI3K)/protein kinase B (AKT)/eNOS signaling pathway (Cheng et al., 2017). Oxygen-free radicals impair NO-mediated coronary vasorelaxation affecting basal and agonist-induced NO release and may lead to endothelial dysfunction (Paolocci et al., 2001). Salvianolate inhibited ROS production by downregulating of transcription factors Smad2 and Smad3 (Smad2/3) and TGF-β1 expression, but high concentrations of salvianolate caused cytotoxicity in mouse cardiomyocytes (Fei et al., 2013). Sal A and Sal B protect HUVECs (Yang et al., 2012a) and human aortic endothelial cells (HAECs) (Yang et al., 2011) from damage and improve blood–brain barrier dysfunction (Yang et al., 2016b) by attenuating the production of ROS.
I/R injury is considered to be the main cause of CHD, which is characterized by aggravation of functional damage, accelerated myocardial cell death, and arrhythmia (Kong et al., 2016; Yu et al., 2016). Studies have demonstrated that the PI3K/Akt pathway is involved in the cardioprotective effects provided by pharmacological pre- and post-conditioning by inhibiting mitochondrial permeability transition pore (mPTP) opening (Tsang et al., 2004; Bopassa et al., 2006; Yuan et al., 2014). Compared with the I/R model group, the group treated with TsIIA (10 mg/kg, IV) prior to reperfusion decreased myocardial infarct size, elevated levels of phosphor-Akt and phosphor-eNOS, and attenuated mitochondrial permeability transition (Yuan et al., 2014). Therefore, pharmacological post conditioning with TsIIA can protect the myocardium from I/R injury by activating PI3K/AKT-eNOS pathway, and the blockage of mPTP opening may be involved in the cardioprotective effect (Yuan et al., 2014). TsIIA also activated the PI3K/AKT/mammalian target of rapamycin (mTOR) signaling pathway to attenuate myocardial I/R injury in rats (Li et al., 2016). Furthermore, TsIIA may inhibit the increased ROS formation caused by myocardial I/R, reduce the expression of the high mobility group box B1 protein (HMGB1), and inhibit inflammation reaction in the myocardial tissue (Hu et al., 2015). Moreover, STS improved I/R-induced myocardial damage by reducing inflammation and apoptosis, enhancing autophagy (Pan et al., 2017). Changes in serum myocardial zymograms (such as creatine kinase-MB, aspartate transaminase, lactate dehydrogenase) can be used as indicators to determine the alterations of membrane integrity and degree of myocardial injury (Panda and Naik, 2008; Wei et al., 2014). STS reduced some consequences of myocardial ischemia, including cardiac antioxidant status, serum myocardial zymogram, microstructural disorders, and cardiac function (Wei et al., 2014). The optimal treatment time window for STS treatment of myocardial I/R injury appears to be within 2 h after reperfusion (Wei et al., 2014). Salvianolate can reduce myocardial I/R injury in rats by reducing mitochondrial DNA oxidative damage, protecting mitochondrial function, and inhibiting cardiomyocyte apoptosis (Yue et al., 2017a). It also can reduce myocardial I/R injury in mice, which involves the extracellular signal-regulated kinase (ERK)1/2 signaling pathway but not the PI3K signaling pathway (Qi et al., 2017). In addition, total salvianolic acid injection (TSI) attenuated I/R-induced myocardial damage by inhibiting oxidative stress, which is related to the activation of Nicotinamide adenine dinucleotide dehydrogenase [ubiquinone] 1 alpha subcomplex 10 (NDUFA10) and succinate dehydrogenase complex, subunit A, and flavoprotein variant (SDHA) by upregulating Sirtuin1 (Sirt1) and Sirtuin3 (Sirt3) (Huang et al., 2019a).
When myocardial cells undergo pathological injury, such as hypoxia injury, I/R injury, cardiac surgery, and diabetic injury, the pathological process can evolve from initial cell edema to degeneration and necrosis of myocardial hypertrophy and fibrosis (Gao et al., 2017). Under the condition of hypoxia/reoxygenation (H/R) injury in rats, TsIIA may alleviate myocardial microvascular endothelial cell (MMEC) apoptosis through inhibiting the Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) signaling pathway and regulating the expressions of tumor suppressor p53, B-cell lymphoma-2 (Bcl-2), Bcl-2-associated X protein (Bax), and caspase-3 (Cui et al., 2016). Salvianolate may reduce oxidative damage of mitochondrial DNA, protect mitochondrial function, and inhibit cardiomyocyte apoptosis, thereby reducing H/R injury of cardiomyocytes (Yue et al., 2017b). In case of cardiac hypertrophy in spontaneously hypertensive rats (SHRs), it was demonstrated that TsIIA may inhibit cardiac hypertrophy by inhibiting the cystatin c (Cys-C)/Wingless (Wnt) signaling pathway (Feng et al., 2017). TsIIA also attenuates the Ang II-induced pathological hypertrophy by estrogen receptors (ERs) in H9c2 cardiomyoblast cells (Chen et al., 2017d). Furthermore, it was reported that TsIIA attenuated MI and cardiac fibrosis in rats by inhibiting Toll-like receptor 4 (TLR4)/myeloid differentiation primary response 88 (MyD88)/NF-κB signaling pathway (Wu et al., 2018). Sal B can alleviate myocardial fibrosis by reducing Ang II-induced NF-κB activation in vitro, thus reversing the process of myocardial fibrosis (Wang et al., 2018a).
Ventricular compensation and secondary pathophysiological responses are accompanied by a series of ventricular myocardial damage and ventricular remodeling for the pathological repair (Gao et al., 2017). A study proved that TsIIA attenuated β-catenin and insulin-like growth factor-II receptor (IGF-2R) pathways, decreased subsequent apoptosis and cardiac remodeling, and promoted survival in H9c2 cardiomyoblasts (Chen et al., 2017c). There is evidence that TsIIA significantly ameliorated myocardial remodeling induced by pressure overload through SIRT1 signaling pathway in TAC rats (Feng et al., 2016). TsIIA is important for the treatment of pathological cardiac remodeling; it can inhibit Ang II-induced extracellular matrix (ECM) remodeling responses in human cardiac fibroblasts (Mao et al., 2016).
Under physiological conditions, coagulation and hemostasis system in the human body are mutually restricted by anticoagulation and fibrinolytic system, but they are in a state of dynamic balance. In pathological conditions, no matter which system is abnormal, it can cause bleeding or thrombosis. There is evidence that TsIIA exerts a significant protective effect against lipopolysaccharide (LPS)-induced disseminated intravascular coagulation (DIC) in rabbits. TsIIA also can improve organ injury and reduce the lethal effects of LPS-treated animals (Wu et al., 2012). TsIIA inhibits platelet aggregation induced by adenosine diphosphate (ADP) and collagen via regulating the acetylation of tubulin and inhibiting ERK2 phosphorylation. Therefore, the compound from SM is a promising drug that can improve blood viscosity and microcirculation to prevent CVDs (Maione et al., 2014).
The vasculature plays a vital role in maintaining blood pressure and providing adequate hemoperfusion based on dynamic physical conditions (Gao et al., 2017). Impaired endothelium-dependent vasodilation has been thought to play a major role in the development of cardiovascular complications of diabetes (Li et al., 2015b). It was demonstrated that TsIIA may improve impaired endothelium-dependent vasodilation induced by diabetes via enhancing eNOS expression and activity (Li et al., 2015b), and this effect was initiated by a variety of mechanisms at the post-transcriptional level of eNOS, including regulation of eNOS mRNA and protein stability, coupling, and serine 1177 phosphorylation (Li et al., 2015b). Furthermore, STS also exerts vasodilation effect, which not only activated large conductance Ca2+-activated K+ (BKCa) channel but also blocked Ca2+ channel and inhibited Ca2+ influx in the VSMCs of rats (Zhang et al., 2018c). Danshen water-soluble extract and Sal B exert their vasorelaxant effects by inhibiting Ca2+ influx in VSMCs, and the opening of K+ channels has minor contribution to their effects, but does not involve endothelium-dependent mechanism (Lam et al., 2006). The vasodilatation of Sal B depends, at least in part, on NO and its vasodilation associated NO-guanylate cyclase (GC)-cyclic guanosine 3′,5′-monophosphate (cGMP) signals (Shou et al., 2012).
VSMCs play a major role in the pathogenesis of diabetic vascular disease; TsIIA treatment significantly attenuated advanced glycation end products (AGEs)-induced proliferation and migration of VSMCs by inhibiting ERK1/2 mitogen-activated protein kinase (MAPK) signaling pathway (Lu et al., 2018). Therefore, TsIIA may be a potential candidate for the prevention of diabetic AS (Lu et al., 2018). Furthermore, STS may inhibit high glucose-induced VSMCs proliferation and migration via AMP-activated protein kinase (AMPK) activation; it exerts anti-proliferative effect through activating the AMPK-p53-p21 signaling pathway and anti-migration effect by inhibiting AMPK/NF-κB (Wu et al., 2014). These facts prove that STS can be used to prevent AS and restenosis after PCI (Wu et al., 2014), and it helps to prevent the development of pulmonary arterial hypertension (PAH) by inhibiting the expression of canonical transient receptor potential (TRPC)1 and TRPC6, leading to normalized basal intracellular Ca2+ concentration ([Ca2+]i) and attenuated the proliferation and migration of pulmonary arterial smooth muscle cells (PASMCs) (Wang et al., 2013a). Sal B, a major component of salvianolate, inhibited stromal cell-derived factor-1α (SDF-1α)-stimulated cell proliferation and VSMCs migration by inhibiting C-X-C chemokine receptor type 4 (CXCR4) receptor (Pan et al., 2012).
AS is an inflammatory disease that causes hardening and thickening of the arterial wall and the formation of plaques, including mesenchymal cells, immune cells, lipids, and ECM (Souilhol et al., 2018). Lesions mainly occur in large and medium elastic muscle arteries, which may cause ischemia of the brain, heart, and extremities, or stroke (Fenyo and Gafencu, 2013). The lesions may exist throughout the entire life of the person through circulation of oxidized low-density lipoprotein (ox-LDL) and other pro-atherosclerotic risk factors (such as hyperhomocysteinemia, hyperglycemia) that trigger multiple major pro-atherogenic events, such as endothelial dysfunction, proliferation, and migration of VSMCs, macrophage-derived foam cell formation, T lymphocyte infiltration, and thrombosis (Fenyo and Gafencu, 2013; Xu et al., 2013; Xu et al., 2014; Tabas et al., 2015; Fang et al., 2018).
TsIIA can affect high-density lipoprotein (HDL) subtractions distribution and the intake and efflux of cholesterol (Jia et al., 2016); inhibit endothelial progenitor cell (EPC) injury (Wang et al., 2015), apoptosis of VSMCs (Wang et al., 2017), proliferation and migration of macrophages (Wang et al., 2017), foam cell formation (Liu et al., 2014b), and vascular inflammation (Yang et al., 2016a; Wang et al., 2017; Xuan et al., 2017); and enhance vulnerable plaque stability (Li et al., 2015a; Zhao et al., 2016). These effects can inhibit the progression of AS. TNF-α impaired EPCs’ proliferation, migration, adhesion, and vasculogenesis ability in vitro and promoted EPC secretion of inflammatory cytokines, including IL-6, soluble form of CD40 ligand (sCD40L), and monocyte chemoattractant protein 1 (MCP-1), but TsIIA can reverse these effects (Wang et al., 2015). TsIIA attenuated ox-LDL-induced apoptosis of VSMCs, inhibited ox-LDL-induced proliferation and migration of RAW264.7 cells, and inhibited upregulation of TNF-α, IL-1β, IL-6, and MCP-1 in RAW264.7 cells treated with ox-LDL (Wang et al., 2017); TsIIA reduced scavenger receptor class A (SR-A)-mediated ox-LDL uptake by inhibiting activator protein-1 and increased ATP-binding cassette transporter A1 (ABCA1)/ABCG1-mediated cholesterol efflux through the ERK/Nrf2/heme oxygenase-1 (HO-1) pathway, ultimately leading to reduced cholesterol accumulation in foam cells and atherosclerotic plaques (Liu et al., 2014b). In HUVECs (Chang et al., 2014) and EPCs (Yang et al., 2016a), the TNF-α-induced VCAM-1 and ICAM-1 expression is regulated by inhibiting TNF-α-induced nuclear translocation of NF-κB and activation of IκB kinases (IKK)/NF-κB signaling pathway. TsIIA can also prevent inflammatory responses induced by Porphyromonas gingivalis infection in apolipoprotein E knockout mice (ApoE-/-) mice, and reduce the expression of inflammatory mediators associated with progression of AS (Xuan et al., 2017). These demonstrated the anti-inflammatory effect of TsIIA in AS. Furthermore, the anti-inflammatory and antioxidant properties of STS in the prevention of AS are mediated by inhibition of CLIC1 expression and membrane translocation (Zhu et al., 2017). TsIIA inhibited dendritic cell (DC) maturation and reduced the expression of pro-inflammatory cytokines while attenuating their ability to stimulate T-cell proliferation and cytokine secretion, which may contribute to the pathophysiological processes involved in atherosclerotic plaque instability (Li et al., 2014). The potential mechanism by which TsIIA stabilized vulnerable plaques in ApoE-/- mice may interfere with AGEs and NF-κB activation, as well as downregulation of downstream inflammatory factors, including ICAM-1, VCAM-1, and matrix-metalloproteinases (MMP)-2, -3, and -9 (Zhao et al., 2016). Salvianolate treatment can dose-dependently reduce AS by reducing the levels of pro-inflammatory cytokines and increasing the number of regulatory T cells (Meng et al., 2014).
TsIIA is currently used in China for the treatment of patients with CHD and ischemic stroke, but TsIIA is not easily absorbed by the intestinal pathway, and then STS injection has been developed to improve the bioavailability of the herbal medicine (Yu et al., 2018). Danshen, Danhong, salvianolate, STS injections, and other SM preparations are widely used in China to treat stable AP (SAP) caused by CHD (Zhang et al., 2018a). Salvianolate injections are composed of water-soluble extract of SM (Han et al., 2011; Li et al., 2019). Danhong injection is a modern patented Chinese medicine extracted from SM and Flos Carthami (Zou et al., 2018; Feng et al., 2019). It was approved by the China Food and Drug Administration (FDA) in 2002 (Zou et al., 2018). Compound Danshen dripping pills (CDDP) are a modern Chinese medicine preparation consisting of SM, Panax notoginseng, and borneol (Jia et al., 2018). It is the first TCM approved by the American FDA for the treatment of CVDs in Phase II clinical trials (Luo et al., 2013; Zhang et al., 2018b). Therefore, the clinical preparations of SM are mainly divided into three categories: simple monomer preparation, such as STS injection; water-soluble complex, such as salvianolate and Danshen injection; and compound preparation of SM, such as CDDP; also the form of SM preparation includes tablets, injections, capsules, formulations, and drop pills (Liu et al., 2007). This article summarizes the scientific literature that reported the effects of SM on clinical CVDs like CHD, hyperlipidemia, and hypertension (Table 3).
AP can be divided into SAP and UAP. UAP is a common coronary syndrome between SAP and acute MI, which can easily lead to MI or sudden death (Tan et al., 2018). Chronic SAP accounts for about 50% of all patients with coronary artery disease (CAD) (Chen et al., 2017a). Symptoms of chronic SAP are highly associated with the development of atherosclerotic plaque, which blocks at least one large epicardial coronary artery and triggers an imbalance between myocardial oxygen supply and demand (Chen et al., 2017a).
In a randomized, single-blinded, placebo-controlled, adaptive clinical trial, 156 patients with SAP were randomized into either the placebo (glucose) group or the SM extract (salvianolate injection and Danshen drop pills) group in a 1:1 ratio (Chen et al., 2017a). Participants were treated with glucose or salvianolate injection (200 mg/250 ml 0.9% saline injection, IV drip, qd) for 10 days during hospitalization, followed by the open-label Danshen drop spill (30 pills/day) in the SM extract group for 60 days after discharge (Chen et al., 2017a). Using assessment tools, including the Seattle Angina Questionnaire (SAQ), frequency of AP, angina grade, consumption of short-acting nitrates, and so forth, it was demonstrated that SM extract is beneficial for SAP (Chen et al., 2017a). In addition, previous study reported that five SM-based preparations were effective in the treatment of SAP with clinical improvement rate of 72.4% to 91.6% and electrocardiogram (ECG) improvement rate of 54.5% to 71.6% (Zhang et al., 2018a). The order of five SM-based preparations was as follows: Danhong injection > salvianolate injection > STS injection > Danshen injection of bioactive compounds > Danshen injection (Zhang et al., 2018a).
In another randomized controlled trial (RCT), 100 UAP patients were randomized into two groups that received STS injection (60 mg/250 ml 0.9% sodium chloride injection, qd, 4 weeks) combined with a loading dose of 300 mg aspirin and a maintenance dose of 100 mg of aspirin plus baseline therapy, or 250 ml 0.9% sodium chloride injection (qd, 4 weeks) combined with the same doses of aspirin and baseline therapy (Yan et al., 2009). The severity of AP ameliorated in 94 patients who completed the treatment, with a significant amelioration in total effective rate in the trial groups (Yan et al., 2009). STS can significantly reduce the AP attacks in patients with UAP, which may be associated with decreased levels of fibrinogen (FIB) (Yan et al., 2009). Moreover, in 17 RCTs involving 1,372 patients, the meta-analysis showed that the combination of STS injection and Western medicine for the treatment of UAP significantly improved the total effective rate and the total effective rate of ECG and reduced the level of CRP, FIB, and whole blood high shear viscosity (Tan et al., 2018). In 22 RCTs involving 2,050 patients, the meta-analysis showed that combination of salvianolate injection and Western medicine in the treatment of UAP improved the total effective rate and the total effective rate of ECG, and increased the serum NO lever (Zhang et al., 2016). Therefore, the combined use of STS injection and salvianolate injection was more effective than Western medicine (Zhang et al., 2016; Tan et al., 2018).
MI, also known as acute MI (AMI), is the most severe manifestation of CAD, which causes more than 4 million deaths in northern Asia and Europe, and more than 2 to 4 million deaths in the United States (Nichols et al., 2014; Gao et al., 2017; Wang et al., 2018b). Atherosclerotic plaque rupture is the cause of approximately 70% MI (Benjamin et al., 2017). Patients who survive from AMI may subsequently suffer HF, manifested as fibrotic scar tissue, thinning of the ventricular wall, and reduced systolic function (Opie et al., 2006; Wang et al., 2018b).
Fifty-two patients with non-ST elevation MI (NSTEMI) undergoing PCI were randomized into two groups that received the conventional therapy (n = 26) or the conventional therapy plus SM (n = 26, 1 g each time, three times per day for 1 month after PCI) (Zhang et al., 2014b). Elevated levels of asymmetric dimethylarginine (ADMA) in serum are associated with cardiovascular events and are one of the important biomarkers for predicting adverse events and patient mortality after PCI (Lu et al., 2003; Derkacz et al., 2011). The plasma ADMA level in the two groups was significantly decreased at day 30 after PCI with statistical difference, but the reduction in the SM treatment group was more obvious (Zhang et al., 2014b). The improvement of prognosis after the application of SM in patients with PCI may be related to the negative regulation of ADMA by SM (Zhang et al., 2014b). One hundred eight patients with AMI undergoing PCI were randomized into two groups that received the routine treatment (n = 46) or the routine treatment plus intravenous infusion of salvianolate injection (n = 62, 200 mg administered once at 24 h before surgery, once a day after surgery, 1 week) (Zhang et al., 2017a). The changes of oxidative stress indexes, hemodynamic indexes, cardiac function indexes, and related biochemical indicators were analyzed in the two groups at 24 h before surgery and the 8th day after surgery (Zhang et al., 2017a). It was found that salvianolate injection can effectively improve oxidative stress, enhance myocardial perfusion volume, and promote cardiac function recovery in the perioperative period of PCI (Zhang et al., 2017a). In a double-blind RCT, 35 patients with STEMI undergoing PCI were eligible for qi-yin deficiency syndrome, and blood stasis syndromes were randomized into two groups that received Western medicine (n = 18) or Western medicine plus American ginseng and SM preparations (n = 17) for 3 months (Qiu et al., 2009). At the state of dobutamine stress, the left ventricular ejection fraction (LVEF) in the treatment group was higher than that in the control group, and the symptoms of TCM were improved (Qiu et al., 2009). Therefore, TCM treatment can improve the clinical symptoms and quality of life of AMI patients undergoing PCI, and is conducive to myocardial microcirculation (Qiu et al., 2009). A statistical study has shown that the mortality of SM preparation plus conventional care AMI patients is approximately halved compared to conventional care alone (Peto odds ratio, 0.46; 95% confidence interval, 0.28–0.75) (Wu et al., 2008).
Hypertension is a complex disease involving multiple organ systems, a primary modifiable risk factor for heart disease (Ramirez and Sullivan, 2018), and one of the most common non-communicable diseases in the world, with an increasing incidence rate in developing countries (Gupta et al., 2016; Anupama et al., 2017; Miao et al., 2018). Hypertension is often termed the “silent killer” because many hypertensive patients do not know they have the disease before the onset (Ramirez and Sullivan, 2018). Uncontrolled hypertension causes many complications including but not limited to HF, heart attacks, kidney failure, aneurysms, strokes, and dementia (Ramirez and Sullivan, 2018). The other symptoms include aging (Thawornchaisit et al., 2013), overweight or obesity (Shihab et al., 2012; Tsujimoto et al., 2012), dyslipidemia (Laaksonen et al., 2008), resting heart rate (RHR) (Aladin et al., 2016), hyperuricemia (Krishnan et al., 2007), impaired glucose regulation (Morio et al., 2013; Talaei et al., 2014), and estimated glomerular filtration rate (eGFR) (Takase et al., 2012); these are considered independent risk factors for the development of hypertension (Huang et al., 2018a).
In a double-blind, placebo-controlled, randomized, single-center clinical trial, 55 patients with uncontrolled mild to moderate dose for hypertension were randomized into two groups that received Fufang Danshen capsule (formula mixture, 1,000 mg, twice daily, n = 30) or placebo capsules (n = 25) for 12 weeks (Yang et al., 2012b). The results showed that the Fufang Danshen extract had reduced systolic blood pressure and pulse rate; also it was found that it was well tolerated in patients with hypertension, and no significant difference in adverse effects between the two groups was found (Yang et al., 2012b).
Cor pulmonale [pulmonary heart disease (PHD)] is a chronic progressive complicated disease that requires continuous treatment and imposes a huge financial burden on individuals and society (Liu et al., 2014a). PHD is defined as right ventricular failure secondary to pulmonary hypertension (PH), which is mainly caused by various lung diseases, such as chronic obstructive pulmonary disease (COPD) or pulmonary vascular disease (Han et al., 2007; Weitzenblum and Chaouat, 2009; Huang et al., 2018b). PH caused by respiratory system diseases and/or chronic hypoxemia is the main pathological mechanism of chronic PHD (Shujaat et al., 2007; Shi et al., 2015). Antibiotics, diuretics, oxygen therapy, vasodilators, and anticoagulants are currently used medicines for the treatment of PHD; also, some studies have shown that the safety and effectiveness of TCM combined with conventional treatment is useful in the treatment of these diseases (Shi et al., 2015).
The results of many clinical trials have indicated that SM and compound Danshen injection may be alternatives to PHD (Liu et al., 2014a). A systematic review of the efficacy and safety of SM and compound Danshen injection in PHD patients involved 2,715 patients identified in 35 RCTs (Liu et al., 2014a). Meta-analysis used I2 test for heterogeneity, and randomized or fixed models were selected based on the heterogeneity of the included studies (Liu et al., 2014a). SM and compound Danshen injection have reached favorable conclusions in reducing blood viscosity, plasma viscosity, hematocrit, and mean pulmonary artery pressure (mPAP) by improving blood partial pressure of oxygen (PaO2) (Liu et al., 2014a). In a study enrolled in five hospitalized inpatients, these patients were suffering from various types of serious PH and did not receive the sufficient benefits from sildenafil treatment for at least 3 months (Wang et al., 2013b). After 8 weeks of STS infusion, the patient’s exercise capacity improved, and the Borg dyspnea score was significantly reduced, demonstrating that STS alone or in combination with sildenafil for PH treatment showed significant effect (Wang et al., 2013b). In an RCT, 20 children with congenital heart defects and PH were randomly assigned to two groups that received placebo (n = 10) or SM (200 mg/kg, IV, after anesthesia induction, and at the time of rewarming, n = 10) before cardiac surgery (Xia et al., 2003). The outcome has indicated that SM helps to reduce the ET-1 response and is associated with increased hemodynamic stability after surgery, thereby exerting potent antioxidant therapeutic effect (Xia et al., 2003). Moreover, another clinical trial demonstrated that SM can significantly attenuate lipid peroxide reaction, regulate the imbalance of three antioxidant enzymes [RBC superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT)], and enhance the body’s defense ability against free radical-induced lipid peroxidation damage (Zhang and Chen, 1994).
Hyperlipidemia is a common disease caused by abnormal blood lipid metabolism, which is considered to be a highly independent risk factor for atherosclerotic cardiovascular and cerebrovascular diseases, such as CHD and stroke (Shenghua et al., 2018). Hyperlipidemia is the result of complex interactions between genetic and environmental factors, which can be treated by altering the diet and drugs that regulate lipid metabolism through many mechanisms (Chu et al., 2015). More than 50 TCM formulas have been used to treat hyperlipidemia, of which SM is thought to be beneficial to patients primarily by improving cardiovascular function (Xie et al., 2012). In an RCT, 81 hyperlipidemia patients with phlegm and blood stasis syndrome were randomized into two groups that received CDDP (n = 40) or simvastatin (n = 41) for 3 months (Zhang et al., 2007). The results of this study have shown that CDDP has the effective action for lowering the blood lipid levels without impairing liver function, and its protective liver function may be related to its role in improving antioxidant and reducing inflammation (Zhang et al., 2007).
TsIIA, the main bioactive component of SM, has many physiological functions, including antioxidative, anti-inflammatory, endothelial protective, myocardial protective, anticoagulation, vasodilation, anti-AS, and reduction of VSMC proliferation and migration. However, TsIIA has poor oral absorption and low bioavailability. It can be used as a water-soluble derivative of STS, for preparation of new dosage forms, such as microemulsion for injection, microspheres, solid dispersions, liposomes, and nanoparticles. Salvianolate, a major hydrophilic compound of Danshen, has a variety of cardiovascular protective effects, including antioxidative, anti-inflammatory, endothelial protection, myocardial protection, vasodilation, and anti-AS. Both TsIIA and salvianolate have cardioprotective effects with significant differences in their action of mechanism and effect (Wang et al., 2011). For example, tanshinone acts early after ischemic injury, mainly by inhibiting intracellular calcium and cell adhesion pathways, whereas salvianolic acid acts primarily by down-regulating apoptosis (Wang et al., 2011). Some problems remain to be resolved and should be studied by targeting water-soluble and lipid-soluble components of SM having more or less effects on other CVDs, and whether their effects are consistent at different pathological stages and intervention mechanism. By reviewing clinical studies, it has been found that SM preparations have a good application in the treatment of CHD, hypertension, hyperlipidemia, PHD, and other diseases. However, there is a slight difference in Danshen preparation as STS injection has curative effect in the treatment of CHD, AP, PHD, and other diseases; The adjuvant medicine for the treatment of CVDs, compound Danshen injection can improve the symptoms of patients especially when combined with Western medicine; salvianolate injection has the function of promoting blood circulation and collaterals, and is often used for the treatment of cardiovascular and cerebrovascular diseases, such as AP and MI; CDDP is one of the typical representatives of compound Danshen preparation, which can be used to treat hypertension, hyperlipidemia, and other diseases. Therefore, SM plays an important role in the treatment of CVDs and has a better understanding of the pharmacological mechanism of the monomers of its active ingredients. The existing clinical research results can only be used as a partial reference. More rigorous scientific clinical research data are needed to support for the selection of Danshen preparations with effective regime in certain CVDs.
TCM contains a variety of active ingredients, which act on multiple targets in a complex disease network. Medicines exert synergistic effects on each target to intervene in the occurrence and development of the disease, and finally achieve therapeutic effects (Gao et al., 2017). At the same time, it is unclear which ingredients have produced practical effects, which makes the monomer of Chinese herbal medicines a hot spot of concern. Both basic research and clinical observation have made progress, which shows that TCM has huge advantages and prospects, but there are still deficiencies, such as the lack of uniform application standards. The composition and target of SM are more complicated, but with less adverse reactions. It is often used in combination with other medicines, which poses a hidden danger for the adverse reactions with combinations. TCMs may be used as a supplement and alternative to primary and secondary prevention of CVDs, but further rigorous design of RCTs is needed to assess the impact of TCMs on total mortality and major adverse cardiovascular events in patients with CVDs (Hao et al., 2017). Multicenter, large samples, and RCTs are also needed to evaluate the safety and efficacy of Danshen preparations for CVDs.
JR and SN reviewed relevant literature and wrote this paper. JR, LF, JZ, and GK revised the manuscript. All the authors listed agreed to the publication of this paper.
This work was supported by National Natural Science Fund of China (81522049, 31571735, and 31270007), the “Dawn” Program of Shanghai Education Commission (16SG38), Shanghai Science and Technology Committee Project (17JC1404300, 15430502700), Zhejiang Provincial Ten Thousands Program for Leading Talents of Science and Technology Innovation, Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ajith, T. A., Jayakumar, T. G. (2018). Omega-3 fatty acids in coronary heart disease: recent updates and future perspectives. Clin. Exp. Pharmacol. Physiol. 46, 11–18. doi: 10.1111/1440-1681.13034
Aladin, A. I., Al Rifai, M., Rasool, S. H., Keteyian, S. J., Brawner, C. A., Michos, E. D., et al. (2016). The association of resting heart rate and incident hypertension: the Henry Ford Hospital Exercise Testing (FIT) project. Am. J. Hypertens. 29, 251–257. doi: 10.1093/ajh/hpv095
Anupama, Y. J., Hegde, S. N., Uma, G., Patil, M. (2017). Hypertension is an important risk determinant for chronic kidney disease: results from a cross-sectional, observational study from a rural population in South India. J. Hum. Hypertens 31, 327–332. doi: 10.1038/jhh.2016.81
Benjamin, E. J., Blaha, M. J., Chiuve, S. E., Cushman, M., Das, S. R., Deo, R., et al. (2017). Heart Disease and Stroke Statistics—2017 update: a report from the American Heart Association. Circulation 135, e146–e603. doi: 10.1161/CIR.0000000000000485
Bopassa, J. C., Ferrera, R., Gateau-Roesch, O., Couture-Lepetit, E., Ovize, M. (2006). PI 3-kinase regulates the mitochondrial transition pore in controlled reperfusion and postconditioning. Cardiovasc. Res. 69, 178–185. doi: 10.1016/j.cardiores.2005.07.014
Cao, W., Guo, X. W., Zheng, H. Z., Li, D. P., Jia, G. B., Wang, J. (2012). Current progress of research on pharmacologic actions of salvianolic acid B. Chin. J. Integr. Med. 18, 316–320. doi: 10.1007/s11655-012-1052-8
Cao, W., Wang, Y., Shi, M., Hao, X., Zhao, W., Wang, Y., et al. (2018). Transcription factor SmWRKY1 positively promotes the biosynthesis of tanshinones in Salvia miltiorrhiza. Front. Plant Sci. 9, 554. doi: 10.3389/fpls.2018.00554
Chan, C. K. W., Zhang, L., Cheng, C. K., Yang, H., Huang, Y., Tian, X. Y., et al. (2017). Recent advances in managing atherosclerosis via nanomedicine. Small 14, 1702793. doi: 10.1002/smll.201702793
Chang, C. C., Chu, C. F., Wang, C. N., Wu, H. T., Bi, K. W., Pang, J. H., et al. (2014). The anti-atherosclerotic effect of tanshinone IIA is associated with the inhibition of TNF-alpha-induced VCAM-1, ICAM-1 and CX3CL1 expression. Phytomedicine 21, 207–216. doi: 10.1016/j.phymed.2013.09.012
Chen, A. D., Wang, C. L., Qin, Y., Tian, L., Chen, L. B., Yuan, X. M., et al. (2017a). The effect of Danshen extract on lipoprotein-associated phospholipase A2 levels in patients with stable angina pectoris: study protocol for a randomized controlled trial—the DOLPHIN study. Trials 18, 606. doi: 10.1186/s13063-017-2336-2
Chen, K. J., Shi, D. Z., Xu, H., Lu, S. Z., Li, T. C., Ke, Y. N., et al. (2006). XS0601 reduces the incidence of restenosis: a prospective study of 335 patients undergoing percutaneous coronary intervention in China. Chin. Med. J. (Engl.) 119, 6–13. doi: 10.1097/00029330-200601010-00002
Chen, L., Guo, Q. H., Chang, Y., Zhao, Y. S., Li, A. Y., Ji, E. S. (2017b). Tanshinone IIA ameliorated endothelial dysfunction in rats with chronic intermittent hypoxia. Cardiovasc. Pathol. 31, 47–53. doi: 10.1016/j.carpath.2017.06.008
Chen, T., Li, M., Fan, X., Cheng, J., Wang, L. (2018). Sodium tanshinone IIA sulfonate prevents angiotensin ii-induced differentiation of human atrial fibroblasts into myofibroblasts. Oxid. Med. Cell. Longev. 2018, 6712585. doi: 10.1155/2018/6712585
Chen, W., Lu, Y., Chen, G., Huang, S. (2013). Molecular evidence of cryptotanshinone for treatment and prevention of human cancer. Anticancer Agents Med. Chem. 13, 979–987. doi: 10.2174/18715206113139990115
Chen, Y. F., Day, C. H., Lee, N. H., Chen, Y. F., Yang, J. J., Lin, C. H., et al. (2017c). Tanshinone IIA inhibits beta-catenin nuclear translocation and IGF-2R activation via estrogen receptors to suppress angiotensin II-induced H9c2 cardiomyoblast cell apoptosis. Int. J. Med. Sci. 14, 1284–1291. doi: 10.7150/ijms.20396
Chen, Y. F., Lee, N. H., Pai, P. Y., Chung, L. C., Shen, C. Y., Rajendran, P., et al. (2017d). Tanshinone-induced ERs suppresses IGFII activation to alleviate Ang II-mediated cardiac hypertrophy. J. Recept. Signal Transduct. Res. 37, 493–499. doi: 10.1080/10799893.2017.1360349
Cheng, Q., Zhao, Y., Li, J. (2017). Sodium tanshinone IIA sulfonate suppresses heat stress-induced endothelial cell apoptosis by promoting NO production through upregulating the PI3K/AKT/eNOS pathway. Mol. Med. Rep. 16, 1612–1618. doi: 10.3892/mmr.2017.6760
Chow, C., Cardona, M., Raju, P. K., Iyengar, S., Sukumar, A., Raju, R., et al. (2007). Cardiovascular disease and risk factors among 345 adults in rural India—the Andhra Pradesh Rural Health Initiative. Int. J. Cardiol. 116, 180–185. doi: 10.1016/j.ijcard.2006.03.043
Chu, S. M., Shih, W. T., Yang, Y. H., Chen, P. C., Chu, Y. H. (2015). Use of traditional Chinese medicine in patients with hyperlipidemia: a population-based study in Taiwan. J. Ethnopharmacol. 168, 129–135. doi: 10.1016/j.jep.2015.03.047
Cui, Z. T., Liu, J. P., Wei, W. L. (2016). The effects of tanshinone IIA on hypoxia/reoxygenation-induced myocardial microvascular endothelial cell apoptosis in rats via the JAK2/STAT3 signaling pathway. Biomed. Pharmacother. 83, 1116–1126. doi: 10.1016/j.biopha.2016.07.054
Deng, C., Hao, X., Shi, M., Fu, R., Wang, Y., Zhang, Y., et al. (2019). Tanshinone production could be increased by the expression of SmWRKY2 in Salvia miltiorrhiza hairy roots. Plant Sci. 284, 1–8. doi: 10.1016/j.plantsci.2019.03.007
Derkacz, A., Protasiewicz, M., Poreba, R., Doroszko, A., Poreba, M., Antonowicz-Juchniewicz, J., et al. (2011). Plasma asymmetric dimethylarginine predicts restenosis after coronary angioplasty. Arch. Med. Sci. 7, 444–448. doi: 10.5114/aoms.2011.23410
Fang, J., Little, P. J., Xu, S. (2018). Atheroprotective effects and molecular targets of tanshinones derived from herbal medicine Danshen. Med. Res. Rev. 38, 201–228. doi: 10.1002/med.21438
Farias, J. G., Molina, V. M., Carrasco, R. A., Zepeda, A. B., Figueroa, E., Letelier, P., et al. (2017). Antioxidant therapeutic strategies for cardiovascular conditions associated with oxidative stress. Nutrients 9, 966. doi: 10.3390/nu9090966
Fei, A. H., Cao, Q., Chen, S. Y., Wang, H. R., Wang, F. L., Pan, S. M., et al. (2013). Salvianolate inhibits reactive oxygen species production in H(2)O(2)-treated mouse cardiomyocytes in vitro via the TGFbeta pathway. Acta Pharmacol. Sin. 34, 496–500. doi: 10.1038/aps.2012.209
Feng, J., Li, S., Chen, H. (2016). Tanshinone IIA inhibits myocardial remodeling induced by pressure overload via suppressing oxidative stress and inflammation: possible role of silent information regulator 1. Eur. J. Pharmacol. 791, 632–639. doi: 10.1016/j.ejphar.2016.09.041
Feng, J., Chen, H. W., Pi, L. J., Wang, J., Zhan, D. Q. (2017). Protective effect of tanshinone IIA against cardiac hypertrophy in spontaneously hypertensive rats through inhibiting the Cys-C/Wnt signaling pathway. Oncotarget 8, 10161–10170. doi: 10.18632/oncotarget.14328
Feng, X., Li, Y., Wang, Y., Li, L., Little, P. J., Xu, S. W., et al. (2019). Danhong injection in cardiovascular and cerebrovascular diseases: pharmacological actions, molecular mechanisms, and therapeutic potential. Pharmacol. Res. 139, 62–75. doi: 10.1016/j.phrs.2018.11.006
Fenyo, I. M., Gafencu, A. V. (2013). The involvement of the monocytes/macrophages in chronic inflammation associated with atherosclerosis. Immunobiology 218, 1376–1384. doi: 10.1016/j.imbio.2013.06.005
Gao, J., Chen, G., He, H., Liu, C., Xiong, X., Li, J., et al. (2017). Therapeutic effects of breviscapine in cardiovascular diseases: a review. Front. Pharmacol. 8, 289. doi: 10.3389/fphar.2017.00289
Gao, S., Liu, Z., Li, H., Little, P. J., Liu, P., Xu, S. (2012). Cardiovascular actions and therapeutic potential of tanshinone IIA. Atherosclerosis 220, 3–10. doi: 10.1016/j.atherosclerosis.2011.06.041
Gilbert, R. E. (2014). The endothelium in diabetic nephropathy. Curr. Atheroscler. Rep. 16, 410. doi: 10.1007/s11883-014-0410-8
Guo, Z., Yan, M., Chen, L., Fang, P., Li, Z., Wan, Z., et al. (2018). Nrf2-dependent antioxidant response mediated the protective effect of tanshinone IIA on doxorubicin-induced cardiotoxicity. Exp. Ther. Med. 16, 3333–3344. doi: 10.3892/etm.2018.6614
Gupta, R., Mohan, I., Narula, J. (2016). Trends in coronary heart disease epidemiology in India. Ann. Glob. Health 82, 307–315. doi: 10.1016/j.aogh.2016.04.002
Han, B., Zhang, X., Zhang, Q., Zhao, G., Wei, J., Ma, S., et al. (2011). Protective effects of salvianolate on microvascular flow in a porcine model of myocardial ischaemia and reperfusion. Arch. Cardiovasc. Dis. 104, 313–324. doi: 10.1016/j.acvd.2011.02.004
Han, M. K., Mclaughlin, V. V., Criner, G. J., Martinez, F. J. (2007). Pulmonary diseases and the heart. Circulation 116, 2992–3005. doi: 10.1161/CIRCULATIONAHA.106.685206
Hao, P., Jiang, F., Cheng, J., Ma, L., Zhang, Y., Zhao, Y. (2017). Traditional Chinese medicine for cardiovascular disease: evidence and potential mechanisms. J. Am. Coll. Cardiol. 69, 2952–2966. doi: 10.1016/j.jacc.2017.04.041
He, Z., Sun, C., Xu, Y., Cheng, D. (2016). Reduction of atrial fibrillation by Tanshinone IIA in chronic heart failure. Biomed. Pharmacother. 84, 1760–1767. doi: 10.1016/j.biopha.2016.10.110
Ho, J. H., Hong, C. Y. (2011). Salvianolic acids: small compounds with multiple mechanisms for cardiovascular protection. J. Biomed. Sci. 18, 30. doi: 10.1186/1423-0127-18-30
Hu, H., Zhai, C., Qian, G., Gu, A., Liu, J., Ying, F., et al. (2015). Protective effects of tanshinone IIA on myocardial ischemia reperfusion injury by reducing oxidative stress, HMGB1 expression, and inflammatory reaction. Pharm. Biol. 53, 1752–1758. doi: 10.3109/13880209.2015.1005753
Huang, D. D., Wei, X. H., Mu, H. N., Pan, C. S., Li, Q., Hu, B. H., et al. (2019a). Total salvianolic acid injection prevents ischemia/reperfusion-induced myocardial injury via antioxidant mechanism involving mitochondrial respiratory chain through the upregulation of Sirtuin1 and Sirtuin3. Shock 51, 745–756. doi: 10.1097/SHK.0000000000001185
Huang, Q., Sun, M., Yuan, T., Wang, Y., Shi, M., Lu, S., et al. (2019b). The AP2/ERF transcription factor SmERF1L1 regulates the biosynthesis of tanshinones and phenolic acids in Salvia miltiorrhiza. Food Chem. 274, 368–375. doi: 10.1016/j.foodchem.2018.08.119
Huang, Y., Li, L., Li, X., Fan, S., Zhuang, P., Zhang, Y. (2018b). Ginseng compatibility environment attenuates toxicity and keeps efficacy in cor pulmonale treated by Fuzi Beimu incompatibility through the coordinated crosstalk of PKA and Epac signaling pathways. Front. Pharmacol. 9, 634. doi: 10.3389/fphar.2018.00634
Huang, Y., Deng, Z., Se, Z., Bai, Y., Yan, C., Zhan, Q., et al. (2018a). Combined impact of risk factors on the subsequent development of hypertension. J. Hypertens. 37, 696-701. doi: 10.1097/HJH.0000000000001956
Jia, C., Han, S., Wei, L., Dang, X., Niu, Q., Chen, M., et al. (2018). Protective effect of compound Danshen (Salvia miltiorrhiza) dripping pills alone and in combination with carbamazepine on kainic acid-induced temporal lobe epilepsy and cognitive impairment in rats. Pharm. Biol. 56, 217–224. doi: 10.1080/13880209.2018.1432665
Jia, L. Q., Zhang, N., Xu, Y., Chen, W. N., Zhu, M. L., Song, N., et al. (2016). Tanshinone IIA affects the HDL subfractions distribution not serum lipid levels: involving in intake and efflux of cholesterol. Arch. Biochem. Biophys. 592, 50–59. doi: 10.1016/j.abb.2016.01.001
Kong, Q., Dai, L., Wang, Y., Zhang, X., Li, C., Jiang, S., et al. (2016). HSPA12B attenuated acute myocardial ischemia/reperfusion injury via maintaining endothelial integrity in a PI3K/Akt/mTOR-dependent mechanism. Sci. Rep. 6, 33636. doi: 10.1038/srep33636
Krishnan, E., Kwoh, C. K., Schumacher, H. R., Kuller, L. (2007). Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertension 49, 298–303. doi: 10.1161/01.HYP.0000254480.64564.b6
Laaksonen, D. E., Niskanen, L., Nyyssonen, K., Lakka, T. A., Laukkanen, J. A., Salonen, J. T. (2008). Dyslipidaemia as a predictor of hypertension in middle-aged men. Eur. Heart J. 29, 2561–2568. doi: 10.1093/eurheartj/ehn061
Lam, F. F., Yeung, J. H., Kwan, Y. W., Chan, K. M., Or, P. M. (2006). Salvianolic acid B, an aqueous component of danshen (Salvia miltiorrhiza), relaxes rat coronary artery by inhibition of calcium channels. Eur. J. Pharmacol. 553, 240–245. doi: 10.1016/j.ejphar.2006.09.030
Li, H. Z., Lu, Y. H., Huang, G. S., Chen, Q., Fu, Q., Li, Z. L. (2014). Tanshinone II A inhibits dendritic cell-mediated adaptive immunity: potential role in anti-atherosclerotic activity. Chin. J. Integr. Med. 20, 764–769. doi: 10.1007/s11655-012-1213-9
Li, L., Sha, Z., Wang, Y., Yang, D., Li, J., Duan, Z., et al. (2019). Pre-treatment with a combination of Shenmai and Danshen injection protects cardiomyocytes against hypoxia/reoxygenation- and H2O2-induced injury by inhibiting mitochondrial permeability transition pore opening. Exp. Ther. Med. 17, 4643–4652. doi: 10.3892/etm.2019.7462
Li, Q., Shen, L., Wang, Z., Jiang, H. P., Liu, L. X. (2016). Tanshinone IIA protects against myocardial ischemia reperfusion injury by activating the PI3K/Akt/mTOR signaling pathway. Biomed. Pharmacother. 84, 106–114. doi: 10.1016/j.biopha.2016.09.014
Li, X., Zhang, J., Huang, J., Ma, A., Yang, J., Li, W., et al. (2013). A multicenter, randomized, double-blind, parallel-group, placebo-controlled study of the effects of qili qiangxin capsules in patients with chronic heart failure. J. Am. Coll. Cardiol. 62, 1065–1072. doi: 10.1016/j.jacc.2013.05.035
Li, Y., Guo, Y., Chen, Y., Wang, Y., You, Y., Yang, Q., et al. (2015a). Establishment of an interleukin-1beta-induced inflammation-activated endothelial cell-smooth muscle cell-mononuclear cell co-culture model and evaluation of the anti-inflammatory effects of tanshinone IIA on atherosclerosis. Mol. Med. Rep. 12, 1665–1676. doi: 10.3892/mmr.2015.3668
Li, Y. H., Xu, Q., Xu, W. H., Guo, X. H., Zhang, S., Chen, Y. D. (2015b). Mechanisms of protection against diabetes-induced impairment of endothelium-dependent vasorelaxation by Tanshinone IIA. Biochim. Biophys. Acta 1850, 813–823. doi: 10.1016/j.bbagen.2015.01.007
Li, Z.M., Xu, S.W., Liu, P.Q. (2018). Salvia miltiorrhiza Burge (Danshen): a golden herbal medicine in cardiovascular therapeutics. Acta Pharmacol. Sin. 39, 802-824. doi: 10.1038/aps.2017.193
Liu, D., Yang, G., Zhao, X., Yang, H. (2018). Effects of probucol on atherosclerotic plaque and soluble thrombomodulin in patients with coronary heart disease. Exp. Ther. Med. 16, 886–890. doi: 10.3892/etm.2018.6264
Liu, M., Li, Y. G., Zhang, F., Yang, L., Chou, G. X., Wang, Z. T., et al. (2007). Chromatographic fingerprinting analysis of Danshen root (Salvia miltiorrhiza Radix et Rhizoma) and its preparations using high performance liquid chromatography with diode array detection and electrospray mass spectrometry (HPLC-DAD-ESI/MS). J. Sep. Sci. 30, 2256–2267. doi: 10.1002/jssc.200700149
Liu, Y., Huang, Y., Zhao, C., Qin, X., Zhu, Q., Chen, S., et al. (2014a). Salvia miltiorrhiza injection on pulmonary heart disease: a systematic review and meta-analysis. Am. J. Chin. Med. 42, 1315–1331. doi: 10.1142/S0192415X14500827
Liu, Z., Wang, J., Huang, E., Gao, S., Li, H., Lu, J., et al. (2014b). Tanshinone IIA suppresses cholesterol accumulation in human macrophages: role of heme oxygenase-1. J. Lipid Res. 55, 201–213. doi: 10.1194/jlr.M040394
Lu, M., Luo, Y., Hu, P., Dou, L., Huang, S. (2018). Tanshinone IIA inhibits AGEs-induced proliferation and migration of cultured vascular smooth muscle cells by suppressing ERK1/2 MAPK signaling. Iran J. Basic Med. Sci. 21, 83–88. doi: 10.22038/IJBMS.2017.20100.5276
Lu, T. M., Ding, Y. A., Lin, S. J., Lee, W. S., Tai, H. C. (2003). Plasma levels of asymmetrical dimethylarginine and adverse cardiovascular events after percutaneous coronary intervention. Eur. Heart J. 24, 1912–1919. doi: 10.1016/j.ehj.2003.08.013
Lu, Z., Kou, W., Du, B., Wu, Y., Zhao, S., Brusco, O. A., et al. (2008). Effect of Xuezhikang, an extract from red yeast Chinese rice, on coronary events in a Chinese population with previous myocardial infarction. Am. J. Cardiol. 101, 1689–1693. doi: 10.1016/j.amjcard.2008.02.056
Luo, J., Xu, H., Chen, K. (2013). Systematic review of compound danshen dropping pill: a chinese patent medicine for acute myocardial infarction. Evid. Based Complement. Alternat. Med. 2013, 808076. doi: 10.1155/2013/808076
Ma, P., Liu, J., Zhang, C., Liang, Z. (2013). Regulation of water-soluble phenolic acid biosynthesis in Salvia miltiorrhiza Bunge. Appl. Biochem. Biotechnol. 170, 1253–1262. doi: 10.1007/s12010-013-0265-4
Ma, P. D., Liu, J. Y., Osbourn, A., Dong, J. N., Liang, Z. S. (2015). Regulation and metabolic engineering of tanshinone biosynthesis. RSC Adv. 5, 18137–18144. doi: 10.1039/C4RA13459A
Maione, F., De Feo, V., Caiazzo, E., De Martino, L., Cicala, C., Mascolo, N. (2014). Tanshinone IIA, a major component of Salvia milthorriza Bunge, inhibits platelet activation via Erk-2 signaling pathway. J. Ethnopharmacol. 155, 1236–1242. doi: 10.1016/j.jep.2014.07.010
Makino, H., Miyamoto, Y., Kikuchi-Taura, A., Soma, T., Taguchi, A., Kishimoto, I. (2015). Decreased levels of circulating CD34(+) cells are associated with coronary heart disease in Japanese patients with type 2 diabetes. J. Diabetes Investig. 6, 473–478. doi: 10.1111/jdi.12310
Mao, S., Li, X., Wang, L., Yang, P. C., Zhang, M. (2015). Rationale and design of sodium tanshinone IIA sulfonate in left ventricular remodeling secondary to acute myocardial infarction (STAMP-REMODELING) trial: a randomized controlled study. Cardiovasc. Drugs Ther. 29, 535–542. doi: 10.1007/s10557-015-6625-2
Mao, S., Li, W., Qa’aty, N., Vincent, M., Zhang, M., Hinek, A. (2016). Tanshinone IIA inhibits angiotensin II induced extracellular matrix remodeling in human cardiac fibroblasts—implications for treatment of pathologic cardiac remodeling. Int. J. Cardiol. 202, 110–117. doi: 10.1016/j.ijcard.2015.08.191
Meng, C., Zhuo, X. Q., Xu, G. H., Liu, J. L. (2014). Protection of salvianolate against atherosclerosis via regulating the inflammation in rats. J. Huazhong Univ. Sci. Technol. Med. Sci. 34, 646–651. doi: 10.1007/s11596-014-1331-z
Miao, C., Chang, J., Zhang, G. (2018). Recent research progress of microRNAs in hypertension pathogenesis, with a focus on the roles of miRNAs in pulmonary arterial hypertension. Mol. Biol. Rep. 45, 2883–2896. doi: 10.1007/s11033-018-4335-0
Morio, M., Inoue, M., Inoue, K., Akimoto, K. (2013). Impaired fasting glucose as an independent risk factor for hypertension among healthy middle-aged Japanese subjects with optimal blood pressure: the Yuport Medical Checkup Centre retrospective cohort study. Diabetol. Metab. Syndr. 5, 81. doi: 10.1186/1758-5996-5-81
Nichols, M., Townsend, N., Scarborough, P., Rayner, M. (2014). Cardiovascular disease in Europe 2014: epidemiological update. Eur. Heart J. 35, 2950–2959. doi: 10.1093/eurheartj/ehu299
Opie, L. H., Commerford, P. J., Gersh, B. J., Pfeffer, M. A. (2006). Controversies in ventricular remodelling. Lancet 367, 356–367. doi: 10.1016/S0140-6736(06)68074-4
Pan, C. H., Chen, C. W., Sheu, M. J., Wu, C. H. (2012). Salvianolic acid B inhibits SDF-1alpha-stimulated cell proliferation and migration of vascular smooth muscle cells by suppressing CXCR4 receptor. Vascul. Pharmacol. 56, 98–105. doi: 10.1016/j.vph.2011.11.008
Pan, Y., Qian, J. X., Lu, S. Q., Chen, J. W., Zhao, X. D., Jiang, Y., et al. (2017). Protective effects of tanshinone IIA sodium sulfonate on ischemia-reperfusion-induced myocardial injury in rats. Iran J. Basic Med. Sci. 20, 308–315. doi: 10.22038/ijbms.2017.8361
Panda, V. S., Naik, S. R. (2008). Cardioprotective activity of Ginkgo biloba phytosomes in isoproterenol-induced myocardial necrosis in rats: a biochemical and histoarchitectural evaluation. Exp. Toxicol. Pathol. 60, 397–404. doi: 10.1016/j.etp.2008.03.010
Pang, J., Hu, P., Wang, J., Jiang, J., Lai, J. (2019). Vorapaxar stabilizes permeability of the endothelial barrier under cholesterol stimulation via the AKT/JNK and NFkappaB signaling pathways. Mol. Med. Rep. 19, 5291–5300. doi: 10.3892/mmr.2019.10211
Paolocci, N., Biondi, R., Bettini, M., Lee, C. I., Berlowitz, C. O., Rossi, R., et al. (2001). Oxygen radical-mediated reduction in basal and agonist-evoked NO release in isolated rat heart. J. Mol. Cell. Cardiol. 33, 671–679. doi: 10.1006/jmcc.2000.1334
Qi, J. Y., Yu, J., Huang, D. H., Guo, L. H., Wang, L., Huang, X., et al. (2017). Salvianolate reduces murine myocardial ischemia and reperfusion injury via ERK1/2 signaling pathways in vivo. Chin. J. Integr. Med. 23, 40–47. doi: 10.1007/s11655-016-2621-z
Qian, C., Ren, Y., Xia, Y. (2017). Sodium tanshinone IIA sulfonate attenuates hemorrhagic shock-induced organ damages by nuclear factor-kappa B pathway. J. Surg. Res. 209, 145–152. doi: 10.1016/j.jss.2016.10.008
Qiang, G., Yang, X., Shi, L., Zhang, H., Chen, B., Zhao, Y., et al. (2015). Antidiabetic effect of salvianolic acid A on diabetic animal models via AMPK activation and mitochondrial regulation. Cell. Physiol. Biochem. 36, 395–408. doi: 10.1159/000430258
Qiu, H., Liu, W., Lan, T., Pan, W., Chen, X., Wu, H., et al. (2018). Salvianolate reduces atrial fibrillation through suppressing atrial interstitial fibrosis by inhibiting TGF-beta1/Smad2/3 and TXNIP/NLRP3 inflammasome signaling pathways in post-MI rats. Phytomedicine 51, 255–265. doi: 10.1016/j.phymed.2018.09.238
Qiu, S. L., Jin, M., Yi, J. H., Zhu, T. G., Quan, X., Liang, Y. (2009). Therapy for replenishing qi, nourishing yin and promoting blood circulation in patients with acute myocardial infarction undergoing percutaneous coronary intervention: a randomized controlled trial. Zhong Xi Yi Jie He Xue Bao 7, 616–621. doi: 10.3736/jcim20090704
Ramirez, L. A., Sullivan, J. C. (2018). Sex differences in hypertension: where we have been and where we are going. Am. J. Hypertens. 31, 1247–1254. doi: 10.1093/ajh/hpy148
Rezaei-Hachesu, P., Oliyaee, A., Safaie, N., Ferdousi, R. (2017). Comparison of coronary artery disease guidelines with extracted knowledge from data mining. J. Cardiovasc. Thorac. Res. 9, 95–101. doi: 10.15171/jcvtr.2017.16
Sanz, J., Fayad, Z. A. (2008). Imaging of atherosclerotic cardiovascular disease. Nature 451, 953–957. doi: 10.1038/nature06803
Shenghua, P., Shuyu, T., Kunping, L., Huixia, Z., Xue, X., Jiao, G. (2018). UPLC-QTOF/MS-based lipidomic profiling of liver Qi-stagnation and spleen-deficiency syndrome in patients with hyperlipidemia. Evid. Based Complement. Alternat. Med. 2018, 4530849. doi: 10.1155/2018/4530849
Shi, L., Xie, Y., Liao, X., Chai, Y., Luo, Y. (2015). Shenmai injection as an adjuvant treatment for chronic cor pulmonale heart failure: a systematic review and meta-analysis of randomized controlled trials. BMC Complement Altern. Med. 15, 418. doi: 10.1186/s12906-015-0939-2
Shi, M., Huang, F., Deng, C., Wang, Y., Kai, G. (2019). Bioactivities, biosynthesis and biotechnological production of phenolic acids in Salvia miltiorrhiza. Crit. Rev. Food Sci. Nutr. 59, 953–964. doi: 10.1080/10408398.2018.1474170
Shihab, H. M., Meoni, L. A., Chu, A. Y., Wang, N. Y., Ford, D. E., Liang, K. Y., et al. (2012). Body mass index and risk of incident hypertension over the life course: the Johns Hopkins Precursors Study. Circulation 126, 2983–2989. doi: 10.1161/CIRCULATIONAHA.112.117333
Shou, Q., Pan, Y., Xu, X., Xu, J., Wang, D., Ling, Y., et al. (2012). Salvianolic acid B possesses vasodilation potential through NO and its related signals in rabbit thoracic aortic rings. Eur. J. Pharmacol. 697, 81–87. doi: 10.1016/j.ejphar.2012.09.044
Shujaat, A., Minkin, R., Eden, E. (2007). Pulmonary hypertension and chronic cor pulmonale in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2, 273–282.
Souilhol, C., Harmsen, M. C., Evans, P. C., Krenning, G. (2018). Endothelial-mesenchymal transition in atherosclerosis. Cardiovasc. Res. 114, 565–577. doi: 10.1093/cvr/cvx253
Stumpf, C., Fan, Q., Hintermann, C., Raaz, D., Kurfurst, I., Losert, S., et al. (2013). Anti-inflammatory effects of danshen on human vascular endothelial cells in culture. Am. J. Chin. Med. 41, 1065–1077. doi: 10.1142/S0192415X13500729
Sun, M., Shi, M., Wang, Y., Huang, Q., Yuan, T., Wang, Q., et al. (2019). The biosynthesis of phenolic acids is positively regulated by the JA-responsive transcription factor ERF115 in Salvia miltiorrhiza. J. Exp. Bot. 70, 243–254. doi: 10.1093/jxb/ery349
Tabas, I., Garcia-Cardena, G., Owens, G. K. (2015). Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 209, 13–22. doi: 10.1083/jcb.201412052
Takase, H., Dohi, Y., Toriyama, T., Okado, T., Tanaka, S., Sonoda, H., et al. (2012). Evaluation of risk for incident hypertension using glomerular filtration rate in the normotensive general population. J. Hypertens. 30, 505–512. doi: 10.1097/HJH.0b013e32834f6a1d
Talaei, M., Sadeghi, M., Mohammadifard, N., Shokouh, P., Oveisgharan, S., Sarrafzadegan, N. (2014). Incident hypertension and its predictors: the Isfahan Cohort Study. J. Hypertens. 32, 30–38. doi: 10.1097/HJH.0b013e32836591d4
Tan, D., Wu, J. R., Zhang, X. M., Liu, S., Zhang, B. (2018). Sodium tanshinone II a sulfonate injection as adjuvant treatment for unstable angina pectoris: a meta-analysis of 17 randomized controlled trials. Chin. J. Integr. Med. 24, 156–160. doi: 10.1007/s11655-017-2424-x
Thawornchaisit, P., De Looze, F., Reid, C. M., Seubsman, S. A., Sleigh, A. C., Thai Cohort Study, T. (2013). Health risk factors and the incidence of hypertension: 4-year prospective findings from a national cohort of 60 569 Thai Open University students. BMJ Open 3, e002826. doi: 10.1136/bmjopen-2013-002826
Tian, G., Sun, Y., Liu, S., Li, C., Chen, S., Qiu, R., et al. (2018). Therapeutic effects of wenxin keli in cardiovascular diseases: an experimental and mechanism overview. Front. Pharmacol. 9, 1005. doi: 10.3389/fphar.2018.01005
Tsang, A., Hausenloy, D. J., Mocanu, M. M., Yellon, D. M. (2004). Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ. Res. 95, 230–232. doi: 10.1161/01.RES.0000138303.76488.fe
Tsujimoto, T., Sairenchi, T., Iso, H., Irie, F., Yamagishi, K., Tanaka, K., et al. (2012). Impact of obesity on incident hypertension independent of weight gain among nonhypertensive Japanese: the Ibaraki Prefectural Health Study (IPHS). J. Hypertens. 30, 1122–1128. doi: 10.1097/HJH.0b013e328352b879
Wang, B., Ge, Z., Cheng, Z., Zhao, Z. (2017). Tanshinone IIA suppresses the progression of atherosclerosis by inhibiting the apoptosis of vascular smooth muscle cells and the proliferation and migration of macrophages induced by ox-LDL. Biol. Open 6, 489–495. doi: 10.1242/bio.024133
Wang, C., Luo, H., Xu, Y., Tao, L., Chang, C., Shen, X. (2018a). Salvianolic acid B-alleviated angiotensin II induces cardiac fibrosis by suppressing NF-kappaB pathway in vitro. Med. Sci. Monit. 24, 7654–7664. doi: 10.12659/MSM.908936
Wang, J., Jiang, Q., Wan, L., Yang, K., Zhang, Y., Chen, Y., et al. (2013a). Sodium tanshinone IIA sulfonate inhibits canonical transient receptor potential expression in pulmonary arterial smooth muscle from pulmonary hypertensive rats. Am. J. Respir. Cell Mol. Biol. 48, 125–134. doi: 10.1165/rcmb.2012-0071OC
Wang, J., Lu, W., Wang, W., Zhang, N., Wu, H., Liu, C., et al. (2013b). Promising therapeutic effects of sodium tanshinone IIA sulfonate towards pulmonary arterial hypertension in patients. J. Thorac. Dis. 5, 169–172. doi: 10.3978/j.issn.2072-1439.2013.02.04
Wang, L., Li, Y., Deng, W., Dong, Z., Li, X., Liu, D., et al. (2018b). Cardio-protection of ultrafine granular powder for Salvia miltiorrhiza Bunge against myocardial infarction. J. Ethnopharmacol. 222, 99–106. doi: 10.1016/j.jep.2018.04.029
Wang, X., Wang, Y., Jiang, M., Zhu, Y., Hu, L., Fan, G., et al. (2011). Differential cardioprotective effects of salvianolic acid and tanshinone on acute myocardial infarction are mediated by unique signaling pathways. J. Ethnopharmacol. 135, 662–671. doi: 10.1016/j.jep.2011.03.070
Wang, X. X., Yang, J. X., Pan, Y. Y., Zhang, Y. F. (2015). Protective effects of tanshinone A on endothelial progenitor cells injured by tumor necrosis factor-alpha. Mol. Med. Rep. 12, 4055–4062. doi: 10.3892/mmr.2015.3969
Wei, B., Li, W. W., Ji, J., Hu, Q. H., Ji, H. (2014). The cardioprotective effect of sodium tanshinone IIA sulfonate and the optimizing of therapeutic time window in myocardial ischemia/reperfusion injury in rats. Atherosclerosis 235, 318–327. doi: 10.1016/j.atherosclerosis.2014.05.924
Weitzenblum, E., Chaouat, A. (2009). Cor pulmonale. Chron. Respir. Dis. 6, 177–185. doi: 10.1177/1479972309104664
Wong, N. D. (2014). Epidemiological studies of CHD and the evolution of preventive cardiology. Nat. Rev. Cardiol. 11, 276–289. doi: 10.1038/nrcardio.2014.26
Wu, D. M., Wang, Y. J., Han, X. R., Wen, X., Li, L., Xu, L., et al. (2018). Tanshinone IIA prevents left ventricular remodelling via the TLR4/MyD88/NF-kappaB signalling pathway in rats with myocardial infarction. J. Cell. Mol. Med. 22, 3058–3072. doi: 10.1111/jcmm.13557
Wu, L. C., Lin, X., Sun, H. (2012). Tanshinone IIA protects rabbits against LPS-induced disseminated intravascular coagulation (DIC). Acta Pharmacol. Sin. 33, 1254–1259. doi: 10.1038/aps.2012.84
Wu, T., Ni, J., Wu, J. (2008). Danshen (Chinese medicinal herb) preparations for acute myocardial infarction. Cochrane Database Syst. Rev., 16, CD004465. doi: 10.1002/14651858.CD004465.pub2
Wu, W. Y., Yan, H., Wang, X. B., Gui, Y. Z., Gao, F., Tang, X. L., et al. (2014). Sodium tanshinone IIA silate inhibits high glucose-induced vascular smooth muscle cell proliferation and migration through activation of AMP-activated protein kinase. PLoS One 9, e94957. doi: 10.1371/journal.pone.0094957
Xia, Z., Gu, J., Ansley, D. M., Xia, F., Yu, J. (2003). Antioxidant therapy with Salvia miltiorrhiza decreases plasma endothelin-1 and thromboxane B2 after cardiopulmonary bypass in patients with congenital heart disease. J. Thorac. Cardiovasc. Surg. 126, 1404–1410. doi: 10.1016/S0022-5223(03)00970-X
Xie, W., Zhao, Y., Du, L. (2012). Emerging approaches of traditional Chinese medicine formulas for the treatment of hyperlipidemia. J. Ethnopharmacol. 140, 345–367. doi: 10.1016/j.jep.2012.01.027
Xu, S., Bai, P., Little, P. J., Liu, P. (2014). Poly(ADP-ribose) polymerase 1 (PARP1) in atherosclerosis: from molecular mechanisms to therapeutic implications. Med. Res. Rev. 34, 644–675. doi: 10.1002/med.21300
Xu, S., Ogura, S., Chen, J., Little, P. J., Moss, J., Liu, P. (2013). LOX-1 in atherosclerosis: biological functions and pharmacological modifiers. Cell. Mol. Life Sci. 70, 2859–2872. doi: 10.1007/s00018-012-1194-z
Xuan, Y., Gao, Y., Huang, H., Wang, X., Cai, Y., Luan, Q. X. (2017). Tanshinone IIA attenuates atherosclerosis in apolipoprotein E knockout mice infected with Porphyromonas gingivalis. Inflammation 40, 1631–1642. doi: 10.1007/s10753-017-0603-8
Yan, F. F., Liu, Y. F., Liu, Y., Zhao, Y. X. (2009). Sulfotanshinone sodium injection could decrease fibrinogen level and improve clinical outcomes in patients with unstable angina pectoris. Int. J. Cardiol. 135, 254–255. doi: 10.1016/j.ijcard.2008.03.020
Yang, J. X., Pan, Y. Y., Ge, J. H., Chen, B., Mao, W., Qiu, Y. G., et al. (2016a). Tanshinone II A attenuates TNF-alpha-induced expression of VCAM-1 and ICAM-1 in endothelial progenitor cells by blocking activation of NF-kappaB. Cell. Physiol. Biochem. 40, 195–206. doi: 10.1159/000452537
Yang, L. L., Li, D. Y., Zhang, Y. B., Zhu, M. Y., Chen, D., Xu, T. D. (2012a). Salvianolic acid A inhibits angiotensin II-induced proliferation of human umbilical vein endothelial cells by attenuating the production of ROS. Acta Pharmacol. Sin. 33, 41–48. doi: 10.1038/aps.2011.133
Yang, M. C., You, F. L., Wang, Z., Liu, X. N., Wang, Y. F. (2016b). Salvianolic acid B improves the disruption of high glucose-mediated brain microvascular endothelial cells via the ROS/HIF-1alpha/VEGF and miR-200b/VEGF signaling pathways. Neurosci. Lett. 630, 233–240. doi: 10.1016/j.neulet.2016.08.005
Yang, T. L., Lin, F. Y., Chen, Y. H., Chiu, J. J., Shiao, M. S., Tsai, C. S., et al. (2011). Salvianolic acid B inhibits low-density lipoprotein oxidation and neointimal hyperplasia in endothelium-denuded hypercholesterolaemic rabbits. J. Sci. Food Agric. 91, 134–141. doi: 10.1002/jsfa.4163
Yang, T. Y., Wei, J. C., Lee, M. Y., Chen, C. M., Ueng, K. C. (2012b). A randomized, double-blind, placebo-controlled study to evaluate the efficacy and tolerability of Fufang Danshen (Salvia miltiorrhiza) as add-on antihypertensive therapy in Taiwanese patients with uncontrolled hypertension. Phytother. Res. 26, 291–298. doi: 10.1002/ptr.3548
Yang, W. S., Jeong, D., Yi, Y. S., Park, J. G., Seo, H., Moh, S. H., et al. (2013). IRAK1/4-targeted anti-inflammatory action of caffeic acid. Mediators Inflamm. 2013, 518183. doi: 10.1155/2013/518183
Yu, H., Zhang, H., Zhao, W., Guo, L., Li, X., Li, Y., et al. (2016). Gypenoside protects against myocardial ischemia-reperfusion injury by inhibiting cardiomyocytes apoptosis via inhibition of CHOP pathway and activation of PI3K/Akt pathway in vivo and in vitro. Cell. Physiol. Biochem. 39, 123–136. doi: 10.1159/000445611
Yu, M. L., Li, S. M., Gao, X., Li, J. G., Xu, H., Chen, K. J. (2018). Sodium tanshinone II A sulfonate for coronary heart disease: a systematic review of randomized controlled trials. Chin. J. Integr. Med. 24, 1–8. doi: 10.1007/s11655-018-2556-7
Yuan, X., Jing, S., Wu, L., Chen, L., Fang, J. (2014). Pharmacological postconditioning with tanshinone IIA attenuates myocardial ischemia-reperfusion injury in rats by activating the phosphatidylinositol 3-kinase pathway. Exp. Ther. Med. 8, 973–977. doi: 10.3892/etm.2014.1820
Yue, R. C., Yang, X. L., Zhang, R. Y., Liu, S., Liu, J., Zeng, J., et al. (2017a). The effects and related mechanism of salvianolate on rats with myocardial ischemia-reperfusion injury. Zhonghua Xin Xue Guan Bing Za Zhi 45, 1072–1077. doi: 10.3760/cma.j.issn.0253-3758.2017.12.012
Yue, R. C., Yang, X. L., Zhang, R. Y., Liu, S., Liu, J., Zeng, J., et al. (2017b). Salvianolate protects H9c2 cells from hypoxia/reoxygenation injury-induced apoptosis by attenuating mitochondrial DNA oxidative damage. Zhonghua Xin Xue Guan Bing Za Zhi 45, 57–63. doi: 10.3760/cma.j.issn.0253-3758.2017.01.011
Zhang, D., Wu, J., Liu, S., Zhang, X., Zhang, B. (2016). Salvianolate injection in the treatment of unstable angina pectoris: a systematic review and meta-analysis. Medicine (Baltimore) 95, e5692. doi: 10.1097/MD.0000000000005692
Zhang, G. X., Zhang, Y. Y., Zhang, X. X., Wang, P. Q., Liu, J., Liu, Q., et al. (2018a). Different network pharmacology mechanisms of Danshen-based Fangjis in the treatment of stable angina. Acta Pharmacol. Sin. 39, 952–960. doi: 10.1038/aps.2017.191
Zhang, H. C., Liu, W., Yuan, H. T., Tang, Y. S. (2014b). Salvia miltiorrhiza reduces plasma levels of asymmetric ADMA in patients with non-ST elevation myocardial infarction undergoing percutaneous coronary intervention. Zhongguo Zhong Xi Yi Jie He Za Zhi 34, 1436–1439. doi: 10.6138/JIT.2013.14.1.10
Zhang, H., Long, M., Wu, Z., Han, X., Yu, Y. (2014a). Sodium tanshinone IIA silate as an add-on therapy in patients with unstable angina pectoris. J. Thorac. Dis. 6, 1794–1799. doi: 10.3978/j.issn.2072-1439.2014.12.37
Zhang, P. T., Chen, Z. R. (1994). Effect of Salvia miltiorrhiza on lipid peroxidation antioxidant enzymes activity in patients with chronic cor pulmonale. Zhongguo Zhong Xi Yi Jie He Za Zhi 14, 474–477. doi: 10.1007/bf02934242
Zhang, Q., Jiao, F., Hua, C. (2017a). Perioperative application of salvianolate on oxidative stress and plasma IMD/ADM2 in patients with acute myocardial infarction undergoing PCI. Exp. Ther. Med. 13, 1475–1479. doi: 10.3892/etm.2017.4114
Zhang, Q., Xiao, X., Zheng, J., Li, M., Yu, M., Ping, F., et al. (2018b). Compound danshen dripping pill inhibits retina cell apoptosis in diabetic rats. Front. Physiol. 9, 1501. doi: 10.3389/fphys.2018.01501
Zhang, S. J., Cheng, Z. X., Lin, Y. W., Qin, J., Cheng, Y. H., Liu, S. L. (2007). Effection of compositie salviae dropping pill on hyperlipemia patients with phlegm and blood stasis syndrome. Zhongguo Zhong Yao Za Zhi 32, 440–443. doi: 10.3321/j.issn:1001-5302.2007.05.022
Zhang, W., Li, Y., Li, R., Wang, Y., Zhu, M., Wang, B., et al. (2017b). Sodium tanshinone IIA sulfonate prevents radiation-induced toxicity in H9c2 cardiomyocytes. Evid. Based Complement. Alternat. Med. 2017, 4537974. doi: 10.1155/2017/4537974
Zhang, X. D., He, C. X., Cheng, J., Wen, J., Li, P. Y., Wang, N., et al. (2018c). Sodium tanshinone II-A sulfonate (DS-201) induces vasorelaxation of rat mesenteric arteries via inhibition of L-type Ca(2+) channel. Front. Pharmacol. 9, 62. doi: 10.3389/fphar.2018.00062
Zhang, Y., Jiang, P., Ye, M., Kim, S. H., Jiang, C., Lu, J. (2012). Tanshinones: sources, pharmacokinetics and anti-cancer activities. Int. J. Mol. Sci. 13, 13621–13666. doi: 10.3390/ijms131013621
Zhao, D., Tong, L., Zhang, L., Li, H., Wan, Y., Zhang, T. (2016). Tanshinone II A stabilizes vulnerable plaques by suppressing RAGE signaling and NF-kappaB activation in apolipoprotein-E-deficient mice. Mol. Med. Rep. 14, 4983–4990. doi: 10.3892/mmr.2016.5916
Zhou, L., Chow, M., Zuo, Z. (2006). Improved quality control method for Danshen products—consideration of both hydrophilic and lipophilic active components. J. Pharm. Biomed. Anal. 41, 744–750. doi: 10.1016/j.jpba.2005.12.032
Zhou, W., Huang, Q., Wu, X., Zhou, Z., Ding, M., Shi, M., et al. (2017). Comprehensive transcriptome profiling of Salvia miltiorrhiza for discovery of genes associated with the biosynthesis of tanshinones and phenolic acids. Sci. Rep. 7, 10554. doi: 10.1038/s41598-017-10215-2
Zhu, J., Xu, Y., Ren, G., Hu, X., Wang, C., Yang, Z., et al. (2017). Tanshinone IIA Sodium sulfonate regulates antioxidant system, inflammation, and endothelial dysfunction in atherosclerosis by downregulation of CLIC1. Eur. J. Pharmacol. 815, 427–436. doi: 10.1016/j.ejphar.2017.09.047
Zhu, Z., Wang, Y., Liao, W., Li, H., Wang, D. (2018). Effect of various Danshen injections on patients with coronary heart disease after percutaneous coronary intervention: a protocol for a systematic review and network meta-analysis. Medicine (Baltimore) 97, e11062. doi: 10.1097/MD.0000000000011062
Zou, J. B., Zhang, X. F., Wang, J., Wang, F., Cheng, J. X., Yang, F. Y., et al. (2018). The therapeutic efficacy of Danhong injection combined with percutaneous coronary intervention in acute coronary syndrome: a systematic review and meta-analysis. Front. Pharmacol. 9, 550. doi: 10.3389/fphar.2018.00550
Keywords: cardiovascular diseases, Salvia miltiorrhiza, antioxidative, atherosclerosis, endothelial protective, myocardial infarction
Citation: Ren J, Fu L, Nile SH, Zhang J and Kai G (2019) Salvia miltiorrhiza in Treating Cardiovascular Diseases: A Review on Its Pharmacological and Clinical Applications. Front. Pharmacol. 10:753. doi: 10.3389/fphar.2019.00753
Received: 30 March 2019; Accepted: 11 June 2019;
Published: 05 July 2019.
Edited by:
Changhua Wang, Wuhan University, ChinaReviewed by:
Pengda Ma, Northwest A&F University, ChinaCopyright © 2019 Ren, Fu, Nile, Zhang and Kai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoyin Kai, Z3VveWlua2FpQHlhaG9vLmNvbQ==; Jun Zhang, emhqQHNobnUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.