- 1Department of Pharmacy, Faculty of Pharmacy, Mahidol University, Bangkok, Thailand
- 2Department of Clinical Pharmacy, Faculty of Pharmacy, Srinakharinwirot University, Nakhon Nayok, Thailand
- 3Department of Pharmacy Practice, Faculty of Pharmaceutical Sciences, Center of Pharmaceutical Outcomes Research, Naresuan University, Phitsanulok, Thailand
- 4Department of Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 5Section for Clinical Epidemiology and Biostatistics, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 6School of Epidemiology and Preventive Medicine, Monash University, Melbourne, VIC, Australia
- 7School of Public Health, Curtin University, Perth, WA, Australia
- 8Division of Cardiology, Department of Internal Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
- 9School of Pharmacy, Monash University Malaysia, Bandar Sunway, Selangor, Malaysia
- 10School of Pharmacy, University of Wisconsin, Madison, WI, United States
- 11Asian Centre for Evidence Synthesis in Population, Implementation and Clinical Outcomes, Health and Well-being Cluster, Global Asia in the 21st Century (GA21) Platform, Monash University Malaysia, Bandar Sunway, Malaysia
Background: Currently, there is a lack of information on the comparative efficacy and safety of non-statin lipid-lowering agents (NST) in cardiovascular (CV) disease risk reduction when added to background statin therapy (ST). This study determine the relative treatment effects of NST on fatal and non-fatal CV events among statin-treated patients.
Methods: A network meta-analysis based on a systematic review of randomized controlled trials (RCTs) comparing non-statin lipid-modifying agents among statin-treated patients was performed. PubMed, EMBASE, CENTRAL, and Clinicaltrial.gov were searched up to April 10, 2018. The primary outcomes were CV and all-cause mortalities. Secondary CV outcomes were coronary heart disease (CHD) death, non-fatal myocardial infarction (MI), any stroke, and coronary revascularization. Risks of discontinuations were secondary safety outcomes.
Results: Sixty-seven RCTs including 259,429 participants with eight interventions were analyzed. No intervention had significant effects on the primary outcomes (CV mortality and all-cause mortality). For secondary endpoints, proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK) plus statin (PCSK/ST) significantly reduced the risk of non-fatal MI (RR 0.82, 95% CI 0.72–0.93, p = 0.003), stroke (RR 0.74, 95% CI 0.65–0.85, p < 0.001), coronary revascularization (RR 0.84, 95% CI 0.75–0.94, p = 0.003) compared to ST. Combinations of ST and all NST except PCSK and ezetimibe showed higher rate of discontinuation due to adverse events compared to ST.
Conclusions: None of NST significantly reduced CV or all-cause death when added to ST. PCSKs and to a lesser extent, ezetimibe may help reduce cardiovascular events with acceptable tolerability profile among broad range of patients.
Introduction
Statins or 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase inhibitors are the cornerstone of atherosclerotic cardiovascular disease (ASCVD) risk reduction therapy for both primary and secondary preventions (Baigent et al., 2005; Taylor et al., 2013; Fulcher et al., 2015). Nevertheless, a significant number of patients do not achieve optimal lipid level or still experience cardiovascular (CV) events despite receiving statin therapy (Fruchart et al., 2008). The concept of adding non-statin lipid-lowering agents (NST) on top of statins has therefore been implemented to achieve the lipid goal with the hope that it may reduce hard clinical outcomes. Despite their lipid modifying effects, when tested in large-scale clinical trials, these agents did not uniformly lead to a reduction in CV events when added to statin therapy. Some agents were shown to have neutral effects (Barter et al., 2007a; Ginsberg et al., 2010; Kromhout et al., 2010; Boden et al., 2011; Schwartz et al., 2012; Landray et al., 2014) while some agents were shown to reduce some forms of cardiovascular outcomes (Yokoyama et al., 2007; Cannon et al., 2015a; Robinson et al., 2015; Sabatine et al., 2015). Up to now, most CV outcome studies involving a combination of lipid-modifying therapies were a comparison of a non-statin lipid-modifying agent plus statin therapy vs. statin monotherapy. There remains insufficient data regarding the comparative efficacy and safety of various non-statin agents among statin-treated patients. As a result, current practice guidelines are making recommendation based on an inferential interpretation without data from direct comparison (Catapano et al., 2016; Lloyd-Jones et al., 2017). Since most trials evaluating NST used statin as a comparator, indirect comparisons across trials based on a common comparator is therefore possible through a network meta-analysis (Mills et al., 2012; Cipriani et al., 2013). Therefore, we conducted a systematic review and a network meta-analysis to evaluate the relative treatment effects and safety of NST on cardiovascular morbidity and mortality among statin users.
Methods
Study Design
This study was performed in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for network meta-analysis (Hutton et al., 2015). The study protocol was registered in PROSPERO with the number of registration of CRD42016052839. Additionally, this study protocol was approved by the Institutional Review Board of Mahidol University (COE.No. MU-DT/PY-IRB 2017/PY055).
Data Sources and Search Strategy
The following databases were used to search for original research articles from inception to April 2018: PubMed, Embase, Cochrane Central Register of Control Trials (CENTRAL), and ClinicalTrials.gov. Combinations of terms of medical subject headings (MeSH) and keywords were used in the search strategy. The MeSH and keywords contain Ezetimibe, “Omega-3 fatty acid,” Fibrate, Niacin, “Bile acid sequestrant,” “Proprotein convertase subtilisin/kexin,” “Cholesteryl ester transfer protein,” Lomitapide, Mipomersen, Phytosterol, Non-statin, statin, name of statin (atorvastatin, simvastatin, pravastatin, fluvastatin, rosuvastatin, pitavastatin, lovastatin), cardiovascular, death, mortality, “myocardial infarction” stroke, and synonymous words. References of papers derived for full text review were screened to identify potential studies not indexed in the above databases. No language restriction was applied (Appendix 1).
Study Selection
We included only randomized controlled trials (RCTs) if they met the inclusion criteria including (1) studied in adults (age ≥18 years), (2) comparing NSTs among statin-treated patients, where statin was used either as monotherapy or as a part of combination therapy, (3) reported any outcome of interest including CV mortality, all-cause mortality, individual (not composite) events of coronary heart disease (CHD) mortality or non-fatal myocardial infarction (MI), any stroke, or coronary revascularization (4) with the entire follow-up duration of ≥24 weeks.
Data Extraction and Quality Assessment
Two reviewers (TC and PD) independently screened the titles and abstracts of retrieved citations to identify potentially relevant studies. Relevant data were abstracted using a standardized extraction form including study characteristics, patient characteristics, interventions, outcomes, and other relevant findings. The Revised Cochrane Risk of Bias Tool for randomized trials (RoB 2.0) was used to assess risk of bias among the included studies (Higgins et al., 2016). The quality assessment was undertaken by two reviewers (T.C. and P.D.) independently. Disagreements were resolved by consensus, or with consultation of a third party.
Interventions
NSTs were bile acid sequestrants (BAS), cholesteryl ester transfer protein inhibitors (CETP), ezetimibe (EZT), fibrates (FBT), microsomal transfer protein inhibitors (MTP), niacin (NIA), omega-3 fatty acids (OMG3), proprotein convertase subtilisin/kexin-9 inhibitors (PCSK), or miscellaneous agents following 2017 ACC-AHA (Lloyd-Jones et al., 2017) and 2016 ESC guidelines of dyslipidemias (Catapano et al., 2016). Combinations of NST were also evaluated. ST was used as the reference for network meta-analysis.
Outcomes of Interest
The primary outcomes were cardiovascular death and all-cause mortality. Secondary cardiovascular endpoints were (1) CHD mortality, (2) non-fatal MI, (3) any stroke, and (4) coronary revascularization. Although composite CV outcome is the common endpoint in CV outcome trials (e.g., any major vascular event (MVE) or any major adverse cardiovascular event (MACE), we did not consider a composite CV outcome because of non-mutually exclusive patients with events and varied definitions of MVE or MACE across studies. For secondary safety endpoints, risks of all-cause discontinuation (acceptability) and discontinuation due to adverse events (tolerability) were also investigated.
Quality of Evidence
Evaluation of evidence quality from both direct and network meta-analysis was performed using GRADEpro® GDT software online version (http://www.guidelinedevelopment.org/ [access April 2018]). There were 4 levels of quality of evidence including, very low, low, moderate, and high (Balshem et al., 2011; Puhan et al., 2014). Grading of evidence for each outcome was performed based on 5 domains including risk of bias, inconsistency, indirectness, imprecision, and publication bias. Two independent reviewers (T.C and P.D) assessed the quality of evidence. When discrepancy cannot be resolved by discussion, the third reviewer was consulted to make a final decision.
Data Synthesis and Statistical Analysis
The relative treatment effects of all outcomes of interest among treatment interventions were estimated using the risk ratio (RR). A direct meta-analysis was applied for pooling RRs across studies using a random-effects model (Dersimonian and Laird, 1986). Cochran Q test and the I-squared statistics were deployed to assess heterogeneity (Higgins et al., 2003). Heterogeneity was present if the Cochrane Q test was significant (P < 0.10) or I2 ≥ 50%.
A network meta-analysis with consistency model was constructed to compare all interventions using ST as the common comparator. This approach assumes “consistency” of treatment effects across all included trials—that is, the direct and indirect estimates are consistent (Lu and Ades, 2004; Caldwell et al., 2005). Global inconsistency test by fitting design-by-treatment in the inconsistency model was used for examining the assumption of inconsistency in the entire network (Dias et al., 2010). Additionally, transitivity was explored by assessing the distribution of clinical and methodological variables that might affect the outcome of interests. These data also were available across treatment comparisons (Cipriani et al., 2013). The rankograms, surface under the cumulative ranking (SUCRA) curves (Salanti et al., 2011), and mean ranks were calculated to rank all interventions in the network meta-analysis model. Comparison-adjusted funnel plot was finally used to evaluate publication bias (Chaimani et al., 2013).
Pre-specified subgroup analyses were performed by several clinical factors including indication of treatment (primary, secondary, or mixed indication), intensity of statin therapy (low/moderate, moderate, or moderate/ high) based on the ACC/AHA 2013 definition (Stone et al., 2014), requirement of statin prior to starting NST (optimal LDL-C level/maximally tolerated dose vs. no optimal LDL-C target/maximally tolerated dose), level of cardiovascular risk (non-high CV risk vs. high CV risk) adapted from the ESC 2016 definition (Catapano et al., 2016), age (<65 vs. ≥65 years), percentage of familial hypercholesterolemia (FH) (≥80 vs. <80%) and baseline lipid level (LDL-C, non-HDL-C, HDL, and TG). Additionally, we conducted a sensitivity analysis by excluding the following conditions of studies; studies with high risk of bias, non-adjudicated CV events, follow-up duration <1 year, and small sample size study (<25 percentile) (Dechartres et al., 2014). All analyses were performed in STATA® version 14.2 (StataCorp, College Station, Texas, USA). A p-value < 0.05 was considered statistically significant.
Results
Study Selection
A total of 20,508 potential studies were identified by searching strategies (eTable 1.1), 68 studies including 259,537 adults were eligible for the qualitative review. However, only 67 studies with 259,429 participants were included for network meta-analysis except for one study reported composite CV outcome but not for individual CV events. The searching results and the PRISMA flowchart were shown in eFigure 1.1.
Study Characteristics
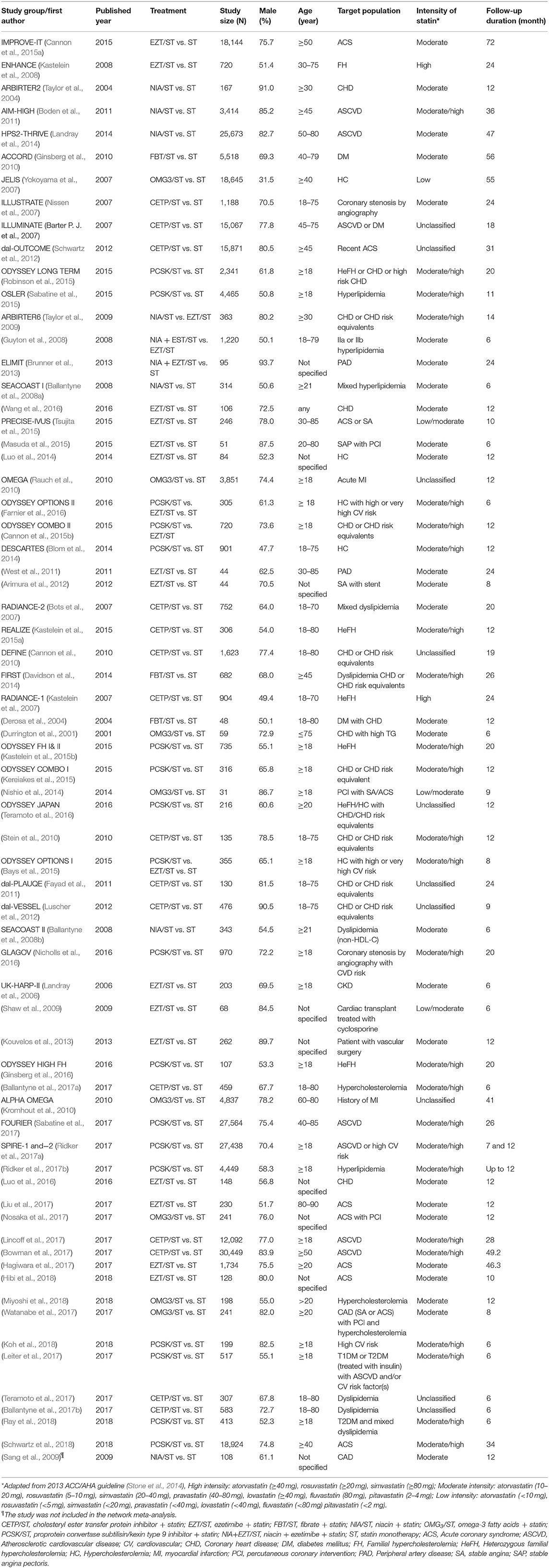
Six different classes of NST including CETP, EZT, FBT, NIA, OMG3, and PCSK were used among 67 included studies. Among these trials, there were 8 interventions including ST, CETP/ST, EZT/ST, FBT/ST, NIA/ST, OMG3/ST, PCSK/ST, and NIA + EZT/ST. Most studies (65 studies) were with 2-arm comparison while the two trials (Bays et al., 2015; Farnier et al., 2016) were with multiple comparisons. ST was mostly used as the comparator (64 in 67) while NST plus ST was used as the comparator in 3 trials (Guyton et al., 2008; Taylor et al., 2009; Cannon et al., 2015b). For trial design, the majority (74%) were double-blind RCT. Studied population in these trials were mostly high risk patients under the age of 65 who were receiving moderate to high intensity statin with mean age ranged from 45.9 to 84.1 years. It is important to note that 40% of the trial used moderate intensity of statin while another 40% used moderate to high intensity of statin. Proportion of male patients ranged from 31.5 to 93.7%. Most trials were secondary prevention or mixed prevention trials with small contribution (9%) of primary prevention trials. Two thirds of the trials were with a follow-up period of ≥1 year with a range of 6–72 (0.5–6 years) months of treatment duration. Summary of all comparisons are shown in Appendix 2 while key characteristics of these trials are shown in Table 1. Additional details of included studies such as number of patients, type of study population and interventions were provided in Appendix 3.
Risk of Bias
Based on the Revised Cochrane Risk of Bias Tool for randomized trials (RoB 2.0) (Higgins et al., 2016), 31, 40, and 29% of studies were considered as at low risk, some concerns, and high risk of bias, respectively (Appendix 4, eFigure 4.1). Among five domains evaluated, inadequate description of allocation concealment and blinding process along with missing outcome data were the three most common reasons for potential bias. For trials with high risk of bias (20 trials with 10,812 patients which represented about 4% of total population), the majority were relatively small trials with <1,000 patients in each trial. Additional details for the assessment of risk of bias were provided in Appendix 4, eTable 4.1.
Effects of Non-statin Therapy on Primary and Secondary Outcomes
Pair-wise meta-analyses were performed for eight outcomes (see Appendix 5), all pooling were with low heterogeneity except six pair-wise comparisons (1 for coronary revascularization, 3 for any discontinuation and 2 for discontinuation from adverse events) in which the I2 ranged from 61.9 to 84%. We explored but could not identify the source of heterogeneity.
Network of eligible comparisons for primary and secondary outcomes were provided in Figure 1 and Appendix 6. Global inconsistency test was performed and found no evidence of inconsistency of treatment effects for the outcomes (Appendix 7). In addition, transitivity was explored by comparing distributions of age, duration of treatments, intensity of statin, and indication of treatment. These indicated no evidence of intransitivity, see Appendix 8. Comparisons among all treatment interventions for the outcomes were demonstrated in Appendix 9. SUCRAs are provided in Appendix 10. The lists of included studies for the network meta-analysis of primary and secondary outcomes were presented in Appendix 11.
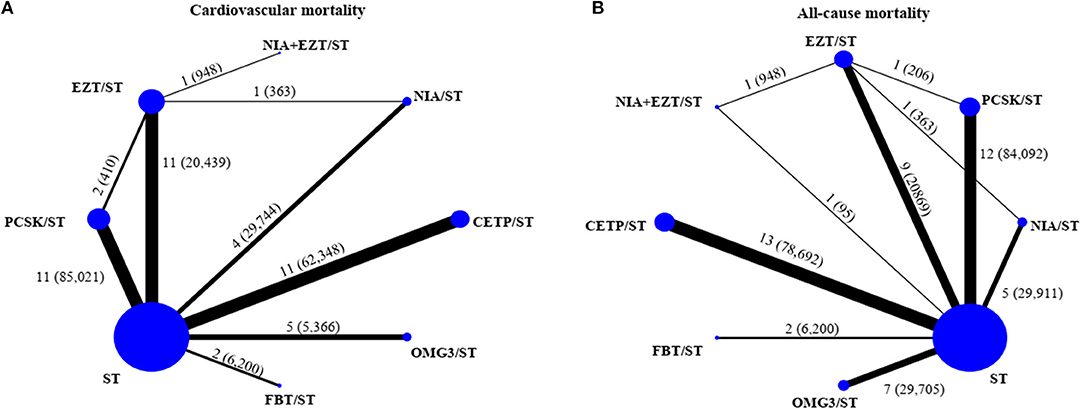
Figure 1. Network of eligible comparisons for primary outcomes [(A) cardiovascular mortality and (B) all-cause mortality]. The size of the node corresponds to the number of individual studies that studied the interventions. The directly compared interventions are linked with a line, the thickness of which corresponds to the number of studies that assess respective comparison. CETP/ST, cholesteryl ester transfer protein inhibitor + statin; EZT/ST, ezetimibe + statin; FBT/ST, fibrate + statin; NIA/ST, niacin + statin; OMG3/ST, omega-3 fatty acids + statin; PCSK/ST, proprotein convertase subtilisin/kexin type 9 inhibitor + statin; NIA+EZT/ST, niacin + ezetimibe + statin; ST, statin monotherapy.
Primary Outcomes
A total of 44 studies (210,179 participants, 5,052 cases with events) and 50 studies (249,196 participants, 11,112 cases with events) were analyzed for the risk of CV death and all-cause death, respectively. Networks of eight treatment interventions for CV and all-cause mortality were mapped as shown in Figure 1. Overall, there were no statistically significant differences in both primary outcomes among various NST compared to ST (Figure 2). Additionally, no significant difference on estimated effects was seen among non-statin therapies for both primary outcomes (eTables 9.1, 9.2). Results of SUCRA rank on both outcomes were shown in eTables 10.1, 10.2.
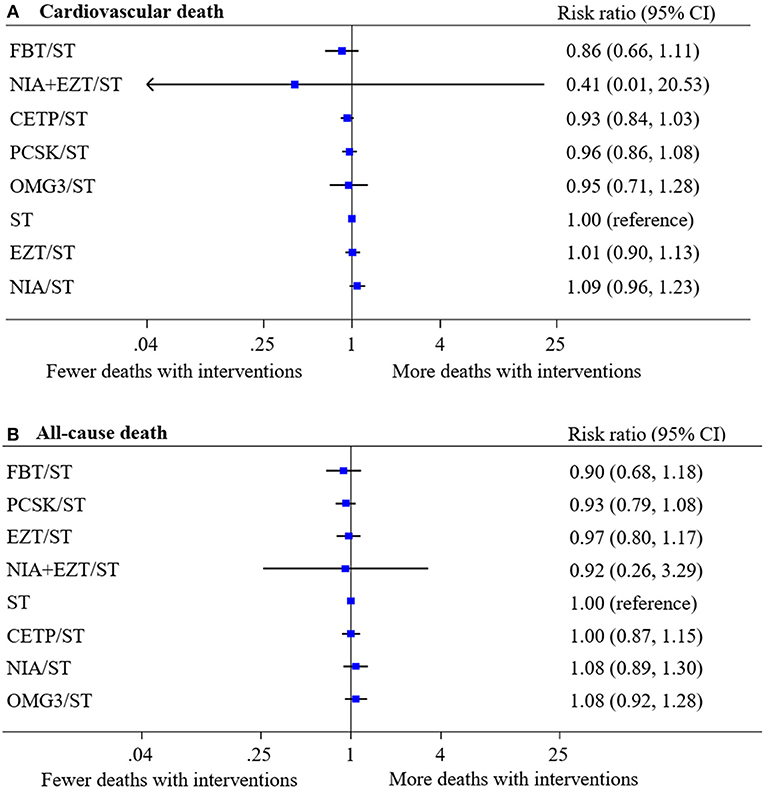
Figure 2. Network meta-analysis of treatment interventions compared with statin monotherapy for primary outcomes. Summary estimate represents risk ratio of (A) cardiovascular death and (B) all-cause death. Interventions were ranked by Surface under the cumulative ranking (SUCRA) values. CI, confidence interval; CETP/ST, cholesteryl ester transfer protein inhibitor + statin; EZT/ST, ezetimibe + statin; FBT/ST, fibrate + statin; NIA/ST, niacin + statin; OMG3/ST, omega-3 fatty acids + statin; PCSK/ST, proprotein convertase subtilisin/kexin type 9 inhibitor + statin; NIA+EZT/ST, niacin + ezetimibe + statin; ST, statin monotherapy.
Secondary Outcomes
Treatment interventions were mapped for CHD mortality, non-fatal MI, stroke, and coronary revascularization using data from 43, 37, 41, and 36 studies, respectively (see eFigures 6.1–6.4).
The treatment effects for these outcomes compared with ST were estimated (Figure 3). Overall, there were no differences in the risk of CHD mortality among all treatment comparisons. However, PCSK/ST was significantly reduced the risks of non-fatal MI (RR 0.82, 95% CI 0.72–0.93, p = 0.003), stroke (RR 0.74, 95% CI 0.65–0.85, p < 0.001) and coronary revascularization (RR 0.84, 95% CI 0.75–0.94, p = 0.003). Additionally, PCSK/ST significantly reduced the risks of stroke when compared to CETP/ST, OMG3/ST, and NIA/ST (RR 0.74 with 95% CI 0.63–0.88, RR 0.74 with 95% CI 0.57–0.95, and RR 0.73 with 95% CI 0.61–0.87, respectively). Also, PCSK/ST was superior to CETP/ST in reducing the risk of coronary revascularization (RR 0.83 with 95% CI 0.71–0.96), see eTables 9.3–9.6. Results of SUCRA rank of these outcomes are listed in eTables 10.3–10.6. Based on these results along with SUCRA rank, PCSK/ST appeared to be the most efficacious regimen to reduce non-fatal MI and coronary revascularization compared to other NST.
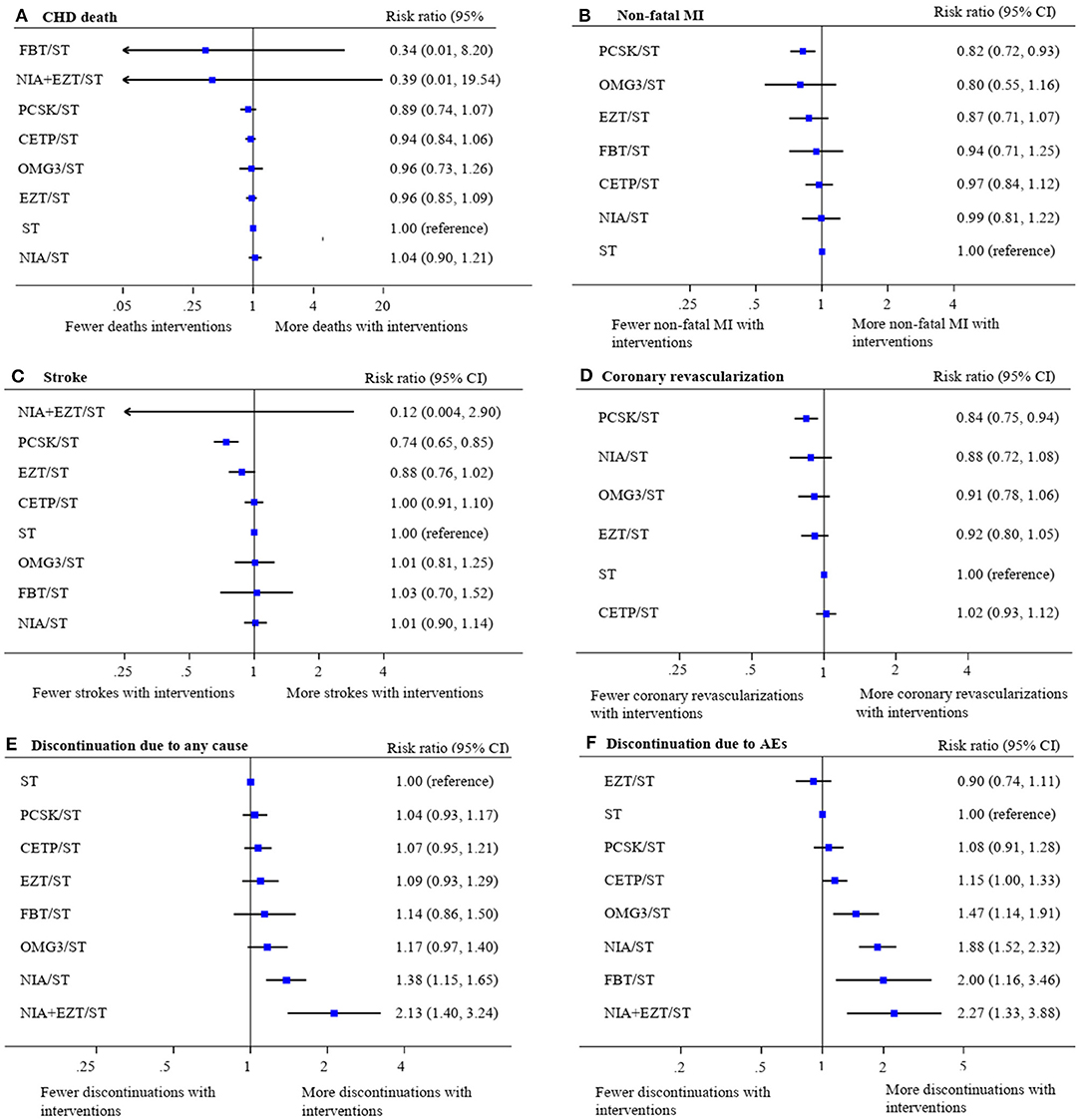
Figure 3. Network meta-analysis of treatment interventions compared with statin monotherapy for secondary cardiovascular endpoints and discontinuations. Summary estimate represents risk ratio of (A) coronary heart disease death, (B) non-fatal myocardial infarction, (C) stroke, (D) coronary revascularization, (E) discontinuation due to any cause, and (F) discontinuation due to adverse events. Interventions were ranked by SUCRA values. AE, adverse event; CHD, coronary heart disease; CI, confidence interval; CETP/ST, cholesteryl ester transfer protein inhibitor + statin; EZT/ST, ezetimibe + statin; FBT/ST, fibrate + statin; MI, myocardial infarction; NIA/ST, niacin + statin; OMG3/ST, omega-3 fatty acids + statin; PCSK/ST, proprotein convertase subtilisin/kexin type 9 inhibitor + statin; NIA+EZT/ST, niacin + ezetimibe + statin; ST, statin monotherapy.
For safety endpoints, the network maps were presented in eFigures 6.5, 6.6. The effects of treatments on all-cause discontinuation (58 studies, 236,043 participants) and discontinuation from any adverse event (56 studies, 209,532 participants) compared with ST were demonstrated in Figure 3. Only NIA/ST and NIA + EZT/ST showed a significant increase in the risk of all-cause discontinuation. Most NST significantly increased the risk of treatment discontinuations due to adverse events except PCSK/ST and EZT/ST compared with ST. Details of network estimates for safety endpoints of all treatment comparisons were presented in eTables 9.7, 9.8. A three-drug combination of NIA + EZT/ST was ranked the lowest for both safety endpoints (see eTables 10.7–10.8).
Subgroup Analyses
We performed subgroup analyses in primary and secondary outcomes with regards to indication of treatment, intensity of statin therapy, requirement of statin prior to starting NST, level of cardiovascular risk, elderly, familial hypercholesterolemia (FH) and lipid level at baseline. Most effect estimates among subgroup analyses on the outcomes were relatively consistent with results in the main analyses (Appendix 12).
Sensitivity Analyses and Publication Bias
We also performed sensitivity analyses by excluding studies with high risk of bias, non-adjudicated CV events, follow-up duration <1 year, and sample size <25 percentile. The effect estimates were generally robust among sensitivity analyses (Appendix 13). Comparison-adjusted funnel plots for all outcomes showed no evidence of asymmetry (Appendix 14). We also identified 7 studies registered in ClinicalTrials.gov but no published reports or results of those studies are available (Appendix 15). However, these trials were mostly small in size in comparison to the total study population. As a result, the chance for these trials to affect the main analysis is very low.
Quality of Evidence
The quality of direct evidence for all outcomes was generally rated as moderate to high quality. When applying GRADE to network meta-analysis evidence, most comparison of interventions were rated as moderate quality for primary and secondary outcomes except safety endpoints as low quality. In addition, a better rating of quality of evidence for non-fatal MI was found. More details of their quality of evidence are presented in Appendix 16.
Discussion
This network meta-analysis offers a single and comprehensive framework for comparison of efficacy and safety outcomes among various NST when added on to statin therapy in a broad range of patient populations. The results showed that none of these agents reduced the risk of CV death or all-cause death when compared with ST. Our findings suggested that PCSKs were the most efficacious agents when added on to statin therapy based on their ability to significantly reduce cardiovascular events including non-fatal MI, stroke and coronary revascularization. Such findings were robust and remain significant in various sensitivity and subgroup analyses. For safety aspects, the tolerability profile of PCSK/ST was similar to ST; therefore, such regimen appears to have a well-balanced efficacy and safety profile.
The reason of why NST did not reduce the risk of CV death and all-cause mortality may derive from several aspects including differences in mechanism of lipid-lowering actions, magnitude of LDL-C lowering effects along with trial design. Previously, a meta-analysis has shown that NST whose mechanisms of action relates to the upregulation of LDL-C receptor reduce CV events while those without this action did not (Silverman et al., 2016). As a result, mechanism of action may play a role in translating biochemical modification into clinical benefit. Trial design may partly explain the lack of mortality benefit of PCSK. A recent meta-analysis of 24 RCTs (Navarese et al., 2015) showed that PCSKs significantly reduced all-cause mortality. However, some of the included studies in the meta-analysis were without background statin therapy which is different from our study. Based on statin trials that demonstrated reduction in risk of mortality, the data showed that event curves started to diverge after 1.5–2.0 years [Scandinavian Simvastatin Survival Study Group, 1994; The Long-Term Intervention with Pravastatin in Ischaemic Disease (Lipid) Study Group, 1998]. Therefore, the duration of followed-up time might be an important factor. For CV outcome trials of PCSK9 inhibitors including FOURIER and ODESSEY Outcomes, the median follow-up time was 2.2 and 2.8 years, respectively (Sabatine et al., 2017; Schwartz et al., 2018). These may explain why the lack of reduction was seen in the trials of PCSK9 inhibitors in spite of dramatic reduction in LDL-C level compared with placebo (Sabatine et al., 2017; Schwartz et al., 2018). Of note, The ODYSSEY Outcomes trial, which had a longer follow-up, demonstrated significant reduction in mortality; however, it was a secondary endpoint of the trial (Schwartz et al., 2018).
For ezetimibe, we did not find significant effects of ezetimibe on clinical outcome in the overall analysis. Nevertheless, the results from the IMPROVE-IT trial showed that ezetimibe reduced non-fatal MI and ischemic stroke in ACS patients during the mean follow-up of 7 years (Cannon et al., 2015a). This may indicate that cardiovascular benefits of ezetimibe require a long period of exposure, potentially due to its modest LDL-C reduction effects. Since our analysis included studies of ezetimibe that were mostly run for no more than 2 years, inclusion of those trials therefore may dilute the effect of ezetimibe in our analysis. However, based on the subgroup and sensitivity analyses, ezetimibe reduced the risk of non-fatal MI and coronary revascularization in patients receiving moderate-intensity statins. Favorable tolerability profile, ease of use and affordability may make ezetimibe a viable option compared to PCSKs. Overall, this study lends a strong support toward the current clinical practice guideline that PCSKs and ezetimibe should be considered when patients failed to reach lipid goals or desired percentage reduction after maximally tolerated statin therapy has been deployed (Lloyd-Jones et al., 2017).
Similar to the results of previous RCTs of niacin and CETPs (Barter et al., 2007b; Boden et al., 2011; Schwartz et al., 2012; Landray et al., 2014; Lincoff et al., 2017), our analysis did not find any benefit of these agents on all CV outcomes of interest. Although previous epidemiological data have shown the association between low HDL-C and increased risk of cardiovascular disease (Barter et al., 2007b), a recent observational cohort study demonstrated that high level of HDL-C have not been associated with lowered risk of CV death (Ko et al., 2016). As a result, the hypothesis of using therapeutic agents to raise HDL-C may need to be carefully reexamined. Recently, anacetrapib, a CETP inhibitor, has been shown in HPS3/TIMI55–REVEAL trial to significantly reduce CV events. However, the effect was modest (Bowman et al., 2017) despite a doubling increase in HDL-C level. With a modest effect coupled with safety concern including blood pressure increase, reduced renal function along with prolonged accumulation of the drug in adipose tissue, this agent is later dropped from entering the market. For safety endpoints, both NIA/ST and CETP/ST were associated with higher risks of discontinuations compared with ST. In summary, these interventions did not demonstrate any benefit yet were associated with increased risk of adverse events, making it very difficult to justify their uses.
Fibrates and OMG3 are NST with predominant triglyceride-lowering effects. Based on our analysis, neither agent has demonstrable effects on clinical outcomes. Based on our inclusion criteria, all trials for fibrate included in our analysis used fenofibrate. The lack of effect in our analysis is consistent with findings from the ACCORD trial (Ginsberg et al., 2010). For OMG3, available evidence from 3 large RCTs are conflicting; with one positive and two neutral trials (Yokoyama et al., 2007; Kromhout et al., 2010; Rauch et al., 2010). Our main analysis showed that OMG3/ST was not superior to any NST or ST. Combination of these agents with statin was also associated with higher risks of discontinuations compared with ST. As a result, justification for use of these agents is quite limited.
The clinical benefit seen with PCSK and the lack of benefit among other therapies may partly be explained by two potential reasons including the magnitude of additional LDL-C lowering effects and the mechanism of LDL-lowering effect (Silverman et al., 2016). A recent two meta-analyses suggested that the risk of CV events was reduced by 19–23% per 1-mmol/L reduction in LDL-C level among ST and NST that reduced LDL-C via the upregulation of LDL-C receptor expression (including PCSK and ezetimibe) (Silverman et al., 2016; Koskinas et al., 2018). Our finding is consistent with their findings except ezetimibe where the inclusion of short-term trials may dilute the effect of ezetimibe as mentioned above.
While our study can be considered as the most comprehensive evaluation for NST, the heterogeneity of trials that came with data gathered for this analysis should be clearly declared and noted. Despite our best attempt with statistical analysis, conclusion drawn from our analysis is still far from being definitive. This stems from the fact that approximately one third of included trials were at high risk of bias while quality of evidence among included data were considered moderate. We therefore caution reader to consider this limitation when interpreting our results.
In addition to the key limitation mentioned above, several other limitations should be noted. First, bile acid sequestrant, mipomersen, lomitapide, or phytosterol were not included in the network meta-analysis. None of clinical studies of these agents met our inclusion criteria due to short follow-up duration, lack of background statin therapy or no reporting of outcomes of interest. Second, since we did not have access to individual patient data, we therefore were unable to perform analysis on composite endpoints such as the standard MACEs. Third, our subgroup analyses were based on aggregated data; consequently, contamination of each subgroup is possible. For certain subgroup, we were unable to compare all 8 interventions due to the lack of data of some interventions on certain subgroups. In addition, we were unable to perform an analysis on diabetes subgroup due to incomplete information for data extraction. Fourth, most studies included in the analysis did not use CV events as primary outcome and follow-up duration of these studies were generally not as long as large-scale clinical studies. Certain therapies may require very long duration of treatment before any effects can be seen. Lastly, although PCSK/ST showed acceptable tolerability in our analysis, this was derived from mostly short-term studies. As a result, long-term safety of this combination needs to be evaluated further. Despite these limitations, our analysis offers a useful comparative data on both efficacy and safety of various NST among statin-treated patients. Such information may be useful to guide clinical decision or formulate clinical practice guideline for dyslipidemia.
Conclusion
In summary, our network meta-analysis suggested that none of NST significantly reduce the risk of CV death and all-cause death when added to moderate to high intensity statin therapy. However, PCSKs and to a lesser extent, ezetimibe may help reduce cardiovascular events with acceptable tolerability profile among broad range of patients. Fibrate, CETPs, niacin, and OMG3 did not show any positive effects on CV outcomes in broad range of high risk patients. Moreover, these agents when combined with statin were associated with higher incidence of adverse reactions. Further research into the risk-benefit along with cost-effectiveness analysis of these therapeutic options should be warranted.
Transparency
The lead authors (TC, SN, NC) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Author Contributions
TC, SN, and NC conceived and designed the study. TC wrote the protocol and the first draft. SN, NC, AT, CR, WW, and PV contributed to the writing of the manuscript. TC and PD screened, extracted the data, and performed the quality assessment and the quality of evidence. SN and NC had access to all the data in the study, analyzed the data and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.00547/full#supplementary-material
References
Arimura, T., Miura, S., Ike, A., Sugihara, M., Iwata, A., Nishikawa, H., et al. (2012). Comparison of the efficacy and safety of statin and statin/ezetimibe therapy after coronary stent implantation in patients with stable angina. J. Cardiol. 60, 111–118. doi: 10.1016/j.jjcc.2012.03.002
Baigent, C., Keech, A., Kearney, P. M., Blackwell, L., Buck, G., Pollicino, C., et al. (2005). Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366, 1267–1278. doi: 10.1016/S0140-6736(05)67394-1
Ballantyne, C. M., Davidson, M. H., Mckenney, J., Keller, L. H., Bajorunas, D. R., and Karas, R. H. (2008a). Comparison of the safety and efficacy of a combination tablet of niacin extended release and simvastatin vs simvastatin monotherapy in patients with increased non-HDL cholesterol (from the SEACOAST I study). Am. J. Cardiol. 101, 1428–1436. doi: 10.1016/j.amjcard.2008.02.092
Ballantyne, C. M., Davidson, M. H., Mckenney, J. M., Keller, L. H., Bajorunas, D. R., and Karas, R. H. (2008b). Comparison of the efficacy and safety of a combination tablet of niacin extended-release and simvastatin with simvastatin 80 mg monotherapy: the SEACOAST II (high-dose) study. J. Clin. Lipidol. 2, 79–90. doi: 10.1016/j.jacl.2008.02.004
Ballantyne, C. M., Shah, S., Kher, U., Hunter, J. A., Gill, G. G., Cressman, M. D., et al. (2017a). Lipid-modifying efficacy and tolerability of anacetrapib added to ongoing statin therapy in patients with hypercholesterolemia or low high-density lipoprotein cholesterol. Am. J. Cardiol. 119, 388–396. doi: 10.1016/j.amjcard.2016.10.032
Ballantyne, C. M., Shah, S., Sapre, A., Ashraf, T. B., Tobias, S. C., Sahin, T., et al. (2017b). A multiregional, randomized evaluation of the lipid-modifying efficacy and tolerability of anacetrapib added to ongoing statin therapy in patients with hypercholesterolemia or low high-density lipoprotein cholesterol. Am. J. Cardiol. 120, 569–576. doi: 10.1016/j.amjcard.2017.03.255
Balshem, H., Helfand, M., Schunemann, H. J., Oxman, A. D., Kunz, R., Brozek, J., et al. (2011). GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 64, 401–406. doi: 10.1016/j.jclinepi.2010.07.015
Barter, P., Gotto, A. M., Larosa, J. C., Maroni, J., Szarek, M., Grundy, S. M., et al. (2007a). HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N. Engl. J. Med. 357, 1301–1310. doi: 10.1056/NEJMoa064278
Barter, P. J., Caulfield, M., Eriksson, M., Grundy, S. M., Kastelein, J. J., Komajda, M., et al. (2007b). Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357, 2109–2122. doi: 10.1056/NEJMoa0706628
Bays, H., Gaudet, D., Weiss, R., Ruiz, J. L., Watts, G. F., Gouni-Berthold, I., et al. (2015). Alirocumab as add-on to atorvastatin versus other lipid treatment strategies: ODYSSEY OPTIONS I randomized trial. J. Clin. Endocrinol. Metab. 100, 3140–3148. doi: 10.1210/jc.2015-1520
Blom, D. J., Hala, T., Bolognese, M., Lillestol, M. J., Toth, P. D., Burgess, L., et al. (2014). A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N. Engl. J. Med. 370, 1809–1819. doi: 10.1056/NEJMoa1316222
Boden, W. E., Probstfield, J. L., Anderson, T., Chaitman, B. R., Desvignes-Nickens, P., Koprowicz, K., et al. (2011). Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365, 2255–2267. doi: 10.1056/NEJMoa1107579
Bots, M. L., Visseren, F. L., Evans, G. W., Riley, W. A., Revkin, J. H., Tegeler, C. H., et al. (2007). Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trial. Lancet 370, 153–160. doi: 10.1016/S0140-6736(07)61088-5
Bowman, L., Hopewell, J. C., Chen, F., Wallendszus, K., Stevens, W., Collins, R., et al. (2017). Effects of anacetrapib in patients with atherosclerotic vascular disease. N. Engl. J. Med. 377, 1217–1227. doi: 10.1056/NEJMoa1706444
Brunner, G., Yang, E. Y., Kumar, A., Sun, W., Virani, S. S., Negi, S. I., et al. (2013). The effect of lipid modification on peripheral artery disease after Endovascular Intervention Trial (ELIMIT). Atherosclerosis 231, 371–377. doi: 10.1016/j.atherosclerosis.2013.09.034
Caldwell, D. M., Ades, A. E., and Higgins, J. P. (2005). Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 331:897. doi: 10.1136/bmj.331.7521.897
Cannon, C. P., Blazing, M. A., Giugliano, R. P., Mccagg, A., White, J. A., Theroux, P., et al. (2015a). Ezetimibe added to statin therapy after acute coronary syndromes. N. Engl. J. Med. 372, 2387–2397. doi: 10.1056/NEJMoa1410489
Cannon, C. P., Cariou, B., Blom, D., Mckenney, J. M., Lorenzato, C., Pordy, R., et al. (2015b). Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur. Heart J. 36, 1186–1194. doi: 10.1093/eurheartj/ehv028
Cannon, C. P., Shah, S., Dansky, H. M., Davidson, M., Brinton, E. A., Gotto, A. M., et al. (2010). Safety of anacetrapib in patients with or at high risk for coronary heart disease. N. Engl. J. Med. 363, 2406–2415. doi: 10.1056/NEJMoa1009744
Catapano, A. L., Graham, I., De Backer, G., Wiklund, O., Chapman, M. J., Drexel, H., et al. (2016). 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur. Heart J. 37, 2999–3058. doi: 10.1093/eurheartj/ehw272
Chaimani, A., Higgins, J. P., Mavridis, D., Spyridonos, P., and Salanti, G. (2013). Graphical tools for network meta-analysis in STATA. PLoS ONE 8:e76654. doi: 10.1371/journal.pone.0076654
Cipriani, A., Higgins, J. P., Geddes, J. R., and Salanti, G. (2013). Conceptual and technical challenges in network meta-analysis. Ann. Intern. Med. 159, 130–137. doi: 10.7326/0003-4819-159-2-201307160-00008
Davidson, M. H., Rosenson, R. S., Maki, K. C., Nicholls, S. J., Ballantyne, C. M., Mazzone, T., et al. (2014). Effects of fenofibric acid on carotid intima-media thickness in patients with mixed dyslipidemia on atorvastatin therapy: randomized, placebo-controlled study (FIRST). Arterioscler. Thromb. Vasc. Biol. 34, 1298–1306. doi: 10.1161/ATVBAHA.113.302926
Dechartres, A., Altman, D. G., Trinquart, L., Boutron, I., and Ravaud, P. (2014). Association between analytic strategy and estimates of treatment outcomes in meta-analyses. JAMA 312, 623–630. doi: 10.1001/jama.2014.8166
Derosa, G., Cicero, A. E., Bertone, G., Piccinni, M. N., Ciccarelli, L., and Roggeri, D. E. (2004). Comparison of fluvastatin + fenofibrate combination therapy and fluvastatin monotherapy in the treatment of combined hyperlipidemia, type 2 diabetes mellitus, and coronary heart disease: a 12-month, randomized, double-blind, controlled trial. Clin. Ther. 26, 1599–1607. doi: 10.1016/j.clinthera.2004.10.008
Dersimonian, R., and Laird, N. (1986). Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188. doi: 10.1016/0197-2456(86)90046-2
Dias, S., Welton, N. J., Caldwell, D. M., and Ades, A. E. (2010). Checking consistency in mixed treatment comparison meta-analysis. Stat. Med. 29, 932–944. doi: 10.1002/sim.3767
Durrington, P. N., Bhatnagar, D., Mackness, M. I., Morgan, J., Julier, K., Khan, M. A., et al. (2001). An omega-3 polyunsaturated fatty acid concentrate administered for one year decreased triglycerides in simvastatin treated patients with coronary heart disease and persisting hypertriglyceridaemia. Heart 85, 544–548. doi: 10.1136/heart.85.5.544
Farnier, M., Jones, P., Severance, R., Averna, M., Steinhagen-Thiessen, E., Colhoun, H. M., et al. (2016). Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular-risk patients: the ODYSSEY OPTIONS II randomized trial. Atherosclerosis 244, 138–146. doi: 10.1016/j.atherosclerosis.2015.11.010
Fayad, Z. A., Mani, V., Woodward, M., Kallend, D., Abt, M., Burgess, T., et al. (2011). Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet 378, 1547–1559. doi: 10.1016/S0140-6736(11)61383-4
Fruchart, J. C., Sacks, F., Hermans, M. P., Assmann, G., Brown, W. V., Ceska, R., et al. (2008). The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in patients with dyslipidemia. Am J Cardiol 102, 1k−34k. doi: 10.1016/j.amjcard.2008.10.002
Fulcher, J., O'connell, R., Voysey, M., Emberson, J., Blackwell, L., Mihaylova, B., et al. (2015). Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 385, 1397–1405. doi: 10.1016/S0140-6736(14)61368-4
Ginsberg, H. N., Elam, M. B., Lovato, L. C., Crouse, J. R. III, Leiter, L. A., Linz, P., et al. (2010). Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 362, 1563–1574. doi: 10.1056/NEJMoa1001282
Ginsberg, H. N., Rader, D. J., Raal, F. J., Guyton, J. R., Baccara-Dinet, M. T., Lorenzato, C., et al. (2016). Efficacy and safety of alirocumab in patients with heterozygous familial hypercholesterolemia and LDL-C of 160 mg/dl or higher. Cardiovasc. Drugs Ther. 30, 473–483. doi: 10.1007/s10557-016-6685-y
Guyton, J. R., Brown, B. G., Fazio, S., Polis, A., Tomassini, J. E., and Tershakovec, A. M. (2008). Lipid-altering efficacy and safety of ezetimibe/simvastatin coadministered with extended-release niacin in patients with type IIa or type IIb hyperlipidemia. J. Am. Coll. Cardiol. 51, 1564–1572. doi: 10.1016/j.jacc.2008.03.003
Hagiwara, N., Kawada-Watanabe, E., Koyanagi, R., Arashi, H., Yamaguchi, J., Nakao, K., et al. (2017). Low-density lipoprotein cholesterol targeting with pitavastatin + ezetimibe for patients with acute coronary syndrome and dyslipidaemia: the HIJ-PROPER study, a prospective, open-label, randomized trial. Eur. Heart J. 38, 2264–2276. doi: 10.1093/eurheartj/ehx162
Hibi, K., Sonoda, S., Kawasaki, M., Otsuji, Y., Murohara, T., Ishii, H., et al. (2018). Effects of ezetimibe-statin combination therapy on coronary atherosclerosis in acute coronary syndrome. Circ. J. 82, 757–766. doi: 10.1253/circj.CJ-17-0598
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327:557. doi: 10.1136/bmj.327.7414.557
Higgins, J. P. T., Sterne, J. A. C., Savović, J., Page, M. J., Hróbjartsson, A., Boutron, I., et al. (2016). “A revised tool for assessing risk of bias in randomized trials,” in Cochrane Methods, eds J. Chandler, J. McKenzie, I. Boutron, and V. Welch (Chichester: Cochrane Database of Systematic Reviews), 29–31.
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 162, 777–784. doi: 10.7326/M14-2385
Kastelein, J. J., Akdim, F., Stroes, E. S., Zwinderman, A. H., Bots, M. L., Stalenhoef, A. F., et al. (2008). Simvastatin with or without ezetimibe in familial hypercholesterolemia. N. Engl. J. Med. 358, 1431–1443. doi: 10.1056/NEJMoa0800742
Kastelein, J. J., Besseling, J., Shah, S., Bergeron, J., Langslet, G., Hovingh, G. K., et al. (2015a). Anacetrapib as lipid-modifying therapy in patients with heterozygous familial hypercholesterolaemia (REALIZE): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet 385, 2153–2161. doi: 10.1016/S0140-6736(14)62115-2
Kastelein, J. J., Ginsberg, H. N., Langslet, G., Hovingh, G. K., Ceska, R., Dufour, R., et al. (2015b). ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur. Heart J. 36, 2996–3003. doi: 10.1093/eurheartj/ehv370
Kastelein, J. J., Van Leuven, S. I., Burgess, L., Evans, G. W., Kuivenhoven, J. A., Barter, P. J., et al. (2007). Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N. Engl. J. Med. 356, 1620–1630. doi: 10.1056/NEJMoa071359
Kereiakes, D. J., Robinson, J. G., Cannon, C. P., Lorenzato, C., Pordy, R., Chaudhari, U., et al. (2015). Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am. Heart. J. 169, 906–915.e913. doi: 10.1016/j.ahj.2015.03.004
Ko, D. T., Alter, D. A., Guo, H., Koh, M., Lau, G., Austin, P. C., et al. (2016). High-density lipoprotein cholesterol and cause-specific mortality in individuals without previous cardiovascular conditions: the CANHEART study. J. Am. Coll. Cardiol. 68, 2073–2083. doi: 10.1016/j.jacc.2016.08.038
Koh, K. K., Nam, C. W., Chao, T. H., Liu, M. E., Wu, C. J., Kim, D. S., et al. (2018). A randomized trial evaluating the efficacy and safety of alirocumab in South Korea and Taiwan (ODYSSEY KT). J. Clin. Lipidol. 12, 162–172.e166. doi: 10.1016/j.jacl.2017.09.007
Koskinas, K. C., Siontis, G. C. M., Piccolo, R., Mavridis, D., Raber, L., Mach, F., et al. (2018). Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: a meta-analysis of randomized trials. Eur. Heart J. 39, 1172–1180. doi: 10.1093/eurheartj/ehx566
Kouvelos, G. N., Arnaoutoglou, E. M., Matsagkas, M. I., Kostara, C., Gartzonika, C., Bairaktari, E. T., et al. (2013). Effects of rosuvastatin with or without ezetimibe on clinical outcomes in patients undergoing elective vascular surgery: results of a pilot study. J. Cardiovasc. Pharmacol. Ther. 18, 5–12. doi: 10.1177/1074248412445506
Kromhout, D., Giltay, E. J., and Geleijnse, J. M. (2010). n-3 fatty acids and cardiovascular events after myocardial infarction. N. Engl. J. Med. 363, 2015–2026. doi: 10.1056/NEJMoa1003603
Landray, M., Baigent, C., Leaper, C., Adu, D., Altmann, P., Armitage, J., et al. (2006). The second United Kingdom Heart and Renal Protection (UK-HARP-II) Study: a randomized controlled study of the biochemical safety and efficacy of adding ezetimibe to simvastatin as initial therapy among patients with CKD. Am. J. Kidney Dis. 47, 385–395. doi: 10.1053/j.ajkd.2005.11.018
Landray, M. J., Haynes, R., Hopewell, J. C., Parish, S., Aung, T., Tomson, J., et al. (2014). Effects of extended-release niacin with laropiprant in high-risk patients. N. Engl. J. Med. 371, 203–212. doi: 10.1056/NEJMoa1300955
Leiter, L. A., Cariou, B., Muller-Wieland, D., Colhoun, H. M., Del Prato, S., Tinahones, F. J., et al. (2017). Efficacy and safety of alirocumab in insulin-treated individuals with type 1 or type 2 diabetes and high cardiovascular risk: the ODYSSEY DM-INSULIN randomized trial. Diabetes Obes. Metab. 19, 1781–1792. doi: 10.1111/dom.13114
Lincoff, A. M., Nicholls, S. J., Riesmeyer, J. S., Barter, P. J., Brewer, H. B., Fox, K., et al. (2017). Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N. Engl. J. Med. 376, 1933–1942. doi: 10.1056/NEJMoa1609581
Liu, Z., Hao, H., Yin, C., Chu, Y., Li, J., and Xu, D. (2017). Therapeutic effects of atorvastatin and ezetimibe compared with double-dose atorvastatin in very elderly patients with acute coronary syndrome. Oncotarget 8, 41582–41589. doi: 10.18632/oncotarget.15078
Lloyd-Jones, D. M., Morris, P. B., Ballantyne, C. M., Birtcher, K. K., Daly, D. D. Jr., Depalma, S. M., et al. (2017). 2017 focused update of the 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the american college of cardiology task force on expert consensus decision pathways. J. Am. Coll. Cardiol. 70, 1785–1822. doi: 10.1016/j.jacc.2017.07.745
Lu, G., and Ades, A. E. (2004). Combination of direct and indirect evidence in mixed treatment comparisons. Stat. Med. 23, 3105–3124. doi: 10.1002/sim.1875
Luo, P., Li, L., Wang, L. X., Zhu, H. H., Du, S., Wu, S. L., et al. (2014). Effects of atorvastatin in combination with ezetimibe on carotid atherosclerosis in elderly patients with hypercholesterolemia. Genet. Mol. Res. 13, 2377–2384. doi: 10.4238/2014.April.3.10
Luo, P., Wang, L., Zhu, H., Du, S., Wang, G., and Ding, S. (2016). Impact of atorvastatin combined with ezetimibe for the treatment of carotid atherosclerosis in patients with coronary heart disease. Acta Cardiol. Sin. 32, 578–585. doi: 10.6515/ACS20151013H
Luscher, T. F., Taddei, S., Kaski, J. C., Jukema, J. W., Kallend, D., Munzel, T., et al. (2012). Vascular effects and safety of dalcetrapib in patients with or at risk of coronary heart disease: the dal-VESSEL randomized clinical trial. Eur. Heart J. 33, 857–865. doi: 10.1093/eurheartj/ehs019
Masuda, J., Tanigawa, T., Yamada, T., Nishimura, Y., Sasou, T., Nakata, T., et al. (2015). Effect of combination therapy of ezetimibe and rosuvastatin on regression of coronary atherosclerosis in patients with coronary artery disease. Int. Heart J. 56, 278–285. doi: 10.1536/ihj.14-311
Mills, E. J., Ioannidis, J. P., Thorlund, K., Schunemann, H. J., Puhan, M. A., and Guyatt, G. H. (2012). How to use an article reporting a multiple treatment comparison meta-analysis. JAMA 308, 1246–1253. doi: 10.1001/2012.jama.11228
Miyoshi, T., Kohno, K., Asonuma, H., Sakuragi, S., Nakahama, M., Kawai, Y., et al. (2018). Effect of intensive and standard pitavastatin treatment with or without eicosapentaenoic acid on progression of coronary artery calcification over 12 months- prospective multicenter study. Circ. J. 82, 532–540. doi: 10.1253/circj.CJ-17-0419
Navarese, E. P., Kolodziejczak, M., Schulze, V., Gurbel, P. A., Tantry, U., Lin, Y., et al. (2015). Effects of proprotein convertase subtilisin/Kexin Type 9 antibodies in adults with hypercholesterolemia: a systematic review and meta-analysis. Ann. Intern. Med. 163, 40–51. doi: 10.7326/M14-2957
Nicholls, S. J., Puri, R., Anderson, T., Ballantyne, C. M., Cho, L., Kastelein, J. J., et al. (2016). Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA 316, 2373–2384. doi: 10.1001/jama.2016.16951
Nishio, R., Shinke, T., Otake, H., Nakagawa, M., Nagoshi, R., Inoue, T., et al. (2014). Stabilizing effect of combined eicosapentaenoic acid and statin therapy on coronary thin-cap fibroatheroma. Atherosclerosis 234, 114–119. doi: 10.1016/j.atherosclerosis.2014.02.025
Nissen, S. E., Tardif, J. C., Nicholls, S. J., Revkin, J. H., Shear, C. L., Duggan, W. T., et al. (2007). Effect of torcetrapib on the progression of coronary atherosclerosis. N. Engl. J. Med. 356, 1304–1316. doi: 10.1056/NEJMoa070635
Nosaka, K., Miyoshi, T., Iwamoto, M., Kajiya, M., Okawa, K., Tsukuda, S., et al. (2017). Early initiation of eicosapentaenoic acid and statin treatment is associated with better clinical outcomes than statin alone in patients with acute coronary syndromes: 1-year outcomes of a randomized controlled study. Int. J. Cardiol. 228, 173–179. doi: 10.1016/j.ijcard.2016.11.105
Puhan, M. A., Schunemann, H. J., Murad, M. H., Li, T., Brignardello-Petersen, R., Singh, J. A., et al. (2014). A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 349:g5630. doi: 10.1136/bmj.g5630
Rauch, B., Schiele, R., Schneider, S., Diller, F., Victor, N., Gohlke, H., et al. (2010). OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation 122, 2152–2159. doi: 10.1161/CIRCULATIONAHA.110.948562
Ray, K. K., Leiter, L. A., Muller-Wieland, D., Cariou, B., Colhoun, H. M., Henry, R. R., et al. (2018). Alirocumab vs usual lipid-lowering care as add-on to statin therapy in individuals with type 2 diabetes and mixed dyslipidaemia: the ODYSSEY DM-DYSLIPIDEMIA randomized trial. Diabetes Obes. Metab. 20, 1479–1489. doi: 10.1111/dom.13257
Ridker, P. M., Revkin, J., Amarenco, P., Brunell, R., Curto, M., Civeira, F., et al. (2017a). Cardiovascular efficacy and safety of bococizumab in high-risk patients. N. Engl. J. Med. 376, 1527–1539. doi: 10.1056/NEJMoa1701488
Ridker, P. M., Tardif, J. C., Amarenco, P., Duggan, W., Glynn, R. J., Jukema, J. W., et al. (2017b). Lipid-reduction variability and antidrug-antibody formation with bococizumab. N. Engl. J. Med. 376, 1517–1526. doi: 10.1056/NEJMoa1614062
Robinson, J. G., Farnier, M., Krempf, M., Bergeron, J., Luc, G., Averna, M., et al. (2015). Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 372, 1489–1499. doi: 10.1056/NEJMoa1501031
Sabatine, M. S., Giugliano, R. P., Keech, A. C., Honarpour, N., Wiviott, S. D., Murphy, S. A., et al. (2017). Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 376, 1713–1722. doi: 10.1056/NEJMoa1615664
Sabatine, M. S., Giugliano, R. P., Wiviott, S. D., Raal, F. J., Blom, D. J., Robinson, J., et al. (2015). Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 372, 1500–1509. doi: 10.1056/NEJMoa1500858
Salanti, G., Ades, A. E., and Ioannidis, J. P. (2011). Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J. Clin. Epidemiol. 64, 163–171. doi: 10.1016/j.jclinepi.2010.03.016
Sang, Z. C., Wang, F., Zhou, Q., Li, Y. H., Li, Y. G., Wang, H. P., et al. (2009). Combined use of extended-release niacin and atorvastatin: safety and effects on lipid modification. Chin. Med. J. 122, 1615–1620. doi: 10.3760/cma.j.issn.0366-6999.2009.14.003
Scandinavian Simvastatin Survival Study Group (1994). Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344, 1383–1389. doi: 10.1016/S0140-6736(94)90566-5
Schwartz, G. G., Olsson, A. G., Abt, M., Ballantyne, C. M., Barter, P. J., Brumm, J., et al. (2012). Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 367, 2089–2099. doi: 10.1056/NEJMoa1206797
Schwartz, G. G., Steg, P. G., Szarek, M., Bhatt, D. L., Bittner, V. A., Diaz, R., et al. (2018). Alirocumab and cardiovascular outcomes after acute coronary syndrome. N. Engl. J. Med. 379, 2097–2107. doi: 10.1056/NEJMoa1801174
Shaw, S. M., Chaggar, P., Ritchie, J., Shah, M. K., Baynes, A. C., O'neill, N., et al. (2009). The efficacy and tolerability of ezetimibe in cardiac transplant recipients taking cyclosporin. Transplantation 87, 771–775. doi: 10.1097/TP.0b013e318198d7d0
Silverman, M. G., Ference, B. A., Im, K., Wiviott, S. D., Giugliano, R. P., Grundy, S. M., et al. (2016). Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA 316, 1289–1297. doi: 10.1001/jama.2016.13985
Stein, E. A., Roth, E. M., Rhyne, J. M., Burgess, T., Kallend, D., and Robinson, J. G. (2010). Safety and tolerability of dalcetrapib (RO4607381/JTT-705): results from a 48-week trial. Eur. Heart J. 31, 480–488. doi: 10.1093/eurheartj/ehp601
Stone, N. J., Robinson, J. G., Lichtenstein, A. H., Bairey Merz, C. N., Blum, C. B., Eckel, R. H., et al. (2014). 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 63, 2889–2934. doi: 10.1016/j.jacc.2013.11.002
Taylor, A. J., Sullenberger, L. E., Lee, H. J., Lee, J. K., and Grace, K. A. (2004). Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 110, 3512–3517. doi: 10.1161/01.CIR.0000148955.19792.8D
Taylor, A. J., Villines, T. C., Stanek, E. J., Devine, P. J., Griffen, L., Miller, M., et al. (2009). Extended-release niacin or ezetimibe and carotid intima-media thickness. N. Engl. J. Med. 361, 2113–2122. doi: 10.1056/NEJMoa0907569
Taylor, F., Huffman, M. D., Macedo, A. F., Moore, T. H., Burke, M., Davey Smith, G., et al. (2013). Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2013:Cd004816. doi: 10.1002/14651858.CD004816.pub5
Teramoto, T., Daida, H., Ikewaki, K., Arai, H., Maeda, Y., Nakagomi, M., et al. (2017). Lipid-modifying efficacy and tolerability of anacetrapib added to ongoing statin therapy in Japanese patients with dyslipidemia. Atherosclerosis 261, 69–77. doi: 10.1016/j.atherosclerosis.2017.03.009
Teramoto, T., Kobayashi, M., Tasaki, H., Yagyu, H., Higashikata, T., Takagi, Y., et al. (2016). Efficacy and safety of alirocumab in Japanese patients with heterozygous familial hypercholesterolemia or at high cardiovascular risk with hypercholesterolemia not adequately controlled with statins- ODYSSEY JAPAN randomized controlled trial. Circ. J. 80, 1980–1987. doi: 10.1253/circj.CJ-16-0387
The Long-Term Intervention with Pravastatin in Ischaemic Disease (Lipid) Study Group (1998). Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N. Engl. J. Med. 339, 1349–1357. doi: 10.1056/NEJM199811053391902
Tsujita, K., Sugiyama, S., Sumida, H., Shimomura, H., Yamashita, T., Yamanaga, K., et al. (2015). Impact of dual lipid-lowering strategy with ezetimibe and atorvastatin on coronary plaque regression in patients with percutaneous coronary intervention: the multicenter randomized controlled PRECISE-IVUS trial. J. Am. Coll. Cardiol. 66, 495–507. doi: 10.1016/j.jacc.2015.05.065
Wang, X., Zhao, X., Li, L., Yao, H., Jiang, Y., and Zhang, J. (2016). Effects of combination of ezetimibe and rosuvastatin on coronary artery plaque in patients with coronary heart disease. Heart Lung Circ. 25, 459–465. doi: 10.1016/j.hlc.2015.10.012
Watanabe, T., Ando, K., Daidoji, H., Otaki, Y., Sugawara, S., Matsui, M., et al. (2017). A randomized controlled trial of eicosapentaenoic acid in patients with coronary heart disease on statins. J. Cardiol. 70, 537–544. doi: 10.1016/j.jjcc.2017.07.007
West, A. M., Anderson, J. D., Meyer, C. H., Epstein, F. H., Wang, H., Hagspiel, K. D., et al. (2011). The effect of ezetimibe on peripheral arterial atherosclerosis depends upon statin use at baseline. Atherosclerosis 218, 156–162. doi: 10.1016/j.atherosclerosis.2011.04.005
Yokoyama, M., Origasa, H., Matsuzaki, M., Matsuzawa, Y., Saito, Y., Ishikawa, Y., et al. (2007). Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 369, 1090–1098. doi: 10.1016/S0140-6736(07)60527-3
Keywords: non-statin lipid-modifying agent, statin-treated patient, cardiovascular morbidity, mortality, network meta-analysis
Citation: Chaiyasothi T, Nathisuwan S, Dilokthornsakul P, Vathesatogkit P, Thakkinstian A, Reid C, Wongcharoen W and Chaiyakunapruk N (2019) Effects of Non-statin Lipid-Modifying Agents on Cardiovascular Morbidity and Mortality Among Statin-Treated Patients: A Systematic Review and Network Meta-Analysis. Front. Pharmacol. 10:547. doi: 10.3389/fphar.2019.00547
Received: 21 February 2019; Accepted: 01 May 2019;
Published: 22 May 2019.
Edited by:
Sandor Kerpel-Fronius, Semmelweis University, HungaryReviewed by:
Yaser Mohammed Al-Worafi, Ajman University of Science and Technology, United Arab EmiratesChin Fen Neoh, Universiti Teknologi MARA, Malaysia
Copyright © 2019 Chaiyasothi, Nathisuwan, Dilokthornsakul, Vathesatogkit, Thakkinstian, Reid, Wongcharoen and Chaiyakunapruk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Surakit Nathisuwan, c3VyYWtpdC5uYXRAbWFoaWRvbC5hYy50aA==; Nathorn Chaiyakunapruk, bmF0aG9ybi5jaGFpeWFrdW5hcHJ1a0Btb25hc2guZWR1
 Thanaputt Chaiyasothi
Thanaputt Chaiyasothi Surakit Nathisuwan
Surakit Nathisuwan Piyameth Dilokthornsakul
Piyameth Dilokthornsakul Prin Vathesatogkit4
Prin Vathesatogkit4 Nathorn Chaiyakunapruk
Nathorn Chaiyakunapruk