- 1Department of Pharmaceutical Biology, Johannes Gutenberg University, Mainz, Germany
- 2Division of Chemotherapy, Faculty of Pharmacy, Keio University, Tokyo, Japan
- 3Medical and Clinical Affairs Phytomedicines, Steigerwald Arzneimittelwerk GmbH, Bayer Consumer Health, Darmstadt, Germany
For decades, natural products represented a significant source of diverse and unique bioactive lead compounds in drug discovery field. In Clinical oncology, complete tumors remission is hampered by the development of drug-resistance. Therefore, development of cytotoxic agents that may overcome drug resistance is urgently needed. Here, the natural benzophenanthridine alkaloid sanguinarine has been studied for its cytotoxic activity against multidrug resistance (MDR) cancer cells. We investigated the role of the ATP-binding cassette (ABC) transporters BCRP/ABCG2, P-glycoprotein/ABCB1 and its close relative ABCB5 in drug resistance. Further drug resistance mechanisms analyzed in this study were the tumor suppressor TP53 and the epidermal growth factor receptor (EGFR). Multidrug resistant cells overexpressing BCRP, ABCB5 and mutated ΔEGFR were not cross-resistant toward sanguinarine. Interestingly, P-gp overexpressing cells were hypersensitive to sanguinarine. Doxorubicin uptake assay carried by flow cytometry revealed that sanguinarine is a potent inhibitor of the P-gp transporter. Moreover, immunoblotting analysis proved that P-gp was downregulated in a dose dependent manner after treating P-gp overexpressing cells with sanguinarine. It was surmised that The inhibition of NFκB activity might explain the collateral sensitivity in CEM/ADR5000 cells. The COMPARE and hierarchical cluster analyses of transcriptome-wide expression profiles of tumor cell lines of the National Cancer Institute identified genes involved in various cellular processes (immune response, inflammation signaling, cell migration and microtubule formation) significantly correlated with log10IC50 values for sanguinarine. In conclusion, sanguinarine may have therapeutic potential for treating multidrug resistant tumors.
Introduction
Sanguinarine is a natural benzophenanthridine alkaloid isolated from Sanguinaria canadensis (Papaveraceae). The roots of the plant, also known as bloodroot because of its red latex, are widely used in North America for cold, cough, sore throat, as a systemic expectorant, antifungal, and ulcers of the skin (Predny and Chamberlain, 2005; Croaker et al., 2016). The plant is applied topically in paste form to treat cancerous growths, warts, boils and polyps in the skin (Howell, 2006).
Sanguinarine exerts several biological activities including, antimicrobial, antioxidant, anti-inflammatory as well as anticancer properties (Jeng et al., 2007). The cytotoxicity of sanguinarine against tumor cells has been reported in vitro and in vivo (Gaziano et al., 2016; Bodle et al., 2017; Wei et al., 2017). Furthermore, sanguinarine induced apoptosis and triggered cell death signaling cascades in numerous cancer cell lines (Lee et al., 2008, 2016; Xu et al., 2012). Several modes of action have been proposed to explain the anticancer activity of sanguinarine, such as activation of the caspase machinery in mitochondria, irreversible microtubule depolymerization, depletion of nuclear topoisomerase II preventing DNA strand break reconnection, inhibition of B-DNA to Z-DNA transition altering DNA supercoiling, capping telomeres inducing rapid apoptosis, depletion of cellular glutathione, and cell cycle arrest (Rich, 1994; Das et al., 1999; Messori et al., 2001; Debiton et al., 2003; Adhami et al., 2004; Bai et al., 2006, 2008; Lopus and Panda, 2006; Nitiss, 2009). Moreover, sanguinarine targets vital cellular compartments including extracellular signal-regulated kinases and NF-κB, which are involved in signal transduction pathways associated with cell proliferation and/or cell death mechanisms (Lee et al., 2008). In addition, it has been shown that sanguinarine inhibits the sodium–potassium ATPase affecting the membrane permeability (Ding et al., 2002).
Drug resistance to clinically established chemotherapeutic agents remains the main obstacle toward achieving complete tumors remission and long-lasting cures. Several mechanisms have been proposed to be responsible for chemotherapy failure. Multidrug resistance (MDR) is the phenomenon in which cancer cells exhibit cross-resistance or loss the sensitivity to various chemotherapeutic agents that are structurally and functionally different, including anthracyclines, Vinca alkaloids, colchicine, epipodophyllotoxins, taxanes, camptothecins, imatinib, and mitoxantrone (Raaijmakers et al., 2006; Efferth et al., 2008; Fletcher et al., 2010). Well-known mechanisms with clinical significance represent the overexpression of transmembrane efflux pumps such as ATP-binding cassette (ABC) transporters (P-gp, BCRP and ABCB5), activation of enzymes of the glutathione detoxification system, mutations in genes involved in tumor development and apoptosis (e.g., EGFR and p53). These mechanisms act in a cooperative manner due to tumor heterogeneity. Therefore, MDR is defined as a multifactorial process. Despite most mechanisms of MDR have been well studied, understanding their role in the clinical setting still represents a significant challenge.
Chemotherapy is considered as a corner stone in cancer treatment worldwide. The development of MDR and the severe side effects are common challenges for the therapeutic efficacy of most clinically used chemotherapeutic agents. Therefore, the identification of novel effective therapeutic agents is urgently needed. In this context, phytochemicals may represent an attractive alternative because of their affordability and low toxicity (Efferth, 2017; Efferth et al., 2017).
The objectives of the present study were, firstly, to investigate whether ABC transporters (P-gp, ABCB5, BCRP) as classical MDR mechanisms also play a role in response to sanguinarine. Secondly, we addressed the question whether the cytotoxic activity of sanguinarine correlated to other molecular determinants in the cell line panel of the National Cancer Institute (NCI, USA). In addition to ABC transporters, we also studied oncogenes and tumor suppressor genes (EGFR, TP53). Furthermore, we performed bioinformatic COMPARE and hierarchical cluster analyses of microarray-based transcriptomic mRNA expression data of the NCI cell lines that were correlated to sanguinarine's response, as well as transcription factor promoter binding motif analysis searching for common transcription factors that might regulate the genes appeared in microarray-and COMPARE analyses. Moreover, we carried in vitro assay detecting the activity of drug resistance-mediating transcription factor NFκB using cells stably expressing reporter gene preceded by NFκB promoter, as well as in silico molecular docking analyses of sanguinarine to NFκB and its regulator IκB.
Materials and Methods
Cell Lines
Two leukemia cell lines, drug-sensitive CCRF-CEM and multidrug-resistant CEM/ADR5000, were cultured in RPMI 1640 medium, supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Darmstadt, Germany) and 1% penicillin (100 U/ml)-streptomycin (100 μg/ml) antibiotic (Invitrogen, Darmstadt, German) and incubated in humidified 5% CO2 atmosphere at 37°C. Human HEK293-ABCB5 embryonic kidney cells transfected with another ABC-transporter, ABCB5, were propagated in DMEM medium supplemented with 10% FBS and 1% penicillin/streptomycin (Invitrogen) (Kawanobe et al., 2012). Breast cancer cells transduced with control vector (MDA-MB-231-pcDNA3) or with a cDNA for the breast cancer resistance protein BCRP (MDA-MB-231BCRP clone 23), human wild-type HCT116 (p53+/+) colon cancer cells as well as knockout clones HCT116 (p53−/−) derived by homologous recombination, non-transduced human U87MG glioblastoma multiforme cells and U87MG cells transduced with an expression vector harboring an epidermal growth factor receptor (EGFR) gene with a genomic deletion of exons 2 through 7 (U87MG.ΔEGFR) were all maintained in DMEM medium, supplemented with 10% FBS and 1% penicillin-streptomycin and incubated under standard conditions as described for leukemia cell lines. The resistance of the different resistant cell lines has been maintained by adding 5,000 ng/ml doxorubicin for CEM/ADR5000, 400 μg/ ml geneticin for U87MG.ΔEGFR and HCT116 (p53−/−) and 800 ng/ml of the same compound for MDA-MB-231 BCRP clone 23.
A panel of 59 human tumor cell lines of the Developmental Therapeutics Program of the NCI (Bethesda, MD, USA) consisted of leukemia, melanoma, non-small cell lung cancer, colon cancer, renal cancer, ovarian cancer, breast cancer, and prostate carcinoma cells as well as tumor cells of the central nervous system (Alley et al., 1988). Cells treated with sanguinarine for 48 h and cytotoxicity was evaluated using a sulforhodamine B assay (Rubinstein et al., 1990).
Resazurin Assay
The non-fluorescent dye resazurin is reduced metabolically by living cells to the strongly-fluorescent dye resorufin (O'Brien et al., 2000). The resazurin (Promega, Mannheim, Germany) reduction assay was performed to assess the cytotoxicity of sanguinarine toward drug-sensitive and -resistant cell lines. Briefly, tumor cells (2 × 104cells/well) were seeded in 96-wells plate in a volume of 100 μL, and varying concentrations of sanguinarine were added to reach the total volume of 200 μL. After 72 h, 20 μL of 0.01% w/v resazurin (Sigma-Aldrich, Schnelldorf, Germany) was added to each well. Cells were incubated for 4 h at 37°C. Fluorescence at excitation wave length 544 nm and emission at 590 nm was measured using Infinite M2000 Pro™ plate reader (Tecan, Crailsheim, Germany). The percentage of viable cells was calculated as follows:
IC50 values were calculated from concentration dependent curves using nonlinear regression analysis tool built in Prism 7 GraphPad software. All IC50 values are expressed as the mean ± standard deviation (SD). Each assay was repeated thrice independently with six replicates each.
Toxicity of Sanguinarine in Normal Cells
Using Histopaque® (Sigma-Aldrich, St. Louis, MO, USA), the human peripheral mononuclear cells (PMNC) were isolated from fresh blood samples of a healthy donor. In brief, three mL blood was layered with three mL Histopaque® and centrifuged (400 × g) for 30 min at 4°C. The buffy coat interface, containing lymphocytes and other mononuclear cells, was transferred into a new tube and washed several times. Isolated PMNCs were kept in Panserin 413 medium (PAN-Biotech, Aidenbach, Germany) supplemented with 2% phytohemagglutinin M (PHA-M, Life Technologies, Darmstadt, Germany). Afterwards, the resazurin assay was carried out as described above.
Doxorubicin Uptake Assay
The uptake of doxorubicin with and without the addition of sanguinarine has been measured using BD FACSCalibur™ (Beckton Dickinson, GmbH, Heidelberg, Germany). Sensitive parental CCRF-CEM leukemia cells, as well as their resistance P-glycoprotein-expressing subline, CEM/ADR5000 were tested. 106 cells/well were seeded in 12-well tissue culture plates (Becton-Dickinson, Heidelberg, Germany) in the incubation medium (RPMI 1640, Invitrogen™) without phenol red. Doxorubicin at a concentration of 20 μM (University Hospital Pharmacy, Mainz, Germany) was used in all sample wells. Verapamil (20 μM, Sigma Aldrich, Taufkirchen, Germany) was used as a known inhibitor of P-gp. Sanguinarine (1 μM, Sigma Aldrich) was used as the test compound. After incubation for at 37°C 24 h, the cells were centrifuged to discard the old incubation medium and resuspended in fresh medium. Mean fluorescence intensity (MFI) of doxorubicin was measured by blue laser at an excitation wave length of 488 nm, and the emitted light was collected with a band pass filter at 530/30 nm. Every 30,000 cells were counted per sample. Dead cells were eliminated by gating the living cells in the forward vs. the side scatter.
COMPARE and Hierarchical Cluster Analyses of Microarray Data
COMPARE analysis is a web-based algorithm correlating between transcriptome-wide mRNA expressions and drug response of the NCI cell line panel (https://dtp.cancer.gov). The method is based on Pearson's rank correlation coefficient (Paull et al., 1989). We performed COMPARE analysis between the IC50 values for sanguinarine and the microarray-based transcriptome-wide mRNA expression levels in the NCI cell lines. Standard and reverse COMPARE were conducted to determine the genes, which were associated with resistance or sensitivity to sanguinarine, respectively.
Using the CIMMINER program (https://discover.nci.nih.gov/cimminer/), We performed agglomerative hierarchical cluster analysis (WARD method) to cluster the mRNA expression of genes identified by COMPARE analysis, and the heatmap was prepared accordingly.
Pearson's correlation test was used to calculate significance values and rank correlation coefficients as a relative measure for the linear dependence of two variables. The chi square (χ2) test was performed using the Excel program to proof bivariate frequency distributions for pairs of nominal scaled variables for dependencies obtained from cluster analysis/heat mapping.
Transcription Factor Gene Promoter Binding Motif Analysis
The top 40 genes that either directly or inversely correlated with log10IC50 values of the NCI cell lines in COMPARE analysis were subjected to binding motif analysis. Promoter sequences 25 kb long upstream of exon 1 of the corresponding genes were retrieved from UCSC Genome Browser Gene Sorter (http://genome.ucsc.edu). Browser Extensible Data (BED) files were created to screen for the possible binding motifs for each gene. Promoter sequences were screened using the SeqPos tool implemented in the Galaxy Cistrome software [72].
Molecular Docking
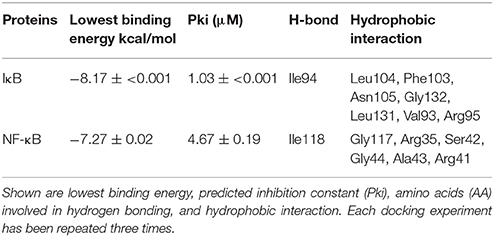
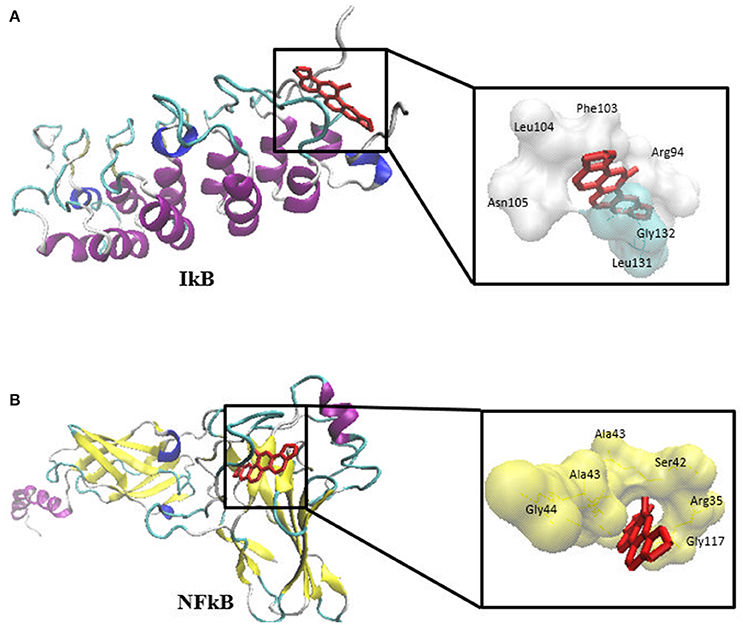
Molecular docking is a predictive algorithm to evaluate the mode of binding of ligands with target macromolecules. X-ray crystallography-based structures of IκB and NF-κB were obtained from Protein Data Bank (http://www.rcsb.org/pdb); IκBα (PDB ID: 1IKN), and NF-κB (PDB ID: 1NFI). To determine the binding geometry of sanguinarine to both targets, a grid box was then constructed to define docking spaces for each protein. Docking parameters were set to 250 runs and 2,500,000 energy evaluations for each cycle. Docking was performed three times independently by Autodock4 and with AutodockTools-1.5.7rc1 using the Lamarckian algorithm. The corresponding lowest binding energies and predicted inhibition constants were obtained from the docking log files (dlg). Mean ± SD of binding energies were calculated from three independent dockings. Visual Molecular Dynamics (VMD) was used to depict the docking poses of sanguinarine for each target protein.
Reporter Cell Line
HEK-Blue-Null1 cells (Invivogen, San Diego, CA, USA) stably express an optimized secreted embryonic alkaline phosphatase (SEAP) reporter gene under the control of the IFN-β promoter fused to NFκB binding site. The protocol was previously described by us (Seo et al., 2016). NFκB activation was detected by measuring SEAP spectrophotometrically at 630 nm (Tecan Teader, Tecan Group Ltd., Maennedorf, Switzerland).
Proteins Analyses by SDS-PAGE and Immunoblotting
CEM/ADR5000 leukemia cells (106 cells/well) were treated with varying concentrations of sanguinarine, harvested after 24 h, and washed with PBS. Total proteins extraction protocol was previously described (Saeed et al., 2015). Protein concentrations were measured by NanoDrop1000 (PEQLAB, Erlangen, Germany). Twenty micrograms of protein were electrophoresed on 10% SDS-polyacrylamide gels. Using wet sandwich blotting, the proteins were transferred onto Polyvinylidene difluoride (Ruti®-PVDF) membrane (Millipore Corporation, Billerica, MA). Membranes were washed using Tris-buffered saline containing 0.5% Tween-20 (TBST), then blocked with 5% (w/v) bovine serum albumin in TBST for 1 h at room temperature. Membranes were incubated at 4°C overnight with primary antibodies including: P-gp (1:1000) (Thermoscientific, Darmstadt, Germany), NFκB (1:1000) (Cell Signaling Technology, Frankfurt, Germany), IκBα (1:1000) (Cell Signaling Technology, Frankfurt, Germany), and β-actin (1:2000) (Cell Signaling Technology, Frankfurt, Germany). After washing membranes three times with TBST, the blots were probed with horseradish peroxidase-linked IgG secondary antibodies (1:2000) for 2 h at room temperature. Finally, LuminataTM Classico Western HRP substrate (Merck Millipore, Schwalbach, Germany) was added for 5 min in the dark. Alpha Innotech FluorChem Q system (Biozym, Oldendorf, Germany) was used for documentation and band analysis (Zhao et al., 2015).
Results
Role of Classical Drug Resistance Mechanisms for Sanguinarine
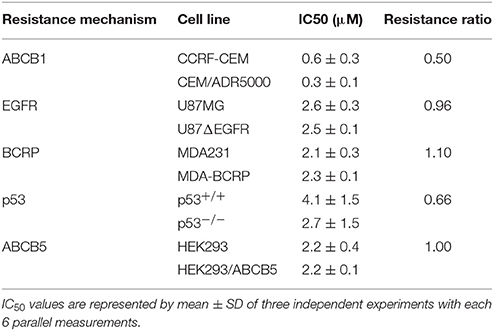
By using the Pearson correlation test, we correlated the log10IC50 values of the NCI cell lines panel for sanguinarine with different of mRNA expression patterns of ABC transporters (ABCB1, ABCG2, and ABCB5), and EGFR, as well as mutations in the TP53 or H-, K-, N-RAS genes - all of which represent known drug resistance mechanisms (Table 1). For each resistance mechanism, positive control drugs have been used. Interestingly, none of the expression patterns or mutations correlated with cellular response to sanguinarine. Indeed, these are favorable results which gave a clue that none of these classical drug resistance mechanisms may hamper the effect of sanguinarine in cancer cells. To confirm the results obtained by using the NCI cell line panel, we performed cytotoxicity assays of sanguinarine in sensitive and drug-resistant cell lines bearing the different drug resistance mechanisms. As shown in Figure 1 and Table 2, sanguinarine has been tested in concentrations range from 10−5 to 102 μM. Interestingly, sanguinarine showed selective toxicity toward P-gp overexpressing cell lines and p53 knockout cell lines, when compared to their corresponding sensitive parent cell lines (resistance ratios of 0.5 and 0.65, respectively) (Figures 1A,D), a phenomenon called collateral sensitivity. Cross-resistance was not observed in the cell lines overexpressing other ABC transporters (ABCB5 and ABCG2) or mutation-activated EGFR, if treated with sanguinarine (Figures 1B,C,E). The sanguinarine inhibited the human peripheral mononuclear cells in 0.611 ± 0.1 μM. This represents two times concentration needed to kill resistant leukemia cells (CEM/ADR5000) (Figure 1F), which considered comparable counterpart as a tumor model.
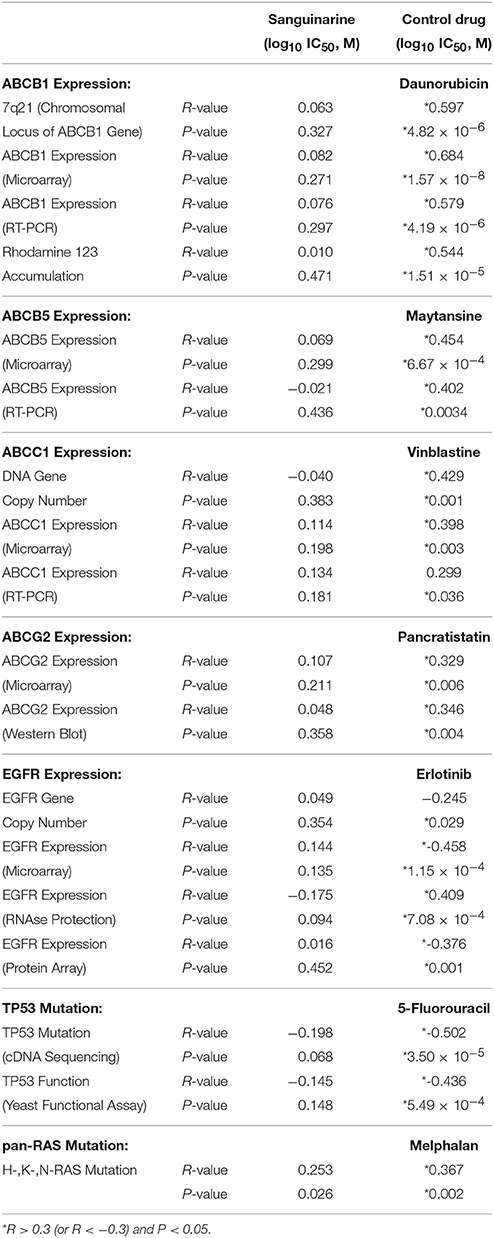
Table 1. Correlation of log10IC50 values for sanguinarine to drug resistance mechanisms (ABCB1, ABCB5, ABCC1, ABCG2, EGFR, TP53, pan-RAS) in the NCI cell line panel.
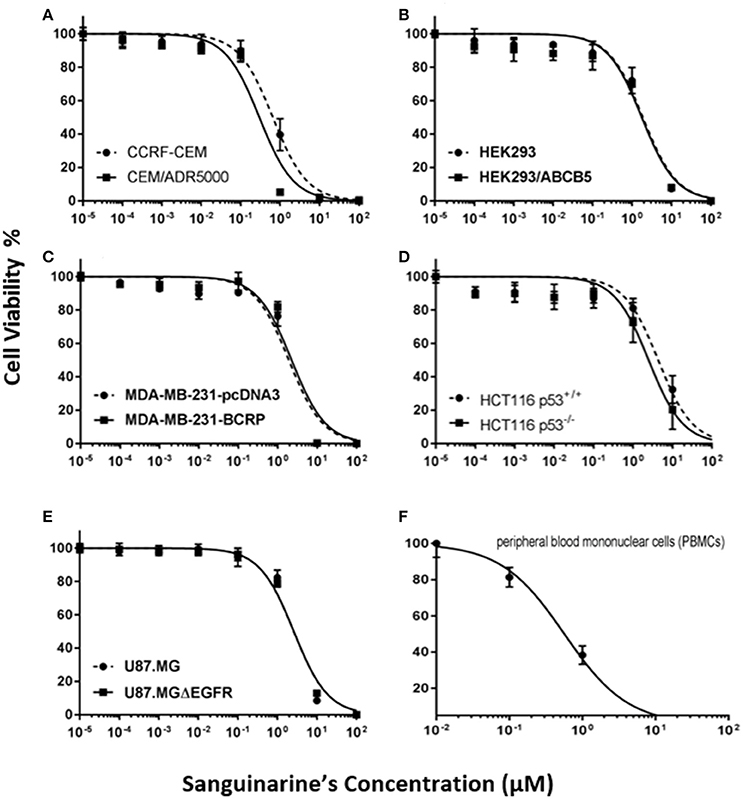
Figure 1. Dose response curves of sanguinarine. (A) Cytotoxicity of sanguinarine toward drug-sensitive parental CCRF-CEM tumor cells and their P-glycoprotein (MDR1/ABCB1)-expressing, multidrug-resistant subline, CEM/ADR5000. (B) Cytotoxicity of sanguinarine toward HEK293 cells and their ABCB5-transfectant subline, HEK293/ABCB5 as determined by resazurin assays. (C) Cytotoxicity of sanguinarine toward MDA-MB-231-pc DNA cells and their BCRP-transduced subline, MDA-MB-231-BCRP as determined by resazurin assays. (D) Cytotoxicity of sanguinarine toward HCT116 p53+/+ cells and their p53−/− knockout subline, HCT116 p53−/− as determined by resazurin assays. (E) Cytotoxicity of sanguinarine toward U87MG cells and their EGFR-transduced subline U87MGΔEGFR, as determined by resazurin assays. (F) Cytotoxicity of sanguinarine toward human peripheral mononuclear cells, as determined by resazurin assays.
Drug Class Profiling
To have a clue about the possible mechanism of action of sanguinarine, we correlated the log10IC50 values of the NCI cell lines to sanguinarine with those of standard drugs Figure 2C. The cellular response of antibiotics and DNA topoisomerase I or II inhibitors drugs significantly correlated with those of sanguinarine (100%). Antimetabolites and platinum compounds were correlated with sanguinarine (79 and 65%).
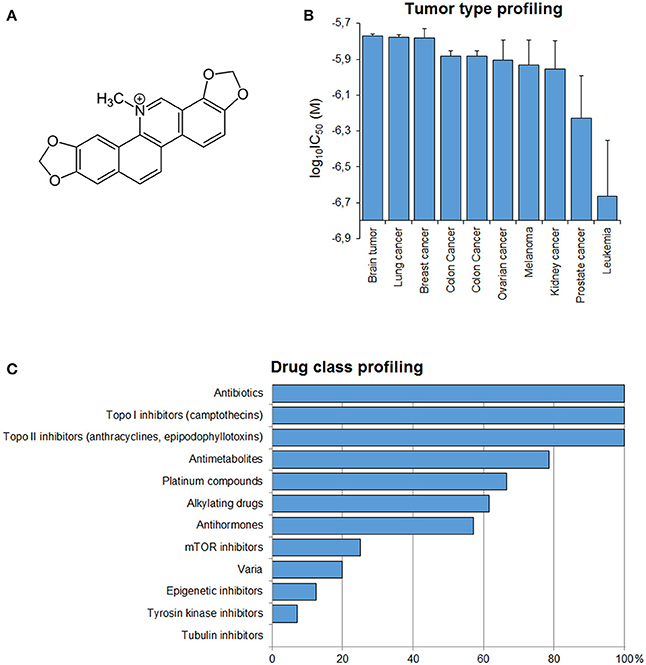
Figure 2. (A) Chemical structure of sanguinarine. (B) Mean log10IC50 values for sanguinarine of the NCI cell lines, mean values and S.E.M. of log10IC50 were grouped according to the tumor type of the cell lines. (C) Percentage of classes of established anticancer drugs, whose log10IC50 values correlate with those for sanguinarine.
Tumor-Type Dependent Response Toward Sanguinarine
If the average of log10IC50 values of al cell lines according to their corresponding tumor type was plotted, it became apparent that leukemia cell lines were most sensitive toward snaguinarine, whereas brain cell lines were the most resistant (Figure 2B).
Inhibition of P-Glycoprotein Function by Sanguinarine
The overexpression of P-glycoprotein in cancer cells mediates extrusion of chemotherapeutic drugs outside the cells leading to sub-lethal intracellular drug concentrations (Efferth, 2001). To maintain a high concentration of the chemotherapeutic drugs inside the cancer cells, P-gp has to be inhibited to enhance drug uptake. Therefore, we analyzed the uptake of doxorubicin by P-glycoprotein-overexpressing cells in the presence or absence of sanguinarine by flow cytometry. A representative histogram is shown in Figure 3A. High doxorubicin uptake was obtained in CCRF-CEM cells, whereas considerably less drug was taken up in CEM/ADR5000 cells due to P-glycoprotein-mediated efflux. The addition of sanguinarine (1 μM) or the well-known P-glycoprotein inhibitor verapamil (20 μM) increased doxorubicin uptake in CEM/ADR5000 cells. The bar diagram in Figure 3B shows that sanguinarine and verapamil significantly increased doxorubicin uptake in CEM/ADR5000 cells. The increase of doxorubicin uptake by P-glycoprotein-overexpressing cells after addition of sanguinarine or verapamil indicates that the transporter function in CEM/ADR5000 cells was specifically inhibited by both compounds.
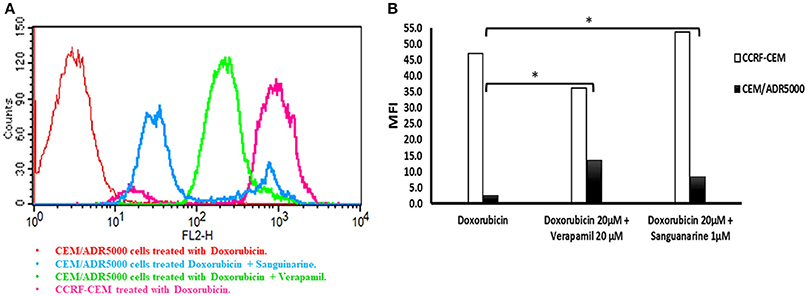
Figure 3. Flow cytometry analysis of the effect of sanguinarine on doxorubicin uptake by cell line that overexpress P-gp in comparison of known transporter's inhibitor, verapamil. (A) Histograms represent doxorubicin fluorescence intensity, CEM/ADR5000 cells treated with doxorubicin appear in red, CCRF-CEM cells treated with doxorubicin appears in pink, CEM/ADR5000 cells treated with doxorubicin in combination with sanguinarine and Verapamil appear in blue and light green respectively. (B) Bar diagram represent doxorubicin mean fluorescence intensity on cell line that overexpress P-gp, white bars represented sensitive cells, whereas resistant cells were represented by black bars. Mean fluorescence intensity (MFI) of doxorubicin has been measured by blue laser at excitation wave length 488 and emitted light was collected at 530/30 band pass. MFI values have been plotted on the y-axis against tested compounds on the x-axis. *P-value < 0.05.
Microarray-Based Expression Profiling to Predict Sensitivity and Resistance to Sanguinarine
To identify possible genes that are associated with sensitivity or resistance of cancer cells toward sanguinarine, we dug the transcriptome-wide mRNA expressions of the NCI cells and correlated them to the log10IC50 values for sanguinarine. We performed a transcriptome-wide COMPARE analysis to generate a ranking list of genes, whose mRNA expression directly or inversely correlated with the log10IC50 values for sanguinarine. Only cut-off values of correlation coefficients of R > 0.5 (direct correlations) or R < −0.5 (inverse correlations) were taken into account. Fourty genes were identified, half of them were directly and the other half were inversely correlated to the log10IC50 values for sanguinarine (Table 3). The proteins encoded by these genes have diverse biological functions (Table 3).
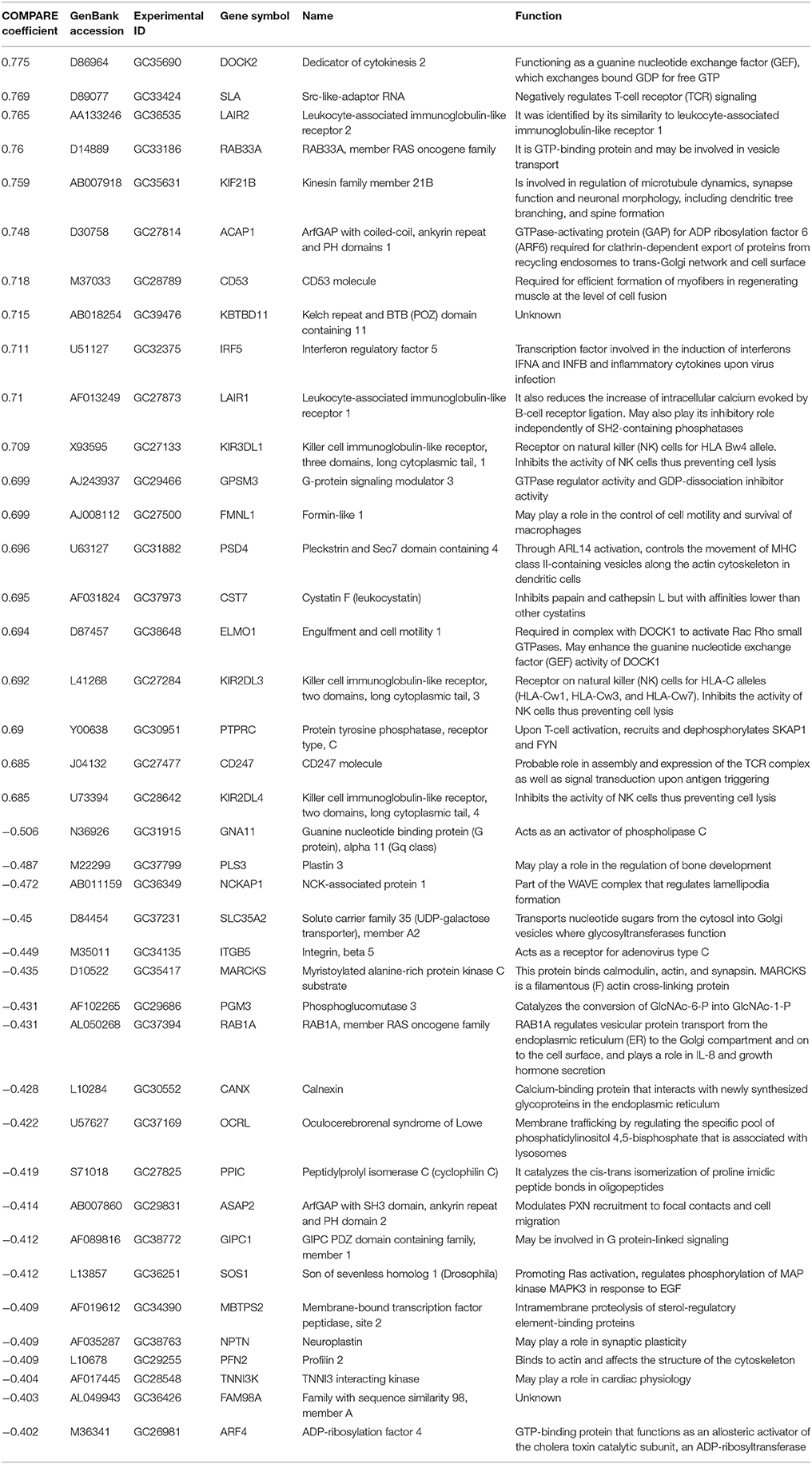
Table 3. Correlation of constitutive mRNA expression of genes identified by COMPARE analysis with log10IC50 values for sanguinarine of the NCI tumor cell lines.
The mRNA expression values of all NCI cell lines for the genes listed in Table 3 were subsequently subjected to hierarchical cluster analysis, in order to find out, whether clusters of cell lines could be identified with similar behavior after exposure to sanguinarine. The dendrogram of the cluster analysis showed five main branches in the cluster tree that depicted in the heatmap (Figure 4). As a next step, the log10IC50 values for sanguinarine, which were not included in the cluster analysis, were assigned to the corresponding position of the cell lines in the cluster tree. The distribution among the five clusters was significantly different from each other (P = 0.03). Clusters 4 and 5 contained in its majority of cell lines resistant to sanguinarine, whereas clusters 1, 2, and 3 contained in its majority sensitive ones. The median value of the log10IC50 values was used as a cutoff value to define cell lines as being sensitive or resistant to sanguinarine.
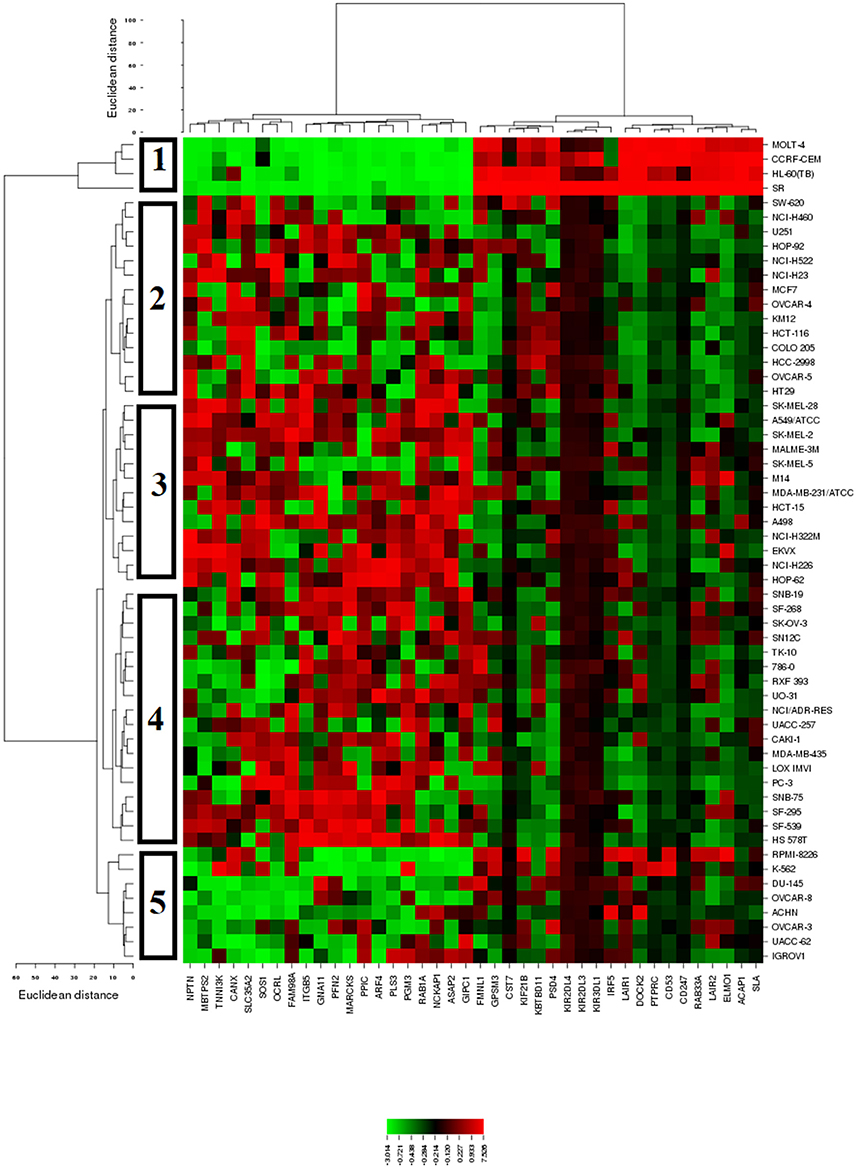
Figure 4. Dendrograms and heatmap of sanguinarine obtained by hierarchical cluster analyses of NCI cells line panel and genes whose mRNA expression directly or inversely correlated with the log10IC50 values for sanguinarine. The dendrogram on the left shows the clustering of cell lines and the dendrogram on the top shows the clustering of genes.
Transcription Factor Gene Promoter Binding Motif Analysis
We subjected the 40 genes identified by COMPARE analysis to binding motif analysis to find possibly common regulatory transcription factors. The theoretical concept behind was that, a wide array of functionally diverse genes might be commonly up-or down-regulated by one or a few transcription factors that are involved in resistance to cytotoxic compounds. Interestingly, the NF-κB DNA binding motif (Rel) was widely distributed in the untranslated promoter regions 25 kb upstream of all identified genes, with 99 hits and a Z-score of −3.8 (Figure 5). This analysis pointed out that NF-κB might play a considerable role in the regulation of genes associated with cellular response to sanguinarine.
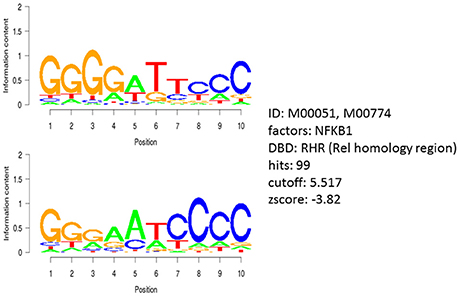
Figure 5. Motif analysis of 25 kb upstream regions of 40 genes identified by COMPARE analysis revealing the significant presence of NF-κB binding motif.
Molecular Docking
The previous results allow speculating that sanguinarine might prevent the degradation of IκBα leading to maintenance of the IκB-NF-κB complex in its idle form (Chaturvedi et al., 1997). In order to substantiate this idea, we performed molecular docking analyses studying the binding affinity of sanguinarine to both proteins. As shown in Table 4, sanguinarine preferably bound to IκBα with a binding energy of −8.2 kcal/mol, whereas sanguinarine exerted lower affinities to NF-κB with a binding energy of −7.2 kcal/mol. These results indicate that sanguinarine has a higher affinity to IκB than to NF-κB. The corresponding docking positions of sanguinarine into binding pockets of IκB and NF-κB are depicted in Figure 6.
NF-κB Reporter Cell Line
Molecular docking studies and motif analysis have predicted the ability of sanguinarine to interact with NFκB and it's regulator IκB. However, since these are speculative findings we could not reach a clear statement whether sanguinarine inhibits or activates the NFκB. Therefore, we performed NFκB reporter assay using SEAP-driven cell line to address this question. HEK-Blue-Null1 cells were treated with varying concentrations of sanguinarine (0.1, 1, and 10 μM). A known inhibitor of NFκB activation, triptolide (1 μM), was used as a positive control. Interestingly, sanguinarine reduced the SEAP levels in a dose dependent manner, indicating that NFκB's activity is blocked Figure 7. This result clearly showed that sanguinarine is an inhibitor of NFκB activity.
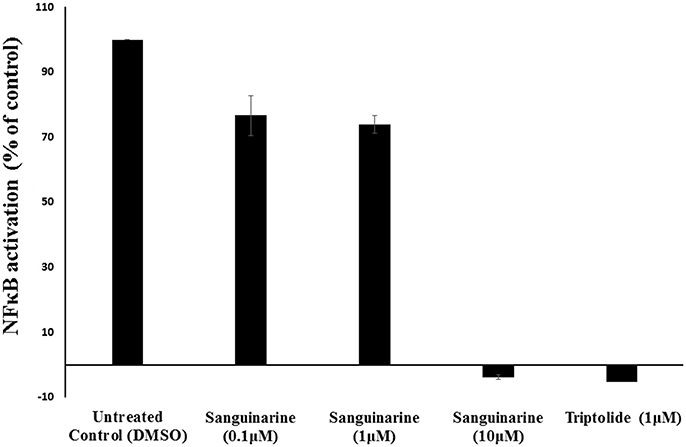
Figure 7. Effect of sanguinarine on NF-κB activity in comparison to the known NF-κB inhibitor, triptolide, as positive control.
Immunoblotting Analysis
We have a clue that the inhibition of NκFB activity may explain the phenomenon of the collateral sensitivity of resistant P-gp overexpressing cells toward sanguinarine. To assess this hypothesis, the P-gp, NFκB, and IκBα expressions were evaluated by immunoblotting analysis. Three concentrations of sanguinarine were selected (0.5 IC50, IC50 and 2x IC50). Interestingly, the expressions of all three proteins were decreased in a dose dependent manner (Figure 8).
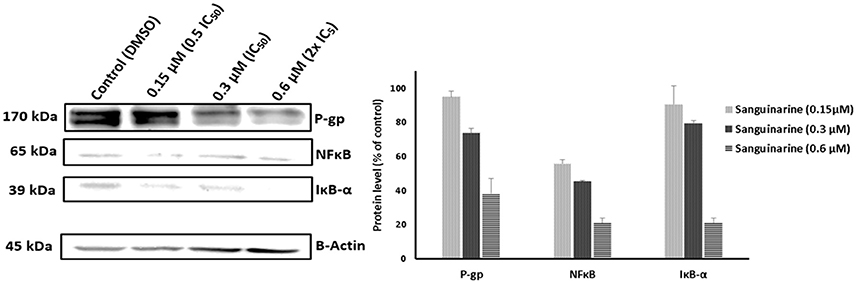
Figure 8. Western blot analysis of the effect of Sanguinarine on CEM/ADR5000 leukemia cells. Evaluation of the P-gp, NFκB, and IκBα expressions. β-actin was used as loading control. Bands were normalized to β-actin in order to obtain numerical values (Mean ± SD).
Discussion
Faced with the general requirement to develop novel cancer drugs with activity against otherwise drug-resistant tumors, we focused on sanguinarine, a natural product from S. canadensis. In the present study, sanguinarine revealed considerable cytotoxicity against several drug-resistant tumor cell lines. The cytotoxic concentrations needed to kill 50% of the cell lines tested were all in the micromolar range. While sanguinarine's cytotoxicity against cancer cells has been previously reported (Ding et al., 2002; Debiton et al., 2003; Choi et al., 2008), it was not known whether or not sanguinarine's activity may be hampered by mechanisms that also confer resistance to clinically established anticancer drugs. Therefore, the main aim of our study was to investigate the capability of sanguinarine to kill otherwise drug-resistant tumor cells. For this reason, we used cell lines overexpressing drug-resistance-conferring ABC-transporters (P-gp/ABCB1, BCRP, or ABCB5), or harboring mutations in the EGFR, RAS, or TP53 genes.
ABC transporters are responsible for chemotherapy failure by expelling drugs outside of tumor cells and, thereby, minimizing doses required for cells killing. From the 49 members of the ABC transporter family, P-gp and BCRP were extensively studied for their contribution to MDR (Ueda et al., 1986; Doyle et al., 1998). A few years ago, a new member of the B-subfamily conferring MDR had been cloned, ABCB5 (Frank et al., 2003). This transporter is closely related to P-gp and exhibits resistance to doxorubicin, paclitaxel, and docetaxel in cancer cells (Frank et al., 2005; Kawanobe et al., 2012). All our cell models that overexpress the ABC transporters mentioned above did not confer resistance to sanguinarine indicating that this compound is not a substrate for these transporters. Interestingly, sanguinarine showed hypersensitivity (collateral sensitivity) to P-gp-overexpressing cells, if compared to their parental drug-sensitive counterparts. From a therapeutic point of view, collateral sensitivity is a favorable phenomenon, since the corresponding cytotoxic agent preferentially kills drug-resistant cells (Hall et al., 2009). This finding led us to the assumption that sanguinarine may interfere with P-glycoprotein's function. Therefore, we performed doxorubicin uptake assays. Indeed, sanguinarine comparably increased doxorubicin uptake, as the known P-gp inhibitor, verapamil, did. This is a remarkable result, since verapamil is not only an inhibitor of drug efflux in P-glycoprotein, it also exerts collateral sensitivity if applied alone (Pluchino et al., 2012). In this respect, sanguinarine behaves comparably as verapamil.
EGFR is a member of the HER family. Upon binding of its ligands, EGF or TGF-α, it forms homo- or hetero-dimers with other HER family members to activate specific downstream signaling cascades after tyrosine phosphorylation. This activation of signaling routes subsequently regulates multiple cellular processes, such as proliferation, survival, and apoptosis. EGFR mutations are known to affect the poor prognosis of patients and mediate drug resistance of tumors. The in-frame deletion of the extracellular EGFR domain results in ligand-independent receptor activations and represents a common mutant type in brain tumors, named ΔEFGR (Shinojima et al., 2003). Sanguinarine inhibited both cell models harboring wtEGFR (sensitive) or ΔEFGR (resistant) at the same concentrations, indicating that ΔEFGR does not confer resistance toward sanguinarine.
P53 is a transcription factor that regulates the expression of p21WAF1/CIP1/SID1, MDM2, Bax, and Gadd45. These proteins play a critical role in cell cycle arrest, DNA repair and induction of apoptosis. P53 mutations occur in almost half cancer types ever known (Hollstein et al., 1991), and loss of p53 function is linked to drug resistance in several tumor entities, including carcinoma of the breast, ovary, and colorectum as well as melanoma, acute lymphoblastic leukemia, neuroblastoma and osteosarcoma (Li et al., 1998; Asada et al., 1999; Lam et al., 1999; Righetti et al., 1999; Berns et al., 2000). Cells lacking alleles of p53 (HCT116 p53−/−) conferred resistance to 5-FU, irinotecan and oxaliplatin (Boyer et al., 2004). Unexpectedly, our results showed that sanguinarine preferentially inhibited the resistant p53 knockout colorectal cancer cells comparably to the cells bearing wt p53. This result indicates that sanguinarine may have another molecular target, which is functionally regulated in the presence or absence of p53. Worth to mention, Webster et al., proved that both p53 and NF-κB inhibited each other's ability to stimulate gene expression by competing for the limited pool of transcriptional co-activator p300 and CREB-binding protein (Webster and Perkins, 1999). This means NF-κB inhibits p53-dependent transactivation, whereas p53 expression down-regulates NF-κB expression leading to diminished drug resistance and prevention of apoptosis and tumorigenesis. Moreover, they concluded that reactivation of wild-type p53 function by coordinated suppression of NF-κB might favor a better clinical outcome. Recently, several studies have affirmed that mutant p53 augmented and prolonged NFκB activation in vitro and in vivo (Scian et al., 2005; Weisz et al., 2007; Schneider et al., 2010; Cooks et al., 2013). Apparently, all these studies gave us a clue that NF-κB might be the target of sanguinarine. Therefore, transcription factor gene promoter binding motif analysis for genes appeared from wide transcriptome microarray data have been carried out. Intriguingly, the NF-κB binding motif was detected in the upstream promoter regions of all genes, confirming that NF-κB may be a master transcription regulator for most—if not all—molecular determinants of sanguinarine's activity in cancer cells.
NF-κB is an ubiquitous transcription factor that regulates the expression of genes involved in inflammation, the immune response, cell proliferation, and apoptosis (Li and Verma, 2002). It is inactive in the cytoplasm bound to its inhibitory protein, IκB. Upon activation by cytokines, IκB subsequently undergoes phosphorylation, ubiquitination, and degradation by the proteasome allowing nuclear translocation of NF-κB (Baldwin, 1996). Sanguinarine has been found to inhibit phosphorylation and degradation of IκB keeping NF-κB in its inactive form (Chaturvedi et al., 1997). This result concurs well with our molecular docking analyses, which showed that sanguinarine has high affinity to IκB compared to NF-κB. Moreover, a recent study reported that PI3K/Akt/NF-κB pathway is involved in the development of MDR in MCF-7/ADR cells through the upregulation of the MDR1 gene and P-gp expression. Accordingly, the MDR could be reversed in P-gp overexpressing cells through inhibition of the PI3K/Akt/NF-κB signaling pathway (Yang et al., 2017). Taking Yang et al.'s study together with our immunoblotting analysis results (Figure 8) into account, these findings gave us clear evidence that sanguinarine not only inhibits the activity of P-gp but also downregulates its expression via inhibition of NFκB.
The COMPARE analysis explores the relationship between the gene expression patterns and the drug responsiveness measured by the NCI developmental therapeutic program. The patterns of drug activity across the 60 NCI cell lines provide a clue on mechanisms of drug action, resistance and sensitivity determinants (Ross et al., 2000). The approach of defining genes whose mRNA expression correlated with sanguinarine's activity is based on Pearson correlation coefficient. These correlation coefficients (Table 3) were calculated for each combination of a gene and a drug by taking the (normalized) level of expression of the gene in each cell line, multiplying it by the corresponding (normalized) sensitivity of the cell to the drug, summing the results over all of the cell lines and renormalizing (Scherf et al., 2000). By combining genome-wide expression profiling with sanguinarine's activity data, a possible genes-drug interaction was explored. This comprehensive approach allowed us to assess information of a large number of genes simultaneously and generate hypotheses about the possible mode of action rather than testing a particular biological hypothesis classically, in which cell characteristics were assessed one gene, gene product or molecular pathway at a time (Weinstein, 1998). The theme of the cluster analysis was to group cell lines depending on the levels of gene expressions which correlated to sanguinarine's activity. The log10IC50 values were excluded from the calculation. As shown in Figure 4, leukemia cells were clustered as the most sensitive ones. Interestingly, this predictive findings harmonized with our in vitro cytotoxic assays in which small IC50 values were obtained on leukemia cells after sanguinarine treatment when compared to other tested solid tumor cells (brain, breast, and colon).
The COMPARE analysis revealed that most of the identified genes are involved in activating the immune response and promoting inflammatory signaling (Dock2, SLA, LAIR2, IRF5, KIR3DL1, FMNL1, PSD4, KIR2DL3, PTPRC, CD247, KIR2DL4, LAIR1, ITGB5, RAB1A, and ARF4). Thus, it is not a coincidence that a prototypical proinflammatory protein such as NF-κB appeared as a presumable master regulator of transcription of genes recognized in microarray-based transcriptomic mRNA expression data. Recently, Wang et al., proved that sanguinarine as anti-inflammatory agent prevented cerebral stroke in vivo via downregulation of the inflammatory cytokines, TNF-α, IL-1β and IL-6 (Wang et al., 2017). Moreover, other genes from diverse functional groups appeared in our analysis, such as signal transducers promoting cancer growth (CD53, RAB33A, GNA11, CANX, OCRL, GIPC1, and SOS1), microtubule formation (KIF21B) and cell migration (ASAP2). These cellular processes may be of specific relevance for the responsiveness of tumor cells to sanguinarine.
In conclusion, sanguinarine exhibited remarkable cytotoxicity against different drug-resistant tumor cell lines, which is possibly dependent on multiple mechanisms. The increased doxorubicin uptake by resistant cells indicates that sanguinarine inhibited the P-glycoprotein transporter. Furthermore, sanguinarine inhibited NF-κB activation. In particular, sanguinarine may be a promising candidate either if used alone or in combination with other chemotherapeutic regimens to treat refractory tumors. Nevertheless, the safety profile of sanguinarine should be well investigated, and the chemical scaffold could be further derivatized to increase the therapeutic index. Therefore, further pharmacological studies are needed.
Author Contributions
MS: Carried out the in vitro experiments and wrote the manuscript; NM: Performed COMPARE and cluster analysis; YS: Provided ABCB5 transfected cell lines; HA-A: Designed the bioassays and edited the manuscript; TE: Supervised the work, provided the facilities for the study and edited the manuscript. All authors read the manuscript and approved the final version.
Conflict of Interest Statement
HA-A was employed by company by Bayer/Steigerwald.
The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer WZ and handling Editor declared their shared affiliation.
References
Adhami, V. M., Aziz, M. H., Reagan-Shaw, S. R., Nihal, M., Mukhtar, H., and Ahmad, N. (2004). Sanguinarine causes cell cycle blockade and apoptosis of human prostate carcinoma cells via modulation of cyclin kinase inhibitor-cyclin-cyclin-dependent kinase machinery. Mol. Cancer Ther. 3, 933–940. Available online at: http://mct.aacrjournals.org/content/3/8/933.long
Alley, M. C., Scudiero, D. A., Monks, A., Hursey, M. L., Czerwinski, M. J., Fine, D. L., et al. (1988). Feasibility of drug screening with panels of human-tumor cell-lines using a microculture tetrazolium assay. Cancer Res. 48, 589–601.
Asada, N., Tsuchiya, H., and Tomita, K. (1999). De novo deletions of p53 gene and wild-type p53 correlate with acquired cisplatin-resistance in human osteosarcoma OST cell line. Anticancer Res. 19, 5131–5137.
Bai, L. P., Hagihara, M., Jiang, Z. H., and Nakatani, K. (2008). Ligand binding to tandem G quadruplexes from human telomeric DNA. Chembiochem 9, 2583–2587. doi: 10.1002/cbic.200800256
Bai, L. P., Zhao, Z. Z., Cai, Z. W., and Jiang, Z. H. (2006). DNA-binding affinities and sequence selectivity of quaternary benzophenanthridine alkaloids sanguinarine, chelerythrine, and nitidine. Bioorg. Med. Chem. 14, 5439–5445. doi: 10.1016/j.bmc.2006.05.012
Baldwin, A. S. Jr. (1996). The NF-κ B and I κ B proteins: new discoveries and insights. Annu. Rev. Immunol. 14, 649–683. doi: 10.1146/annurev.immunol.14.1.649
Berns, E. M., Foekens, J. A., Vossen, R., Look, M. P., Devilee, P., Henzen-Logmans, S. C., et al. (2000). Complete sequencing of TP53 predicts poor response to systemic therapy of advanced breast cancer. Cancer Res. 60, 2155–2162. Available online at: http://cancerres.aacrjournals.org/content/60/8/2155.long
Bodle, C. R., Mackie, D. I., Hayes, M. P., Schamp, J. H., Miller, M. R., Henry, M. D., et al. (2017). Natural products discovered in a high-throughput screen identified as inhibitors of RGS17 and as cytostatic and cytotoxic agents for lung and prostate cancer cell lines. J. Nat. Prod. 80, 1992–2000. doi: 10.1021/acs.jnatprod.7b00112
Boyer, J., McLean, E. G., Aroori, S., Wilson, P., McCulla, A., Carey, P. D., et al. (2004). Characterization of p53 wild-type and null isogenic colorectal cancer cell lines resistant to 5-fluorouracil, oxaliplatin, and irinotecan. Clin. Cancer Res. 10, 2158–2167. doi: 10.1158/1078-0432.CCR-03-0362
Chaturvedi, M. M., Kumar, A., Darnay, B. G., Chainy, G. B., Agarwal, S., and Aggarwal, B. B. (1997). Sanguinarine (pseudochelerythrine) is a potent inhibitor of NP-κ B activation, I κ B α phosphorylation, and degradation. J. Biol. Chem. 272, 30129–30134. doi: 10.1074/jbc.272.48.30129
Choi, W. Y., Kim, G. Y., Lee, W. H., and Choi, Y. H. (2008). Sanguinarine, a benzophenanthridine alkaloid, induces apoptosis in MDA-MB-231 human breast carcinoma cells through a reactive oxygen species-mediated mitochondrial pathway. Chemotherapy 54, 279–287. doi: 10.1159/000149719
Cooks, T., Pateras, I. S., Tarcic, O., Solomon, H., Schetter, A. J., Wilder, S., et al. (2013). Mutant p53 prolongs NF-κ B activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell 23, 634–646. doi: 10.1016/j.ccr.2013.03.022
Croaker, A., King, G. J., Pyne, J. H., Anoopkumar-Dukie, S., and Liu, L. (2016). Sanguinaria canadensis: traditional medicine, phytochemical composition, biological activities and current uses. Int. J. Mol. Sci. 17:E1414. doi: 10.3390/ijms17091414
Das, S., Kumar, G. S., and Maiti, M. (1999). Conversions of the left-handed form and the protonated form of DNA back to the bound right-handed form by sanguinarine and ethidium: a comparative study. Biophys. Chem. 76, 199–218. doi: 10.1016/S0301-4622(98)00238-5
Debiton, E., Madelmont, J. C., Legault, J., and Barthomeuf, C. (2003). Sanguinarine-induced apoptosis is associated with an early and severe cellular glutathione depletion. Cancer Chemother. Pharmacol. 51, 474–482. doi: 10.1007/s00280-003-0609-9
Ding, Z., Tang, S. C., Weerasinghe, P., Yang, X., Pater, A., and Liepins, A. (2002). The alkaloid sanguinarine is effective against multidrug resistance in human cervical cells via bimodal cell death. Biochem. Pharmacol. 63, 1415–1421. doi: 10.1016/S0006-2952(02)00902-4
Doyle, L. A., Yang, W., Abruzzo, L. V., Krogmann, T., Gao, Y., Rishi, A. K., et al. (1998). A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. U.S.A. 95, 15665–15670. doi: 10.1073/pnas.95.26.15665
Efferth, T. (2001). The human ATP-binding cassette transporter genes: from the bench to the bedside. Curr. Mol. Med. 1, 45–65. doi: 10.2174/1566524013364194
Efferth, T. (2017). Cancer combination therapies with artemisinin-type drugs. Biochem. Pharmacol. 139, 56–70. doi: 10.1016/j.bcp.2017.03.019
Efferth, T., Konkimalla, V. B., Wang, Y. F., Sauerbrey, A., Meinhardt, S., Zintl, F., et al. (2008). Prediction of broad spectrum resistance of tumors towards anticancer drugs. Clin. Cancer Res. 14, 2405–2412. doi: 10.1158/1078-0432.CCR-07-4525
Efferth, T., Saeed, M. E. M., Mirghani, E., Alim, A., Yassin, Z., Saeed, E., et al. (2017). Integration of phytochemicals and phytotherapy into cancer precision medicine. Oncotarget 8, 50284–50304. doi: 10.18632/oncotarget.17466
Fletcher, J. I., Haber, M., Henderson, M. J., and Norris, M. D. (2010). ABC transporters in cancer: more than just drug efflux pumps. Nat. Rev. Cancer 10, 147–156. doi: 10.1038/nrc2789
Frank, N. Y., Margaryan, A., Huang, Y., Schatton, T., Waaga-Gasser, A. M., Gasser, M., et al. (2005). ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 65, 4320–4333. doi: 10.1158/0008-5472.CAN-04-3327
Frank, N. Y., Pendse, S. S., Lapchak, P. H., Margaryan, A., Shlain, D., Doeing, C., et al. (2003). Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J. Biol. Chem. 278, 47156–47165. doi: 10.1074/jbc.M308700200
Gaziano, R., Moroni, G., Buè, C., Miele, M. T., Sinibaldi-Vallebona, P., and Pica, F. (2016). Antitumor effects of the benzophenanthridine alkaloid sanguinarine: evidence and perspectives. World J. Gastrointest. Oncol. 8, 30–39. doi: 10.4251/wjgo.v8.i1.30
Hall, M. D., Handley, M. D., and Gottesman, M. M. (2009). Is resistance useless? Multidrug resistance and collateral sensitivity. Trends Pharmacol. Sci. 30, 546–556. doi: 10.1016/j.tips.2009.07.003
Hollstein, M., Sidransky, D., Vogelstein, B., and Harris, C. C. (1991). P53 mutations in human cancers. Science 253, 49–53. doi: 10.1126/science.1905840
Howell, P. (2006). Medicinal Plants of the Southern Appalachians. Mountain City, GA: BotanoLogos Books.
Jeng, J. H., Wu, H. L., Lin, B. R., Lan, W. H., Chang, H. H., Ho, Y. S., et al. (2007). Antiplatelet effect of sanguinarine is correlated to calcium mobilization, thromboxane and cAMP production. Atherosclerosis 191, 250–258. doi: 10.1016/j.atherosclerosis.2006.05.023
Kawanobe, T., Kogure, S., Nakamura, S., Sato, M., Katayama, K., Mitsuhashi, J., et al. (2012). Expression of human ABCB5 confers resistance to taxanes and anthracyclines. Biochem. Biophys. Res. Commun. 418, 736–741. doi: 10.1016/j.bbrc.2012.01.090
Lam, V., Mcpherson, J. P., Salmena, L., Lees, J., Chu, W., Sexsmith, E., et al. (1999). p53 gene status and chemosensitivity of childhood acute lymphoblastic leukemia cells to adriamycin. Leuk. Res. 23, 871–880. doi: 10.1016/S0145-2126(99)00102-2
Lee, B., Lee, S. J., Park, S. S., Kim, S. K., Kim, S. R., Jung, J. H., et al. (2008). Sanguinarine-induced G1-phase arrest of the cell cycle results from increased p27KIP1 expression mediated via activation of the Ras/ERK signaling pathway in vascular smooth muscle cells. Arch. Biochem. Biophys. 471, 224–231. doi: 10.1016/j.abb.2008.01.008
Lee, T. K., Park, C., Jeong, S. J., Jeong, M. J., Kim, G. Y., Kim, W. J., et al. (2016). Sanguinarine induces apoptosis of human oral squamous cell carcinoma KB cells via inactivation of the PI3K/Akt signaling pathway. Drug Dev. Res. 77, 227–240. doi: 10.1002/ddr.21315
Li, G., Tang, L., Zhou, X., Tron, V., and Ho, V. (1998). Chemotherapy-induced apoptosis in melanoma cells is p53 dependent. Melanoma Res. 8, 17–23. doi: 10.1097/00008390-199802000-00004
Li, Q., and Verma, I. M. (2002). NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2, 725–734. doi: 10.1038/nri910
Lopus, M., and Panda, D. (2006). The benzophenanthridine alkaloid sanguinarine perturbs microtubule assembly dynamics through tubulin binding. A possible mechanism for its antiproliferative activity. FEBS J. 273, 2139–2150. doi: 10.1111/j.1742-4658.2006.05227.x
Messori, L., Temperini, C., Piccioli, F., Animati, F., Di Bugno, C., and Orioli, P. (2001). Solution chemistry and DNA binding properties of MEN 10755, a novel disaccharide analogue of doxorubicin. Bioorg. Med. Chem. 9, 1815–1825. doi: 10.1016/S0968-0896(01)00092-X
Nitiss, J. L. (2009). Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 9, 338–350. doi: 10.1038/nrc2607
O'Brien, J., Wilson, I., Orton, T., and Pognan, F. (2000). Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 267, 5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x
Paull, K. D., Shoemaker, R. H., Hodes, L., Monks, A., Scudiero, D. A., Rubinstein, L., et al. (1989). Display and analysis of patterns of differential activity of drugs against human tumor cell lines: development of mean graph and COMPARE algorithm. J. Natl. Cancer Inst. 81, 1088–1092. doi: 10.1093/jnci/81.14.1088
Pluchino, K. M., Hall, M. D., Goldsborough, A. S., Callaghan, R., and Gottesman, M. M. (2012). Collateral sensitivity as a strategy against cancer multidrug resistance. Drug Resistance Updates 15, 98–105. doi: 10.1016/j.drup.2012.03.002
Predny, M. L., and Chamberlain, J. L. (2005). Bloodroot (Sanguinaria Canadensis): An Annotated Bibliography. Asheville, NC: U.S. Department of Agriculture, Forest Service, Southern Research Station.
Raaijmakers, M. H., De Grouw, E. P., Van Der Reijden, B. A., De Witte, T. J., Jansen, J. H., and Raymakers, R. A. (2006). ABCB1 modulation does not circumvent drug extrusion from primitive leukemic progenitor cells and may preferentially target residual normal cells in acute myelogenous leukemia. Clin. Cancer Res. 12, 3452–3458. doi: 10.1158/1078-0432.CCR-05-1945
Rich, A. (1994). Speculation on the biological roles of left-handed Z-DNA. Ann. N. Y. Acad. Sci. 726, 1–16; discussion: 16–17. doi: 10.1111/j.1749-6632.1994.tb52792.x
Righetti, S. C., Perego, P., Corna, E., Pierotti, M. A., and Zunino, F. (1999). Emergence of p53 mutant cisplatin-resistant ovarian carcinoma cells following drug exposure: spontaneously mutant selection. Cell Growth Differ. 10, 473–478.
Ross, D. T., Scherf, U., Eisen, M. B., Perou, C. M., Rees, C., Spellman, P., et al. (2000). Systematic variation in gene expression patterns in human cancer cell lines. Nat. Genet. 24, 227–235. doi: 10.1038/73432
Rubinstein, L. V., Shoemaker, R. H., Paull, K. D., Simon, R. M., Tosini, S., Skehan, P., et al. (1990). Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J. Natl. Cancer Inst. 82, 1113–1118. doi: 10.1093/jnci/82.13.1113
Saeed, M., Jacob, S., Sandjo, L. P., Sugimoto, Y., Khalid, H. E., Opatz, T., et al. (2015). Cytotoxicity of the sesquiterpene lactones neoambrosin and damsin from Ambrosia maritima against multidrug-resistant cancer cells. Front. Pharmacol. 6:267. doi: 10.3389/fphar.2015.00267
Scherf, U., Ross, D. T., Waltham, M., Smith, L. H., Lee, J. K., Tanabe, L., et al. (2000). A gene expression database for the molecular pharmacology of cancer. Nat. Genet. 24, 236–244. doi: 10.1038/73439
Schneider, G., Henrich, A., Greiner, G., Wolf, V., Lovas, A., Wieczorek, M., et al. (2010). Cross talk between stimulated NF-κB and the tumor suppressor p53. Oncogene 29, 2795–2806. doi: 10.1038/onc.2010.46
Scian, M. J., Stagliano, K. E., Anderson, M. A., Hassan, S., Bowman, M., Miles, M. F., et al. (2005). Tumor-derived p53 mutants induce NF-kappaB2 gene expression. Mol. Cell. Biol. 25, 10097–10110. doi: 10.1128/MCB.25.22.10097-10110.2005
Seo, E. J., Saeed, M., Law, B. Y., Wu, A. G., Kadioglu, O., Greten, H. J., et al. (2016). Pharmacogenomics of scopoletin in tumor cells. Molecules 21:496. doi: 10.3390/molecules21040496
Shinojima, N., Tada, K., Shiraishi, S., Kamiryo, T., Kochi, M., Nakamura, H., et al. (2003). Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 63, 6962–6970. Available online at: http://cancerres.aacrjournals.org/content/63/20/6962.long
Ueda, K., Cornwell, M. M., Gottesman, M. M., Pastan, I., Roninson, I. B., Ling, V., et al. (1986). The mdr1 gene, responsible for multidrug-resistance, codes for P-glycoprotein. Biochem. Biophys. Res. Commun. 141, 956–962. doi: 10.1016/S0006-291X(86)80136-X
Wang, Q., Dai, P., Bao, H., Liang, P., Wang, W., Xing, A., et al. (2017). Anti-inflammatory and neuroprotective effects of sanguinarine following cerebral ischemia in rats. Exp. Ther. Med. 13, 263–268. doi: 10.3892/etm.2016.3947
Webster, G. A., and Perkins, N. D. (1999). Transcriptional cross talk between NF-κ B and p53. Mol. Cell. Biol. 19, 3485–3495. doi: 10.1128/MCB.19.5.3485
Wei, G., Xu, Y., Peng, T., Yan, J., Wang, Z., and Sun, Z. (2017). Sanguinarine exhibits antitumor activity via up-regulation of Fas-associated factor 1 in non-small cell lung cancer. J. Biochem. Mol. Toxicol. 31:e21914. doi: 10.1002/jbt.21914
Weinstein, J. N. (1998). Fishing expeditions. Science 282, 628–629. doi: 10.1126/science.282.5389.627g
Weisz, L., Damalas, A., Liontos, M., Karakaidos, P., Fontemaggi, G., Maor-Aloni, R., et al. (2007). Mutant p53 enhances nuclear factor κB activation by tumor necrosis factor α in cancer cells. Cancer Res. 67, 2396–2401. doi: 10.1158/0008-5472.CAN-06-2425
Xu, J. Y., Meng, Q. H., Chong, Y., Jiao, Y., Zhao, L., Rosen, E. M., et al. (2012). Sanguinarine inhibits growth of human cervical cancer cells through the induction of apoptosis. Oncol. Rep. 28, 2264–2270. doi: 10.3892/or.2012.2024
Yang, X., Ding, Y., Xiao, M., Liu, X., Ruan, J., and Xue, P. (2017). Anti-tumor compound RY10-4 suppresses multidrug resistance in MCF-7/ADR cells by inhibiting PI3K/Akt/NF-κB signaling. Chem. Biol. Interact. 278, 22–31. doi: 10.1016/j.cbi.2017.10.008
Keywords: bioinformatics, cancer, drug resistance, microarray, pharmacogenomics, phytotherapy
Citation: Saeed MEM, Mahmoud N, Sugimoto Y, Efferth T and Abdel-Aziz H (2018) Molecular Determinants of Sensitivity or Resistance of Cancer Cells Toward Sanguinarine. Front. Pharmacol. 9:136. doi: 10.3389/fphar.2018.00136
Received: 23 November 2017; Accepted: 07 February 2018;
Published: 26 February 2018.
Edited by:
Vincent Kam Wai Wong, Macau University of Science and Technology, ChinaReviewed by:
Wei Zhang, Macau University of Science and Technology, ChinaWilliam Chi-Shing Tai, Hong Kong Polytechnic University, Hong Kong
Copyright © 2018 Saeed, Mahmoud, Sugimoto, Efferth and Abdel-Aziz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas Efferth, ZWZmZXJ0aEB1bmktbWFpbnouZGU=
Heba Abdel-Aziz, aGViYS5hYmRlbGF6aXpAZ21haWwuY29t
 Mohamed E. M. Saeed
Mohamed E. M. Saeed Nuha Mahmoud1
Nuha Mahmoud1 Thomas Efferth
Thomas Efferth Heba Abdel-Aziz
Heba Abdel-Aziz