
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 30 January 2018
Sec. Ethnopharmacology
Volume 9 - 2018 | https://doi.org/10.3389/fphar.2018.00029
Liver disease is one of the most risk factors threatening human health. It is of great significance to find drugs that can treat liver diseases, especially for acute and chronic hepatitis, non-alcoholic fatty liver disease, and liver cancer. The search for drugs with good efficacy from traditional natural medicines has attracted more and more attention. Tibetan medicine, one of the China's traditional medical systems, has been widely used by the Tibetan people for the prevention and treatment of liver diseases for hundreds of years. The present paper summarized the natural Tibetan medicines that have been used in Tibetan traditional system of medicine to treat liver diseases by bibliographic investigation of 22 Tibetan medicine monographs and drug standards. One hundred and ninety three species including 181 plants, 7 animals, and 5 minerals were found to treat liver diseases in traditional Tibetan medicine system. The most frequently used species are Carthamus tinctorius, Brag-zhun, Swertia chirayita, Swertia mussotii, Halenia elliptica, Herpetospermum pedunculosum, and Phyllanthus emblica. Their names, families, medicinal parts, traditional uses, phytochemicals information, and pharmacological activities were described in detail. These natural medicines might be a valuable gift from the old Tibetan medicine to the world, and would be potential drug candidates for the treatment of liver diseases. Further studies are needed to prove their medicinal values in liver diseases treatment, identify bioactive compounds, elucidate the underlying mechanism of action, and clarify their side effects or toxicity with the help of modern phytochemical, pharmacological, metabonomics, and/or clinical trial methods.
Liver disease, one of the most risk factors threatening human health, is the fifth most common cause of death worldwide after heart disease and stroke (Williams, 2006), mainly including alcoholic liver disease (ALD), non-alcoholic fatty liver disease (NAFLD), chronic viral hepatitis (e.g., hepatitis B virus and hepatitis C virus infections), autoimmune hepatitis (AIH), hepatic schistosomiasis (HS), liver cirrhosis (LC), hepatocellular carcinoma (HCC), and so forth (Wang et al., 2014). In recent years, several types of liver diseases received widespread attention and have become a public health problem. NAFLD, a reported prevalence of 6–35% worldwide (Federico et al., 2016), is often associated with the metabolic syndrome. At present, NAFLD has become an important cause of chronic liver disease in developed countries, and its incidence has been increasing significantly in recent years. HCC accounts for ~75% of liver cancer cases (Petrick et al., 2016). It is one of the most common malignant tumors in the world, especially in Asia, Africa, and Europe. According to World Health Organization (WHO) statistics, the mortality rate of HCC was as high as 95% in 2012. Moreover, hepatitis B virus (HBV) and hepatitis C virus (HCV) infections affect at least 2 and 150 million people worldwide, respectively (Wang et al., 2014).
In recent years, the incidence of various liver diseases among Tibetan people has been reported. Yan et al. (2007) found that the incidence of fatty liver was 29.89% among 696 cadres living in Tibet, China. The prevalence of ALD among adult Tibetans in Lhasa, Tibet, China is 4.87% (Baima et al., 2016). Zhao et al. (2001) reported a high prevalence of HBV infection in Tibet of China, and the average positive rate of hepatitis B surface antigen (HBsAg) (19.1%) among the native Tibetan population was much higher than that of China (10%) and the world (<5%). Moreover, liver cancer and gastric cancer are still the leading causes of cancer deaths in Tibet. These two kinds of cancer account for 75.2% of all cancer deaths in 2004–2005 (Li et al., 2011). On the other hand, hepatic echinococcosis, a common parasitic disease in pastoral areas, is mainly prevalent in Qinghai, Inner Mongolia, Sichuan, Xinjiang, and Tibet of China. The highest prevalence of echinococcosis in the world has been reported from the Tibetan plateau, China (Wang et al., 2008). In Tibet, the overall incidence of hepatic echinococcosis is 5–10% (Zhu et al., 2012). Schantz et al. (2003) reported that 6.6% of the Tibetan volunteers from Qinghai of China had confirmed infection with Echinococcus granulosus. The high incidence of some liver diseases among Tibetans may be related to their special dietary patterns. Due to the cold climate and hypoxia in the residential areas, they like to eat high-calorie foods (e.g., yak meat and mutton), while consuming less fruits and vegetables. Moreover, they love to drink barley wine and butter tea. Long-term high-fat and high-protein diet as well as drinking may contribute to the high incidence of fatty liver and ALD (Yan et al., 2007). Of course, other than dietary patterns, other risk factors need attention. For example, the high incidence of echinococcosis and HBV infection among Tibetan people can be attributed to their actual exposure to larval Echinococcus spp. and hepatitis B virus, respectively (Zhao et al., 2001; Zhu et al., 2012).
Traditional Tibetan medicine (TTM) is one of the world's oldest known medical systems. It has a long history of more than 2000 years. TTM originated from the local folk tradition called Bon that can be traced back to about 300 B.C. Later, TTM has gradually developed into a unique medical system by incorporating the theories of early traditional Chinese medicine, India medicine (Ayurveda), and Arabia medicine. The fundamental theory of TTM is three elements (also known as three humors) theory consisting of “rLung,” “mKhris-pa,” and “Badkan.” TTM believes that the three elements jointly maintain the body's physiological balance. Among them, mKhris-pa represents fire, helping digestion, accelerating the decomposition of waste, absorbing heat energy from food, and producing heat energy (Luo et al., 2015), and so is the source of many functions such as thermoregulation, metabolism, and liver function. In Qinghai-Tibet Plateau of China, TTM plays an important role in the health care system. It has been practiced by Tibetan physicians throughout the Tibetan regions, including Tibet, Qinghai, Gannan State of Gansu, Ganzi State and Aba State of Sichuan, and Diqing State of Yunnan. The number of physicians practicing TTM was over 5,000 (Luo et al., 2015). Similar to traditional Chinese medicine, TTM mainly uses herbs, animals and sometimes minerals to treat diseases. According to the latest statistics (Jia and Zhang, 2016), 3,105 natural medicines including 2,644 plants, 321 animals, and 140 minerals have been used in Tibetan medicine system. TTM has long-term clinical practices and accumulated rich experience in the treatment of various diseases. It has proved particularly beneficial in the treatment of chronic diseases, such as hepatitis, high altitude polycythemia, gastritis, stroke, cholecystitis, and rheumatism. It is worth noting that TTM has been widely used for the treatment of liver diseases in clinical practice. Many TTM monographs and official drug standards recorded a lot of natural medicines and prescriptions that were traditionally used to treat a variety of liver diseases. However, most of these records are scattered, and lack of systematic summary and induction.
In this paper, a bibliographic investigation of TTM monographs and drug standards and data mining were performed to sample information on natural Tibetan medicines used to treat liver diseases. Their names, original species, families, medicinal parts, treated diseases, and reported biological activities are shown in detail. These data can provide a good reference for their development and utilization. Moreover, we reviewed the most frequently used TTM in terms of their original species, traditional uses, active ingredients, and biological/pharmacological activities. These natural medicines might be a valuable gift from the old Tibetan medicine to the world, and would be potential drug candidates for the treatment of liver diseases.
We manually searched 22 Tibetan medicine monographs and drug standards (Table S1), such as “Jing Zhu Materia Medica,” “Dictionary of Chinese Ethnic Medicine,” “Drug Standards of Tibetan Medicine,” “Tibetan Medicine Annals,” “Annotation of Commonly Used Tibetan Medicine Prescription,” and “Chinese Herbalism for Tibetan Medicine,” to obtain the information on natural Tibetan medicines and their prescriptions used for the treatment of a variety of liver diseases. Data collected from these literatures included names, original species, families, medicinal parts, and treated diseases. The botanical names of original plants are mainly from the references, and verified through the “Flora of China (http://frps.eflora.cn/)” database based on their Chinese names. The database of “The Plant List (http://www.theplantlist.org/)” is also used to standardize their Latin names.
In order to know the most frequently used Tibetan medicines for the treatment of liver diseases, data mining was performed to obtain the usage frequency of each medicine in traditional Tibetan prescriptions by using Traditional Chinese Medicine Inheritance Support System (TCMISS) (Version 2.5) (Yan et al., 2016). All collected prescriptions were manually entered into the TCMISS software, and the usage frequency of each medicine was ranked from large to small by clicking on the “Frequency Statistics” module. In addition, we searched the online Chinese databases (e.g., Wanfang, Weipu, and CNKI) and international databases (e.g., ISI Web of Science, MEDLINE, Science Direct, and Google Scholar) to obtain the active ingredients and biological/pharmacological effects of the selected species using their vernacular, English, or Latin names as search keywords.
This paper recorded the uses of 193 species of natural Tibetan medicines for the treatment of various liver diseases in the traditional Tibetan medical system. The scientific name, family, medicinal part, treated disease, and reported biological activities of these natural medicines are given in Table 1 and Table S2 (see Supplementary Material). These medicines were distributed among 54 families. The most common families are: Gentianaceae (14%), Compositae (12%), Papaveraceae (9%), Labiatae (6%), Saxifragaceae (6%), Ranunculaceae (5%), Scrophulariaceae (4%), and Leguminosae (4%) (Figure S1). Moreover, herb (71%) is the primary source of these medicinal plant species, followed by tree and shrub (9% each), animal (7%), vine and mineral (3% each), lichen and fungus (1% each) (Figure S2). Among various plant parts used, the whole plant was the most frequently used (39%), followed by root and flower (9% each), fruit and aerial part (8% each), seed (7%), rhizome and inflorescence (2% each) (Figure S3).
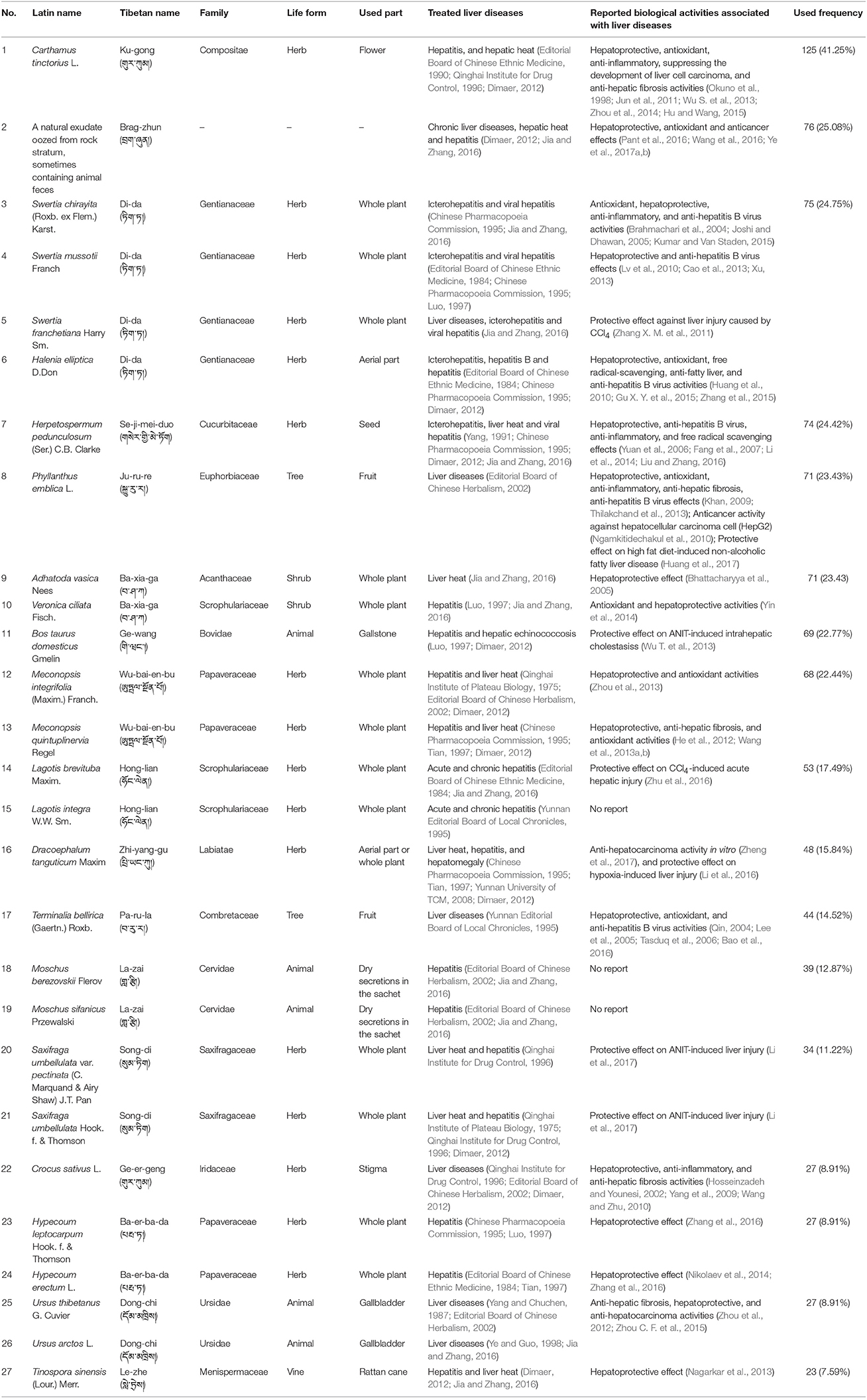
Table 1. The most frequently used Tibetan medicines for the treatment of liver diseases in traditional Tibetan medical system.
By comparison with other articles, we found that most of the species used in the Tibetan medical system to cure liver diseases are different from those reported elsewhere. Chassagne et al. (2017) reported 83 species commonly used by Khmer traditional healers to treat liver disorders in Phnom Penh area, Cambodia. Only one species (i.e., Oroxylum indicum) has a similar use to our article. Similarly, Mukazayire et al. (2011) described 86 herbs used in Southern Rwanda for the treatment of liver diseases. Only one species mentioned by them is included in our study viz. Bidens pilosa. Moradi et al. (2016) reported 26 medicinal plants used for liver disorders in Iranian traditional medicine. Among them, only Rheum palmatum is also mentioned in the present article. In addition, 94, 99, 7 species were found for the treatment of liver diseases in the Southern Regions of Korea, the Maritime region of Togo and Nallamalais, Andhra Pradesh, India, respectively (Kim and Song, 2013; Sabjan et al., 2014; Kpodar et al., 2016). However, these species are quite different from the ones reported in our article. It may be due to the specific mountain flora. Most of the species (e.g., Brag-zhun, Swertia mussotii, Halenia elliptica, and Herpetospermum pedunculosum) reported in the present study are mainly distributed in Tibetan Plateau.
TTM has a unique understanding of the occurrence and development of liver disease. It believes that the pathogenesis of liver disease is divided into external and internal causes (Yutuo, 1983). The external causes include dampness, heat, and epidemic toxin (similar to western medicine's virus), while the internal causes refer to improper diet and overwork (Xizhu, 2010). Long-term or excessive consumption of alcoholic beverages, or salty, sour, spicy, moldy, and greasy foods, or strong work and strenuous exercise will cause the disorder of several basic substances including rLung, mKhris-pa, Badkan, and blood in the body (Bianba and Basang, 2000). Among them, the mKhris-pa disorder will lead to heat toxin invasion of the liver, and eventually cause a variety of liver diseases. In traditional Tibetan medical system, liver disease is divided into several types, such as jaundice liver disease, toxic hepatopathy, and liver heat (Xizhu, 2010). Among them, the liver heat belongs to the category of “mKhris-pa” disease, and its nature belongs to fire. Symptoms of liver heat disease mainly include loss of appetite, liver, and stomach discomfort, abdominal distension, dislike greasy food, and fatigue.
In the present study, we found that 193 Tibetan medicines were used to treat various liver diseases, such as hepatitis, hepatomegaly, viral hepatitis, hepatitis B, toxic hepatopathy, liver heat, and so forth (Figure 1). The results indicated that some species were documented to treat broad liver diseases (e.g., hepatitis and liver heat), while some were clearly indicated for the treatment of specific liver diseases (e.g., hepatitis B). In order to ensure the primordial nature of the information obtained from 22 monographs, we did not incorporate similar disease types in statistics, although the scope of some liver diseases cross each other. For example, viral hepatitis includes hepatitis B, but we still do separate statistics on the two in Figure 1. For liver diseases therapy, the Tibetan people preferred natural medicines most frequently for the treatment of hepatitis using 96 species (37.65%), 46 for icterohepatitis (18.04%), and 45 for liver heat (17.65%) (Figure 1). There were 23 species (9.02%) recorded in the treatment of extensive liver diseases. In addition, 8, 7, and 6 species were described to be able to treat viral hepatitis, hepatomegaly and toxic hepatopathy, respectively. It is noteworthy that 5 Chrysosplenium plants (Chrysosplenium carnosum, Chrysosplenium nudicaule, Chrysosplenium griffithii, Chrysosplenium lanuginosum, and Chrysosplenium nepalense) and Sphaerophysa salsula were used to cure liver cirrhosis. More importantly, 3 Rhododendron plants (Rhododendron anthopogon, Rhododendron anthopogonoides, and Rhododendron primuliflorum) were clearly indicated for the treatment of liver cancer, and three species (H. elliptica, Coriolus versicolor, and Halenia corniculata) for hepatitis B. These information are of great value for the development of potential candidate drugs.
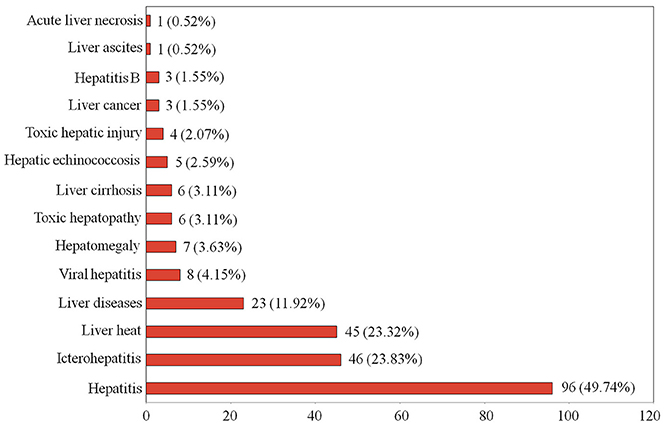
Figure 1. The number and percentage of traditional Tibetan medicines used in the treatment of various liver diseases.
Out of 193 species traditionally used for liver diseases treatment, 51 species (26.42%) have been experimentally demonstrated to have various biological and pharmacological activities associated with liver diseases (Table 1 and Table S2), such as the hepatoprotective, anti-hepatic fibrosis, antioxidant, anti-inflammatory, and anti-hepatitis B virus effects. These findings have proved the validity of these species traditionally used in the treatment of liver diseases. So far, however, there are still 142 species (73.58%) that lack modern experimental evidences. Therefore, more in-depth studies are necessary in order to make better use of these traditional Tibetan medicines.
In order to know the most frequently used Tibetan medicines for the treatment of liver diseases, data mining based on TCMISS software was performed to obtain the usage frequency of these medicines in traditional Tibetan prescriptions. As a result, 303 prescriptions for the treatment of liver diseases were collected from Tibetan monographs and drug standards. Species with used frequencies above 20 are shown in Table 1 and Figure 2. The top five Tibetan medicines are Ku-gong (Carthamus tinctorius) with the used frequency of 125, Brag-zhun with 76, Di-da (Swertia chirayita, S. mussotii, S. franchetiana, and H. elliptica) with 75, Se-ji-mei-duo (H. pedunculosum) with 74, and Ju-ru-re (Phyllanthus emblica) with 71. In the following sections, the names, original species, traditional uses, active ingredients, and biological/pharmacological activities of these five Tibetan medicines have been summarized in detail.

Figure 2. The most frequently used natural Tibetan medicines for the treatment of liver diseases in traditional Tibetan medical system. (A) Carthamus tinctorius, (B) Brag-zhun, (C) Herpetospermum caudigerum, (D) Swertia chirayita, (E) Swertia mussotii, (F) Swertia franchetiana, (G) Halenia elliptica, (H) Phyllanthus emblica, (I) Adhatoda vasica, (J) Veronica ciliata, (K) Meconopsis integrifolia, (L) Meconopsis quintuplinervia, (M) Dracoephalum tanguticum, and (N) Saxifraga umbellulata var. pectinata.
The dried flower of C. tinctorius, known as Ku-gong (Tibetan: 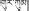 ), Honghua (Chinese name) or safflower (English name), is a commonly used herbal medicine. In China, C. tinctorius is widely cultivated in various locations of Henan, Sichuan, Xinjiang, and Zhejiang provinces. In the traditional theory of Tibetan medicine, Ku-gong is pungent in flavor and warm in nature (Health Bureau of Tibet et al., 1979). It is applied for the treatment of dysmenorrhea, dystocia, traumatic injury, blood stasis, hepatitis, and hepatic heat for thousands of years in Tibetan clinics (Health Bureau of Tibet et al., 1979). So far, many chemical constituents have been isolated from safflower, such as hydroxysafflor yellow A, safflor yellow A, luteolin, kaempferide, and adenosine (Zhou et al., 2014). Among them, hydroxysafflor yellow A is the major bioactive compound, and so is usually used as the marker for controlling the quality of safflower in pharmaceutical industry and drug standards.
), Honghua (Chinese name) or safflower (English name), is a commonly used herbal medicine. In China, C. tinctorius is widely cultivated in various locations of Henan, Sichuan, Xinjiang, and Zhejiang provinces. In the traditional theory of Tibetan medicine, Ku-gong is pungent in flavor and warm in nature (Health Bureau of Tibet et al., 1979). It is applied for the treatment of dysmenorrhea, dystocia, traumatic injury, blood stasis, hepatitis, and hepatic heat for thousands of years in Tibetan clinics (Health Bureau of Tibet et al., 1979). So far, many chemical constituents have been isolated from safflower, such as hydroxysafflor yellow A, safflor yellow A, luteolin, kaempferide, and adenosine (Zhou et al., 2014). Among them, hydroxysafflor yellow A is the major bioactive compound, and so is usually used as the marker for controlling the quality of safflower in pharmaceutical industry and drug standards.
Moreover, it is worth pointing out that phytochemicals or extracts obtained from the safflower have been proved to possess some pharmacological activities associated with liver diseases. For example, Zhang Y. et al. (2011) reported that hydroxysafflor yellow A showed an obvious protective effect against carbon tetrachloride-induced liver fibrosis in rats. Jiang et al. (2014) found that hydroxysafflor yellow A can reduce ischemia/reperfusion-induced acute liver injury by directly attenuating macrophage activation under inflammatory conditions. Carthamus red isolated from safflower was found to have strong antioxidant and hepatoprotective effects against CCl4-induced liver damage in rats (Wu S. et al., 2013). Moreover, the methanol extract of C. tinctorius was demonstrated to have anti-inflammatory action by inducing heme oxygenase-1 expression via NF-E2-related factor translocation and inhibiting NF-κB activity (Jun et al., 2011). Hu and Wang (2015) reported that the water extract of C. tinctorius has significant inhibitory effect on diethylnitrosamine-induced liver cirrhosis in rats. Besides, safflower oil, rich in n-6 polyunsaturated fatty acids, was reported to be able to alter the membrane fatty acid composition of the liver, and suppress the development of liver cell carcinoma induced by diethylnitrosamine in rats (Okuno et al., 1998). These results suggest that Ku-gong (safflower) may serve as a drug candidate for various liver diseases treatment.
Brag-zhun (Tibetan: 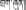 ), one of the commonly used Tibetan medicines, is a well-known natural medicine all over the world recorded by other names, such as Mineral pitch in English, Asphaltum punjabianum in Latin, also known as Baragshun (Mongolian), Tasmayi (Kazakh), Mumlai (Farsi), Shilajit (Sanskrit), Shilajeet (Hindi), and Moomiyo (Russian). It is found predominately in the Altai, Himalaya, and Caucasus mountains. In Ayurveda, the traditional Indian system of medicine, shilajit has been recognized as a rejuvenator because it can prevent ailment and enhance the quality of life (Wilson et al., 2011).
), one of the commonly used Tibetan medicines, is a well-known natural medicine all over the world recorded by other names, such as Mineral pitch in English, Asphaltum punjabianum in Latin, also known as Baragshun (Mongolian), Tasmayi (Kazakh), Mumlai (Farsi), Shilajit (Sanskrit), Shilajeet (Hindi), and Moomiyo (Russian). It is found predominately in the Altai, Himalaya, and Caucasus mountains. In Ayurveda, the traditional Indian system of medicine, shilajit has been recognized as a rejuvenator because it can prevent ailment and enhance the quality of life (Wilson et al., 2011).
Brag-zhun was first recorded in the classic Tibetan book “Yue Wang Yao Zhen (Somaratsa)” compiled in the mid-eighth century. In China, brag-zhun is mainly distributed in the Qinghai-Tibet Plateau, such as Tibet, Qinghai, Ganzi, and Aba of Sichuan, and Shangri-La of Yunnan. There are several schools of thought for the origin of brag-zhun. Most researchers think that brag-zhun is a natural exudate oozed from rock stratum, sometimes containing animal feces (e.g., Trogopterus xanthotis, Ochotona erythrotis; Wilson et al., 2011; Cao et al., 2016). In China, brag-zhun as well as its preparations are commonly used Tibetan medicines for the treatment of hot diseases, especially good at treating liver (e.g., hepatitis, hepatomegaly) and ocular diseases (e.g., conjunctivitis) (Chinese Pharmacopoeia Commission, 1995; Cao et al., 2016).
It has been reported that brag-zhun contains a lot of minerals in ionic form, as well as organic matter (e.g., humic acid and fulvic acid). Moreover, carotenoids, indigoids, amino acids, essential fatty acids, and vitamins were also found in brag-zhun (Wilson et al., 2011; Cao et al., 2015). Modern pharmacological study has demonstrated that the n-butanol extract of brag-zhun has an obvious protective effect against acetaminophen-induced acute liver injury in mice (Ye et al., 2017a). Wang et al. found that the n-butanol extract of brag-zhun significantly reduced the serum levels of ALT, AST, TNF-α, and IFN-γ, increased the activity of SOD in liver tissue, decreased the activation levels of Caspase-3 and Caspase-8 in liver tissue, and reduced the pathological damage of liver tissue in mice with liver injury induced by concanavalin A. It indicated that the mechanisms of the hepatoprotective effect of brag-zhun may be related to the inhibition of release of inflammatory factors, antioxidant activity, and anti-apoptosis (Wang et al., 2016). In addition, brag-zhun as well as its water extract were reported to be able to significantly reduce the liver index and the serum levels of ALT and AST in mice with liver injury initiated by concanavalin A (Ye et al., 2017b). Recently, Pant et al. (2016) reported that mineral pitch (the same substance with brag-zhun) induced apoptosis via the production of ROS, and inhibited proliferation by modulating the expression levels of miRNA-21 and miRNA-22 in hepatic cancer cells (Huh-7).
Di-da (Tibetan: 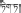 ) is a widely used traditional Tibetan medicine in China. It originates from multiple plant species. S. chirayita, S. mussotii, S. franchetiana, and H. elliptica are the most commonly used species of Di-da in China. In traditional theory, Di-da is bitter in flavor and cold in property (Health Bureau of Tibet et al., 1979). It can clear liver and gallbladder heat, and remove jaundice, and so has been commonly used for the treatment of liver and gallbladder diseases, such as icterohepatitis, viral hepatitis, and cholecystitis.
) is a widely used traditional Tibetan medicine in China. It originates from multiple plant species. S. chirayita, S. mussotii, S. franchetiana, and H. elliptica are the most commonly used species of Di-da in China. In traditional theory, Di-da is bitter in flavor and cold in property (Health Bureau of Tibet et al., 1979). It can clear liver and gallbladder heat, and remove jaundice, and so has been commonly used for the treatment of liver and gallbladder diseases, such as icterohepatitis, viral hepatitis, and cholecystitis.
Swertia chirayita, a well-known herbal medicine in India, Nepal, and China, is widely grown in the temperate regions of Himalayas. Its medicinal usage is reported in various traditional systems of medicines, such as the Tibetan medicine, Ayurveda, Unani, and Siddha under different names (e.g., Swertia chirata, Chiretta, Anaryatikta, Chirrato, and Cherayata; Joshi and Dhawan, 2005). In China, S. chirayita is frequently used to treat damp-heat and quench the fire of the liver and gallbladder in traditional Tibetan medicine system (Fan et al., 2014). Modern investigations have shown that S. chirayita possesses a wide range of biological activities, such as hepatoprotective, anticancer, anti-inflammatory, hypoglycemic, antibacterial, and antiviral activities (Brahmachari et al., 2004; Joshi and Dhawan, 2005; Kumar and Van Staden, 2015). It is noteworthy that the extract of S. chirayita was reported to show protection against hepatotoxicity induced by paracetamol (Nagalekshmi et al., 2011). Mukherjee et al. (1997) found that different doses of S. chirata could improve the liver injury induced by CCl4 in albino rats, and the moderate dose (50 mg/kg body wt) was found to be the most effective. Moreover, the 50% EtOH–H2O extract of S. chirayita was reported to be able to inhibit the secretions of HBsAg and hepatitis B e antigen (HBeAg) (Zhou N. et al., 2015). These pharmacological activities are mainly attributed to the presence of a diverse group of bioactive phytochemicals in S. chirayita, such as swertiamarin, sweroside, gentiopicrin, mangiferin, amaroswerin, oleanolic acid, 3,3′,5-trihydroxybiphenyl, (+)-cycloolivil-4′-O-β-D-glucopyranoside, 1-hydroxy-3,7,8-trimethoxy xanthone, and 1,5,8-trihydroxy-3-methoxy xanthone. Among them, swertiamarin was reported to possess significant antioxidant and hepatoprotective effects against D-galactosamine induced acute liver damage (Jaishree and Badami, 2010). Gentiopicrin could decrease the serum ALT and AST levels, and increase the liver GSH-Px activity in the mice treated with CCl4, indicating significant liver protection property (Liu et al., 2002). Mangiferin showed significant hepatoprotective activity against D-galactosamine induced hepatotoxicity in rats via Nrf2–NFκB pathways (Das et al., 2012). In addition, (+)-cycloolivil-4′-O-β-D-glucopyranoside showed inhibitory activity on HBsAg secretion with IC50 values of 0.31 ± 0.045 mM (Zhou N. et al., 2015).
Swertia mussotii is an important and frequently used Tibetan medicinal plant indigenous to the Qinghai-Tibet Plateau. It is mainly distributed in high altitude areas (2000–3800 m) of Sichuan, Qinghai, Tibet, and Yunnan provinces in China. Qinghai's Yushu and northwest Sichuan plateau are the main producing areas of S. mussotii. In “Drug Standards of Tibetan Medicine,” S. mussotii was recorded with detoxification and clearing liver and gallbladder heat effects. It has been commonly used for the treatment of hepatitis, acute icterohepatitis, cholecystitis, epidemic fever, headache, and blood diseases in traditional Tibetan medicine system. Modern pharmacological experiment has proved that S. mussotii could alleviate the damage of immunological liver injury in mice caused by BCG vaccine and lipopolysaccharide (Xu, 2013). Lv et al. (2010) found that 75% ethanol extract of S. mussotii showed hepatoprotective effect on CCl4-induced acute liver damage in mice. Moreover, the alcohol extract of S. mussotii was reported to significantly reduce lipopolysaccharide-induced cholestatic liver damage in rats (Gao et al., 2014). Similar to S. chirayita, iridoid glycosides, xanthones, and triterpenoids are the major bioactive compounds in S. mussotii. The total iridoids and xanthones extracted from S. mussotii exhibited significant hepatoprotective effect on alpha naphthylisot hiocyanate-induced liver damage in mice (Tian et al., 2014). In addition, Cao et al. (2013) isolated several xanthones from S. mussotii, which exhibited significant anti-hepatitis B virus activity.
Swertia franchetiana, an annual herb, is also widely grown in the Qinghai-Tibet Plateau of China (Tibet, Sichuan, Qinghai, and southern Gansu) at elevations of 2200–3600 m. The whole plant of S. franchetiana is commonly used by Tibetan people for the treatment of various liver diseases, mainly icterohepatitis and viral hepatitis (Jia and Zhang, 2016). In recent years, multiple active phytochemicals belonging to different classes were isolated from S. franchetiana, such as swertiamarin, gentiopicrin, mangiferin, swertianolin, and oleanolic acid. It is worth pointing out that the content of swertiamarin was found to be higher in S. franchetiana than that in S. chirata and S. mussotii (Fan, 2012). Up to now, the pharmacological activities of S. franchetiana have rarely been reported. Zhang X. M. et al. (2011) found that the n-butanol extract of S. Franchetiana could exert a protective effect against liver injury caused by CCl4.
Halenia elliptica is a commonly used traditional herbal medicine. It is mainly distributed across the Tibet, Yunnan, Sichuan, Qinghai, Xinjiang, and Inner Mongolia of China as well as Nepal, Bhutan, and India. In “Drug Standards of Tibetan Medicine,” H. elliptica was described with clearing heat, promoting diuresis, calming the liver, and promoting bile flow functions. Its aerial part was used for the treatment of acute icterohepatitis, cholecystitis, dizziness, headache, and toothache in Tibetan clinics. The widespread uses of H. elliptica in traditional Tibetan medicine have resulted in considerable pharmacological and phytochemical studies of the plant. Huang et al. (2010) reported that administration of the methanolic extract of H. elliptica significantly decreased the serum levels of ALT, AST, ALP, and total bilirubin in rats with liver toxicity caused by CCl4. Similarly, the ethanol extract of H. elliptica was found to have hepatoprotective effect on CCl4-induced chemical hepatic injury in mice (Jin et al., 2007). The main active constituents of H. elliptica are recognized as xanthones (e.g., 1-hydroxy-2,3,5-trimethoxy xanthone and 1-hydroxy-2,3,4,5-tetramethoxy xanthone), flavonoids (e.g., luteolin and apigenin), and pentacyclic triterpenes (e.g., oleanolic acid) (Zhang et al., 2015). Gu X. Y. et al. (2015) reported that the total flavonoids isolated from H. elliptica could improve liver cell damage, decrease the serum level of MDA, and increase SOD level in rats with experimental steatohepatitis induced by high-fat diet.
The dried seeds of H. pedunculosum, known as “Se-ji-mei-duo” (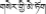 ) in Tibetan or “Bolengguazi” in Chinese, is one of the most representative Tibetan medicines. It is bitter in flavor and cold in property, and frequently used in the traditional Tibetan system of medicine for the treatment of icterohepatitis, liver heat, viral hepatitis, cholecystitis, and dyspepsia in the past few decades. H. pedunculosum, also named H. caudigerum in some literatures, belongs to the Cucurbitaceae family and is widely distributed in Tibet and Yunnan of China, India and Nepal at elevations of about 2300–3500 m.
) in Tibetan or “Bolengguazi” in Chinese, is one of the most representative Tibetan medicines. It is bitter in flavor and cold in property, and frequently used in the traditional Tibetan system of medicine for the treatment of icterohepatitis, liver heat, viral hepatitis, cholecystitis, and dyspepsia in the past few decades. H. pedunculosum, also named H. caudigerum in some literatures, belongs to the Cucurbitaceae family and is widely distributed in Tibet and Yunnan of China, India and Nepal at elevations of about 2300–3500 m.
Previous phytochemical studies have shown that the seeds of H. pedunculosum is rich in lignan compounds (e.g., herpetrione, herpetin, herpetetrone, herpetone, herpetradione, herpepropenal, herpetal, and herpepentol; Liu and Zhang, 2016). They have been proved to possess multiple pharmacological activities such as anti-hepatitis B virus, anti-inflammatory and hepatoprotective effects (Yuan et al., 2006; Yu et al., 2014; Shen et al., 2015; Liu and Zhang, 2016). It was reported that herpetrione displayed promising inhibitory potential against hepatitis b virus, which could reduce the replication and expression of HBsAg and HBeAg (Yuan et al., 2006). Moreover, herpetrione was discovered to be the active ingredient in protecting liver and lowering aminotransferase levels, and its nanosuspensions exhibited a significant hepatoprotective effect against acute liver injury induced by CCl4 in mice (Shen et al., 2013). Similarly, herpetin was found to have significant inhibitory effect on HBV-DNA in vitro (Yuan et al., 2006). Recently, Gu J. et al. (2015) reported that herpetin exhibited certain hepatoprotective activity against carbon tetrachloride-induced liver injury in mice, and this effect could be promoted through pharmaceutical application of liposome.
Moreover, in order to make better use of the seeds of H. pedunculosum in the treatment of liver diseases, some modern pharmacological studies were performed. Li et al. (2014) reported that administration of the seed oil of H. pedunculosum could significantly reduce CCl4-induced liver damage, decrease the serum levels of triglycerides, malondialdehyde, total bilirubin, and hepatic enzyme markers (e.g., alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase), and increase the activities of SOD in rats. The ethyl acetate extract of H. caudigerum was found to show a protective effect on CCl4-induced acute liver injury in mice (Shen et al., 2015). Besides, the total lignans extracted from H. pedunculosun seeds were found to have hepatoprotective effect on concanavalin A-induced immunological liver injury in mice (Gu et al., 2014). These findings mentioned above support the traditional applications of H. pedunculosun seeds by Tibetan medicine practitioners in liver diseases treatment.
The dried fruits of P. emblica, known as “Ju-ru-re” (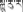 ) in Tibetan, is one of the commonly used Tibetan medicines in China. It is a well-known traditional medicine all over the world recorded by several names, such as Emblica officinalis, Indian gooseberry, Emblic myrobalan, Amla (Hindi), Amba (Nepalese), and Mirabolano emblico (Portuguese) (Khan, 2009). In Indian indigenous system of medicine (e.g., Ayurveda), Amla, also named as E. officinalis or Indian gooseberry, enjoys an important position because of its good resistance to a variety of diseases. Most of the parts of E. officinalis are used for medicinal purposes, for example, the fruits have been widely used in Ayurveda for the treatment of liver disorders, diabetes, diarrhea, jaundice, and inflammation (Khan, 2009; Krishnaveni and Mirunalini, 2010; Khosla and Sharma, 2012).
) in Tibetan, is one of the commonly used Tibetan medicines in China. It is a well-known traditional medicine all over the world recorded by several names, such as Emblica officinalis, Indian gooseberry, Emblic myrobalan, Amla (Hindi), Amba (Nepalese), and Mirabolano emblico (Portuguese) (Khan, 2009). In Indian indigenous system of medicine (e.g., Ayurveda), Amla, also named as E. officinalis or Indian gooseberry, enjoys an important position because of its good resistance to a variety of diseases. Most of the parts of E. officinalis are used for medicinal purposes, for example, the fruits have been widely used in Ayurveda for the treatment of liver disorders, diabetes, diarrhea, jaundice, and inflammation (Khan, 2009; Krishnaveni and Mirunalini, 2010; Khosla and Sharma, 2012).
In traditional Tibetan system of medicine, Ju-ru-re is described as sweet, sour, astringent in flavor and cool in property. It is frequently used for the treatment of liver diseases, blood heat, sore throat, dry mouth, indigestion, abdominal pain, and cough for thousands of years (Health Bureau of Tibet et al., 1979; Chinese Pharmacopoeia Commission, 2015). P. emblica is a deciduous tree and widely grown and sometimes cultivated in subtropical and tropical areas including China, India, Sri Lanka, Indonesia, Malaysia, and Philippines. In China, P. emblica is mainly distributed in Sichuan, Guizhou, Fujian, Guangdong, Hainan, Guangxi, and Yunnan at elevations of 200–2300 m.
Up to now, many chemical constituents belonging to different classes have been isolated from the fruits of P. emblica including tannins, flavanoids, vitamins, amino acids, and carbohydrates. Hydrolyzable tannins (e.g., gallic acid, ellagic acid, corilagin, chebulagic acid, and geraniin) are the dominating active ingredients of the fruits of P. emblica (Yang and Liu, 2014). It was reported that gallic acid could protect the liver from injuries induced by various hepatotoxic agents, including paracetamol, sodium fluoride, cyclophosphamide, nitrosodiethylamine, and carbon tetrachloride in experimental animal models (Jadon et al., 2007; Rasool et al., 2010; Nabavi et al., 2013; Latief et al., 2016; Oyagbemi et al., 2016). Moreover, Hsu and Yen (2007) found that intake of gallic acid could be beneficial for the suppression of high fat diet-induced dyslipidaemia and hepatosteatosis in rats. Recently, gallic acid was found to ameliorate impaired glucose and lipid homeostasis in high fat diet-induced NAFLD mice by using 1H NMR-based metabolomics method (Chao et al., 2014). Similar to gallic acid, ellagic acid has also been reported to show obvious hepatoprotective effects in murine models against a variety of agents, such as paracetamol, carbon tetrachloride, alcohol, D-galactosamine, and concanavalin A (García-Nino and Zazueta, 2015). In addition, ellagic acid exhibited good antiviral properties against HBV and HCV (García-Nino and Zazueta, 2015). As another hydrolyzed tannin, corilagin was found to have hepatoprotective effect on galactosamine/lipopolysaccharide-induced liver injury in rats through suppression of oxidative stress and apoptosis (Kinoshita et al., 2007). Moreover, Hau et al. (2010) found that corilagin was considerably effective to retard the in vivo growth of xenografted Hep3B hepatocellular carcinoma. Given these beneficial biological activities, gallic acid, ellagic acid, and/or corilagin are usually qualitatively analyzed to evaluate the quality of Ju-ru-re by using HPLC method (Zhang et al., 2012). Moreover, the fruits of P. emblica are also rich in flavonoids, such as quercetin, kaempferol, rutin, quercetin 3-β-d-glucopyranoside, and kaempferol 3-β-d-glucopyranoside (Liu et al., 2008).
The widespread uses of the fruits of P. emblica in traditional medicines and food products have resulted in considerable pharmacological studies. A wide range of biological activities and potential health benefits have been reported, including hepatoprotective, gastroprotective, anti-inflammatory, anticancer, cardioprotective, and immunomodulatory effects (Khan, 2009; Krishnaveni and Mirunalini, 2010; Khosla and Sharma, 2012; Thilakchand et al., 2013; Yang and Liu, 2014). In this paper, we mainly focus on its hepatoprotective activity. P. emblica has been proven to have potential in protecting the liver from injuries induced by multiple hepatotoxic agents, such as ethanol, carbon tetrachloride, arsenic, ochratoxins, and antitubercular drugs in experimental models (Jose and Kuttan, 2000; Tasduq et al., 2005; Pramyothin et al., 2006; Panchabhai et al., 2008; Maiti et al., 2014). For exploring anti-viral compounds from P. emblica, eight new sesquiterpenoid glycosides along with three known compounds were isolated and evaluated for their anti-HBV activities. The results found that phyllaemblicin G6 displayed anti-HBV activities with IC50 of 8.53 ± 0.97 and 5.68 ± 1.75 μM for the HBsAg and HBeAg secretion, respectively (Lv et al., 2014). Recently, the water extract of P. emblica fruits was also found to show a protective effect on high fat diet-induced non-alcoholic fatty liver disease (NAFLD) in SD rats (Huang et al., 2017). In addition, pretreatment with the defatted methanolic extract of E. officinalis (100 and 200 mg/kg) significantly inhibited the appearance of γ-GT foci, pathological manifestations and tumor formation induced by the Solt-Farber regimen in the liver of Wistar rats (Sultana et al., 2008). The water extract of P. emblica fruits significantly decreased fat accumulation and ROS production in HepG2 cells, and also inhibited hepatic fibrosis in HSC-T6 cells (Lu et al., 2016). Besides, Ngamkitidechakul et al. (2010) found that the aqueous extract of P. emblica at 50–100 μg/mL significantly inhibited cell growth of six human cancer cell lines including hepatocellular carcinoma cell (HepG2). In summary, these modern pharmacological studies indicated that Ju-ru-re may have a good potential to develop into a candidate drug for the treatment of liver diseases.
Although some positive effects of TTM in the treatment of liver diseases have been reported, attention should also be paid to the potential toxicity of some Tibetan medicines. For example, the aqueous extract of C. tinctorius was found to have toxic effects on mouse spermatogenesis (Mirhoseini et al., 2012). Nobakht et al. (2000) reported that the C. tinctorius extract displayed teratogenic effects on the central nervous system development in mice at the dose of 1.2 mg/kg/day, and its cytotoxic effect on the rat nervous cell culture appeared dose-dependent (Zhou et al., 2014). Besides, C. tinctorius is thought to be potentially harmful to pregnant women (Ernst, 2002).
Furthermore, we should also pay special attention to the hepatotoxicity of some Tibetan medicines. In our survey, Compositae was found as the second most commonly used family for liver diseases treatment, but some medicinal plants in this family (e.g., Senecio scandens) are known to contain pyrrolizidine alkaloids which can induce liver damage (Wang D. et al., 2013). It was reported that and the aqueous extract of S. scandens produced mild hepatotoxicity in rats at a high dose of 20 g/kg, and the toxicity was related to its total alkaloid content (Wang, 2008). Similarly, Lin et al. (2009) found that a single overdose (6 g/kg) of the water extract of S. scandens produced typical pyrrolizidine alkaloids-induced hepatotoxicity in rats. However, no significant hepatotoxic effects were observed in rats at the dose recommended by the Pharmacopeia of China. These findings indicated that the hepatotoxicity of S. scandens is dose related. Moreover, Lithospermum erythrorhizon was also reported to contain some pyrrolizidine alkaloids (Roeder and Rengel, 1990). Therefore, caution should be taken when administering L. erythrorhizon especially at high doses, although no significant toxicity has been observed so far in this herb (Han et al., 2015). In recent years, the safety of Bupleurum chinense and its products has raised general concerns. Lv et al. (2009) found that the long-term administration of the crude extract of B. chinense induced obvious hepatotoxicity in rats. It can not only cause the changes of liver function (ALT and AST), but also the pathological changes in hepatic cell. Besides, it is worth noting that R. palmatum was found to have bidirectional effects of liver protection and hepatotoxicity on CCl4-treated and normal rats. Its hepatotoxic effect could be attributable to the liver cell fibrosis induced by high doses of this herb (Wang et al., 2011).
In summary, despite the good benefits of Tibetan medicines in the treatment of liver diseases were indicated in our article, their potential toxicity should also be given enough attention. It is especially important to take precautions against drug-induced liver injury when selecting Tibetan medicines and their doses. More experiments should be encouraged to identify their side effects or toxicity so that they can be used safely and effectively.
Nature medicines including medicinal plants, animals and minerals are nature's gift to human beings, which play an important role in the fight against various diseases. Many commonly used drugs of modern medicine have originated directly or indirectly from them, such as artemisinin, paclitaxel, and aspirin. Tibetan medicine is an important part of the world's traditional medical system. The Tibetan people living in the Qinghai-Tibet Plateau have accumulated rich experience in medication in their struggle against natural conditions and diseases. It is recognized that traditional Tibetan medicine has a good curative effect in the treatment of liver diseases, rheumatism, acute and chronic mountain sicknesses, cardiovascular and cerebrovascular diseases, and stomach diseases.
In the present study, we have attempted to generalize and congregate the names, original species, families, medicinal parts, traditional uses, and pharmacological information on natural Tibetan medicines traditionally used in the Tibetan system of medicine for liver diseases treatment. The results showed that these Tibetan medicines were mainly distributed among 54 families, and the most frequently used family is Gentianaceae. In addition, herb is the primary source of these medicines, and the whole plant is the most commonly used part. More importantly, we found several natural Tibetan medicines, such as C. tinctorius, Brag-zhun, S. chirayita, S. mussotii, H. elliptica, H. pedunculosum, and P. emblica, which were frequently used to treat liver diseases by bibliographic investigation and data mining. It is worth noting that C. tinctorius is also a widely used traditional Chinese medicine (TCM), which is mainly applied for blood-stasis syndrome with dysmenorrhea, amenorrhea, and postpartum abdominal pain in the clinical practice of TCM (Zhou et al., 2014). However, the present study found that C. tinctorius was frequently used to treat liver diseases in TTM system. The medicinal value of C. tinctorius in treating liver diseases deserves further exploration and utilization. Moreover, it is also need to pay special attention to several active compounds isolated from the most frequently used Tibetan herbs. We think that hydroxysafflor yellow A, swertiamarin, gentiopicrin, herpetrione, herpetin, gallic acid, and ellagic acid may be good and promising drug candidates for treating liver diseases because of their exact hepatoprotective effect as well as high concentration levels in the corresponding species. In order to assess the potential of these compounds for druggability, multidisciplinary approaches should be integrated to perform more pharmacological studies, reveal their mechanisms of action, clarify the pathways of their absorption, distribution, metabolism, and excretion (i.e., the ADME process), and also to assess their potential toxicity.
In addition, the gaps and limitations of current research on these Tibetan medicines also need to be pointed out. First, only 51 species (26.42%) have been demonstrated to have biological activities associated with liver diseases, and most species still lack adequately powered experimental evidences. For example, Lagotis integra, known as “Hong-lian” in Tibetan, is a commonly used Tibetan medicines for the treatment of liver diseases with the used frequency of 53. However, so far, no biological activities or active components related to liver diseases have been reported for this herb. Similarly, Moschus berezovskii and M. sifanicus, named “La-zai” in Tibetan with the used frequency of 39, also lack the liver disease-related study. Given their high frequency of use, these gaps of research need to be tackled urgently. Secondly, although some compounds isolated from Tibetan medicines were found to have biological activities associated with liver diseases, their mechanisms of action and possible synergies with each other have not been explicit enough. Further research is necessary to solve these problems. On the other hand, although some Tibetan herbal medicines (e.g., P. emblica) have been reported to inhibit the proliferation of several human liver cancer cell lines, these in vitro studies are definitely not sufficient to show that they have a positive effect on the treatment of hepatocellular carcinoma because the response of a cell line to an herbal extract may or may not occur in humans (Gertsch, 2009). Rigorous in vivo experiments and even clinical studies involving different mechanisms are still needed to confirm their effectiveness in the treatment of liver diseases.
In conclusion, this study provides the first compilation of data for the ethnomedicinal knowledge of TTM in the treatment of liver diseases. The medicinal species with high frequency of use may signpost the probable existence of valuable active compounds. In order to better develop and utilize these traditional Tibetan medicines, more efforts should be made to evaluate their biological activities in vivo, identify bioactive components, elucidate the underlying mechanism of action, and clarify their side effects or toxicity by using pharmacological, phytochemical, metabonomics, and/or clinical trial methods.
QL: conducted the research, performed data analysis, and wrote the paper; H-JL, HD, TX: collected, organized, and analyzed the data; C-LH: wrote the Tibetan names of natural medicines; GF: conceived and designed the study; and YZ: amended the paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors gratefully acknowledge the financial support from Key Project of Sichuan Provincial Department of Education (no. 17ZA0158), Science Development Fund of Chengdu University of Traditional Chinese Medicine (no. ZRQN1637), Cultivation Program of Outstanding Young Academic and Technological Leaders of Sichuan Province (no. 2014JQ0050), and Major Project of National Social Science Foundation of China (no. 16ZDA238).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2018.00029/full#supplementary-material
Baima, K. Z., Ouzhu, L. B., Ciren, Y. Z., Luobu, Z. D., Yang, L., Qiangba, D. Z., et al. (2016). Prevalence and risk factors of alcoholic liver disease among Tibetan native adults in Lhasa. Chin. J. Public Health 32, 295–298. doi: 10.11847/zgggws2016-32-03-11
Bao, Y. F., Ma, X. Y., Zheng, L. F., and Li, H. Y. (2016). Hepatoprotective effects of the extract of Terminalia billerica (Gaertn.) Roxb. on mice. Lishizhen Med. Mater. Med. Res. 27, 342–345.
Bhattacharyya, D., Pandit, S., Jana, U., Sen, S., and Sur, T. K. (2005). Hepatoprotective activity of Adhatoda vasica aqueous leaf extract on D-galactosamine-induced liver damage in rats. Fitoterapia 76, 223–225. doi: 10.1016/j.fitote.2004.10.014
Bianba, C. R., and Basang, D. J. (2000). Understanding and treatment of Tibetan medicine for liver diseases. Chin. J. Ethn. Ethn. 5, 262.
Brahmachari, G., Mondal, S., Gangopadhyay, A., Gorai, D., Mukhopadhyay, B., Saha, S., et al. (2004). Swertia (Gentianaceae): chemical and pharmacological aspects. Chem. Biodivers. 1, 1627–1651. doi: 10.1002/cbdv.200490123
Cao, T., Geng, C., Ma, Y., He, K., Wang, H., Zhou, N., et al. (2013). Xanthones with anti-hepatitis B virus activity from Swertia mussotii. Planta Med. 79, 697–700. doi: 10.1055/s-0032-1328399
Cao, Y., Gu, R., Ma, Y. Y., Zhong, S. H., Zeng, R., and Lu, X. F. (2015). Material composition research on Tibetan medicine “Brag-zhun”. J. Chin. Med. Mater. 38, 279–283. doi: 10.13863/j.issn1001-4454.2015.02.018
Cao, Y., Gu, R., Zhao, M. M., Ma, Y. Y., Zhong, S. H., Rang, J., et al. (2016). Source and application status investigation of Tibetan medicine “Brag-zhun”. Chin. J. Chin. Mater. Med. 41, 4663–4669. doi: 10.4268/cjcmm20162428
Chao, J., Huo, T. I., Cheng, H. Y., Tsai, J. C., Liao, J. W., Lee, M. S., et al. (2014). Gallic acid ameliorated impaired glucose and lipid homeostasis in high fat diet-induced NAFLD mice. PLoS ONE 9:e96969. doi: 10.1371/journal.pone.0096969
Chassagne, F., Deharo, E., Punley, H., and Bourdy, G. (2017). Treatment and management of liver diseases by Khmer traditional healers practicing in Phnom Penh area, Cambodia. J. Ethnopharmacol. 202, 38–53. doi: 10.1016/j.jep.2017.03.002
Chinese Pharmacopoeia Commission (1995). Drug Standards of Tibetan Medicine. Beijing: Ministry of Health of the People's Republic of China.
Chinese Pharmacopoeia Commission (2015). Pharmacopoeia of the People's Republic of China, Part 1. Beijing: China Medical Science Press.
Das, J., Ghosh, J., Roy, A., and Sil, P. C. (2012). Mangiferin exerts hepatoprotective activity against D-galactosamine induced acute toxicity and oxidative/nitrosative stress via Nrf2–NFκB pathways. Toxicol. Appl. Pharm. 260, 35–47. doi: 10.1016/j.taap.2012.01.015
Dimaer, D. Z. P. C. (2012). Jing Zhu Materia Medica. Shanghai: Shanghai Science and Technology Press.
Editorial Board of Chinese Ethnic Medicine (1984). Chinese Ethnic Medicine Annals (vol. 1). Beijing: People's Medical Publishing Press.
Editorial Board of Chinese Ethnic Medicine (1990). Chinese Ethnic Medicine Annals, Vol. 2. Beijing: People's Medical Publishing Press.
Editorial Board of Chinese Herbalism (2002). Chinese Herbalism for Tibetan Medicine. Shanghai: Shanghai Science and Technology Press.
Ernst, E. (2002). Herbal medicinal products during pregnancy: are they safe? Int. J. Obstet. Gy. 109, 227–235. doi: 10.1111/j.1471-0528.2002.t01-1-01009.x
Fan, G. (2012). Application of 1H NMR-Based Metabolomics for the Species Differentiation and Quality Evaluation of Traditional Chinese Medicine and Tibetan Medicine from Various Species. Dissertation/master's thesis, Chengdu University of Traditional Chinese Medicine: Chengdu.
Fan, G., Luo, W. Z., Luo, S. H., Li, Y., Meng, X. L., Zhou, X. D., et al. (2014). Metabolic discrimination of Swertia mussotii and Swertia chirayita known as “Zangyinchen” in traditional Tibetan medicine by 1H NMR-based metabolomics. J. Pharm. Biomed. Anal. 98, 364–370. doi: 10.1016/j.jpba.2014.06.014
Fang, Q. M., Zhang, H., Cao, Y., and Wang, C. (2007). Anti-inflammatory and free radical scavenging activities of ethanol extracts of three seeds used as “Bolengguazi”. J. Ethnopharmacol. 114, 61–65. doi: 10.1016/j.jep.2007.07.024
Federico, A., Dallio, M., Masarone, M., Persico, M., and Loguercio, C. (2016). The epidemiology of non-alcoholic fatty liver disease and its connection with cardiovascular disease: role of endothelial dysfunction. Eur. Rev. Med. Pharmacol. Sci. 20, 4731–4741.
Gao, Y., Chai, J., Li, S. X., Liu, C., Cheng, Y., Liu, X. C., et al. (2014). Protective effect of alcohol extract of Swertia mussotii Franch on lipopolysaccharideinduced cholestatic liver damage in rats. J. Third Mil. Med. Univ. 36, 769–773. doi: 10.16016/j.1000-5404.2014.08.006
García-Nino, W. R., and Zazueta, C. (2015). Ellagic acid: pharmacological activities and molecular mechanisms involved in liver protection. Pharmacol. Res. 97, 84–103. doi: 10.1016/j.phrs.2015.04.008
Gertsch, J. (2009). How scientific is the science in ethnopharmacology? Historical perspectives and epistemological problems. J. Ethnopharmacol. 122, 177–183. doi: 10.1016/j.jep.2009.01.010
Gu, J., Li, J. C., and Fan, L. N. (2014). The protective effect and its mechanism of total Lignans from Tibetan medicinal Herpetospermum Seeds on Concanavalin A-induced liver injury in mice. J. Southwest Univ. Nat.-Nat. Sci. Edn. 40, 375–387. doi: 10.3969/j.issn.1003-4271.2014.03.10
Gu, J., Yuan, Z., Tan, R., and Zhang, X. (2015). Isolation of herpetin from Herpetospermum seed and hepatoprotective activity of liposomal herpetin against carbon tetrachloride-induced liver injury in mice. Die Pharm.-Int. J. Pharm. Sci. 70, 745–752. doi: 10.1691/ph.2015.5696
Gu, X. Y., Zhu, J. K., and Zhang, L. (2015). Pharmacological effects of total flavonoids from Halenia elliptica on fatty hepatitis model rats. Chin. Tradit. Pat. Med. 37, 2308–2311.
Han, C. T., Kim, M. J., Moon, S. H., Jeon, Y. R., Hwang, J. S., Nam, C., et al. (2015). Acute and 28-day subacute toxicity studies of hexane extracts of the roots of Lithospermum erythrorhizon in Sprague-Dawley rats. Toxicol. Res. 31:403. doi: 10.5487/TR.2015.31.4.403
Hau, D. K. P., Zhu, G. Y., Leung, A. K. M., Wong, R. S. M., Cheng, G. Y. M., Bo-San Lai, P., et al. (2010). In vivo anti-tumour activity of corilagin on Hep3B hepatocellular carcinoma. Phytomedicine 18, 11–15. doi: 10.1016/j.phymed.2010.09.001
He, J., Huang, B., Ban, X., Tian, J., Zhu, L., and Wang, Y. (2012). In vitro and in vivo antioxidant activity of the ethanolic extract from Meconopsis quintuplinervia. J. Ethnopharmacol. 141, 104–110. doi: 10.1016/j.jep.2012.02.006
Health Bureau of Tibet, Qinghai, Sichuan, Gansu, Yunnan, and Xinjiang. (1979). Tibetan Medicine Standards. Xining: Qinghai People's Publishing Press.
Hosseinzadeh, H., and Younesi, H. M. (2002). Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2:7. doi: 10.1186/1471-2210-2-7
Hsu, C. L., and Yen, G. C. (2007). Effect of gallic acid on high fat diet-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats. Br. J. Nutr. 98, 727–735. doi: 10.1017/S000711450774686X
Huang, B., Ban, X., He, J., Zeng, H., Zhang, P., and Wang, Y. (2010). Hepatoprotective and antioxidant effects of the methanolic extract from Halenia elliptica. J. Ethnopharmacol. 131, 276–281. doi: 10.1016/j.jep.2010.06.029
Huang, C., Tung, Y., Hsia, S., Wu, C., and Yen, G. (2017). The hepatoprotective effect of Phyllanthus emblica L. fruit on high fat diet-induced non-alcoholic fatty liver disease (NAFLD) in SD rats. Food Funct. 8, 842–850. doi: 10.1039/C6FO01585A
Hu, Z., and Wang, W. (2015). Effect of Carthamus tinctorius L. extract on diethylnitrosamine-induced liver cirrhosis in rats. Trop. J. Pharm. Res. 14, 1213–1216. doi: 10.4314/tjpr.v14i7.13
Jadon, A., Bhadauria, M., and Shukla, S. (2007). Protective effect of Terminalia belerica Roxb. and gallic acid against carbon tetrachloride induced damage in albino rats. J. Ethnopharmacol. 109, 214–218. doi: 10.1016/j.jep.2006.07.033
Jaishree, V., and Badami, S. (2010). Antioxidant and hepatoprotective effect of swertiamarin from Enicostemma axillare against D-galactosamine induced acute liver damage in rats. J. Ethnopharmacol. 130, 103–106. doi: 10.1016/j.jep.2010.04.019
Jia, M. R., and Zhang, Y. (2016). Dictionary of Chinese Ethnic Medicine. Beijing: China Medical Science and Technology Press.
Jiang, S., Shi, Z., Li, C., Ma, C., Bai, X., and Wang, C. (2014). Hydroxysafflor yellow A attenuates ischemia/reperfusion-induced liver injury by suppressing macrophage activation. Int. J. Clin. Exp. Pathol. 7, 2595–2608.
Jin, L., Ge, Y. B., Luo, G. H., Ding, L., and Chen, Z. (2007). Protective effects of ethanol extract of Halenia elliptica on chemical hepatic injury in mice. Tradit. Chin. Drug Res. Pharmacol. 18, 345–346. doi: 10.3321/j.issn:1003-9783.2007.05.003
Jose, J. K., and Kuttan, R. (2000). Hepatoprotective activity of Emblica officinalis and Chyavanaprash. J. Ethnopharmacol. 72, 135–140. doi: 10.1016/S0378-8741(00)00219-1
Jun, M. S., Ha, Y. M., Kim, H. S., Jang, H. J., Kim, Y. M., Lee, Y. S., et al. (2011). Anti-inflammatory action of methanol extract of Carthamus tinctorius involves in heme oxygenase-1 induction. J. Ethnopharmacol. 133, 524–530. doi: 10.1016/j.jep.2010.10.029
Khosla, S., and Sharma, S. (2012). A short description on pharmacogenetic properties of Emblica officinalis. Spatula DD 2, 187–193. doi: 10.5455/spatula.20121112072137
Kim, H., and Song, M. J. (2013). Ethnomedicinal practices for treating liver disorders of local communities in the southern regions of Korea. Evid. Based Complement. Altern. Med. 2013:869176. doi: 10.1155/2013/869176
Kinoshita, S., Inoue, Y., Nakama, S., Ichiba, T., and Aniya, Y. (2007). Antioxidant and hepatoprotective actions of medicinal herb, Terminalia catappa L. from Okinawa Island and its tannin corilagin. Phytomedicine 14, 755–762. doi: 10.1016/j.phymed.2006.12.012
Kpodar, M. S., Karou, S. D., Katawa, G., Anani, K., Gbekley, H. E., Adjrah, Y., et al. (2016). An ethnobotanical study of plants used to treat liver diseases in the Maritime region of Togo. J. Ethnopharmacol. 181, 263–273. doi: 10.1016/j.jep.2015.12.051
Krishnaveni, M., and Mirunalini, S. (2010). Therapeutic potential of Phyllanthus emblica (amla): the ayurvedic wonder. J. Basic Clin. Physiol. Pharmacol. 21, 93–105.
Kumar, V., and Van Staden, J. (2015). A review of Swertia chirayita (Gentianaceae) as a traditional medicinal plant. Front. Pharmacol. 6:308. doi: 10.3389/fphar.2015.00308
Latief, U., Husain, H., Mukherjee, D., and Ahmad, R. (2016). Hepatoprotective efficacy of gallic acid during Nitrosodiethylamine-induced liver inflammation in Wistar rats. J. Basic Appl. Zool. 76, 31–41. doi: 10.1016/j.jobaz.2016.07.002
Lee, H. S., Won, N. H., Kim, K. H., Lee, H., Jun, W., and Lee, K. W. (2005). Antioxidant effects of aqueous extract of Terminalia chebula in vivo and in vitro. Biol. Pharm. Bull. 28, 1639–1644. doi: 10.1248/bpb.28.1639
Li, G., Wang, X. Y., Suo, Y. R., and Wang, H. L. (2014). Protective effect of seed oil of Herpetospermum pedunculosum against carbon tetrachloride-induced liver injury in rats. Saudi Med. J. 35, 981–987.
Li, S. S., Li, B., Zhang, S. W., Cao, L., Du, X. L., Mu, Z. J., et al. (2017). Comparison of pharmacological actions of crude extracts from six original species of Tibetan medicine Dida on mice with acute intrahepatic cholestasis. Tradit. Chin. Drug Res. Pharmacol. 28, 314–319. doi: 10.19378/j.issn.1003-9783.2017.03.011
Li, Y. H., Li, Y. F., and Yang, M. (2016). Protective effect of Dracocephalum tanguticum Maxim on liver injury of rats under altitude hypoxia. J. High Altitude Med. 26, 6–9.
Li, Y. Q., Ping, C., Li, G. L., Chen, W. Q., Zhao, P., and Zou, X. L. (2011). An investigation on deaths caused by malignancy in Tibet autonomous region, 2004~2005. China Cancer 20, 498–502.
Lin, G., Li, S. L., Li, M., Li, N., Chan, S. S. K., Chan, W. Y., et al. (2009). Qianliguang (Senecio scandens) safety dilemma: dose is the key? Planta Med. 75, 1107–1111. doi: 10.1055/s-0029-1185468
Liu, M. L., and Zhang, M. (2016). Modern research progress of Tibet medicine Bolengguazi. Pharm. Clin. Chin. Mater. Med. 7, 99–102.
Liu, X., Cui, C., Zhao, M., Wang, J., Luo, W., Yang, B., et al. (2008). Identification of phenolics in the fruit of emblica (Phyllanthus emblica L.) and their antioxidant activities. Food Chem. 109, 909–915. doi: 10.1016/j.foodchem.2008.01.071
Liu, Z. W., Chen, C. X., Jin, R. M., Shi, G. Q., Song, C. Q., and Hu, Z. B. (2002). Studies on liver-protection and promoting bile secretion of gentiopicroside. Chin. Tradit. Herb. Drugs 33, 49–52.
Lu, C., Yang, S., Hsia, S., Wu, C., and Yen, G. (2016). Inhibitory effects of Phyllanthus emblica L. on hepatic steatosis and liver fibrosis in vitro. J. Funct. Foods 20, 20–30. doi: 10.1016/j.jff.2015.10.012
Luo, H., Zhong, G. J., Yue, L. F., Wang, Q., Ma, L. N., and Luobu, Z. X. (2015). Traditional Tibetan medicine in China: a systematic overview of randomized clinical trials. Eur. J. Integr. Med. 7, 450–459. doi: 10.1016/j.eujim.2015.05.001
Lv, J., Wang, Y., Zhang, J., Yu, S., Wang, D., Zhu, H., et al. (2014). Anti-hepatitis B virus activities and absolute configurations of sesquiterpenoid glycosides from Phyllanthus emblica. Organ. Biomol. Chem. 12, 8764–8774. doi: 10.1039/C4OB01196A
Lv, L. L., Huang, W., Yu, X., Ren, H. Y., Yang, Q., and Sun, R. (2009). The reseach of hepatotoxicity damage on rats caused by crude extracts of total saikosaponins. Chin. J. Pharmacov. 6, 202–206.
Lv, P., Wei, L. X., Du, Y. Z., Yang, H. X., and Peng, M. (2010). Hepatoprotective and toxic characteristics of the whole herb of traditional Tibetan folk medicine Swertia mussotii Franch. J. Med. Plants Res. 4, 706–709. doi: 10.5897/JMPR10.114
Maiti, S., Chattopadhyay, S., Acharyya, N., Deb, B., and Hati, A. K. (2014). Emblica officinalis (amla) ameliorates arsenic-induced liver damage via DNA protection by antioxidant systems. Mol. Cell. Toxicol. 10, 75–82. doi: 10.1007/s13273-014-0009-8
Mirhoseini, M., Mohamadpour, M., and Khorsandi, L. (2012). Toxic effects of Carthamus tinctorius L. (Safflower) extract on mouse spermatogenesis. J. Assist. Reprod. Genet. 29, 457–461. doi: 10.1007/s10815-012-9734-x
Moradi, M. T., Asadi-Samani, M., Bahmani, M., and Shahrani, M. (2016). Medicinal plants used for liver disorders based on the ethnobotanical documents of Iran: a review. Drugs 26, 33.
Mukazayire, M.-J., Minani, V., Ruffo, C. K., Bizuru, E., Stévigny, C., and Duez, P. (2011). Traditional phytotherapy remedies used in Southern Rwanda for the treatment of liver diseases. J. Ethnopharmacol. 138, 415–431. doi: 10.1016/j.jep.2011.09.025
Mukherjee, S., Sur, A., and Maiti, B. R. (1997). Hepatoprotective effect of Swertia chirata on rat. Indian J. Exp. Biol. 35, 384–388.
Nabavi, S. F., Nabavi, S. M., Habtemariam, S., Moghaddam, A. H., Sureda, A., Jafari, M., et al. (2013). Hepatoprotective effect of gallic acid isolated from Peltiphyllum peltatum against sodium fluoride-induced oxidative stress. Ind. Crop. Prod. 44, 50–55. doi: 10.1016/j.indcrop.2012.10.024
Nagalekshmi, R., Menon, A., Chandrasekharan, D. K., and Nair, C. K. (2011). Hepatoprotective activity of Andrographis paniculata and Swertia chirayita. Food Chem. Toxicol. 49, 3367–3373. doi: 10.1016/j.fct.2011.09.026
Nagarkar, B., Kulkarni, R., Bhondave, P., Kasote, D., Kulkarni, O., Harsulkar, A., et al. (2013). Comparative hepatoprotective potential of Tinospora cordifolia, Tinospora sinensis and Neem-guduchi. Br. J. Pharm. Res. 3, 906–916. doi: 10.9734/BJPR/2013/4003
Ngamkitidechakul, C., Jaijoy, K., Hansakul, P., Soonthornchareonnon, N., and Sireeratawong, S. (2010). Antitumour effects of Phyllanthus emblica L.: induction of cancer cell apoptosis and inhibition of in vivo tumour promotion and in vitro invasion of human cancer cells. Phytother. Res. 24, 1405–1413. doi: 10.1002/ptr.3127
Nikolaev, S. M., Fedorov, A. V., Toropova, A. A., Razuvaeva, I., Sambueva, Z. G., and Lubsandorzhieva, P. B. (2014). Hepatoprotective effect of Hypecoum erectum extract on experimental D-galactosamine-induced damage of rat liver. Eksp. Klin. Farmakol. 77, 18–22.
Nobakht, M., Fattahi, M., Hoormand, M., Milanian, I., Rahbar, N., and Mahmoudian, M. (2000). A study on the teratogenic and cytotoxic effects of safflower extract. J. Ethnopharmacol. 73, 453–459. doi: 10.1016/S0378-8741(00)00324-X
Okuno, M., Tanaka, T., Komaki, C., Nagase, S., Shiratori, Y., Muto, Y., et al. (1998). Suppressive effect of low amounts of safflower and perilla oils on diethylnitrosamine-induced hepatocarcinogenesis in male F344 rats. Nutr. Cancer 30, 186–193. doi: 10.1080/01635589809514662
Oyagbemi, A. A., Omobowale, O. T., Asenuga, E. R., Akinleye, A. S., Ogunsanwo, R. O., and Saba, A. B. (2016). Cyclophosphamide-induced hepatotoxicity in wistar rats: the modulatory role of gallic acid as a hepatoprotective and chemopreventive phytochemical. Int. J. Prev. Med. 7:51. doi: 10.4103/2008-7802.177898
Panchabhai, T. S., Ambarkhane, S. V., Joshi, A. S., Samant, B. D., and Rege, N. N. (2008). Protective effect of Tinospora cordifolia, Phyllanthus emblica and their combination against antitubercular drugs induced hepatic damage: an experimental study. Phytother. Res. 22, 646–650. doi: 10.1002/ptr.2356
Pant, K., Gupta, P., Damania, P., Yadav, A. K., Gupta, A., Ashraf, A., et al. (2016). Mineral pitch induces apoptosis and inhibits proliferation via modulating reactive oxygen species in hepatic cancer cells. BMC Complem. Altern. Med. 16:148. doi: 10.1186/s12906-016-1131-z
Petrick, J. L., Kelly, S. P., Altekruse, S. F., McGlynn, K. A., and Rosenberg, P. S. (2016). Future of hepatocellular carcinoma incidence in the united states forecast through 2030. J. Clin. Oncol. 34, 1787–1794. doi: 10.1200/JCO.2015.64.7412
Pramyothin, P., Samosorn, P., Poungshompoo, S., and Chaichantipyuth, C. (2006). The protective effects of Phyllanthus emblica Linn. extract on ethanol induced rat hepatic injury. J. Ethnopharmacol. 107, 361–364. doi: 10.1016/j.jep.2006.03.035
Qin, X. J. (2004). Anti-HBV Study of Tibetan Medicines and Its Active Components. Dissertation/master's thesis, Chinese Academy of Military Medical Sciences: Beijing.
Qinghai Institute for Drug Control (1996). Chinese Tibetan Medicine Vol. 1–3. Shanghai: Shanghai Science and Technology Press.
Qinghai Institute of Plateau Biology (1975). Drug Illustration of Qinghai-Tibet Plateau Vol. 1-3. Xining: Qinghai People's Publishing Press.
Rasool, M. K., Sabina, E. P., Ramya, S. R., Preety, P., Patel, S., Mandal, N., et al. (2010). Hepatoprotective and antioxidant effects of gallic acid in paracetamol-induced liver damage in mice. J. Pharm. Pharmacol. 62, 638–643. doi: 10.1211/jpp.62.05.0012
Roeder, E., and Rengel, B. (1990). Pyrrolizidine alkaloids from Lithospermum erythrorhizon. Phytochemistry 29, 690–693. doi: 10.1016/0031-9422(90)85153-7
Sabjan, G., Sudarsanam, G., Reddy, D. D., and Rao, D. M. (2014). Ethno-botanical crude drugs used in treatment of liver diseases by Chenchu Tribes in Nallamalais, Andhra Pradesh, India. Am. J. Ethnomed. 1, 115–121.
Schantz, P. M., Wang, H., Qiu, J., Liu, F. J., Saito, E., Emshoff, A., et al. (2003). Echinococcosis on the Tibetan Plateau: prevalence and risk factors for cystic and alveolar echinococcosis in Tibetan populations in Qinghai Province, China. Parasitology 127, S109–S120. doi: 10.1017/S0031182003004165
Shen, B., Chen, H., Shen, C., Xu, P., Li, J., Shen, G., et al. (2015). Hepatoprotective effects of lignans extract from Herpetospermum caudigerum against CCl4-induced acute liver injury in mice. J. Ethnopharmacol. 164, 46–52. doi: 10.1016/j.jep.2015.01.044
Shen, B., Jin, S., Lv, Q., Jin, S., Yu, C., Yue, P., et al. (2013). Enhanced intestinal absorption activity and hepatoprotective effect of herpetrione via preparation of nanosuspensions using pH-dependent dissolving-precipitating/homogenization process. J. Pharm. Pharmacol. 65, 1382–1392. doi: 10.1111/jphp.12103
Sultana, S., Ahmed, S., and Jahangir, T. (2008). Emblica officinalis and hepatocarcinogenesis: a chemopreventive study in Wistar rats. J. Ethnopharmacol. 118, 1–6. doi: 10.1016/j.jep.2007.04.021
Tasduq, S. A., Kaisar, P., Gupta, D. K., Kapahi, B. K., Jyotsna, S., and Johri, R. K. (2005). Protective effect of a 50% hydroalcoholic fruit extract of Emblica officinalis against anti-tuberculosis drugs induced liver toxicity. Phytother. Res. 19, 193–197. doi: 10.1002/ptr.1631
Tasduq, S. A., Singh, K., Satti, N. K., Gupta, D. K., Suri, K. A., and Johri, R. K. (2006). Terminalia chebula (fruit) prevents liver toxicity caused by sub-chronic administration of rifampicin, isoniazid and pyrazinamide in combination. Hum. Exp. Toxicol. 25, 111–118. doi: 10.1191/0960327106ht601oa
Thilakchand, K. R., Mathai, R. T., Simon, P., Ravi, R. T., Baliga-Rao, M. P., and Baliga, M. S. (2013). Hepatoprotective properties of the Indian gooseberry (Emblica officinalis Gaertn): a review. Food Funct. 4, 1431–1441. doi: 10.1039/c3fo60237k
Tian, C., Zhang, T., Wang, L., Shan, Q., and Jiang, L. (2014). The hepatoprotective effect and chemical constituents of total iridoids and xanthones extracted from Swertia mussotii Franch. J. Ethnopharmacol. 154, 259–266. doi: 10.1016/j.jep.2014.04.018
Tian, S. Q. (1997). Commonly Used Tibetan Medicine Annals. Chengdu: Sichuan Science and Technology Press.
Wang, D., Huang, L., and Chen, S. (2013). Senecio scandens Buch.-Ham.: a review on its ethnopharmacology, phytochemistry, pharmacology, and toxicity. J. Ethnopharmacol. 149, 1–23. doi: 10.1016/j.jep.2013.05.048
Wang, F., Fan, J., Zhang, Z., Gao, B., and Wang, H. (2014). The global burden of liver disease: the major impact of China. Hepatology 60, 2099–2108. doi: 10.1002/hep.27406
Wang, J. B., Zhao, H. P., Zhao, Y. L., Jin, C., Liu, D. J., Kong, W. J., et al. (2011). Hepatotoxicity or hepatoprotection? Pattern recognition for the paradoxical effect of the Chinese herb Rheum palmatum L. in treating rat liver injury. PLoS ONE 6:e24498. doi: 10.1371/journal.pone.0024498
Wang, W. Q., Ye, C. P., Zeng, Y., Zhong, S. H., Gu, R., and Rang, J. (2016). Protective effect of n-butanol fraction from Tibetan medicine Brag-zhun on liver injury in mice induced by concanavalin A. J. Chin. Med. Mater. 39, 2849–2852. doi: 10.13863/j.issn1001-4454.2016.12.042
Wang, X. K. (2008). Study on the Liver Toxicity of Senecio scandens. Dissertation/master's thesis. China Academy of Chinese Medical Sciences, Beijing.
Wang, Y., and Zhu, L. Y. (2010). Experimental study on the treatment of rat hepatic fibrosis with saffron. Prog. Mod. Biomed. 10, 3244–3247. doi: 10.13241/j.cnki.pmb.2010.17.012
Wang, Z. H., Wang, X. M., and Liu, X. Q. (2008). Echinococcosis in China, a review of the epidemiology of Echinococcus spp. Ecohealth 5, 115–126. doi: 10.1007/s10393-008-0174-0
Wang, Z. W., Shao, J., Guo, M., Wang, R. Q., and Ren, Y. (2013a). Effect of total flavonoids and alkaloids from Meconopsis quintuplinervia on hepatic fibrosis in rat. Chin. Tradit. Pat. Med. 35, 1125–1128. doi: 10.3969/j.issn.1001-1528.2013.06.004
Wang, Z. W., Wang, R. Q., Guo, M., Shao, J., and Ren, Y. (2013b). Study on liver protection of total flavones of Meconopsis quintuplinervia from Gansu province in mice. Chin. J. Exp. Tradit. Med. Form. 19, 206–209. doi: 10.13422/j.cnki.syfjx.2013.02.006
Williams, R. (2006). Global challenges in liver disease. Hepatology 44, 521–526. doi: 10.1002/hep.21347
Wilson, E., Rajamanickam, G. V., Dubey, G. P., Klose, P., Musial, F., Saha, F. J., et al. (2011). Review on shilajit used in traditional Indian medicine. J. Ethnopharmacol. 136, 1–9. doi: 10.1016/j.jep.2011.04.033
Wu, S., Yue, Y., Tian, H., Li, Z., Li, X., He, W., et al. (2013). Carthamus red from Carthamus tinctorius L. exerts antioxidant and hepatoprotective effect against CCl4-induced liver damage in rats via the Nrf2 pathway. J. Ethnopharmacol. 148, 570–578. doi: 10.1016/j.jep.2013.04.054
Wu, T., Chang, M. J., Xu, Y. J., Li, X. P., Du, G., and Liu, D. (2013). Protective effect of Calculus Bovis Sativus on intrahepatic cholestasis in rats induced by alpha-naphthylisothiocyanate. Am. J. Chin. Med. 41, 1393–1405. doi: 10.1142/S0192415X13500936
Xizhu, J. C. (2010). Understanding of Tibetan medicine for liver and liver diseases. China Tibetol. 4, 155–158.
Xu, M. (2013). Resistance of Swertia mussotii Franch against immunological liver injury of mice. J. Northwest A&F Univ.-Nat. Sci. Ed. 41, 32–36. doi: 10.13207/j.cnki.jnwafu.2013.05.014
Yang, B., and Liu, P. (2014). Composition and biological activities of hydrolyzable tannins of fruits of Phyllanthus emblica. J. Agric. Food Chem. 62, 529–541. doi: 10.1021/jf404703k
Yang, C. X., Li, L. L., Xi, Y., and Qu, P. (2009). Protective effect of Crocus sativus on acute liver injury induced by CCl4 in mice. Mod. Tradit. Chin. Med. 29, 64–65. doi: 10.13424/j.cnki.mtcm.2009.02.035
Yang, J. S., and Chuchen, J. C. (1987). Diqing Tibetan Medicine. Kunming: Nationalities Publishing Press of Yunnan.
Yan, H. F., Dai, X. D., Fan, K. T., and Wang, Y. (2016). Research on medication regularity of traditional Chinese medicine based on hyperuricemia patents. Chin. Herbal Med. 8, 376–381. doi: 10.1016/S1674-6384(16)60066-7
Yan, M., Chen, Y., and Guan, Z. F. (2007). Prevalence and control strategy of fatty liver in Tibet Plateau. Chin. Prev. Med. 2, 116–119. doi: 10.3969/j.issn.1009-6639.2007.02.010
Ye, B. L., and Guo, P. J. (1998). Medicinal Animals of Qinghai-Tibet Plateau. Xi'an: Shaanxi Science and Technology Press.
Ye, C. P., Wang, W. Q., Cao, Y., Zhao, M. M., Zeng, Y., and Gu, R. (2017a). Protective effect of n-butanol extract from Tibetan Medicine Brag-zhun on the acute liver injury induced by acetaminophen in mice. J. Med. Pharm. Chin. Min. 5, 60–62. doi: 10.16041/j.cnki.cn15-1175.2017.05.038
Ye, C. P., Wang, W. Q., Zhao, M. M., Zeng, Y., and Gu, R. (2017b). Comparative study of antiulcerogenic and anti-liver injury effects of different fraction from Tibetan medicine Brag-zhun. Chin. J. Ethnomed. Ethnopharm. 26, 39–41.
Yin, L., Wei, L., Fu, R., Ding, L., Guo, Y., Tang, L., et al. (2014). Antioxidant and hepatoprotective activity of Veronica ciliata Fisch extracts against carbon tetrachloride-induced liver injury in mice. Molecules 19, 7223–7236. doi: 10.3390/molecules19067223
Yuan, H. L., Yang, M., Li, X. Y., You, R. H., Liu, Y., Zhu, J., et al. (2006). Hepatitis B virus inhibiting constituents from Herpetospermum caudigerum. Chem. Pharm. Bull. 54, 1592–1594. doi: 10.1248/cpb.54.1592
Yu, J. Q., Hang, W., Duan, W. J., Wang, X., Wang, D. J., and Qin, X. M. (2014). Two new anti-HBV lignans from Herpetospermum caudigerum. Phytochem. Lett. 10, 230–234. doi: 10.1016/j.phytol.2014.10.001
Yunnan Editorial Board of Local Chronicles (1995). Yunnan Province Annals (Medicine Annals). Kunming: Yunnan People's Publishing Press.
Yunnan University of TCM (2008). Illustrated Handbook of Ethnic Medicine of Shangri-La. Kunming: Yunnan Science and Technology Press.
Zhang, H. Y., Sha, L., Shi, R. B., and Zhang, L. Z. (2012). Determination of gallic acid, corilagin and ellagic acid in Fructus Phyllanthi and tannins effective fraction with RP-HPLC. Chin. J. Tradit. Chin. Med. Pharm. 27, 2834–2838.
Zhang, R. F., Zha, S., Yin, X., Bai, R. F., Li, Y., Tu, P. F., et al. (2016). Phytochemical and pharmacological progress on Tibetan medicine Hypecoi Erecti Herba and plants of Hypecoum L. Chin. Tradit. Herb. Drugs 47, 1217–1224.
Zhang, X. M., Li, M., Deng, Y., Ma, X. M., Zhang, Y. P., Li, X. Y., et al. (2011). Immunoregulation and protective effects of n-butanol extract of Swertia franchetiana on liver injury in rats. Chin. Tradit. Pat. Med. 33, 139–141. doi: 10.3969/j.issn.1001-1528.2011.01.036
Zhang, Y., Guo, J., Dong, H., Zhao, X., Zhou, L., Li, X., et al. (2011). Hydroxysafflor yellow A protects against chronic carbon tetrachloride-induced liver fibrosis. Eur. J. Pharmacol. 660, 438–444. doi: 10.1016/j.ejphar.2011.04.015
Zhang, Z., Bian, Q., Luo, P., and Sun, W. (2015). Ethnopharmacological, chemical, and pharmacological aspects of Halenia elliptica: a comprehensive review. Pharmacogn. Rev. 9, 114–119. doi: 10.4103/0973-7847.162114
Zhao, S. M., Li, H. C., Lou, H., Lu, X. X., Yu, X. F., Gao, D. H., et al. (2001). High prevalence of HBV in Tibet, China. Asian Pac. J. Cancer Prev. 2, 299–304.
Zheng, S. J., Xu, C., Yang, J., Huang, Y., Wen, Y. Z., Song, P., et al. (2017). In vitro anticancer screening of Tibetan medicines. J. Cent. China Norm. Univ.-Nat. Sci. Ed. 51, 328–334. doi: 10.3969/j.issn.1000-1190.2017.03.011
Zhou, C. F., Gao, G. J., and Liu, Y. (2015). Advances in studies on bear bile powder. Chin. J. Chin. Mater. Med. 40, 1252–1258. doi: 10.4268/cjcmm20150706
Zhou, G., Chen, Y., Liu, S., Yao, X., and Wang, Y. (2013). In vitro and in vivo hepatoprotective and antioxidant activity of ethanolic extract from Meconopsis integrifolia (Maxim.) Franch. J. Ethnopharmacol. 148, 664–670. doi: 10.1016/j.jep.2013.05.027
Zhou, J. Y., Yin, Z. Y., Wang, S. Y., Yan, J. H., Zhao, Y. L., Wu, D., et al. (2012). Influence of bear bile on rat hepatocarcinoma induced by diethylnitrosamine. Acta. Pharm. Sin. 47, 1483–1488. doi: 10.16438/j.0513-4870.2012.11.011
Zhou, N., Geng, C., Huang, X., Ma, Y., Zhang, X., Wang, J., et al. (2015). Anti-hepatitis B virus active constituents from Swertia chirayita. Fitoterapia 100, 27–34. doi: 10.1016/j.fitote.2014.11.011
Zhou, X., Tang, L., Xu, Y., Zhou, G., and Wang, Z. (2014). Towards a better understanding of medicinal uses of Carthamus tinctorius L. in traditional Chinese medicine: a phytochemical and pharmacological review. J. Ethnopharmacol. 151, 27–43. doi: 10.1016/j.jep.2013.10.050
Zhu, J. X., Zhang, H. Y., Li, X. W., Wei, C. H., Li, M., Yao, X. L., et al. (2016). Comparison of protective effects of two kinds of lagotises against mice liver injury induced by carbon tetrachloride. Tradit. Chin. Drug Res. Pharmacol. 27, 210–214. doi: 10.3969/j.issn.1003-9783.2016.02.012
Keywords: traditional Tibetan medicine, natural medicines, liver diseases, hepatitis, Carthamus tinctorius, Brag-zhun, Swertia chirayita
Citation: Li Q, Li H-J, Xu T, Du H, Huan Gang C-L, Fan G and Zhang Y (2018) Natural Medicines Used in the Traditional Tibetan Medical System for the Treatment of Liver Diseases. Front. Pharmacol. 9:29. doi: 10.3389/fphar.2018.00029
Received: 08 August 2017; Accepted: 10 January 2018;
Published: 30 January 2018.
Edited by:
Marco Leonti, Università degli studi di Cagliari, ItalyReviewed by:
François Chassagne, IRD UMR152 Pharmacochimie et Biologie Pour le Développement, FranceCopyright © 2018 Li, Li, Xu, Du, Huan Gang, Fan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Fan, ZmFuZ2FuZzExMTFAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.