- 1Center for Respiratory Diseases, JCHO Hokkaido Hospital, Sapporo, Japan
- 2Clinical Research and Medical Innovation Center, Hokkaido University Graduate School of Medicine, Sapporo, Japan
- 3Department of Respiratory Medicine, Obihiro-Kosei General Hospital, Obihiro, Japan
- 4Department of Respiratory Medicine, National Hospital Organization Asahikawa Medical Center, Asahikawa, Japan
- 5Department of Respiratory Medicine, Hirosaki University Graduate School of Medicine, Hirosaki, Japan
- 6Respiratory Center, Asahikawa Medical University Hospital, Asahikawa, Japan
- 7Department of Pulmonary Medicine, Sendai Kousei Hospital, Sendai, Japan
- 8Department of Pulmonary Medicine, Fukushima Medical University, Fukushima, Japan
- 9Department of Respiratory Medicine, Iwamizawa Municipal General Hospital, Iwamizawa, Japan
- 10Division of Pulmonary Medicine, Allergy, and Rheumatology, Department of Internal Medicine, Iwate Medical University, Morioka, Japan
- 11Department of Medical Oncology, Hokkaido University Graduate School of Medicine, Sapporo, Japan
- 12Respiratory Center, KKR Sapporo Medical Center, Sapporo, Japan
- 13First Department of Medicine, Hokkaido University Hospital, Sapporo, Japan
The herbal medicine rikkunshito has the potential to improve chemotherapy-induced nausea and vomiting (CINV) by stimulating ghrelin secretion. We aimed to evaluate the efficacy and safety of rikkunshito in preventing CINV for patients with lung cancer. Two separate prospective, randomized, phase II parallel design studies were conducted in patients with lung cancer. Fifty-eight and sixty-two patients scheduled to receive highly emetogenic chemotherapy (HEC) and moderately emetogenic chemotherapy (MEC), respectively, were randomized 1:1 to receive either standard antiemetic therapy in accordance with international guidelines (S group) or standard antiemetic therapy plus oral rikkunshito (R group). The primary endpoint was overall complete response (CR)—that is, no emesis and rescue medication in the first 120 h post-chemotherapy. Secondary endpoints included CR in the acute (0–24 h) and delayed (>24–120 h) phases and safety. Fifty-seven patients (S group, 28; R group, 29) receiving HEC and sixty-two patients (S group, 30; R group, 32) receiving MEC with comparable characteristics were evaluated. The CR rates were similar across the S and R groups for the HEC study in the overall (67.9% vs. 62.1%), acute (96.4% vs. 89.6%), and delayed (67.9% vs. 62.1%) phases, respectively, and for the MEC study in the overall (83.3% vs. 84.4%), acute (100% vs. 100%), and delayed (83.3% vs. 84.4%) phases, respectively. No severe adverse events were observed. Although rikkunshito was well tolerated, it did not demonstrate an additional preventative effect against CINV in lung cancer patients receiving HEC or MEC.
Clinical Trial Registry Information: This study is registered with the University Hospital Medical Information Network (UMIN) Clinical Trial Registry1, identification numbers UMIN 000014239 and UMIN 000014240.
Introduction
Some of the most prevalent and concerning effects of cancer treatment are chemotherapy-induced nausea and vomiting (CINV) (Hofman et al., 2004; Molassiotis et al., 2008). CINV leads to reduced chemotherapy adherence rates, deteriorated of function and quality of life (QOL), and aggravated anxiety and depression (Schwartzberg, 2007; Hesketh, 2008). Therefore, circumvention of CINV is a critical element of supportive care in cancer.
In recent years, the incidence of CINV has been decreasing through the improvement of antiemetic agents (Warr et al., 2005; Botrel et al., 2011; Hesketh et al., 2014) and refinements of antiemetic guidelines by the American Society of Clinical Oncology (ASCO) (Hesketh et al., 2016), Multinational Association of Supportive Care in Cancer/European Society of Medical Oncology (MASCC/ESMO) (Jordan et al., 2011), National Comprehensive Cancer Network (NCCN) (National Comprehensive Cancer Network [NCCN], 2016), and Japan Society of Clinical Oncology (JSCO) (Japan Society of Clinical Oncology [JSCO], 2015). However, CINV still occurs in approximately half of patients who receive chemotherapy for cancer (Aapro et al., 2012), and additional CINV prevention methods are required.
The emetogenicity of anti-cancer agents has been categorized according to their risk levels in the guidelines set by ASCO (Hesketh et al., 2016), MASCC/ESMO (Jordan et al., 2011), NCCN (National Comprehensive Cancer Network [NCCN], 2016), and JSCO (Japan Society of Clinical Oncology [JSCO], 2015). A chemotherapy regimen that is associated with emesis in ≥90% of patients is considered to have high emetic risk (highly emetogenic chemotherapy, HEC), regimens that cause emesis in 30–90% of patients are considered to have a moderate emetic risk (moderately emetogenic chemotherapy, MEC), those causing emesis in 10–30% of patients have a low emetic risk, and those causing emesis in less than 10% of patients have a minimum emetic risk. In all clinical guidelines, cisplatin (CDDP) is classified as HEC and carboplatin (CBDCA) as MEC. With these regimens, the patients are at risk of developing CINV for up to 120 h after receiving chemotherapy; this 120-h watch period for CINV incorporates an acute phase (0–24 h), delayed phase (>24–120 h), and overall phase (0–120 h).
The treatments recommended by the international antiemetic guidelines of ASCO (Hesketh et al., 2016), MASCC/ESMO (Jordan et al., 2011), NCCN (National Comprehensive Cancer Network [NCCN], 2016), and JSCO (Japan Society of Clinical Oncology [JSCO], 2015) for the prevention of CINV that is associated with HEC are a neurokinin 1 receptor antagonist (NK-1-RA), a serotonin receptor antagonist (5HT3-RA), and dexamethasone (DEX); those for MEC include 5HT3-RA, DEX, and (optionally) NK-1-RA.
Herbal medicines, which were systemically popularized in Japan in the 16th century, have a wide range of indications aimed at maintaining QOL in patients rather than curing them (Mizukami et al., 2009). Herbal medicines are thus intended to boost the body’s own healing power (i.e., immune system) and help restore its natural balance. Rikkunshito, an herbal medicine, has been shown to improve upper gastrointestinal symptoms and anorexia (Tomono et al., 2006; Ohno et al., 2011; Arai et al., 2012; Tominaga et al., 2012); therefore, we hypothesized that this herbal medicine can reduce CINV. Rikkunshito was approved in Japan only as a fixed dose of 7.5 g (2.5 g three times a day).
To the best of our knowledge, there are no prospective studies on the efficacy of herbal medicines in preventing CINV. Herein, we describe the results of two separate prospective, randomized, phase II parallel design studies that evaluated the efficacy and safety of rikkunshito in the prevention of CINV in patients with lung cancer receiving CDDP-based HEC (HOT1402) and CBDCA-based MEC (HOT1403).
Materials and Methods
This study comprised two separate prospective, randomized phase II Hokkaido Lung Cancer Study Group Trial (HOT) investigations that were conducted in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice guidelines (World Medical Association, 1997), and CONSORT guidelines. The protocol was approved by the institutional review boards of all participating institutions, and all patients provided written informed consent before treatment. This study was registered at the University Hospital Medical Information Network (UMIN) Clinical Trials Registry as UMIN000014239 (HOT1402) and UMIN000014240 (HOT1403).
Patient Eligibility
Eligible patients met the following criteria: histologic or cytologic confirmation of lung cancer; age ≥20 years; an Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 0–2; treatment with CDDP-based HEC (HOT1402) or CBDCA-based MEC (HOT1403); adequate bone marrow function (leukocyte count ≥3,000/mm3, neutrophil count ≥1,500/mm3, platelet count ≥100,000/mm3, and hemoglobin content ≥9.0 g/dL); adequate function in other organs (total bilirubin concentration ≤1.5 mg/dL, aspartate transaminase and alanine transaminase levels ≤100 IU/L, and creatinine clearance ≥60 mL/min [HEC] or ≥50 mL/min [MEC]); PaO2 ≥60 Torr, or SpO2 ≥92%; and a life expectancy of 2 months or more. Patients who previously used rikkunshito or had active infectious diseases, serious medical complications (e.g., active peptic ulcer, heart disease, diabetes mellitus, cerebrovascular disease, neuropsychiatric disorder), had symptomatic brain metastasis, were lactating or pregnant, or had active concomitant malignancies were ineligible for the study.
Treatment Plan
Eligible patients were randomized in a 1:1 ratio using a minimization method and were assigned to receive either (1) standard antiemetic therapy in accordance with the ASCO (Hesketh et al., 2016), NCCN (Jordan et al., 2011), MASCC/ESMO (National Comprehensive Cancer Network [NCCN], 2016), or JSCO (Japan Society of Clinical Oncology [JSCO], 2015) guidelines at the investigators’ discretion (the S group) or (2) standard antiemetic therapy plus 2.5 g of oral rikkunshito three times a day on days 1–7 (the R group). The stratification factors included sex, habitual alcohol intake (yes or no), and palonosetron use (yes or no) in the HEC study, and sex, habitual alcohol intake (yes or no), and NK-1-RA use (yes or no) in the MEC study.
Assessment
The efficacy and safety of the antiemetic therapy were evaluated during the 7 days following the administration of the HEC or MEC in the first cycle. The patients recorded episodes of emesis, nausea ratings, and rescue medications taken during the first 120 h, as well as any impairment of eating habits during the first 7 days post-chemotherapy, in a diary. Patients assessed their nausea with a 100-mm horizontal visual analog scale (VAS); scores of ≤5 and ≤25 mm on the VAS scale indicated no nausea or no significant nausea, respectively. The patients also recorded the ratio of dietary intake with a 100-mm horizontal VAS. Adverse events related to the antiemetic treatment were surveyed by the investigators according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Objectives
The primary endpoint was the complete response (CR; i.e., no emesis and no rescue medication) rate in the overall post-chemotherapy phase. The secondary endpoints were (1) the CR rate in the acute and delayed phases; (2) complete protection (CP; i.e., no emesis, no significant nausea, and no rescue medication) rate in the acute, delayed, and overall phases; (3) total control (TC; i.e., no emesis, no nausea, and no rescue medication) rate in the acute, delayed, and overall phases; (4) dietary intake during the 7 days post-chemotherapy; and (5) safety.
Statistical Analysis
These two prospective, randomized phase II studies were designed to assess antiemetic efficacy with regard to the CR rate during the overall phase. The primary endpoint was CR rate in the overall phase among all per-protocol patients. The sample size was determined according to a one-arm binomial design devised by the Southwest Oncology Group. In the HEC study, we estimated the patient accrual number to be 27 assuming that a CR of 80% in eligible patients would indicate potential usefulness while a CR of 55% would be the lower limit of interest (Hesketh et al., 2003; Poli-Bigelli et al., 2003; Longo et al., 2011; Suzuki et al., 2016), with α = 0.05 and β = 0.20. To allow for patient dropouts, we aimed for the enrollment of 58 patients in the HEC study. In the MEC study, the estimated accrual number was 29 patients, assuming that a CR of 75% in eligible patients would indicate potential usefulness while a CR of 50% would be the lower limit of interest (Herrstedt et al., 2009; Aapro et al., 2010; Rapoport et al., 2010), with α = 0.05 and β = 0.20. We aimed to enroll 62 patients in the MEC study to allow for dropouts. Categorical variables were analyzed by using the χ2 or Fisher’s exact tests. All P-values are 2-sided; a P-value of 0.05 indicated statistical significance. Statistical analyses were performed by using Excel 2011 (Microsoft) with the add-in software Statcel 4 (OMS Publishing Inc., Saitama, Japan).
Results
Patient Characteristics
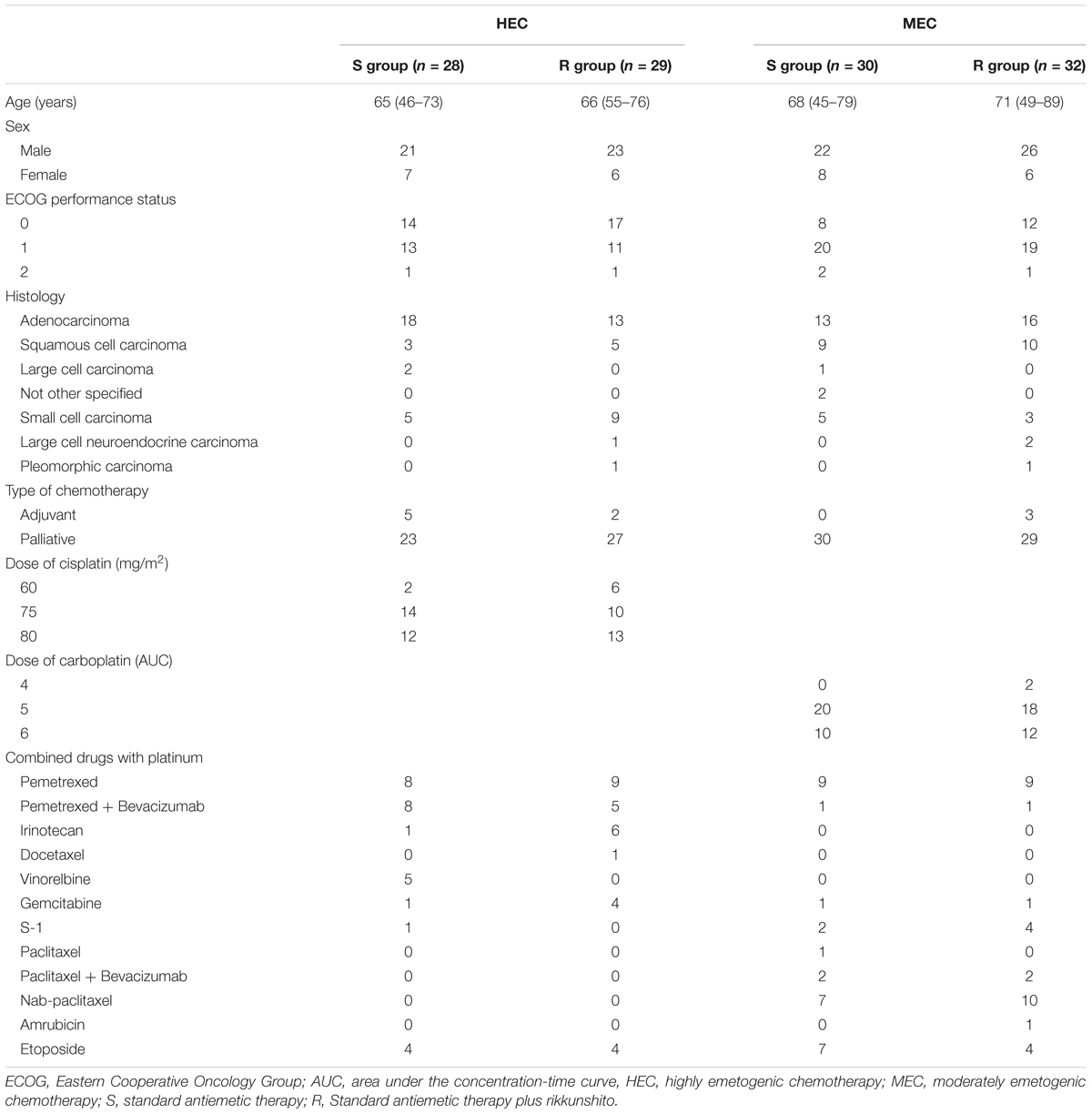
Between July 2014 and January 2016, 58 patients who received HEC (29 each in the S and R groups) and 62 patients who received MEC (30 in the S group and 32 in the R group) were enrolled. One patient from the S group of the HEC study was excluded because of disease progression before the antiemetic treatment; hence, only 57 patients who received HEC were evaluable (Figures 1, 2). All patients enrolled in the two phase II studies had a good nutritional status, and had neither muscle wasting nor weight loss. The patients’ characteristics are summarized in Table 1. The baseline characteristics of the study subjects were similar between the groups in both the HEC and MEC studies. In the HEC study, the median patient age was 65 years (range 46–76 years), of whom 77.2% were men and most (96.5%) had a good ECOG PS (0–1). The most common histology was adenocarcinoma (54.4%), followed by small cell carcinoma (24.6%), and squamous cell carcinoma (14.0%). Most of the patients (87.7%) were treated with palliative chemotherapy. The combined chemotherapeutic agents with CDDP were as follows: pemetrexed (29.8%), pemetrexed plus bevacizumab (22.8%), etoposide (14.0%), vinorelbine (8.8%), irinotecan (12.3%), gemcitabine (8.8%), docetaxel (1.8%), and S-1 (1.8%). As for the MEC study, the median patient age was 70 years (range 45–89 years); 77.4% were men and most (95.2%) had a good ECOG PS of 0–1. The most common histology was adenocarcinoma (46.8%), followed by squamous cell carcinoma (30.6%) and small cell carcinoma (12.9%). Most of the patients (95.2%) were treated with palliative chemotherapy. The combined chemotherapeutic agents with carboplatin were as follows: pemetrexed (29.0%), nab-paclitaxel (27.4%), etoposide (17.7%), S-1 (9.7%), paclitaxel plus bevacizumab (6.5%), pemetrexed plus bevacizumab (3.2%), gemcitabine (3.2%), paclitaxel (1.6%), and amrubicin (1.6%).
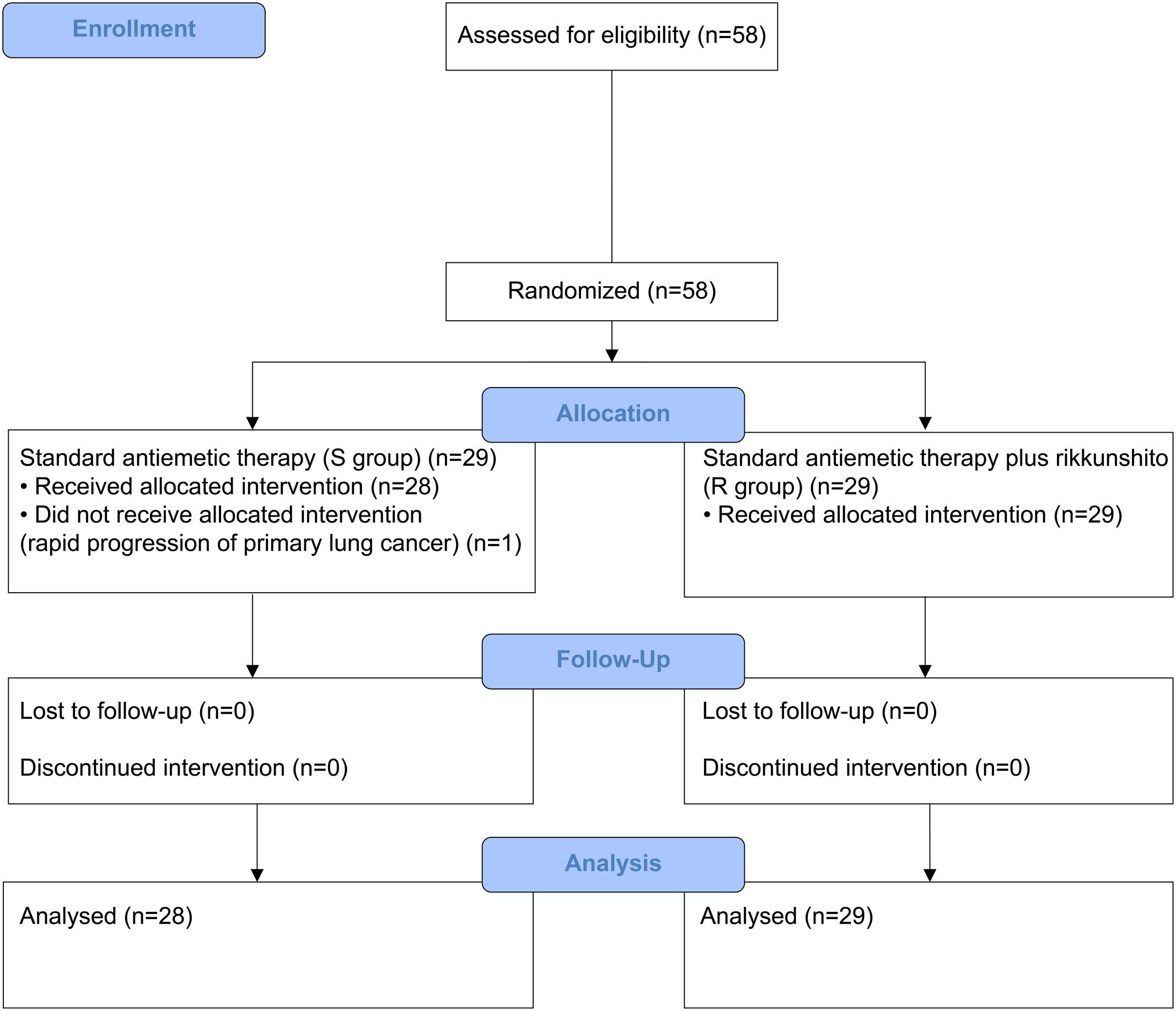
FIGURE 1. CONSORT diagram showing patients disposition in the highly emetogenic chemotherapy (HEC) study.
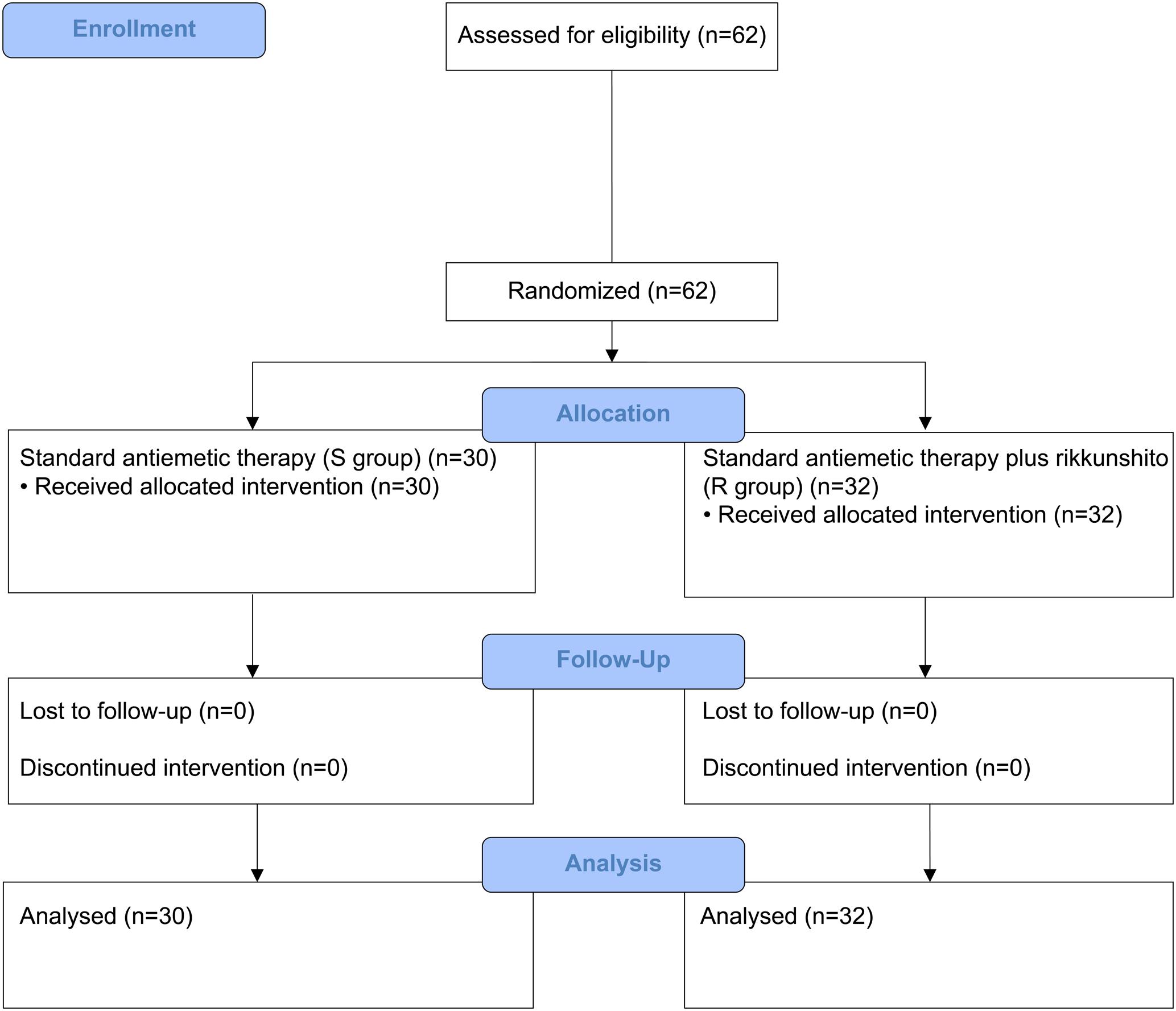
FIGURE 2. CONSORT diagram showing patients disposition in the moderately emetogenic chemotherapy (MEC) study.
Efficacy
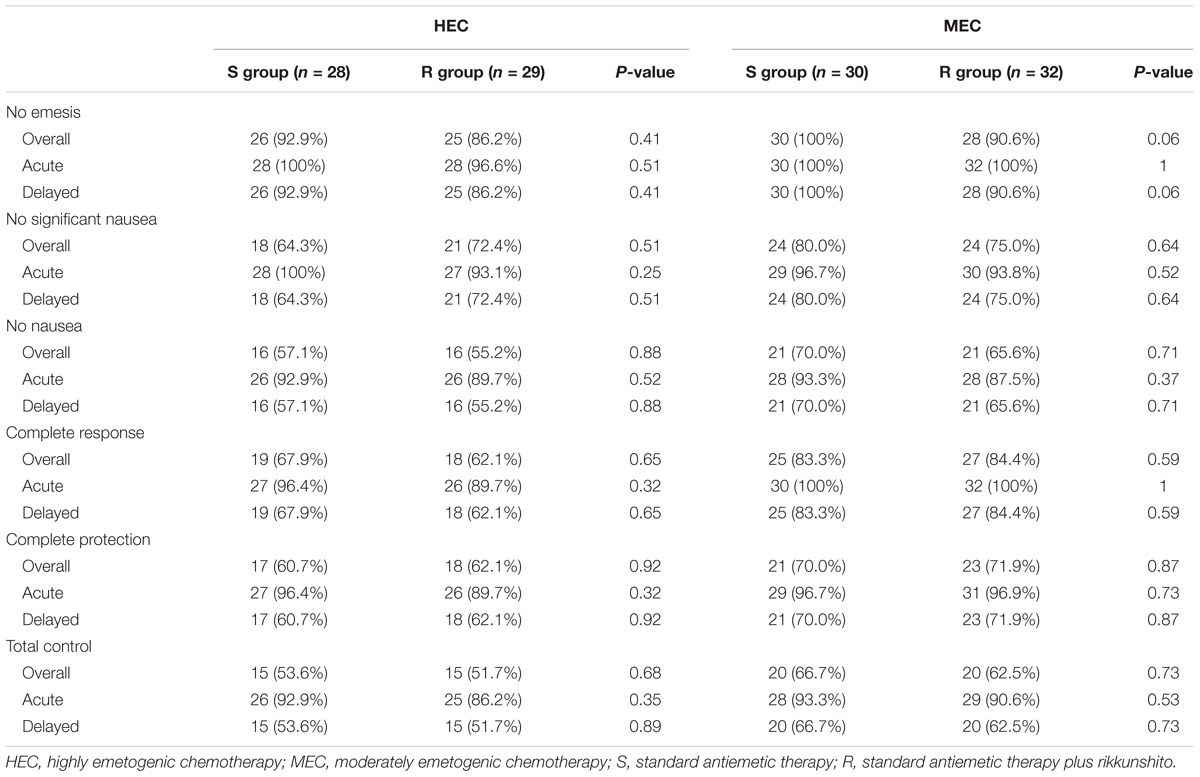
In the HEC study, the CR rates in the overall phase were 67.9% (95% confidence interval [CI], 47.7–84.1) in S group and 62.1% (95% CI, 42.3–79.3) in R group (P = 0.65), which did not meet the primary endpoint. In the MEC study, the CR rates in the overall phase were 83.3% (95% CI, 65.3–94.4) in S group and 84.4% (95% CI, 67.2–94.7) in R group (P = 0.59), which met the primary endpoint (Table 2). In the HEC and MEC studies, rikkunshito did not exhibit additional improvement on CR rates in the overall phase. Furthermore, rikkunshito did not improve CR rates for the acute and delayed phases in either the HEC or MEC study. Rikkunshito administration also did not improve CP or TC rates in the acute, delayed, or overall phases in the HEC and MEC study. The median dietary intakes were also similar between the S and R groups (89 and 89 mm in the HEC study, and 90 and 91 mm in the MEC study, respectively). Subgroup analyses according to sex, age, alcohol intake, smoking status, ECOG PS, body mass index, motion sickness, dexamethasone dose, treatment line, histology, CDDP, or CBDCA dose, and combined drugs revealed no additional benefit for rikkunshito administration on CR rates in the acute, delayed, or overall phases (data not shown).
Safety
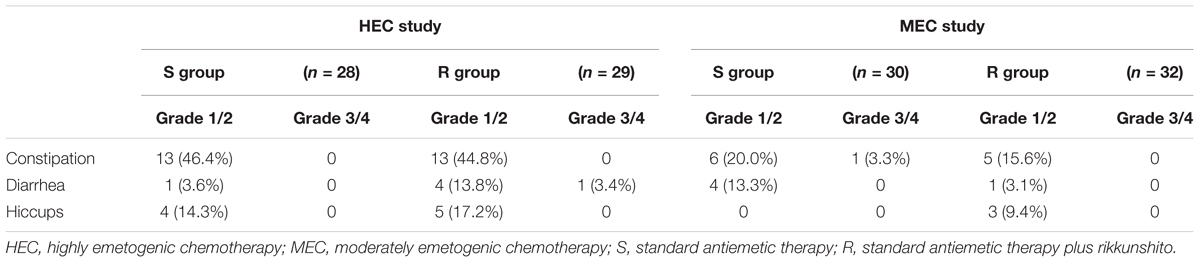
Rikkunshito was well tolerated, with frequencies of treatment-related adverse events similar to those reported in the S groups. Most adverse events were mild and were associated with the patients’ cancer and/or chemotherapy treatment. The most common treatment-related adverse events were constipation, diarrhea, and hiccups. No severe adverse events attributed to antiemetic treatments were reported in either study (Table 3).
Discussion
To the best of our knowledge, this study was the first prospective trial designed to evaluate the efficacy and safety of herbal medicine for the prevention of CINV in patients with lung cancer receiving HEC and MEC. Herbal medicines are inexpensive dietary supplements that can boost the body’s immune system and have the potential to improve anorexia and CINV in cancer patients. The orexigenic hormone ghrelin is a 28-amino acid peptide and has an n-octanoyl modification on Ser3; it was first isolated from rat stomachs and was found to be an endogenous ligand for the receptor of the growth hormone secretagogue. Additionally, ghrelin also has an intense appetite-enhancing effect (Kojima et al., 1999). A decrease in the concentration of circulating ghrelin along with appetite loss has been observed in CDDP-treated rats (Takeda et al., 2008). Administration of exogenous ghrelin peripherally improves anorexia (Liu et al., 2006; Takeda et al., 2008) and vomiting (Rudd et al., 2006) induced by CDDP.
Rikkunshito is an herbal medicine prepared by combining eight herbal medicines: Atractylodis lanceae rhizoma, Ginseng radix, Pinelliae tuber, Hoelen, Zizyphi fructus, Aurantii nobilis pericarpium, Glycyrrhizae radix, and Zingiberis rhizoma (Suzuki et al., 2009; Mochiki et al., 2010). Rikkunshito stimulates ghrelin secretion from the stomach and the response to it in the hypothalamus (Takeda et al., 2008; Fujitsuka et al., 2009). It is widely used in Japan for the treatment of upper gastrointestinal symptoms in patients with functional dyspepsia (Arai et al., 2012), gastroesophageal reflux disease (Tominaga et al., 2012), and chemotherapy-induced nausea for cancer patients (Tomono et al., 2006; Ohno et al., 2011). Based on these findings, we posited that rikkunshito can improve CINV and conducted this prospective study to evaluate its efficacy for the prevention of CINV in patients with lung cancer who were receiving HEC and MEC.
In the HEC study, the CR rates in the overall phase were 67.9% in the S group and 62.1% in the R group, which did not meet the primary endpoint goals. These results were inferior to previous phase III study results that revealed overall CR rates of 59–77% (Hesketh et al., 2003; Poli-Bigelli et al., 2003; Longo et al., 2011; Suzuki et al., 2016). On the other hand, the overall phase CR rates in our MEC study were 83.3% in the S group and 84.4% in R the group, which met the primary endpoint goals. These results were superior compared to previous phase III trial results that yielded overall CR rates of 54–74% (Herrstedt et al., 2009; Aapro et al., 2010; Rapoport et al., 2010). Despite having the CINV symptoms, patients enrolled in these two studies were diligent with fulfilling the requirements for daily-recommended nutrients. Therefore, the patients had well to excellent food intake throughout the course of these two studies.
In the present study, rikkunshito was safe and manageable; however, it did not demonstrate any additional benefits beyond those of standard antiemetic regimens used for the prevention of CINV in patients receiving HEC and MEC for lung cancer. Moreover, rikkunshito did not show additional benefits when performing subset analyses of various clinical factors. One possibility that remains to be investigated is that rikkunshito did not sufficiently increase the plasma levels of acylated ghrelin (the active form of ghrelin) in the patients included in this study. For ethical reasons, we did not examine the level of ghrelin in each patient.
The frequency of CINV in patients receiving HEC remained high, which is a challenge that remains to be solved. Olanzapine, which inhibits multiple neurotransmitters, has been reported to produce favorable results and could be an attractive treatment option for CINV prevention (Navari et al., 2011; Hashimoto et al., 2016). In the present study, olanzapine was not used as part of the standard antiemetic regimen, but it was used as a rescue medication in one patient who was receiving HEC.
Conclusion
Rikkunshito was well tolerated; however, it did not show any additional benefits beyond those of standard antiemetic regimens for the prevention of CINV in patients with lung cancer who were receiving HEC and MEC. Further investigation is required to improve CINV control, especially in patients receiving HEC.
Author Contributions
Conceptualization and design by TH. Data collection by all authors. Data analysis and interpretation done by TH and TA. Manuscript written by TH. Critical review and revisions of manuscript done by all the authors. Finally, all authors have agreed with the content and approve of the manuscript for submission.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
Aapro, M., Fabi, A., Nolè, F., Medici, M., Steger, G., Bachmann, C., et al. (2010). Double-blind, randomized, controlled study of the efficacy and tolerability of palonosetron plus dexamethasone for 1 day with or without dexamethasone on days 2 and 3 in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy. Ann. Oncol. 21, 1083–1088. doi: 10.1093/annonc/mdp584
Aapro, M., Molassiotis, A., Dicato, M., Peleáz, I., Lescure, R. Á., Pastorelly, D., et al. (2012). The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER). Ann. Oncol. 23, 1986–1992. doi: 10.1093/annonc/mds021
Arai, M., Matsumura, T., Tsuchiya, N., Sadakane, C., Inami, R., Suzuki, T., et al. (2012). Rikkunshito improves the symptoms in patients with functional dyspepsia, accompanied by an increase in the level of plasma ghrelin. Hepatogastroenterology 59, 62–66. doi: 10.5754/hge11246
Botrel, T. E. A., Clark, O. A. C., Clark, L., Paladini, L., Faleiros, E., and Pegoretti, B. (2011). Efficacy of palonosetron (PAL) compared to other serotonin inhibitors (5-HT3R) in preventing chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately or highly emetogenic (MoHE) treatment: systematic review and meta-analysis. Support. Care Cancer 19, 823–832. doi: 10.1007/s00520-010-0908-8
Fujitsuka, N., Asakawa, A., Hayashi, M., Sameshima, M., Amitani, H., Kojima, S., et al. (2009). Selective serotonin reuptake inhibitors modify physiological gastrointestinal motor activities via 5-HT2c receptor and acyl ghrelin. Biol. Psychiatry 65, 748–759. doi: 10.1016/j.biopsych.2008.10.031
Hashimoto, H., Yanai, T., Nagashima, K., Tsuda, N. O., Horinouichi, H., and Takiguchi, T. et al. (2016). A double-blind randomized phase II study of 10 versus 5 mg olanzapine for emesis induced by highly emetogenic chemotherapy with cisplatin. J. Clin. Oncol. 34(Suppl.):10111. doi: 10.1200/JCO.2016.34.15-suppl.10111
Herrstedt, J., Apornwirat, W., Shaharyar, A., Aziz, Z., Roila, F., Belle, S. V., et al. (2009). Phase III trial of casopitant, a novel neurokinin-1 receptor antagonist, for the prevention of nausea and vomiting in patients receiving moderately emetogenic chemotherapy. J. Clin. Oncol. 27, 5363–5369. doi: 10.1200/JCO.2009.21.8511
Hesketh, P. J. (2008). Chemotherapy-induced nausea and vomiting. N. Engl. J. Med. 358, 2482–2494. doi: 10.1056/NEJMra0706547
Hesketh, P. J., Bohlke, K., Lyman, G. H., Basch, E., Chesney, M., Clark-Snow, R. A., et al. (2016). Antiemetics: American society of clinical oncology focused guideline update. J. Clin. Oncol. 34, 381–386. doi: 10.1200/JCO.2015.64.3635
Hesketh, P. J., Grunberg, S. M., Gralla, R. J., Warr, D. G., Roila, F., de Wit, R., et al. (2003). The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin–the aprepitant Protocol 052 Study Group. J. Clin. Oncol. 21, 4112–41129. doi: 10.1200/JCO.2003.01.095
Hesketh, P. J., Rossi, G., Rizzi, G., Palmas, M., Alyasova, A., Bondarenko, I., et al. (2014). Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann. Oncol. 25, 1340–1346. doi: 10.1093/annonc/mdu110
Hofman, M., Morrow, G. R., Roscoe, J. A., Hickok, J. T., Mustian, K. M., Moore, D. F., et al. (2004). Cancer patients’ expectations of experiencing treatment related side effects: a university of rochester cancer center-community clinical oncology program study of 938 patients from community practices. Cancer 101, 851–857. doi: 10.1002/cncr20423
Japan Society of Clinical Oncology [JSCO] (2015). Guidelines for Appropriate Use of Antiemetic Drugs, Version 2. Tokyo: Japan Society of Clinical Oncology.
Jordan, K., Roila, F., Molassiotis, A., Maranzano, E., Clark-Snow, R. A., and Feyer, P. (2011). Antiemetics in children receiving chemotherapy MASCC/ESMO guideline update 2009. Support. Care Cancer 9, S37–S42. doi: 10.1007/s00520-010-0994-7
Kojima, M., Hosoda, H., Date, Y., Nakazato, M., Matsuo, H., and Kangawa, K. (1999). Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660. doi: 10.1038/45230
Liu, Y. L., Malik, N. M., Sanger, G. J., and Andrews, P. L. R. (2006). Ghrelin alleviates cancer chemotherapy-associated dyspepsia in rodents. Cancer Chemother. Pharmacol. 58, 326–333. doi: 10.1007/s00280-005-0179-0
Longo, F., Mansueto, G., Lapadula, V., De Sanctis, R., Quadrini, S., Grande, R., et al. (2011). Palonosetron plus 3-day aprepitant and dexamethasone to prevent nausea and vomiting in patients receiving highly emetogenic chemotherapy. Support. Care Cancer 19, 1159–1164. doi: 10.1007/s00520-010-0930-x
Mizukami, K., Asada, T., Kinoshita, T., Tanaka., K., Sonohara, K., et al. (2009). A randomized cross-over study of a traditional Japanese medicine (kampo), yokukansan, in the treatment of the behavioural and psychological symptoms of dementia. Int. J. Neuropsychopahrmacol. 12, 191–199. doi: 10.1017/S146114570800970X
Mochiki, E., Yanai, M., Ohno, T., and Kuwano, H. (2010). The effect of traditional Japanese medicine (Kampo) on gastrointestinal function. Surg. Today 40, 1105–1111. doi: 10.1007/s0095-010-43888
Molassiotis, A., Saunders, M. P., Valle, J., Wilson, G., Lorigan, P., Wardley, A., et al. (2008). A prospective observational study of chemotherapy-related nausea and vomiting in routine practice in a UK cancer centre. Support. Care Cancer 16, 201–208. doi: 10.1007/s00520-007-0343-7
National Comprehensive Cancer Network [NCCN] (2016). Clinical Practice Guidelines in Oncology: Antiemesis, Version 2. Available at: https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf [accessed April 7, 2017].
Navari, R. M., Gray, S. E., and Kerr, A. C. (2011). Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J. Support. Oncol. 9, 188–195. doi: 10.1016/j.suponc.2011.05.002
Ohno, T., Yanai, M., Ando, H., Toyomasu, Y., Ogawa, A., Morita, H., et al. (2011). Rikkunshito, a traditional Japanese medicine, suppresses cisplatin-induced anorexia in humans. Clin. Exp. Gastroenterol. 4, 291–296. doi: 10.2147/CEG.S26297
Poli-Bigelli, S., Rodrigues-Pereira, J., Carides, A. D., Ma, G. J., Eldridge, K., Hipple, A., et al. (2003). Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer 97, 3090–3098. doi: 10.1002/cncr.11433
Rapoport, B. L., Jordan, K., Boice, J. A., Taylor, A., Brown, C., Hardwick, J. S., et al. (2010). Aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: a randomized, double-blind study. Support. Care Cancer 18, 423–431. doi: 10.1007/s00520-009-0680-9
Rudd, J. A., Ngan, M. P., Wai, M. K., King, A. G., Witherington, J., Andrews, P. L. R., et al. (2006). Anti-emetic activity of ghrelin in ferrets exposed to the cytotoxic anti-cancer agent cisplatin. Neurosci. Lett. 392, 79–83. doi: 10.1016/j.neulet.2005.08.062
Schwartzberg, L. S. (2007). Chemotherapy-induced nausea and vomiting: which antiemetic for which therapy? Oncology 21, 946–953.
Suzuki, H., Inadomi, J. M., and Hibi, T. (2009). Japanese herbal medicine in functional gastrointestinal disorders. Neurogastroenterol. Motil. 21, 688–696. doi: 10.1111/j.1365-2982-2009-01290.x
Suzuki, K., Yamanaka, T., Hashimoto, H., Shimada, Y., Arata, K., Matsui, R., et al. (2016). Randomized, double-blind, phase iii trial of palonosetron versus granisetron in the triplet regimen for preventing chemotherapy-induced nausea and vomiting after highly emetogenic chemotherapy: TRIPLE study. Ann. Oncol. 27, 1601–1606. doi: 10.1093/annonc/mdw220
Takeda, H., Sadakane, C., Hattori, T., Katsurada, T., Ohkawara, T., Nagai, K., et al. (2008). Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology 134, 2004–2013. doi: 10.1053/j.gastro.2008.02.078
Tominaga, K., Iwakiri, R., Fujimoto, K., Fujiwara, Y., Tanaka, M., Shimoyama, Y., et al. (2012). Rikkunshito improves symptoms in PPI-refractory GERD patients: a prospective, randomized, multicenter trial in Japan. J. Gastroenterol. 47, 284–292. doi: 10.1007/s00535-011-0488-5
Tomono, H., Ito, Y., and Watanabe, T. (2006). Successful antiemetic treatment of Tsumura rikkunshi-to extract granules for ethical use in addition to other antiemetic agents in neoadjuvant chemotherapy for an advanced breast cancer patient. Gan To Kagaku Ryoho 33, 1129–1131.
Warr, D. G., Grunberg, S. M., Gralla, R. J., Hesketh, P. J., Roila, F., de Wit, R., et al. (2005). The oral NK(1) antagonist aprepitant for the prevention of acute and delayed chemotherapy-induced nausea and vomiting: pooled data from 2 randomised, double-blind, placebo controlled trials. Eur. J. Cancer 41, 1278–1285. doi: 10.1016/j.ejca.2005.01.024
Keywords: herbal medicine, nausea, vomiting, lung cancer, chemotherapy
Citation: Harada T, Amano T, Ikari T, Takamura K, Ogi T, Fujikane T, Fujita Y, Taima K, Tanaka H, Sasaki T, Okumura S, Sugawara S, Yokouchi H, Yamada N, Morikawa N, Dosaka-Akita H, Isobe H and Nishimura M (2018) Rikkunshito for Preventing Chemotherapy-Induced Nausea and Vomiting in Lung Cancer Patients: Results from 2 Prospective, Randomized Phase 2 Trials. Front. Pharmacol. 8:972. doi: 10.3389/fphar.2017.00972
Received: 04 September 2017; Accepted: 20 December 2017;
Published: 16 January 2018.
Edited by:
Ganna Tolstanova, Taras Shevchenko National University of Kyiv, UkraineReviewed by:
Alessandro Laviano, Sapienza Università di Roma, ItalyYanis Boumber, Fox Chase Cancer Center, United States
Copyright © 2018 Harada, Amano, Ikari, Takamura, Ogi, Fujikane, Fujita, Taima, Tanaka, Sasaki, Okumura, Sugawara, Yokouchi, Yamada, Morikawa, Dosaka-Akita, Isobe and Nishimura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Toshiyuki Harada, aGFyYWRhLXRvc2hpeXVraUBob2trYWlkby5qY2hvLmdvLmpw
 Toshiyuki Harada
Toshiyuki Harada Toraji Amano2
Toraji Amano2 Tomoo Ikari
Tomoo Ikari Takaaki Sasaki
Takaaki Sasaki Shunichi Sugawara
Shunichi Sugawara