
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 24 May 2019
Sec. Gastrointestinal and Hepatic Pharmacology
Volume 10 - 2019 | https://doi.org/10.3389/fphar.2019.00509
 Priyanka Saha1
Priyanka Saha1 Anupam Das Talukdar1*
Anupam Das Talukdar1* Rajat Nath1
Rajat Nath1 Satyajit D. Sarker2
Satyajit D. Sarker2 Lutfun Nahar2
Lutfun Nahar2 Jagajjit Sahu3
Jagajjit Sahu3 Manabendra Dutta Choudhury1
Manabendra Dutta Choudhury1The liver is not only involved in metabolism and detoxification, but also participate in innate immune function and thus exposed to frequent target Thus, they are the frequent target of physical injury. Interestingly, liver has the unique ability to regenerate and completely recoup from most acute, non-iterative situation. However, multiple conditions, including viral hepatitis, non-alcoholic fatty liver disease, long term alcohol abuse and chronic use of medications can cause persistent injury in which regenerative capacity eventually becomes dysfunctional resulting in hepatic scaring and cirrhosis. Despite the recent therapeutic advances and significant development of modern medicine, hepatic diseases remain a health problem worldwide. Thus, the search for the new therapeutic agents to treat liver disease is still in demand. Many synthetic drugs have been demonstrated to be strong radical scavengers, but they are also carcinogenic and cause liver damage. Present day various hepatic problems are encountered with number of synthetic and plant based drugs. Nexavar (sorafenib) is a chemotherapeutic medication used to treat advanced renal cell carcinoma associated with several side effects. There are a few effective varieties of herbal preparation like Liv-52, silymarin and Stronger neomin phages (SNMC) against hepatic complications. Plants are the huge repository of bioactive secondary metabolites viz; phenol, flavonoid, alkaloid etc. In this review we will try to present exclusive study on phenolics with its mode of action mitigating liver associated complications. And also its future prospects as new drug lead.
The liver is labeled as the biggest glandular organ that controls diverse physiological and chemical processes in human body. In other words, it plays a central role in metabolic control and detoxification involving metabolism of lipids, carbohydrates, alcohol, and a wide range of drugs as well as toxins (Aseervatham et al., 2018). The liver also participates in innate immune function (Gao et al., 2008). Interestingly, the liver has the unique ability to regenerate and completely recoup from most acute, non-iterative situations (Mosedale and Watkins, 2017; Oliva-Vilarnau et al., 2018). However, multiple conditions, e.g., hepatitis, chronic alcohol consumption, frequent use of antibiotics associated medications, and also even non- alcoholic fatty liver disease, can affect the regenerative efficacy of the hepatocytes which become totally dysfunctional (Forbes and Newsome, 2016), generally witnessed by visible hepatic scaring, apoptosis and entering into the most severe cirrhosis. The liver, when witness to such atrocities, ultimately loses its vitality and thus imbalances the normal metabolic phenomenon, leading to many other fatal conditions (Branco et al., 2016; Defronzo et al., 2016). Despite considerable amounts of research which have been carried out aiming at cure various hepatic ailments across the world, limitations still exist in finding more effective hepatoprotective drugs than the currently available medications. Moreover, fewer medications promise restoration effects.
The Mediterranean-style diet which covers the immense geographical area adjoining the Mediterranean Sea focuses on the use of root legumes, vegetable, fruits, nuts and seeds predominantly (Tuck and Hayball, 2002). Presently there is an arising concern or interest, rather, n exploring the positive effects of a plant-based diet for mitigating various chronic diseases including several hepatic ailments like hepatic cirrhosis, hepatic ulcerative syndrome and fibrosis. It is noteworthy that the Mediterranean diet, which has been allied with many health benefits, is characterized by a high intake of fruits, vegetables and nuts containing several bioactive natural products of plants. One such dietary component common in plant-based diets are natural phenolics, which are particularly plentiful not only in fruits, whole grains, vegetables, and legumes but equally in coffee, tea, cocoa, and also in red wine.
Phenolics are a bulky and heterogeneous group of phytochemicals containing phenol rings, and are divided into several groups, viz; phenolic acids, flavonoids and lignin. Fruits such as pears, grapes, apples, and a range of berries naturally contain good amounts of polyphenols (250–400 mg in 100 g). The most frequent phenolic acids are ferulic acid and caffeic acid of which the major phenolic compounds in coffee and cereals, respectively, are comprised. The most studied stilbene is resveratrol, found in red wine and grape products (Veberic et al., 2008). Other main dietary sources of natural phenolics comprise of chocolate, green tea, and whole grains. Polyphenol contains abundant antioxidants in the diet and these act as natural scavengers for toxic elements and, thus, their intake has been directly connected with a reduced frequency of several hepatic ailments, particularly hepatocellular carcinoma in humans (Turati et al., 2014). Phenolics also exhibit anti-inflammatory effects and influences hepatotoxicity through altered mechanisms discussed in detail in the subsequent paragraphs.
Thus, herbal approach, an alternative to the conventional protocol with a touch of a therapeutic essence, remains a valid option. These strategies, in most cases, not only target the disease but also contain minimal side effects. The majority of the available synthetic drugs for liver diseases are found to be strong pro-oxidant scavengers, but their long-term uses may cause inflammation (Rani et al., 2016; González-Ponce et al., 2018) and cancer. A noteworthy instance is the use of tiopronin, which increases the risk of liver injury ten-fold with its long-term treatment (Tang et al., 2014; Wan and Jiang, 2018). Another well-illustrated detrimental combination is ribavirin and interferon-α (IFN-α), a common medication in liver-related diseases, which is seen to affect hepatitis C patients. Taking into consideration such complications and the high cost of available medicines, researchers are inclined to utilize natural product-based alternative medications for liver diseases, which will have better efficacy, cost-effectiveness, and lower or no toxicity (Zhang et al., 2013; Seeff et al., 2015).
It is evident from the reports of the WHO (WHO 2016) that non-communicable diseases were the cause of 68% of all global death in 2012 (Figure 1), rising from 60% in 2000. Hepatic complications have turned out to be multifactorial diseases that affected a population of around almost 600 million in 2014 (Figure 2), and it is likely to amplify by about 33% over the next two decades (Finkelstein et al., 2012; Dhilleswara Rao et al., 2017).
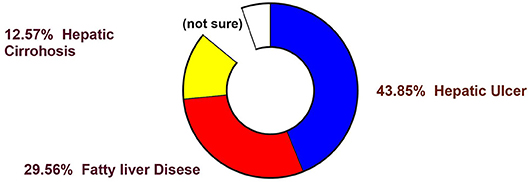
Figure 1. Statistical representation of mortality (in percentage) from various diseases in human (Finkelstein et al., 2012).
Hepatic ailment results in anomalous hypertrophy, expressed phenotypically with surplus adiposity, body fatness and brawny genetic correlation, while its constituent to basal metabolic index and associated health hazard of obesity have also been reported (Locke et al., 2015; Stender et al., 2017).
Various hepatic problems are encountered with a number of synthetic as well as plant-based drugs. Nexavar is a chemotherapeutic drug generally prescribed for complex renal carcinoma (Ravaud et al., 2016; Decker et al., 2017). It is additionally used to treat liver carcinoma. Known adverse effects of Nexavar include dry skin, itching, skin rash, nausea, vomiting, diarrhea, patchy hair loss, loss of appetite, stomach pain, dry mouth, hoarseness, and tiredness (Schmidinger and Bellmunt, 2010). Sorafenib is the first multi-kinase inhibitor (TKI) approved for the treatment of advanced hepatocellular cancer (HCC) metastatic renal cell cancer (RCC), and well-differentiated radioiodine-resistant thyroid cancer (DTC) (Monsuez et al., 2010). It demonstrates targeted activity on several families of receptor and non-receptor tyrosine kinases that are involved in angiogenesis, tumor growth and metastatic progression of cancer (Adnane et al., 2006). Sorafenib is a well-known antihepatotoxic drug available in market but the product of its metabolism has been seen to be toxic, affecting other parts of the body with long-term exposure resulting in renal and pancreatic failure (Randrup Hansen et al., 2017; Balderramo et al., 2018).
A few efficient varieties of herbal preparation like Liv-52, silymarin (Kolasani et al., 2017) and Stronger neomycin phages (SNMC) are in attendance against hepatic complications. All the candidates come up with notable complications. Silymarin is not found effective against chronic liver disease as it fails to modulate the metabolic condition of the liver along with cellular recovery. An effective Japanese preparation like SNMC (Ghiliyal and Bhatt, 2017) also fails to improve the clinical status of liver cirrhosis despite its prominent anti-inflammatory and cytoprotective efficacy. It is successfully used against hepatocellular carcinoma (Luo et al., 2015). Liv-52 is used quite effectively against hepatic damages (Stickel and Hellerbrand, 2015). However, it also fails to demonstrate clinical efficacy in alcoholic liver damages. Various research involving techniques with increasing efficacy of the phytochemicals like nanotechnology, proteomics, and transcriptomics are evident, and efforts being made with herbal preparations are somewhat successful too (Patil et al., 2018). Following the lead of these interesting results, further attempts should be initiated to overcome all the odds of existing drugs, and an initiative may proceed with plant-based natural products. The plants are an enormous repository of bioactive secondary metabolites viz; alkaloids, flavonoids, phenol, etc (Figure 3). This review presents an account of studies on phenolics, with an emphasis on its mechanisms toward hepatotoxicity. Emphasis have been given to understand various pathways through which phenolics exihibit their efficacy. Furthermore, a gene networking model has been constructed in order to gain a clear concise idea of the ways in which natural phenolics contribute to mitigating various hepatic ailments.
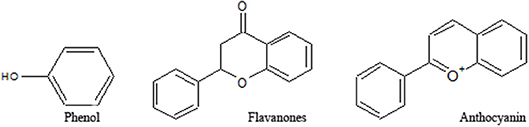
Figure 3. Structures of various groups of phenolic compounds (Hussain, 2016; Mandal et al., 2017; Xi et al., 2018).
With an aim to evaluate the actual sceneries of phenolic compounds for the treatment of various hepatic diseases, a search on the metabolic disease Library and PubMed has been performed matching the keywords “hepatic disease inhibitors treatment,” “target therapy,” and “Hepatic carcinoma,” limited to the English written literature, but with no restriction of time. It was examined and the titles of 202 relevant papers were retrieved.
While performing the search of abstracts and full-text research papers, all unrelated and less important ones were discarded. A selection of the most recent, well-illustrated, full-text, and cited articles were considered regarding similar types of research work from the same institutes at different points in time. We have tried not counting the research papers, whose abstract or full-text is not obtainable. The references for significant and relevant papers have been further sought for other pertinent articles. After such an illustrative survey, around 160 latest bioactivity reports of phenolic compounds mitigating hepatic diseases were brought into light, and around 38 clinical trials have been retrieved gratifying the indispensable criteria for analysis.
The gene networking model and connectivity model were developed by analyzing all the available reports on the hepatoprotective activity of the natural phenolics using the online software Cirrcon and Cytoscape version 3.6.1.
Plant secondary metabolites are well-known for their efficacy in the treatment, as well as prevention, of various fatal diseases. Plant phenolics, e.g., coumarins, flavonoids, lignans, stilbenoids, and tannins, have been studied extensively to provide scientific rationale behind their potential usage against various human ailments. Phenolics are the target group for this review article and subsequent discussions will revolve around exploring the chemical nature and modes of action of these compounds (Smith, 2017; Stander et al., 2017).
Phenolics constitute a major portion of all secondary plant metabolites discovered to date, and there are about 8,000 of such compounds, in both conjugated and free-form, which are distributed in all parts of the plant. Phenolics are generally biosynthesized from acetyl CoA, shikimate, and amino acids (Cseke et al., 2016; Saltveit, 2017). Plant phenolics include simple phenols, phenolic acids, coumarins, lignans, flavonoids, diaryl-alkanoids, stilbenoids, proanthocyanins, tannins, and anthocyanins some alkaloids (Figure 4).
Natural products have been an integral part of medicine since ancient times where around 400 different species of plant and animal origins were then listed. According to the WHO, plant-based therapies are regularly used in places where minerals, plants are common and easily available. Such therapies are used by around 88% of the world's population, who depend on natural products for their primary health care regime. Though the term “drug discovery” sounds contemporary, the story and origin of drug discovery dates back many centuries. Thus, present day uses of plants for both “lead molecule” discovery, which confirms their activity as active natural molecules, and for their structural analog, proves them to be an ideal drug candidate.
Natural products drug discovery is a hot topic as of recent years, with a comeback in mainstream drug discovery protocols. Such comeback is welcomed by academics and pharma companies, mainly due to inherent chemical diversities in natural products, and ease in identification and separation techniques. Noteworthy among natural products are alkaloids, carbohydrates, glycosides, and terpenoids. Phenolics are most studied due to their antioxidant activities. The phenolic moiety is responsible for various pharmacological effects (Sarker and Nahar, 2007).
Phenolic acids are mainly represented as derivatives of benzoic acid and cinnamic acid. The methyl ester of the phenol ring imparts a pharmacophore, which is responsible for interacting with various protein targets present in cell membranes. Gallic, ellagic, vallic, procatecutic, procoumaric, and caffeic acids are important representatives of hydroxyl benzoic acid and hydroxyl cinnamic acid, which are the product of the condensation reaction of phenols under sunlight (Ahmad et al., 2016; De Beer et al., 2017). Flavonoids on the other hand, biosynthesised from cinnamic acids, have two benzene rings (ring A and ring B), and an apyrrole ring (ring C). Plant flavonoids are generally classified into flavan, flavanone, flavanol, flavone, and flavonols (Sarker and Nahar, 2007). Often there are prenylations, glycosidations and conjugation with other ring systems or natural skeletons, as well as dimerisations and oligomerisations which diversify flavonoid structures and provide new pharmacophores. Quercetin, hesperidin, diosmetic, myrectin, and kaempferol are just a few notable examples imparting biological properties (Hussain, 2016; Brodowska, 2017).
Anthocyanidins and anthocyanins are normally plant pigments. Anthocyanidins are grouped into 3-hydroxyanthocyanidins, 3-deoxyanthocyanidins, and O-methylated anthocyanidins. On the other hand, anthocyanins are in the forms of anthocyanidin glycosides and acylated anthocyanins (Sarker and Nahar, 2007). The most common types of anthocyanidins are cyanidin, delphinidin, pelargonidin, peonidin, petunidin, and malvidin (Figures 5, 6) (Wallace and Giusti, 2015; Chorfa et al., 2016; Mäkilä et al., 2016; Stein-Chisholm et al., 2017). The site of glycosylation in anthocyanidins is usually at C-3 (Kay et al., 2017; Rodriguez-Amaya, 2018; Zhang et al., 2018). Acylated anthocyanins are presented with p-coumaric acid, ferulic acid and caffeic acid with attached sugar molecules, in addition to simple acetyl groups (Sigurdson et al., 2017; Zhao et al., 2017).
Phenolic compounds are known for their diverse chemical structures, common antioxidant and specific anti-inflammatory actions. They offer protection against oxidative damages by donating hydrogen or electron to free radicals and thus, in this process, they aid in stabilizing cell membrane networks and inhibiting the formation and expression of inflammatory cytokines like tumor necrosis factor alpha (TNF-α), Transforming Growth Factor beta (TGF-β) and varieties of interleukins (IL-6, IL-2, IL-8) (Parhiz et al., 2015; Taofiq et al., 2015; Zhang and Tsao, 2016; Zhen et al., 2016).
To exert any pharmacological or biological actions, phenolic compounds are initially absorbed in the gastrointestinal tract (GIT) and thus make it bioavailable to circulating system. In the case of inadequate or no absorption through the GIT, they undergo biotransformation in the colon with the help of resident microbiota culture (Figure 7) (Filannino et al., 2015; Heleno et al., 2015; Gómez-Juaristi et al., 2018). Phenolic compounds offer health benefits including treating cancer, oxidative damage and inflammation. Literature supports their effectiveness against chronic pathogenic conditions like neurodegenerative and cardiovascular diseases (Heleno et al., 2015; Rangel-Huerta et al., 2015; Domínguez-Avila et al., 2017).
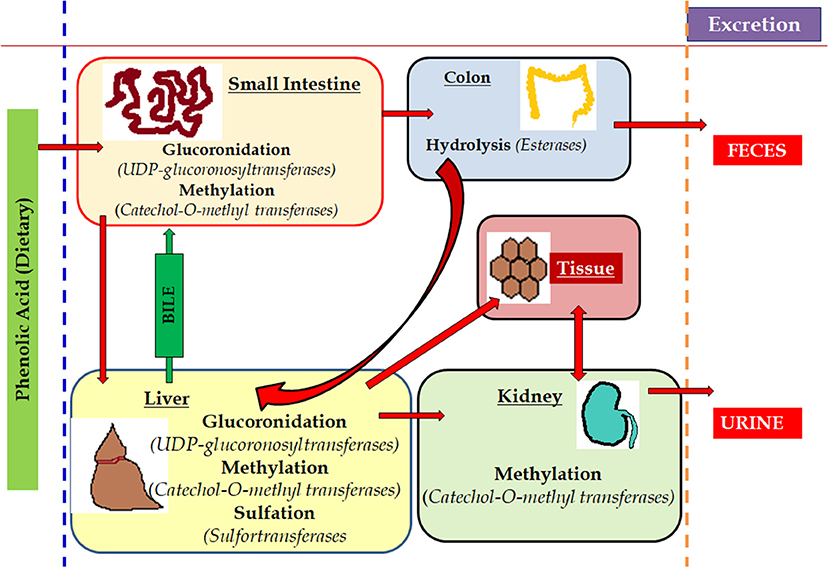
Figure 7. Metabolism of phenols in the living system. The metabolism of the dietary components rich in phenols is easily absorbed by various part of the animal body where the small intestine process and deviates the potent part to hepatic cells and remains are hydrolysed in colon and excreted via feces. Simultaneously, a part of it is methylated in kidney through liver and the last remains are excreted through urination. The red arrows mark is used to show the various route of metabolism of phenols.
When the liver is exposed to alcohol, drugs, and pollutants, its progression toward damage initiates hepato steatosis, fibrosis, and cirrhosis. This exposure results in the death of hepatocytes and, as a consequence, the level of various liver enzymes and metabolites are altered, indicating the anomaly (Sheriff et al., 2017; Balderramo et al., 2018; Hu et al., 2018). Hepatocytes may be injured in various circumstances such as a toxic environment, alcohol, virus, fatty acid metabolism, or chronic antibiotics exposure. Transaminases and glutathione are reported to be prime candidates' marker in the line-up metabolism of bile when hepatocytes are damaged. The clinical condition of the hepatic environment can further be measured by levels of alkaline phosphatase (a key hepatic enzyme) in the serum (Culver, 2016).
Under these surroundings, scarring tissues try to replace the damages, and thus compromise vital liver functions like drug detoxification, secretion of the protein, albumin production, etc. (Anand and Garg, 2015; Baker, 2015). Metabolism, detoxification, and clearing of many drugs are blocked by the impaired liver (Bhattacharyya et al., 2014; Sheriff et al., 2017). Although there are several cited important bioactivity of phenolic compounds, the current discussion will primarily circle around the exploration of detailed mechanisms of actions, and further contributions of phenolics against various liver damages.
The liver, being a keen partner and prime neighbor of the GIT, is usually exposed to toxicity arising from a broad range of drugs, xenobiotics, and the stress mediated by reactive radicals formed during uncontrollable oxidation processes. Being a frequent target of such complex substances, and possessing a unique metabolism system, it hampers itself in the process of breaking them into simpler ones (Cederbaum, 2017). For instance, the large amount of bile acid produced during the oxidation of ethanol produces hepatocellular apoptosis by exciting Fas, an apoptotic element, expressing it from in the plasma membrane, which triggers apoptosis, resulting in cholestatic disease (Cederbaum, 2017). The liver also efficiently expresses main cytochrome P450 isoforms in response to various xenobiotics. CYP2E1 is one such that generates a reactive oxygen family, activates toxicologically central intermediates, and may be the critical alleyway by which these toxic chemical groups cause oxidative stress. Furthermore, kupffer cell, a specialized cell in the liver is activated in this process of metabolism. Both Kupffer cell activation and infiltration of neutrophil release reactive oxygen species (ROS), a range of inflammatory chemokines increasing the fold of hepatotoxicity (Figure 8) (Wang, 2015; Ahadpour et al., 2016).
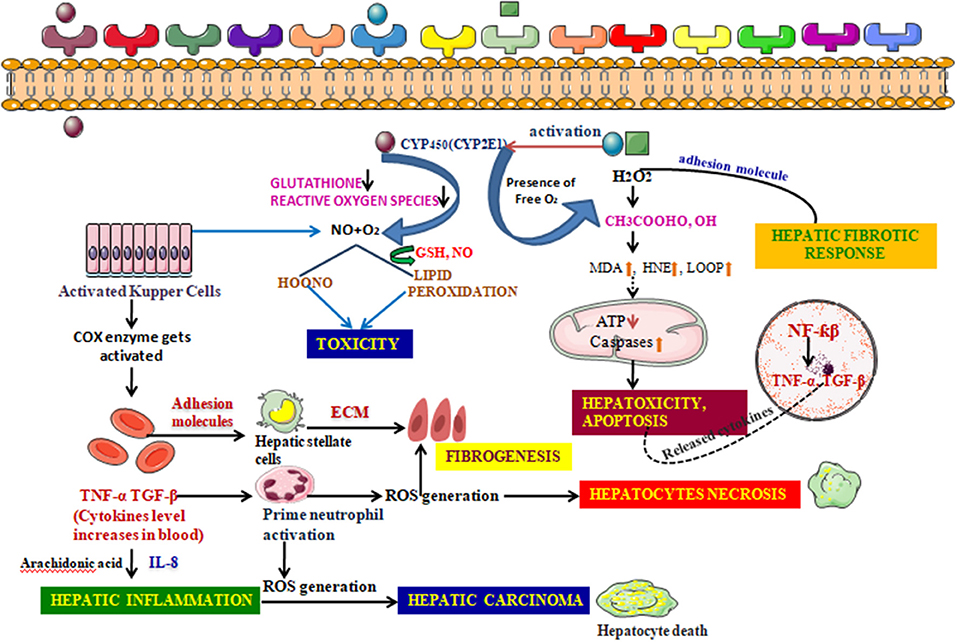
Figure 8. Detailed Mechanism of generation of hepatotoxicity. When the elicitors like alcohol, CCl4, enter the cell membrane they instigate various metabolic reactions activating the CYP systems viz; activating the endogenous glutathione enzyme, hydrogen peroxide. Formation of the reactive oxygen species are responsible for the lipid peroxidation reaction. A conjugation reaction takes place alongside this resulting in the deterioration in the ATP levels and elevation in the caspases levels. This clinical manifestation leads to the building up of hepatotoxicity and induces apoptosis. The nucleus also takes part in such build up by upregulating various transcription factors associated with inflammation. The adhesion molecules present in the cell membrane further create a hepatic fibrotic response by coupling with various reactive oxygen species. The activated Kupffer cells, on the other hand, further activate the prostaglandin by COX-2 and thus increases the cytokines level in the blood. These reactions are catalyzed by arachidonic acid. Such atrocities give rise to associated diseases with inflammation and further fibrogenesis. Hepatic necrosis is another condition imparted by the activated neutrophil which, though inactive during a normal state, increase in number when the cytokine level increases in the blood. The fatal condition, hepatic cirrhosis, is also encountered from hepatotoxicity, which is the additive effect of the prolonged inflammation and interaction with the ROS generation.
In vivo and in vitro studies have demonstrated the promising preventative and therapeutic effects of plant phenolics in a range of liver diseases. Translational studies are extremely vital and indispensable for the application of phenolics in humans with liver diseases. Although literature in the PubMed database about clinical trials of phenolics in liver diseases are limited, encouraging beneficial effects of these phenols have been demonstrated, particularly in Non-Alcoholic Fatty Liver Disease (NAFLD). When working with the high fed diet, the AKT signal molecule responsible for fat metabolism is mutated in the model systems, placebo-inhibited trial of a purified form of anthocyanin in NAFLD patients, treating with the fixed amount of purified anthocyanin for 3 months significantly improved insulin resistance, in liver injury (Zhang et al., 2015a), and clinical evolution in such patients (Bischoff et al., 2018). In another double-blind clinical trial, dihydromyricetin, the main active ingredient of Ampelopsis grossedentata, improved glucose and lipid metabolism and showed anti-inflammatory effects in NAFLD (Chen et al., 2015b; Hou et al., 2015). When working with the hepatotoxic model system, the mice cohort which was treated with thioamino acetic acid showed significant recovery in its MAPK and AMPK level, the two important pathways, which impart cAMP and are a source of energy to the hepatocytes. This recovery was witnessed when a most studied flavonoid, curcumin, was administered at a dose of 118 μg/kg b.wt.
Alcohol hinders the functional aspects of various tissue components and hepatocytes in particular. Alcohol diffuses, crossing the membrane barrier, and is distributed throughout the cell and tissue system, interacting with the major proteins and cellular component present in it (Li et al., 2016). Development of toxic molecules like reactive oxygen species (ROS) is another negative upshot of alcohol. In addition to ROS, it also produces acetaldehyde and nitric oxides, an extremely reactive and toxic by-product that chip into tissue damage (Madrigal-Santillán et al., 2015; Marshall, 2016). Nitric oxide (NO) is recognized as managing mitochondrial respiration and biogenesis amongst organelle. Under conditions of alcohol-mediated hepatic complications, mitochondrial respiration was hindered and, in turn, hypoxia occured. Simultaneously, nuclear factor-kappa β (NF-κβ), a transcription factor activation, takes place, in which it binds to iNOS promoter, an important NO, and aggravates the expression of iNOS (Figure 9) (Iwakiri and Kim, 2015; Starkel et al., 2016). Together, this entire environment amplifies the expression of inducible nitric oxide synthase (iNOS). iNOS joins hands in inducing hepatic fibrosis and the expression of inflammatory cytokines (Tacke and Zimmermann, 2014; Cassini-Vieira et al., 2015). iNOS increases two other factors in this process. Hypoxia-inducible factor-1 and its gene expression aid various connected hepatic anomalies viz; inhibition of mitochondrial respiration, impairment of mitochondrial fatty acid β-oxidation, and mitochondrial DNA damage (Chang et al., 2015; Suraweera et al., 2015).
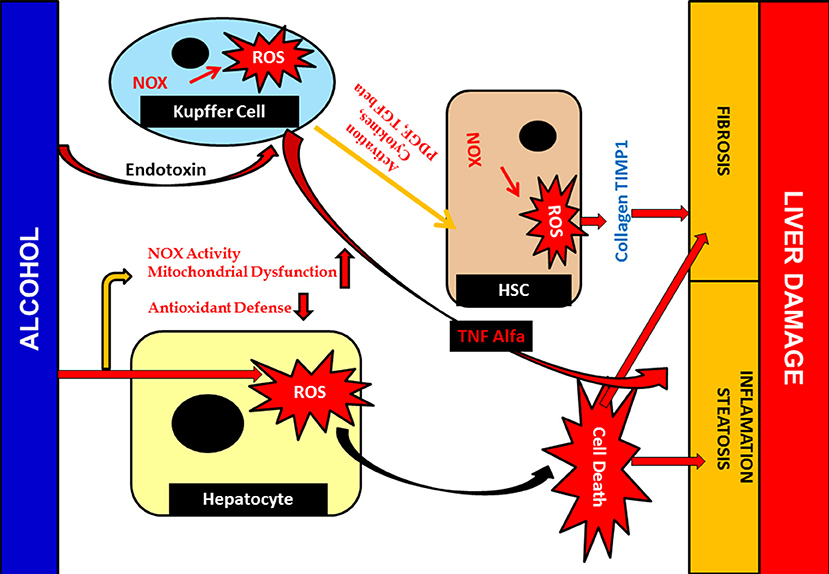
Figure 9. Alcohol mediated Hepatotoxicity. Hepatotoxicity caused by increased production of ROS; due to alcohol damages antioxidant defenses and mitochondrial function as well as structure. It leads to liver inflammation, fibrosis and steatosis. Cellular responses, which are sturdily involved in Kupffer cell may also activated due to action of ROS which contribute to an increase of inflammatory responses, resulting liver injury. Furthermore, activated Kupffer cells release ROS and cytokines that are crucial for HSC activation and inducing the pro-fibrogenic pathway.
Phenolics possess immense potentials in regulating the inflammatory cytokines, which are expressed in clinical conditions such as alcoholic liver diseases (Wan and Jiang, 2018; Xu et al., 2018). Puerarin, a known isoflavone, can excite the AMP-activated protein kinase (AMPK) phosphorylation in H4IIE cell lines suppressing the (m TOR) target proteins and 4E-binding protein (Zhao et al., 2016). This strategy aids in ameliorating the alcohol-based hepatotoxicity. Puerarin can also alleviate the hepatic necrosis due to its role in the AMPK pathway activation, scavenging activity, and lipid peroxidation inhibition (Wang et al., 2018a).
A majority of the metabolic disorders and their physiology related to hepatotoxicity have been studied over the years. Where sharp and clear possible elements that are responsible for chemical-induced toxicity, enzymes and protein-induced complications are considered, yet a fair amount of diseases related to metabolism remain unidentified. Such prognostic parameters include blood pressure, abdominal obesity, or potentially hyperglycaemia. They are collectively termed as the non-alcoholic fatty liver diseases (NAFLD) (Chalasani et al., 2018). This clinical situation is one of the most familiar and dormant forms of liver diseases, which accounts for the preliminary stage, but when left untreated this results in inflammation and, subsequently, can even lead to serious fibrosis and hepatocellular carcinoma (HCC), with high rates of mortality (Chen et al., 2015a).
Until now, the main drugs for the treatment of NAFLD in clinics are lipid regulating agents such as statins, which are not only toxic, but also aggravate the deposition of lipids in the liver, leading to serious liver injury (Arguello et al., 2015). Phenolics such as baicalin, epicatechin, and apigenin (Figure 5) have been reported to protect the liver from NAFLD, which are associated with their effects on insulin resistance and for signaling the way to anti-inflammation as well as antioxidant action (Sen and Chakraborty, 2017; Wan and Jiang, 2018).
Phenolic compounds can significantly regulate these NAFLD conditions. Apigenin, a flavone, is a well-studied phenolic compound that can check the lipid accumulation and oxidative stress induced by high-fat diet. It can abridge the inflammatory mediators but can simultaneously amplify various endogenous antioxidative enzymes actions like superoxide dismutase and glutathione peroxidase in the liver (Feng et al., 2017; Vergani et al., 2017). Dihydromyricetin, another important phenolic, exhibits its therapeutic effect on the improvement of glucose and lipid metabolism in patients with NAFLD, by blocking the phosphatidyl inositol 3-kinase, NF-κβ signaling pathway (Chen et al., 2015b).
Liver inflammation is a state of the reaction in which the liver tissues send a constant stimulus, whether acute or chronic, in response to extrinsic and intrinsic factors hampering the liver status. Acute inflammation is a localized affair, where the liver tries to regain its previous configuration. It is the first line of defense, but when the liver cannot check these associated level of lymphocytes, vascular proliferation and tissue destruction become chronic and ultimately lead to fibrotic condition (Pawlak et al., 2015; Leyva-López et al., 2016).
During such chronic conditions, specialized cells such as macrophages recruit more of the inflammatory mediators including interleukins and tumor necrosis factor (TNF)-α (Seki and Schwabe, 2015; Williams et al., 2016). This amplification altogether results in such a complex state that it leads to many degenerative diseases including severe cirrhosis and hepatic carcinoma (Czaja, 2014). For this reason, slowing down the inflammation process becomes essential. Initially, non-steroidal anti-inflammatory drugs (NSAIDs) are prescribed but the associated side effects include mild gastritis, renal failure and at times allergy due to hypersensitivity (Figure 10) (Pawlak et al., 2015).
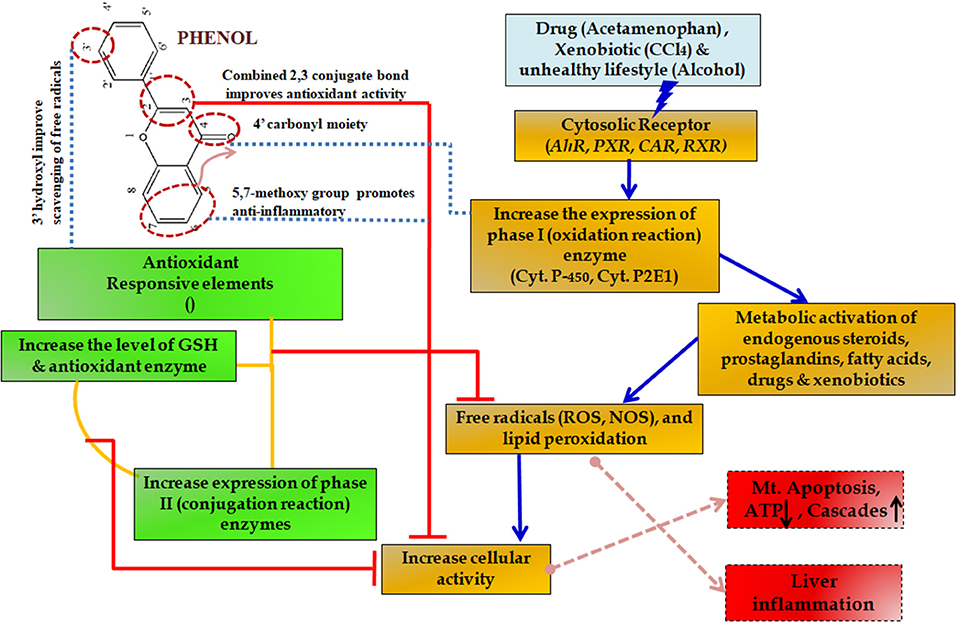
Figure 10. Protective effect of phenols in various metabolic pathways in liver diseases. The upward arrow indicating upregulation and down arrow indicating downregulation of the enzymes.
Recent information on hepatic inflammation demonstrated the role of phenolics in protecting such inflammation. Phenolic compounds like hesperidin can act against inflammation by downregulating liver enzyme biomarkers such as aspartate amino transferase (AST) and alanine aminotransferase (ALT) primarily. It can also hold back oxidative stress and activation of T cells, which is a prime instigator of inflammation (Li et al., 2014). Hesperidin, a common Citrus flavonoid, further aids in the management of various proinflammatory recruiters viz; NF-κβ and α smooth muscle actin (α-SMA). Another well-known flavone, silymarin, is also a subclass of the family of phenolic compounds that works in patients with chronic hepatic carcinoma (González-Gallego et al., 2014).
The chemical nature, physical properties, and dose ratio of a particular drug, along with an individual's gene expression profile, antioxidant status, and the capacity for regeneration are also crucial for cell injury. Several mechanisms are involved in the initiation of liver cell damage and aggravate ongoing injury processes (Guan et al., 2014; Ju and Tacke, 2016). Dysfunction of these vital cell organelles results in the impairment of dynamic equilibrium in homeostatic condition, thus resulting in intracellular oxidative stress with excessive formation of reactive oxygen species (Cannistrà et al., 2016; Ramachandran et al., 2018). Major causes of the hepatotoxic reactions by drugs are elevated ROS generation, oxidative stress and suppressed immune responses. Hepatotoxicity remains a major cause of drug withdrawal from the market. Recent examples in the USA and Europe are ximelagatran, nefazodone, nimesulide, ebrotidine, trovafloxacin, troglitazone, bromfenac, and so forth.
Gene-metabolic networks are an advanced mode to construct a network with genes and metabolites specifically deregulated in different liver disease phenotypes. It compactly gives an overview of genes of interest, representative gene subsets that were involved in regulated signaling pathways, including tumor necrosis factor (TNF), P53, NF-κB, chemokine, peroxisome proliferator activated receptor (PPAR) and Toll-like receptor (TLR) signaling pathways associated with the physiology of various hepatic disease. Detailed information for the clinical status and associated genes in the hepatotoxicity are summarized in gene networking model Figures 11, 12. Gene regulation of a few bioactive phytocompounds is discussed below in Tables 1, 2.
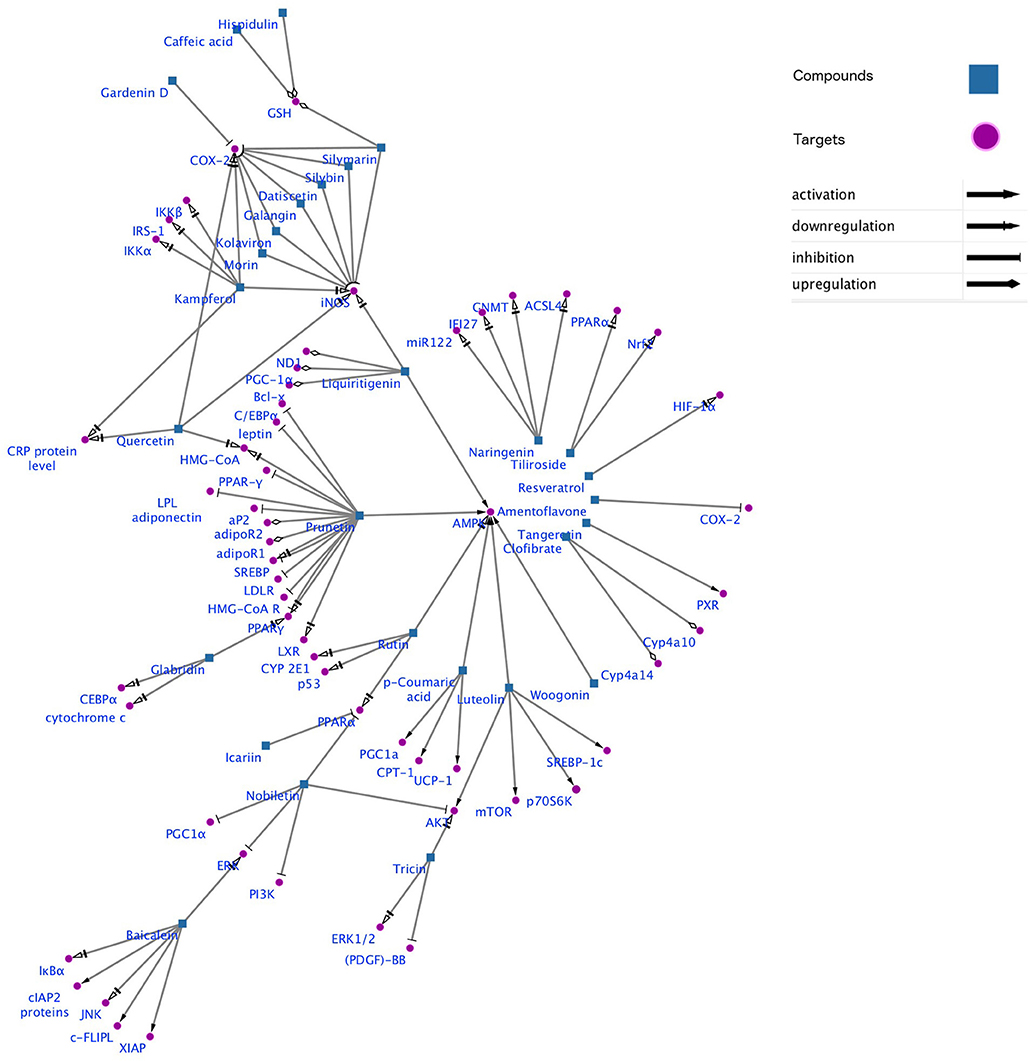
Figure 11. Gene networking showing hepatotoxicity mediated gene expression and subsequent mode of action of various natural products. This network was generated by a software Cytoscape version 3.6.1.
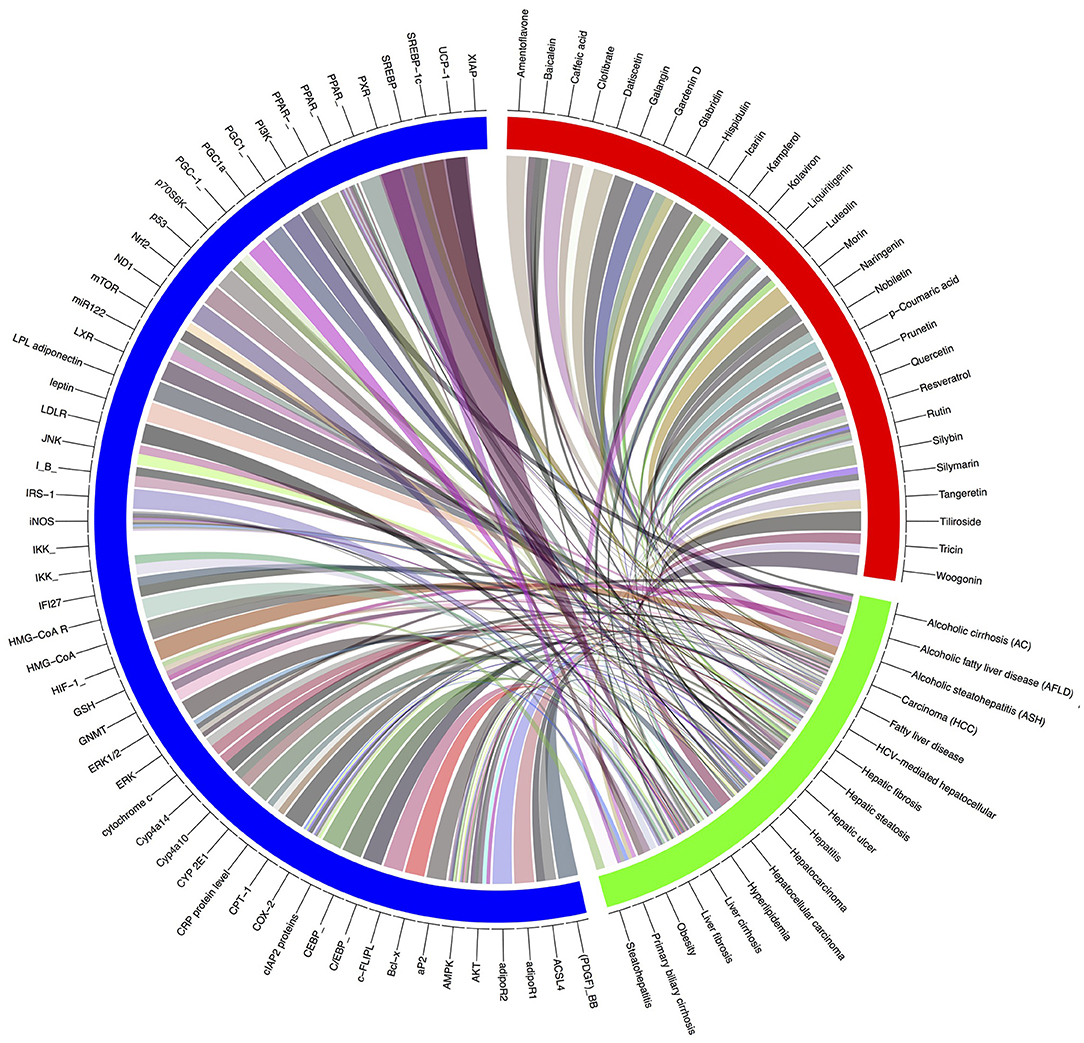
Figure 12. Gene-modeling showing various hepatic diseases and associated genes with it. A tool named Circus on shiny Circos server generated this image. The blue band is showing various genes responsible for pathophysiological conditions, the green showing various hepatic complications and the red band shows the bioactive natural compounds possessive hepatoprotective activity. Various shades indicating the degree of relatedness between the various bands.
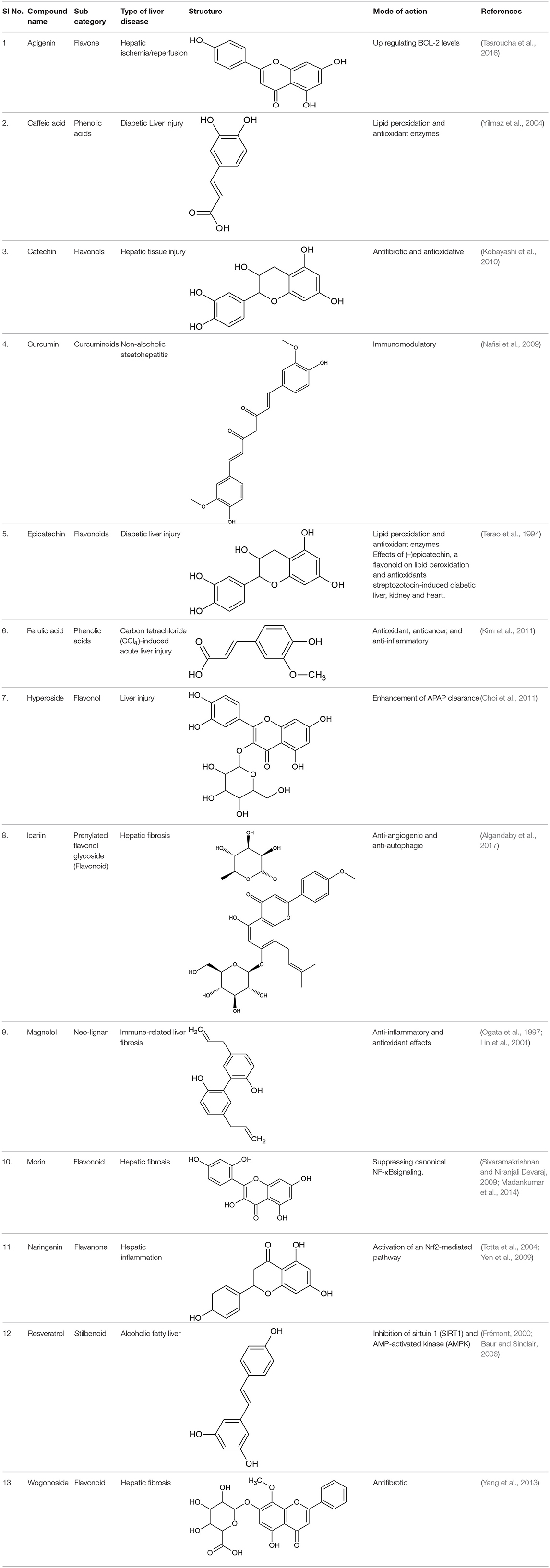
Table 1. List of a few potent natural phenolics and their mode of action imparting hepatoprotective activity.
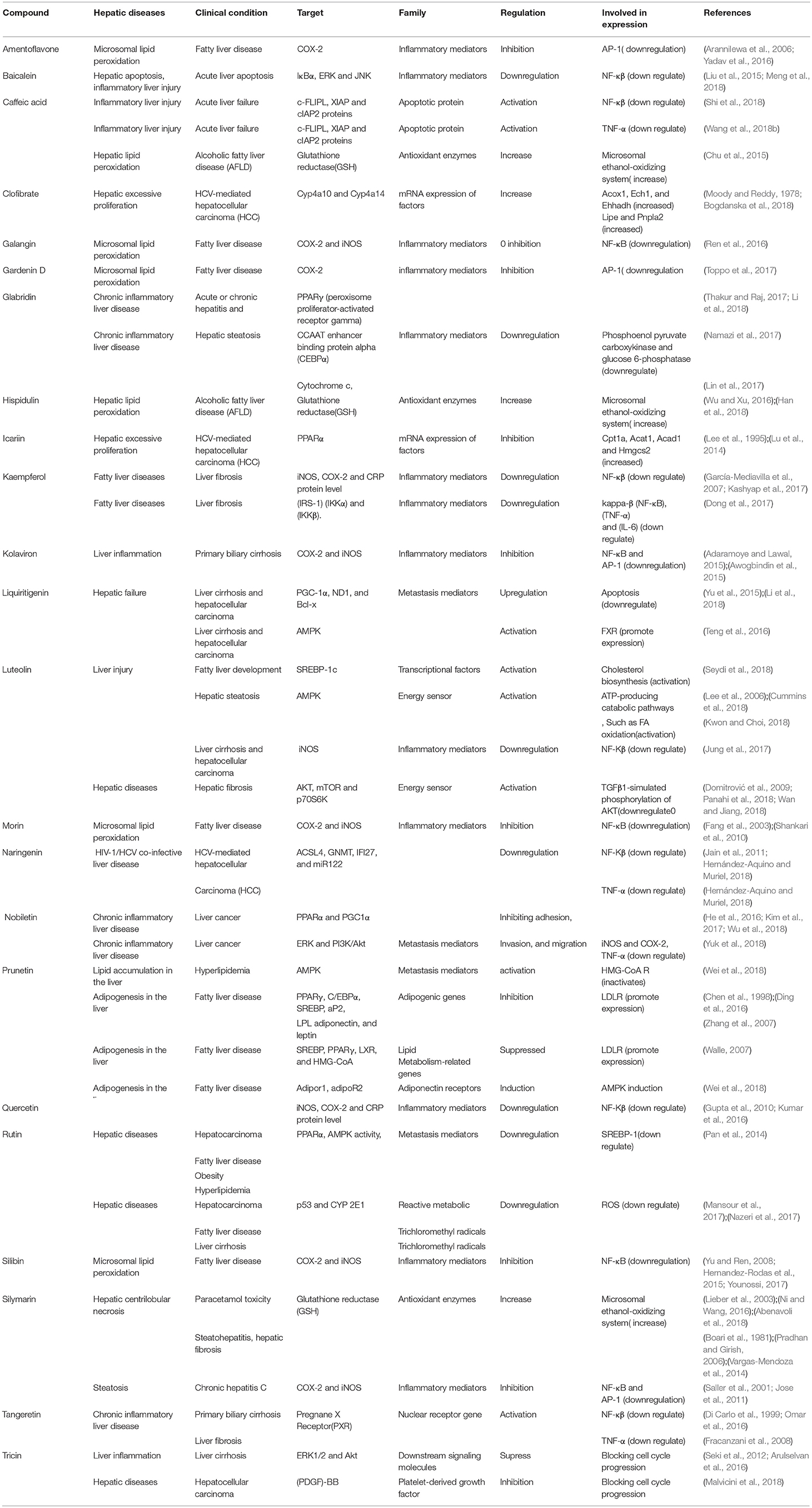
Table 2. Table showing various hepatic diseases and various genes and metabolites associated with it.
Apigenin, a plant flavone, can improve hepatic health during severe liver disease conditions by down-regulating Nrf2-signaling and up-regulatingBCL-2 apoptotic pathway (Tsaroucha et al., 2016).
It is chemically 3,4-dihydroxycinnamic acid that occurs in a diet of fruits, green tea, wine, and coffee bean components. Caffeic acid showed potential antioxidant and anti-inflammatory properties and is effective in treating major liver hitches (Kim et al., 2018). It can modulate the expression of kelch-like ECH-associated protein-1 (Keap1), a hepatic carcinoma factor, by interacting with Nrf2 binding site and restraining it from binding to Keap1 and elevating the expressions of vital antioxidative signals like HO-1 (Yang et al., 2017).
Catechin from green tea extracts, selective seeds, and fruits. It is categorized by the presence of a hydroxyl moiety at C3, C5, and C7 position of at A ring, and again in C3 and C4 of the B ring. Catechin with anti-hyperlipidemic property helps in treating diverse clinical conditions associated with non-alcoholic fatty liver diseases where abnormalities in protein and lipid metabolism play the prime role in pathophysiology of the liver (Sun et al., 2015; Pezeshki et al., 2016).
It exerts its protective and therapeutic effects in oxidative coupled liver diseases by suppressing proinflammatory cytokines, lipid peroxidation products, hepatic stellate cells, and Akt activation. Curcumin ameliorates oxidative stress induced expression of Nrf2, SOD, CAT, and GSH. Curcumin acts as a free-radical scavenger over the activity of different kinds of ROS via its active phenolic pharmacophore, β-diketone and methoxy group (Nabavi et al., 2014).
It is a flavan-3-ol found in edible plant products like cocoa and other varieties of plant foods. Epicatechin plays an important role in lipid metabolism in fatty liver condition and hypercholesterimia (Cordero-Herrera et al., 2015). It can down-regulate important liver enzymes like SGPT and SGOT, which increases its liver anomalies (Shanmugam et al., 2017).
It is the most abundant phenolic acid in plants that has potent antioxidant ability to freeze the activity of the free radicals like NO, . It exhibits prevailing anticholestatic action against liver cholestasis by inhibiting extracellular matrix related gene expression and also by disruption of the Smad signaling pathways and extracellular signal-regulated kinases (Gerin et al., 2016). It sometimes activate the AMPK or the MAPK signaling pathway by enhancing lipid metabolism (Cheng et al., 2018). Several reports also confirmed the mode of action of ferulic acid is mediated by regulating the expression of several physiological factors viz; PPAR-α, CPT-1α toward lipid oxidation and this action is very important in treating fatty liver diseases (Kim et al., 2011).
It is a significant flavonoid that can fuel up the expression of diverse endogenous antioxidant enzymes and can quench free radicals formed during the metabolism of xenobiotics in the liver. Further, the capacity of hyperoside to regulate detoxifying enzymes phase II makes it potent as these enzymes are the prerequisite for liver during the initial round of oxidation. It helps in mitigating liver fibrosis by activating the Nrf2 signaling pathway, meant for neutralizing oxidants, when studied in CCl4−induced hepatotoxicity (Wang et al., 2016; Xie et al., 2016; Zou et al., 2017).
It is reported from genus Epimedium and has been shown to delay the fibronectin and collagen accumulation in renal interstitial tissues and mesengial cells of rat model (Algandaby et al., 2017). Several published reports confirmed its protective role in inflammation blocking TNF-α and IFN-γ signaling pathway (Sinha et al., 2016). Other important protective actions of iccarin comprises of modulating expression of toll-like receptor and inhibition of the mitogen activated protein kinase (MAPK) (Mochizuki et al., 2002).
Magnolol from Magnolia officinalis is an important phenolic compound that maintains the oxidative balance during hepatotoxicity in galactosamine-injured mice models. Magnolin, another phenolics from same plant was reported to have ameliorating activity in lipid build up, insulin resistance and also in hepatic inflammation, when hepatocytes are exposed to free fatty acid in vitro (Tian et al., 2018).
Morin, is a naturally occurring 2′,3,4′5,7-penta-hydroxyflavone, present in mulberry, tartary buckwheat, jackfruit, green tea, orange, and in many dietary plants. It exerts beneficial effects on metabolism by suppressing canonical NF-Kβ signaling (Caselli et al., 2016; Sinha et al., 2016).
Naringenin, a natural flavonoid, possesses antioxidant, anticancer and anti-inflammatory activity (Chtourou et al., 2015). Naringenin exhibits very little antioxidant action directly as a scavenger, yet it helps in upregulating of Nrf2 pathways and thus upholds the normal redox of the cell even in clinical conditions where prooxidants and reactive oxygens are formed as a of damage mechanism in hepatocytes, (Esmaeili and Alilou, 2014).
Resveratrol, a 3,5,4′-trihydroxystilbene polyphenolic compound, is available in edible plants and selected fruits like grapes. It can control a specialized mammalian homolog, sirtuins (SIRT) (Andrade et al., 2014). Over expression of this homolog helps in treating non-alcoholic related fatty liver disease by regulation lipogenesis. Resveratrol is associated with considerable reduction in various liver enzymes, cytokines, and also transcriptional factors like nuclear factor κB. It alleviates the nuclear factor-κB (NF-κB) expression following the stimulation of its inhibitor IκBα (Zhang et al., 2015b).
It is another flavone that imparts hepatoprotective activity via different facilitating lipid metabolism by increasing oxidation process. AMPK signaling to bestow its effectiveness by various modules (Wang et al., 2015).
In silico appraisal presently happens to be a pronounced method of evaluation in various biological research these days. It has the benefit of low cost, fast execution, and the most constructive face of such study is to diminish the animal usage in various toxicity screening. PASS prediction assay (Lagunin et al., 2000), which is highly studied these days, is based on primarily structure-activity relationships investigation of the training set, that generally contains more than 200,000 compounds showing at least 3,700 type of biological actions that interestingly allows to estimate if a phytochemical compound has a particular effect (Dei et al., 2013). Lipinski's Rule of Five (Lipinski, 2004) is another method that can be applied to all the phenolic compounds to evaluate their drug likeness and pharmacological properties. Such information is very helpful in accessing the phenolic compounds as potential drug leads that can act as natural therapeutics. Only the compounds satisfying the Lipinski's criteria are further considered for additional computational operations. Compounds that cleared the Lipinski's barrier were prepared for docking studies by their energy minimization in Marvin Sketch. Receptor-ligand interaction study using the Hex docking tool (Macindoe et al., 2010) are also another mode of interaction study. Various amino acids of the target protein interaction with the lead compound are studied with respect to their bond length and bond angle. Hence, the reported phenolic compounds can thus be studied as good prospective options for their use as medicine that targets various proteins for hepatic treatment. Reports of phosphorylated flavonoids i.e., iccartin is extensively studied for the potent target TGF-β, where the score of molecular docking was reported 0.28 which was more than the marketed standard ursidiol 0.23 (Wheng et al., 2016).
Insilico studies have its implication in various pharmacological studies. From the initial protein, study to gene expression analysis related to any diseases can be carried out by the concept of pharmacogenomics. Phenolic compounds as hepatoprotective have been reported in the work of Kaveri, 2017, with insilico approach. The work was carried out on a group of newly synthesized acetylated phenolics. A good number of target proteins of hepatic anomaly have been reported when target fishing was performed (Liu et al., 2017); which not only predicted the probable important target but directed the study of those prospective targets in understanding the mechanism of that disease. This mode thus supports the traditional uses for hepatic disorders and thus can suggest major bioactive phenolic compounds as contributors to produce ethnopharmacological effect.
Natural products and specially plant phenolics have become a promising therapeutic alternative and prospective replacement of conventional marketed drug in practice due to their effectiveness, minimal side effects, and protective properties. Furthermore, their dietary nature and availability is a bonus, and gives all the more reason to decline those generally available drugs that also cause toxicity to cells. Remarkable phenolics like curcumin and resveratrol are pharmacologically tested chemoprotective agents against treatment of hepatic carcinoma. Though widely held natural products evaluated until now are generally non-toxic in nature, a few studies on toxicity regarding certain natural products are also highlighted these days. As a result, appropriate selection of the natural based drug is also obligatory. All the important phenolics with their derivatives, though studied and well reported for, have not yet been fully analyzed for their immense therapeutic usage, as there are not enough studies available regarding them. Components of such compounds in the diet varies with temperature and cultivation process. Furthermore, variation in the physicochemical properties could result from different modes of production of such plants, including agricultural and environmental factors. Many pharmacological reports have demonstrated that phenols have a variety of therapeutic effects, including anti-cancer, anti-diabetic, anti-obesity, immunomodulatory, cardioprotective, hepatoprotective, and neuroprotective effects through antioxidant and anti-inflammatory activities. However, additional studies are required to understand biological functions and compositions of many phenols, such as iccartin and morin, in more detail. Understanding biological function, composition, and therapeutic effects could help prevent adverse effects from long-term administration of phenolic compounds, and develop health promoting properties. It is envisaged from this presented review that plant based phenolics will not only reduce the risk of hepatopathy, but will also endow a sure substitute that can be used for various hepatotoxicity mediated diseases.
PS prepared the initial draft and graphical representation for figures. AT finalized the manuscript and supervised as a whole. RN worked on the graphical representations and carried out various literature survey studies. JS worked on gene network modeling and the gene expression study. MC worked on bioinformatics and the phenolic study in hepatic disease. SS and LN provided significant input into the chemistry part of this review, editing, and finalizing the draft.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are grateful to DBT (government of india) sponsored institutional biotech hub and bioinformatics infrastructure facility of Assam University.
Abenavoli, L., Izzo, A. A., Milić, N., Cicala, C., Santini, A., and Capasso, R. (2018). Milk thistle (Silybum marianum): a concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother. Res. 32, 2202–2213. doi: 10.1002/ptr.6171
Adaramoye, O. A., and Lawal, S. O. (2015). Kolaviron, a biflavonoid fraction from Garcinia kola, protects against isoproterenol-induced injury by mitigating cardiac dysfunction and oxidative stress in rats. J. Basic Clin. Physiol. Pharmacol. 26, 65–72. doi: 10.1515/jbcpp-2013-0139
Adnane, L., Trail, P. A., Taylor, I., and Wilhelm, S. M. (2006). Sorafenib (BAY 43-9006, Nexavar®), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 407, 597–612. doi: 10.1016/S0076-6879(05)07047-3
Ahadpour, M., Eskandari, M. R., Mashayekhi, V., Haj Mohammad Ebrahim Tehrani, K., Jafarian, I., Naserzadeh, P., et al. (2016). Mitochondrial oxidative stress and dysfunction induced by isoniazid: study on isolated rat liver and brain mitochondria. Drug Chem. Toxicol. 39, 224–232. doi: 10.3109/01480545.2015.1092039
Ahmad, N., Zuo, Y., Lu, X., Anwar, F., and Hameed, S. (2016). Characterization of free and conjugated phenolic compounds in fruits of selected wild plants. Food Chem. 190, 80–89. doi: 10.1016/j.foodchem.2015.05.077
Algandaby, M. M., Breikaa, R. M., Eid, B. G., Neamatallah, T. A., Abdel-Naim, A. B., and Ashour, O. M. (2017). Icariin protects against thioacetamide-induced liver fibrosis in rats: Implication of anti-angiogenic and anti-autophagic properties. Pharmacol. Rep. 69, 616–624. doi: 10.1016/j.pharep.2017.02.016
Anand, A. C., and Garg, H. K. (2015). Approach to clinical syndrome of jaundice and encephalopathy in tropics. J. Clin. Exp. Hepatol. 5, S116–S130. doi: 10.1016/j.jceh.2014.05.007
Andrade, J. M., Paraíso, A. F., De Oliveira, M. V., Martins, A. M., Neto, J. F., Guimarães, A. L., et al. (2014). Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition 30, 915–919. doi: 10.1016/j.nut.2013.11.016
Arannilewa, S., Ekrakene, T., and Akinneye, J. (2006). Laboratory evaluation of four medicinal plants as protectants against the maize weevil, Sitophilus zeamais (Mots). Afr. J. Biotechnol. 5, 2032–2036.
Arguello, G., Balboa, E., Arrese, M., and Zanlungo, S. (2015). Recent insights on the role of cholesterol in non-alcoholic fatty liver disease. Biochim. et Biophys. Acta 1852, 1765–1778. doi: 10.1016/j.bbadis.2015.05.015
Arulselvan, P., Fard, M. T., Tan, W. S., Gothai, S., Fakurazi, S., Norhaizan, M. E., et al. (2016). Role of antioxidants and natural products in inflammation. Oxid. Med. Cell. Longev. 2016, 2016: 5276130. doi: 10.1155/2016/5276130
Aseervatham, G. S. B., Ananth, D. A., and Sivasudha, T. (2018). “The liver: oxidative stress and dietary antioxidants,” in The Liver, eds V. B. Patel, R. Rajendram, and V. R. Preedy (Academic Press), 239–246. doi: 10.1016/B978-0-12-803951-9.00020-3
Awogbindin, I. O., Olaleye, D. O., and Farombi, E. O. (2015). Kolaviron improves morbidity and suppresses mortality by mitigating oxido-inflammation in BALB/c mice infected with influenza virus. Viral Immunol. 28, 367–377. doi: 10.1089/vim.2015.0013
Baker, P. R. (2015). “Pathophysiology of inherited metabolic disease,” in Nutrition Management of Inherited Metabolic Diseases, eds L. E. Bernstein, F. Rohr, and J. R. Helm (Switzerland: Springer International Publishing), 35–45. doi: 10.1007/978-3-319-14621-8_4
Balderramo, D., Prieto, J., Diehl, F., Gonzalez-Ballerga, E., Ferreiro, M., Carrera, E., et al. (2018). Sorafenib for treatment of hepatocellular carcinoma: a survival analysis from the south american liver research network. J. Clin. Gastroenterol. doi: 10.1097/MCG.0000000000001085. [Epub ahead of print].
Baur, J. A., and Sinclair, D. A. (2006). Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 5, 493. doi: 10.1038/nrd2060
Bhattacharyya, S., Sinha, K., and Sil, P. C. (2014). Cytochrome P450s: mechanisms and biological implications in drug metabolism and its interaction with oxidative stress. Curr. Drug Metabol. 15, 719–742. doi: 10.2174/1389200215666141125121659
Bischoff, K., Mukai, M., and Ramaiah, S. K. (2018). “Liver toxicity,” in Veterinary Toxicology, 3rd Ed, ed R. C. Gupta (Breathitt Veterinary Center, Murray State University, Hopkinsville, KY: Elsevier), 239–257. doi: 10.1016/B978-0-12-811410-0.00015-5
Boari, C., Montanari, F. M., Galletti, G. P., Rizzoli, D., Baldi, E., Caudarella, R., et al. (1981). Toxic occupational liver diseases. Therapeutic effects of silymarin. Miner. Medica. 72, 2679–2688.
Bogdanska, J., Trajkovska, K. T., Labudovic, D., Cekovska, S., Topuzovska, S., and Petrusevska, G. (2018). Morphological and biochemical changes in wistar rat livers after clofibrate treatment. J. Morphol. Sci. 1, 46–56. Available online at: http://www.jms.mk/jms/article/view/15
Branco, A. F., Ferreira, A., Simões, R. F., Magalhães-Novais, S., Zehowski, C., Cope, E., et al. (2016). Ketogenic diets: from cancer to mitochondrial diseases and beyond. Eur. J. Clin. Invest. 46, 285–298. doi: 10.1111/eci.12591
Brodowska, K. M. (2017). Natural flavonoids: classification, potential role, and application of flavonoid analogues. Eur. J. Biol. Res. 7, 108–123. doi: 10.5281/zenodo.545778
Cannistrà, M., Ruggiero, M., Zullo, A., Gallelli, G., Serafini, S., Maria, M., et al. (2016). Hepatic ischemia reperfusion injury: a systematic review of literature and the role of current drugs and biomarkers. Int. J. Surg. 33, S57–S70. doi: 10.1016/j.ijsu.2016.05.050
Caselli, A., Cirri, P., Santi, A., and Paoli, P. (2016). Morin: a promising natural drug. Curr. Med. Chem. 23, 774–791. doi: 10.2174/0929867323666160106150821
Cassini-Vieira, P., Araújo, F. A., Da Costa Dias, F. L., Russo, R. C., Andrade, S. P., Teixeira, M. M., et al. (2015). iNOS activity modulates inflammation, angiogenesis, and tissue fibrosis in polyether-polyurethane synthetic implants. Medi. Inflam. (2015) 2015:138461. doi: 10.1155/2015/138461
Cederbaum, A. (2017). “Cytochrome P450 and oxidative stress in the liver,” in Liver Pathophysiology, ed P. Muriel (Mexico: Elsevier), 401–419. doi: 10.1016/B978-0-12-804274-8.00031-X
Chalasani, N., Younossi, Z., Lavine, J. E., Charlton, M., Cusi, K., Rinella, M., et al. (2018). The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67, 328–357. doi: 10.1002/hep.29367
Chang, W. J., Song, L. J., Yi, T., Shen, K. T., Wang, H. S., Gao, X. D., et al. (2015). Early activated hepatic stellate cell-derived molecules reverse acute hepatic injury. World J. Gastroenterol. 21, 4184. doi: 10.3748/wjg.v21.i14.4184
Chen, J., Schenker, S., Frosto, T. A., and Henderson, G. I. (1998). Inhibition of cytochrome c oxidase activity by 4-hydroxynonenal (HNE): role of HNE adduct formation with the enzyme subunits. Biochim. et Biophy. Acta 1380, 336–344. doi: 10.1016/S0304-4165(98)00002-6
Chen, S., Zhao, X., Ran, L., Wan, J., Wang, X., Qin, Y., et al. (2015a). Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: a randomized controlled trial. Digest. Liver Dis. 47, 226–232. doi: 10.1016/j.dld.2014.11.015
Chen, S., Zhao, X., Wan, J., Ran, L., Qin, Y., Wang, X., et al. (2015b). Dihydromyricetin improves glucose and lipid metabolism and exerts anti-inflammatory effects in nonalcoholic fatty liver disease: a randomized controlled trial. Pharmacol. Res. 99, 74–81. doi: 10.1016/j.phrs.2015.05.009
Cheng, Q., Li, Y. W., Yang, C. F., Zhong, Y. J., He, H., Zhu, F. C., et al. (2018). Methyl ferulic acid attenuates ethanol-induced hepatic steatosis by regulating AMPK and FoxO1 pathways in rats and L-02 cells. Chem. Biol. Interac. 291, 180–189. doi: 10.1016/j.cbi.2018.06.028
Choi, J. H., Kim, D. W., Yun, N., Choi, J. S., Islam, M. N., Kim, Y. S., et al. (2011). Protective effects of hyperoside against carbon tetrachloride-induced liver damage in mice. J. Nat. Prod. 74, 1055–1060. doi: 10.1021/np200001x
Chorfa, N., Savard, S., and Belkacemi, K. (2016). An efficient method for high-purity anthocyanin isomers isolation from wild blueberries and their radical scavenging activity. Food Chem. 197, 1226–1234. doi: 10.1016/j.foodchem.2015.11.076
Chtourou, Y., Fetoui, H., Jemai, R., Ben Slima, A., Makni, M., and Gdoura, R. (2015). Naringenin reduces cholesterol-induced hepatic inflammation in rats by modulating matrix metalloproteinases-2, 9 via inhibition of nuclear factor κB pathway. Eur. J. Pharmacol. 746, 96–105. doi: 10.1016/j.ejphar.2014.10.027
Chu, J., Zhang, X., Jin, L., Chen, J., Du, B., and Pang, Q. (2015). Protective effects of caffeic acid phenethyl ester against acute radiation-induced hepatic injury in rats. Environ. Toxicol. Pharmacol. 39, 683–689. doi: 10.1016/j.etap.2015.01.020
Cordero-Herrera, I., Martín, M. Á., Fernández-Millán, E., Álvarez, C., Goya, L., and Ramos, S. (2015). Cocoa and cocoa flavanol epicatechin improve hepatic lipid metabolism in in vivo and in vitro models. Role of PKCζ. J. Funct. Foods 17, 761–773. doi: 10.1016/j.jff.2015.06.033
Cseke, L. J., Kirakosyan, A., Kaufman, P. B., Warber, S., Duke, J. A., and Brielmann, H. L. (2016). Natural Products from Plants. Boca Raton, FL: CRC press.
Cummins, C. B., Wang, X., Nunez Lopez, O., Graham, G., Tie, H.-Y., Zhou, J., et al. (2018). Luteolin-mediated inhibition of hepatic stellate cell activation via suppression of the STAT3 pathway. Int. J. Mol. Sci. 19, 1567. doi: 10.3390/ijms19061567
Czaja, A. J. (2014). Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J. Gastroenterol. 20:2515. doi: 10.3748/wjg.v20.i10.2515
De Beer, D., Joubert, E., Gelderblom, W., and Manley, M. (2017). Phenolic compounds: a review of their possible role as in vivo antioxidants of wine. South Afr. J. Eno. Viticult. 23, 48–61. doi: 10.21548/23-2-2155
Decker, T., Overkamp, F., Rösel, S., Nusch, A., Göhler, T., Indorf, M., et al. (2017). A randomized phase II study of paclitaxel alone versus paclitaxel plus sorafenib in second-and third-line treatment of patients with HER2-negative metastatic breast cancer (PASO). BMC Cancer 17, 499. doi: 10.1186/s12885-017-3492-1
Defronzo, R., Fleming, G. A., Chen, K., and Bicsak, T. A. (2016). Metformin-associated lactic acidosis: current perspectives on causes and risk. Metabolism 65, 20–29. doi: 10.1016/j.metabol.2015.10.014
Dei, A., Zengin, G., Simirgiotis, M., Schafberg, M., Mollica, A., Vodnar, D. C., et al. (2013). Functional constituents of wild and cultivated Goji (L. barbarum L.) leaves: phytochemical characterization, biological profile, and computational studies. J. Enzyme Inhib. Med. Chem. 32, 153–168. doi: 10.1080/14756366.2016.1243535
Dhilleswara Rao, V., Dattatreya, A., Dan, M. M., Sarangi, T., Sasidhar, K., and Rahul, J. (2017). Translational approach in emerging infectious disease treatment: an update. Biomed. Res. 28, 5678–5686.
Di Carlo, G., Mascolo, N., Izzo, A. A., and Capasso, F. (1999). Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci. 65, 337–353. doi: 10.1016/S0024-3205(99)00120-4
Ding, L., Li, J., Song, B., Xiao, X., Zhang, B., Qi, M., et al. (2016). Curcumin rescues high fat diet-induced obesity and insulin sensitivity in mice through regulating SREBP pathway. Toxicol. Appl. Pharmacol. 304, 99–109. doi: 10.1016/j.taap.2016.05.011
Domínguez-Avila, J. A., Wall-Medrano, A., Velderrain-Rodríguez, G. R., Chen, C.-Y. O., Salazar-López, N. J., Robles-Sánchez, M., et al. (2017). Gastrointestinal interactions, absorption, splanchnic metabolism and pharmacokinetics of orally ingested phenolic compounds. Food Func. 8, 15–38. doi: 10.1039/C6FO01475E
Domitrović, R., Jakovac, H., Tomac, J., and Sain, I. (2009). Liver fibrosis in mice induced by carbon tetrachloride and its reversion by luteolin. Toxicol. Appl. Pharmacol. 241, 311–321. doi: 10.1016/j.taap.2009.09.001
Dong, L., Yin, L., Quan, H., Chu, Y., and Lu, J. (2017). Hepatoprotective effects of kaempferol-3-O-α-l-arabinopyranosyl-7-O-α-l-rhamnopyranoside on d-galactosamine and lipopolysaccharide caused hepatic failure in mice. Molecules 22, 1755. doi: 10.3390/molecules22101755
Esmaeili, M. A., and Alilou, M. (2014). Naringenin attenuates CC l4-induced hepatic inflammation by the activation of an Nrf2-mediated pathway in rats. Clin. Exp. Pharmacol. Physiol. 41, 416–422. doi: 10.1111/1440-1681.12230
Fang, S. H., Hou, Y. C., Chang, W. C., Hsiu, S. L., Chao, P. D., and Chiang, B.-L. (2003). Morin sulfates/glucuronides exert anti-inflammatory activity on activated macrophages and decreased the incidence of septic shock. Life Sci. 74, 743–756. doi: 10.1016/j.lfs.2003.07.017
Feng, X., Yu, W., Li, X., Zhou, F., Zhang, W., Shen, Q., et al. (2017). Apigenin, a modulator of PPARγ, attenuates HFD-induced NAFLD by regulating hepatocyte lipid metabolism and oxidative stress via Nrf2 activation. Biochem. Pharmacol. 136, 136–149. doi: 10.1016/j.bcp.2017.04.014
Filannino, P., Bai, Y., Di Cagno, R., Gobbetti, M., and Gänzle, M. G. (2015). Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 46, 272–279. doi: 10.1016/j.fm.2014.08.018
Finkelstein, E. A., Khavjou, O. A., Thompson, H., Trogdon, J. G., Pan, L., Sherry, B., et al. (2012). Obesity and severe obesity forecasts through 2030. Am. J. Prevent. Med. 42, 563–570. doi: 10.1016/j.amepre.2011.10.026
Forbes, S. J., and Newsome, P. N. (2016). Liver regeneration—mechanisms and models to clinical application. Nat. Rev. Gastroenterol. Hepatol. 13:473. doi: 10.1038/nrgastro.2016.97
Fracanzani, A. L., Valenti, L., Bugianesi, E., Andreoletti, M., Colli, A., Vanni, E., et al. (2008). Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology 48, 792–798. doi: 10.1002/hep.22429
Frémont, L. (2000). Biological effects of resveratrol. Life Sci. 66, 663–673. doi: 10.1016/S0024-3205(99)00410-5
Gao, B., Jeong, W. I., and Tian, Z. (2008). Liver: an organ with predominant innate immunity. Hepatology 47, 729–736. doi: 10.1002/hep.22034
García-Mediavilla, V., Crespo, I., Collado, P. S., Esteller, A., Sánchez-Campos, S., Tuñón, M. J., et al. (2007). The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 557, 221–229. doi: 10.1016/j.ejphar.2006.11.014
Gerin, F., Erman, H., Erboga, M., Sener, U., Yilmaz, A., Seyhan, H., et al. (2016). The effects of ferulic acid against oxidative stress and inflammation in formaldehyde-induced hepatotoxicity. Inflammation 39, 1377–1386. doi: 10.1007/s10753-016-0369-4
Ghiliyal, P., and Bhatt, A. (2017). Medicinal plants for treatment of liver disorders. World J. Pharm. Pharmaceutical. Sci. 6, 326–337. doi: 10.20959/wjpps20178-9695
Gómez-Juaristi, M., Martínez-López, S., Sarria, B., Bravo, L., and Mateos, R. (2018). Absorption and metabolism of yerba mate phenolic compounds in humans. Food Chem. 240, 1028–1038. doi: 10.1016/j.foodchem.2017.08.003
González-Gallego, J., García-Mediavilla, M. V., Sánchez-Campos, S., and Tuñón, M. J. (2014). “Anti-inflammatory and immunomodulatory properties of dietary flavonoids,” in Polyphenols in Human Health and Disease (Spain: Elsevier), 435–452. doi: 10.1016/B978-0-12-398456-2.00032-3
González-Ponce, H. A., Rincón-Sánchez, A. R., Jaramillo-Juárez, F., and Moshage, H. (2018). Natural dietary pigments: potential mediators against hepatic damage induced by over-the-counter non-steroidal anti-inflammatory and analgesic drugs. Nutrients 10:E117. doi: 10.3390/nu10020117
Guan, L. Y., Fu, P. Y., Li, P. D., Li, Z. N., Liu, H. Y., Xin, M. G., et al. (2014). Mechanisms of hepatic ischemia-reperfusion injury and protective effects of nitric oxide. World J. Gastrointest. Surg. 6, 122–128. doi: 10.4240/wjgs.v6.i7.122
Gupta, C., Vikram, A., Tripathi, D. N., Ramarao, P., and Jena, G. B. (2010). Antioxidant and antimutagenic effect of quercetin against DEN induced hepatotoxicity in rat. Phytother. Res. 24, 119–128. doi: 10.1002/ptr.2883
Han, M., Gao, H., Ju, P., Gao, M. Q., Yuan, Y. P., Chen, X. H., et al. (2018). Hispidulin inhibits hepatocellular carcinoma growth and metastasis through AMPK and ERK signaling mediated activation of PPARγ. Biomed. Pharmacother. 103, 272–283. doi: 10.1016/j.biopha.2018.04.014
He, B., Nohara, K., Park, N., Park, Y.-S., Guillory, B., Zhao, Z., et al. (2016). The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metabol. 23, 610–621. doi: 10.1016/j.cmet.2016.03.007
Heleno, S. A., Martins, A., Queiroz, M. J., and Ferreira, I. C. (2015). Bioactivity of phenolic acids: Metabolites versus parent compounds: a review. Food Chem. 173, 501–513. doi: 10.1016/j.foodchem.2014.10.057
Hernández-Aquino, E., and Muriel, P. (2018). Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 24, 1679. doi: 10.3748/wjg.v24.i16.1679
Hernandez-Rodas, M., Valenzuela, R., and Videla, L. A. (2015). Relevant aspects of nutritional and dietary interventions in non-alcoholic fatty liver disease. Int. J. Mol. Sci. 16, 25168–25198. doi: 10.3390/ijms161025168
Hou, X. L., Tong, Q., Wang, W. Q., Shi, C., Xiong, W., Chen, J., et al. (2015). Suppression of inflammatory responses by dihydromyricetin, a flavonoid from ampelopsis grossedentata, via inhibiting the activation of NF-κB and MAPK signaling pathways. J. Nat. Products 78, 1689–1696. doi: 10.1021/acs.jnatprod.5b00275
Hu, C., Yang, J., He, Q., Luo, Y., Chen, Z., Yang, L., et al. (2018). CysLTR1 blockage ameliorates liver injury caused by aluminum-overload via PI3K/AKT/mTOR-mediated autophagy activation in vivo and in vitro. Mol. Pharmaceut. 15, 1996–2006. doi: 10.1021/acs.molpharmaceut.8b00121
Hussain, A. (2016). Cellular DNA breakage by flavonoids and other polyphenols in the presence of CU II a structure activity study. BMJ Open Gastroenterol. 3:e000096. doi: 10.3390/ijms161125992
Iwakiri, Y., and Kim, M. Y. (2015). Nitric oxide in liver diseases. Trends Pharmacol, Sci. 36, 524–536. doi: 10.1016/j.tips.2015.05.001
Jain, A., Yadav, A., Bozhkov, A. I., Padalko, V. I., and Flora, S. J. (2011). Therapeutic efficacy of silymarin and naringenin in reducing arsenic-induced hepatic damage in young rats. Ecotoxicol. Environ. Saf. 74, 607–614. doi: 10.1016/j.ecoenv.2010.08.002
Jose, M. A., Abraham, A., and Narmadha, M. P. (2011). Effect of silymarin in diabetes mellitus patients with liver diseases. J. Pharmacol. Pharmacotherapeut. 2, 287–289. doi: 10.4103/0976-500X.85952
Ju, C., and Tacke, F. (2016). Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell. Mol. Immunol. 13, 316–327. doi: 10.1038/cmi.2015.104
Jung, E. H., Lee, J.-H., Kim, S. C., and Kim, Y. W. (2017). AMPK activation by liquiritigenin inhibited oxidative hepatic injury and mitochondrial dysfunction induced by nutrition deprivation as mediated with induction of farnesoid X receptor. Eur. J. Nutr. 56, 635–647. doi: 10.1007/s00394-015-1107-7
Kashyap, D., Sharma, A., Tuli, H. S., Sak, K., Punia, S., and Mukherjee, T. K. (2017). Kaempferol–A dietary anticancer molecule with multiple mechanisms of action: Recent trends and advancements. J. Funct. Foods 30, 203–219. doi: 10.1016/j.jff.2017.01.022
Kaveri, A. (2017). Behavioral alterations are associated with vitamin B12 deficiency in the transcobalamin receptor/CD320 KO mouse. PLoS ONE 12:e0177156. doi: 10.1371/journal.pone.0177156
Kay, C. D., Pereira-Caro, G., Ludwig, I. A., Clifford, M. N., and Crozier, A. (2017). Anthocyanins and flavanones are more bioavailable than previously perceived: a review of recent evidence. Ann. Rev. Food Sci. Technol. 8, 155–180. doi: 10.1146/annurev-food-030216-025636
Kim, H. M., Kim, Y., Lee, E. S., Huh, J. H., and Chung, C. H. (2018). Caffeic acid ameliorates hepatic steatosis and decreased ER stress in high fat diet-induced obese mice by regulating autophagy. Nutrition 55-56, 63–70. doi: 10.1016/j.nut.2018.03.010
Kim, H. Y., Park, J., Lee, K. H., Lee, D. U., Kwak, J. H., Kim, Y. S., et al. (2011). Ferulic acid protects against carbon tetrachloride-induced liver injury in mice. Toxicology 282, 104–111. doi: 10.1016/j.tox.2011.01.017
Kim, Y. J., Choi, M. S., Woo, J. T., Jeong, M. J., Kim, S. R., and Jung, U. J. (2017). Long-term dietary supplementation with low-dose nobiletin ameliorates hepatic steatosis, insulin resistance, and inflammation without altering fat mass in diet-induced obesity. Mol. Nutr. Food Res. 61, 1600889. doi: 10.1002/mnfr.201600889
Kobayashi, H., Tanaka, Y., Asagiri, K., Asakawa, T., Tanikawa, K., Kage, M., et al. (2010). The antioxidant effect of green tea catechin ameliorates experimental liver injury. Phytomedicine 17, 197–202. doi: 10.1016/j.phymed.2009.12.006
Kolasani, B. P., Sasidharan, P., Divyashanthi, C., Jayabal, P., and Rajaseharan, A. (2017). Prescribing pattern of drugs in patients with alcoholic liver disease in a tertiary care teaching hospital. Nat. J. Physiol. Pharm. Pharmacol. 7:538. doi: 10.5455/njppp.2017.7.1233027012017
Kumar, A. N., Bevara, G. B., Kaja, L. K., Badana, A. K., and Malla, R. R. (2016). Protective effect of 3-O-methyl quercetin and kaempferol from Semecarpus anacardium against H 2 O 2 induced cytotoxicity in lung and liver cells. BMC Compl. Alternat. Med. 16:376. doi: 10.1186/s12906-016-1354-z
Kwon, E. Y., and Choi, M. S. (2018). Luteolin targets the toll-like receptor signaling pathway in prevention of hepatic and adipocyte fibrosis and insulin resistance in diet-induced obese mice. Nutrients 10:1415. doi: 10.3390/nu10101415
Lagunin, A., Stepanchikova, A., Filimonov, D., and Poroikov, V. (2000). PASS: prediction of activity spectra for biologically active substances. Bioinformatics 16, 747–748. doi: 10.1093/bioinformatics/16.8.747
Lee, M. K., Choi, Y. J., Sung, S. H., Shin, D. I., Kim, J. W., and Kim, Y. C. (1995). Antihepatotoxic activity of icariin, a major constituent of Epimedium koreanum. Planta Med. 61, 523–526. doi: 10.1055/s-2006-959362
Lee, W. J., Wu, L. F., Chen, W. K., Wang, C. J., and Tseng, T. H. (2006). Inhibitory effect of luteolin on hepatocyte growth factor/scatter factor-induced HepG2 cell invasion involving both MAPK/ERKs and PI3K–Akt pathways. Chem. Biol. Int. 160, 123–133. doi: 10.1016/j.cbi.2006.01.002
Leyva-López, N., Gutierrez-Grijalva, E. P., Ambriz-Perez, D. L., and Heredia, J. B. (2016). Flavonoids as cytokine modulators: a possible therapy for inflammation-related diseases. Int. J. Mol. Sci. 17:921. doi: 10.3390/ijms17060921
Li, G., Chen, M.-J., Wang, C., Nie, H., Huang, W.-J., Yuan, T.-D., et al. (2014). Protective effects of hesperidin on concanavalin A-induced hepatic injury in mice. Int. Immunopharmacol. 21, 406–411. doi: 10.1016/j.intimp.2014.05.018
Li, M., He, Y., Zhou, Z., Ramirez, T., Gao, Y., Gao, Y., et al. (2016). MicroRNA-223 ameliorates alcoholic liver injury by inhibiting the IL-6–p47phox–oxidative stress pathway in neutrophils. Gut. 66:705–715. doi: 10.1136/gutjnl-2016-311861
Li, S., Tan, H. Y., Wang, N., Cheung, F., Hong, M., and Feng, Y. (2018). The potential and action mechanism of polyphenols in the treatment of liver diseases. Oxid. Med. Cell. Longev. 2018:8394818. doi: 10.1155/2018/8394818
Lieber, C. S., Leo, M. A., Cao, Q., Ren, C., and Decarli, L. M. (2003). Silymarin retards the progression of alcohol-induced hepatic fibrosis in baboons. J. Clin. Gastroenterol. 37, 336–339. doi: 10.1097/00004836-200310000-00013
Lin, S. Y., Chang, Y. T., Liu, J. D., Yu, C. H., Ho, Y. S., Lee, Y. H., et al. (2001). Molecular mechanisms of apoptosis induced by magnolol in colon and liver cancer cells. Mol. Carcinog. 32, 73–83. doi: 10.1002/mc.1066
Lin, Y., Kuang, Y., Li, K., Wang, S., Song, W., Qiao, X., et al. (2017). Screening for bioactive natural products from a 67-compound library of Glycyrrhiza inflata. Bioorg. Med. Chem. 25, 3706–3713. doi: 10.1016/j.bmc.2017.05.009
Lipinski, C. A. (2004). Lead-and drug-like compounds: the rule-of-five revolution. Drug Discov. Today Technol. 1, 337–341. doi: 10.1016/j.ddtec.2004.11.007
Liu, A., Wang, W., Fang, H., Yang, Y., Jiang, X., Liu, S., et al. (2015). Baicalein protects against polymicrobial sepsis-induced liver injury via inhibition of inflammation and apoptosis in mice. Eur. J. Pharmacol. 748, 45–53. doi: 10.1016/j.ejphar.2014.12.014
Liu, J., Pu, H., Liu, S., Kan, J., and Jin, C. (2017). Synthesis, characterization, bioactivity and potential application of phenolic acid grafted chitosan: a review. Carbohydrate Polymers 174, 999–1017. doi: 10.1016/j.carbpol.2017.07.014
Locke, A. E., Kahali, B., Berndt, S. I., Justice, A. E., Pers, T. H., Day, F. R., et al. (2015). Genetic studies of body mass index yield new insights for obesity biology. Nature 518:197. doi: 10.1038/nature14177
Lu, Y. F., Xu, Y. Y., Jin, F., Wu, Q., Shi, J. S., and Liu, J. (2014). Icariin is a PPARα activator inducing lipid metabolic gene expression in mice. Molecules 19, 18179–18191. doi: 10.3390/molecules191118179
Luo, J., Zhang, Y., Hu, X., Zhong, S., Chen, G., Wang, Y., et al. (2015). The effects of modified sini decoction on liver injury and regeneration in acute liver failure induced by d-galactosamine in rats. J. Ethnopharmacol. 161, 53–59. doi: 10.1016/j.jep.2014.12.003
Macindoe, G., Mavridis, L., Venkatraman, V., Devignes, M.-D., and Ritchie, D. W. (2010). HexServer: an FFT-based protein docking server powered by graphics processors. Nucleic Acids Res. 38, W445–W449. doi: 10.1093/nar/gkq311
Madankumar, P., Naveenkumar, P., Manikandan, S., Devaraj, H., and Niranjalidevaraj, S. (2014). Morin ameliorates chemically induced liver fibrosis in vivo and inhibits stellate cell proliferation in vitro by suppressing Wnt/β-catenin signaling. Toxicol. Appl. Pharmacol. 277, 210–220. doi: 10.1016/j.taap.2014.03.008
Madrigal-Santillán, E., Bautista, M., Gayosso-De-Lucio, J. A., Reyes-Rosales, Y., Posadas-Mondragón, A., Morales-González, Á., et al. (2015). Hepatoprotective effect of Geranium schiedeanum against ethanol toxicity during liver regeneration. World J. Gastroenterol. 21:7718. doi: 10.3748/wjg.v21.i25.7718
Mäkilä, L., Laaksonen, O., Alanne, A.-L., Kortesniemi, M., Kallio, H., and Yang, B. (2016). Stability of hydroxycinnamic acid derivatives, flavonol glycosides, and anthocyanins in black currant juice. J. Agricul. Food Chem. 64, 4584–4598. doi: 10.1021/acs.jafc.6b01005
Malvicini, M., Gutierrez-Moraga, A., Rodriguez, M. M., Gomez-Bustillo, S., Salazar, L., Sunkel, C., et al. (2018). A tricin derivative from Deschampsia antarctica Desv. inhibits colorectal carcinoma growth and liver metastasis through the induction of a specific immune response. Mol. Cancer Therapeut. 17, 966–976. doi: 10.1158/1535-7163.MCT-17-0193
Mandal, B., Chakraborty, T., Ali, I., Mondal, D., Majee, M. C., Raha, S., et al. (2017). Synthesis, structure, catechol oxidase activity and antibacterial studies of Mn III complex with sterically constrained phenol-based N 2 O 2 ligand. J. Ind. Chem. Soc. 94, 1079–1087.
Mansour, S. Z., El-Marakby, S. M., and Moawed, F. S. (2017). Ameliorative effects of rutin on hepatic encephalopathy-induced by thioacetamide or gamma irradiation. J. Photochem. Photobiol. B: Biol. 172, 20–27. doi: 10.1016/j.jphotobiol.2017.05.005
Marshall, J. (2016). “Alcohol: pharmacokinetics and pharmacodynamics,” in The SAGE Handbook of Drug & Alcohol Studies: Biological Approaches, eds K. Wolff, J. White, and S. Karch (SAGE Publications Ltd.), 63. doi: 10.4135/9781473922143
Meng, X., Li, Y., Li, S., Gan, R. Y., and Li, H. B. (2018). Natural products for prevention and treatment of chemical-induced liver injuries. Comprehen. Rev. Food Sci. Food Safety 17, 472–495. doi: 10.1111/1541-4337.12335
Mochizuki, M., Yamazaki, S.-I., Kano, K., and Ikeda, T. (2002). Kinetic analysis and mechanistic aspects of autoxidation of catechins. Biochim. Biophys. Acta General Subj. 1569, 35–44. doi: 10.1016/S0304-4165(01)00230-6
Monsuez, J. J., Charniot, J. C., Vignat, N., and Artigou, J. Y. (2010). Cardiac side-effects of cancer chemotherapy. Int. J. Cardiol. 144, 3–15. doi: 10.1016/j.ijcard.2010.03.003
Moody, D. E., and Reddy, J. K. (1978). The hepatic effects of hypolipidemic drugs (clofibrate, nafenopin, tibric acid, and Wy-14,643) on hepatic peroxisomes and peroxisome-associated enzymes. Am. J. Pathol. 90:435.
Mosedale, M., and Watkins, P. B. (2017). Drug-induced liver injury: advances in mechanistic understanding that will inform risk management. Clin. Pharmacol. Therapeut. 101, 469–480. doi: 10.1002/cpt.564
Nabavi, S. F., Daglia, M., Moghaddam, A. H., Habtemariam, S., and Nabavi, S. M. (2014). Curcumin and liver disease: from chemistry to medicine. Comprehen. Rev. Food Sci. Food Safety 13, 62–77. doi: 10.1111/1541-4337.12047
Nafisi, S., Adelzadeh, M., Norouzi, Z., and Sarbolouki, M. N. (2009). Curcumin binding to DNA and RNA. DNA Cell Biol. 28, 201–208. doi: 10.1089/dna.2008.0840
Namazi, N., Alizadeh, M., Mirtaheri, E., and Farajnia, S. (2017). The effect of dried glycyrrhiza glabra l. extract on obesity management with regard to PPAR-γ2 (Pro12Ala) gene polymorphism in obese subjects following an energy restricted diet. Adv. Pharmaceut. Bull. 7:221. doi: 10.15171/apb.2017.027
Nazeri, S., Farhangi, M., and Modarres, S. (2017). The effect of different dietary inclusion levels of rutin (a flavonoid) on some liver enzyme activities and oxidative stress indices in rainbow trout, Oncorhynchus mykiss (Walbaum) exposed to Oxytetracycline. Aquacul. Res. 48, 4356–4362. doi: 10.1111/are.13257
Ni, X., and Wang, H. (2016). Silymarin attenuated hepatic steatosis through regulation of lipid metabolism and oxidative stress in a mouse model of nonalcoholic fatty liver disease (NAFLD). Am. J. Transl. Res. 8, 1073–1081.
Ogata, M., Hoshi, M., Shimotohno, K., Urano, S., and Endo, T. (1997). Antioxidant activity of magnolol, honokiol, and related phenolic compounds. J. Am. Oil Chem. Soc. 74, 557–562. doi: 10.1007/s11746-997-0180-3
Oliva-Vilarnau, N., Hankeova, S., Vorrink, S. U., Mkrtchian, S., Andersson, E. R., and Lauschke, V. M. (2018). Calcium signaling in liver injury and regeneration. Front. Med. 5:192. doi: 10.3389/fmed.2018.00192
Omar, H. A., Mohamed, W. R., Arab, H. H., and Arafa, el-S. A. (2016). Tangeretin alleviates cisplatin-induced acute hepatic injury in rats: targeting MAPKs and apoptosis. PloS ONE 11:e0151649. doi: 10.1371/journal.pone.0151649
Pan, P. H., Lin, S. Y., Wang, Y. Y., Chen, W. Y., Chuang, Y. H., Wu, C. C., et al. (2014). Protective effects of rutin on liver injury induced by biliary obstruction in rats. Free Rad. Biol. Med. 3, 106–116. doi: 10.1016/j.freeradbiomed.2014.05.001
Panahi, Y., Kianpour, P., Mohtashami, R., Atkin, S. L., Butler, A. E., Jafari, R., et al. (2018). Efficacy of artichoke leaf extract in non-alcoholic fatty liver disease: A pilot double-blind randomized controlled trial. Phytother. Res. doi: 10.1002/ptr.6073
Parhiz, H., Roohbakhsh, A., Soltani, F., Rezaee, R., and Iranshahi, M. (2015). Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother. Res. 29, 323–331. doi: 10.1002/ptr.5256
Patil, S. N., Kumbar, S. V., Deshpande, A., Jayaprakash, A., Menon, P., Somu, A., et al. (2018). 55. Clinical profile of non-ascitic infections in liver cirrhosis patients and factors affecting outcome in the hospital setting. J. Clin. Exp. Hepatol. 8, S78–S79. doi: 10.1016/j.jceh.2018.06.416
Pawlak, M., Lefebvre, P., and Staels, B. (2015). Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 62, 720–733. doi: 10.1016/j.jhep.2014.10.039
Pezeshki, A., Safi, S., Feizi, A., Askari, G., and Karami, F. (2016). The effect of green tea extract supplementation on liver enzymes in patients with nonalcoholic fatty liver disease. Int. J. Prevent. Med. 7:28. doi: 10.4103/2008-7802.173051
Pradhan, S. C., and Girish, C. (2006). Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Ind. J. Med. Res. 124, 491–504.
Ramachandran, A., Duan, L., Akakpo, J. Y., and Jaeschke, H. (2018). Mitochondrial dysfunction as a mechanism of drug-induced hepatotoxicity: current understanding and future perspectives. J. Clin. Transl. Res. 4:5. doi: 10.18053/jctres.04.201801.005
Randrup Hansen, C., Grimm, D., Bauer, J., Wehland, M., and Magnusson, N. (2017). Effects and side effects of using sorafenib and sunitinib in the treatment of metastatic renal cell carcinoma. Int. J. Mol. Sci. 18:461. doi: 10.3390/ijms18020461
Rangel-Huerta, O. D., Pastor-Villaescusa, B., Aguilera, C. M., and Gil, A. (2015). A systematic review of the efficacy of bioactive compounds in cardiovascular disease: phenolic compounds. Nutrients 7, 5177–5216. doi: 10.3390/nu7075177
Rani, V., Deep, G., Singh, R. K., Palle, K., and Yadav, U. C. (2016). Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 148, 183–193. doi: 10.1016/j.lfs.2016.02.002
Ravaud, A., Motzer, R. J., Pandha, H. S., George, D. J., Pantuck, A. J., Patel, A., et al. (2016). Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N. Engl. J. Med. 375, 2246–2254. doi: 10.1056/NEJMoa1611406
Ren, K., Zhang, W., Wu, G., Ren, J., Lu, H., Li, Z., et al. (2016). Synergistic anti-cancer effects of galangin and berberine through apoptosis induction and proliferation inhibition in oesophageal carcinoma cells. Biomed. Pharmacother. 84, 1748–1759. doi: 10.1016/j.biopha.2016.10.111
Rodriguez-Amaya, D. B. (2018). Update on natural food pigments-A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. doi: 10.1016/j.foodres.2018.05.028
Saller, R., Meier, R., and Brignoli, R. (2001). The use of silymarin in the treatment of liver diseases. Drugs 61, 2035–2063. doi: 10.2165/00003495-200161140-00003
Saltveit, M. E. (2017). Synthesis and metabolism of phenolic compounds. Fruit Veget. Phytochem. Chem. Human Health 2:115. doi: 10.1002/9781119158042.ch5
Sarker, S. D., and Nahar, L. (2007). Chemistry for Pharmacy Students - General, Organic and Natural Product Chemistry. Wiley & Sons.
Schmidinger, M., and Bellmunt, J. (2010). Plethora of agents, plethora of targets, plethora of side effects in metastatic renal cell carcinoma. Cancer Treat. Rev. 36, 416–424. doi: 10.1016/j.ctrv.2010.01.003
Seeff, L. B., Bonkovsky, H. L., Navarro, V. J., and Wang, G. (2015). Herbal products and the liver: a review of adverse effects and mechanisms. Gastroenterology 148:e513. doi: 10.1053/j.gastro.2014.12.004
Seki, E., and Schwabe, R. F. (2015). Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology 61, 1066–1079. doi: 10.1002/hep.27332
Seki, N., Toh, U., Kawaguchi, K., Ninomiya, M., Koketsu, M., Watanabe, K., et al. (2012). Tricin inhibits proliferation of human hepatic stellate cells in vitro by blocking tyrosine phosphorylation of PDGF receptor and its signaling pathways. J. Cell. Biochem. 113, 2346–2355. doi: 10.1002/jcb.24107
Sen, S., and Chakraborty, R. (2017). “Herbs, gastrointestinal protection, and oxidative stress,” in Gastrointestinal Tissue, eds J. Gracia-Sancho and J. Salvadó (Spain: Elsevier), 259–274. doi: 10.1016/B978-0-12-805377-5.00019-9
Seydi, E., Salimi, A., Rasekh, H. R., Mohsenifar, Z., and Pourahmad, J. (2018). Selective cytotoxicity of luteolin and kaempferol on cancerous hepatocytes obtained from rat model of hepatocellular carcinoma: involvement of ROS-mediated mitochondrial targeting. Nutr. Cancer 70, 594–604. doi: 10.1080/01635581.2018.1460679
Shankari, S. G., Karthikesan, K., Jalaludeen, A. M., and Ashokkumar, N. (2010). Hepatoprotective effect of morin on ethanol-induced hepatotoxicity in rats. J. Basic Clin. Physiol. Pharmacol. 21, 277–294. doi: 10.1515/JBCPP.2010.21.4.277
Shanmugam, B., Shanmugam, K. R., Ravi, S., Subbaiah, G. V., Ramakrishana, C., Mallikarjuna, K., et al. (2017). Exploratory studies of (-)-Epicatechin, a bioactive compound of Phyllanthus niruri, on the antioxidant enzymes and oxidative stress markers in D-galactosamine-induced hepatitis in rats: a study with reference to clinical prospective. Pharmacog. Magaz. 13:S56. doi: 10.4103/0973-1296.203973
Sheriff, S. A., Shaik Ibrahim, S., Devaki, T., Chakraborty, S., Agarwal, S., and Pérez-Sánchez, H. (2017). Lycopene prevents mitochondrial dysfunction during d-galactosamine/lipopolysaccharide-induced fulminant hepatic failure in albino rats. J. Proteome Res. 16, 3190–3199. doi: 10.1021/acs.jproteome.7b00176
Shi, L., Hao, Z., Zhang, S., Wei, M., Lu, B., Wang, Z., et al. (2018). Baicalein and baicalin alleviate acetaminophen-induced liver injury by activating Nrf2 antioxidative pathway: The involvement of ERK1/2 and PKC. Biochem. Pharmacol. 150, 9–23. doi: 10.1016/j.bcp.2018.01.026
Sigurdson, G. T., Robbins, R. J., Collins, T. M., and Giusti, M. M. (2017). Effects of hydroxycinnamic acids on blue color expression of cyanidin derivatives and their metal chelates. Food Chem. 234, 131–138. doi: 10.1016/j.foodchem.2017.04.127
Sinha, D., Sarkar, N., Biswas, J., and Bishayee, A. (2016). Resveratrol for breast cancer prevention and therapy: Preclinical evidence and molecular mechanisms. Semin. Cancer Biol. 40–41, 209–232. doi: 10.1016/j.semcancer.2015.11.001
Sivaramakrishnan, V., and Niranjali Devaraj, S. (2009). Morin regulates the expression of NF-κB-p65, COX-2 and matrix metalloproteinases in diethylnitrosamine induced rat hepatocellular carcinoma. Chem. Biol. Inter. 180, 353–359. doi: 10.1016/j.cbi.2009.02.004
Smith, T. (2017). Elucidation of Molecular Mechanisms That may Contribute to Polyphenol-Induced Effects on Neutrophil Chemokinesis. Stellenbosch: Stellenbosch University.
Stander, M. A., Van Wyk, B. E., Taylor, M. J. C., and Long, H. S. (2017). Analysis of phenolic compounds in rooibos tea (Aspalathus linearis) with a comparison of flavonoid-based compounds in natural populations of plants from different regions. J. Agri. Food Chem. 65, 10270–10281. doi: 10.1021/acs.jafc.7b03942
Starkel, P., De Saeger, C., Lanthier, N., De Timary, P., and Leclercq, I. (2016). Expression of pro-inflammatory and hepatoprotective factors at early stages of alcoholic liver disease in humans and the impact of short term abstinence. J. Hepatol. 64:S239. doi: 10.1016/S0168-8278(16)00237-3
Stein-Chisholm, R. E., Beaulieu, J. C., Grimm, C. C., and Lloyd, S. W. (2017). LC–MS/MS and UPLC–UV evaluation of anthocyanins and anthocyanidins during rabbiteye blueberry juice processing. Beverages 3:56. doi: 10.3390/beverages3040056
Stender, S., Kozlitina, J., Nordestgaard, B. G., Tybjærg-Hansen, A., Hobbs, H. H., and Cohen, J. C. (2017). Adiposity amplifies the genetic risk of fatty liver disease conferred by multiple loci. Nat. Genet. 49:842. doi: 10.1038/ng.3855
Stickel, F., and Hellerbrand, C. (2015). Herbs to treat liver diseases: More than placebo? Clin. Liver Dis. 6, 136–138. doi: 10.1002/cld.515
Sun, X., Yamasaki, M., Katsube, T., and Shiwaku, K. (2015). Effects of quercetin derivatives from mulberry leaves: Improved gene expression related hepatic lipid and glucose metabolism in short-term high-fat fed mice. Nutr. Res. Pract. 9, 137–143. doi: 10.4162/nrp.2015.9.2.137
Suraweera, D. B., Weeratunga, A. N., Hu, R. W., Pandol, S. J., and Hu, R. (2015). Alcoholic hepatitis: The pivotal role of Kupffer cells. World J. Gastrointest. Pathophysiol. 6:90. doi: 10.4291/wjgp.v6.i4.90
Tacke, F., and Zimmermann, H. W. (2014). Macrophage heterogeneity in liver injury and fibrosis. J. Hepatol. 60, 1090–1096. doi: 10.1016/j.jhep.2013.12.025
Tang, M. C., Cheng, L., Qiu, L., Jia, R. G., Sun, R. Q., Wang, X. P., et al. (2014). Efficacy of Tiopronin in treatment of severe non-alcoholic fatty liver disease. Eur. Rev. Med. Pharmacol. Sci. 18, 160–164.
Taofiq, O., Calhelha, R. C., Heleno, S., Barros, L., Martins, A., Santos-Buelga, C., et al. (2015). The contribution of phenolic acids to the anti-inflammatory activity of mushrooms: Screening in phenolic extracts, individual parent molecules and synthesized glucuronated and methylated derivatives. Food Res. Int. 76, 821–827. doi: 10.1016/j.foodres.2015.07.044
Teng, H., Chen, M., Zou, A., Jiang, H., Han, J., Sun, L., et al. (2016). Hepatoprotective effects of licochalcone B on carbon tetrachloride-induced liver toxicity in mice. Iran. J. Basic Med. Sci. 19:910.
Terao, J., Piskula, M., and Yao, Q. (1994). Protective effect of epicatechin, epicatechin gallate, and quercetin on lipid peroxidation in phospholipid bilayers. Arch. Biochem. Biophy. 308, 278–284. doi: 10.1006/abbi.1994.1039
Thakur, A., and Raj, P. (2017). Pharmacological perspective of Glycyrrhiza glabra linn.: a mini-review. J. Anal. Pharm. Res. 5:00156. doi: 10.15406/japlr.2017.05.00156
Tian, Y., Feng, H., Han, L., Wu, L., Lv, H., Shen, B., et al. (2018). Magnolol alleviates inflammatory responses and lipid accumulation by AMP-activated protein kinase-dependent peroxisome proliferator-activated receptor α activation. Front. Immunol. 9:147. doi: 10.3389/fimmu.2018.00147
Toppo, E., Darvin, S. S., Esakkimuthu, S., Stalin, A., Balakrishna, K., Sivasankaran, K., et al. (2017). Antihyperlipidemic and hepatoprotective effects of Gardenin A in cellular and high fat diet fed rodent models. Chem. Biol. Int. 269, 9–17. doi: 10.1016/j.cbi.2017.03.013
Totta, P., Acconcia, F., Leone, S., Cardillo, I., and Marino, M. (2004). Mechanisms of naringenin-induced apoptotic cascade in cancer cells: involvement of estrogen receptor a and ß signalling. IUBMB Life 56, 491–499. doi: 10.1080/15216540400010792
Tsaroucha, A. K., Tsiaousidou, A., Ouzounidis, N., Tsalkidou, E., Lambropoulou, M., Giakoustidis, D., et al. (2016). Intraperitoneal administration of apigenin in liver ischemia/reperfusion injury protective effects. Saudi J. Gastroenterol. 22:415. doi: 10.4103/1319-3767.195556
Tuck, K. L., and Hayball, P. J. (2002). Major phenolic compounds in olive oil: metabolism and health effects. J. Nutr. Biochem. 13, 636–644. doi: 10.1016/S0955-2863(02)00229-2
Turati, F., Trichopoulos, D., Polesel, J., Bravi, F., Rossi, M., Talamini, R., et al. (2014). Mediterranean diet and hepatocellular carcinoma. J. Hepatol. 60, 606–611. doi: 10.1016/j.jhep.2013.10.034
Vargas-Mendoza, N., Madrigal-Santillán, E., Morales-González, A., Esquivel-Soto, J., Esquivel-Chirino, C. Y., et al. (2014). Hepatoprotective effect of silymarin. World J. Hepatol. 6:144. doi: 10.4254/wjh.v6.i3.144
Veberic, R., Colaric, M., and Stampar, F. (2008). Phenolic acids and flavonoids of fig fruit (Ficus carica L.) in the northern Mediterranean region. Food Chem. 106, 153–157. doi: 10.1016/j.foodchem.2007.05.061
Vergani, L., Vecchione, G., Baldini, F., Grasselli, E., Voci, A., Portincasa, P., et al. (2017). Polyphenolic extract attenuates fatty acid-induced steatosis and oxidative stress in hepatic and endothelial cells. Eur. J. Nutr. 57, 1–13. doi: 10.1007/s00394-017-1464-5
Wallace, T. C., and Giusti, M. M. (2015). Anthocyanins. Adv. Nutr. 6, 620–622. doi: 10.3945/an.115.009233
Walle, T. (2007). Methylation of dietary flavones greatly improves their hepatic metabolic stability and intestinal absorption. Mol. Pharmaceut. 4, 826–832. doi: 10.1021/mp700071d
Wan, L., and Jiang, J.-G. (2018). Protective effects of plant-derived flavonoids on hepatic injury. J. Funct. Foods 44, 283–291. doi: 10.1016/j.jff.2018.03.015
Wang, K. (2015). Molecular mechanisms of hepatic apoptosis. Cell Death Dis. 5:e996. doi: 10.1038/cddis.2013.499
Wang, L., Yue, Z., Guo, M., Fang, L., Bai, L., Li, X., et al. (2016). Dietary flavonoid hyperoside induces apoptosis of activated human LX-2 hepatic stellate cell by suppressing canonical NF-κB signaling. BioMed Res. Int. 2016:1068528 doi: 10.1155/2016/1068528
Wang, P., Jiang, L., Zhou, N., Zhou, H., Liu, H., Zhao, W., et al. (2018a). Resveratrol ameliorates autophagic flux to promote functional recovery in rats after spinal cord injury. Oncotarget 9:8427. doi: 10.18632/oncotarget.23877
Wang, Q., Wen, R., Lin, Q., Wang, N., Lu, P., and Zhu, X. (2015). Wogonoside shows antifibrotic effects in an experimental regression model of hepatic fibrosis. Digest. Dis. Sci. 60, 3329–3339. doi: 10.1007/s10620-015-3751-4
Wang, Q., Xu, H., and Zhao, X. (2018b). Baicalin inhibits human cervical cancer cells by suppressing protein kinase c/signal transducer and activator of transcription (PKC/STAT3) signaling pathway. Med. Sci. Monitor 24:1955. doi: 10.12659/MSM.909640
Wei, D.-D., Wang, J.-S., Duan, J.-A., and Kong, L.-Y. (2018). Metabolomic assessment of acute cholestatic injuries induced by thioacetamide and by bile duct ligation, and the protective effects of Huang-Lian-Jie-Du-Decoction. Front. Pharmacol. 9:458. doi: 10.3389/fphar.2018.00458
Wheng, I. E., Şener, B., and Musharraf, S. G. (2016). Antioxidant and hepatoprotective activity appraisal of four selected Fumaria species and their total phenol and flavonoid quantities. Exp. Toxicol. Pathol. 64, 205–209. doi: 10.1016/j.etp.2010.08.007
Williams, C. B., Yeh, E. S., and Soloff, A. C. (2016). Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer 2:15025. doi: 10.1038/npjbcancer.2015.25
Wu, J. C., Tung, Y. C., Zheng, Y. N., Tsai, M. L., Lai, C. S., Ho, C. T., et al. (2018). 5-Demethylnobiletin is more effective than nobiletin in preventing AOM/DSS-induced colorectal carcinogenesis in ICR mice. J. Food Bioactiv. 2, 198–103. doi: 10.31665/JFB.2018.2144
Wu, X., and Xu, J. (2016). New role of hispidulin in lipid metabolism: PPARα activator. Lipids 51, 1249–1257. doi: 10.1007/s11745-016-4200-7
Xi, L., Liu, D., Wang, L., Qiao, N., and Liu, J. (2018). Catechol 2, 3-dioxygenase from a new phenolic compound degrader Thauera sp. K11: purification and biochemical characterization. J. Basic Microbiol. 58, 255–262. doi: 10.1002/jobm.201700566
Xie, W., Jiang, Z., Wang, J., Zhang, X., and Melzig, M. F. (2016). Protective effect of hyperoside against acetaminophen (APAP) induced liver injury through enhancement of APAP clearance. Chem. Biol. Int. 246, 11–19. doi: 10.1016/j.cbi.2016.01.004
Xu, G., Huang, K., and Zhou, J. (2018). Hepatic AMP Kinase as a potential target for treating nonalcoholic fatty liver disease: evidence from studies of natural products. Curr. Med. Chem. 25, 889–907. doi: 10.2174/0929867324666170404142450
Yadav, M., Parle, M., Sharma, N., Ghimire, K., and Khare, N. (2016). Role of bioactive phytoconstituents from several traditional herbs as natural neuroprotective agents. Inventi. Rapid: Planta. Activa. 2016:33.
Yang, N., Dang, S., Shi, J., Wu, F., Li, M., Zhang, X., et al. (2017). Caffeic acid phenethyl ester attenuates liver fibrosis via inhibition of TGF-β1/Smad3 pathway and induction of autophagy pathway. Biochem. Biophys. Res. Commun. 486, 22–28. doi: 10.1016/j.bbrc.2017.02.057
Yang, Y. Z., Tang, Y. Z., and Liu, Y. H. (2013). Wogonoside displays anti-inflammatory effects through modulating inflammatory mediator expression using RAW264. 7 cells. J. Ethnopharmacol. 148, 271–276. doi: 10.1016/j.jep.2013.04.025
Yen, F. L., Wu, T. H., Lin, L. T., Cham, T. M., and Lin, C. C. (2009). Naringenin-loaded nanoparticles improve the physicochemical properties and the hepatoprotective effects of naringenin in orally-administered rats with CCl 4-induced acute liver failure. Pharmaceut. Res. 26, 893–902. doi: 10.1007/s11095-008-9791-0
Yilmaz, H. R., Uz, E., Yucel, N., Altuntas, I., and Ozcelik, N. (2004). Protective effect of caffeic acid phenethyl ester (CAPE) on lipid peroxidation and antioxidant enzymes in diabetic rat liver. J. Biochem. Mol. Toxicol. 18, 234–238. doi: 10.1002/jbt.20028
Younossi, Z. M. (2017). Long-term outcomes of nonalcoholic fatty liver disease: from nonalcoholic steatohepatitis to nonalcoholic steatofibrosis. Clin Gastroenterol Hepatol. 15, 1144–1147. doi: 10.1016/j.cgh.2017.05.029
Yu, H. D., and Ren, M. J. (2008). Clinical effect of compound silibin-phosphatidylcholine in patients with non-alcoholic fatty liver disease. Modern Med. Health 13:1153.
Yu, J. Y., Ha, J. Y., Kim, K. M., Jung, Y. S., Jung, J. C., and Oh, S. (2015). Anti-Inflammatory activities of licorice extract and its active compounds, glycyrrhizic acid, liquiritin and liquiritigenin, in BV2 cells and mice liver. Molecules 20, 13041–13054. doi: 10.3390/molecules200713041
Yuk, T., Kim, Y., Yang, J., Sung, J., Jeong, H. S., and Lee, J. (2018). Nobiletin inhibits hepatic lipogenesis via activation of AMP-activated protein kinase. Evid. Based Complement. Alt. Med. 2018:7420265. doi: 10.1155/2018/7420265
Zhang, A., Sun, H., and Wang, X. (2013). Recent advances in natural products from plants for treatment of liver diseases. Eur. J. Med. Chem. 63, 570–577. doi: 10.1016/j.ejmech.2012.12.062
Zhang, H., and Tsao, R. (2016). Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 8, 33–42. doi: 10.1016/j.cofs.2016.02.002
Zhang, J., Zuo, B., Poklar Ulrih, N., Sengupta, P. K., Zheng, X., and Xiao, J. (2018). Structure-affinity relationship of dietary anthocyanin–HSA interaction. J. Berry Res. 8, 1–9. doi: 10.3233/JBR-170167
Zhang, L., Zuo, Z., and Lin, G. (2007). Intestinal and hepatic glucuronidation of flavonoids. Mol Pharmaceut. 4, 833–845. doi: 10.1021/mp700077z
Zhang, P. W., Chen, F. X., Li, D., Ling, W. H., and Guo, H. H. (2015a). A CONSORT-compliant, randomized, double-blind, placebo-controlled pilot trial of purified anthocyanin in patients with nonalcoholic fatty liver disease. Medicine 94:e758. doi: 10.1097/MD.0000000000000758
Zhang, Y., Chen, M. L., Zhou, Y., Yi, L., Gao, Y. X., Ran, L., et al. (2015b). Resveratrol improves hepatic steatosis by inducing autophagy through the cAMP signaling pathway. Mol Nutr. Food Res. 59, 1443–1457. doi: 10.1002/mnfr.201500016
Zhao, C. L., Yu, Y. Q., Chen, Z. J., Wen, G. S., Wei, F. G., Zheng, Q., et al. (2017). Stability-increasing effects of anthocyanin glycosyl acylation. Food Chem. 214, 119–128. doi: 10.1016/j.foodchem.2016.07.073
Zhao, L., Wang, Y., Liu, J., Wang, K., Guo, X., Ji, B., et al. (2016). Protective effects of genistein and puerarin against chronic alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms. J. Agri. Food Chem. 64, 7291–7297. doi: 10.1021/acs.jafc.6b02907
Zhen, J., Villani, T. S., Guo, Y., Qi, Y., Chin, K., Pan, M. H., et al. (2016). Phytochemistry, antioxidant capacity, total phenolic content and anti-inflammatory activity of Hibiscus sabdariffa leaves. Food Chem. 190, 673–680. doi: 10.1016/j.foodchem.2015.06.006
Keywords: gene network, hepatoprotection, phenolics, liver disease, in silico analysis, mechanism, natural product
Citation: Saha P, Talukdar AD, Nath R, Sarker SD, Nahar L, Sahu J and Choudhury MD (2019) Role of Natural Phenolics in Hepatoprotection: A Mechanistic Review and Analysis of Regulatory Network of Associated Genes. Front. Pharmacol. 10:509. doi: 10.3389/fphar.2019.00509
Received: 25 February 2019; Accepted: 24 April 2019;
Published: 24 May 2019.
Edited by:
Raffaele Capasso, University of Naples Federico II, ItalyReviewed by:
Ludovico Abenavoli, Università degli studi Magna Græcia di Catanzaro, ItalyCopyright © 2019 Saha, Talukdar, Nath, Sarker, Nahar, Sahu and Choudhury. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anupam Das Talukdar, YW51cGFtQGJpb2luZm9hdXMuYWMuaW4=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.