- 1Graduate Institute of Basic Medical Science, School of Medicine, College of Medicine, China Medical University, Taichung, Taiwan
- 2Department of Medical Research, School of Chinese Medicine, China Medical University and Hospital, Taichung, Taiwan
- 3Management Office for Health Data, China Medical University Hospital, Taichung, Taiwan
- 4Division of Gastroenterology and Hepatology, Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
- 5Division of Gastroenterology and Hepatology, Department of Internal Medicine, Shuang-Ho Hospital, New Taipei City, Taiwan
- 6Department of Nursing, Asia University, Taichung, Taiwan
- 7Department of Laboratory Medicine, Chang Gung Memorial Hospital, Linkou, Taiwan
- 8Department of Microbiology and Immunology, Graduate Institute of Biomedical Sciences, College of Medicine, Chang Gung University, Taoyuan, Taiwan
- 9Molecular Infectious Disease Research Center, Chang Gung Memorial Hospital, Linkou, Taiwan
- 10Department of Bioinformatics and Medical Engineering, Asia University, Taichung, Taiwan
- 11Department of Nuclear Medicine, PET Center, China Medical University Hospital, Taichung, Taiwan
Prostate cancer (PCa) is one of the most commonly diagnosed cancers in the western world, and the mortality rate from PCa in Asia has been increasing recently. Statins are potent inhibitors of 3-hydroxy-3-methyl glutaryl coenzyme A (HMG-CoA) reductase and are commonly used for treating hyperlipidemia, with beneficial effects for cardiovascular disease and they also exhibit anti-cancer activity. However, the protective effects of statins against PCa are controversial. In this study, we investigated the effect of two types of statins (simvastatin and lovastatin) and the mortality rate of PCa patients by using the Taiwan National Health Insurance Research Database (NHIRD). A total of 15,264 PCa patients with hyperlipidemia records and medical claims from the Registry of Catastrophic Illness were enrolled. The patients were divided into two cohorts based on their statin use before the diagnosis of PCa: statin users (n = 1,827) and non-statin users (n = 1,826). The results showed that patients who used statins exhibited a significantly reduced risk of mortality from PCa [adjusted hazard ratio (HR) = 0.84, 95% CI = 0.73–0.97]. Analysis of the cumulative defined daily dose (DDD) indicated that patients who were prescribed simvastatin ≥ 180 DDD had a dramatically decreased risk of death from PCa (adjusted HR = 0.63; 95% CI = 0.51–0.77). This population-based cohort study demonstrated that statin use significantly decreased the mortality of PCa patients, and that this risk was inversely associated with the cumulative DDD of simvastatin therapy. The results of this study revealed that statins may be used for drug repositioning and in the development of a feasible approach to prevent death from PCa.
Introduction
Prostate cancer (PCa) is one of the most prevalent male cancers in western developed countries, and its incidence is increasing in the Asia-Pacific region (Global Burden of Disease Cancer et al., 2017). Although recent advances in surgical techniques and radiotherapy have improved the disease-free survival rates of men with localized PCa, the recurrence rate after radical prostatectomy, chemotherapy, or radiotherapy is still high (Trock et al., 2008). Men are at higher risk for developing PCa, while the risk factors include having a family history of PCa along with environmental and lifestyle-related factors, such as physical inactivity, obesity, and a high-fat diet (Barnard, 2007; Meyerhardt et al., 2010).
Statins, inhibitors of 3-hydroxy-3-methyl glutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme in cholesterol biosynthesis, are widely used for treating high levels of serum cholesterol (Hebert et al., 1997). The activity of HMG-CoA reductase may be increased due to the activation of transcriptional regulation mediated by sterol regulatory element binding proteins (SREBPs) (Chen et al., 1978). In LNCaP and PC-3 PCa cells, inhibition of SREBP2 activity reduced cell viability (Krycer et al., 2012). Recent studies indicated that statins possess the potential to prevent and treat several types of cancers, including PCa (Hindler et al., 2006; Bonovas et al., 2008; Yu et al., 2014). The protective functions of statins are included reducing the risk of PCa through arrest cell cycle, inducing apoptosis, and inhibiting tumor metastasis (Papadopoulos et al., 2011; Sun Q. et al., 2015). However, some conflicting results about the protective effects of statins against PCa have not been clearly clarified (Moon et al., 2014; Alfaqih et al., 2017).
Although previous studies indicated that statins may decrease the incidence of PCa (Shannon et al., 2005; Flick et al., 2007), there have been no large-scale epidemiologic studies on the effects of statins for treating PCa patients with hyperlipidemia who received different therapeutic modalities including radical prostatectomy, chemotherapy, or radiotherapy. In this study, we conducted a nationwide population-based cohort study to analyze the mortality in PCa patients with hyperlipidemia who were prescribed either simvastatin or lovastatin. The association between the cumulative defined daily dose (DDD) of two types of statins and the risk of PCa mortality was also investigated. Our results showed that statin prescriptions effectively reduced the risk of death from PCa. This study also revealed that cholesterol-lowering agents, such as statins, may be used for drug repurposing to help prevent death in PCa patients who also have hyperlipidemia.
Materials and Methods
Data Source
To examine whether statin use is associated with risk of PCa, a nationwide cohort study was conducted. Data were obtained from the Registry of Catastrophic Illness and Taiwan National Health Insurance Research Database (NHIRD). The National Health Research Institutes (NHRI) in Taiwan is responsible for managing the insurance claims data reported to the Bureau of Health Insurance. For research purposes, the NHRI compiles all medical claims in the National Health Insurance (NHI) program and releases the database annually to the public. Patient consent is not required to access the NHIRD. This study was approved by the Institutional Review Board of China Medical University in Taiwan (CMUH104-REC2-115-CR2).
Population-Based Cohort Study
A total of 15,264 PCa patients with hyperlipidemia (ICD-9-CM 272) were included in this study (Figure 1). The patients were divided into two cohorts, the statin users (either simvastatin or lovastatin) and non-statin users before the diagnosis of PCa. Patients under 20 years old were excluded. In the statin use cohort, 1,827 PCa patients prescribed statins for at least 6 months during January 1, 1998 to December 31, 2010. The non-statin cohort (n = 1,826) was randomly selected from 12,111 PCa patients without receiving statin therapy. Comparison group was established by 1:1 randomly frequency matching (according to age and index year) with PCa patients who did not use any types of statin-based drugs during the study period. The study endpoint was mortality.
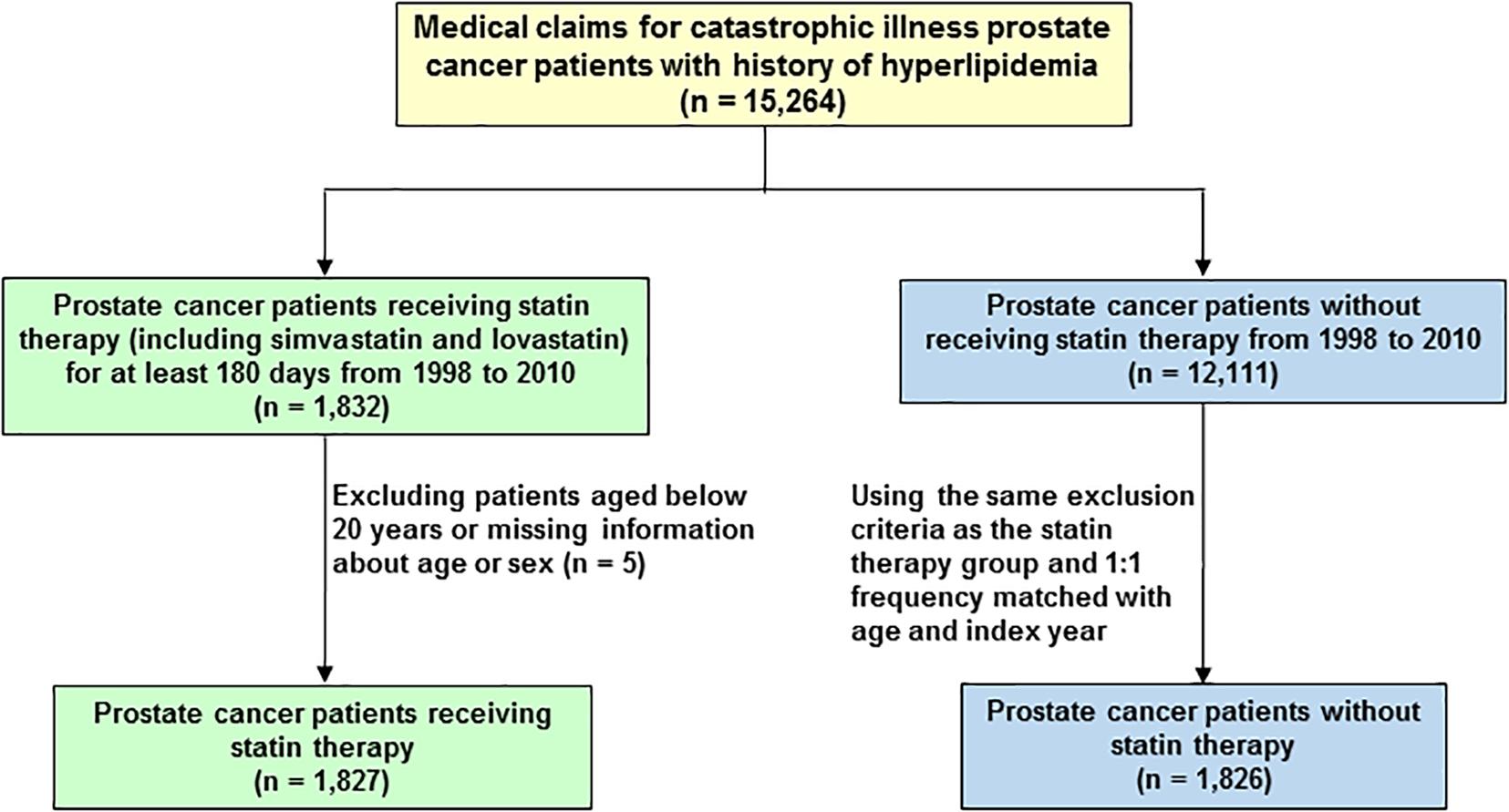
FIGURE 1. Flowchart of the establishment of cohorts, patient selection, identification, and analysis.
Medication and Analysis of Statin Prescription
Hazard ratio (HR) and 95% confidence interval (CI) were adjusted for age, treatment of hormone therapy (including oral and injection), radical prostatectomy, radiotherapy, chemotherapy, and the comorbidities of diabetes (ICD-9-CM codes 250), hypertension (ICD-9-CM codes 401- 405), stroke (ICD-9-CM codes 430-438), cardiovascular disease (CAD; ICD-9-CM codes 410–414) and chronic obstructive pulmonary disease (COPD; ICD-9-CM codes 490–496).
The DDD, recommended by the World Health Organization (WHO), was assumed to be the average maintenance dose per day of a drug and analyzed as described previously (Lin et al., 2017). The cumulative DDD was calculated by deriving the total prescribed DDD of each type of statin, namely simvastatin (ATC C10AA01) and lovastatin (ATC C10AA02), for statin users. For each statin type, the cumulative DDD was partitioned into two levels by setting the cutoff value in the median.
Statistical Analysis
The distributions of demographic characteristics were compared between the statin and non-statin cohorts, and the differences were examined using the Chi-squared test for categorical variables and the Student’s t-test for continuous variables. The multivariate analysis of the mortality rates associated with statin use was performed using the cox proportional hazard regressions. Furthermore, we divided each statin type into two groups according to the quartile of cumulative DDD. Cox proportional hazard regression was used to assess how the statin dose affected the relative risk of mortality. A P-value of < 0.05 indicated statistical significance. All analyses were conducted using SAS statistical software (Version 9.3 for Windows; SAS Institute, Inc., Cary, NC, United States).
Results
Demographic Characteristics of Study Patients
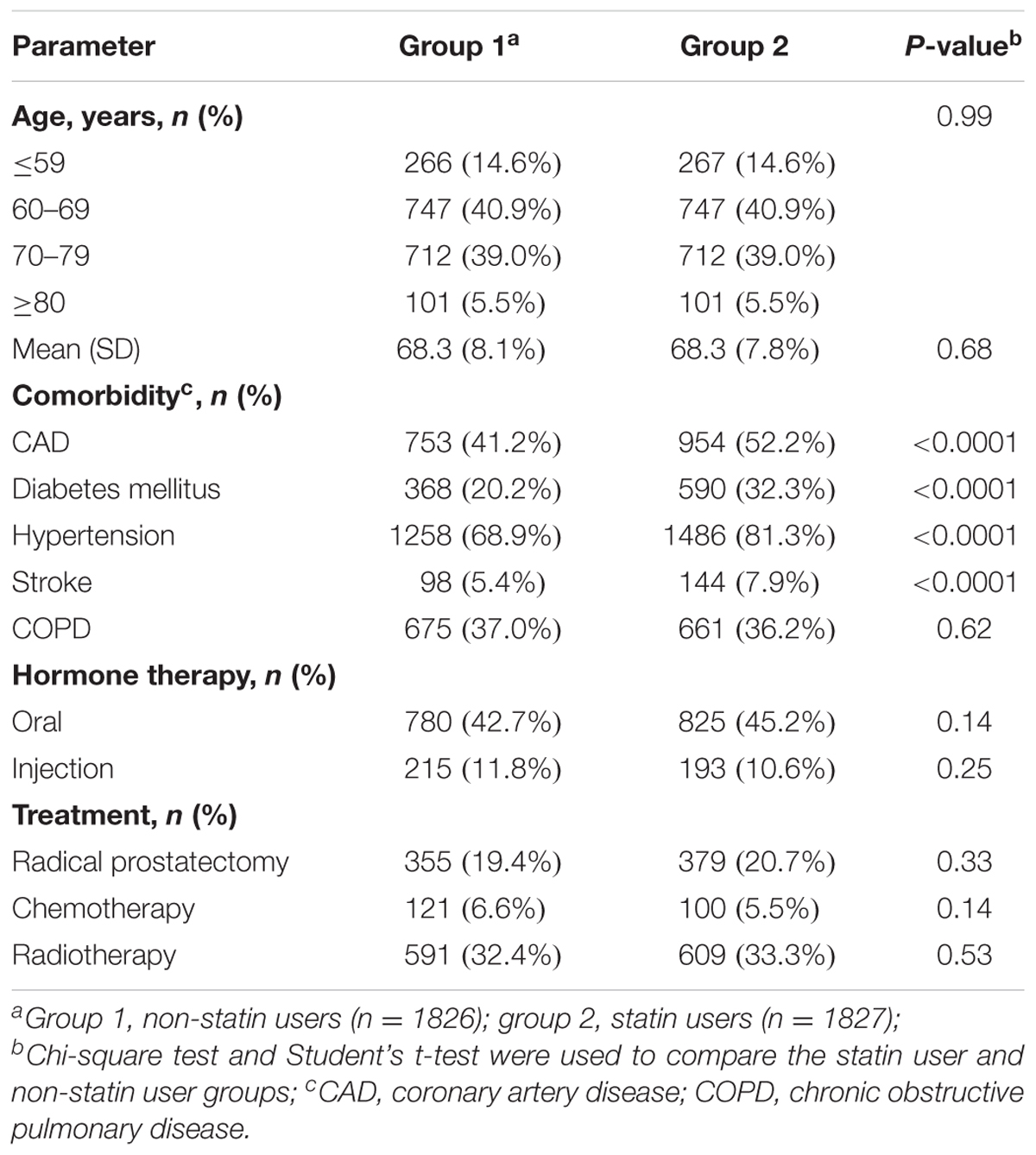
A total of 15,264 PCa patients with hyperlipidemia were enrolled in this study and analyzed. In the population-based cohort study, we first evaluated 3,653 patients with PCa, aged ≥ 20 years (Figure 1). There were 1,827 patients using statin-based drugs (either simvastatin or lovastatin) and 1,826 patients who did not. Table 1 shows the age, comorbidities, hormone therapy, and treatments of the cohorts. Among the patients with PCa, 85.4% of the patients were older than 60 years of age. Compared with non-statin users (Group 1), the statin users (Group 2) were more likely to have coronary artery disease (CAD) (41.2% vs. 52.2%, P < 0.0001), diabetes mellitus (20.2% vs. 32.3%, P < 0.0001), hypertension (68.9% vs. 81.3%, P < 0.0001), and stroke (5.37% vs. 7.88%, P < 0.0001). In the treatments used for PCa including hormone therapy, radical prostatectomy, chemotherapy, and radiotherapy, the statin users and non-statin users did not show statistically significant differences.
Comparison of Incidence and Hazard Ratio (HR) of Mortality in PCa Patients
The person-years (PY), incidence (age, comorbidity, hormone therapy, and treatments), and HR for the risk of mortality among the cohorts were then analyzed. As shown in Table 2, statin users had a significantly lower HR of mortality (adjusted HR = 0.84, 95% CI = 0.73–0.97, P < 0.05) compared with the non-statin users. Among patients who had no comorbidities, statin users exhibited the lowest HR of mortality (adjusted HR = 0.55, 95% CI = 0.31–0.99, P < 0.05). A significantly reduced adjusted HR of mortality was also found in the following groups for patients prescribed statins: treatment with chemotherapy (adjusted HR = 0.60, 95% CI = 0.41–0.87, P < 0.01), non-oral hormone therapy (adjusted HR = 0.81, 95% CI = 0.68–0.98, P < 0.05), and non-prostatectomy (adjusted HR = 0.86, 95% CI = 0.74–0.99, P < 0.05).
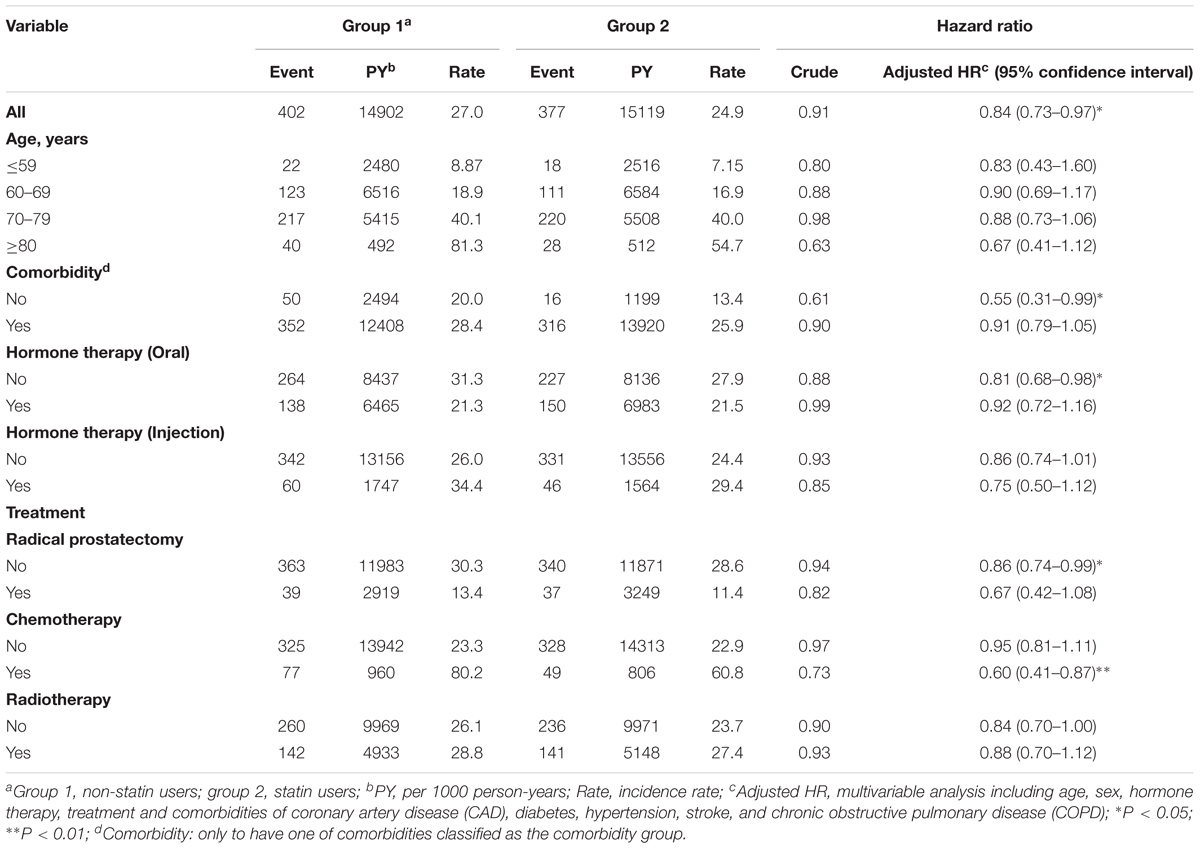
TABLE 2. Hazard ratios and 95% confidence intervals of PCa mortality in statin user and non-statin user groups.
Statin Use Reduces the Risk of PCa Mortality
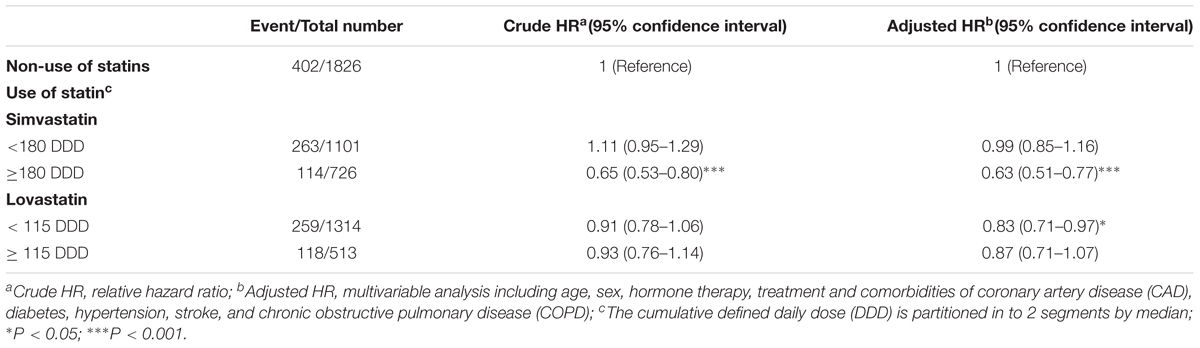
The association between the cumulative DDD of two types of statins and the risk of PCa mortality was further analyzed. Compared with non-statin users, patients who were prescribed simvastatin at a DDD ≥ 180 (adjusted HR = 0.63, 95% CI = 0.51–0.77) exhibited the lowest risk of mortality, which was associated with the high cumulative DDD of prescribed simvastatin (Table 3). Patients who received lovastatin at a DDD < 115 (adjusted HR = 0.83, 95% CI = 0.71–0.97) had a lower adjusted HR of mortality compared with the non-statin user group. The results from these analyses demonstrate that statin use effectively reduced the mortality of PCa patients, and that this risk was associated with a high cumulative DDD of prescribed simvastatin.
Discussion
Prostate cancer is a commonly diagnosed cancer and the second leading cause of cancer-related deaths in US men (Wadajkar et al., 2013). In Taiwan, the prevalence of PCa has gradually increased in recent years (Chiang et al., 2016; Hung et al., 2016). Based on a statistical report from the Ministry of Health and Welfare [MOHW] (2016) of Taiwan in 2016, PCa has become the fifth most prevalent cancer and the seventh leading cause of cancer-related death, indicating that the threat of PCa to men has become severe. Therefore, it is important to pay more attention to the diagnosis and treatment of patients with PCa.
The regulation of lipid rafts (also referred to cholesterol-rich microdomains) is critical for PCa cell survival and growth (Zhuang et al., 2002). PCa cells harbored the activity to synthesize dihydrotestosterone (DHT) from acetic acid, indicating that the entire mevalonate-steroidogenic pathway was functionally intact (Locke et al., 2008). In addition, all enzymes contributed to testosterone and DHT synthesis are presented in many human primary and metastatic PCa (Montgomery et al., 2008). Therefore, new therapeutic agents with lesser toxicity and higher selectivity for preventing recurrence and progression of PCa are urgently required. This population-based cohort analysis revealed that statin therapy significantly decreased the mortality of PCa patients treated with chemotherapy. In addition, the reduced risk was correlated with the high cumulative DDD of prescribed simvastatin. This nationwide population-based study provides evidence that statin therapy not only reduced the risk of cardiovascular disease but may also be used for drug repurposing to prevent death of PCa patients who also have hyperlipidemia.
It has been demonstrated that simvastatin inhibits the proliferation, migration, and invasion of PCa cells via the up-regulation of ANXA10 and inactivation of the AKT/PI3K pathway (Miyazawa et al., 2017). Simvastatin can arrest the cell cycle at G1 in PC-3 cells through the AMPK pathway (Karlic et al., 2017). In addition, lovastatin was found to enhance the efficacy of PRRA-TRAIL via the promotion of tumor suppression and induction of apoptosis (Liu et al., 2015). A recent study also indicated that the induction of the LDL receptor is a possible mechanism of resistance that prostate tumors use to counteract the therapeutic effects of reducing serum cholesterol (Masko et al., 2017). Therefore, statins can be used to delay PCa progression by reducing LDL levels (Murtola et al., 2012).
Clinical studies have been reported that statins significantly reduced the incidence of advanced PCa (Bonovas et al., 2008). A 10-year retrospective cohort study indicated that statin users had a 31% lower risk of PCa (Farwell et al., 2011). The mechanism for statins was the indirect reduction in cellular cholesterol levels in multiple cell types through the lowering of circulating cholesterol, which impacts the membrane microdomains and steroidogenesis (Moon et al., 2014). In addition, statin can prevent against PCa by inhibiting the proliferation and inducing apoptosis of PCa cells, which inhibits angiogenesis, inflammation, and metastasis (Papadopoulos et al., 2011). A recent clinical analysis demonstrated that statin users had a significantly longer median time to progression during androgen deprivation therapy than non-users (Harshman et al., 2015).
Both in vitro and in vivo studies have demonstrated that statins inhibited cancer cell growth and induced apoptosis in a variety of tumor cell types, including PCa, colon adenocarcinoma, pancreatic carcinoma, and gastric cancer cells (Lochhead and Chan, 2013; Babcook et al., 2016; Lin et al., 2016; Gong et al., 2017). A population-based cohort study revealed that not all types of statin were associated with a decreased incidence of PCa, except for simvastatin, atorvastatin, and rosuvastatin (Lustman et al., 2014). Simvastatin was identified to inhibit the migration and invasion of PCa cells (Shah et al., 2016). The inhibitory effect of statin-derivatives on tumorigenicity of castrate-resistant prostate cancer (CRPC) may be mediated via concurrent inhibition of both androgen receptor and AKT signaling pathways, and membrane destabilization (Ingersoll et al., 2016). Furthermore, the mechanism for inhibition of CRPC growth by statins suppressed nuclear factor-κB pathway to induce apoptosis and prevent CRPC development (Park et al., 2013; Kang et al., 2017). Our current study also revealed that statins can function as a radiosensitizer in PCa cells for triggering the CHK1 checkpoint response and promoting DNA double-strand breaks (unpublish data). Together, these findings demonstrated the potential mechanism of statins in the treatment of PCa.
Although we recently reported the protective effects of statins against PCa (Sun L.M. et al., 2015), certain limitations and confounding factors of previous studies are still emerging. In the current study, we further selected patients with hyperlipidemia and divided them into two cohorts based on the prescription of statins. Because statin users may have other comorbidities, several treatments and comorbidities were adjusted for, including hormone therapy, diabetes, hypertension, stroke, CAD, and COPD. In addition, the cause of death among patients with PCa was documented in the Registry of Catastrophic Illness, and thus, mortality from PCa can be precisely analyzed. Therefore, our current findings have been thoroughly investigated and are based on rigorous and valid methods.
Some limitations exist in the current studies, including potential confounders, concomitant use of other chemo-preventive drugs, short enrollment periods, and small sample sizes. A social gradient in statin use may reflect social inequalities in health care use or health care quality, as well as systematic differences in factors such as smoking, nutrition, physical activity, and obesity (Bonovas and Sitaras, 2009). Patients with cardiovascular disease prescribed statins may also take non-steroidal anti-inflammatory drugs (NSAIDs), which have been reported to reduce the risk of PCa (Papadopoulos et al., 2011). Although current studies using population-based analysis consistently report an inverse association between statin use and PCa risk, the detailed mechanism of statins for the primary prevention of PCa requires further investigation.
The Taiwan government has launched an NHI program that has provided citizens with comprehensive coverage since 1995. All insurance claims are scrutinized by medical reimbursement specialists and recorded in the database. The NHIRD enabled appropriately selecting matched patients to represent the underlying population. In addition, we have analyzed data in the NHIRD to perform several studies and have evaluated statin-based drugs for protection against several diseases (Peng et al., 2015; Sun L.M. et al., 2015; Lin et al., 2016). Therefore, these results obtained by using a nationwide cohort study regarding statin use and diagnoses for patients with PCa mortality are reliable.
Conclusion
This study demonstrated that statin prescription has effectively reduced the deaths of PCa patients with hyperlipidemia. The mortality risk of patients with PCa is inversely associated with a high cumulative DDD of simvastatin. Therefore, statins, cholesterol-lowering agents, can be drug repurposed and used in a preventive application for protecting against death from PCa. However, large, long-term clinical analysis and experimental studies on the biological mechanisms of statin therapy are required to further validate statins as a means of reducing PCa mortality.
Author Contributions
C-HL and C-HK: conceived and designed the experiments. Y-AC, Y-JL, C-LL, H-JL, H-YH, Y-CS, and H-YW: performed the experiments and analyzed the data. Y-AC, Y-JL, C-LL, and H-JL: wrote the manuscript. All authors reviewed the final version of the manuscript.
Funding
This work was supported by grants from the Ministry of Health and Welfare, Taiwan (MOHW107-TDU-B-212-123004), China Medical University Hospital, Academia Sinica Stroke Biosignature Project (BM10701010021), MOST Clinical Trial Consortium for Stroke (MOST 106-2321-B-039-005), Tseng-Lien Lin Foundation, Taichung, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan, Taiwan Ministry of Science and Technology (105-2313-B-182-001 and 106-2320-B-182-012-MY3), Chang Gung Memorial Hospital (CMRPD1F0011-3, CMRPD1F0431-3, and BMRPE90), and Tomorrow Medical Foundation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the editor and the reviewers for the editorial assistance and their valuable comments. The authors sincerely appreciate the Bureau of National Health Insurance for providing the NHIRD.
References
Alfaqih, M. A., Allott, E. H., Hamilton, R. J., Freeman, M. R., and Freedland, S. J. (2017). The current evidence on statin use and prostate cancer prevention: are we there yet? Nat. Rev. Urol. 14, 107–119. doi: 10.1038/nrurol.2016.199
Babcook, M. A., Joshi, A., Montellano, J. A., Shankar, E., and Gupta, S. (2016). Statin use in prostate cancer: an update. Nutr. Metab. Insights 9, 43–50. doi: 10.4137/NMI.S38362
Barnard, R. J. (2007). Prostate cancer prevention by nutritional means to alleviate metabolic syndrome. Am. J. Clin. Nutr. 86, s889–s893. doi: 10.1093/ajcn/86.3.889S
Bonovas, S., Filioussi, K., and Sitaras, N. M. (2008). Statin use and the risk of prostate cancer: a metaanalysis of 6 randomized clinical trials and 13 observational studies. Int. J. Cancer 123, 899–904. doi: 10.1002/ijc.23550
Bonovas, S., and Sitaras, N. M. (2009). Statins and cancer risk: a confounded association. Gastroenterology 137, 740; author reply 740–741. doi: 10.1053/j.gastro.2009.02.088
Chen, H. W., Kandutsch, A. A., and Heiniger, H. J. (1978). The role of cholesterol in malignancy. Prog. Exp. Tumor Res. 22, 275–316. doi: 10.1159/000401203
Chiang, C. J., Lo, W. C., Yang, Y. W., You, S. L., Chen, C. J., and Lai, M. S. (2016). Incidence and survival of adult cancer patients in Taiwan, 2002-2012. J. Formos. Med. Assoc. 115, 1076–1088. doi: 10.1016/j.jfma.2015.10.011
Farwell, W. R., D’Avolio, L. W., Scranton, R. E., Lawler, E. V., and Gaziano, J. M. (2011). Statins and prostate cancer diagnosis and grade in a veterans population. J. Natl. Cancer Inst. 103, 885–892. doi: 10.1093/jnci/djr108
Flick, E. D., Habel, L. A., Chan, K. A., Van Den Eeden, S. K., Quinn, V. P., Haque, R., et al. (2007). Statin use and risk of prostate cancer in the California Men’s Health Study cohort. Cancer Epidemiol. Biomarkers Prev. 16, 2218–2225. doi: 10.1158/1055-9965.EPI-07-0197
Global Burden of Disease Cancer, Collaboration, Fitzmaurice, C., Allen, C., Barber, R. M., Barregard, L., Bhutta, Z. A., et al. (2017). Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 3, 524–548. doi: 10.1001/jamaoncol.2016.5688
Gong, J., Sachdev, E., Robbins, L. A., Lin, E., Hendifar, A. E., and Mita, M. M. (2017). Statins and pancreatic cancer. Oncol. Lett. 13, 1035–1040. doi: 10.3892/ol.2017.5572
Harshman, L. C., Wang, X., Nakabayashi, M., Xie, W., Valenca, L., Werner, L., et al. (2015). Statin use at the time of initiation of androgen deprivation therapy and time to progression in patients with hormone-sensitive prostate cancer. JAMA Oncol. 1, 495–504. doi: 10.1001/jamaoncol.2015.0829
Hebert, P. R., Gaziano, J., Chan, K., and Hennekens, C. H. (1997). Cholesterol lowering with statin drugs, risk of stroke, and total mortality: an overview of randomized trials. JAMA 278, 313–321. doi: 10.1001/jama.1997.03550040069040
Hindler, K., Cleeland, C. S., Rivera, E., and Collard, C. D. (2006). The role of statins in cancer therapy. Oncologist 11, 306–315. doi: 10.1634/theoncologist.11-3-306
Hung, C. F., Yang, C. K., and Ou, Y. C. (2016). Urologic cancer in Taiwan. Jpn. J. Clin. Oncol. 46, 605–609. doi: 10.1093/jjco/hyw038
Ingersoll, M. A., Miller, D. R., Martinez, O., Wakefield, C. B., Hsieh, K. C., Simha, M. V., et al. (2016). Statin derivatives as therapeutic agents for castration-resistant prostate cancer. Cancer Lett. 383, 94–105. doi: 10.1016/j.canlet.2016.09.008
Kang, M., Lee, K. H., Lee, H. S., Jeong, C. W., Ku, J. H., Kim, H. H., et al. (2017). Concurrent treatment with simvastatin and NF-kappaB inhibitor in human castration-resistant prostate cancer cells exerts synergistic anti-cancer effects via control of the NF-kappaB/LIN28/let-7 miRNA signaling pathway. PLoS One 12:e0184644. doi: 10.1371/journal.pone.0184644
Karlic, H., Haider, F., Thaler, R., Spitzer, S., Klaushofer, K., and Varga, F. (2017). Statin and bisphosphonate induce starvation in fast-growing cancer cell lines. Int. J. Mol. Sci. 18:e1982. doi: 10.3390/ijms18091982
Krycer, J. R., Phan, L., and Brown, A. J. (2012). A key regulator of cholesterol homoeostasis, SREBP-2, can be targeted in prostate cancer cells with natural products. Biochem. J. 446, 191–201. doi: 10.1042/BJ20120545
Lin, C. J., Liao, W. C., Chen, Y. A., Lin, H. J., Feng, C. L., Lin, C. L., et al. (2017). statin therapy is associated with reduced risk of peptic ulcer disease in the Taiwanese population. Front. Pharmacol. 8:210. doi: 10.3389/fphar.2017.00210
Lin, C. J., Liao, W. C., Lin, H. J., Hsu, Y. M., Lin, C. L., Chen, Y. A., et al. (2016). Statins Attenuate Helicobacter pylori CagA translocation and reduce incidence of gastric cancer: In Vitro and population-based case-control studies. PLoS One 11:e0146432. doi: 10.1371/journal.pone.0146432
Liu, Y., Chen, L., Gong, Z., Shen, L., Kao, C., Hock, J. M., et al. (2015). Lovastatin enhances adenovirus-mediated TRAIL induced apoptosis by depleting cholesterol of lipid rafts and affecting CAR and death receptor expression of prostate cancer cells. Oncotarget 6, 3055–3070. doi: 10.18632/oncotarget.3073
Lochhead, P., and Chan, A. T. (2013). Statins and colorectal cancer. Clin. Gastroenterol. Hepatol. 11, 109–118; quiz e13–e14. doi: 10.1016/j.cgh.2012.08.037
Locke, J. A., Guns, E. S., Lubik, A. A., Adomat, H. H., Hendy, S. C., Wood, C. A., et al. (2008). Androgen levels increase by intratumoral De novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 68, 6407–6415. doi: 10.1158/0008-5472.CAN-07-5997
Lustman, A., Nakar, S., Cohen, A. D., and Vinker, S. (2014). Statin use and incident prostate cancer risk: does the statin brand matter? A population-based cohort study. Prostate Cancer Prostatic Dis. 17, 6–9. doi: 10.1038/pcan.2013.34
Masko, E. M., Alfaqih, M. A., Solomon, K. R., Barry, W. T., Newgard, C. B., Muehlbauer, M. J., et al. (2017). Evidence for feedback regulation following cholesterol lowering therapy in a prostate cancer xenograft model. Prostate 77, 446–457. doi: 10.1002/pros.23282
Meyerhardt, J. A., Ma, J., and Courneya, K. S. (2010). Energetics in colorectal and prostate cancer. J. Clin. Oncol. 28, 4066–4073. doi: 10.1200/JCO.2009.26.8797
Ministry of Health and Welfare [MOHW]. (2016). Taiwan Statistics of Causes of Death 2016. Available at: http://www.mohw.gov.tw/lp-3327-2.html [accessed March 6, 2018].
Miyazawa, Y., Sekine, Y., Kato, H., Furuya, Y., Koike, H., and Suzuki, K. (2017). Simvastatin up-regulates annexin A10 that can inhibit the proliferation, migration, and invasion in androgen-independent human prostate cancer cells. Prostate 77, 337–349. doi: 10.1002/pros.23273
Montgomery, R. B., Mostaghel, E. A., Vessella, R., Hess, D. L., Kalhorn, T. F., Higano, C. S., et al. (2008). Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 68, 4447–4454. doi: 10.1158/0008-5472.CAN-08-0249
Moon, H., Hill, M. M., Roberts, M. J., Gardiner, R. A., and Brown, A. J. (2014). Statins: protectors or pretenders in prostate cancer? Trends Endocrinol. Metab. 25, 188–196. doi: 10.1016/j.tem.2013.12.007
Murtola, T. J., Syvala, H., Pennanen, P., Blauer, M., Solakivi, T., Ylikomi, T., et al. (2012). The importance of LDL and cholesterol metabolism for prostate epithelial cell growth. PLoS One 7:e39445. doi: 10.1371/journal.pone.0039445
Papadopoulos, G., Delakas, D., Nakopoulou, L., and Kassimatis, T. (2011). Statins and prostate cancer: molecular and clinical aspects. Eur. J. Cancer 47, 819–830. doi: 10.1016/j.ejca.2011.01.005
Park, Y. H., Seo, S. Y., Lee, E., Ku, J. H., Kim, H. H., and Kwak, C. (2013). Simvastatin induces apoptosis in castrate resistant prostate cancer cells by deregulating nuclear factor-kappaB pathway. J. Urol. 189, 1547–1552. doi: 10.1016/j.juro.2012.10.030
Peng, Y. C., Lin, C. L., Hsu, W. Y., Chang, C. S., Yeh, H. Z., Tung, C. F., et al. (2015). Statins are associated with a reduced risk of cholangiocarcinoma: a population-based case-control study. Br. J. Clin. Pharmacol. 80, 755–761. doi: 10.1111/bcp.12641
Shah, E. T., Upadhyaya, A., Philp, L. K., Tang, T., Skalamera, D., Gunter, J., et al. (2016). Repositioning “old” drugs for new causes: identifying new inhibitors of prostate cancer cell migration and invasion. Clin. Exp. Metastasis 33, 385–399. doi: 10.1007/s10585-016-9785-y
Shannon, J., Tewoderos, S., Garzotto, M., Beer, T. M., Derenick, R., Palma, A., et al. (2005). Statins and prostate cancer risk: a case-control study. Am. J. Epidemiol. 162, 318–325. doi: 10.1093/aje/kwi203
Sun, L. M., Lin, M. C., Lin, C. L., Chang, S. N., Liang, J. A., Lin, I. C., et al. (2015). Statin use reduces prostate cancer all-cause mortality: a nationwide population-based cohort study. Medicine 94:e1644. doi: 10.1097/MD.0000000000001644
Sun, Q., Arnold, R. S., Sun, C. Q., and Petros, J. A. (2015). A mitochondrial DNA mutation influences the apoptotic effect of statins on prostate cancer. Prostate 75, 1916–1925. doi: 10.1002/pros.23089
Trock, B. J., Han, M., Freedland, S. J., Humphreys, E. B., DeWeese, T. L., Partin, A. W., et al. (2008). Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 299, 2760–2769. doi: 10.1001/jama.299.23.2760
Wadajkar, A. S., Menon, J. U., Tsai, Y. S., Gore, C., Dobin, T., Gandee, L., et al. (2013). Prostate cancer-specific thermo-responsive polymer-coated iron oxide nanoparticles. Biomaterials 34, 3618–3625. doi: 10.1016/j.biomaterials.2013.01.062
Yu, O., Eberg, M., Benayoun, S., Aprikian, A., Batist, G., Suissa, S., et al. (2014). Use of statins and the risk of death in patients with prostate cancer. J. Clin. Oncol. 32, 5–11. doi: 10.1200/JCO.2013.49.4757
Keywords: hyperlipidemia, HMG-CoA reductase, prostate cancer, statin, cohort study
Citation: Chen Y-A, Lin Y-J, Lin C-L, Lin H-J, Wu H-S, Hsu H-Y, Sun Y-C, Wu H-Y, Lai C-H and Kao C-H (2018) Simvastatin Therapy for Drug Repositioning to Reduce the Risk of Prostate Cancer Mortality in Patients With Hyperlipidemia. Front. Pharmacol. 9:225. doi: 10.3389/fphar.2018.00225
Received: 27 October 2017; Accepted: 27 February 2018;
Published: 22 March 2018.
Edited by:
Yuhei Nishimura, Mie University Graduate School of Medicine, JapanReviewed by:
William B. Grant, Sunlight Nutrition and Health Research Center, United StatesChia-Yang Li, Kaohsiung Medical University, Taiwan
Muhammad Imran Qadir, Bahauddin Zakariya University, Pakistan
Copyright © 2018 Chen, Lin, Lin, Lin, Wu, Hsu, Sun, Wu, Lai and Kao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chih-Ho Lai, Y2hsYWlAbWFpbC5jZ3UuZWR1LnR3 Chia-Hung Kao, ZDEwMDQwQG1haWwuY211aC5vcmcudHc=
†These authors have contributed equally to this work.
 Yu-An Chen1†
Yu-An Chen1† Chih-Ho Lai
Chih-Ho Lai Chia-Hung Kao
Chia-Hung Kao