
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 30 November 2018
Sec. Ethnopharmacology
Volume 9 - 2018 | https://doi.org/10.3389/fphar.2018.01413
 Haibo Cheng1†
Haibo Cheng1† Xiaoyin Ge2†
Xiaoyin Ge2† Shiqin Zhuo3
Shiqin Zhuo3 Yanan Gao2
Yanan Gao2 Bo Zhu2
Bo Zhu2 Junfeng Zhang2
Junfeng Zhang2 Wenbin Shang1
Wenbin Shang1 Dakang Xu4,5,6
Dakang Xu4,5,6 Weihong Ge3
Weihong Ge3 Liyun Shi2,5*
Liyun Shi2,5*The inhibitors for EGF receptor tyrosine kinase (EGFR-TKIs) such as gefitinib have been used as a standard treatment for non-small cell lung cancer (NSCLC), but the increasingly occurrence of drug resistance, the associated adverse effects and the enrichment of cancer stem cells significantly impedes its clinical application. β-elemene is a natural sesquiterpene with potent anti-cancer ability, and also it is renowned for its plant-origin, safety and the additive effect with traditional therapies, which prompt us to explore its potential to co-operate with TKIs to achieve greater therapeutic efficacy. Impressively, our study demonstrates that, elemene, in combination of gefitinib, displayed a significantly higher activity in inhibiting lung cancer cellular proliferation, migration and invasion. More importantly, combinative treatment profoundly impaired the epithelial to mesenchymal transition (EMT), the stem-like properties and the self-renewal capacity of lung cancer cells, and hence impeded the in vivo tumor development. We also reveal that the synergistic anti-tumor effect of elemene and gefitinib was largely mediated their regulation of enhancer of zeste homolog 2 (EZH2), an oncogenic histone methyltransferase and gene transcriptional regulator. Thus, our data indicate that combinative treatment of elemene and gefitinib has greater anti-neoplastic activity and greater efficacies in targeting cancer stem-like properties, mainly through regulating the malignant gene modifier and hence the subsequent effector molecules required for cancer progression. The findings may have potential implications for treating aggressive and resistant lung cancers.
Lung cancer is one of the most deadly and the most prevalent cancer worldwide, among which non-small cell lung cancer (NSCLC) accounts for approximately 85% (Chen Z. et al., 2014). In additional to the traditional treatments, such as surgery, radiotherapy and chemotherapy, the target therapy like the inhibitors for epidermal growth factor receptor-tyrosine kinase (EGFR-TKIs) has been approved as a standard first-line therapy in NSCLC patients with sensitive EGFR mutations (Maemondo et al., 2010). However, almost all patients responding to EGFR-TKIs would eventually develop drug resistance in 1 year despite the initial success, significantly impeding the therapeutic efficacy (Li et al., 2014). Moreover, accumulating evidences have indicated that chemotherapy or radiotherapy, if unable to kill cancer cells, would invariably cause the enrichment of cancer stem cells (CSCs) (Reya et al., 2001). CSCs is a small sub-population of cancer cells presumably derived from bulk tumors but adopting the stem-like traits, including the increased motility and invasiveness, the transition from epithelial to mesenchymal phenotypes, the resistance to anti-tumor drugs, and the tumor-initiating potential (Mani et al., 2008; Singh et al., 2010). The induction of stem-like cells is therefore considered as a risk factor for poor prognostics and cancer recurrence, and targeting CSCs become a novel promising therapeutic strategy (Li et al., 2017). In this regarding, combinative therapy that generally targets multiple key signaling molecules may have greater effect than the monotherapy in conquering cancer stem-like phenotypes (Morgillo et al., 2013; Wei et al., 2017).
Another major challenge facing the current cancer treatment is the high toxicity of chemotherapeutic drugs (van Meerbeeck et al., 2011). To achieve the optimal therapeutic efficacy, high doses and sustained application of chemo-drugs are required. This would invariably generate a series of side effects such as anemia, nausea, fatigue and hair loss, etc. Thus, it is of clinical relevance to find the agents that can be combined with conical TKIs or other chemotherapeutic drugs, allowing the drug use at lower dose without affecting their efficacy.
β-elemene (refer to elemene thereafter) is an active compound isolated from traditional Chinese herb Rhizoma Zedoariaem (Jiang et al., 2017). It has been approved to treat a wide spectrum of cancers including brain, breast, prostate, ovarian and lung cancers (Yao et al., 2008). Elemene has been demonstrated to exert a wide range of anti-tumor activities. It can inhibit cellular proliferation, induce progressive apoptosis, and suppress cellular invasion and metastasis (Zhang et al., 2012; Zhao et al., 2015). As a natural plant-derived agent, elemene is renowned for its safety, efficacy and less adverse effects, making it a rational candidate for co-treatment of cancer, In support of this concept, elemene has been shown to potentially reverse the chemo-resistance of cisplatin and oxaliplatin and increase drug accessibility and cellule cytotoxicity (Zhang et al., 2014; Li et al., 2016). Also, elemene was reported to enhance the effect of EGFR inhibitor in glioblastoma multiforme and reduce the resistance of gefitinib, although the exact mechanism remains largely elusive (Mu et al., 2016). Recently, the clinical trial of combination of elemene and EGFR-TKI for advanced EGFR-TKI-resistant NSCLC has been approved in China (ChiCTR-IPR-17012912 and NCT03123484), promoting further evaluation and mechanistic understanding of elemene to be as an adjuvant for the combinative therapy.
In this study, we show that the combination of elemene and gefitinib, both at lower dose, exerted the synergistic effect in inhibiting cellular proliferation, migration and invasion, enhancing the TKI sensitivity in lung cancer cells. Importantly, the combined treatment repressed cancer stem-like phenotypes, reversed the mesenchymal phenotypes and inhibited their sphere-forming ability, and consequentially, inhibited tumor development in xenograft mice. The therapeutic effect is believed to be associated with the down-regulation of the epigenetic modifier, enhancer of zeste homolog 2(EZH2) (Kim and Roberts, 2016; Wang et al., 2017). Thus, we propose that co-administration of elemene and gefitinib has remarkable antitumor potential and warrants further development as a promising therapeutic approach in treating drug-resistant NSCLC.
β-elemene (99.2% purity) was purchased from the Chinese National Institutes for Food and Drug Control, with a molecular formula of C15H24 and molecular weight of 204.35 (Supplementary Figure S1A). The reagent was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, United States) at 20 mg/ml as a stock solution. Gefitinib was provided by AstraZeneca (Gaithersburg, MD, United States). The antibodies for E-Cadherin (cat. 1702-1) and N-Cadherin (cat. 2447-1) were obtained from Abcam (Cambridge, MA, United States). The fluorescein-conjugated antibodies to CD24 (cat. 555428), CD44 (cat. 555478) and the isotype controls were from BD PharMingen (San Diego, CA, United States). EZH2 and PD-L1 antibodies were purchased from Cell Signaling. The EZH2 inhibitor GSK343 was obtained from Sigma-Aldrich. The plasmid pcDNA3.1-EZH2 was provided by the Public Protein/Plasmid Library (PPPL).This Primers used in the study were included in Supplementary Tabel 1.
Data were expressed as mean ± standard deviation (SD) and analyzed by Student’s t-test or by ANOVA with the Dunnet method. Differences were considered significant at p < 0.05.
Detailed methods are included in Supplementary Material.
Since high dose of anti-tumor agents is generally associated with high toxicity and severe advert effects, we sought to identify some combinative regimens that can take advantage of the synergistic effects of the agents at lower dose without affecting their efficacy. To this end, we initially applied a sublethal concentration of elemene and gefitinib to the two human cancer cell lines (Supplementary Figure S1B). It was revealed that the administration of elemene or gefitinib, at concentrations of 40 μg/ml or 5 μM, respectively, only exerted mild effects on cellular viability in both A549 and H1299 cells, the two cancer cell lines proved to be EGFR-TKI resistant (Shi et al., 2017). However, the combination of two agents caused a significant reduction in cellular viability in human lung cancer cells (Figure 1A). The addition of elemene appeared to profoundly increase gefitinib susceptibility and reduce the numbers of living cells following treatment (Figure 1B). Consistent with this, FACS analysis indicated that gefitinib, in combination with elemene, caused an elevation in cellular apoptosis in both A549 and H1299 cells (Figure 1C).
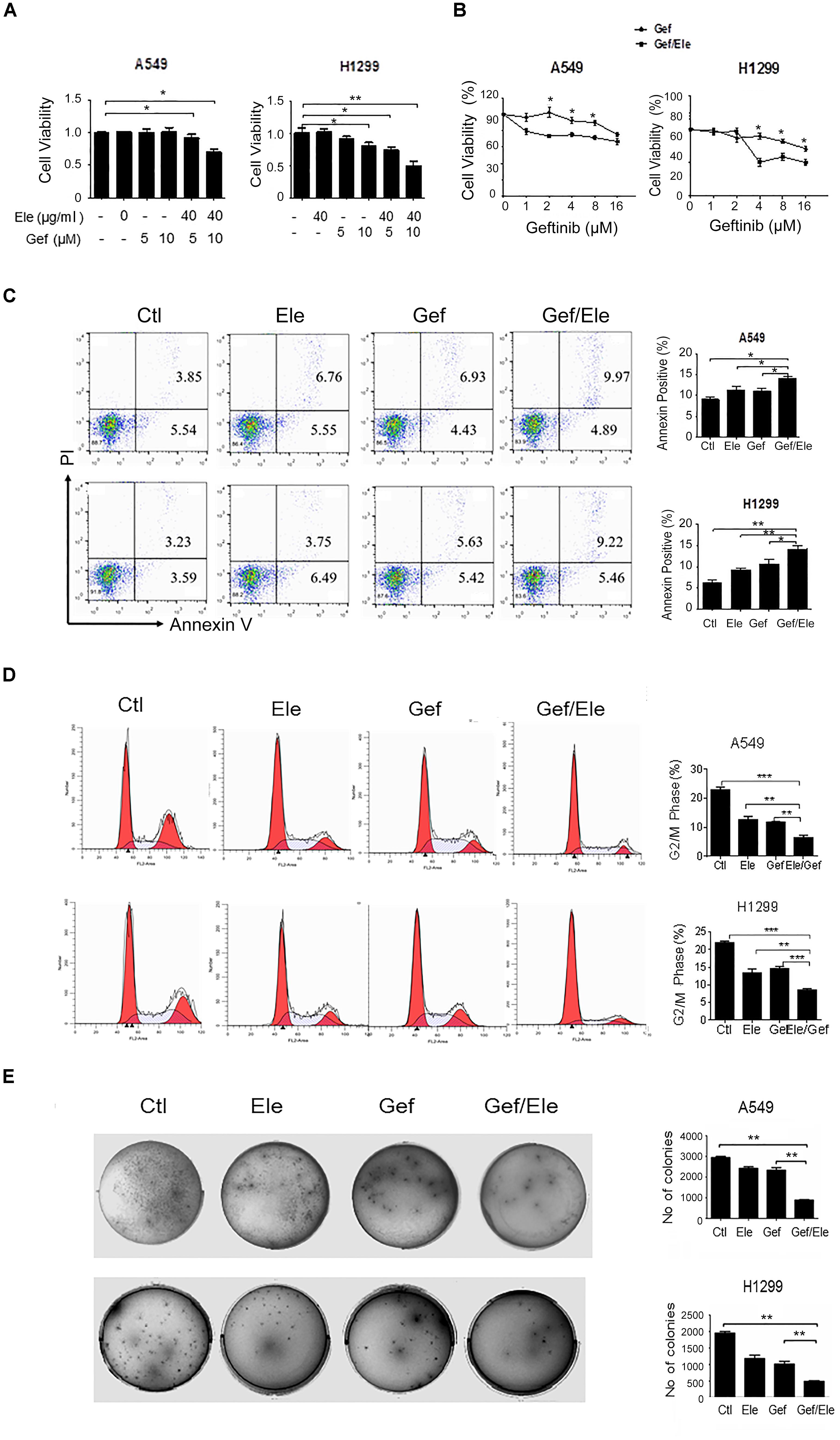
FIGURE 1. Elemene and gefitinib synergistically inhibit the viability and proliferation of lung cancer cells. (A,B) A549 and H1299 cells were treated with elemene, gefitinib or their combination at the indicated concentration; or cells were treated with various doses of gefitinib alone, or gefitinib in combination with elemene (40 μg/ml). Cellular viability was assessed by the MTS method 24 h post treatment. (C,D) A549 and H1299 cells were treated with elemene (40 μg/ml), gefitinib (5 μM) or their combination, respectively, for 24 h. Cellular apoptosis were detected with Annexin V/PI double staining followed by flow cytometry analysis, and cell cycles were detected upon PI staining. The representative images and the quantification data were shown. (E) A549 and H1299 cells were incubated with elemene (40 μg/ml), gefitinib (5 μM) or their combination for 14 days. Cellular colonigenic ability was examined by the colony-forming assay. Representative dishes and quantification of the colony numbers were shown. Data are representative of three independent experiments and presented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 by student t’s test.
We then assessed the effect of combinative treatment on cellular proliferation, and the cell cycles of cancer cells were analyzed firstly. The result showed that, compared with either single agent, combination of elemene with gefitinib caused a marked cycle arrest in A549 and H1299 cells, inhibiting cellular entry to G2/M phase (Figure 1D). More strikingly, the colony-forming ability of lung cancer cells were significantly reduced by co-treatment of elemene or gefitinib, compared with that upon either of single agent treatment (Figure 1E). Together, our data indicated that co-treatment of elemene and gefitinib had a synergistic effect in reducing cellular viability and proliferation in lung cancer.
Since cellular motility and invasion were closely related with cancer aggressiveness, we then explored the effect of the combination of elemene and gefitinib on cellular migration and invasiveness. By using the transwell invasion assays, we demonstrated that the invasive capacities of A549 and H1299 cells were significantly decreased upon co-treatment of elemene and gefitinib, compared with that upon either single agent treatment (Figures 2A,B). Likewise, elemene, in combination with gefitinib, displayed more profound effect in delaying wound closure in lung cancer cells, suggesting an additive effect of this co-treatment on cellular motility and migration (Figures 2C,D).
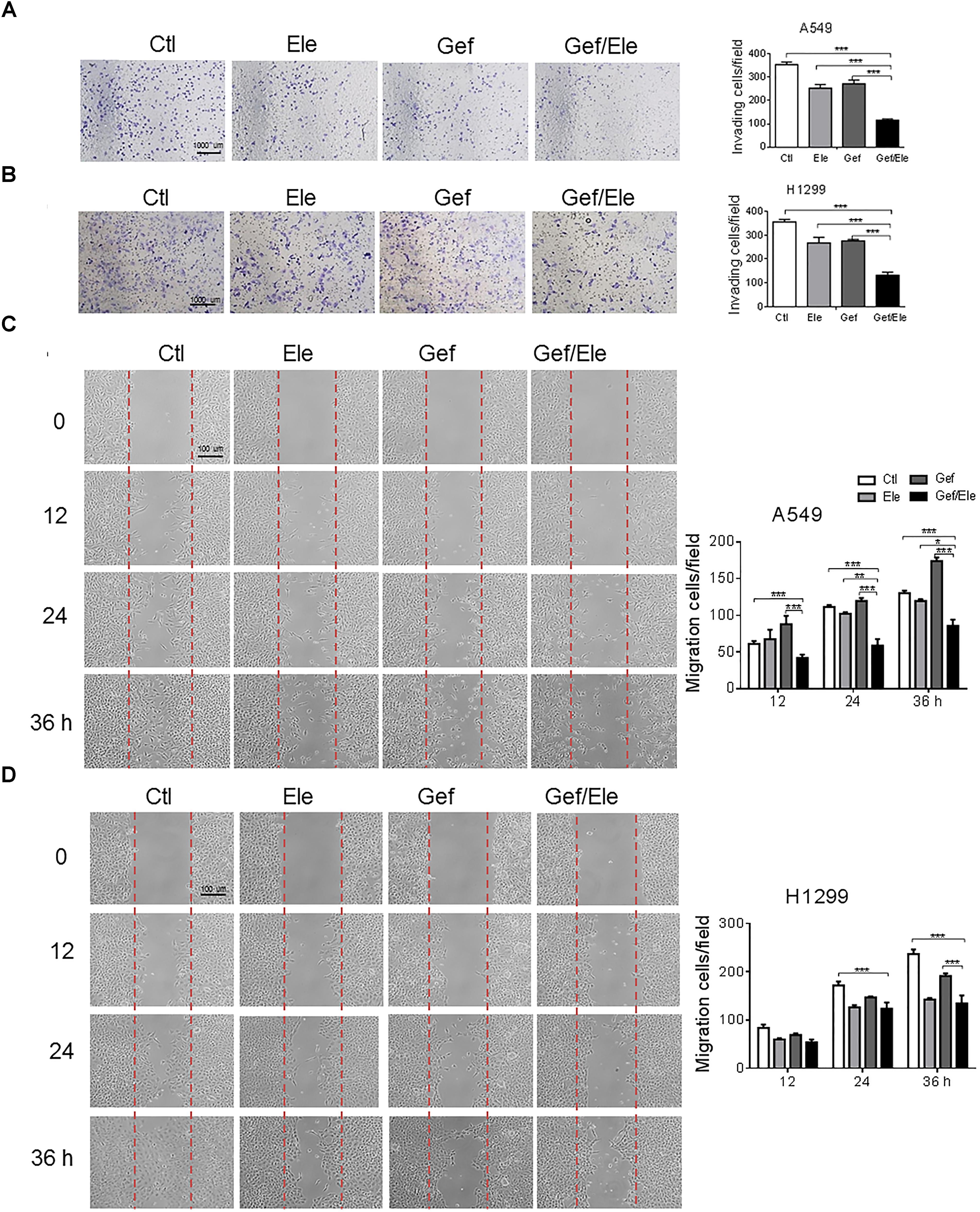
FIGURE 2. Gefitinib combined with elemene suppresses cellular invasion and migration. A549 and H1299 cells were incubated with elemene (40 μg/ml), gefitinib (5 μM) or their combination, respectively. (A,B) The invasive capacity of cells was detected by the Boyden chamber transwell assay. The mean numbers of cells in five fields per membrane were counted. (C,D) The cellular migratory capacity was analyzed by the wound healing assay. Shown are the representative photographs of scratched areas and cell migration indexes at 0, 12, 24, and 36 h post scratching. All the experiments were performed in triplicates. Data are presented as mean ± SD. ∗p < 0.05; ∗∗p < 0.01, ∗∗∗p < 0.001 by student t’s test.
Epithelial to mesenchymal transition (EMT) is a process during which cells undergo morphologic changes from epithelial to mesenchymal phenotype, allowing cells to invade and migrate and therefore contributing to cancer metastasis and progression (Garofalo et al., 2011; De Craene and Berx, 2013). As combination of elemene and gefitinib profoundly affected cellular invasion and migration in lung cancers, we wondered if the co-treatment involved in the modulation of EMT process. Clearly, our data showed that gefitinib, in combination with elemene, significantly increased the level of epithelial marker E-cadherin while suppressing the expression of mesenchymal markers (Wellner et al., 2009; Lamouille et al., 2014), in particular, ZEB1, Snail and Twist (Figures 3A,B). Immunofluorescence staining also showed that the expression of E-cadherin was enhanced while vimentin was repressed in both A549 and H1299 cells following co-treatment (Figures 3C,D). Thus, our data indicated that, compared with single agent treatment, combined therapy effectively reversed the transition from epithelial to mesenchymal phenotypes in lung cancer cells.
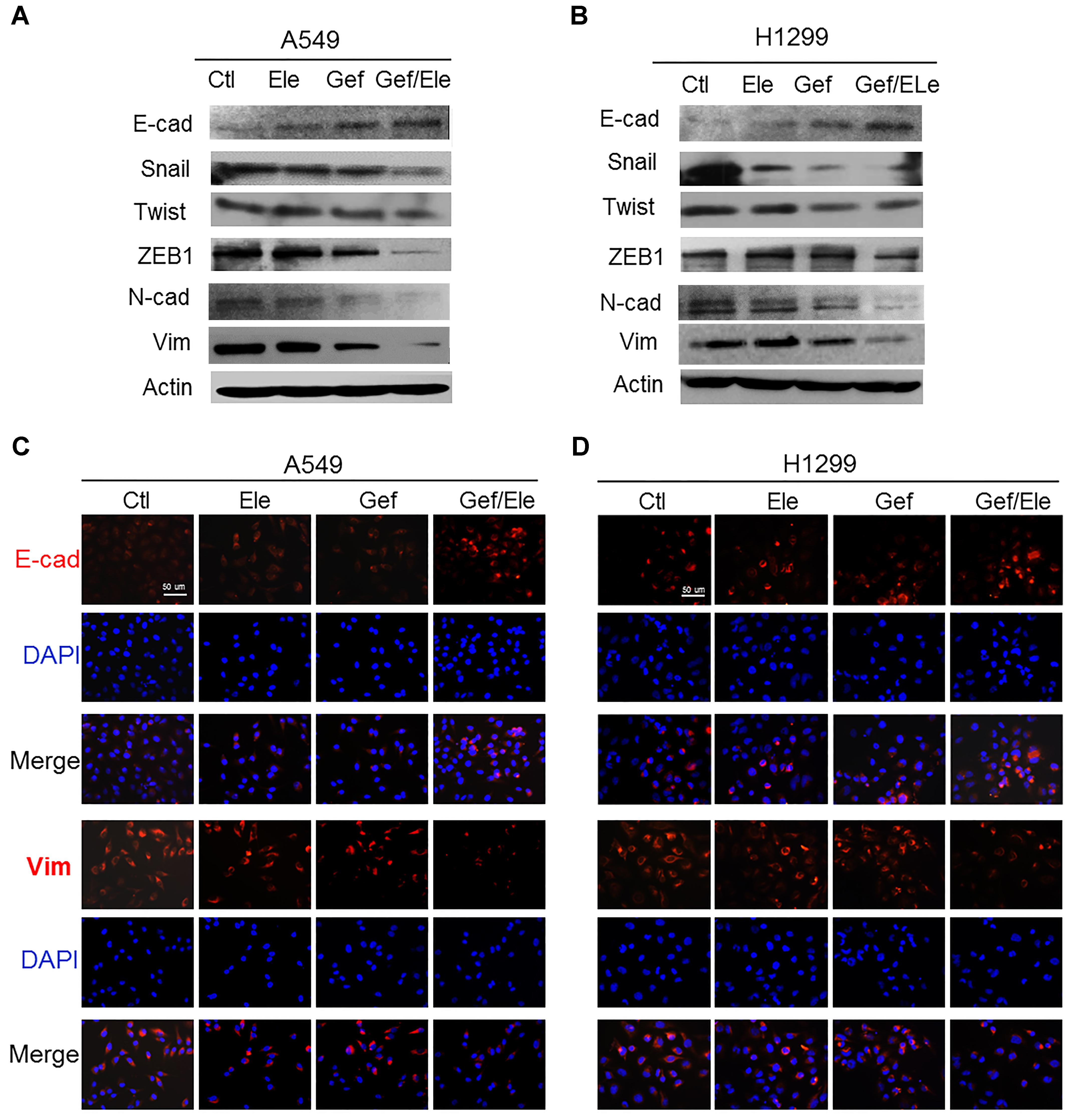
FIGURE 3. Gefitinib synergizes with elemene to reverse the EMT process in lung cancer cells. A549 and H1299 cells were treated, respectively, with elemene (40 μg/ml), gefitinib (5 μM) or their combination for 24 h. (A,B) Immunoblotting analysis of the representative epithelial and mesenchymal markers. (C,D) Immunofluorescence staining of E-cadherin (E-cad) and Vimentin (Vim). Nuclear DNA was stained with DAPI. Scale bars: 50 μm. Data are representative of three independent experiments.
Growing body of evidences have supported the critical link between EMT and cancer stemness. During cancer progression, the activation of EMT program renders cancer cells to acquire stem-like traits such as the self-renewal ability, the expression of stemness markers, the chemo-resistance and the in vivo tumorigenic potential (Chen W.J. et al., 2014). Remarkably, our data indicated that compared with either single agent, co-treatment of elemene and gefitinib caused a marked decrease in the expression of stem-related genes including sonic hedgehog (SHH), hairy and enhancer of split (HES)1, NOTCH1, c-Myc, SRY-box (SOX)2 (though to a less extent) and octamer-binding transcription factor (OCT)4 in lung cancer cells (Figures 4A,B). As a result, elemene and gefitinib synergized to impair the self-renewal ability of lung cells, as evidenced by the reduced formation of mammospheres, both in size and qualities, in A549 and H1299 cells following treatment (Figures 4C,D). Since tumor spheres were primarily composed of CSCs, this finding suggested a profound effect of the combinative treatment on CSCs. Next, we assessed the ratio of CD44+CD24-/low cells, the subpopulation that have been demonstrated to adopt stem-like phenotypes and tumor-initiating potential (Shien et al., 2013; Chao et al., 2014). The result showed that gefitinib synergizing with elemene generated a lower percent of CD44+CD24-/low populations among A549 or H1299 cells than either single agent (Figure 4E). Interestingly, we noted that gefitinib treatment caused an increase rather than a decrease in CD44+CD24-/low population, implying that traditional TKI might induce the enrichment of the drug-resistant stem/progenitor cell populations. Additionally, we examined the activity of aldehyde dehydrogenase (ALDH), a class of detoxifying enzymes required for cancer chemo-resistance and aggressiveness (Ginestier et al., 2007), in cancer cells following treatment. It was revealed that, elemene, in combination with gefitinib, synergistically reduced the ALDHhigh cell population in A549 and H1299 cells while the single agent only had mild effect (Figure 4F). Taken together, our data indicated that co-administration of elemene and gefitinib had a suppressive effect on cancer stem-like properties and reversed the malignant progression in lung cancer.
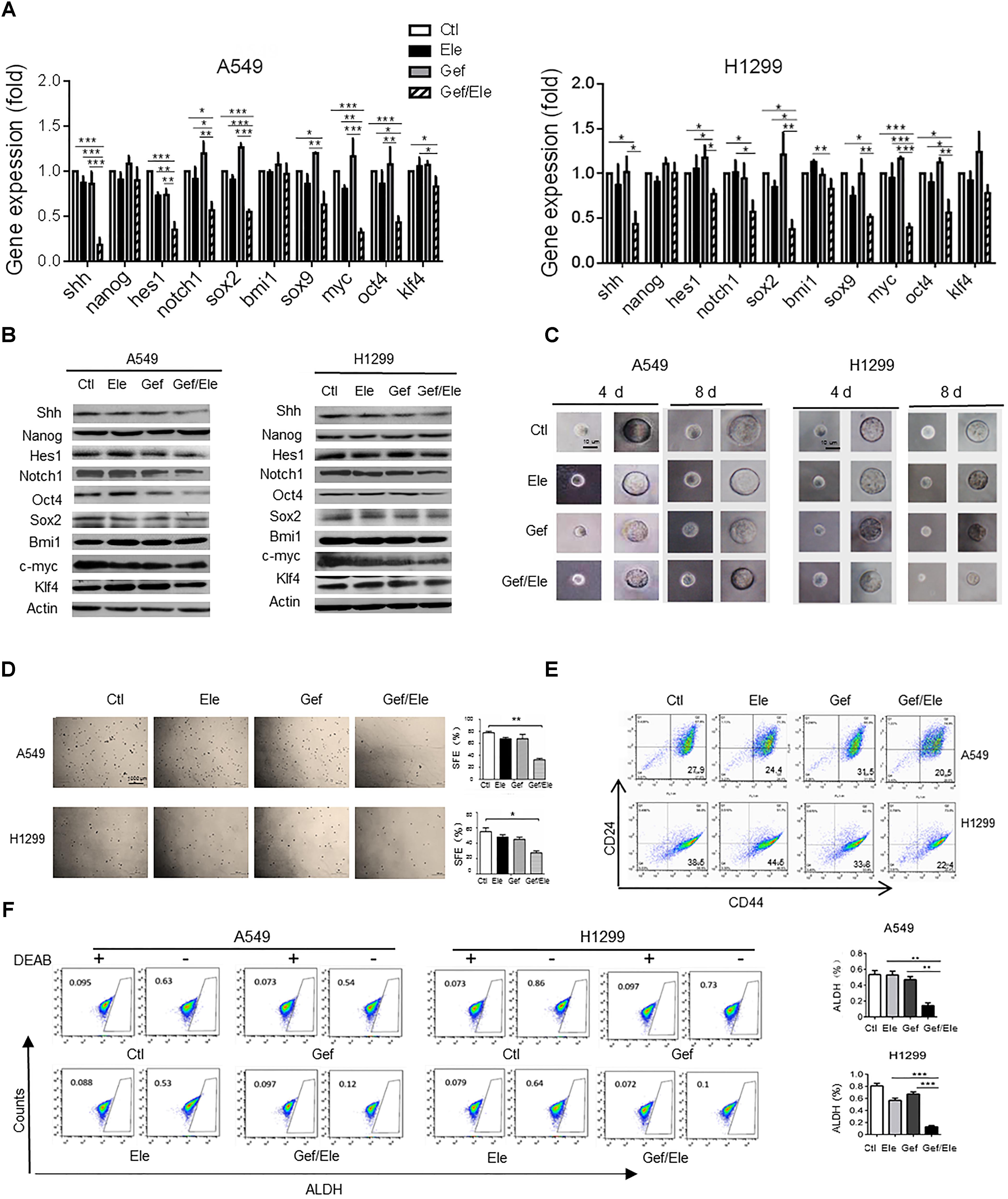
FIGURE 4. Gefitinib and elemene cooperate to repress stem-like properties of lung cancer cells. A549 and H1299 cells were treated with elemene (40 μg/ml), gefitinib (5 μM) or their combination for 24 h. (A,B) The relative mRNA and the protein levels of stem-associated genes were examined. (C,D) The cellular self-renewal capacity was assessed by the spheroid formation assay. Representative phase contrast images of self-renewal tumorspheres (C) and representative dishes of tumorspheres and quantification of primary spheres (D) were shown. Sphere-forming efficiency (SFE) was calculated as described in the section “Materials and Methods.” (E,F) The CD44+CD24- or the ALDHHigh population in A549 or H1299 cells was assessed by flow cytometry. Diethylaminobenzaldehyde (DEAB) was used as a negative control. Images are representative of three independent experiments. Data are presented as mean ± SD. ∗p < 0.05; ∗∗p < 0.01, ∗∗∗p < 0.001 by student t’s test.
Our above data showed that combination of elemene and gefitinib substantially influenced a spectrum of malignant behaviors in lung cancer cells, including cellular growth, survival, motility, invasion, and particularly, cellular EMT process and stemness-like properties. Given the multiple roles of the co-treatment involved, we speculated that the combinative therapy would operate at the upstream of the regulatory pathway that governed a panel of genes required for cancer development and progression. EZH2 is a H3K27 methyltransferase that has been recognized as a gene transcriptional regulator and a cancer driver, mainly through its ability to epigenetically and globally modify tumor-related genes (Kim and Roberts, 2016; Wang et al., 2017). Notably, our data demonstrated that compared with either of the single agent, combination of elemene and gefitinib caused a marked decrease in EZH2 levels both in A549 and H1299 cells (Figures 5A,B). Furthermore, the administration of lung cancer cells with GSK343, a well-established EZH2 inhibitor, led to a significant reduction in cellular motility and migration, indicating a functional relevance of EZH2 in our system (Supplementary Figure S2). To further understand its action mode in tumor treatment, we next introduced the EZH2-expressing plasmids into lung cancer cells following treatment. The result showed that enforced expression of EZH2 substantially reversed the inhibitory effects of combined therapy on the key malignant features such as cellular migratory ability and the colony-forming potential of cancer cells (Figures 5C–F). Moreover, the resumption of EZH2 led to an elevation in tumorsphere formation efficiency both in A549 and H1299 cells, indicating that EZH2 was critically involved in the stem-targeting effect of combinative therapy (Figures 5G,H). Additionally, we noted that the gefitinib/elemene-induced repression of ZEB1, a key mesenchymal marker gene (Chen L. et al., 2014), was increased upon EZH2 overexpression. Accompanied with this, the level of PD-L1, a prototypic checkpoint molecule essential for cancer immune evasion (Akbay et al., 2013), was also enhanced (Figures 5I,J). This implied that combinative therapy might also impact host anti-tumor immune response in a EZH2/PD-L1-dependent manner. Collectively, we herein provided the compelling evidences to show that combination of elemene and gefitinib had a greater anti-tumor activity, which was largely through its regulation of EZH2.
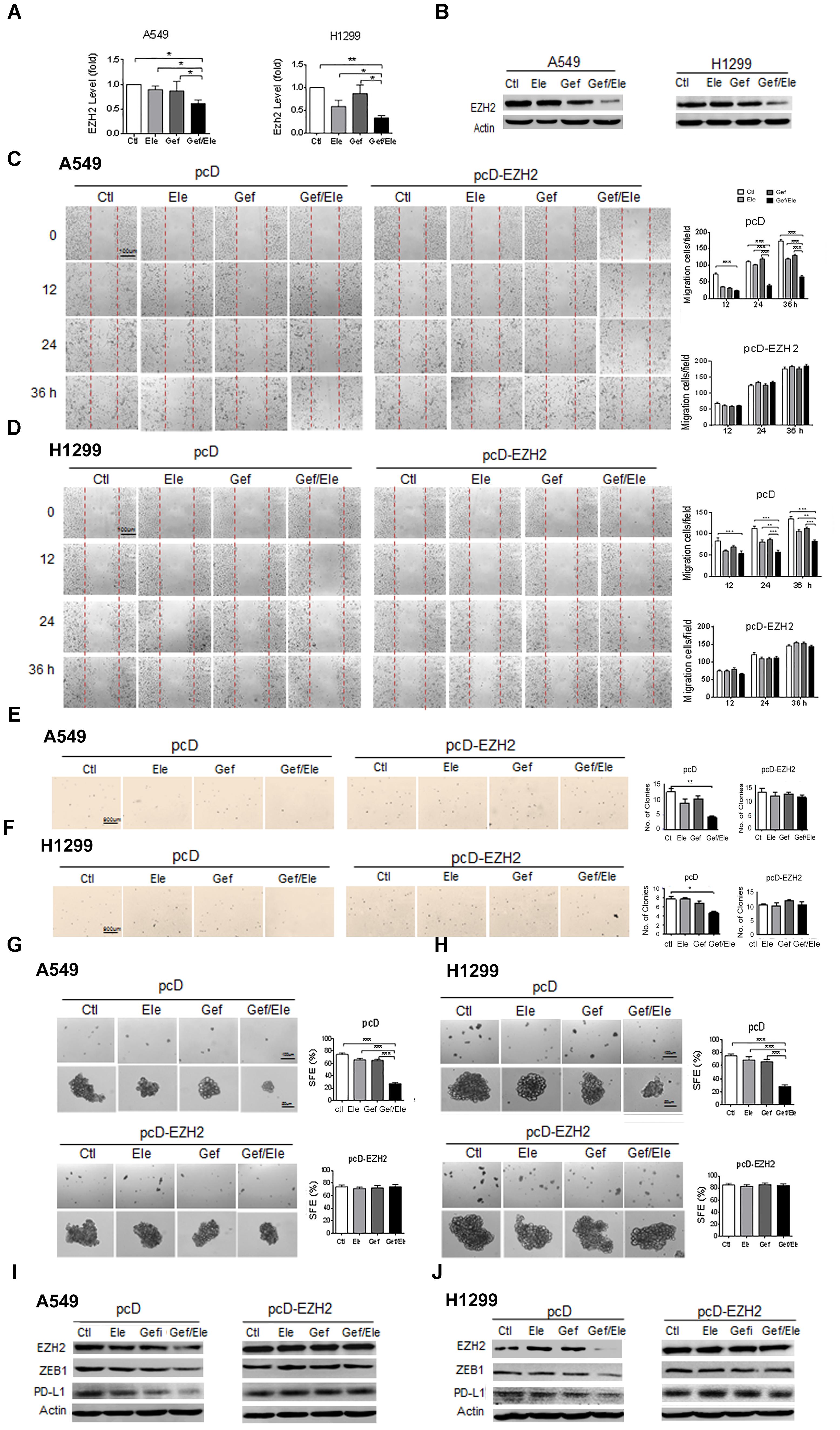
FIGURE 5. The synergistic anti-tumor effect of elemene and gefitinib is largely dependent on the regulation of EZH2. (A,B) The expression of EZH2 was detected by qPCR or the immunoblotting in A549 or H1299 cells treated with elemene (40 μg/ml), gefitinib (5 μM) or the combination treatment. (C–J) A549 and H1299 cells were transfected with EZH2-expressing or the control plasmids for 48 h, followed by the treatment of elemene (40 μg/ml), gefitinib (5 μM) or their combination. Cellular migratory capacity was examined by the scratching assay (C,D). The colony formation efficiency was tested at soft agar (E,F); and cellular self-renewal ability was analyzed by the sphere-forming assay (G,H). The levels of EZH2, PD-L1 and ZEB1 were examined by immune-blotting (I,J). Data from three independent experiments are presented as mean ± SD. ∗p < 0.05; ∗∗p < 0.01 by student t’s test.
To evaluate the in vivo importance of combinatorial therapy, the A549 subcutaneous xenografts were generated in nude mice. Tumor-bearing animals were randomly divided into 4 groups when tumors became palpable, followed by the treatment of elemene, gefitinib or the combination, respectively. Consequentially, combination therapy resulted in a profound tumor regression in xenograft mice (82% decrease, as detected by tumor volume) while the individual treatment caused much weaker effects (about 48% decrease by gefitinib and 59% decrease by elemene) (Figures 6A,B). The analyses on tumor weights confirmed that tumor weight was more significantly reduced by drug combination compared with that upon single agent treatment (Figure 6C). Associated with this, tumor proliferation, as assessed by Ki-67 staining, was more profoundly inhibited by combined therapy compared with single agent treatment. Notably, the level of EZH2, concurrent with PD-L1, was repressed by co-treatment of elemene and gefitinib (Figure 6D). The finding was consistent with the in vitro observation we described above (Figures 5I,J). Interestingly, we noted that the levels of CXCL9 and CXCL10, the two conical chemokines required for efficient T cells infiltration, were remarkably up-regulated in mice receiving combined treatment that receiving single agent treatment (Figure 6E). The finding was congruent with the recent report that Th1 chemokines were susceptible to the EZH2-mediated epigenetic regulation (Peng et al., 2015) and the co-treatment likely caused the de-repression of chemokines from EZH2-dependent restraint by suppressing its expression. Consistently, we observed that the numbers of CD4+ and CD8+ T cells were increased in the gefitinib/elemene-treated mice (Supplementary Figure S3). Taken together, gefitinib, in combination with elemene, potentially repressed lung cancer development in xenograft mice, exhibiting its therapeutic value in devastating NSCLCs.
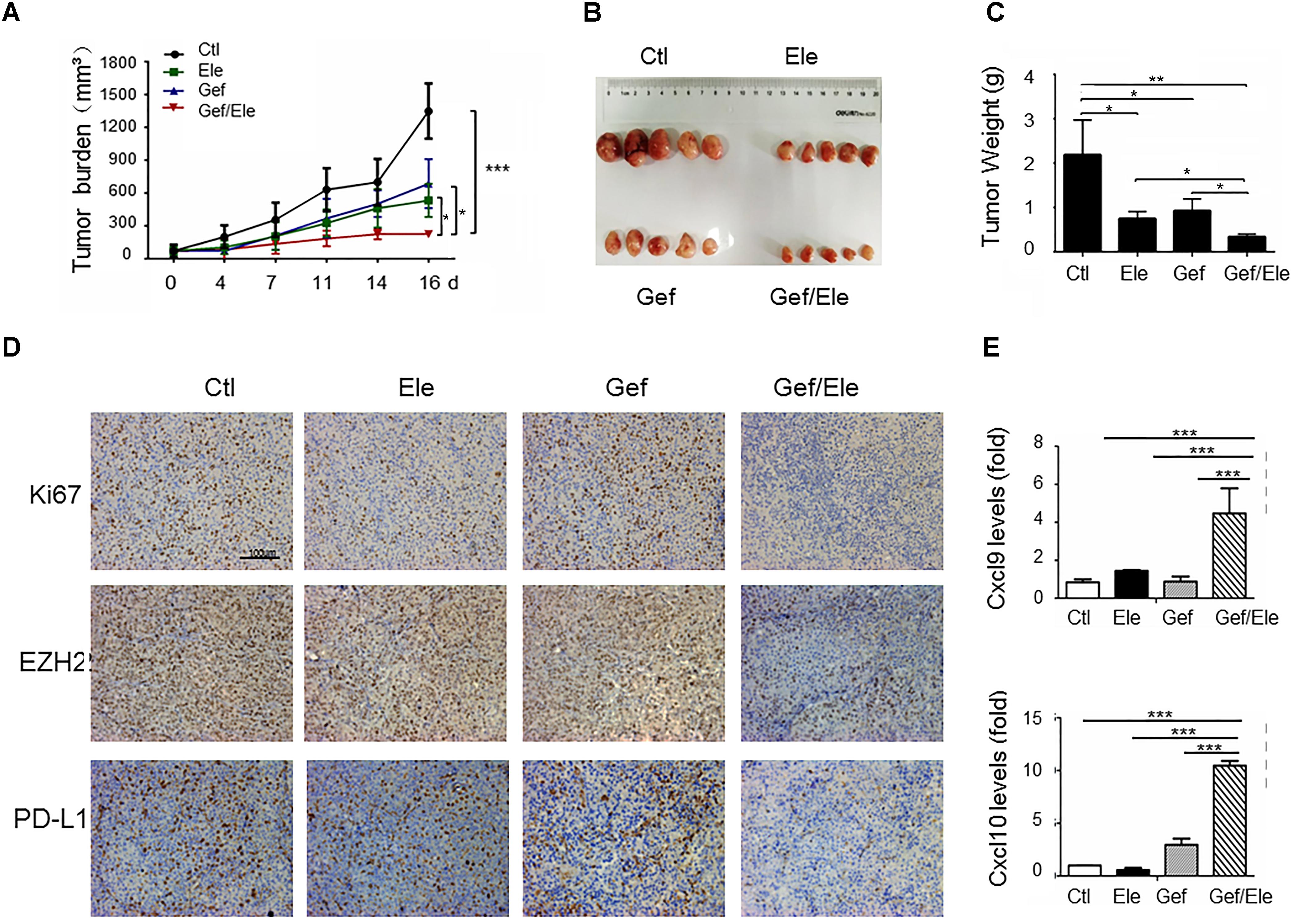
FIGURE 6. Gefitinib combines with elemene to retard tumor growth in xenograft mice. Nude mice (n = 5 each group) were subcutaneously (s.c.) with A549 cells (5 × 106/mice) for 10 days, and then divided into four groups to receive the treatment of vehicle, elemene (40 mg/kg), gefitinib (5 mg/kg) or their combination, respectively. (A) Tumor volume (mm3) was measured twice weekly. (B) Macroscopic appearance of the tumors and (C) tumor weight at 16 d post tumor transplantation. (D) IHC staining of Ki67, EZH2 and PD-L1 on A549 tumor xenografts. (E) qPCR assay of the relative levels of CXCL9 and CXCL10 at tumors. Data are presented as mean ± SD. ∗p < 0.05; ∗∗p < 0.01, ∗∗∗p < 0.001 by student t’s test.
Lung cancer is one of the most prevalent and most deadly cancers worldwide. EGFR-TKI have been used as a first-line treatment against lung cancer, but the increasing occurrence of drug resistance and the associated adverse sides precludes it widespread application (Maemondo et al., 2010; van Meerbeeck et al., 2011; Chen Z. et al., 2014). Elemene is a novel anticancer drug known for its broad anti-tumor effects and lower adverse effects both in vitro and in vivo (Yao et al., 2008; Zhang et al., 2012, 2014; Zhao et al., 2015; Li et al., 2016; Jiang et al., 2017). It has been authorized to treat a panel of solid tumors including lung cancer, but its potential to be as an adjunctive agent for the current standard anti-tumor drug is yet to be explored (Zhang et al., 2012). In the present study, we for the first time demonstrated that administration of elemene significantly enhanced cellular responses to the canonical EGFR-TKI gefitinib, inhibiting cellular proliferation and inducing progressive apoptosis in lung cancer cells. Moreover, combination of elemene and gefitinib suppressed stem-like features in NSCLC cell lines, and reduced tumorigenic potential in xenograft mice. This anti-tumor capability was partially at least, through the disruption of EZH2-dependent oncogenic pathway. We have thus revealed a novel combinative strategy that displays a greater anti-oncogenic activity, and more importantly, it shows a great promise to overcome drug resistance and improve the current treatment for devastating lung cancer.
Evidences have shown that cancer development is a multi-step process that disturbs multiple signaling pathways essential for normal cell activity. The aberrant activation of the tumorigenic pathway like EGFR-driven signaling is frequently intertwined with other oncogenic pathways, such as the signaling driven by VEGF, RAS and PI3-K/Akt (Martinelli et al., 2010; Seguin et al., 2014). In this sense, combinative treatment that simultaneously targets the key signaling molecules would outperform a single agent to fight against cancer cells (Vujic et al., 2015). In our current study, the combination of elemene with gefitinib showed greater effect in restraining cellular proliferation, survival, invasion and migration, the core malignancies associated with EGFR signaling. Our data showed that the addition of elemene boosted cellular response to the conical TKI both in A549 and H1299 cells, the cell lines proved to be refractory to TKI therapy. This finding is of critical clinic implication since drug resistance has become a bottleneck issue for cancer therapy. Combined treatment of elemene and gefitinib may offer a novel strategy for the resistant lung cancer.
Previously, the “synergistic” strategies were primarily focused on the induction of cellular death and tumor eradication. However, the unselective cytotoxicity of the agents would invariably generate serious adverse effects and cause the treatment intolerance among patients, which would eventually come to therapy cession or suspension (Zhou et al., 2009; Sequist et al., 2011). Furthermore, accumulating data demonstrated that cancer treatment would give rise to a minor subpopulation of cells within tumors, called CSCs or cancer initiating cells (CICs) (Chen et al., 2012; Chen Z. et al., 2014). These residual post-treatment populations are relatively resistant to cytotoxic chemo-and radio-therapies and adopt more aggressive phenotypes, contributing to cancer metastasis and recurrent. Based on these two critical issues associated with cancer therapy, we firstly lowered the concentration of elemene and gefitinib to such an extent that either of them exerted only mild cytotoxic effect on lung cancer cells, but the combinative treatment showed greater anti-tumor activity and enhanced TKI sensitivity. Secondly, given the importance of CSCs in tumor initiation and progression, targeting cancer stemness becomes a primary goal for our combinative strategy. As shown in our data, gefitinib, in combination with elemene, significantly repressed cancer EMT process, inhibited the expression of stemness-associated genes, and reduced cellular self-renewal potential. Along with this, the enrichment of CD44+CD24- or ALDHHigh cell populations was reduced by the co-treatment in A549 and H1299 cells. Since the EMT program is closely associated with invasive and migratory capacity of cancer cells, and the enrichment of CD44+CD24- or ALDHHigh cells is thought to be a major event prerequisite for tumor initiation and chemo-resistance, the finding about the profound effect on stem-related features may open a new perspective on combinative treatment. The dual effect of the co-treatment, both enhancing TKI responsiveness and preventing TKI-induced stem-like progression, may make it an ideal strategy to optimize the treat efficacy and minimize the side-effect profiles (Hirsch et al., 2009; Lau et al., 2014; Van Roosbroeck et al., 2017).
In a sought to identify the mechanism responsible for the synergistic effect of the combined treatment, our data demonstrated a striking reduction of EZH2, the epigenetic regulator as well as a cancer driver, by the combinative treatment. EZH2 is a histone methyl transferase subunit of polycomb repressive complex 2 (PRC2) that is proposed to remold chromatin configure and hence modulate gene expression profile (Souroullas et al., 2016). Evidences have demonstrated that EZH2 is capable of reprogramming the gene program essential for the proliferation, stemness and metastasis of cancer cells (Kim and Roberts, 2016; Wang et al., 2017). It has been proved to highly express in a wide variety of human cancers and is regarded as a diagnostic and prognostic marker for poor outcome (Behrens et al., 2013). A recent study using a genetically engineered mouse model confirmed the oncogenic effect of EZH2 and showed that overexpression of EZH2 resulted in aberrant spread of H3K27me3 at the known tumor suppressors in lung cancer, thus pointing to EZH2 as a pressing target for cancer therapy (Zhang et al., 2016). Consistent with our current study, EZH2 inhibitors have been able to repress tumorigenesis and malignant progression. Notably, our data showed that enforced expression of EZH2 largely abrogated the anti-tumor effect of the combination of elemene and gefitinib, indicating that EZH2-dependent mechanism is critically involved in the action of combinative treatment. Moreover, since much effort has been invested to develop drug-like EZH2 inhibitors for cancer treatment, our finding about the EZH2-targeting co-treatment may offer an efficient and relatively safe strategy for cancer treatment (Kim and Roberts, 2016; Jin et al., 2017).
Additionally, our data also showed that combination of elemene and gefitinib caused a marked decrease in PD-L1 in vitro and in vivo, in parallel to the reduction of EZH2. Ectopic expression of EZH2 abolished the inhibitory effect of co-treatment and elevated PD-L1 level, implying that EZH2 had an essential role in promoting PD-L1 expression. This observation is congruent with the very recent report showing a correlation between EZH2 and PD-L1 level on cancer cells (Zingg et al., 2017). Also, we found that the expression of Th1-type chemokines CXCL9 and CXCL10, the molecules presumably subjected to EZH2-dependent epigenetic modification (Martinelli et al., 2010; Nagarsheth et al., 2016), was up-regulated upon combinative therapy. Since CXCL9 and CXCL10 constitute two major molecules for effector T cells infiltration, it is speculated that combination of elemene and gefitinib might have an additional effect on enhancing anti-tumor T cell response. Further studies might be needed to explore the potential role for combinative therapy in enhancing anti-cancer immunity.
Taken together, the combination of gefitinib and elemene exhibits the remarkably enhanced anti-cancer activity, with potential to overcome EGFR-TKI resistance, to inhibit cancer stemness and to repress cancer development capability in xenograft mice. We thus propose that this combinative therapy may become a novel promising strategy for lung (and other potentially) cancer treatment.
All of the animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and with the approval of Animal Care and Use Committee of Nanjing University of Chinese Medicine.
LS designed the study. HC, XG, SZ, YG, and JZ performed the experiments and analyzed the data. YG, BZ, and LS wrote the manuscript. WS and DX contributed to the experimental material and provided the insightful suggestions. LS and WG supervised the program.
This work was supported by National Natural Scientific Funds (81270066, 81470210, and 81770014) and National Key Research and Development Program Project (2012CB911200).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer EL and handling Editor declared their shared affiliation at the time of review.
We would like to thank Dr. Abbas for his editing of the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2018.01413/full#supplementary-material
Akbay, E. A., Koyama, S., Carretero, J., Altabef, A., Tchaicha, J. H., Christensen, C. L., et al. (2013). Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 3, 1355–1363. doi: 10.1158/2159-8290.CD-13-0310
Behrens, C., Solis, L. M., Lin, H., Yuan, P., Tang, X., Kadara, H., et al. (2013). EZH2 protein expression associates with the early pathogenesis, tumor progression, and prognosis of non-small cell lung carcinoma. Clin. Cancer Res. 19, 6556–6565. doi: 10.1158/1078-0432.CCR-12-3946
Chao, C. H., Chang, C. C., Wu, M. J., Ko, H. W., Wang, D., Hung, M. C., et al. (2014). MicroRNA-205 signaling regulates mammary stem cell fate and tumorigenesis. J. Clin. Invest. 124, 3093–3106. doi: 10.1172/JCI73351
Chen, J., Li, Y., Yu, T. S., McKay, R. M., Burns, D. K., Kernie, S. G., et al. (2012). A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 488, 522–526. doi: 10.1038/nature11287
Chen, L., Gibbons, D. L., Goswami, S., Cortez, M. A., Ahn, Y. H., Byers, L. A., et al. (2014). Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat. Commun. 5:5241. doi: 10.1038/ncomms6241
Chen, W. J., Ho, C. C., Chang, Y. L., Chen, H. Y., Lin, C. A., Ling, T. Y., et al. (2014). Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat. Commun. 5:3472. doi: 10.1038/ncomms4472
Chen, Z., Fillmore, C. M., Hammerman, P. S., Kim, C. F., and Wong, K. K. (2014). Non-small-cell lung cancers: a heterogeneous set of diseases. Nat. Rev. Cancer 14, 535–546. doi: 10.1038/nrc3775
De Craene, B., and Berx, G. (2013). Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 13, 97–110. doi: 10.1038/nrc3447
Garofalo, M., Romano, G., Di Leva, G., Nuovo, G., Jeon, Y. J., Ngankeu, A., et al. (2011). EGFR and MET receptor tyrosine kinase-altered microRNA expression induces tumorigenesis and gefitinib resistance in lung cancers. Nat. Med. 18, 74–82. doi: 10.1038/nm.2577
Ginestier, C., Hur, M. H., Charafe-Jauffret, E., Monville, F., Dutcher, J., Brown, M., et al. (2007). ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 1, 555–567. doi: 10.1016/j.stem.2007.08.014
Hirsch, H. A., Iliopoulos, D., Tsichlis, P. N., and Struhl, K. (2009). Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 69, 7507–7511. doi: 10.1158/0008-5472.CAN-09-2994
Jiang, Z., Jacob, J. A., Loganathachetti, D. S., Nainangu, P., and Chen, B. (2017). beta-Elemene: mechanistic studies on cancer cell interaction and its chemosensitization effect. Front. Pharmacol. 8:105. doi: 10.3389/fphar.2017.00105
Jin, X., Kim, L. J. Y., Wu, Q., Wallace, L. C., Prager, B. C., Sanvoranart, T., et al. (2017). Targeting glioma stem cells through combined BMI1 and EZH2 inhibition. Nat. Med. 23, 1352–1361. doi: 10.1038/nm.4415
Kim, K. H., and Roberts, C. W. (2016). Targeting EZH2 in cancer. Nat. Med. 22, 128–134. doi: 10.1038/nm.4036
Lamouille, S., Xu, J., and Derynck, R. (2014). Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell. Biol. 15, 178–196. doi: 10.1038/nrm3758
Lau, Y. K., Du, X., Rayannavar, V., Hopkins, B., Shaw, J., Bessler, E., et al. (2014). Metformin and erlotinib synergize to inhibit basal breast cancer. Oncotarget 5, 10503–10517. doi: 10.18632/oncotarget.2391
Li, L., Han, R., Xiao, H., Lin, C., Wang, Y., Liu, H., et al. (2014). Metformin sensitizes EGFR-TKI-resistant human lung cancer cells in vitro and in vivo through inhibition of IL-6 signaling and EMT reversal. Clin. Cancer Res. 20, 2714–2726. doi: 10.1158/1078-0432.CCR-13-2613
Li, X., Lin, Z., Zhang, B., Guo, L., Liu, S., Li, H., et al. (2016). beta-elemene sensitizes hepatocellular carcinoma cells to oxaliplatin by preventing oxaliplatin-induced degradation of copper transporter 1. Sci. Rep. 6:21010. doi: 10.1038/srep21010
Li, Y., Atkinson, K., and Zhang, T. (2017). Combination of chemotherapy and cancer stem cell targeting agents: preclinical and clinical studies. Cancer Lett. 396, 103–109. doi: 10.1016/j.canlet.2017.03.008
Maemondo, M., Inoue, A., Kobayashi, K., Sugawara, S., Oizumi, S., Isobe, H., et al. (2010). Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 362, 2380–2388. doi: 10.1056/NEJMoa0909530
Mani, S. A., Guo, W., Liao, M. J., Eaton, E. N., Ayyanan, A., Zhou, A. Y., et al. (2008). The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715. doi: 10.1016/j.cell.2008.03.027
Martinelli, E., Troiani, T., Morgillo, F., Rodolico, G., Vitagliano, D., Morelli, M. P., et al. (2010). Synergistic antitumor activity of sorafenib in combination with epidermal growth factor receptor inhibitors in colorectal and lung cancer cells. Clin. Cancer Res. 16, 4990–5001. doi: 10.1158/1078-0432.CCR-10-0923
Morgillo, F., Sasso, F. C., Della Corte, C. M., Vitagliano, D., D’Aiuto, E., Troiani, T., et al. (2013). Synergistic effects of metformin treatment in combination with gefitinib, a selective EGFR tyrosine kinase inhibitor, in LKB1 wild-type NSCLC cell lines. Clin. Cancer Res. 19, 3508–3519. doi: 10.1158/1078-0432.CCR-12-2777
Mu, L., Wang, T., Chen, Y., Tang, X., Yuan, Y., and Zhao, Y. (2016). β-Elemene enhances the efficacy of gefitinib on glioblastoma multiforme cells through the inhibition of the EGFR signaling pathway. Int. J. Oncol. 49, 1427–1436. doi: 10.3892/ijo.2016.3626
Nagarsheth, N., Peng, D., Kryczek, I., Wu, K., Li, W., Zhao, E., et al. (2016). PRC2 epigenetically silences Th1-Type chemokines to suppress effector T-Cell trafficking in colon cancer. Cancer Res. 76, 275–282. doi: 10.1158/0008-5472.CAN-15-1938
Peng, D., Kryczek, I., Nagarsheth, N., Zhao, L., Wei, S., Wang, W., et al. (2015). Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 527, 249–253. doi: 10.1038/nature15520
Reya, T., Morrison, S. J., Clarke, M. F., and Weissman, I. L. (2001). Stem cells, cancer, and cancer stem cells. Nature 414, 105–111. doi: 10.1038/35102167
Seguin, L., Kato, S., Franovic, A., Camargo, M. F., Lesperance, J., Elliott, K. C., et al. (2014). An integrin beta(3)-KRAS-RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat. Cell Biol. 16, 457–468. doi: 10.1038/ncb2953
Sequist, L. V., Waltman, B. A., Dias-Santagata, D., Digumarthy, S., Turke, A. B., Fidias, P., et al. (2011). Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med. 3:75ra26. doi: 10.1126/scitranslmed.3002003
Shi, L., Wang, Y., Lu, Z., Zhang, H., Zhuang, N., Wang, B., et al. (2017). miR-127 promotes EMT and stem-like traits in lung cancer through a feed-forward regulatory loop. Oncogene 36, 1631–1643. doi: 10.1038/onc.2016.332
Shien, K., Toyooka, S., Yamamoto, H., Soh, J., Jida, M., Thu, K. L., et al. (2013). Acquired resistance to EGFR inhibitors is associated with a manifestation of stem cell-like properties in cancer cells. Cancer Res. 73, 3051–3061. doi: 10.1158/0008-5472.CAN-12-4136
Singh, A., Settleman, J., Singh, A., and Settleman, J. (2010). EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29, 4741–4751. doi: 10.1038/onc.2010.215
Souroullas, G. P., Jeck, W. R., Parker, J. S., Simon, J. M., Liu, J. Y., Paulk, J., et al. (2016). An oncogenic Ezh2 mutation induces tumors through global redistribution of histone 3 lysine 27 trimethylation. Nat. Med. 22, 632–640. doi: 10.1038/nm.4092
Van Roosbroeck, K., Fanini, F., Setoyama, T., Ivan, C., Rodriguez-Aguayo, C., Fuentes-Mattei, E., et al. (2017). Combining Anti-Mir-155 with chemotherapy for the treatment of lung cancers. Clin. Cancer Res. 23, 2891–2904. doi: 10.1158/1078-0432.CCR-16-1025
van Meerbeeck, J. P., Fennell, D. A., and De Ruysscher, D. K. (2011). Small-cell lung cancer. Lancet 378, 1741–1755. doi: 10.1016/S0140-6736(11)60165-7
Vujic, I., Sanlorenzo, M., Posch, C., Esteve-Puig, R., Yen, A. J., Kwong, A., et al. (2015). Metformin and trametinib have synergistic effects on cell viability and tumor growth in NRAS mutant cancer. Oncotarget 6, 969–978. doi: 10.18632/oncotarget.2824
Wang, J., Cheng, P., Pavlyukov, M. S., Yu, H., Zhang, Z., Kim, S. H., et al. (2017). Targeting NEK2 attenuates glioblastoma growth and radioresistance by destabilizing histone methyltransferase EZH2. J. Clin. Invest. 127, 3075–3089. doi: 10.1172/JCI89092
Wei, W. J., Sun, Z. K., Shen, C. T., Song, H. J., Zhang, X. Y., Qiu, Z. L., et al. (2017). Obatoclax and LY3009120 efficiently overcome vemurafenib resistance in differentiated thyroid cancer. Theranostics 7, 987–1001. doi: 10.7150/thno.17322
Wellner, U., Schubert, J., Burk, U. C., Schmalhofer, O., Zhu, F., Sonntag, A., et al. (2009). The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell Biol. 11, 1487–1495. doi: 10.1038/ncb1998
Yao, Y. Q., Ding, X., Jia, Y. C., Huang, C. X., Wang, Y. Z., and Xu, Y. H. (2008). Anti-tumor effect of beta-elemene in glioblastoma cells depends on p38 MAPK activation. Cancer Lett. 264, 127–134. doi: 10.1016/j.canlet.2008.01.049
Zhang, H., Qi, J., Reyes, J. M., Li, L., Rao, P. K., Li, F., et al. (2016). Oncogenic deregulation of EZH2 as an opportunity for targeted therapy in lung cancer. Cancer Discov. 6, 1006–1021. doi: 10.1158/2159-8290.CD-16-0164
Zhang, H., Xu, F., Xie, T., Jin, H., and Shi, L. (2012). beta-elemene induces glioma cell apoptosis by downregulating survivin and its interaction with hepatitis B X-interacting protein. Oncol. Rep. 28, 2083–2090. doi: 10.3892/or.2012.2022
Zhang, J., Zhang, H. D., Chen, L., Sun, D. W., Mao, C. F., Chen, W., et al. (2014). beta-elemene reverses chemoresistance of breast cancer via regulating MDR-related microRNA expression. Cell Physiol. Biochem. 34, 2027–2037. doi: 10.1159/000366398
Zhao, S., Wu, J., Zheng, F., Tang, Q., Yang, L., Li, L., et al. (2015). beta-elemene inhibited expression of DNA methyltransferase 1 through activation of ERK1/2 and AMPKalpha signalling pathways in human lung cancer cells: the role of Sp1. J. Cell. Mol. Med. 19, 630–641. doi: 10.1111/jcmm.12476
Zhou, B. B., Zhang, H., Damelin, M., Geles, K. G., Grindley, J. C., and Dirks, P. B. (2009). Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat. Rev. Drug Discov. 8, 806–823. doi: 10.1038/nrd2137
Keywords: elemene, gefitinib, lung cancer, stemness, EZH2
Citation: Cheng H, Ge X, Zhuo S, Gao Y, Zhu B, Zhang J, Shang W, Xu D, Ge W and Shi L (2018) β-Elemene Synergizes With Gefitinib to Inhibit Stem-Like Phenotypes and Progression of Lung Cancer via Down-Regulating EZH2. Front. Pharmacol. 9:1413. doi: 10.3389/fphar.2018.01413
Received: 07 September 2018; Accepted: 16 November 2018;
Published: 30 November 2018.
Edited by:
Vincent Kam Wai Wong, Macau University of Science and Technology, MacauReviewed by:
Songxiao Xu, Artron BioResearch Inc., CanadaCopyright © 2018 Cheng, Ge, Zhuo, Gao, Zhu, Zhang, Shang, Xu, Ge and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liyun Shi, c2hpX2xpeXVuQG1zbi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.