- 1School of Pharmacy, Fudan University, Key Laboratory of Smart Drug Delivery, Ministry of Education, Shanghai, China
- 2Institute of Hematology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Nanomedicines including liposomes, micelles, and nanoparticles based on the enhanced permeability and retention (EPR) effect have become the mainstream for tumor treatment owing to their superiority over conventional anticancer agents. Advanced design of nanomedicine including active targeting nanomedicine, tumor-responsive nanomedicine, and optimization of physicochemical properties to enable highly effective delivery of nanomedicine to tumors has further improved their therapeutic benefits. However, these strategies still could not conquer the delivery barriers of a tumor microenvironment such as heterogeneous blood flow, dense extracellular matrix, abundant stroma cells, and high interstitial fluid pressure, which severely impaired vascular transport of nanomedicines, hindered their effective extravasation, and impeded their interstitial transport to realize uniform distribution inside tumors. Therefore, modulation of tumor microenvironment has now emerged as an important strategy to improve nanomedicine delivery to tumors. Here, we review the existing strategies and approaches for tumor microenvironment modulation to improve tumor perfusion for helping more nanomedicines to reach the tumor site, to facilitate nanomedicine extravasation for enhancing transvascular transport, and to improve interstitial transport for optimizing the distribution of nanomedicines. These strategies may provide an avenue for the development of new combination chemotherapeutic regimens and reassessment of previously suboptimal agents.
Introduction
In recent times, nanomedicine delivery to tumors has attracted extensive attention in the field of tumor treatment (Allen and Cullis, 2004; Peer et al., 2007). The advantage of nanomedicines over free drugs is based on the enhanced permeability and retention (EPR) effect (Fang et al., 2011; Maeda, 2012). The fundamental characteristics of EPR physiology are highly permeable tumor vessels allowing the enhanced permeability (EP) of large particles including proteins, macromolecules, liposomes, micelles, and other particles large enough to avoid renal clearance, into the tumor interstitium combined with impaired lymphatic drainage limiting clearance and causing enhanced retention (ER) of those extravasated particles. Both features result from the rapid growth of a tumor and collapse of the existing blood and lymph vessels in the limited interstitial space (Leu et al., 2000; Dreher et al., 2006). With EPR effect as the main principle for passive targeting strategy, nanomedicine delivery to tumors has achieved success to varying degrees. However, the clinical benefits of the three EPR-based Food and Drug Administration (FAD)-approved nanomedicines including pegylated liposomal doxorubicin (Doxil/Caelyx), liposomal daunorubicin (DaunoXome), and nanoparticle albumin-bound paclitaxel (Abraxane) for the treatment of solid tumors were demonstrated to be only modest (O'Brien et al., 2004; Gradishar et al., 2005; Jain and Stylianopoulos, 2010), posing considerable challenges for the clinical translation of new nanomedicines. Accumulating evidence revealed that EPR-dependent drug delivery was always compromised by the tumor microenvironment including irregular vascular distribution, elevated tumor interstitial fluid pressure (IFP), poor blood flow, rich extracellular matrix (ECM) and abundant tumor stroma cells (Nichols and Bae, 2014). Delivery barriers posed by the tumor microenvironment are the main reasons responsible for the modest survival benefits of FDA-approved nanomedicines (Jain and Stylianopoulos, 2010).
The tumor microenvironment, as an important component of tumor tissues, functions as the soil for the seeds i.e., tumor cells to proliferate, differentiate, and promote tumor growth (Zhang et al., 2015, 2016a). The components of tumor microenvironment include the extracellular matrix (ECM) and different kinds of stromal cells such as tumor-associated fibroblasts (TAF), tumor-associated macrophages, and pericytes (Hanahan and Weinberg, 2011). As a pathologic condition, the tumor microenvironment is remarkably abnormal: tumor blood flow is low, perfusion is uneven in tumors, the tumor vessel permeability is highly heterogeneous, interstitial fluid pressure (IFP) is elevated, and a large number of active stromal cells and ECM are often dense and stiff.
Systemically administrated nanomedicines need to undergo a three-step process in solid tumors to achieve their therapeutic effect: vascular transportation to different areas of the tumor, trans-vascular transport across the vessel wall, and interstitial transport to reach the tumor cells (Wong et al., 2011). Delivery of nanomedicines differs markedly between tumors and normal tissues owing to structural differences. The abnormality in organization and structure of the tumor vasculature leads to heterogeneous blood flow, which directly influences the vascular transport of nanomedicines (Jain, 1994, 2005). Additionally, the vascular hyper-permeability and lack of functional lymphatic vessels inside tumors results in elevated IFP (Boucher et al., 1990), which not only compresses tumor vessels to aggravate the heterogeneous blood flow, but also reduces convective transport of nanomedicines (Jain, 1987). Besides, compression from proliferating tumor cells, stromal cells, and the ECM could also compress tumor vessels (Stylianopoulos et al., 2012). Furthermore, the dense ECM hinders interstitial diffusion of nanomedicines (Jain, 1987). When 90-nm liposomes, approximately the size of liposomal doxorubicin, an approved nanomedicine, was intravenously administered in tumor-bearing mice, these particles leaked out of tumor vessels but did not move far away from the vessel wall (Yuan et al., 1994a). Even directly intra-tumor injected 150-nm particles did not move far from the injection site (McKee et al., 2006). Altogether, the complex tumor microenvironment could negatively affect vascular transport, trans-vascular transport and interstitial transport of nanomedicine, and compromise nanomedicine delivery for tumor treatment.
To improve the therapeutic benefits of nanomedicine, different strategies including active targeting nanomedicine (Zhang et al., 2014a,b), tumor-responsive nanomedicine (Zhu et al., 2012; Huang et al., 2013), as well as optimization of the physiochemical parameters of nanomedicine such as size (Tong et al., 2013; Tang et al., 2014), charge (Han et al., 2015), and shape (Chauhan et al., 2011) have been developed. However, these methods rely on the advanced development of the nanomedicine itself, which could not conquer the above-mentioned delivery barriers of the tumor microenvironment (Chauhan and Jain, 2013). Accordingly, a modification of the tumor microenvironment was recognized as an important tool to improve tumor nanomedicine delivery (Jain and Stylianopoulos, 2010; Miao et al., 2015). In this review, in terms of the three processes including vascular transport, trans-vascular transport and interstitial transport that nanomedicines need to experience before reaching tumor cells and achieving therapeutic benefit, we tried to summarize different strategies of modulating tumor microenvironment to improve tumor nanomedicine delivery from the corresponding three aspects including improving tumor perfusion, facilitating nanomedicine extravasation, and enhancing interstitial transport of nanomedicine.
Nanomedicine Transport Barriers from Tumor Microenvironment
The main transport barriers of a tumor microenvironment include abnormal tumor vasculature, elevated IFP, dense ECM, and stromal cells (Figure 1). These components varied with respect to different tumor types. To better understand and address the complexities of a tumor microenvironment, we used two representative models—highly permeable tumors and highly desmoplastic tumors (Stylianopoulos and Jain, 2013). Highly permeable tumors such as gliomas and melanomas are always rich in vessels and a small amount of pericytes, TAFs, and ECM, wherein tumor cells are in the vicinity of tumor vessels. On the other hand, highly desmoplastic tumors such as pancreatic cancers (Cabral et al., 2011), bladder cancers (Zhang et al., 2014), and some breast cancers (Stylianopoulos and Jain, 2013) are always hypovascular with numerous TAFs, a dense ECM, and high coverage rate of pericytes on the endothelium, such that the tumor cells are isolated into nests by TAF complexed with ECM and are a certain distance from the tumor vessels (Feig et al., 2012; Zhang et al., 2016c). Highly permeable and highly desmoplastic tumors were also referred to as tumors with tumor-vessels architecture and tumors with stroma-vessels architecture, respectively, in some reports (Smith et al., 2013; Miao et al., 2016).
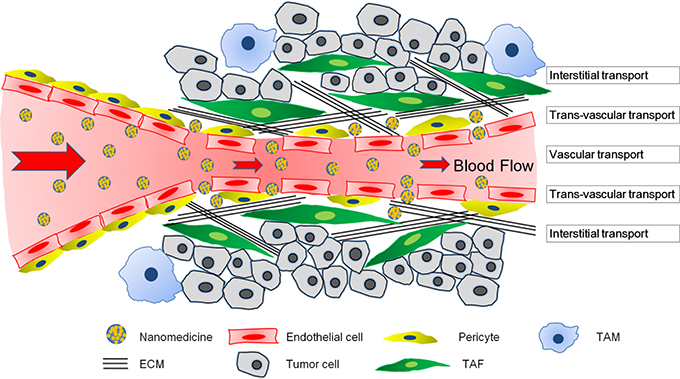
Figure 1. The transport barriers for tumor nanomedicine delivery imposed by a complicated tumor microenvironment including poor blood perfusion, IFP, dense ECM, and a large number of stromal cells. The nanomedicines have to cross the blood vessel walls, penetrate the extravascular space, and eventually reach tumor cells to exert their therapeutic effects.
Abnormal Tumor Vasculature Networks
In normal tissues, an exquisite counterbalance is achieved between the proangiogenic molecules such as VEGF and endogenous antiangiogenic molecules such as sVEGFR1 and thrombospondins (Carmeliet and Jain, 2000; Jain, 2003). In tumor tissues, however, the proangiogenic effect is abnormally upregulated and the pathological angiogenesis occurs in a disorganized manner. Compared to normal vessels, tumor vessels are highly irregular and chaotic in structure with wide endothelial gaps and a heterogeneous basement membrane (Carmeliet and Jain, 2011). The tortuous and leaky nature of tumor vessels contributes to compromised tumor blood flow, as the high tortuosity of tumor vessels results in an elevated geometric resistance that retards the blood flow (Sevick and Jain, 1989a). Furthermore, this sharp drop of blood flow due to the geometric resistance exerts a marked influence on the viscous resistance of blood in tumor vessels (Sevick and Jain, 1989b). Additionally, the leaky nature of tumor vessels allows the permeability of fluid into the interstitium. Fluid loss would increase the hematocrit of tumor blood to elevate its viscosity and further impede tumor blood flow (Sevick and Jain, 1989b; Netti et al., 1996; Sun et al., 2007). This combined effect of tortuosity of and leakage from tumor vessels compromises blood flow in brain tumors to one to three orders of magnitude, slower than that in the pial vessels surrounding normal tissues (Yuan et al., 1994b). Inefficient blood flow in tumors always leads to poor delivery of systemically administered drugs. Therefore, the tumor vascular network poses a major barrier in vascular transportation of nanomedicine, specifically highly permeable tumors with a variety of hyper permeable vessels.
Elevated IFP
IFP is a type of stress exerted by fluids and is uniformly elevated throughout the tumor bulk in many tumors. Fluid flow is involved in three processes including flow along the tumor vessels, through the tumor interstitium, and drainage of excessive fluid by the lymphatic vessels (Jain et al., 2014).
Abnormalities in the tumor microenvironment concerning these three processes lead to elevated IFP. The leaky tumor vessels allow the extravasation of excessive fluid and plasma macromolecules into the tumor interstitium. In normal tissues, excessive fluid could be drained by an effective lymphatic network to maintain a balanced tissue interstitial pressure. However, lymphatic drainage in tumors do not function properly and hence, IFP is elevated in tumor tissues. Apart from abnormality in tumor blood vessels and lymphatic vessels, abnormal hydraulic conductivity is also a regulator of IFP, especially in highly desmoplastic tumors. The hydraulic conductivity depends on the volume fraction, surface charge, chemical composition, and organization of fibers in the tumor interstitial space (Levick, 1987; Stylianopoulos et al., 2008). Tumors abundant in collagen could display an order of magnitude lower in hydraulic conductivity than those with low collagen content (Netti et al., 2000). The negatively charged glycosaminoglycans could increase flow resistance because of their ability to trap water (Levick, 1987). Therefore, depletion of glycosaminoglycans with matrix metalloproteinases-1 and -8 increases the hydraulic conductivity and thus, the interstitial fluid velocity (Mok et al., 2007). Furthermore, a high density of tumor cells and stromal cells might reduce the interstitial space available for fluid flow, thereby increasing fluid resistance and IFP. In normal tissues, IFP is in the range of 0–3 mm Hg. However, experimental and human solid tumors exhibit high IFP, typically ranging from 5 to 40 mmHg, which may reach 75–130 mmHg in highly desmoplastic pancreatic tumors (Milosevic et al., 2004; Provenzano et al., 2012). Therefore, in many tumors, elevated IFP and reduced microvascular pressure (MVP) hinder nanomedicine delivery by the following mechanisms. First, as IFP and MVP also impose fluid stresses on vessel walls, elevated IFP could compress tumor vessels to cause blood stasis and/or vessel collapse to reduce the vascular transport of nanomedicine. Another consequence of high IFP is that interstitial fluid might escape from the tumor periphery into surrounding normal tissue, carrying not only nanomedicine to reduce the trans-vascular transport of nanomedicine but also growth factors or tumor cells to drive tumor metastasis or drug resistance (Chary and Jain, 1989; Netti et al., 1996). Finally, IFP might force the nanomedicine to extravasate by passive diffusion, instead of convention, a much faster transport process, which compromises the interstitial transport of nanomedicine especially larger nanomedicines. To conclude, elevated IFP inside tumors hinders vascular, trans-vascular, and interstitial transport of nanomedicines.
ECM
The ECM is the non-cellular component widely present within all tissues and organs. ECM is mainly composed of two types of macromolecules: proteoglycans (PGs) and fibrous proteins (Järveläinen et al., 2009; Schaefer and Schaefer, 2010). PGs such as hyaluronan fill the majority of the extracellular interstitium of the tissue in the form of a hydrated gel. The fibrous ECM proteins include collagens, elastins, fibronectins, and laminins (Dequidt et al., 2007). ECM is a highly dynamic structure being constantly remodeled, either enzymatically or non-enzymatically, and its final components are controlled by a myriad of post-translational modifications. ECM is tissue-specific and the component varies greatly among different tissues including cancerous ones (Frantz et al., 2010).
Under normal conditions, the unique composition and structure of the ECM functions as a growth regulator. ECM and ECM-associated enzymes and growth factors regulate cell proliferation and differentiation, maintaining cell survival and dynamic homeostasis (Li et al., 2010). However, ECM is commonly deregulated and becomes disorganized in diseases such as cancer. Fibrosis due to excessive ECM production or limited ECM turnover occurs in many types of cancers. Especially in highly desmoplastic tumor such as pancreatic cancer and some breast cancers (Stylianopoulos and Jain, 2013), a dense ECM composed of collagen, hyaluronan, and fibronectin is always found (Feig et al., 2012; Zhang et al., 2016c). In contrast, tumors with abundant vessels always harbor a low level of ECM. Signaling pathways involved in ECM production included transforming growth factor-beta (TGF-β), Hedgehog (Hh) signaling, and platelet-derived growth factor (PDGF). ECM turnover is subjected to enzyme-mediated remolding including heparanase, cysteine proteases, 6-O-sulfatases, urokinase, and many matrix metalloproteinases (MMPs) (Egeblad et al., 2010; Lu et al., 2012). In highly vascularized and permeable tumors such as glioma and melanoma, ECM is always scarce.
The dense ECM in the tumor interstitium not only compressed tumor vasculature and reduced vascular transport of nanomedicine but also isolates tumor cells into nests within a certain distance from collapsed vessels and resists the free penetration and homogeneous distribution of nanomedicine in three main ways (Bailey et al., 2008; Miao et al., 2015). First, limited interstitial volume plus high stromal fraction and large matrix molecules result in a dense network (Padera et al., 2004), effectively reducing blood flow and limiting convection of nanomedicine. Second, the fibrillar structure, mesh size, and collagen thickness directly limit the diffusion of nanomedicine. The diffusion capacity is inversely related to the size of nanomedicine. Matrix mesh size ranges between 20 and 40 nm in solid tumors. Particles larger than the mesh size are completely prevented from diffusing through the ECM, those near the mesh size can be hindered to a certain extent, and only small particles can penetrate almost freely (Nichols and Bae, 2012). Third, the tortuous nature of the interstitial space poses an additional barrier for drugs of all size, because it elongates the diffusion path of the nanomedicine from blood vessels to tumor cells (Chauhan et al., 2009). The resistance of nanomedicine delivery from ECM mainly occurs in highly desmoplastic tumors. For highly permeable tumors with tumor-vessels architecture, the ECM is not as abundant, dense, and stiff as that in highly desmoplastic tumors, the and nanomedicine can penetrate throughout the tumor tissues much more easily after its extravasation from tumor vessels (Cabral et al., 2011). Unfortunately, the ECM is much more denser and thicker in human tumors than in mouse models (Miao and Huang, 2015). In conclusion, rich ECM in tumors resists vascular and interstitial transport of nanomedicine.
Stromal Cells
Stromal cells include TAF, tumor-associated macrophages (TAM), and pericytes. The origin of TAF is still debatable. TAF probably originated from resident tissue fibroblasts, bone marrow-derived mesenchymal stem cells, hematopoietic stem cells, epithelial cells (epithelial-mesenchymal transition; EMT), and endothelial cells (endothelial-mesenchymal transition; EndMT) (Shiga et al., 2015). It was now widely accepted that TAF significantly contributes to cancer progression (Brennen et al., 2012). TAF is abundant in highly desmoplastic tumors and produces large amounts of ECM to isolate tumor cells into a nest. TAF has been regarded as the major component of tumor stroma and contributes to the binding-site barrier for interstitial transport of nanomedicine (Miao et al., 2016). Large numbers of TAF associated with dense ECM also compress tumor vessels to compromise the vascular transport of nanomedicine. Besides, reports have shown that uptake of the anisamide ligand-modified nanomedicine by TAF was 7-fold higher than that of the other cells because of the different expression level of the sigma receptor between TAF and other cells (Miao et al., 2016).
TAM is the major cancer-related inflammatory cell primarily converted from monocytes that are closely associated with the prognosis of many cancer types. Other inflammatory cells include granulocytes, dendritic cells, and myeloid derived suppressor cells, which are also important constituents of the tumor microenvironment (Mocellin et al., 2001; Hu et al., 2016). When polarized toward the anti-inflammatory state by the tumor microenvironment, TAM promotes immune evasion and angiogenesis, thereby driving tumor growth (Cieslewicz et al., 2013). The off-target effect of nanomedicines is inevitable because of the phagocytic properties of inflammatory cells. Roode et al. showed that the association between TAM and NP were 4-fold greater than that of cancer cells despite TAM constituting only 1% of all cells in tumors (Roode et al., 2016). The off-target uptake of nanomedicine by stromal cells including TAF and TAM could certainly reduce the uptake of nanomedicine by tumor cells and therefore the therapeutic benefits.
Pericytes are another important type of stromal cells located mainly in the perivascular space, which also affect nanomedicine delivery. Neither leaky, immature vessels with little coverage nor over-mature vessels with high pericyte coverage are favorable for nanomedicine delivery. Excessively leaky vessels in highly vascularized tumors affect nanomedicine delivery mainly by compromising blood perfusion and thereby the vascular transport of nanomedicine (Stylianopoulos and Jain, 2013). In contrast, high pericyte coverage is always found in highly desmoplastic tumors, which reduce the endothelial gap and limit the trans-vascular transport of nanomedicine, especially for larger nanomedicine (Cabral et al., 2011).
Strategies to Modulate Tumor Microenvironment
In accordance with the three processes including vascular, trans-vascular and interstitial transport that nanomedicines need to experience before reaching tumor cells, strategies of modulating tumor microenvironment to improve nanomedicine delivery for tumor treatment can be divided into three categories: improving tumor perfusion, facilitating nanomedicine extravasation, and enhancing interstitial transport of nanomedicine (Table 1).
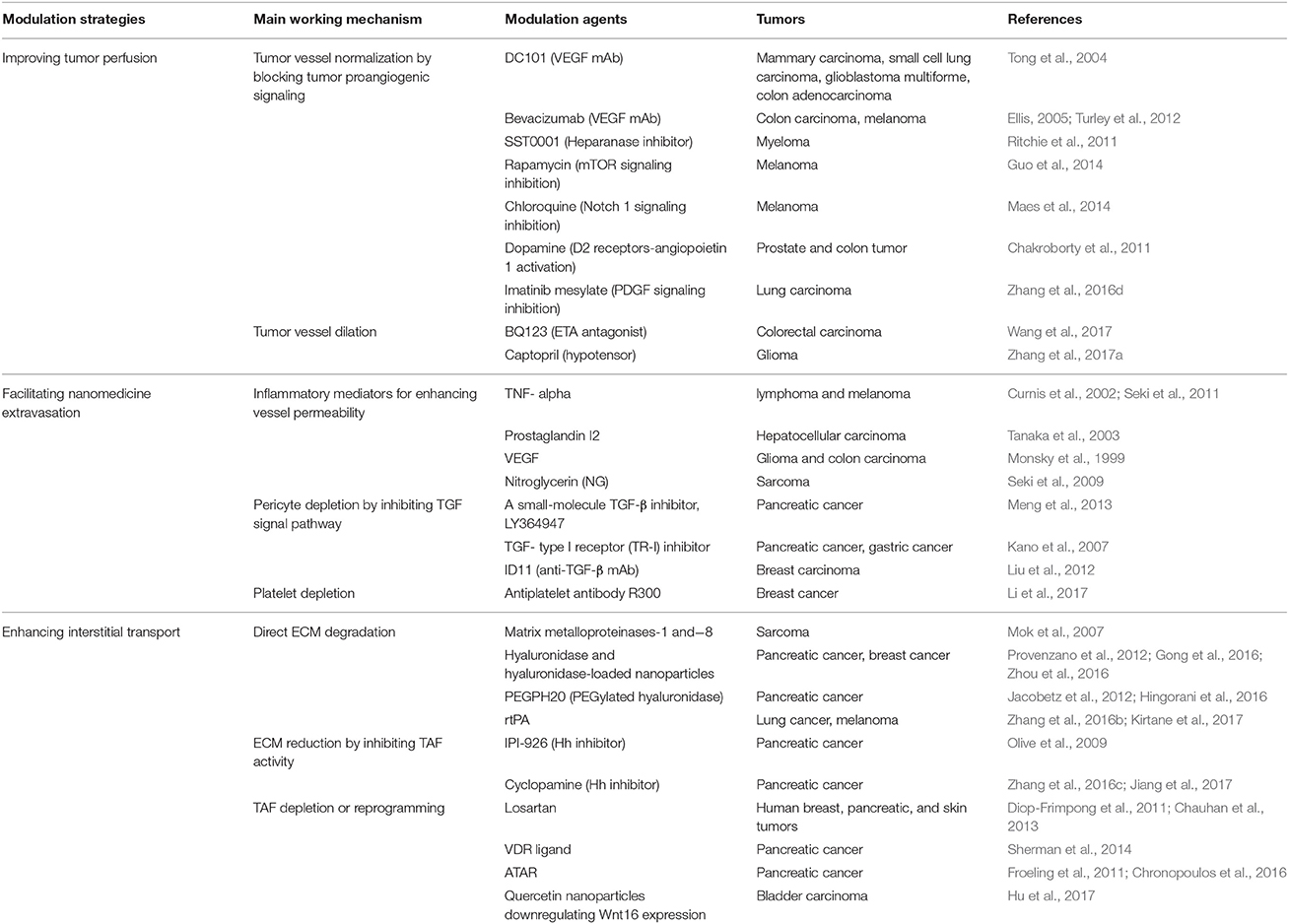
Table 1. Summary of tumor microenvironment modulation strategies for improving tumor nanomedicine delivery.
Improving Tumor Perfusion
Tumor Vasculature Normalization
The newly formed tumor vessels are always tortuous and leaky allowing the extravasation of nanomedicine but simultaneously increasing IFP, which prevents adequate and homogeneous blood flow and vascular transport of nanomedicine. To improve nanomedicine delivery for tumor treatment, normalization of vessels has emerged as an effective approach. Vessel normalization transforms the abnormal phenotype of tumor vessels into a phenotype that closely resembles that of fully functional normal vessels by repairing the basement membrane and increasing coverage rate of pericytes, and ultimately decreasing vessel leakiness. Optimizing the structure of tumor vessels could reduce the extravasation of excessive fluid and lower IFP, and then restore tumor blood flow, thereby improving vascular transport of nanomedicine. Many proangiogenic molecules including VEGF, fibroblast growth factor (FGF), and PDGF are overexpressed in tumors and involved in angiogenesis, which cause chaotic structural development in these newly formed tumor vessels (Goel et al., 2011). Therefore, strategies to block these proangiogenic signaling molecules were designed to repair tumor vessels. For example, VEGF inhibitors Bevacizumab, the FDA-approved antiangiogenic monoclonal antibody (mAb), capable of reverting abnormal structure of tumor vessels toward a more normal phenotype have been applied in the treatment of metastatic colorectal cancer (Salgaller, 2003; Ellis, 2005; Table 1), which were of high potential to improve nanomedicine delivery for tumor treatment. Moreover, some angiogenic signaling pathways such as mTOR signaling (Guo et al., 2014), Notch 1 signaling (Maes et al., 2014), and D2 receptors-angiopoietin 1 signaling (Chakroborty et al., 2011) involved in vessel normalization have also been modulated to improve nanomedicine delivery (Table 1). In our previous research, it was also shown imatinib mesylate (IMA) could normalize the tumor vessels of A549 tumors by inhibiting platelet-derived growth factor signaling pathway (Zhang et al., 2016d). Interestedly, IMA treatment could significantly reduce the accumulation of nanoparticles (NPs) around 110 nm but enhanced the accumulation of micelles around 23 nm. Furthermore, IMA treatment limited the distribution of NPs inside tumors but increased that of micelles with a more homogeneous pattern (Figure 2). Finally, the anti-tumor efficacy study displayed that IMA pretreatment could significantly increase the therapeutic effects of paclitaxel-loaded micelles. As tumor vessel normalization minimized endothelial gap, it could prevent tumor cells shedding into tumor vessels, and reduce the possible tumor metastasis to a certain degree.
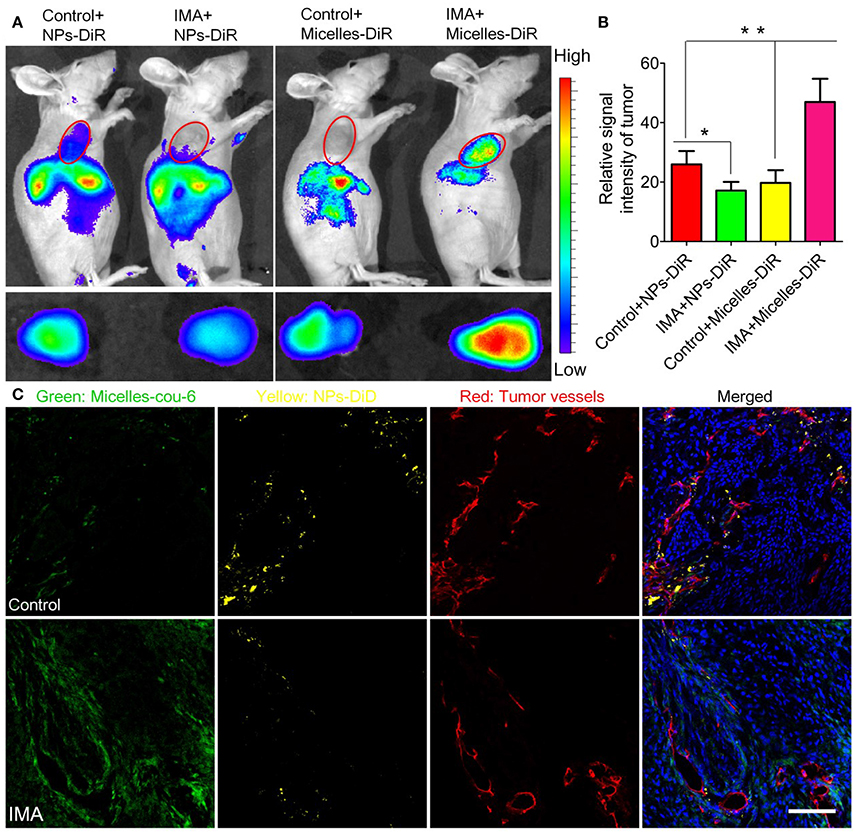
Figure 2. The effects of IMA treatment on tumor nanoparticle delivery. (A) In vivo fluorescence imaging of A549 xenograft-bearing mice (the upper row) treated with IMA or water as a control, ex vivo fluorescence imaging of their corresponding tumor xenografts (the lower row), and (B) the relative signal intensity of tumor tissue 24 h post the injection of DiR-labeled nanoparticles or micelles. *p < 0.05, compared with Control+NP group. **p < 0.01 compared with IMA+Micelles group. (C) In vivo distribution of micelles and nanoparticles in tumor slices from A549 tumor xenograft-bearing mouse models treated with IMA or water at 24 h after i.v. injection of a mixture of DiD-labeled nanoparticles and coumarin-6-labeled micelles. The oral dose of IMA was 50 mg/kg/d for 3 weeks. The dose of both coumarin-6 and DiD was 0.05 mg/kg. The bar indicated 100 μm. Reprinted from reference with permission by copyright holder, Zhiqing Pang.
To utilize vessel normalization strategy to improve nanomedicine delivery for tumor treatment, four concerns should be kept in mind. First, the strategy can only improve the delivery of small molecular weight drugs or relatively smaller nanomedicines ranging from 20 to 40 nm, but reduces the delivery of large nanomedicines around 100 nm as it decreases the endothelial gap of tumor vessels (Chauhan et al., 2012; Zhang et al., 2016d, 2017b). Second, the normalization is transient and the followed nanomedicine should be applied in the normalization window. Third, a judicious dose of vascular normalizer is highly recommended to prevent excessive pruning of tumor vessels, which might impair vascular efficiency and thus the delivery of concurrent therapy (Huang et al., 2012; Jain, 2013). Fourth, as vasculatures are always severely compressed in highly desmoplastic tumors and are refractory to vasculature normalizers (Smith et al., 2013), this strategy might only be used in tumors that are highly permeable but not highly desmoplastic, or at least combined with other strategies capable of reopening compressed vessels.
Tumor Vessel Dilation
Vasoconstrictive endothelin-1 (ET1) and its receptor ETA via which ET-1 mediates vasoconstriction are both abundant in tumor tissues for maintaining the contractile tone of tumor vessels. The expression level of ET1 and ETA in tumor vessels was 13-fold and 5-folder higher than that of size-matched normal vessels, respectively (Sonveaux et al., 2004). BQ123, a selective antagonist against ETA could inhibit ET1-ETA signaling, induce tumor vessel dilation, and trigger a tumor-specific increase in blood flow. The blood flow improvement induced by BQ123 improved the delivery of free drugs to tumors despite an increase in IFP (Martinive et al., 2006). In addition, it was also demonstrated that BQ123 could increase the delivery of photothermal nanomedicine around 100 nm for effective photothermal therapy of tumors (Wang et al., 2017). Some inflammation factors such as bradykinin capable of dilating vessels could also directly increase tumor perfusion. In our previous study, it was shown captopril, a widely used hypotensor in clinics, could dilate tumor blood vessels by increasing bradykinin expression and even increase tumor vessel permeability to enhance nanomedicine delivery for tumor therapy (Zhang et al., 2017a).
Facilitating Nanomedicine Extravasation
Inflammatory Mediators for Enhanced Permeability
Inflammatory mediators such as TNFα (Seki et al., 2011), prostaglandin analogs (Tanaka et al., 2003), VEGF (Monsky et al., 1999), and nitric oxide (NO) donors (Seki et al., 2009), capable of enhancing vascular permeability, have been utilized to increase nanomedicine accumulation in tumors up to 2–6-fold higher than that of the control group (Table 1). Apart from vascular permeability enhancement, vasodilatation and blood-flow improvement by usage of inflammatory mediators were also involved in improving nanomedicine delivery for tumors. However, a series of effects of inflammatory mediators mentioned above could also lead to elevated IFP against nanomedicine delivery. Thus, accumulation of nanomedicine in tumors comprehensively depends on these factors. As inflammation might potentially promote cancer development (Atsumi et al., 2014), local application (Seki et al., 2011) or targeted delivery of inflammatory mediators to the tumor site should be adopted.
Pericyte Depletion
In highly desmoplastic tumor, the coverage rate of pericytes on endothelium was about 70%, much higher than highly permeable tumors, which significantly limit the transvascular movement of nanomedicine into tumor interstium. Therefore, strategies by using low dose of a TGF-β inhibitor, LY364947 was developed to reduce the pericyte coverage of endothelium and increase size gaps between endothelium to increase therapeutic benefits of gemcitabine-loaded liposomes for pancreatic cancer (Cabral et al., 2011; Meng et al., 2013) and Doxil for diffuse-type gastric cancer (Kano et al., 2007).
Platelet Depletion
It is well known that platelets contribute a lot to hemostasis. Apart from its role in thrombus formation, platelets are highly involved in tumor progression and metastasis. In addition, it could also support tumor vascular homeostasis and protect the integrity of tumor vessels (Kisucka et al., 2006; Ho-Tin-Noé et al., 2009). Studies showed that platelets reduction induced bleeding in the tumor site and increased leakiness of tumor vasculature. Therefore, platelets reduction in thrombocytopenic mice increased efficacy of chemotherapy for breast cancer (Demers et al., 2011). To avoid potential bleeding in normal organs caused by low platelet counts, a recent study by Li et al. designed a tumor microenvironment-responsive nanoparticle capable of delivering antiplatelet antibody R300 to selectively deplete platelet in tumor tissues, therefore augmenting vascular permeability and improving nanomedicine delivery for tumors (Li et al., 2017). Platelet depletion represented as a promising approach to augment transvascular delivery of nanomedicine to tumors.
Physical Stimulus
Radiation can improve tumor-targeted delivery of nanomedicine (Davies Cde et al., 2004; Giustini et al., 2012). Some possible mechanisms are as follows: First, radiation could upregulate the level of vascular endothelial growth factor (VEGF) by activating hypoxia inducible factor 1 (HIF1) (Moeller et al., 2004; Stapleton et al., 2016) or via multiple mitogen-activated protein kinase dependent pathways (Park et al., 2001) to increase the permeability of tumor vessels (Kobayashi et al., 2004). Results showed that the tumor vessels' permeability of magnetic resonance imaging-contrast agent with the molecular weight above 200 kDa was increased by 32.8% after irradiation (10 Gy). In addition, radiation can rapidly kill the sensitive tumor cells. The reduced cell density helped to alleviate compression stress from tumor cells, reopen collapsed vessels, and therefore increase tumor blood flow (Nagano et al., 2008; Khawar et al., 2015). The effect of radiation on tumors is complex and dose-, time-, and tumor-type dependent (Garcia-Barros et al., 2003; Fuks and Kolesnick, 2005; Kioi et al., 2010). Milosevic's recent review provides further evidence of the same (Stapleton et al., 2016).
Kong et al. pioneered the use of mild hyperthermia (HT) for nanomedicine extravasation into tumor tissues by improved vascular permeability (Kong et al., 2000, 2001). Studies further demonstrated that mild HT could also help improve tumor perfusion and reduce IFP (Sen et al., 2011; Winslow et al., 2015), probably via creation of vascular fenestrations and perturbation of the vascular endothelium (Kirui et al., 2015), and thus allow deep nanomedicine penetration throughout the tumors, rather than perivascular accumulation (Li et al., 2013). However, there was no direct evidence to demonstrate the pore size change of tumor vessels after HT treatment. The extravasation depth and intensity in tumor interstitium was considered to vary greatly among different types of tumors, which depends on the pattern of the endothelial lining and the intrinsic property of the surrounding tumor microenvironment (Eberhard et al., 2000), such as structure of the interstitial matrix. Apart from tumors with a certain vascular component, highly desmoplastic tumors could also respond well to mild HT treatment (Kirui et al., 2014, 2015). The temperature is a crucial element in the heating method, wherein results showed that 41–43°C was appropriate, because a very high temperature might damage the endothelial lining of tumor vessels and induce coagulation response. Thrombin formation could choke the vessels and compromise nanomedicine delivery. Alternatively, insufficient temperature might exert a very minimal effect on the tumor vessel endothelium to increase the endothelial gap (von Maltzahn et al., 2011; Li et al., 2013).
Ultrasound has been used to improve nanomedicine delivery for tumors by both mechanical and HT effect (Goins et al., 2016). For mechanical effects, many reports showed that gas-filled bubbles could be used to transiently produce pores in blood vessels (Durymanov et al., 2015) or cell membranes (sonoporation) (Yoon et al., 2014; Ma et al., 2016), through which nanomedicines of different types can effectively extravasate tumor vessels or enter into tumor cells (Thakkar et al., 2013), therefore achieving improved delivery of nanomedicine (Rapoport et al., 2013, 2015). Besides, ultrasound also produces heat at an acoustic intensity and in a time-dependent manner. Recently, Frazier used magnetic resonance imaging-guided, high-intensity focused ultrasound (HIFU) to produce a spatially uniform 43°C heating pattern in a xenograft tumor model and improved the accumulation of Evans blue dye in heated tumors to nearly 2-fold higher than in unheated tumors (Frazier et al., 2016).
Improving Interstitial Transport of Nanomedicine
ECM Disruption Strategies
Dense ECM always resists free penetration of nanomedicine throughout tumor tissues to reach tumor cells. Therefore, modification of ECM has been extensively explored to improve the delivery and distribution of nanomedicine in tumor tissues. The ECM modification strategy includes direct ECM disruption and reduction of ECM synthesis by inhibiting TAF activity.
Several studies have shown that different kinds of enzymes directly degrade the components of ECM such as collagen and hyaluronic acid and can improve the delivery of nanomedicine (Table 1). For instance, collagenase-coated nanomedicine could penetrate deeper into the core of in vitro tumor spheroids than control ones (Goodman et al., 2007; Cui et al., 2013). Besides, enzymatic digestion of collagen and decorin facilitates >10-fold increase in the diffusion of macromolecular dextran into tumor tissue, supporting matrix degradation as a useful tool to improve macromolecule distribution (Magzoub et al., 2008). In another study, intravenous injection of collagenase-1 into xenograft-bearing mice models increased the accumulation and gene expression of lipoplex in tumors by 1.5- and 2-fold, respectively, further confirming collagen digestion to be a useful strategy to improve nanomedicine delivery (Kato et al., 2012). Hyaluronan, or hyaluronic acid, a large linear glycosaminoglycan, composed of repeating N-acetyl glucosamine and glucuronic acid units, was also a crucial component of ECM (Provenzano et al., 2012), which was found abundant in non-small cell lung cancer (NSCLC), prostate, pancreatic, and breast cancers. Hyaluronidase was shown to induce a 4-fold increase in the distribution of liposomal doxorubicin in a human osteosarcoma xenograft model (Eikenes et al., 2005). A phase 1b trial of docetaxel combining PEGPH20 in metastatic refractory NSCLC has been completed (NCT02346370) with results pending (Wong et al., 2017). In addition, a nanomedicine combining PEGylated hyaluronidase (PEGPH20) to improve the efficiency of chemotherapeutics for hyaluronan-high pancreatic cancer is currently in phase 3 clinical trial (NCT02715804) (Provenzano et al., 2012; Wong et al., 2017). Lysyl oxidase (LOX) is a key element in the crosslinking of collagen and increasing the stiffness of collagen fibers (Egeblad et al., 2010; Kanapathipillai et al., 2012). LOX-activity inhibition has proven successful in preventing ECM remodeling and stiffening (Levental et al., 2009; Barry-Hamilton et al., 2010), which may overcome the deregulated ECM barrier for nanomedicine delivery (Khawar et al., 2015).
It was noteworthy that ECM disruption was seldom used to increase nanomedicine delivery in highly vascularized tumors, which might be the relative lack of ECM in these tumors. However, our group found that fibrin, a kind of ECM component was rich in tumors harboring rich tumor vessels (Dvorak, 1986). The reason might be due to leakage of coagulation factors from circulation to tumor tissues and the high express level of tissue factor on tumor cells, both of which together contribute to local coagulation response in tumor tissues (Dvorak et al., 1985; Liu et al., 2011). As the end product of coagulation response, fibrin is mostly covalently cross-linked in tumor interstitium as an important component of tumor ECM (Dvorak, 1986; Pilch et al., 2006) and is mainly located in the vicinity tumor vessels (Nakahara et al., 2006), a distinct distribution pattern totally different from that of other components of matrix such as collagen and hyaluronic acid, which are always extensively distributed throughout tumor tissues. The special distribution pattern of fibrin was demonstrated to compress tumor vessels nearby, which reduce blood flow and compromise nanomedicine delivery for tumors. Moreover, as fibrin is always covalently cross-linked near tumor vessels, the penetration of nanomedicines in the tumor interstitium could also be hindered. Treatment with rtPA, a clinically widely used drug, at a dose of 25 mg/kg for 2 weeks, could safely and successfully deplete fibrin deposition, reopen compressed tumor vessels, reduce erythrocytes retention in tumor vessels, improve tumor blood flow, and further enhance the accumulation and penetration of nanoparticles (Figure 3; Zhang et al., 2016b).
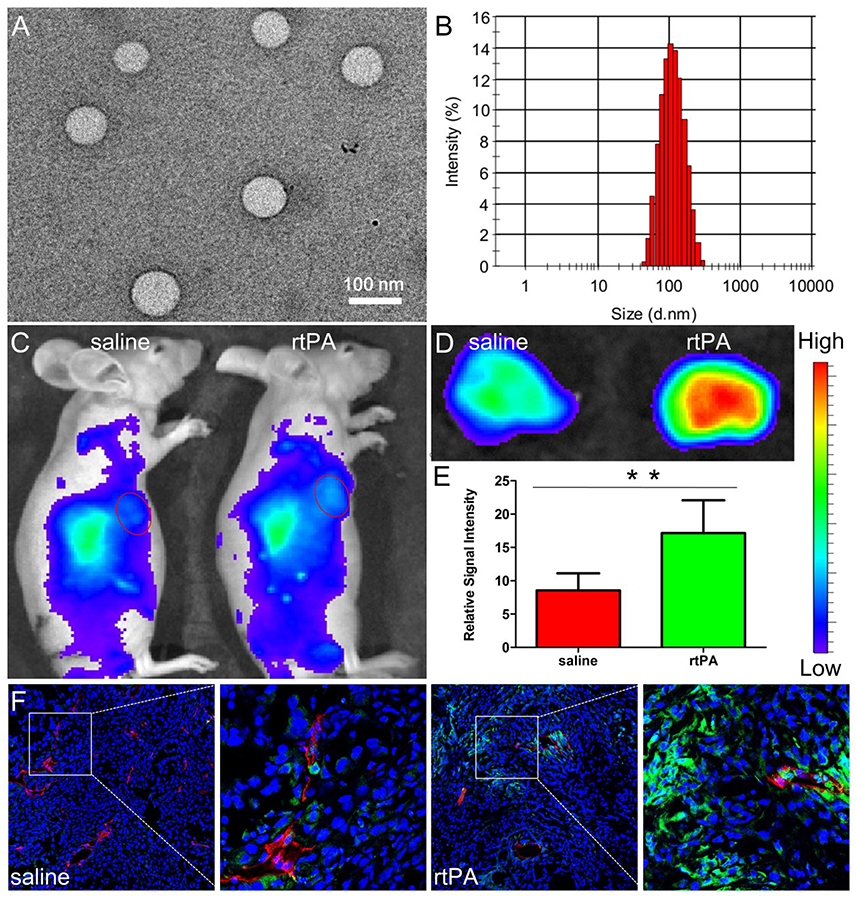
Figure 3. Characterizations of NPs and effects of rtPA treatment on tumor nanoparticle delivery. TEM photograph (A) and size distribution (B) of NPs. Bar: 100 nm. In vivo (C) and ex vivo imaging (D,E) of A549 xenograft-bearing mice treated with 2 weeks of rtPA (25 mg/kg/d) or saline 24 h after the injection of DiR-labeled NPs. **p < 0.01 rtPA vs. saline group. In vivo distribution of NPs in tumor tissues. (F) Original magnification: 120 ×. Reprinted from reference with permission, Copyright Elsevier, 2016.
However, systematic treatment with ECM disruptors such as collagenase, hyaluronidase, and rtPA may cause damage to healthy tissues, and site-specific action might be safer for clinical transformation. Therefore, tumor-specific degradation of ECM was achieved by coating nanomedicines with specific ECM enzymes (Zhou et al., 2016) or PEGlated ECM enzymes (Hingorani et al., 2016). Zhou et al. showed that hyaluronidase modified on the surface of nanoparticles was more effective than free hyaluronidase to help facilitate nanoparticle diffusion and achieved better therapeutic benefits (Zhou et al., 2016). Reports also showed that the dose of hyaluronidase seemed critical, because high doses of hyaluronidase may collapse water-swelling cage structures of hyaluronan rendering the ECM more viscous and less permeable, thereby reducing the diffusion coefficient of nanomedicine (Eikenes et al., 2010). In another research, Bromelain, a crude enzymatic complex purified from pineapple stems that belongs to the peptidase papain family, was decorated to mesoporous silica nanoparticles (Br-MSN), which showed an increased ability to digest and diffuse in tumor ECM in vitro and in vivo (Parodi et al., 2014).
TAF was mainly responsible for ECM production. Resting fibroblasts were transformed to TAF by cancer-derived growth factors such as TGF-β, Hedgehog moiety (Olive et al., 2009; Stylianopoulos et al., 2012), and PDGF (Olson and Hanahan, 2009). This trans-differentiation process of TAF is always characterized by the encoding of ECM-associated components such as collagens, hyaluronan, fibronectin, and MMPs (Cirri and Chiarugi, 2011). Therefore, ECM deregulation can be realized by blocking the growth factors involved in signaling for TAF stimulation. Taking TGF-β-associated signaling as an example, antibodies or other agents capable of blocking TGF-β signaling have proven to inhibit collagen synthesis and enhance nanomedicine delivery in xenograft models. The TGF-β neutralizing antibody ID11 improved tumor tissue delivery of Doxil and obtained better control of mammary carcinoma in xenograft models (Liu et al., 2012). Recently, our group used cyclopamine, a naturally occurring steroidal alkaloid, to inhibit the Hedgehog signaling pathway which contributes a lot to ECM production in pancreatic carcinoma by acting on the Smoothened (SMO) receptor (Heretsch et al., 2010). Compared with the control group, cyclopamine treatment successfully disrupt ECM in in pancreatic cancer, increased functional vessels about 2 folds at a dose of 50 mg/kg for 3 weeks, and significantly improved the accumulation (by a 2.6-fold) and penetration of nanoparticles in tumor tissues (Zhang et al., 2016c).
ECM modulation suits nanomedicine regardless of their size, because ECM modification could reopen compressed vasculatures to improve tumor blood flow and decrease the hindrance of nanomedicine penetrating ECM to reach tumor cells (Chauhan and Jain, 2013). Generally speaking, this strategy most benefits the delivery of larger nanomedicines (Cabral et al., 2011; Jacobetz et al., 2012), as they are more hindered by the ECM (Pluen et al., 2001).
Stromal Cell Reprogramming or Depletion Strategies
Apart from dense ECM, desmoplastic tumors always harbor a high density of stromal cells, among which TAF has been regarded as the major component of tumoral stroma as a potential therapeutic target for nanomedicine delivery. TAF depletion can improve the interstitial transport and distribution of nanomedicine by optimization of the tumor interstitium. Quercetin nanoparticles, capable of suppressing Wnt16 expression could reduce the number of TAF and improve nanomedicine delivery to bladder carcinoma (Hu et al., 2017). Inspired by the close association between cyclooxygenase-2 (COX-2) and tumor-associated angiogenesis, as well as tumor matrix formation, our group explored the tumor microenvironment modulation effect of celecoxib, a special COX-2 inhibitor widely used in clinics. Very interestingly, oral celecoxib treatment at a dose of 200 mg/kg for 2 weeks could successfully normalized the tumor microenvironment, including tumor-associated fibroblast depletion, fibronectin bundle disruption, tumor vessel normalization, and tumor perfusion improvement. Furthermore, it also significantly enhanced the in vivo accumulation and deep penetration of 22-nm micelles rather than 100-nm nanoparticles in tumor tissues and improved the therapeutic efficacy of paclitaxel-loaded micelles in tumor xenograft-bearing mouse models (Zhang et al., 2017b).
Although TAF depletion could undoubtedly modify the tumor microenvironment to improve nanomedicine delivery, recent studies have also indicated that direct TAF depletion might drive tumor metastasis and progression (Özdemir et al., 2014), suggesting a paradoxical effect of TAF depletion. One explanation for this paradoxical effect is that the TAF-depleting strategy runs the risk of eliminating the key element needed for tissue homeostasis (Miao et al., 2015). To avoid this paradox, an alternative approach is to transform activated TAF to a dormant form. Losartan is a antihypertensive agent with anti-fibrosis properties. Research has shown that losartan treatment reprogramed TAF and reduced the collagen and hyaluronan content in desmoplastic models of human breast and pancreatic tumors in mice and improved the distribution and therapeutic effects of systematic administered Doxil (Diop-Frimpong et al., 2011; Chauhan et al., 2013). The antifibrotic effect of losartan was associated with reduced number of activated TAFs and therefore decreased expression of downstream profibrotic factors, such as connective tissue growth factor (CTGF), TGF-β1, and ET-1 (Chauhan et al., 2013), which led to an ongoing clinical trial of losartan combined with chemotherapy in pancreatic tumors (NCT01821729). Vitamin D receptor (VDR) and Wnt-β-Catenin signaling pathway was upregulated in pancreatic stellate cell (PSC), a form of TAF in PDA (Omary et al., 2007). Other studies showed that the VDR ligand and all-trans retinoic acid (ATRA) can act through VDR or Wnt-β-Catenin signaling pathway to reprogram PSC to the quiescent state to reduce the fibrotic content in tumor interstitium (Froeling et al., 2011; Sherman et al., 2014; Chronopoulos et al., 2016), which is promising for the second-wave nanomedicine therapy.
TAMs are prominent components and critical modulators of the tumor microenvironment and contribute to tumor development, invasion, and metastases. Evidences have shown TAMs protect tumor cells from chemotherapy and suppress the immune response of cytotoxic T cells (Jinushi et al., 2011), highlighting the essential of targeting TAMs for cancer treatment. TAM depletion could decrease off-target uptake of nanomedicine by TAMs and thus increase drug delivery to tumor cells. However, the effect of selective TAM depletion on nanomedicine delivery for tumor was seldom reported, and TAM depletion might be a new strategy for tumor microenvironment modulation to enhance tumor nanomedicine delivery.
Future Perspectives
Nanomedicine drug delivery system has attracted extensive attention in the field of tumor treatment. The complex tumor microenvironment including structural abnormalities in tumor vessels, dense ECM structure, and high density of stromal cells as well as physicochemical environment such as elevated IFP pose barriers and compromise the delivery of nanomedicines. In this review, we summarized these barriers and provided strategies to overcome them for improved nanomedicine delivery.
However, some aspects deserve special attention. First, tumors could be generally divided into two types such as those with abundant permeable but uncompressed vessels and tumors with dense ECM and large amount of TAF. More precise classification of tumor microenvironment-like tumor cells might be urgently needed for precise tumor nanomedicine delivery. We need to adopt different strategies according to the characteristics of the tumor microenvironment. For example, tumor vessel normalization is more effective for tumors with abundant, highly permeable but not compressed vessels, but not so appropriate for highly desmoplastic tumors. ECM disruption strategy demonstrates promising prospects to enhance nanomedicine delivery for tumors with abundant ECM even in clinical trials. As for tumors with a certain amount of both vessels and ECM, strategies capable of modulating vessels and ECM should be combined to obtain an optimal effect. In addition, it is important to study new animal models capable of quantitative analysis of parameters involved in the tumor microenvironment, such as IFP to quantify the negative contribution of tumor microenvironment parameters to tumor nanomedicine delivery and help develop corresponding strategies. Furthermore, the tumor microenvironment is too complex and one strategy might have multiple modulation effects on the tumor microenvironment. With respect to ECM disruption, it not only disrupt tumor ECM to optimize interstitial transport of nanomedicines, but also alleviate compression for tumor vessels to improve tumor perfusion to bring more nanomedicines to the tumor site. Another good illustration is the tumor vessel normalization strategy. Tumor vessel normalization could repair tumor vessels structure, which could improve tumor perfusion and reduce IFP to increase the delivery of small nanomedicine around 20–40 nm. However, it inversely compromised the delivery of larger nanomedicine around 100 nm because of the size reduction of endothelial gaps.
Therefore, nanomedicines with suitable qualities should be combined with approaches modulating the tumor microenvironment to overcome nanomedicine transport barriers that the advanced design of nanomedicines cannot conquer. With these in mind, we believe nanomedicines of the future could be far more effective than those available at present.
Author Contributions
ZP and YH conceived the principal idea and revised the manuscripts. BZ and ZP co-wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81773283, 81472757, 81361140344, and 81600175).
References
Allen, T. M., and Cullis, P. R. (2004). Drug delivery systems: entering the mainstream. Science 303, 1818–1822. doi: 10.1126/science.1095833
Atsumi, T., Singh, R., Sabharwal, L., Bando, H., Meng, J., Arima, Y., et al. (2014). Inflammation amplifier, a new paradigm in cancer biology. Cancer Res. 74, 8–14. doi: 10.1158/0008-5472.CAN-13-2322
Bailey, J. M., Swanson, B. J., Hamada, T., Eggers, J. P., Singh, P. K., Caffery, T., et al. (2008). Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin. Cancer Res. 14, 5995–6004. doi: 10.1158/1078-0432.CCR-08-0291
Barry-Hamilton, V., Spangler, R., Marshall, D., McCauley, S., Rodriguez, H. M., Oyasu, M., et al. (2010). Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat. Med. 16, 1009–1017. doi: 10.1038/nm.2208
Boucher, Y., Baxter, L. T., and Jain, R. K. (1990). Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Res. 50, 4478–4484.
Brennen, W. N., Rosen, D. M., Wang, H., Isaacs, J. T., and Denmeade, S. R. (2012). Targeting carcinoma-associated fibroblasts within the tumor stroma with a fibroblast activation protein-activated prodrug. J. Natl. Cancer Inst. 104, 1320–1334. doi: 10.1093/jnci/djs336
Cabral, H., Matsumoto, Y., Mizuno, K., Chen, Q., Murakami, M., Kimura, M., et al. (2011). Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 6, 815–823. doi: 10.1038/nnano.2011.166
Carmeliet, P., and Jain, R. K. (2000). Angiogenesis in cancer and other diseases. Nature 407, 249–257. doi: 10.1038/35025220
Carmeliet, P., and Jain, R. K. (2011). Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307. doi: 10.1038/nature10144
Chakroborty, D., Sarkar, C., Yu, H., Wang, J., Liu, Z., Dasgupta, P. S., et al. (2011). Dopamine stabilizes tumor blood vessels by up-regulating angiopoietin 1 expression in pericytes and Kruppel-like factor-2 expression in tumor endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 108, 20730–20735. doi: 10.1073/pnas.1108696108
Chary, S. R., and Jain, R. K. (1989). Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proc. Natl. Acad. Sci. U.S.A. 86, 5385–5389. doi: 10.1073/pnas.86.14.5385
Chauhan, V. P., and Jain, R. K. (2013). Strategies for advancing cancer nanomedicine. Nat. Mater. 12, 958–962. doi: 10.1038/nmat3792
Chauhan, V. P., Lanning, R. M., Diop-Frimpong, B., Mok, W., Brown, E. B., Padera, T. P., et al. (2009). Multiscale measurements distinguish cellular and interstitial hindrances to diffusion in vivo. Biophys. J. 97, 330–336. doi: 10.1016/j.bpj.2009.03.064
Chauhan, V. P., Martin, J. D., Liu, H., Lacorre, D. A., Jain, S. R., Kozin, S. V., et al. (2013). Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 4:2516. doi: 10.1038/ncomms3516
Chauhan, V. P., Popović, Z., Chen, O., Cui, J., Fukumura, D., Bawendi, M. G., et al. (2011). Fluorescent nanorods and nanospheres for real-time in vivo probing of nanoparticle shape-dependent tumor penetration. Angew. Chem. Int. Ed. 50, 11417–11420. doi: 10.1002/anie.201104449
Chauhan, V. P., Stylianopoulos, T., Martin, J. D., Popovic, Z., Chen, O., Kamoun, W. S., et al. (2012). Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 7, 383–388. doi: 10.1038/nnano.2012.45
Chronopoulos, A., Robinson, B., Sarper, M., Cortes, E., Auernheimer, V., Lachowski, D., et al. (2016). ATRA mechanically reprograms pancreatic stellate cells to suppress matrix remodelling and inhibit cancer cell invasion. Nat. Commun. 7:12630. doi: 10.1038/ncomms12630
Cieslewicz, M., Tang, J., Yu, J. L., Cao, H., Zavaljevski, M., Motoyama, K., et al. (2013). Targeted delivery of proapoptotic peptides to tumor-associated macrophages improves survival. Proc. Natl. Acad. Sci. U.S.A. 110, 15919–15924. doi: 10.1073/pnas.1312197110
Cirri, P., and Chiarugi, P. (2011). Cancer associated fibroblasts: the dark side of the coin. Am. J. Cancer Res. 1, 482–497.
Cui, M., Naczynski, D. J., Zevon, M., Griffith, C. K., Sheihet, L., Poventud-Fuentes, I., et al. (2013). Multifunctional albumin nanoparticles as combination drug carriers for intra-tumoral chemotherapy. Adv. Healthc. Mater. 2, 1236–1245. doi: 10.1002/adhm.201200467
Curnis, F., Sacchi, A., and Corti, A. (2002). Improving chemotherapeutic drug penetration in tumors by vascular targeting and barrier alteration. J. Clin. Invest. 110, 475–482. doi: 10.1172/JCI0215223
Davies Cde, L., Lundstrøm, L. M., Frengen, J., Eikenes, L., Bruland, S. O., Kaalhus, O., et al. (2004). Radiation improves the distribution and uptake of liposomal doxorubicin (caelyx) in human osteosarcoma xenografts. Cancer Res. 64, 547–553. doi: 10.1158/0008-5472.CAN-03-0576
Demers, M., Ho-Tin-Noé, B., Schatzberg, D., Yang, J. J., and Wagner, D. D. (2011). Increased efficacy of breast cancer chemotherapy in thrombocytopenic mice. Cancer Res. 71, 1540–1549. doi: 10.1158/0008-5472.CAN-10-2038
Dequidt, C., Danglot, L., Alberts, P., Galli, T., Choquet, D., and Thoumine, O. (2007). Fast turnover of L1 adhesions in neuronal growth cones involving both surface diffusion and exo/endocytosis of L1 molecules. Mol. Biol. Cell 18, 3131–3143. doi: 10.1091/mbc.E06-12-1101
Diop-Frimpong, B., Chauhan, V. P., Krane, S., Boucher, Y., and Jain, R. K. (2011). Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc. Natl. Acad. Sci. U.S.A. 108, 2909–2914. doi: 10.1073/pnas.1018892108
Dreher, M. R., Liu, W., Michelich, C. R., Dewhirst, M. W., Yuan, F., and Chilkoti, A. (2006). Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J. Natl. Cancer Inst. 98, 335–344. doi: 10.1093/jnci/djj070
Durymanov, M. O., Rosenkranz, A. A., and Sobolev, A. S. (2015). Current approaches for improving intratumoral accumulation and distribution of nanomedicines. Theranostics 5, 1007–1020. doi: 10.7150/thno.11742
Dvorak, H. F. (1986). Tumors: wounds that do not heal: similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 315, 1650–1659.
Dvorak, H. F., Senger, D. R., Dvorak, A. M., Harvey, V. S., and McDonagh, J. (1985). Regulation of extravascular coagulation by microvascular permeability. Science 227, 1059–1061. doi: 10.1126/science.3975602
Eberhard, A., Kahlert, S., Goede, V., Hemmerlein, B., Plate, K. H., and Augustin, H. G. (2000). Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res. 60, 1388–1393.
Egeblad, M., Rasch, M. G., and Weaver, V. M. (2010). Dynamic interplay between the collagen scaffold and tumor evolution. Curr. Opin. Cell Biol. 22, 697–706. doi: 10.1016/j.ceb.2010.08.015
Eikenes, L., Tari, M., Tufto, I., Bruland, O. S., and de Lange Davies, C. (2005). Hyaluronidase induces a transcapillary pressure gradient and improves the distribution and uptake of liposomal doxorubicin (Caelyx) in human osteosarcoma xenografts. Br. J. Cancer 93, 81–88. doi: 10.1038/sj.bjc.6602626
Eikenes, L., Tufto, I., Schnell, E. A., Bjørkøy, A., and De Lange Davies, C. (2010). Effect of collagenase and hyaluronidase on free and anomalous diffusion in multicellular spheroids and xenografts. Anticancer Res. 30, 359–368.
Fang, J., Nakamura, H., and Maeda, H. (2011). The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Delivery Rev. 63, 136–151. doi: 10.1016/j.addr.2010.04.009
Feig, C., Gopinathan, A., Neesse, A., Chan, D. S., Cook, N., and Tuveson, D. A. (2012). The pancreas cancer microenvironment. Clin. Cancer Res. 18, 4266–4276. doi: 10.1158/1078-0432.CCR-11-3114
Frantz, C., Stewart, K. M., and Weaver, V. M. (2010). The extracellular matrix at a glance. J. Cell Sci. 123, 4195–4200. doi: 10.1242/jcs.023820
Frazier, N., Payne, A., de Bever, J., Dillon, C., Panda, A., Subrahmanyam, N., et al. (2016). High intensity focused ultrasound hyperthermia for enhanced macromolecular delivery. J. Control. Release 241, 186–193. doi: 10.1016/j.jconrel.2016.09.030
Froeling, F. E., Feig, C., Chelala, C., Dobson, R., Mein, C. E., Tuveson, D. A., et al. (2011). Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-beta-catenin signaling to slow tumor progression. Gastroenterology 141, 486–497. doi: 10.1053/j.gastro.2011.06.047
Fuks, Z., and Kolesnick, R. (2005). Engaging the vascular component of the tumor response. Cancer Cell 8, 89–91. doi: 10.1016/j.ccr.2005.07.014
Garcia-Barros, M., Paris, F., Cordon-Cardo, C., Lyden, D., Rafii, S., Haimovitz-Friedman, A., et al. (2003). Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 300, 1155–1159. doi: 10.1126/science.1082504
Giustini, A. J., Petryk, A. A., and Hoopes, P. J. (2012). Ionizing radiation increases systemic nanoparticle tumor accumulation. Nanomedicine 8, 818–821. doi: 10.1016/j.nano.2012.05.001
Goel, S., Duda, D. G., Xu, L., Munn, L. L., Boucher, Y., Fukumura, D., et al. (2011). Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 91, 1071–1121. doi: 10.1152/physrev.00038.2010
Goins, B., Phillips, W. T., and Bao, A. (2016). Strategies for improving the intratumoral distribution of liposomal drugs in cancer therapy. Expert Opin. Drug Deliv. 13, 873–889. doi: 10.1517/17425247.2016.1167035
Gong, H., Chao, Y., Xiang, J., Han, X., Song, G., Feng, L., et al. (2016). Hyaluronidase To Enhance Nanoparticle-Based Photodynamic Tumor Therapy. Nano Lett. 16, 2512–2521. doi: 10.1021/acs.nanolett.6b00068
Goodman, T. T., Olive, P. L., and Pun, S. H. (2007). Increased nanoparticle penetration in collagenase-treated multicellular spheroids. Int. J. Nanomedicine 2, 265–274.
Gradishar, W. J., Tjulandin, S., Davidson, N., Shaw, H., Desai, N., Bhar, P., et al. (2005). Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J. Clin. Oncol. 23, 7794–7803. doi: 10.1200/JCO.2005.04.937
Guo, S., Lin, C. M., Xu, Z., Miao, L., Wang, Y., and Huang, L. (2014). Co-Delivery of Cisplatin and Rapamycin For Enhanced Anticancer Therapy Through Synergistic Effects and Microenvironment Modulation. ACS Nano 8, 4996–5009. doi: 10.1021/nn5010815
Han, S. S., Li, Z. Y., Zhu, J. Y., Han, K., Zeng, Z. Y., Hong, W., et al. (2015). Dual-pH Sensitive Charge-Reversal Polypeptide Micelles for Tumor-Triggered Targeting Uptake and Nuclear Drug Delivery. Small 11, 2543–2554. doi: 10.1002/smll.201402865
Hanahan, D., and Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell 144, 646–674. doi: 10.1016/j.cell.2011.02.013
Heretsch, P., Tzagkaroulaki, L., and Giannis, A. (2010). Cyclopamine and hedgehog signaling: chemistry, biology, medical perspectives. Angew. Chem., Int. Ed. 49, 3418–3427. doi: 10.1002/anie.200906967
Hingorani, S. R., Harris, W. P., Beck, J. T., Berdov, B. A., Wagner, S. A., Pshevlotsky, E. M., et al. (2016). Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer. Clin. Cancer Res. 22, 2848–2854. doi: 10.1158/1078-0432.CCR-15-2010
Ho-Tin-Noé, B., Goerge, T., and Wagner, D. D. (2009). Platelets: guardians of tumor vasculature. Cancer Res. 69, 5623–5626. doi: 10.1158/0008-5472.CAN-09-1370
Hu, K., Miao, L., Goodwin, T. J., Li, J., Liu, Q., and Huang, L. (2017). Quercetin remodels the tumor microenvironment to improve the permeation, retention, and antitumor effects of nanoparticles. ACS Nano 11, 4916–4925. doi: 10.1021/acsnano.7b01522
Hu, W., Li, X., Zhang, C., Yang, Y., Jiang, J., and Wu, C. (2016). Tumor-associated macrophages in cancers. Clin. Transl. Oncol. 18, 251–258. doi: 10.1007/s12094-015-1373-0
Huang, S., Shao, K., Liu, Y., Kuang, Y., Li, J., An, S., et al. (2013). Tumor-targeting and microenvironment-responsive smart nanoparticles for combination therapy of antiangiogenesis and apoptosis. ACS Nano 7, 2860–2871. doi: 10.1021/nn400548g
Huang, Y., Yuan, J., Righi, E., Kamoun, W. S., Ancukiewicz, M., Nezivar, J., et al. (2012). Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl. Acad. Sci. U.S.A. 109, 17561–17566. doi: 10.1073/pnas.1215397109
Jacobetz, M. A., Chan, D. S., Neesse, A., Bapiro, T. E., Cook, N., Frese, K. K., et al. (2012). Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 62, 112–120. doi: 10.1136/gutjnl-2012-302529
Jain, R. K. (1987). Transport of molecules in the tumor interstitium: a review. Cancer Res. 47, 3039–3051.
Jain, R. K. (1994). Barriers to drug delivery in solid tumors. Sci. Am. 271, 58–65. doi: 10.1038/scientificamerican0794-58
Jain, R. K. (2003). Molecular regulation of vessel maturation. Nat. Med. 9, 685–693. doi: 10.1038/nm0603-685
Jain, R. K. (2005). Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62. doi: 10.1126/science.1104819
Jain, R. K. (2013). Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J. Clin. Oncol. 31, 2205–2218. doi: 10.1200/JCO.2012.46.3653
Jain, R. K., and Stylianopoulos, T. (2010). Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 7, 653–664. doi: 10.1038/nrclinonc.2010.139
Jain, R. K., Martin, J. D., and Stylianopoulos, T. (2014). The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 16, 321–346. doi: 10.1146/annurev-bioeng-071813-105259
Järveläinen, H., Sainio, A., Koulu, M., Wight, T. N., and Penttinen, R. (2009). Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol. Rev. 61, 198–223. doi: 10.1124/pr.109.001289
Jiang, T., Zhang, B., Shen, S., Tuo, Y., Luo, Z., Hu, Y., et al. (2017). Tumor microenvironment modulation by cyclopamine improved photothermal therapy of biomimetic gold nanorods for pancreatic ductal adenocarcinomas. ACS Appl. Mater. Interfaces 9, 31497–31508. doi: 10.1021/acsami.7b09458
Jinushi, M., Chiba, S., Yoshiyama, H., Masutomi, K., Kinoshita, I., Dosaka-Akita, H., et al. (2011). Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc. Natl. Acad. Sci. U.S.A. 108, 12425–12430. doi: 10.1073/pnas.1106645108
Kanapathipillai, M., Mammoto, A., Mammoto, T., Kang, J. H., Jiang, E., Ghosh, K., et al. (2012). Inhibition of mammary tumor growth using lysyl oxidase-targeting nanoparticles to modify extracellular matrix. Nano Lett. 12, 3213–3217. doi: 10.1021/nl301206p
Kano, M. R., Bae, Y., Iwata, C., Morishita, Y., Yashiro, M., Oka, M., et al. (2007). Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-beta signaling. Proc. Natl. Acad. Sci. U.S.A. 104, 3460–3465. doi: 10.1073/pnas.0611660104
Kato, M., Hattori, Y., Kubo, M., and Maitani, Y. (2012). Collagenase-1 injection improved tumor distribution and gene expression of cationic lipoplex. Int. J. Pharm. 423, 428–434. doi: 10.1016/j.ijpharm.2011.12.015
Khawar, I. A., Kim, J. H., and Kuh, H. J. (2015). Improving drug delivery to solid tumors: priming the tumor microenvironment. J. Control. Release 201, 78–89. doi: 10.1016/j.jconrel.2014.12.018
Kioi, M., Vogel, H., Schultz, G., Hoffman, R. M., Harsh, G. R., and Brown, J. M. (2010). Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J. Clin. Invest. 120, 694–705. doi: 10.1172/JCI40283
Kirtane, A. R., Sadhukha, T., Kim, H., Khanna, V., Koniar, B., and Panyam, J. (2017). Fibrinolytic Enzyme Cotherapy Improves Tumor Perfusion and Therapeutic Efficacy of Anticancer Nanomedicine. Cancer Res. 77, 1465–1475. doi: 10.1158/0008-5472.CAN-16-1646
Kirui, D. K., Celia, C., Molinaro, R., Bansal, S. S., Cosco, D., Fresta, M., et al. (2015). Mild hyperthermia enhances transport of liposomal gemcitabine and improves in vivo therapeutic response. Adv. Healthc. Mater. 4, 1092–1103. doi: 10.1002/adhm.201400738
Kirui, D. K., Koay, E. J., Guo, X., Cristini, V., Shen, H., and Ferrari, M. (2014). Tumor vascular permeabilization using localized mild hyperthermia to improve macromolecule transport. Nanomedicine 10, 1487–1496. doi: 10.1016/j.nano.2013.11.001
Kisucka, J., Butterfield, C. E., Duda, D. G., Eichenberger, S. C., Saffaripour, S., Ware, J., et al. (2006). Platelets and platelet adhesion support angiogenesis while preventing excessive hemorrhage. Proc. Natl. Acad. Sci. U.S.A. 103, 855–860. doi: 10.1073/pnas.0510412103
Kobayashi, H., Reijnders, K., English, S., Yordanov, A. T., Milenic, D. E., Sowers, A. L., et al. (2004). Application of a macromolecular contrast agent for detection of alterations of tumor vessel permeability induced by radiation. Clin. Cancer Res. 10, 7712–7720. doi: 10.1158/1078-0432.CCR-04-1175
Kong, G., Braun, R. D., and Dewhirst, M. W. (2000). Hyperthermia enables tumor-specific nanoparticle delivery: effect of particle size. Cancer Res. 60, 4440–4445.
Kong, G., Braun, R. D., and Dewhirst, M. W. (2001). Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res. 61, 3027–3032.
Leu, A. J., Berk, D. A., Lymboussaki, A., Alitalo, K., and Jain, R. K. (2000). Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res. 60, 4324–4327.
Levental, K. R., Yu, H., Kass, L., Lakins, J. N., Egeblad, M., Erler, J. T., et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906. doi: 10.1016/j.cell.2009.10.027
Levick, J. R. (1987). Flow through interstitium and other fibrous matrices. Q. J. Exp. Physiol. 72, 409–437. doi: 10.1113/expphysiol.1987.sp003085
Li, L., ten Hagen, T. L., Bolkestein, M., Gasselhuber, A., Yatvin, J., van Rhoon, G. C., et al. (2013). Improved intratumoral nanoparticle extravasation and penetration by mild hyperthermia. J. Controlled Release 167, 130–137. doi: 10.1016/j.jconrel.2013.01.026
Li, Q., Hata, A., Kosugi, C., Kataoka, N., and Funaki, M. (2010). The density of extracellular matrix proteins regulates inflammation and insulin signaling in adipocytes. FEBS Lett. 584, 4145–4150. doi: 10.1016/j.febslet.2010.08.033
Li, S., Zhang, Y., Wang, J., Zhao, Y., Ji, T., Zhao, X., et al. (2017). Nanoparticle-mediated local depletion of tumour-associated platelets disrupts vascular barriers and augments drug accumulation in tumours. Nat. Biomed. Eng. 1, 667–679. doi: 10.1038/s41551-017-0115-8
Liu, J., Liao, S., Diop-Frimpong, B., Chen, W., Goel, S., Naxerova, K., et al. (2012). TGF-beta blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc. Natl. Acad. Sci. U.S.A. 109, 16618–16623. doi: 10.1073/pnas.1117610109
Liu, Y., Jiang, P., Capkova, K., Xue, D., Ye, L., Sinha, S. C., et al. (2011). Tissue factor-activated coagulation cascade in the tumor microenvironment is critical for tumor progression and an effective target for therapy. Cancer Res. 71, 6492–6502. doi: 10.1158/0008-5472.CAN-11-1145
Lu, P., Weaver, V. M., and Werb, Z. (2012). The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 196, 395–406. doi: 10.1083/jcb.201102147
Ma, J., Shen, M., Xu, C. S., Sun, Y., Duan, Y. R., and Du, L. F. (2016). Biodegradable double-targeted PTX-mPEG-PLGA nanoparticles for ultrasound contrast enhanced imaging and antitumor therapy in vitro. Oncotarget 7, 80008–80018. doi: 10.18632/oncotarget.13243
Maeda, H. (2012). Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J. Control. Release 164, 138–144. doi: 10.1016/j.jconrel.2012.04.038
Maes, H., Kuchnio, A., Peric, A., Moens, S., Nys, K., De Bock, K., et al. (2014). Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell 26, 190–206. doi: 10.1016/j.ccr.2014.06.025
Magzoub, M., Jin, S., and Verkman, A. S. (2008). Enhanced macromolecule diffusion deep in tumors after enzymatic digestion of extracellular matrix collagen and its associated proteoglycan decorin. FASEB J. 22, 276–284. doi: 10.1096/fj.07-9150com
Martinive, P., De Wever, J., Bouzin, C., Baudelet, C., Sonveaux, P., Grégoire, V., et al. (2006). Reversal of temporal and spatial heterogeneities in tumor perfusion identifies the tumor vascular tone as a tunable variable to improve drug delivery. Mol. Cancer Ther. 5, 1620–1627. doi: 10.1158/1535-7163.MCT-05-0472
McKee, T. D., Grandi, P., Mok, W., Alexandrakis, G., Insin, N., Zimmer, J. P., et al. (2006). Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 66, 2509–2513. doi: 10.1158/0008-5472.CAN-05-2242
Meng, H., Zhao, Y., Dong, J., Xue, M., Lin, Y. S., Ji, Z., et al. (2013). Two-wave nanotherapy to target the stroma and optimize gemcitabine delivery to a human pancreatic cancer model in mice. ACS Nano 7, 10048–10065. doi: 10.1021/nn404083m
Miao, L., and Huang, L. (2015). Exploring the tumor microenvironment with nanoparticles. Cancer Treat. Res. 166, 193–226. doi: 10.1007/978-3-319-16555-4_9
Miao, L., Lin, C. M., and Huang, L. (2015). Stromal barriers and strategies for the delivery of nanomedicine to desmoplastic tumors. J. Control. Release 219, 192–204. doi: 10.1016/j.jconrel.2015.08.017
Miao, L., Newby, J. M., Lin, C. M., Zhang, L., Xu, F., Kim, W. Y., et al. (2016). The binding site barrier elicited by tumor-associated fibroblasts interferes disposition of nanoparticles in stroma-vessel type tumors. ACS Nano 10, 9243–9258. doi: 10.1021/acsnano.6b02776
Milosevic, M., Fyles, A., Hedley, D., and Hill, R. (2004). The human tumor microenvironment: invasive (needle) measurement of oxygen and interstitial fluid pressure. Semin. Radiat. Oncol. 14, 249–258. doi: 10.1016/j.semradonc.2004.04.006
Mocellin, S., Wang, E., and Marincola, F. M. (2001). Cytokines and immune response in the tumor microenvironment. J. Immunother. 24, 392–407. doi: 10.1097/00002371-200109000-00002
Moeller, B. J., Cao, Y., Li, C. Y., and Dewhirst, M. W. (2004). Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell 5, 429–441. doi: 10.1016/S1535-6108(04)00115-1
Mok, W., Boucher, Y., and Jain, R. K. (2007). Matrix metalloproteinases-1 and−8 improve the distribution and efficacy of an oncolytic virus. Cancer Res. 67, 10664–10668. doi: 10.1158/0008-5472.CAN-07-3107
Monsky, W. L., Fukumura, D., Gohongi, T., Ancukiewcz, M., Weich, H. A., Torchilin, V. P., et al. (1999). Augmentation of transvascular transport of macromolecules and nanoparticles in tumors using vascular endothelial growth factor. Cancer Res. 59, 4129–4135.
Nagano, S., Perentes, J. Y., Jain, R. K., and Boucher, Y. (2008). Cancer cell death enhances the penetration and efficacy of oncolytic herpes simplex virus in tumors. Cancer Res. 68, 3795–3802. doi: 10.1158/0008-5472.CAN-07-6193
Nakahara, T., Norberg, S. M., Shalinsky, D. R., Hu-Lowe, D. D., and McDonald, D. M. (2006). Effect of inhibition of vascular endothelial growth factor signaling on distribution of extravasated antibodies in tumors. Cancer Res. 66, 1434–1445. doi: 10.1158/0008-5472.CAN-05-0923
Netti, P. A., Berk, D. A., Swartz, M. A., Grodzinsky, A. J., and Jain, R. K. (2000). Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 60, 2497–2503.
Netti, P. A., Roberge, S., Boucher, Y., Baxter, L. T., and Jain, R. K. (1996). Effect of transvascular fluid exchange on pressure-flow relationship in tumors: a proposed mechanism for tumor blood flow heterogeneity. Microvasc. Res. 52, 27–46. doi: 10.1006/mvre.1996.0041
Nichols, J. W., and Bae, Y. H. (2012). Odyssey of a cancer nanoparticle: from injection site to site of action. Nano Today 7, 606–618. doi: 10.1016/j.nantod.2012.10.010
Nichols, J. W., and Bae, Y. H. (2014). EPR: evidence and fallacy. J. Control. Release 190, 451–464. doi: 10.1016/j.jconrel.2014.03.057
O'Brien, M. E., Wigler, N., Inbar, M., Rosso, R., Grischke, E., Santoro, A., et al. (2004). Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 15, 440–449. doi: 10.1093/annonc/mdh097
Olive, K. P., Jacobetz, M. A., Davidson, C. J., Gopinathan, A., McIntyre, D., Honess, D., et al. (2009). Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461. doi: 10.1126/science.1171362
Olson, P., and Hanahan, D. (2009). Cancer. Breaching the cancer fortress. Science 324, 1400–1401. doi: 10.1126/science.1175940
Omary, M. B., Lugea, A., Lowe, A. W., and Pandol, S. J. (2007). The pancreatic stellate cell: a star on the rise in pancreatic diseases. J. Clin. Invest. 117, 50–59. doi: 10.1172/JCI30082
Özdemir, B. C., Pentcheva-Hoang, T., Carstens, J. L., Zheng, X., Wu, C. C., Simpson, T. R., et al. (2014). Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734. doi: 10.1016/j.ccr.2014.04.005
Padera, T. P., Stoll, B. R., Tooredman, J. B., Capen, D., di Tomaso, E., and Jain, R. K. (2004). Pathology: cancer cells compress intratumour vessels. Nature 427:695. doi: 10.1038/427695a
Park, J. S., Qiao, L., Su, Z. Z., Hinman, D., Willoughby, K., McKinstry, R., et al. (2001). Ionizing radiation modulates vascular endothelial growth factor (VEGF) expression through multiple mitogen activated protein kinase dependent pathways. Oncogene 20, 3266–3280. doi: 10.1038/sj.onc.1204258
Parodi, A., Haddix, S. G., Taghipour, N., Scaria, S., Taraballi, F., Cevenini, A., et al. (2014). Bromelain surface modification increases the diffusion of silica nanoparticles in the tumor extracellular matrix. ACS Nano 8, 9874–9883. doi: 10.1021/nn502807n
Peer, D., Karp, J. M., Hong, S., Farokhzad, O. C., Margalit, R., and Langer, R. (2007). Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2, 751–760. doi: 10.1038/nnano.2007.387
Pilch, J., Brown, D. M., Komatsu, M., Järvinen, T. A., Yang, M., Peters, D., et al. (2006). Peptides selected for binding to clotted plasma accumulate in tumor stroma and wounds. Proc. Natl. Acad. Sci. U.S.A. 103, 2800–2804. doi: 10.1073/pnas.0511219103
Pluen, A., Boucher, Y., Ramanujan, S., McKee, T. D., Gohongi, T., di Tomaso, E., et al. (2001). Role of tumor-host interactions in interstitial diffusion of macromolecules: cranial vs. subcutaneous tumors. Proc. Natl. Acad. Sci. U.S.A. 98, 4628–4633. doi: 10.1073/pnas.081626898
Provenzano, P. P., Cuevas, C., Chang, A. E., Goel, V. K., Von Hoff, D. D., and Hingorani, S. R. (2012). Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429. doi: 10.1016/j.ccr.2012.01.007
Rapoport, N., Nam, K. H., Gupta, R., Gao, Z., Mohan, P., Payne, A., et al. (2015). Ultrasound-mediated tumor imaging and nanotherapy using drug loaded, block copolymer stabilized perfluorocarbon nanoemulsions. J. Control. Release 153, 4–15. doi: 10.1016/j.jconrel.2011.01.022
Rapoport, N., Payne, A., Dillon, C., Shea, J., Scaife, C., and Gupta, R. (2013). Focused ultrasound-mediated drug delivery to pancreatic cancer in a mouse model. J. Ther. Ultrasound 1:11. doi: 10.1186/2050-5736-1-11
Ritchie, J. P., Ramani, V. C., Ren, Y., Naggi, A., Torri, G., Casu, B., et al. (2011). SST0001, a chemically modified heparin, inhibits myeloma growth and angiogenesis via disruption of the heparanase/syndecan-1 axis. Clin. Cancer Res. 17, 1382–1393. doi: 10.1158/1078-0432.CCR-10-2476
Roode, L. E., Brighton, H., Bo, T., Perry, J. L., Parrott, M. C., Kersey, F., et al. (2016). Subtumoral analysis of PRINT nanoparticle distribution reveals targeting variation based on cellular and particle properties. Nanomedicine 12, 1053–1062. doi: 10.1016/j.nano.2015.12.382
Salgaller, M. L. (2003). Technology evaluation: bevacizumab, Genentech/Roche. Curr. Opin. Mol. Ther. 5, 657–667.
Schaefer, L., and Schaefer, R. M. (2010). Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res. 339, 237–246. doi: 10.1007/s00441-009-0821-y
Seki, T., Carroll, F., Illingworth, S., Green, N., Cawood, R., Bachtarzi, H., et al. (2011). Tumour necrosis factor-alpha increases extravasation of virus particles into tumour tissue by activating the Rho A/Rho kinase pathway. J. Control. Release 156, 381–389. doi: 10.1016/j.jconrel.2011.08.022
Seki, T., Fang, J., and Maeda, H. (2009). Enhanced delivery of macromolecular antitumor drugs to tumors by nitroglycerin application. Cancer Sci. 100, 2426–2430. doi: 10.1111/j.1349-7006.2009.01323.x
Sen, A., Capitano, M. L., Spernyak, J. A., Schueckler, J. T., Thomas, S., Singh, A. K., et al. (2011). Mild elevation of body temperature reduces tumor interstitial fluid pressure and hypoxia and enhances efficacy of radiotherapy in murine tumor models. Cancer Res. 71, 3872–3880. doi: 10.1158/0008-5472.CAN-10-4482
Sevick, E. M., and Jain, R. K. (1989a). Geometric resistance to blood flow in solid tumors perfused ex vivo: effects of tumor size and perfusion pressure. Cancer Res. 49, 3506–3512.
Sevick, E. M., and Jain, R. K. (1989b). Viscous resistance to blood flow in solid tumors: effect of hematocrit on intratumor blood viscosity. Cancer Res. 49, 3513–3519.
Sherman, M. H., Yu, R. T., Engle, D. D., Ding, N., Atkins, A. R., Tiriac, H., et al. (2014). Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159, 80–93. doi: 10.1016/j.cell.2014.08.007
Shiga, K., Hara, M., Nagasaki, T., Sato, T., Takahashi, H., and Takeyama, H. (2015). Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers (Basel). 7, 2443–2458. doi: 10.3390/cancers7040902
Smith, N. R., Baker, D., Farren, M., Pommier, A., Swann, R., Wang, X., et al. (2013). Tumor stromal architecture can define the intrinsic tumor response to VEGF-targeted therapy. Clin. Cancer Res. 19, 6943–6956. doi: 10.1158/1078-0432.CCR-13-1637
Sonveaux, P., Dessy, C., Martinive, P., Havaux, X., Jordan, B. F., Gallez, B., et al. (2004). Endothelin-1 is a critical mediator of myogenic tone in tumor arterioles: implications for cancer treatment. Cancer Res. 64, 3209–3214. doi: 10.1158/0008-5472.CAN-03-1291
Stapleton, S., Jaffray, D., and Milosevic, M. (2016). Radiation effects on the tumor microenvironment: implications for nanomedicine delivery. Adv. Drug Deliv. Rev. 109, 119–130. doi: 10.1016/j.addr.2016.05.021
Stylianopoulos, T., and Jain, R. K. (2013). Combining two strategies to improve perfusion and drug delivery in solid tumors. Proc. Natl. Acad. Sci. U.S.A. 110, 18632–18637. doi: 10.1073/pnas.1318415110
Stylianopoulos, T., Martin, J. D., Chauhan, V. P., Jain, S. R., Diop-Frimpong, B., Bardeesy, N., et al. (2012). Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc. Natl. Acad. Sci. U.S.A. 109, 15101–15108. doi: 10.1073/pnas.1213353109
Stylianopoulos, T., Yeckel, A., Derby, J. J., Luo, X. J., Shephard, M. S., Sander, E. A., et al. (2008). Permeability calculations in three-dimensional isotropic and oriented fiber networks. Phys. Fluids (1994). 20, 123601. doi: 10.1063/1.3021477
Sun, C., Jain, R. K., and Munn, L. L. (2007). Non-uniform plasma leakage affects local hematocrit and blood flow: implications for inflammation and tumor perfusion. Ann. Biomed. Eng. 35, 2121–2129. doi: 10.1007/s10439-007-9377-8
Tanaka, S., Akaike, T., Wu, J., Fang, J., Sawa, T., Ogawa, M., et al. (2003). Modulation of tumor-selective vascular blood flow and extravasation by the stable prostaglandin 12 analogue beraprost sodium. J. Drug Target. 11, 45–52. doi: 10.1080/1061186031000086072
Tang, L., Yang, X., Yin, Q., Cai, K., Wang, H., Chaudhury, I., et al. (2014). Investigating the optimal size of anticancer nanomedicine. Proc. Natl. Acad. Sci. U.S.A. 111, 15344–15349. doi: 10.1073/pnas.1411499111
Thakkar, D., Gupta, R., Monson, K., and Rapoport, N. (2013). Effect of ultrasound on the permeability of vascular wall to nano-emulsion droplets. Ultrasound Med. Biol. 39, 1804–1811. doi: 10.1016/j.ultrasmedbio.2013.04.008
Tong, R. T., Boucher, Y., Kozin, S. V., Winkler, F., Hicklin, D. J., and Jain, R. K. (2004). Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 64, 3731–3736. doi: 10.1158/0008-5472.CAN-04-0074
Tong, R., Chiang, H. H., and Kohane, D. S. (2013). Photoswitchable nanoparticles for in vivo cancer chemotherapy. Proc. Natl. Acad. Sci. U.S.A. 110, 19048–19053. doi: 10.1073/pnas.1315336110
Turley, R. S., Fontanella, A. N., Padussis, J. C., Toshimitsu, H., Tokuhisa, Y., Cho, E. H., et al. (2012). Bevacizumab-induced alterations in vascular permeability and drug delivery: a novel approach to augment regional chemotherapy for in-transit melanoma. Clin. Cancer Res. 18, 3328–3339. doi: 10.1158/1078-0432.CCR-11-3000
von Maltzahn, G., Park, J. H., Lin, K. Y., Singh, N., Schwöppe, C., Mesters, R., et al. (2011). Nanoparticles that communicate in vivo to amplify tumour targeting. Nat. Mater. 10, 545–552. doi: 10.1038/nmat3049
Wang, X., Li, H., Liu, X., Tian, Y., Guo, H., Jiang, T., et al. (2017). Enhanced photothermal therapy of biomimetic polypyrrole nanoparticles through improving blood flow perfusion. Biomaterials 143, 130–141. doi: 10.1016/j.biomaterials.2017.08.004
Winslow, T. B., Eranki, A., Ullas, S., Singh, A. K., Repasky, E. A., and Sen, A. (2015). A pilot study of the effects of mild systemic heating on human head and neck tumour xenografts: analysis of tumour perfusion, interstitial fluid pressure, hypoxia and efficacy of radiation therapy. Int. J. Hyperthermia 31, 693–701. doi: 10.3109/02656736.2015.1037800
Wong, C., Stylianopoulos, T., Cui, J., Martin, J., Chauhan, V. P., Jiang, W., et al. (2011). Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl. Acad. Sci. U.S.A. 108, 2426–2431. doi: 10.1073/pnas.1018382108
Wong, K. M., Horton, K. J., Coveler, A. L., Hingorani, S. R., and Harris, W. P. (2017). Targeting the tumor stroma: the biology and clinical development of pegylated recombinant human hyaluronidase (PEGPH20). Curr. Oncol. Rep. 19:47. doi: 10.1007/s11912-017-0608-3
Yoon, Y. I., Kwon, Y. S., Cho, H. S., Heo, S. H., Park, K. S., Park, S. G., et al. (2014). Ultrasound-mediated gene and drug delivery using a microbubble-liposome particle system. Theranostics 4, 1133–1144. doi: 10.7150/thno.9945
Yuan, F., Leunig, M., Huang, S. K., Berk, D. A., Papahadjopoulos, D., and Jain, R. K. (1994a). Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 54, 3352–3356.
Yuan, F., Salehi, H. A., Boucher, Y., Vasthare, U. S., Tuma, R. F., and Jain, R. K. (1994b). Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Res. 54, 4564–4568.
Zhang, B., Jiang, T., Ling, L., Cao, Z., Zhao, J., Tuo, Y., et al. (2016a). Enhanced Antitumor Activity of EGFP-EGF1-Conjugated Nanoparticles by a Multitargeting Strategy. ACS Appl. Mater. Interfaces 8, 8918–8927. doi: 10.1021/acsami.6b00036
Zhang, B., Jiang, T., She, X., Shen, S., Wang, S., Deng, J., et al. (2016b). Fibrin degradation by rtPA enhances the delivery of nanotherapeutics to A549 tumors in nude mice. Biomaterials 96, 63–71. doi: 10.1016/j.biomaterials.2016.04.015
Zhang, B., Jiang, T., Shen, S., She, X., Tuo, Y., Hu, Y., et al. (2016c). Cyclopamine disrupts tumor extracellular matrix and improves the distribution and efficacy of nanotherapeutics in pancreatic cancer. Biomaterials 103, 12–21. doi: 10.1016/j.biomaterials.2016.06.048
Zhang, B., Jiang, T., Tuo, Y., Jin, K., Luo, Z., Shi, W., et al. (2017a). Captopril improves tumor nanomedicine delivery by increasing tumor blood perfusion and enlarging endothelial gaps in tumor blood vessels. Cancer Lett. 410, 12–19. doi: 10.1016/j.canlet.2017.09.007
Zhang, B., Jin, K., Jiang, T., Wang, L., Shen, S., Luo, Z., et al. (2017b). Celecoxib normalizes the tumor microenvironment and enhances small nanotherapeutics delivery to A549 tumors in nude mice. Sci. Rep. 7:10071. doi: 10.1038/s41598-017-09520-7
Zhang, B., Shen, S., Liao, Z., Shi, W., Wang, Y., Zhao, J., et al. (2014a). Targeting fibronectins of glioma extracellular matrix by CLT1 peptide-conjugated nanoparticles. Biomaterials 35, 4088–4098. doi: 10.1016/j.biomaterials.2014.01.046
Zhang, B., Shi, W., Jiang, T., Wang, L., Mei, H., Lu, H., et al. (2016d). Optimization of the tumor microenvironment and nanomedicine properties simultaneously to improve tumor therapy. Oncotarget 7, 62607–62618. doi: 10.18632/oncotarget.11546
Zhang, B., Wang, H., Liao, Z., Wang, Y., Hu, Y., Yang, J., et al. (2014b). EGFP-EGF1-conjugated nanoparticles for targeting both neovascular and glioma cells in therapy of brain glioma. Biomaterials 35, 4133–4145. doi: 10.1016/j.biomaterials.2014.01.071
Zhang, B., Zhang, Y., Liao, Z., Jiang, T., Zhao, J., Tuo, Y., et al. (2015). UPA-sensitive ACPP-conjugated nanoparticles for multi-targeting therapy of brain glioma. Biomaterials 36, 98–109. doi: 10.1016/j.biomaterials.2014.09.008
Zhang, J., Miao, L., Guo, S., Zhang, Y., Zhang, L., Satterlee, A., et al. (2014). Synergistic anti-tumor effects of combined gemcitabine and cisplatin nanoparticles in a stroma-rich bladder carcinoma model. J. Control Release 182, 90–96. doi: 10.1016/j.jconrel.2014.03.016
Zhou, H., Fan, Z., Deng, J., Lemons, P. K., Arhontoulis, D. C., Bowne, W. B., et al. (2016). Hyaluronidase embedded in nanocarrier PEG shell for enhanced tumor penetration and highly efficient antitumor efficacy. Nano Lett. 16, 3268–3277. doi: 10.1021/acs.nanolett.6b00820
Keywords: tumor microenvironment, nanomedicine, tumor nanomedicine delivery, interstitial fluid pressure, tumor perfusion, extracellular matrix
Citation: Zhang B, Hu Y and Pang Z (2017) Modulating the Tumor Microenvironment to Enhance Tumor Nanomedicine Delivery. Front. Pharmacol. 8:952. doi: 10.3389/fphar.2017.00952
Received: 03 November 2017; Accepted: 15 December 2017;
Published: 22 December 2017.
Edited by:
Susan Hua, University of Newcastle, AustraliaReviewed by:
Saraswati Sukumar, School of Medicine, Johns Hopkins University, United StatesJai Prakash, University of Twente, Netherlands
Copyright © 2017 Zhang, Hu and Pang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Hu, ZHJfaHV5dUAxMjYuY29t
Zhiqing Pang, enFwYW5nQGZ1ZGFuLmVkdS5jbg==
 Bo Zhang1,2
Bo Zhang1,2 Zhiqing Pang
Zhiqing Pang