
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 04 April 2018
Sec. Ethnopharmacology
Volume 9 - 2018 | https://doi.org/10.3389/fphar.2018.00234
Qianlie Xiaozheng decoction (QLXZD), a traditional Chinese medicinal formula, has been used clinically to treat advanced prostate cancer (PCa) for more than 10 years. However, experimental evidence supporting its efficacy is lacking. Here, we investigated the anticancer properties and molecular mechanism of QLXZD in vitro in a human PCa cell line (PC3) and in vivo using PC3 xenografts in nude mice. We confirmed the antineoplastic activity of QLXZD by analyzing cell viability and tumor volume growth, which decreased significantly compared to that of the controls. Autophagy following QLXZD treatment was detected morphologically using transmission electron microscopy and was confirmed by measuring the expression of autophagy markers (LC3-II and p62) using fluorescence analysis, flow cytometry, and western blotting. Increasing autophagic flux induced by QLXZD was monitored via pmCherry-GFP-LC3 fluorescence analysis. QLXZD-induced autophagic cell death was alleviated by the autophagy inhibitors, 3-methyl adenine and hydroxychloroquine. We evaluated the total expression and phosphorylation levels of proteins involved in the Akt/mTOR pathway regulating autophagy. Phosphorylation of Akt, mTOR, and p70S6K, but not total protein levels, decreased following treatment. This is the first study to demonstrate the autophagy-related mechanistic pathways utilized during QLXZD-mediated antitumor activity both in vitro and in vivo. These findings support the clinical use of QLXZD for PCa treatment.
Prostate cancer is one of the most prevalent malignant tumors as well as the second leading cause of cancer-related deaths in men in Western countries, with appropriately 15% of men having PCa during their lifetime (Kar et al., 2016). Androgen deprivation therapy, a treatment for PCa, shows obvious effects at the beginning of treatment, but after 14–30 months, most patients develop HIPC (Thy et al., 2015; Raymond et al., 2017). The median survival for HIPC patients is less than 20 months, and there is currently no effective treatment or cure (Raymond et al., 2017). Therefore, it is essential to elucidate the mechanisms underlying the onset of PCa and to develop novel treatment options for advanced PCa to extend patients’ lives.
The development of PCa is a combined result of genetic and epigenetic alterations that result in the formation of multifocal heterogeneous lesions (Datta et al., 2016; Arcila-Ruiz et al., 2017). Evidence shows that autophagy plays a key role in PCa development, and numerous studies focusing on potential anticancer treatments are ongoing (Naponelli et al., 2015; Amaravadi et al., 2016). Autophagy normally is maintained at low levels in cellular homeostasis, but is strongly induced by stressful conditions such as anticancer drug treatments. Notably, autophagy has recently emerged as a key regulator of multiple aspects of cancer (Shintani and Klionsky, 2004; Singh et al., 2017), and its role in both cancer suppression and progression is still not completely clarified. In PCa, whether autophagy contributes to or opposes cell death is context-specific (Bennett et al., 2013; Elio et al., 2013). The pro-autophagic beclin1 gene is allelically deleted in many PCas, suggesting that autophagy may represent a tumor suppression mechanism in the prostate. Additionally, studies performed in epithelial PCa cells showed that autophagy may provide a survival mechanism in the prostate (Naponelli et al., 2015). Together, these data paradoxically indicate that induction or inhibition of autophagy could have effects against PCa, depending on context.
Experimental and clinical data indicate that the mTOR pathway, and its upstream regulators in the PI3K/PTEN/Akt cascade, are altered in a wide variety of human malignancies (Ciuffreda et al., 2010). PI3K/Akt/mTOR complex-1 (mTORC1) and the androgen receptor are two major drivers of PCa, and the PI3K/Akt/mTOR signaling pathway plays a key role in anticancer drug-mediated regulation of autophagy in human PCa cells (Wang et al., 2016; Kim et al., 2017; Lin et al., 2017). Previous studies have also revealed that the degree of activation of Akt/mTOR signaling components is important, as too little or too much signaling has different consequences depending on the PCa state (Farrow et al., 2014). Therefore, targeting critical components in this signaling cascade to regulate autophagy in PCa cells has become an attractive new therapeutic strategy in the treatment of PCa.
Qianlie Xiaozheng decoction (QLXZD), prepared by Prof. Youfang Liu, is a TCM formula that has been used to effectively treat advanced PCa patients (Yin et al., 2015). QLXZD is composed of seven herbs as shown in Table 1. This formula has been used clinically for over 10 years as a means to effectively alleviate the symptoms of advanced PCa, and improve the patient’s lifespan and quality of life. Because QLXZD is widely used in clinical medicine, it is essential to elucidate its underlying mechanisms to understand its function as well as the basis of the PCa itself.
In this study, we examined the mechanism of QLXZD in vitro using the PC3 cell line. We also evaluated its safety and antitumor effect in vivo using healthy and PC3 tumor-bearing nude mice. The objectives of this research were to provide scientific evidence to support the use of QLXZD in the clinical treatment of PCa and to offer insight into its molecular mechanism.
Seven herbs formed QLXZD were provided by Affiliated Hospital of Integrated Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine (Nanjing, Jiangsu, China). The seven crude drugs were morphologically authenticated according to Chinese Pharmacopoeia (2015 Edition). Reference substances including Calycosin-7-O-β-d-glucoside, rutin, calycosin, tubeimoside, formononetin and curdione, ergosterol were purchased from Must Biological Technology Co. Ltd. (Chengdu, China). The purity of each reference compound was over 98% by HPLC. HPLC-grade acetonitrile and methanol were purchased from ROE Scientific Inc. (United States). Deionized water was purified using a Milli-Q water purification system from Millipore (Bedford, MA, United States). The following antibodies were used: LC3B (Abcam, Cambridge, MA, United States); p62, mTOR, phospho-mTOR (Ser2448), p70S6K, phospho-p70S6K (T389), AKT, phospho-AKT (T308) (Cell Signaling Technology, Beverly, MA, United States); polyclonal rabbit anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Bioworld, Technology, Inc., St. Louis Park, MN, United States). MTT (methyl thiazolyl tetrazolium), 3-MA, and HCQ were purchased from Sigma-Aldrich (St. Louis, MO, United States). FlowCellectTM Autophagy LC3 Antibody-based Assay Kit was purchased from Millipore (Bedford, MA, United States). Other chemicals were provided by local commercial sources and were of analytical grade quality.
To prepare QLXZD samples, 15 g of Astragali Radix Praeparata Cum Melle, 10 g of Polygonati Rhizoma, 30 g of Coicis Semen, 9 g of Curcumae Rhizoma, 9 g of Bolbostemmatis Rhizoma, 15 g of Polyporus, 20 g of Oldenlandia diffusa (willd.) roxb were soaked together in 10 volumes of distilled water for 30 min, then refluxed for 2 h (Cheng et al., 2017). The water extract was filtered, and the residue with eight volumes of water refluxed again for 2 h. The combined filtrates were desiccated to 1.08 g crude drug/mL, and stored at -80°C. Before quality control analysis, the extracts were diluted and filtered through a 0.22 μm filter membrane. Qualitative analysis was performed by Waters e2695 Alliance HPLC system (Waters Corp., Milford, MA, United States) with 2489 UV/Vis DAD detector. Chromatographic separation was carried out at 25°C on an Inertsil ODS-SP C18 column (250 mm × 4.6 mm, 5 μm) with 30 μL injection volume. The mobile phase consisted of linear gradients of 0.05% (v/v) phosphoric acid (A) and acetonitrile (B): 0–15 min, 2–5% B (v/v); 15–30 min, 5–10% B; 30–60 min, 10–40% B; 60–70 min, 40–75% B; 70–75 min, 75–100% B; 75–80 min, 100–100% B. The mobile phase flow rate was 1 mL/min and wavelengths were set at 254 and 210 nm. The concentrations of major constituents were determined by an external standard curve.
PC3 was established from a grade 4 prostatic adenocarcinoma from a 62-year-old Caucasian male. DU 145 was isolated from a brain metastases lesion in a 69-year-old Caucasian male with PCa. PC3 and DU 145 cells were purchased from the Cell Bank of the Shanghai Institute of Biochemistry and Cell Biology and maintained in Ham’s F12 and MEM medium (Gibco, Life Technologies, Carlsbad, CA, United States) respectively, which supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, United States). All cells were cultured in a humidified atmosphere with a 5% CO2 incubator at 37°C.
PC3 cells and DU145 cells were incubated in triplicate in a 96-well plate at a density of 1 × 104 cells with 100 μL culture medium per well supplemented with increasing concentration of QLXZD (0–54 mg/mL) for 24 or 48 h. After which, cell viability was determined by the MTT dye uptake method as described earlier (Yang et al., 2016).
Seed PC3 cells in six-well plates at a density of 5 × 105 cells per well, in a CO2 incubator overnight and treated with QLXZD (0–30 mg/mL) in the presence or absence of 3-MA and H-CQ for 24 h. After which, autophagosome-associated LC3 assay by flow cytometry was performed as the protocol of FlowCellectTM autophagy LC3 antibody-based Assay Kit (Millipore, Bedford, MA, United States).
Seed PC3 cells in six-well plates at a density of 5 × 105 cells per well, in a CO2 incubator overnight and treated with QLXZD (0–30 mg/mL) for 24 h. The cells were collected and fixed in glutaraldehyde (2.5% in 0.1 mol/L cacodylate buffer, PH 7. 4) for 2 h at room temperature and postfixed in osmium tetroxide. After dehydration with a graded series of alcohol concentrations, the samples were rinsed in propylene oxide and impregnated with epoxy resins. The ultrathin sections were contrasted with uranyl acetate and lead citrate for electron microscopy. Electron micrographs were observed through a transmission electron microscope (H7650; Hitachi, Tokyo, Japan), according to the method as described earlier (Wang et al., 2013).
PC3 cells were cultured on coverslips for 24 h followed transfected with pmCherry-GFP-LC3 plasmid using LipofectamineTM 2000 (Invitrogen, Carlsbad, CA, United States) according to the manufacturer’s instruction for another 24 h before receiving QLXZD (0–30 mg/mL) treatments. After 24 h QLXZD treatment, the cells were washed with PBS, fixed with 4% paraformaldehyde, nuclei stained with hoechst and imaged with a confocal fluorescence microscopy (Olympus FV10i, Japan). The number of GFP and mRFP dots was determined as the method described earlier (Hariharan et al., 2011).
Seed PC3 cells in six-well plates at a density of 5 × 105 cells per well, in a CO2 incubator overnight and treated with QLXZD (0–30 mg/mL) for 24 h. The total protein were collected as previously described (Liu et al., 2017). The equalized amounts of proteins from each sample were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) followed by transfer to polyvinylidene fluoride (PVDF) membranes. After blocking with 1% (w/v) albumin from bovine serum (BSA) for 2 h, the membranes were incubated overnight at 4°C with primary antibodies, followed by IRDye-conjugated secondary antibodies for 1 h at room temperature and scanned with an Odyssey infrared fluorescent scanner (LI-COR Biosciences). GAPDH was used as the endogenous control.
Human prostatic cancer xenografts were established by injecting 5 × 106 PC3 cells subcutaneously into 4-week-old BALB/c nude male mice. When the tumors reached a volume of 50–100 mm3, the mice were randomly assigned to model, positive control, and treated groups (low-dose group and high-dose group), and there was a control group that did not receive xenografts. The model group and the control group were given 0.2 mL of sterilized water twice 1 day via oral gavage, the positive control group was given 15 mg/kg CTX daily via oral gavage in a volume of 0.2 mL, the low-dose group was given 0.2 mL of a solution containing 108 mg of QLXZD twice per day via oral gavage (10.8 g/kg), and the high-dose group was given 0.2 mL of a solution containing 216 mg of QLXZD twice per day via oral gavage (21.6 g/kg). Body weight and tumor volume were measured individually every 2 days. Tumor volume was calculated using the formula: Volume = (width2 × length)/2 (Li et al., 2014). After 14 days, the mice were sacrificed and the organs and xenografts were removed for H&E staining and immunohistochemical staining, western blot assays, respectively. All animal experiments were conducted in accordance with the U.S. NIH Guidelines for the Care and Use of Laboratory Animals.
Statistical analysis was performed using SPSS SPSS 15.0 (SPSS, Inc., Chicago, IL, United States). Data are expressed as mean ± SD. Unpaired Student’s t-test were used to compare the means of two groups. Tests were two-sided and P < 0.05 was considered to indicate a statistically significant difference.
To analyze and assess the quality of the QLXZD used in this study, chemical fingerprinting and quantitation of phytochemical markers were performed using chromatography. The species and concentrations of the seven herbs found in QLXZD are listed in Table 1. Overlapping chromatograms of the six batches of QLXZD used during this study are shown in Figure 1A. Importantly, the quality of the QLXZD used appeared to remain stable throughout the in vitro and in vivo experiments performed.
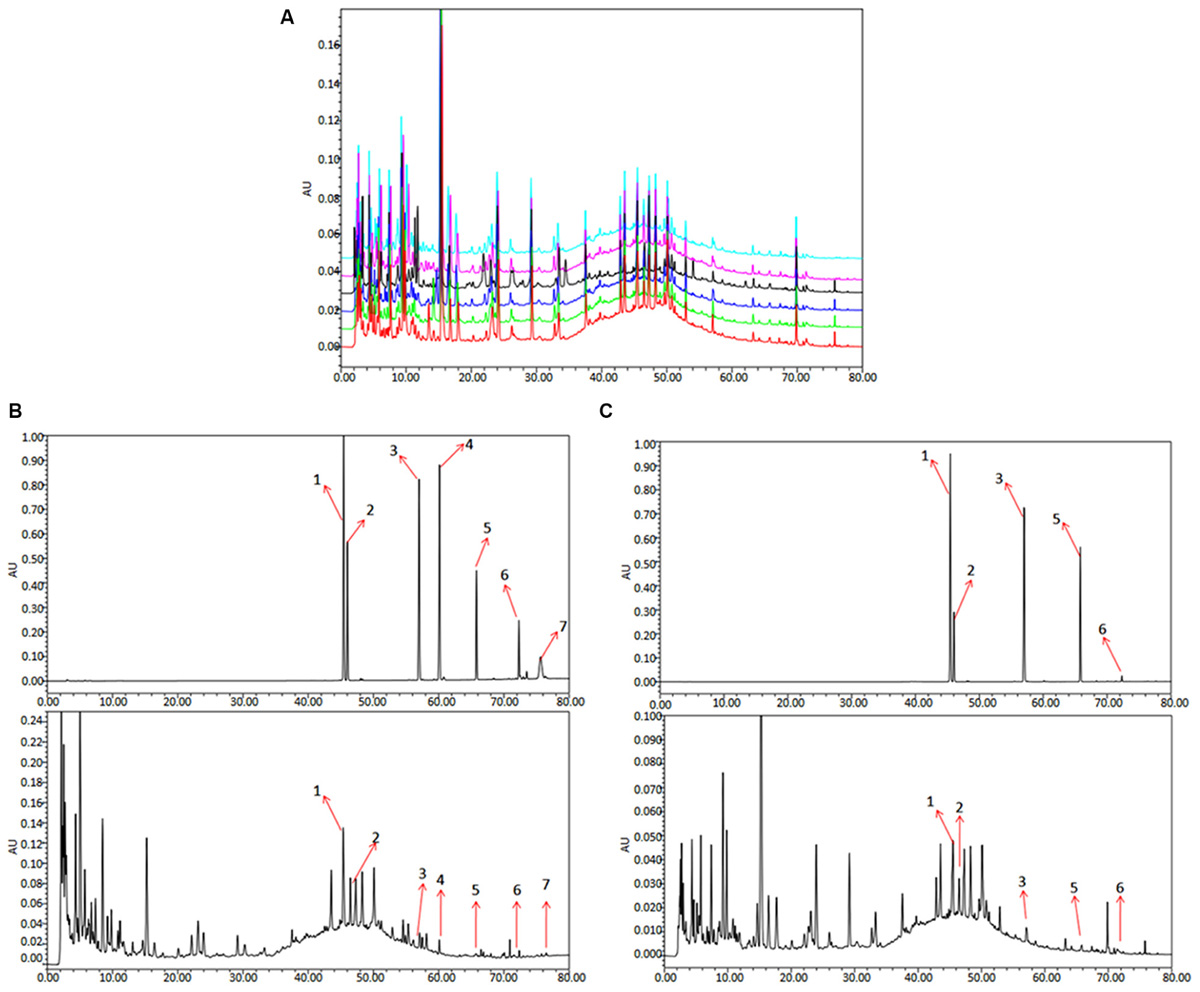
FIGURE 1. HPLC chromatogram of QLXZD. (A) Overlapping chromatograms of six batches of QLXZD. (B) Chromatogram at λmax = 210 nm. The reference standards are shown on top, while QLXZD is shown below. (C) Chromatogram at λmax = 254 nm. The reference standards are shown on top, while QLXZD is shown below.
Chromatograms of the seven herbal constituents of QLXZD were analyzed quantitatively at 210 nm (Figure 1B) and 254 nm (Figure 1C) using an external standard curve, and the calculated concentrations (mg/L) of each compound are summarized in Table 2. The contents of calycosin-7-O-β-D-glucoside, tubeimoside, and rutin were greatest in QLXZD, and come, respectively, from Astragali Radix Praeparata Cum Melle, Bolbostemmatis Rhizoma, and Oldenlandia diffusa (willd.) roxb.
To evaluate the cytotoxicity of QLXZD, PC3 cells were treated with various concentrations of QLXZD for 24 and 48 h. QLXZD inhibited PC3 cell viability significantly in a time- and concentration-dependent manner (Figures 2A,B). The IC50 values of QLXZD for PC3 cells treated for 24 and 48 h were 16.6 and 15.0 mg/mL, respectively. PC3 cells treated with 9.6 mg/mL showed no morphological changes, but suspected autophagic vacuoles (red arrows) were observed, especially in cells treated for 48 h (Figure 2B). As shown in Supplementary Figure S1, treatment with autophagy inhibitors compromised QLXZD-induced cytotoxicity in PC3 cells. The cells appeared obviously lower in density and shrunken following treatment with 27 mg/mL QLXZD.
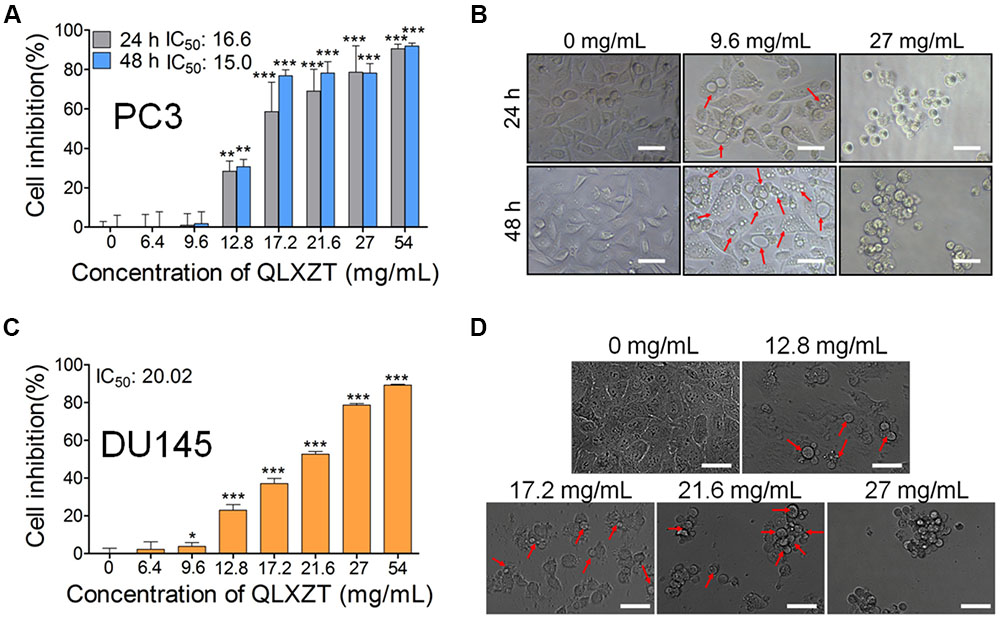
FIGURE 2. Viability of human PCa cell lines treated with QLXZD. (A) PC3 cells were treated with the specified concentrations of QLXZD for 24 and 48 h, and then subjected to MTT assay. Results are expressed as means ± SD (n = 3). Statistical differences between groups were analyzed by Student’s t-test, ∗∗P < 0.01, ∗∗∗P < 0.001 compared to the control (0 mg/mL of QLXZD). (B) Representative images of PC3 cell morphology after treatment with the specified concentrations of QLXZD for 24 and 48 h. Scale bar = 50 μm. (C) DU145 cells were treated with the specified concentrations of QLXZD for 24 h and then subjected to the MTT assay. The results are expressed as means ± SD (n = 3). Statistical differences between groups were analyzed by Student’s t-test, ∗P < 0.05, ∗∗∗P < 0.001 compared to the control (0 mg/mL of QLXZD). (D) Representative images of DU145 cell morphology after treatment with the specified concentrations of QLXZD for 24 h. Scale bars = 50 μm.
QLXZD inhibited DU145 cell viability significantly at 24 h in a concentration-dependent manner (Figures 2C,D). The IC50 value of QLXZD for DU145 cells treated for 24 h was 20.0 mg/mL. DU145 cells treated with 12.8 mg/mL showed no morphological changes, but suspected autophagic vacuoles (red arrows) were observed, especially in cells treated with 17.2 mg/mL (Figure 2D). Cells treated with 21.6 and 27 mg/mL appeared obviously lower in density and shrunken. Based on these findings, a 24 h exposure time and PC3 cells were used for all subsequent experiments.
Transmission electron microscopy can be used to identify autophagic structures morphologically at a resolution in the nm range in their natural environment and cellular position (Klionsky et al., 2016). TEM analysis of QLXZD-treated PC3 cells showed the formation of distinct autophagic vacuoles that appeared to be dependent on the QLXZD concentration (Figure 3A, scale bar = 5 μm). Furthermore, AVi could be differentiated from AVd in QLXZD-treated cells using this analysis (Figure 3A, scale bar = 1 μm).
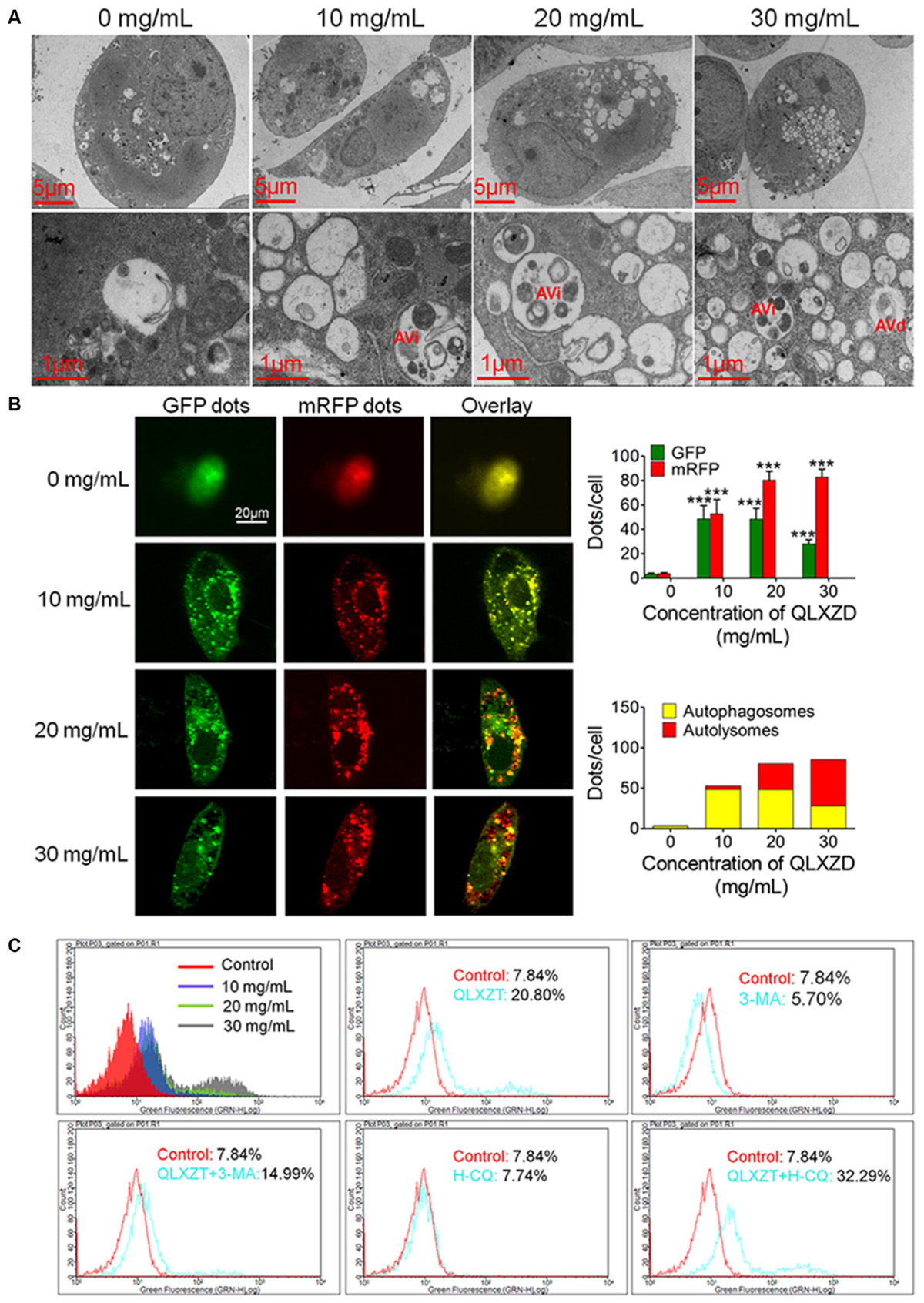
FIGURE 3. Autophagy in PC3 cells induced by QLXZD. (A) Representative TEM images. Autophagic vacuoles were monitored in PC3 cells and increased with the increasing of QLXZD concentration (scale bar = 5 μm). Initial autophagic vacuoles (AVis) and degradative autophagic vacuoles (AVds) are also highlighted (scale bar = 1 μm). (B) Representative confocal microscopy images of PC3 cells transfected with fluorescent Cherry-GFP-LC3 plasmid after QLXZD treatment. QLXZD treatment increased both numbers of GFP and mRFP dots per cell. ∗∗∗P < 0.001. (C) FACS was used to detect GFP-LC3 expression in QLXZD-treated PC3 cells as well as cells treated with 3-MA and QLXZD (which induced a left-ward shift of the histogram) or HCQ and QLXZD (which induced a right-ward shift of the histogram).
Because autophagic flux is a dynamic process, it is imperative to differentiate between increased autophagosome formation and decreased autolysosome clearance to determine the true level of autophagy (Hariharan et al., 2011). pmCherry-GFP-LC3 fluorescence analysis is a well-characterized method used to monitor autophagic flux (Kimura et al., 2007; Jacquin et al., 2017). In this analysis, GFP fluorescence, but not mRFP fluorescence, is quenched at the acidic pH of autolysosomes, while both GFP and mRFP continue to fluoresce in autophagosomes. Therefore, an increase in autophagic flux is indicated by a higher ratio of red (mRFP alone: autolysosomes) to yellow (mRFP and GFP merged: autophagosomes) punctate staining. As shown in Figure 3B, QLXZD treatment increased the total number of both autophagosomes and autolysosomes per cell. Furthermore, treating the cells with 30 mg/mL QLXZD significantly increased the ratio of autolysosomes to autophagosomes (more free red than yellow puncta were seen), suggesting that QLXZD increased autophagic flux.
Flow cytometry was performed to evaluate the autophagy-associated expression of GFP-labeled LC3-II. These data indicated that the percentage of QLXZD-treated cells expressing GFP-LC3-II increased significantly in a concentration-dependent manner compared with that in the control group, as illustrated by the rightward shift of the histogram (Figure 3C).
To confirm that the observed increase in LC3-II-expressing PC3 cells was indeed related to autophagy, the cells were treated with two known autophagic inhibitors: 3-MA and HCQ. Treating PC3 cells with HCQ (30 μM) and QLXZD (20 mg/mL) simultaneously for 24 h resulted in a rightward shift of the histogram compared with PC3 cells treated with QLXZD alone. This indicated inhibition of QLXZD-induced autophagy due to its inhibition of autolysosome degradation. Alternatively, pre-treating cells with 3-MA (5 mM) for 2 h before the addition of QLXZD (20 mg/mL) for 24 h resulted in a leftward shift of the histogram compared with PC3 cells treated with QLXZD alone due to the inhibition of autophagosome formation.
The expression of two key markers of autophagy, LC3-II and p62, was examined by western blotting (Jiang and Mizushima, 2015; Feng et al., 2017). QLXZD treatment appeared to drive the conversion of LC3 from the cytoplasmic form (LC3-I) into the autophagosomic form (LC3-II) while also decreasing the expression of p62 in PC3 cells in a concentration-dependent manner (Figure 4A). PC3 cells treated with 20 mg/mL QLXZD were also treated with HCQ or 3-MA as described above. HCQ treatment inhibited the degradation of LC3-II and p62 in autolysosomes, while 3-MA treatment significantly inhibited the degradation of p62 and conversion of LC3-II (Figure 4B). These data further confirmed that QLXZD induced autophagic cell death in PC3 cells.
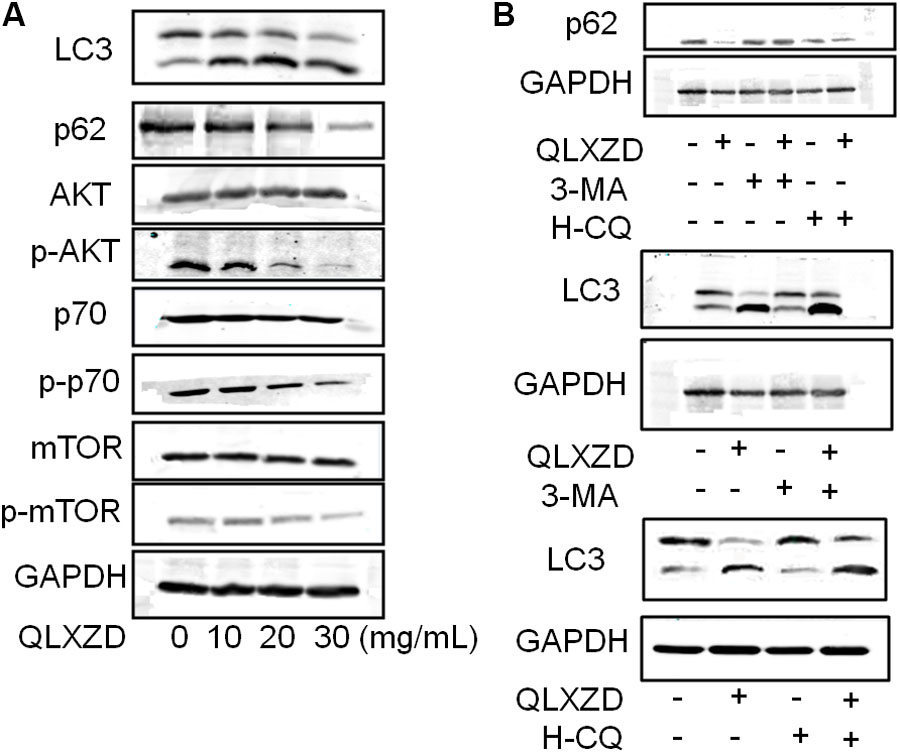
FIGURE 4. Inhibition of the PI3K/Akt/mTOR signaling pathway in PC3 cells. (A) After treatment with QLXZD, the expression of LC3-I/II and p62 were detected by western blotting, a dose-dependent decrease of phosphorylation of proteins involved in the Akt/mTOR signaling pathway were observed. (B) After treatment with QLXZD, 3-MA and HCQ, the expression of LC3-I/II and p62 were detected by western blotting.
To investigate whether QLXZD inhibited the PI3K/Akt/mTOR signaling pathway, we used western blotting to assess the expression of various proteins involved in this signaling cascade (Akt, p-Akt, mTOR, p-mTOR, p70S6K, and p-p70S6K) in PC3 cells treated with QLXZD. As shown in Figure 4A, QLXZD concentration-dependently inhibited the phosphorylation of Akt (T308), mTOR (Ser2448), and p70S6K (T389) in PC3 cells, while the total expression of these proteins was not altered by QLXZD treatment. Inhibiting the phosphorylation-dependent activation of these proteins likely plays a significant role in the observed QLXZD-induced autophagy in PC3 cells.
Mice bearing PC3 xenografts were treated with QLXZD, and their body weights and tumor volumes were measured individually every 2 days. After 14 days of treatment, the mice were sacrificed, and their organs and xenografts were moved and weighed. QLXZD treatment caused a marked inhibition of tumor growth compared with vehicle-treated mice (Figure 5A). Tumors grew rapidly in mice treated with sterilized water. By day 14, the average tumor volume in the model group increased by approximately fourfold, whereas the tumor volume in QLZXD-treated mice increased only twofold. This was approximately 46% less than the control model group, and similar to the CTX-treated group. The inhibition of tumor growth in mice treated with the high concentration of QLXZD was slightly greater than that in the group treated with the low concentration. Differences in tumor volumes of the groups treated with QLXZD (P < 0.01) and CTX (P < 0.001), when compared with that of the model group, were both statistically significant. Changes in the tumor to body weight ratio (Figure 5B) were consistent with the changes in tumor volume. Differences in the tumor to body weight ratio in the CTX-treated group (P < 0.001), high-concentration QLXZD-treated group (P < 0.001), and low-concentration QLXZD-treated group (P < 0.01), when compared with the model group, were statistically significant.
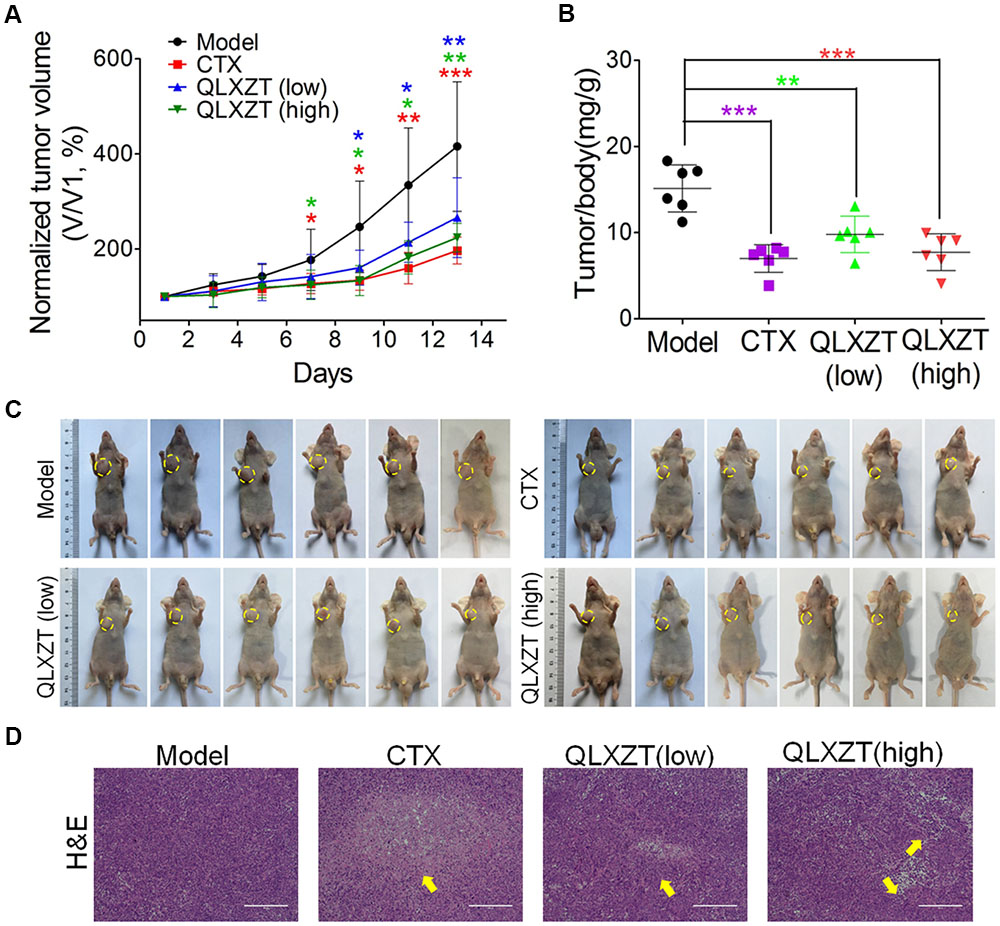
FIGURE 5. Antitumor activity in vivo of QLXZD against PC3 xenografts. The tumor growth curve (A) and the ratio curve of tumor to body weight of nude mice (B). Statistical differences between treating groups and model group were analyzed by Student’s t-test, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, the values are expressed as the mean ± SD (n = 6). (C) The inhibition in the size of the xenografted prostate tumors is shown, yellow circle indicate the tumors on mice. (D) Representative images of H&E stained xenograft tumor tissue indicate that high doses of QLXZD caused obvious tumor inhibition (yellow arrow). Scale bar = 200 μm.
Photographs of complete tumors in mice are shown in Figure 5C (marked with yellow circles). Xenograft tumor tissues were also examined by hematoxylin and eosin staining (Figure 5D). The pathology examination clearly demonstrated tumor necrosis in the CTX- and QLXZD-treated groups (yellow arrows), but not in the model group. The high-concentration QLXZD-treated group showed much larger necrotic regions than the low-concentration QLXZD-treated group; the CTX group had the largest necrotic area (Figure 5D).
Isolated xenograft tumor tissue was subjected to western blotting (Figure 6A) and immunohistochemical analysis (Figure 6B). QLXZD was again observed to increase the conversion of LC3-I to LC3-II and decrease the expression of p62 in a dose-dependent manner. Furthermore, the expression of PI3K/Akt/mTOR pathway-related proteins was also altered in xenograft tumor tissue isolated from QLXZD-treated mice, with the phosphorylation of Akt (T308), mTOR (Ser2448), and p70S6K (T389) being inhibited in the QLXZD-treated group. Similar to our in vitro experiments, total levels of these proteins were not altered regardless of treatment, confirming the role of PI3K/Akt/mTOR pathway inhibition during QLXZD-induced autophagy.
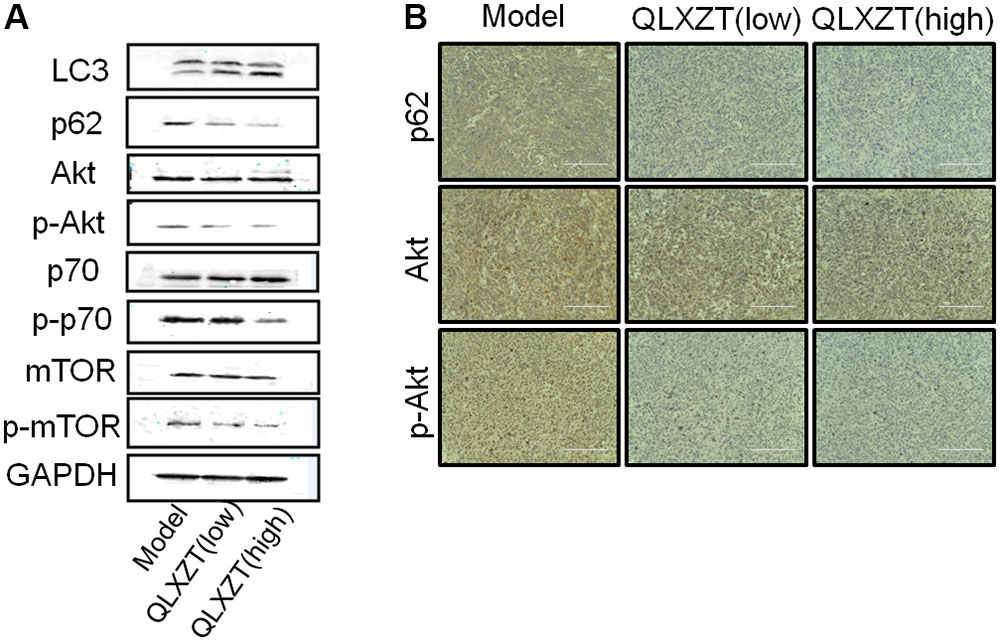
FIGURE 6. Autophagy induced by QLXZD via inhibiting PI3K/Akt/mTOR signaling in PC3 xenografts. (A) Isolated xenograft tumor tissue was subjected to western blotting using anti-LC3 and anti-p62 antibodies, and the expression of Akt, p-Akt, mTOR, p-mTOR, p70S6K, and p-p70S6K was also analyzed with western blot. (B) Representative immunohistochemistry images of the xenograft tumor tissue showing cells that were positive (brown staining) and negative (blue staining) for p62, AKT, p-AKT. Scale bar = 200 μm.
Mice were weighed individually every 2 days over the 14-day course of treatment. The increase in body weight observed in QLXZD-treated mice was consistent with the control group, while CTX-treated mice showed a significant decrease in body weight (Figure 7A). Major organs were harvested immediately after the mice were sacrificed. The organ to body weight ratios showed no significant differences between treatment groups (Figure 7B). No obvious histological differences were found (Figure 7C).
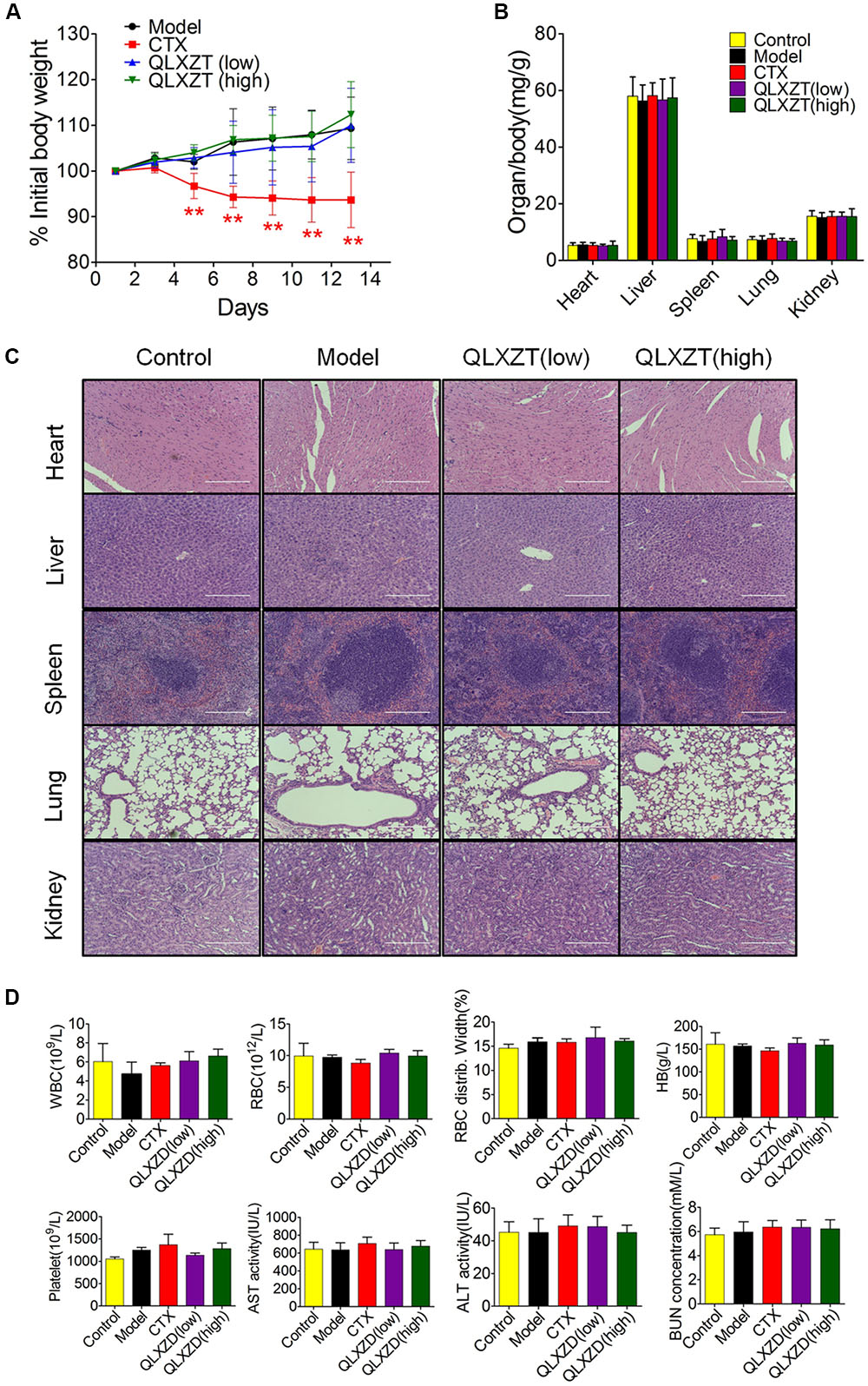
FIGURE 7. In vivo safety evaluation of QLXZD. (A) The body weight curve of all test groups. Statistical differences between treating groups and model group were analyzed by Student’s t-test, ∗∗P < 0.01, the values are expressed as the mean ± SD (n = 6). (B) The ratio of major organs to body weight in all groups. Statistical differences between test groups and control group were analyzed by Student’s t-test, the values are expressed as the mean ± SD (n = 6). (C) Representative images of H&E stained sections of major organs. Scale bar = 200 μm. (D) Hematological parameters and serum levels of AST, ALT, and BUN. Data are shown as the means ± SD (n = 3).
Blood samples were collected before mice were sacrificed and used for routine blood tests within 15 min. We found no physiologically significant differences in any of the important hematology markers or in the blood serum concentrations of aspartate transaminase, alanine transaminase, or blood urea nitrogen in the QLXZD-treated mice (Figure 7D). Taken together, these data suggest these doses of QLXZD were non-toxic in vivo.
According to the serum androgen level of PCa patients, PCa is divided into HDPC and HIPC in WHO classification (Newling et al., 1997). The primary PCa is almost the typical HDPC, androgen is the key growth factor of which, and androgen deprivation therapy shows obvious effects at the beginning of treatment. As the course of the PCa progresses after 14–30 months, most patients develop HIPC and eventually die from it, which is the final form and inevitable result of the development of PCa, and there is currently no effective treatment or cure (Thy et al., 2015; Raymond et al., 2017). Among the various PCa cells, androgen-refractory PC3 and DU 145 cells are widely used in HIPC research. PC3 cell line was isolated from a grade 4 prostatic adenocarcinoma from a 62-year-old Caucasian male. DU 145 cell line was isolated from a brain metastases lesion in a 69-year-old Caucasian male with PCa and 3-year history of lymphocytic leukemia. Studies have shown that the two well-characterized androgen independent cell lines, which lack androgen receptors, exhibit differences in their chemotherapeutic response (Jayakumar et al., 2014). Some sublines of PC3 cell line are highly metastatic when grafted to nude mice, while DU 145 is moderately metastatic in comparison to that of PC3 cells (Janssen et al., 2015).
Traditional Chinese medicine has been used extensively in the treatment of patients with metastatic PCa develop HIPC; it has multiple targets and action mechanisms, and varying degrees of efficacy depending on the synergistic actions of its complex chemical components (Liu et al., 2015). QLXZD prepared by Prof. Youfang Liu is an effective TCM prescription and has been used clinically as a treatment for advanced PCa for more than 10 years. Modern medical research suggests that autophagic modulation by current PCa treatments is a promising therapeutic option (Farrow et al., 2014). QLXZD may be particularly useful for patients with HIPC because it ameliorates urinary urgency, dysuria, and weakness, and decreases the doubling time of prostate-specific antigen levels, thereby benefitting patients. In this study, we performed a quality control analysis of this TCM and evaluated its effects in vitro and in vivo to better understand its underlying mechanism. We demonstrated, for the first time, both the mechanism and efficacy of QLXZD as a treatment option for PCa.
It is important to note that autophagy plays a paradoxical role during PCa progression; it can be neutral, tumor-suppressive, or tumor-promoting in different contexts. In this study, we demonstrate that QLXZD indeed has antitumor capabilities and induces autophagy in PC3 cells in vitro and in PC3 xenografts in vivo with no overt toxicity. Autophagic vacuole formation and autophagic flux were monitored in QLXZD-treated PC3 cells using TEM, and by assessing the protein levels of LC3-I/II and p62. The responses appeared to be largely concentration-dependent and were inhibited by 3-MA and HCQ, and no significant increase of apoptosis was involved in QLXZD-induced effects (Supplementary Figure S2). Indicating that induction of autophagy was the main pathway regulated by QLXZD. These results are consistent with the literature regarding PCa treatment via autophagy induction, but opposite to the autophagy blockade induced by capsaicin (a natural ingredient in peppers) in PCa cells (Jiang et al., 2013; Kumar et al., 2014). These results confirm that autophagy plays a paradoxical role during PCa progression. Thus, the final effect of autophagy in cancer cells is highly variable depending on the integration of extracellular conditions and complex signaling pathways.
To further elucidate the mechanism by which QLXZD regulated autophagy, we performed various molecular studies focusing on the mTOR pathway. Numerous reports have demonstrated that mTOR kinase suppresses autophagy and apoptosis (Wang et al., 2015) via interference with Atg protein-related formation of autophagosomes (Levine and Yuan, 2005). The PI3K/Akt/mTOR signaling pathways are key axes involved in anticancer drug-induced autophagy, including PCa treatment (Kim et al., 2017; Lin et al., 2017; Yu et al., 2017). This largely explains the effectiveness of mTOR inhibitors, such as rapamycin, as anticancer agents. However, rapamycin only inhibits mTORC1, whereas its upstream regulators are still activated (Yori et al., 2014; Fraga et al., 2017). This limits its antineoplastic effects. Our data indicated that QLXZD not only inhibited the activation of mTORC1 but also blocked the phosphorylation of its upstream regulator, namely Akt, which prevented compensatory feedback from its downstream regulator. Therefore, it appears that QLXZD may be a stronger antitumor drug than other conventional therapeutics. Although additional work is necessary to fully characterize the clinical efficacy of QLXZD and other TCMs, these findings highlight a future for this QLXZD formula in the treatment of androgen-independent PCa.
This study is the first to demonstrate the mechanistic pathways utilized during QLXZD-mediated antitumor activity both in vitro (PC3 cells) and in xenografts (Figure 8). These effects are mediated through induction of excessive autophagy at high concentrations of QLXZD, leading to accelerated cell death. QLXZD functions by inhibiting the Akt/mTOR signaling pathway. These findings provide experimental evidence for the clinical use of QLXZD and a foundation for further scientific study of TCMs.
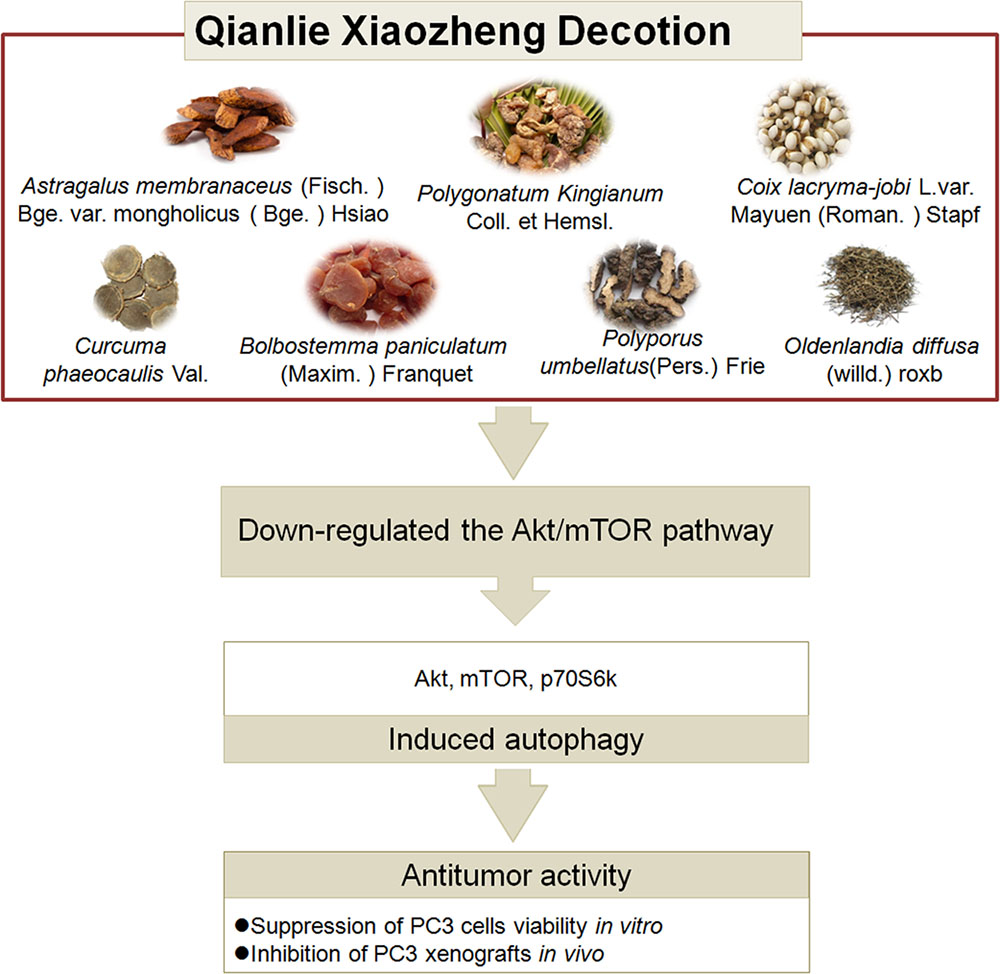
FIGURE 8. The ingredients, antitumor activity against prostate cancer and possible mechanisms of action of QLXZD. The decoction is made from seven different herbs. Its antitumor activity against prostate cancer in vitro and in vivo is proposed to be due to its autophagy induction via down-regulating the AKT/mTOR pathway.
YX obtained funding, design, acquisition of data, analysis of data, and writing of the manuscript. XC design, technical support, proof-reading, and revision of the manuscript. BZ technical support and revision of the manuscript. RF, YJ, and GC technical support, analysis and interpretation of data. ZL obtained funding, conception and design, study supervision, writing and revision of the manuscript. All authors read and approved the final manuscript.
This work was financially supported by Technology Project of Jiangsu Province Administration of Traditional Chinese Medicine (YB2015145), 2017 Technology and Innovation Foundation of Zhenjiang city (SH2017019), and the Science and Technology Development Foundation of Clinical Medicine of Jiangsu University (JLY20160101).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Xiaoyan Sun, Jie Yang, and Qian Zhou in the Laboratory of Cellular and Molecular Biology (Jiangsu Province Academy of Traditional Chinese Medicine) for providing us with experimental guidance.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2018.00234/full#supplementary-material
3-MA, 3-methyladenine; AVd, degradative autophagic vacuole; AVi, initial autophagic vacuoles; CTX, cyclophosphamide; GFP, green fluorescent protein; HCQ, hydroxychloroquine; HDPC, hormone dependent PCa; HIPC, hormone-independent PCa; mRFP, monomeric red fluorescent protein; mTORC1, mechanistic target of rapamycin complex-1; PCa, prostate cancer; PI3K, phosphoinositide 3-kinase; QLXZD, Qianlie Xiaozheng decoction; TCM, traditional Chinese medicine; TEM, transmission electron microscopy.
Amaravadi, R., Kimmelman, A. C., and White, E. (2016). Recent insights into the function of autophagy in cancer. Genes Dev. 30, 1913–1930. doi: 10.1101/gad.287524.116
Arcila-Ruiz, M. J., Assimos, D. G., Brucker, B. M., Chancellor, M. B., Druskin, S. C., Curtis Nickel, J., et al. (2017). Best of the 2017 AUA annual meeting: highlights from the 2017 American urological association annual meeting, May 12-16, 2017, Boston, MA. Rev. Urol. 19, 169–179. doi: 10.3909/riu0763
Bennett, H. L., Stockley, J., Fleming, J. T., Mandal, R., O’Prey, J., Ryan, K. M., et al. (2013). Does androgen-ablation therapy (AAT) associated autophagy have a pro-survival effect in LNCaP human prostate cancer cells? BJU Int. 111, 672–682. doi: 10.1111/j.1464-410X.2012.11409.x
Cheng, X., Huo, J., Wang, D., Cai, X., Sun, X., Lu, W., et al. (2017). Herbal medicine AC591 prevents oxaliplatin-induced peripheral neuropathy in animal model and cancer patients. Front. Pharmacol. 8:344. doi: 10.3389/fphar.2017.00344
Ciuffreda, L., Di, S. C., Incani, U. C., and Milella, M. (2010). The mTOR pathway: a new target in cancer therapy. Curr. Cancer Drug Targets 10, 484–495. doi: 10.2174/156800910791517172
Datta, D., Aftabuddin, M., Gupta, D. K., Raha, S., and Sen, P. (2016). Human prostate cancer hallmarks map. Sci. Rep. 6:30691. doi: 10.1038/srep30691
Elio, Z., Simonetta, P., Sara, M. E., Donatella, S., Silvia, C., Antonio, F., et al. (2013). Autophagy in prostate cancer and androgen suppression therapy. Int. J. Mol. Sci. 14, 12090–12106. doi: 10.3390/ijms140612090
Farrow, J. M., Yang, J. C., and Evans, C. P. (2014). Autophagy as a modulator and target in prostate cancer. Nat. Rev. Urol. 11, 508–516. doi: 10.1038/nrurol.2014.196
Feng, L., Zhang, J., Zhu, N., Ding, Q., Zhang, X., Yu, J., et al. (2017). Ubiquitin ligase SYVN1/HRD1 facilitates degradation of the SERPINA1 Z variant/α-1-antitrypsin Z variant via SQSTM1/p62-dependent selective autophagy. Autophagy 13, 686–702. doi: 10.1080/15548627.2017.1280207
Fraga, A., Ribeiro, R., Coelho, A., Vizcaino, J. R., Coutinho, H., Lopes, J. M., et al. (2017). Genetic polymorphisms in key hypoxia-regulated downstream molecules and phenotypic correlation in prostate cancer. BMC Urol. 17:12. doi: 10.1186/s12894-017-0201-y
Hariharan, N., Zhai, P., and Sadoshima, J. (2011). Oxidative stress stimulates autophagic flux during Ischemia/Reperfusion. Antioxid. Redox Signal. 14, 2179–2190. doi: 10.1089/ars.2010.3488
Jacquin, E., Leclercmercier, S., Judon, C., Blanchard, E., Fraitag, S., and Florey, O. (2017). Pharmacological modulators of autophagy activate a parallel noncanonical pathway driving unconventional LC3 lipidation. Autophagy 13, 854–867. doi: 10.1080/15548627.2017.1287653
Janssen, T., Darro, F., Petein, M., Raviv, G., Pasteels, J. L., Kiss, R., et al. (2015). In vitro characterization of prolactin-induced effects on proliferation in the neoplastic LNCaP, DU145, and PC3 models of the human prostate. Cancer 77, 144–149. doi: 10.1002/(SICI)1097-0142(19960101)77:1<144::AID-CNCR24>3.0.CO;2-4
Jayakumar, S., Kunwar, A., Sandur, S. K., Pandey, B. N., and Chaubey, R. C. (2014). Differential response of DU145 and PC3 prostate cancer cells to ionizing radiation: role of reactive oxygen species, GSH and Nrf2, in radiosensitivity. Biochim. Biophys. Acta 1840, 485–494. doi: 10.1016/j.bbagen.2013.10.006
Jiang, H., Sun, J., Xu, Q., Liu, Y., Wei, J., Young, C. Y. F., et al. (2013). Marchantin M: a novel inhibitor of proteasome induces autophagic cell death in prostate cancer cells. Cell Death Dis. 4:e761. doi: 10.1038/cddis.2013.285
Jiang, P., and Mizushima, N. (2015). LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods 75, 13–18. doi: 10.1016/j.ymeth.2014.11.021
Kar, S. P., Beesley, J., Amin, A. O. A., Michailidou, K., Tyrer, J., Kotejarai, Z., et al. (2016). Genome-wide meta-analyses of breast, ovarian, and prostate cancer association studies identify multiple new susceptibility loci shared by at least two cancer types. Cancer Discov. 6, 1052–1067. doi: 10.1158/2159-8290.CD-15-1227
Kim, K. Y., Park, K. I., Kim, S. H., Yu, S. N., Park, S. G., Kim, Y. W., et al. (2017). Inhibition of autophagy promotes salinomycin-induced apoptosis via reactive oxygen species-mediated PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human prostate cancer cells. Int. J. Mol. Sci. 18:1088. doi: 10.3390/ijms18051088
Kimura, S., Noda, T., and Yoshimori, T. (2007). Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 3, 452–460. doi: 10.4161/auto.4451
Klionsky, D. J., Abdelmohsen, K., Abe, A., Abedin, M. J., Abeliovich, H., Acevedo Arozena, A., et al. (2016). Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12, 1–222. doi: 10.1080/15548627.2015.1100356
Kumar, D., Shankar, S., and Srivastava, R. K. (2014). Rottlerin induces autophagy and apoptosis in prostate cancer stem cells via PI3K/Akt/mTOR signaling pathway. Cancer Lett. 343, 179–189. doi: 10.1016/j.canlet.2013.10.003
Levine, B., and Yuan, J. (2005). Autophagy in cell death: an innocent convict? J. Clin. Invest. 115, 2679–2688. doi: 10.1172/JCI26390
Li, Y., Luo, P., Wang, J., Dai, J., Yang, X., Wu, H., et al. (2014). Autophagy blockade sensitizes the anticancer activity of CA-4 via JNK-Bcl-2 pathway. Toxicol. Appl. Pharmacol. 274, 319–327. doi: 10.1016/j.taap.2013.11.018
Lin, H. J., Liu, H. H., Lin, C. D., Kao, M. C., Chen, Y. A., Chiangni, C., et al. (2017). Cytolethal distending toxin enhances radiosensitivity in prostate cancer cells by regulating autophagy. Front. Cell. Infect. Microbiol. 7:223. doi: 10.3389/fcimb.2017.00223
Liu, J., Wang, H., Gu, J., Deng, T., Yuan, Z., Hu, B., et al. (2017). BECN1-dependent CASP2 incomplete autophagy induction by binding to rabies virus phosphoprotein. Autophagy 13, 739–753. doi: 10.1080/15548627.2017.1280220
Liu, J., Wang, S., Zhang, Y., Fan, H. T., and Lin, H. S. (2015). Traditional Chinese medicine and cancer: history, present situation, and development. Thorac. Cancer 6, 561–569. doi: 10.1111/1759-7714.12270
Naponelli, V., Modernelli, A., Bettuzzi, S., and Rizzi, F. (2015). Roles of autophagy induced by natural compounds in prostate cancer. Biomed. Res. Int. 2015:121826. doi: 10.1155/2015/121826
Newling, D., Fossa, S. D., Andersson, L., Abrahamsson, P. A., Aso, Y., Eisenberger, M. A., et al. (1997). Assessment of hormone refractory prostate cancer. Urology 149(Suppl. 4A), 46–53. doi: 10.1016/S0090-4295(99)80323-9
Raymond, E., O’Callaghan, M. E., Campbell, J., Vincent, A. D., Beckmann, K., Roder, D., et al. (2017). An appraisal of analytical tools used in predicting clinical outcomes following radiation therapy treatment of men with prostate cancer: a systematic review. Radiat. Oncol. 12:56. doi: 10.1186/s13014-017-0786-z
Shintani, T., and Klionsky, D. J. (2004). Autophagy in health and disease: a double-edged sword. Science 306, 990–995. doi: 10.1126/science.1099993
Singh, S. S., Vats, S., Chia, Y. Q., Tan, T. Z., Deng, S., Mei, S. O., et al. (2017). Dual role of autophagy in hallmarks of cancer. Oncogene 37, 1142–1158. doi: 10.1038/s41388-017-0046-6
Thy, P., Sadowski, M. C., Li, H., Richard, D. J., D’Emden, M. C., and Kerry, R. (2015). Advances in hormonal therapies for hormone naïve and castration-resistant prostate cancers with or without previous chemotherapy. Exp. Hematol. Oncol. 5:15. doi: 10.1186/s40164-016-0046-1
Wang, F., Mao, Y., You, Q., Hua, D., and Cai, D. (2015). Piperlongumine induces apoptosis and autophagy in human lung cancer cells through inhibition of PI3K/Akt/mTOR pathway. Int. J. Immunopathol. Pharmacol. 28, 362–373. doi: 10.1177/0394632015598849
Wang, J., Tan, X., Yang, Q., Zeng, X., Zhou, Y., Luo, W., et al. (2016). Inhibition of autophagy promotes apoptosis and enhances anticancer efficacy of adriamycin via augmented ROS generation in prostate cancer cells. Int. J. Biochem. Cell Biol. 77(Pt A), 80–90. doi: 10.1016/j.biocel.2016.05.020
Wang, Q., Zhu, J., Zhang, K., Jiang, C., Wang, Y., Yuan, Y., et al. (2013). Induction of cytoprotective autophagy in PC-12 cells by cadmium. Biochem. Biophys. Res. Commun. 438, 186–192. doi: 10.1016/j.bbrc.2013.07.050
Yang, J., Qian, S., Cai, X., Lu, W., Hu, C., Sun, X., et al. (2016). Chikusetsusaponin IVa butyl ester (CS-IVa-Be), a novel IL6R antagonist, inhibits IL6/STAT3 signaling pathway and induces cancer cell apoptosis. Mol. Cancer Ther. 15, 1190–1200. doi: 10.1158/1535-7163.MCT-15-0551
Yin, X., Liu, D., Kegang, L. I., Zhang, Y., and Song, S. (2015). The effect of qianliexiaozheng decotion on PC-3 prostate cancer stem cells. China J. Chin. Med. 30, 1545–1548. doi: 10.16368/j.issn.1674-8999.2015.05.236
Yori, J. L., Lozada, K. L., Seachrist, D. D., Mosley, J. D., Abdulkarim, F. W., Booth, C. N., et al. (2014). Combined SFK/mTOR inhibition prevents rapamycin-induced feedback activation of AKT and elicits efficient tumor regression. Cancer Res. 74, 4762–4771. doi: 10.1158/0008-5472.CAN-13-3627
Keywords: Qianlie Xiaozheng decoction, autophagy, prostate cancer, Akt/mTOR pathway, PC3 cell
Citation: Xu Y, Cai X, Zong B, Feng R, Ji Y, Chen G and Li Z (2018) Qianlie Xiaozheng Decoction Induces Autophagy in Human Prostate Cancer Cells via Inhibition of the Akt/mTOR Pathway. Front. Pharmacol. 9:234. doi: 10.3389/fphar.2018.00234
Received: 13 January 2018; Accepted: 01 March 2018;
Published: 04 April 2018.
Edited by:
Vincent Kam Wai Wong, Macau University of Science and Technology, ChinaReviewed by:
Songxiao Xu, Artron BioResearch Inc., CanadaCopyright © 2018 Xu, Cai, Zong, Feng, Ji, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongxing Li, bHp4ODYxN0AxNjMuY29t
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.