
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 25 April 2019
Sec. Neuropharmacology
Volume 10 - 2019 | https://doi.org/10.3389/fphar.2019.00432
This article is part of the Research Topic Novel Targets and the Application of Targeting Techniques in the Treatment of Cerebrovascular Disease View all 9 articles
 Beatrice Severino1
Beatrice Severino1 Angela Corvino1
Angela Corvino1 Ferdinando Fiorino1
Ferdinando Fiorino1 Francesco Frecentese1
Francesco Frecentese1 Elisa Perissutti1
Elisa Perissutti1 Giuseppe Caliendo1
Giuseppe Caliendo1 Vincenzo Santagada1
Vincenzo Santagada1 Elisa Magli1
Elisa Magli1 Pasquale Molinaro2
Pasquale Molinaro2 Giuseppe Pignataro2
Giuseppe Pignataro2 Lucio Annunziato3
Lucio Annunziato3 Natalícia J. Antunes4*
Natalícia J. Antunes4* Julio Rojas-Moscoso4
Julio Rojas-Moscoso4 Noedi L. de Freitas4
Noedi L. de Freitas4 Gustavo D. Mendes5,6,7
Gustavo D. Mendes5,6,7 Gilberto De Nucci4,6,8
Gilberto De Nucci4,6,8Neurounina-1 [chemical name: 7-nitro-5-phenyl-1-(pyrrolidin-1-ylmethyl)-1H-benzo[e][1,4]diazepin-2(3H)-one] is a new compound provided with relevant neuroprotective effect during stroke and in neonatal hypoxia by increasing the Na+/Ca2+ exchanger (NCX) isoforms NCX1 and NCX2 activity. This study shows for the first time, the development and validation of a sensitive and selective method for analysis of neurounina-1 in beagle dog plasma by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). The sample preparation consisted of extraction of the analyte and the internal standard (IS) (ropivacaine) from plasma (50 μL) by liquid-liquid extraction using acetonitrile (100 μL). The selected reaction monitoring mode of the positive ion was performed and the precursor to the product ion transitions of m/z 365 > 83 and m/z 275 > 126 were used to measure the derivative of neurounina-1 and ropivacaine. The chromatographic separation was achieved using a Phenomenex C18 Luna (150 mm × 4.6 mm × 5 μm) analytical column with an isocratic mobile phase composed of methanol/acetonitrile/water (50/40/10, v/v/v) + 0.1% formic acid + 1 M ammonium formate. The method was linear over a concentration range of 1–500 ng/mL. The method was applied to evaluate the pharmacokinetics of neurounina-1 after a single intravenous administration of three different doses (0.1 mg/kg, 0.3 mg/kg, and 1 mg/kg) to beagle dogs (n = 5). The mean AUC0-tlast values were 26.10, 115.81, and 257.28 ng∗h/mL following intravenous administration of 0.1, 0.3, and 1 mg/kg, respectively. Linear pharmacokinetics was observed up to 1.0 mg/kg. The neurounina-1 was rapidly eliminated, with mean CL values of 46.24, 47.57, and 69.15 L/h, Vd of 130.31, 154.15, and 210.79 L and t1/2 of 2.14, 2.54, and 2.04 h after intravenous administration of 0.1, 0.3, and 1 mg/kg, respectively. This new analytical method allows the rapid determination of the neurounina-1, a new developed compound, able to exert a remarkable neuroprotective effect in the low nanomolar range.
Stroke is a leading cause of long-lasting injury, disability, and death. Early treatment and preventive measures can reduce the brain damage that occurs as a result of a stroke. Several studies have demonstrated that the activation of the three Na+/Ca2+ exchanger (NCX) isoforms, NCX1, NCX2, and NCX3 exerts a neuroprotective action against stroke injury in adult mice (Pignataro et al., 2004a,b; Jeon et al., 2008; Molinaro et al., 2008, 2016) and against neonatal hypoxic-ischemic encephalopathy damage (Cerullo et al., 2018). Therefore, in recent years, the interest has been focused on the identification of NCX activator aiming to have new therapeutic tools able to limit the extension of ischemic brain damage (Annunziato et al., 2004). With this purpose, the structure of one of the most potent inhibitors, SM-15811, was modified obtaining 7-nitro-5-phenyl-1-(pyrrolidin-1-ylmethyl)-1H-benzo[e][1,4]diazepin-2(3H)-one, a new small molecule named neurounina-1 that was patented in 2012 (Pignataro et al., 2012). Several pharmacological experiments have demonstrated that this compound exerts a remarkable neuroprotective effect during stroke by stimulation of NCX1 and NCX2 activities (Molinaro et al., 2013). Neurounina-1 has a wide therapeutic window, as shown in an in vivo mouse model of transient middle cerebral artery occlusion (tMCAO). When intraperitoneally administered at doses of 0.003 to 30 μg/kg to mouse, neurounina-1 effect was notable even 5 h after ischemia induction, unlike other drugs used to date for treating stroke, which require a much earlier administration. Furthermore, neurounina-1 presents a high lipophilicity index and low toxicity (Molinaro et al., 2013).
To support pre-clinical studies with neurounina-1, the development of a bioanalytical method to monitor its concentration in biological matrices is required. There is no available method for neurounina-1 quantification. Moreover, its half maximal effective concentration (EC50) is in the picomolar to low nanomolar range (Molinaro et al., 2013), which requires a low dose administration and consequently, a sensitive and selective method of quantification.
This study presents for the first time, the development and validation of a sensitive and selective method to quantify neurounina-1 in beagle dog plasma using liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS), with ropivacaine as internal standard (IS). The method showed a lower limit of quantification (LLOQ) of 1.0 ng/mL, which was used to evaluate the pharmacokinetics in plasma after a single intravenous administration of three different doses of neurounina-1 (0.1 mg/kg, 0.3 mg/kg, and 1 mg/kg) to beagle dogs (n = 5).
Ropivacaine was utilized as IS and was supplied by the United States Pharmacopeia (Rockville, MD, United States). Neurounina-1 was synthesized in house according to the previously reported study (Molinaro et al., 2013). Acetonitrile and methanol (HPLC grade) were purchased from J.T Baker (Phillipsburg, NJ, United States); ammonium acetate and hexane (analysis grade) from J.T Baker (Ecatepec, Mexico); ethyl ether (analysis grade) from Mallinckrodt (Phillipsburg, NJ, United States). The water was obtained from the purification system Synergy UV® (Millipore, Molsheim, France).
Stock solutions of neurounina-1 and ropivacaine were prepared in methanol/water (50/50, v/v). Calibration curves for neurounina-1 were prepared by adding the compound to blank plasma to yield final concentrations of 1, 2, 10, 50, 100, 200, 350, and 500 ng/mL. The calibration curves were performed in duplicate for each day’s assays. The quality control (QC) samples were prepared in blank plasma at the concentrations of 1, 1.5, 30, 240, and 400 ng/mL, respectively. For each validation, seven replicates were analyzed for each CQ level (three validations were performed). The spiked plasma samples (standards and QC) were extracted in each analytical batch along with the unknown samples.
Aliquots (0.05 mL) of each plasma sample were added to glass tubes followed by 0.05 mL of IS (ropivacaine 100 ng/mL). The tubes were vortexed for 5 s and 0.1 mL of acetonitrile were added. The samples were vortexed for 40 s, again, and then centrifuged at 2000 ×g for 5 min. The obtained supernatants were transferred to microvials for analysis.
Neuronina-1 was separated in a 150 mm × 4.6 mm x 5 μm Phenomenex C18Luna reversed phase analytical column (Phenomenex Inc., CA, United States). The temperature of the column was maintained constant at 65°C. The mobile phase used was composed by methanol/acetonitrile/water (50/40/10, v/v/v) + 0.1% of formic acid + 1 M ammonium formate at a flow rate of 1 mL/min (split 1:3). The auto-sampler was maintained at room temperature.
The mass spectrometer (Quattro Micro – Micromass, Waters, United Kingdom) equipped with an electrospray source in the ESI positive polarity mode (ES+) was configured for multiple reaction monitoring (MRM) to monitor the transitions 365.3 > 83.9 and 275.2 > 126.0, for neurounina-1 and ropivacaine, respectively. Figure 1 shows the full scan spectra (upper trace) and the product ion spectra (lower trace) obtained by the proposed fragmentation pathways for neurounina-1 (panel A) and ropivacaine (panel B). To optimize all MS parameters, a standard solution of the analyte and IS was infused into the mass spectrometer. The optimized values of ion spray voltage, collision energy, and cone voltage were, respectively 2800 (V), 20 (eV), and 20 (V) for neurounina-1 and 2800 (V), 15 (eV), and 15 (V) for ropivacaine. Data acquisition and analysis were performed using the software Masslynx 4.0 (Waters Corporation, Milford, MA, United States).
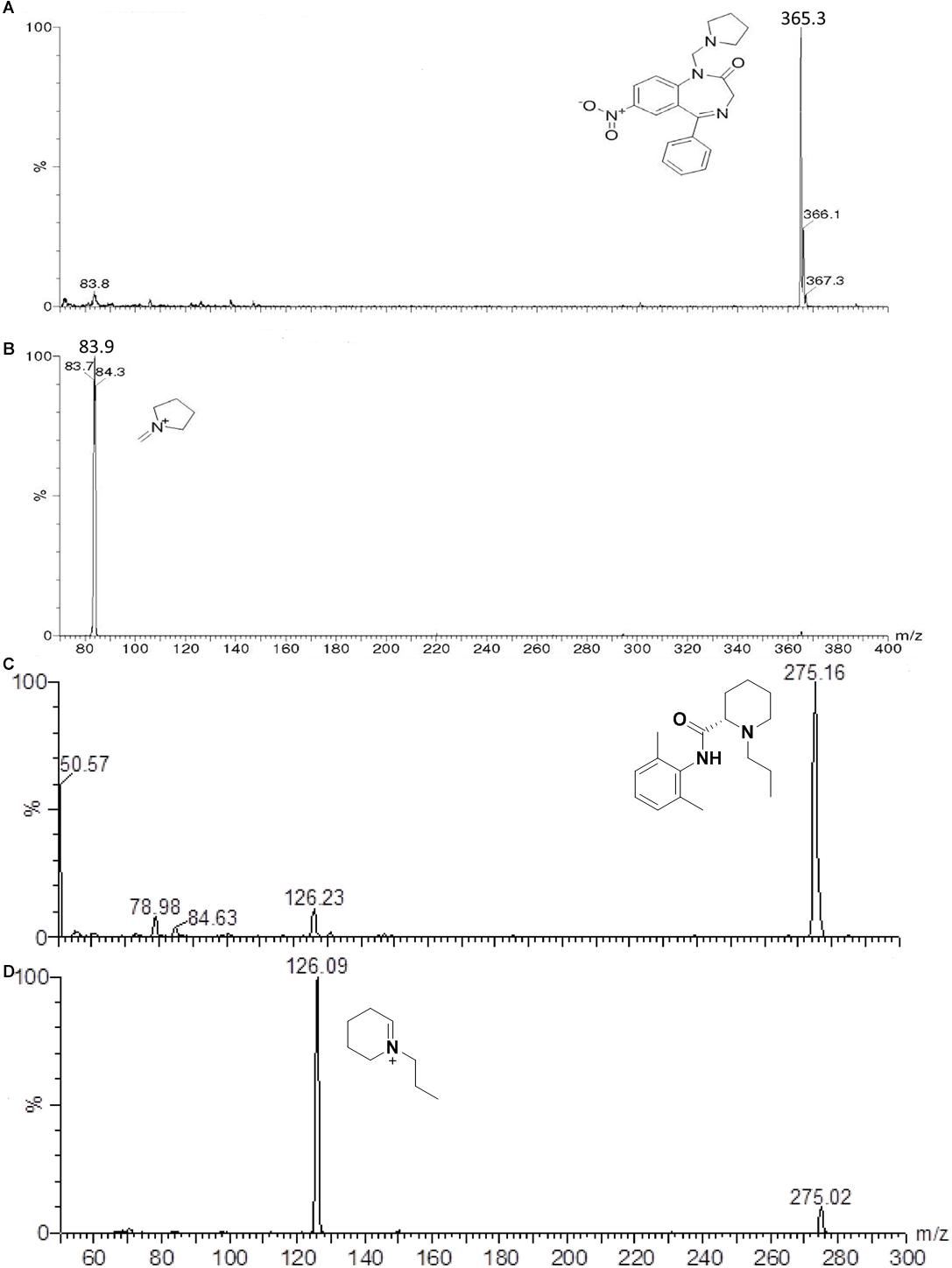
Figure 1. Full scan mass spectrum of the protonated molecular ion of neurounina-1 – m/z = 365.3 (A), ion product of neurounina-1 – m/z = 83.9 (B), protonated molecular ion of ropivacaine – m/z = 275.16 (C), and the ion product of ropivacaine – m/z = 126.09 (D).
The method validation was carried out according to the United States Food and Drug Administration (FDA, 2001) bioanalytical method validation guidance and the Brazilian National Sanitary Surveillance Agency (Agência Nacional de Vigilância Sanitária [ANVISA], 2003).
Calibration curves were prepared by assaying standard plasma samples at eight concentrations of neurounina-1 (1, 2, 10, 50, 100, 200, 350, and 500 ng/mL) and the linearity of each calibration curve was determined by plotting the peak area ratio (y) of neurounina-1/ropivacaine vs. nominal concentration of analyte. The calibration curve was constructed by weighted (1/X) least squares linear regression.
The precision and accuracy of assay was determined at five different concentrations (1.0, 1.5, 30, 240, and 400 ng/mL), selected based on the literature and comparison with the previously established analytical values for similar studies. The following criteria were found to approve precision and intra-race accuracy: (i) for each concentration level, coefficient of variation (CV) that does not exceed 15% for LLOQ and 20% for QC samples; (ii) mean value of the samples at each concentration level, within 85 – 115% of the actual value for LLOQ and 80–120% for QC samples.
The recovery was evaluated by dividing the extracted sample mean response by the unextracted (spiked blank plasma extract) sample mean of the corresponding concentration. The matrix effect experiments were carried out using the ratio between spiked mobile phase solutions and unextracted samples, spiked on plasma residues.
To assess stability, QC plasma samples (1.5 and 400.0 ng/mL) were subjected to short-term (6 h) incubation at room temperature; four freeze/thaw (-20°C) cycles and 52 h in the autosampler at room temperature. Subsequently, the neurounina-1 concentrations were measured and compared with freshly prepared samples.
Beagle dogs (n = 5) of both sexes, aged between 2 and 3 years old, weighing between 10 and 14 kg were provided by Camilo Castelo Branco University, Brazil. The dogs were housed in Domingos Alves Veterinary Hospital in individual rooms and fed with a standard canine food. They had free access to water. The dogs underwent rigorous veterinary control and were all considered healthy based on physical examination and laboratory analysis performed before initiation and after completion of each occasion of the study. At the end of the study, the animals were returned to their habitat. This study was carried out in accordance with the recommendations of the general ethical guidelines established by the Brazilian Society for Laboratory Animal Science (SBCAL). The protocol was approved by the Committee for Ethics in Animal Use – State University of Campinas (CEUA/UNICAMP, protocol n° 3340-1).
After an overnight (8 h) fasting, a single intravenous dose of 0.1 mg/kg, 0.3 mg/kg, or 1 mg/kg neurounina-1 formulation was administered to beagle dogs, with 7 days washout period between each dose administration. Blood samples (5 mL) from a suitable antecubital vein were collected into heparin-containing tubes before and 0.03, 0.08, 0.17, 0.25, 0.33, 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 h after the administration of each dose. A total of 210 mL of blood was collected during the study. The blood samples treated with sodium heparin were centrifuged at approximately 2000 ×g for 10 min at 4°C and the plasma was stored at -20°C until analysis.
Non-compartmental analysis was used to determine the pharmacokinetic parameters of neurounina-1 after the intravenous administration. The concentration at time zero (C0) was estimated by back-extrapolating from the elimination curve. The area under the plasma concentration vs. time curves from zero to the last detectable concentration (AUC0-tlast) were calculated by applying the linear-log trapezoid rule. Extrapolation of these areas to infinity (AUC0-inf) was done by adding the value Clast/ke to the calculated AUC0-tlast (where Clast = the last detectable concentration). Clearance (CL) was calculated by the formula dose/AUC0-inf. Volume of distribution (Vd) was calculated by the formula CL/ke. The WinNonlin software, version 6.4 (Pharsight Corp, Mountain View, CA, United States) was used. Statistical analysis was performed using GraphPad Prism software, version 3.2 (GraphPad Software, San Diego, California, United States).
Direct infusion was used to determine the best mass spectrometry conditions for neurounina-1 and ropivacaine. The spectrum for neurounina-1 showed a protonated molecular ion at m/z 365.3 and its collision-induced dissociation formed a distinctive product at m/z 83.9, corresponding to the 1-methylenepyrrolidinium ion. Ropivacaine showed a base peak ion ([M + H]+) at mass-to-charge ratio (m/z) of 275.16 and a fragmentation product at m/z 126.09, corresponding to the 1-propyl-2,3,4,5-tetrahydropyridinium ion (Figure 1). The selected reaction monitoring is based on the m/z 365.3 > 83.9 and 275.2 > 126.0 transitions, for neurounina-1 and ropivacaine, respectively. The total run time was 3.8 min (Figure 2). Ropivacaine was selected as the IS because of its behavior and structural similarity with neurounina-1.
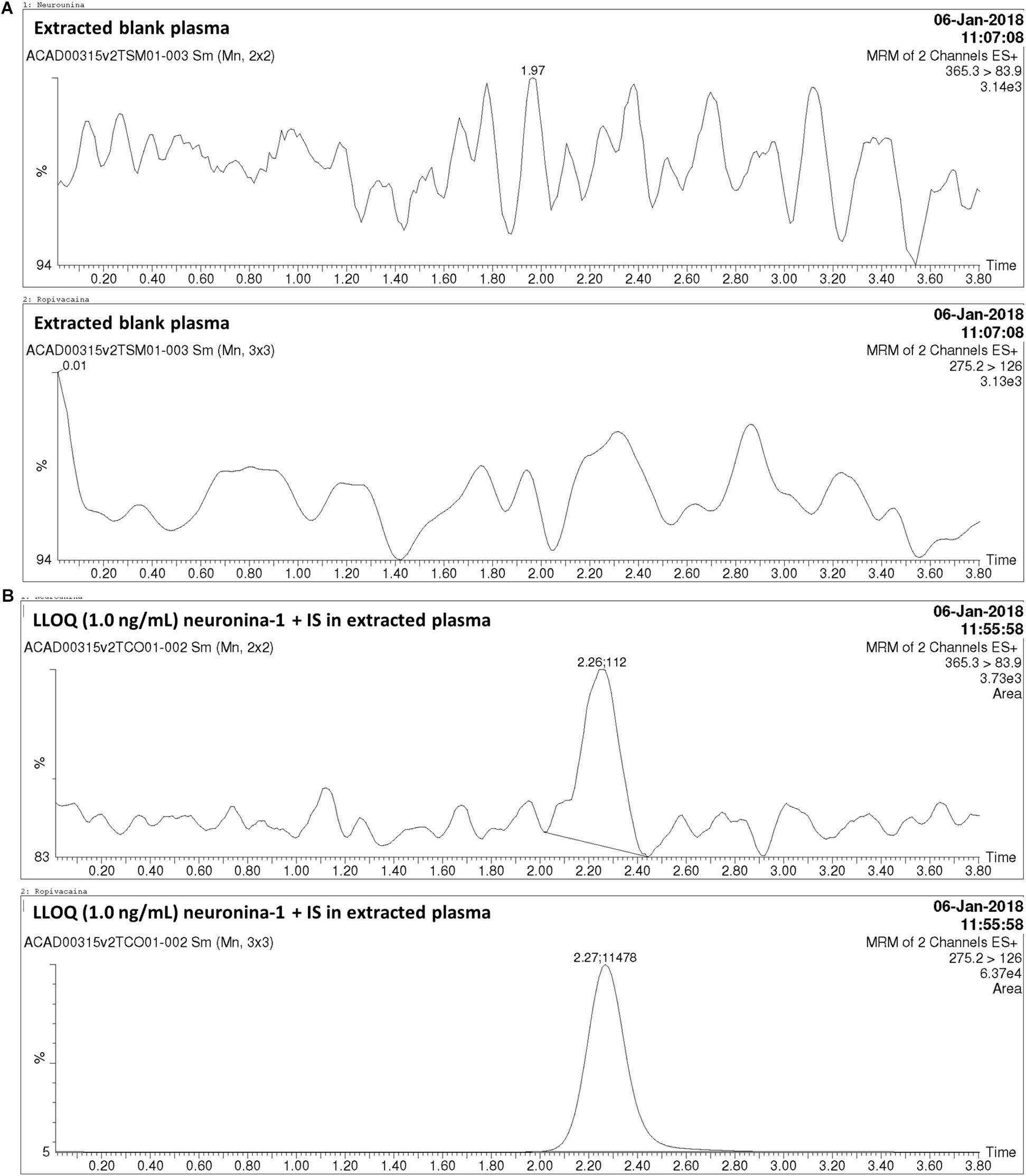
Figure 2. Chromatograms obtained in the analysis of neurounina-1 in beagle dog plasma. (A) Blank beagle dog plasma; (B) Blank beagle dog plasma spiked with neurounina-1 (lower limit of quantification (LLOQ – 1.0 ng/mL) and ropivacaine (internal standard – IS, 100 ng/mL).
The method was linear regression for neurounina-1 concentrations from 1 to 500 ng/mL (calibration curve y = 0.00767312x + 0.00170099, r = 0.997172). A linear regression with a weighting index of 1/x was performed on the peak area ratios of neurounina-1 and the IS vs. neurounina-1 concentrations of the eight beagle dogs plasma standards (in duplicate) to generate a calibration curve. The LLOQ, defined as the lowest concentration at which both the precision and accuracy were <20%, was 1.0 ng/mL. No endogenous peak was observed in the mass chromatogram of blank plasma (Figure 2). The retention times for neurounina-1 and IS were 2.27 min and 2.26 min, respectively (Figure 2).
The within- and between-run precision and accuracy for the LLOQ and QCs, summarized in Table 1, insure the reproducibility and repeatability of the results.
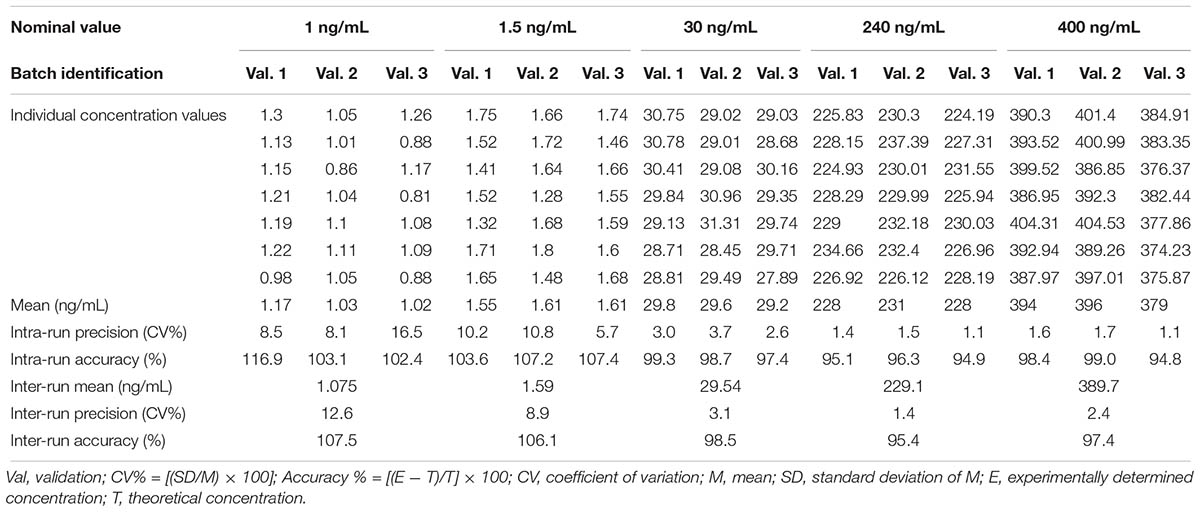
Table 1. Accuracy and precision data for neurounina-1 from the pre-study validation in beagle dog plasma.
The recovery obtained with the method of extraction of neurounina-1 with acetonitrile was higher than 89%. The matrix effect was practically absent in the analysis of neurounina-1 in beagle dog plasma (Table 2). Values >91% were obtained when the peak areas resulting from the injection of standard solutions in the mobile phase were compared with those of standard solutions added to extracts of blank plasma from eight different lots.
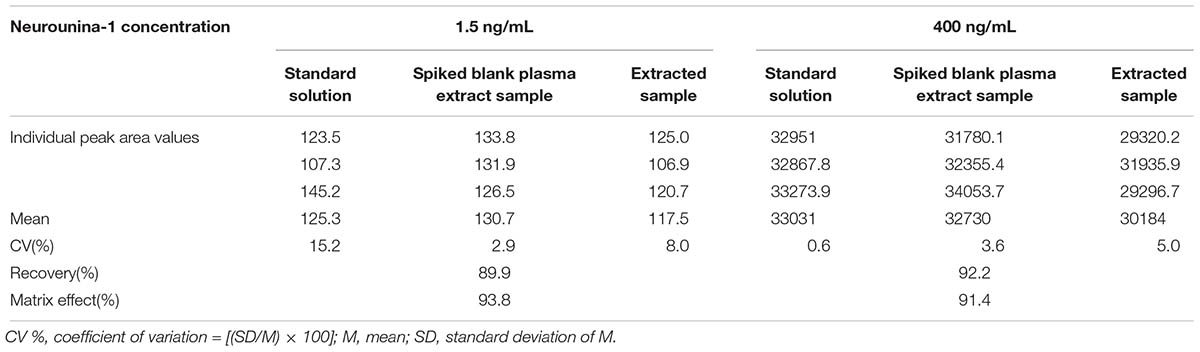
Table 2. Recovery and matrix effect for neurounina-1 in eight different lots of beagle dog plasma (4 normal, 2 lipemic, and 2 hemolysate).
The stability tests indicated that no significant degradation of neurounina-1 occurred during four freezing and thawing cycles, over a period of 6 h at room temperature and after processing in the self-injector for 52 h at 5°C, as shown in Table 3.
The mean neurounina-1 plasma concentration vs. time profiles after a single intravenous dose administration of 0.1, 0.3, and 1 mg/kg neurounina-1 to beagle dogs (n = 5) are shown in Figure 3. The obtained neurounina-1 pharmacokinetic parameters are presented in Table 4. The mean AUC0-tlast values were 26.10, 115.81, and 257.28 ng∗h/mL following intravenous administration of 0.1, 0.3, and 1 mg/kg, respectively. Linear pharmacokinetics was observed up to 1.0 mg/kg. The neurounina-1 was rapidly eliminated, with mean CL values of 46.24, 47.57, and 69.15 L/h, Vd of 130.31, 154.15, and 210.79 L and t1/2 of 2.14, 2.54 and 2.04 h after intravenous administration of 0.1, 0.3 and 1 mg/kg, respectively.
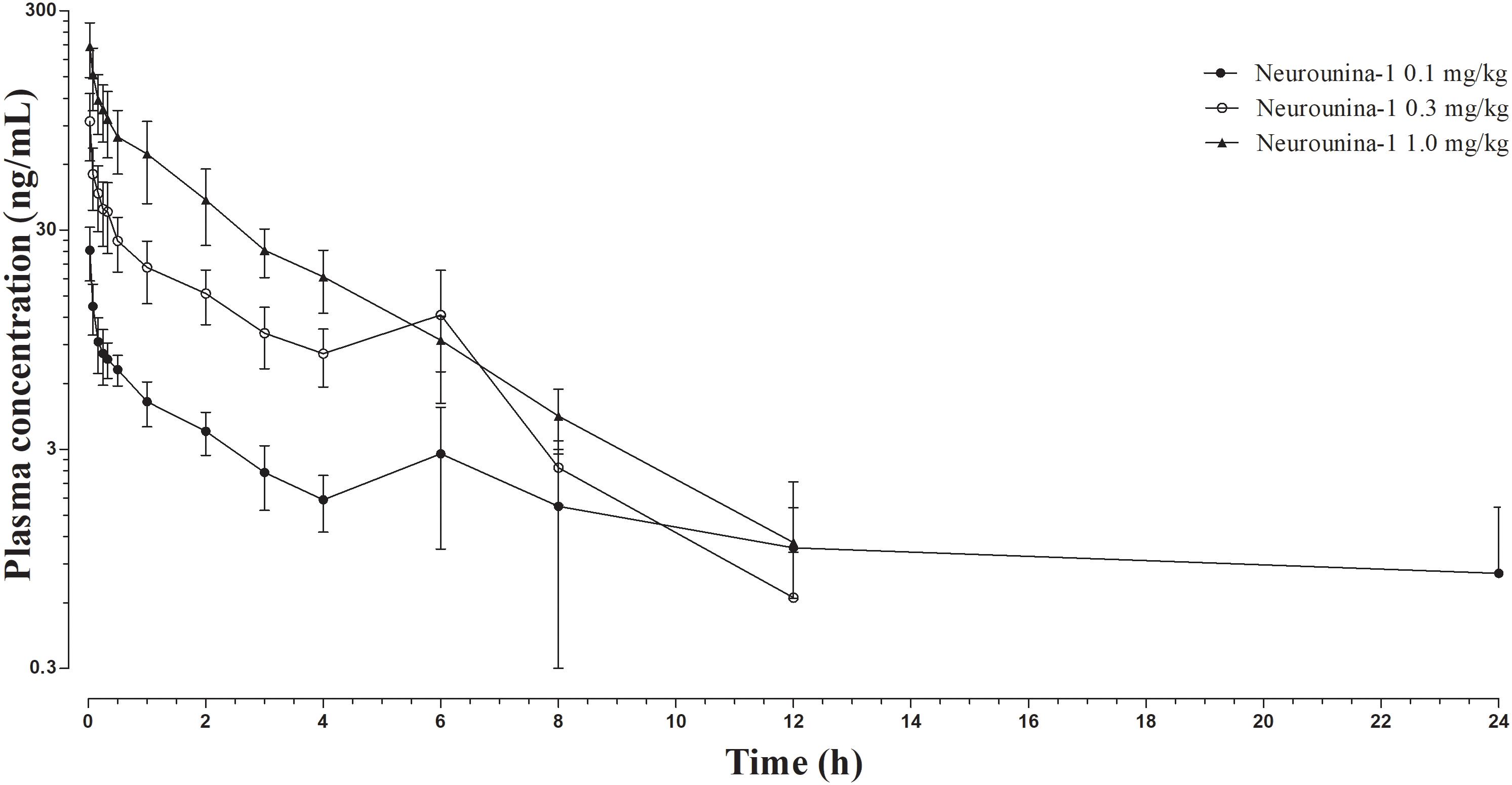
Figure 3. Neurounina-1 plasma concentration vs. time profile obtained after a single intravenous administration of 0.1, 0.3, and 1 mg/kg of neurounina-1 to beagle dogs (n = 5). Data are expressed as mean and standard deviation.
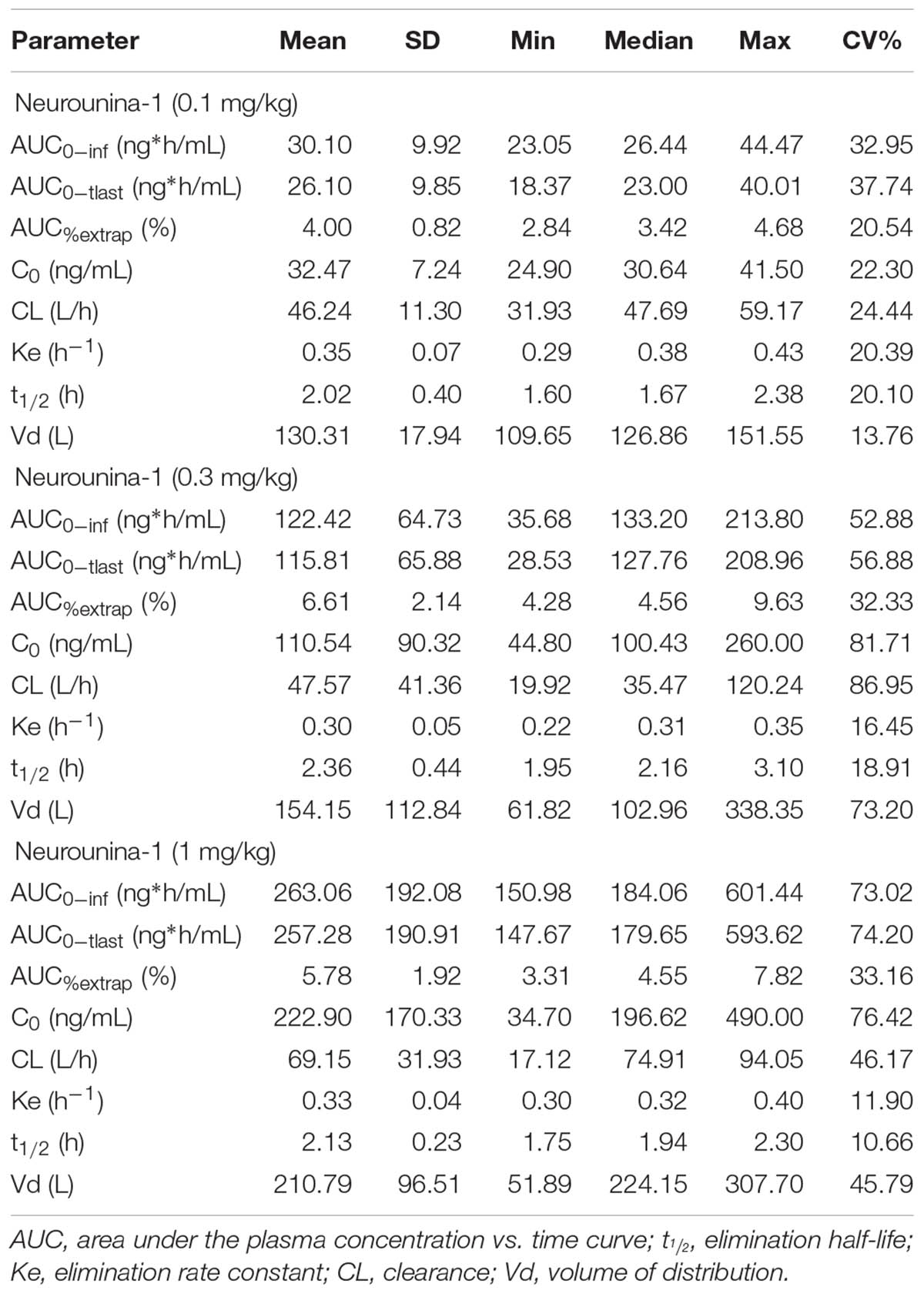
Table 4. Pharmacokinetic parameters after a single intravenous administration of 0.1, 0.3, and 1 mg/kg of neurounina-1 to beagle dogs (n = 5).
The present study shows, for the first time, a selective and sensitive method for analysis of neurounina-1 in beagle dog plasma using LC-MS/MS. One of the most advantages is that this method does not require deuterated analogs.
The LC-MS/MS method presented a good sensitivity (LLOQ of 1 ng/mL) and permits a high throughput. Furthermore, this method is simple and selective for quantification and pharmacokinetic evaluation of neurounina-1 in beagle dog plasma. Indeed, this method was applied to evaluate the pharmacokinetics of neurounina-1 after a single intravenous administration of three different doses (0.1 mg/kg, 0.3 mg/kg, and 1 mg/kg) to beagle dogs. Linear pharmacokinetics was observed up to 1.0 mg/kg. Nevertheless, the high lipophilicity index of neurounina-1, it showed a low half-life with mean CL values of 46.24, 47.57, and 69.15 L/h, Vd of 130.31, 154.15, and 210.79 L and t1/2 of 2.14, 2.54, and 2.04 h after intravenous administration of 0.1, 0.3, and 1 mg/kg, respectively.
Remarkably, prediction analysis revealed that neurounina-1 possesses a high lipophilicity and, thus, a high capability of crossing the blood-brain barrier (BBB), with an estimated log P of 0.87 (n-octanol/water). This aspect is further highlighted by data on the Vd. Indeed, the high Vd values of neurounina-1 confirm the ability of this compound to reach the CNS and support its indication in neurological disorders. As concern, the possible breakdown of BBB after stroke may alter its permeability (Krueger et al., 2017). Therefore, experiments carried out in healthy animals could show lower levels of the drug in the brain tissue. On the other hands, the pharmacokinetic profile determined in beagle dogs correlates with the in vitro and in vivo therapeutic effect observed in neurons exposed to oxygen glucose deprivation (OGD) and in adult mice subjected to transient occlusion of middle cerebral artery and in neonatal hypoxic mice treated intraperitoneally with neurounina-1 (30 μg/kg) (Cerullo et al., 2018). The dosage used in the present study is in a higher range of that exerting a protective effect in mice cortical neurons exposed to OGD. Furthermore, the most effective dosage of neurounina-1 in an animal model of stroke was 0.03 μg/Kg, administered 3 h after stroke induction (Molinaro et al., 2013), much smaller than the dosage range administered to beagle dogs. A protection, less marked than that obtained with 0.03 μg/Kg neurounina-1, was also seen when neurounina-1 was used at a dose of 0.003 μg/Kg (Molinaro et al., 2013). Notably, the administration of neurounina-1 seemed to be well tolerated by the animals and no sign of behavior modification were observed.
A possible major limitation of this study is the relative short half-life of neurounina-1, around 2 h. For its potential use during the acute stroke episode, intravenous infusion of the drug could be eventually done. However, for the sub-acute or chronic administration of the compound, its fast elimination rate could be an important drawback.
The developed and validated method to quantify neurounina-1 in beagle dog plasma using LC-MS/MS presented sensitivity and selectivity, thus allowing the rapid and precise determination of the pharmacokinetics of this neuroprotective compound working in the low nanomolar range.
This study was carried out in accordance with the recommendations of the general ethical guidelines established by the Brazilian Society for Laboratory Animal Science (SBCAL). The protocol was approved by the Committee for Ethics in Animal Use – State University of Campinas (CEUA/UNICAMP, protocol n° 3340-1).
BS, AC, FeF, FrF, EP, GC, VS, EM, PM, GP, and LA participated the drug development. NA and GM performed the pharmacokinetic and statistical analysis. JR-M and NdF performed the drug administration and blood sampling. GDN was the supervisor. BS, LA, NA, and GM wrote the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
This pharmacokinetic trial was supported by Programma Operativo Nazionale (PON_01602 and PON03PE_00146_1) from MIUR, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP – Grant n° 2016/22506-1).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Agência Nacional de Vigilância Sanitária [ANVISA] (2003). Resolution RE n° 899/03. Available at: http://portal.anvisa.gov.br/documents/10181/2718376/RE_899_2003_COMP.pdf/ff6fdc6b-3ad1-4d0f-9af2-3625422e6f4b (accessed April 15, 2019).
Annunziato, L., Pignataro, G., and Di Renzo, G. F. (2004). Pharmacology of brain Na+/Ca2+ exchanger: from molecular biology to therapeutic perspectives. Pharmacol. Rev. 56, 633–654. doi: 10.1124/pr.56.4.5
Cerullo, P., Brancaccio, P., Anzilotti, S., Vinciguerra, A., Cuomo, O., Fiorino, F., et al. (2018). Acute and long-term NCX activation reduces brain injury and restores behavioral functions in mice subjected to neonatal brain ischemia. Neuropharmacology 135, 180–191. doi: 10.1016/j.neuropharm.2018.03.017
FDA (2001). US Department of Health and Human Services, Center for Drug Evaluation and Research Guidance for Industry - Bioanalytical Method Validation. Silver Spring, MD: FDA.
Jeon, D., Chu, K., Jung, K. H., Kim, M., Yoon, B. W., Lee, C. J., et al. (2008). Na(+)/Ca(2+) exchanger 2 is neuroprotective by exporting Ca(2+) during a transient focal cerebral ischemia in the mouse. Cell Calcium 43, 482–491. doi: 10.1016/j.ceca.2007.08.003
Krueger, M., Härtig, W., Frydrychowicz, C., Mueller, W. C., Reichenbach, A., Bechmann, I., et al. (2017). Stroke-induced blood–brain barrier breakdown along the vascular tree – No preferential affection of arteries in different animal models and in humans. J. Cereb. Blood Flow Metab. 37, 2539–2554. doi: 10.1177/0271678X16670922
Molinaro, P., Cantile, M., Cuomo, O., Secondo, A., Pannaccione, A., Ambrosino, P., et al. (2013). Neurounina-1, a novel compound that increases Na+/Ca2+ exchanger activity, effectively protects against stroke damage. Mol. Pharmacol. 83, 142–156. doi: 10.1124/mol.112.080986
Molinaro, P., Cuomo, O., Pignataro, G., Boscia, F., Sirabella, R., Pannaccione, A., et al. (2008). Targeted disruption of Na+/Ca2+ exchanger 3 (NCX3) gene leads to a worsening of ischemic brain damage. J. Neurosci. 28, 1179–1184. doi: 10.1523/JNEUROSCI.4671-07.2008
Molinaro, P., Sirabella, R., Pignataro, G., Petrozziello, T., Secondo, A., Boscia, F., et al. (2016). Neuronal NCX1 overexpression induces stroke resistance while knockout induces vulnerability via Akt. J. Cereb. Blood Flow Metab. 36, 1790–1803. doi: 10.1177/0271678X15611913
Pignataro, G., Annunziato, L., Molinaro, P., Scorziello, A., Secondo, A., Pannaccione, A., et al. (2012). Preparation of 7-nitro-5-phenyl-1-(pyrrolidin-1-ylmethyl)-1H-benzo[e][1,4]diazepin-2(3H)-one and other benzodiazepine derivatives useful in treating cerebral ischemia. Patent No. WO2012072620A1.
Pignataro, G., Gala, R., Cuomo, O., Tortiglione, A., Giaccio, L., Castaldo, P., et al. (2004a). Two sodium/calcium exchanger gene products, NCX1 and NCX3, play a major role in the development of permanent focal cerebral ischemia. Stroke 35, 2566–2570. doi: 10.1161/01.STR.0000143730.29964.93
Pignataro, G., Tortiglione, A., Scorziello, A., Giaccio, L., Secondo, A., Severino, B., et al. (2004b). Evidence for a protective role played by the Na+/Ca2+ exchanger in cerebral ischemia induced by middle cerebral artery occlusion in male rats. Neuropharmacology 46, 439–448. doi: 10.1016/j.neuropharm.2003.09.015
Keywords: neurounina-1, Na+/Ca2+ exchanger (NCX), LC-MS/MS, beagle dog plasma, pharmacokinetics
Citation: Severino B, Corvino A, Fiorino F, Frecentese F, Perissutti E, Caliendo G, Santagada V, Magli E, Molinaro P, Pignataro G, Annunziato L, Antunes NJ, Rojas-Moscoso J, de Freitas NL, Mendes GD and De Nucci G (2019) Development, Validation of LC-MS/MS Method and Determination of Pharmacokinetic Parameters of the Stroke Neuroprotectant Neurounina-1 in Beagle Dog Plasma After Intravenous Administration. Front. Pharmacol. 10:432. doi: 10.3389/fphar.2019.00432
Received: 23 July 2018; Accepted: 04 April 2019;
Published: 25 April 2019.
Edited by:
Anna Rita Bilia, University of Florence, ItalyReviewed by:
Nasiara Karim, University of Malakand, PakistanCopyright © 2019 Severino, Corvino, Fiorino, Frecentese, Perissutti, Caliendo, Santagada, Magli, Molinaro, Pignataro, Annunziato, Antunes, Rojas-Moscoso, de Freitas, Mendes and De Nucci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Natalícia J. Antunes, bmF0YWxpY2lhamFAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.