- 1Neuroscience Discovery Research, Lilly Research Laboratories, Eli Lilly and Company, Indianapolis, IN, United States
- 2Lilly Corporate Center, Eli Lilly and Company, Indianapolis, IN, United States
- 3Discovery Chemistry Research, Lilly Research Laboratories, Eli Lilly and Company, Indianapolis, IN, United States
There is substantial evidence that glutamate can modulate the effects of 5-hydroxytryptamine2A (5-HT2A) receptor activation through stimulation of metabotropic glutamate2/3 (mGlu2/3) receptors in the prefrontal cortex. Here we show that constitutive deletion of the mGlu2 gene profoundly attenuates an effect of 5-HT2A receptor activation using the mouse head twitch response (HTR). MGlu2 and mGlu3 receptor knockout (KO) as well as age-matched ICR (CD-1) wild type (WT) mice were administered (±)1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) and observed for head twitch activity. DOI failed to produce significant head twitches in mGlu2 receptor KO mice at a dose 10-fold higher than the peak effective dose in WT or mGlu3 receptor KO mice. In addition, the mGlu2/3 receptor agonist LY379268, and the mGlu2 receptor positive allosteric modulator (PAM) CBiPES, potently blocked the HTR to DOI in WT and mGlu3 receptor KO mice. Conversely, the mGlu2/3 receptor antagonist LY341495 (10 mg/kg) increased the HTR produced by DOI (3 mg/kg) in mGlu3 receptor KO mice. Finally, the mGlu2 receptor potentiator CBiPES was able to attenuate the increase in the HTR produced by LY341495 in mGlu3 receptor KO mice. Taken together, all of these results are consistent with the hypothesis that that DOI-induced head twitches are modulated by mGlu2 receptor activation. These results also are in keeping with a critical autoreceptor function for mGlu2 receptors in the prefrontal cortex with differential effects of acute vs. chronic perturbation (e.g., constitutive mGlu2 receptor KO mice). The robust attenuation of DOI-induced head twitches in the mGlu2 receptor KO mice appears to reflect the critical role of glutamate in ongoing regulation of 5-HT2A receptors in the prefrontal cortex. Future experiments with inducible knockouts for the mGlu2 receptor and/or selective mGlu3 receptor agonists/PAMs/antagonists could provide an important tools in understanding glutamatergic modulation of prefrontal cortical 5-HT2A receptor function.
Introduction
Metabotropic glutamate receptors (mGluRs) have been implicated in a wide variety of effects with clinical implications. The group II mGlu2 and mGlu3 receptor subtypes have been shown in a wide range of animal studies to have effects in models predictive of antidepressant activity (Chaki et al., 2004; Yasuhara et al., 2006; Nikiforuk et al., 2010; Fell et al., 2011), anxiolytic activity (Klodzinska et al., 2002; Schoepp et al., 2003; Galici et al., 2006) and antipsychotic activity (Moghaddam and Adams, 1998; Johnson et al., 2005; Rorick-Kehn et al., 2007). These preclinical predictions have been confirmed for orthosteric mGlu2/3 receptor agonists in the treatment of schizophrenia, though in a limited subpopulation of patients (Patil et al., 2007; Kinon et al., 2015). In contrast to schizophrenia, safety considerations from preclinical data available at that time precluded registration of the first highly potent orally available orthosteric mGlu2/3 receptor agonist demonstrated to be effective in patients with generalized anxiety disorder (Schoepp et al., 2003; Michelson et al., 2005; Dunayevich et al., 2008).
Suppression of rodent head twitches induced by serotonergic hallucinogens via 5-hydroxytryptamine2A (5-HT2A) receptor activation appears to be a promiscuous preclinical pharmacological screen sensitive to both antipsychotic drugs and many antidepressant drugs (Canal and Morgan, 2012; Marek, 2017). Previous studies have shown that orthosteric mGlu2/3 receptor agonists suppress 2,5-dimethoxy-4-iodoamphetamine (DOI)-induced head twitches in both rats and mice (Gewirtz and Marek, 2000; Klodzinska et al., 2002; Gonzalez-Maeso et al., 2008; Moreno et al., 2011, 2012; Wieronska et al., 2013). A permissive role of the mGlu2 receptor in these effects is supported by several studies where mGlu2 receptor positive allosteric modulators (PAMs) also suppressed head twitches induced by phenethylamine hallucinogens (Benneyworth et al., 2007; Lavreysen et al., 2015; Griebel et al., 2016).
Head twitches induced by 5-HT2A receptor activation are supported by direct infusions of serotonergic hallucinogens into the medial prefrontal cortex (mPFC) of the rat (Willins and Meltzer, 1997). DOI-induced head twitches, in addition to suppression by mGlu2/3 receptor agonists or mGlu2 receptor PAMs, also are suppressed by activation of mGlu4, adenosine A1, and m-opioid receptors (Marek, 2003, 2009; Wieronska et al., 2013). Interestingly, these Gi/Go-coupled receptor agonists/PAMs also suppress excitatory postsynaptic currents/potentials (EPSCs/EPSPs) induced by activation of 5-HT2A receptors in layers I and Va of the mPFC/neocortex (Marek and Aghajanian, 1998; Stutzman et al., 2001; Zhang and Marek, 2007; Slawinska et al., 2013). Thus, 5-HT and serotonergic hallucinogen acts on mPFC layer V pyramidal cells by inducing glutamate release with DOI-induced head twitches as one salient behavioral manifestation (Marek, 2017).
Lesion studies have also suggested that the intralaminar and midline thalamic nuclei, the non-specific thalamic nuclei known to be important in arousal and attention, may be the source of neurons from which 5-HT2A receptor stimulation induces glutamate release onto cortical layer V pyramidal cells (Lambe and Aghajanian, 2001; Marek et al., 2001). These findings are in keeping with (1) the higher expression of mGlu2 mRNA in the thalamic midline and intralaminar nuclei compared to other thalamic nuclei or the prefrontal cortex/neocortex (Ohishi et al., 1993, 1998); (2) the predominant physiological role of mGlu2 receptors as autoreceptors (Marek et al., 2000; Schoepp, 2001); (3) the laminar overlap within the prefrontal cortex of relatively higher densities of 5-HT2A and mGlu2 receptor binding in layers I and Va (Marek et al., 2000, 2001; Schoepp, 2001; Wright et al., 2013); and (4) the known termination of thalamic midline and intralaminar thalamic nuclei in layers I and Va (Berendse and Groenewegen, 1991; Van der Werf et al., 2002). There undoubtedly are effects of 5-HT2A receptor activation independent of this thalamocortical pathway (Celada et al., 2008; Wischhof and Koch, 2016). However, increased thalamocortical connectivity appears to play a role in psychotomimetic effects of LSD and psilocybin in humans (Carhart-Harris et al., 2013; Tagliazucchi et al., 2016; Muller et al., 2017). But given the role of these thalamic nuclei in attention and arousal, coupled with massive feedback from layer V pyramidal neurons as drivers of intralaminar and midline thalamic nuclei activity (Saalmann, 2014), this thalamocortical circuitry likely plays a major role on psychotomimetic effects of serotonergic hallucinogens.
In the context of the pharmacology, circuitry and potential clinical implications of modulating the DOI-induced head twitch response (HTR), we applied genetic strategies studying both mGlu2 and mGlu3 knockouts to understand the role of mGlu2 and mGlu3 receptors in mediating the effects of mGlu2/3 receptor agonists or mGlu2/3 receptor antagonists in modulating DOI-induced head twitches. One question of interest was the contributions of mGlu2/3 receptors to the DOI-induced head twitch model. A second question was whether modulation of mGlu2 and/or mGlu3 receptors would be therapeutic drug candidates. The primary caveat to this approach is that the mGlu2 receptor appears to play an autoreceptor role in modulating DOI-induced head twitches. As discussed earlier, acute or single dose administration of mGlu2/3 receptor agonists suppress while mGlu2/3 receptor antagonists enhance DOI-induced head twitches, consistent with the widespread role of mGlu2 receptors as an autoreceptor on glutamatergic terminals/pre-terminal axons. Thus, this key homeostatic role played by an autoreceptor raises the possibility that differential regulation of DOI-induced head shakes could be observed with acute (single dose) vs. chronic (daily administration). A mGlu2 constitutive knockout would in and of itself constitute a chronic perturbation of the system existing throughout the life of the mutant mouse.
Even at the time these studies were begun, evidence for potential glutamatergic modulation of prefrontal cortical 5-HT2A receptors was present. First, cortical 5-HT2A receptor binding was increased by chemical thalamic lesions (Marek et al., 2001). Second, two daily mGlu2/3 agonist systemic doses prior to treatment with DOI prevented down-regulation of DOI receptor binding in the prefrontal cortex (Marek et al., 2006). Subsequent to these studies, chronic administration of a mGlu2/3 receptor antagonist LY341495 on a daily basis for 21 days decreased both 5-HT2A receptor binding and LSD-induced head twitches in mice (Moreno et al., 2013). This latter result is completely opposite of a previous finding that a single dose of the same mGlu2/3 receptor antagonist increased DOI-induced head twitches in rats (Gewirtz and Marek, 2000). Not surprisingly in the light of these caveats, paradoxical effects with the mGlu2 receptor knockout mice were observed in this manuscript where a dramatic rightward shift in the DOI-induced head twitch dose response. This was in contrast to the lack of any apparent change in the DOI dose-response relationship with the mGlu3 knockout mice. These transgenic mouse studies of the DOI dose-response relationship support a prominent role for mGlu2 receptors in modulating DOI-induced head twitches. The lack of an attenuation of the acute effects of either an mGlu2/3 receptor agonist, an mGlu2 receptor PAMs, or mGlu2/3 receptor antagonists in mGlu3 receptor KO mice in this manuscript is consistent with a potentially necessary and sufficient role for the mGlu2 receptor in modulating DOI-induced head twitches for group II mGlu receptor ligands. Thus, mGlu2 receptors appear to play a major modulatory role in the circuitry controlling DOI-induced head twitches while screening with this behavioral model also suggests mGlu2 receptor agonists and PAMs as candidate antipsychotic treatments.
Materials and Methods
Animals
MGlu2 and mGlu3 receptor knockout mouse strains were generated by homologous recombination as previously described (Linden et al., 2005). Wild type (WT) ICR (CD-1) littermates were used in all experiments employing KO animals. In all experiments using KO mice, animals were used in more than one experiment, with at least a 7-day washout period. A total of 32 mGlu2 receptor KO mice and 40 mGlu3 receptor KO mice were used in these experiments (Taconic Inc., Germantown, NY, United States). Other experiments utilized ICR (CD-1) out bred mice (n = 104; Harlan Inc., Indianapolis, IN, United States). The mice used in these experiments were 4–8 weeks old. Mice were housed 6–10 per cage under a 12:12 light-dark cycle (lights on at 6 a.m.), and food and water were available at all times. All animal protocols were approved by the Eli Lilly and Company Animal Care and Use Committee and followed guidelines recommended by NIH at the time the experiments were conducted.
Head Twitch Test
The test apparatus had eight individual observation chambers and was made of Plexiglas, measuring 10 cm × 10 cm × 12 cm per chamber, through which all animals were observed. An experienced blinded observer watched eight animals in tandem and recorded all head twitches on a multiple counter (Fisher Scientific, Inc., Pittsburgh, PA, United States). Mice were brought from the colony room in groups of eight, weighed, and allowed to acclimate to the test chamber for 15 min prior to testing. Head twitches were induced by DOI (3 mg/kg, i.p.), and mice were observed for 30 min beginning 5 min after dosing. The only exception to this was the initial DOI dose-response determination where the WT mice observation duration was 15 min instead of 30 min. LY379268, LY341495 and CBiPES were administered i.p., 30 min prior to DOI. When CBiPES was administered with LY341495, CBiPES was dosed 5 min prior to LY341495. Each experimental group consisted of eight animals per group except for the experiment testing DOI in mGlu3 receptor KO animals (N = 10), the experiment testing the LY379268 dose-response relationship in WT mice (N = 6) and when LY341495 was tested in mGlu3 receptor KO animals (N = 16). The sample size used for these experiments were determined in part by availability transgenic mice. Separate groups of animals were used for each dose of DOI in all experiments with CD-1 (ICR) mice, and in all experiments utilizing KO/WT animals, mice were used at least twice, with a 7-day washout period between tests.
The methodology used for the DOI-induced HTR was fit for purpose as a relatively modest-high throughput behavioral assay for a range of drug discovery programs at the Lilly Research Laboratories. Statisticians and biologists worked in tandem to validate this in vivo assay (and other in vivo and in vitro assays). This validation included determining the number of animals required for individual behavioral experiments as well as the statistical tests used to evaluate whether a concentration- or dose-related effect was present. A critical part of the validation for the DOI-induced HTR including running these validating experiments with a trained observed (SFC), with substantial personal experience of measuring DOI-induced head twitches under these condition. On occasion, additional validating experiments were carried out such as an experiment where CBiPES statistically suppressed DOI-induced head twitches in a dose-related manner where a significant decrease in rat head twitches induced by DOI (3 mg/kg, i.p.) occurred for a 30 mg/kg CBiPES dose (approximately 10 head twitches with DOI alone down to about 4 head twitches with DOI/CBiPES). Furthermore, this mouse DOI-induced HTR assay was used for optimization of the physicochemical characteristics of mGlu2 receptor PAM SAR that later resulted in mGlu2 receptor PAMs that were both more potent and centrally penetrant. In a set of 12 compounds not described in this manuscript, the mean frequency of head twitches/30 min observation periods induced by DOI alone were 7.9 ± 1.2 (SD) in WT CD1 mice. The range of DOI-induced head twitches was (6.4–10.7). Only one experiment was the mean DOI-induce head twitch frequency lower than the 95% confidence intervals for the set of 12 experiments. Only for two experiments were the mean frequency of DOI-induced head twitches higher than the 95% confidence interval for the entire set of 12 experiments. MGlu2 receptor PAMs significantly reduced the frequency of DOI-induced head twitches in doses ranging from 1 to 30 mg/kg. Additional internal converging validity of the DOI-induced head twitch assay for the mGlu2 receptor PAM program was found in similar results for the same mGlu2 receptor PAMs with respect to potency and maximal efficacy with hyperactivity induced by a NMDA receptor antagonist. Another internal converging validity of the DOI-induced head twitch assay for the mGlu2 receptor PAM was found in similar results with respect to potency and maximal efficacy when testing for antidepressant-like activity in mice or rats on an operant differential-reinforcement-of-low rate (DRL) schedule of reinforcement. A range of other discovery projects (including selective mGlu2 receptor orthosteric agonists, mGlu5 receptor negative allosteric modulators, orexin receptor antagonists) also successfully utilized the DOI-induced head twitch assay either for supporting the development of new projects and/or utility as an early relatively modest-high behavioral throughput assay that was complimented by in vivo receptor occupancy (where available) and other physiological/behavioral assays.
Drugs
(±)-DOI hydrochloride was obtained from a commercial source (Sigma-Aldrich, Inc., St. Louis, MO, United States) and dissolved in dH2O. LY379268 (1R,4R,5S,6R-4-amino-oxabicyclo[3.1.0]hexane-4,6-dicarboxylic acid), LY341495 (2S1′S,2′S)-2-(9-xanthylmethyl)-2-(2′carboxycyclopropyl)glycine and CBiPES (N-(4′-cyano-biphenyl-3-yl)-N-(3-pyridinymethyl)-ethanesulfonamide hydrochloride) were synthesized at Lilly Research Labs (Indianapolis, IN, United States). LY379268 and LY341495 were dissolved in dH2O with the addition of drops of 5 N NaOH to adjust pH to 6–7 while CBiPES was suspended in 1% methylcellulose, 0.25% Tween 80, 0.05% Dow Antifoam, dH20. All compounds were administered by the intraperitoneal (i.p.) route and given in a volume of 10 ml/kg. All compounds were weighed and described in figures as the nominal weight including the associated salt.
Statistics
The data obtained in the experiments are presented as means ± SEM and was analyzed using a one-way ANOVA, followed post hoc by a Dunnett’s test to determine individual difference using p < 0.05 for the significance level. Where a single dose of drug was compared to a vehicle condition, the Student’s t-test was employed using p < 0.05 for the significance level. In addition, all groups in each experiment were analyzed for total number of animals exhibiting head twitch behavior using the Fisher Exact Test with p < 0.05 for significance. All experiments were analyzed using JMP 5.1 (SAS Institute, Inc., Cary, NC, United States).
Results
DOI/mGlu Receptor Effects in CD-1 Mice
(±)-DOI (0.3–10 mg/kg, i.p.), given alone to CD-1 mice, produced an increase in mean head twitches [F(3,20) = 4.09, p < 0.05] to 4.1 in a 15 min observation period, at a dose of 3 mg/kg (Figure 1A). Lower doses produced non-significant increases in head twitches, while a higher dose of 10 mg/kg produced increased variability yet a significant increase in mean head twitches. This 10 mg/kg DOI dose also produced an increase in arousal, manifested by increased locomotion in the observation cage. For this reason, 3 mg/kg (±)-DOI was chosen as the optimal dose for further testing with a 30 min observation period. The mGlu2 receptor PAM, CBiPES (10–30 mg/kg), when given to DOI-treated CD-1 mice, produced a mean of 2.3 head twitches at 30 mg/kg, a 60% reduction from DOI and vehicle-treated animals (Figure 1B). Although this dose was not statistically different from vehicle [F(2,21) = 0.94, p = 0.31], the number of animals emitting head twitches (2/8) was significantly different from vehicle/DOI-treated animals (8/8; Fisher Exact, p = 0.003). Additionally, the 10 mg/kg CBiPES dose also produced a significant decrease in the number of animals emitting head twitches (4/8; Fisher Exact, p = 0.038). LY379268 (Figure 1C), the orthosteric mGlu2/3 receptor agonist, was very potent at decreasing the mean head twitches produced by DOI [F(3,20) = 8.51, p < 0.001]. At a dose of 0.1 mg/kg, there was a significant decrease in mean head twitches to less than 15% of DOI alone. Additionally, all LY379268 doses tested (0.01–1.0 mg/kg) reduced head twitches to less than 50% of the mean number when receiving DOI alone. The mGlu2/3 receptor antagonist, LY341495 (1–3 mg/kg), when tested in the presence of DOI in CD-1 mice, produced a numerical increase in the mean number of head twitches as compared to DOI alone, but the effect did not reach significance [F(2,21) = 1.88, p = 0.17, Figure 1D].
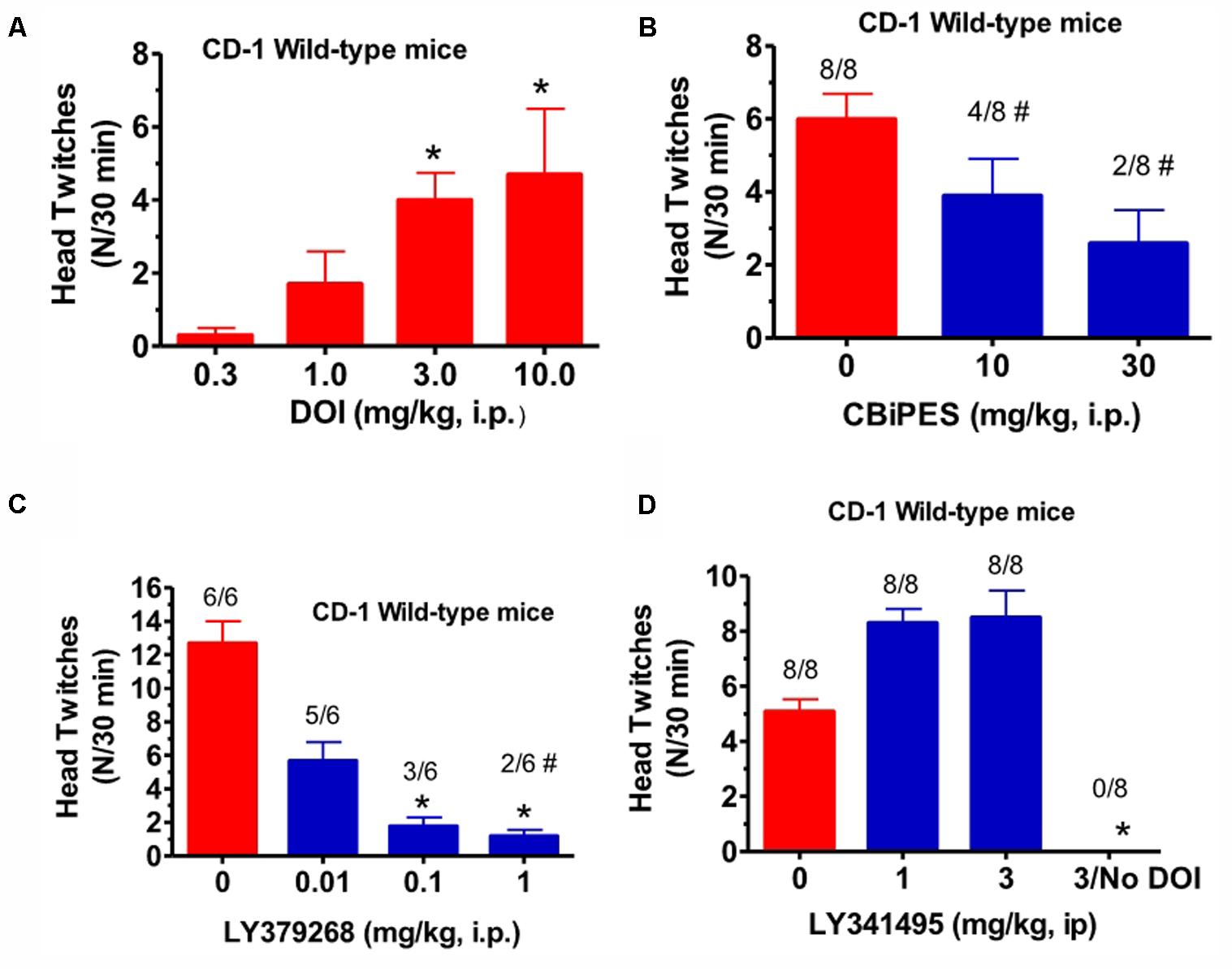
FIGURE 1. (A) The effect of (±)-DOI (0.3–10 mg/kg) on head twitches in CD-1 WT mice observed for 15 min following drug administration. Each bar represents the mean (±SEM) of six animals after i.p. dosing. Significantly different from 0, ∗p < 0.05. (B) The effect of CBiPES (10–30 mg/kg) on head twitches induced by DOI (3 mg/kg) in CD-1 WT mice observed for 30 min following drug administration. CBiPES was administered 30 min prior to DOI. Each bar represents the mean (±SEM) of eight animals after i.p. dosing. Significantly different from 0 or the vehicle condition by Fisher Exact Test, #p < 0.05. (C) The effect of LY379268 (0.01–1 mg/kg) on head twitches induced by DOI (3 mg/kg) in CD-1 WT mice observed for 30 min following drug administration. LY379268 was administered 30 min prior to DOI. Each bar represents the mean (±SEM) of six animals after i.p. dosing. Significantly different from 0 (vehicle) by the Fisher Exact Test, #p < 0.05. (D) The effect of LY341495 (1–3 mg/kg) on head twitches induced by DOI (3 mg/kg) in CD-1 WT mice observed for 30 min following drug administration. LY341495 was administered 30 min prior to DOI. Each bar represents the mean (±SEM) of eight animals after i.p. dosing. Significantly different from 0 (vehicle condition), ∗p < 0.05. Note that the DOI dose-response relationship is the only experiment in this paper where a 15 min observation period was employed, unlike all other experiments using a 30 min observation period.
DOI Effects in mGlu2 or mGlu3 Receptor KO Mice
When DOI (3–30 mg/kg) was administered to mGlu2 receptor KO mice in the same fashion, and compared to the effect of 3 mg/kg given to littermate WT mice, there was a significant reduction in mean head twitches [F(3,28) = 40.5, p < 0.0001] (Figure 2A). In fact, at the highest DOI dose tested (30 mg/kg), only 3 of 8 mGlu2 receptor KO mice exhibited head twitches. DOI did not induce more than five head twitches at either dose level in any of the few mGlu2 receptor KO mice with observed head twitches. Thus, the number of DOI-induced head twitches in these mice was even below the range of the mean DOI-induced head twitches for the entire series of 12 groups of WT mice tested with 3 mg/kg described in the Section “Materials and Methods.” In contrast, when tested in mGlu3 receptor KO mice, DOI (1–3 mg/kg) produced mean head twitches similar to that seen in WT mice. At doses of 1 and 3 mg/kg, there was no significant difference between WT and mGlu3 receptor KO mice when tested with DOI (Figure 2B). Given the surprisingly low number of DOI-induced head twitches in the mGlu2 receptor KO mice, the effects of LY379268, CBiPES and LY379268 were not studied in the mGlu2 receptor KO mice.
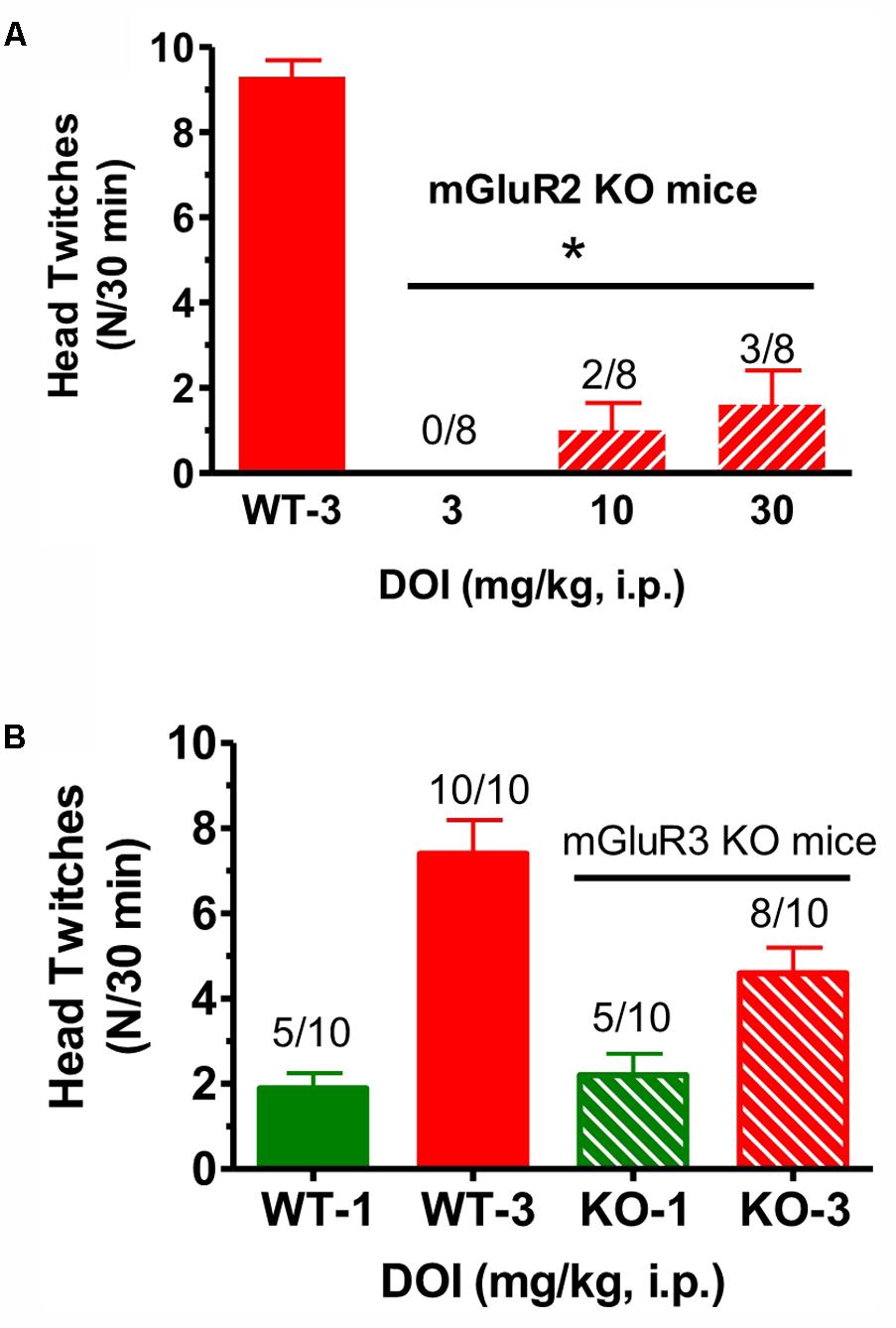
FIGURE 2. (A) The effect of DOI (3 mg/kg) on head twitches in WT and DOI (3–30 mg/kg) in mGlu2 receptor KO mice for 30 min following drug administration. Each bar represents the mean (±SEM) of eight animals after i.p. dosing. Significantly different from WT performance, ∗p < 0.05. WT, wild type; KO, knockout. (B) The effect of DOI (1–3 mg/kg) on head twitches in WT and the effect of DOI (1–3 mg/kg) on head twitches in mGlu3 receptor KO mice for 30 min following drug administration. Each bar represents the mean (±SEM) of 10 animals after i.p. dosing. WT, wild type; KO, knockout. The horizontal line is shown above the data for the mGlu2 receptor KO mice and for the mGlu3 receptor KO mice in the top (A) and lower figures(B), respectively.
mGlu Receptor Drugs in mGlu3 Receptor KO Mice
When the mGlu2 receptor PAM CBiPES (1–10 mg/kg) was injected in mGlu3 receptor KO mice, it produced a significant decrease in mean head twitches [F(3,28) = 5.2, p < 0.05] at doses of 3 and 10 mg/kg compared to vehicle/DOI-treated animals (Figure 3A). The number of animals emitting head twitches at those doses was also significantly reduced (3/8, 2/8, respectively). A CBiPES dose of 1 mg/kg was ineffective in suppressing the DOI-induced HTR. When the orthosteric mGlu2/3 receptor agonist LY379268 (1 mg/kg) was administered to mGlu3 receptor KO mice following DOI, the effect was the same as seen in the WT animals (Figure 3B). LY379268, at a dose of 1 mg/kg was fully effective [F(3,60) = 2.82, p < 0.05] at attenuated head twitches in both the mGlu3 KO and the WT mice. When the mGlu2/3 receptor antagonist LY341495 (1–10 mg/kg) was tested in mGlu3 receptor KO mice, there was a dose-dependent increase in mean head twitches tested (1–10 mg/kg; Figure 3C), an effect that reached statistical significance [F(3,60) = 2.82, p < 0.05]. Post hoc analysis revealed that the 10 mg/kg dose produced a significant increase in mean head twitches as compared to vehicle-treated animals (p = 0.046) and lower doses were not significant different from DOI alone.
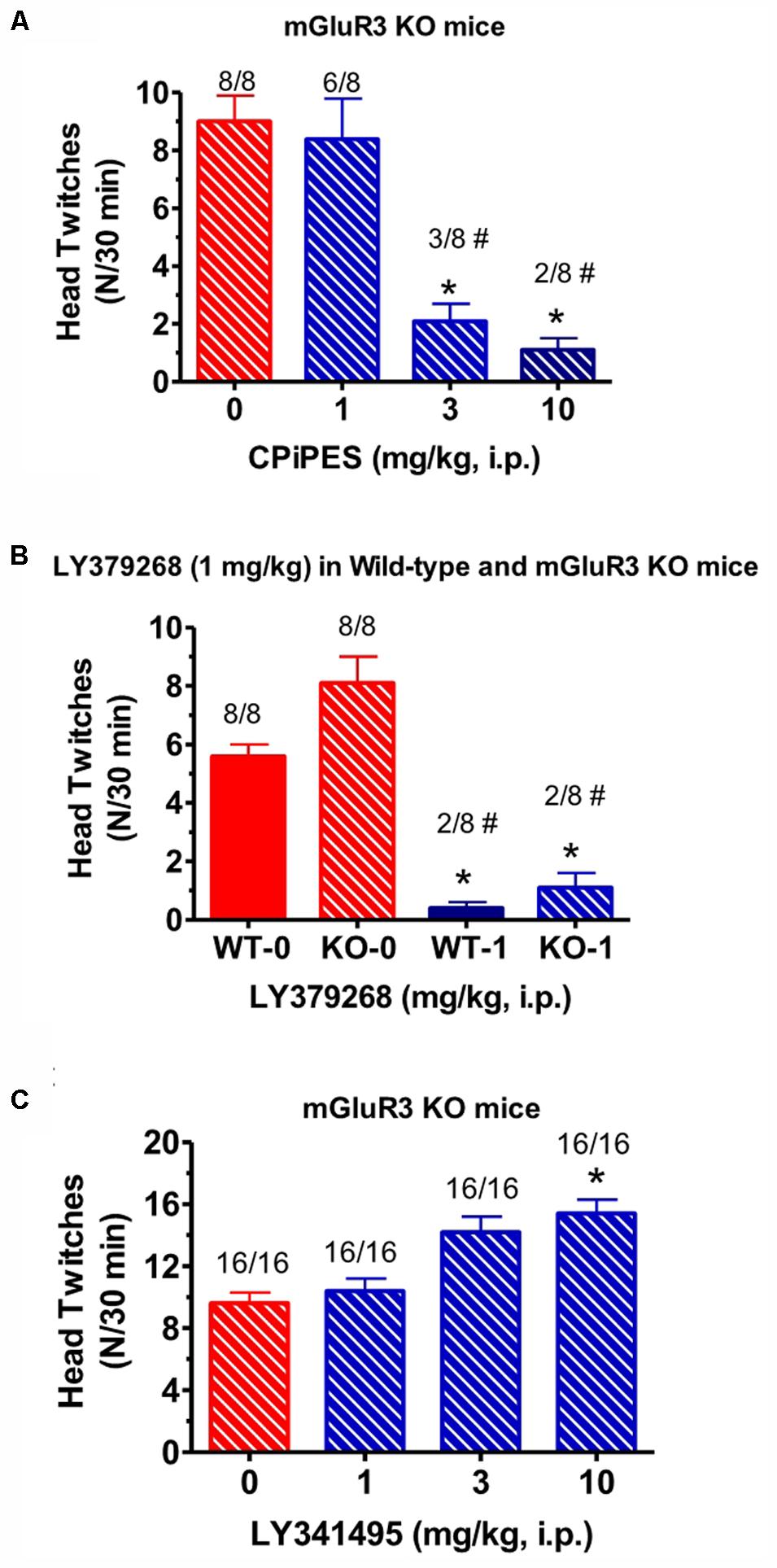
FIGURE 3. (A) Effect of CBiPES (1–10 mg/kg) on head twitches induced by DOI in mGlu3 receptor KO mice for 30 min following drug administration. CBiPES was administered 30 min prior to DOI. Each bar represents the mean (±SEM) of eight animals after i.p. dosing. Significantly different from 0 or the vehicle condition, ∗p < 0.05. Significantly different from 0 (vehicle) by the Fishers Exact Test, #p < 0.05. (B) Effect of LY379268 (1 mg/kg) on head twitches induced by DOI in mGlu3 receptor KO mice for 30 min following drug administration. LY379268 was administered 30 min prior to DOI. Each bar represents the mean (±SEM) of eight animals after i.p. dosing. Significantly different from respective WT, ∗p < 0.05 or KO, ∗∗∗p < 0.05 treated only with DOI. Significantly different from respective WT and KO treated only with DOI by Fishers Exact Test, #p < 0.05. (C) The effect of LY341495 (1–10 mg/kg) on head twitches induced by DOI in mGlu3 receptor KO mice for 30 min following drug administration. LY341495 was administered 30 min prior to DOI. Each bar represents the mean (±SEM) of 16 animals after i.p. dosing. Significantly different from DOI alone (“0” condition in the figure), ∗p < 0.05.
Drug Combinations in mGlu3 Receptor KO Mice
Tests for the combination of the mGlu2 receptor potentiator, CBiPES and the mGlu2/3 receptor antagonist LY341495, can be seen in Figure 4. When DOI (3 mg/kg) and LY341495 (3 mg/kg) were dosed in WT mice, the addition of 30 mg/kg of CBiPES (Figure 4A) reduced the mean head twitches by 60% while 10 mg/kg of CBiPES reduced mean head twitches by almost 50%. However, this effect did not reach statistical significance [F(2,21) = 1.23, p = 0.31]. When the same dosing regimen was given to mGlu3 receptor KO mice (Figure 4B), LY341495 (3 mg/kg) significantly increased mean head twitches in the animals compared to mGlu3 receptor KO mice given DOI alone (t = 2.52, p < 0.05), and when CBiPES (30 mg/kg) was added to DOI + LY341495 (3 mg/kg), mean head twitches were significantly reduced by over 80% (t = 5.4, p < 0.05), and only two of eight mice emitted head twitches while eight of eight animals emitted head twitches when given vehicle + DOI + LY341495. So, while the addition of the mGlu2/3 receptor antagonist increased the number of head twitches produced by DOI in mGlu3 receptor KO mice, CBiPES, the mGlu2 receptor potentiator was able to attenuate the increase produced by the orthosteric antagonist LY341495.
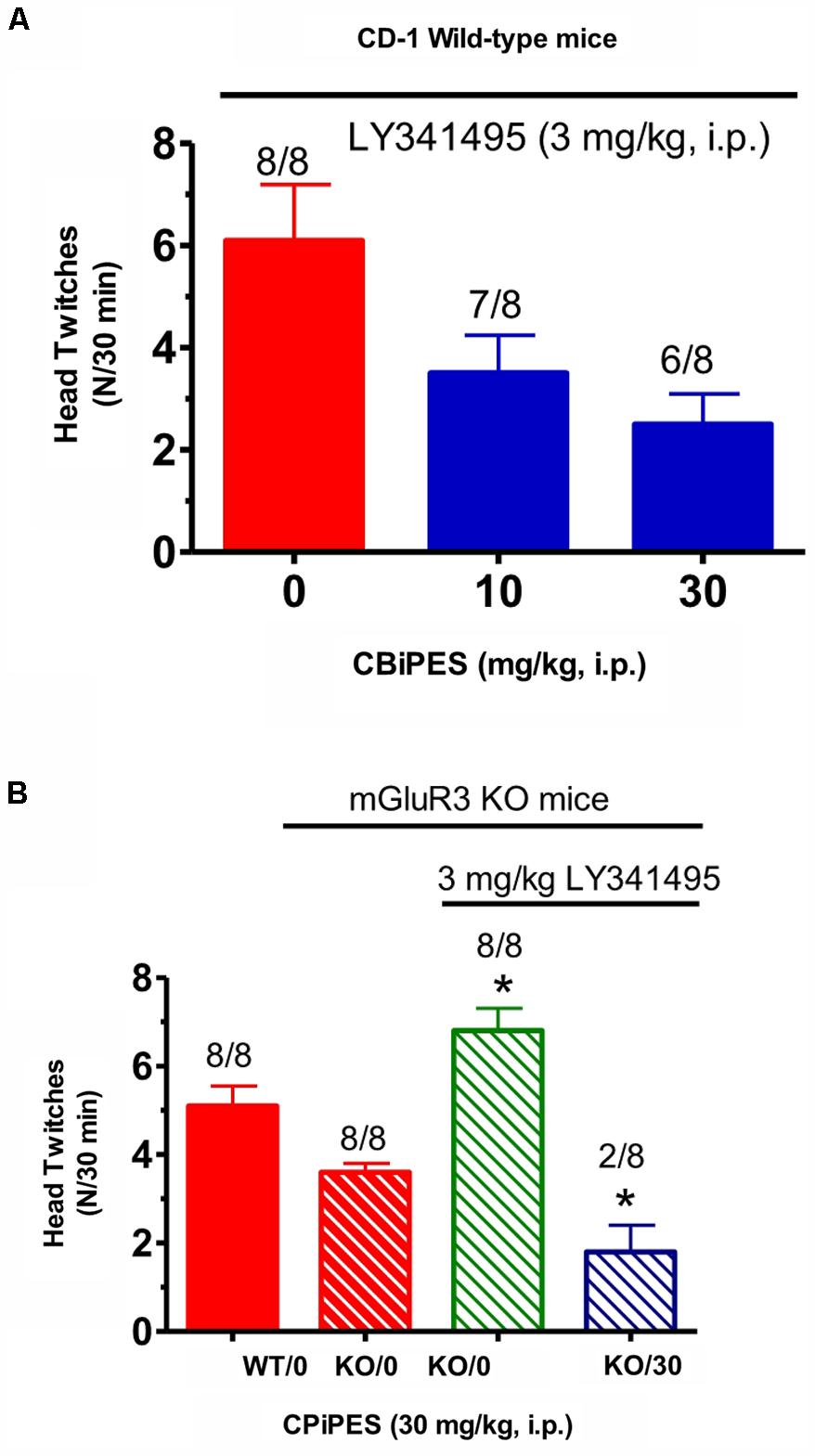
FIGURE 4. (A) The effect of vehicle or CBiPES (10–30 mg/kg) on head twitches induced by the administration of DOI (3 mg/kg) and LY341495 (3 mg/kg) in CD-1 WT mice for 30 min following drug administration. CBiPES was administered 5 min prior to LY341495, which was administered 30 min prior to DOI. Each bar represents the mean (±SEM) of eight animals after i.p. dosing. (B) The effect of vehicle or CBiPES (30 mg/kg) on head twitches induced by DOI (3 mg/kg), with or without the presence of LY341495 (3 mg/kg), in WT and mGlu3 receptor KO mice for 30 min following drug administration. Each bar represents the mean (±SEM) of eight animals after i.p. dosing. KO-LY341495 significantly different from KO without LY341495, ∗p < 0.05 or KO-DOI-LY341495-CBiPES significantly different from KO-DOI-LY341495, ∗p < 0.05.
Discussion
The most striking finding from the present results was the dramatic rightward shift in the DOI-induced HTR in transgenic mice lacking mGlu2 receptors compared to either CD-1 mice, WT mice or transgenic mice lacking mGlu3 receptors. A monotonic increasing DOI-induced HTR was observed in CD-1 mice over a 1–10 mg/kg dose range. In contrast to this, over a 10-fold rightward shift in the dose-response relationship was observed in mGlu2 KO mice. No head twitches were observed in the mGlu2 KO mice for a 3 mg/kg DOI dose while only three of eight mice exhibited head twitches at the 30 mg/kg DOI dose. This finding is consistent with the relative loss of DOI-induced head twitches using a different line of mGlu2 receptor KO mice (Moreno et al., 2011), but extends this finding by examining a wider dose range. This marked rightward shift in the DOI-induced HTR in mGlu2 KO mice has also been confirmed by a pharmacological experiment where subchronic daily treatment (21 days) with the mGlu2/3 receptor antagonist LY341495 also down-regulated 5-HT2A receptor density and the frequency of DOI-induced head twitches in WT mice (Moreno et al., 2013).
This finding of the near loss of DOI-induced HTR in mGlu2 KO mice was previously suggested to be related to heteromeric 5-HT2A/mGlu2 receptors in the prefrontal cortex (Moreno et al., 2011, 2012). However, other interpretations are also plausible. The most likely alternate hypothesis is a down-regulation in the sensitivity of 5-HT2A receptors to agonist stimulation mediating the desensitization of serotonergic hallucinogen-induced head twitches. A single large dose or subchronic smaller doses of phenethylamine hallucinogens or LSD is known to decrease 5-HT2A receptor binding in rodents (Buckholtz et al., 1985, 1988; Leysen et al., 1989; Shi et al., 2008; Buchborn et al., 2015). Accordingly, either multiple injections within a single day or daily dosing over a week result in a tachyphylaxis for hallucinogen-induced head twitches (Leysen et al., 1989; Darmani et al., 1992; Darmani and Gerdes, 1995; Buchborn et al., 2015). Serotonergic hallucinogens have been shown to increase extracellular cortical glutamate in rodents (Scruggs et al., 2003; Muschamp et al., 2004). These three types of serotonergic hallucinogen induced effects are consistent with the finding that a single dose of the mGlu2/3 receptor agonist LY354740 was able to reverse to decrease in prefrontal cortical DOI binding from a subacute regimen of 3 daily DOI doses (Marek et al., 2006). Furthermore, just 3–4 days following an adenoviral incorporation of functional mGlu2 receptors in the prefrontal cortex of mGlu2 receptor KO mice restored a normal appearing DOI-induced HTR (Moreno et al., 2012). Conversely, lesions of the glutamatergic input to the prefrontal cortex and neocortex from the midline and intralaminar thalamic nuclei, along with most other thalamic inputs, resulted in an approximately 20% increase in DOI binding several weeks following the chemical lesion of the thalamus (Marek et al., 2001). This implies that at least some effects of cortical 5-HT2A receptor function/regulation occurs within a context of glutamatergic input from the intralaminar and midline thalamic nuclei (Scruggs et al., 2000; Lambe and Aghajanian, 2001; Marek et al., 2001) as modified by mGlu2 autoreceptors. The critical role of mGlu2 receptors as an autoreceptor on thalamocortical glutamatergic afferents to layer V pyramidal cell apical dendrites appears to provide a biological substrate where homeostasis exists for optimal stimulation of serotonergic and glutamatergic drive at layer V pyramidal cell apical dendrites. The paradoxical effects between acute and chronic perturbation may be a consequence of maintaining a homeostatic state of circuit activity (Figure 5).
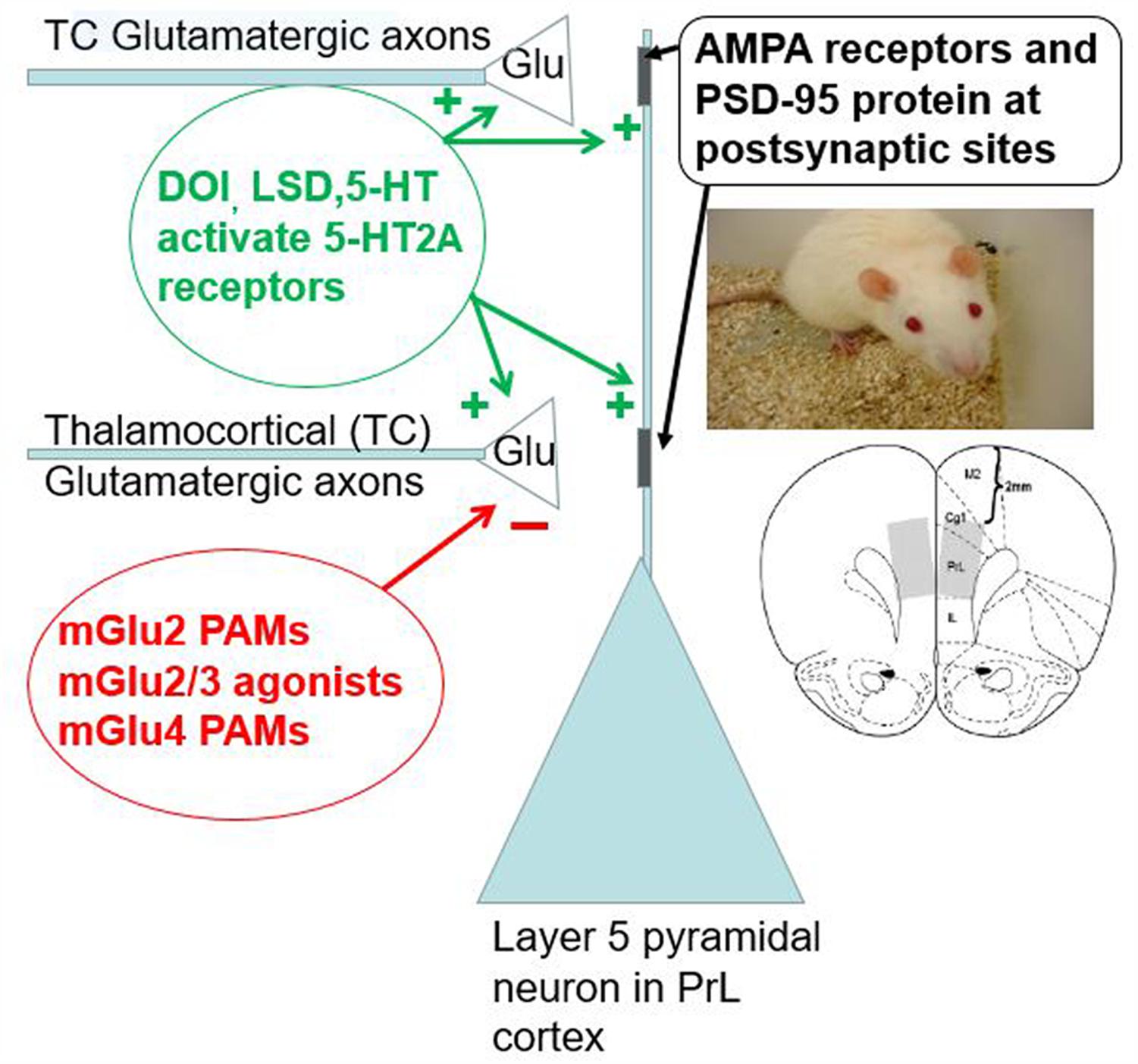
FIGURE 5. The range of serotonergic and glutamatergic modulation in the layer V pyramidal neuron apical dendritic field from the prefrontal cortex/neocortex is shown. 5-HT2A receptors are present at postsynaptic sites to glutamatergic axon terminals and may also be present at presynaptic thalamocortical axon terminals (arising from the midline and thalamic nuclei) relative to the layer V pyramidal neuron apical dendrites. 5-HT2A receptor activation appears to induce glutamate release from these midline and intralaminar thalamic nuclei axons. Due to the presence of both mGlu2 receptors acting as a critical autoreceptor on these thalamocortical terminals, mGlu2/3 receptor agonists and mGlu2 receptor PAMs suppress glutamate release from these terminals in a homeostatic fashion. MGlu4 receptors also may be present on these glutamatergic terminals. Glutamate released by these thalamocortical axons activates AMPA receptors on the layer V pyramidal neuron apical dendrite. The PSD-95 protein is a scaffolding protein that may keep AMPA and postsynaptic 5-HT2A receptors in close physical proximity. The lower insert shows a coronal slice of the mouse brain including the prelimbic region of the medial prefrontal cortex. This region appears to support DOI-induced head twitches in rodents. Thus, this microcircuitry appears very salient for rodent behavior, at least with respect to DOI-induced head twitches.
A second major finding is that both the suppressant action of a mGlu2/3 receptor agonist and a mGlu2 receptor PAM on the DOI-induced HTR was retained in mGlu3 receptor KO mice. Unfortunately, the large rightward shift in the DOI-induced HTR in mGlu2 KO mice effectively precluded testing the effects of mGlu2/3 receptor agonists or mGlu2 receptor PAMs in the mGlu2 receptor KO mice. The down-regulation of DOI-induced head twitches in mGlu2 receptor KO mice coupled with the finding that the suppressant effects of mGlu2/3 receptor agonists or mGlu2 receptor PAMs on the DOI-induced HTR are present in mGlu3 receptor KO mice is consistent with the hypothesis that activation of mGlu2 receptors may be a necessary and sufficient pharmacological action when mGlu2/3 receptor agonists suppress the DOI-induced HTR. However, a subtle role of mGlu3 receptors in modulating the DOI-induced HTR would require testing of selective mGlu3 receptor agonists or PAMs. Nevertheless, the mGlu2 receptor KO finding replicates observations with an independent mouse transgenic strain lacking mGlu2 receptors (Moreno et al., 2011).
Previously, acute treatment with a mGlu2/3 receptor antagonist was found to increase the mean DOI-induced HTR in rats (Gewirtz and Marek, 2000). This effect was present in mGlu3 receptor KO mice, suggesting this increased behavioral DOI response is due to blockade of presumed mGlu2 autoreceptors on thalamic inputs to the cortex. The failure to similarly observe a significant increase in DOI-induced head twitches following administration of a mGlu2/3 receptor antagonist in WT mice may have been related to examining a limited portion of the dose-response relationship (only 1–3 mg/kg of LY341495 for the WT experiment vs. 1–10 mg/kg for the mGlu3 receptor KO experiment) or a smaller sample size (16 vs. 8).
The robust rightward shift in the DOI dose-response relationship for the DOI-induced HTR in mice lacking mGlu2 receptors implies that decreasing feedback regulation of glutamate release plays a major role in the regulation of at least some cortical 5-HT2A receptor responses. Another independent transgenic line also shows a robust decrease in the DOI HTR (Moreno et al., 2011). Mice lacking the serotonin transporter (SERT) respond to DOI with minimal if any head twitches (Qu et al., 2005; Fox et al., 2010), in keeping with the critical importance of SERT for inactivating the synaptic 5-HT signal by reuptake of 5-HT back into the presynaptic terminal. Without SERT, the 5-HT2A receptor is down-regulated by the supra-physiological neuropil 5-HT concentrations.
Conversely, a robust up-regulation or leftward shift in the DOI-induced HTR has also previously been observed in a mutant mouse strain lacking a number of presynaptic 5-HT components (tryptophan hydroxylase2, the serotonin transporter, the vesicular monoamine transporter 2, and aromatic amino acid decarboxylase) (Yadav et al., 2011). Brain 5-HT concentrations for these pet1-/- mutant mice is only 15% of WT mice (Hendricks et al., 2003). These pet1-/- mice exhibit an overactivity of 5-HT2A receptor behavioral function in the prefrontal cortex that is probably due to a decrease in tonic inhibitory 5-HT1A receptor activity (Yadav et al., 2011).
In addition to the presynaptic side of either serotonergic or glutamatergic terminals appearing to cause a profound regulation of the DOI-induced HTR, mutant mice lacking the postsynaptic density protein of 95 kDa (PSD-95), a scaffolding protein associated with glutamatergic synapses, show an attenuated DOI-induced HTR (Abbas et al., 2009). This may be related to a PSD-95 binding motif in the 5-HT2A receptor protein that is postulated to play a role in dendritic targeting of 5-HT2A receptors (Xia et al., 2003a,b). These relationships between presynaptic modulation of both glutamate and serotonin release as well as the postsynaptic side machinery including AMPA receptors (Zhang and Marek, 2008) and 5-HT2A receptors highlights a critical role played by cortical 5-HT2A receptor activity in regulating the output of the prefrontal cortex/neocortex (Figure 5).
A limitation of the present findings may be an underestimation of the total number of head twitches observed during the observation period. For example, the frequency of DOI-induced head twitches may be about two–threefold lower than other examples recent examples of the more frequently used observation of a single mouse at a time (Griebel et al., 2016). This study may also be on the low side compared to other studies cited in the introduction. A second limitation of the study is the inherent variability in the DOI-induced HTR. A two–threefold variation in the mean number of DOI-induced HTR between different experiments was observed in the present studies as well. Other experiments from our laboratory in Sprague-Dawley rats, with mean head twitch frequencies induced by DOI (3 mg/kg, i.p.) in the range of reports using single observations, have found that two mGlu2 receptor PAMs (CBiPES and THIIC) significantly suppressed the frequency of DOI-induced head twitches (Supplementary Figure S1). Similarly, the only other proprietary mGlu2 receptor PAM that was tested in both WT and mGlu3 receptor KO mice demonstrated reliable effects of both DOI and the suppressant action of this mGlu2 receptor PAM in both the WT and mGlu3 receptor KO mice (Supplementary Figure S2). In addition, replicable effects of DOI alone in a series of 12 experiments with proprietary mGlu2 receptor PAMs (see description of the head twitch test in the section “Materials and Methods”) does not suggest that there is a major role for tachyphylaxis of the DOI-induced HTR when employing the currently described methodology in WT mice. Most importantly, the pattern of results obtained with mGlu2/3 receptor agonists, mGlu2 receptor PAMs, and mGlu2/3 receptor antagonists in the present experiments is in good agreement with past results for these compounds in rodents and mice as discussed in the Section “Introduction.”
Clinical implications for these relationships between 5-HT2A receptors and mGlu2 receptors are multifold including the notion that these relationships may paradoxically limit clinical efficacy for 5-HT2A or mGlu2 receptor ligands. First, the antipsychotic-like properties of 5-HT2A receptor inverse agonists or antagonists were clearly demonstrated for patients with Parkinson’s disease psychosis (Meltzer et al., 2010; Cummings et al., 2014). This replicated earlier findings that 5-HT2A receptor antagonists exerted modest antipsychotic properties in patients with schizophrenia (Marder, 1999; Meltzer et al., 2004), in keeping with psychotomimetic effects of LSD, mescaline and psilocybin. Secondly, a role for mGlu2 receptor agonists or PAMs for the treating psychosis is another implication derived in part from a range of interactions between 5-HT2A and mGlu2 receptors. However, antipsychotic efficacy of mGlu2/3 agonists or mGlu2 receptor PAMs may be restricted to those subjects with schizophrenia not previously exposed to atypical antipsychotics with nearly complete 5-HT2A receptor blockade or in those subjects early in their personal trajectory with schizophrenia (Patil et al., 2007; Kinon et al., 2015). Given the pimavanserin clinical profile in Parkinson’s disease psychosis, mGlu2 receptor agonists or PAMs might be useful in patients with neurodegenerative disease suffering from psychotic-like symptoms. Ironically, the earlier hope that the extensive relationships between 5-HT2A receptors and mGlu2 receptors would provide a path toward therapeutic drugs with broad antipsychotic clinical activity may instead mean that antipsychotic efficacy with mGlu2 receptor agonists/PAMs might be restricted to a smaller subpopulation of patients.
Author Contributions
MJB and GM designed the experiments and wrote the manuscript. TB, WH, and JM synthesized the mGlu receptor drugs. MB developed and maintained the transgenic mice. SC performed the experiments. MJB, JM, and GM analyzed and discussed the data.
Funding
This research was supported by the Lilly Neuroscience Discovery Research and Discovery Chemistry Research.
Conflict of Interest Statement
All work was funded by Eli Lilly and Co. and was performed at Lilly Neuroscience Discovery laboratories.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work, in part, was presented as a poster (170.10) at Experimental Biology 2006, April 1–5, San Francisco, CA. MJB, SC, MB, TB, WH, JM, and GM (2006). Attenuation of DOI-induced head twitches in mGluR2 KO mice. The FASEB Journal, 20, A246. (www.fasebj.org/doi/abs/10.1096/fasebj.20.4.A246)
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2018.00208/full#supplementary-material
FIGURE S1 | Two mGlu2 receptor positive allosteric modulator (PAMs) (CBiPES and THIIC) suppress DOI-induced head twitches in Sprague-Dawley rats. Both DOI (3 mg/kg) and the mGlu2 receptor PAMs were administered i.p. CBiPES and THIIC were injected 30 min prior to DOI and then the eight animals/dose cohort were observed immediately afterward for 30 min. The rats were approximately 5–6 weeks old (100–150 g). The individual chamber size was 25% larger in both length and width than the mouse chambers. Eight rats were observed at a single time. The top graph shows the effects of CBiPES while the lower graph provides the results of the THIIC experiment. Each bar represents the mean (±SEM) of eight rats. ∗Significantly different from the DOI alone (0) group, p < 0.05. Red bars display the effects of DOI alone while the blue bars show the effects of different doses of the mGlu2 receptor PAMs.
FIGURE S2 | The suppressant effect of a mGlu2 receptor PAM, compound A, on DOI-induced head twitches in wild-type CD1 mice (top graph) and also in both wild-type and mGlu3 receptor KO mice bottom graph). Compound A was administered 30 min prior to DOI. Each bar represents the mean (±SEM) of eight animals after i.p. dosing. Significantly different from 0 or the vehicle condition, ∗p < 0.05. Significantly different from 0 (vehicle) by the Fishers Exact Test, #p < 0.05. The solid red bars show the effects of DOI alone in the wild-type mice while the solid blue bars display the suppressant effect of compound A on DOI-induced head twitches. The red hatched bars show the effects of DOI alone in the mGlu3 receptor KO mice while the blue hatched bars display the suppressant effect of compound A on DOI-induced head twitches. Significantly different from respective WT, ∗p < 0.05 or KO, ∗p < 0.05 treated only with DOI. Significantly different from respective WT and KO treated only with DOI by Fishers Exact Test, #p < 0.05.
References
Abbas, A. I., Yadav, P. N., Yao, W.-D., Arbuckle, M. I., Grant, S. G. N., Caron, M. G., et al. (2009). PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. J. Neurosci. 29, 7124–7136. doi: 10.1523/JNEUROSCI.1090-09.2009
Benneyworth, M. A., Xiang, Z., Smith, R. L., Garcia, E. E., Conn, P. J., and Sanders-Bush, E. (2007). A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol. Pharmacol. 72, 477–484. doi: 10.1124/mol.107.035170
Berendse, H. W., and Groenewegen, H. J. (1991). Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience 42, 73–102. doi: 10.1016/0306-4522(91)90151-D
Buchborn, T., Schroder, H., Dieterich, D. C., Grecksch, G., and Hollt, V. (2015). Tolerance to LSD and DOB induced shaking behaviour: differential adaptations of frontocortical 5-HT2A and glutamate receptor binding sites. Behav. Brain Res. 281, 62–68. doi: 10.1016/j.bbr.2014.12.014
Buckholtz, N. S., Freedman, D. X., and Middaugh, L. D. (1985). Daily LSD administration selectively decreases serotonin2 receptor binding in rat brain. Eur. J. Pharmacol. 109, 421–425. doi: 10.1016/0014-2999(85)90407-8
Buckholtz, N. S., Zhou, D., and Freedman, D. X. (1988). Serotonin2 agonist administration down-regulates rat brain serotonin2 receptors. Life Sci. 42, 2439–2445. doi: 10.1016/0024-3205(88)90342-6
Canal, C. E., and Morgan, D. (2012). Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test. Anal. 4, 556–576. doi: 10.1002/dta.1333
Carhart-Harris, R. L., Leech, R., Erritzoe, D., Williams, T. M., Stone, J. M., Evans, J., et al. (2013). Functional connectivity measures after psilocybin inform a novel hypothesis of early psychosis. Schizophr. Bull. 39, 1343–1351. doi: 10.1093/schbul/sbs117
Celada, P., Puig, M. V., Diaz-Mataix, L., and Artigas, F. (2008). The hallucinogen DOI reduces low-frequency oscillations in rat prefrontal cortex: reversal by antipsychotic drugs. Biol. Psychiatry 64, 392–400. doi: 10.1016/j.biopsych.2008.03.013
Chaki, S., Yoshikawa, R., Hirota, S., Shimazaki, T., Maeda, M., Kawashima, N., et al. (2004). MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology 46, 457–467. doi: 10.1016/j.neuropharm.2003.10.009
Cummings, J., Isaacson, S., Mills, R., Willams, H., Chi-Burris, K., Corbett, A., et al. (2014). Pimavanserin for patients with Parkinson’s disease psychosis: a randomized, placebo-controlled phase 3 trial. Lancet 383, 533–540. doi: 10.1016/S0140-6736(13)62106-6
Darmani, N. A., and Gerdes, C. F. (1995). Temporal differential adaptation of head-twitch and ear-scratch responses following administration of challenge doses of DOI. Pharmacol. Biochem. Behav. 50, 545–550. doi: 10.1016/0091-3057(94)00340-8
Darmani, N. A., Martin, B. R., and Glennon, R. A. (1992). Behavioral evidence for differential adaptation of the serotonergic system after acute and chronic treatment with (+/-)-1(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) or ketanserin. J. Pharmacol. Exp. Ther. 262, 692–698.
Dunayevich, E., Erickson, J., Levine, L., Landbloom, R., Schoepp, D. D., and Tollefson, G. D. (2008). Efficacy and tolerability of an mGlu2/3 agonist in the treatment of generalized anxiety disorder. Neuropsychopharmacology 33, 1603–1610. doi: 10.1038/sj.npp.1301531
Fell, M. J., Witkin, J. M., Falcone, J. F., Katner, J. S., Perry, K. W., Hart, J., et al. (2011). N-(4-((2-(trifluoromethyl)-3-hydroxy-4-(isobutyryl)phenoxy)methyl)benzyl)-1-methyl-1H-imidazole-4-carboxamide (THIIC), a novel metabotropic glutamate 2 potentiator with potential anxiolytic/antidepressant properties: in vivo profiling suggests a link between behavioral and central nervous system neurochemical changes. J. Pharmacol. Exp. Ther. 336, 165–177. doi: 10.1124/jpet.110.172957
Fox, M. A., Stein, A. R., French, H. T., and Murphy, D. L. (2010). Functional interactions between 5-HT2A and presynaptic 5-HT1A receptor-based responses in mice genetically deficient in the serotonin 5-HT transporter (SERT). Br. J. Pharmacol. 159, 879–887. doi: 10.1111/j.1476-5381.2009.00578.x
Galici, R., Jones, C. K., Hemstapat, K., Nong, Y., Echemendia, N. G., Willams, L. C., et al. (2006). Biphenyl-indanone A, a positive allosteric modulator of the metabotropic glutamate receptor subtype 2, has antipsychotic- and anxiolytic-like effects in mice. J. Pharmacol. Exp. Ther. 318, 173–185. doi: 10.1124/jpet.106.102046
Gewirtz, J. C., and Marek, G. J. (2000). Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology 23, 569–576. doi: 10.1016/S0893-133X(00)00136-6
Gonzalez-Maeso, J., Ang, R. L., Yuen, T., Chan, P., Weisstaub, N. V., Lopez-Gimenez, J. F., et al. (2008). Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452, 93–97. doi: 10.1038/nature06612
Griebel, G., Pichat, P., Boulay, D., Naimoli, V., Potestio, L., Featherstone, R., et al. (2016). The mGluR2 positive allosteric modulator, SAR218645, improves memory and attention deficits in translational models of cognitive symptoms associated with schizophrenia. Sci. Rep. 13:35320. doi: 10.1038/srep35320
Hendricks, T. J., Fyodorov, D. V., Wegman, L. J., Lelutiu, N. B., Pehek, E. A., Yamamoto, B., et al. (2003). Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron 37, 233–247. doi: 10.1016/S0896-6273(02)01167-4
Johnson, M. P., Barda, D., Britton, T. C., Emkey, R., Hornback, W. J., Jagdmann, G. E., et al. (2005). Metabotropic glutamate 2 receptor potentiators: receptor modulation, frequency-dependent synaptic activity, and efficacy in preclinical anxiety and psychosis model(s). Psychopharmacology 179, 271–283. doi: 10.1007/s00213-004-2099-9
Kinon, B. J., Millen, B. A., Zhang, L., and McKinzie, D. L. (2015). Exploratory analysis for a targeted population responsive to the metabotropic glutamate 2/3 receptor agonist pomaglumetad methionil in schizophrenia. Biol. Psychiatry 78, 754–762. doi: 10.1016/j.biopsych.2015.03.016
Klodzinska, A., Bijak, M., Tokarski, K., and Pilc, A. (2002). Group II mGlu receptor agonists inhibit behavioral and electrophysiological effects of DOI in mice. Pharmacol. Biochem. Behav. 73, 327–332. doi: 10.1016/S0091-3057(02)00845-6
Lambe, E. K., and Aghajanian, G. K. (2001). The role of Kv1.2-containing potassium channels in serotonin-induced glutamate release from thalamocortical terminals in rat frontal cortex. J. Neurosci. 21, 9955–9963.
Lavreysen, H., Langlois, X., Donk, L. V., Nunez, J. M., Pype, S., Lutjens, R., et al. (2015). Preclinical evaluation of the antipsychotic potential of the mGlu2-positive allosteric modulator JNJ-40411813. Pharmacol. Res. Perspect. 3:e00097. doi: 10.1002/prp2.97
Leysen, J. E., Janssen, P. F. M., and Niemegeers, C. J. E. (1989). Rapid desensitization and down-regulation of 5-HT2 receptors by DOM treatment. Eur. J. Pharmacol. 163, 145–149. doi: 10.1016/0014-2999(89)90409-3
Linden, A. M., Shannon, H., Baez, M., Yu, J. L., Koester, A., and Schoepp, D. D. (2005). Anxiolytic-like activity of the mGlu2/3 receptor agonist LY354740 in the elevated plus maze test is disrupted in metabotropic glutamate receptor 2 and 3 knock-out mice. Psychopharmacology 179, 284–291. doi: 10.1007/s00213-004-2098-x
Marder, S. R. (1999). Limitations of dopamine-D2 antagonists and the search for novel antipsychotic strategies. Neuropsychopharmacology 21, S117–S121. doi: 10.1016/S0893-133X(99)00105-0
Marek, G. J. (2003). Behavioral evidence for μ-opioid and 5-HT2A receptor interactions. Eur. J. Pharmacol. 474, 77–83. doi: 10.1016/S0014-2999(03)01971-X
Marek, G. J. (2009). Activation of adenosine1 (A1) receptors suppresses head shakes induced by a serotonergic hallucinogen in rats. Neuropharmacology 56, 1082–1087. doi: 10.1016/j.neuropharm.2009.03.005
Marek, G. J. (2017). Interactions of Hallucinogens with the Glutamatergic System: Permissive Network Effects Mediated Through Cortical Layer V Pyramidal Neurons. Current Topics in Behavioral Neuroscience. Berlin: Springer.
Marek, G. J., and Aghajanian, G. K. (1998). 5-HT-induced EPSCs in neocortical layer V pyramidal cells: suppression by μ-opiate receptor activation. Neuroscience 86, 485–497. doi: 10.1016/S0306-4522(98)00043-8
Marek, G. J., Wright, R. A., Gewirtz, J. C., and Schoepp, D. D. (2001). A major role for thalamocortical afferents in serotonergic hallucinogen receptor function in the rat neocortex. Neuroscience 105, 379–392. doi: 10.1016/S0306-4522(01)00199-3
Marek, G. J., Wright, R. A., and Schoepp, D. D. (2006). 5-Hydroxytryptamine2A (5-HT2A) receptor regulation in rat prefrontal cortex: interaction of a phenethylamine hallucinogen and the metabotropic glutamate2/3 receptor agonist LY354740. Neurosci. Lett. 403, 256–260. doi: 10.1016/j.neulet.2006.05.021
Marek, G. J., Wright, R. A., Schoepp, D. D., Monn, J. A., and Aghajanian, G. K. (2000). Physiological antagonism between 5-hydroxytryptamine2A and group II metabotropic glutamate receptors in prefrontal cortex. J. Pharmacol. Exp. Ther. 292, 76–87.
Meltzer, H. Y., Arvanitis, L., Bauer, D., Rein, W., and Group, M.-T. S. (2004). Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorders. Am. J. Psychiatry 161, 975–984. doi: 10.1176/appi.ajp.161.6.975
Meltzer, H. Y., Mills, R., Revell, S., Willams, H., Johnson, A., Bahr, D., et al. (2010). Pimavanserin, a serotonin(2A) receptor inverse agonist, for the treatment of parkinson’s disease psychosis. Neuropsychopharmacology 35, 881–892. doi: 10.1038/npp.2009.176
Michelson, D., Levine, L. R., Dellva, M. A., Mesters, P., Schoepp, D. D., Dunayevich, E., et al. (2005). Clinical studies with mGlu2/3 receptor agonists: LY354740 compared with placebo in patients with generalized anxiety disorder. Neuropharmacology 49(Suppl. 1):257.
Moghaddam, B., and Adams, B. W. (1998). Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonists in rats. Science 281, 1349–1352. doi: 10.1126/science.281.5381.1349
Moreno, J. L., Holloway, T., Albizu, L., Sealfon, S. C., and Gonzalez-Maeso, J. (2011). Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci. Lett. 493, 76–79. doi: 10.1016/j.neulet.2011.01.046
Moreno, J. L., Holloway, T., Rayannavar, V., Sealfon, S. C., and Gonzalez-Maeso, J. (2013). Chronic treatment with LY341495 decreases 5-HT2A receptor binding and hallucinogenic effects of LSD in mice. Neurosci. Lett. 536, 69–73. doi: 10.1016/j.neulet.2012.12.053
Moreno, J. L., Muguruzza, C., Umali, A., Mortillo, S., Holloway, T., Pilar-Cuellar, F., et al. (2012). Identification of three residues essential for 5-hydroxtryptamine 2A-metabotropic glutamate 2 (5-HT2A-mGlu2) receptor heteromerizaiton and its psychoactive behavioral function. J. Biol. Chem. 287, 44301–44319. doi: 10.1074/jbc.M112.413161
Muller, F., Lenz, C., Dolder, P., Lang, U. E., Schmidt, A., Liechti, M., et al. (2017). Increased thalamic resting-state connectivity as a core driver of LSD-induced hallucinations. Acta Psychiatr. Scand. 136, 648–657. doi: 10.1111/acps.12818
Muschamp, J. W., Regina, M. J., Hull, E. M., Winter, J. C., and Rabin, R. A. (2004). Lysergic acid diethylamide and (-)-2,5-dimethoxy-4-methylamphetamine increase extracellular glutamate in rat prefrontal cortex. Brain Res. 1023, 134–140. doi: 10.1016/j.brainres.2004.07.044
Nikiforuk, A., Popik, P., Drescher, K. U., van Gaalen, M., Relo, A.-L., Mezler, M., et al. (2010). Effects of a positive allosteric modulatory of group II metabotropic glutamate receptors, LY487379, on cognitive flexibility and impulsive-like responding in rats. J. Pharmacol. Exp. Ther. 335, 665–673. doi: 10.1124/jpet.110.170506
Ohishi, H., Neki, A., and Mizuno, N. (1998). Distribution of a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat and mouse: an immunohistochemical study with a monoclonal antibody. Neurosci. Res. 30, 65–82. doi: 10.1016/S0168-0102(97)00120-X
Ohishi, H., Shigemoto, R., Nakanishi, S., and Mizuno, N. (1993). Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience 53, 1009–1018. doi: 10.1016/0306-4522(93)90485-X
Patil, S. T., Zhang, L., Martenyi, F., Lowe, S. L., Jackson, K. A., Andreev, B. V., et al. (2007). Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized phase 2 clinical trial. Nat. Med. 13, 1103–1108.
Qu, Y., Villacreses, N., Murphy, D. L., and Rapoport, S. I. (2005). 5-HT2A/2C receptor signaling via phospholipase A2 and arachidonic acid is attenuated in mice lacking the serotonin reuptake transporter. Psychopharmacology 180, 12–20. doi: 10.1007/s00213-005-2231-5
Rorick-Kehn, L. M., Johnson, B. G., Knitowski, K. M., Salhoff, C. R., Witkin, J. M., Perry, K. W., et al. (2007). In vivo pharmacological characterization of the structurally novel, potent, selective mGlu2/3 receptor agonist LY404039 in animal models of psychiatric disorders. Psychopharmacology 193, 121–136. doi: 10.1007/s00213-007-0758-3
Saalmann, Y. B. (2014). Intralaminar and medial thalamic influence on cortical synchrony, information transmission and cognition. Front. Syst. Neurosci. 8:83. doi: 10.3389/fnsys.2014.00083
Schoepp, D. D. (2001). Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J. Pharmacol. Exp. Ther. 299, 12–20.
Schoepp, D. D., Wright, R. A., Levine, L. R., Gaydos, B., and Potter, W. Z. (2003). LY354740, an mGlu2/3 receptor agonist as a novel approach to treat anxiety/stress. Stress 6, 189–197. doi: 10.1080/1025389031000146773
Scruggs, J. L., Patel, S., Bubser, M., and Deutch, A. Y. (2000). DOI-induced activation of the cortex: dependence upon 5-HT2A heteroceptors on thalamocortical glutamatergic neurons. J. Neurosci. 20, 8846–8852.
Scruggs, J. L., Schmidt, D., and Deutch, A. Y. (2003). The hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) increases cortical extracellular glutamate levels in rats. Neurosci. Lett. 346, 137–140. doi: 10.1016/S0304-3940(03)00547-0
Shi, J., Landry, M., Carrasco, G. A., Battaglia, G., and Muma, N. A. (2008). Sustained treatment with a 5-HT(2A) receptor agonist causes functional desensitization and reductions in agonist-labeled 5-HT(2A) receptors despite increases in receptor protein levels in rats. Neuropharmacology 55, 687–692. doi: 10.1016/j.neuropharm.2008.06.001
Slawinska, A., Wieronska, J. M., Stachowicz, K., Marciniak, M., Lason-Tyburkiewicz, M., Gruca, P., et al. (2013). The antipsychotic-like effects of positive allosteric modulators of metabotropic glutamate mGlu4 receptors in rodents. Br. J. Pharmacol. 169, 1824–1839. doi: 10.1111/bph.12254
Stutzman, G. E., Marek, G. J., and Aghajanian, G. K. (2001). Adenosine preferentially suppresses serotonin2A receptor-enhanced excitatory postsynaptic currents in layer V neurons of the rat medial prefrontal cortex. Neurosci. 105, 55–69. doi: 10.1016/S0306-4522(01)00170-1
Tagliazucchi, E., Roseman, L., Kaelen, M., Muthukumaraswamy, S. D., Murphy, K., Laufs, H., et al. (2016). Increased global functional connectivity correlates with LSD-induced ego dissolution. Curr. Biol. 26, 1043–1050. doi: 10.1016/j.cub.2016.02.010
Van der Werf, Y. D., Witter, M. P., and Groenewegen, H. J. (2002). The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res. Rev. 39, 107–140. doi: 10.1016/S0165-0173(02)00181-9
Wieronska, J. M., Slawinska, A., Stachowicz, K., Lason-Tyburkiewicz, M., Gruca, P., Papp, M., et al. (2013). The reversal of cognitive, but not negative or positive symptoms of schizophrenia by the mGlu2/3 receptor agonist, LY379268, is 5-HT1A dependent. Behav. Brain Res. 256, 298–304. doi: 10.1016/j.bbr.2013.08.007
Willins, D. L., and Meltzer, H. Y. (1997). Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J. Pharmacol. Exp. Ther. 282, 699–706.
Wischhof, L., and Koch, M. (2016). 5-HT2A and mGlu2/3 receptor interactions: on their relevance to cognitive function and psychosis. Behav. Pharmacol. 27, 1–11. doi: 10.1097/FBP.0000000000000183
Wright, R. A., Johnson, B. G., Zhang, C., Salhoff, C., Kingston, A. E., Calligaro, D. O., et al. (2013). CNS distribution of metabotropic glutamate 2 and 3 receptors: transgenic mice and [3H]LY459477 autoradiography. Neuropharmacology 66, 89–98. doi: 10.1016/j.neuropharm.2012.01.019
Xia, Z., Gray, J. A., Compton-Toth, B. A., and Roth, B. L. (2003a). A direct interaction of PSD-95 with 5-HT2A serotonin receptors regulates receptor trafficking and signal transduction. J. Biol. Chem. 278, 21901–21908.
Xia, Z., Hufeisen, S. J., Gray, J. A., and Roth, B. L. (2003b). The PDZ-binding domain is essential for the dendritic targeting of 5-HT2A serotonin receptors in cortical pyramidal neurons in vitro. Neuroscience 122, 907–920.
Yadav, P. N., Abbas, A. I., Farrell, M. S., Setola, V., Sciaky, N., Huang, X.-P., et al. (2011). The presynaptic component of the serotonergic system is required for clozapine’s efficacy. Neuropsychopharmacology 36, 638–651. doi: 10.1038/npp.2010.195
Yasuhara, A., Nakamura, M., Sakagami, K., Shimazaki, T., Yoshikawa, R., Chaki, S., et al. (2006). Prodrugs of 3-(3,4-dichlorobenzyloxy)-2-amino-6-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (MGS0039): a potent and orally active group II mGluR antagonist with antidepressant-like potential. Bioorg. Med. Chem. 14, 4193–4207. doi: 10.1016/j.bmc.2006.01.060
Zhang, C., and Marek, G. J. (2007). Group III metabotropic glutamate receptor agonists selectively suppress excitatory synaptic currents in the rat prefrontal cortex induced by 5-hydroxytryptamine2A receptor stimulation. J. Pharmacol. Exp. Ther. 320, 437–447. doi: 10.1124/jpet.106.107490
Keywords: 5-hydroxytryptamine2A (5-HT2A) receptors, DOI, CBiPES, LY379268, LY341495, head twitches, prefrontal cortex (PFC), transgenic mice
Citation: Benvenga MJ, Chaney SF, Baez M, Britton TC, Hornback WJ, Monn JA and Marek GJ (2018) Metabotropic Glutamate2 Receptors Play a Key Role in Modulating Head Twitches Induced by a Serotonergic Hallucinogen in Mice. Front. Pharmacol. 9:208. doi: 10.3389/fphar.2018.00208
Received: 31 October 2017; Accepted: 23 February 2018;
Published: 15 March 2018.
Edited by:
Andrew Robert Gallimore, Okinawa Institute of Science and Technology, JapanReviewed by:
Esa R. Korpi, University of Helsinki, FinlandRobert Warren Gould, Vanderbilt University, United States
Copyright © 2018 Benvenga, Chaney, Baez, Britton, Hornback, Monn and Marek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gerard J. Marek, Z2VyYXJkLm1hcmVrQGFzdGVsbGFzLmNvbQ==
†Present address: Gerard J. Marek, Astellas Pharma Global Development, Inc., Northbrook, IL, United States
 Mark J. Benvenga1,2
Mark J. Benvenga1,2 James A. Monn
James A. Monn Gerard J. Marek
Gerard J. Marek