- 1Departamento de Análises Clínicas, Toxicológicas e Bromatológicas, Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, Brazil
- 2Centro Universitário Barão de Mauá, Ribeirão Preto, Brazil
- 3Departamento de Física e Química, Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, Brazil
- 4Faculté de Pharmacie, Université de Montréal, Montréal, QC, Canada
Over 1 million cases of scorpion stings are estimated every year, whereas current treatment is limited to antivenom serum combined with supportive therapy. Tityus serrulatus scorpion venom (TsV) is composed of diverse molecules, including toxins that induce a catecholamine storm and mediate classical symptoms of scorpion envenomation. However, the same toxins promote an intense inflammatory response coordinated by innate immune cells, such as macrophages, contributing significantly to the lung edema and mortality caused by TsV injection. Macrophages sense TsV via innate immune receptors, including TLR2, TLR4, and CD14 that promote inflammation and mortality via PGE2/cAMP/PKA/NF-κB/IL-1β axis. The scavenger receptor CD36 also recognizes TsV, but in contrast to the other receptors, it drives the production of leukotriene B4 (LTB4). This lipid mediator operates via BLT1 receptor to reduce cAMP production and consequently IL-1β release, which results in resistance to fatal outcomes of experimental scorpion envenomation. EP80317 is an hexapeptide that serves as a ligand for CD36 and features protective effects under conditions such as atherosclerosis and vascular inflammation. In this study, we evaluated the effects of EP80317 treatment during experimental scorpion envenomation. EP80317 treatment suppressed mouse peritoneal macrophage production of IL-1β, IL-6, tumor necrosis factor (TNF-α), CCL3, and PGE2 in vitro. EP80317 treatment also boosted the production of LTB4 and IL-10 in response to TsV. Importantly, EP80317 restrained lung inflammation and mortality caused by TsV in vivo. Taken together, these data indicate a strong therapeutic potential of EP80317 as a supportive treatment to control inflammation induced by scorpion envenomation.
Introduction
Scorpion envenomation affects more than 1 million subjects every year (Chippaux and Goyffon, 2008; Isbister and Bawaskar, 2014). In Brazil, stings by the scorpion Tityus serrulatus contribute significantly for this scenario, whose venom (TsV) is composed by a cocktail of bioactive compounds (Pucca et al., 2015). In recent years, it became clear that beyond the classical neuroexcitatory syndrome, inflammation also underlies the pathology and mortality caused by TsV. We and others have demonstrated that crude TsV or isolated toxins (Ts1, Ts2, Ts6) induce the production of inflammatory mediators by innate immune cells, including cytokines, nitric oxide (NO), and bioactive lipids such as prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) (Petricevich, 2002; Petricevich and Lebrun, 2005; Petricevich et al., 2007; Zoccal et al., 2011, 2013). Macrophages sense TsV via TLR2, TLR4, or CD14, which control the release of PGE2 and interleukin-1β (IL-1β) (Zoccal et al., 2014, 2016, 2018b). PGE2 signals through EP2/4 receptors, increasing intracellular levels of cyclic adenosine monophosphate (cAMP). The cAMP-dependent protein kinase A (PKA) is activated and phosphorylates the nuclear factor-κB (NF-κB), potentiating IL-1β release (Zoccal et al., 2016). IL-1R signaling mediates activation and accumulation of neutrophils in the lung, edema, and eventually death in a mouse model of scorpion envenomation (Zoccal et al., 2016). In stark contrast to other receptors, CD36 promotes intracellular signaling that favors LTB4 release (Zoccal et al., 2018b). This eicosanoid reduces intracellular cAMP via BLT1 receptor and suppresses IL-1β production and mortality due to scorpion envenomation (Zoccal et al., 2016, 2018b). Of importance, molecular mechanisms governing responses to TsV in mouse models are strongly correlated with that of human cell responses (Zoccal et al., 2018b).
Recommended therapy is limited to antivenom serum, and depending on the severity, the serum is combined with supportive treatment such as prazosin, ionotropic agents, atropine, vasodilators, and benzodiazepines (Boyer et al., 2009; Isbister and Bawaskar, 2014). However, current therapeutic strategies do not account for tissue damage and mortality caused by TsV-induced inflammation (Zoccal et al., 2016). EP80317 is a synthetic analog of growth hormone-releasing peptides, which serves as a ligand for CD36 and exhibits cardioprotective, anti-atherosclerotic, hypocholesterolemic, and anticonvulsant effects (Marleau et al., 2005; Bujold et al., 2009, 2013; Harb et al., 2009; Bessi et al., 2012; Lucchi et al., 2017). EP80317 activates the peroxisome proliferator-activated receptor gamma (PPAR-γ) (Bujold et al., 2009), a nuclear receptor that regulates lipid bodies and lipid metabolism in response to TsV (Zoccal et al., 2015). In line with these findings, we investigated whether EP80317 limits TsV-mediated inflammation and mortality in a mouse model.
Materials and Methods
Tityus serrulatus Venom (TsV)
In accordance with the Brazilian Institute of Environment, T. serrulatus scorpions were maintained at the vivarium of the Ribeirão Preto Medical School, University of São Paulo. TsV was obtained by electrical stimulation, lyophilized, weighted, and stored at −80°C. Before experiments, TsV was diluted in PBS, filtered, and tested for LPS contamination as described (Zoccal et al., 2014, 2018b).
Mice and Experimental Settings in vivo
All experiments using animals were approved by the Comissão de Ética no Uso de Animais da Faculdade de Ciências Farmacêuticas de Ribeirão Preto-USP (protocol #14.1.272.53.7). Six- to eight-week-old C57BL/6 female or male mice were weighed before experiments under all conditions. Animals were injected with vehicle (PBS) or a lethal (180 μg/kg, i.p.) or excessive (super dose; 360 μg/kg, i.p.) dose of TsV (Zoccal et al., 2016). Following this, TsV-injected animals were treated with vehicle or EP80317 (0.289 μmol/kg, i.p.) at 0.5 and 2 h after the venom injection (Marleau et al., 2005; Bessi et al., 2012; Bujold et al., 2013). Mice were followed during 8 h and sacrificed upon signs of severe envenomation such as difficult of breathing, unusual head, neck and eye movement, and sweating. Euthanasia was performed with an overdose of chemical anesthetics (100 mg/kg ketamine and 10 mg/kg xylazine). In one set of animals, bronchoalveolar lavage fluid (BALF) was collected for total leukocyte and neutrophil counts. In another set of animals, lungs were removed, weighted, homogenized, and stored at −80°C.
Isolation and Treatment of Murine Peritoneal Macrophages
Resident peritoneal macrophages (PMs) were isolated from naïve C57BL/6 mice by injecting 3 ml of PBS into the abdominal cavity and massaged for 1 min. Peritoneal fluid then collected using a syringe with a needle inserted into the inguinal region as described previously (Zoccal et al., 2013). PMs (2 × 105 cells/well) were cultured at 37°C, 5% CO2 for 2 h. After this period, PMs were incubated with EP80317 (100 nM) or vehicle (PBS) for 2 h, followed by stimulation with TsV (50 μg/ml) for 24 h at 37°C, 5% CO2. Culture supernatants were collected and stored at −20°C for further analysis.
Quantification of Soluble Mediators and NF-κB Phosphorylation
Cell culture supernatants and lung homogenates were used to quantify IL-1β, IL-6, TNF-α, CCL3 (MIP-1α), and IL-10 using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, United States). Quantifications of LTB4 and PGE2 were performed by enzymatic immunoassays (Enzo Life Sciences, NY, United States), after lipid extraction and purification from cell culture supernatants or lung homogenates using Sep-Pak C18 cartridges (Thermo Fisher Scientific, Bellefonte, PA, United States), as described previously (Zoccal et al., 2018b). Total protein was quantified using Coomassie Protein Assay Reagent (Pierce Chemical, Rockford, IL, United States). Nitrite (NO2 −) was measured as an indicator of NO production by obtaining a standard curve using serial NaNO2 dilutions, as described (Zoccal et al., 2011). cAMP was quantified by ELISA, according to the manufacturer’s instructions (Enzo Life Sciences, Farmingdale, NY, United States), as described (Zoccal et al., 2018b). Cell cultures were lysed to measure phospho-NF-κB p65 (Ser536) and total NF-κB p65 using the PathScan Inflammation Multi-Target Sandwich ELISA kit (Cell Signaling, Danvers, MA, United States) as described (Zoccal et al., 2018a).
Statistical Analyses
Data were analyzed with GraphPad Prism 5.0 software (GraphPad, San Diego, CA, United States), using one-way ANOVA followed by Bonferroni’s multi-comparison test or log-rank test. Adjusted p values <0.05 were considered significant.
Results
EP80317 Suppresses TsV-Induced Inflammation and Mortality in vivo
EP80317 mediates protective effects via CD36 (Marleau et al., 2005; Bujold et al., 2009, 2013; Harb et al., 2009; Bessi et al., 2012; Lucchi et al., 2017), a scavenger receptor that drives eicosanoid metabolism toward LTB4 synthesis and represses inflammation and mortality caused by scorpion envenomation (Zoccal et al., 2016, 2018b). This suggests that binding of EP80317 to CD36 could modulate LTB4 metabolism and influence the outcome of scorpion envenomation. To test this hypothesis, C57BL/6 mice were injected with a lethal dose of TsV (180 μg/kg, i.p.) and treated with vehicle or EP80317 (0.289 μmol/kg, i.p.) at 0.5 and 2 h after the experimental envenomation. TsV injection in mice causes significant perturbations in the lung (Zoccal et al., 2016, 2018b), prompting for detailed analysis of inflammatory parameters in this organ. As expected, TsV induced an intense inflammatory response reflected by elevated lung weight (Figure 1A), total protein content (Figure 1B), accumulation of total leukocytes (Figure 1C), and neutrophils (Figure 1D), as by increased levels of IL-1β (Figure 1E), NO (Figure 1F), PGE2 (Figure 1G), and LTB4 (Figure 1H). Strikingly, treatment with EP80317 significantly impacted the inflammatory response, promoting further increase of LTB4 levels (Figure 1H) while reducing all other parameters (Figures 1A–G). These results suggest that EP80317 might restrain the inflammation-mediated mortality induced by TsV. To address this question, C57BL/6 mice were injected with a lethal (180 μg/kg, i.p.) or an excessive (super dose; 360 μg/kg, i.p.) dose of TsV and later treated with vehicle or EP80317 (0.289 μmol/kg, i.p.) at 0.5 and 2 h after the experimental envenomation. We observed 75% mortality of mice injected with a lethal dose of TsV, whereas 100% of mice survived after treatment with EP80317 (Figure 1I). Moreover, excessive TsV dose induced 100% of mortality, independently of EP80317 treatment (Figure 1J). However, non-treated mice died within 4 h of envenomation, while EP80317 significantly extended mice survival.
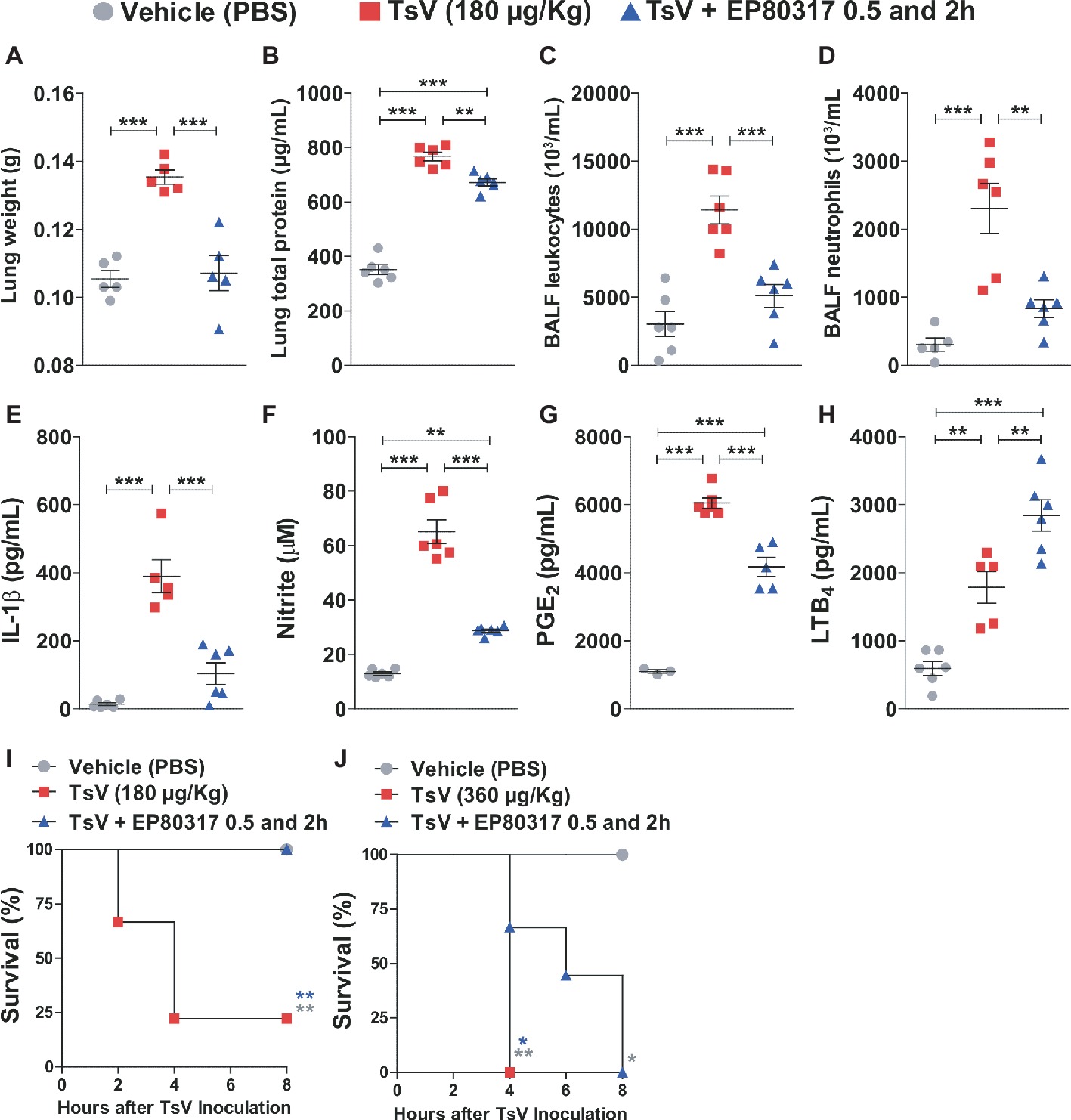
Figure 1. Treatment with EP80317 protects mice from scorpion envenomation. (A–H) C57BL/6 mice were injected with a lethal dose of TsV (180 μg/kg, i.p.) and treated with vehicle (PBS) or EP80317 (0.289 μmol/kg, i.p.) at 0.5 and 2 h after the venom injection. Lungs were removed immediately after death or at 8 h after venom injection. BALF was collected from a different set of animals under the same condition. (A) Lung weight, (B) Lung total protein concentration, (C) BALF total leukocyte counts, (D) BALF neutrophil counts, (E) IL-1β levels, (F) NO levels, (G) PGE2 levels, and (H) LTB4 levels. The experiment was conducted once with six mice per group. Differences were evaluated with one-way ANOVA followed by Bonferroni’s multi-comparison test. (I,J) C57BL/6 mice were injected with a (I) lethal (180 μg/kg, i.p.) or (J) excessive (superdose; 360 μg/kg, i.p.) dose of TsV and treated with vehicle (PBS) or EP80317 (0.289 μmol/kg, i.p.) 0.5 and 2 h after the venom injection. Survival was monitored for 8 h. The experiment was performed once with six mice per group, and the log-rank test was used to analyze significant differences. Data represent mean ± SDs, and significance is given by *p < 0.05, **p < 0.01, and ***p < 0.001.
EP80317 Modulates Inflammatory Pathways in Macrophages Activated by TsV
To determine the molecular mechanisms by which EP80317 restrains inflammation and mortality induced by TsV, peritoneal macrophages were isolated from C57BL/6 mice and incubated or not with EP80317 for 2 h. Following, macrophages were stimulated with TsV for 24 h in the presence of EP80317 throughout the challenge. Macrophages stimulated with TsV produced increased levels of the pro-inflammatory cytokines IL-1β (Figure 2A), IL-6 (Figure 2B), and TNF-α (Figure 2C); the chemokine CCL3 (Figure 2D); the second messenger cAMP (Figure 2E); and the bioactive lipids PGE2 (Figure 2F) and LTB4 (Figure 2G). Of note, exposure to EP80317 potentiated LTB4 synthesis (Figure 2G) and induced the production of the anti-inflammatory cytokine IL-10 (Figure 2H). At the same time, EP80317 abrogated the production of IL-1β (Figure 2A), IL-6 (Figure 2B), and cAMP (Figure 2E) and significantly reduced levels of TNF-α (Figure 2C), CCL3 (Figure 2D), and PGE2 (Figure 2F). The transcription factor NF-κB targets genes coding for these cytokines, chemokine, or even the rate-limiting enzyme involved in PGE2 synthesis, cyclooxygenase-2 (COX-2). This suggests that EP80317 might restrain the production of inflammatory mediators by suppressing NF-κB activity. As expected, exposure to EP80317 significantly reduced NF-κB phosphorylation induced by TsV stimulation (Figure 2I).
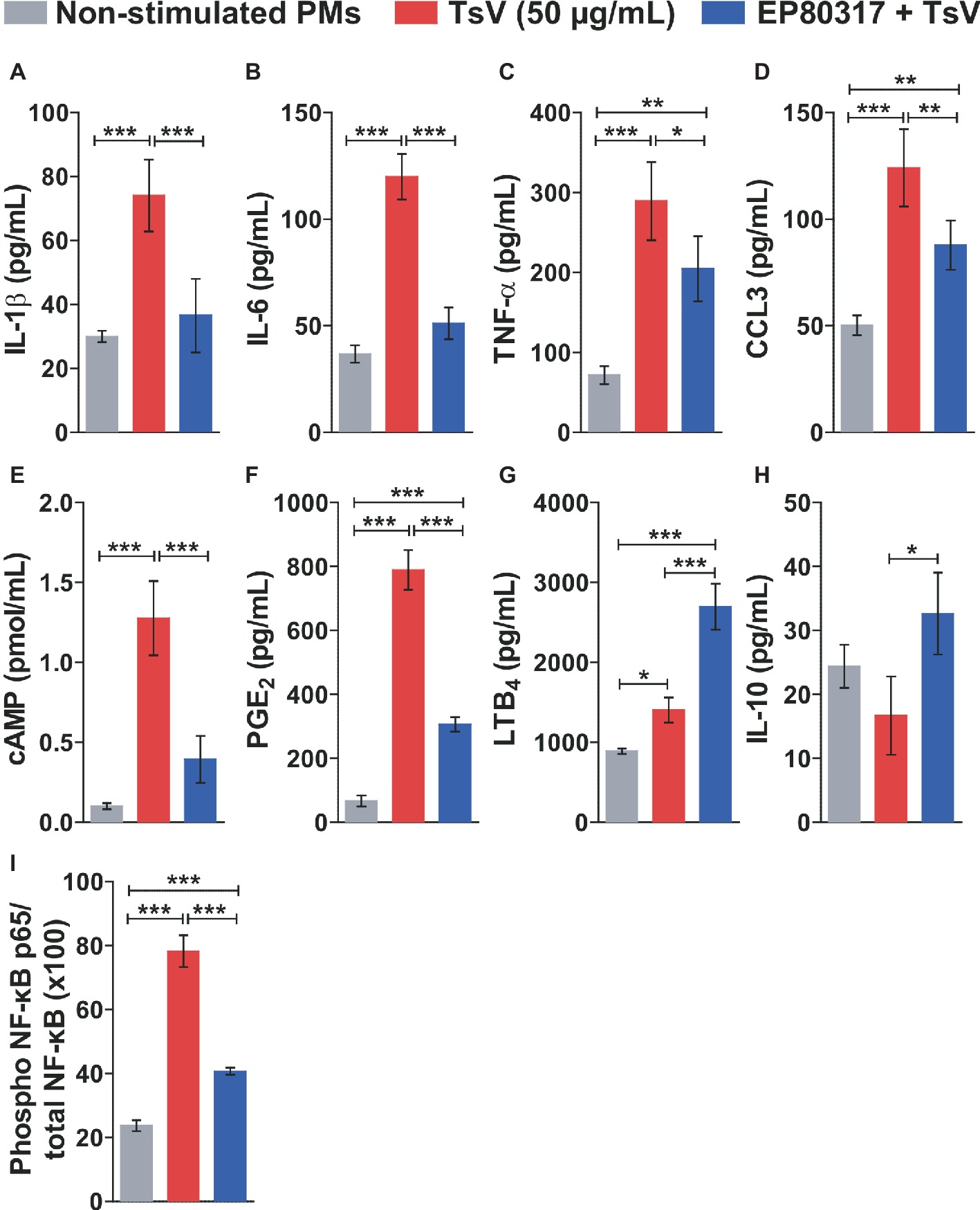
Figure 2. EP80317 inhibits NF-κB activation and production of inflammatory mediators in TsV-stimulated macrophages. (A–H) Peritoneal macrophages (PMs) were incubated or not with EP80317 (100 nM) for 2 h and later stimulated with TsV (50 μg/ml) for 24 h for quantification of cytokines/chemokine, or 5 min. for quantification of cAMP. (A) IL-1β, (B) IL-6, (C) TNF-α, (D) CCL3, (E) cAMP, (F) PGE2, (G) LTB4, and (H) IL-10. (I) PMs were pre-treated or not with EP80317 (100 nM) for 2 h and then stimulated with TsV (50 μg/ml). Cell lysates were obtained 2 h later for phospho-NF-κB p65 (Ser536) and total NF-κB p65 quantification. Data represent one of two independent experiments (n = 4 replicates). Differences were evaluated with one-way ANOVA followed by Bonferroni’s multi-comparison test. Data represent mean ± SDs, and significance is given by *p < 0.05, **p < 0.01, and ***p < 0.001.
Discussion
Here, we explored the therapeutic potential of the CD36 ligand, EP80317, for the control of inflammation-dependent mortality caused by scorpion envenomation. Overall, data point to a model of action in which EP80317 interacts with CD36 and dampens TsV-mediated inflammation via molecular mechanisms that are at least partially dependent on LTB4 production in vitro and in vivo. Signaling via BLT1 receptor inhibits cAMP synthesis and reduces the activation of PKA, consequently diminishing NF-κB phosphorylation and activation (Zoccal et al., 2016). This results in reduced levels of diverse inflammatory mediators, including IL-1β. EP80317 induces intracellular increase of 15-deoxy-delta(12,14)-prostaglandin J2 (15d-PGJ2) via COX-2 pathway in macrophages (Bujold et al., 2009). PPAR-γ negatively regulates NF-κB in macrophages stimulated with TsV (Zoccal et al., 2015), while 15d-PGJ2 activates this transcription factor and might contribute to reduced inflammation. PGE2 and the precursor of 15d-PGJ2, PGD2, exhibit opposite effects on macrophages (Pereira et al., 2018). Thus, PGD2 could also play a significant role in this process by downregulating cAMP levels via DP2 receptor and influence NF-κB activity. Taken together, these data provide important insights into operating molecular mechanisms and potential supportive therapy with EP80317. It also encourages further investigation during envenomation by other scorpions or even other poisonous animals, since similar molecular mechanisms take place upon stimulation of cells with venoms from two species of Bothrops snakes (Zoccal et al., 2018a).
Interestingly, CD36 activates the p130Cas-binding kinase Pyk2 in macrophages stimulated with oxidized phospholipid, whereas previous exposure to EP80317 inhibits Pyk2 phosphorylation (Harb et al., 2009). This correlated with reduced levels of plasma IL-6, as well as reduced expression of NADPH oxidase, inducible nitric oxide synthase and CCL2 in the vasculature of apolipoprotein E-deficient mice fed a high-fat, high-cholesterol diet, and treated daily with EP80317 (Harb et al., 2009). Although we have observed that EP80317 promotes LTB4 synthesis or that CD36 deficiency abrogates the production of this lipid mediator in response to TsV (Zoccal et al., 2018b), the signaling pathways leading to this phenomenon are unknown. Furthermore, we observed previously that CD36 deficiency increases IL-1β release by macrophages stimulated with TsV, but cytokines such as IL-6 and TNF-α were significantly reduced (Zoccal et al., 2018b). With exception of LTB4 and IL-10, treatment with EP80317 significantly reduced all the evaluated inflammatory parameters, suggesting additional anti-inflammatory mechanisms. Indeed, EP80317 could induce TLR heterodimer complex dissociation and suppress TLR-mediated signaling (Triantafilou et al., 2006; Stewart et al., 2010). However, TLR2 deficiency enhances PGE2 production by macrophages upon TsV stimulation (Zoccal et al., 2018b). Beneficial inhibition of the immune response during scorpion envenomation is further supported by the fact that dexamethasone, a potent immunosuppressive compound, completely abrogates IL-1β release by human peripheral blood mononuclear cells in response to TsV (Zoccal et al., 2018b). Collectively, these studies provide a proof of concept for the therapeutic potential of EP80317 during scorpion envenomation, involving multiple signaling pathways in mitigating inflammation induced by TsV.
Data Availability
All datasets generated for this study are included in the manuscript and/or the supplementary files.
Ethics Statement
All experiments using animals were approved by the Comissão de Ética no Uso de Animais da Faculdade de Ciências Farmacêuticas de Ribeirão Preto-USP (protocol 14.1.272.53.7) and carried out in accordance with the ethical principles for animal research adopted by the Sociedade Brasileira de Ciência em Animais de Laboratório.
Author Contributions
KZ designed and performed the experiments. KZ and LG analyzed the data and wrote the manuscript. EA and KB provided the scorpion venom. EA, KB, SM, and HO discussed the data. SM and HO provided the EP80317. LF conceived and supervised the study. All authors read and approved the final manuscript.
Funding
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grant 2014/07125-6 and EMU 2015/00658-1 to LF, 2014/03332-7 to KZ) and from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). LG is supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, process 1746212).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Alyne Fávero Galvão Meirelles, Caroline Fontanari, and Carlos Arterio Sorgi for technical support.
References
Bessi, V. L., Labbé, S. M., Huynh, D. N., Ménard, L., Jossart, C., Febbraio, M., et al. (2012). EP 80317, a selective CD36 ligand, shows cardioprotective effects against post-ischaemic myocardial damage in mice. Cardiovasc. Res. 96, 99–108. doi: 10.1093/cvr/cvs225
Boyer, L. V., Theodorou, A. A., Berg, R. A., Mallie, J., Chávez-Méndez, A., García-Ubbelohde, W., et al. (2009). Antivenom for critically Ill children with neurotoxicity from scorpion stings. N. Engl. J. Med. 360, 2090–2098. doi: 10.1056/NEJMoa0808455
Bujold, K., Mellal, K., Zoccal, K. F., Rhainds, D., Brissette, L., Febbraio, M., et al. (2013). EP 80317, a CD36 selective ligand, promotes reverse cholesterol transport in apolipoprotein E-deficient mice. Atherosclerosis 229, 408–414. doi: 10.1016/j.atherosclerosis.2013.05.031
Bujold, K., Rhainds, D., Jossart, C., Febbraio, M., Marleau, S., and Ong, H. (2009). CD36-mediated cholesterol efflux is associated with PPARgamma activation via a MAPK-dependent COX-2 pathway in macrophages. Cardiovasc. Res. 83, 457–464. doi: 10.1093/cvr/cvp118
Chippaux, J. -P., and Goyffon, M. (2008). Epidemiology of scorpionism: a global appraisal. Acta Trop. 107, 71–79. doi: 10.1016/j.actatropica.2008.05.021
Harb, D., Bujold, K., Febbraio, M., Sirois, M. G., Ong, H., and Marleau, S. (2009). The role of the scavenger receptor CD36 in regulating mononuclear phagocyte trafficking to atherosclerotic lesions and vascular inflammation. Cardiovasc. Res. 83, 42–51. doi: 10.1093/cvr/cvp081
Isbister, G. K., and Bawaskar, H. S. (2014). Scorpion envenomation. N. Engl. J. Med. 371, 457–463. doi: 10.1056/NEJMra1401108
Lucchi, C., Costa, A. M., Giordano, C., Curia, G., Piat, M., Leo, G., et al. (2017). Involvement of PPARγ in the anticonvulsant activity of EP-80317, a ghrelin receptor antagonist. Front. Pharmacol. 8:676. doi: 10.3389/fphar.2017.00676
Marleau, S., Harb, D., Bujold, K., Avallone, R., Iken, K., Wang, Y., et al. (2005). EP 80317, a ligand of the CD36 scavenger receptor, protects apolipoprotein E-deficient mice from developing atherosclerotic lesions. FASEB J. 19, 1869–1871. doi: 10.1096/fj.04-3253fje
Pereira, P. A. T., Assis, P. A., Prado, M. K. B., Ramos, S. G., Aronoff, D. M., de Paula-Silva, F. W. G., et al. (2018). Prostaglandins D2and E2have opposite effects on alveolar macrophages infected with Histoplasma capsulatum. J. Lipid Res. 59, 195–206. doi: 10.1194/jlr.M078162
Petricevich, V. L. (2002). Effect of Tityus serrulatus venom on cytokine production and the activity of murine macrophages. Mediat. Inflamm. 11, 23–31. doi: 10.1080/09629350210308
Petricevich, V. L., Hernández Cruz, A., Coronas, F. I. V., and Possani, L. D. (2007). Toxin gamma from Tityus serrulatus scorpion venom plays an essential role in immunomodulation of macrophages. Toxicon 50, 666–675. doi: 10.1016/j.toxicon.2007.06.001
Petricevich, V. L., and Lebrun, I. (2005). Immunomodulatory effects of the Tityus serrulatus venom on murine macrophage functions in vitro. Mediat. Inflamm. 2005, 39–49. doi: 10.1155/MI.2005.39
Pucca, M. B., Cerni, F. A., Pinheiro Junior, E. L., de Bordon, K. C. F., Amorim, F. G., Cordeiro, F. A., et al. (2015). Tityus serrulatus venom—A lethal cocktail. Toxicon 108, 272–284. doi: 10.1016/j.toxicon.2015.10.015
Stewart, C. R., Stuart, L. M., Wilkinson, K., van Gils, J. M., Deng, J., Halle, A., et al. (2010). CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 11, 155–161. doi: 10.1038/ni.1836
Triantafilou, M., Gamper, F. G. J., Haston, R. M., Mouratis, M. A., Morath, S., Hartung, T., et al. (2006). Membrane sorting of Toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J. Biol. Chem. 281, 31002–31011. doi: 10.1074/jbc.M602794200
Zoccal, K. F., da Bitencourt, C. S., Paula-Silva, F. W. G., Sorgi, C. A., de Castro Figueiredo Bordon, K., Arantes, E. C., et al. (2014). TLR2, TLR4 and CD14 recognize venom-associated molecular patterns from Tityus serrulatus to induce macrophage-derived inflammatory mediators. PLoS One 9:e88174. doi: 10.1371/journal.pone.0088174
Zoccal, K. F., da Bitencourt, C. S., Secatto, A., Sorgi, C. A., de Bordon, K. C. F., Sampaio, S. V., et al. (2011). Tityus serrulatus venom and toxins Ts1, Ts2 and Ts6 induce macrophage activation and production of immune mediators. Toxicon 57, 1101–1108. doi: 10.1016/j.toxicon.2011.04.017
Zoccal, K. F., da Bitencourt, C. S., Sorgi, C. A., de Bordon, K. C. F., Sampaio, S. V., Arantes, E. C., et al. (2013). Ts6 and Ts2 from Tityus serrulatus venom induce inflammation by mechanisms dependent on lipid mediators and cytokine production. Toxicon 61, 1–10. doi: 10.1016/j.toxicon.2012.10.002
Zoccal, K. F., Ferreira, G. Z., Prado, M. K. B., Gardinassi, L. G., Sampaio, S. V., and Faccioli, L. H. (2018a). LTB4 and PGE2 modulate the release of MIP-1α and IL-1β by cells stimulated with Bothrops snake venoms. Toxicon 150, 289–296. doi: 10.1016/j.toxicon.2018.06.066
Zoccal, K. F., Gardinassi, L. G., Sorgi, C. A., Meirelles, A. F. G., Bordon, K. C. F., Glezer, I., et al. (2018b). CD36 shunts eicosanoid metabolism to repress CD14 licensed interleukin-1β release and inflammation. Front. Immunol. 9:890. doi: 10.3389/fimmu.2018.00890
Zoccal, K. F., Paula-Silva, F. W. G., Bitencourt, C. d. S., Sorgi, C. A., de Bordon, K. C. F., Arantes, E. C., et al. (2015). PPAR-γ activation by Tityus serrulatus venom regulates lipid body formation and lipid mediator production. Toxicon 93, 90–97. doi: 10.1016/j.toxicon.2014.11.226
Keywords: scorpion envenomation, EP80317, inflammation, leukotriene B4, mortality
Citation: Zoccal KF, Gardinassi LG, Bordon KCF, Arantes EC, Marleau S, Ong H and Faccioli LH (2019) EP80317 Restrains Inflammation and Mortality Caused by Scorpion Envenomation in Mice. Front. Pharmacol. 10:171. doi: 10.3389/fphar.2019.00171
Edited by:
Orina Belton, University College Dublin, IrelandReviewed by:
Jiiang-Huei Jeng, National Taiwan University, TaiwanCarole L. Wilson, Medical University of South Carolina, United States
Copyright © 2019 Zoccal, Gardinassi, Bordon, Arantes, Marleau, Ong and Faccioli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lúcia H. Faccioli, ZmFjY2lvbGlAZmNmcnAudXNwLmJy
†These authors have contributed equally to this work
 Karina F. Zoccal
Karina F. Zoccal Luiz G. Gardinassi
Luiz G. Gardinassi Karla C. F. Bordon
Karla C. F. Bordon Eliane C. Arantes
Eliane C. Arantes Sylvie Marleau
Sylvie Marleau Huy Ong
Huy Ong Lúcia H. Faccioli
Lúcia H. Faccioli