
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 30 April 2018
Sec. Neuropharmacology
Volume 9 - 2018 | https://doi.org/10.3389/fphar.2018.00440
 Abadi Kahsu Gebre1*
Abadi Kahsu Gebre1* Birhanetensay Masresha Altaye1
Birhanetensay Masresha Altaye1 Tesfay Mehari Atey2
Tesfay Mehari Atey2 Kald Beshir Tuem1
Kald Beshir Tuem1 Derbew Fikadu Berhe1
Derbew Fikadu Berhe1Renin Angiotensin System (RAS) is a hormonal system that regulates blood pressure and fluid balance through a coordinated action of renal, cardiovascular, and central nervous systems. In addition to its hemodynamic regulatory role, RAS involves in many brain activities, including memory acquisition and consolidation. This review has summarized the involvement of RAS in the pathology of Alzheimer’s disease (AD), and the outcomes of treatment with RAS inhibitors. We have discussed the effect of brain RAS in the amyloid plaque (Aβ) deposition, oxidative stress, neuroinflammation, and vascular pathology which are directly and indirectly associated with AD. Angiotensin II (AngII) via AT1 receptor is reported to increase brain Aβ level via different mechanisms including increasing amyloid precursor protein (APP) mRNA, β-secretase activity, and presenilin expression. Similarly, it was associated with tau phosphorylation, and reactive oxygen species generation. However, these effects are counterbalanced by Ang II mediated AT2 signaling. The protective effect observed with angiotensin receptor blockers (ARBs) and angiotensin converting enzyme inhibitors (ACEIs) could be as the result of inhibition of Ang II signaling. ARBs also offer additional benefit by shifting the effect of Ang II toward AT2 receptor. To conclude, targeting RAS in the brain may benefit patients with AD though it still requires further in depth understanding.
Renin Angiotensin System (RAS) is a hormonal system that regulates body fluid, electrolyte homostasis, and vascular tone (Yim and Yoo, 2008; Sparks et al., 2014). These classical functions of RAS are mediated by angiotensin effector peptides including Ang II, III and 1–7 (Atlas, 2007). Ang II, the primary effector peptide, is produced in the blood and exerts a number of effects on kidney, adrenal glands, sympathetic nervous system and baroreceptor reflexes (Reid, 1992; Dasgupta and Zhang, 2011). Studies have also shown the presence of local RAS in many different tissues including brain (Ganten et al., 1983; Wang et al., 1996; Vila-Porcile and Corvol, 1998; Grobe et al., 2010; Ferrão et al., 2014). In the central nervous system, angiotensinogen is synthesized by astrocytes and subsequently cleaved by renin, angiotensin converting enzyme (ACE) and aminopeptidases or ACE2 and Neprilysin (Bodiga and Bodiga, 2013). Despite some speculations, it is not clearly known where these RAS enzymes are locally synthesized in the brain (McKinley et al., 2003).
The angiotensin ligands interact with their receptors including angiotensin (AT) 1A, 1B, 2, 4 and Mas and controls various brain function (Guimond and Gallo-Payet, 2012; Premer et al., 2013). The receptors are differentially expressed in several parts of the brain (Kakar et al., 1992; Braga, 2011). AT1A is expressed in areas mainly involved in regulation of blood pressure and electrolyte balance including subfornical organ, paraventricular nucleus of the hypothalamus, lateral septum, cerebral cortex, and hippocampus (Johren et al., 1995; MacGregor et al., 1995; Lenkei et al., 1997), brainstem baroreflex arc, olivocerebellary system, and preoptic region (Lenkei et al., 1997). While AT1B is expressed in structures which involve in higher brain function and memory including cerebral cortex and hippocampus (Johren et al., 1995).
Activation of AT1 receptors is associated with increase in oxidative stress (Prusty et al., 2017), anxiety and stress (Saavedra et al., 2005; Wincewicz and Braszko, 2014), ischemic brain damage (Panahpour et al., 2014), and cognitive impairment (Nakagawa et al., 2017).
AT2 receptor, on the other hand, is observed in parts of the brain which regulate learning and memory including hippocampus, cingulate cortex, superior colliculus, lateral septum, in thalamic nuclei, in the subthalamic nucleus, in the locus coeruleus, and in the inferior olive (Millan et al., 1991; Lenkei et al., 1996). AT2 receptor is also expressed in brain structures including red nucleus, pedunculopontine tegmental nucleus, bed nucleus of the supraoptic decussation, paragenual nucleus, motor hypoglossal nucleus, cerebellar nuclei (Song et al., 1991, 1992; Tsutsumi and Saavedra, 1991; Lenkei et al., 1996), substantia nigra (Garrido-Gil et al., 2013; Valenzuela et al., 2016), and ventral tegmental area (Garrido-Gil et al., 2013). However, the extent of the receptor expression is limited after the fetal period (AbdAlla et al., 2009). AT2 receptor signaling is suggested to play beneficial role in neurogenesis (Umschweif et al., 2014), cerebral blood flow (Iwai et al., 2004; Fuchtemeier et al., 2015), neuronal plasticity (Namsolleck et al., 2013), and learning and memory (Jing et al., 2012). Activation of the receptor is also reported to attenuate inflammation (Rompe et al., 2010), oxidative stress (Lu et al., 2015) and abnormal neuronal firing (Grammatopoulos et al., 2004; Matsuura et al., 2005) observed as the result of AT1 receptor stimulation (Guimond and Gallo-Payet, 2012).
In addition to AT1 and AT2 receptors, recent evidences show the presence of other receptors in CNS including AT4 and Mas (Singh and Karnik, 2016). AT4 receptor interacts with a different angiotensin ligand called angiotensin IV, and it is reported to regulate learning and memory in brain areas including the hippocampus, neocortex and motor nuclei (Wright et al., 1999; Chai et al., 2000). The receptor is also localized in claustrum, choroid plexus, pontine nucleus, thalamic nuclei, substantia nigra pars compacta and hypothalamus (Zhuo et al., 1998; Chai et al., 2000). It is also suggested for its neuroprotective effect against cerebral ischemia (Faure et al., 2006). Mas receptor also contributes for the diverse actions of RAS in the brain (Jackson et al., 2018). The receptor is mainly localized in the hippocampus, amygdala, anterodorsal thalamic nucleus, cortex, and hypoglossal nucleus (Bunnemann et al., 1990; Becker et al., 2007; Freund et al., 2012; Lazaroni et al., 2012). Activation of the receptor by angiotensin 1–7 was found to strengthen synapses in areas involved in memory (Bunnemann et al., 1990; Hellner et al., 2005; Uekawa et al., 2016).
Brain RAS generally involves in regulating central activities including learning, memory, anxiety, depression, cognition, and emotional stress (Gard, 2004; Paul et al., 2006; de Gasparo et al., 2013), but it also complements functions of the peripheral RAS (McKinley et al., 2003). Importantly, there are growing evidence indicating the contribution of brain RAS in development of neurodegenerative disorders including AD (Zhu et al., 2011; Tian et al., 2012; AbdAlla et al., 2013; Ana Flavia et al., 2017; Takane et al., 2017). However, it is not exactly known how RAS system influences the development and progression of AD. It is not also well understood how medications acting on RAS system affect AD though some studies have shown a link between RAS and accumulation of toxic Aβ peptides (Murphy and LeVine, 2010; Gouras et al., 2015), tau phosphorylation (Tian et al., 2012), oxidative stress (Chrissobolis et al., 2012), mitochondrial dysfunction (Nozoe et al., 2008), neuroinflammation (Vargas et al., 2012) and cholinergic dysfunction (Barnes et al., 1990).
Aβ42 and Aβ40 are the two-predominant Aβ-proteins that are highly susceptible for aggregation to form oligomers, protofibrils, and fibrils (Shin et al., 1997; Ahmed et al., 2010). Under normal physiological conditions, brain eliminates toxic peptides via enzymatic degradation, perivascular drainage and receptor-mediated efflux transport (Higuchi et al., 2005; Wang et al., 2011; Iliff et al., 2012; Provias and Jeynes, 2014; Baranello et al., 2015). Impairment of either of these clearance mechanisms may result in accumulation of Aβ peptide. The accumulation can cause neuronal membrane damage, an increase in oxidative stress, receptor-mediated alteration of signal transduction, alteration of membrane pore, increase in intracellular level of calcium ion and mitochondrial damage (Yankner, 1996; Carrillo-Mora et al., 2014). These changes also trigger persistent loss of cholinergic projections to the neocortex (Supnet and Bezprozvanny, 2010).
Aβ deposition facilitates the formation of pathological phosphorylated tau proteins (Busciglio et al., 1995; Zheng et al., 2002; Bloom, 2014). Accumulation of toxic tau protein could also occur independent of amyloid β (Katsuno et al., 2005). The abnormal aggregation and deposition of tau protein can result in formation of neurofibrillary tangles leading to a progressive loss of neurons (Buee et al., 2000; Hanger et al., 2009; Wolfe, 2012). Tau mediated neurodegeneration could be due to sequestration of tau protein and disturbance of microtubule function (Alonso et al., 2008; Iqbal et al., 2009). This results impairment of normal axon flow and subsequent loss of neurons and their connectivity (Iqbal et al., 2009; Baird and Bennett, 2013).
In vitro studies have shown the role of ACE in the degradation of Aβ peptides halting the halts development of amyloid plaque (Hu et al., 2001; Oba et al., 2005). The enzymatic action of ACE in the breakdown Aβ peptides have demonstrated by several studies (Hemming and Selkoe, 2005; Sun et al., 2008; Zou et al., 2009). Whilst ACE inhibitors were reported to promote Aβ aggregation (Hu et al., 2001). ACE2, a homolog of ACE, was also reported to have a catalytic role in the cleavage of Aβ43 to Aβ40 and this was inhibited by specific ACE2 inhibitor called DX600 (Liu et al., 2014). N domain part of the enzyme was found responsible for hydrolysis Aβ peptides at N-terminal position. ACE hydrolyses the most neurotoxic peptides Aβ43 and Aβ42 (Welander et al., 2009; Brouillette et al., 2012), in to amyloid peptides that are less susceptibility to aggregate and form senile plaques. ACE also metabolizes the most abundant amyloid peptide, Aβ40 with the potential to reduce the Aβ42 oligomerization and deposition (Kim et al., 2007; Murray et al., 2009). ACE reduces amyloid β peptides the main risk factor for the development and progression of AD (Karran et al., 2011) (Table 1). These studies altogether indicate the metabolic action of RAS enzymes in reducing amyloid plaque deposition via degradation of the most toxic form amyloid peptides composed of 40-43 amino acid sequences.
In vitro studies have shown the role of ACE in degradation of Aβ peptides thereby reducing deposition and accumulation of amyloid plaque while inhibition of the enzyme is detrimental (Hemming and Selkoe, 2005; Sun et al., 2008; Zou et al., 2009; Liu et al., 2014). Ramipril (ACE inhibitor) also increased Aβ peptides in ACE10/10 mice with AD (Bernstein et al., 2014). Recent studies, however, does not support the idea that ACEIs increases accumulation of Aβ peptides in AD animal models (Eckman et al., 2006; Hemming et al., 2007; Ferrington et al., 2011, 2012). These studies challenge the notion that ACEIs inhibit degradation of Aβ peptides and favoring amyloid plaque formation. Some ACEIs even reduced Aβ peptide level in animal models of AD (AbdAlla et al., 2013). Moreover, ACEIs showed beneficial effect in reducing AD signs and symptoms (Dong et al., 2011; Tota et al., 2012; AbdAlla et al., 2015). Administration of perindopril (ACEI) has shown an instrumental effect in increasing density of normal neurons and improving learning and memory (Hou et al., 2008). A study on Tg2576 AD model demonstrated the positive role of captopril in preventing signs of neurodegeneration (AbdAlla et al., 2013). These studies support the potential benefit of ACEIs in alleviating sign and symptom of AD; however, with contrasting reports. A study on Tg2576 mice showed increase in deposition of Aβ42 after treatment with captopril (Zou et al., 2007). In line with this study, treatment with ramipril elevated brain level of Aβ42 peptide in AD+ACE (10/10) mice. Most in vivo studies have shown a positive correlation between increased expression of ACE and signs of AD but ACE inhibitors have protective effect against AD (Table 2). The protective effect of ACE inhibitors could be explained partly via suppressing brain derived neurotrophic factor decline and TNF-α release. They were also found to ameliorate oxidonitrosative stress and nitrotyrosine production (Ali et al., 2016) with that in turn reduces amyloidogenesis and subsequent Aβ deposition (Goel et al., 2016). However, further investigations are required to see if the contradicting reports were intrinsic to the specific inherent nature of the drug or methodological issue.
A review by Kehoe indicated Ang II (as with ACE) increased accumulation and deposition Aβ peptides in AD animal models (Kehoe, 2009). Ang II increases Aβ level, promotes cerebrovascular dysfunction, and micro-vascular amyloid deposition which those in turn worsens AD outcome (Faraco et al., 2016). ARBs, e.g., telmisartan, have shown to prevent cognitive decline associated with Aβ40 injection (Mogi et al., 2008). Olmesartan was also associated with improved cognitive function and hippocampal synaptic plasticity (Takeda et al., 2009). Losartan was reported to prevent neuropathological and cognitive deficits observed in AD (Ongali et al., 2014). These studies showed the beneficial roles of ARBs in animal models of AD. The protective effect could be explained in part via suppressing AT1 receptor mediated APP mRNA up regulation, Aβ peptide production and phosphorylated tau induced neurotoxicity (Zhu et al., 2011). The protective effect of these drugs could also be attributed as a result of unopposed action of Ang II on AT2 receptor (Horiuchi et al., 2010; Gallo-Payet et al., 2011) and stimulation of AngIV/AT4R signaling as observed in losartan (Royea et al., 2017). AT2 receptor mediated signaling pathways are known to prevent degeneration of neurons (Li et al., 2005; McCarthy et al., 2009). In line with these reports, valsartan have shown to attenuate oligomerization of Aβ peptides into high molecular weight oligomeric peptides and reduces cognitive deterioration (Wang et al., 2007). However, other studies with the same model have shown that Aβ induces the formation of oligomers of AT2 receptor in the hippocampus that disrupts Ang II mediated signaling. The Aβ- induced AT2 receptor oligomerization was associated with enhanced neurodegeneration. Conversely, inhibition of cross-linked AT2 receptor delayed tau phosphorylation (AbdAlla et al., 2009).
In other studies, however, valsartan or eprosartan (ARBs), did not alter accumulation of Aβ oligomers and phosphorylated tau in triple transgenic mice (Ferrington et al., 2011). The contradiction could be reconciled by the difference in AD animal models used. Variability in the dose of drug, the age and strain of animal used in the experiment could also explain the discrepancy (Ferrington et al., 2012). Despite varying result of RAS on amyloidosis, the overall effects of this system seem to favor amyloidosis. More specifically, the Ang II favors production of Aβ peptides via the most widely expressed angiotensin receptor, AT1 (Hohle et al., 1995). In addition to reduction of Aβ deposition and its consequences, RAS inhibitors have also other beneficial roles including suppression of inflammation (Saavedra, 2012), oxidative stress (Prusty et al., 2017), vascular damage/ischemia (Takeda and Morishita, 2017), and increase in acetylcholine release (Barnes et al., 1990) and glutamate uptake (Ruginsk et al., 2015) (Figure 1).
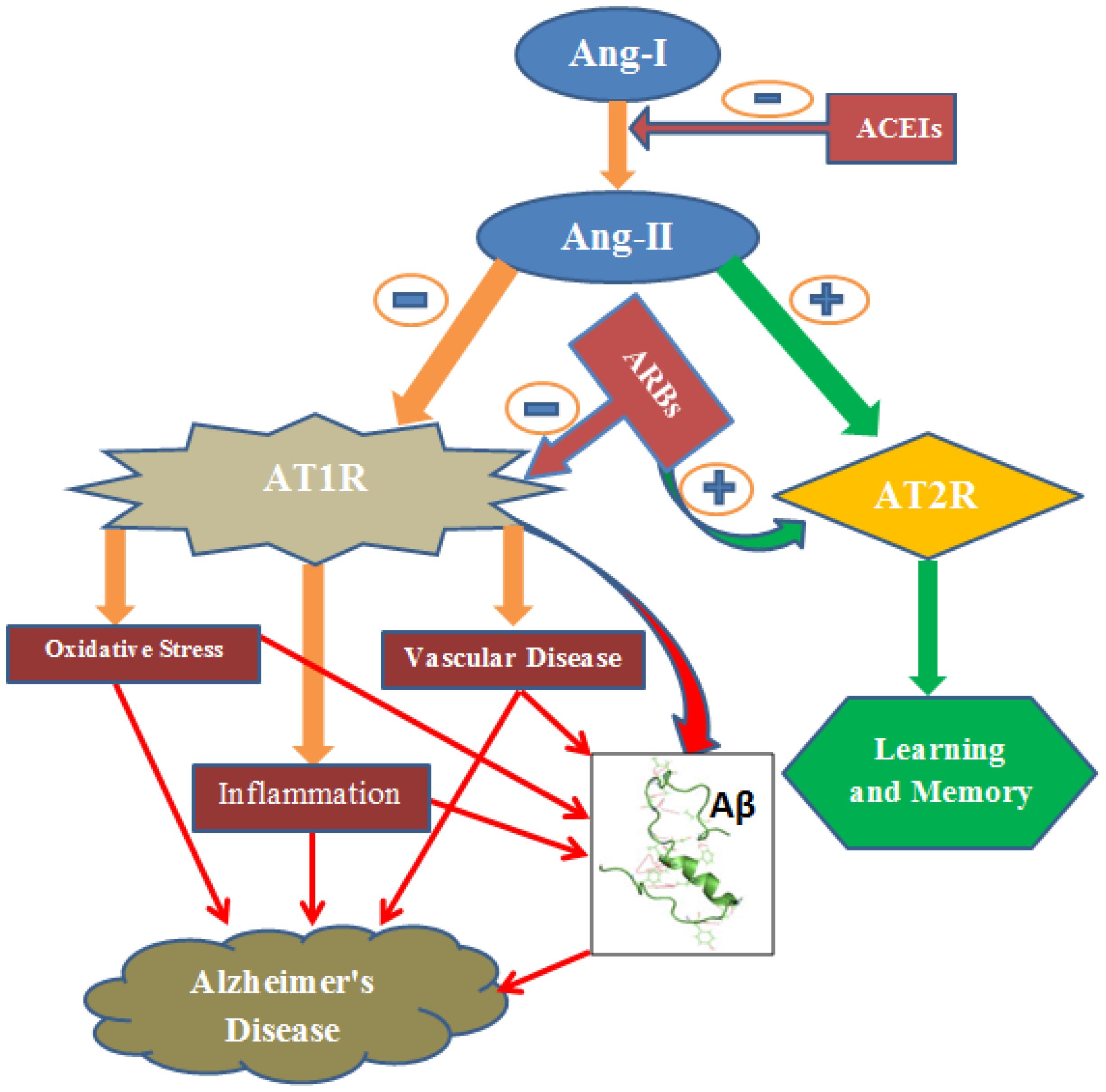
FIGURE 1. Ang-II induces oxidative stress, inflammation and vascular disease via AT1R. Consequently, it causes accumulation of amyloid-β resulting Alzheimer’s disease. However, AT2 R signaling produces beneficial effect including learning and memory. ARBs inhibit AT1R signaling and this shifts the action of Ang-II toward the beneficial pathway (AT2R signaling). ACEIs, Angiotensin converting enzyme inhibitors; ARBs, Angiotensin Receptor blockers; AT1R, Angiotensin 1 Receptor; AT2R, Angiotensin 2 Receptor; Aβ, Amyloid-β; -, negative outcome or blockage; +, positive outcome.
Ang II enhances AT1 receptor mediated brain inflammation. Contrarily, ARBs attenuates the release of proinflammatory mediators (Lanz et al., 2010). Central infusion of Ang II increased hippocampal CD68- positive cells, indicating its hippocampal proinflammatory action (Takane et al., 2017). In contrarily, candesartan (ARB) decreased lipopolysaccharide (LPS) induced and AT1 receptor mediated release of proinflammatory mediators including TNFα, IL-1β, IκBα, iNOS, ICAM-1, and VCAM-1 in cerebral cortex (Benicky et al., 2009). In addition, candesartan attenuated brain level of NF-α, GFAP, COX-2, and NF-κB in the same animal model. They have also demonstrated the advantage of unopposed action of Ang II on AT2 receptor in addition to AT1 receptor blockage mediated amelioration of proinflammatory mediators releasesuggesting the beneficial role of AT2 receptors in reducing neuroinflammation (Goel et al., 2018). Moreover, ARBs prevents impairment and preserves the integrity of blood brain barrier which in turn reduces infiltration of inflammatory mediators observed in many neurodegenerative disease including AD (de Vries et al., 1997; Panahpour et al., 2014; So et al., 2015).
AngII via AT1 receptor is also suggested as effector of oxidative stress (Nickenig and Harrison, 2002; Marchesi et al., 2008; Chan and Chan, 2013; Seifi et al., 2014; Prusty et al., 2017). Ang II increased a reactive oxygen species called superoxide (Takane et al., 2017). On the other hand, telmisartan (ARB) was found to normalize diminished thioredoxin (Trx) system in addition to attenuating thioredoxin-interacting protein (TXNIP) expression. This reduces generation of endogenous reactive oxygen species (Erdi et al., 2016). Similarly, telmisartan reduced advanced glycation end products and 4-hydroxynonenal, which are the markers of oxidative stress and associated with Neurodegeneration (Safciuc et al., 2007; Barone et al., 2017). Candesartan also reduced brain level of free radicals by diminishing Malondialdehyde and increasing glutathione level (Tota et al., 2009). Thus partly alleviates the development and progression of AD (Gustaw-Rothenberg et al., 2010; Saharan and Mandal, 2014). Captopril (Bild et al., 2013) and losartan (Seifi et al., 2015) were also found to ameliorate oxidative stress.
Ang II is also implicated in neurovascular damage and cognitive impairment (Mogi et al., 2012; Bodiga and Bodiga, 2013; Bloch et al., 2015). Candesartan increased cerebral blood flow, reduced infarct size and improved cerebral ischemia (Ito et al., 2002; Engelhorn et al., 2004). Similarly, losartan prevented blood brain barrier disruption and restored blood flow after induction of hemorrhagic stroke. Moreover, telmisartan (Iwanami et al., 2010), valsartan (Takada et al., 2006), and olmesartan (Matsumoto et al., 2009) have shown a beneficial role in prevention of vascular damage via blockage of AT1 receptor. Suggested mechanism of ARBs on cerebral blood flow is in part explained via unblocked AT2 receptor activation (Iwai et al., 2004; Li et al., 2005; Jing et al., 2012). These studies generally show the benefit of ARBs in improving neurovascular network and cerebral blood flow after certain initial insult which in turn prevents onset and progressive neurodegeneration observed in AD (Bell and Zlokovic, 2009; Zlokovic, 2011). In addition to the above mechanisms described, Ang II is also speculated to inhibit acetylcholine release in which the deficiency is responsible for AD (Barnes et al., 1992; Tota et al., 2013). Conversely, pre-treatment with candesartan prevented Ang II induced reduction of acetylcholine level (Tota et al., 2009, 2013). This reduces cognitive impairment observed in AD (Burns, 2003; Herholz, 2008).
Human studies have shown the involvement of RAS in the pathogenesis and progression of AD (Amouyel et al., 2000; Kolsch et al., 2005). Nevertheless, only few studies have shown a link between RAS and AD (Ellul et al., 2007; Davies et al., 2011). ACEIs and ARBs have shown a beneficial effect in slowing and reducing the cognitive impairment associated with AD (Li et al., 2012; Hsu et al., 2013; Saavedra, 2016). In a cross sectional study, patients taking ARBs and ACEIs had lower risk of cognitive deterioration (Jackson et al., 2018).
Central acting RAS inhibitors have shown a superior efficacy which imply brain RAS involvement in development and progression of AD (Hebert et al., 2013; Soto et al., 2013; Wharton et al., 2015; Zhuang et al., 2016). A prospective multicentre cohort study showed slower rate of cognitive decline on older adults taking ACE-Is (Soto et al., 2013). ARBs and ACEIs were generally found to reduce the risk and progression of AD (Hajjar et al., 2008; Li et al., 2010; Davies et al., 2011). The central acting agent including perindopril was significantly associated with a slower rate of functional decline (Davies et al., 2011). Telmisartan reduced cognitive impairment in hypertensive patients with AD (Li et al., 2012). The drug reduced amyloid β, oxidative stress and neuroinflammation. The RAS also activates peroxisome proliferator activated receptor (PPAR) gamma which has a role in prevention of neurodegeneration (Inestrosa et al., 2005; Kume et al., 2012; Li et al., 2012). Other ARBs losartan (Moriwaki et al., 2004; Hong et al., 2010), and olmesartan (Matsumoto et al., 2010) have shown beneficial effect in AD patients. In contrast, in a 4-month of pilot clinical trial ramipril was not associated with reduction of CSF Aβ1-42 level and cognitive impairment (Wharton et al., 2012). This limited effect of ramipril could be attributed to its limited blood brain barrier penetration (Sink et al., 2009). Most of these studies support the beneficial effect of RAS inhibitors in prevention and mitigation of cognitive impairments associated with AD (Table 3).
Genetic studies have also reported for the associate of ACE with AD (Elkins et al., 2004). ACE protein is coded by several genes containing various variants, specifically the insertion/deletion variant (rs1799752) have been associated with AD. Some other variants, including single nucleotide polymorphisms rs4291A > T located 240 base pair from the initiation codon, and rs4343G > A encoding a silent mutation in exon 16 were also thought to be involved in AD (Helbecque et al., 2009; Gaiteri et al., 2016). AD patients with the haplotype of rs1800764 (CC): rs4291 (TT) responded better for ACEIs that can cross the blood brain barrier (captopril or perindopril). However, the response was not significant among independent carriers of rs1800764 or rs429 (de Oliveira et al., 2014). Further stratification showed the benefit of ACEIs among ACE haplotypes (rs1800764 – T and rs4291 – A) and Apolipoprotein (APOE4) – carriers (rs1800764 – T or rs4291 – T). Nevertheless, APOE4+ carriers were non-responsive for ACEIs indicating the role of genetic variation and ACEIs response rate among AD patients (de Oliveira et al., 2018).
Understanding AD in terms of various pathophysiological pathways is worthwhile to unravel the complex nature of the disease process and identifying potential therapeutic targets. The brain RAS is reported to be involved in the development and progression of AD through AT1 receptor via increasing the production of amyloid-β, oxidative stress, inflammatory processes, and decreasing release of acetylcholine. However, RAS also is reported to have protective effect against AD. Through AT2 receptor activation that counterbalances the deleterious effects of AT1 receptor mediated RAS effects. With concept, beneficial effect of ARBs against AD is via the unopposed action of Ang II on AT2 receptors it as AT1 receptor is blocked these drugs increased Ang II concentration to act on AT2 receptor. ACE is reported to be involved in breakdown of amyloid β peptides, but most of the studies have contradicting result. This requires further understanding especially involvement of ACE in cleavage of amyloid β peptides in vivo. In summary, RAS through AT1 receptor is linked with AD pathology through its action on neurovascular change, oxidative stress, and inflammation as evidenced by the protective role of ARBs and ACEIs both in patients and animal models. However, the role of RAS in AD pathology is still not well established and need further in-depth understanding.
AG conducted the review and prepared the first draft while all authors contributed to substantial enhancement of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AbdAlla, S., El Hakim, A., Abdelbaset, A., Elfaramawy, Y., and Quitterer, U. (2015). Inhibition of ACE retards tau hyperphosphorylation and signs of neuronal degeneration in aged rats subjected to chronic mild stress. Biomed Res. Int. 2015:917156.
AbdAlla, S., Langer, A., Fu, X., and Quitterer, U. (2013). ACE inhibition with captopril retards the development of signs of neurodegeneration in an animal model of Alzheimer’s disease. Int. J. Mol. Sci. 14, 16917–16942. doi: 10.3390/ijms140816917
AbdAlla, S., Lother, H., El Missiry, A., Langer, A., Sergeev, P., El Faramawy, Y., et al. (2009). Angiotensin II AT2 receptor oligomers mediate G-protein dysfunction in an animal model of Alzheimer disease. J. Biol. Chem. 284, 6554–6565. doi: 10.1074/jbc.M807746200
Ahmed, M., Davis, J., Aucoin, D., Sato, T., Ahuja, S., Aimoto, S., et al. (2010). Structural conversion of neurotoxic amyloid-beta(1-42) oligomers to fibrils. Nat. Struct. Mol. Biol. 17, 561–567. doi: 10.1038/nsmb.1799
Ali, M. R., Abo-Youssef, A. M., Messiha, B. A., and Khattab, M. M. (2016). Tempol and perindopril protect against lipopolysaccharide-induced cognition impairment and amyloidogenesis by modulating brain-derived neurotropic factor, neuroinflammation and oxido-nitrosative stress. Naunyn Schmiedebergs Arch. Pharmacol. 389, 637–656. doi: 10.1007/s00210-016-1234-6
Alonso, A. C., Li, B., Grundke-Iqbal, I., and Iqbal, K. (2008). Mechanism of tau-induced neurodegeneration in Alzheimer disease and related tauopathies. Curr. Alzheimer Res. 5, 375–384. doi: 10.2174/156720508785132307
Amouyel, P., Richard, F., Berr, C., David-Fromentin, I., and Helbecque, N. (2000). The renin angiotensin system and Alzheimer’s disease. Ann. N. Y. Acad. Sci. 903, 437–441. doi: 10.1111/j.1749-6632.2000.tb06395.x
Ana Flavia, A.-S., Lucas, M. K., and Maria Jose, C.-S. (2017). The renin-angiotensin system and the neurodegenerative diseases: a brief review. Protein Pept. Lett. 24, 841–853.
Atlas, S. A. (2007). The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J. Manag. Care Pharm. 13(8 Suppl B), 9–20. doi: 10.18553/jmcp.2007.13.s8-b.9
Baird, F. J., and Bennett, C. L. (2013). Microtubule defects & neurodegeneration. J. Genet. Syndr. Gene Ther. 4:203. doi: 10.4172/2157-7412.1000203
Baranello, R. J., Bharani, K. L., Padmaraju, V., Chopra, N., Lahiri, D. K., Greig, N. H., et al. (2015). Amyloid-beta protein clearance and degradation (ABCD) pathways and their role in Alzheimer’s disease. Curr. Alzheimer Res. 12, 32–46. doi: 10.2174/1567205012666141218140953
Barnes, J. M., Barnes, N. M., Costall, B., Coughlan, J., Kelly, M. E., Naylor, R. J., et al. (1992). Angiotensin-converting enzyme inhibition, angiotensin, and cognition. J. Cardiovasc. Pharmacol. 19(Suppl. 6), S63–S71. doi: 10.1097/00005344-199219006-00011
Barnes, J. M., Barnes, N. M., Costall, B., Horovitz, Z. P., Ironside, J. W., Naylor, R. J., et al. (1990). Angiotensin II inhibits acetylcholine release from human temporal cortex: implications for cognition. Brain Res. 507, 341–343. doi: 10.1016/0006-8993(90)90294-L
Barone, E., Head, E., Butterfield, D. A., and Perluigi, M. (2017). HNE-modified proteins in down syndrome: Involvement in development of Alzheimer disease neuropathology. Free Radic. Biol. Med. 111, 262–269. doi: 10.1016/j.freeradbiomed.2016.10.508
Becker, L. K., Etelvino, G. M., Walther, T., Santos, R. A., and Campagnole-Santos, M. J. (2007). Immunofluorescence localization of the receptor Mas in cardiovascular-related areas of the rat brain. Am. J. Physiol. Heart Circ. Physiol. 293, H1416–H1424. doi: 10.1152/ajpheart.00141.2007
Bell, R. D., and Zlokovic, B. V. (2009). Neurovascular mechanisms and blood–brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 118, 103–113. doi: 10.1007/s00401-009-0522-3
Benicky, J., Sánchez-Lemus, E., Pavel, J., and Saavedra, J. M. (2009). Anti-inflammatory effects of angiotensin receptor blockers in the brain and the periphery. Cell. Mol. Neurobiol 29, 781–792. doi: 10.1007/s10571-009-9368-4
Bernstein, K. E., Koronyo, Y., Salumbides, B. C., Sheyn, J., Pelissier, L., Lopes, D. H., et al. (2014). Angiotensin-converting enzyme overexpression in myelomonocytes prevents Alzheimer’s-like cognitive decline. J. Clin. Invest. 124, 1000–1012. doi: 10.1172/JCI66541
Bild, W., Hritcu, L., Stefanescu, C., and Ciobica, A. (2013). Inhibition of central angiotensin II enhances memory function and reduces oxidative stress status in rat hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry 43, 79–88. doi: 10.1016/j.pnpbp.2012.12.009
Bloch, S., Obari, D., and Girouard, H. (2015). Angiotensin and neurovascular coupling: beyond hypertension. Microcirculation 22, 159–167. doi: 10.1111/micc.12193
Bloom, G. S. (2014). Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 71, 505–508. doi: 10.1001/jamaneurol.2013.5847
Bodiga, V. L., and Bodiga, S. (2013). Renin angiotensin system in cognitive function and dementia. Asian J. Neurosci. 2013:102602. doi: 10.1155/2013/102602
Braga, V. A. (2011). Differential brain angiotensin-II type I receptor expression in hypertensive rats. J. Vet. Sci. 12, 291–293. doi: 10.4142/jvs.2011.12.3.291
Brouillette, J., Caillierez, R., Zommer, N., Alves-Pires, C., Benilova, I., Blum, D., et al. (2012). Neurotoxicity and memory deficits induced by soluble low-molecular-weight amyloid-beta1-42 oligomers are revealed in vivo by using a novel animal model. J. Neurosci. 32, 7852–7861. doi: 10.1523/JNEUROSCI.5901-11.2012
Buee, L., Bussiere, T., Buee-Scherrer, V., Delacourte, A., and Hof, P. R. (2000). Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Brain Res. Rev. 33, 95–130. doi: 10.1016/S0165-0173(00)00019-9
Bunnemann, B., Fuxe, K., Metzger, R., Mullins, J., Jackson, T. R., Hanley, M. R., et al. (1990). Autoradiographic localization of mas proto-oncogene mRNA in adult rat brain using in situ hybridization. Neurosci. Lett. 114, 147–153. doi: 10.1016/0304-3940(90)90063-F
Burns, A. (2003). Treatment of cognitive impairment in Alzheimer’s disease. Dialogues Clin. Neurosci. 5, 35–43.
Busciglio, J., Lorenzo, A., Yeh, J., and Yankner, B. A. (1995). beta-amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron 14, 879–888. doi: 10.1016/0896-6273(95)90232-5
Carrillo-Mora, P., Luna, R., and Colín-Barenque, L. (2014). Amyloid beta: multiple mechanisms of toxicity and only some protective effects? Oxid. Med. Cell. Longev. 2014:795375. doi: 10.1155/2014/795375
Chai, S. Y., Bastias, M. A., Clune, E. F., Matsacos, D. J., Mustafa, T., Lee, J. H., et al. (2000). Distribution of angiotensin IV binding sites (AT4 receptor) in the human forebrain, midbrain and pons as visualised by in vitro receptor autoradiography. J. Chem. Neuroanat. 20, 339–348. doi: 10.1016/S0891-0618(00)00112-5
Chan, S. H., and Chan, J. Y. (2013). Angiotensin-generated reactive oxygen species in brain and pathogenesis of cardiovascular diseases. Antioxid. Redox Signal. 19, 1074–1084. doi: 10.1089/ars.2012.4585
Chrissobolis, S., Banfi, B., Sobey, C. G., and Faraci, F. M. (2012). Role of Nox isoforms in angiotensin II-induced oxidative stress and endothelial dysfunction in brain. J. Appl. Physiol. 113, 184–191. doi: 10.1152/japplphysiol.00455.2012
Dasgupta, C., and Zhang, L. (2011). Angiotensin II receptors and drug discovery in cardiovascular disease. Drug Discov. Today 16, 22–34. doi: 10.1016/j.drudis.2010.11.016
Davies, N. M., Kehoe, P. G., Ben-Shlomo, Y., and Martin, R. M. (2011). Associations of anti-hypertensive treatments with Alzheimer’s disease, vascular dementia, and other dementias. J. Alzheimers Dis. 26, 699–708.
de Gasparo, M., Speth, R. C., Baltatu, O. C., and Vanderheyden, P. (2013). Brain RAS: hypertension and beyond. Int. J. Hypertens. 2013:157180. doi: 10.1155/2013/157180
de Oliveira, F. F., Bertolucci, P. H., Chen, E. S., and Smith, M. C. (2014). Brain-penetrating angiotensin-converting enzyme inhibitors and cognitive change in patients with dementia due to Alzheimer’s disease. J. Alzheimers Dis. 42(Suppl. 3), S321–S324.
de Oliveira, F. F., Chen, E. S., Smith, M. C., and Bertolucci, P. H. F. (2018). Pharmacogenetics of angiotensin-converting enzyme inhibitors in patients with Alzheimer’s disease dementia. Curr. Alzheimer Res. 15, 386–398. doi: 10.2174/1567205014666171016101816
de Vries, H. E., Kuiper, J., De Boer, A. G., Van Berkel, T. J., and Breimer, D. D. (1997). The blood-brain barrier in neuroinflammatory diseases. Pharmacol. Rev. 49, 143–156.
Dong, Y. F., Kataoka, K., Tokutomi, Y., Nako, H., Nakamura, T., Toyama, K., et al. (2011). Perindopril, a centrally active angiotensin-converting enzyme inhibitor, prevents cognitive impairment in mouse models of Alzheimer’s disease. FASEB J. 25, 2911–2920. doi: 10.1096/fj.11-182873
Eckman, E. A., Adams, S. K., Troendle, F. J., Stodola, B. A., Kahn, M. A., Fauq, A. H., et al. (2006). Regulation of steady-state beta-amyloid levels in the brain by neprilysin and endothelin-converting enzyme but not angiotensin-converting enzyme. J. Biol. Chem. 281, 30471–30478. doi: 10.1074/jbc.M605827200
Elkins, J. S., Douglas, V. C., and Johnston, S. C. (2004). Alzheimer disease risk and genetic variation in ACE: a meta-analysis. Neurology 62, 363–368. doi: 10.1212/01.WNL.0000106823.72493.FF
Ellul, J., Archer, N., Foy, C. M., Poppe, M., Boothby, H., Nicholas, H., et al. (2007). The effects of commonly prescribed drugs in patients with Alzheimer’s disease on the rate of deterioration. J. Neurol. Neurosurg. Psychiatry 78, 233–239. doi: 10.1136/jnnp.2006.104034
Engelhorn, T., Goerike, S., Doerfler, A., Okorn, C., Forsting, M., Heusch, G., et al. (2004). The angiotensin II type 1-receptor blocker candesartan increases cerebral blood flow, reduces infarct size, and improves neurologic outcome after transient cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 24, 467–474. doi: 10.1097/00004647-200404000-00012
Erdi, F., Keskin, F., Esen, H., Kaya, B., Feyzioglu, B., Kilinc, I., et al. (2016). Telmisartan ameliorates oxidative stress and subarachnoid haemorrhage-induced cerebral vasospasm. Neurol. Res. 38, 224–231. doi: 10.1080/01616412.2015.1105626
Faraco, G., Park, L., Zhou, P., Luo, W., Paul, S. M., Anrather, J., et al. (2016). Hypertension enhances Abeta-induced neurovascular dysfunction, promotes beta-secretase activity, and leads to amyloidogenic processing of APP. J. Cereb. Blood Flow Metab. 36, 241–252. doi: 10.1038/jcbfm.2015.79
Faure, S., Chapot, R., Tallet, D., Javellaud, J., Achard, J. M., and Oudart, N. (2006). Cerebroprotective effect of angiotensin IV in experimental ischemic stroke in the rat mediated by AT(4) receptors. J. Physiol. Pharmacol. 57, 329–342.
Ferrão, F. M., Lara, L. S., and Lowe, J. (2014). Renin-angiotensin system in the kidney: What is new? World J. Nephrol. 3, 64–76. doi: 10.5527/wjn.v3.i3.64
Ferrington, L., Miners, J. S., Palmer, L. E., Bond, S. M., Povey, J. E., Kelly, P. A., et al. (2011). Angiotensin II-inhibiting drugs have no effect on intraneuronal Abeta or oligomeric Abeta levels in a triple transgenic mouse model of Alzheimer’s disease. Am. J. Transl. Res. 3, 197–208.
Ferrington, L., Palmer, L. E., Love, S., Horsburgh, K. J., Kelly, P. A., and Kehoe, P. G. (2012). Angiotensin II-inhibition: effect on Alzheimer’s pathology in the aged triple transgenic mouse. Am. J. Transl. Res. 4, 151–164.
Freund, M., Walther, T., and Von Bohlen Und Halbach, O. (2012). Immunohistochemical localization of the angiotensin-(1-7) receptor Mas in the murine forebrain. Cell Tissue Res. 348, 29–35. doi: 10.1007/s00441-012-1354-3
Fuchtemeier, M., Brinckmann, M. P., Foddis, M., Kunz, A., Po, C., Curato, C., et al. (2015). Vascular change and opposing effects of the angiotensin type 2 receptor in a mouse model of vascular cognitive impairment. J. Cereb. Blood Flow Metab. 35, 476–484. doi: 10.1038/jcbfm.2014.221
Gaiteri, C., Mostafavi, S., Honey, C. J., De Jager, P. L., and Bennett, D. A. (2016). Genetic variants in Alzheimer disease - molecular and brain network approaches. Nat. Rev. Neurol. 12, 413–427. doi: 10.1038/nrneurol.2016.84
Gallo-Payet, N., Guimond, M. O., Bilodeau, L., Wallinder, C., Alterman, M., and Hallberg, A. (2011). Angiotensin II, a neuropeptide at the frontier between endocrinology and neuroscience: is there a link between the angiotensin ii type 2 receptor and Alzheimer’s disease? Front. Endocrinol. 2:17. doi: 10.3389/fendo.2011.00017
Ganten, D., Hermann, K., Unger, T., and Lang, R. E. (1983). The tissue renin-angiotensin systems: focus on brain angiotensin, adrenal gland and arterial wall. Clin. Exp. Hypertens. A 5, 1099–1118. doi: 10.3109/10641968309048844
Gard, P. R. (2004). Angiotensin as a target for the treatment of Alzheimer’s disease, anxiety and depression. Expert Opin. Ther. Targets 8, 7–14. doi: 10.1517/14728222.8.1.7
Garrido-Gil, P., Valenzuela, R., Villar-Cheda, B., Lanciego, J. L., and Labandeira-Garcia, J. L. (2013). Expression of angiotensinogen and receptors for angiotensin and prorenin in the monkey and human substantia nigra: an intracellular renin–angiotensin system in the nigra. Brain Struct. Funct. 218, 373–388. doi: 10.1007/s00429-012-0402-9
Goel, R., Bhat, S. A., Hanif, K., Nath, C., and Shukla, R. (2016). Perindopril attenuates lipopolysaccharide-induced amyloidogenesis and memory impairment by suppression of oxidative stress and RAGE activation. ACS Chem. Neurosci. 7, 206–217. doi: 10.1021/acschemneuro.5b00274
Goel, R., Bhat, S. A., Hanif, K., Nath, C., and Shukla, R. (2018). Angiotensin II receptor blockers attenuate lipopolysaccharide-induced memory impairment by modulation of NF-kappaB-Mediated BDNF/CREB expression and apoptosis in spontaneously hypertensive rats. Mol. Neurobiol. 55, 1725–1739. doi: 10.1007/s12035-017-0450-5
Gouras, G. K., Olsson, T. T., and Hansson, O. (2015). beta-Amyloid peptides and amyloid plaques in Alzheimer’s disease. Neurotherapeutics 12, 3–11. doi: 10.1007/s13311-014-0313-y
Grammatopoulos, T. N., Johnson, V., Moore, S. A., Andres, R., and Weyhenmeyer, J. A. (2004). Angiotensin type 2 receptor neuroprotection against chemical hypoxia is dependent on the delayed rectifier K+ channel, Na+/Ca2+ exchanger and Na+/K+ ATPase in primary cortical cultures. Neurosci. Res. 50, 299–306. doi: 10.1016/j.neures.2004.07.010
Grobe, J. L., Grobe, C. L., Beltz, T. G., Westphal, S. G., Morgan, D. A., Xu, D., et al. (2010). The brain renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab. 12, 431–442. doi: 10.1016/j.cmet.2010.09.011
Guimond, M.-O., and Gallo-Payet, N. (2012). The angiotensin II type 2 receptor in brain functions: an update. Int. J. Hypertens. 2012:351758 doi: 10.1155/2012/351758
Gustaw-Rothenberg, K., Kowalczuk, K., and Stryjecka-Zimmer, M. (2010). Lipids’ peroxidation markers in Alzheimer’s disease and vascular dementia. Geriatr. Gerontol. Int. 10, 161–166.
Hajjar, I. M., Keown, M., Lewis, P., and Almor, A. (2008). Angiotensin converting enzyme inhibitors and cognitive and functional decline in patients with Alzheimer’s disease: an observational study. Am. J. Alzheimers Dis. Other Demen. 23, 77–83. doi: 10.1177/1533317507309803
Hanger, D. P., Anderton, B. H., and Noble, W. (2009). Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 15, 112–119. doi: 10.1016/j.molmed.2009.01.003
Hebert, P. L., Mcbean, A. M., O’Connor, H., Frank, B., Good, C., and Maciejewski, M. L. (2013). Time until incident dementia among Medicare beneficiaries using centrally acting or non-centrally acting ACE inhibitors. Pharmacoepidemiol. Drug Saf. 22, 641–648. doi: 10.1002/pds.3449
Helbecque, N., Codron, V., Cottel, D., and Amouyel, P. (2009). An age effect on the association of common variants of ACE with Alzheimer’s disease. Neurosci. Lett. 461, 181–184. doi: 10.1016/j.neulet.2009.06.006
Hellner, K., Walther, T., Schubert, M., and Albrecht, D. (2005). Angiotensin-(1-7) enhances LTP in the hippocampus through the G-protein-coupled receptor Mas. Mol. Cell. Neurosci. 29, 427–435. doi: 10.1016/j.mcn.2005.03.012
Hemming, M. L., and Selkoe, D. J. (2005). Amyloid beta-protein is degraded by cellular angiotensin-converting enzyme (ACE) and elevated by an ACE inhibitor. J. Biol. Chem. 280, 37644–37650. doi: 10.1074/jbc.M508460200
Hemming, M. L., Selkoe, D. J., and Farris, W. (2007). Effects of prolonged angiotensin-converting enzyme inhibitor treatment on amyloid beta-protein metabolism in mouse models of Alzheimer disease. Neurobiol. Dis. 26, 273–281. doi: 10.1016/j.nbd.2007.01.004
Herholz, K. (2008). Acetylcholine esterase activity in mild cognitive impairment and Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 35(Suppl. 1), S25–S29. doi: 10.1007/s00259-007-0699-4
Higuchi, M., Iwata, N., and Saido, T. C. (2005). Understanding molecular mechanisms of proteolysis in Alzheimer’s disease: progress toward therapeutic interventions. Biochim. Biophys. Acta 1751, 60–67. doi: 10.1016/j.bbapap.2005.02.013
Hohle, S., Blume, A., Lebrun, C., Culman, J., and Unger, T. (1995). Angiotensin receptors in the brain. Pharmacol. Toxicol. 77, 306–315. doi: 10.1111/j.1600-0773.1995.tb01032.x
Hong, K. S., Kang, D. W., Bae, H. J., Kim, Y. K., Han, M. K., Park, J. M., et al. (2010). Effect of cilnidipine vs losartan on cerebral blood flow in hypertensive patients with a history of ischemic stroke: a randomized controlled trial. Acta Neurol. Scand. 121, 51–57. doi: 10.1111/j.1600-0404.2009.01299.x
Horiuchi, M., Mogi, M., and Iwai, M. (2010). The angiotensin II type 2 receptor in the brain. J. Renin Angiotensin Aldosterone Syst. 11, 1–6. doi: 10.1177/1470320309347793
Hou, D. R., Wang, Y., Zhou, L., Chen, K., Tian, Y., Song, Z., et al. (2008). Altered angiotensin-converting enzyme and its effects on the brain in a rat model of Alzheimer disease. Chin. Med. J. 121, 2320–2323.
Hsu, C. Y., Huang, C. C., Chan, W. L., Huang, P. H., Chiang, C. H., Chen, T. J., et al. (2013). Angiotensin-receptor blockers and risk of Alzheimer’s disease in hypertension population–a nationwide cohort study. Circ. J. 77, 405–410. doi: 10.1253/circj.CJ-12-0658
Hu, J., Igarashi, A., Kamata, M., and Nakagawa, H. (2001). Angiotensin-converting enzyme degrades Alzheimer amyloid beta-peptide (A beta); retards A beta aggregation, deposition, fibril formation; and inhibits cytotoxicity. J. Biol. Chem. 276, 47863–47868. doi: 10.1074/jbc.M104068200
Iliff, J. J., Wang, M., Liao, Y., Plogg, B. A., Peng, W., Gundersen, G. A., et al. (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4:147ra111. doi: 10.1126/scitranslmed.3003748
Inestrosa, N. C., Godoy, J. A., Quintanilla, R. A., Koenig, C. S., and Bronfman, M. (2005). Peroxisome proliferator-activated receptor gamma is expressed in hippocampal neurons and its activation prevents beta-amyloid neurodegeneration: role of Wnt signaling. Exp. Cell Res. 304, 91–104. doi: 10.1016/j.yexcr.2004.09.032
Iqbal, K., Liu, F., Gong, C. X., Alonso, A. C., and Grundke-Iqbal, I. (2009). Mechanisms of tau-induced neurodegeneration. Acta Neuropathol. 118, 53–69. doi: 10.1007/s00401-009-0486-3
Ito, T., Yamakawa, H., Bregonzio, C., Terron, J. A., Falcon-Neri, A., and Saavedra, J. M. (2002). Protection against ischemia and improvement of cerebral blood flow in genetically hypertensive rats by chronic pretreatment with an angiotensin II AT1 antagonist. Stroke 33, 2297–2303. doi: 10.1161/01.STR.0000027274.03779.F3
Iwai, M., Liu, H. W., Chen, R., Ide, A., Okamoto, S., Hata, R., et al. (2004). Possible inhibition of focal cerebral ischemia by angiotensin II type 2 receptor stimulation. Circulation 110, 843–848. doi: 10.1161/01.CIR.0000138848.58269.80
Iwanami, J., Mogi, M., Tsukuda, K., Min, L. J., Sakata, A., Jing, F., et al. (2010). Low dose of telmisartan prevents ischemic brain damage with peroxisome proliferator-activated receptor-gamma activation in diabetic mice. J. Hypertens. 28, 1730–1737. doi: 10.1097/HJH.0b013e32833a551a
Jackson, L., Eldahshan, W., Fagan, S. C., and Ergul, A. (2018). Within the brain: the renin angiotensin system. Int. J. Mol. Sci. 19:876. doi: 10.3390/ijms19030876
Jing, F., Mogi, M., Sakata, A., Iwanami, J., Tsukuda, K., Ohshima, K., et al. (2012). Direct stimulation of angiotensin II type 2 receptor enhances spatial memory. J. Cereb. Blood Flow Metab. 32, 248–255. doi: 10.1038/jcbfm.2011.133
Johren, O., Inagami, T., and Saavedra, J. M. (1995). AT1A, AT1B, and AT2 angiotensin II receptor subtype gene expression in rat brain. Neuroreport 6, 2549–2552. doi: 10.1097/00001756-199512150-00024
Kakar, S. S., Riel, K. K., and Neill, J. D. (1992). Differential expression of angiotensin II receptor subtype mRNAs (AT-1A and AT-1B) in the brain. Biochem. Biophys. Res. Commun. 185, 688–692. doi: 10.1016/0006-291X(92)91680-O
Karran, E., Mercken, M., and De Strooper, B. (2011). The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 10, 698–712. doi: 10.1038/nrd3505
Katsuno, T., Morishima-Kawashima, M., Saito, Y., Yamanouchi, H., Ishiura, S., Murayama, S., et al. (2005). Independent accumulations of tau and amyloid β-protein in the human entorhinal cortex. Neurology 64, 687–692. doi: 10.1212/01.WNL.0000151958.79884.86
Kehoe, P. G. (2009). Angiotensins and Alzheimer’s disease: a bench to bedside overview. Alzheimers Res. Ther. 1:3. doi: 10.1186/alzrt3
Kim, J., Onstead, L., Randle, S., Price, R., Smithson, L., Zwizinski, C., et al. (2007). Abeta40 inhibits amyloid deposition in vivo. J. Neurosci. 27, 627–633. doi: 10.1523/JNEUROSCI.4849-06.2007
Kolsch, H., Jessen, F., Freymann, N., Kreis, M., Hentschel, F., Maier, W., et al. (2005). ACE I/D polymorphism is a risk factor of Alzheimer’s disease but not of vascular dementia. Neurosci. Lett. 377, 37–39. doi: 10.1016/j.neulet.2004.11.062
Kume, K., Hanyu, H., Sakurai, H., Takada, Y., Onuma, T., and Iwamoto, T. (2012). Effects of telmisartan on cognition and regional cerebral blood flow in hypertensive patients with Alzheimer’s disease. Geriatr. Gerontol. Int. 12, 207–214. doi: 10.1111/j.1447-0594.2011.00746.x
Lanz, T. V., Ding, Z., Ho, P. P., Luo, J., Agrawal, A. N., Srinagesh, H., et al. (2010). Angiotensin II sustains brain inflammation in mice via TGF-beta. J. Clin. Invest. 120, 2782–2794. doi: 10.1172/JCI41709
Lazaroni, T. L., Raslan, A. C., Fontes, W. R., De Oliveira, M. L., Bader, M., Alenina, N., et al. (2012). Angiotensin-(1-7)/Mas axis integrity is required for the expression of object recognition memory. Neurobiol. Learn. Mem. 97, 113–123. doi: 10.1016/j.nlm.2011.10.003
Lenkei, Z., Palkovits, M., Corvol, P., and Llorens-Cortes, C. (1996). Distribution of angiotensin II type-2 receptor (AT2) mRNA expression in the adult rat brain. J. Comp. Neurol. 373, 322–339. doi: 10.1002/(SICI)1096-9861(19960923)373:3<322::AID-CNE2>3.0.CO;2-4
Lenkei, Z., Palkovits, M., Corvol, P., and Llorens-Cortes, C. (1997). Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front. Neuroendocrinol. 18, 383–439. doi: 10.1006/frne.1997.0155
Li, J., Culman, J., Hortnagl, H., Zhao, Y., Gerova, N., Timm, M., et al. (2005). Angiotensin AT2 receptor protects against cerebral ischemia-induced neuronal injury. FASEB J. 19, 617–619. doi: 10.1096/fj.04-2960fje
Li, N.C., Lee, A., Whitmer, R.A., Kivipelto, M., Lawler, E., Kazis, L.E., et al. (2010). Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ 340:b5465. doi: 10.1136/bmj.b5465
Li, W., Zhang, J. W., Lu, F., Ma, M. M., Wang, J. Q., Suo, A. Q., et al. (2012). [Effects of telmisartan on the level of Abeta1-42, interleukin-1beta, tumor necrosis factor alpha and cognition in hypertensive patients with Alzheimer’s disease]. Zhonghua Yi Xue Za Zhi 92, 2743–2746.
Liu, S., Liu, J., Miura, Y., Tanabe, C., Maeda, T., Terayama, Y., et al. (2014). Conversion of Abeta43 to Abeta40 by the successive action of angiotensin-converting enzyme 2 and angiotensin-converting enzyme. J. Neurosci. Res. 92, 1178–1186. doi: 10.1002/jnr.23404
Lu, J., Wu, L., Jiang, T., Wang, Y., Zhao, H., Gao, Q., et al. (2015). Angiotensin AT2 receptor stimulation inhibits activation of NADPH oxidase and ameliorates oxidative stress in rotenone model of Parkinson’s disease in CATH.a cells. Neurotoxicol. Teratol. 47, 16–24. doi: 10.1016/j.ntt.2014.11.004
MacGregor, D. P., Murone, C., Song, K., Allen, A. M., Paxinos, G., and Mendelsohn, F. A. (1995). Angiotensin II receptor subtypes in the human central nervous system. Brain Res. 675, 231–240. doi: 10.1016/0006-8993(95)00076-3
Marchesi, C., Paradis, P., and Schiffrin, E. L. (2008). Role of the renin–angiotensin system in vascular inflammation. Trends Pharmacol. Sci. 29, 367–374. doi: 10.1016/j.tips.2008.05.003
Matsumoto, S., Shimodozono, M., Miyata, R., and Kawahira, K. (2009). Benefits of the angiotensin II receptor antagonist olmesartan in controlling hypertension and cerebral hemodynamics after stroke. Hypertens. Res. 32, 1015–1021. doi: 10.1038/hr.2009.143
Matsumoto, S., Shimodozono, M., Miyata, R., and Kawahira, K. (2010). The angiotensin II type 1 receptor antagonist olmesartan preserves cerebral blood flow and cerebrovascular reserve capacity, and accelerates rehabilitative outcomes in hypertensive patients with a history of stroke. Int. J. Neurosci. 120, 372–380. doi: 10.3109/00207450903389362
Matsuura, T., Kumagai, H., Onimaru, H., Kawai, A., Iigaya, K., Onami, T., et al. (2005). Electrophysiological properties of rostral ventrolateral medulla neurons in angiotensin II 1a receptor knockout mice. Hypertension 46, 349–354. doi: 10.1161/01.HYP.0000173421.97463.ac
McCarthy, C. A., Vinh, A., Callaway, J. K., and Widdop, R. E. (2009). Angiotensin AT2 receptor stimulation causes neuroprotection in a conscious rat model of stroke. Stroke 40, 1482–1489. doi: 10.1161/STROKEAHA.108.531509
McKinley, M. J., Albiston, A. L., Allen, A. M., Mathai, M. L., May, C. N., Mcallen, R. M., et al. (2003). The brain renin-angiotensin system: location and physiological roles. Int. J. Biochem. Cell Biol. 35, 901–918. doi: 10.1016/S1357-2725(02)00306-0
Millan, M. A., Jacobowitz, D. M., Aguilera, G., and Catt, K. J. (1991). Differential distribution of AT1 and AT2 angiotensin II receptor subtypes in the rat brain during development. Proc. Natl. Acad. Sci. U.S.A. 88, 11440–11444. doi: 10.1073/pnas.88.24.11440
Mogi, M., Iwanami, J., and Horiuchi, M. (2012). Roles of brain angiotensin II in cognitive function and dementia. Int. J. Hypertens. 2012:169649. doi: 10.1155/2012/169649
Mogi, M., Li, J. M., Tsukuda, K., Iwanami, J., Min, L. J., Sakata, A., et al. (2008). Telmisartan prevented cognitive decline partly due to PPAR-gamma activation. Biochem. Biophys. Res. Commun. 375, 446–449. doi: 10.1016/j.bbrc.2008.08.032
Moriwaki, H., Uno, H., Nagakane, Y., Hayashida, K., Miyashita, K., and Naritomi, H. (2004). Losartan, an angiotensin II (AT1) receptor antagonist, preserves cerebral blood flow in hypertensive patients with a history of stroke. J. Hum. Hypertens. 18, 693–699. doi: 10.1038/sj.jhh.1001735
Murphy, M. P., and LeVine, H. (2010). Alzheimer’s disease and the β-Amyloid peptide. J. Alzheimers Dis. 19, 311–323. doi: 10.3233/JAD-2010-1221
Murray, M. M., Bernstein, S. L., Nyugen, V., Condron, M. M., Teplow, D. B., and Bowers, M. T. (2009). Amyloid β-protein: Aβ40 Inhibits Aβ42 Oligomerization. J. Am. Chem. Soc. 131, 6316–6317. doi: 10.1021/ja8092604
Nakagawa, T., Hasegawa, Y., Uekawa, K., Senju, S., Nakagata, N., Matsui, K., et al. (2017). Transient mild cerebral ischemia significantly deteriorated cognitive impairment in a mouse model of Alzheimer’s disease via angiotensin AT1 receptor. Am. J. Hypertens. 30, 141–150. doi: 10.1093/ajh/hpw099
Namsolleck, P., Boato, F., Schwengel, K., Paulis, L., Matho, K. S., Geurts, N., et al. (2013). AT2-receptor stimulation enhances axonal plasticity after spinal cord injury by upregulating BDNF expression. Neurobiol. Dis. 51, 177–191. doi: 10.1016/j.nbd.2012.11.008
Nickenig, G., and Harrison, D. G. (2002). The AT1-type angiotensin receptor in oxidative stress and atherogenesis: part I: oxidative stress and atherogenesis. Circulation 105, 393–396. doi: 10.1161/hc0302.102618
Nozoe, M., Hirooka, Y., Koga, Y., Araki, S., Konno, S., Kishi, T., et al. (2008). Mitochondria-derived reactive oxygen species mediate sympathoexcitation induced by angiotensin II in the rostral ventrolateral medulla. J. Hypertens. 26, 2176–2184. doi: 10.1097/HJH.0b013e32830dd5d3
Oba, R., Igarashi, A., Kamata, M., Nagata, K., Takano, S., and Nakagawa, H. (2005). The N-terminal active centre of human angiotensin-converting enzyme degrades Alzheimer amyloid beta-peptide. Eur. J. Neurosci. 21, 733–740. doi: 10.1111/j.1460-9568.2005.03912.x
O’Caoimh, R., Healy, L., Gao, Y., Svendrovski, A., Kerins, D. M., Eustace, J., et al. (2014). Effects of centrally acting angiotensin converting enzyme inhibitors on functional decline in patients with Alzheimer’s disease. J. Alzheimers Dis. 40, 595–603.
Ongali, B., Nicolakakis, N., Tong, X. K., Aboulkassim, T., Papadopoulos, P., Rosa-Neto, P., et al. (2014). Angiotensin II type 1 receptor blocker losartan prevents and rescues cerebrovascular, neuropathological and cognitive deficits in an Alzheimer’s disease model. Neurobiol. Dis. 68, 126–136. doi: 10.1016/j.nbd.2014.04.018
Panahpour, H., Nekooeian, A. A., and Dehghani, G. A. (2014). Candesartan attenuates ischemic brain edema and protects the blood-brain barrier integrity from ischemia/reperfusion injury in rats. Iran. Biomed. J. 18, 232–238.
Paul, M., Poyan Mehr, A., and Kreutz, R. (2006). Physiology of local renin-angiotensin systems. Physiol. Rev. 86, 747–803. doi: 10.1152/physrev.00036.2005
Premer, C., Lamondin, C., Mitzey, A., Speth, R. C., and Brownfield, M. S. (2013). Immunohistochemical localization of, and angiotensin II receptor subtypes in the rat adrenal, pituitary, and brain with a perspective commentary. Int. J. Hypertens. 2013:175428. doi: 10.1155/2013/175428
Provias, J., and Jeynes, B. (2014). The role of the blood-brain barrier in the pathogenesis of senile plaques in Alzheimer’s disease. Int. J. Alzheimers Dis. 2014:191863. doi: 10.1155/2014/191863
Prusty, S. K., Sahu, P. K., and Subudhi, B. B. (2017). Angiotensin mediated oxidative stress and neuroprotective potential of antioxidants and AT1 receptor blockers. Mini Rev. Med. Chem. 17, 518–528. doi: 10.2174/1389557516666161025094539
Reid, I. A. (1992). Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am. J. Physiol. 262, E763–E778. doi: 10.1152/ajpendo.1992.262.6.E763
Rompe, F., Artuc, M., Hallberg, A., Alterman, M., Stroder, K., Thone-Reineke, C., et al. (2010). Direct angiotensin II type 2 receptor stimulation acts anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor kappaB. Hypertension 55, 924–931. doi: 10.1161/HYPERTENSIONAHA.109.147843
Royea, J., Zhang, L., Tong, X. K., and Hamel, E. (2017). Angiotensin IV receptors mediate the cognitive and cerebrovascular benefits of losartan in a mouse model of Alzheimer’s disease. J. Neurosci. 37, 5562–5573. doi: 10.1523/JNEUROSCI.0329-17.2017
Ruginsk, S., Vechiato, F., Elias, L., Antunes-Rodrigues, J., and Cruz, J. (2015). Angiotensin II reduces mRNA expression for glutamate transporters and glutamine synthetase in cultured hypothalamic astrocytes. FASEB J. 29, 968.919.
Saavedra, J. M. (2012). Angiotensin II AT(1) receptor blockers as treatments for inflammatory brain disorders. Clin. Sci. 123, 567–590. doi: 10.1042/CS20120078
Saavedra, J. M. (2016). Evidence to consider angiotensin II receptor blockers for the treatment of early Alzheimer’s disease. Cell. Mol. Neurobiol. 36, 259–279. doi: 10.1007/s10571-015-0327-y
Saavedra, J. M., Ando, H., Armando, I., Baiardi, G., Bregonzio, C., Juorio, A., et al. (2005). Anti-stress and anti-anxiety effects of centrally acting angiotensin II AT1 receptor antagonists. Regul. Pept. 128, 227–238. doi: 10.1016/j.regpep.2004.12.015
Safciuc, F., Constantin, A., Manea, A., Nicolae, M., Popov, D., Raicu, M., et al. (2007). Advanced glycation end products, oxidative stress and metalloproteinases are altered in the cerebral microvasculature during aging. Curr. Neurovasc. Res. 4, 228–234. doi: 10.2174/156720207782446351
Saharan, S., and Mandal, P. K. (2014). The emerging role of glutathione in Alzheimer’s disease. J. Alzheimers Dis. 40, 519–529.
Seifi, B., Kadkhodaee, M., Bakhshi, E., Ranjbaran, M., Ahghari, P., and Rastegar, T. (2014). Enhancement of renal oxidative stress by injection of angiotensin II into the paraventricular nucleus in renal ischemia-reperfusion injury. Can. J. Physiol. Pharmacol. 92, 752–757. doi: 10.1139/cjpp-2014-0108
Seifi, B., Kadkhodaee, M., Bakhshi, E., Ranjbaran, M., Zahmatkesh, M., Sedaghat, Z., et al. (2015). Angiotensin II in paraventricular nucleus contributes to sympathoexcitation in renal ischemia-reperfusion injury by AT1 receptor and oxidative stress. J. Surg. Res. 193, 361–367. doi: 10.1016/j.jss.2014.06.042
Shin, R. W., Ogino, K., Kondo, A., Saido, T. C., Trojanowski, J. Q., Kitamoto, T., et al. (1997). Amyloid beta-protein (Abeta) 1-40 but not Abeta1-42 contributes to the experimental formation of Alzheimer disease amyloid fibrils in rat brain. J. Neurosci. 17, 8187–8193. doi: 10.1523/JNEUROSCI.17-21-08187.1997
Singh, K. D., and Karnik, S. S. (2016). Angiotensin receptors: structure, function, signaling and clinical applications. J. Cell Signal. 1:111
Sink, K. M., Leng, X., Williamson, J., Kritchevsky, S. B., Yaffe, K., Kuller, L., et al. (2009). Angiotensin converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the cardiovascular health study. Arch. Intern. Med. 169, 1195–1202. doi: 10.1001/archinternmed.2009.175
So, G., Nakagawa, S., Morofuji, Y., Hiu, T., Hayashi, K., Tanaka, K., et al. (2015). Candesartan improves ischemia-induced impairment of the blood-brain barrier in vitro. Cell. Mol. Neurobiol. 35, 563–572. doi: 10.1007/s10571-014-0152-8
Song, K., Allen, A. M., Paxinos, G., and Mendelsohn, F. A. (1991). Angiotensin II receptor subtypes in rat brain. Clin. Exp. Pharmacol. Physiol. 18, 93–96. doi: 10.1111/j.1440-1681.1991.tb01414.x
Song, K., Allen, A. M., Paxinos, G., and Mendelsohn, F. A. (1992). Mapping of angiotensin II receptor subtype heterogeneity in rat brain. J. Comp. Neurol. 316, 467–484. doi: 10.1002/cne.903160407
Soto, M. E., Van Kan, G. A., Nourhashemi, F., Gillette-Guyonnet, S., Cesari, M., Cantet, C., et al. (2013). Angiotensin-converting enzyme inhibitors and Alzheimer’s disease progression in older adults: results from the Reseau sur la Maladie d’Alzheimer Francais cohort. J. Am. Geriatr. Soc. 61, 1482–1488. doi: 10.1111/jgs.12415
Sparks, M. A., Crowley, S. D., Gurley, S. B., Mirotsou, M., and Coffman, T. M. (2014). Classical renin-angiotensin system in kidney physiology. Compr. Physiol. 4, 1201–1228. doi: 10.1002/cphy.c130040
Sun, X., Becker, M., Pankow, K., Krause, E., Ringling, M., Beyermann, M., et al. (2008). Catabolic attacks of membrane-bound angiotensin-converting enzyme on the N-terminal part of species-specific amyloid-beta peptides. Eur. J. Pharmacol. 588, 18–25. doi: 10.1016/j.ejphar.2008.03.058
Supnet, C., and Bezprozvanny, I. (2010). The dysregulation of intracellular calcium in Alzheimer disease. Cell Calcium 47, 183–189. doi: 10.1016/j.ceca.2009.12.014
Takada, J., Ibayashi, S., Ooboshi, H., Ago, T., Ishikawa, E., Kamouchi, M., et al. (2006). Valsartan improves the lower limit of cerebral autoregulation in rats. Hypertens. Res. 29, 621–626. doi: 10.1291/hypres.29.621
Takane, K., Hasegawa, Y., Lin, B., Koibuchi, N., Cao, C., Yokoo, T., et al. (2017). Detrimental effects of centrally administered angiotensin II are enhanced in a mouse model of Alzheimer disease independently of blood pressure. J. Am. Heart Assoc. 6:e004897. doi: 10.1161/JAHA.116.004897
Takeda, S., and Morishita, R. (2017). Angiotensin receptor blocker protects Alzheimer’s disease brain from ischemic insult. Am. J. Hypertens. 30, 110–111. doi: 10.1093/ajh/hpw158
Takeda, S., Sato, N., Takeuchi, D., Kurinami, H., Shinohara, M., Niisato, K., et al. (2009). Angiotensin receptor blocker prevented beta-amyloid-induced cognitive impairment associated with recovery of neurovascular coupling. Hypertension 54, 1345–1352. doi: 10.1161/HYPERTENSIONAHA.109.138586
Tian, M., Zhu, D., Xie, W., and Shi, J. (2012). Central angiotensin II-induced Alzheimer-like tau phosphorylation in normal rat brains. FEBS Lett. 586, 3737–3745. doi: 10.1016/j.febslet.2012.09.004
Tota, S., Goel, R., Pachauri, S. D., Rajasekar, N., Najmi, A. K., Hanif, K., et al. (2013). Effect of angiotensin II on spatial memory, cerebral blood flow, cholinergic neurotransmission, and brain derived neurotrophic factor in rats. Psychopharmacology 226, 357–369. doi: 10.1007/s00213-012-2913-8
Tota, S., Kamat, P. K., Awasthi, H., Singh, N., Raghubir, R., Nath, C., et al. (2009). Candesartan improves memory decline in mice: involvement of AT1 receptors in memory deficit induced by intracerebral streptozotocin. Behav. Brain Res. 199, 235–240. doi: 10.1016/j.bbr.2008.11.044
Tota, S., Kamat, P. K., Saxena, G., Hanif, K., Najmi, A. K., and Nath, C. (2012). Central angiotensin converting enzyme facilitates memory impairment in intracerebroventricular streptozotocin treated rats. Behav. Brain Res. 226, 317–330. doi: 10.1016/j.bbr.2011.07.047
Tsutsumi, K., and Saavedra, J. M. (1991). Characterization and development of angiotensin II receptor subtypes (AT1 and AT2) in rat brain. Am. J. Physiol. 261, R209–R216. doi: 10.1152/ajpregu.1991.261.1.R209
Uekawa, K., Hasegawa, Y., Senju, S., Nakagata, N., Ma, M., Nakagawa, T., et al. (2016). Intracerebroventricular infusion of angiotensin-(1-7) ameliorates cognitive impairment and memory dysfunction in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 53, 127–133. doi: 10.3233/JAD-150642
Umschweif, G., Liraz-Zaltsman, S., Shabashov, D., Alexandrovich, A., Trembovler, V., Horowitz, M., et al. (2014). Angiotensin receptor type 2 activation induces neuroprotection and neurogenesis after traumatic brain injury. Neurotherapeutics 11, 665–678. doi: 10.1007/s13311-014-0286-x
Valenzuela, R., Costa-Besada, M. A., Iglesias-Gonzalez, J., Perez-Costas, E., Villar-Cheda, B., Garrido-Gil, P., et al. (2016). Mitochondrial angiotensin receptors in dopaminergic neurons. Role in cell protection and aging-related vulnerability to neurodegeneration. Cell Death Dis. 7:e2427. doi: 10.1038/cddis.2016.327
Vargas, R., Rincon, J., Pedreanez, A., Viera, N., Hernandez-Fonseca, J. P., Pena, C., et al. (2012). Role of angiotensin II in the brain inflammatory events during experimental diabetes in rats. Brain Res. 1453, 64–76. doi: 10.1016/j.brainres.2012.03.021
Vila-Porcile, E., and Corvol, P. (1998). Angiotensinogen, prorenin, and renin are Co-localized in the secretory granules of all glandular cells of the rat anterior pituitary: an immunoultrastructural study. J. Histochem. Cytochem. 46, 301–311. doi: 10.1177/002215549804600303
Wang, B. R., Shi, J. Q., Zhang, Y. D., Zhu, D. L., and Shi, J. P. (2011). Angiotensin II does not directly affect Abeta secretion or beta-/gamma-secretase activity via activation of angiotensin II type 1 receptor. Neurosci. Lett. 500, 103–107. doi: 10.1016/j.neulet.2011.06.014
Wang, J., Ho, L., Chen, L., Zhao, Z., Zhao, W., Qian, X., et al. (2007). Valsartan lowers brain beta-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J. Clin. Invest. 117, 3393–3402. doi: 10.1172/JCI31547
Wang, J. M., Slembrouck, D., and Potter, W. D. (1996). Expression of angiotensinogen mRNA and localization of angiotensin II and renin in peripheral adrenergic neurons in primary culture. Biochem. Biophys. Res. Commun. 229, 876–881. doi: 10.1006/bbrc.1996.1895
Welander, H., Franberg, J., Graff, C., Sundstrom, E., Winblad, B., and Tjernberg, L. O. (2009). Abeta43 is more frequent than Abeta40 in amyloid plaque cores from Alzheimer disease brains. J. Neurochem. 110, 697–706. doi: 10.1111/j.1471-4159.2009.06170.x
Wharton, W., Goldstein, F. C., Zhao, L., Steenland, K., Levey, A. I., and Hajjar, I. (2015). Renin-angiotensin-system modulation may slow the conversion from mild cognitive impairment to Alzheimer’s disease. J. Am. Geriatr. Soc. 63, 1749–1756. doi: 10.1111/jgs.13627
Wharton, W., Stein, J. H., Korcarz, C., Sachs, J., Olson, S. R., Zetterberg, H., et al. (2012). The effects of ramipril in individuals at risk for Alzheimer’s disease: results of a pilot clinical trial. J. Alzheimers Dis. 32, 147–156.
Wincewicz, D., and Braszko, J. J. (2014). Telmisartan attenuates cognitive impairment caused by chronic stress in rats. Pharmacol. Rep. 66, 436–441. doi: 10.1016/j.pharep.2013.11.002
Wolfe, M. S. (2012). The role of tau in neurodegenerative diseases and its potential as a therapeutic target. Scientifica 2012:796024. doi: 10.6064/2012/796024
Wright, J. W., Stubley, L., Pederson, E. S., Kramar, E. A., Hanesworth, J. M., and Harding, J. W. (1999). Contributions of the brain angiotensin IV-AT4 receptor subtype system to spatial learning. J. Neurosci. 19, 3952–3961. doi: 10.1523/JNEUROSCI.19-10-03952.1999
Yankner, B. A. (1996). Mechanisms of neuronal degeneration in Alzheimer’s disease. Neuron 16, 921–932. doi: 10.1016/S0896-6273(00)80115-4
Yim, H. E., and Yoo, K. H. (2008). Renin-angiotensin system - Considerations for hypertension and kidney. Electrolyte Blood Press. 6, 42–50. doi: 10.5049/EBP.2008.6.1.42
Zheng, W. H., Bastianetto, S., Mennicken, F., Ma, W., and Kar, S. (2002). Amyloid beta peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neuroscience 115, 201–211. doi: 10.1016/S0306-4522(02)00404-9
Zhu, D., Shi, J., Zhang, Y., Wang, B., Liu, W., Chen, Z., et al. (2011). Central angiotensin II stimulation promotes beta amyloid production in sprague dawley rats. PLoS One 6:e16037. doi: 10.1371/journal.pone.0016037
Zhuang, S., Wang, H. F., Wang, X., Li, J., and Xing, C. M. (2016). The association of renin-angiotensin system blockade use with the risks of cognitive impairment of aging and Alzheimer’s disease: a meta-analysis. J. Clin. Neurosci. 33, 32–38. doi: 10.1016/j.jocn.2016.02.036
Zhuo, J., Moeller, I., Jenkins, T., Chai, S. Y., Allen, A. M., Ohishi, M., et al. (1998). Mapping tissue angiotensin-converting enzyme and angiotensin AT1, AT2 and AT4 receptors. J. Hypertens. 16, 2027–2037. doi: 10.1097/00004872-199816121-00026
Zlokovic, B. V. (2011). Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 12, 723–738. doi: 10.1038/nrn3114
Zou, K., Maeda, T., Watanabe, A., Liu, J., Liu, S., Oba, R., et al. (2009). Aβ42-to-Aβ40- and angiotensin-converting activities in different domains of angiotensin-converting enzyme. J. Biol. Chem. 284, 31914–31920. doi: 10.1074/jbc.M109.011437
Keywords: RAS, ARB, ACEI, amyloid β, oxidative stress, vascular disease, inflammation, AD
Citation: Gebre AK, Altaye BM, Atey TM, Tuem KB and Berhe DF (2018) Targeting Renin–Angiotensin System Against Alzheimer’s Disease. Front. Pharmacol. 9:440. doi: 10.3389/fphar.2018.00440
Received: 24 January 2018; Accepted: 13 April 2018;
Published: 30 April 2018.
Edited by:
Hanting Zhang, West Virginia University, United StatesReviewed by:
Luigia Trabace, University of Foggia, ItalyCopyright © 2018 Gebre, Altaye, Atey, Tuem and Berhe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abadi Kahsu Gebre, YWJhZGkua2Foc3VAbXUuZWR1LmV0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.