- 1GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands
- 2Department of Obstetrics and Gynaecology, Maastricht University Medical Centre, Maastricht, Netherlands
- 3Forendo Pharma Ltd., Turku, Finland
Our understanding of the intracrine (or local) regulation of estrogen and other steroid synthesis and degradation expanded in the last decades, also thanks to recent technological advances in chromatography mass-spectrometry. Estrogen responsive tissues and organs are not passive receivers of the pool of steroids present in the blood but they can actively modify the intra-tissue steroid concentrations. This allows fine-tuning the exposure of responsive tissues and organs to estrogens and other steroids in order to best respond to the physiological needs of each specific organ. Deviations in such intracrine control can lead to unbalanced steroid hormone exposure and disturbances. Through a systematic bibliographic search on the expression of the intracrine enzymes in various tissues, this review gives an up-to-date view of the intracrine estrogen metabolisms, and to a lesser extent that of progestogens and androgens, in the lower female genital tract, including the physiological control of endometrial functions, receptivity, menopausal status and related pathological conditions. An overview of the intracrine regulation in extra gynecological tissues such as the lungs, gastrointestinal tract, brain, colon and bone is given. Current therapeutic approaches aimed at interfering with these metabolisms and future perspectives are discussed.
Introduction
The term “intracrinology,” coined in 1988 by prof Labrie, refers to the ability of peripheral tissues to use blood precursors and generate steroids (Labrie, 1991). Several studies have been published but several controversies still exist and relate to the following technical and biological aspects: (a) some intracrine enzymes in peripheral tissues have low expression (300–50,000-times lower than in endocrine glands Stoffel-Wagner, 2001; Murakami et al., 2006, close to the detection limit of standard methods like western blotting and immunohistochemistry -IHC); (b) the technology to robustly quantify steroids (liquid-/gas-chromatography tandem mass-spectrometry -LC-MS or GC-MS), became available during the last 5–10 years only (Rosner et al., 2013); (c) intracrine pathways are highly complex.
This review summarizes our knowledge of intracrinology in peripheral tissues like the endometrium, lungs, gastrointestinal tract (GIT), bone and central nervous system (CNS), with special attention to the metabolism of estrogens. Drug development and potential therapeutic approaches are discussed. In this review, the enzymes involved in steroid deactivation/clearance (Rižner, 2013, 2016; with the exclusion of steroid sulphotransferases) and those involved in the transport of conjugated steroids through the plasma membrane (Rižner et al., 2017) are not described. Studies on serum/tissue steroid levels are reported and discussed only if based on gold standard GC/LC-MS.
From Ovarian Estrogen Synthesis to Intracrinology
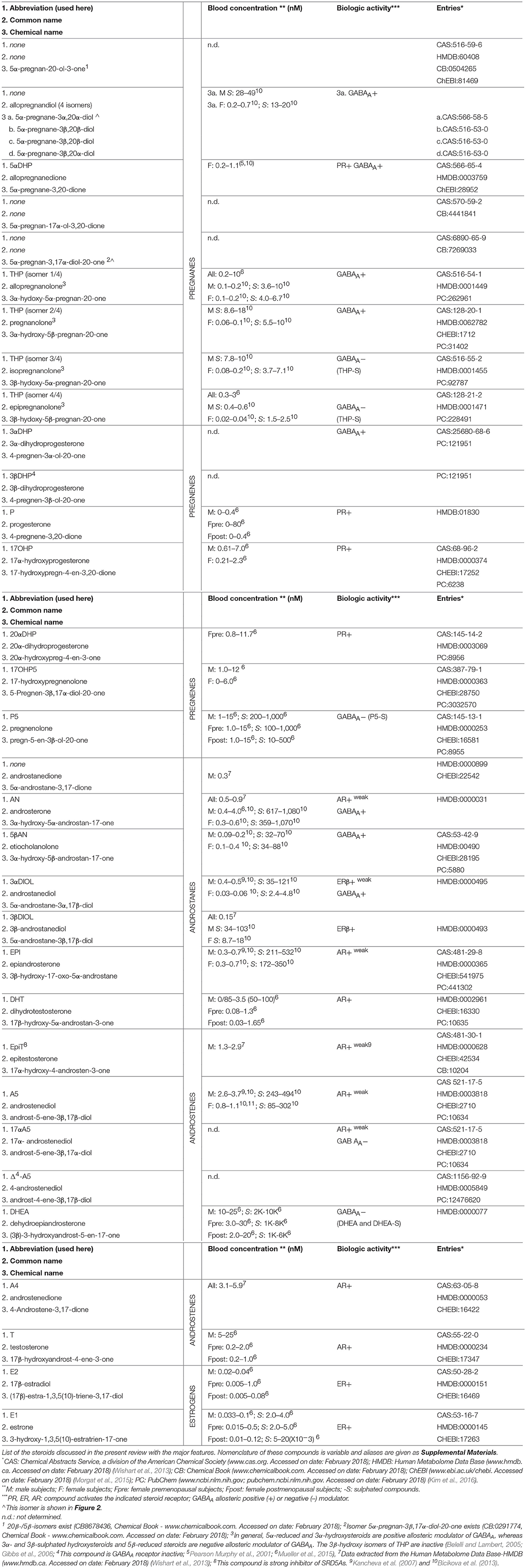
Local steroid metabolism is possible because those enzymes responsible for steroid synthesis in classical glands (ovaries, adrenals, testes) are expressed in peripheral tissues, where additional and alternative routes for metabolizing steroids are present and make intracrine networks intricate and flexible (Figures 1, 2, Tables 1, 2). In particular, several compounds generated through these pathways, although not being estrogens, can have estrogenic action, because able to bind and activate the estrogen receptors. The biologic activity of the various compounds is given in Table 1, and in Figure 2, by the color codes.
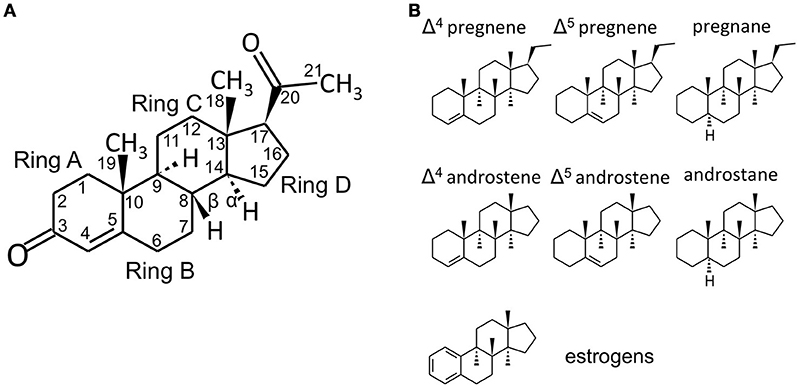
Figure 1. Steroid structure. (A) structure of the C21 steroid progesterone (P, used as an example), with carbon numbering and steroid ring numbering. In the storied graphics in Figures 1B and 2, the H groups and the relative bonds will be omitted (with the exclusion of the H in 5α-reduced steroids - androstanes and pregnanes). Methyl groups will be indicated by the bonds only without the CH3 group. (B) structures of C21 pregnene (Δ4 and Δ5, i.e., double bond between C4 and C5 or between C5 and C6, respectively), pregnane (5α-reduced steroid), C19 androstene (Δ4, Δ5) and androstane and C18 (A-ring)-aromatic estrogens. Chemical structures were designed with the aid of Sketcher V2.4 (Ihlenfeldt et al., 2009), available online at PubChem (www.ncbi.nlm.nih.gov; pubchem.ncbi.nlm.nih.gov) (Kim et al., 2016).
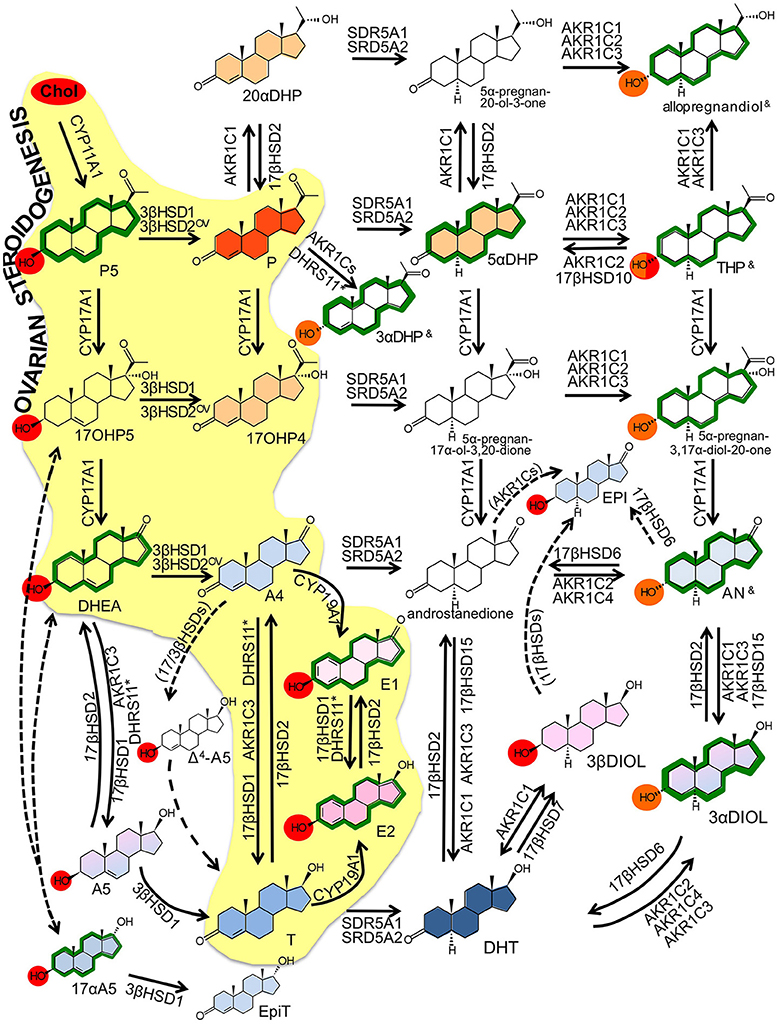
Figure 2. Intracrine networks. Major intracrine networks metabolizing steroids. In this figure, each reaction reports the catalyzing enzymes whose role in that specific reaction is established based on robust evidences (in vitro, ex vivo, in vivo). Additional enzymes whose involvement in the same reactions is less robustly demonstrated or based only on in silico or cell-free assay are reported in Table 2. The role of 17βHSD3 is disregarded in this figure because restricted to tissues that are not assessed in the present review (testes, prostate, Table 2).
Color codes:
OV ovarian specific referring to 3β-HSD2 (see text); — dotted arrows indicate reactions that are not fully demonstrated to occur or for which the responsible enzyme is not identified yet; (enzyme name) enzymes indicated by brackets are supposed to catalyze the indicated reaction based on the theoretical assumptions, no experimental proof is yet available; & these compounds (THP, 3αDHP and allopregnandiol) exist as various hydroxyl α/β isomers (3, 5, 17) with no activity, classic action or neuroactivity (see Table 2); * the role of DHRS11 in steroid metabolism is reported only recently by one publication (Endo et al., 2016).
Ovarian Steroidogenesis
Transformation of cholesterol to 17β-estradiol (E2) involves first the production of dehydroepiandrosterone (DHEA) in theca cells through the action of steroidogenic acute regulatory protein (StAR) that facilitates the transport of cholesterol into mitochondria, followed by CYP11A1 (rate-limiting) and CYP17A1 (Figure 2); the ovarian pathway is indicated by the yellow background; reviewed by (Miller and Auchus, 2011; Andersen and Ezcurra, 2014). CYP11A1 is a type I CYP localized in mitochondria that uses nicotine-adenine-dinucleotide-phosphate (NADPH) and ferredoxin (Fdx)/ferredoxin reductase (FdR) to cleave the cholesterol side chain and produce pregnenolone (P5). Type II CYP17A1, localized in the endoplasmic reticulum (EndRet), has both 17α-hydroxylase and 17,20-lyase activities. It uses NADPH and P450 oxidoreductase (POR) to first hydroxylate P5 to 17α-hydroxypregnenolone (17OHP5) (17α-hydroxylase action), followed by 17,20-lyase action to release DHEA. Gonad specific type 2 3β-hydroxysteroid dehydrogenase (3βHSD2) has 3β-dehydrogenase and Δ5 to Δ4 isomerase activities and converts DHEA to androstenedione (A4). Next, CYP19A1 catalyzes the oxidative demethylation of C19 androgens to C18 estrogens, with A-ring aromatisation; hence A4 is converted to estrone (E1). The final conversion of E1 (with low affinity for the estrogen-receptors -ERs) to E2 (high affinity for ERs and high estrogenic potency) is catalyzed by 17βHSD1 that reduces 17-keto to 17β-hydroxyl steroids. In the ovary, the 17-keto group of A4 can be reduced to 17β-hydroxyl by AKR1C3/17βHSD5 yielding testosterone (T) that is converted to E2 by CYP19A1. Upon ovulation, high 3βHSD2 levels in the corpus luteum lead to high progesterone (P) generation from P5.
Intracrine Steroidogenesis
The expression of StAR, CYP11A1 and CYP17A1 is demonstrated in a limited number of peripheral tissues (see later and Tables 6–8). However, pregnenes, pregnanes, androstenes and androstanes generated from these initial steps (but also abundantly available as circulating precursors) can be further metabolized locally thus generating a plethora of compounds with various biological activities (estrogenic, androgenic, progestogenic and neuroactive; Tables 1, 2 and Figure 2). The Δ5 to Δ4 isomerization of androstenes (DHEA, androstenediol -A5- and 17αA5) and pregnenes (P5, 17OHP5) is catalyzed by 3βHSD1, which is the peripheral counterpart of ovarian 3βHSD2. Also 3βHSD2, whose expression was initially considered to be restricted to endocrine tissues, is detected peripherally in recent reports (Stoffel-Wagner, 2001; Tsai et al., 2001; Attar et al., 2009; Huhtinen et al., 2014; Osinski et al., 2018). Due to the high concentration of DHEA (both in blood and tissues), its conversion to A4 by 3βHSDs is relevant to the formation of downstream androgens and of estrogens. Additionally, 3βHSDs convert A5 and the isomer 17αA5 to T and epitestosterone (EpiT). Although minor, in the context of women's health, these pathways are relevant. A5, together with 3α and 3βDIOL (generated by AKR1Cs from DHT and AN, see below) activate both ERs and have estrogenic action (especially 3βDIOL, a potent ERβ binder). A5 possesses immune stimulatory activity whereas its 17α isomer (17αA5) has androgenic, antitumor and neuroactivity. Additionally, EpiT is a weak AR binder and a strong endogenous inhibitor of SRD5As (Loria and Graf, 2012). The endogenous occurrence of 17αA5 is demonstrated in humans (Laatikainen et al., 1971) but its route of synthesis is unclear (Shimizu, 1979). A 17αHSD able to convert A4 to EpiT and DHEA to 17αA5 is characterized in mice (Bellemare et al., 2005) but no human homologous is described yet. Similarly to the ovaries, androgen to estrogen conversion is catalyzed by CYP19A1.
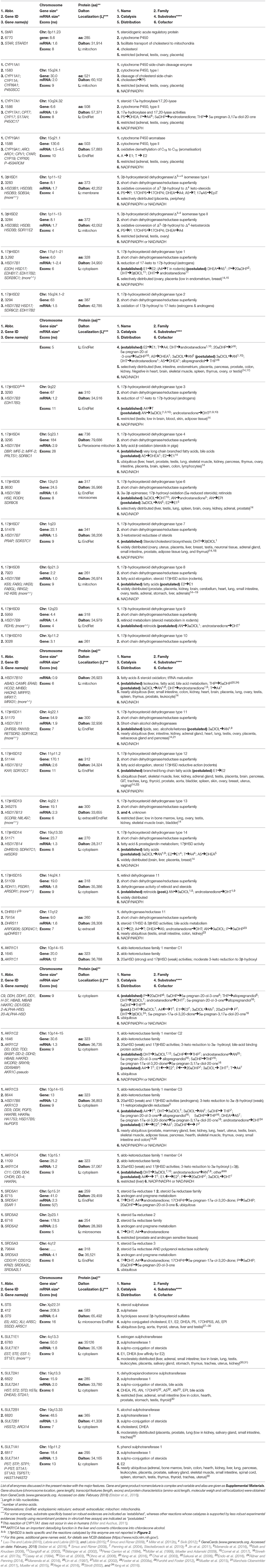
A particularly important reaction is controlled by oxidative and reductive 17βHSDs, which interconvert 17-keto and 17β-hydroxysteroids. Since 17β-hydroxysteroids (T and E2) have higher affinity for the receptors than the keto-steroids (A4 and E1), this balance determines the final androgenic/estrogenic activity. Fourteen 17βHSDs exist, whose specificity is determined by tissue distribution, intracellular localization and biochemistry (Table 2); reviewed thoroughly in (Mindnich et al., 2004; Moeller and Adamski, 2006, 2009; Prehn et al., 2009; Miller and Auchus, 2011). Unpublished data also refer to a 15th 17βHSD (see Table 2; reported in Luu-The et al., 2008) with a putative role in androgen metabolism. With the exclusion of 17βHSD5 (AKR1C3, see below), all other 17βHSDs belong to the short-chain dehydrogenase (SRD) family.
Although all 17βHSDs have been postulated to use steroids as substrates based on cell-free or in vitro assays, recent investigations based on substrate specificity (Laplante et al., 2009) and knock-out (KO) models (Table 4) better clarified their roles. Type 1 17βHSD is the estrogenic enzyme and coverts E1 to E2 both in the ovary and in peripheral tissue. Type 2 17βHSD oxidizes 17-hydroxyl groups (E2 and T) to the 17-keto forms (E1 and A4), and possesses also a 20α-hydroxyl oxidative action, through which this enzyme generates P from 20αDHP. Type 6 17βHSD uses 5α-reduced androgens and has 17-hydroxyl oxidative activity (converting androsterone -AN- to androstanedione) and 3-hydroxyl oxidative activity (converting 3αDIOL to the most potent androgen dihydrotestosterone -DHT). Additional catalytic actions for 17βHSD6 (epimerase or 17-hydroxydehydrogenase) are demonstrated in vitro (Table 2). Type 14 17βHSD is postulated to have 17β-hydroxyl oxidative action on various steroids, type 7 is involved in cholesterol metabolism as indicated by KO mice (Table 4), whereas there is apparently little/no in vivo role of types 8, 9, 10, 11 and 12 17βHSDs on steroid metabolism (Table 2 and indicated by KO mice, Table 4). Recently, a novel SRD, DHRS11, was shown to possess in vitro 17-keto to 17β-hydroxyl reductive action (able to use E1, Δ5 or Δ4 androstenes, androstanes), plus reductive 3βHSD activity toward Δ4 pregnenes and other compounds (5β-steroids, bile acids; Table 2 and Figure 2; Endo et al., 2016).
Androgens and progestogens can be further metabolized by aldo-ketoreductases (AKRs) and 5α-reductases (SRD5As; Figure 2). Cytoplasmic AKRs (AKR1C1, 1C2, 1C3/17βHSD5 and 1C4) have broad substrate specificity with non-stereo-selective 3α/3βHSD, 17- and 20-ketosteroid reductase activities (Table 2; Penning et al., 2004; Steckelbroeck et al., 2010). Together with the fact that they have wide tissue distribution (only AKR1C4 is restricted), AKR1Cs contribute to make intracrine networks flexible and intricate (Rižner and Penning, 2014; Sinreih et al., 2014).
SRD5As convert 3-keto Δ4 androstene and pregnene to 5α-reduced steroids (androstanes and pregnanes), hence they are important in progestogen, androgen (DHT production) and neurosteroid metabolism (Di Costanzo et al., 2009). SRD5A1 and 3 are widely expressed, in contrast to SRD5A2. Human 5β-reductase activity, catalyzed by AKR1D1, is restricted to the liver, where 5β-steroids are directed to clearance/catabolism. However, some 5β-compounds are neuroactive and recent studies indicate the presence of AKR1D1 in placenta and myometrium (Jin et al., 2011). With the exclusion of their neuroactivity (Paragraph 4.6), 5β-steroids will not be further considered.
The sulphatase pathway is finally responsible for the balance between sulpho-conjugated and free steroids. Sulpho-conjugated steroids (-S) possess higher water solubility, increased stability and longer half-life than unconjugated compounds (e.g., 10–12 h vs. 20–30 min for estrogens), and although they cannot bind steroid-receptors, they serve as a reservoir for the formation of biologically active steroids (Reed et al., 2005). Sulphotransferases (SULTs) are phase-I detoxifying enzymes that use bis-phospho-nucleotide 3′-phospho-adenosine-5′-phosphate- (PAP)-sulfate as donor to conjugate 3β-hydroxyl steroids (e.g., estrogens, DHEA, P5, cholesterol; red circles in Figure 2) with a sulfate group (Strott, 2002; Rižner, 2016). Distinct SULTs have different specificities toward substrates, with SULT1E1 being the major estrogen sulphating enzyme (with little contribution of SULT1A1), and SULT2A1 being specific for DHEA (but also for P5, 17OHP5 and A5) (Table 2). Steroid sulphatase (STS) is a membrane-bound microsomal enzyme that catalyzes the hydrolysis of sulfate ester bonds from sulphated-steroids (cholesterol-S, P5-S, 17OHP5-S, DHEA-S, E1-S) (Mueller et al., 2015; Rižner, 2016), thus releasing unconjugated compounds.
Although sulphated-3α-hydroxysteroids are not thoroughly studied, they are detected in biospecimens (AN-S, 3αDIOL-S; Table 1 and orange circles in Figure 2). They are most likely produced by SULT2A1 (active on 3α-hydroxy bile acids) (Strott, 2002; Rižner, 2016) but no 3α-stereo specific sulphatase is known to date. Some intracellular sulphated-steroids are converted to other compounds without prior desulphation (Sánchez-Guijo et al., 2016).
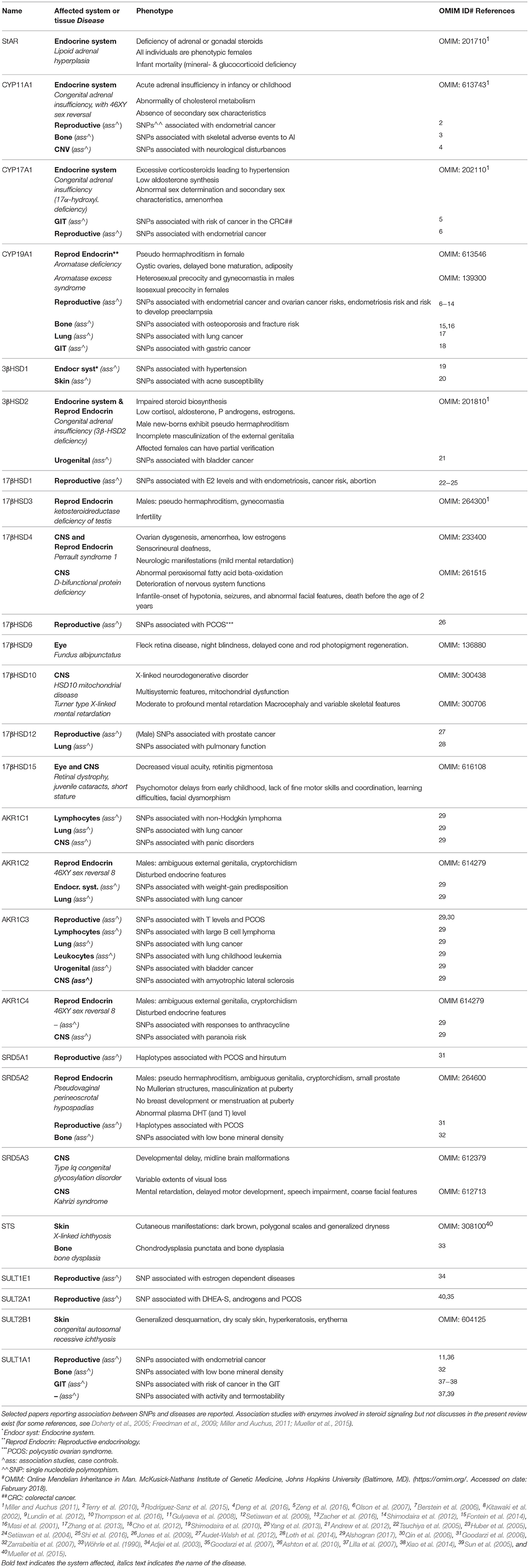
In conclusions, intracrinology presents redundant and complex pathways, which generate compounds with various activities. Genetic variants in intracrine genes are associated with various diseases (classically endocrine and not; Table 5). Even in the absence of the enzymatic machinery to metabolize cholesterol (StAR, steroidogenic factor, CYP17A1 and CYP11A1), DHEA, P5 and especially their sulphated-conjugates have high blood concentrations (Table 1), and are used to generate all other steroids in peripheral tissues.
Drug Development
Natural hormones have been historically used as drugs, and depending on definitions, approximately 90 marketed drugs share a steroidal core (see https://www.drugbank.ca). Steroids (T, E2, cortisol, DHEA), simple derivatives (ethinylestrogen, prednisolone) or more complex analogs (abiraterone, fulvestrant) are used in various conditions. This old-and-proven steroidal chemistry based approach is used even in modern era.
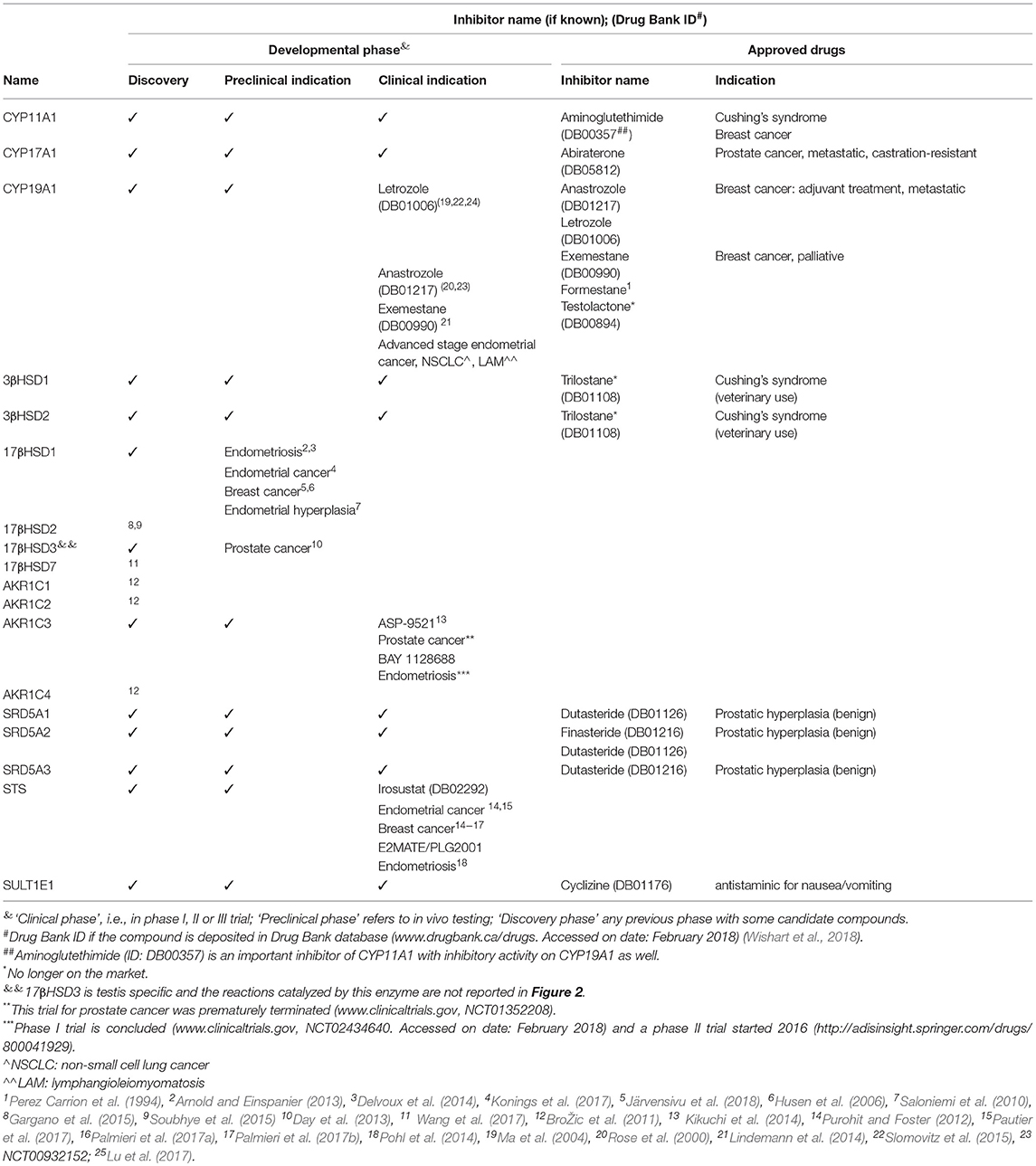
By targeting steroid intracrine metabolism, the effects of steroids can be modulated locally. Table 3 overviews the available drugs targeting intracrine enzymes and their developmental status. CYP19A1 (aromatase) inhibitors, currently at their third generation, started to be used for breast cancer during the 80's of last century (Lønning and Eikesdal, 2013), and was followed by drugs able to target other enzymes (CYP11A1, CYP17A1, SRD5As; Table 3).
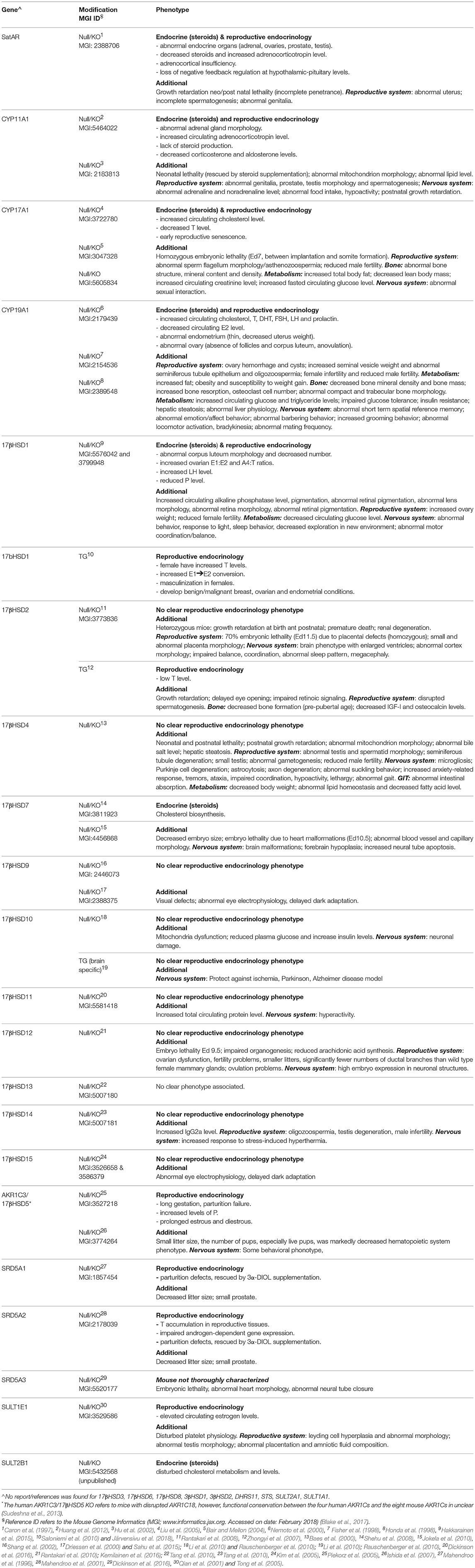
Table 4. Mouse models (knockouts - KO or transgenic-TG, i.e., ubiquitous expression of the gene, unless specified) for intracrine enzymes.
More recently, there is a re-emerging interest in developing novel intracrine drugs. A number of compounds are in their clinical phases, like STS inhibitors (Maltais and Poirier, 2011; Woo et al., 2011; Purohit and Foster, 2012; Pohl et al., 2014; Pautier et al., 2017) or inhibitors of AKR1C3/17βHSD5, which are of particular interest because this enzyme has crucial role in androgen/estrogen and prostaglandin biosynthesis (Penning, 2017). Bayer's AKR1C3/17βHSD5 inhibitor BAY 1128688 has a modified estrogen core, it interferes with both pathways, and is in phase II clinical trial for endometriosis (Bothe et al., 2017). Astellas Pharma potent and selective AKR1C3/17βHSD5 inhibitor ASP-9521 had only modest effect in a phase II study on prostate cancer as single drug, but combination therapy approaches remain to be studied (Kikuchi et al., 2014; Loriot et al., 2014).
HSD inhibitors are being studied in the area of hormone-dependent diseases, with 11βHSD inhibitors being in clinical trials for metabolic disorders (Ye et al., 2017) and 17βHSD inhibitors approaching the clinical phase for a number of gynecological indications (Table 3; Abdelsamie et al., 2017).
Intracrinology in Peripheral Tissues
In this paragraph, intracrinology of endometrium, GIT, bone, lungs, and CNS is reviewed. To comprehensively understand the ability of these tissues and systems to generate estrogens and other steroids, we have performed a systematic search of all original papers published in English until June 2018 that described the levels of intracrine enzymes (those indicated in Table 2-mRNA, protein or activity) in healthy tissues. In total 177 if the four extra ref are allowed papers were reviewed, and for details of this search, see Supplemental panel: “Systematic Review.” The results of this systematic review are summarized in Tables 6–8 and are briefly overviewed in each section dedicated to the distinct tissues or systems. Reports describing the enzymes in cultured cells or cell lines were excluded (may have been discussed elsewhere, though). Each section follows then with a non-systematic overview of the role of intracrinology in pathophysiology. A brief non-systematic description of the intracrinology of the skin, immune system and adipose tissue is also given. We will not describe the intracrinology of breast, prostate and liver (where steroid catabolism is the most relevant aspect), and we redirect the reader to recent reviews (Foster et al., 2008a; Luu-The et al., 2008; Luu-The and Labrie, 2010; Labrie and Labrie, 2013; Labrie, 2015; Mueller et al., 2015; Zhao et al., 2016; Hilborn et al., 2017; Penning, 2017).
Endometrium
The actions of steroid hormones in the endometrium are mediated by hormone-receptors via the classical mechanisms, although non-genomic and rapid signaling are also present (Groothuis et al., 2007; Zwart et al., 2011; Flach and Zwart, 2016; Hewitt et al., 2016). Estrogens and P control the menstrual cycle (Groothuis et al., 2007; Andersen and Ezcurra, 2014) and the endometrium during the window of implantation (WOI), occurring in the mid-luteal phase (Wang and Dey, 2006).
In rats, the WOI is characterized by high E2 plasma levels, and endometrial ERα and PR expression shows specific and varying cytosolic/nuclear patterns (Singh et al., 1996). ERα and PR expression decreases after ovulation and in preimplantation stages in both mice (Vasquez and DeMayo, 2013) and primates (Macaca mulatta) (Ghosh et al., 1999).
Rodent genetic models unraveled some molecular mechanisms underlying the estrogen-dependency of these processes. ERα-KO mice are infertile, no implantation occurs, endometrium is hypoplastic and estrogen response is absent (Couse and Korach, 1999; Walker and Korach, 2004). Not only its absence, but also sustained estrogen signaling has deleterious effects on endometrial receptivity, as recapitulated by mice with uterine COUP-TFII ablation. These mice exhibit increased estrogen signaling and asynchrony between embryo competency and uterine receptivity with consequent implantation defects. This effect is rescued by treatment with the antiestrogen ICI-182780 (Lee et al., 2010). Additionally, the duration of E2 exposure and its dosage affect endometrial receptivity and WOI length in mice (Ma et al., 2003).
Available human data, mostly obtained in the context of assisted reproduction technologies (ART), also indicate that steroid stimulation retards or shortens the luteal phase, the WOI, causes shifts in the appearance of pinopodes (a classical WOI marker) and causes asynchrony between ovarian and menstrual cycles (Devroey et al., 2004).
Intracrinology in Healthy Endometrium–Systematic Search
Initial studies on steroid hormone metabolism in the endometrium date back to 1965 with first demonstration of the STS activity, followed by investigation on the oxidative and reductive 17βHSD activities (Table 6).
Both pre and postmenopausal tissues possess oxidative and reductive 17βHSD activities and the expression of 17βHSD1, 2, 4, 6, 7, 8, 10, 12, 14, and AKR1C3/17βHSD5 was detected at the mRNA or protein levels. Sulphatase pathway (STS and SULT1E1; recently reviewed by Rižner, 2016), CYP19A1, 3βHSDs, SRD5As and AKR1Cs are also present, indicating that human endometrium can metabolize sulphated-compounds and DHEA to form androgens and estrogens.
Few 17βHSDs have been characterized by IHC. The low expression of 17βHSD1 poses sensitivity problems using standard detection methods (Cornel et al., 2017), and few authors reported endometrial absence of 17βHSD1 (Table 6). Type 1 17βHSD localizes in the cytoplasm of epithelial cells (Dassen et al., 2007; Colette et al., 2013; Mori et al., 2015; Sinreih et al., 2017a) and it is also detected in primary stroma cells cultured in vitro (Aghajanova et al., 2009; Mori et al., 2015). Type 2 17βHSD, AKR1C3/17βHSD5 and 3βHSD1 give strong reactivity in the glandular epithelium (Rhee et al., 2003; Ito et al., 2006; Dassen et al., 2007; Vani et al., 2007; Smuc and Rizner, 2009; Zakharov et al., 2010; Colette et al., 2013; Mori et al., 2015; Sinreih et al., 2017a).
CYP19A1 as well has low expression and some authors detected this enzyme only in association with diseases (see below and recently reviewed by Rižner, 2013). Although CYP19A1 immunoreactivity was initially associated with stroma cells (Watanabe et al., 1995), subsequent investigations showed also glandular expression (Kitawaki et al., 1999; Hudelist et al., 2007) and laser-capture-microdissected stroma/epithelial components detected CYP19A1 mRNA in both cell types (Matsuzaki et al., 2006).
The mRNA of those enzymes converting cholesterol to DHEA (CYP11A1, CYP17A1, StAR) and (ovarian) 3βHSD2 was reported in recent studies, suggesting that the endometrium can produce steroids from cholesterol (Table 6).
Intratissue Steroid Levels
Endometrial steroid levels were recently profiled by LC-MS. E2 levels differ between tissue and serum during the menstrual cycle, being up to five-times higher in tissue than in serum during the proliferative phase and 1.5-fold higher in the luteal period (Huhtinen et al., 2012a, 2014). T levels were lower in tissue than in serum with no cyclic changes. The levels P and P5 (and their 17-hydroxy derivatives) did not vary between serum and tissue, indicating that, contrarily to estrogens, progestogen intra-tissue levels are determined by passive diffusion from the blood (Huhtinen et al., 2014).
Intracrinology and Reproduction
Animal models show not only that intracrine enzymes are expressed in the endometrium, but also they vary the expression levels during the endometrial phases and during implantation, as shown already during the 80's in rhesus monkeys for the oxidizing 17βHSD activity (Kreitmann et al., 1979).
In rodents, STS activity measured with [3H]E1-S in 6-days pregnant rats was lower around the implantation site compared with non-implantation sites (Loza, 1995). In situ hybridisation signal of 17βHSD7 mRNA varied spatio-temporally throughout implantation and early gestation, being initially detected on luminal epithelium around the implantation site and absent in decidua (embryonic day, Ed5.5). At Ed8 and Ed9.5, 17βHSD7 expression increased in the decidua capsularis (the part that interacts with the trophoblast) and later (after E9) in the junctional zone of the developing placenta and in the spongiotrophoblasts (Nokelainen et al., 2000).
A brilliant study in mice showed that decidualization is dependent on local E2 produced through CYP19A1. CYP19A1 expression increased during pregnancy and decidualization was unaffected by ovariectomy. In contrast, treatment with the aromatase inhibitor (AI) letrozole impaired decidualization and decreased decidual marker expression (e.g., PRP, BMP2 and CX43) (Das et al., 2009).
In human endometrium, 17βHSD2 and SULT1E1 are induced by P as their expression peaks in the luteal phase (Rubin et al., 1999; Tseng and Mazella, 2002; Utsunomiya et al., 2004; Dassen et al., 2007; Huhtinen et al., 2012a; Colette et al., 2013; Piccinato et al., 2016b). Since both enzymes decrease intra-tissue estrogen levels, their up-regulation is one of the mechanisms of the uterine antiestrogenic effects of P. The P-dependency of 17βHSD2 and SULT1E1 was recapitulated in vitro using explant cultures and primary cells (Tseng and Mazella, 2002; Dassen et al., 2007; Piccinato et al., 2016b). Luteal peak expression of other SULTs (1A1 and 2B1) was also reported (Rubin et al., 1999; Koizumi et al., 2010). Some reports also suggested that STS expression increased in the luteal phase (Tanaka et al., 2003; Piccinato et al., 2016b) with a potential role during decidualization (Tseng and Mazella, 2002). Mid-luteal phase endometrium shows also peaking expression of 3βHSD1 (mRNA and protein) (Rhee et al., 2003; Vani et al., 2007).
Two studies on human ectopic pregnancies explored the endometrium around the implanted blastocyst. Expression of 3βHSD1 (mRNA and protein) was highest in decidua obtained from ectopic pregnancies (Rhee et al., 2003) and in a study on 23 tubal pregnancies, 17βHSD1 showed highest immunoreactivity at the fetal-maternal interface (Li et al., 2003), suggestive for a role of these enzymes in the nidation site.
Endometriosis
Endometriosis, an estrogen-dependent benign disorder affecting up to 10% of reproductive-aged women, is associated with pelvic pain, infertility, decreased life-quality and important health care/social costs (Simoens et al., 2011, 2012; De Graaff et al., 2013, 2015, 2016; Vercellini et al., 2014). Endometriosis is characterized by the growth of endometrium-like tissue outside the uterus (ectopic locations), beside the ovaries (endometrioma), as peritoneal implants, or as deep-lesions infiltrating peritoneal organs (deep endometriosis).
The expression of intracrine enzymes in endometriosis was reviewed in 2012, (Huhtinen et al., 2012b) and among other studies, 20 papers published between 1996 and 2009 specifically described the levels of intracrine enzymes in eutopic and ectopic endometrium from patients and control women. With the exclusion of one study that included over 100 patients (Colette et al., 2009), the rest included small study populations, and in most cases, the various endometriosis types (ovarian, peritoneal and deep infiltrating) were pooled together. Various techniques were used (RT-qPCR, immunohistochemistry, enzyme activity assay). Overall, no clear conclusion could be drawn from these studies. Comparing endometriosis with controls, CYP19A1 was up-regulated (six studies), unchanged (three studies) and one study found no expression of this gene. With respect to oxidative and reductive 17βHSDs, 17βHSD1 was reported up-regulated (three studies), 17βHSD2 was reported down-regulated or unchanged and two studies reported an up-regulation of 17βHSD7 and 12 in endometriosis vs. controls (Huhtinen et al., 2012b).
Subsequent investigations also continued to report inconsistent results. No change in mRNA (Delvoux et al., 2014) or increased expression of CYP19A1 in ovarian endometriosis vs. controls (Huhtinen et al., 2012a) were reported. An increased expression of CYP19A1 was also described using in vitro spheroids derived from endometrial stroma cells from patients compared with controls (Mori et al., 2015).
The mRNA expression of 17βHSD1 was higher in endometriosis compared with normal tissue using patient biopsies as well as spheroid cultures derived from endometrial stroma cells of patients and controls (Delvoux et al., 2014; Mori et al., 2015). One study assessing the three endometriosis types separately (60 patients in total) described that the increased 17βHSD1 level was restricted to endometrioma during the secretory phase of the menstrual cycle (Huhtinen et al., 2012a), whereas a second study on 79 patients and 41 controls, found no change in 17βHSD1 level, but described an increased 17βHSD1/2 ratio (Colette et al., 2013).
Regarding 17βHSD2, recent investigations reported both unchanged (Delvoux et al., 2014) and down-regulated mRNA in patient biopsies compared with controls (Huhtinen et al., 2012a; Colette et al., 2013). No variations were found in 17βHSD4, 5, 7 and 12 (Smuc et al., 2009; Delvoux et al., 2014) but an increased level of 17βHSD6 mRNA was detected in endometriosis compared with controls (Huhtinen et al., 2012a).
A few studies reported detectable levels of the enzymes involved in the generation of DHEA from cholesterol (StAR, CYP11A1 and CYP17A1) in endometriosis (Tsai et al., 2001; Rhee et al., 2003; Bukulmez et al., 2008a; Attar et al., 2009; Sinreih et al., 2013, 2017b; Huhtinen et al., 2014), suggesting that, in contrast to eutopic endometrium, endometriosis is able to produce steroids from cholesterol. However, it has also been argued that the presence of paracrine confounders of ovarian origin in studies using endometriomas could bias the results (Noël et al., 2011).
The contribution of STS, SULT1E1 and other SULTs was investigated by numerous studies and also in this case, conclusions are unclear (recently reviewed, Rižner, 2016). A recent investigation using 78 specimens described increased STS levels in endometriosis vs. control samples and found that the overall balance between STS and SULT1E1 differed between eutopic and ectopic tissue, implying an unbalanced flux of sulpho-conjugated estrogens in this disease (Piccinato et al., 2016b). The same research group also described an aberrant regulation of the enzymes involved in the estrogen oxidative metabolism in endometriosis (Piccinato et al., 2016a).
Although the level of the single enzymes in the intracrine machinery varies with apparently no clear association with the disease condition, the intracrinological nature of endometriosis was recently proven by comparison between serum and tissue levels of steroids in 60 patients (eutopic and ectopic endometrium) and 16 controls. Although E2 changed cyclically in eutopic tissue, E2 levels remained constant in the lesions and inversely correlated with the mRNA level of 17βHSD2 and 17βHSD6 suggesting an impairment in E2 deactivation to E1. P levels were equal in serum and control tissues, but resulted higher in patients and correlated with high 3βHSD2 mRNA. T, low in the tissue of controls, was over 13-times more concentrated at ectopic locations and correlated with low expression of SRD5A3 (Huhtinen et al., 2012a, 2014).
Endometrial Cancer (EC)
EC is the most common gynecological malignancy in western society and 80% of all cases are estrogen-driven (Amant et al., 2005; Morice et al., 2015). Major serum steroids are increased in patients with EC, including several substrates for intracrine E2 synthesis (Lépine et al., 2010; Audet-Walsh et al., 2011). In addition, tissue-steroid levels differ between cancer, normal tissue and serum and correlate with the levels of specific intracrine enzymes (see below) (Tanaka et al., 2015).
A systematic review recently explored all studies published between 1990 and 2017 assessing the expression of 17βHSD1, 2, STS, SULT1E1, and CYP19A1, with results that describe unbalanced intracrine regulation and important inter-patient variability (Cornel et al., 2018). Most studies compared cases with controls or tumor tissue with adjacent normal endometrium. Compared with normal tissue (from controls or adjacent to tumor), 17βHSD1 was found increased in EC (Cornel et al., 2012), decreased (Smuc and Rizner, 2009; Lépine et al., 2010) and undetected (Utsunomiya et al., 2001, 2003); 17βHSD2 was found decreased (Utsunomiya et al., 2003, 2004) or increased (Lépine et al., 2010; Cornel et al., 2012; Sinreih et al., 2013); AKR1C3/17βHSD5 was found unchanged (Cornel et al., 2012; Sinreih et al., 2013), increased (Ito et al., 2016) and decreased (Zakharov et al., 2010); 17βHSD7 both decreased (Smuc and Rizner, 2009) and unchanged (Lépine et al., 2010; Cornel et al., 2012) and 17βHSD12 was unchanged (Smuc and Rizner, 2009; Cornel et al., 2012) or increased in tumors vs. controls (Lépine et al., 2010). One recent report described decreased 17βHSD14 levels in tumor compared with adjacent tissue (Sinreih et al., 2017a). Controversial results apply to CYP19A1, described as increased (Watanabe et al., 1995; Utsunomiya et al., 2001, 2004; Smuc and Rizner, 2009) and unchanged (Jongen et al., 2005; Pathirage et al., 2006; Cornel et al., 2012). STS/SULT1E1 expression is also inconsistent in different studies (recently reviewed in Mueller et al., 2015; Rižner, 2016).
Recent studies exploring the association between enzyme levels and tumor characteristics found a correlation between STS with tumor grade and lymphovascular invasion (Sinreih et al., 2017a) and described an association between high CYP19A1 or 17βHSD1 and poor patient prognosis (Segawa et al., 2005; Cornel et al., 2017).
Other investigations emphasized the potential antiestrogenic and protective roles of androgens and P. Formation of DHT (via conversion of A4 to T by AKR1C3/17βHSD5 and of T to DHT by SRD5As) has potential antiestrogenic action because it devoids tissue from T (substrate of CYP19A1 yielding E2) and because it has direct endometrial antiproliferative effects via AR (Ito et al., 2016). Similar to the AKR1C3/17βHSD5 data reported earlier, results on SDR5A expression are inconclusive as SRD5A2 was down-regulated in a study on 47 tumor specimens compared with adjacent normal tissue (Sinreih et al., 2013), but both SRD5A1 and SRD5A2 resulted unchanged in another study on 122 tumors (although only five controls were studied) (Tanaka et al., 2015). This last study found however increased androgen levels (T and DHT) in tissue vs. blood. High DHT levels were restricted to samples with high SRD5A1 immunohistochemical staining. In addition, AR and SRD5A1 positivity was associated with good patient prognosis (Tanaka et al., 2015). The prognostic value of AR is confirmed by independent investigations (Tangen et al., 2016).
P is well-known for its antiestrogenic action, PR positivity is a good prognostic marker (Tangen et al., 2014) and P synthesis and metabolism are disturbed in EC (Sinreih et al., 2013). Interestingly, in a study on 47 tumors and adjacent normal tissues, EC had decreased StAR and CYP11A1 mRNA levels, indicative of diminished de novo steroid synthesis (Sinreih et al., 2013, 2017b). At the same time, EC showed decreased SRD5A2 and increased 17βHSD2 indicative of a diminished rate of conversion of P to 5αDHP and of 20αDHP to 5α-pregnan-20-ol-3-one, but increased conversion of 20αDHP to P (see Figure 2).
Other Endometrial/Gynecological Disorders
Although literature is scarce, a potential role of intracrinology is postulated for ovarian cancer (Ito et al., 2016), for adenomyosis and fibroids (Rižner, 2016), for sarcoma, where CYP19A1 expression may have prognostic significance (Kitaoka et al., 2004) and among infertile women (Brosens et al., 2004).
Intracrine Drug Targets
Endometriosis: blocking the systemic estrogen signaling via P, or GnRH agonist is standard care (Vercellini et al., 2014). Blocking the intracrine E2 generation is the future approach with on-going preclinical/clinical research.
STS inhibition showed promising results. Irosustat (Table 3) inhibited up to 100% the formation of free steroids using ex-vivo material from 27 patients (Purohit et al., 2008) and STS inhibition showed good results in a mouse model of endometriosis, where decreased size and weight of the lesions was observed (Colette et al., 2011). A phase-I clinical trial on 24 volunteers proved the safety of the STS inhibitor E2MATE (PLG2001), which reduced STS activity by over 90% and induced changes in endometrial markers (both alone or co-administered with norethindrone acetate) (Pohl et al., 2014).
Inhibitors of 17βHSD1 are in preclinical phase, and promising results are described using a primate model of endometriosis, where decreased behavior/pain symptoms were reported (Arnold and Einspanier, 2013) and using ex-vivo material from endometriosis patient (over 70% of the patients showed over 80% of enzyme inhibition) (Delvoux et al., 2014).
AKR1C3/17βHSD5 inhibition can interfere with E2, androgen synthesis, and reduce prostaglandin-associated inflammation/proliferation and an inhibitor has recently entered a phase II trial for endometriosis (Table 3). Overall, AIs have limited efficacy for endometriosis (Ferrero et al., 2011; Dunselman et al., 2014),
EC: only in case of advanced stage/metastatic disease hormonal care is given (progestogen, tamoxifen or AIs). AIs alone have limited efficacy with low response rates (Rose et al., 2000; Ma et al., 2004; Lindemann et al., 2014). Promising data were obtained using dual regimen (AI and mTOR inhibitor; Slomovitz et al., 2015) and additional trials on combinatory regimen are on-going. STS inhibitors showed promising results in a mouse subcutaneous model of EC, with decreased tumor growth by 48–67% (Foster et al., 2008b). However, a phase II trial on advanced stage EC was stopped because of the absence of added benefit compared with progestogen treatment (Purohit and Foster, 2012; Pautier et al., 2017).
Preclinical studies on 17βHSD1 inhibitors showed promising results in a mouse model of endometrial hyperplasia (Saloniemi et al., 2010; Järvensivu et al., 2015) and in various models of EC (Konings et al., 2018).
Endometrium: Conclusions
The ability to synthesize DHEA from cholesterol (reported by few studies) needs confirmation. However, the endometrium possesses the enzymatic machinery to metabolize sulphated-compounds and DHEA and form androgens and estrogens, (although this contention is wrangled by other authors: Labrie and Labrie, 2013; Labrie, 2015). Further, the endometrium can metabolize androgens and progestogens via AKR1Cs and SRD5As to produce a wide range of compounds, including estrogens (Table 6 and Figure 3). The morphological changes during the menstrual cycle are accompanied by cyclic changes in intracrine steroid and enzyme levels, indicating that steroid exposure needs to be cyclically regulated to support endometrial physiology.
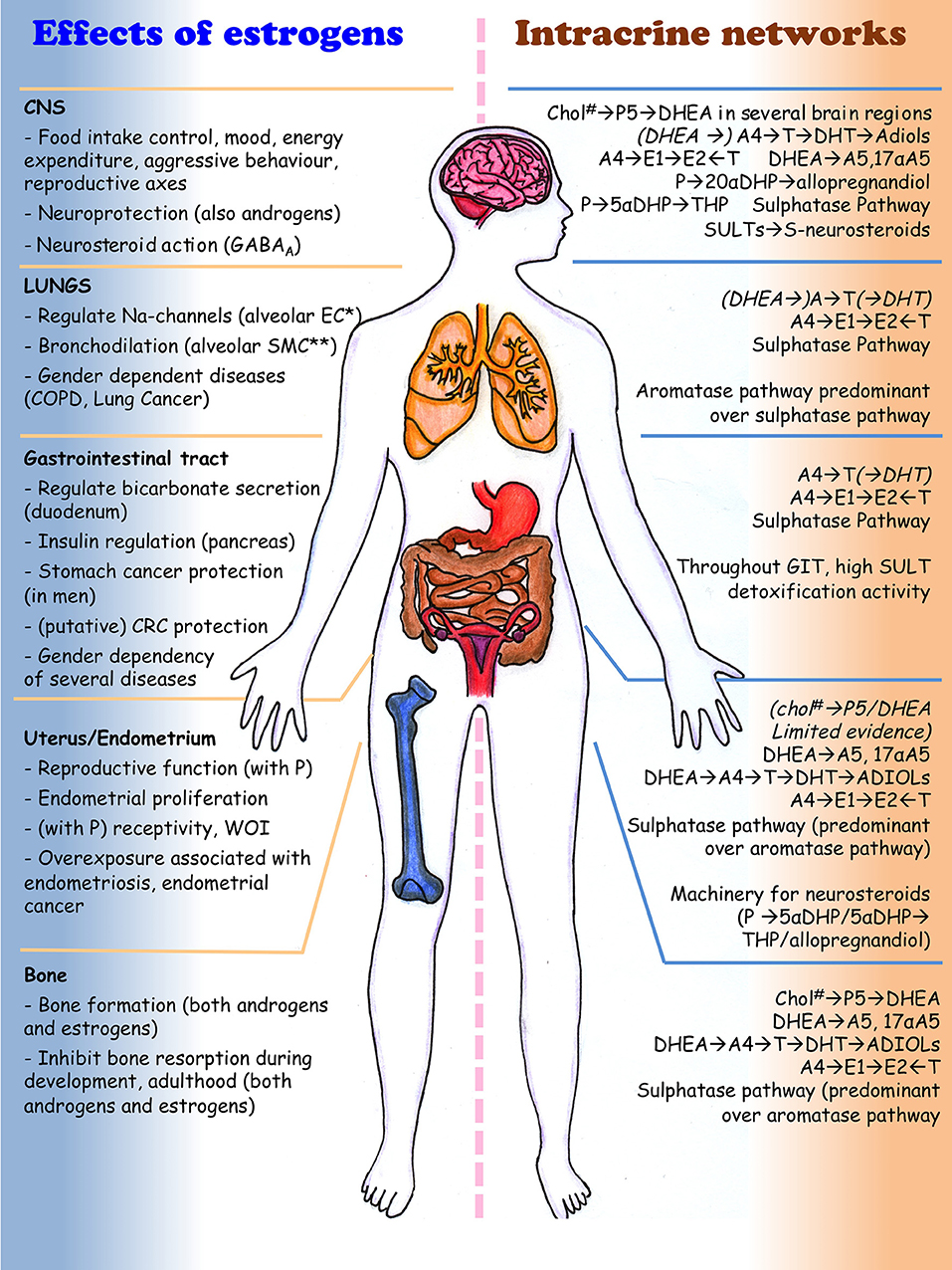
Figure 3. Effect of steroids (mainly estrogens) and intracrine networks in central nervous system, lungs, digestive system, uterus and bone. Italics and by brackets are those metabolism/reactions that need conformation by independent authors (because validated at the mRNA level only or in few studies). * EC, epithelial cells; **SMC, smooth muscle cells; #Chol, cholesterol. The drawing was kindly generated by Dr. Margaretha A. Skowron (Department of Urology, University Düsseldorf, Germany) for this review.
Gastrointestinal Tract (GIT) and Digestive System (DS)
ERα and ERβ are expressed throughout the GIT and DS (esophagus, stomach, colon, gallbladder, pancreas) and epidemiological studies show important influence of sex hormones in DS physiology and disturbances, with a clear gender-dependency. In the duodenum, estrogens regulate bicarbonate secretion (Nayeb-Hashemi and Kaunitz, 2009; Tuo et al., 2011). This is an important defense mechanism of the mucosa against acids discharged from the stomach, and men develop duodenal ulcer two/three-times more often than premenopausal women (Wu et al., 2008). Such estrogen protective effect is recapitulated in animal studies exposed to estrogens and anti-estrogens, and is mediated by a rapid action (i.e., non genomic) of ERα on membrane ion channels (Smith et al., 2008).
ERα, ERβ and GPER mediate important effects on the pancreatic beta-cells during adaptation to insulin resistance periods (e.g., pregnancy, puberty, obesity; Nadal et al., 2011). In mice, ERα signaling regulates proliferation of beta-cell during development and after injury (Yuchi et al., 2015).
Men are also more likely than women to develop cancer in the esophagus, stomach and colon. Accordingly, estrogen treatment for prostate cancer decreases the incidence of gastric cancer and menopausal status in women is associated with colorectal cancer CRC risk (Freedman et al., 2007; Kennelly et al., 2008; Hogan et al., 2009; Duell et al., 2010). ERβ results oncoprotective at several GIT sites (Kennelly et al., 2008; Barzi et al., 2013; Caiazza et al., 2015) and low expression correlate with high CRC stage in mice and with poor differentiated gallbladder cancer in humans (Hogan et al., 2009).
The association between estrogens and DS cancer risk is however controversial. The Women's Health Initiative and other large studies showed that combined estrogens plus P hormone replacement therapy (HRT) decreases CRC risk, but increases that of gallbladder. In addition, CRC during HRT has a higher grade (Kennelly et al., 2008; Hogan et al., 2009; Rennert et al., 2009; Foster, 2013; Mueller et al., 2015). However, a recent randomized, placebo-controlled trial enrolling over 10,000 women receiving estrogens alone vs. placebo found no difference in CRC incidence (Lavasani et al., 2015). Such complexity is recapitulated in animal studies where estrogens and androgens can have distinct and opposite effects on colitis and CRC (Amos-Landgraf et al., 2014; Heijmans et al., 2014). Overall, the association between DS disturbances/cancers with estrogens depends on the moment in life, extent and nature (endogenous or exogenous) of exposure and is influenced by the relative balance of the receptors (Foster, 2013). Similarly, androgens influence DS pathophysiology via complex and unclear mechanisms involving classical, membrane signaling, level of free and SHBG bound T (Roshan et al., 2016).
The lack of clear conclusion and the fact that the levels of circulating endogenous estrogens in women do not influence CRC risk indicates that intracrine steroids may have a predominant role irrespective of their circulating levels (Sato et al., 2009; Falk et al., 2015).
Intracrinology in Healthy GIT–Systematic Search
In total, 29 original papers were retrieved that described the levels of the intracrine enzymes in the GIT, published from the late 80's (Table 7 and Supplemental panel: “Systematic Review”).
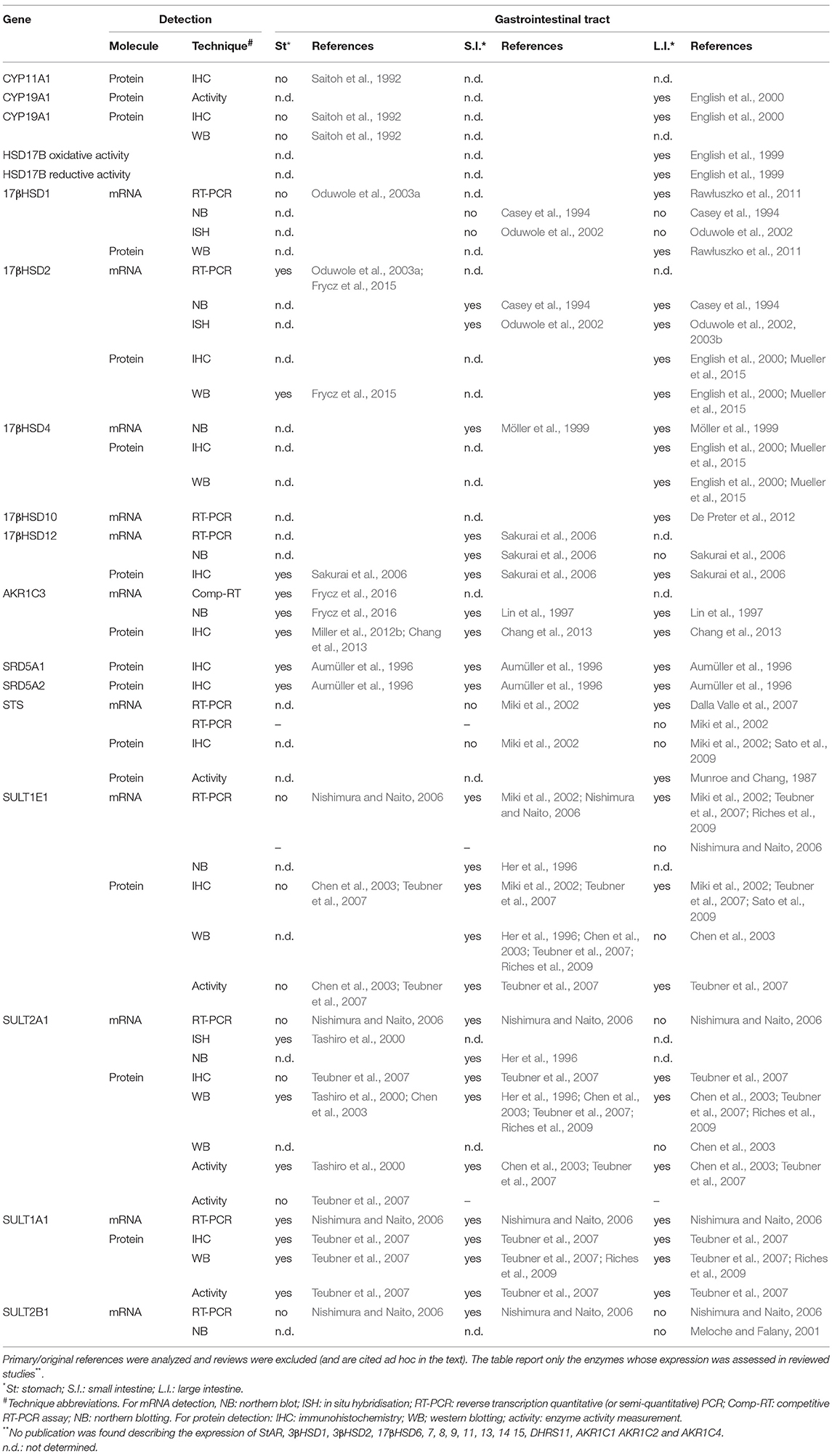
Table 7. Expression of intracrine enzymes in the gastrointestinal tract (GIT)-results of the systematic search.
Stomach intracrinology.
The stomach is an endocrine tissue, and in rodents it produces steroids starting at birth and throughout adulthood (Kobayashi et al., 2013). Human gastric mucosa expresses 17βHSD1, 2, 12 and AKR1C3/17βHSD5 (Table 7). The mRNA for 17βHSD2 in mucosa surface and glandular epithelium inversely correlates with age in both genders (Oduwole et al., 2003a). Luminal gastric mucosa has strong AKR1C3/17βHSD5 immunoreactivity that decreases toward the gastric pits (Chang et al., 2013). Weak immunoreactivity for 17βHSD12 localizes in the fundic glands and in the squamous epithelium of the esophagus (Sakurai et al., 2006).
Sulphatases in parietal cells of the gastric glands have a protective role in detoxification. Estrogenic SULT1E1 is not expressed whereas data for SULT2A1 are inconsistent. SULT2A1 was detected in the gastric mucosa in a study on seven subjects (Tashiro et al., 2000), but it was low/absent in other studies on 39 (Teubner et al., 2007) and 23 subjects (Chen et al., 2003).
Small intestine: duodenum–jejunum–ileum.
Due to its high exposure to food components and harmful xenobiotics, the duodenum expresses several phase I/II enzymes including DHEA/estrogenic SULT1E1, 2A1, 1A1 (Table 7). Protein and enzyme activity of SULT1E1 and 2A1 are present in human jejunum and ileum but absent in duodenum (Teubner et al., 2007), mRNA and protein levels vary with no relation either with age or gender (Her et al., 1996; Nishimura and Naito, 2006). In a study on 23 subjects, SULT1E1 and 2A1 varied inter-individually and between different intestine tracts (Chen et al., 2003). The duodenal mucosa expresses 17βHSD2, but not 17βHSD1 (Casey et al., 1994; Oduwole et al., 2003a) and shows strong luminal AKR1C3/17βHSD5 (Chang et al., 2013) and weak 17βHSD12 immunoreactivity (Sakurai et al., 2006) that decreases toward the Brunner's gland (Chang et al., 2013).
Large intestine: colon, cecum, rectum
The intracrinology of healthy colon mucosa and its relation to CRC was recently reviewed (Foster, 2013). Studies dating from 1987 demonstrated the presence of CYP19A1, 17βHSD reductive and oxidative enzymatic activities, plus the expression of 17βHSD1, 2, 4, CYP19A1, STS and SULT1E1 (Table 7). Most 17βHSDs tend to have higher levels at the surface than in cryptal epithelial cells as indicated for 17βHSD2 mRNA (Oduwole et al., 2002; Foster, 2013), and for the immunoreactivity of AKR1C3/17βHSD5 (very strong; Chang et al., 2013) and 17βHSD12 (weak; Sakurai et al., 2006).
Pancreas
Radiolabelled substrates demonstrated the presence of CYP19A1 and SRD5A activities in human pancreatic tissue (Iqbal et al., 1983), which expresses 17βHSD2, 12, STS, SULT1E1 (Casey et al., 1994; Miki et al., 2002; Sakurai et al., 2006; Dalla Valle et al., 2007). High levels of AKR1C3/17βHSD5 localized in pancreatic ductules (acini and islets of Langerhans resulted negative; Chang et al., 2013).
Association With Diseases
SNPs in genes controlling estrogen synthesis, response and deactivation are associated with GIT cancers (Freedman et al., 2009; Cho et al., 2012; Zeng et al., 2016) and AKR1C4 is a candidate gene in hereditary CRC (Gylfe et al., 2013; Table 5). Also variations in the expression of these genes associate with GIT disturbances. Low 17βHSD10 levels are associated with aberrant butyrate β-oxidation and ulcerative colitis (De Preter et al., 2012). The epithelial 17βHSD2 level is low in case of stomach, duodenal cancer and chronic gastritis, though it is high in regenerating epithelium close to active gastritis and ulcers (Oduwole et al., 2003a). In a study on 34 gastric tumors and adjacent healthy tissue, the mRNA and protein levels of 17βHSD2 and AKR1C3/17βHSD5 were down-regulated in cancer (Frycz et al., 2015, 2016). Some studies showed lower oxidative 17βHSD activity and mRNA level of 17βHSD2 (and 4) in CRC vs. adjacent normal tissue, suggesting a protective role of estrogen deactivation. However, another study on 35 women and 39 men found that high 17βHSD2 levels were associated with poor prognosis in female patients with distal CRC (reviewed in Foster, 2013). Also 17βHSD1 level measured by RT-qPCR and western blotting in specimens from 52 patients was lower in CRC than adjacent normal mucosa (Rawłuszko et al., 2011). CRC show also higher CYP19A1 mRNA compared with adjacent normal mucosa (n = 31) (Sato et al., 2012).
Although no clear target for drugs has been identified in the intracrine network, intracrine enzymes showed some values as biomarkers. In CRC, high STS/SULT1E1 ratio correlates with poor prognosis (Foster, 2013) and AKR1C3/17βHSD5 expression with lymph-node metastasis (Nakarai et al., 2015). In addition, AKR1C1 and AKR1C3/17βHSD5 associate with cisplatin resistance in CRC, hence inhibitors of these AKR1Cs may be used to re-sensitize patients to chemotherapy (Matsunaga et al., 2013). In a study were the levels of E1, E2 and DHEA-S were measured in CRC specimens and adjacent normal mucosa of men and women by LC-MS, intra-tumor estrogens were elevated and (in particular E1) correlated with poor prognosis. In line with an unfavorable role of intra-tissue estrogens, absence on STS was associated with long survival (Sato et al., 2009).
GIT: Conclusions
Human GIT/DS is unable to metabolize cholesterol and there is no clear evidence that it expresses 3βHSDs, hence DHEA cannot be used to generate androgens and estrogens (Table 7 and Figure 3). Several SULTs are expressed throughout GIT and involved in detoxification and STS is regulated by estrogen in vitro via non-classical GPER signaling (Gilligan et al., 2017).
The role of steroids in pathology is complex, with divergent effects that depend on time, length and extent of exposure. In line with this, intracrine networks have unclear roles in pathogenesis. In the GIT these networks are strongly involved in the metabolisms of fatty acids and bile acids (outside the scope of this review).
Bone Tissue and Skeletal System
Bones consist of mineralized connective tissue with structural and supportive functions. The hard exterior part (cortical bone) and the trabecular and spongy cancellous tissue filling the bone interior are identical but differ in the level of mineralization. Osteoblasts, derived from multipotent mesenchymal stem cells, build the bone tissue through deposition of Type-I collagen and through the release of ions that combine chemically forming the bone mineral. Osteoclasts differentiate from hematopoietic stem cells and cause resorption of the mineralized bone mass. The balance between osteoblasts and osteoclasts regulates mineral deposition and resorption. Sex steroid hormones contribute to control bone development during puberty, contribute to bone physiology, bone mass maintenance and regulate the rate of mineral bone deposition and resorption (Svoboda et al., 2010).
The presence of the ERs as well as other hormone-receptors in normal osteoblastic cells, osteoclasts and osteoblasts is documented (Gruber et al., 2002) and estrogens and androgens stimulate bone formation and inhibit bone resorption in both males and females. During human puberty and throughout adulthood, E2 and T induce osteoblast proliferation (Kassem et al., 1998), which is mediated by IGF and GH (Riggs et al., 2002; Svoboda et al., 2010). Such human effects are well recapitulated in animal models. ERα-KO (Vidal et al., 2000) and CYP19A1-KO mice (Oz et al., 2000) exhibit low BMD in both genders and E2 treatment rescues the CYP19A1-KO phenotype (Miyaura et al., 2001). Additionally, ovariectomy stimulates osteoclast differentiation through (indirect) increased levels of IL-1, 6 and TNF in osteoblasts and other bone-derived stromal cells (Gruber et al., 2002; Svoboda et al., 2010).
Accelerated bone loss and increased osteoporotic fractures are associated with postmenopausal estrogen deficiency and low sex steroid levels elicit similar manifestations in men (Compston, 2001; Riggs et al., 2002; Syed and Khosla, 2005). Free E2 levels are associated with low lumbar spine and femoral neck bone mineral density (BMD) in both genders (Zarrabeitia et al., 2007) and estrogen therapy reduces bone loss and the risk of fracture in women with osteoporosis (Gruber et al., 2002).
Intracrinology in Healthy Bone–Systematic Search
Bone expresses CYP19A1 and 17βHSD1, and mRNA in situ hybridisation and immunohistochemistry signals were seen in lining cells, osteoblasts, chondrocytes of articular cartilage, and adipocytes adjacent to bone trabeculae in both male and female tibiae. CYP19A1 mRNA was also widely present in various bones (ribs, femurs) with inter-individual variability, but no relation with gender or age (Sasano et al., 1997). STS and 17βHSD activities were demonstrated by recovery of [3H]E1 and [3H]E2 after incubating femur-head fragments with [3H]E1-S (15 women and 12 men with osteoarthritis indicated for hip replacement). No gender-related differences were observed and E2 formation from androgens was lower than that from E1-S, indicating a predominant role of the sulphatase pathway in bone estrogen supply (Muir et al., 2004). Subsequent studies also demonstrated the presence of CYP11A1, CYP17A1, 17βHSD reductive and oxidative activity in bone tissues (Table 8). Overall, however, only six papers describing the level of intracrine enzymes in bone tissues were retrieved by the systemic search (Table 8) and most studies on bone intracrinology used in vitro cell cultures. In vitro studies were not included in our systematic review, but those on bone are briefly described in the next paragraph. These studies demonstrate the presence of a complex intracrine networks.
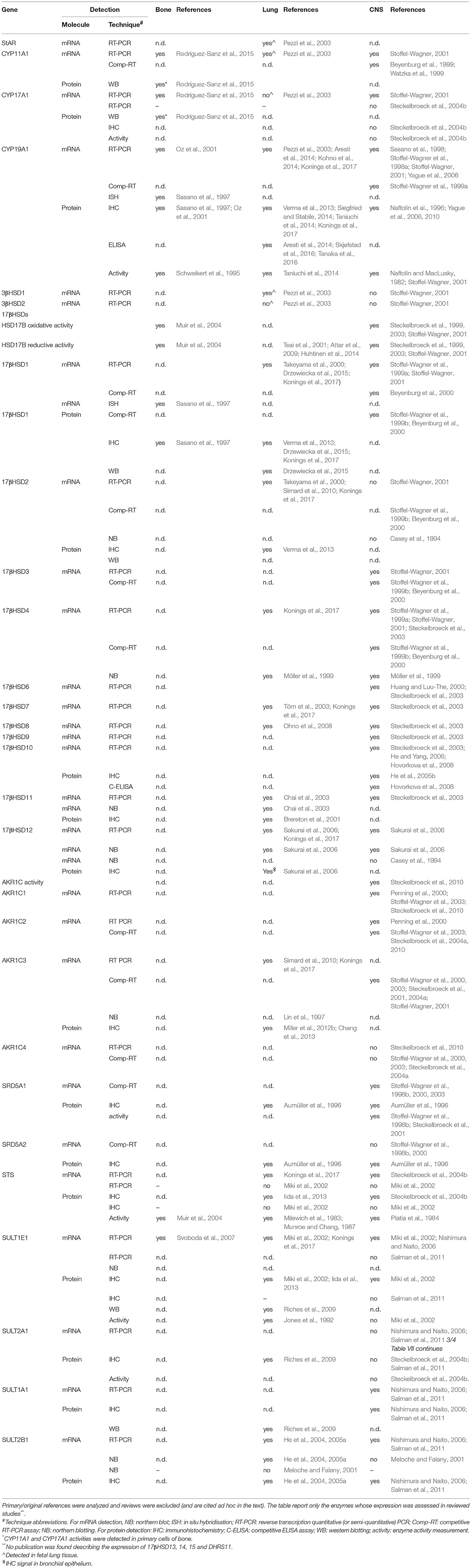
Table 8. Expression of intracrine enzymes in bone, lungs and central nervous system (CNS) – results of the systematic search.
Bone Intracrinology: in vitro and in vivo
From early ‘90s, various isotopic techniques demonstrated the presence of CYP19A1, 17βHSD reductive/oxidative, 3βHSD and STS activities and the mRNA expression of 17βHSD1, 2, 4, STS, SULT1E1, CYP19A1 and SDR5A in human osteoblastic (e.g., HOS, U20S, HTB-96 and MG63) and osteosarcoma cell lines like CRL-1543 (Purohit et al., 1992; Fujikawa et al., 1997; Jakob et al., 1997; Dong et al., 1998; Saito and Yanaihara, 1998; Janssen et al., 1999; Muir et al., 2004; Svoboda et al., 2007; Dias and Selcer, 2014).
In vitro evidence using osteoblastic cells show that E2 has mitogenic effects, which is blocked by the ERα antagonist fulvestrant. Since both E1-S and DHEA-S elicit effects similar to E2, which are blocked by STS inhibition (Selcer and Difrancesca, 2012; Dias and Selcer, 2014), these studies demonstrate that conjugated steroids are activated and that DHEA is converted to E2. Studies in rat osteoblast with [14C]T demonstrated that T is converted by SRD5As and AKR1Cs to 3α/3βDIOLs, which induce proliferation via activation of ER (and not AR) (Enríquez et al., 2013).
In vitro models of osteoblast differentiation showed that various differentiation stages are accompanied by declines in STS, CYP19A1 and 17βHSD1 (Janssen et al., 1999; Dias and Selcer, 2016).
In rats, during and after sexual maturation, in situ hybridization showed that ERα and ERβ localize in osteoblasts, osteoclasts and osteocytes covering the tibia metaphysis (responsible for elongation of long bones), and co-localize with STS. Starting at sexual maturation (e.g., 7-week-old), ERs also co-localize with CYP19A1, 17βHSD1, 2 and SRD5A1 (van der Eerden et al., 2004). In addition, male transgenic mice overexpressing 17βHSD2 show disturbed IGF-I/steroid actions in bone, with growth retardation, decreased bone formation at prepuberty and decreased serum levels of IGF-I, osteocalcin and T (Shen et al., 2008).
Diseases and Treatments
Genetic variants of estrogen and intracrine pathways are associated with bone disturbances (Table 5). Defects in the CYP19A1 and ERα are associated with low BMD and other skeletal disturbances (e.g., high stature, delayed bone age) and estrogen therapy ameliorates some bone abnormalities caused by CYP19A1 deficiencies in men (Smith et al., 1994; Morishima et al., 1995; Carani et al., 1997; Mullis et al., 1997; Bilezikian et al., 1998). In lumbar vertebrae, CYP19A1 levels correlate with changes in osteoporotic degree (Sasano et al., 1997).
Inhibitors of 17βHSD2 attracted attention as potential drugs to oppose the effects of low E2 on BMD, fracture and osteoporosis. Ovariectomised female macaques receiving a 17βHSD2 inhibitor display desirable bone balance, bone strength and lower bone resorption compared with untreated controls (Bagi et al., 2008). Several compounds targeting this enzyme have been developed and their use and challenges in osteoporosis were recently reviewed (Soubhye et al., 2015).
In a study on 35 chondrosarcoma biopsies (a malignant bone cancer occurring in middle aged patients), ERα (mRNA and IHC) and CYP19A1 (mRNA and activity) were demonstrated in the majority of the samples, and the AI exemestane impaired the E2- and androgen-induced proliferation of primary chondrosarcoma cells (Cleton-Jansen et al., 2005). Although AIs were proposed as novel drugs to treat this condition (Bovée et al., 2005), a pilot study on six patients with progressive disease showed no benefit of exemestane in progression-free survival compared with untreated patients (Meijer et al., 2011).
In a study of 28 osteosarcoma specimens (one of the most common bone cancers developing at young age) strong ERβ and PR immunoreactivity was seen in over 80% of the samples (and also correlated with Ki67). ERα and AR staining was seen in 30% of the samples, whereas CYP19A1 was undetected (Dohi et al., 2008). In another study, 20 osteosarcoma specimens, including 11 good responders to chemotherapy and nine poor responders, were subjected to cDNA microarray and 17βHSD10 resulted unregulated in the poor responder group. Results were further confirmed by IHC on 69 archival biopsies, hence targeting 17βHSD10 may be a valuable approach for drug (re)sensitisation (Salas et al., 2009).
Additional intracrine imbalances are described in bone diseases, such as higher androgen reducing 17βHSD activity in benign vs. malignant tumors, declines of CYP19A1 from normal bone to osteosarcoma and expression of SULT1E1 in the majority of the skeletal benign and malignant lesions, originated in bones or from primary tumors elsewhere (Svoboda et al., 2007).
Bone Tissue: Conclusions
In vitro, animal and human studies show that intracrinology controls bone development, benign and malignant conditions, and offer novel potential drug targets (Table 8 and Figure 3). Steroids can be synthesized in situ from cholesterol (Rodríguez-Sanz et al., 2015) and can be recruited from the serum via the sulphatase pathway. DHEA is substrate for androgen and estrogen production. The action of androgens is partly mediated by their conversion to estrogens via CYP19A1 or to estrogenic 3α/βDIOLs (Vanderschueren et al., 2008).
Lungs
Sex steroids play an important role in lung development and homeostasis. Androgens, progestogens and estrogens are present and exert genomic and non-genomic actions via their hormone-receptors. Classical ERs (with ERβ as predominant form) and membrane GPER are expressed (Couse et al., 1997; Prossnitz and Barton, 2011; Konings et al., 2017). Sex steroids remain active in the lungs throughout lifetime and modulate lung function in both a beneficial or detrimental way, extensively reviewed (González-Arenas and Agramonte-Hevia, 2012; Townsend et al., 2012; Sathish et al., 2015).
E2 and P regulate epithelial sodium channel expression in alveolar epithelial cells (Luo et al., 2015). In alveolar smooth muscle cells, E2 induces bronchodilation via the reduction of intracellular Ca2+ (Townsend et al., 2010).
Both human and animal studies support a promoting role for estrogens and inhibitory role for androgens in lung development and maturation. During gestation and neonatal period, AR is expressed in mesenchymal and epithelial cells. Androgens inhibit the production of surfactants, which starts later in male than in female neonatal lungs (Carey et al., 2007), but also support the developing lung during branching morphogenesis (Kimura et al., 2003).
Lung Intracrinology in Lungs–Systematic Search
Adult human lungs express CYP19A1 and most 17βHSDs (1, 2, 4, 7, 8, 11, 12, 17βHSD5/AKR1C3; Table 8). STS, SULT and 17βHSD1, 12 and 17βHSD5/AKR1C3 immunoreactivity localizes in the bronchial epithelium (weak for types 1 and 12, strong for type 17βHSD5) and alveolar macrophages (Sakurai et al., 2006; Miller et al., 2012b; Chang et al., 2013; Konings et al., 2017).
Intracrinology controls lung development and maturation as shown in various animal models (Boucher et al., 2009) and intracrine enzymes are expressed already during fetal stages. Human fetal lungs possess StAR, CYP11A1, 3βHSD1 mRNA (Pezzi et al., 2003), SULT1E1 activity (Jones et al., 1992) and show 17βHSD1 and 2 mRNAs expression at 13 and 20 weeks of gestational age (Takeyama et al., 2000). High mRNA levels of AR, 17βHSD2 and 17βHSD5/AKR1C3 in mid-late gestation period and adult lungs indicate the present of androgen metabolism (Simard et al., 2010). Immunoreactivity for 17βHSD11 is detected in bronchioles of 14 and 31 weeks old fetuses, whereas other structures are negative (e.g., alveoli, ciliated epithelium, acini of the trachea). The expression of 17βHSD11 increases during the second half of pregnancy and maintains similar patterns in neonatal (14 days) and adult lugs (Brereton et al., 2001).
Intracrinology and Lung Diseases
Altered intracrinology is involved in lung disorders already from neonatal stages toward adulthood, and SNPs in intracrine genes are associated with the onset of diseases (Zhang et al., 2013). Higher concentration of estrogens were measured by LC-MS in women with multiple-synchronous-lung adenocarcinoma compared with single adenocarcinoma (Ikeda et al., 2016) and in neoplastic tissue compared with adjacent normal lungs (Niikawa et al., 2008; Verma et al., 2013). Type 1 17βHSD mRNA, protein and activity are present in various non-small-cell-lung-cancer (NSCLC) cell lines where the mitogenic effect of E1 is abrogated by 17βHSD1 knockdown (Drzewiecka and Jagodzinski, 2012; Verma et al., 2013). In specimens from 48 NSCLC patients, 17βHSD1 expression was associated with squamous cell carcinoma and stage 3A disease (Drzewiecka et al., 2015). In another study on 103 NSCLC specimens, high 17βHSD1 immunoreactivity was associated with low intratumoural E1 and high E2:E1 ratio, whereas higher 17βHSD2 immunoreactivity was associated with high intratumoural E1. Multivariate regression analysis also demonstrated that increased 17βHSD1 immunoreactivity in tumors was an independent negative prognostic factor (Verma et al., 2013).
CYP19A1 is expressed in lung cancer and has potential therapeutic value (Niikawa et al., 2008; Verma et al., 2011; Siegfried and Stabile, 2014). A recent IHC study on 335 NSCLC specimens found an inverse association between CYP19A1 expression with disease specific survival (Skjefstad et al., 2016). Similar data, although restricted to women only, were confirmed in an independent study on 150 primary lung adenocarcinoma specimens, where CYP19A1 was found as the main driver of local estrogen supply (Tanaka et al., 2016). Another study on 110 lung adenocarcinoma specimens found an association between CYP19A1 mRNA (RT-qPCR) and poor prognosis in females, never-smokers and harboring EGFR mutations (Kohno et al., 2014). However, a recent mRNA study on 96 NSCLC patients showed that CYP19A1 in combination with ER is a good prognostic marker (Aresti et al., 2014).
STS and SULT1E1 immunoreactivity is detected in the majority of NSCLC cases, and STS is a good prognostic marker (Iida et al., 2013).
Lymphangioleiomyomatosis (LAM) is a rare, potentially fatal disease affecting predominantly young women. It is strongly hormone sensitive and it is hypothesized to originate from the uterus as lung metastasis (Prizant and Hammes, 2016). The levels of ERs, PR, AR, CYP19A1, STS, 17βHSD1 and SRD5A2 were recently assessed among 30 LAM biopsies. CYP19A1 expression resulted a useful classification marker with implication for potential therapy (Adachi et al., 2015). A recent study on specimens from 73 patients with chronic obstructive pulmonary disease (COPD) and 48 controls described an association between both CYP19A1 and 17βHSD1 with COPD (Konings et al., 2017). CYP19A1 is also implicated in interstitial pneumonia interstitial pneumonia, where local E2 concentration and CYP19A1 activity and immunoreactivity were elevated in diseased compared with normal tissue (Taniuchi et al., 2014).
Potential Novel Treatments
Blocking the estrogen signaling showed promising preclinical results in animal models of lung cancer (Verma et al., 2011). In humans, antiestrogen treatments (ER antagonists, GnRH, oophorectomy, P) have been used in LAM (Taveira-DaSilva and Moss, 2014) and lung cancer patients (Verma et al., 2011; Kohno et al., 2014). A phase II study on advanced NSCLC patients non-responsive to platinum-based drugs tested the dual-regimen mTOR/CYP19A1 inhibitors. Unfortunately, this study was prematurely terminated due to high toxicity (Singhal et al., 2015) and one additional trial using ER antagonist plus AI (fulvestrant and anastrozole) as consolidation therapy in postmenopausal women with advanced NSCLC (NCT00932152) was terminated due to poor recruitment.
Better results were obtained using the AI letrozole as single agent or in combination with rapamycin in a phase II trial on 17 postmenopausal women with LAM (NCT01353209). AI treatment was safe and well tolerated also in the dual drug regimen (Lu et al., 2017).
Lungs: Conclusions
Steroids are involved in lung maturation, development and in susceptibility to diseases. Most 17βHSDs, STS/SULT1E1, CYP19A1 are expressed indicating the lung's ability to metabolize androgens, estrogens and progestogens. Evidence of 3βHSDs is limited to fetal tissues (Table 8 and Figure 3). Approaches aimed at decreasing local estrogens may offer future novel treatments for various lung diseases.
Brain and Central Nervous System (CNS)
One of the first CNS actions of sex steroids to be described is the hypothalamus-pituitary-gonadal axes control (Andersen and Ezcurra, 2014). The identification of steroid-receptors outside the hypothalamus, like hippocampus (controlling memory), prefrontal cortex, cerebellum and dopaminergic system regulation indicated that sex steroids have complex and widespread effects in the CNS. They control aggressive behavior, cognitive functions, mood, food intake, appetite, addiction, blood pressure, fine motor skills, motor coordination, pain circuit and both estrogens and androgens are neuroprotective (López and Tena-Sempere, 2015; Soma et al., 2015; McEwen and Milner, 2017). Estrogen deprivation in animals and humans is associated with development of metabolic disorders and estrogen administration has a general catabolic effect (López and Tena-Sempere, 2015). Animal experiments and KO models show that ERα mediates the major actions of estrogens in the CNS, like the metabolic control functions (Musatov et al., 2007) and the negative-feedback on the hypothalamus-pituitary-gonadal axes (Couse et al., 2003). However, both nuclear and non-nuclear ERs are relevant in distinct CNS regions (Almey et al., 2015; López and Tena-Sempere, 2015; McEwen and Milner, 2017).
Local steroid synthesis in the CNS is demonstrated in animal studies. CYP19A1-KO mice have increased ischemic damages compared with ovariectomised wild-type mice, indicating a local action of CYP19A1 (McEwen and Milner, 2017). Similar conclusions were drawn for the estrogen protective effects on stroke, Alzheimer (AD), Parkinson diseases, aggressive behavior (Soma et al., 2015; McEwen and Milner, 2017) and mice with ablation in various 17βHSDs show neuronal defects (Table 4). In rodents, CNS regions like the hippocampus and the hypothalamus express the enzymes involved in the local generation of steroids, like StAR, CYP11A1, CYP17A1, 3βHSD1, CYP19A1, 17βHSD1, SRD5A1 and 2 (mRNA and protein by immunohistochemistry or western blot), and can produce pregnenolone, DHEA, androgens and estrogens from cholesterol, as confirmed by HPLC using radiolabelled substrates and tissue cultures of brain slices (Mukai et al., 2006; Murakami et al., 2006). CYP enzymes of rat hippocampus co-localize in pyramidal neurons (CA1–CA3 regions) and granule cells (dentate gyrus) (Mukai et al., 2006; Murakami et al., 2006). Regulation of intracrine enzymes varies during development and sexual maturation, as indicated by mRNA expression (RT-qPCR) of 20 intracrine enzymes analyzed in rat hippocampus post-natal and throughout early (1 week) development (Kimoto et al., 2010).
Intracrinology in the CNS is particularly relevant because, beside the traditional pathway via the receptors, several steroids have neuroactivity and are allosteric modulators of GABAA receptors (Figure 2). Such actions are possessed also by steroids that are unable to activate the steroid hormone receptors, such as 3β- and 3α-hydroxyl sulpho-conjugates (P5-S and DHEA-S), 5β-reduced steroids (5βAN, etiocholanolone and 5β-THP isomers; Table 1), which are all GABAA negative modulators (in contrast to unconjugated 3α-hydroxysteroids) (Stoffel-Wagner, 2001; Belelli and Lambert, 2005; Agís-Balboa et al., 2006; Gibbs et al., 2006; Reddy, 2010; Steckelbroeck et al., 2010).
Intracrinology in CNS–Systematic Search
Intracrine enzymes are widely expressed in human CNS (Table 8) and intratissue concentrations of steroids in distinct regions differ between regions and from the levels in the blood (Mukai et al., 2006; Murakami et al., 2006; Jäntti et al., 2010). In contrast to rodents, however, the presence of the complete steroid biosynthetic pathway is not clearly demonstrated in the human CNS and contrasting data were reported (Table 8). CYP11A1 mRNA was detected in the temporal, frontal neocortex and subcortical white matter of men, women and children (Stoffel-Wagner, 2001). Low mRNA levels of CYP17A1 were detected in the hippocampus, amygdala, caudate nucleus, cerebellum, corpus callosum, spinal cord and thalamus (Stoffel-Wagner, 2001; Yu et al., 2002), but other authors found no expression of this enzyme (Steckelbroeck et al., 2004b, 2010; MacKenzie et al., 2008). No 3βHSD1 or 2 was detected in temporal lobes, hippocampus, thalamus and amygdala (Stoffel-Wagner, 2001; Steckelbroeck et al., 2010), although other authors detected low levels in amygdala, caudate nucleus, cerebellum, corpus callosum, hippocampus, spinal cord and thalamus (Yu et al., 2002).
The temporal lobes (both neocortex and white matter) have 17βHSD oxidative and reductive activities, CYP19A1 mRNA expression and activity, which is also present in hippocampus (Stoffel-Wagner et al., 1999a; Stoffel-Wagner, 2001). Temporal lobe specimens from 10 men to 12 women indicated that 17βHSD estrogen-oxidative and DHEA-reductive metabolisms are predominant, thus producing E1 and A5, respectively (Stoffel-Wagner, 2001). Regarding the different 17βHSDs, type 1, 3, and 4 mRNAs (but not type 2) were demonstrated by competitive reverse transcription-PCR in specimens from 34 women, 32 men and 10 children (Casey et al., 1994; Beyenburg et al., 2000). Subsequent studies confirmed the expression of types 4, 7, 8, 10, 11 17βHSD and AKR1C3/17βHSD5 in temporal lobes and hippocampus (Stoffel-Wagner, 2001; Steckelbroeck et al., 2003). In particular 17βHSD10 is involved in the deactivation of THP to 5αDHP, and it is an important regulator of neurological functions (Yang et al., 2016).
Production of 5α-androstane and pregnane neurosteroids is mediated by the action of SRD5As and AKR1Cs (Figure 2). SRD5A1 (not type 2) mRNA and enzyme activity were demonstrated in temporal neocortex and subcortical white matter, hippocampus, cerebellum, hypothalamus (Steckelbroeck et al., 2001; Stoffel-Wagner, 2001), and AKR1C4 mRNA was detected in both hippocampus and temporal lobe (Stoffel-Wagner, 2001). AKR1C1 and AKR1C2 are widely expressed in CNS and since no specific inhibitors directed against AKR1C1 to 4 could completely inhibit AKR1C brain activity, the involvement of an unidentified enzyme is suggested (Steckelbroeck et al., 2010). Isomeric 5β-neurosteroids require the action of AKR1D1, and it is unknown whether AKR1D1 is expressed in CNS, or liver 5β-steroids reach peripheral regions via the circulation (Jin et al., 2011).
The sulphatase pathway in the CNS is relevant because (although recent studies are revisiting this paradigm; Qaiser et al., 2017), sulphated-steroids do not cross the blood-brain barrier. Therefore, sulphated neurosteroids like DHEA-S and P5-S need to be generated locally, and in line with this, their level in the CNS is independent from the level in the blood (Rajkowski et al., 1997) and varies throughout distinct brain regions (especially hippocampus and hypothalamus) (Jäntti et al., 2010).
STS and SULTs are widely expressed, with no gender-related differences (Table 8) (Kríz et al., 2008a,b; Mueller et al., 2015). SULT1A1 has high expression especially in specimens isolated from cerebellum, occipital and frontal lobes (Salman et al., 2009). No brain region expresses SULT2A1, whereas contrasting data exist for SULT2B1 and SULT1E1 (Table 8).
Diseases and Treatments
Steroid metabolism is deviated in schizophrenia (Bicikova et al., 2013) and aberrations and unbalances of intracrine enzymes are associated with neurological disorders (Luchetti et al., 2011 and see Table 5). In a study of 49 patients with AD, prefrontal cortex mRNA levels of 17βHSD1, CYP19A1 and AKR1C2 increased at late stages (Luchetti et al., 2011). STS and SULT activities, measured by radioimmunoassay and GC-MS in 55 human brain tumor specimens, varied between tumor types (Kríz et al., 2008b). Immunoreactivity for AKR1C3/17βHSD5 was low in medulloblastomas (n = 10 analyzed), high in 37 glial neoplasms and 18 meningiomas and was absent in intracranial schwannoma (n = 7) (Park et al., 2010). A recent screening of a chemical library of steroid inhibitors using three low grade pediatric glioma cell lines found that inhibition of 17βHSD3 blocked cell growth and induced apoptosis in vitro (Ajeawung et al., 2013)
Type 10 17βHSD is associated with AD and is a potential target in diseases like AD, Parkinson, and an X-linked mental retardation, that may arise from the impaired degradation of branched chain fatty acid, isoleucine or aberrant neurosteroid (THP) metabolism (Lim et al., 2011; Yang et al., 2016).
STS has been implicated in ADHD and a recent mouse study indicates that genetic and pharmacological manipulations of the STS axis influence the inhibitory processes and give rise to improvements in response control (Davies et al., 2014). A recent animal experiment using a model of autoimmune encephalomyelitis showed high SULT1A1 mRNA expression in laser-captured-micro-dissected white matter astrocytes, suggesting that deactivation of estrogens (and other phenolic substrates) may be responsible for the resistance to anti-neuro-inflammatory treatments in these cells and could be possibly used as new treatments to protect CNS from inflammatory injuries (Guillot et al., 2015).
CNS: Conclusions
CNS can synthesize steroids from cholesterol, although this is restricted to few brain regions. Steroid metabolism in the CNS is particular complex due to the formation of both 5α-/β-reduced and sulpho-conjugated neurosteroids (Table 8 and Figure 3).
Intracrinology in Other Tissues and Systems
Steroid metabolism is also important in the immune system, skin and adipose tissue. A thorough review of these systems is outside the scope of this study, however, a brief mention is given below.
Immune System and Inflammation
Beside corticosteroids, several other steroids affect the immune system and inflammation. A5 induces white blood cells and platelets production in bone marrow (Chen et al., 2004); estrogens and androgens control B-lymphocyte development in a sex-dependent way and modulate autoimmune diseases (McCarthy, 2000; Calippe et al., 2010; Sakiani et al., 2013).
Lipopolysaccharide-mediated proinflammatory pathway in macrophages and NF-κB activation are blocked by estrogens, which induce T-helper (Th) type 2 responses, whereas androgens stimulate type 1 responses (Iwasa et al., 2015). DHEA and DHEA-S also regulate the maturation of Th1 or Th2 cells. It was shown that plasma Th2 lymphocytes and its major secreted cytokine IL6 increase with age, and this is reversed in mice upon administration of DHEA or DHEA-S (Reed et al., 2005). Such effect was recapitulated in vitro by DHEA but not DHEA-S implicating the involvement of macrophage STS in lymphoid tissues where Th cell maturation occurs. In line with this, the effect of DHEA-S, but not DHEA, was impaired in vivo by an STS inhibitor (Reed et al., 2005). These data prompted to propose STS inhibition as a therapeutic approach for diseases associated with inappropriate immune responses and excess Th1 cytokines such as rheumatoid arthritis (Reed et al., 2005). Whether the action of DHEA is secondary to its conversion to androgens or estrogens is currently unclear. STS activity of peripheral blood leukocytes is higher in women during the follicular phase of the menstrual cycle than in women in the luteal phase or in men and it becomes highest during pregnancy, suggesting a role for P in regulating STS activity (Reed et al., 2005; Mueller et al., 2015). In vitro studies also demonstrated that STS activity is induced by cytokines such as IL6 and TNF (Mueller et al., 2015).
Opposite deregulation of the sulphatase pathway is seen in other chronic inflammatory diseases/cell types. Vascular smooth muscle cells show higher STS activity in women with mild atherosclerosis compared with women with severe disease (and male), whereas SULT1E1 activity is lower in women with severe disease (Mueller et al., 2015).
CYP19A1 is also expressed in macrophages (Konings et al., 2017) and KO mice have increased numbers of peripheral blood and bone marrow cells and inflammatory renal lesions (Shim et al., 2004). CYP19A1 inhibitors exacerbate the autoimmune lesions in a murine model of Sjögren syndrome and estrogen administration reverses such phenotype (Iwasa et al., 2015; Park et al., 2015). Opposite effects are observed in prostate, where elevated intracrine estrogens due to CYP19A1 overexpression induce inflammation and pre-malignant pathology (Ellem et al., 2009) as well as in adipose tissue (Reed et al., 2005).
Skin
The skin is the largest human organ and first barrier against pathogens where important immune functions interconnected with intracrine steroid metabolism take place (Slominski et al., 2013). Keratinocytes and sebocytes express ERs, intracrine enzymes, and the activity of sebaceous glands is influenced by steroids as indicated by the sebum production at andrenarche (Slominski et al., 2013). CYP17A1, CYP19A1, 17βHSD1, 2, 3, 4 (and enzymes metabolizing corticosteroid - outside the scope of this review) are detected in human skin. Some genes are under the influence of vitamin D and sebocytes can synthesize T from adrenal precursors (Hughes et al., 1997; Thiboutot et al., 1998; Slominski et al., 2013). Low 17βHSD oxidative metabolism characterizes sebaceous glands from skin areas prone to develop acne compared with other locations, suggesting a protective role of the oxidative metabolism against androgen excess (Fritsch et al., 2001). Sulphatase pathway is present in the skin (Reed et al., 2005; Simard et al., 2005), and genetic variants in STS and SULTs are associated with skin disturbances, most likely because of unbalanced steroid accumulation (Table 5).
Adipose Tissue
The adipose tissue is one of the most complex endocrine organs that besides secreting leptin and adiponectin, is a site of steroid metabolism, it establishes interaction with the CNS for glucose and lipid metabolism control, energy homeostasis and inflammation. The implication of sex steroids in adipose tissue is demonstrated by the different fat distribution that characterizes men and women (Mauvais-Jarvis et al., 2013; Varlamov et al., 2014; López and Tena-Sempere, 2015, 2016; Palmer and Clegg, 2015). ER-KO and CYP19A1-KO mice develop obesity with human-like phenotypes (López and Tena-Sempere, 2015). Estrogens protect against metabolic syndrome and men lacking endogenous estrogens (CYP19A1 or ER-α deficiency) develop hypertriglyceridemia, glucose intolerance and insulin resistance (Kim et al., 2014). In adipose tissue of men, 17βHSD2 levels and androgen inactivation correlate with BMI (Fouad Mansour et al., 2015). A mouse study also showed that increased unsulphated-estrogen availability due to loss of SULT1E1 improved metabolic function in a model of type 2 diabetes, which leads to speculations about a potential role of SULT1E1 inhibition for this disease - at least in women (Gao et al., 2012).
Fat consists of different tissue types (white and brown) and different regional depots with distinct physiological, intracrinological characteristics and distinct relations with pathologies and metabolic disorders (Blouin et al., 2009; Mauvais-Jarvis et al., 2013). White adipose tissue is mainly subcutaneous (abdomen) or visceral (surrounding the inner organs), this last being associated with metabolic risks. A plethora of investigations demonstrated the ability of adipose tissue to aromatise androgens into estrogens and that the intra-tissue steroid levels are higher than the levels in blood (Bélanger et al., 2002). Androgenic and estrogenic 17βHSD activity and the mRNA for 17βHSD1, 2, 3, 7, 12, AKR1C3/17βHSD5 were detected in both intra-abdominal and subcutaneous fat (Bélanger et al., 2002; Quinkler et al., 2004; Bellemare et al., 2009; Wang et al., 2013).
Both subcutaneous and visceral fat tissue of women expresses the androgenic 17βHSD3 (generally considered testis specific) indicating that adipose tissue in women is substantially androgenic. Such characteristic in the visceral depot increased with increasing BMI, suggesting a link between central obesity and metabolic diseases (Corbould et al., 2002).
Additionally, several enzymes (AKR1C2, AKR1C3/17βHSD5, CYP19A1, STS and SULT1E1) vary throughout adipocyte differentiation and maturation (Quinkler et al., 2004; Bellemare et al., 2009; Blouin et al., 2009; Mueller et al., 2015).
Conclusions and Recommendations
Intracrinology consists of a complex and intricate network of alternative and redundant pathways that generate, deactivate steroids in peripheral tissues and ultimately control steroid exposure in a tissue specific manner. A number of compounds have that ability to bind and activate more than one nuclear receptors thus exerting multiple biological actions. Blood steroids represent a reservoir of substrates that support these intracrine networks. Studies retrieved by the systematic search demonstrated that most investigations rely on RT-PCR or IHC to detect enzyme and protein, and frequently without multiple-technique confirmation of the data. Since both techniques present limitations, and antibodies for IHC often perform sub-optimally (detection limit is not sufficient to detect some intracrine enzymes, crossreactivity between isoforms) these techniques are not always suitable to infer the real biological role of a reaction/enzyme.
However, the recent technological advances in steroid profiling together with an improved knowledge of intracrine enzymes and the possibility to validate data using multiple approaches (RNA, protein, activity, steroid profiling) create today unprecedented opportunities to expand our understanding of intracrinology, its relation with endocrinology and to exploit this knowledge in patient care. Improved multiplex platforms allowing to profile in peripheral tissues all steroids depicted in Figure 2 are awaited and will elucidate the relevant tissue-specific networks. It is envisaged that novel prognostic markers and drug targets will become of clinical relevance soon.
We should however be aware that the redundant actions of intracrine enzymes, their substrate promiscuity, the existence of alternative pathways and the patient-to-patient variability might result in drug insensitivity. Dual/triple inhibitors will help solving this problem. In addition, in order to optimize research on novel drugs, the classical preclinical drug discovery pipelines (safety, pharmacokinetics and dynamics), should encompass parallel research lines to learn how to pre-select potentially responsive patients.
Finally, since we know that steroidal and intracrine drugs might have profound effects on the CNS, it is desirable to have in depth research on the neurological effects of potential novel drugs during the nonclinical phase of drug development. This will facilitate to select suitable compounds to the clinical development.
Author Contributions
GK drafted the study, prepared figures, tables, intermediate versions, final version and approved final version. LB drafted part of the study, contributed to intermediate versions and approved final version. KC drafted part of the study, contributed to intermediate versions and approved final version. BD contributed to intermediate versions and approved final version. TL drafted part of the study, contributed to intermediate versions and approved final version. PK contributed to intermediate versions and approved final version. MB contributed to intermediate versions and approved final version. RK contributed to intermediate versions and approved final version. SX drafted part of the study, contributed to intermediate versions and approved final version. AR drafted the study, prepared figures, tables, intermediate versions, final version and approved final version.
We also would like to thank the management and curator of the online databases we made use of, namely: the database of chemical molecules PubChem (www.ncbi.nlm.nih.gov; pubchem.ncbi.nlm.nih.gov) maintained by the National Center for Biotechnology Information (NCBI; National Library of Medicine/United States National Institutes of Health - NIH); Chemical Abstracts Service (CAS), maintained by the American Chemical Society (www.cas.org); Human Metabolome Data Base (HMDB, www.hmdb.ca), funded and maintained by Genome Canada; Chemical Book (www.chemicalbook.com), funded by industrial partners; Chemical Entities of Biological Interest (ChEBI; www.ebi.ac.uk/chebi/init.do), curated by the European Bioinformatics Institute of the European Molecular Biology Laboratory (EMBL); drug and drug target database Drugbank (www.drugbank.ca/drugs), University of Alberta and The Metabolomics Innovation Centre; GeneCards (www.genecards.org), developed and maintained by the Crown Human Genome Center at the Weizmann Institute of Science; Online Mendelian Inheritance in Man® (OMIM®, https://omim.org/), McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University (Baltimore, MD); Mouse Genome Informatics (MGI; www.informatics.jax.org), Mouse Genome Database at the Mouse Genome Informatics website, The Jackson Laboratory, Bar Harbor, Maine.
Funding
The present study was sponsored by the Dutch Cancer Society (KWF Kankerbestrijding: www.kwf.nl), contract number UM-13-5782 granted to AR.
Conflict of Interest Statement
PK and TL are employees of Forendo Pharma Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Dr. Margaretha A Skowron (Department of Urology, University Düsseldorf, Germany) for graphic arts.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2018.00940/full#supplementary-material
Abbreviations
[3H], tritiated hydrogen; [14C], radioactive carbon; AD, Alzheimer disease; ADHD, attention deficit hyperactivity disorder; AI, aromatase inhibitor; AKR, aldo-ketoreductase; AR, androgen-receptor; ART, assisted reproduction technology; BMD, bone mineral density; BMP-2, bone morphogenetic protein 2; COPD, chronic obstructive pulmonary disease; COUP-TFII, chicken-ovalbumin-upstream-promoter-transcription-factor II; CNS, central nervous system; CRC, colorectal cancer; CX43, connexin 43; DS, digestive system; Ed, embryonic day; EC, endometrial cancer; EndRet, endoplasmic reticulum; ER, estrogen-receptor; Fdx, ferredoxin; FdR, ferredoxin reductase; GC-MS, gas-chromatography tandem mass-spectrometry; GnRH, gonadotropin releasing hormone; GH, growth hormone; GIT, gastrointestinal tract; GPER, G protein-coupled estrogen-receptor; HPLC, high performance liquid-chromatography; HRT, hormone-replacement-therapy; hCG, human chorionic gonadotropin; IGF1, insulin-like growth factor 1; IHC, immunohistochemistry; IL-1, interleukin-1; IL-6, interleukin-6; KO, knock-out; LC-MS, liquid-chromatography tandem mass-spectrometry; mTOR, mammalian target of rapamycin; NAPDH, nicotine-adenine-dinucleotide-phosphate; NSCLC, non-small cell lung cancer; PAIN, phenomena of pan-assay interfering compounds; PAP, bis-phospho-nucleotide-3′-phospho-adenosine-5′-phosphate; PCOC, poly cystic ovarian syndrome; POR, P450 oxidoreductase; RT-qPCR, reverse-transcriptase quantitative polymerase-chain-reaction; SF1, steroidogenic factor 1; SHBG, sex hormone binding globulin; SNP, single nucleotide polymorphism; SRD, short-chain dehydrogenase; Th, T-helper; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor; WB, western blot/blotting; WOI, window of implantation.
References
Abdelsamie, A. S., van Koppen, C. J., Bey, E., Salah, M., Börger, C., Siebenbürger, L., et al. (2017). 17β-HSD1 inhibitor with sub-nanomolar IC50 for a proof-of-principle study. Eur. J. Med. Chem. 127, 944–957. doi: 10.1016/j.ejmech.2016.11.004
Acién, P., Velasco, I., Gutiérrez, M., and Martínez-Beltrán, M. (2007). Aromatase expression in endometriotic tissues and its relationship to clinical and analytical findings. Fertil. Steril. 88, 32–38. doi: 10.1016/j.fertnstert.2006.11.188
Adachi, K., Miki, Y., Saito, R., Hata, S., Yamauchi, M., Mikami, Y., et al. (2015). Intracrine steroid production and mammalian target of rapamycin pathways in pulmonary lymphangioleiomyomatosis. Hum. Pathol. 46, 1685–1693. doi: 10.1016/j.humpath.2015.02.019
Adessi, G. L., Prost, O., Agnani, G., Petitjean, A., and Burnod, J. (1984). Estrone sulfatase activity in normal and abnormal endometrium. Arch. Gynecol. 236, 13–18. doi: 10.1007/BF02114864
Adjei, A. A., Thomae, B. A., Prondzinski, J. L., Eckloff, B. W., Wieben, E. D., and Weinshilboum, R. M. (2003). Human estrogen sulfotransferase (SULT1E1) pharmacogenomics: Gene resequencing and functional genomics. Br. J. Pharmacol. 139, 1373–1382. doi: 10.1038/sj.bjp.0705369
Aghajanova, L., Hamilton, A., Kwintkiewicz, J., Vo, K. C., and Giudice, L. C. (2009). Steroidogenic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women with versus without endometriosis. Biol. Reprod. 80, 105–114. doi: 10.1095/biolreprod.108.070300
Agís-Balboa, R. C., Pinna, G., Zhubi, A., Maloku, E., Veldic, M., Costa, E., et al. (2006). Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 103, 14602–14607. doi: 10.1073/pnas.0606544103
Ajeawung, N. F., Maltais, R., Jones, C., Poirier, D., and Kamnasaran, D. (2013). Viability screen on pediatric low grade glioma cell lines unveils a novel anti-cancer drug of the steroid biosynthesis inhibitor family. Cancer Lett. 330, 96–105. doi: 10.1016/j.canlet.2012.11.034
Almey, A., Milner, T. A., and Brake, W. G. (2015). Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm. Behav. 74, 125–138. doi: 10.1016/j.yhbeh.2015.06.010
Alshogran, O. Y. (2017). Pharmacogenetics of aldo-keto reductase 1C (AKR1C) enzymes. Expert Opin. Drug Metab. Toxicol. 13, 1063–1073. doi: 10.1080/17425255.2017.1376648
Amant, F., Moerman, P., Neven, P., Timmerman, D., Van Limbergen, E., and Vergote, I. (2005). Endometrial cancer. Lancet 366, 491–505. doi: 10.1016/S0140-6736(05)67063-8
Amos-Landgraf, J. M., Heijmans, J., Wielenga, M. C., Dunkin, E., Krentz, K. J., Clipson, L., et al. (2014). Sex disparity in colonic adenomagenesis involves promotion by male hormones, not protection by female hormones. Proc. Natl. Acad. Sci. U.S.A. 111, 16514–16519. doi: 10.1073/pnas.1323064111
Andersen, C. Y., and Ezcurra, D. (2014). Human steroidogenesis: implications for controlled ovarian stimulation with exogenous gonadotropins. Reprod. Biol. Endocrinol. 12:128. doi: 10.1186/1477-7827-12-128
Andrew, A. S., Hu, T., Gu, J., Gui, J., Ye, Y., Marsit, C. J., et al. (2012). HSD3B and gene-gene interactions in a pathway-based analysis of genetic susceptibility to bladder cancer. PLoS ONE 7:e51301. doi: 10.1371/journal.pone.0051301
Aresti, U., Carrera, S., Iruarrizaga, E., Fuente, N., Marrodan, I., de Lobera, A. R., et al. (2014). Estrogen receptor 1 gene expression and its combination with estrogen receptor 2 or aromatase expression predicts survival in non-small cell lung cancer. PLoS ONE 9:e109659. doi: 10.1371/journal.pone.0109659
Arnold, C., and Einspanier, A. (2013). Medical treatment improves social behavior in a primate endometriosis model (Callithrix jacchus). J. Med. Primatol. 42, 112–119. doi: 10.1111/jmp.12042
Ashton, K. A., Proietto, A., Otton, G., Symonds, I., McEvoy, M., Attia, J., et al. (2010). Polymorphisms in genes of the steroid hormone biosynthesis and metabolism pathways and endometrial cancer risk. Cancer Epidemiol. 34, 328–337. doi: 10.1016/j.canep.2010.03.005
Attar, E., Tokunaga, H., Imir, G., Yilmaz, M. B., Redwine, D., Putman, M., et al. (2009). Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J. Clin. Endocrinol. Metab. 94, 623–631. doi: 10.1210/jc.2008-1180
Audet-Walsh, É., Bellemare, J., Lacombe, L., Fradet, Y., Fradet, V., Douville, P., et al. (2012). The impact of germline genetic variations in hydroxysteroid (17-beta) dehydrogenases on prostate cancer outcomes after prostatectomy. Eur. Urol. 62, 88–96. doi: 10.1016/j.eururo.2011.12.021
Audet-Walsh, E., Lepine, J., Gregoire, J., Plante, M., Caron, P., Tetu, B., et al. (2011). Profiling of endogenous estrogens, their precursors, and metabolites in endometrial cancer patients: association with risk and relationship to clinical characteristics. J. Clin. Endocrinol. Metab. 96, E330–E339. doi: 10.1210/jc.2010-2050
Aumüller, G., Eicheler, W., Renneberg, H., Adermann, K., Vilja, P., and Forssmann, W. G. (1996). Immunocytochemical evidence for differential subcellular localization of 5 alpha-reductase isoenzymes in human tissues. Acta Anat. (Basel). 156, 241–252. doi: 10.1159/000147852
Bacallao, K., Leon, L., Gabler, F., Soto, E., Romero, C., Valladares, L., et al. (2008). In situ estrogen metabolism in proliferative endometria from untreated women with polycystic ovarian syndrome with and without endometrial hyperplasia. J. Steroid Biochem. Mol. Biol. 110, 163–169. doi: 10.1016/j.jsbmb.2008.03.031
Baes, M., Huyghe, S., Carmeliet, P., Declercq, P. E., Collen, D., Mannaerts, G. P., et al. (2000). Inactivation of the peroxisomal multifunctional protein-2 in mice impedes the degradation of not only 2-methyl-branched fatty acids and bile acid intermediates but also of very long chain fatty acids. J. Biol. Chem. 275, 16329–16336. doi: 10.1074/jbc.M001994200
Bagi, C. M., Wood, J., Wilkie, D., and Dixon, B. (2008). Effect of 17β-hydroxysteroid dehydrogenase type 2 inhibitor on bone strength in ovariectomized cynomolgus monkeys. J. Musculoskelet. Neuronal Interact. 8, 267–280.
Bair, S. R., and Mellon, S. H. (2004). Deletion of the mouse P450c17 gene causes early embryonic lethality. Mol. Cell. Biol. 24, 5383–5390. doi: 10.1128/MCB.24.12.5383-5390.2004
Balk, S. P., and Knudsen, K. E. (2008). AR, the cell cycle, and prostate cancer. Nucl. Recept. Signal. 6:e001.
Barzi, A., Lenz, A. M., Labonte, M. J., and Lenz, H. J. (2013). Molecular pathways: estrogen pathway in colorectal cancer. Clin. Cancer Res. 19, 5842–5848. doi: 10.1158/1078-0432.CCR-13-0325
Bélanger, C., Luu-The, V., Dupont, P., and Tchernof, A. (2002). Adipose tissue intracrinology: potential importance of local androgen/estrogen metabolism in the regulation of adiposity. Horm. Metab. Res. 34, 737–745. doi: 10.1055/s-2002-38265
Belelli, D., and Lambert, J. J. (2005). Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat. Rev. Neurosci. 6, 565–575. doi: 10.1038/nrn1703
Bellemare, V., Faucher, F., Breton, R., and Luu-The, V. (2005). Characterization of 17α-hydroxysteroid dehydrogenase activity (17α-HSD) and its involvement in the biosynthesis of epitestosterone. BMC Biochem. 6:12. doi: 10.1186/1471-2091-6-12
Bellemare, V., Laberge, P., Noel, S., Tchernof, A., and Luu-The, V. (2009). Differential estrogenic 17β-hydroxysteroid dehydrogenase activity and type 12, 17β-hydroxysteroid dehydrogenase expression levels in preadipocytes and differentiated adipocytes. J. Steroid Biochem. Mol. Biol. 114, 129–134. doi: 10.1016/j.jsbmb.2009.01.002
Berstein, L., Zimarina, T., Imyanitov, E., Kovalevskij, A., Maximov, S., Pujol, P., et al. (2006). Hormonal imbalance in two types of endometrial cancer and genetic polymorphism of steroidogenic enzymes. Maturitas 54, 352–355. doi: 10.1016/j.maturitas.2006.04.017
Beyenburg, S., Stoffel-Wagner, B., Watzka, M., Blumcke, I., Bauer, J., Schramm, J., et al. (1999). Expression of cytochrome P450scc mRNA in the hippocampus of patients with temporal lobe epilepsy. Neuroreport 10, 3067–3070. doi: 10.1097/00001756-199909290-00035
Beyenburg, S., Watzka, M., Blumcke, I., Schramm, J., Bidlingmaier, F., Elger, C. E., et al. (2000). Expression of mRNAs encoding for 17β-hydroxisteroid dehydrogenase isozymes 1, 2, 3 and 4 in epileptic human hippocampus. Epilepsy Res. 41, 83–91. doi: 10.1016/S0920-1211(00)00130-3
Bicikova, M., Hill, M., Ripova, D., Mohr, P., and Hampl, R. (2013). Determination of steroid metabolome as a possible tool for laboratory diagnosis of schizophrenia. J. Steroid Biochem. Mol. Biol. 133, 77–83. doi: 10.1016/j.jsbmb.2012.08.009
Bilezikian, J. P., Morishima, A., Bell, J., and Grumbach, M. M. (1998). Increased bone mass as a result of estrogen therapy in a man with aromatase deficiency. N. Engl. J. Med. 339, 599–603. doi: 10.1056/NEJM199808273390905
Blake, J. A., Eppig, J. T., Kadin, J. A., Richardson, J. E., Smith, C. L., Bult, C. J., et al. (2017). Mouse Genome Database (MGD)-2017: community knowledge resource for the laboratory mouse. Nucleic Acids Res. 45, D723–D729. doi: 10.1093/nar/gkw1040
Blouin, K., Nadeau, M., Mailloux, J., Daris, M., Lebel, S., Luu-The, V., et al. (2009). Pathways of adipose tissue androgen metabolism in women: depot differences and modulation by adipogenesis. Am. J. Physiol. Endocrinol. Metab. 296, E244–E255. doi: 10.1152/ajpendo.00039.2008
Bothe, U., Busemann, M., Steinmeyer, A., Droescher, P., Fischer, O. M., Peters, M., et al. (2017). “First time disclosure of BAY 1128688: a novel AKR1C3 inhibitor for the treatment of endometriosis,” in 254th ACS National Meeting & Exposition (Washington, DC).
Boucher, E., Provost, P. R., Plante, J., and Tremblay, Y. (2009). Androgen receptor and 17β-HSD type 2 regulation in neonatal mouse lung development. Mol. Cell. Endocrinol. 311, 109–119. doi: 10.1016/j.mce.2009.06.012
Bovée, J. V., Cleton-Jansen, A. M., Taminiau, A. H., and Hogendoorn, P. C. (2005). Emerging pathways in the development of chondrosarcoma of bone and implications for targeted treatment. Lancet Oncol. 6, 599–607. doi: 10.1016/S1470-2045(05)70282-5
Brereton, P., Suzuki, T., Sasano, H., Li, K., Duarte, C., Obeyesekere, V., et al. (2001). Pan1b (17βHSD11)-enzymatic activity and distribution in the lung. Mol. Cell. Endocrinol. 171, 111–117. doi: 10.1016/S0303-7207(00)00417-2
Brosens, J., Verhoeven, H., Campo, R., Gianaroli, L., Gordts, S., Hazekamp, J., et al. (2004). High endometrial aromatase P450 mRNA expression is associated with poor IVF outcome. Hum. Reprod. 19, 352–356. doi: 10.1093/humrep/deh075
BroŽic, P., Turk, S., Rizner, T. L., and Gobec, S. (2011). Inhibitors of aldo-keto reductases AKR1C1-AKR1C4. Curr. Med. Chem. 18, 2554–2565. doi: 10.2174/092986711795933713
Bukulmez, O., Hardy, D. B., Carr, B. R., Auchus, R. J., Toloubeydokhti, T., Word, R. A., et al. (2008a). Androstenedione up-regulation of endometrial aromatase expression via local conversion to estrogen: potential relevance to the pathogenesis of endometriosis. J. Clin. Endocrinol. Metab. 93, 3471–3477. doi: 10.1210/jc.2008-0248
Bukulmez, O., Hardy, D. B., Carr, B. R., Word, R. A., and Mendelson, C. R. (2008b). Inflammatory status influences aromatase and steroid receptor expression in endometriosis. Endocrinology 149, 1190–1204. doi: 10.1210/en.2007-0665
Bulun, S. E., Economos, K., Miller, D., and Simpson, E. R. (1994). CYP19 (aromatase cytochrome P450) gene expression in human malignant endometrial tumors. J. Clin. Endocrinol. Metab. 79, 1831–1834.
Bulun, S. E., Mahendroo, M. S., and Simpson, E. R. (1993). Polymerase chain reaction amplification fails to detect aromatase cytochrome P450 transcripts in normal human endometrium or decidua. J. Clin. Endocrinol. Metab. 76, 1458–1463.
Caiazza, F., Ryan, E. J., Doherty, G., Winter, D. C., and Sheahan, K. (2015). Estrogen receptors and their implications in colorectal carcinogenesis. Front. Oncol. 5:19. doi: 10.3389/fonc.2015.00019
Calippe, B., Douin-Echinard, V., Delpy, L., Laffargue, M., Lelu, K., Krust, A., et al. (2010). 17β-estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J. Immunol. 185, 1169–1176. doi: 10.4049/jimmunol.0902383
Carani, C., Qin, K., Simoni, M., Faustini-Fustini, M., Serpente, S., Boyd, J., et al. (1997). Effect of testosterone and estradiol in a man with aromatase deficiency. N. Engl. J. Med. 337, 91–95. doi: 10.1056/NEJM199707103370204
Carey, M. A., Card, J. W., Voltz, J. W., Arbes, S. J. Jr., Germolec, D. R., Korach, K. S., et al. (2007). It's all about sex: gender, lung development and lung disease. Trends Endocrinol. Metab. 18, 308–313. doi: 10.1016/j.tem.2007.08.003
Carneiro, M. M., Morsch, D. M., Camargos, A. F., Reis, F. M., and Spritzer, P. M. (2008). Androgen receptor and 5α-reductase are expressed in pelvic endometriosis. BJOG 115, 113–117. doi: 10.1111/j.1471-0528.2007.01521.x
Carneiro, M. M., Morsch, D. M., Camargos, A. F., Spritzer, P. M., and Reis, F. M. (2007). Expression of 17β-hydroxysteroid dehydrogenase type 2 in pelvic endometriosis. Gynecol. Endocrinol. 23, 188–192. doi: 10.1080/09513590701200850
Caron, K. M., Soo, S. C., Wetsel, W. C., Stocco, D. M., Clark, B. J., and Parker, K. L. (1997). Targeted disruption of the mouse gene encoding steroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. Proc. Natl. Acad. Sci. U.S.A. 94, 11540–11545. doi: 10.1073/pnas.94.21.11540
Casey, M. L., MacDonald, P. C., and Andersson, S. (1994). 17 beta-Hydroxysteroid dehydrogenase type 2: chromosomal assignment and progestin regulation of gene expression in human endometrium. J. Clin. Invest. 94, 2135–2141. doi: 10.1172/JCI117569
Chai, Z., Brereton, P., Suzuki, T., Sasano, H., Obeyesekere, V., Escher, G., et al. (2003). 17β-hydroxysteroid dehydrogenase type XI localizes to human steroidogenic cells. Endocrinology 144, 2084–2091. doi: 10.1210/en.2002-221030
Chang, T. S., Lin, H. K., Rogers, K. A., Brame, L. S., Yeh, M. M., Yang, Q., et al. (2013). Expression of aldo-keto reductase family 1 member C3 (AKR1C3) in neuroendocrine tumors & adenocarcinomas of pancreas, gastrointestinal tract, and lung. Int. J. Clin. Exp. Pathol. 6, 2419–2429.
Chen, F., Knecht, K., Leu, C., Rutledge, S. J., Scafonas, A., Gambone, C., et al. (2004). Partial agonist/antagonist properties of androstenedione and 4-androsten-3β,17β-diol. J. Steroid Biochem. Mol. Biol. 91, 247–257. doi: 10.1016/j.jsbmb.2004.04.009
Chen, G., Zhang, D., Jing, N., Yin, S., Falany, C. N., and Radominska-Pandya, A. (2003). Human gastrointestinal sulfotransferases: identification and distribution. Toxicol. Appl. Pharmacol. 187, 186–197. doi: 10.1016/S0041-008X(02)00073-X
Cho, L. Y., Yang, J. J., Ko, K. P., Ma, S. H., Shin, A., Choi, B. Y., et al. (2012). Genetic susceptibility factors on genes involved in the steroid hormone biosynthesis pathway and progesterone receptor for gastric cancer risk. PLoS ONE 7:e47603. doi: 10.1371/journal.pone.0047603
Ciuffi, M., Savino, L., Santini, D., Buzzoni, P., Scarselli, G., and Mazzei, T. (1982). Estradiol and progestin receptors, 17-beta-hydroxysteroid-dehydrogenase and histopathologic grade in endometrial carcinoma. Tumori 68, 217–221. doi: 10.1177/030089168206800305
Cleton-Jansen, A. M., van Beerendonk, H. M., Baelde, H. J., Bovee, J. V., Karperien, M., and Hogendoorn, P. C. (2005). Estrogen signaling is active in cartilaginous tumors: implications for antiestrogen therapy as treatment option of metastasized or irresectable chondrosarcoma. Clin. Cancer Res. 11, 8028–8035. doi: 10.1158/1078-0432.CCR-05-1253
Colette, S., Defrere, S., Lousse, J. C., Van Langendonckt, A., Gotteland, J. P., Loumaye, E., et al. (2011). Inhibition of steroid sulfatase decreases endometriosis in an in vivo murine model. Hum. Reprod. 26, 1362–1370. doi: 10.1093/humrep/der079
Colette, S., Defrere, S., Van Kerk, O., Van Langendonckt, A., Dolmans, M. M., and Donnez, J. (2013). Differential expression of steroidogenic enzymes according to endometriosis type. Fertil. Steril. 100, 1642–1649. doi: 10.1016/j.fertnstert.2013.08.003
Colette, S., Lousse, J. C., Defrere, S., Curaba, M., Heilier, J. F., Van Langendonckt, A., et al. (2009). Absence of aromatase protein and mRNA expression in endometriosis. Hum. Reprod. 24, 2133–2141. doi: 10.1093/humrep/dep199
Compston, J. E. (2001). Sex steroids and bone. Physiol. Rev. 81, 419–447. doi: 10.1152/physrev.2001.81.1.419
Corbould, A. M., Bawden, M. J., Lavranos, T. C., Rodgers, R. J., and Judd, S. J. (2002). The effect of obesity on the ratio of type 3, 17β-hydroxysteroid dehydrogenase mRNA to cytochrome P450 aromatase mRNA in subcutaneous abdominal and intra-abdominal adipose tissue of women. Int. J. Obes. Relat. Metab. Disord. 26, 165–175. doi: 10.1038/sj.ijo.0801886
Cornel, K. M., Krakstad, C., Delvoux, B., Xanthoulea, S., Jori, B., Bongers, M. Y., et al. (2017). High mRNA levels of 17β-hydroxysteroid dehydrogenase type 1 correlate with poor prognosis in endometrial cancer. Mol. Cell. Endocrinol. 442, 51–57. doi: 10.1016/j.mce.2016.11.030
Cornel, K. M., Kruitwagen, R. F., Delvoux, B., Visconti, L., Van de Vijver, K. K., Day, J. M., et al. (2012). Overexpression of 17β-Hydroxysteroid Dehydrogenase Type 1 Increases the exposure of endometrial cancer to 17β-Estradiol. J. Clin. Endocrinol. Metab. 97, E591–E601. doi: 10.1210/jc.2011-2994
Cornel, K. M. C., Kruitwagen, R. P. F. M., and Romano, A. (2018). Local estrogen metabolism (intracrinology) in endometrial cancer: a systematic review. Mol. Cell. Endocrinol. Accepted for publication.
Couse, J. F., and Korach, K. S. (1999). Estrogen receptor null mice: what have we learned and where will they lead us? Endocr. Rev. 20, 358–417.
Couse, J. F., Lindzey, J., Grandien, K., Gustafsson, J. A., and Korach, K. S. (1997). Tissue distribution and quantitative analysis of estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) messenger ribonucleic acid in the wild-type and ERα-knockout mouse. Endocrinology 138, 4613–4621. doi: 10.1210/endo.138.11.5496
Couse, J. F., Yates, M. M., Walker, V. R., and Korach, K. S. (2003). Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERα but not ERβ. Mol. Endocrinol. 17, 1039–1053. doi: 10.1210/me.2002-0398
Dalla Valle, L., Toffolo, V., Nardi, A., Fiore, C., Armanini, D., Belvedere, P., et al. (2007). The expression of the human steroid sulfatase-encoding gene is driven by alternative first exons. J. Steroid Biochem. Mol. Biol. 107, 22–29. doi: 10.1016/j.jsbmb.2007.05.004
Das, A., Mantena, S. R., Kannan, A., Evans, D. B., Bagchi, M. K., and Bagchi, I. C. (2009). De novo synthesis of estrogen in pregnant uterus is critical for stromal decidualization and angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 106, 12542–12547. doi: 10.1073/pnas.0901647106
Dassen, H., Punyadeera, C., Kamps, R., Delvoux, B., Van Langendonckt, A., Donnez, J., et al. (2007). Estrogen metabolizing enzymes in endometrium and endometriosis. Hum. Reprod. 22, 3148–3158. doi: 10.1093/humrep/dem310
Davies, W., Humby, T., Trent, S., Eddy, J. B., Ojarikre, O. A., and Wilkinson, L. S. (2014). Genetic and pharmacological modulation of the steroid sulfatase axis improves response control; comparison with drugs used in ADHD. Neuropsychopharmacology 39, 2622–2632. doi: 10.1038/npp.2014.115
Day, J. M., Foster, P. A., Tutill, H. J., Schmidlin, F., Sharland, C. M., Hargrave, J. D., et al. (2013). STX2171, a 17β-hydroxysteroid dehydrogenase type 3 inhibitor, is efficacious in vivo in a novel hormone-dependent prostate cancer model. Endocr. Relat. Cancer 20, 53–64. doi: 10.1530/ERC-12-0231
De Graaff, A. A., D'Hooghe, T. M., Dunselman, G. A., Dirksen, C. D., Hummelshoj, L., Consortium, W. E., et al. (2013). The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum. Reprod. 28, 2677–2685. doi: 10.1093/humrep/det284
De Graaff, A. A., Dirksen, C. D., Simoens, S., De Bie, B., Hummelshoj, L., D'Hooghe, T. M., et al. (2015). Quality of life outcomes in women with endometriosis are highly influenced by recruitment strategies. Hum. Reprod. 30, 1331–1341. doi: 10.1093/humrep/dev084
De Graaff, A. A., Van Lankveld, J., Smits, L. J., Van Beek, J. J., and Dunselman, G. A. (2016). Dyspareunia and depressive symptoms are associated with impaired sexual functioning in women with endometriosis, whereas sexual functioning in their male partners is not affected. Hum. Reprod. 31, 2577–2586. doi: 10.1093/humrep/dew215
De Preter, V., Arijs, I., Windey, K., Vanhove, W., Vermeire, S., Schuit, F., et al. (2012). Impaired butyrate oxidation in ulcerative colitis is due to decreased butyrate uptake and a defect in the oxidation pathway. Inflamm. Bowel Dis. 18, 1127–1136. doi: 10.1002/ibd.21894
Delvoux, B., D'Hooghe, T., Kyama, C., Koskimies, P., Hermans, R. J., Dunselman, G. A., et al. (2014). Inhibition of type 1, 17β-hydroxysteroid dehydrogenase impairs the synthesis of 17β-estradiol in endometriosis lesions. J. Clin. Endocrinol. Metab. 99, 276–284. doi: 10.1210/jc.2013-2851
Delvoux, B., Groothuis, P., D'Hooghe, T., Kyama, C., Dunselman, G., and Romano, A. (2009). Increased production of 17β-estradiol in endometriosis lesions is the result of impaired metabolism. J. Clin. Endocrinol. Metab. 94, 876–883. doi: 10.1210/jc.2008-2218
Delvoux, B., Husen, B., Aldenhoff, Y., Koole, L., Dunselman, G., Thole, H., et al. (2007). A sensitive HPLC method for the assessment of metabolic conversion of estrogens. J. Steroid Biochem. Mol. Biol. 104, 246–251. doi: 10.1016/j.jsbmb.2007.03.006
Deng, H. Z., You, C., Xing, Y., Chen, K. Y., and Zou, X. B. (2016). A Family-Based Association Study of CYP11A1 and CYP11B1 Gene Polymorphisms With Autism in Chinese Trios. J. Child Neurol. 31, 733–737. doi: 10.1177/0883073815620672
Devroey, P., Bourgain, C., Macklon, N. S., and Fauser, B. C. (2004). Reproductive biology and IVF: ovarian stimulation and endometrial receptivity. Trends Endocrinol. Metab. 15, 84–90. doi: 10.1016/j.tem.2004.01.009
Dheenadayalu, K., Mak, I., Gordts, S., Campo, R., Higham, J., Puttemans, P., et al. (2002). Aromatase P450 messenger RNA expression in eutopic endometrium is not a specific marker for pelvic endometriosis. Fertil. Steril. 78, 825–829. doi: 10.1016/S0015-0282(02)03324-1
Di Costanzo, L., Penning, T. M., and Christianson, D. W. (2009). Aldo-keto reductases in which the conserved catalytic histidine is substituted. Chem. Biol. Interact. 178, 127–133. doi: 10.1016/j.cbi.2008.10.046
Dias, N. J., and Selcer, K. W. (2014). Steroid sulfatase mediated growth Sof human MG-63 pre-osteoblastic cells. Steroids 88, 77–82. doi: 10.1016/j.steroids.2014.07.001
Dias, N. J., and Selcer, K. W. (2016). Steroid sulfatase in the human MG-63 preosteoblastic cell line: Antagonistic regulation by glucocorticoids and NFkappaB. Mol. Cell. Endocrinol. 420, 85–96. doi: 10.1016/j.mce.2015.11.029
Dickinson, M. E., Flenniken, A. M., Ji, X., Teboul, L., Wong, M. D., White, J. K., et al. (2016). High-throughput discovery of novel developmental phenotypes. Nature 537, 508–514. doi: 10.1038/nature19356
Doherty, J. A., Weiss, N. S., Freeman, R. J., Dightman, D. A., Thornton, P. J., Houck, J. R., et al. (2005). Genetic factors in catechol estrogen metabolism in relation to the risk of endometrial cancer. Cancer Epidemiol. Biomarkers Prev. 14, 357–366. doi: 10.1158/1055-9965.EPI-04-0479
Dohi, O., Hatori, M., Suzuki, T., Ono, K., Hosaka, M., Akahira, J., et al. (2008). Sex steroid receptors expression and hormone-induced cell proliferation in human osteosarcoma. Cancer Sci. 99, 518–523. doi: 10.1111/j.1349-7006.2007.00673.x
Dong, Y., Qiu, Q. Q., Debear, J., Lathrop, W. F., Bertolini, D. R., and Tamburini, P. P. (1998). 17β-hydroxysteroid dehydrogenases in human bone cells. J. Bone Miner. Res. 13, 1539–1546. doi: 10.1359/jbmr.1998.13.10.1539
Driessen, C. A., Winkens, H. J., Hoffmann, K., Kuhlmann, L. D., Janssen, B. P., Van Vugt, A. H., et al. (2000). Disruption of the 11-cis-retinol dehydrogenase gene leads to accumulation of cis-retinols and cis-retinyl esters. Mol. Cell. Biol. 20, 4275–4287. doi: 10.1128/MCB.20.12.4275-4287.2000
Drzewiecka, H., Galecki, B., Jarmolowska-Jurczyszyn, D., Kluk, A., Dyszkiewicz, W., and Jagodzinski, P. P. (2015). Increased expression of 17-beta-hydroxysteroid dehydrogenase type 1 in non-small cell lung cancer. Lung Cancer 87, 107–116. doi: 10.1016/j.lungcan.2014.12.012
Drzewiecka, H., and Jagodzinski, P. P. (2012). Conversion of estrone to 17-beta-estradiol in human non-small-cell lung cancer cells in vitro. Biomed. Pharmacother. 66, 530–534. doi: 10.1016/j.biopha.2012.02.006
Duell, E. J., Travier, N., Lujan-Barroso, L., Boutron-Ruault, M. C., Clavel-Chapelon, F., Palli, D., et al. (2010). Menstrual and reproductive factors, exogenous hormone use, and gastric cancer risk in a cohort of women from the European Prospective Investigation Into Cancer and Nutrition. Am. J. Epidemiol. 172, 1384–1393. doi: 10.1093/aje/kwq321
Dunselman, G. A., Vermeulen, N., Becker, C., Calhaz-Jorge, C., D'Hooghe, T., De Bie, B., et al. (2014). ESHRE guideline: management of women with endometriosis. Hum. Reprod. 29, 400–412. doi: 10.1093/humrep/det457
Ellem, S. J., Wang, H., Poutanen, M., and Risbridger, G. P. (2009). Increased endogenous estrogen synthesis leads to the sequential induction of prostatic inflammation (prostatitis) and prostatic pre-malignancy. Am. J. Pathol. 175, 1187–1199. doi: 10.2353/ajpath.2009.081107
Endo, S., Miyagi, N., Matsunaga, T., Hara, A., and Ikari, A. (2016). Human dehydrogenase/reductase (SDR family) member 11 is a novel type of 17β-hydroxysteroid dehydrogenase. Biochem. Biophys. Res. Commun. 472, 231–236. doi: 10.1016/j.bbrc.2016.01.190
English, M. A., Hughes, S. V., Kane, K. F., Langman, M. J., Stewart, P. M., and Hewison, M. (2000). Oestrogen inactivation in the colon: analysis of the expression and regulation of 17β-hydroxysteroid dehydrogenase isozymes in normal colon and colonic cancer. Br. J. Cancer 83, 550–558. doi: 10.1054/bjoc.2000.1324
English, M. A., Kane, K. F., Cruickshank, N., Langman, M. J., Stewart, P. M., and Hewison, M. (1999). Loss of estrogen inactivation in colonic cancer. J. Clin. Endocrinol. Metab. 84, 2080–2085. doi: 10.1210/jcem.84.6.5772
Enríquez, J., Larrea, F., Santillan, R., Hernandez, A., Herrero, B., Perez-Palacios, G., et al. (2013). Neonatal rat osteoblasts bioconvert testosterone to non-phenolic metabolites with estrogen-like effects on bone cell proliferation and differentiation. Horm. Mol. Biol. Clin. Investig. 13, 41–49. doi: 10.1515/hmbci-2012-0035
Falk, R. T., Dallal, C. M., Lacey, J. V. Jr., Bauer, D. C., Buist, D. S., Cauley, J. A., et al. (2015). Estrogen metabolites are not associated with colorectal cancer risk in postmenopausal women. Cancer Epidemiol. Biomarkers Prev. 24, 1419–1422. doi: 10.1158/1055-9965.EPI-15-0541
Ferrero, S., Gillott, D. J., Venturini, P. L., and Remorgida, V. (2011). Use of aromatase inhibitors to treat endometriosis-related pain symptoms: a systematic review. Reprod. Biol. Endocrinol. 9, 89. doi: 10.1186/1477-7827-9-89
Fisher, C. R., Graves, K. H., Parlow, A. F., and Simpson, E. R. (1998). Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc. Natl. Acad. Sci. U.S.A. 95, 6965–6970. doi: 10.1073/pnas.95.12.6965
Flach, K. D., and Zwart, W. (2016). The first decade of estrogen receptor cistromics in breast cancer. J. Endocrinol. 229 R43–56. doi: 10.1530/JOE-16-0003
Fontein, D. B., Houtsma, D., Nortier, J. W., Baak-Pablo, R. F., Kranenbarg, E. M., van der Straaten, T. R., et al. (2014). Germline variants in the CYP19A1 gene are related to specific adverse events in aromatase inhibitor users: a substudy of Dutch patients in the TEAM trial. Breast Cancer Res. Treat. 144, 599–606. doi: 10.1007/s10549-014-2873-2
Foster, P. A. (2013). Oestrogen and colorectal cancer: mechanisms and controversies. Int. J. Colorectal Dis. 28, 737–749. doi: 10.1007/s00384-012-1628-y
Foster, P. A., Reed, M. J., and Purohit, A. (2008a). Recent developments of steroid sulfatase inhibitors as anti-cancer agents. Anticancer. Agents Med. Chem. 8, 732–738. doi: 10.2174/187152008785914815
Foster, P. A., Woo, L. W., Potter, B. V., Reed, M. J., and Purohit, A. (2008b). The use of steroid sulfatase inhibitors as a novel therapeutic strategy against hormone-dependent endometrial cancer. Endocrinology 149, 4035–4042. doi: 10.1210/en.2008-0223
Fouad Mansour, M., Pelletier, M., Boulet, M. M., Mayrand, D., Brochu, G., Lebel, S., et al. (2015). Oxidative activity of 17β-hydroxysteroid dehydrogenase on testosterone in male abdominal adipose tissues and cellular localization of 17β-HSD type 2. Mol. Cell. Endocrinol. 414, 168–176. doi: 10.1016/j.mce.2015.06.016
Freedman, N. D., Ahn, J., Hou, L., Lissowska, J., Zatonski, W., Yeager, M., et al. (2009). Polymorphisms in estrogen- and androgen-metabolizing genes and the risk of gastric cancer. Carcinogenesis 30, 71–77. doi: 10.1093/carcin/bgn258
Freedman, N. D., Chow, W. H., Gao, Y. T., Shu, X. O., Ji, B. T., Yang, G., et al. (2007). Menstrual and reproductive factors and gastric cancer risk in a large prospective study of women. Gut 56, 1671–1677. doi: 10.1136/gut.2007.129411
Fritsch, M., Orfanos, C. E., and Zouboulis, C. C. (2001). Sebocytes are the key regulators of androgen homeostasis in human skin. J. Invest. Dermatol. 116, 793–800. doi: 10.1046/j.1523-1747.2001.01312.x
Frycz, B. A., Murawa, D., Borejsza-Wysocki, M., Marciniak, R., Murawa, P., Drews, M., et al. (2015). Expression of 17β-hydroxysteroid dehydrogenase type 2 is associated with some clinicopathological features in gastric cancer. Biomed. Pharmacother. 70, 24–27. doi: 10.1016/j.biopha.2014.12.042
Frycz, B. A., Murawa, D., Borejsza-Wysocki, M., Wichtowski, M., Spychala, A., Marciniak, R., et al. (2016). Transcript level of AKR1C3 is down-regulated in gastric cancer. Biochem. Cell Biol. 94, 138–146. doi: 10.1139/bcb-2015-0096
Fujikawa, H., Okura, F., Kuwano, Y., Sekizawa, A., Chiba, H., Shimodaira, K., et al. (1997). Steroid sulfatase activity in osteoblast cells. Biochem. Biophys. Res. Commun. 231, 42–47. doi: 10.1006/bbrc.1996.6038
Gangloff, A., Shi, R., Nahoum, V., and Lin, S. X. (2003). Pseudo-symmetry of C19 steroids, alternative binding orientations, and multispecificity in human estrogenic 17β-hydroxysteroid dehydrogenase. FASEB J. 17, 274–276. doi: 10.1096/fj.02-0397fje
Gao, J., He, J., Shi, X., Stefanovic-Racic, M., Xu, M., O'Doherty, R. M., et al. (2012). Sex-specific effect of estrogen sulfotransferase on mouse models of type 2 diabetes. Diabetes 61, 1543–1551. doi: 10.2337/db11-1152
Gargano, E. M., Allegretta, G., Perspicace, E., Carotti, A., Van Koppen, C., Frotscher, M., et al. (2015). 17β-Hydroxysteroid dehydrogenase Type 2 inhibition: discovery of selective and metabolically stable compounds inhibiting both the human enzyme and its murine ortholog. PLoS ONE 10:e0134754. doi: 10.1371/journal.pone.0134754
Ghosh, D., Dhara, S., Kumar, A., and Sengupta, J. (1999). Immunohistochemical localization of receptors for progesterone and oestradiol-17 beta in the implantation site of the rhesus monkey. Hum. Reprod. 14, 505–514. doi: 10.1093/humrep/14.2.505
Gibbs, T. T., Russek, S. J., and Farb, D. H. (2006). Sulfated steroids as endogenous neuromodulators. Pharmacol. Biochem. Behav. 84, 555–567. doi: 10.1016/j.pbb.2006.07.031
Gibson, D. A., McInnes, K. J., Critchley, H. O., and Saunders, P. T. (2013). Endometrial Intracrinology–generation of an estrogen-dominated microenvironment in the secretory phase of women. J. Clin. Endocrinol. Metab. 98, E1802–E1806. doi: 10.1210/jc.2013-2140
Gilligan, L. C., Gondal, A., Tang, V., Hussain, M. T., Arvaniti, A., Hewitt, A. M., et al. (2017). Estrone sulfate transport and steroid sulfatase activity in colorectal cancer: implications for hormone replacement therapy. Front. Pharmacol. 8:103. doi: 10.3389/fphar.2017.00103
González-Arenas, A., and Agramonte-Hevia, J. (2012). Sex steroid hormone effects in normal and pathologic conditions in lung physiology. Mini Rev. Med. Chem. 12, 1055–1062. doi: 10.2174/138955712802762194
Goodarzi, M. O., Antoine, H. J., and Azziz, R. (2007). Genes for enzymes regulating dehydroepiandrosterone sulfonation are associated with levels of dehydroepiandrosterone sulfate in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 92, 2659–2664. doi: 10.1210/jc.2006-2600
Goodarzi, M. O., Shah, N. A., Antoine, H. J., Pall, M., Guo, X., and Azziz, R. (2006). Variants in the 5α-reductase type 1 and type 2 genes are associated with polycystic ovary syndrome and the severity of hirsutism in affected women. J. Clin. Endocrinol. Metab. 91, 4085–4091. doi: 10.1210/jc.2006-0227
Groothuis, P. G., Dassen, H. H., Romano, A., and Punyadeera, C. (2007). Estrogen and the endometrium: lessons learned from gene expression profiling in rodents and human. Hum. Reprod. Update 13, 405–417. doi: 10.1093/humupd/dmm009
Gruber, C. J., Tschugguel, W., Schneeberger, C., and Huber, J. C. (2002). Production and actions of estrogens. N. Engl. J. Med. 346, 340–352. doi: 10.1056/NEJMra000471
Guillot, F., Garcia, A., Salou, M., Brouard, S., Laplaud, D. A., and Nicot, A. B. (2015). Transcript analysis of laser capture microdissected white matter astrocytes and higher phenol sulfotransferase 1A1 expression during autoimmune neuroinflammation. J. Neuroinflammation 12, 130. doi: 10.1186/s12974-015-0348-y
Gulyaeva, L. F., Mikhailova, O. N., PustyInyak, V. O., Kim, I. V. IV., Gerasimov, A. V., Krasilnikov, S. E., et al. (2008). Comparative analysis of SNP in estrogen-metabolizing enzymes for ovarian, endometrial, and breast cancers in Novosibirsk, Russia. Adv. Exp. Med. Biol. 617, 359–366. doi: 10.1007/978-0-387-69080-3_34
Gylfe, A. E., Katainen, R., Kondelin, J., Tanskanen, T., Cajuso, T., Hanninen, U., et al. (2013). Eleven candidate susceptibility genes for common familial colorectal cancer. PLoS Genet. 9:e1003876. doi: 10.1371/journal.pgen.1003876
Hakkarainen, J., Jokela, H., Pakarinen, P., Heikela, H., Katkanaho, L., Vandenput, L., et al. (2015). Hydroxysteroid (17β)-dehydrogenase 1-deficient female mice present with normal puberty onset but are severely subfertile due to a defect in luteinization and progesterone production. FASEB J. 29, 3806–3816. doi: 10.1096/fj.14-269035
He, D., Frost, A. R., and Falany, C. N. (2005a). Identification and immunohistochemical localization of Sulfotransferase 2B1b (SULT2B1b) in human lung. Biochim Biophys Act. 1724, 119–126. doi: 10.1016/j.bbagen.2005.03.018
He, D., Meloche, C. A., Dumas, N. A., Frost, A. R., and Falany, C. N. (2004). Different subcellular localization of sulphotransferase 2B1b in human placenta and prostate. Biochem. J. 379, 533–540. doi: 10.1042/bj20031524
He, W., Gauri, M., Li, T., Wang, R., and Lin, S. X. (2016). Current knowledge of the multifunctional 17β-hydroxysteroid dehydrogenase type 1 (HSD17B1). Gene 588, 54–61. doi: 10.1016/j.gene.2016.04.031
He, X. Y., Wegiel, J., Yang, Y. Z., Pullarkat, R., Schulz, H., and Yang, S. Y. (2005b). Type 10, 17β-hydroxysteroid dehydrogenase catalyzing the oxidation of steroid modulators of gamma-aminobutyric acid type A receptors. Mol. Cell. Endocrinol. 229, 111–117. doi: 10.1016/j.mce.2004.08.011
He, X. Y., and Yang, S. Y. (2006). Roles of type 10, 17β-hydroxysteroid dehydrogenase in intracrinology and metabolism of isoleucine and fatty acids. Endocr. Metab. Immune Disord. Drug Targets 6, 95–102. doi: 10.2174/187153006776056639
Heijmans, J., Wielenga, M. C., Rosekrans, S. L., van Lidth de Jeude, J. F., Roelofs, J., Groothuis, P., et al. (2014). Oestrogens promote tumorigenesis in a mouse model for colitis-associated cancer. Gut 63, 310–316. doi: 10.1136/gutjnl-2012-304216
Her, C., Szumlanski, C., Aksoy, I. A., and Weinshilboum, R. M. (1996). Human jejunal estrogen sulfotransferase and dehydroepiandrosterone sulfotransferase: immunochemical characterization of individual variation. Drug Metab. Dispos. 24, 1328–1335.
Hevir, N., Ribic-Pucelj, M., and Lanisnik Rizner, T. (2013). Disturbed balance between phase I and II metabolizing enzymes in ovarian endometriosis: a source of excessive hydroxy-estrogens and ROS? Mol. Cell. Endocrinol. 367, 74–84. doi: 10.1016/j.mce.2012.12.019
Hevir, N., Sinkovec, J., and Rizner, T. L. (2011a). Disturbed expression of phase I and phase II estrogen-metabolizing enzymes in endometrial cancer: lower levels of CYP1B1 and increased expression of S-COMT. Mol. Cell. Endocrinol. 331, 158–167. doi: 10.1016/j.mce.2010.09.011
Hevir, N., Vouk, K., Sinkovec, J., Ribic-Pucelj, M., and Rizner, T. L. (2011b). Aldo-keto reductases AKR1C1, AKR1C2 and AKR1C3 may enhance progesterone metabolism in ovarian endometriosis. Chem. Biol. Interact. 191, 217–226. doi: 10.1016/j.cbi.2011.01.003
Hewitt, S. C., Winuthayanon, W., and Korach, K. S. (2016). What's new in estrogen receptor action in the female reproductive tract. J. Mol. Endocrinol. 56 R55–71. doi: 10.1530/JME-15-0254
Hilborn, E., Stal, O., and Jansson, A. (2017). Estrogen and androgen-converting enzymes 17β-hydroxysteroid dehydrogenase and their involvement in cancer: with a special focus on 17β-hydroxysteroid dehydrogenase type 1, 2, and breast cancer. Oncotarget 8, 30552–30562. doi: 10.18632/oncotarget.15547
Hogan, A. M., Collins, D., Baird, A. W., and Winter, D. C. (2009). Estrogen and gastrointestinal malignancy. Mol. Cell. Endocrinol. 307, 19–24. doi: 10.1016/j.mce.2009.03.016
Honda, S., Harada, N., Ito, S., Takagi, Y., and Maeda, S. (1998). Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem. Biophys. Res. Commun. 252, 445–449. doi: 10.1006/bbrc.1998.9672
Hovorkova, P., Kristofikova, Z., Horinek, A., Ripova, D., Majer, E., Zach, P., et al. (2008). Lateralization of 17β-hydroxysteroid dehydrogenase type 10 in hippocampi of demented and psychotic people. Dement. Geriatr. Cogn. Disord. 26, 193–198. doi: 10.1159/000151778
Hu, M. C., Hsu, N. C., El Hadj, N. B., Pai, C. I., Chu, H. P., Wang, C. K., et al. (2002). Steroid deficiency syndromes in mice with targeted disruption of Cyp11a1. Mol. Endocrinol. 16, 1943–1950. doi: 10.1210/me.2002-0055
Huang, C. C., Shih, M. C., Hsu, N. C., Chien, Y., and Chung, B. C. (2012). Fetal glucocorticoid synthesis is required for development of fetal adrenal medulla and hypothalamus feedback suppression. Endocrinology 153, 4749–4756. doi: 10.1210/en.2012-1258
Huang, X. F., and Luu-The, V. (2000). Molecular characterization of a first human 3(α->β)-hydroxysteroid epimerase. J. Biol. Chem. 275, 29452–29457. doi: 10.1074/jbc.M000562200
Huber, A., Keck, C. C., Hefler, L. A., Schneeberger, C., Huber, J. C., Bentz, E. K., et al. (2005). Ten estrogen-related polymorphisms and endometriosis: a study of multiple gene-gene interactions. Obstet. Gynecol. 106, 1025–1031. doi: 10.1097/01.AOG.0000185259.01648.41
Hudelist, G., Czerwenka, K., Keckstein, J., Haas, C., Fink-Retter, A., Gschwantler-Kaulich, D., et al. (2007). Expression of aromatase and estrogen sulfotransferase in eutopic and ectopic endometrium: evidence for unbalanced estradiol production in endometriosis. Reprod. Sci. 14, 798–805. doi: 10.1177/1933719107309120
Hughes, S. V., Robinson, E., Bland, R., Lewis, H. M., Stewart, P. M., and Hewison, M. (1997). 1,25-dihydroxyvitamin D3 regulates estrogen metabolism in cultured keratinocytes. Endocrinology 138, 3711–3718. doi: 10.1210/endo.138.9.5406
Huhtinen, K., Desai, R., Stahle, M., Salminen, A., Handelsman, D. J., Perheentupa, A., et al. (2012a). Endometrial and endometriotic concentrations of estrone and estradiol are determined by local metabolism rather than circulating levels. J. Clin. Endocrinol. Metab. 97, 4228–4235. doi: 10.1210/jc.2012-1154
Huhtinen, K., Saloniemi-Heinonen, T., Keski-Rahkonen, P., Desai, R., Laajala, D., Stahle, M., et al. (2014). Intra-tissue steroid profiling indicates differential progesterone and testosterone metabolism in the endometrium and endometriosis lesions. J. Clin. Endocrinol. Metab. 99 E2188–2197. doi: 10.1210/jc.2014-1913
Huhtinen, K., Stahle, M., Perheentupa, A., and Poutanen, M. (2012b). Estrogen biosynthesis and signaling in endometriosis. Mol. Cell. Endocrinol. 358, 146–154. doi: 10.1016/j.mce.2011.08.022
Husen, B., Huhtinen, K., Poutanen, M., Kangas, L., Messinger, J., and Thole, H. (2006). Evaluation of inhibitors for 17β-hydroxysteroid dehydrogenase type 1 in vivo in immunodeficient mice inoculated with MCF-7 cells stably expressing the recombinant human enzyme. Mol. Cell. Endocrinol. 248, 109–113. doi: 10.1016/j.mce.2005.11.042
Ihlenfeldt, W. D., Bolton, E. E., and Bryant, S. H. (2009). The PubChem chemical structure sketcher. J. Cheminform. 1:20. doi: 10.1186/1758-2946-1-20
Iida, S., Kakinuma, H., Miki, Y., Abe, K., Sakurai, M., Suzuki, S., et al. (2013). Steroid sulphatase and oestrogen sulphotransferase in human non-small-cell lung carcinoma. Br. J. Cancer 108, 1415–1424. doi: 10.1038/bjc.2013.84
Ikeda, K., Shiraishi, K., Yoshida, A., Shinchi, Y., Sanada, M., Motooka, Y., et al. (2016). Synchronous multiple lung adenocarcinomas: estrogen concentration in peripheral lung. PLoS ONE 11:e0160910. doi: 10.1371/journal.pone.0160910
Iqbal, M. J., Greenway, B., Wilkinson, M. L., Johnson, P. J., and Williams, R. (1983). Sex-steroid enzymes, aromatase and 5 alpha-reductase in the pancreas: a comparison of normal adult, foetal and malignant tissue. Clin. Sci. 65, 71–75. doi: 10.1042/cs0650071
Ishida, M., Choi, J. H., Hirabayashi, K., Matsuwaki, T., Suzuki, M., Yamanouchi, K., et al. (2007). Reproductive phenotypes in mice with targeted disruption of the 20α-hydroxysteroid dehydrogenase gene. J. Reprod. Dev. 53, 499–508. doi: 10.1262/jrd.18125
Ito, K., Miki, Y., Suzuki, T., McNamara, K. M., and Sasano, H. (2016). In situ androgen and estrogen biosynthesis in endometrial cancer: focus on androgen actions and intratumoral production. Endocr. Relat. Cancer 23 R323–335. doi: 10.1530/ERC-15-0470
Ito, K., Suzuki, T., Akahira, J., Moriya, T., Kaneko, C., Utsunomiya, H., et al. (2002). Expression of androgen receptor and 5α-reductases in the human normal endometrium and its disorders. Int. J. Cancer 99, 652–657. doi: 10.1002/ijc.10394
Ito, K., Utsunomiya, H., Suzuki, T., Saitou, S., Akahira, J., Okamura, K., et al. (2006). 17β-hydroxysteroid dehydrogenases in human endometrium and its disorders. Mol. Cell. Endocrinol. 248, 136–140. doi: 10.1016/j.mce.2005.11.038
Iwasa, A., Arakaki, R., Honma, N., Ushio, A., Yamada, A., Kondo, T., et al. (2015). Aromatase controls Sjogren syndrome-like lesions through monocyte chemotactic protein-1 in target organ and adipose tissue-associated macrophages. Am. J. Pathol. 185, 151–161. doi: 10.1016/j.ajpath.2014.09.006
Jakob, F., Siggelkow, H., Homann, D., Kohrle, J., Adamski, J., and Schutze, N. (1997). Local estradiol metabolism in osteoblast- and osteoclast-like cells. J. Steroid Biochem. Mol. Biol. 61, 167–174. doi: 10.1016/S0960-0760(97)80009-X
Janssen, J. M., Bland, R., Hewison, M., Coughtrie, M. W., Sharp, S., Arts, J., et al. (1999). Estradiol formation by human osteoblasts via multiple pathways: relation with osteoblast function. J. Cell. Biochem. 75, 528–537. doi: 10.1002/(SICI)1097-4644(19991201)75:3<528::AID-JCB16>3.0.CO;2-3
Jäntti, S. E., Tammimaki, A., Raattamaa, H., Piepponen, P., Kostiainen, R., and Ketola, R. A. (2010). Determination of steroids and their intact glucuronide conjugates in mouse brain by capillary liquid chromatography-tandem mass spectrometry. Anal. Chem. 82, 3168–3175. doi: 10.1021/ac902321z
Järvensivu, P., Heinosalo, T., Hakkarainen, J., Kronqvist, P., Saarinen, N., and Poutanen, M. (2018). HSD17B1 expression induces inflammation-aided rupture of mammary gland myoepithelium. Endocr. Relat. Cancer 25, 393–406. doi: 10.1530/ERC-17-0476
Järvensivu, P., Saloniemi-Heinonen, T., Awosanya, M., Koskimies, P., Saarinen, N., and Poutanen, M. (2015). HSD17B1 expression enhances estrogen signaling stimulated by the low active estrone, evidenced by an estrogen responsive element-driven reporter gene in vivo. Chem. Biol. Interact. 234, 126–134. doi: 10.1016/j.cbi.2015.01.008
Jeon, Y. T., Park, S. Y., Kim, Y. B., Kim, J. W., Park, N. H., Kang, S. B., et al. (2007). Aromatase expression was not detected by immunohistochemistry in endometrial cancer. Ann. N. Y. Acad. Sci. 1095, 70–75. doi: 10.1196/annals.1397.010
Jin, Y., Mesaros, A. C., Blair, I. A., and Penning, T. M. (2011). Stereospecific reduction of 5β-reduced steroids by human ketosteroid reductases of the AKR (aldo-keto reductase) superfamily: role of AKR1C1-AKR1C4 in the metabolism of testosterone and progesterone via the 5β-reductase pathway. Biochem. J. 437, 53–61. doi: 10.1042/BJ20101804
Jokela, H., Rantakari, P., Lamminen, T., Strauss, L., Ola, R., Mutka, A. L., et al. (2010). Hydroxysteroid (17β) dehydrogenase 7 activity is essential for fetal de novo cholesterol synthesis and for neuroectodermal survival and cardiovascular differentiation in early mouse embryos. Endocrinology 151, 1884–1892. doi: 10.1210/en.2009-0928
Jones, A. L., Hume, R., Bamforth, K. J., and Coughtrie, M. W. (1992). Estrogen and phenol sulfotransferase activities in human fetal lung. Early Hum. Dev. 28, 65–77. doi: 10.1016/0378-3782(92)90007-4
Jones, M. R., Mathur, R., Cui, J., Guo, X., Azziz, R., and Goodarzi, M. O. (2009). Independent confirmation of association between metabolic phenotypes of polycystic ovary syndrome and variation in the type 6, 17β-hydroxysteroid dehydrogenase gene. J. Clin. Endocrinol. Metab. 94, 5034–5038. doi: 10.1210/jc.2009-0931
Jongen, V. H., Thijssen, J. H., Hollema, H., Donker, G. H., Santema, J. G., Van der Zee, A. G., et al. (2005). Is aromatase cytochrome P450 involved in the pathogenesis of endometrioid endometrial cancer? Int. J. Gynecol. Cancer 15, 529–536. doi: 10.1111/j.1525-1438.2005.15320.x
Kancheva, L., Hill, M., Vcelakova, H., Vrbikova, J., Pelikanova, T., and Starka, L. (2007). The identification and simultaneous quantification of neuroactive androstane steroids and their polar conjugates in the serum of adult men, using gas chromatography-mass spectrometry. Steroids 72, 792–801. doi: 10.1016/j.steroids.2007.06.006
Kassem, M., Okazaki, R., Harris, S. A., Spelsberg, T. C., Conover, C. A., and Riggs, B. L. (1998). Estrogen effects on insulin-like growth factor gene expression in a human osteoblastic cell line with high levels of estrogen receptor. Calcif. Tissue Int. 62, 60–66. doi: 10.1007/s002239900395
Kemilainen, H., Adam, M., Maki-Jouppila, J., Damdimopoulou, P., Damdimopoulos, A. E., Kere, J., et al. (2016). The hydroxysteroid (17β) dehydrogenase family gene HSD17B12 Is involved in the prostaglandin synthesis pathway, the ovarian function, and regulation of fertility. Endocrinology 157, 3719–3730. doi: 10.1210/en.2016-1252
Kennelly, R., Kavanagh, D. O., Hogan, A. M., and Winter, D. C. (2008). Oestrogen and the colon: potential mechanisms for cancer prevention. Lancet Oncol. 9, 385–391. doi: 10.1016/S1470-2045(08)70100-1
Kikuchi, A., Furutani, T., Azami, H., Watanabe, K., Niimi, T., Kamiyama, Y., et al. (2014). In vitro and in vivo characterisation of ASP9521: a novel, selective, orally bioavailable inhibitor of 17β-hydroxysteroid dehydrogenase type 5 (17βHSD5; AKR1C3). Invest. New Drugs 32, 860–870. doi: 10.1007/s10637-014-0130-5
Kim, J. H., Cho, H. T., and Kim, Y. J. (2014). The role of estrogen in adipose tissue metabolism: insights into glucose homeostasis regulation. Endocr. J. 61, 1055–1067. doi: 10.1507/endocrj.EJ14-0262
Kim, S., Thiessen, P. A., Bolton, E. E., Chen, J., Fu, G., Gindulyte, A., et al. (2016). PubChem substance and compound databases. Nucleic Acids Res. 44, D1202–D1213. doi: 10.1093/nar/gkv951
Kim, T. S., Maeda, A., Maeda, T., Heinlein, C., Kedishvili, N., Palczewski, K., et al. (2005). Delayed dark adaptation in 11-cis-retinol dehydrogenase-deficient mice: a role of RDH11 in visual processes in vivo. J. Biol. Chem. 280, 8694–8704. doi: 10.1074/jbc.M413172200
Kimoto, T., Ishii, H., Higo, S., Hojo, Y., and Kawato, S. (2010). Semicomprehensive analysis of the postnatal age-related changes in the mRNA expression of sex steroidogenic enzymes and sex steroid receptors in the male rat hippocampus. Endocrinology 151, 5795–5806. doi: 10.1210/en.2010-0581
Kimura, Y., Suzuki, T., Kaneko, C., Darnel, A. D., Akahira, J., Ebina, M., et al. (2003). Expression of androgen receptor and 5α-reductase types 1 and 2 in early gestation fetal lung: a possible correlation with branching morphogenesis. Clin. Sci. 105, 709–713. doi: 10.1042/CS20030236
Kitaoka, Y., Kitawaki, J., Koshiba, H., Inoue, S., Ishihara, H., Teramoto, M., et al. (2004). Aromatase cytochrome P450 and estrogen and progesterone receptors in uterine sarcomas: correlation with clinical parameters. J. Steroid Biochem. Mol. Biol. 88, 183–189. doi: 10.1016/j.jsbmb.2003.11.013
Kitawaki, J., Kado, N., Ishihara, H., Koshiba, H., Kitaoka, Y., and Honjo, H. (2002). Endometriosis: the pathophysiology as an estrogen-dependent disease. J. Steroid Biochem. Mol. Biol. 83, 149–155. doi: 10.1016/S0960-0760(02)00260-1
Kitawaki, J., Koshiba, H., Ishihara, H., Kusuki, I., Tsukamoto, K., and Honjo, H. (2000). Progesterone induction of 17β-hydroxysteroid dehydrogenase type 2 during the secretory phase occurs in the endometrium of estrogen-dependent benign diseases but not in normal endometrium. J. Clin. Endocrinol. Metab. 85, 3292–3296. doi: 10.1210/jcem.85.9.6829
Kitawaki, J., Kusuki, I., Koshiba, H., Tsukamoto, K., Fushiki, S., and Honjo, H. (1999). Detection of aromatase cytochrome P-450 in endometrial biopsy specimens as a diagnostic test for endometriosis. Fertil. Steril. 72, 1100–1106. doi: 10.1016/S0015-0282(99)00424-0
Knapp, P., Chabowski, A., Blachnio-Zabielska, A., Walentowicz-Sadlecka, M., Grabiec, M., and Knapp, P. A. (2013). Expression of estrogen receptors (alpha, beta), cyclooxygenase-2 and aromatase in normal endometrium and endometrioid cancer of uterus. Adv. Med. Sci. 58, 96–103. doi: 10.2478/v10039-012-0055-1
Kobayashi, H., Yoshida, S., Sun, Y. J., Shirasawa, N., and Naito, A. (2013). Postnatal development of gastric aromatase and portal venous estradiol-17β levels in male rats. J. Endocrinol. 218, 117–124. doi: 10.1530/JOE-13-0074
Kohno, M., Okamoto, T., Suda, K., Shimokawa, M., Kitahara, H., Shimamatsu, S., et al. (2014). Prognostic and therapeutic implications of aromatase expression in lung adenocarcinomas with EGFR mutations. Clin. Cancer Res. 20, 3613–3622. doi: 10.1158/1078-0432.CCR-13-2683
Koizumi, M., Momoeda, M., Hiroi, H., Hosokawa, Y., Tsutsumi, R., Osuga, Y., et al. (2010). Expression and regulation of cholesterol sulfotransferase (SULT2B1b) in human endometrium. Fertil. Steril. 93, 1538–1544. doi: 10.1016/j.fertnstert.2009.01.075
Konings, G. F., Cornel, K. M., Xanthoulea, S., Delvoux, B., Skowron, M. A., Kooreman, L., et al. (2018). Blocking 17β-hydroxysteroid dehydrogenase type 1 in endometrial cancer: a potential novel endocrine therapeutic approach. J. Pathol. 244, 203–214. doi: 10.1002/path.5004
Konings, G. F., Reynaert, N. L., Delvoux, B., Verhamme, F. M., Bracke, K. R., Brusselle, G. G., et al. (2017). Increased levels of enzymes involved in local estradiol synthesis in chronic obstructive pulmonary disease. Mol. Cell. Endocrinol. 443, 23–31. doi: 10.1016/j.mce.2016.12.001
Kreitmann, O., Kreitmann-Gimbal, B., Bayard, F., and Hodgen, G. D. (1979). 17 beta-hydroxysteroid dehydrogenase in monkey endometrium: characterization of enzyme activity, and effects of estradiol alone or in combination with progesterone. Steroids 34, 693–703. doi: 10.1016/0039-128X(79)90139-9
Kríz, L., Bicikova, M., and Hampl, R. (2008a). Roles of steroid sulfatase in brain and other tissues. Physiol. Res. 57, 657–668.
Kríz, L., Bicikova, M., Mohapl, M., Hill, M., Cerny, I., and Hampl, R. (2008b). Steroid sulfatase and sulfuryl transferase activities in human brain tumors. J. Steroid Biochem. Mol. Biol. 109, 31–39. doi: 10.1016/j.jsbmb.2007.12.004
Laatikainen, T., Laitinen, E. A., and Vihko, R. (1971). Secretion of free and sulfate-conjugated neutral steroids by the human testis. Effect of administration of human chorionic gonadotropin. J. Clin. Endocrinol. Metab. 32, 59–64. doi: 10.1210/jcem-32-1-59
Labrie, F. (1991). Intracrinology. Mol. Cell. Endocrinol. 78, C113–C118. doi: 10.1016/0303-7207(91)90116-A
Labrie, F. (2015). All sex steroids are made intracellularly in peripheral tissues by the mechanisms of intracrinology after menopause. J. Steroid Biochem. Mol. Biol. 145, 133–138. doi: 10.1016/j.jsbmb.2014.06.001
Labrie, F., and Labrie, C. (2013). DHEA and intracrinology at menopause, a positive choice for evolution of the human species. Climacteric 16, 205–213. doi: 10.3109/13697137.2012.733983
Lane, G. (1990). The histochemistry of isocitric and oestradiol-17 beta dehydrogenases in the endometrium of postmenopausal women treated with oestrogens and progestogens. Histochem. J. 22, 45–50. doi: 10.1007/BF01962878
Laplante, Y., Rancourt, C., and Poirier, D. (2009). Relative involvement of three 17β-hydroxysteroid dehydrogenases (types 1, 7 and 12) in the formation of estradiol in various breast cancer cell lines using selective inhibitors. Mol. Cell. Endocrinol. 301, 146–153. doi: 10.1016/j.mce.2008.08.026
Lavasani, S., Chlebowski, R. T., Prentice, R. L., Kato, I., Wactawski-Wende, J., Johnson, K. C., et al. (2015). Estrogen and colorectal cancer incidence and mortality. Cancer 121, 3261–3271. doi: 10.1002/cncr.29464
Lee, D. K., Kurihara, I., Jeong, J. W., Lydon, J. P., DeMayo, F. J., Tsai, M. J., et al. (2010). Suppression of ERα activity by COUP-TFII is essential for successful implantation and decidualization. Mol. Endocrinol. 24, 930–940. doi: 10.1210/me.2009-0531
Lépine, J., Audet-Walsh, E., Gregoire, J., Tetu, B., Plante, M., Menard, V., et al. (2010). Circulating estrogens in endometrial cancer cases and their relationship with tissular expression of key estrogen biosynthesis and metabolic pathways. J. Clin. Endocrinol. Metab. 95, 2689–2698. doi: 10.1210/jc.2010-2648
Li, C., Chen, P., Palladino, A., Narayan, S., Russell, L. K., Sayed, S., et al. (2010). Mechanism of hyperinsulinism in short-chain 3-hydroxyacyl-CoA dehydrogenase deficiency involves activation of glutamate dehydrogenase. J. Biol. Chem. 285, 31806–31818. doi: 10.1074/jbc.M110.123638
Li, Y., Qin, L., Xiao, Z. J., Wang, Y. L., Herva, R., Leng, J. H., et al. (2003). Expression of P450 aromatase and 17β-hydroxysteroid dehydrogenase type 1 at fetal-maternal interface during tubal pregnancy. J. Steroid Biochem. Mol. Biol. 87, 241–246. doi: 10.1016/j.jsbmb.2003.09.013
Lilla, C., Risch, A., Verla-Tebit, E., Hoffmeister, M., Brenner, H., and Chang-Claude, J. (2007). SULT1A1 genotype and susceptibility to colorectal cancer. Int. J. Cancer 120, 201–206. doi: 10.1002/ijc.22156
Lim, Y. A., Grimm, A., Giese, M., Mensah-Nyagan, A. G., Villafranca, J. E., Ittner, L. M., et al. (2011). Inhibition of the mitochondrial enzyme ABAD restores the amyloid-beta-mediated deregulation of estradiol. PLoS ONE 6:e28887. doi: 10.1371/journal.pone.0028887
Lin, H. K., Jez, J. M., Schlegel, B. P., Peehl, D. M., Pachter, J. A., and Penning, T. M. (1997). Expression and characterization of recombinant type 2, 3 α-hydroxysteroid dehydrogenase (HSD) from human prostate: demonstration of bifunctional 3α/17β-HSD activity and cellular distribution. Mol. Endocrinol. 11, 1971–1984.
Lindemann, K., Malander, S., Christensen, R. D., Mirza, M. R., Kristensen, G. B., Aavall-Lundqvist, E., et al. (2014). Examestane in advanced or recurrent endometrial carcinoma: a prospective phase II study by the Nordic Society of Gynecologic Oncology (NSGO). BMC Cancer 14:68. doi: 10.1186/1471-2407-14-68
Liu, Y., Yao, Z. X., Bendavid, C., Borgmeyer, C., Han, Z., Cavalli, L. R., et al. (2005). Haploinsufficiency of cytochrome P450, 17β-hydroxylase/17,20 lyase (CYP17) causes infertility in male mice. Mol. Endocrinol. 19, 2380–2389. doi: 10.1210/me.2004-0418
Lønning, P. E., and Eikesdal, H. P. (2013). Aromatase inhibition 2013: clinical state of the art and questions that remain to be solved. Endocr. Relat. Cancer 20, R183–R201. doi: 10.1530/ERC-13-0099
López, M., and Tena-Sempere, M. (2015). Estrogens and the control of energy homeostasis: a brain perspective. Trends Endocrinol. Metab. 26, 411–421. doi: 10.1016/j.tem.2015.06.003
López, M., and Tena-Sempere, M. (2016). Estradiol and brown fat. Best Pract. Res. Clin. Endocrinol. Metab. 30, 527–536. doi: 10.1016/j.beem.2016.08.004
Loria, R. M., and Graf, M. R. (2012). 17α-androstenediol-mediated oncophagy of tumor cells by different mechanisms is determined by the target tumor. Ann. N. Y. Acad. Sci. 1262, 127–133. doi: 10.1111/j.1749-6632.2012.06602.x
Loriot, Y., Fizazi, K., Jones, R. J., Van den Brande, J., Molife, R. L., Omlin, A., et al. (2014). Safety, tolerability and anti-tumour activity of the androgen biosynthesis inhibitor ASP9521 in patients with metastatic castration-resistant prostate cancer: multi-centre phase I/II study. Invest. New Drugs 32, 995–1004. doi: 10.1007/s10637-014-0101-x
Loth, D. W., Soler Artigas, M., Gharib, S. A., Wain, L. V., Franceschini, N., Koch, B., et al. (2014). Genome-wide association analysis identifies six new loci associated with forced vital capacity. Nat. Genet. 46, 669–677. doi: 10.1038/ng.3011
Loza, M. C. (1995). Hydrolysis of estrone sulfate in uterine minces of the 6-days pregnant rat. J. Steroid Biochem. Mol. Biol. 52, 277–280. doi: 10.1016/0960-0760(94)00177-N
Lu, C., Lee, H. S., Pappas, G. P., Dilling, D. F., Burger, C. D., Shifren, A., et al. (2017). A Phase II Clinical Trial of an Aromatase Inhibitor for Postmenopausal Women with Lymphangioleiomyomatosis. Ann. Am. Thorac. Soc. 14, 919–928. doi: 10.1513/AnnalsATS.201610-824OC
Luchetti, S., Bossers, K., Van de Bilt, S., Agrapart, V., Morales, R. R., Frajese, G. V., et al. (2011). Neurosteroid biosynthetic pathways changes in prefrontal cortex in Alzheimer's disease. Neurobiol. Aging 32, 1964–1976. doi: 10.1016/j.neurobiolaging.2009.12.014
Lundin, E., Wirgin, I., Lukanova, A., Afanasyeva, Y., Krogh, V., Axelsson, T., et al. (2012). Selected polymorphisms in sex hormone-related genes, circulating sex hormones and risk of endometrial cancer. Cancer Epidemiol. 36, 445–452. doi: 10.1016/j.canep.2012.04.006
Luo, L., Deng, J., Wang, D. X., He, J., and Deng, W. (2015). Regulation of epithelial sodium channel expression by oestradiol and progestogen in alveolar epithelial cells. Respir. Physiol. Neurobiol. 216, 52–62. doi: 10.1016/j.resp.2015.06.001
Luu-The, V., Belanger, A., and Labrie, F. (2008). Androgen biosynthetic pathways in the human prostate. Best Pract. Res. Clin. Endocrinol. Metab. 22, 207–221. doi: 10.1016/j.beem.2008.01.008
Luu-The, V., and Labrie, F. (2010). The intracrine sex steroid biosynthesis pathways. Prog. Brain Res. 181, 177–192. doi: 10.1016/S0079-6123(08)81010-2
Ma, B. B., Oza, A., Eisenhauer, E., Stanimir, G., Carey, M., Chapman, W., et al. (2004). The activity of letrozole in patients with advanced or recurrent endometrial cancer and correlation with biological markers–a study of the National Cancer Institute of Canada Clinical Trials Group. Int. J. Gynecol. Cancer 14, 650–658. doi: 10.1111/j.1048-891X.2004.14419.x
Ma, W. G., Song, H., Das, S. K., Paria, B. C., and Dey, S. K. (2003). Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc. Natl. Acad. Sci. U.S.A. 100, 2963–2968. doi: 10.1073/pnas.0530162100
MacKenzie, S. M., Dewar, D., Stewart, W., Fraser, R., Connell, J. M., and Davies, E. (2008). The transcription of steroidogenic genes in the human cerebellum and hippocampus: a comparative survey of normal and Alzheimer's tissue. J. Endocrinol. 196, 123–130. doi: 10.1677/JOE-07-0427
Maentausta, O., Peltoketo, H., Isomaa, V., Jouppila, P., and Vihko, R. (1990). Immunological measurement of human 17β-hydroxysteroid dehydrogenase. J. Steroid. Biochem. 36, 673–680.
Mäentausta, O., Sormunen, R., Isomaa, V., Lehto, V. P., Jouppila, P., and Vihko, R. (1991). Immunohistochemical localization of 17 beta-hydroxysteroid dehydrogenase in the human endometrium during the menstrual cycle. Lab. Invest. 65, 582–587.
Mahendroo, M. S., Cala, K. M., Hess, D. L., and Russell, D. W. (2001). Unexpected virilization in male mice lacking steroid 5 alpha-reductase enzymes. Endocrinology 142, 4652–4662. doi: 10.1210/endo.142.11.8510
Mahendroo, M. S., Cala, K. M., and Russell, D. W. (1996). 5 alpha-reduced androgens play a key role in murine parturition. Mol. Endocrinol. 10, 380–392.
Maia, H. Jr., Pimentel, K., Casoy, J., Correia, T., Freitas, L. A., Zausner, B., et al. (2007). Aromatase expression in the eutopic endometrium of myomatous uteri: the influence of the menstrual cycle and oral contraceptive use. Gynecol. Endocrinol. 23, 320–324. doi: 10.1080/09513590701321565
Maia, H. Jr., Pimentel, K., Silva, T. M., Freitas, L. A., Zausner, B., Athayde, C., et al. (2006). Aromatase and cyclooxygenase-2 expression in endometrial polyps during the menstrual cycle. Gynecol. Endocrinol. 22, 219–224. doi: 10.1080/09513590600585955
Maltais, R., and Poirier, D. (2011). Steroid sulfatase inhibitors: a review covering the promising 2000-2010 decade. Steroids 76, 929–948. doi: 10.1016/j.steroids.2011.03.010
Manenda, M. S., Hamel, C. J., Masselot-Joubert, L., Picard, M. E., and Shi, R. (2016). Androgen-metabolizing enzymes: A structural perspective. J. Steroid Biochem. Mol. Biol. 161, 54–72. doi: 10.1016/j.jsbmb.2016.02.021
Marchais-Oberwinkler, S., Wetzel, M., Ziegler, E., Kruchten, P., Werth, R., Henn, C., et al. (2011). New drug-like hydroxyphenylnaphthol steroidomimetics as potent and selective 17β-hydroxysteroid dehydrogenase type 1 inhibitors for the treatment of estrogen-dependent diseases. J. Med. Chem. 54, 534–547. doi: 10.1021/jm1009082
Masi, L., Becherini, L., Gennari, L., Amedei, A., Colli, E., Falchetti, A., et al. (2001). Polymorphism of the aromatase gene in postmenopausal Italian women: distribution and correlation with bone mass and fracture risk. J. Clin. Endocrinol. Metab. 86, 2263–2269. doi: 10.1210/jc.86.5.2263
Matsunaga, T., Hojo, A., Yamane, Y., Endo, S., El-Kabbani, O., and Hara, A. (2013). Pathophysiological roles of aldo-keto reductases (AKR1C1 and AKR1C3) in development of cisplatin resistance in human colon cancers. Chem. Biol. Interact. 202, 234–242. doi: 10.1016/j.cbi.2012.09.024
Matsuzaki, S., Canis, M., Pouly, J. L., Dechelotte, P. J., and Mage, G. (2006). Analysis of aromatase and 17β-hydroxysteroid dehydrogenase type 2 messenger ribonucleic acid expression in deep endometriosis and eutopic endometrium using laser capture microdissection. Fertil. Steril. 85, 308–313. doi: 10.1016/j.fertnstert.2005.08.017
Mauvais-Jarvis, F., Clegg, D. J., and Hevener, A. L. (2013). The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 34, 309–338. doi: 10.1210/er.2012-1055
McCarthy, M. (2000). The “gender gap” in autoimmune disease. Lancet 356, 1088. doi: 10.1016/S0140-6736(05)74535-9
McEwen, B. S., and Milner, T. A. (2017). Understanding the broad influence of sex hormones and sex differences in the brain. J. Neurosci. Res. 95, 24–39. doi: 10.1002/jnr.23809
Meijer, D., Gelderblom, H., Karperien, M., Cleton-Jansen, A. M., Hogendoorn, P. C., and Bovee, J. V. (2011). Expression of aromatase and estrogen receptor alpha in chondrosarcoma, but no beneficial effect of inhibiting estrogen signaling both in vitro and in vivo. Clin. Sarcoma Res. 1:5. doi: 10.1186/2045-3329-1-5
Meloche, C. A., and Falany, C. N. (2001). Expression and characterization of the human 3 beta-hydroxysteroid sulfotransferases (SULT2B1a and SULT2B1b). J. Steroid Biochem. Mol. Biol. 77, 261–269. doi: 10.1016/S0960-0760(01)00064-4
Miki, Y., Nakata, T., Suzuki, T., Darnel, A. D., Moriya, T., Kaneko, C., et al. (2002). Systemic distribution of steroid sulfatase and estrogen sulfotransferase in human adult and fetal tissues. J. Clin. Endocrinol. Metab. 87, 5760–5768. doi: 10.1210/jc.2002-020670
Milewich, L., Garcia, R. L., and Johnson, A. R. (1983). Steroid sulfatase activity in human lung tissue and in endothelial pulmonary cells in culture. J. Clin. Endocrinol. Metab. 57, 8–14. doi: 10.1210/jcem-57-1-8
Miller, P. B., Parnell, B. A., Bushnell, G., Tallman, N., Forstein, D. A., Higdon, H. L III., et al. (2012a). Endometrial receptivity defects during IVF cycles with and without letrozole. Hum. Reprod. 27, 881–888. doi: 10.1093/humrep/der452
Miller, V. L., Lin, H. K., Murugan, P., Fan, M., Penning, T. M., Brame, L. S., et al. (2012b). Aldo-keto reductase family 1 member C3 (AKR1C3) is expressed in adenocarcinoma and squamous cell carcinoma but not small cell carcinoma. Int. J. Clin. Exp. Pathol. 5, 278–289.
Miller, W. L., and Auchus, R. J. (2011). The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 32, 81–151. doi: 10.1210/er.2010-0013
Mindnich, R., Moller, G., and Adamski, J. (2004). The role of 17 beta-hydroxysteroid dehydrogenases. Mol. Cell. Endocrinol. 218, 7–20. doi: 10.1016/j.mce.2003.12.006
Miyaura, C., Toda, K., Inada, M., Ohshiba, T., Matsumoto, C., Okada, T., et al. (2001). Sex- and age-related response to aromatase deficiency in bone. Biochem. Biophys. Res. Commun. 280, 1062–1068. doi: 10.1006/bbrc.2001.4246
Moeller, G., and Adamski, J. (2006). Multifunctionality of human 17β-hydroxysteroid dehydrogenases. Mol. Cell. Endocrinol. 248, 47–55. doi: 10.1016/j.mce.2005.11.031
Moeller, G., and Adamski, J. (2009). Integrated view on 17β-hydroxysteroid dehydrogenases. Mol. Cell. Endocrinol. 301, 7–19. doi: 10.1016/j.mce.2008.10.040
Möller, G., Leenders, F., van Grunsven, E. G., Dolez, V., Qualmann, B., Kessels, M. M., et al. (1999). Characterization of the HSD17B4 gene: D-specific multifunctional protein 2/17β-hydroxysteroid dehydrogenase IV. J. Steroid Biochem. Mol. Biol. 69, 441–446. doi: 10.1016/S0960-0760(99)00066-7
Morgat, A., Axelsen, K. B., Lombardot, T., Alcantara, R., Aimo, L., Zerara, M., et al. (2015). Updates in Rhea–a manually curated resource of biochemical reactions. Nucleic Acids Res. 43, D459–D464. doi: 10.1093/nar/gku961
Mori, T., Ito, F., Matsushima, H., Takaoka, O., Koshiba, A., Tanaka, Y., et al. (2015). Dienogest reduces HSD17β1 expression and activity in endometriosis. J. Endocrinol. 225, 69–76. doi: 10.1530/JOE-15-0052
Morice, P., Leary, A., Creutzberg, C., Abu-Rustum, N., and Darai, E. (2015). Endometrial cancer. Lancet 387, 1094–1108. doi: 10.1016/S0140-6736(15)00130-0
Morishima, A., Grumbach, M. M., Simpson, E. R., Fisher, C., and Qin, K. (1995). Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J. Clin. Endocrinol. Metab. 80, 3689–3698.
Mueller, J. W., Gilligan, L. C., Idkowiak, J., Arlt, W., and Foster, P. A. (2015). The regulation of steroid action by sulfation and desulfation. Endocr. Rev. 36, 526–563. doi: 10.1210/er.2015-1036
Muir, M., Romalo, G., Wolf, L., Elger, W., and Schweikert, H. U. (2004). Estrone sulfate is a major source of local estrogen formation in human bone. J. Clin. Endocrinol. Metab. 89, 4685–4692. doi: 10.1210/jc.2004-0049
Mukai, H., Tsurugizawa, T., Ogiue-Ikeda, M., Murakami, G., Hojo, Y., Ishii, H., et al. (2006). Local neurosteroid production in the hippocampus: influence on synaptic plasticity of memory. Neuroendocrinology 84, 255–263. doi: 10.1159/000097747
Mullis, P. E., Yoshimura, N., Kuhlmann, B., Lippuner, K., Jaeger, P., and Harada, H. (1997). Aromatase deficiency in a female who is compound heterozygote for two new point mutations in the P450arom gene: impact of estrogens on hypergonadotropic hypogonadism, multicystic ovaries, and bone densitometry in childhood. J. Clin. Endocrinol. Metab. 82, 1739–1745. doi: 10.1210/jc.82.6.1739
Munroe, D. G., and Chang, P. L. (1987). Tissue-specific expression of human arylsulfatase-C isozymes and steroid sulfatase. Am. J. Hum. Genet. 40, 102–114.
Murakami, G., Tanabe, N., Ishii, H. T., Ogiue-Ikeda, M., Tsurugizawa, T., Mukai, H., et al. (2006). Role of cytochrome p450 in synaptocrinology: endogenous estrogen synthesis in the brain hippocampus. Drug Metab. Rev. 38, 353–369. doi: 10.1080/03602530600724068
Musatov, S., Chen, W., Pfaff, D. W., Mobbs, C. V., Yang, X. J., Clegg, D. J., et al. (2007). Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc. Natl. Acad. Sci. U.S.A. 104, 2501–2506. doi: 10.1073/pnas.0610787104
Nadal, A., Alonso-Magdalena, P., Soriano, S., Ripoll, C., Fuentes, E., Quesada, I., et al. (2011). Role of estrogen receptors alpha, beta and GPER1/GPR30 in pancreatic beta-cells. Front Biosci (Landmark Ed) 16, 251–260. doi: 10.2741/3686
Naftolin, F., Horvath, T. L., Jakab, R. L., Leranth, C., Harada, N., and Balthazart, J. (1996). Aromatase immunoreactivity in axon terminals of the vertebrate brain. An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology 63, 149–155. doi: 10.1159/000126951
Naftolin, F., and MacLusky, N. J. (1982). Aromatase in the central nervous system. Cancer Res 42, 3274s−3276s.
Nakarai, C., Osawa, K., Akiyama, M., Matsubara, N., Ikeuchi, H., Yamano, T., et al. (2015). Expression of AKR1C3 and CNN3 as markers for detection of lymph node metastases in colorectal cancer. Clin. Exp. Med. 15, 333–341. doi: 10.1007/s10238-014-0298-1
Nayeb-Hashemi, H., and Kaunitz, J. D. (2009). Gastroduodenal mucosal defense. Curr. Opin. Gastroenterol. 25, 537–543. doi: 10.1097/MOG.0b013e328330da7b
Nemoto, Y., Toda, K., Ono, M., Fujikawa-Adachi, K., Saibara, T., Onishi, S., et al. (2000). Altered expression of fatty acid-metabolizing enzymes in aromatase-deficient mice. J. Clin. Invest. 105, 1819–1825. doi: 10.1172/JCI9575
Niikawa, H., Suzuki, T., Miki, Y., Suzuki, S., Nagasaki, S., Akahira, J., et al. (2008). Intratumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clin. Cancer Res. 14, 4417–4426. doi: 10.1158/1078-0432.CCR-07-1950
Nishimura, M., and Naito, S. (2006). Tissue-specific mRNA expression profiles of human phase I metabolizing enzymes except for cytochrome P450 and phase II metabolizing enzymes. Drug Metab. Pharmacokinet. 21, 357–374. doi: 10.2133/dmpk.21.357
Noble, L. S., Simpson, E. R., Johns, A., and Bulun, S. E. (1996). Aromatase expression in endometriosis. J. Clin. Endocrinol. Metab. 81, 174–179.
Noble, L. S., Takayama, K., Zeitoun, K. M., Putman, J. M., Johns, D. A., Hinshelwood, M. M., et al. (1997). Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J. Clin. Endocrinol. Metab. 82, 600–606. doi: 10.1210/jc.82.2.600
Noël, J. C., Anaf, V., Borghese, B., Vaiman, D., Fayt, I., and Chapron, C. (2011). The steroidogenic factor-1 protein is not expressed in various forms of endometriosis but is strongly present in ovarian cortical or medullary mesenchymatous cells adjacent to endometriotic foci. Fertil. Steril. 95, 2655–2657. doi: 10.1016/j.fertnstert.2011.01.131
Nokelainen, P., Peltoketo, H., Mustonen, M., and Vihko, P. (2000). Expression of mouse 17β-hydroxysteroid dehydrogenase/17-ketosteroid reductase type 7 in the ovary, uterus, and placenta: localization from implantation to late pregnancy. Endocrinology 141, 772–778. doi: 10.1210/endo.141.2.7309
Oduwole, O. O., Isomaa, V. V., Nokelainen, P. A., Stenback, F., and Vihko, P. T. (2002). Downregulation of estrogen-metabolizing 17 beta-hydroxysteroid dehydrogenase type 2 expression correlates inversely with Ki67 proliferation marker in colon-cancer development. Int. J. Cancer 97, 1–6. doi: 10.1002/ijc.1567
Oduwole, O. O., Makinen, J. M., Isomaa, V. V., Karttunen, T. J., and Vihko, P. T. (2003a). Sex steroid metabolism in human gastric mucosa: 17 beta-hydroxysteroid dehydrogenase type 2 in normal, inflamed and neoplastic gastric tissues. Anticancer Res. 23, 3889–3897.
Oduwole, O. O., Makinen, M. J., Isomaa, V. V., Pulkka, A., Jernvall, P., Karttunen, T. J., et al. (2003b). 17β-hydroxysteroid dehydrogenase type 2: independent prognostic significance and evidence of estrogen protection in female patients with colon cancer. J. Steroid Biochem. Mol. Biol. 87, 133–140. doi: 10.1016/j.jsbmb.2003.08.008
Ohno, S., Nishikawa, K., Honda, Y., and Nakajin, S. (2008). Expression in E. coli and tissue distribution of the human homologue of the mouse Ke 6 gene, 17β-hydroxysteroid dehydrogenase type 8. Mol. Cell. Biochem. 309, 209–215. doi: 10.1007/s11010-007-9637-9
Olson, S. H., Bandera, E. V., and Orlow, I. (2007). Variants in estrogen biosynthesis genes, sex steroid hormone levels, and endometrial cancer: a HuGE review. Am. J. Epidemiol. 165, 235–245. doi: 10.1093/aje/kwk015
Osinski, M., Wirstlein, P., Wender-Ozegowska, E., Mikolajczyk, M., Jagodzinski, P. P., and Szczepanska, M. (2018). HSD3B2, HSD17B1, HSD17B2, ESR1, ESR2 and AR expression in infertile women with endometriosis. Ginekol. Pol. 89, 125–134. doi: 10.5603/GP.a2018.0022
Oz, O. K., Millsaps, R., Welch, R., Birch, J., and Zerwekh, J. E. (2001). Expression of aromatase in the human growth plate. J. Mol. Endocrinol. 27, 249–253. doi: 10.1677/jme.0.0270249
Oz, O. K., Zerwekh, J. E., Fisher, C., Graves, K., Nanu, L., Millsaps, R., et al. (2000). Bone has a sexually dimorphic response to aromatase deficiency. J. Bone Miner. Res. 15, 507–514. doi: 10.1359/jbmr.2000.15.3.507
Palmer, B. F., and Clegg, D. J. (2015). The sexual dimorphism of obesity. Mol. Cell. Endocrinol. 402, 113–119. doi: 10.1016/j.mce.2014.11.029
Palmieri, C., Stein, R. C., Liu, X., Hudson, E., Nicholas, H., Sasano, H., et al. (2017a). IRIS study: a phase II study of the steroid sulfatase inhibitor Irosustat when added to an aromatase inhibitor in ER-positive breast cancer patients. Breast Cancer Res. Treat. 165, 343–353. doi: 10.1007/s10549-017-4328-z
Palmieri, C., Szydlo, R., Miller, M., Barker, L., Patel, N. H., Sasano, H., et al. (2017b). IPET study: an FLT-PET window study to assess the activity of the steroid sulfatase inhibitor irosustat in early breast cancer. Breast Cancer Res. Treat. 166, 527–539. doi: 10.1007/s10549-017-4427-x
Park, A. L., Lin, H. K., Yang, Q., Sing, C. W., Fan, M., Mapstone, T. B., et al. (2010). Differential expression of type 2, 3α/type 5, 17β-hydroxysteroid dehydrogenase (AKR1C3) in tumors of the central nervous system. Int. J. Clin. Exp. Pathol. 3, 743–754.
Park, Y. S., Gauna, A. E., and Cha, S. (2015). Mouse models of primary Sjogren's syndrome. Curr. Pharm. Des. 21, 2350–2364. doi: 10.2174/1381612821666150316120024
Pathirage, N., Di Nezza, L. A., Salmonsen, L. A., Jobling, T., Simpson, E. R., and Clyne, C. D. (2006). Expression of aromatase, estrogen receptors, and their coactivators in patients with endometrial cancer. Fertil. Steril. 86, 469–472. doi: 10.1016/j.fertnstert.2005.12.057
Pautier, P., Vergote, I., Joly, F., Melichar, B., Kutarska, E., Hall, G., et al. (2017). A Phase 2, randomized, open-label study of irosustat versus megestrol acetate in advanced endometrial cancer. Int. J. Gynecol. Cancer 27, 258–266. doi: 10.1097/IGC.0000000000000862
Pearson Murphy, B. E., Steinberg, S. I., Hu, F. Y., and Allison, C. M. (2001). Neuroactive ring A-reduced metabolites of progesterone in human plasma during pregnancy: elevated levels of 5 alpha-dihydroprogesterone in depressed patients during the latter half of pregnancy. J. Clin. Endocrinol. Metab. 86, 5981–5987. doi: 10.1210/jcem.86.12.8122
Pelletier, G., Luu-The, V., Tetu, B., and Labrie, F. (1999). Immunocytochemical localization of type 5, 17β-hydroxysteroid dehydrogenase in human reproductive tissues. J. Histochem. Cytochem. 47, 731–738. doi: 10.1177/002215549904700602
Penning, T. M. (2017). Aldo-Keto Reductase (AKR) 1C3 inhibitors: a patent review. Expert Opin. Ther. Pat. 27, 1329–1340. doi: 10.1080/13543776.2017.1379503
Penning, T. M., Burczynski, M. E., Jez, J. M., Hung, C. F., Lin, H. K., Ma, H., et al. (2000). Human 3α-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem. J. 351, 67–77. doi: 10.1042/bj3510067
Penning, T. M., Jin, Y., Steckelbroeck, S., Lanisnik Rizner, T., and Lewis, M. (2004). Structure-function of human 3 alpha-hydroxysteroid dehydrogenases: genes and proteins. Mol. Cell. Endocrinol. 215, 63–72. doi: 10.1016/j.mce.2003.11.006
Perez Carrion, R., Alberola Candel, V., Calabresi, F., Michel, R. T., Santos, R., Delozier, T., et al. (1994). Comparison of the selective aromatase inhibitor formestane with tamoxifen as first-line hormonal therapy in postmenopausal women with advanced breast cancer. Ann. Oncol. 5(Suppl. 7), S19–S24.
Pezzi, V., Mathis, J. M., Rainey, W. E., and Carr, B. R. (2003). Profiling transcript levels for steroidogenic enzymes in fetal tissues. J. Steroid Biochem. Mol. Biol. 87, 181–189. doi: 10.1016/j.jsbmb.2003.07.006
Piccinato, C. A., Neme, R. M., Torres, N., Sanches, L. R., Cruz Derogis, P. B., Brudniewski, H. F., et al. (2016a). Increased expression of CYP1A1 and CYP1B1 in ovarian/peritoneal endometriotic lesions. Reproduction 151, 683–692. doi: 10.1530/REP-15-0581
Piccinato, C. A., Neme, R. M., Torres, N., Sanches, L. R., Derogis, P., Brudniewski, H. F., et al. (2016b). Effects of steroid hormone on estrogen sulfotransferase and on steroid sulfatase expression in endometriosis tissue and stromal cells. J. Steroid Biochem. Mol. Biol. 158, 117–126. doi: 10.1016/j.jsbmb.2015.12.025
Piekorz, R. P., Gingras, S., Hoffmeyer, A., Ihle, J. N., and Weinstein, Y. (2005). Regulation of progesterone levels during pregnancy and parturition by signal transducer and activator of transcription 5 and 20α-hydroxysteroid dehydrogenase. Mol. Endocrinol. 19, 431–440. doi: 10.1210/me.2004-0302
Platia, M. P., Fencl, M. D., Elkind-Hirsch, K. E., Canick, J. A., and Tulchinsky, D. (1984). Estrone sulfatase activity in the human brain and estrone sulfate levels in the normal menstrual cycle. J. Steroid Biochem. 21, 237–241. doi: 10.1016/0022-4731(84)90275-9
Pohl, O., Bestel, E., and Gotteland, J. P. (2014). Synergistic effects of E2MATE and norethindrone acetate on steroid sulfatase inhibition: a randomized phase I proof-of-principle clinical study in women of reproductive age. Reprod. Sci. 21, 1256–1265. doi: 10.1177/1933719114522526
Pollow, K., Lubbert, H., Jeske, R., and Pollow, B. (1975a). Studies on 17β-hydroxysteroid dehydrogenase in human endometrium and endometrial carcinoma. Acta Endocrinol. 79, 146–156.
Pollow, K., Lubbert, H., and Pollow, B. (1975b). Studies on 17 beta-hydroxysteroid dehydrogenase in human endometrium and endometrial carcinoma. III. Partial purification and characterization of the microsomal enzyme. Acta Endocrinol (Copenh) 80, 355-364.
Pollow, K., Lubbert, H., and Pollow, B. (1976). On the mitochondrial 17β-hydroxysteroid dehydrogenase from human endometrium and endometrial carcinoma: characterization and intramitochondrial distribution. J. Steroid Biochem. 7, 45–50. doi: 10.1016/0022-4731(76)90163-1
Polow, K., Lubbert, H., Boquoi, E., Kreutzer, G., Jeske, R., and Pollow, B. (1975). Studies on 17β-hydroxysteroid dehydrogenase in human endometrium and endometrial carcinoma I. Subcellular distribution and variations of specific enzyme activity. Acta Endocrinol (Copenh) 79, 134–145.
Prehn, C., Moller, G., and Adamski, J. (2009). Recent advances in 17β-hydroxysteroid dehydrogenases. J. Steroid Biochem. Mol. Biol. 114, 72–77. doi: 10.1016/j.jsbmb.2008.12.024
Prizant, H., and Hammes, S. R. (2016). Minireview: lymphangioleiomyomatosis (LAM): the “Other” steroid-sensitive cancer. Endocrinology 157, 3374–3383. doi: 10.1210/en.2016-1395
Prossnitz, E. R., and Barton, M. (2011). The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 7, 715–726. doi: 10.1038/nrendo.2011.122
Prost, O., and Adessi, G. L. (1983). Estrone and dehydroepiandrosterone sulfatase activities in normal and pathological human endometrium biopsies. J. Clin. Endocrinol. Metab. 56, 653–661. doi: 10.1210/jcem-56-4-653
Purohit, A., Flanagan, A. M., and Reed, M. J. (1992). Estrogen synthesis by osteoblast cell lines. Endocrinology 131, 2027–2029. doi: 10.1210/endo.131.4.1396346
Purohit, A., and Foster, P. A. (2012). Steroid sulfatase inhibitors for estrogen- and androgen-dependent cancers. J. Endocrinol. 212, 99–110. doi: 10.1530/JOE-11-0266
Purohit, A., Fusi, L., Brosens, J., Woo, L. W., Potter, B. V., and Reed, M. J. (2008). Inhibition of steroid sulphatase activity in endometriotic implants by 667 COUMATE: a potential new therapy. Hum. Reprod. 23, 290–297. doi: 10.1093/humrep/dem308
Qaiser, M. Z., Dolman, D. E. M., Begley, D. J., Abbott, N. J., Cazacu-Davidescu, M., Corol, D. I., et al. (2017). Uptake and metabolism of sulphated steroids by the blood-brain barrier in the adult male rat. J. Neurochem. 142, 672–685. doi: 10.1111/jnc.14117
Qian, Y. M., Sun, X. J., Tong, M. H., Li, X. P., Richa, J., and Song, W. C. (2001). Targeted disruption of the mouse estrogen sulfotransferase gene reveals a role of estrogen metabolism in intracrine and paracrine estrogen regulation. Endocrinology 142, 5342–5350. doi: 10.1210/endo.142.12.8540
Qin, K., Ehrmann, D. A., Cox, N., Refetoff, S., and Rosenfield, R. L. (2006). Identification of a functional polymorphism of the human type 5, 17β-hydroxysteroid dehydrogenase gene associated with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 91, 270–276. doi: 10.1210/jc.2005-2012
Quinkler, M., Sinha, B., Tomlinson, J. W., Bujalska, I. J., Stewart, P. M., and Arlt, W. (2004). Androgen generation in adipose tissue in women with simple obesity–a site-specific role for 17β-hydroxysteroid dehydrogenase type 5. J. Endocrinol. 183, 331–342. doi: 10.1677/joe.1.05762
Rajkowski, K. M., Robel, P., and Baulieu, E. E. (1997). Hydroxysteroid sulfotransferase activity in the rat brain and liver as a function of age and sex. Steroids 62, 427–436. doi: 10.1016/S0039-128X(97)00013-5
Rantakari, P., Lagerbohm, H., Kaimainen, M., Suomela, J. P., Strauss, L., Sainio, K., et al. (2010). Hydroxysteroid (17{beta}) dehydrogenase 12 is essential for mouse organogenesis and embryonic survival. Endocrinology 151, 1893–1901. doi: 10.1210/en.2009-0929
Rantakari, P., Strauss, L., Kiviranta, R., Lagerbohm, H., Paviala, J., Holopainen, I., et al. (2008). Placenta defects and embryonic lethality resulting from disruption of mouse hydroxysteroid (17-beta) dehydrogenase 2 gene. Mol. Endocrinol. 22, 665–675. doi: 10.1210/me.2007-0257
Rauschenberger, K., Scholer, K., Sass, J. O., Sauer, S., Djuric, Z., Rumig, C., et al. (2010). A non-enzymatic function of 17β-hydroxysteroid dehydrogenase type 10 is required for mitochondrial integrity and cell survival. EMBO Mol. Med. 2, 51–62. doi: 10.1002/emmm.200900055
Rawłuszko, A. A., Horbacka, K., Krokowicz, P., and Jagodzinski, P. P. (2011). Decreased expression of 17β-hydroxysteroid dehydrogenase type 1 is associated with DNA hypermethylation in colorectal cancer located in the proximal colon. BMC Cancer 11:522. doi: 10.1186/1471-2407-11-522
Reddy, D. S. (2010). Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog. Brain Res. 186, 113–137. doi: 10.1016/B978-0-444-53630-3.00008-7
Reed, M. J., Purohit, A., Woo, L. W., Newman, S. P., and Potter, B. V. (2005). Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr. Rev. 26, 171–202. doi: 10.1210/er.2004-0003
Rege, J., Karashima, S., Lerario, A. M., Smith, J. M., Auchus, R. J., Kasa-Vubu, J. Z., et al. (2016). Age-dependent increases in adrenal cytochrome b5 and serum 5-androstenediol-3-sulfate. J. Clin. Endocrinol. Metab. 101, 4585–4593. doi: 10.1210/jc.2016-2864
Rennert, G., Rennert, H. S., Pinchev, M., Lavie, O., and Gruber, S. B. (2009). Use of hormone replacement therapy and the risk of colorectal cancer. J. Clin. Oncol. 27, 4542–4547. doi: 10.1200/JCO.2009.22.0764
Rhee, H. S., Oh, S. H., Ko, B. J., Han, D. M., Jeon, B. H., Park, H., et al. (2003). Expression of 3β-hydroxysteroid dehydrogenase and P450 side chain cleavage enzyme in the human uterine endometrium. Exp. Mol. Med. 35, 160–166. doi: 10.1038/emm.2003.22
Riches, Z., Stanley, E. L., Bloomer, J. C., and Coughtrie, M. W. (2009). Quantitative evaluation of the expression and activity of five major sulfotransferases (SULTs) in human tissues: the SULT “pie”. Drug Metab. Dispos. 37, 2255–2261. doi: 10.1124/dmd.109.028399
Riggs, B. L., Khosla, S., and Melton, L. J. III. (2002). Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 23, 279–302. doi: 10.1210/edrv.23.3.0465
Rižner, T. L. (2013). Estrogen biosynthesis, phase I and phase II metabolism, and action in endometrial cancer. Mol. Cell. Endocrinol. 381, 124–139. doi: 10.1016/j.mce.2013.07.026
Rižner, T. L. (2016). The important roles of steroid sulfatase and sulfotransferases in gynecological diseases. Front. Pharmacol. 7:30. doi: 10.3389/fphar.2016.00030
Rižner, T. L., and Penning, T. M. (2014). Role of aldo-keto reductase family 1 (AKR1) enzymes in human steroid metabolism. Steroids 79, 49–63. doi: 10.1016/j.steroids.2013.10.012
Rizner, T. L., Smuc, T., Rupreht, R., Sinkovec, J., and Penning, T. M. (2006). AKR1C1 and AKR1C3 may determine progesterone and estrogen ratios in endometrial cancer. Mol. Cell. Endocrinol. 248, 126–135. doi: 10.1016/j.mce.2005.10.009
Rižner, T. L., Thalhammer, T., and Ozvegy-Laczka, C. (2017). The importance of steroid uptake and intracrine action in endometrial and ovarian cancers. Front. Pharmacol. 8:346. doi: 10.3389/fphar.2017.00346
Rodríguez-Sanz, M., Garcia-Giralt, N., Prieto-Alhambra, D., Servitja, S., Balcells, S., Pecorelli, R., et al. (2015). CYP11A1 expression in bone is associated with aromatase inhibitor-related bone loss. J. Mol. Endocrinol. 55, 69–79. doi: 10.1530/JME-15-0079
Rose, P. G., Brunetto, V. L., VanLe, L., Bell, J., Walker, J. L., and Lee, R. B. (2000). A phase II trial of anastrozole in advanced recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol. 78, 212–216. doi: 10.1006/gyno.2000.5865
Roshan, M. H., Tambo, A., and Pace, N. P. (2016). The role of testosterone in colorectal carcinoma: pathomechanisms and open questions. EPMA J. 7, 22. doi: 10.1186/s13167-016-0071-5
Rosner, W., Hankinson, S. E., Sluss, P. M., Vesper, H. W., and Wierman, M. E. (2013). Challenges to the measurement of estradiol: an endocrine society position statement. J. Clin. Endocrinol. Metab. 98, 1376–1387. doi: 10.1210/jc.2012-3780
Rubin, G. L., Harrold, A. J., Mills, J. A., Falany, C. N., and Coughtrie, M. W. (1999). Regulation of sulphotransferase expression in the endometrium during the menstrual cycle, by oral contraceptives and during early pregnancy. Mol. Hum. Reprod. 5, 995–1002. doi: 10.1093/molehr/5.11.995
Sahu, B., Sun, W., Perusek, L., Parmar, V., Le, Y. Z., Griswold, M. D., et al. (2015). Conditional ablation of retinol dehydrogenase 10 in the retinal pigmented epithelium causes delayed dark adaption in mice. J. Biol. Chem. 290, 27239–27247. doi: 10.1074/jbc.M115.682096
Saito, H., and Yanaihara, T. (1998). Steroid formation in osteoblast-like cells. J. Int. Med. Res. 26, 1–12. doi: 10.1177/030006059802600101
Saitoh, Y., Sasano, H., Naganuma, H., Ohtani, H., Sasano, N., Ohuchi, A., et al. (1992). De novo expression of aromatase in gastric carcinoma. Light and electron microscopic immunohistochemical and immunoblot study. Pathol. Res. Pract. 188, 53–60. doi: 10.1016/S0344-0338(11)81156-8
Sakiani, S., Olsen, N. J., and Kovacs, W. J. (2013). Gonadal steroids and humoral immunity. Nat. Rev. Endocrinol. 9, 56–62. doi: 10.1038/nrendo.2012.206
Sakurai, N., Miki, Y., Suzuki, T., Watanabe, K., Narita, T., Ando, K., et al. (2006). Systemic distribution and tissue localizations of human 17β-hydroxysteroid dehydrogenase type 12. J. Steroid Biochem. Mol. Biol. 99, 174–181. doi: 10.1016/j.jsbmb.2006.01.010
Salas, S., Jezequel, P., Campion, L., Deville, J. L., Chibon, F., Bartoli, C., et al. (2009). Molecular characterization of the response to chemotherapy in conventional osteosarcomas: predictive value of HSD17B10 and IFITM2. Int. J. Cancer 125, 851–860. doi: 10.1002/ijc.24457
Salman, E. D., Faye-Petersen, O., and Falany, C. N. (2011). Hydroxysteroid sulfotransferase 2B1b expression and localization in normal human brain. Horm. Mol. Biol. Clin. Investig. 8, 445–454. doi: 10.1515/HMBCI.2011.117
Salman, E. D., Kadlubar, S. A., and Falany, C. N. (2009). Expression and localization of cytosolic sulfotransferase (SULT) 1A1 and SULT1A3 in normal human brain. Drug Metab. Dispos. 37, 706–709. doi: 10.1124/dmd.108.025767
Saloniemi, T., Jarvensivu, P., Koskimies, P., Jokela, H., Lamminen, T., Ghaem-Maghami, S., et al. (2010). Novel hydroxysteroid (17β) dehydrogenase 1 inhibitors reverse estrogen-induced endometrial hyperplasia in transgenic mice. Am. J. Pathol. 176, 1443–1451. doi: 10.2353/ajpath.2010.090325
Sánchez-Guijo, A., Neunzig, J., Gerber, A., Oji, V., Hartmann, M. F., Schuppe, H. C., et al. (2016). Role of steroid sulfatase in steroid homeostasis and characterization of the sulfated steroid pathway: Evidence from steroid sulfatase deficiency. Mol. Cell. Endocrinol. 437, 142–153. doi: 10.1016/j.mce.2016.08.019
Sasano, H., Takashashi, K., Satoh, F., Nagura, H., and Harada, N. (1998). Aromatase in the human central nervous system. Clin. Endocrinol. (Oxf). 48, 325–329. doi: 10.1046/j.1365-2265.1998.00390.x
Sasano, H., Uzuki, M., Sawai, T., Nagura, H., Matsunaga, G., Kashimoto, O., et al. (1997). Aromatase in human bone tissue. J. Bone Miner. Res. 12, 1416–1423. doi: 10.1359/jbmr.1997.12.9.1416
Sathish, V., Martin, Y. N., and Prakash, Y. S. (2015). Sex steroid signaling: implications for lung diseases. Pharmacol. Ther. 150, 94–108. doi: 10.1016/j.pharmthera.2015.01.007
Sato, R., Suzuki, T., Katayose, Y., Miura, K., Shiiba, K., Miki, Y., et al. (2012). Aromatase in colon carcinoma. Anticancer Res. 32, 3069–3075.
Sato, R., Suzuki, T., Katayose, Y., Miura, K., Shiiba, K., Tateno, H., et al. (2009). Steroid sulfatase and estrogen sulfotransferase in colon carcinoma: regulators of intratumoral estrogen concentrations and potent prognostic factors. Cancer Res. 69, 914–922. doi: 10.1158/0008-5472.CAN-08-0906
Schweikert, H. U., Wolf, L., and Romalo, G. (1995). Oestrogen formation from androstenedione in human bone. Clin. Endocrinol. (Oxf). 43, 37–42. doi: 10.1111/j.1365-2265.1995.tb01890.x
Scublinsky, A., Marin, C., and Gurpide, E. (1976). Localization of estradiol 17β dehydrogenase in human endometrium. J. Steroid Biochem. 7, 745–747. doi: 10.1016/0022-4731(76)90174-6
Segawa, T., Shozu, M., Murakami, K., Kasai, T., Shinohara, K., Nomura, K., et al. (2005). Aromatase expression in stromal cells of endometrioid endometrial cancer correlates with poor survival. Clin. Cancer Res. 11, 2188–2194. doi: 10.1158/1078-0432.CCR-04-1859
Selcer, K. W., and Difrancesca, H. M. (2012). Characterization of steroid sulfatase in the MC3T3-E1 mouse pre-osteoblastic cell line. Steroids 77, 696–702. doi: 10.1016/j.steroids.2012.02.024
Setiawan, V. W., Doherty, J. A., Shu, X. O., Akbari, M. R., Chen, C., De Vivo, I., et al. (2009). Two estrogen-related variants in CYP19A1 and endometrial cancer risk: a pooled analysis in the epidemiology of endometrial cancer consortium. Cancer Epidemiol. Biomarkers Prev. 18, 242–247. doi: 10.1158/1055-9965.EPI-08-0689
Setiawan, V. W., Hankinson, S. E., Colditz, G. A., Hunter, D. J., and De Vivo, I. (2004). HSD17B1 gene polymorphisms and risk of endometrial and breast cancer. Cancer Epidemiol. Biomarkers Prev. 13, 213–219. doi: 10.1158/1055-9965.EPI-03-0241
Shang, E., Lai, K., Packer, A. I., Paik, J., Blaner, W. S., de Morais Vieira, M., et al. (2002). Targeted disruption of the mouse cis-retinol dehydrogenase gene: visual and nonvisual functions. J. Lipid Res. 43, 590–597.
Shehu, A., Mao, J., Gibori, G. B., Halperin, J., Le, J., Devi, Y. S., et al. (2008). Prolactin receptor-associated protein/17β-hydroxysteroid dehydrogenase type 7 gene (Hsd17b7) plays a crucial role in embryonic development and fetal survival. Mol. Endocrinol. 22, 2268–2277. doi: 10.1210/me.2008-0165
Shen, Z., Peng, Z., Sun, Y., Vaananen, H. K., and Poutanen, M. (2008). Overexpression of human hydroxysteroid (17β) dehydrogenase 2 induces disturbance in skeletal development in young male mice. J. Bone Miner. Res. 23, 1217–1226. doi: 10.1359/jbmr.080322
Shi, L., Yang, X., Dong, X., and Zhang, B. (2016). Polymorphism of HSD17B1 Ser312Gly with Cancer Risk: Evidence from 66,147 Subjects. Twin Res. Hum. Genet. 19, 136–145. doi: 10.1017/thg.2016.6
Shim, G. J., Warner, M., Kim, H. J., Andersson, S., Liu, L., Ekman, J., et al. (2004). Aromatase-deficient mice spontaneously develop a lymphoproliferative autoimmune disease resembling Sjogren's syndrome. Proc. Natl. Acad. Sci. U.S.A. 101, 12628–12633. doi: 10.1073/pnas.0405099101
Shimizu, K. (1979). Metabolism of [17-2H]pregnenolone into 5-[17β-2H, 17α-18O]androstene-3β, 17α-diol and other products by incubation with the microsomal fraction of boar testis under 18O2 atmosphere. Biochim. Biophys. Acta 575, 37–45. doi: 10.1016/0005-2760(79)90128-0
Shimodaira, M., Nakayama, T., Sato, I., Sato, N., Izawa, N., Mizutani, Y., et al. (2012). Estrogen synthesis genes CYP19A1, HSD3B1, and HSD3B2 in hypertensive disorders of pregnancy. Endocrine 42, 700–707. doi: 10.1007/s12020-012-9699-7
Shimodaira, M., Nakayama, T., Sato, N., Aoi, N., Sato, M., Izumi, Y., et al. (2010). Association of HSD3B1 and HSD3B2 gene polymorphisms with essential hypertension, aldosterone level, and left ventricular structure. Eur. J. Endocrinol. 163, 671–680. doi: 10.1530/EJE-10-0428
Siegfried, J. M., and Stabile, L. P. (2014). Estrongenic steroid hormones in lung cancer. Semin. Oncol. 41, 5–16. doi: 10.1053/j.seminoncol.2013.12.009
Simard, J., Ricketts, M. L., Gingras, S., Soucy, P., Feltus, F. A., and Melner, M. H. (2005). Molecular biology of the 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase gene family. Endocr. Rev. 26, 525–582. doi: 10.1210/er.2002-0050
Simard, M., Plante, J., Boucher, M., Provost, P. R., and Tremblay, Y. (2010). Type 2 and 5, 17β-hydroxysteroid dehydrogenases and androgen receptor in human fetal lungs. Mol. Cell. Endocrinol. 319, 79–87. doi: 10.1016/j.mce.2009.12.007
Simoens, S., Dunselman, G., Dirksen, C., Hummelshoj, L., Bokor, A., Brandes, I., et al. (2012). The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum. Reprod. 27, 1292–1299. doi: 10.1093/humrep/des073
Simoens, S., Hummelshoj, L., Dunselman, G., Brandes, I., Dirksen, C., and D'Hooghe, T. (2011). Endometriosis cost assessment (the EndoCost study): a cost-of-illness study protocol. Gynecol. Obstet. Invest. 71, 170–176. doi: 10.1159/000316055
Singh, M. M., Trivedi, R. N., Chauhan, S. C., Srivastava, V. M., Makker, A., Chowdhury, S. R., et al. (1996). Uterine estradiol and progesterone receptor concentration, activities of certain antioxidant enzymes and dehydrogenases and histoarchitecture in relation to time of secretion of nidatory estrogen and high endometrial sensitivity in rat. J. Steroid Biochem. Mol. Biol. 59, 215–224. doi: 10.1016/S0960-0760(96)00102-1
Singhal, N., Vatandoust, S., and Brown, M. P. (2015). Phase II study evaluating efficacy and safety of everolimus with letrozole for management of advanced (unresectable or metastatic) non-small cell lung cancer after failure of platinum-based treatment: a preliminary analysis of toxicity. Cancer Chemother. Pharmacol. 75, 325–331. doi: 10.1007/s00280-014-2644-0
Sinreih, M., Anko, M., Zukunft, S., Adamski, J., and Rizner, T. L. (2014). Important roles of the AKR1C2 and SRD5A1 enzymes in progesterone metabolism in endometrial cancer model cell lines. Chem. Biol. Interact. 234, 297–308. doi: 10.1016/j.cbi.2014.11.012
Sinreih, M., Hevir, N., and Rizner, T. L. (2013). Altered expression of genes involved in progesterone biosynthesis, metabolism and action in endometrial cancer. Chem. Biol. Interact. 202, 210–217. doi: 10.1016/j.cbi.2012.11.012
Sinreih, M., Knific, T., Anko, M., Hevir, N., Vouk, K., Jerin, A., et al. (2017a). The significance of the sulfatase pathway for local estrogen formation in endometrial cancer. Front. Pharmacol. 8:368. doi: 10.3389/fphar.2017.00368
Sinreih, M., Stupar, S., Cemazar, L., Verdenik, I., Frkovic Grazio, S., Smrkolj, S., et al. (2017b). STAR and AKR1B10 are down-regulated in high-grade endometrial cancer. J. Steroid Biochem. Mol. Biol.. doi: 10.1016/j.jsbmb.2017.02.015
Sivik, T. (2012). Elucidating the Role of 17β Hydroxysteroid Dehydrogenase Type 14 in Normal Physiology and in Breast Cancer. Department of Clinical and Experimental Medicine: Linköping University (Linköping).
Skjefstad, K., Grindstad, T., Khanehkenari, M. R., Richardsen, E., Donnem, T., Kilvaer, T., et al. (2016). Prognostic relevance of estrogen receptor α, β and aromatase expression in non-small cell lung cancer. Steroids 113, 5–13. doi: 10.1016/j.steroids.2016.05.008
Slominski, A., Zbytek, B., Nikolakis, G., Manna, P. R., Skobowiat, C., Zmijewski, M., et al. (2013). Steroidogenesis in the skin: implications for local immune functions. J. Steroid Biochem. Mol. Biol. 137, 107–123. doi: 10.1016/j.jsbmb.2013.02.006
Slomovitz, B. M., Jiang, Y., Yates, M. S., Soliman, P. T., Johnston, T., Nowakowski, M., et al. (2015). Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. J. Clin. Oncol. 33, 930–936. doi: 10.1200/JCO.2014.58.3401
Smith, A., Contreras, C., Ko, K. H., Chow, J., Dong, X., Tuo, B., et al. (2008). Gender-specific protection of estrogen against gastric acid-induced duodenal injury: stimulation of duodenal mucosal bicarbonate secretion. Endocrinology 149, 4554–4566. doi: 10.1210/en.2007-1597
Smith, E. P., Boyd, J., Frank, G. R., Takahashi, H., Cohen, R. M., Specker, B., et al. (1994). Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N. Engl. J. Med. 331, 1056–1061. doi: 10.1056/NEJM199410203311604
Smuc, T., Hevir, N., Ribic-Pucelj, M., Husen, B., Thole, H., and Rizner, T. L. (2009). Disturbed estrogen and progesterone action in ovarian endometriosis. Mol. Cell. Endocrinol. 301, 59–64. doi: 10.1016/j.mce.2008.07.020
Smuc, T., Pucelj, M. R., Sinkovec, J., Husen, B., Thole, H., and Lanisnik Rizner, T. (2007). Expression analysis of the genes involved in estradiol and progesterone action in human ovarian endometriosis. Gynecol. Endocrinol. 23, 105–111. doi: 10.1080/09513590601152219
Smuc, T., and Rizner, T. L. (2009). Aberrant pre-receptor regulation of estrogen and progesterone action in endometrial cancer. Mol. Cell. Endocrinol. 301, 74–82. doi: 10.1016/j.mce.2008.09.019
Smuc, T., Rupreht, R., Sinkovec, J., Adamski, J., and Rizner, T. L. (2006). Expression analysis of estrogen-metabolizing enzymes in human endometrial cancer. Mol. Cell. Endocrinol. 248, 114–117. doi: 10.1016/j.mce.2005.10.013
Soma, K. K., Rendon, N. M., Boonstra, R., Albers, H. E., and Demas, G. E. (2015). DHEA effects on brain and behavior: insights from comparative studies of aggression. J. Steroid Biochem. Mol. Biol. 145, 261–272. doi: 10.1016/j.jsbmb.2014.05.011
Soubhye, J., Alard, I. C., van Antwerpen, P., and Dufrasne, F. (2015). Type 2, 17-beta hydroxysteroid dehydrogenase as a novel target for the treatment of osteoporosis. Future Med. Chem. 7, 1431–1456. doi: 10.4155/fmc.15.74
Steckelbroeck, S., Jin, Y., Gopishetty, S., Oyesanmi, B., and Penning, T. M. (2004a). Human cytosolic 3alpha-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3beta-hydroxysteroid dehydrogenase activity: implications for steroid hormone metabolism and action. J. Biol. Chem. 279, 10784–10795. doi: 10.1074/jbc.M313308200
Steckelbroeck, S., Lutjohann, D., Bauman, D. R., Ludwig, M., Friedl, A., Hans, V. H., et al. (2010). Non-stereo-selective cytosolic human brain tissue 3-ketosteroid reductase is refractory to inhibition by AKR1C inhibitors. Biochim. Biophys. Act. 1801, 1221–1231. doi: 10.1016/j.bbalip.2010.07.005
Steckelbroeck, S., Nassen, A., Ugele, B., Ludwig, M., Watzka, M., Reissinger, A., et al. (2004b). Steroid sulfatase (STS) expression in the human temporal lobe: enzyme activity, mRNA expression and immunohistochemistry study. J. Neurochem. 89, 403–417. doi: 10.1046/j.1471-4159.2004.02336.x
Steckelbroeck, S., Stoffel-Wagner, B., Reichelt, R., Schramm, J., Bidlingmaier, F., Siekmann, L., et al. (1999). Characterization of 17β-hydroxysteroid dehydrogenase activity in brain tissue: testosterone formation in the human temporal lobe. J. Neuroendocrinol. 11, 457–464. doi: 10.1046/j.1365-2826.1999.00363.x
Steckelbroeck, S., Watzka, M., Reichelt, R., Hans, V. H., Stoffel-Wagner, B., Heidrich, D. D., et al. (2001). Characterization of the 5α-reductase-3α-hydroxysteroid dehydrogenase complex in the human brain. J. Clin. Endocrinol. Metab. 86, 1324–1331. doi: 10.1210/jcem.86.3.7325
Steckelbroeck, S., Watzka, M., Reissinger, A., Wegener-Toper, P., Bidlingmaier, F., Bliesener, N., et al. (2003). Characterisation of estrogenic 17β-hydroxysteroid dehydrogenase (17β-HSD) activity in the human brain. J. Steroid Biochem. Mol. Biol. 86, 79–92. doi: 10.1016/S0960-0760(03)00251-6
Stelzer, G., Rosen, N., Plaschkes, I., Zimmerman, S., Twik, M., Fishilevich, S., et al. (2016). The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinformatics 54, 1.30, 31–31, 30, 33. doi: 10.1002/cpbi.5
Stoffel-Wagner, B. (2001). Neurosteroid metabolism in the human brain. Eur. J. Endocrinol. 145, 669–679. doi: 10.1530/eje.0.1450669
Stoffel-Wagner, B., Beyenburg, S., Watzka, M., Blumcke, I., Bauer, J., Schramm, J., et al. (2000). Expression of 5α-reductase and 3α-hydroxisteroid oxidoreductase in the hippocampus of patients with chronic temporal lobe epilepsy. Epilepsia 41, 140–147. doi: 10.1111/j.1528-1157.2000.tb00133.x
Stoffel-Wagner, B., Watzka, M., Schramm, J., Bidlingmaier, F., and Klingmuller, D. (1999a). Expression of CYP19 (aromatase) mRNA in different areas of the human brain. J. Steroid Biochem. Mol. Biol. 70, 237–241. doi: 10.1016/S0960-0760(99)00114-4
Stoffel-Wagner, B., Watzka, M., Steckelbroeck, S., Ludwig, M., Clusmann, H., Bidlingmaier, F., et al. (2003). Allopregnanolone serum levels and expression of 5 alpha-reductase and 3 alpha-hydroxysteroid dehydrogenase isoforms in hippocampal and temporal cortex of patients with epilepsy. Epilepsy Res. 54, 11–19. doi: 10.1016/S0920-1211(03)00036-6
Stoffel-Wagner, B., Watzka, M., Steckelbroeck, S., Schramm, J., Bidlingmaier, J. F., and Klingmuller, D. (1999b). Expression of 17β-hydroxysteroid dehydrogenase types 1, 2, 3 and 4 in the human temporal lobe. J. Endocrinol. 160, 119–126. doi: 10.1677/joe.0.1600119
Stoffel-Wagner, B., Watzka, M., Steckelbroeck, S., Schwaab, R., Schramm, J., Bidlingmaier, F., et al. (1998a). Expression of CYP19 (aromatase) mRNA in the human temporal lobe. Biochem. Biophys. Res. Commun. 244, 768–771. doi: 10.1006/bbrc.1998.8337
Stoffel-Wagner, B., Watzka, M., Steckelbroeck, S., Wickert, L., Schramm, J., Romalo, G., et al. (1998b). Expression of 5α-reductase in the human temporal lobe of children and adults. J. Clin. Endocrinol. Metab. 83, 3636–3642.
Strott, C. A. (2002). Sulfonation and molecular action. Endocr. Rev. 23, 703–732. doi: 10.1210/er.2001-0040
Sudeshna, T., Anand, K., and Medhamurthy, R. (2013). Analysis of 20α-hydroxysteroid dehydrogenase expression in the corpus luteum of the buffalo cow: effect of prostaglandin F2-α treatment on circulating 20α-hydroxyprogesterone levels. Reprod. Biol. Endocrinol. 11:111. doi: 10.1186/1477-7827-11-111
Sun, X. F., Ahmadi, A., Arbman, G., Wallin, A., Asklid, D., and Zhang, H. (2005). Polymorphisms in sulfotransferase 1A1 and glutathione S-transferase P1 genes in relation to colorectal cancer risk and patients' survival. World J. Gastroenterol. 11, 6875–6879. doi: 10.3748/wjg.v11.i43.6875
Svoboda, M., Hamilton, G., and Thalhammer, T. (2010). Steroid hormone metabolizing enzymes in benign and malignant human bone tumors. Expert Opin. Drug Metab. Toxicol. 6, 427–437. doi: 10.1517/17425251003592129
Svoboda, M., Thalhammer, T., Aust, S., Arrich, F., Assadian, O., and Toma, C. D. (2007). Estrogen sulfotransferase (SULT1E1) expression in benign and malignant human bone tumors. J. Surg. Oncol. 95, 572–581. doi: 10.1002/jso.20748
Syed, F., and Khosla, S. (2005). Mechanisms of sex steroid effects on bone. Biochem. Biophys. Res. Commun. 328, 688–696. doi: 10.1016/j.bbrc.2004.11.097
Taga, S., Yoshida, N., and Sekiba, K. (1990). Distribution and cyclic change of aromatase cytochrome P-450 activity in human uteri. J. Steroid Biochem. Mol. Biol. 37, 741–745. doi: 10.1016/0960-0760(90)90359-S
Takeyama, J., Suzuki, T., Hirasawa, G., Muramatsu, Y., Nagura, H., Iinuma, K., et al. (2000). 17β-hydroxysteroid dehydrogenase type 1 and 2 expression in the human fetus. J. Clin. Endocrinol. Metab. 85, 410–416. doi: 10.1210/jcem.85.1.6323
Tanaka, K., Kubushiro, K., Iwamori, Y., Okairi, Y., Kiguchi, K., Ishiwata, I., et al. (2003). Estrogen sulfotransferase and sulfatase: Roles in the regulation of estrogen activity in human uterine endometrial carcinomas. Cancer Sci. 94, 871–876. doi: 10.1111/j.1349-7006.2003.tb01369.x
Tanaka, K., Shimizu, K., Kakegawa, S., Ohtaki, Y., Nagashima, T., Kaira, K., et al. (2016). Prognostic significance of aromatase and estrogen receptor beta expression in EGFR wild-type lung adenocarcinoma. Am. J. Transl. Res. 8, 81–97.
Tanaka, S., Miki, Y., Hashimoto, C., Takagi, K., Doe, Z., Li, B., et al. (2015). The role of 5α-reductase type 1 associated with intratumoral dihydrotestosterone concentrations in human endometrial carcinoma. Mol. Cell. Endocrinol. 401, 56–64. doi: 10.1016/j.mce.2014.11.022
Tang, T., Li, L., Tang, J., Li, Y., Lin, W. Y., Martin, F., et al. (2010). A mouse knockout library for secreted and transmembrane proteins. Nat. Biotechnol. 28, 749–755. doi: 10.1038/nbt.1644
Tangen, I. L., Onyango, T. B., Kopperud, R., Berg, A., Halle, M. K., Oyan, A. M., et al. (2016). Androgen receptor as potential therapeutic target in metastatic endometrial cancer. Oncotarget 7, 49289–49298. doi: 10.18632/oncotarget.10334
Tangen, I. L., Werner, H. M., Berg, A., Halle, M. K., Kusonmano, K., Trovik, J., et al. (2014). Loss of progesterone receptor links to high proliferation and increases from primary to metastatic endometrial cancer lesions. Eur J Cancer 50, 3003–3010. doi: 10.1016/j.ejca.2014.09.003
Taniuchi, S., Fujishima, F., Miki, Y., Abe, K., Nakamura, Y., Sato, S., et al. (2014). Tissue concentrations of estrogens and aromatase immunolocalization in interstitial pneumonia of human lung. Mol. Cell. Endocrinol. 392, 136–143. doi: 10.1016/j.mce.2014.05.016
Tashiro, A., Sasano, H., Nishikawa, T., Yabuki, N., Muramatsu, Y., Coughtrie, M. W., et al. (2000). Expression and activity of dehydroepiandrosterone sulfotransferase in human gastric mucosa. J. Steroid Biochem. Mol. Biol. 72, 149–154. doi: 10.1016/S0960-0760(00)00020-0
Taveira-DaSilva, A. M., and Moss, J. (2014). Management of lymphangioleiomyomatosis. F1000Prime Rep. 6, 116. doi: 10.12703/P6-116
Terry, K., McGrath, M., Lee, I. M., Buring, J., and De Vivo, I. (2010). Genetic variation in CYP11A1 and StAR in relation to endometrial cancer risk. Gynecol. Oncol. 117, 255–259. doi: 10.1016/j.ygyno.2010.02.002
Teubner, W., Meinl, W., Florian, S., Kretzschmar, M., and Glatt, H. (2007). Identification and localization of soluble sulfotransferases in the human gastrointestinal tract. Biochem. J. 404, 207–215. doi: 10.1042/BJ20061431
Thiboutot, D., Martin, P., Volikos, L., and Gilliland, K. (1998). Oxidative activity of the type 2 isozyme of 17β-hydroxysteroid dehydrogenase (17β-HSD) predominates in human sebaceous glands. J. Invest. Dermatol. 111, 390–395. doi: 10.1046/j.1523-1747.1998.00322.x
Thompson, D. J., O'Mara, T. A., Glubb, D. M., Painter, J. N., Cheng, T., Folkerd, E., et al. (2016). CYP19A1 fine-mapping and Mendelian randomization: estradiol is causal for endometrial cancer. Endocr. Relat. Cancer 23, 77–91. doi: 10.1530/ERC-15-0386
Tong, M. H., Jiang, H., Liu, P., Lawson, J. A., Brass, L. F., and Song, W. C. (2005). Spontaneous fetal loss caused by placental thrombosis in estrogen sulfotransferase-deficient mice. Nat. Med. 11, 153–159. doi: 10.1038/nm1184
Törn, S., Nokelainen, P., Kurkela, R., Pulkka, A., Menjivar, M., Ghosh, S., et al. (2003). Production, purification, and functional analysis of recombinant human and mouse 17β-hydroxysteroid dehydrogenase type 7. Biochem. Biophys. Res. Commun. 305, 37–45. doi: 10.1016/S0006-291X(03)00694-6
Townsend, E. A., Miller, V. M., and Prakash, Y. S. (2012). Sex differences and sex steroids in lung health and disease. Endocr. Rev. 33, 1–47. doi: 10.1210/er.2010-0031
Townsend, E. A., Thompson, M. A., Pabelick, C. M., and Prakash, Y. S. (2010). Rapid effects of estrogen on intracellular Ca2+ regulation in human airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 298 L521–530. doi: 10.1152/ajplung.00287.2009
Tsai, S. J., Wu, M. H., Lin, C. C., Sun, H. S., and Chen, H. M. (2001). Regulation of steroidogenic acute regulatory protein expression and progesterone production in endometriotic stromal cells. J. Clin. Endocrinol. Metab. 86, 5765–5773. doi: 10.1210/jcem.86.12.8082
Tseng, L., and Gurpide, E. (1974). Estradiol and 20α-dihydroprogesterone dehydrogenase activities in human endometrium during the menstrual cycle. Endocrinology 94, 419–423. doi: 10.1210/endo-94-2-419
Tseng, L., Gusberg, S. B., and Gurpide, E. (1977). Estradiol receptor and 17 beta-dehydrogenase in normal and abnormal human endometrium. Ann. N. Y. Acad. Sci. 286, 190–198. doi: 10.1111/j.1749-6632.1977.tb29416.x
Tseng, L., and Mazella, J. (2002). Endometrial cell specific gene activation during implantation and early pregnancy. Front. Biosci. 7, d1566–d1574. doi: 10.2741/A861
Tseng, L., Mazella, J., Mann, W. J., and Chumas, J. (1982). Estrogen synthesis in normal and malignant human endometrium. J. Clin. Endocrinol. Metab. 55, 1029–1031. doi: 10.1210/jcem-55-5-1029
Tsuchiya, M., Nakao, H., Katoh, T., Sasaki, H., Hiroshima, M., Tanaka, T., et al. (2005). Association between endometriosis and genetic polymorphisms of the estradiol-synthesizing enzyme genes HSD17B1 and CYP19. Hum. Reprod. 20, 974–978. doi: 10.1093/humrep/deh726
Tuo, B., Wen, G., Wei, J., Liu, X., Wang, X., Zhang, Y., et al. (2011). Estrogen regulation of duodenal bicarbonate secretion and sex-specific protection of human duodenum. Gastroenterology 141, 854–863. doi: 10.1053/j.gastro.2011.05.044
Utsunomiya, H., Ito, K., Suzuki, T., Kitamura, T., Kaneko, C., Nakata, T., et al. (2004). Steroid sulfatase and estrogen sulfotransferase in human endometrial carcinoma. Clin. Cancer Res. 10, 5850–5856. doi: 10.1158/1078-0432.CCR-04-0040
Utsunomiya, H., Suzuki, T., Ito, K., Moriya, T., Konno, R., Sato, S., et al. (2003). The correlation between the response to progestogen treatment and the expression of progesterone receptor B and 17β-hydroxysteroid dehydrogenase type 2 in human endometrial carcinoma. Clin. Endocrinol. (Oxf). 58, 696–703. doi: 10.1046/j.1365-2265.2003.01766.x
Utsunomiya, H., Suzuki, T., Kaneko, C., Takeyama, J., Nakamura, J., Kimura, K., et al. (2001). The analyses of 17β-hydroxysteroid dehydrogenase isozymes in human endometrial hyperplasia and carcinoma. J. Clin. Endocrinol. Metab. 86, 3436–3443. doi: 10.1210/jcem.86.7.7661
van der Eerden, B. C., Lowik, C. W., Wit, J. M., and Karperien, M. (2004). Expression of estrogen receptors and enzymes involved in sex steroid metabolism in the rat tibia during sexual maturation. J. Endocrinol. 180, 457–467. doi: 10.1677/joe.0.1800457
Vanderschueren, D., Gaytant, J., Boonen, S., and Venken, K. (2008). Androgens and bone. Curr. Opin. Endocrinol. Diabetes Obes. 15, 250–254. doi: 10.1097/MED.0b013e3282fe6ca9
Vani, S., McDonald, S. E., Williams, A. R., Mason, J. I., Thong, K. J., and Critchley, H. O. (2007). Mid-luteal endometrial intracrinology following controlled ovarian hyperstimulation involving use of a gonadotrophin releasing hormone antagonist. Hum. Reprod. 22, 2981–2991. doi: 10.1093/humrep/dem269
Varlamov, O., Bethea, C. L., and Roberts, C. T. Jr. (2014). Sex-specific differences in lipid and glucose metabolism. Front. Endocrinol. (Lausanne). 5:241. doi: 10.3389/fendo.2014.00241
Vasquez, Y. M., and DeMayo, F. J. (2013). Role of nuclear receptors in blastocyst implantation. Semin. Cell Dev. Biol. 24, 724–735. doi: 10.1016/j.semcdb.2013.08.004
Velasco, I., Rueda, J., and Acien, P. (2006). Aromatase expression in endometriotic tissues and cell cultures of patients with endometriosis. Mol. Hum. Reprod. 12, 377–381. doi: 10.1093/molehr/gal041
Vercellini, P., Vigano, P., Somigliana, E., and Fedele, L. (2014). Endometriosis: pathogenesis and treatment. Nat. Rev. Endocrinol. 10, 261–275. doi: 10.1038/nrendo.2013.255
Verma, M. K., Miki, Y., Abe, K., Suzuki, T., Niikawa, H., Suzuki, S., et al. (2013). Intratumoral localization and activity of 17β-hydroxysteroid dehydrogenase type 1 in non-small cell lung cancer: a potent prognostic factor. J. Transl. Med. 11, 167. doi: 10.1186/1479-5876-11-167
Verma, M. K., Miki, Y., and Sasano, H. (2011). Aromatase in human lung carcinoma. Steroids 76, 759–764. doi: 10.1016/j.steroids.2011.02.020
Vidal, O., Lindberg, M. K., Hollberg, K., Baylink, D. J., Andersson, G., Lubahn, D. B., et al. (2000). Estrogen receptor specificity in the regulation of skeletal growth and maturation in male mice. Proc. Natl. Acad. Sci. U.S.A. 97, 5474–5479. doi: 10.1073/pnas.97.10.5474
Vouk, K., Smuc, T., Guggenberger, C., Ribic-Pucelj, M., Sinkovec, J., Husen, B., et al. (2011). Novel estrogen-related genes and potential biomarkers of ovarian endometriosis identified by differential expression analysis. J. Steroid Biochem. Mol. Biol. 125, 231–242. doi: 10.1016/j.jsbmb.2011.03.010
Walker, V. R., and Korach, K. S. (2004). Estrogen receptor knockout mice as a model for endocrine research. ILAR J. 45, 455–461. doi: 10.1093/ilar.45.4.455
Wang, F., Vihma, V., Soronen, J., Turpeinen, U., Hamalainen, E., Savolainen-Peltonen, H., et al. (2013). 17β-Estradiol and estradiol fatty acyl esters and estrogen-converting enzyme expression in adipose tissue in obese men and women. J. Clin. Endocrinol. Metab. 98, 4923–4931. doi: 10.1210/jc.2013-2605
Wang, H., and Dey, S. K. (2006). Roadmap to embryo implantation: clues from mouse models. Nat. Rev. Genet. 7, 185–199. doi: 10.1038/nrg1808
Wang, X. Q., Aka, J. A., Li, T., Xu, D., Doillon, C. J., and Lin, S. X. (2017). Inhibition of 17β-hydroxysteroid dehydrogenase type 7 modulates breast cancer protein profile and enhances apoptosis by down-regulating GRP78. J. Steroid Biochem. Mol. Biol. 172, 188–197. doi: 10.1016/j.jsbmb.2017.06.009
Warren, J. C., and French, A. P. (1965). DISTRIBUTION OF STEROID SULFATASE IN HUMAN TISSUES. J. Clin. Endocrinol. Metab. 25, 278–282. doi: 10.1210/jcem-25-2-278
Watanabe, K., Sasano, H., Harada, N., Ozaki, M., Niikura, H., Sato, S., et al. (1995). Aromatase in human endometrial carcinoma and hyperplasia. Immunohistochemical, in situ hybridization, and biochemical studies. Am. J. Pathol. 146, 491–500.
Watzka, M., Bidlingmaier, F., Schramm, J., Klingmuller, D., and Stoffel-Wagner, B. (1999). Sex- and age-specific differences in human brain CYP11A1 mRNA expression. J. Neuroendocrinol. 11, 901–905. doi: 10.1046/j.1365-2826.1999.00407.x
Wishart, D. S., Feunang, Y. D., Guo, A. C., Lo, E. J., Marcu, A., Grant, J. R., et al. (2018). DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 46, D1074–D1082. doi: 10.1093/nar/gkx1037
Wishart, D. S., Jewison, T., Guo, A. C., Wilson, M., Knox, C., Liu, Y., et al. (2013). HMDB 3.0–the human metabolome database in 2013. Nucleic Acids Res. 41, D801–D807. doi: 10.1093/nar/gks1065
Wöhrle, D., Barbi, G., Schulz, W., and Steinbach, P. (1990). Heterozygous expression of X-linked chondrodysplasia punctata. Complex chromosome aberration including deletion of MIC2 and STS. Hum. Genet. 86, 215–218. doi: 10.1007/BF00197708
Woo, L. W., Purohit, A., and Potter, B. V. (2011). Development of steroid sulfatase inhibitors. Mol. Cell. Endocrinol. 340, 175–185. doi: 10.1016/j.mce.2010.12.035
Wu, H. C., Tuo, B. G., Wu, W. M., Gao, Y., Xu, Q. Q., and Zhao, K. (2008). Prevalence of peptic ulcer in dyspeptic patients and the influence of age, sex, and Helicobacter pylori infection. Dig. Dis. Sci. 53, 2650–2656. doi: 10.1007/s10620-007-0177-7
Xiao, J., Zheng, Y., Zhou, Y., Zhang, P., Wang, J., Shen, F., et al. (2014). Sulfotransferase SULT1A1 Arg213His polymorphism with cancer risk: a meta-analysis of 53 case-control studies. PLoS ONE 9:e106774. doi: 10.1371/journal.pone.0106774
Yague, J. G., Azcoitia, I., DeFelipe, J., Garcia-Segura, L. M., and Munoz, A. (2010). Aromatase expression in the normal and epileptic human hippocampus. Brain Res. 1315, 41–52. doi: 10.1016/j.brainres.2009.09.111
Yague, J. G., Munoz, A., de Monasterio-Schrader, P., Defelipe, J., Garcia-Segura, L. M., and Azcoitia, I. (2006). Aromatase expression in the human temporal cortex. Neuroscience 138, 389–401. doi: 10.1016/j.neuroscience.2005.11.054
Yamaki, J., Yamamoto, T., and Okada, H. (1985). Aromatization of androstenedione by normal and neoplastic endometrium of the uterus. J. Steroid Biochem. 22, 63–66. doi: 10.1016/0022-4731(85)90142-6
Yamamoto, T., Fukuoka, M., Fujimoto, Y., Kitawaki, J., Nakakoshi, M., Yoshihama, M., et al. (1990a). Inhibitory effect of a new androstenedione derivative, 14α-hydroxy-4-androstene-3,6,17-trione (14α-OHAT) on aromatase activity of human uterine tumors. J. Steroid Biochem. 36, 517–21. doi: 10.1016/0022-4731(90)90167-Q
Yamamoto, T., Kitawaki, J., Urabe, M., Honjo, H., Tamura, T., Noguchi, T., et al. (1993a). Estrogen productivity of endometrium and endometrial cancer tissue; influence of aromatase on proliferation of endometrial cancer cells. J. Steroid Biochem. Mol. Biol. 44, 463–468. doi: 10.1016/0960-0760(93)90251-Q
Yamamoto, T., Noguchi, T., Tamura, T., Kitawaki, J., and Okada, H. (1993b). Evidence for estrogen synthesis in adenomyotic tissues. Am. J. Obstet. Gynecol. 169, 734–738. doi: 10.1016/0002-9378(93)90654-2
Yamamoto, T., Urabe, M., Naitoh, K., Kitawaki, J., Honjo, H., and Okada, H. (1990b). Estrone sulfatase activity in human uterine leiomyoma. Gynecol. Oncol. 37, 315–318. doi: 10.1016/0090-8258(90)90358-R
Yanaihara, Yanaihara, T., Toma, Y., Shimizu, Y., Saito, H., Okai, T., et al. (2001). Localization and expression of steroid sulfatase in human fallopian tubes. Steroids 66, 87–91. doi: 10.1016/S0039-128X(00)00204-X
Yang, W., Wu, G., Broeckel, U., Smith, C. A., Turner, V., Haidar, C. E., et al. (2016). Comparison of genome sequencing and clinical genotyping for pharmacogenes. Clin. Pharmacol. Ther. 100, 380–388. doi: 10.1002/cpt.411
Yang, X. Y., Wu, W. J., Yang, C., Yang, T., He, J. D., Yang, Z., et al. (2013). Association of HSD17B3 and HSD3B1 polymorphisms with acne vulgaris in Southwestern Han Chinese. Dermatology 227, 202–208. doi: 10.1159/000353581
Ye, X. Y., Chen, S. Y., Wu, S., Yoon, D. S., Wang, H., Hong, Z., et al. (2017). Discovery of Clinical Candidate 2-((2S,6S)-2-Phenyl-6-hydroxyadamantan-2-yl)-1-(3'-hydroxyazetidin-1-yl)ethanone [BMS-816336], an orally active novel selective 11β-Hydroxysteroid dehydrogenase type 1 inhibitor. J. Med. Chem. 60, 4932–4948. doi: 10.1021/acs.jmedchem.7b00211
Yu, L., Romero, D. G., Gomez-Sanchez, C. E., and Gomez-Sanchez, E. P. (2002). Steroidogenic enzyme gene expression in the human brain. Mol. Cell. Endocrinol. 190, 9–17. doi: 10.1016/S0303-7207(02)00041-2
Yuchi, Y., Cai, Y., Legein, B., De Groef, S., Leuckx, G., Coppens, V., et al. (2015). Estrogen receptor α regulates β-cell formation during pancreas development and following injury. Diabetes 64, 3218–3228. doi: 10.2337/db14-1798
Zacher, A., Kaulich, K., Stepanow, S., Wolter, M., Kohrer, K., Felsberg, J., et al. (2016). Molecular diagnostics of gliomas using next generation sequencing of a glioma-tailored gene panel. Brain Pathol. 27, 146–159. doi: 10.1111/bpa.12367
Zakharov, V., Lin, H. K., Azzarello, J., McMeekin, S., Moore, K. N., Penning, T. M., et al. (2010). Suppressed expression of type 2, 3α/type 5, 17β-hydroxysteroid dehydrogenase (AKR1C3) in endometrial hyperplasia and carcinoma. Int. J. Clin. Exp. Pathol. 3, 608–617.
Zarrabeitia, M. T., Hernandez, J. L., Valero, C., Zarrabeitia, A., Amado, J. A., Gonzalez-Macias, J., et al. (2007). Adiposity, estradiol, and genetic variants of steroid-metabolizing enzymes as determinants of bone mineral density. Eur. J. Endocrinol. 156, 117–122. doi: 10.1530/eje.1.02318
Zeitoun, K., Takayama, K., Sasano, H., Suzuki, T., Moghrabi, N., Andersson, S., et al. (1998). Deficient 17β-hydroxysteroid dehydrogenase type 2 expression in endometriosis: failure to metabolize 17β-estradiol. J. Clin. Endocrinol. Metab. 83, 4474–4480.
Zeng, C., Matsuda, K., Jia, W. H., Chang, J., Kweon, S. S., Xiang, Y. B., et al. (2016). Identification of susceptibility loci and genes for colorectal cancer risk. Gastroenterology 150, 1633–1645. doi: 10.1053/j.gastro.2016.02.076
Zhang, J., Yin, Y., Niu, X. M., Liu, Y., Garfield, D., Chen, S. F., et al. (2013). CYP19A1 gene polymorphisms and risk of lung cancer. J. Int. Med. Res. 41, 735–742. doi: 10.1177/0300060513477291
Zhao, H., Zhou, L., Shangguan, A. J., and Bulun, S. E. (2016). Aromatase expression and regulation in breast and endometrial cancer. J. Mol. Endocrinol. 57 R19–33. doi: 10.1530/JME-15-0310
Zhongyi, S., Rantakari, P., Lamminen, T., Toppari, J., and Poutanen, M. (2007). Transgenic male mice expressing human hydroxysteroid dehydrogenase 2 indicate a role for the enzyme independent of its action on sex steroids. Endocrinology 148, 3827–3836. doi: 10.1210/en.2007-0365
Keywords: intracrinology, endometrium, estrogens, lungs, gastrointestinal tract, central nervous system, bone
Citation: Konings G, Brentjens L, Delvoux B, Linnanen T, Cornel K, Koskimies P, Bongers M, Kruitwagen R, Xanthoulea S and Romano A (2018) Intracrine Regulation of Estrogen and Other Sex Steroid Levels in Endometrium and Non-gynecological Tissues; Pathology, Physiology, and Drug Discovery. Front. Pharmacol. 9:940. doi: 10.3389/fphar.2018.00940
Received: 28 March 2018; Accepted: 02 August 2018;
Published: 19 September 2018.
Edited by:
Walter Jäger, Universität Wien, AustriaReviewed by:
Ashwini Chand, Olivia Newton-John Cancer Research Institute, AustraliaPhilippa Saunders, University of Edinburgh, United Kingdom
Copyright © 2018 Konings, Brentjens, Delvoux, Linnanen, Cornel, Koskimies, Bongers, Kruitwagen, Xanthoulea and Romano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Romano, YS5yb21hbm9AbWFhc3RyaWNodHVuaXZlcnNpdHkubmw=
†These authors have contributed equally to this work
 Gonda Konings
Gonda Konings Linda Brentjens
Linda Brentjens Bert Delvoux1,2
Bert Delvoux1,2 Pasi Koskimies
Pasi Koskimies Andrea Romano
Andrea Romano