
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Pharmacol. , 31 August 2018
Sec. Neuropharmacology
Volume 9 - 2018 | https://doi.org/10.3389/fphar.2018.00994
There is a substantive clinical literature on classical hallucinogens, most commonly lysergic acid diethylamide (LSD) for the treatment of alcohol use disorder. However, there has been no published research on the effect of LSD on alcohol consumption in animals. This study evaluated the effect of LSD in mice using a two-bottle choice alcohol drinking paradigm. Adult male C57BL/6J mice were exposed to ethanol to develop preference and divided into three groups of equal ethanol consumption, and then treated with single intraperitoneal injection of saline or 25 or 50 μg/kg LSD and offered water and 20% ethanol. The respective LSD-treated groups were compared to the control group utilizing a multilevel model for repeated measures. In mice treated with 50 μg/kg LSD ethanol consumption was reduced relative to controls (p = 0.0035), as was ethanol preference (p = 0.0024), with a group mean reduction of ethanol consumption of 17.9% sustained over an interval of 46 days following LSD administration. No significant effects on ethanol consumption or preference were observed in mice treated with 25 μg/kg LSD. Neither total fluid intake nor locomotor activity in the LSD-treated groups differed significantly from controls. These results suggest that classical hallucinogens in the animal model merit further study as a potential approach to the identification of targets for drug discovery and investigation of the neurobiology of addiction.
Alcohol-related disorders accounted for approximately 88,000 deaths annually and one in ten deaths in working age adults in the US from 2006 to 2010 (Stahre et al., 2014), and are estimated to cause 5.9% of deaths worldwide (World Health Organization, 2014). The 2012-2013 National Epidemiologic Survey on Alcohol and Related Conditions III found a 12-month prevalence of alcohol use disorder (AUD) of 13.9% (Grant et al., 2015), and the economic cost of alcohol misuse in the US in 2010 was estimated at $249 billion (Sacks et al., 2015).
A class of serotonin type 2A receptor (5-HT2AR) agonists with a distinctive domain of subjective effects, referenced as “classical hallucinogens” (Nichols, 2004), “serotonergic hallucinogens” (Halberstadt, 2015), or “psychedelics” (Nichols, 2016), are reported to be effective in some clinical studies of AUD (Krebs and Johansen, 2012; Bogenschutz et al., 2015), and other substance use disorders (Bogenschutz and Johnson, 2016). Among the classical hallucinogens, LSD, the prototype of this group of compounds, has the most extensive history of use in clinical research on AUD (Krebs and Johansen, 2012; Liechti et al., 2017). The literature on LSD for the treatment of AUD extends back to the 1950s, and a meta-analysis that included 6 randomized controlled trials (RCTs) involving a total of 536 subjects found that treatment with a single dose of LSD significantly reduced alcohol misuse for up to three months (Krebs and Johansen, 2012). This meta-analysis retrieved an additional 23 non-RCT open-label studies, case reports, or studies that did not otherwise meet inclusion criteria, indicating the considerable extent of the historical interest in clinical research on LSD for the treatment of AUD.
There is apparently no previously published work on the effects of LSD on the self-administration of alcohol or other abused substances in animals. In view of the elements of possible relevance to addiction in the signaling cascade downstream from the binding sites of LSD (Lopez-Gimenez and Gonzalez-Maeso, 2018), an effect of LSD on the self-administration of alcohol in animals would provide support for the animal model as a possible approach to target identification and drug discovery. This present research focuses on the effect of LSD on alcohol intake in a two-bottle choice drinking paradigm in C57BL/6J mice, an inbred alcohol-preferring strain (Melo et al., 1996).
This research was conducted in accordance with the National Institutes of Health and Nathan Kline Institute Animal Care and Use Committee’s guidelines. All experiments were conducted in adult male C57BL/6J mice (10–18 weeks old; Jackson Laboratory, United States). Mice were given unlimited access to standard mouse chow and water throughout the entire study.
The effect of LSD on ethanol consumption was assessed using a two-bottle choice drinking paradigm. Mice were housed singly and habituated to reverse light-dark cycle for at least a week. The mice were then exposed to ethanol to develop preference with two bottles containing water and 20% ethanol offered for 24 h in the beginning of the dark phase for five days a week (Monday through Friday) for 4 weeks. The mice were then divided into three groups of equal ethanol intake based on the amount of ethanol consumed on the day before administration of either saline or LSD (25 or 50 μg/kg body weight, i.p.) once before the onset of the dark cycle. LSD was supplied by the NIDA Drug Supply Program, Bethesda, MD, United States. Water bottles were replaced with bottles containing water and ethanol after 10 min of treatment. Amounts of ethanol and water consumption were measured every 24 h. The positions of water and ethanol bottles were alternated every day to avoid place preference. Two bottles containing water and 20% ethanol were placed in a cage without a mouse to control for spillage and evaporation.
Ambulatory activity was measured as ambulatory counts (interruption of the total number of beams, on both the x and y-axis) with an infrared beam-based activity sensor (ATM3; Columbus Instrument, Columbus, OH, United States) over a 24-h period beginning at the dark cycle at 1 and 8 days after the administration of 50 μg/kg LSD or saline to ethanol-naïve mice.
Statistical analyses were performed with SAS® software Version 9.4, utilizing a multilevel model (MLM) for repeated measures with PROC MIXED (SAS Institute Inc., 2018), and t-tests (one-tailed) with p-value = 0.05 as the threshold for significance.
The interval of observation of ethanol intake was continued until self-administration amounts of ethanol had reached equivalence, defined as two consecutive days on which ethanol consumption in the LSD-treated group equaled or exceeded the control group. For the group treated with 25 μg/kg, equivalence was reached at day 23; for the group treated with 50 μg/kg LSD it had not been reached at the time the study was terminated on day 46. For the group treated with 25 μg/kg LSD, the MLM independent variables included time, group and the interaction between them (to account for the change over time in the treated group). For the group treated with 50 μg/kg LSD, the interaction was not included because there was little evidence of change over time.
Ethanol consumption was decreased relative to controls in the group treated with 50 μg/kg LSD (F = 11.99, df = 15, p = 0.0035; Figure 1B), without a significant effect with 25 μg/kg LSD (F = 3.08, df = 17, p = 0.0974; Figure 1A). Notably, the effect appears to have been sustained across the 46-day interval of observation. Likewise, ethanol preference, defined as ethanol consumption/total fluid intake, (where total fluid is defined as 20% ethanol solution + water) was decreased relative to controls in the 50 μg/kg LSD group (F = 13.32, df = 15, p = 0.0024; Figure 1D), with no difference from control for the group treated with 25 μg/kg LSD (F = 0.53, df = 17, p = 0.475; Figure 1C). Total fluid intake did not differ from control in either the 25 μg/kg LSD (F = 0.68, df = 17, p = 0.4204; Figure 1E) or 50 μg/kg LSD (F = 1.30, df = 15, p = 0.2722; Figure 1F).
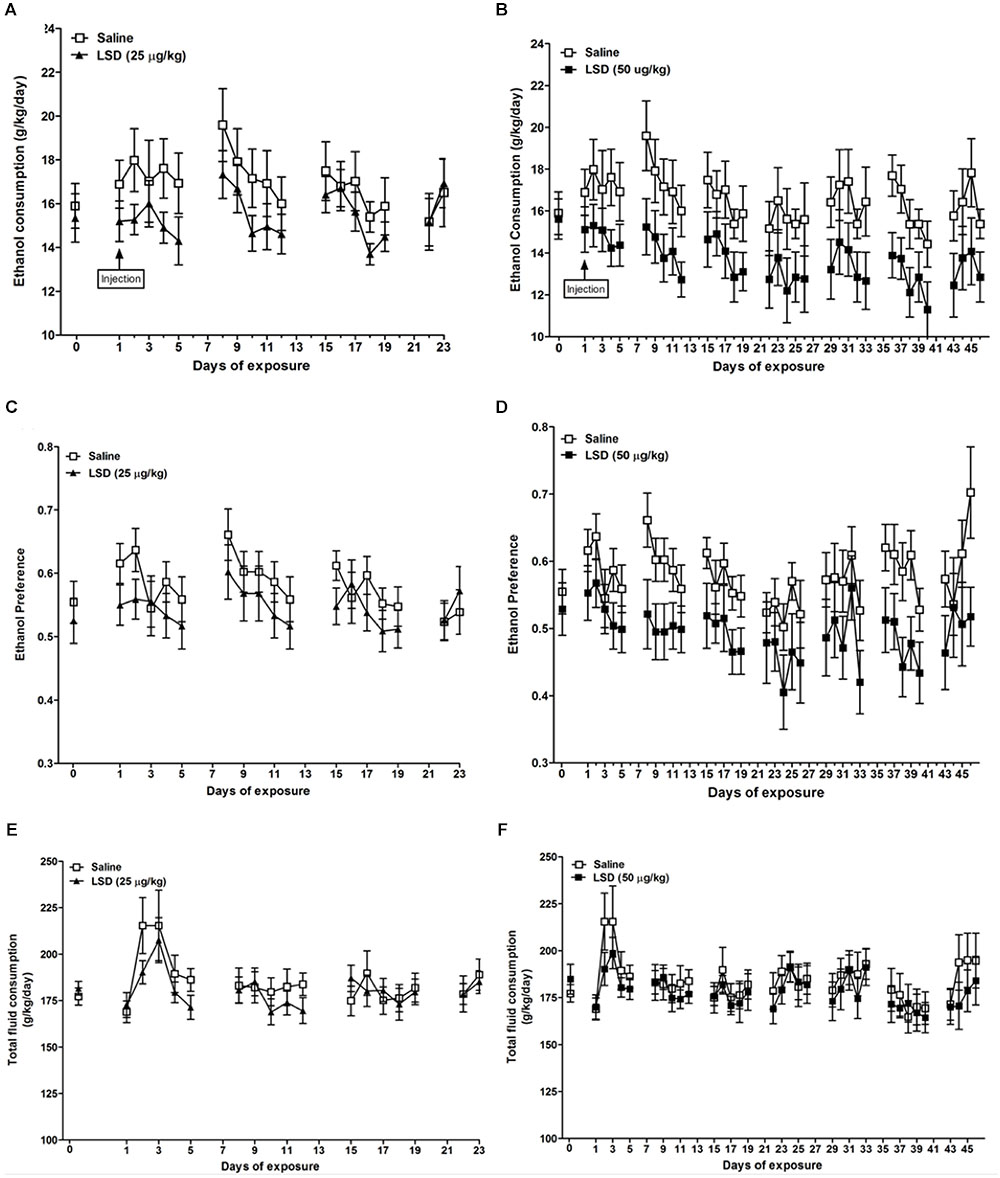
FIGURE 1. Ethanol consumption (A,B), ethanol preference (C,D), and total fluid consumption (E,F) in mice treated with single dosages of LSD of 25 (left column) or 50 (right column) μg/kg body weight, i.p. Data are presented as mean ± SEM (n = 8–10 per group). Horizontal axis indicates the number of days following the administration of LSD. The mice were divided into three groups of equal ethanol consumption based on the amount of ethanol consumed on the day before LSD administration (day 0). LSD dosages were administered on day 1 [“injection,” as indicated in the top row panels (A,B)]. In the group treated with 50 μg/kg LSD, MLM for repeated measures indicated significant reductions versus saline-treated controls in ethanol consumption (p = 0.0035) and preference (p = 0.0024). Differences from control were not significant for the group treated with 25 μg/kg. Total fluid intake did not differ significantly from controls in either LSD-treated group (E,F) (See “Results” in text).
In the group treated with 50 μg/kg LSD, mean daily ethanol consumption was 13.62 ± 3.02 g/kg/day compared to 16.60 ± 3.52 g/kg/day (mean ± SD) in controls (t = -1.86, df = 15, p = 0.0414), and ethanol preference was 0.493 ± 0.104 versus 0.580 ± 0.091 in controls (t = -1.83, df = 15, p = 0.0436), a 17.9% reduction in the LSD-treated animals, without a significant difference in mean daily total fluid intake. The group treated with 25 μg/kg LSD did not differ from control group with regard to mean daily ethanol consumption or preference, or total fluid intake.
To evaluate a potential effect of LSD on ambulatory activity, a group of ethanol-naïve mice were treated with 50 μg/kg LSD or saline, and ambulatory counts were measured on day 1 and 8 (Figure 2). The result showed no significant difference in locomotor activity comparisons between control versus the LSD-treated group utilizing a MLM for hourly ambulatory counts on day 1 (F = 0.74, df = 14, p = 0.4054) or day 8 (F < 0.01, df = 14, p = 0.9907).
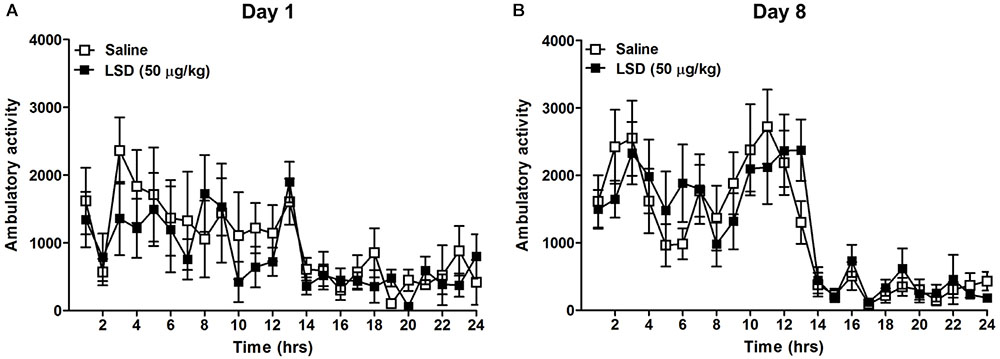
FIGURE 2. Home cage ambulatory activity (beams broken X-Y) was measured for 24 h following the administration of LSD 50 μg/kg weight, i.p. to ethanol-naïve mice on day 1 immediately following LSD administration (A) and 8 days later (B). Data are presented as mean ± SEM (n = 8 per group). LSD administration did not significantly alter the ambulatory activity compared to saline treated mice on either day (See “Results” in text).
This study found reductions in both ethanol consumption and preference in the group of mice treated with the LSD dosage of 50 μg/kg. Although the magnitude of effect on ethanol consumption was limited, with a group mean reduction of 17.9%, it was sustained across a time interval of 46 days. Total fluid intake and locomotor activity did not differ among the control and LSD-treated groups, indicating that the observed effect on ethanol self-administration was not an artifact of a global effect on water or total fluid intake or possible differences in physically approaching or self-administering fluid.
This is apparently the first study to report on the effects of LSD on the self-administration of alcohol in animals. The literature reporting on the effect of classical hallucinogens on animal drug or alcohol self-administration is apparently limited to a single study that found the classical hallucinogen 1-(2,5-dimethoxy-4 iodophenyl)-2-aminopropane (DOI) administered intraperitoneally 15 min prior to a 12 h test session diminished alcohol preference in a two-lever water versus ethanol choice procedure in rats (Maurel et al., 1999). However, DOI is not frequently used by humans and has some features such as a very long half-life that would limit its generalizability to LSD, and the available evidence raises questions regarding its safety in humans (Kowalski and Salzman, 2012) relative to that of LSD (Nichols and Grob, 2018).
Mechanisms that could logically be proposed to explain the findings reported here include effects on G-protein coupled signal transduction and downstream effectors, second messengers, and gene expression (Halberstadt, 2015; Nichols, 2016; Lopez-Gimenez and Gonzalez-Maeso, 2018; Martin and Nichols, 2018), and a systematic exploration of these possibilities is well beyond the scope of this discussion. Classical hallucinogens produce 5-HT2AR desensitization (Nichols, 2016), and it has been suggested that persistently reduced signaling in downstream pathways linked to the 5-HT2AR might explain sustained effects observed in clinical studies on substance use (Bogenschutz and Johnson, 2016) or mood and anxiety symptoms (Vollenweider and Kometer, 2010; Ross et al., 2016).
Diminished signaling in downstream pathways linked to the 5-HT2AR could increase transmission via metabotropic glutamate receptors 2 and 3 (mGluR2/3) due to the reciprocally inhibitory relationship between 5-HT2AR and GluR2/3 (Nichols, 2016). Increased transmission through mGluR2/3, which are presynaptic and autoinhibitory on glutaminergic neurons, might be expected to diminish alcohol intake in view of evidence for mGluR2/3 agonists as possible pharmacotherapy for alcohol dependence (Goodwani et al., 2017; Windisch and Czachowski, 2018). Extracellular signal-regulated kinases 1 and 2 (ERK1/2) are positively coupled to the 5-HT2AR, suggesting that a diminution of 5-HT2AR signaling could have anti-addictive effects in view of evidence linking ERK1/2 activation to neuroadaptations and animal behaviors putatively associated with addiction (Girault et al., 2007; Pascoli et al., 2014).
Work that could logically follow includes neurochemical analysis aimed at identifying signaling pathways which might mediate the effect of LSD on alcohol intake observed in this study. Coupling among G proteins, phospholipase effectors and the 5-HT2AR subsumes a set questions of current interest in research on psychedelics. Although 5-HT2ARs classically couple to Gαq/11, the effects of classical hallucinogens also apparently involve Gαi/o (Lopez-Gimenez and Gonzalez-Maeso, 2018). Gi/o signaling interacts with brain region, for example alcohol intake in C57BL/6 mice is decreased by activation of Gi/o protein that is regionally expressed in the dorsal striatum (Robins et al., 2018). Investigative approaches to identifying the signaling pathways and cell populations involved may include measurement of protein expression and function as well as intracerebral injection of LSD in specific brain sites. Future studies may utilize alcohol-related behaviors such as drinking-in-the dark, intermittent access, or withdrawal to model AUD phenotypes (Leeman et al., 2010).
The finding of reduced ethanol preference with LSD in a rodent model suggests that the efficacy of LSD for the treatment of AUD reported in clinical studies may be not fully explained by psychological factors and involves a biologically mediated effect. The settings in which LSD has been administered as treatment for AUD are varied, including an RCT reporting positive results in which patients were strapped to a bed in a hospital room (Smart et al., 1966), suggesting that the effect of LSD on alcohol misuse may have involved a pharmacological determinant distinct from psychotherapeutic set and setting.
In view of the lack of prior published animal self-administration studies using LSD, reference to other animal behavioral paradigms may help contextualize the dosages of LSD used in this present research. Two behavioral paradigms appear relevant, the head twitch response (HTR) (Halberstadt and Geyer, 2013) and discrimination (Nichols, 2016). Reported ED50 dosages for correct responding to LSD in discrimination studies in mice range from approximately 170 to 200 μg/kg (Benneyworth et al., 2005; Winter et al., 2005; Krall et al., 2008). Similarly, in studies of the HTR, a rapid side-to-side rotational head movement in response to administered classical hallucinogens that is thought to correspond with compounds that produce psychedelic effects in humans, the maximum HTR to LSD is seen at 200 μg/kg, and 50 μg/kg appears to be approximately ED50 (Halberstadt and Geyer, 2013). The data reported from discrimination and HTR studies of LSD suggest that the dosages used in this study might not represent the maximum of the dose response curve, which should be addressed by evaluating higher dosages in future work.
In summary, this study found that LSD administered as a single dose reduces ethanol consumption and preference in C57BL/6J mice. These findings indicate that the apparent effect of LSD in AUD observed in clinical samples may be modeled in animals, suggesting the involvement of a biologically mediated determinant, and support the potential utility of classical hallucinogens in the animal model as a paradigm for target identification in drug discovery and investigation of the neurobiology of addiction.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
KA originated the study and wrote the first draft of the manuscript. BD and RS performed the experiments. KA, HS, and KYV conceived and designed the study. KYV performed the experiments, wrote sections of the manuscript, and performed statistical analysis. All authors contributed to manuscript revision, read and approved the submitted version.
Funding for this research was provided by the Research Foundation for Mental Hygiene, Inc. (RFMH) in honor of Robert Cancro, M.D., and a donation from Carey and Claudia Turnbull.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Peter Flom, Ph.D. for providing statistical consultation, David Nichols, Ph.D. for helpful discussion and NIDA (Bethesda, MD) for the supply of LSD.
Benneyworth, M. A., Smith, R. L., Barrett, R. J., and Sanders-Bush, E. (2005). Complex discriminative stimulus properties of (+)lysergic acid diethylamide (LSD) in C57BL/6J mice. Psychopharmacology 179, 854–862. doi: 10.1007/s00213-004-2108-z
Bogenschutz, M. P., Forcehimes, A. A., Pommy, J. A., Wilcox, C. E., Barbosa, P. C., and Strassman, R. J. (2015). Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J. Psychopharmacol. 29, 289–299. doi: 10.1177/0269881114565144
Bogenschutz, M. P., and Johnson, M. W. (2016). Classic hallucinogens in the treatment of addictions. Prog. Neuropsychopharmacol. Biol. Psychiatry 64, 250–258. doi: 10.1016/j.pnpbp.2015.03.002
Girault, J. A., Valjent, E., Caboche, J., and Herve, D. (2007). ERK2: a logical AND gate critical for drug-induced plasticity? Curr. Opin. Pharmacol. 7, 77–85. doi: 10.1016/j.coph.2006.08.012
Goodwani, S., Saternos, H., Alasmari, F., and Sari, Y. (2017). Metabotropic and ionotropic glutamate receptors as potential targets for the treatment of alcohol use disorder. Neurosci. Biobehav. Rev. 77, 14–31. doi: 10.1016/j.neubiorev.2017.02.024
Grant, B. F., Goldstein, R. B., Saha, T. D., Chou, S. P., Jung, J., Zhang, H., et al. (2015). Epidemiology of DSM-5 alcohol use disorder: results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiatry 72, 757–766. doi: 10.1001/jamapsychiatry.2015.0584
Halberstadt, A. L. (2015). Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav. Brain Res. 277, 99–120. doi: 10.1016/j.bbr.2014.07.016
Halberstadt, A. L., and Geyer, M. A. (2013). Characterization of the head-twitch response induced by hallucinogens in mice: detection of the behavior based on the dynamics of head movement. Psychopharmacology 227, 727–739. doi: 10.1007/s00213-013-3006-z
Kowalski, J. M., and Salzman, M. S. (2012). Pseudo-ergotism resulting in quadruple amputation after DOI abuse. Clin. Toxicol. 50, 709–710.
Krall, C. M., Richards, J. B., Rabin, R. A., and Winter, J. C. (2008). Marked decrease of LSD-induced stimulus control in serotonin transporter knockout mice. Pharmacol. Biochem. Behav. 88, 349–357. doi: 10.1016/j.pbb.2007.09.006
Krebs, T. S., and Johansen, P. O. (2012). Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials. J. Psychopharmacol. 26, 994–1002. doi: 10.1177/0269881112439253
Leeman, R. F., Heilig, M., Cunningham, C. L., Stephens, D. N., Duka, T., and O’Malley, S. S. (2010). Ethanol consumption: how should we measure it? Achieving consilience between human and animal phenotypes. Addict. Biol. 15, 109–124. doi: 10.1111/j.1369-1600.2009.00192.x
Liechti, M. E., Dolder, P. C., and Schmid, Y. (2017). Alterations of consciousness and mystical-type experiences after acute LSD in humans. Psychopharmacology 234, 1499–1510. doi: 10.1007/s00213-016-4453-0
Lopez-Gimenez, J. F., and Gonzalez-Maeso, J. (2018). Hallucinogens and serotonin 5-HT2A receptor-mediated signaling pathways. Curr. Top. Behav. Neurosci. 36, 45–73. doi: 10.1007/7854_2017_478
Martin, D. A., and Nichols, C. D. (2018). The effects of hallucinogens on gene expression. Curr. Top. Behav. Neurosci. 36, 137–158. doi: 10.1007/7854_2017_479
Maurel, S., De Vry, J., and Schreiber, R. (1999). 5-HT receptor ligands differentially affect operant oral self-administration of ethanol in the rat. Eur. J. Pharmacol. 370, 217–223. doi: 10.1016/s0014-2999(99)00125-9
Melo, J. A., Shendure, J., Pociask, K., and Silver, L. M. (1996). Identification of sex specific quantitative trait loci controlling alcohol preference in C57BL/6 mice. Nat. Genet. 13, 147–153. doi: 10.1038/ng0696-147
Nichols, D. E. (2004). Hallucinogens. Pharmacol. Ther. 101, 131–181. doi: 10.1016/j.pharmthera.2003.11.002
Nichols, D. E., and Grob, C. S. (2018). Is LSD toxic? Forensic Sci. Int. 284, 141–145. doi: 10.1016/j.forsciint.2018.01.006
Pascoli, V., Cahill, E., Bellivier, F., Caboche, J., and Vanhoutte, P. (2014). Extracellular signal-regulated protein kinases 1 and 2 activation by addictive drugs: a signal toward pathological adaptation. Biol. Psychiatry 76, 917–926. doi: 10.1016/j.biopsych.2014.04.005
Robins, M. T., Chiang, T., Mores, K. L., Alongkronrusmee, D., and van Rijn, R. M. (2018). Critical role for Gi/o-protein activity in the dorsal striatum in the reduction of voluntary alcohol intake in C57BL/6 mice. Front. Psychiatry 9:112. doi: 10.3389/fpsyt.2018.00112
Ross, S., Bossis, A., Guss, J., Agin-Liebes, G., Malone, T., Cohen, B., et al. (2016). Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J. Psychopharmacol. 30, 1165–1180. doi: 10.1177/0269881116675512
Sacks, J. J., Gonzales, K. R., Bouchery, E. E., Tomedi, L. E., and Brewer, R. D. (2015). 2010 national and state costs of excessive alcohol consumption. Am. J. Prev. Med. 49, e73–e79. doi: 10.1016/j.amepre.2015.05.031
SAS Institute Inc. (2018). The MIXED Procedure. SAS/STAT 14.3 User’s Guide. Available at: http://documentation.sas.com/?docsetId=statug&docsetTarget=statug_mixed_syntax01.htm&docsetVersion=14.3&locale=en
Smart, R. G., Storm, T., Baker, E. F., and Solursh, L. (1966). Controlled study of lysergide in treatment of alcoholism 1. The effects on drinking behavior. Q. J. Stud. Alcohol 27, 469–482.
Stahre, M., Roeber, J., Kanny, D., Brewer, R. D., and Zhang, X. (2014). Contribution of excessive alcohol consumption to deaths and years of potential life lost in the united states. Prev. Chronic Dis. 11:E109. doi: 10.5888/pcd11.130293
Vollenweider, F. X., and Kometer, M. (2010). The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat. Rev. Neurosci. 11, 642–651. doi: 10.1038/nrn2884
Windisch, K. A., and Czachowski, C. L. (2018). Effects of group II metabotropic glutamate receptor modulation on ethanol- and sucrose-seeking and consumption in the rat. Alcohol 66, 77–85. doi: 10.1016/j.alcohol.2017.07.011
Winter, J. C., Kieres, A. K., Zimmerman, M. D., Reissig, C. J., Eckler, J. R., Ullrich, T., et al. (2005). The stimulus properties of LSD in C57BL/6 mice. Pharmacol. Biochem. Behav. 81, 830–837. doi: 10.1016/j.pbb.2005.05.014
Keywords: lysergic acid diethylamide, psychedelic, hallucinogen, serotonin receptor agonists, alcohol, mouse, substance-related and addictive disorders
Citation: Alper K, Dong B, Shah R, Sershen H and Vinod KY (2018) LSD Administered as a Single Dose Reduces Alcohol Consumption in C57BL/6J Mice. Front. Pharmacol. 9:994. doi: 10.3389/fphar.2018.00994
Received: 12 May 2018; Accepted: 13 August 2018;
Published: 31 August 2018.
Edited by:
Brian McCool, Wake Forest School of Medicine, United StatesReviewed by:
Alan Rosenwasser, University of Maine, FranceCopyright © 2018 Alper, Dong, Shah, Sershen and Vinod. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kenneth Alper, a2VubmV0aC5hbHBlckBueXVtYy5vcmc= K. Yaragudri Vinod, dmlub2QueWFyYWd1ZHJpQG55dW1jLm9yZw==; Vmlub2QuWWFyYWd1ZHJpQE5LSS5yZm1oLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.