
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 11 May 2018
Sec. Ethnopharmacology
Volume 9 - 2018 | https://doi.org/10.3389/fphar.2018.00465
Moringa is a sole genus of Moringaceae family with 13 species distributed in the tropical and sub-tropical regions. Among them, Moringa peregrina is one of the species which has wide range of traditional, nutritional, industrial, and medicinal values. The plant parts are used in folk medicine for many human health care purposes including diabetes, wound healing, disinfectant, fever, constipation, muscle pains, slimness, burns, labor pain, hypertension, malaria, stomach disorder, asthma, skin problems, and to expel a retained placenta. In addition to medicinal value, M. peregrina has cultural, spiritual, and religious connections with the native people of Arabian Peninsula. M. peregrina plant parts were tested for many pharmacological activities viz, antioxidant, anti-microbial, anti-diabetic, anti-spasmodic, hypertension, hepatotoxicity, lipid lowering activity, anti-inflammatory, anti-cancer, and memory disorders. Few active molecules belong to the class isothiocyanate, flavonoid, triterpenoid, phytosterol, polyphenol, and glycoside were also isolated, identified and reported for anti-microbial, anti-oxidant, anthelmintic, anti-mutagenic, neuroprotective, anti-cancer, anti-hypertensive, anti-diabetic, anti-infective, anti-allergic, anti-inflammatory, herbicidal, lipid lowering potential, anti-trypanosomal, and cytotoxic activities. So, the aim of the present review is to provide comprehensive information from recognized sources on the traditional uses, pharmacological efficacy and phytochemistry of the desert medicinal plant, M. peregrina. The information provided in this review will be very useful for further studies to develop novel therapeutic drugs.
Plants play a vital role in cultural, social, religious, environmental, and nutritional aspects. Among all other purposes, the use of plants as medicine for human health originated ~60,000 years ago in the mid-Paleolithic age (Solecki, 1975). To date, 391,000 vascular plants had been identified (Willis and Bachman, 2016). Of these, only about 6% of plants were screened for their biological activity and 15% for their phytoconstituents (Verpoorte, 2000). Among those, Moringa is one of the most important genuses with outstanding economic importance. This genus is potentially used in traditional medicine, pharmacological screening and chemical constituents identification. The genus Moringa consist of 13 species viz., M. arborea, M. borziana, M. concanensis, M. drouhardii, M. hildebrandtii, M. longituba, M. oleifera, M. ovalifolia, M. peregrina, M. pygmaea, M. rivae, M. ruspoliana, and M. stenopetala. The history of Moringa dates back to 150 BC. The taxon name Moringa was derived from the Tamil word “murunggi” or the Malayalam word “muringa” (Quattrocchi, 2000). Historical proof showed that various civilizations viz., Indian, Greek, and Egyptian were using Moringa for thousands of years for several purposes. They preferred to take the leaves and fruits of Moringa in their diet to maintain their skin health and mental fitness. In the warfront, the ancient Maurian warriors of India were fed the leaf extracts of Moringa as it was believed that the decoction relieves them from the pain and stress incurred during the war. Moreover, the drink provides added energy in the war field (Jahn, 1996; Fuglie, 2001; Manzoor et al., 2007). Edible oil with pleasant taste (Ben oil) from the seeds of Moringa was highly valued by the civilizations of ancient Greek, Roman, and Egyptian for protecting their skin and making perfume. Since the middle and old kingdoms (3000–2000 BC), the ben oil was used by the Egyptians (Miller and Morris, 1988; ICUN, 2005).
The previous studies on the Moringa genus were mainly concentrated on M. oleifera (Gilani et al., 1994; Pal et al., 1995, 1996; Anwar et al., 2007; Santos et al., 2012; Stohs and Hartman, 2015; Goswami et al., 2016; Leone et al., 2016; Saini et al., 2016; Asensi et al., 2017; Kalappurayil and Joseph, 2017; Mallya et al., 2017; Mangundayao and Yasurin, 2017) since the species is common in Africa and Asia where the common people are search for nutritional foods in an inexpensive way to meet their demand of food sources (Wangcharoen and Gomolanee, 2011). Recently, M. peregrina is gaining more attention due to traditional, nutritional, industrial and medicinal values. As this plant has wide range of medicinal uses, it has been screened for various pharmacological activities in the past few decades (Marwah et al., 2007; Soltan and Zaki, 2009; Koheil et al., 2011; Dehshahri et al., 2012; Lalas et al., 2012; Al-Owaisi et al., 2014; Moustafa et al., 2014; Majali et al., 2015; Safaeian et al., 2015; Ullah et al., 2015; Alrayes et al., 2016; El-Awady et al., 2016; Azim et al., 2017; Elabd et al., 2017; Saleh et al., 2017). Few active molecules were also isolated, identified and reported for various pharmacological activities. An overview of health applications and salient modes of actions of phytoconstituents from M. peregrina are illustrated in Figure 1. Recently, Robiansyah et al. (2014) reviewed the current status of M. peregrina on its nutrient content, medicinal properties, phenotypic and genetic variation and conservation status. But there was no much information about traditional medicinal values, pharmacological activities and phytochemistry of this plant. Therefore, the present review is aimed to summarize the up-to-date information on the traditional uses, pharmacological activities and phytochemistry of M. peregrina.
M. peregrina is a deciduous tree belonging to the family of Moringaceae. It is a fastest growing tree among the other Moringa species (Abd El-Wahab, 1995) with 3–10 m height and grayish green bark adapted to high aridity. The leaves are 30–40 cm long, alternate, obovate and deciduous. One of the unique features of M. peregrina is the falling of their leaflets when the leaves mature, leaving leaf rachises naked (Robiansyah et al., 2014; Olson et al., 2016). The plant has axillary inflorescence with much branched panicle (18–30 cm long). Flowers are 10–15 mm long, hermaphrodite, zygomorphic, pentamerous, and pinkish white in color with white sepals. A single tree of M. peregrina may produce up to 1,000 pods per year and length of the pods may vary from 20 to 40 cm. Each pod contains 8–15 ovoid, un-winged, trigonous seeds (Afsharypuor et al., 2010). Another unique feature of M. peregrina is the formation of root tuber in the seedling phase (Munyanziza and Yongabi, 2007).
M. peregrina was originated in Arabian Peninsula (Bellostas et al., 2010) and is well-adapted to extreme environmental conditions (Robiansyah et al., 2014). The plant grows in wide geographic range from tropical Africa to East India (Sengupta and Gupta, 1970; Al-Kahtani, 1995; Ghahreman, 2001; Hegazy et al., 2008; Singh et al., 2013). M. peregrina is mostly distributed in South and North Hijaz of Saudi Arabia (Migahid, 1978). Jahn et al. (1986) stated that the plant is indigenous as well as cultivated in Sudan. It also grows in Baluchestan, Southeast and Sistan provience of Iran (Ghahreman, 2001). The plant is widely distributed in Yemen, Somalia, Syria, Palestine (Somali et al., 1984), Jordan (Al-Dabbas et al., 2010), and Oman (Al-Owaisi et al., 2014).
Moringa and its healing potential were documented for the first time around 5000 years ago in the Vedic litrature in India (Patwardhan, 2000). In folk medicine, M. peregrina leaf extract is rubbed over skin to treat paralysis and skin rashes (Ghazanfar and Al-Al-Sabahi, 1993). The pod oil is used to treat the convulsions or infantile paralysis in the northern region of Oman (Miller and Morris, 1988). Its seeds are most commonly used to control diabetes in Sultanate of Oman (Al-Kahtani, 1995; Reddy et al., 2015). It is also effectively used for the diabetes related symptoms such as hyperlipidemia and hyperglycemia in the Indian sub continent. The young leaves of M. peregrina are used traditionally in folk medicine as antioxidant and wound healing in Arab countries. The bark juice is also used as disinfectant (Marwah et al., 2007) and also to treat fever, headache, constipation, back and muscle pains, slimness, burns and labor pain (Boulos, 2000; Elbatran et al., 2005; Tahany et al., 2010). The leaves are used for wound healing (Nawash and Al-Horani, 2011) and seeds are used for abdominal pain (Van der Vossen and Mkamilo, 2007). The roots and leaves of M. peregrina are mixed together with water and used to treat hypertension, malaria, asthma, stomach disorders, diabetes, and to expel a retained placenta (Mekonnen et al., 1999). Traditionally, the oil of this plant is used to treat skin problems such as freckles, itches, and scabies (Al-Dhaheri, 2016).
In addition to their medicinal importance, M. peregrina has significant nutritional importance. The young leaves of M. peregrina can be used as a vegetable (Al-Dhaheri, 2016). The immature seeds are eaten in India and mature seeds are consumed either roasted or fried in Malawi (FAO, 1988; Elbatran et al., 2005; Afsharypuor et al., 2010). In traditional herbal medication, the seeds of the plant are mixed with other herbs and used as food for anti-malnutrition (MPCP, 2006). In addition, M. peregrina is one of the important native trees in the UAE as it has cultural, spiritual, and religious connections. Locally, the leaves of the plant are used to flavor the meat during smoked meat (tanour) preparation. This traditional practice is still followed by the native people of the UAE (Al-Dhaheri, 2016).
M. peregrina parts were tested for broad range of pharmacological activities viz, antioxidant, antimicrobial, anti-diabetic, anti-spasmodic, hypertension, hepatotoxicity, lipid lowering activity, anti-inflammatory, anticancer, and memory disorders (Table 1).
Reactive oxygen species (ROS) are responsible for the initiation and progression of number of human diseases such as cancer, diabetes mellitus, atherosclerosis, cardiovascular diseases, aging, and cirrhosis (Taniyama and Griendling, 2003). The previous studies indicated that, extracts from plants could prevent or delay the above mentioned diseases owing to their redox properties, which allow them to act as free radical scavengers, reducing agents, and hydrogen donors (Robards et al., 1999; Govindarajan et al., 2005). Along these lines, various extracts of M. peregrina were studied for their antioxidant potential. Marwah et al. (2007) studied the antioxidant potential of some plants growing in Sultanate of Oman including M. peregrina, which are edible and used for wound healing activity. The aqueous and ethanol extracts of the plant showed a good DPPH scavenging potential with the inhibition up to 87.8% and the IC50 value of 7.6 μg/ml. The total antioxidant potential as gallic acid equivalents of ethanol extracts of M. peregrina was 814 mg/g. Though, DPPH scavenging potential assay was widely accepted to determine the antioxidant activity of plant extracts, different test methods should be adopted to confirm the potential of the extracts. This single assay can give only a reductive suggestion, because the crude extracts may contain multiple number of compounds with different functional groups (Sacchetti et al., 2005).
Koheil et al. (2011) studied the antioxidant potential of aqueous and ethanol extracts of M. peregrina seeds. Reducing power, chelating effect of ferrous ions, DPPH free radical scavenging potential, superoxide anion scavenging potential, hydrogen peroxide scavenging activity, and hydroxyl radical scavenging ability were investigated to find out the antioxidant ability of both ethanol and aqueous extracts of M. peregrina. It was observed that the reducing power of the extracts was proportionally increased when the concentration was increased equally. At a concentration of 20 μg/ml, the ethanol and aqueous extracts of M. peregrina showed similar reducing power potential similar to that of positive control α-tocopherol (20 μg/ml). The results of the chelating potential of the extracts showed that the activity increased when the concentration was enhanced and chelating activities of ethanol and aqueous extracts were 60 and 37%, respectively at the dose level of 1.50 mg/ml at 90 mts. However, at 1.0 mg/ml concentration the chelating activity of ethanol extract was nearly equal to the positive control EDTA. The ethanol extract of M. peregrina showed the highest DPPH radical scavenging activity at the concentration of 6 mg/ml when compared to control. Whereas, 6 mg/ml concentration of aqueous extract showed free radical scavenging potential which was nearly equal to trolox. Superoxide anion scavenging potential of ethanol and aqueous extracts of M. peregrina were studied at different concentrations viz. 0.1, 0.5, and 1.0 mg/ml and the activity was compared at the same concentration of BHA, ascorbic acid, and trolox. The results revealed that both the extracts showed good superoxide anion scavenging ability than BHA and nearly equal activity to ascorbic acid and trolox. Hydrogen peroxide radical scavenging potential of both the extracts of M. peregrina indicated that the activity was in the manner of concentration dependent. Ethanol and aqueous extracts scavenged 79 and 65% of hydrogen peroxide radicals respectively at a dose of 100 μg/ml. Whereas, at the same concentration, control α-tocopherol, BHA, and BHT scavenged 75, 35, and 28%, respectively. It was observed that, both the extracts showed same hydroxyl radical scavenging potential at the concentration of 20 and 40 μg/ml. On the other hand, 20–40 μg/ml concentration of M. peregrina ethanol extract showed good hydroxyl radical scavenging potential than the ascorbic acid.
Methanol extract of M. peregrina leaves was studied for DPPH free radical and superoxide anion scavenging potential (Dehshahri et al., 2012). The results revealed that the extract scavenged the DPPH radical and superoxide anion radicals with the IC50 values of 8.06 and 47.93 μg/ml, respectively. In vitro antioxidant activity of hexane, chloroform, ethyl acetate and methanol extracts of M. peregrina leaves was studied through the DPPH and H2O2 scavenging potential. All the extracts showed dose dependent DPPH scavenging potential with the IC50 values of 22.36 (hexane), 17.44 (chloroform), 21.87 (ethyl acetate), and 17.07 μg/mL (methanol). The hexane, chloroform, ethyl acetate, and methanol extracts of M. peregrina showed significant H2O2 potential when compared to control. The highest H2O2 radical scavenging potential was observed at 100 μg/mL of all extracts (Al-Owaisi et al., 2014). The antioxidant activity of the plant samples may also be influenced by the solvents used (Abrahim et al., 2012). Furthermore, polar paradox and polar antioxidants are more potent in lipophilic media whereas nonpolar antioxidants are more active in the polar media (Ramadan and Moersel, 2006).
Moustafa et al. (2014) studied the antioxidant activity of methanol extract of M. peregrina along with 199 other wild and cultivated plants in Egypt. DPPH free radical scavenging assay was used to screen the extracts for preliminary antioxidant potential. The methanol extract of M. peregrina showed good antioxidant potential (EC50 values of 4.4 μg/mL). Antioxidant activity of hydro-alcoholic extract of M. peregrina was reported by Ullah et al. (2015). The extract scavenged the ABTS∙+ radical in dose dependent manner and the IC50 value was 20.56 μg/mL. Elabd et al. (2017) reported that the seed oil of M. peregrina showed DPPH scavenging potential at 172 mMol Trolox equivalent/kg.
In comparative study, antioxidant activity (viz. DPPH free radical scavenging potential and total antioxidant capacity) of methanol and aqueous extracts of M. peregrina were studied and compared with M. oleifera (El-Awady et al., 2016). Among the extracts studied, the methanol extract of M. peregrina showed high DPPH scavenging activity (165.49 mg ascorbic acid equivalent g extract) and high reducing power potential (335.89 mg ascorbic acid equivalent/g extract). The leaf extract of M. peregrina showed good DPPH free radical scavenging potential in concentration dependent manner. The IC50 value of the leaf extract was 7.1 μg/ml. While the positive control, ascorbic acid showed the IC50 value of 4.6 μg/ml (Azim et al., 2017). The extract of M. peregrina young leaves showed 74.78% of DPPH free radical inhibition (Juhaimi et al., 2017). It is evident that the reduction of ROS might have helped the management of degenerative diseases (Valko et al., 2006).
Globally, infectious diseases are the predominant cause of the loss of life. Currently, synthetic antibiotics are widely used to prevent or cure several infectious diseases. The indiscriminate use of synthetic antibiotics poses a serious threat to humans (Lin et al., 2018) as multidrug resistance is developed among the disease causing microbes. Therefore, scientists are more focused on plant based drugs which are no/least toxic to treat the infectious diseases. Moreover, it may help to overcome the emergence of multidrug resistance problem. Therefore, extracts of M. peregrina were studied for antiviral, antibacterial and antifungal activities.
In 1997, Mehdi et al. studied the in vitro anti hepatitis B viral activity of ethanol extract of M. peregrina together with 18 other plants parts against HepG2.2.15 cell line (Mehdi et al., 1997). The results showed that the extract of M. peregrina did not inhibit the cell line and survival rate was 100%. Whereas, Soltan and Zaki (2009) screened 42 Egyptian medicinal plants including M. peregrina for their antiviral activity and the authors found that the hydro-alcoholic extract of M. peregrina demonstrated antiviral potential against herpes simplex-1 virus at concentrations range between 50 and 100 μg/mL with Rf 104. But, the extract inhibited the host cells growth though the experiment was conducted at the maximum concentration of 100 μg/mL. M. peregrina extract was found to be inactive against poliomyelitis-1 and vesicular stomatitis viruses.
Disk diffusion method and the determination of minimum inhibitory concentrations were employed to study the antimicrobial potential of M. peregrina seed oil against Staphylococcus epidermidis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella pneumoniae, Candida albicans, C. tropicalis, and C. glabrata. The activities were compared with the standard antibiotics. The results indcated that the oil was effective against all the tested microorganisms. C. glabrata was observed as a most resistant strain among the bacterial and fungal strains. The MIC values of the above mentioned microorganisms were 3.35, 3.50, 4.95, 4.38, 4.80, 4.30, 5.70, 3.30, and 3.25 mg/ml, respectively (Lalas et al., 2012).
Antimicrobial activity of ethanol extract of leaves, seed coat and endosperm of M. peregrina were studied by agar well diffusion assay against bacterial (Bacillus subtilis, Micrococcus luteus, S. aureus, E. coli, P. aeruginosa, and K. pneumonia) and fungal strains (Aspergillus flavus, Aspergillus fumigatus, Aspergillus niger, and C. albicans; Hajar and Gumgumjee, 2014). The leaf extract of M. peregrina showed good antibacterial activity (B. subtilis = 20.0 mm; M. luteus = 23.67 mm; S. aureus = 27.66 mm; E. coli = 19.67 mm; P. aeruginosa = 26.67 mm; and K. pneumonia = 20.67 mm) followed by seed coat (B. subtilis = 18.67 mm; M. luteus = 20.33 mm; S. aureus = 24.0 mm; E. coli = 19.33 mm; P. aeruginosa = 20.67 mm; and K. pneumonia = 13.33 mm) and endosperm (M. luteus = 13.33 mm; E. coli = 17.67 mm; and P. aeruginosa = 16.33 mm. The ethanol extract of M. peregrina endosperm had no activity against B. subtilis, S. aureus, and K. pneumonia. The ethanolic leaf extract also showed good antifungal activity against the tested fungal strains (Leaf extract—A. flavus = 23.33 mm; A. fumigatus = 22.67 mm; A. niger = 18.67 mm; and C. albicans = 24.67 mm: seed coat—A. flavus = 21.67 mm; A. fumigatus = 22.33 mm; A. niger = 17.67 mm and C. albicans = 22.67 mm: and endosperm—A. flavus = 17.33 mm; A. fumigatus = 21.33 mm; A. niger = 14.67 mm; and C. albicans = 20.67 mm). El-Awady et al. (2015) reported the comparative antibacterial activity of M. peregrina and M. oleifera leaf extracts. The methanol and ethanol extracts of both the plants showed antibacterial activity against E. coli, S. aureus, Enterococcus sps, Aeromonas hydrophila and P. aeruginosa. But, when compared to M. oleifera leaf extracts, M. peregrina had less activity.
The antibacterial activity of ethanol extracts of leaves, roots and seeds of M. peregrina extracts were investigated against E. coli, S. aureus, and Klebsiella pneumonia (Majali et al., 2015). The results on the inhibition of studied bacterial strains were concentration dependent. The root extract showed good antibacterial activity against E. coli (18–42 mm), K. pneumonia (44–59 mm), and S. aureus (34–45 mm) followed by ethanol extract of leaf (E. coli = 14–30 mm; K. pneumonia = 8–19 mm; and S. aureus = 9– 22 mm) and seed extract (E. coli = 16–38 mm; K. pneumonia = 6–32 mm; and S. aureus = 6–18 mm). The minimum inhibitory concentrations values of the M. peregrina extracts were 12.0 (E. coli), 15.0 (K. pneumonia) and 18 mg/ml (S. aureus) for leaf extract, 13.0 (E. coli), 7.0 (K. pneumonia), and 9.0 mg/ml (S. aureus) for seed extract and 3 (E. coli), 5.0 (K. pneumonia), and 2.0 mg/ml (S. aureus) for roots extracts.
Different extracts (viz. acetone, methanol, ethanol, and aqueous) of both in vitro plantlets and field grown samples were studied for their antibacterial activity against K. oxytoca, Salmonella typhimurium, Methicillin resistant S. aureus, K. pneumonia, Proteus vulgaris, Proteus mirabilis, Enterobacter aerogenes, P. aeruginosa, E. coli O157:H7, S. aureus, Salmonella paratyphi, and E. coli ATCC 29522. The results revealed that in vitro plantlets of M. peregrina showed significant antibacterial potential when compared to field grown samples. At a concentration of 40 mg/100 μl, the ethanol extract of in vitro plantlets of M. peregrina showed the highest zone of inhibition against S. aureus (Alrayes et al., 2016). Aqueous extract of M. peregrina seeds was investigated for antibacterial activity against clinically isolated multidrug resistant Salmonella species (Saleh et al., 2017). The results showed that the extracts exhibited good antibacterial activity against the multidrug resistant Salmonella isolates. The minimum inhibitory concentration of the extract ranged between 109.37 and 437.5 mg/mL. These results support the use of M. peregrina as disinfectant in the folk medicine and further studies can be focused on the isolation of novel antimicrobial molecules to treat the infections caused by microbes.
Diabetes mellitus is one of the most common metabolic disorders that resulted in significant morbidity and mortality rate (Deshpande et al., 2008). The chronic hyperglycemia of diabetes is associated with prolonged dysfunction, damage, and failure of different organs particularly kidneys, heart, eyes, and blood vessels. There is an increasing demand for traditionally used medicinal plants to manage the diabetes mellitus and its complications since the use of insulin and oral hypoglycemic agents are associated with side effects (Holman and Turner, 1991; Rao et al., 2001). Furthermore, medicinal plants are inexpensive, easily accessible and less or no toxic. Previously, many medicinal plants including M. peregrina were reported for hypoglycemic properties (Ahmed et al., 2010).
El-Haddad et al. (2002) reported the antidiabetic activity of hydroalcoholic extract fraction of M. peregrina seeds on streptozotocin induced diabetic rats. The administration of hydroalcoholic extract decreased the blood glucose level significantly at the dose of 200 mg/kg b.w. Also the chloroform and petroleum ether fractions decreased the blood glucose level. Furthermore, the histopathological study indicated that the hepatocytes of chloroform treated rats were non toxicated and regenerated the streptozotocin induced diabetic effect. The antidiabetic effect of aerial parts of M. peregrina ethanolic extract on streptozotocin induced diabetic rats was reported by Elbatran et al. (2005). The extract significantly decreased the levels of serum glucose, aspartate aminotransferase, and alanine aminotransferase. Also the administration of ethanolic extract of M. peregrina has decreased the serum triglycerides, cholesterol and low density lipoprotein. Whereas, the extract increased the level of high density lipoprotein. In toxicological study, the M. peregrina extract increased the respiration rate, general depression, mucous membrane cyanoses, righting reflex loss, convulsion and death. The LD50 value of alcoholic extract was 113.4 mg/100 g body weight.
The ethanol and aqueous extracts of M. peregrina seeds were studied for their anti-diabetic potential in streptozotocin induced diabetic rats through tissue lipid peroxides and enzymatic antioxidant (Koheil et al., 2013). Both the extracts were treated by oral administration at a dose of 150 mg/kg body weight. The results indicated that the blood glucose levels were reduced in the rats administrated with M. peregrina seed extracts and glibenclamide (anti-diabetic drug) when compared to untreated diabetic rats. The results on the levels of thiobarbaturic acid reactive substances, nitric oxide, reduced glutathione and hydroperoxides in liver and kidney of treated rats proved that the administration of aqueous ethanolic extracts of M. peregrina and glibenclamide were tend to bring down the nitric oxide and reduced glutathione values near to the normal level. The enzymatic antioxidants such as catalase, superoxide dismutase, glutathione peroxidase, glutathione-S-transferase were significantly low in liver and kidney of diabetic control rats when compared to the diabetic rats administrated with ethanol and aqueous extracts of M. peregrina seeds and glibenclamide. The diabetic rats administrated with the extracts of M. peregrina and glibenclamide showed a decreased level of glycosylated hemoglobin, increased levels of total hemoglobin and plasma insulin when compared to the diabetic control level.
The hydro-alcoholic extract from the dried leaves of M. peregrina showed inhibitory potential against three in vitro model enzyme assays viz. α-glucosidase, α-amylase, and dipeptidyl peptidase IV (Ullah et al., 2015). The results on pancreatic α-amylase inhibitory activity of M. peregrina extract suggested that the enzyme responded to the extract when the concentration was increased. The IC50 value of the extract was 1335.89 μg/mL. M. peregrina extract demonstrated moderate mammalian intestinal α-Glucosidase enzyme inhibitory potential with the IC50 value of 3256.68 μg/mL. Whereas, the extract gradually inhibited the activity of mammalian DPP IV enzyme in a dose dependent manner (IC50 value of 1218.12 μg/mL).
Antispasmodic drugs are prescribed frequently for numerous gastrointestinal illnesses (N'Guessan et al., 2015). Most of the antispasmodic drugs contain antimuscarinic compounds and calcium channel blockers (Farhadi et al., 2001; Pasricha, 2001) and the consumption of these drugs may associate with unwanted side effects. Medicinal plants which are used in folk and traditional medicine for gastrointestinal disorders have been validated through pharmacological studies for antispasmodic activity (Hajhashemi et al., 2000; Sadraei et al., 2003). The results showed that the investigated medicinal plants recorded significant antispasmodic potential (Cortés et al., 2006; Cechinel-Filho et al., 2007). Similarly, the anti-spasmodic potential of hydroalcoholic extract from the leaves and seeds of M. peregrina was studied by Sadraei et al. (2015) by ileum contractions induced by 80 mM KCl, 250 μM of acetylcholine (ACh) and electrical field stimulation (EFS). Both the extracts have an inhibitory potential on ileum contractions. The seeds extract of M. peregrina had more potential inhibitory effect of ileum contraction induced by KCl (IC50 = 87 ± 18 μg/ml); ACh (IC50 = 118 ± 18 μg/ml), and EFS (I IC50 = 230 ± 51 μg/ml). Whereas, the leaf extract also showed inhibitory effect of ileum contraction (KCl—IC50 = 439 ± 108 μg/ml; ACh—IC50 = 365 ± 61 μg/ml; EFS—IC50 = 314 ± 92 μg/ml). Further investigation on bio assay guided isolation is required to identify the active molecule which could be an alternative and safer anti-spasmodic molecule for future use.
Hypertension is a cardio vascular disease and it is one of the leading causes of death worldwide. Various anti-hypertensive drugs have been developed for the treatment of hypertension. But the drugs showed efficacy along with associated side effects (Alamgeer et al., 2017). Investigations on edible and medicinal plants remain important since it has potential benefits (Kalia, 2005). Based on the edible importance as well as the traditional uses, the hydroalcoholic extract of M. peregrina was investigated on blood pressure and oxidative status in hypertensive rats induced with dexamethasone. Systolic blood pressure, thymus weight, body weight, plasma hydrogen peroxide concentration, plasma ferric reducing antioxidant power were measured after the treatment. The results of the prevention study proved that the extract of M. peregrina prevents the rise of systolic blood pressure at 400 mg/kg dose level. Whereas, the reversal study indicated that M. peregrina extract failed to lower the SBP in dexamethasone induced hypertension in rats. The oral administration of M. peregrina extract had no significant effect on the loss of thymus weight and also the extract was botched to prevent the body weight changes. In contrast, Rouhi-Broujeni et al. (2013) reported that the hydroalcoholic extract from the seeds of M. peregrina decreased the mean body weight. In the prevention study, treatment with 200 and 400 mg/kg of extract prevented the rise of H2O2 concentration. Whereas, in the reversal study, a dose of 400 mg/kg M. peregrina extract reduced the elevated plasma hydrogen peroxide concentration. In prevention as well as reversal study, the rats administrated with 400 mg/kg of M. peregrina extract significantly reduced the plasma ferric reducing antioxidant power (Safaeian et al., 2015). So, the antihypertensive activity might be linked with the availability of antioxidant molecules present in M. peregrina. The antioxidant molecules showed significant role in reducing the level of blood pressure (Duarte et al., 2001; Jalili et al., 2006).
The liver is an important organ and plays vital functions in the human body by regulating many biochemical pathways (Sharma et al., 1991). Hepatotoxicity caused by certain drugs/antibiotics, chemicals, microbial infections, and consumption of alcohol is a major concern. Protection of liver using medicinal plants is the best alternative and many plants were reported for anti-hepatotoxicity effect. The seed oil of M. peregrina was used for its proteceive effect against doxorubicin induced hepatotoxicity in mice. The reduction in caspase-3 immunoreactivity and apoptotic index were noted in M. peregrina seed oil treated mice. Seed oil with the dose of 150 mg/kg treatment reduced the liver damage induced by doxorubicin (Sliai and Abdel-Rahman, 2016). The seed oil of M. peregrina was studied along with other two Moringa species for liver tissue oxidative stress state in high fat diet induced liver damage. Hepatic marker enzymes, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, total serum cholesterol, triacylglycerol, glucose, lipid peroxidation, antioxidant enzymes such as catalase, superoxide dismutase, and glutathione peroxidase were analyzed after the treatment. The results indicated that glucose, total serum cholesterol, alanine transaminase, aspartate transaminase, and body weights significantly increased in the rats fed with high fat diet. The post administration of seed oil was significantly improved the liver enzymes, lipid profile, and glucose content (Elabd et al., 2017). The hepatoprotective effect of ethanol leaf extract of M. peregrina through oral administration showed that the extract significantly decreased the activities of serum hepatic marker enzymes. The effect of M. peregrina leaf extract on oxidative stress markers of acetaminophen induced hepataotoxicity showed that the administration of extract was successful in replenishing the reduced glutathione level in the liver, blood, and brain. In the meantime, the superoxide dismutase, catalase, and glutathione peroxidase activities were reduced significantly in the rats intoxicated with acetaminophen. The superoxide dismutase, catalase and glutathione peroxidase activities enhanced in successful way when the M. peregrina leaf extract was administrated (Azim et al., 2017). In literature, triterpenoids have been reported as one of the most important anti-hepatotoxic agents. In the past few decades, more than 350 triterpenoids have been reported for hepatoprotective potential (Xu et al., 2018). So, structure-based investigations are advised to isolate a potent hepatoprotective agent from M. peregrina.
Hyperlipidemia is closely associated with the coronary heart disease. Hence, lipid lowering therapy alone with the management of other risk factors is advised to prevent the cardiovascular diseases (Jessani et al., 2006). Several drugs are available in the market for lipid related disorders. However, maintenance of lipid homeostasis after the treatment and avoidance of it's the side effect is not an easy task (Pahan, 2006). Thus, plants with medicinal importance are the promising source of lipid lowering active molecule. Hydroalcoholic extract of M. peregrina seeds were studied for lipid lowering activity on hyperlipidemic rats (Rouhi-Broujeni et al., 2013) by determining the profile of serum lipid, malondyaldehide, level of thiol, antioxidant capacity, cardiopulmonary resuscitation, ferritin and atherogenic index. The results revealed that the extract of M. peregrina significantly reduced the lipid levels such as total cholesterol of plasma, level of LDL-C and VLDL and increased the level of HDL-C in hyperlipidemic rats which is comparable with the lipid lowering activity of the control drug, atorvastatin. Also the level of thiol and carbonyl in the rats administrated with M. peregrina extract were same as drug treated rats. The high level of antioxidant capacity and a decreased atherogenic index were also observed in the rats treated with M. peregrina extract.
In many physiological processes, inflammation is an essential part in response to host defense and the damage of tissues. After injury, the wound healing process starts immediately and the processes comprise of three phases viz. inflammation, proliferation, and maturation. The first phase provides resistance to the microbial contaminations (Kondo, 2007) and the anti-inflammatory activity is essential to minimize the healing period (Shimizu et al., 2000). Ethanol and aqueous extracts of M. peregrina were studied for anti-inflammatory potential using fresh egg albumin induced inflammation (oedema) in rats (Koheil et al., 2011). The results revealed that the aqueous and ethanol extracts significantly reduced the acute inflammation induced by fresh egg albumin. At a dose level of 300 mg/kg, aqueous and ethanol extracts reduced the inflammation by 72.96 and 81.01%, respectively at the third hour after the oedema was induced. Whereas, the control drug diclofenac at the dose level of 100 mg/kg reduced the inflammation by 100% at the third hour.
Resistance in cancer therapy is a serious issue and it remains as a major cause of death (Batist et al., 2011). The resistance can be developed through various biological mechanisms including reduced drug uptake, increased drug efflux and cellular pathway changes (Tan et al., 2016). It is well-known that plant molecules can be an alternative to the synthetic anticancer drugs to overcome its resistance. Globally, more than 3000 plants have been studied for anticancer properties (Solowey et al., 2014) including M. peregrina. In vitro anti-cancer properties of seed oil of M. peregrina was studied on various cell lines such as MCF-7 (breast cancer cell line), HepG2 (liver cancer cell line), CACO-2 (colon cancer cell line), HeLa (cervical cancer cell line), and L929 (mouse fibroblasts). A significant cytotoxic potential was observed against all the cell lines tested and activity was dose dependent manner. One milligram of the seed oil showed the highest cytotoxic potential against the tested cell lines. Cell viability decreased to 24.65, 24.18, 42.51, 46.57, and 32.11% and the IC50 values of the oil were 366.3, 604.3, 850.9, 721.7, and 935.8 μg/mL for HeLa, HepG2, MCF-7, CACO-2, and L929 cell lines, respectively (Elsayed et al., 2016). Based on these results, extensive investigation on the isolation of anticancer molecule is recommended. It could help to overcome the resistance issue as well the lowering the treatment cost.
Age related neurodegenerative diseases namely Parkinson's, Huntington's, and Alzheimer's diseases are increased among the human population (Aruoma et al., 2003). Recently, investigations are ongoing to develop new strategies to reduce the disease progression since there is no effective cure for above mentioned disorders (Abushouk et al., 2017). Recent studies indicate that medicinal plants showed good neuroprotection (de Rus Jacquet et al., 2017; Zhang et al., 2017). The neuroprotective effect of aqueous extract from the leaves of M. peregrina was investigated and reported by studying the learning capacity and memory in mice (Elsaey et al., 2016). Four doses of the extract were administrated and memory test was performed at two different Zeitgeber times (3:00-rest phase and 15:00-active phase). Insulin was administrated intranasal were treated as positive control. The results on the memory performance showed that intranasal administration of the extract improved the functions of memory close to the positive control insulin. The subchronic administration of the extract at the dose of 25 mg/kg showed significant differences at the Zeitgeber time 3:00 on memory and learning. Whereas, subchronic administration had no significant difference on memory and learning at the Zeitgeber time 15:00. Also it was observed that, in acute administration, there was no locomotor activity observed after intranasal administration of single dose of M. peregrina extract neither with any dose nor at both Zeitgeber times. Also there was no significant difference on locomotor activity in sub-chronic administration of the extract. Based on the results, it was concluded that the aqueous extract of M. peregrina enhanced the memory function of scopolamine induced amnesia in mice.
The desirable therapeutic effects of plant extracts may typically result from the combination of two or more compounds. So, the preliminary phytochemical quantification is essential to correlate the biological activity and it may also help to conduct further studies to discover the particular classes of secondary metabolites. Ullah et al. (2015) screened the hydro alcoholic extract from the dried leaves of M. peregrina for its phytochemical compounds. The preliminary quantification analyses showed the presence of major classes of compounds such as alkaloids, tannins, phenolics, and saponins at different concentrations. These phytochemicals are considered to possess extensive range of biological activities (Ramawat et al., 2009). Saponin was recorded in high concentration in the hydroalcoholic extract of M. peregrina when compared to other compounds. Dehshahri et al. (2012) reported the presence of flavonoid glycoside (rutin) in the air dried methanol extract of M. peregrina. But the authors were abortive to provide the detailed spectral and structure information.
A study was made to quantify the total phenol and flavonoid contents in hexane, chloroform, ethyl acetate, and methanol extracts of M. peregrina leaves. The total content of phenols in chloroform, ethyl acetate and methanol were 75.53, 81.26, and 94.56 GAE/g of dry extract, respectively. The results on the total content of flavonoids revealed that 6.55, 8.39, and 20.81 mg of QE/g were present in chloroform, ethyl acetate, and methanol extracts of M. peregrina leaves, respectively. On the other hand, phenol and flavonoid contents were not detected in the hexane extract of M. peregrina. Also hexane, chloroform, ethyl acetate, and methanol extracts of M. peregrina leaves were analyzed by GC-MS to identify the presence of chemical compounds. A total of 32 compounds were identified in all the extracts and all the major chemical compounds namely ethanone, 1-cyclohexyl- (27.26%), pentacosane (17.11%), hexacosane (16.57%), tetracosane (15.45%), heptacosane (13.02), tricosane (11.79%), octacosane (9.10%), cyclopentanol, 1-methyl (8.08%), and 2-heptanone, 3-methyl (7.36%) were identified in hexane extract of M. peregrina leaf extracts except p-xylene (10.67%) which was identified in methanol extract (Al-Owaisi et al., 2014).
Safaeian et al. (2015) quantified the total phenol content of dried leaves of M. peregrina by Folin-ciocalteu method. The results showed that the dried leaves of M. peregrina had 2.3 mg TAE/g of total phenol content. The bark and seed oils of M. peregrina contain 454 and 12.6 mg/kg of total phenol content, respectively (Marwah et al., 2007; Elabd et al., 2017). The quantification of total phenol and flavonoid contents of aqueous and methanol extracts of M. peregrina were reported by El-Awady et al. (2016) Methanol extract showed a higher amount of total phenol content (137.53 mg gallic acid equivalent/g extract) when compared to aqueous extract (92.26 mg gallic acid equivalent/g extract). The methanol extract also showed high amount of total flavonoid content (33.40 mg quercetin equivalent/g extract) compared to aqueous extract (9.59 mg quercetin equivalent/g extract) of M. peregrina.
Saleh et al. (2017) reported the presence of oleic acid-3 hydroxy propyl ester in oily aqueous extract of M. peregrina seeds. Though, the authors claimed to successfully purify the compound by GLC and TLC techniques and identified by IR, NMR, and GC-MS, the structure details were not provided. Total flavonoid and phenol content of young leaves of M. peregrina were quantified by Juhaimi et al. (2017). The extracts had 35.50 mg catechol gDW−1 of total flavonoid and 45.90 mg gallic acid gDW−1 of total phenolic compounds. Moreover, the phenolic compounds such as gallic acid (0.930 mg/100 g/DW), protocatechuic acid (0.070 mg/100 g/DW), catechin (0.120 mg/100 g/DW), 4-hydroxybenzoic acid (0.740 mg/100 g/DW), caffeic acid (0.250 mg/100 g/DW), syringic acid (0.08 mg/100 g/DW), rutin trihydrate (0.020 mg/100 g/DW), trans p-coumaric acid (0.140 mg/100 g/DW), chlorogenic acid (0.030 mg/100 g/DW), trans-ferulic acid (0.19 mg/100 g/DW), fisetin (0.030 mg/100 g/DW), trans-resveratrol (0.11), quercetin (0.020 mg/100 g/DW), trans-cinnamic acid (0.270 mg/100 g/DW), naringenin (0.050 mg/100 g/DW), and isorhamnetin (0.330 mg/100 g/DW) were also deducted in M. peregrina extracts.
Azim et al. (2017) reported the presence of flavonoids and phenolic compounds in the leaves of M. peregrina by HPLC. The highest concentration of flavonoid, reported in mg/100 g dry extract, was rutin (487.3) followed by naringin (45.43), vitexin (16.52), quercetin (14.32), quercetrin (6.96), apigenin (5.43), rosmarinic (3.67), hesperetin (2.27), kaempferol (1.82), naringenin (1.1) and 7-OH flavone (0.31). Whereas 3-OH-tyrosol was recorded in the highest concentration (1763.74 mg/100 g) among phenolic compounds followed by acid vanillic (485.25), protochatecuic acid (444.43), epicatechin (413.1), pyrogallol (243.14), catechol (165.65), salicylic acid (157.65), chicoric (96.9), chlorogenic acid (93.42), benzoic acid (73.57), caffeine (64.31), ellagic acid (60.82), ferulic acid (52.1), p-OH-benzoic (51.72), caffeic acid (51.44), iso-ferulic acid (29.8), 4-amino-benzoic acid (23.1), resveratrol (19.5), gallic acid (15.1), p-coumaric acid (12.1), 3,4,5-methoxy-cinnamic acid (10.5), coumarin (8.24), cinnamic acid (6.02), and p-coumaric acid (1.96). All values were expressed in mg/100 g dry weight.
Essential oil obtained from the medicinal and aromatic plants gained attention as a potential source in pharmaceutical and food industry due to its efficacy and safety. The volatile oils of leaf and seed kernel of M. peregrina were analyzed by GC and GC-MS for its chemical composition. Isobutyl isothiocyanate was identified as a major chemical constituent in both leaf and seed kernel oil with 88.5 and 94%, respectively. Other chemical compounds in leaf oil were isopropyl isothiocyanate (10.2%), n-butyl isothiocyanate (0.4%), and hexadecanoic acid (0.2%). Trace amount of sec-butyl isothiocyanate was also deducted in the leaf volatile oil of M. peregrina. In seed kernel oil, 4.9% of isopropyl isothiocyanate, 0.5% of sec-butyl isothiocyanate, and n-butyl isothiocyanate were identified. In addition, trace amount of n-tridecane, dihydro-α-curcumene, benzyl isothiocyanate, n-pentadecane and hexadecanoic acid were also identified in the volatile oil of seed kernel. These compounds were not present in leaf volatile oil of M. peregrina except hexadecanoic acid (Afsharypuor et al., 2010).
Salehi et al. (2014) studied the effect of salinity on volatile oil composition of shoot and root of M. peregrina. Different levels of salinity viz. 2, 4, 6, 8, 12, and 14 dS/m were used to treat the plants and the oil composition was examined by GC-MS. The results indicated that the salinity levels altered the quantity and composition of volatile oil. In control plants, 1,2-benzenedicarboxylic acid, bis (2-methyl propyl) ether was identified as the major compound (29.02%) in the shoots. But in roots, thiocyanic acid, phenylmethyl ester was the major compound. Benzyl isothiocyanate (29.6%) was identified as the major compound in the root samples of M. peregrina in all salinity levels. In shoots of M. peregrina, isobutyl isothiocyanate was identified as the major compound at 2 and 4 dS/m. Despite in other salinity levels n-butylisothiocyanate was identified as the major compound.
The seed oil of M. peregrina was studied by several researchers for its characteristics and chemical composition, mainly on fatty acid. GC analysis of fatty acid methyl esters of M. peregrina seed oil showed the presence of 9 fatty acids viz., myristic acid (trace), palmitic acid (9.3%), lauric acid (2.4%) stearic acid (3.5%), oleic acid (78.0%), linoleic acid (0.6%), linolenic acid (1.6%), arachidic acid (1.8%), and behenic acid (2.6%). Among the fatty acids detected, 84.7% were unsaturated fatty acids and 14.7% were saturated fatty acids (Somali et al., 1984). Gas-liquid Chromatography analysis of aerial parts of M. peregrina revealed the presence of 13 fatty acids. Palmitic acid was recorded as the major fatty acid (24.68%) followed by myristic acid (16.43%) and linoleic acid (13.60%). Heptadecanoic acid was identified as the lowest (1.38%) fatty acid content. The unsaponifiable matter analysis of M. peregrina indicated that β-sitosterol as the major (28.79%) steroidal component (Elbatran et al., 2005).
In 1998, Tsaknis reported the full characterization and chemical composition of M. peregria seed oil collected from the Kondom of Saudi Arabia (Tsaknis, 1998). The degummed oil of M. peregria had ten fatty acids namely capric acid (0.08%), myristic acid (0.10%), palmitic acid (8.90%), stearic acid (3.82%), oleic acid (70.52%), linoleic acid (0.62%), arachidic acid (1.94%), paullinic acid (1.50%), behenic acid (2.36%), and erucic acid (0.49%). Similar results were obtained with the oil of the seeds collected from Wadi Fenan, Jordan (Al-Dabbas et al., 2010). The analysis of the seed oil showed the presence of high amount of unsaturated fatty acids (83.5%) than saturated fatty acids (16.53%). Among that oleic acid was identified as the major one (74.81%) followed by palmitic acid (8.95), oleic acid (3.72%), stearic acid (3.08%), behenic acid (2.59%), palmitoleic acid (2.28%), arachidic acid (1.73%), eicosenoic acid (1.62%), lingnoceric acid (0.46%), linoleic acid (0.46%), margaric acid (0.12%), myristic acid (0.08%), margaroleic acid (0.08%), and linolenic acid (0.05%). Furthermore, sterol composition analysis of both the oils showed that β-sitosterol as the major one followed by stigmasterol, campesterol and δ-5-avenasterol.
GC analysis showed that the seed oil of Iranian M. peregrina contains 82.6% of unsaturated and 17.2% of saturated fatty acid contents. Oleic acid (77.9%) was found to be the major fatty acid followed by palmitic acid (9.3%), stearic acid (3.5%), behenic acid (2.6%) acid, palmitoleic acid (2.5%), arachidic acid (1.8%), linolenic acid (1.6%), and linoleic acid (0.6%) (Gharibzahedi et al., 2013).
Fatty acid composition analysis of M. peregrina seed oil by Al-Rawashdeh et al. (2013) revealed that a total of 13 fatty acids were detected in the oil. Oleic acid (78.79%) was identified as the dominant fatty acid, followed by palmitic acid (8.17%), stearic acid (3.85%), behenic acid (2.60%), arachidoic acid (1.99%), palmitoleic acid (1.87%), gadoleic acid (1.57%), linoleic acid (0.47%), lignoceric acid (0.42%), heptadecanoic acid (0.11%), myristic acid (0.07%), heptadecenoic acid (0.07%), and linolenic acid (0.02%).
Fatty acid composition of M. peregrina leaf oil was investigated along with two other Moringa species (Al-Juhaimi et al., 2016). A total of 12 fatty acids were detected in the leaf oil and linolenic acid recorded the highest (32.53%). Furthermore, considerable amount of linoleic acid (19.5%), palmitic acid (17.6%), oleic acid (7.14%), and arachidic (4.85%) were also detected by GC analysis. Other fatty acids present in the leaf oil of M. peregrina were myristic acid, stearic acid, palmitoleic acid, lignoceric acid, behenic acid, and erucic acid with 2.27, 1.96, 1.42, 1.37, 1.19, and 0.76%, respectively. The fatty acid composition analysis (Elabd et al., 2017) revealed that the seed oil of M. peregrina contained palmitic acid (10%), linoleic acid (0.42%), oleic acid (78.33%), linolenic acid (2.75%), stearic acid (4.85%), arachidic acid (1.42%), and behenic acid (2.15%).
Medicinal plants are the dominant resource for wide range of molecule structures which helps for the discovery and development of new therapeutic drugs. The therapeutic value of the medicinal plants completely depends on the presence of phytoconstituents and the major group of bio-active compounds present in the plants such as alkaloids, glycosides, flavonoids, proanthocyanidins, tannins, terpenoids, phenylpropanoids, resins, lignans, furocoumarines, naphthodianthrones, proteins, and peptides (Bernhoft, 2010).
The literature indicates so far only few studies were made to isolate and identify the phytochemicals from M. peregrina. Chemical constituents isolated from M. peregrina, plant parts used for isolation, chemical nature and its reported pharmacological activities are illustrated in Table 2 and the molecular structure of the isolated compounds are given in Figure 2. Kær et al. (1979) reported eight isothiocyanates viz. benzyl isothiocyanate (1), 2-propyl isothiocyanate (2), 2-butyl isothiocyanate (3), 2-methylpropyl isothiocyanate (4), 4(α-L-rhamnosyloxy) benzyl isothiocyanate (5), 4-(4′-O-Acetyl-α-L-rhamnosyloxy benzyl isothiocyanate (6), glucosinolate (7) and 5,5-dimethyl-oxazolidine-2-thione (8) from the seeds of M. peregrina. But, no biological potential of these compounds were demonstrated. However, benzyl isothiocyanate was reported for anthelmintic (Kermanshai et al., 2001), vascular contraction (Wilson et al., 2002), and antibacterial (Jang et al., 2010) activities. Antimicrobial (Padla et al., 2012), productive effect on spinal cord trauma (Giacoppo et al., 2015) and neuroprotective (Galuppo et al., 2015) activities were also reported for 4-(α-L rhamnosyloxy) benzyl isothiocyanate. Vig et al. (2009) extensively reviewed the antimicrobial, antioxidant, herbicidal, antiproliferative and antimutagenic potential of Glucosinolate. El-Haddad et al. (2002) isolated three nitrile glycosides viz, niazirin (9), niazirinin (10) and 4-(4′- O-methyl-α-L-rhamnosyloxy benzyl nitrile (11) from the seeds of M. peregrina. However, the pharmacological potential of niazirin, niazirinin and 4-(4′- O-methyl-α-L-rhamnosyloxy benzyl nitrile were not extensively studied. The aerial parts of M. peregrina yielded four flavonoid compounds namely quercetin (12), 6,8,3′,5′-tetramethoxy apigenin (13), chrysoeriol-7-O-rhamnoside (14), and rutin (15) (Elbatran et al., 2005). Wild distribution of flavonoid compounds in plant kingdom were noted in several studies over the past decades and its concentration varied from plant to plant or even in different parts of the same plant (Justesen and Knethsen, 2001; Dinelli et al., 2006). Flavonoids provids significant health care benefits such as anti-oxidant, antimicrobial, anti-inflammatory, anti-allergic, anti-artherogenic, and cardioprotective (Manach et al., 2005). Quercetin isolated from the aerial parts of M. peregrina is one of the important bioflavonoids present in many plants and its various biological activities were already proved in many studies which were complied in several review articles (Sultana and Anwar, 2008; Salvamani et al., 2014; David et al., 2016). Rutin has also received more attention due to its pharmacological activities such as anti-microbial, anti-allergic, anti-diabetes, anti-hypertension, and anti-cancer (Sharma et al., 2013; Gullon et al., 2017).
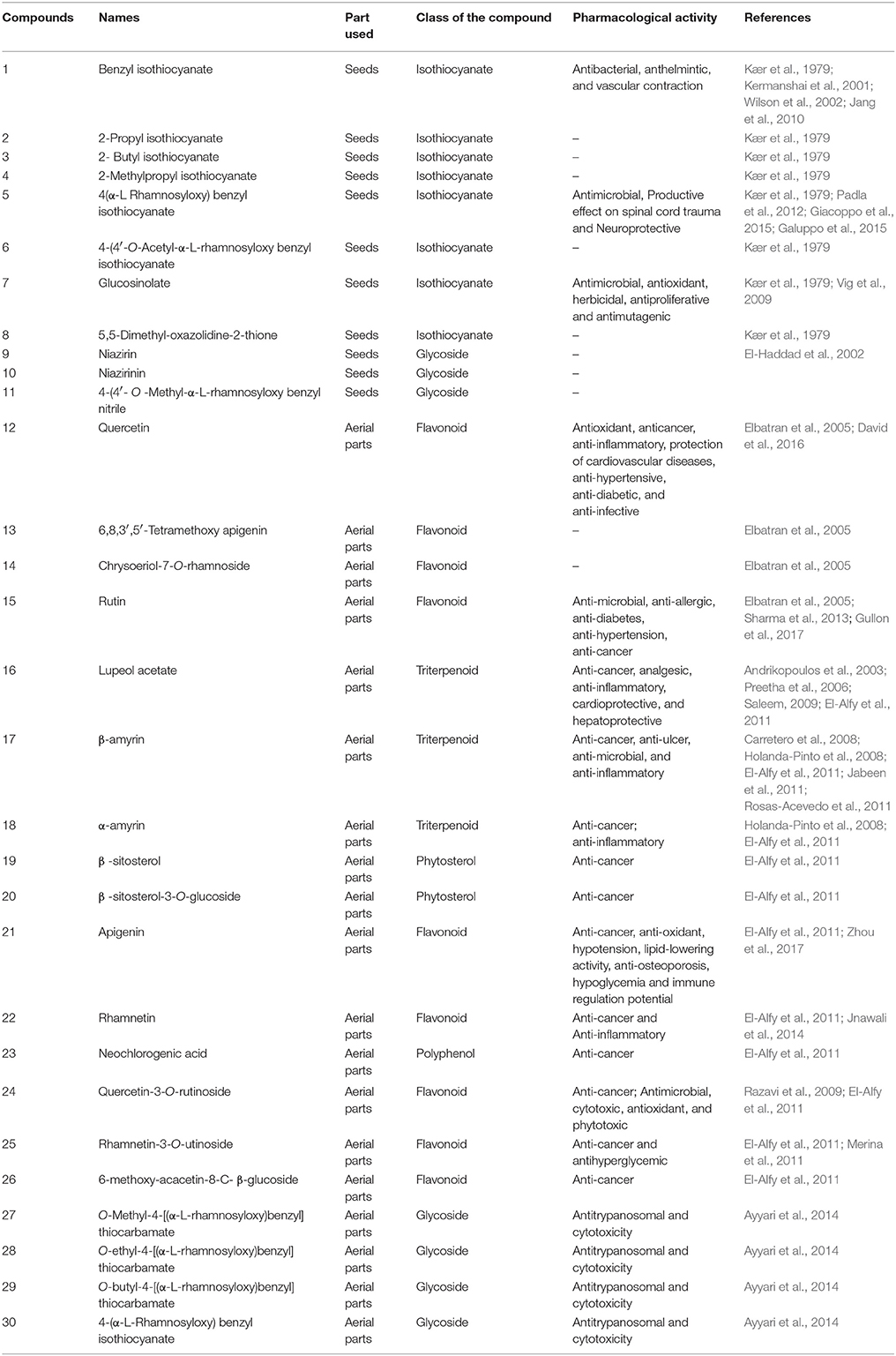
Table 2. Name of the chemical constituents isolated from M. peregrina, parts used, chemical nature and its reported pharmacological activities.
In 2011, El-Alfy et al. isolated bio-active molecules which include seven flavonoids [quercetin (12), chrysoeriol-7-O-rhamnoside (14), apigenin (21), rhamnetin (22), Quercetin-3 -O-rutinoside (24), Rhamnetin-3-O-utinoside (25) and 6-methoxy-acacetin-8-C- β-glucoside (26)], three triterpenoids [lupeol acetate (16), β-amyrin (17) and α-amyrin (18)], two thytosterols [β-sitosterol (19) and β-sitosterol-3-O-glucoside (20)] and one polyphenol [neochlorogenic acid (23)]. The isolated compounds were also investigated for anti-cancer activity against breast and colon cancer cell lines and all the compounds found to be good cytotoxic potential on the cell lines tested. Various other pharmacological activities of the above mentioned compounds have been well-documented in the previous studies. For example, lupeol acetate was reported for analgesic, anti-inflammatory, cardioprotective and hepatoprotective potentials (Andrikopoulos et al., 2003; Preetha et al., 2006; Saleem, 2009). β-amyrin and α-amyrin molecules had close structural relationship with lupeol acetate and known for biological activities such as anti-cancer, anti-ulcer, anti-microbial, and anti-inflammatory (Carretero et al., 2008; Holanda-Pinto et al., 2008; El-Alfy et al., 2011; Jabeen et al., 2011; Rosas-Acevedo et al., 2011). Apigenin is a natural flavonoid and has many pharmacological activities (anti-oxidant, hypotension, lipid-lowering activity, anti-osteoporosis, hypoglycemia, and immune regulation potential) which are recently reviewed by Zhou et al. (2017). A study performed by Razavi et al. (2009) indicated that quercetin-3-O-rutinoside had antimicrobial, cytotoxic, antioxidant and phytotoxic potential. Rhamnetin-3-O-rutinoside also a flavonoid showed good antihyperglycemic activity on alloxan induced diabetic rats (Merina et al., 2011). Ayyari et al. (2014) isolated three thiocarbamate [O-methyl-4-[(α-L-rhamnosyloxy)benzyl] thiocarbamate (27), O-ethyl-4-[(α-L-rhamnosyloxy)benzyl] thiocarbamate (28) and O-butyl-4-[(α-L-rhamnosyloxy)benzyl] thiocarbamate (29)] and one isothiocyanate [4-(α-L-rhamnosyloxy) benzyl isothiocyanate (30)] glycosides from the aerial parts of M. peregrina. The isolated compounds were studied for in vitro cytotoxicity in rat skeletal myoblasts and anti-trypanosomal efficacy against Trypanosoma brucei rhodesiense. The results revealed that thiocarbamate glycosides had moderate in vitro effect, whereas, 4-(α-L-rhamnosyloxy) benzyl isothiocyanate showed significant anti-trypanosomal and cytotoxic potential. Many plant molecules have been isolated and reported for biological activities. But, only few compounds were successfully forwarded from the laboratory to clinical trials. This is due to inadequate information on structure characterization and pharmacological efficacy (Kannathasan et al., 2011). Therefore, sufficient studies on the biological and cytotoxic potential of isolated molecules from M. peregrina are suggested for their safe clinical use.
It is well-known that the researchers always try to discover potent bioactive molecules with least cytotoxicity, as the plant based molecules play a vital role in the development of new modern medicines. M. peregrina has rich cultural heritage of traditional healing practices among the people of Arabian Peninsula to treat multiple disorders. Available literature demonstrated that M. peregrina were tested for pharmacological activities which are related to traditional uses. Also, different classes of active molecules were also reported in the past few decades. In this review, a comprehensive informations about the traditional uses, pharmacological efficacy and isolated bioactive molecules from M. peregrina are documented in order to give collective information for future research. In conclusion, the scientific evidences showed that M. peregrina is not fully validated for its pharmacological potential. Moreover, most of the studies were mainly focused on seeds and leaves for pharmacological activities. Since the other plant parts have therapeutic properties, future investigations should be done to evaluate wide range of biological activities of tubers, flowers and seeds. In addition, the phytochemical investigations from the M. peregrina are also very limited. Only few molecules were isolated, identified and studied for biological activity. More investigations are needed to explore the efficacy of the medicinal plant and extensive investigations should be carried out to find out the active molecules found in this plant. And also to ensure the safety of the isolated compounds in clinical practice, further investigations are needed to understand the mode of action and toxic potential on host cells.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by the Research Affairs, United Arab Emirates University (UAEU), through UPAR Grant to AC as PI (Grant No. 31F092).
Abd El-Wahab, R. (1995). Reproduction Ecology of Wild Trees and Shrubs in Southern Sinai, Egypt. Master thesis, Botany Department, Faculty of Science, Suez Canal University, Ismailia.
Abrahim, N. N., Kanthimathi, M., and Abdul-Aziz, A. (2012). Piper betle shows antioxidant activities, inhibits MCF-7 cell proliferation and increases activities of catalase and superoxide dismutase. BMC Complement. Altern. Med. 12:220. doi: 10.1186/1472-6882-12-220
Abushouk, A. I., Negida, A., Ahmed, H., and Abdel-Daim, M. M. (2017). Neuroprotective mechanisms of plant extracts against MPTP induced neurotoxicity: future applications in Parkinson's disease. Biomed. Pharmacother. 85, 635–645. doi: 10.1016/j.biopha.2016.11.074
Afsharypuor, S., Asghari, G., Mohagheghzadeh, A., and Dehshahri, S. (2010). Volatile constituents of the seed kernel and leaf of Moringa peregrina (Forssk.) Fiori, Agricolt. Cultivated in Chabahar (Iran). Iran J. Pharm. Res. 6, 141–144.
Ahmed, M. F., Kazim, S. M., Ghori, S. S., Mehjabeen, S. S., Ahmed, S. R., Ali, S. M., et al. (2010). Antidiabetic activity of Vinca rosea extracts in alloxan-induced diabetic rats. Int. J. Endocrinol. 2010:841090. doi: 10.1155/2010/841090
Alamgeer, I. S., Asif, H., and Saleem, M. (2017). Evaluation of antihypertensive potential of Ficus carica fruit. Pharm. Biol. 55, 1047–1053. doi: 10.1080/13880209.2017.1278611
Al-Dabbas, M. M., Ahmad, R., Ajo, R. Y., Abulaila, K., Akash, M., and Al-Ismail, K. (2010). Chemical composition and oil components in seeds of Moringa peregrina (Forssk) Fiori. Crop Res. 40, 161–167.
Al-Dhaheri, S. M. (2016). In vitro re Generation and Marker Assisted Evaluation of Genetic Fidelity in Endangered Tree Species Moringa peregrina (Forsk) Fiori. Master thesis, United Arab Emirates University, Al Ain Abu Dhabi.
Al-Juhaimi, F., Babiker, E. E., Ghafoor, K., and Özcan, M. M. (2016). Fatty acid composition of three different Moringa leave oils. Riv. Ital. Sostanze Gr. 93, 111–113.
Al-Kahtani, H. (1995). Moringa perigrina (Al-yassar or Al-ban) seeds oil from northwest Saudi Arabia. J. King Saud Univ. Agric. Sci. 7, 31–45.
Al-Owaisi, M., Al-Hadiwi, N., and Khan, S. A. (2014). GC-MS analysis, determination of total phenolics, flavonoid content and free radical scavenging activities of various crude extracts of Moringa peregrina (Forssk.) Fiori leaves. Asian Pac. J. Trop. Biomed. 4, 964–970. doi: 10.12980/APJTB.4.201414B295
Al-Rawashdeh, N. Q., Al-Rawashdeh, I. M., and Hourni, A. M. (2013). Fatty acid comparison between oil of Moringa Peregrina and Olea europaea. J. Ethnobiol. Tradit. Med. 118, 264–268.
Alrayes, L. M. H., Al Khateeb, W. M. H., and Shatnawi, M. A. M. (2016). Clonal propagation and antibacterial activity of Moringa peregrina (Forssk) fiori plant. J. Adv. Biotechnol. 6, 787–797.
Andrikopoulos, N. K., Kaliora, A. C., Assimopoulou, A. N., and Papapeorgiou, V. P. (2003). Biological activity of some naturally occurring resins, gums and pigments against in vitro LDL oxidation. Phytother Res. 17, 501–507. doi: 10.1002/ptr.1185
Anwar, F., Latif, S., Ashraf, M., and Gilani, A. H. (2007). Moringa oleifera: a food plant with multiple medicinal uses. Phytother. Res. 21, 17–25. doi: 10.1002/ptr.2023
Aruoma, O. I., Bahorun, T., and Jen, L. S. (2003). Neuroprotection by bioactive components in medicinal and food plant extracts. Mutat Res. 544, 203–215. doi: 10.1016/j.mrrev.2003.06.017
Asensi, G. D., Villadiego, A. M. D., and Berruezo, G. R. (2017). Moringa oleifera: a review of food applications. Arch. Latinoam. Nutr. 67, 86–97.
Ayyari, M., Salehi, P., Ebrahimi, S. N., Zimmermann, S., Portmann, L., Krauth-Siegel, R. L., et al. (2014). Antitrypanosomal isothiocyanate and thiocarbamate glycosides from Moringa peregrina. Planta Med. 80, 86–89. doi: 10.1055/s-0033-1351102
Azim, S. A. A., Abdelrahem, M. T., Said, M. M., and Khattab, A. (2017). Protective effect of Moringa peregrina leaves extract on acetaminophen -induced liver toxicity in albino rats. Afr. J. Trad. Complement. Altern. Med. 14, 206–216. doi: 10.21010/ajtcam.v14i2.22
Batist, G., Wu, J. H., Spatz, A., Miller, W. H., Cocolakis, E., Rousseau, C., et al. (2011). Resistance to cancer treatment: the role of somatic genetic events and the challenges for targeted therapies. Front. Pharmacol. 2:59. doi: 10.3389/fphar.2011.00059
Bellostas, N., Sørensen, J. C., Nikiema, A., Sørensen, H., Pasternak, D., and Kumar, S. (2010). Glucosinolates in leaves of Moringa species grown and disseminated in Niger. Afr. J. Agric. Res. 5, 1338–1340.
Bernhoft, A. (ed.). (2010). “A brief review on bioactive compounds in plants,” in Bioactive Compounds in Plants – benefits and Risks for Man and Animals, (Oslo: The Norwegian Academy of Science and Letters), 11–17.
Carretero, M. E., López-Pérez, J. L., Abad, M. J., Bermejo, P., Tillet, S., Israel, et al. (2008). Preliminary study of the anti-inflammatory activity of hexane extract and fraction from Bursera simaruba (Linneo) Sarg. (Burseraceae) leaves. J. Ethnopharmacol. 116, 11–15. doi: 10.1016/j.jep.2007.10.034
Cechinel-Filho, V., Zampirolo, J. A., Stulzer, H. K., and Schlemper, V. (2007). Antispasmodic effects of Persea cordata bark fractions on guinea pig ileum. Fitoterapia 78, 125–128. doi: 10.1016/j.fitote.2006.10.005
Cortés, A. R., Delgadillo, A. J., Hurtado, M., Domínguez-Ramírez, A. M., Medina, J. R., and Aoki, K. (2006). The antispasmodic activity of Buddleja scordioides and Buddleja perfoliata on isolated intestinal preparations. Biol. Pharm Bull. 29, 1186–1190. doi: 10.1248/bpb.29.1186
David, A. V., Arulmoli, R., and Parasuraman, S. (2016). Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn. Rev. 10, 84–89. doi: 10.4103/0973-7847.194044
Dehshahri, S., Wink, M., Afsharypuor, S., Asghari, G., and Mohagheghzadeh, A. (2012). Antioxidant activity of methanolic leaf extract of Moringa peregrina (Forssk.) Fiori. Res. Pharm. Sci. 7, 111–118.
de Rus Jacquet, A., Timmers, M., Ma, S. Y., Thieme, A., McCabe, G. P., Vest, J. H. C., et al. (2017). Lumbee traditional medicine: neuroprotective activities of medicinal plants used to treat Parkinson's disease-related symptoms. J. Ethnopharmacol. 206, 408–425. doi: 10.1016/j.jep.2017.02.021
Deshpande, A. D., Harris-Hayes, M., and Schootman, M. (2008). Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 88, 1254–1264. doi: 10.2522/ptj.20080020
Dinelli, G., Bonetti, A., Minelli, M., Marotti, I., Catizone, P., and Mazzanti, A. (2006). Content of flavonols in Italian bean (Phaseolus vulgaris L.) ecotypes. Food Chem. 90, 105–114. doi: 10.1016/j.foodchem.2005.07.028
Duarte, J., Pérez-Palencia, R., Vargas, F., Ocete, M. A., Pérez-Vizcaino, F., Zarzuelo, A., et al. (2001). Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br. J. Pharmacol. 133, 117–124. doi: 10.1038/sj.bjp.0704064
Elabd, E. M. Y., Zahran, H. A., and Abdalla, A. M. (2017). A comparative study of the effects of three Moringa species on obesity- induced oxidative stress state in liver tissue. Int. J. Pharma. Bio Sci. 8, 572–584. doi: 10.22376/ijpbs.2017.8.2.b572-584
El-Alfy, T. S., Ezzat, S. M., Hegazy, A. K., Amer, A. M., and Kamel, G. M. (2011). Isolation of biologically active constituents from Moringa peregrina (Forssk.) Fiori. (family: Moringaceae) growing in Egypt. Pharmacogn. Mag. 7, 109–115. doi: 10.4103/0973-1296.80667
El-Awady, M. A., Hassan, M. M., Abdel-Hameed, E.-S. S., and Gaber, A. (2015). Comparison of the antimicrobial activities of the leaves-crude extracts of Moringa peregrina and Moringa oleifera in Saudi Arabia. Int. J. Curr. Microbiol. App. Sci. 4, 1–9.
El-Awady, M. A., Hassan, M. M., El-Sayed, S. A. H., and Gaber, A. (2016). Comparison of the antioxidant activities, phenolic and flavonoids contents of the leaves-crud extracts of Moringa peregrine and Moringa oleifera. Int. J. Biosci. 8, 55–62. doi: 10.12692/ijb/8.1.55-62
Elbatran, S. A., Abdel-Salam, O. M., Abdelshfeek, K. A., Nazif, N. M., Ismail, S. I., and Hammouda, F. M. (2005). Phytochemical and pharmacological investigations on Moringa Peregrina (Forssk) Fiori. Nat. Prod. Sci. 11, 199–206.
El-Haddad, A. E., Koheil, M. A., El-Khalik, S. M. A., and Osman, S. (2002). “Antihyperglycemic activity and nitrile glycosides of Moringa peregrina (Forssk.) seeds,” in Fourth Euro-Mediterranean Conference of Natural Products and Drug Discovery: Back to Mother Nature (Cairo/Sharm El-Sheikh: BioNat-IV).
Elsaey, M. A., Sallam, A. E.-D., Hassaneen, E., and Zaghloul, M. S. (2016). Circadian phase modulates the enhancing effect of the Egyptian Moringa peregrina extract on learning and memory in mice. Biol. Rhythm Res. 47, 703–715. doi: 10.1080/09291016.2016.1183880
Elsayed, E. A., Sharaf-Eldin, M. A., El-Enshasy, H. A., and Wadaan, M. (2016). In vitro assessment of anticancer properties of Moringa peregrina essential seed oil on different cell lines. Pak. J. Zool. 48, 853–859.
FAO (1988). Traditional Food Plants - A Resource Book for Promoting the Exploitation and Consumption of Food Plants In Arid, Semi-arid and Sub-Humid Lands of Eastern Africa. Food and Agriculture Organization of the United Nations, Rome, Italy.
Farhadi, A., Bruninga, K., Fields, J., and Keshavarzian, A. (2001). Irritable bowel syndrome: an update on therapeutic modalities. Expert Opin. Investig. Drugs 10, 1211–1222. doi: 10.1517/13543784.10.7.1211
Fuglie, L. J. (2001). The Miracle Tree: Moringa oleifera: Natural Nutrition for the Tropics. Training Manual. Dakar: Church World Service.
Galuppo, M., Giacoppo, S., Iori, R., De Nicola, G. R., Bramanti, P., and Mazzon, E. (2015). Administration of 4-(α-L-Rhamnosyloxy)-benzyl isothiocyanate delays disease phenotype in SOD1G93A rats: a transgenic model of amyotrophic lateral sclerosis. Bio Med. Res. Int. 2015:259417. doi: 10.1155/2015/259417
Gharibzahedi, S. M. T., Ansarifard, I., Hasanabadi, Y. S., Ghahderijani, M., and Yousefi, R. (2013). Physicochemical properties of Moringa peregrina seed and its oil. Qual. Assur. Saf. Crop. Foods 5, 303–309. doi: 10.3920/QAS2012.0172
Ghazanfar, S. A., and Al-Al-Sabahi, A. M. (1993). Medicinal plants of Northern and Central Oman (Arabia). Econ. Bot. 47, 89–98. doi: 10.1007/BF02862209
Giacoppo, S., Galuppo, M., De Nicola, G. R., Iori, R., Bramanti, P., and Mazzon, E. (2015). 4(α-l-rhamnosyloxy)-benzyl isothiocyanate, a bioactive phytochemical that attenuates secondary damage in an experimental model of spinal cord injury. Bioorgan. Med. Chem. 23, 80–88. doi: 10.1016/j.bmc.2014.11.022
Gilani, A. H., Aftab, K., Suria, A., Siddiqui, S., Salem, R., Siddiqui, B. S., et al. (1994). Pharmacological studies on hypotensive and spasmolytic activities of pure compounds from Moringa oleifera. Phytother. Res. 8, 87–91. doi: 10.1002/ptr.2650080207
Goswami, S. K., Inamdar, M. N., Dethe, S. M., Gururaj, G. M., Jamwal, R., Bhaskar, A., et al. (2016). Erectogenic and Aphrodisiac Property of Moringa oleifera: involvement of soluble epoxide hydrolase enzyme. Phytother. Res. 30, 1119–1127. doi: 10.1002/ptr.5614
Govindarajan, R., Vijayakumar, M., and Pushpangadan, P. (2005). Antioxidant approach to disease management and the role of ‘Rasayana’ herbs of ayurveda. J. Ethnopharmacol. 99, 165–178. doi: 10.1016/j.jep.2005.02.035
Gullon, B., Lú-Chau, T. A., Moreira, M. T., Lema, J. M., and Eibes, G. (2017). Rutin: a review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Tech. 67, 220–235. doi: 10.1016/j.tifs.2017.07.008
Hajar, A. S., and Gumgumjee, N. M. (2014). Antimicrobial activities and evaluation of genetic effects of Moringa peregrina (Forsk.) Fiori using molecular techniques. Int. J. Plant Anim. Environ. Sci. 4, 65–72.
Hajhashemi, V., Sadraei, H., Ghannadi, A. R., and Mohseni, M. (2000). Antispasmodic and anti-diarrhoeal effect of Satureja hortensis L. essential oil. J. Ethnopharmacol. 71, 187–192. doi: 10.1016/S0378-8741(99)00209-3
Hegazy, A. K., Hammouda, O., Lovett-Doust, J., and Gomaa, N. H. (2008). Population dynamics of Moringa peregrina along an altitudinal gradient in the northwestern sector of the Red Sea. J. Arid. Environ. 72, 1537–1551. doi: 10.1016/j.jaridenv.2008.03.001
Holanda-Pinto, S. A., Pinto, L. M. S., Cunha, G. M. A., Chaves, M. H., Santos, F. A., and Rao, V. S. (2008). Anti-inflammatory effect of α, β-Amyrin, a pentacyclic triterpene from Protium heptaphyllum in rat model of acute periodontitis. Inflammopharmacology 16, 48–52. doi: 10.1007/s10787-007-1609-x
Holman, R. R., and Turner, R. C. (1991). “Oral agents and insulin in the treatment of NIDDM” in Text Book of Diabetes, ed J. Pickup and G. Williams (Oxford, UK: Blackwell), 467–469.
ICUN (2005). A Guide to Medicinal Plants in South Africa. IUCN Centre for Mediterranean Cooperation, Campanillas, Málaga.
Jabeen, K., Javaid, A., Ahmad, E., and Athar, M. (2011). Antifungal compounds from Melia azederach leaves for management of Ascochyta rabiei, the cause of chickpea blight. Nat. Prod. Res. 25, 264–276. doi: 10.1080/14786411003754298
Jahn, S. A. A. (1996). On the introduction of a tropical multipurpose tree to China traditional and potential utilisation of Moringa oleifera Lamark. Senckenb. Biol. 75, 243–254.
Jahn, S. A., Musnad, H. A., and Heinz, B. (1986). The tree that purifies water: cultivating multipurpose Moringaceae in the Sudan. Unasylva. 38, 23–28.
Jalili, T., Carlstrom, J., Kim, S., Freeman, D., Jin, H., Wu, T. C., et al. (2006). Quercetin-supplemented diets lower blood pressure and attenuate cardiac hypertrophy in rats with aortic constriction. J. Cardiovas. Pharmacol. 47, 531–541. doi: 10.1097/01.fjc.0000211746.78454.50
Jang, M., Hong, E., and Kim, G. H. (2010). Evaluation of antibacterial activity of 3-butenyl, 4-pentenyl, 2-phenylethyl, and benzyl isothiocyanate in Brassica vegetables. J. Food Sci. 75, M412–M416. doi: 10.1111/j.1750-3841.2010.01725.x
Jessani, S., Watson, T., Cappuccio, F. P., and Lip, G. Y. (2006). Prevention of cardiovascular disease in clinical practice: The Joint British Societies' (JBS 2) guidelines. J. Hum. Hypertens. 20, 641–645. doi: 10.1038/sj.jhh.1002058
Jnawali, H. N., Lee, E., Jeong, K.-W., Shin, A., Heo, Y.-S., and Kim, Y. (2014). Anti-inflammatory activity of rhamnetin and a model of its binding to c-Jun NH2-terminal kinase 1 and p38 MAPK. J. Nat. Prod. 77, 258–263. doi: 10.1021/np400803n
Juhaimi, F. A. L., Ghafoor, K., Ahmed, I. A. M., Babiker, E. E., and Özcan, M. M. (2017). Comparative study of mineral and oxidative status of Sonchus oleraceus, Moringa oleifera and Moringa peregrina leaves. J. Food Meas. Charact. 11, 1745–1751. doi: 10.1007/s11694-017-9555-9
Justesen, U., and Knethsen, P. (2001). Composition of flavonoids in fresh herbs and calculation of flavonoids intake by use of herbs in traditional danish dishes. Food Chem. 73, 245–250. doi: 10.1016/S0308-8146(01)00114-5
Kær, A., Malver, O., El-menshawi, B., and Reischt, J. (1979). Isothiocyanates in myrosinase-treated seed extracts of Moringa peregrina. Phytochem. 18, 1485–1487. doi: 10.1016/S0031-9422(00)98480-2
Kalappurayil, T. M., and Joseph, B. P. (2017). A review of pharmacognostical studies on Moringa oleifera Lam. Flowers Phcog. J. 9, 1–7. doi: 10.5530/pj.2017.1.1
Kannathasan, K., Senthilkumar, A., and Venkatesalu, V. (2011). Mosquito larvicidal activity of methyl-p-hydroxybenzoate isolated from the leaves of Vitex trifolia Linn. Acta Trop. 120, 115–118. doi: 10.1016/j.actatropica.2011.07.001
Kermanshai, R., Mc Carry, B. E., Rosenfeld, J., Summers, P. S., Weretilnyk, E. A., and Sorger, G. J. (2001). Benzyl isothiocyanate is the chief or sole antihelmintic in papaya seed extracts. Phytochemistry 57, 427–435. doi: 10.1016/S0031-9422(01)00077-2
Koheil, M. A., Hussein, M. A., Othman, S. M., and El-Haddad, A. (2011). Anti-inflammatory and antioxidant activities of Moringa peregrina Seeds. Free Radical Antioxid. 1, 49–61. doi: 10.5530/ax.2011.2.10
Koheil, M. A., Hussein, M. A., Othman, S. M., and El-Haddad, A. (2013). In-vivo antioxidant activity of Moringa peregrina against STZ – induced oxidative stress in type 2 diabetic rats. Mol. Clin. Pharmacol. 4, 65–75.
Lalas, S., Gortzi, O., Athanasiadis, V., Tsaknis, J., and Chinou, I. (2012). Determination of antimicrobial activity and resistance to oxidation of Moringa peregrina seed oil. Molecules 17, 2330–2334. doi: 10.3390/molecules17032330
Leone, A., Spada, A., Battezzati, A., Schiraldi, A., Aristil, J., and Bertoli, S. (2016). Moringa oleifera seeds and oil: characteristics and uses for human health. Int. J. Mol. Sci. 17:2141. doi: 10.3390/ijms17122141
Lin, L., Liu, Y.-C., Huang, J.-L., Liu, X.-B., Qing, Z.-X., Zeng, J.-G., et al. (2018). Medicinal plants of the genus Macleaya (Macleaya cordata, Macleaya microcarpa): a review of their phytochemistry, pharmacology, and toxicology. Phytother. Res. 32, 19–48. doi: 10.1002/ptr.5952
Majali, I. S., Oran, S. A., Khleifat, K. M. A., Qaralleh, H., Rayyan, W. A., and Althunibat, O. Y. (2015). Assessment of the antibacterial effects of Moringa peregrina extracts. Afr. J. Microbiol. Res. 9, 2410–2414. doi: 10.5897/AJMR2015.7787
Mallya, R., Chatterjee, P. K., Vinodini, N. A., Chatterjee, P., and Mithra, P. (2017). Moringa oleifera leaf extract: Beneficial effects on cadmium induced toxicities - A review. J. Clin. Diagn. Res. 11, CE01–CE04. doi: 10.7860/JCDR/2017/21796.9671
Manach, C., Mazur, A., and Scalbert, A. (2005). Polyphenols and prevention of cardiovascular diseases. Curr. Opin. Lipidol. 16, 77–84. doi: 10.1097/00041433-200502000-00013
Mangundayao, K., and Yasurin, P. (2017). Bioactivity of Moringa oleifera and its applications: a review. J. Pure Appl. Microbiol. 11, 43–50. doi: 10.22207/JPAM.11.1.07
Manzoor, M., Anwar, F., Iqbal, T., and Bhnager, M. I. (2007). Physico-chemical characterization of Moringa concanensis seeds and seed oil. J. Am. Oil Chem. Soc. 84, 413–419. doi: 10.1007/s11746-007-1055-3
Marwah, R. G., Fatope, M. O., Al Mahrooqi, R., Varma, G. B., Al Abadi, H., and Al-Burtamani, S. K. S. (2007). Antioxidant capacity of some edible and wound healing plants in Oman. Food Chem. 101, 465–470. doi: 10.1016/j.foodchem.2006.02.001
Mehdi, H., Tan, G. T., Pezzuto, J. M., Fong, H. H., Farnsworth, N. R., El-Feraly, F. S., et al. (1997). Cell culture assay system for the evaluation of natural product-mediated anti-Hepatitis B virus activity. Phytomedicine 3, 369–377. doi: 10.1016/S0944-7113(97)80011-6
Mekonnen, Y., Yardley, V., Rock, P., and Croft, S. (1999). In vitro antitrypanosomal activity of Moringa stenopetala leaves and roots. Phytother. Res. 13, 538–539.
Merina, A. J., Kesavan, D., and Sulochana, D. (2011). Isolation and antihyperglycemic activity of flavonoid from flower petals of Opuntia stricta. Pharm. Chem. J. 45, 317–321. doi: 10.1007/s11094-011-0625-8
Migahid, A. M. (1978). Flora of Soudi Arabia. Vol. 1, Dicotyledon. Riyadh: Riyadh University Publication.
Miller, A. G., and Morris, M. (1988). Plants of Dhofar. The Southern Region of Oman: Traditional, Economic, and Medicinal Uses. Muscat: The office of the advisor for conservation of the Environment, Diwan of Royal Court.
Moustafa, S. M. A., Menshawi, B. M., Wassel, G. M., Mahmoud, K., and Mounier, M. M. (2014). Screening of some wild and cultivated Egyptian plants for their free radical scavenging activity. Int. J. PharmTech. Res. 6, 1271–1278.
MPCP (2006). Conservation and Sustainable Use of Medicinal Plants Project. National survey: 2 – Medicinal plants in North Sinai. Final report.
Munyanziza, E., and Yongabi, K. A. (2007). “Moringa peregrina (Forssk.) Fiori.” in Vegetable oils/Ol é agineux [CD-Rom], eds H. A. M. van der Vossen and G. S. Mkamilo (Wageningen: PROTA 14: PROTA).
N'Guessan, B. B., Dosso, K., Gnangoran, B. N., Amoateng, P., Asiedu-Gyekye, I. J., and Yapo, A. P. (2015). Antibacterial and antispasmodic activities of a dichloromethane fraction of an ethanol extract of stem bark of Piliostigma reticulatum. J. Pharm. Bioallied Sci. 7, 128–135. doi: 10.4103/0975-7406.154439
Nawash, S. O., and Al-Horani, S. A. (2011). The most important medicinal plants in Wadi Araba desert in South West Jordan: a review article. Adv. Environ. Biol. 5, 418–425.
Olson, M. E., Sankaran, R. P., Fahey, J. W., Grusak, M. A., Odee, D., and Nouman, W. (2016). Leaf protein and mineral concentrations across the “Miracle Tree” genus Moringa. PLoS ONE 11:e0159782. doi: 10.1371/journal.pone.0159782
Padla, E. P., Solis, L. T., Levida, R. M., Shen, C. C., and Ragasa, C. Y. (2012). Antimicrobial isothiocyanates from the seeds of Moringa oleifera Lam. Z Naturforsch C. 67, 557–564. doi: 10.5560/ZNC.2012.67c0557
Pahan, K. (2006). Lipid-lowering drugs. Cell Mol. Life Sci. 63, 1165–1178. doi: 10.1007/s00018-005-5406-7
Pal, S. K., Mukherjee, P. K., and Saha, B. P. (1995). Studies on the antiulcer activity of Moringa oleifera leaf extract on gastric ulcer models in rats. Phytother. Res. 9, 463–465. doi: 10.1002/ptr.2650090618
Pal, S. K., Mukherjee, P. K., Saha, K., Pal, M., and Saha, B. P. (1996). Studies on some psychopharmacological actions of Moringa oleifera Lam. (Moringaceae) leaf extract. Phytother. Res. 10, 402–405.
Pasricha, P. (2001). “Prokinetic agents, antiemetics and agents used in irritable bowel syndrome” in Goodman and Gilman's the Pharmacological Basis of Therapeutics, eds J. Hardman, L. Limbird, and G. A. Gilman (New York, NY: McGraw Hill), 1021–1036.
Preetha, S. P., Kanniappan, M., Selvakumar, E., Nagaraj, M., and Varalakshmi, P. (2006). Lupeol ameliorates aflatoxin B1-induced peroxidative hepatic damage in rats. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 143, 333–339. doi: 10.1016/j.cbpc.2006.03.008
Quattrocchi, U. (2000). CRC World Dictionary of Grasses: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology, Vol. 3. Florida, FL: CRC Press.
Ramadan, M. F., and Moersel, J. T. (2006). Screening of the antiradical action of vegetable oils. J. Food Compos. Anal. 19, 838–842. doi: 10.1016/j.jfca.2006.02.013
Ramawat, K. G., Dass, S., and Mathur, M. (2009). “The chemical diversity of bioactive molecules and therapeutic potential of medicinal plants” in Herbal Drugs: Ethnomedicine to Modern Medicine, ed K. G. Ramawat (Berlin; Heidelberg: Springer-Verlag), 7–32.
Rao, B. R., Kesavulu, M. M., and Apparao, C. (2001). Antihyperglycemic activity of Momordica cymbalaria in alloxan diabetic rats. J. Ethnopharmacol. 78, 67–71. doi: 10.1016/S0378-8741(01)00324-5
Razavi, S. M., Zahri, S., Zarrini, G., Nazemiyeh, H., and Mohammadi, S. (2009). Biological activity of quercetin-3-O-glucoside, a known plant flavonoid. Russian J. Bioorg. Chem. 35, 414–416. doi: 10.1134/S1068162009030133
Reddy, S. H., Al-Neeri, I. S., Al-Issaei, H. K., and Al-Jabri, S. A. (2015). Effect of selective medicinal plant extract on blood glucose, sperm shape and various physiological parameters. Am. J. Plant Sci. 6, 1109–1115. doi: 10.4236/ajps.2015.68115
Robards, K., Prenzler, P. D., Tucker, G., Swaitsitang, P., and Glover, W. (1999). Phenolic compounds and their role in oxidative processes. Food Chem. 66, 401–436. doi: 10.1016/S0308-8146(99)00093-X
Robiansyah, I., Hajar, A. S., Al-kordy, M. A., and Ramadan, A. (2014). Current status of economically important plant Moringa peregrina (Forrsk.) Fiori in Saudi Arabia: a review. Int. J. Theor. Appl. Sci. 6, 79–86.
Rosas-Acevedo, H., Terrazas, T., González-Trujano, M. E., Guzmán, Y., and Soto-Hernández, M. (2011). Anti-ulcer activity of Cyrtocarpa procera analogous to that of Amphipterygium adstringens, both assayed on the experimental gastric injury in rats. J. Ethnopharmacol. 134, 67–73. doi: 10.1016/j.jep.2010.11.057
Rouhi-Broujeni, H., Heidarian, E., Darvishzadeh-Broojeni, P., Rafieian-Kopaei, M., and Gharipour, M. (2013). Lipid lowering activity of Moringa peregrina seeds in rat: a comparison between the extract and atorvastatin. Res. J. Biol. Sci. 8, 150–154.
Sacchetti, G., Maietti, S., Muzzoli, M., Scaglianti, M., Manfredini, S., Radice, M., et al. (2005). Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 91, 621–632. doi: 10.1016/j.foodchem.2004.06.031
Sadraei, H., Asghari, G., and Farahnaki, F. (2015). Assessment of hydroalcoholic extract of seeds and leaves of Moringa peregrina on ileum spasm. Res. Pharm. Sci. 10, 252–258.
Sadraei, H., Asghari, G., and Hekmatti, A. A. (2003). Antispasmodic effect of three fractions of hydroalcoholic extract of Pycnocycla spinosa. J. Ethnopharmacol. 86, 187–190. doi: 10.1016/S0378-8741(03)00077-1
Safaeian, L., Asghari, G., Javanmard, S. H., and Heidarinejad, A. (2015). The effect of hydroalcoholic extract from the leaves of Moringa peregrina (Forssk.) Fiori. on blood pressure and oxidative status in dexamethasone-induced hypertensive rats. Adv. Biomed. Res. 4:101. doi: 10.4103/2277-9175.156681
Saini, R. K., Sivanesan, I., and Keum, Y.-S. (2016). Phytochemicals of Moringa oleifera: a review of their nutritional, therapeutic and industrial significance. 3 Biotech. 6:203. doi: 10.1007/s13205-016-0526-3
Saleem, M. (2009). Lupeol, A novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 285, 109–115. doi: 10.1016/j.canlet.2009.04.033
Saleh, N. M., Mabrouk, M. I., Salem-Bekhit, M. M., and Hafez, E. H. (2017). Challenge of Moringa peregrina Forssk as an antimicrobial agent against multi-drug-resistant Salmonella sp. Med. Biotechnol. 31, 380–386. doi: 10.1080/13102818.2016.1262750
Salehi, M., Badi, H. N., Hosseini, M. N., and Mazaheri, D. (2014). Determination of volatile oil constituents in root and shoot of Moringa peregrina (Forssk.) Fiori in saline soil. J Med. Plants 13, 87–96.
Salvamani, S., Gunasekaran, B., Shaharuddin, N. A., Ahmad, S. A., and Shukor, M. Y. (2014). Antiartherosclerotic effects of plant flavonoids. Biomed. Res. Int. Id. 2014:480258. doi: 10.1155/2014/480258
Santos, A. F. S., Argolo, A. C. C., Paiva, P. M. G., and Coelho, L. C. B. B. (2012). Antioxidant activity of Moringa oleifera tissue extracts. Phytother. Res. 26, 1366–1370. doi: 10.1002/ptr.4591
Sengupta, A., and Gupta, M. P. (1970). Studies on the seed fat composition of moringacea family. Eur. J. Lipid Sci. Technol. 72, 6–10.
Sharma, A., Chakraborti, K. K., and Handa, S. S. (1991). Anti-hepatotoxic activity of some Indian herbal formulations as compared to silymarin. Fitoterapia 62, 229–235.
Sharma, S., Sahni, J. K., Ali, J., and Baboota, S. (2013). Patent perspective for potential antioxidant compounds-rutin and quercetin. Rec. Pat. Nanomed. 3, 62–68. doi: 10.2174/18779123112029990002
Shimizu, N., Watanabe, T., Arakawa, T., Fujiwara, Y., Higuchi, K., and Kuroki, T. (2000). Pentoxifylline accelerates gastric ulcer healing in rats: roles of tumor necrosis factor alpha and neutrophils during the early phase of ulcer healing. Digestion 61, 157–164. doi: 10.1159/000007752
Singh, R. S. G., Negi, P. S., and Radha, C. (2013). Phenolic composition, antioxidant and antimicrobial activities of free and bound phenolic extracts of Moringa oleifera seed flour. J. Funct. Foods 5, 1883–1891. doi: 10.1016/j.jff.2013.09.009
Sliai, A. M., and Abdel-Rahman, G. H. (2016). Protective effect of Moringa peregrina seed oil against doxorubicin-induced hepatotoxicity in male mice. Res. Opin. Anim. Vet. Sci. 6, 69–73. doi: 10.20490/ROAVS/16-011
Solecki, R. S. (1975). Shanidar IV, a Neanderthal flower burial in northan Iraq. Science 190, 880–881. doi: 10.1126/science.190.4217.880
Solowey, E., Lichtenstein, M., Sallon, S., Paavilainen, H., Solowey, E., and HLorberboum-Galski, H. (2014). Evaluating medicinal plants for anticancer activity. Sci. World J. 2014:721402. doi: 10.1155/2014/721402
Soltan, M. M., and Zaki, A. K. (2009). Antiviral screening of forty-two Egyptian medicinal plants. J. Ethnopharmacol. 126, 102–107. doi: 10.1016/j.jep.2009.08.001
Somali, M. A., Bajneid, M. A., and Al Fhaimani, S. S. (1984). Chemical composition and characteristics of Moringa peregrina seeds and seed oil. J. Am. Oil. Chem. Soc. 61, 85–86. doi: 10.1007/BF02672051
Stohs, S. J., and Hartman, M. J. (2015). Review of the safety and efficacy of Moringa oleifera. Phytother. Res. 29, 796–804. doi: 10.1002/ptr.5325
Sultana, B., and Anwar, F. (2008). Flavonols (Kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. 10, 879–884. doi: 10.1016/j.foodchem.2007.11.053
Tahany, M. A., Hegazy, A. K., Sayed, A. M., Kabiel, H. F., El-Alfy, T., and El-Komy, S. M. (2010). Study on combined antimicrobial activity of some biologically active constituents from wild Moringa peregrina Forssk. J. Yeast Fungal Res. 1, 15–24.
Tan, H.-L., Chan, K.-G., Pusparajah, P., Saokaew, S., Duangjai, A., Lee, L.-H., et al. (2016). Anti-cancer properties of the naturally occurring Aphrodisiacs: Icariin and its derivatives. Front. Pharmacol. 7:191. doi: 10.3389/fphar.2016.00191
Taniyama, Y., and Griendling, K. K. (2003). Reactive oxygen species in the vasculature. Hypertension 42, 1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F
Tsaknis, J. (1998). Characterisation of Moringa peregrina Arabia seed oil. Grasas y Aceites 49, 170–176. doi: 10.3989/gya.1998.v49.i2.717
Ullah, M. F., Bhat, S. H., and Abuduhier, F. M. (2015). Antidiabetic potential of hydro-alcoholic extract of Mringa peregrina leaves: implication as functional food for prophylactic intervention in prediabetic stage. J. Food Biochem. 39, 360–367. doi: 10.1111/jfbc.12140
Valko, M., Rhodes, C. J., Moncol, J., Izakovic, M., and Mazur, M. (2006). Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 160, 1–40. doi: 10.1016/j.cbi.2005.12.009
Van der Vossen, H. A. M., and Mkamilo, G. S. (2007). Porta 14: Vegetable Oils. Wgageningen: Backhuys Publishers.
Verpoorte, R. (2000). Pharmacognosy in the new millennium: lead finding and biotechnology. J. Pharm. Pharmacol. 52, 253–262. doi: 10.1211/0022357001773931
Vig, A. P., Rampal, G., Thind, T. S., and Arora, S. (2009). Bio-protective effects of glucosinolates – A review. LWT Food Sci. Technol. 42, 1561–1572. doi: 10.1016/j.lwt.2009.05.023
Wangcharoen, W., and Gomolanee, S. (2011). Antioxidant capacity and total phenolic content of Moringa oleifera grown in Chiang Mai, Thailand. Thai J. Agric. Sci. 44, 118–124.
Willis, K. J., and Bachman, S. (2016). State of the World's Plants Report. Royal Botanic Garden, Kew.
Wilson, R. K., Kwan, T. K. Y., Kwan, C., and Sorger, G. J. (2002). Effects of papaya seed extract and benzyl isothiocyanate on vascular contraction. Life Sci. 71, 497–507. doi: 10.1016/S0024-3205(02)01708-3
Xu, G. B., Xiao, Y. H., Zhang, Q. Y., Zhou, M., and Liao, S. G. (2018). Hepatoprotective natural triterpenoids. Eur. J. Med. Chem. 145, 691–716. doi: 10.1016/j.ejmech.2018.01.011
Zhang, H., Lai, Q., Li, Y., Liu, Y., and Yang, M. (2017). Learning and memory improvement and neuroprotection of Gardenia jasminoides (Fructus gardenia) extract on ischemic brain injury rats. J. Ethnopharmacol. 196, 225–235. doi: 10.1016/j.jep.2016.11.042
Keywords: Moringaceae, medicinal plant, Moringa peregrina, traditional uses, pharmacology, phytochemistry
Citation: Senthilkumar A, Karuvantevida N, Rastrelli L, Kurup SS and Cheruth AJ (2018) Traditional Uses, Pharmacological Efficacy, and Phytochemistry of Moringa peregrina (Forssk.) Fiori. —A Review. Front. Pharmacol. 9:465. doi: 10.3389/fphar.2018.00465
Received: 03 November 2017; Accepted: 20 April 2018;
Published: 11 May 2018.
Edited by:
Lyndy Joy McGaw, University of Pretoria, South AfricaReviewed by:
Kuldeep Dhama, Indian Veterinary Research Institute (IVRI), IndiaCopyright © 2018 Senthilkumar, Karuvantevida, Rastrelli, Kurup and Cheruth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdul J. Cheruth, YWJkdWwuamFsZWVsQHVhZXUuYWMuYWU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.