- 1Anatomical Sciences Research Center, Kashan University of Medical Sciences, Kashan, Iran
- 2Department of Internal Medicine, Kashan University of Medical Sciences, Kashan, Iran
- 3Research Center for Biochemistry and Nutrition in Metabolic Diseases, Kashan University of Medical Sciences, Kashan, Iran
- 4Department of Internal Medicine, Semnan University of Medical Sciences, Semnan, Iran
- 5Department of Urology, School of Medicine, Alborz University of Medical Sciences, Karaj, Iran
Objective: This study was carried out to determine the effects of vitamin D supplementation on signaling pathway of inflammation and oxidative stress in diabetic hemodialysis (HD) patients.
Methods: This randomized double-blind placebo-controlled clinical trial was conducted among 60 diabetic HD patients. Subjects were randomly allocated into two groups to intake either vitamin D supplements at a dosage of 50,000 IU (n = 30) or placebo (n = 30) every 2 weeks for 12 weeks. Gene expression of inflammatory cytokines and biomarkers of oxidative stress were assessed in peripheral blood mononuclear cells (PBMCs) of diabetic HD patients with RT-PCR method.
Results: Results of RT-PCR indicated that after the 12-week intervention, compared to the placebo, vitamin D supplementation downregulated gene expression of interleukin (IL)-1β (P = 0.02), tumor necrosis factor alpha (TNF-α) (P = 0.02) and interferon gamma (IFN-γ) (P = 0.03) in PBMCs of diabetic HD patients. Additionally, vitamin D supplementation, compared to the placebo, downregulated gene expression of transforming growth factor beta (TGF-β) (P = 0.04), protein kinase C (PKC) (P = 0.001), and mitogen-activated protein kinases 1 (MAPK1) (P = 0.02) in PBMCs of diabetic HD patients. Although not significant, vitamin D supplementation let to a reduction of nuclear factor kappa B (NF-kB) (p = 0.75) expression in PBMCs isolated from diabetic patients compared to the placebo group. There was no statistically significant change following supplementation with vitamin D on gene expression of interleukin (IL)-4, IL-6, and vascular endothelial growth factor (VEGF) in PBMCs of diabetic HD patients.
Conclusions: Overall, we found that vitamin D supplementation for 12 weeks among diabetic HD patients had beneficial effects on few gene expression related to inflammation and oxidative stress.
Clinical trial registration: IRCT201701035623N101. Registered on January 8, 2017.
Introduction
Increased inflammatory cytokines as a necessary part of chronic kidney disease (CKD) has been identified and associated with cardiovascular disease (CVD), protein-energy malnutrition, all-cause mortality, and decreased glomerular filtration rate (GFR) (Stenvinkel et al., 1999; Akchurin and Kaskel, 2015). Increased biomarkers of inflammation, such as interleukin-1beta (IL-1β) and tumor necrosis factor alpha (TNF-α) were negatively linked to the measures of kidney action and directly with albuminuria (Gupta et al., 2012). Furthermore, various factors, including increased oxygen metabolism, uremic toxicity, increased inflammatory factors, lack of antioxidant vitamins and microelements, and dialysis procedure in patients with CKD would result in oxidative stress (Stepniewska et al., 2015).
Prior studies have documented that hypovitaminosis D is very prevalent in individuals with CKD (de Boer, 2008; Cupisti et al., 2015). In a study by Bhan et al. (2010) hypovitaminosis D was reported 50–90% in dialysis subjects. On the other hand, beneficial effects of vitamin D on biochemical variables have reported in hemodialysis (HD) patients (Ibrahim et al., 2015), subjects with type 2 diabetes mellitus (T2DM) (Calvo-Romero and Ramiro-Lozano, 2016) and polycystic ovary syndrome (Maktabi et al., 2017). Few studies have evaluated the effects of vitamin D on gene expression related to inflammation and oxidative stress. Choi et al. (2013) shown significant reductions in gene expression of interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) in vitamin D-treated rats. In addition, in vitro studies have shown that the release of TNF-α can be inhibited by calcitriol in a dose-dependent fashion (Müller et al., 1992). Another study indicated that vitamin D through vitamin D receptor (VDR) suppressed TNF-α-induced nuclear factor kappa B (NF-κB) activation and IL-6 up-regulation in epithelial cell isolated from transgenic mice and colitis animal model (Liu et al., 2013). However, another study supported no significant changes in TNF-α and IL-6 levels in high-dose vitamin D administration in healthy overweight individuals following a 12-week progressive resistance exercise training program (Carrillo et al., 2012).
The molecular mechanism of vitamin D in modulating inflammatory cytokines and oxidative stress are still unclear. Mitogen-activated protein kinases (MAPKs) participate in signaling mechanisms in responses to pro-inflammatory factors as well as NF-κB are thought to play an important function in the regulation of pro-inflammatory cytokines in cellular responses (Baldassare et al., 1999). To our knowledge, data on the effects of vitamin D supplementation on signaling pathway of inflammation and oxidative stress in diabetic HD patients are limited and controversial. The purpose of this study was, therefore, conducted to evaluate the effects of vitamin D supplementation on signaling pathway of inflammation and oxidative stress in diabetic HD patients.
Methods
Trial Design and Participants
This randomized double-blind placebo-controlled clinical trial, registered in the Iranian website for registration of clinical trials as http://www.irct.ir:IRCT201701035623N101, was carried out among 60 diabetic HD subjects aged 18-80 years who were referred to the Akhavan Clinic in Kashan, Iran, from January 2017 to April 2017. This research was carried out in accordance with the Declaration of Helsinki and the Research Ethics Committee of Kashan University of Medical Sciences (KAUMS) approved the study protocol, and informed consent was taken from all subjects. The main exclusion criteria from the study were as follows: taking vitamin D, antioxidant and/or anti-inflammatory supplements such as vitamins E and C, omega-3 fatty acids, and taking immunosuppressive medications within 3 months prior to the enrollment in the study.
Study Design
At first, all patients were randomized into two groups to receive either 50,000 IU of vitamin D or placebo (n = 30 in each group) every 2 weeks for 12 weeks. Vitamin D supplements and placebos were produced by Zahravi Pharmaceutical Company, Tabriz, Iran, and Barij Essence Pharmaceutical Company, Kashan, Iran, respectively. In the current study, we used the above-mentioned dose of vitamin D based on the tolerable upper intake level (4,000 IU/day) of vitamin D (Ross et al., 2011), as well as dosage recommended in previous studies among HD patients (Armas et al., 2013; Mose et al., 2014). Compliance to the vitamin D consumption was evaluated through determination of serum 25 (OH) vitamin D values and asking subjects to return the medication containers. Both dietary 3-day food records [analyzed by nutritionist IV software (First Databank, San Bruno, CA)] and physical activity records were taken at baseline, weeks 3, 6, 9, and 12 of the intervention.
Assessment of Anthropometric Measures
Body weight and height were quantified in a fasting status using a digital scale (Seca, Hamburg, Germany) at baseline and after the 12-week intervention. Body mass index (BMI) was calculated by weight and height measurements [weight (kg)/height (m2)].
Assessment of Outcomes
Primary outcomes were inflammatory cytokines expression and secondary outcomes were biomarkers of oxidative stress expression. We selected the following inflammatory cytokines and proteins [IL-1β, IL-4, IL-6,TNF-α, interferon gamma (IFN-γ), transforming growth factor beta (TGF-β), protein kinase C (PKC), MAPK1, vascular endothelial growth factor (VEGF) and NF-κB] as they play a very important role in signaling pathways of oxidative stress (Geraldes and King, 2010; Baker et al., 2011; Tanti et al., 2012; Chen et al., 2015).
Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
At baseline and endpoint of the intervention, 15 mL samples of fasting blood were taken at reference laboratory of KAUMS, Kashan, Iran. PBMCs were isolated from blood by centrifugation through a Ficoll-Hypaque (Denholm and Wolber, 1991) followed by Percoll. In summary, 10 mL of blood with an equal volume of phosphate-buffered saline (PBS) was mixed (Dagur and McCoy, 2015). Layer diluted blood over Ficoll-Hypaque solution, using 3 parts diluted blood to 2 parts Ficoll-Hypaque and centrifuged 30 min at 500 × g, 25°C. Then, using a sterile Pasteur pipet, carefully collected PBMCs, and located at the interface between the plasma (upper layer) and Ficoll-Hypaque solution (Dagur and McCoy, 2015). Cells to a 50 mL centrifuge tube were transferred, then after adding 10 mL PBS, were centrifuged 10 min at 400 × g, 4°C. The supernatant was discarded and was resuspended cells in 4 mL PBS. Following previous step, 1.65 mL of 10× Hanks balanced salt solution with 10 mL Percoll was mixed. Eight mL Percoll solution with 4 mL mononuclear cells in a silanized 10 × 1.5 cm round-bottom polypropylene tube was mixed (Dagur and McCoy, 2015). Mixing thoroughly by inverting tube three or four times was done. Then, 25 min at 370 × g, 25°C was centrifuged. PBMCs were aspirated by gentle pipetting into a clean test tube. After centrifugation, monocytes appear as a cloudy layer in the top 5 mm of the gradient (Dagur and McCoy, 2015).
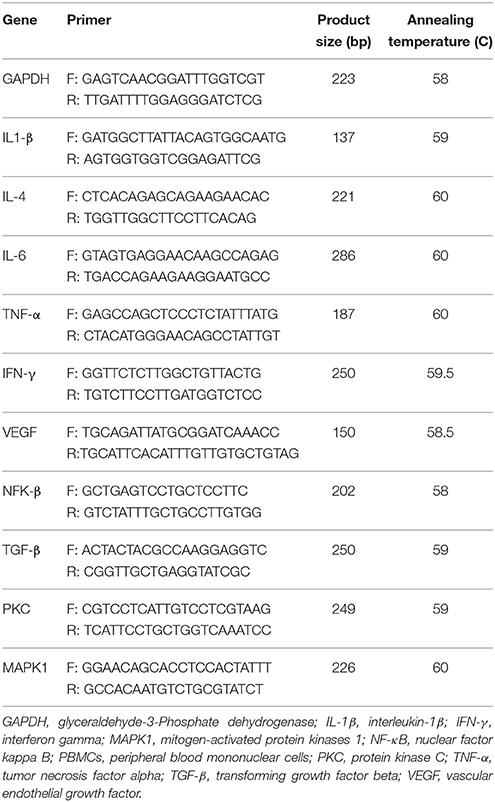
RNA Extraction and Real-Time PCR
To RNA extraction, we used the RNX-plus kit (Cinnacolon, Tehran, Iran). RNA were extracted just after lymphocyte extraction from fresh 5 mL peripheral blood and less than 1 million cells was used for RNA extraction with the trizol reagent (Invitrogen, USA). Following extraction of the total RNAs from each sample, RNA quantification were performed by UV spectrophotometer. Each samples OD 260/280 ratio between 1.7 and 2.1 was intended showing no contamination with both protein and DNA (Dunkley et al., 2008). The isolated RNA was reverse transcribed to cDNA library using moloney murine leukemia virus reverse transcriptase. Gene expression of IL-1β, IL-4, IL-6,TNF-α, IFN-γ, TGF-β, PKC, MAPK1, VEGF, and NF-κB were evaluated by quantitative RT-PCR, using the LightCycler technology (Roche Diagnostics, Rotkreuz, Switzerland) with SYBR green detection and Amplicon Kit (Table 1). Glyceraldehyde-3-phosphate dehydrogenase primers were used as housekeeping gene. To design primers, Primer Express Software (Applied Biosystems, Foster City) and Beacon designer software (Takaposizt, Tehran, Iran) were used. Relative transcription levels were calculated by the method of Pffafi or 2−ΔΔCT. In the current study, we quantified gene expression levels related to inflammation and oxidative stress in PBMCs from diabetic HD patients. PBMCs from venous blood samples are the most available tissue for analysis of gene expression (Mizuarai et al., 2010). In addition, gene expression levels related to inflammation and oxidative stress in PBMCs are more accurate and may provide more valuable information than plasma concentrations (Mizuarai et al., 2010). Few studies have previously assessed gene expression levels of inflammation and oxidative stress in PBMCs from diabetic patients (Xavier et al., 2015; Mazloom et al., 2016).
Randomization
Randomization assignment was carried out using computer-generated random numbers. Randomization and allocation concealment were conducted by the researchers and participants and were carried out by a trained staff at the clinic.
Statistical Methods
The Kolmogorov-Smirnov test was applied to control the normal distribution of variables. Independent sample t-test was used to establish changes in anthropometric measures and dietary intakes between the two groups. To determine the effects of vitamin D administration on inflammation and oxidative stress expression, we used independent sample t-test. P < 0.05 were considered statistically significant. All statistical analyses conducted using the Statistical Package for Social Science version 18 (SPSS Inc., Chicago, Illinois, USA).
Results
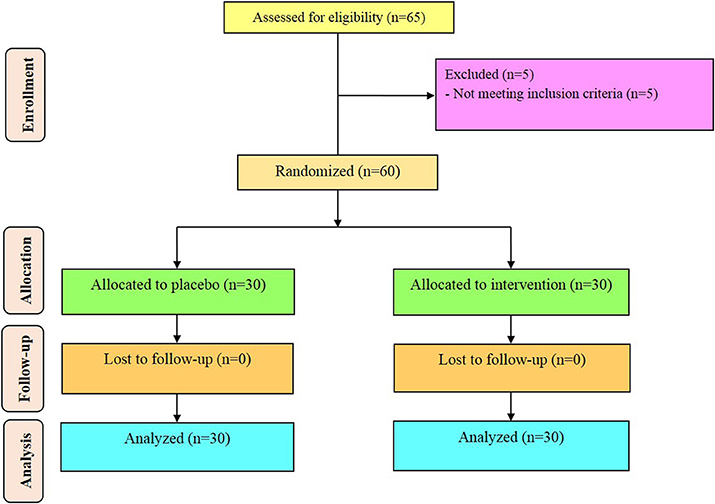
Sixty diabetic HD patients [vitamin D and placebo (n = 30 each group)] completed the trial (Figure 1). On average, the rate of compliance in our study was high, such that more than 100% of capsules were taken throughout the study in both groups. No side effects were reported following supplementation of vitamin D in diabetic HD patients throughout the study. Potential side effects of vitamin D supplementation monitored by evaluating the serum levels of calcium every month, as well as, monitoring clinical symptoms among participants including, anorexia, nausea, and vomiting.
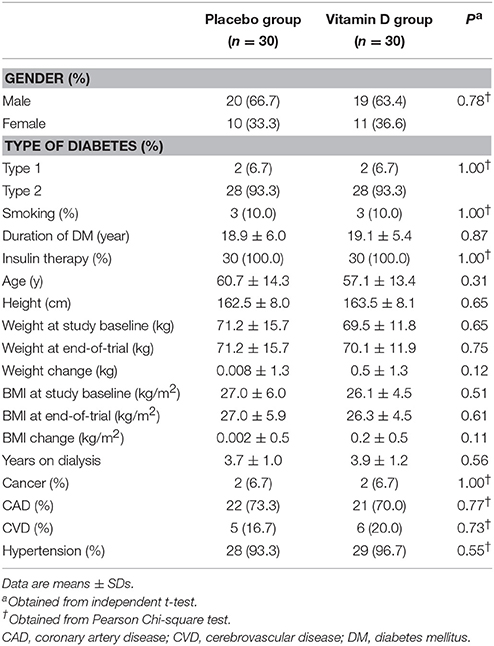
Distribution of gender, participants' mean age, height, baseline weight and BMI as well as their change, and years of dialysis of study participants were not statistically different between the two groups (Table 2).
Considering the 3-day dietary records obtained during the intervention, there was no significant difference in terms of dietary macro- and micro-nutrient intakes between vitamin D and placebo groups (Data not shown).
After 12 weeks of intervention, compared to the placebo, vitamin D supplementation significantly increased serum 25-OH-vitamin D (+11.1 ± 4.2 vs. +0.5 ± 3.9 ng/mL, P < 0.001). Results of RT-PCR indicated that compared to the placebo, vitamin D supplementation downregulated gene expression of IL-1β (P = 0.02), TNF-α (P = 0.02), and IFN-γ (P = 0.03) in PBMCs of diabetic HD patients (Figure 2). There was no statistically significant change following supplementation with vitamin D on gene expression of IL-4 and IL-6 in PBMCs of diabetic HD patients.
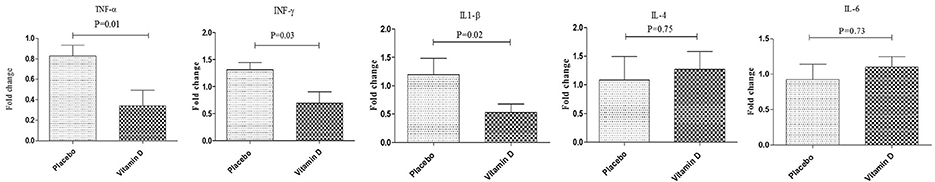
Figure 2. Effect of 12-week supplementation with vitamin D or placebo on expression ratio of TNF-α, IFN-γ, IL-1β, IL-4, and IL-6 genes in PBMCs of diabetic HD subjects. P-value was obtained from independent sample t-test. Data are means ± standard deviation.
Additionally, vitamin D supplementation, compared to the placebo, downregulated gene expression of TGF-β (P = 0.04), PKC (P = 0.001), and MAPK1 (P = 0.02) in PBMCs of diabetic HD patients (Figure 3). Although not significant, vitamin D supplementation let to a reduction of NF-kB (p = 0.75) expression in PBMCs isolated from diabetic patients compared to the placebo group. We did not observe any significant change following supplementation with vitamin D on gene expression of VEGF in PBMCs of diabetic HD patients.
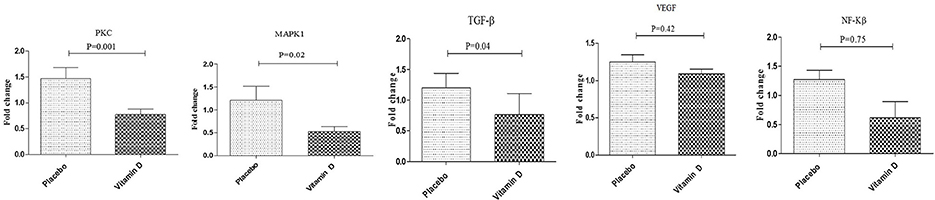
Figure 3. Effect of 12-week supplementation with vitamin D or placebo on expression ratio of PKC, MAPK1, TGF-β, NF-κB, and VEGF genes in PBMCs of diabetic HD subjects. P-value was obtained from independent sample t-test. Data are means ± standard deviation.
Discussion
We found that vitamin D supplementation for 12 weeks among diabetic HD patients had beneficial effects on few gene expression related to inflammation and oxidative stress. To our knowledge, this is the first report of the effects of vitamin D supplementation on gene expression related to inflammation and oxidative stress in diabetic HD patients.
HD subjects are susceptible to several metabolic complications, including increased inflammation and oxidative stress (Kotur-Stevuljevic et al., 2012). Our data demonstrated that vitamin D supplementation for 12 weeks to diabetic HD subjects downregulated gene expression of IL-1β, TNF-α, and IFN-γ compared to the placebo, but did not affect gene expression of IL-4 and IL-6. A study in healthy endurance-trained runners has demonstrated the inverse relationship between vitamin D and TNF-α levels (Willis et al., 2012). Furthermore, in line with our findings, 50 mug/day of vitamin D supplementation for 9 months was able to suppress the production of TNF-α in subjects with congestive heart failure (Schleithoff et al., 2006). 1,25-(OH)2D3 dose-dependently also suppressed the production of IL-α, IL-6, and TNF-α by Escherichia coli lipopolysaccharide-stimulated monocytes (Müller et al., 1992). However, one study has documented no significant changes in TNF-α and IL-6 concentrations in high-dose vitamin D supplementation-healthy overweight populations after 12 weeks (Carrillo et al., 2012). Chronic inflammation in CKD is not only associated with macro- and micro-cardiovascular outcomes, such as atherosclerosis, but is also one of the main players in the progress of malnutrition/protein-energy wasting, which in turn resulted in the description of the malnutrition-inflammation-cachexia syndrome in CKD/ESRD patients (Kalantar-Zadeh, 2005). Hypoalbuminemia result from chronic inflammation and malnutrition is strongly related to mortality in dialysis populations (Kalantar-Zadeh et al., 2005; de Mutsert et al., 2009). Pro-inflammatory factors may directly lead to anorexia via the effect on the brain. Furthermore, inflammatory cytokines, particularly IL-6, may be related to depression in CKD/ESRD patients, which by itself is a predictor of morbidity and mortality (Taraz et al., 2015) and may result in decreasing nutrient intake. Therefore, vitamin D due to its useful effects on inflammatory cytokines may be benefit to decrease clinical and metabolic symptoms in diabetic HD patients.
This study demonstrated that vitamin D supplementation for 12 weeks downregulated gene expression of TGF-β, PKC, and MAPK1 in PBMCs of diabetic HD patients, but did not influence gene expression of VEGF and NF-Kβ. Supporting our study, vitamin D administration at a dosage of 50,000 IU/week for 8 weeks in VD-deficient subjects with PCOS significantly decreased the bioavailability of TGF-β1 (Irani et al., 2015). In addition, 1,25-(OH)2D3 decreased gene expression of MAPK phosphorylation in macrophages (Xu et al., 2016). In another study, vitamin D protected human endothelial cells from ionizing radiation induced/oxidative stress by modulating the MAPKs/SirT1 axis (Marampon et al., 2016). Doxercalciferol also significantly decreased PKCα levels suggesting that PKC-mediated cardiac hypertrophy may be related to vitamin D deficiency (Choi et al., 2011). Excessive evidence exists suggesting that MAPK signaling pathways are critical for the synthesis and amplification of inflammatory factors (Carter et al., 1999). Especially, MAPK activation was closely associated with cytokine transcription and translation, which in turn make the target of anti-inflammatory treatment (Kaminska, 2005). However, calcitriol treatment had no significant effect on gene expression of TGF-β in microcardiovascular endothelial cells (Gonzalez-Curiel et al., 2016). In addition, both the low (1 nM) and the high (100 nM) doses of vitamin D3 used in vitro for 48 h did not able to restore the decreased gene expression of PKC isoenzymes in the T cells of systemic lupus erythematosus subjects (Czifra et al., 2014). Oxidative stress in patients with CKD may generate by uremic toxicity, persistent inflammatory state, deficiency of vitamins and microelements, and dialysis procedure itself (Stepniewska et al., 2015). Increased biomarkers of oxidative stress and free radicals in HD subjects are correlated with increased CVD risk and a large number of diseases related to uremia (Taccone-Gallucci et al., 2010).
Conclusions
Overall, we found that vitamin D supplementation for 12 weeks among diabetic HD patients had beneficial effects on some gene expression related to inflammation and oxidative stress. This suggests that vitamin D supplementation may confer advantageous therapeutic potential for HD patients. Further research is needed in other participants and for longer periods to determine the efficacy of vitamin D supplementation.
Limitations
The limitations of our findings include short duration. Long-term interventions might result in better effects in other gene expression related to inflammation and oxidative stress. In addition, due to funding limitations in developing countries, we did not evaluate some gene expression related to insulin and lipid, and reactive oxygen species levels. Further investigations are required to evaluate this outcome. We did not know baseline levels of vitamin D among our participants, therefore, we were not able to comment on their commended dosage of vitamin D by the Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines. This limitation should be considered in the interpretation of our findings.
Availability of Data and Materials
The primary data for this study is available from the authors on direct request.
Author Contributions
ZA contributed in conception, data collection and manuscript drafting; HH, ES, HN, AS, MS, FK, and MT contributed in conception, data collection and manuscript drafting; All authors read and approved the final version of the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The present study was supported by a grant from the Vice-chancellor for Research, Kashan University of Medical Sciences, Kashan, Iran.
Abbreviations
HD, hemodialysis; PBMCs, peripheral blood mononuclear cells; IL, interleukin; TNF-α, tumor necrosis factor alpha; IFN-γ, interferon gamma; TGF-β, transforming growth factor beta; PKC, protein kinase C; MAPK1, mitogen-activated protein kinases 1; VEGF, vascular endothelial growth factor; NF-κB, nuclear factor kappa B; CVD, cardiovascular disease; CKD, chronic kidney disease; GFR, glomerular filtration rate; VDR, vitamin D receptor; BMI, Body mass index.
References
Akchurin, O. M., and Kaskel, F. (2015). Update on inflammation in chronic kidney disease. Blood Purif. 39, 84–92. doi: 10.1159/000368940
Armas, L. A., Zena, M., Lund, R., and Heaney, R. P. (2013). Calcium absorption response to cholecalciferol supplementation in hemodialysis. Clin. J. Am. Soc. Nephrol. 8, 1003–1008. doi: 10.2215/CJN.08610812
Baker, R. G., Hayden, M. S., and Ghosh, S. (2011). NF-κB, inflammation, and metabolic disease. Cell Metab. 13, 11–22. doi: 10.1016/j.cmet.2010.12.008
Baldassare, J. J., Bi, Y., and Bellone, C. J. (1999). The role of p38 mitogen-activated protein kinase in IL-1 beta transcription. J. Immunol. 162, 5367–5373.
Bhan, I., Burnett-Bowie, S. A., Ye, J., Tonelli, M., and Thadhani, R. (2010). Clinical measures identify vitamin D deficiency in dialysis. Clin. J. Am. Soc. Nephrol. 5, 460–467. doi: 10.2215/CJN.06440909
Calvo-Romero, J. M., and Ramiro-Lozano, J. M. (2016). Metabolic effects of supplementation with vitamin D in type 2 diabetic patients with vitamin D deficiency. Diabetes Metab. Syndr. 10, 72–74. doi: 10.1016/j.dsx.2015.09.008
Carrillo, A. E., Flynn, M. G., Pinkston, C., Markofski, M. M., Jiang, Y., Donkin, S. S., et al. (2012). Vitamin D supplementation during exercise training does not alter inflammatory biomarkers in overweight and obese subjects. Eur. J. Appl. Physiol. 112, 3045–3052. doi: 10.1007/s00421-011-2279-3
Carter, A. B., Monick, M. M., and Hunninghake, G. W. (1999). Both Erk and p38 kinases are necessary for cytokine gene transcription. Am. J. Respir. Cell Mol. Biol. 20, 751–758. doi: 10.1165/ajrcmb.20.4.3420
Chen, L., Chen, R., Wang, H., and Liang, F. (2015). Mechanisms linking inflammation to insulin resistance. Int. J. Endocrinol. 2015:508409. doi: 10.1155/2015/508409
Choi, J. H., Ke, Q., Bae, S., Lee, J. Y., Kim, Y. J., Kim, U. K., et al. (2011). Doxercalciferol, a pro-hormone of vitamin D, prevents the development of cardiac hypertrophy in rats. J. Card. Fail. 17, 1051–1058. doi: 10.1016/j.cardfail.2011.08.006
Choi, M., Park, H., Cho, S., and Lee, M. (2013). Vitamin D3 supplementation modulates inflammatory responses from the muscle damage induced by high-intensity exercise in SD rats. Cytokine 63, 27–35. doi: 10.1016/j.cyto.2013.03.018
Cupisti, A., Vigo, V., Baronti, M. E., D'Alessandro, C., Ghiadoni, L., and Egidi, M. F. (2015). Vitamin D status and cholecalciferol supplementation in chronic kidney disease patients: an Italian cohort report. Int. J. Nephrol. Renovasc. Dis. 8, 151–157. doi: 10.2147/IJNRD.S90968
Czifra, G., Tóth, B., Kovács, I., Bíró, T., Griger, Z., Baróth, S., et al. (2014). The in vitro treatment with vitamin D3 is ineffective on the expression of PKC isoenzymes, but decreases further the impaired production of IL-2 in the T lymphocytes of SLE patients. Rheumatol. Int. 34, 717–720. doi: 10.1007/s00296-013-2751-y
Dagur, P. K., and McCoy, J. P. Jr. (2015). Collection, storage, and preparation of human blood cells. Curr. Protoc. Cytom. 73, 1–16. doi: 10.1002/0471142956
Denholm, E. M., and Wolber, F. M. (1991). A simple method for the purification of human peripheral blood monocytes: A substitute for Sepracell-MN. J. Immunol. Methods. 144, 247–251. doi: 10.1016/0022-1759(91)90092-T
de Boer, I. H. (2008). Vitamin D and glucose metabolism in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 17, 566–572. doi: 10.1097/MNH.0b013e32830fe377
de Mutsert, R., Grootendorst, D. C., Indemans, F., Boeschoten, E. W., Krediet, R. T., and Dekker, F. W. (2009). Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J. Ren. Nutr. 19, 127–135. doi: 10.1053/j.jrn.2008.08.003
Dunkley, P. R., Jarvie, P. E., and Robinson, P. J. (2008). A rapid percoll gradient procedure for preparation of synaptosomes. Nat. Protoc. 3, 1718–1728. doi: 10.1038/nprot.2008.171
Geraldes, P., and King, G. L. (2010). Activation of protein kinase C isoforms and its impact on diabetic complications. Circ. Res. 106, 1319–1331. doi: 10.1161/CIRCRESAHA.110.217117
Gonzalez-Curiel, I., Marin-Luevano, P., Trujillo, V., Enciso-Moreno, J. A., Gonzalez-Castillo, C., and Rivas-Santiago, B. (2016). Calcitriol prevents inflammatory gene expression in macrovascular endothelial cells. Br. J. Biomed. Sci. 73, 74–78. doi: 10.1080/09674845.2016.1162376
Gupta, J., Mitra, N., Kanetsky, P. A., Devaney, J., Wing, M. R., Reilly, M., et al. (2012). Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin. J. Am. Soc. Nephrol. 7, 1938–1946. doi: 10.2215/CJN.03500412
Ibrahim, M. A., Sarhan, I. I., Halawa, M. R., Afify, E. N., Hebah, H. A., Al-Gohary, E. A., et al. (2015). Study of the effect of vitamin D supplementation on glycemic control in type 2 diabetic prevalent hemodialysis patients. Hemodial. Int. 19(Suppl. 3), S11–S19. doi: 10.1111/hdi.12347
Irani, M., Seifer, D. B., Grazi, R. V., Julka, N., Bhatt, D., Kalgi, B., et al. (2015). Vitamin D supplementation decreases TGF-β1 bioavailability in PCOS: a randomized placebo-controlled trial. J. Clin. Endocrinol. Metab. 100, 4307–4314. doi: 10.1210/jc.2015-2580
Kalantar-Zadeh, K. (2005). Recent advances in understanding the malnutrition-inflammation-cachexia syndrome in chronic kidney disease patients: what is next? Semin. Dial. 18, 365–369. doi: 10.1111/j.1525-139X.2005.00074.x
Kalantar-Zadeh, K., Kilpatrick, R. D., Kuwae, N., McAllister, C. J., Alcorn, H. Jr., Kopple, J. D., et al. (2005). Revisiting mortality predictability of serum albumin in the dialysis population: time dependency, longitudinal changes and population-attributable fraction. Nephrol. Dial. Transplant 20, 1880–1888. doi: 10.1093/ndt/gfh941
Kaminska, B. (2005). MAPK signalling pathways as molecular targets for anti-inflammatory therapy–from molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 1754, 253–262. doi: 10.1016/j.bbapap.2005.08.017
Kotur-Stevuljevic, J., Simic-Ogrizovic, S., Dopsaj, V., Stefanovic, A., Vujovic, A., Ivanic-Corlomanovic, T., et al. (2012). A hazardous link between malnutrition, inflammation and oxidative stress in renal patients. Clin. Biochem. 45, 1202–1205. doi: 10.1016/j.clinbiochem.2012.04.021
Liu, W., Chen, Y., Golan, M. A., Annunziata, M. L., Du, J., Dougherty, U., et al. (2013). Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J. Clin. Invest. 123, 3983–3996. doi: 10.1172/JCI65842
Maktabi, M., Chamani, M., and Asemi, Z. (2017). The effects of vitamin D supplementation on metabolic status of patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Horm. Metab. Res. 49, 493–498. doi: 10.1055/s-0043-107242
Marampon, F., Gravina, G. L., Festuccia, C., Popov, V. M., Colapietro, E. A., Sanità, P., et al. (2016). Vitamin D protects endothelial cells from irradiation-induced senescence and apoptosis by modulating MAPK/SirT1 axis. J. Endocrinol. Invest. 39, 411–422. doi: 10.1007/s40618-015-0381-9
Mazloom, H., Alizadeh, S., Esfahani, E. N., Razi, F., and Meshkani, R. (2016). Decreased expression of microRNA-21 is associated with increased cytokine production in peripheral blood mononuclear cells (PBMCs) of obese type 2 diabetic and non-diabetic subjects. Mol. Cell. Biochem. 419, 11–17. doi: 10.1007/s11010-016-2743-9
Mizuarai, S., Irie, H., and Kotani, H. (2010). Gene expression-based pharmacodynamic biomarkers: the beginning of a new era in biomarker-driven anti-tumor drug development. Curr. Mol. Med. 10, 596–607. doi: 10.2174/1566524011009060596
Mose, F. H., Vase, H., Larsen, T., Kancir, A. S., Kosierkiewic, R., Jonczy, B., et al. (2014). Cardiovascular effects of cholecalciferol treatment in dialysis patients–a randomized controlled trial. BMC Nephrol. 15:50. doi: 10.1186/1471-2369-15-50
Müller, K., Haahr, P. M., Diamant, M., Rieneck, K., Kharazmi, A., and Bendtzen, K. (1992). 1,25-dihydroxyvitamin D3 inhibits cytokine production by human blood monocytes at the post-transcriptional level. Cytokine 4, 506–512. doi: 10.1016/1043-4666(92)90012-G
Ross, A. C., Manson, J. E., Abrams, S. A., Aloia, J. F., Brannon, P. M., Clinton, S. K., et al. (2011). The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J. Clin. Endocrinol. Metab. 96, 53–58. doi: 10.1210/jc.2010-2704
Schleithoff, S. S., Zittermann, A., Tenderich, G., Berthold, H. K., Stehle, P., and Koerfer, R. (2006). Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 83, 754–759.
Stenvinkel, P., Heimburger, O., Paultre, F., Diczfalusy, U., Wang, T., Berglund, L., et al. (1999). Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 55, 1899–1911. doi: 10.1046/j.1523-1755.1999.00422.x
Stepniewska, J., Golembiewska, E., Dolegowska, B., Domanski, M., and Ciechanowski, K. (2015). Oxidative stress and antioxidative enzyme activities in chronic kidney disease and different types of renal replacement therapy. Curr. Protein Pept. Sci. 16, 243–248. doi: 10.2174/1389203716666150224150508
Taccone-Gallucci, M., Noce, A., Bertucci, P., Fabbri, C., Manca-di-Villahermosa, S., Della-Rovere, F. R., et al. (2010). Chronic treatment with statins increases the availability of selenium in the antioxidant defence systems of hemodialysis patients. J. Trace Elem. Med. Biol. 24, 27–30. doi: 10.1016/j.jtemb.2009.06.005
Tanti, J. F., Ceppo, F., Jager, J., and Berthou, F. (2012). Implication of inflammatory signaling pathways in obesity-induced insulin resistance. Front. Endocrinol. 3:181. doi: 10.3389/fendo.2012.00181
Taraz, M., Taraz, S., and Dashti-Khavidaki, S. (2015). Association between depression and inflammatory/anti-inflammatory cytokines in chronic kidney disease and end-stage renal disease patients: a review of literature. Hemodial. Int. 19, 11–22. doi: 10.1111/hdi.12200
Willis, K. S., Smith, D. T., Broughton, K. S., and Larson-Meyer, D. E. (2012). Vitamin D status and biomarkers of inflammation in runners. Open Access J. Sports Med. 3, 35–42. doi: 10.2147/OAJSM.S31022
Xavier, D. J., Takahashi, P., Evangelista, A. F., Foss-Freitas, M. C., Foss, M. C., Donadi, E. A., et al. (2015). Assessment of DNA damage and mRNA/miRNA transcriptional expression profiles in hyperglycemic versus non-hyperglycemic patients with type 2 diabetes mellitus. Mutat. Res. 776, 98–110. doi: 10.1016/j.mrfmmm.2015.01.016
Keywords: vitamin D supplementation, hemodialysis, signaling pathway, inflammation, oxidative stress
Citation: Haddad Kashani H, Seyed Hosseini E, Nikzad H, Soleimani A, Soleimani M, Tamadon MR, Keneshlou F and Asemi Z (2018) The Effects of Vitamin D Supplementation on Signaling Pathway of Inflammation and Oxidative Stress in Diabetic Hemodialysis: A Randomized, Double-Blind, Placebo-Controlled Trial. Front. Pharmacol. 9:50. doi: 10.3389/fphar.2018.00050
Received: 11 November 2017; Accepted: 15 January 2018;
Published: 02 February 2018.
Edited by:
Li-Long Pan, Fudan University, ChinaReviewed by:
Annalisa Trenti, Università degli Studi di Padova, ItalyEmanuela Marcantoni, New York University, United States
Copyright © 2018 Haddad Kashani, Seyed Hosseini, Nikzad, Soleimani, Soleimani, Tamadon, Keneshlou and Asemi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zatollah Asemi, YXNlbWlfckB5YWhvby5jb20=
 Hamed Haddad Kashani
Hamed Haddad Kashani Elahe Seyed Hosseini1
Elahe Seyed Hosseini1 Zatollah Asemi
Zatollah Asemi