
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 11 September 2018
Sec. Gastrointestinal and Hepatic Pharmacology
Volume 9 - 2018 | https://doi.org/10.3389/fphar.2018.01009
 Xin Ma1,2†
Xin Ma1,2† Yicong Chang1†
Yicong Chang1† Yuanyuan Zhang1
Yuanyuan Zhang1 Ishfaq Muhammad1
Ishfaq Muhammad1 Chenxi Shi1
Chenxi Shi1 Rui Li1,3
Rui Li1,3 Changwen Li2
Changwen Li2 Zhi Li1
Zhi Li1 Yuexia Lin1
Yuexia Lin1 Qing Han1
Qing Han1 Fangping Liu1,3*
Fangping Liu1,3*In this study, acetaminophen (APAP)-induced acute liver injury mice model was used to investigate the effects of C2-ceramide and oltipraz on hepatocyte nuclear factor 1 (HNF-1) and glutathione S-transferase A1 (GSTA1). Notably, C2-ceramide caused alteration in mice serum transaminases and liver tissue indexes, and aggravated hepatic injury, while oltipraz alleviated hepatic injury. By screening, the optimal concentrations of C2-ceramide and oltipraz were confirmed to be 120 and 150 μmol/L, respectively. In histopathology, karyolysis and more necrotic cells and bleeding spots were appeared on administration of C2-ceramide, but only a small amount of inflammatory cells infiltration was seen after oltipraz treatment. In addition, RT-PCR and western blot results revealed that the mRNA and protein expression levels of HNF-1 and GSTA1 in liver were significantly decreased (p < 0.01) with the administration of 120 μmol/L C2-ceramide. Meanwhile, GSTA1 content in serum increased up to 1.27-fold. In contrast, 150 μmol/L oltipraz incorporation to APAP model mice resulted in obvious elevation (p < 0.01) in the mRNA and protein expression levels of HNF-1 and GSTA1 in liver, and serum GSTA1 content decreased up to 0.77-fold. In conclusion, C2-ceramide could down-regulate the expression of HNF-1 and GSTA1 which exacerbated hepatic injury, while oltipraz could up-regulate the expression of HNF-1 and GSTA1 which mitigated hepatic injury.
Drug-induced liver injury (DILI) is associated with oxidative stress induced via the drug itself and/or its metabolites or the body’s allergic reaction to certain drugs (Kaplowitz, 2004). DILI is a key issue in drug development and clinical drug safety, which can be manifested as acute, chronic liver disease, and liver dysfunction (Kaplowitz, 2004). Due to lack of specificity, it is not easy to perform clinical diagnosis and treatment (Reuben et al., 2010). Studies reported that drug induced hepatotoxicity and the injury mechanisms bewitch the interest of scientists due to the increasing number of incidence of liver damage (Ilavenil et al., 2016; Cheng et al., 2017; Kim et al., 2017). Acute liver injury is frequently related with increased enzyme activity without clinical symptoms and results in acute fulminant hepatic failure (Michaut et al., 2016). The acute liver injury model induced by acetaminophen (APAP) is used as the most typical DILI model in clinical trials. In rodents, especially in mice, it is common to study APAP induced liver injury model in vivo (Yuan et al., 2010). Currently, the evaluation of liver injury relies on the determination of various indicators in serum (aminotransferases) (Ozer et al., 2008) and in liver tissues (SOD, MDA, GSH, etc) (Hsieh et al., 2015). Previously, GSTA1 was identified as a more sensitive indicator than aminotransferases by using APAP-induced hepatic injury (Liu et al., 2014).
Glutathione S-transferase A1, a key phase II enzyme involved in the detoxification reactions (Iorio et al., 2014), is one of the important members of GST family (Ma et al., 2017), which is generally believed as a key factor in detoxification pathways. GSTA1 is an important component of antioxidant defense system, which can catalyze a number of exogenous chemicals such as carcinogens, environmental toxins, certain drugs combined with GSH, promote their degradation in the intracellular clearance and protect body systems (Board and Menon, 2013; Liu et al., 2016). GSTA1 has non-selenium-dependent GSH-Px activity, scavenge lipid free radicals and oxidative stress reaction products, and contains the function of anti-lipid peroxidation (Singhal et al., 2015). GSTA1 is released into serum from liver for detoxification on the onset of DILI (Shi et al., 2017). Meanwhile, GSTA1 can activate some molecular mechanisms, regulate the signal transduction and prevent cell apoptosis (Adnan et al., 2012). The detoxification of GSTA1 to extraneous materials and reactive oxygen species may represent an important cellular reaction process in oxidative stress.
Hepatocyte nuclear factor (HNF) is a type of transcription factor that regulates gene expression in the liver, contains an evolutionarily conserved DNA-binding domain, regulates the expression of the target genes by binding to cis-acting elements, and plays an important role in the differentiation and metabolism of hepatocytes at transcriptional level (Hayashi et al., 1999). HNF-1 has two forms, namely HNF-1 and variant HNF-1 (vHNF-1) (Pontoglio, 2000). HNF-1 accounts for 95% of the total HNF1-like protein in liver, while vHNF-1 expression is very low (Pontoglio, 2000). HNF-1 plays an important role in promoting the development of liver and maintaining the biological function of hepatocytes, also controls the expression of liver specific genes (Boudreau et al., 2001; Michels and Hagen, 2009).
Ceramide is an endogenous bioactive lipid second messenger and a member of sphingolipid family that plays a key role in mediating cell proliferation, differentiation, migration, senescence, and apoptosis (Dany and Ogretmen, 2015; Li and Zhang, 2015). Furthermore, ceramide exhibits significant combination therapy efficacy when used in combination with other drugs due to its involvement in various tumor suppressor signaling pathways including apoptosis, autophagy, and metastasis (Thayyullathil et al., 2014; Li and Zhang, 2015). C2 is a synthetic short-chain ceramide compound with endogenous ceramide activity (Zhu et al., 2014). Oltipraz is a kind of extract in cruciferous vegetables with anti-cancer effect (Langouet et al., 1995). Oltipraz has been extensively studied as a cancer chemopreventive agent, and it is also used for the treatment of liver cirrhosis (Kang et al., 2002, 2003). Kang et al. (2002) have shown that oltipraz can increase the survival rate of liver cirrhosis in rats through improvement of liver function, regeneration of liver cirrhosis and reduction of liver fibrosis. Therefore, the present study aimed to investigate the effects of C2 and oltipraz on HNF-1 and GSTA1 expression in APAP-induced acute hepatic injury to clarify the roles of HNF-1 and GSTA1 in liver injury.
APAP was purchased from Yong An Chemical Industry (Anhui, China). C2 and Oltipraz were purchased from Sigma-Aldrich Trading Co., Ltd. (Shanghai, China). The detection kits of ALT, AST, SOD, MDA, GSH and GSH-Px were obtained from the Nanjing Jiancheng Institute of Biotechnology (Nanjing, China). The GSTA1 detection kit was obtained from American RapidBio (RB) company. TRIzol reagent was provided by Life Technologies Corporation (Cal., United States). Reverse Transcription kit was purchased from Takara Corp (Dalian, China). TransStart Top Green qPCR SuperMix kit was obtained from TransGen Biotech Co., Ltd. (Beijing, China). Polyclonal antibodies of GSTA1 and HNF-1 were bought from ABclonal Biotechnology Co., Ltd. (Boston, MA, United States).
Adult male Kunming mice (8-week-old, 18–22 g) were obtained from the Harbin Pharmaceutical Group Co. Ltd. General Pharm. Factory, laboratory animal centre. The animals were housed in a controlled environment under standard conditions at a temperature (20 ± 2°C), 12 h light/dark cycle, a relative humidity (40–60%), and allowed free access to food (standard mice pellets), water and were acclimatized for at least 1 week before use. This study was carried out in accordance with the recommendations of the China National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The protocol was approved by the Harbin Veterinary Research Institute Animal Ethics Committee (approval number was SYXK (Hei) 2012-2067).
(1) Mice were randomly divided into five groups (n = 8), including control group, APAP (200 mg/kg) model group, C2 (100, 120, and 140 μmol/L) + APAP groups. (2) Mice were randomly divided into five groups (n = 8), including control group, APAP (200 mg/kg) model group, oltipraz (OL) (140, 150, and 160 μmol/L) + APAP groups. (3) Mice were randomly divided into seven groups (n = 8), including control group, C2 (100, 120, and 140 μmol/L) groups and oltipraz (140, 150, and 160 μmol/L) groups. C2 and oltipraz were dissolved in pure DMSO, and diluted in physiological saline to result in final concentrations. The doses corresponding to the concentrations of C2 (100, 120, and 140 μmol/L) are 342, 410, and 478 μg/kg, respectively. The doses corresponding to the concentrations of oltipraz (140, 150, and 160 μmol/L) are 317, 340, and 362 μg/kg, respectively. Ten milliliters solution was given to mice per kilogram of body weight. The control group received an equal volume of DMSO and physiological saline. After administration of different drugs for 12 h, mice were subsequently anesthetized with ether (Shenyang Chemical Reagent Factory) and sacrificed by cervical dislocation, following serum separation for the determination of ALT and AST.
The animals were randomly divided into six groups (n = 8), including control group, APAP model group, C2-ceramide group (C2), C2-ceramide + APAP group (C2+APAP), oltipraz group (OL) and oltipraz + APAP group (OL+APAP). C2 and C2+APAP groups were administered 120 μmol/L (410 μg/kg) C2, OL and OL+APAP groups were administered 150 μmol/L (340 μg/kg) oltipraz. Then APAP, C2+APAP and OL+APAP groups were administered 200 mg/kg APAP by gavage, and control group was given an equal volume of physiological saline and DMSO. After 12 h, mice were subsequently anesthetized with ether and sacrificed by cervical dislocation, following which serum was separated for the determination of ALT, AST and GSTA1. Then, the liver was isolated and stored at -80°C for analysis of other indicators, real-time RT-PCR and western blot, except for the left lobe, which was used for histopathological studies.
Alanine aminotransferase and AST activities in serum were determined using detection kits according to the manufacturer’s instructions.
Malondialdehyde, SOD, GSH, and GSH-Px in liver were determined using detection kits according to the manufacturer’s instructions.
Formalin-fixed specimens were embedded in paraffin and sectioned for 5 μm thickness according to the routine procedure. After hematoxylin and eosin (H&E) staining, the slides were observed for conventional morphological evaluation under a light microscope and photographed at 400× magnification.
Total RNA was prepared from 50 to 100 mg of liver tissue using TRIzol reagent according to the manufacturer’s instructions. Isolated RNA (1 μg/20 μl reaction volume) was used for first-strand cDNA synthesis using Reverse Transcription Kit. The levels of mRNA expression were quantified by real-time RT-PCR with SYBR®Premix Ex TaqTM II (Tli RNaseH Plus) and LightCycler® 96 System. A real-time RT-PCR reaction was performed in a 20 μl volume containing 10 μl SYBR Premix Ex TaqTM and 5 μM of each primer. Thermocycling was performed as follows: 94°C, 5 min for denaturation and then 40 cycles of PCR (94°C, 30 s; 58°C, 40 s; 72°C, 40 s) and 72°C, 5 min. The mRNA levels were normalized against β-actin mRNA. Primer sequences used for real-time RT-PCR are shown in Table 1.
Total protein was extracted from 20 mg of liver tissue using 200 μl lysis solution according to the manufacturer’s instructions. Protein concentrations were measured by using the NANODROP 2000c Spectrophotometer to keep all sample protein concentrations consistent. After SDS polyacrylamide gel electrophoresis, protein was transferred onto NC membrane by transmembrane. The NC membrane was incubated with primary antibody overnight, and then incubated with secondary antibody for 1 h. ECL droplets were deposited on the NC membrane and the NC membrane was placed in the Tanon 5200 system to develop. Finally, the expression of HNF-1 and GSTA1 protein was analyzed by Image J software.
Glutathione S-transferase A1 concentration in serum was detected with a Mouse GSTA1 ELISA Kit that used Purified Mouse GSTA1 to coat microtiter plate wells, making a solid-phase antibody. The procedure was performed according to the manufacturer’s instructions.
Values were expressed as the mean ± SD, and statistical significance was determined by one-way analysis of variance using the SPSS software 19.0. The Tukey’s multiple comparison test in post hoc Multiple Comparisons was used to examine statistical significance (p < 0.05 and p < 0.01) between groups.
The results of screening for optimal C2 and oltipraz concentrations were presented in Figure 1. Compared with control group, significant increase (p < 0.01) in serum AST and ALT activities were observed in model group. Compared to model group, serum AST and ALT activities were significantly increased (p < 0.01) with 120 and 140 μmol/L C2 concentration. Meanwhile, serum AST and ALT activities were obviously decreased (p < 0.05) with 140 μmol/L oltipraz concentration, and significantly decreased (p < 0.01) with 150and 160 μmol/L oltipraz concentration.
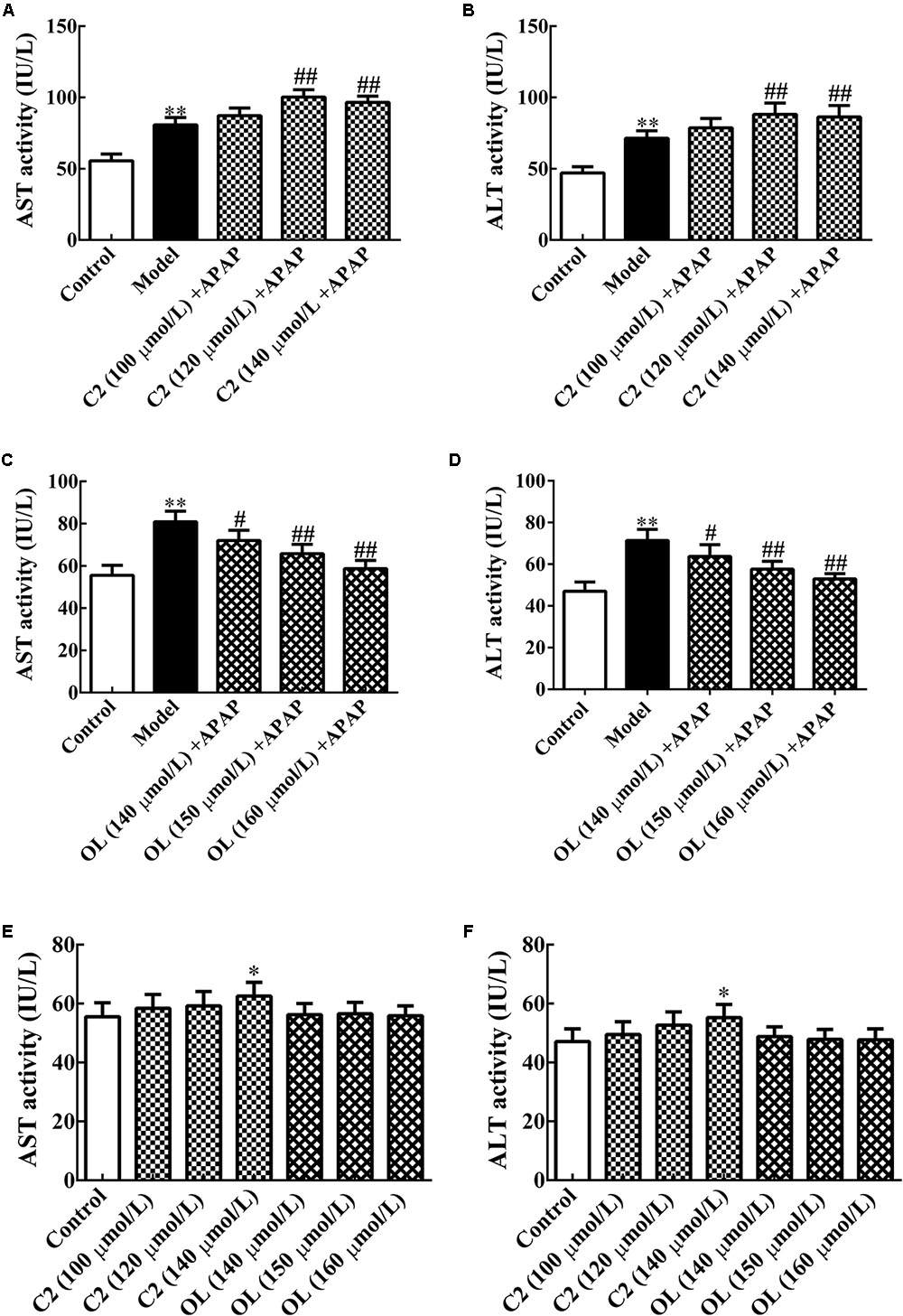
FIGURE 1. (A,B) Effects of different concentrations of C2-ceramide on AST and ALT activities in APAP-induced hepatic injury. (C,D) Effects of different concentrations of oltipraz on AST and ALT activities in APAP-induced hepatic injury. (E,F) Effects of different concentrations of C2-ceramide and oltipraz on AST and ALT activities in normal mice. Values are expressed as the mean ± SD for each group (n = 8). ∗p < 0.05, ∗∗p < 0.01 compared with control group; #p < 0.05, ##p < 0.01 compared with model group.
Compared with control group, it has been showed that serum AST and ALT activities were significantly increased (p < 0.05) with 140 μmol/L of C2 which indicated that the optimum concentration of C2 was chosen less than 140 μmol/L. Meanwhile, there were no obvious changes in other C2 groups and oltipraz groups. Thus, we chose 120 μmol/L for C2 and 150 μmol/L for oltipraz, which cannot affect normal mice to perform subsequent experiments.
The results of transaminases in serum and hepatic oxidative stress indicators in liver were presented in Figure 2. Serum ALT and AST activities and liver MDA content were significantly increased (p < 0.01). Meanwhile, SOD and GSH-Px activities and GSH content in liver were obviously decreased (p < 0.01) in model group with respect to control group. Compared with model group, it has been noted that serum ALT and AST activities and liver MDA content were significantly increased (p < 0.01). Besides, liver SOD, GSH-Px, and GSH levels were markedly decreased (p < 0.01) in C2+APAP group. In contrast, serum AST and ALT activities and liver MDA content were significantly decreased (p < 0.01). Moreover, liver SOD, GSH-Px, and GSH levels were obviously increased (p < 0.01) in OL+APAP group.
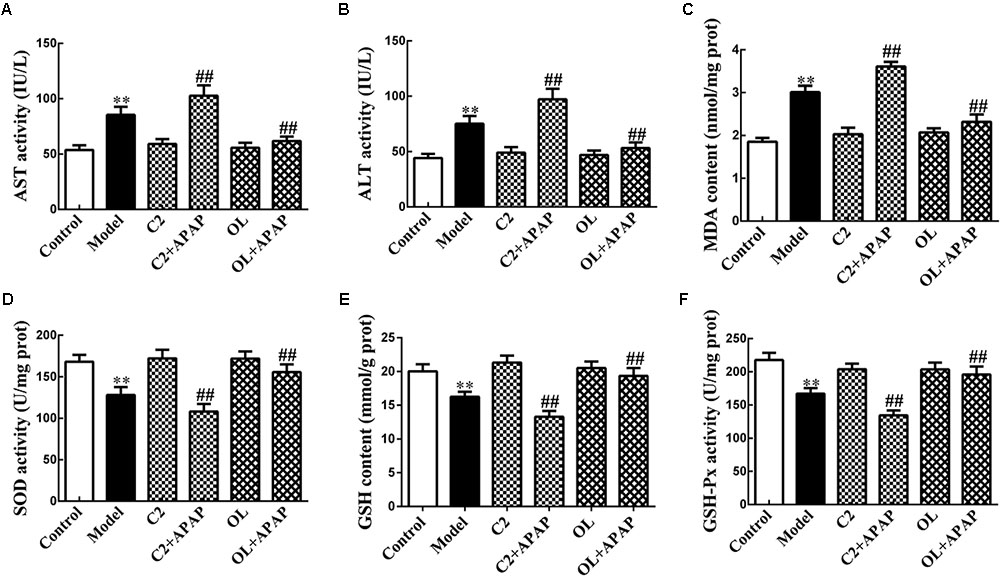
FIGURE 2. The effects of C2-ceramide and oltipraz on serum AST (A) and ALT (B) activities, liver MDA content (C), SOD activity (D), GSH content (E), and GSH-Px activity (F) in APAP-induced hepatic injury. Values are expressed as the mean ± SD for each group (n = 8). ∗∗p < 0.01 compared with control group; ##p < 0.01 compared with model group.
Pathological section results of each group were presented in Figure 3. In model group (Figure 3B), the hepatic cords were obscure and severely damaged, multiple inflammatory infiltrates and hemorrhages were found in the hepatocytes. The hepatocytes morphology and hepatic cords in C2 and OL groups (Figures 3C,E) were normal and clear as observed in control group (Figure 3A). More lesions appeared in the C2+APAP group (Figure 3D) in comparison to model group, which showed the blurred hepatic cord, serious bleeding, karyolysis, more necrotic cells and inflammatory cells infiltration. OL+APAP group (Figure 3F) was close to the control group, but there was still a small amount of inflammatory cells infiltration in liver tissues.
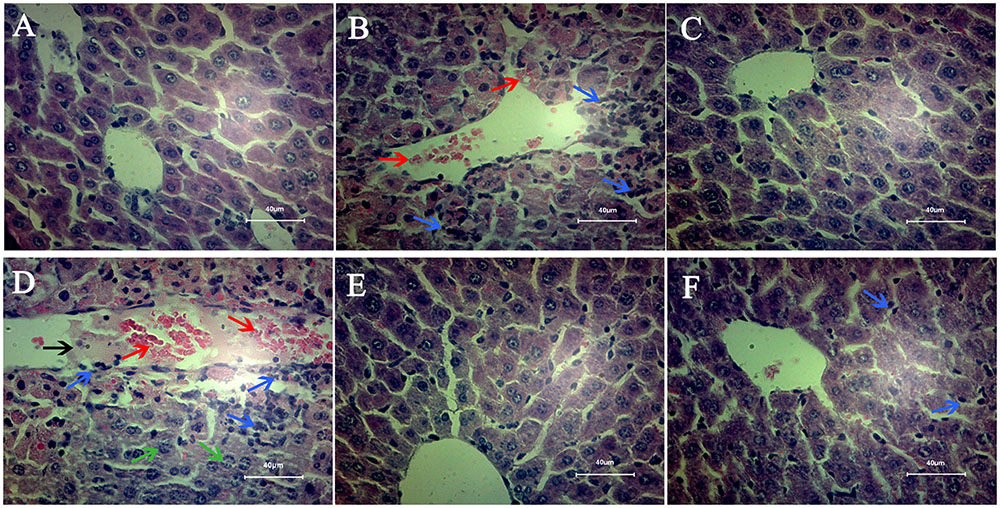
FIGURE 3. Mice liver pathology. Liver sections were stained by hematoxylin and eosin. All the liver sections were examined by light microscopy and the images were displayed at 400× the original magnification. (A) Control group; (B) model group; (C) C2-ceramide group; (D) C2-ceramide + APAP group; (E) Oltipraz group; (F) Oltipraz + APAP group. The bleeding was pointed out by the red arrows. The inflammatory cells infiltration was marked out by blue arrows. The karyolysis was pointed out by the green arrows. Black arrow meant that there is exudate in the blood vessels.
The changes of serum GSTA1 content in APAP-induced hepatic injury were presented in Figure 4. Compared with control group, a significant increase (p < 0.01) in serum GSTA1 content was observed in model group. Moreover, a significant increase (p < 0.01) in serum GSTA1 concentration was found in C2+APAP group compared to model group. In contrast, a significant decrease (p < 0.01) has been noted in serum GSTA1 content in OL+APAP group.
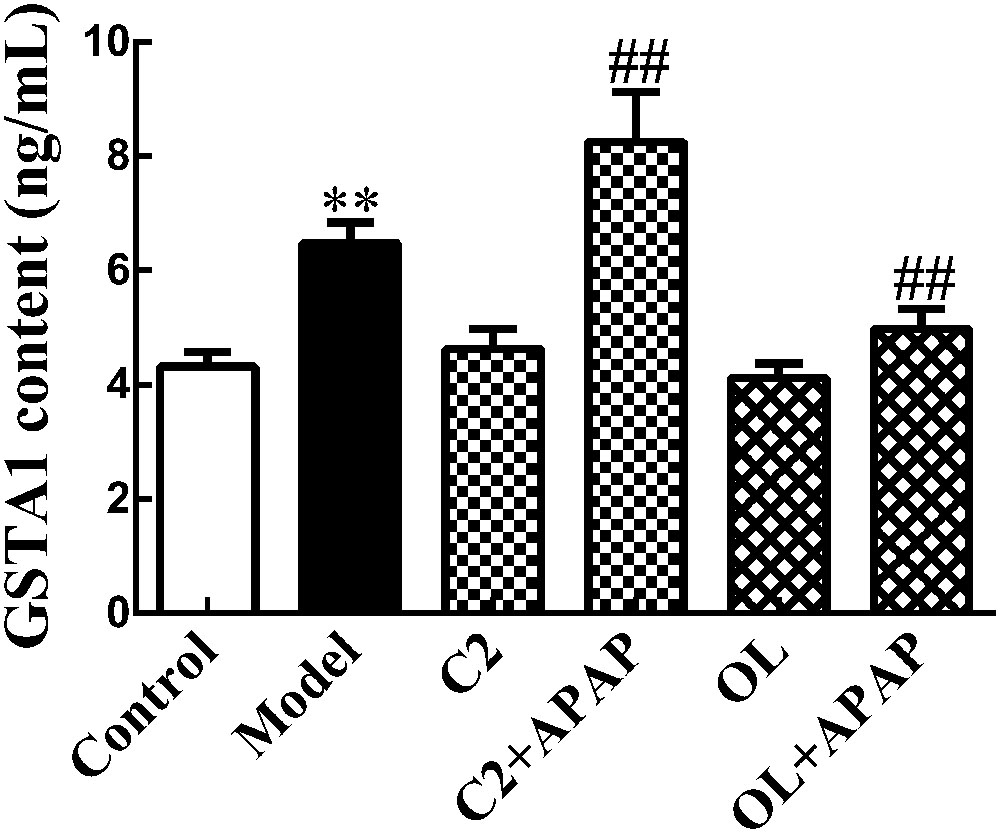
FIGURE 4. Effects of C2-ceramide and oltipraz on serum GSTA1 content in APAP-induced hepatic injury. Values are expressed as the mean ± SD for each group (n = 8). ∗∗p < 0.01 compared with control group; ##p < 0.01 compared with model group.
The results of HNF-1 and GSTA1 mRNA expression by Real-time RT-PCR were presented in Figure 5 in the presence of C2 and oltipraz. Compared with control group, the expression of HNF-1 and GSTA1 mRNA in model group were significantly decreased (p < 0.01). Significant decrease (p < 0.01) in the expression of HNF-1 and GSTA1 mRNA were observed in C2+APAP group in comparison with model group. In contrast, the expression of HNF-1 and GSTA1 mRNA were significantly increased (p < 0.01) in OL+APAP group. Intriguingly, the expression levels of GSTA1 mRNA were consistent with the trend of HNF-1 in each group.
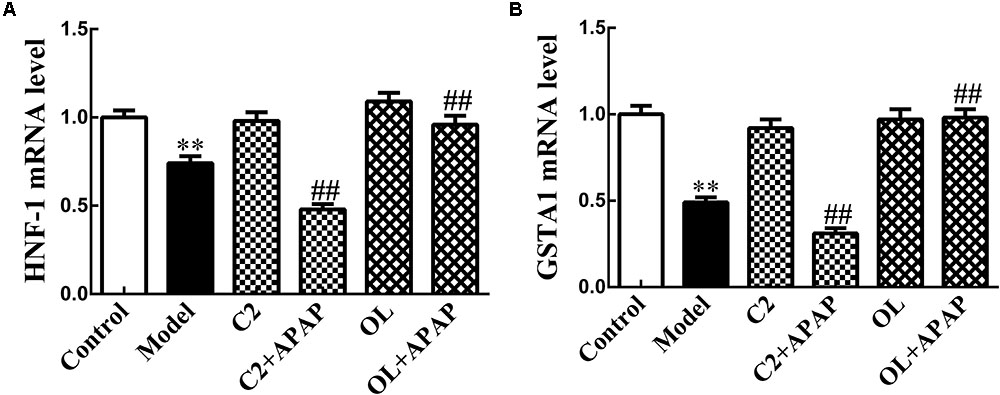
FIGURE 5. Effects of C2-ceramide and oltipraz on liver HNF-1 (A) and GSTA1 (B) mRNA expression in APAP-induced hepatic injury. Values are expressed as the mean ± SD for each group (n = 8). ∗∗p < 0.01 compared with control group; ##p < 0.01 compared with model group.
The protein expression level of HNF-1 and GSTA1 were quantified in liver and displayed in Figure 6. Compared with control group, the expression of HNF-1 and GSTA1 protein were significantly decreased (p < 0.01) in model group. Compared to model group, the expression of HNF-1 and GSTA1 protein were significantly decreased (p < 0.01) in C2+APAP group. While, in OL+APAP group, HNF-1 and GSTA1 showed significant increase in protein expression (p < 0.01). Surprisingly, similar trends were found in GSTA1 and HNF-1 protein expression.
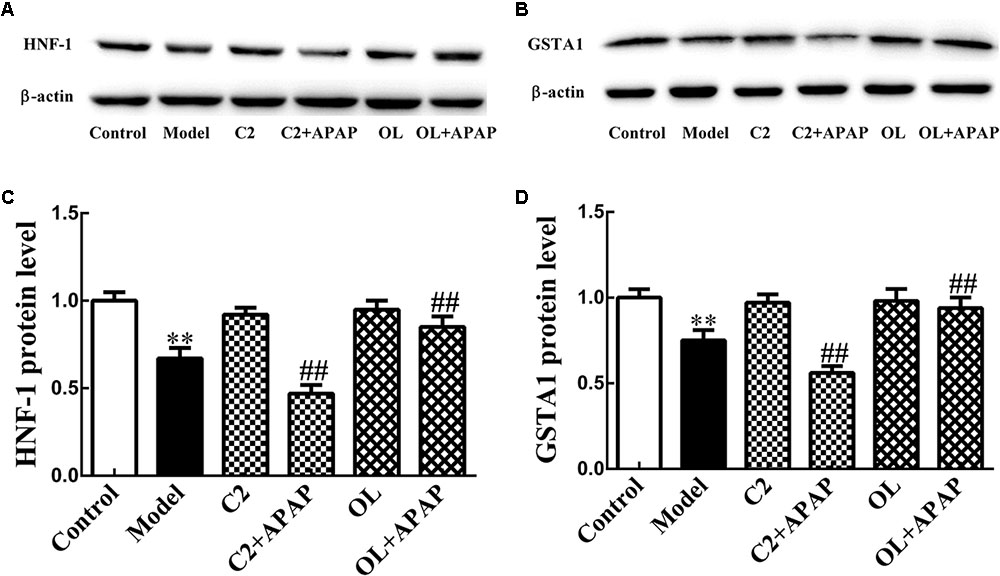
FIGURE 6. Original blots for HNF-1 (A) and GSTA1 (B). Effects of C2-ceramide and oltipraz on liver HNF-1 (C) and GSTA1 (D) protein expression in APAP-induced hepatic injury. Values are expressed as the mean ± SD for each group (n = 8). ∗∗p < 0.01 compared with control group; ##p < 0.01 compared with model group.
Previously, our research group proved that GSTA1 was a more sensitive and accurate indicator to represent liver injury than ALT in vivo and in vitro (Liu et al., 2014; Chang et al., 2017; Li et al., 2017). Lu et al. (2012) reported that GSTA1 was reduced in CCl4-induced acute liver injury and dioscin could protect liver with increased GSTA1 expression. Similarly, we investigated the hepatoprotective effects of Folium Syringae against APAP-induced hepatic injury in vitro and in vivo, and the results showed that Folium Syringae could protect the integrity of hepatocytes and thus reduce the release of liver GSTA1, which contributed to improve liver detoxification (Shi et al., 2017). HNF-1, a transcription factor located on chromosome 12, involved in the regulation of the expression of several liver-specific genes (Jawaid et al., 2016). Although HNF-1 is highly expressed in liver (Bjorkhaug et al., 2005), research about the role of HNF-1 in hepatic injury is insufficient. Therefore, the aim of our study was to investigate the effects of C2 and oltipraz on HNF-1 and GSTA1 expression and the roles of HNF-1 and GSTA1 in APAP-induced acute hepatic injury.
Ceramide is a central molecule in the biosynthetic pathway of sphingolipids and a potent tumor suppressor by regulating cell proliferation, differentiation, senescence, and apoptosis (Yabu et al., 2015). Jiang et al. (2017) have shown that the combination of C2 and sorafenib provides synergistic inhibition of hepatocellular carcinoma (HCC) cells through cell cycle arrest, ROS production, and caspase-dependent apoptosis. Moreover, C2 and sorafenib combination treatment increased ROS accumulation in Bel7402 cells and scavenging ROS by antioxidant tocopherol and lipoic acid could restore cell viability (Jiang et al., 2017). In general, phase II enzymes mediate the coupling reaction to help eliminate toxic materials. If the phase II response is insufficient, activated poisons may cause tissue damage, inflammation, genetic alterations, and/or inactivation of tumor suppressor genes (Choi et al., 2010). Oltipraz is a member of a novel class of dithiothreitol, many of which are capable of preventing cells from mitochondrial dysfunction induced by arachidonic acid (AA) + iron (Shin and Kim, 2009). Studies have shown that dithiothreitol, including oltipraz and some of its metabolites, induces transactivation of genes encoding phase II enzymes (Egner et al., 1994; Petzer et al., 2003). Moreover, oltipraz has an antioxidant effect at least in part by inducing phase II enzyme and/or Mn-SOD genes (Kwon et al., 2009). Oltipraz metabolites increase the cellular GSH content (Kwon et al., 2009). In addition, oltipraz can inhibit AA + iron-induced ROS production and its metabolites can abolish the ability of AA-induced mitochondrial superoxide production, thereby protecting cells from ROS-induced apoptosis (Kwon et al., 2009; Shin and Kim, 2009).
Firstly, we screened the optimum concentrations of optimal C2 and oltipraz. It has been found that AST and ALT activities were significantly increased (p < 0.01) in APAP-induced hepatic injury with 120 and 140 μmmol/L of C2, while these were obviously decreased (p < 0.05 or p < 0.01) with 140, 150, and 160 μmmol/L of oltipraz relative to model group. Meanwhile, 120 μmmol/L of C2 and all concentrations of oltipraz could not affect normal mice. Therefore, we chose 120 μmmol/L of C2 and 150 μmmol/L of oltipraz for subsequent experiments. Then, APAP-induced liver injury mice model was treated with C2 and oltipraz to study the alterations in liver indexes. Compared to model group, serum transaminases (ALT, AST) and liver MDA were obviously increased and liver SOD, GSH, and GSH-Px were significantly decreased in C2+APAP group. In contrast, serum AST and ALT and liver MDA were significantly reduced. While liver SOD, GSH, and GSH-Px were obviously enhanced in OL+APAP group. Similarly, the results of histopathological observation showed that there was less inflammatory cell infiltration in OL+APAP group, almost close to control group. While, more necrotic cells and karyolysis appeared in C2+APAP group relative to model group. Meanwhile, there were no obvious lesions and all indexes were not changed in C2 and OL groups. These findings suggested that C2 aggravated liver injury and oltipraz mitigated liver injury.
Transcription factor HNF-1 contains DNA binding regions that are evolutionarily conserved, regulates the expression of target genes through the combination of cis-acting elements and plays an important role in the regulation of hepatocyte differentiation and detoxification (Mosialou et al., 2011; David-Silva et al., 2013). In this study, the expression level of HNF-1 mRNA and protein were determined by real-time fluorescence quantitative analysis and western blot. It was found that the relative expression of HNF-1 mRNA and protein in model group were significantly lower than those in control group, which proved that APAP induced acute liver injury could reduce HNF-1 expression. The relative expression of HNF-1 mRNA and protein decreased obviously in C2+APAP group. In contrast, HNF-1 mRNA and protein level significantly increased in OL+APAP group relative to model group. While, compared to control group, there was no significant difference in C2 and OL groups. These results suggested that C2 could down-regulate the expression of HNF-1 and oltipraz could up-regulate the expression of HNF-1 in the presence of APAP-induced liver injury. Moreover, there was no effect on HNF-1 expression under the optimum concentrations of C2 and oltipraz. However, higher studies are needed to investigate the molecular mechanism behind the stimulation of HNF-1 expression with C2 and oltipraz treatment in different concentrations.
Glutathione S-transferase A1 is a key enzyme in the glutathione binding reaction and plays an important role in the metabolic process of APAP. GSTA1 can catalyze the reaction of nucleophilic glutathione with metabolites of APAP and degrade the toxic substances in the body (Bailey et al., 2012). The increased expression of GSTA1 can reduce the proliferation of cancer cells and protect cells against the damage of lipid peroxidation (Matic et al., 2013), which has been an important mechanism of anti-oxidative stress in detoxification of toxic substances and reactive oxygen species (Marcus et al., 2012; Sohn et al., 2013). In this study, the expression level of GSTA1 mRNA and protein in model group were significantly lower than those in the control group, and GSTA1 content in serum was obviously increased. Thus, it has been demonstrated that GSTA1 was released from liver to serum for detoxification in APAP-induced acute liver injury. There was no significant difference in C2 and OL groups in GSTA1 content relative to control group. Our data showed that the relative expression of GSTA1 mRNA and protein in C2+APAP group were obviously decreased and GSTA1 content in serum was significantly increased. While, contrasting results have been found in OL+APAP group. More importantly, these results were consistent with the trend of HNF-1 mRNA and protein expression. Therefore, there might be a certain intrinsic link between them and further molecular studies are needed to investigate the mechanism behind the regulation of transcription factor HNF-1 and its downstream gene GSTA1 in APAP-induced liver injury.
C2 could aggravate liver damage and oltipraz could alleviate liver damage in APAP-induced acute hepatic injury in mice. Moreover, HNF-1 and GSTA1 expression could be down-regulated by C2 which exacerbated hepatic injury, while up-regulated by oltipraz which mitigated hepatic injury. Accordingly, HNF-1 and GSTA1 are involved in acute hepatic injury induced by APAP, and there might be a certain intrinsic molecular association between HNF-1 and GSTA1.
FL supervised the whole experiments. XM and YC performed the practical work and completed the experiments. YZ helped to analyzed the data for the work and revised the article. CS, RL, CL, ZL, YL, and QH provided help during the experiments. IM helped in improving language expression.
This work was supported by the National Natural Science Foundation of China (Grant No. 31472241).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ALT, alanine aminotransferase; APAP, N-acetyl-p-aminophenol/acetaminophen/paracetamol; AST, aspartate aminotransferase; C2, C2-ceramide; DILI, drug-induced liver injury; DMEM, dulbecco’s modified eagle medium; DMSO, dimethyl sulfoxide; GSH, glutathione; GSH-Px, glutathione peroxidase; GST, glutathione S-transferases; GSTA1, glutathione S-transferase A1; HNF-1, hepatocyte nuclear factor 1; MDA, malondialdehyde; NAPQI, N-acetyl-p-benzoquinone imine; OL, oltipraz; SOD, superoxide dismutase.
Adnan, H., Quach, H., MacIntosh, K., Antenos, M., and Kirby, G. M. (2012). Low levels of GSTA1 expression are required for Caco-2 cell proliferation. PLoS One 7:e51739. doi: 10.1371/journal.pone.0051739
Bailey, W. J., Holder, D., Patel, H., Devlin, P., Gonzalez, R. J., Hamilton, V., et al. (2012). A performance evaluation of three drug-induced liver injury biomarkers in the rat: alpha-glutathione S-transferase, arginase 1, and 4-hydroxyphenyl-pyruvate dioxygenase. Toxicol. Sci. 130, 229–244. doi: 10.1093/toxsci/kfs243
Bjorkhaug, L., Bratland, A., Njolstad, P. R., and Molven, A. (2005). Functional dissection of the HNF-1alpha transcription factor: a study on nuclear localization and transcriptional activation. DNA Cell Biol. 24, 661–669. doi: 10.1089/dna.2005.24.661
Board, P. G., and Menon, D. (2013). Glutathione transferases, regulators of cellular metabolism and physiology. Biochim. Biophys. Acta 1830, 3267–3288. doi: 10.1016/j.bbagen.2012.11.019
Boudreau, F., Zhu, Y., and Traber, P. G. (2001). Sucrase-isomaltase gene transcription requires the hepatocyte nuclear factor-1 (HNF-1) regulatory element and is regulated by the ratio of HNF-1 alpha to HNF-1 beta. J. Biol. Chem. 276, 32122–32128. doi: 10.1074/jbc.M102002200
Chang, Y. C., Liu, F. P., Ma, X., Li, M. M., Li, R., Li, C. W., et al. (2017). Glutathione S-transferase A1 - a sensitive marker of alcoholic injury on primary hepatocytes. Hum. Exp. Toxicol. 36, 386–394. doi: 10.1177/0960327116650013
Cheng, D., Xu, W., Yuan, L., and Zhang, X. (2017). Investigation of drug-induced hepatotoxicity and its remediation pathway with reaction-based fluorescent probes. Anal. Chem. 89, 7693–7700. doi: 10.1021/acs.analchem.7b01671
Choi, S. H., Kim, Y. M., Lee, J. M., and Kim, S. G. (2010). Antioxidant and mitochondrial protective effects of oxidized metabolites of oltipraz. Exp. Opin. Drug Metab. Toxicol. 6, 213–224. doi: 10.1517/17425250903427972
Dany, M., and Ogretmen, B. (2015). Ceramide induced mitophagy and tumor suppression. Biochim. Biophys. Acta 1853(10 Pt B), 2834–2845. doi: 10.1016/j.bbamcr.2014.12.039
David-Silva, A., Freitas, H. S., Okamoto, M. M., Sabino-Silva, R., Schaan, B. D., and Machado, U. F. (2013). Hepatocyte nuclear factors 1alpha/4alpha and forkhead box A2 regulate the solute carrier 2A2 (Slc2a2) gene expression in the liver and kidney of diabetic rats. Life Sci. 93, 805–813.
Egner, P. A., Kensler, T. W., Prestera, T., Talalay, P., Libby, A. H., Joyner, H. H., et al. (1994). Regulation of phase 2 enzyme induction by oltipraz and other dithiolethiones. Carcinogenesis 15, 177–181.
Hayashi, Y., Wang, W., Ninomiya, T., Nagano, H., Ohta, K., and Itoh, H. (1999). Liver enriched transcription factors and differentiation of hepatocellular carcinoma. Mol. Pathol. 52, 19–24.
Hsieh, Y. L., Yeh, Y. H., Lee, Y. T., and Huang, C. Y. (2015). Protective effects of Cholestin on ethanol induced oxidative stress in rats. J. Sci. Food Agric. 95, 799–808. doi: 10.1002/jsfa.6904
Ilavenil, S., Al-Dhabi, N. A., Srigopalram, S., Ock Kim, Y., Agastian, P., Baru, R., et al. (2016). Acetaminophen induced hepatotoxicity in wistar rats—A proteomic approach. Molecules 21:161. doi: 10.3390/molecules21020161
Iorio, A., Piacentini, S., Polimanti, R., De Angelis, F., Calderon, R., and Fuciarelli, M. (2014). Functional variability of glutathione S-transferases in Basque populations. Am. J. Hum. Biol. 26, 361–366. doi: 10.1002/ajhb.22520
Jawaid, P., Rehman, M. U., Hassan, M. A., Zhao, Q. L., Li, P., Miyamoto, Y., et al. (2016). Effect of platinum nanoparticles on cell death induced by ultrasound in human lymphoma U937 cells. Ultrason Sonochem 31, 206–215. doi: 10.1016/j.ultsonch.2015.12.013
Jiang, S., Wang, Q., Feng, M., Li, J., Guan, Z., An, D., et al. (2017). C2-ceramide enhances sorafenib-induced caspase-dependent apoptosis via PI3K/AKT/mTOR and Erk signaling pathways in HCC cells. Appl. Microbiol. Biotechnol. 101, 1535–1546. doi: 10.1007/s00253-016-7930-9
Kang, K. W., Cho, I. J., Lee, C. H., and Kim, S. G. (2003). Essential role of phosphatidylinositol 3-kinase-dependent CCAAT/enhancer binding protein beta activation in the induction of glutathione S-transferase by oltipraz. J. Natl. Cancer Inst. 95, 53–66.
Kang, K. W., Kim, Y. G., Cho, M. K., Bae, S. K., Kim, C. W., Lee, M. G., et al. (2002). Oltipraz regenerates cirrhotic liver through CCAAT/enhancer binding protein-mediated stellate cell inactivation. FASEB J. 16, 1988–1990. doi: 10.1096/fj.02-0406fje
Kaplowitz, N. (2004). Drug-induced liver injury. Clin. Infect. Dis 38(Suppl. 2), S44–S48. doi: 10.1086/381446
Kim, J.-H., Nam, W. S., Kim, S. J., Kwon, O. K., Seung, E. J., Jo, J. J., et al. (2017). Mechanism investigation of rifampicin-induced liver injury using comparative toxicoproteomics in mice. Int. J. Mol. Sci. 18, 1417. doi: 10.3390/ijms18071417
Kwon, Y. N., Shin, S. M., Cho, I. J., and Kim, S. G. (2009). Oxidized metabolites of oltipraz exert cytoprotective effects against arachidonic acid through AMP-activated protein kinase-dependent cellular antioxidant effect and mitochondrial protection. Drug Metab. Dispos. 37, 1187–1197. doi: 10.1124/dmd.108.025908
Langouet, S., Coles, B., Morel, F., Becquemont, L., Beaune, P., Guengerich, F. P., et al. (1995). Inhibition of CYP1A2 and CYP3A4 by oltipraz results in reduction of aflatoxin B1 metabolism in human hepatocytes in primary culture. Cancer Res. 55, 5574–5579.
Li, F., and Zhang, N. (2015). Ceramide: therapeutic Potential in Combination Therapy for Cancer Treatment. Curr. Drug Metab. 17, 37–51.
Li, R., Liu, F., Chang, Y., Ma, X., Li, M., Li, C., et al. (2017). Glutathione S-transferase A1 (GSTA1) as a marker of acetaminophen-induced hepatocyte injury in vitro. Toxicol. Mech. Methods 27, 401–407. doi: 10.1080/15376516.2017.1320457
Liu, F., Lin, Y., Li, Z., Ma, X., Han, Q., Liu, Y., et al. (2014). Glutathione S-transferase A1 (GSTA1) release, an early indicator of acute hepatic injury in mice. Food Chem. Toxicol. 71, 225–230. doi: 10.1016/j.fct.2014.06.011
Liu, F. P., Ma, X., Li, M. M., Li, Z., Han, Q., Li, R., et al. (2016). Hepatoprotective effects of Solanum nigrum against ethanol-induced injury in primary hepatocytes and mice with analysis of glutathione S-transferase A1. J. Chin. Med. Assoc. 79, 65–71. doi: 10.1016/j.jcma.2015.08.013
Lu, B., Xu, Y., Xu, L., Cong, X., Yin, L., Li, H., et al. (2012). Mechanism investigation of dioscin against CCl4-induced acute liver damage in mice. Environ. Toxicol. Pharmacol. 34, 127–135. doi: 10.1016/j.etap.2012.03.010
Ma, X., Liu, F., Li, M., Li, Z., Lin, Y., Li, R., et al. (2017). Expression of glutathione S-transferase A1, a phase II drug-metabolizing enzyme in acute hepatic injury on mice. Exp. Ther. Med. 14, 3798–3804. doi: 10.3892/etm.2017.4957
Marcus, N. Y., Blomenkamp, K., Ahmad, M., and Teckman, J. H. (2012). Oxidative stress contributes to liver damage in a murine model of alpha-1-antitrypsin deficiency. Exp. Biol. Med. 237, 1163–1172. doi: 10.1258/ebm.2012.012106
Matic, M., Pekmezovic, T., Djukic, T., Mimic-Oka, J., Dragicevic, D., Krivic, B., et al. (2013). GSTA1, GSTM1, GSTP1, and GSTT1 polymorphisms and susceptibility to smoking-related bladder cancer: a case-control study. Urol. Oncol. 31, 1184–1192. doi: 10.1016/j.urolonc.2011.08.005
Michaut, A., Le Guillou, D., Moreau, C., Bucher, S., McGill, M. R., Martinais, S., et al. (2016). A cellular model to study drug-induced liver injury in nonalcoholic fatty liver disease: application to acetaminophen. Toxicol. Appl. Pharmacol. 292, 40–55. doi: 10.1016/j.taap.2015.12.020
Michels, A. J., and Hagen, T. M. (2009). Hepatocyte nuclear factor 1 is essential for transcription of sodium-dependent vitamin C transporter protein 1. Am. J. Physiol. Cell Physiol. 297, C1220–C1227. doi: 10.1152/ajpcell.00348.2009
Mosialou, I., Krasagakis, K., and Kardassis, D. (2011). Opposite regulation of the human apolipoprotein M gene by hepatocyte nuclear factor 1 and Jun transcription factors. J. Biol. Chem. 286, 17259–17269. doi: 10.1074/jbc.M110.200659
Ozer, J., Ratner, M., Shaw, M., Bailey, W., and Schomaker, S. (2008). The current state of serum biomarkers of hepatotoxicity. Toxicology 245, 194–205. doi: 10.1016/j.tox.2007.11.021
Petzer, J. P., Navamal, M., Johnson, J. K., Kwak, M. K., Kensler, T. W., and Fishbein, J. C. (2003). Phase 2 enzyme induction by the major metabolite of oltipraz. Chem. Res. Toxicol. 16, 1463–1469. doi: 10.1021/tx034154e
Pontoglio, M. (2000). Hepatocyte nuclear factor 1, a transcription factor at the crossroads of glucose homeostasis. J. Am. Soc. Nephrol. 11(Suppl. 16), S140–S143.
Reuben, A., Koch, D. G., and Lee, W. M. (2010). Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 52, 2065–2076. doi: 10.1002/hep.23937
Shi, C. X., Lin, Y. X., Liu, F. P., Chang, Y. C., Li, R., Li, C. W., et al. (2017). Hepatoprotective effects of ethanol extracts from Folium Syringae against acetaminophen-induced hepatotoxicity in vitro and in vivo. J. Chin. Med. Assoc. 80, 623–629. doi: 10.1016/j.jcma.2017.03.007
Shin, S. M., and Kim, S. G. (2009). Inhibition of arachidonic acid and iron-induced mitochondrial dysfunction and apoptosis by oltipraz and novel 1,2-dithiole-3-thione congeners. Mol. Pharmacol. 75, 242–253. doi: 10.1124/mol.108.051128
Singhal, S. S., Singh, S. P., Singhal, P., Horne, D., Singhal, J., and Awasthi, S. (2015). Antioxidant role of glutathione S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated signaling. Toxicol. Appl. Pharmacol. 289, 361–370. doi: 10.1016/j.taap.2015.10.006
Sohn, S. W., Jung, J. W., Lee, S. Y., Kang, H. R., Park, H. W., Min, K. U., et al. (2013). Expression pattern of GSTP1 and GSTA1 in the pathogenesis of asthma. Exp. Lung Res. 39, 173–181. doi: 10.3109/01902148.2013.789572
Thayyullathil, F., Rahman, A., Pallichankandy, S., Patel, M., and Galadari, S. (2014). ROS-dependent prostate apoptosis response-4 (Par-4) up-regulation and ceramide generation are the prime signaling events associated with curcumin-induced autophagic cell death in human malignant glioma. FEBS Open Bio 4, 763–776. doi: 10.1016/j.fob.2014.08.005
Yabu, T., Shiba, H., Shibasaki, Y., Nakanishi, T., Imamura, S., Touhata, K., et al. (2015). Stress-induced ceramide generation and apoptosis via the phosphorylation and activation of nSMase1 by JNK signaling. Cell Death. Differ. 22, 258–273. doi: 10.1038/cdd.2014.128
Yuan, H. D., Jin, G. Z., and Piao, G. C. (2010). Hepatoprotective effects of an active part from Artemisia sacrorum Ledeb. against acetaminophen-induced toxicity in mice. J. Ethnopharmacol. 127, 528–533. doi: 10.1016/j.jep.2009.10.002
Keywords: GSTA1, HNF-1, C2-ceramide, oltipraz, acetaminophen, liver injury
Citation: Ma X, Chang Y, Zhang Y, Muhammad I, Shi C, Li R, Li C, Li Z, Lin Y, Han Q and Liu F (2018) Effects of C2-Ceramide and Oltipraz on Hepatocyte Nuclear Factor-1 and Glutathione S-Transferase A1 in Acetaminophen-Mediated Acute Mice Liver Injury. Front. Pharmacol. 9:1009. doi: 10.3389/fphar.2018.01009
Received: 12 June 2018; Accepted: 20 August 2018;
Published: 11 September 2018.
Edited by:
Raffaele Capasso, Università degli Studi di Napoli Federico II, ItalyReviewed by:
Huiyong Yin, Shanghai Institutes for Biological Sciences (CAS), ChinaCopyright © 2018 Ma, Chang, Zhang, Muhammad, Shi, Li, Li, Li, Lin, Han and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fangping Liu, ZmFuZ3BpbmdsaXVAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.